This review covers the diving response of mammals.
Abstract
The mammalian diving response is a remarkable behavior that overrides basic homeostatic reflexes. It is most studied in large aquatic mammals but is seen in all vertebrates. Pelagic mammals have developed several physiological adaptations to conserve intrinsic oxygen stores, but the apnea, bradycardia, and vasoconstriction is shared with those terrestrial and is neurally mediated. The adaptations of aquatic mammals are reviewed here as well as the neural control of cardiorespiratory physiology during diving in rodents.
The mammalian diving response is an amalgam of three independent reflexes inducing physiological changes that counter normal homeostatic control. This remarkable behavior is called the diving response (DR) since it was first studied in pelagic pinnepeds (106, 108, 215), but all aquatic mammals, including whales and dolphins, posses this response. Moreover, numerous studies have shown that all mammals, including those terrestrial (Panneton WM, unpublished observations), have a DR, from the primitive platypus (10, 112) to humans (73, 132), and can be extended to include all vertebrates (63, 218). For example the common laboratory rat maintains a brisk DR to underwater submersion (67, 159, 185); in our hands, the response is seen in 100% of rats, 100% of the time. The hypothesis that the purpose of the DR is to conserve intrinsic oxygen stores, no matter what the species, appears evident to us.
The DR is based in respiration where the animals are rendered apneic by underwater submersion. The source of the organisms' oxygen–a mammal cannot breathe underwater or it will drown–is lost underwater. Thus, to survive, underwater mammals must rely on intrinsic oxygen stores bound mostly in blood to hemoglobin and in muscle to myoglobin. The conservative use of intrinsic oxygen stores maintains aerobic metabolism; when these stores are depleted, the diving animal has reached its aerobic dive limit (ADL), a metabolic threshold where diving duration goes beyond intrinsic oxygen stores and is marked by lactate concentration in blood increasing above resting levels (22, 118, 124, 200). The cardiovascular system helps remedy this problem of anoxia. A controlled reflex of onset bradycardia, a parasympathetic response, is foremost and reduces cardiac output dramatically, which by itself would induce a precipitous drop in arterial blood pressure. Thus the sympathetic nervous system counteracts the ensuing pressure drop, and a massive peripheral vasoconstriction commences redistributing circulating blood by reducing blood flow in cutaneous, muscular, and splanchnic circulations, but a maintained or augmented flow to the central nervous system and heart (15, 94, 105, 256). Since these reflex behaviors, collectively coined the DR, are found in all vertebrates studied (69, 119), they may be the ultimate weapon organisms posses to maintain life during asphyxia.
This review will cover only the DR of mammals, although birds, especially ducks and penguins, commonly are studied. The pioneering studies on birds by Scholander (215), Folkow (70), Blix (14), Butler and Jones (25), and their colleagues have been reviewed previously (15, 26, 27, 63, 119, 123, 167, 198). It might be noted, however, that the avian responses are somewhat dissimilar to those seen in mammals in that the bradycardia and peripheral vascular responses are slower in onset than those in mammals and may be more the result of slower activation of arterial chemoreceptors rather than the rapid activation of nerves innervating the face.
Adaptations of Aquatic Mammals
Anatomical Adaptations
Several anatomical adaptations that promote diving efficiency are found in seals. Pinnepeds possess an elastic and bulbous ascending aorta (aortic bulb) hypothesized to maintain arterial pressure during the long diastolic intervals during diving bradycardia (53). The high volume of blood usually seen in aquatic mammals also must be shunted somewhere after massive peripheral vasoconstriction. Many seals have developed large venous retes in continuity with the inferior vena cava as well as an extradural intravertebral vein (169) to store such blood. The heads of dolphins also have extensive venous plexi (47), possibly for the same purpose. Many seals also have developed a caval sphincter of striated musculature near the diaphragm to regulate venous return to the heart (64). These anatomical adaptations have been reviewed previously (15, 27, 63).
Physiological Adaptations
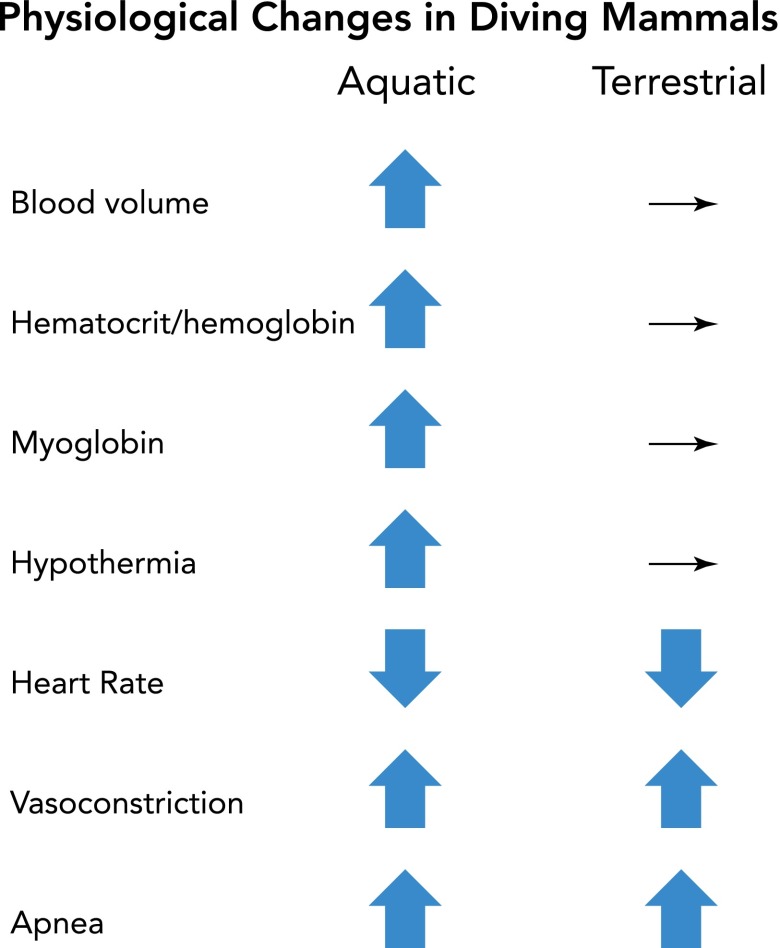
If the rationale behind the DR is to preserve intrinsic oxygen stores (FIGURE 1), it is especially important for diving aquatic mammals, which spend up to 80% of their time submerged (77, 95), to bank as much oxygen as possible. Indeed, diving mammals do this several ways. Oxygen conservation is enhanced by gliding behavior (251) or descent approaches (146) in many diving species. Behavior coordinates with physical adaptations as the animal matures; physiological factors limiting dive duration are correlated with animal size and mass (21, 100, 122). Physiological adaptations (FIGURE 2) of diving animals include increased blood volume and elevated hematocrit, hemoglobin, and myoglobin, whereas oxygen-use rates are minimized via regulation of metabolism, heart rate, and peripheral vasoconstriction (26, 27, 63, 118, 119, 121).
FIGURE 1.
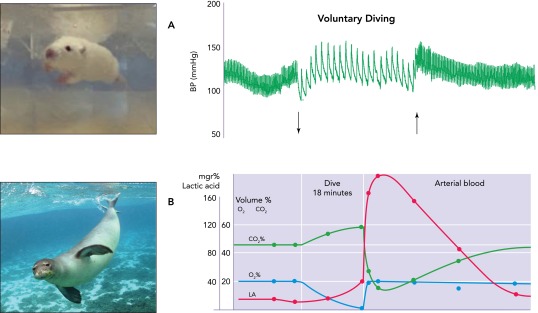
Homeostatic control of cardiovascular hemodynamics and respiration are disrupted during underwater submersion
A: note the ∼80% drop in HR when a laboratory rat (top left photograph) volunteers to dive underwater (down arrow), which persists until it surfaces (up arrow). The transitory increase in arterial blood pressure due to sympathetic activation can also be seen. B: respirations also cease during diving, inducing radical changes in blood chemistry. Po2 falls while underwater, whereas Pco2 rises dramatically. Despite this, respiratory chemoreceptors that normally would increase ventilation are muted. The hypoxia in tissues deprived of blood after the selective peripheral vasoconstriction induces anerobic metabolism, with an increase in lactic acid as by-product. Note, however, that its release into the bloodstream does not occur until after the animal surfaces, when the stringent vasoconstriction of muscular, splanchnic, and cutaneous circulations is released. B is adapted from Ref. 215 (and is used with permission) showing such changes in a seal (bottom left photomicrograph) during a dive.
FIGURE 2.
Aquatic mammals develop several changes to augment their intrinsic oxygen stores
The changes aquatic mammals develop to increase oxygen stores and decrease its utilization include increased blood volume, enhanced hematocrit, hemoglobin, and myoglobin, as well as hypothermia. Although such adaptations seldom are seen in land-bound mammals, both aquatic and terrestrial mammals share the bradycardia, vasoconstriction, and apnea characterizing the diving response.
Oxygen Storage
The size of oxygen stores as well as their rate of utilization limits aerobic dive capacity; the mammals that dive for the longest durations have the largest oxygen stores. Bert (15) first noted that diving ducks had a much larger blood volume than barnyard hens, an observation confirmed in several species (128, 177). Indeed, blood volume is at least three times greater in diving mammals. This volume is augmented by the fact that considerably more oxygen is bound to hemoglobin and myoglobin of diving mammals than in terrestrial humans (15, 63), such that ∼9.5 times more hemoglobin is found in diving mammals than in terrestrial mammals (252). Phocid seals store ∼65% of intrinsic oxygen in blood, 28% in muscle myoglobin, and 7% in the lungs (119, 166). Despite the numerous interpretational caveats for determining hematocrit and hemoglobin of mammals during diving (see Ref. 30), both increase during diving in numerous species, including those aquatic (36, 102, 246) and human (5, 66, 103, 212). The increase of hematocrit and hemoglobin during diving (202) is largely from reflex-induced contraction of the spleen, which is quite large and capable of storing inordinate amounts of red blood cells in diving mammals (28, 30, 33, 202, 246). Both hematocrit and hemoglobin also increase in response to seasons and training in semi-aquatic muskrats (137, 138, 139), suggesting increased blood oxygen stores. Besides the increase in hemoglobin and myoglobin in diving mammals, their brains contain other globins (neuroglobin and cytoglobin) that are elevated compared with terrestrial mammals (214, 252). This prompted Williams et al. (252) to propose that marine mammals recruit globins to fight hypoxia by increasing globins in their brains. Since aquatic mammals seldom dive beyond their ADL, however, diving species must utilize their oxygen stores efficiently (35), with globin and hematocrit concentrations varying depending on behavior.
Investigations have shown the larger the myoglobin oxygen store, the greater the aerobic dive duration (119, 172). Muscle biomass is large in aquatic mammals, and its metabolism is dependent on oxygen. Myoglobin is an oxygen-binding protein found in muscle tissue and is especially abundant in diving mammals, reaching 10–30 times that of terrestrial animals (38, 92, 116, 119, 215, 225). Moreover, myoglobin stores of oxygen are especially prominent in active diving muscles of aquatic mammals (115, 116). Myoglobin was found to increase significantly in cultured muscle cells from a Weddell seal in hypoxia (52) vs. control cells, suggesting skeletal muscle cells of the seal have a unique response to hypoxia than a terrestrial mammalian cell line. DeMiranda et al. (52) speculated that a combination of hypoxia, activity, and lipids act in concert to increase myoglobin stores. If diving mammals have resting oxygen saturation levels close to 100 Torr, hemoglobin has a P50 (Po2 at 50% saturation) of ∼27 Torr, whereas myoglobin has a P50 of 3 Torr (49). Thus myoglobin-bound oxygen during aerobic metabolism will be used only if the muscle becomes very hypoxic; peripheral vasoconstriction makes muscles ischemic and the resulting hypoxia promotes the myoglobin-bound oxygen to be utilized first. The DR thus maximizes the ADL at low levels of exertion. It is unknown whether myoglobin increases in laboratory rats after dive training, however. Thus “globins” increase in hypoxia, whether it be in an athlete training at high altitude or in diving mammals at sea. Utilization of intrinsic oxygen stores recently has been reviewed elegantly by Ponganis et al. (201).
Hypometabolism and Hypothermia
Hypometabolism has been suggested in diving mammals, despite the difficulty in measuring it during undersea excursions (87, 236). Diving mammals experience extreme hypoxic conditions, such that adequate levels of oxygen to supply tissues, especially the brain (205, 214, 252), are compromised. In this regard, hypothermia has been suggested as a metabolic adaptation to decreased oxygen availability (23, 24). Indeed, temperature drops in the brain during diving in seals (16, 174, 217), as does aortic blood temperature (16, 94), slowing metabolism while promoting survival. The depressed metabolism in diving also induces increases in antioxidant defenses to counteract generation of detrimental reactive oxygen species (74, 75).
Although all mammals possess a DR, not all mammals have all these physical and physiological adaptations.
Ontogeny of the Diving Response
Aquatic mammals must quickly develop both behavioral and physiological adaptations to cope with their environment. Seal pups generally explore their aquatic environment first with short shallow dives, but develop longer, deeper dives over time, which aid their foraging skills as adults (4, 17, 21, 114, 137, 157). Behavioral ontogenetic studies in fur seals indicate that diving capabilities are dependent on age for the first 6 mo of life and then generally on body mass (9, 100, 218). As aquatic mammals mature behaviorally and physically, they also mature physiologically to adapt to their environments.
Blood pH is maintained within a tight range by buffers, which neutralize effects of small amounts of acid or base. Diving mammals, however, can experience large increases in lactic acid while submerged, especially if they reach their ADL. The buffering capacity of blood of neonatal seals is comparable to that of adults, suggesting that hypoxic intrauterine environments stimulate buffering capacity prenatally (129). Immature cetaceans, pinnepeds, and penguins have only 9–31% of adult myoglobin stores (2, 173), and thus lack concentrations required for extended underwater submersion. However, myoglobin increases with age to adult stores in all species, being completed especially after extended foraging behavior underwater (173). It is thought that physical activity, thermal demands (shivering), and hypoxia all contribute to increased myoglobin. Total oxygen stores also increase seasonally in the semi-aquatic feral muskrat (137, 138), whose increased dependence on underwater diving and hypoxic winter lodges promotes myoglobin formation. However, most of these gains were in blood oxygen capacity vs. insignificant increases in myoglobin (139).
Physiological Responses to Diving
All mammals have nervous systems that regulate homeostatic control over breathing, heart rate (HR), and arterial blood pressure (ABP), but these controls are altered dramatically during diving. Mammals become apneic upon submergence and show an abrupt bradycardia with peripheral vasoconstriction that is maintained during submersion (94). Studying the physiological adaptations of pinnipeds and cetaceans are restricted, however, by limitations of working in pelagic domains on very large animals of many different species with different ontogeny in different environments. Despite these impediments, our interest has been the neural organization regulating these autonomic functions during diving. The question has been raised (49), however, as to why the physiological perturbations to cardiovascular behavior exist at all, considering that most aquatic mammals dive within their aerobic dive limit. Nevertheless, change in HR is both dramatic and temporally linked to diving behavior. Those interested in studying the physiological responses of diving may be better served by studying diving behavior in a rodent such as the common laboratory rat, a small mammal with a reliable diving response (159, 185) and about which much physiology is known. Such studies circumvent the technical impediments imposed on studies of large, pelagic diving mammals (34).
Neural Control of Diving Physiology
Data suggests that the DR consists of three independent neural reflexes regulating respiration, HR, and ABP, respectively. Pharmacological studies using antagonists/agonists show that HR and ABP responses can be blocked selectively while preserving the other two reflexes (62, 168, 254), suggesting the independence of these reflexes after peripheral blockade but implying nothing about their central integration. The three reflexes comprising the DR act harmoniously toward preserving vital oxygen stores and are initiated by activating peripheral receptors. Early studies (54, 60, 79, 106, 108, 125, 130, 213, 244, 249) noted that submersion or wetting of nasal areas was important to induce the DR, and this has been confirmed by others numerous times.
Peripheral Receptive Fields
The autonomic reflexes of the mammalian DR can be induced with only snout immersion, suggesting that primary afferent fibers innervating paranasal areas may be important. Paranasal areas (FIGURE 3), including the anterior nasal mucosa, are innervated by branches of the infraorbital nerve as well as the anterior ethmoidal nerve (AEN) from maxillary and ophthalmic divisions of the trigeminal, respectively (250). Innervation of the nasal mucosa is via free nerve endings from small-diameter fibers (39), many of which contain peptides, notably calcitonin gene-related peptide and substance P (76, 150, 151, 197, 228, 230, 237, 238), derived from trigeminal ganglion neurons (104, 151, 211, 230). Most of these fibers are sensory in function, and many respond as chemoreceptors to local chemical changes induced by inhalation of noxious gases or inflammatory processes (43, 44, 45, 89, 101).
FIGURE 3.
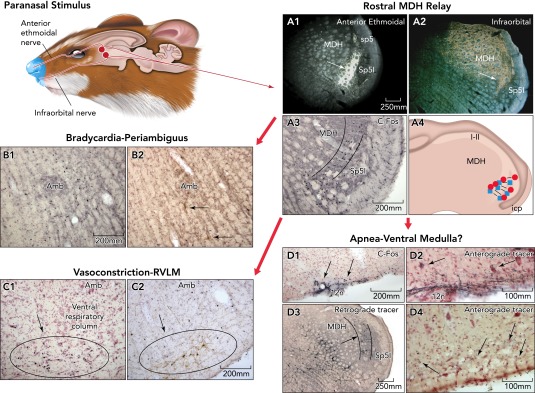
Work on diving rodents suggest paranasal areas (shaded blue) innervated by the anterior ethmoidal and infraorbital nerves are important for initiating the diving response
These nerves project [A1 and A2 show transport of an HRP cocktail (colored gold) transported transganglionically from the anterior ethmoidal and infraorbital nerves, respectively] into the rostral medullary dorsal horn (MDH) overlapping the caudal subnucleus interpolaris (Sp5I). Note the band of neuropil just dorsal to the Sp5I (arrows) is labeled from either nerve. Neurons activated with cFos (A3; small black nuclei) induced by diving are found in similar neuropil. Moreover, small, bilateral injections of lidocaine (blue squares) or kynurenate (red circles) made into similar locations (A4) blocked the cardiorespiratory responses of nasal stimulation. The hallmark of the diving response is the dramatic bradycardia (see FIGURE 1A); many neurons surrounding the rostral nucleus ambiguus are labeled with cFos after diving (B1), and some of these are preganglionic cardiac motoneurons (B2; arrows point to double-labeled neurons containing cFos and a retrograde tracer injected into the pericardial sac). There also is a massive but selective peripheral vasoconstriction during diving in rats mediated by neurons in the rostral ventrolateral medulla (C1 shows cFos-labeled neurons in the RVLM induced by diving); many such neurons are monoaminergic (C2 showing double labeled neurons with antibodies against cFos and tyrosine hydroxylase). The third neuronal reflex induced by underwater submergence is a profound apnea, which is maintained despite gross disruption of blood chemistry, suggesting inhibition of the respiratory chemoreceptor reflex. Few neurons were activated in the medullary ventral respiratory column (see C1, C2), but projections from the MDH to the ventral surface of the caudal medulla at the spinomedullary junction (D2; approximately −14.6 mm from bregma) overlap where neurons/glia activated by diving are found (D1, arrows; small black profiles show cFos activation). Arrows in D2 point to presumptive neurons with juxtaposed BDA fibers. Injection of a retrograde tracer, which included the retrotrapezoid nucleus labeled small neurons in neuropil similar to that labeled by paranasal primary afferent fibers (D3, arrow). Anterograde transport of tracers injected into these areas of the MDH resulted in extremely small labeled fibers with swellings (D4, arrows) in the retrotrapzoid nucleus ventral to the facial motor nucleus. Similar neurons/glia have long been suspected to be chemoreceptors sensitive to high Pco2, but details of how they interact with central respiratory neurons is lacking. Other studies (188) have shown the neuronal circuitry driving the diving response is contained within the medulla and spinal cord.
The AEN is considered the “gatekeeper” nerve by us since it is the first to sense noxious gases or water entering the nasal passages. Indeed, transection of the AEN eliminates the bradycardia and attenuates the apnea and ABP changes to nasal stimulation (210). This nerve contains both mechanoreceptors and chemoreceptors (136, 165, 221, 222, 223, 224, 229, 248) responsive to a variety of stimuli, with fibers of small diameter in the Aγ or C range (7, 161) that reach between the epithelial cells toward tight junctions (76, 237). The central fibers of the AEN descend in the ventral third of the spinal trigeminal tract (178, 184) and send fibers into the trigeminal sensory complex. The infra-orbital nerve also sends central fibers to all trigeminal sensory nuclei (190), but its distribution is much wider than the AEN. Although currently unknown, it is probable that similar distributions exist in aquatic mammals.
Central Integration
The sensory stimulus is linked to motor output via a reflex arc, “a route followed by nerve impulses in the production of a reflex act, from the peripheral receptor organ through the afferent nerve to the central nervous system synapse and then through the efferent nerve to the effector organ.” However, peripheral physiologists, who know the stimulus (underwater submersion) as well as the output (apnea, bradycardia, peripheral vasoconstriction) seldom explore central integration.
The trigeminal sensory complex is the principal relay for somatosensory afferent fibers innervating structures in the head (6, 140, 141, 149, 182, 227). The central projections of the AEN have been studied in several nonaquatic species (3, 98, 135, 178, 184, 220). These studies (3, 135, 178, 184, 210) generally show dense projections into superficial laminae of the medullary dorsal horn (MDH) of the spinal trigeminal nucleus (FIGURE 3A1), with less dense projections to rostral parts of the trigeminal sensory complex (178, 184). The central projections of the infraorbital nerve (FIGURE 3A2) partially overlap those of the AEN in the rostral MDH (190). Panneton et al. (191) further showed transganglionic transport of herpes simplex virus (HSV-1, strain 29) from the AEN to similar trigeminal areas, as well as transneuronal projections to brain stem autonomic nuclei in the muskrat.
The MDH is an important relay in autonomic reflexes such as the DR (179, 193, 254) and trigeminal depressor response (126, 127, 245, 255). Indeed, underwater submersion activates numerous neurons immunolabeled with cFos in the MDH (158, 183, 186) in locations similar to the termination of primary afferent fibers contained within the AEN and infraorbital nerve (FIGURE 3A3). Moreover, Panneton et al. (179, 193) showed that small injections into similar MDH areas of either lidocaine or kynurenate selectively inhibited the cardiorespiratory sequelae of nasal stimulation (FIGURE 3A4). These data promote this area of the rostral MDH as an important nexus in the reflex circuit driving the DR.
Respiration
Mammals become apneic during diving such that oxygen saturation in the blood drops routinely from 95 to 20% (128, 166, 183, 199, 202) while the animals become increasingly hypercapnic (FIGURE 1). Both changes robustly increase ventilation in animals on land, yet diving animals do not breathe. Diving mammals remain apneic despite gross alterations in blood chemistry (FIGURE 1B) that can exceed their ADL (183), suggesting the homeostatic respiratory chemoreceptor reflex is overridden. Indeed, rats submerged underwater had their PaCO2 reach 79.2 Torr (normal ∼32 Torr) and PaO2 reach 15.7 Torr (normal ∼95 Torr), yet the rats did not breathe (183). Numerous laboratories are exploring for neurons mediating the respiratory chemoreceptor circuit, but this circuit has not been characterized to date. The neuronal circuitry driving respiration is complexly organized, and its efficiency in fulfilling physiological needs is incompletely understood (72), but a reflex apnea induced with nasal stimulation persists despite truncating the brain at the pontomedullary junction (188), suggesting the neurons are contained within the medulla and spinal cord. Although influences over reflex behavior are manifested by many suprabulbar neurons, including those important in apneic reflexes (41, 55, 56, 57, 58, 203, 204, 247), we consider them modulators rather than intrinsic to the reflex diving circuit.
Indeed, respiratory behavior persists in many slice preparations of only the medulla (11, 72, 206). The medullary ventral respiratory column (8, 71) holds many respiratory neurons, and one part of it, the PreBötzinger complex, is where many neurons generating rhythm lie (11, 72, 206). Neuroanatomical projections from the part of the MDH that receives paranasal afferent fibers (184) were relatively dense to caudal parts of the ventral respiratory column where expiratory neurons dominate. More sparse projections from the MDH also were seen in the pre-Bötzinger complex (184), the retrotrapezoid nucleus (FIGURE 3D3 AND D4), and the ventral surface of the caudal medulla near the spinomedullary junction (FIGURE 3D2); the latter two groups overlapped with cells labeled with Fos in diving rats (FIGURE 3D1; Ref.183). Although there were but few neurons labeled with cFos in the ventral respiratory column (FIGURE 3C1) after underwater submersion (183), more anterograde labeling was juxtaposed to cFos-labeled “epi-glia” cells (183), and in the retrotrapezoid nucleus (FIGURE 3D4), where potential general and respiratory chemoreceptors were found throughout the medulla's ventral surface and activated by underwater submersion. Such putative chemoreceptors are linked by gap junctions (51, 235) and may provide a fast link to the brain stem respiratory network. Idiosyncrasies of the cFos technique must be considered, however (see Ref. 186 for discussion).
The inhibition of respiration while submerged despite gross fluctuations in blood gases is powerful. A persistent apnea also is created when somatostatin neurons in the pre-Bötzinger complex are silenced (241), and these same somatostatin-expressing neurons project throughout the ventral respiratory column as well as at other sites including the retrotrapezoid nucleus (242). Although the projection from the MDH to the pre-Bötzinger complex is relatively sparse, it is possible that it plays a role in inhibiting the respiratory network by both inducing and maintaining an apnea, but this must still be proven.
Heart Rate
The dramatic bradycardia seen with underwater submersion (FIGURE 1A) is mediated via the parasympathetic vagus nerve. Most parasympathetic preganglionic cardiac motoneurons are found in the external formation of the nucleus ambiguus (99, 192, 243) that provide B-fibers to principal neurons in cardiac ganglia (42, 156, 207). Double-labeling cardiac motoneurons (181) with cFos after voluntary diving and cholera toxin after retrograde transport showed most of them surrounding the rostral nucleus ambiguus (FIGURE 3B). Although cardiac motoneurons maintain several peptide and glutamate receptors (12, 13, 46, 110, 148), work in in vitro slices show preganglionic cardiac motoneurons activated by stimulation of the trigeminal tract are modulated by serotonin (84) and acetylcholine (85) receptors. However, our functional anatomical studies provide little information concerning from where cardiac motoneurons receive their input. Although our earlier studies (179, 193) suggest a relay from superficial neurons in the rostral MDH as important for mediating HR, ABP, and respiration to underwater submersion (see FIGURE 3A), the function of primary afferent terminals of the anterior ethmoidal nerve, which project beyond the trigeminal sensory complex (178, 184) into the reticular formation near the rostral nucleus ambiguus and rostral ventrolateral medulla, is still unknown.
Although the function of these fibers must still be explored, apparently many contain calcitonin gene receptor peptide and TRPV1 (40); some may provide direct projections to cardiac motoneurons. Moreover, investigations on postganglionic cardiac motoneurons driven by nasotrigeminal stimulation have commenced (155). McAllen et al. (155) recorded from principal cells in the cardiac ganglia of a working heart-brain stem preparation and showed their activation after applying cold Ringer's solution to the snout. They also concluded that convergence occurs before the postganglionic principal cells from various reflexes influencing cardiac function (arterial baroreceptor, peripheral chemoreceptor, and diving), probably within the brain stem.
Regional Blood Flow
The redistribution of blood supply in mammals in response to diving shunts blood away from hypoxia tolerant tissues to those with greater oxygen need, including in diving rats (175, 176). Blood supply to tissues/organs of diving animals is exercised at the level of arteries rather than precapillary arterioles (29), reducing the effect of vasodilator metabolites released by ischemic tissues. Indeed, near-zero conductance through the abdominal aorta has been shown in seals during diving (18). The delayed release of lactic acid during recovery (FIGURE 1B) is attributed to a striking redistribution of blood away from muscle during submersion and reperfusion after emersion. It is well known that the sympathetic nervous system controls such distribution of blood.
Numerous studies have shown that neurons in the rostral ventrolateral medulla (RVLM) regulate ABP by maintaining sympathetic tone. Moreover, studies have implicated the RVLM as the brain stem relay to the spinal cord for the baroreceptor reflex (88, 164, 219) and somatosympathetic reflexes (20). The early increase in ABP with underwater submersion activates the baroreflex but does not include baroreceptive circuitry until the RVLM since bilateral injections of kynurenate made either into the dorsolateral solitary nucleus or the caudal ventrolateral medulla (164) greatly attenuate the baroreflex but fail to modify responses from nasal stimulation. However, similar injections into the RVLM greatly reduce effects of nasal stimulation on sympathetic nerve discharge but not effects from baroreflex activation (164), since baroreceptor circuits at this level utilize GABA as a transmitter. The RVLM contains the C1 adrenergic cell group (209), and many of these neurons (FIGURE 3C2) are activated by underwater submersion (163). However, both adrenergic and nonadrenergic spinally projecting neurons in the RVLM are responsive to nasal stimulation (164). Moreover, ∼62% of the same baroreceptive RVLM neurons normally silenced by increases in ABP are excited by nasal stimulation despite increases in ABP, suggesting that the homeostatic baroreceptor reflex is overridden. The fact that the vasoconstriction imposed by underwater submersion is not universal to all tissues suggests that a general sympathetic activation does not occur. Moreover, regional specificities to different vascular beds have been proposed in the RVLM (48, 153, 154). Smaller diving mammals such as rats are compatible with laboratory experiments (159, 185) and can be utilized to explore this phenomenon better.
Suprabulbar Control of the Diving Response
Aquatic mammals have repeatedly shown that they “control” the bradycardia induced, despite the fact the heart is a visceral organ generally considered under autonomic, or automatic, control. Indeed, we initially decided to study the mammalian DR after seeing that seals showed an abrupt bradycardia before underwater submersion and a tachycardia prior to emersion (32). Such control over their “autonomic” nervous system has been noticed by diving physiologists for some time (15, 16). Indeed, seals often either show little bradycardia when diving voluntarily, reduce heart rate in anticipation of underwater submersion (32), induce a bradycardia to auditory, photic, or painful stimulation (108), or show an anticipatory tachycardia before emerging (15, 32). Moreover, sea lions conditioned to adjust their autonomic nervous systems to auditory or visual commands suggest they may “will” bradycardia (200, 208), probably from suprabulbar sites. Control of bradycardia develops through ontogeny (37, 68, 86, 90, 100, 107, 157, 170), and minimum HR is increased in older animals compared with younger cohorts (86, 170) but has a caveat in that dive duration is generally longer in mature animals that have more mature adaptations in blood volume, myoglobin, etc. Bradycardia of diving is also variable in dolphins depending on behavior. If a submerged mammal is exercising, the degree of bradycardia developed is dependent on exercise levels (50, 171); the diving bradycardia is modulated by behavior and exercise in a predictable manner (171).
Since underwater submergence is the usual stimulus inducing the DR in awake animals, many investigators performed “forced” submersions, where the animals were tethered on boards or placed in cages and dunked underwater (54, 59, 94, 111, 113, 120, 125, 130, 131, 147, 185, 213, 216). Many of these studies noted the hemodynamic responses were subtly dissimilar to voluntary submersions (94, 111, 162, 244). HR generally reaches a nadir quickly in forced submersions and remains depressed until the animal surfaces. However, the bradycardia of aquatic mammals diving voluntarily and with but moderate exercise is more variable and less intense than that seen in forced diving (54, 111, 119, 162, 185), but the bradycardia of involuntarily dunked rats is not different than voluntarily diving rats (185). Although such differences suggest neural control beyond the level of reflex and implicate suprabulbar control in large aquatic mammals, it suggests the response in the rodent is more reflexive in nature.
It is possible that preventing an organism from deciding its own fate by involuntary submersion may induce either fear or stress in several species (31, 78, 231, 233, 234, 257), and these emotions may alter normal reflex responses. McCulloch and colleagues (160) concluded that forced submergence is stressful to both naive and trained rats, but voluntary diving in trained rats is no more stressful than being handled by humans. The bradycardia seen in rats is locked tightly to the time submerged, but hemodynamic changes were more variable in dunked naive rats and included more arrhythmias (183). It is of interest that co-activation of both parasympathetic and sympathetic cardiac nerves induces cardiac arrhythmias (194, 195, 226). Although the bradycardia of voluntary diving is vagally mediated and dominant, forced underwater submersion stresses the animal and probably also activates the sympathetic nervous system from sources beyond reflex diving. Perhaps the numerous arrhythmias seen during forced diving are induced by suprabulbar sources and counter the bradycardia of underwater submersion.
It is of interest that cetaceans and pinnepeds have brains that approach human brains in complexity with highly convoluted cortices (65, 96, 97, 142, 143, 144, 145). Although the DR apparently has minimal suprabulbar modulation in rodents, we suggest suprabulbar neurons in higher species may indeed direct autonomic behavior seen in the DR. The importance of such control highlights deflection from dogma that control of the “autonomic” nervous system is involuntary. Harnessing the suprabulbar circuits of origin that control diving physiology may promote feedback therapies designed to remedy high HR and hypertension associated with anxiety.
Summary and Perspectives
The autonomic changes resulting from underwater submersion are dramatic and swift, suggesting reflex circuitry with little integration and few synapses in rodents. If the area of the rostral MDH where primary afferent fibers innervating paranasal areas project is considered the locus from which the cardiac, blood pressure, and respiratory consequences of underwater immersion emanate, the route of such fibers toward the brain stem targets mediating the responses must be considered. First, the very small neurons found in the aforementioned MDH neuropil are considered the rostral extension of the substantia gelatinosa (laminae I and II), which has been misplaced dorsally and medially by the caudal pole of the subnucleus interpolaris. Lamina II neurons are very small, and very few have been shown to be projection neurons. However, many with paranasal receptive fields indeed are projection neurons going to cardiorespiratory brain stem nuclei, implicating the importance of the DR and supporting a role in early mammalian evolution. We show numerous projections from these neurons (see Ref. 184 and FIGURE 3D3) to areas of the medulla regulating cardiorespiratory behavior. These small neurons also probably have very small axons see (FIGURE 3D4); if they are below 1 μm in diameter, they are beyond the resolution of the light microscope, which implies their identify must be obtained by other means.
That said, the increase in arterial blood pressure with diving probably is mediated by very small fibers projecting from neurons in the rostral MDH to the RVLM. The caudal pressor area (239, 240) also receives such projections (187) but apparently does not mediate the increase in arterial blood pressure since pressure increases are still seen with nasal stimulation after inhibiting injections of glycine, muscimol, or ibotenic acid (189) are placed in this caudal pressor area. Although retrograde transport of tracers injected into the RVLM is found in numerous neurons in this paranasal part of the MDH, such projections were not matched in intensity by the relatively sparse anterorgrade transport from the MDH (184). This perhaps also may be due to the extremely small axons of these MDH neurons, making them difficult to see with the light microscope. The ventral respiratory column and other associated respiratory-related nuclei (e.g., ventrolateral solitary nucleus, retrotrapezoid nucleus) were all labeled using retrograde and anterograde transport techniques, albeit sparsely with anterograde methods and mostly by small-diameter fibers. Although there were but few neurons labeled with cFos in the ventral respiratory column, numerous neurons considered presumptive chemoreceptors were labeled. We suggest that apnea of diving is induced by inhibitory MDH projections to the pre-Bötzinger complex, but this still must be proven. The bradycardia of diving must at least partially be relayed through the MDH, since it can be inhibited after injections there but with the caveat that injections of ibotenic acid into the nearby caudal pressor area also eliminate the bradycardia to nasal stimulation. The role played by the extratrigeminal projections of the AEN into the rostral and caudal ventrolateral medulla must still be deciphered, but these also may augment the cardiovascular responses.
The DR in humans often is deployed in biology/physiology laboratories since HR is easily monitored (19, 93). The HR responses of such exercises, however, are variable, perhaps because of suprabulbar control over this reflex behavior (253). Nevertheless, the DR is prominent in human neonates (82, 196) and is suggested as the etiology of “cold water drownings” (80, 83, 91), the Sudden Infant Death Syndrome (1, 133, 134, 152, 232), as well as important in apnea attacks in children (61, 81, 117). The DR is the most powerful autonomic reflex known, and further study of this phenomenon, especially its neural control (180), may be fruitful for understanding how the brain controls autonomic behavior. It is also important to explain why the DR is universal in all vertebrates. Is the purpose of this enigmatic response designed to preserve life by conserving oxygen? Is it the “master switch of life” (216)? Although neurophilosophers can debate these issues, physiologists perhaps could explore genetic databases to determine whether code was developed very early in the evolution of vertebrates to promote species survival. The remarkable mammalian diving response is a chapter worth studying.
Acknowledgments
This work would not have been possible without the technical help of Qi Gan and Rajko Juric as well as numerous students of the nervous system. In the author's opinion, Physiology is not referenced enough.
Footnotes
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-38471 and HL-64772, and an IRSF grant from Saint Louis University.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: W.M.P. conception and design of research; W.M.P. performed experiments; W.M.P. analyzed data; W.M.P. interpreted results of experiments; W.M.P. prepared figures; W.M.P. drafted manuscript; W.M.P. edited and revised manuscript; W.M.P. approved final version of manuscript.
References
- 1.Allen LG, Howard G, Smith JB, McCubbin JA, Weaver RL. Infant heart rate responses to trigeminal airstream stimulation: determination of normal and deviant values. Pediatr Res 13: 184–187, 1979 [DOI] [PubMed] [Google Scholar]
- 2.Andrews RD, Jones DR, Williams JD, Thorson PH, Oliver GW, Costa DP, Le Boeuf BJ. Heart rates of northern elephant seals diving at sea and resting on the beach. J Exp Biol 200: 2083–2095, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Anton F, Peppel P. Central projections of trigeminal primary afferents innervating the nasal mucosa: a horseradish peroxidase study in the rat. Neuroscience 41: 617–628, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Baker JD, Donohue MJ. Ontogeny of swimming and diving in northern fur seal (Callorhinus ursinus) pups. Can J Zool 78: 100–109, 2000 [Google Scholar]
- 5.Bakovic D, Valic Z, Eterovic D, Vukovic I, Obad A, Marinovic Terzic I, Dujic Z. Spleen volume and blood flow response to repeated breath-hold apneas. J Appl Physiol 95: 1460–1466, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Beckstead RM, Norgren R. An autoradiographic examination of the central distribution of the trigeminal, facial, glossopharyngeal, and vagal nerves in the monkey. J Comp Neurol 184: 455–472, 1979 [DOI] [PubMed] [Google Scholar]
- 7.Beidenbach MA, Beuerman RW, Brown AC. Graphic-digitizer analysis of axon spectra in ethmoidal and lingual branches of the trigeminal nerve. Cell Tiss Res 157: 341–352, 1975 [DOI] [PubMed] [Google Scholar]
- 8.Benarroch EE. Brainstem respiratory sensitivity: new insights and clinical implications. Neurol 68: 2140–2143, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bennett KA, McConnell BJ, Moss SEW, Speakman JR, Pomeroy PR, fedak MA. Effects of age and body mass on development of diving capabilities of gray seal pups: costs and benefits of the postweaning fast. Physiol Biochem Zool 83: 911–923, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Bethge P, Munks S, Otley H, Nicol S. Diving behaviour, dive cycles and aerobic dive limit in the platypus Ornithorhynchus anatinus. Comp Biochem Physiol A Mol Integr Physiol 136: 799–809, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–46, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Blinder KJ, Johnson TA, Massari VJ. Enkephalins and functionally specific vagal preganglionic neurons to the heart: ultrastructural studies in the cat. Auton Neurosci 120: 52–61, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Blinder KJ, Moore CT, Johnson TA, Massari VJ. Central control of atrio-ventricular conduction and left ventricular contractility in the cat heart: synaptic interactions of vagal preganglionic neurons in the nucleus ambiguus with neuropeptide Y-immunoreactive nerve terminals. Auton Neurosci 131: 57–64, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Blix AS. The importance of asphyxia for the development of diving bradycardia in ducks. Acta Physiol Scand 95: 41–45, 1975 [DOI] [PubMed] [Google Scholar]
- 15.Blix AS, Folkow B. Cardiovascular adjustments to diving in mammals and birds. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, vol. III, pt. 2, chapt. 25, p. 917–945 [Google Scholar]
- 16.Blix AS, Wallře L, Messelt EB, Folkow L. Selective brain cooling and its vascular basis in diving seals. J Exp Biol 213: 2610–2613, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Bowen WD, Boness DJ, Iverson SJ. Diving behaviour of lactating harbour seals and their pups during maternal foraging trips. Can J Zool 77: 978–988, 1999 [Google Scholar]
- 18.Bron KM, Murdaugh HV, Millen JE, Lentall R, Rzaskin P, Robin ED. Arterial constrictor response in a diving mammal. Science 152: 540–543, 1966 [DOI] [PubMed] [Google Scholar]
- 19.Bruce DS, Speck DF. Human simulated diving experiments. Physiologist 5: 39–40, 1979 [PubMed] [Google Scholar]
- 20.Burke PGR, Neale JKWS, McMullan SGAK. Patterning of somatosympathetic reflexes reveals nonuniform organization of presympathetic drive from C1 and non-C1 RVLM neurons. Am J Physiol Regul Integr Comp Physiol 301: R1112–R1122, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Burns JM. The development of diving behavior in juvenile Weddell seals: pushing physiological limits in order to survive. Can J Zool 77: 737–747, 1999 [Google Scholar]
- 22.Burns JM, Castellini MA. Physiological and behavioral determinants of the aerobic dive limit in Weddell seal (Leptonychotes weddellii) pups. J Comp Physiol B 166: 473–483, 1996 [Google Scholar]
- 23.Butler PJ. Metabolic regulation in diving birds and mammals. Respir Physiol Neurobiol 141: 297–315, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Butler PJ, Bevan RM, Woakes AJ, Croxall JP, Boyd IL. The use of data loggers to determine the energetics and physiology of aquatic birds and mammals. Braz J Med Biol Res 28: 1307–1317, 1995 [PubMed] [Google Scholar]
- 25.Butler PJ, Jones DR. Onset of recovery from diving bradycardia in ducks. J Physiol 196: 255–272, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler PJ, Jones DR. The comparative physiology of diving in vertebrates. Adv Comp Physiol Biochem 8: 179–364, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Butler PJ, Jones DR. Physiology of diving of birds and mammals. Physiol Rev 77: 837–899, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Cabanac A, Folkow LP, Blix AS. Volume capacity and contraction control of the seal spleen. J Appl Physiol 82: 1989–1994, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Cabanac A, Folkow LP, Blix AS. Effects of adrenergic and cholinergic drugs on splenic arteries and veins from hooded seals (Cystophora cristata). Comp Biochem Physiol 120: 277–281, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Cabanac AJ. Blood volume in hooded seals: implications for diving capacity. Can J Zool 78: 1293–1299, 2000 [Google Scholar]
- 31.Carrive P. Conditioned fear to environmental context: cardiovascular and behavioral components in the rat. Brain Res 858: 440–445, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Casson DM, Ronald K. The harp seal, Pagophilus groenlandicus. XIV. Cardiac arrhthmias. Comp Biochem Physiol A 50: 307–314, 1975 [DOI] [PubMed] [Google Scholar]
- 33.Castellini JM, Castellini MA. Estimation of splenic volume and its relationship to long-duration apnea in seals. Physiol Zool 66: 619–627, 1993 [Google Scholar]
- 34.Castellini M. History of polar whaling: insights into the physiology of the great whales. Comp Biochem Physiol A 126: 153–159, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Castellini MA, Baskurt O, Castellini JM, Meiselman HJ. Blood rheology in marine animals. Front Physiol 1: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castellini MA, Costa DP, Huntley A. Hematocrit variation during sleep apnea in elephant seal pups. Am J Physiol Regul Integr Comp Physiol 251: R429–R431, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Castellini MA, Milsom WK, Berger RJ, Costa DP, Jones DR, Castellini JM, Rea LD, Bharma S, Harris M. Patterns of respiration and heart rate during wakefulness and sleep in elephant seal pups. Am J Physiol Regul Integr Comp Physiol 266: R863–R869, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Castellini MA, Somero GN. Buffering capacity of vertebrate muscle: correlations with potentials for anaerobic function. J Comp Physiol B 143: 191–198, 1981 [Google Scholar]
- 39.Cauna N, Hinderer KH, Wentges RT. Sensory receptor organs of the human nasal respiratory mucosa. Am J Anat 124: 187–210, 1969 [DOI] [PubMed] [Google Scholar]
- 40.Cavanaugh DJ, Chesler AT, Bráz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in non-peptidergic neurons. J Neurosci 31: 10119–10127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chamberlin NL, Saper CB. A brainstem network mediating apneic reflexes in the rat. J Neurosci 18: 6048–6056, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng ZX, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol 424: 588–606, 2000 [PubMed] [Google Scholar]
- 43.Cometto-Muniz JE, Cain WS, Abraham MH. Nasal pungency and odor of homologous aldehydes and carbosylic acids. Exp Brain Res 118: 180–188, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Cometto-Muniz JE, Cain WS, Abraham MH, Gola JMR. Ocular and nasal trigeminal detection of butyl acetate and toluene presented singly and in mixtures. Toxicol Sci 63: 233–244, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Cometto-Muńiz JE, Cain WS. Agonistic sensory effects of airborne chemicals in mixtures: Odor, nasal pungency, and eye irritation. Percept Psychophys 59: 665–674, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Corbett EKA, Saha S, Deuchars J, McWilliam PN, Batten TFC. Ionotropic glutamate receptor subunit immunoreactivity of vagal preganglionic neurones projecting to the rat heart. Auton Neurosci 105: 105–117, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Costidis A, Rommel SA. Vascularization of air sinuses and fat bodies in the head of Bottlenose dolphin (Tursiops truncatus): morphological implications on physiology. Front Physiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dampney RAL, McAllen RM. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J Physiol 395: 41–56, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis RW, Polasek L, Watson R, Fuson A, Williams TM, Kanatous SB. The diving paradox: new insights into the role of the dive response in air-breathing vertebrates. Comp Biochem Physiol A Mol Integr Physiol 138: 263–268, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Davis RW, Williams TM. The marine mammal dive response is exercise modulated to maximize aerobic dive duration. J Comp Physiol A 198: 583–591, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Dean JB, Ballantyne D, Cardone DL, Erlichman JS, Solomon IC. Role of gap junctions in CO2 chemoreception and respiratory control. Am J Physiol Lung Cell Mol Physiol 283: L665–L670, 2002 [DOI] [PubMed] [Google Scholar]
- 52.DeMiranda MA, Schlater AE, Green TL, Kanatous SB. In the face of hypoxia: myoglobin increases in response to hypoxic conditions and lipid supplementation in cultured Weddell seal skeletal muscle cells. J Exp Biol 215: 808–813, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Drabek CM, Burns JM. Heart and aorta morphology of the deep-diving hooded seal (Cystophora cristata). Can J Zool 80: 2030–2036, 2002 [Google Scholar]
- 54.Drummond PC, Jones DR. The initiation and maintenance of bradycardia in a diving mammal, the muskrat, Ondatra zibethica. J Physiol 290: 253–271, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dutschmann M, Herbert H. The Kölliker-Fuse nucleus mediates the trigeminally induced apnoea in the rat. Neuroreport 7: 1432–1436, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Dutschmann M, Herbert H. Fos expression in the rat parabrachial and Kölliker-Fuse nuclei after electrical stimulation of the trigeminal ethmoidal nerve and water stimulation of the nasal mucosa. Exp Brain Res 117: 97–110, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Dutschmann M, Herbert H. NMDA and GABAA receptors in the rat Kolliker-Fuse area control cardiorespiratory responses evoked by trigeminal ethmoidal nerve stimulation. J Physiol 510: 793–804, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dutschmann M, Herbert H. Pontine cholinergic mechanisms enhance trigeminally evoked respiratory suppression in the anesthetized rat. J Appl Physiol 87: 1059–1065, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Dykes RW. Factors related to the dive reflex in harbor seals: respiration, immersion, bradycardia, and lability of the heart rate. Can J Physiol Pharmacol 52: 248–257, 1974 [DOI] [PubMed] [Google Scholar]
- 60.Dykes RW. Factors related to the dive reflex in harbor seals: sensory contributions from the trigeminal region. Can J Physiol Pharmacol 52: 259–265, 1974 [DOI] [PubMed] [Google Scholar]
- 61.Edner A, Ericson M, Milerad J, Katz-Salamon M. Abnormal heart rate response to hypercapnia in boys with an apparent life-threatening event. Acta Paediatr 91: 1318–1323, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Elliott NM, Andrews RD, Jones DR. Pharmacological blockade of the dive response: effects on heart rate and diving behaviour in the harbour seal (Phoca vitulina). J Exp Biol 205: 3757–3765, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Elsner R, Gooden B. Diving and Asphyxia: A Comparative Study of Animals and Man. New York: Cambridge Univ. Press, 1983, p. 1–168 [PubMed] [Google Scholar]
- 64.Elsner R, Hanafee WN, Hammond DD. Angiography of the inferior vena cava of the harbor seal during simulated diving. Am J Physiol 220: 1155–1157, 1971 [DOI] [PubMed] [Google Scholar]
- 65.Eriksen N, Pakkenberg B. Total neocortical cell number in the mysticete brain. Anat Rec (Hoboken) 290: 83–95, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Espersen K, Frandsen H, Lorentzen T, Kanstrup IL, Christensen NJ. The human spleen as an erythrocyte reservoir in diving-related interventions. J Appl Physiol 92: 2071–2079, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Fahlman A, Bostrom BL, Dillon KH, Jones DR. The genetic component of the forced diving bradycardia response in mammals. Front Physiol 2: 63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falabella V, Lewis M, Campagna C. Development of cardiorespiratory patterns associated with terrestrial apneas in free-ranging southern elephant seals. Physiol Biochem Zool 72: 64–70, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Farrell AP. Tribute to PL Lutz: a message from the heart: why hypoxic bradycardia in fishes? J Exp Biol 210: 1715–1725, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Feigl E, Folkow B. Cardiovascular responses in “diving” and during brain stimulation in ducks. Acta Physiol Scand 57: 99–110, 1963 [DOI] [PubMed] [Google Scholar]
- 71.Feldman JL. Neurophysiology of breathing in mammals. In: Handbook of Physiology. The Nervous System. Intrinsic Regulatory Systems of the Brain. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 1, vol. IV, chapt. 9, p. 463–524 [Google Scholar]
- 72.Feldman JL, Del Negro CA, Gray PA. Understanding the rhytm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferretti G. Extreme human breath-hold diving. Eur J Appl Physiol 84: 254–271, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Filho DW, Torres MA, Marcon JL, Fraga CG, Boveris A. Comparative antioxidant defences in vertebrates-emphasis on fish and mammals. Trends Comp Biochem Physiol 7: 33–45, 2000 [Google Scholar]
- 75.Filho WD, Sell F, Ribeiro L, Ghislandi M, Carrasquedo F, Fraga CG, Wallauer JP, Simoes-Lopes PC, Uhart MM. Comparison between the antioxidant status of terrestrial and diving mammals. Comp Biochem Physiol A 133: 885–892, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Finger TE, St Jeor VL, Kinnamon JC, Silver WL. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol 294: 293–305, 1990 [DOI] [PubMed] [Google Scholar]
- 77.Folkow LP, Blix LP. Diving behavior of hooded seals (Cystophora cristata) in the Greenland and Norwegian Seas. Polar Biol 22: 61–74, 1999 [Google Scholar]
- 78.Gabrielsen G, Kanwisher J, Steen JB. “Emotional” bradycardia: a telemetry study on incubating willow grouse (Lagopus laopus). Acta Physiol Scand 100: 255–257, 1977 [DOI] [PubMed] [Google Scholar]
- 79.Gandevia SC, McCloskey DI, Potter EK. Reflex bradycardia occurring in response to diving, nasopharyngeal stimulation and ocular pressure, and its modification by respiration and swallowing. J Physiol 276: 383–394, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giesbrecht GG. Cold stress, near drowning and accidental hypothermia: a review. Aviat Space Environ Med 71: 733–52, 2000 [PubMed] [Google Scholar]
- 81.Girling DJ. Changes in heart rate, blood pressure, and pulse pressure during apnoeic attacks in newborn babies. Arch Dis Child 47: 405–410, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goksör E, Rosengren L, Wennergren G. Bradycardic response during submersion in infant swimming. Acta Paediatr 91: 307–312, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Golden FS, Tipton MJ, Scott RC. Immersion, near-drowning and drowning. Br J Anaesth 79: 214–225, 1997 [DOI] [PubMed] [Google Scholar]
- 84.Gorini C, Jameson HS, Mendelowitz D. Serotonergic modulation of the trigeminocardiac reflex neurotransmission to cardiac vagal neurons in the nucleus ambiguus. J Neurophysiol 102: 1443–50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gorini C, Philbin K, Bateman R, Mendelowitz D. Endogenous inhibition of the trigeminally evoked neurotransmission to cardiac vagal neurons by muscarinic acetylcholine receptors. J Neurophysiol 104: 1841–1848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greaves DK, Schreer JF, Hammill MO, Burns JM. Diving heart rate development in postnatal harbour seals, Phoca vitulina. Physiol Biochem Zool 78: 9–17, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Guppy M, Hill RD, Schneider RC, Qvist J, Liggins GC, Sapol WM, Hochachka PW. Microcomputer-assisted metabolic studies of voluntary diving of Weddell seals. Am J Physiol Regul Integr Comp Physiol 250: R175–R187, 1986 [DOI] [PubMed] [Google Scholar]
- 88.Guyenet PG. Role of the ventrolateteral medulla oblongata in blood pressure regulation. In: Central Regulation of Autonomic Function, edited by Loewy AD, Spyer KM. New York: Oxford Univ. Press, 1990, p. 145–167 [Google Scholar]
- 89.Handwerker HO, Kobal G. Psychophysiology of experimentally induced pain. Physiol Rev 73: 639–671, 1993 [DOI] [PubMed] [Google Scholar]
- 90.Harrison RJ, Tomlinson JDW. Normal and experimental diving in the common seal (Phoca vitulina). Mammalia 24: 386–399, 1960 [Google Scholar]
- 91.Hayward JS, Hay C, Matthews BR, Overweel CH, Radford DD. Temperature effect on the human dive response in relation to cold water near-drowning. J Appl Physiol 56: 202–206, 1984 [DOI] [PubMed] [Google Scholar]
- 92.Helbo S, Fago A. Functional properties of myoglobins from five whale species with different diving capacities. J Exp Biol 215: 3403–3410, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Hiebert SM, Burch E. Simulated human diving and heart rate: making the most of the diving response as a laboratory exercise. Adv Physiol Educ 27: 130–145, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Hill RD, Schneider RC, Liggins GC, Schuette AH, Elliott RL, Guppy M, Hochachka PW, Qvist J, Falke KJ, Zapol WM. Heart rate and body temperature during free diving of Weddell seals. Am J Physiol Regul Integr Comp Physiol 253: R344–R351, 1987 [DOI] [PubMed] [Google Scholar]
- 95.Hindell MA, Slip DJ, Burton HR. The diving behavior of adult male and female southern elephant seals, Mirounga leonina (Pinnipedia: Phocidae). Austr J Zool 39: 595–619, 1991 [Google Scholar]
- 96.Hof PR, Chanis R, Marino L. Cortical complexity in cetacean brains. Anat Rec A Discov Mol Cell Evol Biol 287: 1142–1152, 2005 [DOI] [PubMed] [Google Scholar]
- 97.Hof PR, Van der Gucht E. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae). Anat Rec (Hoboken) 290: 1–31, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Hollandsworth MP, DiNovo KM, McCulloch PF. Unmyelinated fibers of the anterior nerve of the rat co-localize with neurons in the medullary dorsal horn and ventrolateral medulla activated by nasal stimulation. Brain Res 1298: 131–144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hopkins DA, Armour JA. Brainstem cells of origin of physiologically identified cardiopulmonary nerves in the rhesus monkey (Macaca mulatta). J Auton Nerv Syst 68: 21–32, 1998 [DOI] [PubMed] [Google Scholar]
- 100.Horning M, Trillmich F. Ontogeny of diving behaviour in the Galapagos fur seal. Behaviour 134: 1211–1257, 1997 [Google Scholar]
- 101.Hummel T, Mohammadian P, Marchl R, Kobal G, Loetsch J. Pain in the trigeminal system: irritation of the nasal mucosa using short- and long-lasting stimuli. Int J Psychophysiol 47: 147–158, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Hurford WE, Hochachka PW, Schneider RC, Guyton GP, Stanek KS, Zapol DG, Liggins GC, Zapol WM. Splenic contraction, catecholamine release, and blood volume redistribution during diving in the Weddell seal. J Appl Physiol 80: 298–306, 1996 [DOI] [PubMed] [Google Scholar]
- 103.Hurford WE, Hong SK, Park YS, Ahn DW, Shiraki K, Mohri M, Zapol WM. Splenic contraction during breath-hold diving in the Korean ama. J Appl Physiol 69: 932–936, 1990 [DOI] [PubMed] [Google Scholar]
- 104.Ichikawa H, Mitani S, Hijiya H, Nakago T, Jacobowitz DM, Sugimoto T. Calretinin-immunoreactivity in trigeminal neurons innervating the nasal mucosa of the rat. Brain Res 629: 231–238, 1993 [DOI] [PubMed] [Google Scholar]
- 105.Irving L. Changes in the blood flow through the brain and muscles during the arrest of breathing. Am J Physiol 122: 207–214, 1938 [Google Scholar]
- 106.Irving L. Respiration in diving mammals. Physiol Rev 19: 112–134, 1939 [Google Scholar]
- 107.Irving L, Peyton LJ, Bahn CH, Peterson RS. Action of the heart and breathing during the development of fur seals (Callorhinus usinus). Physiol Zool 36: 1–20, 1963 [Google Scholar]
- 108.Irving L, Scholander PF, Grinnell SW. The regulation of arterial blood pressure in the seal during diving. Am J Physiol 135: 557–566, 1942 [Google Scholar]
- 109.Itaya SK, Williams TH, Engel EL. Anterograde transport of horseradish peroxidase enhanced by poly-l-ornithine. Brain Res 150: 170–176, 1978 [DOI] [PubMed] [Google Scholar]
- 110.Izzo PN, Deuchars J, Spyer KM. Localization of cardiac vagal preganglionic motoneurons in the rat: immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. J Comp Neurol 327: 572–583, 1993 [DOI] [PubMed] [Google Scholar]
- 111.Jobsis PD, Ponganis PJ, Kooyman GL. Effects of training on forced submersion responses in harbor seals. J Exp Biol 204: 3877–3885, 2001 [DOI] [PubMed] [Google Scholar]
- 112.Johansen K, Lenfant C, Grigg GC. Respiratory properties of blood and responses to diving of the platypus, Ornithorhynchus anatinus (Shaw). Comp Biochem Physiol 18: 597–608, 1966 [DOI] [PubMed] [Google Scholar]
- 113.Jones DR, Furilla RA, Heieis MR, Cabbott A, Smith FM. The effect of the stress of forcible submergence on the diving response in muskrats (Ondatra zibethica). Can J Zool 60: 187–193, 1982 [Google Scholar]
- 114.Jorgensen C, Lydersen C, Brix O, Kovacs KA. Diving development in nursing harbour seal pups. J Exp Biol 204: 3993–4004, 2001 [DOI] [PubMed] [Google Scholar]
- 115.Kanatous SB, Davis RW, Watson R, Polasek L, Williams TM, Mathieu-Costello O. Aerobic capacities in the skeletal muscles of Weddell seals: key to longer dive durations? J Exp Biol 205: 3601–3608, 2002 [DOI] [PubMed] [Google Scholar]
- 116.Kanatous SB, DiMichele LV, Cowan DF, Davis RW. High aerobic capacities in the skeletal muscles of pinnipeds: adaptations to diving hypoxia. J Appl Physiol 86: 1247–1256, 1999 [DOI] [PubMed] [Google Scholar]
- 117.Katz-Salamon M. Delayed chemoreceptor responses in infants with apnoea. Arch Dis Child 89: 261–266, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kooyman GL. Physiology without restraint in diving mammals. Mar Mammal Sci 1: 166–178, 1985 [Google Scholar]
- 119.Kooyman GL. Diverse Divers: Physiology and Behavior. Berlin, Germany: Springer-Verlag, 1989 [Google Scholar]
- 120.Kooyman GL, Campbell WB. Heart rates in freely diving Weddell seals. Comp Biochem Physiol A 43: 31–36, 1972 [DOI] [PubMed] [Google Scholar]
- 121.Kooyman GL, Castellini MA, Davis RW. Physiology of diving in marine mammals. Annu Rev Physiol 43: 343–356, 1981 [DOI] [PubMed] [Google Scholar]
- 122.Kooyman GL, Castellini MA, Davis RW, Maue RA. Aerobic dive limits in immature Weddell seals. J Comp Physiol A 151: 171–174, 1983 [Google Scholar]
- 123.Kooyman GL, Ponganis PJ. The physiological basis of diving to depth: Birds and mammals. Ann Rev Physiol 60: 19–32, 1998 [DOI] [PubMed] [Google Scholar]
- 124.Kooyman GL, Wahrenbrock EA, Castellini MA, Davis RW, Sinnett EE. Aerobic and anaerobic metabolism during diving in Weddell seals: evidence of preferred pathways from blood chemistry and behavior. J Comp Physiol A 138: 335–346, 1980 [Google Scholar]
- 125.Koppányi T, Dooley MS. Submergence and postural apnea in the muskrat. Am J Physiol 88: 592–595, 1929 [Google Scholar]
- 126.Kumada M, Dampney RAL, Reis DJ. The trigeminal depressor response: a cardiovascular reflex originating from the trigeminal system. Brain Res 92: 485–489, 1975 [DOI] [PubMed] [Google Scholar]
- 127.Kumada M, Dampney RAL, Reis DJ. The trigeminal depressor response: a novel vasodepressor response originating from the trigeminal system. Brain Res 119: 305–326, 1977 [DOI] [PubMed] [Google Scholar]
- 128.Lenfant C, Johansen K, Torrence JD. Gas transport and oxygen storage capacity in some pinnepeds and the sea otter. Resp Physiol 9: 277–286, 1970 [DOI] [PubMed] [Google Scholar]
- 129.Lestyk KC, Folkow LP, Blix AS, Hammill MO, Burns JM. Development of myoglobin concentration and acid buffering capacity in harp (Pagophilus groenlandicus) and hooded (Cysophora cristata) seals from birth to maturity. J Comp Physiol B 179: 985–996, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Lin YC. Autonomic nervous control of cardiovascular response during diving in the rat. Am J Physiol 227: 601–605, 1974 [DOI] [PubMed] [Google Scholar]
- 131.Lin YC, Baker DG. Cardiac output and its distribution during diving in the rat. Am J Physiol 228: 733–737, 1975 [DOI] [PubMed] [Google Scholar]
- 132.Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol 106: 284–292, 2009 [DOI] [PubMed] [Google Scholar]
- 133.Lobban CD. The oxygen-conserving dive reflex re-examined as the principal contributory factor in sudden infant death. Med Hypotheses 44: 273–277, 1995 [DOI] [PubMed] [Google Scholar]
- 134.Lobban CDR. The human dive reflex as a primary cause of SIDS: a review of the literature. Med J Aust 155: 561–563, 1991 [PubMed] [Google Scholar]
- 135.Lucier GE, Egizii R. Central projections of the ethmoidal nerve of the cat as determined by the horseradish peroxidase tracer technique. J Comp Neurol 247: 123–132, 1986 [DOI] [PubMed] [Google Scholar]
- 136.Lucier GE, Egizii R. Characterization of cat nasal afferents and brain stem neurones receiving ethmoidal input. Exp Neurol 103: 83–89, 1989 [DOI] [PubMed] [Google Scholar]
- 137.MacArthur RA. Seasonal changes in the oxygen storage capacity and aerobic dive limits of the muskrat (Ondatra zibethicus). J Comp Physiol B 160: 593–599, 1990 [Google Scholar]
- 138.MacArthur RA, Humphries MM, Fines GA, Campbell KL. Body oxygen stores, aerobic dive limits, and the diving abilities of juvenile and adult muskrats (Ondatra zibethicus). Physiol Biochem Zool 74: 178–190, 2001 [DOI] [PubMed] [Google Scholar]
- 139.MacArthur RA, Weseen GL, Campbell KL. Diving experience and the aerobic dive capacity of muskrats: does training produce a better diver? J Exp Biol 206: 1153–1161, 2003 [DOI] [PubMed] [Google Scholar]
- 140.Marfurt CF. The central projections of trigeminal primary afferent neurons in the cat as determined by the transganglionic transport of horseradish peroxidase. J Comp Neurol 203: 785–798, 1981 [DOI] [PubMed] [Google Scholar]
- 141.Marfurt CF, Rajchert DM. Trigeminal primary afferent projections to “non-trigeminal” areas of the rat central nervous system. J Comp Neurol 303: 489–511, 1991 [DOI] [PubMed] [Google Scholar]
- 142.Marino L. A comparison of encephalization between odontocete cetaceans and anthropoid primates. Brain Behav Evol 51: 230–238, 1998 [DOI] [PubMed] [Google Scholar]
- 143.Marino L, Murphy TL, Deweerd AL, Morris JA, Fobbs AJ, Humblot N, Ridgway SH, Johnson JI. Anatomy and three-dimensional reconstructions of the brain of the white whale (Delphinapterus leucas) from magnetic resonance images. Anat Rec 262: 429–439, 2001 [DOI] [PubMed] [Google Scholar]
- 144.Marino L, Rilling JK, Lin SK, Ridgway SH. Relative volume of the cerebellum in dolphins and comparison with anthropoid primates. Brain Behav Evol 56: 204–211, 2000 [DOI] [PubMed] [Google Scholar]
- 145.Marino L, Subheimer K, Mclellan WA, Johnson JI. Neuroanatomical structure of the spinner dolphin (Stenella longirostris orientalis) brain from magnetic resonance images. Anatomical Record Part A 279: 601–610, 2004 [DOI] [PubMed] [Google Scholar]
- 146.Martin AR, Smith TG. Strategy and capability of wild belugas, Delphinapterus leucas, during deep, benthic diving. Can J Zool 77: 1783–1793, 1999 [Google Scholar]
- 147.Martner J, Wadenvik H, Lisander B. Apnoea and bradycardia from submersion in “chronically” decerebrated cats. Acta Physiol Scand 101: 476–480, 1977 [DOI] [PubMed] [Google Scholar]
- 148.Massari VJ, Johnson TA, Llewellyn-Smith IJ, Gatti PJ. Substance P nerve terminals synapse upon negative chronotropic vagal motoneurons. Brain Res 660: 275–287, 1994 [DOI] [PubMed] [Google Scholar]
- 149.Matesz C. Termination areas of primary afferent fibers of the trigeminal nerve in the rat. Acta Biol Hung 34: 31–44, 1983 [PubMed] [Google Scholar]
- 150.Matsuda H, Kusakabe T, Hayashida Y, Furukawa M, Kawakami T, Takenaka T, Tsukuda M. Substance P- and calcitonin gene-related peptide-containing nerve fibers in the nasal mucosa of chronically hypoxic rats. Brain Res Bull 45: 563–569, 1998 [DOI] [PubMed] [Google Scholar]
- 151.Matsuda H, Tsukuda M, Kadota T, Kusunoki T, Kishida R. Coexistence of galanin and substance P in the mouse nasal mucosa, including the vomeronasal organ. Neurosci Lett 173: 55–58, 1994 [DOI] [PubMed] [Google Scholar]
- 152.Matturri L, Ottaviani G, Lavezzi AM. Sudden infant death triggered by dive reflex. J Clin Pathol 58: 77–80, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McAllen RM, Dampney RAL. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neurosci Lett 110: 91–96, 1990 [DOI] [PubMed] [Google Scholar]
- 154.McAllen RM, May CN. Differential drives from rostral ventrolateral medullary neurons to three identified sympathetic outflows. Am J Physiol Regul Integr Comp Physiol 267: R935–R944, 1994 [DOI] [PubMed] [Google Scholar]
- 155.McAllen RM, Salo LM, Paton JFR, Picikering AE. Processing of central and reflex vagal drives by rat cardiac ganglion neurons: an intracellular analysis. J Physiol 589: 5801–5818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McAllen RM, Spyer KM. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J Physiol 282: 353–364, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McCafferty DJ, Boyd IL, Taylor RI. Diving behaviour of Antarctic fur seal (Arctocephalus gazella) pups. Can J Zool 76: 513–520, 1998 [Google Scholar]
- 158.McCulloch PF. Activation of the trigeminal medullary dorsal horn during voluntary diving in rats. Brain Res 1051: 194–198, 2005 [DOI] [PubMed] [Google Scholar]
- 159.McCulloch PF. Animal models for investigating the central control of the mammalian diving response. Front Physiol 3: 169, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McCulloch PF, Dinovo KM, Connolly TM. The cardiovascular and endocrine responses to voluntary and forced diving in trained and untrained rats. Am J Physiol Regul Integr Comp Physiol 298: R224–R234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McCulloch PF, Faber KM, Panneton WM. Electrical stimulation of the anterior ethmoidal nerve produces the diving response. Brain Res 830: 24–31, 1999 [DOI] [PubMed] [Google Scholar]
- 162.McCulloch PF, Jones DR. Cortical influences on diving bradycardia in muskrats (Ondatra zibethicus). Physiol Zool 63: 1098–1117, 1990 [Google Scholar]
- 163.McCulloch PF, Panneton WM. Activation of brainstem catecholaminergic neurons during voluntary diving in rats. Brain Res 984: 42–53, 2003 [DOI] [PubMed] [Google Scholar]
- 164.McCulloch PF, Panneton WM, Guyenet PG. The rostral ventrolateral medulla mediates the sympathoactivation produced by chemical stimulation of the nasal mucosa. J Physiol 516: 471–484, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McKeegan DEF, Demmers TGM, Wathes CM, Jones RB, Gentle MJ. Response characteristics of nasal trigeminal nociceptors in Gallus domesticus. Neuroreport 13: 1033–1035, 2002 [DOI] [PubMed] [Google Scholar]
- 166.Meir JU, Champagne CD, Costa DP, Williams CL, Ponganis PJ. Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am J Physiol Regul Integr Comp Physiol 297: R927–R939, 2009 [DOI] [PubMed] [Google Scholar]
- 167.Meir JU, Stockard TK, Williams CL, Ponganis KV, Ponganis PJ. Heart rate regulation and extreme bradycardia in diving emperor penguins. J Exp Biol 211: 1169–1179, 2008 [DOI] [PubMed] [Google Scholar]
- 168.Murdaugh HV, Cross CE, Millen JE, Gee JBL, Robin ED. Dissociation of bradycardia and arterial constriction during diving in the seal (Phoca vitulina). Science 162: 364–365, 1968 [DOI] [PubMed] [Google Scholar]
- 169.Nordgarden U, Folkow LP, Walloe L, Blix AS. On the direction and velocity of blood flow in the extradural intravertebral vein of harp seals (Phoca groenlandica) during simulated diving. Acta Physiol Scand 168: 271–276, 2000 [DOI] [PubMed] [Google Scholar]
- 170.Noren SR, Cuccurullo V, Williams TM. The development of diving bradycardia in bottlenose dolphins (Tursiops truncatus). J Comp Physiol B 174: 139–147, 2004 [DOI] [PubMed] [Google Scholar]
- 171.Noren SR, Kendall T, Cuccurullo V, Williams TM. The dive response redefined: underwater behavior influences cardiac variability in freely diving dolphins. J Exp Biol 215: 2735–2741, 2012 [DOI] [PubMed] [Google Scholar]
- 172.Noren SR, Williams TM. Body size and skeletal muscle myoglobin of cetaceans: adaptations for maximizing dive duration. Comp Biochem Physiol A 126: 181–191, 2000 [DOI] [PubMed] [Google Scholar]
- 173.Noren SR, Williams TM, Pabst DA, McLellan WA, Dearolf JL. The development of diving in marine endotherms: preparing the skeletal muscles of dolphins, penguins, and seals for activity during submergence. J Comp Physiol B 171: 127–134, 2001 [DOI] [PubMed] [Google Scholar]
- 174.Odden A, Folkow LP, Caputa M, Hotvedt R, Blix AS. Brain cooling in diving seals. Acta Physiol Scand 166: 77–78, 1999 [DOI] [PubMed] [Google Scholar]
- 175.Ollenberger GP, Matte G, Wilkinson AA, West NH. Relative distribution of blood flow in rats during surface and submerged swimming. Comp Biochem Physiol A 119: 271–277, 1998 [DOI] [PubMed] [Google Scholar]
- 176.Ollenberger GP, West NH. Distribution of regional cerebral blood flow in voluntarily diving rats. J Exp Biol 201: 549–558, 1998 [DOI] [PubMed] [Google Scholar]
- 177.Packer BS, Altman M, Cross CE, Murdaugh HV, Linta JM, Robin E. Adaptations to diving in the harbor seal: oxygen stores and supply. Am J Physiol 217: 903–906, 1969 [DOI] [PubMed] [Google Scholar]
- 178.Panneton WM. Primary afferent projections from the upper respiratory tract in the muskrat. J Comp Neurol 308: 51–65, 1991 [DOI] [PubMed] [Google Scholar]
- 179.Panneton WM. Trigeminal mediation of the diving response in the muskrat. Brain Res 560: 321–325, 1991 [DOI] [PubMed] [Google Scholar]
- 181.Panneton WM, Anch MA, Gan Q. Topography of preganglionic parasympathetic cardiac motor neurons labeled after underwater submersion. FASEB J 21: 952.6, 2007 [Google Scholar]
- 182.Panneton WM, Burton H. Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J Comp Neurol 199: 327–344, 1981 [DOI] [PubMed] [Google Scholar]
- 183.Panneton WM, Gan Q, Dahms TE. Cardiorespiratory and neural consequences of rats brought past their aerobic dive limit. J Appl Physiol 109: 1256–1269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Panneton WM, Gan Q, Juric R. Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience 141: 889–906, 2006 [DOI] [PubMed] [Google Scholar]
- 185.Panneton WM, Gan Q, Juric R. The rat: a laboratory model for studies of the diving response. J Appl Physiol 108: 811–820, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Panneton WM, Gan Q, Le J, Livergood RS, Clerc P, Juric R. Activation of brainstem neurons by underwater diving in the rat. Front Physiol. First published May 3, 2012; 10.3389/fphys.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Panneton WM, Gan Q, Livergood R. A trigeminoreticular pathway: Implications in pain. PLos One 6: e24499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Panneton WM, Gan Q, Sun DW. Persistence of the nasotrigeminal reflex after pontomedullary transection. Respir Physiol Neurobiol 180: 230–236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Panneton WM, Gan Q, Sun W. Pressor responses to nasal stimulation are unaltered after disrupting the caudalmost ventrolateral medulla. Auto Neurosci 144: 13–21, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Panneton WM, Hsu H, Gan Q. Distinct central representations for sensory fibers innervating either the conjunctiva or cornea of the rat. Exp Eye Res 90: 388–396, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Panneton WM, McCulloch PF, Sun W. Trigemino-autonomic connections in the muskrat: the neural substrate for the diving response. Brain Res 874: 48–65, 2000 [DOI] [PubMed] [Google Scholar]
- 192.Panneton WM, McCulloch PF, Tan Y, Tan YX, Yavari P. Brainstem origin of preganglionic cardiac motoneurons in the muskrat. Brain Res 738: 342–346, 1996 [DOI] [PubMed] [Google Scholar]
- 193.Panneton WM, Yavari P. A medullary dorsal horn relay for the cardiorespiratory responses evoked by stimulation of the nasal mucosa in the muskrat, Ondatra zibethicus: evidence for excitatory amino acid transmission. Brain Res 691: 37–45, 1995 [DOI] [PubMed] [Google Scholar]
- 194.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Rev 49: 555–565, 2005 [DOI] [PubMed] [Google Scholar]
- 195.Paton JFR, Nalivaiko E, Boscan P, Pickering AE. Reflexly evoked coactivation of cardiac vagal and sympathetic motor outflows: observations and functional implications. Clin Exper Pharmacol Physiol 33: 1245–1250, 2006 [DOI] [PubMed] [Google Scholar]
- 196.Pedroso FS, Riesgo RS, Gatiboni T, Rotta NT. The diving reflex in healthy infants in the first year of life. J Child Neurol 27: 168–171, 2012 [DOI] [PubMed] [Google Scholar]
- 197.Petersson G, Malm L, Ekman R, Håkanson R. Capsaicin evokes secretion of nasal fluid and depletes substance P and calcitonin gene-related peptide from the nasal mucosa in the rat. Br J Pharmacol 98: 930–936, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ponganis PJ, Kooyman GL. Diving physiology of birds: a history of studies on polar species. Comp Biochem Physiol A 126: 143–151, 2000 [DOI] [PubMed] [Google Scholar]
- 199.Ponganis PJ, Kooyman GL, Castellini MA. Determinants of aerobic dive limit of Weddell seals: analysis of diving metabolic rates, postidve end tidal Po2s, and blood and muscle oxygen stores. Physiol Zool 66: 732–749, 1993 [Google Scholar]
- 200.Ponganis PJ, Kooyman GL, Winter LM, Starke LN. Heart rate and plasma lactate responses during submerged swimming and trained diving in California sea lions, Zalophus californianus. J Comp Physiol B 167: 9–16, 1997 [DOI] [PubMed] [Google Scholar]
- 201.Ponganis PJ, Meir JU, Williams CL. In pursuit of Irving and Scholander: a review of oxygen store management in seals and penguins. J Exp Biol 214: 3325–3339, 2012 [DOI] [PubMed] [Google Scholar]
- 202.Qvist J, Hill RD, Schneider RC, Falke KJ, Liggins GC, Guppy M, Elliot RL, Hochachka PW, Zapol WM. Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. J Appl Physiol 61: 1560–1569, 1986 [DOI] [PubMed] [Google Scholar]
- 203.Radulovacki M, Pavlovic S, Carley DW. Pontine intertrigeminal region attenuates sleep apneas in rats. Sleep 27: 383–387, 2004 [DOI] [PubMed] [Google Scholar]
- 204.Radulovacki M, Pavlovic S, Saponjic J, Carley DW. Intertrigeminal region attenuates reflex apnea and stabilizes respiratory pattern in rats. Brain Res 975: 66–72, 2003 [DOI] [PubMed] [Google Scholar]
- 205.Ramirez JM, Folkow LP, Blix AS. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Ann Rev Physiol 69: 1–31, 2007 [DOI] [PubMed] [Google Scholar]
- 206.Rekling JC, Feldman JL. Prebötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Ann Rev Physiol 60: 385–405, 1998 [DOI] [PubMed] [Google Scholar]
- 207.Rentero N, Cividjian A, Trevaks D, Pequignot JM, Quintin L, McAllen RM. Activity patterns of cardiac vagal motoneurons in rat nucleus ambiguus. Am J Physiol Regul Integr Comp Physiol 283: R1327–R1334, 2002 [DOI] [PubMed] [Google Scholar]
- 208.Ridgway SH, Carder DA, Clark W. Conditioned bardycardia in the sea lion Zalophus californianus. Nature 256: 37–38, 1975 [DOI] [PubMed] [Google Scholar]
- 209.Ruggiero DA, Ross CA, Anwar M, Park DH, Joh TH, Reis DJ. Distribution of neurons containing phenylethanolamine N-methyltransferase in medulla and hypothalamus of rat. J Comp Neurol 239: 127–154, 1985 [DOI] [PubMed] [Google Scholar]
- 210.Rybka EJ, McCulloch PF. The anterior ethmoidal nerve is necessary for the initiation of the nasopharyngeal response in the rat. Brain Res 1075: 122–132, 2006 [DOI] [PubMed] [Google Scholar]
- 211.Schaefer ML, Boettger B, Silver WL, Finger TE. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. J Comp Neurol 444: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 212.Schagatay E, Andersson JP, Nielsen B. Hematological response and diving response during apnea and apnea with face immersion. Eur J Appl Physiol 101: 125–132, 2007 [DOI] [PubMed] [Google Scholar]
- 213.Schagatay E, Van Kampen M. Apneic snout immersion in trained pigs elicits a “diving response”. Adv Exp Med Biol 393: 73–76, 1995 [PubMed] [Google Scholar]
- 214.Schneuer M, Flachsbarth S, Czech-Damal NU, Folkow LP, Siebert U, Burmester T. Neuroglobin of seals and whales: evidence for a divergent role in the diving brain. Neuroscience 223: 35–44, 2012 [DOI] [PubMed] [Google Scholar]
- 215.Scholander PF. Experimental investigations on the respiratory function in diving mammals and birds. Hvalrådets Skrifter 22: 1–131, 1940 [Google Scholar]
- 216.Scholander PF. The master switch of life. Sci Am 209: 92–106, 1963 [DOI] [PubMed] [Google Scholar]
- 217.Scholander PF, Irving L, Grinnell SW. On the temperature and metabolism of the seal during diving. J Cell Comp Physiol 19: 67–78, 1942 [Google Scholar]
- 218.Schreer JF, Kovacs KM. Allometry of diving capacity in air-breathing vertebrates. Can J Zool 75: 339–358, 1997 [Google Scholar]
- 219.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 387: 524–536, 1997 [DOI] [PubMed] [Google Scholar]
- 220.Segade LA. Trigeminal primary afferent projections to the peribrachial area in the guinea pig. Neurosci Lett 351: 63–66, 2003 [DOI] [PubMed] [Google Scholar]
- 221.Sekizawa S, Tsubone H. Nasal receptors responding to noxious chemical irritants. Respir Physiol 96: 37–48, 1994 [DOI] [PubMed] [Google Scholar]
- 222.Sekizawa S, Tsubone H, Kuwahara M, Sugano S. Nasal receptors responding to cold and l-menthol airflow in the guinea pig. Respir Physiol 103: 211–219, 1996 [DOI] [PubMed] [Google Scholar]
- 223.Sekizawa S, Tsubone H, Kuwahara M, Sugano S. Does histamine stimulate trigeminal nasal afferents? Respir Physiol 112: 13–22, 1998 [DOI] [PubMed] [Google Scholar]
- 224.Sekizawa SI, Tsubone H. Nasal mechanoreceptors in guinea pigs. Respir Physiol 106: 223–230, 1996 [DOI] [PubMed] [Google Scholar]
- 225.Shaffer SA, Costa DP, Williams TM, Ridgeway SH. Diving and swimming performance of white whales, Delphinapterus leucas: an assessment of plasma lactate and blood gas levels and respiratory rates. J Exp Biol 200: 3091–3099, 1997 [DOI] [PubMed] [Google Scholar]
- 226.Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way todie during cold water immersion? J Physiol 590: 3219–3230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shigenaga Y, Chen IC, Suemune S. Oral and facial representation within the medullary and upper cervical dorsal horns in the cat. J Comp Neurol 243: 388–408, 1986 [DOI] [PubMed] [Google Scholar]
- 228.Silver WL, Farley LG, Finger TE. The effects of neonatal capsaicin administration on trigeminal nerve chemoreceptors in the rat nasal cavity. Brain Res 561: 212–216, 1991 [DOI] [PubMed] [Google Scholar]
- 229.Silver WL, Mason JR, Adams MA, Smeraski CA. Nasal trigeminal chemoreception: responses to n-aliphatic alcohols. Brain Res 376: 221–229, 1986 [DOI] [PubMed] [Google Scholar]
- 230.Silverman JD, Kruger L. Calcitonin-gene-related-peptide-immunoreactive innervation of the rat head with emphasis on specialized sensory structures. J Comp Neurol 280: 303–330, 1989 [DOI] [PubMed] [Google Scholar]
- 231.Smith EN, Johnson C, Martin KJ. Fear bradycardia in captive eastern chipmunk, Tamias striatus. Comp Biochem Physiol 70: 529–532, 1981 [Google Scholar]
- 232.Smith JB, McCubbin JA, McClean WT, Toole JF. The diving reflex in infants: a preliminary report. Trans Amer Neurol Soc 101: 294–295, 1976 [PubMed] [Google Scholar]
- 233.Smith NE, Tobey EW. Heart rate response to forced and voluntary diving in swamp rabbits, Sylvilagus aquaticus. Physiol Zool 56: 632–638, 1983 [Google Scholar]
- 234.Smith NE, Woodruff RA. Fear bradycardia in free ranging woodchucks, Marmota monax. J Mammal 61: 750–753, 1980 [Google Scholar]
- 235.Solomon IC, Halat TJ, El-Maghrabi MR, O'Neal MHI. Localization of connexin 26 and connexin32 in putative CO2-chemosensitive brainstem regions in rat. Resp Physiol 129: 101–121, 2001 [DOI] [PubMed] [Google Scholar]
- 236.Sparling CE, Fedak MA. Metabolic rates of captive grey seals during voluntary diving. J Exp Biol 207: 1615–1624, 2004 [DOI] [PubMed] [Google Scholar]
- 237.Spit BJ, Bretschneider F, Hendriksen EGJ, Kuper CF. Ultrastructure of free nerve endings in respiratory and squamous epithelium on the rat nasal septum. Cell Tissue Res 274: 329–335, 1993 [DOI] [PubMed] [Google Scholar]
- 238.Stjärne P, Lundblad L, Änggård A, Hökfelt T, Lundberg JM. Tachykinins and calcitonin gene-related peptide: co-existence in sensory nerves of the nasal mucosa and effects on blood flow. Cell Tissue Res 256: 439–446, 1989 [DOI] [PubMed] [Google Scholar]
- 239.Sun W, Panneton WM. The caudal pressor area of the rat: its precise location and projections to the ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 283: R768–R778, 2002 [DOI] [PubMed] [Google Scholar]
- 240.Sun W, Panneton WM. Defining projections from the caudal pressor area of the caudal ventrolateral medulla. J Comp Neurol 482: 273–293, 2005 [DOI] [PubMed] [Google Scholar]
- 241.Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rats. Nat Neurosci 11: 538–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of PreBötzinger complex neurons in adult rats. J Comp Neurol 518: 1862–1878, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Taylor T, Al-Ghamdi MS, Ihmied IH, Wang T, Abe AS. The neuranatomical basis of central control of cardiorespiratory interactions in vertebrates. Exp Physiol 86: 771–776, 2001 [DOI] [PubMed] [Google Scholar]
- 244.Tchobroutsky C, Merlet C, Rey P. The diving reflex in rabbit, sheep and newborn lamb and its afferent pathways. Resp Physiol 8: 108–117, 1969 [DOI] [PubMed] [Google Scholar]
- 245.Terui N, Numao Y, Kumada M, Reis DJ. Identification of the primary afferent fiber group and adequate stimulus initiating the trigeminal depressor response. J Auton Nerv Syst 4: 1–16, 1981 [DOI] [PubMed] [Google Scholar]
- 246.Thornton SJ, Spielman DM, Pelc NJ, Block WF, Crocker DE, Costa DP, LeBoeuf BJ, Hochachka PW. Effects of forced diving on the spleen and hepatic sinus in northern elephant seal pups. Proc Natl Acad Sci USA 98: 9413–9418, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Topchiy I, Radulovacki M, Waxman J, Carley DW. Cardiorespiratory effects of intertrigeminal area stimulation in vagotomized rats. Brain Res 1250: 120–129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wallois F, Macron JM, Jounieaux V, Duron B. Trigeminal nasal receptors related to respiration and to various stimuli in cats. Resp Physiol 85: 111–125, 1991 [DOI] [PubMed] [Google Scholar]
- 249.Whishaw IQ, Schallert T. Hippocampal RSA (theta), apnea, bradycardia, and effects of atropine during underwater swimming in the rat. Electroencephalogr Clin Neurophysiol 42: 389–396, 1977 [DOI] [PubMed] [Google Scholar]
- 250.Williams PL, Warwick R. Gray's Anatomy. Philadelphia, PA: Saunders, 1980 [Google Scholar]
- 251.Williams TM, Davis RW, Fuiman LA, Francis J, Le Boeuf BL, Horning M, Calambokidis J, Croll DA. Sink or swim: strategies for cost-efficient diving by marine mammals. Science 288: 133–136, 2000 [DOI] [PubMed] [Google Scholar]
- 252.Williams TM, Zavenelli M, Miller MA, Goldbeck RA, Morledge M, Casper D, Pabst DA, McLellan W, Cantin LP, Kliger DS. Running, swimming and diving modifies neurprotecting globins in the mammalian brain. Proc R Soc B 275: 751–758, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wolf S. Psychophysiological influences on the dive reflex in man. In: Neural Mechanisms in Cardiac Arrythmias, edited by Schwartz PJ, Brown AM, Malliani A, Zanchetti A. New York: Raven, 1978, p. 237–250 [Google Scholar]
- 254.Yavari P, McCulloch PF, Panneton WM. Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst 61: 195–200, 1996 [DOI] [PubMed] [Google Scholar]
- 255.Yu YH, Blessing WW. Constriction of the ear pinna vascular bed accompanies the trigeminal depressor response in rabbits. Neurosci Lett 255: 172–174, 1998 [DOI] [PubMed] [Google Scholar]
- 256.Zapol WM, Liggins GC, Schneider RC, Qvist J, Snider MT, Creasy RK, Hochachka PW. Regional blood flow during simulated diving in the conscious Weddell seal. J Appl Physiol 47: 968–973, 1979 [DOI] [PubMed] [Google Scholar]
- 257.Zhang WN, Murphy CA, Feldon J. Behavioural and cardiovascular responses during latent inhibition of conditioned fear: measurement by telemetry and conditioned freezing. Behav Brain Res 154: 199–209, 2004 [DOI] [PubMed] [Google Scholar]