Abstract
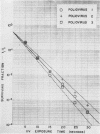
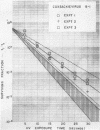
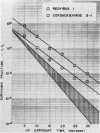
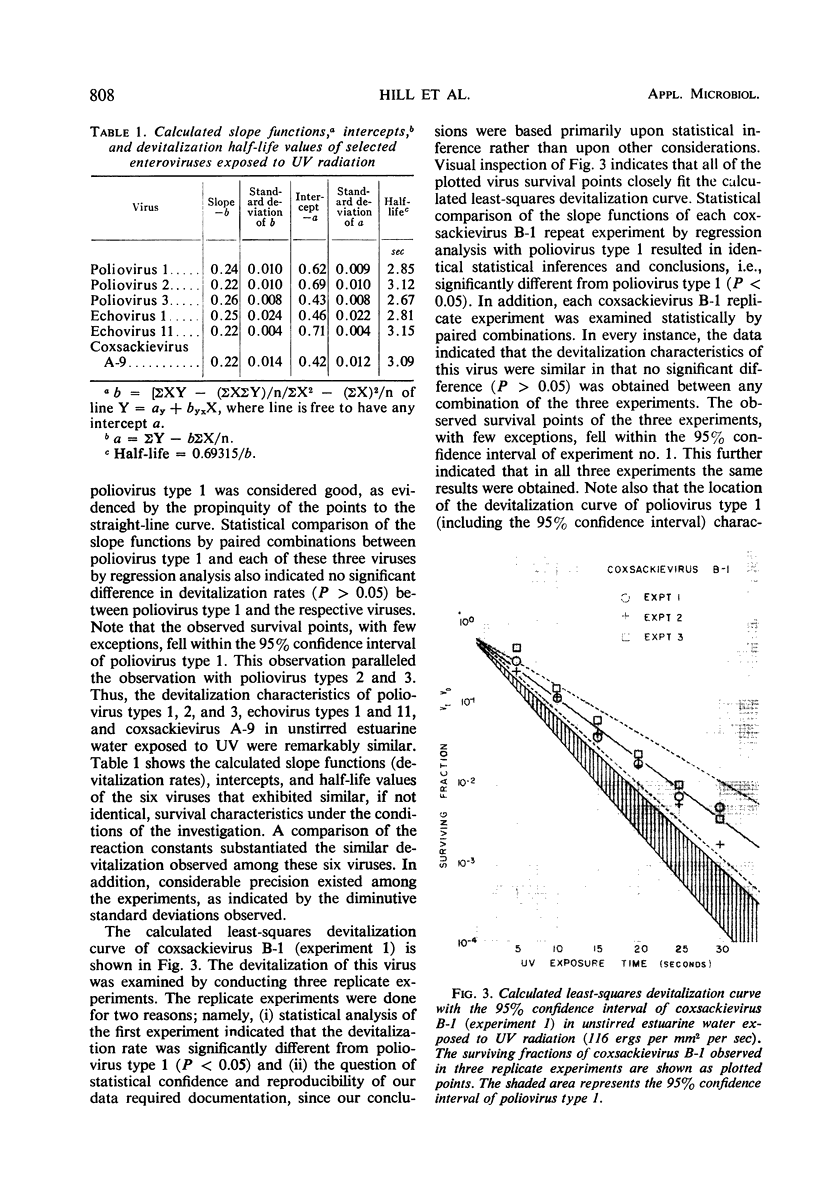
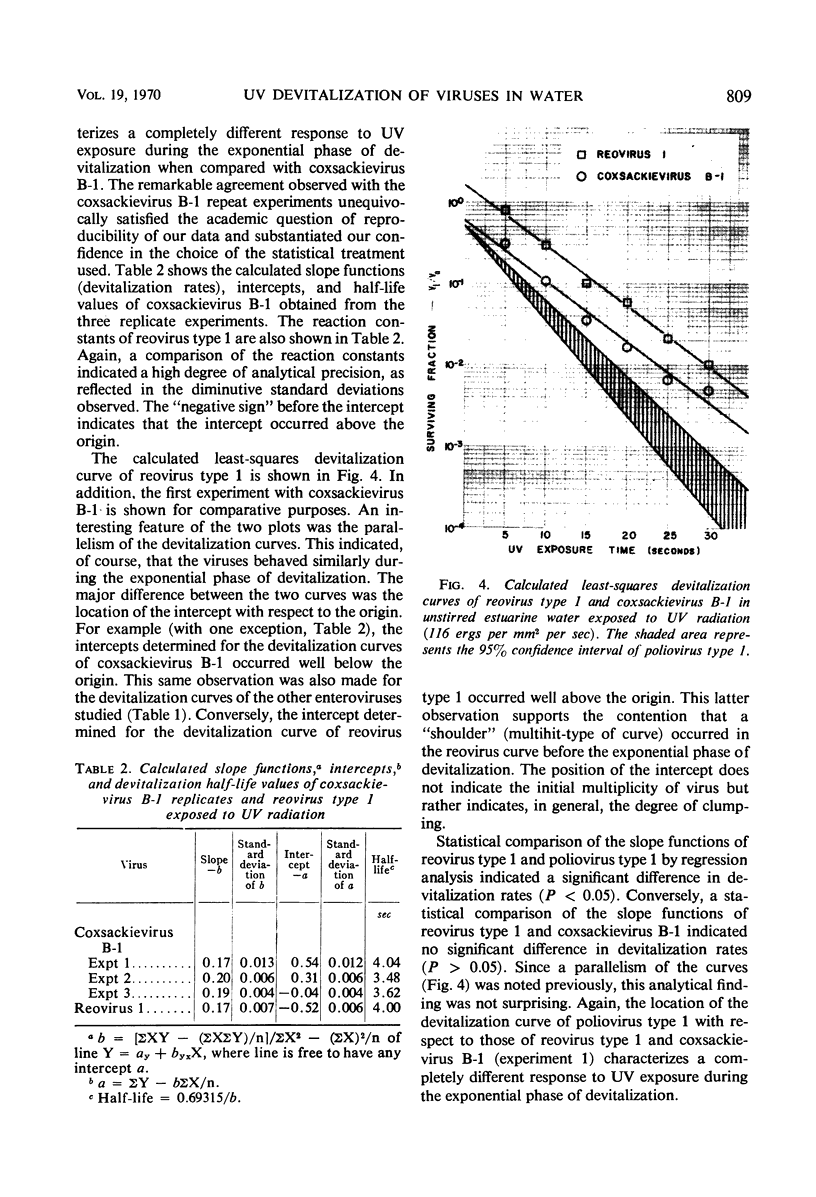
The effect of ultraviolet (UV) radiation on the devitalization of eight selected enteric viruses suspended in estuarine water was determined. The surviving fractions of each virus were calculated and then plotted against the UV exposure time for purposes of comparison. Analytical assessment of the survival data for each virus consisted of least squares regression analysis for determination of intercepts and slope functions. All data were examined for statistical significance. When the slope function of each virus was compared against the slope function of poliovirus type 1, the analytical findings indicated that poliovirus types 2 and 3, echovirus types 1 and 11, and coxsackievirus A-9 exhibited similar devitalization characteristics in that no statistically significant difference was found (P > 0.05). Conversely, the devitalization characteristics of coxsackievirus B-1 and reovirus type 1 were dissimilar from those of poliovirus type 1 in that a statistically significant difference was found between the slope functions (P < 0.05). This observed difference in devitalization of coxsackievirus B-1 and reovirus type 1 was attributed primarily to the frequency distribution of single and aggregate virions, the geometric configuration, the size of the aggregates, and the severity of aggregation. The devitalization curve of coxsackievirus B-1 was characteristic of a retardant die-away curve. The devitalization curve of reovirus type 1 was characteristic of a multihittype curve. The calculated devitalization half-life values for poliovirus types 1, 2, and 3; echovirus types 1 and 11; coxsackievirus types A-9 and B-1; and reovirus type 1 were 2.8, 3.1, 2.7, 2.8, 3.2, 3.1, 4.0, 4.0 sec, respectively. These basic data should facilitate an operative extrapolation of the findings to the applied situation. It was concluded that UV can be highly effective and provide a reliable safety factor in treating estuarine water.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERG G., CHANG S. L., HARRIS E. K. DEVITALIZATION OF MICROORGANISMS BY IODINE. I. DYNAMICS OF THE DEVITALIZATION OF ENTEROVIRUSES BY ELEMENTAL IODINE. Virology. 1964 Apr;22:461–481. doi: 10.1016/0042-6822(64)90068-6. [DOI] [PubMed] [Google Scholar]
- BERG G., HARRIS E. K., CHANG S. L., BUSCH K. A. QUANTITATION OF VIRUSES BY THE PLAQUE TECNHIQUE. J Bacteriol. 1963 Mar;85:691–700. doi: 10.1128/jb.85.3.691-700.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. L. Water borne viral infections and their prevention. Bull World Health Organ. 1968;38(3):401–414. [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasso G. J., Sharp D. G. Effect of particle aggregation on the survival of irradiated vaccinia virus. J Bacteriol. 1965 Oct;90(4):1138–1142. doi: 10.1128/jb.90.4.1138-1142.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossgebauer K. Assessment of virucidal ability of chemical disinfectants. Appl Microbiol. 1967 Mar;15(2):316–318. doi: 10.1128/am.15.2.316-318.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Plaque formation with poliomyelitis, Coxsackie, and orphan (echo) viruses in bottle cultures of monkey epithelial cells. Virology. 1955 Dec;1(5):533–535. doi: 10.1016/0042-6822(55)90041-6. [DOI] [PubMed] [Google Scholar]
- Hamblet F. E., Hill W. F., Akin E. W. Effect of plaque assay diluent upon enumeration of poliovirus type 1. Appl Microbiol. 1967 Jan;15(1):208–208. doi: 10.1128/am.15.1.208-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F., Jr, Hamblet F. E., Benton W. H. Inactivation of poliovirus type 1 by the Kelly-Purdy ultraviolet seawater treatment unit. Appl Microbiol. 1969 Jan;17(1):1–6. doi: 10.1128/am.17.1.1-6.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY C. B. Disinfection of sea water by ultraviolet radiation. Am J Public Health Nations Health. 1961 Nov;51:1670–1680. doi: 10.2105/ajph.51.11.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METCALF T. G., STILES W. C. THE ACCUMULATION OF ENTERIC VIRUSES BY THE OYSTER, CRASSOSTREA VIRGINICA. J Infect Dis. 1965 Feb;115:68–76. doi: 10.1093/infdis/115.1.68. [DOI] [PubMed] [Google Scholar]
- Metcalf T. G., Stiles W. C. Enteroviruses within an estuarine environment. Am J Epidemiol. 1968 Nov;88(3):379–391. doi: 10.1093/oxfordjournals.aje.a120898. [DOI] [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Morales F., Powell J., Melnick J. L. Plaque enhancement of enteroviruses by magnesium chloride, cysteine, and pancreatin. J Bacteriol. 1966 May;91(5):1932–1935. doi: 10.1128/jb.91.5.1932-1935.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]