Abstract
Background
Vitamins and minerals are essential for growth and metabolism. The World Health Organization estimates that more than 2 billion people are deficient in key vitamins and minerals. Groups most vulnerable to these micronutrient deficiencies are pregnant and lactating women and young children, given their increased demands. Food fortification is one of the strategies that has been used safely and effectively to prevent vitamin and mineral deficiencies.
Methods
A comprehensive search was done to identify all available evidence for the impact of fortification interventions. Studies were included if food was fortified with a single, dual or multiple micronutrients and impact of fortification was analyzed on the health outcomes and relevant biochemical indicators of women and children. We performed a meta-analysis of outcomes using Review Manager Software version 5.1.
Results
Our systematic review identified 201 studies that we reviewed for outcomes of relevance. Fortification for children showed significant impacts on increasing serum micronutrient concentrations. Hematologic markers also improved, including hemoglobin concentrations, which showed a significant rise when food was fortified with vitamin A, iron and multiple micronutrients. Fortification with zinc had no significant adverse impact on hemoglobin levels. Multiple micronutrient fortification showed non-significant impacts on height for age, weight for age and weight for height Z-scores, although they showed positive trends. The results for fortification in women showed that calcium and vitamin D fortification had significant impacts in the post-menopausal age group. Iron fortification led to a significant increase in serum ferritin and hemoglobin levels in women of reproductive age and pregnant women. Folate fortification significantly reduced the incidence of congenital abnormalities like neural tube defects without increasing the incidence of twinning. The number of studies pooled for zinc and multiple micronutrients for women were few, though the evidence suggested benefit. There was a dearth of evidence for the impact of fortification strategies on morbidity and mortality outcomes in women and children.
Conclusion
Fortification is potentially an effective strategy but evidence from the developing world is scarce. Programs need to assess the direct impact of fortification on morbidity and mortality.
Keywords: Child, Food, Fortification, Nutrition, Supplementation, Women
Background
Vitamins and minerals are essential for growth and metabolism. The World Health Organization (WHO) estimates that more than 2 billion people are deficient in key vitamins and minerals, particularly vitamin A, iodine, iron and zinc [1]. Most of these affected populations are from developing countries where multiple micronutrient (MMN) deficiencies coexist.
The population groups most vulnerable to these micronutrient deficiencies are pregnant and lactating women and young children, given their increased demands [2,3]. According to recent WHO estimates, globally about 190 million preschool children and 19.1 million pregnant women are vitamin A deficient (that is, have serum retinol <0.70 μmol/l) [4], approximately 100 million women of reproductive age (WRA) have iodine deficiency [5], and an estimated 82% of pregnant women worldwide have inadequate zinc intakes to meet the normal needs of pregnancy [6]. Iron deficiency is widespread and globally about 1.62 billion people are anemic, with the highest prevalence among preschool children (47%) followed by pregnant women (42%) [7]. Suboptimal vitamin B6 and B12 status have also been observed in many developing countries [8]. These micronutrient deficiencies are also associated with increased incidence and severity of infectious illness and mortality from diarrhea, measles, malaria and pneumonia [9]. The consequences of micronutrient deficiencies are not limited to health parameters alone but have far-reaching effects on economies through secondary physical and mental disabilities and altered work productivity.
Several strategies have been employed to supplement micronutrients to women and children [10-13]. These include education, dietary modification, food rationing, supplementation and fortification. Food fortification is one of the strategies that has been used safely and effectively to prevent micronutrient deficiencies and has been practiced in developed countries for well over a century now. In the early 20th century, salt iodization began in Switzerland; vitamin A-fortified margarine was introduced in Denmark in 1918; and in the 1930s, vitamin A-fortified milk and iron and B complex flour was introduced in a number of developed countries. These fortification strategies are now almost universal in the developed world and increasingly deployed in many middle-income countries. Relatively few of these programs have been adequately evaluated to assess their impact on population health [14].
WHO categorizes food fortification strategies into three possible approaches: mass, targeted, and market driven [14]. Mass fortification involves foods that are widely consumed, such as wheat, salt, sugar; targeted approaches fortify foods consumed by specific age groups like infant complementary foods; and the market-driven approach is when a food manufacturer fortifies a specific brand for a particular consumer niche. Food vehicles commonly used can be grouped into three broad categories: staples (wheat, rice, oils), condiments (salt, soy sauce, sugar), and processed commercial foods (noodles, infant complementary foods, dairy products).
Food fortification is an attractive public health strategy and has the advantage of reaching wider at-risk population groups through existing food delivery systems, without requiring major changes in existing consumption patterns [13,15]. Compared with other interventions, food fortification may be cost-effective and, if fortified foods are regularly consumed, has the advantage of maintaining steady body stores [15].
There are a number of systemic reviews and meta-analyses analyzing the effect of food fortification on health outcomes. An extensive review of zinc fortification in children described an overall significant impact on serum zinc levels with no adverse effects [16]. Reviews on MMN fortification in children showed improved micronutrient status and reduced anemia prevalence [10,17]. A review of iron fortification in the general population also showed an increase in serum hemoglobin levels and decreased anemia prevalence [18]. Although iron, zinc and MMN fortification has been consistently analyzed, literature on vitamin A, iodine and vitamin D fortification in children has not been sufficiently assessed. Furthermore, iron and MMN fortification in women of different age groups has not been adequately evaluated. We therefore feel that the current evidence of certain micronutrients and population groups may not be sufficient for providing a way forward. Hence we undertook a systematic review of the current evidence to assess the effectiveness of food fortification with single micronutrients (iron, folic acid, vitamin A, vitamin D, iodine, zinc) as well as MMN when compared with no fortification on the health and nutrition of women and children.
Conceptual framework
We analyzed the impact of micronutrient fortification strategies - single, dual or multiple - on various outcomes guided by our conceptual framework (Figure 1). These micronutrients were administered through one of the three food vehicles (staples, condiments or processed foods) to reach the population targeted. For the scope of this review, we focused on a priori defined population groups of infants, children and adolescents under 18 years of age, WRA and post-menopausal women. The outcomes analyzed were broadly categorized into biochemical indicators, hematologic markers, anthropometric indicators, pregnancy outcomes, and relevant morbidity and mortality.
Figure 1.
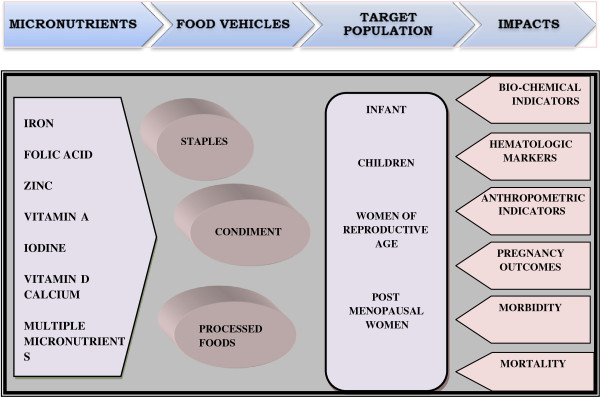
Conceptual framework.
Methods
Search strategy
All available evidence for the impact of fortification interventions was systematically retrieved and analyzed. A comprehensive search was done for key words including Medical Subject Headings and free text terms for all the micronutrients included in this review. We searched MEDLINE, PubMed, POPLINE, Literatura Latino Americana em Ciências da Saúde, Cumulative Index to Nursing and Allied Health Literature, Cochrane Library, British Library for Development Studies at the International Development Statistics, WHO regional databases and the IDEAS database of unpublished working papers, Google and Google Scholar. Detailed manual searches were undertaken, including cross-references and bibliographies of available data and publications. Existing relevant reviews were used to identify additional sources of information. The search was extended to review the gray literature in non-indexed and non-electronic sources. The bibliographies of books with relevant sections were also searched manually to identify relevant reports and publications. We did not apply any language or date restriction and the date of last search was 1 November 2012.
Inclusion and exclusion criteria
We included randomized controlled trials (RCTs), quasi- experimental and before-after studies. In addition, other less rigorous study designs like observational studies (cohort and case–control), program evaluations and descriptive studies were also reviewed to understand the context in which these interventions were implemented. Studies were included if food was fortified with a single, dual or multiple micronutrients and the impact of fortification was analyzed on the health outcomes and relevant biochemical indicators of women and children. We included studies with children and adolescents of all age groups - infants and preschool children (ages 2 to 5 years), school-going children (ages of above 5 years) and adolescents till 18 years of age. For women, we included studies on pre-pregnant women, WRA, and post-menopausal women. The control groups in the included trials either received unfortified foods or regular diets. Studies were included if they measured the relevant outcomes according to our conceptual framework described above and if the data for these outcomes were presented in a manner that could be included in the meta-analysis. Studies were not considered that focused on home fortification with micronutrient powders, food contents, intake levels, bioavailability, comparisons between different food vehicles or comparisons among compounds of the same micronutrient, comparisons between fortification and supplementation, bio-fortification and studies evaluating the sensory impacts of fortification.
Study selection process
All the available studies underwent triage with standardized criteria for evaluating outputs from primary screening. Following an agreement on the search strategy, the abstracts and full texts were screened by two independent abstractors to identify studies adhering to the objectives. Any disagreements on selection of studies between the two primary abstractors were resolved by the third reviewer.
Data extraction
After retrieval of the full texts of all the relevant studies, each study was double data abstracted into a standardized form, which included the following details.
General information
Title, author, year of publication, country of intervention, study design.
Intervention
Study duration, characteristics of intervention and comparison groups.
Fortification
Micronutrient fortified, food vehicle used, fortificant compound and its concentration in the food.
Key outcome measures
Outcome measures were identified and evaluated separately for the various micronutrients. Relevant biochemical indicators included serum micronutrient levels and hematologic markers (anemia, iron deficiency anemia, hemoglobin). Anthropometric indicators were stunting; wasting; underweight; and changes in Z-scores for height for age (HAZ), weight for age (WAZ) and weight for height (WHZ). Pregnancy outcomes included twinning and congenital abnormalities. Morbidity outcomes included diarrhea, pneumonia, malaria, urinary tract infections (UTI), fever and mortality (Table 1). These were reported as means, standard deviations, number of events or other usable outcomes that could be pooled. Where information was missing or incomplete, the authors were contacted for clarification and access to data. In cases where it was not possible, the outcome was not considered for further analysis.
Table 1.
Outcomes for various micronutrients analyzed
| Micronutrient | Outcomes analyzed |
|---|---|
|
Zinc |
Serum zinc concentration, height velocity, alkaline phosphatase, hemoglobin and serum copper concentration |
|
Folate |
Serum folate concentration, red blood cell folate concentration, folate deficiency, twinning, incidence of neural tube defects, anencephaly and spina bifida |
|
Vitamin A |
Serum vitamin A concentration, vitamin A deficiency and hemoglobin |
|
Vitamin D/calcium |
Serum 25-hydroxy vitamin D3 concentration, alkaline phosphatase and serum parathyroid hormone |
|
Iodine |
Serum thyroxin levels, prevalence of hypothyroidism and urinary iodine concentration |
|
Iron |
Serum ferritin, serum transferrin, hemoglobin and anemia |
| Multiple micronutrients | Serum ferritin, serum zinc, serum retinol, vitamin A deficiency, iron deficiency anemia, hemoglobin levels, height for age Z-scores, weight for age Z-scores, weight for height Z-scores and morbidity. |
Data analysis
We performed meta-analyses of all outcomes using Review Manager Software version 5.1. For dichotomous data, we presented results as summary risk ratio (RR) and odds ratio (OR) and their respective 95% confidence intervals (CI). For continuous data, we used the standard mean difference (SMD) if outcomes were comparable. We performed separate meta-analyses for RCTs or quasi experimental and before-after studies to maintain the quality of evidence. Results of before-after meta-analyses were only reported if there were no RCTs or quasi-experimental studies in a particular category of micronutrient fortification. For analyzing and pooling cluster randomized trial data, the entire cluster was used as the unit of randomization and the analysis was adjusted for design. The data of cluster-randomized trials were incorporated using a generic inverse variance method in which logarithms of RR estimates were used along with the standard error of the logarithms of RR estimates. Subgroup analyses were performed according to the different age groups, countries, population characteristics, type of food fortified and the duration of intervention. For the outcomes where only medians were reported, medians were converted to approximate of the means and then pooled for analysis [19].
The level of attrition was noted for each study and its impact on the overall assessment of treatment effect explored by using sensitivity analysis. Heterogeneity between trials was assessed using the I-squared statistic (I2 >30) and a p-value <0.1 (on chi-square) and by visual inspection of forest plots, and a random effect model was used.
Risk of bias and quality assessment of evidence
We used the Cochrane Collaboration’s tool for assessing risk of bias for the included RCTs and quasi-experimental studies [20]. It is a two-part tool addressing the seven specific domains, namely sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and ‘other issues’. Within each entry, the first part of the tool describes what was reported to have happened in the study in sufficient detail to support a judgment about the risk of bias. The second part of the tool assigns a judgment relating to the risk of bias for that entry. This is achieved by assigning a judgment of ‘low risk’, ‘high risk’ or ‘unclear risk’.
We summarized the evidence by outcome, including qualitative assessments of study quality and quantitative measures, according to the standard guidelines. For each outcome, the quality of the evidence was assessed independently by two review authors using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which involves consideration of within-study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias. A grade of ‘high’, ‘moderate’, ‘low’ and ‘very low’ was used for grading the overall evidence indicating the strength of an effect on specific health outcome [21-24].
Results
A total of 201 studies were identified for inclusion in this review. Of these, 121 [25-145] studies were on infants and children and 79 [146-224] were on women, while one study had both women and children as their study population [33] (Figure 2). Furthermore 125 [25-28,30,32-39,41-44,47,48,51,53],[54,56,59,60,62,63,65-73,75-77],[81-83,85,86,88-90,92,93,95-104,107-109],[111-117,119-127,129,130,132-136,139-144,223,225-228] of these trials were RCTs; the rest were quasi-experimental [46,55,61,74,78,87,91] and before-after studies [29,31,40,45,49,50,52,57],[58,79,80,84,94,95,105,131],[137,138,149,150,153,157,159-169,172],[174,177,181,183-186,189-191,193,195-200,202],[205-211,213,219-222,224]. Various micronutrients were used as fortificants in these studies including iron, zinc, vitamin A, folate, iodine, calcium and vitamin D alone and in combination. Food vehicles chosen varied for each micronutrient fortification and are described in detail in the relevant section. For the 125 RCTs included in this review, randomization and allocation concealment were adequate in 41 of the studies [33-37,56,60,63,65,68,69,72],[75,77,83,88,92,97,99,101],[103,107,108,112,113,115,116,122],[125,127,132,135,139,140,142,143],[146,147,188,214,217], information regarding attrition rates was adequately discussed with reasons in 110 studies [33-39,41-44,47,48,51,53,54,56],[59,60,62,63,65,67-73,75-77,81-83],[85,86,88-90,92,93,96-104,107-109,111-117],[119-127,129,130,132-136,139-144,171,173,175],[176,178-180,182,187,188,192,194,201],[212,214-218,223] and blinding was described adequately in 62 studies [25,26,28,30,32,35,38,39],[47,48,51,53,54,56,59,60],[62,65,67-72,75-77,81,83,86,93],[96,99,100,103,108,109,113,116],[119,123-125,130,133,134,140-144,146,170],[171,179,180,182,187,192,194,201]. The studies provided insufficient information on selective reporting, which limited us from making any judgment. We analyzed the outcomes separately for women and children and the outcomes analyzed for each micronutrient fortification are outlined in Table 1. Definitions of various outcomes used are outlined in Table 2. The characteristics of the included studies and quality assessment of the evidence are summarized below and in Tables 3, 4, 5, 6, 7 and 8 and further details along with the forest plots are provided in Additional files 1, 2 and 3.
Figure 2.
Summary of search strategy.
Table 2.
Definitions of various micronutrient deficiencies
| Term | Definition |
|---|---|
| Anemiaa |
Children 6 to 59 months of age: Hb less than 110 g/l |
| Children 5 to 11 years of age: Hb less than 115 g/l | |
| Children 12 to 14 years of age: Hb less than 120 g/l | |
| Women 15 years and older: Hb less than 120 g/l | |
| Vitamin A deficiencyb |
Plasma (serum) retinol concentration of less than 20 μg/dl |
| Zinc deficiencyc |
Serum zinc concentration of less than 10.7 μmol/L |
| Asymptomatic zinc deficiency | Serum zinc concentration of less than 10.7 μmol/L with the absence of clinical signs and symptoms. |
a Adapted from WHO VIMNIS (available from http://www.who.int/vmnis/indicators/haemoglobin/en/) [229].
b Adapted from WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005 [230].
c Adapted from WHO. Trace elements in human nutrition and health. Geneva, Switzerland: WHO, 1996 [231] Hb, hemoglobin.
Table 3.
Summary of results for iron fortification in children
| Outcome/quality of evidence | Combined effect |
Population |
Age groups |
Country |
Fortificant type |
Food vehicle |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General/healthy population (studies/ participants) | Deficient population | Infants | Preschool and school- going children | Low- and lower middle income countries | Upper middle- and higher-income countries | Ferrous sulfate | NaFeEDTA | Processed food | Staples | Condiments | ||
|
Hemoglobin levels |
SMD: 0.55 (95% CI: 0.34, 0.76) |
SMD: 0.50 (95% CI: 0.28, 0.71) |
SMD: 0.79 (95% CI: 0.19, 1.38) |
SMD: 0.81 (95% CI: 0.31, 1.31) |
SMD: 0.46 (95% CI: 0.24, 0.67) |
SMD: 0.32 (95% CI: 0.14, 0.50) |
SMD: 0.67 (95% CI: 0.36, 0.97) |
SMD: 0.50 (95% CI: 0.14, 0.86) |
SMD: 0.76 (95% CI: 0.06, 1.47) |
SMD: 0.55 (95% CI: 0.29, 0.82) |
SMD: 0.44 (95% CI: 0.10, 0.79) |
SMD: 1.14 (95% CI: -0.09, 2.37) |
|
Quality of evidence: Moderate |
|
25 studies 5,083 participants |
6 studies 1,184 participants |
12 studies 1,834 participants |
16 studies 3,832 participants |
10 studies 1,937 participants |
20 studies 4,176 participants |
9 studies 1,076 participants |
6 studies 1,172 participants |
18 studies 3,317 participants |
8 studies 1,941 participants |
3 studies 705 participants |
|
Serum ferritin levels |
SMD: 0.91 (95% CI: 0.38, 1.44) |
SMD: 0.63 (95% CI: 0.28, 0.98) |
SMD: 1.37 (95% CI: 0.01, 2.74) |
SMD: 0.63 (95% CI: 0.28, 0.98) |
SMD: 1.37 (95% CI: 0.01, 2.74) |
SMD: 1.37 (95% CI: 0.01, 2.74) |
SMD: 0.68 (95% CI: 0.49, 0.87) |
SMD: 0.84 (95% CI: 0.60, 1.09) |
SMD: 1.83 (95% CI: -0.08, 3.73) |
SMD: 0.66 (95% CI: 0.36, 0.97) |
SMD: 0.48 (95% CI: 0.17, 0.78) |
SMD: 2.80 (95% CI: 2.43, 3.17) |
|
Quality of evidence: Moderate |
|
4 studies 952 participants |
3 studies 555 participants |
4 studies 952 participants |
3 studies 555 participants |
3 studies 555 participants |
3 studies 547 participants |
3 studies 539 participants |
2 studies 385 participants |
5 studies 1,097 participants |
1 study 170 participants |
1 study 240 participants |
|
Effect on anemia |
RR: 0.55 (95% CI: 0.42, 0.72) |
RR: 0.64 (95% CI: 0.50, 0.82) |
RR: 0.23 (95% CI: 0.04, 1.25) |
RR: 0.42 (95% CI: 0.24, 0.72) |
RR: 0.60 (95% CI: 0.43, 0.84) |
RR: 0.91 (95% CI: 0.72, 1.14) |
RR: 0.41 (95% CI: 0.28, 0.61) |
RR: 0.52 (95% CI: 0.34, 0.79) |
RR: 0.46 (95% CI: 0.17, 1.23) |
RR: 0.46 (95% CI: 0.28, 0.76) |
RR: 0.81 (95% CI: 0.60, 1.10) |
RR: 0.12 (95% CI: 0.01, 2.16) |
| Quality of evidence: Moderate | 15 studies 3,096 participants | 3 studies 555 participants | 6 studies 1,234 participants | 10 studies 2,013 participants | 6 studies 1,171 participants | 11 studies 2,396 participants | 4 studies 630 participants | 4 studies 800 participants | 10 studies 1,617 participants | 5 studies 1,312 participants | 2 studies 568 participants | |
Estimates in bold show significant impacts. CI, confidence interval; NaFeEDTA, sodium iron ethylenediaminetetraacetate RR: relative risk; SMD: standard mean difference.
Table 4.
Summary of results for zinc fortification in children
|
Outcome/ quality of evidence |
Combined effect |
Age groups |
Zinc compound used for fortification |
Food vehicle |
Duration |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Newborns with very low birth weight | Full term healthy infants | Infants at risk of stunting | Malnourished infants | School-going children | School-going children with asymptomatic zinc deficiency | Zinc sulfate | Zinc chloride | Zinc acetate | Milk products | Cereal products | Less than 6 months | 6 months or more | ||
|
Serum zinc levels |
SMD: 1.28 (95% CI: 0.56, 2.01) |
SMD: 0.51 (95% CI: -0.32, 1.34) |
SMD: 3.49 (95% CI: -0.36, 7.33) |
SMD: 0.09 (95% CI: -0.24, 0.41) |
SMD: 0.12 (95% CI: -0.55, 0.78) |
SMD: 1.12 (95% CI: -0.21, 2.46) |
SMD: 2.77 (95% CI: 1.43, 4.11) |
No studies identified |
No studies identified |
No studies identified |
SMD: 1.46 (95% CI: 0.26, 2.66) |
SMD: 1.16 (95% CI: 0.08, 2.24) |
SMD: 1.48 (95% CI: 0.57, 2.39) |
SMD: 1.51 (95% CI: 0.13, 2.89) |
|
Quality of evidence: Moderate |
|
2 studies 64 participants |
2 studies 71 infants |
1 study 147 infants |
1 study 35 infants |
2 studies 347 participants |
1 study 19 children |
|
|
|
5 studies 176 participants |
4 studies 513 participants |
4 studies 381 participants |
4 studies 155 participants |
|
Hemoglobin level |
SMD: -0.11 (95% CI: -0.52, 0.31) |
No studies identified |
No studies identified |
No studies identified f |
No studies identified |
No studies identified |
No studies identified |
SMD: 0.00 (95% CI: -0.67, 0.67) |
SMD: -0.39 (95% CI: -1.03, 0.24) |
SMD: 0.28 (95% CI: -0.62, 1.19) |
SMD: -0.21 (95% CI: -0.67, 0.25) |
SMD: 0.28 (95% CI: -0.62, 1.19) |
No studies identified |
No studies identified |
|
Quality of evidence: Low |
|
|
|
|
|
|
|
1 study 34 children |
1 study 39 children |
1 study 19 children |
2 studies 73 participants |
1 study 19 participants |
|
|
|
Serum copper levels |
SMD: 0.57 (95% CI: -0.91, 2.06) |
No studies identified |
SMD: 0.75 (95% CI: -1.65, 3.16) |
No studies identified |
SMD: 0.21 (95% CI: -0.46, 0.87) |
No studies identified |
No studies identified |
No studies identified |
No studies identified |
No studies identified |
SMD: 0.57 (95% CI: -0.91, 2.06) |
SMD: -0.61 (95% CI: -1.54, 0.31) |
No studies identified |
No studies identified |
|
Quality of evidence: Very low |
|
|
3 studies 126 infants |
|
1 study 35 infants |
|
|
|
|
|
3 studies 142 participants |
1 study 19 participants |
|
|
|
Serum alkaline phosphatase levels |
SMD: 0.94 (95% CI: -0.29, 2.17) |
No studies identified |
SMD: 0.68 (95% CI: -0.90, 2.25) |
No studies identified |
No studies identified |
No studies identified |
SMD: 1.56 (95% CI: 0.50, 2.62) |
No studies identified |
No studies identified |
No studies identified |
SMD: 0.68 (95% CI: -0.90, 2.25) |
SMD: 1.56 (95% CI: 0.50, 2.62) |
No studies identified |
No studies identified |
|
Quality of evidence: Low |
|
|
2 studies 100 infants |
|
|
|
1 study 19 children |
|
|
|
2 studies 100 participants |
1 study 19 participants |
|
|
|
Weight gain |
SMD: 0.50 (95% CI: -0.12, 1.11) |
SMD: 0.33 (95% CI: -0.33, 0.99) |
SMD: 1.48 (95% CI: -1.49, 4.45) |
SMD: 0.00 (95% CI: -0.30, 0.30) |
SMD: -0.10 (95% CI: -0.73, 0.52) |
SMD: 0.25 (95% CI: -0.17, 0.67) |
No studies identified |
No studies identified |
No studies identified |
No studies identified |
SMD: 0.77 (95% CI: -0.43, 1.96) |
SMD: 0.08 (95% CI:-0.16, 0.33) |
No studies identified |
No studies identified |
|
Quality of evidence: Moderate |
|
1 study 36 infants |
2 studies 80 infants |
1 study 176 infants |
1 study 39 infants |
1 study 88 children |
|
|
|
|
4 studies 155 participants |
2 studies 264 participants |
|
|
|
Height growth |
SMD: 0.52 (95% CI: 0.01, 1.04) |
SMD: 0.70 (95% CI: 0.02, 1.37) |
SMD: -0.48 (95% CI: -2.45, 1.48) |
SMD: 0.05 (95% CI: -0.25, 0.35) |
SMD: 0.16 (95% CI: -0.47, 0.79) |
SMD: 0.31 (95% CI: -0.11, 0.74) |
No studies identified |
No studies identified |
No studies identified |
No studies identified |
SMD: -0.09 (95% CI: -1.17, 0.99) |
SMD: 0.14 (95% CI: -0.11, 0.38) |
No studies identified |
No studies identified |
| Quality of evidence: Moderate | 1 study 36 infants | 2 studies 80 infants | 1 study 176 infants | 1 study 39 infants | 1 study 88 children | 5 studies 187 participants | 2 studies 264 participants | |||||||
CI, confidence interval; SMD: Standard mean difference. Estimates in bold show significant impacts.
Table 5.
Summary of results for iodine, Vitamin A and calcium/vitamin D fortification in children
| |
|
Iodine fortification |
||
|---|---|---|---|---|
| Outcome/quality of evidence | Combined effect | |||
|
Serum thyroxin levels |
SMD: 0.45 (95% CI: -1.15, 2.06) |
2 studies 1,131 participants reported this outcome. The studies were conducted in Africa on school-going children. |
||
|
Quality of evidence: Low |
|
|||
|
Urinary iodine concentrations |
SMD: 6.39 (95% CI: 2.69, 10.08) |
2 studies 1,016 participants reported this outcome. The studies were conducted in Africa on school-going children. |
||
|
Quality of evidence: Moderate |
|
|||
| |
|
Vitamin A fortification |
||
|
Outcome/quality of evidence |
Combined effect |
|
||
|
Hemoglobin levels |
SMD: 0.48 (95% CI: 0.07, 0.89) |
2 studies with1,538 participants reported the outcome. The study by Zhang et al.[132] used four different comparison groups. The first group received low amounts of vitamin A-fortified biscuits for 9 months daily. The second group received high amount of vitamin A-fortified biscuits for 3 months daily. The third group received very high amounts of vitamin A-fortified biscuits for 3 months weekly. The standard mean differences for the three groups were 0.73 (95% CI: 0.42, 1.04), 0.62 (95% CI: 0.38, 0.85) and 0.63 (95% CI: 0.38, 0.88) respectively. |
||
|
Quality of evidence: Low | ||||
|
Serum vitamin A concentration |
SMD: 0.61 (95% CI: 0.39, 0.83) |
3 studies with 2,362 participants reported the outcome. The study by Zhang et al.[132] used four different comparison groups as explained in comments for hemoglobin levels. The standard mean differences for the three groups were 0.52 (95% CI: 0.21, 0.82), 0.73 (95% CI: 0.49, 0.97) and 0.44 (95% CI: 0.22, 0.66). |
||
|
Quality of evidence: Low | ||||
|
Vitamin A deficiency |
RR: 0.39 (95% CI: 0.09, 1.74) |
2 studies with 1,465 participants reported the outcome. The study by Zhang et al.[132] showed positive impacts in reducing vitamin A deficiency whereas the study by Solon et al.[110] showed negative impacts. The latter used monosodium glutamate for vitamin A fortification and the quantity of vitamin A used was much less than that used by Zhang et al. |
||
|
Quality of evidence: Moderate | ||||
|
Calcium and vitamin D fortification | ||||
|
Outcome/quality of evidence |
Combined effect |
Calcium only |
Vitamin D only |
Calcium and vitamin D |
|
Serum parathyroid hormone levels |
SMD: -0.40 (95% CI: -0.56, -0.24) |
SMD: -0.28 (95% CI: -0.50, -0.06) |
No studies identified |
SMD: -0.52 (95% CI: -0.74, -0.29) |
|
Quality of evidence: Low |
2 studies 317 participants |
2 studies 327 participants |
||
|
Serum vitamin D levels |
SMD: 1.23 (95% CI: 0.35, 2.11) |
SMD: -0.15 (95% CI: -0.41, 0.10) |
SMD: 1.76 (95% CI: 0.37, 3.15) |
SMD: 1.58 (95% CI: 1.28, 1.87) |
|
Quality of evidence: Moderate |
1 study 233 participants |
2 studies 651 participants |
1 study 235 participants |
|
|
Serum calcium levels |
SMD: -0.40 (95% CI: -0.59, -0.20) |
SMD: -0.30 (95% CI: -0.56, -0.04) |
No studies identified |
SMD: -0.50 (95% CI: -0.76, -0.24) |
| Quality of evidence: Low | 1 study 231 participants | 1 study 235 participants | ||
Estimates in bold show significant impacts. CI, confidence intervals; RR: relative risk; SMD: standard mean difference.
Table 6.
Summary of results for multiple micronutrient fortification in children
| Outcome/quality of evidence | Combined effect | |
Age groups |
Country |
Number of micronutrients used |
Duration of fortification |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General/healthy population | Deficient population | Infants | Preschool and school-going children | Low- and lower middle-income countries | Upper middle- and higher-income countries | 5 or less | 6 to 12 | More than 12 | Less than 6 months | 6 to 11 months | 12 months or more | ||
|
Hemoglobin levels |
SMD: 0.75 (95% CI: 0.41, 1.08) |
SMD: 0.72 (95% CI: 0.37, 1.07) |
SMD: 1.06 (95% CI: 0.75, 1.38) |
SMD: 1.05 (95% CI: 0.48, 1.63) |
SMD: 0.45 (95% CI: 0.12, 0.79) |
SMD: 0.50 (95% CI: 0.21, 0.78) |
SMD: 1.25 (95% CI: 0.45, 2.06) |
SMD: 1.50 (95% CI: -0.23, 3.23) |
SMD: 0.51 (95% CI: 0.24, 0.78) |
SMD: 0.71 (95% CI: 0.32, 1.09) |
SMD: 0.07 (95% CI: -0.17, 0.31) |
SMD: 1.05 (95% CI: 0.55, 1.56) |
SMD: 0.52 (95% CI: 0.07, 0.98) |
|
Quality of evidence: Moderate |
|
13 studies 2,876 participants |
1 study 175 participants |
7 studies 1,508 participants |
7 studies 1,543 participants |
8 studies 1,769 participants |
6 studies 1,282 participants |
4 studies 747 participants |
6 studies 1,449 participants |
4 studies 855 participants |
2 studies 310 participants |
8 studies 2,025 participants |
4 studies 716 participants |
|
Serum ferritin levels |
SMD: 0.37 [0.13, 0.62) |
SMD: 0.37 (95% CI: 0.13, 0.62) |
No studies identified |
SMD: 0.43 (95% CI: 0.17, 0.68) |
SMD: 0.06 (95% CI: -0.17, 0.29) |
SMD: 0.47 (95% CI: 0.14, 0.80) |
SMD: 0.18 (95% CI: -0.03, 0.40) |
SMD: 0.45 (95% CI: 0.00, 0.90) |
SMD: 0.62 (95% CI: 0.18, 1.06) |
SMD: 0.17 (95% CI: -0.16, 0.50) |
SMD: 0.45 (95% CI: 0.00, 0.90) |
SMD: 0.48 (95% CI: 0.07, 0.89) |
SMD: 0.20 (95% CI: -0.23, 0.64) |
|
Quality of evidence: Low |
|
7 studies 1,848 participants |
|
6 studies 1,547 participants |
1 study 301 participants |
4 studies 1,380 participants |
3 studies 468 participants |
1 study 79 participants |
2 studies 748 participants |
4 studies 1,021 participants |
1 study 79 participants |
3 studies 1,049 participants |
3 studies 7,20 participants |
|
Serum zinc levels |
SMD: 0.08 [−0.02, 0.19) |
SMD: 0.08 (95% CI: -0.02, 0.19) |
No studies identified |
SMD: 0.04 (95% CI: -0.10, 0.17) |
SMD: 0.17 (95% CI: 0.04, 0.30) |
SMD: 0.12 (95% CI: 0.01, 0.23) |
SMD: -0.02 (95% CI: -0.26, 0.23) |
SMD: -0.12 (95% CI: -0.75, 0.51) |
SMD: 0.13 (95% CI: -0.02, 0.29) |
SMD: 0.06 (95% CI: -0.05, 0.17) |
SMD: 0.31 (95% CI: 0.06, 0.57) |
SMD: 0.06 (95% CI: -0.09, 0.21) |
SMD: 0.02 (95% CI: -0.11, 0.15) |
|
Quality of evidence: Moderate |
|
11 studies 2,972 participants |
|
7 studies 1,780 participants |
4 studies 1,192 participants |
7 studies 1,960 participants |
4 studies 1,012 participants |
2 studies 232 participants |
4 studies 1,820 participants |
5 studies 920 participants |
1 study 223 children |
6 studies 1,128 participants |
4 studies 1,221 participants |
|
Serum retinol levels |
SMD: -0.05 [−0.23, 0.13) |
SMD: 0.05 (95% CI: -0.23, 0.13) |
No studies identified |
SMD: 0.04 (95% CI: -0.22, 0.30) |
SMD: -0.21 (95% CI: -0.34, -0.07) |
SMD: -0.06 (95% CI: -0.25, 0.14) |
SMD: 0.01 (95% CI: -0.63, 0.64) |
SMD: -0.28 (95% CI: -0.45, -0.11) |
SMD: -0.03 (95% CI: -0.33, 0.27) |
SMD: 0.02 (95% CI: -0.27, 0.31) |
No studies identified |
SMD: -0.08 (95% CI: -0.29, 0.13) |
SMD: 0.03 (95% CI: -0.37, 0.43) |
|
Quality of evidence: Low |
|
8 studies 1,927 participants |
|
4 studies 964 participants |
4 studies 963 participants |
6 studies 1,320 participants |
to 2 studies 607 participants |
2 studies 533 participants |
5 studies 878 participants |
3 studies 516 participants |
|
3 studies 1,676 participants |
3 studies 251 participants |
|
Effect on anemia |
RR: 0.55 (95% CI: 0.42, 0.71) |
RR: 0.59 (95% CI: 0.46, 0.75) |
RR: 0.20 (95% CI: 0.10, 0.40) |
RR: 0.59 (95% CI: 0.50, 0.70) |
RR: 0.45 (95% CI: 0.22, 0.89) |
RR: 0.50 (95% CI: 0.37, 0.67) |
RR: 0.69 (95% CI: 0.41, 1.15) |
No studies identified |
No studies identified |
No studies identified |
RR: 0.77 (95% CI: 0.43, 1.36) |
RR: 0.54 (95% CI: 0.36, 0.83) |
RR: 0.50 (95% CI: 0.33, 0.77) |
|
Quality of evidence: Low |
|
9 studies 2,880 participants |
1 study 175 participants |
5 studies 1809 participants |
5 studies 1246 participants |
7 studies 2,229 participants |
3 studies 826 participants |
|
|
|
1 study 223 children |
4 studies 1,097 participants |
5 studies 1,726 participants |
|
Effect on vitamin A deficiency |
RR: 0.90 (95% CI: 0.76, 1.06) |
RR: 0.90 (95% CI: 0.76, 1.06) |
No studies identified |
RR: 0.85 (95% CI: 0.69, 1.03) |
RR: 1.02 (95% CI: 0.76, 1.38) |
RR: 0.88 (95% CI: 0.73, 1.07) |
RR: 0.95 (95% CI: 0.66, 1.36) |
No studies identified |
No studies identified |
No studies identified |
No studies identified |
RR: 0.85 (95% CI: 0.71, 1.03) |
RR: 1.14 (95% CI: 0.76, 1.71) |
|
Quality of evidence: Low |
|
6 studies 2,036 participants |
|
3 studies 687 participants |
3 studies 1349 participants |
4 studies 1,391 participants |
2 studies 645 participants |
|
|
|
|
3 studies 1,308 participants |
3 studies 728 participants |
|
Height-for-age Z-score |
SMD: 0.13 (95% CI: -0.04, 0.29) |
SMD: 0.12 (95% CI: -0.09, 0.32) |
SMD: 0.22 (95% CI: -0.04, 0.47) |
SMD: 0.26 (95% CI: 0.12, 0.40) |
SMD: -0.01 (95% CI: -0.21, 0.20) |
SMD: 0.09 (95% CI: -0.17, 0.34) |
SMD: 0.18 (95% CI: 0.02, 0.34) |
SMD: -0.00 (95% CI: -0.19, 0.18) |
SMD: 0.11 (95% CI: -0.12, 0.34) |
SMD: 0.18 (95% CI: -0.07, 0.42) |
SMD: 0.01 (95% CI: -0.13, 0.15) |
SMD: 0.11 (95% CI: -0.12, 0.34) |
SMD: 0.39 (95% CI: 0.24, 0.55) |
|
Quality of evidence: Low |
|
6 studies 1,853 participants |
2 studies 245 participants |
5 studies 1,083 participants |
3 studies 1,009 participants |
5 studies 1,485 participants |
3 studies 607 participants |
2 studies 624 participants |
1 study 288 participants |
5 studies 1,180 participants |
5 studies 1,170 participants |
1 study 288 participants |
2 studies 634 participants |
|
Weight-for-age Z-score |
SMD: -0.12 (95% CI: -0.43, 0.20) |
SMD: 0.05 (95% CI: -0.09, 0.18) |
SMD: 0.18 (95% CI: -0.14, 0.49) |
SMD: -0.28 (95% CI: -0.85, 0.29) |
SMD: 0.09 (95% CI: -0.16, 0.33) |
SMD: 0.10 (95% CI: -0.13, 0.32) |
SMD: -0.48 (95% CI: -1.45, 0.49) |
SMD: -0.02 (95% CI: -0.28, 0.24) |
SMD: -0.03 (95% CI: -0.26, 0.20) |
SMD: -0.22 (95% CI: -0.76, 0.33) |
SMD: -0.25 (95% CI: -0.90, 0.41) |
SMD: 0.06 (95% CI: -0.09, 0.21) |
SMD: -0.68 (95% CI: -2.79, 1.42) |
|
Quality of evidence: Low |
|
6 studies 1,853 participants |
2 studies 245 participants |
5 studies 1083 participants |
3 studies 1,009 participants |
5 studies 1,485 participants |
3 studies 607 participants |
2 studies 624 participants |
1 study 288 participants |
5 studies 1,180 participants |
5 studies 1,170 participants |
1 study 288 participants |
2 studies 634 participants |
|
Weight-for-height Z-score |
SMD: -0.11 (95% CI: -0.40, 0.17) |
SMD: -0.17 (95% CI: -0.56, 0.22) |
SMD: -0.56 (95% CI: -1.42, 0.31) |
SMD: 0.08 (95% CI: -0.06, 0.21) |
SMD: -0.39 (95% CI: -1.06, 0.28) |
SMD: 0.07 (95% CI: -0.07, 0.20) |
SMD: -0.40 (95% CI: -1.17, 0.38) |
SMD: -0.01 (95% CI: -0.29, 0.26) |
SMD: -0.14 (95% CI: -0.37, 0.09) |
SMD: -0.16 (95% CI: -0.66, 0.34) |
SMD: -0.10 (95% CI: -0.75, 0.56) |
SMD: -0.23 (95% CI: -0.64, 0.18) |
SMD: 0.18 (95% CI: 0.02, 0.34) |
| Quality of evidence: Low | 6 studies 1,853 participants | 2 studies 245 participants | 5 studies 1,083 participants | 3 studies 1,009 participants | 5 studies 1,485 participants | 3 studies 607 participants | 2 studies 624 participants | 1 study 288 participants | 5 studies 1,180 participants | 5 studies 1,170 participants | 1 study 288 participants | 2 studies 634 participants | |
Estimates in BOLD show significant impacts. CI, confidence intervals; RR: relative risk; SMD: standard mean difference.
Table 7.
Summary of results for iron, folate and calcium/vitamin D fortification in women
| Iron fortification | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Outcome/quality of evidence |
Combined effect |
Population |
Country |
Fortificant type |
Food vehicle |
|||||
|
General/healthy population |
Deficient population |
Low- and lower middle-income countries |
Upper middle- and higher-income countries |
Ferrous sulfate |
NaFeEDTA |
Processed food |
Staples |
Condiments |
||
|
Hemoglobin levels |
SMD: 0.62 (95% CI: 0.36, 0.89) |
SMD: 0.66 (95% CI: 0.30, 1.02) |
SMD: 0.59 (95% CI: 0.34, 0.83) |
SMD: 0.52 (95% CI: -0.12, 1.16) |
SMD: 0.66 (95% CI: 0.31, 1.00) |
SMD: 0.44 (95% CI: 0.21, 0.67) |
SMD: 0.48 (95% CI: 0.22, 0.73) |
SMD: 0.50 (95% CI: 0.30, 0.70) |
SMD: 0.90 (95% CI: -0.10, 1.90) |
SMD: 0.45 (95% CI: 0.22, 0.68) |
|
Quality of evidence: Moderate |
|
7 studies 2,819 participants |
4 studies 404 participants |
2 studies 524 participants |
8 studies 2,588 participants |
3 studies 310 participants |
4 studies 1,588 participants |
4 studies 398 participants |
3 studies 1,211 participants |
4 studies 1,614 participants |
|
Serum ferritin levels |
SMD: 0.41 (95% CI: 0.23, 0.60) |
SMD: 0.23 (95% CI: 0.08, 0.38) |
SMD: 0.75 (95% CI: 0.47, 1.03) |
SMD: 0.48 (95% CI: 0.31, 0.66) |
SMD: 0.39 (95% CI: 0.17, 0.61) |
SMD: 0.42 (95% CI: 0.05, 0.78) |
SMD: 0.45 (95% CI: 0.21, 0.69) |
SMD: 0.46 (95% CI: 0.10, 0.82) |
SMD: 0.17 (95% CI: -0.15, 0.49) |
SMD: 0.50 (95% CI: 0.33, 0.68) |
|
Quality of evidence: Low |
|
6 studies 2,609 participants |
4 studies 400 participants |
2 studies 525 participants |
8 studies 2,484 participants |
1 study 117 participants |
4 studies 1,559 participants |
5 studies 1,390 participants |
to 2 studies 993 participants |
3 studies 626 participants |
|
Effect on anemia |
RR: 0.68 (95% CI: 0.49, 0.93) |
RR: 0.65 (95% CI: 0.40, 1.04) |
RR: 0.74 (95% CI: 0.61, 0.90) |
RR: 0.74 (95% CI: 0.61, 0.90) |
RR: 0.67 (95% CI: 0.39, 1.17) |
RR: 0.57 (95% CI: 0.34, 0.94) |
RR: 0.74 (95% CI: 0.61, 0.90) |
RR: 0.51 (95% CI: 0.21, 1.25) |
RR: 0.67 (95% CI: 0.39, 1.17) |
RR: 0.74 (95% CI: 0.61, 0.90) |
|
Quality of evidence: Low |
|
3 studies 1,180 participants |
1 study 136 participants |
1 study 136 participants |
2 studies 1,056 participants |
2 studies 199 participants |
1 study 136 participants |
1 study 124 participants |
2 studies 1,056 participants |
1 study 136 participants |
|
Folate fortification | ||||||||||
|
Outcome/quality of evidence |
Combined effect |
Quantity |
Duration |
|||||||
|
40 μg/100 g |
More than 100 μg/100 g |
Less than 1 year |
Greater than 1 year |
|||||||
|
Neural tube defects |
RR: 0.57 (95% CI: 0.45, 0.73) |
RR: 0.80 (95% CI: 0.75, 0.86) |
RR: 0.55 (95% CI: 0.41, 0.75) |
RR: 0.52 (95% CI: 0.28, 0.97) |
RR: 0.60 (95% CI: 0.50, 0.72) |
|||||
|
Quality of evidence: Moderate |
|
3 studies |
2 studies |
3 studies |
4 studies |
|||||
|
Spina bifida |
RR: 0.64 (95% CI: 0.57, 0.71) |
RR: 0.72 (95% CI: 0.65, 0.80) |
RR: 0.51 (95% CI: 0.41, 0.65) |
RR: 0.72 (95% CI: 0.65, 0.80) |
RR: 0.56 (95% CI: 0.49, 0.65) |
|||||
|
Quality of evidence: Moderate |
|
6 studies |
3 studies |
3 studies |
7 studies |
|||||
|
Anencephaly |
RR: 0.76 (95% CI: 0.68, 0.85) |
RR: 0.81 (95% CI: 0.74, 0.88) |
RR: 0.67 (95% CI: 0.43, 1.04) |
RR: 0.85 (95% CI: 0.79, 0.93) |
RR: 0.70 (95% CI: 0.59, 0.83) |
|||||
|
Quality of evidence: Moderate |
|
6 studies |
2 studies |
3 studies |
7 studies |
|||||
|
Serum folate levels |
SMD: 1.38 (95% CI: -0.20, 2.95) |
No studies identified |
No studies identified |
SMD: 1.65 (95% CI: -0.51, 3.82) |
SMD: 0.45 (95% CI: -0.06, 0.97) |
|||||
|
Quality of evidence: Low |
|
|
|
3 studies |
2 studies |
|||||
|
Iodine fortification | ||||||||||
|
Outcome/quality of evidence |
Combined effect |
|||||||||
|
Effect on hypothyroidism |
RR: 1.42 (95% CI: 1.11, 1.82) |
|||||||||
|
Quality of evidence: Low |
2 studies |
|||||||||
|
Urinary iodine concentrations |
SMD: 7.16 (95% CI: 1.00, 13.31) |
|||||||||
|
Quality of evidence: Moderate |
4 studies |
|||||||||
|
Calcium and vitamin D fortification | ||||||||||
|
Outcome/quality of evidence |
Women of reproductive age |
Post-menopausal women |
||||||||
|
Vitamin D only |
Calcium and vitamin D |
Calcium only |
Vitamin D only |
|||||||
|
Serum parathyroid hormone levels |
SMD: 0.10 (95% CI: -0.28, 0.48) |
SMD: -0.23 (95% CI: -0.78, 0.32) |
No studies identified |
No studies identified |
||||||
|
Quality of evidence: Low |
2 studies 108 participants |
1 study 52 participants |
|
|
||||||
|
Serum vitamin D levels |
SMD: 0.26 (95% CI: -0.22, 0.75) |
SMD: -2.50 (95% CI: -3.22, -1.78) |
SMD: 0.69 (95% CI: 0.38, 1.00) |
No studies identified |
||||||
| Quality of evidence: Moderate | 1 study 66 participants | 1 study 55 participants | 3 studies 228 | |||||||
Estimates in BOLD show significant impacts. CI, confidence intervals; NaFeEDTA: sodium iron ethylenediaminetetraacetate; RR: relative risk; SMD: standard mean difference.
Table 8.
Summary of results for multiple micronutrient fortification in women
| Outcome/quality of evidence | Combined effect |
|---|---|
|
Hemoglobin levels |
SMD: 0.31 (95% CI: 0.13, 0.48) |
|
Quality of evidence: Moderate |
1 study 516 women |
|
Serum ferritin levels |
SMD: 0.47 (95% CI: 0.36, 0.58) |
|
Quality of evidence: Moderate |
2 studies 1,214 women |
|
Serum zinc levels |
SMD: 0.50 (95% CI: 0.38, 0.61) |
|
Quality of evidence: Moderate |
2 studies 1214 women |
|
Serum retinol levels |
SMD: 0.47 (95% CI: 0.30, 0.65) |
|
Quality of evidence: Moderate |
1 study 516 women |
|
Effect on anemia |
RR: 0.76 (95% CI: 0.48, 1.21) |
| Quality of evidence: Moderate | 1 study 516 women |
Estimates in bold show significant impacts. RR: relative risk; SMD: standard mean difference.
Children
Iron fortification
A total of 41 [25,26,28,30-32,35,39,48,49],[54,56,57,65,70,77,80,81],[83,85,88,89,97,99,101,104],[106-108,112-114,116,120,124,126],[127,129,135,136,139] studies reported outcomes for impact of iron fortification. Four of the studies were before-after studies and the rest were RCTs. Nineteen of these studies had infants as their study population, fortifying formula milk, cow’s milk and complementary baby foods. Condiments such as curry powder, fish sauce and soy sauce were the vehicles used in a few studies targeting Asian populations. Drinking water, food bars, candies, noodles and fruit juices were also assessed as potential vehicles by various studies. Seven studies used sodium iron ethylenediaminetetraacetate (NaFeEDTA) and 15 used ferrous sulfate as the fortificant. Other forms of iron used were ferrous pyrophosphate, unspecified elemental iron and hydrogen-reduced iron. Twenty-two of the studies were carried out in upper middle income countries (UMIC) and higher income countries (HIC), and 18 were from lower middle income countries (LMIC) and lower income countries (LIC). Eight of the studies were carried out in populations with some form of iron deficiency or a comorbidity. A summary of results is presented in Table 3.
Pooled analyses from the RCTs demonstrated a significant increase in hemoglobin concentration (SMD: 0.55 (95% CI: 0.34, 0.76)), serum ferritin levels (SMD: 0.91 (95% CI: 0.38, 1.44)) and reduction in anemia (RR: 0.55 (95% CI: 0.42, 0.72)). A few studies reported the effect of iron fortification on cognition, but these could not be pooled together because the reported outcomes varied significantly.
Subgroup analysis according to the various age groups (infants, preschool and school going), countries (LIC/LMIC and UMIC/HIC) and baseline micronutrient status (deficient and normal populations) showed consistent findings for all the outcomes assessed. The impacts on serum ferritin levels and anemia were significant for all the three food vehicles, that is, processed food, condiments and staples, whereas the impact on hemoglobin levels was significant for processed food and staples only. The effect of NaFeEDTA when used as the fortificant was significant on hemoglobin levels and anemia but was non-significant on serum ferritin levels. The impacts of ferrous sulfate when used as the fortificant were significant for all the three outcomes assessed.
Zinc
Our search strategy identified 10 studies examining zinc fortification [34,41,44,53,59,78,91,100],[103,125], seven on infants, where infant formula feeds or milk were fortified, and three on school children, where porridge or bread was fortified [53,59,91]. Eight of the studies were RCTs and two were quasi-experimental designs. The studies varied in the duration of intervention ranging from 1 month to 12 months. Five studies employed zinc sulfate as the fortificant; other compounds used were zinc oxide, zinc chloride and zinc acetate. A summary of results is presented in Table 4.
Results from the RCTs showed a significant impact of zinc fortification on increasing serum zinc concentration (SMD: 1.28 (95% CI: 0.56, 2.01)), whereas non-significant impacts were observed for height velocity (SMD: 0.08 (95% CI: -0.53, 0.69)), weight gain (SMD: 0.50 (95% CI: -0.12, 1.11)), serum alkaline phosphatase (SMD: 0.94 (95% CI: -0.29, 2.17)), hemoglobin levels (SMD: -0.11, (95% CI: -0.52 to 0.31)) and serum copper levels (SMD: 0.22 (95% CI: -1.14 to 1.59)). Visual inspection of the forest plot for height velocity showed that the study by Salmenpera et al.[98] was the main outlier; after sensitivity analysis, it showed significant improvements (SMD: 0.52 (95% CI: 0.01, 1.04)).
Subgroup analysis showed a consistent positive effect on serum zinc levels for both milk- and cereal-based products, and for various durations of fortification (less than and more than six months). For the various age groups assessed, the effect was significant for only school-going children with an asymptomatic deficiency at baseline. The impact on weight gain was non-significant across all the various age groups while the effect on height gain was significant only for infants with very low birth weight.
Vitamin D and calcium
A total of seven RCTs, one quasi-experimental and two before-after studies were identified for vitamin D and calcium fortification [33,42,45,51,55,60,66,96],[133,134]. Milk was the preferred food vehicle and the amount of micronutrient used varied significantly among the studies. One study was on preterm infants while the rest were on children and adolescents ranging from 6 years to 18 years of age. A summary of results is presented in Table 5.
Analysis of the results from RCTs showed that fortification with vitamin D significantly increased serum concentration of 25-hydroxy-vitamin D3 (SMD: 1.23 (95% CI: 0.35, 2.11)) and reduced serum parathyroid hormone concentration (SMD: -0.40 (95% CI: -0.56, -0.24)).
Vitamin A
A total of three RCTs, one quasi-experimental and four before-after studies were identified for vitamin A fortification [29,36,79,84,95,110,111,132]. The studies used monosodium glutamate, sugar and flour as the fortificant. These studies spanned from 6 months to 2 years in duration. Five studies had children of ages ranging from 1 to 6 years, and three studies had a range of 6 to 16 years of age. A summary of results is presented in Table 5.
Analysis of the RCTs showed significant impacts on serum retinol concentration (SMD: 0.61 (95% CI: 0.39, 0.83)) and hemoglobin levels (SMD: 0.48 (95% CI: 0.07, 0.89)). A non-significant effect was shown on prevalence of vitamin A deficiency (RR: 0.39 (95% CI: 0.09, 1.74)).
Iodine
Iodine fortification was identified in seven studies [40,52,58,94,131,137,138]. All were before-after study designs evaluations of mass salt fortification programs.
The analysis showed a significant effect on median urinary iodine concentrations (SMD: 6.39 (95% CI: 2.69, 10.08)) whereas the effect on serum thyroxin levels was non-significant (SMD: 0.45 (95% CI: -1.15, 2.06)). A summary of results is presented in Table 5.
Dual fortification
Ten studies were identified to study dual fortification [27,90,109,123,128,140-144]. Nine of the studies were RCTs and one was a quasi-experimental design and included children ranging from 5 to 17 years. Iron and iodine were the most commonly used micronutrients with salt as the food vehicle. Our analysis showed a significant impact of iron and iodine fortification on hemoglobin concentration (SMD: 0.53 (95% CI: 0.18, 0.89)) and on reducing the prevalence of anemia (RR: 0.47 (95% CI: 0.34, 0.66)).
Multiple micronutrients
MMN fortification was considered when three or more micronutrients were fortified together. A total of 34 studies were identified that reported outcomes on children [36-38,43,46,47,50,61-64],[67-69,71-76,82,86,87,92,93,98],[102,105,115,117,118,121,122,130]. Three of these were before-after studies and the rest were RCTs and quasi-experimental studies. Eleven studies had infants as their study population, others included children up to 15 years of age. The duration of fortification ranged from 3 months to 4 years. All the studies employed targeted fortification as their fortification strategy. A summary of results is presented in Table 6.
Analysis of the RCTs showed a significant effect of MMN fortification on hemoglobin levels (SMD: 0.75 (95% CI: 0.41, 1.08)), serum ferritin concentration (SMD: 0.37 (95% CI: 0.13, 0.62)) and on reducing anemia prevalence (RR: 0.55 (95% CI: 0.42, 0.71)). The impacts were non-significant on serum zinc concentration, serum retinol concentration and vitamin A deficiency. MMN fortification also had a non-significant impact on HAZ, WAZ and WHZ with a SMD of 0.13 (95% CI: -0.04, 0.29), 0.11 (95% CI: -0.06, 0.28) and 0.04 (95% CI: -0.08, 0.16) respectively, although showing positive trends. Analysis showed a non-significant effect on overall morbidity (including fever, UTI, diarrhea and respiratory infections) with a RR of 1.19 (95% CI: 0.98, 1.44). There was a non-significant impact on diarrhea and respiratory infections but the incidence of UTIs was significantly reduced, although the evidence of UTIs was from a single study.
Subgroup analyses for various age groups demonstrated a significant impact on hemoglobin levels, serum ferritin levels, anemia prevalence and HAZ in infants. Significant effects were observed in hemoglobin levels, serum ferritin levels and anemia prevalence for preschool and school-going children. Subgroup analyses according to countries showed that MMN fortification in LMIC/LIC and UMIC/HIC had significant impacts on hemoglobin levels, whereas in LIC/LMIC, the significant impact was on serum ferritin levels and serum zinc levels.
Women
Iron fortification
A total of 13 studies, including 11 RCTs, reported outcomes for the impact of iron fortification in women [35,151,154-156,173,175,178,203,204],[216,218,223]. Various fortificants, including NaFeEDTA, ferrous sulfate and ferrous pyrophosphate, were used. Ten of the studies were carried out in UMIC/HIC and three studies were from LIC/LMIC. Four of the studies were carried out in populations with some form of micronutrient deficiency or comorbidity. The most commonly reported outcomes included hemoglobin levels, serum ferritin levels and anemia. A summary of results is presented in Table 7.
Analysis of the RCTs showed a significant effect of iron fortification on hemoglobin concentration (SMD: 0.64 (95% CI: 0.30, 0.97)), serum ferritin levels (SMD: 0.41 (95% CI: 0.23, 0.60)) and on prevalence of anemia (RR: 0.68 (95% CI: 0.49, 0.93)).
Subgroup analyses showed that there was a significant impact on hemoglobin and ferritin levels for both the healthy and iron-deficient population, whereas the impact on anemia was only significant for iron-deficient population. Further subgroup analyses based on countries showed significant impacts of iron fortification on anemia and serum ferritin levels in LIC/LMIC and UMIC/HIC whereas hemoglobin was significantly affected only in UMIC/HIC. All different types of fortificants used showed positive impact on anemia prevalence and hemoglobin levels.
Folate
Thirty one before-after mass fortification studies were identified [149,150,153,157,159,164,168,169],[172,174,177,181,184-186,189,193,195-197],[199,200,202,205,206,209-211,221,222],[224]. Flour was used as the food vehicle with the level of fortification varying from 40 μg/100 g to 500 μg/100 g. Duration of intervention varied between 1 and 10 years. A summary of results is presented in Table 7. Although anencephaly and spina bifida are two specific forms of neural tube defects, some studies described results for neural tube defects without mentioning the specific type. Thus we have pooled anencephaly, spina bifida and neural tube defects separately as mentioned in studies to avoid any misinterpretation.
Our analysis showed that folate fortification had a significant impact in reducing neural tube defects (RR: 0.57 (95% CI: 0.45, 0.73)), spina bifida (RR: 0.64 (95% CI: 0.57, 0.71)) and anencephaly (RR: 0.80 (95% CI: 0.73, 0.87)). The impacts were non-significant for red blood cell folate levels (SMD: 1.26 (95% CI: -0.49, 3.01)), serum folate concentration (SMD: 1.38 (95% CI: -0.20, 2.95)) and twinning (RR: 1.06 (95% CI: 0.92, 1.22)).
Subgroup analyses showed that use of various concentration of folate (40 μg/100 g or more than 100 μg/100 g) had consistent significant impacts in reducing neural tube defects and spina bifida, whereas only 40 μg/100 g folate fortification was effective in reducing incidence of anencephaly. In a subgroup analysis based on duration of intervention of 1 year or more than 1 year, both had significant impacts in reducing neural tube defects, spina bifida and anencephaly.
Iodine
A total of 16 studies were identified investigating iodine fortification [160-163,165-167,183,190,191,198,207,208],[213,219,220]. All of these were before-after studies that used mass fortification as the strategy with salt being the most commonly used food vehicle.
The analysis showed that iodine fortification had a significant impact on median urinary iodine concentrations with a SMD of 7.16 (95% CI: 1.00, 13.31) and on the incidence of hypothyroidism with a RR of 1.42 (95% CI: 1.11, 1.82). The effect was non-significant on serum thyroxin levels with a SMD of 0.45 (95% CI: -1.15, 2.06).
Vitamin D and calcium
A total of 13 RCTs and one before-after study were identified for vitamin D and calcium fortification [146,147,158,170,171,179,180,182],[187,192,194,201,212,215]. The level of fortification varied significantly. Fortification durations varied from 2 weeks to 2 years. Reported outcomes included serum parathyroid hormone levels, serum vitamin D levels, serum calcium levels and bone mineral density. Target populations included WRA and post-menopausal women. A summary of results is presented in Table 7.
The analysis showed that vitamin D and calcium fortification in WRA had a non-significant impact on vitamin D3 levels with a SMD of −1.10 (95% CI: -3.81, 1.60) and serum parathyroid hormone levels with SMD of −0.01 (95% CI: -0.32, 0.30). For the post-menopausal women, pooled analysis showed significant impacts on serum concentration of 25-hydroxy-D3 with a SMD of 0.82 (95% CI: 0.30, 1.34) and on serum parathyroid hormone concentration with SMD of −2.53 (95% CI: -4.42, -0.65).
Results also indicated a significant impact of vitamin D and calcium fortification on reducing the serum levels of bone resorption markers: P1NP and CTx. Specifically, pooled analyses showed significantly reduced serum levels of P1NP (three studies; SMD of −3.36 (95% CI: -6.37, -0.35)) and CTx (four studies; SMD of −4.93 (95% CI: -7.78, -2.08)) in both WRA and post-menopausal women
Multiple micronutrients
Five RCTs were identified for the impact of MMN fortification on women [148,176,188,214,217]. Varying number (3 to 20) of micronutrients were used and the duration of intervention ranged from 6 months to 2 years. The study population included WRA, pregnant and post-menopausal women. A summary of results is presented in Table 8.
Analysis showed that MMN fortification significantly improved hemoglobin levels (SMD: 0.31 (95% CI: 0.13, 0.48)), serum ferritin (SMD: 0.47 (95% CI: 0.36, 0.58)), serum zinc (SMD: 0.50 (95% CI: 0.38, 0.61)) and serum retinol (SMD: 0.47 (95% CI: 0.30, 0.65)), and reduced anemia prevalence (RR: 0.76 (95% CI: 0.48, 1.21)). However, these findings should be interpreted with caution as only a few studies were pooled for each outcome.
Discussion
Various strategies have been pursued globally to alleviate nutritional deficiencies including food programs, diet modification, supplementation and fortification. In this review, we have attempted to broadly quantify the impacts of fortification of various commodities with micronutrients, singly or in combination, and to compare benefits.
Our review of fortification strategies among children does show significant impacts on increasing respective serum micronutrient concentrations, which could indirectly be used to assess impact at population level. Zinc fortification led to a significant increase in serum zinc concentration, and the same was observed with vitamin A and vitamin D fortification on respective serum micronutrient concentrations. Fortification with iron or MMN fortification was associated with a significant increase in serum ferritin, whereas the effect of MMN fortification on serum retinol and serum zinc was non-significant. Other hematologic markers also improved following food fortification among children with vitamin A, iron and MMN, suggesting some conjoint benefits. Comparable to the findings by others [65], the observed lack of negative effects of zinc fortification on hemoglobin concentrations suggests that that the quantities of zinc present in fortified foods is not high enough to alter the absorption of iron. Although benefits of zinc fortification were seen on linear growth, MMN fortification was not associated with any significant benefits on HAZ, WAZ and WHZ.
Our analysis of the impact of fortification among women indicates that calcium and vitamin D fortification do not lead to an increase in serum vitamin D3 levels in WRA but has a significant effect in post-menopausal age groups. Iron fortification led to a significant increase in serum ferritin and hemoglobin levels in WRA and pregnant women. Folate fortification significantly reduced the incidence of congenital abnormalities like anencephaly, spinal bifida and neural tube defects. Similarly, fortification of salt with iodine decreased the incidence of hypothyroidism and led to higher median urinary iodine concentrations. This evidence suggests that mass fortification strategies can be extremely productive and beneficial for numerous health outcomes. The total number of studies pooled for zinc and MMN fortification in women were insufficient to draw firm conclusions.
Few studies in our review reported the direct impact of fortification on morbidity. However, as many supplementation trials have already proven the effects of improved micronutrient status on morbidity, it could be inferred that these improvements in micronutrient status, reflected in changes in serum concentrations, could also have beneficial effects on health outcomes including morbidity and mortality. However, this cannot be definitely concluded without large scale studies measuring the direct impact of fortification on such outcomes or direct comparisons of fortification and supplementation strategies.
Notwithstanding the conclusions above, we are aware that biochemical responses to specific micronutrient fortification strategies may differ depending on the underlying nutritional conditions of the individuals or populations, the micronutrient compound used for fortification, and the food vehicle chosen. To cater to these variations, which are inherent to this strategy, we undertook diverse subgroup analyses to confirm and account for the differences, where possible. For this, we divided the studies and reported separate estimates based on the various age groups targeted, baseline micronutrient status, compound and foods chosen, concentration of the micronutrient in the food, the duration of intervention, and whether the evidence was from HIC or LMIC. Our analyses suggest that the impact of food fortification were stronger in populations with nutritional deficiencies at baseline, as there was greater hematological impact from food fortification with iron and MMNs in marginalized at-risk populations. We also analyzed the impact of food fortification on infants alone and the results indicated that iron fortification was associated with a significant increase in serum ferritin and also a reduction in the incidence of anemia, although effects on hemoglobin concentrations were non-significant. Zinc fortification in infants raised serum zinc concentrations without any observed benefits on linear growth and weight gain. MMN fortification significantly improved hematological parameters and was also associated with significant gains in HAZ scores, whereas no beneficial effects were observed for WAZ and WHZ.
The choice of food and fortificant are critically important. The fortificant must be stable, have a long shelf life and should not alter the color, taste and appearance of food. The food matrix should not interfere with the absorption of the micronutrient, thereby affecting its bioavailability, for example through phytates. Apart from the scientific aspects, the choice of food should be such that it is readily available to the target population and the quantity consumed should be enough to match the dietary requirements. This is particularly true for children, who typically do not consume the same foods and staples. A variety of food vehicles have been chosen for fortification, broadly classified as staples, condiments and commercially processed foods. Our review shows that, overall, staples have been the primary choice as they are widely consumed by the population, whereas processed foods and cereals have been chosen when infants were the target population. Iodine fortification almost exclusively used salt as a traditional and proven method, folate fortification was commonly employed through grains or grain products, and milk has been the carrier of choice for vitamin D and calcium. Of the outcomes analyzed in this review, we could not conclude whether a specific compound was better for impacting individual micronutrient status, as consistent results were observed for iron sulfate and NaFeEDTA when used in iron fortification, and zinc sulfate, zinc chloride and zinc oxide when used in zinc fortification. However, we did not compare costs or the cost effectiveness of various approaches.
The process of fortification comes with significant costs, which have to be ultimately borne by the end consumer or the state. As the poor have limited purchasing power, funding agencies and public private partnerships are needed to provide costs subsidies and cost-sharing to ensure that the products reach those who need them most. Additional demand creation and social marketing may be necessary through campaigns to ensure adequate marketing pull factors and consumption.
Fortification promises to tackle a multitude of micronutrient deficiencies through a single intervention: a one-window solution. Although the results are encouraging, more is needed. The evidence from these subgroup analyses for different fortification vehicles and compounds was not conclusive, and suggests that further trials are needed with defined functional and biological outcomes. Most national programs analyzed were from developed countries and data from the developing world were relatively scarce. However, where available, the results showed comparable benefits. This scarcity of studies from developing countries is due to the fact that national fortification programs require large resources; robust scientific and research facilities are required to identify the ideal food, micronutrient compound and industrial support. The experience of commodities such as pre-fortified ready-to-use fortified foods indicates that global procurement and supply as well as local production is possible.
Our review has many limitations because we included a range of studies of varying sizes and scientific rigor. This was due to the fact that large scale fortification programs are, in general, before-after evaluations. Many studies used different foods and concentrations of micronutrients and the frequency of feeding/intake was not uniform. Limited information on confounding factors like age and nutritional status at the initiation of the intervention also limited inference of outcomes by age bands and nutrition categories, and the duration of the intervention period evaluated also varied across studies. There is limited evidence available for the direct impact of fortification on anthropometric measures, morbidity and mortality and these are essential to evaluate future benefits and effective strategies. We propose that further future trials are warranted, which should take into consideration the limitations identified in this review and draw upon this, so that we have a pool of appropriate studies with good quality study designs to impute the actual impacts of this promising strategy.
Conclusion
Fortification, though promising, is not an answer to the global widespread nutritional deficiencies. With high burdens of diarrhea and enteropathy, widespread malabsorption may be a barrier to this strategy taking maximal effect. Integration of fortification and supplementation strategies together with other mother and child health and prevention programs may be the answer to address the widespread global under-nutrition and to ensure sustainable benefits. Community education and promotion campaigns should also be implemented parallel to the primary fortification programs to increase awareness, acceptability and equity. Fortification is potentially an effective strategy but evidence from the developing world is scarce and future programs also need to assess the direct impact of fortification on morbidity and mortality.
Abbreviations
CI: Confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; HAZ: Height for age Z-scores; HIC: High-income countries; LIC: Lower-income countries; LMIC: Lower middle-income countries; MMN: Multiple micronutrient; OR: Odds ratio; RCT: Randomized controlled trial; RR: Relative risk; SMD: Standard mean difference; UMIC: Upper middle-income countries; UTI: Urinary tract infections; WAZ: Weight for age Z-scores; WHZ: Weight for height Z-scores; WHO: World Health Organization; WRA: Women of reproductive age.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZAB was responsible for designing and coordinating the review. JKD, RAS and RK were responsible for data collection, screening the search results, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, entering data into RevMan, analysis and interpretation of data and writing the review. ZAB and JKD critically reviewed and modified the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Characteristics of included studies.
Forest plots.
Summary of findings and quality of outcomes.
Contributor Information
Jai K Das, Email: jai.das@aku.edu.
Rehana A Salam, Email: rehana.salam@aku.edu.
Rohail Kumar, Email: rohailkumar@gmail.com.
Zulfiqar A Bhutta, Email: zulfiqar.bhutta@aku.edu.
Acknowledgments
We would like to acknowledge Sohni Dean and Zohaib Sohail, who helped us in the search and abstraction of data for the iron and iodine fortification components respectively. We would also like to thanks our Research Assistant Talha Mirza for his administrative support. We are grateful to the Nestle Nutrition Institute for its unrestricted support towards the genesis of this review and its external assessment in an advisory group meeting in Zurich in October 2011.
References
- WHO. World Health Report. Geneva: World Health Organization; 2000. [Google Scholar]
- Black RE. Micronutrients in pregnancy. Br J Nutr. 2001;85(S2):S193–S197. doi: 10.1079/bjn2000314. [DOI] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, De Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO global database on vitamin A deficiency. Geneva: World Health Organization; 2009. [Google Scholar]
- Leslie J. Women’s nutrition: the key to improving family health in developing countries? Health Policy Plan. 1991;6(1):1. [Google Scholar]
- Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am Journal Clin Nutr. 1998;68(2):499S. doi: 10.1093/ajcn/68.2.499S. [DOI] [PubMed] [Google Scholar]
- Benoist B, McLean E, Egll I, Cogswell M. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva: World Health Organization; 2008. [Google Scholar]
- McLean E, De Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29(Supplement 1):38–51. doi: 10.1177/15648265080292S107. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA. Micronutrient needs of malnourished children. Curr Opin Clin Nutr Metab Care. 2008;11(3):309. doi: 10.1097/MCO.0b013e3282fbf5a0. [DOI] [PubMed] [Google Scholar]
- Best C, Neufingerl N, Del Rosso JM, Transler C, van den Briel T, Osendarp S. Can multi micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review. Nutr Rev. 2011;69(4):186–204. doi: 10.1111/j.1753-4887.2011.00378.x. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HPS. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- Dewey KG, Yang Z, Boy E. Systematic review and meta analysis of home fortification of complementary foods. Matern Child Nutr. 2009;5(4):283–321. [Google Scholar]
- Serdula M. Maximizing the impact of flour fortification to improve vitamin and mineral nutrition in populations. Food Nutr Bull. 2010;31(1 Suppl):S86–S93. doi: 10.1177/15648265100311s108. [DOI] [PubMed] [Google Scholar]
- Allen LD, de Benoist B, Dary O, Hurrell RE. Guidelines on food fortification with micronutrients. Geneva: World Health Organization/Food and Agriculture Organization; 2006. [Google Scholar]
- Serdula M. The opportunity of flour fortification: building on the evidence to move forward. Food Nutr Bull. 2010;31(1 Suppl):S3. doi: 10.1177/15648265100311s101. [DOI] [PubMed] [Google Scholar]
- Hess SY, Brown KH. Impact of zinc fortification on zinc nutrition. Food Nutr Bull. 2009;30(1 Suppl):S79–S107. doi: 10.1177/15648265090301S106. [DOI] [PubMed] [Google Scholar]
- Eichler K, Wieser S, Ruthemann I, Brugger U. Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review. BMC Public Health. 2012;12:506. doi: 10.1186/1471-2458-12-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gera T, Sachdev HS, Boy E. Effect of iron-fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials. Am J Clin Nutr. 2012;96(2):309–324. doi: 10.3945/ajcn.111.031500. [DOI] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editor. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011] Chichester, UK: The Cochrane Collaboration and John Wiley & Sons Ltd; 2011. [Google Scholar]
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH. GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, Schunemann HJ. Going from evidence to recommendations. BMJ. 2008;336(7652):1049–1051. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abizari AR, Moretti D, Zimmermann MB, Armar-Klemesu M, Brouwer ID. Whole cowpea meal fortified with NaFeEDTA reduces iron deficiency among Ghanaian school children in a malaria endemic area. J Nutr. 2012;142(10):1836–1842. doi: 10.3945/jn.112.165753. [DOI] [PubMed] [Google Scholar]
- Andang’o PE, Osendarp SJ, Ayah R, West CE, Mwaniki DL, De Wolf CA, Kraaijenhagen R, Kok FJ, Verhoef H. Efficacy of iron-fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet. 2007;369(9575):1799–1806. doi: 10.1016/S0140-6736(07)60817-4. [DOI] [PubMed] [Google Scholar]
- Andersson M, Thankachan P, Muthayya S, Goud RB, Kurpad AV, Hurrell RF, Zimmermann MB. Dual fortification of salt with iodine and iron: a randomized, double-blind, controlled trial of micronized ferric pyrophosphate and encapsulated ferrous fumarate in southern India. Am J Clin Nutr. 2008;88(5):1378–1387. doi: 10.3945/ajcn.2008.26149. [DOI] [PubMed] [Google Scholar]
- Arcanjo FP, Amancio OM, Braga JA, de Paula Teixeira Pinto V. Randomized controlled trial of iron-fortified drinking water in preschool children. J Am Coll Nutr. 2010;29(2):122–129. doi: 10.1080/07315724.2010.10719825. [DOI] [PubMed] [Google Scholar]
- Arroyave G, Mejia LA, Aguilar JR. The effect of vitamin A fortification of sugar on the serum vitamin A levels of preschool Guatemalan children: a longitudinal evaluation. Am J Clin Nutr. 1981;34(1):41–49. doi: 10.1093/ajcn/34.1.41. [DOI] [PubMed] [Google Scholar]
- Barbosa TN, Taddei JA, Palma D, Ancona-Lopez F, Braga JA. Double-blind randomized controlled trial of rolls fortified with microencapsulated iron. Rev Assoc Med Bras. 2012;58(1):118–124. [PubMed] [Google Scholar]
- Beinner MA, Lamounier JA, Tomaz C. Effect of iron-fortified drinking water of daycare facilities on the hemoglobin status of young children. J Am Coll Nutr. 2005;24(2):107–114. doi: 10.1080/07315724.2005.10719451. [DOI] [PubMed] [Google Scholar]
- Beinner MA, Velasquez-Meléndez G, Pessoa MC, Greiner T. Iron-fortified rice is as efficacious as supplemental iron drops in infants and young children. J Nutr. 2010;140(1):49–53. doi: 10.3945/jn.109.112623. [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Carrie AL, Ferrari S, Clavien H, Slosman D, Theintz G, Rizzoli R. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Investig. 1997;99(6):1287–1294. doi: 10.1172/JCI119287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KH, Lopez de Romana D, Arsenault JE, Peerson JM, Penny ME. Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity, and plasma zinc concentrations of young Peruvian children. Am J Clin Nutr. 2007;85(2):538–547. doi: 10.1093/ajcn/85.2.538. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhao X, Zhang X, Yin S, Piao J, Huo J, Yu B, Qu N, Lu Q, Wang S. Studies on the effectiveness of NaFeEDTA-fortified soy sauce in controlling iron deficiency: a population-based intervention trial. Food Nutr Bull. 2005;26(2):177–186. doi: 10.1177/156482650502600201. [DOI] [PubMed] [Google Scholar]
- Chen K, Li TY, Chen L, Qu P, Liu YX. Effects of vitamin A, vitamin A plus iron and multiple micronutrient-fortified seasoning powder on preschool children in a suburb of Chongqing. China. J Nutr Sci Vitaminol. 2008;54(6):440–447. doi: 10.3177/jnsv.54.440. [DOI] [PubMed] [Google Scholar]
- Chen K, Liu YF, Chen L, Zhang X, Liu YX, Chen J, Li TY. Effects of vitamin A, vitamin A plus iron and multiple micronutrient-fortified seasoning powder on iron metabolic homeostasis. Zhonghua Er Ke Za Zhi. 2011;49(12):926–932. [PubMed] [Google Scholar]
- Chen K, Zhang X, Li T, Chen L, Wei X, Qu P, Liu Y. Effect of vitamin A, vitamin A plus iron and multiple micronutrient-fortified seasoning powder on infectious morbidity of preschool children. Nutrition. 2011;27(4):428–434. doi: 10.1016/j.nut.2010.04.004. [DOI] [PubMed] [Google Scholar]
- de Almeida CA, Dutra-De-Oliveira JE, Crott GC, Cantolini A, Ricco RG, Del Ciampo LA, Baptista ME. Effect of fortification of drinking water with iron plus ascorbic acid or with ascorbic acid alone on hemoglobin values and anthropometric indicators in preschool children in day-care centers in Southeast Brazil. Food Nutr Bull. 2005;26(3):259–265. doi: 10.1177/156482650502600302. [DOI] [PubMed] [Google Scholar]
- Delange F, De Benoist B, Alnwick D. Risks of iodine-induced hyperthyroidism after correction of iodine deficiency by iodized salt. Thyroid. 1999;9(6):545–556. doi: 10.1089/thy.1999.9.545. [DOI] [PubMed] [Google Scholar]
- Diaz-Gomez NM, Domenech E, Barroso F, Castells S, Cortabarria C, Jimenez A. The effect of zinc supplementation on linear growth, body composition, and growth factors in preterm infants. Pediatr. 2003;111(5):1002–1009. doi: 10.1542/peds.111.5.1002. [DOI] [PubMed] [Google Scholar]
- Du X, Zhu K, Trube A, Zhang Q, Ma G, Hu X, Fraser DR, Greenfield H. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br J Nutr. 2004;92(01):159–168. doi: 10.1079/BJN20041118. [DOI] [PubMed] [Google Scholar]
- Faber M, Kvalsvig JD, Lombard CJ, Benade AJ. Effect of a fortified maize-meal porridge on anemia, micronutrient status, and motor development of infants. Am J Clin Nutr. 2005;82(5):1032–1039. doi: 10.1093/ajcn/82.5.1032. [DOI] [PubMed] [Google Scholar]
- Friel JK, Andrews WL, Matthew JD, Long DR, Cornel AM, Cox M, McKim E, Zerbe GO. Zinc supplementation in very-low-birth-weight infants. J Pediatr Gastroenterol Nutr. 1993;17(1):97–104. doi: 10.1097/00005176-199307000-00015. [DOI] [PubMed] [Google Scholar]
- Ganmaa D, Tserendolgor U, Frazier L, Nakamoto E, Jargalsaikhan N, Rich-Edwards J. Effects of vitamin D fortified milk on vitamin D status in Mongolian school age children. Asia Pac J Clin Nutr. 2008;17(1):68–71. [PubMed] [Google Scholar]
- Gershoff SN, McGandy RB, Suttapreyasri D, Promkutkao C, Nondasuta A, Pisolyabutra U, Tantiwongse P, Viravaidhaya V. Nutrition studied in Thailand. II. Effects of fortification of rice with lysine, threonine, thiamin, riboflavin, vitamin A, and iron on preschool children. Am J Clin Nutr. 1977;30(7):1185–1195. doi: 10.1093/ajcn/30.7.1185. [DOI] [PubMed] [Google Scholar]
- Gibson RS, Kafwembe E, Mwanza S, Gosset L, Bailey KB, Mullen A, Baisley K, Filteau S. A micronutrient-fortified food enhances iron and selenium status of Zambian infants but has limited efficacy on zinc. J Nutr. 2011;141(5):935–943. doi: 10.3945/jn.110.135228. [DOI] [PubMed] [Google Scholar]
- Gill DG, Vincent S, Segal DS. Follow-on formula in the prevention of iron deficiency: a multicentre study. Acta Paediatr. 1997;86(7):683–689. doi: 10.1111/j.1651-2227.1997.tb08568.x. [DOI] [PubMed] [Google Scholar]
- Giorgini E, Fisberg M, De Paula RA, Ferreira AM, Valle J, Braga JA. The use of sweet rolls fortified with iron bis-glycinate chelate in the prevention of iron deficiency anemia in preschool children. Arch Latinoam Nutr. 2001;51(1):48–53. [PubMed] [Google Scholar]
- Goyle A, Prakash S. Effect of supplementation of micronutrient fortified biscuits on haemoglobin and serum iron levels of adolescent girls from Jaipur city. India. Nutr Food Sci. 2010;40(5):477–484. [Google Scholar]
- Graham D, Kira G, Conaglen J, McLennan S, Rush E. Vitamin D status of Year 3 children and supplementation through schools with fortified milk. Public Health Nutr. 2009;12(12):2329–2334. doi: 10.1017/S1368980008004357. [DOI] [PubMed] [Google Scholar]
- Guttikonda K, Burgess JR, Hynes K, Boyages S, Byth K, Parameswaran V. Recurrent iodine deficiency in Tasmania, Australia: a salutary lesson in sustainable iodine prophylaxis and its monitoring. J Clin Endocrinol Metab. 2002;87(6):2809–2815. doi: 10.1210/jcem.87.6.8600. [DOI] [PubMed] [Google Scholar]
- Hambidge KM, Chavez MN, Brown RM, Walravens PA. Zinc nutritional status of young middle-income children and effects of consuming zinc-fortified breakfast cereals. Am J Clin Nutr. 1979;32(12):2532–2539. doi: 10.1093/ajcn/32.12.2532. [DOI] [PubMed] [Google Scholar]
- Haschke F, Pietschnig B, Vanura H, Heil M, Steffan I, Hobiger G, Schuster E, Camaya Z. Iron intake and iron nutritional status of infants fed iron-fortified beikost with meat. Am J Clin Nutr. 1988;47(1):108–112. doi: 10.1093/ajcn/47.1.108. [DOI] [PubMed] [Google Scholar]
- Ho SC, Guldan GS, Woo J, Yu R, Tse MM, Sham A, Cheng J. A prospective study of the effects of 1-year calcium-fortified soy milk supplementation on dietary calcium intake and bone health in Chinese adolescent girls aged 14 to 16. Osteoporos Int. 2005;16(12):1907–1916. doi: 10.1007/s00198-005-1963-8. [DOI] [PubMed] [Google Scholar]
- Huo J, Sun J, Miao H, Yu B, Yang T, Liu Z, Lu C, Chen J, Zhang D, Ma Y. Therapeutic effects of NaFeEDTA-fortified soy sauce in anaemic children in China. Asia Pac J Clin Nutr. 2002;11(2):123–127. doi: 10.1046/j.1440-6047.2002.00277.x. [DOI] [PubMed] [Google Scholar]
- Iost C, Jeppsen RB, Ashmead HDW. Repleting hemoglobin in iron deficiency anemia in young children through liquid milk fortification with bioavailable iron amino acid chelate. J Am Coll Nutr. 1998;17(2):187–194. doi: 10.1080/07315724.1998.10718745. [DOI] [PubMed] [Google Scholar]
- Jooste P, Zimmermann MB. Progress towards eliminating iodine deficiency in South Africa. S Afr J Clin Nutr. 2008;21(1):08–14. [Google Scholar]
- Kiliç I, Özalp I, Coskun T, Tokatli A, Emre S, Saldamli I, Köksel H, Ozboy O. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr. 1998;26(2):167–171. doi: 10.1097/00005176-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Koo WWK, Hockman EM. Posthospital discharge feeding for preterm infants: effects of standard compared with enriched milk formula on growth, bone mass, and body composition. Am J Clin Nutr. 2006;84(6):1357–1364. doi: 10.1093/ajcn/84.6.1357. [DOI] [PubMed] [Google Scholar]
- Kumar MV, Rajagopalan S. Multiple micronutrient fortification of salt and its effect on cognition in Chennai school children. Asia Pac J Clin Nutr. 2007;16(3):505–511. [PubMed] [Google Scholar]
- Kuusipalo H, Maleta K, Briend A, Manary M, Ashorn P. Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: a preliminary trial. J Pediatr Gastroenterol Nutr. 2006;43(4):525–532. doi: 10.1097/01.mpg.0000235981.26700.d3. [DOI] [PubMed] [Google Scholar]
- Lartey A, Manu A, Brown KH, Peerson JM, Dewey KG. A randomized, community-based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. Am J Clin Nutr. 1999;70(3):391–404. doi: 10.1093/ajcn/70.3.391. [DOI] [PubMed] [Google Scholar]
- Layrisse M, Chaves JF. Early response to the effect of iron fortification in the Venezuelan population. Am J Clin Nutr. 1996;64(6):903–907. doi: 10.1093/ajcn/64.6.903. [DOI] [PubMed] [Google Scholar]
- Le Huong T, Inge B, Jan B, Khan N, Frans K. Efficacy of iron fortification compared to iron supplementation among Vietnamese schoolchildren. Nutr J. 2006;5:32. doi: 10.1186/1475-2891-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen-Veromaa M, Mottonen T, Leino A, Heinonen OJ, Rautava E, Viikari J. Prospective study on food fortification with vitamin D among adolescent females in Finland: minor effects. Br J Nutr. 2008;100(2):418–423. doi: 10.1017/S0007114508894469. [DOI] [PubMed] [Google Scholar]
- Lein, Shigeru Y, te Biesebeke R. Impact of milk consumption on performance and health of primary school children in rural Vietnam. Asia Pac J Clin Nutr. 2009;18(3):326–334. [PubMed] [Google Scholar]
- Lin J, Lee I. Plasma folate, vitamin B-6, vitamin B-12, and risk of breast cancer in women. Am J Clin Nutr. 2008;87(3):734–743. doi: 10.1093/ajcn/87.3.734. [DOI] [PubMed] [Google Scholar]
- Liu D, Bates CJ, Yin TA, Wang XB, Lu C. Nutritional efficacy of a fortified weaning rusk in a rural area near Beijing [published erratum appears in Am J Clin Nutr 1993 Oct; 58 (4): 582] Am J Clin Nutr. 1993;57(4):506–511. doi: 10.1093/ajcn/57.4.506. [DOI] [PubMed] [Google Scholar]
- Longfils P, Monchy D, Weinheimer H, Chavasit V, Nakanishi Y, Schumann K. A comparative intervention trial on fish sauce fortified with NaFe-EDTA and FeSO4+ citrate in iron deficiency anemic school children in Kampot, Cambodia. Asia Pac J Clin Nutr. 2008;17(2):250–257. [PubMed] [Google Scholar]
- Lopriore C, Guidoum Y, Briend A, Branca F. Spread fortified with vitamins and minerals induces catch-up growth and eradicates severe anemia in stunted refugee children aged 3–6 y. Am J Clin Nutr. 2004;80(4):973–981. doi: 10.1093/ajcn/80.4.973. [DOI] [PubMed] [Google Scholar]
- Loui A, Raab A, Wagner M, Weigel H, Grüters-Kieslich A, Brätter P, Obladen M. Nutrition of very low birth weight infants fed human milk with or without supplemental trace elements: a randomized controlled trial. J Pediatr Gastroenterol Nutr. 2004;39(4):346–353. doi: 10.1097/00005176-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Lucas A, Fewtrell MS, Morley R, Lucas PJ, Baker BA, Lister G, Bishop NJ. Randomized outcome trial of human milk fortification and developmental outcome in preterm infants. Am J Clin Nutr. 1996;64(2):142–151. doi: 10.1093/ajcn/64.2.142. [DOI] [PubMed] [Google Scholar]
- Lutter CK, Rodríguez A, Fuenmayor G, Avila L, Sempertegui F, Escobar J. Growth and micronutrient status in children receiving a fortified complementary food. J Nutr. 2007;138(2):379–388. doi: 10.1093/jn/138.2.379. [DOI] [PubMed] [Google Scholar]
- Manger MS, McKenzie JE, Winichagoon P, Gray A, Chavasit V, Pongcharoen T, Gowachirapant S, Ryan B, Wasantwisut E, Gibson RS. A micronutrient-fortified seasoning powder reduces morbidity and improves short-term cognitive function, but has no effect on anthropometric measures in primary school children in northeast Thailand: a randomized controlled trial. Am J Clin Nutr. 2008;87(6):1715–1722. doi: 10.1093/ajcn/87.6.1715. [DOI] [PubMed] [Google Scholar]
- Manno D, Kowa PK, Bwalya HK, Siame J, Grantham-McGregor S, Baisley K, de Stavola BL, Jaffar S, Filteau S. Rich micronutrient fortification of locally produced infant food does not improve mental and motor development of Zambian infants: a randomised controlled trial. Br J Nutr. 2011;1(1):1–11. doi: 10.1017/S0007114511003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A, Long H, Stierwalt E. Comparative hematologic response to iron fortification of a milk formula for infants. Pediatr. 1959;24:404–412. [PubMed] [Google Scholar]
- Matsuda I, Higashi A, Ikeda T, Uehara I, Kuroki Y. Effects of zinc and copper content of formulas on growth and on the concentration of zinc and copper in serum and hair. J Pediatr Gastroenterol Nutr. 1984;3(3):421–425. doi: 10.1097/00005176-198406000-00020. [DOI] [PubMed] [Google Scholar]
- Mejia LA, Arroyave G. The effect of vitamin A fortification of sugar on iron metabolism in preschool children in Guatemala. Am J Clin Nutr. 1982;36(1):87–93. doi: 10.1093/ajcn/36.1.87. [DOI] [PubMed] [Google Scholar]
- Miglioranza LH, Breganà JW, Dichi I, Matsuo T, Dichi JB, Barbosa DS. Effectiveness of fortification of corn flour-derived products with hydrogen-reduced elemental iron on iron-deficiency anaemia in children and adolescents in southern Brazil. Public Health Nutr. 2009;12(02):244–248. doi: 10.1017/S1368980008003704. [DOI] [PubMed] [Google Scholar]
- Moretti D, Zimmermann MB, Muthayya S, Thankachan P, Lee TC, Kurpad AV, Hurrell RF. Extruded rice fortified with micronized ground ferric pyrophosphate reduces iron deficiency in Indian schoolchildren: a double-blind randomized controlled trial. Am J Clin Nutr. 2006;84(4):822–829. doi: 10.1093/ajcn/84.4.822. [DOI] [PubMed] [Google Scholar]
- Morgan EJ, Heath ALM, Szymlek-Gay EA, Gibson RS, Gray AR, Bailey KB, Ferguson EL. Red meat and a fortified manufactured toddler milk drink increase dietary zinc intakes without affecting zinc status of New Zealand toddlers. J Nutr. 2010;140(12):2221–2226. doi: 10.3945/jn.109.120717. [DOI] [PubMed] [Google Scholar]
- Morley R, Abbott R, Fairweather-Tait S, MacFadyen U, Stephenson T, Lucas A. Iron fortified follow on formula from 9 to 18 months improves iron status but not development or growth: a randomised trial. Arch Dis Child. 1999;81(3):247–252. doi: 10.1136/adc.81.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhilal P. Vitamin A-fortified monosodium glutamate and health, growth, and survival of children: a controlled field trial. Am J Clin Nutr. 1988;48(5):1271–1276. doi: 10.1093/ajcn/48.5.1271. [DOI] [PubMed] [Google Scholar]
- Muthayya S, Thankachan P, Hirve S, Amalrajan V, Thomas T, Lubree H, Agarwal D, Srinivasan K, Hurrell RF, Yajnik CS, Kurpad AV. Iron fortification of whole wheat flour reduces iron deficiency and iron deficiency anemia and increases body iron stores in Indian school-aged children. J Nutr. 2012;142(11):1997–2003. doi: 10.3945/jn.111.155135. [DOI] [PubMed] [Google Scholar]
- Nesamvuni AE, Vorster HH, Margetts BM, Kruger A. Fortification of maize meal improved the nutritional status of 1–3-year-old African children. Public Health Nutr. 2005;8(05):461–467. doi: 10.1079/phn2005782. [DOI] [PubMed] [Google Scholar]
- Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Furr H, Wieringa FT. Multi-micronutrient-fortified biscuits decreased prevalence of anemia and improved micronutrient status and effectiveness of deworming in rural Vietnamese school children. J Nutr. 2009;139(5):1013–1021. doi: 10.3945/jn.108.099754. [DOI] [PubMed] [Google Scholar]
- Nogueira Arcanjo FP, Costa Arcanjo C, Nogueira Arcanjo FC, de Albuquerque CL, Silverio Amancio OM, Pellegrini Braga JA. Milk-based cornstarch porridge fortified with iron is effective in reducing anemia: a randomized, double-blind, placebo-controlled trial. J Trop Pediatr. 2012;58(5):370–374. doi: 10.1093/tropej/fms003. [DOI] [PubMed] [Google Scholar]
- Nogueira Arcanjo FP, Roberto Santos P, Arcanjo CP, Amancio OM, Braga JA. Use of iron-fortified rice reduces anemia in infants. J Trop Pediatr. 2012;58(6):475–480. doi: 10.1093/tropej/fms021. [DOI] [PubMed] [Google Scholar]
- Nugroho HW, Lestari ED, Salimo H, Dewi M. Effects of iron and zinc fortified milk supplementation on working memory of underweight poor-urban school children: A randomized double blind controlled trial. Paediatr Indones. 2010;50(3):92–95. [Google Scholar]
- Ohiokpehai OMD, Dorcus, Kamau, Joyce. Serum zinc levels of school children on a corn-soy blend feeding trial in primary schools in Suba district, Kenya. J Appl Biosci. 2009;17:904–912. [Google Scholar]
- Osei AK, Rosenberg IH, Houser RF, Bulusu S, Mathews M, Hamer DH. Community-level micronutrient fortification of school lunch meals improved vitamin A, folate, and iron status of schoolchildren in Himalayan villages of India. J Nutr. 2010;140(6):1146–1154. doi: 10.3945/jn.109.114751. [DOI] [PubMed] [Google Scholar]
- Ouédraogo HZ, Traoré T, Zèba AN, Dramaix-Wilmet M, Hennart P, Donnen P. Effect of an improved local ingredient-based complementary food fortified or not with iron and selected multiple micronutrients on Hb concentration. Public Health Nutr. 2006;1(1):1–8. doi: 10.1017/S1368980010000911. [DOI] [PubMed] [Google Scholar]
- Pongpaew P, Tungtrongchitr R, Phonrat B, Supawan V, Schelp EP, Intarakhao C, Mahaweerawat U, Saowakontha S. Nutritional status of school children in an endemic area of iodine deficiency disorders (IDD) after one year of iodine supplementation. Southeast Asian J Trop Med Public Health. 1998;29:50–57. [PubMed] [Google Scholar]
- Ribaya-Mercado JD, Solomons NW, Medrano Y, Bulux J, Dolnikowski GG, Russell RM, Wallace CB. Use of the deuterated-retinol-dilution technique to monitor the vitamin A status of Nicaraguan schoolchildren 1 y after initiation of the Nicaraguan national program of sugar fortification with vitamin A. Am J Clin Nutr. 2004;80(5):1291–1298. doi: 10.1093/ajcn/80.5.1291. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Ganmaa D, Kleinman K, Sumberzul N, Holick MF, Lkhagvasuren T, Dulguun B, Burke A, Frazier AL. Randomized trial of fortified milk and supplements to raise 25-hydroxyvitamin D concentrations in schoolchildren in Mongolia. Am J Clin Nutr. 2011;94(2):578–584. doi: 10.3945/ajcn.110.008771. [DOI] [PubMed] [Google Scholar]
- Rim HY, Kim SH, Sim BC, Gang HY, Kim HY, Kim YR, Kim RC, Yang MH, Kim SP. Effect of iron fortification of nursery complementary food on iron status of infants in the DPR Korea. Asia Pac J Clin Nutr. 2008;17(2):264–269. [PubMed] [Google Scholar]
- Rivera JA, Shamah T, Villalpando S, Monterrubio E. Effectiveness of a large-scale iron-fortified milk distribution program on anemia and iron deficiency in low-income young children in Mexico. Am J Clin Nutr. 2010;91(2):431–439. doi: 10.3945/ajcn.2009.28104. [DOI] [PubMed] [Google Scholar]
- Rohner F, Zimmermann MB, Amon RJ, Vounatsou P, Tschannen AB, N’Goran EK, Nindjin C, Cacou MC, Té-Bonlé MD, Utzinger J. In a randomized controlled trial of iron fortification, anthelmintic treatment, and intermittent preventive treatment of malaria for anemia control in Ivorian children, only anthelmintic treatment shows modest benefit. J Nutr. 2010;140(3):635–641. doi: 10.3945/jn.109.114256. [DOI] [PubMed] [Google Scholar]
- Salmenpera L, Perheentupa J, Pakarinen P, Siimes MA. Zinc supplementation of infant formula. Am J Clin Nutr. 1994;59(5):985–989. doi: 10.1093/ajcn/59.5.985. [DOI] [PubMed] [Google Scholar]
- Sari M, Bloem MW, de Pee S, Schultink WJ, Sastroamidjojo S. Effect of iron-fortified candies on the iron status of children aged 4–6 y in East Jakarta, Indonesia. Am J Clin Nutr. 2001;73(6):1034–1039. doi: 10.1093/ajcn/73.6.1034. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Dhingra U, Dhingra P, Hiremath G, Sarkar A, Dutta A, Menon VP, Black RE. Micronutrient fortified milk improves iron status, anemia and growth among children 1–4 years: a double masked, randomized, controlled trial. PloS One. 2010;5(8):e12167. doi: 10.1371/journal.pone.0012167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L, Arevalo M, Arredondo S, Diaz M, Lonnerdal B, Stekel A. Effect of a zinc-fortified formula on immunocompetence and growth of malnourished infants. Am J Clin Nutr. 1992;56(3):491–498. doi: 10.1093/ajcn/56.3.491. [DOI] [PubMed] [Google Scholar]
- Schümann K, Romero-Abal ME, Mäurer A, Luck T, Beard J, Murray-Kolb L, Bulux J, Mena I, Solomons NW. Haematological response to haem iron or ferrous sulphate mixed with refried black beans in moderately anaemic Guatemalan pre-school children. Public Health Nutr. 2005;8(06):572–581. doi: 10.1079/phn2004708. [DOI] [PubMed] [Google Scholar]
- Seal A, Kafwembe E, Kassim IA, Hong M, Wesley A, Wood J, Abdalla F, van den Briel T. Maize meal fortification is associated with improved vitamin A and iron status in adolescents and reduced childhood anaemia in a food aid-dependent refugee population. Public Health Nutr. 2008;11(7):720–728. doi: 10.1017/S1368980007001486. [DOI] [PubMed] [Google Scholar]
- Semba RD, Moench-Pfanner R, Sun K, de Pee S, Akhter N, Rah JH, Campbell AA, Badham J, Bloem MW, Kraemer K. Iron-fortified milk and noodle consumption is associated with lower risk of anemia among children aged 6–59 mo in Indonesia. Am J Clin Nutr. 2010;92(1):170–176. doi: 10.3945/ajcn.2010.29254. [DOI] [PubMed] [Google Scholar]
- Shamah-Levy T, Villalpando S, Rivera-Dommarco JA, Mundo-Rosas V, Cuevas-Nasu L, Jiménez-Aguilar A. Ferrous gluconate and ferrous sulfate added to a complementary food distributed by the Mexican nutrition program Oportunidades have a comparable efficacy to reduce iron deficiency in toddlers. J Pediatr Gastroenterol Nutr. 2008;47(5):660–666. doi: 10.1097/mpg.0b013e318167b03b. [DOI] [PubMed] [Google Scholar]
- Singhal A, Morley R, Abbott R, Fairweather-Tait S, Stephenson T, Lucas A. Clinical safety of iron-fortified formulas. Pediatr. 2000;105(3):e38. doi: 10.1542/peds.105.3.e38. [DOI] [PubMed] [Google Scholar]
- Sivakumar B, Nair KM. Double fortified salt at crossroads. Indian J Pediatr. 2002;69(7):617–623. doi: 10.1007/BF02722691. [DOI] [PubMed] [Google Scholar]
- Solon FS, Fernandez TL, Latham MC, Popkin BM. Planning, implementation, and evaluation of a fortification program. Control of vitamin A deficiency in the Philippines. J Am Diet Assoc. 1979;74(2):112–118. [PubMed] [Google Scholar]
- Solon FS, Klemm RDW, Sanchez L, Darnton-Hill I, Craft NE, Christian P, West KP. Efficacy of a vitamin A–fortified wheat-flour bun on the vitamin A status of Filipino schoolchildren. Am J Clin Nutr. 2000;72(3):738–744. doi: 10.1093/ajcn/72.3.738. [DOI] [PubMed] [Google Scholar]
- Stekel A, Olivares M, Cayazzo M, Chadud P, Llaguno S, Pizarro F. Prevention of iron deficiency by milk fortification. II. A field trial with a full-fat acidified milk. The Am J Clin Nutr. 1988;47(2):265–269. doi: 10.1093/ajcn/47.2.265. [DOI] [PubMed] [Google Scholar]
- Stevens D, Nelson A. The effect of iron in formula milk after 6 months of age. Arch Dis Child. 1995;73(3):216–260. doi: 10.1136/adc.73.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Guo Y, Wang S, Sun J. Social marketing improved the consumption of iron-fortified soy sauce among women in China. J Nutr Educ Behav. 2007;39(6):302–310. doi: 10.1016/j.jneb.2007.03.090. [DOI] [PubMed] [Google Scholar]
- Torrejón CS, Castillo-Durán C, Hertrampf ED, Ruz M. Zinc and iron nutrition in Chilean children fed fortified milk provided by the Complementary National Food Program. Nutr. 2004;20(2):177–180. doi: 10.1016/j.nut.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Tuthill DP, Cosgrove M, Dunstan F, Stuart ML, Wells JCK, Davies DP. Randomized double-blind controlled trial on the effects on iron status in the first year between a no added iron and standard infant formula received for three months. Acta Paediatr. 2002;91(2):119–124. doi: 10.1080/080352502317285072. [DOI] [PubMed] [Google Scholar]
- Van Phu P, van Hoan N, Salvignol B, Treche S, Wieringa FT, Khan NC, Tuong PD, Berger J. Complementary foods fortified with micronutrients prevent iron deficiency and anemia in Vietnamese infants. J Nutr. 2010;140(12):2241–2247. doi: 10.3945/jn.110.123711. [DOI] [PubMed] [Google Scholar]
- Van Stuijvenberg ME, Dhansay MA, Smuts CM, Lombard CJ, Jogessar VB, Benadé AJ. Long-term evaluation of a micronutrient-fortified biscuit used for addressing micronutrient deficiencies in primary school children. Public Health Nutr. 2001;4(6):1201–1210. doi: 10.1079/phn2001179. [DOI] [PubMed] [Google Scholar]
- Van Stuijvenberg ME, Kvalsvig JD, Faber M, Kruger M, Kenoyer DG, Benade AJ. Effect of iron-, iodine-, and beta-carotene-fortified biscuits on the micronutrient status of primary school children: a randomized controlled trial. Am J Clin Nutr. 1999;69(3):497–503. doi: 10.1093/ajcn/69.3.497. [DOI] [PubMed] [Google Scholar]
- Van Stuijvenberg ME, Smuts CM, Lombard CJ, Dhansay MA. Fortifying brown bread with sodium iron EDTA, ferrous fumarate, or electrolytic iron does not affect iron status in South African schoolchildren. J Nutr. 2008;138(4):782–786. doi: 10.1093/jn/138.4.782. [DOI] [PubMed] [Google Scholar]
- Varma JL, Das S, Sankar R, Mannar MGV, Levinson FJ, Hamer DH. Community-level micronutrient fortification of a food supplement in India: a controlled trial in preschool children aged 36–66 mo. Am J Clin Nutr. 2007;85(4):1127–1133. doi: 10.1093/ajcn/85.4.1127. [DOI] [PubMed] [Google Scholar]
- Villalpando S, Shamah T, Rivera JA, Lara Y, Monterrubio E. Fortifying milk with ferrous gluconate and zinc oxide in a public nutrition program reduced the prevalence of anemia in toddlers. J Nutr. 2006;136(10):2633–2637. doi: 10.1093/jn/136.10.2633. [DOI] [PubMed] [Google Scholar]
- Vinodkumar M, Rajagopalan S, Bhagwat IP, Singh S, Parmar BS, Mishra OP, Upadhyay SS, Bhalia NB, Deshpande SR. A multicenter community study on the efficacy of double-fortified salt. Food Nutr Bull. 2007;28(1):100–108. doi: 10.1177/156482650702800111. [DOI] [PubMed] [Google Scholar]
- Virtanen MA, Svahn CJE, Viinikka LU, Raiha NCR, Siimes MA, Axelsson IEM. Iron-fortified and unfortified cow’s milk: Effects on iron intakes and iron status in young children. Acta Paediatr. 2001;90(7):724–731. [PubMed] [Google Scholar]
- Walravens PA, Hambidge KM. Growth of infants fed a zinc supplemented formula. Am J Clin Nutr. 1976;29(10):1114–1121. doi: 10.1093/ajcn/29.10.1114. [DOI] [PubMed] [Google Scholar]
- Walter T, Dallman PR, Pizarro F, Velozo L, Pena G, Bartholmey SJ, Hertrampf E, Olivares M, Letelier A, Arredondo M. Effectiveness of iron-fortified infant cereal in prevention of iron deficiency anemia. Pediatr. 1993;91(5):976–982. [PubMed] [Google Scholar]
- Walter T, Hertrampf E, Pizarro F, Olivares M, Llaguno S, Letelier A, Vega V, Stekel A. Effect of bovine-hemoglobin-fortified cookies on iron status of schoolchildren: a nationwide program in Chile. Am J Clin Nutr. 1993;57(2):190–194. doi: 10.1093/ajcn/57.2.190. [DOI] [PubMed] [Google Scholar]
- Wegmüller R, Camara F, Zimmermann MB, Adou P, Hurrell RF. Salt dual-fortified with iodine and micronized ground ferric pyrophosphate affects iron status but not hemoglobin in children in Cote d’Ivoire. J Nutr. 2006;136(7):1814–1820. doi: 10.1093/jn/136.7.1814. [DOI] [PubMed] [Google Scholar]
- Williams J, Wolff A, Daly A, MacDonald A, Aukett A, Booth IW. Iron supplemented formula milk related to reduction in psychomotor decline in infants from inner city areas: randomised study. Bmj. 1999;318(7185):693–697. doi: 10.1136/bmj.318.7185.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winichagoon P, McKenzie JE, Chavasit V, Pongcharoen T, Gowachirapant S, Boonpraderm A, Manger MS, Bailey KB, Wasantwisut E, Gibson RS. A multimicronutrient-fortified seasoning powder enhances the hemoglobin, zinc, and iodine status of primary school children in North East Thailand: a randomized controlled trial of efficacy. J Nutr. 2006;136(6):1617–1623. doi: 10.1093/jn/136.6.1617. [DOI] [PubMed] [Google Scholar]
- Yamamah G, Wahba S, Ghanem K. Monitoring iodine deficiency of female children after introducing iodized salt in El-Farafra Oasis. Med J Cairo Univ. 2003;4(Suppl 11):269–273. [Google Scholar]
- Zhang X, Chen K, Qu P, Liu YX, Li TY. Effect of biscuits fortified with different doses of vitamin A on indices of vitamin A status, haemoglobin and physical growth levels of pre-school children in Chongqing. Public Health Nutr. 2010;13(09):1462–1471. doi: 10.1017/S1368980010000820. [DOI] [PubMed] [Google Scholar]
- Zhu K, Du X, Cowell CT, Greenfield H, Blades B, Dobbins TA, Zhang Q, Fraser DR. Effects of school milk intervention on cortical bone accretion and indicators relevant to bone metabolism in Chinese girls aged 10–12 y in Beijing. Am J Clin Nutr. 2005;81(5):1168–1175. doi: 10.1093/ajcn/81.5.1168. [DOI] [PubMed] [Google Scholar]
- Zhu K, Greenfield H, Zhang Q. Effects of two year’s milk supplementation on site-corrected bone mineral density of Chinese girls. Asia Pac J Clin Nutr. 2008;17(S1):147–150. [PubMed] [Google Scholar]
- Ziegler EE, Nelson SE, Jeter JM. Iron status of breastfed infants is improved equally by medicinal iron and iron-fortified cereal. Am J Clin Nutr. 2009;90(1):76–78. doi: 10.3945/ajcn.2008.27350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MB, Chassard C, Rohner F, N’Goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr. 2010;92(6):1406–1415. doi: 10.3945/ajcn.110.004564. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Hess SY, Adou P, Toresanni T, Wegmuller R, Hurrell RF. Thyroid size and goiter prevalence after introduction of iodized salt: a 5-y prospective study in schoolchildren in Cote d’Ivoire. Am J Clin Nutr. 2003;77(3):663–667. doi: 10.1093/ajcn/77.3.663. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Jooste PL, Mabapa NS, Mbhenyane X, Schoeman S, Biebinger R, Chaouki N, Bozo M, Grimci L, Bridson J. Treatment of iodine deficiency in school-age children increases insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations and improves somatic growth. J Clin Endocrinol Metab. 2006;92(2):437–442. doi: 10.1210/jc.2006-1901. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Muthayya S, Moretti D, Kurpad A, Hurrell RF. Iron fortification reduces blood lead levels in children in Bangalore. India. Pediatr. 2006;117(6):2014–2021. doi: 10.1542/peds.2005-2440. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Wegmueller R, Zeder C, Chaouki N, Rohner F, Saïssi M, Torresani T, Hurrell RF. Dual fortification of salt with iodine and micronized ferric pyrophosphate: a randomized, double-blind, controlled trial. Am J Clin Nutr. 2004;80(4):952–959. doi: 10.1093/ajcn/80.4.952. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Zeder C, Chaouki N, Saad A, Torresani T, Hurrell RF. Dual fortification of salt with iodine and microencapsulated iron: a randomized, double-blind, controlled trial in Moroccan schoolchildren. Am J Clin Nutr. 2003;77(2):425–432. doi: 10.1093/ajcn/77.2.425. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Zeder C, Chaouki N, Torresani T, Saad A, Hurrell RF. Addition of microencapsulated iron to iodized salt improves the efficacy of iodine in goitrous, iron-deficient children: a randomized, double-blind, controlled trial. Eur J Endocrinol. 2002;147(6):747–753. doi: 10.1530/eje.0.1470747. [DOI] [PubMed] [Google Scholar]
- Zlotkin S, Arthur P, Antwi KY, Yeung G. Treatment of anemia with microencapsulated ferrous fumarate plus ascorbic acid supplied as sprinkles to complementary (weaning) foods. Am J Clin Nutr. 2001;74(6):791–795. doi: 10.1093/ajcn/74.6.791. [DOI] [PubMed] [Google Scholar]
- Zlotkin S, Arthur P, Schauer C, Antwi KY, Yeung G, Piekarz A. Home-fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. J Nutr. 2003;133(4):1075–1080. doi: 10.1093/jn/133.4.1075. [DOI] [PubMed] [Google Scholar]
- El Hamdouchi A, El Kari K, Rjimati L, El Haloui N, El Mzibri M, Aguenaou H, Mokhtar N. Impact of flour fortification with elemental iron on the prevalence of anaemia among preschool children in Morocco. East Mediterr Health J. 2010;16(11):1148–1152. [PubMed] [Google Scholar]
- Chee WSS, Suriah AR, Chan SP, Zaitun Y, Chan YM. The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos Int. 2003;14(10):828–834. doi: 10.1007/s00198-003-1448-6. [DOI] [PubMed] [Google Scholar]
- Adolphi B, Scholz-Ahrens KE, de Vrese M, Açil Y, Laue C, Schrezenmeir J. Short-term effect of bedtime consumption of fermented milk supplemented with calcium, inulin-type fructans and caseinphosphopeptides on bone metabolism in healthy, postmenopausal women. Eur J Nutr. 2009;48(1):45–53. doi: 10.1007/s00394-008-0759-y. [DOI] [PubMed] [Google Scholar]
- Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr. 2007;86(2):412–420. doi: 10.1093/ajcn/86.2.412. [DOI] [PubMed] [Google Scholar]
- Alasfoor D, Elsayed MK, Mohammed AJ. Spina bifida and birth outcome before and after fortification of flour with iron and folic acid in Oman. East Mediterr Health J. 2010;16(5):533–538. [PubMed] [Google Scholar]
- Amarin ZO, Obeidat AZ. Effect of folic acid fortification on the incidence of neural tube defects. Paediatr Perinat Epidemiol. 2010;24(4):349–351. doi: 10.1111/j.1365-3016.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- Andersson M, Theis W, Zimmermann MB, Foman JT, Jakel M, Duchateau GS, Frenken LG, Hurrell RF. Random serial sampling to evaluate efficacy of iron fortification: a randomized controlled trial of margarine fortification with ferric pyrophosphate or sodium iron edetate. Am J Clin Nutr. 2010;92(5):1094–1104. doi: 10.3945/ajcn.2010.29523. [DOI] [PubMed] [Google Scholar]
- Badii A, Nekouei N, Fazilati M, Shahedi M, Badiei S. Effect of consuming zinc-fortified bread on serum zinc and iron status of zinc-deficient women: a double blind, randomized clinical trial. Int J Prev Med. 2012;3(Suppl 1):S124–S130. [PMC free article] [PubMed] [Google Scholar]
- Bar-Oz B, Koren G, Nguyen P, Kapur BM. Folate fortification and supplementation–Are we there yet? Reprod Toxicol. 2008;25(4):408–412. doi: 10.1016/j.reprotox.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Biebinger R, Zimmermann MB, Al-Hooti SN, Al-Hamed N, Al-Salem E, Zafar T, Kabir Y. Efficacy of wheat-based biscuits fortified with microcapsules containing ferrous sulfate and potassium iodate or a new hydrogen-reduced elemental iron: a randomised, double-blind, controlled trial in Kuwaiti women. Br J Nutr. 2009;102(09):1362–1369. doi: 10.1017/S0007114509990353. [DOI] [PubMed] [Google Scholar]
- Blanco-Rojo R, Pérez-Granados AM, Toxqui L, González-Vizcayno C, Delgado MA, Vaquero MP. Efficacy of a microencapsulated iron pyrophosphate-fortified fruit juice: a randomised, double-blind, placebo-controlled study in Spanish iron-deficient women. Br J Nutr. 2011;105(11):1652–1659. doi: 10.1017/S0007114510005490. [DOI] [PubMed] [Google Scholar]
- Blanco-Rojo R, Perez-Granados AM, Toxqui L, Zazo P, de la Piedra C, Vaquero MP. Relationship between vitamin D deficiency, bone remodelling and iron status in iron-deficient young women consuming an iron-fortified food. Eur J Nutr. 2012;52(2):695–703. doi: 10.1007/s00394-012-0375-8. [DOI] [PubMed] [Google Scholar]
- Bol KA, Collins JS, Kirby RS. Survival of infants with neural tube defects in the presence of folic acid fortification. Pediatrics. 2006;117(3):803–813. doi: 10.1542/peds.2005-1364. [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Benoit V, Pourchaire O, Ferry M, Rousseau B, Souberbielle JC. Inhibition of markers of bone resorption by consumption of vitamin D and calcium-fortified soft plain cheese by institutionalised elderly women. Br J Nutr. 2009;102(07):962–966. doi: 10.1017/S0007114509371743. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, Robbins JM, Meyer R, Canfield MA, Mulinare J. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82(7):527–532. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- Bulow Pedersen I, Knudsen N, Carle A, Vejbjerg P, Jorgensen T, Perrild H, Ovesen L, Banke Rasmussen L, Laurberg P. A cautious iodization program bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol (Oxf) 2011. Epub ahead of print. [DOI] [PubMed]
- Bülow Pedersen I, Laurberg P, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Rasmussen LB. An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J Clin Endocrinol Metab. 2007;92(8):3122–3127. doi: 10.1210/jc.2007-0732. [DOI] [PubMed] [Google Scholar]
- Burgess JR, Seal JA, Stilwell GM, Reynolds PJ, Taylor ER, Parameswaran V. A case for universal salt iodisation to correct iodine deficiency in pregnancy: another salutary lesson from Tasmania. Med J Aust. 2007;186(11):574–576. doi: 10.5694/j.1326-5377.2007.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Camargo RYA, Tomimori EK, Neves SC, Rubio GSI, Galrão AL, Knobel M, Medeiros-Neto G. Thyroid and the environment: exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo. Brazil. Eur J Endocrinol. 2008;159(3):293–299. doi: 10.1530/EJE-08-0192. [DOI] [PubMed] [Google Scholar]
- Canfield MA, Mai CT, Kirby RS, Pearson K, Devine O, Mulinare J. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi-state population-based study. Birth Defects Res A Clin Mol Teratol. 2005;73:679–689. doi: 10.1002/bdra.20210. [DOI] [PubMed] [Google Scholar]
- Carle A, Laurberg P, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Jorgensen T. Epidemiology of subtypes of hypothyroidism in Denmark. Eur J Endocrinol. 2006;154(1):21–28. doi: 10.1530/eje.1.02068. [DOI] [PubMed] [Google Scholar]
- Cerqueira C, Knudsen N, Ovesen L, Laurberg P, Perrild H, Rasmussen LB, Jørgensen T. Doubling in the use of thyroid hormone replacement therapy in Denmark: association to iodization of salt? Eur J Epidemiol. 2011;26:1–7. doi: 10.1007/s10654-011-9590-5. [DOI] [PubMed] [Google Scholar]
- Cerqueira C, Knudsen N, Ovesen L, Perrild H, Rasmussen LB, Laurberg P, Jorgensen T. Association of Iodine Fortification with Incident Use of Antithyroid Medication–A Danish Nationwide Study. J Clin Endocrinol Metab. 2009;94(7):2400. doi: 10.1210/jc.2009-0123. [DOI] [PubMed] [Google Scholar]
- Chen BH, Carmichael SL, Selvin S, Abrams B, Shaw GM. NTD prevalences in central California before and after folic acid fortification. Birth Defects Res A Clin Mol Teratol. 2008;82(8):547–552. doi: 10.1002/bdra.20466. [DOI] [PubMed] [Google Scholar]
- Chen LT, Rivera MA. The Costa Rican experience: reduction of neural tube defects following food fortification programs. Nutr Rev. 2004;62:S40–S43. doi: 10.1111/j.1753-4887.2004.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Ferrar L, van der Hee RM, Berry M, Watson C, Miret S, Wilkinson J, Bradburn M, Eastell R. Effects of calcium-fortified ice cream on markers of bone health. Osteoporos Int. 2010;22:2721–2731. doi: 10.1007/s00198-010-1513-x. [DOI] [PubMed] [Google Scholar]
- Green TJ, Skeaff CM, Rockell JE. Milk fortified with the current adequate intake for vitamin D (5 g) increases serum 25-hydroxyvitamin D compared to control milk but is not sufficient to prevent a seasonal decline in young women. Asia Pac J Clin Nutr. 2010;19(2):195–199. [PubMed] [Google Scholar]
- Hertrampf E, Cortés F, Erickson JD, Cayazzo M, Freire W, Bailey LB, Howson C, Kauwell G, Pfeiffer C. Consumption of folic acid–fortified bread improves folate status in women of reproductive age in Chile. J Nutr. 2003;133(10):3166–3169. doi: 10.1093/jn/133.10.3166. [DOI] [PubMed] [Google Scholar]
- Hoa PT, Khan NC, van Beusekom C, Gross R, Conde WL, Khoi HD. Milk fortified with iron or iron supplementation to improve nutritional status of pregnant women: An intervention trial from rural Vietnam. Food Nutr Bull. 2005;26(1):32–38. doi: 10.1177/156482650502600104. [DOI] [PubMed] [Google Scholar]
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LYC. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- Hotz C, Porcayo M, Onofre G, GarcÃa-Guerra A, Elliott T, Jankowski S, Greiner T. Efficacy of iron-fortified Ultra Rice in improving the iron status of women in Mexico. Food Nutr Bull. 2008;29(2):140–149. doi: 10.1177/156482650802900208. [DOI] [PubMed] [Google Scholar]
- Huo J, Sun J, Huang J, Li W, Wang L, Selenje L, Gleason GR, Yu X. The effectiveness of fortified flour on micro-nutrient status in rural female adults in China. Asia Pac J Clin Nutr. 2011;20(1):118–124. [PubMed] [Google Scholar]
- Ionescu-Ittu R, Marelli AJ, Mackie AS, Pilote L. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec. Canada. BMJ. 2009;338:b1673. doi: 10.1136/bmj.b1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl JP, Lieberman HR, Cable SJ, Williams KW, Young AJ, McClung JP. Randomized, double-blind, placebo-controlled trial of an iron-fortified food product in female soldiers during military training: relations between iron status, serum hepcidin, and inflammation. Am J Clin Nutr. 2010;92(1):93–100. doi: 10.3945/ajcn.2010.29185. [DOI] [PubMed] [Google Scholar]
- Kruger MC, Booth CL, Coad J, Schollum LM, Kuhn-Sherlock B, Shearer MJ. Effect of calcium fortified milk supplementation with or without vitamin K on biochemical markers of bone turnover in premenopausal women. Nutrition. 2006;22(11–12):1120–1128. doi: 10.1016/j.nut.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Kruger MC, Schollum LM, Kuhn-Sherlock B, Hestiantoro A, Wijanto P, Li-Yu J, Agdeppa I, Todd JM, Eastell R. The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South East Asia. Bone. 2010;46(3):759–767. doi: 10.1016/j.bone.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Kucik J, Correa A. Trends in twinning rates in metropolitan Atlanta before and after folic acid fortification. J Reprod Med. 2004;49(9):707–712. [PubMed] [Google Scholar]
- Lau EM, Woo J, Lam V, Hong A. Milk supplementation of the diet of postmenopausal Chinese women on a low calcium intake retards bone loss. J Bone Miner Res. 2001;16(9):1704–1709. doi: 10.1359/jbmr.2001.16.9.1704. [DOI] [PubMed] [Google Scholar]
- Laurberg P, Jorgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, Rasmussen LB, Carle A, Vejbjerg P. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol. 2006;155(2):219–228. doi: 10.1530/eje.1.02210. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Watkins ML, Chiu V, Erickson JD, Petitti DB. Food fortification with folic acid and rate of multiple births, 1994–2000. Birth Defects Res A Clin Mol Teratol. 2004;70(12):948–952. doi: 10.1002/bdra.20088. [DOI] [PubMed] [Google Scholar]
- Liu S, West R, Randell E, Longerich L, O’Connor K, Scott H, Crowley M, Lam A, Prabhakaran V, McCourt C. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childbirth. 2004;4(1):20. doi: 10.1186/1471-2393-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Camelo JS, Orioli IM, Dutra MG, Nazer Herrera J, Rivera N, Ojeda ME, Canessa A, Wettig E, Fontannaz AM, Mellado C. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. Am J Med Genet A. 2005;135(2):120–125. doi: 10.1002/ajmg.a.30651. [DOI] [PubMed] [Google Scholar]
- Manios Y, Moschonis G, Panagiotakos DB, Farajian P, Trovas G, Lyritis GP. Changes in biochemical indices of bone metabolism in post menopausal women following a dietary intervention with fortified dairy products. J Hum Nutr Diet. 2009;22(2):156–165. doi: 10.1111/j.1365-277X.2008.00934.x. [DOI] [PubMed] [Google Scholar]
- Mardones F, Urrutia MT, Villarroel L, Rioseco A, Castillo O, Rozowski J, Tapia JL, Bastias G, Bacallao J, Rojas I. Effects of a dairy product fortified with multiple micronutrients and omega-3 fatty acids on birth weight and gestation duration in pregnant Chilean women. Public Health Nutr. 2008;11(1):30–40. doi: 10.1017/S1368980007000110. [DOI] [PubMed] [Google Scholar]
- Modjadji SE, Alberts M, Mamabolo RL. Folate and iron status of South African non-pregnant rural women of childbearing age, before and after fortification of foods. S Afr Clin Nutr. 2008;20(3):89–93. [Google Scholar]
- Moleti M, Di Bella B, Giorgianni G, Mancuso A, De Vivo A, Alibrandi A, Trimarchi F, Vermiglio F. Maternal thyroid function in different conditions of iodine nutrition in pregnant women exposed to mild-moderate iodine deficiency: an observational study. Clin Endocrinol (Oxf) 2011;74(6):762–768. doi: 10.1111/j.1365-2265.2011.04007.x. [DOI] [PubMed] [Google Scholar]
- Moleti M, Lo Presti VP, Campolo MC, Mattina F, Galletti M, Mandolfino M, Violi MA, Giorgianni G, De Domenico D, Trimarchi F. Iodine prophylaxis using iodized salt and risk of maternal thyroid failure in conditions of mild iodine deficiency. J Clin Endocrinol Metab. 2008;93(7):2616–2621. doi: 10.1210/jc.2008-0352. [DOI] [PubMed] [Google Scholar]
- Moschonis G, Manios Y. Skeletal site-dependent response of bone mineral density and quantitative ultrasound parameters following a 12-month dietary intervention using dairy products fortified with calcium and vitamin D: the Postmenopausal Health Study. Br J Nutr. 2006;96(06):1140–1148. doi: 10.1017/bjn20061977. [DOI] [PubMed] [Google Scholar]
- Murphy M, Whiteman D, Stone D, Botting B, Schorah C, Wild J. Dietary folate and the prevalence of neural tube defects in the British Isles: the past two decades. BJOG. 2000;107(7):885–889. doi: 10.1111/j.1471-0528.2000.tb11087.x. [DOI] [PubMed] [Google Scholar]
- Natri AM, Salo P, Vikstedt T, Palssa A, Huttunen M, Kärkkäinen MUM, Salovaara H, Piironen V, Jakobsen J, Lamberg-Allardt CJ. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J Nutr. 2006;136(1):123–127. doi: 10.1093/jn/136.1.123. [DOI] [PubMed] [Google Scholar]
- Orioli IM, Lima Do Nascimento R, López Camelo JS, Castilla EE. Effects of folic acid fortification on spina bifida prevalence in Brazil. Birth Defects Res A Clin Mol Teratol. 2011;91(9):831–835. doi: 10.1002/bdra.20830. [DOI] [PubMed] [Google Scholar]
- Pacheco SS, Braga C, Souza AI, Figueiroa JN. Effects of folic acid fortification on the prevalence of neural tube defects. Rev Saude Publica. 2009;43(4):565–571. doi: 10.1590/s0034-89102009005000033. [DOI] [PubMed] [Google Scholar]
- Persad VL, van den Hof MC, Dubé JM, Zimmer P. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. Can Med Assoc J. 2002;167(3):241–245. [PMC free article] [PubMed] [Google Scholar]
- Rasmussen LB, Carlé A, Jørgensen T, Knudsen N, Laurberg P, Pedersen IB, Perrild H, Vejbjerg P, Ovesen L. Iodine intake before and after mandatory iodization in Denmark: results from the Danish Investigation of Iodine Intake and Thyroid Diseases (DanThyr) study. Br J Nutr. 2008;100(01):166–173. doi: 10.1017/S0007114507886387. [DOI] [PubMed] [Google Scholar]
- Ray JG. Folic acid food fortification in Canada. Nutr Rev. 2004;62:S35–S39. doi: 10.1111/j.1753-4887.2004.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Ray JG, Meier C, Vermeulen MJ, Cole DEC, Wyatt PR. Prevalence of trisomy 21 following folic acid food fortification. Am J Med Genet A. 2003;120(3):309–313. doi: 10.1002/ajmg.a.20161. [DOI] [PubMed] [Google Scholar]
- Recker RR, Heaney RP. The effect of milk supplements on calcium metabolism, bone metabolism and calcium balance. Am J Clin Nutr. 1985;41(2):254–263. doi: 10.1093/ajcn/41.2.254. [DOI] [PubMed] [Google Scholar]
- Robbins JM, Tilford JM, Bird TM, Cleves MA, Reading JA, Hobbs CA. Hospitalizations of newborns with folate-sensitive birth defects before and after fortification of foods with folic acid. Pediatr. 2006;118(3):906–915. doi: 10.1542/peds.2005-2784. [DOI] [PubMed] [Google Scholar]
- Sadighi J, Mohammad K, Sheikholeslam R, Amirkhani MA, Torabi P, Salehi F, Abdolahi Z. Anaemia control: lessons from the flour fortification programme. Public Health. 2009;123(12):794–799. doi: 10.1016/j.puhe.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Sadighi J, Sheikholeslam R, Mohammad K, Pouraram H, Abdollahi Z, Samadpour K, Kolahdooz F, Naghavi M. Flour fortification with iron: a mid-term evaluation. Public Health. 2008;122(3):313–321. doi: 10.1016/j.puhe.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Safdar OY, Al-Dabbagh AA, AbuElieneen WA, Kari JA. Decline in the incidence of neural tube defects after the national fortification of flour (1997–2005) Saudi Med J. 2007;28(8):1227–1229. [PubMed] [Google Scholar]
- Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost benefit in South Africa. Birth Defects Res A Clin Mol Teratol. 2008;82(4):211–216. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- Seal JA, Doyle Z, Burgess JR, Taylor R, Cameron AR. Iodine status of Tasmanians following voluntary fortification of bread with iodine. Med J Aust. 2007;186(2):69–71. doi: 10.5694/j.1326-5377.2007.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Sebotsa MLD, Dannhauser A, Jooste PL, Joubert G. Iodine status as determined by urinary iodine excretion in Lesotho two years after introducing legislation on universal salt iodization. Nutrition. 2005;21(1):20–24. doi: 10.1016/j.nut.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Nelson V, Selvin S, Schaffer DM. Food fortification with folic acid and twinning among California infants. Am J Med Genet A. 2003;119(2):137–140. doi: 10.1002/ajmg.a.20145. [DOI] [PubMed] [Google Scholar]
- Signore C, Mills JL, Cox C, Trumble AC. Effects of folic acid fortification on twin gestation rates. Obstet Gynecol. 2005;105(4):757–762. doi: 10.1097/01.AOG.0000154886.40318.5e. [DOI] [PubMed] [Google Scholar]
- Simmons CJ, Mosley BS, Fulton Bond CA, Hobbs CA. Birth defects in Arkansas: is folic acid fortification making a difference? Birth Defects Res A Clin Mol Teratol. 2004;70(9):559–564. doi: 10.1002/bdra.20063. [DOI] [PubMed] [Google Scholar]
- Storm D, Porter REES, Musgrave K, Vereault D, Patton C, Kessenich C, Mohan S, Chen T, Holick MF, Rosen CJ. Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 1998;83(11):3817–3825. doi: 10.1210/jcem.83.11.5289. [DOI] [PubMed] [Google Scholar]
- Sultanalieva RB, Mamutova S, van der Haar F. The current salt iodization strategy in Kyrgyzstan ensures sufficient iodine nutrition among school-age children but not pregnant women. Public Health Nutr. 2010;13(05):623–630. doi: 10.1017/S136898000999200X. [DOI] [PubMed] [Google Scholar]
- Tapola NS, Karvonen HM, Niskanen LK, Sarkkinen ES. Mineral water fortified with folic acid, vitamins B6, B12, D and calcium improves folate status and decreases plasma homocysteine concentration in men and women. Eur J Clin Nutr. 2004;58(2):376–385. doi: 10.1038/sj.ejcn.1601795. [DOI] [PubMed] [Google Scholar]
- Tenta R, Moschonis G, Koutsilieris M, Manios Y. Calcium and vitamin D supplementation through fortified dairy products counterbalances seasonal variations of bone metabolism indices: the Postmenopausal Health Study. Eur J Nutr. 2010;50:341–349. doi: 10.1007/s00394-010-0142-7. [DOI] [PubMed] [Google Scholar]
- Thuy PV, Berger J, Davidsson L, Khan NC, Lam NT, Cook JD, Hurrell RF, Khoi HH. Regular consumption of NaFeEDTA-fortified fish sauce improves iron status and reduces the prevalence of anemia in anemic Vietnamese women. Am J Clin Nutr. 2003;78(2):284–290. doi: 10.1093/ajcn/78.2.284. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Olson B, Bakun P, Dallal GE, Selhub J, Rosenberg IH. Breakfast cereal fortified with folic acid, vitamin B-6, and vitamin B-12 increases vitamin concentrations and reduces homocysteine concentrations: a randomized trial. Am J Clin Nutr. 2004;79(5):805–811. doi: 10.1093/ajcn/79.5.805. [DOI] [PubMed] [Google Scholar]
- Van Thuy P, Berger J, Nakanishi Y, Khan NC, Lynch S, Dixon P. The use of NaFeEDTA-fortified fish sauce is an effective tool for controlling iron deficiency in women of childbearing age in rural Vietnam. J Nutr. 2005;135(11):2596–2601. doi: 10.1093/jn/135.11.2596. [DOI] [PubMed] [Google Scholar]
- Vejbjerg P, Knudsen N, Perrild H, Carle A, Laurberg P, Pedersen IB, Rasmussen LB, Ovesen L, Jorgensen T. Effect of a mandatory iodization program on thyroid gland volume based on individuals’ age, gender, and preceding severity of dietary iodine deficiency: a prospective, population-based study. J Clin Endocrinol Metab. 2007;92(4):1397–1401. doi: 10.1210/jc.2006-2580. [DOI] [PubMed] [Google Scholar]
- Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Carlé A, Pedersen IB, Rasmussen LB, Ovesen L, Jørgensen T. Lower prevalence of mild hyperthyroidism related to a higher iodine intake in the population: prospective study of a mandatory iodization programme. Clin Endocrinol. 2009;71(3):440–445. doi: 10.1111/j.1365-2265.2008.03493.x. [DOI] [PubMed] [Google Scholar]
- Waller DK, Tita ATN, Annegers JF. Rates of twinning before and after fortification of foods in the US with folic acid, Texas, 1996 to 1998. Paediatr Perinat Epidemiol. 2003;17(4):378–383. doi: 10.1046/j.1365-3016.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Mai CT, Edmonds LD, Shaw GM, Kirby RS, Hobbs CA, Sever LE, Miller LA, Meaney FJ, Levitt M. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66(1):33–39. doi: 10.1002/tera.10060. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Winichagoon P, Gowachirapant S, Hess SY, Harrington M, Chavasit V, Lynch SR, Hurrell RF. Comparison of the efficacy of wheat-based snacks fortified with ferrous sulfate, electrolytic iron, or hydrogen-reduced elemental iron: randomized, double-blind, controlled trial in Thai women. Am J Clin Nutr. 2005;82(6):1276–1282. doi: 10.1093/ajcn/82.6.1276. [DOI] [PubMed] [Google Scholar]
- Shuaibi AM, House JD, Sevenhuysen GP. Folate status of young Canadian women after folic acid fortification of grain products. J Am Diet Assoc. 2008;108(12):2090–2094. doi: 10.1016/j.jada.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Ash DM, Tatala SR, Frongillo EA Jr, Ndossi GD, Latham MC. Randomized efficacy trial of a micronutrient-fortified beverage in primary school children in Tanzania. Am J Clin Nutr. 2003;77(4):891–898. doi: 10.1093/ajcn/77.4.891. [DOI] [PubMed] [Google Scholar]
- Ballot DE, MacPhail AP, Bothwell TH, Gillooly M, Mayet FG. Fortification of curry powder with NaFe(111)EDTA in an iron-deficient population: report of a controlled iron-fortification trial. Am J Clin Nutr. 1989;49(1):162–169. doi: 10.1093/ajcn/49.1.162. [DOI] [PubMed] [Google Scholar]
- Colman N, Larsen JV, Barker M, Barker EA, Green R, Metz J. Prevention of folate deficiency by food fortification. V. A pilot field trial of folic acid-fortified maize meal. S Afr Med J. 1974;48(41):1763–1766. [PubMed] [Google Scholar]
- Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. A randomized controlled trial of the effect of antihelminthic treatment and micronutrient fortification on health status and school performance of rural primary school children. Ann Trop Paediatr. 2001;21(4):319–333. doi: 10.1080/07430170120093508. [DOI] [PubMed] [Google Scholar]
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- WHO. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO global database on vitamin A deficiency. Geneva: WHO; 2009. p. 283. [Google Scholar]
- Mills CF. Trace elements in human nutrition and health. Geneva: WHO; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of included studies.
Forest plots.
Summary of findings and quality of outcomes.