The translation of bioengineering strategies pioneered in cardiac electrophysiology is driving fundamental new insights into gastrointestinal motility in health and disease.
Abstract
A key discovery in gastrointestinal motility has been the central role played by interstitial cells of Cajal (ICC) in generating electrical slow waves that coordinate contractions. Multielectrode mapping and multiscale modeling are two emerging interdisciplinary strategies now showing translational promise to investigate ICC function, electrophysiology, and contractions in the human gut.
Gastrointestinal (GI) motility is coordinated by an elegant interplay of neurogenic, myogenic, and hormonal mechanisms that cooperate to mix and propel contents (41). In health, these functions are tightly regulated in accordance with the volume and nutrient content of digesta, and in concert with the absorptive capability of the gut. However, in modern society, disorders of gut function have become remarkably common, accounting for substantial suffering (101).
Among the mechanisms regulating GI motility, slow waves generated by interstitial cells of Cajal (ICC) perform a fundamental role. Slow waves are oscillating changes in membrane potential that serve to depolarize smooth muscle cells (SMC) from a resting potential between −75 and −55 mV to a peak potential of between −40 and −25 mV, thereby increasing the propensity for voltage-dependent Ca2+-channel opening and contraction (89). ICC form anastomosing networks within and between GI smooth muscle layers, and variation in their distribution contributes to distinct functions among gut organs (38). Slow waves alone may elicit sufficient excitation to generate contractions in SMC, but functionally significant contractions typically require other co-regulatory signals, such as postprandial neural input (88). Besides their pacemaker function, ICC serve other roles, notably mechanotransduction, mediation of neurotransmission, and modulation of smooth muscle cell membrane potential gradients (44).
The significance of ICC was only established relatively recently (43, 102), and intense research interest now centers on what roles ICC play in dysmotility (32). In notable examples, ICC depletion has become a recognized hallmark of gastroparesis and slow-transit constipation, and recent evidence has linked ICC/SMC channelopathies to irritable bowel syndrome (37, 65, 87). However, challenges arise in interpreting the importance of these associations for whole-organ function and in achieving meaningful clinical translation.
This review is focused on multi-electrode mapping and multi-scale modeling, two emerging interdisciplinary strategies now being successfully applied in translational studies to investigate GI electrical activity and motility in humans (FIGURE 1).
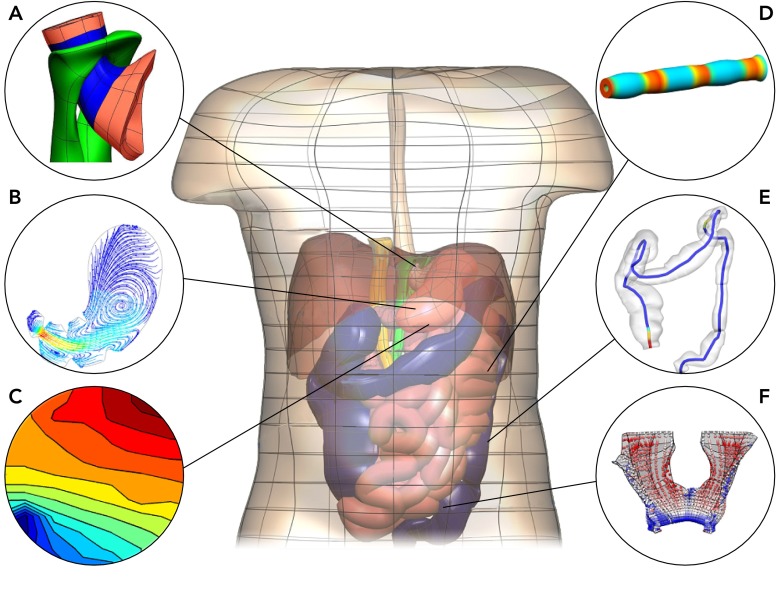
FIGURE 1.
Anatomically realistic model of the human abdomen constructed from Visible Human Project data
Included are the esophagus, stomach, small intestine, and colon. Insets show examples of mapping and modeling techniques applied to investigate gastrointestinal motility: finite elasticity model of the gastroesophageal junction (A; Ref. 107), computational fluid dynamics model of flow in the stomach (B; Ref. 33; courtesy of Dr. Maria Ferrua), gastric slow wave activation map (C; Ref. 73), coupled electro-mechanical model of an intestine segment (D; Ref. 26), visualization of colonic manometry measurements overlaid on an anatomical colon model (E; Refs. 17, 33; courtesy of Dr. Phil Dinning), visualization of the extension ratios in the levator ani muscle due to a “bear-down” maneuver (F; Ref. 70).
Multi-Electrode Mapping the Stomach
Gastric slow-wave propagation is facilitated by an intrinsic frequency gradient within ICC that declines in the aboral direction. In intact stomach, all ICC “entrain” to the node of highest frequency within the syncytium, at ∼3 cycles/min (cpm) in humans (39, 103). If distal regions are isolated, they autonomously generate slow waves at lower frequencies (39, 47).
Classically, researchers attempted to reconstruct patterns of slow-wave entrainment using sparse arrangements of extracellular electrodes (2, 39). Although this strategy enabled a basic outline of activation that still informs current textbooks, it is now clear that sparse slow-wave sampling may mislead due to spatial aliasing and cannot reliably quantify wavefront initiation, propagation, and interaction (62, 73). High-resolution (HR) spatiotemporal detail is required to accurately track slow-wave activation, achieved by using multi-electrode arrays (72). HR mapping of the GI tract was introduced in 1993 on rabbit duodenum by Lammers et al., who were motivated by the striking success of this strategy in cardiac electrophysiology (52). Progress is now accelerating with the advent of mass-produced flexible printed-circuit board arrays and semi-automated analysis software (23, 28, 29, 108).
Recent in vivo HR mapping studies have now substantially revised descriptions of normal gastric slow-wave activation (27, 61, 73). In contrast to previous assumptions, there is not a gradual increase in slow-wave velocity and amplitude in the stomach. Instead, distinct regions are recognizable: the pacemaker and distal antrum regions show rapid activation of high amplitude, whereas multiple wavefronts propagate through the corpus at a constant slow velocity (∼3 mm/s). FIGURE 2 summarizes this new understanding of the normal human gastric slow-wave pattern.
FIGURE 2.
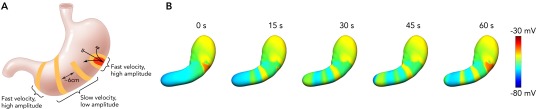
Human gastric slow wave conduction based on HR mapping
A: human gastric slow-wave conduction based on HR mapping (73). Slow waves originate from a pacemaker region on the greater curvature of the corpus and propagate rapidly in the circumferential axis, forming ring wavefronts in the mid corpus. Multiple wavefronts accrue in the corpus at a spacing dependent on the period (∼20 s) and velocity (∼3 mm/s). There is a transition to rapid activation in the distal antrum. These patterns are closely concordant with contraction patterns revealed by dynamic MRI (67, 80). B: simulated whole-organ gastric slow-wave activity. The blue color represents resting membrane potential, and the red color represents the peak slow-wave potential. The simulation demonstrates the existence of multiple wavefronts as “rings” around the gastric lumen. Each cycle of slow waves takes ∼1 min to propagate from the pacemaker region to the pylorus and is demonstrated by the arrow head (22, 76). (For animation refer to: http://www.youtube.com/v/ROeH9LGeD54.)
Following clarification of normal activation, HR mapping has progressed toward investigating human gastric dysrhythmias. Gastric dysrhythmias have been investigated for many years, commonly studied by cutaneous electrogastrography (EGG) (2). EGG reliably reflects a summative slow-wave frequency (96) and has well established associations with several motility disorders (49, 109); however, clinical use has been constrained by uncertainty in its interpretation, low specificity, and sensitivity to noise (7, 104). Consequently, dysrhythmia is largely neglected in current GI practice.
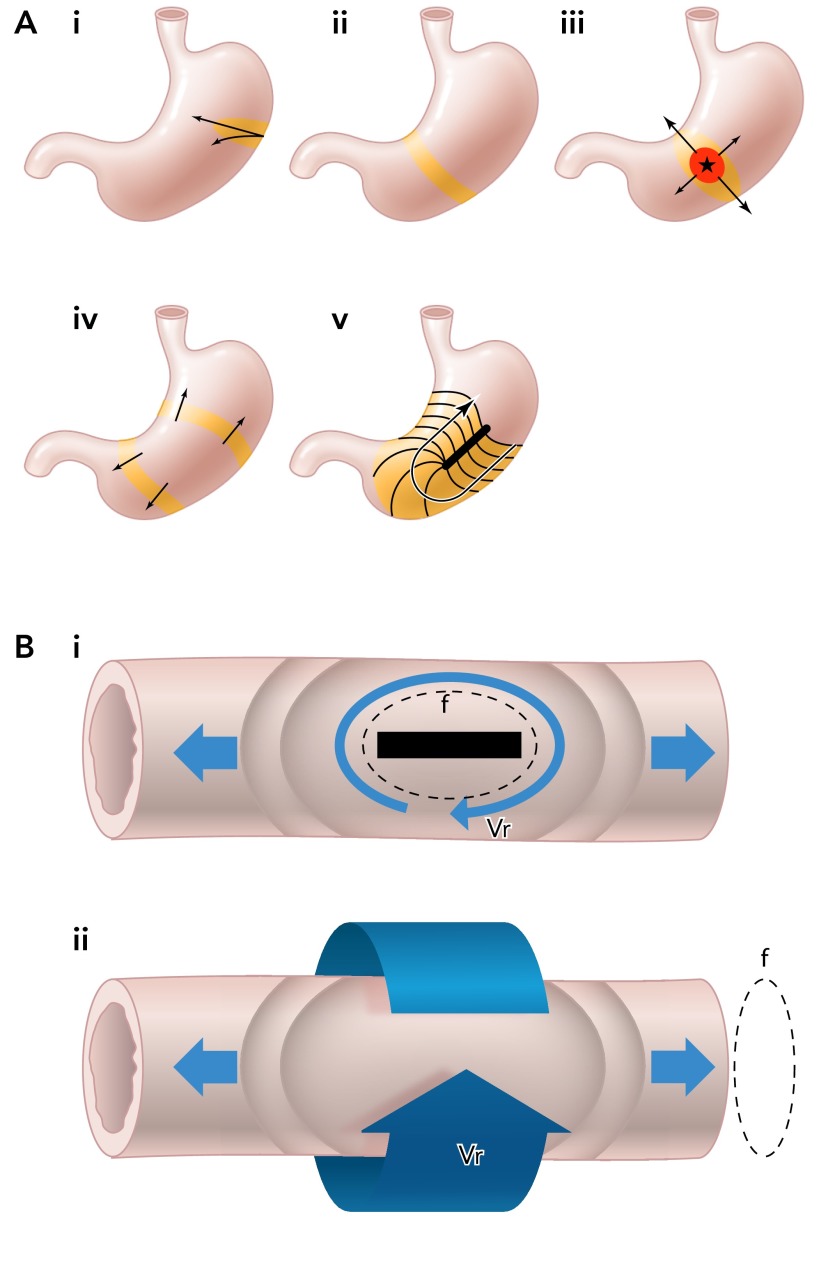
HR mapping may now be establishing a new era in which dysrhythmic patterns and mechanisms are known and applied in translational studies (51, 71). Using EGG, dysrhythmias were predominantly defined by their frequency, e.g., bradygastria of <2.5 cpm and tachygastria of >3.75 cpm (81). Based on HR data, a new trend is emerging toward classifying specific patterns by spatiotemporal criteria. A surprising complexity underlying gastric dysrhythmia is being revealed, necessitating adaption of terms from cardiology, including focal ectopic activities and reentry as mechanisms of tachygastria and conduction block and retrograde escape rhythms in bradygastria (62, 77) (FIGURE 3A).
FIGURE 3.
Schematic diagram illustrating principles of dysrhythmia and reentry in the stomach and intestine
A: roles of anisotropy in normal and dysrhythmic slow-wave propagation (62, 71, 77). i: normal pacemaker with rapid circumferential occurs at the normal site of initiation. ii: normal propagation with ring wavefronts formed in corpus that propagate longitudinally, and circumferential conduction ceases. iii: ectopic pacemaker with rapid circumferential propagation accompanying ectopic initiation, inducing elliptical wavefronts. iv: organized ring wavefronts generated from an ectopic source may propagate both antegrade and retrograde. v: rapid circumferential propagation and retrograde propagation occurring around an incomplete conduction block. B: illustrations of functional (i) and anatomical (ii) reentry (large arrows) in an intestine segment with both antegrade and retrograde waves arising. These events are capable of establishing antegrade and retrograde waves at a frequency dependent on the velocity (Vr) of the reentrant wave and the diameter (f) of the reentrant circuit. In each case, the wavefront (arrow head) follows the refractory tail of the previous cycle (tail of arrow). A zone of excitable tissue must be present in between the wavefront and refractory tail (4, 58).
An important milestone has been the publication of the first GI HR mapping study in a human disease state, gastroparesis (71). This work has enabled the first spatial classification of human dysrhythmias, adopting the classic cardiac framework of abnormalities of initiation and conduction (105) (FIGURE 3A). The marked level of slow-wave disorganization shown in gastroparesis patients is likely a result of ICC loss and injury in this disease (37, 71), presenting a structure-function pathway that could explain the motility abnormalities. Another major discovery from this study was that highly disorganized dysrhythmic patterns routinely occurred at normal frequencies in human stomach, providing insight into a key limitation of traditional EGG diagnostic approaches (71).
A further important outcome of human HR mapping has been to clarify the role of anisotropy in gastric slow-wave activation, recently enabled by new spatial analysis methods (82, 83). Anisotropic gastric conduction had been known from studies in isolated tissue strips (40) and from in vivo gastric pacing studies (75), but has now been quantified in humans: slow waves travel ∼2.5 times faster circumferentially than longitudinally (∼7.3 vs. 2.9 mm/s) (71). In normal propagation, rapid circumferential conduction is only observed at the pacemaker region, because ring wavefronts are formed within corpus that propagate exclusively longitudinally (73, 76) (FIGURE 2). However, circumferential conduction reemerges during dysrhythmia, either because the normal ring wavefronts are disrupted by conduction defects or because of aberrant initiation within resting tissue fields. The reemergence of fast circumferential conduction is then a major determinant of dysrhythmic patterns; it may act in a homeostatic manner to rapidly restore organized ring wavefronts, but the ring wavefronts generated may also move retrogradely, disrupting normal gastric pacemaking (71, 76) (FIGURE 3A). Rapid circumferential conduction may result from differing rates of slow-wave conduction through coupled ICC layers (76).
The “Virtual Stomach”
A key challenge in interpreting and integrating the wealth of new data concerning ICC, slow waves, and motility, such as the emerging pathophysiological pathways of gastroparesis, lies in quantifying structure-function relationships across vast spatiotemporal horizons. Therefore, a “scale gap” must be bridged between (sub)cellular events, spanning micrometers and milliseconds, to whole gut function, where motility patterns routinely span meters and minutes (11, 24). The IUPS Physiome Project was developed to help overcome such challenges and has contributed to steady progress in applying mathematical modeling to develop a spatiotemporally integrative “Virtual Physiological Human” (45, 50).
Modeling Gastric Electrophysiology
At the cellular scale, a great expansion in experimental data coupled with physiome computational techniques have facilitated creation of biophysically based GI computational models of ICC and SMC (15, 16, 63). These models quantitatively integrate vast sets of experimental data while also enabling predictive in silico experiments by simulation (20, 85). In early gastric computational models, electrical activation was represented as moving dipole sources in simple cylindrical- or conical-shaped approximations of the stomach (30, 68). However, as detailed anatomical images and finite-element fitting procedures became available, anatomically realistic stomach geometric meshes were created using Visible Human Project, CT, or MRI data (10, 86). Biophysically based SMC and ICC models can be embedded into these geometries, and their activation sequences coupled to HR mapping data, to realistically reconstruct the entire slow wave activation sequences in silico in 3D (22). The dipole representations of earlier studies have been improved by vectorial summation of the individual dipoles formed by each solution point in the stomach model (22, 48). This combination of approaches enables sophisticated and translational simulations that span physiology from cell to body surface, for example, to quantify the physical transmission of summated gastric slow-wave potentials to torso EGG electrodes (22).
The mechanisms of slow-wave dysrhythmias are also beginning to be addressed within virtual frameworks (24, 34), again adapting successful strategies from cardiology (13, 95). For example, Du et al. recently used multi-scale simulations to quantify the impact of ICC depletion in myenteric plexus ICC networks (25). The results showed that ICC depletion could induce haphazard entrainment within the networks, reducing velocity as well as reducing current density and intracellular calcium release in affected tissues. These insights are also of translational value, being consistent with, and potentially explaining, electrophysiological abnormalities mapped in patients with gastroparesis (71).
Multi-scale, whole-organ models of gastric dysrhythmia are currently limited to simple patterns, such as uncoupling due to partial retrograde entrainment (8). An important future step will be to link disease mechanisms at the cellular scales and spatial data from tissue scales to improve whole-organ dysrhythmia models. Such models are anticipated to help define fundamental influences on dysrhythmia genesis and maintenance, inform their impact on motility, and lead to new EGG signatures that improve noninvasive diagnostics (22).
Modeling Gastric Motility
Ultimately, the stomach serves to break down ingesta by chemical and physical means, delivering chyme to the duodenum at a regulated rate. Different imaging techniques have been employed to study the movements of the stomach and stomach contents (99). Scintigraphy was introduced in 1966 to estimate the rate of gastric emptying (36) and remains the gold-standard method for evaluating gastric emptying due to its direct, noninvasive, and validated approach (99). However, MRI is also increasingly being applied to noninvasively evaluate gastric motility and mixing (67).
In exciting work that further extends the virtual stomach, MRI data is being applied to derive and validate computational fluid dynamic (CFD) models of human gastric flow and mixing (92). One of the aims of such an approach is to understand how different food types and physiological conditions can be related to the pressure, flow fields, shear stresses in dispersing food particles. Studies from several groups have now applied different CFD models and methods for this purpose (33, 46, 79). A notable recent approach by Imai et al. applied an anatomically accurate model and MRI-derived contractions to demonstrate how the J-shape gastric geometry facilitates antral recirculation, with high-pressure retropulsive flow directed away from the pylorus and compensatory antegrade flow occurring along the greater curvature (46).
MRI techniques are also being used to quantitatively assess changes in gastric volumes and contraction patterns in dysmotility, such as in idiopathic rapid gastric emptying (6). An important future avenue will be to couple such data (and HR mapping dysrhythmia data) to generate mechanical and CFD models of gastric disease states to help define impacts of electrical and motor abnormalities on gastric flow, mixing, and emptying (26). The development of gastric CFD models is also motivated by their potential use in the nutrition and drug industries, as such models have the ability to investigate the rate of food and pharmaceutical breakdown and absorption (33).
Multi-Electrode Mapping the Small Intestine
In small intestine, slow waves modulate the frequency, direction, and velocity of excitation, but motor patterns also substantially depend on concurrent enteric nervous system (ENS) control (3). The predominant motor patterns are peristalsis and segmentation (9); peristalsis involves sequential ring contractions that propel intraluminal contents, thereby also often providing a mixing function (41), whereas segmentation occurs when stationary ring contractions divide and mix contents without their net movement (106).
As in stomach, HR intestinal mapping is driving important insights into control mechanisms. Two approaches have been used. For several years, Lammers et al. have been applying dense arrays of silver-wire electrodes to small areas of one side of the intestine, or surgically “unfurled” segments, achieving high-quality recordings from focused regions in mouse, rat, cat, and dog animal models (53, 60). Recently, Angeli et al. developed an alternative approach using flexible printed circuit board electrodes housed in silicone cradles, achieving an inferior signal quality but enabling mapping around the highly curved intestinal geometry in vivo (5, 23).
It is well known that an initial pacemaker site in proximal duodenum is followed by a declining frequency gradient along the intestine, organized in stepwise plateaus from 11–12 cpm in duodenum to ∼8 cpm in ileum in humans (12, 18, 100). Alternative hypotheses for this gradient have been posited, and HR mapping studies have been instructive. In vivo canine and porcine recordings show a hierarchy of competing pacemakers along the intestinal tract (5, 18, 60), thought to reflect ICC within successive plateaus entraining to the highest-order pacemaker in their network that is compatible with their maximal intrinsic rate (3). Alternatively, a HR mapping study of the feline intestine in vitro study demonstrated that individual slow waves can propagate the entire length of the tract, with plateaus resulting from successive blocks to antegrade wavefronts (57). However, tissue isolation could have altered frequencies and gradients in this study (78, 98).
HR mapping has also shown that slow-wave activity is anisotropic in the feline and porcine intestine. Slow waves propagate ∼1.3 times faster in the circumferential axis compared with the longitudinal axis (4, 59). As occurs in the stomach, this propagation anisotropy causes slow waves to emerge from the pacemaker sites in an elliptical pattern, then rapidly spread around the circumference of the bowel and form ring wavefronts that subsequently propagate longitudinally.
HR mapping is also generating new interest in the nascent field of intestinal dysrhythmias. To date, disorganized intestinal propagation has been studied in few contexts, such as ischemia, where conduction block, ectopic pacemakers have been described (54, 93). Interestingly, both in vitro and in vivo studies have also now shown functional reentry to be a prominent dysrhythmic pattern in intestine (4, 58), a state well known from cardiology where activation occurs continuously in a loop around a functional block (4) (FIGURE 3B). This form of reentry has also been observed underlying tachygastria, although not yet in humans (62, 77). An additional form of anatomical reentry, unique to the gut, has also recently been described in intestine, where activation occurs continuously around the luminal axis (“circumferential reentry”) (4) (FIGURE 3B). Both reentry patterns have been shown to establish antegrade and retrograde waves of a frequency determined primarily by the velocity of the reentrant activity and diameter of the reentrant circuit. It is unknown whether reentrant patterns play any pathophysiological role, but it is possible because tachyarrhythmias have been proposed to occur in clinical disease states (91, 93). Now that circumferential reentry has been established in the intestine, it is of further interest to speculate on whether the similar processes could also occur in the stomach as a mechanism of regular tachygastria (4).
“Spikes” are smooth muscle action potentials that may accompany slow waves. They serve to increase excitation and are associated with elevated cytoplasmic Ca2+ and stronger contractile force (88). HR mapping has been applied to track spike sequences occurring during slow-wave propagation (53, 56). These studies have shown that, although intestinal spike activation sequences are associated with slow waves, they may also occur directionally independently of slow waves and with a characteristically different spatial pattern (3, 55). In the intestine, spikes spread at high velocity in overlapping patches following slow waves, with these patches spreading predominantly in either longitudinal or circumferential orientations, relating to their SMC layer derivation (53). The detailed mechanisms controlling spike patch propagation are largely unknown.
The “Virtual Intestine”
Virtual intestine modeling is relatively less advanced than in stomach, but progress is noteworthy. At the cellular level, Poh et al. recently simulated the effect of Nav1.5 sodium channel mutations on slow-wave function in virtual ICC and SM cells, thereby quantifying a possible causal pathway between channelopathies and irritable bowel syndrome (84). The same group has also presented a detailed biophysically based model of human jejunal SMC capable of accurately simulating experimental data (85), which will be of utility in further extending multi-scale simulations of intestine function.
A key issue for organ-level intestinal modeling is to accurately represent frequency plateaus. Early numerical studies modeled intestinal entrainment using a chain of coupled oscillators, applying an intrinsic frequency gradient (69, 90). More recently, a nonlinear oscillator model that explicitly represented intestinal ICC and SMC activity was presented (the Aliev model) (1), which has also been applied in a multi-scale framework to simulate 1D entrainment, using a visible human intestinal geometry (64). The Aliev model has also been coupled with the Pennes bio-heat equation in cylindrical intestine simulations to study how falling temperature may destabilize slow-wave frequency and conduction, leading to turbulent dysrhythmias (35). These studies demonstrate a necessary trend toward integrating disparate biological data, mathematical descriptions, and physical laws under a unified framework.
As in stomach, CFD techniques have also been applied to intestinal relationships between contraction, propulsion, mixing, and absorption (66, 92, 97). Commonly, it is explained that efficient mucosal absorption is mainly a consequence of the increased surface area provided by the villi. However, in a recent example of applied modeling, it was postulated that an important dynamic influence also arises from micro-scale mixing produced by the moving villi interacting with the macro-scale luminal flow. Coupled electro-mechanical models of the intestine are also now beginning to emerge (21). The next step will be to unify electro-mechanical and CFD models within a common virtual framework, offering a powerful approach to integrate, extend, and apply experimental observations currently emerging on intestinal wall motion, intraluminal pressure, flow, and absorption (19).
Future Directions
Mapping and modeling are currently contributing to a time of rapid progress in the physiology of GI motility, and a key challenge now is to translate this progress into clinical benefit.
For HR mapping, an essential step is to develop minimally invasive mapping systems for routine diagnostic applications that move the field beyond EGG. Laparoscopic devices, wireless acquisition systems, and endoscopic systems with few electrodes are already available (14, 31, 74), but the essential step must be to develop endoscopic HR mapping devices suitable for routine diagnosis. Importantly, the application of such translational devices must continue to be coupled with basic physiological studies in sound animal models that continue to grow our embryonic understanding of gut dysrhythmias at fundamental levels.
We also anticipate accelerated growth of biophysically based, anatomically accurate, multi-scale modeling of GI motility within the robust frameworks provided by the Physiome/Virtual Physiological Human Projects (24). As well as refining current gastric and small intestine models, it is anticipated that “virtual colon” models will also evolve. Colonic modeling is an attractive strategy due to the high complexity of the control systems operating, including dual ICC networks that work in tandem with the ENS to generate coordinated motor patterns (42). More detailed experimental data is required at all biological scales to guide GI modeling, but particularly so in colon. However, early studies are now beginning to emerge, with recent examples including visible human geometries applied to map manometric pressure profiles in health and slow transit constipation (17) and CFD modeling of colonic flow (94).
Ultimately, the success of the above efforts will depend on the continued close interdisciplinary collaboration of engineers, physiologists, and clinicians to the benefit of patients who suffer the burden of motility disorders.
Acknowledgments
The authors acknowledge the contributions from the Auckland GI research group and the mentorship provided by Prof. Andrew Pullan in previous years.
Footnotes
The work is funded in part by grants from the New Zealand (NZ) Health Research Council, the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-64775), NZ Marsden Fund, and the NZ Postdoctoral Fellowship.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: L.K.C., P.D., and G.O. drafted figures; L.K.C., P.D., and G.O. drafted manuscript; L.K.C., P.D., and G.O. edited and revised manuscript; L.K.C., P.D., and G.O. approved final version of manuscript.
References
- 1.Aliev RR, Richards W, Wikswo JP. A simple nonlinear model of electrical activity in the intestine. J Theor Biol 204: 21–28, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez WC. The electrogastrogram and what it shows. JAMA 78: 1116–1119, 1922 [Google Scholar]
- 3.Angeli TR, O'Grady G, Lammers WJ. The electrical regulation of gi motility at the whole-organ level. In: New Advances in Gastrointestinal Motility Research, edited by Cheng LK, Farrugia G, Pullan AJ. New York: Springer, 2013, p. 95–112 [Google Scholar]
- 4.Angeli TR, O'Grady G, Du P, Paskaranandavadivel N, Pullan AJ, Bissett IP, Cheng LK. Circumferential and functional re-entry of in-vivo slow wave activity in the porcine small intestine. Neurogastroenterol Motil 25: 304–314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angeli TR, O'Grady G, Paskaranandavadivel N, Erickson JC, Du P, Pullan AJ, Bissett IP, Cheng LK. Experimental and automated analysis techniques for high resolution electrical mapping of small intestine slow wave activity. J Neurogastroenterol Motil 19: 179–179, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharucha AE, Manduca A, Lake DS, Fidler J, Edwards P, Grimm RC, Zinsmeister AR, Riederer SJ. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol Motil 23: 617–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bortolotti M. Electrogastrography: a seductive promise, only partially kept. Am J Gastroenterol 93: 1791–1794, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Buist ML, Cheng LK, Sanders KM, Pullan AJ. Multiscale modelling of human gastric electric activity: can the electrogastrogram detect functional electrical uncoupling? Exp Physiol 91: 383–390, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Cannon WB. The movements of the intestines studied by means of the Rontgen rays. Am J Physiol 6: 249–282, 1902 [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng LK, Komuro R, Austin TM, Buist ML, Pullan AJ. Anatomically realistic multiscale models of normal and abnormal gastrointestinal electrical activity. World J Gastroenterol 13: 1378–1383, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng LK, O'Grady G, Du P, Egbuji J, Windsor JA, Pullan AJ. Gastrointestinal system. Wiley Interdisciplin Rev Syst Biol Med 2: 65–79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen J, Schedl HP, Clifton JA. The small intestinal basic electrical rhythm (slow wave) frequency gradient in normal men and in patients with variety of diseases. Gastroenterology 50: 309–315, 1966 [PubMed] [Google Scholar]
- 13.Clancy CE, Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature 400: 566–569, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Coleski R, Hasler WL. Coupling and propagation of normal and dysrhythmic gastric slow waves during acute hyperglycaemia in healthy humans. Neurogastroenterol Motil 21: 492–499, e1–e2, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Corrias A, Buist ML. A quantitative model of gastric smooth muscle cellular activation. Ann Biomed Eng 35: 1595–1607, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Corrias A, Buist ML. Quantitative cellular description of gastric slow wave activity. Am J Physiol Gastrointest Liver Physiol 294: G989–G995, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Davidson JB, O'Grady G, Arkwright JW, Zarate N, Scott SM, Pullan AJ, Dinning PG. Anatomical registration and three-dimensional visualization of low and high-resolution pan-colonic manometry recordings. Neurogastroenterol Motil 23: 387–390, e171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamant NE, Bortoff A. Nature of the intestinal slow-wave frequency gradient. Am J Physiol 216: 301–307, 1969 [DOI] [PubMed] [Google Scholar]
- 19.Dinning PG, Arkwright JW, Costa M, Wiklendt L, Hennig G, Brookes SJ, Spencer NJ. Temporal relationships between wall motion, intraluminal pressure, and flow in the isolated rabbit small intestine. Am J Physiol Gastrointest Liver Physiol 300: G577–G585, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Du P, Li S, O'Grady G, Cheng LK, Pullan AJ, Chen JDZ. Effects of electrical stimulation on isolated rodent gastric smooth muscle cells evaluated via a joint computational simulation and experimental approach. Am J Physiol Gastrointest Liver Physiol 297: G672–G680, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du P, Lim J, Cheng LK. A model of electromechanical coupling in the small intestine. In: Studies in Mechanobiology, Tissue Engineering and Biomaterials. Berlin: Springer, 2013 [Google Scholar]
- 22.Du P, O'Grady G, Cheng LK, Pullan AJ. A multi-scale model of the electrophysiological basis of the human electrogastrogram. Biophys J 99: 2784–2792, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du P, O'Grady G, Egbuji JU, Lammers WJ, Budgett D, Nielsen P, Windsor JA, Pullan AJ, Cheng LK. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. Ann Biomed Eng 37: 839–846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du P, O'Grady G, Gao J, Sathar S, Cheng LK. Toward the virtual stomach: progress in multiscale modeling of gastric electrophysiology and motility. Wiley Interdiscip Rev Syst Biol Med 5: 481–493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du P, O'Grady G, Gibbons SJ, Yassi R, Lees-Green R, Farrugia G, Cheng LK, Pullan AJ. Tissue-specific mathematical models of slow wave entrainment in wild-type and 5-HT2B knockout mice with altered interstitial cell of Cajal networks. Biophys J 98: 1772–1781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du P, Poh Y, Lim J, Gajendiran V, O'Grady G, Buist ML, Pullan AJ, Cheng LK. A preliminary model of gastrointestinal electromechanical coupling. IEEE Trans Biomed Eng 58: 3491–3495, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egbuji JU, O'Grady G, Du P, Cheng LK, Lammers WJEP, Windsor JA, Pullan AJ. Origin, propagation and regional characteristics of porcine gastric slow wave activity determined by high-resolution mapping. Neurogastroenterol Motil 22: 292–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson JC, O'Grady G, Du P, Egbuji JU, Pullan AJ, Cheng LK. Automated cycle partitioning and visualization of high-resolution activation time maps of gastric slow wave recordings: the Region Growing Using Polynomial Surface-estimate stabilization (REGROUPS) Algorithm. Ann Biomed Eng 39: 469–483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erickson JC, O'Grady G, Du P, Obioha C, Qiao W, Richards WO, Bradshaw LA, Pullan AJ, Cheng LK. Falling-edge, variable threshold (FEVT) method for the automated detection of gastric slow wave events in serosal high-resolution electrical recordings. Ann Biomed Eng 38: 1511–1529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Familoni BO, Abell TL, Bowes KL. A model of gastric electrical activity in health and disease. IEEE Trans Biomed Eng 42: 647–657, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Farajidavar A, O'Grady G, Rao SM, Cheng LK, Abell T, Chiao JC. A miniature bidirectional telemetry system for in vivo gastric slow wave recordings. Physiol Meas 33: N29–N37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil 20, Suppl 1: 54–63, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Ferrua MJ, Singh RP. Modeling the fluid dynamics in a human stomach to gain insight of food digestion. J Food Sci 75: R151–R162, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Du P, Archer R, O'Grady G, Gibbons SJ, Farrugia G, Cheng LK, Pullan AJ. A stochastic multi-scale model of electrical function in normal and depleted interstitial cell of Cajal networks. IEEE Trans Biomed Eng 58: 3451–3455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gizzi A, Cherubini C, Migliori S, Alloni R, Portuesi R, Filippi S. On the electrical intestine turbulence induced by temperature changes. Phys Biol 7: 16011, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Griffith GH, Owen GM, Kirkman S, Shields R. Measurement of rate of gastric emptying using chromium-51. Lancet 1: 1244–1245, 1966 [DOI] [PubMed] [Google Scholar]
- 37.Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, Unalp-Arida A, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Hamilton FA, Pasricha PJ. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 140: 1575–1585, e8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanani M, Farrugia G, Komuro T. Intercellular coupling of interstitial cells of Cajal in the digestive tract. Int Rev Cytol 18: 249–282, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surg 133: 29–33, 1977 [DOI] [PubMed] [Google Scholar]
- 40.Hirst GD, Edwards FR. Electrical events underlying organized myogenic contractions of the guinea pig stomach. J Physiol 576: 659–665, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huizinga JD, Lammers WJEP. Gut peristalsis is coordinated by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol 296: G1–G8, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Huizinga JD, Martz S, Gil V, Wang XY, Jimenez M, Parsons S. Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci 5: 93, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology 137: 1548–1556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter PJ, Borg TK. Integration from proteins to organs: the Physiome Project. Nat Rev Mol Cell Biol 4: 237–243, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Imai Y, Kobayashi I, Ishida S, Ishikawa T, Buist M, Yamaguchi T. Antral recirculation in the stomach during gastric mixing. Am J Physiol Gastrointest Liver Physiol 304: G536–G542, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Kelly KA, Code CF. Canine gastric pacemaker. Am J Physiol 220: 112–118, 1971 [DOI] [PubMed] [Google Scholar]
- 48.Kim JH, Bradshaw LA, Pullan AJ, Cheng LK. Characterization of gastric electrical activity using magnetic field measurements: a simulation study. Ann Biomed Eng 38: 177–186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koch KL. The electrifying stomach. Neurogastroenterol Motil 23: 815–818, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Kohl P, Noble D. Systems biology and the virtual physiological human. Mol Syst Biol 5: 292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lammers WJ. Arrhythmias in the gut. Neurogastroenterol Motil 25: 353–357, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Lammers WJ, al-Kais A, Singh S, Arafat K, el-Sharkawy TY. Multielectrode mapping of slow-wave activity in the isolated rabbit duodenum. J Appl Physiol 74: 1454–1461, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Lammers WJ, Donck LV, Schuurkes JA, Stephen B. Longitudinal and circumferential spike patches in the canine small intestine in vivo. Am J Physiol Gastrointest Liver Physiol 285: G1014–G1027, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Lammers WJ, el-Kays A, Manefield GW, Arafat K, el-Sharkawy TY. Disturbances in the propagation of the slow wave during acute local ischaemia in the feline small intestine. Eur J Gastroenterol Hepatol 9: 381–388, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Lammers WJ, Slack JR. Of slow waves and spike patches. News Physiol Sci 16: 138–144, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Lammers WJ, Slack JR, Stephen B, Pozzan O. The spatial behaviour of spike patches in the feline gastroduodenal junction in vitro. Neurogastroenterol Motil 12: 467–473, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Lammers WJ, Stephen B. Origin and propagation of individual slow waves along the intact feline small intestine. Exp Physiol 93: 334–346, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Lammers WJ, Stephen B, Karam SM. Functional reentry and circus movement arrhythmias in the small intestine of normal and diabetic rats. Am J Physiol Gastrointest Liver Physiol 302: G684–G689, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Lammers WJ, Stephen B, Slack JR, Dhanasekaran S. Anisotropic propagation in the small intestine. Neurogastroenterol Motil 14: 357–364, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Lammers WJ, Ver Donck L, Schuurkes JA, Stephen B. Peripheral pacemakers and patterns of slow wave propagation in the canine small intestine in vivo. Can J Physiol Pharmacol 83: 1031–1043, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Lammers WJ, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol 296: G1200–G1210, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology 135: 1601–1611, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Lees-Green R, Du P, O'Grady G, Beyder A, Farrugia G, Pullan AJ. Biophysically based modeling of the interstitial cells of Cajal: current status and future perspectives. Front Physiol 2: 29, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin AS, Buist ML, Smith NP, Pullan AJ. Modelling slow wave activity in the small intestine. J Theor Biol 242: 356–362, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Lyford GL, He CL, Soffer E, Hull TL, Strong SA, Senagore AJ, Burgart LJ, Young-Fadok T, Szurszewski JH, Farrugia G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 51: 496–501, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macagno EO, Christensen J, Lee CL. Modeling the effect of wall movement on absorption in the intestine. Am J Physiol Gastrointest Liver Physiol 243: G541–G550, 1982 [DOI] [PubMed] [Google Scholar]
- 67.Marciani L. Assessment of gastrointestinal motor functions by MRI: a comprehensive review. Neurogastroenterol Motil 23: 399–407, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Mintchev MP, Bowes KL. Conoidal dipole model of electrical field produced by the human stomach. Med Biol Eng Comput 33: 179–184, 1995 [DOI] [PubMed] [Google Scholar]
- 69.Nelsen TS, Becker JC. Simulation of the electrical and mechanical gradient of the small intestine. Am J Physiol 214: 749–757, 1968 [DOI] [PubMed] [Google Scholar]
- 70.Noakes KF, Bissett IP, Pullan AJ, Cheng LK. Anatomically realistic three-dimensional meshes of the pelvic floor and anal canal for finite element analysis. Ann Biomed Eng 36: 1060–1071, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Grady G, Angeli T, Du P, Lahr C, Lammers WJ, Windsor JA, Abell TL, Farrugia G, Pullan AJ, Cheng LK. Abnormal initiation and conduction of slow wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology 143: 589–598, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Grady G, Angeli T, Lammers WJ. The principles and practice of gastrointestinal high-resolution mapping. In: New Advances in Gastrointestinal Motility Research, edited by Cheng LK, Farrugia G, Pullan AJ. New York: Springer, 2013, p. 51–69 [Google Scholar]
- 73.O'Grady G, Du P, Cheng LK, Egbuji JU, Lammers WJ, Windsor JA, Pullan AJ. The origin and propagation of human gastric slow wave activity defined by high-resolution mapping. Am J Physiol Gastrointest Liver Physiol 299: G585–G592, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Grady G, Du P, Egbuji JU, Lammers WJ, Wahab A, Pullan AJ, Cheng LK, Windsor JA. A novel laparoscopic device for measuring gastrointestinal slow-wave activity. Surg Endosc 23: 2842–2848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O'Grady G, Du P, Lammers WJ, Egbuji JU, Mithraratne P, Chen JDZ, Windsor JA, Cheng LK, Pullan AJ. High-resolution entrainment mapping for gastric pacing: a new analytic tool. Am J Physiol Gastrointest Liver Physiol 298: G314–G321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Grady G, Du P, Paskaranandavadivel N, Angeli T, Lammers WJEP, Farrugia G, Asirvatham S, Windsor JA, Pullan AJ, Cheng LK. Rapid high-amplitude circumferential slow wave conduction during normal gastric pacemaking and dysrhythmia. Neurogastroenterol Motil 24: e299–e312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Grady G, Egbuji JU, Du P, Lammers WJ, Cheng LK, Windsor JA, Pullan AJ. High-resolution spatial analysis of slow wave initiation and conduction in porcine gastric dysrhythmia. Neurogastroenterol Motil 239: e345–e355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Grady G, Pullan AJ, Cheng LK. The analysis of human gastric pacemaker activity. J Physiol 590: 1299–1300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pal A, Brasseur JG, Abrahamsson B. A tomach road or “Magenstrasse” for gastric emptying. J Biomech 40: 1202–1210, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Pal A, Indireshkumar K, Schwizer W, Abrahamsson B, Fried M, Brasseur JG. Gastric flow and mixing studied using computer simulation. Proc Biol Sci 271: 2587–2594, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil 2003: 89–102, 2003 [DOI] [PubMed] [Google Scholar]
- 82.Paskaranandavadivel N, Cheng LK, Du P, O'Grady G, Pullan AJ. Improved signal processing techniques for the analysis of high resolution serosal slow wave activity in the stomach. Conf Proc IEEE Eng Med Biol Soc 2011: 1737–1740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paskaranandavadivel N, O'Grady G, Du P, Pullan AJ, Cheng LK. An improved method for the estimation and visualization of velocity fields from gastric high-resolution electrical mapping. IEEE Trans Biomed Eng 59: 882–889, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poh YC, Beyder A, Strege PR, Farrugia G, Buist ML. Quantification of gastrointestinal sodium channelopathy. J Theor Biol 293: 41–48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poh YC, Corrias A, Cheng N, Buist ML. A quantitative model of human jejunal smooth muscle cell electrophysiology. PLos One 7: e42385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pullan AJ, Cheng LK, Yassi R, Buist ML. Modelling gastrointestinal bioelectrical activity. Prog Biophys Mol Biol 85: 523–550, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Saito YA, Strege PR, Tester DJ, Locke GR, Talley NJ, Bernard CE, Rae JL, Makielski JC, Ackerman MJ, Farrugia G. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol 296: G211–G218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil 20, Suppl 1: 39–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol 68: 307–343, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Sarna SK, Daniel EE, Kingma YJ. Simulation of slow-wave electrical activity of small intestine. Am J Physiol 221: 166–175, 1971 [DOI] [PubMed] [Google Scholar]
- 91.Scheffer RC, Smout AJ. Tachyduodenia in mitochondrial neurogastrointestinal encephalomyopathy. Neurogastroenterol Motil 23: 408–410, 2011 [DOI] [PubMed] [Google Scholar]
- 92.Schulze K. Imaging and modelling of digestion in the stomach and the duodenum. Neurogastroenterol Motil 18: 172–183, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Seidel SA, Hegde SS, Bradshaw LA, Ladipo JK, Richards WO. Intestinal tachyarrhythmias during small bowel ischemia. Am J Physiol Gastrointest Liver Physiol 277: G993–G999, 1999 [DOI] [PubMed] [Google Scholar]
- 94.Sinnott MD, Cleary PW, Arkwright JW, Dinning PG. Investigating the relationships between peristaltic contraction and fluid transport in the human colon using smoothed particle hydrodynamics. Comput Biol Med 42: 492–503, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Smith NP, Hunter PJ, Paterson DJ. The cardiac physiome: at the heart of coupling models to measurement. Exp Physiol 94: 469–471, 2009 [DOI] [PubMed] [Google Scholar]
- 96.Smout AJPM, Van der schee EJ, Grashuis JL. What is measured in electrogastrography? Dig Dis Sci 25: 179–187, 1980 [DOI] [PubMed] [Google Scholar]
- 97.Stavitsky D, Macagno EO, Christensen J. Finite-element analysis of flow induced by contractions like those of the intestine. J Biomech 14: 183–193, 1981 [DOI] [PubMed] [Google Scholar]
- 98.Suzuki N, Prosser CL, DeVos W. Waxing and waning of slow waves in intestinal musculature. Am J Physiol Gastrointest Liver Physiol 250: G28–G34, 1986 [DOI] [PubMed] [Google Scholar]
- 99.Szarka LA, Camilleri M. Methods for measurement of gastric motility. Am J Physiol Gastrointest Liver Physiol 296: G461–G475, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Szurszewski JH, Elveback LR, Code CF. Configuration and frequency gradient of electric slow wave over canine small bowel. Am J Physiol 218: 1468–1473, 1970 [DOI] [PubMed] [Google Scholar]
- 101.Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil 20, Suppl 1: 121–129, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol 71: 1–130, 1982 [PubMed] [Google Scholar]
- 103.van Helden DF, Laver DR, Holdsworth J, Imtiaz MS. The generation and propagation of gastric slow waves. Clin Exp Pharmacol Physiol 37: 516–524, 2010 [DOI] [PubMed] [Google Scholar]
- 104.Verhagen MAMT, van Schelven LJ, Samsom M, Smout AJPM. Pitfalls in the analysis of electrogastrographic recordings. Gastroenterology 177: 453–460, 1999 [DOI] [PubMed] [Google Scholar]
- 105.Waldo AL, Wit AL. Mechanisms of cardiac arrhythmias. Lancet 341: 1189–1193, 1993 [DOI] [PubMed] [Google Scholar]
- 106.Weisbrodt NW. Patterns of intestinal motilty. Annu Rev Physiol 43: 21–31, 1981 [DOI] [PubMed] [Google Scholar]
- 107.Yassi R, Cheng LK, Al-Ali S, Sands G, Gerneke D, LeGrice I, Pullan AJ, Windsor JA. Three-dimensional high-resolution reconstruction of the human gastro-oesophageal junction. Clin Anat 23: 287–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yassi R, O'Grady G, Paskaranandavadivel N, Du P, Angeli T, Pullan AJ, Cheng LK, Erickson J. The Gastrointestinal Electrical Mapping Suite (GEMS): software for analysing and visualising gastrointestinal multi-electrode recordings. BMC Gastroenterol 12: 60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yin J, Chen JD. Electrogastrography: methodology, validation and applications. J Neurogastroenterol Motil 19: 5–17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]