Abstract
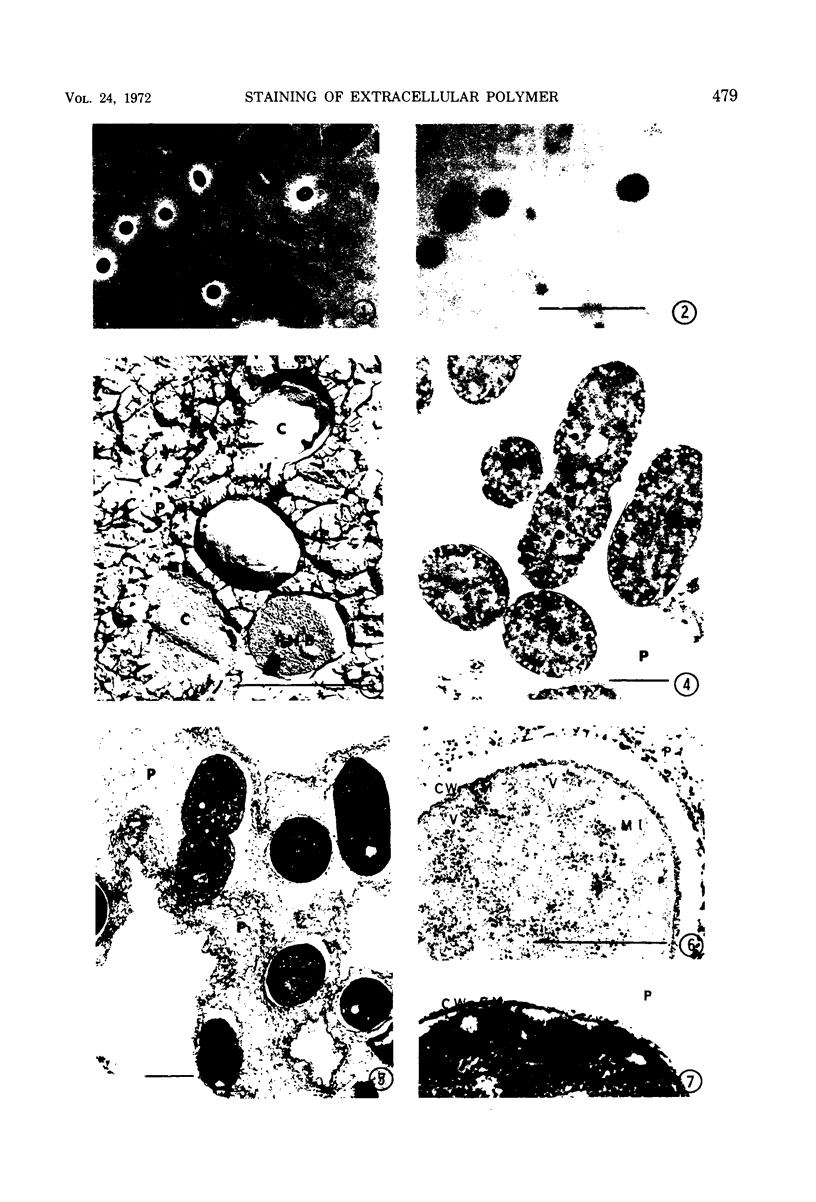
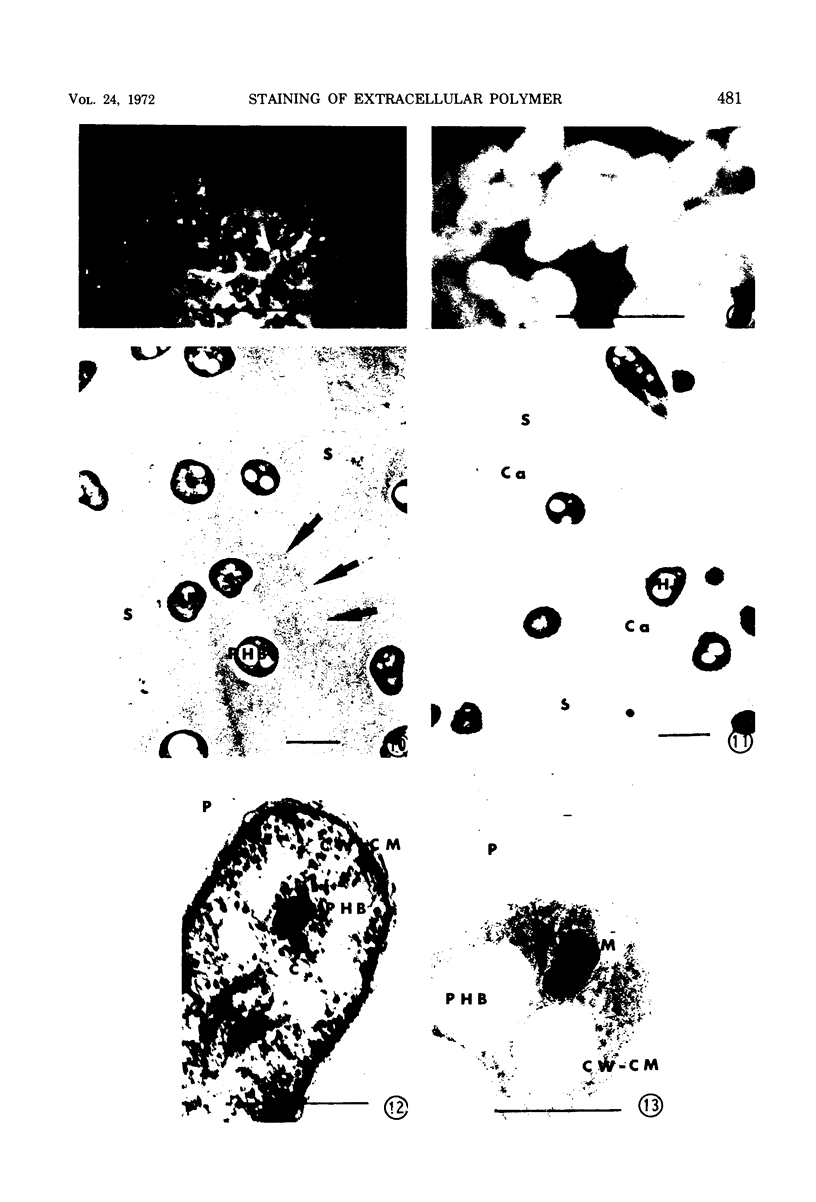
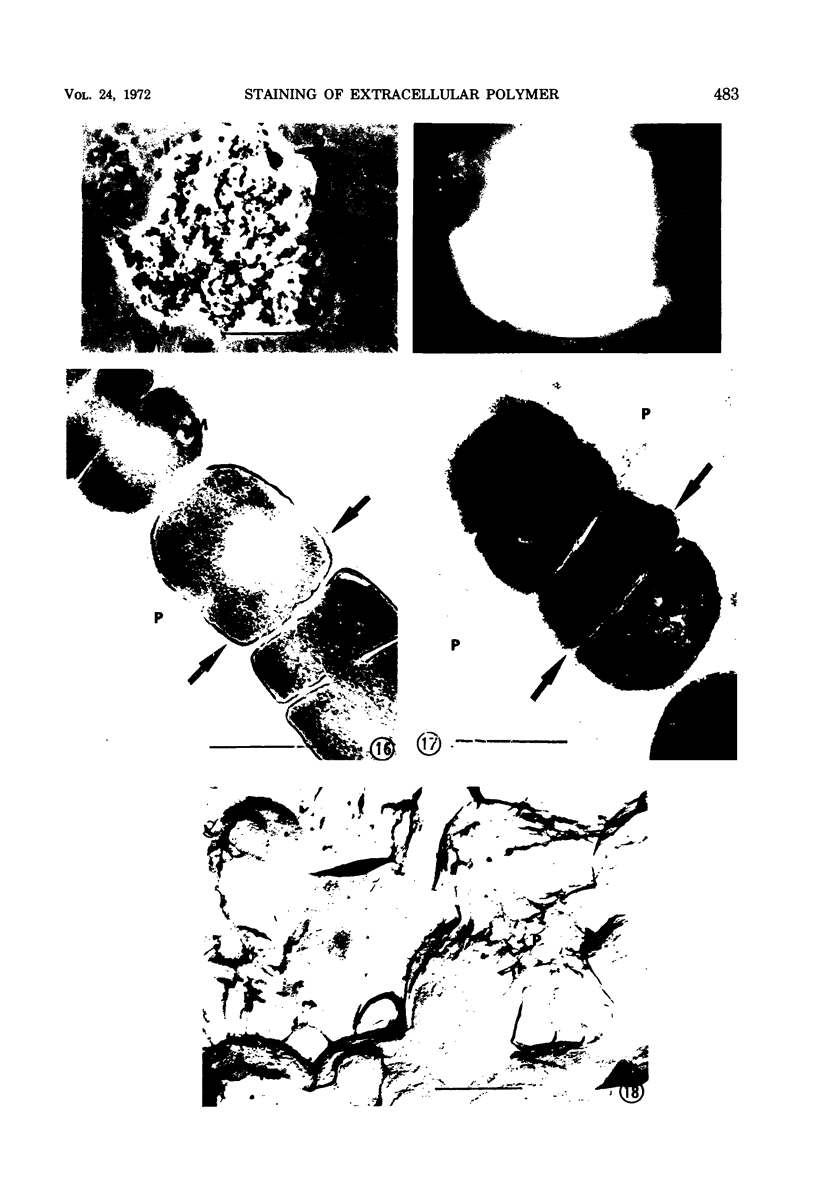
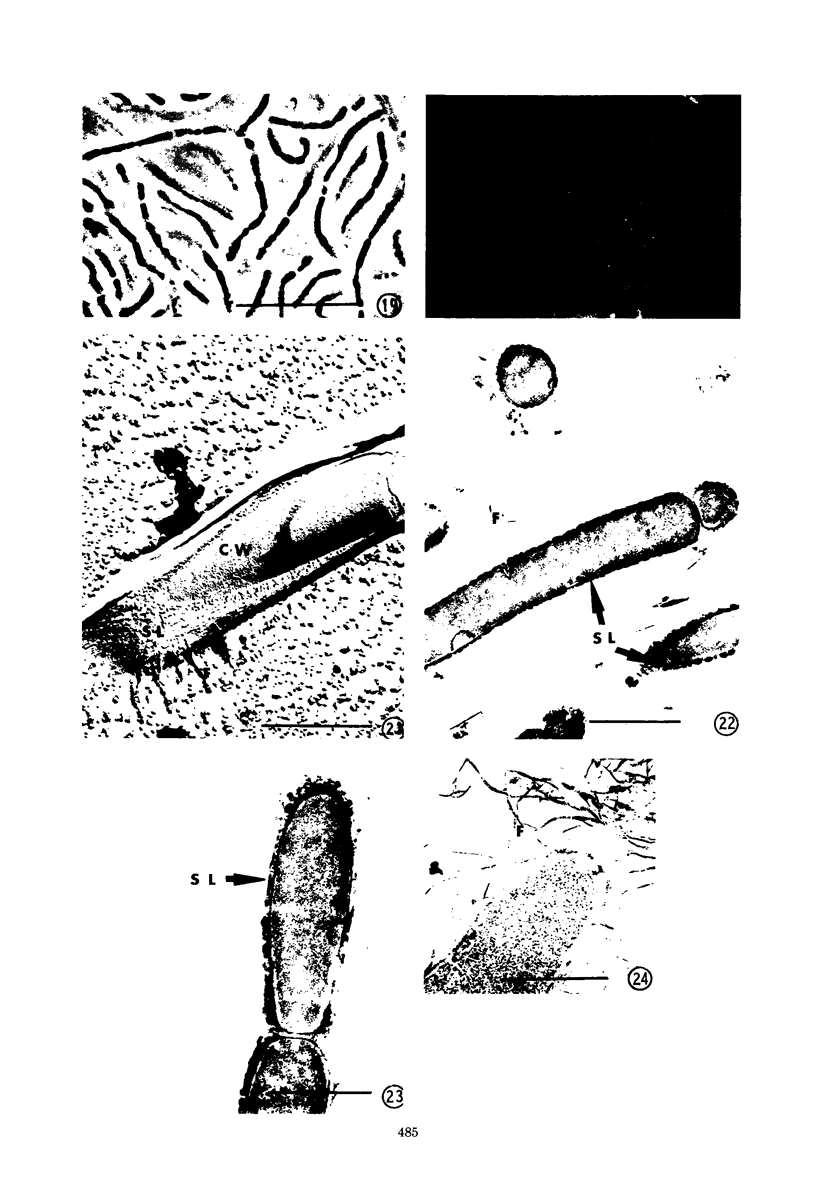
Phase contrast, ultraviolet microscopy, and freeze-etching were used to determine the amount of exocellular polymer surrounding unfixed cells of four genera of bacteria: Azotobacter vinelandii, Zoogloea ramigera, Leuconostoc mesenteroides, and an acid-tolerant, floc-forming Bacillus species. Thin-sectional electron microscopy was employed to measure the effectiveness of a modified ruthenium red staining method. The results obtained with this modification of ruthenium red staining technique were compared to results obtained when previously proposed ruthenium red methods of fixation were employed. The results of these relations were then compared to the amounts of exocellular material as determined with phase-contrast microscopy, ultraviolet microscopy, and freeze-etching. The data obtained indicate that improved fixation of exocellular polymer is achieved when cells are pretreated with ruthenium red as described herein. In addition, the modified methods also reveal cytological detail not apparent when other methods of ruthenium fixation are employed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRABTREE K., MCCOY E., BOYLE W. C., ROHLICH G. A. ISOLATION, IDENTIFICATION, AND METABOLIC ROLE OF THE SUDANOPHILIC GRANULES OF ZOOGLOEA RAMIGERA. Appl Microbiol. 1965 Mar;13:218–226. doi: 10.1128/am.13.2.218-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D., Vela G. R. Giant cysts and cysts with multiple central bodies in Azotobacter vinelandii. J Bacteriol. 1971 Jul;107(1):315–319. doi: 10.1128/jb.107.1.315-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan P. R., MacMillan C. B., Pfister R. M. Aerobic heterotrophic bacteria indigenous to pH 2.8 acid mine water: predominant slime-producing bacteria in acid streamers. J Bacteriol. 1970 Mar;101(3):982–988. doi: 10.1128/jb.101.3.982-988.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B. A., Dugan P. R., Pfister R. M., Remsen C. C. Fine structure and composition of the zoogloeal matrix surrounding Zoogloea ramigera. J Bacteriol. 1968 Dec;96(6):2144–2153. doi: 10.1128/jb.96.6.2144-2153.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL C. E. Electron densitometry of stained virus particles. J Biophys Biochem Cytol. 1955 Jan;1(1):1–12. doi: 10.1083/jcb.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B., GOMEZ C. G., NOYES H. E., HOUSEWRIGHT R. D. Production of glutamyl polypeptide by Bacillus subtilis. J Bacteriol. 1954 Sep;68(3):307–315. doi: 10.1128/jb.68.3.307-315.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani E., Ametani T. Substructure of microtubules in brain nerve cells as revealed by ruthenium red. J Cell Biol. 1970 Jul;46(1):159–165. doi: 10.1083/jcb.46.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]