Significance
We determined the transcriptional program that is rapidly provoked to counteract heat-induced stress and uncovered the broad range of molecular mechanisms that maintain cellular homeostasis under hostile conditions. Because transcriptional responses are directed in the complex chromatin environment that undergoes dramatic changes during the cell cycle progression, we identified the genomewide transcriptional response to stress also in cells where the chromatin is condensed for mitotic division. Our results highlight the importance of the cell cycle phase in provoking cellular responses and identify molecular mechanisms that direct transcription during the progression of the cell cycle.
Keywords: ChIP-seq, ENCODE, human genome, proteostasis
Abstract
Heat shock factors (HSFs) are the master regulators of transcription under protein-damaging conditions, acting in an environment where the overall transcription is silenced. We determined the genomewide transcriptional program that is rapidly provoked by HSF1 and HSF2 under acute stress in human cells. Our results revealed the molecular mechanisms that maintain cellular homeostasis, including HSF1-driven induction of polyubiquitin genes, as well as HSF1- and HSF2-mediated expression patterns of cochaperones, transcriptional regulators, and signaling molecules. We characterized the genomewide transcriptional response to stress also in mitotic cells where the chromatin is tightly compacted. We found a radically limited binding and transactivating capacity of HSF1, leaving mitotic cells highly susceptible to proteotoxicity. In contrast, HSF2 occupied hundreds of loci in the mitotic cells and localized to the condensed chromatin also in meiosis. These results highlight the importance of the cell cycle phase in transcriptional responses and identify the specific mechanisms for HSF1 and HSF2 in transcriptional orchestration. Moreover, we propose that HSF2 is an epigenetic regulator directing transcription throughout cell cycle progression.
Cells exposed to proteotoxic conditions provoke a rapid and transient response to maintain homeostasis. The stress response induces profound cellular adaptation as cytoskeleton and membranes are reorganized, cell cycle progression is stalled, and the global transcription and translation are silenced (1, 2). Despite the silenced chromatin environment, the stressed cell mounts a transcriptional program that involves induction of genes coding for heat shock proteins (HSPs). HSPs are molecular chaperones and proteases that assist in protein folding and maintain cellular structures and molecular functions (3).
Heat shock factor 1 (HSF1) is an evolutionarily well-conserved transcription factor that is rapidly activated by stress and absolutely required for the stress-induced HSP expression (4). Aberrant HSF1 levels are associated with stress sensitivity, aging, neurodegenerative diseases, and cancer (5–9). Instead of a single HSF in yeasts and invertebrates, vertebrates contain a family of four members, HSF1–4. HSF2 and HSF4 are involved in corticogenesis, spermatogenesis, and formation of sensory epithelium, and they have primarily been considered as developmental factors (10–14). HSF1 and HSF2 share high sequence homology of the DNA-binding and oligomerization domains and are able to form heterotrimers at the chromatin (15, 16). Moreover, HSF2 participates in the regulation of stress-responsive genes and is required for proper protein clearance also at febrile temperatures (17, 18). Although HSF1 and HSF2 have been shown to interplay on the heat shock elements (HSEs) of the target loci, their impacts on transcription of chaperone genes are remarkably different; HSF1 is a potent inducer of transcription, whereas HSF2 is a poor transactivator of HSPs on heat stress (19–21). HSF1 and HSF2 are also subjected to distinct regulatory mechanisms, because HSF1 is a stable protein that undergoes rapid posttranslational modifications (PTMs), and HSF2 is predominantly regulated at the level of expression (22, 23).
The rapid and robust chaperone expression has served as a model for inducible transcriptional responses (24). However, the previous studies have almost exclusively concentrated on the expression of a handful of HSP genes in unsynchronized cell populations (17, 25–28). Currently, comprehensive knowledge on the target genes for HSF1 and HSF2 and their cooperation during stress responses is missing. Moreover, the cell cycle progression creates profoundly different environments for transcription depending on whether the chromatin undergoes replication or division or whether the cell resides in the gap phases. For transcription factors, the synthesis phase provides an opportunity to access the transiently unwound DNA, whereas in mitosis, most factors are excluded from the condensed chromatin (29–32). Importantly, throughout the cell cycle progression, epigenetic cues are required to maintain the cellular identity and fate (33).
In this study, we investigated the genomewide transcriptional response that is provoked in the acute phase of heat stress in freely cycling cells and in cells arrested in mitosis. We characterized the transactivator capacities and the genomewide target loci for HSF1 and HSF2 and analyzed chromatin landmarks at the HSF target sites. By comparing transcriptional responses in cycling versus mitotic cells, we determined the ability of mitotic cells to respond to proteotoxic insults and the capacity of transcription factors to interact with chromatin that is condensed for cell division. We discovered the broad range of molecular mechanisms that maintain cellular homeostasis in stressed cells and provide unique mechanistic insights into the regulation of gene expression during the cell cycle progression. Our results revealed the cooperation of HSF1 and HSF2 in orchestrating gene expression in stressed cycling cells and identified their profoundly distinct capacities to coordinate transcription in cells where the chromatin is compacted for cell division.
Results
Genomewide Identification of Target Sites for HSF1 and HSF2 in Cycling and Mitotic Cells.
ChIP coupled to massively parallel sequencing (ChIP-seq) is a powerful method that enables genomewide mapping of protein binding sites in a high-resolution and unbiased manner (34–36). We used ChIP-seq to characterize the binding sites for HSF1 and HSF2 in cycling and mitotic cells that were either untreated or heat treated for 30 min at 42 °C. As the model system, we chose human K562 erythroleukemia cells, where HSF1 and HSF2 levels and regulatory mechanisms are well characterized (17, 20, 37), and chromatin landmarks have been identified by the Encyclopedia of DNA Elements (ENCODE) consortium (38). The efficiency of cell cycle arrest was improved by collecting the cells in S-phase before nocodazole treatment (39). Histograms of cells based on the DNA content are shown in Fig. 1A, confirming the mitotic arrest (5% of cells in G1 and 85% in G2/M) compared with freely cycling cells (45% in G1 and 15% in G2/M). For sequencing, 10 PCR-verified ChIP-replicates were collected per sample, and two controls, IgG and input, were included (Fig. S1).
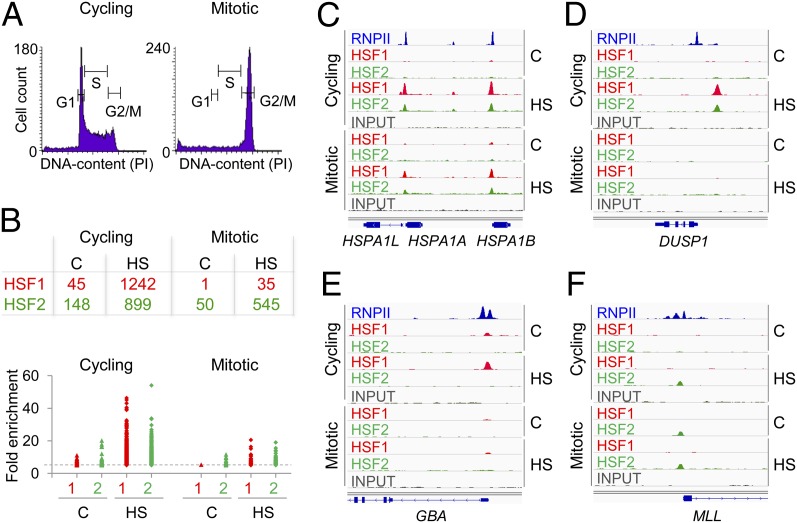
Fig. 1.
Binding sites for HSF1 and HSF2 in cycling and mitotic K562 cells. (A) Histograms of cycling and mitotic cells based on the DNA content. (B) (Upper) Number of HSF1 (red) and HSF2 (green) target loci in cycling and mitotic cells. (Lower) Fold enrichment over input of each identified HSF1 (red) and HSF2 (green) target locus. The dashed gray line indicates fold enrichment five, which was set as cutoff criterion for HSF target sites. (C–F) Enrichments of HSF1 (red) and HSF2 (green) on indicated target genes in cycling and mitotic cells. (C) HSPA1A and HSPA1B promoters are bound by HSF1 and HSF2 in cycling and mitotic cells. (D) DUSP1 promoter harbors prominent HSF1 and HSF2 enrichments in stressed cycling cells only. (E) GBA is an HSF1-specific and (F) MLL is an HSF2-specific target promoter. Distribution of RNPII in nontreated cycling cells (blue) is recovered from the ENCODE project (wgEncodeEH000616; Snyder Laboratory, Yale University). The scale for each HSF or input sample is set to 0–100, except at the HSPA1 locus, where, due to high enrichments, the scale is set to 0–250 in cycling and 0–125 in mitotic cells. C, control; HS, heat shock.
The ChIP-seq provided high-resolution maps of HSF1 and HSF2 target loci in the human genome (Dataset S1; Gene Expression Omnibus accession no. GSE43579). Under optimal growth conditions in cycling cells, 45 target sites were identified for HSF1 and 148 for HSF2. On acute stress, both the number of the target sites (1242 for HSF1 and 899 for HSF2) and the fold enrichments of the targets were considerably higher, indicating a rapid recruitment of HSF1 and HSF2 to their target loci in heat-stressed cycling cells (Fig. 1B; Dataset S1). In mitosis, the ability of HSFs to bind chromatin was clearly different; HSF2 occupied 50 loci under optimal conditions and 545 loci on acute stress. In contrast, HSF1 interacted only with the promoter of HSPA1B/HSP70.2 in the absence of stress, and with 35 loci on heat stress (Fig. 1B; Dataset S1). Although effectively excluded from the dividing chromatin, HSF1 displayed prominent heat-induced occupancy on certain loci, including promoters of HSPA1A/HSP70.1, HSPA1B/HSP70.2, HSPH1/HSP110, MRPS6 (mitochondrial ribosome protein 6), and DNAJB6 (Fig. 1C; Dataset S1). Enrichments of HSF1 and HSF2 on selected target genes are illustrated in Fig. 1 C–F.
The HSPA1/HSP70 locus was strongly bound by HSF1 and HSF2 in heat-treated cycling and mitotic cells (Fig. 1C), whereas DUSP1 (dual specific phosphatase 1) was occupied by HSF1 and HSF2 in cycling cells only, demonstrating the importance of the cell cycle phase in transcriptional responses (Fig. 1D). In Fig. 1 E and F, the capacity of HSF1 and HSF2 to bind their individual target loci is indicated with promoters of GBA (glucosidase β acid) and MLL (myeloid/lymphoid or mixed-lineage leukemia). Intriguingly, HSF2 occupied the promoter of MLL in unstressed mitotic cells, although in cycling cells the binding was strictly induced by heat shock (Fig. 1F). Promoter-proximally paused RNA polymerase II (RNPII) was originally identified at the HSP70 promoter (40, 41), and it is estimated to poise ∼30% of human genes for rapid or synchronous activation (42). We investigated the status of RNPII at selected HSF target promoters using an existing ChIP-seq data on RNPII (wgEncodeEH000616; Snyder Laboratory, Yale University). ChIP cannot determine transcriptional engagement, but recent global-run-on sequencing (GRO-seq) experiments revealed that the majority of promoter-associated polymerases are transcriptionally engaged and competent for elongation (43). Accordingly, we refer to RNPII whose enrichment on a promoter site exceeds at least five times the overall signal on the gene as paused RNPII (see below). In Fig. 1 C–F, the distribution of RNPII is visualized.
HSF1 and HSF2 Recognize Similar Consensus DNA Sequences but Display Distinct Binding Profiles in the Human Genome.
Binding of HSF2 to target genes on stress has been considered to occur in an HSF1-dependent manner (16, 17). However, we identified target genes that are specific for either HSF1 or HSF2 (Fig. 1 E and F; Fig. S2A; Dataset S1). In heat-treated cycling cells, HSF1 and HSF2 shared a majority of their target sites, but under optimal growth conditions and in mitosis, they displayed strikingly different localization to the human genome (Fig. S2A). Despite their distinct binding sites, HSF1 and HSF2 recognized similar HSEs both in cycling and mitotic cells (Fig. S2B), demonstrating that the DNA sequence alone is not sufficient to determine the binding site for HSF1 vs. HSF2.
Given that promoters and exons account for <2% of the human genome (Human Genome Project), HSF1 and HSF2 were enriched on gene promoters and protein coding sequences (Fig. S3A). For example, 19% of HSF1 and 22% of HSF2 target loci were found within promoters in heat-treated cycling cells (Fig. S3A). On average, the enrichments of HSF1 and HSF2 were higher on promoter regions than on coding sequences (Fig. S3B), but prominent HSF1 and HSF2 targets were found also within introns, exons, and intergenic regions as illustrated with PRKCA and PRKCB (protein kinase C α and β, respectively), KCNN1 (calcium-activated channel N1), and an intergenic region upstream of HSPB1/HSP27 (Fig. S3C).
HSF1 and HSF2 Bind Selected Chaperone Gene Promoters.
HSF1 and HSF2 are best known for their stress-related regulatory functions on certain chaperone genes, such as HSPA1A/HSP70.1, HSPC/HSP90, HSPB1/HSP27, and DNAJB1/HSP40 (4, 17, 26, 27). The ChIP-seq enabled unbiased analyses of HSF1 and HSF2 on every chaperone gene encoded in the human genome and revealed HSF occupancy on 70% of HSP, 90% of chaperonin, and 13% of DNAJ genes (Table 1; Table S1). A majority of the HSF-targeted chaperone genes were bound by both HSF1 and HSF2 on promoters that contained paused RNPII (Table 1; Table S1). Exceptions were the small HSPs HSPB2/HSP27-2, HSPB5/CRYAB, and HSPB9, whose promoters lacked paused RNPII (Table 1). To address the impact of HSF1 and HSF2 on transcription, we depleted either HSF1 or HSF2, or both factors together, by shRNA-mediated degradation and analyzed a set of HSF-targeted chaperone genes for their mRNA expression during heat shock (Fig. 2 A and B; Fig. S4A). In freely cycling cells, HSPH1/HSP110, HSPH2, and DNAJB6 were identified as unique HSF-regulated genes whose heat-induced expression was strictly dependent on HSF1 (Fig. 2B; Fig. S4A). CCT1 (chaperonin 1) has previously been shown to be regulated by HSFs (44), which we corroborated in K562 cells (Fig. 2B). The HSPH1/HSP110 mRNA expression rapidly increased to sevenfold, whereas the levels of DNAJB6 and CCT1 mRNA doubled during the acute heat stress (Fig. 2B). DNAJB6 is the most potent chaperone in inhibiting protein aggregation, and HSPH1/HSP110 remodels the HSP70-HSP40 machinery to solubilize and refold aggregated proteins in metazoan cells (45, 46). These results indicate a considerably wider repertoire of HSF-induced chaperones than earlier anticipated and highlight the importance of managing aggregated proteins under proteotoxic conditions.
Table 1.
HSF1 and HSF2 occupancy on HSPA, HSPB, HSPC, HSPH, and chaperonin genes
| Family | Gene | Alias | HSF1 |
HSF2 |
Binding site | Paused RNPII* | ||||||
| Cycling |
Mitosis |
Cycling |
Mitosis |
|||||||||
| C | HS | C | HS | C | HS | C | HS | |||||
| HSPA | HSPA1A | HSP70-1 | 2.8 | 40.4 | 2.5 | 16.3 | 5.2 | 25.6 | 3.3 | 10.8 | Promoter | Yes |
| HSPA1B | HSP70-2 | 4.3 | 45.1 | 5.3 | 20.5 | 5.4 | 26.8 | 5.4 | 15.5 | Promoter | Yes | |
| HSPA1L | HSP70-1L | 2.8 | 40.4 | 2.5 | 16.3 | 5.2 | 25.6 | 3.3 | 10.8 | Promoter | Yes | |
| HSPA2 | HSPA3 | — | — | — | — | — | — | — | — | — | No | |
| HSPA5 | BIP, GRP78 | — | — | — | — | — | — | — | — | — | Yes | |
| HSPA6 | HSP70B' | — | 15.2 | — | — | — | 7.3 | — | — | Promoter | Yes | |
| HSPA7 | HSP70 | — | 3.0 | — | — | — | — | — | — | Promoter | No | |
| HSPA8 | HSC70 | 4.3 | 29.5 | 2.8 | 5.3 | 20.2 | 26.8 | 8.3 | 12.3 | Promoter | Yes | |
| HSPA9 | GRP75 | — | 6.6 | — | — | — | 5.9 | — | — | Promoter | Yes | |
| HSPA12A | FLJ13874 | — | — | — | — | — | — | — | — | — | No | |
| HSPA12B | RP23-32L15.1 | — | — | — | — | — | — | — | — | — | No | |
| HSPA13 | Stch | — | — | — | — | — | — | — | — | — | Yes | |
| HSPA14 | HSP70-4 | — | — | — | — | — | — | — | — | — | Yes | |
| HSPB | HSPB1 | HSP27-1 | — | 23.8 | — | 6.3 | — | 16.5 | 3.5 | 5.6 | Promoter | Yes |
| HSPB2 | HSP27-2 | — | 14.1 | — | — | — | 10.4 | — | — | Promoter | No | |
| HSPB3 | HSPL27 | — | — | — | — | — | — | — | — | — | No | |
| HSPB4 | CRYAA | — | — | — | — | — | — | — | — | — | No | |
| HSPB5 | CRYAB | — | 14.1 | — | — | — | 10.4 | — | — | Promoter | No | |
| HSPB6 | HSP20 | — | — | — | — | — | — | — | — | — | No | |
| HSPB7 | cvHSP | — | — | — | — | — | — | — | — | — | No | |
| HSPB8 | HSP22 | — | — | — | — | — | — | — | — | — | No | |
| HSPB9 | FLJ27437 | — | 12.3 | — | 3.5 | 7.0 | 19.0 | — | 6.0 | Promoter | No | |
| HSPB10 | ODF1 | — | — | — | — | — | — | — | — | — | NA | |
| HSPB11 | HSP16.2 | — | — | — | — | — | — | 3.1 | — | Promoter | Yes | |
| HSPC | HSPC1 | HSP90AA1 | — | 13.2 | — | 6.0 | — | 10.5 | 3.2 | 5.3 | Promoter | Yes |
| HSPC2 | HSP90AA2 | — | — | — | — | — | — | — | — | — | NA | |
| HSPC3 | HSP90AB1 | — | 23.8 | — | 8.2 | 6.8 | 14.1 | — | 9.9 | Promoter | Yes | |
| HSPC4 | HSP90B1 | — | — | — | — | — | — | — | — | — | Yes | |
| HSPC5 | TRAP1, HSP90L | — | — | — | — | — | — | — | — | — | Yes | |
| HSPD | HSPD1 | HSP60, GROEL | 5.8 | 43.0 | 3.5 | 15.1 | 17.8 | 33.4 | 6.0 | 9.5 | Promoter | Yes |
| HSPE | HSPE1 | HSP10, GROES | 5.8 | 43.0 | 3.5 | 15.1 | 17.8 | 33.4 | 6.0 | 9.5 | Promoter | Yes |
| HSPH | HSPH1 | HSP105/110 | — | 32.6 | — | 8.8 | — | 19.0 | — | 6.3 | Promoter | Yes |
| HSPH2 | HSPA4 | — | 22.5 | — | 4.2 | — | 20.9 | — | 4.2 | Promoter | Yes | |
| HSPH3 | HSPA4L | — | 9.1 | — | 4.2 | — | 5.5 | — | 3.5 | Promoter | Yes | |
| HSPH4 | HYOU1 | — | — | — | — | — | — | — | — | — | Yes | |
| TRiC | CCT1 | TCP1, CCTA | 3.5 | 31.8 | — | 4.6 | 8.5 | 21.5 | — | 5.2 | Promoter | Yes |
| CCT2 | CCTB | — | 8.7 | — | — | — | 6.3 | — | — | Prom/Int | Yes | |
| CCT3 | CCTG | — | 8.5 | — | — | — | 8.5 | 3.9 | — | Prom/Int | Yes | |
| CCT4 | CCTD | — | 12.4 | — | 3.9 | 4.7 | 8.5 | — | — | Promoter | Yes | |
| CCT5 | CCTE | — | 14.0 | — | 2.7 | — | 15.3 | — | 4.9 | Promoter | Yes | |
| CCT6A | CCTZ | — | — | — | — | — | 5.7 | — | — | Promoter | Yes | |
| CCT6B | CCTZ2 | — | — | — | — | — | — | — | — | — | Yes | |
| CCT7 | CCTH | — | 12.5 | — | 3.5 | — | 11.4 | — | 3.9 | Promoter | Yes | |
| CCT8 | CCTQ | — | 5.0 | — | — | — | 7.1 | — | — | Promoter | Yes | |
Fold enrichments ≥2 are shown. The nomenclature of HSPs is according to ref. 85. C, control; HS, heat shock; NA, not analyzed; Prom/Int, promoter or intron depending on the transcript variant.
*The RNPII density signal is recovered from ENCODE (wgEncodeEH000616; Snyder Laboratory, Yale University).
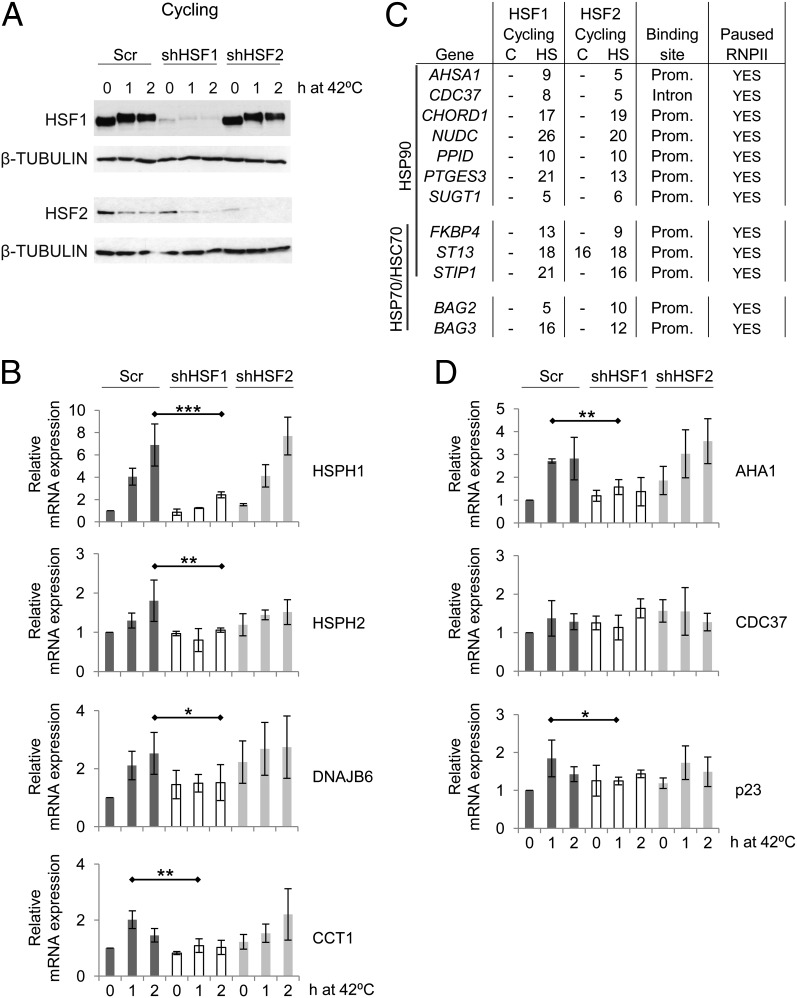
Fig. 2.
HSFs define the set of chaperones and cochaperones induced in response to acute heat stress. (A) HSF1 and HSF2 protein levels during the 2-h heat stress in scrambled-transfected (Scr), HSF1-depleted (shHSF1), and HSF2-depleted (shHSF2) cycling cells. HSF2 levels decline on heat stress as previously reported (25). (B) mRNA levels of chaperones HSPH1, HSPH2, DNAJB6, and chaperonin CCT1 during heat stress in the presence or absence of HSF1 or HSF2. (C) Cochaperone genes bound by HSF1 and HSF2 in cycling cells. Fold enrichments over input as detected with ChIP-seq are indicated. (D) mRNA levels of HSP90 cochaperones AHA1, CDC37, and p23 in nontreated and heat-treated cycling cells with or without HSF1 or HSF2. In B and D, SDs of a minimum of three biological replicates are shown. The statistical analyses were conducted with two-tailed independent Student t test and asterisks denote statistical significance between indicated scrambled-transfected and HSF-depleted cells. *P < 0.1; **P < 0.05; ***P < 0.01.
HSF1 Is Required for Heat-Induced Cochaperone Expression.
As shown in Fig. 2C and Dataset S1, HSF1 and HSF2 occupied many cochaperone genes, which have not previously been demonstrated to be under control of HSFs. Most cochaperones possess intrinsic chaperone activity but are termed cochaperones because they determine the activities of the HSP70 and/or HSP90 protein complexes (47, 48). As summarized in Fig. 2C, HSF1 and HSF2 showed a strong tendency to bind cochaperone gene promoters that contain the paused RNPII. We determined the mRNA levels of the HSP90 cochaperones AHSA1 (encoding AHA1), CDC37, and PTGES3 (encoding p23) in the presence and absence of HSF1 or HSF2 (Fig. 2D). AHA1, which stimulates the ATPase cycle and is the most potent activator of the HSP90 protein complex (49), showed a threefold heat-induced expression that required the presence of HSF1 (Fig. 2D). CDC37 and p23 are involved in client protein recruitment and maturation, and they inhibit the HSP90 ATPase cycle (48, 50, 51). We did not detect any significant change in CDC37 mRNA levels during the 2-h heat shock but found a twofold HSF1-dependent increase in p23 mRNA (Fig. 2D). Curiously, HSF1 and HSF2 bound the promoters of AHSA1 and PTGES3, but the first intron of the noninducible CDC37 (Fig. 2C). We conclude that in addition to controlling the set of chaperones induced by acute heat stress, HSFs also determine the expression patterns of cochaperones, which are critical for the recognition and fate of the clients, as well as for the chaperoning efficacy.
Stress-Generated Demand for Protein Clearance Is Mitigated by HSF1-Dependent Ubiquitin Expression.
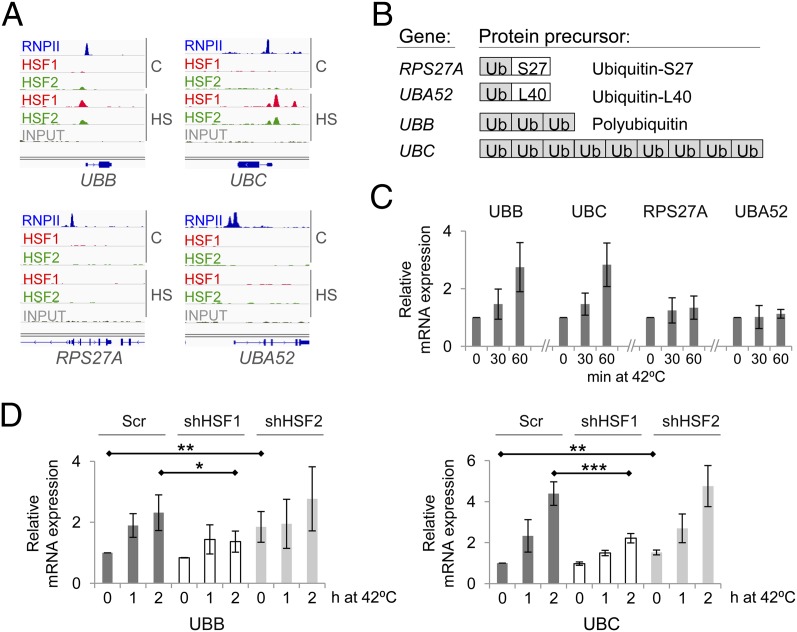
On heat stress, damaged and aggregated proteins accumulate and generate an increased demand for protein clearance. The vast majority of proteins destined for degradation are marked by ubiquitin, which is encoded by four genes in humans (52). As revealed by ChIP-seq, HSF1 and HSF2 selectively bound to the promoters of polyubiquitin genes UBB and UBC but were absent on the monoubiquitin genes RPS27A and UBA52 (Fig. 3A). Transcription of UBB or UBC enables a prompt increase in the ubiquitin levels because their corresponding mRNAs encode a precursor for three and nine ubiquitin proteins, respectively (Fig. 3B). In comparison, transcription of RPS27A or UBA52 would generate one ribosomal protein per each ubiquitin produced (Fig. 3B). In heat-treated cycling cells, the mRNA levels of UBB and UBC rapidly increased to two- and fourfold, respectively, whereas the levels of RPS27A and UBA52 mRNA remained unchanged (Fig. 3 C and D). Depletion of HSF1 severely compromized the heat-induced ubiquitin expression in cycling cells (Fig. 3D), revealing that HSF1 localization to the promoters of polyubiquitin genes is required for the cell to generate adequate levels of ubiquitin mRNA under proteotoxic stress.
Fig. 3.
HSF1 induces polyubiquitin gene expression in cycling cells. (A) HSF1 and HSF2 bind to polyubiquitin (UBB and UBC) but not to monoubiquitin (RPS27A, UBA52) genes as shown by ChIP-seq. (B) Schematic illustration of the four ubiquitin coding genes in the human genome. (C and D) Heat stress leads to increased expression of polyubiquitin but not monoubiquitin genes in cycling cells, and the heat-induced ubiquitin expression is abolished in HSF1-depleted cells. The mRNA levels and statistical significances were analyzed as in Fig. 2.
HSF1- and HSF2-Driven Transcriptional Programming Extends to Genes Encoding Transcriptional and Translational Regulators, Cell Cycle Determinants, and Core Signaling Components.
To investigate the biological processes associated with HSF1 and HSF2 target genes in acute stress, we performed gene ontology analysis using Database for Annotation, Visualization and Integrated Discovery (DAVID) (53). Because heat shock leads to a rapid transcriptional response, we sought to understand whether HSFs are recruited to genomic loci that harbor RNPII before stress (wgEncodeEH000529; Iyer Laboratory, University of Texas) and whether the presence or absence of RNPII characterizes HSF target genes associated with a specific function. Interestingly, 90% of HSF1- or HSF2-targeted loci on promoters were occupied by RNPII, and at the gene bodies, approximately half of the HSF1 or HSF2 target sites harbored RNPII (Fig. 4A).
Fig. 4.
HSFs control a broad range of cellular processes during stress. (A) Gene ontology analyses of HSF1 and HSF2 target genes in heat-treated cycling cells in respect to genomic region and to presence or absence of RNPII. Promoter is defined as −1,200 to +300 bp from the TSS, and gene body is coding sequence from +301 bp to the end of the gene. HSF1-specific target gene groups are indicated in red, HSF2-specific target gene groups are in green, and their shared target gene groups are in blue. (Lower) Fractions of HSF1 or HSF2 target sites that localize to RNPII-occupied sites. RNPII-containing sites in nontreated cycling cells are recovered from the ENCODE project (wgEncodeEH000529; Iyer Laboratory, University of Texas). (B) mRNA levels of HSF target genes during heat shock. mRNA levels and statistical significances were analyzed as in Fig. 2.
In heat-shocked cycling cells, HSF1 and HSF2 co-occupied promoters of chaperones and cochaperones, transcriptional and translational regulators, and mediators of cell cycle progression (Fig. 4A; Dataset S2). On the gene bodies, HSF1 occupied RNPII-containing loci of genes that mediate transcriptional repression, methylation, and inhibition of mRNA metabolism. As a comparison, HSF2 localized to the coding sequences of genes involved in activation of diverse cellular processes, specifically on the sites that lacked RNPII (Fig. 4A; Dataset S2). Among the promoter-targeted genes, we verified the mRNA levels of transcriptional regulators TAF7 (TATA-box associated factor 7) and MLL, translational components EEF1G (eukaryote elongation factor 1γ) and MRPS6, as well as mitotic factors NUDC (nuclear distribution C homolog) and BANF1 (barrier to autointegration factor 1). Among the genes that harbored HSF on the coding sequence, mRNA levels of CTCF (CCCTC-binding factor) and PRKCA were investigated. In response to heat stress, an HSF1-dependent increase to 3-fold was detected for TAF7, 2-fold for EEF1G, and 1.5-fold for NUDC (Fig. 4B). Depletion of HSF2 did not hamper the heat-inducible expression of any of the studied genes, but it disrupted the expression kinetics of MLL (Fig. 4B). Moreover, absence of HSF2 caused more than a twofold increase in mRNA levels of BANF1 and PRKCA under optimal growth conditions (Fig. 4B).
Mitosis Inhibits Transcriptional Activation and Renders Chromatin Inaccessible for HSF1.
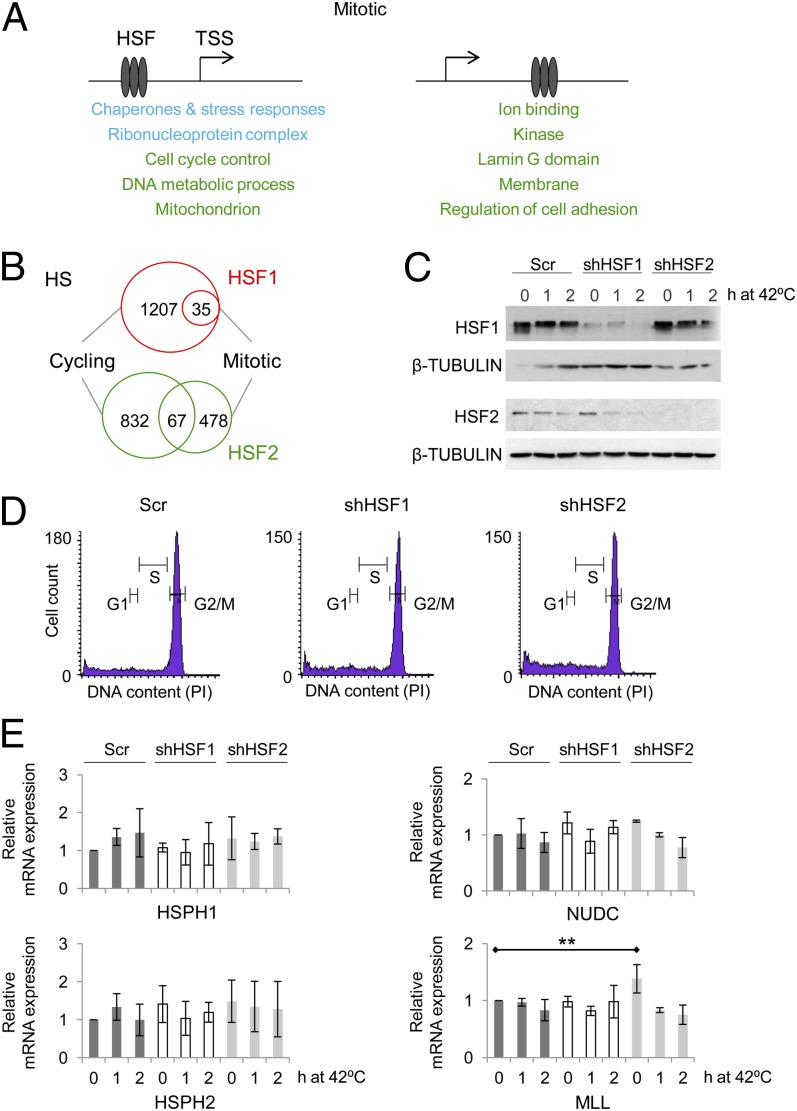
HSF1 bound to 35 target loci in heat-treated mitotic cells, mainly on the promoters of chaperones and translational components (Fig. 5 A and B; Datasets S1 and S2). It is noteworthy that HSF1 was unable to bind to 1,207 loci, which it occupied in heat-stressed cycling cells (Fig. 5B). Among the genes that lacked HSF1 in stressed mitotic cells were UBB and UBC (Fig. S4B), and subsequently, the ubiquitin gene expression remained unchanged during heat treatment of mitotic cells (Fig. S4C). In contrast to the radically limited capacity of HSF1 to interact with the mitotic chromatin, HSF2 occupied hundreds of target loci both in cycling and mitotic cells (Figs. 1B and 5B; Dataset S1). However, depending on the phase of the cell cycle, HSF2 localized to a distinct set of targets (Fig. 5 A and B; Datasets S1 and S2). We confirmed that depletion of HSF1 or HSF2 did not interfere with synchronization of cells to mitosis (Fig. 5 C and D) and investigated whether binding of HSF1 or HSF2 leads to induced transcription of their target genes in mitotic cells (Fig. 5E; Fig. S4D). Although HSF1 was a potent transactivator in cycling cells (Figs. 2–4), no significant induction of any of the studied HSF1 and HSF2 target genes, HSPH1/HSP110, HSPH2, NUDC, DNAJB6, or MRPS6, was detected in mitosis (Fig. 5E; Fig. S4D). These results indicate that besides displaying an impaired ability to bind to the mitotic chromatin, the occupancy of HSF1 at promoters does not induce transcription in the mitotic environment, where the promoters likely lack RNPII and the condensed chromatin generates barriers for transcriptional initiation and elongation (30). Moreover, mitotic cells have been shown to be highly susceptible to heat-induced stress (54, 55), which is in agreement with increased cell death that we observed in heat-treated mitotic cells (Fig. S4E).
Fig. 5.
HSF2 interacts with mitotic chromatin. (A) Gene ontology analyses of HSF1 and HSF2 target loci in mitosis. The genomic regions were defined as in Fig. 4. (B) The number of HSF1 (red) and HSF2 (green) target loci in cycling and mitotic heat-treated cells. (C) HSF1 and HSF2 protein levels in scrambled-transfected (Scr), HSF1-depleted (shHSF1), and HSF2-depleted (shHSF2) mitotic cells during heat stress. (D) Histograms of scrambled-transfected (Scr), HSF1-depleted (shHSF1), and HSF2-depleted (shHSF2) mitotic cells after a 1-h heat treatment, indicating that neither transfection nor lack of HSF1 or HSF2 compromises the synchronization of cells to G2/M. (E) mRNA levels of HSPH1, HSPH2, NUDC, and MLL in mitotic cells with or without HSF1 or HSF2. mRNA levels and statistical significances were analyzed as in Fig. 2.
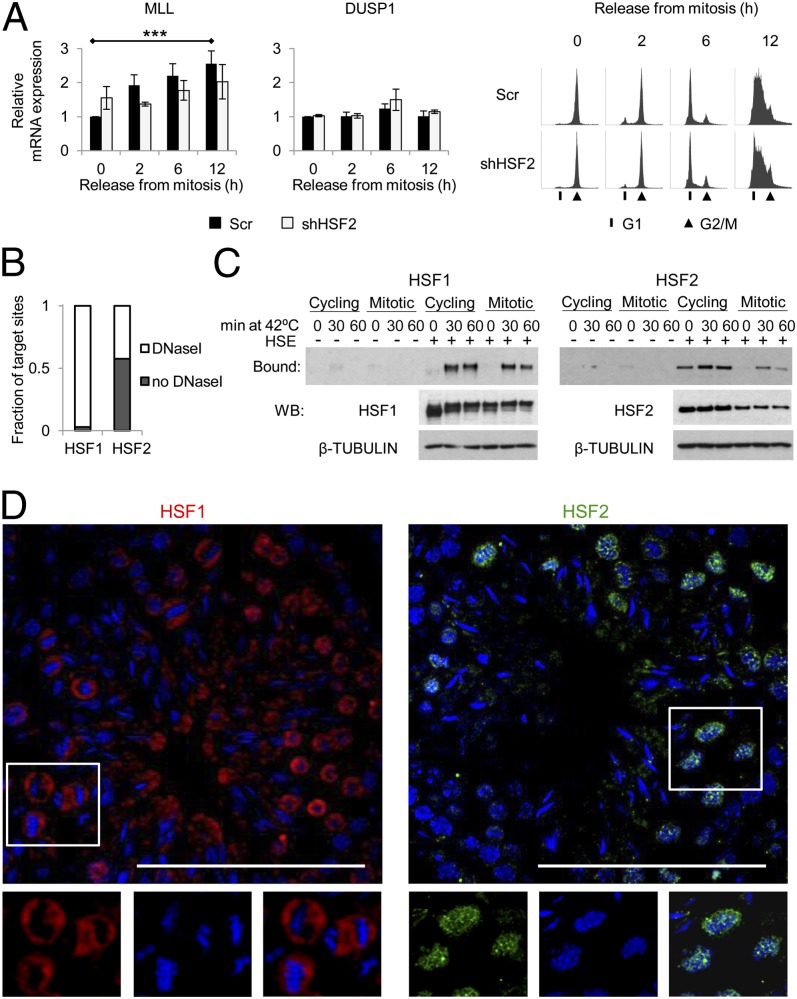
The mRNA levels of the HSF2-specific target gene, MLL, remained relatively constant in the mitotic cells exposed to acute heat stress. However, depletion of HSF2 led to increased MLL expression under nonstress conditions (Fig. 5E), suggesting that HSF2 either inhibits transcription or modifies the chromatin environment of MLL. To elucidate the role of HSF2 in mitosis, when the overall transcription is silenced (30), we monitored the expression kinetics of MLL after the non–heat-shocked cells had been released from the mitotic arrest (Fig. 6A). In scrambled-transfected cells, the mRNA levels of MLL gradually doubled as the cells progressed from mitosis to G1 phase (Fig. 6A). These results are in agreement with a previous study where the MLL protein levels were shown to increase after mitosis (56). However, the postmitotic induction of MLL expression in HSF2-depleted cells was only minute and showed delayed kinetics (Fig. 6A). As a control, we used DUSP1, which was not bound by HSFs in the mitotic cells (Fig. 1D). DUSP1 is a heat-inducible gene (57), and no elevation in DUSP1 mRNA expression was detected after the mitotic release, nor did the absence of HSF2 affect the DUSP1 expression during cell cycle progression (Fig. 6A). In conclusion, the ability of HSF2 to bind to the MLL promoter in mitosis (Fig. 1D) and to direct its postmitotic expression (Fig. 6A) indicates the involvement of HSF2 in restoring the transcription of MLL after the mitotic silencing.
Fig. 6.
Chromatin compaction during mitosis and meiosis renders DNA inaccessible for HSF1. (A) mRNA levels of MLL and DUSP1 in the presence (Scr) or absence (shHSF2) of HSF2. The analyses were conducted in non–heat shock conditions at indicated times points after releasing the cells from mitotic arrest. mRNA levels and statistical significances were analyzed as in Fig. 2. (Right) Progression of cells from mitotic arrest is illustrated with FACS profiles. (B) Fraction of HSF1 or HSF2 target sites in mitosis that occur within DNaseI hypersensitive regions. The DNaseI sensitive regions in G2/M are recovered from the ENCODE project (wgEncodeEH003472; Crawford Laboratory, Duke University). (C) Oligonucleotide-mediated pull-down in cycling and mitotic cells. Bound fraction indicates binding of HSF1 or HSF2 to the HSE-containing oligonucleotide (+). The scrambled oligonucleotide sequence (−) lacks an HSE. WB indicates the total HSF1 and HSF2 protein levels in the respective samples. (D) Single sections of confocal microscope images showing localization of HSF1 (red) and HSF2 (green) in mouse male germ cells during meiotic division I. DNA (stained with Hoechst) is shown in blue. (Scale bar, 100 µm.)
The binding of HSF2 to mitotic chromatin (Figs. 1B and 5B; Fig. S2A) argued that a great number of HSEs are accessible for transcription factors during cell division. We compared the HSF target sites in heat-treated mitotic cells to DNaseI hypersensitive regions in mitosis (wgEncodeEH003472; Crawford Laboratory, Duke University) and found that open chromatin is a prerequisite for HSF1 binding, as 97% of its target sites occurred within DNaseI sensitive regions (Fig. 6B). Instead, 42% of HSF2 targets in mitosis were found within open chromatin (Fig. 6B). Although HSF2 was able to bind both to DNaseI hypersensitive and insensitive regions, this did not explain why HSF1 was absent from 195 DNaseI hypersensitive loci that contained HSF2. Next, we addressed whether the exclusion of HSF1 from the mitotic chromatin is mediated by inhibiting the intrinsic DNA-binding capacity of HSF1 or by rendering the chromatin inaccessible for HSF1. As analyzed with oligonucleotide-mediated pull-down, both HSF1 and HSF2 bound to the exogenous HSE-containing oligonucleotides in cycling and mitotic heat-treated cells (Fig. 6C). Curiously, HSF2 bound to DNA also in untreated cycling cells, and its levels declined in mitosis (Fig. 6C). Besides binding to the exogenous HSE, hyperphosphorylation of HSF1 was detected in mitosis (Fig. 6C), indicating HSF1 transactivating capacity (22). These results demonstrate that HSF1 was indeed capable of binding to DNA in mitosis, but its access to chromatin was dramatically reduced from 1,242 sites in heat-treated cycling cells to only 35 sites in cells arrested to mitosis.
An intriguing congruency has been reported during meiotic division in mouse spermatocytes, where HSF1 was shown to bind to the exogenous HSE on heat stress but no binding to the HSP70 promoter could be detected (11, 58–60). Because HSF2-deficient mice display meiotic defects (61), we investigated by confocal microscopy the subcellular localization of HSF1 and HSF2 in metaphase spermatocytes. HSF2 was abundant at the chromatin in meiotic divisions, whereas HSF1, albeit highly expressed, was detected only outside the dividing chromatin (Fig. 6D). These results suggest a common mechanism in cell division that selectively allows HSF2 to bind mitotic and meiotic chromatin from which HSF1 is efficiently excluded.
Discussion
Characterization of the genomewide binding sites and transactivating capacities for HSF1 and HSF2 discovered the extent of HSF-mediated transcriptional reprogramming under acute heat stress. The results uncovered molecular mechanisms that maintain homeostasis, highlighting the myriad of different cellular processes that are orchestrated under protein-damaging conditions. In stressed freely cycling cells, HSFs induced the expression of polyubiquitin genes, which is a rapid means to produce a large amount of ubiquitin (Fig. 3). Ubiquitin is a small signaling molecule with versatile functions in the cell, and it is required for the proteasomal degradation of damaged proteins (52). Polyubiquitin genes have been shown to be induced on exposure to heat (52, 62), and here we provide evidence for the direct involvement of HSF1 and HSF2 in the regulation of polyubiquitin gene expression. HSFs also defined the expression of chaperones (Table 1; Table S1; Fig. 2B) and cochaperones (Fig. 2 C and D) that facilitate protein folding. Previously, a subset of chaperone genes has been used as a model for induced transcriptional responses, but here we characterized the complete set of human chaperones that are transcriptionally regulated by HSF1 and HSF2 (Table 1; Table S1). Importantly, we discovered that HSF1 and HSF2 also determine the cochaperones that are induced during heat stress, demonstrating that HSFs orchestrate the whole machinery that maintains proteostasis. Beyond the protein quality control, HSFs directed genes encoding transcriptional and translational regulators, mitotic determinants, and core components of various signaling pathways (Fig. 4; Dataset S2). For example, TAF7 has been suggested to function as a transcriptional checkpoint regulator by inhibiting RNPII phosphorylation at the promoter (63, 64), whereas BANF1 and NUDC are key regulators in mitosis required for correct spindle assembly, cytokinesis, and nuclear envelope assembly (65–68). The stress response has dramatic effects on cellular physiology, but the basic mechanisms of the adaptation processes such as the transcriptional and translational silencing or the stalling of the cell cycle progression are not understood. The characterized HSF1- and HSF2-driven transcriptional reprogramming will aid in elucidating the detailed mechanisms that rapidly adjust cellular processes during stress, as well as clarify how the processes are reestablished once favorable conditions for proliferation are again restored.
Functions of HSF1 and HSF2 Are Tightly Interlinked, but Their Effects on Promoters Are Profoundly Distinct.
Previous genomewide studies in yeast (69), fly (70, 71), and humans (8, 72) identified the HSE for HSF1, showing a striking conservation of the HSF1-recognized DNA sequence over evolution. Our analyses corroborated the importance of inverted nGAAn pentamers for HSF1 binding both in cycling and mitotic cells (Fig. S2B). By revealing the HSF2-targeted HSEs in a genomewide scale, we expected to find a major difference between the DNA sequences preferred by HSF1 vs. HSF2. Despite their unique target sites in the human genome (Figs. 1 B, E, and F and 5B; Fig. S2A), HSF1 and HSF2 recognized a staggeringly similar HSE (Fig. S2B). Earlier footprinting studies have revealed that HSF2 is able to bind shorter HSEs than HSF1 (19, 37). The ability of HSF2 to bind less extensive HSEs could provide an advantage when accessing HSEs in the compacted chromatin regions. The overlapping genomic coordinates and highly similar binding profiles of HSF1 and HSF2 at the shared target sites (Figs. 1 C and D and 3A; Fig. S3C; Dataset S1) suggest their intimate interplay in transcriptional regulation. The resolution of our ChIP-seq analyses, however, cannot distinguish HSF1-HSF2 heterotrimers from homotrimers on adjacent pentamers of the same or nearby HSE.
Cancer cells display a nononcogene addiction to HSF1 as a result of ameliorated proteotoxic stress (5, 73). Moreover, the binding profile of HSF1 in nontransformed cells radically differs from that in cancer cells with high malignant potential (8). Because K562 cells are an erythroleukemia cell line and express high basal levels of HSPs, HSF1 could have cancer-specific target genes also in these cells. However, the low fold enrichments and the limited number of HSF1 target sites in nonstressed K562 cells (Fig. 1B) are in accordance with the HSF1 binding profiles in nontransformed cells and in cells with low malignant potential (8).
The detailed analyses of target gene expression revealed HSF1 as a potent transactivator that responds instantly to stress (Figs. 1B and 2–4). Also HSF2 rapidly localized to the target sites (Fig. 1B), but it did not enhance transcription during 2 h of heat stress (Figs. 2–4). HSF1 and HSF2 show similar recruitment kinetics to the HSP70 promoter, but HSF2 is gradually degraded from the HSE after 30 min of heat stress (25), indicating the need for delicate regulation of the HSF1-HSF2 composition at the promoter. Our preliminary results show an HSF2-mediated effect on transcription during prolonged stress when HSF2 levels have already declined (Fig. S4F) (25) and suggest that HSF2 modifies the chromatin landscape for transcriptional responses. HSF2 is also involved in many developmental processes when the epigenetic features of the cells are determined (10, 12, 61), and it has been shown to affect the chromatin compaction and integrity (10). These results argue for an HSF2-mediated establishment of the transcriptional environment and indicate profoundly distinct mechanisms for HSF1 and HSF2 during their dynamic interplay in driving gene expression. The versatility of cellular processes directed by HSF1 and HSF2 underscore the importance of establishing their specific activators and inhibitors, PTM signatures, and chromatin-associated factors that direct HSFs to their individual target loci.
Is HSF2 an Epigenetic Regulator of Cell Fate?
An unexpected finding of our studies was the ability of HSF2 to interact with the dividing chromatin where HSF1 was hardly detected (Figs. 1B and 6D). HSF2 has been reported to bind HSP promoters in mitosis and suggested to mediate bookmarking of stress-responsive genes (74, 75). We identified both HSF1 and HSF2 on chaperone genes in mitosis, including promoters of HSPA1A/HSP70.1, HSPA1B/HSP70.2, HSPH1/HSP110, and HSPE1/HSP10 (Fig. 1C; Dataset S1). However, we did not detect HSF1-induced transcription in mitosis (Fig. 5E; Fig. S4D), indicating an impaired ability of dividing cells to provoke transcriptional responses. HSF1 has been shown to recruit chromatin modifiers and facilitate RNPII loading (76), which could explain why HSF1 localized to 25 target gene promoters in heat-treated mitotic cells without inducing transcription (Fig. 5; Fig. S4D; Dataset S1). The unbiased analyses by ChIP-seq identified a broad range of mitotic target genes for HSF2 both under optimal growth and stress conditions (Fig. 5A; Datasets S1 and S2). One prominent unique HSF2 target gene in mitosis is MLL, a trithorax homolog and a transcriptional coactivator that has been found to interact with promoters in mitotic human cells, marking the genes for early activation in G1 (77, 78). We discovered that HSF2 controls the cell cycle phase–dependent expression of MLL, which is prerequisite for an efficient induction of MLL after mitotic silencing (Fig. 6A). Moreover, recent computational analyses found MLL target promoters to be enriched with HSEs (77), arguing for either cooperation or competition of MLL and HSFs on the genome. Several studies have addressed how the memory of cell fate is maintained during mitosis when most of the transcriptional regulators are erased from the chromatin (33). To this end, the capacity of HSF2 to bind mitosis-specific target genes both under optimal growth conditions and on stress (Figs. 1B and 5B; Dataset S1) indicates that HSF2 directs gene expression throughout cell cycle progression.
Genomewide analyses in high resolution enable broad views on the interplay of transcriptional regulators and chromatin state in different cell types, cell cycle phases, and in response to various stimuli. In this study, we characterized the HSF1- and HSF2-driven transcriptional response to acute stress in cycling and mitotic cells. Our results revealed the molecular mechanisms that maintain cellular homeostasis in cycling cells and identified a dramatically impaired capacity of mitotic cells to provoke transcriptional responses, even when challenged with proteotoxicity. We characterized the complete set of chaperones, cochaperones, and ubiquitin genes that is directed by HSFs under acute stress and highlighted the various processes that are transcriptionally reprogrammed in proteotoxic conditions. We uncovered the strikingly distinct mechanisms for HSF1 and HSF2 in orchestrating transcription and discovered HSF2 as an epigenetic regulator that controls transcription throughout cell cycle progression. Future studies, expanding beyond acute heat shock, will establish whether cells are able to epigenetically remember past environmental exposures, such as stress.
Materials and Methods
Cell Culture, Synchronization of Cells to Mitosis, and Heat Treatments.
Human K562 erythroleukemia cells were maintained at 37 °C in a humidified 5% CO2 atmosphere and cultured in RPMI medium (Sigma) containing 10% (vol/vol) FCS, 2 mM l-glutamate, and streptomycin/penicillin. Cells were arrested to mitosis using thymidine and nocodazole (39). Briefly, after 16 h in 2 mM thymidine (Sigma), the cells were washed with PBS to allow cell cycle progression for 8 h. The cells were collected in S-phase by a second thymidine treatment for 24 h. After cell cycle progression for 5 h, the cells were arrested in mitosis with nocodazole (100 ng/mL; Fluka) for 12 h. The nocodazole was removed, and the cells were harvested, allowed to proceed the cell cycle at 37 °C, or heat treated. The heat shock treatments were conducted in a water bath at 42 °C. The DNA content of the cells was determined by propidium iodide (PI) staining (40 µg/mL; Sigma) and fluorescence-mediated counting by FACSCalibur (BD Biosciences). The FACS histograms were generated using Cell Quest Pro-6.0 (BD Biosciences) and Flowing Software 2.5 (Turku Centre for Biotechnology). The error bars in statistical analyses indicate SDs.
ChIP.
HSF1- and HSF2-bound DNA fragments were isolated by ChIP using a previously described protocol (11, 17) and the following ChIP-verified antibodies: HSF1 (Spa-901; Enzo) and HSF2 (17). IgG (sc-2027; Santa Cruz) was used as a negative antibody and AcH4 (06-866; Upstate) was used as a positive antibody. PCR primers are listed in Table S2.
High-Throughput Sequencing and Data Analysis.
For sequencing, 10 ChIP replicates per sample were collected. Sequencing libraries were generated using New England BioLabs NEBNext kits, and adapters and primers were from a Bioo Scientific AIR DNA Barcodes kit. Template amplification and cluster generation were performed using the cBot and Truseq SR Cluster kit for cBot v, and 36 nucleotides were sequenced with Illumina Genome Analyzer IIx using v5 TruSeq SBS sequencing kits. After quality trim and removal of duplicates, the reads were mapped to the human genome (hg19) with Bowtie (79). The peaks were called with MACS 1.4 (80), and a minimum fold enrichment of five times over input was set as a cutoff criterion for target sites. Any site that exceeded the cutoff in the negative IgG sample was discarded.
Identification of HSF1- and HSF2-Targeted DNA Sequences and Genomic Regions and Functional Annotation of Target Genes.
The consensus DNA sequences for HSF1 and HSF2 were identified by motif analysis of large DNA datasets (MEME-ChIP) (81) using a 120-bp region centered on the summit point. HSF1 and HSF2 target sites were annotated to genomic regions using exon and intron coordinates provided by RefSeq and by defining a core promoter to span from −1,200 to +300 bp from the transcriptional start site (TSS). Fifty percent of peak length was centered on the summit point, and peaks that fell on exon-intron boundaries are indicated as exons. Biological processes associated with HSF1 or HSF2 target genes in cycling and mitotic cells were analyzed with DAVID (53), which uses Fisher's exact test for calculation of the P value for enriched gene ontology terms. The density signals of HSF1, HSF2, and RNPII on the human genome were visualized with the Integrative Genomics Viewer (82).
Depletion of HSF1 and HSF2 with RNAi by shRNA.
HSF1 and HSF2 were depleted from the cells using shRNA constructs ligated into pSUPER vectors (Oligoengine) as previously described (17). The vector-encoded oligonucleotides were specific for HSF1 (GCT CAT TCA GTT CCT GAT C) or HSF2 (CAG GCG AGT ACA ACA GCA T). A scrambled sequence (GCG CGC TTT GTA GGA TTC G) was used as a control. The shRNA constructs were transfected into cells by electroporation (970 µF, 220 mV), and the cells were incubated for 72 h prior to harvesting. Synchronization of cells to mitosis was initiated after a 7-h recovery from the transfection, allowing for simultaneous sample preparation of the cycling and mitotic cells.
Quantitative Real-Time RT-PCR.
RNA was isolated using the RNeasy kit (Qiagen), and 1 μg of total RNA was DNaseI treated (Promega) and reverse transcribed with Moloney murine leukemia virus reverse transcriptase RNase H(–) (Promega). Real-time RT-PCR reactions were prepared and run as described earlier (17) using ABI Prism 7900 (Applied Biosystems). The primers were purchased from Oligomer (Helsinki) and the probes were purchased from Roche Applied Science. Primer and probe sequences are listed in Table S3. Relative quantities of the target gene mRNAs were normalized to GAPDH, and the fold inductions were calculated against the respective mRNA level in nontreated cells. All reactions were made in triplicate for samples derived from at least three biological replicates. SDs were calculated and are shown in the graphs. An independent two-tailed Student t test was used to determine the P value when comparing mRNA levels between scrambled-transfected and shHSF1- or shHSF2-transfected cells.
Western Blotting.
Cells were lysed with buffer C [25% (vol/vol) glycerol, 20 mM Hepes, pH 7.4, 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA, 0.5 mM PMSF, and 0.5 mM DTT], and the protein concentration in the soluble fraction was measured using Bradford analysis. Twenty micrograms of total protein was boiled in Laemmli sample buffer and subjected to SDS/PAGE followed by transfer to nitrocellulose membrane (Protran nitrocellulose; Schleicher & Schuell). Proteins were analyzed with primary antibodies against HSF1 (Spa-901; Enzo), HSF2 (3E2; Upstate), and β-TUBULIN (ab6046; Abcam). The secondary antibodies were HRP conjugated (GE Healthcare), and the blots were developed using an enhanced chemiluminescence method (ECL kit; GE Healthcare).
Oligonucleotide-Mediated Pull-Down Assay.
The oligonucleotide-mediated pull-down assay was performed as described previously (83) using biotinylated oligonucleotides (Oligomer). The double-stranded HSE contained the sequence 5′-biotin-TCG ACT AGA AGC TTC TAG AAG CTT CTA G-3′ and the scrambled control 5′-biotin-AAC GAC GGT CGC TCC GCC TGG CT-3′. Buffer C extracts of 100–400 μg total protein were annealed to oligonucleotide (0.5 μM) in binding buffer [20 mM Tris⋅HCl, pH 7.5, 100 mM NaCl, 2 mM EDTA, and 10% (vol/vol) glycerol] containing salmon sperm DNA (0.5 μg/μL). The samples were precleared, and the oligonucleotides were precipitated with a 50% (vol/vol) slurry of UltraLink streptavidin beads (Pierce). Bound fractions were washed three times with binding buffer containing 0.1% Triton X-100. DNA-bound proteins were eluted with Laemmli buffer and detected by SDS/PAGE and Western blotting.
Immunofluorescence of HSF1 and HSF2 in Dividing Male Spermatocytes.
Testes of 60- to 80-d-old C57BL/6N mice were isolated, fixed in 4% (wt/vol) paraformaldehyde, and sectioned to 4 µm. All mice were handled according to the institutional animal care policies of the Åbo Akademi University (Turku, Finland). Confocal immunofluorescence analyses were performed as previously described (11) using primary antibodies against HSF1 and HSF2 (20) and secondary antibodies conjugated to Alexa 488 or Alexa 568 (Invitrogen). The DNA was stained with Hoechst 33342 (H-1399; Molecular Probes). Images for all channels were sequentially captured from a single confocal section using a Zeiss Meta510 confocal microscope. For analyzing the meiotic divisions, stage XII of the mouse seminiferous cycle was selected, and dividing spermatocytes in meiotic division I were imaged. The channels were merged using ImageJ (84).
Supplementary Material
Acknowledgments
We thank Michael J. Guertin for insightful discussions and for sharing his expertise in bioinformatics. The Functional Genomics Unit (University of Helsinki) is acknowledged for performing the high-throughput sequencing, Jukka Lehtonen for expert assistance in computational analyses, and Diana M. Toivola, Johanna K. Björk, and Eva Henriksson for constructive advice during manuscript preparation. This work was financially supported by the Academy of Finland, the Sigrid Jusélius Foundation, the Finnish Cancer Organizations, Åbo Akademi University Foundation, the Tor, Joe, and Pentti Borg Foundation, BioCenter Finland, and the Turku Doctoral Program of Biomedical Sciences (A.V. and A.N.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The high-throughput sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE43579).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305275110/-/DCSupplemental.
References
- 1.Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: Intricate molecular relationships. EMBO J. 2011;30(13):2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter K, Haslbeck M, Buchner J. The heat shock response: Life on the verge of death. Mol Cell. 2010;40(2):253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 4.Åkerfelt M, Morimoto RI, Sistonen L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130(6):1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimoto M, et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280(41):34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 7.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273(13):7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 8.Mendillo ML, et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150(3):549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santagata S, et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci USA. 2011;108(45):18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Åkerfelt M, et al. Promoter ChIP-chip analysis in mouse testis reveals Y chromosome occupancy by HSF2. Proc Natl Acad Sci USA. 2008;105(32):11224–11229. doi: 10.1073/pnas.0800620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Åkerfelt M, et al. Heat shock transcription factor 1 localizes to sex chromatin during meiotic repression. J Biol Chem. 2010;285(45):34469–34476. doi: 10.1074/jbc.M110.157552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang Y, et al. Role of heat-shock factor 2 in cerebral cortex formation and as a regulator of p35 expression. Genes Dev. 2006;20(7):836–847. doi: 10.1101/gad.366906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto M, et al. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004;23(21):4297–4306. doi: 10.1038/sj.emboj.7600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaki E, et al. Maintenance of olfactory neurogenesis requires HSF1, a major heat shock transcription factor in mice. J Biol Chem. 2006;281(8):4931–4937. doi: 10.1074/jbc.M506911200. [DOI] [PubMed] [Google Scholar]
- 15.Loison F, et al. Up-regulation of the clusterin gene after proteotoxic stress: Implication of HSF1-HSF2 heterocomplexes. Biochem J. 2006;395(1):223–231. doi: 10.1042/BJ20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandqvist A, et al. Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol Biol Cell. 2009;20(5):1340–1347. doi: 10.1091/mbc.E08-08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Östling P, Björk JK, Roos-Mattjus P, Mezger V, Sistonen L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J Biol Chem. 2007;282(10):7077–7086. doi: 10.1074/jbc.M607556200. [DOI] [PubMed] [Google Scholar]
- 18.Shinkawa T, et al. Heat shock factor 2 is required for maintaining proteostasis against febrile-range thermal stress and polyglutamine aggregation. Mol Biol Cell. 2011;22(19):3571–3583. doi: 10.1091/mbc.E11-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroeger PE, Sarge KD, Morimoto RI. Mouse heat shock transcription factors 1 and 2 prefer a trimeric binding site but interact differently with the HSP70 heat shock element. Mol Cell Biol. 1993;13(6):3370–3383. doi: 10.1128/mcb.13.6.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13(3):1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshima T, Yura T, Yanagi H. Heat shock factor 1 mediates hemin-induced hsp70 gene transcription in K562 erythroleukemia cells. J Biol Chem. 1998;273(39):25466–25471. doi: 10.1074/jbc.273.39.25466. [DOI] [PubMed] [Google Scholar]
- 22.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 23.Björk JK, Sistonen L. Regulation of the members of the mammalian heat shock factor family. FEBS J. 2010;277(20):4126–4139. doi: 10.1111/j.1742-4658.2010.07828.x. [DOI] [PubMed] [Google Scholar]
- 24.Guertin MJ, Petesch SJ, Zobeck KL, Min IM, Lis JT. Drosophila heat shock system as a general model to investigate transcriptional regulation. Cold Spring Harb Symp Quant Biol. 2010;75:1–9. doi: 10.1101/sqb.2010.75.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahlskog JK, et al. Anaphase-promoting complex/cyclosome participates in the acute response to protein-damaging stress. Mol Cell Biol. 2010;30(24):5608–5620. doi: 10.1128/MCB.01506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jedlicka P, Mortin MA, Wu C. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J. 1997;16(9):2452–2462. doi: 10.1093/emboj/16.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984;311(5981):81–84. doi: 10.1038/311081a0. [DOI] [PubMed] [Google Scholar]
- 28.Yao J, Munson KM, Webb WW, Lis JT. Dynamics of heat shock factor association with native gene loci in living cells. Nature. 2006;442(7106):1050–1053. doi: 10.1038/nature05025. [DOI] [PubMed] [Google Scholar]
- 29.Alabert C, Groth A. Chromatin replication and epigenome maintenance. Nat Rev Mol Cell Biol. 2012;13(3):153–167. doi: 10.1038/nrm3288. [DOI] [PubMed] [Google Scholar]
- 30.Delcuve GP, He S, Davie JR. Mitotic partitioning of transcription factors. J Cell Biochem. 2008;105(1):1–8. doi: 10.1002/jcb.21806. [DOI] [PubMed] [Google Scholar]
- 31.Fisher D, Méchali M. Vertebrate HoxB gene expression requires DNA replication. EMBO J. 2003;22(14):3737–3748. doi: 10.1093/emboj/cdg352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor JH. Nucleic acid synthesis in relation to the cell division cycle. Ann N Y Acad Sci. 1960;90:409–421. doi: 10.1111/j.1749-6632.1960.tb23259.x. [DOI] [PubMed] [Google Scholar]
- 33.Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10(3):192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- 34.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316(5830):1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 35.Park PJ. ChIP-seq: Advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10(10):669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pepke S, Wold B, Mortazavi A. Computation for ChIP-seq and RNA-seq studies. Nat Methods. 2009;6(11) Suppl:S22–S32. doi: 10.1038/nmeth.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sistonen L, Sarge KD, Phillips B, Abravaya K, Morimoto RI. Activation of heat shock factor 2 during hemin-induced differentiation of human erythroleukemia cells. Mol Cell Biol. 1992;12(9):4104–4111. doi: 10.1128/mcb.12.9.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Consortium EP. ENCODE Project Consortium A user’s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 2011;9(4):e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitfield ML, et al. Stem-loop binding protein, the protein that binds the 3′ end of histone mRNA, is cell cycle regulated by both translational and posttranslational mechanisms. Mol Cell Biol. 2000;20(12):4188–4198. doi: 10.1128/mcb.20.12.4188-4198.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen EB, Lis JT. In vivo transcriptional pausing and cap formation on three Drosophila heat shock genes. Proc Natl Acad Sci USA. 1993;90(17):7923–7927. doi: 10.1073/pnas.90.17.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rougvie AE, Lis JT. The RNA polymerase II molecule at the 5′ end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988;54(6):795–804. doi: 10.1016/s0092-8674(88)91087-2. [DOI] [PubMed] [Google Scholar]
- 42.Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: Emerging roles in metazoans. Nat Rev Genet. 2012;13(10):720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Core LJ, et al. Defining the status of RNA polymerase at promoters. Cell Rep. 2012;2(4):1025–1035. doi: 10.1016/j.celrep.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubota H, Matsumoto S, Yokota S, Yanagi H, Yura T. Transcriptional activation of mouse cytosolic chaperonin CCT subunit genes by heat shock factors HSF1 and HSF2. FEBS Lett. 1999;461(1-2):125–129. doi: 10.1016/s0014-5793(99)01437-4. [DOI] [PubMed] [Google Scholar]
- 45.Hageman J, et al. A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol Cell. 2010;37(3):355–369. doi: 10.1016/j.molcel.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Rampelt H, et al. Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 2012;31(21):4221–4235. doi: 10.1038/emboj.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11(8):579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Soroka J, Buchner J. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochim Biophys Acta. 2012;1823(3):624–635. doi: 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Retzlaff M, et al. Asymmetric activation of the hsp90 dimer by its cochaperone aha1. Mol Cell. 2010;37(3):344–354. doi: 10.1016/j.molcel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Ali MM, et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440(7087):1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taipale M, et al. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150(5):987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimura Y, Tanaka K. Regulatory mechanisms involved in the control of ubiquitin homeostasis. J Biochem. 2010;147(6):793–798. doi: 10.1093/jb/mvq044. [DOI] [PubMed] [Google Scholar]
- 53.Dennis G, Jr, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):3. [PubMed] [Google Scholar]
- 54.Hut HM, Kampinga HH, Sibon OC. Hsp70 protects mitotic cells against heat-induced centrosome damage and division abnormalities. Mol Biol Cell. 2005;16(8):3776–3785. doi: 10.1091/mbc.E05-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez-Balbás MA, Dey A, Rabindran SK, Ozato K, Wu C. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell. 1995;83(1):29–38. doi: 10.1016/0092-8674(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Cheng EH, Hsieh JJ. Bimodal degradation of MLL by SCFSkp2 and APCCdc20 assures cell cycle execution: A critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 2007;21(19):2385–2398. doi: 10.1101/gad.1574507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992;359(6396):644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- 58.Sarge KD, Bray AE, Goodson ML. Altered stress response in testis. Nature. 1995;374(6518):126. doi: 10.1038/374126a0. [DOI] [PubMed] [Google Scholar]
- 59.Vydra N, et al. Spermatocyte-specific expression of constitutively active heat shock factor 1 induces HSP70i-resistant apoptosis in male germ cells. Cell Death Differ. 2006;13(2):212–222. doi: 10.1038/sj.cdd.4401758. [DOI] [PubMed] [Google Scholar]
- 60.Widlak W, et al. Inducible 70 kDa heat shock protein does not protect spermatogenic cells from damage induced by cryptorchidism. Int J Androl. 2007;30(2):80–87. doi: 10.1111/j.1365-2605.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 61.Kallio M, et al. Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J. 2002;21(11):2591–2601. doi: 10.1093/emboj/21.11.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee HS, Simon JA, Lis JT. Structure and expression of ubiquitin genes of Drosophila melanogaster. Mol Cell Biol. 1988;8(11):4727–4735. doi: 10.1128/mcb.8.11.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gegonne A, et al. TFIID component TAF7 functionally interacts with both TFIIH and P-TEFb. Proc Natl Acad Sci USA. 2008;105(14):5367–5372. doi: 10.1073/pnas.0801637105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gegonne A, Weissman JD, Zhou M, Brady JN, Singer DS. TAF7: A possible transcription initiation check-point regulator. Proc Natl Acad Sci USA. 2006;103(3):602–607. doi: 10.1073/pnas.0510031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aumais JP, et al. Role for NudC, a dynein-associated nuclear movement protein, in mitosis and cytokinesis. J Cell Sci. 2003;116(Pt 10):1991–2003. doi: 10.1242/jcs.00412. [DOI] [PubMed] [Google Scholar]
- 66.Bradley CM, Ronning DR, Ghirlando R, Craigie R, Dyda F. Structural basis for DNA bridging by barrier-to-autointegration factor. Nat Struct Mol Biol. 2005;12(10):935–936. doi: 10.1038/nsmb989. [DOI] [PubMed] [Google Scholar]
- 67.Haraguchi T, et al. Live cell imaging and electron microscopy reveal dynamic processes of BAF-directed nuclear envelope assembly. J Cell Sci. 2008;121(Pt 15):2540–2554. doi: 10.1242/jcs.033597. [DOI] [PubMed] [Google Scholar]
- 68.Nishino M, et al. NudC is required for Plk1 targeting to the kinetochore and chromosome congression. Curr Biol. 2006;16(14):1414–1421. doi: 10.1016/j.cub.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 69.Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol. 2004;24(12):5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonsalves SE, Moses AM, Razak Z, Robert F, Westwood JT. Whole-genome analysis reveals that active heat shock factor binding sites are mostly associated with non-heat shock genes in Drosophila melanogaster. PLoS ONE. 2011;6(1):e15934. doi: 10.1371/journal.pone.0015934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guertin MJ, Lis JT. Chromatin landscape dictates HSF binding to target DNA elements. PLoS Genet. 2010;6(9):e1001114. doi: 10.1371/journal.pgen.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trinklein ND, Murray JI, Hartman SJ, Botstein D, Myers RM. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol Biol Cell. 2004;15(3):1254–1261. doi: 10.1091/mbc.E03-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130(6):986–988. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 74. Wilkerson DC, Skaggs HS, Sarge KD (2007) HSF2 binds to the Hsp90, Hsp27, and c-Fos promoters constitutively and modulates their expression. Cell StressChap 12(3):283–290. [DOI] [PMC free article] [PubMed]
- 75.Xing H, et al. Mechanism of hsp70i gene bookmarking. Science. 2005;307(5708):421–423. doi: 10.1126/science.1106478. [DOI] [PubMed] [Google Scholar]
- 76.Fujimoto M, et al. RPA assists HSF1 access to nucleosomal DNA by recruiting histone chaperone FACT. Mol Cell. 2012;48(2):182–194. doi: 10.1016/j.molcel.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 77.Blobel GA, et al. A reconfigured pattern of MLL occupancy within mitotic chromatin promotes rapid transcriptional reactivation following mitotic exit. Mol Cell. 2009;36(6):970–983. doi: 10.1016/j.molcel.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tyagi S, Chabes AL, Wysocka J, Herr W. E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol Cell. 2007;27(1):107–119. doi: 10.1016/j.molcel.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 79.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng J, Liu T, Qin B, Zhang Y, Liu XS. Identifying ChIP-seq enrichment using MACS. Nat Protoc. 2012;7(9):1728–1740. doi: 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Machanick P, Bailey TL. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics. 2011;27(12):1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson JT, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anckar J, et al. Inhibition of DNA binding by differential sumoylation of heat shock factors. Mol Cell Biol. 2006;26(3):955–964. doi: 10.1128/MCB.26.3.955-964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kampinga HH, et al. (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell StressChap 14(1):105–111. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.