Abstract
Background and Aims
Evidence shows that antioxidant supplements may increase mortality. Our aims were to assess whether different doses of beta-carotene, vitamin A, and vitamin E affect mortality in primary and secondary prevention randomized clinical trials with low risk of bias.
Methods
The present study is based on our 2012 Cochrane systematic review analyzing beneficial and harmful effects of antioxidant supplements in adults. Using random-effects meta-analyses, meta-regression analyses, and trial sequential analyses, we examined the association between beta-carotene, vitamin A, and vitamin E, and mortality according to their daily doses and doses below and above the recommended daily allowances (RDA).
Results
We included 53 randomized trials with low risk of bias (241,883 participants, aged 18 to 103 years, 44.6% women) assessing beta-carotene, vitamin A, and vitamin E. Meta-regression analysis showed that the dose of vitamin A was significantly positively associated with all-cause mortality. Beta-carotene in a dose above 9.6 mg significantly increased mortality (relative risk (RR) 1.06, 95% confidence interval (CI) 1.02 to 1.09, I2 = 13%). Vitamin A in a dose above the RDA (> 800 µg) did not significantly influence mortality (RR 1.08, 95% CI 0.98 to 1.19, I2 = 53%). Vitamin E in a dose above the RDA (> 15 mg) significantly increased mortality (RR 1.03, 95% CI 1.00 to 1.05, I2 = 0%). Doses below the RDAs did not affect mortality, but data were sparse.
Conclusions
Beta-carotene and vitamin E in doses higher than the RDA seem to significantly increase mortality, whereas we lack information on vitamin A. Dose of vitamin A was significantly associated with increased mortality in meta-regression. We lack information on doses below the RDA.
Background
All essential compounds to stay healthy cannot be synthesized in our body. Therefore, these compounds must be taken through our diet or obtained in other ways [1]. Oxidative stress has been suggested to cause a variety of diseases [2]. Therefore, it is speculated that antioxidant supplements could have a potential role in preventing diseases and death. Despite the fact that a normal diet in high-income countries may provide sufficient amounts of antioxidants [3,4], more than one third of adults regularly take antioxidant supplements [5,6].
Introduction
The preventive potential of antioxidants has been studied in a large number of trials [7]. The results so far have remained largely inconclusive [7]. Systematic reviews of randomized clinical trials have shown that some antioxidant supplements may increase mortality [7,8].
The present study is based on the results of our recently updated Cochrane systematic review of primary and secondary prevention randomized clinical trials with low risk of bias showing that all-cause mortality was significantly increased by the administration of beta-carotene, vitamin E, and possibly vitamin A [8]. The review results urged us to assess whether doses of beta-carotene, vitamin E, and vitamin A influenced all-cause mortality, using meta-regression analyses. We also compared the influence of daily dose within the recommended dietary (daily) allowance (RDA) (low dose) to daily dose above the RDA (high dose) of beta-carotene, or vitamin A, or vitamin E on all-cause mortality in meta-analyses. To control the risks of type I and type II errors in meta-analyses, we used trial sequential analyses to assess whether the level of evidence was sufficient to make conclusions (i.e., whether the required information size was reached or if any of the trial sequential monitoring boundaries were crossed before the required information size was reached) [9–13].
Methods
Review methods are described in detail in two earlier publications [7,8]. Briefly, we identified relevant randomized clinical trials by searching The Cochrane Library, MEDLINE, Embase, LILACS, Science Citation Index Expanded, and Conference Proceedings Citation Index-Science from inception to February 2011 [8]. We scanned bibliographies of relevant publications and asked experts and pharmaceutical companies for additional trials. We considered for inclusion primary and secondary prevention randomized clinical trials in adults (aged ≥ 18 years) comparing beta-carotene, vitamin A, vitamin C, vitamin E, and selenium at any dose, duration, and route of administration versus placebo. The antioxidants could have been administered separately or in any combination, or in combination with other vitamins or trace elements without antioxidant function. Concomitant interventions were allowed when used equally in all the intervention groups of the trial.
For the present study we selected only the randomized primary or secondary prevention trials with low risk of bias where beta-carotene, vitamin A, and vitamin E were compared with placebo. This was in order to assess trials with the lowest risk of bias, that is overestimation of benefits and underestimation of harms (see below) [8]. Trials including general and healthy populations were defined as primary prevention [14]. Trials in which investigators mentioned a specific disease as inclusion criterion for the participants were defined as secondary prevention [14]. We excluded tertiary prevention (treatment) trials, like trials on acute, infectious, or malignant diseases, except for non-melanoma skin cancer [8]. Our only outcome was all-cause mortality at maximum follow-up. Two review authors independently assessed trial eligibility without blinding of the study authors. We listed excluded trials with the reasons for exclusion. Disagreement was resolved by discussion or in consultation with a third author. We contacted authors of the trials for missing information.
Due to the risk of overestimation of beneficial intervention effects and underestimation of harms in randomized clinical trials with unclear or inadequate methodological quality, our present analyses only included trials with low risk of bias [15–18]. The risk of bias was assessed in regard to generation of the allocation sequence, allocation concealment, blinding, incomplete outcome data, outcome reporting, and other bias like industry or academic bias [15–19]. The allocation sequence generation was classified as adequate if based on a table of random numbers or was computer-generated. The allocation concealment was classified as adequate if the allocation of participants involved a central independent unit, on-site locked computer, or identically appearing, numbered, drug bottles prepared by an independent investigator. Sealed envelopes were considered adequate if they were opaque and serially numbered. Blinding was classified as adequate if participants, personnel, and outcome assessors were blinded and the assessment of outcomes was not likely to be influenced by lack of blinding. The description of incomplete outcome data was classified as adequate if missing data were unlikely to make treatment effects depart from plausible values. Outcome reporting was classified as adequate if reported outcomes were pre-defined or clinically relevant and expected. We also considered whether the trial was free of other components that could put it at risk of bias. Trials with adequate assessments in all of the mentioned bias risks domains were considered as having low risk of bias.
Doses of antioxidant supplements
We used the RDA provided by the Institute of Medicine of the National Academies [20,21] to separate trials of low experimental dose (≤ RDA) from trials of high experimental dose (> RDA). The RDA is the dietary intake level that is sufficient to meet the nutrient requirement of nearly all healthy individuals in a particular life stage and sex group [22]. The RDA is determined based upon the estimated average requirement, defined as a nutrient intake value that is estimated to meet the requirements of half the healthy individuals in a group plus twice the standard deviation [22].
RDAs for vitamin A and vitamin E are displayed in Table 2. No RDA has been established for beta-carotene [20,21]. The Institute of Medicine has proposed a retinol activity equivalent (RAE) of 12 mg of beta-carotene, equivalent to the activity of 1000 µg of all-trans retinol [22]. Therefore, we used the dose of 9.6 mg beta-carotene as the ‘RDA’ for beta-carotene equivalent to a dose of vitamin A of 800 µg. This RDA is close to the vitamin A RDA for men and women. We also recorded how many trials used doses above the tolerable upper intake level (TUIL), defined as the highest level of nutrient intake, likely to pose no risk of adverse health effects for almost all individuals in the general population [22,23].
Statistical analyses
We conducted the systematic review according to the recommendations of The Cochrane Handbook for Systematic Reviews of Interventions [15]. For the present analyses, we used RevMan 5.2 (The Nordic Cochrane Centre, Copenhagen) [24], Trial Sequential Analysis version 0.9 beta (The Copenhagen Trial Unit, Copenhagen) [25], STATA 8.2 (STATA Corp, College Station, Tex), and Sigma Stat 3.0 (SPSS Inc, Chicago, Ill). We analyzed the data with a random-effects model meta-analysis calculating the relative risk (RR) with 95% confidence intervals (CI). Heterogeneity was explored using a chi-squared test, and the quantity of heterogeneity was measured using the I2 statistic [26]. Random-effects meta-regression analyses were performed to assess potential covariates that could predict intertrial heterogeneity, i.e., the covariates that were statistically associated with estimated intervention effects. The included covariates were daily dose of beta-carotene, daily dose of vitamin A, daily dose of vitamin E, type of prevention (primary or secondary), and the way of taking the supplements, i.e., singly or combined. We calculated weighted averages for duration of intervention and duration of the follow-up period.
Trial sequential analyses (TSA)
A meta-analysis may result in type I errors and type II errors if data are sparse or if there is repeated testing for significance when new trials are added to it [9–13,27,28]. In a single trial, interim analyses increase the risk of type I errors. To avoid type I errors, group sequential monitoring boundaries [29] are constructed to decide whether a trial could be terminated early in the case of a sufficiently small P value, i.e., what we may see is a cumulative Z-curve crossing the sequential monitoring boundary either for benefit or harm. Trial sequential monitoring boundaries can, in a comparable way, be applied to meta-analysis [9–13]. Trial sequential monitoring boundaries are based on the calculation of a required information size and the formation of trial sequential monitoring boundaries for benefit, harm, and futility [9–13,28]. The methods for adjusting thresholds for significance (i.e., controlling the type I error) are built upon the ‘group sequential analysis’ methodology [10]. An interim look at the data of a meta-analysis is necessitated when data from new clinical trials are added. This happens at arbitrary intervals, and the number of added patients varies and cannot be predicted. Lan and DeMets extended the ‘group sequential’ methodology of repeated significance testing in a single randomized trial to ‘sequential analysis’ in order to allow for flexible, unplanned interim analyses [10]. This flexibility has been used for trial sequential analyses of meta-analyses, where trials are added in the sequence they are published (by year and alphabetically according to the family name of the first author if more trials are published in the same year). With the TSA program, trial sequential monitoring boundaries for benefits, harms, and futility are constructed [10]. If the cumulative Z-curve crosses any of the trial sequential monitoring boundaries for benefit, harm, or futility, sufficient evidence is reached and no further trials may be needed. If the cumulative Z-curve does not cross the trial sequential monitoring boundaries for benefit, or harm, or futility, there is insufficient evidence to reach any conclusion on the intervention effect. If the cumulative Z-curve does not reach the trial sequential monitoring boundary for futility, we have too sparse data to declare that the intervention does not cause benefit or harm [30].
We conducted trial sequential analyses to assess the risk of random errors due to sparse data and repetitive testing of cumulative meta-analyses [10,30]. The diversity (D2)-adjusted required information size (DARIS) and the adjacent trial sequential monitoring boundaries were calculated assuming a mortality of 10% in the placebo group [8]; an anticipated intervention effect of 5% relative risk reduction in the antioxidant supplemented group; a type I error of 5%; a type II error of 20% (80% power); and the diversity of the meta-analysis [28]. In the case when a trial sequential analysis was difficult to interpret because the distance between the accrued information size and the DARIS was too large, we adjusted the assumptions by increasing the anticipated intervention effect (by increasing relative risk reduction) to decrease the distance between the accrued information size and the DARIS (post hoc analyses). This was only conducted to make the TSA figures more easily interpretable. This reduces the apparent gap of required number of participants in further trials and does not affect our conclusions.
Results
We identified a total of 15,545 references of possible interest through searching the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 3456), MEDLINE (n = 3388), Embase (n = 2124), Science Citation Index Expanded (n = 6515), Conference Proceedings Citation Index-Science (n = 28), and reference lists (n = 34) (Figure 1) [8]. We excluded 14,134 duplicates and 401 clearly irrelevant references through reading the abstracts. Accordingly, 1010 references describing 615 trials were retrieved for further assessment. Of these, we excluded 47 studies because they were not randomized trials or did not fulfill our inclusion criteria. We included 568 randomized trials. The authors of 481 trials did not report mortality. The majority of these were small phase I or phase II trials, with a short duration of follow-up and without assessment of clinical outcomes. In nine trials [31–39] there were deaths reported, but the authors did not report in which group of the trial and did not respond to our requests for additional information [8].
Figure 1. PRISMA flow diagram of identification of randomized clinical trials for inclusion.
In total, 78 randomized trials with 296,707 participants reported on mortality (Figure 1). Fifty-six trials (244,056 participants) were with low risk of bias [8]. Twenty-two trials (52,651 participants) were with high risk of bias and were excluded [40–61]. . [8]
Fifty-three randomized trials with low risk of bias assessed beta-carotene, vitamin A, and vitamin E singly, in any combination, or in combination with other vitamins and trace elements. They included 241,883 participants, aged 18 to 103 years, and 44.6% were women. Beta-carotene was assessed in 26 trials (173,006 participants), vitamin A in 12 trials (41,144 participants), and vitamin E in 46 trials (171,244 participants) (Table 1) [62–114]. All antioxidant supplements were administered orally. Beta-carotene was tested in a daily dose of 1.2 to 72.0 mg (mean 21 mg; median 19.5 mg); vitamin A was tested in a daily dose of 400 to 7500 µg (mean 2056 µg; median 800 µg); and vitamin E was tested in a daily dose of 10 to 5000 mg (mean 470 mg; median 350 mg). Two beta-carotene trials [63,79], two vitamin A trials [75,98], and two vitamin E trials [77,93] used doses above the TUIL. The antioxidant supplements were administered daily or on alternate days for 1 day to 12 years; weighted mean 6.9 years. The weighted mean duration of follow-up was 8.5 years (range 2.8 months to 14.1 years) (Table 1; Table 2).
Table 1. Characteristics of 53 Included Randomized Clinical Trials with Low Risk of Bias.
| Trial | Design | No. of Participants | Women % | Mean Age | Suppl. (Y) | Follow-up (Y) | BC, mg | Vit A µg | Vit C mg | Vit E mg | Selenium µg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AREDS [62] 2001 | 2x2 | 4757 | 56 | 68 | 6.3 | 6.3 | 15 | 500 | 400 | ||
| ARMDS [63] 1996 | Parallel | 71 | 7 | 72 | 1.5 | 1.5 | 72 | 750 | 165 | 50 | |
| Allsup et al [64], 2004 | Parallel | 164 | 63 | 83 | 0.15 | 0.5 | 800 | 120 | 60 | 60 | |
| Avenell et al [65], 2005 | Parallel | 910 | 47 | 72 | 1 | 1 | 800 | 60 | 10 | ||
| Boaz et al [66], 2000 | Parallel | 196 | 31 | 65 | 1.42 | 1.42 | 537 | ||||
| Brown et al [67], 2001 | 2x2 | 160 | 13 | 53 | 3 | 3 | 25 | 1000 | 537 | 100 | |
| Chylack et al [68], 2002 | Parallel | 297 | 59 | 68 | 3 | 3 | 18 | 750 | 600 | ||
| Collins et al [69], 2003 | 2x2 | 52 | 2 | 67 | 0.5 | 2.5 | 400 | ||||
| Cook et al [70], 2007 | 2x2x2 | 8171 | 100 | 61 | 9.4 | 9.4 | 25 | 500 | 221 | ||
| Correa et al [71], 2000 | 2x2x2 | 976 | 54 | 51 | 6 | 6 | 30 | 2000 | |||
| Desnuelle et al [72], 2001 | Parallel | 288 | 45 | 64 | 1 | 1 | 1000 | ||||
| Garbagnati et al [73], 2009 | Parallel | 72 | 35 | 65 | 1 | 1 | 19 | 240 | 290 | ||
| Girodon et al [74], 1999 | 2x2 | 725 | 74 | 84 | 2 | 2 | 6 | 120 | 15 | 100 | |
| Goodman et al [75], 2004 | Parallel | 18314 | 34 | 58 | 4 | 10 | 30 | 7500 | |||
| Graat et al [76], 2002 | 2x2 | 652 | 50 | N/A | 1 | 1 | 1.2 | 600 | 60 | 210 | 25 |
| Graf et al [77], 2005 | Parallel | 160 | 35 | 58 | 1.5 | 1.5 | 5000 | ||||
| Green et al [78], 1999 | 2x2 | 1621 | 56 | 49 | 4.5 | 4.5 | 30 | ||||
| Greenberg et al [79], 1990 | Parallel | 1805 | 30 | N/A | 5 | 5 | 50 | ||||
| Greenberg et al [80], 1994 | 2x2 | 864 | 21 | 61 | 4 | 4 | 25 | 1000 | 400 | ||
| Grieger et al [81], 2009 | Parallel | 115 | 65 | NA | 0.5 | 0.5 | 3 | 75 | 12,2 | ||
| Hennekens et al [82], 1996 | 2x2 | 22071 | 0 | 53 | 12 | 12.9 | 25 | ||||
| Hercberg et al [83], 2010 | Parallel | 13017 | 61 | 49 | 7.54 | 7.54 | 6 | 120 | 30 | 100 | |
| Hodis et al [84], 2002 | Parallel | 353 | 52 | 56 | 3 | 3 | 364 | ||||
| Jacobson et al [85], 2000 | Parallel | 112 | 42 | 42 | 0.5 | 0.5 | 12 | 500 | 268 | ||
| Lee et al [86], 2005 | 2x2 | 39876 | 100 | 55 | 10.1 | 10.1 | 25 | 221 | |||
| Li et al [87], 1993 | Parallel | 3318 | 56 | 54 | 6 | 6 | 15 | 3000 | 180 | 54 | 50 |
| Lippman et al [88], 2008 | 2x2 | 35533 | 0 | 62 | 5.5 | 5.5 | 400 | 200 | |||
| Liu et al [89], 2007 | Parallel | 763 | 68 | 85 | 1.6 | 1.6 | 16 | 400 | 80 | 44 | 20 |
| Lonn et al [90], 2005 | 2x2 | 9541 | 27 | 66 | 4.5 | 7 | 294 | ||||
| Magliano et al [91], 2006 | Parallel | 409 | 55 | 63 | 4 | 4 | 336 | ||||
| Manuel-y-Keenoy et al [92], 2004 | Parallel | 24 | 14 | 51 | 0.5 | 4.5 | 503 | ||||
| Marras et al [93], 2005 | 2x2 | 800 | 34 | 61 | 2.6 | 13 | 2000 | ||||
| McNeil et al [94], 2004 | Parallel | 1193 | 56 | 66 | 4 | 4 | 500 | ||||
| Meydani et al [95], 2004 | Parallel | 617 | 73 | 84 | 1 | 1 | 182 | 100 | |||
| Mezey et al [96], 2004 | Parallel | 51 | 33 | 48 | 0.25 | 1 | 1000 | ||||
| Milman et al [97], 2008 | Parallel | 1434 | 52 | 69 | 1.5 | 1.5 | 268 | ||||
| Moon et al [98], 1997 | Parallel | 2297 | 30 | 63 | 3.8 | 3.8 | 7500 | ||||
| Mooney et al [99], 2005 | Parallel | 284 | 45 | 37 | 1.25 | 1.25 | 500 | 364 | |||
| HPS [100] 2002 | 2x2 | 20536 | 25 | N/A | 5 | 5 | 20 | 250 | 600 | ||
| Murphy et al [101], 1992 | Parallel | 109 | N/A | N/A | 0.003 | 0.25 | 667 | ||||
| Pike et al [102], 1995 | Parallel | 47 | 72 | 69 | 1 | 1 | 800 | 90 | 45 | ||
| Plummer et al [103], 2007 | Parallel | 1980 | 53 | NA | 3 | 3 | 18 | 750 | 600 | ||
| Prince et al [104], 2003 | Cross-over | 61 | 92 | 58 | 0.25 | 0.25 | 3 | 150 | 50 | 75 | |
| Richer et al [105], 2004 | Parallel | 61 | 4 | 75 | 1 | 1 | 9 | 750 | 1500 | 413 | 200 |
| Salonen et al [106], 2003 | 2x2 | 520 | 51 | N/A | 6 | 6 | 250 | 182 | |||
| Sesso et al [107], 2008 | 2x2x2 | 14641 | 0 | 64 | 8 | 8 | 25 | 1050 | 560 | 182 | 20 |
| Stephens et al [108], 1996 | Parallel | 2002 | 16 | 62 | 1.4 | 1.4 | 403 | ||||
| Tam et al [109], 2005 | Parallel | 39 | 100 | 46 | 0.23 | 2.67 | 500 | 661 | |||
| Virtamo et al [110], 2003 | 2x2 | 29133 | 0 | 57 | 6.1 | 14.1 | 20 | 50 | |||
| Waters et al [111], 2002 | 2x2 | 423 | 100 | 65 | 3 | 3 | 1000 | 800 | |||
| White et al [112], 2002 | Parallel | 100 | 42 | 63 | 0.23 | 0.23 | 1000 | 200 | |||
| Witte et al [113], 2005 | Parallel | 32 | N/A | N/A | 0.75 | 0.75 | 800 | 500 | 400 | 50 | |
| Wluka et al [114], 2002 | Parallel | 136 | 45 | 64 | 2 | 2 | 368 |
Abbreviations: N/A, Not Available; Suppl, Duration of Supplementation; Y, year; BC, Beta-carotene; Vit, Vitamin.
Table 2. Recommended dietary allowances, tolerable upper intake levels, and experimental doses used of antioxidant supplement.
| Antioxidant supplements | RDA** | TUIL*** | Experimental doses | Median doses | ||
| Men | Women | |||||
| Beta-carotene | 9.6 mg* | 36 mg* | 1.2 to 50 mg | 19.5 mg | ||
| Vitamin A | 900 µg | 700 µg | 3000 µg | 400 to 60000 µg | 800 µg | |
| Vitamin E | 15 mg |
15 mg | 1000 mg**** | 10 to 5000 mg | 350 mg | |
*Calculated based on retinol equivalents.
** RDA The recommended dietary allowance is the average daily dietary intake level that is sufficient to meet the nutrient requirement of nearly all (97 to 98 per cent) healthy individuals in a particular life stage and gender group [20,21].
***TUIL Tolerable upper intake level is the highest level of nutrient intake that is likely to pose no risk of adverse health effects for almost all individuals [22].
****The European Commission Scientific Committee on Food published its opinion on the tolerable upper intake level of vitamin E. The TUIL was established as 270 mg for adults, rounded to 300 mg [23].
Univariate and multivariate meta-regression analyses revealed that only the daily dose of vitamin A was significantly associated with the estimated intervention effect on mortality (univariate RR 1.00002, 95% CI 1.000004 to 1.00004, P = 0.017; multivariate RR 1.00002, 95% CI 1.000001 to 1.00004, P = 0.039) (Table 3). None of the other covariates (daily dose of beta-carotene; daily dose of vitamin E; primary or secondary prevention, single or combined administration) were significantly associated with the estimated intervention effect on mortality (Table 3).
Table 3. Meta-regression analyses on trials with low risk of bias.
| Covariates | Trials (N) |
Univariate
|
Multivaraite
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| RR |
95% CI
|
P | RR |
95% CI
|
P | ||||
| Dose of beta-carotene | 26 | 1.005 | 0.999 | 1.01 | 0.12 | 1.005 | 0.998 | 1.012 | 0.15 |
| Type of prevention* | 1.032 | 0.933 | 1.142 | 0.54 | 1.021 | 0.923 | 1.130 | 0.68 | |
| Administered singly or combined | 1.003 | 0.907 | 1.109 | 0.95 | 0.990 | 0.900 | 1.089 | 0.83 | |
| Dose of vitamin A | 12 | 1.00002 | 1.000004 | 1.00004 | 0.017 | 1.00002 | 1.000001 | 1.00004 | 0.039 |
| Type of prevention* | 1.111 | 0.880 | 1.403 | 0.38 | 1.156 | 0.910 | 1.470 | 0.24 | |
| Administered singly or combined | 1.105 | 0.733 | 1.665 | 0.63 | 1.161 | 0.748 | 1.803 | 0.51 | |
| Dose of vitamin E | 46 | 1.00002 | 0.999 | 1.0001 | 0.68 | 1.00002 | 0.999 | 1.0001 | 0.70 |
| Type of prevention* | 0.989 | 0.924 | 1.059 | 0.76 | 0.982 | 0.905 | 1.067 | 0.67 | |
| Administered singly or combined | 1.028 | 0.960 | 1.100 | 0.44 | 1.039 | 0.964 | 1.120 | 0.31 | |
Abbreviations: RR-relative risk; CI-confidence interval.
*Either primary prevention or secondary prevention.
Meta-analyses and trial sequential analyses
Beta-carotene trials
Beta-carotene used singly versus placebo (7 trials, 43,019 participants) significantly increased mortality (RR 1.06, 95% CI 1.02 to 1.10, I2 = 0%) (Table 4).
Table 4. Type of antioxidant supplement, dose, all-cause mortality, accrued information size (AIS), and diversity-adjusted required information size (DARIS).
| Antioxidants | Dose range and divided according to RDA* | Trials N | All-cause mortality (RR, 95% CI) | AIS | DARIS |
|---|---|---|---|---|---|
| Beta-carotene singly | 25-50 mg | 7 | 1.06 [1.02, 1.10] | 43,019 | 110,505 |
| Beta-carotene singly or combined | 1.2-50 mg | 26 | 1.05 [1.01, 1.09] | 173,006 | 261,708 |
| ≤ 9.6 mg* | 6 | 0.90 [0.69, 1.17] | 14,285 | 267,631 | |
| > 9.6 mg* | 20 | 1.06 [1.02, 1.09] | 158,721 | 190,906 | |
| Vitamin A singly | 667-7500 µg | 2 | 1.18 [0.83, 1.68] | 1323 | 110,505 |
| Vitamin A singly or combined | 400-60000 µg | 12 | 1.07 [0.97, 1.18] | 41,144 | 394,010 |
| ≤ 800 µg* | 8 | 1.05 [0.65, 1.69] | 1415 | 456,748 | |
| > 800 µg* | 4 | 1.08 [0.98, 1.19] | 38,570 | 415,996 | |
| Vitamin E singly | 50-5000 mg | 20 | 1.02 [0.98, 1.05] | 58,904 | 110,505 |
| Vitamin E singly or combined | 10-5000 mg | 46 | 1.03 [1.00, 1.05] | 70,836 | 110,505 |
| ≤ 15 mg* | 2 | 1.32 [0.51, 3.46] | 563 | 119,364 | |
| > 15 mg* | 44 | 1.03 [1.00, 1.05] | 170,219 | 110,505 |
Abbreviations: RDA - recommended dietary allowance; CI – confidence interval; N – number of trials; n – number of participants; AIS – accrued information size; DARIS – diversity-adjusted required information size; n/a – not available
*calculated based on retinol equivalents
Beta-carotene used singly or in combination with other antioxidants versus placebo (26 trials, 173,006 participants) significantly increased mortality (RR 1.05, 95% CI 1.01 to 1.09, I2 = 21%) (Table 4).
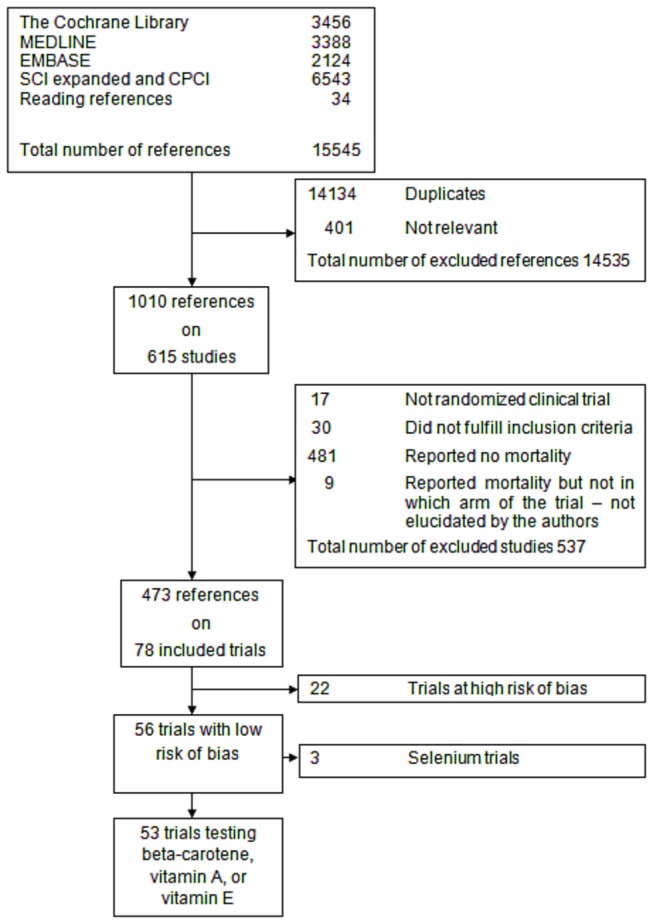
Beta-carotene used singly or in combination with other antioxidants in a dose at or below 9.6 mg (considered to be the RDA) versus placebo (6 trials, 14,285 participants) had no significant effect on mortality (RR 0.90, 95% CI 0.69 to 1.17, I2 = 23%) (Table 4). There was diversity (D2 = 59%). The DARIS was 267,631 participants (Table 4). Accordingly, only 5.3% of the DARIS were randomized. The trial sequential analysis of the six trials revealed that the cumulative Z-curve (blue line) did neither cross the conventional boundaries for significance (green lines) nor the trial sequential monitoring boundaries for harm or benefit (inward sloping red lines) (Figure 2). Neither did the cumulative Z-curve reach the trial sequential monitoring boundaries for futility (not even drawn by the program due to the large difference between the accrued information size and the DARIS).
Figure 2. Trial sequential analysis of 6 trials assessing beta-carotene in a dose at or below 9.6 mg daily versus placebo.
The diversity-adjusted required information size (DARIS = 267,631 participants) was based on a proportion of deaths of 10% in the placebo group; a relative risk reduction of 5% in the beta-carotene group; an alpha of 5%; a beta of 20%; and a diversity of 59%. The blue line represents the cumulative Z-score of the meta-analysis. The green lines represent the conventional statistical boundaries. The red lines represent the truncated trial sequential monitoring boundaries.
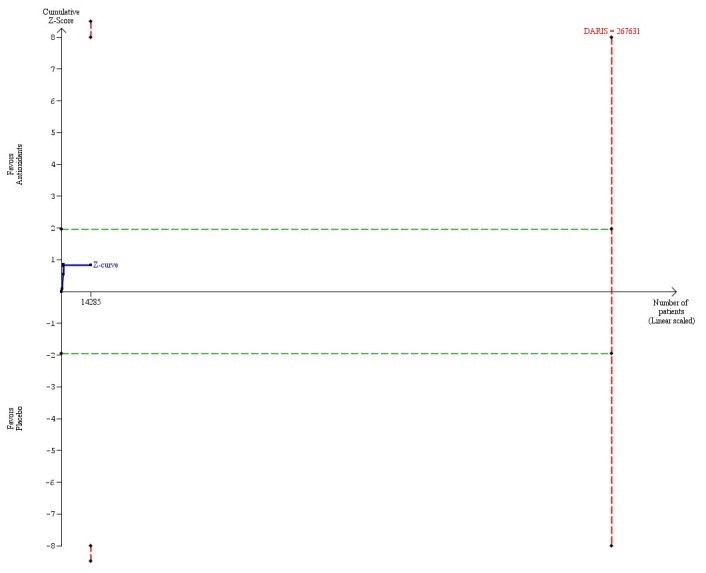
Beta-carotene in a dose above 9.6 mg (considered to be the RDA) versus placebo (20 trials, 158,721 participants) significantly increased mortality (RR 1.06, 95% CI 1.02 to 1.09, I2 = 13%) (Table 4). There was diversity (D2 = 42%). The DARIS was 190,906 participants (Table 4; Figure 3). The trial sequential analysis revealed that the cumulative Z-curve (blue line) crossed both the conventional significant boundary for harm in 2003 during the 13th trial (green line) and the trial sequential monitoring boundary for harm (inward sloping red line) also in 2003 during the 13th trial (Figure 3).
Figure 3. Trial sequential analysis of 20 trials assessing beta-carotene in a dose above 9.6 mg daily versus placebo.
The diversity-adjusted required information size (DARIS = 190,906 participants) was based on a proportion of deaths of 10% in the placebo group; a relative risk reduction of 5% in the beta-carotene group; an alpha of 5%; a beta of 20%; and a diversity of 42%. The blue line represents the cumulative Z-score of the meta-analysis. The green lines represent the conventional statistical boundaries. The red inward sloping lines represent the trial sequential monitoring boundaries. The red outward sloping lines represents the area of futility.
The difference between the estimate of the effect of beta-carotene on mortality in trials using a dose of beta-carotene within the RDA and trials using a dose of beta-carotene above the RDA was not statistically significant (Chi2 = 1.48, P = 0.22).
Vitamin A trials
Vitamin A used singly versus placebo (2 trials, 2406 participants) had no significant effect on mortality (RR 1.18, 95% CI 0.83 to 1.68, I2 = 0%) (Table 4).
Vitamin A used singly or in combination with other antioxidants versus placebo (12 trials, 41,144 participants) had no significant effect on mortality (RR 1.07, 95% CI 0.97 to 1.18, I2 = 27%) (Table 4).
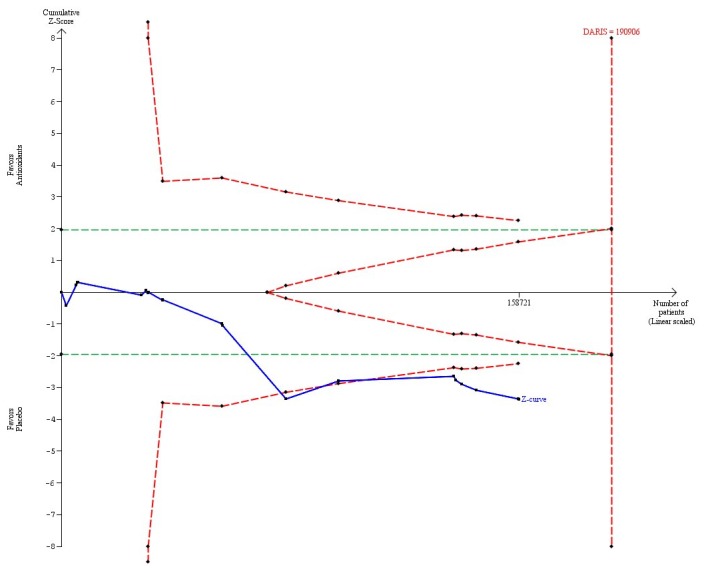
Vitamin A in a dose at or below the RDA (≤ 800 µg) versus placebo (8 trials, 2574 participants) had no significant effect on mortality (RR 1.05, 95% CI 0.65 to 1.69, I2 = 15%) (Table 4). There was diversity (D2 = 76%). The DARIS was 48,417 participants based upon a relative risk reduction of 15% (post hoc estimation) and 456,748 participants based upon a priory relative risk reduction of 5% (Table 4; Figure 4). Accordingly, only 5.6% of the latter number of participants were randomized. The trial sequential analysis showed that the cumulative Z-curve (blue line) neither crossed the conventional boundaries for significance (green lines) nor the trial sequential monitoring boundaries for harm or benefit (inward sloping red lines). Neither did the cumulative Z-curve reach the trial sequential monitoring boundaries for futility (not even drawn by the program due to the large difference between the accrued information size and the DARIS even in the post hoc analysis) (Figure 4).
Figure 4. Trial sequential analysis of 8 trials assessing vitamin A in a dose at or below RDA (≤ 800 µg) daily versus placebo.
The diversity-adjusted required information size (DARIS = 48,417 participants) was based on a proportion of deaths of 10% in the placebo group; a relative risk reduction of 15% in the vitamin A group; an alpha of 5%; a beta of 20%; and a diversity of 76%. The blue line represents the cumulative Z-score of the meta-analysis. The green lines represent the conventional statistical boundaries. The red lines represent the truncated trial sequential monitoring boundaries. Had we used a relative risk reduction of 5% as planned, the DARIS would have been 456,748 participants and the program could not have drawn the trial sequential analysis due to the fact that the number of randomized patients out of the DARIS is too small. This is why, we post hoc decided to construct the trial sequential analysis with a larger relative risk reduction.
Vitamin A in doses above the RDA (> 800 µg) versus placebo (4 trials, 38,570 participants) had no significant effect on mortality (RR 1.08, 95% CI 0.98 to 1.19, I2 = 53%) (Table 4). There was diversity (D2 = 73%). The DARIS was 415,996 participants (Table 4; Figure 5). Accordingly, only 9.2% of the DARIS were randomized. The trial sequential analysis showed that the cumulative Z-curve (blue line) neither crossed the conventional boundaries for significance (green lines) nor the trial sequential monitoring boundaries for harm or benefit (inward sloping red lines). Neither did the cumulative Z-curve reach the trial sequential monitoring boundaries for futility (not even drawn by the program due to the large difference between the accrued information size and the DARIS) (Figure 5).
Figure 5. Trial sequential analysis of 4 trials assessing vitamin A in a dose above the RDA (> 800 µg) daily versus placebo.
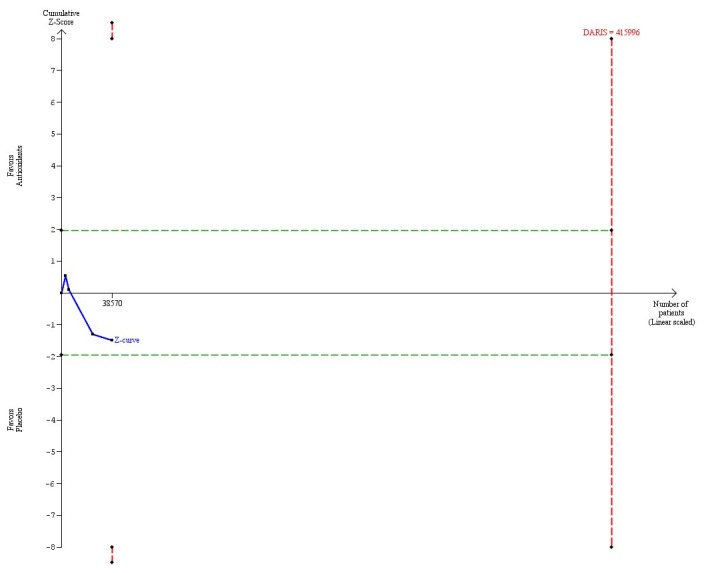
The diversity-adjusted required information size (DARIS = 415,996 participants) was based on a proportion of deaths of 10% in the placebo group; a relative risk reduction of 5% in the vitamin A group; an alpha of 5%; a beta of 20%; and a diversity of 73%. The blue line represents the cumulative Z-score of the meta-analysis. The green lines represent the conventional statistical boundaries. The red lines represent the truncated trial sequential monitoring boundaries.
The difference between the estimate of the effect of vitamin A on mortality in trials using a dose of vitamin A within the RDA and trials using a dose of vitamin A above the RDA was not significant (Chi2 = 0.01, P = 0.92).
Vitamin E trials
Vitamin E used singly versus placebo (20 trials, 58,904 participants) had no significant effect on mortality (RR 1.02, 95% CI 0.98 to 1.05, I2 = 0%) (Table 4).
Vitamin E used singly or in combination with other antioxidants versus placebo (46 trials, 171,244 participants) significantly increased mortality (RR 1.03, 95% CI 1.00 to 1.05, I2 = 0%) (Table 4).
Vitamin E in a dose at or below the RDA (≤ 15 mg) versus placebo (2 trials, 1025 participants) had no significant effect on mortality (RR 1.32, 95% CI 0.51 to 3.46, I2 = 7%) (Table 4). There was diversity (D2 = 7%). The DARIS was 119,364 participants (Table 4; Figure 6). Accordingly, only 0.9% of the DARIS were randomized. The trial sequential analysis showed that the cumulative Z-curve (blue line) neither crossed the conventional significant boundaries (green lines) nor the trial sequential monitoring boundaries for harm or benefit (inward sloping red lines). Neither did the cumulative Z-curve reach the trial sequential monitoring boundaries for futility (which were not even drawn by the program due to the large difference between the accrued information size and the DARIS even in the post hoc analysis) (Figure 6).
Figure 6. Trial sequential analysis of 2 trials assessing vitamin E in a dose at or below RDA (≤ 15 mg) daily versus placebo.
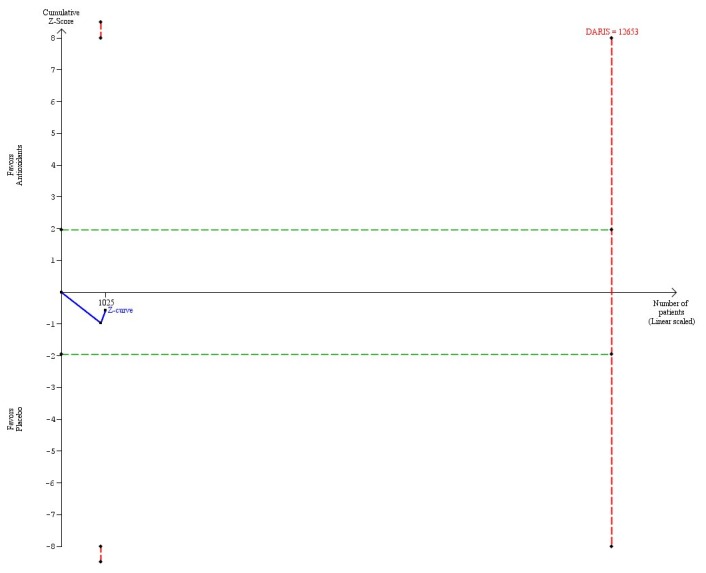
The diversity-adjusted required information size (DARIS = 12,563 participants) was based on a proportion of deaths of 10% in the placebo group; a relative risk reduction of 15% in the vitamin E group; an alpha of 5%; a beta of 20%; and a diversity of 7%. The blue line represents the cumulative Z-score of the meta-analysis. The green lines represent the conventional statistical boundaries. The red lines represent the truncated trial sequential monitoring boundaries. Had we used a relative risk reduction of 5% as planned, the DARIS would have been 119,364 participants and the program could not have drawn the trial sequential analysis due to the fact that the number of randomized patients out of the DARIS is too small. This is why, we post hoc decided to construct the trial sequential analysis with a larger relative risk reduction.
Vitamin E in a dose above the RDA (> 15 mg) versus placebo (44 trials, 170,219 participants) significantly increased mortality (RR 1.03, 95% CI 1.00 to 1.05, I2 = 0%) (Table 4). There was no diversity (D2 = 0%). The DARIS was 110,505 participants (Table 4; Figure 7). The trial sequential analysis showed that the cumulative Z-curve (blue line) crossed the conventional boundary for harm in 2003 during the 21th trial and crossed the trial sequential monitoring boundary for harm (inward sloping red line) in 2005 during the 34th trial (Figure 7).
Figure 7. Trial sequential analysis of 44 trials assessing vitamin E in a dose above the RDA (> 15 mg) daily versus placebo.
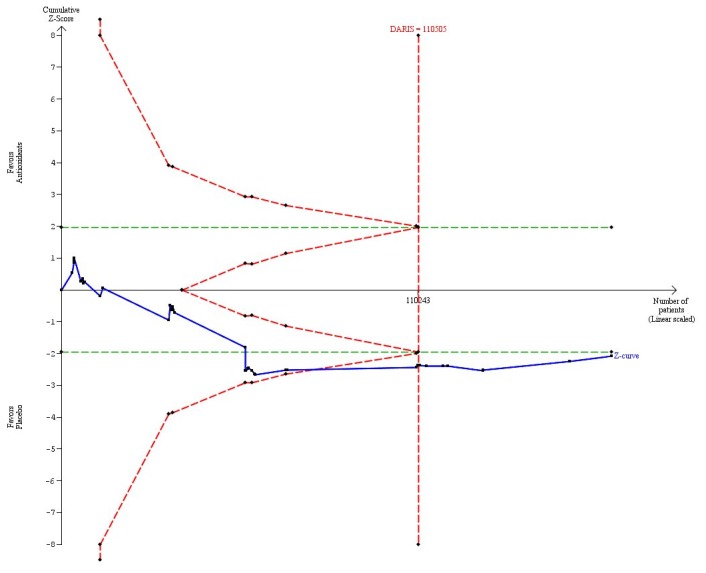
The diversity-adjusted required information size (DARIS = 110,505 participants) was based on a proportion of deaths of 10% in the placebo group; a relative risk reduction of 5% in the vitamin E group; an alpha of 5%; a beta of 20%; and a diversity of 0%. The blue line represents the cumulative Z-score of the meta-analysis. The green lines represent the conventional statistical boundaries. The red inward sloping lines represent the trial sequential monitoring boundaries. The red outward sloping lines represent the area of futility.
The difference between the estimate of the effect of vitamin E on mortality in trials using a dose within the RDA and trials using a dose above the RDA was not significant (Chi2 = 0.27, P = 0.60).
Discussion
Our study contains a number of new findings. Beta-carotene and vitamin E used singly or in combination with other antioxidants significantly increased mortality when administered in doses above the RDA. These findings were supported by trial sequential analyses, leaving out random errors. We could not draw any firm conclusion if doses of vitamin A above the RDA were harmful or not. The trial sequential analysis for vitamin A demonstrated that we do not have sufficient evidence, as the cumulative Z-curve did not cross the conventional boundaries, the trial sequential monitoring boundaries for benefit or harm, or the trial sequential monitoring boundaries for futility. Accordingly, the lack of benefit or harm could be a random error [13,28]. On the other hand, our meta-regression analysis showed that the dose of vitamin A was significantly associated with the estimated intervention effect on mortality. In support of this observation, when we meta-analyzed only the trials in which interaction or effect modification from the factorial design could be excluded, we observed a significant increase in mortality from vitamin A (RR 1.14, 95% CI 1.07 to 1.20; 10 trials, 26,015 participants), in accordance with our previous observations [7,8].
The effects of beta-carotene, vitamin A, and vitamin E on mortality seemed neutral when administered in doses within the RDAs. Again, the trial sequential analyses showed that we have insufficient information to show if the antioxidants within the RDAs provide benefits, harms, or have neutral effects. Absence of evidence is not evidence of absence of effect, be it beneficial or harmful [115]. The fact that we cannot find any significant differences between the intervention effects of meta-analyses of doses above the RDAs when compared to the intervention effects of meta-analyses of doses within the RDAs underlines further that we cannot assume lack of harm from smaller doses.
Strengths
Our present study represents a comprehensive review of 53 randomized clinical trials with low risk of bias including almost a quarter million participants. This increases the precision and power of our analyses [15]. The outcome of all-cause mortality has been the primary outcome of our systematic review since publication of the first protocol in 2003 [8]. Previous meta-analyses of preventive trials of antioxidant supplements have included less information [116–118]. We only focused on trials with low risk of bias, leaving out trials that may overestimate benefits and underestimate harms [15–19]. Our meta-analyses had little trial heterogeneity. This further increases the trustworthiness of our findings. We also performed trial sequential analyses to estimate the risk of random errors in the cumulative meta-analyses and to prevent premature statements of superiority or inferiority of antioxidant supplements [9,11–13,27,28]. Hereby, we were able to assess the risks of random errors [9,11–13,27,28]. One should discuss how much evidence one would require when dealing with potential harm or harmful effects. On the one hand, harmful effects may occur due to random errors, and, therefore, sufficient information needs to be assessed to demonstrate harm beyond reasonable doubt. We have excluded such random error risks for doses above the RDA for beta-carotene and vitamin E. On the other hand, when the evidence is pointing towards harm, then we think, that ethical considerations should prevail and proof beyond any doubt should be avoided [119,120].
Limitations
Our present study has several limitations. As with all systematic reviews, our findings and interpretations are limited by the quality and quantity of available evidence on the effects of specific supplements on mortality. A number of trials did not report mortality, although mortality is a very important part of serious adverse events and ought to be reported in all randomised trials [8]. We have extensively examined the influence of trials without any deaths on our results and found no noticeable effects [8]. The examined populations varied. The effects of supplements were assessed in general population or in patients with gastrointestinal, cardiovascular, neurological, skin, ocular, renal, endocrinological, rheumatoid, and undefined diseases in a stable phase [8]. We did not observe any differences in the effects of the beta-carotene, vitamin A, and vitamin E singly or in different combinations on mortality in these two groups [8]. These populations mostly came from countries without overt deficiencies of specific supplements. Accordingly, we are unable to assess how the antioxidant supplements affected mortality in populations with specific nutritional needs. Most trials assessed combinations of different supplements, which reflects the way supplements are marketed, sold, and taken by people [6,121,122]. As a result, we have compared antioxidants with different properties, given at different doses, and for different periods of time, either singly or combined. We are aware of the potential risks in assessing together the effects of different types of antioxidants with different mechanisms of action, biotransformation, and bioavailability. The methodological quality of some of the trials was assessed using the published reports, which may not reflect the actual design and risk of bias of the trials. Only some authors responded to our requests for further information [8]. Furthermore, our review includes several trials in which we cannot exclude interaction or effect modification by other interventions examined in these trials [8]. However, when we removed trials with potential interaction or effect modification then a statistically significant effect of vitamin A on all-cause mortality was revealed [8].
Most trials investigated the effects of supplements administered at higher doses than those commonly found in a balanced diet using doses well above the RDA. Some trials assessed doses even above the upper tolerable intake levels (Table 2) [20,21]. Too low intake of certain vitamins and minerals may lead to specific deficiency syndromes. However, too high intakes are associated with adverse health effects [123].
Our results extend the evidence in previous reviews suggesting that beta-carotene, vitamin A, and vitamin E may be harmful [117,118,124]. There are several possible explanations for the negative effect of antioxidant supplements on mortality [125,126]. Although oxidative stress has a hypothesized role in the pathogenesis of many chronic diseases, it may be the consequence of pathologic conditions [127,128]. By eliminating free radicals from our organism, we interfere with some essential defensive mechanisms like apoptosis, phagocytosis, and detoxification [125,126,129]. Antioxidant supplements are synthetic and not subjected to the same rigorous toxicity studies like other pharmaceutical agents [130]. Better understanding of mechanisms of action, biotransformation, bioavailability, safety, and appropriate dosage of antioxidants in relation to potential disease prevention is needed. [131] A balanced diet can provide sufficient and healthy amounts of vitamins [3,4]. Today, more than one half of adults in high-income countries ingest dietary supplements [6,132], and most frequently in the form of multivitamins with or without minerals [122]. When combined with dietary intake, the total intake of vitamin A and vitamin E of antioxidant supplement users in the United States exceeds 100% of the estimated average requirement, with the intake of vitamin E exceeding 700% of the estimated average requirement [122,133,134]. Bailey et al. recently reported that 5% of adults in the United States older than 50 years exceed the vitamin A tolerable upper intake level [132]. Therefore, there is a clear need for a risk assessment of antioxidant supplements because the total daily intake of certain vitamins and minerals may reach critical levels. [135] One should consider the U-shaped relation between vitamin status and mortality risk [123,136,137]. Antioxidant vitamins may have adverse health effects at both too low and too high intakes. Lack of a vitamin due to inadequate intake, malabsorption, or increased excretion may lead to deficiency. In this situation, it seems rational to use vitamin supplementation. However, excessive supplementation in people already well saturated with antioxidants through their diet seem to provoke adverse health effects [123,138,139].
Our results are consistent with the 2010 Dietary Guidelines for Americans, as well as conclusions of a National Institutes of Health-sponsored State-of-the-Science Conference that there is no evidence to support the use of multivitamin/mineral supplements in the primary prevention of chronic diseases [140,141]. There is no convincing evidence showing benefits of antioxidant supplements on disease prevention; cancer occurrence; cardiovascular outcomes; or quality of life.
Conclusions
The current evidence calls for a shift in attitude towards antioxidant supplements, with beta-carotene, vitamin A, and vitamin E in specific. The current evidence on the effects of these antioxidants on all-cause mortality, disease occurrence, and quality of life does not support the use of these antioxidant supplements in a generally well-nourished population [142–144]. Beta-carotene, vitamin A, and vitamin E in lower doses may have neutral or beneficial effects on mortality, but according to our accumulated evidence we cannot exclude harmful effects either. We simply lack such knowledge.
Acknowledgments
We thank Rosa G. Simonetti and Lise Lotte Gluud for our collaboration on the Cochrane Systematic review which forms the basis of the present study.
Funding Statement
The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark, provided monetary support for the study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rosenfeld L (1997) Vitamin e - vitamin. The early years of discovery. Clin Chem 43: 680-685. PubMed: 9105273. [PubMed] [Google Scholar]
- 2. Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82: 291-295. PubMed: 9129943. [DOI] [PubMed] [Google Scholar]
- 3. Dwyer JT, Costello RB (2013) Assessment of dietary supplement use. In: Coulston AM, Boushey CJ, Ferruzzi MG. Nutrition in the prevention and treatment of disease. Amsterdam, Boston, Heidelberg, London, New York. Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo: Oxford: Academic Press; . pp. 47-64 [Google Scholar]
- 4. Herbert V, Subak-Sharpe G, Hammock DA (1990) The Mount Sinai School of Medicine Complete Book of Nutrition. New York: St Martin’s Press. [Google Scholar]
- 5. Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT (2013) Why US adults use dietary supplements. JAMA Intern Med 173: 355-361. doi:10.1001/jamainternmed.2013.2299. PubMed: 23381623. [DOI] [PubMed] [Google Scholar]
- 6. Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS et al. (2011) Dietary supplement use in the United States, 2003-2006. J Nutr 141: 261-266. doi:10.3945/jn.110.133025. PubMed: 21178089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention. JAMA 297: 842-857. doi:10.1001/jama.297.8.842. PubMed: 17327526. [DOI] [PubMed] [Google Scholar]
- 8. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (2012) Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 310: 1002/14651858.CD007176.pub2 PubMed: 2241932018425980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brok J, Thorlund K, Wetterslev J, Gluud C (2009) Apparently conclusive meta-analyses may be inconclusive--Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 38: 287-298. doi:10.1093/ije/dyn188. PubMed: 18824466. [DOI] [PubMed] [Google Scholar]
- 10. Thorlund K, Engstrom J, Wetterslev J, Brok J, Imberger G et al. (2011) User manual for trial sequential analysis (TSA). Available: www ctu dk/tsa.
- 11. Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP et al. (2009) Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses. Int J Epidemiol 38: 276-286. doi:10.1093/ije/dyn179. PubMed: 18824467. [DOI] [PubMed] [Google Scholar]
- 12. Thorlund K, Imberger G, Walsh M, Chu R, Gluud C et al. (2011) The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis- a simulation study. PLOS ONE 6: e25491. doi:10.1371/journal.pone.0025491. PubMed: 22028777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wetterslev J, Thorlund K, Brok J, Gluud C (2008) Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 61: 64-75. doi:10.1016/j.jclinepi.2007.03.013. PubMed: 18083463. [DOI] [PubMed] [Google Scholar]
- 14. Starfield B, Hyde J, Gérvas J, Heath I (2008) The concept of prevention: a good idea gone astray? J Epidemiol Community Health 62: 580-583. doi:10.1136/jech.2007.071027. PubMed: 18559439. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 [updated March 2011]. The Cochrane Colloboration.
- 16. Savović J, Jones H, Altman D, Harris R, Jűni P et al. (2012) Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol Assess 16: 1-82. PubMed: 22989478. [DOI] [PubMed] [Google Scholar]
- 17. Savović J, Jones HE, Altman DG, Harris RJ, Jüni P et al. (2012) Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 157: 429-438. PubMed: 22945832. [DOI] [PubMed] [Google Scholar]
- 18. Wood L, Egger M, Gluud LL, Schulz KF, Jüni P et al. (2008) Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 336: 601-605. doi:10.1136/bmj.39465.451748.AD. PubMed: 18316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L (2012) Industry sponsorship and research outcome. Cochrane Database Syst Rev MR, 12: 000033. doi: 10.1002/14651858.MR000033.pub2. PubMed: 23235689. [DOI] [PubMed] [Google Scholar]
- 20. Institute of Medicine, National Academy of Sciences (2000) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. In: .Washington, DC: National Academy Press; pp. 1-506. [Google Scholar]
- 21. Institute of Medicine, National Academy of Sciences (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. In: .Washington, DC: National Academy Press; pp. 1-773. [PubMed] [Google Scholar]
- 22. Standing Committee on the Scientific Evaluation of Dietary (1998). Reference Intakes. Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients. In: The National Academies Press; pp. 1-71. PubMed; : 231936252084556523115811 [PubMed] [Google Scholar]
- 23. Scientific Committee on Food, Scientific Panel on Dietetic Products, Nutrition and Allergies (2006) Tolerable upper intake levels for vitamins and minerals. In: European Food Safety Authority; pp. 1-480. [Google Scholar]
- 24. the Nordic Cochrane Centre The Cochrane Collaboration (2012) Review Manager (RevMan), version 5.2 Copenhagen [computer program].
- 25.Trial Copenahgen. Unit; (2011)Trial Sequential Analysis, version 0.9 beta [computer program] [Google Scholar]
- 26. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539-1558. doi:10.1002/sim.1186. PubMed: 12111919. [DOI] [PubMed] [Google Scholar]
- 27. Brok J, Thorlund K, Gluud C, Wetterslev J (2008) Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 61: 763-769. doi:10.1016/j.jclinepi.2007.10.007. PubMed: 18411040. [DOI] [PubMed] [Google Scholar]
- 28. Wetterslev J, Thorlund K, Brok J, Gluud C (2009) Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 9: 86: 86-. doi:10.1186/1471-2288-9-86. PubMed: 20042080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lan KK, DeMets D (1983) Discrete sequential monitoring boundaries for clinical trials. Biometrika 70: 659-663. doi:10.1093/biomet/70.3.659. [Google Scholar]
- 30. Wetterslev J, Engstrřm J, Gluud C (2012) Trial sequential analysis: methods and software for cumulative meta-analyses. Cochrane Methods Cochrane Database Syst Rev Suppl 1: 29-31. [Google Scholar]
- 31. Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD (2005) Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 46: 166-172. doi:10.1016/j.jacc.2005.02.089. PubMed: 15992652. [DOI] [PubMed] [Google Scholar]
- 32. Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW et al. (1993) A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol 111: 761-772. doi:10.1001/archopht.1993.01090060049022. PubMed: 8512476. [DOI] [PubMed] [Google Scholar]
- 33. Bogden JD, Oleske JM, Lavenhar MA, Munves EM, Kemp FW et al. (1990) Effects of one year of supplementation with zinc and other micronutrients on cellular immunity in the elderly. J Am Coll Nutr 9: 214-225. PubMed: 2358617. [DOI] [PubMed] [Google Scholar]
- 34. Bussey HJ, DeCosse JJ, Deschner EE, Eyers AA, Lesser ML et al. (1982) A randomized trial of ascorbic acid in polyposis coli. Cancer 50: 1434-1439. doi:10.1002/1097-0142(19821001)50:7. PubMed: 7049351. [DOI] [PubMed] [Google Scholar]
- 35. Chandra RK (2001) Effect of vitamin and trace-element supplementation on cognitive function in elderly subjects. Nutrition 17: 709-712. doi:10.1016/S0899-9007(01)00610-4. PubMed: 11527656. [DOI] [PubMed] [Google Scholar]
- 36. MacLennan R, Macrae F, Bain C, Battistutta D, Chapuis P et al. (1995) Randomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomas. The Australian Polyp Prevention Project. J Natl Cancer Inst 87: 1760-1766. PubMed: 7473832. [DOI] [PubMed] [Google Scholar]
- 37. Muñoz N, Wahrendorf J, Bang LJ, Crespi M, Thurnham DI et al. (1985) No effect of riboflavine, retinol, and zinc on prevalence of precancerous lesions of oesophagus. Randomised double-blind intervention study in high-risk population of China. Lancet 2: 111-114. PubMed: 2862315. [DOI] [PubMed] [Google Scholar]
- 38. Roncucci L, Di Donato P, Carati L, Ferrari A, Perini M et al. (1993) Antioxidant vitamins or lactulose for the prevention of the recurrence of colorectal adenomas. Colorectal Cancer Study Group of the University of Modena and the Health Care District 16. Dis Colon Rectum 36: 227-234. doi:10.1007/BF02053502. PubMed: 8449125. [DOI] [PubMed] [Google Scholar]
- 39. Stich HF, Hornby AP, Mathew B, Sankaranarayanan R, Nair MK (1988) Response of oral leukoplakias to the administration of vitamin A. Cancer Lett 40: 93-101. doi:10.1016/0304-3835(88)90266-2. PubMed: 3370632. [DOI] [PubMed] [Google Scholar]
- 40. Stevic Z, Nicolic A, Blagojevic D, Saicia ZS, Kocev N et al. (2001) A controlled trial of combination of methionine and antioxidants in ALS patients. Yug Med Bioch 20: 223-228. [Google Scholar]
- 41. Takagi H, Kakizaki S, Sohara N, Sato K, Tsukioka G et al. (2003) Pilot clinical trial of the use of alpha-tocopherol for the prevention of hepatocellular carcinoma in patients with liver cirrhosis. Int J Vitam Nutr Res 73: 411-415. doi:10.1024/0300-9831.73.6.411. PubMed: 14743544. [DOI] [PubMed] [Google Scholar]
- 42. Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K et al. (1997) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 336: 1216-1222. doi:10.1056/NEJM199704243361704. PubMed: 9110909. [DOI] [PubMed] [Google Scholar]
- 43. de la Maza MP, Petermann M, Bunout D, Hirsch S (1995) Effects of long-term vitamin E supplementation in alcoholic cirrhotics. J Am Coll Nutr 14: 192-196. PubMed: 7790695. [DOI] [PubMed] [Google Scholar]
- 44. Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R et al. (2005) Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352: 2379-2388. doi:10.1056/NEJMoa050151. PubMed: 15829527. [DOI] [PubMed] [Google Scholar]
- 45. McKeown-Eyssen G, Holloway C, Jazmaji V, Bright-See E, Dion P et al. (1988) A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res 48: 4701-4705. PubMed: 3293777. [PubMed] [Google Scholar]
- 46. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 354: 447-455. [PubMed] [Google Scholar]
- 47. de Waart FG, Kok FJ, Smilde TJ, Hijmans A, Wollersheim H et al. (2001) Effect of glutathione S-transferase M1 genotype on progression of atherosclerosis in lifelong male smokers. Atherosclerosis 158: 227-231. doi:10.1016/S0021-9150(01)00420-8. PubMed: 11500195. [DOI] [PubMed] [Google Scholar]
- 48. Penn ND, Purkins L, Kelleher J, Heatley RV, Mascie-Taylor BH et al. (1991) The effect of dietary supplementation with vitamins A, C and E on cell-mediated immune function in elderly long-stay patients: a randomized controlled trial. Age Ageing 20: 169-174. doi:10.1093/ageing/20.3.169. PubMed: 1853789. [DOI] [PubMed] [Google Scholar]
- 49. Hogarth MB, Marshall P, Lovat LB, Palmer AJ, Frost CG et al. (1996) Nutritional supplementation in elderly medical in-patients: a double-blind placebo-controlled trial. Age Ageing 25: 453-457. doi:10.1093/ageing/25.6.453. PubMed: 9003882. [DOI] [PubMed] [Google Scholar]
- 50. ter Riet G, Kessels AG, Knipschild PG (1995) Randomized clinical trial of ascorbic acid in the treatment of pressure ulcers. J Clin Epidemiol 48: 1453-1460. doi:10.1016/0895-4356(95)00053-4. PubMed: 8543959. [DOI] [PubMed] [Google Scholar]
- 51. Bonelli L, Camoriano A, Ravelli P, Missale G, Bruzzi P, Aste H (1998) Reduction of the incidence of metachronous adenomas of the large bowel by means of antioxidants. In: Proceedings of International Selenium Tellurium Development Association. In: Palmieri Y. pp. 91–94. ScottsdaleAZ: [Google Scholar]
- 52. Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S et al. (1993) Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 85: 1483-1492. doi:10.1093/jnci/85.18.1483. PubMed: 8360931. [DOI] [PubMed] [Google Scholar]
- 53. Gillilan RE, Mondell B, Warbasse JR (1977) Quantitative evaluation of vitamin E in the treatment of angina pectoris. Am Heart J 93: 444-449. doi:10.1016/S0002-8703(77)80406-7. PubMed: 320856. [DOI] [PubMed] [Google Scholar]
- 54. Burns A, Marsh A, Bender DA (1989) A trial of vitamin supplementation in senile dementia. Int J Geriatr Psychiatry 4: 333-338. doi:10.1002/gps.930040606. [Google Scholar]
- 55. Sasazuki S, Sasaki S, Tsubono Y, Okubo S, Hayashi M et al. (2003) The effect of 5-year vitamin C supplementation on serum pepsinogen level and Helicobacter pylori infection. Cancer Sci 94: 378-382. doi:10.1111/j.1349-7006.2003.tb01450.x. PubMed: 12824908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Girodon F, Lombard M, Galan P, Brunet-Lecomte P, Monget AL et al. (1997) Effect of micronutrient supplementation on infection in institutionalized elderly subjects: a controlled trial. Ann Nutr Metab 41: 98-107. doi:10.1159/000177984. PubMed: 9267584. [DOI] [PubMed] [Google Scholar]
- 57. You WC, Brown LM, Zhang L, Li JY, Jin ML et al. (2006) Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst 98: 974-983. doi:10.1093/jnci/djj264. PubMed: 16849680. [DOI] [PubMed] [Google Scholar]
- 58. Chandra RK (1992) Effect of vitamin and trace-element supplementation on immune responses and infection in elderly subjects. Lancet 340: 1124-1127. doi:10.1016/0140-6736(92)93151-C. PubMed: 1359211. [DOI] [PubMed] [Google Scholar]
- 59. Takamatsu S, Takamatsu M, Satoh K, Imaizumi T, Yoshida H et al. (1995) Effects on health of dietary supplementation with 100 mg d-alpha-tocopheryl acetate, daily for 6 years. J Int Med Res 23: 342-357. PubMed: 8529777. [DOI] [PubMed] [Google Scholar]
- 60. Clinical Trial of Nutritional Supplements and Age-Related Cataract Study Group, Maraini G, Williams SL, Sperduto RD, Ferris F, (2008) A randomized, double-masked, placebo-controlled clinical trial of multivitamin supplementation for age-related lens opacities. Clinical trial of nutritional supplements and age-related cataract report no. 3. Ophtalmology 115: 599-607. doi:10.1016/j.ophtha.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 61. Collaborative Group of the Primary Prevention (2001) Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 357: 89-95 doi:10.1016/S0140-6736(00)03539-X. PubMed: 11197445. [DOI] [PubMed] [Google Scholar]
- 62. Eye Age-Related Disease Study Research Group (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS Report no. 9 Arch Ophthalmol 119: 1439-1452 [DOI] [PMC free article] [PubMed]
- 63. Age Related Macular Degeneration Study Group; (1996) Multicenter ophthalmic and nutritional age-related macular degeneration study - part 2: antioxidant intervention and conclusions. J Am Optom Assoc 67: 30-49. PubMed: 8825017. [PubMed] [Google Scholar]
- 64. Allsup SJ, Shenkin A, Gosney MA, Taylor S, Taylor W et al. (2004) Can a short period of micronutrient supplementation in older institutionalized people improve response to influenza vaccine? A randomized, controlled trial. J Am Geriatr Soc 52: 20-24. doi:10.1111/j.1532-5415.2004.52005.x. PubMed: 14687310. [DOI] [PubMed] [Google Scholar]
- 65. Avenell A, Campbell MK, Cook JA, Hannaford PC, Kilonzo MM, McNeill G et al. (2005) Effect of multivitamin and multimineral supplements on morbidity from infections in older people (MAVIS trial): pragmatic, randomised, double blind, placebo controlled trial. BMJ 331: 324-329. doi:10.1136/bmj.331.7512.324. PubMed: 16081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U et al. (2000) Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet 356: 1213-1218. doi:10.1016/S0140-6736(00)02783-5. PubMed: 11072938. [DOI] [PubMed] [Google Scholar]
- 67. Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC et al. (2001) Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 345: 1583-1592. doi:10.1056/NEJMoa011090. PubMed: 11757504. [DOI] [PubMed] [Google Scholar]
- 68. Chylack LTJ, Brown NP, Bron A, Hurst M, Köpcke W et al. (2002) The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthal Epidemiol 9: 49-80. doi:10.1076/opep.9.1.49.1717. PubMed: 11815895. [DOI] [PubMed] [Google Scholar]
- 69. Collins EG, Edwin Langbein W, Orebaugh C, Bammert C, Hanson K et al. (2003) PoleStriding exercise and vitamin E for management of peripheral vascular disease. Med Sci Sports Exerc 35: 384-393. doi:10.1097/00005768-200305001-02138. PubMed: 12618567. [DOI] [PubMed] [Google Scholar]
- 70. Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J et al. (2007) A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med 167: 1610-1618. doi:10.1001/archinte.167.15.1610. PubMed: 17698683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B et al. (2000) Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst 92: 1881-1888. doi:10.1093/jnci/92.23.1881. PubMed: 11106679. [DOI] [PubMed] [Google Scholar]
- 72. Desnuelle C, Dib M, Garrel C, Favier A (2001) A double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. ALS Riluzole-Tocopherol Study Group. Amyotroph Lateral Scler Other Mot Neuron Disord 2: 9-18. doi:10.1080/146608201300079364. [DOI] [PubMed] [Google Scholar]
- 73. Garbagnati F, Cairella G, De Martino A, Multari M, Scognamiglio U et al. (2009) Is antioxidant and n-3 supplementation able to improve functional status in poststroke patients? Results from the Nutristroke Trial. Cerebrovasc Dis 27: 375-383. doi:10.1159/000207441. PubMed: 19276620. [DOI] [PubMed] [Google Scholar]
- 74. Girodon F, Galan P, Monget AL, Boutron-Ruault MC, Brunet-Lecomte P et al. (1999) Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN. VIT. AOX. Geriatric Network. Arch Intern Med 159: 748-754. [DOI] [PubMed] [Google Scholar]
- 75. Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FLJ et al. (2004) The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst 96: 1743-1750. doi:10.1093/jnci/djh320. PubMed: 15572756. [DOI] [PubMed] [Google Scholar]
- 76. Graat JM, Schouten EG, Kok FJ (2002) Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. JAMA 288: 715-721. doi:10.1001/jama.288.6.715. PubMed: 12169075. [DOI] [PubMed] [Google Scholar]
- 77. Graf M, Ecker D, Horowski R, Kramer B, Riederer P et al. (2005) High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind study. J Neural Transm 112: 649-660. doi:10.1007/s00702-004-0220-1. PubMed: 15517433. [DOI] [PubMed] [Google Scholar]
- 78. Green A, Williams G, Neale R, Hart V, Leslie D et al. (1999) Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 354: 723-729. doi:10.1016/S0140-6736(98)12168-2. PubMed: 10475183. [DOI] [PubMed] [Google Scholar]
- 79. Greenberg ER, Baron JA, Stukel TA, Stevens MM, Mandel JS et al. (1990) A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin The Skin Cancer Prevention Study Group. N Engl J Med 323: 789-795 [DOI] [PubMed]
- 80. Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Beck GJ et al. (1994) A clinical trial of antioxidant vitamins to prevent colorectal adenoma Polyp Prevention Study Group. N Engl J Med 331: 141-147 [DOI] [PubMed]
- 81. Grieger JA, Nowson CA, Jarman HF, Malon R, Ackland LM (2009) Multivitamin supplementation improves nutritional status and bone quality in aged care residents. Eur J Clin Nutr 63: 558-565. doi:10.1038/sj.ejcn.1602963. PubMed: 18043700. [DOI] [PubMed] [Google Scholar]
- 82. Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B et al. (1996) Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 334: 1145-1149. doi:10.1056/NEJM199605023341801. PubMed: 8602179. [DOI] [PubMed] [Google Scholar]
- 83. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, Touvier M, Favier A et al. (2010) Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer 127: 1875-1881. doi:10.1002/ijc.25201. PubMed: 20104528. [DOI] [PubMed] [Google Scholar]
- 84. Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A et al. (2002) Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation 106: 1453-1459. doi:10.1161/01.CIR.0000029092.99946.08. PubMed: 12234947. [DOI] [PubMed] [Google Scholar]
- 85. Jacobson JS, Begg MD, Wang LW, Wang Q, Agarwal M et al. E (2000) Effects of a 6-month vitamin intervention on DNA damage in heavy smokers. Cancer Epidemiol Biomarkers Prev Sentyabr: 1303-1311. PubMed: 11142415 [PubMed] [Google Scholar]
- 86. Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM et al. (2005) Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA 294: 56-65. doi:10.1001/jama.294.1.56. PubMed: 15998891. [DOI] [PubMed] [Google Scholar]
- 87. Li JY, Taylor PR, Li B, Dawsey S, Wang GQ et al. (1993) Nutrition Intervention Trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst 85: 1492-1498. doi:10.1093/jnci/85.18.1492. PubMed: 8360932. [DOI] [PubMed] [Google Scholar]
- 88. Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM et al. (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301: 39-51. doi:10.1001/jama.2008.864. PubMed: 19066370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu BA, McGeer A, McArthur MA, Simor AE, Aghdassi E et al. (2007) Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc 55: 35-42. doi:10.1111/j.1532-5415.2006.01033.x. PubMed: 17233683. [DOI] [PubMed] [Google Scholar]
- 90. Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J et al. (2005) Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293: 1338-1347. doi:10.1001/jama.293.11.1338. PubMed: 15769967. [DOI] [PubMed] [Google Scholar]
- 91. Magliano D, McNeil J, Branley P, Shiel L, Demos L et al. (2006) The Melbourne Atherosclerosis Vitamin E Trial (MAVET): a study of high dose vitamin E in smokers. Eur J Cardiovasc Prev Rehabil 13: 341-347. doi:10.1097/01.hjr.0000219108.10167.46. PubMed: 16926662. [DOI] [PubMed] [Google Scholar]
- 92. Manuel-Y-Keenoy B, Vinckx M, Vertommen J, Van Gaal L, De Leeuw I (2004) Impact of vitamin E supplementation on lipoprotein peroxidation and composition in type 1 diabetic patients treated with atorvastatin. Atherosclerosis 175: 369-376. doi:10.1016/j.atherosclerosis.2004.04.005. PubMed: 15262194. [DOI] [PubMed] [Google Scholar]
- 93. Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G et al. (2005) Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology 64: 87-93. doi:10.1212/01.WNL.0000148603.44618.19. PubMed: 15642909. [DOI] [PubMed] [Google Scholar]
- 94. McNeil JJ, Robman L, Tikellis G, Sinclair MI, McCarty CA et al. (2004) Vitamin E supplementation and cataract: randomized controlled trial. Ophthalmology 111: 75-84. doi:10.1016/j.ophtha.2003.04.009. PubMed: 14711717. [DOI] [PubMed] [Google Scholar]
- 95. Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT et al. (2004) Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA 292: 828-836. doi:10.1001/jama.292.7.828. PubMed: 15315997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A (2004) A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol 40: 40-46. doi:10.1016/S0168-8278(04)90110-9. PubMed: 14672612. [DOI] [PubMed] [Google Scholar]
- 97. Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R et al. (2008) Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol 28: 341-347. PubMed: 18032779. [DOI] [PubMed] [Google Scholar]
- 98. Moon TE, Levine N, Cartmel B, Bangert JL, Rodney S et al. (1997) Effect of retinol in preventing squamous cell skin cancer in moderate-risk subjects: a randomized, double-blind, controlled trial Southwest Skin Cancer Prevention Study Group. Cancer; Epidemiol Biomarkers Prev 6: 949-956 [PubMed]
- 99. Mooney LA, Madsen AM, Tang D, Orjuela MA, Tsai WY et al. (2005) Antioxidant vitamin supplementation reduces benzo(a)pyrene-DNA adducts and potential cancer risk in female smokers. Cancer Epidemiol Biomarkers Prev 14: 237-242. PubMed: 15668500. [PubMed] [Google Scholar]
- 100. MRC/BHF Heart Protection Study Group; (2002) MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360: 23-33. doi:10.1016/S0140-6736(02)11807-1. PubMed: 12114037. [DOI] [PubMed] [Google Scholar]
- 101. Murphy S, West KPJ, Greenough WB, Cherot E, Katz J et al. (1992) Impact of vitamin A supplementation on the incidence of infection in elderly nursing-home residents: a randomized controlled trial. Age Ageing 21: 435-439. doi:10.1093/ageing/21.6.435. PubMed: 1471582. [DOI] [PubMed] [Google Scholar]
- 102. Pike J, Chandra RK (1995) Effect of vitamin and trace element supplementation on immune indices in healthy elderly. Int J Vit Nutr Res 65: 117-121. PubMed: 7591530. [PubMed] [Google Scholar]
- 103. Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S et al. (2007) Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst 99: 137-146. doi:10.1093/jnci/djk017. PubMed: 17227997. [DOI] [PubMed] [Google Scholar]
- 104. Prince MI, Mitchison HC, Ashley D, Burke DA, Edwards N et al. (2003) Oral antioxidant supplementation for fatigue associated with primary biliary cirrhosis: results of a multicentre, randomized, placebo-controlled, cross-over trial. Aliment Pharmacol Ther 17: 137-143. doi:10.1046/j.1365-2036.2003.01398.x. PubMed: 12492743. [DOI] [PubMed] [Google Scholar]
- 105. Richer S, Stiles W, Statkute L, Pulido J, Frankowski J et al. (2004) Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 75: 216-230. doi:10.1016/S1529-1839(04)70049-4. PubMed: 15117055. [DOI] [PubMed] [Google Scholar]
- 106. Salonen RM, Nyyssönen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S et al. (2003) Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation 107: 947-953. doi:10.1161/01.CIR.0000050626.25057.51. PubMed: 12600905. [DOI] [PubMed] [Google Scholar]
- 107. Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C et al. (2008) Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA 300: 2123-2133. doi:10.1001/jama.2008.600. PubMed: 18997197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K et al. (1996) Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 347: 781-786. doi:10.1016/S0140-6736(96)90866-1. PubMed: 8622332. [DOI] [PubMed] [Google Scholar]
- 109. Tam LS, Li EK, Leung VY, Griffith JF, Benzie IF et al. (2005) Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: a double blind, placebo controlled pilot study. J Reumatol 32: 275-282. [PubMed] [Google Scholar]
- 110. Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N et al. (2003) Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA 290: 476-485. doi:10.1001/jama.290.4.476. PubMed: 12876090. [DOI] [PubMed] [Google Scholar]
- 111. Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR et al. (2002) Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA 288: 2432-2440. doi:10.1001/jama.288.19.2432. PubMed: 12435256. [DOI] [PubMed] [Google Scholar]
- 112. White KL, Chalmers DM, Martin IG, Everett SM, Neville PM et al. (2002) Dietary antioxidants and DNA damage in patients on long-term acid-suppression therapy: a randomized controlled study. Br J Nutr 88: 265-271. doi:10.1079/BJN2002619. PubMed: 12207836. [DOI] [PubMed] [Google Scholar]
- 113. Witte KK, Nikitin NP, Parker AC, von Haehling S, Volk HD et al. (2005) The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J 26: 2238-2244. doi:10.1093/eurheartj/ehi442. PubMed: 16081469. [DOI] [PubMed] [Google Scholar]
- 114. Wluka AE, Stuckey S, Brand C, Cicuttini FM (2002) Vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2 year double blind randomized placebo controlled study. J Rheumatol 29: 2585-2591. PubMed: 12465157. [PubMed] [Google Scholar]
- 115. Anonymous. (2012). ermi Paradox. Available: www crystalinks Com/fermiparadox html [Google Scholar]
- 116. Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ (2011) Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 4: 158-170. doi:10.2174/1874609811104020158. PubMed: 21235492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA et al. (2005) Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142: 37-46. doi:10.7326/0003-4819-142-1-200501040-00110. PubMed: 15537682. [DOI] [PubMed] [Google Scholar]
- 118. Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ (2003) Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361: 2017-2023. doi:10.1016/S0140-6736(03)13637-9. PubMed: 12814711. [DOI] [PubMed] [Google Scholar]
- 119. DeMets DL, Pocock SJ, Julian DG (1999) The agonising negative trend in monitoring of clinical trials. Lancet 354: 1983-1988. doi:10.1016/S0140-6736(99)03464-9. PubMed: 10622312. [DOI] [PubMed] [Google Scholar]
- 120. Wittes J, Barrett-Connor E, Braunwald E, Chesney M, Cohen HJ et al. (2007) Monitoring the randomized trials of the Women’s Health Initiative: the experience of the Data and Safety Monitoring Board. Clin Trials 4: 218-234. doi:10.1177/1740774507079439. PubMed: 17715247. [DOI] [PubMed] [Google Scholar]
- 121. Murphy SP, White KK, Park SY, Sharma S (2007) Multivitamin-multimineral supplements’ effect on total nutrient intake. Am J Clin Nutr 85: 280S-284S. PubMed: 17209210. [DOI] [PubMed] [Google Scholar]
- 122. Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C et al. (2004) Dietary Supplement Use by US Adults: Data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol 160: 339-349. doi:10.1093/aje/kwh207. PubMed: 15286019. [DOI] [PubMed] [Google Scholar]
- 123. Verkaik-Kloosterman J, McCann MT, Hoekstra J, Verhagen H (2012) Vitamins and minerals: issues associated with too low and too high population intakes. Foods Nutr Res 56.doi: 10.3402/fnr.v56i0.5728. PubMed: 22489212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cortés-Jofré M, Rueda JR, Corsini-Muńoz G, Fonseca-Cortés C, Caraballoso M et al. (2012) Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. doi:10.1002/14651858.CD002141.pub2. [DOI] [PubMed] [Google Scholar]
- 125. Ristow M, Schmeisser S (2011) Extending life span by increasing oxidative stress. Free Radic Biol Med 51: 327-336. doi:10.1016/j.freeradbiomed.2011.05.010. PubMed: 21619928. [DOI] [PubMed] [Google Scholar]
- 126. Watson J (2013) Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol 3. doi:10.1098/rsob.120144. PubMed: 23303309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gutteridge JMC, Halliwell B (2010) Antioxidants: Molecules, medicines, and myths. Biochem Biophys Res Commun 393: 561-564. doi:10.1016/j.bbrc.2010.02.071. PubMed: 20171167. [DOI] [PubMed] [Google Scholar]
- 128. Halliwell B (2013) The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol 75: 637-644. PubMed: 22420826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Salganik RI (2001) The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr 20: 464S-472S. doi:10.1080/07315724.2001.10719185. PubMed: 11603657. [DOI] [PubMed] [Google Scholar]
- 130. Haenen GR, Bast A (2002) The use of vitamin supplements in self-medication. Therapie 57: 119-122. PubMed: 12185958. [PubMed] [Google Scholar]
- 131. Bast A, Haenen GR (2002) The toxicity of antioxidants and their metabolites. Environl Toxicol Pharmacol 11: 251-258. doi:10.1016/S1382-6689(01)00118-1. PubMed: 21782609. [DOI] [PubMed] [Google Scholar]
- 132. Bailey RL, Fulgoni VL III, Keast DR, Dwyer JT (2012) Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet 112: 657-663. doi:10.1016/j.jand.2012.01.026. PubMed: 22709770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Archer SL, Stamler J, Moag-Stahlberg A, Van Horn L, Garside D et al. (2005) Association of dietary supplement use with specific micronutrient intakes among middle-aged american men and women: The INTERMAP Study. J Am Diet Assoc 105: 1106-1114. doi:10.1016/j.jada.2005.04.010. PubMed: 15983530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ford ES, Ajani UA, Mokdad AH (2005) Brief Communication: The prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann Intern Med 143: 116-120. doi:10.7326/0003-4819-143-2-200507190-00010. PubMed: 16027453. [DOI] [PubMed] [Google Scholar]
- 135. Walter P (2001) Towards ensuring the safety of vitamins and minerals. Toxicol Lett 120: 83-87. doi:10.1016/S0378-4274(01)00286-7. PubMed: 11323165. [DOI] [PubMed] [Google Scholar]
- 136. Ovesen L (1987) Vitamin therapy in the absence of obvious deficiency. What is the evidence? Drugs 27: 148-170. [DOI] [PubMed] [Google Scholar]
- 137. Bjelakovic G, Gluud C (2011) Vitamin and mineral supplement use in relation to all-cause mortality in the Iowa Women’s Health Study. Arch Intern Med 171: 1633-1634. doi:10.1001/archinternmed.2011.459. PubMed: 21987193. [DOI] [PubMed] [Google Scholar]
- 138. Marik PE, Flemmer M (2012) Do dietary supplements have beneficial health effects in industrialized nations: what is the evidence? JPEN J Parenter Enteral Nutr 36: 159-168. doi:10.1177/0148607111416485. PubMed: 22275325. [DOI] [PubMed] [Google Scholar]
- 139. Martínez ME, Jacobs ET, Baron JA, Marshall JR, Byers T (2012) Dietary supplements and cancer prevention: balancing potential benefits against proven harms. J Natl Cancer Inst 104: 732-739. doi:10.1093/jnci/djs195. PubMed: 22534785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. NIH State-of-the Science Panel; (2007) National Institutes of Health State-of-the-Science Conference Statement: multivitamin/mineral supplements and chronic disease prevention. Am J Clin Nutr 85: 257S-264S. PubMed: 17209206. [DOI] [PubMed] [Google Scholar]
- 141.US Department of Agriculture and US Department of Health and Human Services; (2010) Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. Available: www cnpp usda gov/DGAs2010-DGACReport htm. [Google Scholar]
- 142. Myung SK, Ju W, Cho B, Oh SW, Park SM et al. (2013) Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ 346: f10. doi:10.1136/bmj.f10. PubMed: 23335472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Myung SK, Kim Y, Ju W, Choi HJ, Bae WK (2010) Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol 21: 166-179. doi:10.1093/annonc/mdp286. PubMed: 19622597. [DOI] [PubMed] [Google Scholar]
- 144. Ye Y, Li J, Yuan Z (2013) Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLOS ONE 8: e56803. doi:10.1371/journal.pone.0056803. PubMed: 23437244. [DOI] [PMC free article] [PubMed] [Google Scholar]


