Summary
A dynamic interplay between intrinsic regional molecular cues and extrinsic factors from the thalamus shape multiple features of early cortical development. It remains uncertain and controversial, however, whether the initial formation of cortical columns depends on neuronal activity, and there is little evidence that cortical lamination or neuronal differentiation is influenced by extrinsic activity. We examined the role of thalamic-derived factors in cortical development by selectively eliminating glutamatergic synaptic transmission from thalamocortical neurons in mice, and found that eliminating thalamocortical neurotransmission prevented the formation of ‘barrel’ columns in somatosensory cortex. Interestingly, based on cytoarchitectonic criteria and genetic markers, blocking thalamocortical neurotransmission also perturbed the development of superficial cortical lamina and the morphological development of neurons. These experiments demonstrate that barrels and aspects of the layer-dependent pattern of cortical cytoarchitecture, gene expression and neuronal differentiation depend on thalamocortical neurotransmission, extending the apparent influence of extrinsic, presumably activity-dependent factors, on cortical development.
Introduction
A central framework for the study of cortical development concerns the relative role of intrinsic and extrinsic factors in shaping cortical development (Grove and Fukuchi-Shimogori, 2003; O’Leary and Sahara, 2008; Rakic et al., 2009; Sur and Rubenstein, 2005). Cortical arealization, lamination and neuronal differentiation are generally thought to be intrinsic features of the developing cortex governed by genetic factors (Rakic et al., 2009). For instance, the development of distinct cortical areas is under the control of diffusible morphogens that govern the specification of frontal, parietal and occipital regions of the elaborating neuroepithelium (O’Leary and Sahara, 2008). Similarly, the familiar six-layered laminar structure of the neocortex forms as a result of the inside-out chronological migration of newly born postmitotic neurons from the proliferative zone to the nascent cortical plate, with different neuronal subtypes in these layers emerging as the consequence of the combinatorial expression of distinct transcription factors during successive rounds of cell division and migration (Molyneaux et al., 2007; Kwan et al., 2012).
In contrast, some cortical features that emerge later in development, such as aspects of thalamocortical and intracortical neuronal connectivity and the distribution and spacing of cortical columns, are markedly shaped by the sensory periphery during critical periods of development, presumably through activity-dependent mechanisms (Hensch, 2004). For instance, whisker removal or monocular deprivation during an early ‘critical period’ shifts the anatomical and functional properties of neurons in the cortex to favor the remaining non-deprived whiskers or eye. It remains uncertain and controversial, however, whether the initial formation of cortical columns representing peripheral whiskers (so called ‘barrel columns’) in the somatosensory cortex, or ocular dominance columns in the visual cortex, are dependent on neuronal activity (Huberman et al., 2008; Li and Crair, 2011), and there is rather limited evidence that migration, lamination or the molecular and morphologic elaboration of neurons are sensitive to activity (De Marco García et al., 2011) or extrinsic influences from the thalamus (Miyashita-Lin et al., 2003; Zhou et al., 2010; Sato et al., 2012).
We sought to determine the role of extrinsic, thalamic derived factors on multiple features of cortical development by examining the effect of eliminating glutamatergic neurotransmission from thalamocortical neurons on cortical development. We found that glutamate release from thalamocortical neurons was absolutely essential for cortical barrel column development. Remarkably, the differentiation of neurons and the elaboration of superficial layers in the cortex were also disrupted upon removal of excitatory input from the thalamus. These experiments help define limits on the role of intrinsic factors in cortical development, and establish a role for extrinsic, presumably activity-dependent factors on cortical columnar, laminar and neuronal morphological development.
Results
Thalamocortical neurotransmission is eliminated in ThVGdKO mice
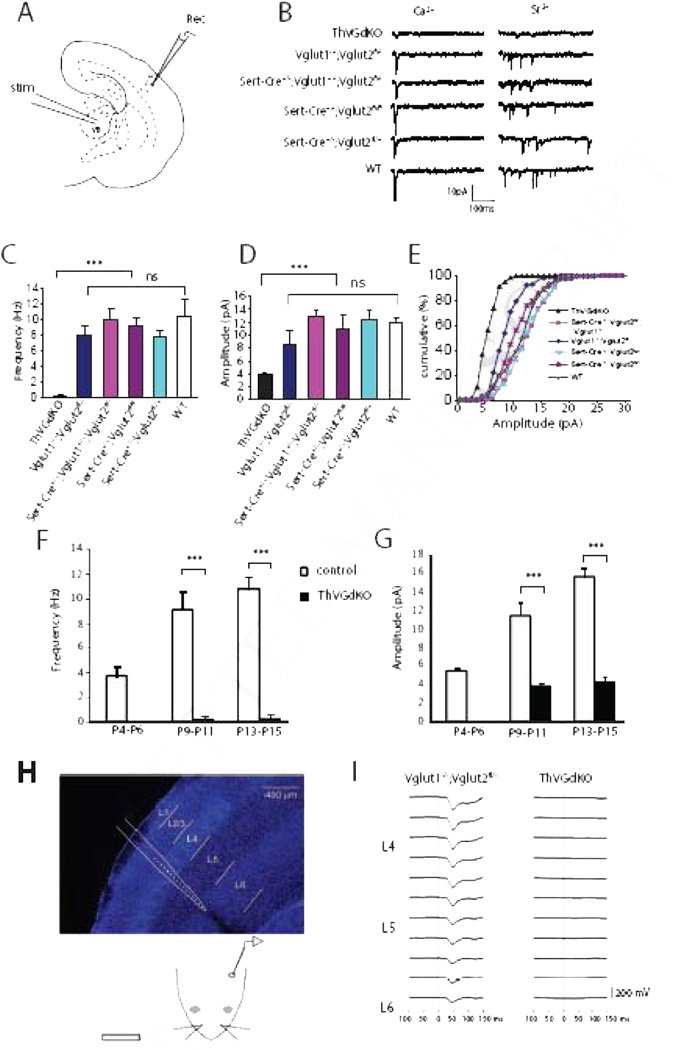
To examine the role of thalamocortical neurotransmission in cortical development, we generated mice in which glutamatergic release is disrupted in thalamocortical neurons using a Cre/loxP recombination approach. We focused on vesicular glutamate transporters, of which there are three known genetic forms in mice (Vglut1-3). Vglut3 is expressed weakly and sporadically in the brain, while Vglut2 and Vglut1 have strong and largely complimentary expression patterns (Fremeau et al., 2004), with Vglut2 robustly expressed in the thalamus and Vglut1 to a lesser extent. Since Vglut2 null mice die at birth (Moechars et al., 2006), we crossed floxed Vglut2 mice (Vglut2fl/fl, Hnasko et al., 2010) with the Sert-Cre driver line (Zhuang et al., 2005) to delete Vglut2 from thalamocortical projection neurons. Somewhat to our surprise, thalamocortical neurotransmission in these mice was indistinguishable from control mice (Fig. 1A–E). Reasoning that Vglut1 may compensate for the absence of Vglut2 in thalamic neurons, we generated mice that lacked Vglut1 and Vglut2 in the thalamus by crossing Sert-Cre mice with Vglut1+/−;Vglut2fl/fl mice to generate Vglut1 and Vglut2 double knockout mice (Sert-Cre+/−;Vglut1−/−;Vglut2fl/flor ThVGdKO). ThVGdKO mice had severely disrupted thalamocortical neurotransmission, whereas all littermate control mice, even those with just a single copy of Vglut1 or Vglut2, had thalamocortical neurotransmission that was grossly indistinguishable from wild type mice (Fig. 1).
Figure 1. Thalamocortical neurotransmission is completely disrupted in ThVGdKO mice.
(A) Schematic diagram of the in vitro thalamocortical slice used to examine thalamocortical neurotransmission. Stimulating electrode (stim) was placed in the ventrobasal thalamus (VB) and whole-cell recording electrode (Rec) in L4 of cortex. (B) Example whole-cell recordings from ThVGdKO and littermate controls at P9-P11. To isolate thalamocortical synapse mini-EPSCs, Ca2+ (left side) was replaced by Sr2+ (right side) in the extracellular medium, which desynchronizes neurotransmitter release. (C) Evoked mini-EPSC frequency in P9-P11 ThVGdKO and their littermate controls (mean ± SEM). n=6 for ThVGdKO, n=3 for each control genotype. *** P < 0.001, ns: non-significant. (D) Quantification of mini-EPSC amplitude in P9-P11 ThVGdKO mice in comparison to littermate controls, *** P < 0.01. Data are presented as mean ± SEM. (E) Cumulative frequency plot of the amplitude of evoked mini-EPSCs at thalamocortical synapses in P9-11 ThVGdKO mice and their littermate controls. (F) Comparison of evoked mini-EPSCs frequency in control and ThVGdKO mice between P4 and P15 (mean ± SEM). No thalamocortical response could be evoked at P4-P6 in ThVGdKO mice, and the frequency of evoked mini-EPSCs remains very low in ThVGdKO in comparison to littermate controls through P15. n=4 for ThVGdKO at P4-P6, n=6 at P9-P11, n=3 at P13-P15. n=5 for controls at P4-P6, n=12 at P9-P11 and n=3 at P13-P15, *** P < 0.001. (G) Comparison of evoked mini-EPSC amplitude in ThVGdKO and control mice between P4 and P15. In ThVGdKO mice, mini-EPSC amplitudes were much smaller than in control mice through P15, *** P < 0.001. (H) Schematic drawing of in vivo local field potential (LFP) recording using a multi-site silicon probe in the somatosensory cortex (upper panel) while air puffs were delivered to the contralateral snout (lower panel). (I) Multiphasic LFP events were recorded during air puff stimulation of contralateral whiskers in Vglut1−/−;Vglut2 fl /−and ThVGdKO mice. No stimulated LFP signal was detected in the somatosensory cortex of ThVGdKO mice.
We measured the effect of Vglut deletion on thalamocortical neurotransmission in two ways. First, we used in vitro electrophysiological techniques to examine miniature excitatory postsynaptic current (mini-EPSC) amplitude and frequency in thalamocortical brain slices (Crair and Malenka, 1995) across a range of ages (P4-P15). Mini-EPSCs were measured using whole-cell patch clamp recordings from layer 4 (L4) neurons following thalamic stimulation after replacing Ca2+ with Sr2+ in the extracellular medium to desynchronize neurotransmitter release (Iwasato et al. 2008). In 5 out of 11 ThVGdKO mice at P9-P11, we could not evoke a measurable thalamocortical response. In the remaining 6 ThVGdKO mice at P9-P11 (Fig. 1C–E), evoked mini-EPSC amplitude (3.9 ± 0.18 pA) and frequency (0.28 ± 0.24 Hz) were much smaller in comparison to littermate controls (p < 0.01). Neither single knock out of Vglut1 (Vglut1−/−;Vglut2fl/−; amplitude: 8.44 ± 1.78 pA; frequency: 8.0 ± 1.18 Hz; n=6) nor thalamic deletion of Vglut2 (Sert-Cre+/−;Vglut1+/−;Vglut2fl/fl, amplitude: 12.92 ± 0.99 pA, frequency: 10.24 ± 1.51 Hz, n=5) significantly reduced thalamocortical neurotransmission in comparison to WT mice (amplitude: 11.84 ± 0.84 pA; frequency: 10.47 ± 2.14 Hz; n=3). We did not detect any thalamocortical synaptic response at P4-P6 in ThVGdKO mice (n=4), and detected only very weak response in some slices at P13-P15 that was similar in amplitude and frequency to that observed at P9-P11 and much smaller than observed in control littermates (P < 0.001) (Fig. 1F, G). These results indicate that Vglut1 and Vglut2 can both contribute to glutamatergic neurotransmission at thalamocortical synapses, and elimination of both Vglut1 and Vglut2 in ThVGdKO mice nearly completely abolishes thalamocortical neurotransmission.
We confirmed these results using in vivo electrophysiological techniques in P9-P12 mice (Fig. 1H, I). Local field potentials (LFPs) recorded with extracellular multi-site silicon array electrodes in somatosensory cortex in response to peripheral whisker stimulation typically produce brief multiphasic events that are dominated by an initial negative-going waveform with greatest amplitude in L4 (Quairiaux et al., 2007). Stimulus-triggered waveform averages in control (Vglut1−/−;Vglut2fl/−) mice showed robust evoked LFPs (Fig. 1I, left panel; maximum negative amplitudes of 207 µV and 209 µV; maxima at 38 ms and 51 ms, respectively, after stimulus onset; waveform widths at half maximum were 23 ms and 31 ms). The same experimental procedure in ThVGdKO mice failed to elicit evoked potentials (n = 4). Indeed, the stimulus-triggered waveform averages revealed no stimulus-related activity in the LFPs at all (Fig. 1I, right panel). Histology confirmed that the recording probes were placed in similar locations within somatosensory cortex in both groups of mice (data not shown). Together, these results indicate that glutamatergic neurotransmission at thalamocortical synapses in ThVGdKO somatosensory cortex was largely, if not completely abolished.
ThVGdKO mice lack barrels in the somatosensory cortex
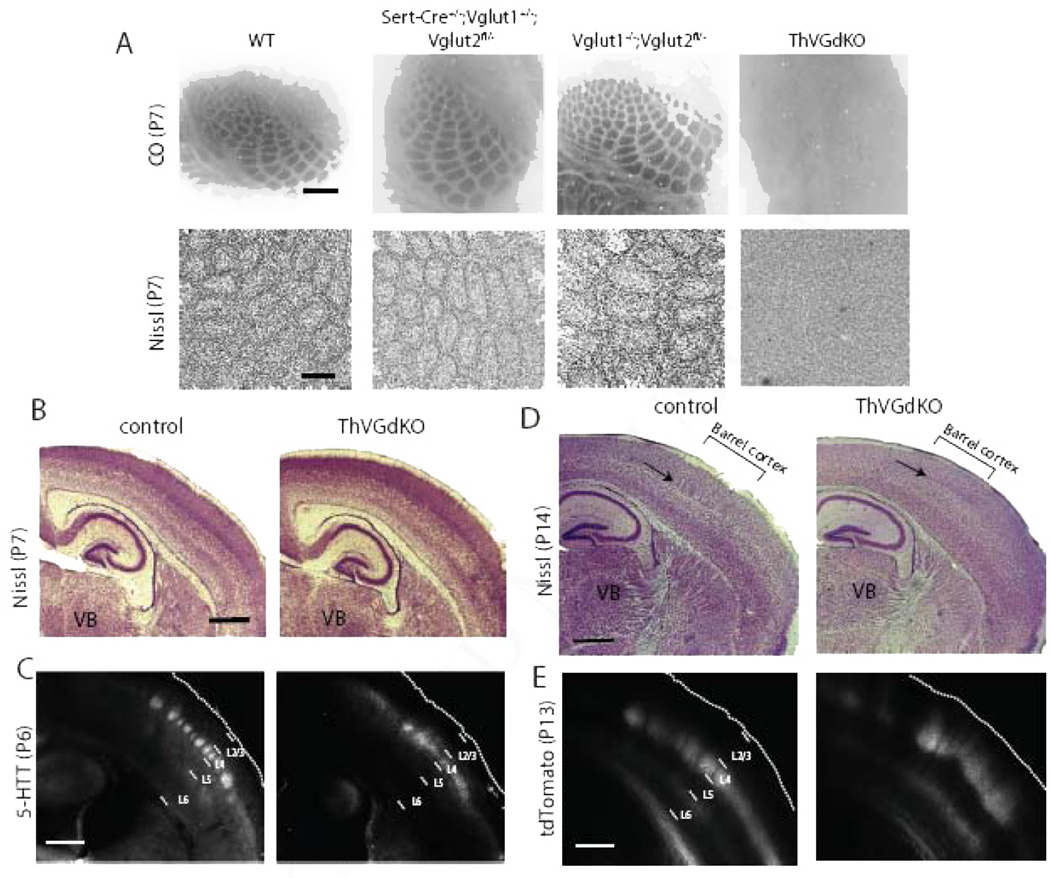
Barrels in the somatosensory cortex of mice are composed of clusters of thalamocortical axon arbors in L4 surrounded by rings of spiny stellate neuron somata whose dendrites are oriented toward the center of the barrel to synapse with thalamocortical afferents relaying information from a single whisker (Li and Crair, 2011). We used Cytochrome Oxidase (CO) histochemistry and Nissl staining to examine whether cortical barrel formation was dependent on thalamocortical glutamatergic neurotransmission. In flattened tangential sections through somatosensory cortex, clear CO barrel patterns were present in Vglut1−/−,Vglut2fl/− and all other control mice, while a barrel pattern was not detectable in ThVGdKO mice (Fig. 2A, upper panels and Suppl. Fig. S1A). This suggests that thalamocortical afferents fail to cluster into barrels in ThVGdKO mice. Using Nissl staining in flattened tangential sections through L4 to examine cortical cytoarchitecture, barrels were again absent in ThVGdKO mice, while clear barrels were evident in all control mice (Fig. 2A, lower panels). Nissl-stained thalamocortical sections from ThVGdKO mice at P7 and P14 were grossly normal, with the obvious exception of L4 in somatosensory cortex of ThVGdKO mice, which lacked barrels (Fig. 2B, D, arrows in D). Thalamocortical axon innervation of the somatosensory cortex was also grossly normal, as revealed by immunolabeling for serotonin transporter (5-HTT) in thalamocortical axons at P6 (Fig. 2C) and direct imaging of thalamocortical afferents at P14 following the injection of a floxed-tdTomato viral construct into the thalamus of Sert-Cre mice (Fig. 2E), again with the obvious exception of disrupted barrel clusters in somatosensory cortex of ThVGdKO mice. The formation of cortical barrels is contingent on intact barrel structures in the thalamus (barreloids) and brainstem (barelettes) (Li and Crair, 2011), but CO staining in coronal sections through the ventrobasal thalamus and brainstem showed typical barrel patterns in these structures (Suppl. Fig. S1B). These results indicate that the emergence of cortical cytoarchitecture and the clustering of thalamocortical afferents into a barrel pattern depend critically on glutamatergic neurotransmission in thalamocortical neurons, suggesting a key role for extrinsic, presumably activity-dependent factors in cortical columnar development.
Figure 2. Barrels are completely disrupted in ThVGdKO mice.
(A) Cytochrome oxidase (CO) histochemistry in flattened sections through L4 of ThVGdKO somatosensory cortex and littermate controls (upper panels). Scale bar: 400µm. Nissl stain in flattened sections through L4 of ThVGdKO somatosensory cortex and littlermate controls (lower panels). Scale bar: 150 µm. Note that barrels are absent in ThVGdKO mice when viewing (presynaptic) thalamocortical afferents (CO) or (postsynaptic) L4 neurons (Nissl). (B) Low magnification image of Nissl stained thalamocortical sections at P7 in control (left) and ThVGdKO mice (right). Cortical lamination appears normal. Scale bar: 500µm. (C) 5-HTT immunostaining of thalamocortical axons shows normal innervation of L4 in cortex of ThVGdKO mice at P7 (right), except axons are not clustered into barrels as in control mice (left). Scale bar: 500µm. (D) Low magnification image of Nissl stained thalamocortical sections at P15 show no barrels in ThVGdKO mice (arrow in right panel) and apparently reduced superficial layers (particularly L4) in comparison to control mice (left). Scale bar: 500µm. (E) Thalamocortical axon targeting of cortex, revealed through AAV-tdTomato injection into thalamus, shows grossly normal cortical innervation in ThVGdKO mice (right panel) at P15 in comparison to control mice (left), though thalamocortical axon clustering into barrels is not apparent, and axon arbors are shifted toward the pial surface in ThVGdKO mice in comparison to control mice. Scale bar: 500 µm. See also Fig. S1.
Cortical lamination defects in ThVGdKO mice
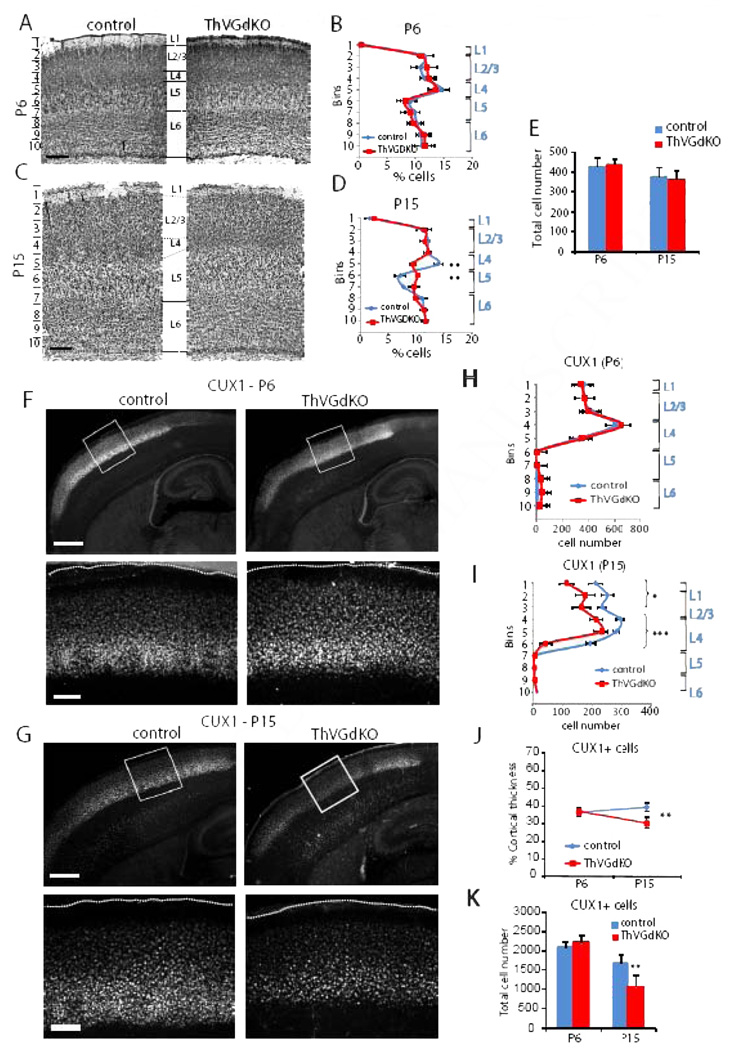
The absence of barrels in the somatosensory cortex of ThVGdKO mice is consistent with previous reports showing that cortical barrel topography is sensitively dependent on the presence, number and arrangement of whiskers on the contralateral snout, and specifically implicates thalamocortical neurotransmission in communicating the peripheral sensory pattern onto the cortex (Van der Loos and Woolsey, 1973; Welker and Van der Loos, 1986). We wondered whether the elimination of thalamocortical glutamatergic neurotransmission would disrupt cortical laminar organization since the distinctive granular nature of L4 is unique to sensory areas of cortex that receive extensive thalamic innervation. At postnatal day 6 (P6), when barrels have just formed, Nissl staining showed that cortical thickness and lamination in ThVGdKO mice was no different than littermate controls (Fig. 2B; Fig. 3A, B; Suppl. Fig. S1D, E). To our surprise, noticeable differences in cortical lamination emerged in the second week after birth, when superficial layers of the cortex undergo their most dramatic elaboration (Fig. 2D; Fig. 3C, D; Suppl. Fig. S1F). In particular, the characteristic dense band of granular cells (L4) at mid-cortical depths was blurred in ThVGdKO mice at P15 and replaced by a relatively cell-sparse layer resembling L5a. These changes were evidenced by a significantly reduced density of cells in ThVGdKO mice at a depth corresponding to L4 in comparison to all littermate control mice (bin 5 in Fig. 3D and Suppl. Fig. S1F), and a significantly higher density of cells at a depth corresponding to L5a (bin 6 in Fig. 3D). Both at P6 and P15, the total number of Nissl-stained cells (Fig. 3E) (P6: control: 438 ± 35, ThVGdKO: 446 ± 26, P = 0.3; P15: control: 378.6 ± 42, ThVGdKO: 365 ± 45, P = 0.15) and caspase-3 positive cells (data not shown) were not different in control and ThVGdKO mice, indicating there was no obvious cell proliferation or apoptosis defects in ThVGdKO mice.
Figure 3. Cortical lamination is disrupted in ThVGdKO somatosensory cortex at P15.
(A) Nissl stained coronal sections through barrel cortex of control (left) and ThVGdKO mice (right) at P6. Scale bar: 150µm. (B) Distribution of Nissl stained cells as a function of depth (in indicated 100 µm bins from pial surface) at P6 (mean ± SEM). n=5 for controls, n=5 for ThVGdKO. (C) Nissl stained coronal sections through barrel cortex at P15. Scale bar: 150µm. (D) Distribution of Nissl stained cells as a function of depth (in indicated 150 µm bins from pial surface) at P15 (mean ± SEM). n=13 for controls, n=7 for ThVGdKO. ** P < 0.01. Note the significant difference in cell density at depths corresponding to L4 and upper L5. (E) There was no difference in total cell number per barrel column at P6 and P15 between control and ThVGdKO mice (mean ± SEM). (F) Representative images of CUX1 immunostaining in control and ThVGdKO somatosensory cortex at P6. Upper panels: low magnification images of thalamocortical sections. Lower panels: high magnification images of framed area in upper panels. Scale bar: Upper panel: 400µm. Lower panel: 100µm (G) Representative images of CUX1 immunostaining in control and ThVGdKO somatosensory cortex at P15. Upper panels: low magnification images of thalamocortical sections. Lower panels: high magnification images of framed area in upper panels. Scale bar: Upper panel: 400µm. Lower panel: 100µm. (H) Distribution of CUX1+ cells as a function of depth in somatosensory cortex at P6 (mean ± SEM). n > 10 for each group. (I) Distribution of CUX1+ cells as a function of depth in somatosensory cortex at P15 (mean ± SEM). n > 10 for each group, * P < 0.05, *** P> 0.001. (J) CUX1+ cells form a similar fraction of cortical depth at P6 but decrease by P15 in ThVGdKO mice in comparison to controls (mean ± SEM), ** P < 0.01. (K) Total CUX1 cell number per barrel column at P15 (mean ± SEM). ** P < 0.01. See also Fig. S2.
CUX1 (aka CUTL1 or CDP) is a transcription factor expressed in superficial layers of somatosensory cortex that clearly delineates the bottom of L4 (Nieto et al., 2004). As with Nissl staining, there was no difference in the laminar expression of CUX1 at P6 (Fig. 3F, H). However, there were fewer cells labeled with CUX1 at P15 (Fig. 3G, I, K), and the thickness of CUX1-expressing superficial layers was significantly reduced in ThVGdKO mice (control: 39 ± 3% of cortical thickness; ThVGdKO: 30 ± 4%, P < 0.01, Fig. 3G, I, J), consistent with the lamination defects observed with Nissl stain. These results suggest that in the prolonged absence of glutamatergic input from the thalamus, the relative thickness of infragranular layers (L5) of the cortex expands at the expense of granular and supragranular layers (L2/3 and L4) during the second week after birth.
Since Sert-Cre is expressed in all the thalamic sensory relay nuclei (Zhuang et al., 2005), including the visual thalamus (dorsal lateral geniculate nucleus or dLGN) and the auditory thalamus (medial geniculate nucleus or MGN), we wondered whether laminar development in visual and auditory cortex was similarly impaired as somatosensory cortex. However, we did not observe any obvious cortical laminar cytoarchitecture defects in the visual or auditory cortex of ThVGdKO mice (Suppl. Fig. S2A – F). Sert-Cre expression is much weaker in the dLGN and MGN in comparison to the somatosensory thalamus (ventrobasal or VB) (Suppl. Fig. S3A–O), and accordingly Vglut2 mRNA and VGLUT2 protein levels were only modestly decreased in the dLGN (68.9% of control mRNA levels) and MGN (48.4% of control mRNA levels) of ThVGdKO mice at P12. In contrast, Vglut2 mRNA in the VB was only 13.5% of control levels (P < 0.001 for the difference between dLGN, MGN and VB), and VGLUT2 protein levels were down to 20% of control already at P4. This is consistent with the earlier and stronger expression of SERT in the VB relative to the other thalamic relay nuclei (Lebrand et al., 1998), and is probably responsible for sparing the auditory cortex and visual cortex from the laminar changes observed in the somatosensory cortex of ThVGdKO mice
Lamination defects are not a necessary consequence of barrel defects
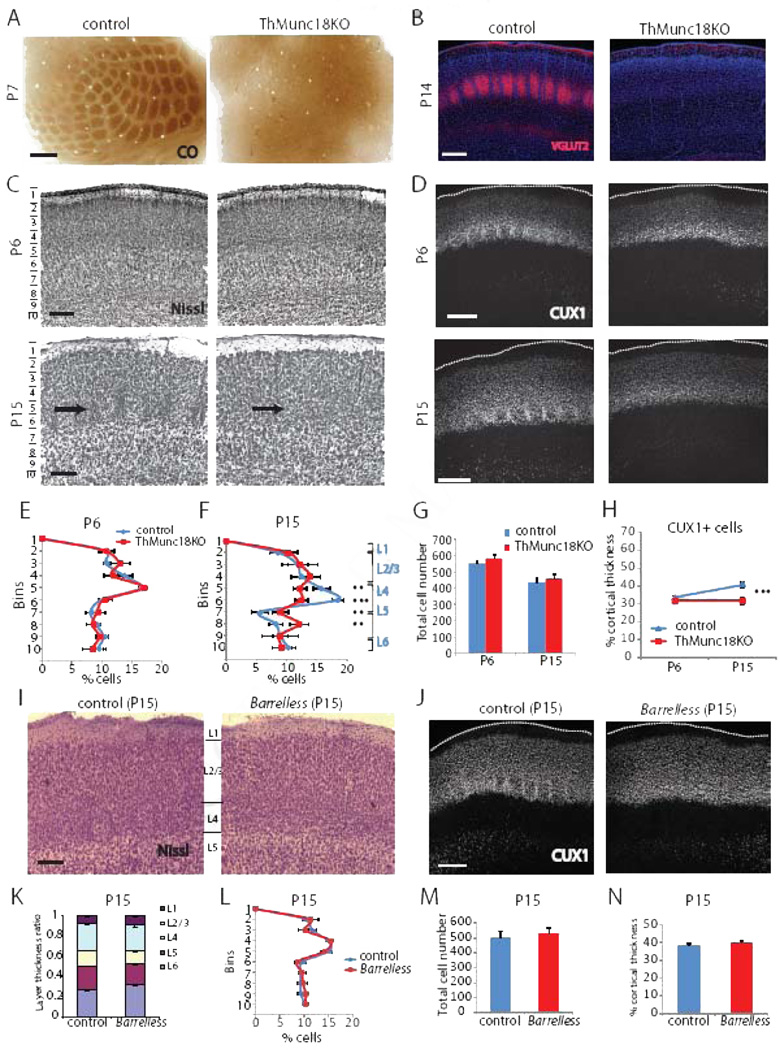
We generated a second model of disrupted neurotransmitter release to confirm and expand our understanding of the role of thalamocortical neurotransmission on somatosensory cortex development. In this case we deleted the gene encoding syntaxin binding protein 1 (Stxbp1 or Munc18–1), which is essential for Ca2+-stimulated neurotransmitter release (Toonen and Verhage, 2007), from neurons in somatosensory thalamus by crossing Sert-Cre mice with floxed Munc18– 1 mice (Dudok et al., 2011) to conditionally delete Munc18–1 in the thalamus (ThMunc18KO mice). Like ThVGdKO mice, barrels did not form in the somatosensory cortex of ThMunc18KO mice (Fig. 4A, C), but cortical lamination was normal at P6 (Fig. 4C, D, E). Moreover, ThMunc18KO mice developed cortical lamination defects at P15 that were similar to those observed in ThVGdKO mice (Fig. 4C, D, F, H), with cell number and cell density significantly reduced in L4, but significantly increased in L5 (Fig. 4F). Deletion of Munc18–1 had a rather severe effect on thalamic neurons, leading to the death and the eventual degeneration of the somatosensory (VB) thalamus between P0 and P7 (Suppl. Fig. S4), and the absence of cortical innervation by thalamic axons as demonstrated by VGLUT2 immunostaining (Fig. 4B). This effect was not observed in ThVGdKO mice (Fig. 2B, D). Generally, it was difficult to distinguish L4 from L2/3 and L5 in ThMunc18 mice at P15 (Fig. 4C), though cortical lamination appeared normal in ThMunc18KO somatosensory cortex at P6 (Fig. 4C), as in ThVGdKO mice. The progressive changes in cortical lamination observed in ThVGdKO and ThMunc18KO mice were probably not to due to a progressive deletion of Vglut2 or Munc18, as thalamocortical neurotransmission was already absent at P6 in ThVGdKO mice and got no worse thereafter (Fig. 1F, G), while thalamocortical neuron degeneration in ThMunc18KO mice occurred between P0 and P7 (Suppl. Fig. S4). Thus, thalamocortical innervation had little effect on the initial wave of cortical neuron migration and laminar formation in the first week after birth, though the absence of thalamocortical neurotransmission disrupted barrel formation. We were curious whether the cortical lamination defects observed in ThVGdKO and ThMunc18KO somatosensory cortex were a necessary consequence of abnormal barrel formation, so we also examined cortical laminar development in barrelless mice. Barrelless is a classic mutant with a spontaneous loss-of-function mutation in adenylate cyclase 1 (Adcy1) that causes deficits in thalamocortical synapse development and the complete absence of barrels (Lu et al., 2003). Unlike ThVGdKO and ThMunc18KO mice, there was no difference in cortical lamination in barrelless mice in comparison to controls (Fig. 4I–N). These results suggest that cortical lamination defects, as observed in ThVGdKO and ThMunch18KO mice, occur as a consequence of the complete disruption of thalamocortical synaptic communication and are not a necessary consequence of simply disrupting barrel formation.
Figure 4. Cortical lamination is disrupted in ThMunc18KO but not Barreless mice at P15.
(A) CO histochemistry shows that barrels do not form in ThMunc18KO mice (right). Scale bar: 200µm. (B) Immunostaining for VGLUT2 reveals that thalamocortical axons do not innervate barrel cortex in ThMunc18KO mice at P14 (right). Scale bar: 300µm. (C) Nissl histochemistry shows that cortical lamination in ThMunc18KO somatosensory cortex (top right) at P6 is similar to controls (top left). Scale bar: 150µm. By P15, cortical lamination in ThMunc18KO somatosensory cortex (bottom right) is disrupted in comparison to controls (bottom left). Scale bar: 150µm. (D) CUX1 immunostaining of somatosensory cortex in ThMunc18KO mice at P6 (top right) and controls (top left) is similar. By P15, CUX1 labeled cells form a smaller fraction of the cortical depth in ThMunc18KO mice (bottom right) in comparison to controls (bottom left). Scale bar: 200µm. (E) Quantification of laminar distribution of Nissl stained cells in control and ThMunc18KO mice at P6. Data presented as mean ± SEM. n=4 for each genotype. (F) Quantification of laminar distribution of Nissl stained cells in control and ThMunc18KO mice at P15. Data presented as mean ± SEM. ** P < 0.01, ***P < 0.001. n=4 for ThMunc18KO, n=6 for controls. (G) Total number of Nissl stained cells per barrel column in ThMunc18KO and littermate controls at P6 and P15 (mean ± SEM). (H) CUX1+ cells form a similar fraction of the total cortical thickness at P6 but a smaller fraction at P15 in Munc18KO mice in comparison to controls (mean ± SEM). *** P<0.001. (I) Nissl histochemistry shows that cortical lamination in somatosensory cortex is similar in Barrelless mice (right) as controls (left), with the obvious exception that barrels are absent in Barrelless. Scale bar: 150µm. (J) CUX1 immunostaining of somatosensory cortex in Barrelless (right) mice and controls (left) is similar. Scale bar: 200µm. (K) The laminar distribution of Nissl stained cells as a fraction of total thickness in Barrelless and control somatosensory cortex at P15 is similar. (L) The laminar distribution of Nissl stained cells as a function of depth in Barrelless and control somatosensory cortex at P15 is similar. (M) Total number of Nissl stained cells per barrel column in somatosensory cortex is similar in Barrelless and control mice. (N) The fraction of barrel cortex thickness covered by CUX1 positive cells is similar in Barrelless and control somatosensory cortex.
L4 neurons have abnormal pyramidal morphology in ThVGdKO mice
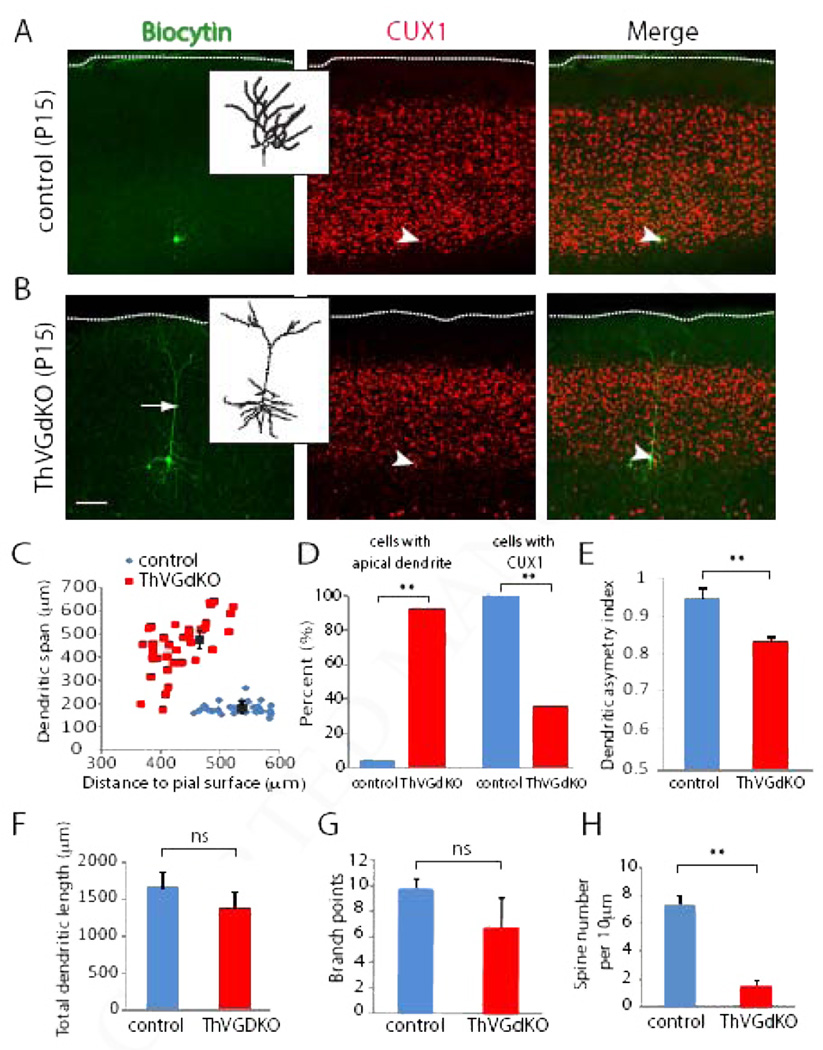
Changes in granular layer development suggest that neuronal differentiation of L4 neurons is disrupted in ThVGdKO mice. In rodent somatosensory cortex, most L4 excitatory neurons are spiny stellate cells, which are local circuit neurons with a small cell body and compact spiny dendrites (Simons and Woolsey, 1984; Lund, 1984). Spiny stellate neurons are largely confined to primary sensory areas of cortex and are common synaptic targets of thalamocortical axons (Benshalom and White, 1986). Mature L4 spiny stellate cells lack the apical process typical of pyramidal neurons in non-granular layers. Some studies suggest that the development of cortical L4 neuron morphology is dependent on sensory experience (Callaway and Borrell, 2011; Harris and Woolsey, 1981; McMullen et al., 1988). To investigate the role of thalamocortical glutamatergic neurotransmission on the development of spiny stellate cell morphology, we filled L4 cells with biocytin and digitally reconstructed their dendrites. We carefully limited our analysis to neurons that were confined to the bottom of the CUX1-positive band marking L4 of cortex. In P15 control mice (n=25 neurons in 4 mice), L4 neurons expressed CUX1, had typical spiny stellate morphology without an apical dendrite, and compact, asymmetric, spiny dendritic trees (Fig. 5). In contrast, neurons in L4 of ThVGdKO mice (n=36 from 5 mice) often did not express CUX1, had distinct apical dendrites that extended toward the pial surface, with large dendritic spans, relatively symmetric basal dendrites, and many fewer spines than control mice (Fig. 5C–E, H). Total dendritic length and the number of branch points were not significantly different in ThVGdKO and control neurons (Fig. 5F, G). These results suggest that in the absence of thalamocortical glutamatergic neurotransmission, L4 development and the emergence of characteristic spiny stellate (granular cell) morphology are compromised.
Figure 5. Neurons in L4 of ThVGdKO mice have pyramidal, not granular morphology.
(A) Representative biocytin filled L4 spiny stellate neuron in control animal (left). Inset shows digitally reconstructed dendritic tree. Immunostaining for CUX1 (center) was used to identify the laminar location of the filled cell. Merged image is on the right. Arrow head indicates position of the filled neuron. Scale bar: 100µm; 30 µm for inset. (B) Representative biocytin filled L4 neuron in ThVGdKO animal (left). Arrow shows an apical dendrite that extends all the way to the pial surface. Inset shows digitally reconstructed dendritic tree. Immunostaining for CUX1 (center) was used to identify the laminar location of the filled cell. Merged image is on the right. Arrowhead indicates position of the filled neuron. Only neurons in the lower band of CUX1 immunostain (within 150 µm of the lower edge) were considered for further analysis. Scale bar: 100 µm; 30 µm for inset. (C) The dendritic span of reconstructed neurons in ThVGdKOs is significantly greater than controls. Note that the dendritic span is consistently small for control animals, regardless of the cortical depth of the labeled neuron, while the dendritic span in ThVGdKO mice is larger for neurons that are deeper, consistent with an apical dendrite that extends to the pial surface regardless of depth. n=36 neurons from 5 animals in ThVGdKO; n=25 neurons from 4 animals for controls. (D) Only 1 of 25 neurons in L4 of control mice had an apical dendrite, while 32 out of 36 neurons in ThVGdKO had a distinct apical dendrite. 25 out of 25 L4 neurons in control mice expressed CUX1, while only 13 out of 36 neurons in ThVGdKO mice expressed CUX1. (E) Dendrites in L4 neurons in ThVGdKO mice are more symmetric than control neurons; **P < 0.01. (F) Total dendritic length in ThVGdKO mice is not significantly different from controls (mean ± SEM). (G) The number of dendritic branch points in ThVGdKO mice is not significantly different from controls (mean ± SEM). (H) Spine density in ThVGdKO L4 neurons is significantly lower in ThVGdKO relative to control mice (mean ± SEM).
Abnormal expression of the L4 marker Dcdc2a in ThVGdKO mice
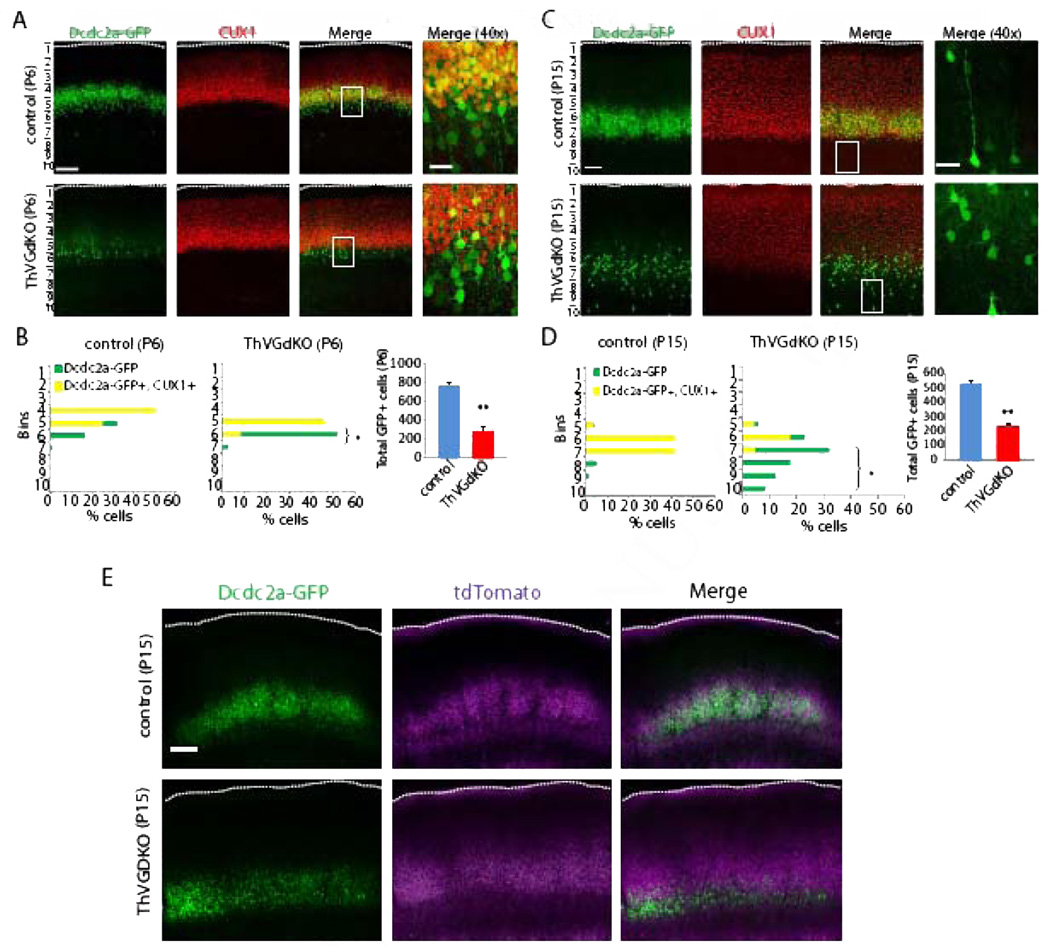
We next turned to molecular markers of cortical lamination to determine the extent of lamination defects in ThVGdKO mice. To visualize L4 neurons in the somatosensory cortex, we used the Dcdc2a–Gfp transgenic reporter mouse generated by the GENSAT project (Gong et al., 2004). Dcdc2a is one of a family of genes containing two doublecortin domains, which bind tubulin and enhance microtubule polymerization (Kerjan and Gleeson, 2007). In humans, genetic variants in Dcdc2a have been associated with susceptibility to developmental dyslexia (Meng et al., 2005; McGrath et al. 2006), and functional analysis in Dcdc2a mutant mice suggest a role in neuronal migration during cortical development (Meng et al., 2005) that is partially redundant with doublecortin (Dcx) (Wang et al., 2011). In Dcdc2a–Gfp mice, GFP is largely confined to L4 neurons in the barrel cortex and, to a lesser extent, L5a pyramidal shaped neurons that are distributed more broadly in the neocortex (Fig. 6A, B). In ThVGdKO mice at P6, there were significantly fewer GFP positive cells than in control mice (Fig. 6A, C), and most cells expressing GFP in ThVGdKO mice were arranged just below the dense band of CUX1 neurons in L4. This results in a significantly smaller fraction of GFP expressing neurons double-labeled with CUX1 in ThVGdKO mice relative to control mice (Fig. 6C). At P15, the difference in expression was even more dramatic (Fig. 6B, D), with many fewer neurons expressing GFP in ThGVdKO mice, and these cells were largely distributed deeper in cortex, corresponding to L5a, than in control mice and did not express CUX1 (Fig. 6D). Thalamocortical axon terminal arbors at P15 completely overlapped with the layer of neurons expressing GFP (Fig. 6E), consistent with the dominant expression of Dcdc2a–Gfp in L4 of control mice. In contrast, in ThVGdKO mice GFP neurons were present mainly below the bulk of thalamocortical axon terminal arbors (Fig. 6E). These data suggest that the normal maintenance in L4 and down regulation in L5a of Dcdc2a expression is disrupted in ThVGdKO mice, possibly due to disruptions in postnatal neuronal position or changes in laminar expression of the Dcdc2a–Gfp reporter.
Figure 6. Laminar distribution of Dcdc2a–GFP neurons is altered in ThVGdKO mice.
(A) In control mice at P6 (top panels), Dcdc2a–GFP+ cells are distributed at the bottom edge and largely colocalized with CUX1 expressing neurons in somatosensory cortex. In ThVGdKO mice at P6 (bottom panels), there are fewer Dcdc2a GFP+ cells, and these cells tend to reside below the layer of CUX1 expressing neurons. Scale bar: 100µm; 20µm for 40X pictures. (B) Quantification of laminar distribution of Dcdc2a–GFP+ cells in somatosensory cortex at P6. Total number of GFP+ cells (right panel) is significantly reduced at P6 (mean ± SEM). ** P < 0.01, n=3 for each genotype. (C) At P15, the difference between ThVGdKO mice and control mice is more striking, with many fewer Dcdc2a–GFP+ neurons in ThVGdKO mice (bottom) in comparison to control mice (top), and these neurons are below the CUX1 layer and do not colocalize with CUX1. Scale bar: 100µm, 20µm for 40X pictures. (D) Quantification of laminar distribution of Dcdc2a–GFP+ cells in somatosensory cortex of control (left) and ThVGdKO mice (middle). Total number of Dcdc2a–GFP+ cells (right) is significantly reduced at P15 (mean ± SEM). * P< 0.05; ** P< 0.01, n=3 for each genotypes. (E) In control mice at P15 (top panels), dcdc2aGFP expressing neurons (left) largely overlap with thalamocortical afferent arbors (middle) in L4. In contrast, in ThVGdKO mice (bottom) Dcdc2a–GFP expressing neurons (left) and thalamocortical arbors labeled with tdTomato (middle) overlap weakly (right). Scale bar: 200 µm.
Further genetic evidence of lamination defects in ThVGdKO mice
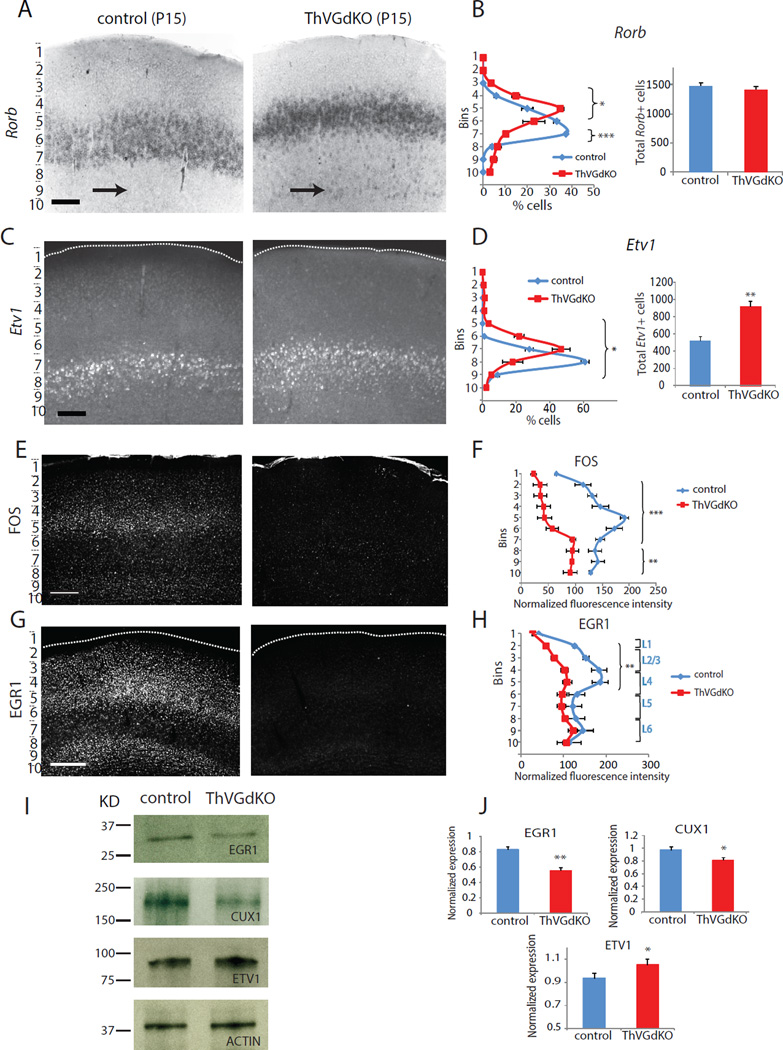
We examined the expression of a number of genes with layer specific expression patterns in ThVGdKO somatosensory cortex at P15 and consistently observed changes in and around L4. As already described, the expression of the predominantly superficial layer gene Cux1 in ThVGdKO mice was significantly reduced (Fig. 3G–K), as was SatB2 (Suppl. Fig. S5A, B). The expression of the L4 transcription factor Rorb (RORβ; Schaeren-Wiemers et al, 1997), and the L5a transcription factor Etv1 (aka Er81; (Yoneshima et al, 2006), changed reciprocally (Fig. 7A–D). In ThVGdKO, a dense band of Rorb positive cells that corresponds to L4 was shifted upward (Fig. 7A, B), consistent with the Nissl staining (Fig. 3C, D). We also observed a number of Rorb positive cells throughout L5 and L6 in ThVGdKO mice, which was more unusual in controls (Fig. 7A, black arrows; Suppl. Fig. S6A, B, white arrows). In control mice, neurons expressing Rorb were mostly confined to L4 and co-expressed CUX1, whereas in ThVGdKO mice Rorb expression extended to L5 where it was not co-expressed with CUX1 (Suppl. Fig. S7). The domain of Etv1 expression spread towards the pial surface in ThVGdKO mice (Fig. 7C, D), again consistent with the expansion of L5 observed with Nissl staining. The expression of L5b (Ctip2, Fezf2) and L6 (FoxP2, Tbr1) markers were largely undisturbed in somatosensory cortex of ThVGdKO mice (Suppl. Fig. S5C–G, and data not shown), and changes in laminar-specific gene expression observed in somatosensory cortex did not occur in motor cortex of ThVGdKO mice (Suppl. Fig. S6C, D). None of these aberrant expression patterns were apparent at P6 (Suppl. Fig. S6E). The changes in layer-specific gene expression observed in ThVGdKO cortex at P15 are consistent with the differences observed histologically, and imply an unexpected degree of activity-dependent thalamic influence on laminar development of somatosensory cortex.
Figure 7. Changes in L4 and L5 molecular markers in ThVGdKO mice.
(A) In situ hybridization for Rorb at P15 shows a dense band of labeled cells that is shifted toward the pial surface in ThVGdKO mice (right) relative to controls (left). Arrows mark sparse L5 cells, which are more prominent in ThVGdKO mice. Scale bar: 150µm. (B) Quantitative analysis of the laminar distribution of Rorb+ cells across layers (mean ± SEM) shows that the dense band of Rorb+ cells is shifted upward and there are an increased number of Rorb+ cells in deeper layers in ThVGdKO somatosensory cortex. The total number of Rorb+ cells is not different in the two genotypes (right panel). * P< 0.05; ***P< 0.001, n=4 for each genotype. (C) In situ hybridization for Etv1 at P15 in ThVGdKO (right) and littermate control (left) mice. Scale bar: 150µm. (D) The laminar distribution of Etv1+ cells in ThVGdKO mice is shifted toward the pial surface relative to control mice, and there are many more Etv1+ cells in ThVGdKO mice (mean ± SEM). *P< 0.05; **P< 0.01, n=5 for each genotype. (E) C-FOS expression is dramatically reduced in ThVGdKO mice (right) relative to control mice (left) at P14. (F) Laminar distribution of FOS expression (mean ± STDEV) shows reduced expression throughout cortex, particularly in superficial layers. ***P< 0.001; **P< 0.01, n=2. (G) EGR1 immunostaining in somatosensory cortex of ThVGdKO (right) and littermate controls (left) at P15. Scale bar: 300 µm. (H) EGR1 expression is reduced in ThVGdKO mice relative to littermate control mice, particularly in superficial layers (mean ± SEM). ** P< 0.01. (I) Western blot analysis shows that EGR1 and CUX1 expression are reduced in ThVGdKO somatosensory cortex, while ETV1 expression is increased. (J) Quantification of western blots for indicated proteins. * P< 0.05; ** P< 0.01, n=3 for controls and ThVGdKOs. See also Fig. S7.
Changes in the expression of activity-dependent transcription factors in ThVGdKO mice
A number of transcription factors are regulated by activity in the cortex, particularly during experience dependent circuit remodeling that occurs throughout development and in the adult (Flavell and Greenberg, 2008). We sought to examine whether such an activity-dependent signaling cascade might mediate the developmental changes we observed in barrel cortex of ThVGdKO mice. The expression of FOS (c-Fos), a prototypical activity-dependent transcription factor, is dramatically reduced in ThVGdKO mice in comparison to controls, particularly in superficial layers of cortex (Fig. 7E, F). The Egr family of transcription factors are also regulated by activity during sensory cortex development (Mataga et al., 2001; Patra et al., 2004), and of the four known variants in the family (Egr1–4), the expression of EGR1 was significantly reduced in the superficial layers of somatosensory cortex of ThVGdKO mice (Fig. 7G–J, data not shown). Interestingly, Cux1, whose expression is reduced in superficial layers of ThVGdKO somatosensory cortex (Fig. 3; Fig 7I, J), and Etv1, whose expression is increased (Fig. 7C, D), both regulate dendritogenesis (Abe et al., 2012), dendrite branching and spine morphology of pyramidal neurons in the upper layers of the cortex (Cubelos et al., 2010). This suggests that signaling mechanisms under the direct or indirect control of activity-dependent transcription factors may regulate late stages in the elaboration of cortical lamination and neuronal morphogenesis, particularly in L4 stellate cells of the somatosensory cortex.
Discussion
We examined the role of neurotransmitter release by thalamocortical neurons on the emergence of distinctive areal and laminar features during cortical development. Through the manipulation and elimination of vesicular glutamate from somatosensory thalamic nuclei, we identified a range of cortical attributes that were dependent on thalamocortical neurotransmission. In particular, the development of cortical ‘barrel’ columns relied completely on glutamate released from thalamocortical neurons. Surprisingly, we also observed that aspects of cortical laminar cytoarchitecture and gene expression, particularly associated with the emergence of the ‘granular’ L4, were disrupted in the absence of thalamocortical neurotransmission. Finally, the paucity of compact stellate (granular) cells and the persistence of pyramidal neuron dendritic morphology in L4 neurons of the somatosensory cortex of ThVGdKO mice indicates that the emergence of gross neuronal morphology is also influenced by activity-dependent factors. These results expand the apparent influence of neuronal activity in cortical development, suggesting that aspects of columnar development, lamination and neuronal differentiation rely on thalamocortical neurotransmission.
Barrel development requires glutamate release from thalamocortical neurons
Barrels are composed of cell sparse hollows filled with clusters of thalamocortical axon arbors surrounded by cell dense walls of spiny stellate neurons. The dendrites of spiny stellate neurons that ring a barrel are oriented into the hollow to form synapses with thalamocortical afferents relaying information from a single whisker. Genetic mutations in mice that disrupt barrel development typically disrupt only the columnar distribution of neurons in L4 and leave the clustering of thalamocortical axons intact (Li and Crair, 2011). A handful of the most severe barrel map mutants, including barrelless mice and GAP-43 KO mice, have no hint of either thalamocortical axon clustering into barrels or L4 cytoarchitecture resembling barrel walls. Previous experiments that disrupted neuronal activity or cortical glutamatergic signaling pharmacologically or genetically had mixed effects on barrel development (Li and Crair, 2011). For instance, interfering with cortical glutamatergic receptors (Schlaggar et al., 1993; Iwasato et al., 2000; Wijetunge et al., 2008) disrupts cortical barrel cytoarchitecture, but has no effect on thalamocortical axon clustering into a barrel pattern. Similarly, interfering with neuronal activity pharmacologically (Chiaia et al., 1992) or disrupting thalamocortical neurotransmission genetically (Lu et al., 2006; Narboux-Neme et al., 2012) interferes with the emergence of cortical barrel cytoarchitecture but has no effect on thalamocortical axon clustering. Notably however, the interventions employed in these studies did not completely block thalamocortical glutamatergic neurotransmission, but rather interfered with restricted subsets of glutamate receptors, or decreased the probability of neurotransmitter release without eliminating thalamocortical neurotransmission or changing synaptic strength. A likely consequence of the incomplete nature of these manipulations is that barrel cytoarchitecture is disrupted, but thalamocortical axon clustering and cortical laminar cytoarchitecture are preserved. In contrast to these previous studies, the manipulation we reported here nearly completely blocks thalamocortical neurotransmission (ThMunc18KO mice) or nearly completely prevents thalamocortical neurons from releasing glutamate (ThVGdKO mice). We suggest that the more comprehensive disruption of thalamocortical glutamatergic neurotransmission we achieved produced the correspondingly more dramatic effects on cortical barrel, laminar and neuronal cytoarchitectural development.
We observed that Vglut1 was capable of compensating for the absence of Vglut2 in thalamocortical neurons in vivo. The same is not true in cultured neurons, where thalamic cells that lack only Vglut2 have dramatically disrupted neurotransmitter release (Moechars et al., 2006). Neurons in the ventrobasal thalamus are known to express both Vglut1 and Vglut2 in a dynamic fashion through the course of development (Barroso-Chinea et al., 2008; Nakamura et al., 2005), as do single axon terminals in L4 of barrel cortex during the first week after birth (Nakamura et al., 2005). The observed difference in compensation by Vglut1 for Vglut2 may reflect a difference in the dynamic regulation of these two Vglut gene family members in vivo and in vitro.
Defects in cortical lamination in somatosensory cortex of ThVGdKO mice
It is notable that the initial wave of cortical lamination and lamina-specific gene expression that occurs through the first week after birth appears largely normal in ThVGdKO and ThMunc18KO mice, which suggests that initial migration cues guiding cells from the ventricular zone to the pial surface are intact in ThVGdKO and ThMunc18KO mice, but local cues responsible for distributing neurons into barrel walls (columns) are disrupted. This is consistent with previous studies in mice lacking extrinsic connections (Miyashita-Lin et al., 1999; Zhou et al., 2010) or after thalamic ablation (Windrem and Finlay, 1991) and conforms with a classic ‘protomap’ view of development in which cortical development (arealization, lamination) is self organized (Rakic et al., 2009), but specific local features of cortical patterning (barrel columns) are sensitive to extrinsic influences. Subsequent to the initial wave of normal migration, the elaboration of superficial cortical lamina and lamina-specific gene expression in the second week after birth is markedly disrupted in ThVGdKO and ThMunc18KO mice. These defects may be due to ‘local’ positioning errors, analogous to the errors that produce barrel wall defects, and/or a disruption in the morphologic and molecular elaboration of superficial layer neuron identity, particularly in L4. The elaboration of features of cortical organization that emerge during the second postnatal week may be much more sensitive to the influence of extrinsic factors, such as thalamocortical activity, which is more consistent with a classic ‘protocortex’ view of development (O’Leary, 1989). The deficits in barrel formation, superficial cortical lamination and neuronal morphological development apparent in ThVGdKO mice provide a clear demarcation between specific features of cortical development that are dependent on extrinsic, activity-dependent influences and features of cortical development that are principally self-organizing.
It is also notable that the cortical lamination defects we observed in ThVGdKO mice appear restricted to somatosensory cortex and do not encompass other cortical areas with distinct granular layers (L4), such as auditory cortex and visual cortex. We believe this is due to the significantly more effective deletion of Vglut2 from somatosensory thalamus (VB) than the visual thalamus (dLGN) or auditory thalamus (MGN) in ThVGdKO mice (Supp. Fig. S3). It is also possible that there is something unique about somatosensory cortex that makes it more sensitive to the elimination of glutamate release from thalamocortical neurons. For instance, the development of auditory cortex and visual cortex are delayed relative to somatosensory cortex by a few days, but this difference wouldn’t appear to be substantial enough to account for the absence of a lamination phenotype in these cortical areas at P15, when cortical elaboration should be reasonably complete everywhere.
Requirement of thalamic glutamate for development of L4 spiny stellate morphology
The compact, ‘granular’ morphology of L4 is a characteristic feature of mammalian sensory cortex (Staiger et al., 2004). The typical spiny stellate (granular) morphology of L4 excitatory neurons is thought to arise from a common cortical pyramidal cell template after the elimination of a developmentally precocious pial-projecting apical dendrite (Callaway and Borrell, 2011). The conspicuous absence of spiny stellate neurons in ThVGdKO mice, and the persistence of pyramidal-like L4 cells with apical dendrites that extend to the pial surface, is consistent with a model in which cortical excitatory neurons adopt a pyramidal cell morphology by default (Lu et al., 2013), and the emergence of spiny stellate morphology is an activity-dependent process under thalamic guidance (Callaway and Borrell, 2011). This is a clear example of the morphological development of a distinct cell type typical of only one cortical layer that is regulated by thalamus-derived factors (Sato et al., 2012; Lombardo et al., 1995), presumably through a transcription factor expression cascade under the direct or indirect influence of thalamocortical activity. Similar activity-dependent transcription factor cascades may account for aspects of the distinct laminar, neuronal and circuit wiring properties characteristic of different areas of neocortex.
Activity-dependent expression of laminar and molecular markers
Recent experiments indicate that a wide number and variety of genes in the brain are transcribed in an activity-dependent manner (Kim et al., 2010). Activity regulated gene transcription is important for synapse formation (West and Greenberg, 2011), axon branching (Hayano and Yamamoto, 2008), dendritic development (Whitford et al., 2002), and even interneuron migration and development (De Marco García et al., 2011). The results described here suggest that the positioning and morphologic development of cortical glutamatergic neurons is also subject to activity-dependent regulation under the specific influence of the thalamus. We observed that genes typically associated with granular and supragranular layers, such as Cux1 and Satb2, have reduced expression in ThVGdKO mice, while genes typically expressed in the deepest layers of cortex, such as Bcl11b (aka Ctip2), Fezf2 (aka Fezl, Zfp312) and FoxP2 were not altered in ThVGdKO mice. Genes normally enriched in L5a neurons, such as Etv1 were increased in ThVGdKO mice, and many cells in L5 spuriously co-expressed Rorb, typically associated with L4. Interestingly, the expression of Tbr1, a transcription factor that is mainly expressed in L6 but also expressed to a lesser extent in L4, is increased in L4 but not in L6 of ThVGdKO mice (Suppl. Fig. S5). Thus, it appears that the expression and distribution of genes in and around L4 of ThVGdKO somatosensory cortex is specifically altered, presumably as a consequence of changes in activity-dependent transcriptional regulation. For example, we observed that the immediate early gene Egr1, which binds to the promoter for Cux1 (Champion ChiP Transcription Factor Search Portal (http://www.sabiosciences.com/chipqpcrsearch.php), is suppressed in ThVGdKO mice (Fig. 7). In turn, Cux1 has multiple binding sites on Etv1 and may act as a transcriptional repressor. Etv1 is spuriously expressed in L4 neurons of ThVGdKO mice (Fig. 7C–D) and is known to regulate dendritogenesis (Abe et al., 2012), which is atypical in L4 neurons in ThVGdKO mice (Fig. 5). This and/or other activity-dependent signaling mechanisms or transcription factors, including Fos, may regulate late stages of lamination and neuronal morphogenesis, particularly for stellate cells in L4 of the somatosensory cortex.
How does thalamocortical glutamate act to modulate barrel cortex development?
We favor a model in which thalamocortical neurons convey the arrangement of whiskers on the snout to the cortex to form barrels in L4 through the effect of their correlated pattern of activity on the development of granular (spiny stellate) neurons. Similarly, alterations in cortical lamination observed in ThVGdKO mice are a consequence of the elimination of glutamatergic synaptic drive on developing L4 neurons. In this model, glutamate acts directly at thalamocortical synapses of spiny stellate neurons to modulate activity and direct the local migration of neurons into barrels, modify gene expression and influence cell morphological development. We suggest that the gradual emergence of a laminar and cell morphologic phenotype in the second week after birth in ThMunc18KO and ThVGdKO mice reflects the progressive nature of cortical development that becomes increasingly influenced by activity as the brain matures, rather than a frank ‘respecification’ of neuron laminar or morphologic identity. Alternatively, respecification (or ‘fate conversion’) of postmitotic superficial layer neurons may occur in the absence of glutamatergic drive from the thalamus even as late as the first postnatal week (De la Rossa et al., 2013).
The experimental manipulation we performed blocked glutamate release from thalamocortical neurons, but did not specifically modulate neuronal activity or exclusively synaptic activity. For instance, glutamate receptors expressed in glial cells or extrasynaptically in neurons could potentially cause the phenotypes we observed. Moreover, activity throughout barrel cortex is likely reduced in the absence of glutamatergic drive by thalamocortical axons onto L4 neurons in ThVGdKO mice. It is possible that extrasynaptic glutamate or altered activity patterns throughout the cortex mediate the effects on barrel cortex development we observed in ThVGdKO mice. In any case, the striking effects of eliminating thalamocortical neurotransmitter release on cortical columnar, laminar and neuronal morphological development suggests that these events are modulated by factors extrinsic to the cortex that are sensitive to ongoing thalamic activity. These extrinsic factors may play a role in the emergence of areal differences seen in the cortex during normal development, such as the presence of a dense granular L4 in sensory cortex and an expanded L5 in motor cortex. Thus, abnormal patterns of activity during development, or disruptions in activity-dependent transcription factor cascades, may account for some of the laminar, morphological and synaptic defects observed in a variety of neurodevelopmental disorders.
Experimental Procedures
Animals
All animals were treated in compliance with Yale IACUC and U. S. Department of Health and Human Services guidelines. We maintained and bred Sert-Cre+/−;Vglut1+/−;Vglut2fl/+ , Sert-Cre+/−;Vglut1+/−;Vglut2fl/−, and Vglut1+/−;Vglut2fl/fl mice on a mixed C57B/6J and CD1 background and used Vglut1−/−;Vglut2fl/− mice as littermate controls for ThVGdKO (Sert-Cre+/− ;vglut1−/−;vglut2fl/fl and Sert-Cre+/−;Vglut1−/−;Vglut2fl/−) mice throughout unless otherwise explicitly stated. Dcdc2a–Gfp and Fezf2-Gfp transgenic mice were obtained from GENSAT.
Histology
As previously described (Iwasato et al., 2008), cytochrome oxidase (CO) and Nissl stain was performed on flattened tangential sections through barrel cortex. CO was visualized using a solution of 3mg cytochlomec, 0.4g sucrose and one 3,3’-Diaminobenzidine tablet (Sigma) in 10ml PBS. Nissl bodies were visualized with a 2% cresyl violet solution. Stereological quantification of Nissl sections was performed on mounted slides at high magnification (40X or 63X) with Neurolucida Software (MicroBrightfield, Inc. USA) blind to genotype. Statistical analysis was performed with two tailed Student t-tests and one way ANOVA. Significance level was set at P < 0.05. 1µl of Cre dependent AAV2/9 CAG.FLEX.tdTomato.WPRE.bGH virus (University of Pennsylvania Vector Core Cat# AV-9-ALL864) was injected into the thalamus using a Nanoject (Drummond Scientific) for visualization of thalamocortical afferents with tdTomato. Biocytin labeling of L4 neurons was performed on acute thalamocortical slices using whole-cell patch pipettes that contained 10 mM Biocytin in addition to the standard whole cell solution. Labeled neurons were visualized with confocal and multiphoton laser microscopy (LSM duo710, Zeiss) and reconstructed using Neurolucida (MBF Bioscience). In situ hybridization was performed with Digoxigenin-11-UTP and/or Flourescence-12-UTP (Roche) probes on 60 µm free-floating coronal sections. Immunohistochemistry was performed on free-floating 60nm thick thalamocortical or coronal sections and images for fluorescence quantification were acquired with a Zeiss Axio Imager.Z2 or LSM 510 Meta microscope using the same exposure time and background subtraction for all genotypes. Quantification of laminar distribution was perfomed on images with the pial surface at the upper edge and the cortex depth divided into 10 equal bins below the pial surface. Cells in each bin were counted using Image J (NIH) and Volocity (PerkinElmer) software and reported as a percentage of total cells counted blind to genotype. Statistical analysis was performed with two tailed Sutdent’s t-tests and one was ANOVA with significance level set at P < 0.05.
Electrophysiology
In vitro whole-cell patch-clamp electrophysiology was performed on acute thalamocortical brain slices as previously described (Lu et al., 2001). AMPA “evoked mini-EPSCs” were recorded at −70 mV holding potential after the exchange of Ca2+ for Sr2+in the ACSF, and mini-EPSCs were analyzed with Mini Analysis (Synaptosoft). In vivo electrophysiology was performed on P9-P12 mice using a 16-site linear silicon probe (NeuroNexus Technologies) and analyzed using Spike2 (Cambridge Electronic Design). Whisker stimulation with puffs of air was applied using a Picospritzer III (Parker).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Y. Zhang for her excellent technical support and members of the Crair lab for their continual feedback and valuable comments on the manuscript. This work was supported by a Brown-Coxe fellowship to H.L., NIH grants K01 DA026504 to T.H., R01 MH50712 to R.E., R01 NS054273 to N.S., R01 EY015788, T32 NS007224 and R01 MH062639 to M.C.C., and the family of William Ziegler III.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abe H, Okazawa M, Nakanishi S. Gene regulation via excitation and BDNF is mediated by induction and phosphorylation of the Etv1 transcription factor in cerebellar granule cells. Proc. Natl. Acad. Sci. 2012;109:8734–8739. doi: 10.1073/pnas.1206418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Lanciego JL. Expression of vesicular glutamate transporters 1 and 2 in the cells of origin of the rat thalamostriatal pathway. J Chem Neuroanat. 2008;35:101–107. doi: 10.1016/j.jchemneu.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Benshalom G, White EL. Quantification of thalamocortical synapses with spiny stellate neurons in layer IV of mouse somatosensory cortex. J Comp Neurol. 1986;253:303–314. doi: 10.1002/cne.902530303. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Borrell V. Developmental sculpting of dendritic morphology of layer 4 neurons in visual cortex: influence of retinal input. J. Neurosci. 2011;31:7456–7470. doi: 10.1523/JNEUROSCI.5222-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaia NL, Fish SE, Bauer WR, Bennett-Clarke CA, Rhoades RW. Postnatal blockade of cortical activity by tetrodotoxin does not disrupt the formation of vibrissa-related patterns in the rat’s somatosensory cortex. Brain Res. 1992;66:244–250. doi: 10.1016/0165-3806(92)90086-c. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Sebastián-Serrano A, Beccari L, Calcagnotto ME, Cisneros E, Kim S, Dopazo A, Alvarez-Dolado M, Redondo JM, Bovolenta P, Walsh C, Nieto M. Cux1 and Cux2 regulate dendritic branching, spine morphology, and synapses of the upper layer neurons of the cortex. Neuron. 2010;66:523–535. doi: 10.1016/j.neuron.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rossa A, Bellone C, Golding B, Vitali I, Moss J, Toni N, Lüscher C, Jabaudon D. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nat Neurosci. 2013;16:193–200. doi: 10.1038/nn.3299. [DOI] [PubMed] [Google Scholar]
- De Marco García NV, Karayannis T, Fishell G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011;472:351–355. doi: 10.1038/nature09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudok JJ, Groffen AJA, Toonen RFT, Verhage M. Deletion of Munc18-1 in 5-HT neurons results in rapid degeneration of the 5-HT system and early postnatal lethality. PloS one. 2011;6:e28137. doi: 10.1371/journal.pone.0028137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu. Rev. Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Dendritic plasticity in mouse barrel cortex following postnatal vibrissa follicle damage. J. Comp. Neurol. 1981;196:357–376. doi: 10.1002/cne.901960302. [DOI] [PubMed] [Google Scholar]
- Hayano Y, Yamamoto N. Activity-dependent thalamocortical axon branching. Neuroscientist. 2008;14:359–368. doi: 10.1177/1073858408317272. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu. Rev. Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu. Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knöpfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Inan M, Kanki H, Erzurumlu RS, Itohara S, Crair MC. Cortical adenylyl cyclase 1 is required for thalamocortical synapse maturation and aspects of layer IV barrel development. J. Neurosci. 2008;28:5931–5943. doi: 10.1523/JNEUROSCI.0815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjan G, Gleeson JG. Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly. Trends Genet. 2007;23:623–30. doi: 10.1016/j.tig.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kim T-K, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Sestan N, Anton ES. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development. 2012;139:1535–1546. doi: 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrlé R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J. Comp. Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Li H, Crair MC. How do barrels form in somatosensory cortex? Ann. N Y Acad. Sci. 2011;1225:119–129. doi: 10.1111/j.1749-6632.2011.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo A, Rabacchi SA, Cremisi F, Pizzorusso T, Cenni MC, Possenti R, Barsacchi G, Maffei L. A developmentally regulated nerve growth factor-induced gene, VGF, is expressed in geniculocortical afferents during synaptogenesis. Neuroscience. 1995;65:997–1008. doi: 10.1016/0306-4522(94)00538-g. [DOI] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Lu HC, She W-C, Plas DT, Neumann PE, Janz R, Crair MC. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical “barrel” map development. Nat. Neurosci. 2003;6:939–947. doi: 10.1038/nn1106. [DOI] [PubMed] [Google Scholar]
- Lu HC, Butts DA, Kaeser PS, She WC, Janz R, Crair MC. Role of efficient neurotransmitter release in barrel map development. J Neurosci. 2006;26:2692–703. doi: 10.1523/JNEUROSCI.3956-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Bushong EA, Shih TP, Ellisman MH, Nicoll RA. The cell-autonomous role of excitatory synaptic transmission in the regulation of neuronal structure and function. Neuron. 2013;78:433–439. doi: 10.1016/j.neuron.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS. Spiny stellate neurons. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 1. New York: Cellular components of the cerebral cortex; 1984. pp. 255–308. [Google Scholar]
- Mataga N, Fujishima S, Condie BG, Hensch TK. Experience-dependent plasticity of mouse visual cortex in the absence of the neuronal activity-dependent marker egr1/zif268. J. Neurosci. 2001;21:9724–9732. doi: 10.1523/JNEUROSCI.21-24-09724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath LM, Smith SD, Pennington BF. Breakthroughs in the search for dyslexia candidate genes. Trends Mol Med. 2006;12:333–41. doi: 10.1016/j.molmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- McMullen NT, Goldberger B, Suter CM, Glaser EM. Neonatal deafening alters nonpyramidal dendrite orientation in auditory cortex: a computer microscope study in the rabbit. J. Comp. Neurol. 1988;267:92–106. doi: 10.1002/cne.902670107. [DOI] [PubMed] [Google Scholar]
- Meng H, Smith SD, Hager K, Held M, Liu J, Olson RK, Pennington BF, DeFries JC, Gelernter J, O’Reilly-Pol T, et al. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc Natl Acad Sci U S A. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita-Lin E, Hevner R, Wassarman KM, Martinez S, Martin GR, Rubenstein JL. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J. Neurosci. 2006;26:12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol. 2005;492:263–288. doi: 10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- Narboux-Neme N, Evrard A, Ferezou I, Erzurumlu RS, Kaeser PS, Laine J, Rossier J, Ropert N, Sudhof TC, Gaspar P. Neurotransmitter Release at the Thalamocortical Synapse Instructs Barrel Formation But Not Axon Patterning in the Somatosensory Cortex. J. Neurosci. 2012;32:6183–6196. doi: 10.1523/JNEUROSCI.0343-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J. Comp. Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- O’Leary DD. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989;12:400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr. Opin. Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra RC, Blue ME, Johnston MV, Bressler J, Wilson MA. Activity-dependent expression of Egr1 mRNA in somatosensory cortex of developing rats. J. Neurosci. Res. 2004;78:235–244. doi: 10.1002/jnr.20243. [DOI] [PubMed] [Google Scholar]
- Quairiaux C, Armstrong-James M, Welker E. Modified sensory processing in the barrel cortex of the adult mouse after chronic whisker stimulation. J. Neurophysiol. 2007;97:2130–2147. doi: 10.1152/jn.00338.2006. [DOI] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Fukutani Y, Yamamoto Y, Tatara E, Takemoto M, Shimamura K, Yamamoto N. Thalamus-derived molecules promote survival and dendritic growth of developing cortical neurons. J Neurosci. 2012;32:15388–15402. doi: 10.1523/JNEUROSCI.0293-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, André E, Kapfhammer JP, Becker-André M. The expression pattern of the orphan nuclear receptor RORbeta in the developing and adult rat nervous system suggests a role in the processing of sensory information and in circadian rhythm. Eur. J. Neurosci. 1997;9:2687–2701. doi: 10.1111/j.1460-9568.1997.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Fox K, O’Leary DD. Postsynaptic control of plasticity in developing somatosensory cortex. Nature. 1993;364:623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J. Comp. Neurol. 1984;230:119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Flagmeyer I, Schubert D, Zilles K, Kötter R, Luhmann HJ. Functional diversity of layer IV spiny neurons in rat somatosensory cortex: quantitative morphology of electrophysiologically characterized and biocytin labeled cells. Cereb. cortex. 2004;14:690–701. doi: 10.1093/cercor/bhh029. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Toonen RFG, Verhage M. Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30:564–572. doi: 10.1016/j.tins.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin X, Rosen G, Gabel L, Guadiana SM, Sarkisian MR, Galaburda AM, Loturco JJ. Dcdc2 knockout mice display exacerbated developmental disruptions following knockdown of doublecortin. Neuroscience. 2011;190:398–408. doi: 10.1016/j.neuroscience.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J. Neurosci. 1986;6:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb. Perspect Biol. 2011;3(6):a005744. doi: 10.1101/cshperspect.a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford KL, Dijkhuizen P, Polleux F, Ghosh A. Molecular control of cortical dendrite development. Annu. Rev. Neurosci. 2002;25:127–149. doi: 10.1146/annurev.neuro.25.112701.142932. [DOI] [PubMed] [Google Scholar]
- Wijetunge LS, Till SM, Gillingwater TH, Ingham CA, Kind PC. mGluR5 regulates glutamate-dependent development of the mouse somatosensory cortex. J. Neurosci. 2008;28:13028–13037. doi: 10.1523/JNEUROSCI.2600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Finlay BL. Thalamic ablations and neocortical development: alterations of cortical cytoarchitecture and cell number. Cereb.Cortex. 1991;1:230–240. doi: 10.1093/cercor/1.3.230. [DOI] [PubMed] [Google Scholar]
- Yoneshima H, Yamasaki S, Voelker CCJ, Molnár Z, Christophe E, Audinat E, Takemoto M, Nishiwaki M, Tsuji S, Fujita I, et al. Er81 is expressed in a subpopulation of layer 5 neurons in rodent and primate neocortices. Neuroscience. 2006;137:401–412. doi: 10.1016/j.neuroscience.2005.08.075. [DOI] [PubMed] [Google Scholar]
- Zhou L, Gall D, Qu Y, Prigogine C, Cheron G, Tissir F, Schiffmann SN, Goffinet AM. Maturation of “neocortex isole” in vivo in mice. J. Neurosci. 2010;30:7928–7939. doi: 10.1523/JNEUROSCI.6005-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J. Neurosci. Meth. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.