Abstract
The Gadd45 proteins have been intensively studied, in view of their important role in key cellular processes. Indeed, the Gadd45 proteins stand at the crossroad of the cell fates by controlling the balance between cell (DNA) repair, eliminating (apoptosis) or preventing the expansion of potentially dangerous cells (cell cycle arrest, cellular senescence), and maintaining the stem cell pool. However, the biogerontological aspects have not thus far received sufficient attention. Here we analyzed the pathways and modes of action by which Gadd45 members are involved in aging, longevity and age-related diseases. Because of their pleiotropic action, a decreased inducibility of Gadd45 members may have far-reaching consequences including genome instability, accumulation of DNA damage, and disorders in cellular homeostasis – all of which may eventually contribute to the aging process and age-related disorders (promotion of tumorigenesis, immune disorders, insulin resistance and reduced responsiveness to stress). Most recently, the dGadd45 gene has been identified as a longevity regulator in Drosophila. Although further wide-scale research is warranted, it is becoming increasingly clear that Gadd45s are highly relevant to aging, age-related diseases (ARDs) and to the control of life span, suggesting them as potential therapeutic targets in ARDs and pro-longevity interventions.
Keywords: Gadd45 genes and proteins, Regulatory networks, Aging, Age-related diseases, Longevity
1. Introduction
A decreased ability to cope with stress is one of the hallmarks of aging. Resistance to stress is one of the important determinants of animal survival and longevity (Jazwinski, 1998). Indeed, within a wide variety of species, individuals or strains with longer life spans generally demonstrate a higher resistance to environmental and/or physiological stress and vice versa (Sohal et al., 1990; Lithgow et al., 1995; Murakami and Johnson, 1996; Lin et al., 1998; Guarente and Kenyon, 2000; Fabrizio et al., 2001; Landis et al., 2003; Brown-Borg, 2006; Masse et al., 2008; Ungvari et al., 2008; Labinskyy et al., 2009; Perez et al., 2009; Amrit et al., 2010; Slack et al., 2010). For example, selection for stress resistance in Drosophila increases its life span (Rose et al., 1992; Harshman et al., 1999). The ability to resist oxidative stress was used as a major criterion for identifying long-lived worm mutants in a genome-wide scale RNAi screen for new longevity regulators (Kim and Sun, 2007). Mutations which contribute to a longer life span in yeast (Saccharomyces cerevisiae), worms (Caenorhabditis elegans), flies (Drosophila melanogaster), and mice (Mus musculus) are generally accompanied by increased resistance to starvation, oxidative stress, and heat shock (Migliaccio et al., 1999; Fabrizio et al., 2001; Johnson et al., 2001; Longo, 2003; Rea et al., 2005; Perez et al., 2009). On the other hand, the short-lived mutants of various model organisms show a reduced capacity to cope with unfavorable environmental insults (Vermeulen et al., 2005). In many cases, overexpression of stress-related proteins (e.g., sirtuins, FOXO, HSP70, HSP22, superoxide dismutase, catalase) in certain tissues results in a longer life span of model organisms (Saunders and Verdin, 2009). Reduced resistance to stress was suggested to be both a cause and an effect of aging (Pandolf, 1997; Ikeyama et al., 2002). Further strengthening the links between stress and longevity is the observation that moderate exposures to harmful factors such as thermal or oxidative stress, ionizing radiation, and hypergravity can stimulate protective mechanisms and eventually lead to increased longevity (“longevity hormesis”) (Crawford and Davies, 1994; Moskalev, 2007; Moskalev et al., 2009; Rattan et al., 2009; Saunders and Verdin, 2009; Rattan, 2010).
Among the stress-associated genes, the members of the Gadd45 family play an important role in the integration of cellular response to a wide variety of stressors in mammals (Liebermann and Hoffman, 1998; Zhang et al., 1999; Fornace et al., 2002). At basal conditions, the expression levels of the Gadd45 family members are relatively low, but they are highly inducible upon a wide plethora of stressful stimuli, both physiological and environmental. This is summarized in Table 1. Of note, the median half-life of the Gadd45 mRNA is unusually short (less than 1 h), suggesting a regulatory rather than metabolic function for Gadd45 proteins (Sharova et al., 2009). Genotoxic and oxidative stress can rapidly induce their transcription (Fornace et al., 1989; Papathanasiou et al., 1991) and increase the mRNA stability of the Gadd45α (Jackman et al., 1994). Expression of Gadd45α is stimulated by physical stress such as UV-radiation (Fornace et al., 1988), X-rays (Papathanasiou et al., 1991), γ-radiation (Papathanasiou et al., 1991; Gajdusek et al., 2001), low-frequency electromagnetic fields (Nikolova et al., 2005), as well as by hypoxia (Price and Calderwood, 1992), peroxynitrite free radicals (Oh-Hashi et al., 2001), hyperosmotic (Drews-Elger et al., 2009) and oncogenic stress (Bulavin et al., 2003), low pH (Duggan et al., 2006), arachidonic acid metabolite and growth inhibitor Delta12-prostaglandin J2 (Ohtani-Fujita et al., 1998), a component of the C5b-9 complement system (Pippin et al., 2003), xenobiotics such as arsenic (Bower et al., 2006), Cr(VI) compounds (Ceryak et al., 2004), cisplatinum (DeHaan et al., 2001), alkylating agent methyl methane sulfonate (Zhang et al., 2001), ethanol (Ji et al., 2005), cigarette smoke condensate (Fields et al., 2005), and many other soil, air, and water pollutants (Sen et al., 2007). Different inducers of oxidative stress including sodium arsenite, carbon tetrachloride, bacterial lipopolysaccharides, inflammatory cytokines IL-6, IL-12 and IL-18, pro-apoptotic cytokines TGFβ and TNFα stimulate the expression of Gadd45β (Selvakumaran et al., 1994; De Smaele et al., 2001; Lu et al., 2001; Yang et al., 2001; Takekawa et al., 2002; Amanullah et al., 2003; Yoo et al., 2003; Zhang et al., 2005). Gadd45γ is induced by bacterial lipopolysaccharides that trigger inflammation, and also in response to pro-inflammatory cytokines IL-2 and IL-6 (Zhang et al., 1999; Altemeier et al., 2005).
Table 1.
Induction of Gadd45 expression by various stresses.
| Stress | Model | Gadd45 member | Reference |
|---|---|---|---|
| UV-radiation | Chinese hamster cells | Gadd45α | Fornace et al. (1988) |
| HPV-immortalized oral keratinocytes | Gadd45α | Gujuluva et al. (1994) | |
| HPV-positive cervical cancer cell lines | Gadd45α | Butz et al. (1999) | |
| Human skin keratinocytes and human skin in vivo | Gadd45α | Wan et al. (2000) | |
| M1 myeloblastic leukemia and H1299 lung carcinoma cells | Gadd45α/β/γ | Zhang et al. (2001) | |
| Human keratinocytes | Gaddd45α Gadd45α/β |
Maeda et al. (2003) Thyss et al. (2005) |
|
| Human Caucasian melanocytes | Gaddd45α | Marrot et al. (2005) | |
| X-rays | Human lymphoblast and fibroblast lines | Gadd45α | Papathanasiou et al. (1991) |
| Rat seminiferous tubules | Gadd45α | West et al. (2002) | |
| α-Radiation | HPV-positive cervical cancer cell lines | Gadd45α | Butz et al. (1999) |
| Aortic endothelial cells | Gadd45α | Gajdusek et al. (2001) | |
| M1 myeloblastic leukemia and H1299 lung carcinoma cell lines | Gadd45α/β/γ | Zhang et al. (2001) | |
| ML-1 human myeloid tumor cell line | Gadd45α | Fornace et al. (2002) | |
| Low-frequency electromagnetic fields | Mouse embryonic stem cells | Gadd45α | Nikolova et al. (2005) |
| Hypoxia | NIH-3T3 cells | Gadd45α | Price and Calderwood (1992) |
| H460 human lung cancer cell line | Gadd45α | Corn and El-Deiry (2007) | |
| Peroxynitrite free radicals | SH-SY5Y human neuroblastoma cells | Gadd45α | Oh-Hashi et al. (2001) |
| Hyperosmotic stress | Mouse inner medullary collecting duct cells | Gadd45α Gadd45α/β/γ |
Kültz et al. (1998) Chakravarty et al. (2002) |
| RKO cells | Gadd45α/β | Zumbrun et al. (2009) | |
| Mouse NFAT5−/− T lymphocytes | Gadd45α | Drews-Elger et al. (2009) | |
| Oncogenic stress | Mouse embryo fibroblasts | Gadd45α | Bulavin et al. (2003) |
| Low pH | OE-33 oesophageal cells | Gadd45α | Duggan et al. (2006) |
| Delta12-prostaglandin J2 | HeLa cells | Gadd45α | Ohtani-Fujita et al. (1998) |
| Component of the C5b-9 complement system | Rat podocytes in vitro and differentiated postmitotic mouse podocytes in vivo | Gadd45α | Pippin et al. (2003) |
| Arsenic As(III) | Offspring of DBA2 female with C57BL/6 male mice | Gadd45α | Simeonova et al. (2000) |
| BEAS-2B human bronchial epithelial cell line | Gadd45α |
Chen et al. (2001) Bower et al. (2006) |
|
| Male 129/Sv mice | Gadd45α | Liu et al. (2001) | |
| MiaPaCa2 and PANC-1 cells | Gadd45α | Li et al. (2003) | |
| AsPC-1 human pancreatic, HT-29colonic, and MCF-7 breast cancer cells | Gadd45α | Li et al. (2004) | |
| Sodium arsenite | CT7 mouse BAC clone | Gadd45β | Zhang et al. (2005) |
| Chromium Cr(VI) | Human lung fibroblasts | Gadd45α | Ceryak et al. (2004) |
| Cisplatin | Human ovarian cancer cell lines | Gadd45α |
De Feudis et al. (1997) Delmastro et al. (1997) |
| HPV-positive cervical cancer cell lines | Gadd45α | Butz et al. (1999) | |
| Methyl methanesulfonate | Human lymphoblast and human fibroblast lines | Gadd45α | Papathanasiou et al. (1991) |
| M1 myeloblastic leukemia and H1299 lung carcinoma cell lines | Gadd45α/β/γ | Zhang et al. (2001) | |
| Ethanol | Wild-type mice | Gadd45α | Ji et al. (2005) |
| Cigarette smoke condensate | MCF10A breast epithelial cell line | Gadd45α | Narayan et al. (2004) |
| NHBE cells | Gadd45α | Fields et al. (2005) | |
| Mitomycin C | HPV-positive cervical cancer cell lines | Gadd45α | Butz et al. (1999) |
| Bacterial lipopolysaccharides | CT7 mouse BAC clone | Gadd45β | Zhang et al. (2005) |
| C57BL/6 male mice | Gadd45γ | Altemeier et al. (2005) | |
| Pro-inflammatory cytokines IL-6, IL-12, and IL-18; | Th1cells | Gadd45β Gadd45β |
Yang et al. (2001) Du et al. (2008) |
| IL-2 and IL-6 | M1 mouse myeloid leukemia cell line and M1p53 cell line | Gadd45γ | Zhang et al. (1999) |
| Pro-apoptotic cytokines TGFβ and TNFα | M1 myeloblastic leukemic cells | Gadd45β | Selvakumaran et al. (1994) |
| HeLa, COS-7 and C2C12 cells | Gadd45β | Takekawa et al. (2002) | |
| AML12 mouse hepatocytes | Gadd45β | Yoo et al. (2003) | |
| Th1 cells | Gadd45β | Du et al. (2008) | |
| CT7 mouse BAC clone | Gadd45β | Zhang et al. (2005) |
The Gadd45 proteins are implicated in many basic processes shown to be intimately linked to aging and age-related diseases (ARDs), including DNA repair (Smith et al., 1994; Vairapandi et al., 1996), maintaining genome stability (Hollander et al., 1999), epigenetic regulation (Muñoz-Najar and Sedivy, 2011), cell cycle arrest (Beadling et al., 1993; Hollander et al., 1999; Wang et al., 1999; Zhang et al., 1999), cellular senescence (Tront et al., 2006), apoptosis (Harkin et al., 1999; Vairapandi et al., 2000; Azam et al., 2001; Takekawa et al., 2002; Yoo et al., 2003), cell survival (Smith et al., 1996; De Smaele et al., 2001; Zazzeroni et al., 2003; Papa et al., 2004; Gupta et al., 2005), inflammatory responses and immunity (Lu et al., 2001, 2004; Yang et al., 2001), and embryogenesis (Hoffman and Liebermann, 2009). Recently, we have shown for the first time that overexpression of the dGadd45 gene in the nervous system of Drosophila leads to the extension of the maximum life span, without compromising such life quality factors as physical activity and female fertility (Plyusnina et al., 2011). Given that longevity-associated genes are also deeply involved in major ARDs and aging-associated conditions (Budovsky et al., 2009; Wolfson et al., 2009; Tacutu et al., 2010), studying the mammalian Gadd45 family may provide potential therapeutic targets for combating ARDs and promoting longevity.
Our knowledge regarding the impact of Gadd45 proteins on ARDs and aging-associated conditions has not yet been systematically reviewed. With this in mind, we have undertaken extensive analysis of relevant literature and using the systems biology tools highlighted the Gadd45-associated pathways relevant to aging, longevity and ARDs.
2. Gadd45 family: structure, partners, and evolutionary conservation
The Gadd45 abbreviated name stands for Growth Arrest and DNA Damage-inducible (Fornace et al., 1989; Papathanasiou et al., 1991). Currently, three mammalian genes of the Gadd45 family, Gadd45α, Gadd45β and Gadd45γ are known. The Gadd45α gene was discovered as a rapidly induced transcript in response to UV-irradiation of the Chinese hamster ovary cells (Fornace et al., 1988). Another gene of the Gadd45 family, Gadd45β (originally designated as MyD118) was cloned as a primary response gene in myeloid differentiation (Abdollahi et al., 1991). Gadd45γ was found in mice as the ortholog of the human CR6 gene encoding an acute phase response protein expressed upon the interleukin-2 stimulation (Zhang et al., 1999).
In humans, the Gadd45 genes are localized to three different chromosomes (chr 1, 19, and 9 for Gadd45α, Gadd45β, and Gadd45γ, respectively). Of four Gadd45α transcripts, only two are protein-coding. Gadd45β encodes for one protein and Gadd45γ produces three transcripts of which two are protein-coding. Distinct isoforms for Gadd45α and Gadd45γ are produced through alternative splicing (Flicek et al., 2011). It has not yet been established whether there is any functional difference between these isoforms.
The proteins of the Gadd45 family are relatively small (about 18 kDa), acidic (pH 4.0–4.2), with a high degree of homology among them (55–57% of identical amino acid sequence), and predominantly localized to the nucleus (Abdollahi et al., 1991; Zhang et al., 1999; Vairapandi et al., 2002). Gadd45 proteins are able to form homo- and hetero-dimers with other Gadd45 members, as well as oligomers, dimers being the predominant form (Kovalsky et al., 2001; Schrag et al., 2008). Each of the Gadd45 genes is expressed in multiple types of mammalian tissues including heart, brain, spleen, lungs, liver, skeletal muscles, kidneys, and testes (Zhang et al., 1999). Specifically, Gadd45γ is highly expressed in placenta.
Although the Gadd45 family members have much in common, they still differ in their regulation and function (Liebermann and Hoffman, 2003). This could be attributed to some diversity in the spectrum of binding transcription factors, 3D structure [resolved thus far for Gadd45α (Sánchez et al., 2008) and Gadd45γ (Schrag et al., 2008)], and in interacting partners, described in Fig. 1. Until now, several regulatory elements have been found in the promoters of Gadd45 genes, including binding sites for transcription factors Egr1 (early growth response 1), WT1 (Wilms tumor 1), Oct-1 (POU2F1, POU class 2 homeobox 1), NFYA (nuclear transcription factor Y, alpha; a subunit of a trimeric complex which also contains the NFYB and NFYC), and NF-κB (Zhan et al., 1998; Jin et al., 2001; Fan et al., 2002; Daino et al., 2003). In addition, there are binding motifs in the third intron of Gadd45α for p53, AP-1 (a heterodimeric complex composed of c-Fos, c-Jun, ATF and JDP proteins), NF-AT, HNF-3 and KLF (Jin et al., 2001; Daino et al., 2003, 2006).
Fig. 1.
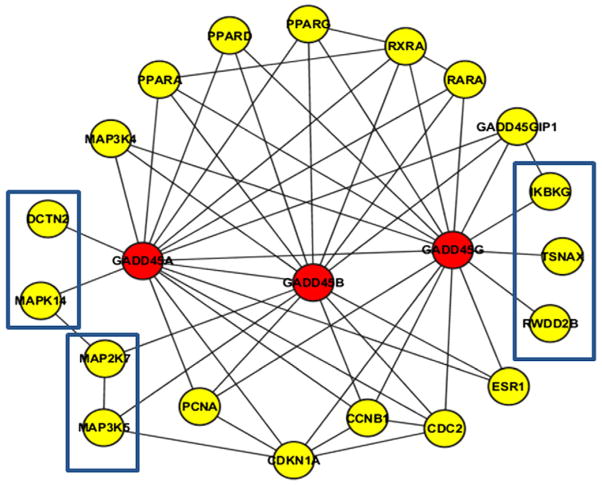
The protein–protein interaction network (PPI) of Gadd45α, β and γ with their first-order partners. The PPI data were extracted from the BioGRID database (Stark et al., 2011; http://thebiogrid.org), human interactome release 3.1.71, using the YABNA (Yet Another Biological Networks Analyzer) software program, previously described (Tacutu et al., 2010; http://www.netage-project.org). The graphical output was generated using Cytoscape 2.8.0 (Shannon et al., 2003; http://www.cytoscape.org/). Note that the vast majority of Gadd45 partners form connections with all three Gadd45 proteins. Nodes enclosed in rectangles – unique partners of Gadd45α, β or γ. For the details on gene/protein abbreviations, see Appendix B.
Gadd45 proteins contain the highly conserved L7Ae/L30e/S12e/Gadd45 domain, found in archaea, eubacteria and eukaryota. This domain confers the RNA binding properties and characteristics of a ribonucleoprotein particle to Gadd45α (Sytnikova et al., 2011). Our analysis using the InParanoid database (Ostlund et al., 2010) showed that Gadd45 orthologs first appeared in mollusks, and was also found in anemones, polychaete worms, insects, fish, amphibians and mammals. The number of Gadd45 proteins in each species varies from one in lower organisms to 5–6 in fish, and decreases to 2–3 in amphibians and mammals. This indicates that the Gadd45 family is relatively “young” and has undergone duplications and deletions in the course of evolution.
3. Gadd45 involvement in stress responses and maintaining the cellular homeostasis
3.1. GADD45 in DNA repair and epigenetic regulation
As mentioned above (see Table 1), the genotoxic stress of various natures could induce the transcriptional activity of Gadd45 genes, thus evoking Gadd45-mediated DNA repair. Several tumor suppressors involved in DNA repair and the FOXO transcription factors are particularly important in linking the stressful signals with Gadd45 repair activity. It is important to stress that since Gadd45 proteins are non-enzymatic, all their actions are realized through the interactions with either their protein partners or modulation of DNA/RNA accessibility to other proteins.
One of the central DNA damage sensors – p53, a tumor suppressor protein which regulates cell cycle, DNA repair and apoptosis, is involved in the genotoxic stress-induced upregulation of Gadd45α (Kastan et al., 1992; Guillouf et al., 1995; Vairapandi et al., 1996; el-Deiry, 1998). For example, upon benzene treatment, the mouse bone marrow p53+/+ cells express more Gadd45α than the p53+/−cells (Boley et al., 2002). Further supporting the role of p53 in the regulation of Gadd45 expression is that the Gadd45-mediated cell cycle arrest is not observed in the Li-Fraumeni fibroblast culture bearing p53 gene mutation (Wang et al., 1999) (see also Section 4). Another tumor suppressor protein p33 (ING1) which along with p53 stimulates DNA repair, was also shown to interact with Gadd45α (Cheung et al., 2001). The member of p53 family p73 protein is capable of stimulating Gadd45α expression as well (De Laurenzi and Melino, 2000). The tumor suppressor BRCA1 was shown to mediate the Gadd45α upregulation triggered by ionizing radiation (MacLachlan et al., 2000).
Another important group of regulators that evoke Gadd45-mediated DNA repair activity are the transcription factors of the FOXO family. The FOXO proteins regulate many mammalian genes associated with longevity and resistance to various types of stress including oxidative stress (Sedding, 2008). The Gadd45 gene promoter contains FOXO-binding motifs and activation of AFX/FOXO4 and FKHRL1/FOXO3A transcription factors upon oxidative stress results in Gadd45 upregulation with subsequent stimulation of DNA repair and blockage of the transition between G2/M cell cycle phases (Furukawa-Hibi et al., 2002; Tran et al., 2002). In contrast, the disrupted expression of Gadd45β caused by the hepatitis C viral infection suppresses the DNA excision repair (Higgs et al., 2010).
While it is not completely clear how Gadd45γ participates in the repair of DNA, there is strong evidence indicating an important role of Gadd45α and Gadd45β in DNA excision repair through their interaction with the proliferating cell nuclear antigen (PCNA), one of the key molecules in cell cycle regulation (Smith et al., 1994, 2000) and in the stabilization of DNA replication and repair machinery (Essers et al., 2005). Specifically, PCNA stabilizes binding of DNA polymerase delta (involved in resynthesis of excised damaged DNA strands during DNA repair) to DNA. The PCNA proteins encircles DNA and forms a sliding clamp that holds the DNA replication and repair complexes close to DNA (Ellison and Stillman, 2003).
Strongly supporting the role of Gadd45 in DNA repair are studies on the Gadd45a−/− mice which were found to exhibit genomic instability, reduced nucleotide excision repair, an increased level of mutations and high susceptibility to chemically induced tumorigenesis (Hollander et al., 1999, 2001) (this mouse model is also discussed in Section 4.1. The list with description of phenotypic features of Gadd45 knockouts is presented in Table 2).
Table 2.
Phenotypic characteristics of Gadd45 knockout mice.
| Targeted Gadd45 member | Observed phenotype | Reference |
|---|---|---|
| Gadd45α−/− | Genomic instability, abnormal nucleotide- and base-excision DNA repair, increased sensitivity to carcinogenesis | Hollander et al. (1999, 2001), Tront et al. (2006), Maeda et al. (2005) |
| Impaired ability for cell differentiation and increased sensitivity to the induction of apoptosis | Gupta et al. (2006) | |
| Increased sensitivity to skin irradiation | Hildesheim et al. (2002) | |
| Premature death, auto-immunity | Salvador et al. (2002) | |
| Abnormal hematopoiesis | Salvador et al. (2002), Gupta et al. (2005) | |
| Depletion of stem cell pool; decreased clonogenic potential of myeloid progenitors | Hoffman and Liebermann (2007) | |
| Nervous, reproductive and renal disorders | Hollander et al. (1999), Salvador et al. (2002) | |
| Double KO: Gadd45α−/−, XPC−/− | Premature death and increased tumorigenesis | Hollander et al. (2005) |
| Double KO: Gadd45α−/−, Cdkn1a−/− | Premature death, auto-immunity, abnormal hematopoiesis, impaired homeostasis, renal disorders | Salvador et al. (2002) |
| Abnormal chromosome number, increased cell proliferation | Hollander et al. (1999) | |
| Decreased cellular sensitivity to ultraviolet irradiation, normal nucleotide-excision repair | Maeda et al. (2005) | |
| Double KO: Gadd45α−/−, Brca1−/− | Exencephaly at embryonic days 9.5–10.5, complete prenatal lethality | Wang et al. (2004) |
| Gadd45β−/− | Impaired immunity and anti-tumor immune response; increased susceptibility to ionizing radiation and chemical carcinogens | Ju et al. (2009), Lu et al. (2004) |
| Autoimmune conditions | Liu et al. (2005) | |
| Impaired ability for differentiation and increased sensitivity to the induction of apoptosis | Gupta et al. (2006) | |
| Abnormal hematopoiesis | Gupta et al. (2005) | |
| Depletion of stem cell pool; decreased clonogenic potential of myeloid progenitors | Hoffman and Liebermann (2007) | |
| Gadd45γ−/− | Impaired immunity | Lu et al. (2001), Lu et al. (2004) |
| Normal hematopoiesis and proliferative response to IL-2 | Hoffmeyer et al. (2001) | |
| Abnormal cell physiology | Cai et al. (2006) | |
| Double KO: Gadd45β−/−, Gadd45γ−/− | Impaired immunity and anti-tumor immune response; Autoimmune conditions |
Ju et al. (2009), Lu et al. (2004) Liu et al. (2005) |
| Triple KO: Gadd45α−/−, Gadd45β−/−, Gadd45γ−/− | Increased cell proliferation in the renal outer medullary cells | Cai et al. (2006) |
| Ras-over expressing mice: Gadd45α+/− or Gadd45α−/− | Ras-driven breast tumorigenesis is Gadd45α-dependent; Gadd45α inhibits the onset and growth of mammary tumors | Tront et al. (2006) |
Involvement of Gadd45 in DNA repair is directly connected to its ability to facilitate the physical approach of several proteins to DNA. This allows epigenetic activation of genes through the repair-mediated active DNA demethylation (Barreto et al., 2007; Rai et al., 2008; Sen et al., 2010; Cortellino et al., 2011). The key role of Gadd45 in linking DNA repair and epigenetic regulation is highlighted by the coupling of cytosine deamination with base and nucleotide excision repair (Ma et al., 2009; Schmitz et al., 2009). This coupling results in active DNA demethylation which is partially attributed to the ability of Gadd45 proteins to bind histones and modify accessibility of DNA on damaged chromatin (Carrier et al., 1999). Gadd45 was also shown to participate in chromatin decondensation (Carrier et al., 1999; Ma et al., 2009).
While the exact mechanisms of Gadd45-mediated demethylation remain unclear, they could involve nucleotide excision repair (NER) associated with the endonuclease activity of xeroderma pigmentosum group G (XPG). Specifically, 5-methylcytosine containing nucleotides could be recognized and removed through Gadd45–XPG complex, ultimately resulting in the demethylation of CpG dinucleotides (Ma et al., 2009; Schmitz et al., 2009; Le May et al., 2010; Schäfer et al., 2010). Of note, both Gadd45 and XPG are also involved in base excision repair (BER), which could be another DNA repair mechanism associated with removal of methylated DNA (Jung et al., 2007).
Recently, Gadd45α was shown to bind RNA, forming ribonucleoprotein particles (Sytnikova et al., 2011). Gadd45 was detected inside nuclear speckles which are sites of active transcription, RNA splicing and processing. Thus, Gadd45 could exert its epigenetic effects both through active DNA demethylation, chromatin remodeling and post-transcriptional RNA regulation, which are intimately linked to Gadd45-mediated DNA repair.
3.2. Gadd45 in cell cycle arrest and cellular senescence
The damaged cell often enters a cell cycle arrest in order to allow time for DNA repair. Transient delays of cell division occur to prevent both replication of a damaged DNA template and segregation of damaged chromosomes. If the repair is successful, the cell cycle progresses. Otherwise, a non-reparable damage results in the prolonged or permanent cell cycle arrest (cellular senescence) or in apoptosis. Gadd45 is essential for a DNA damage-induced G2/M cell cycle arrest in both human and mouse fibroblasts (Wang et al., 1999). Microinjection of the Gadd45α expression vector into human primary fibroblasts arrests the cells in G2/M phase (Wang et al., 1999). The same Gadd45γ-dependent effect was achieved by withdrawal of growth stimuli (serum deprivation) in HeLa cells (Zhang et al., 2001).
Ectopic expression of Gadd45α, Gadd45β or Gadd45γ in cancer cells (M1 human myeloblastic leukemia and H1299 lung carcinoma) leads to the accumulation of cells arrested in the G1 phase (Zhang et al., 2001). In contrast to the cells arrested in the G2/M phase, the above G1 arrested cells later underwent apoptosis (Zhang et al., 2001).
Gadd45 proteins achieve cycle arrest by physically interacting with several proteins including protein kinase cell division cycle 2 (Cdc2), cyclin B1, and p53-inducing proteins such as PCNA and cyclin-dependent kinase inhibitor p21 (Liebermann and Hoffman, 2003). The interaction of Gadd45 with the Cdc2/cyclin B1 kinase complex is responsible for the G2/M cell cycle arrest through dissociation of the kinase complex (Zhan et al., 1999; Zhang et al., 1999; Vairapandi et al., 2002) and inhibition of Cdc2 kinase activity. The interaction of Gadd45 with p21 may induce the G1 arrest (Xiong et al., 1993; Zhang et al., 1993; el-Deiry, 1998).
One of the possible outcomes of DNA damage is the commitment of cells to cellular senescence, an irreversible cell cycle arrest in the G1 phase with subsequent unresponsiveness to growth factors. Gadd45 proteins are intimately involved in this process. Cellular senescence in cultures of human fibroblasts is associated with a p53-dependent induction of Gadd45α (Jackson and Pereira-Smith, 2006). Of note, in senescent diploid fibroblasts, p53 predominantly binds to the promoters of p21 and Gadd45, the cell growth arrest genes, rather than to other p53 targets involved in regulation of apoptosis (such as TNFRSF10B, TNFRSF6, and PUMA) (Jackson and Pereira-Smith, 2006). This may in part explain the resistance of senescent cells to apoptosis.
Gadd45α is involved in cellular senescence induced by diverse factors (Hollander et al., 1999; Bulavin et al., 2003). For example, a significant increase in Gadd45α expression was observed upon stress-induced cellular senescence triggered by H2O2 (Duan et al., 2005). This process was also characterized by an increased expression of p21, involving mitochondrial dysfunction and generation of reactive oxygen species through the Gadd45/p38 MAPK/GRB2/TGFBR2/TGFβ signaling pathway, induced by prolonged activation of p21 as a result of the DNA damage (Passos et al., 2010).
3.3. Dual role of Gadd45 in apoptosis and cell survival
As mentioned above (Section 3.2), the excessive DNA damage beyond a certain threshold may trigger apoptosis. In the case of irreparable damage, Gadd45 proteins exert a pro-apoptotic function. For example, the Gadd45 proteins mediate the endoplasmic reticulum stress-induced apoptosis in mouse liver cells (Ji et al., 2005). Another example includes the ectopic expression of Gadd45 in human leukemic cells or in mouse hepatocytes, which triggers apoptosis via the TGFβ/MEKK4/p38/JNK pathway, whereas blockage of the Gadd45β expression suppresses the apoptosis induced by TGFβ (Selvakumaran et al., 1994; Yoo et al., 2003).
However, in the case of moderate DNA damage, the Gadd45α and Gadd45β proteins act as anti-apoptotic agents and, for example, increase hematopoietic cell survival under UV-irradiation or treatment with certain chemotherapeutic drugs (Gupta et al., 2005). Bone marrow cells obtained from Gadd45α−/− and Gadd45β−/− mice show an impaired ability for differentiation and increased sensitivity to the induction of apoptosis after being stimulated by cytokines (Gupta et al., 2006). Anti-apoptotic function of Gadd45 is realized through two different ways: via activation of the p38/NF-κB anti-apoptotic pathway by Gadd45α (Gupta et al., 2006) and inhibition of the MKK7/JNK pro-apoptotic pathway by Gadd45β (Papa et al., 2004; Tornatore et al., 2008). Consistent with the idea that interactions of Gadd45 proteins with PCNA may promote cell survival by enhancing DNA repair is the observation that Gadd45/PCNA complexes inhibit apoptosis (Vairapandi et al., 2000; Azam et al., 2001).
3.4. GADD45 in cell differentiation and maintenance of the quiescent stem cell pool
Gadd45 family members play an important role in cell differentiation, both during embryonic development and in the mature organism. Moreover, there is evidence that the expression of Gadd45 members may be cell type-specific, as was recently shown by Kaufmann et al. (2011) in mouse embryos.
In many cases, the cell differentiation is preceded by cell cycle arrest in G1 phase and is consistently accompanied by an increased expression of Gadd45 proteins. For example, Gadd45γ is a primary transcription target of Ascl1, a transcription factor involved in neuronal differentiation (Huang et al., 2010). The differentiation-promoting role of XGadd45γ during neurogenesis was also shown in Xenopus embryos (de la Calle-Mustienes et al., 2002).
In another study on zebrafish, it was shown that Gadd45β expression in the anterior presomitic mesoderm is required for somite segmentation (Kawahara et al., 2005). Gadd45β was also found to play an important role in the differentiation of chondrocytes. It was identified as an early response gene induced by the bone morphohenic protein BMP-2 via a Smad1/Runx2-dependent pathway (Ijiri et al., 2005). In turn, it was shown that during the neural development in chicken embryo, an increased expression of Gadd45s induced by retinoic acid, promotes proteasomal degradation of Smad1 (Sheng et al., 2010). Based on this, it is attractive to suggest the existence of a Gadd45-Smad1 regulatory loop.
Treatment of myeloid cells with IL-3, GM-CSF, G-CSF, or M-CSF cytokines known as important players in myeloid differentiation rapidly induces the expression of all three Gadd45 genes (Zhang et al., 1999). Furthermore, induction of the Gadd45 genes in response to various cytokines at the initial stage of myeloid differentiation suggests that Gadd45 proteins play a role in hematopoiesis (Abdollahi et al., 1991).
The Gadd45 molecules effect not only differentiation but also the maintenance of a pool of myeloid quiescent stem cells. Though the mechanisms underlying these effects are still unknown, Gadd45α or β deletion was shown to either directly suppress the quiescent stem cell population or lower the survival rate of progenitor cells, thus leading to the depletion of the stem cell compartment (Hoffman and Liebermann, 2007). Gadd45 deletion also affects clonogenic potential of myeloid progenitor cells (Hoffman and Liebermann, 2007).
A possible hint to the regulatory mechanism could be the observation that the Nucleus accumbens-1 (Nac1, or NAC-1) protein which is important for self-regeneration and pluripotency of embryonic stem cells, negatively regulates the expression of Gadd45γ-interacting protein 1 (Gadd45γ-ip1), thus preventing its suppressive activity towards Gadd45γ (Jinawath et al., 2009). All the accumulated data indicate that Gadd45 proteins are important for promoting cell differentiation and maintaining the stem cell pool.
3.5. Gadd45 in immunity
Apart from their activities at the cellular level, the Gadd45 proteins also contribute to organismal survival by modulating the immune responses. The Gadd45 proteins stimulate proliferation of T helper 1 (Th1) cells which belong to the acquired branch of the immune system (Yang et al., 2001). Besides, Gadd45β and Gadd45γ induce production of the IFNγ by Th1 cells, thus further boosting the activation of the immune system (Yang et al., 2001). The essential role of Gadd45 in elevating the level of IFNγ is clearly evidenced by the disruption of this process in Gadd45γ - (Lu et al., 2001) and Gadd45β-deficient mice (Ju et al., 2009). Also, the study on Gadd45β and Gadd45β/Gadd45γ double knockout mice revealed the crucial importance of Gadd45 members, and particularly Gadd45β, in Th1-mediated anti-tumor immune responses (Ju et al., 2009) and in regulating autoimmunity (Liu et al., 2005). Gadd45β has recently been identified as an immunological tolerance (anergy)-associated gene (Safford et al., 2005). Activation of its transcription by the Notch signaling associated protein Deltex 1 (DTX1) results in induction of the T cell-mediated anergy. On the other hand, deletion of DTX1 decreases (but not abolishes) the expression of Gadd45β and promotes the maturation of T cells, giving rise to enhanced production of antibodies and increased inflammation (Hsiao et al., 2009). Collectively, Gadd45 contributes to enhancing the immune surveillance of tumors and elimination of cells that might display autoimmune behavior.
3.6. Gadd45 in stress signaling
The involvement of Gadd45 in stress signaling is partly discussed in previous sections. This issue was intensively reviewed (for example, see: Gao et al., 2009; Yang et al., 2009) and its detailed discussion is beyond the scope of this paper. Here we would like to emphasize that the involvement of Gadd45 proteins in various cell activities (see Sections 3.1–3.5) depends on different combinations of interactions with their partners (see Figs. 1 and 2). Nevertheless, the common basis of action for all three Gadd45 proteins lies in connecting an upstream sensor module (MAPK-associated cascade) to the downstream transcription regulatory module (NF-κB molecule). In fact, the MAPK/Gadd45/NF-κB axis responds to a variety of extracellular stimuli, converting them to intracellular responses. In most cases, in response to stress, the Gadd45 proteins stimulate the p38/JNK MAPK signaling pathway, thus increasing the sensitivity of cells to growth arrest, apoptosis or survival (Takekawa and Saito, 1998; Harkin et al., 1999; Lu et al., 2001; Hildesheim et al., 2002; Yoo et al., 2003). For example, Gadd45γ and Gadd45β bind to MEK kinase 4 (MEKK4) and promote phosphorylation and activation of the p38 and JNK MAP kinases by MEKK4 (Takekawa and Saito, 1998). The effect of Gadd45 on stress kinases is cell type-specific: activation of p38 and JUNK MAPKs by Gadd45 is associated with apoptosis in endothelial and epithelial cells (Harkin et al., 1999; Hildesheim et al., 2002), whereas it increases survival of hematopoietic cells (Platanias, 2003). The opposite effect of Gadd45β on JNK activity was observed in other types of cells: induction of Gadd45β by NF-κB downregulates pro-apoptotic JNK signaling in mouse embryonic fibroblasts (De Smaele et al., 2001) and in hepatocytes during liver regenerations after partial hepatectomy (Papa et al., 2008). Of note, Gadd45 proteins not only modulate the activity of stress kinases, but the expression of Gadd45 is also under the control from p38 and JUNK MAPKs. The latter is supported by the observation that the specific inhibitor of p38 MAPK SB202190 suppresses the expression of all three Gadd45 genes (Oh-Hashi et al., 2001). Thus, it seems plausible that Gadd45 and stress kinases form a feedback regulatory loop.
Fig. 2.
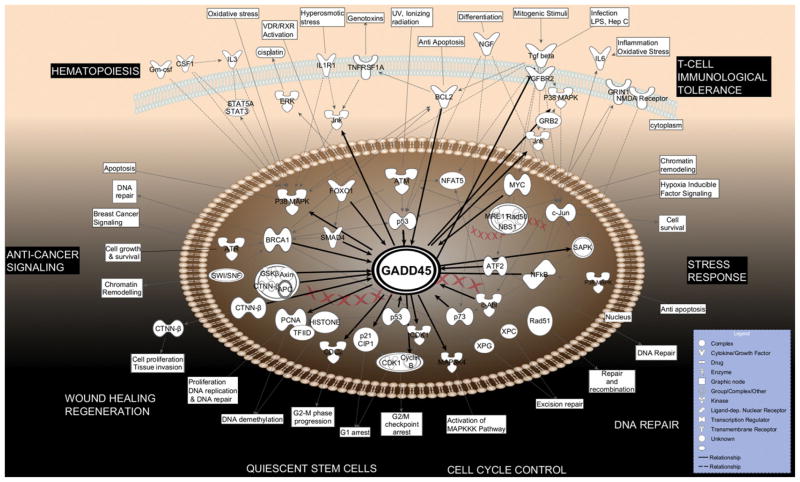
Map of molecular interactions involving GADD45 proteins and cell signaling pathways generated using Ingenuity Pathway Analysis software (IPA). Top functions identified by IPA were Cell Death, Cell Cycle and Cell Growth and Proliferation. Each of the node shapes denotes the function of the interacting protein or group of proteins. Molecular interactions are indicated by the lines between nodes. For the details on gene/protein abbreviations, see Appendix B.
Gadd45 is directly involved in modulating the cell protective effect of NF-κB, well known for its anti-apoptotic activity along with reduction in free radical formation (Zhang et al., 2005). Gadd45α stimulates degradation of the NF-κB inhibitor IκB, followed by relocalization of NF-κB into the nucleus and activation of its target genes (Gupta et al., 2006). Importantly, NF-κB can stimulate the expression of Gadd45 and simultaneously acts as its regulatory target, thus (till a certain threshold) creating a positive feedback regulatory loop. However, a constitutive activation of NF-κB decreases the expression of Gadd45α and Gadd45β (Zerbini et al., 2004).
As previously mentioned (Sections 3.1–3.5), Gadd45 family members are important regulators in several other pathways (p53, FOXO, TGFβ, Wnt, etc.), in addition to stress MAPKs/NF-κB pathway. All these pathways substantially overlap, and to a great extent shape the fate of the stressed cell.
4. Gadd45 in age-related pathologies and aging-associated conditions
4.1. Cancer
Aging is the major risk factor for cancer, and incidence of breast, prostate, colon, lung, stomach, bladder and skin cancer dramatically increases with age (Hoeijmakers, 2009). Aging-associated conditions such as oxidative stress, chronic inflammation and immunosenescence predispose to tumorigenesis (Chung et al., 2009).
The anti-tumor activity of Gadd45 was shown both in vitro and in vivo studies. For example, ectopic expression of Gadd45 members blocks cell growth by arresting the cells at the G2/M phase (Zhu et al., 2009) and/or induces apoptosis in several human tumor cell lines such as M1 myeloblastic leukemia, H1299 lung carcinoma, HeLa cervical cancer, RKO colon carcinoma, and in non-transformed NIH3T3 mouse embryonic fibroblasts (Zhan et al., 1994; Vairapandi et al., 1996; Zhang et al., 2001; Sun et al., 2003; Jiang and Wang, 2004; Ying et al., 2005). Also, Gadd45 was shown as being involved in fibroblast growth factor-2 (FGF-2)-induced differentiation of SK-N-MC human neuroblastoma cells via promoting the cell cycle arrest in G1/G0 phase (Higgins et al., 2009). The anti-cancer activity of Gadd45α is strongly supported by studies on the mouse knockout models. Mice with a Gadd45α gene deletion show genomic instability and increased sensitivity to carcinogenesis (Hollander et al., 1999; Tront et al., 2006). The Gadd45α knockout mice are also more prone to DMBA-induced ovarian tumors, hepatocellular tumors in males, and vascular tumors in both sexes (Hollander et al., 2001). The increased rate of tumorigenesis in these mice could be attributed to a very low efficiency of the thymidine dimer and nucleotide excision repair as already described in previous sections of this work (Hollander et al., 2001; Maeda et al., 2002). Mice with the Gadd45β gene knockout are more susceptible to ionizing radiation and chemical carcinogens and display a lower immune response against implanted melanoma cells (Ju et al., 2009). In the in vivo model of Ras-overexpressing mice which differ in the levels of Gadd45α expression (Ras/Gadd45α+/+, Ras/Gadd45α+/−, and Ras/Gadd45α−/−), it was shown that Ras-driven breast tumorigenesis is Gadd45α-dependent (Tront et al., 2006). The authors concluded that Gadd45α inhibits the onset and growth of mammary tumors via induction of JNK-activated apoptosis and p38-mediated cellular senescence (Tront et al., 2006). On the other hand, the loss of the Gadd45α gene accelerates the mammary tumor growth (Tront et al., 2006). These findings coincide well with the clinical observations that Gadd45α is frequently deleted in breast cancer (Hoggard et al., 1995). Furthermore, disruption in the Gadd45 expression was observed in multiple types of solid and hematopoietic malignancies including nasopharyngeal, breast, lung and prostate cancer, hepatocellular tumors, pituitary adenoma, and lymphoma (Qiu et al., 2003, 2004; Sun et al., 2003; Jiang and Wang, 2004; Ying et al., 2005; Cretu et al., 2009; Na et al., 2010).
The silencing of Gadd45 in cancers could be greatly attributed to epigenetic changes that typically occur with advanced age. In particular, promoters of many tumor suppressors were found to be hypermethylated in aging, with subsequent transcriptional gene silencing (Muñoz-Najar and Sedivy, 2011). A similar situation was observed in many cancers (Muñoz-Najar and Sedivy, 2011). Thus, this common epigenetic modification could be important in linking cancer and aging. With regard to this notion, the methylation of the Gadd45γ promoter was found to be significantly higher in different types of cancer than in normal tissues (Zhang et al., 2010). Nevertheless, treatment of cancer cells with DNA methyltransferase inhibitors can restore Gadd45β expression to its level in the non-tumorous cells (Qiu et al., 2004).
One of the mechanisms which could lead to the increased methylation of Gadd45 in aging may be attributed to a constitutive activation of NF-κB, a quite typical age-related condition. This transcription factor induces the expression of proto-oncogene c-Myc (Zerbini et al., 2004; Zerbini and Libermann, 2005), which binds to the GC-rich sites of the Gadd45 promoters and significantly reduces the Gadd45 expression in response to genotoxic stress (Amundson et al., 1998). It seems plausible that c-Myc-mediated repression of Gadd45 expression occurs through recruitment of DNA methyltransferase Dnmt3a, as was reported for p21 (Brenner et al., 2005). The inhibition of NF-κB in cancer cells leads to the Gadd45α- and γ-dependent induction of apoptosis and reduction in tumor growth (Zerbini et al., 2004), further supporting the role of NF-κB in the regulation of Gadd45 expression. The epigenetic coupling between cell death and the expression levels of Gadd45 is also exemplified by histone deacetylase inhibitors such as trichostatin and butyrate, which induce Gadd45α and Gadd45β expression and cause apoptosis in human carcinomas (Chen et al., 2002; Campanero et al., 2008).
Other examples connecting the Gadd45 expression levels to the induction of apoptosis in cancer cells include several pharmacological agents. A widely known drug Troglitazone which possesses the anti-cancer, anti-diabetic and anti-inflammatory activities inhibits proliferation and induces apoptosis in MCF7 human breast carcinoma cells (Yin et al., 2004). Remarkably, out of the 23 Troglitazone-induced genes, not only had Gadd45α the highest expression level, but its knockdown abrogated the Troglitazone-induced apoptosis (Yin et al., 2004). Stimulation of Gadd45 expression by arsenic trioxide (Li et al., 2003) or an anti-tumor flavonoid quercetin (Yoshida et al., 2005) also leads to apoptosis in cancer cells.
Apart from the “canonical” anti-cancer activity of Gadd45 proteins through the induction of apoptosis in cancer cells, Gadd45α was found to be an important negative regulator of two oncogenes commonly over-expressed in epithelial tumors, p63 and β-catenin (Hildesheim et al., 2004). Gadd45α prevents translocation of β-catenin (the effector molecule of Wnt signaling) into the nucleus where it functions as a transcription factor involved in the induction of cell proliferation. Among its targets are also endopeptidases and matrix metalloproteinases (MMP3 and MMP9). These enzymes are important players in the remodeling of extracellular matrix enabling the invasion and metastasis of cancer cells. Thus, not only are Gadd45s involved in killing the cancer cells, but they also suppress cancer progression by inhibiting the cell migration. The latter has recently been substantiated by a study on cancer cells with different migration potentials (Trabucco, 2010). The migration levels of the BL185 murine cell line with the stable shRNA Gadd45α knockdowns were significantly increased versus the parental non-metastasizing line. Furthermore, hepatocellular carcinoma (HCC) cell lines with high migration ability exhibited a reduced expression of Gadd45α.
In view of the established role of Wnt signaling and MMPs in epithelial-mesenchymal transition (EMT) (Przybylo and Radisky, 2007), its inhibition may be another putative mechanism by which Gadd45s may exert its tumor suppressor activity. This phenomenon is characterized by the downregulation of epithelial markers and loss of intercellular junctions with subsequent breakage of epithelial polarity, loss of contact inhibition, and acquisition of mesenchymal features that eventually contribute to cancer initiation and progression (Christiansen and Rajasekaran, 2006). This possible aspect of Gadd45 anti-tumor activity has not as yet been directly addressed.
In contrast to its well established anti-cancer role, in some cases, Gadd45α may exert an opposite action, depending on the type of the oncogenic stimuli. For example, while Ras-driven breast tumorigenesis is suppressed by Gadd45α, the Myc-driven breast cancer is promoted by Gadd45α (Tront et al., 2010). In the case of c-Myc-driven breast carcinogenesis, activation of Gadd45α results in a dramatic decrease in the level of MMP10, an enzyme promoting angiogenesis. Consequently, its inhibition by Gadd45α results in stimulation of tumor vascularization. In the presence of Myc, loss of Gadd45α was accompanied by activation of either apoptosis or cellular senescence, an outcome opposite to that observed in the case of Ras-driven breast tumorigenesis (Tront et al., 2010).
4.2. Other age-related diseases
While most of our knowledge with regard to the involvement of Gadd45 in the age-related pathologies comes from cancer studies, some evidence has been accumulated pointing to its role in other age-related conditions as well. For example, Gadd45 is highly expressed in neurons of Alzheimer’s disease patients and protects the neurons from apoptosis induced by extracellular accumulation of β-amyloid (Torp et al., 1998; Santiard-Baron et al., 1999, 2001). The upregulation of Gadd45 was also observed in the in vitro model (human neuroblastoma cells) of dopamine-induced neurotoxicity (Stokes et al., 2002). This observation could be relevant to the suggested role of ROS and reactive quinones, produced during dopamine oxidation, in pathogenesis of both Parkinson’s disease and normal brain aging (Stokes et al., 2002). An increased expression of Gadd45 in age-related neurodegeneration is likely a protective mechanism aimed at coping with the neurotoxic stress.
The same could also be the case in another major ARD, atherosclerosis. As was shown in primary cultures of human endothelial cells derived from atherosclerotic aorta or coronary arteries, activation of the lectin-like oxLDL receptor (LOX-1) leads to DNA damage and to a 4-fold increase in Gadd45 expression (Thum and Borlak, 2008). A comparable increase in Gadd45 transcription of aortic endothelial cells was also observed in a mouse model of atherosclerosis (ApoE knockout mice fed with a high lipid diet for >4 months) (Thum and Borlak, 2008).
The remarkable induction of Gadd45β by anti-diabetic drug Troglitazone (see Section 4.1) may point to the anti-diabetic potential of Gadd45 molecules. Indeed, an increased expression of Gadd45β may contribute to the amelioration of chronic insulin resistance, one of the major complications of diabetes. The underlying molecular mechanism could be linked to the Gadd45β-induced inhibition of TNFα cytotoxicity by suppressing TNFα-induced JNK signaling pathway, whose activation leads to chronic insulin resistance (Tuncman et al., 2006). Interestingly, insulin induces Gadd45β transcription by activating the mTOR pathway (Bortoff et al., 2010), well known for its association with aging, longevity, and ARDs (Blagosklonny, 2008; Zoncu et al., 2011).
There is direct and indirect evidence of the involvement of Gadd45 in major aging-associated conditions including oxidative stress, chronic inflammation, immunosenescence and fibroproliferative repair that, in turn, largely contribute to the development of ARDs and aging progression. The disbalance between pro- and anti-oxidant activities inclining towards elevated levels of oxidant species has long been considered a causative factor of aging. The age-related effects of oxidative stress on Gadd45 expression have not yet been fully addressed. It seems however that aging, at least in certain tissues, negatively affects the ability of cells to express Gadd45 proteins in response to oxidative stress. For example, when subjected to paraquat which promotes generation of superoxide radicals, cardiomyocytes of young mice increase expression of all three Gadd45s, whereas this does not occur in the myocardium of old animals (Edwards et al., 2004).
Another major condition underlying aging and ARDs is chronic inflammation which is largely attributed to an age-related increase in pro-inflammatory cytokines (TNFα, IL-1β, IL-6, etc.) and NF-κB (Finch, 1990; Chung et al., 2009). In view of the links between these pro-inflammatory pathways and Gadd45s (described in Sections 3.3 and 3.6), the involvement of Gadd45 family in chronic inflammation is highly plausible. As an example, the induction of Gadd45 was observed in the course of liver inflammation (Gant et al., 2003). An additional source of the pro-inflammatory molecules are senescent cells which are accumulated in aging (Coppé et al., 2010). To what extent Gadd45s contribute to this process remains to be explored.
Both oxidative stress and chronic inflammation contribute to a process known as immunosenescence, in which the defects in T cells play a major role (Cannizzo et al., 2011; Larbi et al., 2011). Immunosenescence manifests in a decreased immune responsiveness to foreign and self-antigens, leading to an increased susceptibility to infection, cancer and autoimmune diseases. A decreased ability to maintain tolerance against self-antigens may result in autoimmune disorders. Mice with deficiency in Gadd45β and Gadd45γ spontaneously develop signs of autoimmune lymphoproliferative syndrome and systemic lupus erythematosus (Liu et al., 2005). In view of the key role of Gadd45β and Gadd45γ in maintaining the immunological tolerance (see Section 3.5), the reduced inducibility of Gadd45s in immune cells may stand behind an increased frequency of autoimmune conditions in aging (Liu et al., 2005).
One of the few examples where increased levels of Gadd45 may sustain the age-related immune dysfunctions is rheumatoid arthritis, whose incidence increases with advanced age (Lindstrom and Robinson, 2010). It is known that infiltrated Th1 cells in the synovial fluid of patients with rheumatoid arthritis are resistant to apoptosis. This resistance could be partially explained by the high levels of Gadd45β found in Th1 cells of synovial fluid and be primarily attributed to the stimulation of Gadd45β expression by pro-inflammatory cytokines TNFα and IL-12 (Du et al., 2008). As a result, the activated Th1cells avoid removal from the inflamed joints, thus contributing to chronic inflammation and tissue destruction. The important role of Gadd45β in this process also follows from the finding that silencing of Gadd45β by RNA interference abolished the anti-apoptotic effect of rheumatoid arthritis synovial fluid.
Pro-inflammatory cytokines and ROS, whose levels progressively increase in aging, may induce EMT, in particular, through activation of transcription factor NF-κB (López-Novoa and Nieto, 2009). In fact, EMT is the convergence point between inflammation, oxidative stress and the progression of degenerative fibrotic diseases and cancer. As mentioned above (see Section 4.1), Gadd45 closely cooperates with NF-κB and other EMT key players, β-catenin and MMPs, and by this way may thus contribute to EMT and associated pathologies.
4.3. Gadd45 protein partners and ARDs
It has been reported thus far that Gadd45s directly interact with 19 proteins (depicted in Fig. 1, Section 2). The links between Gadd45s and ARDs may to a great extent be realized through the interactions of Gadd45s with their first-order protein partners and regulators. As shown in Table 3, most of Gadd45 partners have been established as being strongly involved in at least one ARD (17 Gadd45 partners are involved in cancer, 8 in atherosclerosis, 7 in diabetes, and 6 in Alzheimer’s disease), with several being involved in all or almost all major human ARDs. Important examples include the peroxisome proliferator-activated receptor family (PPARs) and CDKN1A (p21). The nuclear hormone receptor PPARγ has been implicated in the pathogenesis of numerous diseases including obesity, diabetes, atherosclerosis, Alzheimer’s disease and cancer (Zieleniak et al., 2008; Schmidt et al., 2010). PPARγ is activated by thiazolidinediones (TZDs), insulin-sensitizing agents used for the treatment of type 2 diabetes. These drugs have also been shown to induce apoptosis in the vascular muscle cells through the upregulation of Gadd45 by activated PPARγ, and by this way may reduce the formation of atherosclerotic lesions (Bruemmer et al., 2003). PPARγ possesses anti-aging and anti-inflammatory activities (Chung et al., 2008). To what extent they are mediated by Gadd45 remains a point for future investigations. The involvement of PPARγ in the regulation of NF-κB signaling (Cabrero et al., 2002), and interactions of Gadd45 with both of them strengthen this suggestion.
Table 3.
The involvement of Gadd45 direct proteins partners in age-related diseases.
| Genea | Cancer | Atherosclerosis | Diabetes | ADb | LAGsc |
|---|---|---|---|---|---|
| CCNB1 | + | ||||
| CDC2 | + | + | |||
| CDKN1A | + | + | + | + | |
| DCTN2 | + | ||||
| ESR1 | + | + | + | + | |
| GADD45GIP1 | + | ||||
| IKBKG | + | ||||
| MAP2K7 | + | ||||
| MAP3K4 | + | ||||
| MAP3K5 | + | + | |||
| MAPK14 | + | + | + | + | |
| PCNA | + | + | |||
| PPARA | + | + | + | + | |
| PPARD | + | + | + | ||
| PPARG | + | + | + | + | + |
| RARA | + | ||||
| RWDD2B | + | ||||
| RXRA | + | + | |||
| TSNAX |
For the details on gene/protein abbreviations, see Appendix B.
Alzheimer’s disease.
Longevity-associated genes were extracted from http://genomics.senescence.info/species/ (de Magalhaes et al., 2009) and http://netage-project.org/ (Tacutu et al., 2010).
Another example is CDKNA1 (p21). This important regulator of cell cycle is deeply involved in cellular senescence, cancer, atherosclerosis, and diabetes (Chang et al., 2000). Its interaction with Gadd45 was shown to inhibit cell growth and as such, could be part of p21-mediated tumor suppressor activity (Zhao et al., 2000).
Apart from direct protein–protein interactions, there are numerous regulatory links between Gadd45 and well known players in ARDs, such as p53, p38, JNK, FOXO, SIRT1, HDAC, and others (see previous sections and Fig. 3).
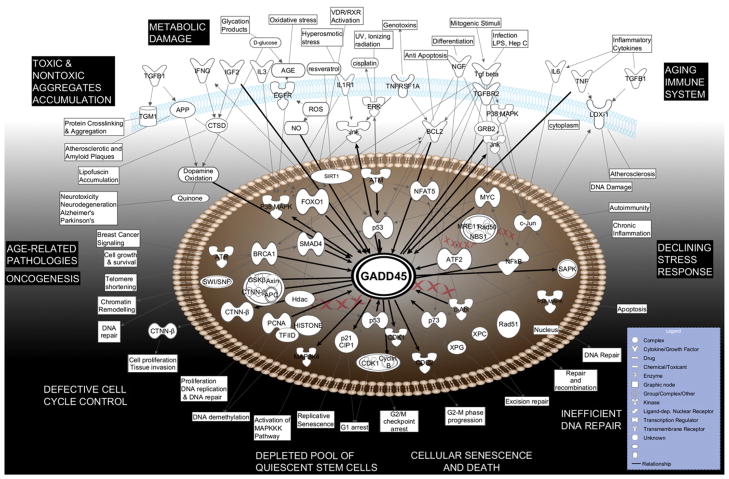
Fig. 3.
Map of molecular interactions of GADD45 proteins related to aging processes. Generated using Ingenuity Pathway Analysis software. Top functions and the node shapes as indicated in Fig. 2. For the details on gene/protein abbreviations, see Appendix B.
4.4. GADD45 and longevity
As mentioned in previous sections, among the Gadd45 partners are several well-known longevity regulators. Furthermore, Gadd45 is among the targets of a number of established longevity-associated genes (LAGs). An example is the regulation of Gadd45 expression by two well established LAGs, SIRT1 and FOXO (see also Section 3). Depletion of SIRT1 expression by RNA interference results in a marked inhibition of FOXO-mediated Gadd45 induction in response to oxidative stress (Kobayashi et al., 2005).
The links between LAGs and Gadd45s make them highly probable candidates for the control of life span. In view of its functions (described in previous sections), it seems reasonable that over-expression of Gadd45 might promote longevity, in particular, by increasing the efficiency of DNA repair. Indeed, an opposite situation caused by mutations in the DNA repair genes may lead to hereditary syndromes of premature aging (e.g., Werner syndrome) (Navarro et al., 2006).
With the above in mind, we have recently developed the transgenic fruit flies overexpressing dGadd45 in the nervous system, using the ELAV-Switch neuronal specific GAL4 driver (Plyusnina et al., 2011). By means of this model system, we have shown for the first time that the constitutive or conditional overexpression of dGadd45 in the nervous system increases median and maximum Drosophila life span, without affecting fertility or physical activity. The important point is that the dGadd45-induced life span extension was accompanied by a considerable decrease (by approximately 25%) in the number of single-stranded breaks in DNA of Drosophila larvae neuroblast, as compared to their wild-type counterparts (Plyusnina et al., 2011). It should also be noted that in a wild-type fruit flies (Canton-S line), dGadd45 undergoes dramatic downregulation during the second half of life (our unpublished data). Altogether, these finding suggests that the longevity-promoting effect of Gadd45 overexpression could be causatively related to a more efficient detection and correction of spontaneous DNA damage.
5. Concluding remarks
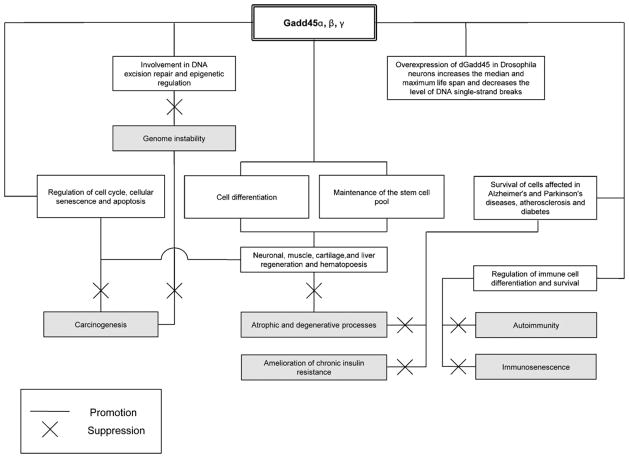
Because of their pleiotropic action, a decreased inducibility of Gadd45 members may have far-reaching consequences including genome instability, accumulation of DNA damage, and disorders in cellular homeostasis – all of which may eventually contribute to the aging process, promotion of tumorigenesis, immune disorders, insulin resistance and reduced responsiveness to stress (Fig. 4). Gadd45s stand at the crossroad of the cell fates by controlling the balance between cell (DNA) repair, eliminating (apoptosis) or preventing the expansion of potentially dangerous cells (cell cycle arrest, cellular senescence), and maintaining the stem cell pool. Furthermore, Gadd45s are unique in coupling the DNA repair to epigenetic regulation. An additional and still unexplored possibility, with potential relevance to aging and ARDs, is that Gadd45s could be involved in the regulation of cell polarity. Gadd45s contain RNA-binding domains and are part of ribonucleoprotein particles (Sytnikova et al., 2011) which are often involved and play an important role in polar processes (Herr et al., 2008; Mili and Macara, 2009). Our analysis showed that RNA-binding proteins are a significantly enriched category among the polarity- and longevity-associated genes (Budovsky et al., 2011).
Fig. 4.
Summary scheme: the main anti-aging and pro-longevity activities of Gadd45 family.
Despite the fact that the regulation of Gadd45s is much more complex in mammals than in lower organisms, our recent discovery that the dGadd45 gene is a longevity regulator in Drosophila (Plyusnina et al., 2011) offers hope that controlled manipulations of Gadd45s and its interacting partners may also bring benefits to humans. Remarkably, among the pro-longevity genes found in the lower model organisms that normally do not develop tumors, many are orthologous to human tumor suppressors (Budovsky et al., 2009). Given a strong association between cancer and aging, based on the common evolutionary and molecular mechanisms, it seems plausible that factors able to suppress cancer could also inhibit aging and vice verse (Blagosklonny, 2008). Thus, the anti-cancer activity of Gadd45s may presumably contribute to slowing or delaying the aging process. This could be also true with regard to another major human ARD – diabetes, as Gadd45β offers the potential of being a target for ameliorating the diabetes complications (Bortoff et al., 2010). Whether Gadd45s operate in the same manner in other ARDs and aging-associated conditions is still an unresolved issue and should be a point for future investigations.
It is important to note that Gadd45s are particularly suitable for manipulations as they are rapidly induced by various stressful stimuli. Furthermore, the moderate stimulation of cellular mechanisms of stress response often results in pro-longevity effects, even when being activated in the absence of the stress.
While Gadd45 family has been intensively studied, the biogerontological aspects have not as yet received a sufficient attention. Although further wide-scale research is warranted, it is becoming increasingly clear that Gadd45s are highly relevant to aging, ARDs and to the control of life span, suggesting them as potential therapeutic targets in ARDs and pro-longevity interventions.
Acknowledgments
This work was supported from the Presidium of the Russian Academy of Science, grant number 09-P-4-1021 (to A.A.M.) and by the European Commission FP7 Health Research, grant number HEALTH-F4-2008-202047 (to V.E.F.). We appreciate the assistance of Caroline Simon and Dmitri Taranukha in the preparation of this manuscript.
Appendix A. Construction and pathway analysis of GADD45-associated regulatory networks, with the focus on cell signaling and links to aging
To generate graphs of the Gadd45 family regulatory networks, we used the web-based bioinformatics software, Ingenuity Pathway Analysis (IPA, Ingenuity Systems, www.ingenuity.com). IPA is a curated database generated from more than 1.7 million published peer-reviewed scientific publications. Molecules are represented as nodes, and the biological relationship between two nodes is represented as an edge (line). All edges are supported by at least one reference from the literature or from canonical information stored in the Ingenuity Pathways Knowledge Base. Human, mouse, and rat orthologs of a gene are stored as separate objects in the Ingenuity Pathways Knowledge Base, but are represented as a single node in the network. Nodes are displayed using various shapes that represent the functional class of the gene product (see the legends for Figs. 2 and 3). The Path Designer module of IPA was used for creation of the graphs and cell art.
Using IPA software, we have designed graphical representations of the Gadd45-centered networks. These networks depict interactions of Gadd45 molecules with signal transduction and other intracellular regulatory pathways. In addition to IPA, we also used peer-reviewed literature to search for experimentally confirmed interactions of Gadd45s with small molecules, environmental and other factors that affect Gadd45 expression.
The constructed Gadd45α, β and γ interaction networks displayed 100, 88, and 88 direct molecular interactions, respectively. The majority of regulatory interactions of Gadd45 proteins are with transcription factors/regulators, small molecules (drugs or chemicals), cytokines, kinases, steroid hormone receptors, growth factors and miRNAs. Additionally, the following canonical IPA signal transduction pathways, associated with Gadd45s, have been represented: ATM Signaling; Hereditary Breast Cancer Signaling; Cell cycle: DNA Damage Checkpoint Regulation; p53 Signaling; Role of BRCA1 in DNA Damage Response; SAPK-JNK Signaling; and VDR-RXR Activation.
For the sake of simplicity, we labeled the central molecule of the graphs as “GADD45”, even though the molecular interactions represent all three GADD45 family members. In order to achieve the most optimal visual representation and avoid figure overcrowding, a selection process was applied to display only the key interactions for inducers of GADD45 (listed mainly on the top of the figures) or intracellular responders (listed mainly towards the bottom). Besides information available from IPA, we have also overlaid Fig. 3 with data concerning the age-related functional alterations, diseases and conditions.
Appendix B. The list of genes/proteins represented in Figs. 1–3 and Table 3
ABL1, c-abl oncogene 1; APC/APC2, adenomatous polyposis coli/adenomatosis polyposis coli 2; ATF2, activating transcription factor 2; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; AXIN1, axin 1; BCL2, B-cell CLL/lymphoma2; BRCA1, breast cancer 1; CCNB1, cyclin B1; CDK1, cyclin-dependent kinase 1 (CDC2); CDKN1A, cyclin-dependent kinase inhibitor 1A (p21CIP1); CSF1, colony stimulating factor 1; CTNNB1, catenin (cadherin-associated protein), beta 1; Cyclin B; DCTN2, dynactin 2 (p50); ERCC5, excision repair cross-complementing rodent repair deficiency, complementation group 5 (XPG, XPGC); ERK, mitogen-activated protein kinase 1 (MAPK1); ESR1, estrogen receptor 1; FOXO1, forkhead box O1; GADD45, growth arrest and DNA-damage-inducible gene 45; GADD45GIP1, growth arrest and DNA-damage-inducible, gamma interacting protein 1; GM-CSF, granulocyte-macrophage colony-stimulating-factor; GRB2, growth factor receptor-bound protein 2; GRIN1, glutamate receptor, ionotropic, NMDA 1; GSK3B, glycogen synthase kinase 3 beta; GSK3B, glycogen synthase kinase 3 beta; IKBKG, inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase gamma; IL1R1, interleukin 1 receptor type I; IL3, interleukin 3; IL6, interleukin 6; Jnk, c-Jun NH2-terminal kinase; JUN, jun proto-oncogene; MAP2K7, mitogen-activated protein kinase kinase 7; MAP3K4, mitogen-activated protein kinase 4; MAP3K5, mitogen-activated protein kinase kinase kinase 5; MAPK14, mitogen-activated protein kinase 14; MRE11A, MRE11 meiotic recombination 11 homolog A; MYC, my proto-oncogene protein; NBN, nibrin; NBS1; Nijmegen breakage syndrome 1; NFAT5, nuclear factor of activated T-cells 5; NF-κB, nuclear factor kappa-B; NGF, nerve growth factor; NMDA Receptor, P38 MAPK, p38 mitogen-activated protein kinase; PCNA, proliferating cell nuclear antigen; PPARA, peroxisome proliferator-activated receptor alpha; PPARD, peroxisome proliferator-activated receptor delta; PPARG, peroxisome proliferator-activated receptor gamma; RAD50, DNA repair protein Rad50; RAD51, DNA repair protein Rad51; RARA, retinoic acid receptor, alpha; RWDD2B, RWD domain containing 2B; RXRA, retinoid X receptor, alpha; Sapk, mitogen-activated protein kinase 9 (Jun kinase, MAPK9); SMAD4, MAD homolog 4; STAT3, signal transducer and activator of transcription 3 (acute-phase response factor); STAT5A, signal transducer and activator of transcription 5A; SWI/SNF, matrix-associated actin-dependent chromatin remodeling complex; TFIID, transcription initiation factor TFIID; Tgf beta, transforming growth factor, beta; TGFBR2, transforming growth factor, beta receptor II; TNFRSF1A, tumor necrosis factor receptor superfamily, member 1A; TSNAX, translin-associated factor X; TP53, tumor suppressor protein p53; TP73, tumor protein p73; XPC, xeroderma pigmentosum, complementation group C.
Footnotes
Conflict of interest
All of authors have no conflict of interest.
References
- Abdollahi A, Lord KA, Hoffman-Liebermann B, Liebermann DA. Sequence and expression of a cDNA encoding MyD118: a novel myeloid differentiation primary response gene induced by multiple cytokines. Oncogene. 1991;6:165–167. [PubMed] [Google Scholar]
- Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol. 2005;175:3369–3376. doi: 10.4049/jimmunol.175.5.3369. [DOI] [PubMed] [Google Scholar]
- Amanullah A, Azam N, Balliet A, Hollander C, Hoffman B, Fornace A, Liebermann D. Cell signalling (communication arising): cell survival and a Gadd45-factor deficiency. Nature. 2003;424:741. doi: 10.1038/424741b. (Discussion 742) [DOI] [PubMed] [Google Scholar]
- Amrit FR, Boehnisch CM, May RC. Phenotypic covariance of longevity, immunity and stress resistance in the caenorhabditis nematodes. PLoS ONE. 2010;5:e9978. doi: 10.1371/journal.pone.0009978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson SA, Zhan Q, Penn LZ, Fornace AJ., Jr Myc suppresses induction of the growth arrest genes gadd34, gadd45, and gadd153 by DNA-damaging agents. Oncogene. 1998;17:2149–2154. doi: 10.1038/sj.onc.1202136. [DOI] [PubMed] [Google Scholar]
- Azam N, Vairapandi M, Zhang W, Hoffman B, Liebermann DA. Interaction of CR6 (GADD45γ) with proliferating cell nuclear antigen impedes negative growth control. J Biol Chem. 2001;276:2766–2774. doi: 10.1074/jbc.M005626200. [DOI] [PubMed] [Google Scholar]
- Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, Niehrs C. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- Beadling C, Johnson KW, Smith KA. Isolation of interleukin 2-induced immediate-early genes. Proc Natl Acad Sci USA. 1993;90:2719–2723. doi: 10.1073/pnas.90.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther. 2008;7:1520–1524. doi: 10.4161/cbt.7.10.6663. [DOI] [PubMed] [Google Scholar]
- Boley SE, Wong VA, French JE, Recio L. p53 heterozygosity alters the mRNA expression of p53 target genes in the bone marrow in response to inhaled benzene. Toxicol Sci. 2002;66:209–215. doi: 10.1093/toxsci/66.2.209. [DOI] [PubMed] [Google Scholar]
- Bortoff KD, Keeton AB, Franklin JL, Messina JL. Anti-inflammatory action of insulin via induction of Gadd45-β transcription by the mTOR signaling pathway. Hepat Med. 2010;2001:79–85. doi: 10.2147/HMER.S7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JJ, Leonard SS, Chen F, Shi X. As(III) transcriptionally activates the gadd45a gene via the formation of H2O2. Free Radic Biol Med. 2006;41:285–294. doi: 10.1016/j.freeradbiomed.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Brenner C, Deplus R, Didelot C, Loriot A, Vire E, De Smet C, Gutierrez A, Danovi D, Bernard D, Boon T, Pelicci PG, Amati B, Kouzarides T, de Launoit Y, Di Croce L, Fuks F. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 2005;24:336–346. doi: 10.1038/sj.emboj.7600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM. Longevity in mice: is stress resistance a common factor? Age (Dordr) 2006;28:145–162. doi: 10.1007/s11357-006-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruemmer D, Yin F, Liu J, Berger JP, Sakai T, Blaschke F, Fleck E, Van Herle AJ, Forman BM, Law RE. Regulation of the growth arrest and DNA damage-inducible gene 45 (GADD45) by peroxisome proliferator-activated receptor γ in vascular smooth muscle cells. Circ Res. 2003;93:e38–e47. doi: 10.1161/01.RES.0000088344.15288.E6. [DOI] [PubMed] [Google Scholar]
- Budovsky A, Fraifeld VE, Aronov S. Linking cell polarity, aging and rejuvenation. Biogerontology. 2011;12:167–175. doi: 10.1007/s10522-010-9305-4. [DOI] [PubMed] [Google Scholar]
- Budovsky A, Tacutu R, Yanai H, Abramovich A, Wolfson M, Fraifeld V. Common gene signature of cancer and longevity. Mech Ageing Dev. 2009;130:33–39. doi: 10.1016/j.mad.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Kovalsky O, Hollander MC, Fornace AJ., Jr Loss of oncogenic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45a. Mol Cell Biol. 2003;23:3859–3871. doi: 10.1128/MCB.23.11.3859-3871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz K, Whitaker N, Denk C, Ullmann A, Geisen C, Hoppe-Seyler F. Induction of the p53-target gene GADD45 in HPV-positive cancer cells. Oncogene. 1999;18:2381–2386. doi: 10.1038/sj.onc.1202557. [DOI] [PubMed] [Google Scholar]
- Cabrero A, Laguna JC, Vazquez M. Peroxisome proliferator-activated receptors and the control of inflammation. Curr Drug Targets Inflamm Allergy. 2002;1:243–248. doi: 10.2174/1568010023344616. [DOI] [PubMed] [Google Scholar]
- Cai Q, Dmitrieva NI, Ferraris JD, Michea LF, Salvador JM, Hollander MC, Fornace AJ, Jr, Fenton RA, Burg MB. Effects of expression of p53 and Gadd45 on osmotic tolerance of renal inner medullary cells. Am J Physiol Renal Physiol. 2006;291:F341–F349. doi: 10.1152/ajprenal.00518.2005. [DOI] [PubMed] [Google Scholar]
- Campanero MR, Herrero A, Calvo V. The histone deacetylase inhibitor trichostatin A induces GADD45γ expression via Oct and NF-Y binding sites. Oncogene. 2008;27:1263–1272. doi: 10.1038/sj.onc.1210735. [DOI] [PubMed] [Google Scholar]
- Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 2011 doi: 10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Carrier F, Georgel PT, Pourquier P, Blake M, Kontny HU, Antinore MJ, Gariboldi M, Myers TG, Weinstein JN, Pommier Y, Fornace AJ., Jr Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol. 1999;19:1673–1685. doi: 10.1128/mcb.19.3.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceryak S, Zingariello C, O‘Brien T, Patierno SR. Induction of pro-apoptotic and cell cycle-inhibiting genes in chromium (VI)-treated human lung fibroblasts: lack of effect of ERK. Mol Cell Biochem. 2004;255:139–149. doi: 10.1023/b:mcbi.0000007270.82431.3e. [DOI] [PubMed] [Google Scholar]
- Chakravarty D, Cai Q, Ferraris JD, Michea L, Burg MB, Kultz D. Three GADD45 isoforms contribute to hypertonic stress phenotype of murine renal inner medullary cells. Am J Physiol Renal Physiol. 2002;283:F1020–F1029. doi: 10.1152/ajprenal.00118.2002. [DOI] [PubMed] [Google Scholar]
- Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci USA. 2000;97:4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zhang Z, Leonard SS, Shi X. Contrasting roles of NF-κB and JNK in arsenite-induced p53-independent expression of GADD45α. Oncogene. 2001;20:3585–3589. doi: 10.1038/sj.onc.1204442. [DOI] [PubMed] [Google Scholar]
- Chen Z, Clark S, Birkeland M, Sung CM, Lago A, Liu R, Kirkpatrick R, Johanson K, Winkler JD, Hu E. Induction and superinduction of growth arrest and DNA damage gene 45 (GADD45) α and β messenger RNAs by histone deacetylase inhibitors trichostatin A (TSA) and butyrate in SW620 human colon carcinoma cells. Cancer Lett. 2002;188:127–140. doi: 10.1016/s0304-3835(02)00322-1. [DOI] [PubMed] [Google Scholar]
- Cheung KJ, Jr, Mitchell D, Lin P, Li G. The tumor suppressor candidate p33ING1 mediates repair of UV-damaged DNA. Cancer Res. 2001;61:4974–4977. [PubMed] [Google Scholar]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Seo AY, Chung SW, Kim MK, Leeuwenburgh C, Yu BP, Chung HY. Molecular mechanism of PPAR in the regulation of age-related inflammation. Ageing Res Rev. 2008;7:126–136. doi: 10.1016/j.arr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn PG, El-Deiry WS. Microarray analysis of p53-dependent gene expression in response to hypoxia and DNA damage. Cancer Biol Ther. 2007;6:1858–1866. doi: 10.4161/cbt.6.12.5330. [DOI] [PubMed] [Google Scholar]
- Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, Abramowitz LK, Bartolomei MS, Rambow F, Bassi MR, Bruno T, Fanciulli M, Renner C, Klein-Szanto AJ, Matsumoto Y, Kobi D, Davidson I, Alberti C, Larue L, Bellacosa A. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DR, Davies KJ. Adaptive response and oxidative stress. Environ Health Perspect. 1994;102 (Suppl 10):25–28. doi: 10.1289/ehp.94102s1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretu A, Sha X, Tront J, Hoffman B, Liebermann DA. Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther. 2009;7:268–276. [PMC free article] [PubMed] [Google Scholar]
- Daino K, Ichimura S, Nenoi M. Comprehensive search for X-ray-responsive elements and binding factors in the regulatory region of the GADD45a gene. J Radiat Res (Tokyo) 2003;44:311–318. doi: 10.1269/jrr.44.311. [DOI] [PubMed] [Google Scholar]
- Daino K, Ichimura S, Nenoi M. Both the basal transcriptional activity of the GADD45A gene and its enhancement after ionizing irradiation are mediated by AP-1 element. Biochim Biophys Acta. 2006;1759:458–469. doi: 10.1016/j.bbaexp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- De Feudis P, Debernardis D, Beccaglia P, Valenti M, Graniela Sire E, Arzani D, Stanzione S, Parodi S, D’Incalci M, Russo P, Broggini M. DDP-induced cytotoxicity is not influenced by p53 in nine human ovarian cancer cell lines with different p53 status. Br J Cancer. 1997;76:474–479. doi: 10.1038/bjc.1997.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Calle-Mustienes E, Glavic A, Modolell J, Gómez-Skarmeta JL. Xiro homeoproteins coordinate cell cycle exit and primary neuron formation by upregulating neuronal-fate repressors and downregulating the cell-cycle inhibitor XGadd45-gamma. Mech Dev. 2002;119:69–80. doi: 10.1016/s0925-4773(02)00296-4. [DOI] [PubMed] [Google Scholar]
- De Laurenzi V, Melino G. Evolution of functions within the p53/p63/p73 family. Ann N Y Acad Sci. 2000;926:90–100. doi: 10.1111/j.1749-6632.2000.tb05602.x. [DOI] [PubMed] [Google Scholar]
- de Magalhaes JP, Budovsky A, Lehmann G, Costa J, Li Y, Fraifeld V, Church GM. The Human Ageing Genomic Resources: online databases and tools for biogerontologists. Aging Cell. 2009;8:65–72. doi: 10.1111/j.1474-9726.2008.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- DeHaan RD, Yazlovitskaya EM, Persons DL. Regulation of p53 target gene expression by cisplatin-induced extracellular signal-regulated kinase. Cancer Chemother Pharmacol. 2001;48:383–388. doi: 10.1007/s002800100318. [DOI] [PubMed] [Google Scholar]
- Delmastro DA, Li J, Vaisman A, Solle M, Chaney SG. DNA damage inducible-gene expression following platinum treatment in human ovarian carcinoma cell lines. Cancer Chemother Pharmacol. 1997;39:245–253. doi: 10.1007/s002800050568. [DOI] [PubMed] [Google Scholar]
- Drews-Elger K, Ortells MC, Rao A, Lopez-Rodriguez C, Aramburu J. The transcription factor NFAT5 is required for cyclin expression and cell cycle progression in cells exposed to hypertonic stress. PLoS ONE. 2009;4:e5245. doi: 10.1371/journal.pone.0005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Wang L, Zhang Y, Jiang W, Sheng H, Cao Q, Wu J, Shen B, Shen T, Zhang JZ, Bao C, Li D, Li N. Role of GADD45β in the regulation of synovial fluid T cell apoptosis in rheumatoid arthritis. Clin Immunol. 2008;128:238–247. doi: 10.1016/j.clim.2008.03.523. [DOI] [PubMed] [Google Scholar]
- Duan J, Zhang Z, Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol. 2005;37:1407–1420. doi: 10.1016/j.biocel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Duggan SP, Gallagher WM, Fox EJ, Abdel-Latif MM, Reynolds JV, Kelleher D. Low pH induces co-ordinate regulation of gene expression in oesophageal cells. Carcinogenesis. 2006;27:319–327. doi: 10.1093/carcin/bgi211. [DOI] [PubMed] [Google Scholar]
- Edwards MG, Sarkar D, Klopp R, Morrow JD, Weindruch R, Prolla TA. Impairment of the transcriptional responses to oxidative stress in the heart of aged C57BL/6 mice. Ann N Y Acad Sci. 2004;1019:85–95. doi: 10.1196/annals.1297.017. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345–357. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- Ellison V, Stillman B. Biochemical characterization of DNA damage checkpoint complexes: clamp loader and clamp complexes with specificity for 5′ recessed DNA. PLoS Biol. 2003;1:E33. doi: 10.1371/journal.pbio.0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, Kanaar R, Vermeulen W. Nuclear dynamics of PCNA in DNA replication and repair. Mol Cell Biol. 2005;25:9350–9359. doi: 10.1128/MCB.25.21.9350-9359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- Fan W, Jin S, Tong T, Zhao H, Fan F, Antinore MJ, Rajasekaran B, Wu M, Zhan Q. BRCA1 regulates GADD45 through its interactions with the OCT-1 and CAAT motifs. J Biol Chem. 2002;277:8061–8067. doi: 10.1074/jbc.M110225200. [DOI] [PubMed] [Google Scholar]
- Fields WR, Leonard RM, Odom PS, Nordskog BK, Ogden MW, Doolittle DJ. Gene expression in normal human bronchial epithelial (NHBE) cells following in vitro exposure to cigarette smoke condensate. Toxicol Sci. 2005;86:84–91. doi: 10.1093/toxsci/kfi179. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. University of Chicago Press; Chicago: 1990. [Google Scholar]
- Flicek P, Amode MR, Barrell D, Beal K, Brent S, Chen Y, Clapham P, Coates G, Fairley S, Fitzgerald S, Gordon L, Hendrix M, Hourlier T, Johnson N, Kahari A, Keefe D, Keenan S, Kinsella R, Kokocinski F, Kulesha E, Larsson P, Longden I, McLaren W, Overduin B, Pritchard B, Riat HS, Rios D, Ritchie GR, Ruffier M, Schuster M, Sobral D, Spudich G, Tang YA, Trevanion S, Vandrovcova J, Vilella AJ, White S, Wilder SP, Zadissa A, Zamora J, Aken BL, Birney E, Cunningham F, Dunham I, Durbin R, Fernandez-Suarez XM, Herrero J, Hubbard TJ, Parker A, Proctor G, Vogel J, Searle SM. Ensembl 2011. Nucleic Acids Res. 2011;39:D800–D806. doi: 10.1093/nar/gkq1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace AJ, Jr, Alamo I, Jr, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci USA. 1988;85:8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace AJ, Jr, Amundson SA, Do KT, Meltzer P, Trent J, Bittner M. Stress-gene induction by low-dose gamma irradiation. Mil Med. 2002;167:13–15. [PubMed] [Google Scholar]
- Fornace AJ, Jr, Nebert DW, Hollander MC, Luethy JD, Papathanasiou M, Fargnoli J, Holbrook NJ. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989;9:4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N. FOXO forkhead transcription factors induce G2-M checkpoint in response to oxidative stress. J Biol Chem. 2002;277:26729–26732. doi: 10.1074/jbc.C200256200. [DOI] [PubMed] [Google Scholar]
- Gajdusek C, Onoda K, London S, Johnson M, Morrison R, Mayberg M. Early molecular changes in irradiated aortic endothelium. J Cell Physiol. 2001;188:8–23. doi: 10.1002/jcp.1091. [DOI] [PubMed] [Google Scholar]
- Gant TW, Baus PR, Clothier B, Riley J, Davies R, Judah DJ, Edwards RE, George E, Greaves P, Smith AG. Gene expression profiles associated with inflammation, fibrosis, and cholestasis in mouse liver after griseofulvin. EHP Toxicogenomics. 2003;111:37–43. [PubMed] [Google Scholar]
- Gao M, Guo N, Huang C, Song L. Diverse roles of GADD45alpha in stress signaling. Curr Protein Pept Sci. 2009;10:388–394. doi: 10.2174/138920309788922216. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Guillouf C, Grana X, Selvakumaran M, De Luca A, Giordano A, Hoffman B, Liebermann DA. Dissection of the genetic programs of p53-mediated G1 growth arrest and apoptosis: blocking p53-induced apoptosis unmasks G1 arrest. Blood. 1995;85:2691–2698. [PubMed] [Google Scholar]
- Gujuluva CN, Baek JH, Shin KH, Cherrick HM, Park NH. Effect of UV-irradiation on cell cycle, viability and the expression of p53, gadd153 and gadd45 genes in normal and HPV-immortalized human oral keratinocytes. Oncogene. 1994;9:1819–1827. [PubMed] [Google Scholar]
- Gupta M, Gupta SK, Balliet AG, Hollander MC, Fornace AJ, Hoffman B, Liebermann DA. Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene. 2005;24:7170–7179. doi: 10.1038/sj.onc.1208847. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Gupta M, Hoffman B, Liebermann DA. Hematopoietic cells from gadd45a-deficient and gadd45b-deficient mice exhibit impaired stress responses to acute stimulation with cytokines, myeloablation and inflammation. Oncogene. 2006;25:5537–5546. doi: 10.1038/sj.onc.1209555. [DOI] [PubMed] [Google Scholar]
- Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD, Haber DA. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Moore KM, Sty MA, Magwire MM. Stress resistance and longevity in selected lines of Drosophila melanogaster. Neurobiol Aging. 1999;20:521–529. doi: 10.1016/s0197-4580(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Herr JC, Chertihin O, Digilio L, Jha KN, Vemuganti S, Flickinger CJ. Distribution of RNA binding protein MOEP19 in the oocyte cortex and early embryo indicates pre-patterning related to blastomere polarity and trophectoderm pecification. Dev Biol. 2008;314:300–316. doi: 10.1016/j.ydbio.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S, Wong SH, Richner M, Rowe CL, Newgreen DF, Werther GA, Russo VC. Fibroblast growth factor 2 reactivates G1 checkpoint in SK-N-MC cells via regulation of p21, inhibitor of differentiation genes (Id1–3), and epithelium-mesenchyme transition-like events. Endocrinology. 2009;150:4044–4055. doi: 10.1210/en.2008-1797. [DOI] [PubMed] [Google Scholar]
- Higgs MR, Lerat H, Pawlotsky JM. Downregulation of Gadd45β expression by hepatitis C virus leads to defective cell cycle arrest. Cancer Res. 2010;70:4901–4911. doi: 10.1158/0008-5472.CAN-09-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildesheim J, Belova GI, Tyner SD, Zhou X, Vardanian L, Fornace AJ., Jr Gadd45a regulates matrix metalloproteinases by suppressing ΔNp63α and β-catenin via p38 MAP kinase and APC complex activation. Oncogene. 2004;23:1829–1837. doi: 10.1038/sj.onc.1207301. [DOI] [PubMed] [Google Scholar]
- Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L, Fornace AJ., Jr Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002;62:7305–7315. [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Hoffman B, Liebermann DA. Role of gadd45 in myeloid cells in response to hematopoietic stress. Blood Cells Mol Dis. 2007;39:344–347. doi: 10.1016/j.bcmd.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B, Liebermann DA. Gadd45 modulation of intrinsic and extrinsic stress responses in myeloid cells. J Cell Physiol. 2009;218:26–31. doi: 10.1002/jcp.21582. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer A, Piekorz R, Moriggl R, Ihle JN. Gadd45gamma is dispensable for normal mouse development and T-cell proliferation. Mol Cell Biol. 2001;21:3137–3143. doi: 10.1128/MCB.21.9.3137-3143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard N, Hey Y, Brintnell B, James L, Jones D, Mitchell E, Weissenbach J, Varley JM. Identification and cloning in yeast artificial chromosomes of a region of elevated loss of heterozygosity on chromosome 1p31.1 in human breast cancer. Genomics. 1995;30:233–243. doi: 10.1006/geno.1995.9882. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Kovalsky O, Salvador JM, Kim KE, Patterson AD, Haines DC, Fornace AJ., Jr Dimethylbenzanthracene carcinogenesis in Gadd45α-null mice is associated with decreased DNA repair and increased mutation frequency. Cancer Res. 2001;61:2487–2491. [PubMed] [Google Scholar]
- Hollander MC, Philburn RT, Patterson AD, Velasco-Miguel S, Friedberg EC, Linnoila RI, Fornace AJ., Jr Deletion of XPC leads to lung tumors in mice and is associated with early events in human lung carcinogenesis. Proc Natl Acad Sci USA. 2005;102:13200–13205. doi: 10.1073/pnas.0503133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro TA, Kim KE, Tolosa E, Ashwell JD, Rosenberg MP, Zhan Q, Fernandez-Salguero PM, Morgan WF, Deng CX, Fornace AJ., Jr Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- Hsiao HW, Liu WH, Wang CJ, Lo YH, Wu YH, Jiang ST, Lai MZ. Deltex1 is a target of the transcription factor NFAT that promotes T cell anergy. Immunity. 2009;31:72–83. doi: 10.1016/j.immuni.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Huang HS, Kubish GM, Redmond TM, Turner DL, Thompson RC, Murphy GG, Uhler MD. Direct transcriptional induction of Gadd45γ by Ascl1 during neuronal differentiation. Mol Cell Neurosci. 2010;44:282–296. doi: 10.1016/j.mcn.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri K, Zerbini LF, Peng H, Correa RG, Lu B, Walsh N, Zhao Y, Taniguchi N, Huang XL, Otu H, Wang H, Wang JF, Komiya S, Ducy P, Rahman MU, Flavell RA, Gravallese EM, Oettgen P, Libermann TA, Goldring MB. A novel role for GADD45β as a mediator of MMP-13 gene expression during chondrocyte terminal differentiation. J Biol Chem. 2005;280:38544–38555. doi: 10.1074/jbc.M504202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeyama S, Kokkonen G, Shack S, Wang XT, Holbrook NJ. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. FASEB J. 2002;16:114–116. doi: 10.1096/fj.01-0409fje. [DOI] [PubMed] [Google Scholar]
- Jackman J, Alamo I, Jr, Fornace AJ., Jr Genotoxic stress confers preferential and coordinate messenger RNA stability on the five gadd genes. Cancer Res. 1994;54:5656–5662. [PubMed] [Google Scholar]
- Jackson JG, Pereira-Smith OM. p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res. 2006;66:8356–8360. doi: 10.1158/0008-5472.CAN-06-1752. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. Genetics of longevity. Exp Gerontol. 1998;33:773–783. doi: 10.1016/s0531-5565(98)00027-8. [DOI] [PubMed] [Google Scholar]
- Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Wang Z. Gadd45γ is androgen-responsive and growth-inhibitory in prostate cancer cells. Mol Cell Endocrinol. 2004;213:121–129. doi: 10.1016/j.mce.2003.10.050. [DOI] [PubMed] [Google Scholar]
- Jin S, Fan F, Fan W, Zhao H, Tong T, Blanck P, Alomo I, Rajasekaran B, Zhan Q. Transcription factors Oct-1 and NF-YA regulate the p53-independent induction of the GADD45 following DNA damage. Oncogene. 2001;20:2683–2690. doi: 10.1038/sj.onc.1204390. [DOI] [PubMed] [Google Scholar]
- Jinawath N, Vasoontara C, Yap KL, Thiaville MM, Nakayama K, Wang TL, Shih IM. NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene. 2009;28:1941–1948. doi: 10.1038/onc.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, de Castro E, Hegi de Castro S, Cypser J, Henderson S, Tedesco P. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp Gerontol. 2001;36:1609–1617. doi: 10.1016/s0531-5565(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Ju S, Zhu Y, Liu L, Dai S, Li C, Chen E, He Y, Zhang X, Lu B. Gadd45b and Gadd45g are important for anti-tumor immune responses. Eur J Immunol. 2009;39:3010–3018. doi: 10.1002/eji.200839154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Kim EH, Mun JY, Park S, Smith ML, Han SS, Seo YR. Base excision DNA repair defect in Gadd45a-deficient cells. Oncogene. 2007;26:7517–7525. doi: 10.1038/sj.onc.1210557. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann LT, Gierl MS, Niehrs C. Gadd45a, Gadd45b and Gadd45g expression during mouse embryonic development. Gene Exp Patterns. 2011 doi: 10.1016/j.gep.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Che YS, Hanaoka R, Takeda H, Dawid IB. Zebrafish GADD45beta genes are involved in somite segmentation. Proc Natl Acad Sci USA. 2005;102:361–366. doi: 10.1073/pnas.0408726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sun H. Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal lifespan. Aging Cell. 2007;6:489–503. doi: 10.1111/j.1474-9726.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16:237–243. [PubMed] [Google Scholar]
- Kovalsky O, Lung FD, Roller PP, Fornace AJ., Jr Oligomerization of human Gadd45a protein. J Biol Chem. 2001;276:39330–39339. doi: 10.1074/jbc.M105115200. [DOI] [PubMed] [Google Scholar]
- Kültz D, Madhany S, Burg MB. Hyperosmolality causes growth arrest of murine kidney cells. Induction of GADD45 and GADD153 by osmosensing via stress-activated protein kinase 2. J Biol Chem. 1998;273:13645–13651. doi: 10.1074/jbc.273.22.13645. [DOI] [PubMed] [Google Scholar]
- Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol. 2009;296:H946–H956. doi: 10.1152/ajpheart.00693.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis GN, Bhole D, Tower J. A search for doxycycline-dependent mutations that increase Drosophila melanogaster life span identifies the VhaSFD, Sugar baby, filamin, fwd and Cctl genes. Genome Biol. 2003;4:R8. doi: 10.1186/gb-2003-4-2-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ, Fulop T. Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res Rev. 2011;10:370–378. doi: 10.1016/j.arr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Le May N, Egly JM, Coin F. True lies: the double life of the nucleotide excision repair factors in transcription and DNA repair. J Nucleic Acids. 2010 doi: 10.4061/2010/616342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ding X, Adrian TE. Arsenic trioxide induces apoptosis in pancreatic cancer cells via changes in cell cycle, caspase activation, and GADD expression. Pancreas. 2003;27:174–179. doi: 10.1097/00006676-200308000-00011. [DOI] [PubMed] [Google Scholar]
- Li X, Ding X, Adrian TE. Arsenic trioxide causes redistribution of cell cycle, caspase activation, and GADD expression in human colonic, breast, and pancreatic cancer cells. Cancer Invest. 2004;22:389–400. doi: 10.1081/cnv-200029068. [DOI] [PubMed] [Google Scholar]
- Liebermann DA, Hoffman B. MyD genes in negative growth control. Oncogene. 1998;17:3319–3329. doi: 10.1038/sj.onc.1202574. [DOI] [PubMed] [Google Scholar]
- Liebermann DA, Hoffman B. Myeloid differentiation (MyD) primary response genes in hematopoiesis. Blood Cells Mol Dis. 2003;31:213–228. doi: 10.1016/s1079-9796(03)00160-8. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Lindstrom TM, Robinson WH. Rheumatoid arthritis: a role for immunosenescence? J Am Geriatr Soc. 2010;58:1565–1575. doi: 10.1111/j.1532-5415.2010.02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kadiiska MB, Liu Y, Lu T, Qu W, Waalkes MP. Stress-related gene expression in mice treated with inorganic arsenicals. Toxicol Sci. 2001;61:314–320. doi: 10.1093/toxsci/61.2.314. [DOI] [PubMed] [Google Scholar]
- Liu L, Tran E, Zhao Y, Huang Y, Flavell R, Lu B. Gadd45β and Gadd45γ are critical for regulating autoimmunity. J Exp Med. 2005;202:1341–1347. doi: 10.1084/jem.20051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD. The Ras and Sch9 pathways regulate stress resistance and longevity. Exp Gerontol. 2003;38:807–811. doi: 10.1016/s0531-5565(03)00113-x. [DOI] [PubMed] [Google Scholar]
- López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Ferrandino AF, Flavell RA. Gadd45β is important for perpetuating cognate and inflammatory signals in T cells. Nat Immunol. 2004;5:38–44. doi: 10.1038/ni1020. [DOI] [PubMed] [Google Scholar]
- Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA. GADD45γ mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–590. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Ma DK, Guo JU, Ming GL, Song H. DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle. 2009;8:1526–1531. doi: 10.4161/cc.8.10.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan TK, Somasundaram K, Sgagias M, Shifman Y, Muschel RJ, Cowan KH, El-Deiry WS. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J Biol Chem. 2000;275:2777–2785. doi: 10.1074/jbc.275.4.2777. [DOI] [PubMed] [Google Scholar]
- Maeda T, Espino RA, Chomey EG, Luong L, Bano A, Meakins D, Tron VA. Loss of p21WAF1/Cip1 in Gadd45-deficient keratinocytes restores DNA repair capacity. Carcinogenesis. 2005;26:1804–1810. doi: 10.1093/carcin/bgi140. [DOI] [PubMed] [Google Scholar]
- Maeda T, Hanna AN, Sim AB, Chua PP, Chong MT, Tron VA. GADD45 regulates G2/M arrest, DNA repair, and cell death in keratinocytes following ultraviolet exposure. J Invest Dermatol. 2002;119:22–26. doi: 10.1046/j.1523-1747.2002.01781.x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Sim AB, Leedel DA, Chua PP, Chomey EG, Luong L, Tron VA. UV induces GADD45 in a p53-dependent and -independent manner in human keratinocytes. J Cutan Med Surg. 2003;7:119–123. doi: 10.1007/s10227-002-0108-3. [DOI] [PubMed] [Google Scholar]
- Marrot L, Belaidi JP, Jones C, Perez P, Meunier JR. Molecular responses to stress induced in normal human caucasian melanocytes in culture by exposure to simulated solar UV. Photochem Photobiol. 2005;81:367–375. doi: 10.1562/2004-10-13-RA-343. [DOI] [PubMed] [Google Scholar]
- Masse I, Molin L, Mouchiroud L, Vanhems P, Palladino F, Billaud M, Solari F. A novel role for the SMG-1 kinase in lifespan and oxidative stress resistance in Caenorhabditis elegans. PLoS ONE. 2008;3:e3354. doi: 10.1371/journal.pone.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Mili S, Macara IG. RNA localization and polarity: from A(PC) to Z(BP) Trends Cell Biol. 2009;19:156–164. doi: 10.1016/j.tcb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalev A. Radiation-induced life span alteration of Drosophila lines with genotype differences. Biogerontology. 2007;8:499–504. doi: 10.1007/s10522-007-9090-x. [DOI] [PubMed] [Google Scholar]
- Moskalev A, Shaposhnikov M, Turysheva E. Life span alteration after irradiation in Drosophila melanogaster strains with mutations of Hsf and Hsps. Biogerontology. 2009;10:3–11. doi: 10.1007/s10522-008-9147-5. [DOI] [PubMed] [Google Scholar]
- Muñoz-Najar U, Sedivy JM. Epigenetic control of aging. Antioxid Redox Signal. 2011;14:241–259. doi: 10.1089/ars.2010.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Johnson TE. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics. 1996;143:1207–1218. doi: 10.1093/genetics/143.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na YK, Lee SM, Hong HS, Kim JB, Park JY, Kim DS. Hypermethylation of growth arrest DNA-damage-inducible gene 45 in non-small cell lung cancer and its relationship with clinicopathologic features. Mol Cells. 2010;30:89–92. doi: 10.1007/s10059-010-0092-1. [DOI] [PubMed] [Google Scholar]
- Narayan S, Jaiswal AS, Kang D, Srivastava P, Das GM, Gairola CG. Cigarette smoke condensate-induced transformation of normal human breast epithelial cells in vitro. Oncogene. 2004;23:5880–5889. doi: 10.1038/sj.onc.1207792. [DOI] [PubMed] [Google Scholar]
- Navarro CL, Cau P, Levy N. Molecular bases of progeroid syndromes. Hum Mol Genet. 2006;(15 Spec No 2):R151–R161. doi: 10.1093/hmg/ddl214. [DOI] [PubMed] [Google Scholar]
- Nikolova T, Czyz J, Rolletschek A, Blyszczuk P, Fuchs J, Jovtchev G, Schuderer J, Kuster N, Wobus AM. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J. 2005;19:1686–1688. doi: 10.1096/fj.04-3549fje. [DOI] [PubMed] [Google Scholar]
- Oh-Hashi K, Maruyama W, Isobe K. Peroxynitrite induces GADD34, 45, and 153 VIA p38 MAPK in human neuroblastoma SH-SY5Y cells. Free Radic Biol Med. 2001;30:213–221. doi: 10.1016/s0891-5849(00)00461-5. [DOI] [PubMed] [Google Scholar]
- Ohtani-Fujita N, Minami S, Mimaki S, Dao S, Sakai T. p53-Independent activation of the gadd45 promoter by Delta12-prostaglandin J2. Biochem Biophys Res Commun. 1998;251:648–652. doi: 10.1006/bbrc.1998.9511. [DOI] [PubMed] [Google Scholar]
- Ostlund G, Schmitt T, Forslund K, Köstler T, Messina DN, Roopra S, Frings O, Sonnhammer EL. InParanoid 7: new algorithms and tools for eukaryotic orthology analysis. Nucleic Acids Res. 2010;38:D196–D203. doi: 10.1093/nar/gkp931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolf KB. Aging and human heat tolerance. Exp Aging Res. 1997;23:69–105. doi: 10.1080/03610739708254027. [DOI] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Fu YX, Bubici C, Alvarez K, Dean K, Christiansen PA, Anders RA, Franzoso G. Gadd45β promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J Clin Invest. 2008;118:1911–1923. doi: 10.1172/JCI33913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G. Linking JNK signaling to NF-κB: a key to survival. J Cell Sci. 2004;117:5197–5208. doi: 10.1242/jcs.01483. [DOI] [PubMed] [Google Scholar]
- Papathanasiou MA, Kerr NC, Robbins JH, McBride OW, Alamo I, Jr, Barrett SF, Hickson ID, Fornace AJ., Jr Induction by ionizing radiation of the gadd45 gene in cultured human cells: lack of mediation by protein kinase C. Mol. Cell Biol. 1991;11:1009–1016. doi: 10.1128/mcb.11.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippin JW, Durvasula R, Petermann A, Hiromura K, Couser WG, Shankland SJ. DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J Clin Invest. 2003;111:877–885. doi: 10.1172/JCI15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- Plyusnina EN, Shaposhnikov MV, Moskalev AA. Increase of Drosophila melanogaster lifespan due to D-GADD45 overexpression in the nervous system. Biogerontology. 2011;12:211–226. doi: 10.1007/s10522-010-9311-6. [DOI] [PubMed] [Google Scholar]
- Price BD, Calderwood SK. Gadd45 and Gadd153 messenger RNA levels are increased during hypoxia and after exposure of cells to agents which elevate the levels of the glucose-regulated proteins. Cancer Res. 1992;52:3814–3817. [PubMed] [Google Scholar]
- Przybylo JA, Radisky DC. Matrix metalloproteinase-induced epithelial–mesenchymal transition: tumor progression at Snail’s pace. Int J Biochem Cell Biol. 2007;39:1082–1088. doi: 10.1016/j.biocel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Qiu W, David D, Zhou B, Chu PG, Zhang B, Wu M, Xiao J, Han T, Zhu Z, Wang T, Liu X, Lopez R, Frankel P, Jong A, Yen Y. Down-regulation of growth arrest DNA damage-inducible gene 45β expression is associated with human hepatocellular carcinoma. Am J Pathol. 2003;162:1961–1974. doi: 10.1016/s0002-9440(10)64329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Zhou B, Zou H, Liu X, Chu PG, Lopez R, Shih J, Chung C, Yen Y. Hypermethylation of growth arrest DNA damage-inducible gene 45 β promoter in human hepatocellular carcinoma. Am J Pathol. 2004;165:1689–1699. doi: 10.1016/s0002-9440(10)63425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008;135:1201–1212. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SI. Targeting the age-related occurrence, removal, and accumulation of molecular damage by hormesis. Ann N Y Acad Sci. 2010;1197:28–32. doi: 10.1111/j.1749-6632.2010.05193.x. [DOI] [PubMed] [Google Scholar]
- Rattan SI, Fernandes RA, Demirovic D, Dymek B, Lima CF. Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation. Dose Response. 2009;7:90–103. doi: 10.2203/dose-response.08-014.Rattan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MR, Vu LN, Park SU, Graves JL., Jr Selection on stress resistance increases longevity in Drosophila melanogaster. Exp Gerontol. 1992;27:241–250. doi: 10.1016/0531-5565(92)90048-5. [DOI] [PubMed] [Google Scholar]
- Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, Powell JD. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- Salvador JM, Hollander MC, Nguyen AT, Kopp JB, Barisoni L, Moore JK, Ashwell JD, Fornace AJ., Jr Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity. 2002;16:499–508. doi: 10.1016/s1074-7613(02)00302-3. [DOI] [PubMed] [Google Scholar]
- Sánchez R, Pantoja-Uceda D, Torres D, Prieto J, Campos-Olivas R, Blanco FJ. NMR assignment and secondary structure of human growth arrest and DNA damage alpha protein (Gadd45 alpha) Biomol NMR Assign. 2008;2:139–142. doi: 10.1007/s12104-008-9105-9. [DOI] [PubMed] [Google Scholar]
- Santiard-Baron D, Gosset P, Nicole A, Sinet PM, Christen Y, Ceballos-Picot I. Identification of beta-amyloid-responsive genes by RNA differential display: early induction of a DNA damage-inducible gene, gadd45. Exp Neurol. 1999;158:206–213. doi: 10.1006/exnr.1999.7076. [DOI] [PubMed] [Google Scholar]
- Santiard-Baron D, Lacoste A, Ellouk-Achard S, Soulie C, Nicole A, Sarasin A, Ceballos-Picot I. The amyloid peptide induces early genotoxic damage in human preneuron NT2. Mutat Res. 2001;479:113–120. doi: 10.1016/s0027-5107(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Saunders LR, Verdin E. Cell biology. Stress response and aging. Science. 2009;323:1021–1022. doi: 10.1126/science.1170007. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Schomacher L, Barreto G, Döderlein G, Niehrs C. Gemcitabine functions epigenetically by inhibiting repair mediated DNA demethylation. PLoS ONE. 2010;5:e14060. doi: 10.1371/journal.pone.0014060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Brune B, von Knethen A. The nuclear hormone receptor PPARγ as a therapeutic target in major diseases. ScientificWorldJournal. 2010;10:2181–2197. doi: 10.1100/tsw.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz KM, Schmitt N, Hoffmann-Rohrer U, Schäfer A, Grummt I, Mayer C. TAF12 recruits Gadd45a and the nucleotide excision repair complex to the promoter of rRNA genes leading to active DNA demethylation. Mol Cell. 2009;33:344–353. doi: 10.1016/j.molcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Schrag JD, Jiralerspong S, Banville M, Jaramillo ML, O’Connor-McCourt MD. The crystal structure and dimerization interface of GADD45γ. Proc Natl Acad Sci USA. 2008;105:6566–6571. doi: 10.1073/pnas.0800086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedding DG. FoxO transcription factors in oxidative stress response and ageing—a new fork on the way to longevity? Biol Chem. 2008;389:279–283. doi: 10.1515/BC.2008.033. [DOI] [PubMed] [Google Scholar]
- Selvakumaran M, Lin HK, Sjin RT, Reed JC, Liebermann DA, Hoffman B. The novel primary response gene MyD118 and the proto-oncogenes myb, myc, and bcl-2 modulate transforming growth factor beta 1-induced apoptosis of myeloid leukemia cells. Mol Cell Biol. 1994;14:2352–2360. doi: 10.1128/mcb.14.4.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Mahadevan B, DeMarini DM. Transcriptional responses to complex mixtures: a review. Mutat Res. 2007;636:144–177. doi: 10.1016/j.mrrev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–567. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharova LV, Sharov AA, Nedorezov T, Piao Y, Shaik N, Ko MS. Database for mRNA half-life of 19 977 genes obtained by DNA microarray analysis of pluripotent and differentiating mouse embryonic stem cells. DNA Res. 2009;16:45–58. doi: 10.1093/dnares/dsn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng N, Xie Z, Wang C, Bai G, Zhang K, Zhu Q, Song J, Guillemot F, Chen YG, Lin A, Jing N. Retinoic acid regulates bone morphogenic protein signal duration by promoting the degradation of phosphorylated Smad1. Proc Natl Acad Sci USA. 2010;107:18886–18891. doi: 10.1073/pnas.1009244107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova PP, Wang S, Toriuma W, Kommineni V, Matheson J, Unimye N, Kayama F, Harki D, Ding M, Vallyathan V, Luster MI. Arsenic mediates cell proliferation and gene expression in the bladder epithelium: association with activating protein-1 transactivation. Cancer Res. 2000;60:3445–3453. [PubMed] [Google Scholar]
- Slack C, Werz C, Wieser D, Alic N, Foley A, Stocker H, Withers DJ, Thornton JM, Hafen E, Partridge L. Regulation of lifespan, metabolism, and stress responses by the Drosophila SH2B protein, Lnk. PLoS Genet. 2010;6:e1000881. doi: 10.1371/journal.pgen.1000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, Kastan MB, O‘Connor PM, Fornace AJ., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266:1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Smith ML, Ford JM, Hollander MC, Bortnick RA, Amundson SA, Seo YR, Deng CX, Hanawalt PC, Fornace AJ., Jr p53-mediated DNA repair responses to UV radiation: studies of mouse cells lacking p53, p21, and/or gadd45 genes. Mol Cell Biol. 2000;20:3705–3714. doi: 10.1128/mcb.20.10.3705-3714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Kontny HU, Zhan Q, Sreenath A, O’Connor PM, Fornace AJ., Jr Antisense GADD45 expression results in decreased DNA repair and sensitizes cells to u. v.-irradiation or cisplatin. Oncogene. 1996;13:2255–2263. [PubMed] [Google Scholar]
- Sohal RS, Sohal BH, Brunk UT. Relationship between antioxidant defenses and longevity in different mammalian species. Mech Ageing Dev. 1990;53:217–227. doi: 10.1016/0047-6374(90)90040-m. [DOI] [PubMed] [Google Scholar]
- Stark C, Breitkreutz BJ, Chatr-Aryamontri A, Boucher L, Oughtred R, Livstone MS, Nixon J, Van Auken K, Wang X, Shi X, Reguly T, Rust JM, Winter A, Dolinski K, Tyers M. The BioGRID Interaction Database: 2011 update. Nucleic Acids Res. 2011;39:D698–D704. doi: 10.1093/nar/gkq1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes AH, Freeman WM, Mitchell SG, Burnette TA, Hellmann GM, Vrana KE. Induction of GADD45 and GADD153 in neuroblastoma cells by dopamine-induced toxicity. Neurotoxicology. 2002;23:675–684. doi: 10.1016/S0161-813X(02)00093-1. [DOI] [PubMed] [Google Scholar]
- Sun L, Gong R, Wan B, Huang X, Wu C, Zhang X, Zhao S, Yu L. GADD45γ, down-regulated in 65% hepatocellular carcinoma (HCC) from 23 Chinese patients, inhibits cell growth and induces cell cycle G2/M arrest for hepatoma Hep-G2 cell lines. Mol Biol Rep. 2003;30:249–253. doi: 10.1023/a:1026370726763. [DOI] [PubMed] [Google Scholar]
- Sytnikova YA, Kubarenko AV, Schafer A, Weber AN, Niehrs C. Gadd45a is an RNA binding protein and is localized in nuclear speckles. PLoS ONE. 2011;6:e14500. doi: 10.1371/journal.pone.0014500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacutu R, Budovsky A, Wolfson M, Fraifeld VE. MicroRNA-regulated protein-protein interaction networks: how could they help in searching for pro-longevity targets? Rejuvenation Res. 2010;13:373–377. doi: 10.1089/rej.2009.0980. [DOI] [PubMed] [Google Scholar]
- Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95:521–530. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- Takekawa M, Tatebayashi K, Itoh F, Adachi M, Imai K, Saito H. Smad-dependent GADD45βexpression mediates delayed activation of p38 MAP kinase by TGF-β. EMBO J. 2002;21:6473–6482. doi: 10.1093/emboj/cdf643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T, Borlak J. LOX-1 receptor blockade abrogates oxLDL-induced oxidative DNA damage and prevents activation of the transcriptional repressor Oct-1 in human coronary arterial endothelium. J Biol Chem. 2008;283:19456–19464. doi: 10.1074/jbc.M708309200. [DOI] [PubMed] [Google Scholar]
- Thyss R, Virolle V, Imbert V, Peyron JF, Aberdam D, Virolle T. NF-κB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J. 2005;24:128–137. doi: 10.1038/sj.emboj.7600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore L, Marasco D, Dathan N, Vitale RM, Benedetti E, Papa S, Franzoso G, Ruvo M, Monti SM. Gadd45β forms a homodimeric complex that binds tightly to MKK7. J Mol Biol. 2008;378:97–111. doi: 10.1016/j.jmb.2008.01.074. [DOI] [PubMed] [Google Scholar]
- Torp R, Su JH, Deng G, Cotman CW. GADD45 is induced in Alzheimer’s disease, and protects against apoptosis in vitro. Neurobiol Dis. 1998;5:245–252. doi: 10.1006/nbdi.1998.0201. [DOI] [PubMed] [Google Scholar]
- Trabucco S. Gadd45-α and Metastatic Hepatocellular Carcinoma Cell Migration. Polytechnic Institute; Worcester: 2010. p. 26. [Google Scholar]
- Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr, DiStefano PS, Chiang LW, Greenberg ME. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- Tront JS, Hoffman B, Liebermann DA. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 2006;66:8448–8454. doi: 10.1158/0008-5472.CAN-06-2013. [DOI] [PubMed] [Google Scholar]
- Tront JS, Huang Y, Fornace AJ, Jr, Hoffman B, Liebermann DA. Gadd45a functions as a promoter or suppressor of breast cancer dependent on the oncogenic stress. Cancer Res. 2010;70:9671–9681. doi: 10.1158/0008-5472.CAN-10-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci USA. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Krasnikov BF, Csiszar A, Labinskyy N, Mukhopadhyay P, Pacher P, Cooper AJ, Podlutskaya N, Austad SN, Podlutsky A. Testing hypotheses of aging in long-lived mice of the genus Peromyscus: association between longevity and mitochondrial stress resistance, ROS detoxification pathways, and DNA repair efficiency. Age (Dordr) 2008;30:121–133. doi: 10.1007/s11357-008-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vairapandi M, Azam N, Balliet AG, Hoffman B, Liebermann DA. Characterization of MyD118, Gadd45, and proliferating cell nuclear antigen (PCNA) interacting domains. PCNA impedes MyD118 AND Gadd45-mediated negative growth control. J Biol Chem. 2000;275:16810–16819. doi: 10.1074/jbc.275.22.16810. [DOI] [PubMed] [Google Scholar]
- Vairapandi M, Balliet AG, Fornace AJ, Jr, Hoffman B, Liebermann DA. The differentiation primary response gene MyD118, related to GADD45, encodes for a nuclear protein which interacts with PCNA and p21WAF1/CIP1. Oncogene. 1996;12:2579–2594. [PubMed] [Google Scholar]
- Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- Vermeulen CJ, Van De Zande L, Bijlsma R. Resistance to oxidative stress induced by paraquat correlates well with both decreased and increased lifespan in Drosophila melanogaster. Biogerontology. 2005;6:387–395. doi: 10.1007/s10522-005-4903-2. [DOI] [PubMed] [Google Scholar]
- Wan Y, Wang Z, Shao Y, Xu Y, Voorhees J, Fisher G. UV-induced expression of GADD45 is mediated by an oxidant sensitive pathway in cultured human keratinocytes and in human skin in vivo. Int J Mol Med. 2000;6:683–688. doi: 10.3892/ijmm.6.6.683. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang RH, Li W, Xu X, Hollander MC, Fornace AJ, Jr, Deng CX. Genetic interactions between Brca1 and Gadd45a in centrosome duplication, genetic stability, and neural tube closure. J Biol Chem. 2004;279:29606–29614. doi: 10.1074/jbc.M312279200. [DOI] [PubMed] [Google Scholar]
- Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, Hollander MC, O’Connor PM, Fornace AJ, Jr, Harris CC. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA. 1999;96:3706–3711. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A, Priante G, Lahdetie J. Stage-specific expression of Gadd45 induced by X-irradiation in rat spermatogenesis. Int J Radiat Biol. 2002;78:29–39. doi: 10.1080/09553000110089982. [DOI] [PubMed] [Google Scholar]
- Wolfson M, Budovsky A, Tacutu R, Fraifeld V. The signaling hubs at the crossroad of longevity and age-related disease networks. Int J Biochem Cell Biol. 2009;41:516–520. doi: 10.1016/j.biocel.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45β required in cytokine-induced, but not TCR-induced, IFN-γ production. Nat Immunol. 2001;2:157–164. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- Yang Z, Song L, Huang C. Gadd45 proteins as critical signal transducers linking NF-κB to MAPK cascades. Curr Cancer Drug Targets. 2009;9:915–930. doi: 10.2174/156800909790192383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Bruemmer D, Blaschke F, Hsueh WA, Law RE, Herle AJ. Signaling pathways involved in induction of GADD45 gene expression and apoptosis by troglitazone in human MCF-7 breast carcinoma cells. Oncogene. 2004;23:4614–4623. doi: 10.1038/sj.onc.1207598. [DOI] [PubMed] [Google Scholar]
- Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK, Ambinder R, Tao Q. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res. 2005;11:6442–6449. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ, Jr, Liebermann DA, Bottinger EP, Roberts AB. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem. 2003;278:43001–43007. doi: 10.1074/jbc.M307869200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Maeda A, Horinaka M, Shiraishi T, Nakata S, Wakada M, Yogosawa S, Sakai T. Quercetin induces gadd45 expression through a p53-independent pathway. Oncol Rep. 2005;14:1299–1303. [PubMed] [Google Scholar]
- Zazzeroni F, Papa S, Algeciras-Schimnich A, Alvarez K, Melis T, Bubici C, Majewski N, Hay N, De Smaele E, Peter ME, Franzoso G. Gadd45β mediates the protective effects of CD40 costimulation against Fas-induced apoptosis. Blood. 2003;102:3270–3279. doi: 10.1182/blood-2003-03-0689. [DOI] [PubMed] [Google Scholar]
- Zerbini LF, Libermann TA. Life and death in cancer. GADD45 α and γ are critical regulators of NF-κB mediated escape from programmed cell death. Cell Cycle. 2005;4:18–20. doi: 10.4161/cc.4.1.1363. [DOI] [PubMed] [Google Scholar]
- Zerbini LF, Wang Y, Czibere A, Correa RG, Cho JY, Ijiri K, Wei W, Joseph M, Gu X, Grall F, Goldring MB, Zhou JR, Libermann TA. NF-κB-mediated repression of growth arrest- and DNA-damage-inducible proteins 45α and γ is essential for cancer cell survival. Proc Natl Acad Sci USA. 2004;101:13618–13623. doi: 10.1073/pnas.0402069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q, Antinore MJ, Wang XW, Carrier F, Smith ML, Harris CC, Fornace AJ., Jr Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18:2892–2900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]
- Zhan Q, Chen IT, Antinore MJ, Fornace AJ., Jr Tumor suppressor p53 can participate in transcriptional induction of the GADD45 promoter in the absence of direct DNA binding. Mol Cell Biol. 1998;18:2768–2778. doi: 10.1128/mcb.18.5.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q, Lord KA, Alamo I, Jr, Hollander MC, Carrier F, Ron D, Kohn KW, Hoffman B, Liebermann DA, Fornace AJ., Jr The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993;4:897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Ahsan MH, Zhu L, Sambucetti LC, Purchio AF, West DB. NF-κB and not the MAPK signaling pathway regulates GADD45β expression during acute inflammation. J Biol Chem. 2005;280:21400–21408. doi: 10.1074/jbc.M411952200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Bae I, Krishnaraju K, Azam N, Fan W, Smith K, Hoffman B, Liebermann DA. CR6: A third member in the MyD118 and Gadd45 gene family which functions in negative growth control. Oncogene. 1999;18:4899–4907. doi: 10.1038/sj.onc.1202885. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hoffman B, Liebermann DA. Ectopic expression of MyD118/Gadd45/CR6 (Gadd45β/α/γ) sensitizes neoplastic cells to genotoxic stress-induced apoptosis. Int J Oncol. 2001;18:749–757. [PubMed] [Google Scholar]
- Zhang W, Li T, Shao Y, Zhang C, Wu Q, Yang H, Zhang J, Guan M, Yu B, Wan J. Semi-quantitative detection of GADD45-gamma methylation levels in gastric, colorectal and pancreatic cancers using methylation-sensitive high-resolution melting analysis. J Cancer Res Clin Oncol. 2010;136:1267–1273. doi: 10.1007/s00432-010-0777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Jin S, Antinore MJ, Lung FD, Fan F, Blanck P, Roller P, Fornace AJ, Jr, Zhan Q. The central region of Gadd45 is required for its interaction with p21/WAF1. Exp Cell Res. 2000;258:92–100. doi: 10.1006/excr.2000.4906. [DOI] [PubMed] [Google Scholar]
- Zhu N, Shao Y, Xu L, Yu L, Sun L. Gadd45-α and Gadd45-γ utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol Biol Rep. 2009;36:2075–2085. doi: 10.1007/s11033-008-9419-9. [DOI] [PubMed] [Google Scholar]
- Zieleniak A, Wojcik M, Wozniak LA. Structure and physiological functions of the human peroxisome proliferator-activated receptor γ. Arch Immunol Ther Exp (Warsz) 2008;56:331–345. doi: 10.1007/s00005-008-0037-y. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrun SD, Hoffman B, Liebermann DA. Distinct mechanisms are utilized to induce stress sensor gadd45b by different stress stimuli. J Cell Biochem. 2009;108:1220–1231. doi: 10.1002/jcb.22354. [DOI] [PubMed] [Google Scholar]