Abstract
Patricia S. Goldman-Rakic (1937–2003) transformed the study of the prefrontal cortex (PFC) and the neural basis of mental representation, the basic building block of abstract thought. Her pioneering research first identified the dorsolateral PFC (dlPFC) region essential for spatial working memory, and the extensive circuits of spatial cognition. She discovered the cellular basis of working memory, illuminating the dlPFC microcircuitry underlying spatially tuned, persistent firing, whereby precise information can be held “in mind”: persistent firing arises from recurrent excitation within glutamatergic pyramidal cell circuits in deep layer III, while tuning arises from GABAergic lateral inhibition. She was the first to discover that dopamine is essential for dlPFC function, particularly through D1 receptor actions. She applied a host of technical approaches, providing a new paradigm for scientific inquiry. Goldman-Rakic's work has allowed the perplexing complexities of mental illness to begun to be understood at the cellular level, including atrophy of the dlPFC microcircuits subserving mental representation. She correctly predicted that impairments in dlPFC working memory activity would contribute to thought disorder, a cardinal symptom of schizophrenia. Ten years following her death, we look back to see how she inspired an entire field, fundamentally changing our view of cognition and cognitive disorders.
Keywords: dopamine, mental representation, prefrontal cortex, schizophrenia, working memory
Introduction
How does the brain create thought? This weighty question has perplexed philosophers and scientists alike, and many still surmise that it is a quandary beyond the scope of scientific inquiry. How do we think about something that is not actually stimulating our senses? How does the brain generate its own activity—creating goals and visions—and how does it maintain this information despite distractions and interruptions? The brain's ability to create mental representations is the foundation of abstraction, a process that liberates us from our environment, liberates us from conditioned responses, the foot-in-the-door that is free will. It is extraordinary that this vital process has now begun to be understood at the cellular level, in large part due to the groundbreaking research of Patricia Shoer Goldman-Rakic.
Patricia Shoer was born on 22 April 1937 in Salem, Massachusetts. It was a year after the publication of the very first work to uncover the critical role of the dorsolateral prefrontal cortex (dlPFC) in the generation of thought, the research of Carlyle Jacobsen at Yale (Fig. 1). The chairman of Jacobsen's department was John Fulton, an expert primate neurosurgeon, who helped Jacobsen create lesions to different parts of the cerebral cortex. Jacobsen discovered that the monkeys with bilateral lesions to the dlPFC could solve even difficult puzzles if the information needed was present in the environment, while even a short delay that required information to be held in mind, reduced performance to chance (Jacobsen 1936). He wrote: “The animal without the frontal association area learns and retains sensory-motor habits and visual discriminations but it is unable to remember for even a few seconds under which of two cups a piece of food is concealed … It is as if ‘out of sight, out of mind’ were literally applicable” (Jacobsen 1936), a reference to Ferrier's generalized description of subjects with frontal lesions (Ferrier 1886). Jacobsen also speculated about the possible cellular basis for this critical ability, writing that the answer “must be supplied by the subject either through some sustained activity during the period of delay or by recall from past experience…” (Jacobsen 1936). This speculation would be supported some 40 years later when neuroscientists began to record from prefrontal neurons.
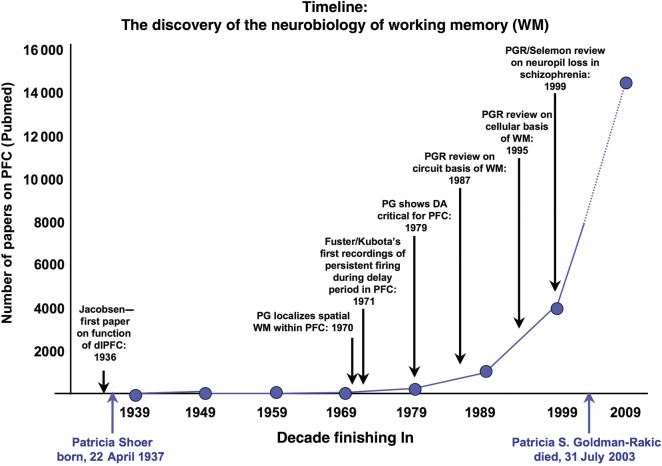
Figure 1.
Timeline of the discoveries of the PFC role in working memory (WM) and the key contributions of Goldman-Rakic. The graph shows the number of papers cited on PubMed using the search term “prefrontal cortex” for each decade ending in the year noted. Key publications by Goldman-Rakic and other early pioneers are indicated.
Following the interruption of World War II, there ensued an era of extraordinary lesion studies, much of which has become invisible to today's researchers, as the data were often published in books or journals not captured in PubMed, for example, in The Frontal Granular Cortex and Behavior edited by Warren and Akert and published by McGraw-Hill in 1964. Lesion studies in monkeys have become prohibitively expensive, but they reveal the essential contributions of a brain area in ways that functional imaging and even neuronal recordings do not. Functional imaging and physiology can reflect indirect activity from other, interconnected brain regions, while lesion studies reveal what is uniquely lost. These early lesion studies showed that monkeys with PFC ablations or cooling of the PFC to induce a functional lesion were easily distracted (Grueninger and Pribram 1969), inflexible, perseverative (Mishkin 1964; Butter 1968), and hyperactive (Kennard et al. 1941; Ruch and Shenkin 1943), with lesions to the orbital PFC altering emotional responses (Butter and Snyder 1972) and that to the dorsolateral aspects altering cognition (Fuster and Bauer 1974). The beginnings of circuit contributions were also apparent in lesion studies, for example, showing that the most prominent deficits on spatial working memory tasks were found with frontal lesions, but more subtle deficits could be seen following lesions to such areas as the caudate and hippocampus (Rosvold and Szwarcbart 1964).
Early in her career at the NIH, Patricia Shoer Goldman worked with Rosvold to continue the work of Jacobsen and refined the region of dlPFC necessary for visuospatial working memory. She determined that the cortex surrounding the caudal two-thirds of the principal sulcus was essential for spatial working memory, and that monkeys with principal sulcal lesions could perform visuospatial tasks that did not require memory, or perform memory tasks that did use visuospatial information, but could not perform tasks that required memory of visuospatial information (Goldman and Rosvold 1970; Goldman et al. 1971). This information not only defined the “bull's eye” for the cortex underlying spatial working memory, but also gave the first hints of the parallel organization underlying cognitive operations.
The Circuit Basis for Working Memory
Currently, researchers often use magnetic resonance imaging (MRI) methods to try to reveal connectivity in human brains, for example, examining the cohesion of white matter tracks, or correlations between activated areas. Many are unaware of the wealth of anatomical tracing studies of the monkey brain, some of which continue to this day. With the development of sensitive track tracing methods in the 1970's, the detailed connections between brain regions could be revealed for the first time. Pat collaborated with Walle Nauta at MIT to learn these new techniques and found the first evidence of columnar organization of cortical–cortical connections in the dlPFC, similar to what had been traced in the primary visual cortex (Goldman and Nauta 1977). These columns suggested that the methods being applied to the primary visual cortex to reveal the circuit and cellular basis of visual perception could be applied to the PFC to explore the neuronal basis of thought. This affirmation of strategy served as a talisman to Pat, as the attitude at the time (and even sometimes today) was that the processes underlying thought were beyond the scope of science, and that rigorous scientific pursuits could only be applied to sensory-motor functions. Pat's work revolutionized this view, demonstrating that the neurobiology of cognition was tractable if approached in a manner that respected component processes and revealed the inherent neural organization.
Pat married Pasko Rakic in 1979 and they both came to Yale to create the Section of Neuroanatomy (later called the Department of Neurobiology). Goldman-Rakic and Rakic went on to found this journal, Cerebral Cortex, in 1991. On arriving at Yale, Goldman-Rakic performed an intensive series of anatomical tracing studies with colleagues such as Schwartz, Selemon, and Cavada, to identify the circuit basis of spatial cognition. They found that the principal sulcul PFC shared reciprocal projections with area 7a/7lip of the parietal association cortex, a region known to perform high-order processing of visuospatial information (Cavada and Goldman-Rakic 1989), as well as intensive connections across the corpus callosum with its counterpart in the opposite hemisphere (Schwartz and Goldman-Rakic 1984). These connections terminated in columns (Schwartz and Goldman-Rakic 1984), and appeared before birth (Schwartz and Goldman-Rakic 1991), establishing a genetic mediation for cortical connectivity. Remarkably, the 2 regions projected to many of the same brain areas (Selemon and Goldman-Rakic 1988), forming a complex and beautifully organized pattern of connections summarized in Figure 2. Thus, both the dlPFC and the posterior parietal cortex shared projections to a large number of cortical areas, including those involved with visuospatial processing (area 19, medial parietal cortex), auditory information and sensory integration (superior temporal cortex), motor response (premotor cortex and frontal eye fields), reward and punishment (orbital and insular PFC), memory (parahippocampal gyrus and presubiculum), and error detection (anterior cingulate cortex). Interestingly, they also interconnect with regions now considered part of the so-called “Default Network,” for example, the anterior and posterior cingulate cortices and the retrosplenial cortex, which is involved in episodic memory, navigation, imagination, and planning for the future (Vann et al. 2009). There were also shared projections to subcortical structures that are not shown in Figure 2, including extensive projections to striatum, thalamus, and the cerebellum via the pontine nuclei (Selemon and Goldman-Rakic 1985, 1988). Thus, a picture began to build of the coordinated, long-range circuits for spatial cognition.
Figure 2.
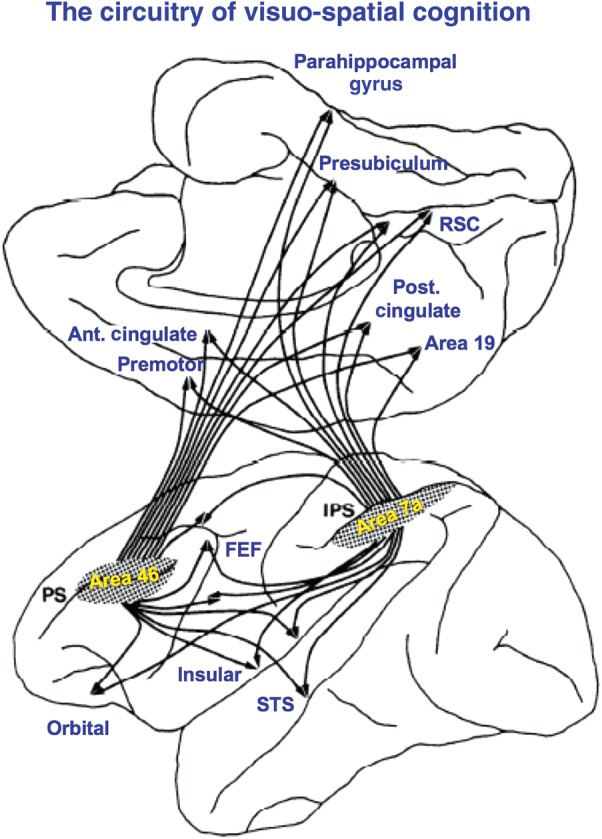
The cortical circuitry for spatial cognition, based on the work of Goldman-Rakic and Selemon. Note that both the dlPFC (area 46) and parietal cortex have many shared connections to subcortical structures that are not shown in this illustration, as well as “nonshared” connections that are not included in this diagram. Figure used with the permission of L. Selemon. Ant. Cingulate: anterior cingulate; FEF: frontal eye fields; IPS: intraparietal sulcus; Post. Cingulate: posterior cingulate; PS: principal sulcus; RSC: retrosplenial cortex; STS: superior temporal sulcus.
But what about working memory for other sensory modalities? The work of Haxby, Ungerleider, and colleagues had identified parallel processing streams for the processing of visual space versus visual features (Haxby et al. 1991). Goldman-Rakic saw that these streams remained in parallel as they projected into distinct subregions of the dlPFC (Goldman-Rakic 1987; Fig. 3). Further work showed that these parallels extended to auditory processing as well, creating a dorsal zone for spatial aspects of working memory, and a more ventral zone for working memory of sensory features (Romanski et al. 1999). As with the posterior cortical streams, these areas are extensively interconnected (Barbas and Pandya 1989), thus providing a cohesive experience of reality. In contrast to the sensory projections to the dlPFC, affective and interoceptive information projected into the orbital and medial PFC, which, in turn, projected to limbic structures such as the amygdala, hypothalamus, and brainstem (Price et al. 1996; Ghashghaei and Barbas 2002). Thus, there is a topographic organization to the circuitry, and therefore the functions, of the primate PFC.
Figure 3.
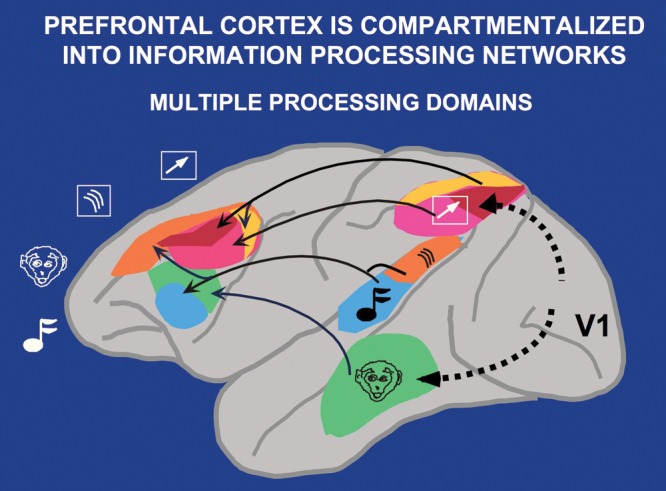
The parallel cortical circuits for space versus features in the visual and auditory domains. Parallel visual pathways for the processing of visual space and visual features emerge from the primary visual cortex, area V1. These pathways remain in parallel as they project into the PFC. Similar parallel projections were observed for the auditory spatial and feature streams. The visuospatial circuit is shown in pink/red/yellow; the auditory spatial circuit in orange; the visual feature circuit is shown in green, and the auditory feature circuit is shown in blue. Figure from a Goldman-Rakic presentation for Yale undergraduates with the permission of P. Rakic. Note that projections from the PFC back to the sensory cortex are not illustrated in this figure, but likely play an important role in top-down regulation of attention and sensory processing.
The Cellular Basis of Working Memory
Fuster (Fuster and Alexander 1971; Fuster 1973) and Kubota (Kubota and Niki 1971) were the first to record from neurons in the dlPFC as monkeys performed working memory tasks. They used classical, manual versions of these tasks and discovered neurons with a variety of properties: Those that responded to the sensory cue, many that responded in anticipation of or during the motor response, and most intriguingly, neurons that were able to maintain persistent firing across the delay period, the sustained neural activity that was predicted by Jacobsen years before. Fuster (Fuster 1985, 2008) realized that, with these “memory cells” he had captured that foot-in-the-door, the neural process that integrated perception with action, the temporal bridge that wedded the past to the future: “the bridging of cross-temporal contingencies of behavior, in other words, the adjustment of the actions of the organism to temporally distant events and objectives” thus generating “short-term memory, preparatory set, and control of interference” (Fuster 1985).
Pat built on this work with Funahashi and Bruce, adapting a delayed saccade task (Hikosaka and Wurtz 1983) that allowed precise knowledge of the retinotopic position of 8 spatial cues. These recordings revealed that the persistent firing across the delay period in the spatial working memory task was spatially tuned (Funahashi et al. 1989), representing a specific portion of the visual field (Fig. 4A1). Thus, a “Delay cell” will show elevated firing across the delay period for the memory of one particular location (usually in the contralateral visual field), and actually inhibit its firing during the delay period for other directions, creating a so-called “memory field” (Fig. 4A2). The location of a neuron's memory field is stable day-to-day (Fig. 4B), as would be needed for mental representation. Furthermore, tiny lesions within this area of dlPFC produced “mnemonic scotomas,” impairments in remembering just that specific area of visual space, with no effect on visually guided eye movements (Funahashi, Bruce, et al. 1993). Neurons representing visual features, for example faces, could be found more ventrally in the area of PFC that receives information from the ventral stream (Wilson et al. 1993; O'Scalaidhe et al. 1997). Taken together, these findings had uncovered the cellular basis for mental representation: “I have maintained that the prefrontal neuron's capacity for sustained activation in the absence of external stimulation is the cellular basis of mental representation and the essential building block for information processing systems in the human brain. This is the neural mechanism presumably disrupted in the condition: ‘out of sight-out of mind’ that Sir John Ferrier used to describe monkeys with prefrontal lesions (Ferrier 1886) and so often been used to describe patients with prefrontal lesions” (Goldman-Rakic 2002).
Figure 4.
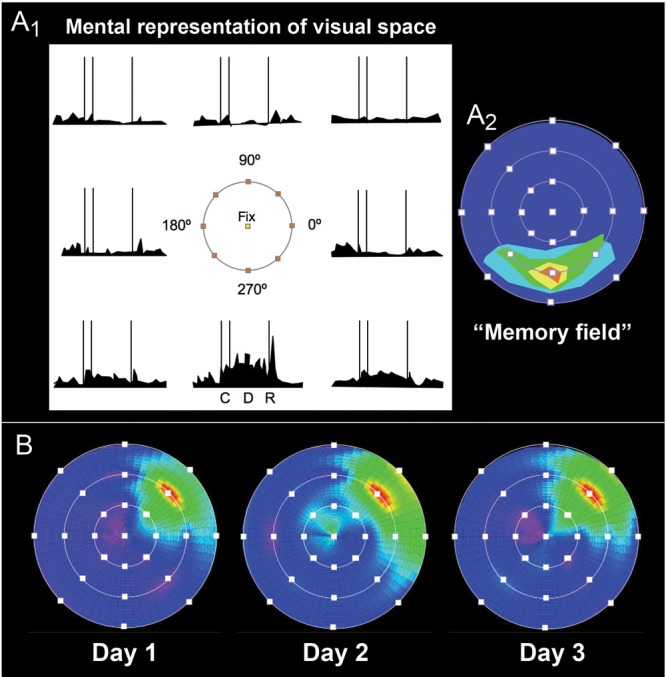
The physiology of mental representation in dlPFC. (A1) A schematic representation of a neuron with spatially tuned, persistent firing during the oculomotor delayed-response task. The possible spatial locations for cues are shown in the center of this figure, with the fixation point indicated in yellow. This neuron has persistent firing for the memory of a cue at 270°, but has less persistent firing for the memory of nearby locations, and actually inhibits firing during the delay period following cues distant to the neuron's “preferred location.” Goldman-Rakic considered this the cellular representation of visual space, the fundamental building block of mental representation. C: cue period; D: delay period; R: signal for saccadic response. (A2) The neuronal firing patterns of the neuron depicted in (A1) shown as a “memory field,” where dark blue represents low levels of firing during the delay period and brighter colors signify progressively higher firing rates. This method provides a more intuitive process for depicting the strength and precision of a neuron's spatial tuning. (B) The same neuron recorded on 3 separate days shows stable spatial tuning for 45°, as would be needed for the mental representation of visual space. This figure is from a Goldman-Rakic presentation for Yale undergraduates with the permission of P. Rakic; the data are from O’Scalaidhe and Goldman-Rakic, unpublished.
This “essential building block” can be seen contributing to other PFC executive operations in recordings from monkeys performing related tasks. For example, spatially tuned persistent firing underlies behavioral inhibition, in which the monkey has to look away from a remembered stimulus (Funahashi, Chafee, et al. 1993). Similarly, it is essential for goal-directed attention and resistance to distraction. For example, dlPFC neurons can maintain persistent firing across the delay period despite distractions, in contrast to more posterior cortices where distraction interrupts firing (Miller et al. 1996). How do PFC circuits generate this robust, highly specific, persistent firing to represent events and goals for action?
The dlPFC Microcircuits That Generate Mental Representations
Goldman-Rakic combined anatomical tracing methods with multiple electrode recordings to reveal the circuitry underlying spatially tuned, persistent firing by dlPFC Delay cells.
Persistent Firing
Kritzer and Goldman-Rakic (1995) examined the intrinsic circuitry of the dlPFC by making very small injections of a retrograde tracer within a distinct layer. They found that neurons in deep layer IIIc had the most extensive horizontal connections, consistent with recurrent excitatory connections in this sublayer (Fig. 5A). These horizontal connections extended 2–7 mm and terminated in a series of columns in deep layer III. There was also evidence of horizontal connections between neurons in the superficial part of layer V (Va), connecting with both layer III and superficial layer V cells. In contrast, the other layers and sublayers showed more typical, vertical labeling. The depiction of deep layer III horizontal, recurrent excitatory connections is schematically illustrated by Goldman-Rakic in Figure 5B. The finding of extensive horizontal projections with a columnar pattern within deep layer III fits with the previous data, showing columnar inputs of visuospatial information from the parietal association cortex (Schwartz and Goldman-Rakic 1984) and also with subsequent physiological recordings showing clusters of neurons with similar spatial tuning and timing consistent with monosynaptic excitatory connections (Constantinidis et al. 2001). Pyramidal cells intersynapse onto dendritic spines, and our more recent data have shown very long and thin spines in deep layer III (Arnsten et al. 2012; Paspalas et al. 2012). We have also shown that the persistent firing of Delay cells depends on glutamate stimulation of N-methyl-D-aspartic acid (NMDA) receptors with slow, NR2B subunits that can be found in the postsynaptic density on spines in deep layer III (Wang et al. 2013). Thus, the persistent firing needed to sustain a mental representation without sensory stimulation arises from recurrent glutamate NMDAR pyramidal cell excitation, likely in deep layer III and possibly superficial layer V.
Figure 5.
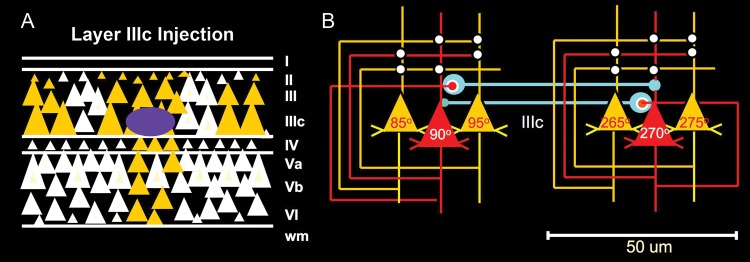
The dlPFC microcircuits underlying mental representation. (A) Microinjections of an anatomical tracer (purple) into layer IIIc of the primate dlPFC-labeled horizontal connections (gold) consistent with recurrent excitation between pyramidal cells. (B) Goldman-Rakic's schematic depiction of the primate layer IIIc microcircuits that provide the cellular basis for mental representation. Pyramidal cells are depicted by triangles; they excite each other through glutamatergic synapses on spines (white circles). GABAergic interneurons providing lateral inhibition are shown in blue. Note that although connections between pyramidal cells are depicted on the apical dendrites for the sake of clarity, they are likely most concentrated on the basal dendrites. Figures from a Goldman-Rakic presentation for Yale undergraduates with the permission of P. Rakic.
Spatial Tuning
The circuit basis for the spatial tuning of dlPFC Delay cells arises from the lateral inhibition provided by fast-spiking, parvalbumin-containing, GABAergic interneurons (basket and chandelier cells), for example, the basket cell seen in Figure 6A (Rao et al. 1999; Constantinidis and Goldman-Rakic 2002). A schematic illustration of this lateral inhibition is portrayed in Figure 5B, where the 90° pyramidal cells activate an interneuron (represented in blue) to inhibit the firing of the 270° pyramidal cells, and vice versa. An example of simultaneous, multiple electrode recordings from a fast-spiking neuron (presumed parvalbumin-containing GABAergic interneurons) and a regular-spiking neuron (presumed pyramidal cells) is shown in Figure 6; note that the spatial tuning of the presumed pyramidal cell (Fig. 6C1) is opposite to that of the presumed GABAergic interneuron (Fig. 6B1), and that the pyramidal cell increased its firing (Fig. 6C2) as the interneuron reduced its firing (Fig. 6B2). Furthermore, local application of a gamma-aminobutyric acid (GABA) antagonist eroded the spatial tuning of dlPFC Delay cells (Rao et al. 2000), consistent with this working model. Thus, lateral inhibition from GABAergic interneurons is important for enhancing contrast and allowing more precise information to be held in working memory stores.
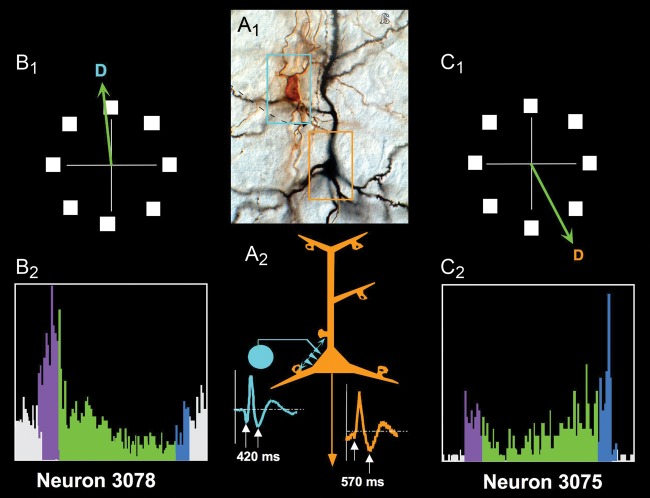
Figure 6.
An example of the reciprocal relationship between pyramidal cells and GABAergic interneurons in the dlPFC. (A1) Photograph of a GABAergic basket cell (within blue rectangle) and a pyramidal cell (within orange rectangle) in the primate dlPFC. (A2) A schematic diagram of the likely connections between these neurons, whereby the basket cell (blue, fast-spiking with thin waveform) inhibits the pyramidal cell (orange, regular-spiking with longer waveform) through connections on the soma (shown) and proximal primary dendrites (not shown). (B1) The preferred direction of the presumed GABAergic interneuron during the delay period. (B2) The firing pattern of a fast-spiking, presumed GABAergic interneuron during the initial fixation (gray), cue presentation (purple), delay period (green), and response epochs (blue). (C1) The preferred direction of the regular-spiking, presumed pyramidal cell during the delay period. Note that it is opposite to the preferred direction of the interneuron. (C2) The firing pattern of the presumed pyramidal cell during the initial fixation (gray), cue presentation (purple), delay period (green), and response epochs (blue). Note that the firing of the pyramidal cell increases as the firing of the GABAergic interneuron decreases. Figures from a Goldman-Rakic presentation for Yale undergraduates with the permission of P. Rakic.
Recordings down the depth of the principal sulcus (Fig. 7), as well as from the surface of the dlPFC (Constantinidis et al. 2001), showed the progressive representation of the visual field as the electrode advanced. These extensive physiological assessments of the dlPFC revealed a microcolumnar architecture, as schematically depicted in Figure 5B and described by Goldman-Rakic in 2002:
“Thus, using multiple electrodes in vivo, we have shown that neurons that lie in close proximity to each other not only are likely to have shared spatial tuning, i.e., to be iso-directionally related (Constantinidis et al. 2001), but also to be monosynaptically connected (Constantinidis et al. 2000). In contrast, neurons at wider distances, e.g., within 200–300 μm of each other, are more likely to have wide disparities in their spatial tuning and to be cross-directionally tuned, suggestive of a modular organization for visuo-spatial information processing. The striking local circuit and functional arrangements between adjacent and separated pyramidal and fast-spiking interneurons support a microcolumnar functional architecture in the dorsolateral prefrontal cortex for spatial memory fields and hence for psychic functions, similar to that found in other areas of cortex for sensory receptive fields.” (Goldman-Rakic 2002)
Figure 7.
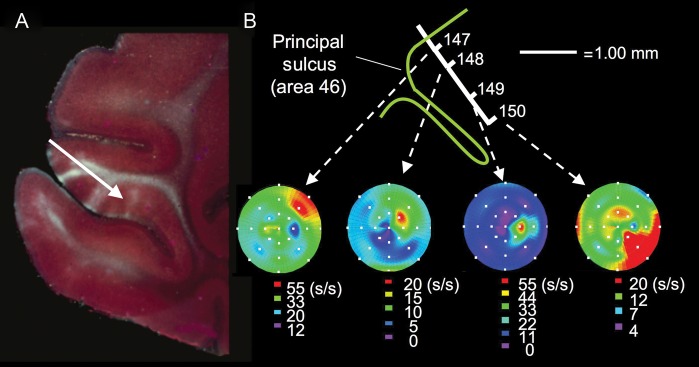
Representation of the visual field in principal sulcal dlPFC. (A) An image of the rostral principal sulcus that Goldman-Rakic used to illustrate the concept of progressive recordings down the sulcus as indicated by the white arrow. The autoradiographic image of the dlPFC is from an earlier anatomical study with Nauta showing columns of labeling from connections with the contralateral dlPFC. The actual recordings were performed in a more caudal region of the principal sulcal cortex. (B) Recordings by O’Scalaidhe and Goldman-Rakic (unpublished) down the length of the caudal principal sulcal cortex show progressive changes in the preferred direction of each neuron, thus providing comprehensive representation of the entire, contralateral visual field. Figures from a Goldman-Rakic presentation for Yale undergraduates with the permission of P. Rakic.
Thus, Goldman-Rakic revealed the basic microcircuitry for mental representation in the dlPFC. This building block of cognition can be used to construct ever-higher functions—representations of representations—the foundation of abstract thought.
The Key Role of Dopamine and Neuromodulation
The higher cognitive functioning of the dlPFC is especially sensitive to its neuromodulatory environment, a finding discovered by Pat in 1979. In her groundbreaking study with Brozoski et al. (1979), Pat showed that depletion of catcholamines from the monkey dlPFC was as devastating to spatial working memory performance as removing the cortex itself. This work has served as a beacon for all subsequent studies on the neuromodulation of dlPFC, illuminating the critical importance of molecular state to higher cognitive function, and helping to explain both the etiology of cognitive disorders and possibilities for their treatment.
It is remarkable that the finding of dopamine's importance to the primate dlPFC was published in 1979, years before parallel cognitive studies were performed in rodents (Bubser and Schmidt 1990). The dopamine innervation of the rat cortex was first mapped in the 1970's, showing a selective projection of dopamine fibers to the PFC but not other cortical areas (Berger et al. 1976). Pat and Roger Brown were the first to measure monoamine concentrations in the primate cortex, and found that dopamine levels and synthesis were very high in the primate PFC, but that unlike the rodent, dopamine was also prevalent in other cortical areas as well (Brown et al. 1979). Working with Brozoski, they examined the functional contribution of dopamine to the primate dlPFC by infusing the catecholamine neurotoxin, 6-OHDA, into the dlPFC, with or without desmethylimipramine (DMI), supposed to protect noradrenergic fibers. DMI was not very effective in protecting norepinephrine, but it did facilitate uptake into dopaminergic fibers to enhance depletion. Thus, they created lesions with very large dopaminergic (and large noradrenergic) depletions restricted to the dlPFC. These lesions markedly impaired spatial working memory performance, similar to that seen with dlPFC ablations. Performance was improved by catecholaminergic drugs: The catecholamine precursor l-3,4-dihydroxyphenylalanine, the dopamine D2 receptor agonist, apomorphine, and (in a footnote), the α2 noradrenergic agonist, clonidine. Since noradrenergic depletion with minimal dopamine depletion had little effect on working memory performance, the authors concluded that dopamine was the key factor. However, it is now known that both dopamine and norepinephrine are critical for dlPFC function, and it is likely that one can substitute for the other in long-term lesion studies such as the one performed by Brozoski et al. Indeed, that footnote on clonidine led to studies showing that noradrenergic stimulation of postsynaptic, α2 adrenergic receptors is essential to dlPFC function (Arnsten and Goldman-Rakic 1985) via functional strengthening of pyramidal cell circuits (Wang et al. 2007), and α2A receptor agonists such as guanfacine are now in widespread clinical use to treat PFC cognitive disorders (Hunt et al. 1995; Scahill et al. 2001, 2006; Biederman et al. 2008; McAllister et al. 2011; Connor et al. 2013). Goldman-Rakic also began to explore other modulatory influences on dlPFC, including serotonin (e.g. Lidow et al. 1989; Williams et al. 2002); and acetylcholine (e.g. Mrzljak et al. 1993). But her primary focus remained on dopamine. She worked with Mark Williams to identify the midbrain source of dopamine to the PFC (Williams and Goldman-Rakic 1998), and to map the dopaminergic fibers innervating the frontal lobe (Williams and Goldman-Rakic 1993). The dopamine-containing fibers in the dlPFC are actually rather sparse (Fig. 8A), emphasizing that quantity does not always correlate with efficacy.
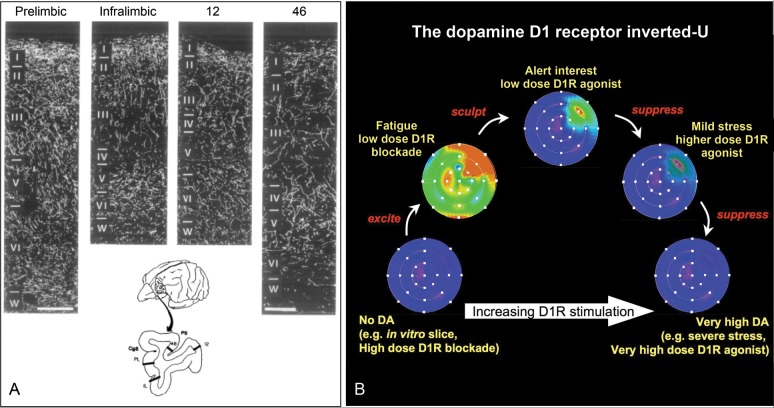
Figure 8.
The key role of dopamine in the primate dlPFC. (A) The dopaminergic innervation of the primate PFC, including the dlPFC area 46, as visualized using an antibody directed against dopamine. Note the relatively sparse labeling in the dlPFC, a region that critically depends on dopamine actions. (From Williams and Goldman-Rakic 1993.) (B) A schematic illustration of the dopamine D1 receptor inverted-U influence on the pattern of Delay cell firing in the dlPFC. The memory fields of dlPFC neurons are shown under conditions of increasing levels of D1 receptor stimulation. Either very low or very high levels of D1 receptor stimulation markedly reduce delay-related firing. Low levels of D1 receptor stimulation are associated with noisy neuronal representations of visual space, while optimal levels reduce noise and enhance spatial tuning. The high levels of D1 receptor stimulation during stress exposure would reduce delay-related firing for all directions. Brighter colors indicate higher firing rates during the delay period. This figure is a schematic illustration of the physiological data presented in Williams and Goldman-Rakic (1995); Vijayraghavan et al. (2007); and Arnsten et al. (2009) and is consistent with the behavioral data from Arnsten et al. (1994); Murphy et al. (1996); Zahrt et al. (1997); and Arnsten and Goldman-Rakic (1998).
D1 versus D2 Receptor Actions
The advent of selective D1 versus D2 receptor antagonists allowed the exploration of dopamine's actions at these differing receptor families. The D1 receptor family (D1 and D5) was most prevalent in the dlPFC, with dense binding in superficial and deep layers, whereas D2 receptor binding was sparse and concentrated in layer V (Goldman-Rakic et al. 1990; Lidow et al. 1991). These findings were later confirmed by in situ hybridization histochemistry, where mRNA for D2 receptors was again focused in layer V neurons (Lidow et al. 1998), and by immunoelectron microscopy, where D1 receptors were most prevalent on dendritic spines (Smiley et al. 1994).
D1 Receptor Beneficial Actions
Given the extensive D1 receptor binding in the dlPFC, Goldman-Rakic's initial studies focused on D1 receptor actions. Working with Sawaguchi, she found that infusion of a D1 receptor antagonist into the dlPFC impaired spatial working memory performance, but had no effect on visually guided saccades (Sawaguchi and Goldman-Rakic 1991, 1994). Infusion of a D2 receptor antagonist had no effect on spatial working memory, although this may have been due to the fact that ceiling effects precluded improvements in performance. The impairment following D1 receptor antagonist infusion was consistent with the subsequent physiological data, showing that iontophoretic application of a high dose of D1 receptor antagonist onto dlPFC Delay neurons markedly reduced neuronal firing (Williams and Goldman-Rakic 1995). Taken together, these data showed that dopamine has an important beneficial influence on dlPFC spatial working memory function through D1 receptor actions.
The Discovery of the D1 Receptor “inverted-U” Dose–Response
Although the research had emphasized the beneficial influences of dopamine, behavioral data provided the first indication that high levels of dopamine release, such as occurs during stress exposure (Deutch and Roth 1990), could be detrimental to dlPFC function through excessive stimulation of D1 receptors (Arnsten and Goldman-Rakic 1990, 1998; Arnsten et al. 1994; Murphy et al. 1996; Arnsten 1998). This was also the first evidence that exposure to uncontrollable stress could impair PFC function (Arnsten and Goldman-Rakic 1990, 1998; Murphy et al. 1996; Arnsten 1998), a finding of immediate relevance to the etiology of mental illness. With the advent of dopamine D1 receptor agonists, the inverted-U D1 receptor dose–response was confirmed (Arnsten et al. 1994; Zahrt et al. 1997). The inverted-U was also seen at the physiological level, where either too little or too much dopamine D1 receptor stimulation reduced neuronal firing (schematically illustrated in Fig. 8B; Williams and Goldman-Rakic 1995; Vijayraghavan et al. 2007). At optimal levels of D1 receptor stimulation, D1 receptors reduce “noise,” that is, neuronal firing for the memory of nonpreferred spatial inputs, while low doses of D1 receptor antagonist produce the converse pattern of increased firing for nonpreferred inputs (Vijayraghavan et al. 2007; Fig. 8B). Currently available D1 receptor agonists have high affinity for D1 receptors, and it is likely that compounds that better mimic dopamine's gentler interactions with the D1 receptor will be needed to visualize dopamine's excitatory actions in vivo, as has been documented in vitro where bath application allows more rapid removal of drug (Seamans et al. 2001; Seong and Carter 2012).
In contrast to most biological systems where the inverted-U is seen at the extremes of physiological conditions, the D1 inverted-U occurs within a normal, relatively narrow range of physiological conditions, that is, within the parameters of daily life (e.g. fatigue and mild stress). The D1 inverted-U has translated well to humans, where it has helped to explain cognitive variations in humans based on the COMT genotype (e.g. Egan et al. 2001; Meyer-Lindenberg et al. 2005; Bertolino et al. 2006; Williams-Gray et al. 2007; Papaleo et al. 2008; Jacobs and D'Esposito 2011), and in response to dopamine drugs (e.g. Gibbs and D'Esposito 2006), thus explaining otherwise perplexing findings.
The D2 Receptor Family
The D2 receptor family (D2, D3, and D4) is also of great interest, especially in regard to the etiology and treatment of schizophrenia. Immunoelectron microscopy has revealed D2 receptors concentrated on the dendritic shafts of pyramidal cells but not on spines (Paspalas et al. 2006), while D4 receptors are enriched on GABAergic interneurons (Mrzljak et al. 1996). Just before she died, Goldman-Rakic completed a study with Wang and Vijayraghavan showing that D2 receptor stimulation increases the firing of dlPFC Response cells, with no effect on Delay cell firing (Wang et al. 2004). These data are consistent with the idea that Response cells likely reside in layer V, the site of the greatest D2 receptor mRNA, and the neurons that project most strongly to the caudate nucleus (Yeterian and Pandya 1994). Intriguingly, many of the Response cells influenced by D2 receptor stimulation fired during or after the saccadic response, suggesting that D2 receptor stimulation may be altering corollary discharge (also called “efference copy”), the mental tag that tracks and provides feedback about an internal response. Reduced corollary discharge from the dlPFC has been associated with auditory hallucinations in patients with schizophrenia (Ford et al. 2002), suggesting a potential link between altered Response cell modulation and the positive symptoms of schizophrenia.
The Neurobiological Foundations of Schizophrenia
The cognitive deficits of schizophrenia involve profound dysfunction of the dlPFC, including deficits in working memory (Weinberger et al. 1986; Park and Holzman 1992; Barch et al. 2001; Keefe et al. 2006; Barch and Ceaser 2012). Goldman-Rakic collaborated with Driesen and Krystal to adapt a spatial working memory task to human functional MRI (fMRI) imaging and found that patients with schizophrenia had reduced dlPFC activation during the delay epoch when information was held in mind (Driesen et al. 2008). Importantly, fMRI studies had also shown that working memory deficits and reduced activation of the dlPFC correlate with symptoms of thought disorder in patients with schizophrenia, thus linking cognitive impairment to a classic symptom of the illness (Perlstein et al. 2001). Goldman-Rakic had predicted this finding in her earlier writings (Goldman-Rakic 1991), saying “a defect in working memory—the ability to guide behavior by representations—may be the fundamental impairment leading to schizophrenic thought disorder” (Goldman-Rakic 1994).
Insults to dlPFC Microcircuitry
Neuropathological studies of the brains of patients with schizophrenia have demonstrated marked atrophy in the dlPFC microcircuits needed for mental representation. Selemon, Rajkowska, and Goldman-Rakic discovered increased neuronal density corresponding to a loss of neuropil in the dlPFC (Fig. 9; Selemon et al. 1995, 1998), and overall smaller PFC gray matter volume (Selemon et al. 2002). Consonant findings were observed by the Lewis lab, which found reduced numbers of dendritic spines specifically in deep layer IIIc of the dlPFC, but not in the primary visual cortex or more superficial layers of the dlPFC (Glantz and Lewis 2000; Glausier and Lewis 2012). Based on what we have learned from Goldman-Rakic's studies in monkeys, loss of spines in layer IIIc pyramidal cell microcircuits should decrease persistent firing and weaken the ability to maintain information “in mind.” The Lewis lab has also found that layer III dlPFC microcircuits show signs of weakened GABAergic function (Gonzalez-Burgos et al. 2010), which may be a compensation for a loss of excitatory pyramidal cell drive (Lewis and Gonzalez-Burgos 2006). Much of the field has focused on the consequences of weaker GABA leading to disruptions in network oscillations and cortical timing (Gonzalez-Burgos et al. 2010). However, the Goldman-Rakic data suggest that weaker GABA would also lead to weaker lateral inhibition and, thus, less precise representations of the information held in working memory. Overall, the loss of spines and weaker GABA would lead to poor maintenance of unclear information, eroding the basic building block of mentation. Layer V pyramidal cells in the dlPFC also seem to be affected, having smaller basilar dendrites (Black et al. 2004). We do not know if these are Delay cells (e.g. ramp-up Delay cells that likely inform motor structures of the goal for action) and/or Response cells, but the findings suggest that the output from and/or feedback to the dlPFC is likely impaired as well. Thus, the circuits needed to represent information in memory stores and to provide guidance for actions are especially altered in schizophrenia (Fig. 9). The work of Goldman-Rakic allowed this most complex and devastating of cognitive disorders to begin to be understood at the cellular level.
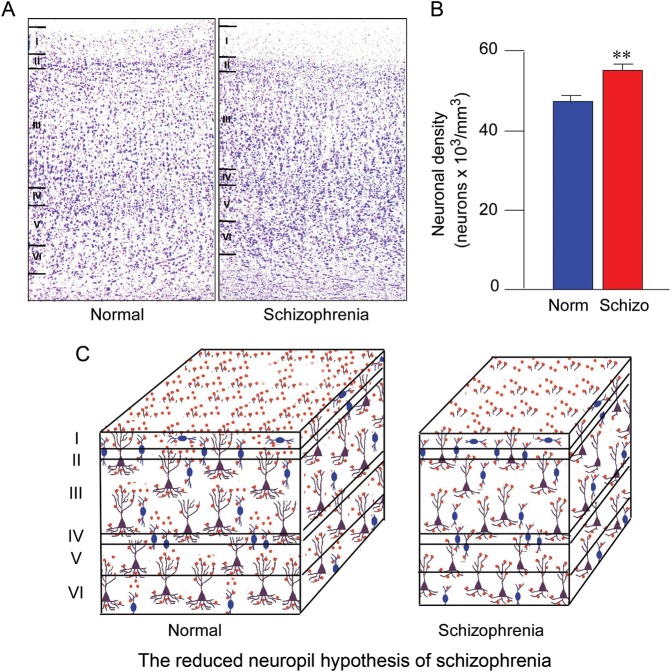
Figure 9.
Reduced neuropil in the dlPFC in the brains of patients with schizophrenia. (A) Examples of Nissl-stained coronal sections of the dlPFC from a normal control subject and a subject with schizophrenia. (B) Neuronal density measured across all cortical layers is greater in the dlPFC in patients with schizophrenia. (C) Schematic illustration of greater cell packing in schizophrenia, that is, the same number of neurons is present in a smaller volume, suggesting that the intervening space containing neuropil is diminished. Reduced neuropil in the cortex of patients with schizophrenia suggests that impoverished connectivity of the dlPFC is a neuropathologic correlate of the disease. Figure generously provided by L. Selemon.
Interestingly, the loss of spines and dendrites in the dlPFC of patients with schizophrenia is mimicked by amphetamine sensitization in monkeys (Selemon et al. 2007), which also recreates some of the symptoms of schizophrenia (Castner and Goldman-Rakic 1999). Amphetamine increases both norepinephrine and dopamine in the PFC (Berridge et al. 2006; Berridge and Devilbiss 2011), similar to what is seen with stress exposure (Deutch and Roth 1990; Finlay et al. 1995; Miner et al. 2006). As stress can also cause spine loss and dendritic atrophy of PFC neurons (Cook and Wellman 2004; Radley et al. 2006, 2008), it is possible that dysregulation of the catecholamine stress response may contribute to dlPFC atrophy in schizophrenia. In this regard, it is of interest that a D1 antagonist reversed dendritic atrophy caused by amphetamine sensitization (Selemon et al. 2010).
Dopamine and Schizophrenia
How is dopamine altered in the dlPFC of patients with schizophrenia? This is a surprisingly difficult question to answer, as the dopamine innervation of PFC is too delicate for reliable imaging in vivo. This contrasts with studies of dopamine in the heavily innervated striatum, where there is strong evidence of increased dopamine release in schizophrenia (Laruelle et al. 1996). Postmortem studies of the dlPFC from patients with schizophrenia show reduced tyrosine hydroxylase staining, which is a likely an indication of reduced dopamine levels (Akil et al. 1999), but could also be a sign of reduced tyrosine hydroxylase expression due to excessive dopamine creating negative feedback on its synthetic enzyme. Data from monkeys show that there is a hyperinnervation by dopamine of layer III in adolescence (Rosenberg and Lewis 1994, 1995), but it is not known if this also occurs in humans. If so, a hyperdopaminergic state in adolescence could promote a psychotic break and loss of spines, which could be followed by a deficit state as the disease progresses. There has been more success with imaging D1 receptors in the dlPFC. These studies show an increase in D1 receptor expression in the dlPFC early in the disease, prior to medication (Abi-Dargham et al. 2002, 2012). This may reflect a needed compensation for reduced dopamine, and/or may magnify the stress response.
Pat's great hope was that D1 agonists would help normalize cognition in patients with schizophrenia. Studies in monkeys encourage this possibility: The cognitive deficits induced by the NMDA antagonist, ketamine, were ameliorated by D1 agonist treatment (Roberts et al. 2010; Nakako et al. 2013). D1 agonists are currently being tested in patients with schizophrenia and those with schizotypal symptoms. Thus, we will soon learn whether this hope will be realized.
The Enduring Influence of Patricia Goldman-Rakic
Goldman-Rakic sparked a revolution in the study and appreciation of the PFC. Prior to her work, there were few studies published on the PFC; now, it has become a major focus on Neuroscience and Neuropsychiatry (Fig. 1). She eloquently explained why prefrontal mental representations were fundamental to cognition, and illuminated the cellular basis for this elemental function, inspiring many others to pursue the next generations of ideas. Goldman-Rakic also inspired a new “top-down” strategy for research in general, where one first asks an important scientific question and then finds multiple, appropriate techniques to try to integrate an answer, rather than finding a question to fit one's established technique. (Clearly, this expert on the PFC had remarkable prefrontal function!) But challenges remain. Goldman-Rakic's discoveries are still not taught in many medical schools, despite their immediate relevance to serious cognitive disorders such as schizophrenia. Thus, many psychiatrists are still unaware of her work, and the neural basis of the disorders they treat. As time goes by, the ever-expanding accumulation of human imaging studies and molecular studies in mice has also obscured her groundbreaking work in primates, with many still not knowing that key aspects of cortical neural connectivity have already been discovered. Goldman-Rakic showed us that the most perplexing and clinically important questions were open to scientific inquiry, and revealed the roadmap of cognition. “‘We're at the edge,’ Goldman-Rakic said, ‘making discoveries that are of great moment for understanding humans’” (Horgan 1999).
Funding
Funding to pay the Open Access publication charges for this article was provided by AFTA.
Notes
I would like to thank Lynn Selemon, Constantinos Paspalas, Min Wang, and Pasko Rakic for their guidance and inspiration in writing this review. Conflict of Interest: Yale University and AFTA receive royalties from the sale of extended release guanfacine (Intuniv™) from Shire Pharmaceuticals..
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban NB, Narendran R, Hwang DR, Laruelle M, Slifstein M. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J Psychopharmacol. 2012;26:794–805. doi: 10.1177/0269881111409265. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. The biology of feeling frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–369. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Stress impairs prefrontal cortex cognitive function in monkeys: role of dopamine. Soc Neurosci Abstr. 1990;16:164. [Google Scholar]
- Arnsten AFT, Vijayraghavan S, Wang M, Gamo NJ, Paspalas CD. Dopamine's influence on prefrontal cortical cognition: actions and circuits in behaving primates. In: Bjorklund A, Dunnett S, Iversen L, Iversen S, editors. Dopamine handbook. Oxford (UK): Oxford University Press; 2009. pp. 230–249. [Google Scholar]
- Arnsten AFT, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald Ar, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Thierry AM, Tassin JP, Moyne MA. Dopaminergic innervation of the rat prefrontal cortex: a fluorescence histochemical study. Brain Res. 1976;106:133–145. doi: 10.1016/0006-8993(76)90078-0. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines and attention deficit hyperactivity disorder. Biol Psychiatry. 2011;69:e101–e111. doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, Scherer N, Group SS. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Brown RM, Crane AM, Goldman PS. Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res. 1979;168:133–150. doi: 10.1016/0006-8993(79)90132-x. [DOI] [PubMed] [Google Scholar]
- Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Bubser M, Schmidt W. 6-OHDA lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterupted tasks in the radial maze. Behav Brain Res. 1990;37:157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- Butter C. Perseveration in extinction and in discrimination reversal following selective frontal ablations in Macaca mulatta. Physiol Behav. 1968;4:163–171. [Google Scholar]
- Butter CM, Snyder DR. Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiol Exp (Wars) 1972;32:525–565. [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Long-lasting psychotomimetic consequences of repeated low-dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology. 1999;20:10–28. doi: 10.1016/S0893-133X(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Connor DF, Grasso DJ, Slivinsky MD, Pearson GS, Banga A. An open-label study of guanfacine extended release for traumatic stress related symptoms in children and adolescents. J Child Adolesc Psychopharmacol. 2013;23:244–251. doi: 10.1089/cap.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. Coding specificity in cortical microcircuits: a multiple-electrode analysis of primate prefrontal cortex. J Neurosci. 2001;21:3646–3655. doi: 10.1523/JNEUROSCI.21-10-03646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. Psychophysical performance reflected in mnemonic activity of primate prefrontal cortex during a spatial working memory task. Soc Neurosci Abstr. 2000;26 Abstr 365.11. [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol. 2002;88:3487–3497. doi: 10.1152/jn.00188.2002. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- Driesen NR, Leung HC, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, Skudlarski P, Goldman-Rakic PS, Krystal JH. Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64:1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier J. The functions of the brain. NY: Putnam; 1886. [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic "scotomas". J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. San Diego (CA): Academic Press; 2008. [Google Scholar]
- Fuster JM. The prefrontal cortex, mediator of cross-temporal contingencies. Hum Neurobiol. 1985;4:169–179. [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed response performance: neuronal correlates of transient memory. J Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bauer RH. Visual short-term memory deficit from hypothermia of frontal cortex. Brain Res. 1974;81:393–400. doi: 10.1016/0006-8993(74)90838-5. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gibbs SE, D'Esposito M. A functional magnetic resonance imaging study of the effects of pergolide, a dopamine receptor agonist, on component processes of working memory. Neuroscience. 2006;139:359–371. doi: 10.1016/j.neuroscience.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.04.044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman PS, Nauta WJ. Columnar distribution of cortico-cortical fibers in the frontal association, limbic, and motor cortex of the developing rhesus monkey. Brain Res. 1977;122:393–413. doi: 10.1016/0006-8993(77)90453-x. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp Neurol. 1970;27:291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Vest B, Galkan TW. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J Comp Phys Psych. 1971;77:212–220. doi: 10.1037/h0031649. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the primate prefrontal cortex and the regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology, the nervous system, higher functions of the brain. Bethesda: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory. In: Carroll BJ, Barrett JE, editors. Psychopathology and the brain. New York: Raven Press; 1991. pp. 1–23. [Google Scholar]
- Goldman-Rakic PS. The "psychic cell" of Ramón y Cajal. Prog Brain Res. 2002;136:427–434. doi: 10.1016/s0079-6123(02)36035-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotonergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueninger WE, Pribram KH. Effects of spatial and nonspatial distractors on performance latency of monkeys with frontal lesions. J Comp Physiol Psychol. 1969;68:203–209. doi: 10.1037/h0027498. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider L, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Nat Acad Sci. 1991;88:1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Horgan J. The undiscovered mind: how the human brain defies replication, medication, and explanation. NY: Simon and Schuster; 1999. [Google Scholar]
- Hunt RD, Arnsten AFT, Asbell MD. An open trial of guanfacine in the treatment of attention deficit hyperactivity disorder. J Am Acad Child Adoles Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Jacobs E, D'Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CF. Studies of cerebral function in primates. Comp Psychol Monogr. 1936;13:1–68. [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res. 2006;88:26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kennard MA, Spencer S, Fountain G. Hyperactivity in monkeys following lesions of the frontal lobes. J Neurophysiology. 1941;4:512–524. [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol. 1995;359:131–143. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiology. 1971;34:337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos GR. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone, and [3H]SCH 23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Quantitative autoradiographic mapping of serotonin 5-HT1 and 5-HT2 receptors and uptake sites in neocortex of rhesus monkey. J Comp Neurol. 1989;280:27–42. doi: 10.1002/cne.902800104. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Wang F, Cao Y, Goldman-Rakic PS. Layer V pyramidal neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse. 1998;28:10–20. doi: 10.1002/(SICI)1098-2396(199801)28:1<10::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- McAllister TW, McDonald BC, Flashman LA, Ferrell RB, Tosteson TD, Yanofsky NN, Grove MR, Saykin AJ. Alpha-2 adrenergic challenge with guanfacine one month after mild traumatic brain injury: altered working memory and BOLD response. Int J Psychophysiol. 2011;82:107–114. doi: 10.1016/j.ijpsycho.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M. Perseveration of central sets after frontal lesions in monkeys. In: Warren JM, Akert K, editors. The frontal granular cortex and behavior. New York: McGraw-Hill; 1964. pp. 219–241. [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Levenson R, Huff R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci USA. 1993;90:5194–5198. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AFT, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Nat Acad Sci USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakako T, Murai T, Ikejiri M, Ishiyama T, Taiji M, Ikeda K. Effects of a dopamine D1 agonist on ketamine-induced spatial working memory dysfunction in common marmosets. Behav Brain Res. 2013;249:109–115. doi: 10.1016/j.bbr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- O'Scalaidhe SP, Wilson FA, Goldman-Rakic PS. Areal segregation of face-processing neurons in prefrontal cortex. Science. 1997;278:1135–1138. doi: 10.1126/science.278.5340.1135. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psych. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Min Wang M, Arnsten AFT. Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex—potential substrate for working memory deficits in schizophrenia. Cereb Cortex. 2012;23:1643–1654. doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Rakic P, Goldman-Rakic PS. Internalization of D2 dopamine receptors is clathrin-dependent and select to dendro–axonic appositions in primate prefrontal cortex. Eur J Neurosci. 2006;24:1395–1403. doi: 10.1111/j.1460-9568.2006.05023.x. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex: a substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Roberts BM, Seymour PA, Schmidt CJ, Williams GV, Castner SA. Amelioration of ketamine-induced working memory deficits by dopamine D1 receptor agonists. Psychopharmacology. 2010;210:407–418. doi: 10.1007/s00213-010-1840-9. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Szwarcbart MK. Neural structures involved in delayed response performance. In: Warren JM, Akert K, editors. The frontal granular cortex and behavior. NY: McGraw-Hill; 1964. [Google Scholar]
- Ruch TC, Shenkin HA. The relation of area 13 on orbital surface of frontal lobes to hyperactivity and hyperphagia in monkeys. J Neurophys. 1943;6:349–360. [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptors in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Scahill L, Aman MG, McDougle CJ, McCracken JT, Tierney E, Dziura J, Arnold LE, Posey D, Young C, Shah B, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16:589–598. doi: 10.1089/cap.2006.16.589. [DOI] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AFT, Cohen DJ, Leckman JF. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Callosal and intrahemispheric connectivity of the prefrontal association cortex in rhesus monkey: relation between intraparietal and principal sulcal cortex. J Comp Neurol. 1984;226:403–420. doi: 10.1002/cne.902260309. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Prenatal specification of callosal connections in rhesus monkey. J Comp Neurol. 1991;307:144–162. doi: 10.1002/cne.903070113. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Begović A, Goldman-Rakic PS, Castner SA. Amphetamine sensitization alters dendritic morphology in prefrontal cortical pyramidal neurons in the non-human primate. Neuropsychopharmacology. 2007;32:919–931. doi: 10.1038/sj.npp.1301179. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Begović A, Williams GV, Castner SA. Reversal of neuronal and cognitive consequences of amphetamine sensitization following chronic treatment with a D1 antagonist. Pharmacol Biochem Behav. 2010;96:325–332. doi: 10.1016/j.pbb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Kleinman JE, Herman MM, Goldman-Rakic PS. Smaller frontal gray matter volume in postmortem schizophrenic brains. Am J Psychiatry. 2002;159:1983–1991. doi: 10.1176/appi.ajp.159.12.1983. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- Seong HJ, Carter AG. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J Neurosci. 2012;32:10516–10521. doi: 10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos B, Paspalas C, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley AG, Nou E, et al. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang X-J, Arnsten AF. NMDA Receptors subserve working memory persistent neuronal firing in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Blockade of dopamine D1 receptors enhances memory fields of prefrontal neurons in primate cerebral cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cereb Cortex. 1993;3:199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Laminar origin of striatal and thalamic projections of the prefrontal cortex in rhesus monkeys. Exp Brain Res. 1994;99:383–398. doi: 10.1007/BF00228975. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of dopamine D1 receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]