Abstract
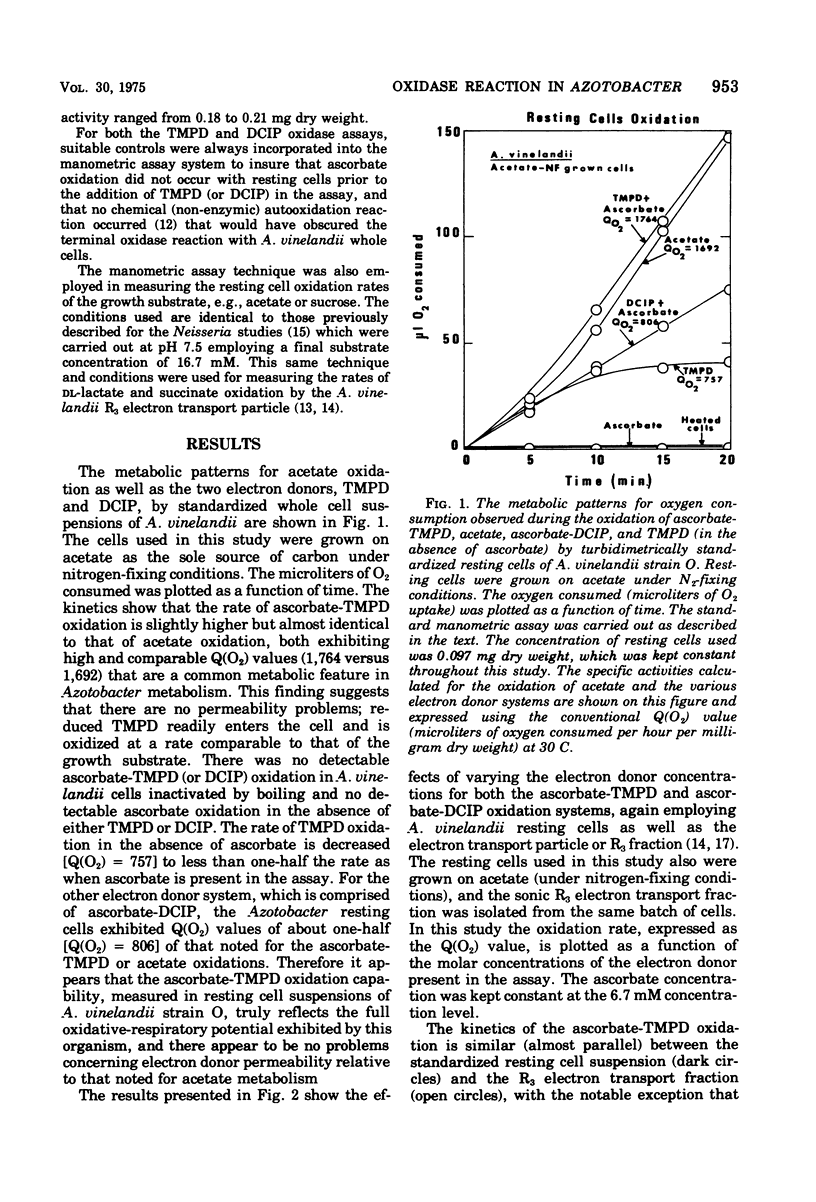
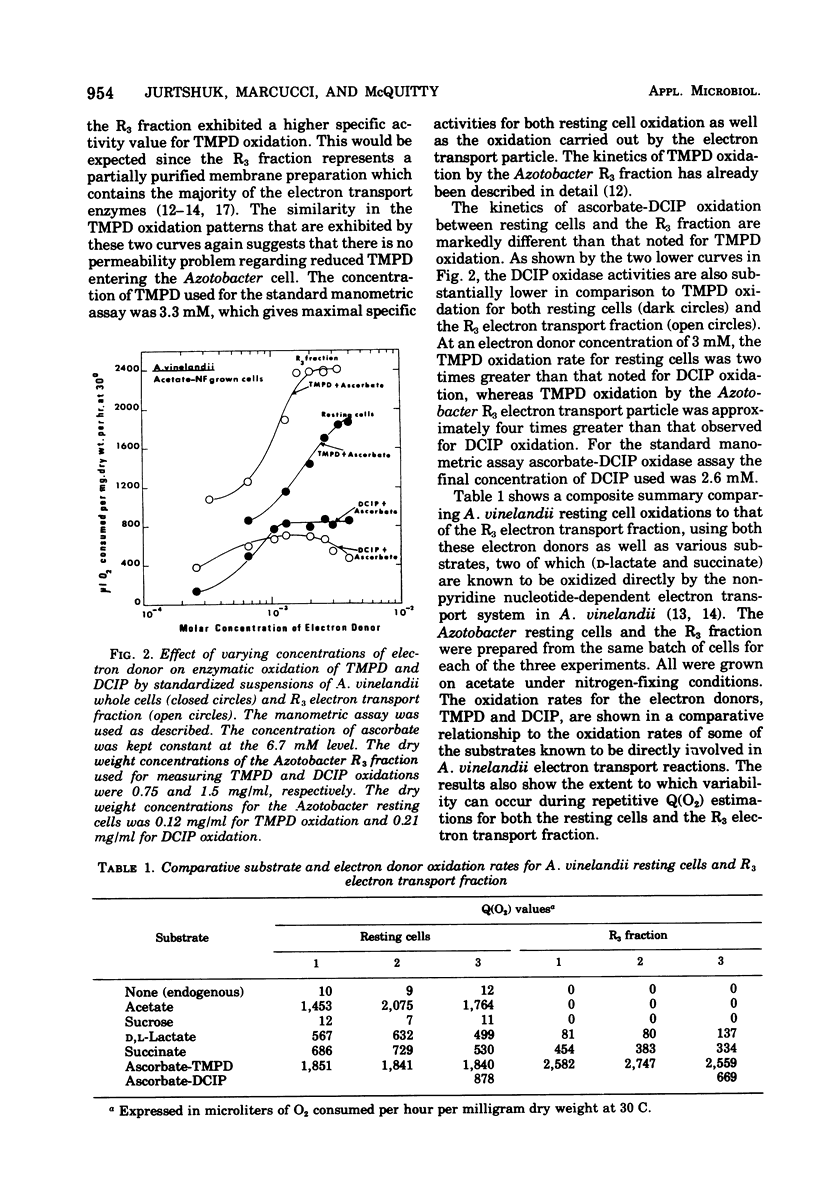
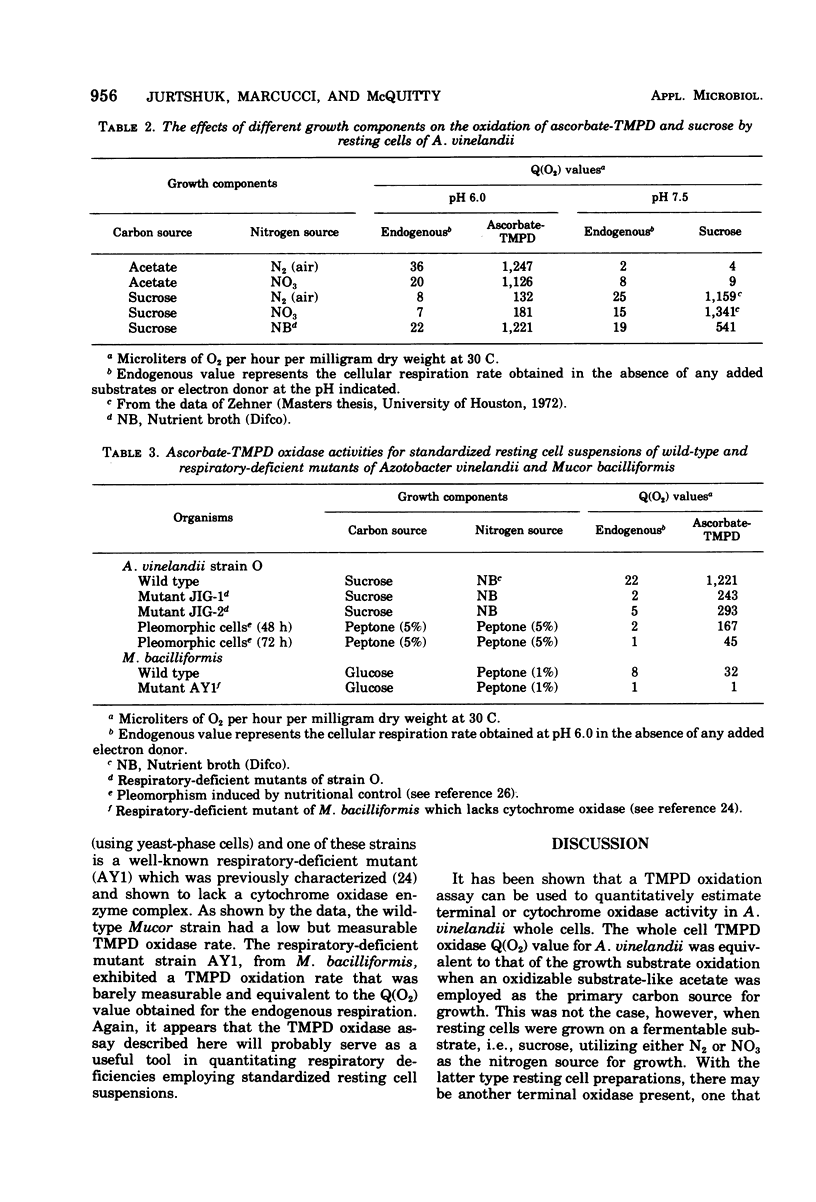
It was possible to quantitate the tetramethyl-p-phenylenediamine (TMPD) oxidase reaction in Azotobacter vinelandii strain O using turbidimetrically standarized resting cell suspensions. The Q(O2) value obtained for whole cell oxidation of ascorbate-TMPD appeared to reflect the full measure of the high respiratory oxidative capability usually exhibited by this genera of organisms. The Q(O2) value for the TMPD oxidase reaction ranged from 1,700 to 2,000 and this value was equivalent to that obtained for the oxidation of the growth substrate, e.g., acetate. The kinetic analyses for TMPD oxidation by whole cells was similar to that obtained for the "particulate" A. vinelandii electron transport particle, that fraction which TMPD oxidase activity is exclusively associated with. Under the conditions used, there appeared to be no permeability problems; TMPD (reduced by ascorbate) readily penetrated the cell and oxidized at a rate comparable to that of the growth substrate. This, however, was not true for the oxidation of another electron donor, 2,6-dichloroindophenol, whose whole cell Q(O2) values, under comparable conditions, were twofold lower. The TMPD oxidase activity in A. vinelandii whole cells was found to be affected by the physiological growth conditions, and resting cells obtained from cells grown on sucrose, either under nitrogen-fixing conditions or on nitrate as the combined nitrogen source, exhibited low TMPD oxidase rates. Such low TMPD oxidase rates were also noted for chemically induced pleomorphic A. vinelandii cells, which suggests that modified growth conditions can (i) alter the nature of the intracellular terminal oxidase formed (or induced), or (ii) alter surface permeability, depending upon the growth conditions used. Preliminary studies on the quantitative TMPD oxidation reaction in mutant whole cells of both Azotobacter and a well-known Mucor bacilliformis strain AY1, deficient in cytochrome oxidase activity, showed this assay can be very useful for detecting respiratory deficiencies in the metabolism of whole cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOREI H. G., BJORKLUND U. Oxidation through the cytochrome system of substituted phenylenediamines. Biochem J. 1953 Jun;54(3):357–362. doi: 10.1042/bj0540357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GABY W. L., HADLEY C. Practical laboratory test for the identification of Pseudomonas aeruginosa. J Bacteriol. 1957 Sep;74(3):356–358. doi: 10.1128/jb.74.3.356-358.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E. Phosphorylation coupled to electron transport initiated by substituted phenylenediamines. Biochem Biophys Res Commun. 1960 Nov;3:536–539. doi: 10.1016/0006-291x(60)90170-4. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Preparation of red and green electron transport particles from Azotobacter vinelandii. Biochim Biophys Acta. 1967 Sep 6;143(2):354–362. doi: 10.1016/0005-2728(67)90089-8. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. The cytochrome system of Azotobacter vinelandii. Biochim Biophys Acta. 1967 Sep 6;143(2):340–353. doi: 10.1016/0005-2728(67)90088-6. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Aston P. R., Old L. Enzymatic oxidation of tetramethyl-p-phenylenediamine and p-phenylenediamine by the electron transport particulate fraction of Azotobacter vinelandii. J Bacteriol. 1967 Mar;93(3):1069–1078. doi: 10.1128/jb.93.3.1069-1078.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Harper L. Oxidation of D(minus) lactate by the electron transport fraction of Azotobacter vinelandii. J Bacteriol. 1968 Sep;96(3):678–686. doi: 10.1128/jb.96.3.678-686.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Mueller T. J., Acord W. C. Bacterial terminal oxidases. CRC Crit Rev Microbiol. 1975 May;3(4):399–468. doi: 10.3109/10408417509108757. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., May A. K., Pope L. M., Aston P. R. Comparative studies on succinate and terminal oxidase activity in microbial and mammalian electron-transport systems. Can J Microbiol. 1969 Jul;15(7):797–807. doi: 10.1139/m69-139. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Preliminary characterization studies on the Neisseria catarrhalis respiratory electron transport chain. J Bacteriol. 1974 Oct;120(1):552–555. doi: 10.1128/jb.120.1.552-555.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Quantitation of the tetramethyl-p-phenylenediamine oxidase reaction in Neisseria species. Appl Microbiol. 1974 Dec;28(6):1079–1081. doi: 10.1128/am.28.6.1079-1081.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Old L. Cytochrome c oxidation by the electron transport fraction of Azotobacter vinelandii. J Bacteriol. 1968 May;95(5):1790–1797. doi: 10.1128/jb.95.5.1790-1797.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACS N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 1956 Sep 29;178(4535):703–703. doi: 10.1038/178703a0. [DOI] [PubMed] [Google Scholar]
- SCHILS D. J., HOVENKAMP H. G., COLPA-BOONSTRA J. P. Studies on menadione reduction and reoxidation with Azotobacter vinelandii. Biochim Biophys Acta. 1960 Sep 9;43:129–131. doi: 10.1016/0006-3002(60)90416-9. [DOI] [PubMed] [Google Scholar]
- Storck R., Morrill R. C. Respiratory-deficient, yeastlike mutant of Mucor. Biochem Genet. 1971 Oct;5(5):467–479. doi: 10.1007/BF00487136. [DOI] [PubMed] [Google Scholar]
- Vela G. R., Rosenthal R. S. Effect of peptone on Azotobacter morphology. J Bacteriol. 1972 Jul;111(1):260–266. doi: 10.1128/jb.111.1.260-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


