Abstract
Purpose
The epidermal growth factor receptor gene (EGFR) is most frequently amplified and overexpressed, along with its deletion mutant, EGFRvIII, in glioblastoma. We tested the preclinical efficacy of the recombinant immunotoxin, D2C7-(scdsFv)-PE38KDEL, which is reactive with a 55-amino acid (AA) region present in the extracellular domain of both EGFRwt (583–637 AAs) and EGFRvIII (292–346 AAs) proteins.
Experimental Design
The binding affinity and specificity of D2C7-(scdsFv)-PE38KDEL for EGFRwt and EGFRvIII were measured by surface-plasmon resonance and flow cytometry. In vitro cytotoxicity of D2C7-(scdsFv)-PE38KDEL was measured by protein synthesis inhibition in human EGFRwt-transfected NR6 (NR6W), human EGFRvIII-transfected NR6 (NR6M), EGFRwt-overexpressing A431-epidermoid-carcinoma, and glioblastoma xenograft cells (43, D08-0493MG, D2159MG and D270MG). In vivo anti-tumor efficacy of D2C7-(scdsFv)-PE38KDEL was evaluated with 43, NR6M, and D270MG orthotopic tumor models.
Results
The KD of D2C7-(scdsFv)-PE38KDEL for EGFRwt and EGFRvIII was 1.6×10−9 M and 1.3×10−9 M, respectively. Flow cytometry with NR6W and NR6M cells confirmed the specificity of D2C7-(scdsFv)-PE38KDEL for EGFRwt and EGFRvIII. The D2C7-(scdsFv)-PE38KDEL IC50 was 0.18–2.5 ng/ml on cells expressing EGFRwt (NR6W, A431, 43, and D08-0493MG). The D2C7-(scdsFv)-PE38KDEL IC50 was ≈0.25 ng/ml on EGFRvIII-expressing cells (NR6M) and on EGFRwt- and EGFRvIII-expressing glioblastoma xenograft cells (D2159MG and D270MG). Significantly, in intracranial tumor models of 43, NR6M, and D270MG, D2C7-(scdsFv)-PE38KDEL treatment by convection-enhanced delivery (CED) prolonged survival by 310% (P=0.006), 28% (P=0.002), and 166% (P=0.001), respectively.
Conclusions
In preclinical studies, the D2C7-(scdsFv)-PE38KDEL immunotoxin exhibited significant potential for treating brain tumors expressing EGFRwt, EGFRvIII, and both EGFRwt and EGFRvIII.
Keywords: epidermal growth factor receptor, EGFRvIII, glioblastoma multiforme, recombinant immunotoxin, single-chain disulfide Fv
Introduction
Gliomas are the most common primary tumors of the central nervous system (1). The most frequent and most malignant type of glioma, glioblastoma multiforme, accounts for about 16% of all brain tumors in the United States (2). Current treatment for patients with glioblastoma includes surgery followed by radiation and chemotherapy; however, the median survival for glioblastoma patients until the early 1990s was less than a year (3, 4). Recent trials incorporating temozolomide or the monoclonal antibody (mAb), bevacizumab, have resulted in only modest extensions in survival (5, 6). Clearly, new and more efficient therapeutic approaches are needed to improve glioblastoma patient survival.
In the past decade, mAb-based clinical trials have increasingly focused on genetically engineered single-chain variable-region antibody fragments (scFvs) consisting of the heavy- and light-chain variable regions (VH and VL) fused to immunotoxins (ITs); these scFv-ITs target antigens expressed specifically by brain tumor cells. Because an scFv-IT fusion protein is smaller than an intact immunoglobulin (IgG), it should have greater tumor penetration and therefore lead to enhanced therapeutic efficacy when delivered intrathecally, intratumorally, or intracerebrally (7–11).
The epidermal growth factor receptor (EGFR), a 170-kDa, transmembrane receptor tyrosine kinase (RTK), is frequently overexpressed in a wide variety of human cancers (12), including brain tumors (13, 14). EGFR is the most frequently amplified gene in glioblastoma (15) and EGFR amplification defines the classical glioblastoma subtype (16); in contrast, the level of EGFR in normal brain is undetectable or extremely low (12). Correlating with the gene amplification, the protein is overexpressed in about 60–90% of glioblastoma cases. Even in the absence of gene amplification, protein overexpression has been observed in 12–38% of glioblastoma patients (17), which may be due to aberrant translational and post-translational mechanisms. Preclinical studies have shown that EGFR activation—in addition to protecting tumor cells from apoptosis—also induces several tumorigenic processes, including proliferation, angiogenesis, and invasiveness (18).
EGFR amplification is often associated with gene rearrangements. Several EGFR deletion mutants have been identified, the most common being EGFRvIII, which is present in 67% of glioblastomas with EGFRwt amplification (19). EGFRvIII contains a deletion of exons 2–7 of the EGFR gene, (20) and this in-frame deletion creates a novel glycine residue at the fusion junction at position 6, between amino acid residues 5 and 274, generating a tumor-specific epitope that is expressed specifically on tumor cells, but not on normal tissues. EGFRvIII is a constitutively active RTK that is not further activated by EGFR ligands (21). Like its wild-type counterpart, EGFRvIII is widely expressed in malignant gliomas (22) and carcinomas, including that of the head and neck (23) and breast (24). Overexpression of EGFRvIII induces resistance in glioma cells to radiation and chemotherapy (25).
Several anti-EGFR mAbs are in clinical trials for various human cancers, including head and neck, colorectal, pancreatic, lung, renal cell, and prostate carcinoma or high-grade glioma (7, 26). The anti-EGFRwt mAbs EGFR1, H17E2, and 425 were the first to be introduced in targeted radiotherapy trials that involved systemic injection of radiolabeled mAbs in patients with malignant gliomas (27–29). Furthermore, in a Phase I clinical trial with TP-38, a recombinant EGFR-ligand (transforming growth factor alpha) Pseudomonas exotoxin fusion protein, an overall median survival of 23 weeks for all 20 glioblastoma patients enrolled was observed (30). Also, a recombinant human EGF diphtheria toxin fusion protein (DT-EGF) inhibited tumor growth in an in vivo xenograft model with EGFR-expressing U87MG glioma cells, and 75% of the treated animals remained tumor free 60 days post treatment (31).
Several mAbs and scFv constructs specific for EGFRvIII, including L8A4, Y10, MR1, MR1-1, and 14E1, are well described in previous studies (24, 32–34). Among the various antibody constructs, MR1-1 scFv, derived from a mouse scFv library, has significant potential (32, 33). A Phase I clinical study with the MR1-1 Pseudomonas IT delivered by convection-enhanced delivery (CED), is currently underway at Duke University for treating patients with EGFRvIII-expressing glioblastoma tumors (http://clinicaltrials.gov/ct2/show/NCT01009866).
Due to the high prevalence of EGFRvIII mutation in tumors that have EGFRwt amplification (19), it would be advantageous to have antibodies that could target both antigens for glioblastoma therapy. Co-targeting these two antigens could promote greater killing of tumor cells than that which is achieved by antibodies specific for a single antigen. Cetuximab, an unarmed EGFRwt- and EGFRvIII-reactive antibody, has demonstrated limited activity (progression-free survival of <6 months) in a Phase II trial in recurrent, high-grade glioma patients with EGFRwt amplification (35). Our study focuses on D2C7, a novel mAb that reacts with both EGFRwt and EGFRvIII proteins (36). In comparison to the established specific mAbs (anti-EGFRwt mAb, EGFR1 or anti-EGFRvIII mAb, L8A4), D2C7 demonstrated a significantly higher tumor localization in tumors expressing EGFRwt or EGFRvIII proteins (36). Significantly, in immunohistochemical analysis of 101 adult glioblastoma samples, the D2C7 mAb positively stained virtually all cells in 100% (50/50) of the samples that had amplification of the EGFRwt gene and in 76% (39/51) of the cases without this amplification (36).
Here, we summarize the in vitro and in vivo results of our investigation of D2C7-(scdsFv)-PE38KDEL, a recombinant scFv IT that binds to both EGFRwt and EGFRvIII and has potential clinical application for the therapy of brain tumors expressing these proteins.
Material and Methods
Cell lines
The cell lines used were, the human epidermoid-carcinoma cell line, A43l (expressing EGFRwt), the murine Swiss 3T3 fibroblast cell line NR6 transfected with human EGFRwt (NR6W) (21), and human EGFRvIII (NR6M) (21). The parental murine Swiss 3T3 fibroblast cell line, NR6 (kindly provided by Dr. Harvey Herschman, University of California, Los Angeles), which lacks expression of murine or human EGFRwt and EGFRvIII, was used as control. All cell lines were cultured in complete zinc option (ZO)-10% fetal bovine serum (FBS; Improved Modified Eagle Medium ZO [Richter’s Modification, Cat. No. 10373-017] liquid; Invitrogen, San Diego, CA) and passed at confluence with 0.05% Trypsin-EDTA (Invitrogen). All cell lines were authenticated by periodic morphology assessment and by testing for mycoplasma infection.
D2C7 epitope mapping by ELISA
Different concentrations (0.5, 0.25, 0.125 and 0.0625 μg/ml in 0.1M carbonate buffer pH 10) of Nus-Tag EGFRvIII ECD deletion constructs along with the Nus-Tag protein alone were added to 96-well plates and incubated for 1 h at 37°C. The plates were then blocked with SuperBlock buffer (Pierce, Rockford, IL) for 30 min at 37°C and incubated with 1.0 μg/ml of D2C7 mAb for 1 h at 37°C. The plates were then incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody for 1 h at 37°C, followed by the addition of TMB substrate solution (Pierce). The reaction was stopped by the addition of stop solution (Pierce) and the optical density was measured at 450 nm.
Disaggregation of xenograft tumor samples
Xenograft tissue derived from glioblastoma samples D270MG (expressing EGFRwt and EGFRvIII), D2159MG (expressing EGFRwt and EGFRvIII), 43 (expressing EGFRwt) (kindly provided by Dr. C. David James, University of California, San Francisco), and D08-0493MG (expressing EGFRwt), was finely minced and digested with 100 μg Liberase (Roche, Indianapolis, IN) at 37°C for 10 min. The dissociated cells were filtered, and the reaction was inhibited with the addition of 5% HSA solution. The cells were washed with ZO medium, further treated with Ficoll-Hypaque to remove any red blood cells, and then washed again in ZO medium.
Preparation of recombinant immunotoxins
D2C7 and P588 ITs were generated by fusing the specific scFv with the sequences for domains II and III of Pseudomonas exotoxin A (PE38) according to the protocol described in a previous publication (37). The specific scFv IT was expressed under control of the T7 promoter in E. coli BL21 (λ DE3) (Stratagene, La Jolla, CA). All recombinant proteins accumulated in the inclusion bodies. The ITs were then reduced, refolded, and further purified as monomers (64 kDa) by ion exchange and size exclusion chromatography to >95% purity, as described in another study (37).
Flow cytometry
Indirect fluorescence activated cell sorting (FACS) analysis was performed with the D2C7-(scdsFv)-PE38KDEL immunotoxin. Briefly, 1×106 cells (NR6, NR6W, or NR6M) were suspended in 500 μl of phosphate-buffered saline pH 7.4 (PBS) (Invitrogen) containing 5% FBS (Invitrogen) (5% FBS/PBS). The D2C7-(scdsFv)-PE38KDEL IT or negative control, P588-(scdsFv)-PE38KDEL, was added to the cells at a concentration of 10 μg/ml, and the samples were incubated for 40 min at 4°C. After washing, cells were incubated with rabbit anti-Pseudomonas exotoxin A antibody (Sigma, St. Louis, MO) followed by goat anti-rabbit-IgG-FITC antibody (Zymed). Stained cells were washed with 1X PBS and analyzed on a Becton Dickinson FACSort instrument equipped with CellQuest software (BD Biosciences). Glioblastoma (43 and D2159MG) xenograft cells (5×105), were suspended in 500 μl of 5% FBS/PBS. Human CD133-APC (AC133 and 293C3) (Miltenyi Biotec, Auburn, CA) and D2C7-AF488 antibodies were added to the cells and the samples were incubated for 40 min at 4°C. APC-conjugated and AF488-conjugated mouse IgG isotype antibodies were used as controls. Stained cells were washed with 1X PBS and analyzed on a Becton Dickinson FACSort instrument equipped with CellQuest software (BD Biosciences).
In vitro cytotoxicity assay
The cytotoxicity of the ITs on cultured cell lines (NR6W, NR6M, and A431) and cells freshly isolated from glioblastoma xenografts (43, D08-0493MG, D270MG, and D2159MG) was assayed by inhibition of protein synthesis as described in an earlier study (32). The cytotoxic activity was defined by IC50, which was the toxin concentration that suppressed incorporation of radioactivity by 50% as compared to the radioactivity measured in cells that were not treated with toxin.
In vivo intracranial tumor model and convection-enhanced delivery
Male NOD scid gamma (NSG) mice (≈30 g; 8–12 weeks, Duke University, Division of Laboratory Animal Resources) were anesthetized by an intraperitoneal injection of a solution of ketamine 50 mg/kg (Fort Dodge Animal Health, Overland Park, Kansas), and xylazine 10 mg/kg (Akorn, Inc., Decatur, Illinois). The anterior cranial region was shaved under sterile conditions and an incision ≈1 cm in length was made in the skin over the skull, and a small burr hole was drilled at coordinates 2.5 mm left lateral of the sagittal and 1 mm anterior to the bregma. A 25-gauge 10-μl Hamilton needle was inserted vertically to a depth of 2.5 mm from the dura mater. All mice were implanted with 105 43 or D270MG xenograft cells in 3 μl of 1X PBS or NR6M cells in 3 μl of BD PuraMatrix peptide hydrogel (BD Biosciences, Bedford, MA) into the cortex.
Six (43 xenograft), five (NR6M) or eleven (D270MG) days after tumor implantation, animals were randomized into four groups (sham n=4/5, others n=8–10), Sham (no pump), 0.2% PBS-HSA, P588-(scdsFv)-PE38KDEL, and D2C7-(scdsFv)-PE38KDEL. One microgram of D2C7-(scdsFv)-PE38KDEL or P588-(scdsFv)-PE38KDEL diluted in 100 μl of 0.2% PBS-HSA was delivered via alzet osmotic mini pumps (Durect Corporation, Cupertino, California) over a three day (43 xenograft) or seven day (NR6M and D270MG) period. The mice were followed to assess tumor development and death.
Evaluation of intracranial xenograft response to treatment
The response of intracranial (ic) xenografts to treatment was assessed by percentage increase in time to a specific neurologic endpoint (seizure activity, repetitive circling, or other subtle changes such as grooming or decrease in appetite) or to death. Statistical analysis was performed using the Wilcoxon rank order test as previously described (38, 39). Animals were observed twice daily for signs of distress or development of neurologic symptoms, at which time, the mice were euthanized.
Results
Determination of D2C7 epitope on EGFRvIII/EGFRwt
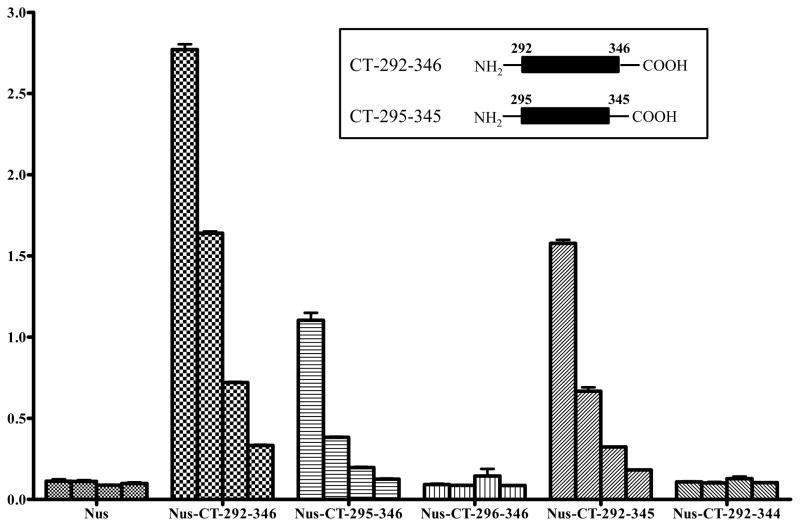
Since several EGFR deletion mutants have been described in gliomas (19), determining the epitope of D2C7 on EGFRwt/EGFRvIII will aid in the identification of specific EGFR mutations that could be therapeutically targeted with the D2C7 mAb. Accordingly, several deletion mutants of human EGFRvIII extracellular domain (ECD) were generated. The different EGFRvIII ECD proteins were expressed as Nus-Tag fusion proteins and purified as described in “Supplementary Methods.” D2C7 recognized three types of C-terminal (CT) protein constructs (CT 179–356 amino acids, CT 267–356 amino acids, and CT 287–356 amino acids) but not the N-terminal (NT) protein construct (NT 1–178 amino acids) in enzyme linked immunosorbent assay (data not shown). Further deletions of the EGFRvIII ECD peptide fragment CT 287–356 amino acids demonstrated strong reactivity of D2C7 to CT 292–346 amino acids and a weak reactivity to CT 295–345 amino acids (Fig. 1). These results indicated that the minimum epitope of D2C7 is 51 amino acids (CT 295–345), but 55 amino acids (CT 292–346) are essential for strong binding by D2C7 (Fig. 1 Inset).
Figure 1.
Determination of the epitope of D2C7 on EGFRvIII ECD. Different concentrations (0.5 μg, 0.25 μg, 0.125 μg, and 0.0625 μg) of Nus-Tag alone or Nus-Tag conjugated to EGFRvIII ECD deletion mutants (CT 292–346, CT 295–346, CT 296–346, CT 292–345, and CT 292–344) were immobilized on 96-well plates. The wells were blocked with superblock and incubated with D2C7 mAb followed by peroxidase conjugated anti-mouse IgG antibody. Following the addition of TMB substrate solution, and stop solution, the optical density was measured at 450 nm. The ELISA assay with different concentrations of Nus-Tag alone or Nus-Tag conjugated to EGFRvIII ECD deletion mutants was performed in triplicate for each epitope. Results are presented as mean ± standard deviation where n > 2. Inset represents the EGFRvIII ECD peptides essential for minimum (CT 295–345) and strong binding (CT 292–346) of D2C7.
Cloning of the VH and VL domain of D2C7 IgG1κ
VH and VL cDNAs were isolated from the D2C7 hybridoma as described in “Supplementary Methods.” The VH and VL domains were cloned and sequenced, and the fragments were 360 and 321 bp, respectively. The deduced amino acid sequences of the D2C7 VH and VL domains are shown in Supplementary Fig. S1. Sequence analysis of the VH and VL amino acids, using the NIH database of germ-line genes (http://www.ncbi.nlm.nih.gov/igblast/), revealed that the sequences were derived from different germ-line V genes, with a similarity of 70–75%.
Construction, expression, and purification of D2C7-(scdsFv)-PE38KDEL immunotoxin
The C-terminus of the D2C7 VH domain was connected to the amino terminus of the VL domain by the 15-amino-acid peptide (Gly4Ser)3 linker. To obtain a stable IT, it is essential to ensure that during renaturation, VH is positioned near VL. This was achieved by mutating a single key residue in each chain to cysteine for the stabilizing disulfide bond to form. Following procedures used for developing other dsFv-recombinant ITs, we chose to mutate residue 44 in the framework region 2 (FR2) of VH and residue 100 in the FR4 of VL (according to the Kabat numbering) to cysteine (40). Thus, we prepared an Fv containing both a peptide linker and a disulfide bond generated by cysteine residues that replace Ser44 of VH and Gly100 of VL. The D2C7-(scdsFv) PCR fragment was then fused to DNA for domains II and III of Pseudomonas exotoxin A. The Pseudomonas exotoxin A version used here, PE38KDEL, has a modified C-terminus that increases its intracellular retention, thereby enhancing its cytotoxicity. The D2C7-(scdsFv)-PE38KDEL was expressed and purified as described in “Material and Methods” to >95% purity (Supplementary Fig. S2).
Antigen binding characteristics of D2C7-(scdsFv)-PE38KDEL antibody
The antigen-binding capability of the D2C7-(scdsFv)-PE38KDEL IT was assessed by surface plasmon resonance (Biacore, GE Healthcare, Piscataway, NJ). The purified D2C7-(scdsFv)-PE38KDEL was applied to sensor chips coated with either purified recombinant EGFRwt or EGFRvIII ECD proteins (Supplementary Fig. S3). The D2C7-(scdsFv)-PE38KDEL bound to both the EGFRwt- and the EGFRvIII-ECD-protein-coated chips. The KD of D2C7-(scdsFv)-PE38KDEL on the EGFRwt- and EGFRvIII-coated chips were 1.6×10−9 M and 1.3×10−9 M, respectively. Thus, the cloned D2C7-(scdsFv)-PE38KDEL IT bound with similar kinetics to both the wild-type and the mutant EGFR proteins.
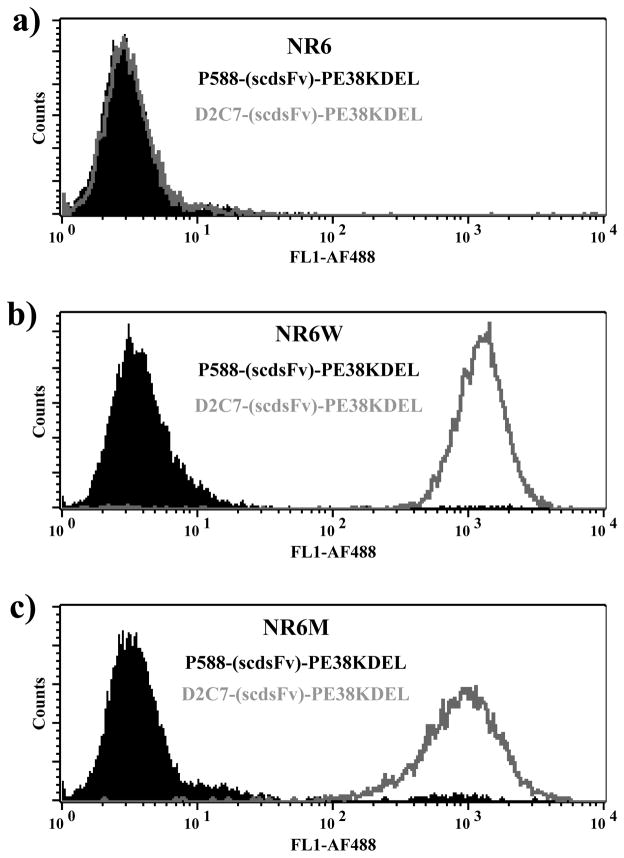
To determine whether the D2C7-(scdsFv)-PE38KDEL IT binds to native EGFRwt and EGFRvIII proteins expressed on the cell surface, indirect flow cytometric analysis was performed (Fig. 2). FACS analysis revealed that the D2C7-(scdsFv)-PE38KDEL bound to both the EGFRwt-expressing NR6W cells (Fig. 2b) and the EGFRvIII-expressing NR6M cells (Fig. 2c). The parental NR6 cells (Fig. 2a) and the nonspecific IT p588-(scdsFv)-PE38KDEL were used as negative controls, which confirmed the binding specificity of D2C7-(scdsFv)-PE38KDEL. These results demonstrate that the D2C7-(scdsFv)-PE38KDEL IT binds to both purified EGFRwt and EGFRvIII proteins on a chip and to native protein molecules expressed on transfected cells.
Figure 2.
Flow cytometric analysis of D2C7-(scdsFv)-PE38KDEL to determine reactivity of the D2C7 IT. (a) Parental NR6 cells were used as control. Indirect FACS analysis demonstrates the reactivity of D2C7-(scdsFv)-PE38KDEL IT with cells expressing EGFRwt (NR6W) (b) or EGFRvIII (NR6M) (c). Cells were stained with D2C7-(scdsFv)-PE38KDEL (grey open peaks) or a non-specific scFv derived immunotoxin (P588-(scdsFv)-PE38KDEL) control (filled black peaks).
Cytotoxicity of D2C7-(scdsFv)-PE38KDEL on transfected cells and cancer cells
We next examined the effects of the D2C7-(scdsFv)-PE38KDEL IT on EGFRwt- or EGFRvIII-transfected NR6W and NR6M cell lines, respectively. The ability of the D2C7-(scdsFv)-PE38KDEL to inhibit protein synthesis was used as a measure of its cytotoxic effect. The cytotoxicity of the D2C7-(scdsFv)-PE38KDEL IT was compared to that of a known EGFRwt-specific IT, TP-38 (30), and that of a known EGFRvIII-specific IT, MR1-1 (32). We initially evaluated the cytotoxicity of the various ITs to the EGFRwt-expressing NR6W cells. The IC50 of the D2C7-(scdsFv)-PE38KDEL IT on NR6W cells was 10 times lower than that of the EGFRwt-specific IT, TP-38 (0.467 vs. 4.6) (Table 1). Even on the NR6M cells, D2C7-(scdsFv)-PE38KDEL IT had an IC50 value 1.6 times lower than that of the EGFRvIII-specific IT, MR1-1 (0.253 vs. 0.413) (Table 1).
Table 1.
Cytotoxicity of D2C7 (EGFRwt and EGFRvIII), TP-38 (EGFRwt) and MR1-1 (EGFRvIII) immunotoxins toward EGFRwt/EGFRvIII transfected cell lines*
| Cell line | D2C7 IC50 ng/ml |
TP-38 IC50 ng/ml |
MR1-1 IC50 ng/ml |
|---|---|---|---|
| NR6W | 0.467 ± 0.18 | 4.6 ± 2.2 | 74.7 ± 17.9 |
| NR6M | 0.253 ± 0.04 | 101.7 ± 14.4 | 0.413 ± 0.03 |
Cytotoxicity data are given as an IC50 value, the concentration of immunotoxin that causes a 50% inhibition of protein synthesis after a 20-h incubation with immunotoxin. All the assays were performed in triplicate for each cell line. Results are presented as mean ± standard deviation where n ≥ 2.
The cytotoxic effects of D2C7-(scdsFv)-PE38KDEL were also tested on various EGFRwt- and EGFRvIII-positive human cancer cells. The A431-epidermoid-carcinoma cell line overexpresses wild-type EGFR protein. Freshly isolated cells from glioblastoma xenografts, 43 and D08-0493MG express EGFRwt protein and D2159MG and D270MG express both EGFRwt and EGFRvIII proteins. Prominently, FACS analysis demonstrated the co-expression of cancer stem cell marker CD133 and D2C7 on 71% and 86% of cells from glioblastoma xenografts 43 and D2159MG, respectively (Supplementary Fig. S4). As shown in Table 2, the D2C7-(scdsFv)-PE38KDEL was highly effective, with an IC50 of 0.18–2.5 ng/ml, in killing the A431 human cancer cell line and all of the glioblastoma xenograft cells tested.
Table 2.
Cytotoxicity of D2C7 immunotoxin toward various cancer cells*
| Cell line | Cancer type | D2C7-(scdsFv)-PE38KDEL IC50 ng/ml |
|---|---|---|
| A431 (EGFRwt) | Epidermoid carcinoma | 0.180 ± 0.014 |
| 43 (EGFRwt) | Glioblastoma | 2.28 ± 0.85 |
| D08-0493MG (EGFRwt) | Glioblastoma | 2.5 ± 0.99 |
| D2159MG (EGFRwt and EGFRvIII) | Glioblastoma | 0.204 ± 0.220 |
| D270MG (EGFRwt and EGFRvIII) | Glioblastoma | 0.265 ± 0.134 |
All the cells are of human origin. Cytotoxicity data are given as an IC50 value; the concentration of immunotoxin that causes a 50% inhibition of protein synthesis after a 20-h incubation with immunotoxin. All the assays were performed in triplicate for each cell line. Results are presented as mean ± standard deviation where n ≥ 2.
Stability of D2C7-(scdsFv)-PE38KDEL IT
The therapeutic efficacy of an IT is greatly influenced by its stability. Hence, the stability of D2C7-(scdsFv)-PE38KDEL was determined at two different concentrations over a seven day period at 37°C by measuring its cytotoxic activity against NR6M cells as described in “Supplementary Methods.” Statistical analysis (P = 0.103) of the seven day IC50 values established D2C7-(scdsFv)-PE38KDEL to be highly stable throughout the assay period (Supplementary Fig. S5).
Efficacy of D2C7-(scdsFv)-PE38KDEL in intracranial tumor models
The D2C7-(scdsFv)-PE38KDEL is human specific and does not react with murine EGFR (Supplementary Fig. S6). As a model for tumors expressing EGFRwt, glioblastoma xenograft 43 was chosen; as a model for tumors expressing both EGFRwt and EGFRvIII, glioblastoma xenograft D270MG was chosen. Since the EGFRvIII mutation is most frequently reported in glioblastomas with EGFR amplification (19), there is no true glioblastoma xenograft that expresses EGFRvIII only. Therefore, we choose the fibroblast cell line NR6M, transfected with the human EGFRvIII as a model for EGFRvIII expressing tumor. The survival curve graph for 43 (Supplementary Fig. S7a), NR6M (Supplementary Fig. S7b), and D270MG (Supplementary Fig. S7c) demonstrated that 100% death occurred at day 18, day 15, and day 36 post-tumor implantation for 43, NR6M, and D270MG, respectively. Hence, day 6 (43 xenograft), day 5 (NR6M), and day 11 (D270MG) post-tumor implantation were chosen as the optimal days to study the efficacy of D2C7-(scdsFv)-PE38KDEL. Further, toxicity studies in NSG mice with different concentrations of the D2C7-(scdsFv)-PE38KDEL (1–10 μg/100 μl) demonstrated that, 1 μg of D2C7-(scdsFv)-PE38KDEL had no toxicity-associated mortality (Supplementary Fig. S8).
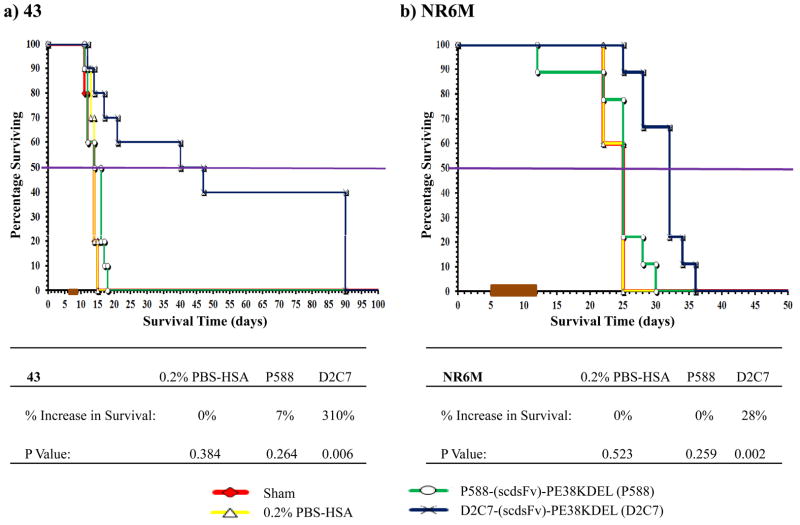
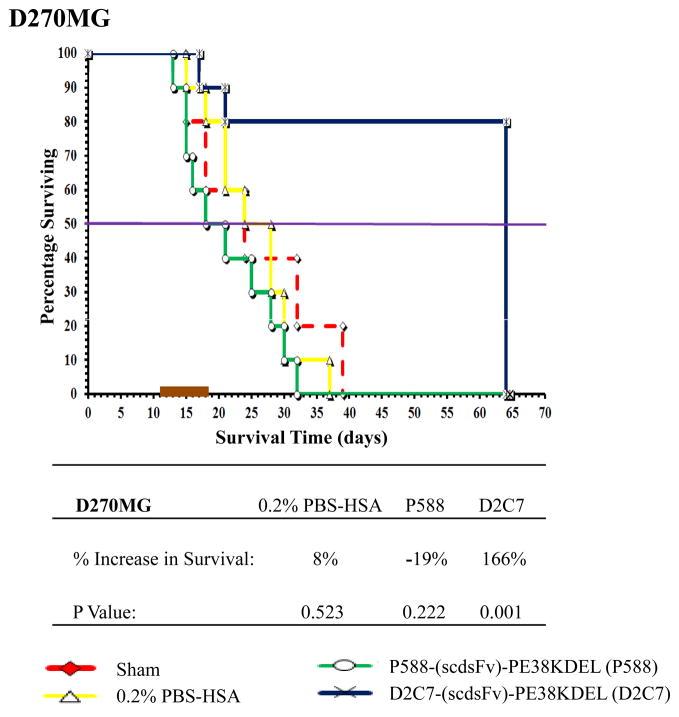
In the 43 intracranial tumor model overexpressing EGFRwt protein in the absence of EGFRwt amplification, orthotopic delivery of D2C7-(scdsFv)-PE38KDEL prolonged the survival by 310% (P=0.006) (Fig. 3a). The group treated with the control IT, P588-(scdsFv)-PE38KDEL, showed only a 7% increase in survival with a P value of 0.264. Four out of ten 43 tumor-bearing mice in the D2C7-(scdsFv)-PE38KDEL treatment group were still alive at the termination of the study. Similarly, in the EGFRvIII-expressing NR6M orthotopic tumor model, the control IT, P588-(scdsFv)-PE38KDEL, failed to show any response while D2C7-(scdsFv)-PE38KDEL treatment demonstrated a statistically significant (P=0.002) increase in survival by 28% (Fig. 3b). Likewise, in the D270MG intracranial tumor model expressing both EGFRwt and EGFRvIII, delivery of D2C7-(scdsFv)-PE38KDEL by CED prolonged the survival by 166% (P=0.001) (Fig. 4). Notably, 8/10 of the D270MG tumor-bearing mice in the D2C7-(scdsFv)-PE38KDEL treatment group were still healthy and alive at the termination of the study.
Figure 3.
Effect of D2C7-(scdsFv)-PE38KDEL on 43 and NR6M intracranial tumors in NSG mice. Male NSG mice (≈ 30 g; 8–12 weeks) bearing 43 (a) or NR6M (b) tumors were randomized into four groups, Sham, 0.2% PBS/HSA, P588-(scdsFv)-PE38KDEL, and D2C7-(scdsFv)-PE38KDEL. The test mice were treated over a three (43 xenograft) or seven (NR6M) day period with a total of 1 μg of D2C7-(scdsFv)-PE38KDEL diluted in 100 μl 0.2% PBS-HSA. Control mice were either left untreated (Sham) or treated with 0.2% PBS-HSA or 1 μg of the control immunotoxin, P588-(scdsFv)-PE38KDEL, diluted in 100 μl 0.2% PBS-HSA.
Figure 4.
Effect of D2C7-(scdsFv)-PE38KDEL on D270MG intracranial tumors in NSG mice. Male NSG mice (≈ 30 g; 8–12 weeks) bearing D270MG tumors were randomized into four groups, Sham, 0.2% PBS/HSA, P588-(scdsFv)-PE38KDEL, and D2C7-(scdsFv)-PE38KDEL. The test mice were treated over a seven day period with a total of 1 μg of D2C7-(scdsFv)-PE38KDEL diluted in 100 μl 0.2% PBS-HSA. Control mice were either left untreated (Sham) or treated with 0.2% PBS-HSA or 1 μg of the control immunotoxin, P588-(scdsFv)-PE38KDEL, diluted in 100 μl 0.2% PBS-HSA.
Assessment of D2C7-(scdsFv)-PE38KDEL tumor distribution after CED
Sufficiently high concentrations of D2C7-(scdsFv)-PE38KDEL in the tumor area are achieved by CED. However, the anti-tumor efficacy of D2C7-(scdsFv)-PE38KDEL depends on its homogenous distribution within the tumor area. Tumor distribution of D2C7-(scdsFv)-PE38KDEL administered by CED in D270MG intracranial model was studied by immunohistochemistry. Tumor sections from the D270MG-sham group and the D270MG-D2C7-(scdsFv)-PE38KDEL group were used as the negative control (Supplementary Fig. S9a) and the test specimen (Supplementary Fig. S9c), respectively. The D270MG-D2C7-(scdsFv)-PE38KDEL group, pre-stained with D2C7-(scdsFv)-PE38KDEL, served as the positive control (Supplementary Fig. S9b). Supplementary Figures S9b and S9c clearly demonstrate the identical staining pattern of both the positive control and the test specimen, thereby establishing the uniform distribution of D2C7-(scdsFv)-PE38KDEL within the tumor area.
Discussion
To achieve the clinical goal of successful immunotherapy for solid intracranial tumors, investigators have begun developing mAbs or recombinant scFvs against tumor antigens. One such antibody, D2C7, recognizes both the EGFRwt and the mutant EGFRvIII, two proteins that are overexpressed in glioblastoma. The D2C7 antibody exhibited significant reactivity against both EGFRwt and EGFRvIII on tissue sections from adult glioblastoma patients. It also showed significant affinity by surface plasmon resonance and specificity by flow cytometry (36). The high affinity, specificity, and reactivity of this mAb make it an ideal candidate for the construction of a recombinant IT that targets tumors overexpressing EGFRwt and EGFRvIII proteins. Due to the prevalence of different EGFR deletion mutants in glioblastoma, we deemed it necessary to identify the epitope for D2C7 on its target EGFRwt/EGFRvIII proteins. The 55 amino acid D2C7 epitope (EGFRwt 583–637 amino acids) is known to be present in EGFR deletion mutants C-958, Δ959–1030, Δ6–185, I, III– VII (19, 41), thereby increasing the number of antigenic targets for D2C7.
Subsequently, we cloned an EGFRwt/EGFRvIII specific IT, D2C7-(scdsFv)-PE38KDEL, from the D2C7 hybridoma and characterized its efficacy using in vitro and in vivo models. The in vitro cytotoxicity data showed that D2C7-(scdsFv)-PE38KDEL effectively inhibits protein synthesis in a variety of EGFRwt- or EGFRwt- and EGFRvIII-expressing glioblastoma xenograft cells and human tumor cell line. Notably, in the intracranial animal models of EGFRwt-expressing glioma xenograft 43, EGFRvIII-expressing NR6M, and EGFRwt- and EGFRvIII-expressing glioblastoma xenograft D270MG, D2C7-(scdsFv)-PE38KDEL demonstrated significant increase in survival, 310% (P=0.006), 28% (P=0.002), and 166% (P=0.001), respectively. To the best of our knowledge, this is the first report demonstrating that an IT can target both the wild-type EGFR and the mutant EGFRvIII in glioma models.
In order to demonstrate significant therapeutic efficacy when administered to brain tumor patients, an IT has to be considerably stable over long periods of time. Hence, in addition to the conventional 15-amino-acid peptide linker present between the VH and VL domain of D2C7 scFv, we introduced an inter-chain disulfide bond between the structurally conserved framework regions of the D2C7 VH and VL domains, to construct a new disulfide-stabilized IT termed D2C7-(scdsFv)-PE38KDEL. The disulfide-stabilized D2C7-(scdsFv)-PE38KDEL was highly stable over a seven day period, with a P value of 0.103.
In in vitro studies, D2C7-(scdsFv)-PE38KDEL inhibited protein synthesis in EGFRvIII-expressing NR6M cells at a concentration 402 times lower than TP-38, an EGFRwt-specific transforming growth factor alpha-based IT. Further, D2C7-(scdsFv)-PE38KDEL inhibited protein synthesis at a concentration 160 times lower than MR1-1, an EGFRvIII-specific IT, in EGFRwt-expressing NR6W cells. Any therapy that fails to target the EGFRwt-expressing cells or constitutively autophosphorylated EGFRvIII-expressing cells will lead to enhanced proliferation and migration of these tumor cells. Further, any surviving EGFRvIII-only-expressing tumor cells in tumors that have both EGFRwt and EGFRvIII will promote resistance to EGFR antibody therapy (23). In our in vitro studies with EGFRwt- or both EGFRwt- and EGFRvIII-expressing human cancer cells, D2C7-(scdsFv)-PE38KDEL demonstrated significant inhibition of protein synthesis. Further, the D2C7-(scdsFv)-PE38KDEL IT prolonged survival in in vivo tumor models with the 43 xenograft, NR6M, and D270MG xenograft. Examination of brain sections from the 8 euthanized mice from the D270MG-D2C7-(scdsFv)-PE38KDEL treatment group by hematoxylin and eosin staining revealed no tumor cells in 8/8 mice. Macrophage or monocyte infiltration was observed in 4/8 mice. No expression of EGFRwt (EGFR1 mAb), EGFRvIII (L8A4 mAb), or EGFRwt and EGFRvIII (D2C7 mAb) was observed in 8/8 mice, indicating that all the 8 mice in the D270MG-D2C7-(scdsFv)-PE38KDEL treatment group were cured. Hence, we believe that D2C7-(scdsFv)-PE38KDEL is likely to be more efficacious in treating glioblastoma patients whose tumors express both EGFRwt and EGFRvIII or either EGFRwt or EGFRvIII alone. Further, EGFR mutants form homo/heterodimers and currently available EGFR mAbs cetuximab, matuzumab, and panitumumab are unable to block activation of these dimers (42). Since the D2C7 epitope is present on a wide variety of these EGFR deletion mutants, D2C7-(scdsFv)-PE38KDEL treatment should prove efficacious for glioblastoma patients harboring these mutations.
For effective in vivo therapy of glioblastoma it is essential to achieve high local concentration and homogeneous distribution of the IT at the tumor site. In the 43, NR6M, and D270MG models, D2C7-(scdsFv)-PE38KDEL was administered by continuous intracranial delivery through osmotic mini-pumps. This method of continuous intracranial delivery will aid in achieving elevated concentrations and uniform distribution of D2C7-(scdsFv)-PE38KDEL at the tumor site, which would be expected to optimize its anti-tumor activity. By this method we were able to achieve significant increase in survival at a very low dose of 1 μg of D2C7-(scdsFv)-PE38KDEL.
D2C7-(scdsFv)-PE38KDEL might cause human skin and liver toxicity if the immunotoxin were administered by systemic injection. However, since we propose to administer the IT by CED directly into intracranial tumors, any systemic toxicity due to low and clinically insignificant amounts of immunotoxin gaining access to systemic organs and tissues would be minimized by this localized treatment. The regional drug-delivery technique of CED has been used successfully to deliver recombinant toxins targeting EGFRwt (30) and EGFRvIII (43)in malignant glioma patients, without any liver or other systemic organ toxicity.
The in vitro internalization studies demonstrated rapid uptake of D2C7-(scdsFv)-PE38KDEL by NR6W and NR6M cells within 1–2 hours of treatment (data not shown). We hypothesize a similar uptake of D2C7-(scdsFv)-PE38KDEL by tumor cells upon infusion and fast clearance of the immunotoxin within the tumor area. Moreover, our immunohistochemistry studies in the D270MG orthotopic model clearly demonstrate that homogenous distribution of the D2C7-(scdsFv)-PE38KDEL is essential to generating a significant anti-tumor response. Consequently, tumor cells that failed to encounter D2C7-(scdsFv)-PE38KDEL might regrow and repopulate the tumor area. Thus, tumor recurrence in our in vivo models might be due to both the swift depletion of the low levels of D2C7-(scdsFv)-PE38KDEL (1 μg) and lack of uniform distribution, and not due to escape from immunotoxin efficacy.
Due to the recurrent nature of malignant gliomas, as well as the diversity of antigens populating the glioma cell surface, there is a need for innovative immunotherapeutic approaches. In an earlier study, Schmidt et al. described a recombinant IT, scFv(14E1)-ETA, which bound both EGFRwt and EGFRvIII (34). The activity of scFv(14E1)-ETA was demonstrated both in vitro and in vivo, but only on the epidermoid carcinoma cell line A431 or the EGFRwt- or EGFRvIII-transfected murine renal carcinoma cells (Renca-lacZ/EGFR or Renca-lacZ/EGFRvIII) and not on EGFRwt- and EGFRvIII-expressing human glioma models. Moreover, the Schmidt et al. study did not include immunohistochemical analyses that would show the binding of their construct to human glioma tissue or any other human cancer tissue. In this regard, D2C7-(scdsFv)-PE38KDEL is a novel IT that can target both the EGFRwt and the EGFRvIII proteins that are frequently overexpressed in malignant gliomas.
Monoclonal antibodies 528 and 806, with dual specificity for wild-type and mutant EGFR proteins expressed on different cell lines, have been well described in previous studies (44, 45). Combination therapy using mAbs 528 and 806 demonstrated a significant decrease in tumor volume of xenografts expressing EGFRwt or EGFRvIII (46)_ENREF_43. Such combination therapies targeting multiple tumor-associated molecules involve high development, manufacturing, and treatment costs. These intrinsic difficulties associated with combination antibody therapies will be circumvented with our EGFRwt/EGFRvIII specific D2C7-(scdsFv)-PE38KDEL.
In conclusion, we have created a scFv molecule that is capable of mediating selective in vitro and in vivo tumor targeting. We believe this to be the first significant evidence demonstrating enhanced glioblastoma tumor targeting with high selectivity and specificity by an antibody specific for two glioma-associated antigens. Taken together, our results suggest that D2C7-(scdsFv)-PE38KDEL should be clinically efficacious against brain tumors expressing EGFRwt or EGFRvIII alone or together. A GLP clinical preparation of D2C7-(scdsFv)-PE38KDEL has been prepared, after which we will obtain an FDA IND. NCI funding (P01 CA154291-01) for Phase I and II trials of D2C7-(scdsFv)-PE38KDEL by CED in glioblastoma patients has been obtained. A previous, randomized trial of an IL13-PE38 immunotoxin in recurrent glioblastoma patients failed to show a survival advantage over Gliadel wafers (47). There was no imaging of delivery in that trial, and later studies showed that the IL13 receptor is only expressed on about 40% of glioblastoma (48). Moreover, there was heterogeneous expression among glioblastoma cells within 40% of positive cases. It is likely that the IL13-PE38 trial failed because of poor delivery from the lack of imaging during delivery, as well as the low, heterogeneous expression of the IL13 receptor on a minority of glioblastoma cases.
We have recently demonstrated successful imaging of an immunotoxin, MR1-1-PE38, similar in size to our D2C7-(scdsFv)-PE38KDEL, in a clinical trial with CED. Successful imaging of the delivery of D2C7-(scdsFv)-PE38KDEL will greatly improve treatment efficacy (43, 49). As discussed above, the high levels of homogeneous expression of the D2C7-(scdsFv)-PE38KDEL molecular targets, wild-type EGFR and EGFRvIII (both of which are major glioblastoma driver oncogenes), in more than 95% of newly diagnosed glioblastoma tumors also will improve D2C7-(scdsFv)-PE38KDEL efficacy over other immunotoxins previously used in glioblastoma clinical trials.
Supplementary Material
Translational Relevance.
Glioblastoma multiforme is the most malignant and most frequently occurring brain tumor. The epidermal growth factor receptor (EGFRwt) and its mutant, EGFRvIII, which is not found on normal tissues, are overexpressed in 60–90% and 58–61% of all glioblastomas, respectively. D2C7-(scdsFv)-PE38KDEL is a recombinant immunotoxin (IT), targeting both EGFRwt and the tumor-specific EGFRvIII.
We show that D2C7-(scdsFv)-PE38KDEL has significant anti-tumor activity due to its affinity for EGFRwt and EGFRvIII. This IT is efficacious in in vitro cytotoxicity assays and in in vivo orthotopic models of glioblastoma xenograft cells that express EGFRwt, EGFRvIII, or both EGFRwt and EGFRvIII.
The dual-specific activity of this new toxin should translate well into the clinical setting. The specificity and high binding affinity toward both targets indicate that D2C7-(scdsFv)-PE38KDEL is likely to show greater efficiency in treating brain tumors than monospecific therapeutics. Funding has been awarded for Phase I and II clinical trials of D2C7-(scdsFv)-PE38KDEL in glioblastoma patients.
Acknowledgments
Grant sponsor: National Institute of Neurological Disorders and Stroke (NIH); National Cancer Institute (NIH); Grant numbers: P50 NS020023-29, P01 CA154291-01, R37 CA011898-42 (D.D. Bigner); P30 CA014236-39 (M.B. Kastan); Grant sponsor: Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (I.H. Pastan).
The present study was funded in part by the following NIH grants: P50 NS020023-29, P01 CA154291-01, R37 CA011898-42 (NCI Merit Award) (D.D. Bigner), and P30 CA014236-39 (M.B. Kastan). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (I.H. Pastan). We thank Janet Parsons and Lauren Albritton for editorial review of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest: Ira H. Pastan holds patents on immunotoxins, all of which have been assigned to the National Institutes of Health. Darell Bigner, Chien-Tsun Kuan, and Charles Pegram hold submitted patents on D2C7-(scdsFv)-PE38KDEL, which have been assigned to Duke University.
References
- 1.Louis DN, Gusella JF. A tiger behind many doors: multiple genetic pathways to malignant glioma. Trends Genet. 1995;11:412–5. doi: 10.1016/s0168-9525(00)89125-8. [DOI] [PubMed] [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2005–2009. Neuro-oncology. 2012;14 (Suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker MD, Alexander E, Jr, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 4.Levin VA, Wara WM, Davis RL, Vestnys P, Resser KJ, Yatsko K, et al. Phase III comparison of BCNU and the combination of procarbazine, CCNU, and vincristine administered after radiotherapy with hydroxyurea for malignant gliomas. J Neurosurg. 1985;63:218–23. doi: 10.3171/jns.1985.63.2.0218. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–36. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 6.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 7.Boskovitz A, Wikstrand CJ, Kuan CT, Zalutsky MR, Reardon DA, Bigner DD. Monoclonal antibodies for brain tumour treatment. Expert Opin Biol Ther. 2004;4:1453–71. doi: 10.1517/14712598.4.9.1453. [DOI] [PubMed] [Google Scholar]
- 8.Archer GE, Sampson JH, Lorimer IA, McLendon RE, Kuan CT, Friedman AH, et al. Regional treatment of epidermal growth factor receptor vIII-expressing neoplastic meningitis with a single-chain immunotoxin, MR-1. Clin Cancer Res. 1999;5:2646–52. [PubMed] [Google Scholar]
- 9.Pastan IH, Archer GE, McLendon RE, Friedman HS, Fuchs HE, Wang QC, et al. Intrathecal administration of single-chain immunotoxin, LMB-7 [B3(Fv)-PE38], produces cures of carcinomatous meningitis in a rat model. Proc Natl Acad Sci U S A. 1995;92:2765–9. doi: 10.1073/pnas.92.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandramohan V, Bao X, Kato Kaneko M, Kato Y, Keir ST, Szafranski SE, et al. Recombinant anti-podoplanin (NZ-1) immunotoxin for the treatment of malignant brain tumors. International journal of cancer Journal international du cancer. 2013;132:2339–48. doi: 10.1002/ijc.27919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandramohan V, Sampson JH, Pastan I, Bigner DD. Toxin-based targeted therapy for malignant brain tumors. Clinical & developmental immunology. 2012;2012:480429. doi: 10.1155/2012/480429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 13.Arita N, Hayakawa T, Izumoto S, Taki T, Ohnishi T, Yamamoto H, et al. Epidermal growth factor receptor in human glioma. J Neurosurg. 1989;70:916–9. doi: 10.3171/jns.1989.70.6.0916. [DOI] [PubMed] [Google Scholar]
- 14.Libermann TA, Razon N, Bartal AD, Yarden Y, Schlessinger J, Soreq H. Expression of epidermal growth factor receptors in human brain tumors. Cancer research. 1984;44:753–60. [PubMed] [Google Scholar]
- 15.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaffanet M, Chauvin C, Laine M, Berger F, Chedin M, Rost N, et al. EGF receptor amplification and expression in human brain tumours. Eur J Cancer. 1992;28:11–7. doi: 10.1016/0959-8049(92)90374-b. [DOI] [PubMed] [Google Scholar]
- 18.Huang SM, Harari PM. Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest New Drugs. 1999;17:259–69. doi: 10.1023/a:1006384521198. [DOI] [PubMed] [Google Scholar]
- 19.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer research. 2000;60:1383–7. [PubMed] [Google Scholar]
- 20.Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci U S A. 1990;87:8602–6. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batra SK, Castelino-Prabhu S, Wikstrand CJ, Zhu X, Humphrey PA, Friedman HS, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ. 1995;6:1251–9. [PubMed] [Google Scholar]
- 22.Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A. 1990;87:4207–11. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–73. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 24.Wikstrand CJ, Hale LP, Batra SK, Hill ML, Humphrey PA, Kurpad SN, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer research. 1995;55:3140–8. [PubMed] [Google Scholar]
- 25.Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, et al. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19:713–23. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laskin JJ, Sandler AB. Epidermal growth factor receptor: a promising target in solid tumours. Cancer Treat Rev. 2004;30:1–17. doi: 10.1016/j.ctrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Brady LW, Miyamoto C, Woo DV, Rackover M, Emrich J, Bender H, et al. Malignant astrocytomas treated with iodine-125 labeled monoclonal antibody 425 against epidermal growth factor receptor: a phase II trial. Int J Radiat Oncol Biol Phys. 1992;22:225–30. doi: 10.1016/0360-3016(92)91009-c. [DOI] [PubMed] [Google Scholar]
- 28.Epenetos AA, Courtenay-Luck N, Pickering D, Hooker G, Durbin H, Lavender JP, et al. Antibody guided irradiation of brain glioma by arterial infusion of radioactive monoclonal antibody against epidermal growth factor receptor and blood group A antigen. Br Med J (Clin Res Ed) 1985;290:1463–6. doi: 10.1136/bmj.290.6480.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalofonos HP, Pawlikowska TR, Hemingway A, Courtenay-Luck N, Dhokia B, Snook D, et al. Antibody guided diagnosis and therapy of brain gliomas using radiolabeled monoclonal antibodies against epidermal growth factor receptor and placental alkaline phosphatase. J Nucl Med. 1989;30:1636–45. [PubMed] [Google Scholar]
- 30.Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. Journal of neuro-oncology. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 31.Liu TF, Hall PD, Cohen KA, Willingham MC, Cai J, Thorburn A, et al. Interstitial diphtheria toxin-epidermal growth factor fusion protein therapy produces regressions of subcutaneous human glioblastoma multiforme tumors in athymic nude mice. Clin Cancer Res. 2005;11:329–34. [PubMed] [Google Scholar]
- 32.Beers R, Chowdhury P, Bigner D, Pastan I. Immunotoxins with increased activity against epidermal growth factor receptor vIII-expressing cells produced by antibody phage display. Clin Cancer Res. 2000;6:2835–43. [PubMed] [Google Scholar]
- 33.Kuan CT, Reist CJ, Foulon CF, Lorimer IA, Archer G, Pegram CN, et al. 125I-labeled anti-epidermal growth factor receptor-vIII single-chain Fv exhibits specific and high-level targeting of glioma xenografts. Clin Cancer Res. 1999;5:1539–49. [PubMed] [Google Scholar]
- 34.Schmidt M, Maurer-Gebhard M, Groner B, Kohler G, Brochmann-Santos G, Wels W. Suppression of metastasis formation by a recombinant single chain antibody-toxin targeted to full-length and oncogenic variant EGF receptors. Oncogene. 1999;18:1711–21. doi: 10.1038/sj.onc.1202489. [DOI] [PubMed] [Google Scholar]
- 35.Neyns B, Sadones J, Joosens E, Bouttens F, Verbeke L, Baurain JF, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20:1596–603. doi: 10.1093/annonc/mdp032. [DOI] [PubMed] [Google Scholar]
- 36.Zalutsky MR, Boskovitz A, Kuan CT, Pegram CN, Ayriss J, Wikstrand CJ, et al. Radioimmunotargeting of malignant glioma by monoclonal antibody D2C7 reactive against both wild-type and variant III mutant epidermal growth factor receptors. Nucl Med Biol. 2012;39:23–34. doi: 10.1016/j.nucmedbio.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchner J, Pastan I, Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992;205:263–70. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- 38.Friedman HS, Colvin OM, Skapek SX, Ludeman SM, Elion GB, Schold SC, Jr, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer research. 1988;48:4189–95. [PubMed] [Google Scholar]
- 39.Gehan EA. A Generalized Wilcoxon Test for Comparing Arbitrarily Singly-Censored Samples. Biometrika. 1965;52:203–23. [PubMed] [Google Scholar]
- 40.Reiter Y, Brinkmann U, Lee B, Pastan I. Engineering antibody Fv fragments for cancer detection and therapy: disulfide-stabilized Fv fragments. Nat Biotechnol. 1996;14:1239–45. doi: 10.1038/nbt1096-1239. [DOI] [PubMed] [Google Scholar]
- 41.Rasheed BK, Wiltshire RN, Bigner SH, Bigner DD. Molecular pathogenesis of malignant gliomas. Curr Opin Oncol. 1999;11:162–7. doi: 10.1097/00001622-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Gajadhar AS, Bogdanovic E, Munoz DM, Guha A. In situ analysis of mutant EGFRs prevalent in glioblastoma multiforme reveals aberrant dimerization, activation, and differential response to anti-EGFR targeted therapy. Mol Cancer Res. 2012;10:428–40. doi: 10.1158/1541-7786.MCR-11-0531. [DOI] [PubMed] [Google Scholar]
- 43.Sampson JH, Brady M, Raghavan R, Mehta AI, Friedman AH, Reardon DA, et al. Colocalization of gadolinium-diethylene triamine pentaacetic acid with high-molecular-weight molecules after intracerebral convection-enhanced delivery in humans. Neurosurgery. 2011;69:668–76. doi: 10.1227/NEU.0b013e3182181ba8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johns TG, Stockert E, Ritter G, Jungbluth AA, Huang HJ, Cavenee WK, et al. Novel monoclonal antibody specific for the de2-7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. International journal of cancer Journal international du cancer. 2002;98:398–408. doi: 10.1002/ijc.10189. [DOI] [PubMed] [Google Scholar]
- 45.Masui H, Kawamoto T, Sato JD, Wolf B, Sato G, Mendelsohn J. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer research. 1984;44:1002–7. [PubMed] [Google Scholar]
- 46.Perera RM, Narita Y, Furnari FB, Gan HK, Murone C, Ahlkvist M, et al. Treatment of human tumor xenografts with monoclonal antibody 806 in combination with a prototypical epidermal growth factor receptor-specific antibody generates enhanced antitumor activity. Clin Cancer Res. 2005;11:6390–9. doi: 10.1158/1078-0432.CCR-04-2653. [DOI] [PubMed] [Google Scholar]
- 47.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-oncology. 2010;12:871–81. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer research. 2007;67:7983–6. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 49.Ding D, Kanaly CW, Bigner DD, Cummings TJ, Herndon JE, 2nd, Pastan I, et al. Convection-enhanced delivery of free gadolinium with the recombinant immunotoxin MR1-1. Journal of neuro-oncology. 2010;98:1–7. doi: 10.1007/s11060-009-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.