1. Introduction
In 1893, Alfred Werner described the structure of octahedral transition metal complexes and provided the basis for assigning coordination number and oxidation state to what were then known as double salts.1 This term arose from the observation that transition metal ions appeared to form bonds not only to anion ligands with which to neutralize their charge, but also to additional species which seemed unnecessary since neutrality was already achieved. This work was the origin of modern coordination chemistry and greatly expanded the field of inorganic chemistry. By understanding the preferred coordination geometry about a metal ion, rational synthetic methodologies to install specific ligands was now possible. The past 119 years have witnessed a tremendous growth in coordination chemistry, leading to advances in our understanding of the synthesis, structure and reactivity of novel complexes and materials from simple metal-ligand complexes to organometallic catalysts and extended inorganic polymers. In recent decades, two new branches of coordination chemistry have emerged—metal-organic frameworks (MOFs) and supramolecular coordination complexes (SCCs). The former is comprised of infinite networks of metal centers or inorganic clusters bridged by simple organic linkers through metal-ligand coordination bonds. The latter encompasses discrete systems in which carefully-selected metal centers undergo self-assembly with ligands containing multiple binding sites oriented with specific angularity to generate a finite supramolecular complex. On the most basic level, both SCCs and MOFs share the design of metal nodes linked by organic ligands and such constructs can be broadly defined as metal-organic materials (MOMs).
1.1 Supramolecular Coordination-Driven Self-Assembly
Supramolecular polygons and polyhedra based on metal-ligand coordination emerged in part as a result of studies in the 1960s by Pedersen and coworkers which demonstrated that complementary small molecules could exhibit intermolecular recognition via noncovalent interactions.2 Early molecular-recognition systems were simple: crown ethers could by synthesized and selectively accommodate simple guest ions. New host/guest systems quickly followed, leading to more complex ensembles such as cryptand and spherand hosts with small molecule guests, pioneered by Lehn3 and Cram.4 The non-covalent interactions governing host/guest formation were then applied to construct large entities from molecular components. These constructs, held together by intramolecular hydrogen bonding, π-π interactions, van der Waals forces and other weak interactions were dubbed “supermolecules.” Supramolecular chemistry is a broad field, owing to the vast number of diverse structures which can be formed by using a variety of noncovalent intermolecular interactions. Notable examples include biologically relevant enzyme mimics,5 molecular devices including light harvesters,6 sensors,7 wires8 and rectifiers,9 liquid crystals,10 molecular flasks11 and more.12 One subset of this chemistry is the self-assembly of coordination compounds.
Supramolecular coordination complexes are discrete constructs, typically obtained by mixing soluble metal and ligand precursors which spontaneously form metal-ligand bonds to generate a single thermodynamically-favored product. Since coordination bonds are the impetus for formation, this process is often referred to as coordination-driven self-assembly. A pioneering example of macrocycles formed from coordination bonds was given by Verkade and coworkers in 1983.13 By mixing a diphosphine bridging ligand, P(OCH2)3P,14 with either Cr, Mo or W carbonyl precursors, 20-membered tetranuclear rings were obtained (Figure 1). Exploiting the directionality of transition metal coordination spheres and rigid organic ligands was largely overlooked until the early 1990s when Fujita15 and Stang16 provided examples of rationally-designed supramolecular squares with Pd and Pt. Over the next two decades, various methodologies for the rational design of polygons, polyhedra and prisms were developed, led by the groups of Stang,17 Raymond,11c,d,18 Fujita,11a,19 Mirkin20 and Cotton.21 From squares came the rationale to generate triangles,22 rectangles23 and higher polygons,24 as well as extensions to 3D systems including tetrahedra,25 cubes,26 octahedra,27 cuboctahedra,28 dodecahedra29 and others.30 As a subset of supramolecular chemistry, SCCs have been employed in a range of applications including those listed above.
Figure 1.
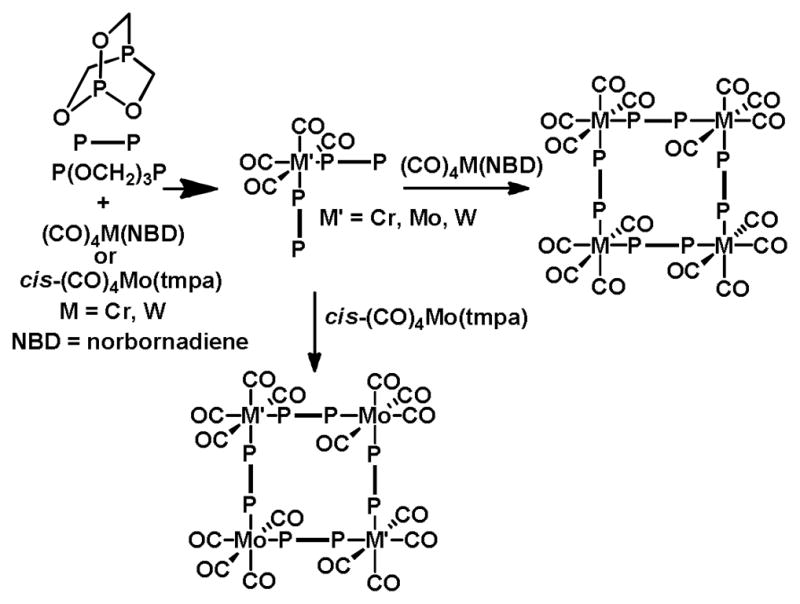
An early example of coordination-driven self-assembly. Square metallacycles may be formed upon combination of a metal precursor with a linear ligand with two bridgehead phosphorus atoms. An intermediate monomer species is isolable, permitting the construction of both homo and hetero-tetranuclear squares.13
1.2 Coordination Polymers and Metal-Organic Frameworks
Like SCCs, metal-organic frameworks owe their development to coordination chemistry. In a broad sense, MOFs have a rich history beginning with the development of coordination polymers.31 Coordination polymers are a subset of inorganic polymers which contain metal-ligand bonds as the primary design feature. The term coordination polymer has been traced back to a 1964 review by J. C. Bailar which was concerned with polymeric structures comprised of metals and ligands. The motivation behind early interest in inorganic and coordination polymers was application-driven from the very beginning. Inorganic materials were recognized to potentially withstand thermal and oxidative stress better than their organic counterparts.31 The induction time between the first coordination polymers and modern MOF chemistry is much greater. The synthetic pigment commonly known as Prussian Blue has been in use since the early 1700s. Its structure was determined by X-ray diffraction in 1977 to reveal a mixed-valent Fe(II)/Fe(III) network with Fe(II)-carbon distances of 1.92 Å and Fe(III)-nitrogen distances of 2.03 Å (Figure 2).32
Figure 2.
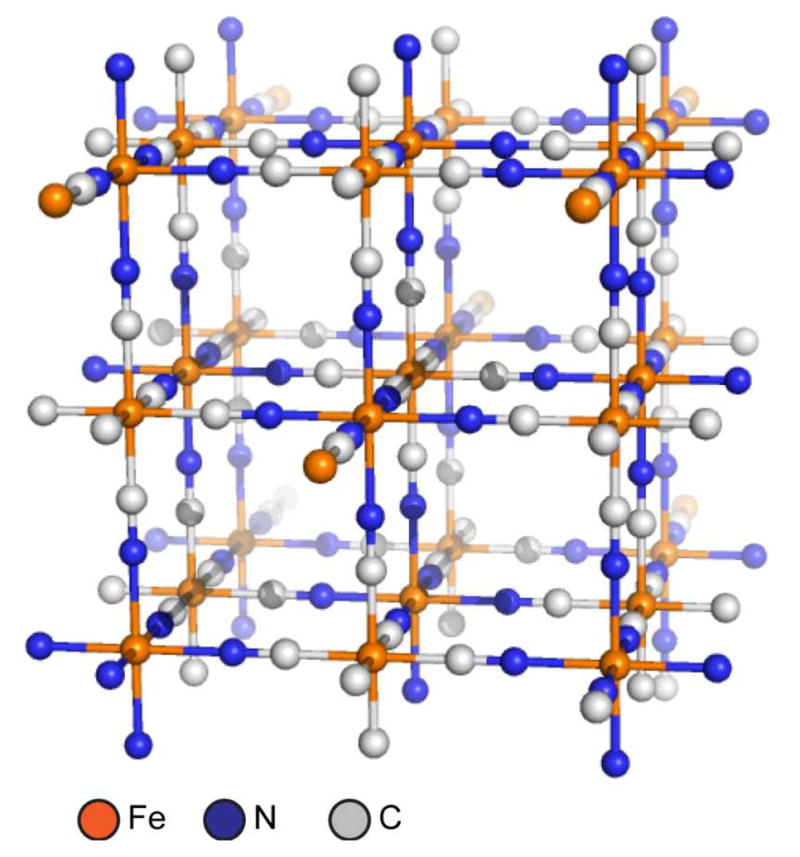
Idealized structure of Prussian Blue, the first synthetic coordination polymer. Alternating octahedral sites of Fe(II) and Fe(III) ions are bridged by cyanide ligands to generate a cubic 3D array.32
In 1897, Hoffman and coworkers discovered that the addition of benzene into a solution of Ni(CN)2 in ammonia furnished a coordination network but early work on these polymers was hindered by a lack of structural characterization techniques. In fact, the structure of the Hoffman complex was not fully understood until X-ray studies by Powell and coworkers over half a century after the initial synthesis was reported.34 The material, Ni(CN)2(NH3)·C6H6 shared similar structural elements with Prussian Blue; the extended structure consisted of metal nodes bridged by cyanide ligands. However, the Ni(CN)2 network did not extend into three dimensions. Instead, parallel 2D sheets with ammine-capped nickel sites were layered to give benzene-containing channels between independent arrays. These exemplary coordination polymers provided the motivation to explore alternative bridging ligands, guests, and capping moieties. As characterization techniques became more refined, efforts to define, classify, and synthesize such materials accelerated throughout the 1960s, cementing coordination polymers as a distinct area of research.35 Early MOMs consisted of Hoffman type topologies in which the metal centers, capping ligands and guests were systematically swapped with similar species, for example by replacing the original benzene guests with biphenyl (Figure 3).33 From the late 1960s into the 1980s, a variety of such species were reported. The final exodus from these early materials occurred when the cyanide ligand was replaced with alternative organic ligands which rapidly led to the discovery of a myriad of new MOMs.
Figure 3.
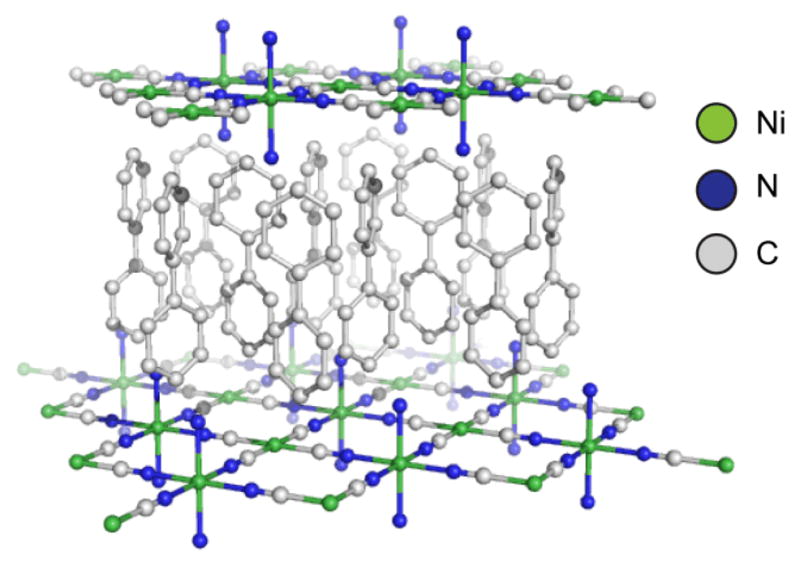
The structure of a Hoffman-type complex, Ni(CN)2(NH3)(C12H10)2. Hydrogen atoms omitted for clarity.33
A hallmark discovery was made by Robson and coworkers in 1989, in which Cu(I) centers were linked with 4,4′,4″,4‴-tetracyanotetraphenylmethane (tctpm; Figure 4).36 This tetratopic organic donor adopted a tetrahedral geometry due to the central carbon atom. The four nitrogen atoms each coordinate to a unique Cu(I) center, which are themselves coordinated to three other tctpm ligands. The extended network adopts a diamond-like topology comprised of repeating adamantanoid subunits. This was a key material in that it set the foundation for the use of tunable organic ligands in coordination polymers; such materials were not limited to cyanide bridges.
Figure 4.
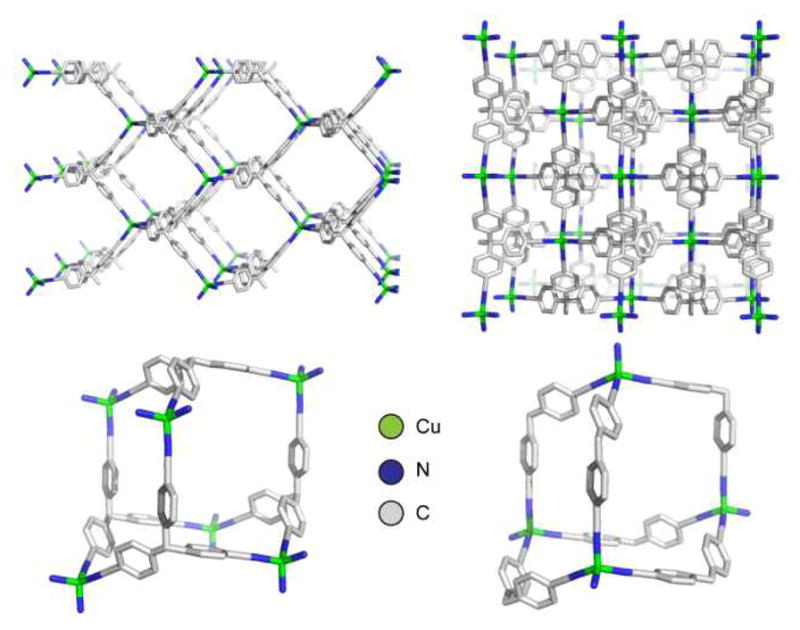
The slow evaporation of a solution of a tetranitrile ligand with a Cu(I) precursor deposits a coordination polymer containing tetrahedral metal nodes with tetrahedral tetratopic spacers. Two views are shown (top) with hydrogen atoms omitted for clarity revealing a diamond-like network comprised of repeating adamantanoid units (bottom).36
Soon thereafter, ligand diversity continued to increase, from cyanide, to organic nitriles, to pyridyl-based donors. Of the pyridyl ligands, 4,4′-bipyridine (4,4′-bpy) is the most iconic. The early adoption of 4,4′-bpy as a building block for both MOFs and SCCs can be attributed to its suitability as a ligand for a variety of metals, its structural rigidity as a linear, ditopic donor, and its ready availability from a number of commercial sources. Four years after demonstrating its use in forming a supramolecular square, Fujita and coworkers utilized 4,4′-bpy in the assembly of square grids.37 Treatment of Cd(NO3)2 with 4,4′-bipy in a 1:2 ratio in aqueous ethanol led to the deposition of colorless crystals characterized as [Cd(4,4′-bpy)2(NO3)2] (Figure 5). Motivated by materials utilizing the ditopic 4,4′-bpy, the field then moved towards polytopic donors with varying geometries. In the mid 1990s, the exploration of carboxylate-based materials garnered significant interest, building upon the milestone discovery of permanently microporous pyridyl-based MOMs by Kitagawa and coworkers38 with an example of carboxylate-based porosity by Yaghi and coworkers.39 These coordination polymers distinguished themselves by possessing reversible gas adsorption and adopted the name metal-organic frameworks. The term metal-organic framework is technically suitable for any extended array comprised of metal nodes (either mono- or polynuclear), however it’s not uncommon for the term MOF to be reserved for materials which are characterized as micro-crystalline, well-defined materials containing polynuclear clusters and often showing permanent porosity. The clusters themselves are stabilized by bridging ligands and the networks of clusters are supported by the strong covalent bonds found within the organic components of the MOFs40
Figure 5.
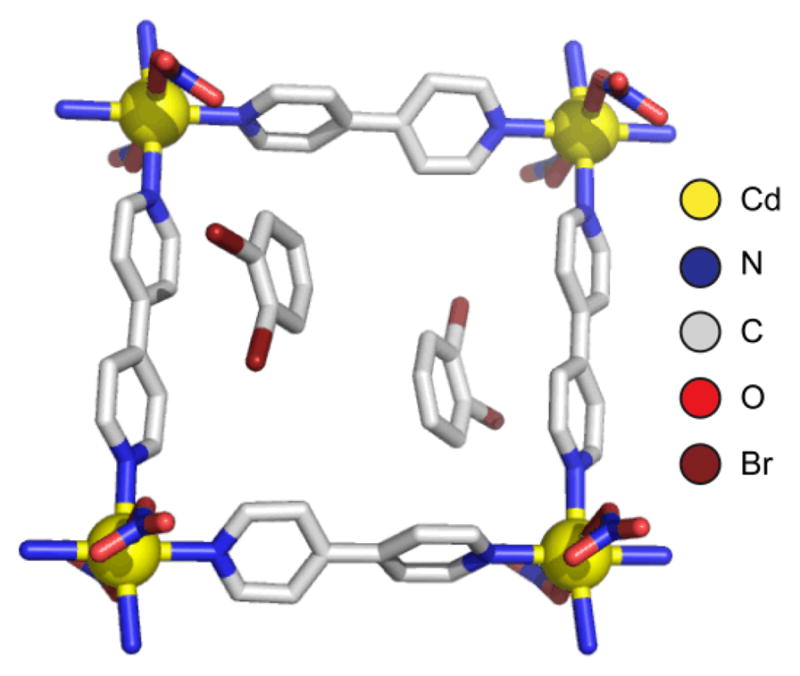
The structure of [Cd(4,4′-bpy)2(NO3)2], a square lattice with channels capable of accommodating guests such as o-dibromobenzene. Hydrogen atoms omitted for clarity. 37
Modern MOF synthesis is driven in part by a goal to generate extremely porous materials often accompanied by very large internal surface areas. This is accomplished by a careful selection of molecular precursors and reaction conditions which dictate the thermodynamically-favored architecture. In addition to ongoing synthetically-focused research, a major hallmark of MOF chemistry results from the properties afforded by the aforementioned surface area and porosities. MOFs are increasingly applied towards applications which require efficient uptake of substrates, such as energy storage41, chemical purification,42 sensing,43 and more.44
1.3 Scope of this Review
The coordination bond between a metal center and organic ligands is the fundamental theme underpinning the synthesis of both MOFs and SCCs. Because of this common element, there are many similarities including the specific building blocks used, the structural topologies and the possible applications. However, the two fields have also diverged due to the differences—sometimes subtle, other times straightforward—associated with targeting infinite arrays versus discrete molecular systems. While some groups have provided elegant examples that bridge the gap between MOFs and SCCS, sometimes even using the same molecular building blocks, a wider appreciation of the relationship between the two fields would be beneficial to the chemical community at large. This comprehensive review provides a discussion of the commonality and differences of SCCs and MOFs, in order to present a linkage between these two important fields and share concepts and applications which may be useful to both. To achieve this, we compare four different aspects of a collection of reported SCCs and MOFs: design methodologies, synthetic conditions, post-assembly modifications and applications. It is important to note that both SCCs and MOFs have grown to contain a gigantic body of work. Independent reviews detailing SCCs or MOFs are themselves typically limited to one area, be it a particular class of building blocks, design methodology or application. As such, this review does not attempt to exhaustively present all known MOFs and SCCs, which would require several hundred pages and many thousands of references, resulting in little practical value. Instead, representative examples are given in each section which best highlight the relationship of the two fields, from pioneering work to contemporary discoveries. The reader will also be directed to a number of relevant manuscripts and reviews which will guide further investigations into a particular aspect of SCC or MOF chemistry which may be of interest.
2. Design Methodologies
2.1 Classifying Networks
Every MOF and SCC necessarily contains at least two components, a ligand and a metal center. While the complexity of these units can vary widely from one assembly to the next, they can often be simplified conceptually. Important fundamental characteristics of a given material include the number of binding sites available on the metal or metal clusters, the relative orientation of these binding sites, the number of Lewis-basic sites on a ligand, the modes of coordination of these sites, and the relative angularity of these sites. Modern researchers often think of MOFs and SCCs in terms of their constituent building blocks. This methodology has origins as early as the mid-1950s, during which time A. F. Wells developed a description of the structure of inorganic polymers by reducing the components to nodes and spacers.45
By viewing an extended network as an array of nodes connected by linear spacers it became possible to assign a given structure to a specific network, or net. Since this method requires that all spacers are linear in nature, all divergent sites become nodes, including sites on organic ligands which break linearity. For example, in Robson’s coordination polymer introduced, the central carbon of the tctpm ligand would be described as a 4-conneted node with the 4-cyanophenyl fragments acting as linear spacers to a second type of node, the Cu centers (Figure 6).
Figure 6.
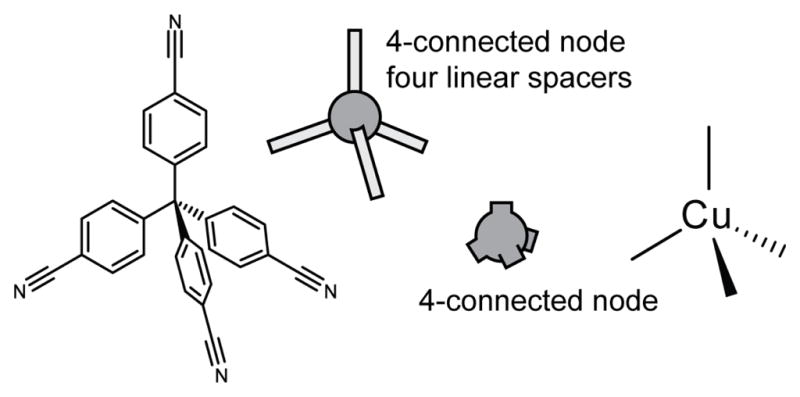
The Cu coordination network of Robson and coworkers36 can be broken down to nodes and spacers. The tetrahedral organic ligand is deconstructed to a four-connected node and linear spacers while the Cu centers represent a second four-connected node.
Since Well’s introductory papers in the 50s, the net approach has been widely applied and reviewed.46 There are a few common ways to name nets which are based on a few parameters: the connectivity of the nodes, the number of nodes in the smallest loop containing a spacer-node-spacer unit, and the size of these loops (sometimes referred to as shortest circuits). When a net possesses both one type of node and identically sized loops, it is dubbed a Platonic uniform net. Wells introduced the (n,p) nomenclature to describe nets of this type, wherein n is the number of nodes in the smallest loop and p is the connectivity of the nodes. For example, Figure 7(a) shows a honeycomb lattice which is comprised of three-connected nodes with six nodes comprising the smallest loop containing a unique spacer-node-spacer motif. Since all nodes are the same and all shortest circuits are identical, this is a Platonic uniform net designated (6,3). Nets (b) and (c) are not Platonic uniform. This is readily apparent in net (c) since it contains both six-connected and three-connected nodes. Net (b) contains only one type of four-connected node, but there are two unique loops. If the spacer-node-spacer unit contains cis-spacers, the loop contains four nodes. However, taking a trans-spacer orientation results in a six-node shortest loop. These unique loops appear as outlines in Figure 7, with the cis or trans spacer motifs highlighted in red. Since this is not technically a Platonic uniform net due to the two different loops, the common designation as a (4,4) net is not correct and a more detailed naming scheme is needed.
Figure 7.
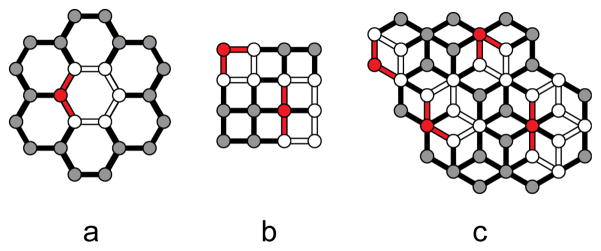
Inorganic polymers may be classified as nets consisting of nodes and linear spacers. For these three examples of 2-periodic nets, the shortest circuits containing unique spacer-node-spacer motifs are outlined, with the motifs highlighted in red.
A second naming system uses Schläfli symbols and can also be used for non-Platonic uniform nets. This system also indicates the number of nodes in a shortest loop and then indicates the number of times this loop occurs as a superscripted number. For example, in Figure 7(b), the cis-oriented spacer-node-spacer motif repeats four times at each node, giving rise to four independent four-node loops. The trans motif occurs twice for each node, one set oriented vertically and the other horizontally. This results in the Schläfli symbol 44.62. When the topology increases in complexity with multiple types of nodes, for example in Figure 7(c), the Schläfli symbol still indicates all unique shortest circuits with the unique nodes separated by parenthesis, such as (43)2(46.66.83). Though it can be applied to any net, the Schläfli notation is still limited in that two different geometric nets can still have the same symbol. In addition, it can be very difficult to convert from a complicated Schläfli symbol to a corresponding topology. Because of the disconnect between the symbols and a spacial description, many nets are simply named in analogy to well-known topologies.
Schläfli symbols also appropriately describe discrete polyhedra and thus have relevance to SCCs in addition to extended networks. In fact, the symbols originate in the realm of geometry rather than chemistry and took their name from Ludwig Schläfli, a mathematician who studied multidimensional geometry.47 When describing polygons, the notation indicates regular shapes as {n} where n is the number of edges. Regular polyhedra are represented by {p,q} where p is the number of sides of the polygons acting as faces and q is the number of faces meeting at each vertex. For example, {4} represents a square, whereas {4,3} represents a cube, which consists of vertices at which three squares meet. While used even less in the description of SCCs than for MOFs, Schläfli symbols are nonetheless intriguing in their ability to mathematically describe both infinite tessellations (i.e. extended networks) as well as discrete polytopes. It has long been known from a geometric standpoint that increasing the dimensionality of a vertex figure requires a change to the angle defect. That is, if the angles of a vertex do not sum to 360° or 180°, the figure folds around itself to close around itself. This is exactly what occurs when moving from an infinite square lattice (2D) to a cube (3D). Chemists forming MOFs and SCCs exploit this by designing ligands and metal nodes with specific angularity, oftentimes without realizing the relevance to multidimensional geometry. It remains to be seen whether the mathematical tools utilized in exploring polytopes, such as Schläfli symbols, will be useful in the design of new MOMs.
It is important to note that the classification of a network concerns only topology. The methods described above are naming tools to sort existing frameworks into common groups. As a topological description, two very different geometric arrays will sometimes share a net type and a single array can be described multiple ways, depending on how one defines the nodes and spacers (Figure 8).35 A honeycomb lattice contains the same shortest circuits as an array containing offset rectangular loops. It may be useful to recall that topology is the study of properties that are preserved upon deformation of a system. Deformation of a net does not affect the connectivity of the nodes, nor the number of components in the shortest circuits. For example, compressing the nodes in a hexagonal grid so that offset rectangular loops are formed does not result in any topological change and such nets thus share the same name (see the (6,3) nets in Figure 8). Likewise, shearing a square lattice into one containing repeating rhomboids does not change the topological description despite the obvious geometric differences in these nets (Figure 8). This is, of course, problematic when used as a synthetic tool. Since the properties and potential applications of a material are intimately linked to its size and shape, especially for supramolecular applications which so often rely on host/guest phenomena, topology is too general to guide complex designs. While the connectivity of a node is undoubtedly important, rational design strategies emerge only when considering other fundamental parameters of a MOM. From a synthetic standpoint it is advantageous to consider organic ligands as complete building blocks which need not be linear or ditopic. Metal clusters can be represented as single nodes with specific connectivities, rather than considering each metal ion as an independent node. The size of the ligands and clusters cannot be ignored, nor can the angularity and coordination geometries of these species.
Figure 8.
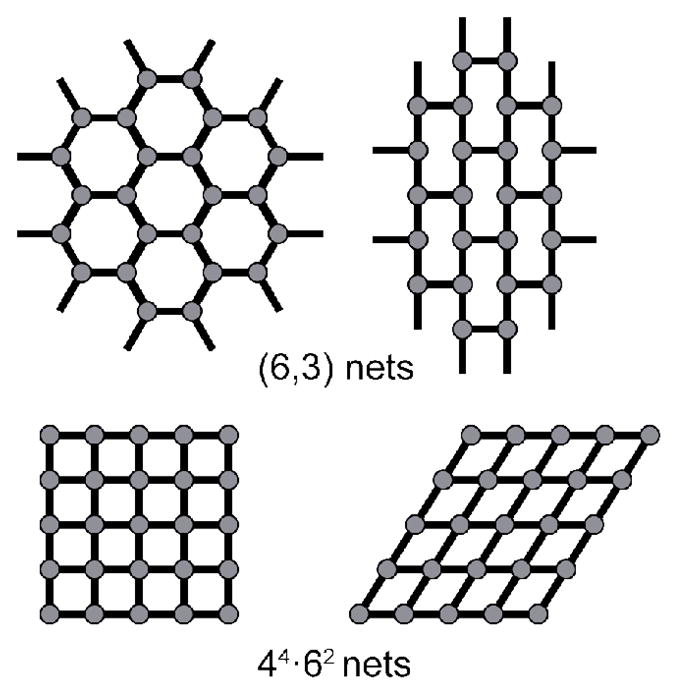
Net descriptions are based on topology and can result in distinct arrays sharing a common name.
2.2 Directional Bonding and Reticular MOFs
While the net approach was used to classify inorganic structures, it was not until the 1990s that its node-and-spacer foundations would be used to develop synthetic methodologies to construct discrete and infinite self-assemblies. The realization that the concept of nodes and spacers could be exploited not for the description of structures, but for the rational design of new constructs, would later grow into a suite of methodologies and a myriad of novel SCCs and MOFs.
The early 1990s reports of molecular squares by Stang and Fujita using Pt and Pd, respectively, using 4,4′-bipyridine as linear donors to link four metal centers were the start of a design methodology based on distilling a target construct to rigid molecular precursors encoded with the proper directionality.15–16 Using Well’s description, these squares are classified as 0-periodic nets, as phosphine or amine “caps” enforce discrete, closed constructs. Over the next decades, Stang used these early squares as motivation for what would become known as the “directional bonding” approach to self-assembly.17a
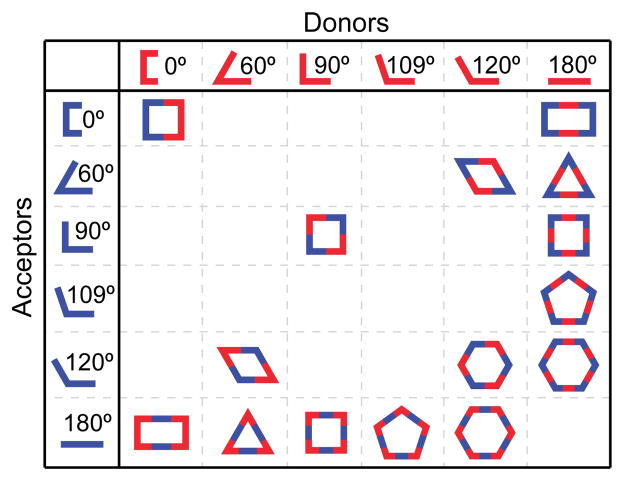
Beginning with 2D architectures, this emerging methodology was used to construct macrocycles in the shape of selected polygons by rationally designing organic ligands and metal-containing precursors to occupy the edges and vertices of the target shape. As such, this design approach is sometimes referred to as “edge-directed self-assembly,” in contrast to “face-directed self-assembly” which uses molecular precursors as panels to occupy the faces of a given 3D shape. Regular convex polygons were logical early targets for edge-directed self-assembly since satisfying the geometric requirements was possible by employing traditional organic and organometallic syntheses. To refresh, a regular convex polygon requires identical internal angles of less than 180° and sides of uniform length. By breaking such a polygon along its edges, each component consists of a fragment with no higher than a two-fold symmetry axis (see Figure 9).12,17a The internal angles found in these shapes are serendipitously found in simple molecules and were employed by Stang and others to generate a suite of polygons. Fused aromatics and benzene ring substitutions are especially useful, as are ethynyl groups to provide additional spacing and a site for organometallic carbon-metal bond formation.
Figure 9.
Ditopic building blocks generate a suite of 2D convex polygons via self-assembly.
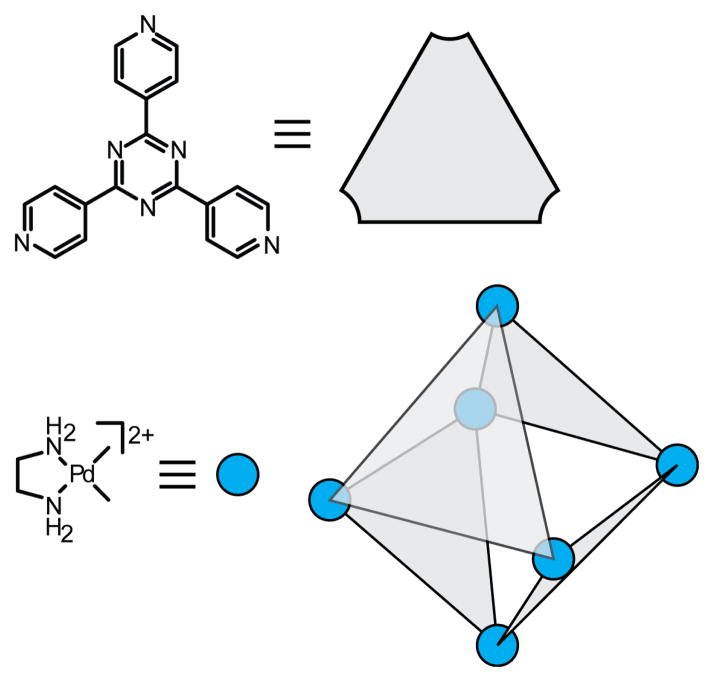
Examples from the molecular library of directional bonding components populated by Stang and others are shown in Figure 10. An angle of 0°, found in the so-called molecular clips, can be encoded by functionalization of the 1 and 8 position of anthracene.48 A 60° precursor is similarly exemplified by a dinuclear organoplatinum complex in which the anthracene is replaced by a 3,6-substituted phenanthrene.49 The iconic 90° acceptor used by the Stang group is a cis-capped mononuclear Pt complex found in a number of SCCs similar to the use of diamine-capped Pd precursors popularized by Fujita.12 Angles of 120° are readily encoded by meta substitution of a benzene ring.24b Lastly, para substitution of a benzene ring provides a scaffold for dinuclear 180° acceptor.50 The current molecular library of rigid building blocks far exceeds the Pt-based examples shown here. Many of these angles are readily encoded using other functionalities, such as the 120° orientation enforced by sp2 carbonyl carbon atoms. Two-component SCCs are often referred to as an [m + n] self-assembly, wherein m and n denote the total number of acceptor and donor units in a single discrete ensemble.
Figure 10.
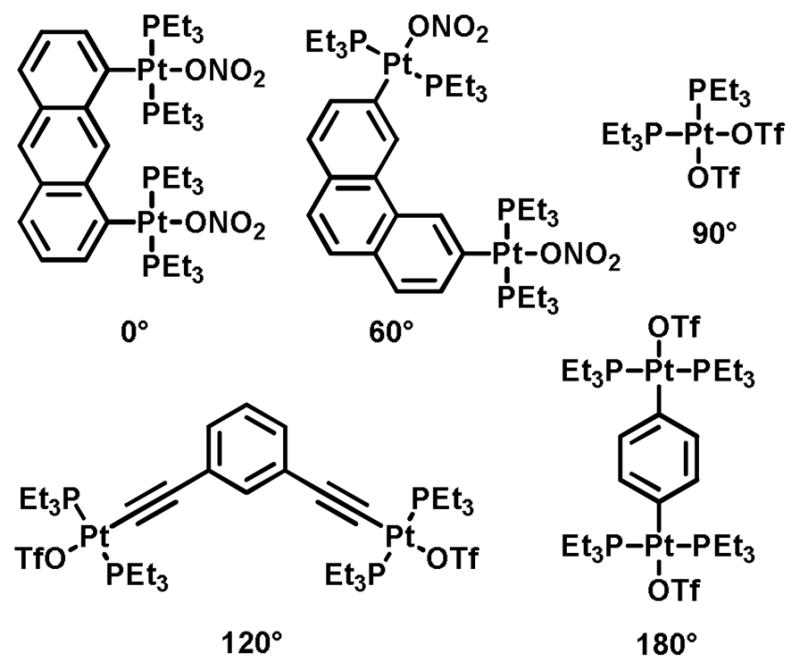
Metal-based molecular precursors possessing rigid directionality for coordination-driven self-assembly.
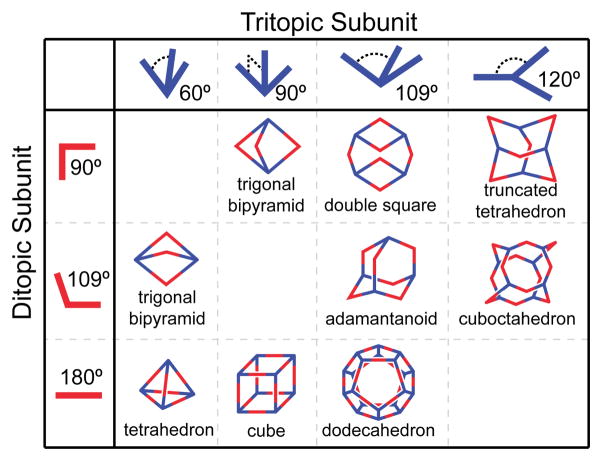
When a molecular component possesses a symmetry axis that is higher than two-fold, more complex geometries can be achieved. There are a few ways to encode this higher symmetry. The method most similar to that used to form the 2D polygons above is to simply increase the number of binding sites on a donor, the number of labile coordination sites on a metal, or the number of metals in an organometallic acceptor. When tritopic building blocks are combined with simpler ditopic precursors, the species formed are 3D metallacages (Figure 11).12,17a There are a few notable points illustrated by this expansion from 2D to 3D systems. The first is that the ditopic subunits may be chemically identical in both cases. The same molecules developed to make 2D assemblies are readily adapted to 3D ensembles when paired with a different donor or acceptor. A second point to note is that the specific identity of the acceptor and donor is not important. If the precursor is designed such that metal ligand bond formation can occur, the identity of the organic fragment and metal-containing fragment can be exchanged, provided that the necessary angles are preserved. That is, for a given [n + m] two-component assembly, suitable precursors can often be synthesized such that an [m + n] assembly will generate a geometrically similar ensemble. A hexagon can thus be assembled from the [6 + 6] combination of a linear, ditopic organic donor and a dinuclear metal acceptor with 120° between metal sites or from the combination of a linear metal acceptor with a ditopic, 120° ligand. This versatility allows for a variety of unique chemical compositions for a single structure type. However, synthetic feasibility often guides the identity of one component over another. For example, 90° angles are readily available from square planar metal coordination geometries but are substantially rarer for organic-only molecules.
Figure 11.
The combination of ditopic and tritopic building blocks results in 3D polygons.
The versatility of directional-bonding becomes apparent when considering that the same strategy used by Stang and coworkers to construct [2 + 2] rectangles48 and [3 + 3] triangles49 comprised of four and six total building blocks has been used to design [8 + 12] cuboctahedra of twenty individual units28a and [20 + 30] self-assembled dodecahedra consisting of fifty total precursors.29b Once properly encoded ligands and metal acceptors are in hand, there is fundamentally little difference between these self-assembly reactions (Figure 12). In theory, the two components are simply mixed under suitable reaction conditions and the thermodynamics of the system delivers the desired closed structure.
Figure 12.
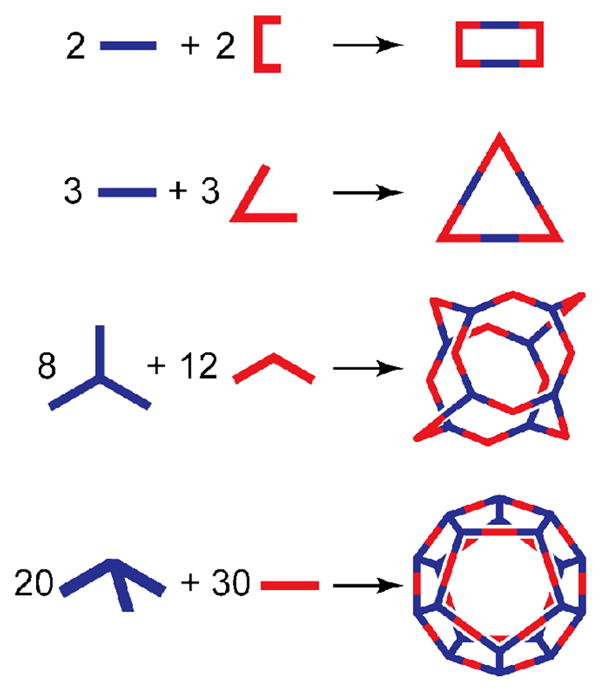
The versatility of the directional bonding approach to SCC formation allows the same methodology used to make simple rectangles and triangles to be applied to far more complex structures, such as a cuboctahedron or dodecahedron.
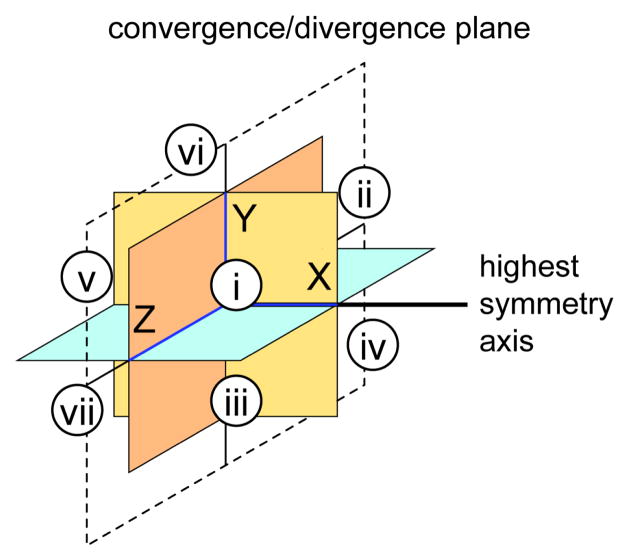
A noteworthy consideration for edge-directed self-assembly which has implications for SCC versus MOF chemistry is the relationship of the angularity of the two components. In order to generate a closed structure, either one or both components must have a bite angle of less than 180 degrees. One way to visualize this is to consider 3D Cartesian coordinate axes (Figure 13). Each Lewis-basic site of a polytopic ligand or coordination site of a metal can be used to orient a coordination vector. That is, the vector collinear with a metal-ligand bond involving that site. If the bisector of these vectors is placed at the origin, it is easy to predict which combinations of ligands will give closed structures by considering which octants the coordination vectors occupy. If their highest symmetry axes are placed along the positive x-axis, one or both of the building blocks must have coordination vectors which extend only into the four octants with positive x-values (octants i, ii, iii, and iv in Figure 13). If the bite angle is exactly 180 degrees such that the building block is planar, the only way for a convergent structure to be realized is for the other component to have an angle of less than 180°. This may appear to be a needless analysis for the simple polygons shown in Figure 9, but it illustrates why capping ligands are oftentimes used for metal centers which can extend coordination vectors into a variety of directions. Ditopic building blocks contain either two Lewis basic sites or two subsitutionally labile ligands on a metal. Such molecules never have a symmetry axis greater than two-fold, and thus either are planar, or have bite angles of less than 180 degrees. Most transition metal coordination spheres have more than two ligands, introducing higher order symmetry axes. This can be problematic when trying to generate closed structures, since extension into all eight octants is not conducive to closed structures. The archetypal Pt(II) and Pd(II) ions used in coordination-driven self-assembly of SCCs adopt square planar geometries. By capping specific sites, a level of control is afforded since the number of coordination vectors is reduced and the relative angularity of these vectors is controlled. An octahedral geometry which extends vectors in all three dimensions is harder to force into convergent structures. As such, octahedral building blocks are more commonly found in MOFs. That said, it is possible to confine the coordination vectors of an octahedron to the positive x octants. A fac-capped octahedral metal center has three coordination vectors oriented with 90 degree angularity, meaning closed structures are possible. Indeed, hexanuclear cubes have been synthesized by using a three-site capping ligand.
Figure 13.
A Cartesian axis defines octants (eighth octant is hidden) which can be useful for determining if a convergent (discrete SCC) or divergent (MOF) construct will be obtained for a two-component self-assembly of well-defined building blocks.
Using uncapped transition metals or metal clusters is more difficult from a design perspective, leading to an increasing in complexity when using planar coordination environments (trigonal and square), to tetrahedral and octahedral geometries which are inherently divergent due to the orientation of their coordination vectors. Since the planar geometries do not extend beyond the positive x octants, it is possible to form closed structures when a suitably angled ditopic building block is also used. This is the theory behind the formation of [8 + 12] cuboctahedra, first reported by Stang and coworkers.28a A planar, trinuclear organoplatinum precursor will assemble with a ditopic ligand to give a closed structure if the bite angle of the ligand is 108°. In practice, it is easier to synthesize a ligand based around a tetrahedral carbon center, and structural distortion easier accommodates the ~109.5° disposition of the coordination vectors. Interestingly, a tritopic planar tripyridyl donor will assemble with a dinuclear organoplatinum acceptor with a bite angle of 120 degrees. It was theorized that the large size of the assembly allowed for the necessary structural distortions to still furnish a closed cuboctahedron despite the non-ideal angle of the acceptor unit.
Elegant examples of uncapped square planar metal centers used for coordination-driven self-assembly of SCCs can be found in recent reports by Fujita and coworkers on the construction of a suite of “molecular spheres” based on homoleptic Pd-pyridine self-assembly. These impressive examples of edge-directed assembly draw upon the observation that MnL2n constructs are well suited for the construction of entropically favored regular and semi-regular polyhedra for finite values of n. Such species are designed so that the four-coordinate metal centers occupy the vertices of the structure with ditopic ligands occupying the edges (Figure 14). Four-coordinate metal centers with ditopic bridging ligands are special in that closed structures consisting of these two components will always utilize twice as many ligands as metal nodes. The smallest example occurs when n = 6, the easily recognized octahedron consisting of six vertices and twelve edges.26c The next value for which the number of edges is twice the number of vertices occurs when n = 12.51 This describes a cuboctahedron. The next largest assembly of this type is a M24L48 rhombicuboctahedron. Fujita and coworkers described this 72 component self-assembly by carefully tuning the angularity between the binding sites of a dipyridyl donor.52 Subsequent studies concerning these MnL2n complexes highlights the importance of the careful angular control required of directional bonding.53 When a furan-based dipyridyl donor was employed, a M12L24 cuboctahedral sphere was obtained. This ligand possess a bite angle (defined as the angle between the two coordination vectors of the pyridine groups) of 127°. When this angle was expanded to 149° by replacing the furan with a thiophene, the system shifted to favor M24L48 spheres. A systematic tuning of angles between 127° and 149° revealed that no intermediate structure was possible. Expansion of the ligand only eight degrees to a pyrrole-based 135° ligand resulted in exclusively M24L48 spheres.53
Figure 14.
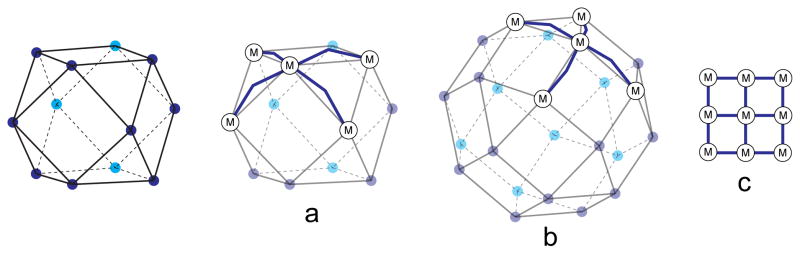
Molecular spheres of general formula MnL2n may be formed for specific values of n by using homoleptic square planar metal nodes joined by ditopic ligands. The specific polyhedron formed is sensitive to the angularity encoded in the ligand: a cuboctahedron (a) has four-connected nodes, but the dihedral angle is ~125 degrees. Square planar metal nodes thus require angled ligands to close the structure. This angle is reduced for a rhombicuboctahedron (b). If this angle is flattened to 180 degrees, a closed structure is not possible and a square lattice is produced (c).
In the case of uncapped square planar geometries and ditopic ligands, as the ligand bite angle approaches 180 degrees, the ideal MnL2n closed structure becomes larger and larger (reducing the curvature of the sphere) until ultimately resulting in no curvature. An MnL2n material with zero curvature is readily recognizable as a 2D square lattice. Fujita and coworkers first reported such a material by replacing the Pd centers with Cd(II) ions and utilizing 4,4′-bpy as a linear, ditopic donor, as mentioned in Section 1.2. When the uncapped, square planar Cd(II) ions assembled with the dipyridyl donor in a 1:2 ratio, a repeating network of [Cd(4,4′-bpy)2]2+ was generated.37 Other examples of square MnL2n lattices are known with 4,4′-bpy,54 as well as larger diptopic pyridyl-based ligands.55
Other researchers have found creative ways to utilize uncapped metal centers to generate closed, discrete SCCs despite the divergent nature of naked metal ions. In all cases, the methodology is highly dependent on carefully understanding the directionality and angularity of the subunits.
The directional bonding approach developed to construct SCCs translates well into synthetic approaches for MOF formation, though there has been a divergence in terminology. One of the major design strategies which has fueled MOF synthesis since its development in the mid 1990s has been termed reticular synthesis by Yaghi and coworkers.56 Generally speaking, this term refers to the formation of periodic networks. The fundamentals are more or less identical to the directional bonding paradigm developed by Stang and coworkers, but with its own unique nomenclature and nuances.
Ligands are categorized by their number and orientation of Lewis-basic sites and are combined with metal centers or clusters with well-defined geometries. These ligands and metal nodes can be combined to give predictable arrays via coordination-driven self-assembly. The term Secondary Building Unit (SBU) was borrowed from zeolite chemistry to refer to metal nodes and clusters used in an array and are analogous to the metal acceptors used in SCC formation. While oftentimes chemically distinct from the metallic building blocks used for SCCs, the various SBUs used in MOF formation are themselves an extension of the molecular library of the directional bonding approach. These SBUs are rigid, well-defined molecular entities which maintain their directionality through the self-assembly process.
For a given ligand, it is easy to conceptually transition between SCCs and MOFs simply by replacing a “capped” metal-center iconic of SCC formation with its uncapped analogue. For example, a 90° cis-capped square planar metal center which is the basis for molecular squares will become a four-coordinate square node, resulting in a square grid. In practice, however, reticular MOF synthesis typically employs metal clusters rather than single metal ions (see Figure 15).57 As discussed above, naked metal ions are challenging to use in SCC synthesis since they have inherently divergent coordination vectors and for a given geometry, the orientation may differ from one site to the next. Certain SCCs circumvent this through the use of capping ligands which, in addition to preventing infinite array formation, add directionality. A cis-capped metal precursor will have two and only two sites for incoming ligands and these sites are oriented with a 90° angle. While a divergent coordination sphere is well suited for MOF formation, the lack of orientation associated with single metal-centers can lead to low-quality MOFs and higher instances of defect sites. In addition, the chelating ligands which are used in many clusters afford structural stability which is advantageous from an application standpoint in which permanent pores are sought. When multiple metal ions are arranged as a cluster, usually using carboxylate linkers, rigid well-defined metal SBUs can be accessed which avoid the geometric ambiguity of naked metal ions.
Figure 15.
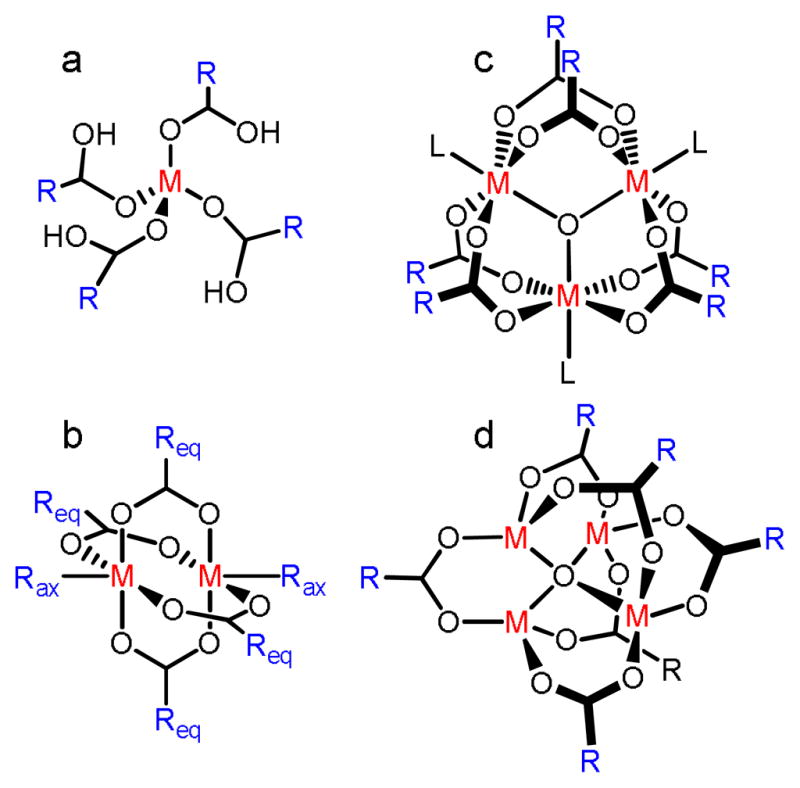
Some common SBUs used in reticular MOF synthesis: a) a mononuclear tetrahedral center with four monodentate acetate ligands; b) a dinuclear paddlewheel center with four bridging acetates and two axial ligands; c) a trinuclear basic chromium acetate structure with six bridging acetates and three terminal ligands; d) a tetranuclear basic zinc acetate structure with six bridging acetate ligands
While SBUs based on metal-nodes can be described by the directional-bonding method of noting the number and orientation of their attachment sites, these building blocks are often formed in situ during MOF synthesis since the individual clusters are oftentimes not isolable. As such, the molecular library of isolable building blocks for MOF synthesis is populated predominantly by organic linkers and has substantial overlap with the building blocks of SCCs. The use of metal-based SBUs focuses more on elucidating the synthetic conditions and precursors needed to generate the metal clusters during the self-assembly process. Conditions are chosen such that the organic linkers act solely as ligands and maintain their structural integrity during the synthesis. It is important to note that the geometry of an SBU is determined by the carboxylate carbons of the organic ligands. Many inorganic and organometallic precursors for SCC formation possess substitutionally labile ligands which are displaced by organic donors. In MOF chemistry, the bridging ligands are an important structural part of the cluster. Using carboxylate clusters as an example, the carboxylate carbons are the points from which the MOF will extend.
A common design feature of both reticular MOF synthesis and directional-bonding formation of SCCs is “expansion.” That is, for a given structure, increasing the distance between nodes can often be achieved simply by employing extended spacers.58 Most commonly this is done by adding phenyl or ethynyl groups, which rigidly and linearly increase the distance between Lewis basic sites of a polytopic ligand while maintaining the angularity between sites (Figure 17). A series of reticular MOFs which have the same topology is most easily formed simply by expanding a ligand for use with a particular SBU. Yaghi and coworkers grouped together sets of isoreticular MOFs (IRMOF), which share a net type, typically assigning each as IRMOF-n where n is simply a unique integer designator for members of the series.
Figure 17.
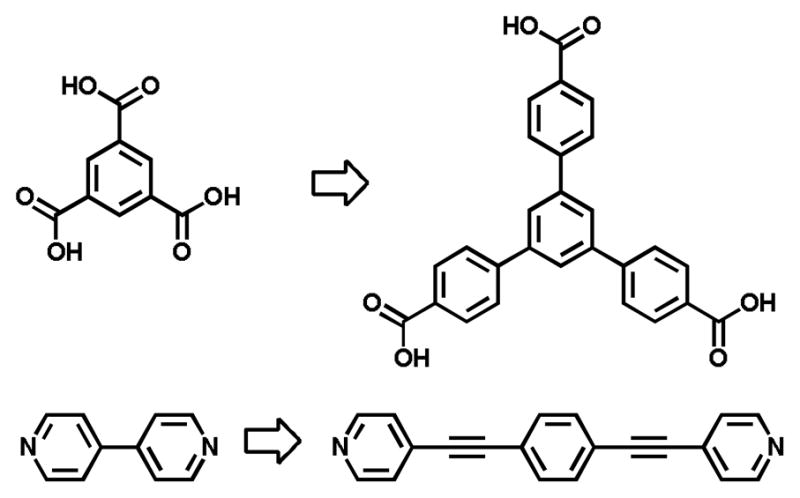
By adding rigid phenyl or ethynyl spacers, the pore size of MOFs and inner cavities of SCCs can be expanded without significant effects on the synthetic conditions used for self-assembly
A further ramification of the use of clusters rather than single metal sites for MOF synthesis is the concept of decoration, which refers to the replacement of a vertex in a periodic net with a group of vertices.58 This is shown in Figure 18 wherein a square lattice comprised of four-connected nodes and linear spacers is expanded by replacing the nodes with square clusters. The Schläfli symbols of Figure 18(a) and Figure 18(b) are 44.62 and 4.82, respectively. While these are obviously very different nets in terms of topology, the reticular MOF approach takes advantage of the synthetic similarities between the two systems. If conditions to make a suitable square SBU in situ are known, the square precursors simply replace whatever mononuclear node was used previously. In this way, decoration allows for the rapid formation of many topologically different frameworks without having to completely reinvent synthetic routes. Whereas expansion is typically a ligand-centered modification, preserving the metallic SBU used across an isoreticular series, decoration allows one to use the same ligand while altering the SBU.
Figure 18.
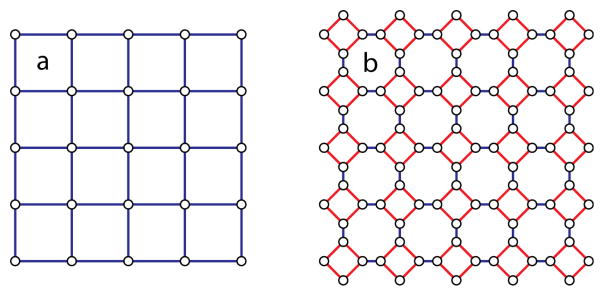
Decoration of a net replaces a vertex with a group of vertices. The four-connected nodes of (a) are replaced by square clusters of four nodes in (b).
The decoration of a net need not be limited to replacing mononuclear sites with metal clusters. Metal clusters can themselves be replaced by larger and larger constructs, which has led to the use of SCCs as building blocks for MOFs, described as supramolecular building blocks (SBBs) by Zaworotko and coworkers in a critical review.59 This recent strategy for MOF formation typically uses metal-organic polyhedra (MOPs) which are well-suited to act as building blocks since they are modular, highly symmetric and compatible with the synthetic conditions used in MOF formation. MOPs are a subset of SCCs constructed using metal-nodes more commonly employed for MOF synthesis. A recent paper on SBBs coauthored by Zaworotko observes the overlap in building blocks between MOF and SCC chemistry.60 Yaghi and coworkers developed a series of MOPs after extensive experience with MOF chemistry, so it follows that studies of such materials often employ the jargon of MOF chemistry. The synthetic approach to these materials follows the same systematic analysis of angularity and number of binding sites enumerated by Stang’s directional bonding strategy. As such, a molecular library of SBUs and linkers has been developed to generate a number of polyhedra.61
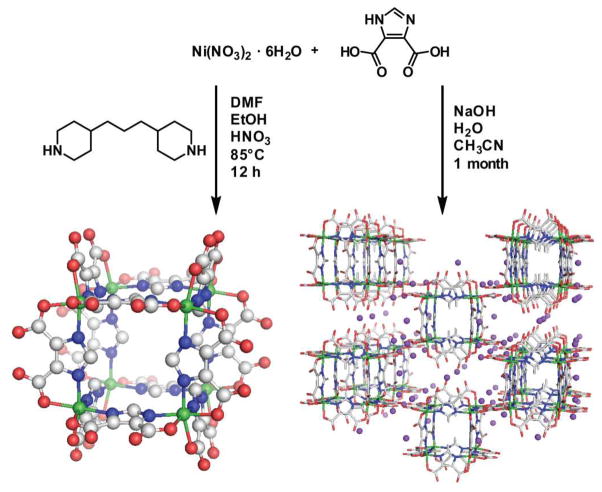
In some cases, discrete polyhedra can be isolated or incorporated into frameworks depending on synthetic conditions. For example, Eddaoudi and coworkers showed that the combination of Ni2+ ions and 4,5-imidazoledicarboxylic acid generates a M8L12 cube in an edge-directed assembly when a base such as dipiperdine is present to partially deprotonate the carboxylic acid.62a This base then occupies a structural role in the solid state, bridging the cubes through hydrogen bonding interactions. Xu and coworkers later carried out the self-assembly of the same ligand with Ni2+ in the presence of NaOH which led not to discrete cubes, but rather a framework of deprotonated cubes linked by sodium ions (Figure 19).62b
Figure 19.
Treatment of nickel nitrate with 4,5-Imidazoledicarboxylic acid generates cubic clusters. Depending on the reaction conditions, a discrete cube (left) or an extended cubic network bridged by sodium atoms (right) can be isolated.62
In other cases, discrete polyhedra are formed with terminal capping ligands. By replacing specific ligands with bridging analogues, extended networks comprised of the MOPs are generated. For instance, Fe-based polyhedra can be formed by capping three sites of Fe3O(CO2)6 clusters with sulfate groups to form triangular building blocks.63 The angularity of the remaining sites is suitable for the formation of closed structures, either truncated tetrahedra or truncated heterocubanes, when combined with linear ditopic or trigonal tritopic carboxylate ligands, respectively. The formation of these MOPs requires the presence of pyridine, which is incorporated into the Fe3O(RCO2)3(SO4)3(py)3 clusters (R = linking polyphenyl moiety). By replacing pyridine with cis-1,2-bis-4-pyridylethane in the assembly process, the resulting MOPs were linked into an extended framework dubbed MOF-500.64
While the MOF-500 formation was best achieved as a one-pot synthesis due to the insolubility of the MOP precursor, examples of stepwise interconversion between MOPs and MOFs are known. Zhou and coworkers described the synthesis of solvated [Cu2(CDC)2(DMA)(EtOH)]6 (CDC = 9H-carbazole-3,6-dicarboxylate; DMA = N,N-dimethylacetamide) which adopts a supramolecular octahedral geometry.65 This polyhedron possessed somewhat rare solubility in organic solvents which made it well suited as a precursor for further chemistry. When solutions of the octahedral-like MOP were treated with 4,4′-bipy, a twofold-interpenetrated framework was formed. In addition to being an interesting example of MOP to MOF conversion, the framework could be deconstructed back to discrete polyhedra when dissolved in DEF/py solvent mixtures (Figure 20).
Figure 20.
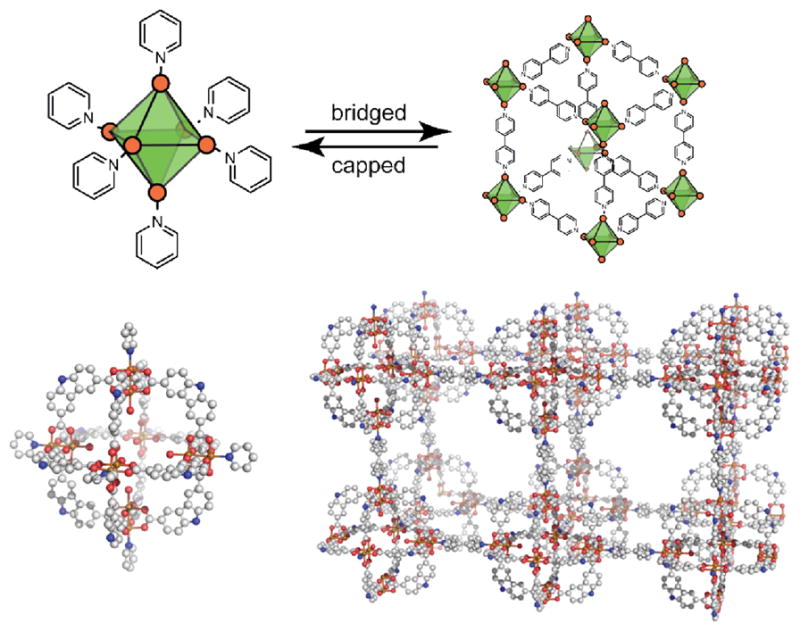
Discrete octahedra containing capping ligands are closely related to cubic networks of bridged octahedral sites. In some cases, such SCCs can be used as precursors to generate MOFs when suitable conditions may be found to replace capping ligands with bridging ligands. Solvated paddlewheel complexes of the type [Cu2(CDC)2(DMA)(EtOH)]6 may be converted to a pyridine-capped analog (left) or a 4,4′-bipy bridged network (right, only one of the two interpenetrating networks shown).65
A versatile MOP SBB was reported by Zaworotko and coworkers based on anionic “nanoballs” of the formula [Cu2(BDC)2L2]12 (L = pyridine, substituted pyridine, MeOH).66 These rhombihexahedra have multiple sites of possible modification, either at the axial ligation sites of Cu, or on the BDC organic linkers. When sulfonated BDC was employed to the 24- charged [Cu2(5-SO3-bdc)2(4-methoxypyridine)0.50(MeOH)x(H2O)1.50-x]12 MOP, the sulfonate groups were nicely disposed to bind additional metal ions. Upon treatment with a Cu(II) source, a framework was generated comprised of nanoballs bridged by cross-linking [Cu(methoxypyridine)4]2+ ions. If methoxy-substituted BDC is used in lieu of the sulfonated form, a neutral rhombihexahedron is formed. The Lewis-basic methoxy sites form double cross links between nanoballs, resulting in 1D chains.67
These examples nicely illustrated the close relationship between SCCs and MOFs in that for certain groups of SCCs which utilized capped nodes, freeing these capped sites will generate extended frameworks. That is, if synthetic conditions can be found which generated closed, capped MOPs, an analogous synthesis of MOFs can sometimes involve simply replacing the capping reagent with a suitable bridging ligand.
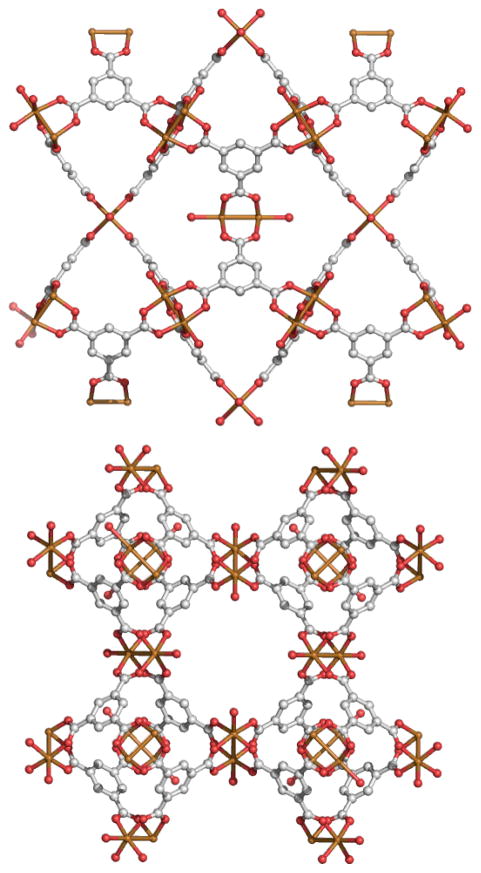
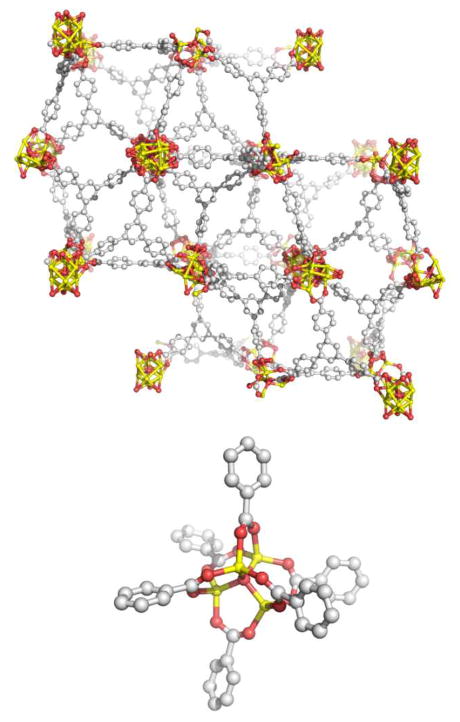
A second class of MOFs is comprised of MOP building blocks which share polyhedral faces, rather than having discrete linkers joining the polyhedra together. In 1999, Williams and coworkers reported the combination of copper nitrate with H3BTC in an aqueous ethanol solution.68 In order to avoid lower dimensionality frameworks previously observed when mild conditions were used,69 the mixture was heated to 180°C for 12 hours in a high-pressure vessel. This produced crystals of face-sharing rhombihexahedra dubbed HKUST-1 (Figure 21). Zawortko and coworkers observed that this type of fused structure does not utilize the MOP as a node and is therefore distinct from the concept of MOPs as SBBs. These fused faces and resulting extended frameworks are difficult to approach from a rational design standpoint and rationally-designed constructs of this type are relatively rare.
Figure 21.
Mixtures which gave low-dimensionality frameworks under mild reaction conditions generated fused Cu-based rhombihexahedra (HKUST-1) with solvothermal techniques. Atom (color): copper (copper), carbon (grey), oxygen (red). Hydrogen atoms omitted for clarity.68
2.3 Symmetry-Interaction Self-Assembly
The symmetry interaction approach to forming SCCs oftentimes employs uncapped, tetrahedral metal centers as nodes. As such, very careful ligand design is necessary to ensure that single, discrete thermodynamic products can be favored over unwanted side-products. Due to the divergent nature of a tetrahedral coordination environment, combining tetrahedral centers with non-chelating ligands will not produce discrete, closed structures. Simple examples are diamond-like networks consisting of tetrahedral nodes and linear spacers which give repeating adamantanoid units. To favor discrete systems without using capping ligands, the spacers themselves must enforce structural convergence, achieved by carefully designing polytopic chelating ligands which occupy all available coordination sites of the metal nodes while still maintaining strict directionality.
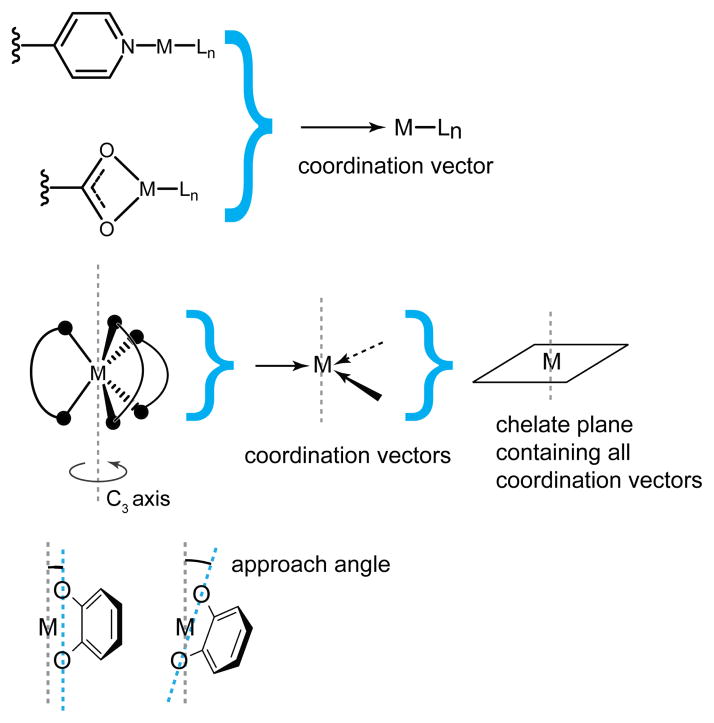
This methodology introduces and expands upon concepts from edge-directed self-assembly. The coordination vectors that were critical to defining the angularity and directionality of molecular building blocks remain an important parameter when designing ligands for symmetry-interaction self-assembly (see Figure 22). As was discussed previously, for monodentate ligands the coordination vector is simply the vector collinear with the M-L bond. For chelating ligands, it bisects the chelating atoms and is oriented towards the metal. When multiple chelating ligands are bound to a single metal center, the coordinate vectors define the chelate plane, which is the plane containing all of these vectors. A final parameter required for rational design using the symmetry interaction approach is the approach angle. This angle is found between the principle rotation axis of the metal center and the line defined by the two coordinating atoms of the chelating ligand.
Figure 22.
The symmetry interaction approach defines a coordination vector collinear to the metal-ligand bond of a monodentate ligand and bisecting the metal-ligand bonds of a chelate ligand. A collection of coplanar coordination vectors defines the chelate plane of a metal node. The angle between the highest order symmetry axis and the line defined by the chelate atoms is known as the approach angle.
As pioneers of this strategy, Raymond and coworkers have demonstrated the formation of triple helicates,70 triple mesocates,71 M4L6 clusters,72 M4L4 clusters73 and two-metal clusters.74 In each of these designs, a careful assessment of the required symmetries of metals and ligands guides the selection of building blocks. Rigid, polychelating ligands with predictable configurations of coordinate vectors are at the heart of this design strategy. While the metal centers are, of course, important, many structures feature homoleptic octahedral or tetrahedral geometries. Since many different metals can adopt these geometries, a particular structure can oftentimes be accessed using a suite of different metals.
To this point, the methodologies discussed have focused on edge-directed self-assembly. However, for 3D polyhedra and prisms, it is possible to design ligands to occupy the faces of a given shape, oriented by metal nodes at the vertices. Thus, a distinction may be made between edge-directed and face-directed self-assembly. Some species, such as supramolecular cubes, have been constructed using both approaches. An edge-directed cube assembles from eight tritopic building blocks with 90° angles between sites and twelve linear ditopic units. Alternatively, a face-directed cube may be constructed from six tetratopic panels held together by twelve 90° ditopic units, as shown in Figure 24.
Figure 24.
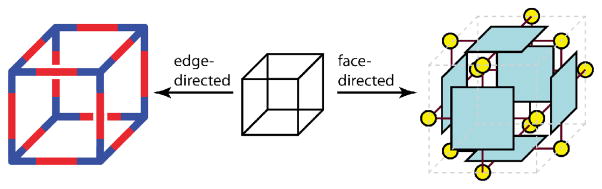
A supramolecular cube can be constructed from a linear ditopic and 90° tritopic building blocks using a edge-directed method (left) or from six tetratopic panels joined by twelve 90° ditopic building blocks using a face or panel-based approach (right).
Symmetry-interaction self-assembly is well-suited for face-directed assembly with uncapped metal centers. A face-directed assembly requires that the ligands match the symmetry elements found affecting the faces of a desired geometry. The faces of a tetrahedron have 3-fold symmetry, which can be built into a tritopic ligand (Figure 25). This route yields M4L4 tetrahedra. The orientation of the coordination vectors must be maintained since the metal nodes are identical between M4L4 and M4L6 tetrahedra. The same octahedral tris-chelate metal centers are used in both designs, which is one advantage of this approach over traditional directional bonding. Since almost all structural information is encoded in the ligand, a single metal center may be used for a variety of structures and swapping out the metal for a different element does not require new syntheses. In contrast, the conditions used to cap a square planar or octahedral metal center may not work for a different metal ion.
Figure 25.
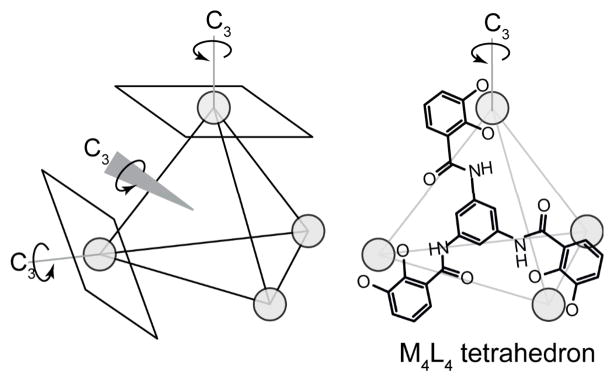
A M4L4 face-directed tetrahedron can be constructed from four ligands reflecting the facial symmetry of the four triangular panels with the proper orientation between coordination vectors.
The geometry of a carboxylate cluster SBU for MOF synthesis is not determined by the coordination environment of the metal, but rather by the carboxylate carbons which are the points where the node is extended. This geometric analysis is also applicable to the SCC structures formed by the symmetry-interaction approach. Returning to the Cartesian octant analysis of two-component assembly, it is readily apparent that a tetrahedral metal ion extends coordination sites on both sides of the convergence/divergence plane described in Figure 13. However, the points of extension of a symmetry-interaction self-assembly are not the metal ions, but rather the metal-ligand fragments. That is, the determination of the space group and the highest rotation axis must be determined for the resulting coordination sphere. This makes it somewhat harder to apply the symmetry interaction approach to extended networks. This is related to the relatively small number of MOF structure types compared to the theoretical possibilities. Certain SBUs orient a specific number of points of extension into space and these common fragments (see Figure 15) end up being shared among most MOFs. The clusters that have delivered stable, robust frameworks are naturally favored by chemists such that they dominate the known materials. That said, an analysis of the symmetry of a given framework will provide guidelines for the symmetry elements required of the bridging ligands. For instance, one type of honeycomb lattice has nodes with D3h symmetry so the construction of such a MOF requires SBUs with the associated symmetry elements. The C2 axes dictate that linear ditopic links must join these D3h SBUs. Alternatively, a hexatopic fragment with D6h symmetry will also fulfill the symmetry of a similar looking lattice. In this case, the D6h SBUs would again require linear ditopic spacers to maintain the mirror plane and C2 axes, while it fulfilled the requirements for C6 axes at the center of each node (Figure 26). Similar analyses can of course be made on 3D lattices; all symmetry elements of a given lattice must logically manifest themselves in the building blocks used. While the use of the symmetry interaction method has not yet been exploited by MOF chemists, it is nonetheless a potential tool, and in some cases is used without realization. A diamondoid network necessarily contains adamantane-like units. The network of Robson and coworkers36 which has been previously discussed in terms of tetrahedral metal centers and tetratopic, tetrahedral-based ligands is easily broken down to its symmetry elements. Each adamantanoid has Td symmetry and thus building blocks used to construct diamondoid must reflect this. That is, they must have C3, C2, S4 and σd elements in accordance with the Td space group. A quick analysis of the Cu and organic fragments used in the aforementioned example confirms this to be the case.
Figure 26.
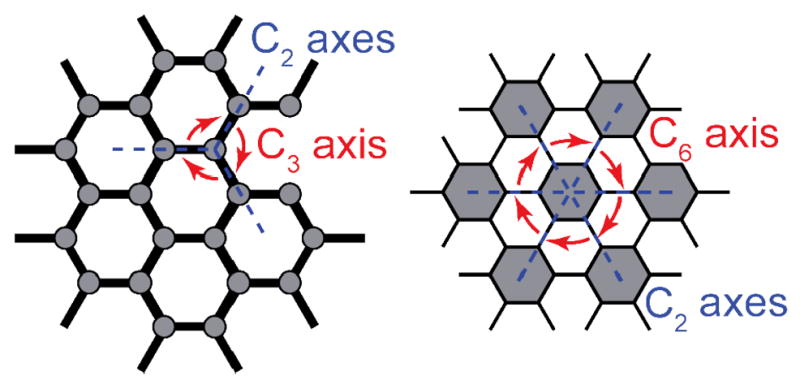
The symmetry interaction approach can be applied to extended networks. In this case, the symmetry elements of the entire lattice must appear as local symmetry elements of the building blocks.
2.4 Face-Directed Self-Assembly: Molecular Paneling
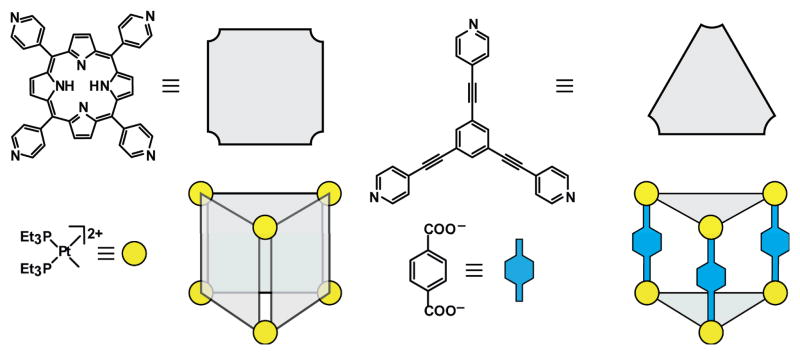
Briefly introduced in Section 2.3, face-directed assembly is a subset of directional bonding that deconstructs 3D architectures to their faces rather than edges. This technique has been used to construct a wide range of structures, from relatively small prisms and truncated tetrahedra to the significantly larger cuboctahedra.
It is not always necessary to occupy all the faces of a given polyhedron with panels. For example, rather than constructing all eight faces of an octahedron, trigonal panels can be joined by 90° metal centers to cover alternating faces, as shown in Figure 27. Since the alternating faces destroy the C4 rotational axis of symmetry, the resulting structure is a truncated tetrahedron with Td symmetry. The organic ligands that comprise the faces of these cages oftentimes do not occupy the full space implied by their representation as panels. While this gives rise to opening in the cages, such constructs are often well-suited for host/guest chemistry, even in the case of a truncated tetrahedron with alternating trigonal faces.
Figure 27.
Polytopic organic linkers can be represented as panels for the construction of 3D SCCs, such as truncated tetrahedra
Truncated polyhedra are excellent examples of applying the reticular MOF concept of decoration to SCCs. A special case of decoration is the replacement of an n-connected node with a group of n nodes, a process which is known as augmentation. A tetrahedron contains four vertices which are most simply represented as three-connected nodes. If each of these three vertices are replaced by three new nodes, an augmented structure results. In the case of a tetrahedron, augmentation delivers a truncated tetrahedron, converting the structure from a Platonic solid to an Archimedean solid (see Figure 28). A truncated tetrahedron is an eight-sided solid with four hexagonal faces and four triangular faces. From a synthetic standpoint, only the hexagonal faces share edges, making them the logical choice for panels to be joined by two-connected nodes. If the depth of the truncation is large enough such that the new vertices overlap, the resulting solid is octahedral with eight trigonal faces. However, if the faces corresponding to the previously hexagonal sites are occupied with the remaining trigonal faces still open the symmetry of the solid remains Td. This augmentation may also be applied to octahedra to generate truncated octahedra. Unlike the tetrahedral case, however, when the depth of the augmentation is such that the new nodes overlap, an entirely new solid results, known as a cuboctahedron.
Figure 28.
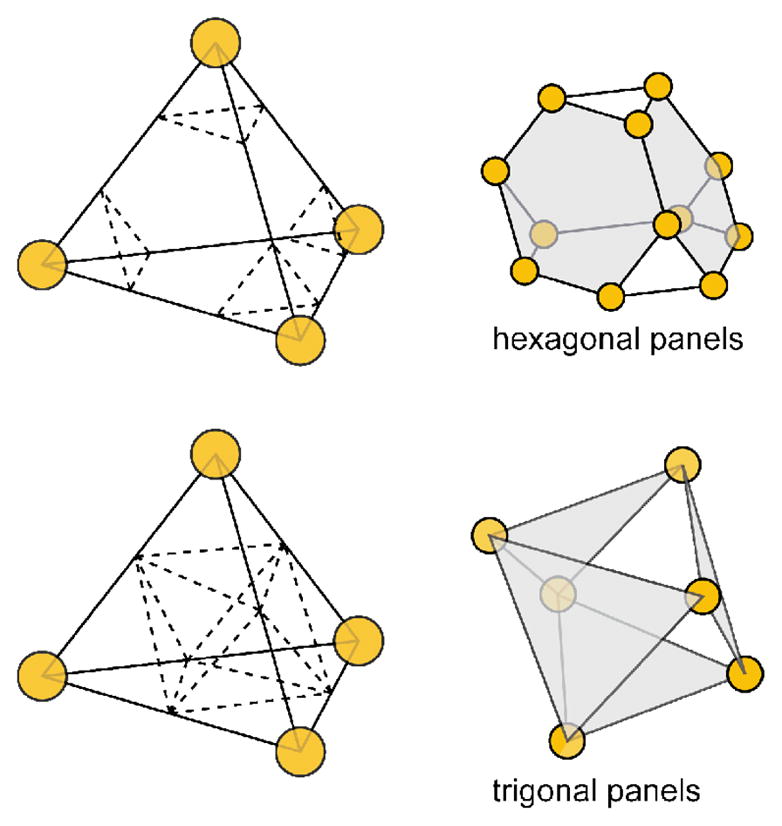
Depending on the depth of the cuts, truncated tetrahedra contain hexagonal (top) or trigonal (bottom) faces. Note that if all faces of the bottom truncated tetrahedron are occupied, the structure is an octahedron.
Aside from the Platonic and Archimedean solids, a number of prisms have also been formed which contain panels or faces in their design. As shown in Figure 29, a trigonal prism can be constructed either by combining three square panels with six ditopic donors or by using two triangular panels with ditopic nodes spanned by linear ligands acting as pillars. The former design requires a two-component self-assembly and delivers an open-faced prism. The latter requires a third building block for a multi-component self-assembly. It is important to note that the steric bulk surrounding the metal nodes can play a role in determining overall structure. If large phosphines are used, rather than assembling a trigonal prism, the angles between panels can be expanded to give other prismatic shapes. For instance, open hexagonal barrels are formed when tetrapyridyl porphyrin is combined with Pt(dppf)(OTf)2 (dppf=1,1′-bis(diphenylphosphino)ferrocene).75 Multicomponent self-assembly is a relatively new technique for the formation of SCCs and as demonstrated by Fujita76 and Mukherjee,77 such structures can require a fourth species to act as a template for prism formation.78 Despite the need for a template in some syntheses, template-free prism formation can occur, as demonstrated by Stang79 and Mukherjee.77,80 Multicomponent self-assembly is especially appealing since structures of higher complexity, if controllable, are promising for a variety of applications. This topic is the focus of a recent tutorial review by Ward and Raithby in which supramolecular assemblies are used to illustrate current design methods for multicomponent structures, potential applications are highlighted, and future directions are proposed.81
Figure 29.
The panel approach to forming SCCs provides a logical route to two types of trigonal prisms. One using a two-component assembly with a square or rectangular panel, the other a three component using a triangular panel and linear pillars.
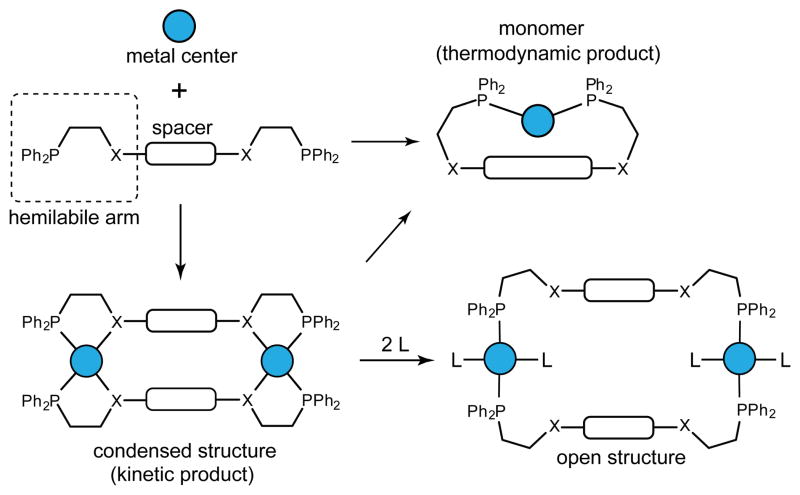
2.5 Weak-Link Approach
The design principles discussed thus far focused on obtaining singular, thermodynamic products by the careful selection of building blocks. The weak-link approach, as developed by Mirkin and coworkers, utilizes hemilabile ligands such that the initial SCCs formed can undergo post-self-assembly modifications to access new structures.20b The majority of SCCs made using this approach are 2D macrocycles containing asymmetric chelating ligands in which one donor atom of the chelate forms a stronger M-L bond than the other. The moniker stems from the displacement of this weaker donor, rendering the bis-chelator into a bis-monodentate bridging ligand upon addition of a suitable exogenous ligand. As the chelating coordination mode is broken, the macrocycle expands and the metal centers incorporate new ancillary ligands en route to the formation of a new thermodynamic product.
Upon expansion, the resulting macrocycle is typically conformationally flexible, owing to the once-chelating ligand sites which are necessarily non-rigid. The conversion from a condensed to expanded structure has been shown to trigger catalytic activity and useful applications in sensing.
It is important to note that the initial condensed structure is not always the thermodynamically favored product in the primary self-assembly reaction. By carefully selecting reaction conditions the kinetic condensed structure can be isolated preferentially. Treatment of this SCC with an additional ligand alters the system such that the expanded structure is now the favored product (Figure 30). The demonstration of post-self-assembly modifications of the ligand sphere of a metal node has since been applied to SCCs formed using directional bonding in the conversion of 2D and 3D constructs to entirely new topologies. At elevated temperatures, the condensed structure can be driven to the thermodynamic monomer species, however in practice this requires temperatures of over 100°C and the stability of the kinetic product is thus high enough to circumvent the unwanted thermodynamic sink.82
Figure 30.
The weak-link approach generates condensed structures as kinetic products. These [2 + 2] macrocycles can convert to [1 + 1] monomeric species under thermodynamic control, or be expanded to open structures upon treatment with strong ligands.
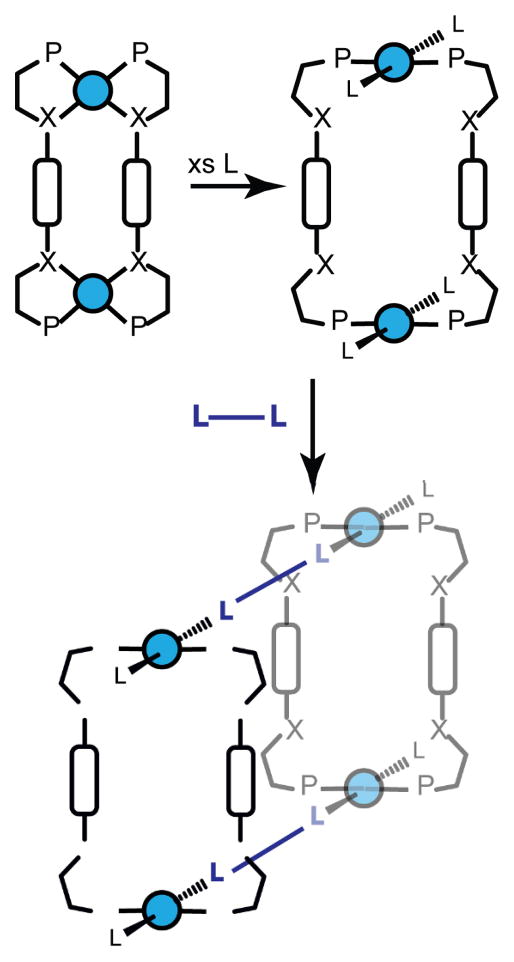
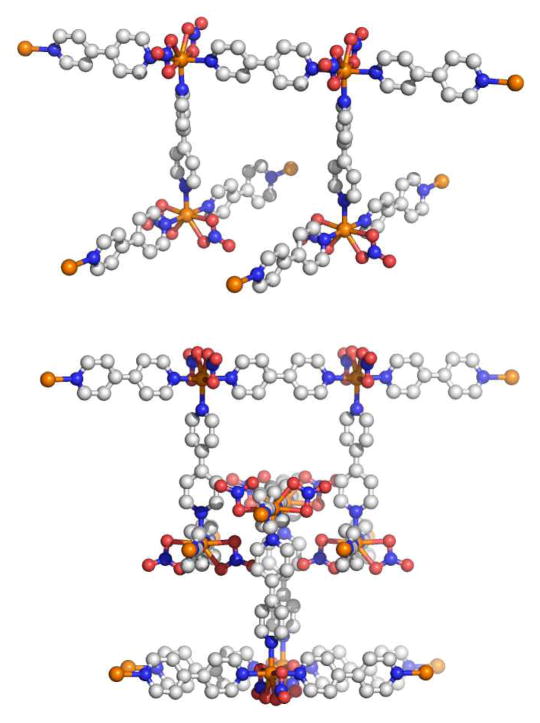
Since the ligands which initial the expansion from the condensed structure to the open structure can be multitopic, it is possible to use condensed structures as precursors to larger constructs and potentially networks (Figure 31). For example, Mirkin and coworkers describe the formation of a molecular cylinder by linking two open structures using either 4,4′-biphenyldicarbonitrile or 4,4′-biphenyldiisocyanide.83 The tetranuclear cylinders were examined by single crystal X-ray diffraction to confirm the tetranuclear structure comprised of two linked macrocycles capped by either CO or acetonitrile. Since each end of the cylinder could conceivably be uncapped and treated with further equivalents of bridging ligands, the weak-link approach could be used to generate extended MOF tubes.
Figure 31.
The weak-link approach can be used to extend 2D structures into 3D cylinders consisting of two macrocycles linked by ditopic ligands. In theory, the remaining monodentate ligands could be replaced by additional bridging ligands to give extended frameworks.
2.6 Dimetallic Building Block Self-Assembly
The dimetallic building block approach wonderfully illustrates the intimate relationship between MOFs and SCCs in that the paddlewheel metal units used to form SCCs are widely employed in MOF syntheses as well. Paddlewheel complexes are characterized as two metal centers bridged by four ligands. Depending on the metal centers used, direct metal-metal bonding can occur. The bridging ligands occupy equatorial coordination sites. If present, ligands that cap the remaining coordination sites which fall along the metal-metal axis are assigned as axial ligands.
The dinuclear paddlewheel building block is versatile in that individual units can be fused either by linkages between equatorial sites, linkages between axial sites, or both. As demonstrated most prolifically by Cotton and coworkers,21 a variety of different linking ligands can be used in controllable stoichiometries to furnish rhomboidal “loops,” triangles, squares and polygons. In most cases, a dinuclear precursor is utilized in which two or more equatorial sites are occupied by easily displaced acetonitrile ligands. The remaining equatorial sites are capped with bridging ligands, commonly N,N’-di(p-anisyl)formamidinate, which stabilize the dinuclear building blocks and prevent oligimerization.
Since there is synthetic control over which ligands occupy which site, there are a variety of different outcomes when using a dimetallic SBU. As shown in Figure 32, paddlewheel complexes can theoretically lead to 3D cubic grids, 2D square lattices, linear wires and supramolecular squares. When all sites are used, the building block can be represented by an octahedron. A square SBU results either from capping both axial sites, or capping two equatorial sites trans to one-another (the axial sites then complete two vertices of the square). A trans-capped square requires all sites to be capped save for two trans equatorial sites or by capping all equatorial sites and instead using the axial positions. Alternatively, all equatorial sites can be capped and the dinuclear sites linked through the axial positions instead. Lastly, a cis-capped square can be made either by capping all but two cis-oriented equatorial sites or by capping all but one equatorial and one axial site. In practice, such species usually use two cis equatorial sites.
Figure 32.
Paddlewheel dimetallic building blocks can be used to make 3D MOFs (a), 2D MOFs (b), 1D wires (c) or supramolecular squares (d) depending on how the six sites of the equatorial and axial ligands are used: either as bridges to neighboring dinuclear sites or as caps to prevent propagation along that vector. Note that capped squares can be achieved by alternate capping methods, as discussed in the text.
Dinuclear building blocks were described previously in the system described by Zhou and coworkers which can convert between discrete octahedra and a bridged cubic network. This conversion was possible due to the versatility of the dinuclear building block. By extending all four equatorial sites using a ligand with a 90 degree turn, the discrete octahedron was formed. In this case, one axial site from each of the six dinuclear centers was oriented inward, with the remaining axial sites capped by pyridine. By extending not only from the equatorial sites, but also from the axial positions, the octahedra were linked into a MOF.65
The first chelate-carboxylate SCC, dubbed MOP-1 contains dinuclear paddlewheel building blocks, specifically copper ions bridged by meta-BDC.66c While similar in composition to MOFs (hence the MOP moniker), MOP-1 is readily soluble in refluxing DMF which allowed traditional recystallization methods and afforded single crystals suitable for X-ray diffraction studies. Since the vast majority of SCCs to this point utilized pyridyl-based ligands, the discovery that chelating carboxylate ligands could be used to form discrete structures set the state for a myriad of SCCs sharing carboxylate-based chelating ligands and SBUs more traditionally found in MOF synthesis.
2.7 Alternative MOMs
Strictly speaking, the various design methodologies described above for forming both SCCs and MOFs are all based on directional bonding first proposed and described by Stang and coworkers.84 The only way to rationally design a material comprised of nodes and spacers to possess a given topology is to enforce specific interactions between building blocks. However, the different approaches have led to different methodologies which oftentimes employ unique nomenclatures, ideally to distinguish between strategies but sometimes to distinguish between researchers. While this review focuses primarily on MOFs and SCCs, there are other coordination polymers that merit note:
Metal-Organic Zeolites (MOZs) are a subset of coordination polymers which are MOF-like in that they are microporous extended networks comprised of metal and organic building blocks. In fact, MOZs are perhaps best described as a subset of MOFs in which the building blocks are chosen to closely mimic the directionality and topology of existing zeolite materials. For example, the tetrahedral Si, Al, etc. sites of known zeolites can be replaced by common transition metals such as Zn, Co or Cu. Zeolites often contain Si-O-Si motifs which have an angle of 145 degrees. This angularity is also present in bridged M-Im-M imidazolate moieties. As such, a number of zoelitic imidazolate frameworks (ZIFs) have been developed with over eleven unique topologies. A pioneering example of such a material was reported in 2002 by You and coworkers with the synthesis of [Co5(im)10·2 MB] (im = imidazolate, MB = 3-methyl-1-butanol).85 Originally these MOFs were known as metal-azolate frameworks (MAFs) before the term ZIF was popularized in a paper by Yaghi and coworkers in 2006 in which twelve unique frameworks were synthesized and characterized.86 Nonetheless, the term MAF persists, especially in a recent review by Chen and coworkers which offers an excellent analysis of these frameworks.87 Recent work by Feng, Bu and coworkers has explored the use of trifunctional ligands such as BTC in the formation of zeolite-type MOFs.88 By breaking the paradigm of tetrahedral nodes and ditopic ligands, the library of potential ligands is greatly expanded which may prove promising in the discovery of new zeolite mimics.
A second class of MOF-like materials has been dubbed infinite coordination polymers (ICPs) by Mirkin and coworkers. ICPs are distinguished by an emphasis not only on the building blocks used, but also additional factors such as size dispersity and intramolecular interactions. ICPs encompass a range of materials, spanning between crystalline (MOF-like) and amorphous structure types. Since a goal of ICP chemistry is to arrest network formation to give controllable particle size, synthetic methods often rely on triggering precipitation of the material from a mixture of its building blocks. These building blocks have some overlap with traditional MOF and SCC chemistry, with Lewis binding sites oftentimes consisting of carboxylates, amines or N-heterocyclic donors. Unlike SCC and MOF chemistry which can employ diffraction techniques for characterization, ICPs rely heavily on microscopy due to their oftentimes amorphous nature. Unlike traditional nanoparticles which are constructed from simple elemental precursors, ICPs enjoy the tunability and modularity associated with MOF chemistry due to the similarity in building blocks. An informative tutorial review of ICPs has been written by Mirkin and coworkers and is an excellent resource on this emerging field.89
It is important to note that the use of metal nodes is attractive from a synthetic standpoint due to the ease of self-assembly afforded by metal-ligand coordination. These metal sites also can impart interesting functional characteristics if redox, photophysical or catalytically active metals are used. Of course, the very moniker metal-organic materials requires their presence in order to be an accurate description. That said, from a structural standpoint, if proper angles can be encoded into purely organic-based building blocks, metal sites are not required for the formation of extended frameworks. Such is the case in the covalent organic frameworks recently reviewed by Jiang and coworkers,90 which offers an interesting look at a related class of materials to which traditional MOF and SCC chemists may be underexposed.
3. Synthetic Conditions and Characterization
The conditions used for SCC and MOF synthesis are necessarily dictated by the chemical properties of the building blocks and final materials. Forming large, high-quality crystalline porous MOFs which are stable to solvent loss require robust metal-ligand bonds. Because labile bonds are detrimental to crystal integrity, MOFs are often inert at ambient conditions. Conversely, SCCs are often soluble constructs which are isolated like more traditional coordination compounds, either as powders or small crystals. If single crystals are desired, either for characterization or a specific application, these crystals need not be formed upon the initial synthesis. The solubility of SCCs allows standard methods of crystallization to be applied and if suitable quality is not obtained, the material can be recycled and used again. Thus, the major distinction is that MOF synthesis contains strong metal-ligand bonds throughout the entire material in the solid state, while SCCs are subject to the same relatively weak intramolecular interactions which are found in small molecule chemistry.
The synthesis of SCCs and MOFs typically target thermodynamic products. One major advantage of coordination-driven self-assembly is that metal-ligand bonds are oftentimes substitutionally labile. If a rigid ligand coordinates in an improper orientation such that a closed structure will not form, the ligand can dissociate. In a sense, SCCs and MOFs are self-healing during their assembly, automatically rectifying structural defects en route to a thermodynamic minimum (see Figure 33). Thus, a major characteristic of coordination-driven self-assembly, both for SCCs and MOFs, is kinetic reversibility. If kinetic products are inert or sluggish to self-correct the reaction time may become unfeasible and the product mixture may be plagued by impurities. One particular issue can be insoluble kinetic products. If an incomplete or incorrectly oriented fragment precipitates out of solution there is no way for ligand dissociations and substitutions to occur to remedy the defects. As such, synthetic conditions must be determined such that all kinetic intermediates are free to transform further.
Figure 33.
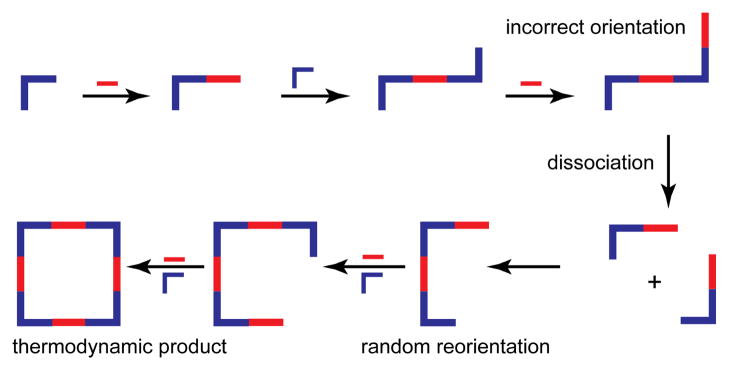
The self-assembly of a square may proceed through incorrectly oriented intermediate species. As these fragments associate and dissociate in solution, they will eventually funnel to the thermodynamically favored square, automatically healing any defects.
Since SCCs possess weak intermolecular forces, conditions to maintain kinetic reversibility are oftentimes more mild than for MOF chemistry. Since the structures are relatively smaller than fragments of MOFs, solubility can oftentimes be maintained and extremely high yields can be realized. The substitutional lability of Pt-based SCCs has been explored by Stang and coworkers, revealing that Pt-pyridine systems continue to undergo ligand self-exchange over the course of days in solution at room temperature,91 complementing earlier investigations on ligand exchange which rationalize faster rates for Pd versus Pt on the basis of the activation enthalpies for the associative substitution mechanisms.92 This exchange was observed for both rectangular and triangular systems by employing deuterium-labeled 4,4′-bipy ligands primarily monitored by high-resolution mass spectrometry and supported by NMR studies. For this system, the exchange was sensitive to temperature, solvent and counterions. This is to be expected given the associative mechanism of exchange on square planar complexes. When weakly coordinating nitrate anions were replaced with non-coordinating hexafluorophosphate, exchange was no longer observed. The role of solvent was thought to modulate the ability of the counterion to coordinate and initiate substitution. The rate of exchange decreased as the concentration of water was added, suggesting that hydrogen bonding between nitrate and water attenuated the weakly coordinating nature of the anion.
This exchange has also been investigated by Fujita and coworkers using Pd-based cuboctahedra.93 Rather than isotopically labeling the ligands, two endo-functionalized 120° dipyridyl ligands were used with alkoxy moieties of different lengths. Two Pd12L24 cuboctahedra were independently formed using the two ligands in which the alkoxy groups were determined to be non-interacting. The ligand exchange between cages was then monitored by mass spectrometry to reveal that the cages were highly stable. The half-lives of exchange were on the order of 20 days, about 105 times longer than Pd monomers with similar coordination environments.94 The attenuated exchange rate for spheres relative to small molecules is likely due to the closed nature of the polyhedra. Each Pd center is liked to four others and therefore even if ligand substitution is initiated such that one bridging ligand is removed, the overall structure will remain intact. Deconstruction of an M12L24 sphere, which contains 48 Pd-pyridyl bonds, would require an unfeasible number of rapid substitutions or dissociations without reformation of the thermodynamically preferred structure, which is unlikely. The fact that smaller Pd-pyridyl species are more substitutionally labile helps large systems funnel towards single thermodynamic products. As intermediates spontaneously form, the better they resemble the final polyhedra the slower their exchange kinetics will be.
These studies by Stang and Fujita indicate why SCCs can be formed rapidly and quantitatively provided that all intermediate species remain soluble throughout the assembly process. If kinetic intermediates precipitate out of solution, self-correction cannot occur, yield necessarily drops and stoichiometric control is lost. This requirement can often be met by careful selection of the solvent rather than using elevated temperatures. The library of polygons, polyhedra and prisms formed by the Stang group typically form either at room temperature or with mild heating using common solvents with assembly times spanning from hours to overnight.95 Even the [20 + 30] self-assembly of large dodecahedra occurs at room temperature in an acetone/CH2Cl2 mixture. The [12 + 24] and [24 + 48] assemblies of molecular spheres (cuboctahedra and rhombicuboctahedra, respectively) occur remarkably quickly when heated to 70°C in DMSO, finishing in around three hours.53 Fujita and coworkers utilized continuous-flow techniques to monitor the self assembly of M12L24 species, observing a number of intermediate M(12-n)L(24-m) species. Since these species remained in solution, their dynamic nature drove the reaction mixture to the thermodynamic product. Most impressively, Fujita reported the quantitative formation of a sphere-in-a-sphere double-cuboctahedron which formed over six hours of stirring at 80°C in DMSO.96 This twenty-four Pd construct contains 96 Pd-pyridyl bonds yet still forms as the sole reaction product due to the kinetic reversibility of the undoubtedly countless number of intermediates formed en route to the final construct.
The strong metal-ligand bonds found throughout the entirety of a MOF structure mean that kinetic reversibility can be more difficult to maintain during self-assembly. The chelating carboxylate-metal bonds often exploited to form robust materials are useful for maintaining structural integrity but make kinetic intermediates a larger concern. Single crystals of MOFs are highly desirable both from a characterization and application standpoint, which is problematic in traditional crystallization methods are not applicable to inert, insoluble framework. Thus, crystals must be formed concurrently with self-assembly and conditions must be found to maximize the quality of the materials.
There are a few methods which have been effectively employed to avoid defect sites in MOF synthesis. When carboxylate ligands are used, one approach is to throttle the self-assembly by using carboxylic acid building blocks rather than carboxylate salts. By either slowly introducing an organic base or heating the solutions at elevated temperatures in high-pressure vessels, the rate of deprotonation and metal-ligand bond formation can be controlled such that proper cluster formation and long range ordering is correct from the onset. This “slow growth” method largely circumvents kinetic intermediates, providing high-quality materials.57
If hydrothermal techniques are used, kinetic intermediates are likely unavoidable, but due to the relatively harsh conditions, the system is reversible and defect sites can be resolved. Under these conditions anionic ligands are also used in their acidic form to attenuate the rate of coordination. Once the material is isolated from the elevated temperatures and pressures all ligand exchange ceases and the quality is fixed, since the material is typically insoluble in common solvents. For example, in an early use of 1,3,5-benzenetricarboxylate (BTC) for MOF synthesis Yaghi and coworkers mixed aqueous solutions of metal salts (Co, Ni or Zn) with protonated BTC.97 This mixture was heated to 140°C for 24 hours, and then slowly stepped down in temperature for a total reaction time of 34+ hours. This technique produced 1D chains in the form of crystalline rods, insoluble in all common solvents save for limited solubility in methanol.
The same ligand was also used to form 2D sheets upon the slow diffusion of pyridine into alcohol solutions of cobalt nitrate and protonated BDC.98 Over the course of three days, large crystals insoluble in common solvents deposited from solution containing three equivalents of BTC per Co, with one BTC fully deprotonated and the other two remaining in carboxylic acid form. In this case, the pyridine was found integrated into the MOF, with one pyridine coordinating to cobalt and a second equivalent occupying the pores. This guest pyridine could be removed by heating to 200°C. Characterization of samples held at 350°C indicated that the coordinated pyridine could also be reversibly removed from the material.
The inclusion and identity of the organic base plays a large role in the topology of the resulting MOF. For the examples above, the absence of any base produced 1D chains whereas the presence of pyridine resulted in 2D sheets incorporating the base with partial deprotonation of acidic BTC. If pyridine is replaced by the poorly-coordinating but strongly basic triethylamine, a third topology is obtained.99 The slow diffusion of triethylamine into a mixture of zinc nitrate and the acid form of BTC over six days results in the formation of large cubic crystals. Yaghi and coworkers characterized this material as a 3D framework of two unique Zn environments bridged by three carboxylates belonging to three different BTC moieties.
While high quality materials can be obtained by hydro- or solvo-thermal methods, seeking alternative synthetic pathways can be useful in obtaining more functional materials. A good example of this may be found in a recent paper by Matzger and coworkers100 in which a room temperature synthesis of Zn4O(ndc)3 (ndc = naphthalene-2,6,-dicarboxylate) resulted in a bulk material which was less interpenetrated than the same framework obtained via solvothermal routes.101 The MOF thus obtained was in good agreement with theoretical predictions for a non-interpenetrated framework (4400 m2 g−1).
Ionothermal techniques have also been employed in the formation of MOFs. Such methods utilize ionic liquids that can fulfill a dual role of solvent and template agent. This is advantageous in that the number of species involved in framework formation is minimized, which in theory can deliver high quality materials. An excellent review by Parnham and Morris explore ionothermal methods for the formation of zeolites and MOFs.102
For some self-assemblies of SCCs, a non-coordinating template species is needed to maximize the formation of a single species as was described earlier in the formation of 3D cages.77–78 In one interesting example, the choice of template agent can direct the assembly either to a discrete SCC or infinite MOF. In 2005, Eddaoudi and coworkers described the assembly of In(NO3)3·2H2O with 2,5-pyridinedicarboxylic acid (2,5-H2PDC) in an aqueous ethanol solution.103 When this reaction was carried out in the presence of 4,4′-trimethylenedipiperdine (TMPD) a 2D framework was obtained with a Kagomé topology. If this same reaction was instead treated with 1,2-diaminocyclohexane (1,2-DACH) a discrete octahedron was formed. Both species were isolated after 12 hours of stirring at 85°C with both materials reported as insoluble in organic solvents. This example not only shows the close relationship between SCCs and MOFs, but also demonstrates the importance of reaction conditions in the formation of such species.
The characterization of MOFs and SCCs shares considerations with their syntheses. That is, the discrete soluble nature of SCCs allows certain techniques to be employed which may be difficult to apply towards extended networks. While the crystallization of SCCs shares methodologies with small molecules, for example vapor diffusion or layering, it is not always possible to obtain crystals of sufficient quality for single crystal X-ray diffraction. Because of this, most papers reporting the characterization of SCCs use mass spectrometry with a variety of ionization methods. Electro-spray and cold-spray ionization have been widely used and oftentimes allow for the observation of intact SCCs, with various charge states arising from the loss of counterions. Since charge states corresponding to the loss of counterions still reflect intact SCCs, these peaks are used to confirm the stoichiometry of assembly. For example, the characterization of dendrimer-functionalized hexagons by Stang, Muddiman and coworkers revealed [M – 4OTf]4+ and [M – 5OTf]5+ peaks which were consistent with the sole formation of [3 + 3] assemblies.104
Computational studies play a significant role in both MOF and SCC research. When sufficient resources are available, calculations popularized by small molecules chemistry have been applied towards SCCs, for example geometry optimizations using DFT and time-dependent DFT to afford photophysical information. That said, the large size of even simplified model systems oftentimes makes molecular mechanics calculations a more sensible options, especially when qualitative structural information is desired. Computational information is not limited, however, to structural calculations. A recent paper by Calero, López and coworkers includes a combination of Monte Carlo simulations, DFT and molecular dynamics in order to elucidate the degradation of isoreticular MOFs in the presence of water molecules.105 These studies, performed on MOF-5 (referred to as IRMOF-1 in the manuscript), identified that lattice disruption occurs via displacement of a terephthalic acid ligand by water, leading to five-coordinate Zn sites. Yoneya and coworkers used molecular mechanics to study the assembly of Fujita’s M6L8 nanospheres by simulating a random orientation of building blocks and letting the ensemble spontaneously aggregate into discrete SCCs.106 This study offers a unique look at the self-assembly process and supports that ligand-exchange is extremely important in allowing an evolution from randomly oriented intermediate fragments to final assemblies through reversible metal-ligand coordination.
The insoluble nature of many MOFs means that solution-based characterization techniques are not as useful. As such, most labs equipped to study such materials instead use infrared spectroscopy which is readily performed on solid samples. Crystallography, both powder and single crystal, is oftentimes employed as the ultimate method for structural determination, but requires materials of suitable quality.
4. Molecular Building Blocks
As was discussed previously, the cyanide bridging ligands of Prussian blue and the Hoffman-type complexes set the stage for such arrays to be generalized to metal sites connected by specific organic donors. The molecular library of precursors popularized by Stang for the rational design of SCCs by directional bonding can be joined with the suite of SBUs and ligands inspired by Yaghi’s development of reticular MOFs to offer an impressive number of building blocks which continued to grow throughout the 1990s through today, providing new topologies and functionalities to MOMs of all types.
Scheme 1 and Scheme 2 demonstrate the two most widely used classes of ligands for SCC and MOF formation, N-donors and O-donors, with pyridyl and carboxylate-based ligands dominating these two groups, respectively. From these two schemes and the exemplary references given in Tables 1 and 2, it is clear that N-containing ligands dominate SCC syntheses, while carboxylate ligands are more commonly used to construct MOFs. This is expected, given the differences in synthetic methodologies discussed above. MOFs require the strong metal-ligand bonds associated with chelating carboxylate ligands so that the frameworks remain structurally sound even when solvent molecules are removed. The substitutional lability of pyridyl ligands, on the other hand, is perfect for SCCs in which kinetic intermediates can be rectified under the mild conditions of self-assembly. Both classes of ligands, however, are dominated by rigid phenyl and/or ethynyl containing molecules. This rigidity is fundamental to directional bonding.
Scheme 1.
N-donor-based ligands as building blocks for SCCs and MOFs
Scheme 2.
O-donor-based building blocks for SCCs and MOFs.
4.1. Pyridyl-based MOMs
From the early discoveries of cyanide acting as a linear bridge between two metal sites, as described above, a number of ditopic linear donors have been used, ranging from the simple and iconic 4,4′-bpy, to more elaborate species shown in Scheme 1. Despite being the simplest of the donors used in MOF and SCC synthesis, linear ligands can be used to construct a wide variety of topologies. We will begin the discussion with 4,4′-bpy, which deserves special distinction since it has been used in both MOF and SCC chemistry from the very start, and as such has been used to construct a number of different materials and provides nice examples of syntheses of both SCCs and MOFs.
The combination of 4,4′-bipy with a suitable capped Pd or Pt precursor generates molecular squares, best exemplified by [(en)Pd(4,4′-bpy)]48+ and [(dppp)Pt(4,4′-bpy)4]8+, as reported by the labs of Fujita15 and Stang,16 respectively. The amine-capped Pd square was synthesized by mixing (en)Pd(NO3)2 and 4,4′-bpy in a mixture of methanol and water (Scheme 3). The water-soluble supramolecular square fully formed during 10 minutes of stirring at room temperature. The Pt-based square, [(dppp)Pt(4,4′-bpy)4]8+, forms under similar conditions. Mixing the (dppp)Pt(OTf)2 precursor with 4,4′-bpy in CH2Cl2 results in the self-assembled supramolecular square (Scheme 4). These synthesis demonstrate the kinetic reversibility of metal-pyridyl chemistry under mild conditions.
Scheme 3.
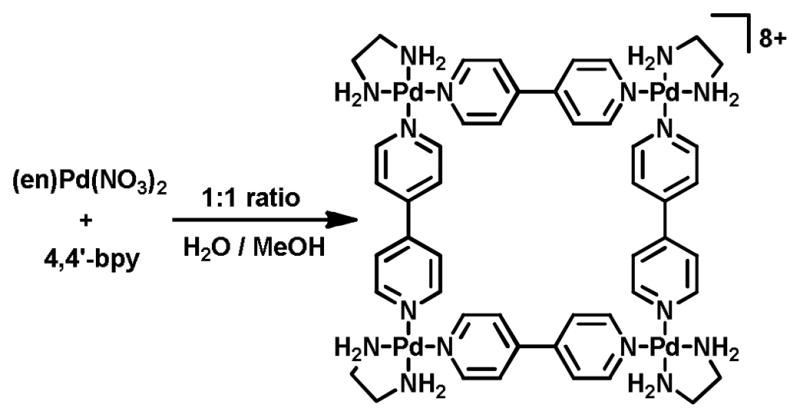
Scheme 4.
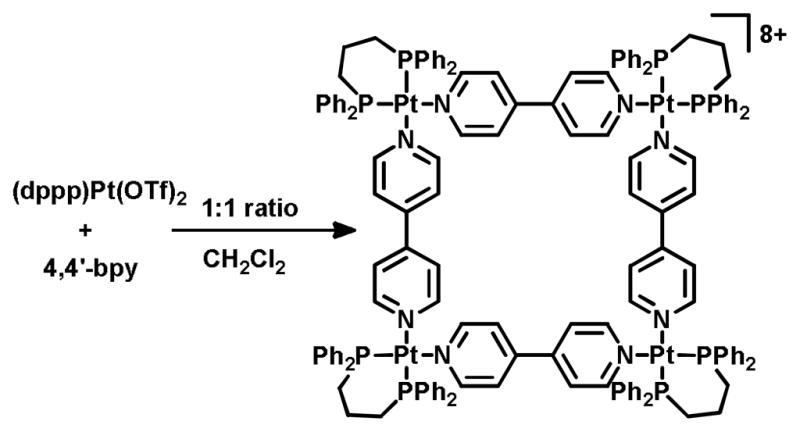
This square unit can be extended to an infinite 2D lattice when the square planar metal node is uncapped. For example, when Cd(II) ions replace Pd or Pt, a repeating network of [Cd(4,4′-bpy)2]2+ is generated.37 Experimentally, this material is obtained by treating Cd(NO3)2 with 4,4′-bipy in a 1:2 ratio. Over the course of 24 hours, colorless crystals of the 2D network fall out of the H2O/EtOH solution.
Since 4,4′-bpy is a linear ligand, it necessarily falls in a plane and thus any additional dimensionality must be encoded via the metal nodes. As such, these 2D constructs (square and square grid) can be extended to three dimensions by using octahedral centers. An example of a discrete 3D assembly is given by the Ru-based cube of Thomas and coworkers, reported in 1998.26a When [Ru([9]ane-S3)Cl2(DMSO)]2+ is combined with 4,4′-bpy, the chlorides and DMSO ligands are displaced. The fac-capping nature of the [9]ane-S3 ligand means the 4,4′-bpy occupies the remaining three binding sites of the octahedral Ru coordination environment, which are held at 90° with respect to one another. Unlike [(en)Pd(4,4′-bpy)]48+ and [(dppp)Pt(4,4′-bpy)4]8+, the supramolecular cube is not rapidly formed, but rather assembles slowly over the course of two weeks in solution, presumably due to slower ligand exchange kinetics. Each Ru site acts as the vertices of a cube, with the 4,4′-bpy molecules comprising the edges (see Scheme 5).
Scheme 5.
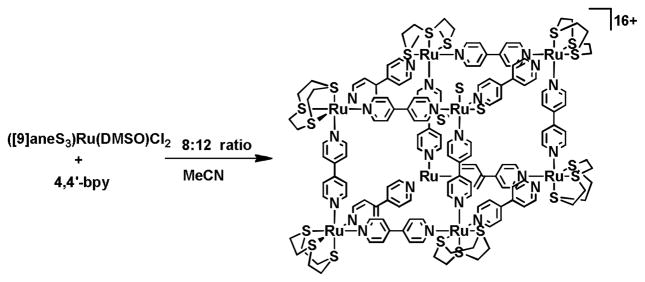
In analogy to the discrete Pt/Pd bpy square expanding to a 2D Cd grid by uncapping the metal nodes, the supramolecular cube can be generalized to an infinite 3D array by utilizing uncapped octahedral or tetrahedral metal nodes. The first reports of such materials were given by Zaworotko and coworkers and Yaghi and coworkers. Zaworotko et al described the synthesis of a [Zn(4,4′-bpy)2(SiF6)]n network in which 2D Zn-4,4′-bipy arrays were connected by bridging SiF6 molecules to generate a 3D topology (Figure 34).243 The MOF was synthesized by first dehydrating [Zn(OH2)6]SiF6 after which 4,4′-bpy was added and the mixture refluxed in DMF and dioxane. Upon cooling, crystals of the framework could be collected however they would desolvate and lose crystallinity rapidly upon isolation.
Figure 34.
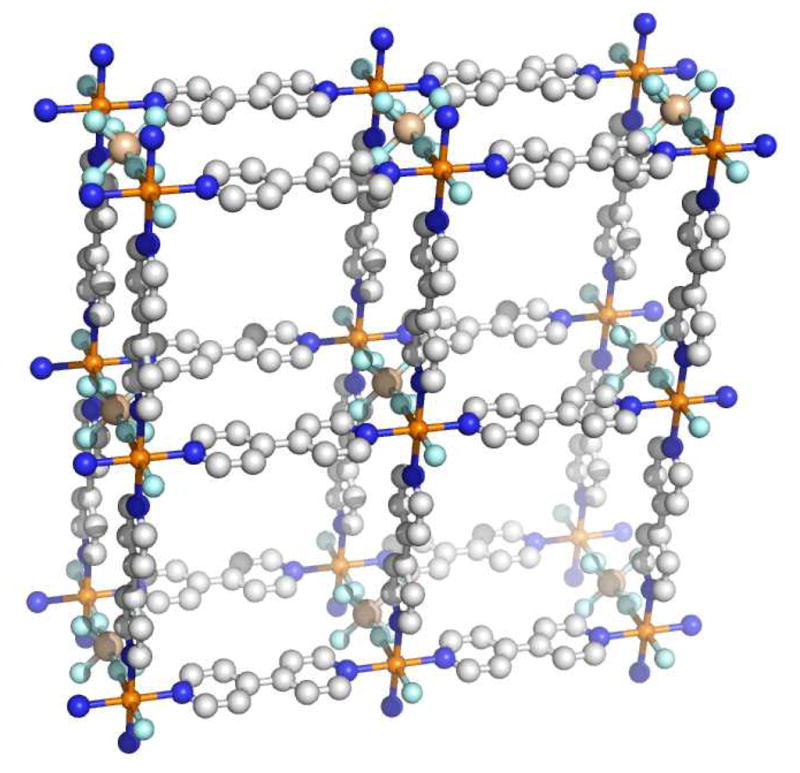
The [Zn(4,4′-bpy)2(SiF6)]n network consists of 2D Zn(4,4′-bpy)2 square lattices bridged by SiF6 to give a 3D array. Element (color): Zn (orange), N (blue), C (grey), Si (tan), F (teal). Hydrogen atoms omitted.243
It is noteworthy that no examples of a cubic lattice consisting of only octahedral metal centers and 4,4′-bpy exist. Though removing the capping ligands of a cube like that formed by Thomas and coworkers should conceptually give a cubic network, a homoleptic hexapyridyl complex is not known and therefore such nodes are not practical. There are, however, many examples of octahedral-shaped polynuclear SBUs which give cubic networks with linear bridging ligands, such as the paddlewheel complexes described in Figure 32. There has also been research into Molybdenum-halide clusters of the type [Mo6X8]4+ which arrange into an octahedral clusters, originally studied in the 1980s due to their interesting redox properties.244 Such clusters are isolable when capped with six triflate ligands to generate dianionic species. Shriver and coworkers treated the Mo6Cl8 cluster with 4,4′-bipy to generate a xerogel which was used as evidence of a rigid, 3D structure. While single crystal X-ray diffraction was not possible, a suite of spectroscopic techniques were used to support a cubic network of clusters bridged by 4,4′-bpy.245 Discrete clusters capped by six pyridine ligands have also been isolated, indicating that a hexapyridyl-cluster coordination environment is feasible.246
The rapid decomposition of extended solids based on 4,4′-bpy is another reason for the paucity of pyridyl-based MOFs relative to the more stable carboxylate version. That said, there are examples of porous 4,4′-bpy and pyridyl-based networks. The first such frameworks were reported by Kitagawa and coworkers in 1997 using Co, Ni and Zn as metal nodes.38 By combining 4,4′-bpy with the hydrated nitrate salts of the metals, crystalline frameworks of ([M2(4,4′-bipy)3(NO3)4]·H2O)n (M = Co, Ni, Zn) deposited out of solution over the course of a week. The resulting frameworks possessed unique interpenetrated double-sheets with channels imparting gas adsorption functionality to the materials (Figure 35).
Figure 35.
Structure of {[Co2(4,4′-bpy)3(NO3)4]· 4 H2O, the first porous MOF using 4,4′-bpy as an organic linker. The extended structure consists of double-sheets (top) which interpenetrate in the solid state (bottom), leaving small voids for guest inclusion. Element (color): Co (orange), N (blue), C (grey), O (red). Hydrogen atoms and waters omitted.38
A second example of a porous MOF using pyridyl-based ligands can be found in the 2006 report of a 2D framework comprised of tetrapyridyl porphyrin ligands with Cu-based paddlewheel complexes.173j Treatment of the free-base porphyrin with copper acetate generates the framework shown in Figure 36. Since the sheets do not interpenetrate, there are significant channels which run through the structure which can be used for gas absorption once the MOF is evacuated. This material also nicely illustrates the relationship between SCC and MOF synthesis since both porphyrin and paddlewheel complexes can be applied to both types of materials depending on the connectivity. In this case, all equatorial sites of the paddlewheel copper acetate are capped (the acetate in unfunctionalized). This leaves the axial sites open and allows the complex to act as a linear, two-connect node (see Figure 32). The porphyrin in this case is fully functionalized with four pyridyl groups and thus acts as a planar tetratopic donor. The directional bonding approach treats this system like the Cd(4,4′-bpy)2 network since both contain a tetratopic square building block bridged by a linear ditopic building block. Chemically, the roles of the metal acceptor and organic donor are flipped, with little consequence on the overall structure.
Figure 36.
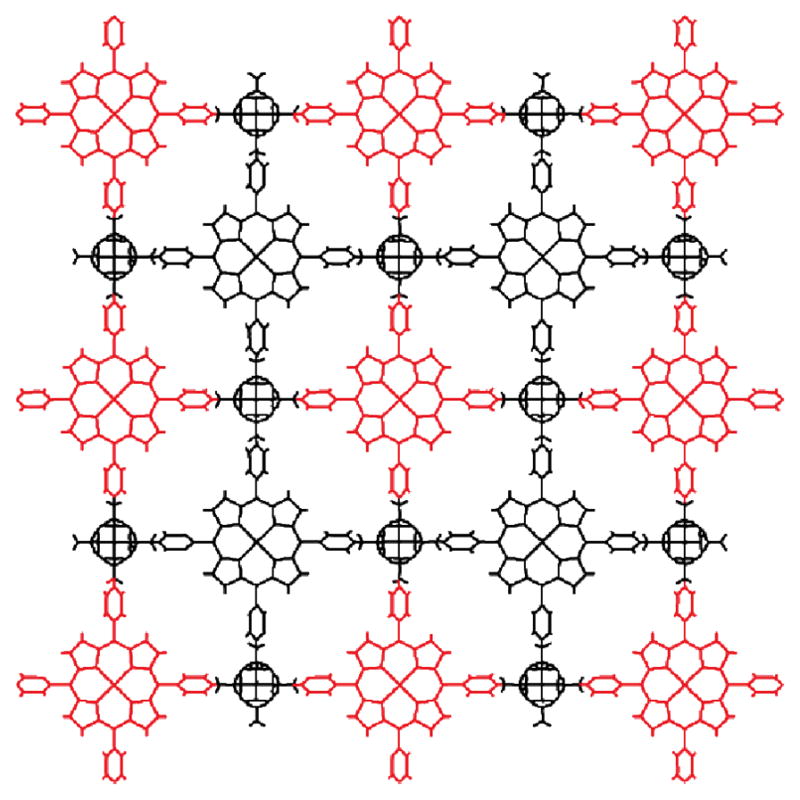
Tetrapyridyl porphyrin assembles into staggered 2D square lattices when combined with copper acetate. The layered sheets, shown here as black and red, are separated by 7.12 Å.173j
These pyridyl-based MOFs have their origins in SCCs.247 By reducing the square tetratopic ligand to one of lower symmetry, closed structures are possible. A trans-dipyridyl porphyrin represents a linear ditopic donor and a cis-pyridyl porphyrin is a 90° donor. Suitable complementary building blocks can be found in Figure 9 to generate closed macrocycles. Both Stang248 and Lehn249 have reported supramolecular squares in which both cis and trans dipyridyl porphyrins have been used. Three different types of squares can be formed from these porphyrins, depending on the type of metal node used. Like with the two square lattices described above, the building blocks acting as donor and acceptor can be freely swapped with little consequence to the overall structure. As depicted in Figure 37, a cis-pyridyl porphyrin can act as a 90° corner unit with a linear metal acceptor, or the metal acceptor can act as the corner unit with a linear dipyridyl porphyrin to give two types of related [4 + 4] assemblies. Alternatively, the square can be attenuated by using both metal and ligand as 90° units, resulting in a [2 + 2] assembly.
Figure 37.
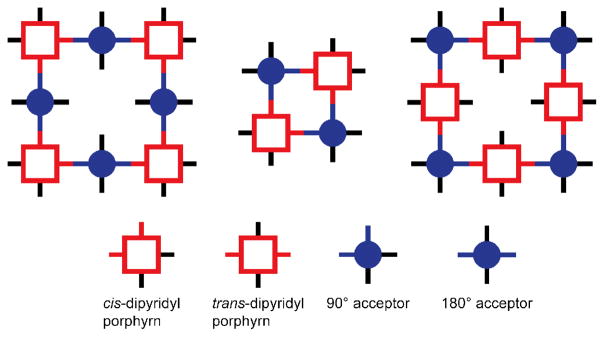
Three different supramolecular squares have formed by assembling dipyridyl porphyrins with Pt or Pd-based acceptors.
The materials described above have all focused on square and octahedral metal coordination environments which limits the resulting constructs to 180° and 90° angles. There is a rich chemistry of 4,4′-bpy and related pyridyl-based ligands which extends beyond square and cubic SCCs and MOFs.
For example, in 1994, Zaworotko and coworkers described the formation of two unique MOFs based on linear pyridyl ligands and Cu(I) centers. When 4,4′-bpy was combined with [Cu(CH3CN)4]PF6, repeating adamantoid units formed four interpenetrating diamondoid frameworks of [Cu(4,4′-bpy)2]PF6. Each Cu(I) center adopted a tetrahedral geometry with four pyridyl ligands. Diamondoid frameworks of this type are a common thermodynamic sink for tetrahedral nodes and linear spacers. Interestingly, the synthesis of a 2D honeycomb lattice using pyrazine (pz) as a linear ditopic donor can be found in the same manuscript.250 While the same Cu precursor was used, it adopted a trigonal coordination environment, furnishing [Cu2(pz)3]SiF6 as a two dimensional honeycomb framework. The very different topologies between the pz and 4,4′-bpy-based materials were attributed to the role of the noncoordinating anions. The PF6− of [Cu(4,4′-bpy)2]PF6 occupied channels which placed them on the four-fold crystallographic axes. It was observed that if [Cu2(pz)3]SiF6 would have formed a similar diamondoid framework, the shorter length of the pz ligand would result in a 1-fold rather than 4-fold interpenetration. Even with SiF62− occupying the voids, this would have resulted in considerably more free space. Instead, by adopting a woven honeycomb framework, the SiF62− anions pack better.
Around the same time, Yaghi and coworkers reported the hydrothermal synthesis of an array [Cu(4,4′-bpy)1.5(NO3)(H2O)] sites.174j Unlike the diamond-like network of Zaworotko’s tetrahedral [Cu(4,4′-bpy)2]PF6, the material obtained by Yahgi possessed trigonal-planar Cu(I) centers which formed six unique interpenetrating arrays. Despite the higher degree of interpenetration, the tolopogy of [Cu(4,4′-bpy)1.5(NO3)(H2O)] results in significant voids, organized as rectangular channels running through the MOF. These three unique materials are interesting in that they each contain Cu(I) and a linear N-heterocyclic bridging ligand, yet each adopts a very different topology.
These examples reinforce why MOF chemistry has diverged from SCCs by using metal clusters rather than single metal ions. All three materials used Cu(I) centers with linear, ditopic ligands, yet three different topologies resulted. Single metal ions give rise to too much structural diversity to allow rational designs of MOFs, either by adopting different coordination geometries or entirely different extended structures even for the same coordination geometry at the metal center.
4.2 Carboxylate-based MOMs
The anionic, chelating nature of carboxylate ligands is desirable for more than just the robust metal-ligand coordination it affords. The anionic charge neutralizes some or all of the cationic charge imparted by the metal nodes used in SCCs and MOFs. This is especially important for host/guest materials in which these anions will lower the space available in the internal channels and cavities.
The discovery of the first permanently porous MOF in 1998 by Yahgi and coworkers marked an obvious shift towards carboxylate ligands.251 The carboxylate-based linear donor counterpart to 4,4′-bpy is 1,4-benzenedicarboxylate (BDC). While similar to 4,4′-bpy in that it contains two Lewis-basic moieties, BDC is more versatile as a ligand since it has multiple possible binding modes. As shown in Scheme 6, carboxylates are commonly found in three binding modes: a) monodentate, which is noteworthy in that the 4-position of phenylcarboxylates is held collinearly to the metal-oxygen bond; b) bidentate, in which the 4-position is held collinearly to the chelate vector bisecting the two metal-oxygen bonds; c) bridging, in which two metal centers are joined in close-contact. The monodentate mode is most analogous to 4,4′-bpy, the obvious differences being the formal charge imparted by carboxylate and the different steric profile. Thus, many topologies constructed from 4,4′-bpy may also be achieved with BDC. The other two binding modes are significantly different. Chelation of a carboxylate ligand occupies two coordination sites, unlocking new topologies.
Scheme 6.
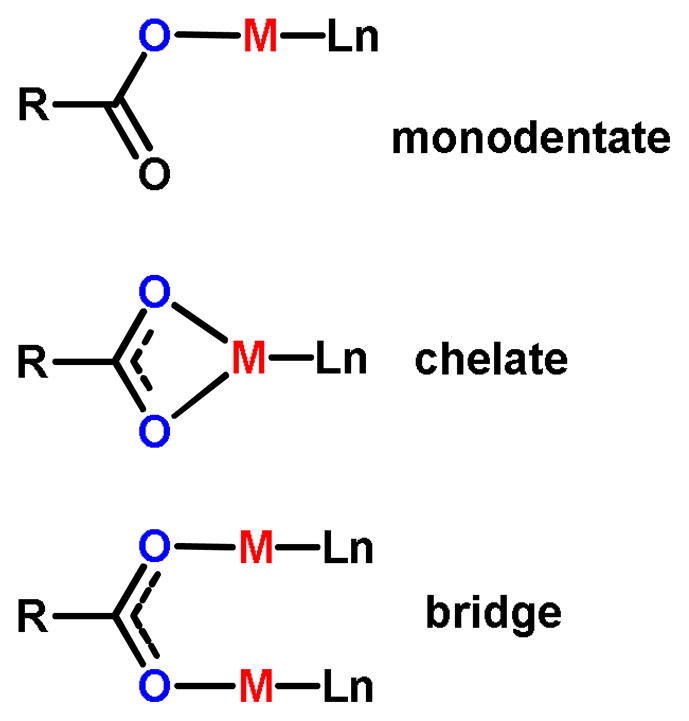
Arguably the best known example of a BDC MOF is given by Yaghi and coworkers assembly of Zn4O(BDC)3·(DMF)8(C6H5Cl), commonly referred to as MOF-5.252 The notoriety of this framework stems from its permanently porous nature upon guest removal by heating at temperatures up to 350°C. The BDC ligands link together Zn nodes possessing a basic zinc acetate structure to generate a cubic network. The unprecedented Langmuir surface area of 2900 m2/g of this framework solidified a reputation that MOFs are highly desirable as new materials for applications requiring high surface areas. MOF-5 is also the simplest of a series of sixteen isoreticular MOFs which demonstrate the power of expansion of a ligand.253 By employing a suite of related dicarboxylate ligands, the pore size and gas absorption properties of the resulting cubic MOFs could by systematically tuned without affecting the overall topology.
The versatile binding modes of the carboxylate functionality make carboxylate-based ligands suitable for a wide number of SBUs, including both mononuclear and polynuclear clusters (Figure 15; Scheme 6.) As such, the same BDC ligand used in MOF-5 readily forms alternative frameworks when used in conjunction with metal precursors and reaction conditions to form different SBUs during assembly. For example, Férey and coworkers combined H2BDC with Cr(NO3)3·9H2O with HF in water under hydrothermal conditions (8 hours at 220°C) resulting in the formation of hydrated Cr3F(H2O)2O[(BDC)]3 (dubbed MIL-101) containing trinuclear clusters in a fundamentally different topology to MOF-5.
The tritopic analogue of BDC, BTC was used in the formation of HKUST-1 by Williams and coworkers, as discussed above.68 An expanded form of BTC in which phenyl spacers are added, 1,3,5-benzenetribenzoate (BTB), can be used to link basic zinc acetate nodes to form a framework called MOF-177 (Figure 38).234a This MOF was synthesized by combining H3BTB with Zn(NO3)2·6H2O in DEF and heating to 100°C for 23 hours. This framework was especially novel when first reported, since it possessed a record-breaking surface area of 4500 m2/g. Like BDC, tricarboxylate ligands are certainly not limited to basic zinc acetate SBUs. The same trinuclear Cr cluster used to form MIL-101 was used a year prior with BTC to generate MIL-100 by combining metallic chromium in an aqueous HF solution with H3BTC and heating for 96 hours before ramping back to room temperature.254 This resulted in the formation of a green powder, characterized as hydrated Cr3F(H2O)3O[C6H3-(CO2)3]2.
Figure 38.
The basic Zn acetate SBU found in MOF-5 can assemble with the tritopic carboxylate ligand 1,3,5-benzenetribenzoate to furnish the highly porous MOF-177.234a The disordered metal nodes (top) are comprised of basic Zn acetate scaffolds (bottom, single part of disorder shown) Atom (color): Zn (yellow), C (grey), O (red). Hydrogen atoms omitted for clarity.
Despite the widespread use of carboxylate ligands for MOFs, a relatively small number of SCCs have employed such species. The first example of a Pt-carboxylate SCC was given by Stang and coworkers in the form of molecular rectangles in which BDC was used to bridge dinuclear organometallic complexes.255 Because of the milder synthetic conditions possible for SCC chemistry, the BDC was added as its sodium salt to an aqueous acetone solution of the diplatinum precursor. Hor and coworkers pioneered the use of mixed pyridyl-carboxylate ligands as linear spacers in the formation of a suite of Pt and Pd squares, triangles, and rectangles.256 Expanded forms of the archetypal 4-pyridylcarboxylic acid were later used by Stang and coworkers to generate a series of rectangles either using mononuclear bis-phosphine platinum nodes or the same dinuclear clips used in the carboxylate-only rectangles described above.257 Additional studies by Mukherjee and coworkers emerged which continued to illustrate the preference for heteroligation in mixed pyridyl-carboxylate and related systems.190,258 The fact that both Pt and Pd centers were observed to contain heteroligated pyridyl-carboxylate coordination in almost all cases led to the realization that using carboxylate ligands and pyridyl ligands in the same synthesis could generate multicomponent assemblies as single thermodynamic products. In 2010, Mukherjee and coworkers reported the first example of a multicomponent Pd-prism,77 while Stang and coworkers provided a suite of 2D and 3D multicomponent Pt-based polygons and cages.79a
The multicomponent approach to panel-directed SCC formation has yet to take advantage of a number of polytopic planar carboxylate ligands, as shown in Scheme 2. In theory, a number of these polycarboxylates could serve as panels to be joined either using dinuclear molecular clips or through multicomponent assembly with a second type of ligand acting as pillars. Since multicomponent assembly is a relatively new development to SCCs, it is likely that carboxylate-based panels will enjoy future use in such assemblies.
Multicomponent MOFs are much more common than multicomponent SCCs. MOFs have the advantage of being extended frameworks in which mixed ligands can disperse throughout the structure. In 2010, Yaghi and coworkers synthesized a number of MOF-5-based frameworks containing up to eight functionalities in a single phase.198f Crystallographic studies in tandem with NMR experiments on segments of large single crystals indicated that macroscopic domains of specific functional ligands were not present. Other examples of multicomponent MOFs have been given by Baiker,259 Burrows,213b and Cohen.213j Quantitative formation of mixed-functionalities is more difficult for SCCs because if two ligands differ only by appended functionalities while possessing the same directionality and type of Lewis-basic sites, statistical mixtures will result. Instead of a single thermodynamic product an assembly will produce all iterations of ligand and metal node combinations. This is why quantitative formation of multicomponent SCCs requires either: i) a driving force for heteroligation using two different Lewis-basic groups; ii) ligands of drastically different directionality, such as for the formation of pyridyl-based prisms; iii) asymmetric ligands designed to have one bulky end which prevent two such ends from coordination to the same metal site.
5. Functionalized MOFs and SCCs
Since both MOFs and SCCs are characterized by having internal cavities or pores, both classes of materials are well-suited for applications involving adsorption or host/guest chemistry. These include gas storage, drug delivery, sensing and more. For certain applications, the extended solid nature of MOFs makes them more attractive, such as hydrogen storage or CO2 uptake where the goal is to maximize the density of the substrate. In other applications, the molecular nature of SCCs imparts an advantage, for instance when solubility is a requirement. The ability to tune the hydrophobic/hydrophilic pockets of an SCC can allow host/guest chemistry and provides a strategy to solubilize drug molecules for targeted delivery or transport, or act as biological sensors.
The functionality of both types of materials is not limited to their inherent pores and cavities, however. Both MOFs and SCCs employ rigid organic ligands as a structural element. These organic linkers can oftentimes be modified with functional groups that can be carefully chosen to not interfere with the self-assembly process. Once the structures are formed, these functional groups can impart unique properties relative to the unfunctionalized materials.
5.1. Chirality and MOMs
Both SCCs and MOFs can be constructed using building blocks that impart chirality on the final materials. There are a few strategies which can be used to obtain chiral structures. The use of chiral bridging ligands or metal nodes is perhaps the most straightforward, wherein the chirality can either be inherent to the ligand itself, or a chiral molecule can be appended to an existing ligand through coupling chemistry. Templated self-assembly in the presence of chiral molecules can also be used. There are also rare examples of homochiral frameworks from achiral building blocks,180a though typically such materials are generated as racemic mixtures.
Achiral building blocks can deliver homochiral frameworks in the presence of chiral coligands which will dictate the handedness of certain structures. For example, Rosseinsky and coworkers260 described the use of 1,2-propanediol as a bidentate coligand in the formation of MOFs comprised of octahedral metal centers and BTC. The coligand binds to in the equatorial plane of the metal nodes and directs the formation of helices, which in the case of 1,2-propanediol results in a chiral framework. Desolvation of the resulting material furnishes chiral pore cavities.
The first examples of chiral SCCs were reported by Stang and coworkers in the form of molecular squares made using carefully selected bidentate donors (2,6-diazaanthracene and 2,6-dizaanthracene-9,10-dione).261 These molecules were oriented orthogonally to the plane of the square and while they still acted as linear, ditopic donors, the relative location of the N-atoms imparted C2h symmetry, rather than the more common D2d or D2h symmetry found in typical ditopic linear ligands. This resulted in two specific face-orientations of the ligands, of which there were four in each molecular square, generating six diasteromeric possibilities. The same group also demonstrated chiral SCCs by appending stereocenters onto tripyridyl methane donors which could then be used to form trigonal prisms.139a
A pioneering example of a porous homochiral MOF built from enantiopure SBUs was provided by Kimoon Kim and coworkers who synthesized chiral building blocks based on tartaric acid.262 When these ligands were combined with zinc ions, trimetallic clusters formed which were held together by Zn-pyridyl bonds. Chirality does not need to be an inherent part of the ligand backbone, but rather can be included via coupling schemes. For example, Stoddart, Yaghi and coworkers appended chiral recognition sites in the form of enantiopure crown ethers with binaphthyl moieties onto linear dicarboxylates.263 The resulting MOFs were thus decorated with numerous chiral sites for host/guest chemistry. The rigidity of binaphthyl groups allows their direct incorporation into the bridging ligands of a MOF, as demonstrated by Lin and coworkers who used dipyridyl binaphthyl ligands to link 1D chains of cadmium chloride.264 The same group had previously reported the use of analogous dipyridyl binaphthyl ligands into SCCs rather than MOFs by linking four fac-capped carbonyl rhenium chloride centers to yield chiral molecular squares.169 These squares possessed enantioselective emission quenching in the presence of 2-amino-1-propanol. The versatility of this ligand type was further illustrated by Lin and coworkers265 in the formation of chiral triangles. In this case, three cis-capped Pt centers were joined by diethynyl binaphthyl ligands and were successful in catalyzing enantioselective additions of diethylzinc to aromatic aldehydes, generating chiral secondary alcohols. Lin’s expertise in generating homochiral frameworks is evident in a 2005 paper which focuses on the synthesis and study of such materials with an emphasis on their use and design.266
In some cases, chiral centers can be incorporated into a MOF scaffold without playing a structure role. For instance, Duan and coworkers267 recently prepared photoactive chiral frameworks by forming 2D sheets of Zn ions with 4,4′,4″-nitrilotribenzoic acid. When L- or D-pyrrolidine-2ylimidazole was present, these proline derivatives occupied the cavities of the MOF, bound to open coordination sites on the Zn centers and assisted in the photochemical asymmetric alkylation of aldehydes.
5.2. Post-Self-Assembly Modifications (PSMs)
Functionalization of both MOFs and SCCs can occur either by preserving or disrupting the overall topology of the MOMs. The ability of MOFs (and by extension, SCCs) to undergo such transformations was predicted by Robson and coworkers, stemming from the observations that reagents could pass through the porous frameworks to access reactive sites. While a relatively new chemistry of MOFs, post-synthetic modifications (PSMs) have been effective enough to warrant a critical review.268 Early examples of MOF modifications include the reversible crystal-to-crystal transformation of Ag(TEB)(OTf)·2C6H6 (TEB = 1,3,5-tris(3-ethynylbenzonitrile) shown in 1995 by Lee and coworkers to release four equivalents of benzene molecules upon heating while maintaining structural integrity.269 The loss of guest molecules following assembly is an important aspect of MOF chemistry through which porous materials may be accessed.38
One of the simplest forms of PSM is anion exchange. It is typically more facile to exchange the anions of an SCC versus a MOF since the former are typically more soluble and the counterions are not trapped within an infinite array. In many cases, anion exchange reactions of SCCs are identical to those of small coordination complexes. A suitable solvent is selected in which the SCC is soluble and a salt containing the desired anion is added after which either the SCC or salt is precipitated from solution. Pt-based assemblies initially isolated as triflate salts can be converted to their corresponding hexafluorophosphate forms by treatment with a PF6− salt. For example, a truncated tetrahedron possessing a 24+ cationic charge has been formed by Stang and coworkers by combining twelve equivalents of Pt(PEt3)2(OTf)2 with four equivalents of a hexapyridyl donor. Treatment of the triflate complex with KPF6 in aqueous acetone solution replaces all twenty four triflate counterions with hexafluorophosphate, causing the material to precipitate out of solution.270 This approach has been successful for both 2D and 3D SCCs with both pyridyl and carboxylate ligands.79a
Anion exchanges in MOFs may occur, however whereas salt metathesis occurs within minutes for SCCs, the reaction time is often longer for frameworks in which the anions are embedded throughout and the materials are not soluble. For example, Oliver and coworkers have shown reversible anion exchange on Cu and Ag-based MOFs of the type M2(4,4′-bipy)2(EDS)·4H2O (M = Ag, Cu; EDS = O3SCH2CH2SO3).174c The ethanedisulfonate anions could be replaced by either nitrate or perchlorate by soaking the crystalline material in aqueous 0.1 M solutions of the salts. The exchange took seven days for static mixtures and up to three days for stirred solutions, after which the crystals could be collected and washed. The EDS anions could be replaced by subsequent three-day stirring in a 0.1 M EDS solution.
Long and coworkers have demonstrated cation exchange in MOFs by exploiting charge-balancing Mn2+ guest ions in Mn3[(Mn4Cl)3(BTT)8(CH3OH)10]2 (BTT = 1,3,5-benzenetristetrazolate).271 The three equivalents of Mn2+ were replaced with Fe, Co, Ni,Cu, Zn, Li, and Cu by immersing the parent Mn MOF in concentrated methanolic solutions of the metal chloride salts. Fresh solutions were added three times over the course of one month to achieve cation exchange, after which the crystals were washed with methanol. Exchange of the Mn2+ with singly charged Li+ or Cu+ was problematic and incomplete exchange was observed, attributed to the lack of available space to accommodate six equivalents of monocation per manganese site.
If a MOM possesses uncoordinated Lewis-basic sites, PSM can involve treatment with an exogenous metal source to induce coordination. Lin and coworkers described a Cd-based MOF constructed with a binaphthyl bipyridine ligand with two hydroxy groups. The pyridyl functionalities acted as primary ligation sites leaving the hydroxy groups free to bind titanium ions upon treatment with Ti(OiPr)4.264 This bimetallic MOF was an active catalyst for enantioselective addition of diethylzinc to aromatic aldehydes. In a similar design Stang and coworkers have described the synthesis of polygons with uncoordinated pyridyl sites, however no metallations have been reported using these materials.272
The incorporation of metal centers has been demonstrated on MOFs even when an obvious site for PSM is not present. In 2008, Long and coworkers hypothesized that the BDC ligands of MOF-5 could bind metals via η6 coordination of their benzene rings.273 This was achieved by treatment of MOF-5 with Cr(CO)6 as a mixture in ether a sealed ampoule for five days at 140°C. The framework was stable to guest solvent removal after which the Cr could be used for gas uptake upon photolysis, liberating CO and opening up coordination sites.
It is also possible to initiate PSM of MOFs by treatment with exogenous ligands rather than metals. In 2008, Hupp and coworkers described the removal of DMF from [Zn2(btba)(DMF)2]n(DMF)m (btba = 4,4′,4,4-benzene-1,2,4,5-tetrayltetrabenzoic acid) from its position on the axial sites of the Zn paddlewheel SBUs.240a This was accomplished by heating the material at 150°C for 24 h under vacuum. Treatment of activated MOF in CH2Cl2 solutions of five different pyridyl ligands resulted in uptake of the exogenous Lewis bases over 24 hours. Characterization of the resulting materials indicated that all axial sites of the Zn paddlewheel nodes were occupied, resulting in enhanced CO2/N2 uptake selectivity in the materials.274
Covalent transformations of MOMs typically involve PSM using well-established organic reactions which take place on functionalities built into the organic linkers (Figure 40). One early example of this type of PSM is given by Cohen and coworkers who systematically investigated amide couplings by using amine-functionalized SBUs in the construction of porous MOFs.275 For example, they used monoaminated BDC (NH2-BDC) as a linker for Zn-based MOFs which could be modified by one of two methods. The first involved treatment of vacuum-dried material with organic anhydrides in chloroform solution. After three days the solid material was collected and washed for three additional days before being isolated and dried. The second method involved heating the MOF/anhydride mixtures in chloroform for 24 hours, replacing the anhydride solutions, and reheating for 24 hours. This resulted in the formation of amido-functionalized materials. This reactivity was demonstrated for a variety of alkyl anhydrides, revealing that short-chain anhydrides were effective amide-coupling reagents, giving quantitative modifications, whereas long-chain anhydrides (hexane or longer) greatly reduced the efficacy of coupling. The eighteen-carbon chain anhydride modified less than 10% of the amine sites.208n
Figure 40.
Covalent post-synthetic modifications are possible when SCCs or MOFs are constructed with ligands possessing reactive sites.
In some cases, amine-functionalized MOFs can produced multi-functional species. After demonstrating a series of individual functionalizations using various reagents for amide couplings, Cohen and coworkers treated IRMOF-3 (an amine-functionalized derivative of MOF-5) with a mix of anhydrides and isocyanates in a stepwise fashion.276 By utilizing smaller, highly reactive reagents in the final functionalization steps, such as crotonic anhydride, it was possible to generate a MOF containing up to four different amides. These couplings were performed by treatment of crystalline samples of IRMOF-3 with the desired coupling agent in CHCl3 for up to three days, refreshing the CHCl3 solutions every 24 hours.
A PSM need not be entirely functional in nature. A recent report by Zhang and coworkers277 describes the incorporation of rigid pillars into a MOF which tunes both the stability and porosity of the resulting frameworks. By treating Zn and Cu-carboxylate-based MOFs with 4,4′-bipy in solution at elevated temperatures for 2–3 days, the bipyridine molecules were taken up into the MOFs and rigidified the structures. In the case of the Cu-based framework, this increased rigidity resulted in a notable change in CO2 over N2 selective gas absorption.
Covalent PSMs using SCCs are rarer than their MOF counterparts. However, since similar ligands can be employed, form a practical standpoint such reactions may be more facile for SCCs given that reagents can more effectively reach reactive sites in the discrete structures and conditions can be found in which all materials are soluble. Stang and coworkers have demonstrated similar amide-couplings using amine-functionalized SCCs. A amine or succinimide-decorated benzenedicarboxylate ligand was used in the construction of supramolecular hexagonal prisms.149a Treatment of the amine-functionalized prisms with isocyanate or maleic anhydride for 12 hours in nitromethane solutions effected coupling transformations in greater than 70% isolated yields. The succinimide prism was decorated with a ferrocene-containing moiety upon a Diels-Alder reaction with (9-methylene anthracenyl)-1-ferrocenoate.
These covalent transformations are not limited to amide couplings. The same NH2-BDC used in Cohen’s PSMs has been employed by Yaghi and coworkers for a condensation reaction with aldehydes to furnish imine-containing MOFs.278 The (Zn4O)3(BDC-NH2)3(BTB)4 framework (BTB = benzene tribenzoate) was treated with 2-pyridinecarboxaldehyde in toluene for five days to form the imine MOF. This material was suitable for further PSM since the imine sites could bind additional metal centers. As such, the addition of 1.5 equivalents of Pd(MeCN)2Cl2 afforded quantitative metallation of the iminopyridine sites with Pd ions.
Another well-known small-molecule coupling that has been used for PSM is the azide-alkyne Huisgen cycloaddition279 popularized under the moniker “click chemistry,”280 first demonstrated on MOFs by Sada and coworkers.281 They described the synthesis of an azide-decorated framework by combining a diazide phenylcarboxylates ligand with Zn(II) ions. The resulting MOF was analogous to the undecorated analog dubbed MOF-16 by Yaghi and coworkers and is an expanded form of MOF-5.253 Treatment of the isolated framework with small alkynes was carried out at 80°C in the presence of CuBr in N,N-diethylformamide. As the reaction progressed, the stretching band of the azides observable in IR spectroscopy vanished, indicating a quantitative yield. Observations of crystals of the framework throughout the cycloaddition indicated that the material did not dissolve in order to facilitate coupling.
Shortly thereafter, Hupp and coworkers applied this transformation to a Zn-cornered MOF containing two types of bridging ligands, a 2,6-naphthalenedicarboxylate and a TMS-protected acetylene-functionalized dipyridylethene compound.222d The resulting material formed a doubly interweaved network in the solid state which was subjected to TMS deprotection using THF solutions of tetrabutyl ammonium fluoride (TBAF). After treatment with TBAF, the MOF was combined with ethidium bromide monoazide to install a fluorescent handle to assess successful cycloaddition. Complete characterization indicated that while click chemistry had occurred, less than 0.8% of the acetylene sites were modified. The low conversion was attributed to the chemistry being limited to the surface of the MOF. While the material was microporous (BET surface area of ~510 m2 g−1), the pore width of 5.1 Å was apparently too small to allow efficient diffusion of reagents throughout the material.
These experiments set the groundwork for a general method of click chemistry on MOFs utilizing amino-decorated frameworks as precursors. Farrusseng and coworkers described “one pot click” reactions on MOFs utilizing the NH2-BDC SBU.208i Treatment of the amino-MOF with tBuONO and TMS-N3 in THF for 24 hours generated the azide-decorated framework. This material was used without workup for click reactions with phenylacetylene with a copper catalyst. After 24 hours of stirring the azide stretching band was no longer observable, supporting quantitative click chemistry had occurred, as in the Sada system.
Recently, the first example of a Huisgen cycloaddition using an SCC scaffold was reported by Stang and coworkers who utilized copper-free click chemistry on cyclooctyne-functionalized rhomboids.282 Given that the PSM chemistry of SCCs is similar to that of MOFs, it is likely that such reactions will find growing use to decorate the organic ligands of various discrete constructs. In fact, since the functional sites of the ligands are more accessible in SCCs, as described above, such reactions are likely to meet or exceed the efficiency of similar transformations on MOFs. The challenge of suitable reagents being limited by pore size is a fundamental issue concerning PSM of MOFs.283 A second concern which is valid for both MOFs and SCCs is that the functionalities used for PSM must not interfere with the self-assembly process. This can be an issue when such sites can act as competing ligands for the metal nodes. On a related note, the reagents added to the assembled materials must be carefully chosen to not disrupt structural integrity due to competitive binding with labile SBUs.
5.3 Applications
The popularization of MOFs starting in the 1990s was spurred by the promising applications of permanently porous, high surface area materials, a motivation that continues today.284 As described in the introduction, metal-organic frameworks were synthesized well prior to the relatively recent emergence of MOF chemistry. It wasn’t until examples of catalysis, gas storage, and other uses, however, that the field of MOFs reached its current level of rigorous and systematic study. The main advantage of MOFs over alternative materials is the tunability of pore size and shape, topology, and functionality. This is a direct result of the modularity of the node-and-spacer design in which building blocks can be judiciously selected to deliver a framework best suited to address a particular application. In addition to the tunability resulting from the directionality of the SBUs and organic linkers, a systematic examination of various building blocks has revealed design considerations for frameworks that can retain crystallinity under a variety of conditions, including solvent or guest removal or post-assembly chemical reactions.
SCCs share the same features of node-and-spacer design and tunability and as such, hold similar promise in their use in practical applications. While some common uses are sought, these applications diverge as a result of fundamental difference between MOFs and SCCs, their infinite versus discrete structures. Since MOFs are typically synthesized as large macroscopic crystals, they are much better suited to take up guest molecules into their internal pores, resulting in applications in gas separation, purification and storage. These applications fall under the general category of solid phase guest storage which requires high porosity to maximize uptake as well as continuous networks to minimize the amount of wasted space in a given volume. Such applications make little sense for soluble, discrete MOMs. While also adept for host/guest chemistry, there is little advantage to using discrete supramolecules which lack the extra stability afforded by an infinite network topology and whose solid-state forms may be easily dissolved. Because the entirety of a solid sample of a MOF is the framework itself, surface area is maximized. A crystal of an SCC will have solvent-occupied voids and regions of intermolecular interactions which are not defined or tunable like the internal cavity. As such, applications of SCCs are logically more geared towards uses which can exploit individual supramolecule/guest interactions, namely solution phase guest storage. For example, the delivery of biological agents or other in vivo applications which require soluble, biologically compatible materials. Both SCCs and MOFs are excellent candidates for catalysis either by the materials themselves for by guest catalyst molecules. MOFs make practical sense for high-throughput heterogeneous applications while SCCs offer better opportunities to characterize the chemical transformations taking place, since their soluble nature allows traditional solution-based techniques to be used while still allowing for crystallizations to employ diffraction techniques.
The application most synonymous with MOF chemistry is gas uptake and storage. As such, the design of MOFs has evolved to target the uptake of specific gases. Many excellent summaries of the interactions of MOFs with specific molecules exist. Recently, Long and coworkers reviewed the uptake of CO2, including selective CO2 sequestration from pre- and post-combustion mixtures.285 The topic of PSM has also recently proven relevant to gas uptake in a paper by Zaworotko and coworkers in which biphenyl-3,4′,5-tricarboxylate and tetrapyridyl porphyrin was used in the formation of a Cd-based MOF.286 This MOF underwent single-crystal-to-single-crystal transformations upon addition of simple metal chloride salts through which the metal cations were taken up into the framework. This PSM resulted in a change in the CO2/CH4 binding isotherms, revealing that the metal-containing frameworks were up to 42% more selective for CO2 (for the Mn2+ PSM). Another recent review on hydrogen storage in MOFs was given by Suh and coworkers which focused on MOFs formed with various ligand types, from carboxylates to imidazolates and mixed-ligand systems.41 The uptake of alcohols and hydrocarbons is also of interest, as evidenced by two reviews by Li and coworkers287 and Snurr and coworkers.288 The former focuses on commensurate absorption in which the guest molecule reaches an uptake equilibrium that is consistent with the internal cavity size and symmetry of the MOF. The latter highlights computation studies on the interactions of MOFs specifically with methane, acetylene and hydrogen.
While the solubility and host/guest capabilities of SCCs make them well-suited for biomedical applications, there has been growing progress in the use of MOFs in such applications as well. Serre, Horcajada and coworkers recently summarized biological MOF chemistry.44a Their review stresses the lack of toxicological studies of MOFs and discusses the various synthetic methods available to create particle sizes and delivery mechanisms which are required if such materials are to be effective medicinal agents. While much work remains to find biocompatible and stable MOFs, preliminary work has shown that certain frameworks can obtain high loadings of pharmaceuticals for drug delivery. By expanding the basic structure of Ni2(dhtp)(H2O)2·8H2O (dhtp = dihydroxylterephthalic acid), first discovered289 and dubbed CPO-27 by Dietzel and coworkers and later renamed MOF-74, to an isoreticular series, Yaghi and coworkers created MOFs with extremely large pore dimensions were obtained. MOF-74 is constructed from a trinuclear metal-oxide cluster and dioxidoterphthalate (DOT). Like BDC, DOT may be expanded by including additional phenyl rings. The larger MOFs of this isoreticular series are capable of encapsulating proteins such as myoglobin in the hexagonal channels found in the extended network.290 The formation of large pore sizes is not trivial since MOFs have a tendency to interpenetrate. That is, unique arrays will thread into each other to occupy void spaces in a crystal. A major challenge in forming large pore diameter networks is avoiding such interpenetration which obviates any practical use of such internal cavities. By virtue of being discrete, soluble constructs, SCCs tend to circumvent interpenetration due to the entropic penalties associated with fusing two cages together. As such, large cavities in SCCs have been achieved, with the formation of dodecahedra and cuboctahedra by Stang and coworkers as pioneering examples.28a,29b Fujita and coworkers have made recent progress towards protein encapsulation within a cage with a report on the formation of a protein-tethered ligand which is poised to self-assemble into a molecular sphere (M12L24 cuboctahedron) with one protein per cavity.291
The biomedical applications of SCCs is by no means a mature field, however, significant inroads have been made. Interactions of supramolecules with DNA have been reviewed by Hannon.292 Therrien and coworkers have established the significance of Ru-based self-assembly in biological applications with studies on interactions between tetragonal prisms and DNA293 as well as anticancer studies.175q Pt-based squares have been shown to bind to G-quadruplex DNA and act as telomerase inhibitors, as first described by Sleiman and coworkers 294 and later explored by Mao and coworkers.295 Chi and coworkers have recently been studying a suite of Ru-based rectangles and prisms which have shown promise as anticancer agents,110e,296 and well as the interaction of such species with DNA.297
Both MOFs and SCCs have shown promise in sensing and other photophysical-based applications, since the binding of an analyte to their internal cavities can oftentimes induce electronic structure changes which result in emission changes. Hupp and coworkers have described Zn-based ZIF type MOFs in the sensing of chemical vapors and gases, such as propane.298 Such sensing is oftentimes carried out by forming a thin MOF film, either by sequential immersion of a substrate in solutions of the molecular precursors210l or by particle deposition.299 In some cases, MOF films can form within gel layers.208j Hupp and coworkers recently reviewed the use of MOFs in sensing applications, encompassing photoluminescence-based sensors as well as more exotic techniques such as electromechanical techniques such as surface acoustic wave devices and microcantilevers.43 That said, host/guest sensing is not the only photophysical-based application of such materials. Qian and coworkers300 recently described the nonlinear optical properties of an In-based MOF capable of orienting an organic dipolar chromophore into an ordered array. The resulting host/guest ensemble is an interesting proof-of-concept for the potential development of nonlinear optical materials. This approach affords a higher degree of control over chromophore-based ligands which may be directly incorporated into a framework in a manner which does not necessarily optimize the optical properties of the MOF due to non-ideal orientations of the chromophores, which are forced to specific arrangements due to their role as structural elements..44b
Photoluminescence quenching by SCCs is well documented. Severin and coworkers demonstrated that chiral squares may be quenched by amino acids in organic solutions.169 Mirkin and coworkers showed that chiral assemblies made via the weak-link approach can be used as enantioselective sensors using a Cu-based assembly with mandelic acid as an example.301 Stang, Chi and coworkers also used photoluminescence as the basis for the detection of nitroaromatics using trigonal prismatic cages constructed from Ru-based precursors.141i Mukherjee and coworkers have reported both MOF302 and SCC sensors for nitroaromatic-containing explosives, as well.7j The former consisted of micron sized particles of a Zn carboxylate MOF dispersed in ethanol which could be recycled after use by centrifugation and subsequent washing. The latter consisted of trigonal prismatic cage which was soluble in organic solvents and whose emission was quenched in the presence of nitroaromatic-containing compounds.
Catalysis has been carried out in both MOFs and SCCs with success both in achieving efficient transformations as well as the characterization of reactive intermediates. A recent review by Kim and coworkers focuses on homochiral MOFs which may be used for asymmetric catalysis under heterogeneous conditions.303 Twenty seven different frameworks are described, carrying out a range of transformations from the ring-opening of epoxides to Diels-Alder reactions. Corma and coworkers have also recently reviewed heterogeneous MOF catalysis with a broad focus from hydrogenations, CO oxidation and photocatalysis44c. This review categorizes catalytically active MOFs based on those with reactive metal centers, reactive functional groups, and those with cavity-based reactivity.
The internal cavities of SCCs are also well-suited for such transformations. Examples of Diels-Alder reactions have been given by Fujita and coworkers,137o,304 as well as Mukherjee and coworkers.305 Raymond and coworkers have demonstrated cavity controlled catalytic reactions using cages made via symmetry-interaction self-assembly, including examples of aza-Cope rearrangements306 and Nazarov cyclizations.307 Raymond has also reported interesting examples of acid-catalyzed reactivity in basic solutions, such as the hydrolysis of orthoformates.308 Hupp and coworkers have also described selective olefin epoxidations and enantioselective sulfoxidations reactivity using interesting SCCs constructed from eighteen porphyrin units and containing four Zn trimers, two Sn dimers, and a single Mn dimer.309 The stabilization of otherwise reactive intermediates can be achieved by using suitably-sized prisms. Raymond and coworkers showed that 16-electron organometallic species can be stabilized for weeks while remaining an active species demonstrated via reaction with CO.310 Similarly, Fujita and coworkers showed that a truncated tetrahedron is also capable of stabilizing unusual organometallic intermediates via in situ crystallographic techniques.311 An excellent summary of SCCs used for catalysis and characterization of reactive intermediates can be found in the general review of SCCs by Chakrabarty and coworkers.12
6. Conclusion
The rich ongoing research of SCCs and MOFs is a testament to the structural and functional versatility of coordination chemistry. While the fundamental themes behind both were established a century ago with the birth of coordination chemistry, the pioneering studies motivating modern metal-organic materials emerged merely decades ago. In a short time, both fields have matured and grown to encompass the rich chemistry described above, with topics ranging from design methodologies to post-self-assembly chemistry to functional applications. This rapid development attests to the power of coordination-driven self-assembly and directional bonding, which are central to all metal-organic materials. Each newly discovered molecular building block further extends the library of possible structures, increasing the complexity and functionality of materials without commensurate increases to synthetic difficulty. As such, the ongoing development of new scaffolds and design strategies together with the adaptation and application of existing small molecule chemistry to SCCs and MOFs ensures that both will enjoy continued growth.
As shown in this review, the interplay between the two fields is significant but understated. There are common themes spanning all aspects of the two classes of metal-organic materials, from design strategies and synthesis to structure and function. A solid understanding of both fields is beneficial in that concepts from one can be adapted or in some cases seamlessly applied to the other. Emerging strategies such as multi-component assembly and post-self-assembly modifications can be studied on both systems, and the lessons learned from one field can accelerate studies in the other. In fact, as larger SCCs are developed and new MOFs created with SCCs as primary building blocks, the division between fields is blurred. This review is intended to illustrate the commonalities in design, building blocks and applications while also pointing out how and why the chemistry of MOFs and SCCs is unique. It should serve as an introduction to the origin of coordination-driven self-assembly at the heart of both materials and inspire new directions in MOF and SCC chemistry.
Figure 16.
Simple ditopic, tritopic and tetratopic organic linkers represented by 1,4-benzenedicarboxylate, 1,3,5-benzenetricarboxylate and 1,3,5,7-adamantanetetracarboxylate. These basic ligands can be tuned both spatially and functionally while maintaining the same rigid directionality of binding sites.
Figure 23.
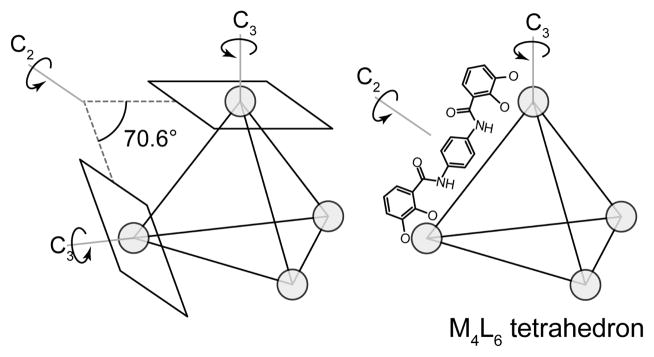
M4L6 tetrahedra can be constructed by designing C2 symmetric ligands with the proper angle between coordination vectors such that all chelation falls on the four chelate planes.
Figure 39.
A binaphthyl moiety provides a rigid, functionalizable platform from which linear bridging ligands can be constructed which are appropriate for both SCC and MOF formation as one strategy to obtain chiral materials.
Table 1.
N-donor-based ligands for SCC and MOF formation
| Ligand | Binding sites | Symmetrya | References |
|---|---|---|---|
| N1 | 2 | D2h | 107 |
| N2 | 2 | Cs | 7k,108 |
| N3 | 2 | D2h | 109 |
| N4 | 2 | D2h | 110 |
| N5 | 2 | D2h | 110e,111 |
| N6 | 2 | C2h | 112 |
| N7 | 2 | D2h | 113 |
| N8 | 2 | C2v | 7a |
| N9 | 2 | D2h | 114 |
| N10 | 2 | D2h | 115 |
| N11 | 2 | D2h | 116 |
| N12 | 2 | C2v | 117 |
| N13 | 2 | C2v | 118 |
| N14 | 2 | C2v | 119 |
| N15 | 2 | C2v | 24a,119–120 |
| N16 | 2 | C2v | 51–52,121 |
| N17 | 2 | C2v | 122 |
| N18 | 2 | C2v | 123 |
| N19 | 2 | C2v | 124 |
| N20 | 2 | C2v | 125 |
| N21 | 2 | C1 | 126 |
| N22 | 2 | C2v | 127 |
| N23 | 2 | C2v | 128 |
| N24 | 2 | C2v | 129 |
| N25 | 2 | C2v | 130 |
| N26 | 2 | C2v | 131 |
| N27 | 2 | C2h | 132 |
| N28 | 2 | C2v | 26c |
| N29 | 2 | C2v | 132a,133 |
| N30 | 2 | C2 | 134 |
| N31 | 2 | C2v | 120d,135 |
| N32 | 3 | D3h | 136 |
| N33 | 3 | C3h | 137 |
| N34 | 3 | C3h | 132a,133a,137g,138 |
| N35 | 3 | C3v | 29b,c,120c,124,139 |
| N36 | 3 | C3v | 140 |
| N37 | 3 | D3h | 141 |
| N38 | 3 | C3v | 139d,139f,g,142 |
| N39 | 3 | C3h | 143 |
| N40 | 4 | D2h | 144 |
| N41 | 4 | C1 | 141a,145 |
| N42 | 4 | D2h | 125a,146 |
| N43 | 4 | D2h | 79a,147 |
| N44 | 4 | D2h | 148 |
| N45 | 6 | D6h | 149 |
| N46 | 2 | D2h | 150,151 |
| N47 | 2 | D2h | 152, 95j, 100b, 133, 24a,49,79b,115j,120b,153 |
| N48 | 2 | C2h | 154 |
| N49 | 2 | D2h | 110a,110e,154d,155 |
| N50 | 2 | D2h | 113,154b,156 |
| N51 | 2 | D2h | 110e,132g,151c,154d,157 |
| N52 | 2 | D2h | 113,152b,156b,c,158 |
| N53 | 2 | D2h | 157b,157g,159 |
| N54 | 2 | C2h | 160 |
| N55 | 2 | C2h | 161, 147b |
| N56 | 2 | D2h | 162, 115j |
| N57 | 2 | C2v | 163, 7h,i,164 |
| N58 | 2 | C2v | 165 |
| N59 | 2 | C2v | 166,167 |
| N60 | 2 | C2v | 168, 169 |
| N61 | 2 | C2v | 170, 171 |
| N62 | 2 | D2h | 172, 172c |
| N63 | 4 | D4h | 150ag,173, 75,79a |
| N64 | 3 | D3h | 152c,152h,152j,152m–q,152t,174, 175 |
| N65 | 3 | C3h | 141g,176 |
| N66 | 4 | Td | 177 |
| N67 | 2 | C2h | 178 |
| N68 | 2 | C2h | 179 |
| N69 | 2 | C2v | 180 |
| N70 | 2 | C2v | 181 |
| N71 | 3 | C3h | 182 |
| N72 | 3 | C3h | 183 |
| N73 | 3 | C3h | 184 |
| N74 | 3 | C3h | 179d,185 |
| N75 | 3 | D3h | 186 |
| N76 | 4 | C2v | 187 |
| N77 | 4 | C2v | 188 |
The symmetry of certain pyridyl-containing ligands depends on the relative orientation of the pyridyl-groups, especially for 3-pyridyl species. As such, the point groups assigned may reflect a particular conformation of a ligand which can change upon free rotation about certain bonds, altering the symmetry.
Table 2.
O-donor-based ligands for SCC and MOF formation
| Ligand | Binding sites | Symmetrya | References |
|---|---|---|---|
| O1 | 2 | C2h | 189 |
| O2 | 2 | Cs | 190 |
| O3 | 2 | C2h | 191 |
| O4 | 2 | Cs | 192 |
| O5 | 2 | Cs | 67 |
| O6 | 2 | C2v | 65,193 |
| O7 | 2 | C2v | 194 |
| O8 | 4 | C4v | 195 |
| O9 | 2 | Ci | 196, 197 |
| O10 | 2 | C2h | 152c,152u,174k,198, 151o,199 |
| O11 | 2 | Cs | 198z,200, 151p,193c,200p,201 |
| O12 | 2 | C2h | 202, 203 |
| O13 | 2 | C2h | 204, 194 |
| O14 | 2 | C2h | 198p,200e,205, 197b |
| O15 | 2 | Cs | 152n,198ae,206, 207 |
| O16 | 2 | Cs | 152j,198f,198p,198w,208, 147b |
| O17 | 2 | C2v | 165b,198p,198x,198z,200p,208h,208o,209, 149b |
| O18 | 3 | C3h | 98,198z,198ae,208h,208j,210, 211 |
| O19 | 2 | C2v | 58,212 |
| O20 | 2 | Cs | 174d,198f,198p,198w,208b,208d,e,208h,213 |
| O21 | 2 | Cs | 198v,210s,214 |
| O22 | 2 | C2v | 208h,213d,215 |
| O23 | 2 | Cs | 209p,q,216 |
| O24 | 2 | C2v | 206b,217 |
| O25 | 2 | Cs | 218 |
| O26 | 2 | C2v | 219 |
| O27 | 2 | C2v | 198z,208h,220 |
| O28 | 2 | C2v | 63 |
| O29 | 2 | C2v | 221 |
| O30 | 2 | C2h | 58,165b,198u,198z,209e,209l,209n,o,222 |
| O31 | 2 | C2h | 200p,223 |
| O32 | 2 | C2v | 209h,224 |
| O33 | 2 | Cs | 200f,225 |
| O34 | 2 | Cs | 200b,200f,g,200p,205f,217u,226 |
| O35 | 4 | Cs | 227 |
| O36 | 2 | C2v | 228 |
| O37 | 2 | C2 | 229 |
| O38 | 3 | Cs | 230 |
| O39 | 3 | C3 | 177c,231 |
| O40 | 3 | C3 | 198r,232 |
| O41 | 3 | C3h | 209e,233 |
| O42 | 3 | C3h | 58,198r,198v,198y,198ae,209g,213i,213l,222g,234 |
| O43 | 4 | C2v | 235 |
| O44 | 4 | C2v | 236 |
| O45 | 4 | C2v | 234d,237 |
| O46 | 4 | C2h | 200i |
| O47 | 4 | C2v | 238 |
| O48 | 4 | C4h | 152b,152d,172a,239 |
| O49 | 4 | C2v | 156c,161,172b,240 |
| O50 | 4 | Td | 58,198z,241 |
| O51 | 6 | C6h | 242 |
The symmetry of carboxylic acid-containing ligands oftentimes depends on the relative orientations of the acid moieties. Rotation about Cα-Cβ bonds can oftentimes result in a different molecular symmetry. As such, the point groups assigned here are not rigorous and a practical use of these ligands requires a consideration of how the carboxylate groups may bind and the ramifications that monodentate coordination versus chelation have on local symmetry.
Acknowledgments
P.J.S. thanks the NIH and NSF for continued financial support over the years of our studies in coordination-driven self-assembly (NIH- GM-057052; NSF-CHE 0820955).
Abbreviations
- 4,4′-bpy
4,4′-bipyridine
- ATC
1,3,5,7-adamantanetetracarboxylate
- BDC
1,4-benzenedicarboxylate
- BTB
1,3,5-benzenetribenzoate
- BTC
1,3,5-benzenetricarboxylate
- CDC
9H-carbazole-3,6-dicarboxylate
- dhtp
dihydroxylterephthalic acid
- DMA
N,N-dimethylacetamide
- DOT
dioxidoterphthalate
- dppf
1,1′-bis(diphenylphosphino)ferrocene
- ICP
infinite coordination polymer
- im
imidazolate
- IRMOF
isoreticular metal-organic framework
- MAFs
metal-azolate frameworks
- MB
3-methyl-1-butanol
- MOF
metal-organic framework
- MOM
metal-organic material
- MOP
metal-organic polyhedron
- MOZ
metal-organic zeolite
- NDB
norbornadiene
- SBB
supramolecular building block
- SBU
secondary building unit
- SCC
supramolecular coordination complex
- tctpm
4,4′,4″,4‴-tetracyanotetraphenylmethane
- tmpa
N,N,N′,N′-tetramethyl-1,3-diaminopropane
- ZIF
zeolite imidazolate framework
Biographies

Timothy R. Cook was born in 1982 and grew up in Moretown, Vermont. In 2001, he began the first of nine years in the Boston area as an undergraduate at Boston University where he was first introduced to inorganic research in the laboratory of Prof. John P. Caradonna. After graduating in 2005 with a B.A. in Chemistry, Tim made a short move across the Charles River to begin graduate research under the guidance of Prof. Daniel G. Nocera at MIT. His doctoral research focused on exploring molecular strategies for solar energy conversion, specifically investigating photo-driven processes to store energy in chemical bonds. After graduating with a Ph.D. in Inorganic Chemistry in 2010, Tim moved to Salt Lake City, Utah, joining the laboratory of Prof. Peter J. Stang where he has been investigating coordination-driven self-assembly for the construction of molecular polygons, polyhedra and prisms with a growing emphasis on the biological applications of such materials.

Yaorong Zheng was born in 1983 in Guangdong, China. He received his B.S. degree in Chemistry from Peking University in 2006. Shortly after, he joined the laboratory of Professor Peter J. Stang in the chemistry department of University of Utah, where he focused his graduate research on multi-component coordination supramolecular systems. After receiving his Ph.D. degree in 2011, he moved to Boston to work with Professor Stephen J. Lippard as a postdoctoral researcher in the Department of Chemistry of the Massachusetts Institute of Technology.

Peter J. Stang obtained his Ph.D. at UC-Berkeley in 1966 and after a two year postdoc at Princeton joined the faculty at the University of Utah in 1969 where is he currently a Distinguished Professor of Chemistry. Since 2002 he has served as the Editor of JACS. He is a fellow of the American Academy of Arts and Sciences, a member of the U.S. National Academy of Sciences and a foreign member of the Chinese, Hungarian and Russian Academies of Sciences. In 2011, he was awarded the U.S. National Medal of Science.
References
- 1.Bowman-James K. Acc Chem Res. 2005;38:671. doi: 10.1021/ar040071t. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen CJ. J Am Chem Soc. 1967;89:7017. [Google Scholar]
- 3.Lehn JM. Pure Appl Chem. 1978;50:871. [Google Scholar]
- 4.Cram DJ, Cram JM. Science. 1974;183:803. doi: 10.1126/science.183.4127.803. [DOI] [PubMed] [Google Scholar]
- 5.(a) Dong SD, Breslow R. Tetrahedron Lett. 1998;39:9343. [Google Scholar]; (b) Breslow R, Dong SD. Chem Rev. 1998;98:1997. doi: 10.1021/cr970011j. [DOI] [PubMed] [Google Scholar]; (c) French RR, Holzer P, Leuenberger MG, Woggon WD. Angew Chem, Int Ed. 2000;39:1267. doi: 10.1002/(sici)1521-3773(20000403)39:7<1267::aid-anie1267>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]; (d) Murakami Y, Kikuchi J-i, Hisaeda Y, Hayashida O. Chem Rev. 1996;96:721. doi: 10.1021/cr9403704. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ahrens MJ, Sinks LE, Rybtchinski B, Liu W, Jones BA, Giaimo JM, Gusev AV, Goshe AJ, Tiede DM, Wasielewski MR. J Am Chem Soc. 2004;126:8284. doi: 10.1021/ja039820c. [DOI] [PubMed] [Google Scholar]; (b) Kumar CV, Duff MR. J Am Chem Soc. 2009;131:16024. doi: 10.1021/ja904551n. [DOI] [PubMed] [Google Scholar]; (c) Warnan J, Pellegrin Y, Blart E, Odobel F. Chem Commun. 2012;48:675. doi: 10.1039/c1cc16066d. [DOI] [PubMed] [Google Scholar]; (d) Seth J, Palaniappan V, Wagner RW, Johnson TE, Lindsey JS, Bocian DF. J Am Chem Soc. 1996;118:11194. [Google Scholar]; (e) Balzani V, Ceroni P, Maestri M, Vicinelli V. Curr Opin Chem Biol. 2003;7:657. doi: 10.1016/j.cbpa.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.(a) Resendiz MJE, Noveron JC, Disteldorf H, Fischer S, Stang PJ. Org Lett. 2004;6:651. doi: 10.1021/ol035587b. [DOI] [PubMed] [Google Scholar]; (b) Vajpayee V, Song YH, Lee MH, Kim H, Wang M, Stang PJ, Chi KW. Chem–Eur J. 2011;17:7837. doi: 10.1002/chem.201100242. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Carr DJ, Lambert L, Hibbs ED, Hursthouse BM, Abdul Malik MK, Tucker HRJ. Chem Commun. 1997:1649. [Google Scholar]; (d) Xu M, Wu S, Zeng F, Yu C. Langmuir. 2009;26:4529. doi: 10.1021/la9033244. [DOI] [PubMed] [Google Scholar]; (e) Palacios MA, Nishiyabu R, Marquez M, Anzenbacher P. J Am Chem Soc. 2007;129:7538. doi: 10.1021/ja0704784. [DOI] [PubMed] [Google Scholar]; (f) Dsouza RN, Pischel U, Nau WM. Chem Rev. 2011;111:7941. doi: 10.1021/cr200213s. [DOI] [PubMed] [Google Scholar]; (g) Hargrove AE, Nieto S, Zhang T, Sessler JL, Anslyn EV. Chem Rev. 2011;111:6603. doi: 10.1021/cr100242s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Shanmugaraju S, Joshi SA, Mukherjee PS. Inorg Chem. 2011;50:11736. doi: 10.1021/ic201745y. [DOI] [PubMed] [Google Scholar]; (i) Shanmugaraju S, Bar AK, Joshi SA, Patil YP, Mukherjee PS. Organometallics. 2011;30:1951. [Google Scholar]; (j) Ghosh S, Mukherjee PS. Organometallics. 2008;27:316. [Google Scholar]; (k) Ghosh S, Chakrabarty R, Mukherjee PS. Inorg Chem. 2009;48:549. doi: 10.1021/ic801381p. [DOI] [PubMed] [Google Scholar]; (l) Anslyn EV. J Am Chem Soc. 2010;132:15833. doi: 10.1021/ja108349y. [DOI] [PubMed] [Google Scholar]
- 8.(a) Wessendorf F, Grimm B, Guldi DM, Hirsch A. J Am Chem Soc. 2010;132:10786. doi: 10.1021/ja101937w. [DOI] [PubMed] [Google Scholar]; (b) Puigmartí-Luis J, Minoia A, Uji-i H, Rovira C, Cornil J, De Feyter S, Lazzaroni R, Amabilino DB. J Am Chem Soc. 2006;128:12602. doi: 10.1021/ja0640288. [DOI] [PubMed] [Google Scholar]; (c) Zhang W, Jin W, Fukushima T, Saeki A, Seki S, Aida T. Science. 2011;334:340. doi: 10.1126/science.1210369. [DOI] [PubMed] [Google Scholar]; (d) Sumpter BG, Meunier V, Valeev EF, Lampkins AJ, Li H, Castellano RK. J Phys Chem C. 2007;111:18912. [Google Scholar]
- 9.(a) Mukherjee B, Mohanta K, Pal AJ. Chem Mater. 2006;18:3302. [Google Scholar]; (b) Matino F, Arima V, Piacenza M, Della Sala F, Maruccio G, Phaneuf RJ, Del Sole R, Mele G, Vasapollo G, Gigli G, Cingolani R, Rinaldi R. ChemPhysChem. 2009;10:2633. doi: 10.1002/cphc.200900371. [DOI] [PubMed] [Google Scholar]; (c) Wimbush KS, Reus WF, van der Wiel WG, Reinhoudt DN, Whitesides GM, Nijhuis CA, Velders AH. Angew Chem. 2010;122:10374. doi: 10.1002/anie.201003286. [DOI] [PubMed] [Google Scholar]
- 10.(a) Ungar G, Liu Y, Zeng X, Percec V, Cho WD. Science. 2003;299:1208. doi: 10.1126/science.1078849. [DOI] [PubMed] [Google Scholar]; (b) Percec V. Phil Trans R Soc A. 2006;364:2709. doi: 10.1098/rsta.2006.1848. [DOI] [PubMed] [Google Scholar]
- 11.(a) Yoshizawa M, Klosterman JK, Fujita M. Angew Chem, Int Ed. 2009;48:3418. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]; (b) Maurizot V, Yoshizawa M, Kawano M, Fujita M. Dalton Trans. 2006:2750. doi: 10.1039/b516548m. [DOI] [PubMed] [Google Scholar]; (c) Pluth MD, Bergman RG, Raymond KN. Acc Chem Res. 2009;42:1650. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; (d) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc Chem Res. 2004;38:349. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarty R, Mukherjee PS, Stang PJ. Chem Rev. 2011;111:6810. doi: 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stricklen P, Verkade J. J Am Chem Soc. 1983;105:2494. [Google Scholar]
- 14.Kim Y, Verkade JG. J Organomet Chem. 2003;669:32. [Google Scholar]
- 15.Fujita M, Yazaki J, Ogura K. J Am Chem Soc. 1990;112:5645. [Google Scholar]
- 16.Stang PJ, Cao DH. J Am Chem Soc. 1994;116:4981. [Google Scholar]
- 17.(a) Leininger S, Olenyuk B, Stang PJ. Chem Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (b) Li SS, Northrop BH, Yuan QH, Wan LJ, Stang PJ. Acc Chem Res. 2008;42:249. doi: 10.1021/ar800117j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Northrop BH, Yang HB, Stang PJ. Chem Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluth MD, Raymond KN. Chem Soc Rev. 2007;36:161. doi: 10.1039/b603168b. [DOI] [PubMed] [Google Scholar]
- 19.Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]
- 20.(a) Oliveri CG, Ulmann PA, Wiester MJ, Mirkin CA. Acc Chem Res. 2008;41:1618. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gianneschi NC, Masar MS, Mirkin CA. Acc Chem Res. 2005;38:825. doi: 10.1021/ar980101q. [DOI] [PubMed] [Google Scholar]
- 21.(a) Cotton FA, Lin C, Murillo CA. Acc Chem Res. 2001;34:759. doi: 10.1021/ar010062+. [DOI] [PubMed] [Google Scholar]; (b) Cotton FA, Lin C, Murillo CA. Proc Natl Acad Sci U S A. 2002;99:4810. doi: 10.1073/pnas.012567599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zangrando E, Casanova M, Alessio E. Chem Rev. 2008;108:4979. doi: 10.1021/cr8002449. [DOI] [PubMed] [Google Scholar]
- 23.(a) Wurthner F, You CC, Saha-Moller CR. Chem Soc Rev. 2004;33:133. doi: 10.1039/b300512g. [DOI] [PubMed] [Google Scholar]; (b) Thanasekaran P, Liao RT, Liu YH, Rajendran T, Rajagopal S, Lu KL. Coord Chem Rev. 2005;249:1085. [Google Scholar]
- 24.(a) Stang PJ, Persky NE, Manna J. J Am Chem Soc. 1997;119:4777. [Google Scholar]; (b) Leininger S, Schmitz M, Stang PJ. Org Lett. 1999;1:1921. doi: 10.1021/ol9911425. [DOI] [PubMed] [Google Scholar]
- 25.(a) Saalfrank RW, Stark A, Peters K, von Schnering HG. Angew Chem, Int Ed Engl. 1988;27:851. [Google Scholar]; (b) Saalfrank RW, Stark A, Bremer M, Hummel HU. Angew Chem, Int Ed Engl. 1990;29:311. [Google Scholar]; (c) Beissel T, Powers RE, Raymond KN. Angew Chem, Int Ed Engl. 1996;35:1084. [Google Scholar]; (d) Ward MD. Chem Commun. 2009:4487. doi: 10.1039/b906726b. [DOI] [PubMed] [Google Scholar]
- 26.(a) Roche S, Haslam C, Heath LS, Thomas AJ. Chem Commun. 1998:1681. [Google Scholar]; (b) Lang JP, Xu QF, Chen ZN, Abrahams BF. J Am Chem Soc. 2003;125:12682. doi: 10.1021/ja036995d. [DOI] [PubMed] [Google Scholar]; (c) Suzuki K, Tominaga M, Kawano M, Fujita M. Chem Commun. 2009:1638. doi: 10.1039/b822311d. [DOI] [PubMed] [Google Scholar]
- 27.(a) Hiraoka S, Harano K, Shiro M, Ozawa Y, Yasuda N, Toriumi K, Shionoya M. Angew Chem, Int Ed. 2006;45:6488. doi: 10.1002/anie.200601431. [DOI] [PubMed] [Google Scholar]; (b) Ronson TK, Fisher J, Harding LP, Hardie MJ. Angew Chem, Int Ed. 2007;46:9086. doi: 10.1002/anie.200703903. [DOI] [PubMed] [Google Scholar]
- 28.(a) Olenyuk B, Whiteford JA, Fechtenkotter A, Stang PJ. Nature. 1999;398:796. doi: 10.1038/19740. [DOI] [PubMed] [Google Scholar]; (b) Argent SP, Adams H, Riis-Johannessen T, Jeffery JC, Harding LP, Ward MD. J Am Chem Soc. 2005;128:72. doi: 10.1021/ja056993o. [DOI] [PubMed] [Google Scholar]
- 29.(a) Paquette LA, Balogh DW, Usha R, Kountz D, Christoph GG. Science. 1981;211:575. doi: 10.1126/science.211.4482.575. [DOI] [PubMed] [Google Scholar]; (b) Olenyuk B, Levin MD, Whiteford JA, Shield JE, Stang PJ. J Am Chem Soc. 1999;121:10434. [Google Scholar]; (c) Levin MD, Stang PJ. J Am Chem Soc. 2000;122:7428. [Google Scholar]
- 30.(a) Mukherjee PS, Das N, Stang PJ. J Org Chem. 2004;69:3526. doi: 10.1021/jo049772u. [DOI] [PubMed] [Google Scholar]; (b) Kryschenko YK, Seidel SR, Muddiman DC, Nepomuceno AI, Stang PJ. J Am Chem Soc. 2003;125:9647. doi: 10.1021/ja030209n. [DOI] [PubMed] [Google Scholar]
- 31.Stone FGA, Graham WAG, editors. Inorganic Polymers. Academic Press; New York: 1962. [Google Scholar]
- 32.Buser HJ, Schwarzenbach D, Petter W, Ludi A. Inorg Chem. 1977;16:2704. [Google Scholar]
- 33.Iwamoto T, Miyoshi T, Sasaki Y. Acta Crystallogr B. 1974;30:292. [Google Scholar]
- 34.Rayner JH, Powell HM. J Chem Soc. 1952:319. [Google Scholar]
- 35.MacGillivray LR, editor. Metal-Organic Frameworks - Design and Application. John Wiley & Sons; Hoboken: 2010. [Google Scholar]
- 36.Hoskins BF, Robson R. J Am Chem Soc. 1989;111:5962. [Google Scholar]
- 37.Fujita M, Kwon YJ, Washizu S, Ogura K. J Am Chem Soc. 1994;116:1151. [Google Scholar]
- 38.Kondo M, Yoshitomi T, Matsuzaka H, Kitagawa S, Seki K. Angew Chem, Int Ed Engl. 1997;36:1725. [Google Scholar]
- 39.Li H, Eddaoudi M, Groy TL, Yaghi OM. J Am Chem Soc. 1998;120:8571. [Google Scholar]
- 40.O’Keeffe M. Chem Soc Rev. 2009;38:1215. doi: 10.1039/b802802h. [DOI] [PubMed] [Google Scholar]
- 41.Suh MP, Park HJ, Prasad TK, Lim DW. Chem Rev. 2011;112:782. doi: 10.1021/cr200274s. [DOI] [PubMed] [Google Scholar]
- 42.Li JR, Sculley J, Zhou HC. Chem Rev. 2011;112:869. doi: 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- 43.Kreno LE, Leong K, Farha OK, Allendorf M, Van Duyne RP, Hupp JT. Chem Rev. 2011;112:1105. doi: 10.1021/cr200324t. [DOI] [PubMed] [Google Scholar]
- 44.(a) Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, Férey G, Morris RE, Serre C. Chem Rev. 2011;112:1232. doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]; (b) Wang C, Zhang T, Lin W. Chem Rev. 2011;112:1084. doi: 10.1021/cr200252n. [DOI] [PubMed] [Google Scholar]; (c) Corma A, García H, Llabrés i Xamena FX. Chem Rev. 2010;110:4606. doi: 10.1021/cr9003924. [DOI] [PubMed] [Google Scholar]
- 45.(a) Wells AF. Acta Crystallogr. 1954;7:545. [Google Scholar]; (b) Wells AF. Acta Crystallogr. 1954;7:535. [Google Scholar]; (c) Wells AF, Sharpe RR. Acta Crystallogr. 1963;16:857. [Google Scholar]
- 46.(a) Robson R. Dalton Trans. 2000:3735. [Google Scholar]; (b) O’Keeffe M, Eddaoudi M, Li H, Reineke T, Yaghi OM. J Solid State Chem. 2000;152:3. [Google Scholar]; (c) Ohrstrom L, Larsson K. Dalton Trans. 2004:347. doi: 10.1039/b310330g. [DOI] [PubMed] [Google Scholar]
- 47.Weisstein EW, editor. CRC Concise Encyclopedia of Mathematics. Chapman & Hall/CRC; Boca Raton, Florida: 2003. [Google Scholar]
- 48.Kuehl CJ, Mayne CL, Arif AM, Stang PJ. Org Lett. 2000;2:3727. doi: 10.1021/ol006638x. [DOI] [PubMed] [Google Scholar]
- 49.Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J Am Chem Soc. 2003;125:5193. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]
- 50.Yang HB, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J Am Chem Soc. 2007;129:2120. doi: 10.1021/ja066804h. [DOI] [PubMed] [Google Scholar]
- 51.Tominaga M, Suzuki K, Kawano M, Kusukawa T, Ozeki T, Sakamoto S, Yamaguchi K, Fujita M. Angew Chem, Int Ed. 2004;43:5621. doi: 10.1002/anie.200461422. [DOI] [PubMed] [Google Scholar]
- 52.Sun QF, Iwasa J, Ogawa D, Ishido Y, Sato S, Ozeki T, Sei Y, Yamaguchi K, Fujita M. Science. 2010;328:1144. doi: 10.1126/science.1188605. [DOI] [PubMed] [Google Scholar]
- 53.Bunzen J, Iwasa J, Bonakdarzadeh P, Numata E, Rissanen K, Sato S, Fujita M. Angew Chem, Int Ed. 2012;51:3161. doi: 10.1002/anie.201108731. [DOI] [PubMed] [Google Scholar]
- 54.Gable RW, Hoskins BF, Robson R. Chem Commun. 1990:1677. [Google Scholar]
- 55.Biradha K, Hongo Y, Fujita M. Angew Chem, Int Ed. 2000;39:3843. doi: 10.1002/1521-3773(20001103)39:21<3843::AID-ANIE3843>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 56.Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Nature. 2003;423:705. doi: 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- 57.Eddaoudi M, Moler DB, Li H, Chen B, Reineke TM, O’Keeffe M, Yaghi OM. Acc Chem Res. 2001;34:319. doi: 10.1021/ar000034b. [DOI] [PubMed] [Google Scholar]
- 58.Kim J, Chen B, Reineke TM, Li H, Eddaoudi M, Moler DB, O’Keeffe M, Yaghi OM. J Am Chem Soc. 2001;123:8239. doi: 10.1021/ja010825o. [DOI] [PubMed] [Google Scholar]
- 59.Perry JJ, Perman JA, Zaworotko MJ. Chem Soc Rev. 2009;38:1400. doi: 10.1039/b807086p. [DOI] [PubMed] [Google Scholar]
- 60.Wang XS, Chrzanowski M, Gao WY, Wojtas L, Chen YS, Zaworotko MJ, Ma S. Chem Sci. 2012;3:2823. [Google Scholar]
- 61.Tranchemontagne DJ, Ni Z, O’Keeffe M, Yaghi OM. Angew Chem, Int Ed. 2008;47:5136. doi: 10.1002/anie.200705008. [DOI] [PubMed] [Google Scholar]
- 62.(a) Liu Y, Kravtsov V, Walsh RD, Poddar P, Srikanth H, Eddaoudi M. Chem Commun. 2004:2806. doi: 10.1039/b409459j. [DOI] [PubMed] [Google Scholar]; (b) Zou RQ, Jiang L, Senoh H, Takeichi N, Xu Q. Chem Commun. 2005:3526. doi: 10.1039/b503667d. [DOI] [PubMed] [Google Scholar]
- 63.Sudik AC, Millward AR, Ockwig NW, Côté AP, Kim J, Yaghi OM. J Am Chem Soc. 2005;127:7110. doi: 10.1021/ja042802q. [DOI] [PubMed] [Google Scholar]
- 64.Sudik AC, Côté AP, Wong-Foy AG, O’Keeffe M, Yaghi OM. Angew Chem, Int Ed. 2006;45:2528. doi: 10.1002/anie.200600175. [DOI] [PubMed] [Google Scholar]
- 65.Li JR, Timmons DJ, Zhou HC. J Am Chem Soc. 2009;131:6368. doi: 10.1021/ja901731z. [DOI] [PubMed] [Google Scholar]
- 66.(a) Moulton B, Lu J, Mondal A, Zaworotko MJ. Chem Commun. 2001:863. [Google Scholar]; (b) Abourahma H, Coleman AW, Moulton B, Rather B, Shahgaldian P, Zaworotko MJ. Chem Commun. 2001:2380. doi: 10.1039/b106592k. [DOI] [PubMed] [Google Scholar]; (c) Eddaoudi M, Kim J, Wachter JB, Chae HK, O’Keeffe M, Yaghi OM. J Am Chem Soc. 2001;123:4368. doi: 10.1021/ja0104352. [DOI] [PubMed] [Google Scholar]
- 67.McManus GJ, Wang Z, Zaworotko MJ. Cryst Growth Des. 2004;4:11. [Google Scholar]
- 68.Chui SSY, Lo SMF, Charmant JPH, Orpen AG, Williams ID. Science. 1999;283:1148. doi: 10.1126/science.283.5405.1148. [DOI] [PubMed] [Google Scholar]
- 69.(a) Pech R, Pickardt J. Acta Crystallogr C. 1988;44:992. [Google Scholar]; (b) Chui SSY, Siu A, Williams ID. Acta Crystallogr C. 1999;55:194. [Google Scholar]
- 70.(a) Kersting B, Meyer M, Powers RE, Raymond KN. J Am Chem Soc. 1996;118:7221. [Google Scholar]; (b) Meyer M, Kersting B, Powers RE, Raymond KN. Inorg Chem. 1997;36:5179. [Google Scholar]
- 71.Xu J, Parac TN, Raymond KN. Angew Chem, Int Ed. 1999;38:2878. doi: 10.1002/(sici)1521-3773(19991004)38:19<2878::aid-anie2878>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 72.Caulder DL, Powers RE, Parac TN, Raymond KN. Angew Chem, Int Ed. 1998;37:1840. [Google Scholar]
- 73.Brückner C, Powers RE, Raymond KN. Angew Chem, Int Ed. 1998;37:1837. [Google Scholar]
- 74.Sun X, Johnson DW, Caulder DL, Powers RE, Raymond KN, Wong EH. Angew Chem, Int Ed. 1999;38:1303. doi: 10.1002/(SICI)1521-3773(19990503)38:9<1303::AID-ANIE1303>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 75.Bar AK, Chakrabarty R, Mostafa G, Mukherjee PS. Angew Chem, Int Ed. 2008;47:8455. doi: 10.1002/anie.200803543. [DOI] [PubMed] [Google Scholar]
- 76.Yoshizawa M, Nagao M, Kumazawa K, Fujita M. J Organomet Chem. 2005;690:5383. [Google Scholar]
- 77.Bar AK, Mostafa G, Mukherjee PS. Inorg Chem. 2010;49:7647. doi: 10.1021/ic101139s. [DOI] [PubMed] [Google Scholar]
- 78.Yoshizawa M, Nakagawa J, Kumazawa K, Nagao M, Kawano M, Ozeki T, Fujita M. Angew Chem, Int Ed. 2005;44:1810. doi: 10.1002/anie.200462171. [DOI] [PubMed] [Google Scholar]
- 79.(a) Zheng YR, Zhao Z, Wang M, Ghosh K, Pollock JB, Cook TR, Stang PJ. J Am Chem Soc. 2010;132:16873. doi: 10.1021/ja106251f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang M, Zheng YR, Ghosh K, Stang PJ. J Am Chem Soc. 2010;132:6282. doi: 10.1021/ja100889h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.(a) Samanta D, Shanmugaraju S, Joshi SA, Patil YP, Nethaji M, Mukherjee PS. Chem Commun. 2012;48:2298. doi: 10.1039/c2cc16345d. [DOI] [PubMed] [Google Scholar]; (b) Bar AK, Mohapatra S, Zangrando E, Mukherjee PS. Chemistry – A European Journal. 2012;18:9571. doi: 10.1002/chem.201201077. [DOI] [PubMed] [Google Scholar]
- 81.Ward MD, Raithby PR. Chem Soc Rev. 2012 doi: 10.1039/C2CS35123D. [DOI] [Google Scholar]
- 82.Farrell JR, Eisenberg AH, Mirkin CA, Guzei IA, Liable-Sands LM, Incarvito CD, Rheingold AL, Stern CL. Organometallics. 1999;18:4856. [Google Scholar]
- 83.Farrell JR, Mirkin CA, Liable-Sands LM, Rheingold AL. J Am Chem Soc. 1998;120:11834. [Google Scholar]
- 84.Stang PJ, Olenyuk B. Acc Chem Res. 1997;30:502. [Google Scholar]
- 85.Tian YQ, Cai CX, Ji Y, You XZ, Peng SM, Lee GH. Angew Chem, Int Ed. 2002;41:1384. doi: 10.1002/1521-3773(20020415)41:8<1384::aid-anie1384>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 86.Park KS, Ni Z, Côté AP, Choi JY, Huang R, Uribe-Romo FJ, Chae HK, O’Keeffe M, Yaghi OM. Proc Natl Acad Sci U S A. 2006;103:10186. doi: 10.1073/pnas.0602439103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang JP, Zhang YB, Lin JB, Chen XM. Chem Rev. 2011;112:1001. doi: 10.1021/cr200139g. [DOI] [PubMed] [Google Scholar]
- 88.Zheng ST, Mao C, Wu T, Lee S, Feng P, Bu X. J Am Chem Soc. 2012;134:11936. doi: 10.1021/ja305181y. [DOI] [PubMed] [Google Scholar]
- 89.Spokoyny AM, Kim D, Sumrein A, Mirkin CA. Chem Soc Rev. 2009;38:1218. doi: 10.1039/b807085g. [DOI] [PubMed] [Google Scholar]
- 90.Feng X, Ding X, Jiang D. Chem Soc Rev. 2012;41:6010. doi: 10.1039/c2cs35157a. [DOI] [PubMed] [Google Scholar]
- 91.Zheng YR, Stang PJ. J Am Chem Soc. 2009;131:3487. doi: 10.1021/ja809788x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.(a) Beret EC, Martínez JM, Pappalardo RR, Marcos ESn, Doltsinis NL, Marx D. J Chem Theory Comput. 2008;4:2108. doi: 10.1021/ct800010q. [DOI] [PubMed] [Google Scholar]; (b) Helm L, Merbach AE. Chem Rev. 2005;105:1923. doi: 10.1021/cr030726o. [DOI] [PubMed] [Google Scholar]
- 93.Sato S, Ishido Y, Fujita M. J Am Chem Soc. 2009;131:6064. doi: 10.1021/ja900676f. [DOI] [PubMed] [Google Scholar]
- 94.Pazderski L, Szłyk E, Sitkowski J, Kamieński B, Kozerski L, Toušek J, Marek R. Magn Reson Chem. 2006;44:163. doi: 10.1002/mrc.1740. [DOI] [PubMed] [Google Scholar]
- 95.Seidel SR, Stang PJ. Acc Chem Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]
- 96.Sun QF, Murase T, Sato S, Fujita M. Angew Chem, Int Ed. 2011;50:10318. doi: 10.1002/anie.201104670. [DOI] [PubMed] [Google Scholar]
- 97.Yaghi OM, Li H, Groy TL. J Am Chem Soc. 1996;118:9096. [Google Scholar]
- 98.Yaghi OM, Li G, Li H. Nature. 1995;378:703. [Google Scholar]
- 99.Yaghi OM, Davis CE, Li G, Li H. J Am Chem Soc. 1997;119:2861. [Google Scholar]
- 100.Feldblyum JI, Wong-Foy AG, Matzger AJ. Chem Commun. 2012;48:9828. doi: 10.1039/c2cc34689c. [DOI] [PubMed] [Google Scholar]
- 101.Siberio-Pérez DY, Wong-Foy AG, Yaghi OM, Matzger AJ. Chem Mater. 2007;19:3681. [Google Scholar]
- 102.Parnham ER, Morris RE. Acc Chem Res. 2007;40:1005. doi: 10.1021/ar700025k. [DOI] [PubMed] [Google Scholar]
- 103.Liu Y, Kravtsov VC, Beauchamp DA, Eubank JF, Eddaoudi M. J Am Chem Soc. 2005;127:7266. doi: 10.1021/ja051259q. [DOI] [PubMed] [Google Scholar]
- 104.Yang HB, Northrop BH, Zheng YR, Ghosh K, Lyndon MM, Muddiman DC, Stang PJ. J Org Chem. 2009;74:3524. doi: 10.1021/jo900067v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bellarosa L, Castillo JM, Vlugt T, Calero S, López N. Chem–Eur J. 2012;18:12260. doi: 10.1002/chem.201201212. [DOI] [PubMed] [Google Scholar]
- 106.Yoneya M, Yamaguchi T, Sato S, Fujita M. J Am Chem Soc. 2012;134:14401. doi: 10.1021/ja303542r. [DOI] [PubMed] [Google Scholar]
- 107.Mueller C, Whiteford JA, Stang PJ. J Am Chem Soc. 1998;120:9827. [Google Scholar]
- 108.Ghosh S, Mukherjee PS. Dalton Trans. 2007:2542. doi: 10.1039/b617513a. [DOI] [PubMed] [Google Scholar]
- 109.(a) Ferrer M, Gutierrez A, Mounir M, Rossell O, Ruiz E, Rang A, Engeser M. Inorg Chem. 2007;46:3395. doi: 10.1021/ic062373s. [DOI] [PubMed] [Google Scholar]; (b) Ferrer M, Mounir M, Rossell O, Ruiz E, Maestro MA. Inorg Chem. 2003;42:5890. doi: 10.1021/ic034489j. [DOI] [PubMed] [Google Scholar]; (c) Lopez-Vidal EM, Blanco V, Garcia MD, Peinador C, Quintela JM. Org Lett. 2012;14:580. doi: 10.1021/ol203191b. [DOI] [PubMed] [Google Scholar]
- 110.(a) Olive AGL, Parkan K, Givelet C, Michl J. J Am Chem Soc. 2011;133:20108. doi: 10.1021/ja209051t. [DOI] [PubMed] [Google Scholar]; (b) Sun SS, Lees AJ. J Am Chem Soc. 2000;122:8956. [Google Scholar]; (c) Sun SS, Lees AJ. Inorg Chem. 1999;38:4181. [Google Scholar]; (d) Vajpayee V, Song YH, Cook TR, Kim H, Lee Y, Stang PJ, Chi KW. J Am Chem Soc. 2011;133:19646. doi: 10.1021/ja208495u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Vajpayee V, Song YH, Yang YJ, Kang SC, Cook TR, Kim DW, Lah MS, Kim IS, Wang M, Stang PJ, Chi KW. Organometallics. 2011;30:6482. doi: 10.1021/om200908c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yamauchi Y, Yoshizawa M, Fujita M. J Am Chem Soc. 2008;130:5832. doi: 10.1021/ja077783+. [DOI] [PubMed] [Google Scholar]
- 111.Vajpayee V, Kim H, Mishra A, Mukherjee PS, Stang PJ, Lee MH, Kim HK, Chi KW. Dalton Trans. 2011;40:3112. doi: 10.1039/c0dt01481h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weilandt T, Troff RW, Saxell H, Rissanen K, Schalley CA. Inorg Chem. 2008;47:7588. doi: 10.1021/ic800334k. [DOI] [PubMed] [Google Scholar]
- 113.Dinolfo PH, Williams ME, Stern CL, Hupp JT. J Am Chem Soc. 2004;126:12989. doi: 10.1021/ja0473182. [DOI] [PubMed] [Google Scholar]
- 114.You CC, Wuerthner F. J Am Chem Soc. 2003;125:9716. doi: 10.1021/ja029648x. [DOI] [PubMed] [Google Scholar]
- 115.(a) Ashton PR, Ballardini R, Balzani V, Constable EC, Credi A, Kocian O, Langford SJ, Preece JA, Prodi L, Schofield ER, Spencer N, Stoddart JF, Wenger S. Chem—Eur J. 1998;4:2413. [Google Scholar]; (b) Ashton PR, Langford SJ, Spencer N, Stoddart JF, White AJP, Williams DJ. Chem Commun. 1996:1387. [Google Scholar]; (c) Blanco V, Garcia MD, Peinador C, Quintela JM. Chem Sci. 2011;2:2407. [Google Scholar]; (d) Diskin-Posner Y, Patra GK, Goldberg I. Eur J Inorg Chem. 2001:2515. [Google Scholar]; (e) Holy P, Sehnal P, Tichy M, Zavada J, Cisarova I. Tet Asymm. 2003;14:245. [Google Scholar]; (f) Koshkakaryan G, Parimal K, He J, Zhang X, Abliz Z, Flood AH, Liu Y. Chem—Eur J. 2008;14:10211. doi: 10.1002/chem.200801590. [DOI] [PubMed] [Google Scholar]; (g) Peinador C, Blanco V, Quintela JM. J Am Chem Soc. 2009;131:920. doi: 10.1021/ja8088372. [DOI] [PubMed] [Google Scholar]; (h) Sehnal P, Holy P, Tichy M, Zavada J, Cisarova I. Collect Czech Chem Commun. 2002;67:1236. [Google Scholar]; (i) Stang PJ, Cao DH, Chen K, Gray GM, Muddiman DC, Smith RD. J Am Chem Soc. 1997;119:5163. [Google Scholar]; (j) Stang PJ, Cao DH, Saito S, Arif AM. J Am Chem Soc. 1995;117:6273. [Google Scholar]; (k) Stang PJ, Olenyuk B, Fan J, Arif AM. Organometallics. 1996;15:904. [Google Scholar]; (l) Stulz E, Scott SM, Bond AD, Teat SJ, Sanders JKM. Chem—Eur J. 2003;9:6039. doi: 10.1002/chem.200305265. [DOI] [PubMed] [Google Scholar]
- 116.Sautter A, Schmid DG, Jung G, Wuerthner F. J Am Chem Soc. 2001;123:5424. doi: 10.1021/ja004360y. [DOI] [PubMed] [Google Scholar]
- 117.(a) Baxter PNW, Khoury RG, Lehn JM, Baum G, Fenske D. Chem—Eur J. 2000;6:4140. doi: 10.1002/1521-3765(20001117)6:22<4140::aid-chem4140>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]; (b) Hall JR, Loeb SJ, Shimizu GKH, Yap GPA. Angew Chem, Int Ed. 1998;37:121. [Google Scholar]; (c) Knaust JM, Inman C, Keller SW. Chem Commun. 2004:492. doi: 10.1039/b309255k. [DOI] [PubMed] [Google Scholar]; (d) Li SH, Huang HP, Yu SY, Li YZ, Huang H, Sei Y, Yamaguchi K. Dalton Trans. 2005:2346. doi: 10.1039/b504639d. [DOI] [PubMed] [Google Scholar]; (e) Yu SY, Huang H, Liu HB, Chen ZN, Zhang R, Fujita M. Angew Chem, Int Ed. 2003;42:686. doi: 10.1002/anie.200390190. [DOI] [PubMed] [Google Scholar]
- 118.(a) Galstyan A, Shen WZ, Freisinger E, Alkam H, Hiller W, Sanz MPJ, Schuermann M, Lippert B. Chem—Eur J. 2011;17:10771. doi: 10.1002/chem.201101381. [DOI] [PubMed] [Google Scholar]; (b) Schnebeck RD, Freisinger E, Lippert B. Eur J Inorg Chem. 2000:1193. [Google Scholar]; (c) Schnebeck RD, Randaccio L, Zangrando E, Lippert B. Angew Chem, Int Ed. 1998;37:119. [Google Scholar]
- 119.Schmitz M, Leininger S, Fan J, Arif AM, Stang PJ. Organometallics. 1999;18:4817. [Google Scholar]
- 120.(a) Braverman MA, Nettleman JH, Supkowski RM, La DRL. Inorg Chem. 2009;48:4918. doi: 10.1021/ic900331m. [DOI] [PubMed] [Google Scholar]; (b) Ghosh K, Yang HB, Northrop BH, Lyndon MM, Zheng YR, Muddiman DC, Stang PJ. J Am Chem Soc. 2008;130:5320. doi: 10.1021/ja711502t. [DOI] [PubMed] [Google Scholar]; (c) Ghosh S, Batten SR, Turner DR, Mukherjee PS. Organometallics. 2007;26:3252. [Google Scholar]; (d) Jude H, Disteldorf H, Fischer S, Wedge T, Hawkridge AM, Arif AM, Hawthorne MF, Muddiman DC, Stang PJ. J Am Chem Soc. 2005;127:12131. doi: 10.1021/ja053050i. [DOI] [PubMed] [Google Scholar]; (e) Whiteford JA, Stang PJ, Huang SD. Inorg Chem. 1998;37:5595. doi: 10.1021/ic980727c. [DOI] [PubMed] [Google Scholar]
- 121.Fujita D, Takahashi A, Sato S, Fujita M. J Am Chem Soc. 2011;133:13317. doi: 10.1021/ja2059236. [DOI] [PubMed] [Google Scholar]
- 122.Kuehl CJ, Huang SD, Stang PJ. J Am Chem Soc. 2001;123:9634. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]
- 123.Fujita M, Aoyagi M, Ogura K. Inorg Chim Acta. 1996;246:53. [Google Scholar]
- 124.Zheng YR, Yang HB, Ghosh K, Zhao L, Stang PJ. Chem—Eur J. 2009;15:7203. doi: 10.1002/chem.200900230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.(a) Amoroso AJ, Thompson AMWC, Maher JP, McCleverty JA, Ward MD. Inorg Chem. 1995;34:4828. [Google Scholar]; (b) Kikuchi T, Murase T, Sato S, Fujita M. Supramol Chem. 2008;20:81. [Google Scholar]; (c) Lee J, Ghosh K, Stang PJ. J Am Chem Soc. 2009;131:12028. doi: 10.1021/ja903330j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu J, Turner DR, Harding LP, Byrne LT, Baker MV, Batten SR. J Am Chem Soc. 2009;131:10372. doi: 10.1021/ja9041912. [DOI] [PubMed] [Google Scholar]
- 127.Stang PJ, Chen K. J Am Chem Soc. 1995;117:1667. [Google Scholar]
- 128.Jeong KS, Kim SY, Shin US, Kogej M, Hai NTM, Broekmann P, Jeong N, Kirchner B, Reiher M, Schalley CA. J Am Chem Soc. 2005;127:17672. doi: 10.1021/ja053781i. [DOI] [PubMed] [Google Scholar]
- 129.(a) Leininger S, Fan J, Schmitz M, Stang PJ. Proc Natl Acad Sci U S A. 2000;97:1380. doi: 10.1073/pnas.030264697. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cammidge AN, Berber G, Chambrier I, Hough PW, Cook MJ. Tetrahedron. 2005;61:4067. [Google Scholar]; (c) Iengo E, Milani B, Zangrando E, Geremia S, Alessio E. Angew Chem, Int Ed. 2000;39:1096. doi: 10.1002/(sici)1521-3773(20000317)39:6<1096::aid-anie1096>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 130.(a) Childs LJ, Pascu M, Clarke AJ, Alcock NW, Hannon MJ. Chem—Eur J. 2004;10:4291. doi: 10.1002/chem.200400169. [DOI] [PubMed] [Google Scholar]; (b) Childs LJ, Alcock NW, Hannon MJ. Angew Chem, Int Ed. 2002;41:4244. doi: 10.1002/1521-3773(20021115)41:22<4244::AID-ANIE4244>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 131.Yang HB, Das N, Huang F, Hawkridge AM, Diaz DD, Arif AM, Finn MG, Muddiman DC, Stang PJ. J Org Chem. 2006;71:6644. doi: 10.1021/jo0608117. [DOI] [PubMed] [Google Scholar]
- 132.(a) Chand DK, Balaji G, Manivannan R, Athilakshmi J. Tetrahedron Lett. 2006;47:2867. [Google Scholar]; (b) Debata NB, Tripathy D, Ramkumar V, Chand DK. Tetrahedron Lett. 2010;51:4449. [Google Scholar]; (c) Kasai K, Fujita M. Chem—Eur J. 2007;13:3089. doi: 10.1002/chem.200501067. [DOI] [PubMed] [Google Scholar]; (d) Kasai K, Sato M. Chem—Asian J. 2006;1:344. doi: 10.1002/asia.200600054. [DOI] [PubMed] [Google Scholar]; (e) Sahoo HS, Chand DK, Debata NB. Inorg Chim Acta. 2007;360:31. [Google Scholar]; (f) Fujita M, Nagao S, Iida M, Ogata K, Ogura K. J Am Chem Soc. 1993;115:1574. [Google Scholar]; (g) Yamashita K-i, Sato K-i, Kawano M, Fujita M. New J Chem. 2009;33:264. [Google Scholar]
- 133.(a) Chand DK, Fujita M, Biradha K, Sakamoto S, Yamaguchi K. Dalton Trans. 2003:2750. [Google Scholar]; (b) Chand DK, Biradha K, Fujita M. Chem Commun. 2001:1652. doi: 10.1039/b104853h. [DOI] [PubMed] [Google Scholar]
- 134.Weilandt T, Kiehne U, Bunzen J, Schnakenburg G, Luetzen A. Chem—Eur J. 2010;16:2418. doi: 10.1002/chem.200902993. [DOI] [PubMed] [Google Scholar]
- 135.(a) Janka M, Anderson GK, Rath NP. Organometallics. 2004;23:4382. [Google Scholar]; (b) Manna J, Kuehl CJ, Whiteford JA, Stang PJ, Muddiman DC, Hofstadler SA, Smith RD. J Am Chem Soc. 1997;119:11611. [Google Scholar]; (c) Manna J, Whiteford JA, Stang PJ, Muddiman DC, Smith RD. J Am Chem Soc. 1996;118:8731. [Google Scholar]; (d) Whiteford JA, Lu CV, Stang PJ. J Am Chem Soc. 1997;119:2524. [Google Scholar]
- 136.Marquis-Rigault A, Dupont-Gervais A, Baxter PNW, Van DA, Lehn JM. Inorg Chem. 1996;35:2307. doi: 10.1021/ic951362o. [DOI] [PubMed] [Google Scholar]
- 137.(a) Chen HL, Li MX, He X, Wang ZX, Shao M, Zhu SR. Inorg Chim Acta. 2010;363:3186. [Google Scholar]; (b) Freudenreich J, Furrer J, Suss-Fink G, Therrien B. Organometallics. 2011;30:942. [Google Scholar]; (c) Govindaswamy P, Suess-Fink G, Therrien B. Organometallics. 2007;26:915. [Google Scholar]; (d) Hung CY, Singh AS, Chen CW, Wen YS, Sun SS. Chem Commun. 2009:1511. doi: 10.1039/b820234f. [DOI] [PubMed] [Google Scholar]; (e) Inokuma Y, Yoshioka S, Fujita M. Angew Chem, Int Ed. 2010;49:8912. doi: 10.1002/anie.201004781. [DOI] [PubMed] [Google Scholar]; (f) Kilbas B, Mirtschin S, Riis-Johannessen T, Scopelliti R, Severin K. Inorg Chem. 2012;51:5795. doi: 10.1021/ic300330p. [DOI] [PubMed] [Google Scholar]; (g) Kubota Y, Sakamoto S, Yamaguchi K, Fujita M. Proc Natl Acad Sci U S A. 2002;99:4854. doi: 10.1073/pnas.082643499. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Liu HB, Yu SY, Huang H, Zhang ZX. Aust J Chem. 2003;56:671. [Google Scholar]; (i) Orita A, Jiang L, Nakano T, Ma N, Otera J. Chem Commun. 2002:1362. [Google Scholar]; (j) Tashiro S, Fujita M. Bull Chem Soc Jpn. 2006;79:833. [Google Scholar]; (k) Tashiro S, Tominaga M, Yamaguchi Y, Kato K, Fujita M. Angew Chem, Int Ed. 2006;45:241. doi: 10.1002/anie.200502802. [DOI] [PubMed] [Google Scholar]; (l) Therrien B. J Organomet Chem. 2011;696:637. [Google Scholar]; (m) Zhang N, Li MX, Wang ZX, Shao M, Zhu SR. Inorg Chim Acta. 2009;363:8. [Google Scholar]; (n) Fujita M, Su SY, Kusukawa T, Funaki H, Ogura K, Yamaguchi K. Angew Chem, Int Ed. 1998;37:2082. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2082::AID-ANIE2082>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]; (o) Yoshizawa M, Tamura M, Fujita M. Science. 2006;312:251. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]
- 138.(a) Chand DK, Biradha K, Fujita M, Sakamoto S, Yamaguchi K. Chem Commun. 2002:2486. [Google Scholar]; (b) Fujita M, Nagao S, Ogura K. J Am Chem Soc. 1995;117:1649. doi: 10.1021/ja00110a026. [DOI] [PubMed] [Google Scholar]; (c) Hiraoka S, Kubota Y, Fujita M. Chem Commun. 2000:1509. [Google Scholar]
- 139.(a) Chi KW, Addicott C, Kryschenko YK, Stang PJ. J Org Chem. 2004;69:964. doi: 10.1021/jo035607n. [DOI] [PubMed] [Google Scholar]; (b) Cotton FA, Dikarev EV, Petrukhina MA, Schmitz M, Stang PJ. Inorg Chem. 2002;41:2903. doi: 10.1021/ic020051s. [DOI] [PubMed] [Google Scholar]; (c) Schweiger M, Seidel SR, Schmitz M, Stang PJ. Org Lett. 2000;2:1255. doi: 10.1021/ol005781n. [DOI] [PubMed] [Google Scholar]; (d) Yang HB, Ghosh K, Das N, Stang PJ. Org Lett. 2006;8:3991. doi: 10.1021/ol0614626. [DOI] [PubMed] [Google Scholar]; (e) Yang HB, Ghosh K, Northrop BH, Stang PJ. Org Lett. 2007;9:1561. doi: 10.1021/ol070371l. [DOI] [PubMed] [Google Scholar]; (f) Zheng YR, Yang HB, Northrop BH, Ghosh K, Stang PJ. Inorg Chem. 2008;47:4706. doi: 10.1021/ic800038j. [DOI] [PubMed] [Google Scholar]; (g) Kuehl CJ, Kryschenko YK, Radhakrishnan U, Seidel SR, Huang SD, Stang PJ. Proc Natl Acad Sci U S A. 2002;99:4932. doi: 10.1073/pnas.012540799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zheng YR, Ghosh K, Yang HB, Stang PJ. Inorg Chem. 2010;49:4747. doi: 10.1021/ic100330c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Radhakrishnan U, Schweiger M, Stang PJ. Org Lett. 2001;3:3141. doi: 10.1021/ol0164684. [DOI] [PubMed] [Google Scholar]
- 141.(a) Caskey DC, Michl J. J Org Chem. 2005;70:5442. doi: 10.1021/jo050409c. [DOI] [PubMed] [Google Scholar]; (b) Crowley JD, Goshe AJ, Bosnich B. Chem Commun. 2003:2824. doi: 10.1039/b307385h. [DOI] [PubMed] [Google Scholar]; (c) Easwaramoorthi S, Jang SY, Yoon ZS, Lim JM, Lee CW, Mai CL, Liu YC, Yeh CY, Vura-Weis J, Wasielewski MR, Kim D. J Phys Chem A. 2008;112:6563. doi: 10.1021/jp801626s. [DOI] [PubMed] [Google Scholar]; (d) Ghosh K, Hu J, White HS, Stang PJ. J Am Chem Soc. 2009;131:6695. doi: 10.1021/ja902045q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kim D, Paek JH, Jun MJ, Lee JY, Kang SO, Ko J. Inorg Chem. 2005;44:7886. doi: 10.1021/ic0508369. [DOI] [PubMed] [Google Scholar]; (f) Lee SJ, Mulfort KL, O’Donnell JL, Zuo X, Goshe AJ, Wesson PJ, Nguyen ST, Hupp JT, Tiede DM. Chem Commun. 2006:4581. doi: 10.1039/b610025b. [DOI] [PubMed] [Google Scholar]; (g) Schweiger M, Yamamoto T, Stang PJ, Blaeser D, Boese R. J Org Chem. 2005;70:4861. doi: 10.1021/jo050469i. [DOI] [PubMed] [Google Scholar]; (h) Vajpayee V, Yang YJ, Kang SC, Kim H, Kim IS, Wang M, Stang PJ, Chi KW. Chem Commun. 2011;47:5184. doi: 10.1039/c1cc10167f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wang M, Vajpayee V, Shanmugaraju S, Zheng YR, Zhao Z, Kim H, Mukherjee PS, Chi KW, Stang PJ. Inorg Chem. 2011;50:1506. doi: 10.1021/ic1020719. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Youm KT, Nguyen SBT, Hupp JT. Chem Commun. 2008:3375. doi: 10.1039/b800063h. [DOI] [PubMed] [Google Scholar]
- 142.Yang HB, Ghosh K, Arif AM, Stang PJ. J Org Chem. 2006;71:9464. doi: 10.1021/jo061779j. [DOI] [PubMed] [Google Scholar]
- 143.(a) Harano K, Hiraoka S, Shionoya M. J Am Chem Soc. 2007;129:5300. doi: 10.1021/ja0659727. [DOI] [PubMed] [Google Scholar]; (b) Hiraoka S, Harano K, Nakamura T, Shiro M, Shionoya M. Angew Chem, Int Ed. 2009;48:7006. doi: 10.1002/anie.200902652. [DOI] [PubMed] [Google Scholar]; (c) Hiraoka S, Harano K, Shiro M, Shionoya M. J Am Chem Soc. 2008;130:14368. doi: 10.1021/ja804885k. [DOI] [PubMed] [Google Scholar]; (d) Hiraoka S, Nakamura T, Shiro M, Shionoya M. J Am Chem Soc. 2010;132:13223. doi: 10.1021/ja1069135. [DOI] [PubMed] [Google Scholar]
- 144.Yamanoi Y, Sakamoto Y, Kusukawa T, Fujita M, Sakamoto S, Yamaguchi K. J Am Chem Soc. 2001;123:980. doi: 10.1021/ja003043o. [DOI] [PubMed] [Google Scholar]
- 145.(a) Caskey DC, Yamamoto T, Addicott C, Shoemaker RK, Vacek J, Hawkridge AM, Muddiman DC, Kottas GS, Michl J, Stang PJ. J Am Chem Soc. 2008;130:7620. doi: 10.1021/ja710715e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Johannessen SC, Brisbois RG, Fischer JP, Grieco PA, Counterman AE, Clemmer DE. J Am Chem Soc. 2001;123:3818. doi: 10.1021/ja004007s. [DOI] [PubMed] [Google Scholar]
- 146.Manimaran B, Thanasekaran P, Rajendran T, Liao RT, Liu YH, Lee GH, Peng SM, Rajagopal S, Lu KL. Inorg Chem. 2003;42:4795. doi: 10.1021/ic034172j. [DOI] [PubMed] [Google Scholar]
- 147.(a) Kilbas B, Mirtschin S, Scopelliti R, Severin K. Chem Sci. 2012;3:701. doi: 10.1021/ic300330p. [DOI] [PubMed] [Google Scholar]; (b) Wang M, Zheng YR, Cook TR, Stang PJ. Inorg Chem. 2011;50:6107. doi: 10.1021/ic2002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fujita N, Biradha K, Fujita M, Sakamoto S, Yamaguchi K. Angew Chem, Int Ed. 2001;40:1718. [PubMed] [Google Scholar]
- 149.(a) Wang M, Lan WJ, Zheng YR, Cook TR, White HS, Stang PJ. J Am Chem Soc. 2011;133:10752. doi: 10.1021/ja204155r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao Z, Zheng YR, Wang M, Pollock JB, Stang PJ. Inorg Chem. 2010;49:8653. doi: 10.1021/ic1014219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.(a) Boldog I, Gaspar AB, Martinez V, Pardo-Ibanez P, Ksenofontov V, Bhattacharjee A, Guetlich P, Real JA. Angew Chem, Int Ed. 2008;47:6433. doi: 10.1002/anie.200801673. [DOI] [PubMed] [Google Scholar]; (b) Carlucci L, Ciani G, Proserpio DM, Sironi A. J Am Chem Soc. 1995;117:4562. [Google Scholar]; (c) Chuang YC, Liu CT, Sheu CF, Ho WL, Lee GH, Wang CC, Wang Y. Inorg Chem. 2012;51:4663. doi: 10.1021/ic202626c. [DOI] [PubMed] [Google Scholar]; (d) Clegg JK, Lindoy LF, McMurtrie JC, Schilter D. Dalton Trans. 2006:3114. doi: 10.1039/b517274h. [DOI] [PubMed] [Google Scholar]; (e) Gao C, Liu S, Xie L, Sun J, Ren Y, Feng D, Su Z. CrystEngComm. 2009;11:177. [Google Scholar]; (f) Ghosh SK, Ribas J, Bharadwaj PK. CrystEngComm. 2004;6:250. [Google Scholar]; (g) Groeneman RH, MacGillivray LR, Atwood JL. Chem Commun. 1998:2735. [Google Scholar]; (h) Han YF, Fei Y, Jin GX. Dalton Trans. 2010;39:3976. doi: 10.1039/b925098k. [DOI] [PubMed] [Google Scholar]; (i) Han YF, Jia WG, Lin YJ, Jin GX. Angew Chem, Int Ed. 2009;48:6234. doi: 10.1002/anie.200805949. [DOI] [PubMed] [Google Scholar]; (j) Horike S, Matsuda R, Tanaka D, Matsubara S, Mizuno M, Endo K, Kitagawa S. Angew Chem, Int Ed. 2006;45:7226. doi: 10.1002/anie.200603196. [DOI] [PubMed] [Google Scholar]; (k) Ingleson MJ, Barrio JP, Bacsa J, Steiner A, Darling GR, Jones JTA, Khimyak YZ, Rosseinsky MJ. Angew Chem, Int Ed. 2009;48:2012. doi: 10.1002/anie.200804196. [DOI] [PubMed] [Google Scholar]; (l) Kaito A, Hatano M, Tajiri A. J Am Chem Soc. 1977;99:5241. [Google Scholar]; (m) Kar P, Haldar R, Gomez-Garcia CJ, Ghosh A. Inorg Chem. 2012;51:4265. doi: 10.1021/ic2027362. [DOI] [PubMed] [Google Scholar]; (n) Liu HY, Wu H, Ma JF, Yang J, Liu YY. Dalton Trans. 2009:7957. doi: 10.1039/b905483a. [DOI] [PubMed] [Google Scholar]; (o) Mahmoudi G, Morsali A. CrystEngComm. 2009;11:1868. [Google Scholar]; (p) Manson JL, Conner MM, Schlueter JA, Lancaster T, Blundell SJ, Brooks ML, Pratt FL, Papageorgiou T, Bianchi AD, Wosnitza J, Whangbo MH. Chem Commun. 2006:4894. doi: 10.1039/b608791d. [DOI] [PubMed] [Google Scholar]; (q) Manson JL, Lapidus SH, Stephens PW, Peterson PK, Carreiro KE, Southerland HI, Lancaster T, Blundell SJ, Steele AJ, Goddard PA, Pratt FL, Singleton J, Kohama Y, McDonald RD, Del SRE, Smith NA, Bendix J, Zvyagin SA, Kang J, Lee C, Whangbo MH, Zapf VS, Plonczak A. Inorg Chem. 2011;50:5990. doi: 10.1021/ic102532h. [DOI] [PubMed] [Google Scholar]; (r) Manson JL, Lecher JG, Gu J, Geiser U, Schlueter JA, Henning R, Wang X, Schultz AJ, Koo HJ, Whangbo MH. Dalton Trans. 2003:2905. [Google Scholar]; (s) Manson JL, Schlueter JA, Funk KA, Southerland HI, Twamley B, Lancaster T, Blundell SJ, Baker PJ, Pratt FL, Singleton J, McDonald RD, Goddard PA, Sengupta P, Batista CD, Ding L, Lee C, Whangbo MH, Franke I, Cox S, Baines C, Trial D. J Am Chem Soc. 2009;131:6733. doi: 10.1021/ja808761d. [DOI] [PubMed] [Google Scholar]; (t) Mohapatra S, Maji TK. Dalton Trans. 2010;39:3412. doi: 10.1039/b922039a. [DOI] [PubMed] [Google Scholar]; (u) Nadeem MA, Thornton AW, Hill MR, Stride JA. Dalton Trans. 2011;40:3398. doi: 10.1039/c0dt01531h. [DOI] [PubMed] [Google Scholar]; (v) Ohba M, Yoneda K, Kitagawa S. CrystEngComm. 2010;12:159. [Google Scholar]; (w) Panda T, Pachfule P, Banerjee R. Chem Commun. 2011;47:7674. doi: 10.1039/c1cc12278a. [DOI] [PubMed] [Google Scholar]; (x) Ovens JS, Leznoff DB. Dalton Trans. 2011;40:4140. doi: 10.1039/c0dt01772h. [DOI] [PubMed] [Google Scholar]; (y) Sakamoto H, Matsuda R, Kitagawa S. Dalton Trans. 2012;41:3956. doi: 10.1039/c2dt12012g. [DOI] [PubMed] [Google Scholar]; (z) Shestopalov MA, Cordier S, Hernandez O, Molard Y, Perrin C, Perrin A, Fedorov VE, Mironov YV. Inorg Chem. 2009;48:1482. doi: 10.1021/ic8018277. [DOI] [PubMed] [Google Scholar]; (aa) Thallapally PK, Fernandez CA, Motkuri RK, Nune SK, Liu J, Peden CHF. Dalton Trans. 2010;39:1692. doi: 10.1039/b921118g. [DOI] [PubMed] [Google Scholar]; (ab) Tokuhiro T, Fraenkel G. J Am Chem Soc. 1969;91:5005. [Google Scholar]; (ac) Vitillo JG, Groppo E, Bordiga S, Chavan S, Ricchiardi G, Zecchina A. Inorg Chem. 2009;48:5439. doi: 10.1021/ic9004664. [DOI] [PubMed] [Google Scholar]; (ad) Wang XF, Wang Y, Zhang YB, Xue W, Zhang JP, Chen XM. Chem Commun. 2012;48:133. doi: 10.1039/c1cc15891k. [DOI] [PubMed] [Google Scholar]; (ae) Yan B, Xu Y, Goh NK, Chia LS. Chem Commun. 2000:2169. [Google Scholar]; (af) Yin P, Zheng LM, Gao S, Xin XQ. Chem Commun. 2001:2346. doi: 10.1039/b106780j. [DOI] [PubMed] [Google Scholar]; (ag) Zhang WH, Song YL, Ren ZG, Li HX, Li LL, Zhang Y, Lang JP. Inorg Chem. 2007;46:6647. doi: 10.1021/ic700762x. [DOI] [PubMed] [Google Scholar]; (ah) Zhou H, Yuan AH, Qian SY, Song Y, Diao GW. Inorg Chem. 2010;49:5971. doi: 10.1021/ic100518b. [DOI] [PubMed] [Google Scholar]
- 151.(a) Han YF, Jia WG, Lin YJ, Jin GX. Organometallics. 2008;27:5002. [Google Scholar]; (b) Han YF, Li H, Weng LH, Jin GX. Chem Commun. 2010;46:3556. doi: 10.1039/b923335k. [DOI] [PubMed] [Google Scholar]; (c) Han YF, Lin YJ, Jia WG, Jin GX. Organometallics. 2008;27:4088. [Google Scholar]; (d) Han YF, Lin YJ, Jin GX. Dalton Trans. 2011;40:10370. doi: 10.1039/c1dt10506j. [DOI] [PubMed] [Google Scholar]; (e) Jia WG, Han YF, Lin YJ, Weng LH, Jin GX. Organometallics. 2009;28:3459. [Google Scholar]; (f) Kumazawa K, Biradha K, Kusukawa T, Okano T, Fujita M. Angew Chem, Int Ed. 2003;42:3909. doi: 10.1002/anie.200351797. [DOI] [PubMed] [Google Scholar]; (g) Li H, Han YF, Jin GX. Dalton Trans. 2011;40:4982. doi: 10.1039/c0dt01377c. [DOI] [PubMed] [Google Scholar]; (h) Lin YJ, Han YF, Jin GX. J Organomet Chem. 2012;708–709:31. [Google Scholar]; (i) Liu S, Zhang J, Wang X, Jin GX. Dalton Trans. 2006:5225. doi: 10.1039/b608934h. [DOI] [PubMed] [Google Scholar]; (j) Ozaki Y, Kawano M, Fujita M. Chem Commun. 2009:4245. doi: 10.1039/b906674h. [DOI] [PubMed] [Google Scholar]; (k) Wang GL, Lin YJ, Jin GX. J Organomet Chem. 2010;695:1225. [Google Scholar]; (l) Wang JQ, Ren CX, Jin GX. Eur J Inorg Chem. 2006:3274. [Google Scholar]; (m) Wang JQ, Ren CX, Jin GX. Organometallics. 2006;25:74. [Google Scholar]; (n) Xiao XQ, Jia AQ, Lin YJ, Jin GX. Organometallics. 2010;29:4842. [Google Scholar]; (o) Yu WB, Han YF, Lin YJ, Jin GX. Organometallics. 2010;29:2827. [Google Scholar]; (p) Yu WB, Han YF, Lin YJ, Jin GX. Organometallics. 2011;30:3090. [Google Scholar]; (q) Yu WB, Han YF, Lin YJ, Jin GX. Chem—Eur J. 2011;17:1863. doi: 10.1002/chem.201002986. [DOI] [PubMed] [Google Scholar]; (r) Yu WB, Lin YJ, Jin GX. Organometallics. 2011;30:3905. [Google Scholar]; (s) Yu XY, Maekawa M, Kondo M, Kitagawa S, Jin GX. Chem Lett. 2001:168. [Google Scholar]; (t) Yu XY, Maekawa M, Morita T, Chang HC, Kitagawa S, Jin GX. Bull Chem Soc Jpn. 2002;75:267. [Google Scholar]
- 152.(a) Amabilino DB, Ashton PR, Balzani V, Brown CL, Credi A, Frechet JMJ, Leon JW, Raymo FM, Spencer N, Stoddart JF, Venturi M. J Am Chem Soc. 1996;118:12012. [Google Scholar]; (b) Burnett BJ, Barron PM, Hu C, Choe W. J Am Chem Soc. 2011;133:9984. doi: 10.1021/ja201911v. [DOI] [PubMed] [Google Scholar]; (c) Chen B, Liang C, Yang J, Contreras DS, Clancy YL, Lobkovsky EB, Yaghi OM, Dai S. Angew Chem, Int Ed. 2006;45:1390. doi: 10.1002/anie.200502844. [DOI] [PubMed] [Google Scholar]; (d) Choi EY, Barron PM, Novotny RW, Son HT, Hu C, Choe W. Inorg Chem. 2009;48:426. doi: 10.1021/ic801677y. [DOI] [PubMed] [Google Scholar]; (e) De LDT, De BDA, Cahill CL. Inorg Chem. 2007;46:3960. doi: 10.1021/ic062019u. [DOI] [PubMed] [Google Scholar]; (f) Fei H, Bresler MR, Oliver SRJ. J Am Chem Soc. 2011;133:11110. doi: 10.1021/ja204577p. [DOI] [PubMed] [Google Scholar]; (g) Fu R, Hu S, Wu X. Inorg Chem. 2007;46:9630. doi: 10.1021/ic700597k. [DOI] [PubMed] [Google Scholar]; (h) Gudbjartson H, Biradha K, Poirier KM, Zaworotko MJ. J Am Chem Soc. 1999;121:2599. [Google Scholar]; (i) Hu S, He KH, Zeng MH, Zou HH, Jiang YM. Inorg Chem. 2008;47:5218. doi: 10.1021/ic800050u. [DOI] [PubMed] [Google Scholar]; (j) Jiang HL, Tatsu Y, Lu ZH, Xu Q. J Am Chem Soc. 2010;132:5586. doi: 10.1021/ja101541s. [DOI] [PubMed] [Google Scholar]; (k) Jiao C, Zhang J, Wang S, Si X, You W, Li Z, Wang Z, Yu H, Gabelica Z, Zhou HY, Sun L, Xu F. Inorg Chem. 2012;51:5022. doi: 10.1021/ic202769e. [DOI] [PubMed] [Google Scholar]; (l) Leblanc N, Bi W, Mercier N, Auban-Senzier P, Pasquier C. Inorg Chem. 2010;49:5824. doi: 10.1021/ic901525p. [DOI] [PubMed] [Google Scholar]; (m) Lo SMF, Chui SSY, Shek L-Y, Lin Z, Zhang XX, Wen G-h, Williams ID. J Am Chem Soc. 2000;122:6293. [Google Scholar]; (n) MacGillivray LR, Groeneman RH, Atwood JL. J Am Chem Soc. 1998;120:2676. [Google Scholar]; (o) Moya-Barrios R, Cozens FL. J Am Chem Soc. 2006;128:14836. doi: 10.1021/ja064779+. [DOI] [PubMed] [Google Scholar]; (p) Pan L, Liu H, Lei X, Huang X, Olson DH, Turro NJ, Li J. Angew Chem, Int Ed. 2003;42:542. doi: 10.1002/anie.200390156. [DOI] [PubMed] [Google Scholar]; (q) Roy X, Hui JKH, Rabnawaz M, Liu G, MacLachlan MJ. Angew Chem, Int Ed. 2011;50:1597. doi: 10.1002/anie.201005537. [DOI] [PubMed] [Google Scholar]; (r) Verduzco JM, Chung H, Hu C, Choe W. Inorg Chem. 2009;48:9060. doi: 10.1021/ic9009916. [DOI] [PubMed] [Google Scholar]; (s) Wang XL, Qin C, Wang EB, Li YG, Su ZM, Xu L, Carlucci L. Angew Chem, Int Ed. 2005;44:5824. doi: 10.1002/anie.200501373. [DOI] [PubMed] [Google Scholar]; (t) Yaghi OM, Li H. J Am Chem Soc. 1996;118:295. [Google Scholar]; (u) Zhang J, Wojtas L, Larsen RW, Eddaoudi M, Zaworotko MJ. J Am Chem Soc. 2009;131:17040. doi: 10.1021/ja906911q. [DOI] [PubMed] [Google Scholar]
- 153.(a) Dinolfo PH, Hupp JT. J Am Chem Soc. 2004;126:16814. doi: 10.1021/ja045457d. [DOI] [PubMed] [Google Scholar]; (b) Fujita M, Aoyagi M, Ibukuro F, Ogura K, Yamaguchi K. J Am Chem Soc. 1998;120:611. [Google Scholar]; (c) Lin R, Yip JHK, Zhang K, Koh LL, Wong KY, Ho KP. J Am Chem Soc. 2004;126:15852. doi: 10.1021/ja0456508. [DOI] [PubMed] [Google Scholar]; (d) Lusby PJ, Muller P, Pike SJ, Slawin AMZ. J Am Chem Soc. 2009;131:16398. doi: 10.1021/ja907297z. [DOI] [PubMed] [Google Scholar]
- 154.(a) Callejo LM, de lPN, Madariaga G, Fidalgo L, Lezama L, Cortes R. Cryst Growth Des. 2010;10:4874. [Google Scholar]; (b) Felloni M, Blake AJ, Hubberstey P, Wilson C, Schroder M. Cryst Growth Des. 2009;9:4685. [Google Scholar]; (c) Slone RV, Hupp JT, Stern CL, Albrecht-Schmitt TE. Inorg Chem. 1996;35:4096. doi: 10.1021/ic960464r. [DOI] [PubMed] [Google Scholar]; (d) Uehara K, Kasai K, Mizuno N. Inorg Chem. 2010;49:2008. doi: 10.1021/ic100011a. [DOI] [PubMed] [Google Scholar]; (e) Wang R, Zhou Y, Sun Y, Yuan D, Han L, Lou B, Wu B, Hong M. Cryst Growth Des. 2005;5:251. [Google Scholar]
- 155.(a) Bartual-Murgui C, Salmon L, Akou A, Ortega-Villar NA, Shepherd HJ, Munoz MC, Molnar G, Real JA, Bousseksou A. Chem—Eur J. 2012;18:507. doi: 10.1002/chem.201102357. [DOI] [PubMed] [Google Scholar]; (b) Marin G, Andruh M, Madalan AM, Blake AJ, Wilson C, Champness NR, Schroeder M. Cryst Growth Des. 2008;8:964. [Google Scholar]; (c) Rajendran T, Manimaran B, Liao RT, Lin RJ, Thanasekaran P, Lee GH, Peng SM, Liu YH, Chang IJ, Rajagopal S, Lu KL. Inorg Chem. 2003;42:6388. doi: 10.1021/ic034099x. [DOI] [PubMed] [Google Scholar]; (d) Song LC, Jin GX, Wang HT, Zhang WX, Hu QM. Organometallics. 2005;24:6464. [Google Scholar]; (e) Welsch S, Lescop C, Scheer M, Reau R. Inorg Chem. 2008;47:8592. doi: 10.1021/ic801222j. [DOI] [PubMed] [Google Scholar]; (f) Zhao L, Ghosh K, Zheng Y, Lyndon MM, Williams TI, Stang PJ. Inorg Chem. 2009;48:5590. doi: 10.1021/ic900649m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zhao L, Northrop BH, Stang PJ. J Am Chem Soc. 2008;130:11886. doi: 10.1021/ja805770w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.(a) Chang Z, Zhang DS, Chen Q, Li RF, Hu TL, Bu XH. Inorg Chem. 2011;50:7555. doi: 10.1021/ic2004485. [DOI] [PubMed] [Google Scholar]; (b) Chung H, Barron PM, Novotny RW, Son HT, Hu C, Choe W. Cryst Growth Des. 2009;9:3327. [Google Scholar]; (c) Farha OK, Malliakas CD, Kanatzidis MG, Hupp JT. J Am Chem Soc. 2010;132:950. doi: 10.1021/ja909519e. [DOI] [PubMed] [Google Scholar]; (d) Lu ZZ, Zhang R, Li YZ, Guo ZJ, Zheng HG. J Am Chem Soc. 2011;133:4172. doi: 10.1021/ja109437d. [DOI] [PubMed] [Google Scholar]; (e) Lu ZZ, Zhang R, Pan ZR, Li YZ, Guo ZJ, Zheng HG. Chem—Eur J. 2012;18:2812. doi: 10.1002/chem.201101963. [DOI] [PubMed] [Google Scholar]; (f) Park HJ, Cheon YE, Suh MP. Chem—Eur J. 2010;16:11662. doi: 10.1002/chem.201001549. [DOI] [PubMed] [Google Scholar]; (g) Varughese S, Draper SM. Cryst Growth Des. 2010;10:2571. [Google Scholar]; (h) Xue M, Ma S, Jin Z, Schaffino RM, Zhu GS, Lobkovsky EB, Qiu SL, Chen B. Inorg Chem. 2008;47:6825. doi: 10.1021/ic800854y. [DOI] [PubMed] [Google Scholar]; (i) Yamauchi Y, Hanaoka Y, Yoshizawa M, Akita M, Ichikawa T, Yoshio M, Kato T, Fujita M. J Am Chem Soc. 2010;132:9555. doi: 10.1021/ja103180z. [DOI] [PubMed] [Google Scholar]
- 157.(a) Biradha K, Fujita M. Dalton Trans. 2000:3805. [Google Scholar]; (b) Biradha K, Fujita M. Chem Commun. 2001:15. doi: 10.1039/b104853h. [DOI] [PubMed] [Google Scholar]; (c) Cui J, Lu Z, Li Y, Guo Z, Zheng H. Cryst Growth Des. 2012;12:1022. [Google Scholar]; (d) Faust TB, Bellini V, Candini A, Carretta S, Lorusso G, Allan DR, Carthy L, Collison D, Docherty RJ, Kenyon J, Machin J, McInnes EJL, Muryn CA, Nowell H, Pritchard RG, Teat SJ, Timco GA, Tuna F, Whitehead GFS, Wernsdorfer W, Affronte M, Winpenny REP. Chem—Eur J. 2011;17:14020. doi: 10.1002/chem.201101785. [DOI] [PubMed] [Google Scholar]; (e) Fujita M, Sasaki O, Mitsuhashi T, Fujita T, Yazaki J, Yamaguchi K, Ogura K. Chem Commun. 1996:1535. [Google Scholar]; (f) Knight LK, Vukotic VN, Viljoen E, Caputo CB, Loeb SJ. Chem Commun. 2009:5585. doi: 10.1039/b911889f. [DOI] [PubMed] [Google Scholar]; (g) Langner A, Tait SL, Lin N, Rajadurai C, Ruben M, Kern K. Proc Natl Acad Sci U S A. 2007;104:17927. doi: 10.1073/pnas.0704882104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Lin MJ, Jouaiti A, Kyritsakas N, Hosseini MW. CrystEngComm. 2009;11:189. [Google Scholar]; (i) Men YB, Sun J, Huang ZT, Zheng QY. Chem Commun. 2010;46:6299. doi: 10.1039/c0cc01491e. [DOI] [PubMed] [Google Scholar]; (j) Qin L, Hu JS, Li YZ, Zheng HG. Cryst Growth Des. 2012;12:403. [Google Scholar]; (k) Sun D, Yan ZH, Liu M, Xie H, Yuan S, Lu H, Feng S, Sun D. Cryst Growth Des. 2012;12:2902. [Google Scholar]; (l) Takaoka K, Kawano M, Hozumi T, Ohkoshi SI, Fujita M. Inorg Chem. 2006;45:3976. doi: 10.1021/ic052138u. [DOI] [PubMed] [Google Scholar]
- 158.(a) Ma BQ, Mulfort KL, Hupp JT. Inorg Chem. 2005;44:4912. doi: 10.1021/ic050452i. [DOI] [PubMed] [Google Scholar]; (b) Takashima Y, Martinez VM, Furukawa S, Kondo M, Shimomura S, Uehara H, Nakahama M, Sugimoto K, Kitagawa S. Nat Commun. 2011;2:1. doi: 10.1038/ncomms1170. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Usov PM, Fabian C, D’Alessandro DM. Chem Commun. 2012;48:3945. doi: 10.1039/c2cc30568b. [DOI] [PubMed] [Google Scholar]
- 159.(a) Biradha K, Fujita M. Chem Commun. 2002:1866. doi: 10.1039/b203025j. [DOI] [PubMed] [Google Scholar]; (b) Collin JP, Durola F, Frey J, Heitz V, Reviriego F, Sauvage JP, Trolez Y, Rissanen K. J Am Chem Soc. 2010;132:6840. doi: 10.1021/ja101759w. [DOI] [PubMed] [Google Scholar]; (c) Liang H, Sun W, Jin X, Li H, Li J, Hu X, Teo BK, Wu K. Angew Chem, Int Ed. 2011;50:7562. doi: 10.1002/anie.201101477. [DOI] [PubMed] [Google Scholar]; (d) Lin MJ, Jouaiti A, Kyritsakas N, Hosseini MW. CrystEngComm. 2011;13:776. [Google Scholar]
- 160.(a) Carlucci L, Ciani G, Proserpio DM. Chem Commun. 2004:380. doi: 10.1039/b314322h. [DOI] [PubMed] [Google Scholar]; (b) Dhal PK, Arnold FH. J Am Chem Soc. 1991;113:7417. [Google Scholar]; (c) Fan J, Slebodnick C, Angel R, Hanson BE. Inorg Chem. 2005;44:552. doi: 10.1021/ic0487528. [DOI] [PubMed] [Google Scholar]; (d) Hoskins BF, Robson R, Slizys DA. J Am Chem Soc. 1997;119:2952. [Google Scholar]; (e) Imaz I, Maspoch D, Rodriguez-Blanco C, Perez-Falcon JM, Campo J, Ruiz-Molina D. Angew Chem, Int Ed. 2008;47:1857. doi: 10.1002/anie.200705263. [DOI] [PubMed] [Google Scholar]; (f) Li C, Chen S, Li J, Han K, Xu M, Hu B, Yu Y, Jia X. Chem Commun. 2011;47:11294. doi: 10.1039/c1cc14829j. [DOI] [PubMed] [Google Scholar]; (g) Madhu V, Das SK. Dalton Trans. 2011;40:12901. doi: 10.1039/c1dt10814j. [DOI] [PubMed] [Google Scholar]; (h) Mallik S, Johnson RD, Arnold FH. J Am Chem Soc. 1993;115:2518. [Google Scholar]; (i) Ross TM, Neville SM, Innes DS, Turner DR, Moubaraki B, Murray KS. Dalton Trans. 2010;39:149. doi: 10.1039/b913234a. [DOI] [PubMed] [Google Scholar]; (j) Song L, Li J, Lin P, Li Z, Li T, Du S, Wu X. Inorg Chem. 2006;45:10155. doi: 10.1021/ic061142i. [DOI] [PubMed] [Google Scholar]; (k) Wang PF, Duan Y, Cao DK, Li YZ, Zheng LM. Dalton Trans. 2010;39:4559. doi: 10.1039/b927100g. [DOI] [PubMed] [Google Scholar]; (l) Zang SQ, Liang R, Fan YJ, Hou HW, Mak TCW. Dalton Trans. 2010;39:8022. doi: 10.1039/c0dt00374c. [DOI] [PubMed] [Google Scholar]
- 161.Mulfort KL, Farha OK, Stern CL, Sarjeant AA, Hupp JT. J Am Chem Soc. 2009;131:3866. doi: 10.1021/ja809954r. [DOI] [PubMed] [Google Scholar]
- 162.(a) Bojdys MJ, Wohlgemuth SA, Thomas A, Antonietti M. Macromolecules. 2010;43:6639. [Google Scholar]; (b) Sumida K, Foo ML, Horike S, Long JR. Eur J Inorg Chem. 2010:3739. [Google Scholar]
- 163.Kilpin KJ, Gower ML, Telfer SG, Jameson GB, Crowley JD. Inorg Chem. 2011;50:1123. doi: 10.1021/ic1020059. [DOI] [PubMed] [Google Scholar]
- 164.(a) Ghosh S, Gole B, Bar AK, Mukherjee PS. Organometallics. 2009;28:4288. doi: 10.1039/b911622m. [DOI] [PubMed] [Google Scholar]; (b) Johnson AM, Hooley RJ. Inorg Chem. 2011;50:4671. doi: 10.1021/ic2001688. [DOI] [PubMed] [Google Scholar]; (c) Liao P, Langloss BW, Johnson AM, Knudsen ER, Tham FS, Julian RR, Hooley RJ. Chem Commun. 2010;46:4932. doi: 10.1039/c0cc00234h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.(a) Gong Y, Li J, Qin JB, Wu T, Cao R, Li JH. Cryst Growth Des. 2011;11:1662. [Google Scholar]; (b) Gong Y, Zhou YC, Liu TF, Lue J, Proserpio DM, Cao R. Chem Commun. 2011;47:5982. doi: 10.1039/c1cc10411j. [DOI] [PubMed] [Google Scholar]; (c) Han LW, Gong Y, Lin ZJ, Lue J, Cao R. Dalton Trans. 2012;41:4146. doi: 10.1039/c2dt11899h. [DOI] [PubMed] [Google Scholar]; (d) Kim K, Park S, Park KM, Lee SS. Cryst Growth Des. 2011;11:4059. [Google Scholar]; (e) Qin Z, Jennings MC, Puddephatt RJ. Inorg Chem. 2003;42:1956. doi: 10.1021/ic020322z. [DOI] [PubMed] [Google Scholar]; (f) Shanmugaraju S, Bar AK, Chi KW, Mukherjee PS. Organometallics. 2010;29:2971. [Google Scholar]
- 166.Morris GA, Zhou H, Stern CL, Nguyen ST. Inorg Chem. 2001;40:3222. doi: 10.1021/ic010090o. [DOI] [PubMed] [Google Scholar]
- 167.Sun SS, Stern CL, Nguyen SBT, Hupp JT. J Am Chem Soc. 2004;126:6314. doi: 10.1021/ja037378s. [DOI] [PubMed] [Google Scholar]
- 168.Wu CD, Lin W. Angew Chem, Int Ed. 2007;46:1075. doi: 10.1002/anie.200602099. [DOI] [PubMed] [Google Scholar]
- 169.Lee SJ, Lin W. J Am Chem Soc. 2002;124:4554. doi: 10.1021/ja0256257. [DOI] [PubMed] [Google Scholar]
- 170.Cui Y, Lee SJ, Lin W. J Am Chem Soc. 2003;125:6014. doi: 10.1021/ja029926s. [DOI] [PubMed] [Google Scholar]
- 171.Lee SJ, Kim JS, Lin W. Inorg Chem. 2004;43:6579. doi: 10.1021/ic0497937. [DOI] [PubMed] [Google Scholar]
- 172.(a) Farha OK, Shultz AM, Sarjeant AA, Nguyen ST, Hupp JT. J Am Chem Soc. 2011;133:5652. doi: 10.1021/ja111042f. [DOI] [PubMed] [Google Scholar]; (b) Shultz AM, Farha OK, Hupp JT, Nguyen ST. J Am Chem Soc. 2009;131:4204. doi: 10.1021/ja900203f. [DOI] [PubMed] [Google Scholar]; (c) Splan KE, Stern CL, Hupp JT. Inorg Chim Acta. 2004;357:4005. [Google Scholar]
- 173.(a) Bagchi V, Bandyopadhyay D. J Organomet Chem. 2009;694:1259. [Google Scholar]; (b) Carlucci L, Ciani G, Proserpio DM, Porta F. Angew Chem, Int Ed. 2003;42:317. doi: 10.1002/anie.200390106. [DOI] [PubMed] [Google Scholar]; (c) DeVries LD, Barron PM, Hurley EP, Hu C, Choe W. J Am Chem Soc. 2011;133:14848. doi: 10.1021/ja2032822. [DOI] [PubMed] [Google Scholar]; (d) Hagrman D, Hagrman PJ, Zubieta J. Angew Chem, Int Ed. 1999;38:3165. doi: 10.1002/(sici)1521-3773(19991102)38:21<3165::aid-anie3165>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]; (e) Li Y, Xiao J, Shubina TE, Chen M, Shi Z, Schmid M, Steinruck HP, Gottfried JM, Lin N. J Am Chem Soc. 2012;134:6401. doi: 10.1021/ja300593w. [DOI] [PubMed] [Google Scholar]; (f) Lin KJ. Angew Chem, Int Ed. 1999;38:2730. [PubMed] [Google Scholar]; (g) Lipstman S, Goldberg I. Acta Crystallogr C. 2009;C65:o447. doi: 10.1107/S0108270109030753. [DOI] [PubMed] [Google Scholar]; (h) Lipstman S, Goldberg I. Cryst Growth Des. 2010;10:1823. [Google Scholar]; (i) Liu B, Qian DJ, Huang HX, Wakayama T, Hara S, Huang W, Nakamura C, Miyake J. Langmuir. 2005;21:5079. doi: 10.1021/la050064t. [DOI] [PubMed] [Google Scholar]; (j) Ohmura T, Usuki A, Fukumori K, Ohta T, Ito M, Tatsumi K. Inorg Chem. 2006;45:7988. doi: 10.1021/ic060358h. [DOI] [PubMed] [Google Scholar]; (k) Shi S, Zhou J, Han S, Peng Y, Ye J. J Nanosci Nanotechnol. 2008;8:1359. [PubMed] [Google Scholar]; (l) Smelser AM, Li YF, Carmichael CN, Zhao HY, Pan WP, Parkin S, Yan B. Inorg Chim Acta. 2011;375:122. [Google Scholar]; (m) Sun D, Tham FS, Reed CA, Boyd PDW. Proc Natl Acad Sci U S A. 2002;99:5088. doi: 10.1073/pnas.072602399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Yucesan G, Golub V, O’Connor CJ, Zubieta J. CrystEngComm. 2004;6:323. [Google Scholar]; (o) Zheng N, Zhang J, Bu X, Feng P. Cryst Growth Des. 2007;7:2576. [Google Scholar]
- 174.(a) Ballesteros-Rivas M, Ota A, Reinheimer E, Prosvirin A, Valdes-Martinez J, Dunbar KR. Angew Chem, Int Ed. 2011;50:9703. doi: 10.1002/anie.201101658. [DOI] [PubMed] [Google Scholar]; (b) Dai F, He H, Sun D. J Am Chem Soc. 2008;130:14064. doi: 10.1021/ja805920t. [DOI] [PubMed] [Google Scholar]; (c) Fei H, Rogow DL, Oliver SRJ. J Am Chem Soc. 2010;132:7202. doi: 10.1021/ja102134c. [DOI] [PubMed] [Google Scholar]; (d) Henke S, Fischer RA. J Am Chem Soc. 2011;133:2064. doi: 10.1021/ja109317e. [DOI] [PubMed] [Google Scholar]; (e) Huang SH, Lin CH, Wu WC, Wang SL. Angew Chem, Int Ed. 2009;48:6124. doi: 10.1002/anie.200901744. [DOI] [PubMed] [Google Scholar]; (f) Kitaura R, Seki K, Akiyama G, Kitagawa S. Angew Chem, Int Ed. 2003;42:428. doi: 10.1002/anie.200390130. [DOI] [PubMed] [Google Scholar]; (g) Roberts JM, Fini BM, Sarjeant AA, Farha OK, Hupp JT, Scheidt KA. J Am Chem Soc. 2012;134:3334. doi: 10.1021/ja2108118. [DOI] [PubMed] [Google Scholar]; (h) Thallapally PK, Tian J, Kishan MR, Fernandez CA, Dalgarno SJ, McGrail PB, Warren JE, Atwood JL. J Am Chem Soc. 2008;130:16842. doi: 10.1021/ja806391k. [DOI] [PubMed] [Google Scholar]; (i) Vaidhyanathan R, Bradshaw D, Rebilly JN, Barrio JP, Gould JA, Berry NG, Rosseinsky MJ. Angew Chem, Int Ed. 2006;45:6495. doi: 10.1002/anie.200602242. [DOI] [PubMed] [Google Scholar]; (j) Yaghi OM, Li H. J Am Chem Soc. 1995;117:10401. [Google Scholar]; (k) Yuan W, Friscic T, Apperley D, James SL. Angew Chem, Int Ed. 2010;49:3916. doi: 10.1002/anie.200906965. [DOI] [PubMed] [Google Scholar]
- 175.(a) Alessio E, Casanova M, Zangrando E, Iengo E. Chem Commun. 2012;48:5112. doi: 10.1039/c2cc31420g. [DOI] [PubMed] [Google Scholar]; (b) Barry NPE, Therrien B. Eur J Inorg Chem. 2009:4695. [Google Scholar]; (c) Fujita M, Fujita N, Ogura K, Yamaguchill K. Nature. 1999;400:52. [Google Scholar]; (d) Fujita M, Oguro D, Miyazawa M, Oka H, Yamaguchi K, Ogura K. Nature. 1995;378:469. [Google Scholar]; (e) Gardner JS, Harrison RG, Lamb JD, Dearden DV. New J Chem. 2006;30:1276. [Google Scholar]; (f) Heine J, Schmedt adGJ, Dehnen S. J Am Chem Soc. 2011;133:10018. doi: 10.1021/ja2030273. [DOI] [PubMed] [Google Scholar]; (g) Icli B, Sheepwash E, Riis-Johannessen T, Schenk K, Filinchuk Y, Scopelliti R, Severin K. Chem Sci. 2011;2:1719. [Google Scholar]; (h) Inokuma Y, Arai T, Fujita M. Nat Chem. 2010;2:780. doi: 10.1038/nchem.742. [DOI] [PubMed] [Google Scholar]; (i) Kobayashi Y, Kawano M, Fujita M. Chem Commun. 2006:4377. doi: 10.1039/b612562j. [DOI] [PubMed] [Google Scholar]; (j) Kusukawa T, Fujita M. J Am Chem Soc. 2002;124:13576. doi: 10.1021/ja020712k. [DOI] [PubMed] [Google Scholar]; (k) Mattsson J, Govindaswamy P, Furrer J, Sei Y, Yamaguchi K, Suss-Fink G, Therrien B. Organometallics. 2008;27:4346. [Google Scholar]; (l) Nishioka Y, Yamaguchi T, Kawano M, Fujita M. J Am Chem Soc. 2008;130:8160. doi: 10.1021/ja802818t. [DOI] [PubMed] [Google Scholar]; (m) Ono K, Yoshizawa M, Kato T, Fujita M. Chem Commun. 2008:2328. doi: 10.1039/b801701h. [DOI] [PubMed] [Google Scholar]; (n) Osuga T, Murase T, Ono K, Yamauchi Y, Fujita M. J Am Chem Soc. 2010;132:15553. doi: 10.1021/ja108367j. [DOI] [PubMed] [Google Scholar]; (o) Pitto-Barry A, Barry NPE, Zava O, Deschenaux R, Therrien B. Chem—Asian J. 2011;6:1595. doi: 10.1002/asia.201100136. [DOI] [PubMed] [Google Scholar]; (p) Sun SS, Lees AJ. Chem Commun. 2001:103. [Google Scholar]; (q) Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. Angew Chem, Int Ed. 2008;47:3773. doi: 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]; (r) Yamashita K-i, Kawano M, Fujita M. Chem Commun. 2007:4102. doi: 10.1039/b712529a. [DOI] [PubMed] [Google Scholar]; (s) Yi JW, Barry NPE, Furrer MA, Zava O, Dyson PJ, Therrien B, Kim BH. Bioconjugate Chem. 2012;23:461. doi: 10.1021/bc200472n. [DOI] [PubMed] [Google Scholar]; (t) Yoshizawa M, Miyagi S, Kawano M, Ishiguro K, Fujita M. J Am Chem Soc. 2004;126:9172. doi: 10.1021/ja047612u. [DOI] [PubMed] [Google Scholar]; (u) Zava O, Mattsson J, Therrien B, Dyson PJ. Chem—Eur J. 2010;16:1428. doi: 10.1002/chem.200903216. [DOI] [PubMed] [Google Scholar]
- 176.(a) Kaminker R, Motiei L, Gulino A, Fragala I, Shimon LJW, Evmenenko G, Dutta P, Iron MA, van dBME. J Am Chem Soc. 2010;132:14554. doi: 10.1021/ja105518n. [DOI] [PubMed] [Google Scholar]; (b) Vartanian M, Lucassen ACB, Shimon LJW, van dBME. Cryst Growth Des. 2008;8:786. [Google Scholar]
- 177.(a) Clegg JK, Iremonger SS, Hayter MJ, Southon PD, MacQuart RB, Duriska MB, Jensen P, Turner P, Jolliffe KA, Kepert CJ, Meehan GV, Lindoy LF. Angew Chem, Int Ed. 2010;49:1075. doi: 10.1002/anie.200905497. [DOI] [PubMed] [Google Scholar]; (b) Deng ZP, Huo LH, Xu H, Zhao H, Ng SW, Gao S. Dalton Trans. 2011;40:1224. doi: 10.1039/c0dt01153c. [DOI] [PubMed] [Google Scholar]; (c) Fang Q, Zhu G, Xue M, Sun J, Tian G, Wu G, Qiu S. Dalton Trans. 2004:2202. doi: 10.1039/B402715A. [DOI] [PubMed] [Google Scholar]; (d) Fang Q, Zhu G, Xue M, Sun J, Wei Y, Qiu S, Xu R. Angew Chem, Int Ed. 2005;44:3845. doi: 10.1002/anie.200462260. [DOI] [PubMed] [Google Scholar]; (e) Fang QR, Zhu GS, Xue M, Zhang QL, Sun JY, Guo XD, Qiu SL, Xu ST, Wang P, Wang DJ, Wei Y. Chem—Eur J. 2006;12:3754. doi: 10.1002/chem.200500963. [DOI] [PubMed] [Google Scholar]; (f) Hazra S, Sarkar B, Naiya S, Drew MGB, Ribas J, Diaz C, Ghosh A. Inorg Chem Commun. 2011;14:1860. [Google Scholar]; (g) Sapchenko SA, Samsonenko DG, Dybtsev DN, Melgunov MS, Fedin VP. Dalton Trans. 2011;40:2196. doi: 10.1039/c0dt00999g. [DOI] [PubMed] [Google Scholar]; (h) Wu H, Dong XW, Liu HY, Ma JF, Liu YY, Liu YY, Yang J. Inorg Chim Acta. 2011;373:19. [Google Scholar]; (i) Xue M, Zhu G, Ding H, Wu L, Zhao X, Jin Z, Qiu S. Cryst Growth Des. 2009;9:1481. [Google Scholar]; (j) Zheng G-l, Zhang H-J, Song S-Y, Li Y-Y, Guo H-D. Eur J Inorg Chem. 2008:1756. [Google Scholar]
- 178.(a) Miyasaka H, Campos-Fernandez CS, Galan-Mascaros JR, Dunbar KR. Inorg Chem. 2000;39:5870. doi: 10.1021/ic0007097. [DOI] [PubMed] [Google Scholar]; (b) Ouyang X, Campana C, Dunbar KR. Inorg Chem. 1996;35:7188. doi: 10.1021/ic960713b. [DOI] [PubMed] [Google Scholar]
- 179.(a) Choi HJ, Dinca M, Dailly A, Long JR. Energy Environ Sci. 2010;3:117. [Google Scholar]; (b) Choi HJ, Dinca M, Long JR. J Am Chem Soc. 2008;130:7848. doi: 10.1021/ja8024092. [DOI] [PubMed] [Google Scholar]; (c) Lu Y, Tonigold M, Bredenkoetter B, Volkmer D, Hitzbleck J, Langstein G. Z Anorg Allg Chem. 2008;634:2411. [Google Scholar]; (d) Poirier E, Dailly A. Langmuir. 2009;25:12169. doi: 10.1021/la901680p. [DOI] [PubMed] [Google Scholar]
- 180.(a) Ezuhara T, Endo K, Aoyama Y. J Am Chem Soc. 1999;121:3279. [Google Scholar]; (b) Ezuhara T, Endo K, Matsuda K, Aoyama Y. New J Chem. 2000;24:609. [Google Scholar]
- 181.(a) Huang YQ, Ding B, Song HB, Zhao B, Ren P, Cheng P, Wang HG, Liao DZ, Yan SP. Chem Commun. 2006:4906. doi: 10.1039/b610185b. [DOI] [PubMed] [Google Scholar]; (b) Li W, Li MX, Shao M, Wang ZX, Liu HJ. Inorg Chem Commun. 2008;11:954. [Google Scholar]; (c) Li W, Shao M, Liu HJ, Li MX. Acta Crystallogr E. 2007;E63:o3224. [Google Scholar]; (d) Li XW, Chen F, Xu WF, Li YZ, Chen XT, Xue ZL. Inorg Chem Commun. 2011;14:1673. [Google Scholar]; (e) Liang SW, He X, Shao M, Li MX. J Coord Chem. 2008;61:2999. [Google Scholar]; (f) Liang SW, Li MX, Shao M, Liu HJ. Inorg Chem Commun. 2007;10:1347. [Google Scholar]; (g) Liu YY, Huang YQ, Shi W, Cheng P, Liao DZ, Yan SP. Cryst Growth Des. 2007;7:1483. [Google Scholar]; (h) Wang Y, Ding B, Cheng P, Liao DZ, Yan SP. Inorg Chem. 2007;46:2002. doi: 10.1021/ic060855y. [DOI] [PubMed] [Google Scholar]
- 182.Demessence A, D’Alessandro DM, Foo ML, Long JR. J Am Chem Soc. 2009;131:8784. doi: 10.1021/ja903411w. [DOI] [PubMed] [Google Scholar]
- 183.(a) Hasegawa S, Horike S, Matsuda R, Furukawa S, Mochizuki K, Kinoshita Y, Kitagawa S. J Am Chem Soc. 2007;129:2607. doi: 10.1021/ja067374y. [DOI] [PubMed] [Google Scholar]; (b) Hong S, Zou Y, Moon D, Lah MS. Chem Commun. 2007:1707. doi: 10.1039/b702216f. [DOI] [PubMed] [Google Scholar]; (c) Tzeng BC, Chen BS, Yeh HT, Lee GH, Peng SM. New J Chem. 2006;30:1087. [Google Scholar]
- 184.(a) Deng YF. Z Kristallogr - New Cryst Struct. 2010;225:331. [Google Scholar]; (b) Fan J, Gan L, Kawaguchi H, Sun WY, Yu KB, Tang WX. Chem—Eur J. 2003;9:3965. doi: 10.1002/chem.200204298. [DOI] [PubMed] [Google Scholar]; (c) Fan J, Shu M-H, Okamura T-a, Li Y-Z, Sun W-Y, Tang W-X, Ueyama N. New J Chem. 2003;27:1307. [Google Scholar]; (d) Fan J, Sun W-Y, Okamura T-a, Tang W-X, Ueyama N. Inorg Chem. 2003;42:3168. doi: 10.1021/ic0206847. [DOI] [PubMed] [Google Scholar]; (e) Ji CC, Li J, Li YZ, Guo ZJ, Zheng HG. CrystEngComm. 2011;13:459. [Google Scholar]; (f) Pan ZR, Yao XQ, Zheng HG, Li YZ, Guo ZJ, Batten SR. CrystEngComm. 2009;11:605. [Google Scholar]; (g) Su Z, Chen MS, Fan J, Chen M, Chen SS, Luo L, Sun WY. CrystEngComm. 2010;12:2040. [Google Scholar]; (h) Su Z, Fan J, Okamura T-a, Chen M-S, Chen S-S, Sun W-Y, Ueyama N. Cryst Growth Des. 2010;10:1911. [Google Scholar]; (i) Su Z, Fan J, Okamura T-a, Sun W-Y, Ueyama N. Cryst Growth Des. 2010;10:3515. [Google Scholar]; (j) Su Z, Song Y, Bai ZS, Fan J, Liu GX, Sun WY. CrystEngComm. 2010;12:4339. [Google Scholar]; (k) Su Z, Xu J, Fan J, Liu DJ, Chu Q, Chen MS, Chen SS, Liu GX, Wang XF, Sun WY. Cryst Growth Des. 2009;9:2801. [Google Scholar]; (l) Wang XF, Lv Y, Okamura TA, Kawaguchi H, Wu G, Sun WY, Ueyama N. Cryst Growth Des. 2007;7:1125. [Google Scholar]; (m) Zhao W, Fan J, Song Y, Kawaguchi H, Okamura T-a, Sun W-Y, Ueyama N. Dalton Trans. 2005:1509. doi: 10.1039/b419418g. [DOI] [PubMed] [Google Scholar]
- 185.(a) Biswas S, Maes M, Dhakshinamoorthy A, Feyand M, De VDE, Garcia H, Stock N. J Mater Chem. 2012;22:10200. [Google Scholar]; (b) Li MN, Du DY, Yang GS, Li SL, Lan YQ, Shao KZ, Qin JS, Su ZM. Cryst Growth Des. 2011;11:2510. [Google Scholar]; (c) Liao J-H, Chen W-T, Tsai C-S, Yang C-C, Wang C-C. CrystEngComm. 2010;12:3033. [Google Scholar]; (d) Poloni R, Smit B, Neaton JB. J Phys Chem A. 2012;116:4957. doi: 10.1021/jp302190v. [DOI] [PubMed] [Google Scholar]; (e) Zhou W, Yildirim T. J Phys Chem C. 2008;112:8132. [Google Scholar]
- 186.Dinca M, Dailly A, Tsay C, Long JR. Inorg Chem. 2008;47:11. doi: 10.1021/ic701917w. [DOI] [PubMed] [Google Scholar]
- 187.(a) Dolomanov OV, Cordes DB, Champness NR, Blake AJ, Hanton LR, Jameson GB, Schroeder M, Wilson C. Chem Commun. 2004:642. doi: 10.1039/b315243j. [DOI] [PubMed] [Google Scholar]; (b) Lin JG, Qiu L, Wang FM, Lu CS, Meng QJ, Wu PH. Inorg Chem Commun. 2010;13:175. [Google Scholar]; (c) Lin JG, Su Y, Tian ZF, Qiu L, Wen LL, Lu ZD, Li YZ, Meng QJ. Cryst Growth Des. 2007;7:2526. [Google Scholar]; (d) Lin JG, Xu YY, Qiu L, Zang SQ, Lu CS, Duan CY, Li YZ, Gao S, Meng QJ. Chem Commun. 2008:2659. doi: 10.1039/b802992j. [DOI] [PubMed] [Google Scholar]; (e) Qiu L, Lin J, Xu Y. Inorg Chem Commun. 2009;12:986. [Google Scholar]
- 188.Klein C, Graf E, Wais HM, De CA. New J Chem. 2001;25:207. [Google Scholar]
- 189.(a) Caulder DL, Brueckner C, Powers RE, Koenig S, Parac TN, Leary JA, Raymond KN. J Am Chem Soc. 2001;123:8923. doi: 10.1021/ja0104507. [DOI] [PubMed] [Google Scholar]; (b) Fiedler D, Pagliero D, Brumaghim JL, Bergman RG, Raymond KN. Inorg Chem. 2004;43:846. doi: 10.1021/ic035105s. [DOI] [PubMed] [Google Scholar]; (c) Lambert JB, Singer SR. J Organomet Chem. 2004;689:2293. [Google Scholar]
- 190.Bar AK, Chakrabarty R, Chi KW, Batten SR, Mukherjee PS. Dalton Trans. 2009:3222. doi: 10.1039/b900118b. [DOI] [PubMed] [Google Scholar]
- 191.Mukherjee PS, Das N, Kryschenko YK, Arif AM, Stang PJ. J Am Chem Soc. 2004;126:2464. doi: 10.1021/ja039235b. [DOI] [PubMed] [Google Scholar]
- 192.(a) Grote A, Scopelliti R, Severin K. Angew Chem, Int Ed. 2003;42:3821. doi: 10.1002/anie.200351623. [DOI] [PubMed] [Google Scholar]; (b) Grote Z, Bonazzi S, Scopelliti R, Severin K. J Am Chem Soc. 2006;128:10382. doi: 10.1021/ja0637806. [DOI] [PubMed] [Google Scholar]; (c) Lehaire ML, Scopelliti R, Piotrowski H, Severin K. Angew Chem, Int Ed. 2002;41:1419. doi: 10.1002/1521-3773(20020415)41:8<1419::aid-anie1419>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]; (d) Piotrowski H, Hilt G, Schulz A, Mayer P, Polborn K, Severin K. Chem—Eur J. 2001;7:3196. doi: 10.1002/1521-3765(20010803)7:15<3196::aid-chem3196>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; (e) Piotrowski H, Polborn K, Hilt G, Severin K. J Am Chem Soc. 2001;123:2699. doi: 10.1021/ja005804t. [DOI] [PubMed] [Google Scholar]; (f) Piotrowski H, Severin K. Proc Natl Acad Sci U S A. 2002;99:4997. doi: 10.1073/pnas.062663899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.(a) Li JR, Yakovenko AA, Lu W, Timmons DJ, Zhuang W, Yuan D, Zhou HC. J Am Chem Soc. 2010;132:17599. doi: 10.1021/ja1080794. [DOI] [PubMed] [Google Scholar]; (b) Li JR, Zhou HC. Angew Chem, Int Ed. 2009;48:8465. doi: 10.1002/anie.200904722. [DOI] [PubMed] [Google Scholar]; (c) Li JR, Zhou HC. Nat Chem. 2010;2:893. doi: 10.1038/nchem.803. [DOI] [PubMed] [Google Scholar]
- 194.Das N, Stang PJ, Arif AM, Campana CF. J Org Chem. 2005;70:10440. doi: 10.1021/jo0517085. [DOI] [PubMed] [Google Scholar]
- 195.Cotton FA, Lei P, Lin C, Murillo CA, Wang X, Yu SY, Zhang ZX. J Am Chem Soc. 2004;126:1518. doi: 10.1021/ja0396899. [DOI] [PubMed] [Google Scholar]
- 196.(a) Chandrasekhar V, Thirumoorthi R. Dalton Trans. 2010;39:2684. doi: 10.1039/b922044e. [DOI] [PubMed] [Google Scholar]; (b) Guo D, Zhang B-g, Duan C-y, Cao X, Meng Q-j. Dalton Trans. 2003:282. [Google Scholar]; (c) Hirai K, Uehara H, Kitagawa S, Furukawa S. Dalton Trans. 2012;41:3924. doi: 10.1039/c2dt12304e. [DOI] [PubMed] [Google Scholar]; (d) Huo J, Wang L, Irran E, Yu H, Gao J, Fan D, Li B, Wang J, Ding W, Amin AM, Li C, Ma L. Angew Chem, Int Ed. 2010;49:9237. doi: 10.1002/anie.201004745. [DOI] [PubMed] [Google Scholar]; (e) Kuehnert J, Rueffer T, Ecorchard P, Braeuer B, Lan Y, Powell AK, Lang H. Dalton Trans. 2009:4499. doi: 10.1039/b821407g. [DOI] [PubMed] [Google Scholar]; (f) Liu D, Liu H, Hu N. J Phys Chem B. 2012;116:1700. doi: 10.1021/jp209788g. [DOI] [PubMed] [Google Scholar]; (g) Yang H-C, Lin S-Y, Yang H-C, Lin C-L, Tsai L, Huang S-L, Chen IW-P, Chen C-h, Jin B-Y, Luh T-Y. Angew Chem, Int Ed. 2006;45:726. doi: 10.1002/anie.200503406. [DOI] [PubMed] [Google Scholar]
- 197.(a) Ohashi M, Yagyu A, Yamagata T, Mashima K. Chem Commun. 2007:3103. doi: 10.1039/b703730a. [DOI] [PubMed] [Google Scholar]; (b) Tanaka S, Yagyu A, Kikugawa M, Ohashi M, Yamagata T, Mashima K. Chem—Eur J. 2011;17:3693. doi: 10.1002/chem.201002808. [DOI] [PubMed] [Google Scholar]
- 198.(a) Arslan HK, Shekhah O, Wieland DCF, Paulus M, Sternemann C, Schroer MA, Tiemeyer S, Tolan M, Fischer RA, Woll C. J Am Chem Soc. 2011;133:8158. doi: 10.1021/ja2037996. [DOI] [PubMed] [Google Scholar]; (b) Centrone A, Yang Y, Speakman S, Bromberg L, Rutledge GC, Hatton TA. J Am Chem Soc. 2010;132:15687. doi: 10.1021/ja106381x. [DOI] [PubMed] [Google Scholar]; (c) Chen B, Zhao X, Putkham A, Hong K, Lobkovsky EB, Hurtado EJ, Fletcher AJ, Thomas KM. J Am Chem Soc. 2008;130:6411. doi: 10.1021/ja710144k. [DOI] [PubMed] [Google Scholar]; (d) Choi KM, Jeon HJ, Kang JK, Yaghi OM. J Am Chem Soc. 2011;133:11920. doi: 10.1021/ja204818q. [DOI] [PubMed] [Google Scholar]; (e) Das MC, Guo Q, He Y, Kim J, Zhao CG, Hong K, Xiang S, Zhang Z, Thomas KM, Krishna R, Chen B. J Am Chem Soc. 2012;134:8703. doi: 10.1021/ja302380x. [DOI] [PubMed] [Google Scholar]; (f) Deng H, Doonan CJ, Furukawa H, Ferreira RB, Towne J, Knobler CB, Wang B, Yaghi OM. Science. 2010;327:846. doi: 10.1126/science.1181761. [DOI] [PubMed] [Google Scholar]; (g) Feng PL, Perry JJIV, Nikodemski S, Jacobs BW, Meek ST, Allendorf MD. J Am Chem Soc. 2010;132:15487. doi: 10.1021/ja1065625. [DOI] [PubMed] [Google Scholar]; (h) Friscic T, Reid DG, Halasz I, Stein RS, Dinnebier RE, Duer MJ. Angew Chem, Int Ed. 2010;49:712. doi: 10.1002/anie.200906583. [DOI] [PubMed] [Google Scholar]; (i) Gould SL, Tranchemontagne D, Yaghi OM, Garcia-Garibay MA. J Am Chem Soc. 2008;130:3246. doi: 10.1021/ja077122c. [DOI] [PubMed] [Google Scholar]; (j) Graham AJ, Allan DR, Muszkiewicz A, Morrison CA, Moggach SA. Angew Chem, Int Ed. 2011;50:11138. doi: 10.1002/anie.201104285. [DOI] [PubMed] [Google Scholar]; (k) Gu X, Lu ZH, Jiang HL, Akita T, Xu Q. J Am Chem Soc. 2011;133:11822. doi: 10.1021/ja200122f. [DOI] [PubMed] [Google Scholar]; (l) Gu ZY, Yan XP. Angew Chem, Int Ed. 2010;49:1477. doi: 10.1002/anie.200906560. [DOI] [PubMed] [Google Scholar]; (m) Han SB, Wei YH, Valente C, Lagzi I, Gassensmith JJ, Coskun A, Stoddart JF, Grzybowski BA. J Am Chem Soc. 2010;132:16358. doi: 10.1021/ja1074322. [DOI] [PubMed] [Google Scholar]; (n) Hausdorf S, Baitalow F, Boehle T, Rafaja D, Mertens FORL. J Am Chem Soc. 2010;132:10978. doi: 10.1021/ja1028777. [DOI] [PubMed] [Google Scholar]; (o) Hermes S, Witte T, Hikov T, Zacher D, Bahnmueller S, Langstein G, Huber K, Fischer RA. J Am Chem Soc. 2007;129:5324. doi: 10.1021/ja068835i. [DOI] [PubMed] [Google Scholar]; (p) Horcajada P, Salles F, Wuttke S, Devic T, Heurtaux D, Maurin G, Vimont A, Daturi M, David O, Magnier E, Stock N, Filinchuk Y, Popov D, Riekel C, Ferey G, Serre C. J Am Chem Soc. 2011;133:17839. doi: 10.1021/ja206936e. [DOI] [PubMed] [Google Scholar]; (q) Hughes JT, Navrotsky A. J Am Chem Soc. 2011;133:9184. doi: 10.1021/ja202132h. [DOI] [PubMed] [Google Scholar]; (r) Koh K, Wong-Foy AG, Matzger AJ. J Am Chem Soc. 2010;132:15005. doi: 10.1021/ja1065009. [DOI] [PubMed] [Google Scholar]; (s) Li H, Eddaoudi M, O’Keeffe M, Yaghi M. Nature. 1999;402:276. [Google Scholar]; (t) Li MY, Dinca M. J Am Chem Soc. 2011;133:12926. doi: 10.1021/ja2041546. [DOI] [PubMed] [Google Scholar]; (u) Li Y, Yang RT. J Am Chem Soc. 2006;128:726. doi: 10.1021/ja056831s. [DOI] [PubMed] [Google Scholar]; (v) Mohideen MIH, Xiao B, Wheatley PS, McKinlay AC, Li Y, Slawin AMZ, Aldous DW, Cessford NF, Duren T, Zhao X, Gill R, Thomas KM, Griffin JM, Ashbrook SE, Morris RE. Nat Chem. 2011;3:304. doi: 10.1038/nchem.1003. [DOI] [PubMed] [Google Scholar]; (w) Ni Z, Masel RI. J Am Chem Soc. 2006;128:12394. doi: 10.1021/ja0635231. [DOI] [PubMed] [Google Scholar]; (x) Nijem N, Veyan JF, Kong L, Li K, Pramanik S, Zhao Y, Li J, Langreth D, Chabal YJ. J Am Chem Soc. 2010;132:1654. doi: 10.1021/ja908817n. [DOI] [PubMed] [Google Scholar]; (y) Park TH, Hickman AJ, Koh K, Martin S, Wong-Foy AG, Sanford MS, Matzger AJ. J Am Chem Soc. 2011;133:20138. doi: 10.1021/ja2094316. [DOI] [PubMed] [Google Scholar]; (z) Rosi NL, Kim J, Eddaoudi M, Chen B, O’Keeffe M, Yaghi OM. J Am Chem Soc. 2005;127:1504. doi: 10.1021/ja045123o. [DOI] [PubMed] [Google Scholar]; (aa) Shekhah O, Wang H, Paradinas M, Ocal C, Schuepbach B, Terfort A, Zacher D, Fischer RA, Woell C. Nat Mater. 2009;8:481. doi: 10.1038/nmat2445. [DOI] [PubMed] [Google Scholar]; (ab) Tsao CS, Yu MS, Chung TY, Wu HC, Wang CY, Chang KS, Chen HL. J Am Chem Soc. 2007;129:15997. doi: 10.1021/ja0752336. [DOI] [PubMed] [Google Scholar]; (ac) Xiang SC, Zhang Z, Zhao CG, Hong K, Zhao X, Ding DR, Xie MH, Wu CD, Das MC, Gill R, Thomas KM, Chen B. Nat Commun. 2011;2:1206/1. doi: 10.1038/ncomms1206. [DOI] [PubMed] [Google Scholar]; (ad) Zhao Y, Zhang J, Han B, Song J, Li J, Wang Q. Angew Chem, Int Ed. 2011;50:636. doi: 10.1002/anie.201005314. [DOI] [PubMed] [Google Scholar]; (ae) Zheng ST, Wu T, Chou C, Fuhr A, Feng P, Bu X. J Am Chem Soc. 2012;134:4517. doi: 10.1021/ja2118255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rigby N, Jacobs T, Reddy JP, Hardie MJ. Cryst Growth Des. 2012;12:1871. [Google Scholar]
- 200.(a) Eubank JF, Wojtas L, Hight MR, Bousquet T, Kravtsov VC, Eddaoudi M. J Am Chem Soc. 2011;133:17532. doi: 10.1021/ja203898s. [DOI] [PubMed] [Google Scholar]; (b) Guo J, Ma JF, Liu B, Kan WQ, Yang J. Cryst Growth Des. 2011;11:3609. [Google Scholar]; (c) He H, Collins D, Dai F, Zhao X, Zhang G, Ma H, Sun D. Cryst Growth Des. 2010;10:895. [Google Scholar]; (d) He J, Yu J, Zhang Y, Pan Q, Xu R. Inorg Chem. 2005;44:9279. doi: 10.1021/ic051143v. [DOI] [PubMed] [Google Scholar]; (e) Hulvey Z, Furman JD, Turner SA, Tang M, Cheetham AK. Cryst Growth Des. 2010;10:2041. [Google Scholar]; (f) Lin JD, Wu ST, Li ZH, Du SW. Dalton Trans. 2010;39:10719. doi: 10.1039/c0dt00390e. [DOI] [PubMed] [Google Scholar]; (g) Ling Y, Yang F, Deng M, Chen Z, Liu X, Weng L, Zhou Y. Dalton Trans. 2012;41:4007. doi: 10.1039/c2dt12059c. [DOI] [PubMed] [Google Scholar]; (h) Liu JQ, Huang YS, Zhao YY, Jia ZB. Cryst Growth Des. 2011;11:569. [Google Scholar]; (i) Liu Y, Eubank JF, Cairns AJ, Eckert J, Kravtsov VC, Luebke R, Eddaoudi M. Angew Chem, Int Ed. 2007;46:3278. doi: 10.1002/anie.200604306. [DOI] [PubMed] [Google Scholar]; (j) Qin YY, Zhang J, Li ZJ, Zhang L, Cao XY, Yao YG. Chem Commun. 2008:2532. doi: 10.1039/b800017d. [DOI] [PubMed] [Google Scholar]; (k) Ren P, Shi W, Cheng P. Cryst Growth Des. 2008;8:1097. [Google Scholar]; (l) Sudik AC, Cote AP, Yaghi OM. Inorg Chem. 2005;44:2998. doi: 10.1021/ic050064g. [DOI] [PubMed] [Google Scholar]; (m) Tanaka D, Henke A, Albrecht K, Moeller M, Nakagawa K, Kitagawa S, Groll J. Nat Chem. 2010;2:410. doi: 10.1038/nchem.627. [DOI] [PubMed] [Google Scholar]; (n) Vodak DT, Braun ME, Kim J, Eddaoudi M, Yaghi OM. Chem Commun. 2001:2534. [Google Scholar]; (o) Xiao B, Byrne PJ, Wheatley PS, Wragg DS, Zhao X, Fletcher AJ, Thomas KM, Peters L, Evans JSO, Warren JE, Zhou W, Morris RE. Nat Chem. 2009;1:289. doi: 10.1038/nchem.254. [DOI] [PubMed] [Google Scholar]; (p) Zheng ST, Zuo F, Wu T, Irfanoglu B, Chou C, Nieto RA, Feng P, Bu X. Angew Chem, Int Ed. 2011;50:1849. doi: 10.1002/anie.201006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.(a) Bonar-Law RP, McGrath TD, Singh N, Bickley JF, Steiner A. Chem Commun. 1999:2457. [Google Scholar]; (b) Ke Y, Collins DJ, Zhou HC. Inorg Chem. 2005;44:4154. doi: 10.1021/ic050460z. [DOI] [PubMed] [Google Scholar]
- 202.(a) Baati T, Horcajada P, Gref R, Couvreur P, Serre C. J Pharm Biomed Anal. 2011;56:758. doi: 10.1016/j.jpba.2011.07.011. [DOI] [PubMed] [Google Scholar]; (b) Friscic T, Fabian L. CrystEngComm. 2009;11:743. [Google Scholar]; (c) Lin J, Wu P, Kang L, Lu C, Meng Q. Solid State Sci. 2011;13:1538. [Google Scholar]; (d) Sbircea L, Sharma ND, Clegg W, Harrington RW, Horton PN, Hursthouse MB, Apperley DC, Boyd DR, James SL. Chem Commun. 2008:5538. doi: 10.1039/b812366g. [DOI] [PubMed] [Google Scholar]; (e) Wissmann G, Schaate A, Lilienthal S, Bremer I, Schneider AM, Behrens P. Microporous Mesoporous Mater. 2012;152:64. [Google Scholar]; (f) Yang Q, Chen S, Gao S. Inorg Chem Commun. 2009;12:1224. [Google Scholar]; (g) Zhang KL, Liang W, Chang Y, Yuan LM, Ng SW. Polyhedron. 2009;28:647. [Google Scholar]; (h) Zhao L, Xu GF, Tang J. J Mol Struct. 2010;979:160. [Google Scholar]; (i) Zhu WH, Wang ZM, Gao S. Gaodeng Xuexiao Huaxue Xuebao. 2011;32:532. [Google Scholar]
- 203.Bar AK, Chakrabarty R, Mukherjee PS. Organometallics. 2008;27:3806. [Google Scholar]
- 204.(a) Bae YS, Farha OK, Spokoyny AM, Mirkin CA, Hupp JT, Snurr RQ. Chem Commun. 2008:4135. doi: 10.1039/b805785k. [DOI] [PubMed] [Google Scholar]; (b) Farha OK, Spokoyny AM, Mulfort KL, Galli S, Hupp JT, Mirkin CA. Small. 2009;5:1727. doi: 10.1002/smll.200900085. [DOI] [PubMed] [Google Scholar]; (c) Huang SL, Lin YJ, Yu WB, Jin GX. ChemPlusChem. 2012;77:141. [Google Scholar]
- 205.(a) Chen B, Yang Y, Zapata F, Qian G, Luo Y, Zhang J, Lobkovsky EB. Inorg Chem. 2006;45:8882. doi: 10.1021/ic060568u. [DOI] [PubMed] [Google Scholar]; (b) Chen SC, Zhang ZH, Chen Q, Gao HB, Liu Q, He MY, Du M. Inorg Chem Commun. 2009;12:835. [Google Scholar]; (c) Cheng ML, Zhu E, Liu Q, Chen SC, Chen Q, He MY. Inorg Chem Commun. 2011;14:300. [Google Scholar]; (d) Chun H, Dybtsev DN, Kim H, Kim K. Chem—Eur J. 2005;11:3521. doi: 10.1002/chem.200401201. [DOI] [PubMed] [Google Scholar]; (e) Hulvey Z, Sava DA, Eckert J, Cheetham AK. Inorg Chem. 2011;50:403. doi: 10.1021/ic101153c. [DOI] [PubMed] [Google Scholar]; (f) Koeberl M, Cokoja M, Bechlars B, Herdtweck E, Kuehn FE. Dalton Trans. 2011;40:11490. doi: 10.1039/c1dt11249j. [DOI] [PubMed] [Google Scholar]; (g) MacNeill CM, Day CS, Marts A, Lachgar A, Noftle RE. Inorg Chim Acta. 2011;365:196. [Google Scholar]; (h) Orthaber A, Seidel C, Belaj F, Albering JH, Pietschnig R, Ruschewitz U. Inorg Chem. 2010;49:9350. doi: 10.1021/ic1009829. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Seidel C, Ahlers R, Ruschewitz U. Cryst Growth Des. 2011;11:5053. [Google Scholar]; (j) Yoon JH, Choi SB, Oh YJ, Seo MJ, Jhon YH, Lee TB, Kim D, Choi SH, Kim J. Catal Today. 2007;120:324. [Google Scholar]; (k) Zacher D, Yusenko K, Betard A, Henke S, Molon M, Ladnorg T, Shekhah O, Schuepbach B, de lAT, Krasnopolski M, Meilikhov M, Winter J, Terfort A, Woell C, Fischer RA. Chem—Eur J. 2011;17:1448. doi: 10.1002/chem.201002381. [DOI] [PubMed] [Google Scholar]
- 206.(a) Chen WT, Zeng XR, Liu DS, Ying SM, Liu JH. Chin J Chem. 2008;26:1678. [Google Scholar]; (b) Cheng JW, Zheng ST, Ma E, Yang GY. Inorg Chem. 2007;46:10534. doi: 10.1021/ic700893w. [DOI] [PubMed] [Google Scholar]; (c) Dulare R, Bharty MK, Kushawaha SK, Singh NK. J Mol Struct. 2011;985:323. [Google Scholar]; (d) Feng W, Xu Y, Zhou G, Zhang C, Zheng X. Inorg Chem Commun. 2007;10:49. [Google Scholar]; (e) Gu X, Lu ZH, Xu Q. Chem Commun. 2010;46:7400. doi: 10.1039/c0cc02808h. [DOI] [PubMed] [Google Scholar]; (f) Han S, Huang Y, Watanabe T, Dai Y, Walton KS, Nair S, Sholl DS, Meredith JC. ACS Comb Sci. 2012;14:263. doi: 10.1021/co3000192. [DOI] [PubMed] [Google Scholar]; (g) Huang L, Han L, Feng W, Zheng L, Zhang Z, Xu Y, Chen Q, Zhu D, Niu S. Cryst Growth Des. 2010;10:2548. [Google Scholar]; (h) Li X, Guo X, Weng XL, Lin S. CrystEngComm. 2012;14:1412. [Google Scholar]; (i) Lian TT, Chen SM. Inorg Chem Commun. 2012;18:8. [Google Scholar]; (j) Liu D, Huang X, Huang C, Huang G, Chen J. J Solid State Chem. 2009;182:1899. [Google Scholar]; (k) Nagarkar SS, Chaudhari AK, Ghosh SK. Inorg Chem. 2012;51:572. doi: 10.1021/ic202102m. [DOI] [PubMed] [Google Scholar]; (l) Pachfule P, Chen Y, Jiang J, Banerjee R. Chem—Eur J. 2012;18:688. doi: 10.1002/chem.201102295. [DOI] [PubMed] [Google Scholar]; (m) Rosado PJ, Ruhlandt-Senge K. J Coord Chem. 2011;64:186. [Google Scholar]; (n) Sekiya R, Nishikiori S-i. Chem Commun. 2012;48:5022. doi: 10.1039/c2cc31586f. [DOI] [PubMed] [Google Scholar]; (o) Sun J, Weng L, Zhou Y, Chen J, Chen Z, Liu Z, Zhao D. Angew Chem, Int Ed. 2002;41:4471. doi: 10.1002/1521-3773(20021202)41:23<4471::AID-ANIE4471>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]; (p) Tran DT, Fan X, Brennan DP, Zavalij PY, Oliver SRJ. Inorg Chem. 2005;44:6192. doi: 10.1021/ic050024c. [DOI] [PubMed] [Google Scholar]; (q) Wang YT, Fan HH, Wang HZ, Chen XM. Inorg Chem. 2005;44:4148. doi: 10.1021/ic0504137. [DOI] [PubMed] [Google Scholar]; (r) Wei Q, Nieuwenhuyzen M, Meunier F, Hardacre C, James SL. Dalton Trans. 2004:1807. doi: 10.1039/B404485A. [DOI] [PubMed] [Google Scholar]; (s) Zhang KL, Zhou F, Gao HY, Pan ZC, Lin JG, Guo R. J Coord Chem. 2008;61:1494. [Google Scholar]
- 207.Chi KW, Addicott C, Arif AM, Stang PJ. J Am Chem Soc. 2004;126:16569. doi: 10.1021/ja045542l. [DOI] [PubMed] [Google Scholar]
- 208.(a) Ahnfeldt T, Guillou N, Gunzelmann D, Margiolaki I, Loiseau T, Ferey G, Senker J, Stock N. Angew Chem, Int Ed. 2009;48:5163. doi: 10.1002/anie.200901409. [DOI] [PubMed] [Google Scholar]; (b) Devic T, Horcajada P, Serre C, Salles F, Maurin G, Moulin B, Heurtaux D, Clet G, Vimont A, Greneche JM, Le OB, Moreau F, Magnier E, Filinchuk Y, Marrot J, Lavalley JC, Daturi M, Ferey G. J Am Chem Soc. 2010;132:1127. doi: 10.1021/ja9092715. [DOI] [PubMed] [Google Scholar]; (c) Fu Y, Sun D, Chen Y, Huang R, Ding Z, Fu X, Li Z. Angew Chem, Int Ed. 2012;51:3364. doi: 10.1002/anie.201108357. [DOI] [PubMed] [Google Scholar]; (d) Grzesiak AL, Uribe FJ, Ockwig NW, Yaghi OM, Matzger AJ. Angew Chem, Int Ed. 2006;45:2553. doi: 10.1002/anie.200504312. [DOI] [PubMed] [Google Scholar]; (e) Kim M, Cahill JF, Su Y, Prather KA, Cohen SM. Chem Sci. 2012;3:126. [Google Scholar]; (f) Liu B, Ma M, Zacher D, Betard A, Yusenko K, Metzler-Nolte N, Woll C, Fischer RA. J Am Chem Soc. 2011;133:1734. doi: 10.1021/ja1109826. [DOI] [PubMed] [Google Scholar]; (g) Rossini AJ, Zagdoun A, Lelli M, Canivet J, Aguado S, Ouari O, Tordo P, Rosay M, Maas WE, Coperet C, Farrusseng D, Emsley L, Lesage A. Angew Chem, Int Ed. 2012;51:123. doi: 10.1002/anie.201106030. [DOI] [PubMed] [Google Scholar]; (h) Rowsell JLC, Yaghi OM. J Am Chem Soc. 2006;128:1304. doi: 10.1021/ja056639q. [DOI] [PubMed] [Google Scholar]; (i) Savonnet M, Bazer-Bachi D, Bats N, Perez-Pellitero J, Jeanneau E, Lecocq V, Pinel C, Farrusseng D. J Am Chem Soc. 2010;132:4518. doi: 10.1021/ja909613e. [DOI] [PubMed] [Google Scholar]; (j) Schoedel A, Scherb C, Bein T. Angew Chem, Int Ed. 2010;49:7225. doi: 10.1002/anie.201001684. [DOI] [PubMed] [Google Scholar]; (k) Song YF, Cronin L. Angew Chem, Int Ed. 2008;47:4635. doi: 10.1002/anie.200801631. [DOI] [PubMed] [Google Scholar]; (l) Vermoortele F, Maes M, Moghadam PZ, Lennox MJ, Ragon F, Boulhout M, Biswas S, Laurier KGM, Beurroies I, Deboyel R, Roeffaers M, Stock N, Duren T, Serre C, De VDE. J Am Chem Soc. 2011;133:18526. doi: 10.1021/ja207287h. [DOI] [PubMed] [Google Scholar]; (m) Volkringer C, Cohen SM. Angew Chem, Int Ed. 2010;49:4644. doi: 10.1002/anie.201001527. [DOI] [PubMed] [Google Scholar]; (n) Tanabe KK, Wang Z, Cohen SM. J Am Chem Soc. 2008;130:8508. doi: 10.1021/ja801848j. [DOI] [PubMed] [Google Scholar]; (o) Wang XL, Qin C, Wu SX, Shao KZ, Lan YQ, Wang S, Zhu DX, Su ZM, Wang EB. Angew Chem, Int Ed. 2009;48:5291. doi: 10.1002/anie.200902274. [DOI] [PubMed] [Google Scholar]; (p) Wang Z, Cohen SM. Angew Chem, Int Ed. 2008;47:4699. doi: 10.1002/anie.200800686. [DOI] [PubMed] [Google Scholar]
- 209.(a) An J, Geib SJ, Rosi NL. J Am Chem Soc. 2009;131:8376. doi: 10.1021/ja902972w. [DOI] [PubMed] [Google Scholar]; (b) Cho SH, Ma B, Nguyen ST, Hupp JT, Albrecht-Schmitt TE. Chem Commun. 2006:2563. doi: 10.1039/b600408c. [DOI] [PubMed] [Google Scholar]; (c) Fang QR, Zhu GS, Jin Z, Ji YY, Ye JW, Xue M, Yang H, Wang Y, Qiu SL. Angew Chem, Int Ed. 2007;46:6638. doi: 10.1002/anie.200700537. [DOI] [PubMed] [Google Scholar]; (d) Fang QR, Zhu GS, Jin Z, Xue M, Wei X, Wang DJ, Qiu SL. Angew Chem, Int Ed. 2006;45:6126. doi: 10.1002/anie.200601668. [DOI] [PubMed] [Google Scholar]; (e) Farha OK, Mulfort KL, Thorsness AM, Hupp JT. J Am Chem Soc. 2008;130:8598. doi: 10.1021/ja803097e. [DOI] [PubMed] [Google Scholar]; (f) Furukawa H, Kim J, Ockwig NW, O’Keeffe M, Yaghi OM. J Am Chem Soc. 2008;130:11650. doi: 10.1021/ja803783c. [DOI] [PubMed] [Google Scholar]; (g) Furukawa H, Ko N, Go YB, Aratani N, Choi SB, Choi E, Yazaydin AO, Snurr RQ, O’Keeffe M, Kim J, Yaghi OM. Science. 2010;329:424. doi: 10.1126/science.1192160. [DOI] [PubMed] [Google Scholar]; (h) Hafizovic CJ, Jakobsen S, Olsbye U, Guillou N, Lamberti C, Bordiga S, Lillerud KP. J Am Chem Soc. 2008;130:13850. doi: 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]; (i) Han SS, Choi SH, van DACT. Chem Commun. 2010;46:5713. doi: 10.1039/c0cc01132k. [DOI] [PubMed] [Google Scholar]; (j) Jin XH, Sun JK, Cai LX, Zhang J. Chem Commun. 2011;47:2667. doi: 10.1039/c0cc04084c. [DOI] [PubMed] [Google Scholar]; (k) Lan A, Li K, Wu H, Olson DH, Emge TJ, Ki W, Hong M, Li J. Angew Chem, Int Ed. 2009;48:2334. doi: 10.1002/anie.200804853. [DOI] [PubMed] [Google Scholar]; (l) Lohe MR, Gedrich K, Freudenberg T, Kockrick E, Dellmann T, Kaskel S. Chem Commun. 2011;47:3075. doi: 10.1039/c0cc05278g. [DOI] [PubMed] [Google Scholar]; (m) Nijem N, Thissen P, Yao Y, Longo RC, Roodenko K, Wu H, Zhao Y, Cho K, Li J, Langreth DC, Chabal YJ. J Am Chem Soc. 2011;133:12849. doi: 10.1021/ja2051149. [DOI] [PubMed] [Google Scholar]; (n) Rosi NL, Eddaoudi M, Kim J, O’Keeffe M, Yaghi OM. Angew Chem, Int Ed. 2002;41:284. doi: 10.1002/1521-3773(20020118)41:2<284::aid-anie284>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; (o) Surble S, Serre C, Mellot-Draznieks C, Millange F, Ferey G. Chem Commun. 2006:284. doi: 10.1039/b512169h. [DOI] [PubMed] [Google Scholar]; (p) Wang C, Lin W. J Am Chem Soc. 2011;133:4232. doi: 10.1021/ja111197d. [DOI] [PubMed] [Google Scholar]; (q) Wang C, Xie Z, de KKE, Lin W. J Am Chem Soc. 2011;133:13445. doi: 10.1021/ja203564w. [DOI] [PubMed] [Google Scholar]; (r) Zhang J, Wu H, Emge TJ, Li J. Chem Commun. 2010;46:9152. doi: 10.1039/c0cc02942d. [DOI] [PubMed] [Google Scholar]
- 210.(a) Allendorf MD, Houk RJT, Andruszkiewicz L, Talin AA, Pikarsky J, Choudhury A, Gall KA, Hesketh PJ. J Am Chem Soc. 2008;130:14404. doi: 10.1021/ja805235k. [DOI] [PubMed] [Google Scholar]; (b) Britt D, Tranchemontagne D, Yaghi OM. Proc Natl Acad Sci U S A. 2008;105:11623. doi: 10.1073/pnas.0804900105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Decoste JB, Peterson GW, Smith MW, Stone CA, Willis CR. J Am Chem Soc. 2012;134:1486. doi: 10.1021/ja211182m. [DOI] [PubMed] [Google Scholar]; (d) Horcajada P, Serre C, Vallet-Regi M, Sebban M, Taulelle F, Ferey G. Angew Chem, Int Ed. 2006;45:5974. doi: 10.1002/anie.200601878. [DOI] [PubMed] [Google Scholar]; (e) Jeong NC, Samanta B, Lee CY, Farha OK, Hupp JT. J Am Chem Soc. 2012;134:51. doi: 10.1021/ja2110152. [DOI] [PubMed] [Google Scholar]; (f) Ma FJ, Liu SX, Sun CY, Liang DD, Ren GJ, Wei F, Chen YG, Su ZM. J Am Chem Soc. 2011;133:4178. doi: 10.1021/ja109659k. [DOI] [PubMed] [Google Scholar]; (g) Maes M, Alaerts L, Vermoortele F, Ameloot R, Couck S, Finsy V, Denayer JFM, De VDE. J Am Chem Soc. 2010;132:2284. doi: 10.1021/ja9088378. [DOI] [PubMed] [Google Scholar]; (h) Maes M, Trekels M, Boulhout M, Schouteden S, Vermoortele F, Alaerts L, Heurtaux D, Seo YK, Hwang YK, Chang JS, Beurroies I, Denoyel R, Temst K, Vantomme A, Horcajada P, Serre C, De VDE. Angew Chem, Int Ed. 2011;50:4210. doi: 10.1002/anie.201100050. [DOI] [PubMed] [Google Scholar]; (i) Murray LJ, Dinca M, Yano J, Chavan S, Bordiga S, Brown CM, Long JR. J Am Chem Soc. 2010;132:7856. doi: 10.1021/ja1027925. [DOI] [PubMed] [Google Scholar]; (j) Nohra B, El MH, Rodriguez ALM, Mialane P, Marrot J, Mellot-Draznieks C, O’Keeffe M, Ngo BR, Lemaire J, Keita B, Nadjo L, Dolbecq A. J Am Chem Soc. 2011;133:13363. doi: 10.1021/ja201165c. [DOI] [PubMed] [Google Scholar]; (k) Ok KM, Sung J, Hu G, Jacobs RMJ, O’Hare D. J Am Chem Soc. 2008;130:3762. doi: 10.1021/ja800395q. [DOI] [PubMed] [Google Scholar]; (l) Shekhah O, Wang H, Kowarik S, Schreiber F, Paulus M, Tolan M, Sternemann C, Evers F, Zacher D, Fischer RA, Woell C. J Am Chem Soc. 2007;129:15118. doi: 10.1021/ja076210u. [DOI] [PubMed] [Google Scholar]; (m) Shoaee M, Anderson MW, Attfield MP. Angew Chem, Int Ed. 2008;47:8525. doi: 10.1002/anie.200803460. [DOI] [PubMed] [Google Scholar]; (n) Sun CY, Liu SX, Liang DD, Shao KZ, Ren YH, Su ZM. J Am Chem Soc. 2009;131:1883. doi: 10.1021/ja807357r. [DOI] [PubMed] [Google Scholar]; (o) Sun LB, Li JR, Park J, Zhou HC. J Am Chem Soc. 2012;134:126. doi: 10.1021/ja209698f. [DOI] [PubMed] [Google Scholar]; (p) Szelagowska-Kunstman K, Cyganik P, Goryl M, Zacher D, Puterova Z, Fischer RA, Szymonski M. J Am Chem Soc. 2008;130:14446. doi: 10.1021/ja8069743. [DOI] [PubMed] [Google Scholar]; (q) Taylor KML, Rieter WJ, Lin W. J Am Chem Soc. 2008;130:14358. doi: 10.1021/ja803777x. [DOI] [PubMed] [Google Scholar]; (r) Volkringer C, Popov D, Loiseau T, Guillou N, Ferey G, Haouas M, Taulelle F, Mellot-Draznieks C, Burghammer M, Riekel C. Nat Mater. 2007;6:760. doi: 10.1038/nmat1991. [DOI] [PubMed] [Google Scholar]; (s) Zhang J, Bu JT, Chen S, Wu T, Zheng S, Chen Y, Nieto RA, Feng P, Bu X. Angew Chem, Int Ed. 2010;49:8876. doi: 10.1002/anie.201003900. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Zhang Z, Zhang L, Wojtas L, Eddaoudi M, Zaworotko MJ. J Am Chem Soc. 2012;134:928. doi: 10.1021/ja208256u. [DOI] [PubMed] [Google Scholar]; (u) Zheng ST, Wu T, Irfanoglu B, Zuo F, Feng P, Bu X. Angew Chem, Int Ed. 2011;50:8034. doi: 10.1002/anie.201103155. [DOI] [PubMed] [Google Scholar]; (v) Zheng ST, Wu T, Zuo F, Chou C, Feng P, Bu X. J Am Chem Soc. 2012;134:1934. doi: 10.1021/ja209800x. [DOI] [PubMed] [Google Scholar]; (w) Zou RQ, Zhong RQ, Han SB, Xu HW, Burrell AK, Henson N, Cape JL, Hickmott DD, Timofeeva TV, Larson TE, Zhao YS. J Am Chem Soc. 2010;132:17996. doi: 10.1021/ja101440z. [DOI] [PubMed] [Google Scholar]
- 211.(a) Zheng YR, Lan WJ, Wang M, Cook TR, Stang PJ. J Am Chem Soc. 2011;133:17045. doi: 10.1021/ja207217t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dai FR, Wang Z. J Am Chem Soc. 2012;134:8002. doi: 10.1021/ja300095j. [DOI] [PubMed] [Google Scholar]
- 212.(a) Lin JL, Zhu HL, Zhang J, Zhao JM, Zheng YQ. J Mol Struct. 2011;995:91. [Google Scholar]; (b) Serre C, Marrot J, Ferey G. Inorg Chem. 2005;44:654. doi: 10.1021/ic0487373. [DOI] [PubMed] [Google Scholar]
- 213.(a) Biswas S, Ahnfeldt T, Stock N. Inorg Chem. 2011;50:9518. doi: 10.1021/ic201219g. [DOI] [PubMed] [Google Scholar]; (b) Burrows AD, Fisher LC, Richardson C, Rigby SP. Chem Commun. 2011;47:3380. doi: 10.1039/c1cc10143a. [DOI] [PubMed] [Google Scholar]; (c) Eddaoudi M, Kim J, O’Keeffe M, Yaghi OM. J Am Chem Soc. 2002;124:376. doi: 10.1021/ja017154e. [DOI] [PubMed] [Google Scholar]; (d) Eddaoudi M, Kim J, Vodak D, Sudik A, Wachter J, O’Keeffe M, Yaghi OM. Proc Natl Acad Sci U S A. 2002;99:4900. doi: 10.1073/pnas.082051899. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Garibay SJ, Cohen SM. Chem Commun. 2010;46:7700. doi: 10.1039/c0cc02990d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Gascon J, Hernandez-Alonso MD, Almeida AR, van KGPM, Kapteijn F, Mul G. ChemSusChem. 2008;1:981. doi: 10.1002/cssc.200800203. [DOI] [PubMed] [Google Scholar]; (g) Henke S, Schmid R, Grunwaldt JD, Fischer RA. Chem—Eur J. 2010;16:14296. doi: 10.1002/chem.201002341. [DOI] [PubMed] [Google Scholar]; (h) Kandiah M, Nilsen MH, Usseglio S, Jakobsen S, Olsbye U, Tilset M, Larabi C, Quadrelli EA, Bonino F, Lillerud KP. Chem Mater. 2010;22:6632. [Google Scholar]; (i) Kim M, Boissonnault JA, Allen CA, Dau PV, Cohen SM. Dalton Trans. 2012;41:6277. doi: 10.1039/c2dt30120b. [DOI] [PubMed] [Google Scholar]; (j) Kim M, Cahill JF, Prather KA, Cohen SM. Chem Commun. 2011;47:7629. doi: 10.1039/c1cc12101d. [DOI] [PubMed] [Google Scholar]; (k) Kim M, Garibay SJ, Cohen SM. Inorg Chem. 2011;50:729. doi: 10.1021/ic102436b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Padmanaban M, Mueller P, Lieder C, Gedrich K, Gruenker R, Bon V, Senkovska I, Baumgaertner S, Opelt S, Paasch S, Brunner E, Glorius F, Klemm E, Kaskel S. Chem Commun. 2011;47:12089. doi: 10.1039/c1cc14893a. [DOI] [PubMed] [Google Scholar]; (m) Wang S. Cryst Res Technol. 2008;43:894. [Google Scholar]; (n) Winston EB, Lowell PJ, Vacek J, Chocholousova J, Michl J, Price JC. Phys Chem Chem Phys. 2008;10:5188. doi: 10.1039/b808104b. [DOI] [PubMed] [Google Scholar]
- 214.(a) Mahata P, Prabu M, Natarajan S. Inorg Chem. 2008;47:8451. doi: 10.1021/ic800621q. [DOI] [PubMed] [Google Scholar]; (b) Rieter WJ, Taylor KML, An H, Lin W, Lin W. J Am Chem Soc. 2006;128:9024. doi: 10.1021/ja0627444. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang Z, Xiang S, Rao X, Zheng Q, Fronczek FR, Qian G, Chen B. Chem Commun. 2010;46:7205. doi: 10.1039/c0cc01236j. [DOI] [PubMed] [Google Scholar]
- 215.Sagara T, Klassen J, Ortony J, Ganz E. J Chem Phys. 2005;123:014701. doi: 10.1063/1.1944730. [DOI] [PubMed] [Google Scholar]
- 216.(a) Blake AJ, Champness NR, Easun TL, Allan DR, Nowell H, George MW, Jia J, Sun XZ. Nat Chem. 2010;2:688. doi: 10.1038/nchem.681. [DOI] [PubMed] [Google Scholar]; (b) Bloch ED, Britt D, Lee C, Doonan CJ, Uribe-Romo FJ, Furukawa H, Long JR, Yaghi OM. J Am Chem Soc. 2010;132:14382. doi: 10.1021/ja106935d. [DOI] [PubMed] [Google Scholar]; (c) Gustafsson M, Su J, Yue H, Yao Q, Zou X. Cryst Growth Des. 2012;12:3243. [Google Scholar]; (d) Huh S, Jung S, Kim Y, Kim SJ, Park S. Dalton Trans. 2010;39:1261. doi: 10.1039/b916176g. [DOI] [PubMed] [Google Scholar]; (e) Jacobs T, Hardie MJ. Chem—Eur J. 2012;18:267. doi: 10.1002/chem.201101998. [DOI] [PubMed] [Google Scholar]; (f) Liu H, Yin B, Gao Z, Li Y, Jiang H. Chem Commun. 2012;48:2033. doi: 10.1039/c2cc16790e. [DOI] [PubMed] [Google Scholar]; (g) Szeto KC, Kongshaug KO, Jakobsen S, Tilset M, Lillerud KP. Dalton Trans. 2008:2054. doi: 10.1039/b719766g. [DOI] [PubMed] [Google Scholar]
- 217.(a) Bai HY, Ma JF, Yang J, Zhang LP, Ma JC, Liu YY. Cryst Growth Des. 2010;10:1946. [Google Scholar]; (b) Cheng JW, Zheng ST, Yang GY. Inorg Chem. 2007;46:10261. doi: 10.1021/ic701468q. [DOI] [PubMed] [Google Scholar]; (c) Du M, Jiang XJ, Zhao XJ. Inorg Chem. 2007;46:3984. doi: 10.1021/ic062098+. [DOI] [PubMed] [Google Scholar]; (d) Huang FP, Tian JL, Gu W, Liu X, Yan SP, Liao DZ, Cheng P. Cryst Growth Des. 2010;10:1145. [Google Scholar]; (e) Kan WQ, Yang J, Liu YY, Ma JF. Polyhedron. 2011;30:2106. [Google Scholar]; (f) Li X, Zha MQ, Wang XW, Cao R. Inorg Chim Acta. 2009;362:3357. [Google Scholar]; (g) Li YW, Ma H, Chen YQ, He KH, Li ZX, Bu XH. Cryst Growth Des. 2012;12:189. [Google Scholar]; (h) Lightfoot P, Snedden A. J Chem Soc, Dalton Trans. 1999:3549. [Google Scholar]; (i) Luo F, Che YX, Zheng JM. J Coord Chem. 2008;61:2097. [Google Scholar]; (j) Mahata P, Ramya KV, Natarajan S. Inorg Chem. 2009;48:4942. doi: 10.1021/ic9003356. [DOI] [PubMed] [Google Scholar]; (k) Mohandes F, Davar F, Salavati-Niasari M, Saberyan K. Curr Nanosci. 2011;7:260. [Google Scholar]; (l) Mu Y, Fu J, Song Y, Li Z, Hou H, Fan Y. Cryst Growth Des. 2011;11:2183. [Google Scholar]; (m) Pizon D, Henry N, Loiseau T, Roussel P, Abraham F. J Solid State Chem. 2010;183:1943. [Google Scholar]; (n) Qi Y, Che Y, Luo F, Batten SR, Liu Y, Zheng J. Cryst Growth Des. 2008;8:1654. [Google Scholar]; (o) Qi Y, Luo F, Che Y, Zheng J. Cryst Growth Des. 2008;8:606. [Google Scholar]; (p) Sun CY, Li LC, Jin LP. Polyhedron. 2006;25:3017. [Google Scholar]; (q) Wang XL, Bi YF, Lin HY, Liu GC. Cryst Growth Des. 2007;7:1086. [Google Scholar]; (r) Xie XF, Chen SP, Xia ZQ, Gao SL. Polyhedron. 2009;28:679. [Google Scholar]; (s) Xu C, Guo Q, Wang X, Hou H, Fan Y. Cryst Growth Des. 2011;11:1869. [Google Scholar]; (t) Zevaco TA, Maennle D, Walter O, Dinjus E. Appl Organomet Chem. 2007;21:970. [Google Scholar]; (u) Zhang LP, Ma JF, Yang J, Liu YY, Wei GH. Cryst Growth Des. 2009;9:4660. [Google Scholar]; (v) Zhang LP, Yang J, Ma JF, Jia ZF, Xie YP, Wei GH. CrystEngComm. 2008;10:1410. [Google Scholar]; (w) Zhu X, Zhao JW, Li BL, Song Y, Zhang YM, Zhang Y. Inorg Chem. 2010;49:1266. doi: 10.1021/ic902404b. [DOI] [PubMed] [Google Scholar]
- 218.(a) Deng ZP, Zhu ZB, Zhang XF, Huo LH, Zhao H, Gao S. CrystEngComm. 2011;13:3895. [Google Scholar]; (b) Li X, Xie Z, Lin J, Cao R. J Solid State Chem. 2009;182:2290. [Google Scholar]; (c) Yang Y, Du P, Ma JF, Kan WQ, Liu B, Yang J. Cryst Growth Des. 2011;11:5540. [Google Scholar]
- 219.Tanaka K, Oda S, Shiro M. Chem Commun. 2008:820. doi: 10.1039/b714083e. [DOI] [PubMed] [Google Scholar]
- 220.Williams CA, Blake AJ, Wilson C, Hubberstey P, Schroeder M. Cryst Growth Des. 2008;8:911. [Google Scholar]
- 221.(a) Furman JD, Warner AY, Teat SJ, Mikhailovsky AA, Cheetham AK. Chem Mater. 2010;22:2255. [Google Scholar]; (b) Yue Q, Yan L, Zhang JY, Gao EQ. Inorg Chem. 2010;49:8647. doi: 10.1021/ic100558x. [DOI] [PubMed] [Google Scholar]
- 222.(a) Das MC, Xu H, Wang Z, Srinivas G, Zhou W, Yue YF, Nesterov VN, Qian G, Chen B. Chem Commun. 2011;47:11715. doi: 10.1039/c1cc12802g. [DOI] [PubMed] [Google Scholar]; (b) Dinca M, Long JR. J Am Chem Soc. 2005;127:9376. doi: 10.1021/ja0523082. [DOI] [PubMed] [Google Scholar]; (c) Du Y, Thompson AL, O’Hare D. Chem Commun. 2008:5987. doi: 10.1039/b814180k. [DOI] [PubMed] [Google Scholar]; (d) Gadzikwa T, Lu G, Stern CL, Wilson SR, Hupp JT, Nguyen ST. Chem Commun. 2008:5493. doi: 10.1039/b805101a. [DOI] [PubMed] [Google Scholar]; (e) Gong HY, Rambo BM, Cho W, Lynch VM, Oh M, Sessler JL. Chem Commun. 2011;47:5973. doi: 10.1039/c1cc10272a. [DOI] [PubMed] [Google Scholar]; (f) Huh S, Kwon TH, Park N, Kim SJ, Kim Y. Chem Commun. 2009:4953. doi: 10.1039/b905138d. [DOI] [PubMed] [Google Scholar]; (g) Klein N, Senkovska I, Gedrich K, Stoeck U, Henschel A, Mueller U, Kaskel S. Angew Chem, Int Ed. 2009;48:9954. doi: 10.1002/anie.200904599. [DOI] [PubMed] [Google Scholar]; (h) Tsao CS, Yu MS, Wang CY, Liao PY, Chen HL, Jeng US, Tzeng YR, Chung TY, Wu HC. J Am Chem Soc. 2009;131:1404. doi: 10.1021/ja802741b. [DOI] [PubMed] [Google Scholar]
- 223.Yang J, Ma JF, Batten SR, Su ZM. Chem Commun. 2008:2233. doi: 10.1039/b800199e. [DOI] [PubMed] [Google Scholar]
- 224.(a) Liu B, Yang Q, Xue C, Zhong C, Smit B. Phys Chem Chem Phys. 2008;10:3244. doi: 10.1039/b801494a. [DOI] [PubMed] [Google Scholar]; (b) Nelson AP, Farha OK, Mulfort KL, Hupp JT. J Am Chem Soc. 2009;131:458. doi: 10.1021/ja808853q. [DOI] [PubMed] [Google Scholar]
- 225.(a) Li XD, Shi ZJ. Z Kristallogr - New Cryst Struct. 2010;225:393. [Google Scholar]; (b) Liang YF, Zhang YR, Han ZB. Z Anorg Allg Chem. 2012;638:423. [Google Scholar]; (c) Ma LF, Meng QL, Wang LY, Liang FP. Inorg Chim Acta. 2010;363:4127. [Google Scholar]; (d) Ma LF, Wang LY, Lu DH, Batten SR, Wang JG. Cryst Growth Des. 2009;9:1741. [Google Scholar]; (e) Ma LF, Wang YY, Liu JQ, Yang GP, Du M, Wang LY. CrystEngComm. 2009;11:1800. [Google Scholar]; (f) Ma LF, Wang YY, Wang LY, Lu DH, Batten SR, Wang JG. Cryst Growth Des. 2009;9:2036. [Google Scholar]; (g) Ma S, Sun D, Wang XS, Zhou HC. Angew Chem, Int Ed. 2007;46:2458. doi: 10.1002/anie.200604353. [DOI] [PubMed] [Google Scholar]; (h) Pan L, Parker B, Huang X, Olson DH, Lee J, Li J. J Am Chem Soc. 2006;128:4180. doi: 10.1021/ja057667b. [DOI] [PubMed] [Google Scholar]; (i) Wang X, Liu Y, Xu C, Guo Q, Hou H, Fan Y. Cryst Growth Des. 2012;12:2435. [Google Scholar]; (j) Xu YQ, Wang XF, Ji JW, Han ZB. Russ J Coord Chem. 2011;37:748. [Google Scholar]
- 226.(a) Hu J, Huang L, Yao X, Qin L, Li Y, Guo Z, Zheng H, Xue Z. Inorg Chem. 2011;50:2404. doi: 10.1021/ic102207n. [DOI] [PubMed] [Google Scholar]; (b) Hu J-S, Shang Y-J, Yao X-q, Qin L, Li Y-Z, Guo Z-J, Zheng H-G, Xue Z-L. Cryst Growth Des. 2010;10:2676. [Google Scholar]; (c) Li X, Cai Y, Fang Z, Wu L, Wei B, Lin S. Cryst Growth Des. 2011;11:4517. [Google Scholar]; (d) Li XJ, Wang XY, Gao S, Cao R. Inorg Chem. 2006;45:1508. doi: 10.1021/ic050999x. [DOI] [PubMed] [Google Scholar]; (e) Li YT, Zhang DJ, Guo YN, Guan BY, Tang DH, Liu YL, Huo QS. Chem Commun. 2011;47:7809. doi: 10.1039/c1cc12479j. [DOI] [PubMed] [Google Scholar]; (f) Ren H, Song TY, Xu JN, Jing SB, Yu Y, Zhang P, Zhang LR. Cryst Growth Des. 2009;9:105. [Google Scholar]
- 227.Nouar F, Eubank JF, Bousquet T, Wojtas L, Zaworotko MJ, Eddaoudi M. J Am Chem Soc. 2008;130:1833. doi: 10.1021/ja710123s. [DOI] [PubMed] [Google Scholar]
- 228.(a) Jung S, Cho W, Lee HJ, Oh M. Angew Chem, Int Ed. 2009;48:1459. doi: 10.1002/anie.200804816. [DOI] [PubMed] [Google Scholar]; (b) Jung S, Oh M. Angew Chem, Int Ed. 2008;47:2049. doi: 10.1002/anie.200704209. [DOI] [PubMed] [Google Scholar]; (c) Liu X. Angew Chem, Int Ed. 2009;48:3018. doi: 10.1002/anie.200805972. [DOI] [PubMed] [Google Scholar]; (d) Park S-j, Cho W, Oh M. CrystEngComm. 2010;12:1060. [Google Scholar]
- 229.Jeon YM, Armatas GS, Kim D, Kanatzidis MG, Mirkin CA. Small. 2009;5:46. doi: 10.1002/smll.200801160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.(a) Guo Z, Li G, Zhou L, Su S, Lei Y, Dang S, Zhang H. Inorg Chem. 2009;48:8069. doi: 10.1021/ic901056d. [DOI] [PubMed] [Google Scholar]; (b) Li L, Luo J, Wang S, Sun Z, Chen T, Hong M. Cryst Growth Des. 2011;11:3744. [Google Scholar]; (c) Li L, Ma J, Song C, Chen T, Sun Z, Wang S, Luo J, Hong M. Inorg Chem. 2012;51:2438. doi: 10.1021/ic202406f. [DOI] [PubMed] [Google Scholar]; (d) Lim CS, Schnobrich JK, Wong-Foy AG, Matzger AJ. Inorg Chem. 2010;49:5271. doi: 10.1021/ic100378p. [DOI] [PubMed] [Google Scholar]; (e) Wong-Foy AG, Lebel O, Matzger AJ. J Am Chem Soc. 2007;129:15740. doi: 10.1021/ja0753952. [DOI] [PubMed] [Google Scholar]; (f) Zhang Z, Zhang L, Wojtas L, Nugent P, Eddaoudi M, Zaworotko MJ. J Am Chem Soc. 2012;134:924. doi: 10.1021/ja209643b. [DOI] [PubMed] [Google Scholar]
- 231.(a) de Lill DT, Cahill CL. Chem Commun. 2006:4946. doi: 10.1039/b610012k. [DOI] [PubMed] [Google Scholar]; (b) Fang QR, Zhu GS, Xue M, Sun JY, Qiu SL. Dalton Trans. 2006:2399. doi: 10.1039/b517400g. [DOI] [PubMed] [Google Scholar]; (c) Min KS, Suh MP. Chem—Eur J. 2001;7:303. doi: 10.1002/1521-3765(20010105)7:1<303::aid-chem303>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]; (d) Pan L, Woodlock EB, Wang X, Zheng C. Inorg Chem. 2000;39:4174. doi: 10.1021/ic000209d. [DOI] [PubMed] [Google Scholar]; (e) Zheng SL, Gembicky M, Messerschmidt M, Dominiak PM, Coppens P. Inorg Chem. 2006;45:9281. doi: 10.1021/ic0609709. [DOI] [PubMed] [Google Scholar]
- 232.(a) Cheon YE, Suh MP. Angew Chem, Int Ed. 2009;48:2899. doi: 10.1002/anie.200805494. [DOI] [PubMed] [Google Scholar]; (b) Lee EY, Jang SY, Suh MP. J Am Chem Soc. 2005;127:6374. doi: 10.1021/ja043756x. [DOI] [PubMed] [Google Scholar]; (c) Suh MP, Cheon YE, Lee EY. Chem—Eur J. 2007;13:4208. doi: 10.1002/chem.200601534. [DOI] [PubMed] [Google Scholar]
- 233.(a) Ma S, Sun D, Ambrogio M, Fillinger JA, Parkin S, Zhou HC. J Am Chem Soc. 2007;129:1858. doi: 10.1021/ja067435s. [DOI] [PubMed] [Google Scholar]; (b) Ma S, Wang XS, Manis ES, Collier CD, Zhou HC. Inorg Chem. 2007;46:3432. doi: 10.1021/ic070338v. [DOI] [PubMed] [Google Scholar]; (c) Ma S, Wang XS, Yuan D, Zhou HC. Angew Chem, Int Ed. 2008;47:4130. doi: 10.1002/anie.200800312. [DOI] [PubMed] [Google Scholar]; (d) Ma S, Yuan D, Chang JS, Zhou HC. Inorg Chem. 2009;48:5398. doi: 10.1021/ic900475q. [DOI] [PubMed] [Google Scholar]; (e) Ma S, Yuan D, Wang XS, Zhou HC. Inorg Chem. 2009;48:2072. doi: 10.1021/ic801948z. [DOI] [PubMed] [Google Scholar]; (f) Ma S, Zhou HC. J Am Chem Soc. 2006;128:11734. doi: 10.1021/ja063538z. [DOI] [PubMed] [Google Scholar]; (g) Park YK, Choi SB, Kim H, Kim K, Won BH, Choi K, Choi JS, Ahn WS, Won N, Kim S, Jung DH, Choi SH, Kim GH, Cha SS, Jhon YH, Yang JK. Angew Chem, Int Ed. 2007;46:8230. doi: 10.1002/anie.200702324. [DOI] [PubMed] [Google Scholar]; (h) Sun D, Ke Y, Collins DJ, Lorigan GA, Zhou HC. Inorg Chem. 2007;46:2725. doi: 10.1021/ic0624773. [DOI] [PubMed] [Google Scholar]; (i) Sun D, Ma S, Ke Y, Collins DJ, Zhou HC. J Am Chem Soc. 2006;128:3896. doi: 10.1021/ja058777l. [DOI] [PubMed] [Google Scholar]; (j) Sun D, Ma S, Ke Y, Petersen TM, Zhou HC. Chem Commun. 2005:2663. doi: 10.1039/b502007g. [DOI] [PubMed] [Google Scholar]
- 234.(a) Chae HK, Siberio-Perez DY, Kim J, Go YB, Eddaoudi M, Matzger AJ, O’Keeffe M, Yaghi OM. Nature. 2004;427:523. doi: 10.1038/nature02311. [DOI] [PubMed] [Google Scholar]; (b) Chen B, Eddaoudi M, Hyde ST, O’Keeffe M, Yaghi OM. Science. 2001;291:1021. doi: 10.1126/science.1056598. [DOI] [PubMed] [Google Scholar]; (c) Choi SB, Seo MJ, Cho M, Kim Y, Jin MK, Jung DY, Choi JS, Ahn WS, Rowsell JLC, Kim J. Cryst Growth Des. 2007;7:2290. [Google Scholar]; (d) Dang D, Wu P, He C, Xie Z, Duan C. J Am Chem Soc. 2010;132:14321. doi: 10.1021/ja101208s. [DOI] [PubMed] [Google Scholar]; (e) Devic T, Serre C, Audebrand N, Marrot J, Ferey G. J Am Chem Soc. 2005;127:12788. doi: 10.1021/ja053992n. [DOI] [PubMed] [Google Scholar]; (f) Gedrich K, Senkovska I, Klein N, Stoeck U, Henschel A, Lohe MR, Baburin IA, Mueller U, Kaskel S. Angew Chem, Int Ed. 2010;49:8489. doi: 10.1002/anie.201001735. [DOI] [PubMed] [Google Scholar]; (g) Han D, Jiang FL, Wu MY, Chen L, Chen QH, Hong MC. Chem Commun. 2011;47:9861. doi: 10.1039/c1cc12858b. [DOI] [PubMed] [Google Scholar]; (h) Hou L, Zhang JP, Chen XM. Cryst Growth Des. 2009;9:2415. [Google Scholar]; (i) Ibarra IA, Lin X, Yang S, Blake AJ, Walker GS, Barnett SA, Allan DR, Champness NR, Hubberstey P, Schroeder M. Chem—Eur J. 2010;16:13671. doi: 10.1002/chem.201000926. [DOI] [PubMed] [Google Scholar]; (j) Jung DW, Yang DA, Kim J, Kim J, Ahn WS. Dalton Trans. 2010;39:2883. doi: 10.1039/b925088c. [DOI] [PubMed] [Google Scholar]; (k) Klein N, Senkovska I, Baburin IA, Gruenker R, Stoeck U, Schlichtenmayer M, Streppel B, Mueller U, Leoni S, Hirscher M, Kaskel S. Chem—Eur J. 2011;17:13007. doi: 10.1002/chem.201101383. [DOI] [PubMed] [Google Scholar]; (l) Lin Q, Wu T, Bu X, Feng P. Dalton Trans. 2012;41:3620. doi: 10.1039/c2dt12392d. [DOI] [PubMed] [Google Scholar]; (m) Pham MH, Vuong GT, Fontaine FG, Do TO. Cryst Growth Des. 2012;12:1008. [Google Scholar]; (n) Sava DF, Rohwer LES, Rodriguez MA, Nenoff TM. J Am Chem Soc. 2012;134:3983. doi: 10.1021/ja211230p. [DOI] [PubMed] [Google Scholar]; (o) Sumida K, Hill MR, Horike S, Dailly A, Long JR. J Am Chem Soc. 2009;131:15120. doi: 10.1021/ja9072707. [DOI] [PubMed] [Google Scholar]; (p) Tanabe KK, Allen CA, Cohen SM. Angew Chem, Int Ed. 2010;49:9730. doi: 10.1002/anie.201004736. [DOI] [PubMed] [Google Scholar]; (q) Yao Q, Sun J, Li K, Su J, Peskov MV, Zou X. Dalton Trans. 2012;41:3953. doi: 10.1039/c2dt12088g. [DOI] [PubMed] [Google Scholar]; (r) Zou R, Abdel-Fattah AI, Xu H, Burrell AK, Larson TE, McCleskey TM, Wei Q, Janicke MT, Hickmott DD, Timofeeva TV, Zhao Y. Cryst Growth Des. 2010;10:1301. [Google Scholar]
- 235.(a) Chen B, Ockwig NW, Fronczek FR, Contreras DS, Yaghi OM. Inorg Chem. 2005;44:181. doi: 10.1021/ic048612y. [DOI] [PubMed] [Google Scholar]; (b) Chen B, Ockwig NW, Millward AR, Contreras DS, Yaghi OM. Angew Chem, Int Ed. 2005;44:4745. doi: 10.1002/anie.200462787. [DOI] [PubMed] [Google Scholar]; (c) Ibarra IA, Yang S, Lin X, Blake AJ, Rizkallah PJ, Nowell H, Allan DR, Champness NR, Hubberstey P, Schroeder M. Chem Commun. 2011;47:8304. doi: 10.1039/c1cc11168j. [DOI] [PubMed] [Google Scholar]; (d) Lin Q, Wu T, Zheng ST, Bu X, Feng P. Chem Commun. 2011;47:11852. doi: 10.1039/c1cc14836b. [DOI] [PubMed] [Google Scholar]; (e) Lin X, Jia J, Zhao X, Thomas KM, Blake AJ, Walker GS, Champness NR, Hubberstey P, Schroeder M. Angew Chem, Int Ed. 2006;45:7358. doi: 10.1002/anie.200601991. [DOI] [PubMed] [Google Scholar]; (f) Lin X, Telepeni I, Blake AJ, Dailly A, Brown CM, Simmons JM, Zoppi M, Walker GS, Thomas KM, Mays TJ, Hubberstey P, Champness NR, Schroder M. J Am Chem Soc. 2009;131:2159. doi: 10.1021/ja806624j. [DOI] [PubMed] [Google Scholar]
- 236.(a) Ma L, Falkowski JM, Abney C, Lin W. Nat Chem. 2010;2:838. doi: 10.1038/nchem.738. [DOI] [PubMed] [Google Scholar]; (b) Ma L, Wu CD, Wanderley MM, Lin W. Angew Chem, Int Ed. 2010;49:8244. doi: 10.1002/anie.201003377. [DOI] [PubMed] [Google Scholar]; (c) Wanderley MM, Wang C, Wu CD, Lin W. J Am Chem Soc. 2012;134:9050. doi: 10.1021/ja302110d. [DOI] [PubMed] [Google Scholar]
- 237.(a) Cheng X, Duan X-y, Liu T, Wang F-m, Lu C-s, Meng Q-j. Inorg Chem Commun. 2010;13:818. [Google Scholar]; (b) Cheng X, Liu T, Duan X, Wang F, Meng Q, Lu C. CrystEngComm. 2011;13:1314. [Google Scholar]; (c) Duan X, Li Y, Su Y, Zang S, Zhu C, Meng Q. CrystEngComm. 2007;9:758. [Google Scholar]; (d) Su S, Chen W, Qin C, Song S, Guo Z, Li G, Song X, Zhu M, Wang S, Hao Z, Zhang H. Cryst Growth Des. 2012;12:1808. [Google Scholar]; (e) Su S, Guo Z, Li G, Deng R, Song S, Qin C, Pan C, Guo H, Cao F, Wang S, Zhang H. Dalton Trans. 2010;39:9123. doi: 10.1039/c001655a. [DOI] [PubMed] [Google Scholar]; (f) Su S, Qin C, Song S, Guo Z, Deng R, Chen W, Song X, Wang S, Li G, Zhang H. CrystEngComm. 2011;13:6057. [Google Scholar]; (g) Wang XS, Ma S, Forster PM, Yuan D, Eckert J, Lopez JJ, Murphy BJ, Parise JB, Zhou HC. Angew Chem, Int Ed. 2008;47:7263. doi: 10.1002/anie.200802087. [DOI] [PubMed] [Google Scholar]; (h) Xiong S, Li S, Wang S, Wang Z. CrystEngComm. 2011;13:7236. [Google Scholar]
- 238.(a) Cairns AJ, Perman JA, Wojtas L, Kravtsov VC, Alkordi MH, Eddaoudi M, Zaworotko MJ. J Am Chem Soc. 2008;130:1560. doi: 10.1021/ja078060t. [DOI] [PubMed] [Google Scholar]; (b) Perman JA, Cairns AJ, Wojtas L, Eddaoudi M, Zaworotko MJ. CrystEngComm. 2011;13:3130. [Google Scholar]
- 239.(a) Barron PM, Wray CA, Hu C, Guo Z, Choe W. Inorg Chem. 2010;49:10217. doi: 10.1021/ic101459j. [DOI] [PubMed] [Google Scholar]; (b) Choi EY, Wray CA, Hu C, Choe W. CrystEngComm. 2009;11:553. [Google Scholar]; (c) George S, Lipstman S, Goldberg I. Cryst Growth Des. 2006;6:2651. [Google Scholar]; (d) Jahan M, Bao Q, Loh KP. J Am Chem Soc. 2012;134:6707. doi: 10.1021/ja211433h. [DOI] [PubMed] [Google Scholar]; (e) Lee CY, Farha OK, Hong BJ, Sarjeant AA, Nguyen SBT, Hupp JT. J Am Chem Soc. 2011;133:15858. doi: 10.1021/ja206029a. [DOI] [PubMed] [Google Scholar]; (f) Motoyama S, Makiura R, Sakata O, Kitagawa H. J Am Chem Soc. 2011;133:5640. doi: 10.1021/ja110720f. [DOI] [PubMed] [Google Scholar]
- 240.(a) Farha OK, Mulfort KL, Hupp JT. Inorg Chem. 2008;47:10223. doi: 10.1021/ic8018452. [DOI] [PubMed] [Google Scholar]; (b) Gadzikwa T, Farha OK, Malliakas CD, Kanatzidis MG, Hupp JT, Nguyen ST. J Am Chem Soc. 2009;131:13613. doi: 10.1021/ja904189d. [DOI] [PubMed] [Google Scholar]; (c) Lee CY, Bae YS, Jeong NC, Farha OK, Sarjeant AA, Stern CL, Nickias P, Snurr RQ, Hupp JT, Nguyen SBT. J Am Chem Soc. 2011;133:5228. doi: 10.1021/ja200553m. [DOI] [PubMed] [Google Scholar]; (d) Mulfort KL, Farha OK, Malliakas CD, Kanatzidis MG, Hupp JT. Chem—Eur J. 2010;16:276. doi: 10.1002/chem.200902104. [DOI] [PubMed] [Google Scholar]; (e) Shultz AM, Farha OK, Adhikari D, Sarjeant AA, Hupp JT, Nguyen ST. Inorg Chem. 2011;50:3174. doi: 10.1021/ic101952y. [DOI] [PubMed] [Google Scholar]
- 241.Chen B, Eddaoudi M, Reineke TM, Kampf JW, O’Keeffe M, Yaghi OM. J Am Chem Soc. 2000;122:11559. [Google Scholar]
- 242.(a) Han Y, Fu L, Mafra L, Shi FN. J Solid State Chem. 2012;186:165. [Google Scholar]; (b) Taylor KML, Jin A, Lin W. Angew Chem, Int Ed. 2008;47:7722. doi: 10.1002/anie.200802911. [DOI] [PubMed] [Google Scholar]; (c) Volkringer C, Henry N, Grandjean S, Loiseau T. J Am Chem Soc. 2012;134:1275. doi: 10.1021/ja209915n. [DOI] [PubMed] [Google Scholar]
- 243.Subramanian S, Zaworotko MJ. Angew Chem, Int Ed Engl. 1995;34:2127. [Google Scholar]
- 244.Schöllhorn R. Angew Chem, Int Ed Engl. 1980;19:983. [Google Scholar]
- 245.Bain RL, Shriver DF, Ellis DE. Inorg Chim Acta. 2001;325:171. [Google Scholar]
- 246.Méry D, Plault L, Nlate S, Astruc D, Cordier S, Kirakci K, Perrin C. Z Anorg Allg Chem. 2005;631:2746. [Google Scholar]
- 247.Lee SJ, Hupp JT. Coord Chem Rev. 2006;250:1710. [Google Scholar]
- 248.(a) Fan J, Whiteford JA, Olenyuk B, Levin MD, Stang PJ, Fleischer EB. J Am Chem Soc. 1999;121:2741. [Google Scholar]; (b) Stang JP, Fan J, Olenyuk B. Chem Commun. 1997:1453. [Google Scholar]
- 249.Drain CM, Lehn JM. J Chem Soc, Chem Commun. 1994:2313. [Google Scholar]
- 250.MacGillivray LR, Subramanian S, Zaworotko MJ. Chem Commun. 1994 [Google Scholar]
- 251.Eddaoudi M, Li H, Reineke T, Fehr M, Kelley D, Groy TL, Yaghi OM. Top Catal. 1999;9:105. [Google Scholar]
- 252.Li H, Eddaoudi M, O’Keeffe M, Yaghi OM. Nature. 1999;402:276. [Google Scholar]
- 253.Eddaoudi M, Kim J, Rosi N, Vodak D, Wachter J, O’Keeffe M, Yaghi OM. Science. 2002;295:469. doi: 10.1126/science.1067208. [DOI] [PubMed] [Google Scholar]
- 254.Férey G, Serre C, Mellot-Draznieks C, Millange F, Surblé S, Dutour J, Margiolaki I. Angew Chem, Int Ed. 2004;43:6296. doi: 10.1002/anie.200460592. [DOI] [PubMed] [Google Scholar]
- 255.Das N, Mukherjee PS, Arif AM, Stang PJ. J Am Chem Soc. 2003;125:13950. doi: 10.1021/ja037788g. [DOI] [PubMed] [Google Scholar]
- 256.(a) Teo P, Koh LL, Hor TSA. Inorg Chem. 2008;47:6464. doi: 10.1021/ic8007238. [DOI] [PubMed] [Google Scholar]; (b) Teo P, Koh LL, Hor TSA. Inorg Chem. 2003;42:7290. doi: 10.1021/ic034690u. [DOI] [PubMed] [Google Scholar]
- 257.Chi KW, Addicott C, Arif AM, Stang PJ. J Am Chem Soc. 2004;126:16569. doi: 10.1021/ja045542l. [DOI] [PubMed] [Google Scholar]
- 258.(a) Ghosh S, Turner DR, Batten SR, Mukherjee PS. Dalton Trans. 2007:1869. doi: 10.1039/b702353g. [DOI] [PubMed] [Google Scholar]; (b) Bar AK, Chakrabarty R, Lee HM, Mukherjee PS. Inorg Chim Acta. 2011;372:313. [Google Scholar]
- 259.Kleist W, Jutz F, Maciejewski M, Baiker A. Eur J Inorg Chem. 2009;2009:3552. [Google Scholar]
- 260.Kepert CJ, Prior TJ, Rosseinsky MJ. J Am Chem Soc. 2000;122:5158. [Google Scholar]
- 261.Stang PJ, Olenyuk B. Angew Chem, Int Ed Engl. 1996;35:732. [Google Scholar]
- 262.Seo JS, Whang D, Lee H, Jun SI, Oh J, Jeon YJ, Kim K. Nature. 2000;404:982. doi: 10.1038/35010088. [DOI] [PubMed] [Google Scholar]
- 263.Valente C, Choi E, Belowich ME, Doonan CJ, Li Q, Gasa TB, Botros YY, Yaghi OM, Stoddart JF. Chem Commun. 2010;46:4911. doi: 10.1039/c0cc00997k. [DOI] [PubMed] [Google Scholar]
- 264.Wu CD, Hu A, Zhang L, Lin W. J Am Chem Soc. 2005;127:8940. doi: 10.1021/ja052431t. [DOI] [PubMed] [Google Scholar]
- 265.Lee SJ, Hu A, Lin W. J Am Chem Soc. 2002;124:12948. doi: 10.1021/ja028099s. [DOI] [PubMed] [Google Scholar]
- 266.Lin W. J Solid State Chem. 2005;178:2486. [Google Scholar]
- 267.Wu P, He C, Wang J, Peng X, Li X, An Y, Duan C. J Am Chem Soc. 2012;134:14991. doi: 10.1021/ja305367j. [DOI] [PubMed] [Google Scholar]
- 268.Wang Z, Cohen SM. Chem Soc Rev. 2009;38:1315. doi: 10.1039/b802258p. [DOI] [PubMed] [Google Scholar]
- 269.Venkataraman D, Gardner GB, Lee S, Moore JS. J Am Chem Soc. 1995;117:11600. [Google Scholar]
- 270.Zheng YR, Zhao Z, Kim H, Wang M, Ghosh K, Pollock JB, Chi KW, Stang PJ. Inorg Chem. 2010;49:10238. doi: 10.1021/ic1018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Dinca M, Long JR. J Am Chem Soc. 2007;129:11172. doi: 10.1021/ja072871f. [DOI] [PubMed] [Google Scholar]
- 272.Zheng YR, Wang M, Kobayashi S, Stang PJ. Tetrahedron Lett. 2011;52:2188. doi: 10.1016/j.tetlet.2010.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kaye SS, Long JR. J Am Chem Soc. 2008;130:806. doi: 10.1021/ja7102108. [DOI] [PubMed] [Google Scholar]
- 274.Bae YS, Farha OK, Hupp JT, Snurr RQ. J Mater Chem. 2009;19:2131. [Google Scholar]
- 275.Wang Z, Tanabe KK, Cohen SM. Inorg Chem. 2008;48:296. doi: 10.1021/ic801837t. [DOI] [PubMed] [Google Scholar]
- 276.Garibay SJ, Wang Z, Tanabe KK, Cohen SM. Inorg Chem. 2009;48:7341. doi: 10.1021/ic900796n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tan YX, He YP, Zhang J. Inorg Chem. 2012;51:9649. doi: 10.1021/ic300778m. [DOI] [PubMed] [Google Scholar]
- 278.Doonan CJ, Morris W, Furukawa H, Yaghi OM. J Am Chem Soc. 2009;131:9492. doi: 10.1021/ja903251e. [DOI] [PubMed] [Google Scholar]
- 279.Huisgen R. Proc Chem Soc. 1961:357. [Google Scholar]
- 280.Hansen TV, Wu P, Sharpless WD, Lindberg JG. J Chem Educ. 2005;82:1833. [Google Scholar]
- 281.Goto Y, Sato H, Shinkai S, Sada K. J Am Chem Soc. 2008;130:14354. doi: 10.1021/ja7114053. [DOI] [PubMed] [Google Scholar]
- 282.Chakrabarty R, Stang PJ. J Am Chem Soc. 2012;134:14738. doi: 10.1021/ja3070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Farrusseng D, editor. Metal-Organic Frameworks. Wiley-VCH; Weinheim, Germany: 2011. [Google Scholar]
- 284.Farha OK, Eryazici I, Jeong NC, Hauser BG, Wilmer CE, Sarjeant AA, Snurr RQ, Nguyen ST, Yazaydin AÖ, Hupp JT. J Am Chem Soc. 2012;134:15016. doi: 10.1021/ja3055639. [DOI] [PubMed] [Google Scholar]
- 285.Sumida K, Rogow DL, Mason JA, McDonald TM, Bloch ED, Herm ZR, Bae TH, Long JR. Chem Rev. 2011;112:724. doi: 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- 286.Zhang Z, Gao WY, Wojtas L, Ma S, Eddaoudi M, Zaworotko MJ. Angew Chem, Int Ed. 2012;51:9330. doi: 10.1002/anie.201203594. [DOI] [PubMed] [Google Scholar]
- 287.Wu H, Gong Q, Olson DH, Li J. Chem Rev. 2012;112:836. doi: 10.1021/cr200216x. [DOI] [PubMed] [Google Scholar]
- 288.Getman RB, Bae YS, Wilmer CE, Snurr RQ. Chem Rev. 2011;112:703. doi: 10.1021/cr200217c. [DOI] [PubMed] [Google Scholar]
- 289.Dietzel PDC, Panella B, Hirscher M, Blom R, Fjellvag H. Chem Commun. 2006:959. doi: 10.1039/b515434k. [DOI] [PubMed] [Google Scholar]
- 290.Deng H, Grunder S, Cordova KE, Valente C, Furukawa H, Hmadeh M, Gándara F, Whalley AC, Liu Z, Asahina S, Kazumori H, O’Keeffe M, Terasaki O, Stoddart JF, Yaghi OM. Science. 2012;336:1018. doi: 10.1126/science.1220131. [DOI] [PubMed] [Google Scholar]
- 291.Fujita D, Suzuki K, Sato S, Yagi-Utsumi M, Kurimoto E, Yamaguchi Y, Kato K, Fujita M. Chem Lett. 2012;41:313. [Google Scholar]
- 292.Hannon MJ. Chem Soc Rev. 2007;36:280. doi: 10.1039/b606046n. [DOI] [PubMed] [Google Scholar]
- 293.Barry NPE, Abd Karim NH, Vilar R, Therrien B. Dalton Trans. 2009:10717. doi: 10.1039/b913642h. [DOI] [PubMed] [Google Scholar]
- 294.Kieltyka R, Englebienne P, Fakhoury J, Autexier C, Moitessier N, Sleiman HF. J Am Chem Soc. 2008;130:10040. doi: 10.1021/ja8014023. [DOI] [PubMed] [Google Scholar]
- 295.Zheng XH, Zhong YF, Tan CP, Ji LN, Mao ZW. Dalton Trans. 2012;41:11807. doi: 10.1039/c2dt31303k. [DOI] [PubMed] [Google Scholar]
- 296.(a) Vajpayee V, Song YH, Yang YJ, Kang SC, Kim H, Kim IS, Wang M, Stang PJ, Chi KW. Organometallics. 2011;30:3242. doi: 10.1021/om200294x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Vajpayee V, Song YH, Jung YJ, Kang SC, Kim H, Kim IS, Wang M, Cook TR, Stang PJ, Chi KW. Dalton Trans. 2012;41:3046. doi: 10.1039/c2dt11811d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mishra A, Ravikumar S, Hong SH, Kim H, Vajpayee V, Lee H, Ahn B, Wang M, Stang PJ, Chi KW. Organometallics. 2011;30:6343. doi: 10.1021/om200802v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lu G, Hupp JT. J Am Chem Soc. 2010;132:7832. doi: 10.1021/ja101415b. [DOI] [PubMed] [Google Scholar]
- 299.Demessence A, Horcajada P, Serre C, Boissiere C, Grosso D, Sanchez C, Ferey G. Chem Commun. 2009:7149. doi: 10.1039/b915011k. [DOI] [PubMed] [Google Scholar]
- 300.Yu J, Cui Y, Wu C, Yang Y, Wang Z, O’Keeffe M, Chen B, Qian G. Angew Chem, Int Ed. 2012:1. doi: 10.1002/anie.201204160. [DOI] [PubMed] [Google Scholar]
- 301.Heo J, Mirkin CA. Angew Chem, Int Ed. 2006;45:941. doi: 10.1002/anie.200503343. [DOI] [PubMed] [Google Scholar]
- 302.Gole B, Bar AK, Mukherjee PS. Chem Commun. 2011;47:12137. doi: 10.1039/c1cc15594f. [DOI] [PubMed] [Google Scholar]
- 303.Yoon M, Srirambalaji R, Kim K. Chem Rev. 2011;112:1196. doi: 10.1021/cr2003147. [DOI] [PubMed] [Google Scholar]
- 304.Murase T, Horiuchi S, Fujita M. J Am Chem Soc. 2010;132:2866. doi: 10.1021/ja9107275. [DOI] [PubMed] [Google Scholar]
- 305.Samanta D, Mukherjee S, Patil YP, Mukherjee PS. Chem–Eur J. 2012;18:12322. doi: 10.1002/chem.201201679. [DOI] [PubMed] [Google Scholar]
- 306.Fiedler D, Bergman RG, Raymond KN. Angew Chem, Int Ed. 2004;43:6748. doi: 10.1002/anie.200461776. [DOI] [PubMed] [Google Scholar]
- 307.Hastings CJ, Pluth MD, Bergman RG, Raymond KN. J Am Chem Soc. 2010;132:6938. doi: 10.1021/ja102633e. [DOI] [PubMed] [Google Scholar]
- 308.Pluth MD, Bergman RG, Raymond KN. J Am Chem Soc. 2008;130:11423. doi: 10.1021/ja802839v. [DOI] [PubMed] [Google Scholar]
- 309.Lee SJ, Cho SH, Mulfort KL, Tiede DM, Hupp JT, Nguyen ST. J Am Chem Soc. 2008;130:16828. doi: 10.1021/ja804014y. [DOI] [PubMed] [Google Scholar]
- 310.Fiedler D, Bergman RG, Raymond KN. Angew Chem, Int Ed. 2006;45:745. doi: 10.1002/anie.200501938. [DOI] [PubMed] [Google Scholar]
- 311.Kawano M, Kobayashi Y, Ozeki T, Fujita M. J Am Chem Soc. 2006;128:6558. doi: 10.1021/ja0609250. [DOI] [PubMed] [Google Scholar]