Abstract
BACKGROUND
To evaluate the hypothesis that functionally over-expressing alleles of the serotonin transporter gene SLC6A4 are present in Tourette disorder (TD), just as we have found in obsessive compulsive disorder (OCD), we evaluated TD probands (N=151) and controls (N=858).
METHODS
We genotyped the refined 5-HTTLPR/rs25531 locus and the associated rs25532 variant in the SLC6A4 promoter plus the rare coding variant SERT I425V.
RESULTS
The higher-expressing 5-HTTLPR/rs25531 LA allele was more prevalent in TD probands than controls (χ2=5.75, p=0.017, OR=1.35), and in a secondary analysis, surprisingly found to be significantly more frequent in probands with TD alone than in those with TD plus OCD (Fisher's exact test, p=0.0006, OR=2.29). Likewise, the higher-expressing LAC haplotype (5-HTTLPR/rs25531/rs25532) was more frequent in TD probands than controls (p=0.024, OR=1.33) and likewise in the TD alone versus TD plus OCD group (p=0.0013, OR=2.14). Further, the rare gain-of-function SERT I425V variant was found in three male siblings with TD and/or OCD and in their father. The cumulative count of SERT I425V thus becomes 1.57% in OCD/TD spectrum conditions vs. 0.15% in controls, with a recalculated, family-adjusted significance of χ2=15.03, p < 0.0001, OR=9.0 (total worldwide genotyped=2914).
CONCLUSIONS
This report provides a unique combination of common and rare variants in one gene in TD, all found to be associated with SLC6A4 gain of function. Thus, altered SERT activity represents a potential contributor to serotonergic abnormalities in TD. Present results call for replication in a similarly intensively evaluated sample.
Keywords: SLC6A4, SERT, Tourette, serotonin, 5-HTTLPR, SERT I425V
INTRODUCTION
Tourette disorder (TD) is a complex neurodevelopmental disorder characterized by chronic, fluctuating, involuntary motor and vocal tics. Comorbidity is a hallmark feature of TD, particularly with respect to obsessive-compulsive disorder (OCD) (up to 60%) and attention deficit hyperactivity disorder (ADHD) (~40%)1–3. These comorbidity patterns are striking in that individuals with a primary OCD or ADHD diagnosis have lower rates of comorbid TD (<10%, although still relatively high compared to the general population)1–4. Family studies show that tic disorders are increased in frequency in relatives of OCD probands and that the frequency of TD, TD plus OCD and also OCD alone are higher in the relatives of TD probands5. Taken together, these data suggest a possible etiological connection between these three disorders. Better understanding of this connection is of key importance since TD (0.3 – 1%), OCD (2.5%) and ADHD (5–10% in children, 2% in adults) are major health burdens, affecting all populations worldwide. While TD-related tics seem to diminish over the lifespan, OCD and to some extent ADHD can be life-long, impairing disorders which are only partially responsive to present treatments6–8.
Among specific genes recently investigated in neuropsychiatric disorders including OCD and ADHD, the serotonin transporter (SERT) gene, SLC6A4 stands out. SLC6A4 maps to chromosome 17q11.2 and is composed of 14 exons spanning 40 kb. The protein, SERT, has 603 amino acids and twelve transmembrane domains regulating the neurotransmitter reuptake and serotonin accumulation in multiple cell types. A 43 bp indel in the promoter region, the 5-HTTLPR (serotonin transporter-linked polymorphic region) modulates SLC6A4 expression. At the 5-HTTLPR locus, the short (S) allele shows lower expression compared to the long (L) allele9. When the G allele of rs25531 is present with the 5-HTTLPR Long (L) allele, the resulting LG allele has reduced expression comparable to the short (S) allele, rendering the LA allele as the only truly high-expressing variant10. Additional functional refinement of SLC6A4 promoter variants was suggested by us in 2008, when we showed that the functional C>T rs25532 further modulates 5-HTTLPR/rs25531 activity, indicating that LAC is the truly highest-expressing haplotype11. Other functional polymorphisms known to affect SLC6A4 expression and function in humans and other species include alternative splicing involving exons 1A, B and C; an intronic (STin2) VNTR, miR-15a/miR-16-mediated regulation as well as additional variants12–15. Since the first report describing the SLC6A4 5-HTTLPR affecting expression and being associated with anxiety-related personality traits9, there has been a constantly growing literature describing altered SERT expression and function associated with multiple disorders including anxiety spectrum disorders like OCD, bipolar disorder, depression, ADHD, autism and neurodevelopmental and peripherally-based disorders of cardiovascular, bone, gastrointestinal, endocrine and other systems10, 15–18.
The present report provides the first comprehensive assessment of TD probands and healthy controls for functional variants in the SLC6A4 gene. Based upon our prior investigations of these variants in OCD, we evaluated the primary hypothesis that TD and OCD might share similar gain-of-function contributions from these functional SLC6A4 gene variants. We investigated the multiallelic 5-HTTLPR/rs25531 locus found to be associated with OCD in case-control and family studies9, 10, 19. We also evaluated contributions to TD from rs25532, investigated in OCD and also shown to have a greater-functioning C allele11. Finally, we genotyped the rare SLC6A4 coding variant SERT I425V that produces enhanced SERT expression, serotonin uptake and impaired regulation20–24 Thus, we considered whether a combination of common and rare alleles in SLC6A4 might comprise plausible contributions to TD.
METHODS
Subjects
Unrelated TD probands (N=151) were all European ancestry subjects from the New Jersey Center for Tourette Syndrome Sharing Repository maintained by the Rutgers University Cell and DNA Repository25. Methods for clinical evaluations are described elsewhere25. Briefly, consenting subjects (or their legal guardians) completed a self-report questionnaire which included sub-scale assessments on tic disorders (adapted from YGTSS)26, OC disorders (adapted from the Dimensional Yale-Brown Obsessive-Compulsive scale)27 and ADHD checklists (see25 for details). Final DSM-IV-TR diagnoses were made by an experienced child psychiatrist based upon review of the self-report questionnaires and by direct semi-structured interview. Cases were routinely “flagged” for atypical TD presentation or for potentially confounding conditions such as congenital abnormalities or others, as previously described25. Healthy controls were from three independent sources: (1) human random control panels 1 and 2 (N=192) consisting of apparently healthy, UK European ancestry blood donors obtained from the European Collection of Cell Cultures (Sigma-Aldrich, St. Louis, MO, USA); (2) self-declared healthy US European ancestry subjects (N=200) obtained from the Coriell cell repository (Camden, NJ, USA), and (3) self-declared healthy volunteer undergraduate students of European ancestry (N=466) from a large Southeastern university who participated in a separate study of genes and personality in return for partial course credit. All studies were conducted under protocols approved by the Rutgers University Institutional Review Board for the TD probands and by the Human Subjects Committee at Florida State University. Written informed consent was obtained from all adult participants (or, at Rutgers, their legal guardians, with written assent for minors). As only the third control group was evaluated by self-report and standard scales, we cannot rule out the occurrence of TD or OCD within the control groups; however frequencies above the general population prevalence (0.3–1% and 2–3%, respectively) are unlikely and therefore should not have a significant impact on our results. None of the SLC6A4 polymorphisms (specifically including the 5-HTTLPR/rs25531 variants) significantly deviated from Hardy–Weinberg equilibrium as determined by contingency table statistics (data not shown). There were no differences in allele frequency across the three control populations, suggesting no evidence for population stratification. A definitive analysis of multiple ancestry-informative markers or through family-based studies to analyze population substructure was not feasible in the present work.
Genotyping
Deoxyribonucleic acid extraction and genotyping procedures for the 5-HTTLPR/rs25531 locus, rs25532 and I425V have been published previously by our group11, 28. The overall genotype completion rate exceeded 98% for each assay. Samples analyzed in duplicate and no-template-controls consistently yielded expected results.
Statistics
We analyzed 5-HTTLPR/rs25531 as a biallelic locus, treating LA as one group and the combined Lg or S alleles as another. LA alleles markedly differ from LG on the basis of mRNA measurements and reporter expression activity that indicate LG as functionally equivalent to S, complicating previous evaluations of `L' alone10, 11,28, 29. Haplotyping and conditional analyses were done as previously described11. Statistical analyses were performed using Fisher's Exact Test (or the Chi-Square Test for larger sample groups) with significance set at p < 0.05 in two-tailed tests. For the I425V frequency estimate, a correction was made using the within-family coefficient of estimate as previously described by our group11. All test results are presented as nominal (uncorrected) p-values.
RESULTS
Three noteworthy, novel findings emerged from this first reported large study of SLC6A4 functional variants in TD probands (N=151) compared to controls (N=858):
-
A)
An increased frequency of the greater-expressing LA variant was found in TD probands compared to healthy controls (Table 1, χ2 =5.75, p=0.017, OR=1.35, 95% CI: 1.06 to 1.73)10, 11. To investigate whether this association might be different in the presence of comorbid OCD, genotypes of TD probands with (N=63, 42%) or without (N=88, 58%) OCD were compared in a secondary analysis. The LA allele was more frequent in probands with TD alone compared to those with TD plus OCD (Fisher's exact test, p=0.0006, OR=2.29), indicating that the association between SLC6A4 and TD may be dominantly-driven by TD diagnosis (LA allele frequency in TD plus OCD= 0.44; TD alone= 0.64). Significant associations for TD probands were not found at rs25532 with either allele; however, the LAC haplotype was more frequent in TD probands than controls (Table 1, p=0.024, OR=1.33). To evaluate whether this association might be different in the presence of comorbid OCD, we performed a secondary analysis comparing genotypes in those TD probands with or without OCD. The greater expressing LAC haplotype was more frequent in probands with TD alone compared to those with TD plus OCD (p=0.0013, OR=2.14), like that for the LA variant alone11. Likewise, there was a greater frequency of the rs25532 C allele in the TD alone group vs. the TD plus OCD group (p=0.018, OR=2.31).
-
B)
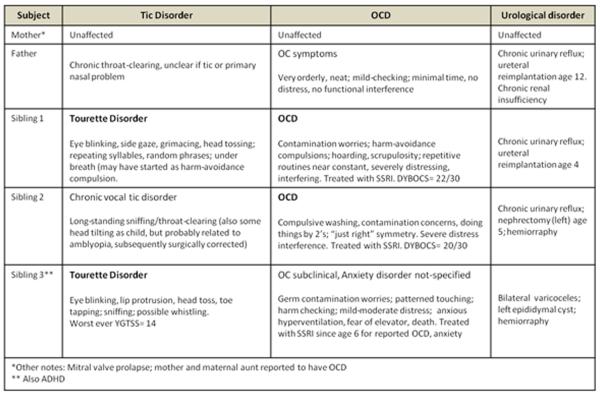
The rare SERT I425V variant that confers gain-of-function and impaired regulation was found in a TD proband with an additional diagnosis of OCD. Follow-up genotyping of this family indicated that his two male siblings were also I425V carriers: a sibling with OCD and chronic phonic tics not meeting full TD criteria, and a sibling with TD, ADHD, and subclinical OC symptoms not meeting full criteria for OCD (Figure 1). Their father, who had subclinical OC and TD symptoms, also had SERT I425V. Of note, all three siblings and their father had both the greater-expressing LA allele and the LAC haplotype, with two being rs25532 CC homozygotes (Figure 1). Without exception in the present study, as in all previous studies, SERT I425V carriers were also LA (or L) carriers24, 30, 31. These findings contribute to the compilation of all publication reports to date of SERT I425V being reported in 27 individuals of whom 15 individuals from 8 families received diagnoses of OCD, OC personality disorder or TD. The cumulative count of SERT I425V thus becomes 1.57% in OCD/TD spectrum conditions vs. 0.15% in controls, with a recalculated, family-adjusted significance of χ2=15.03, p < 0.0001, OR=9.0 (total worldwide genotyped=2914).
-
C)
An unusual aggregation of congenital renal disorders with SERT I425V was observed. Upon closer review of the clinical description of the family segregating SERT I425V, we noted that the father and two of the siblings of this family were originally flagged for congenital urological anomalies. These original designations were “congenital renal syndrome” and “congenital familial renal atrophy”. This family was re-contacted to clarify their renal disorders as well as their tic and OC symptoms (See Figure 1 for updated descriptions). Two siblings were found to have chronic urinary reflux requiring ureteral re-implantation and one, a nephrectomy in childhood. Their father had long-term vesiculoureteral reflux requiring ureteral reimplantation, with continuing reduced (~60%) renal function and hypertension.
TABLE 1.
Summary of Genoytpes and Alleles in TD Probands and Controls; Alleles and Haplotype Analysis by Functional Grouping (see Methods).
| TD Probands | Controls | Statistic | ||
|---|---|---|---|---|
| Total individuals | 150 | 855 | ||
| N (%) | N (%) | |||
| LALA | 51 (34.0) | 203 (23.7) | ||
| LALG | 6 (4.0) | 60 (7.0) | ||
| LGLG | 0 | 4 (0.5) | ||
| LAS | 61 (40.7) | 369 (43.0) | ||
| LGS | 7 (4.7) | 48 (5.6) | ||
| SS | 25 (16.7) | 171 (19.9) | ||
| Total alleles | 300 | 1710 | ||
| LA | 169 (56.3) | 835 (48.8) | ||
| S, LG | 131 (43.7) | 875 (54.2) | χ2=5.75, OR=1.35 | p=0.017 |
| Haplotypes | ||||
| LAC | (49.3) | (38.3) | ||
| LAT, S, LG | (50.6) | (61.7) | χ2=5.08, OR=1.33 | p=0.024 |
Figure 1.
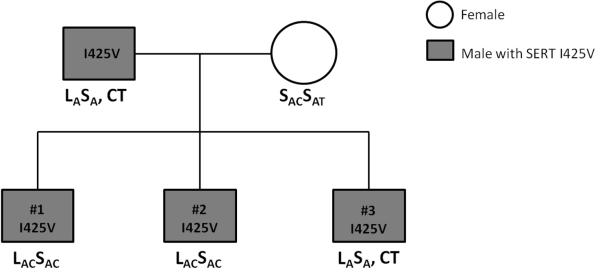
Pedigree of Family D with SLC6A4 SERT I425V in three siblings with TD/OCD and in their father, showing also SLC6A4 5-HTTLPR/rs25531/rs25532 variants.
DISCUSSION
This report describes associations between TD and SLC6A4 at two different levels: first, there is an association of TD with the SLC6A4 greater-expressing promoter region LA allele, and also a haplotypic association of TD with the LAC variant (5-HTTLPR/ rs25531/rs25532). Also, connections between SLC6A4 and TD were observed with the new finding of the rare variant, SERT I425V, in three siblings, one with TD and OCD, one with TD alone, and one with OCD alone, and in their father (with subclinical OC symptoms). In addition, congenital renal system anomalies, all requiring surgical treatment, were found in two of these siblings and also in their father, all with SERT I425V.
Numerous genes involved in neurotransmitter systems and developmental sequences seem highly relevant to TD, OCD and ADHD, but only relatively few have been investigated. These include glutamate, dopamine, serotonin, and other transmitter system genes, neurotrophic factor genes, and genes suggested from animal models of TD-, OCD- and ADHD-related behaviors. Alterations in the serotonergic systems have been suggested in TD since very early cerebrospinal fluid studies and other neurobiological and neuropharmacological studies32–34. The present findings suggesting specific involvement of the SLC6A4 gene and thus SERT activity in TD individuals do not seem to have been previously considered according to recent reviews35–39. Two previous studies reported no association of the 5-HTTLPR and TD40, 41; however, in both studies the genotyping methodology only evaluated L vs. S variants in 5-HTTLPR that are now known to be erroneous due to the substantial modulatory effects of rs25531 and rs2553210, 11, 15.
In most investigations of TD, dopaminergic dysfunctions and more recently histamine system abnormalities have been evaluated, but the serotonin system appears relatively neglected42, 43. The present study thus provides new support for the notion of altered serotonergic neurotransmission in TD32. Both the SLC6A4 LA variant and LAC haplotype have previously been shown to confer increased SLC6A4 expression by our group and colleagues10, 11. By increasing serotonin clearance, both higher expressing SLC6A4 alleles and the gain-of-function SERT I425V variant would confer an hyposerotonergic status, condition that has been suggested in some studies beginning two decades ago44, 45; however, see34. Such a hyposerotonergic state could upregulate postsynaptic 5-HT2A receptors, as reported in TD46. These changes in 5-HT2A receptors could in turn facilitate dopamine release, and this would thus be in convergence with the dopamine hypothesis of TD, postulated by Singer and others47. Almost all studies to date have suggested impairments of the serotonergic system in TD. In addition, multiple investigations support contributions from dopamine and serotonin to abnormalities in the cortico-striato-thalamo-cortical loop in both OCD and TD. Further research is needed to fully answer how common and rare SLC6A4 variants conferring increased SERT expression and function might contribute to TD.
Human SLC6A4 expression was originally ascertained as being regulated by the 5-HTTLPR promoter, in addition to coding region (I425V, G56A) and other variants20, 21, 48–50. The impact of additional polymorphisms such as rs25531, rs25532, rs1696562811, alternative splicing forms and miR15a/16-mediated translational control12, 13 might be responsible for the lack of replication in early human genetics association reports regarding initially identified SLC6A4 polymorphisms considered alone20, 48, 49; in addition, gene x gene interactions related to the consequences of other genes observed to interact with SLC6A4 (e.g., ITGB3, BDNF, BMP) may have contributed to mixed single gene evaluations51, 52.
In regard to SERT I425V in families with OCD, TD and other neuropsychiatric disorders, the total clinically-evaluated (N=5) or non-evaluated (N=2) first degree relatives of OCD or TD probands now includes 7 of 716 (0.97%) individuals genotyped24, 30, a significantly greater proportion than found in controls (3 of 1958, 0.15%; χ2=7.48; p < 0.006; OR=1.5). In addition, five individuals with SERT I425V were fathers or siblings of individuals with SERT I425V who declined or could not participate in these studies ( e.g., due to suicide N=4); three others with SERT I425V were each parents of two to four siblings with OCD in independent families24, 30, 31. Also of relevance, three individuals with SERT I42V have been diagnosed with anorexia nervosa, three with autism or Asperger's syndrome and three with tic disorder, all disorders with overlapping features of perseverative, repetitive behaviors like OCD and TD24, 30. Among all controls genotyped (N=1958), one diagnostically evaluated and two non-evaluated individuals from independent families had I425V (0.15%, Table 2). This compares to a figure of 0.084% in the 1000 Genomes Project for SERT I425V (rs28914832) (http://www.1000genomes.org/)53. A recent report described three parents with SERT I425V, none of whom transmitted this variant to their OCD-affected children31. One parent had OCD, one OCPD and the other skin-picking disorder. Of note, skin-picking disorder co-occurs with OCD in some instances and sometimes occurs as part of a constellation of grooming disorders considered as OCD spectrum disorders genetically associated with familial OCD54–56. As summarized in Table 2, these new data add support to the notion that TD and OCD have some genetic overlap as spectrum conditions in which SERT I425V as well as common SLC6A4 polymorphisms (5-HTTLPR, rs25531 and rs25532) may be relevant, contributing, and perhaps even causative gene variants associated with TD, OCD and related neuropsychiatric disorders.
TABLE 2.
SERT I425V in Genotyped OCD, TD and Controls
| Total | SERT I425V | % | |
|---|---|---|---|
| Total OCD/OCPD or TD | 956 | 15 | 1.57** |
| Total diagnostically-evaluated [N=1] healthy or non-evaluated [n=2] controls | 1958 | 3 | 0.15 |
| OCD/OCPD or TD (with family correction) vs. Controls: | |||
| χ2=15.03, p < 0.0001, OR=9.0 |
SERT I425V leads to greater SERT surface availability, increased transport of serotonin, disrupted regulation via a PKG/cGMP-linked pathway, and also altered binding of some inhibitory ligands20, 21, 46, 48. However, the molecular consequences of SERT I425V have thus far been only investigated in heterologous expression cell systems (HeLa and COS-7 cells)20, 21, 48. The recently available transgenic SERT I425V mice should make possible the study of this variant in vivo [Ramamoorthy, S et. al., in preparation]. These mice with expected SERT over-expression and altered SERT regulation will be especially valuable in developmental studies as well as providing means to explore additional SERT-related disorders, including TD.
This is the first reported association of common SLC6A4 alleles and a haplotype plus the rare, highly penetrant SERT I425V with TD. Three of four individuals with SERT I425V also had in a congenital renal system disorder. Present results call for examination of this possible connection between SERT I425V and renal anomalies in other families with this variant, perhaps related to the existence of a `intrarenal serotonin system' that includes serotonin synthesis in the proximal tubule, SERT-mediated excretion and multiple renal serotonin receptors57–59.
We did not correct our results for multiple testing since we and others have previously shown that the SLC6A4 variants investigated here are not independent: The rs25532 T allele has not been observed on rs25531 G background (i.e. SG or LG when considering 5-HTTLPR/rs25531)11. Of note, the minor G allele of rs25531 has been found to occur almost always in phase with the L allele of 5-HTTLPR10, 11. In all previous reports, and just as we have found in the present results, the SERT I425V variant has been always found in subjects having the 5-HTTLPR/rs2551 LA (or L) allele20–23, 30, 31.
An important limitation in our case-control association study is its susceptibility to population stratification. We addressed this issue by matching ethnicities between cases and controls. Although considered by some authors to be relatively uncommon60, spurious associations can only be definitively ruled out by genotyping multiple ancestry-informative markers or through family-based studies, which were not feasible in the present work.
A complex impact of serotonin changes on early development in rodents has been investigated for some years61. The deleterious consequences of a single gene knockout of SERT or of specific serotonin receptors and studies of prenatal or early postnatal exposure to serotonin reuptake inhibitors have been established in mice62; [Andrews et al., under review]. These include altered extinction fear plus stress- and anxiety-related responses, head twitch responses (which may be tic-like)63, motor dysfunction and more -all of which may be relevant to neuropsychiatric disorders including TD, OCD and ADHD49, 62, 64. OCD has been most clearly associated with the 5-HTTLPR/rs25531, plus rs25532 and SERT I425V variants (as has ADHD, to some extent), although earlier reports suggested mixed results, particularly when only 5-HTTLPR was studied or when TD was excluded from the OCD samples10, 11, 31, 65, 66. The present findings indicate that multiple variants in SLC6A4 are involved in TD, just like those found in OCD – two disorders characterized by obligatory/unwanted movements, behaviors or thoughts. These new results call for replication in a similarly intensively evaluated sample. A number of recent reviews have strengthened the case for SLC6A4 contributions to different neuropsychiatric disorders including affective disorders and suicide plus anxiety related-traits, particularly when life events and other environmental antecedents are taken into account16, 67–69. A related G × E hypothesis might apply to TD: the combined burden of TD associated with OCD or ADHD in addition might provide compounded gene/multiple disorders/environmental interactions akin to that found with the combinations of SLC6A4 variants with traumatic/stressful life events on the risk for behavioral disorder outcomes, as recently examined in several reviews16, 67–69.
ACKNOWLEDGEMENTS
This study was supported by the NIMH Intramural Research Program. F.J. McMahon is also supported by an Independent Investigator Award from NARSAD. G.A. Heiman, R.A. King and J.A. Tischfield are supported by grants from the New Jersey Center for Tourette Syndrome and Associated Disorders and NIMH (R01MH092293). We also thank Teresa Tolliver, Nawei Sun, Lily Deng, Ruby Fried and Su-jan Huang for assay genotyping and mutation sequencing assistance, and Theresa Deguzman for editorial and graphic contributions.
Footnotes
CONFLICT OF INTEREST Jens R. Wendland is a full-time employee of Pfizer, Inc. No conflict of interest for all other authors.
REFERENCES
- 1.Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29(3):175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Grados MA, Mathews CA. Clinical phenomenology and phenotype variability in Tourette syndrome. J Psychosom Res. 2009;67(6):491–496. doi: 10.1016/j.jpsychores.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 3.LaSalle VH, Cromer KR, Nelson KN, Kazuba D, Justement L, Murphy DL. Diagnostic interview assessed neuropsychiatric disorder comorbidity in 334 individuals with obsessive-compulsive disorder. Depress Anxiety. 2004;19(3):163–173. doi: 10.1002/da.20009. [DOI] [PubMed] [Google Scholar]
- 4.Worbe Y, Mallet L, Golmard JL, et al. Repetitive behaviours in patients with Gilles de la Tourette syndrome: tics, compulsions, or both? PLoS One. 2010;5(9):e12959. doi: 10.1371/journal.pone.0012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauls DL, Towbin KE, Leckman JF, Zahner GE, Cohen DJ. Gilles de la Tourette's syndrome and obsessive-compulsive disorder. Evidence supporting a genetic relationship. Arch Gen Psychiatry. 1986;43(12):1180–1182. doi: 10.1001/archpsyc.1986.01800120066013. [DOI] [PubMed] [Google Scholar]
- 6.State MW. The genetics of Tourette disorder. Curr Opin Genet Dev. 2011;21(3):302–309. doi: 10.1016/j.gde.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shmelkov SV, Hormigo A, Jing D, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16(5):598–602. doi: 10.1038/nm.2125. 591p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 9.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 10.Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wendland JR, Moya PR, Kruse MR, et al. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008;17(5):717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- 12.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329(5998):1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 13.Moya PR, Wendland JR, Salemme J, Fried RL, Murphy DL. miR-15a and miR-16 regulate serotonin transporter expression in human placental and rat brain raphe cells. Int J Neuropsychopharmacol. 2012:1–9. doi: 10.1017/S1461145712000454. [DOI] [PubMed] [Google Scholar]
- 14.Ye R, Blakely RD. Natural and engineered coding variation in antidepressant-sensitive serotonin transporters. Neuroscience. 2011;197:28–36. doi: 10.1016/j.neuroscience.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy DL, Moya PR. Human serotonin transporter gene (SLC6A4) variants: their contributions to understanding pharmacogenomic and other functional GxG and GxE differences in health and disease. Curr Opin Pharmacol. 2011;11(1):3–10. doi: 10.1016/j.coph.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 17.Wendland JR, DeGuzman TB, McMahon F, Rudnick G, Detera-Wadleigh SD, Murphy DL. SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive disorder. Psychiatr Genet. 2008;18(1):31–39. doi: 10.1097/YPG.0b013e3282f08a06. [DOI] [PubMed] [Google Scholar]
- 18.Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4(2):109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- 19.Bengel D, Greenberg BD, Cora-Locatelli G, et al. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Mol Psychiatry. 1999;4(5):463–466. doi: 10.1038/sj.mp.4000550. [DOI] [PubMed] [Google Scholar]
- 20.Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol. 2003;64(2):440–446. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YW, Gesmonde J, Ramamoorthy S, Rudnick G. Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J Neurosci. 2007;27(40):10878–10886. doi: 10.1523/JNEUROSCI.0034-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad HC, Zhu CB, McCauley JL, et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2005;102(32):11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy DL, Moya PR, Wendland JR, Timpano KR. Genetic contributions to obessive-compulsive disorder (OCD) and OCD-related disorders. In: Nurnberger J, Berrettini W, editors. Principles of Psychiatric Genetics. Cambridge University Press; in press. [Google Scholar]
- 24.Ozaki N, Goldman D, Kaye WH, et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8(11):933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- 25.Heiman GA, King RA, Tischfield JA. New Jersey Center for Tourette Syndrome sharing repository: methods and sample description. BMC Med Genomics. 2008;1:58. doi: 10.1186/1755-8794-1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Rosario-Campos MC, Miguel EC, Quatrano S, et al. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry. 2006;11(5):495–504. doi: 10.1038/sj.mp.4001798. [DOI] [PubMed] [Google Scholar]
- 28.Wendland JR, Kruse MR, Cromer KR, Murphy DL. A large case-control study of common functional SLC6A4 and BDNF variants in obsessive-compulsive disorder. Neuropsychopharmacology. 2007;32(12):2543–2551. doi: 10.1038/sj.npp.1301394. [DOI] [PubMed] [Google Scholar]
- 29.Wendland JR, Kruse MR, Murphy DL. Functional SLITRK1 var321, varCDfs and SLC6A4 G56A variants and susceptibility to obsessive-compulsive disorder. Mol Psychiatry. 2006;11(9):802–804. doi: 10.1038/sj.mp.4001848. [DOI] [PubMed] [Google Scholar]
- 30.Delorme R, Betancur C, Wagner M, et al. Support for the association between the rare functional variant I425V of the serotonin transporter gene and susceptibility to obsessive compulsive disorder. Mol Psychiatry. 2005;10(12):1059–1061. doi: 10.1038/sj.mp.4001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voyiaziakis E, Evgrafov O, Li D, et al. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol Psychiatry. 2011;16(1):108–120. doi: 10.1038/mp.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen DJ, Shaywitz BA, Caparulo B, Young JG, Bowers MB., Jr. Chronic, multiple tics of Gilles de la Tourette's disease. CSF acid monoamine metabolites after probenecid administration. Arch Gen Psychiatry. 1978;35(2):245–250. doi: 10.1001/archpsyc.1978.01770260123015. [DOI] [PubMed] [Google Scholar]
- 33.Cohen DJ, Shaywitz BA, Young JG, et al. Central biogenic amine metabolism in children with the syndrome of chronic multiple tics of Gilles de la Tourette: norepinephrine, serotonin, and dopamine. J Am Acad Child Psychiatry. 1979;18(2):320–341. doi: 10.1016/s0002-7138(09)61046-3. [DOI] [PubMed] [Google Scholar]
- 34.Leckman JF, Goodman WK, Anderson GM, et al. Cerebrospinal fluid biogenic amines in obsessive compulsive disorder, Tourette's syndrome, and healthy controls. Neuropsychopharmacology. 1995;12(1):73–86. doi: 10.1038/sj.npp.1380241. [DOI] [PubMed] [Google Scholar]
- 35.Deng H, Gao K, Jankovic J. The genetics of Tourette syndrome. Nat Rev Neurol. 2012;8(4):203–213. doi: 10.1038/nrneurol.2012.26. [DOI] [PubMed] [Google Scholar]
- 36.Felling RJ, Singer HS. Neurobiology of tourette syndrome: current status and need for further investigation. J Neurosci. 2011;31(35):12387–12395. doi: 10.1523/JNEUROSCI.0150-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jankovic J, Kurlan R. Tourette syndrome: evolving concepts. Mov Disord. 2011;26(6):1149–1156. doi: 10.1002/mds.23618. [DOI] [PubMed] [Google Scholar]
- 38.O'Rourke JA, Scharf JM, Yu D, Pauls DL. The genetics of Tourette syndrome: a review. J Psychosom Res. 2009;67(6):533–545. doi: 10.1016/j.jpsychores.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119–125. doi: 10.1097/WCO.0b013e328344648c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu SG, Zhang XH, Yin YY, Wang MJ, Che FY, Ma X. An association analysis between 5-HTTLPR polymorphism and obsessive-compulsive disorder, Tourette syndrome in a Chinese Han population. CNS Neurosci Ther. 2011;17(6):793–795. doi: 10.1111/j.1755-5949.2011.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavallini MC, Di Bella D, Catalano M, Bellodi L. An association study between 5-HTTLPR polymorphism, COMT polymorphism, and Tourette's syndrome. Psychiatry Res. 2000;97(2–3):93–100. doi: 10.1016/s0165-1781(00)00220-1. [DOI] [PubMed] [Google Scholar]
- 42.Steeves TD, Ko JH, Kideckel DM, et al. Extrastriatal dopaminergic dysfunction in tourette syndrome. Ann Neurol. 2010;67(2):170–181. doi: 10.1002/ana.21809. [DOI] [PubMed] [Google Scholar]
- 43.Grados MA. The genetics of obsessive-compulsive disorder and Tourette's syndrome: what are the common factors? Curr Psychiatry Rep. 2009;11(2):162–166. doi: 10.1007/s11920-009-0025-x. [DOI] [PubMed] [Google Scholar]
- 44.Leckman JF, Anderson GM, Cohen DJ, et al. Whole blood serotonin and tryptophan levels in Tourette's disorder: effects of acute and chronic clonidine treatment. Life Sci. 1984;35(25):2497–2503. doi: 10.1016/0024-3205(84)90435-1. [DOI] [PubMed] [Google Scholar]
- 45.Cath DC, Spinhoven P, Landman AD, van Kempen GM. Psychopathology and personality characteristics in relation to blood serotonin in Tourette's syndrome and obsessive-compulsive disorder. J Psychopharmacol. 2001;15(2):111–119. doi: 10.1177/026988110101500208. [DOI] [PubMed] [Google Scholar]
- 46.Wong DF, Brasic JR, Singer HS, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33(6):1239–1251. doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer HS, Butler IJ, Tune LE, Seifert WE, Jr., Coyle JT. Dopaminergic dsyfunction in Tourette syndrome. Ann Neurol. 1982;12(4):361–366. doi: 10.1002/ana.410120408. [DOI] [PubMed] [Google Scholar]
- 48.Sutcliffe JS, Delahanty RJ, Prasad HC, et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77(2):265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veenstra-Vanderweele J, Muller CL, Iwamoto H, et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109(14):5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacKenzie A, Quinn J. A serotonin transporter gene intron 2 polymorphic region, correlated with affective disorders, has allele-dependent differential enhancer-like properties in the mouse embryo. Proc Natl Acad Sci U S A. 1999;96(26):15251–15255. doi: 10.1073/pnas.96.26.15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118(4):1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren-Patterson RF, Cochran LW, Holmes A, Lesch KP, Lu B, Murphy DL. Gender-dependent modulation of brain monoamines and anxiety-like behaviors in mice with genetic serotonin transporter and BDNF deficiencies. Cell Mol Neurobiol. 2006;26(4–6):755–780. doi: 10.1007/s10571-006-9048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Consortium TGP. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bienvenu OJ, Samuels JF, Wuyek LA, et al. Is obsessive-compulsive disorder an anxiety disorder, and what, if any, are spectrum conditions? A family study perspective. Psychol Med. 2012;42(1):1–13. doi: 10.1017/S0033291711000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grant JE, Odlaug BL, Kim SW. A clinical comparison of pathologic skin picking and obsessive-compulsive disorder. Compr Psychiatry. 2010;51(4):347–352. doi: 10.1016/j.comppsych.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Lovato L, Ferrao YA, Stein DJ, et al. Skin picking and trichotillomania in adults with obsessive-compulsive disorder. Compr Psychiatry. 2011 doi: 10.1016/j.comppsych.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev. 2012;64(2):359–388. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Yao B, Fan X, Langworthy MM, Zhang MZ, Harris RC. Characterization of a putative intrarenal serotonergic system. Am J Physiol Renal Physiol. 2007;293(5):F1468–1475. doi: 10.1152/ajprenal.00246.2007. [DOI] [PubMed] [Google Scholar]
- 59.Gidener S, Kirkali Z, Guven H. Influence of serotonin on the human ureter: an in vitro pharmacological study. Urol Int. 1995;55(4):202–204. doi: 10.1159/000282786. [DOI] [PubMed] [Google Scholar]
- 60.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361(9357):598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- 61.Deneris ES, Wyler SC. Serotonergic transcriptional networks and potential importance to mental health. Nat Neurosci. 2012;15(4):519–527. doi: 10.1038/nn.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9(2):85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 63.Hayslett RL, Tizabi Y. Effects of donepezil, nicotine and haloperidol on the central serotonergic system in mice: implications for Tourette's syndrome. Pharmacol Biochem Behav. 2005;81(4):879–886. doi: 10.1016/j.pbb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 64.Wellman CL, Izquierdo A, Garrett JE, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27(3):684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landaas ET, Johansson S, Jacobsen KK, et al. An international multicenter association study of the serotonin transporter gene in persistent ADHD. Genes Brain Behav. 2010;9(5):449–458. doi: 10.1111/j.1601-183X.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- 66.Sonuga-Barke EJ, Kumsta R, Schlotz W, et al. A functional variant of the serotonin transporter gene (SLC6A4) moderates impulsive choice in attention-deficit/hyperactivity disorder boys and siblings. Biol Psychiatry. 2011;70(3):230–236. doi: 10.1016/j.biopsych.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15(1):18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 69.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]