Abstract
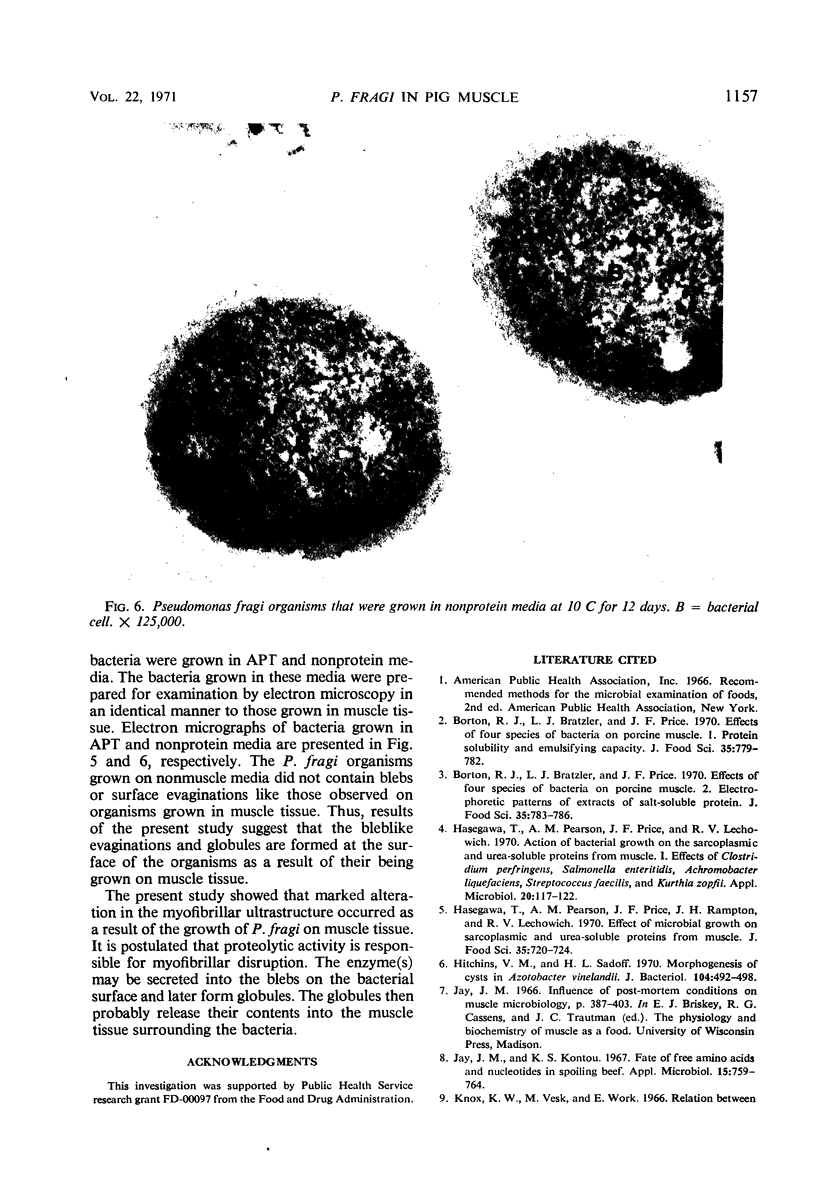
Myofibrils from pig muscle inoculated and incubated with Pseudomonas fragi showed an extremely disrupted appearance as compared to uninoculated controls. There was an almost complete absence of material in the H zone, marked disruption of the A band (probably myosin), and some loss of dense material from the Z line. These changes indicated that marked proteolysis had occurred. Bacteria observed in spoiled muscle tissue exhibited protrusions or blebs on the outer surface of the cell walls. The blebs appeared to form detached globules that migrated into the muscle mass. Bacteria grown in non-muscle-containing media did not produce blebs, which indicates the blebs were induced by growth on muscle tissue. The possibility that the blebs and globules may contain a proteolytic enzyme responsible for myofibrillar disruption is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hasegawa T., Pearson A. M., Price J. F., Lechowich R. V. Action of bacterial growth on the sarcoplasmic and urea-soluble proteins from muscle. I. Effects of Clostridium perfringens, Salmonella enteritidis, Achromobacter liquefaciens, Streptococcus faecalis, and Kurthia zopfi. Appl Microbiol. 1970 Jul;20(1):117–122. doi: 10.1128/am.20.1.117-122.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins V. M., Sadoff H. L. Morphogenesis of cysts in Azotobacter vinelandii. J Bacteriol. 1970 Oct;104(1):492–498. doi: 10.1128/jb.104.1.492-498.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay J. M., Kontou K. S. Fate of free amino acids and nucleotides in spoiling beef. Appl Microbiol. 1967 Jul;15(4):759–764. doi: 10.1128/am.15.4.759-764.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant P. J., Pearson A. M., Price J. F., Lechowich R. V. Action of Pseudomonas fragi on the proteins of pig muscle. Appl Microbiol. 1971 Aug;22(2):224–228. doi: 10.1128/am.22.2.224-228.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe W. J., Chapman G. B. Fine structure of selected marine pseudomonads and achromobacters. J Bacteriol. 1968 May;95(5):1862–1873. doi: 10.1128/jb.95.5.1862-1873.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe W. J., Chapman G. B. Variation in the fine structure of a marine achromobacter and a marine pseudomonad grown under selected nutritional and temperature regimes. J Bacteriol. 1968 May;95(5):1874–1886. doi: 10.1128/jb.95.5.1874-1886.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]








