Abstract
Background
Event-related oscillations (EROs) are increasingly being used to assess neuro-cognitive functioning in normal and clinical populations. The current study compares different frequency activities in offspring of alcoholics (OA) and in normal controls (NC) in order to examine whether the OA group exhibits any abnormality in oscillatory activity while performing a Go/NoGo task.
Methods
The S-Transform algorithm was employed to decompose the EEG signals into different time-frequency bands, and the oscillatory responses in the P300 time window (300–700 ms) was statistically analyzed in both groups.
Results
The OA group manifested significantly decreased activity in delta (1–3 Hz), theta (4–7 Hz), and alpha1 (8–9 Hz) bands during the NoGo condition as well as reduced delta and theta activity during the Go condition. This reduction was more prominent in the NoGo than in the Go condition.
Conclusions
The decreased response in delta, theta, and alpha1 oscillations, especially during the NoGo condition in high risk individuals is perhaps suggestive of cognitive and neural disinhibition, and may serve as an endophenotypic marker in the development of alcoholism and/or other disinhibitory disorders.
Keywords: Event-related oscillations, Go/NoGo, Offspring of Alcoholics, Delta, Theta, Endophenotype
Introduction
The electroencephalographic (EEG) features that underlie sensory, motor or cognitive events are called event-related oscillations (EROs). These brain oscillations are the basic measures of neuroelectric processes and play a critical role in understanding neuro-cognitive functioning (Karakas et al 2000a, 2000b). During the cognitive processing, multiple, superimposed and/or parallel oscillations are evoked and transferred to distributed structures in the brain with various degrees of intensity, synchronization, duration and delay (Basar et al 2001a). Some researchers suggest that the event-related potentials (ERPs) are generated from the oscillatory changes in the dynamics of ongoing EEG rhythms of different frequency bands, a process known as ‘phase resetting’ (e.g. Basar 1980; Karakas et al 2000a, 2000b; Makeig et al 2002; Jansen et al 2003; Klimesch et al 2004). This view has recently been challenged with evidence that ERPs are produced by processes separate from and additive to ongoing brain activity (Yeung et al 2004; Fell et al 2004; Makinen et al 2005).
Oscillatory responses of different frequency bands have been associated with various cognitive functions. For example, delta responses are assumed to mediate signal detection and decision making (e.g. Basar 1999, Basar et al 2001a; Schurmann et al 2001), while theta rhythms are associated with different cognitive processes such as conscious awareness, episodic retrieval, recognition memory, and frontal inhibitory control (e.g. Klimesch et al 1994, 2001a, 2001b; Doppelmayr et al 1998; Karakas et al 2000a, 2000b; Basar et al 2001c; Kamarajan et al 2004). The slow alpha rhythm (8–10 Hz) has been reported to be modulated as a function of attentional demands (e.g. Basar et al 1997; Klimesch 1997b; Klimesch et al 1998), and fast alpha activity (10–12 Hz) has been found to mediate semantic memory processes as well as stimulus-related aspects (e.g. Klimesch 1996; Klimesch et al 1994, 1997a, 1997b). Further, oscillatory gamma responses have been shown to be involved in visual perception, cognitive integrative function such as ‘binding’, and frontal input during sensory processing (top-down processing) (e.g. Basar-Eroglu et al 1996a, 1996b; Schurmann et al 1997; Basar et al 2001a, 2001b; Karakas et al 2001). It is suggested that higher frequency oscillations are involved in more localized neural networks, whereas the long range communication networks (e.g., posterior to anterior sites) are activated through slow oscillations (von Stein and Sarnthein 2000; Kopell et al 2000; Steriade 2001; Csicsvari et al 2003; Buzsaki and Draguhn 2004). ERO analysis has also proven to be an important tool in measuring the neuro-cognitive dysfunctions in clinical conditions such as schizophrenia (Kissler et al 2000; Green et al 2003; Gonzalez-Hernandez et al 2003; Gallinat et al 2004) and ADHD (Yordanova et al 2001).
Recently, we showed that alcoholics have deficient delta and theta oscillatory responses during the NoGo condition in a Go/NoGo task (Kamarajan et al 2004). Further, decreased NoGo-P3 amplitude has also been reported in alcoholics (Cohen et al 1997a; Kamarajan et al, 2005b) as well as in children of alcoholics (Cohen et al 1997b; Kamarajan et al, 2005a). There is a good deal of evidence that P3 responses are primarily the outcome of oscillatory changes in delta and theta rhythms during stimulus processing (Stampfer and Basar 1985; Basar-Eroglu et al 1991, 2001; Roschke and Fell 1997; Devrim et al 1999; Demiralp et al 2001). In addition, children of alcoholics at high risk have been found to have lower P3 amplitude than children of non-alcoholics under various task conditions (for reviews, Porjesz and Begleiter 1990, 1991, 2003; Porjesz et al, 2005). Therefore, the present study has attempted to examine the oscillatory responses in the offspring of alcoholics in the time window of 300–700 milliseconds when P3 is maximum.
This study also attempts to identify the electrophysiological marker(s) of frontal inhibitory control in high risk subjects, which can serve as biological endophenotypes for alcoholism and related disorders. Since alcoholism has been associated with a spectrum of externalizing/disinhibitory disorders (Gorenstein and Newman 1980; Krueger et al 2002; Iacono et al 2002, 2003; Kendler et al 2003; Hicks et al 2004), and similar electrophysiological features have been reported in individuals with diverse disinhibitory clinical conditions (e.g., Bauer and Hesselbrock 1999a, 1999b; van der Stelt et al 2001; Iacono et al 2002, 2003), identifying electrophysiological markers in the OA subjects may provide useful measures for the study of neurogenetic etiology of alcoholism and/or other disinhibitory disorders. As oscillatory responses during the Go/NoGo paradigm has proven to be quite useful in studying cognitive and neural disinhibition in alcoholics, we were interested in determining whether the children of alcoholics would also exhibit deficient oscillatory responses, particularly during the NoGo condition.
Methods and Materials
Subjects
The sample consisted of 50 offspring of alcoholics with an age-range of 18–25 years (Mean = 20.72; S.D. = 2.06), and 50 normal controls (NC) matched for age (Mean = 20.34; S.D. = 1.93; Range = 18–24 years) and gender (29 males and 21 females in either group) were selected. Demographic and clinical characteristics of the sample are shown in Table 1. The ethnicity composition (in count) based on self-report of the (NC + OA) groups is as follows: African American, not of Hispanic origin (25 + 24); African American, of Hispanic origin (3 + 4); Caucasian, not of Hispanic origin (9 + 7); Caucasian, of Hispanic origin (4 + 8); Asian (2 + 0); Pacific Islander (4 + 0); Other (2 + 5); and more than one race (1 + 2). The group difference, however, is not significant (χ2 = 0.227). All subjects were right-handed and were recruited through newspaper advertisements and notices. The OA subjects had at least one of their biological parents diagnosed with alcohol dependence. A semi-structured interview was conducted (by trained clinicians) to diagnose the participants for alcoholism and other major psychiatric disorders using DSM-IV criteria, namely the Bard/Porjesz adult alcoholism battery, a semi-structured clinical assessment schedule based on DSM IV criteria for the evaluation of clinical details of alcohol dependence and alcohol-related medical problems, while a detailed check-list was used for documenting personal and family history of alcoholism, drug use, and other psychiatric disorders. The individuals with major neurological and psychiatric conditions (inclusive of alcohol/drug dependence) and/or with concurrent psychotropic medications were excluded from the study. However, social drinkers (17 in the OA group, 13 in the NC group) were included in the study. Further, individuals with concurrent or past history of externalizing disorders [such as conduct disorder (CD), anti-social personality disorder (ASPD), oppositional defiant disorder (ODD), and attention-deficit hyperactivity disorder (ADHD)] were also included in the OA group, but not in the NC group. The rationale for including the externalizing disorders in the OA group is that these disorders are theorized to be part of a disinhibitory spectrum in which alcoholism is one of the etiological outcomes, and therefore, was considered that these disorders are part of a unified group at “high risk” for developing alcoholism and/or other disinhibitory disorders (Krueger et al 2002; Kendler et al 2003; Iacono et al 2002, 2003; Hicks et al 2004). All the subjects were screened for organicity (i.e., possible brain damage), using the Mini Mental State Examination (MMSE; Folstein et al 1975). The subjects were also excluded for their recent (i.e., past 48 hours) drug/alcohol use, based on Breath-analyzer and urine screen. No subjects had hearing or visual impairments. Informed consent was obtained from each individual, and the experimental procedures and ethical guidelines were in accordance with the Institutional Review Board (IRB).
Table 1.
Demographic and clinical characteristics of the sample. The mean, standard deviation (SD), t-value, and p-value are shown.
| VARIABLE | NC (N =50) | OA (N =50) | t-value | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Range | Mean | S.D. | Range | |||
| Age (in years) | 20.34 | 1.93 | 18–24 | 20.72 | 2.06 | 18–25 | −0.951 | 0.344 |
| Education (in years) | 13.36 | 2.04 | 9–17 | 12.42 | 1.33 | 0–16 | 2.734 | 0.007** |
| No. of drinking days per month | 1.79 | 2.83 | 0–12 | 4.14 | 6.05 | 0–20 | −1.791 | 0.079 |
| No. of drinks† per drinking day | 1.36 | 1.75 | 0–6 | 1.65 | 1.69 | 0–6 | −0.605 | 0.548 |
| No. of cigarettes per smoking day | 0.80 | 1.89 | 0–10 | 3.29 | 5.37 | 0–20 | −3.059 | 0.003** |
p < 0.01
One drink = 1 shot glass of hard liquor; 1 glass of wine; 1 bottle of beer.
Experimental Paradigm
The experimental paradigm is identical to our previous studies and has been described elsewhere (Kamarajan et al 2004, 2005a, 2005b). There were three visual stimuli in the task: (i) a cross (fixation stimulus), (ii) a circle (Go or NoGo stimulus), and (iii) a dollar sign (reinforcement sign). These stimuli subtended a visual angle of approximately 1º, and were presented on a computer monitor. The Go and NoGo stimuli were always preceded by a fixation stimulus that appeared at the center of the monitor. The position of the circle differentiated between the NoGo (no response) and the Go (quick response) condition. The circles that appeared at the top right and bottom left corners served as Go stimuli, to which the subjects responded quickly by pressing a button. The NoGo stimuli appeared at the top left and bottom right corners, to which the subjects were asked to withhold their response. The dollar-sign appeared whenever there was a correct button-press response to indicate a reward. The probabilities of occurrence of Go and NoGo stimuli were equal (50/50), and the order of these stimuli was randomized.
The experiment consisted of a practice phase and a recording phase. The practice phase consisted of twenty Go and NoGo trials, respectively. An auditory feedback signal (‘beep’) alerted the subject if they responded incorrectly; the practice phase did not accrue any reward. The EEG activity was recorded only during the recording phase which consisted of 100 trials (50 Go and 50 NoGo stimuli). The appearance of a dollar sign in this phase indicated a reward of 25 cents for each correct button-press response, while there was no feedback signal provided for the incorrect responses. The total amount gained as reward was not displayed during the stimulus presentation1.
EEG Data Acquisition
The subjects were comfortably seated in front of the computer monitor placed 1m away. EEG activity was recorded on a Neuroscan system (Version 4.1) [Compumedics USA, Ltd, El Paso, TX] using a 61-channel electrode cap which included 19 electrodes of the 10–20 International System and 42 additional electrode sites. The electrodes were referenced to the tip of the nose and the ground electrode was at the forehead (frontal midline). Eye movements were recorded using a supraorbital vertical lead and a horizontal lead on the external canthus of the left eye. Electrode impedance was maintained below 5 kΩ. The EEG signals were recorded continuously with a bandpass at 0.02–100 Hz and amplified 10,000 times using a set of amplifiers (Sensorium, Charlotte, VT). The data consisted of sampling rates of either 256 or 512 Hz, and were resampled at 256 Hz during the signal analysis for the sake of uniformity.
ERO energy estimation
A recently developed method for time-frequency representation called the S-transform (ST) was used to obtain reliable estimates of localized power of nonstationary evoked potential time series (Stockwell et al 1996; Chu 1996; Theophanis and Queen 2000). This method has been recently employed in a genetic linkage study of delta and theta band (Jones et al 2004) as well as in the analysis of early evoked gamma activity in alcoholics (Padmanabhapillai et al, in press) during the visual oddball paradigm. The S-transform is a generalization of the Gabor transform (Gabor 1946) and an extension to the continuous wavelet transform. The S-transform generates a time-frequency representation (TFR) of a signal by integrating the signal at each time point with a series of windowed harmonics of various frequencies as follows:
where h(t) is the signal, f is frequency, τ is a translation parameter, the first exponential is the window function, and the second exponential is the harmonic function. The S-transform TFR is computed by shifting the window function down the signal in time by τ across a range of frequencies. The window function is Gaussian with 1/ f 2 variance and scales in width according to the examined frequency. This inverse dependence of the width of the Gaussian window with frequency provides the frequency-dependent resolution. The amplitude envelope of the complex-valued S-transform TFR is calculated by taking the absolute value | ST( f, τ ) |.
The total energy response was acquired by calculation of the average of individual trials containing time-frequency-energy distributions. Baseline-adjusted poststimulus activity (1000 ms) were obtained by subtracting the prestimulus (125 ms) energy values from the original values. The energy curves were also plotted for eight predetermined frequency bands: Delta (1–3 Hz), Theta (4–7 Hz), Alpha 1 (8–9), Alpha 2 (10–12), Beta 1 (13–16 Hz), Beta 2 (17–20 Hz), Beta 3 (21–28 Hz), and Gamma (29–45 Hz). The trials containing >100 μV were removed for artifacts. The minimum number of artifact-free trials was kept at 20 for the analysis. The trials with incorrect responses (i.e., NoGo trails with button-press response and Go trials without button-press response) were removed from the analyses. The data of individuals who had either “contaminated” waveforms or “no-response like” damped waveforms were also excluded from the study. For the purpose of statistical analysis, mean values were calculated from the TFR amplitude envelope within time-frequency regions of interest (TFROI’s) (Lachaux 2003) corresponding to 300–700 ms time window range separately for each of the frequency bands.
Statistical analyses
The electrodes were grouped into six scalp regions for the statistical analyses as described in our previous study (Kamarajan et al 2005a). The behavioral data were analyzed using t-test. Initially, the Repeated Measures Analysis of Variance (RMANOVA; full-factorial model) was performed on the mean energy values for each of the frequency bands separately by having task condition, regions, and electrodes as within-subject variables and group as a between-subject variable. Greenhouse-Geisser correction was done for the within-subjects factors and interactions wherever applicable. As a second stage of analysis, the mean energy values were compared between groups using the Multivariate Analysis of Variance (MANOVA) for each of the frequency bands and for each region separately. The between-subjects factor in this model was group while 6 electrodes (from respective scalp regions) were entered as within-subjects factors. Only 6 representative electrodes from each of the regions were taken into the analysis as described in our previous study (Kamarajan et al. 2005a). Having equal number of electrodes in each region makes the comparisons among regions more viable and meaningful, and this fits well with the RMANOVA design. Moreover, the grouping of electrodes into six scalp regions was based on the lobe-wise divisions of the cerebral cortex. This regional grouping may help in interpreting the findings in terms of the functional significance of different lobes of the cortex. Furthermore, many previous studies have used this lobe-wise grouping (e.g., Cohen et al., 1997a; Kamarajan et al., 2004; de Bruin et al., 2004).
Results
Behavioral and Clinical Data
The behavioral performance scores between NC and OA are shown in Table 2. It was observed that the subjects in the OA group committed more errors and had longer reaction time than that of the NC group, although none of these measures was statistically significant. The clinical variables that formed the subgroups such as social drinking and externalizing disorders were neither analyzed separately nor included in the analysis of electrophysiological data.
Table 2.
The performance scores between NC and OA group. The mean, standard deviation (SD), t-value, and p-value are shown.
| VARIABLE | NC | OA | t-value | p-value | ||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |||
| MMSE score | 28.74 | 1.94 | 28.10 | 2.04 | 1.608 | 0.111 |
| Reaction time | 301.69 | 27.89 | 311.68 | 31.35 | −1.683 | 0.096 |
| Error (Go) | 4.56 | 2.49 | 6.04 | 5.07 | −1.852 | 0.067 |
| Error (NoGo) | 1.70 | 1.59 | 1.94 | 1.77 | −0.713 | 0.477 |
| Error (Total) | 6.26 | 2.97 | 7.98 | 5.45 | −1.958 | 0.053 |
Electrophysiological data
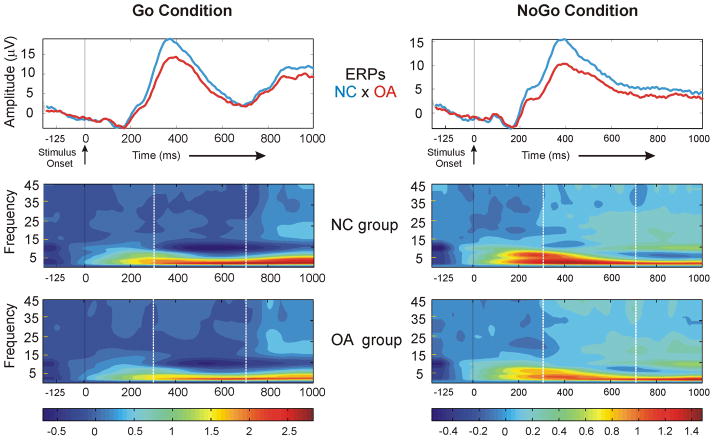
The time-frequency representation of Go and NoGo conditions has been illustrated in Figure 1. It is obvious that the OA group has a relatively decreased P3 amplitude of the ERP as well as a decrease in the low frequency activity. The ERD/ERS energy curves of Go and NoGo condition for each group are shown in Figure 2. These energy curves, as mentioned earlier, are the amplitude envelope of spectral activity. As shown, OA subjects have lower amplitude in delta and theta band during NoGo as well as Go conditions. The mean amplitude values (between 300–700 ms) obtained from the energy curves at different electrode sites were statistically analyzed. The group main effects as well as the interaction effects of group × condition and group × condition × region as found in the RMANOVA are shown in Table 3. It was found that the main effect of group were significant only in the delta and theta bands. The group × condition was significant in the theta and alpha1 bands, whereas the group × condition × region was significant in the delta, theta, and beta3 bands. Further analysis showed that there were no gender differences (main effect) in EROs in the regions of interest. Other effects that showed statistical significance are as follows.
Figure 1.
The ERPs and time-frequency representation of Go and NoGo condition for the NC and OA groups at CZ electrode. Note the lower P3 amplitude and decreased energy in low frequency activity in both Go and NoGo conditions in the OA compared to the NC group. The time window of 300–700 ms is marked with dotted white line in the time-frequency plots. The color scale represents the decomposed energy for both groups.
Figure 2.
The energy curves showing the comparison of NC and OA groups in Go and NoGo condition. These curves were obtained from the amplitude envelope of a particular spectral activity derived from S-Transform.
Table 3.
The results of the RMANOVA showing the Group main effect, and the interaction effects for Group × Condition and Group × Condition × Region. The F-values (df = 1, 98), p-values and the level of significance are shown.
| Band | Group | Group × Condition | Group × Condition × Region | |||
|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | F-value | p-value | |
| Delta | 12.226 | 0.0007*** | 0.859 | 0.3563 | 5.086 | 0.0004*** |
| Theta | 9.162 | 0.0032** | 7.563 | 0.0071** | 2.823 | 0.0202* |
| Alpha1 | 3.106 | 0.0811 | 6.395 | 0.0130* | 1.612 | 0.1644 |
| Alpha2 | 0.530 | 0.4681 | 2.508 | 0.1165 | 0.903 | 0.4824 |
| Beta1 | 1.865 | 0.1751 | 0.483 | 0.4886 | 1.223 | 0.3044 |
| Beta2 | 2.785 | 0.0983 | 1.585 | 0.2111 | 1.590 | 0.1704 |
| Beta3 | 2.887 | 0.0925 | 0.396 | 0.5306 | 3.182 | 0.0107* |
| Gamma | 0.205 | 0.6516 | 0.202 | 0.6542 | 1.046 | 0.3952 |
p < 0.05
p < 0.01
p < 0.001
Delta band: condition (p = 0.003); region (p = 0.000); electrode (p = 0.004); condition × region (p = 0.000); condition × electrode (p = 0.000); condition × electrode × group (p = 0.000); region × electrode (p = 0.000); and condition × region × electrode (p = 0.000).
Theta band: condition (p = 0.001); region (p = 0.000); electrode (p = 0.000); condition × region (p = 0.000); condition × electrode (p = 0.000); condition × electrode × group (p = 0.006); region × electrode (p = 0.000); and condition × region × electrode (p = 0.000).
Alpha1 band: condition (p = 0.000); region (p = 0.000); electrode (p = 0.011); condition × region (p = 0.000); condition × electrode (p = 0.021); region × electrode (p = 0.000); and condition × region × electrode (p = 0.000).
The MANOVA showed that the significant group effects were observed only in delta, theta and alpha1 bands. The significant differences between NC and OA groups during the NoGo and Go conditions in each of the scalp regions are shown in Tables 4 and 5 respectively. It can be observed that the group differences were more obvious in the NoGo condition than in the Go condition (Figure 3). During the Go condition, it was found that the OA group showed significantly less delta energy at parietal, occipital, and right-temporal regions, and less theta activity only at the parietal region (Table 4). On the other hand, during the NoGo condition, the OA group showed significantly less delta energy at frontal, central, parietal, occipital, and left-and right-temporal regions, less theta activity at frontal, central, parietal, occipital, and left-temporal regions, and less alpha1 activity at frontal, central, parietal, occipital regions (Table 5). The topography of delta, theta, and alpha1 activities between 300–700 ms during the NoGo condition is illustrated in Figure 4. The activity level (based on the color scale) represents the mean amplitude (during the P3 time window) across all electrode sites. It is observed that the topography of delta and theta activity is similar in NC and OC groups. However, the OA group showed lower activity in delta and theta bands as well as weaker anterior-posterior communication in alpha1 band as compared to controls.
Table 4.
The comparison of Delta, Theta, and Alpha1 activity between NC and OA groups during the Go condition (using MANOVA). The mean (M), standard deviation (SD), F-values (df = 1, 98), p-values and the level of significance are shown.
| Frequency | Measure | Frontal | Central | Parietal | Occipital | Left-Temporal | Right-Temporal |
|---|---|---|---|---|---|---|---|
| Delta | M ± SE (NC) | 0.677 ± 0.073 | 0.612 ± 0.047 | 0.609 ± 0.041 | 0.529 ± 0.041 | 0.502 ± 0.047 | 0.513 ± 0.049 |
| M ± SE (OA) | 0.436 ± 0.073 | 0.414 ± 0.047 | 0.402 ± 0.041 | 0.335 ± 0.041 | 0.316 ± 0.047 | 0.313 ± 0.049 | |
| F-value | 1.422 | 2.014 | 3.319 | 2.763 | 2.185 | 2.434 | |
| p-value | 0.2143 | 0.0714 | 0.0052** | 0.0162* | 0.0512 | 0.0313* | |
| Theta | M ± SE (NC) | 0.442 ± 0.053 | 0.387 ± 0.042 | 0.333 ± 0.040 | 0.310 ± 0.040 | 0.231 ± 0.043 | 0.289 ± 0.045 |
| M ± SE (OA) | 0.344 ± 0.053 | 0.251 ± 0.042 | 0.228 ± 0.040 | 0.224 ± 0.040 | 0.159 ± 0.043 | 0.188 ± 0.045 | |
| F-value | 1.012 | 0.879 | 2.641 | 1.426 | 1.570 | 0.929 | |
| p-value | 0.4224 | 0.5137 | 0.0207* | 0.2128 | 0.1645 | 0.4783 | |
| Alpha1 | M ± SE (NC) | 0.046 ± 0.036 | −0.020 ± 0.042 | −0.062 ± 0.045 | 0.019 ± 0.046 | −0.032 ± 0.041 | −0.076 ± 0.037 |
| M ± SE (OA) | 0.037 ± 0.036 | −0.054 ± 0.042 | −0.083 ± 0.045 | 0.030 ± 0.046 | −0.016 ± 0.041 | −0.023 ± 0.037 | |
| F-value | 0.604 | 0.254 | 0.730 | 0.923 | 0.837 | 0.826 | |
| p-value | 0.7261 | 0.9565 | 0.6265 | 0.4824 | 0.5446 | 0.5528 |
p < 0.05
p < 0.01
p < 0.001
Table 5.
The comparison of Delta, Theta, and Alpha1 activity between NC and OA groups during the NoGo condition (using MANOVA). The mean (M), standard deviation (SD), F-values (df = 1, 98), p-values and the level of significance are shown.
| Frequency | Measure | Frontal | Central | Parietal | Occipital | Left-Temporal | Right-Temporal |
|---|---|---|---|---|---|---|---|
| Delta | M ± SE (NC) | 0.727 ± 0.078 | 0.587 ± 0.049 | 0.511 ± 0.043 | 0.406 ± 0.043 | 0.424 ± 0.05 | 0.415 ± 0.054 |
| M ± SE (OA) | 0.359 ± 0.078 | 0.292 ± 0.049 | 0.276 ± 0.043 | 0.226 ± 0.043 | 0.202 ± 0.050 | 0.205 ± 0.054 | |
| F-value | 4.658 | 3.981 | 4.741 | 4.237 | 2.249 | 2.901 | |
| p-value | 0.0003*** | 0.0013** | 0.0003*** | 0.0008*** | 0.0451* | 0.0122* | |
| Theta | M ± SE (NC) | 0.985 ± 0.109 | 0.684 ± 0.067 | 0.524 ± 0.059 | 0.415 ± 0.058 | 0.455 ± 0.068 | 0.423 ± 0.072 |
| M ± SE (OA) | 0.481 ± 0.109 | 0.335 ± 0.067 | 0.245 ± 0.059 | 0.169 ± 0.058 | 0.171 ± 0.068 | 0.148 ± 0.072 | |
| F-value | 2.771 | 2.804 | 2.915 | 3.068 | 2.550 | 1.805 | |
| p-value | 0.0159* | 0.0149* | 0.0119* | 0.0087** | 0.0248* | 0.1064 | |
| Alpha1 | M ± SE (NC) | 0.351 ± 0.049 | 0.246 ± 0.040 | 0.221 ± 0.046 | 0.197 ± 0.046 | 0.223 ± 0.044 | 0.145 ± 0.047 |
| M ± SE (OA) | 0.163 ± 0.049 | 0.084 ± 0.040 | 0.051 ± 0.046 | 0.061 ± 0.046 | 0.083 ± 0.044 | 0.037 ± 0.047 | |
| F-value | 2.233 | 2.276 | 2.384 | 4.280 | 1.819 | 0.954 | |
| p-value | 0.0466* | 0.0429* | 0.0345* | 0.007** | 0.1037 | 0.4609 |
p < 0.05
p < 0.01
p < 0.001
Figure 3.
The bar graphs showing mean amplitude values (300–700 ms) between NC (green line) and OA (red line) groups in Go and NoGo condition. As seen, the group difference in NoGo activity is more robust in delta (A), theta (B), and alpha1 (C) activity at frontal (F), central (C), and parietal (P) regions. (The error bars represent standard error).
Figure 4.

The topographic mapping of the mean NoGo activity (in μV) at delta, theta, and alpha1 bands in NC and OA groups. The activity represents the average of all the amplitude values between 300–700 ms post-stimulus. The OA group shows lower activity in delta and theta bands, while showing a weaker anterior-posterior communication in alpha1 band.
Discussion
The present study attempts to examine the neuro-cognitive dysfunctions in subjects who are at high-risk to develop alcoholism (i.e., OA group) as elicited by the oscillatory responses in a Go/No-Go paradigm. The statistical analysis of time-frequency profile and the energy curves of different frequency bands yielded three major findings: (1) the OA subjects displayed a significantly lower responses in delta, theta and alpha1 oscillations during the NoGo condition as well as in delta and theta oscillations during the Go condition, suggesting dysfunctions in response inhibition as well as in response activation, (2) the reduction in oscillatory activity of the OA group was more robust in the No-Go than in the Go condition, suggesting a more prominent dysfunction in the frontal inhibitory control, and (3) The anterior-posterior communication (at 300–700 ms) as reflected in the topography of the slow alpha activity during the NoGo condition was much weaker in the OA group as compared to controls, suggesting a possible impairment in the long-range neural circuits.
Results also showed that there is a near significant difference between the groups in reaction time and error scores, suggesting that these probable behavioral deficits could have been the external manifestation of the neuro-electric dysfunctions observed in the study. However, the finding showing significant neuro-electric deficiencies but not so significant behavioral deficits in the OA group indicates that neuro-electric measures such as ERPs and EROs are more subtle and sensitive to tap the deficiencies in the risk group. As described by Begleiter and Porjesz (1999), a heritable biological endophenotype can help identify those individuals at genetic risk in the absence of overt manifest symptoms. In the present study, the dysfunction in response inhibition (during the NoGo condition) in the OA group is explained in terms of cognitive and neural disinhibition, and we suggest that disinhibition, as a trait, is perhaps genetically mediated in causing alcoholism and related disinhibitory disorders. In our results, more robust group differences are found in the NoGo than Go condition, showing dysfunction in response inhibition (NoGo) related oscillatory activity in OA subjects. Many fMRI and lesion studies implicate the prefrontal areas as the neural basis for response inhibition (e.g., Konishi et al 1999, 2003; Liddle et al 2001; Watanabe et al 2002). We suggest that this frontal dysfunction may be due to the dysregulation in inhibitory rhythms (theta oscillations) and/or potentials (No-Go P3, P3a, N2, etc), and this dysfunction is common across a variety of disinhibitory spectrum disorders. This is why we refer this phenomenon as “cognitive and neural disinhibition”.
In our previous study in alcoholics (Kamarajan et al 2004), we demonstrated that alcoholics had lower delta and theta activity in the NoGo condition, and reduced delta activity in the Go condition. In the present study, the OA subjects who are at high risk to develop alcoholism manifest these deficits in EROs and additionally manifest decreased theta activity in the Go condition, and markedly reduced activity in the theta and alpha1 bands in the NoGo condition. It is possible that since the current study has employed a different methodology both in terms of matching of groups for age, gender, education and handedness, as well as in using the S-Transform as a signal analysis method (rather than the Matching Pursuits method that was used in the study in alcoholics); these perhaps accounts for the additional differences in oscillatory activity. However, as described in alcoholics, the OA group displays stronger NoGo differences in the present study.
It has been reported that the P3 component of the ERPs primarily consists of delta and theta oscillations with a higher portion of posterior delta activity and the fronto-central theta oscillations (Basar-Eroglu et al 1992; Yordanova and Kolev 1996; Basar et al 1999; Karakas et al 2000a, 2000b) as well as a small portion of slow alpha oscillations (e.g., Basar-Eroglu et al 1991). Therefore, it is possible that the decrements primarily observed in the theta and delta activity in ‘at high risk’ population, as our current study has found, can in part be explained by the reduced P3 amplitudes reported in alcoholics and children of alcoholics (for recent reviews, Porjesz and Begleiter 2003; Porjesz et al, 2005). As these low frequency activities involve long range cortical communication, the deficient activity (in either P3 or these low frequency activities) could also imply impairment in the fronto-parietal circuitry in children of alcoholics as reported by an fMRI study by Rangaswamy et al. (2004). This view is strengthened by our finding that the OA group has a weaker fronto-parietal interaction as observed in the topography of slow alpha activity (8–9 Hz) (see Figure 4). It is also known that children of alcoholics, similar to adult alcoholics, manifest neuropsychological or cognitive deficits that precede the onset of alcoholism (Schaeffer et al 1984; Drejer et al 1985; Tarter et al 1989; Pihl et al 1990, Pihl and Bruce 1995; Peterson et al 1992; Knop et al 1993). Therefore, we propose that the neuro-cognitive deficits observed in the OA group, as elicited by decreased P3 amplitude and weaker oscillatory responses in low frequency bands perhaps predispose these high risk individuals to develop alcoholism and related disorders.
Our study has observed dysfunction in both response inhibition and response activation in the OA group. This finding is similar to that of a P3 study in children of alcoholics using a Go/NoGo task reported by Cohen et al. (1997b). Our earlier P3 study using the identical task as the present study (Kamarajan et al, 2005a) had a comparable finding, namely that the OA subjects manifested dysfunction primarily in response inhibition, and there was a trend toward lower P3 amplitudes in the Go condition. It is suggested that our current finding of decrease in ERO activity is more robust in the NoGo than the Go condition, supporting the view that the predominant dysfunction in children of alcoholics is related to frontal inhibitory control and hence they are more vulnerable to develop disinhibitory disorders including alcoholism, as alcoholism is also considered to be a part of the disinhibitory/externalizing spectrum of disorders (Gorenstein and Newman 1980; Krueger et al 2002; Iacono et al 2002, 2003; Kendler et al 2003). Further. as mentioned in our earlier paper (Kamarajan et al. 2004), the explanation of theta activity in terms of inhibitory control comes from several sources of evidence including the following: (1) theta activity at the cellular level is produced by the inhibitory interneurons of the hippocampus (Chapman and Lacaille, 1999); (2) It has also been suggested that frontal midline theta serves a response controlling function (cf. Basar et al., 2001c); and (3) it was also reported that the reduced theta and low alpha indicated the failure of the inhibitory control at the cognitive level (Klimesch et al., 2000), a notion that supports the disinhibition hypothesis.
The implication of these findings highlights the following major points: (1) the deficient oscillatory activity in OA subjects may indicate possible deficits in cognitive functions as indexed by these oscillations, viz., signal detection and decision-making as elicited by delta (Basar et al 1999; Schurmann et al 2001), conscious awareness, recognition memory, and episodic retrieval as marked by theta (Klimesch et al 1994, 2001a, 2001c; Doppelmayr et al 1998; Karakas et al 2000a, 2000b; Basar et al 2001c), and attentional processing as attributed to slow alpha activity (Basar et al 1997; Klimesch 1997; Klimesch et al 1998); (2) The decreased oscillatory responses during the NoGo condition, in particular, may suggest deficient inhibitory control and hence a dysfunctional frontal executive mechanism as evidenced by other findings showing such impairments in alcoholics as well as children of alcoholics (Giancola et al 1993, Giancola and Moss 1998; Finn et al 1999; Ihara et al 2000; Noel et al 2001; Ratti et al 2002; Kamarajan et al, 2005a), (3) the weaker anterior-posterior communication in alpha1 band during the NoGo processing may suggest a dysfunctional long-range neural circuitry in the OA group as reported by Rangaswamy et al (2004) and Kamarajan et al (2005a), and (4) the deficient oscillatory responses and/or weaker cognitive response control, as found in naïve children of alcoholics, may be considered as an endophenotypic marker in predisposing to alcoholism and other disinhibitory disorders.
We suggest that the deficient oscillatory responses observed in OA subjects is a trait (rather than a state) variable, as the findings that the ERP features and EEG oscillations are highly heritable (e.g., van Beijsterveldt et al 1996, 1998, 2001; Almasy et al 1999; Begleiter et al 1998; Porjesz et al 2002a, 2002b) and the neurotransmitter systems (e.g., cholinergic and GABAergic systems) that underlie delta and theta oscillations do have a genetic basis. It is reported that the theta and delta rhythms are produced in the interactions between the GABA and cholinergic neurotransmitter systems (Fellous and Sejnowski 2000; Tiesinga et al 2001). Recent findings from the Collaborative Study on Genetics of Alcoholism (COGA) project indicate that the cholinergic muscarinic receptor genes (CHRM2) on chromosome 7 are involved in the production of theta and delta oscillations that are elicited during the P300 time window of the visual oddball task (Jones et al 2004). However, as a note of caution, it should also be mentioned that although several research studies (e.g., Traub et al 1996; Whittington et al 2000) have shown that excitatory pyramidal neurons as well as inhibitory interneurons involve in the production of different oscillations (theta, gamma), it is not clear as to how these excitatory/inhibitory influences are related to the scalp-recorded (summated) oscillatory phenomenon, genes, and to the cognitive functions such as response activation/inhibition.
In conclusion, our findings suggest that the deficient oscillatory responses in lower frequency bands (especially during the NoGo condition) in offspring of alcoholics may indicate dysfunction in the neurocognitive mechanisms that underlie the neural circuits subserving frontal executive functions. Neuroelectric features in general are highly heritable and therefore the dysfunction in oscillatory response may predispose the high risk individuals to develop alcoholism and/or other disinhibitory disorders. It is proposed that the deficient oscillatory response in delta and theta bands (with the possible inclusion of slow alpha activity), like decreased P3 amplitude, can serve as a valuable endophenotypic marker for disinhibitory disorders in general and alcoholism in particular. A heritable biological endophenotype can help identify those individuals at genetic risk in the absence of overt manifest symptoms (Begleiter and Porjesz 1999). The implications of our findings may therefore support the model by Begleiter and Porjesz (1999) who proposed that a genetically mediated cognitive and neural disinhibition as indexed by electrophysiological anomalies forms the core of a predisposition for alcoholism and related disorders.
Acknowledgments
This study was supported by the NIH grant # 5 RO1 AA005524 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The authors are grateful to the valuable technical assistance of Glenn Murawski, Tracy Crippen, Eric Talbert, Carlene Haynes, and Joyce Alonzia.
Footnotes
However, based on ethical considerations, the subjects received the full amount (without deductions for incorrect responses) at the end of the experiment, although they were not informed of this while performing the experiment.
References
- Almasy L, Porjesz B, Blangero J, et al. Heritability of event-related brain potentials in families with a history of alcoholism. Am J Med Genet. 1999;88:383–390. [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E, Schmielau F. P300 in freely moving cats with intracranial electrodes. Int J Neurosci. 1991;60:215–226. doi: 10.3109/00207459109167034. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Demiralp T, Schurmann M, Basar E. Topological distribution of oddball ‘P300’ responses. Int J Psychophysiol. 2001;39:213–220. doi: 10.1016/s0167-8760(00)00142-2. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Struber D, Kruse P, Basar E, Stadler M. Frontal gamma-band enhancement during multistable visual perception. Int J Psychophysiol. 1996a;24:113–125. doi: 10.1016/s0167-8760(96)00055-4. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E. Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int J Psychophysiol. 1996b;24:101–112. doi: 10.1016/s0167-8760(96)00051-7. [DOI] [PubMed] [Google Scholar]
- Basar E. EEG brain dynamics: Relation between EEG and brain evoked potentials. Amsterdam: Elsevier; 1980. [Google Scholar]
- Basar E. Brain Oscillations: Principles and Approaches. Berlin: Springer; 1999. Brain Function and Oscillations. II. [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001a;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Demiralp T. Selectively distributed gamma band system of the brain. Int J Psychophysiol. 2001b;39:129–135. doi: 10.1016/s0167-8760(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Sakowitz O. The selectively distributed theta system: functions. Int J Psychophysiol. 2001c;39:197–212. doi: 10.1016/s0167-8760(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Karakas S. Alpha oscillations in brain functioning: an integrative theory. Int J Psychophysiol. 1997;26:5–29. doi: 10.1016/s0167-8760(97)00753-8. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999a;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: effects on P300 during the stroop test. Neuropsychopharmacology. 1999b;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, et al. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol. 1998;108:244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Lacaille JC. Intrinsic theta-frequency membrane potential oscillations in hippocampal CA1 interneurons of stratum lacunosum-moleculare. J Neurophysiol. 1999;81:1296–1307. doi: 10.1152/jn.1999.81.3.1296. [DOI] [PubMed] [Google Scholar]
- Chu PC. The S-transform for obtaining localized spectra. MTS J. 1996;29:28–38. [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neuroelectric correlates of response production and inhibition in individuals at risk to develop alcoholism. Biol Psychiatry. 1997a;42:57–67. doi: 10.1016/S0006-3223(96)00221-1. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997b;21:1398–1406. [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- de Bruin EA, Bijl S, Stam CJ, Bocker KB, Kenemans JL, Verbaten MN. Abnormal EEG synchronisation in heavily drinking students. Clin Neurophysiol. 2004;115:2048–2055. doi: 10.1016/j.clinph.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Basar-Eroglu C, Basar E. Wavelet analysis of oddball P300. Int J Psychophysiol. 2001;39:221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Devrim M, Demiralp T, Ademoglu A, Kurt A. A model for P300 generation based on responses to near-threshold visual stimuli. Brain Res Cogn Brain Res. 1999;8:37–43. doi: 10.1016/s0926-6410(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Auinger P, Winkler T. Theta synchronization in the human EEG and episodic retrieval. Neurosci Lett. 1998;257:41–44. doi: 10.1016/s0304-3940(98)00805-2. [DOI] [PubMed] [Google Scholar]
- Drejer K, Theilgaard A, Teasdale TW, Schulsinger F, Goodwin DW. A prospective study of young men at high risk for alcoholism: neuropsychological assessment. Alcohol Clin Exp Res. 1985;9:498–502. doi: 10.1111/j.1530-0277.1985.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Fell J, Dietl T, Grunwald T, et al. Neural Bases of Cognitive ERPs: More than Phase Reset. J Cogn Neurosci. 2004;16:1595–1604. doi: 10.1162/0898929042568514. [DOI] [PubMed] [Google Scholar]
- Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5–2 Hz), theta (5–12 Hz), and gamma (35–70 Hz) bands. Hippocampus. 2000;10:187–197. doi: 10.1002/(SICI)1098-1063(2000)10:2<187::AID-HIPO8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology (Berl) 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gabor D. Theory of communications. J Inst Elec Eng. 1946;93:429–457. [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Dev Alcohol. 1998;14:227–251. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Peterson JB, Pihl RO. Risk for alcoholism, antisocial behavior, and response perseveration. J Clin Psychol. 1993;49:423–428. doi: 10.1002/1097-4679(199305)49:3<423::aid-jclp2270490317>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez JA, Cedeno I, Pita-Alcorta C, Galan L, Aubert E, Figueredo-Rodriguez P. Induced oscillations and the distributed cortical sources during the Wisconsin card sorting test performance in schizophrenic patients: new clues to neural connectivity. Int J Psychophysiol. 2003;48:11–24. doi: 10.1016/s0167-8760(03)00019-9. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;87:301–315. [PubMed] [Google Scholar]
- Green MF, Mintz J, Salveson D, et al. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003;53:1113–1119. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiol. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Ihara H, Berrios GE, London M. Group and case study of the dysexecutive syndrome in alcoholism without amnesia. J Neurol Neurosurg Psychiatry. 2000;68:731–737. doi: 10.1136/jnnp.68.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen BH, Agarwal G, Hegde A, Boutros NN. Phase synchronization of the ongoing EEG and auditory EP generation. Clin Neurophysiol. 2003;114:79–85. doi: 10.1016/s1388-2457(02)00327-9. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, et al. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, et al. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int J Psychophysiol. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, et al. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005a;116:1049–1061. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go Task. Biol Psychol. 2005b;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas S, Basar-Eroglu C, Ozesmi C, Kafadar H, Erzengin OU. Gamma response of the brain: a multifunctional oscillation that represents bottom-up with top-down processing. Int J Psychophysiol. 2001;39:137–150. doi: 10.1016/s0167-8760(00)00137-9. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000a;285:45–48. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol. 2000b;111:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kissler J, Muller MM, Fehr T, Rockstroh B, Elbert T. MEG gamma band activity in schizophrenia patients and healthy subjects in a mental arithmetic task and at rest. Clin Neurophysiol. 2000;111:2079–2087. doi: 10.1016/s1388-2457(00)00425-9. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol. 1997;26:319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997a;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Russegger H. Event-related desynchronization in the alpha band and the processing of semantic information. Brain Res Cogn Brain Res. 1997b;6:83–94. doi: 10.1016/s0926-6410(97)00018-9. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: independent phenomena? Clin Neurophysiol. 2000;111:781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Stadler W, Pollhuber D, Sauseng P, Rohm D. Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neurosci Lett. 2001a;302:49–52. doi: 10.1016/s0304-3940(01)01656-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Wimmer H, et al. Theta band power changes in normal and dyslexic children. Clin Neurophysiol. 2001b;112:1174–1185. doi: 10.1016/s1388-2457(01)00545-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, et al. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain Res Cogn Brain Res. 2001c;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Brain Res Cogn Brain Res. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Knop J, Goodwin DW, Jensen P, et al. A 30-year follow-up study of the sons of alcoholic men. Acta Psychiatr Scand Suppl. 1993;370:48–53. doi: 10.1111/j.1600-0447.1993.tb05360.x. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Konishi S, Jimura K, Asari T, Miyashita Y. Transient activation of superior prefrontal cortex during inhibition of cognitive set. J Neurosci. 2003;23:7776–7782. doi: 10.1523/JNEUROSCI.23-21-07776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Lachaux JP, Chavez M, Lutz A. A simple measure of correlation across time, frequency and space between continuous brain signals. Journal of Neuroscience Methods. 2003;123:175–188. doi: 10.1016/s0165-0270(02)00358-8. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Makinen V, Tiitinen H, May P. Auditory event-related responses are generated independently of ongoing brain activity. Neuroimage. 2005;24:961–968. doi: 10.1016/j.neuroimage.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, Schmidt N, et al. Supervisory attentional system in nonamnesic alcoholic men. Arch Gen Psychiatry. 2001;58:1152–1158. doi: 10.1001/archpsyc.58.12.1152. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Porjesz B, Ranganathan M, et al. Suppression of early evoked gamma band response in male alcoholics during a visual oddball task. Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2005.03.026. (in press) [DOI] [PubMed] [Google Scholar]
- Peterson JB, Finn PR, Pihl RO. Cognitive dysfunction and the inherited predisposition to alcoholism. J Stud Alcohol. 1992;53:154–160. doi: 10.15288/jsa.1992.53.154. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Bruce KR. Cognitive impairments in children of alcoholics. Alcohol Health Res World. 1995;19:142–147. [PMC free article] [PubMed] [Google Scholar]
- Pihl RO, Peterson J, Finn P. Inherited predisposition to alcoholism: characteristics of sons of male alcoholics. J Abnorm Psychol. 1990;99:291–301. doi: 10.1037//0021-843x.99.3.291. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Individuals at risk for alcoholism: Neurophysiologic processes. In: Cloninger CR, Begleiter H, editors. Genetics and biology of alcoholism. New York: Cold Spring Harbor Laboratory Press; 1990. pp. 137–182. [Google Scholar]
- Porjesz B, Begleiter H. Neurophysiological factors in individuals at risk for alcoholism. Recent Dev Alcohol. 1991;9:53–67. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Alcoholism and human electrophysiology. Alcohol Res Health. 2003;27:153–160. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, et al. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, et al. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: which executive functions are imparied? Acta Neurol Scand. 2002;105:276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Roschke J, Fell J. Spectral analysis of P300 generation in depression and schizophrenia. Neuropsychobiology. 1997;35:108–114. doi: 10.1159/000119400. [DOI] [PubMed] [Google Scholar]
- Schaeffer KW, Parsons OA, Yohman JR. Neuropsychological differences between male familial and nonfamilial alcoholics and nonalcoholics. Alcohol Clin Exp Res. 1984;8:347–351. doi: 10.1111/j.1530-0277.1984.tb05678.x. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Basar E. Gamma responses in the EEG: elementary signals with multiple functional correlates. Neuroreport. 1997;8:1793–1796. doi: 10.1097/00001756-199705060-00045. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Kolev V, Basar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. Int J Psychophysiol. 2001;39:229–239. doi: 10.1016/s0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Stampfer HG, Basar E. Does frequency analysis lead to better understanding of human event related potentials. Int J Neurosci. 1985;26:181–196. doi: 10.3109/00207458508985616. [DOI] [PubMed] [Google Scholar]
- Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S-transform. IEEE Trans Sig Proc. 1996;44:998–1001. [Google Scholar]
- Tarter RE, Jacob T, Bremer DL. Specific cognitive impairment in sons of early onset alcoholics. Alcohol Clin Exp Res. 1989;13:786–789. doi: 10.1111/j.1530-0277.1989.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Theophanis MR, Queen J. Color display of the localized spectrum. Geophysics. 2000;65:1330–1340. [Google Scholar]
- Tiesinga PH, Fellous JM, Jose JV, Sejnowski TJ. Computational model of carbachol-induced delta, theta, and gamma oscillations in the hippocampus. Hippocampus. 2001;11:251–274. doi: 10.1002/hipo.1041. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Genetic and environmental influences on EEG coherence. Behav Genet. 1998;28:443–453. doi: 10.1023/a:1021637328512. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC, Molenaar PC, Boomsma DI, de Geus EJ. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behav Genet. 2001;31:533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, van der Molen M, Boudewijn Gunning W, Kok A. Neuroelectrical signs of selective attention to color in boys with attention-deficit hyperactivity disorder. Brain Res Cogn Brain Res. 2001;12:245–264. doi: 10.1016/s0926-6410(01)00055-6. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, et al. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Cohen JD. Detection of synchronized oscillations in the electroencephalogram: An evaluation of methods. Psychophysiology. 2004;41:822–832. doi: 10.1111/j.1469-8986.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Banaschewski T, Kolev V, Woerner W, Rothenberger A. Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder--evidence from event-related gamma oscillations. Clin Neurophysiol. 2001;112:1096–1108. doi: 10.1016/s1388-2457(01)00524-7. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Brain theta response predicts P300 latency in children. Neuroreport. 1996;8:277–280. doi: 10.1097/00001756-199612200-00055. [DOI] [PubMed] [Google Scholar]