Abstract
Epilepsy is a condition affecting 1–2% of the population, characterized by the presence of spontaneous, recurrent seizures. The most common type of acquired epilepsy is temporal lobe epilepsy (TLE). Up to 30% of patients with TLE are refractory to currently available compounds, and there is an urgent need to identify novel targets for therapy. Here, we utilized the in-vitro CA3 burst preparation to examine alterations in network excitability, characterized by changes in interburst interval and bursting thresholds. Specifically, we show that bath application of three different sodium channel blockers—diphenytoin, riluzole, and lidocaine—slow spontaneous CA3 bursts. This in turn, decreased the epileptiform activity. These compounds work at different sites on voltage-gated sodium channels, but produce a similar network phenotype of decreased excitability. In the case of diphenytoin and riluzole, the change in network activity (i.e., increased interburst intervals) was persistent following drug washout. Lidocaine application, however, only increased the CA3 interburst interval when it was in the bath solution. Thus, its action was not permanent and resulted in returning CA3 bursting to baseline levels. These data demonstrate that the CA3 burst preparation provides a relatively easy and quick platform for identifying compounds that can decrease network excitability, providing the initial screen for further and more complex in-vivo, freely-behaving animal studies.
Keywords: epilepsy, hyperexcitability, CA3, diphenytoin, riluzole, lidocaine
1. INTRODUCTION
Although several antiepileptic drugs (AED) have been introduced in the last decade, about 30% of patients with epilepsy are still treatment insensitive for their seizures. This results in significant morbidity and mortality for these patients (Brodie, 2005; Kwan and Brodie, 2000; Stables et al., 2003; Devinsky, 1999). The current methods that are used to screen new AEDs are very labor intensive and expensive, making it difficult to identify potential compounds in a timely manner. Nonetheless, these screening methods have yielded promising compounds. Here, we used a relatively quick and effective screening method – extracellular recordings of spontaneous CA3 bursts – to evaluate currently available and novel AEDs. We propose that this relatively inexpensive and initial screening method may be able to validate and identify promising pharmacologic or molecular targets that stop interictal burst-like activity in the hippocampal slice prior to performing the more complex in-vivo screening techniques needed to confirm effectiveness.
The CA3 hippocampal network is a frequently studied model of synchronous network activity (Traub and Wong, 1982). Specifically, when a single CA3 pyramidal cell is excited, the entire network can synchronize due to its positive feedback comprised of action potential-dependent glutamate release and result in a “burst” (Miles and Wong, 1983; Traub and Dingledine, 1990). This type of bursting can be elicited by removal of GABAA inhibition, alteration of extracellular ion concentration (e.g., increase extracellular potassium), or high-frequency stimulation (Bains et al., 1999; Dulla et al., 2005; Dulla et al., 2009; Hellier et al., 2007; Miles and Wong, 1983; Stasheff et al., 1989; Stasheff et al., 1985; Yee et al., 2003). Spontaneous CA3 burst activity resembles interictal electroencephalogram (EEG) spikes observed in humans with epilepsy, and is an in-vitro model of interictal spikes (Traub and Miles, 1991). Previously, we have used this model of interictal spikes to determine mechanisms for: 1) burst termination, 2) burst initiation, and 3) modulation of burst frequency (Bains et al., 1999; Hellier et al., 2007; Jones et al., 2007; Staley et al., 1998, 2001; Yee et al., 2003).
The pattern of CA3 bursting is of interest because it reflects network parameters including the strength of the recurrent collateral synapses, the degree of inhibition, and the degree of recurrent collateral connectivity (Bains et al., 1999; Behrens et al., 2005; Cohen et al., 2006; Staley et al., 1998; Traub et al., 1987; Yee et al., 2003). Further, it has been hypothesized that interictal spikes drive epileptogenesis (i.e., strengthening of synaptic connections between neurons in epileptic foci measured by interictal spikes), thus sustaining an epileptic/hyperexcitable phenotype (Staley et al., 2005; Staley and Dudek, 2006). By modulating CA3 bursting, we may be able to provide insight into novel anticonvulsant and/or antiepileptogenic targets. Therefore, the in-vitro slice preparation is poised as a platform for initial screening and/or identification of novel molecular targets to treat epilepsy.
Here, we test the hypothesis that CA3 burst timing is altered by three compounds that act upon sodium channels: diphenytoin (DPH), riluzole (RLZ), and lidocaine (LIDO). We analyzed changes in interburst interval, burst length, and CA3 burst synchronization. In general, DPH has been identified as a prototypic, sodium channel, anticonvulsant compound and has been used clinically for partial and generalized seizures (Griffith and Taylor, 1988a; Johannessen, 2002; Korn et al., 1987; Levine and Chang, 1990; Oliver et al., 1977; Schneiderman and Evans, 1986; Schneiderman and Schwartzkroin, 1982). RLZ has been shown to block the persistent sodium current (INaP; Cheah et al., 2010; Harvey et al., 2006; Miles et al., 2005; Theiss et al., 2007; Urbani and Belluzzi, 2000) whereas, LIDO has demonstrated the ability to inhibit voltage-gated sodium channels during abnormal membrane depolarization (Ragsdale et al., 1996). From these different mechanisms of blocking hippocampal sodium channels, we predict that each of these compounds will slow CA3 bursts.
2. METHODS
2.1 Electrophysiological slice preparation
The care and use of all animals were in accordance with the guidelines prepared by the Institutional Committee on Care and Use of Laboratory Animals, University of Colorado Anschutz Medical Campus. Hippocampal slices were obtained from naïve adult (4–6 week old) male Sprague Dawley rats (Harlan). Animals were anesthetized with sodium pentobarbital and then quickly decapitated. The hippocampus was dissected from the whole brain and 400-micron slices were cut from the middle third of the hippocampus with a McIlwain tissue chopper. Slices were initially transferred to a submersion-holding chamber and maintained at 33 degrees C to equilibrate.
After at least 1 hour, slices were transferred to a 1-ml submersion recording chamber where they were placed on a nylon net and superfused at 2ml/min with artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 2.5 KCl, 2.0 MgCl2, 2.0 CaCl2, 1.2 NaH2PO4, 1 glucose, and 26.0 NaHCO3. ACSF was saturated with 95% O2 - 5% CO2.
2.2 Field potential recordings and data analysis
Extracellular recordings were made using glass pipettes pulled on a Narishige electrode puller (Tokyo, Japan) and filled with 150 mM NaCl. For recording spontaneous bursts, a bipolar stimulating electrode and the recording electrode were placed under visual guidance in the stratum pyramidal of the CA3 region (Figure 1). Electrodes were adjusted so that large-amplitude population spikes were elicited as an indication of slice viability and proper electrode positioning. The placement of the recording electrode remained unchanged for the duration of the experiment. Recordings using a Mod DAM 5a Differential Preamplifier (WPI) were digitized at 2 kHz on a PCI-DAS 1602/16 Board (Measurement Computing, Middleboro, MA) using routines written in Visual Basic 6.0 (Microsoft, Seattle, WA) by Kevin Staley, MD.
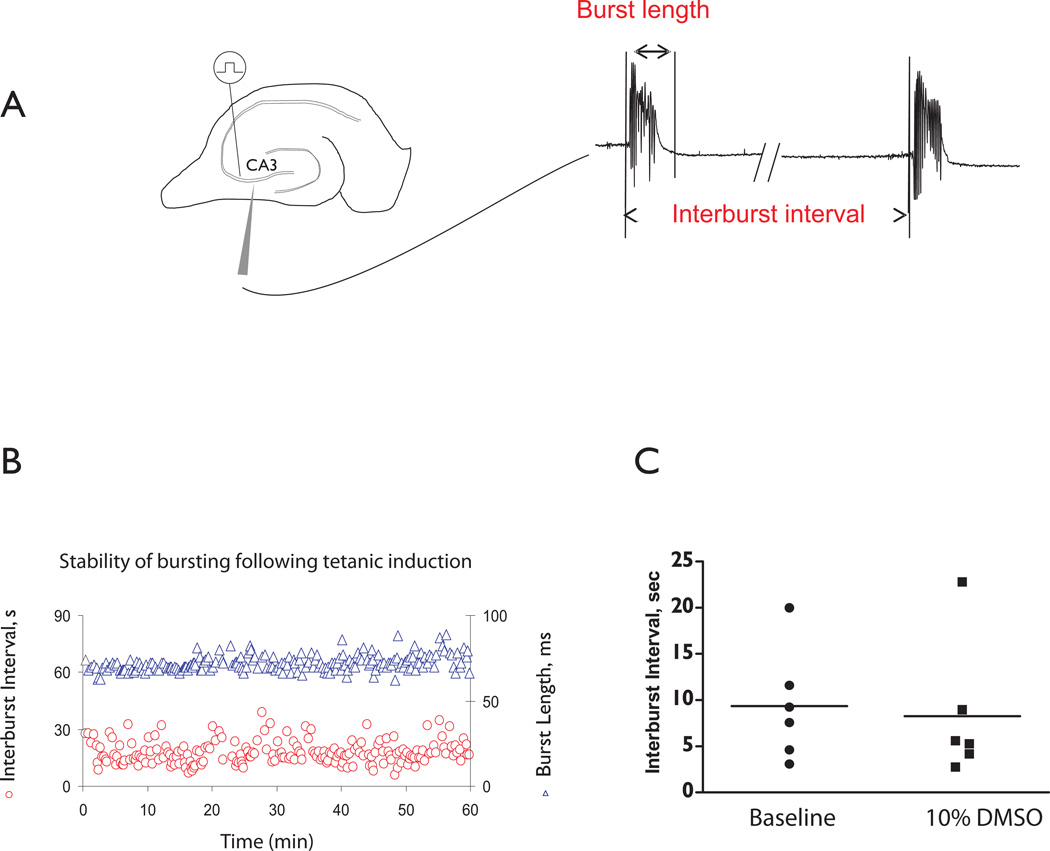
Figure 1. Properties of CA3 population bursts.
A. Left panel: Extracellular recording setup to measure CA3 population bursts. We induced spontaneous bursting of the CA3 pyramidal cell network using tetanic stimulation. Right panel: CA3 network activity in terms of interburst interval and burst length. B. A representative experiment showing that CA3 bursts are stable for up to one hour following tetanic stimuli. C. Interburst interval is not altered when 10% DMSO is added to the ACSF compared to baseline bursting (n=6, Paired T-test, p=0.16).
2.3 Drug preparation
Stock solutions of the DPH were dissolved in dimethyl sulfoxide (DMSO) then diluted with ACSF making a final concentration of 20 to 40ìM DPH. The working concentration of DMSO was kept below 0.01% for each drug application. We examined the role of DMSO on interburst interval for doses up to 10%. There was no significant difference in the interburst interval (n=6 slices; Fig. 1C). Subsequently, we conducted experiments using 0.01% DMSO as this allowed us to make aliquots of higher concentration prior to adding to the bathing solution. After a baseline period, generally 30–60 minutes, 20 or 40ìM DPH was bath-applied to a slice. In all experiments, DPH application continued for at least 50 minutes and was followed by a washout period of at least 20 minutes but most experiments had > 60 min of wash.
RLZ was dissolved in water to make a 10mM stock solution and LIDO was dissolved in water to make a 100mM stock solution. RLZ was applied to the slices at either 5 or 10ìM while LIDO was applied at either 10 or 100ìM. Each drug concentration was delivered to a single slice experiment with a Razel pump into the ACSF bath solution. Washout of these drugs were between 20–60 min long with most experiments having >50 min of wash.
2.4 Burst induction
Tetanic stimulation (100 Hz, 1 second) of recurrent CA3 synapses produced a long-term increase in the strength of recurrent CA3 synapses and induced stable spontaneous CA3 bursting (Bains et al., 1999; Hellier et al., 2007; Yee et al., 2003). If a single tetanus did not induce bursting, it was repeated after a 10-min interval. When tetanus was used to induce bursting, ACSF was modified with: 1.3 mM Ca2+, 0.9 mM Mg2+, and 3.3 mM K+. This preparation allowed for examination of AEDs in the setting of intact excitatory and inhibitory synaptic transmission. We have previously shown that bursting following tetanic stimulation produces bursting that is stable for several hours (Hellier et al., 2007; Yee et al., 2003).
2.5 Data analysis
2.5.1 Calculation of interburst interval and burst lengths
Calculations of interburst interval and burst lengths were determined as previously described (Hellier et al., 2007; Staley et al., 1998; Yee et al., 2003). Generally, burst length was calculated as the time during which the absolute value of the burst was above three times baseline noise, while interburst interval was calculated as the time from the start of a burst to the beginning of the next burst (Fig. 1A, right panel).
2.5.2 Statistical analysis
CA3 burst length and interburst intervals vary between slices (Bragdon et al., 1986; Hellier et al., 2007, 2009; Korn et al., 1987; Mueller and Dunwiddie, 1983; Sagratella, 1998; Scharfman, 1994; Swartzwelder and Wilson, 1987; Whittington and Jefferys, 1994; Yee et al., 2003); thus, we normalized the data to the baseline mean of each experiment. Means ± standard error (s.e.m.) were calculated from the last 10 minutes of each paradigm, when interburst intervals and burst lengths were stable. Additionally, percent change from the normalized data was calculated as [100 * (drug-control)/control]. Statistical analyses were performed using GraphPad Prism 5 software. One-way ANOVA with Tukey’s Multiple Comparison Test assessed significant differences between normalized baseline, drug application, and wash for: 1) interburst intervals, and 2) burst lengths. Spontaneous bursting was defined as “stopped” if no further spontaneous bursting occurred after a 10-minute observation period. Student’s T-test was performed when comparing interburst intervals between baseline and 10% DMSO (Figure 1C) as well as for “stopped” grouped experiments. Significance for all statistical analyses was accepted when p < 0.05.
3. RESULTS
3.1 DPH mediates long-term alterations in CA3 interburst intervals and burst lengths
DPH is a broad-spectrum anticonvulsant compound and has been previously shown to block high frequency firing of voltage-gated sodium channels (Tunnicliff, 1996). Thus based upon its mechanisms of action, we hypothesized that DPH would slow CA3 bursts (i.e., increase interburst intervals) in a use dependent fashion, as spontaneous CA3 bursts are comprised of a depolarization wave with an overriding barrage of action potentials.
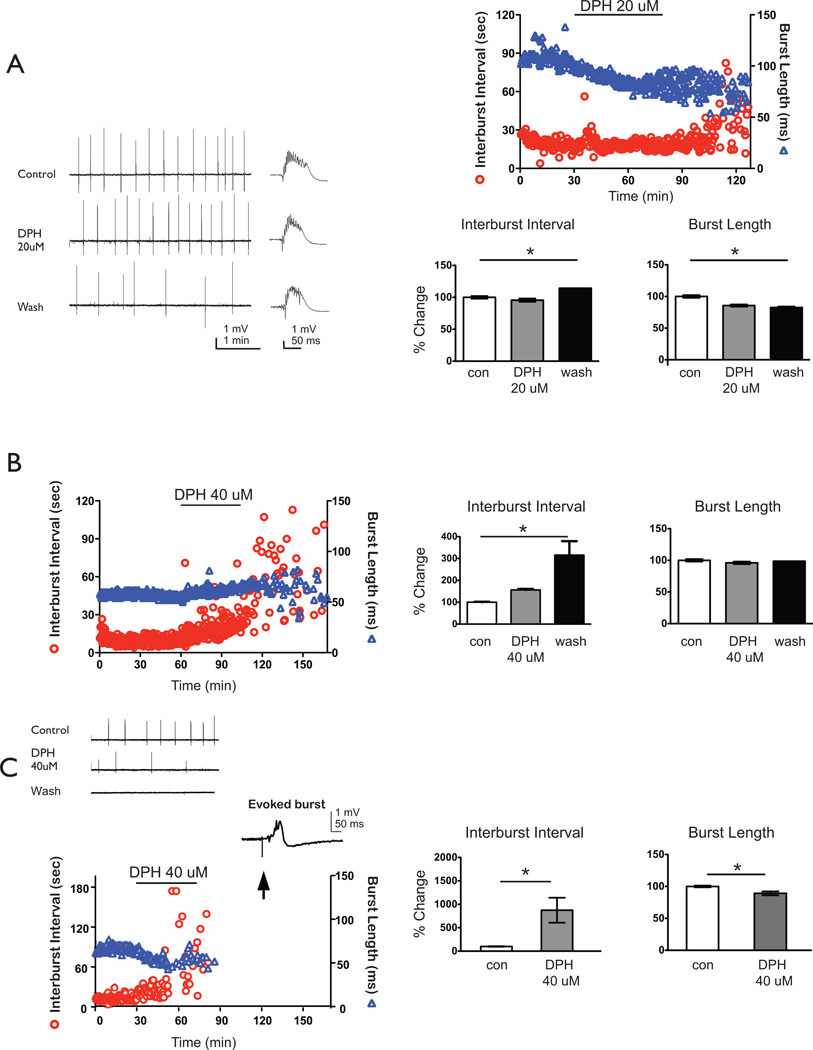
Following application and washout of 20ìM DPH, CA3 interburst intervals significantly increased while burst lengths significantly decreased compared to baseline bursting (n = 10; Fig. 2A). However, when 40ìM DPH was applied to bursting hippocampal slices, two sets of results were observed either a slowing (n=4 of 13; 31% of slices) or a stopping (n=9 of 13; 69% of slices) of bursts. In the slowing group during the washout period, interburst intervals significantly increased and burst lengths remained relatively the same compared to baseline bursts (Fig. 2B). For the stopping group, however, both interburst intervals and burst lengths were significantly altered compared to baseline prior to the spontaneous termination of bursting (Fig. 2C). To ensure that the slices were still viable, we stimulated 30 min after cessation of bursting and recorded robust field potentials.
Figure 2. Interburst interval of CA3 bursts increases following application of DPH and is most pronounced during washout periods.
A. Representative raw tracings of CA3 population burst during baseline, DPH application, or wash conditions. Single bursts on an enlarged timescale are displayed to the right of the prolonged tracing. 20 ìM DPH significantly increased interburst intervals from baseline to wash by 14 ± 1% mean ± s.e.m.] and significantly decreased burst length by 18 ± 1% (ANOVA, Tukey, p<0.05, n = 10). B. In a subset of experiments, 40 ìM DPH application significantly slowed bursting from baseline to wash (interburst interval: 316 ± 64%, ANOVA, Tukey, p<0.05, n=4) but did not decrease burst lengths (9 ± 2%). C. In the remaining experiments, 40 ìM DPH application stopped bursting, and interburst intervals significantly increased by 1198 ± 375% from baseline while burst length decreased by 11 ± 5% (ANOVA, Tukey, p<0.05, n = 9). Representative data set of interburst intervals (depicted as circles; left axis) and burst length (triangles; right axis). Representative raw tracings of CA3 population burst during baseline, DPH application, or wash conditions. Single evoked burst on an enlarged timescale is displayed to the right of the prolonged tracing to show slice viability after spontaneous bursting stopped. Arrow shows the stimulus artifact. Summary bar graphs depict pooled normalized data with % changes from baseline in the interburst interval (left) and burst length right). Solid lines show statistical differences: baseline (con) vs. wash. * denotes p<0.05.
3.2 RLZ also mediates long-term alterations in CA3 interburst intervals and burst lengths
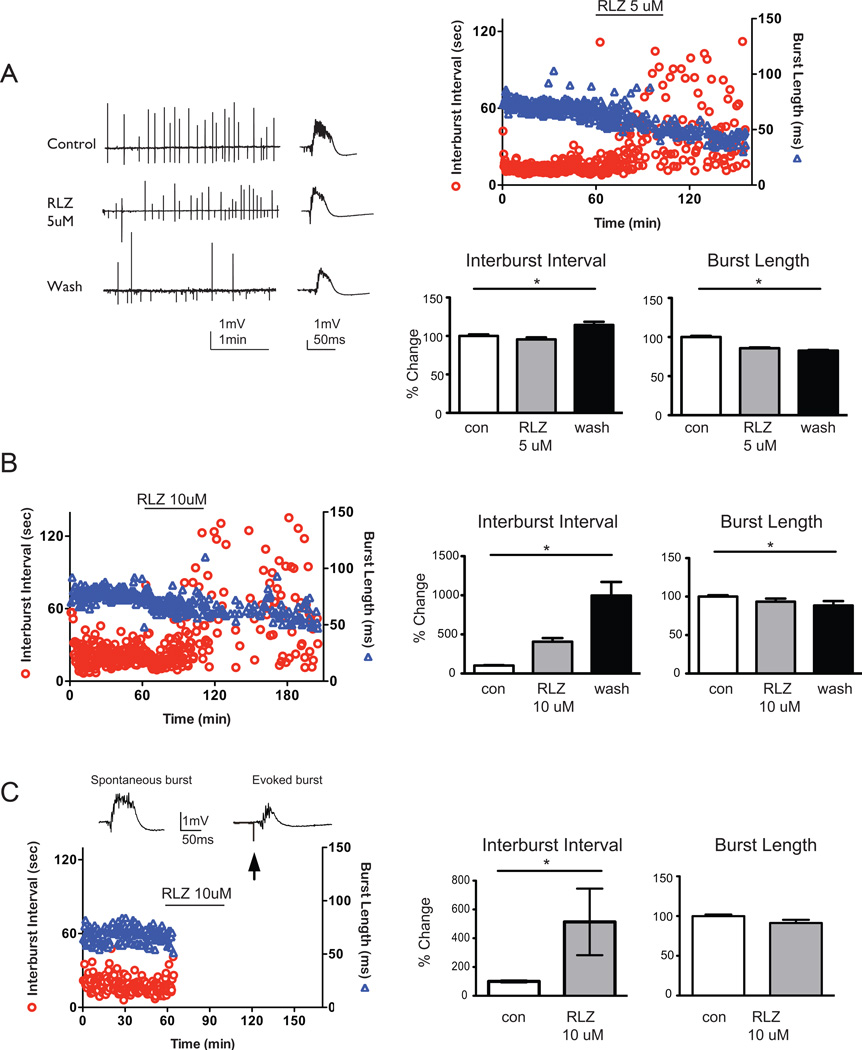
We next examined the role of RLZ, which is a sodium channel blocker and is used clinically as a neuroprotective agent. RLZ preferentially blocks the INaP (Spadoni et al., 2002), thus we hypothesize that bursting will be slower as the persistent sodium current (INaP) is prevented from activating. As observed with DPH, application and washout of 5 ìM RLZ significantly increased interburst intervals and decreased burst length compared to baseline bursting (n=10, Figure 3A). When a higher dose of RLZ (10 ìM) was applied to spontaneous CA3 bursts, two sets of results were seen either a slowing (n=3 of 9; 33% of slices) or a stopping (n=6 of 9; 67% of slices) of bursts (Figure 3B,C). In the slowing group following wash of 10 ìM RLZ, similar results previously seen in 5 ìM RLZ were also observed with significant alterations in interburst interval and burst length compared to baseline bursting (n=3, Figure 3B). In the stopping group, bursting ceased within the first 20 min of 10 ìM RLZ application. Prior to cessation of bursting with 10 ìM RLZ, there was a significant change in the interburst interval but not in the burst duration compared to baseline. After 10 minutes of no bursting, we evoked field responses to test for slice viability, and in each case, a robust field response was present (n=6, Figure 3C). These data suggest that the application and washout of RLZ induced a stabilization of CA3 synaptic strength reflected by persistent increase in interburst intervals (i.e., the interburst intervals did not return to baseline conditions) as well as shorter burst lengths.
Figure 3. Interburst interval of CA3 bursts increases following application of RLZ.
A. Representative raw tracings of CA3 population burst during baseline, RLZ application, or wash conditions. Single bursts on an enlarged timescale are displayed to the right of the prolonged tracing. 5 ìM RLZ significantly increased interburst intervals from baseline by 217% ± 26% [mean ± s.e.m.] and significantly decreased burst length by 9% ± 1% (ANOVA, Tukey, p<0.05, n = 10). B. In a subset of experiments, 10 ìM Riluzole application significantly slowed bursting from baseline to wash (interburst interval: 316 ± 64%, ANOVA, Tukey, p<0.05, n=3) and decreased burst lengths (316 ± 64%, ANOVA, Tukey, p<0.05). C. In the remaining experiments, 10ìM RLZ application stopped bursting, and interburst intervals significantly increased by 500 ± 88% from baseline while burst length decreased by 9 ± 1% (ANOVA, Tukey, p<0.05, n = 6). Spontaneous and an evoked burst (after spontaneous bursting stopped) are shown above the representative data set (interburst intervals depicted as circles; and burst length depicted as triangles). Arrow shows the stimulus artifact. Summary bar graphs depict pooled normalized data with % changes from baseline in the interburst interval (left) and burst length (right). Solid lines show statistical differences: baseline (con) vs. wash. * denotes p<0.05.
3.3 LIDO mediates temporary changes in CA3 interburst intervals and burst lengths
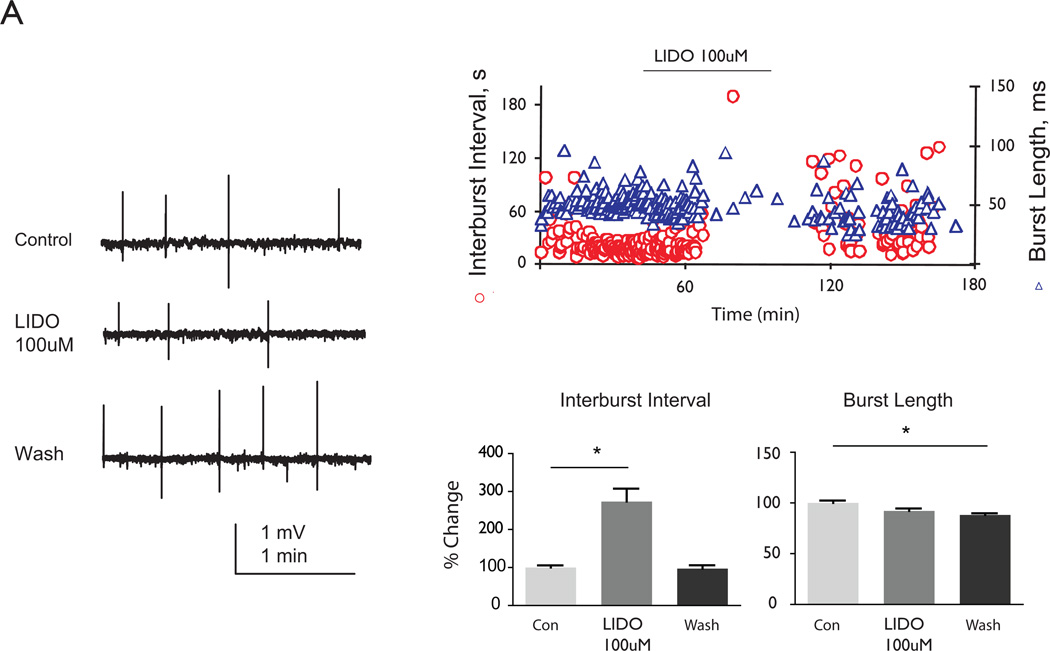
Lastly, we examined the role of LIDO, which blocks sodium channels and is used clinically to treat seizures. It is also used as a local anesthetic and anti-arrhythmic agent, and has been recently examined for its role in treating neonatal and childhood SE (Stoelting and Hillier, 2006; Hattori et al., 2008; Yamamoto et al., 2007). When 100ìM LIDO was bath applied to CA3 bursts, interburst intervals significantly increased compared to baseline while burst length significantly decreased (Figure 4). During the wash, interburst intervals returned to baseline levels; however, burst lengths remained shorter. These data suggest that the application of LIDO induced a temporary change in CA3 synaptic strength reflected by the increase in interburst intervals only during drug application and not washout. However, there was a lasting decrease in burst length following wash of LIDO.
Figure 4. LIDO transiently increases interburst interval of CA3 bursts.
Representative raw tracings of CA3 population burst during baseline, LIDO application, or wash conditions. A.100 ìM LIDO application significantly increased interburst intervals compared to baseline while burst length decreased significantly (274% ± 34% and 9% ± 2% respectively; ANOVA, Tukey, p<0.05, n=10, Fig. 6). During the wash, interburst intervals returned to baseline levels. Solid lines show statistical differences: baseline (con) vs. LIDO and baseline vs. wash. * denotes p<0.05.
4. DISCUSSION
4.1 Summary
We show that the CA3 in-vitro slice model is a relatively quick and easy method to screen novel targets for epileptiform activity. Specifically, we use three voltage-gated sodium channel blockers each with a different mechanism of action to slow CA3 bursts. Here we show that DPH and RLZ produced prolonged changes in the CA3 network as measured by increasing interburst interval. LIDO, however, did not have persistent change in CA3 burst intervals.
The CA3 in-vitro bursting preparation is useful to examine changes of network excitability. Because the extracellular output of this system is either burst or quiescence, we are able to utilize changes in interburst interval as a measure of network excitability. We analyzed the effects of three different sodium channel compounds on spontaneous CA3 population bursts. In general, each drug significantly increased interburst intervals and decreased burst length, but only DPH and RLZ altered CA3 network bursting such that following washout of each drug, interburst intervals did not return to baseline intervals, instead they remained increased. These results suggest a long-term alteration in CA3 network activity. In the presence of 40ìM DPH, CA3 bursts either significantly slowed during application or ceased entirely (Figures 2B–C). Similarly, 5ìM RLZ significantly slows CA3 population bursts where 10ìM RLZ stops bursting in 67% (6 of 9) slices (Figure 3C). Finally, we demonstrate that 100ìM LIDO slows bursting, but returns to baseline interburst intervals following wash (Figure 4).
4.2 Sodium Channel blockers with different mechanisms of action slow spontaneous CA3 bursting
Voltage-gated sodium channels are responsible for the rising phase of the action potential (Ragsdale and Avoli, 1998). In general, these channels are closed at resting membrane potential and open within 200 ìs following membrane depolarization (Ragsdale and Avoli, 1998). Extracellular sodium enters the open channel and further depolarizes the membrane until the channel enters the non-conducting inactivated state. Most voltage-gated sodium channels rarely open from the inactivated state and only repolarization of the membrane resets the channel back to its original resting state, thus preparing the channel to open again for the next membrane depolarization (Ragsdale and Avoli, 1998).
DPH is use clinically for partial, generalized onset seizures and SE. It is believed to work primarily on voltage-dependent sodium channels (Tunnicliff, 1996). In primary dissociated mouse spinal cord neurons, DPH inhibits high frequency, repetitive firing of action potentials evoked by prolonged depolarizing current pulses without affecting the amplitude or duration of spontaneous neuronal activity (McLean and Macdonald, 1983). In addition to postsynaptic effects on sodium channels, DPH may act by modulating synaptic transmission, specifically reversibly depressing field excitatory postsynaptic potentials (fEPSPs) in CA1 hippocampus and decreasing synaptic conductance in mossy fiber to CA3 synapses (Griffith and Taylor, 1988b). Further, DPH has been shown to arrest penicillin-induced bursting by decreasing CA1 fEPSPs without altering resting membrane potential, input resistance, amplitude or duration of action potentials of CA3-CA1 pyramidal cells (Schneiderman and Schwartzkroin, 1982). Thus, DPH’s mechanism of action for blocking sodium channels is increased at depolarized states and during high frequency neuronal activities, which both occur during spontaneous CA3 bursting and conditions such as seizures or SE.
Our data shows that 40ìM DPH slows or stops CA3 bursting, suggesting a sustained alteration of the CA3 network toward a less excitability phenotype, as bursting did not ever return to baseline levels. However, stock solutions of DPH were initially dissolved in DMSO, but the final concentration of DMSO at the slice was below 0.01%. We cannot confirm with absolute certainty that all drug was washed-out as the DMSO makes the DPH lipophilic, which in turn would increase the concentration of DPH in the neuronal membranes. If the increase in interburst interval was due to poor washout of DPH, we would expect some plateauing of the interburst interval during the washout period. Nonetheless, further investigations to determine underlying mechanisms, however, are needed to: 1) demonstrate if interburst interval changes are due to pre or postsynaptic effects in CA3, 2) discover if sodium channel blockers preferentially change glutamatergic vs. GABAergic synaptic transmission in CA3, and 3) find if there are associated changes in sodium channel subunit number or distribution in CA3. Future studies will be required to elucidate the underlying cellular mechanism(s) to explain the slowing in CA3 bursts during the application and washout of DPH.
RLZ is used clinically as a neuroprotective agent in neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS) and traumatic spinal cord injury (Schwartz and Fehlings, 2001). It also acts on the INaP (Bensimon et al., 1994; Urbani and Belluzzi, 2000; Wu et al., 2001). For the treatment of ALS, RLZ is given at 100 mg/day, which translates to 0.1–100ìM in mammalian in-vitro systems (Boireau et al., 2000; Du et al., 2007; Lacomblez et al., 1996). RLZ has also been shown to inhibit glutamate release from rat cerebral cortex nerve synaptosomes resulting in enhancing glutamate transporter activity (Fumagalli et al., 2008; Wang et al., 2004). With its effect of blocking the INaP, we hypothesized and showed that CA3 population bursts significantly slowed at clinically relevant doses (Figure 3). Complete blockage of INaP may also modulate release of glutamate and in turn affect NMDA receptor activity, which has been associated with CA3 burst frequency (Bains et al. 1999; Hellier et al. 2007; Lamanauskas and Nistri, 2008). A recent study suggests that RLZ is effective in blocking seizure activity in the pilocarpine-induced seizure model via an anti-glutamatergic action (i.e., reducing fEPSP slope) and our findings would be consistent with this hypothesis (Kim et al., 2007; Figure 3).
We chose RLZ because it is a sodium channel blocker that is currently approved by the U.S. Food and Drug Administration (FDA) for human conditions other than epilepsy such as ALS and spinal cord injury. We sought to find compounds already used clinically that could also be used as anti-interictal spike agents, because emerging data suggest that blocking interictal spike-like activity might be useful to block epileptogenesis (Dudek and Staley, 2011; Chauvière et al., 2012). However, RLZ’s efficacy in epilepsy has not been fully explored. Based on its mechanism of blocking sodium channels and neuroprotective activity (e.g., antiglutamatergic; Wilson and Fehlings, 2013), we hypothesized that it would be a good compound to reduce interictal spike activity. Here we show that RLZ did slow CA3 bursting (Figure 3). To fully identify the mechanism that caused the bursting to slow, further experiments (e.g., whole-cell patch clamp and whole animal) must be performed.
Both DPH and RLZ begin to slow bursting (i.e., increasing interburst intervals) during the initial application of the drug to CA3 bursts. In most cases, this increase of interburst intervals was observed within 10–30 minutes of drug application compared to control bursting. Furthermore, the slowing of bursts was augmented during the washout period. We anticipate that extending the time of drug application may further increase interburst intervals, as we did not see a plateauing of the increased interburst interval rate with 60 minutes of drug application.
LIDO is a local anesthetic and Class 1 cardiac anti-arrhythmic agent, which inhibits voltage-gated sodium channels during abnormal membrane depolarization (Ragsdale et al., 1996). Its mechanism of action is stabilizing the open state of voltage-dependent sodium channel without altering the resting membrane potential or threshold potential when applied to in-vitro brain slices at 10–100ìM (Armogida et al., 2010; Castaneda-Castellanos et al., 2002; Fried et al., 1995; Niiyama et al., 2005; Stoelting and Hillier, 2006). In CA3, the result is slowing of spontaneous bursts, but unlike the cases for DPH and RLZ, the interburst interval returned to baseline following washout of LIDO (Figure 4). From our experiments, LIDO would not be a good drug for stabilizing CA3 synaptic strength as reflected by persistently increased interburst intervals. However, the prediction from this work is that compounds which block sodium channel activation and slow CA3 bursting – an in vitro model for interictal spikes – may be compounds that subsequently alter the course of interictal spikes and possibly epileptogenesis (Staley et al. 2005; Staley and Dudek 2006). Specifically, RLZ may be a novel target for inactivation of interictal spikes by its ability to block the INaP.
4.3 Assumptions of this model
These experiments have all been conducted in naïve animals and developed on the assumption that epilepsy treatments may be identified by induced seizures in normal brains (Stables et al., 2003). This in-vitro preparation requires further validation in animal models with genetically-induced spontaneous seizures and/or chronic epilepsy models, such as epilepsy induced by the chemoconvulsants (e.g. pilocarpine or kainic acid; Hellier and Dudek, 2005; Hellier et al., 1998). We have shown in a previous study that CA3 bursting in chronically-epileptic rats can be altered by NMDA selective antagonists, but we did not test the effects of sodium channel blockers at that time (Hellier et al., 2009). Another study examining the effects of spontaneous bursting in a combined hippocampal/entorhinal cortex slice preparation in kainate-treated versus control animals suggested that in-vitro slices from kainate-treated animals are an appropriate model for pharmacoresistant epilepsy (Smith et al., 2007). However, using animal models of epilepsy increases the time before pharmacological studies can be performed as it takes several weeks to months before the animal is truly epileptic (Hellier et al., 1998; Williams et al., 2009). Thus, using our spontaneously bursting CA3 slice experiment allows relatively quick analysis of drug studies to determine if a new compound is feasible and efficacious. This allows researchers to test only the more promising compounds on animal models of epilepsy.
5.0 Conclusions
The benefits of in-vitro slice studies are the relative ease of preparation and the potential for multiple slices used from each animal, either naïve or following a manipulation to make the animal chronically epileptic; thus, the slice preparation has potential for moderate throughput screening of compounds. Because of the relatively simple anatomy of the CA3 circuit, it is also one amenable to computer modeling, with empirically collected data corroborating computer output, another area of potential exploration for more investigations of new anticonvulsant targets (Swiercz et al., 2007).
The spontaneous bursting of CA3 slice preparation represents network activity where inhibitory and excitatory systems can be intact or preferentially blocked. The anatomy of CA3 recurrent collaterals provides ample positive feedback to all of the neighboring CA3 pyramidal cells, and mimics, in naïve animals, the recurrent excitatory pathways generated in the dentate gyrus following chronic epilepsy (Wuarin and Dudek, 1996, 2001). Alteration of sodium channels has been shown to inhibit glutamate transmission, which would have ramifications for treatment of epilepsy, in which network excitability is increased. Further exploration to determine if the compounds examined here act in pre- or post-synaptic fashion will be needed.
In prior work, we demonstrated that the CA3 network responded differently to the same compound if the initial network excitability was altered (i.e., bursting induced by high extracellular potassium versus tetanic stimulation; Yee et al., 2003). The efficacy of these compounds at higher network excitability (e.g., 8.5mM extracellular potassium) has yet to be determined. However, for this study we wanted to ensure that extracellular potassium was maintained at physiological levels so that it would not interfere with sodium influxes as we were specifically targeting and blocking the sodium channel. In-vitro studies have demonstrated that CA3 bursting can potentiate individual synapses as well as “speed up” the electrical phenotype of this network (Bains et al., 1999; Debanne et al., 2006). Depending upon their baseline level of excitability, differential effects of compounds on neural networks also merit further exploration in epilepsy, as a demonstrated mechanism of epilepsy is aberrant excitation (Buckmaster and Dudek, 1997; Scharfman et al., 2002).
The CA3 burst preparation has promise to be used as a moderate throughput screening of novel anti343 spike and/or antiepileptogenic compounds (e.g., acute slices from control vs. epileptic animals or slice culture systems). We anticipate concurrent use of CA3 bursting as an electrophysiological marker coupled with genetic techniques to provide further understanding of the molecular/genetic underpinnings of epilepsy and epileptogenesis.
HIGHLIGHTS.
Three different Na+-channel blockers decrease network CA3 bursting.
Diphenytoin persistently slows spontaneous CA3 bursts.
Riluzole persistently slows spontaneous CA3 bursts.
Lidocaine transiently slows spontaneous CA3 bursts.
ACKNOWLEDGEMENTS
This work was funded by an NINDS K08 Award to Audrey S. Yee. We acknowledge past members of the Staley lab for helpful scientific discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors’ have no conflicts of interest to disclose.
References
- Armogida M, Giustizieri M, Zona C, Piccirilli S, Nistico R, Mercuri NB. N-ethyl lidocaine (QX-314) protects striatal neurons against ischemia: an in vitro electrophysiological study. Synapse. 2010;64:161–168. doi: 10.1002/syn.20735. [DOI] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nature Neuroscience. 1999;2:720–726. doi: 10.1038/11184. [DOI] [PubMed] [Google Scholar]
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave ripple complexes in vitro and reorganization of hippocampal networks. Nature Neuroscience. 2005;8:1560–1567. doi: 10.1038/nn1571. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. New England Journal of Medicine. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Boireau A, Dubedat P, Bordier F, Imperato A, Moussaoui S. The protective effect of riluzole in the MPTP model of Parkinson's disease in mice is not due to a decrease in MPP(+) accumulation. Neuropharmacology. 2000;39:1016–1020. doi: 10.1016/s0028-3908(99)00188-4. [DOI] [PubMed] [Google Scholar]
- Bragdon AC, Taylor DM, Wilson WA. Potassium-induced epileptiform activity in area CA3 varies markedly along the septotemporal axis of the rat hippocampus. Brain Research. 1986;378:169–173. doi: 10.1016/0006-8993(86)90300-8. [DOI] [PubMed] [Google Scholar]
- Brodie MJ. Diagnosing and predicting refractory epilepsy. Acta Neurologica Scandinavica. Supplementum. 2005;181:36–39. doi: 10.1111/j.1600-0404.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. Journal of Comparative Neurology. 1997;385:385–404. [PubMed] [Google Scholar]
- Castaneda-Castellanos DR, Nikonorov I, Kallen RG, Recio-Pinto E. Lidocaine stabilizes the open state of CNS voltage-dependent sodium channels. Brain Research. Molecular Brain Research. 2002;99:102–113. doi: 10.1016/s0169-328x(01)00340-0. [DOI] [PubMed] [Google Scholar]
- Chauvière L, Doublet T, Ghestem A, Siyoucef SS, Wendling F, Huys R, Jirsa V, Bartolomei F, Bernard C. Changes in interictal spike features precede the onset of temporal lobe epilepsy. Annals of Neurology. 2012;71(6):805–814. doi: 10.1002/ana.23549. [DOI] [PubMed] [Google Scholar]
- Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Current Medicinal Chemistry. 2010;17 doi: 10.2174/092986710791163939. 1942-1199. [DOI] [PubMed] [Google Scholar]
- Cohen I, Huberfeld G, Miles R. Emergence of disinhibition-induced synchrony in the CA3 region of the guinea pig hippocampus in vitro. Journal of Physiology. 2006;570:583–594. doi: 10.1113/jphysiol.2005.097899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Thompson SM, Gahwiler BH. A brief period of epileptiform activity strengthens excitatory synapses in the rat hippocampus in vitro. Epilepsia. 2006;47:247–256. doi: 10.1111/j.1528-1167.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Devinsky O. Patients with refractory seizures. New England Journal of Medicine. 1999;340:1565–1570. doi: 10.1056/NEJM199905203402008. [DOI] [PubMed] [Google Scholar]
- Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Jr, Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Staley KJ. The time course of acquired epilepsy: implications for therapeutic intervention to suppress epileptogenesis. Neuroscience Letters. 2011;497(3):240–246. doi: 10.1016/j.neulet.2011.03.071. [DOI] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla CG, Frenguelli BG, Staley KJ, Masino SA. Intracellular acidification causes adenosine release during states of hyperexcitability in the hippocampus. Journal of Neurophysiology. 2009;102:1984–1993. doi: 10.1152/jn.90695.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried E, Amorim P, Chambers G, Cottrell JE, Kass IS. The importance of sodium for anoxic transmission damage in rat hippocampal slices: mechanisms of protection by lidocaine. J Physiol. 1995;489(Pt 2):557–565. doi: 10.1113/jphysiol.1995.sp021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. European Journal of Pharmacology. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Griffith WH, Taylor L. Phenytoin reduces excitatory synaptic transmission and post-tetanic potentiation in the in vitro hippocampus. Journal of Pharmacology and Experimental Therapeutics. 1988a;246:851–858. [PubMed] [Google Scholar]
- Griffith WH, Taylor L. Sodium valproate decreases synaptic potentiation and epileptiform activity in hippocampus. Brain Research. 1988b;474:155–164. doi: 10.1016/0006-8993(88)90678-6. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. Journal of Neurophysiology. 2006;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori H, Yamano T, Hayashi K, Osawa M, Kondo K, Aihara M, Haginoya K, Hamano S, Izumi T, Kaneko K, Kato I, Matsukura M, Minagawa K, Miura T, Ohtsuka Y, Sugai K, Takahashi T, Yamanouchi H, Yamamoto H, Yoshikawa H. Effectiveness of lidocaine infusion for status epilepticus in childhood: a retrospective multi-institutional study in Japan. Brain Dev. 2008;30(8):504–512. doi: 10.1016/j.braindev.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Chemoconvulsant model of chronic spontaneous seizures. Curr Protoc Neurosci. 2005;Chapter 9(Unit 9):19. doi: 10.1002/0471142301.ns0919s31. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Grosshans DR, Coultrap SJ, Jones JP, Dobelis P, Browning MD, Staley KJ. Nmda Receptor Trafficking at Recurrent Synapses Stabilizes the State of the Ca3 Network. Journal of Neurophysiology. 2007 doi: 10.1152/jn.00346.2007. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Research. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Hellier JL, White A, Williams PA, Edward Dudek F, Staley KJ. NMDA receptor-mediated long-term alterations in epileptiform activity in experimental chronic epilepsy. Neuropharmacology. 2009;56:414–421. doi: 10.1016/j.neuropharm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen SI. Laboratory Monitoring of Antiepileptic Drugs. In: Levy RH, editor. Antiepileptic drugs. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 104. [Google Scholar]
- Jones J, Stubblefield EA, Benke TA, Staley KJ. Desynchronization of glutamate release prolongs synchronous CA3 network activity. Journal of Neurophysiology. 2007;97:3812–3818. doi: 10.1152/jn.01310.2006. [DOI] [PubMed] [Google Scholar]
- Kim J-E, Kim D-S, Kwak S-E, Choi H-C, Song H-K, Choi S-Y, Kwon O-S, Kim Y-I, Kang T-C. Anti-glutamatergic effect of riluzole: comparison with valproic acid. Neuroscience. 2007;147:136–145. doi: 10.1016/j.neuroscience.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Korn SJ, Giacchino JL, Chamberlin NL, Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. Journal of Neurophysiology. 1987;57:325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Powe L, Durrleman S, Delumeau JC, Meininger V. A confirmatory dose-ranging study of riluzole in ALS. ALS/Riluzole Study Group-II. Neurology. 1996;47:S242–S250. doi: 10.1212/wnl.47.6_suppl_4.242s. [DOI] [PubMed] [Google Scholar]
- Lamanauskas N, Nistri A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. European Journal of Neuroscience. 2008;27:2501–2514. doi: 10.1111/j.1460-9568.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- Levine M, Chang T. Therapeutic drug monitoring of phenytoin. Rationale and current status. Clinical Pharmacokinetics. 1990;19:341–358. doi: 10.2165/00003088-199019050-00001. [DOI] [PubMed] [Google Scholar]
- McLean MJ, Macdonald RL. Multiple actions of phenytoin on mouse spinal cord neurons in cell culture. Journal of Pharmacology and Experimental Therapeutics. 1983;227:779–789. [PubMed] [Google Scholar]
- Miles GB, Dai Y, Brownstone RM. Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones. Journal of Physiology. 2005;566:519–532. doi: 10.1113/jphysiol.2005.086033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Wong RK. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983;306:371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Dunwiddie TV. Anticonvulsant and proconvulsant actions of alpha- and betanoradrenergic agonists on epileptiform activity in rat hippocampus in vitro. Epilepsia. 1983;24:57–64. doi: 10.1111/j.1528-1157.1983.tb04866.x. [DOI] [PubMed] [Google Scholar]
- Niiyama S, Tanaka E, Tsuji S, Murai Y, Satani M, Sakamoto H, Takahashi K, Kuroiwa M, Yamada A, Noguchi M, Higashi H. Neuroprotective mechanisms of lidocaine against in vitro ischemic insult of the rat hippocampal CA1 pyramidal neurons. Neuroscience Research. 2005;53:271–278. doi: 10.1016/j.neures.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Oliver AP, Hoffer BJ, Wyatt RJ. The hippocampal slice: a system for studying the pharmacology of seizures and for screening anticonvulsant drugs. Epilepsia. 1977;18:543–548. doi: 10.1111/j.1528-1157.1977.tb05002.x. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, Avoli M. Sodium channels as molecular targets for antiepileptic drugs. Brain Research. Brain Research Reviews. 1998;26:16–28. doi: 10.1016/s0165-0173(97)00054-4. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagratella S. Characterization of the in vitro antiepileptic activity of new and old anticonvulsant drugs. General Pharmacology. 1998;30:153–160. doi: 10.1016/s0306-3623(97)00266-8. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Synchronization of area CA3 hippocampal pyramidal cells and non-granule cells of the dentate gyrus in bicuculline-treated rat hippocampal slices. Neuroscience. 1994;59:245–257. doi: 10.1016/0306-4522(94)90593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Smith KL, Jackson MB, Goodman JH. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. The journal of comparative Neurology. 2002;454:424–439. doi: 10.1002/cne.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman JH, Evans JC. Effects of anticonvulsants on penicillin-induced bursting in guinea pig hippocampal slices. Epilepsia. 1986;27:347–353. doi: 10.1111/j.1528-1157.1986.tb03552.x. [DOI] [PubMed] [Google Scholar]
- Schneiderman JH, Schwartzkroin PA. Effects of phenytoin on normal activity and on penicillin induced bursting in the guinea pig hippocampal slice. Neurology. 1982;32:730–738. doi: 10.1212/wnl.32.7.730. [DOI] [PubMed] [Google Scholar]
- Schwartz G, Fehlings MG. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. Journal of Neurosurgery. 2001;94:245–256. doi: 10.3171/spi.2001.94.2.0245. [DOI] [PubMed] [Google Scholar]
- Smith MD, Adams AC, Saunders GW, White HS, Wilcox KS. Phenytoin- and carbamazepine-resistant spontaneous bursting in rat entorhinal cortex is blocked by retigabine in vitro. Epilepsy Research. 2007;74:97–106. doi: 10.1016/j.eplepsyres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Spadoni F, Hainsworth AH, Mercuri NB, Caputi L, Martella G, Lavaroni F, Bernardi G, Stefani A. Lamotrigine derivatives and riluzole inhibit INa,P in cortical neurons. Neuroreport. 2002;13:1167–1170. doi: 10.1097/00001756-200207020-00019. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram E, Dudek FE, Holmes G, Mathern G, Pitkanen A, White HS. Therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics: summary of the NIH/NINDS/AES models II workshop. Epilepsia. 2003;44:1472–1478. doi: 10.1111/j.0013-9580.2003.32803.x. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE, Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Bains JS, Yee A, Hellier J, Longacher JM. Statistical model relating CA3 burst probability to recovery from burst-induced depression at recurrent collateral synapses. Journal of Neurophysiology. 2001;86:2736–2747. doi: 10.1152/jn.2001.86.6.2736. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr. 2006;6:199–202. doi: 10.1111/j.1535-7511.2006.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nature Neuroscience. 1998;1:201–209. doi: 10.1038/651. [erratum appears in Nat Neurosci 1998 Aug;1(4)331] [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Anderson WW, Clark S, Wilson WA, Stasheff SF, Anderson WW, Clark S, Wilson WA. NMDA antagonists differentiate epileptogenesis from seizure expression in an in vitro model. Science. 1989;245:648–651. doi: 10.1126/science.2569762. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Bragdon AC, Wilson WA. Induction of epileptiform activity in hippocampal slices by trains of electrical stimuli. Brain Research. 1985;344:296–302. doi: 10.1016/0006-8993(85)90807-8. [DOI] [PubMed] [Google Scholar]
- Stoelting RK, Hillier S. Pharmacology & physiology in anesthetic practice. Philadelphia: Lippincott Williams & Wilkins; 2006. Local anesthetics; pp. 179–207. [Google Scholar]
- Swartzwelder HS, Wilson WA. Differential effects of phenobarbital and pentobarbital on stimulus train-induced bursting in the hippocampal slice. Epilepsia. 1987;28:207–213. doi: 10.1111/j.1528-1157.1987.tb04209.x. [DOI] [PubMed] [Google Scholar]
- Swiercz W, Cios K, Hellier J, Yee A, Staley K. Effects of synaptic depression and recovery on synchronous network activity. Journal of Clinical Neurophysiology. 2007;24:165–174. doi: 10.1097/WNP.0b013e318033756f. [DOI] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ. Persistent inward currents in rat ventral horn neurones. J Physiol. 2007;580:507–522. doi: 10.1113/jphysiol.2006.124123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Dingledine R. Model of synchronized epileptiform bursts induced by high potassium in CA3 region of rat hippocampal slice. Role of spontaneous EPSPs in initiation. Journal of Neurophysiology. 1990;64:1009–1018. doi: 10.1152/jn.1990.64.3.1009. [DOI] [PubMed] [Google Scholar]
- Traub RD, Miles R. Neuronal Networks of the Hippocampus. New York: Cambridge University Press; 1991. [Google Scholar]
- Traub RD, Miles R, Wong RK, Schulman LS, Schneiderman JH. Models of synchronized hippocampal bursts in the presence of inhibition. II. Ongoing spontaneous population events. Journal of Neurophysiology. 1987;58:752–764. doi: 10.1152/jn.1987.58.4.752. [DOI] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G. Basis of the antiseizure action of phenytoin. General Pharmacology. 1996;27:1091–1097. doi: 10.1016/s0306-3623(96)00062-6. [DOI] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. European Journal of Neuroscience. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Wang KY, Wang WC. Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes) Neuroscience. 2004;125:191–201. doi: 10.1016/j.neuroscience.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Jefferys JG. Epileptic activity outlasts disinhibition after intrahippocampal tetanus toxin in the rat. J Physiol. 1994;481(Pt 3):593–604. doi: 10.1113/jphysiol.1994.sp020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. Journal of Neuroscience. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Fehlings MG. Riluzole for Acute Traumatic Spinal Cord Injury: A Promising Neuroprotective Treatment Strategy. World Neurosurgery. 2013 doi: 10.1016/j.wneu.2013.01.001. pii: S1878-8750(13), 00008-9. [DOI] [PubMed] [Google Scholar]
- Wu N, Hsiao CF, Chandler SH. Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: participants in burst generation. Journal of Neuroscience. 2001;21:3729–3739. doi: 10.1523/JNEUROSCI.21-11-03729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. Journal of Neuroscience. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainite treatment. Journal of Neurophysiology. 2001;85:1067–1077. doi: 10.1152/jn.2001.85.3.1067. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Aihara M, Niijima S, Yamanouchi H. Treatments with midazolam and lidocaine for status epilepticus in neonates. Brain and Development. 2007;29:559–564. doi: 10.1016/j.braindev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Yee AS, Longacher JM, Staley KJ. Convulsant and anticonvulsant effects on spontaneous CA3 population bursts. Journal of Neurophysiology. 2003;89:427–441. doi: 10.1152/jn.00594.2002. [DOI] [PubMed] [Google Scholar]