Abstract
Objective
To create a predictive model for preterm birth (PTB) from available clinical data and serum analytes.
Study Design
Serum analytes, routine pregnancy screening plus cholesterol and corresponding health information were linked to birth certificate data for a cohort of 2699 Iowa women with serum sampled in the first and second trimester. Stepwise logistic regression was used to select the best predictive model for PTB.
Results
Serum screening markers remained significant predictors of PTB even after controlling for maternal characteristics. The best predictive model included maternal characteristics, first trimester total cholesterol (TC), TC change between trimesters and second trimester alpha-fetoprotein and inhibin A. The model showed better discriminatory ability than PTB history alone and performed similarly in subgroups of women without past PTB.
Conclusions
Using clinical and serum screening data a potentially useful predictor of PTB was constructed. Validation and replication in other populations, and incorporation of other measures that identify PTB risk, like cervical length, can be a step towards identifying additional women who may benefit from new or currently available interventions.
Keywords: cholesterol, prediction, preterm birth, serum screening
Introduction
Preterm birth (PTB) is the leading cause of morbidity and mortality for newborns both worldwide and in the United States.1 Numerous risk factors are known to be related to PTB, but identifying at risk pregnancies before the onset of labor is difficult.2 Maternal obstetric history of past PTB is the most easily implemented and widely used screening measure. Cervical length measurement is also beneficial, though not currently universally implemented, despite evidence for its use in all singleton pregnancies.3,4 Interventions in response to both short cervical length and past PTB have proven to be effective.5,6 Many other screening measures and biomarkers have been proposed but none are used ubiquitously or justify additional testing, especially in low-risk individuals.7 Thus, a screening tool based on existing and easily available clinical and laboratory data may be useful.
Cholesterol is a common and inexpensive serum test and has been associated with PTB. A recent report indicated that both high (>90th percentile) and low (<10th percentile) cholesterol levels during the second trimester of pregnancy may identify women at risk for PTB.8 Each type of abnormal cholesterol measurement conferred an increased odds of PTB by a factor >2.5. A similar finding was subsequently reported using pre-pregnancy lipids indicating a “U-shaped” relationship between cholesterol and PTB.9 These findings are intriguing given that low-density lipoprotein (LDL) is the precursor to progesterone synthesis during pregnancy.10 In addition, commonly obtained analytes used in maternal serum screening have yielded similar risk profiles for PTB. Alpha-fetoprotein (AFP) and Inhibin A >2.0 multiples of the median (MoM) each more than double the odds of subsequent PTB.11,12
There is an evident need to stratify women at risk for PTB to offer additional screening, or test possible interventions if appropriate. A non-invasive screening tool applicable at the population-level and able to improve upon obstetric history alone should be of clinical interest. This study proposes a model for predicting PTB derived from data collected prospectively in a cohort of Iowa women who underwent routine serum screening and who had cholesterol measured twice during pregnancy.
Materials and Methods
Population & Data Sources
All Iowa women undergoing routine prenatal testing in the first and/or second trimester through the Iowa Maternal Serum Screening Program, from May 2009 until November 2010, were included in the initial cohort (12,057 women). Risk factors and outcome information on included women came from two distinct sources. The first was serum screening measurements and medical information collected at the time of screening. This included maternal race, ethnicity, weight (both trimesters), age and gestational age at sampling (both trimesters). Serum measurements captured as part of routine screening included, pregnancy associated plasma protein A (PAPP-A) and human chorionic gonadotropin (hCG) in the first trimester and estriol, AFP, inhibin A and hCG in the second trimester. These analytes were expressed as adjusted multiples of the median (MoM). Clinical and demographic data was extracted from the neonatal birth certificate. Relevant maternal information included education, ethnicity, race, smoking status, height, previous live births, previous PTB, diabetes (pre-pregnancy and gestational), pre-pregnancy hypertension and sexually transmitted diseases. Information about treatment during pregnancy and delivery outcomes included cerclage, tocolysis, labor onset, prelabor rupture of membranes (PROM), induction, fetal presentation, congenital anomalies, birth weight, gestational age and plurality. There was no information available for cervical length, fetal fibronectin, maternal characteristics or serum analytes not collected as part of the maternal serum screening program or recorded on the birth certificate. This study received approval from the University of Iowa Institutional Review Board (IRB 200812784) and from the Iowa Department of Public Health-Congenital and Inherited Disorders Advisory Committee and State Vital Statistics.
Laboratory Testing
Maternal serum was sent to the State of Iowa Hygienic Laboratory (SHL) for testing of the routine pregnancy analytes discussed above. An additional lipid panel including total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides (TG) was measured on excess sera. Lipid levels were measured using a Roche Diagnostics c111 Cobas Analyzer (Basel, Switzerland) at a single laboratory. Women were not routinely fasted before these samples were collected. Medical information obtained at the time of serum collection, results of maternal serum screening, cholesterol and triglyceride levels were combined using unique identifiers with the corresponding birth certificate data. This dataset was then provided to the investigators without personal identifiers and stored in a Progeny database (Progeny Software, Delray Beach, Florida).
Inclusion and Exclusion Criteria
In Iowa all infants born alive at ≥20 weeks GA receive a birth certificate and thus were eligible for inclusion in this cohort. Thus, six infants with a birth weight <500 g were included. Only women with serum analyte results collected in both the first and second trimester were included in this analysis. Many women received only the second trimester screening (quad screen) and this reduced the available cohort for analysis from 12,057 women with at least one screen to 2976 with two screenings. Additional exclusion criteria included multiple gestations (n=173, 5.8%) or congenital anomaly (n=17, 0.6%) as these would have different management as at-risk pregnancies; unknown birth weight (n=1, <0.01%) or birth weight for gestational age >3 standard deviations from the mean (n=18, 0.6%) considered to be unreliable birth records; any serious infection (gonorrhea, syphilis, hepatitis, etc.) (n=48, 1.6%), and the second pregnancy of any mothers with more than one pregnancy during collection (n=10, 0.3%). Also, term births that were treated with either cerclage (n=1, <0.01%) or tocolysis (n=6, 0.02%) were excluded. Finally, three women were excluded by inspection for aberrant cholesterol measures between trimesters (TC decrease of 170 mg/dL, LDL increase of 184 mg/dL and HDL decrease of 57 mg/dL). This left a total of 2699 women.
Outcome Definitions
PTB was the primary outcome for our predictive model. PTB was defined as birth <37 weeks completed gestational age and term birth (TB) was considered ≥37 completed weeks gestational age as indicated on the birth certificate. There were 2499 TB and 200 PTB in the complete cohort. In addition to the primary outcome of PTB, non-spontaneous PTB was identified as follows:
Delivered vaginally with induction but without preterm PROM (PPROM), tocolysis or precipitous labor.
Delivered via cesarean section after labor by induction without PPROM, tocolysis or precipitous labor.
Delivered by cesarean section without labor and no history of cesarean section, tocolysis or breech presentation.
All PTB remaining after excluding the above non-spontaneous PTBs were considered to be spontaneous PTB (sPTB). The predictive model was also assessed for predicting sPTB and 153 of the 200 total PTB were classified as sPTB. Defining sPTB in this way has not yet been validated but is meant as a sensitivity analysis to test prediction in a population less likely to have pre-eclampsia or indicated PTB. However, sPTB being 76.5% of all total PTB is similar to the 70% figure reported in recent reviews.2
Statistical Analysis
Covariate Adjustment
Many of the serum analytes examined in this study are related to gestational age at the time of sampling. To adjust for this relationship covariates were standardized to the mean gestational age (in days) at sampling. First, they were examined graphically and then linear regression, with a log transformation of the analyte as the outcome variable, and gestational age (GA) in days as the explanatory variable was used for standardization. If the regression coefficient was significant at the p=0.10 level, the analyte measurement was adjusted to the mean GA at sampling before being tested in the predictive model. Again, these were visually inspected to see that the linear relationship was no longer present. This was done for all lipid analytes (TC, LDL, HDL, TG) in each semester and for the serum screening measures of PAPP-A in the first trimester and AFP in the second trimester.
Serum Analysis
Before selecting covariates for inclusion in a final predictive model the lipid measurements (TC, LDL, HDL and TG) and routine screening analytes were examined to characterize any relation to PTB. The highest and lowest quartiles of each lipid measurement were compared to the middle quartiles to assess if the previously reported U-shaped relationship existed in the current cohort. Linear relationships were considered as well, both with log transformation and without. For routine screening analytes, model fit, based upon Aikike Information Criterion (AIC), was compared between specifying them as dichotomous vs. continuous variables. Again, linear relationships were considered, both with log transformation and without.
Covariate Selection
All covariates potentially related to PTB were screened for entry into a final predictive model. All covariates related to PTB at the p<0.10 level using a chi-squared test or simple logistic regression were considered for selection of the final model. The following covariates, all reported to be related to PTB in previous studies, underwent initial screening: The lipid markers as described above (TC, LDL, HDL and TG in the first and second trimesters, as well as change between trimesters for each), pre-pregnancy HTN, maternal age, maternal education, smoking status, pre-pregnancy diabetes, gestational diabetes, previous PTB, previous birth outcomes, maternal body mass index (BMI, both low and high; as indicated by past research), weight change between trimesters, infertility treatment, hCG (both trimesters and change), PAPP-A, AFP, estriol, Inhibin-A, time between pregnancies, maternal race and maternal ethnicity.
Model Selection and Performance
Multivariable logistic regression using a logit link function was used to determine which covariates significantly predicted PTB. The final model was determined using forward, backward and stepwise selection with AIC being used to assess model fit. Parameter estimates for predictive covariates were determined in the entire cohort and these were used for testing the model in all subgroups. Model performance was assessed based upon sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and area under the receiver operating curve (AUC). The predictive model was compared to obstetric history of prior PTB as the only predictor, both in the entire cohort and the subgroup of women with a past live birth (where obstetric history would be most useful). This comparison is justified given that a past PTB is sufficient to initiate treatment to prevent PTB and thus our model may identify a population similarly at risk. For ease of comparison with past PTB, the predictive model’s performance was evaluated at equal specificity. Performance was also calculated setting specificity equal to 90% for the model. In addition, the model was assessed in the subgroup of nulliparous women and those with only past term births. Here, specificity was given a lower bound of 90% or 95% and the other performance metrics evaluated.
Sensitivity Analysis & Reclassification
To test the stability of the predictive model for different theoretical populations a bootstrapping procedure was performed. The cohort was sampled with replacement, creating a new cohort of equal size 1000 times and the estimated AUC and 95% confidence interval for the complete model using all predictors (maternal characteristics, serum screening and cholesterol) were calculated. In addition, all analyses were performed using sPTB as the outcome variable. To assess whether the predictive model assigned higher probabilities of PTB to cases versus controls, AUC was compared from four prediction scenarios. Model I consisted of past PTB alone, Model II past PTB plus other maternal characteristics found to be significant predictors, Model III all predictors from Model II plus results from routine serum screening (excluding cholesterol) and Model IV all predictors from Model III plus cholesterol measures. AUC was compared between models using a standard method for correlated AUCs.13 Continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were also compared between models.14,15 Continuous NRI between two models can be thought of as the percentage of individuals whose probability of PTB moves in the correct direction (i.e. increases for cases, decreases for controls) minus the percentage that moved in the wrong direction (i.e. decreases for cases, increases for controls). IDI is the increase, from one predictive model to another, of the difference in average predicted probabilities of PTB between cases and controls.
All analysis was performed in R version 2.15 with the aid of packages predictABEL, pROC and ROCR.16–18
Results
The average birth weights for TB vs. PTB are 3449 g (standard deviation [SD]: 444 g, Range: 1960-4805 g) vs. 2334 g (SD: 828 Range: 275-3914 g). The average GA for TB vs. PTB are 39.1 wks (SD: 1.1 wks, Range: 37-43 wks) vs. 33.7 wks (SD: 3.6 wks, Range: 20-36 wks).
Results from serum lipid association with PTB are available in Table 1. Neither the highest or lowest quartile of any lipid measurement were significantly associated with PTB (p>0.05). Thus, no U-shaped relationship was observed for any serum lipids. Only first trimester cholesterol, as a continuous variable, showed a trend for association with PTB and was eligible for inclusion in the final model. Using a log transformation did not improve model fit for any of the lipid measurements.
Table 1. Lipid association with preterm birth (PTB).
Q1-Q4 = Quartiles 1-4 for each lipid measurement. There is no evidence of a U-shaped relationship with any of the lipid measurements. Only total cholesterol during the first trimester showed a trend for association with PTB. Log transformation did not improve the fit of any of the lipid measurements association with PTB. Odds ratios for continuous lipid measurements are expressed as a one standard deviation increase of the lipid measurement under investigation.
| Lipid Concentration First Trimester (range mg/dL) |
PTB (<37 wk) | Lipid Concentration Second Trimester (range mg/dL) |
PTB (<37 wk) | ||
|---|---|---|---|---|---|
| Categorical Variables | |||||
| Total Cholesterol | OR (95% CI) | P | Total Cholesterol | OR (95% CI) | P |
| Q4 (193-359) | 1.14 (0.81, 1.61) | 0.440 | Q4 (216-358) | 1.00 (0.70, 1.42) | 0.996 |
| Q2-Q3 (152-192) | Reference | Q2-Q3 (173-215) | Reference | ||
| Q1 (86-151) | 0.90 (0.62, 1.30) | 0.574 | Q1 (96-172) | 1.00 (0.70, 1.42) | 0.996 |
| LDL Cholesterol | LDL Cholesterol | ||||
| Q4 (111-290) | 1.06 (0.75, 1.51) | 0.726 | Q4 (130-265) | 1.08 (0.76, 1.54) | 0.671 |
| Q2-Q3 (77-110) | Reference | Q2-Q3 (91-129) | Reference | ||
| Q1 (28-76) | 1.02 (0.72, 1.45) | 0.918 | Q1 (33-90) | 1.25 (0.88, 1.76) | 0.210 |
| HDL Cholesterol | HDL Cholesterol | ||||
| Q4 (68-121) | 0.89 (0.62, 1.29) | 0.533 | Q4 (72-131) | 0.93 (0.64, 1.34) | 0.679 |
| Q2-Q3 (50-67) | Reference | Q2-Q3 (53-71) | Reference | ||
| Q1 (19-49) | 1.16 (0.82, 1.62) | 0.403 | Q1 (20-52) | 1.21 (0.86, 1.70) | 0.266 |
| Triglycerides | Triglycerides | ||||
| Q4 (163-655) | 1.20 (0.85, 1.69) | 0.310 | Q4 (196-690) | 1.03 (0.72, 1.47) | 0.859 |
| Q2-Q3 (98-162) | Reference | Q2-Q3 (121-195) | Reference | ||
| Q1 (38-97) | 1.12 (0.79, 1.60) | 0.514 | Q1 (42-120) | 1.10 (0.78, 1.56) | 0.592 |
| Continuous Variables | |||||
| Lipid Measurement First Trimester |
OR (95% CI) | P | Lipid Measurement Second Trimester |
OR (95% CI) | P |
| Total Cholesterol | 1.14 (0.99, 1.31) | 0.068 | Total Cholesterol | 1.03 (0.89, 1.19) | 0.681 |
| LDL Cholesterol | 1.12 (0.97, 1.29) | 0.114 | LDL Cholesterol | 1.01 (0.88, 1.17) | 0.856 |
| HDL Cholesterol | 0.91 (0.79, 1.06) | 0.218 | HDL Cholesterol | 0.96 (0.83, 1.11) | 0.583 |
| Triglycerides | 1.10 (0.96, 1.25) | 0.161 | Triglycerides | 1.02 (0.88, 1.17) | 0.821 |
Routine serum analytes were also assessed and AFP and inhibin A were significantly associated with PTB. (Table 2) Specifying each as a categorical variable showed a trend for increasing odds of PTB with increasing levels of each analyte. The best model fit for each analyte was as a continuous variable; log transformation did not improve the fit for either analyte.
Table 2. Serum Analytes.
MoM=multiples of the median, AFP=alpha fetoprotein. Both AFP and inhibin A show evidence of a linear relationship with the risk of PTB as their measured value increases.
| Serum Analytes - Categorical | |||||
|---|---|---|---|---|---|
| AFP (MoM) | OR (95% CI) | P | Inhibin A (MoM) | OR (95% CI) | P |
| AFP < 1.0 | Reference | Inhibin A < 1.0 | Reference | ||
| 1.0 ≤ AFP < 1.5 | 1.44 (1.04, 1.98) | 0.026 | 1.0 ≤ Inhibin A < 1.5 | 1.28 (0.91, 1.80) | 0.163 |
| 1.5 ≤ AFP < 2.0 | 2.45 (1.51, 3.99) | <0.001 | 1.5 ≤ Inhibin A < 2.0 | 1.68 (1.05, 2.69) | 0.030 |
| 2.0 ≤ AFP < 2.5 | 6.27 (2.93, 13.4) | <0.001 | 2.0 ≤ Inhibin A < 2.5 | 2.37 (1.24, 4.53) | 0.009 |
| AFP ≥ 2.5 | 6.99 (1.78, 27.5) | 0.005 | Inhibin A ≥ 2.5 | 3.19 (1.68, 6.04) | <0.001 |
| Model Fit - Continuous | |||||
| AFP (MoM) | P | AIC | Inhibin A (MoM) | P | AIC |
| AFP | 5.81 × 10−9 | 1398 | Inhibin A | 2.13 × 10−5 | 1343 |
| log(AFP) | 1.08 × 10−7 | 1402 | log(Inhibin A) | 9.63 × 10−5 | 1346 |
After initial screening of potential predictor variables, those with a p-value<0.10 were eligible for testing in the final prediction model and are presented in Table 3. Some variables with p<0.10 were not included due to high correlation with other explanatory variables. In each case, the predictor with a better model fit, according to AIC, was included. This resulted in choosing maternal education over maternal age, smoking status during the second trimester in place of smoking status at other time points, measured BMI during the first trimester over self-reported pre-pregnancy BMI. Using the variables identified in the initial screening procedure forward, backward and stepwise selection of logistic regression models was performed. All selection algorithms converged to the same final model. The parameter estimates and odds ratios of the predictors that were included in the final model were similar to their univariate estimates showing no evidence of confounding or multicollinearity. Parameter estimates were also stable after including different subsets of all final explanatory variables. This model was then tested in the subgroup of women that had no previous PTB. The results of these models are shown in Table 4. The serum analytes remain predictive of PTB even after controlling for clinical risk factors. This is also true in the cohort of women without a previous PTB. The predictors listed in Table 4 constitute the predictive model described in further analysis. These include maternal characteristics (maternal degree, pre-pregancy diabetes, previous PTB, previous live birth, and maternal BMI), routine serum analytes (AFP and inhibin A), and cholesterol (first trimester TC and TC change between trimesters [second TC trimester – first trimester TC]). The two cholesterol measures were not correlated (Pearson correlation coefficient = −0.12) and thus were kept as independent predictors in the model. Increased first trimester TC was associated with PTB while decreases in TC change were also associated with PTB.
Table 3. Explanatory variables eligible for the final prediction model.
Maternal degree is included an associate’s degree or higher. HTN=Hypertension, DM=Diabetes Mellitus and MoM=multiples of the median. Smoking is a continuous variable of cigarettes per day during the second trimester. Weight change is from before pregnancy until measured in the second trimester. The p-values listed for continuous variables are based on simple logistic regression.
| Variable | Term (n=2499) |
PTB (n=200) |
P |
|---|---|---|---|
| Dichotomous Variables - % (n) | |||
| Pre-pregnancy HTN | 2.3 (57) | 4.5 (9) | 0.086 |
| Maternal Post-Secondary Degree | 48.9 (1222) | 37.6 (74) | 0.003 |
| Pre-pregnancy DM | 1.8 (45) | 5.5 (11) | 0.001 |
| Previous PTB | 3.0 (75) | 11.5 (23) | <0.001 |
| Previous Live Birth | 59.4 (1484) | 51.5 (103) | 0.035 |
| Race (Black) | 5.4 (135) | 9.5 (19) | 0.025 |
| 1st Trimester BMI<18.5 | 2.3 (53) | 5.6 (10) | 0.011 |
| 1st Trimester BMI>40 | 6.0 (146) | 11.6 (22) | 0.004 |
| Continuous Variables - mean (sd) | |||
| Smoking – 2nd Trimester | 1.1 (3.5) | 1.8 (4.8) | 0.011 |
| Weight Change to 2nd Trimester | 9.0 (12.6) | 10.7 (13.9) | 0.072 |
| AFP (MoM) | 1.0 (0.3) | 1.2 (0.5) | <0.001 |
| Inhibin A (MoM) | 1.1 (0.6) | 1.3 (1.0) | <0.001 |
| TC 1st Trimester | 173.8 (30.4) | 177.9 (35.7) | 0.068 |
| TC Change 1st to 2nd Trimester | 22.4 (18.3) | 19.3 (19.5) | 0.022 |
Table 4. Final prediction model.
TC=total cholesterol, DM=diabetes mellitus, PTB=preterm birth, BMI=body mass index, AFP=alpha fetoprotein, MoM=multiples of the median. The betas are the coefficients resulting from the logistic regression fit. Some variables that remained as significant predictors had missing values and thus those individuals are not included in the model. For the entire cohort there were missing values in TBs for BMI (1), Inhibin A (176) and maternal degree (2). There were also missing values in PTBs for maternal degree (3) and inhibin A (8). The odds ratios (OR) for the cholesterol variables are expressed using their standard deviation as the unit of change; 30.9 mg/dL for 1st trimester TC and 18.5 for TC change.
| Explanatory Variable | Beta | OR (95% CI) | P |
|---|---|---|---|
| Entire Cohort | |||
| Intercept | −4.32 | ||
| TC 1st Trimester (mg/dL) | 0.005 | 1.17 (1.01,1.36) | 0.032 |
| TC Change 1st to 2nd Trimester (mg/dL) |
−0.008 | 0.87 (0.74,1.01) | 0.072 |
| Maternal Degree | −0.411 | 0.66 (0.48,0.91) | 0.012 |
| Pre-pregnancy DM | 1.140 | 3.13 (1.38,6.51) | 0.004 |
| Previous PTB | 1.440 | 4.25 (2.34,7.44) | <0.001 |
| Previous Live Birth | −0.494 | 0.61 (0.44,0.84) | 0.003 |
| 1st Trimester BMI<18.5 | 1.058 | 2.88 (1.31,5.77) | 0.005 |
| 1st Trimester BMI>40 | 0.375 | 1.46 (0.84,2.41) | 0.161 |
| AFP (MoM) | 0.880 | 2.41 (1.71,3.40) | <0.001 |
| Inhibin A (MoM) | 0.308 | 1.36 (1.11,1.68) | 0.004 |
| No Previous PTB | |||
| TC 1st Trimester (mg/dL) | 1.17 (1.00,1.38) | 0.049 | |
| TC Change 1st to 2nd Trimester (mg/dL) |
0.85 (0.72,0.99) | 0.044 | |
| Maternal Degree | 0.69 (0.49,0.95) | 0.026 | |
| Pre-pregnancy DM | 3.36 (1.43,7.17) | 0.003 | |
| Previous Live Birth | 0.61 (0.44,0.84) | 0.003 | |
| 1st Trimester BMI<18.5 | 2.92 (1.33,5.84) | 0.004 | |
| 1st Trimester BMI>40 | 1.33 (0.73,2.29) | 0.322 | |
| AFP (MoM) | 2.24 (1.57,3.20) | <0.001 | |
| Inhibin A (MoM) | 1.33 (1.07,1.67) | 0.011 | |
The predictive ability of the model was assessed and compared to prediction with only past PTB in the entire cohort and in the subgroup of women with a past live birth. (Table 5) The model was also assessed in the subgroups of nulliparous women and those with only past TB.
Table 5. Predictive ability of the final model.
PPV=Positive predictive value, NPV=Negative predictive value, AUC=Area under the receiver operating curve (ROC). The Past Live Birth subgroup contained only women with a past live birth where a previous preterm birth (PTB) would be a more useful predictor; this subgroup consisted of 1369 term birth (TB) and 96 PTB. The Nulliparous subgroup is women with no past live births; this subgroup consisted of 951 TB and 93 PTB. The Past Term Birth subgroup consists of women who have had a past live birth and have never had a PTB; this subgroup contained 1299 TB and 76 PTB.
| Entire Cohort (n=2509) | Past Live Birth Subgroup (n=1465) | |||||
|---|---|---|---|---|---|---|
| Past PTB |
Model | Model (Spec>90%) |
Past PTB |
Model | Model (Spec>90%) |
|
| Prevalence | 7.5 | 7.5 | 7.5 | 6.6 | 6.6 | 6.6 |
| Sensitivity | 10.6 | 17.5 | 31.2 | 20.8 | 26.0 | 35.4 |
| Specificity | 97.0 | 97.2 | 90.6 | 94.9 | 95.3 | 90.8 |
| PPV | 22.2 | 33.7 | 21.3 | 22.2 | 27.8 | 21.2 |
| NPV | 93.0 | 93.5 | 94.2 | 94.5 | 94.8 | 95.2 |
| AUC | 53.8 | 69.5 | 69.5 | 57.9 | 70.3 | 70.3 |
| Nulliparous Subgroup (n=1044) | Past Term Birth Subgroup (n=1375) | |||
|---|---|---|---|---|
| Model (Spec>95%) |
Model (Spec>90%) |
Model (Spec>95%) |
Model (Spec>90%) |
|
| Prevalence | 8.9 | 8.9 | 5.5 | 5.5 |
| Sensitivity | 12.9 | 26.9 | 18.4 | 26.3 |
| Specificity | 96.6 | 90.9 | 96.5 | 91.2 |
| PPV | 27.3 | 22.3 | 23.3 | 14.9 |
| NPV | 91.9 | 92.7 | 95.3 | 95.5 |
| AUC | 65.7 | 65.7 | 65.9 | 65.9 |
The predictive model had higher sensitivity and a higher PPV in the entire cohort and in women with a past live birth with equal specificity compared to using past PTB alone. Allowing for a specificity of ~90%, nearly 1/3 of PTBs can be identified. In the subgroup of nulliparous women, over 25% of women who went on to have a PTB were identified with a specificity >90%. This is also true when considering women with only past term births.
Sensitivity analysis indicated the stability of the predictive model using the bootstrapping procedure. The estimated AUC was 69.5 with a relatively narrow 95% confidence interval of (65.0,72.9). In addition, all analyses were performed using sPTB with similar results. Data for the outcome of sPTB is available in Supplemental Table 1. The results from the reclassification analysis are presented in Supplemental Table 2. The predictive model using all characteristics (Model IV) shows a statistically significantly larger AUC than all other model specifications. (p=0.03). This increasing AUC and the trade-off between sensitivity and specificity can be seen graphically in Figure 1. The receiver operating characteristic (ROC) curves for the four different prediction scenarios are each represented. The full predictive model (Model IV) also correctly reclassifies nearly 40% of individuals according to NRI with a 3.3% actual increase in the predicted probability difference between cases and controls according to IDI when compared to past PTB and maternal characteristics alone.
Figure 1. ROC Curve.
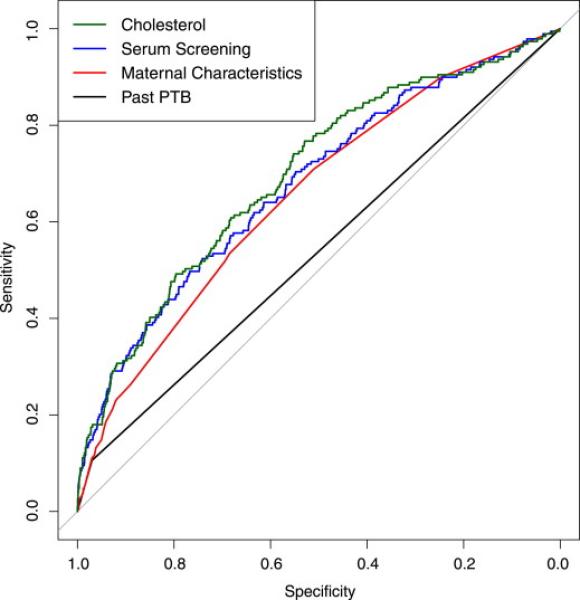
The figured depicts the receiver operating characteristic (ROC) curve for four prediction scenarios: past PTB alone (black), all maternal characteristics, including past PTB (red), maternal characteristics plus routine serum analytes (blue), and finally the full prediction model including cholesterol (green). The trade-off between sensitivity and specificity can be seen and the full prediction model has a statistically significantly greater AUC (p=0.03) than all other scenarios.
Comment
In this study we seek to create a useful screening tool for identifying women at risk of delivering preterm. The predictive model presented here identifies more women who had PTBs (i.e. greater sensitivity), with fewer false positives (i.e. greater positive predictive value) when compared to obstetric history alone. Notably, the model performs similarly well in nulliparous women or those with only past TB. This suggests a screening mechanism that makes use of information readily available during pregnancy that warrants further investigation, refinement and validation in other cohorts.
The current study and this predictive model have several positive attributes. First, the PTB rate for singleton pregnancies in this cohort (7.4%) is very similar to figures by the Iowa Department of Public Health (7.7%), indicating a fairly representative sample of Iowa women.19 It is based on routinely collected maternal history and on readily testable serum analytes, many of which are current standard of care. This increases the ease to which it could be adapted for clinical use, its cost-effectiveness, and its verifiability in broader more diverse populations. The prospective enrollment of the cohort allowed for inference about how this screening method would perform if implemented on a population scale. The study makes use of cholesterol levels during pregnancy as well. While previous reports have linked serum cholesterol measures to pregnancy risk this is the first, to our knowledge, to utilize cholesterol change during pregnancy as a risk factor.
Our study does have limitations that will need to be addressed in future research. The sample size and population demographics limit the generalizability of the study considerably and there was no available validation set. Only ~2500 women had complete information from both trimesters as well as pregnancy outcome data. The Iowa population was >82% white and >88% non-Hispanic. The subset receiving screening in both trimesters differs from the remaining cohort undergoing second trimester screening only. Those with both screening measures were older, more educated, more likely to have diabetes, less likely to smoke and less likely to be black. However, birth outcomes (birth weight, PTB rates), pregnancy histories (previous live births, past PTBs) and screening measurements and their relationship to PTB were not significantly different between populations. All outcome information was obtained from birth certificate data, which can be of variable quality. While past studies indicate that outcome information is typically valid, assessment of risk factors is more difficult.20 This was an unavoidable limitation, and should be addressed by future validation studies. Also, definitive classification of PTB etiology was not possible from birth certificate data. However, the model still performed well using the alternative, although unvalidated, sPTB outcome. Other commonly used screening measures, such as cervical length or fetal fibronectin measurements were unavailable for assessment in combination with this predictive model.
The use of serum markers for risk prediction in this study deserves further comment. Four serum screening measures remained significant predictors even after controlling for maternal characteristics. This study confirmed that increased levels of cholesterol measured during the first trimester of pregnancy confer increased risk of PTB. However, the U-shaped relationship reported in the past was not observed. This could potentially be due to the different study methods. In predictive studies such as this, the goal is not to isolate the individual association of cholesterol and PTB like previous reports.8,9 Instead it is to see which risk factors, in the entire relevant population, give the best predictive ability. In this cohort, first trimester cholesterol as a continuous risk factor was the best predictor. In addition, a smaller change in cholesterol, from first to second trimester, during pregnancy was identified as a potentially new risk and area of interest. There exists a plausible biological link for low cholesterol change being related to PTB. Progesterone, a hormone essential to pregnancy maintenance, and cholesterol levels both rise throughout pregnancy21,22 and cholesterol is the precursor to progesterone synthesis.10 Further investigation into the role of cholesterol and progesterone interventions during pregnancy, especially in longitudinal studies, is warranted. The other serum markers, AFP and inhibin A, were utilized in a slightly different manner than previous reports. Several studies document that levels above 2.0 MoM of these analytes indicate an at-risk pregnancy.11,12,23 However, these papers typically treat measurements as dichotomous outcomes <2.0 vs. ≥2.0 MoM implying that a measurement of 3.0 or 4.0 MoM indicates equivalent risk to 2.0 MoM. Data from the current cohort indicate that the risk of PTB continued to increase as AFP and inhibin A increased even above 2.0 MoM and that a measurement of 2 MoM indicated greater risk than a measurement of 1 MoM. Treating these measures as continuous variables leads to a more complex model but with greater predictive ability and more accurate risk assessment. How cholesterol and other serum measures can be used in combination to predict PTB deserves further investigation.
The emphasis of this report has been on screening for PTB at the population level but, the presented model should not currently be put into practice. However, there is compelling evidence that with future research and validation a similar model may be useful for clinicians. It utilizes existing clinical and serum information that requires little or no additional testing and can be used in all singleton pregnancies. When compared directly to past PTB alone the model shows better sensitivity with fewer false positives. Also, if clinicians are willing to accept 90% specificity nearly 1/3 of PTBs can be identified. The reclassification statistics show that the addition of serum screening measures improves its performance over PTB and maternal characteristics alone. Unfortunately, a direct comparison with cervical length screening was not possible in this study. However, comparing the model to the performance of cervical length screening in past studies is instructive. There have been recommendations to perform cervical length screening on all pregnancies by 24 weeks gestational age and offer treatment to women with a cervical length ≤20mm.3 Iams et al. performed screening on women by 24 weeks and found that cervical length ≤20mm predicted PTB before 35 weeks gestational age with a sensitivity of 23% and a specificity of 97%.24 If the current model, based on data available before 24 weeks, is used to predict the outcome of PTB before 35 weeks it has a sensitivity of 25% with a specificity of 97%. A more recent cohort yields an additional comparison with cervical length assessment. Hassan et al. examined a cohort of 6877 births, 689 with PTB (<37 wks) and 6188 TB.25 There were 64 women with a cervical length of ≤20mm, 39 of which went on to have a PTB. This yields a sensitivity of 6% with a specificity above 99%. The current model has a sensitivity of 17% and specificity of 97% for PTB below 37 weeks. The model derived in the current report does exhibit low sensitivity (depending on the desired level of specificity) for a screening test. However, the sensitivity appears to be greater than other screening procedures (cervical length and past PTB) that are advocated at the population level. It also has the potential to work synergistically with cervical length screening to improve prediction beyond either method alone. Thus, this non-invasive screening tool should be of clinical interest, if, and only if, it can be validated by future studies.
This study is not unique in attempting to create a multi-marker test for PTB. For example, in a prospective study of women sampled during the 2nd trimester McLean et al. combined corticotropin-releasing hormone, AFP and clinical risk factors to predict PTB with 37% sensitivity, 95% specificity and 37% PPV. However, the cohort consisted of only 860 women and AFP was only tested in a subsection of controls making this effectively a case-control study.26 In another case-control study of mostly black women, with the outcome of birth <35 weeks GA, Goldenberg et al. used a collection of five tests to predict PTB. These tests included fetal fibronectin, cervical length, AFP, alkaline phosphatase and granulocyte colony-stimulating factor. Having 3 of 5 positive test results identified 15% of the women delivering before 35 weeks with no false positives.27 Finally, Vogel et al. used a multi-marker test consisting of serum TNF-alpha, cervicovaginal sIL-6R-alpha and cervical length to predict PTB (GA<35 weeks) in 62 women with a history of PTB. Using this test they reported: sensitivity, 69%, specificity, 95%, positive predictive value, 82%, and negative predictive value, 91%.28 The current study differs from past attempts in two important aspects. First, this study was a population-based prospective cohort study, not case-control, including low-risk women, leading to more generalizable estimates of model prediction characteristics. Second, the current study uses <37 weeks GA as the definition for PTB because the majority of PTBs occur between 35-36 weeks. However, this study agrees with previous findings; that developing a multi-marker test for PTB is possible. While the currently proposed test is promising in that it utilizes only readily available clinical and laboratory information it is not currently appropriate for clinical use. It will need to be validated in other populations and potentially improved with the addition of other clinically relevant variables.
Conclusions
Many individual risk factors have been identified for PTB. However, past PTB has remained one of the few predictors both easily available and used at the population level for identifying future PTB and a primary indication to offer treatment. This study shows that by combining several readily available risk markers a screening tool with better characteristics than PTB history alone can be created. Also, it compares favorably to past studies examining cervical length measurement as a screening tool. The model needs verification in separate populations and may be improved by adding other clinical variables. However, it shows that a low-cost, non-invasive screening tool may detect at-risk pregnancies. It is hoped that a similar validated screening measure may help to motivate further testing and possibly contribute to preventing future PTB.
Supplementary Material
Acknowledgements
The authors wish to thank Barbara Shirazi, Michelle Sexton and Stanton Berberich from the State Hygienic Laboratory at the University of Iowa; Donna Johnson and Joann Muldoon from the Iowa Department of Public Health; Val Stille, Jim Smith and Roger Williamson for all their assistance. The above acknowledged have no funding or conflicts of interest to disclose.
Financial Support: Bill & Melinda Gates Millennium Grant OPP52256 RSDP 5K12 HD-000849-23 National Institutes of Health: R01 HD-57192, R01 HD-52953 University of Iowa MSTP: T32 5 GM007337 University of Iowa CTSA: 2 UL1 TR000442-06 March of Dimes: #6-FY11-261, #11-FY10-180
Footnotes
Disclosure: None of the authors have a conflict of interest
Condensation of the paper: A predictive model for preterm birth is proposed by combining clinical risk factors, routine serum screening analytes and cholesterol levels.
Contributor Information
Brandon W. ALLEMAN, Iowa City, Iowa, Department of Pediatrics, University of Iowa
Amanda R. SMITH, Iowa City, Iowa, Department of Pediatrics, University of Iowa
Heather M. BYERS, Iowa City, Iowa, Department of Obstetrics and Gynecology, University of Iowa
Bruce BEDELL, Iowa City, Iowa Department of Obstetrics and Gynecology, University of Iowa
Kelli K. RYCKMAN, Iowa City, Iowa, Department of Pediatrics, University of Iowa.
Jeffrey C. MURRAY, Iowa City, Iowa, Department of Pediatrics, University of Iowa
Kristi S. BOROWSKI, Iowa City, Iowa, Department of Obstetrics and Gynecology, University of Iowa
References
- 1.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The Lancet. 2012;6736(12):1–11. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berghella V. Universal Cervical Length Screening for Prediction and Prevention of Preterm Birth. Obstetrical & Gynecological Survey. 2012;67(10):653–657. doi: 10.1097/OGX.0b013e318270d5b2. [DOI] [PubMed] [Google Scholar]
- 4.Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. American Journal of Obstetrics & Gynecology. 2010;202(6):548.e1–8. doi: 10.1016/j.ajog.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iams JD, Berghella V. Care for women with prior preterm birth. American Journal of Obstetrics and Gynecology. 2010;203(2):89–100. doi: 10.1016/j.ajog.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. American Journal of Obstetrics and Gynecology. 2012;206(2):124.e1–19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honest H, Forbes CA, Durée KH, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technology Assessment. 2009;13(43):1–627. doi: 10.3310/hta13430. [DOI] [PubMed] [Google Scholar]
- 8.Edison RJ, Berg K, Remaley A, et al. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007;120(4):723–33. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 9.Catov JM, Ness RB, Wellons MF, Jacobs DR, Roberts JM, Gunderson EP. Prepregnancy lipids related to preterm birth risk: the coronary artery risk development in young adults study. Clinical Endocrinology. 2010;95(8):3711–8. doi: 10.1210/jc.2009-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26(4):273–81. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Dugoff L, Hobbins JC, Malone FD, et al. Quad Screen as a Predictor of Adverse Pregnancy Outcome. Obstetrics & Gynecology. 2005;106(2):260–267. doi: 10.1097/01.AOG.0000172419.37410.eb. [DOI] [PubMed] [Google Scholar]
- 12.Yuan W, Chen L, Bernal AL. Is elevated maternal serum alpha-fetoprotein in the second trimester of pregnancy associated with increased preterm birth risk? A systematic review and meta-analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2009;145(1):57–64. doi: 10.1016/j.ejogrb.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 13.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 14.Pencina MJ, Agostino (I) RBD, Agostino (II) RBD, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in Medicine. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kundu S, Aulchenko YS, Van Duijn CM, Janssens a CJW. PredictABEL: an R package for the assessment of risk prediction models. European Journal of Epidemiology. 2011;26(4):261–4. doi: 10.1007/s10654-011-9567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12(1):77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Development Core Team R: A Language and Environment for Statistical Computing. 2011.
- 19.Iowa Environmental Public Health Tracking Network Nationally Consistent Data Measures for Vital Statistics: Premature Births. 2012. pp. 1–8.
- 20.DiGiuseppe DL, Aron DC, Ranbom L, Harper DL, Rosenthal GE. Reliability of birth certificate data: a multi-hospital comparison to medical records information. Maternal and Child Health Journal. 2002;6(3):169–79. doi: 10.1023/a:1019726112597. [DOI] [PubMed] [Google Scholar]
- 21.Diareme M, Karkalousos P, Theodoropoulos G, Strouzas S, Lazanas N. Lipid Profile of Healthy Women During Normal Pregnancy. Journal of Medical Biochemistry. 2009;28(3):152–160. [Google Scholar]
- 22.O’Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal Assessment of Changes in Reproductive Hormones during Normal Pregnancy. Clinical Chemistry. 1991;37(5):667–672. [PubMed] [Google Scholar]
- 23.Conde-Agudelo A, Papageorghiou a T, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG. 2011;118(9):1042–54. doi: 10.1111/j.1471-0528.2011.02923.x. [DOI] [PubMed] [Google Scholar]
- 24.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. The New England Journal of Medicine. 1996;44(3):292–4. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 25.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length ≤15 mm have nearly a 50% risk of early spontaneous preterm delivery. American Journal of Obstetrics & Gynecology. 2000;182(6):1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 26.McLean M, Bisits A, Davies J, et al. Predicting risk of preterm delivery by second-trimester measurement of maternal plasma corticotropin-releasing hormone and alpha-fetoprotein concentrations. American Journal of Obstetrics & Gynecology. 1999;181(1):207–15. doi: 10.1016/s0002-9378(99)70461-8. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg RL, Iams JD, Mercer BM, et al. The Preterm Prediction Study: toward a multiple-marker test for spontaneous preterm birth. American Journal of Obstetrics & Gynecology. 2001;185(3):643–51. doi: 10.1067/mob.2001.116752. [DOI] [PubMed] [Google Scholar]
- 28.Vogel I, Goepfert AR, Thorsen P, et al. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. Journal of Reproductive Immunology. 2007;75(2):133–40. doi: 10.1016/j.jri.2007.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


