Abstract
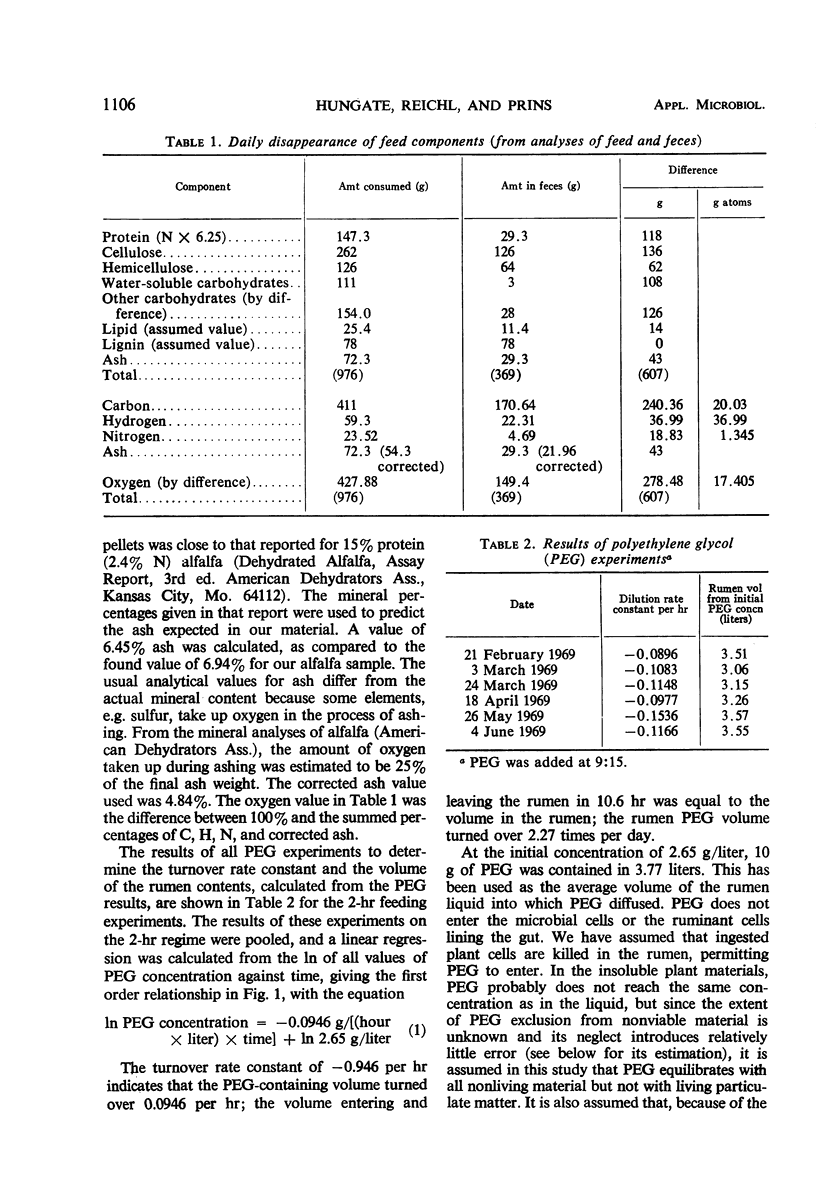
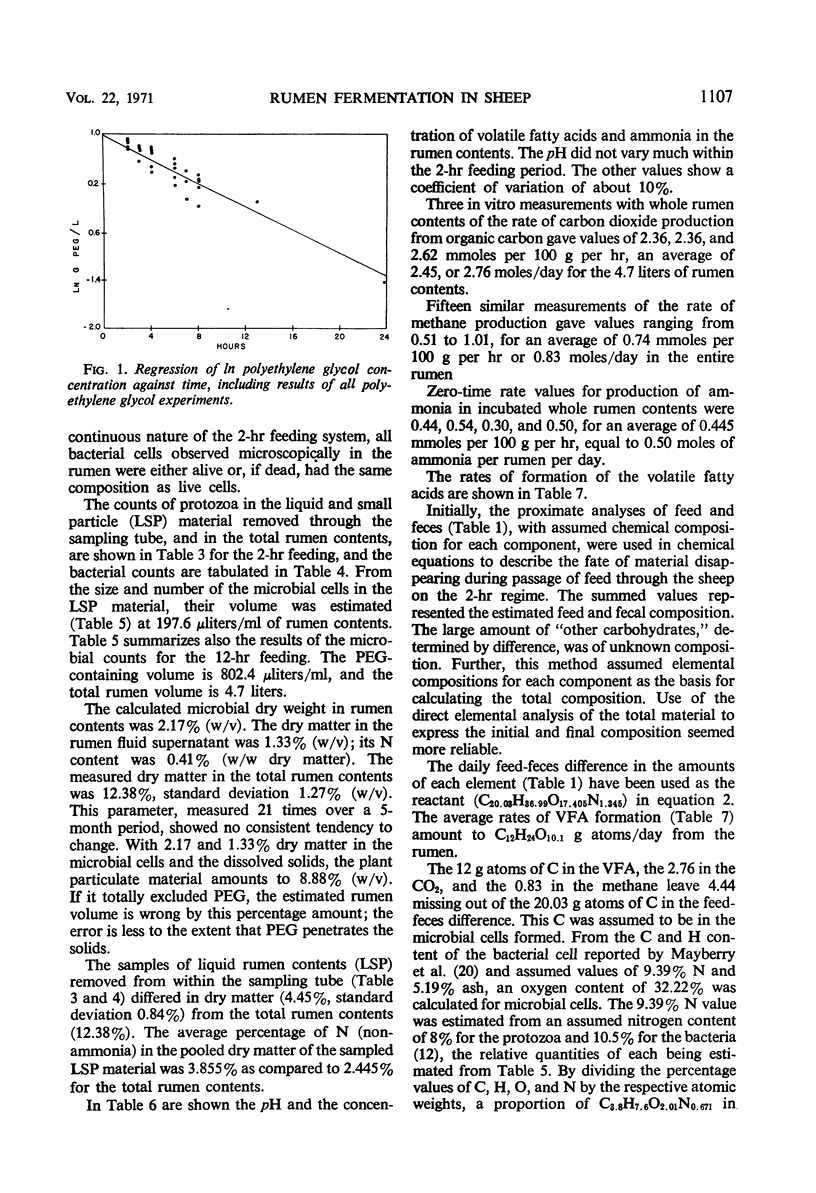
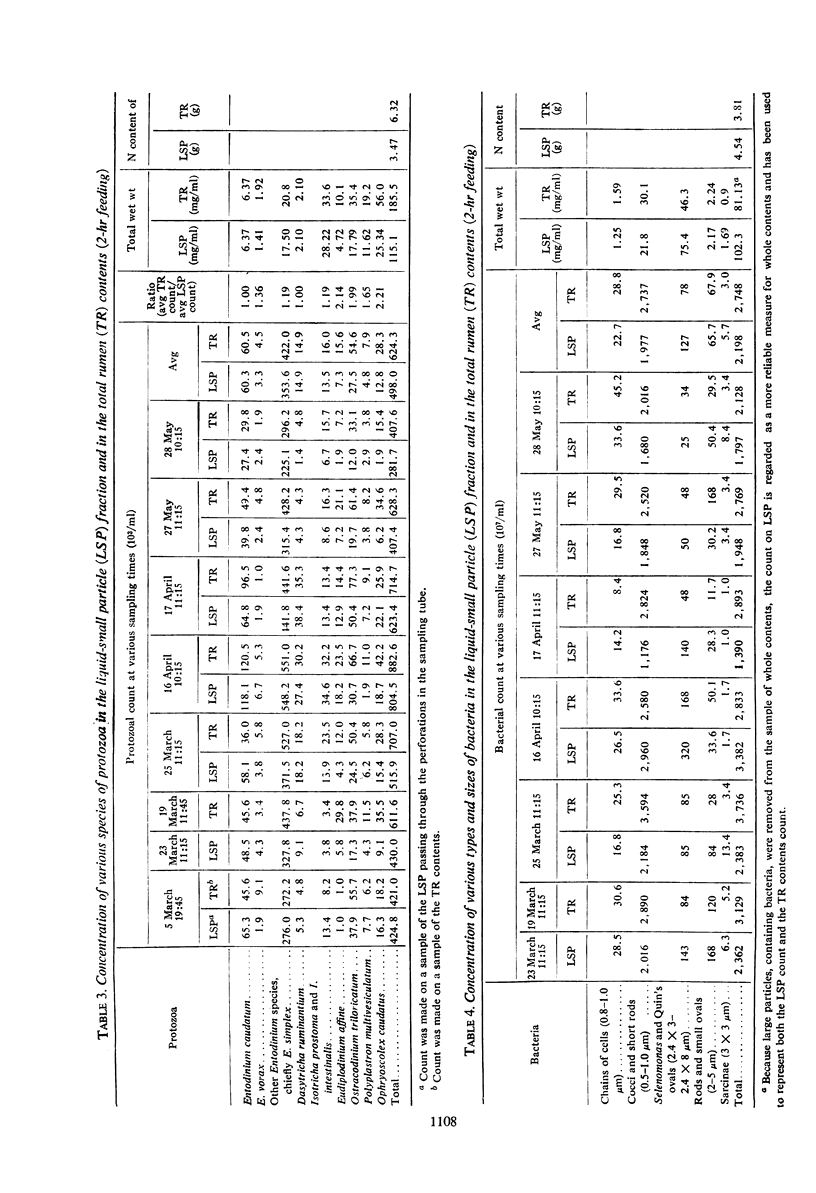
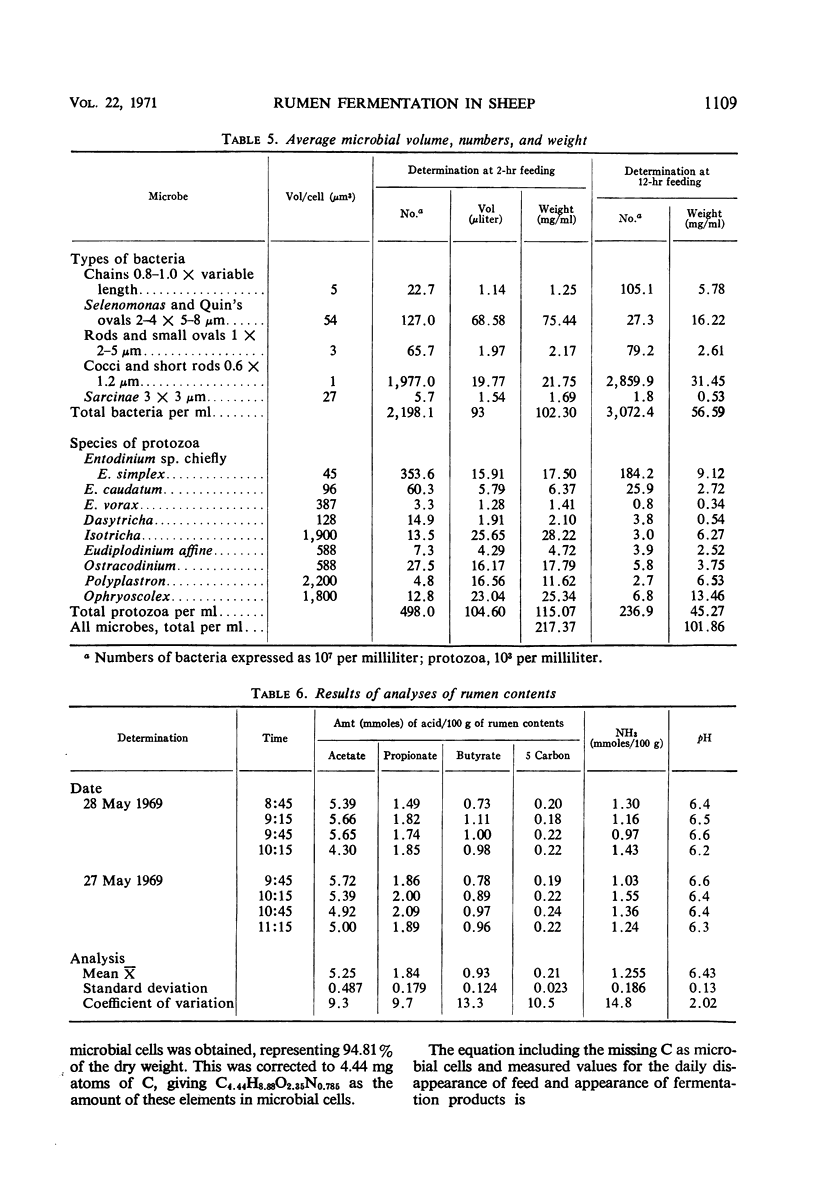
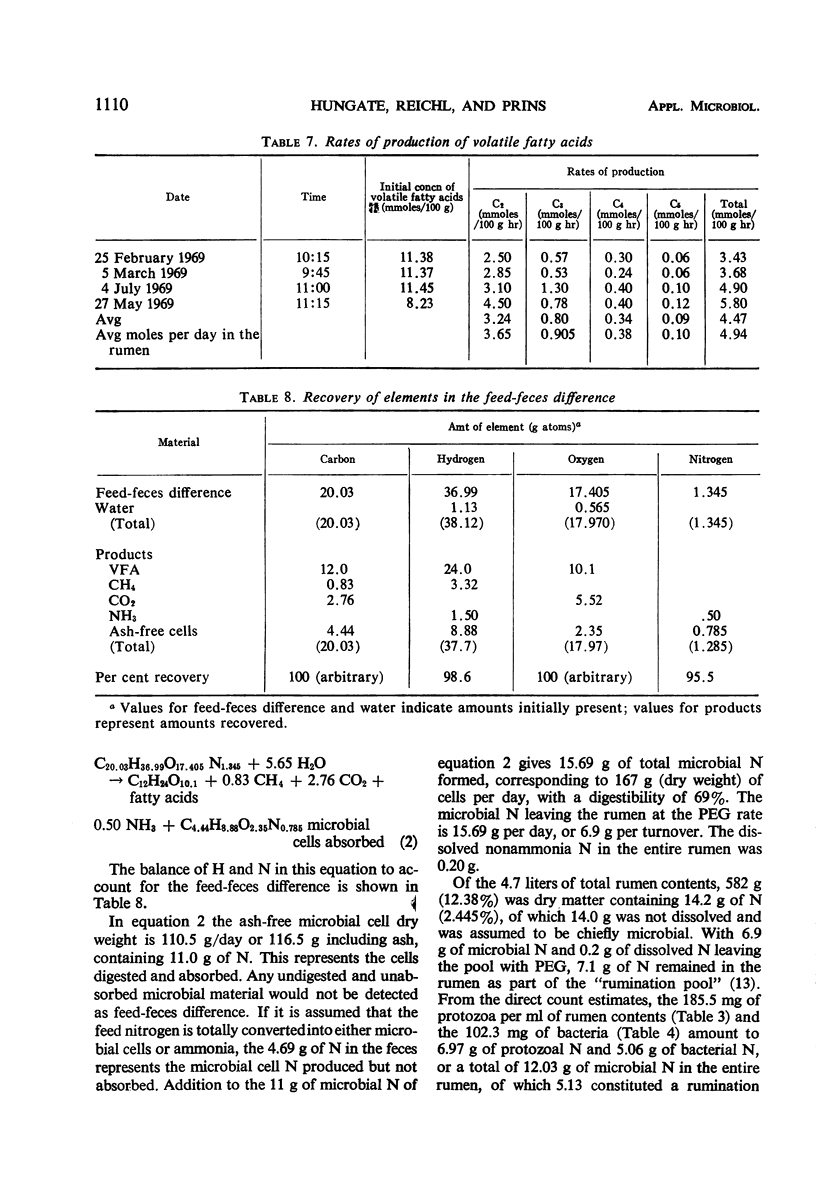
The feed and feces of a continuously fed sheep were analyzed for carbon, hydrogen, and nitrogen, with oxygen as the remainder. The daily feed-feces weight difference was used as the reactant in an equation representing the rumen fermentation. The measured products were the daily production of volatile fatty acids (VFA), CH4, CO2, and ammonia. The carbon unaccounted for was assumed to be in the microbial cell material produced in the rumen and absorbed before reaching the feces. The ratio of C to H, O, and N in bacteria was used to represent the elemental composition of the microbes formed in the rumen fermentation, completing the following equation:C20.03H36.99O17.406N1.345 + 5.65 H2O → C12H24O10.1 + 0.83 CH4 VFA + 2.76 CO2 + 0.50 NH3 + C4.44H8.88O2.35N0.785 microbial cells absorbed With C arbitrarily balanced and O balanced by appropriate addition of water, any error is reflected in the H. The H recovery was 98.5%. The turnover rate constant for rumen liquid equilibrating with polyethylene glycol (PEG) was 2.27 per day. Direct counts and volume measurements of the individual types of bacteria and protozoa in the rumen were used to calculate the total microbial cell volume in the rumen, not equilibrating with it. The dry matter in the rumen (582 g) and the nitrogen content (12.05) of the microbes in the rumen were estimated, the latter constituting 85% of the measured N in the rumen. Calculations for rumen dry matter and nitrogen turning over at the PEG rate introduce big discrepancies with other parameters; a rumination pool must be postulated. Its size and composition are estimated. Arguments are presented to support the view that dry matter and some of the microbes, chiefly the protozoa, do not leave the rumen at the PEG rate. One experiment with the same sheep fed twice daily showed significantly less production of microbial cells than did the continuous (each 2 hr) feeding. Analysis of the microbial cell yield suggests that, on the basis of 11 mg of cells per adenosine triphosphate molecule, a maximum of six adenosine triphosphate molecules could have been formed from each molecule of hexose fermented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL E. J., HUNGATE R. E. The magnitude of the microbial fermentation in the bovine rumen. Appl Microbiol. 1954 Jul;2(4):205–214. doi: 10.1128/am.2.4.205-214.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E., DOUGHERTY R. W., BRYANT M. P., CELLO R. M. Microbiological and physiological changes associated with acute indigestion in sheep. Cornell Vet. 1952 Oct;42(4):423–449. [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P. N., Summers R. The continuous culture of anaerobic bacteria. J Gen Microbiol. 1967 Apr;47(1):53–65. doi: 10.1099/00221287-47-1-53. [DOI] [PubMed] [Google Scholar]
- Hungate R. E., Smith W., Bauchop T., Yu I., Rabinowitz J. C. Formate as an intermediate in the bovine rumen fermentation. J Bacteriol. 1970 May;102(2):389–397. doi: 10.1128/jb.102.2.389-397.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAH R. A. FACTORS INFLUENCING THE IN VITRO CULTURE OF THE RUMEN CILIATE OPHRYOSCOLEX PURKYNEI STEIN. J Protozool. 1964 Nov;11:546–552. doi: 10.1111/j.1550-7408.1964.tb01796.x. [DOI] [PubMed] [Google Scholar]
- Mayberry W. R., Prochazka G. J., Payne W. J. Factors derived from studies of aerobic growth in minimal media. J Bacteriol. 1968 Oct;96(4):1424–1426. doi: 10.1128/jb.96.4.1424-1426.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Pilgrim A. F., Gray F. V., Belling G. B. Production and absorption of ammonia in the sheep's stomach. Br J Nutr. 1969 Aug;23(3):647–655. doi: 10.1079/bjn19690072. [DOI] [PubMed] [Google Scholar]
- Pirt S. J., Kurowski W. M. An extension of the theory of the chemostat with feedback of organisms. Its experimental realization with a yeast culture. J Gen Microbiol. 1970 Nov;63(3):357–366. doi: 10.1099/00221287-63-3-357. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLER R. A., PILGRIM A. F., GRAY F. V. Digestion of foodstuffs in the rumen of the sheep and the passage of digesta through its compartments. 3. The progress of nitrogen digestion. Br J Nutr. 1962;16:83–90. doi: 10.1079/bjn19620009. [DOI] [PubMed] [Google Scholar]


