Abstract
Background
Disrupted neuroplasticity may be an important aspect of the neural basis of schizophrenia. We used event-related brain potentials (ERPs) to assay neuroplasticity after auditory conditioning in chronic schizophrenia patients (SZ) and matched healthy control subjects (HC).
Methods
Subjects (15 HC, 14 SZ) performed an auditory oddball task during electroencephalogram recording before and after auditory tetanic stimulation (Pre/Post Blocks). Each oddball block consisted of 1000-Hz and 1500-Hz standards and 400-Hz targets. During tetanic conditioning, 1000-Hz tones were presented at 11 Hz for 2.4 min. We analyzed the standard trials, comparing the ERPs evoked by the tetanized stimuli (1000 Hz tones: TS+) and untetanized stimuli (1500 Hz tones: TS−) in the Post Blocks with ERPs from the Pre Blocks (averaged into Baseline ERPs).
Results
In Post Block 1 in HC, TS+ tones evoked a negative shift (60–350 msec) at right temporal electrodes relative to Baseline. No pre-/post-tetanus effects were found in SZ. In Post Block 2 in HC, TS+ tones evoked a positive shift (200–300 msec) at bilateral frontal electrodes. In SZ, TS+ tones evoked a positive shift (100–400 msec) at right frontotemporal electrodes. No pre-/post-tetanus effects were found in either subject group for the TS− tones. The right temporal Post Block 1 and 2 effects were correlated in SZ, suggesting a trade-off in the expression of these effects.
Conclusions
These results suggest that stimulus-specific auditory neuroplasticity is abnormal in schizophrenia. The electrophysiologic assessment of stimulus-specific plasticity may yield novel targets for drug treatment in schizophrenia.
Keywords: Auditory processing, ERP, event-related brain potential, NMDA receptor, plasticity, schizophrenia
Abnormalities in the structure and function of the auditory cortex figure prominently in schizophrenia. The volume of gray matter in primary and secondary auditory cortical areas is reduced (1,2), and neuropathologic studies have documented abnormalities in the microcircuitry of auditory cortex (3,4). Functionally, the amplitudes of auditory event-related brain potentials (ERPs) are reduced (5,6), schizophrenia patients have deficits in auditory perception (7), and auditory hallucinations are a hallmark symptom of this disorder (8). However, to our knowledge, the plasticity of auditory cortical circuits has not yet been examined in schizophrenia.
Plasticity is a fundamental property of neural systems that enables them to alter their structure and dynamics in response to changes in their inputs and hence underlies learning and memory (9). Disturbances of plasticity-related mechanisms could thus underlie the abnormal development, structure, and function of neural circuits in neuropsychiatric disorders such as schizophrenia (10,11). For example, the induction of cortical plasticity through long-term potentiation (LTP) is dependent on the integrity of N-methyl-D-aspartate receptors (12), which are also implicated in the patho-physiology of schizophrenia (13–15). Consistent with this hypothesis, reduced motor cortex plasticity in schizophrenia patients has been found using transcranial magnetic stimulation (16,17).
We examined the plasticity of auditory cortex in schizophrenia by measuring event-related brain potentials (ERPs) in a stimulus-specific plasticity (SSP) paradigm based on the study of Clapp and colleagues (18). Investigations of SSP in mouse visual cortex (19) have demonstrated that this phenomenon shares the same cellular and molecular substrates as LTP, so SSP may serve as a noninvasive measure of LTP-associated plasticity mechanisms in humans. In our SSP paradigm subjects performed an oddball task with two standard tones of different frequencies presented in each block. One of these tones (the tetanized stimulus or TS+) was also presented during a tetanic stimulation block. Tetanic stimulation is the presentation of a train of stimuli at a high rate (typically ≥10 Hz) and is used to induce LTP. For example, in classical studies of LTP in the hippocampus, tetanic stimulation consists of a train of electrical pulses and results in increased neuronal responses to a test stimulus. In SSP studies, tetanic stimulation consists of a train of brief sensory stimuli (e.g., tones or visual stimuli) that results in response enhancements that are specific to the TS+ compared with an untetanized stimulus (TS−). In this study, we assessed the ERPs to an auditory TS+ and TS− before and after tetanic stimulation in healthy control subjects (HC) and chronic schizophrenia patients (SZ). We report evidence that the plasticity of auditory processing mechanisms is abnormal in schizophrenia.
Methods and Materials
Subjects
This study was approved by the Institutional Review Boards of the Veterans Affairs Boston Healthcare System and Harvard Medical School. After a complete description of the study to the subjects, written informed consent was obtained. All subjects were paid for their participation.
Seventeen chronic SZ (aged 21–54 years; mean age = 42) and 15 HC (aged 21–54 years; mean age = 42) participated in the study. The SZ subjects were diagnosed according to DSM-IV criteria. Subjects were selected without regard for ethnicity and met our standard inclusion criteria: 1) aged between 18 and 55 years; 2) right-handed (so that possible hemispheric lateralization effects would not be obscured by left-handers with reduced or reversed functional laterality); 3) no history of electroconvulsive treatment; 4) no history of neurologic illness, including epilepsy; 5) no history of alcohol or drug dependence or abuse within the past year, nor long duration (>1 year) of past abuse (DSM-IV criteria); 6) no present medication for medical disorders that would have deleterious electroencephalogram (EEG), neurological, or cognitive functioning consequences; 7) verbal IQ above 75; 8) no alcohol use in the 24 hours before testing; and 9) English as a first language.
The data from 3 SZ were excluded because of technical problems, bad channels, or excessive artifacts, resulting in a final sample of 14 SZ and 15 HC. Demographic and clinical data are presented in Table 1. Schizophrenic symptoms were assessed using the Scale for the Assessment of Positive Symptoms (SAPS) (20) and the Scale for the Assessment of Negative Symptoms (21). The final SZ and HC groups did not differ in gender (all male), age, parental socioeconomic status (22), nor handedness (all right-handed) (23).The diagnostic composition of the SZ group was 7 paranoid, 4 undifferentiated, 2 schizoaffective, and 1 disorganized. Information on antipsychotic medication at the time of the experiment was available for 11 of the 14 SZ. Of these 11 patients, all were receiving atypical antipsychotics, and 1 patient was also receiving a typical antipsychotic. Antipsychotic medication dosages were converted to chlorpromazine equivalents using the values from Stoll (24) for typical and Woods (25) for atypical antipsychotics.
Table 1.
Demographic, Clinical, and Task Performance Data and Between-Group Comparisons for the Healthy Control (HC) and Schizophrenia Patient (SZ) Groups
| HC (n = 15) | SZ (n = 14) | Statistic | p | |
|---|---|---|---|---|
| Age (Years) | 42.1 ± 9.7 | 42.6 ± 10.6 | t[027] = −.14 | .89 |
| Parental Socioeconomic Status | 2.1 ± 1.2 | 2.9 ± 1.1 | t[25] = −1.6 | .13 |
| Age of Onset (Years) | 23.7 ± 5.7 | |||
| Positive Symptom Total (SAPS) | 9.5 ± 3.7 | |||
| Negative Symptom Total (SANS) | 10.9 ± 7.2 | |||
| Medication Dosage | 257 ± 184 | |||
| (Chlorpromazine Equivalents) | Range: 50–650 | |||
| % Error | ||||
| Pre Block 1 | 3.9 ± 4.9 | 17.0 ± 25.3 | ||
| Pre Block 2 | 5.0 ± 5.9 | 21.4 ± 24.0 | ||
| Baseline | 4.4 ± 4.9 | 19.2 ± 23.3 | ||
| Post Block 1 | 3.3 ± 4.2 | 22.0 ± 28.4 | ||
| Post Block 2 | 4.4 ± 10.0 | 16.1 ± 20.1 | ||
| Reaction Time (ms) | ||||
| Pre Block 1 | 372 ± 61 | 397 ± 69 | ||
| Pre Block 2 | 374 ± 64 | 423 ± 93 | ||
| Baseline | 373 ± 61 | 410 ± 76 | ||
| Post Block 1 | 381 ± 59 | 412 ± 83 | ||
| Post Block 2 | 381 ± 70 | 419 ± 67 |
Mean ± SD are given for each variable.
SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms.
Stimuli and Experimental Design
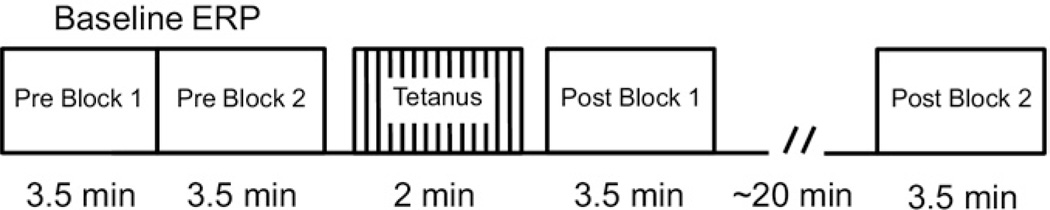
The structure of the SSP paradigm is diagrammed in Figure 1. Subjects performed an auditory oddball task while seated in a quiet room in a comfortable chair in front of a computer monitor. Stimuli were presented binaurally through headphones (50-msec duration, 70-dB sound pressure level, 800-msec stimulus onset asyn-chrony [SOA]). In the oddball task, subjects were instructed to look at the fixation cross on the monitor and to press a button with their right hand to target tones (440 Hz, 24 trials per block), which were embedded in a sequence with 1000 Hz (TS+) and 1500 Hz (TS−) standard tones (120 trials each per block). Subjects performed 2 pretetanus oddball blocks (Pre Blocks 1 and 2) before the tetanic stimulation block. During tetanic stimulation, 1000-Hz tones were presented at 11 Hz (91 msec SOA) for 2.4 min (1560 stimuli total) while subjects looked at the fixation cross on the monitor. Subjects then performed an oddball block immediately after the tetanic stimulation block (Post Block 1). After performing a visual discrimination task for ~20 min, subjects performed a final oddball block (Post Block 2).
Figure 1.
Experimental paradigm. In each block, an auditory oddball task was performed in which the standard stimuli consisted of tetanized (1000 Hz, TS+) and untetanized (1500 Hz, TS−) stimulus tones. Two blocks were performed both before and after the tetanus block. During the tetanus block, TS+ tones were presented at a rapid rate. Subjects performed an irrelevant visual discrimination task during the period between Post Blocks 1 and 2. The event-related potentials (ERPs) from Pre Blocks 1 and 2 were averaged into Baseline ERPs.
Electrophysiologic Recording and Processing
The EEG was recorded (.01 –100 Hz, 500-Hz digitization) with a Neuroscan Synamp amplifier using sintered Ag/Ag-Cl electrodes in an electrode cap at 64 standard scalp sites (26), nosetip, and left mastoid, referenced to the right mastoid, and grounded at AFz. Electrode impedances were < 10 kΩ. Bipolar vertical and horizontal electro-oculograms were recorded from electrodes above and below the right eye and at the left and right outer canthi, respectively. Because of electrode problems, 11 channels were excluded from further processing (F7/8, FT9/10, T9/T10, CP5/6, P9/10, and nosetip), leaving 54 scalp channels. Single-trial epochs were extracted from −250 to +772 msec relative to stimulus onset and corrected for ocular artifacts with independent component analysis (27). Next, epochs containing other artifacts were removed. The artifact criteria were as follows: 1) greater than ± 90 µ,V change in one time point and 2) amplitude range within an epoch exceeding 200 (µV. These criteria were visually tested and verified. Finally, the retained artifact-free single epochs were re-referenced to the average reference. The number of epochs retained per subject was (mean ± SD) 227 ± 10 for HC and 223 ± 9 for SZ, resulting in more than 100 trials for each standard (TS+ and TS−) on average.
Average ERPs were computed for the TS+ and TS− trials in each oddball block. Because the ERPs in Pre Blocks 1 and 2 did not differ (see Figures S1 and S2 in Supplement 1), these ERPs were averaged together into a single Baseline ERP for each tone condition. Difference waves for each tone condition were computed for each posttetanus block ERP minus the Baseline ERPs (Post Block 1 − Baseline, Post Block 2 − Baseline).
Statistical Analysis
Separate statistical analyses were conducted for each Post Block because visual inspection revealed that the ERP effects in each Post Block had very different spatial and temporal characteristics. Average ERP amplitudes were computed at channels and latency windows for effects of interest following visual inspection of the grand average ERPs. These measures were analyzed in mixed-design analyses of variance (ANOVAs) with the general design Group × Tone (TS+/TS−) × Pre/Post-Tetanus × Electrode (relevant electrodes) × Hemisphere. Significant interactions involving the factor Pre/Post in the omnibus ANOVAs were decomposed further in follow-up ANOVAs. The same analysis strategy was used for the task performance data (error rate and median reaction time). The Green-house-Geisser correction for inhomogeneity of variance (28) was applied for factors with more than two levels and is reflected in the reported p values. The nonparametric Spearman’s ρ was used for correlation analyses (two-tailed).
Results
Task Performance Data
The error rate and median reaction time data are presented in Table 1. In the Post Block 1 and Post Block 2 comparisons versus Baseline (average of Pre Block 1 and 2 data), SZ made more errors overall than HC [F(1,27) = 6.076, p < .05], but there were no plasticity effects (main or interaction effects involving the Pre/Post-Tetanus factor). For median reaction time, there were no significant between- or within-subject effects. One SZ was identified as an outlier because of excessive error rates (> 2 SD), but exclusion of this patient’s data did not alter any of the task performance results.
Baseline ERPs
The Pre Block 1 and 2 ERPs for each tone condition were characterized by the typical sequence of auditory ERPs (P50, N100, and P200 components), which did not differ between tones or blocks (see Figures S1 and S2 in Supplement 1). Therefore, the Pre Block 1 and 2 ERPs were averaged together into Baseline ERPs.
Post Block 1: Initial Plasticity Effects
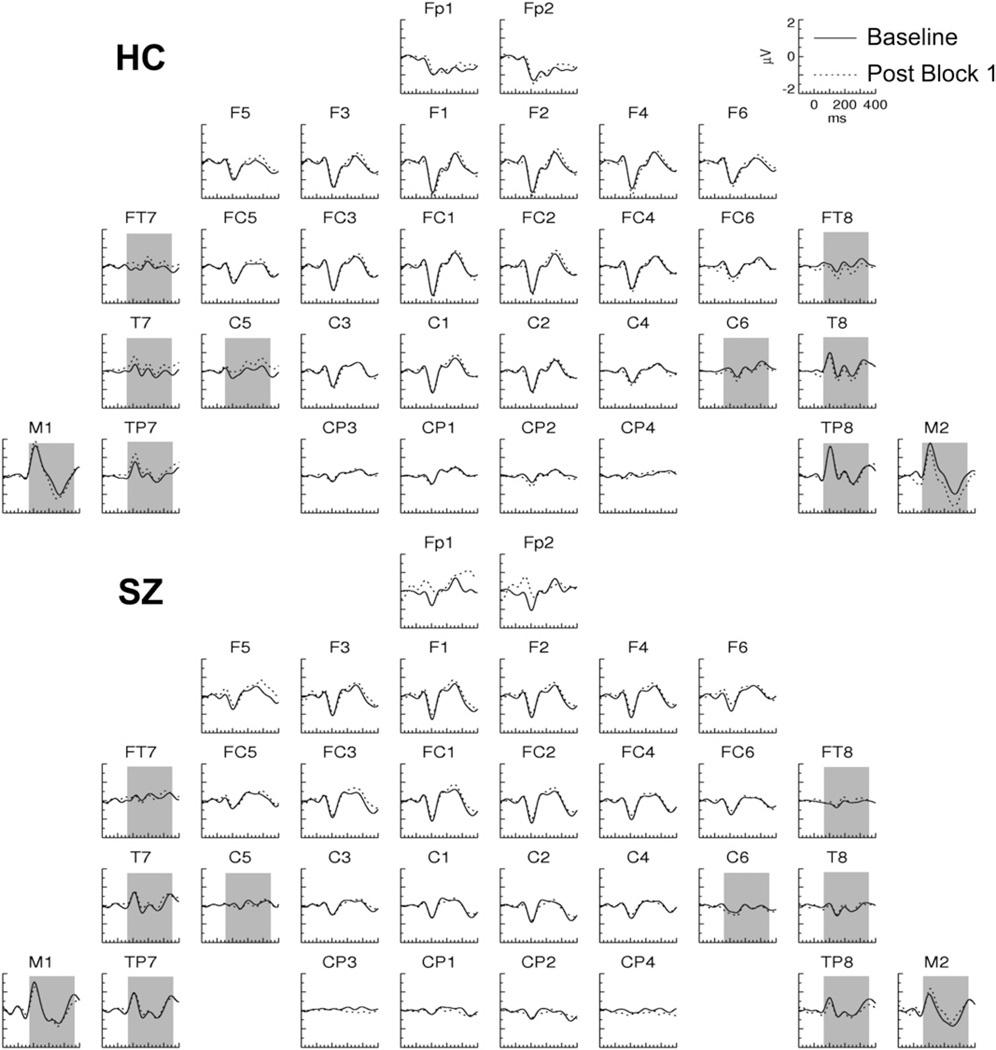
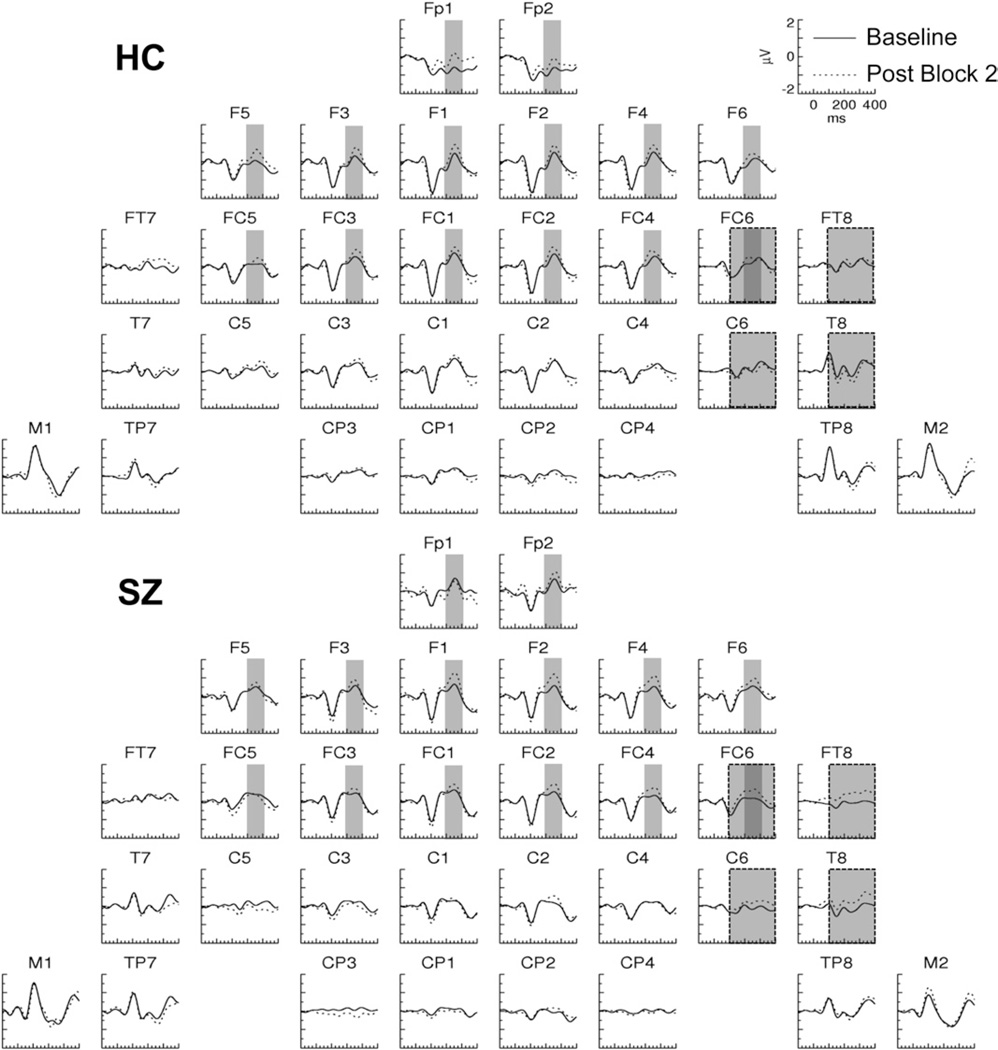
In Post Block 1, the ERPs evoked by the TS+ in HC were characterized by long-lasting shifts relative to the Baseline ERP (Figure 2). These shifts were positive at left frontotemporal electrodes and negative at right temporal electrodes and were not present in SZ (Figures 2 and 3A) nor in either group for the TS− (Figure 4). To assess these effects, ERP average amplitude was measured in the 60- to 350-msec period at frontotemporal (FT7/8), central (C5/6), temporal (T7/8), temporoparietal (TP7/8), and mastoid (M1/2) electrodes. In the omnibus ANOVA, there was a significant four-way interaction [Group × Tone × Pre/Post × Hemisphere: F(1,27) = 5.84, p < .05]. Decomposing this interaction for each tone condition, we found a significant interaction of Group × Pre/Post × Hemisphere for the TS+ [F(1,27) = 4.26, p < .05], but there were no significant interactions involving the Pre/Post factor for the TS−.
Figure 2.
Grand average event-related potentials evoked by the TS+ tones in Post Block 1 and the Baseline in healthy control (HC) and schizophrenia (SZ) subjects. The shaded area (60–350 msec) indicates the latency range and electrodes in which the plasticity effect was analyzed (see also Figure 4).
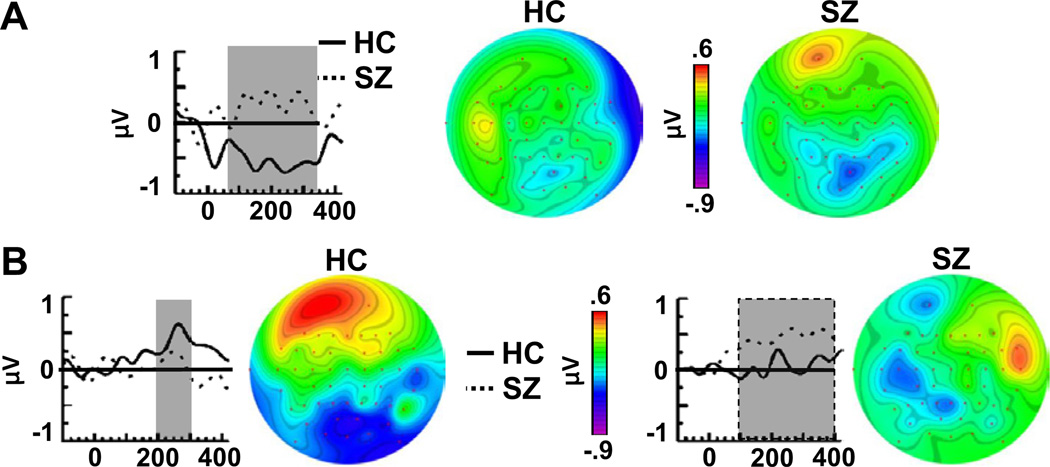
Figure 3.
Difference waves and topographic maps of stimulus-specific plasticity effects. (A) Tetanized (TS+) Post Block 1 minus Baseline difference waves for healthy control (HC) and schizophrenia (SZ) subjects at the right mastoid electrode (M2). (B) TS+ Post Block 2 minus Baseline difference waves for HC and SZ, plotted for the HC effect (left; F5 electrode) and the SZ effect (right; FT8 electrode).
Figure 4.
Grand average event-related potentials evoked by the untetanized tones in Post Block 1 and the baseline in healthy control (HC) and schizophrenia (SZ) subjects. The shaded area is as in Figure 2.
The three-way interaction for the TS+ condition was then separately decomposed for each group in two-factor ANOVAs. There was a significant Pre/Post × Hemisphere interaction for HC [F(1,14) = 7.10, p < .05] but not SZ [F(1,13) = .130, p = .724], who did not have any significant effects involving the Pre/Post factor. The main effect of Pre/Post was significant for HC at right hemisphere [F(1,14) = 5.20, p < .05] but not left hemisphere [F(1,14) = 1.21, p = .291] electrodes.
In the topographic maps of the Pre/Post effect in Figure 3A, a positive effect was apparent in SZ at the Fp1 electrode. To assess the significance of this effect, ERP average amplitude was measured in the 60- to 120-msec period at frontopolar (Fp1/2) sites. This effect, however, was not significant [Pre/Post in SZ: F(1,13) = .660, p = .431].
These results demonstrate that soon after tetanic stimulation, a plasticity effect appeared in HC that was not present in SZ. This plasticity effect occurred only for the TS+ and not the TS−, indicating that it was stimulus-specific. The topography of this SSP effect is consistent with a source in the right lateral temporal cortex.
Post Block 2: Delayed Plasticity Effects
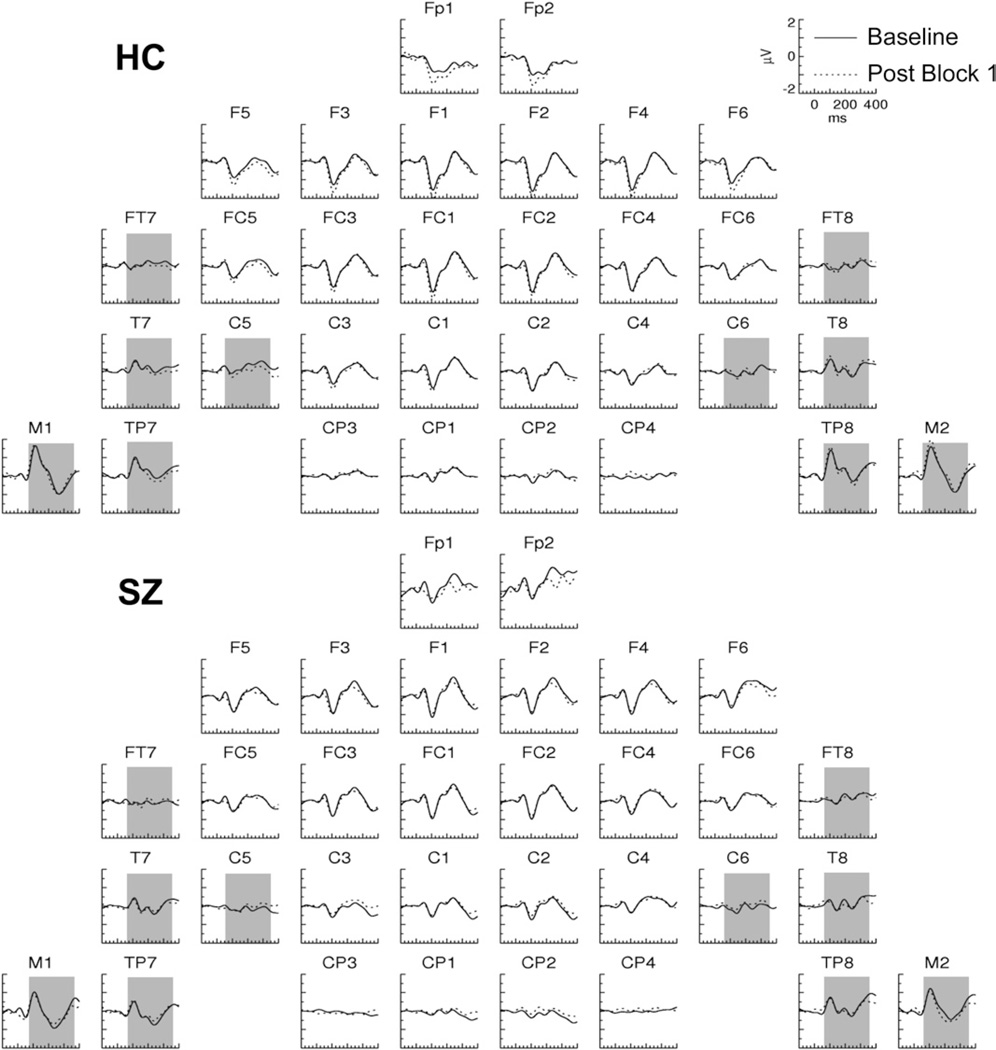
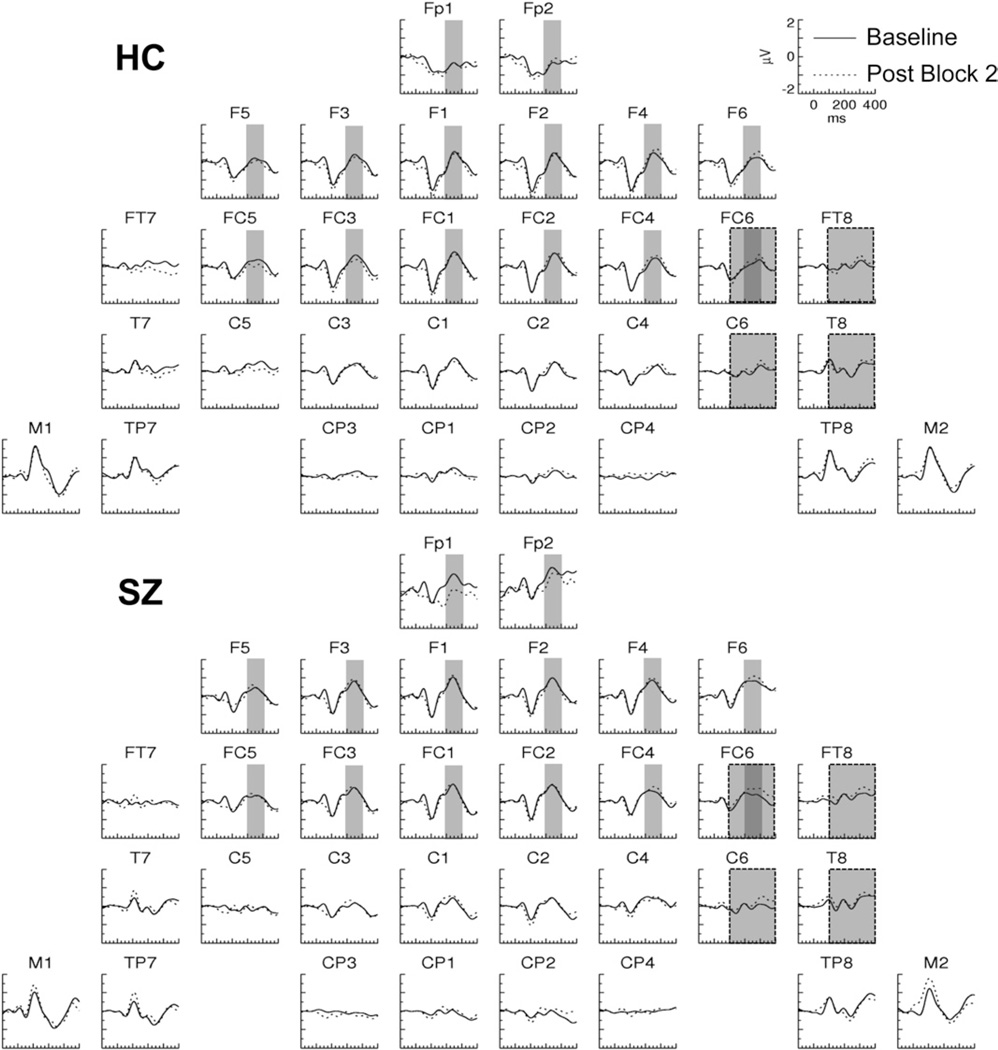
In Post Block 2, SSP effects with positive polarity and later onsets than the Post Block 1 effect were observed in both HCand SZ for the ERPs evoked by the TS+ (Figure 5). In HC, an effect was present at frontopolar sites, whereas in SZ, an effect was present at right frontotemporal sites (Figure 3B). No plasticity effects were apparent in the ERPs evoked by the TS− (Figure 6).
Figure 5.
Grand average event-related potentials evoked by the tetanized tones in Post Block 2 and the Baseline in healthy control (HC) and schizophrenia (SZ) subjects. The narrow and wide shaded areas represent the latency range and electrodes at which the respective HC (200–300 msec) and SZ (100–400 msec) plasticity effects were measured.
Figure 6.
Grand average event-related potentials evoked by the untetanized tones in Post Block 2 and the baseline in healthy control (HC) and schizophrenia (SZ) subjects. The shaded areas are as in Figure 5.
ERP average amplitude was first measured in a set of frontopolar (Fp1/2), frontal (F1/2, F3/4, F5/6), and frontocentral (FC1/2, FC3/4, FC5/6) electrode sites in the 200- to 300-msec period. The omnibus ANOVA yielded a significant two-way interaction [F(1,27) = 4.83, p <.05; Tone × Pre/Post]. Decomposition of this interaction revealed a significant Pre/Post effect for theTS + [F(1,27) = 7.56, p < .05] but not the TS− [F(1,27) = .010, p = .921 ]. The Pre/Post effect for TS+ was significant for HC [F(1,14) = 6.60, p < .05]. For SZ the Pre/Post main effect on the TS+ was not significant [F(1,14) = 2.12, p = .17] but a Pre/Post × Hemisphere interaction approached significance [F(1,13) = 3.62, p = .079].
Because the largest voltage differences in SZ were lateralized, ERP average amplitude was next measured in both subject groups in a SZ-optimized subset of lateral electrodes (FC5/6, FT7/8, C5/6, and T7/8) in the 100- to 400-msec period in which this effect was maximal. The omnibus ANOVA revealed a four-way interaction [Group ×Tone × Pre/Post × Hemisphere:F(1,27) = 6.181, p < .05]. For the TS+ but not the TS−, there was a significant interaction of Group × Pre/Post × Hemisphere [F(1,27) = 7.158, p < .05]. This interaction was further decomposed, revealing a significant Pre/ Post × Hemisphere interaction for SZ [F(1,27) = 8.453, p < .05] but not for HC [F(1,27) = 1.215, p = .289]. In SZ there was a Pre/Post effect at right hemisphere electrodes [F(1,13) = 8.587, p < .05] but not left hemisphere electrodes [F(1,13) = .217, p = .649]. (We note that this pattern of results remained significant when the SZ with the most positive effect was excluded as a potential outlier.)
These results demonstrate that approximately 20 min after tetanic stimulation, HC expressed an SSP effect that had a frontal topography, contrasting with the right temporal topography of their initial SSP effect. Although this frontal effect was not significant in SZ, the absence of a significant interaction with Group prevented us from concluding that this effect was absent in SZ.The SZ group did demonstrate an SSP effect at right frontotemporal electrodes that was absent in the HC group.
SSP Effect Correlations
Next we examined whether any of the SSP effects could be correlated with demographic variables or clinical measures: age and parental socioeconomic status for the HC and SZ groups; age of onset and medication dosage (in chlorpromazine equivalents) for SZ; and total Scale for the Assessment of Positive Symptoms (20) and Scale for the Assessment of Negative Symptoms (21) scores for SZ. There were no significant correlations for HC nor were there any correlations with clinical symptom ratings for SZ. Because SZ had a higher error rate than HC, we examined correlations between SSP effects and error rate in SZ, but none of these were significant (ps < |.497|,ps > .071).
Finally, we asked whether the SSP effects in Post Blocks 1 and 2 might be related to each other within the subject groups. Within each group, we examined correlations between 1)the right temporal Post Block 1 and 2 effects, 2) the right temporal Post Block 1 and frontal Post Block 2 effects, and 3) the frontal and right temporal Post Block 2 effects. In HC, none of the correlations reached significance. In SZ, however, there was a significant correlation between the right temporal Post Block 1 and 2 effects (ρ = .723, p < .05 Bonferroni-corrected). Inspection of the scatterplot (Figure S3 in Supplement 1) revealed that SZ with Post Block 1 values in the HC range had the lowest Post Block 2 values and vice versa. Thus, across SZ there appears to have been a tradeoff in the expression of right temporal SSP effects in Post Blocks 1 and 2. (Note that the polarity of the Post Block 1 effect in HC was negative, rendering the trade-off a positive rather than negative correlation.)
Discussion
Our findings suggest that auditory neuroplasticity is abnormal in chronic schizophrenia. In HC, we found two phases of SSP effects: 1) shortly after tetanic stimulation (Post Block 1), an initial SSP effect appeared at right temporal electrodes with an onset of ~60 msec. 2) Approximately 20 min after tetanic stimulation (Post Block 2), a later SSP effect was found with a bilateral frontal topography beginning at ~200 msec. In SZ, different patterns were observed: 1) no SSP effects were apparent in Post Block 1. 2) In Post Block 2, an SSP effect appeared at right frontotemporal electrodes, similar to the HC Post Block 1 effect. This effect had a somewhat later onset (~100 msec) and opposite polarity compared with the initial HC effect. These patterns suggest that in SZ, auditory SSP detected at right temporal electrodes took longer to develop than in HC. The right temporal SSP effect in SZ differed in latency and polarity from HC. It is not known whether the late frontal SSP effect found in HC might have appeared later in SZ.
Neuroplasticity effects have been observed in humans with different methodologies in a variety of experimental paradigms, including classical conditioning (29–31), perceptual learning (32,33), paired associative conditioning using transcranial magnetic stimulation (34), and tetanic stimulation (18,35–39), the latter of which is commonly used in the study of LTP in animal models (40). Tetanic stimulation has been shown to increase the amplitude of sensory- evoked activity in the visual (36–38) and auditory (18,41) modalities in humans (see Kirket al. for review [42]). In the visual modality, SSP effects on sensory-evoked activity have been reported for gratings of different orientations (37) or spatial frequencies following tetanic stimulation (43). To our knowledge, the present results are the first human ERP findings of auditory SSP following tetanic stimulation, because previous studies of neuroplasticity in humans using auditory tetanic stimulation (18,41) did not include a TS– as a control condition.
The auditory plasticity effect due to tetanic stimulation found by Clapp et al. (18) consisted of an increase in N1 amplitude. In contrast, the SSP effects observed here did not closely coincide with either the timing or the topography of classical auditory ERP components, so these effects cannot be clearly attributed to modulations of auditory evoked responses. Although our experimental paradigm was designed to detect SSP effects, we would have been able to observe the same posttetanus N1 amplitude enhancement as did Clapp et al. Because our study and Clapp et al. (18) are the only ERP studies of which we are aware to examine plasticity in the auditory system as a result of tetanic stimulation, the discrepancy between our HC findings and those of Clapp et al. needs to be explained.
The most obvious difference between the two studies is the addition of the TS− in our paradigm. One possibility is that the TS− interacted with plasticity mechanisms affecting the representation of the TS+, perhaps influencing changes to auditory cortex tono-topic representations, but we are not aware of any evidence that might address this hypothesis. Additionally, there were differences in sample size (15 HC here vs. 6 subjects per group in Experiment 1 of Clapp et al.), subject age (our HC were 42.1 years of age, the subjects of Clapp et al. were 28.6 years), tetanus frequency (11 Hz here vs. 13 Hz in Clapp et al.), SOA in the oddball blocks (800 msec here, 2200 on average in Clapp et al.), and SOA randomization (SOAs were constant here but randomized from 1800–2200 msec in Clapp et al.). Both studies used the average reference, but differences in the electrode montage from which this reference is computed could in theory result in different plasticity effect topographies. Further research is necessary to fully characterize the effects of tetanus-induced plasticity on auditory ERPs.
The functional significance of the SSP effects we observed is not clear and needs to be investigated in future studies. One question is the degree to which these effects are related to auditory perception. Several studies have found that the auditory P2 component is modulated by perceptual learning (33,44–46), and the frontal Post Block 2 effect in HC bears similarity to some of these P2 effects in terms of direction, latency, and topography. Also, we cannot exclude the possibility that tetanic stimulation influenced the salience of the TS+ to the subjects, possibly leading to differences in the allocation of attention to the TS+ and the TS−, although the SSP effects did not resemble any attentional effects of which we are aware.
The right temporal Post Block 1 and 2 SSP effects were correlated across SZ, showing evidence of a trade-off: patients with normal-range Post Block 1 effects had small Post Block 2 effects and vice versa. No such pattern was found for HC. Thus, the degree to which SZ showed a delayed right temporal plasticity effect with reversed polarity in Post Block 2 varied depending on the degree of “preservation” of their Post Block 1 effect. No such pattern of individual differences was found for the frontal Post Block 2 effect, which was not significant in SZ.One interpretation of these findings is that the delayed right temporal Post Block 2 effect in SZ represents a compensatory plasticity mechanism, perhaps invoked by homeostatic plasticity processes.
Some limitations of this study are immediately apparent. First, the sample size was relatively small and did not include any women. The present findings need to be replicated with a larger sample size and more balanced sample with regard to gender. Also, the use of data-driven and randomization-based analysis approaches would be helpful to increase sensitivity in detecting plasticity effects, because this is a novel paradigm.
Rapidly induced and noninvasively measurable from the scalp, ERP measures of SSP may be useful for studying and assessing neuroplasticity in schizophrenia (47– 49). Abnormal neuroplasticity has been hypothesized to be an important substrate of schizophrenia (10,11), and neuropathologic evidence points to disturbed plasticity at the synaptic level (50), but to date there have been relatively few methods available for assessing neuroplasticity mechanisms directly in living patients. The present study demonstrates that auditory SSP may be impaired in schizophrenia and that auditory SSP effects may be useful biomarkers of not only neuroplasticity mechanisms but also antipsychotic treatment efficacy. Furthermore, these data add to the considerable body of evidence of auditory system abnormalities in schizophrenia.
Supplementary Material
Acknowledgments
This study was supported by grants to KMS from the U.S. Department of Veterans Affairs (Merit Review Award CX000154) and the U.S. National Institutes of Health (Grant Nos. R03 MH076760, R01 MH080187).
Footnotes
Preliminary results were presented at the 2010 and 2011 Annual Meetings of the Society for Biological Psychiatry.
KMS designed and conducted the study. RPM and KMS analyzed the data. RPM and KMS wrote the paper.
RPM reported no biomedical financial interests or potential conflicts of interest. KMS reported receiving consultation fees from Galenea Inc. and Bristol-Myers Squibb.
Supplementary material cited in this article is available online.
References
- 1.Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: A magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chance SA, Casanova MF, Switala AE, Crow TJ. Auditory cortex asymmetry, altered minicolumn spacing and absence of ageing effects in schizophrenia. Brain. 2008;131:3178–3192. doi: 10.1093/brain/awn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia—a critical review. Psychiatry Res. 2008;161:259–274. doi: 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 7.Javitt DC. When doors of perception close: Bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayani TH, David AS. The auditory hallucination: A phenomenological survey. Psychol Med. 1996;26:177–189. doi: 10.1017/s003329170003381x. [DOI] [PubMed] [Google Scholar]
- 9.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 10.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 11.Stephan KE, Friston KJ, Frith CD. Dysconnection inschizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 13.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: The final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: Toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 15.Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann NY Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- 16.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry. 2008;65:378–385. doi: 10.1001/archpsyc.65.4.378. [DOI] [PubMed] [Google Scholar]
- 17.Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex. 2008;18:990–996. doi: 10.1093/cercor/bhm151. [DOI] [PubMed] [Google Scholar]
- 18.Clapp WC, Kirk IJ, Hamm JP, Shepherd D, Teyler TJ. Induction of LTP in the human auditory cortex by sensory stimulation. Eur J Neurosci. 2005;22:1135–1140. doi: 10.1111/j.1460-9568.2005.04293.x. [DOI] [PubMed] [Google Scholar]
- 19.Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30:16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: The University of Iowa; 1984. [Google Scholar]
- 21.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: The University of Iowa; 1983. [Google Scholar]
- 22.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Stoll AL. The Psychopharmacology Reference Card. Belmont, MA: McLean Hospital; 2001. [Google Scholar]
- 25.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 26.Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am J EEG Technol. 1985;25:83–92. [Google Scholar]
- 27.Makeig S, Bell AJ, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. In: Touretzky DS, editor. Advances in Neural Information Processing Systems. Cambridge, MA: MIT Press; 1996. pp. 145–151. [Google Scholar]
- 28.Keselman HJ, Rogan JC. Repeated measures F tests and psycho-physiological research: Controlling the number of false positives. Psy-chophysiology. 1980;17:499–503. doi: 10.1111/j.1469-8986.1980.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 29.Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc Biol Sci. 1998;265:649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moses SN, Martin T, Houck JM, Ilmoniemi RJ, Tesche CD. The C50m response: Conditioned magnetocerebral activity recorded from the human brain. Neuroimage. 2005;27:778–788. doi: 10.1016/j.neuroimage.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Stolarova M, Keil A, Moratti S. Modulation of the C1 visual event-related component by conditioned stimuli: Evidence for sensory plasticity in early affective perception. Cereb Cortex. 2006;16:876–887. doi: 10.1093/cercor/bhj031. [DOI] [PubMed] [Google Scholar]
- 32.Bao M, Yang L, Rios C, He B, Engel SA. Perceptual learning increases the strength of the earliest signals in visual cortex. J Neurosci. 2010;30:15080–15084. doi: 10.1523/JNEUROSCI.5703-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-David BM, Campeanu S, Tremblay KL, Alain C. Auditory evoked potentials dissociate rapid perceptual learning from task repetition without learning. Psychophysiology. 2011;48:797–807. doi: 10.1111/j.1469-8986.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 34.Bliem B, Muller-Dahlhaus JF, Dinse HR, Ziemann U. Homeostatic metaplasticity in the human somatosensory cortex. J Cogn Neurosci. 2008;20:1517–1528. doi: 10.1162/jocn.2008.20106. [DOI] [PubMed] [Google Scholar]
- 35.van den Broeke EN, van Rijn CM, Biurrun Manresa JA, Andersen OK, Arendt-Nielsen L, Wilder-Smith OH. Neurophysiological correlates of nociceptive heterosynaptic long-term potentiation in humans. J Neurophysiol. 2010;103:2107–2113. doi: 10.1152/jn.00979.2009. [DOI] [PubMed] [Google Scholar]
- 36.Clapp WC, Zaehle T, Lutz K, Marcar VL, Kirk IJ, Hamm JP, Teyler TJ, Corballis MC, Jancke L. Effects of long-term potentiation in the human visual cortex: A functional magnetic resonance imaging study. NeuroReport. 2005;16:1977–1980. doi: 10.1097/00001756-200512190-00001. [DOI] [PubMed] [Google Scholar]
- 37.Ross RM, McNair NA, Fairhall SL, Clapp WC, Hamm JP, Teyler TJ, Kirk IJ. Induction of orientation-specific LTP-like changes in human visual evoked potentials by rapid sensory stimulation. Brain Res Bull. 2008;76:97–101. doi: 10.1016/j.brainresbull.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Teyler TJ, Hamm JP, Clapp WC, Johnson BW, Corballis MC, Kirk IJ. Long-term potentiation of human visual evoked responses. Eur J Neurosci. 2005;21:2045–2050. doi: 10.1111/j.1460-9568.2005.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: A combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaehle T, Clapp WC, Hamm JP, Meyer M, Kirk IJ. Induction of LTP-like changes in human auditory cortex by rapid auditory stimulation: An FMRI study. Restor Neurol Neurosci. 2007;25:251–259. [PubMed] [Google Scholar]
- 42.Kirk IJ, McNair NA, Hamm JP, Clapp WC, Mathalon DH, Cavus I, Teyler TJ. Long-term potentiation of human sensory-evoked potentials. WIREs Cogn Sci. 2010;1:766–773. doi: 10.1002/wcs.62. [DOI] [PubMed] [Google Scholar]
- 43.McNair NA, Clapp WC, Hamm JP, Teyler TJ, Corballis MC, Kirk IJ. Spatial frequency-specific potentiation of human visual-evoked potentials. NeuroReport. 2006;17:739–741. doi: 10.1097/01.wnr.0000215775.53732.9f. [DOI] [PubMed] [Google Scholar]
- 44.Alain C, Campeanu S, Tremblay K. Changes in sensory evoked responses coincide with rapid improvement in speech identification performance. J Cogn Neurosci. 2009;22:392–403. doi: 10.1162/jocn.2009.21279. [DOI] [PubMed] [Google Scholar]
- 45.Ross B, Tremblay K. Stimulus experience modifies auditory neuromagnetic responses in young and older listeners. Hear Res. 2009;248:48–59. doi: 10.1016/j.heares.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liebenthal E, Desai R, Ellingson MM, Ramachandran B, Desai A, Binder JR. Specialization along the left superior temporal sulcus for auditory categorization. Cereb Cortex. 2010;20:2958–2970. doi: 10.1093/cercor/bhq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clapp WC, Hamm JP, Kirk IJ, Teyler TJ. Translating long term potentiation from animals to humans: a novel method for noninvasive assessment of cortical plasticity. Biol Psychiatry. 2012;71:496–502. doi: 10.1016/j.biopsych.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.C¸ avus¸ I, Reinhart R, Roach BJ, Gueorguieva R, Teyler TJ, Clapp WC, et al. Impaired visual cortical plasticity in schizophrenia. Biol Psychiatry. 2012;71:512–520. doi: 10.1016/j.biopsych.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooke SF, Bear MF. Stimulus-selective response plasticity in the visual cortex:an assay for the assessment of pathophysiology and treatment of cognitive impairment associated with psychiatric disorders. Biol Psychiatry. 2012;71:487–495. doi: 10.1016/j.biopsych.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Fung SJ, Sivagnanasundaram S, Weickert CS. Lack of change in markers of presynaptic terminal abundance alongside subtle reductions in markers of presynaptic terminal plasticity in prefrontal cortex of schizophrenia patients. Biol Psychiatry. 2011;69:71–79. doi: 10.1016/j.biopsych.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.