Abstract
Ehrlichia are small gram-negative obligately intracellular bacteria in the order Rickettsiales that are transmitted by ticks and associated with emerging life-threatening human zoonoses. Vaccines are not available for human ehrlichiosis, and therapeutic options are limited to a single antibiotic class. Ehrlichia are able to subvert both innate and adaptive host defenses, and advances in understanding molecular Ehrlichia-eukaryotic host cell interactions and the cellular and immunologic basis of disease are important for developing effective next generation therapies. New technologies for exploring host-pathogen interactions have yielded recent advances in understanding the molecular interactions between these intracellular pathogens and host cell components and identified new targets for therapeutic and vaccine development including those that target pathogen virulence mechanisms or disrupt the processes associated with ehrlichial effector proteins. Animal models have also provided insight into immunopathologic mechanisms that contribute significantly to understanding severe disease manifestations that will lead to the development of immunomodulatory approaches for treating patients nearing or experiencing severe disease states. In this review, we discuss the recent advances in our understanding of molecular and cellular pathobiology and the immunobiology of Ehrlichia infection. We identify new molecular host-pathogen interactions that can be targets of new therapeutics and prospects for treating immunologic dysregulation that occurs during acute infection leading to life-threatening complications.
Keywords: Ehrlichia, ehrlichiosis, zoonosis, tick, macrophage, innate immunity, secretion system, effector proteins, immunopathology, immunomodulation, therapeutic
Ehrlichia were first associated with veterinary diseases in Africa in 1925 by Cowdry who identified Ehrlichia ruminantium in cattle and a decade later by Donatien and Lestoquard who described E. canis in Algerian dogs (Refs. 1,2). Ehrlichioses continue to be significant diseases of veterinary and agricultural importance and are now associated with newly identified human tick-borne zoonoses. At the end of the twentieth century a new tick-borne disease, human monocytotropic ehrlichiosis (HME), emerged in humans and a novel etiologic agent (Ehrlichia chaffeensis) was identified (Refs. 3). Twelve years later, E. ewingii, a recognized canine pathogen that infects granulocytes, was detected in four immunocompromised patients with symptoms of ehrlichiosis (Refs. 4,5). Nearly a century after Cowdry’s discovery, Ehrlichia are firmly established as zoonotic human pathogens of public health importance. HME is considered one of the most prevalent life-threatening tick-borne diseases in the United States, and ewingii ehrlichiosis is an important clinically indistinguishable disease in immunocompromised patients (Refs. 4,5).
The order Rickettsiales contains two families of arthropod-transmitted obligately intracellular bacteria that cause human diseases including spotted fever rickettsiosis, typhus, scrub typhus, anaplasmosis, and ehrlichiosis. The genus Ehrlichia is a member of the family Anaplasmataceae, which also includes genera Anaplasma, Wolbachia and Neorickettsia. In addition, the family Rickettsiaceae and respective genera Rickettsia and Orientia are also members of Rickettsiales. The Ehrlichia genus consists of six formally named members (E. canis, E. chaffeensis, E. muris, E. ruminantium, E. ewingii, and E. ovis). E. chaffeensis and E. ewingii are recognized as human zoonotic pathogens that also cause significant disease in the animal hosts (Refs. 6,7). E. canis is a globally distributed pathogen, the etiologic agent of canine monocytic ehrlichiosis, and recently associated with human infections (Refs. 8,9). E. ruminantium is strictly a veterinary pathogen that causes a severe acute infection known as heartwater in domestic ruminants localized primarily to sub-Saharan Africa (Refs. 10).
Ehrlichia have small genomes, yet have evolved elaborate and complex molecular strategies that enable adaptation to distinct hosts (invertebrate and vertebrate) and intracellular survival in innate immune effector cells. Utilizing Ehrlichia as a model to understand the pathogen-eukaryotic host cell interactions provides an attractive and manageable system to study molecular interactions involved in this inter-kingdom relationship, advancing our knowledge of the molecular pathobiology of intracellular microbes (plant and animal), as well as the molecular biology of the eukaryotic cell. This review will focus on the molecular and cellular interactions of ehrlichiae and immunologic responses that have clinical implications with regard to development of novel antimicrobial therapeutics or molecular countermeasures, and immunomodulatory approaches.
Physical characteristics and intracellular developmental biology
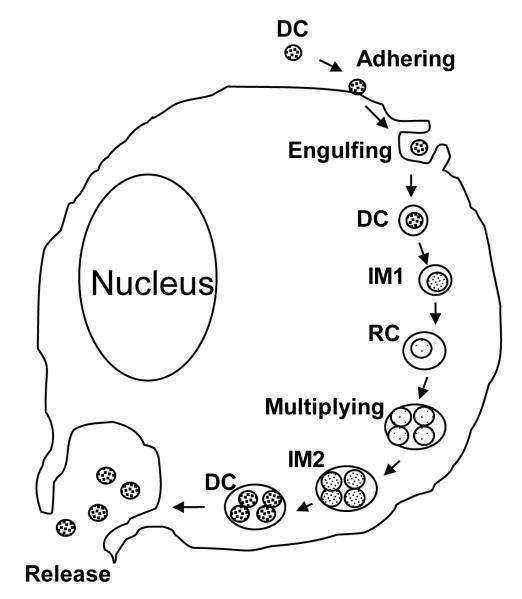
E. chaffeensis is confined to cytoplasmic membrane-bound vacuoles within monocytes/macrophages and dendritic cells (Refs. 11), replicating to form microcolonies called morulae that contain 1 to >400 organisms (Refs. 12). Morphologically individual ehrlichiae are coccoid and coccobacillary and exhibit two ultrastructural cell types, a larger reticulate cell (RC) and a smaller dense-cored cell (DC) (Fig. 1). Morphologic comparisons to other intracellular bacteria such as Chlamydia have suggested that Ehrlichia RC and DC represent analogous replicating and infectious forms of the organism. This hypothesis was confirmed by a recent study of ehrlichial developmental biology that demonstrated that the DC form of E. chaffeensis binds to the host cell surface where it is rapidly (< 1 hr) internalized and completes the developmental cycle within 72 hrs (Refs. 13) (Fig. 2).
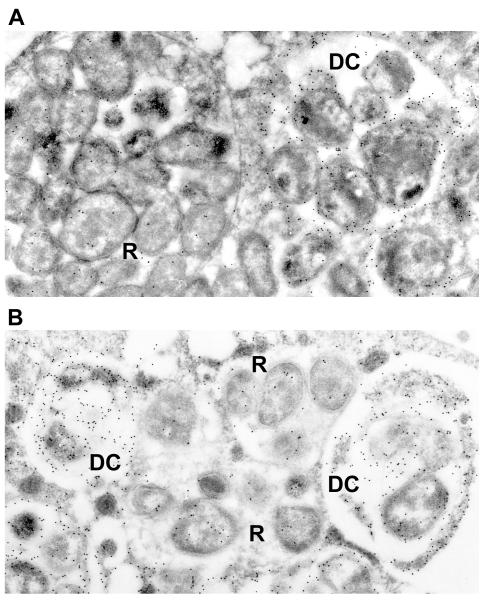
Figure 1.
Immunogold-labeled electronmicrograph of E. chaffeensis TRP47 illustrating differential expression and secretion associated with morulae containing dense-cored (DC) (0.4 to 0.6 μm in diameter) morphological forms compared with reticulate cell (RC) forms (0.4 to 0.6 μm by 0.7 to 1.9 μm). RC and DC can be distinguished by two differentially expressed tandem repeat containing surface proteins, TRP47 and TRP120 that are found only on DC ehrlichiae (Refs. 22,27). The intramorular space in some morulae contains a fibrillar matrix of ehrlichial origin (Refs. 105). (Reproduced with permission from the American Society for Microbiology, license 2499420679397).
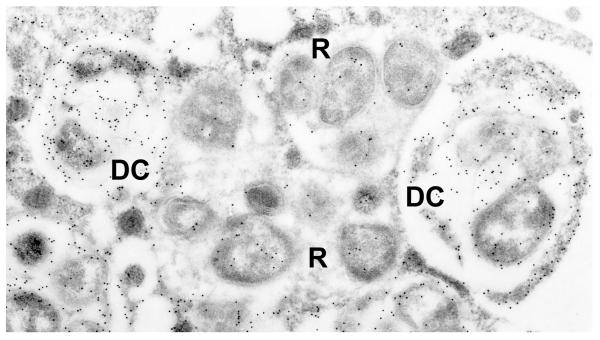
Figure 2.
Developmental cycle of E. chaffeensis in the eukaryotic host cell (Reproduced with permission from the John Wiley and Sons, license 2546680582714). Infectious dense cored (DC) ehrlichiae attaches and enters the host cell thorough receptor mediated endocytosis and within 1 hr after entry transforms into the intermediate (IM)-1 then into the reticulate cell (RC). During the next 48 hrs RC replicates, doubling every 8 hrs, and then transforms into IM-2 and matures to DC within 72 hr after initial cell contact (Refs. 13). Dense-cored ehrlichiae obtained 72 hrs post-infection exhibited much higher infectivity than E. chaffeensis RC obtained 24 hr post infection. RC and DC can be distinguished by two differentially expressed tandem repeat proteins, TRP47 and TRP120 that are found only on DC ehrlichiae, and the major outer membrane protein p28-19, which is only expressed on RC (Refs. 13). However, intermediate, presumably transitional, forms that co-express these proteins have also been described (Refs. 13). Although ehrlichiae and chlamydiae have similar morphologic forms and developmental cycles, homologous genes for the histone H1 homolog proteins (Hc1 and Hc2) involved in condensation and decondensation of the chlamydial nucleoid are not present in the E. chaffeensis genome (Refs. 13).
Genome insight to host-pathogen interactions
The intracellular niche occupied by Ehrlichia has resulted in reductive evolutionary processes and corresponding severe loss of genes associated with metabolic processes provided by the host cell. Hence the genome sizes (~1-1.5 Mb) of Ehrlichia are relatively small compared to extracellular bacteria. The genomes of three Ehrlichia species have been sequenced (Refs. 14,15,16) and exhibit a high degree of genomic synteny, low G+C content (~30%) and one of the smallest genome coding ratios that is attributed to long non-coding regions and numerous long tandemly repeated sequences (TRs) (Refs. 17). These long non-coding regions and low G+C content in other related Rickettsiales members are speculated to represent degraded genes in the final stages of elimination and excess GC-to-AT mutations (Refs. 18,19,20). Another feature of Ehrlichia genomes is the presence of a large number of long period TRs that appear to have evolved after divergence of the species (Refs. 21). The TRs appear to be locally occurring independent events actively created and deleted through a mechanism compatible with DNA slippage (Refs. 21). The generation of TRs by Ehrlichia appears to be a mechanism of adaptation to the host. TRs of different Ehrlichia species have no phylogenetic relationships and suggest that duplication occurred after diversification of the repeat-encoding DNA (Refs. 21).
Features identified in Ehrlichia genomes associated with host-pathogen interactions include genes that encode tandem and ankyrin repeat-containing proteins, actin polymerization proteins, a multigene family encoding outer membrane proteins, and a group of poly(G-C) tract (short sequence repeats)-containing proteins and absence of genes for the biosynthesis of peptidoglycan and lipopolysaccharide (Refs. 15), major pathogen-associated molecular patterns recognized by the innate immune system. Tandem repeats are associated with regulation of gene expression and phase variation, and Ehrlichia species exhibit two types of tandem repeats, small (12 bp) and large (100-300 bp) period repeats (Refs. 21). Secretion of effector proteins into the host cell requires secretion systems, and such delivery mechanisms have been identified, including many of the known type IV secretion system (T4SS) components (Refs. 14,15,16). There is no evidence of a type III secretion system although other intracellular bacteria such as Chlamydia have this system, and some secreted ehrlichial tandem repeat proteins (TRPs) are predicted to have an N-terminal type III secretion transport signal. The Sec-dependent and Sec-independent protein export pathways for secretion of proteins across the inner membrane as well as a putative type I secretion system have also been identified. The Ehrlichia genomes also have genes that encode three response regulator two-component systems (TCS), a family of signal sensor, transduction, and response regulatory systems, composed of a sensor histidine kinase and a response regulator, that allow bacteria to sense signals and respond to changes in their environment through specific gene activation or repression (Refs. 14).
Proteins associated with host-pathogen interactions
Tandem repeat proteins
The presence of long period tandem repeats distributed in intergenic regions of Ehrlichia is well recognized and is associated with expansion and contraction of these regions. Interestingly, long period tandem repeats are also found in a small subset of proteins, many of which are strongly immunoreactive, suggesting that they are surface-exposed and/or secreted. TRPs in pathogenic bacteria have been associated with host-pathogen interactions such as adhesion and internalization (Refs. 22,23), actin nucleation (Refs. 24) and immune evasion (Refs. 25). Many of these proteins in Ehrlichia have been molecularly characterized, and major continuous species-specific antibody epitope(s) have been mapped to the acidic serine-rich TRs of E. chaffeensis, TRP120, TRP47, and TRP32 (Refs. 26,27,28), and in TRs of the E. canis orthologs TRP140, TRP36, and TRP19, respectively (Refs. 28,27,29). Immunoelectron microscopy has identified these TRPs, in addition to a non-differentially expressed TRP32, that are secreted by the ehrlichiae and associated with the morular fibrillar matrix and the morula membrane (Refs. 22,26,27). Other bacteria have effector TRPs that are secreted by the type III secretion system (T3SS). However, the absence of an identifiable T3SS in these organisms suggests that another mechanism such as the type 1 system is involved. We have explored the possibility of these TRPs as T4SS substrates, but they were not secreted in the well characterized model of the VirB/VirD4-dependent T4SS of Agrobacterium tumefaciens (Refs. 30)
The functional role of the tandem repeats in E. chaffeensis TRPs is not fully understood. However, homology between TRs and other functional protein domains and motifs of eukaryotic origin have been reported. The E. chaffeensis TRP47 contains seven 19-mer (ASVSEGDAVVNAVSQETPA) TRs that dominate the C-terminal region of the protein, and approximately half of the TRP47 is represented by the TR domain (Refs. 31). The TRP47 TR region exhibits homology with eukaryotic proteins including renin receptor/ATP6AP2/CAPER protein, DNA polymerase III subunits gamma and tau-conserved domain, and ribonuclease E, suggesting similar functional characteristics (Refs. 31). The TRP47 also has several N-terminal tyrosine and serine/threonine residues that are predicted sites of phosphorylation, and tyrosine phosphorylation has been detected on TRP47 with anti-phosphotyrosine antibodies (Refs. 32). Other TRPs may be phosphorylated, such as TRP32, which has an unusually high frequency of tyrosine residues (20%) in the C-terminal tail (Refs. 26). The TR domains of the TRP120 and TRP32 do not have homology with other conserved protein domains; however, recent studies have reported that the TR region of the TRP120 directly binds host cell DNA (Refs. 33).
Ankryin repeat proteins
The ankyrin repeat (Ank) is a ubiquitous eukaryote motif that mediates protein-protein interactions. Ank domains may occur in combinations with other types of domains and cooperatively fold into structures that mediate molecular recognition via protein-protein interactions. Ehrlichia spp. are among only a few prokaryotes that are known to have ankryin repeat (Ank)-containing proteins. The most extensively studied Ank protein in E. chaffeensis is a 200 kDa protein (Ank200) that has a central domain that contains 19 Anks flanked by acidic (pI 4 to 5) C- and N-terminal domains with a predominance of glutamate and aspartate residues (Refs. 34). In addition, like the TRPs, the E. canis and E. chaffeensis Ank200s have a high proportion of polar amino acids, including serine and threonine (Refs. 35,34). Multiple species-specific antibody eptiopes have been mapped to acidic terminal domains of the Ank200s (Refs. 35,34). Our unpublished data suggest that E. chaffeensis Ank200 is not secreted by the T4SS (Refs. 30); however, A. phagocytophilum, AnkA appears to be secreted by this mechanism (Refs. 36). Although E. chaffeensis and A. phagocytophilum are closely related, they are different in many aspects such as tropism for different cell types, residence in different cytoplasmic compartments (Refs. 37) and distinct immune evasion mechanisms (Refs. 38,39,40). In addition, an A. phagocytophilum VirD4, the T4S substrate coupling protein, exhibits a higher identity with Agrobacterium tumefaciens VirD4 than with that of E. chaffeensis (Refs. 41,42).
Major outer membrane proteins
A superfamily of immunoreactive outer membrane proteins has been identified in the family Anaplasmataceae that are members of Pfam PF01617. E. chaffeensis has a paralogous family of 22 major outer membrane proteins (OMP-1/p28) arranged in single locus upstream from the secA gene and downstream from a hypothetical transcriptional regulator gene (Refs. 43). They were originally proposed to be involved in antigenic variation of the organism. Although recombination of the major outer membrane proteins of closely related Anaplasma spp. occurs to create antigenic diversity, there is no evidence that recombination of the Ehrlichia OMP-1 family occurs. Differential expression of the OMP-1 genes in ticks and animal hosts has been reported, suggesting that they play a role in host adaptation (Refs. 44,45). Expression of only one OMP-1 gene (OMP-1B) has been reported in ticks and tick cell lines and appears to involve a temperature sensitive regulation mechanism (Refs. 44,46). In contrast, all OMP-1 family members are expressed in mammalian hosts and cells, and antibodies against all OMP-1 proteins have been detected in experimentally infected dogs (Refs. 44,47). Although the role of omps in antigenic variation and immune evasion is still uncertain, other characteristics have been identified for the OMP-1 proteins, including transmembrane β-strand structural features and porin activity, suggesting that they may facilitate nutrient acquisition (Refs. 48).
2. Molecular and Cellular Biology of Infection
Entry and characteristics of the ehrlichial vacuole
Infection of the host cell involves DC ehrlichiae that express TRP120 on the surface. TRP120 plays an important role in the binding and entry process (Refs. 49,23), and a potential role has also been demonstrated for TRP47 in attachment to tick cells using the E. ruminantium orthologs (Erum1110) (Refs. 50). The stability of TRP120 and invasion of E. chaffeensis are regulated by the bacterial second messenger, cyclic di-GMP and activity of ehrlichial surface serine protease HtrA (Refs. 23). Binding to the host cell occurs through receptors such as E and L selectin and other glycosylphosphatidylinositol (GPI)-anchored proteins located in caveolae (Refs. 51,52) triggering receptor-mediated endocytosis that involves signaling events including transglutamination, tyrosine phosphorylation, phospholipase C-γ2 activation, IP3 production and increases in intracellular calcium (Refs. 53). Removal of surface-exposed GPI-anchored proteins by phosphatidylinositol-specific phospholipase C prevents the development of early inclusions (Refs. 52). The vacuoles in which the organism enters contain caveolin-1, GM1 ganglioside and phospholipase C-γ2, and later (>3 hr) after infection vacuoles that contain replicating ehrlichiae manifest characteristics of early endosomes such as presence of Rab5, early endosomal antigen 1 (EEA1) and vacuolar (H+) ATPase and accumulate transferrin and transferrin receptor (Refs. 12). Replicative vacuoles of closely related Anaplasma phagocytophilum do not express these markers (Refs. 54). Other molecules found in Ehrlichia inclusions are vesicle-associated membrane protein 2 (VAMP2), and major histocompatibilty class II and β2 microglobulin (Refs. 12,54). Ehrlichia vacuoles appear to be maintained in a caveolar trafficking system that interacts with recycling endosomal pathways (Refs. 52).
Modulation of host cell gene expression
E. chaffeensis appears to actively modulate host cell gene transcription and function through multiple mechanisms including interactions with host chromatin and modulation of host signaling. One mechanism that has been identified involves the inhibition of host MAP kinases by E. chaffeensis leading to the downregulation of transcription factors and transcription of target genes related to host defense (Refs. 55). The discovery of DNA binding proteins (TRP120 and Ank200) of Ehrlichia provides another mechanism by which host cell gene transcription can be modulated (Refs. 56,33).
The transcription levels of a relatively small percentage (5%) of host cell genes are altered significantly within the first 24 hrs post infection (Refs. 57). This transcriptional profile has provided new information on host cell processes targeted by Ehrlichia and revealed key themes in pathobiology and disease pathogenesis. Specific cellular processes that appear to be modulated are apoptosis inhibitors, regulation of cell cycle and differentiation, signal transduction, and expression of proinflammatory cytokines, biosynthetic and metabolic proteins, and membrane trafficking proteins. Host genes modulated during E. chaffeensis infection are distinct from those observed in infections by other intracellular bacteria, illustrating the complexity and diversity of intracellular pathogen-host interactions and survival strategies (Refs. 57).
Survival in mononuclear phagocytes requires the ability to evade innate and adaptive immune responses. E. chaffeensis represses the transcription of cytokines involved in the early innate immune response and cell-mediated immune response to intracellular microbes, including host cell cytokines that modulate innate and adaptive immunity to intracellular bacteria. Early in infection, genes for proinflammatory cytokines IL-1β, IL-8 and TNF-β are upregulated, and others such as TNF-α, are not induced (Refs. 57). In contrast, most cytokines and receptors are downregulated including IL-15, IL-18, and various chemokine receptors. These cytokines play fundamental roles in stimulating NK cells and T helper 1 cells to produce gamma interferon (IFN-γ), which then activates macrophages to kill phagocytized bacteria. IL-12 and IL-15 also activate NK cells and cytotoxic T lymphocytes to kill cells infected with intracellular bacteria. Thus, active modulation of genes associated with the immune response appears to be essential to the survival of E. chaffeensis.
Modulating genes associated with inhibition of apoptosis and cell cycle regulators is observed early during E. chaffeensis infection, and is likely necessary for delaying host cell death. Apoptosis is an innate mechanism of host defense used to prevent proliferation of internalized bacteria. E. chaffeensis infection upregulates transcription of apoptosis inhibitors such as IER3 (immediately early response 3), BirC3 (baculoviral IAP repeat-containing protein 3), and BCL2, but inhibits apoptosis inducers such as BIK (BCL2-interacting killer) and BNIP3L (BCL2/adenovirus E1B 19-kDa interacting protein 3-like) during the early stage of infection, thus impairing host cell apoptosis and maintaining a prolonged growth opportunity for ehrlichiae (Refs. 57).
E. chaffeensis lives in an early endosome and inhibits the maturation of the endosome to evade destruction by lysosomal enzymes (Refs. 37). In an effort to modulate this process, E. chaffeensis inhibits the transcription of genes involved in membrane trafficking and lysosomal fusion. These proteins form a complex that juxtaposes the two membranes to be fused, and this interaction is regulated by Rab5, a small GTPase of the Rab family. E. chaffeensis represses the production of Rab5, synaptosome-associated protein 23 (SNAP23), and syntaxin 16 (STX16) during infection, most dramatically during the first hour of infection. Interestingly, Rab5 is associated with E. chaffeensis inclusions. Thus, E. chaffeensis appears to modulate genes associated with phagosome-lysosome fusion. Depletion of Rab5 inhibits the fusion of the phagosome containing Listeria monocytogenes with lysosomes (Refs. 58).
Acquisition of iron and cholesterol
Ehrlichia survival depends on an available supply of intracellular iron (Refs. 59), and this is also demonstrated by the fact that anti-ehrlichial activity of IFN-γ is mediated by limiting the available cytoplasmic iron (Refs. 60). Interestingly, cytoplasmic vacuoles containing replicating ehrlichiae accumulate transferrin receptor, and transferrin receptor mRNA is upregulated during ehrlichial infection (Refs. 59), which may serve to counteract the reduction of surface transferrin receptor by IFN-γ. How Ehrlichia acquires iron from the host is not fully understood, but a conserved iron acquisition mechanism has been identified in Ehrlichia that belongs to the ATP-binding cassette transporter family and is shared by a diversity of bacterial species (Refs. 61). However, unlike most bacteria in which the genes encoding iron acquision are found in an operon, Ehrlichia iron acquisition genes are not part of a functional operon (Refs. 61). The iron binding protein (Fbp) of Ehrlichia is conserved among other known iron binding proteins in bacteria, and similarly it binds Fe (III). Notably, Ehrlichia Fbp has been observed outside the bacterium within the morular space containing dense-cored cells suggesting that iron is obtained from intracellular pools derived from transferrin and shuttled by Fbp to the bacterium (Refs. 61).
Although Ehrlichia lack structural membrane components such as peptidoglycan and lipopolysaccahride, they appear to utilize cholesterol as a structural substitute (Refs. 62). Ultrastructural changes are observed in Ehrlichia in the presence of cholesterol extraction reagents. Cholesterol is incorporated as a component of the outer membrane, and Ehrlichia have an unidentified, but specific, direct uptake mechanism (Refs. 62). The structural integrity of the organism is dependent on cholesterol, and it is essential for maintaining an infectious state (Refs. 62).
Evading innate host defenses
Ehrlichia have evolved strategies that allow them to survive in phagocytes and evade innate host defense mechanisms. Ehrlichia manipulate innate immune defense mechanisms including production of reactive oxygen species, apoptosis, lysosomal fusion, and IFN-γ responsiveness. A major host defense mechanism is production of reactive oxygen species that have strong antimicrobial effects. E. chaffeensis is highly sensitive to O2-killing (Refs. 63) and lacks genes involved in ROS detoxification (Refs. 14). However, E. chaffeensis actively blocks O2-generation in monocytes stimulated with phorbol myristate acetate and causes degradation of NADPH subunit p22phox, in monocytes, but not neutrophils (Refs. 63). Degradation of p22phox appears to involve non-proteosomal proteolysis and heat labile factors or proteins from E. chaffeensis. Degradation can be detected in isolated monocyte membrane fractions, suggesting that intracellular signaling events are not required (Refs. 63).
Apoptosis is an innate cellular defense mechanism against microbes that is modulated by many bacterial pathogens, and there is new information that indicates that Ehrlichia also modulate host cell death. In nature Ehrlichia survive by persistently infecting vertebrate hosts, so delaying or preventing apoptosis could be a means of enhancing survival by preventing host cell death and subsequent immune recognition. For most intracellular pathogens, induction of apoptosis leads to pathogen killing and clearance of infection, and this thought to be beneficial to the host and enhance the immune response to the infection. In the case of Ehrlichia, there appear to be multiple mechanisms involved in apoptosis modulation by Ehrlichia. E. chaffeensis upregulates transcription of genes related to anti-apoptotic activity, cell cycle and cyclin dependent kinase (CDK) expression in THP-1 cells (Refs. 57). E. ewingii delays apoptosis in neutrophils by stabilization of host cell mitochondria (Refs. 64)
Phagolysosomes represent another important innate host defense mechanism against pathogens. Ehrlichia are able to inhibit fusion of the phagosome containing ehrlichiae with lysosomes and thus prevent their destruction by this defense mechanism (Refs. 12). Although very little is known about how Ehrlichia avoid this host defense mechanism, recent studies have demonstrated that functioning two-component systems play an important role in preventing lysomsomal fusion (Refs. 65). Two-component systems consist of a sensor with a histidine kinase that detects environmental signals and a response regulator that has DNA binding activity and regulates gene transcription (Refs. 66). E. chaffeensis has three two component systems, PleC-PleD, NtrY-NtrX, and CckA-CtrA (Refs. 67). Cells treated with closantel and incubated with E. chaffeensis have increased colocalization between E. chaffeensis and lysosomal glycoprotein, LAMP-1, and E. chaffeensis infection is completely inhibited by pretreatment with closantel (Refs. 67).
The macrophage activating cytokine, interferon-γ (IFN-γ), plays an important role in innate and adaptive immune responses against intracellular pathogens. The activation of macrophages by IFN-γ induces several antimicrobial effector mechanisms including regulation of iron homeostasis (Refs. 68) that are necessary for production of antimicrobial effectors including reactive oxygen species and nitrogen radicals (Refs. 69). The ability to acquire iron is important for the survival of intracellular pathogens such as Ehrlichia, Salmonella, Listeria, and Mycobacterium (Refs. 69,70). With respect to E. chaffeensis, macrophages that are stimulated with IFN-γ before infection or early in infection readily kill E. chaffeensis (Refs. 60). However, E. chaffeensis has developed strategies to circumvent the actions of IFN-γ. E. chaffeensis upregulates transferrin receptor expression to counteract the downregulation by the action of IFN-γ (Refs. 59). The inhibitory effects of IFN-γ on E. chaffeensis infection can be reversed by the addition of iron-saturated transferrin, and E. chaffeensis infection is inhibited by the intracellular iron chelator, deferoxamine (Refs. 60), demonstrating the critical role of iron for E. chaffeensis infection and the role that IFN-γ has in regulating its availability. After E. chaffensis infection is established, IFN-γ is no longer effective possibly because of the ability of E. chaffeensis to upregulate host iron acquisition mechanisms. The ability of E. chaffeensis to block the antimicrobial mechanisms of IFN-γ is mediated at least in part by its ability to block the Jak/Stat IFN-γ signal transduction pathway (Refs. 71). Others have also suggested that avoiding IFN-γ activity may be influenced by transcriptional mechanisms on Jak/Stat expression mediated by the nuclear effector, Ank200 (Refs. 56). Binding of a protein component of E. chaffeensis to the host cell blocks tyrosine phosphorylation of Jak1 and Stat1-α normally induced by IFN-γ (Refs. 71). Jak/Stat inhibition has been linked to the upregulation of protein kinase A by E. chaffeensis (Refs. 71).
E. chaffeensis also appears to modulate the host innate immune response by influencing cell signaling pathways. Antimicrobial activities of macrophages become progressively less responsive during E. chaffeensis infection in association with downregulation of pattern recognition receptors (PRR) including CD14, TLR2 and TLR4 and activity of the associated PRR transcription factor PU.1 (Refs. 55). Activation of PU.1 has been linked to a p38 MAPK-dependent pathway, and p38-specific MAPK inhibitor exhibits similar effects on PU.1 and PRR activity and expression (Refs. 72). Ehrlichia also appears to target the host tyrosine kinases and phosphatases (Fyn and PTPN2; described in more detail below), but the downstream processes effected by these interactions are not known (Refs. 31).
3. E. chaffeensis effectors and molecular host interactions
Significant progress has been made in the identification of host cell processes that are modulated by Ehrlichia in order to survive in phagocytes. However, the effector proteins involved in manipulating these host cell processes have been largely undetermined. Recent genome sequencing efforts and new biotechnology approaches to investigate molecular protein-protein interactions have focused attention on two small groups of Ehrlichia-encoded proteins that contain tandem and/or ankryin repeats. These proteins appear to be effectors involved in novel, complex and multidimensional molecular strategies to reprogram host cell defense mechanisms (Refs. 73). Two proteins, TRP47 and Ank200, have been the focus of recent studies demonstrating interactions with host cell DNA and molecular interactions with host proteins associated with distinct host cell processes.
E. chaffeensis TRP47
Ehrlichia have a small family of TRPs, many of which are strongly recognized by the host immune response, including the TRP47 (Refs. 27,74,75). TRP47 is differentially expressed on DC ehrlichiae and is found extracellularly indicating that it is secreted (Fig. 1). A recent study to examine molecular interactions between TRP47 and the host identified multiple interactions with specific host cell targets including polycomb group ring finger 5 (PCGF5), Src protein tyrosine kinase Fyn, protein tyrosine phosphatase non-receptor type 2 (PTPN2), adenylate cyclase-associated protein 1 (CAP1), and immunoglobulin lambda-like polypeptide 1 (IGLL1) with distinct cellular functions associated with signaling, transcriptional regulation, vesicle trafficking, and cellular proliferation and differentiation (Refs. 76) (Fig. 3). Notably, none of these host targets had been identified by previous studies related to the cell biology of Ehrlichia infection. The interactions between TRP47 and the host cell illustrate the complexity and diversity of pathogen-host interactions that occur and will require additional research to fully comprehend. Although the relevance of these ehrlichiae-host molecular interactions in the context of ehrlichial pathobiology remains to be determined, the host targets identified suggest that TRP47 is a multifunctional effector that plays an important role in establishing bacterial infection and promoting intracellular survival (Fig. 4).
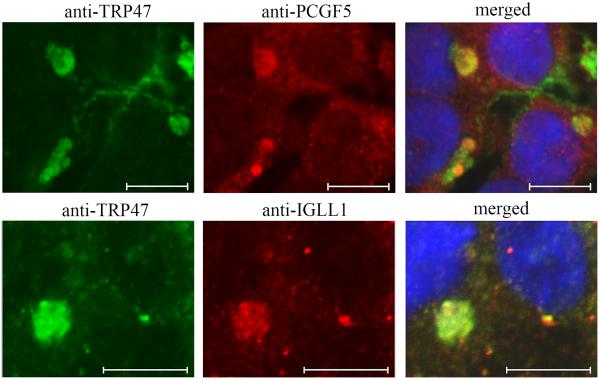
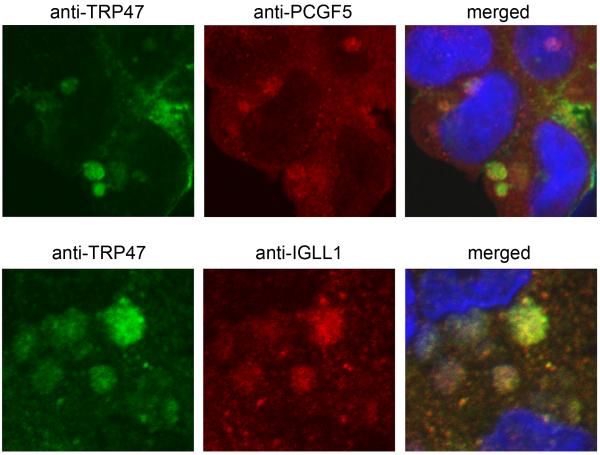
Figure 3.
Colocalization of PCGF5 and IgLL1 with E. chaffeensis expressing TRP47 with in infected THP-1 cells. E. chaffeensis-infected cells were dually-labeled with anti-TRP47 (green) and PCGF5 or IgLL1 (red) and examined by confocal microscopy. PCGF5 and IgLL1 colocalize with E. chaffeensis TRP47 labeled morulae (yellow, right merged panels). Molecular interactions between PCGF5, IGLL1, Fyn, CAP1 and PTPN2 were first reported by Wakeel et. al., 2009).
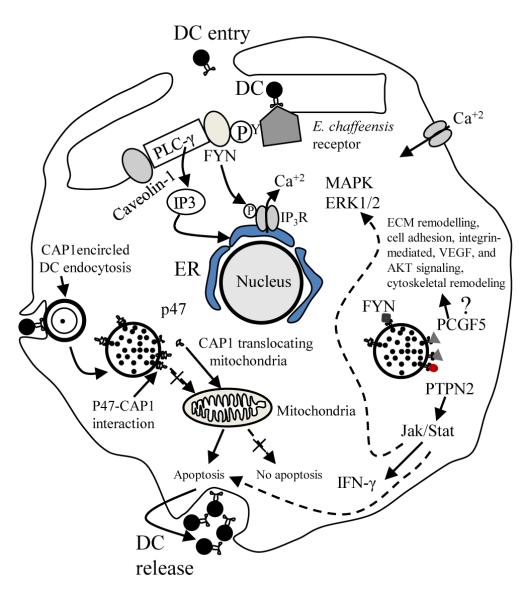
Figure 4.
A proposed model of TRP47 effector function in E. chaffeensis infection of the macrophage based on defined molecular interactions study and previous reports (Refs. 106,107,82,108,109). Binding of E. chaffeensis to its receptor directly or indirectly activates FYN. Activated FYN tyrosine kinase phosphorylates and activates PLC-γ, which hydrolyzes membrane phospholipid PIP2 (phosphatidylinositol biphosphate), resulting in increased level of IP3 (inositol 1,4,5 triphosphate) and release of Ca2+ from intracellular stores, and Ca2+ influx. FYN may regulate the function of IP3 receptor by phosphorylation and promoting release of Ca2+ from the endoplasmic reticulum. FYN also phosphorylates caveolin-1 involved in ehrlichial entry. TRP47-PTPN2 interaction modulates cytokine signaling events by exerting negative feedback on the Jak/Stat pathway by dephosphorylation of Jaks and Stats involved in intravacuolar maintenance and survival of E. chaffeensis. TRP47-PCGF5 interaction modulates gene transcription associated with cell signaling and remodeling of cytoskeleton facilitating and supporting intracellular survival of E. chaffeensis. CAP1 promotes rapid actin dynamics in conjunction with ADF/cofilin and is required for cell morphology, migration, and endocytosis. Mitochondrial shuttling of CAP1 promotes actin- and coflin-dependent apoptosis, which may play important role in release of DC ehrlichiae from the monocyte (Reproduced with permission from the American Society for Microbiology, license 2541950462120).
The strongest Ehrlichia protein-host protein interaction observed has been between TRP47 and interacting partner, PCGF5 (Refs. 31) (Fig. 3). E. chaffeensis is known to modulate host cell gene transcription, and PCGF5 has been associated with DNA-dependent regulation of transcription, metal ion binding, and protein-protein interactions. PCGF5 is related to the polycomb group proteins (transcriptional repressors) Bmi-1/PCGF4 and Mel-18/PCGF2 that play important roles in the regulation of Hox gene expression, X-chromosome inactivation, tumorigenesis, self-renewal, maintenance of pluripotency of stem cells, and stimulation of E3 ubiquitin ligase activity. Thus, it appears that TRP47-expressing DC ehrlichiae may interact with PCGF5 in order to modulate host cell gene expression to favor survival (Fig. 4).
Another interesting interaction that has been characterized is between TRP47 and IGLL1, the surrogate light chain associated with the pre-B cell receptors (Refs. 77) (Fig. 3). The pre-B cell receptor is involved in transduction of signals for cellular proliferation, differentiation from the pro-B cell to the pre-B cell stage, allelic exclusion at the Ig heavy chain gene locus, and promotion of Ig light chain gene rearrangements (Refs. 77). Thus, the significance of the interaction between TRP47 and IGLL1 might involve signaling and development, but suggests a novel role for IGLL1 in the macrophage and one that will require further study to understand.
The location of TRP47 on the surface of DC ehrlichiae suggests that this protein may be involved in host cell attachment and/or entry. The association with Fyn tyrosine kinase suggests that such a role is possible (Fig. 4). Tyrosine kinases are known to be involved in ehrlichial entry; however, the specific kinases involved have not been determined (Refs. 78). In addition, phosphorylation of host and/or bacterial proteins has been implicated in signaling pathways triggering the entry of many intracellular pathogens. Ehrlichia enter through caveolae, and Fyn is known to specifically phosphorylate caveolin-1 and is required for coxsackievirus internalization and infection via caveolin-associated vesicles of polarized epithelial cells (Refs. 79). Evidence of tyrosine phophorlyation of TRP47 has been reported, suggesting that there is a functional significance and that Fyn may be responsible (Refs. 32).
TRP47 also interacts strongly with PTPN2, also known as T cell PTP (TC-PTP), which catalyzes the dephosphorylation of phosphotyrosine peptides and regulates phosphotyrosine levels in signal transduction pathways. It is ubiquitously expressed with particularly high expression in hematopoietic tissues and appears to broadly influence hematopoietic cell development, but recent findings also demonstrate a role in several human illnesses from autoimmune disease to cancer (Refs. 80). Multiple substrates of PTPN2 include CSF-1R, EGFR, PDGFR, IR, p52Shc, Jak1, Jak3, Stat1, Stat3, Stat5a/b, and Stat6 (Refs. 81). The Jak/Stat pathway is inhibited by monocytotropic E. chaffeensis (Refs. 82), supporting the possibility that TRP47 may not only be involved in the inhibition of IFN-γ-induced tyrosine phosphorylation of Stat1, Jak1, and Jak2 by interacting with PTPN2, but also in the regulation of cellular development (Fig. 4). Ehrlichia may favor PTPN2 upregulation because the loss of PTPN2 results in Stat5 hyperactivation, increased production of gamma interferon (IFN-γ), TNF-α, IL-12, and inducible nitric oxide synthase (iNOS), increased tyrosine phosphorylation, recruitment of a Grb2/Gab2/Shp2 complex to the CSF-1 receptor, and enhanced activation of ERK, and may affect transcription factor PU.1 signaling. Thus, TRP47 may influence a large number of important signal transduction pathways by interacting with PTPN2 (Fig. 4).
Ehrlichia reside in membrane-bound vacuoles in the host cell cytoplasm that interact with endosomal recycling pathways. A specific interaction between TRP47 and the multifunctional protein CAP1 has been defined that appears to occur at the morula membrane interface, where CAP1 localizes with the DC morulae adjacent to the morula boundaries (membrane) (Refs. 76). CAP1 is a highly conserved monomeric actin binding protein that contains binding domains for actin (C-terminal), adenylyl cyclase and cofilin (N-terminal), and profilin (central region), and it plays an active role in actin turnover (Refs. 83). Genetic studies in yeast have implicated CAP1 in vesicle trafficking and endocytosis. In mammalian cells, CAP1 is associated with SH3 domain-dependent mAbp1-dynamin complex involved in receptor-mediated endocytosis (Refs. 84). Thus, in an effort to survive in the intracellular niche Ehrlichia may manipulate the mononuclear phagocyte cytoskeletal components such as actin by modulating CAP1 (Fig. 4). Interestingly, CAP1 has also been implicated in promoting apoptosis by functioning as an actin shuttle to mitochondria. Similar to cofilin, BAD, and BAX, CAP1 rapidly translocates to mitochondria independent of caspase activation where it promotes apoptosis (Refs. 85). Associations between ehrlichial morulae and mitochondria have been consistently observed (Refs. 86). Thus, the TRP47 and CAP1 interaction may be multifunctional by facilitating endocytosis and vesicle trafficking, and promoting apoptosis in the late stages of infection (Fig. 4).
E. chaffeensis Ank200 interaction with host Alu elements
There are four genes in the E. chaffeensis genome that encode proteins with Ank repeats. One of these proteins, Ank200, is a well characterized major immunoreactive protein that has multiple species-specific antibody epitopes located primarily in acidic N- and C-terminal domains (Refs. 87). The E. chaffeensis Ank200 lacks a signal peptide, but is predicted to be secreted by a leaderless secretion system (Secretome 2.0), and it has a T4SS motif (Refs. 88). Ank200 has recently been detected in host cell nuclei of E. chaffeensis-infected cells where it interacts with an adenine-rich motif in promoter and intronic Alu elements (Refs. 56). Alu elements are short interspersed mobile DNA elements distributed in a nonrandom manner that comprise approximately 5-10% of the human genome and are thought to be involved in transcriptional regulation as a carrier of cis regulatory elements (Refs. 89,90). Alu elements have known transcription factor binding sites including all MEF2 family members, HNF1.03, OC.2, BARX2 and PAX4 (Refs. 91). The global analysis of binding sites of Ank200 demonstrates that this protein binds to multiple regions distributed on nearly every chromosome via direct DNA interaction or with other DNA-binding proteins.
The host cell processes targeted by Ank200 have been classified, and include genes associated with transcriptional regulation, apoptosis, ATPase activity, and structural associations with the nucleus (Refs. 56) (Table 1). Interestingly, genes associated with host cell processes known to be altered during E. chaffeensis infection were found to be targets of Ank200. In addition, Ank200 also appears to bind genes associated with transcription, and there is evidence that a large number of genes associated with transcription are modulated during E. chaffeensis infection (Refs. 57).
Table 1.
Selected E. chaffeensis Ank200 target genes identified by ChIP-chip
| Gene | Chromosome | GeneBank(accession no) | Description |
|---|---|---|---|
| ZNF703 | 8 | NM_025069 | zinc finger protein 703 |
| ZNF513 | 2 | NM_144631 | zinc finger protein 513 |
| CD48 | 1 | NM_001778 | CD48 molecule |
| FGFRL1 | 4 | NM_021923 | fibroblast growth factor receptor-like 1 |
| PTK2B | 8 | NM_004103 | PTK2B protein tyrosine kinase 2 beta |
| STAT1 | 2 | NM_139266 | signal transducer and activator of transcription |
| PRCC | 1 | NM_199416 | papillary renal cell carcinoma |
| EFHC1 | 6 | NM_018100 | EF-hand domain (C-terminal) containing 1 |
| TNF-α | 6 | NM_000594 | tumor necrosis factor α |
| JAK2 | 9 | NM_004972 | Janus kinase 2 (a protein tyrosine kinase) |
| DIO1 | 1 | NM_000792 | deiodinase, iodothyronine, type I |
| ANKZF1 | 2 | NM_018089 | ankyrin repeat and zinc finger domain containing 1 |
| NDOR1 | 9 | NM_014434 | NADPH dependent diflavin oxidoreductase 1 |
| TDP1 | 14 | NM_018319 | tyrosyl-DNA phosphodiesterase 1 |
| USF1 | 1 | NM_007122 | upstream transcription factor 1 |
A number of Ank200 target genes have been linked to pathogenesis and immune evasion, including TNF-α, Jak2 and CD48. TNF-α expression is not induced early in infection (<48 hr) (Refs. 92,93), but expression is upregulated approximately 30-fold by day 5 post infection (Refs. 56). E. chaffeensis Ank200 may contribute to the induction of TNF-α by binding directly with the promoter and upregulating gene transcription. Studies have demonstrated that overproduction or high serum concentration of TNF-α on day 7 post infection is closely associated with fatality in severe monocytotropic ehrlichiosis (Refs. 94,95). One of the primary mechanisms by which E. chaffeensis survives in the host cell appears to be the ability to block macrophage responsiveness to IFN-γ (Refs. 60). Furthermore, Jak2 transcription appears to be silenced during E. chaffeensis infection, and Jak/Stat genes are also Ank200 targets, suggesting that E. chaffeensis uses multiple strategies, including directly modulating genes associated with the Jak/Stat pathway (Refs. 96).
4. Immunopathologic mechanisms and disease
During E. chaffeensis infection, there is a relatively low bacterial burden in the blood and tissues in non-immunocompromised patients. However, the clinical manifestations which include fever, multiorgan failure and adult respiratory distress syndrome, suggest that the pathogenesis of ehrlichiosis may involve immunopathologic responses that are described as a toxic shock-like syndrome (Refs. 97,98). Studies using a fatal murine model and a surrogate unnamed monocytic ehrlichia isolated from Ixodes ovatus ticks in Japan suggest that such a mechanism is involved (Refs. 99). Mice inoculated with IOE develop histopathologic lesions resembling those observed in HME patients, and a similar disease course is observed in the IOE murine model. Lethal infections with IOE are accompanied by extremely high levels of serum TNF-α, a high frequency of splenic TNF-α producing CD8+ T cells, decreased Ehrlichia-specific CD4+ T lymphocyte proliferation, low IL-12 levels in the spleen, and a 40-fold decrease in the number of ehrlichial antigen-specific IFN-γ producing CD4+ Th1 cells (Refs. 95,100). Furthermore, mice lacking TNF receptors I/II are resistant to IOE-induced liver injury (an apparent effect of reduced immunopathology), but exhibit higher bacterial burdens (indicating reduced protective immunity) (Refs. 101). Others have also demonstrated immunopathologic responses linked to CD8 T cells (Refs. 94). Interestingly, fatal memory responses against homologous but not heterologous challenge are associated with decreased bacterial burden, enhanced inflammatory response in the liver, decreased T cell responses, and defective maintenance of IFN-γ producing T cells (Refs. 102). CD1d-restricted NKT cells appear to be instrumental in the induction of immunopathologic responses (Refs. 103).
5. Clinical implications and applications
Ehrlichiosis manifests as an undifferentiated febrile illness that is difficult to diagnose, and delayed treatment can lead to serious complications and poor prognosis. There are no vaccines for human rickettsial diseases including ehrlichiosis, and therapeutic options are limited. The rapid growth in antibiotic resistance among microbes suggests that a potential for ineffective antibiotics for ehrlichial and rickettsial infections exists in the future. The lack of broader therapeutic options is complicated by the fact that there are no effective vaccines available to prevent disease in susceptible populations. Recent advances in our understanding of molecular pathogen-host interactions, virulence mechanisms, effector proteins, cellular basis of infection and the immunopathologic basis of clinical manifestations have provided new targets for therapies that block pathogen virulence mechanisms, host-pathogen interactions, or interrupt eukaryotic cell processes and identified additional approaches involving immunomodulatory therapies that can be considered.
Antibodies that block interactions between effector proteins and host target can be used therapeutically. For example, passive transfer of E. chaffeensis TRP120 epitope-specific antibodies reduces ehrlichial burden and demonstrates the potential of therapeutic antibodies against ehrlichial effector proteins (Refs. 104). These studies also suggest that vaccines containing defined antigens are feasible and will be developed in the future. Additionally, drugs that target specific pathogen virulence mechanisms such as secretion systems, signal transduction systems or inhibiting acquisition of essential components for pathogen structural integrity and function may be viable alternative therapies that can be utilized for patient treatment. Defining molecular host-pathogen interactions and ultimately the three-dimensional structures of effector molecules will allow development of small molecule inhibitors that can disrupt the ability of the pathogen to manipulate host cell processes resulting in pathogen clearance by the immune system.
Managing patients that are experiencing manifestations and complications associated with severe disease requires understanding of the immune dysregulation involved to develop rational strategies to alleviate the effects of the immunologic consequences. The over- production of inflammatory cytokines/chemokines is responsible for serious clinical manifestations that can be managed by targeted immunotherapies that block cytokine production or neutralize cytokine effects by blocking a specific cytokine/receptor interaction and immunopathologic consequences. Such patient management strategies can be considered as the immune effectors are comprehensively defined.
6. Research in progress and outstanding research questions
The complexity of the pathogen-host relationship between Ehrlichia and the eukaryotic host cell is becoming better understood, and the mechanisms used by the organism to reprogram cellular processes are becoming more defined. New pathogen-host interactions such as nuclear translocated proteins that bind host chromatin have been recently described, but much more research is needed to fully appreciate how these effector proteins modulate host cell transcription and whether they can be useful therapeutic targets. Other effector proteins and secretion mechanisms have been identified, but the mechanisms utilized by these effectors and how they modulate targeted host cell processes are areas of active research that will provide useful information for development of therapeutics. Much research on the cellular processes that are affected during infection has been accomplished, and advancing knowledge regarding host cell processes that are targeted by the organism will be beneficial for designing therapeutic interventions for Ehrlichia as well as other rickettsial pathogens.
Development of immunomodulatory strategies for patient management will become more practical in the future, and research to understand the immunopathologic mechanisms involved has taken large steps forward. Questions that need to be addressed are why do some patients respond immunologically in a detrimental way and how do we accurately identify and manage these patients. More research needs to be devoted to development of biomarkers for disease for diagnosis and development of rational strategies for patient management and therapeutic decisions.
Acknowledgements and funding
The authors thank all current and former laboratory members for discussions and scientific contributions towards understanding the molecular, cellular and immunobiologic aspects of Ehrlichia infections. We also appreciate the helpful comments and suggestions provided by the referees to improve the review. Jere W. McBride and David H. Walker are supported by grants (AI0071145 and AI069270 to JWM; AI 31431 to DHW) from the National Institute of Allergy and Infectious Diseases (NIAID) and jointly by the Clayton Foundation for Research.
References
- 1.Donatien A, Lestoquard F. Existence en Algerie d’uen rickettsia du chien. Bull Soc path exot. 1935;28:418–419. [Google Scholar]
- 2.Cowdry EV. Studies on the etiology of heartwater. J Exp Med. 1925;42 doi: 10.1084/jem.42.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BE, et al. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buller RS, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Eng J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 5.Paddock CD, et al. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2001;33:1586–1594. doi: 10.1086/323981. [DOI] [PubMed] [Google Scholar]
- 6.Breitschwerdt EB, Hegarty BC, Hancock SI. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman EE, et al. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J Vet Intern Med. 1998;12:61–70. doi: 10.1111/j.1939-1676.1998.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 8.Perez M, et al. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci. 2006;1078:110–117. doi: 10.1196/annals.1374.016. [DOI] [PubMed] [Google Scholar]
- 9.Keefe TJ, et al. Distribution of Ehrlichia canis among military working dogs in the world and selected civilian dogs in the United States. J Am Vet Med Assoc. 1982;181:236–238. [PubMed] [Google Scholar]
- 10.Uilenberg G. Heartwater (Cowdria ruminantium infection): current status. Adv Vet Sci Comp Med. 1983;27:427–480. [PubMed] [Google Scholar]
- 11.Koh YS, et al. MyD88-dependent signaling contributes to host defense against ehrlichial infection. PLoS One. 2010;5:e11758. doi: 10.1371/journal.pone.0011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnewall RE, Rikihisa Y, Lee EH. Ehrlichia chaffeensis inclusions are early endosomes which selectively accumulate transferrin receptor. Infect Immun. 1997;65:1455–1461. doi: 10.1128/iai.65.4.1455-1461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JZ, et al. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell Microbiol. 2007;9:610–618. doi: 10.1111/j.1462-5822.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 14.Dunning Hotopp JC, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mavromatis K, et al. The genome of the obligately intracellular bacterium Ehrlichia canis reveals themes of complex membrane structure and immune evasion strategies. J Bacteriol. 2006;188:4015–4023. doi: 10.1128/JB.01837-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins NE, et al. The genome of the heartwater agent Ehrlichia ruminantium contains multiple tandem repeats of actively variable copy number. Proc Natl Acad Sci U S A. 2005;102:838–843. doi: 10.1073/pnas.0406633102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frutos R, et al. Comparative genomic analysis of three strains of Ehrlichia ruminantium reveals an active process of genome size plasticity. J Bacteriol. 2006;188:2533–2542. doi: 10.1128/JB.188.7.2533-2542.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson JO, Andersson SG. Insights into the evolutionary process of genome degradation. Curr Opin Genet Dev. 1999;9:664–671. doi: 10.1016/s0959-437x(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 19.Andersson JO, Andersson SG. Genome degradation is an ongoing process in Rickettsia. Mol Biol Evol. 1999;16:1178–1191. doi: 10.1093/oxfordjournals.molbev.a026208. [DOI] [PubMed] [Google Scholar]
- 20.Andersson SG, et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 21.Frutos R, et al. Ehrlichia ruminantium: genomic and evolutionary features. Trends Parasitol. 2007;23:414–419. doi: 10.1016/j.pt.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Popov VL, Yu XJ, Walker DH. The 120-kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb Path. 2000;28:71–80. doi: 10.1006/mpat.1999.0327. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai Y, et al. Cyclic di-GMP signaling regulates invasion of Ehrlichia chaffeensis into human monocytes. J Bacteriol. 2010;192:4122–4133. doi: 10.1128/JB.00132-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewett TJ, et al. Chlamydial TARP is a bacterial nucleator of actin. Proc Natl Acad Sci U S A. 2006;103:15599–15604. doi: 10.1073/pnas.0603044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gravekamp C, et al. Variation in repeat number within the alpha C protein of group B streptococci alters antigenicity and protective epitopes. Infect Immun. 1996;64:3576–3583. doi: 10.1128/iai.64.9.3576-3583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo T, et al. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect Immun. 2008;76:1572–1580. doi: 10.1128/IAI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle CK, et al. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect Immun. 2006;74:711–720. doi: 10.1128/IAI.74.1.711-720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo T, Zhang X, McBride JW. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin Vaccine Immunol. 2009;16:982–990. doi: 10.1128/CVI.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride JW, et al. Identification of a glycosylated Ehrlichia canis 19-kilodalton major immunoreactive protein with a species-specific serine-rich glycopeptide epitope. Infect Immun. 2007;75:74–82. doi: 10.1128/IAI.01494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakeel A, et al. Investigation of Ehrlichia chaffeensis secreted tandem repeat and Ank proteins in a type IV secretion system model. The 23rd Meeting of the American Society for Rickettsiology; Hilton Head, SC. August 15-18, 2009; 2009. Presented at: [Google Scholar]
- 31.Wakeel A, Kuriakose JA, McBride JW. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect Immun. 2009;77:1734–1745. doi: 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakeel A, Zhang X, McBride JW. Mass spectrometric analysis of Ehrlichia chaffeensis tandem repeat proteins reveals evidence of phosphorylation and absence of glycosylation. PLoS One. 2010;5:e9552. doi: 10.1371/journal.pone.0009552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu B, McBride JW. Ehrlichia chaffeensis tandem repeat protein binds GC-rich host DNA. The 110th General Meeting of the American Society for Microbiology; San Diego, CA. May 23-27, 2010; 2010. Presented at: [Google Scholar]
- 34.Luo T, et al. Molecular characterization of antibody epitopes of Ehrlichia chaffeensis ankyrin protein 200 and tandem repeat protein 47 and evaluation of synthetic immunodeterminants for serodiagnosis of human monocytotropic ehrlichiosis. Clin Vaccine Immunol. 2010;17:87–97. doi: 10.1128/CVI.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nethery KA, et al. Ehrlichia canis gp200 contains dominant species-specific antibody epitopes in terminal acidic domains. Infect Immun. 2007;75:4900–4908. doi: 10.1128/IAI.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin M, et al. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 37.Mott J, Barnewall RE, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbet AF, et al. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect Immun. 2000;68:6133–6138. doi: 10.1128/iai.68.11.6133-6138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbet AF, et al. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect Immun. 2003;71:1706–1718. doi: 10.1128/IAI.71.4.1706-1718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, et al. Rapid sequential changeover of expressed p44 genes during the acute phase of Anaplasma phagocytophilum infection in horses. Infect Immun. 2004;72:6852–6859. doi: 10.1128/IAI.72.12.6852-6859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotopp JC, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohashi N, Rikihisa Y, Unver A. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect Immun. 2001;69:2083–2091. doi: 10.1128/IAI.69.4.2083-2091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unver A, et al. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect Immun. 2002;70:4701–4704. doi: 10.1128/IAI.70.8.4701-4704.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo GM, et al. Total, membrane, and immunogenic proteomes of macrophage- and tick cell-derived Ehrlichia chaffeensis evaluated by liquid chromatography-tandem mass spectrometry and MALDI-TOF methods. Infect Immun. 2008;76:4823–4832. doi: 10.1128/IAI.00484-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unver A, et al. Transcriptional analysis of p30 major outer membrane multigene family of Ehrlichia canis in dogs, ticks, and cell culture at different temperatures. Infect Immun. 2001;69:6172–6178. doi: 10.1128/IAI.69.10.6172-6178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang JZ, et al. Expression of members of the 28-kilodalton major outer membrane protein family of Ehrlichia chaffeensis during persistent infection. Infect Immun. 2004;72:4336–4343. doi: 10.1128/IAI.72.8.4336-4343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumagai Y, Huang H, Rikihisa Y. Expression and porin activity of P28 and OMP-1F during intracellular Ehrlichia chaffeensis development. J Bacteriol. 2008;190:3597–3605. doi: 10.1128/JB.02017-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popov VL, Yu X, Walker DH. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb Pathog. 2000;28:71–80. doi: 10.1006/mpat.1999.0327. [DOI] [PubMed] [Google Scholar]
- 50.de la Fuente J, et al. Adhesion of outer membrane proteins containing tandem repeats of Anaplasma and Ehrlichia species (Rickettsiales: Anaplasmataceae) to tick cells. Vet Microbiol. 2004;98:313–322. doi: 10.1016/j.vetmic.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JZ, McBride JW, Yu XJ. L-selectin and E-selectin expressed on monocytes mediating Ehrlichia chaffeensis attachment onto host cells. FEMS Microbiol Lett. 2003;227:303–309. doi: 10.1016/S0378-1097(03)00696-7. [DOI] [PubMed] [Google Scholar]
- 52.Lin M, Rikihisa Y. Obligatory intracellular parasitism by Ehrlichia chaffeensis and Anaplasma phagocytophilum involves caveolae and glycosylphosphatidylinositol-anchored proteins. Cell Microbiol. 2003;5:809–820. doi: 10.1046/j.1462-5822.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- 53.Lin M, Zhu MX, Rikihisa Y. Rapid activation of protein tyrosine kinase and phospholipase C-gamma2 and increase in cytosolic free calcium are required by Ehrlichia chaffeensis for internalization and growth in THP-1 cells. Infect Immun. 2002;70:889–898. doi: 10.1128/IAI.70.2.889-898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mott J, Barnewall RE, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin M, Rikihisa Y. Ehrlichia chaffeensis downregulates surface Toll-like receptors 2/4, CD14 and transcription factors PU.1 and inhibits lipopolysaccharide activation of NF-kappa B, ERK 1/2 and p38 MAPK in host monocytes. Cell Microbiol. 2004;6:175–186. doi: 10.1046/j.1462-5822.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhu B, et al. Nuclear translocated Ehrlichia chaffeensis ankyrin protein interacts with the mid A-stretch of host promoter and intronic Alu elements. Infect Immun. 2009;77:4243–4255. doi: 10.1128/IAI.00376-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang JZ, et al. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect Immun. 2004;72:498–507. doi: 10.1128/IAI.72.1.498-507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alvarez-Dominguez C, et al. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J Biol Chem. 1996;271:13834–13843. doi: 10.1074/jbc.271.23.13834. [DOI] [PubMed] [Google Scholar]
- 59.Barnewall RE, Ohashi N, Rikihisa Y. Ehrlichia chaffeensis and E. sennetsu, but not the human granulocytic erhlichiosis agent, colocalize with transferrin receptor and up-regulate transferrin receptor mRNA by activating iron-responsive protein 1. Infect Immun. 1999;67:2258–2265. doi: 10.1128/iai.67.5.2258-2265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnewall RE, Rikihisa Y. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron-transferrin. Infect Immun. 1994;62:4804–4810. doi: 10.1128/iai.62.11.4804-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doyle CK, et al. An immunoreactive 38-kilodalton protein of Ehrlichia canis shares structural homology and iron-binding capacity with the ferric ion-binding protein family. Infect Immun. 2005;73:62–69. doi: 10.1128/IAI.73.1.62-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin M, Rikihisa Y. Degradation of p22phox and inhibition of superoxide generation by Ehrlichia chaffeensis in human monocytes. Cell Microbiol. 2007;9:861–874. doi: 10.1111/j.1462-5822.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 64.Xiong Q, et al. Ehrlichia ewingii infection delays spontaneous neutrophil apoptosis through stabilization of mitochondria. J Infect Dis. 2008;197:1110–1118. doi: 10.1086/533457. [DOI] [PubMed] [Google Scholar]
- 65.Kumagai Y, et al. Biochemical activities of three pairs of Ehrlichia chaffeensis two-component regulatory system proteins involved in inhibition of lysosomal fusion. Infect Immun. 2006;74:5014–5022. doi: 10.1128/IAI.00735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parkinson JS, Kofoid EC. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 67.Cheng Z, et al. Intra-leukocyte expression of two-component systems in Ehrlichia chaffeensis and Anaplasma phagocytophilum and effects of the histidine kinase inhibitor closantel. Cell Microbiol. 2006;8:1241–1252. doi: 10.1111/j.1462-5822.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 68.Collins HL. Withholding iron as a cellular defence mechanism--friend or foe? Eur J Immunol. 2008;38:1803–1806. doi: 10.1002/eji.200838505. [DOI] [PubMed] [Google Scholar]
- 69.Collins HL. The role of iron in infections with intracellular bacteria. Immunol Lett. 2003;85:193–195. doi: 10.1016/s0165-2478(02)00229-8. [DOI] [PubMed] [Google Scholar]
- 70.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 71.Lee EH, Rikihisa Y. Protein kinase A-mediated inhibition of gamma interferon-induced tyrosine phosphorylation of Janus kinases and latent cytoplasmic transcription factors in human monocytes by Ehrlichia chaffeensis. Infect Immun. 1998;66:2514–2520. doi: 10.1128/iai.66.6.2514-2520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JM, Lai MZ, Yang-Yen HF. Interleukin-3 stimulation of mcl-1 gene transcription involves activation of the PU.1 transcription factor through a p38 mitogen-activated protein kinase-dependent pathway. Mol Cell Biol. 2003;23:1896–1909. doi: 10.1128/MCB.23.6.1896-1909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wakeel A, et al. New insights into molecular Ehrlichia chaffeensis-host interactions. Microbes Infect. 2010;12:337–345. doi: 10.1016/j.micinf.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo T, Zhang X, McBride JW. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin Vaccine Immunol. 2009;16:982–990. doi: 10.1128/CVI.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo T, et al. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect Immun. 2008;76:1572–1580. doi: 10.1128/IAI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wakeel A, Kuriakose JA, McBride JW. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect Immun. 2009;77:1734–1745. doi: 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 78.Lin M, Zhu MX, Rikihisa Y. Rapid activation of protein tyrosine kinase and phospholipase C-gamma2 and increase in cytosolic free calcium are required by Ehrlichia chaffeensis for internalization and growth in THP-1 cells. Infect Immun. 2002;70:889–898. doi: 10.1128/IAI.70.2.889-898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 80.Doody KM, Bourdeau A, Tremblay ML. T-cell protein tyrosine phosphatase is a key regulator in immune cell signaling: lessons from the knockout mouse model and implications in human disease. Immunol Rev. 2009;228:325–341. doi: 10.1111/j.1600-065X.2008.00743.x. [DOI] [PubMed] [Google Scholar]
- 81.Stuible M, Doody KM, Tremblay ML. PTP1B and TC-PTP: regulators of transformation and tumorigenesis. Cancer Metastasis Rev. 2008;27:215–230. doi: 10.1007/s10555-008-9115-1. [DOI] [PubMed] [Google Scholar]
- 82.Lee EH, Rikihisa Y. Protein kinase A-mediated inhibition of gamma interferon-induced tyrosine phosphorylation of Janus kinases and latent cytoplasmic transcription factors in human monocytes by Ehrlichia chaffeensis. Infect Immun. 1998;66:2514–2520. doi: 10.1128/iai.66.6.2514-2520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hubberstey AV, Mottillo EP. Cyclase-associated proteins: CAPacity for linking signal transduction and actin polymerization. FASEB J. 2002;16:487–499. doi: 10.1096/fj.01-0659rev. [DOI] [PubMed] [Google Scholar]
- 84.Kessels MM, et al. Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J Cell Biol. 2001;153:351–366. doi: 10.1083/jcb.153.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang C, et al. Mitochondrial shuttling of CAP1 promotes actin- and cofilin-dependent apoptosis. J Cell Sci. 2008;121:2913–2920. doi: 10.1242/jcs.023911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Popov VL, et al. Ultrastructural variation of cultured Ehrlichia chaffeensis. J Med Microbiol. 1995;43:411–421. doi: 10.1099/00222615-43-6-411. [DOI] [PubMed] [Google Scholar]
- 87.Luo T, et al. Molecular characterization of antibody epitopes of Ehrlichia chaffeensis ankyrin protein 200 and tandem repeat protein 47 and evaluation of synthetic immunodeterminants for serodiagnosis of human monocytotropic ehrlichiosis. Clin Vaccine Immunol. 2010;17:87–97. doi: 10.1128/CVI.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rikihisa Y, Lin M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol. 2010;13:59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomilin NV, et al. Differential binding of human nuclear proteins to Alu subfamilies. Nucleic Acids Res. 1992;20:2941–2945. doi: 10.1093/nar/20.12.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matera AG, Hellmann U, Schmid CW. A transpositionally and transcriptionally competent Alu subfamily. Mol Cell Biol. 1990;10:5424–5432. doi: 10.1128/mcb.10.10.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polak P, Domany E. Alu elements contain many binding sites for transcription factors and may play a role in regulation of developmental processes. BMC Genomics. 2006;7:133. doi: 10.1186/1471-2164-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee EH, Rikihisa Y. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1beta, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect Immun. 1996;64:4211–4219. doi: 10.1128/iai.64.10.4211-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee EH, Rikihisa Y. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IkappaB-alpha and activation of NF-kappaB. Infect Immun. 1997;65:2890–2897. doi: 10.1128/iai.65.7.2890-2897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bitsaktsis C, Winslow G. Fatal recall responses mediated by CD8 T cells during intracellular bacterial challenge infection. J Immunol. 2006;177:4644–4651. doi: 10.4049/jimmunol.177.7.4644. [DOI] [PubMed] [Google Scholar]
- 95.Ismail N, et al. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol. 2004;172:1786–1800. doi: 10.4049/jimmunol.172.3.1786. [DOI] [PubMed] [Google Scholar]
- 96.Zhang JZ, et al. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect Immun. 2004;72:498–507. doi: 10.1128/IAI.72.1.498-507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fichtenbaum CJ, Peterson LR, Weil GJ. Ehrlichiosis presenting as a life-threatening illness with features of the toxic shock syndrome. Am J Med. 1993;95:351–357. doi: 10.1016/0002-9343(93)90302-6. [DOI] [PubMed] [Google Scholar]
- 98.Maeda K, et al. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 99.Sotomayor E, et al. Animal model of fatal human monocytotropic ehrlichiosis. Am J Path. 2001;158:757–769. doi: 10.1016/S0002-9440(10)64018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ismail N, et al. Relative importance of T-cell subsets in monocytotropic ehrlichiosis: a novel effector mechanism involved in Ehrlichia-induced immunopathology in murine ehrlichiosis. Infect Immun. 2007;75:4608–4620. doi: 10.1128/IAI.00198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ismail N, Stevenson HL, Walker DH. Role of tumor necrosis factor alpha (TNF-alpha) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection. Infect Immun. 2006;74:1846–1856. doi: 10.1128/IAI.74.3.1846-1856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thirumalapura NR, et al. Protective heterologous immunity against fatal ehrlichiosis and lack of protection following homologous challenge. Infect Immun. 2008;76:1920–1930. doi: 10.1128/IAI.01293-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stevenson HL, et al. Regulatory roles of CD1d-restricted NKT cells in the induction of toxic shock-like syndrome in an animal model of fatal ehrlichiosis. Infect Immun. 2008;76:1434–1444. doi: 10.1128/IAI.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuriakose JA, et al. Reduction of Ehrlichia chaffeensis burden by epitope specific TRP120 antibody. 24th Meeting of the American Society for Rickettsiology; Stevenson, WA. July 31-August 3, 2010; 2010. Presented at: [Google Scholar]
- 105.Popov VL, et al. Ultrastructural variation of cultured Ehrlichia chaffeensis. J Med Microbiol. 1995;43:411–421. doi: 10.1099/00222615-43-6-411. [DOI] [PubMed] [Google Scholar]
- 106.Lin M, Rikihisa Y. Ehrlichia chaffeensis downregulates surface Toll-like receptors 2/4, CD14 and transcription factors PU.1 and inhibits lipopolysaccharide activation of NF-kappa B, ERK 1/2 and p38 MAPK in host monocytes. Cell Microbiol. 2004;6:175–186. doi: 10.1046/j.1462-5822.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- 107.Zhang JZ. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect Immun. 2004;72:498–507. doi: 10.1128/IAI.72.1.498-507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin M, Rikihisa Y. Obligatory intracellular parasitism by Ehrlichia chaffeensis and Anaplasma phagocytophilum involves caveolae and glycosylphosphatidylinositol-anchored proteins. Cell Microbiol. 2003;5:809–820. doi: 10.1046/j.1462-5822.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- 109.Zhang JZ, et al. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell Microbiol. 2007;9:610–618. doi: 10.1111/j.1462-5822.2006.00812.x. [DOI] [PubMed] [Google Scholar]
Further reading, resources and contacts
- Rikihisa Y. Molecular events involved in cellular invasion by Ehrlichia chaffeensis and Anaplasma phagocytophilum. Veterinary Parastiology. 2010;167:155–166. doi: 10.1016/j.vetpar.2009.09.017. This is a recent review describing the molecular events of cellular invasion from the perspective of the host cell.
- Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nature Reviews Microbiology. 2010;8:328–339. doi: 10.1038/nrmicro2318. This recent review describes the cellular processes that are subverted or hijacked by Ehrlichia.
- Ganta RR, Peddireddi L, Seo GM, Dedonder SE, Cheng C, Chapes SK. Molecular characterization of Ehrlichia interactions with tick cells and macrophages. Frontiers in Bioscience. 2009;14:3259–73. doi: 10.2741/3449. This review describes some of the more recent findings related to interactions with tick cells.
- McBride JW, Walker DH. Progress and obstacles in vaccine development for the ehrlichioses. Expert Reviews in Vaccines. 2010;9:1071–1082. doi: 10.1586/erv.10.93. This review provides summary of recent advances in vaccine development.
- Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Review of Anti-Infective Therapy. 2009;7:709–22. doi: 10.1586/eri.09.44. This review covers epidemiologic and clinical aspects related to these diseases and suggestions for potential future research.