Abstract
Abuse of psychoactive substances can lead to drug addiction. In animals, addiction is best modeled by drug self-administration paradigms. It has been proposed that the crucial common denominator for the development of drug addiction is the ability of drugs of abuse to increase extracellular concentrations of dopamine in the nucleus accumbens (NAcc). Studies using in vivo microdialysis and chronoamperometry in the behaving animal have demonstrated that drugs of abuse increase tonic dopamine concentrations in the NAcc. However, it is known that dopamine neurons respond to reward-related stimuli on a subsecond timescale. Thus, it is necessary to collect neurochemical information with this level of temporal resolution, as achieved with in vivo fast-scan cyclic voltammetry (FSCV), to fully understand the role of phasic dopamine release in normal behavior and drug addiction. We review studies that investigated the effects of drugs of abuse on NAcc dopamine levels in freely-moving animals using in vivo microdialysis, chronoamperometry and FSCV. After a brief introduction of dopamine anatomy and signal transduction, and a section on current theories of dopamine in natural goal-directed behavior, a discussion of techniques for the in vivo assessment of extracellular dopamine behaving animals is presented. Then, we review studies using these techniques to investigate changes in phasic and tonic dopamine signaling in the NAcc during 1) response-dependent and –independent administration of abused drugs, 2) drug-conditioned stimuli and operant behavior in self-administration paradigms, 3) drug withdrawal, and 4) cue-induced reinstatement of drug seeking. These results are then integrated with current ideas on the role of dopamine in addiction with an emphasis on a model illustrating phasic and tonic NAcc dopamine signaling during different stages of drug addiction. This model predicts that phasic dopamine release in response to drug-related stimuli will be enhanced over stimuli associated with natural reinforcers, which may result in aberrant goal-directed behaviors contributing to drug addiction.
1 The dopamine system: Implication in normal behavior and addiction
1.1 Drug addiction and dopamine neurotransmission in humans
Abuse of psychoactive substances can lead to drug addiction, a maladaptive behavioral pattern of drug use that is often accompanied by drug tolerance and withdrawal symptoms and causes impairment, distress, and the habitual intake of the drug regardless of the devastating consequences (Diagnostic and Statistical Manual of Mental Disorders (DSM-IV); APA 2000). An estimated 5% of the world’s population aged 15 to 64 years used illicit psychoactive drugs in the past year (UN world drug report 2008). Legal psychoactive drugs, such as alcohol and tobacco, are used by at least one quarter of the world’s adult population (UN world drug report 2008). Drug addiction is considered to be a chronic disorder because addicts are rarely able to maintain abstinence for extended periods of time despite an expressed desire to stay drug-free. This is because stress, re-exposure to the drug itself, and drug-associated cues often trigger the resumption of drug taking (Kalivas and McFarland 2003). Such cues can even promote drug seeking behavior outside of awareness that eventually results in relapse to drug taking (Tiffany and Carter 1998).
Evidence shows that drugs of abuse affect dopamine neurotransmission. Imaging studies revealed that drug-naïve individuals show enhanced dopamine levels in the striatum upon exposure to psychostimulants such as cocaine and amphetamine (Volkow et al. 1997a; Drevets et al. 2001). In contrast, decreased striatal dopamine responses were reported in detoxified cocaine abusers (Volkow et al. 1997b). Furthermore, individuals with a history of abuse of alcohol (Volkow et al. 1996), cocaine (Volkow et al. 1990), heroin (Wang et al. 1997), or methamphetamine (Volkow et al. 2001) display lower levels of dopamine receptor binding compared to non-abusers (Volkow et al. 2004). Together, these imaging studies suggest an involvement of dopamine neurotransmission in the acute and long-term effects of abused drugs.
1.2 Drug self-administration as an animal model for drug addiction
To better understand the neurobiology of drug abuse and addiction in humans, several animal models have been developed to investigate different aspects of drug addiction (Koob and Le Moal 2008). Among these models, paradigms that incorporate self-administration of drugs are thought to best capture the human condition because animals are allowed to voluntarily seek the drug and because drugs that are self-administered by animals correspond well with those that have high abuse potential in humans (Koob and Le Moal 2008). However, a great number of studies have examined the effects of drugs of abuse upon passive injection by the experimenter (non-contingent or response-independent administration). Therefore, in this chapter, we will review studies that have used contingent or response-dependent (self-administration), as well as non-contingent or response-independent drug injections.
1.3 Drug-self-administration and dopamine in the nucleus accumbens
Dopamine neurotransmission is highly implicated in the regulation of reinforcement in rodent drug self-administration paradigms. The dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc) has been identified as a critical substrate for the expression of drug reinforcement (Ritz et al. 1987; Wise and Bozarth 1987; Koob and Bloom 1988; Kalivas 2003). For example, direct dopamine receptor agonists are self-administered systemically as well as locally into the NAcc (Yokel and Wise 1978; Woolverton et al. 1984; Carlezon et al. 1995). Dopamine receptor antagonists administered systemically in low doses increase the rate of operant responding for cocaine in animals (DeWit and Wise 1977; Ettenberg et al. 1982; Roberts and Vickers 1984; Britton et al. 1991; Corrigall and Coen 1991; Caine and Koob 1994; Hemby et al. 1996), but decrease the motivation to carry out high work requirements to obtain an infusion of cocaine (Hubner and Moreton 1991; Richardson et al. 1993). Similarly, intra-cerebral administration of dopamine antagonists into the NAcc increases the rate of psychostimulant self-administration (Maldonado et al. 1993; Phillips et al. 1994), but decreases responding when work requirements for the drug infusion increase (McGregor and Roberts 1993). These effects have been interpreted as an attenuation of the reinforcing properties of the drug, such that more drug is taken to achieve the same level of reward and that less effort is invested into a drug infusion with decreased rewarding properties. In contrast, lesion or inactivation of the mesolimbic dopamine system in the VTA (Roberts and Koob 1982; Shoaib et al. 1998; Xi and Stein 1999) or the NAcc (Roberts et al. 1977, 1980; Lyness et al. 1979; Pettit et al. 1984; Corrigall et al. 1992; Shoaib et al. 1998) attenuate cocaine, amphetamine, heroin, and nicotine self-administration in rats. These findings further underline the critical importance of the mesolimbic dopamine system in drug taking. However, there are some conflicting reports on whether dopamine antagonists or lesions of the mesolimbic dopamine system affect responding for all drugs of abuse (Pettit et al. 1984; Hemby et al. 1996; Di Chiara 2000; Czachowski et al. 2001). Notably, the response of the NAcc dopamine system to drug administration (see below) differs significantly depending on whether the animal actively self-administers a drug of abuse or whether the animal receives it independent of a response (Hemby et al. 1995, 1997; Lecca et al. 2007).
1.4 Anatomy of the dopamine system and dopamine signal transduction: Phasic and tonic release
The VTA and the neighboring substantia nigra are the primary dopamine-producing nuclei in the brain (Swanson 1982). From these midbrain nuclei, relatively distinct dopamine cell groups innervate different functional domains of the striatum, i.e. sensorimotor, associative, and limbic circuits that are mainly defined by their differential cortical inputs (Carlsson et al. 1962; Dahlstrom and Fuxe 1964; Alexander and Crutcher 1990; Joel and Weiner 2000). The VTA predominantly projects to the limbic striatum including the NAcc. More than three quarters of this mesolimbic projection stems from dopaminergic neurons (Swanson 1982).
Dopamine acts on D1- or D2-type dopamine receptors upon release from dopaminergic fibers that densely innervate the striatum (Doucet et al. 1986; Ritz et al. 1987; Groves et al. 1994). Dopamine receptors are found at symmetric boutons formed by dopaminergic terminals on the neck or shaft of the spines of striatal projection neurons (Levey et al. 1993; Groves et al. 1994; Hersch et al. 1995; Caille et al. 1996). However, often dopamine receptors are also found on striatal dendrites outside such synapses, in the vicinity of asymmetric boutons on the head of dendritic spines that are contacted by glutamatergic terminals (Levey et al. 1993; Caille et al. 1996). Stimulation of dopamine receptors is terminated by reuptake of extracellular dopamine into the presynaptic terminals. Most dopamine reuptake sites are located on dopaminergic fibers outside synaptic contacts of dopaminergic terminals (Nirenberg et al. 1996; Hersch et al. 1997), similar to the dopamine receptors. Together, this distribution of receptors and reuptake sites allows for ‘volume transmission’; i.e., the activation of extrasynaptic dopamine receptors by dopamine that diffuses a few micrometers away from its release into the synaptic cleft (Garris et al. 1994; Garris and Rebec 2002; Rice and Cragg 2008). Therefore, the measurement of extracellular dopamine concentrations is a meaningful indicator of dopamine signaling.
Dopamine neurons are either hyperpolarized/quiescent or display different patterns of discharge activity: single-spike firing (2-10 Hz) or bursts of two to six action potentials (15-30 Hz) (Grace and Bunney 1984a, b; Freeman and Bunney 1987). Individual dopamine neurons can switch from one of these patterns to the other, as shown in freely-moving rats (Freeman and Bunney 1987; Hyland et al. 2002). Salient sensory stimuli can evoke a transient increase in firing rate and burst firing (Freeman and Bunney 1987; Mirenowicz and Schultz 1996). The ‘tonic’ extracellular concentration of dopamine, ranging from 5-20 nM depending on the target area, is thought to arise from basal dopamine neuron firing patterns, which predominantly consists of single-spike firing (Bunney et al. 1991; Grace 1991; Parsons and Justice 1992; Keefe et al. 1993; Floresco et al. 2003). Conversely, burst firing of dopamine neuron populations is thought to give rise to ‘phasic’ dopamine events which can reach as high as 1 μM (Gonon 1988; Chergui et al. 1994; Garris et al. 1997). In support of this assertion, it has been shown that electrical stimulations mimicking burst firing are much more potent in triggering dopamine overflow than single pulse stimulations (Wightman and Zimmerman 1990; Chergui et al. 1996; Gonon and Sundstrom 1996). In summary, the signaling modes that govern communication between dopamine neurons and their target cells are rapid and transient increases in dopamine concentrations (phasic dopamine) on top of a low, slowly changing dopaminergic tone (tonic dopamine).
1.5 Proposed functions of dopamine in the NAcc
Since the identification of dopamine over 50 years ago (Carlsson et al. 1957), a number of ideas have been developed to explain the role of dopamine in behavior. These ideas (some of which are presented below) are not necessarily mutually exclusive, but rather focus on different aspects of dopamine function in behavior. The most uncontroversial of ideas is that dopamine is implicated in motor function (Berridge 2007, Salamone and Correa 2002), as selective degeneration of dopamine neurons in Parkinson’s disease patients or animals treated with neurotoxins causes motor deficits (Sundstrom et al. 1990; Schwarting and Huston 1996; Galvan and Wichmann 2008). However, these motor deficits are considered to be caused primarily by compromised dopamine signaling in the dorsal striatum, whereas dopamine in the ventral striatum including the NAcc is assumed to be part of a ‘limbic motor interface’ (Mogenson et al. 1980). This idea is based on the fact that NAcc neurons receive input from limbic brain regions and send output to motor brain regions. For example, dopamine infused into the NAcc caused an increase in locomotion that can be inhibited by blocking the effect of NAcc output in one of its motor output nuclei (Jones and Mogenson, 1980), supporting the proposition that NAcc dopamine serves as a modulator in the translation of motivation into action (Mogenson et al. 1980).
1.5.1 Dopamine and motivated behavior
Mogenson’s pioneering work inspired research that produced many lines of evidence supporting a critical role of dopamine in motivation and effort (Salamone and Correa 2002; Berridge 2007). For example, the impact of manipulations that impair dopamine signaling in the NAcc on food-seeking behavior is critically dependent upon the work requirements of the task (e.g., Salamone et al. 1991; Cousins and Salamone 1994). Thus, food-seeking that requires low effort is largely unaffected by partial NAcc dopamine depletions, whereas food-seeking that requires high effort is substantially impaired (e.g., Salamone et al. 1994; Denk et al. 2005). This suggests that dopamine may be implicated in overcoming the motivational costs required for completing tasks that involve a high level of effort (Salamone and Correa 2002; Phillips et al. 2007). Similarly, we recently suggested that NAcc dopamine, influenced by internal deprivation states (e.g., hunger and thirst), plays a key role in overcoming response costs by modulating activity originating from the frontal cortical systems that assess costs and rewards (Phillips et al. 2007). This may enable mesolimbic dopamine to energize goal seeking and affect cost-benefit decision making.
The ‘incentive-salience’ hypothesis of dopamine also builds upon the involvement of dopamine in motivation (Robinson and Berridge 1993; Berridge 2007). In short, incentive salience is the neural representation of motivational value in response to a reward-related stimulus that drives behavior. Dopamine in the NAcc is thought to modulate the incentive value of such reward-related stimuli (Berridge 2007). The hypothesis distinguishes between ‘liking’ of rewards (hedonic value) and ‘wanting’ of rewards (incentive value) (Berridge 2007). For example, enhancing dopamine levels in dopamine transporter knock-down mice increases the ‘wanting’ of food reinforcers (Pecina et al. 2003; Cagniard et al. 2006a), whereas ‘liking’ of food reinforcers is not affected by insults to the function of the dopamine system (Berridge et al. 1989; Pecina et al. 1997). This supports the proposition that NAcc dopamine is implicated in ‘wanting’ or the ‘incentive value’ of a stimulus.
1.5.2 Dopamine and reinforcement learning
The most compelling line of evidence for a role of dopamine in reinforcement learning comes from the firing patterns of dopamine neurons during the presentation of conditioned and unconditioned stimuli (Schultz 1997). Initially, these neurons show synchronous firing patterns in response to the delivery of unpredicted rewards. After repeated pairing with a stimulus that predicts reward, dopamine neurons cease firing to reward delivery and instead respond to the cue that predicts its availability (Schultz et al. 1997; Waelti et al. 2001). This firing pattern of dopamine cells is consistent with a reward prediction signal that provides the organism with the capacity to compare the expected outcome to the actual outcome, in order to maximize reward (Montague et al. 2004). An unexpected reward following a neutral cue is a positive error and favors learning. The omission of an expected reward after a predictive cue or action is a negative error and favors extinction. In support, it has been shown that dopamine neuron firing correlates with the magnitude of the reward (Tobler et al. 2005). However, some have argued it is unlikely that dopamine serves as a teaching signal given the limited availability of afferent sensory processing and the precise timing of dopamine signals (Redgrave and Gurney 2006). Instead, it has been suggested that dopamine may contribute to a more simple, low-computation process, leading to the identification of which aspects of context and behavioral output are crucial in causing unpredicted events (Redgrave and Gurney 2006).
All of the above presented evidence and theoretical framework implicate NAcc dopamine in motivation and/or reinforcement learning. What becomes evident after examining these different lines of research is that the mesolimbic dopamine system is associated with a diverse array of behaviors, which illustrate that dopamine may mediate various functions depending upon the temporal dynamics, location, and context of its release (Schultz 2007). Specifically, the functional impact of phasic and tonic dopamine release may differ, as we will explore below.
2 Dopamine detection in the behaving animal: In vivo microdialysis, chronoamperometry, and fast-scan cyclic voltammetry (FSCV)
A number of analytical techniques are utilized to chemically detect extracellular dopamine in vivo. These techniques differ in their time resolution ranging from milliseconds to hours. Thus, some are best suited to detect tonic changes, while others are optimized for isolating phasic dopamine release events.
2.1 In vivo microdialysis
Microdialysis is one of the most commonly used methods for the in vivo detection of dopamine and has excellent analyte selectivity and sensitivity. In microdialysis, extracellular dialysates of the brain are sampled through a membrane that is permeable to water and small solutes (Bito et al. 1966). The inside of the microdialysis probe inserted into the brain region of interest is continuously flushed with an isomolar solution that lacks the analyte of interest. The analyte of interest is sampled after diffusion from the extracellular space across the membrane into the microdialysis probe and thus changes in concentration can be detected. Another variant of this technique used for determining the absolute basal analyte concentrations is no-net flux microdialysis, which involves perfusion of known concentrations of the analyte of interest through the probe to establish when an equilibrium between the inside and the outside of the probe is reached (Parsons and Justice 1992). Despite the considerable size of the microdialysis probe (0.2-0.5 mm in diameter; 1-2 mm working length), several experimental studies indicate that the damage to the blood brain-barrier is minimal (e.g., Westerink and De Vries 1988; Tossman and Ungerstedt 1986).
Microdialysis is a sampling technique that is not directly coupled to any particular method of chemical analysis. The vast majority of studies uses high performance liquid chromatography in conjunction with electrochemical or fluorescence detection to analyze the very small amount of chemicals in the dialysate. Due to this small amount of dialysate, the sampling time-resolution is usually between 5 to 20 minutes. Even though there are now technical advances that will enable sample collection in intervals significantly shorter than a minute (Bowser and Kennedy 2001), most microdialysis experiments still operate on relatively low temporal resolution and are best suited for the quantitative analysis of basal and slowly changing tonic dopamine concentrations.
2.2 Electrochemical techniques
It is known that dopamine neuron activity responds to reward-related stimuli on a subsecond timescale (Schultz 1997). To fully understand control of behavior by dopamine in the NAcc and its role in drug addiction, we require neurochemical information with this level of temporal resolution. Electrophysiological recordings provide excellent temporal resolution but usually do not determine the projection target of recorded neurons and, thus, cannot inform us on neurotransmission in specific terminal structures. In contrast, electrochemical or voltammetric techniques combine sampling of dopamine neurotransmission in specific terminal structures with excellent temporal resolution.
Although various voltammetric methods have been developed over the years, the basic principle underlying each of these variations is the application of a modest electrical potential sufficient to drive electrolysis of the analyte of interest in brain extracellular fluid (Adams 1976; Stamford 1986; Kawagoe et al. 1993). The current produced by this electrolysis can be measured at the electrode and is proportional to the number of molecules undergoing oxidation, and therefore to the concentration of analyte at the electrode surface (Adams 1976; Stamford 1986; Kawagoe et al. 1993). Adams and coworkers pioneered voltammetric recordings of dopamine in the 1970s (Adams 1976). However, the presence of high concentrations of electroactive neurotransmitter metabolites, as well as ascorbic acid and uric acid, interfered with their recordings (Marsden et al. 1988). Technical advances such as the utilization of modified electrodes and more complex input voltage command waveform to improve selectivity (e.g., Gonon 1981; Gonzalez-Mora et al. 1991) have led to the development of three predominant techniques for high-temporal resolution monitoring of dopamine using carbon-fiber microelectrodes: a) amperometry, b) high-speed chronoamperometry, and c) FSCV (Garris and Rebec 2002). These techniques are now often referred to as voltammetric techniques. They are very attractive tools for chemical monitoring in the brain because measurements can be made with a small probe (5-30 μm in diameter; less than 200 μm working length) that causes minimal tissue damage and allows for sampling in precise brain areas. Considering the size of the carbon fiber electrode and the size of the synaptic cleft (15-25 nm; Savtchenko and Rusakov 2007), it is evident that voltammetry monitors the dopamine overflow in the extrasynaptic extracellular space and not dopamine in the synaptic cleft. Of these techniques, (constant-potential) amperometry is the fastest and ‘simplest’ as it applies a continuous, constant potential to the electrode. Although this variant has microsecond temporal resolution, it offers little chemical selectivity since current produced by oxidation of any compound will be detected. Thus, amperometry is of great utility in studying for example fast release and uptake kinetics of single cells in brain slices (e.g., Chow et al. 1992), but has found little use in behaving animals. The remaining two techniques are reviewed below.
2.2.1 In vivo chronoamperometry
In chronoamperometric recordings, the potential of the working electrode is stepped up, held at this higher potential, and then stepped back down, while the resulting oxidation and reduction currents from faradic processes occurring at the electrode are monitored. Such currents have either been analyzed on the level of seconds (for simplicity here termed: high time resolution) or averaged to achieve a better signal-to-noise ratio with a time resolution of minutes to hours (low time resolution). Because electroactive species have different chemical reversibilities, a ratio of oxidation and reduction currents (redox ratios) can assist in identifying the primary substance contributing to changes in the electrochemical signal (e.g., Gratton et al. 1989). Chemical sensitivity is improved further in this technique by using electrodes coated with Nafion, an ion-selective polymer, (Gerhardt et al. 1984) that reduces the contribution of anionic species such as ascorbic acid and the dopamine metabolite dihydroxyphenylacetic acid (DOPAC) to the signal.
2.2.2 In vivo FSCV
Compared to chronoamperometry, FSCV is a more selective electrochemical method, because it utilizes a triangle input waveform (and not a step function) to separate electrolysis from different analytes into temporally-resolved peaks in the output current. Since the voltage is swept gradually to an oxidizing potential and back, current is generated over time, during oxidation and reduction processes, whereby producing multiple electrochemical peaks for an ideal compound. This allows for the recording of a chemical signature, the voltammogram, that serves to identify the species detected, separating the signal from changes in pH and ‘other noise’ and making the chemical resolution more robust (e.g., Baur et al. 1988, Michael et al. 1998). The voltammogram has sufficient information that it can be used with high-powered statistical analysis and provides standardized identification of the dopamine signal (Heien et al. 2004). For this chemometric approach, a so-called ‘training set’ of phasic dopamine events of different amplitude spanning the concentration range of interest is collected (stimulated electrically with varying pulse rate and frequency). This training set is then used to perform a principal component analysis of the signal to statistically identify dopamine events.
3 Effects of drugs of abuse on extracellular dopamine concentration in the NAcc
3.1 The dopamine hypothesis of addiction
It has been proposed that the critical mechanism for the development of addiction is drug-induced activation of dopamine transmission in the NAcc, also referred to as the ‘dopamine hypothesis of addiction’ (Fibiger et al. 1987; Wise and Bozarth 1987; Di Chiara, 1988). Electrophysiological studies have shown that acute exposure to many drugs of abuse affect the firing properties of dopamine neurons in the VTA despite their many distinct actions in the brain (for review, see Wanat et al. in press). However, to directly study dopamine signaling in the NAcc and to fully characterize the functions of dopamine in this target region, it is essential to study dopamine release into the extracellular space (see below) in addition to dopamine cell firing.
3.2 Drug effects on dopamine signaling measured over the course of minutes: Microdialysis studies
In the following sections, we will focus on studies investigating the effects of abused drugs on dopamine levels in the NAcc in the freely moving animal. Microdialysis studies in the behaving animal demonstrated that response-independent, systemic administration of cocaine, amphetamine, heroin, cannabinoids, nicotine, and ethanol increase dopamine levels in the NAcc (DiChiara 1986; Imperato and Di Chiara 1986; Imperato et al. 1986; Di Chiara and Imperato 1988; Kalivas and Duffy 1990; Kuczesnki et al. 1991; Yoshimoto et al. 1992; Pontieri et al. 1996; Tanda et al. 1997). The dose of the drug administered and the concentration of striatal dopamine following drug administration are positively correlated, as demonstrated for cocaine and ethanol (Nicolaysen et al. 1988; Bradberry 2002). Similar to response-independent drug administration, self-administered drugs of abuse, including cocaine, amphetamine, heroin and ethanol, induce increases in concentrations of dopamine in the NAcc (Hurd et al. 1989, 1990; Pettit and Justice 1989, 1991; Weiss et al. 1992, 1993; Di Ciano et al. 1995; Wise et al. 1995a, b). Conversely, drugs with low potential for abuse do not affect dopamine overflow (Di Chiara and Imperato 1988). These findings are in agreement with the dopamine hypothesis of addiction (see Sect. 3.1), since drugs of abuse increase tonic concentrations of extracellular dopamine in the NAcc.
During psychostimulant self-administration, animals learn to “load up” drug concentrations with an initial burst of operant responses before settling into a slower, more regular pattern of responding with inter-response rates varying between two and twenty minutes (Carelli and Deadwyler 1996). Response rates are inversely related to the infusion dose of cocaine or amphetamine, thus, the lower the dose the higher the number of responses (Pickens and Thompson 1968; Wilson et al. 1971; Yokel and Pickens 1973; Wise and Bozarth 1987; Di Ciano et al. 1995). However, the total intake of these drugs is elevated with higher doses. This intake pattern seems not to be due to aversive drug effects at high doses, since monkeys will choose infrequent high doses in preference to more frequent low doses (Iglauer et al. 1976) and rats show no reliable preference for one over the other (Di Ciano et al. 1995). Furthermore, animals will adjust their response rates to meet increased operant response demands (Roberts et al. 1989). Together, this suggests that animals titrate their drug intake to achieve a preferred level of intoxication.
Cocaine-induced increases in extracellular NAcc dopamine concentrations are thought to be the principal neurochemical event associated with the drug’s positive reinforcing action (see Sect. 1.3; Kuhar et al. 1991; Wise and Bozarth 1987; Roberts et al., 1977; Ritz et al., 1987). For example, psychostimulants are self-administered directly into the NAcc (Hoebel et al. 1983; MCkinzie et al. 1999), and morphine and ethanol into the VTA (e.g., Bozarth and Wise 1981; Gatto et al. 1994). With increased drug dose the dopamine “maintenance” concentration in the NAcc is also held at an increased level (Pettit and Justice 1991). Importantly, microdialysis studies have shown that animals will maintain the increased NAcc dopamine concentration during cocaine, amphetamine, and heroin self-administration sessions at a steady level over days, just like they maintain the drug concentrations at a steady level (Pettit and Justice 1989; Pettit et al. 1990; Wise et al. 1995a, b; Ranaldi et al. 1999). Furthermore, in a microdialysis study with advanced temporal resolution that sampled dopamine every minute, Wise and colleagues (1995b) demonstrated that the self-administration pattern of cocaine closely followed NAcc dopamine levels within sessions. This study demonstrated that cocaine dose-dependently increased dopamine concentrations after an infusion and that rats self-administered the next infusion as soon as the dopamine concentration diminished past a certain threshold. Consistent with this finding, it has been proposed that functional dopamine depletion in the NAcc represents a neurochemical correlate of drug craving (Dackis and Gold 1985; Koob et al. 1989). The findings presented above suggest that fluctuation in tonic dopamine concentration in the NAcc is a common denominator between different abused drugs that can regulate drug self-administration behavior.
3.3 Drug effects on dopamine signaling measured over the course of seconds to hours: Chronoamperometry studies
In vivo chronoamperometry studies in the behaving animal have demonstrated that experimenter-administered ethanol, cocaine, and amphetamine increase extracellular concentrations of dopamine over the course of minutes to hours (Kiyatkin 1994; Di Ciano et al. 1998b; Sabeti et al. 2003). Similarly, self-administration of heroin and psychostimulants caused an increase in dopamine concentrations in the NAcc on this time scale (Gratton 1996; Di Ciano et al. 2001, 2002). Thus, chronoamperometry studies with low time resolution confirmed microdialysis findings on the basic effects of abused drugs on extracellular concentration of dopamine in the NAcc.
To further investigate the temporal dynamics of dopamine signaling in animals self-administering drug, chronoamperometry with high time resolution was used to monitor dopamine on the order of seconds. NAcc dopamine concentrations were found to gradually increase preceding and to drop immediately following response-dependent (and – independent) intravenous injections of cocaine, before rising again around 4-6 min after infusion (Kiyatkin and Stein 1994, 1995). These post-response decreases in signal were dose-dependent, absent when the infusion was withheld, and the pre- response increases became increasingly bigger when the access to the lever was blocked (Kiyatkin et al. 1993; Kiyatkin and Gratton 1994; Gratton 1996). Furthermore, chronoamperometry studies reported similar response patterns during operant behavior maintained by other reinforcers such as heroin (Kiyatkin et al. 1993) and food (Kiyatkin and Gratton 1994), supporting the assumption that dopamine is the principal contributor to the reported biphasic signal fluctuations. In summary, the described high time resolution chronoamperometric data are in conflict with microdialysis findings which found that dopamine levels in the NAcc rise and fall in unison with oscillating blood/brain stimulant levels (Pettit and Justice 1989; Koob and Bloom 1988; Wise and Bozarth 1987; see Sect. 3.2).
The validity of chronoamperometry measurements has been challenged on basis of chemical sensitivity (Salamone 1996; Di Chiara 2002; Wightman and Robinson 2002). First, even though the electrodes are much less sensitive to DOPAC than to dopamine, the DOPAC concentration is several hundred times higher than dopamine and fluctuations in DOPAC concentration may contribute to the signal (Dayton et al. 1981; Gonon et al. 1984). Second, the voltage input step used to measure current changes that were assumed to be due to the oxidation/reduction of dopamine also measures changes in pH that often accompany dopamine signaling (Heien et al. 2004). The argument that chemical species other than dopamine are contributing to the chronoamperometry signal is supported by the fact that the detected task-related signaling changed dramatically during the development of self-administration behavior (Gratton 1996), an observation not reported for heroin or by microdialysis studies with cocaine. Furthermore, chronoamperometry studies report drug-induced elevations in dopamine concentrations last nearly twice as long as that measured with microdialysis (Di Ciano et al. 1995). Although these results were replicated and the reported electrochemical changes were shown to be task-related, one cannot be sure about the chemical specificity of the signal (Wightman and Robinson 2002), especially in light of evidence from a microdialysis study reporting the opposite finding (Wise et al. 1995b; see Sect. 3.2).
In summary, microdialysis and low time resolution chronoamperometry studies have convincingly demonstrated a link between tonic dopamine concentrations in the NAcc and the reinforcing effects of drugs of abuse. However, both techniques also raised questions. For example, what is the detailed temporal composition of dopamine signals? In contrast, chronoamperometry with high temporal resolution leaves the question of the chemical identity of observed phasic changes unanswered. These questions and some of the above described issues are not resolvable with these techniques, as the need for a combination of high temporal and chemical resolution is not satisfied by either of these techniques alone.
3.4 Drug effects on dopamine signaling measured on a subsecond time scale: FSCV studies
3.4.1 Changes in phasic dopamine signaling to response-independent drug administration
FSCV provides sufficient temporal and chemical resolution to study phasic dopamine signals. For example, a single phasic dopamine event in response to either electrical stimulation or presentation of a salient stimulus can be assessed. Furthermore, FSCV can examine the effects of drugs of abuse on phasic dopamine in both in vitro (slice) and in vivo (anesthetized, awake, and during self-administration) preparations, and can separate dopamine release and uptake. In addition to the amplitude and duration of phasic dopamine changes, the frequency of ‘spontaneous’ dopamine transients (i.e., phasic release events found in the awake animal that are not attributed to specific events in the animal’s environment) can also be measured. Thus, findings obtained with FSCV illustrate a greater complexity of dopamine signaling than previously described by data from other techniques (see Sect. 3.2 and 3.3).
The phasic NAcc dopamine response to ethanol and cannabinoids illustrates the complexity in the profile of subsecond dopamine signaling. Ethanol showed no effect on dopamine uptake in striatal brain slices collected from drug-naïve rats (Samson et al. 1997; Budygin et al. 2001b; Mathews et al. 2006), but enhanced dopamine uptake in animals chronically treated with ethanol, possibly due to a compensatory mechanism resulting from elevated dopamine levels (Budygin et al. 2007). Consistent with this, both ethanol and cannabinoids attenuate electrically stimulated dopamine release in the intact animal, possibly due to increased tonic dopamine levels (see Sect. 3.2) that impair phasic dopamine release due to activation of release-regulating autoreceptors (Budygin et al. 2001a; Cheer et al. 2004). In contrast, intravenous infusions of ethanol and cannabinoids increased the amplitude and/or frequency of spontaneous phasic dopamine transients in awake, behaving animals (Cheer et al. 2004, 2007). These findings suggest that the complexity conferred by multiple mechanisms make it difficult to parsimoniously use findings from in vitro preparations and artificial electrical stimulations to make net predictions concerning the effect of drugs on phasic dopamine signaling in awake, behaving rodents. In agreement with results presented in section 3.2 that indicated enhanced tonic dopamine concentrations in the NAcc, the findings presented above demonstrate increased frequency of spontaneous phasic dopamine signals by ethanol and cannabinoids.
A number of FSCV studies have examined the effect of nicotine on phasic dopamine release in both in vitro and in vivo preparations. In contrast to ethanol, acute in vivo nicotine exposure enhances dopamine uptake in the striatum of the anesthetized rat (Middleton et al. 2004). Nicotine exerts frequency-dependent effects on electrically stimulated phasic dopamine release in vitro; at low firing rates dopamine release is attenuated, but at high firing rates nicotine enhances dopamine release (Zhang and Sulzer 2004). Intravenous infusions of nicotine also increase the frequency and amplitude of spontaneous phasic dopamine release events in the behaving rat (Cheer et al. 2007). Together, both in vitro and in vivo studies consistently show enhanced phasic dopamine signaling in response to nicotine.
Consistent with findings from other abused substances, intravenous infusions of cocaine increase amplitude and frequency of spontaneous phasic dopamine release events in the NAcc (Heien et al. 2005; Stuber et al. 2005a, b; Cheer et al. 2007; Wightman et al. 2007; Aragona et al. 2008), as well as the amplitude of electrically stimulated release (Wu et al. 2001). An increase in amplitude can be explained by decreased reuptake due to the pharmacological action of cocaine. However, it is not clear why cocaine increases the frequency of dopamine release events. It seems likely that more phasic signals exceed the FSCV detection threshold due to their increased amplitude and thus become ‘visible’ under drug exposure. Another explanation is that drug-induced behavioral hyperactivity may stimulate afferents to the VTA and therefore may increase the firing frequency of dopamine neurons projecting to the NAcc. Notably, these findings indicate that the strong increases in tonic dopamine levels described in section 3.2 do not appear to result in significant autoreceptor-mediated inhibition of dopamine neurons, thus, one could describe the phasic signals as ‘riding on a tonic dopamine wave’ during drug exposure.
Interestingly, endogenous cannabinoids modulate the cocaine-, nicotine-, and ethanol-mediated increases in phasic dopamine release, as the effects of drugs on phasic dopamine release are attenuated by systemic cannabinoid receptor antagonism (Cheer et al. 2007). While the locus of this effect is yet to be determined, it is speculated that cannabinoid receptor activation in the VTA reduces GABA release on dopamine neurons (Riegel and Lupica 2004). These findings suggest that abused drugs exert similar effects on phasic dopamine release even though their respective pharmacological and cellular effects are quite distinct.
3.4.2 Changes in phasic dopamine signaling during cocaine self-administration: The role of operant behavior and conditioned stimuli
Pavlovian and operant conditioning paradigms using non-drug reinforcers have demonstrated that conditioned stimuli (CS) can elicit phasic dopamine release (Roitman et al. 2004; Day et al. 2007; Stuber et al. 2008; Owesson-White et al. 2008). In studies using drug reinforcers, it has been shown that repeated CS presentation with drug delivery subsequently can elicit an electrochemical response when the CS is presented alone (Kiyatkin et al. 1993; Kiyatkin and Gratton 1994; Di Ciano et al. 1998a). This response develops over time, as its development requires at least 10-50 pairings of the drug with the CS (Gratton 1996), and therefore indicates that these dopamine signals reflect a learning process. Studies that probed the contribution of CS to phasic dopamine in response to drug taking will be discussed below.
Contingent and non-contingent cocaine administration produce differential long-term effects on synaptic plasticity in VTA dopamine neurons (Chen et al. 2008). The effect of cocaine infusions on phasic dopamine release also depends on whether the drug administration is contingent upon an operant response or not, as shown with FSCV (Stuber et al. 2005a). For example, in rats pressing a lever for an intravenous infusion of cocaine, rapid changes in dopamine concentrations are time-locked to specific aspects of this behavior (Phillips et al. 2003; Stuber et al. 2005a, b; Fig. 1a), whereas no changes in dopamine levels are observed within 10 seconds of a response-independent cocaine administration to awake, but idle rats (Stuber et al. 2005a). In contrast, following these initial 10 seconds, the frequency of spontaneous dopamine transients increases dramatically independent of whether the administration of the drug was response-independent or –dependent (Stuber et al. 2005a). However, there is an ongoing debate as to what the exact latency of the pharmacological effects of cocaine is after intravenous infusion is (Espana et al. 2008; Wise et al. 2008). The considerable variance in the onset of these transients may be due to variability in the latency of drug delivery to the brain. Together, this suggests that a) early dopamine release events (first 10 seconds) in animals that self-administered cocaine may be related to learned associations, and b) the increase in spontaneous dopamine transients 10 seconds after the beginning of the infusion may be a consequence of the pharmacological effects of cocaine that is not related to learning. Consistent with this idea, these spontaneous transients are correlated with cocaine levels and the animal’s locomotion (Stuber et al. 2005a). In summary, cocaine self-administration is accompanied by early phasic dopamine release that is time-locked to the onset of drug infusion but cannot be attributed to the pharmacological effects of the drug.
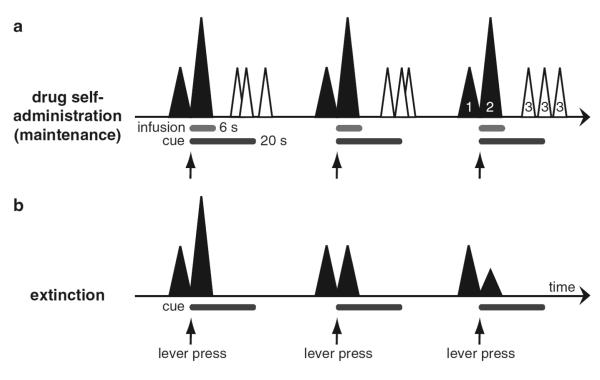
Figure 1.
Phasic dopamine signaling in the NAcc associated with drug seeking and taking (a) Phasic dopamine signaling consists of multiple phasic events (triangles). The first event is elicited by the animal’s approach of the operant lever (pre-response signal; 1), whereas a bigger second event is associated with the onset of the audiovisual cue (dark gray bar) that is presented in response to the lever press (post-response signal; 2). A set of peaks (3) that is observed with an onset of approximately 10 seconds after the lever press and the beginning of the drug infusion (light gray bar), is thought to be a direct consequence of the pharmacological effect of the drug. The latency of this pharmacological effect relative to the operant response appears to be more variable than for the post-response signal. (b) During extinction, the audiovisual stimulus presented after the lever press is not accompanied by a drug infusion. As a consequence, the post-response dopamine signal becomes smaller with repeated non-reinforced responding. In contrast, the pre-response signal remains relatively stable during extinction. This suggests that the pre-response signal reflects the motivation to obtain drug, whereas the post-response encodes the expectation of the drug infusion.
Phillips et al. (2003) demonstrated that the largest change in dopamine concentration time-locked to cocaine self-administration behavior occurs immediately upon completion of the operant response (Fig. 1a). This effect was conditioned to an audiovisual stimulus (presented on the completion of the lever response) and was not due to the pharmacological actions of cocaine since it persisted during initial extinction trials where cocaine is replaced with saline (Stuber et al. 2005b; Fig. 1b). If the CS denoting the onset of the cocaine infusion was presented non-contingently during the period between operant responses, a similar dopamine signal could be evoked. Furthermore, this signal diminished gradually when the associative strength between the stimuli and cocaine is weakened during extinction (Stuber et al. 2005b; Fig. 1b). This phenomenon is identical to what has been observed with non-drug reinforcers, where cues that predict availability of these reinforcers are able to elicit phasic dopamine release on their own (Roitman et al. 2004, Owesson-White et al. 2008). Therefore, the change in extracellular dopamine that occurs at completion of the operant response (Fig. 1a) may encode the association of cue and drug delivery, and thus the expectation of cocaine delivery.
Phasic dopamine release has also been demonstrated just prior to the operant response for a self-administered infusion of cocaine (Phillips et al. 2003, Stuber et al. 2005a, b; Fig. 1a). These changes tended to be smaller than those following the operant response, but consistently preceded the animal’s approach to the response lever. In agreement with these antecedent neurochemical signals, dopamine neuron activation also occurs immediately before subsequent injections in rats trained to bar press for intravenous heroin (Kiyatkin and Rebec 2001). Furthermore, chronoamperometric studies detected a slow increase in dopamine-related signals (over the course of multiple seconds) in rats before they approached the lever for the next drug self-administration (Kiyatkin and Stein 1995). This temporal correlation with the drug seeking (lever approach) suggests that this component of the neurochemical signal might be causally linked to the behavior (Phillips et al. 2003, Stuber et al. 2005a, b). The temporal proximity of the signal precludes testing its role in drug seeking using conventional pharmacological approaches, since blockade of this signal will perturb other phasic dopamine signals or the tonic baseline level of dopamine. However, an electrically-evoked dopamine signal of similar brevity was sufficient to promote the lever approach (drug seeking), as such brief changes in dopamine, heavily influenced drug seeking by biasing the animal to initiate this behavior (Phillips et al. 2003). This may indicate a role for phasic dopamine in drug seeking or at least biasing the animal’s decision-making policies towards selecting actions leading to drug taking, rather than a direct role in drug reward. In contrast to the post-response signal, this pre-response signal does not appear to be a learned or conditioned process because it does not disappear during extinction trials, where the CS was presented contingent to the operant response but without drug infusion (Stuber et al. 2005b; Fig. 1b). Together, these findings indicate that the pre-response dopamine signal may be involved in initiating approach behavior.
In summary, these findings underline the multiple roles of phasic dopamine in operant responding for cocaine. Phasic dopamine release associated with operant behavior is comprised of signals in relation to approach, conditioned cues, and the pharmacological effects of the drug (Fig. 1a). Dopamine signaling is likely to play a role in motivation but probably also encodes other information about goal-directed behavior including learned association betweens cues and drug reward. The extinction-resistant pre-response signal seems to be related to drug seeking and therefore may have a motivational quality. In contrast, the post-response signal is linked to a learned association (lever-press and cue signaling the drug infusion) and therefore may be related to the expectation of drug delivery (see Sect. 3.4.2). Compared to natural reinforcers, such behavior-related dopamine release is enhanced due to the pharmacological properties of abused drugs. Therefore, the normal function of these signals is potentially corrupted, which may explain dysregulated goal-directed behavior in addiction (see Sect. 7.2).
3.5 Summary
The findings obtained in FSCV studies examining the effects of different drugs of abuse on phasic dopamine signaling in the NAcc are in general agreement with findings made with microdialysis and low time resolution chronoamperometry (see Sect. 3.2 and 3.3), as each of these techniques have shown that response-dependent and –independent administration of abused drugs increase dopamine levels in the NAcc. Thus, both phasic and tonic concentrations of dopamine in the NAcc are enhanced by drugs, which is in agreement with the postulate of the dopamine hypothesis of addiction that drugs of abuse converge on the mesolimbic dopamine pathway. These findings also raise the question to what extent such phasic events contribute to the observed changes in tonic dopamine levels. Some evidence indicates that spontaneous dopamine transients that presumably reflect the pharmacological effects of drugs are likely to alter overall tonic dopamine concentrations (see Sect. 3.4.2). Future studies are required to investigate a possible interaction between phasic and tonic dopamine signaling and to examine phasic dopamine release during the self-administration of a wider range of abused substances.
4 Effects of withdrawal from drugs of abuse on the NAcc dopamine system
While the acute effects of drugs on the dopamine system are well cataloged, the long-term effects after cessation of drug intake are less well studied and understood. This disconnect in the research is partly because the latter effects show considerable variance that arises from the drug studied, how the drug is administered (dose, frequency, and route), and duration of abstinence after drug experience (Wanat et al. in press). It has been documented that repeated exposure to a drug of abuse causes structural changes in VTA dopamine neurons, as repeated opiate exposure decreases the size and caliber of dendrites and soma of VTA dopamine neurons (Sklair-Tavron et al. 1996). Along with such structural changes, many studies support the notion that multiple drug exposures lead to an altered function of the dopamine system (Koob and Le Moal 2008), though the timing of this effect can vary.
4.1 Tonic dopamine during withdrawal
Microdialysis studies have demonstrated that the discontinuation of chronic treatment with ethanol (Rossetti et al. 1991, 1992a, b; Diana et al. 1993; Weiss et al. 1996), morphine (Acquas et al. 1991; Pothos et al. 1991; Acquas and Di Chiara 1992; Rossetti et al. 1992a, b; Crippens and Robinson 1994), nicotine (Rahman et al. 2004), amphetamine (Rossetti et al. 1992a), or cocaine (Parsons et al. 1991; Robertson et al. 1991; Imperato et al. 1992; Rossetti et al. 1992a, b; Segal and Kuczenski 1992; Weiss et al. 1992; Diana et al. 1993; Chefer and Shippenberg 2002; Zhang et al. 2003) decreases the basal extracellular concentration of dopamine in the NAcc.
However, there have been conflicting reports for withdrawal from psychostimulants. For example, several studies demonstrated a lack of change in basal concentrations of NAcc dopamine after withdrawal from amphetamine (Segal and Kuczenski 1992; Crippens et al. 1993; Crippens and Robinson 1994; Paulson and Robinson 1996). In the case of cocaine, some studies reported that withdrawal does not change tonic NAcc dopamine (Robinson et al. 1988; Kalivas and Duffy 1993; Hooks et al. 1994; Meil et al. 1995; Kuczenski et al. 1997), and yet others found an increase in basal dopamine levels (Imperato et al. 1992; Weiss et al. 1992; Johnson and Glick 1993; Heidbreder et al. 1996). While some studies reported changes in basal dopamine levels depending upon the time of cocaine withdrawal (Imperato et al. 1992; Heidbreder et al. 1996), these observed changes have not been consistent across studies. For example, some report that withdrawal after chronic cocaine exposure decreases basal dopamine levels in as early as a few hours (Zhang et al. 2003) to as long as 10 days (Parsons 1991), while others found increases in basal dopamine levels during 1 – 4 days of withdrawal (Imperato et al. 1992; Weiss et al. 1992; Heidbreder et al. 1996), and it was reported to have no effect on basal dopamine levels after 24 hours and 2 weeks withdrawal (Kalivas and Duffy 1993; Meil et al. 1995). Thus, at least for cocaine, no clear temporal effect of withdrawal on basal dopamine levels can be inferred from these studies. The studies described above utilized either conventional microdialysis or the more accurate method of determining exact basal dopamine levels, no-net flux microdialysis (Parsons and Justice 1992). The majority of studies employing no-net flux microdialysis did not observe changes in dopamine levels after withdrawal from cocaine treatment, strongly supporting no or minor effects of cocaine withdrawal on tonic dopamine concentration (Crippens et al. 1993; Kalivas and Duffy 1993; Heidbreder et al. 1996; Chefer and Shippenberg 2002).
Together, the effects of withdrawal from chronic drug treatment on NAcc dopamine levels depend on many factors, including the drug studied and the dose of the drug administered during the chronic treatment (Kalivas and Duffy 1993). In addition, it is known that tonic dopamine levels in the NAcc are affected by drug cues (see Sect. 5.1) and by contextual cues associated with aversive stimuli (Mark et al. 1991; Pezze et al. 1991; Saulskaya et al. 1995). Re-exposure to a drug-paired environment can either be rewarding or aversive depending on drug dose and time of drug-cue pairing after drug administration (Ettenberg 2004). Differences in these parameters may explain some of the discrepancies between studies presented here. In summary, according to microdialysis studies the NAcc dopamine system may undergo a depression after withdrawal from abused substances, however, this finding remains controversial regarding psychostimulants such as cocaine.
4.2 Phasic dopamine during (short-term) withdrawal
Only one study using FSCV has examined how phasic dopamine release associated with drug taking is affected by withdrawal from drug self-administration. Stuber et al. (2005b) showed that the dopamine post-response signal associated with the completion of the operant response gradually decreases during an extinction session immediately after completion of a cocaine self-administration session, whereas the pre-response signal remained relatively stable (Fig. 1b). Thus, the overall phasic dopamine release associated with the operant response for drug delivery decreases during withdrawal. However, this study does not reveal how phasic dopamine signals are affected by long-term withdrawal. Furthermore, the self-administration behavior underwent extinction instead of abstinence, a better model for the human condition because humans do not usually undergo extinction during drug withdrawal. The term ‘abstinence’ will be used here to describe withdrawal from drug taking without extinction of drug taking behavior. We are aware of the fact that this term is not an ideal description of animal behavior since the animal does not refrain from drug taking voluntarily. Future studies should investigate changes in phasic dopamine signaling during long-term withdrawal after extinction or abstinence from drug taking.
Collectively, these studies suggest that acute exposure to drugs of abuse activates phasic and tonic dopamine signaling in the NAcc (see Sect. 3), but withdrawal from chronic drug exposure can dampen phasic and tonic dopamine levels in the absence of drug (see Sect. 4). However, withdrawal from psychostimulants does not necessarily lead to decreased tonic levels. Decreased tonic levels of dopamine in the NAcc and firing of VTA dopamine neurons during drug withdrawal have been shown to return to and above basal levels by a subsequent drug re-exposure with ethanol (Diana et al. 1993; Weiss et al. 1996), amphetamine (Robinson et al. 1988), cocaine (Pettit et al. 1990), and morphine (Sklair-Taylor et al. 1996; Diana et al. 1999). Similarly, the extinction-induced decrease of the phasic post-response dopamine signal can be reversed to previous amplitudes during a drug-induced reinstatement session, as shown by FSCV (Stuber et al. 2005b). Returning dampened dopamine levels to ‘baseline’ may be one of the driving forces in relapse to drug taking behavior, since evidence presented in section 3.2 indicates that animals pursue the next drug infusion when tonic dopamine levels in the NAcc decrease past a certain threshold.
5 Stimulus-induced NAcc dopamine release in the absence of drug: Implications for reinstatement of drug seeking
5.1 Effects of drug cues on tonic dopamine concentration in the NAcc
In the previous sections, we reviewed the powerful effects of abused drugs on dopamine signaling in the NAcc during drug taking and withdrawal. This leads to the question of whether the NAcc dopamine system is also involved in reinstatement of drug seeking behavior after abstinence or extinction of drug taking. Re-exposure to drugs of abuse on a single occasion can promote relapse to drug seeking behavior in abstinent human drug users (Jaffe et al. 1989). Similarly, exposure to a CS associated with self-administered drugs can elicit subjective states such as craving (Grant et al. 1996; Childress et al. 1999; Garavan et al. 2000), as well as drug seeking and relapse in humans (Stewart et al. 1984; Avants et al. 1995) and experimental animals (Markou et al. 1993, Robinson and Berridge 1993). Furthermore, even after extinction of self-administration behavior, drug seeking can be reinstated in animals by presentation of conditioned drug cues (e.g., de Wit and Stewart 1981; Weiss et al. 2000).
It has been proposed that the ability of conditioned drug cues to increase dopamine is critical for reinstatement of drug seeking (Stewart et al., 1984), and support for this assumption is provided by findings in animals (see Sect. 3.4.2). Further evidence for the involvement of NAcc dopamine signaling in drug-induced reinstatement is provided by findings demonstrating that microinjection of amphetamine into the NAcc can reinstate heroin self-administration (Stewart and Vezina 1988) and that such drug seeking can be attenuated by drugs that decrease dopamine neuron activity (Di Ciano and Everitt 2003, 2004; Bossert et al. 2004). In contrast, a decrease in the concentration of extracellular dopamine in the NAcc may contribute to some drug withdrawal symptoms (Rossetti et al. 1992b). Decreasing withdrawal symptoms by increasing dopamine levels may therefore possibly promote the motivation to reinstate drug taking (Koob and Le Moal 2008). Therefore, we will now focus on studies examining drug cue-induced changes in dopamine release and how this relates to reinstatement of drug seeking.
Several studies have observed increases in tonic dopamine concentrations in the NAcc following the non-contingent presentation of a psychostimulant-paired CS in the absence of drug. Such CS-induced effects were demonstrated after pairing of the CS with response-independent (Fontana et al. 1993; Di Ciano et al. 1998b) as well as with response-dependent (Gratton and Wise 1994; Kiyatkin and Stein 1996; Di Ciano et al. 1998a; Ito et al. 2000) drug administration. Similar conditioned changes in dopamine have been shown in the NAcc following the non-contingent presentation of stimuli previously paired with food (Young et al. 1998), heroin (Gratton 1996), or footshock (Wilkinson et al. 1998). In contrast to non-contingent presentation, response-contingent presentation of a CS failed to produce changes in tonic NAcc dopamine (Neisewander et al. 1996; Ito et al. 2000). In fact, one study found no difference in NAcc dopamine concentrations during cocaine self-administration without concurrent presentation of the CS as compared to self-administration of cocaine plus contingent presentation of a CS (Bradberry et al. 2000). However, it should be noted that other studies have found no change in tonic dopamine efflux in response to the non-contingent presentation of drug cues (Brown and Fibiger 1992; Bradberry et al. 2000). This discrepancy may be due to the proximity of changes in dopamine concentrations to the microdialysis detection limit, where minor differences in the study design, for example the number of stimulus-reward pairings or CS presentations, may cause different outcomes. Overall, these findings suggest that non-contingent (but not contingent) presentation of drug-paired cues can contribute to overall changes in tonic NAcc dopamine.
Few studies have examined dopamine release during actual cue-induced reinstatement of drug-seeking after abstinence or extinction of the operant behavior. One study found elevations in tonic NAcc dopamine after extinction in conjunction with robust cocaine-seeking behavior elicited by sustained (60 minutes) presentation of a salient discriminate stimulus that previously signaled the availability of cocaine (Weiss et al. 2000). In contrast, reinstatement elicited by brief non-contingent presentation of a visual drug cue during or after extinction did not produce significant changes in dopamine efflux, although drug-induced reinstatement showed both a behavioral and a neurochemical response (Neisewander et al. 1996; Di Ciano et al. 2001). This suggests that repeated or sustained non-contingent presentation of drug-associated cues may be necessary to elicit changes in tonic dopamine concentrations during reinstatement of drug seeking that are detectable with microdialysis.
5.2 Effects of drug cues on phasic dopamine signaling in the NAcc
There are no studies to date that have examined cue-induced reinstatement of drug seeking with FSCV. However, Stuber et al. (2005b) demonstrated that the phasic dopamine component that occurs following a lever-press for cocaine infusion (post-response) gradually diminishes during extinction, whereas the signal that occurs just prior to the lever-press (pre-response) seems resistant to extinction (Fig. 1b, see Sect. 4.2). During drug-induced reinstatement, post-response but phasic dopamine release returns to the previous pre-extinction amplitude, suggesting that it encodes a learned association between cue and drug delivery (Stuber et al. 2005b). In addition, studies recording neuronal firing in the NAcc during within-session extinction have shown that neurons with a post-response discharge pattern are less active during extinction and return their activity to pre-extinction levels after reinstatement (Carelli and Ijames 2000). The absence of the post-response signal after extinction in both striatal dopamine release and NAcc cell firing makes it an unlikely candidate as the driving force behind the initiation of the reinstatement of drug seeking. Moreover, the increase in post-response dopamine does not cause further cocaine seeking in self-administration maintenance sessions, as it would be expected if this aspect of the dopamine signal is involved in approach behavior or drug seeking. Instead the animals typically engage in stereotypies during the immediate post-response phase before seeking the next infusion (Stuber et al. 2005a). However, abstinent human addicts generally do not undergo extinction and thus drug cues may retain their capacity to elicit dopamine release. Furthermore, the post-response signal may reappear during subsequent extinction sessions, as drug seeking in response to drug cues is not permanently extinguished after a single extinction session, as shown in many studies (Shalev et al. 2002).
In contrast to the post-response dopamine signal, the pre-response dopamine signal may be an essential component of reinstatement of drug seeking since it remains relatively stable during extinction (Fig. 1b), and an electrically evoked dopamine signal of similar brevity is sufficient to induce drug seeking (Phillips et al. 2003). Additionally, animals will approach and press a lever for an infusion of dopamine into the NAcc (Dworkin et al. 1986). Such a dopamine-induced approach response may be more reliably triggered in a drug-related environment where the approach is directed towards a familiar goal.
Together, the findings presented in sections 5.1 and 5.2 indicate that tonic and phasic dopamine concentrations in the NAcc can be increased during (non-contingent) cue-induced reinstatement of drug seeking. However, there have been few studies of this effect and their findings are somewhat inconsistent, which may be reflect the highly dynamic quality of the phasic dopamine post-response that fades quickly under extinction conditions. The lack of reinstatement-related tonic dopamine changes after brief exposure to drug cues under extinction conditions may thus be attributable to a diminished amount of phasic dopamine per lever approach (decreased post-response dopamine), whereas higher tonic dopamine levels can be achieved with sustained cue exposure. These findings suggest that the more stable pre-response component of the dopamine signal (Fig. 1b) may be of greater significance in eliciting reinstatement of drug seeking.
6 The role of NAcc dopamine in drug addiction
Altered dopamine signaling has been implicated in all stages of drug addiction, from induction to maintenance to relapse. Unlike natural reinforcers, drugs act directly on the mesolimbic dopamine system thereby bypassing sensory processing and adaptive mechanisms that normally control NAcc dopamine release. Most theories of drug addiction postulate that this ‘direct access’ to dopamine signaling results in abnormal shaping of synaptic efficiency. However, addiction theories differ regarding the effect that these processes have on the organism (see below).
6.1 Motivation and addiction
The ‘incentive-sensitization’ theory of addiction proposed by Robinson and Berridge (1993) emphasizes the motivational function of NAcc dopamine. It states that hedonic processes (‘liking’) associated with drug intake are not mediated by the mesolimbic dopamine projection, which instead is involved in the attribution of incentive salience to stimuli associated with rewards (‘wanting’) (as described in Sect. 1.5.1). Thus, this theory postulates that NAcc dopamine mediates the motivation to pursue rewards. Addictive drugs are assumed to render these brain reward systems hypersensitive (i.e., ‘sensitized’) to drugs and drug-related stimuli, causing pathological wanting of drugs. According to this view, relapse to drug seeking and compulsive aspects of drug taking are mediated by the sensitized dopamine efflux in the NAcc in response to a drug-paired CS.
6.2 Associative learning and addiction
Dopamine receptors regulate intracellular signaling cascades that alter the expression of genes, such as immediate-early genes, which are considered to be one of the first molecular steps that ultimately result in stable neuroadaptations and behavioral changes (for review, see Davis and Squire 1984; Stork and Welzl 1999; Tischmeyer and Grimm 1999). In the striatum, such mechanisms have been shown to play a central role in learning-related changes in protein synthesis (Teather et al. 2005; Hernandez et al. 2006). Consistent with associative learning theories of addiction, psychostimulants engage a set of molecular mechanisms normally implicated in learning and memory including D1 receptors and downstream intracellular messenger cascades that may cause synaptic rearrangements (Berke and Hyman 2000; Everitt et al. 2001). Thus, psychostimulant-induced dopamine release may alter learning-related molecular changes by activating common signal transduction pathways. In fact, the effects of psychostimulants on procedural memory consolidation have been demonstrated in many studies (e.g., Puglisi-Allegra et al. 1994; Castellano et al. 1996; Cestari and Castellano 1996; Packard and White 1991). Importantly, recent studies found D1 receptor-dependent effects of cocaine on procedural learning in association with molecular changes in the striatum (Willuhn and Steiner 2006, 2008). A current influential hypothesis that incorporates these findings would suggest that addiction is due to drug-induced neuroadaptations in reward-related learning and memory processes in the NAcc (Berke and Hyman 2000; Everitt et al. 2001). Such neuroadaptations are believed to cause hypersensitivity to cocaine-associated cues (Di Chiara and Bassareo 2007; Everitt and Wolf, 2002) and abnormal habit-like behaviors (White 1996) that become insensitive to adverse consequences with chronic drug exposure and lead to compulsive drug intake (Wolffgramm and Heyne 1995; Deroche-Gamonet et al. 2004).
7 Different functions for phasic and tonic dopamine transmission in addiction
How can the different modes of dopamine transmission, phasic and tonic signaling, be synthesized with different theories of addiction? Most experiments do not distinguish between these two modes of neurotransmission. For example, in vivo microdialysis and low time resolution chronoamperometry measure changes in tonic levels of dopamine, but it is not clear how much phasic signaling contributes to tonic extracellular concentrations of dopamine, and, thus, these techniques potentially measure the sum of both tonic and phasic dopamine. Similarly, pharmacological manipulations of the dopamine system modify both tonic and phasic aspects of dopamine neurotransmission, as both modes presumably utilize the same mechanisms of release and reuptake. Interestingly, it is both experimentally and conceptually challenging to separate the different functions of dopamine on a behavioral level. As described in section 1.5, dopamine signaling is thought to be implicated in both reinforcement learning and motivation. Dopamine is thought to facilitate reinforcement learning by “stamping in” stimulus-reward associations (Wise 2004), providing a prediction error (Montague et al. 2004), and/or by biasing action selection (Redgrave and Gurney 2006). Alternatively, dopamine is thought to facilitate motivation by enhancing the energizing effect of reward or reward-predicting cues through assignment of incentive salience (Robinson and Berridge 1993, 2008) and/or by maintaining behavior when response costs are high (Salamone and Correa 2002). Below, we argue that the different temporal modes of dopamine signaling fulfill both learning and motivational functions.
Recent research, using a genetic approach, indicates that phasic and tonic dopamine signaling may indeed subserve different functions. Specifically, reduced expression of the dopamine transporter in the striatum and thus reduced clearance of released dopamine has been shown to cause increased motivation in a previously learned task in the absence of new learning (Cagniard et al. 2006b). Importantly, mice carrying this inducible knockdown of the dopamine transporter showed increased non-bursting activity of dopamine neurons (presumably driving tonic dopamine concentration in the striatum) but no change in burst firing (presumably driving phasic dopamine release). Thus, these mice learned a behavioral task and were then rendered tonically hyperdopaminergic, which led to a better performance in this task without affecting reinforcement learning subsequently tested in another task (Cagniard et al. 2006b). This important finding suggests that tonic dopamine signaling may mediate motivational aspects of behavior. Conversely, it has been suggested that phasic signaling is particularly well suited for transmitting rapid time-specific information and thus provide the temporal resolution necessary to represent the contingencies in reinforcement learning (Grace 1991; Schultz 2007). FSCV recordings in the NAcc were made from rats during Pavlovian reinforcement learning support this role for phasic dopamine release (Day et al. 2007; Sunsay and Rebec 2008; Stuber et al. 2008). Early in training, phasic dopamine responses are observed primarily with reward delivery (unconditioned stimulus, US). With continued training, dopamine release is elicited by the presentation of the CS, while the response to the US is attenuated, suggesting a transfer of the phasic dopamine response from the US to the CS (Day et al. 2007; Sunsay and Rebec 2008). Such a role for dopamine in the learning of stimulus-reward associations has also been demonstrated in electrophysiological studies (Schultz 1997; see Sect. 1.5.2) and cocaine self-administration studies using FSCV, as discussed in section 3.4.2 (Phillips et al. 2003; Stuber et al. 2005a, b). However, phasic dopamine is also associated with initiating goal-directed behaviors, and thus may have a motivational impact as well (see Sect. 3.4.2; Phillips et al. 2003). Therefore, phasic signaling, time-locked to drug intake and drug-predicting stimuli, may contribute to both the motivational aspects of drug taking and associative learning related to drug taking. Together, these findings suggest that different time scales of dopamine transmission may have different functions, where different aspects of phasic signaling is related to both reinforcement learning and approach behavior (motivation), and tonic signaling enables motivational and motor systems but not reinforcement learning.
7.1 Dopamine signaling in the drug-naïve state (Fig. 2a)
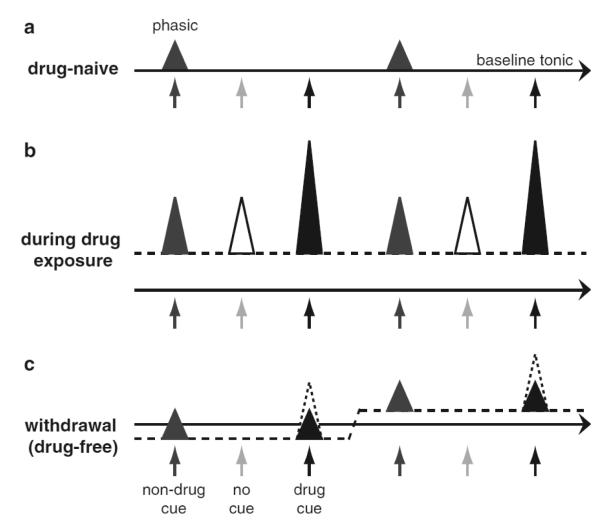
Figure 2.
Drug-induced changes in phasic and tonic dopamine transmission in the NAcc (a) In the drug-naïve state, phasic (triangles) and tonic (solid horizontal line) dopamine signaling in the NAcc is normal. Few spontaneous phasic dopamine events (no cue) are observed. Salient stimuli (non-drug cues) can elicit phasic dopamine release and goal-directed behavior. (b) Drugs of abuse enhance tonic (dashed line) and phasic (triangles) dopamine signaling. Stimuli not associated with drug (non-drug cues) and drug-related cues both elicit phasic dopamine events, but the latter cause more robust release due to the temporal proximity to the drug administration. Furthermore, the number of spontaneous phasic dopamine events is increased. This may lead to aberrant learning of drug-cue associations and thus abnormal goal-directed behavior such as compulsive drug taking. (c) Effects of drug withdrawal on dopamine signaling are variable. For example, dampened tonic dopamine concentrations during withdrawal can be returned to and above basal concentrations by exposure to drug cues and drug context (left to right). Such drug cues may also elicit more phasic dopamine release (dashed triangle) compared to non-drug cues because 1) drugs represent a higher reward magnitude than natural reinforcers and/or 2) extended withdrawal results in incubation of drug craving. As a consequence, independent of tonic dopamine levels, seeking for drugs is more prevalent than seeking for natural reinforcers, which may promote relapse to drug taking.
In Figure 2, we summarize data reviewed in this chapter in a simplified manner and discuss it in light of the theoretical framework of NAcc dopamine function reviewed in sections 1.5 and 6. In a drug-naïve state (Fig. 2a), it is assumed that NAcc neurons receive physiological levels of receptor stimulation by tonic dopamine release providing normal motivational function of the organism. Phasic dopamine release and subsequent postsynaptic dopamine receptor stimulation may alter synaptic plasticity on striatal projection neurons (in concert with glutamate signals) in response to behaviorally-relevant novel stimuli and natural reinforcers. Together this allows for normal goal-directed behavior and reinforcement learning. In this drug-naïve state (Fig. 2a), stimuli that are specifically associated with drug intake, such as drug paraphernalia or cues predicting drug availability, will not affect dopamine neuron firing or release because the organism has not yet been exposed to the drug.
7.2 Immediate effects of drug exposure (Fig. 2b)
Thus far, we have reviewed data demonstrating that acute exposure to drugs of abuse increases phasic (see Sect. 3.4) and tonic dopamine concentrations (see Sect. 3.2 and 3.3) in the NAcc. What are the potential behavioral consequences of this enhanced dopamine signaling? During drug exposure, cues predicting reinforcer availability may elicit greater amounts of dopamine due to the drug-induced increase in the amplitude of phasic dopamine signals, similar to cocaine-induced increases in electrically stimulated release (Wu et al., 2001; Fig. 2a,b). Notably, exposure to cocaine and amphetamine also affects the processing of cues that are not related to drug intake (e.g., Puglisi-Allegra et al. 1994; Castellano et al. 1996; Cestari and Castellano 1996; Packard and White 1991). Additionally, the number of spontaneous phasic dopamine release events is increased dramatically due to the pharmacological effect of the drug which possibly leads to an association of an increased number of environmental stimuli with the drug experience (see Sect. 3.4). Together, this may explain how contextual cues not directly predicting drug administration become powerfully associated with the drug experience. In comparison to natural reinforcers and contextual cues, drug-associated cues are even more robustly ‘consolidated’ due to the high contiguity and contingency of drug administration and these cues (i.e., the drug-induced facilitation of dopamine signaling is strongest immediately after intake and drug cues are never experienced separately from drug administration) (Fig. 2b). Consistent with this idea, learning associations between cues and natural reinforcers transiently affects the synaptic properties of dopamine neurons, while drug experience promotes long-lasting changes to the intrinsic and synaptic properties of dopamine neurons (Chen et al. 2008; Stuber et al. 2008). Such facilitation in Pavlovian learning may then promote the development of abnormal levels of dopamine release by drug-conditioned stimuli upon re-exposure (Redish 2004; Di Chiara and Bassareo 2007).
With repeated training on a Pavlovian learning task using a natural reinforcer, the phasic dopamine signal shifts from reward delivery to the cue that predicts it (Day et al. 2007; Schultz et al. 1997). In contrast, dopamine signals in response to drug delivery in a drug self-administration task may not attenuate or may attenuate slower than with natural reinforcement because drugs directly activate the dopamine system. Therefore, this teaching signal may be constantly exhibited to both drug cues and the drug delivery itself. Thus, the brain continues to perceive drug delivery as a novel reward or positive prediction error despite repeated use (Redish 2004). Additionally, drug reward may be experienced as a reward of exaggerated magnitude and thus the positive prediction error may be extraordinarily large (Tobler et al. 2005). Together, this may lead to aberrant reinforcement learning and eventual fixation on pursuit of drugs and compulsive intake.
If dopamine acts to promote the repetition of actions that immediately precede rewarding events (Redgrave and Gurney 2006), drug exposure would immensely facilitate operant behavior that leads to drug administration. Alternatively, such drug-enhanced phasic dopamine signaling could also lead to the sensitized attribution of incentive salience to drug-related cues (Robinson and Berridge 1993). Thus, enhanced phasic signaling may promote abnormal responding to drug cues, whether due to aberrant learning and memory or motivation. Enhanced tonic dopamine concentrations may produce more exploratory activity and thus greater exposure of the organism to the drug environment including drug-related cues possibly promoting continued drug intake. This role for tonic dopamine fits well with the proposition that dopamine may have a function in overcoming the motivational costs required for completing tasks (Salamone and Correa 2002; Phillips et al. 2007). Tonically elevated dopamine concentrations may therefore keep the motivational cost for pursuing drug rewards minimal.
Together, we posit that the drug-induced enhancement of phasic dopamine signaling will increase phasic release in response to previously weak or neutral stimuli and specifically strengthen associations of drug cues and drug delivery, whereas increased tonic dopamine levels may maintain the organisms motivation to continue drug intake by promoting seeking of cues/environments associated with the drug (Fig. 2b).
7.3 Long-term effects of drug exposure during drug withdrawal (Fig. 2c)
Withdrawal after chronic drug exposure has variable effects on tonic NAcc dopamine levels (see Sect. 4.1). Some studies report decreased dopamine concentrations while others have found no change or even increased basal levels. Lowered tonic dopamine levels during drug withdrawal may be associated with a reduced motivational state, leading to an enhanced susceptibility to drug seeking elicited by drug cues. Drug seeking could result in further exposure to drug cues, and thus eventually lead to elevated tonic levels of dopamine, as non-contingent presentation of drug-associated cues are known to cause elevations in tonic dopamine levels (see Sect. 5.1; Fig 2c). Increases in tonic dopamine could then, in turn, facilitate seeking behavior.
Although little is known about the effect of drug withdrawal on the frequency or size of phasic dopamine release, we assume based on the findings discussed above that phasic signals to drug cues will be greater compared to (non-drug) cues associated with natural reinforcers (Fig. 2c), due to the abnormally strong association between drug and drug-predicting cues that develops during drug exposure (see Sect. 7.2; Fig 2b). One explanation for enhanced phasic dopamine release to drug cues is that drugs of abuse produce an exaggerated reward magnitude, which is known to be reflected in dopamine signaling (Tobler et al. 2005). Another possibility is that the dopamine signal to drug cues escalates over time due to incubation of drug craving during abstinence (Grimm et al. 2001). In support of this proposition, it has been demonstrated that the activation of NAcc neurons by drug-associated cues is potentiated after one month of abstinence from cocaine self-administration (Hollander et al. 2007). Because phasic dopamine release is associated with the initiation of goal-directed behaviors (Roitman et al. 2004; Phillips et al. 2003; Stuber et al. 2005a, b; see Sect. 3.4.2 and 4.2), it follows that promotion of drug seeking in response to drug-related stimuli is more likely than seeking of natural rewards in response to associated non-drug cues. This assumption is consistent with the DSM-IV criterion that human drug addiction normally constitutes a progressive “narrowing” of the behavioral repertoire to that controlled by drug reinforcement rather than that guided by natural reinforcers, such as food or sex. Taken together, we propose that independent of tonic NAcc dopamine concentrations during withdrawal exposure to drug-associated stimuli activates phasic dopamine release more than non-drug related stimuli and thus leads to fixation on drug-related behavior and eventually relapse to drug taking.
8 Summary
In this chapter, we review the current state of knowledge on how abused drugs, drug-associated cues, drug seeking, and drug withdrawal affect phasic and tonic dopamine signaling in the NAcc in animal models of addiction. For the sake of simplicity, we have neglected to address the core and shell subdivisions of the NAcc (e.g., Zahm and Heimer 1990) and referred to the NAcc as a whole. The vast majority of the reviewed studies sampled dopamine concentrations in the NAcc core. However, greater increases in phasic and tonic dopamine concentrations in the NAcc shell compared to the core have been identified in response to psychostimulants, morphine and ethanol (e.g., Pontieri et al. 1995; Ito et al. 2000; Lecca et al. 2007; Aragona et al. 2008; Howard et al. 2008). Furthermore, it has been shown that dopamine in the shell increases following self-administration of cocaine, but not following presentation of CS associated with the drug, whereas such CS predicting cocaine caused dopamine release in the core (Ito et al. 2000). These data suggest that the NAcc shell may be mainly implicated in the primary reinforcing effects of psychostimulants, whereas the core is preferentially involved in conditioned drug responses.
The reviewed results are integrated with current ideas on the role of dopamine in addiction with an emphasis on a model illustrating phasic and tonic NAcc dopamine signaling during different stages of drug addiction. Each theory of dopamine function briefly outlined in this chapter has merits and it is not our intention to verify or falsify any of them, but rather to combine their different perspectives. The purpose of this chapter is not to provide a new psychology of addiction, but rather to give an updated perspective on potential neurochemical mechanisms underlying addiction. We have only focused on dopamine signaling here, although many other neurochemical systems have been identified as important contributors to addiction. Our model predicts that phasic dopamine release in response to drug-related stimuli will be enhanced over stimuli associated with natural reinforcers, which may result in aberrant goal-directed behaviors contributing to drug addiction.
References
- Acquas E, Carboni E, Di Chiara G. Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol. 1991;193:133–134. doi: 10.1016/0014-2999(91)90214-b. [DOI] [PubMed] [Google Scholar]
- Acquas E, Di Chiara G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J Neurochem. 1992;58:1620–1625. doi: 10.1111/j.1471-4159.1992.tb10033.x. [DOI] [PubMed] [Google Scholar]
- Adams RN. Probing brain chemistry with electroanalytical techniques. Anal Chem. 1976;48:1126A–1138A. doi: 10.1021/ac50008a001. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and statistical manual of mental disorders. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants SK, Margolin A, Kosten TR, Cooney NL. Differences between responders and nonresponders to cocaine cues in the laboratory. Addict Behav. 1995;20:215–224. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Baur JE, Kristensen EW, May LJ, Wiedemann DJ, Wightman RM. Fast-scan voltammetry of biogenic amines. Anal Chem. 1988;60:1268–1272. doi: 10.1021/ac00164a006. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci. 1989;103:36–45. doi: 10.1037//0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- Bito L, Davson H, Levin E, Murray M, Snider N. The concentrations of free amino acids and other electrolytes in cerebrospinal fluid, in vivo dialysate of brain, and blood plasma of the dog. J Neurochem. 1966;13:1057–1067. doi: 10.1111/j.1471-4159.1966.tb04265.x. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser MT, Kennedy RT. In vivo monitoring of amine neurotransmitters using microdialysis with on-line capillary electrophoresis. Electrophoresis. 2001;22:3668–3676. doi: 10.1002/1522-2683(200109)22:17<3668::AID-ELPS3668>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Dose-dependent effect of ethanol on extracellular dopamine in mesolimbic striatum of awake rhesus monkeys: comparison with cocaine across individuals. Psychopharmacology (Berl) 2002;165:67–76. doi: 10.1007/s00213-002-1233-9. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DR, Curzon P, Mackenzie RG, Kebabian JW, Williams JE, Kerkman D. Evidence for involvement of both D1 and D2 receptors in maintaining cocaine self-administration. Pharmacol Biochem Behav. 1991;39:911–915. doi: 10.1016/0091-3057(91)90052-4. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Cocaine-induced conditioned locomotion: absence of associated increases in dopamine release. Neuroscience. 1992;48:621–629. doi: 10.1016/0306-4522(92)90406-r. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 2007;193:495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001a;297:27–34. [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Wightman RM, Jones SR. Terminal effects of ethanol on dopamine dynamics in rat nucleus accumbens: an in vitro voltammetric study. Synapse. 2001b;42:77–79. doi: 10.1002/syn.1101. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Chiodo LA, Grace AA. Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse. 1991;9:79–94. doi: 10.1002/syn.890090202. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006a;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006b;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Caille I, Dumartin B, Bloch B. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 1996;730:17–31. doi: 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Dose-dependent transitions in nucleus accumbens cell firing and behavioral responding during cocaine self-administration sessions in rats. J Pharmacol Exp Ther. 1996;277:385–393. [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Nucleus accumbens cell firing during maintenance, extinction, and reinstatement of cocaine self-administration behavior in rats. Brain Res. 2000;866:44–54. doi: 10.1016/s0006-8993(00)02217-4. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Falck B, Hillarp NA. Cellular localization of brain monoamines. Acta Physiol Scand Suppl. 1962;56:1–28. [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- Castellano C, Zocchi A, Cabib S, Puglisi-Allegra S. Strain-dependent effects of cocaine on memory storage improvement induced by post-training physostigmine. Psychopharmacology (Berl) 1996;123:340–345. doi: 10.1007/BF02246644. [DOI] [PubMed] [Google Scholar]
- Cestari V, Castellano C. Caffeine and cocaine interaction on memory consolidation in mice. Arch Int Pharmacodyn Ther. 1996;331:94–104. [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Changes in basal and cocaine-evoked extracellular dopamine uptake and release in the rat nucleus accumbens during early abstinence from cocaine: quantitative determination under transient conditions. Neuroscience. 2002;112:907–919. doi: 10.1016/s0306-4522(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Nomikos GG, Mathe JM, Gonon F, Svensson TH. Burst stimulation of the medial forebrain bundle selectively increase Fos-like immunoreactivity in the limbic forebrain of the rat. Neuroscience. 1996;72:141–156. doi: 10.1016/0306-4522(95)00513-7. [DOI] [PubMed] [Google Scholar]
- Chergui K, Suaud-Chagny MF, Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–645. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Cocaine self-administration is increased by both D1 and D2 dopamine antagonists. Pharmacol Biochem Behav. 1991;39:799–802. doi: 10.1016/0091-3057(91)90168-2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Crippens D, Camp DM, Robinson TE. Basal extracellular dopamine in the nucleus accumbens during amphetamine withdrawal: a ‘no net flux’ microdialysis study. Neurosci Lett. 1993;164:145–148. doi: 10.1016/0304-3940(93)90878-o. [DOI] [PubMed] [Google Scholar]
- Crippens D, Robinson TE. Withdrawal from morphine or amphetamine: different effects on dopamine in the ventral-medial striatum studied with microdialysis. Brain Res. 1994;650:56–62. doi: 10.1016/0006-8993(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25:1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Gold MS. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev. 1985;9:469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Dayton MA, Ewing AG, Wightman RM. Evaluation of amphetamine-induced in vivo electrochemical response. Eur J Pharmacol. 1981;75:141–144. doi: 10.1016/0014-2999(81)90074-1. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- De Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Preferential stimulation of dopamine release in the nucleus accumbens by opiates, alcohol, and barbiturates: studies with transcerebral dialysis in freely moving rats. Ann N Y Acad Sci. 1986;473:367–381. doi: 10.1111/j.1749-6632.1986.tb23629.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur J Neurosci. 1998a;10:1121–1127. doi: 10.1046/j.1460-9568.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. The relation between dopamine oxidation currents in the nucleus accumbens and conditioned increases in motor activity in rats following repeated administration of d-amphetamine or cocaine. Eur J Neurosci. 1998b;10:1113–1120. doi: 10.1046/j.1460-9568.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine efflux associated with extinction, CS-induced and d-amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res. 2001;120:147–158. doi: 10.1016/s0166-4328(00)00373-9. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Inhibition of dopamine efflux in the rat nucleus accumbens during abstinence after free access to d-amphetamine. Behav Brain Res. 2002;128:1–12. doi: 10.1016/s0166-4328(01)00265-0. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Coury A, Depoortere RY, Egilmez Y, Lane JD, Emmett-Oglesby MW, Lepiane FG, Phillips AG, Blaha CD. Comparison of changes in extracellular dopamine concentrations in the nucleus accumbens during intravenous self-administration of cocaine or d-amphetamine. Behav Pharmacol. 1995;6:311–322. [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci. 2003;117:952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Contribution of the ventral tegmental area to cocaine-seeking maintained by a drug-paired conditioned stimulus in rats. Eur J Neurosci. 2004;19:1661–1667. doi: 10.1111/j.1460-9568.2004.03232.x. [DOI] [PubMed] [Google Scholar]
- Diana M, Muntoni AL, Pistis M, Melis M, Gessa GL. Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J Neurosci. 1999;11:1037–1041. doi: 10.1046/j.1460-9568.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Descarries L, Garcia S. Quantification of the dopamine innervation in adult rat neostriatum. Neuroscience. 1986;19:427–445. doi: 10.1016/0306-4522(86)90272-1. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Goeders NE, Smith JE. The reinforcing and rate effects of intracranial dopamine administration. NIDA Res Monogr. 1986;67:242–248. [PubMed] [Google Scholar]
- Espana RA, Roberts DC, Jones SR. Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience. 2008;155:250–257. doi: 10.1016/j.neuroscience.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl) 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, LePiane FG, Jakubovic A, Phillips AG. The role of dopamine in intracranial self-stimulation of the ventral tegmental area. J Neurosci. 1987;7:3888–3896. doi: 10.1523/JNEUROSCI.07-12-03888.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Post RM, Pert A. Conditioned increases in mesolimbic dopamine overflow by stimuli associated with cocaine. Brain Res. 1993;629:31–39. doi: 10.1016/0006-8993(93)90477-5. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Bunney BS. Activity of A9 and A10 dopaminergic neurons in unrestrained rats: further characterization and effects of apomorphine and cholecystokinin. Brain Res. 1987;405:46–55. doi: 10.1016/0006-8993(87)90988-7. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garris PA, Christensen JR, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem. 1997;68:152–161. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Rebec GV. Modeling fast dopamine neurotransmission in the nucleus accumbens during behavior. Behav Brain Res. 2002;137:47–63. doi: 10.1016/s0166-4328(02)00284-x. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984;290:390–395. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon F, Buda M, Cespuglio R, Jouvet M, Pujol JF. Voltammetry in the striatum of chronic freely moving rats: detection of catechols and ascorbic acid. Brain Res. 1981;223:69–80. doi: 10.1016/0006-8993(81)90807-6. [DOI] [PubMed] [Google Scholar]
- Gonon F, Sundstrom L. Excitatory effects of dopamine released by impulse flow in the rat nucleus accumbens in vivo. Neuroscience. 1996;75:13–18. doi: 10.1016/0306-4522(96)00320-x. [DOI] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Gonon FG, Navarre F, Buda MJ. In vivo monitoring of dopamine release in the rat brain with differential normal pulse voltammetry. Anal Chem. 1984;56:573–575. doi: 10.1021/ac00267a060. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mora JL, Guadalupe T, Fumero B, Mas M. Mathematical resolution of mixed in vivo voltammetry signals. Models, equipment, assessment by simultaneous microdialysis sampling. J Neurosci Methods. 1991;39:231–244. doi: 10.1016/0165-0270(91)90102-6. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984a;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984b;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton A. In vivo analysis of the role of dopamine in stimulant and opiate self-administration. J Psychiatry Neurosci. 1996;21:264–279. [PMC free article] [PubMed] [Google Scholar]
- Gratton A, Hoffer BJ, Gerhardt GA. In vivo electrochemical studies of monoamine release in the medial prefrontal cortex of the rat. Neuroscience. 1989;29:57–64. doi: 10.1016/0306-4522(89)90332-1. [DOI] [PubMed] [Google Scholar]
- Gratton A, Wise RA. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci. 1994;14:4130–4146. doi: 10.1523/JNEUROSCI.14-07-04130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Linder JC, Young SJ. 5-hydroxydopamine-labeled dopaminergic axons: three-dimensional reconstructions of axons, synapses and postsynaptic targets in rat neostriatum. Neuroscience. 1994;58:593–604. doi: 10.1016/0306-4522(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J Pharmacol Exp Ther. 1995;273:591–598. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [PubMed] [Google Scholar]
- Hernandez PJ, Schiltz CA, Kelley AE. Dynamic shifts in corticostriatal expression patterns of the immediate early genes Homer 1a and Zif268 during early and late phases of instrumental training. Learn Mem. 2006;13:599–608. doi: 10.1101/lm.335006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol. 1997;388:211–227. [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology (Berl) 1983;81:158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Duffy P, Striplin C, Kalivas PW. Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology (Berl) 1994;115:265–272. doi: 10.1007/BF02244782. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Moreton JE. Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat. Psychopharmacology (Berl) 1991;105:151–156. doi: 10.1007/BF02244301. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob G, Ungerstedt U. The influence of cocaine self-administration on in vivo dopamine and acetylcholine neurotransmission in rat caudate-putamen. Neurosci Lett. 1990;109:227–233. doi: 10.1016/0304-3940(90)90568-t. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res. 1989;498:199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Iglauer C, Llewellyn ME, Woods JH. Concurrent schedules of cocaine injection in rhesus monkeys: dose variations under independent and non-independent variable-interval procedures. Pharmacol Rev. 1975;27:367–383. [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Imperato A, Mele A, Scrocco MG, Puglisi-Allegra S. Chronic cocaine alters limbic extracellular dopamine. Neurochemical basis for addiction. Eur J Pharmacol. 1992;212:299–300. doi: 10.1016/0014-2999(92)90349-9. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Glick SD. Dopamine release and metabolism in nucleus accumbens and striatum of morphine-tolerant and nontolerant rats. Pharmacol Biochem Behav. 1993;46:341–347. doi: 10.1016/0091-3057(93)90362-w. [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ. Nucleus accumbens to globus pallidus GABA projection subserving ambulatory activity. Am J Physiol. 1980;238:R65–69. doi: 10.1152/ajpregu.1980.238.1.R65. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Zimmerman JB, Wightman RM. Principles of voltammetry and microelectrode surface states. J Neurosci Methods. 1993;48:225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Zigmond MJ, Abercrombie ED. In vivo regulation of extracellular dopamine in the neostriatum: influence of impulse activity and local excitatory amino acids. J Neural Transm Gen Sect. 1993;91:223–240. doi: 10.1007/BF01245233. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Changes in dopamine-dependent electrochemical signal in the nucleus accumbens associated with repeated cocaine injections in rats. Brain Res. 1994;642:228–236. doi: 10.1016/0006-8993(94)90926-1. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Gratton A. Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Res. 1994;652:225–234. doi: 10.1016/0006-8993(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102:565–580. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Behavior-associated changes in blood pressure during heroin self-administration. Pharmacol Biochem Behav. 1993;46:561–567. doi: 10.1016/0091-3057(93)90544-4. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Biphasic changes in mesolimbic dopamine signal during cocaine self-administration. Neuroreport. 1994;5:1005–1008. doi: 10.1097/00001756-199404000-00038. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience. 1995;64:599–617. doi: 10.1016/0306-4522(94)00436-9. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Conditioned changes in nucleus accumbens dopamine signal established by intravenous cocaine in rats. Neurosci Lett. 1996;211:73–76. doi: 10.1016/0304-3940(96)12731-2. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Todd PK. Behavioral sensitization and extracellular dopamine responses to amphetamine after various treatments. Psychopharmacology (Berl) 1997;134:221–229. doi: 10.1007/s002130050445. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ, et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci U S A. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Friedle NM, Moore KE. Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav. 1979;11:553–556. doi: 10.1016/0091-3057(79)90040-6. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Robledo P, Chover AJ, Caine SB, Koob GF. D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav. 1993;45:239–242. doi: 10.1016/0091-3057(93)90112-7. [DOI] [PubMed] [Google Scholar]
- Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Marsden CA, Joseph MH, Kruk ZL, Maidment NT, O’Neill RD, Schenk JO, Stamford JA. In vivo voltammetry--present electrodes and methods. Neuroscience. 1988;25:389–400. doi: 10.1016/0306-4522(88)90247-3. [DOI] [PubMed] [Google Scholar]
- Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60:288–294. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Gatto GJ, Levy AD, Lumeng L, Li TK. Serotonin and dopamine systems regulating alcohol intake. Alcohol Alcohol Suppl. 1991;1:411–416. [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res. 1993;624:245–252. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Rodd-Henricks ZA, Dagon CT, Murphy JM, McBride WJ. Cocaine is self-administered into the shell region of the nucleus accumbens in Wistar rats. Ann N Y Acad Sci. 1999;877:788–791. doi: 10.1111/j.1749-6632.1999.tb09323.x. [DOI] [PubMed] [Google Scholar]
- Meil WM, Roll JM, Grimm JW, Lynch AM, See RE. Tolerance-like attenuation to contingent and noncontingent cocaine-induced elevation of extracellular dopamine in the ventral striatum following 7 days of withdrawal from chronic treatment. Psychopharmacology (Berl) 1995;118:338–346. doi: 10.1007/BF02245964. [DOI] [PubMed] [Google Scholar]
- Michael D, Travis ER, Wightman RM. Color images for fast-scan CV measurements in biological systems. Anal Chem. 1998;70:586A–592A. doi: 10.1021/ac9819640. [DOI] [PubMed] [Google Scholar]
- Middleton LS, Cass WA, Dwoskin LP. Nicotinic receptor modulation of dopamine transporter function in rat striatum and medial prefrontal cortex. J Pharmacol Exp Ther. 2004;308:367–377. doi: 10.1124/jpet.103.055335. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, Kilpatrick MR, Wightman RM. Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci. 2004;24:1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castaneda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15:506–514. doi: 10.1016/S0893-133X(96)00097-8. [DOI] [PubMed] [Google Scholar]
- Nicolaysen LC, Pan HT, Justice JB., Jr. Extracellular cocaine and dopamine concentrations are linearly related in rat striatum. Brain Res. 1988;456:317–323. doi: 10.1016/0006-8993(88)90234-x. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci U S A. 2008;105:11957–11962. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav. Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr. Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J Neurochem. 1992;58:212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr. Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Regional differences in the effects of amphetamine withdrawal on dopamine dynamics in the striatum. Analysis of circadian patterns using automated on-line microdialysis. Neuropsychopharmacology. 1996;14:325–337. doi: 10.1016/0893-133X(95)00141-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC, Parker LA. Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacol Biochem Behav. 1997;58:801–811. doi: 10.1016/s0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr. Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr. Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991;539:94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Pan HT, Parsons LH, Justice JB., Jr. Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Heidbreder CA, Feldon J, Murphy CA. Selective responding of nucleus accumbens core and shell dopamine to aversively conditioned contextual and discrete stimuli. Neuroscience. 2001;108:91–102. doi: 10.1016/s0306-4522(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Robbins TW, Everitt BJ. Isolation rearing impairs the reinforcing efficacy of intravenous cocaine or intra-accumbens d-amphetamine: impaired response to intra-accumbens D1 and D2/D3 dopamine receptor antagonists. Psychopharmacology (Berl) 1994;115:419–429. doi: 10.1007/BF02245085. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology (Berl) 2007;191:483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991;566:348–350. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cestari V, Cabib S, Castellano C. Strain-dependent effects of post-training cocaine or nomifensine on memory storage involve both D1 and D2 dopamine receptors. Psychopharmacology (Berl) 1994;115:157–162. doi: 10.1007/BF02244766. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Engleman EA, Corrigall WA. Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience. 2004;129:415–424. doi: 10.1016/j.neuroscience.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neurosci. 1999;19:4102–4109. doi: 10.1523/JNEUROSCI.19-10-04102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Piercey MF, Svensson K, Collins RJ, Myers JE, Roberts DC. Antagonism of cocaine self-administration by the preferential dopamine autoreceptor antagonist, (+)-AJ 76. Brain Res. 1993;619:15–21. doi: 10.1016/0006-8993(93)91591-f. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Vickers G. Atypical neuroleptics increase self-administration of cocaine: an evaluation of a behavioural screen for antipsychotic activity. Psychopharmacology (Berl) 1984;82:135–139. doi: 10.1007/BF00426397. [DOI] [PubMed] [Google Scholar]
- Robertson MW, Leslie CA, Bennett JP., Jr. Apparent synaptic dopamine deficiency induced by withdrawal from chronic cocaine treatment. Brain Res. 1991;538:337–339. doi: 10.1016/0006-8993(91)90451-z. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Schultz W. Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol. 1990;63:592–606. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992a;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Gessa GL. Marked decrease of extraneuronal dopamine after alcohol withdrawal in rats: reversal by MK-801. Eur J Pharmacol. 1991;200:371–372. doi: 10.1016/0014-2999(91)90600-u. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Gessa GL. Dramatic depletion of mesolimbic extracellular dopamine after withdrawal from morphine, alcohol or cocaine: a common neurochemical substrate for drug dependence. Ann N Y Acad Sci. 1992b;654:513–516. doi: 10.1111/j.1749-6632.1992.tb26016.x. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The behavioral neurochemistry of motivation: methodological and conceptual issues in studies of the dynamic activity of nucleus accumbens dopamine. J Neurosci Methods. 1996;64:137–149. doi: 10.1016/0165-0270(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Erickson HL, Niehus JS, Gerhardt GA, Kalivas PW, Floyd EA. The effects of local application of ethanol in the n. accumbens on dopamine overflow and clearance. Alcohol. 1997;14:485–492. doi: 10.1016/s0741-8329(96)00216-9. [DOI] [PubMed] [Google Scholar]
- Saulskaya N, Marsden CA. Conditioned dopamine release: dependence upon N-methyl-D-aspartate receptors. Neuroscience. 1995;67:57–63. doi: 10.1016/0306-4522(95)00007-6. [DOI] [PubMed] [Google Scholar]
- Savtchenko LP, Rusakov DA. The optimal height of the synaptic cleft. Proc Natl Acad Sci U S A. 2007;104:1823–1828. doi: 10.1073/pnas.0606636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Repeated cocaine administration induces behavioral sensitization and corresponding decreased extracellular dopamine responses in caudate and accumbens. Brain Res. 1992;577:351–355. doi: 10.1016/0006-8993(92)90297-m. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW. The GABAB agonist baclofen modifies cocaine self-administration in rats. Behav Pharmacol. 1998;9:195–206. [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA. In vivo voltammetry: some methodological considerations. J Neurosci Methods. 1986;17:1–29. doi: 10.1016/0165-0270(86)90031-2. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Stewart J, Vezina P. A comparison of the effects of intra-accumbens injections of amphetamine and morphine on reinstatement of heroin intravenous self-administration behavior. Brain Res. 1988;457:287–294. doi: 10.1016/0006-8993(88)90698-1. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H. Memory formation and the regulation of gene expression. Cell. Mol. Life Sci. 1999;55:575–592. doi: 10.1007/s000180050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005a;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005b;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Sundstrom E, Fredriksson A, Archer T. Chronic neurochemical and behavioral changes in MPTP-lesioned C57BL/6 mice: a model for Parkinson’s disease. Brain Res. 1990;528:181–188. doi: 10.1016/0006-8993(90)91656-2. [DOI] [PubMed] [Google Scholar]
- Sunsay C, Rebec GV. Real-time dopamine efflux in the nucleus accumbens core during Pavlovian conditioning. Behav Neurosci. 2008;122:358–367. doi: 10.1037/0735-7044.122.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Teather LA, Packard MG, Smith DE, Ellis-Behnke RG, Bazan NG. Differential induction of c-Jun and Fos-like proteins in rat hippocampus and dorsal striatum after training in two water maze tasks. Neurobiol Learn Mem. 2005;84:75–84. doi: 10.1016/j.nlm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell Mol Life Sci. 1999;55:564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tossman U, Ungerstedt U. Microdialysis in the study of extracellular levels of amino acids in the rat brain. Acta Physiol Scand. 1986;128:9–14. doi: 10.1111/j.1748-1716.1986.tb07943.x. [DOI] [PubMed] [Google Scholar]
- UN . World Drug Report. United Nations Publications; New York, NY: 2008. [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997a;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997b;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Current Drug Abuse Reviews. doi: 10.2174/1874473710902020195. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Paulus MP, Lorang MT, Koob GF. Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: effects of acute and repeated administration. J Neurosci. 1992;12:4372–4380. doi: 10.1523/JNEUROSCI.12-11-04372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH, De Vries JB. Characterization of in vivo dopamine release as determined by brain microdialysis after acute and subchronic implantations: methodological aspects. J Neurochem. 1988;51:683–687. doi: 10.1111/j.1471-4159.1988.tb01798.x. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Humby T, Killcross AS, Torres EM, Everitt BJ, Robbins TW. Dissociations in dopamine release in medial prefrontal cortex and ventral striatum during the acquisition and extinction of classical aversive conditioning in the rat. Eur J Neurosci. 1998;10:1019–1026. doi: 10.1046/j.1460-9568.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Steiner H. Motor-skill learning-associated gene regulation in the striatum: effects of cocaine. Neuropsychopharmacology. 2006;31:2669–2682. doi: 10.1038/sj.npp.1300995. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Steiner H. Motor-skill learning in a novel running-wheel task is dependent on D1 dopamine receptors in the striatum. Neuroscience. 2008;153:249–258. doi: 10.1016/j.neuroscience.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, Hitomi M, Schuster CR. Psychomotor stimulant self administration as a function of dosage per injection in the rhesus monkey. Psychopharmacologia. 1971;22:271–281. doi: 10.1007/BF00401789. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995a;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995b;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS ONE. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther. 1984;230:678–683. [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci. 2001;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther. 1999;290:1369–1374. [PubMed] [Google Scholar]
- Yokel RA, Pickens R. Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther. 1973;187:27–33. [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Amphetamine-type reinforcement by dopaminergic agonists in the rat. Psychopharmacology (Berl) 1978;58:289–296. doi: 10.1007/BF00427393. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Young AM, Ahier RG, Upton RL, Joseph MH, Gray JA. Increased extracellular dopamine in the nucleus accumbens of the rat during associative learning of neutral stimuli. Neuroscience. 1998;83:1175–1183. doi: 10.1016/s0306-4522(97)00483-1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schlussman SD, Ho A, Kreek MJ. Effect of chronic “binge cocaine” on basal levels and cocaine-induced increases of dopamine in the caudate putamen and nucleus accumbens of C57BL/6J and 129/J mice. Synapse. 2003;50:191–199. doi: 10.1002/syn.10251. [DOI] [PubMed] [Google Scholar]