Abstract
Telomeres are essential structures at the ends of eukaryotic chromosomes. Work on their structure and function began almost 70 years ago in plants and flies, continued through the Nobel Prize winning work on yeast and ciliates, and goes on today in many model and non-model organisms. The basic molecular mechanisms of telomeres are highly conserved throughout evolution, and our current understanding of how telomeres function is a conglomeration of insights gained from many different species. This review will compare the current knowledge of telomeres in plants with other organisms, with special focus on the functional length of telomeric DNA, the search for TRF homologs, the family of POT1 proteins, and the recent discovery of members of the CST complex.
Keywords: Telomere, Telomerase, Arabidopsis, Genome stability, Plant
1. Introduction
Telomeres form the ends of linear eukaryotic chromosomes, providing them with properties that are essential for long term genome stability. Telomeres play a dual role in the maintenance of linear genomes. First, their unique mode of replication, which involves the highly specialized enzyme telomerase, provides a solution to the so-called end replication problem, the inability of polymerases to fully synthesize 5′ ends of DNA. Second, telomeres shield the natural chromosome ends from DNA damage recognition machinery which normally responds to DNA breaks occurring within the chromosome. Owing to the evolutionary conservation of the telomere–telomerase machinery, telomere research has benefited tremendously from studies in highly diverged organisms. In fact, this field of research is one of the remarkable examples where studies in model organisms representing all the major branches of the tree of life are combined to gain understanding of the functional mechanisms and evolution of a fundamental molecular machine. While telomeres are most extensively studied in protozoan ciliates, yeast, mouse and human cell lines, other systems such as fruit flies, worms and Arabidopsis have also provided important insights. Plants hold some remarkable firsts in telomere biology: the first evidence for the existence of telomerase activity in the form of chromosome healing by McClintock in the 40s [1], the first cloning of higher eukaryotic telomeric DNA by Richards in the late 80s [2], and, along with humans, the identification of CTC1 last year [3]. This review aims to summarize recent progress in understanding how telomeres work in plants as compared to other organisms by discussing individual components of telomeric chromatin.
2. Telomeric DNA
At the sequence level, canonical telomeres are composed of simple repeats of a G-rich sequence that, depending on the organism, extend for tens of basepairs to as much as 150 kilobasepairs. The sequence repeat varies surprisingly little between organisms from diverse kingdoms. The strong conservation of telomeric repeats is likely a result of the interaction between telomeric DNA and telomere-specific binding proteins (discussed below). In vertebrates, the telomeric repeat is TTAGGG while most plants, with several notable exceptions, have TTTAGGG repeats [4,5]. In the model algae Chlamydomonas, telomeres are TTTTAGGG [6], and several plants, including onion and a few species of the Solanaceae family, lack canonical repeats; the exact structure of telomeres in these plants remains unknown [7,8]. Interestingly, in a phylogenetic clade of Asparagales that consists of ∼6300 species, a single nucleotide change occurred in the canonical plant telomere sequence causing a switch to the human TTAGGG sequence [5]. This was likely caused by a mutation altering the RNA template subunit of telomerase, and this event is dated to ∼80 Mya [9].
In plants as well as in other organisms, the G-rich strand at the 3′ end of the chromosome is longer than the C strand, forming a 3′ G-overhang. Interestingly, early experiments suggested that G-overhangs exist on only a portion of the telomeres within a plant [10]. Thus, alternative end-structures may be present in plants, as has recently been demonstrated in Ceanorhabditis elegans [11]. The G-overhang is important for forming a tertiary structure termed the t-loop, where the G-overhang folds back and invades duplex telomeric DNA, effectively hiding the end of the chromosome. T-Loops have been observed in humans, protozoans, and plants, but so far have not been demonstrated in yeast [12–14].
While the variation in telomere length varies by three orders of magnitude between species, each species maintains its telomeres within a narrower size range. Despite this, it is clear from numerous studies that telomeres continue to be functional at lengths much below the size of a species’ wild type minima. A major question remaining in telomere biology is what is the actual minimal length at which telomeres are still functional? Two primary approaches have been employed in attempts to define the minimal functional length: PCR based measurements of the shortest telomeres in telomerase deficient cells, and analysis of the number of telomere repeats recovered in cloned telomeric fusion events [15,16]. The logic behind these techniques is that once a telomere reaches a length that is no longer functional, it will be recruited into a fusion, and no longer be amplified by PCR. Conversely, the size of the shortest functional telomeres should be close to the size of the longest array of telomeric repeats retained at fusion junctions. Experiments in Arabidopsis showed that the shortest telomeres detected from late generation tert plants were approximately 350 bp; the absolute shortest telomere detected was 260 bp. Complementary analysis of sequences at fusion junctions revealed that the amount of telomeric DNA ranged from 120 bp to 450 bp, with an average of 260 bp [15]. These data suggest that the minimal amount of telomeric DNA required for protection from chromosome end-to-end fusion is within the range of 260–450 nt. Further work indicated that the first telomeric fusions became detectable when the shortest telomere within a plant dips below 1 kb, suggesting that Arabidopsis has an approximately 500 bp range within which telomeres are metastable [17].
Similar studies have also been performed in human cells. Xu and Blackburn discovered that a significant fraction of telomeres in some telomerase positive human cancers have extremely short telomeres termed T-stumps, which range in size from 90 to 300 bp [18]. These data suggest that in the presence of telomerase, t-stumps do not trigger a senescence signal, implying that telomerase itself may provide a protective function independent of its catalytic activity. Telomere length and the structure of fusion junctions were also studied in telomerase negative fibroblasts transformed with HPV which abrogates p53 and Rb function [16]. In these cells, the XpYp and 17p telomeres shortened to a minimal length of 54 and 18 bp, respectively. In contrast to an average of 260 bp of telomeric DNA retained at fusion junctions in Arabidopsis, the largest block of telomeric DNA retained in these cells was only 78 bp. Thus, while the minimal functional length is estimated to be 260–450 bp in Arabidopsis, in humans it is suggested to be below 100 bp. It was proposed that the minimal length in Arabidopsis reflected requirements to form a t-loop [15], while in humans the minimal length appears to be the shortest amount of DNA needed to bind TRF1 and TRF2 [16]. Besides the difference in species, there are two key differences that must be considered for these studies, namely, the presence of telomerase and the status of checkpoint pathways. In the Arabidopsis studies, telomerase was absent and checkpoints should have been intact. The studies in humans covered a broader range of conditions, but the only situation mirroring Arabidopsis tert mutants are primary untransformed fibroblasts, which, without further manipulation, are destined to enter senescence in response to dysfunctional telomeres. In the presence of telomerase, these short telomeres are still present, but do not lead to senescence [18]. Also, in the absence of checkpoint machinery, these telomeres are stable and are not recruited into fusions [16]. Thus, the functional length may be different depending on the presence of telomerase and checkpoint machinery.
3. Plant telomerase and its regulation
Composition of the plant telomerase holoenzyme appears to be similar to vertebrate telomerase. Genes encoding telomerase reverse transcriptase (TERT) can readily be identified in plant genomes, and they are more related to the TERTs of vertebrates than to those in yeast and ciliates [19–21]. Rapid divergence of the RNA subunit (TR) of telomerase precluded their in silico identification in plant genomes, but biochemical purification of Arabidopsis telomerase led to the recent discovery of two functional TRs in Arabidopsis (D. Shippen, personal communication). This breakthrough finding is expected to facilitate cloning of TR genes in other plant species as well.
Dyskerin is another conserved member of the telomerase holoenzyme that is shared in vertebrates and Arabidopsis. Dyskerin is a member of the H/ACA snoRNP gene family which have important functions in rRNA processing and production. It binds the H/ACA box of human TR and is involved in TR processing and stabilization [22]. Mutations in human dyskerin cause dyskeratosis congenita, a genetic disorder associated with aberrant telomere maintenance and reduced levels of hTR [23]. In Arabidopsis, dyskerin is also associated with telomerase in an RNA dependent manner and it interacts specifically with POT1A [24]. Arabidopsis dyskerin is an essential gene, but introduction of one of the mutations causing dyskeratosis congenita results in decreased telomerase activity in vitro and leads to short but stable telomeres in vivo. This, together with the observation that dyskerin and TERT co-localize in the nucleolus, suggests that similar processes may govern telomerase maturation in Arabidopsis and vertebrates.
Similar to it’s activity in humans, telomerase activity in plants is restricted to highly proliferative tissues and the germline [25–27]. Furthermore, studies in tobacco cell culture have shown that telomerase activity is regulated in a cell cycle dependent manner with a peak level in S-phase. This regulation is potentiated by the plant hormone auxin and antagonized by another phytohormone, abscisic acid [28,29].
In Arabidopsis, telomerase regulation appears to be achieved primarily at the level of transcription of TERT, as transcripts are undetectable in tissues lacking telomerase activity [19,20]. Further molecular insights into pathways regulating TERT expression have been uncovered in Arabidopsis. A genetic screen of activation tag lines led to the identification of a zinc-finger transcription factor, TAC1, whose overexpression results in ectopic telomerase activity in leaves [30]. Interestingly, TAC1 appears to also be involved in auxin signaling. TAC1 does not directly bind the TERT promoter, but instead binds to and activates the promoter of BT2, a calmodulin binding protein [31]. Overexpression of BT2 is itself sufficient for activating telomerase activity in leaves, and increased levels of calcium are able to overcome the requirement for wild type levels of auxin in TAC1 mediated regulation of telomerase. It is currently unclear how the TAC1/BT2 pathway activates TERT expression and which transcription factors are directly recruited to the TERT promoter.
While the profile of telomerase activity in rice is similar, transcription does not appear to be the major regulatory mechanism. Multiple splice variants of the rice TERT gene are present in all tissues, but the correlation of particular splice variants with telomerase activity is disputed [32,33]. Splice variants have also been detected in Arabidopsis. A truncated splice variant of AtTERT is able to interact with AtPOT1A, which may represent an additional form of regulation through protein–protein interactions of the truncated TERT protein [34].
Regulation of telomerase activity at the telomere is much more complicated, likely involving multiple proteins and a negative feedback loop such as the protein counting mechanism discovered in yeast [35]. Similar to other organisms, telomerase in Arabidopsis has a preference for elongating shorter telomeres within a plant [36,37]. While some of the molecular details of this pathway have been elucidated in yeast, little is known in higher eukaryotes. Est1p from yeast is a telomerase holoenzyme component involved in recruiting telomerase to telomeres. In higher eukaryotes, EST1 homologs are primarily involved in the nonsense-mediated RNA decay (NMD) pathway [38]. Although downregulation of human EST1 homologs (SMG6, SMG5) have telomere maintenance phenotypes, these are caused by insufficient decay of telomeric transcripts [39]. Two EST1 homologs have also been identified in Arabidopsis (SMG7, SMG7L), and while their deficiency leads to interesting developmental defects, they have no apparent role in telomere biology [40]. Interestingly, an Est1-like phenotype was recently described in Arabidopsis plants with a mutation in one of the POT1 homologs (discussed below) indicating that plants may utilize a different machinery for telomerase recruitment to telomeres [41].
4. The search for plant shelterin components
The regulation of telomere synthesis by telomerase and protection of the chromosome end are implemented through the combined action of numerous proteins that associate with telomeres. The key elements of telomeric chromatin are specific duplex telomere binding proteins that nucleate the assembly of additional proteins. The bulk of telomeric DNA in mammals is coated with a six-protein complex called shelterin [42]. The specific binding of shelterin to telomeres is mediated by TRF1 and TRF2. TIN1 fulfils a bridging function in the shelterin complex as it binds to TRF1 and TRF2 and recruits TPP1 and POT1. The last component of shelterin is RAP1 which associates with telomeres via an interaction with TRF2 [43]. Shelterin is assumed to exist in different sub-stochiometric complexes that recruit a number of other proteins to telomeres. These shelterin accessory factors mainly consist of proteins involved in DNA damage response and repair [44]. The major telomere binding complex in Saccharomyces cerevisiae is very different; the only component of shelterin present in S. cerevisiae is Rap1p. In contrast to mammals, Rap1p directly binds to duplex telomeric DNA. The C-terminal domain of Rap1p recruits either Rif1p and Rif2p, proteins that regulate telomerase activity, or Sir3p and Sir4p, proteins that promote heterochromatin assembly in telomere adjacent regions [45]. The differences between telomere constituents in mammals and budding yeast indicated a relatively rapid evolution of the major telomere binding complex. However, the telomere-binding complex in fission yeast appears to be functionally and structurally equivalent to mammalian shelterin [46,47]. This complex is tethered to telomeric DNA via the Taz1 protein which shares homology with TRF1 and TRF2. Thus, the composition of the shelterin complex may be more conserved than was originally hypothesized. This idea is further fuelled by the presence of the TRF1/TRF2/Taz1 related protein Tbp1 in S. cerevisiae. Although Tbp1p does not appear to play a role at telomeres, it has a binding affinity to human type telomeric DNA. Thus, Tbp1p may represent a relict of the original shelterin-like complex in budding yeast.
The high degree of conservation between plant and vertebrate telomeric sequences predicts the existence of a shelterin-like complex in plants. However, the functional counterparts of telomere-binding proteins such as TRF1/TRF2 have not yet been identified. The search for these proteins is impeded by the presence of a short telomeric sequence, TTAGGGTTT, in the promoters of many plant genes [48,49]. Although numerous plant proteins with in vitro telomeric DNA binding activity have been characterized, many of them do not appear to act at telomeres, and may rather represent transcription factors [50]. Two strategies have been employed to identify plant telomere binding proteins. The first strategy utilizes identification of proteins based on their affinity to telomeric DNA. Biochemical purification of protein complexes that bind to single-stranded (ss) telomeric DNA led to the identification of the GTBP1 protein in tobacco and the STEP1 protein in Arabidopsis [51,52]. Both proteins contain conserved RNA binding motifs and it is currently unclear whether they play any role in telomere metabolism. A protein with affinity to duplex telomeric DNA was found by Southwestern screening of an Arabidopsis expression library [49]. The protein is homologous to mammalian Purα, which is a ubiquitous multifunctional protein involved in transcription, translation and DNA replication [53]. AtPurα interacts with the transcriptional regulators E2F and TCP20 and it was suggested to regulate transcription of promoters with telomeric motifs [54,55]. The function of AtPurα at telomeres has not been examined.
The second strategy is based on in silico searches for proteins harboring a Myb/SANT domain that is conserved in TRF1, TRF2, Taz1 and Tbp1p. The characteristic feature of this protein family is a consensus sequence, VDLKDKWRT, in the third helix of the Myb/SANT domain that mediates telomeric sequence-specific contacts in the DNA major groove [56,57]. Three classes of proteins carrying the single Myb/SANT domain have been identified in plants. The so called SMH (single myb histone) family is represented by rather small proteins (30–35 kDa) that contain the Myb/SANT domain at the N-terminus [58]. The Arabidopsis genome encodes at least three SMH-like proteins (AtTRB1-3), while five SMH proteins have been identified in maize. Although the SMH-like proteins specifically bind telomeric DNA in vitro, there is so far no functional evidence for their role at telomeres.
In addition to the SMH-like family, a survey of the Arabidopsis genome revealed 12 other proteins carrying a single Myb/SANT domain [59]. These proteins contain the Myb/SANT motif at the C-terminus, and therefore are referred to as TRF-like (TRFL) proteins. The TRFL proteins can further be divided into two groups. The family II proteins (TRFL3, 5, 6, 7, 8, 10) do not form homodimers and fail to bind telomeric DNA in vitro [59]. The second group of proteins (family I) consists, in Arabidopsis, of six closely related proteins (TBP1, TRP1, TRFL1, TRFL2, TRFL4 and TRFL9) [59–61]. Homologous proteins have also been identified in rice (RTBP1) and tobacco (NgTRF1) [62,63]. Characteristic features of this family are a ubiquitin-like domain in the central region and a 40 amino acid extension of the Myb/SANT domain at the C-terminus. The family I proteins form homodimers and all of them bind duplex telomeric DNA in gel-shift assays. Interestingly, the Myb extension motif is absolutely essential for in vitro DNA binding. Deletion of the C-terminal extension abolishes DNA binding of AtTRFL1, while its heterologous fusion to the Myb domain of AtTRFL3 (family II protein) was sufficient to confer affinity to plant telomeric DNA [59]. Structural studies of AtTRP1, NgTRF1 and RTBP1 revealed that the extension domain forms a helix that stabilizes the three helix bundle of the Myb/SANT domain (Fig. 1) [64–66]. Comparison of NgTRF1 and human TRF1 structures showed that recognition of telomeric sequences is well conserved. Most of the specific interactions are mediated by the third helix of the Myb/SANT domain with bases located in the major groove of the DNA helix (Fig. 1). The extension domain provides an additional interaction with the minor groove that is likely responsible for specific binding to plant telomeric DNA (TTTAGGG) (Fig. 1) [64,66].
Fig. 1.
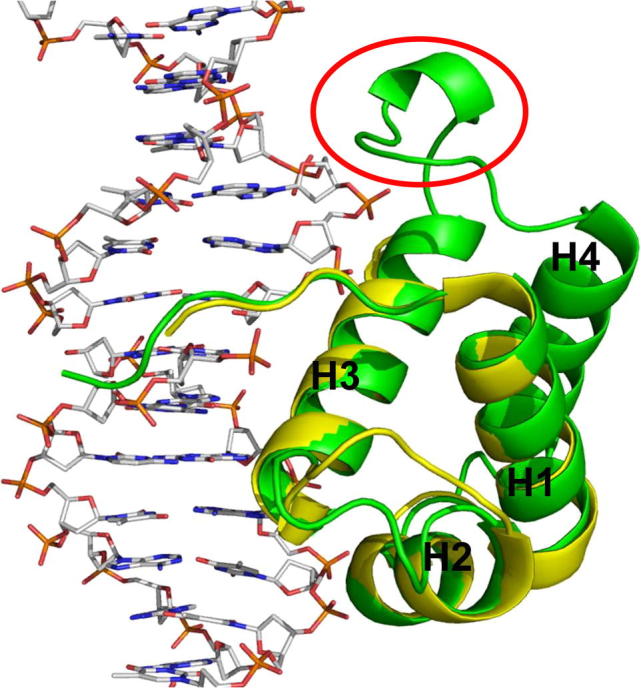
Structural comparison of the DNA binding domains of NgTRF1 in green and human TRF1 in yellow. Circled in red is the loop between helix 3 and 4 which directly contacts the DNA minor groove and provides binding specificity to the plant telomeric sequence TTTAGGG. Reprinted from Ko et al. (2008) Nucl. Acids Res. 36, 2739–2755 with permission from Oxford University Press.
Although the family I TRFL proteins are capable of binding telomeric DNA in vitro, examination of Arabidopsis mutant lines deficient for either TRFL1, 2 or 4 did not reveal any phenotype expected to be associated with altered telomere metabolism [59]. Disruption of AtTBP1 leads to gradual telomere lengthening from 2–4 kb to 10 kb [67]. No other phenotypes were associated with AtTBP1 dysfunction and mutant plants were fertile. Telomere lengthening was also observed in tobacco cell lines in which NgTRF1 was downregulated by siRNA. Furthermore, overexpression of NgTRF1 in tobacco cells resulted in telomere shortening, gradual retardation of proliferation and elevated cell death after prolonged cultivation [68]. It was not reported whether the cell growth defects were associated with telomere dysfunction. Disruption of the RTBP1 gene in rice also led to telomere elongation. However, the rtbp1 plants also exhibited growth defects and reduced fertility [69]. These phenotypes progressively worsened in subsequent generations and their severity correlated with an increasing frequency of anaphase bridges in meiotic cells. This suggests that aberrant telomere maintenance may be the underlying cause of the growth retardation associated with the rtbp1 mutation. Gene redundancy is a likely explanation for the less severe phenotypes in Arabidopsis than in rice. While the rice genome encodes only two family I TRFL proteins, the ancestral genome of Arabidopsis underwent several rounds of duplication resulting in the presence of at least six genes of this family [59,70]. Telomere lengthening associated with TRFL deficiency in Arabidopsis, rice, and tobacco is analogous to deficiency of TRF1 in mammals or Taz1 in S. pombe. However, the absence of plant proteins is less detrimental for telomere function and survival of the organism than lack of TRF1 or Taz1. Even the growth retardation observed in rice rtbp1 mutants is relatively mild in comparison to the embryonic lethality of TRF1 or TRF2 knock-out mice. Therefore, it is likely that the list of proteins that bind duplex telomeric DNA in plants is far from complete, and proteins essential for the assembly of telomeric chromatin remain to be discovered.
5. POT1 proteins
Another evolutionarily conserved component of shelterin is POT1. POT1 proteins are characterized by two oligosaccharide/oligonucleotide binding motifs (OB-fold) at the N-terminus that mediate interaction with G-rich single stranded telomeric DNA. POT1 is thought to fulfil its function through direct association with the telomeric G-strand, while interaction with TPP1 and the shelterin complex may facilitate its efficient recruitment to telomeres. Pot1 is absolutely essential for telomere protection in fission yeast and its inactivation leads to a rapid loss of telomeric DNA [71]. In vertebrates, POT1 represses ATR dependent DNA damage signaling presumably by preventing RPA binding to single-stranded telomeric DNA [72,73]. The POT1-TPP1 heterodimer has also been proposed to regulate telomerase activity in both a positive and negative manner [74,75]. Interestingly, while the majority of mammalian species, including human, have only one POT1 gene, rodent genomes contain two closely related POT1 paralogs. These genes partially diverged in their functions; POT1a is more important for inhibiting the DNA damage response while POT1b protects the telomeric C-strand from resection [76].
POT1 homologs have also been identified in plants. Arabidopsis encodes three POT1 proteins – POT1A, POT1B and POT1C [41,77,78]. The Arabidopsis POT1 proteins are structurally and functionally more divergent than their mouse counterparts. While POT1B and POT1C are implicated in chromosome end protection, POT1A is associated with telomerase and is required for telomere synthesis. The telomere capping role of POT1B was inferred from an experiment in which a C-terminal truncated version of the POT1B protein harboring only the N-terminal OB domains was expressed in wild-type plants [77]. Expression of the truncated protein led to stunted growth in combination with telomere shortening and genome instability. In contrast to non-plant POT1 proteins, Arabidopsis POT1B does not bind single stranded telomeric DNA in vitro [79]. Hence, the protective function of POT1B may not require direct interaction with DNA and it may be tethered to telomeres through other proteins. An attractive candidate is POT1C that has also been suggested to play a role in chromosome end protection [80]. Arabidopsis POT1A does not appear to play any role in telomere protection, but instead acts as a positive regulator of telomerase. Plants expressing a truncated allele of POT1A lacking the N-terminal OB motifs have, on average, 1–1.5 kb shorter telomeres [77], and the complete absence of POT1A results in an ever shorter telomeres (EST) phenotype, identical to the inactivation of telomerase [41]. Telomeres in pot1a mutants shorten at the same rate as telomeres in tert mutants and genetic analysis revealed that POT1A and telomerase act in the same pathway. Furthermore, POT1A interacts with telomerase in vivo. This interaction may be mediated either through direct binding to TERT or the RNA subunit of telomerase [34,41] (D. Shippen, personal communication). Chromatin IP showed that POT1A associates with telomeres only transiently during S-phase, suggesting that POT1A acts as a recruitment factor that brings telomerase to G-overhangs. However, POT1A does not bind to single stranded telomeric DNA in vitro, and hence, an additional bridging protein would be required for this function. Alternatively, POT1A may have a direct stimulatory role on the enzymatic activity of telomerase RNP. This view is supported by the observation of reduced telomerase activity in Arabidopsis pot1a mutants [41].
The data in Arabidopsis suggests that the duplication of POT1 genes in the plant lineage led to a well defined functional diversification among POT1 paralogs, with POT1B playing a predominant role in chromosome end protection, while POT1A is exclusively involved in telomerase regulation. However, this division of labor must have occurred relatively recently as only one POT1 gene was found in the genomes of many land plants, including papaya, which diverged from the lineage leading to Arabidopsis 70 Mya [70,80]. Duplication of the POT1 genes was estimated to occur 34 Mya, when the last common ancestor of the Brassicaceae family underwent a whole genome duplication. Evolution of the Arabidopsis POT1 proteins represents an extraordinary example of rapid neofunctionalization within the context of the otherwise highly conserved chromosome end protection machinery.
6. The CST complex
Budding yeast lack POT1, and the CST complex is the major G-overhang binding complex that performs essential function in telomere protection and replication. CST is a heterotrimeric complex that, in S. cerevisiae, consists of Cdc13p and two associated single OB-fold proteins, Stn1 and Ten1. These three proteins form an RPA like particle that specifically binds to telomeric G-overhangs to provide telomere protection [81,82]. Mutations in any component of the CST complex are lethal due to massive degradation of the telomeric C-strand and telomere dysfunction [83–85]. Cdc13p plays a central role in telomere metabolism as it recruits either the telomerase RNP or the Stn1/Ten1 heterodimer to the G-overhang. In addition, Cdc13p and Stn1p physically and functionally interact with primase, suggesting a role for the CST complex in synthesis of the telomeric C-strand [86].
Initially, it was believed that the CST complex was unique to budding yeast and that other organisms primarily use POT1 to mediate G-overhang protection. However, identification of Stn1 and Ten1 homologs in fission yeast suggested that the telomeric function of CST is evolutionarily more conserved than was originally thought [87]. S. pombe Stn1/Ten1 colocalize with Pot1 and strains deficient for either Stn1 or Ten1 exhibited pot1-like phenotypes; namely, rapid degradation of telomeric DNA and chromosome circularization. Despite these similarities, Pot1 does not appear to substitute for Cdc13 in fission yeast because it does not interact with Stn1/Ten1 [87]. Using the yeast sequence as a query, Stn1 homologs were also found in Arabidopsis and humans [88,89]. These studies indicated a widespread distribution of the CST complex in all branches of eukaryotic life, but the homology searches did not reveal genes orthologous to Cdc13. Two different approaches led to the identification of functional counterparts of Cdc13 in these organisms. A forward genetic screen in Arabidopsis identified a mutant that displayed telomere deprotection phenotypes such as chromosome end-to-end fusions and aberrant telomeric recombination [3]. Genetic mapping led to a locus encoding a 142 kDa protein that was named Conserved Telomere Maintenance Component 1 (CTC1). This protein interacts with STN1 and harbors two C-terminal OB-folds with homology to RPA70 and an N-terminal OB-fold remotely related to POT1 proteins. The Arabidopsis STN1/CTC1 complex co-localizes with telomeres, but it remains to be established whether this occurs through direct association with G-overhangs [3,88]. PSI-BLAST using Arabidopsis CTC1 uncovered homologous proteins in many vertebrates, including human. In a parallel study, human CTC1 was found as a protein associated with STN1 [89]. Human CTC1 forms a trimeric complex with STN1/TEN1, but in contrast to yeast CST, this complex does not exhibit increased in vitro binding affinity to the telomeric G-strand.
Interestingly, the consequences of CTC1 and STN1 dysfunction in human cells and Arabidopsis are not as dramatic as in yeast. Human cells in which CTC1 or STN1 are downregulated by siRNA exhibit accumulation of single stranded telomeric DNA and in some experimental settings also increased the frequency of dysfunctional telomeres [3,89]. While the relatively mild phenotypes in human cells may be due to the presence of residual proteins in siRNA experiments, genetic analysis in Arabidopsis showed that the CST complex, while important for proper telomere maintenance, is not absolutely essential for survival. Phenotypes associated with CTC1 and STN1 gene disruptions are very similar and include telomere shortening, long G-overhangs, chromosome end-to-end fusions, and aberrant telomeric recombination that is manifested by excision of extrachromosomal telomeric circles (t-circles) [3,88]. Nevertheless, Arabidopsis stn1 and ctc1 mutants are still alive and even semifertile, and these phenotypes do not worsen in ctc1 stn1 double mutants. Thus, unlike in yeast, other components of telomeric chromatin are likely to provide sufficient chromosome end protection to support plant viability. The obvious candidates are the POT1 proteins as suggested by the synergistic effect of POT1 and STN1 downregulation on telomere protection in human cell lines [89]. CST may also act in a partially redundant manner with RPA. Arabidopsis possesses several paralogous genes for each RPA subunit and this genetic redundancy allows isolation of viable mutants. A role for the Arabidopsis RPA complex in telomere metabolism is supported by the observation that plants carrying a mutation in the RPA70a gene have longer telomeres [90].
7. DNA repair proteins
Although the major function of telomeres is to restrict DNA repair activities at chromosome termini, many proteins involved in DNA double strand break (DSB) repair and signaling are present at telomeres. It has been suggested that transient recognition of a telomere as a DSB during or immediately after DNA replication is important for both telomere extension by telomerase and formation of the proper telomere capping structure [91,92]. DSB recognition and initiation of a DNA damage response largely relies on the MRN/X (Mre11/Rad50/Nbs1 in mammals and Mre11/Rad50/Xrs2 in yeast) complex and the ATM kinase. Studies in yeast indicate that this signaling pathway promotes extension of telomeres, presumably by enhancing recruitment and processivity of telomerase at the shortest telomeres [93,94]. MRN/X is also implicated in nucleolytic processing of telomeres to generate G-overhangs [95,96].
The MRN complex as well as the ATM and ATR kinases appear to be vital for telomere function in plants as well. Arabidopsis lacking RAD50 or MRE11 are viable, but exhibit various genome instabilities that include chromosome end-to-end fusions [97,98]. Unlike in yeast, lack of MRN in Arabidopsis does not alter overall telomere length homeostasis. However, cytogenetic analysis indicates a massive loss of telomeric sequence on chromosome termini involved in fusions [97]. Absence of telomerase further exacerbates the occurrence of chromosome end-to-end fusions in rad50 mutants. Chromosome end-to-end fusions spontaneously occurring without any obvious change in telomere length were also observed in atm atr double mutant plants [99]. Interestingly, genetic interactions with telomerase revealed distinct functions for ATM and ATR at telomeres. While telomeres in atr tert double mutants underwent accelerated attrition, which was accompanied by an early onset of genome instability, the rate of telomere shortening in atm tert mutants was unaltered. Nevertheless, these plants still exhibited an earlier onset of telomere-dysfunction associated developmental defects. Strikingly, these growth defects could be linked to a single critically shortened telomere that was involved in the majority of fusions, while other telomeres appeared to be functional. It was proposed that the short telomere was generated by a rare deletion event that would be normally eliminated. However, the absence of an ATM-dependent checkpoint allowed propagation of the affected cells through the germline, which had detrimental consequences for the following generations [100]. Another DNA repair complex whose mutation enhances genome instability in the absence of telomerase is the structure specific nuclease XPF/ERCC1. Inactivation of Arabidopsis XPF (RAD1) or ERCC1 in late generation telomerase mutants leads to formation of large extrachromosomal fragments that are proposed to arise from recombination between dysfunctional telomeres and interstitial telomeric sequences located mainly in the vicinity of centromeres [101]. These data support a model suggested from a study of TRF2 depleted human cells, in which the XPF/ERCC1 nuclease prevents ectopic recombination by removing 3′ G-overhangs from dysfunctional telomeres [102].
One of the most enigmatic DNA repair components of telomeric chromatin is Ku, a heterodimer consisting of the Ku70 and Ku80 subunits. Ku acts in early steps of the non-homologous end joining (NHEJ) DNA repair pathway where it binds and stabilizes broken chromosome ends and facilitates their ligation. Paradoxically, although fully protected telomeres suppress NHEJ activity, Ku is an intrinsic part of telomeric chromatin and its function is required for many aspects of telomere metabolism [103,104]. Ku plays a dual role at telomeres in S. cerivisiae: it suppresses nucleolytic resection of the 3′ chromosome end and promotes recruitment of telomerase to telomeres [105–107]. Mouse Ku knock-out strains exhibit telomere length deregulation, chromosome end-to-end fusions and accelerated aging [108,109]. The consequence of Ku dysfunction was also studied in plants. Disruption of KU70 or KU80 genes in Arabidopsis leads to telomerase dependent telomere elongation, but not to any adverse effects on genome stability or plant growth and development [110–113]. However, concomitant inactivation of Ku and telomerase leads to accelerated telomere shortening and early onset of genome instability. This rapid loss of telomeric DNA is attributed to nucleolytic resection of 5′ chromosome ends and to an increased frequency of telomere rapid deletion (TRD) events that can be inferred from a high level of t-circles in Ku deficient plants [112,114]. Excision of t-circles is assumed to be a consequence of aberrant intrachromatid recombination and/or t-loop resolution. Further evidence supporting this suggestion comes from experiments that analyzed long-term survival of telomerase deficient cells. While yeast and animal cell lines overcome telomere attrition due to the absence of telomerase by employing a recombination mechanism for telomere maintenance (ALT, alternative lengthening of telomeres), ALT cells were never detected in Arabidopsis TERT deficient cell cultures [115,116]. However, ALT was efficiently activated in cell lines derived from tert ku70 mutants [114,116]. Thus, supression of recombination may be a key function of Ku at Arabidopsis telomeres. Interestingly, massive t-circle excision has recently been shown to be responsible for lethal loss of telomeric DNA in human cells in which both KU80 alleles were disrupted by targeted deletions [117]. While TRD is often observed in mutant backgrounds, it also occurs with high frequency in wild type plants with dramatically elongated telomeres. A measurable frequency of TRD has also been detected at telomeres within the wild type length range, suggesting that TRD can also function as a telomere length regulator [37].
8. Conclusions
On the surface, telomeres look like a highly conserved molecular structure. However, comparative studies across multiple model organisms revealed that the exact means by which organisms have adapted this machinery to achieve chromosome end protection and replication varies, in some cases remarkably. Recent progress in plant telomere research highlights several such examples. Among the most striking is the finding that while plant genomes possess POT1-like proteins, most of them have lost telomeric DNA binding activity [80]. The Arabidopsis POT1A protein may instead be a part of the telomerase complex. POT1A deficiency leads to an ever shorter telomeres phenotype (EST), typical for mutants lacking the TERT or TR subunits of telomerase. Mutations in other genes resulting in EST phenotypes, have so far only been found in budding yeast (EST1, EST3, CDC13) [118,119], worms (MRT-2) [120] and Arabidopsis (POT1A) [41]. Interestingly, these genes are structurally unrelated, indicating that the pathways regulating telomerase activity at telomeres may be, in evolutionary terms, very flexible.
On the other hand, many evolutionarily conserved proteins may provide essentially the same molecular function at telomeres, but their deficiency may have very different consequences depending on the organisms. Good examples are CST and Ku, which are required for protection of the telomeric C-strand in yeast and Arabidopsis. While the CST complex is essential in yeast, its inactivation in Arabidopsis and human cells produces much milder phenotypes [3,88,89]. This is likely due to additional mechanisms, such as t-loop formation, that may play a primary capping function in higher eukaryotes, whereas protection via CST is the predominant pathway in yeast. Remarkably, Cdc13p is dispensable in budding yeast that maintain telomeres through recombination, which further underscores the flexibility in utilization of chromosome end capping pathways [121]. Deficiency in Ku also results in dramatically different outcomes for viability in Arabidopsis and humans, although the key telomeric function of Ku in both organisms is to inhibit t-circle excision [114,117]. In human cells, t-circle excision results in extremely rapid loss of telomeric DNA. In Arabidopsis this process is efficiently counteracted by telomerase, whose activity is further enhanced in the absence of Ku. Nevertheless, the recent characterization of Ku deficient rice indicates that additional factors besides telomere extension by telomerase are likely important as well. While both Arabidopsis and rice Ku deficient plants have elongated telomeres, Arabidopsis plants are healthy whereas rice mutants exhibit growth retardation caused by aberrant telomere function [122]. It will be interesting to determine which factors modulate this distinct response to Ku dysfunction.
While the basic details of plant telomeres are becoming clearer, much still remains unknown. The top priority for the near future is to determine the composition of the plant shelterin complex. The best candidates for TRF homologs are AtTBP1 in Arabidopsis, RTBP1 in rice, and NgTRF1 in tobacco. However, the phenotypes associated with mutations in these genes are not as severe as expected for depleting the major telomere binding complex. Thus, while the high degree of conservation in telomere structure predicts the existence of TRF couterparts in plants, the examples of rapid functional diversification discussed in this review suggest that non-TRF related proteins should not be excluded from the list of putative candidates. Regardless of the proteins that will eventually be found at plant telomeres, the knowledge and experience gained in their identification and research into the mechanisms by which they function will certainly provide insight not only into the telomere biology of plants, but telomere biology in general.
Acknowledgements
We thank Hyun-Soo Cho for providing Fig. 1, Dorthy Shippen for sharing unpublished data, and Anita Kazda for critical reading of the manuscript. Our work on telomeres is supported by the Austrian Science Fund.
References
- 1.McClintock B. The stability of broken ends of chromosomes in Zea mays. Genetics. 1941;26:234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards E.J., Ausubel F.M. Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell. 1988;53:127–136. doi: 10.1016/0092-8674(88)90494-1. [DOI] [PubMed] [Google Scholar]
- 3.Surovtseva Y.V. Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol. Cell. 2009;36:207–218. doi: 10.1016/j.molcel.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riha K., Shippen D.E. Telomere structure, function and maintenance in Arabidopsis. Chromosome Res. 2003;11:263–275. doi: 10.1023/a:1022892010878. [DOI] [PubMed] [Google Scholar]
- 5.Fajkus J., Sykorova E., Leitch A.R. Telomeres in evolution and evolution of telomeres. Chromosome Res. 2005;13:469–479. doi: 10.1007/s10577-005-0997-2. [DOI] [PubMed] [Google Scholar]
- 6.Petracek M.E., Lefebvre P.A., Silflow C.D., Berman J. Chlamydomonas telomere sequences are A+T-rich but contain three consecutive G-C base pairs. Proc. Natl. Acad. Sci. U.S.A. 1990;87:8222–8226. doi: 10.1073/pnas.87.21.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pich U., Fuchs J., Schubert I. How do Alliaceae stabilize their chromosome ends in the absence of TTTAGGG sequences? Chromosome Res. 1996;4:207–213. doi: 10.1007/BF02254961. [DOI] [PubMed] [Google Scholar]
- 8.Sykorova E., Lim K.Y., Chase M.W., Knapp S., Leitch I.J., Leitch A.R., Fajkus J. The absence of Arabidopsis-type telomeres in Cestrum and closely related genera Vestia and Sessea (Solanaceae): first evidence from eudicots. Plant J. 2003;34:283–291. doi: 10.1046/j.1365-313x.2003.01731.x. [DOI] [PubMed] [Google Scholar]
- 9.Adams S.P., Hartman T.P., Lim K.Y., Chase M.W., Bennett M.D., Leitch I.J., Leitch A.R. Loss and recovery of Arabidopsis-type telomere repeat sequences 5′-(TTTAGGG)(n)-3′ in the evolution of a major radiation of flowering plants. Proc. R. Soc. Lond. B Biol. Sci. 2001;268:1541–1546. doi: 10.1098/rspb.2001.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riha K., McKnight T.D., Fajkus J., Vyskot B., Shippen D.E. Analysis of the G-overhang structures on plant telomeres: evidence for two distinct telomere architectures. Plant J. 2000;23:633–641. doi: 10.1046/j.1365-313x.2000.00831.x. [DOI] [PubMed] [Google Scholar]
- 11.Raices M., Verdun R.E., Compton S.A., Haggblom C.I., Griffith J.D., Dillin A., Karlseder J. C. elegans telomeres contain G-strand and C-strand overhangs that are bound by distinct proteins. Cell. 2008;132:745–757. doi: 10.1016/j.cell.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Griffith J.D., Comeau L., Rosenfield S., Stansel R.M., Bianchi A., Moss H., de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 13.Munoz-Jordan J.L., Cross G.A., de Lange T., Griffith J.D. t-Loops at trypanosome telomeres. EMBO J. 2001;20:579–588. doi: 10.1093/emboj/20.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesare A.J., Quinney N., Willcox S., Subramanian D., Griffith J.D. Telomere looping in P. sativum (common garden pea) Plant J. 2003;36:271–279. doi: 10.1046/j.1365-313x.2003.01882.x. [DOI] [PubMed] [Google Scholar]
- 15.Heacock M., Spangler E., Riha K., Puizina J., Shippen D.E. Molecular analysis of telomere fusions in Arabidopsis: multiple pathways for chromosome end-joining. EMBO J. 2004;23:2304–2313. doi: 10.1038/sj.emboj.7600236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capper R., Britt-Compton B., Tankimanova M., Rowson J., Letsolo B., Man S., Haughton M., Baird D.M. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–2508. doi: 10.1101/gad.439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heacock M.L., Idol R.A., Friesner J.D., Britt A.B., Shippen D.E. Telomere dynamics and fusion of critically shortened telomeres in plants lacking DNA ligase IV. Nucleic Acids Res. 2007;35:6490–6500. doi: 10.1093/nar/gkm472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L., Blackburn E.H. Human cancer cells harbor T-stumps, a distinct class of extremely short telomeres. Mol. Cell. 2007;28:315–327. doi: 10.1016/j.molcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald M.S., Riha K., Gao F., Ren S., McKnight T.D., Shippen D.E. Disruption of the telomerase catalytic subunit gene from Arabidopsis inactivates telomerase and leads to a slow loss of telomeric DNA. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14813–14818. doi: 10.1073/pnas.96.26.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oguchi K., Liu H., Tamura K., Takahashi H. Molecular cloning and characterization of AtTERT, a telomerase reverse transcriptase homolog in Arabidopsis thaliana. FEBS Lett. 1999;457:465–469. doi: 10.1016/s0014-5793(99)01083-2. [DOI] [PubMed] [Google Scholar]
- 21.Sykorova E., Leitch A.R., Fajkus J. Asparagales telomerases which synthesize the human type of telomeres. Plant Mol. Biol. 2006;60:633–646. doi: 10.1007/s11103-005-5091-9. [DOI] [PubMed] [Google Scholar]
- 22.Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat. Rev. Mol. Cell Biol. 2006;7:484–494. doi: 10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell J.R., Wood E., Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 24.Kannan K., Nelson A.D., Shippen D.E. Dyskerin is a component of the Arabidopsis telomerase RNP required for telomere maintenance. Mol. Cell Biol. 2008;28:2332–2341. doi: 10.1128/MCB.01490-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald M.S., McKnight T.D., Shippen D.E. Characterization and developmental patterns of telomerase expression in plants. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14422–14427. doi: 10.1073/pnas.93.25.14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller K., Kilian A., Piatyszek M.A., Kleinhofs A. Telomerase activity in plant extracts. Mol. Gen. Genet. 1996;252:342–345. doi: 10.1007/BF02173780. [DOI] [PubMed] [Google Scholar]
- 27.Riha K., Fajkus J., Siroky J., Vyskot B. Developmental control of telomere lengths and telomerase activity in plants. Plant Cell. 1998;10:1691–1698. doi: 10.1105/tpc.10.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura K., Liu H., Takahashi H. Auxin induction of cell cycle regulated activity of tobacco telomerase. J. Biol. Chem. 1999;274:20997–21002. doi: 10.1074/jbc.274.30.20997. [DOI] [PubMed] [Google Scholar]
- 29.Yang S.W., Jin E., Chung I.K., Kim W.T. Cell cycle-dependent regulation of telomerase activity by auxin, abscisic acid and protein phosphorylation in tobacco BY-2 suspension culture cells. Plant J. 2002;29:617–626. doi: 10.1046/j.0960-7412.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- 30.Ren S., Johnston J.S., Shippen D.E., McKnight T.D. Telomerase activator1 induces telomerase activity and potentiates responses to auxin in Arabidopsis. Plant Cell. 2004;16:2910–2922. doi: 10.1105/tpc.104.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren S., Mandadi K.K., Boedeker A.L., Rathore K.S., McKnight T.D. Regulation of telomerase in Arabidopsis by BT2, an apparent target of telomerase activator1. Plant Cell. 2007;19:23–31. doi: 10.1105/tpc.106.044321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heller-Uszynska K., Schnippenkoetter W., Kilian A. Cloning and characterization of rice (Oryza sativa L) telomerase reverse transcriptase, which reveals complex splicing patterns. Plant J. 2002;31:75–86. doi: 10.1046/j.1365-313x.2001.01337.x. [DOI] [PubMed] [Google Scholar]
- 33.Oguchi K., Tamura K., Takahashi H. Characterization of Oryza sativa telomerase reverse transcriptase and possible role of its phosphorylation in the control of telomerase activity. Gene. 2004;342:57–66. doi: 10.1016/j.gene.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Rossignol P., Collier S., Bush M., Shaw P., Doonan J.H. Arabidopsis POT1A interacts with TERT-V(I8), an N-terminal splicing variant of telomerase. J. Cell Sci. 2007;120:3678–3687. doi: 10.1242/jcs.004119. [DOI] [PubMed] [Google Scholar]
- 35.Marcand S., Gilson E., Shore D. A protein-counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 36.Shakirov E.V., Shippen D.E. Length regulation and dynamics of individual telomere tracts in wild-type Arabidopsis. Plant Cell. 2004;16:1959–1967. doi: 10.1105/tpc.104.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson J.M., Shippen D.E. Telomere rapid deletion regulates telomere length in Arabidopsis thaliana. Mol. Cell Biol. 2006 doi: 10.1128/MCB.02059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conti E., Izaurralde E. Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. 2005;17:316–325. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Azzalin C.M., Reichenbach P., Khoriauli L., Giulotto E., Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 40.Riehs N. Arabidopsis SMG7 protein is required for exit from meiosis. J. Cell Sci. 2008;121:2208–2216. doi: 10.1242/jcs.027862. [DOI] [PubMed] [Google Scholar]
- 41.Surovtseva Y.V., Shakirov E.V., Vespa L., Osbun N., Song X., Shippen D.E. Arabidopsis POT1 associates with the telomerase RNP and is required for telomere maintenance. EMBO J. 2007;26:3653–3661. doi: 10.1038/sj.emboj.7601792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 43.Li B., Oestreich S., de Lange T. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101:471–483. doi: 10.1016/s0092-8674(00)80858-2. [DOI] [PubMed] [Google Scholar]
- 44.Palm W., de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi A., Shore D. How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol. Cell. 2008;31:153–165. doi: 10.1016/j.molcel.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Miyoshi T., Kanoh J., Saito M., Ishikawa F. Fission yeast Pot1- Tpp1 protects telomeres and regulates telomere length. Science. 2008;320:1341–1344. doi: 10.1126/science.1154819. [DOI] [PubMed] [Google Scholar]
- 47.Tomita K., Cooper J.P. Fission yeast Ccq1 is telomerase recruiter and local checkpoint controller. Genes Dev. 2008;22:3461–3474. doi: 10.1101/gad.498608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manevski A., Bertoni G., Bardet C., Tremousaygue D., Lescure B. In synergy with various cis-acting elements, plant interstitial telomere motifs regulate gene expression in Arabidopsis root meristems. FEBS Lett. 2000;483:43–46. doi: 10.1016/s0014-5793(00)02056-1. [DOI] [PubMed] [Google Scholar]
- 49.Tremousaygue D., Manevski A., Bardet C., Lescure N., Lescure B. Plant interstitial telomere motifs participate in the control of gene expression in root meristems. Plant J. 1999;20:553–561. doi: 10.1046/j.1365-313x.1999.00627.x. [DOI] [PubMed] [Google Scholar]
- 50.Zellinger B., Riha K. Composition of plant telomeres. Biochim. Biophys. Acta. 2007;1769:399–409. doi: 10.1016/j.bbaexp.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Hirata Y., Suzuki C., Sakai S. Characterization and gene cloning of telomere-binding protein from tobacco BY-2 cells. Plant Physiol. Biochem. 2004;42:7–14. doi: 10.1016/j.plaphy.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Kwon C., Kwon K., Chung I.K., Kim S.Y., Cho M.H., Kang B.G. Characterization of single stranded telomeric DNA-binding proteins in cultured soybean (Glycine max) cells. Mol. Cell. 2004;17:503–508. [PubMed] [Google Scholar]
- 53.White M.K., Johnson E.M., Khalili K. Multiple roles for Puralpha in cellular and viral regulation. Cell Cycle. 2009;8:1–7. doi: 10.4161/cc.8.3.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossignol P., Stevens R., Perennes C., Jasinski S., Cella R., Tremousaygue D., Bergounioux C. AtE2F-a and AtDP-a, members of the E2F family of transcription factors, induce Arabidopsis leaf cells to re-enter S phase. Mol. Genet. Genomics. 2002;266:995–1003. doi: 10.1007/s00438-001-0624-7. [DOI] [PubMed] [Google Scholar]
- 55.Tremousaygue D., Garnier L., Bardet C., Dabos P., Herve C., Lescure B. Internal telomeric repeats and ‘TCP domain’ protein-binding sites cooperate to regulate gene expression in Arabidopsis thaliana cycling cells. Plant J. 2003;33:957–966. doi: 10.1046/j.1365-313x.2003.01682.x. [DOI] [PubMed] [Google Scholar]
- 56.Brun C., Marcand S., Gilson E. Proteins that bind to double-stranded regions of telomeric DNA. Trends Cell Biol. 1997;7:317–324. doi: 10.1016/S0962-8924(97)01092-1. [DOI] [PubMed] [Google Scholar]
- 57.Court R., Chapman L., Fairall L., Rhodes D. How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high- resolution crystal structures. EMBO Rep. 2005;6:39–45. doi: 10.1038/sj.embor.7400314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marian C.O. The maize Single myb histone 1 gene, Smh1, belongs to a novel gene family and encodes a protein that binds telomere DNA repeats in vitro. Plant Physiol. 2003;133:1336–1350. doi: 10.1104/pp.103.026856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karamysheva Z.N., Surovtseva Y.V., Vespa L., Shakirov E.V., Shippen D.E. A C-terminal Myb extension domain defines a novel family of double-strand telomeric DNA-binding proteins in Arabidopsis. J. Biol. Chem. 2004;279:47799–47807. doi: 10.1074/jbc.M407938200. [DOI] [PubMed] [Google Scholar]
- 60.Chen C.M., Wang C.T., Ho C.H. A plant gene encoding a Myb-like protein that binds telomeric GGTTAG repeats in vitro. J. Biol. Chem. 2001;276:16511–16519. doi: 10.1074/jbc.M009659200. [DOI] [PubMed] [Google Scholar]
- 61.Hwang M.G., Chung I.K., Kang B.G., Cho M.H. Sequence-specific binding property of Arabidopsis thaliana telomeric DNA binding protein 1 (AtTBP1) FEBS Lett. 2001;503:35–40. doi: 10.1016/s0014-5793(01)02685-0. [DOI] [PubMed] [Google Scholar]
- 62.Yang S.W., Kim D.H., Lee J.J., Chun Y.J., Lee J.H., Kim Y.J., Chung I.K., Kim W.T. Expression of the telomeric repeat binding factor gene NgTRF1 is closely coordinated with the cell division program in tobacco BY-2 suspension culture cells. J. Biol. Chem. 2003;278:21395–21407. doi: 10.1074/jbc.M209973200. [DOI] [PubMed] [Google Scholar]
- 63.Yu E.Y., Kim S.E., Kim J.H., Ko J.H., Cho M.H., Chung I.K. Sequence-specific DNA recognition by the Myb-like domain of plant telomeric protein RTBP1. J. Biol. Chem. 2000;275:24208–24214. doi: 10.1074/jbc.M003250200. [DOI] [PubMed] [Google Scholar]
- 64.Ko S. Structure of the DNA-binding domain of NgTRF1 reveals unique features of plant telomere-binding proteins. Nucleic Acids Res. 2008;36:2739–2755. doi: 10.1093/nar/gkn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sue S.C., Hsiao H.H., Chung B.C., Cheng Y.H., Hsueh K.L., Chen C.M., Ho C.H., Huang T.H. Solution structure of the Arabidopsis thaliana telomeric repeat-binding protein DNA binding domain: a new fold with an additional C-terminal helix. J. Mol. Biol. 2006;356:72–85. doi: 10.1016/j.jmb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Ko S. Solution structure of the DNA binding domain of rice telomere binding protein RTBP1. Biochemistry. 2009;48:827–838. doi: 10.1021/bi801270g. [DOI] [PubMed] [Google Scholar]
- 67.Hwang M.G., Cho M.H. Arabidopsis thaliana telomeric DNA-binding protein 1 is required for telomere length homeostasis and its Myb-extension domain stabilizes plant telomeric DNA binding. Nucleic Acids Res. 2007;35:1333–1342. doi: 10.1093/nar/gkm043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang S.W., Kim S.K., Kim W.T. Perturbation of NgTRF1 expression induces apoptosis-like cell death in tobacco BY-2 cells and implicates NgTRF1 in the control of telomere length and stability. Plant Cell. 2004 doi: 10.1105/tpc.104.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong J.P., Byun M.Y., Koo D.H., An K., Bang J.W., Chung I.K., An G., Kim W.T. Suppression of rice telomere binding protein 1 results in severe and gradual developmental defects accompanied by genome instability in rice. Plant Cell. 2007;19:1770–1781. doi: 10.1105/tpc.107.051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shakirov E.V., Salzberg S.L., Alam M., Shippen D.E. Analysis of Carica papaya telomeres and telomere-associated proteins: insights into evolution of telomere maintenance in Brassicales. Tropical Plant Biol. 2008:202–215. doi: 10.1007/s12042-008-9018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baumann P., Cech T.R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science. 2001;292:1171–1175. doi: 10.1126/science.1060036. [DOI] [PubMed] [Google Scholar]
- 72.Denchi E.L., de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 73.Churikov D., Price C.M. Pot1 and cell cycle progression cooperate in telomere length regulation. Nat. Struct. Mol. Biol. 2008;15:79–84. doi: 10.1038/nsmb1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xin H., Liu D., Wan M., Safari A., Kim H., Sun W., O’Connor M.S., Songyang Z. TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature. 2007;445:559–562. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
- 75.Wang F., Podell E.R., Zaug A.J., Yang Y., Baciu P., Cech T.R., Lei M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- 76.Hockemeyer D., Daniels J.P., Takai H., de Lange T. Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126:63–77. doi: 10.1016/j.cell.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 77.Shakirov E.V., Surovtseva Y.V., Osbun N., Shippen D.E. The Arabidopsis Pot1 and Pot2 proteins function in telomere length homeostasis and chromosome end protection. Mol. Cell Biol. 2005;25:7725–7733. doi: 10.1128/MCB.25.17.7725-7733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tani A., Murata M. Alternative splicing of Pot1 (Protection of telomere)-like genes in Arabidopsis thaliana. Genes Genet. Syst. 2005;80:41–48. doi: 10.1266/ggs.80.41. [DOI] [PubMed] [Google Scholar]
- 79.Shakirov E.V., McKnight T.D., Shippen D.E. POT1-independent single-strand telomeric DNA binding activities in Brassicaceae. Plant J. 2009;58:1004–1015. doi: 10.1111/j.1365-313X.2009.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shakirov E.V., Song X., Joseph J.A., Shippen D.E. POT1 proteins in green algae and land plants: DNA-binding properties and evidence of co-evolution with telomeric DNA. Nucleic Acids Res. 2009;37:7455–7467. doi: 10.1093/nar/gkp785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao H., Cervantes R.B., Mandell E.K., Otero J.H., Lundblad V. RPA-like proteins mediate yeast telomere function. Nat. Struct. Mol. Biol. 2007;14:208–214. doi: 10.1038/nsmb1205. [DOI] [PubMed] [Google Scholar]
- 82.Sun J., Yu E.Y., Yang Y., Confer L.A., Sun S.H., Wan K., Lue N.F., Lei M. Stn1-Ten1 is an Rpa2-Rpa3-like complex at telomeres. Genes Dev. 2009;23:2900–2914. doi: 10.1101/gad.1851909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grandin N., Damon C., Charbonneau M. Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13. EMBO J. 2001;20:1173–1183. doi: 10.1093/emboj/20.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grandin N., Reed S.I., Charbonneau M. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 1997;11:512–527. doi: 10.1101/gad.11.4.512. [DOI] [PubMed] [Google Scholar]
- 85.Nugent C.I., Hughes T.R., Lue N.F., Lundblad V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science. 1996;274:249–252. doi: 10.1126/science.274.5285.249. [DOI] [PubMed] [Google Scholar]
- 86.Qi H., Zakian V.A. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated est1 protein. Genes Dev. 2000;14:1777–1788. [PMC free article] [PubMed] [Google Scholar]
- 87.Martin V., Du L.L., Rozenzhak S., Russell P. Protection of telomeres by a conserved Stn1–Ten1 complex. Proc. Natl. Acad. Sci. U.S.A. 2007;104:14038–14043. doi: 10.1073/pnas.0705497104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song X., Leehy K., Warrington R.T., Lamb J.C., Surovtseva Y.V., Shippen D.E. STN1 protects chromosome ends in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19815–19820. doi: 10.1073/pnas.0807867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miyake Y., Nakamura M., Nabetani A., Shimamura S., Tamura M., Yonehara S., Saito M., Ishikawa F. RPA-like mammalian Ctc1–Stn1–Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell. 2009;36:193–206. doi: 10.1016/j.molcel.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Takashi Y., Kobayashi Y., Tanaka K., Tamura K. Arabidopsis replication protein A 70a is required for DNA damage response and telomere length homeostasis. Plant Cell. Physiol. 2009;50:1965–1976. doi: 10.1093/pcp/pcp140. [DOI] [PubMed] [Google Scholar]
- 91.Verdun R.E., Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 92.Gilson E., Geli V. How telomeres are replicated. Nat. Rev. Mol. Cell Biol. 2007;8:825–838. doi: 10.1038/nrm2259. [DOI] [PubMed] [Google Scholar]
- 93.Sabourin M., Tuzon C.T., Zakian V.A. Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol. Cell. 2007;27:550–561. doi: 10.1016/j.molcel.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang M., Arneric M., Lingner J. Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev. 2007;21:2485–2494. doi: 10.1101/gad.1588807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larrivee M., LeBel C., Wellinger R.J. The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev. 2004;18:1391–1396. doi: 10.1101/gad.1199404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng Y., Guo X., Ferguson D.O., Chang S. Multiple roles for MRE11 at uncapped telomeres. Nature. 2009;460:914–918. doi: 10.1038/nature08196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vannier J.B., Depeiges A., White C., Gallego M.E. Two roles for Rad50 in telomere maintenance. EMBO J. 2006;25:4577–4585. doi: 10.1038/sj.emboj.7601345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Puizina J., Siroky J., Mokros P., Schweizer D., Riha K. Mre11 deficiency in Arabidopsis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell. 2004;16:1968–1978. doi: 10.1105/tpc.104.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vespa L., Couvillion M., Spangler E., Shippen D.E. ATM and ATR make distinct contributions to chromosome end protection and the maintenance of telomeric DNA in Arabidopsis. Genes Dev. 2005;19:2111–2115. doi: 10.1101/gad.1333805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vespa L., Warrington R.T., Mokros P., Siroky J., Shippen D.E. ATM regulates the length of individual telomere tracts in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:18145–18150. doi: 10.1073/pnas.0704466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vannier J.B., Depeiges A., White C., Gallego M.E. ERCC1/XPF protects short telomeres from homologous recombination in Arabidopsis thaliana. PLoS Genet. 2009;5:e1000380. doi: 10.1371/journal.pgen.1000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu X.D., Niedernhofer L., Kuster B., Mann M., Hoeijmakers J.H., de Lange T. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol. Cell. 2003;12:1489–1498. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]
- 103.Riha K., Heacock M.L., Shippen D.E. The role of the nonhomologous end-Joining DNA double-strand break repair pathway in telomere biology. Annu. Rev. Genet. 2006;40:237–277. doi: 10.1146/annurev.genet.39.110304.095755. [DOI] [PubMed] [Google Scholar]
- 104.Fisher T.S., Zakian V.A. Ku: a multifunctional protein involved in telomere maintenance. DNA Repair (Amst) 2005;4:1215–1226. doi: 10.1016/j.dnarep.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 105.Gravel S., Larrivee M., Labrecque P., Wellinger R.J. Yeast Ku as a regulator of chromosomal DNA end structure. Science. 1998;280:741–744. doi: 10.1126/science.280.5364.741. [DOI] [PubMed] [Google Scholar]
- 106.Fisher T.S., Taggart A.K., Zakian V.A. Cell cycle-dependent regulation of yeast telomerase by Ku. Nat. Struct. Mol. Biol. 2004;11:1198–1205. doi: 10.1038/nsmb854. [DOI] [PubMed] [Google Scholar]
- 107.Stellwagen A.E., Haimberger Z.W., Veatch J.R., Gottschling D.E. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 2003;17:2384–2395. doi: 10.1101/gad.1125903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.d’Adda di Fagagna F., Hande M.P., Tong W.M., Roth D., Lansdorp P.M., Wang Z.Q., Jackson S.P. Effects of DNA nonhomologous end-joining factors on telomere length and chromosomal stability in mammalian cells. Curr. Biol. 2001;11:1192–1196. doi: 10.1016/s0960-9822(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 109.Espejel S., Franco S., Rodriguez-Perales S., Bouffler S.D., Cigudosa J.C., Blasco M.A. Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J. 2002;21:2207–2219. doi: 10.1093/emboj/21.9.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bundock P., van Attikum H., Hooykaas P. Increased telomere length and hypersensitivity to DNA damaging agents in an Arabidopsis KU70 mutant. Nucleic Acids Res. 2002;30:3395–3400. doi: 10.1093/nar/gkf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Riha K., Watson J.M., Parkey J., Shippen D.E. Telomere length deregulation and enhanced sensitivity to genotoxic stress in Arabidopsis mutants deficient in Ku70. EMBO J. 2002;21:2819–2826. doi: 10.1093/emboj/21.11.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Riha K., Shippen D.E. Ku is required for telomeric C-rich strand maintenance but not for end-to-end chromosome fusions in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:611–615. doi: 10.1073/pnas.0236128100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gallego M.E., Jalut N., White C.I. Telomerase dependence of telomere lengthening in Ku80 mutant Arabidopsis. Plant Cell. 2003;15:782–789. doi: 10.1105/tpc.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zellinger B., Akimcheva S., Puizina J., Schirato M., Riha K. Ku suppresses formation of telomeric circles and alternative telomere lengthening in Arabidopsis. Mol. Cell. 2007;27:163–169. doi: 10.1016/j.molcel.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 115.Watson J.M., Bulankova P., Riha K., Shippen D.E., Vyskot B. Telomerase-independent cell survival in Arabidopsis thaliana. Plant J. 2005;43:662–674. doi: 10.1111/j.1365-313X.2005.02479.x. [DOI] [PubMed] [Google Scholar]
- 116.Akimcheva S., Zellinger B., Riha K. Genome stability in Arabidopsis cells exhibiting alternative lengthening of telomeres. Cytogenet. Genome Res. 2008;122:388–395. doi: 10.1159/000167827. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y., Ghosh G., Hendrickson E.A. Ku86 represses lethal telomere deletion events in human somatic cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:12430–12435. doi: 10.1073/pnas.0903362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lendvay T.S., Morris D.K., Sah J., Balasubramanian B., Lundblad V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lundblad V., Szostak J.W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 120.Ahmed S., Hodgkin J. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature. 2000;403:159–164. doi: 10.1038/35003120. [DOI] [PubMed] [Google Scholar]
- 121.Larrive M., Wellinger R.J. Telomerase- and capping-independent yeast survivors with alternate telomere states. Nat. Cell Biol. 2006;8:741. doi: 10.1038/ncb1429. [DOI] [PubMed] [Google Scholar]
- 122.Hong J.P., Byun M.Y., An K., Yang S.J., An G., Kim W.T. OsKu70 is associated with developmental growth and genome stability in rice. Plant Physiol. 2010;152:374–387. doi: 10.1104/pp.109.150391. [DOI] [PMC free article] [PubMed] [Google Scholar]


