Abstract
The lipid analogues of pyridinium p-toluenesulfonate (PPTS) were examined for catalyzing the condensation of an equimolar mixture of carboxylic acids and alcohols under mild conditions without removal of water. Although PPTS is a poor catalyst, the introduction of a lipid chain and nitro group significantly improved the activity of PPTS and led to selectivity at suppressing elimination side reactions of alcohols. 2-Oleamido-5-nitro-pyridinium p-toluenesulfonate (6) is a lead catalyst that promoted various esterification reactions with yields up to 99%.
INTRODUCTION
During recent years, there is increasing interest in exploring the use of organic catalysts for promoting acid-alcohol esterification reactions. For example, the employment of diarylammonium arenesulfonates1 and pyrosulfates2 as dehydrative condensation catalysts and use of p-dodecylbenzenesulfonic acid for catalytic esterification reactions in water were reported.3 Histidine sulfonamide,4 N-alkyl-4-boronopyridinium iodide5, iodosodilactone6, proline7 and carbenes8 were also examined as catalysts for promoting esterification reactions. One of the advantages of using organic esterification catalysts over metal catalysts9 is that an organic catalyst can be removed out of reaction mixtures more easily than a metal counterpart during work-up. In a pharmaceutical process, repeated recrystallization steps or multiple chromatography purifications are needed to remove a leached metal catalyst out of the drug intermediate since the metal content has to be controlled under several ppm. The adoption of an organic esterification catalyst will avoid the metal contamination problem in a drug production process.
Pyridinium p-Toluenesulfonate (PPTS) has been extensively explored as an organic catalyst for promoting chemical transformations such as synthesis and cleavage of acetals,10 and deprotection of silyl or tetrahydropyranyl groups.11 This pyridinium salt can be facilely prepared from pyridine and p-toluenesulfonic acid and has good solubility in organic solvents like methylene chloride, chloroform, acetone and ethanol. PPTS is a mild acid which is especially useful when a reaction substrate is unstable to strong acids. However, PPTS typically shows weak catalytic activity in promoting esterification condensation reactions and the use of PPTS in esterification reactions, to the best of our knowledge, has not yet been widely reported.
RESULTS AND DISCUSSION
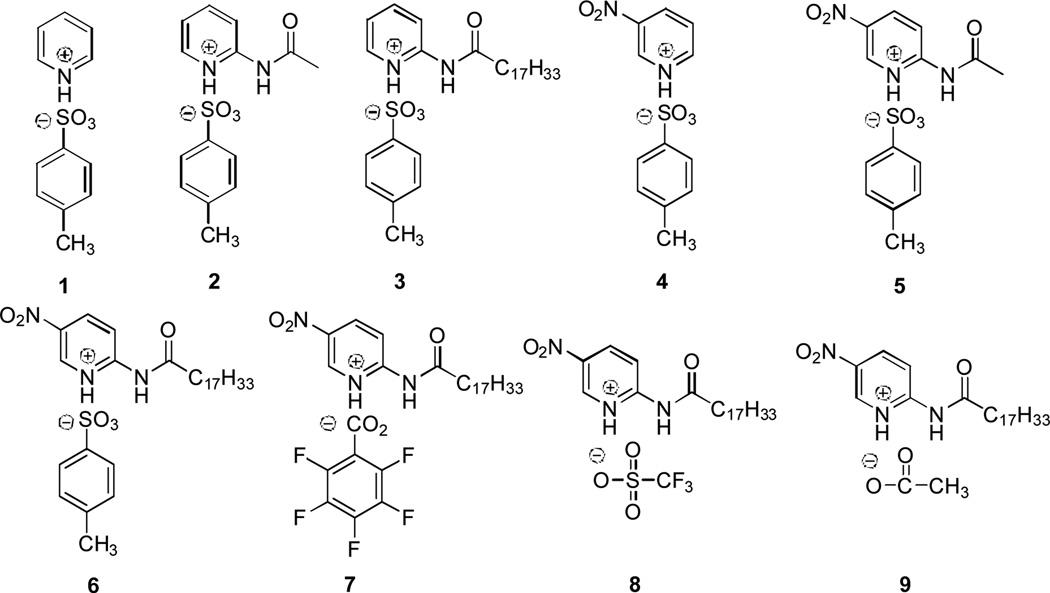
In this communication, we report the design, synthesis and investigation of a group of lipid analogues of PPTS (1) (Figure 1) for catalyzing esterification reactions. An esterification process involves the condensation of an acid and an alcohol and water is generated as the byproduct. Continuous removal of water out of the reaction mixture with the assistance of molecular sieves, evaporative distillation, etc., is a common strategy to shift the reaction equilibrium towards the ester product for a better reaction yield.12 Our approach to improve the activity of PPTS involves the attachment of a lipid chain onto the pyridinium ring to create a local hydrophobic environment that has less affinity to water molecules. Such a hydrophobic reaction center will help shift the equilibrium towards the ester product. In addition, steric hindrance created by the long lipid chain could also lead to selectivity towards some reaction substrates and intermediates.
Figure 1.
PPTS (1) and Its Lipid Analogues (2–9).
PPTS and its lipid analogues in Figure 1 were synthesized via simple chemical transformations and the details of our synthesis protocols are reported in the Supporting Information (available online). Catalysts (5 mol%) were evaulated using 4-phenylbutyric acid (2 mmol) and 1-octanol (2 mmol) at 25 °C in 4 mL of isooctane (Table 1). The reaction progress was monitored by GC and TLC analyses. After 72 hours, the products were isolated, purified by flash chromatography and characterized by 1H NMR, 13C NMR, IR and high-resolution mass spectrometry. Table 1 lists the GC yields of the ester products after 72 hours. PPTS (1) is a weak catalyst that failed to generate the ester product (entry 1) and no esterification product was determined by our GC experiments at ambient temperature and 80 °C. The short C2 acetyl chain on the pyridinium ring of 2 did not improve the activity of 2 as no formation of the esterification product was detected (entry 2). A longer C18 chain derived from oleic acid in catalyst 3 led to a yield of 16% (entry 3). The introduction of a nitro group at the meta-position in the pyridinium ring of 4 improved its catalytic activity as 48% of the esterification product was uncovered (entry 4). −NO2 is an electron-withdrawing group that increases the acidity of 4 and thus improving its activity. The acetyl chain in 5 gave a yield of 49% (entry 5)—which re-confirms that a short chain of C2 does not help improve the activity of the catalyst. However, a much longer C18 chain in catalyst 6 significantly boosted its activity. A yield of 76% was observed for 6 at 25 °C after 72 hours (entry 6) while a higher yield of 99% was obtained when the reaction temperature was raised to 80 °C. Pyridinium salts with an alternative anionic group like pentafluorobenzoate in 7 (entry 7), or trifluoromethanesulfonate in 8 (entry 8) or acetate in 9 (entry 9) all failed to generate detectable esterification products.
Table 1.
Evaluation of the Catalytic Activities of PPTS Lipid Analogues (Figure 1) using an Esterification Reaction of 4-Phenylbutyric Acid with 1-Octanola
|
| ||
|---|---|---|
| entry | catalyst | yield of ester (%)b |
| 1 | PPTS (1) | n.d.c |
| 2 | 2 | n.d. |
| 3 | 3 | 16 |
| 4 | 4 | 48 |
| 5 | 5 | 49 |
| 6 | 6 | 76/99d |
| 7 | 7 | n.d. |
| 8 | 8 | n.d. |
| 9 | 9 | n.d. |
Conditions: 4-phenylbutyric acid (2 mmol), 1-octanol (2 mmol), and catalyst (5 mol%) in isooctane (4 mL) at 25 °C for 72 h.
Determined by GC analysis.
Not detected by GC experiments.
The yield of 76% at 25 °C and 99% at 80 °C, respectively.
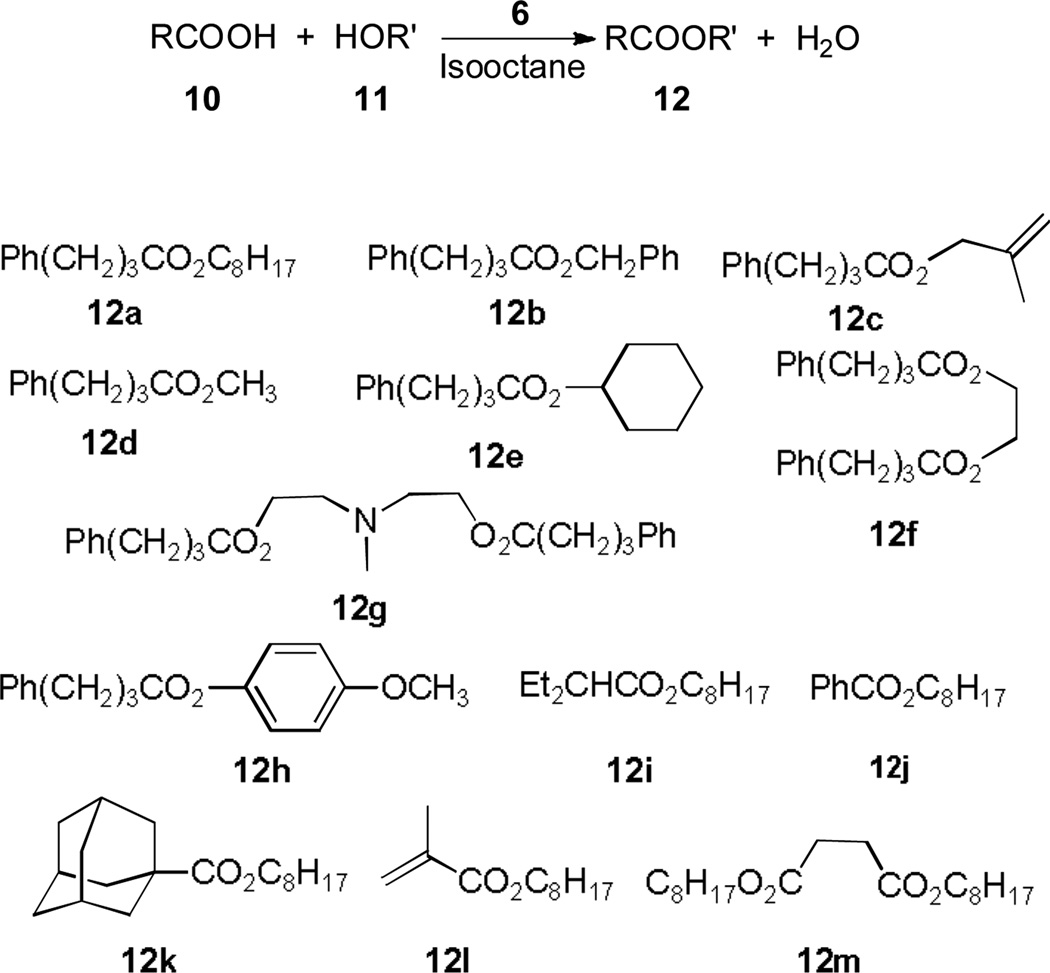
The higher activity of catalyst 6 over that of compound 4 in Table 1 confirms that a long C18 alkyl chain can help improve the activity of pyridinium salts. Catalyst 6 was further explored for facilitating a group of esterification reactions of an equimolar mixture of carboxylic acids and alcohols with diverse functional groups (Figure 2). A typical procedure of these reactions (Table 2) involves the addition of 6 (1–10 mol%) to a mixture of an acid (2 mmol) and an alcohol (2 mmol) in 4 mL of isooctane under various temperatures. These esters in Figure 2 were synthesized from 4-phenylbytric acid (12a-h), bulky acids like 2-ethylbutanoic acid (12i) and 1-adamantanecarboxylic acid (12k), benzoic acid (12j), a vinyl acid (12l) and a di-acid (12m). The alcohols used for constructing esters include methanol (12d), 1-octanol (12a, 12i-m), phenol (12h), an allylic alcohol (12c), a secondary alcohol (12e), a diol (12f) and an amino-diol (12g). Moderate to excellent yields ranging from 40% to 99% were observed via our GC experiments (Table 2). The esterification products were further isolated, purified via flash chromatography and extensively characterized.
Figure 2.
Esterification Products Synthesized under 6.
Table 2.
| entry | RCOOR’ 12 |
GC yield of ester (%) |
isolated yield of ester (%) |
reaction condition |
|---|---|---|---|---|
| 1 | 12a | 99 | 95 | catalyst 1 mol%, 80 °C, 5 h |
| 2 | 12b | 97 | 83 | catalyst 1 mol%, 80 °C, 6 h |
| 3 | 12c | 70 | 61 | catalyst 1 mol%, 80 °C, 30 h |
| 4 | 12d | 78 | 76 | catalyst 5 mol%, 25 °C, 24 h |
| 5 | 12e | 99 | 93 | catalyst 1 mol%, reflux, 16 h |
| 6 | 12f | 81 | 81 | catalyst 1 mol%, 2 eq. acid, reflux, 30 h |
| 7 | 12g | 92 | 83 | catalyst 1 mol%, 2 eq. acid, reflux, 48 h |
| 8 | 12h | 55 | 59 | catalyst 10 mol%, reflux, 96 h |
| 9 | 12i | 61 | 50 | catalyst 1 mol%, reflux, 48 h |
| 10 | 12j | 40 | 33 | catalyst 10 mol%, reflux, 144 h |
| 11 | 12k | 99 | 93 | catalyst 1 mol%, reflux, 96 h |
| 12 | 12l | 74 | 64 | catalyst 10 mol%, reflux, 40 h |
| 13 | 12m | 84 | 78 | catalyst 10 mol%, 2 eq. alcohol, reflux, 48 h |
Unless otherwise noted, an acid (2 mmol), an alcohol (2 mmol) in isooctane (4 mL).
It is noteworthy that no significant formation (<1%) of dehydrative elimination byproducts from alcohols, especially from cyclohexanol (entry 5) was detected by our GC experiments. Steric hindrance created by the alkyl chain suppresses the elimination of water from the alcohol—a competitive side reaction frequently encountered in the acid mediated-esterification reaction of secondary alcohols.1 A yield of 99% for the esterification of 1-adamantanecarboxylic acid (entry 11) suggests that 6 is a robust catalyst that can accommodate a large number of substrates including a very bulky acid. However, yields were lower for less active acids like benzoic acid (entry 10) and 2-methyl-propenoic acid (entry 12).
CONCLUSIONS
In conclusion, a group of lipid analogues of PPTS were synthesized and examined for catalyzing esterification reactions. PPTS itself is a weak catalyst, but the introduction of a lipid C18 chain and a nitro group on the pyridinium ring significantly improved its catalytic activity. 2-Oleamido-5-nitro-pyridinium p-toluenesulfonate (6) is a lead catalyst that facilitated the synthesis of a large number of esters from diverse substrates. This catalyst also demonstrated selectivities in suppressing competitive elimination of water molecules from alcohols. Our investigations here suggest that rational modifications of PPTS can lead to unusual activities and selectivities. Further studies to elucidate the detailed structure and mechanism of PPTS lipid analogues and their applications to other types of organic transformations are now underway.
EXPERIMENTAL
Most chemicals were purchased from Acros Organics (Somerville, NJ), and Aldrich (Milwaukee, WI) and used as received without further purification. Amino acids were bought from Bachem Bioscience (King of Prussia, PA). Water was obtained from a Milli-Q reagent water system purchased from Millipore Corporation (Milford, MA). The heavy metal and bacterial contaminant levels in Milli-Q water are below 10 ppb. 1H NMR and 13C data were obtained on a Varain VXR-300 system with an Oxford wide-bore magnet and the chemical shifts were reported in parts per million (ppm) downfield relative to tetramethylsilane using the residual proton resonance of solvents as the references (1H NMR): CDCl3 δ 7.27; CD2Cl2 δ 5.32 and (13C NMR): CDCl3 δ 77.2; CD2Cl2 δ 54.0. Elemental analyses were done in Galbraith Laboratories, Inc. (Knoxville, TN). GC analyses were carried out on a Varian 3900 system equipped with a TCD detector. A Varian Factorfour Capillary Column VF-1ms, 15 mx 0.25 mm. LC-MS experiments were carried out in the Mass Spectrometry Laboratory in the University of Illinois at Urbana-Champaign.
General procedure for preparing catalysts
A mixture of pyridine or its analogues (1 mmole) with an acid (1 mmole) in in 10 mL toluene was refluxed for 2 hours. Then, the solution was cooled down to ambient temperature and the solvent was removed in vacuo to yield a pyridinium acid salt as a catalyst for our esterification investigations.
General procedure for catalytic esterification reactions
To a mixture of an acid (1 eq.) and an alcohol (1 eq.) in isooctane (4 mL) was added a catalyst ranging from 1–10 mol%. The resultant reactions were carried out at various temperatures and the reaction progresses were monitored by GC and TLC analyses. Then, the solvent was removed in vacuo and the products were isolated, purified by flash chromatography and characterized by 1H NMR, 13C NMR, IR and high-resolution mass spectrometry.
Supplementary Material
Acknowledgement
We thank Narsimha Sattenapally for collecting some preliminary laboratory data. This work was supported in part by the NIH (1R15EB007074-01, 2R15EB007074-02), and NSF (CAREER Award CHE-0343440).
Footnotes
[Supplementary materials are available for this article. Go to the publisher’s online edition of Synthetic Communications® for the following free supplemental resource(s): Full experimental and spectral details.]
SUPPORTING INFORMATION
Full experimental details, spectroscopic characterizations (1H & 13C NMR, IR and high-resolution mass spectrometry) of all compounds. This material can be found via the Supplemental Content section of this article’s Web page.
Supporting Information Available Online: Characterization data of new compounds.
REFERENCES
- 1.Ishihara K, Nakagawa S, Sakakura A. J. Am. Chem. Soc. 2005;127:4168–4169. doi: 10.1021/ja050223v. [DOI] [PubMed] [Google Scholar]
- 2.Sakakura A, Koshikari Y, Akakura M, Ishihara K. Org. Lett. 2012;14:30–33. doi: 10.1021/ol2027366. [DOI] [PubMed] [Google Scholar]
- 3.Manabe K, Limura S, Sun XM, Kobayashi S. J. Am Chem. Soc. 2002;124:11971–11978. doi: 10.1021/ja026241j. [DOI] [PubMed] [Google Scholar]
- 4.Ishihara K, Kosugi Y, Umemura S, Sakakura A. Org. Lett. 2008;10:3191–3194. doi: 10.1021/ol801007m. [DOI] [PubMed] [Google Scholar]
- 5.Maki T, Ishihara K, Yamamoto H. Org. Lett. 2005;7:5047–5050. doi: 10.1021/ol052061d. [DOI] [PubMed] [Google Scholar]
- 6.Tian J, Gao WC, Zhou DM, Zhang C. Org. Lett. 2012;14:3020–3023. doi: 10.1021/ol301085v. [DOI] [PubMed] [Google Scholar]
- 7.Lipshutz BH, Ghorai S. Org. Lett. 2012;14:422–425. doi: 10.1021/ol203242r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noonan C, Baragwanath L, Connon SJ. Tetrahedron Lett. 2008;49:4003–4006. [Google Scholar]
- 9. For some leading references on metal-mediated esterification reactions: Sato A, Nakamura Y, Maki T, Ishihara K, Yamamoto H. Adv. Synth. Catal. 2005;347:1337–1340. Mantri K, Komura K, Sugi Y. Green Chem. 2005;7:677–682. Houston TA, Wilkinson BL, Blanchfield JT. Org. Lett. 2004;6:679–681. doi: 10.1021/ol036123g. Bartoli G, Boeglin J, Bosco M, Locatelli M, Massaccesi M, Melchiorre P, Sambri L. Adv. Synth. Catal. 2005;347:33–38. Mikami K, Mikami Y, Matsumoto Y, Nishikido J, Yamamoto F, Nakajima H. Terhedron Lett. 2001;42:289–292. Dyke AC, Bryson TA. Tetrahedron Lett. 2001;42:3959–3961.
- 10.Fernández E, Castillón S. Tetrahedron Lett. 1994;35:2361–2364. [Google Scholar]
- 11.Miyashita M, Yoshikoshi a, Grieco PA. J. Org. Chem. 1977;42:3772–3774. [Google Scholar]
- 12.Otera J, Nishikido J. Esterification: Methods, Reactions, and Applications. 2nd ed. Weinheim: Wiley-VCH; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.