Abstract
Objectives:
This study aimed to characterize the prevalence of antibiotic resistance in Escherichia coli isolates from the fecal samples of fishery workers who work in fish farms and often use antibiotics for the feeding fishes.
Methods:
Seventy-three E. coli strains isolated from the fecal samples of fishery workers and 180 isolates from a control group of restaurant workers were tested for antibiotic resistance by agar disk diffusion with 16 antimicrobial agents.
Results:
About 30% of isolates from each group showed antimicrobial resistance to ampicillin, and 60% of isolates from fishery workers and 41% from restaurant workers were resistant to tetracycline. The isolates showed higher resistance to cephalothin and cefoxitin than to other cephem antibiotics and to gentamicin than to other aminogycosides. Our data indicated that fecal E. coli isolates from fishery workers showed higher antibiotic resistance than those of non-fishery workers (restaurant workers), especially to cephalothin, tetracycline, and trimethoprim–sulfamethoxazole (p < 0.05). However, rates of multidrug resistance were similar among the fishery workers and restaurant workers.
Conclusion:
Frequent use of antibiotics may cause increased antibiotic resistance in the human microbiome.
Keywords: antimicrobial resistance, Escherichia coli, fishery workers
1. Introduction
Microorganisms with increased antibiotic resistant are a significant health problem that may be worsened by frequent use of antibiotics in hospital [1,2] or in animal feed [3–6]. However, the antibiotic resistance of microorganisms isolated from humans who add antibiotics to animal feed is not well known. Therefore, we have studied the antibiotic resistance of commensal Escherichia coli in volunteers over several years and have demonstrated that higher resistance to the antibiotics most frequently prescribed for diarrhea was found in the isolates of patients with diarrhea and apparently healthy individuals [7] and in livestock workers who often use antibiotics [8].
Here, we report the prevalence of antibiotic resistance in fecal E. coli isolates from healthy fishery workers who often use antibiotics for the feeding fishes and compare these data with isolates obtained from a control group of healthy individuals from the same region.
2. Materials and Methods
2.1. Fecal samples and E. coli isolated from samples
We collected fecal samples from 73 fishery workers and 180 healthy restaurant workers in the same region for comparison (Table 1). The fishery workers were 30–50 years of age and the restaurant workers were 20–50 years of age. The samples were plated on to MacConkey agar directly or occasionally after enrichment in tryptic soy broth (TSB) containing vancomycin (Sigma Chemical Co., St. Louis, MO, USA). Bacteria found on MacConkey agar were assayed using the API20E system (bioMérieux, Marcy l’Étoile, France). For individual samples, one or two E. coli isolates were selected randomly for the purpose of determining susceptibility.
Table 1.
Fecal samples collected for this study
| Age groups (years) | Fishery workers (n = 73)
|
Healthy individuals (n = 180)
|
||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 1–10 | 0 | 0 | 0 | 0 |
| 11–20 | 0 | 0 | 0 | 0 |
| 21–30 | 2 | 0 | 4 | 6 |
| 31–40 | 16 | 0 | 9 | 35 |
| 41–50 | 23 | 2 | 13 | 62 |
| 51–60 | 18 | 3 | 10 | 32 |
| 61+ | 8 | 1 | 2 | 7 |
| Total | 67 | 6 | 38 | 142 |
2.2. Antimicrobial susceptibility test
Antibiotic susceptibility was determined by disk diffusion according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (formerly NCCLS) [9]. Antimicrobial susceptibility was determined by agar disk diffusion (Kirby-Bauer method) using MuellereHinton agar (Difco, MI, USA). The following antibiotics (Oxoid, Hampshire, UK) were tested: ampicillin–sulbactam, ampicillin, tetracycline, aztreonam, cefotetan, cefepime, cefoxitin, cefotaxime, tobramycin, trimethoprim/sulfamethoxazole, cephalothin, imipenem, gentamicin, amikacin, piperacillin/tazobactam, and netilamicin. E. coli ATCC 25922 and E. coli ATCC 35218 were used as quality controls.
2.3. Statistical analysis
The antimicrobial susceptibility data are expressed as percentages or frequencies of the avian or human isolates. A one-way analysis of variance or χ2 test was used to estimate overall difference between the percentages or frequencies of resistance between avian and human E. coli isolates. In all cases, p < 0.05 was regarded as statistically significant.
3. Results
A total of 258 isolates derived from 73 fishery workers and 180 isolates from restaurant workers were used to examine the antibiotic resistance of commensal E. coli strains. Among the fishery workers, more samples were collected from men than from women, while among the restaurant workers, more samples were collected from women than from men (Table 1).
About 30% of isolates from each group showed antimicrobial resistance to ampicillin and 60% of isolates from fishery workers and 41% from restaurant workers were resistant to tetracycline. The isolates of fishery workers showed higher resistance to cephalothin than to other cephem antibiotics (36%). However, the isolates of restaurant workers showed higher resistance to cefoxitin than to other antibiotics (13%). High resistance to trimethoprim/sulfamethoxazole was found in the isolates of fishery workers but not in the isolates of restaurant workers. Among aminoglycosides, the highest resistance was to gentamicin. There was a trend towards higher resistance among fishery workers than restaurant workers, especially to cephalothin, tetracycline, and trimethoprim–sulfamethoxazole (p < 0.05) (Table 2).
Table 2.
Antibiotic resistance rates of the isolates in each group
| Antimicrobial agents | Antibiotic resistances (%) of isolates
|
|
|---|---|---|
| Fishery workers | Control groups | |
| β-Lactams | ||
| Ampicillin | 29 | 34 |
| β-Lactam/β-lactamase inhibitor combinations | ||
| Ampicillin–sulbactam | 14 | 23 |
| Piperacillin–tazobactam | 0 | 0 |
| Cephems | ||
| Cephalothin* | 36 | 0 |
| Cefepime | 0 | 0 |
| Cefotetan | 1 | 0 |
| Cefotaxime | 0 | 1 |
| Cefoxitin | 5 | 13 |
| Carbapenems | ||
| Imipenem | 0 | 0 |
| Aminoglycosides | ||
| Amikacin | 0 | 0 |
| Gentamicin | 15 | 9 |
| Tobramycin | 1 | 5 |
| Netilamicin | 1 | 0 |
| Tetracyclines | ||
| Tetracycline* | 60 | 41 |
| Monobactams | ||
| Aztreonam | 0 | 2 |
| Folate pathway inhibitors | ||
| Trimethoprim–sulfamethoxazole* | 29 | 1 |
p < 0.05.
As shown in Figure 1, the multiple resistance patterns in the E. coli isolates of fishery workers are similar to those of restaurant workers. Overall, 55% of fishery workers and 49% of restaurant workers showed resistance to two or more antibiotics. Approximately 12% of the isolates showed resistance to four or more antibiotics. The most frequently observed resistance patterns in the fishery workers were to tetracycline/sulfamethoxazole (6 isolates) and to tetracycline/sulfa-methoxazole/cephalothin (6 isolates). In the restaurant workers, the most frequently observed resistance pattern was to cefoxitin/tetracycline (10 isolates). The samples with resistance to the most antibiotics were found in a fishery worker isolate (resistance to 6 antibiotics: ampicillin–sulbactam, ampicillin, tetracycline, sulfa-methoxazole, cefoxitin, and gentamicin) and in the isolates of four restaurant workers (resistance to 6 antibiotics: ampicillin, tetracycline, tobramycin, sulfa-methoxazole, cefoxitin, and gentamicin).
Figure 1.
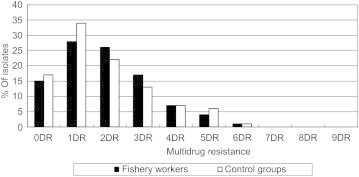
Antibiotic multi-resistance patterns of Escherichia coli strains isolated from fishery workers and restaurant workers. DR = drug resistance.
4. Discussion
Antimicrobial resistance of intestinal bacteria isolated from food animals due to antibiotic usage is an increasing global problem in livestock environments [6,10,11]. The fishery industry is a significant economic force in Korea but there is little information regarding the antibiotic resistance of fecal E. coli isolates from fishery workers. Therefore, in this study, the prevalence of antibiotic resistance in fecal E. coli isolates from healthy fishery workers was examined.
As shown, fecal E. coli isolates from fishery workers showed higher antibiotic resistance than those of nonfishery workers (restaurant workers), especially to cephalothin, tetracycline, and trimethoprim-sulfamethoxazole (p < 0.05). This may be partly due to frequent feeding of antibiotics to fish by the fishery workers. Tetracycline is a common first-line antibiotic for many domestic animals and is used as a growth promoter and infection control agent, and is often used before the antibiotic resistance profile of a pathogen has been determined [12–14].This result agrees with the findings of other research [8,15].In this study, we found that fishery workers often use neomycin, destomycin A, and hygromycin B with feeds. The finding of a higher prevalence of resistance in fishery worker isolates suggests that antimicrobial use in fish may be a factor in the emergence of antimicrobial resistance in the human fecal E. coli isolates.
Other studies by us have reported that there is higher multidrug resistance in the E. coli isolates of patients with diarrhea [7] and in livestock workers who often use antibiotics for the feeding animals [8]. However, in this study, the multiple resistance patterns in the E. coli isolates of fishery workers are similar to those of restaurant workers.
In conclusion, the higher resistance in fishery workers may be explained by the greater exposure of these workers to antibiotics. The information gathered may help us manage the evolution of antimicrobial resistance in the future.
References
- 1.van den Bogaard AE, Stobberingh EE. Antibiotic usage in animals – impact on bacterial resistance and public health. Drugs. 1999 Oct;58(4):589–607. doi: 10.2165/00003495-199958040-00002. [DOI] [PubMed] [Google Scholar]
- 2.Witte W. Medical consequences of antibiotic use in agriculture. Science. 1998 Feb 13;279(53535):996–7. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- 3.Alexander TW, Yanke LJ, Topp E, et al. Effect of subtherapeutic administration of antibiotics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Appl Environ Microbiol. 2008 Jul;74(14):4405–16. doi: 10.1128/AEM.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham JP, Boland JJ, Silbergeld E. Growth promoting antibiotics in food animal production. Public Health Rep. 2007;122:79–87. doi: 10.1177/003335490712200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bywater RJ. Veterinary use of antimicrobials and emergence of resistance in zoonotic and sentinel bacteria in the EU. J Vet Med B. 2004 Oct-Nov;5(8–9):361–3. doi: 10.1111/j.1439-0450.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 6.Teuber M. Veterinary use and antibiotic resistance. Curr Opin Microbiol. 2001 Oct;4(5):493–9. doi: 10.1016/s1369-5274(00)00241-1. [DOI] [PubMed] [Google Scholar]
- 7.Cho S-H, Lim Y-S, Park MS, et al. Prevalence of antibiotic resistance in Escherichia coli fecal isolates from healthy persons and patients with diarrhea. Osong Pub Health Rsrch Persp. 2011 Jun;2(1):41–5. doi: 10.1016/j.phrp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho S-H, Lim Y-S, Kang Y-H. Comparison of antimicrobial resistance in Escherichia coli strains isolated from healthy poultry and swine farm workers using antibiotics in Korea. Osong Pub Health Rsrch Persp. 2012 Sep;3(3):151–5. doi: 10.1016/j.phrp.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. Twenty-Second Informational Supplement. CLSI Document M100-MS19. Wayne, PA: CLSI; 2012. [Google Scholar]
- 10.Smith J, Drum DJV, Dai Y, et al. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl Environ Microbiol. 2007 Mar;73(5):1404–14. doi: 10.1128/AEM.01193-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder CM, Zhao C, DebRoy C, et al. Antimicrobial resistance of Escherichia coli O157:H7 isolated from humans, cattle, swine and food. Appl Environ Microbiol. 2002 Feb;68(2):576–81. doi: 10.1128/AEM.68.2.576-581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chopra I. New developments in tetracycline antibiotics: glycylcyclines and tetracycline efflux pump inhibitors. Drug Resist Updat. 2002 Jul-Aug;5(3–4):119–25. doi: 10.1016/s1368-7646(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 13.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001 Jun;65(2):232–60. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy SB. Antibiotic resistance: consequences of action. Clin Infect Dis. 2001 Sep 15;33(Suppl. 3):S124–9. doi: 10.1086/321837. [DOI] [PubMed] [Google Scholar]
- 15.van den Bogaard AE, London N, Driessen C, Stobberingh EE. Antibiotic resistance of fecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J Antimicrob Chemother. 2001 Jun;47(6):763–71. doi: 10.1093/jac/47.6.763. [DOI] [PubMed] [Google Scholar]


