Abstract
A growing body of evidence implicates human oral bacteria in the etiology of oral and gastrointestinal cancers. Epidemiological studies consistently report increased risks of these cancers in men and women with periodontal disease or tooth loss, conditions caused by oral bacteria. More than 700 bacterial species inhabit the oral cavity, including at least 11 bacterial phyla and 70 genera. Oral bacteria may activate alcohol and smoking-related carcinogens locally or act systemically, through chronic inflammation. High-throughput genetic-based assays now make it possible to comprehensively survey the human oral microbiome, the totality of bacteria in the oral cavity. Establishing the association of the oral microbiome with cancer risk may lead to significant advances in understanding of cancer etiology, potentially opening a new research paradigm for cancer prevention.
Keywords: Human microbiome, Oral and gastrointestinal cancer, Assay, Epidemiology
Introduction
The NIH Human Microbiome Project, launched as a part of the NIH Roadmap for Medical Research, pointed to the need to accelerate our understanding of how our bodies and microorganisms interact to influence health and disease [1]. It is hypothesized that the human microbiome is associated with human health and that dysbiosis can lead to a variety of diseases. Until recently, studies of the human microbiota have been based on bacterial culture, which we now know is limited and insensitive, because large numbers of nonculturables (up to 80%) cannot be studied in culture [2, 3]. The development of high-throughput genetic-based microbiome assays expedited studies to comprehensively examine the human microbiome, the totality of human microbiota, including nonculturable organisms. In the context of these developments, it is becoming possible to test the hypothesis that the oral microbiome and its imbalances are associated etiologically with cancers of the oral and gastrointestinal tracts.
Biological plausibility of the oral bacteria: oral and gastrointestinal cancer relationship
Epidemiological study of periodontal disease
It is well established that oral bacteria are critical to the development of periodontal disease and tooth loss [4], and these oral diseases have been related in a number of studies to the risk of oral and gastrointestinal cancers, with the most consistent increased risks noted in studies of oral and esophageal cancers, followed by evidence for pancreatic and gastric cancer (reviewed in [5, 6]); these relationships tend to persist after taking confounding factors into account—e.g., smoking, body mass index, and socioeconomic status [5–9]. The underlying mechanism for the associations between oral health status and these cancers is not completely understood, yet it is possible that these associations of cancers with oral disease may reflect a stronger underlying association of cancer with as yet unexamined oral microbiome profiles.
Local metabolism of carcinogens by oral microbiota
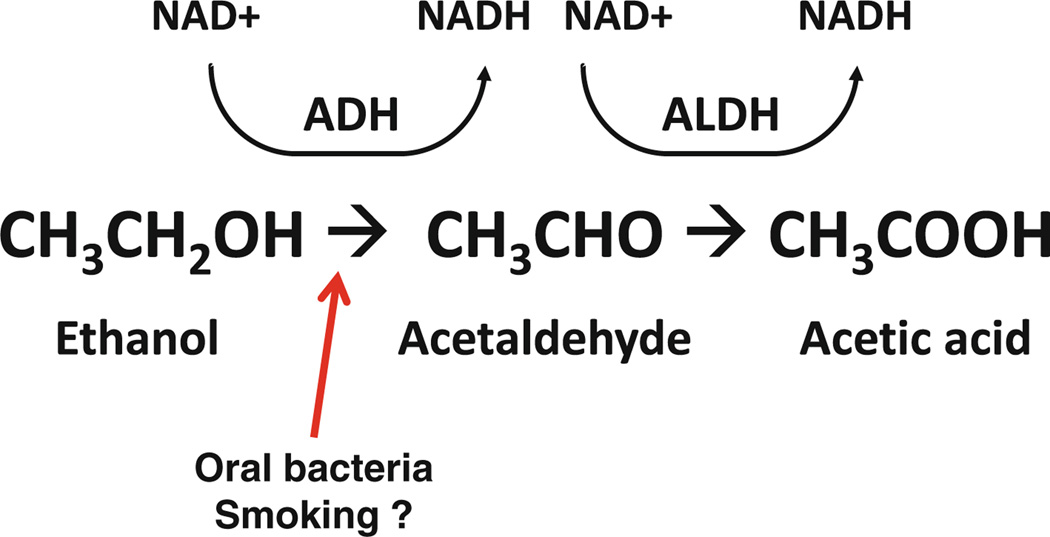
Oral microbiota may affect oral and gastrointestinal cancer risk by local activation of alcohol and smoking-related carcinogens, two well-established risk factors for oral and certain gastrointestinal cancer types [10]. While ethanol (alcohol) itself is not strongly carcinogenic, oral bacteria have the capacity to convert ethanol to acetaldehyde, which is an in vitro [11] and in vivo genotoxin [12] and recognized human carcinogen [13], thus leading to direct carcinogenic acetaldehyde exposure of the oral and gastrointestinal tract, following alcohol use (Fig. 1) [14]. Mutagenic amounts of acetaldehyde can be detected in saliva after ingestion of moderate doses of ethanol, while rinsing the mouth with antibacterial chlorhexidine prior to ethanol exposure reduces salivary acetaldehyde levels by 50%, in parallel with a marked decrease in microbe counts [15]. In addition, oral bacteria may play a role in increased activation of carcinogenic nitrosamines from tobacco smoking [16]; in vitro common oral microbes activate the tobacco smoke nitrosamine, nitrosodiethylamine (NDEA), to its carcinogenic (IARC, Group 2A), adduct-forming hydroxylated product [17]. A role for oral bacteria in carcinogen metabolism is further supported by observation that oral antiseptic mouthwash treatment (chlorhexidine) significantly reduces nitrosoamino acid formation and excretion in saliva (locally) and urine (systemically; each by about 30%) [18]. Smoking also potentiates the alcohol-related production of acetaldehyde by oral bacteria [14], potentially contributing to alcohol–tobacco interactions in carcinogenesis. Taken together, these data suggest oral microbial potential for local metabolism of alcohol and smoking-related carcinogens and a potential role in oral and gastrointestinal carcinogenesis.
Fig. 1.
Oral bacteria in alcohol metabolism. Under normal physiological conditions, ethanol is metabolized to acetaldehyde by alcohol dehydrogenase (ADH), and acetaldehyde is further metabolized to acetic acid by aldehyde dehydrogenase (ALDH). Oral bacteria have the capacity to convert ethanol to acetaldehyde, a genotoxin, leading to extended acetaldehyde exposure of the oral and gastrointestinal tract, following alcohol use, and possibly potentiated by smoking
Systemic effects of oral microbiota
Associations of periodontal disease and tooth loss with cancers at distant sites, including stomach [19, 20] and pancreas cancer [8, 21, 22], suggest that systemic mechanisms may also be involved in oral microbiome-related carcinogenesis. It is becoming increasingly clear that periodontal disease is associated with systemic effects [23, 24], including consistent relationships with cardiovascular disease [25] and diabetes [24]. Oral bacteria were found in atherosclerotic plaque, and importantly, successful treatment for periodontal disease, leads to reversal of systemic markers for these diseases, including improved endothelial function [26], decrease in inflammatory markers [26–28], and improved glycemic control in diabetics [29], providing strong evidence that periodontal disease is causally associated with these systemic effects. Although oral and gut microbiome community structures differ in the same individuals [30], certain oral bacteria are able to reach the GI tract (Ahn unpublished data). Alternatively, oral bacteria are sources of repeated transient systemic bacteremia after mastication, tooth-brushing, and dental procedures [31–35]. Furthermore, bacteria can provide a source of ligands for toll-like receptors (TLRs) [36] at target organ membrane receptors; TLRs are receptors on innate immune cells that bind structurally conserved molecules derived from microbes, collectively denoted pathogen-associated molecular patterns (PAMPs), and thereby potentially link inflammatory response and downstream cell signaling to a wide spectrum of human bacteria. Evidence is building that inflammation due to immunologic response to chronic exposure to bacteria and their toxins may play an important role in oral and gastrointestinal carcinogenesis [24, 37, 38].
Diversity at sampling sites
The oral cavity provides a diversity of environments for bacterial communities and consequently microbiome profiles differ for various intraoral surfaces. The microbiota of subgingival and supragingival plaque adherent to tooth structure tend to be similar, although anaerobes tend to predominate subgingivally. There is also variability in microbiota of the dorsal and lateral tongue and between epithelial covering of soft and bony tissues [39]. Salivary microbial profiles tend to reflect the prevalence of bacterial pathogens in adherent oral biofilms and to be associated with risk for dental disease and pathogen transmission between individuals; also, a decrease in the salivary count of pathogens can serve as an indicator of therapeutic effectiveness in the treatment of oral disease [40]. Thus, salivary microbial assessment may serve as a surrogate sample source for oral pathogens related to cancer risk.
Assays for the oral microbiome
Assays
Significant advances have been made in laboratory assay for genetic-based microbiome assessment, independent of bacterial culture [41]. Current high-throughput approaches employ genetic sequences of 16S ribosomal RNA (or 16S rRNA), a component of the 30S subunit of prokaryotic ribosome. 16S rRNA is used in genetic microbiome assay because components of this sequence are highly conserved between different species of bacteria and archaea, while other type-specific components are highly variable. 16S rRNA structure is employed in the terminal restriction fragment length polymorphism (TRFLP) assay, in microarrays based on gene hybridization, and in 16S rRNA sequencing.
Terminal restriction fragment length polymorphism (TRFLP) is a molecular profiling of microbial communities based on the position of a restriction site closest to a labeled end of the amplified 16S rRNA gene [42]. Following PCR of the16S rRNA gene, the mixture of amplicons is subjected to a restriction reaction. The mixture of fragments is separated using either capillary or polyacrylamide electrophoresis and the sizes of the different terminal fragments are determined by fluorescence detection. This method is a crude way to compare the molecular profiles of bacterial communities; however, it is not suitable for the identification of specific bacteria. A further limitation is that any two distinct sequences which share a terminal restriction site will result in one peak and will be indistinguishable.
16S rRNA gene pyrosequencing and the Human Oral Microbe Identification Microarray (HOMIM) [43] are two common high-throughput oral microbiome assays that provide rich microbiome assessment beyond the capacity of RFLPs. HOMIM uses specially designed probes to detect ~ 300 of the most prevalent oral bacterial species. Since this method is based on a preconstructed microarray, the community structure identified is limited to the specific hybridization probes selected for previously identified bacterial DNA sequences, but it has the advantages of lower cost and standardized data analysis. 16S rRNA gene pyrosequencing is a broad-based sequencing approach, using PCR primers to highly conserved regions for amplification of a segment of the 16S rRNA gene, followed by DNA pyrosequencing to identify unique sequence reads. Compared to traditional sequencing techniques like Sanger sequencing, pyrosequencing provides a larger number of reads and greater depth of coverage in a cost-efficient manner. Although pyrosequencing from 454 or Illumina provide shorter reads than Sanger sequencing, this next generation sequencing method is a significant advance to generate high-throughput, massively parallel processed sequencing, allowing the detection of greater microbial diversity due to the large number of reads and greater coverage depth.
We found that human oral microbiome community profiles assessed by 16S rRNA pyrosequencing and HOMIM were highly correlated at the phylum level and, for the more common taxa, at the genus level [44]. Although the pyrosequencing method detects a greater number of rare genera, this differential may not be decisive in moderate-sized epidemiologic studies where power is limited to detect risks associated with relatively rare exposures. We consider both methods currently suitable for high-throughput epidemiologic investigations relating the oral microbiome to disease risk [44].
In addition to methods employing 16S rRNA gene diversity for taxonomic classification by bacterial type, it is becoming cost-efficient to sequence the entire genomic material in samples, allowing the assembly of whole microbiome communities, including the ability to assess functional and phenotypic relationships for gene families [45]. Because of sequencing costs, computational challenges, and the identification of new genomic sequences with either unknown function or poor quality annotation [49], these studies are currently limited primarily to small-scale explorations. This metagenomic approach is still in development for large-scale studies. The pros and cons of 16S rRNA pyrosequencing, HOMIM, and metagenomic sequencing are summarized in Table 1.
Table 1.
Strengths and limitations of human oral microbe identification microarray (HOMIM) assay, 16S rRNA gene pyrosequencing, and metagenomic approach
| HOMIM: microarray-based 16AS rRNA hybridization | Pyrosequencing: partial 16S rRNA gene sequencing | Metagenomics: entire microbiome community gene sequencing |
| Focused detection of common known species | Broad detection range of taxa | Broad detection range of taxa |
| Custom array-based approach, covered by reference sequences | Detection of unclassified microbes | Possible to infer functional and phenotypic relationships for gene families |
| Quantification based on relative intensity score | Quantification based on sequence reads | Quantification based on sequence reads |
| Relatively low assay cost | Relatively high assay cost | Highest assay cost |
| Relatively less labor intensive | Relatively more labor intensive | Most labor/data intensive |
Human oral microbiome community structure
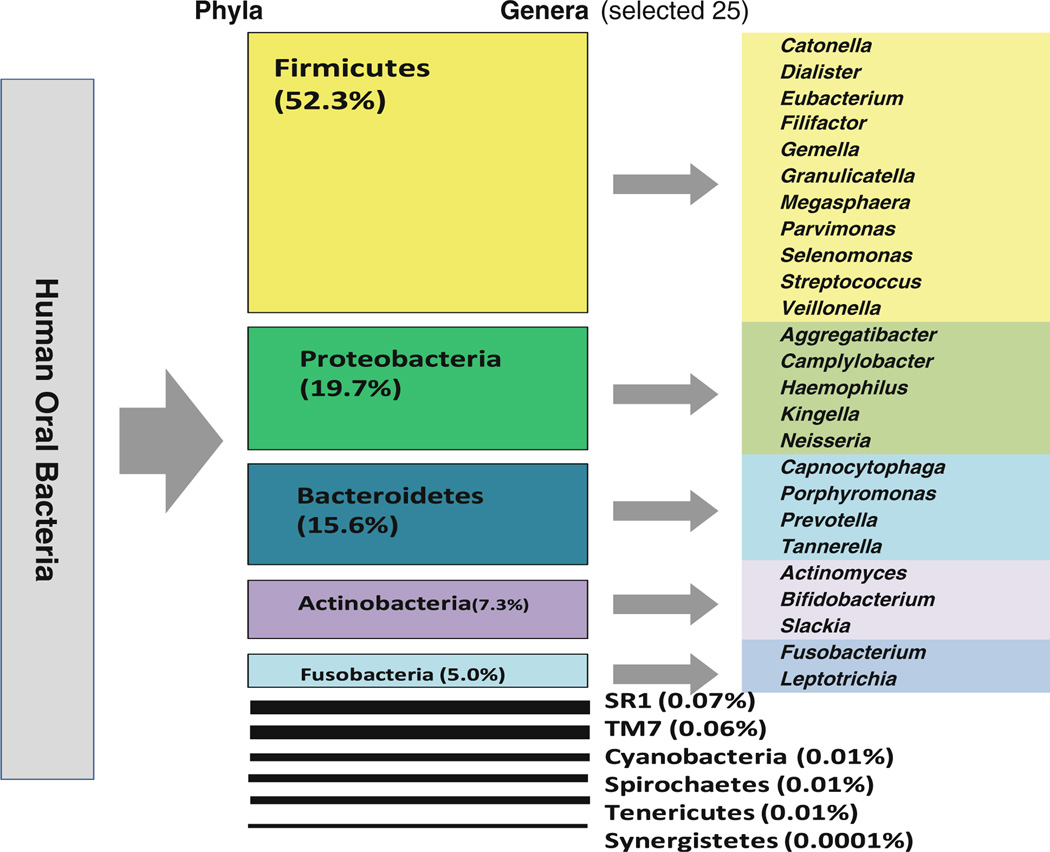
Taxonomic analyses include sequence alignment to the reference rRNA database and further classification by taxonomy. The Human Oral Microbiome Database (HOMD http://www.homd.org/) and 16S rRNA gene reference sequences, such as RDP (http://rdp.cme.msu.edu/) and Silva (http://www.arb-silva.de/) are currently available [2]. We have recently characterized 11 bacterial phyla and 77 genera in human salivary samples using the 16S rRNA gene pyrosequencing assay, based on RDP [44]. Of these phyla, five (Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, and Fusobacteria) predominated (99%). Relative abundance of phyla and the 25 most common genera are shown in Fig. 2.
Fig. 2.
Human oral microbiome structure. 11 phyla and 77 genera were observed from ~ 79,000 sequences. Alignment was done using RDPII
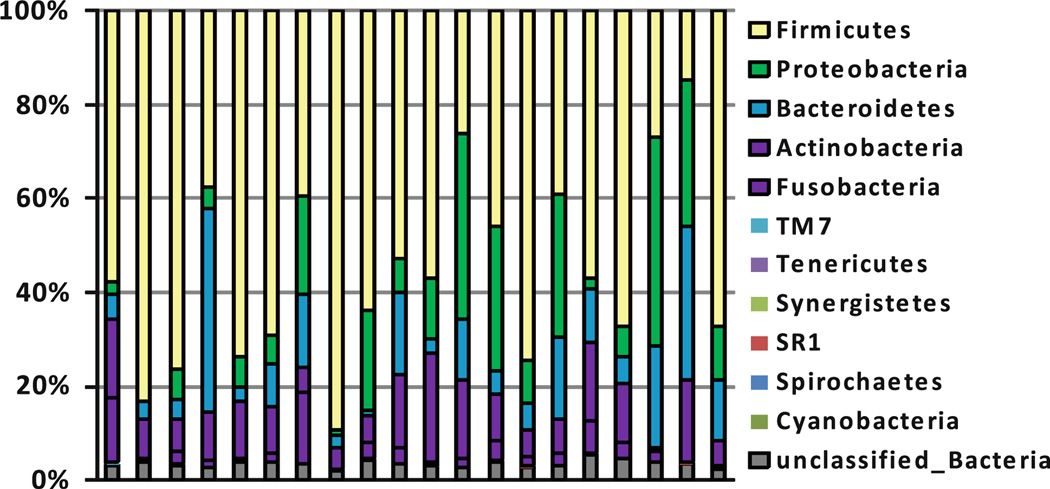
Oral microbiome profiles tend to show patterns of relative intraindividual stability over time and clear interindividual differences. One study examined temporal stability using 4 repeated oral microbiome profiles measured up to 6 months apart from the same individuals and found samples from same subject clustered, suggesting stable microbial profiles over time [46]. These findings were also replicated in another study [47]. We also observed interindividual differentials in the oral microbiome in 20 subjects (Fig. 3). The expectation of high temporal stability and substantial interindividual variability in the composition of individual bacterial communities is currently also being evaluated for forensic identification [48]. Significant interindividual oral microbiome differentials have also been shown for groups characterized by periodontal disease [43] and root caries [49]. The relative intraindividual stability over time and clear interindividual differences suggest that human microbiome profiles may serve as useful biomarkers for disease in population-based studies for disease phenotypes.
Fig. 3.
The relative abundance of human oral bacteria phyla. The relative abundances of human bacterial phyla in 20 healthy subjects. 16S rRNA sequencing assay was conducted and alignment was done using RDPII
Conclusion and future directions
High-throughput microbiome assay technology has opened the door for “microbiomic” epidemiology; initial efforts have provided testable hypotheses using these high-throughput microbiome assays, relating the oral microbiome to risk for oral cancer [16] and esophageal microbiome to premalignant Barrett’s esophagus [50]. Yang et al. [50] examined whether esophagus microbiome is associated with esophagitis and Barrett’s esophagus in tissue samples from 34 subjects. They identified a “type I” microbiome dominated by the genus Streptococcus and concentrated in the normal esophagus and a “type II” microbiome containing a greater proportion of gram-negative anaerobes/microaerophiles and primarily correlated with esophagitis (OR = 15.4) andBarrett’s esophagus (OR = 16.5), suggesting the feasibility to classify microbiome associated with this premalignant disease. In a small case–control study of oral microbiome with oral cancer [16] (10 cases and 10 controls), oral squamous cell cancer/leukoplakia was associated with an apparent decrease in the relative abundance of streptococcus (22.3%) compared with nonsmoking (39.4%) and smoking controls (40.1%).
While initial steps are promising [51], multi-disciplinary collaborations in epidemiology, microbiology, genetics, immunology, and bioinformatics will be needed to broaden our understanding of the relationship of oral bacteria to cancer risk [1]. Establishing the association of the oral microbiome with cancer may lead to significant advances in understanding of cancer etiology, potentially opening a new research paradigm for these diseases. The identified oral bacterial profiles may also serve as readily accessible, noninvasive biomarkers for the identification of high risk for cancer, complementing known risk factors for these diseases. If these relationships are confirmed as causal, findings may also lead to microbial prophylactic cancer prevention in clinical practice.
Acknowledgments
This work was supported by Grants R01 CA159036 and P30 CA016087 from National Cancer Institute.
Contributor Information
Jiyoung Ahn, Email: Jiyoung.Ahn@nyumc.org.
Calvin Y. Chen, Email: Calvin.Y.Chen@nyumc.org.
Richard B. Hayes, Email: Richard.B.Hayes@nyumc.org.
References
- 1.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, et al. The NIH human microbiome project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, et al. The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford): baq013. 2010 doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 5.Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. J Dent. 2010;38:83–95. doi: 10.1016/j.jdent.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550–558. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13:312–316. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 9.Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17:1222–1227. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 10.Schottenfeld D, Fraumeni JF. Cancer epidemiology and prevention. chapter 13. New York: Oxford University Press; 2006. alcohol and chapter 14, Tobacco. [Google Scholar]
- 11.Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, et al. Identification of DNA adducts of acetaldehyde. Chem Res Toxicol. 2000;13:1149–1157. doi: 10.1021/tx000118t. [DOI] [PubMed] [Google Scholar]
- 12.Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–58. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- 13.IARC. Monographs on the evaluation of carcinogenic risks to humans; re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. 1999 [PMC free article] [PubMed] [Google Scholar]
- 14.Salaspuro M. Acetaldehyde: a cumulative carcinogen in humans. Addiction. 2009;104:551–553. doi: 10.1111/j.1360-0443.2009.02546.x. [DOI] [PubMed] [Google Scholar]
- 15.Homann N, Jousimies-Somer H, Jokelainen K, Heine R, Salaspuro M. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathogenetic implications. Carcinogenesis. 1997;18:1739–1743. doi: 10.1093/carcin/18.9.1739. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Ganly I, Morris L, Palmer F, Deng H, et al. Relevance of microbiome to cigarette smoking and oral cancer. J Dent Res. 2011;90:120. [Google Scholar]
- 17.Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro KB, Hotchkiss JH, Roe DA. Quantitative relationship between oral nitrate-reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food Chem Toxicol. 1991;29:751–755. doi: 10.1016/0278-6915(91)90183-8. [DOI] [PubMed] [Google Scholar]
- 19.Abnet CC, Kamangar F, Dawsey SM, Stolzenberg-Solomon RZ, Albanes D, et al. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol. 2005;40:681–687. doi: 10.1080/00365520510015430. [DOI] [PubMed] [Google Scholar]
- 20.Abnet CC, Qiao YL, Mark SD, Dong ZW, Taylor PR, et al. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control. 2001;12:847–854. doi: 10.1023/a:1012290009545. [DOI] [PubMed] [Google Scholar]
- 21.Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171–175. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 22.Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, Virtamo J, Taylor PR, et al. Tooth loss, pancreatic cancer, and Helicobacter Pylori. Am J Clin Nutr. 2003;78:176–181. doi: 10.1093/ajcn/78.1.176. [DOI] [PubMed] [Google Scholar]
- 23.Lamster IB, DePaola DP, Oppermann RV, Papapanou PN, Wilder RS. The relationship of periodontal disease to diseases and disorders at distant sites: communication to health care professionals and patients. J Am Dent Assoc. 2008;139:1389–1397. doi: 10.14219/jada.archive.2008.0051. [DOI] [PubMed] [Google Scholar]
- 24.Pizzo G, Guiglia R, Lo Russo L, Campisi G. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010;21:496–502. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!“—epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Tse HF, Yiu KH, Li LS, Jin L. Effect of periodontal treatment on circulating CD34(+) cells and peripheral vascular endothelial function: a randomized controlled trial. J Clin Periodontol. 2011;38:146–156. doi: 10.1111/j.1600-051X.2010.01651.x. [DOI] [PubMed] [Google Scholar]
- 28.Moura Foz A, Alexandre Romito G, Manoel Bispo C, Luciancencov Petrillo C, Patel K, et al. Periodontal therapy and biomarkers related to cardiovascular risk. Minerva Stomatol. 2010;59:271–283. [PubMed] [Google Scholar]
- 29.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33:421–427. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koren O, Spor A, Felin J, Fak F, Stombaugh J, et al. Microbes and health sackler colloquium: human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108:4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwai T. Periodontal bacteremia and various vascular diseases. J Periodontal Res. 2009;44:689–694. doi: 10.1111/j.1600-0765.2008.01165.x. [DOI] [PubMed] [Google Scholar]
- 32.Crasta K, Daly CG, Mitchell D, Curtis B, Stewart D, et al. Bacteraemia due to dental flossing. J Clin Periodontol. 2009;36:323–332. doi: 10.1111/j.1600-051X.2008.01372.x. [DOI] [PubMed] [Google Scholar]
- 33.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahrani-Mougeot FK, Paster BJ, Coleman S, Ashar J, Barbuto S, et al. Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol. 2008;46:2129–2132. doi: 10.1128/JCM.02004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen I. Update on bacteraemia related to dental procedures. Transfus Apher Sci. 2008;39:173–178. doi: 10.1016/j.transci.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Chinen T, Volchkov PY, Chervonsky AV, Rudensky AY. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J Exp Med. 2010;207:2323–2330. doi: 10.1084/jem.20101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meurman J. Oral microbiota and cancer. J Oral Microbiol. 2010;2:1–10. doi: 10.3402/jom.v2i0.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers AB, Fox JG. Inflammation and cancerI. Rodent models of infectious gastrointestinal and liver cancer. Am J Physiol Gastrointest Liver Physiol. 2004;286:G361–G366. doi: 10.1152/ajpgi.00499.2003. [DOI] [PubMed] [Google Scholar]
- 39.Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003;30:644–654. doi: 10.1034/j.1600-051x.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 40.Slots J, Slots H. Bacterial and viral pathogens in saliva: disease relationship and infectious risk. Periodontol. 2011;55:48–69. doi: 10.1111/j.1600-0757.2010.00361.x. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pozhitkov AE, Beikler T, Flemmig T, Noble PA. High-throughput methods for analysis of the human oral microbiome. Periodontol. 2011;55:70–86. doi: 10.1111/j.1600-0757.2010.00380.x. 2000. [DOI] [PubMed] [Google Scholar]
- 42.Sanders ER, Miller JH, Herbold C. I Microbiologist, a discovery-based course in microbial ecology and molecular evolution. Washington: ASM press; 2010. [Google Scholar]
- 43.Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–1432. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn J, Yang Y, Paster BJ, Ganly I, Morris L, et al. Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison. PLoS One. 2011;6:e22788–e22795. doi: 10.1371/journal.pone.0022788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J. Metagenomic pyrosequencing and microbial identification. Clin Chem. 2009;55:856–866. doi: 10.1373/clinchem.2008.107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazarevic V, Whiteson K, Hernandez D, Francois P, Schrenzel J. Study of inter- and intra-individual variations in the salivary microbiota. BMC Genomics. 2010;11:523. doi: 10.1186/1471-2164-11-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fierer N, Lauber CL, Zhou N, McDonald D, Costello EK, et al. Forensic identification using skin bacterial communities. Proc Natl Acad Sci USA. 2010;107:6477–6481. doi: 10.1073/pnas.1000162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preza D, Olsen I, Willumsen T, Boches SK, Cotton SL, et al. Microarray analysis of the microflora of root caries in elderly. Eur J Clin Microbiol Infect Dis. 2009;28:509–517. doi: 10.1007/s10096-008-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L, Lu X, Nossa CW, Francois F, Peek RM, et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Human Microbiome Research Conference; 31 August–2 September, 2010; St Louis, Missouri. [Google Scholar]