Abstract
During the last decade, our view on the skeleton as a mere solid physical support structure has been transformed, as bone emerged as a dynamic, constantly remodeling tissue with systemic regulatory functions including those of an endocrine organ. Reflecting this remarkable functional complexity, distinct classes of humoral and intracellular regulatory factors have been shown to control vital processes in the bone. Among these regulators, nuclear receptors (NRs) play fundamental roles in bone development, growth, and maintenance. NRs are DNA-binding transcription factors that act as intracellular transducers of the respective ligand signaling pathways through modulation of expression of specific sets of cognate target genes. Aberrant NR signaling caused by receptor or ligand deficiency may profoundly affect bone health and compromise skeletal functions. Ligand dependency of NR action underlies a major strategy of therapeutic intervention to correct aberrant NR signaling, and significant efforts have been made to design novel synthetic NR ligands with enhanced beneficial properties and reduced potential negative side effects. As an example, estrogen deficiency causes bone loss and leads to development of osteoporosis, the most prevalent skeletal disorder in postmenopausal women. Since administration of natural estrogens for the treatment of osteoporosis often associates with undesirable side effects, several synthetic estrogen receptor ligands have been developed with higher therapeutic efficacy and specificity. This review presents current progress in our understanding of the roles of various nuclear receptor-mediated signaling pathways in bone physiology and disease, and in development of advanced NR ligands for treatment of common skeletal disorders.
I. INTRODUCTION
Bone is a central element in skeletal tissues supporting the body and is a place of mineral deposit in vertebrate organisms throughout the life (152, 405). During the morphogenesis and growth stages, bone patterns and structures are formed and developed in a spatiotemporal manner under complex and highly coordinated mechanisms (6). Growing bone supports and limits overall growth of the organism. Once the skeleton is developmentally matured, the major role of bone is shifted to an endocrine function, primarily controlling mineral homeostasis through bone metabolism (130).
The skeleton consists of several types of bone cells that have been traditionally classified into three primary classes: osteoblasts, osteocytes, and osteoclasts. Recently, an additional class of bone cells has been distinguished as “bone lining cells” that represents quiescent osteoblasts. Osteocytes are terminally differentiated osteoblastic lineage cells that are embedded into a matrix of deposited minerals (32). Osteoblasts localize on bone surfaces and promote bone formation by secreting proteins, such as osteocalcin and osteopontin, to anchor serum minerals within the bone matrix (208). The mesenchymal bone marrow cells are the osteoblast precursor cells, although these pluripotent cells can also differentiate into other cell types like adipocytes, chondrocytes, and muscle cells (75, 497). Osteoclasts are derived from macrophage lineage precursor cells (391, 475). Mature osteoclasts are multinucleated macrophage-like bone-resorptive cells and play a key role in bone remodeling (483, 490, 542). As bone remodeling is a dynamic process that also controls serum mineral levels, tight coordination between osteoblastic and osteoclastic cellular functions and cell numbers is considered essential in the maintenance of both the skeleton and systemic homeostasis (120, 565). Reflecting numerous processes in bone formation and remodeling, multiple regulatory molecules operate to harmonize distinct processes ongoing in the bone. Distinct classes of such regulators have been identified, including humoral and cellular factors. Among cellular regulators, DNA-binding transcription factors have been shown to play pivotal roles in bone development and metabolism (100, 236, 340). Cellular proliferation, cell lineage determination, and differentiation of bone cells are directed by distinct sets of transcription factors that are functional at specific stages of cell differentiation and in specific bone cell types (315, 476, 549). Physiological significance of various transcription factors in the bone has been revealed by the link between loss-of-function mutations in their genes and genetic diseases in humans that, in certain cases, has also been recapitulated by genetic manipulation of responsible genes in mouse models (209, 483). In this respect, members of the nuclear receptor (NR) superfamily are remarkable in bone biology, and their physiological significance in bone formation and remodeling has been successfully illustrated in mammalian experimental systems. Notably, the roles of NRs have been uncovered in pathological progression of skeletal disorders, like in the cases including estrogen deficiency-induced osteoporosis in postmenopausal women (343, 344, 408).
NRs are DNA-binding transcription factors and belong to the gene superfamily composed of 48 members in humans (299). NRs bind to specific DNA elements in the promoters and other regulatory regions of target genes (299). Activated by binding specific ligands, NRs control expression of the target genes at the transcriptional level. NR ligands include steroid/thyroid hormones, fat-soluble vitamins A and D, some endogenous metabolites, as well as synthetic chemical compounds.
Synthetic ligands for NRs may be applicable to attenuate initiation and development of some common bone diseases. It has been clinically proven, for example, that synthetic estrogen analogs called selective estrogen receptor modulators (SERMs) (221, 405) effectively ameliorate conditions of patients with osteoporosis, the most prevalent bone disease in the developed countries (343). As androgens exhibit anabolic action in the musculoskeletal organs, several synthetic androgens with desirable effects, selective androgen receptor modulators (SARMs, by analogy with SERMs) are under clinical trials for their efficacy to induce bone mass increase and attenuate muscle loss (sarcopenia) in elderly people (82, 487).
In this review, we summarize recent progress in our understanding of the roles of NRs in the bone physiology. Molecular links between dysfunction of NRs with common bone diseases are also discussed, together with characterization of some synthetic analogs of NR ligands that have been clinically used for the treatment of patients with bone diseases.
II. PHYSIOLOGY OF BONE METABOLISM
Bone tissue provides the skeleton its strength, permits efficient movement of locomotive organs, maintains posture, and protects internal organs and the central nervous system from high-energy impact trauma. Bone tissue also acts as a reservoir for storage of minerals, particularly calcium (Ca) and phosphorus (P) (152). Moreover, recent studies have revealed that the bone functions as an endocrine organ, producing several secreted proteins important for the homeostasis (130). Physiology of the bone tissue is controlled by a wide range of regulatory factors, including steroid hormones, vitamin D, parathyroid hormone (PTH), various cytokines, and growth factors (39, 391).
A. Bone Cell Differentiation
1. Osteoblasts
The osteoblast functions are crucial for the bone formation, producing extracellular bone matrix proteins, type I collagen, and noncollagenous proteins such as osteocalcin and osteopontin. Osteoblast-secreted noncollagenous proteins contribute to mineralization of the bone matrix with hydroxyapatite by binding calcium and phosphate, thereby regulating depositions of minerals. Osteoblasts are differentiated from mesenchymal stem cells. It has been reported that their differentiation is tightly regulated by several transcription factors such as Runx2, Osterix, ATF4, and some others (209).
Runx2 was identified as the first osteoblast-specific transcription factor and is a member of the Runt-containing transcription factor family (100, 236). The family members bind to DNA through their Runt domain and can also interact with various nuclear proteins through other structural domains. The significance of Runx2 function in osteoblast differentiation was confirmed by the phenotypes of mouse mutants lacking Runx2 that lacked osteoblasts and bone formation (236). Moreover, the analyses of human genetic studies revealed that Runx2 is the dominant gene in cleidocranial dysplasia (257, 333).
Runx2 exerts its regulatory action as a transcription factor, the function of which can be enhanced by interaction with other nuclear proteins. For example, Schnurri-3 is a zinc finger-containing protein involved in the recombination of immunoglobulin genes. Schnurri-3-deficient mice exhibited increased bone mass attributed to accelerated bone formation due to accumulation of Runx2 proteins in osteoblasts (198).
Osterix is another essential zinc finger-containing transcription factor that is important in osteoblast differentiation. The transcription of Osterix appears to be regulated by Runx2, since Osterix is not expressed in Runx2-deficient cells. Moreover, Osterix is a key factor in the differentiation from osteoblast progenitor cells to osteoblasts. Indeed, Osterix-deficient mice lack mature osteoblasts and exhibit severe impairment in bone formation (340). In addition, the nuclear factor of activated T cells cytoplasmic 1 (Nfatc1), a pivotal regulator in differentiation of bone resorbing osteoclasts, and Osterix form a complex that binds to DNA, and this association is crucial for the transcriptional activity of Osterix. Thus Nfatc1 and Osterix appear to coordinately regulate osteoblastic bone formation. (235). Furthermore, NO66, a Jumonji C domain-containing protein, directly interacts with Osterix and inhibits Osterix-mediated transcription by modulating the status of histone modifications in promoter regions (439). This shows that transcriptional activity of Osterix can be regulated by transcriptional cofactors including epigenetic regulators.
ATF4 is a transcription factor that belongs to the CREB family of B ZIP proteins. Apparently, ATF4 is important for the maintenance of osteoblastic functions (549), since impairments of ATF4 function can cause human genetic skeletal diseases such as Coffin-Lowry syndrome and neurofibromatosis type I (107, 549).
2. Osteocytes
Osteocytes are located within the bone matrix (31). Osteocytes differentiate from osteoblasts. However, in contrast to osteoblast differentiation from mesenchymal stem cells, the precise molecular mechanisms of “osteocytogenesis” are essentially unknown, especially in regard to key regulatory transcription factors. Although osteocytes constitute more than 90% of the cells in bone, their function is not fully understood, nor is the mechanism of their differentiation. Some reports have suggested that osteocytes can differentiate directly from osteoblasts in the absence of positive regulation (301). Osteocytes differ from osteoblasts in their morphology and array of expressed proteins, as well as in their location. Bone matrix embedded osteocytes form dendritic processes that are extended to the mineralization front and are important for the extensive osteocyte-osteocyte interaction. Osteocytes appear to function as mechanosensing cells communicating through this complex dendritic network. Bone mass can be increased by appropriate intensity, frequency, and amount of loading (404, 411, 496). At the same time, mechanical loading or unloading affects gene expression profiles of osteocytes in vivo (143, 442), supporting the conclusion that osteocytes may act as mechanosensors in addition to their various functions in the maintenance of bone and mineral metabolism (32, 342, 488). For example, mineralizing and mature osteocytes can produce dentin matrix protein 1 (Dmp1), which controls fibroblast growth factor 23 (FGF23) expression in osteocytes (119, 280). FGF23 plays an important role in phosphate metabolism (64, 440, 559) through its binding with Klotho as a coreceptor (498). Patients with Dmp1 loss-of-function mutations exhibited autosomal recessive hypophosphatemia rickets (ARHR) with an increased serum level of FGF23 (119, 280). Consistently, Dmp1 null mice recapitulate the ARHR phenotypes (119, 280). Furthermore, osteocytes can express the sclerostin protein, a product of the SOST gene. Sclerostin acts as an extracellular Wnt signal antagonist by binding with LRP5/6, a Wnt coreceptor in osteoblasts (77, 541). In this way, osteocytes can negatively regulate bone formation through inhibition of Wnt signaling. It has been recently reported that osteocytes express receptor activator of NF-κB ligand (RANKL), an essential cytokine for osteoclastogenesis, and facilitate osteoclast formation (341, 538). Thus osteocytes might regulate bone and mineral metabolisms by both systemic and local influences.
3. Osteoclasts
Osteoclasts are multinucleated cells that can resorb bone matrix. Osteoclasts are derived from hematopoietic stem cells, specifically the monocyte/macrophage lineage of cells. Macrophage-colony stimulating factor (M-CSF) is indispensable for osteoclast differentiation and function (234, 555). M-CSF can induce receptor activator NF-κB (RANK) expression in osteoclast progenitor cells. RANKL is a key factor in stimulation of osteoclast differentiation, while osteoprotegerin (OPG) inhibits osteoclastogenesis acting as a soluble decoy receptor of RANK (475). RANKL and OPG were originally identified as the osteoclast differentiation factors expressed by osteoblasts (466, 551). However, recent studies have revealed that RANKL can be expressed by various types of cells including osteoblasts, osteocytes, and T-cells (237, 238) in both physiological and pathological conditions. The binding of RANKL with RANK induces trimerization of RANK followed by the recruitment of TRAF (TNF receptor-associated factor) family adaptor molecules, with particular TRAF6 known as the major adaptor protein (277, 338). Next, activation of TRAF6 by RANKL-RANK stimulation leads to activation of NF-κB and mitogen-activated protein kinases (MAPKs), such as p38 and JNK (JUN NH2-terminal kinase). These RANKL-RANK-induced signaling pathways stimulate complex formation of transcription factors, such as activator protein 1 (AP1) by inducing one of a member of AP1 complex, c-Fos (315, 477). The induction of c-Fos is regulated by CaMK IV, CREB (cAMP response element binding protein), and NF-κB activation (417). RANKL stimulates the expression of Nfatc1, a master transcription factor for osteoclast differentiation, through both the TRAF and c-Fos pathways. NFATc1 can induce the expression of a number of osteoclast differentiation marker genes such as those encoding tartrate resistant acid phosphatase (TRAP) and cathepsin K (Ctsk), enzymes critical for the osteoclastic bone resorbing functions, as well as NFATc1 itself (475). NFATc1 was originally identified in T cells, as suggested by its name. However, it has been shown that NFATc1 has an important and essential role in osteoclastogenesis (476). The overlap of molecules and signaling pathways between two research fields, i.e., bone biology and immunology, generated a new research field, “osteoimmunology” (475). The concept of osteoimmunology is helpful to understand both the complex immunological mechanisms in bone biology as well as involvement of bone cells in immunological reactions and processes.
Multinucleated mature osteoclasts are formed by differentiation of fused mononuclear osteoclast progenitor cells. This multinucleation step is essential for the bone resorbing function of osteoclasts. Yagi et al. (542) identified a key molecule of osteoclastic fusion and named it dendritic cell-specific transmembrane protein (DC-STAMP). Monocytes/macrophages derived from DC-STAMP null mice can be differentiated into osteoclast progenitors, but cannot undergo fusion and cannot differentiate into mature multinucleated osteoclasts even in the presence of RANKL. Interestingly, DC-STAMP null mice exhibit high bone mass similar to those observed in patients with osteopetrosis (542).
B. Bone Remodeling
During the embryogenesis, skeletal formation involves bone modeling and growth. In a mature skeleton, bones continue to undergo remodeling by osteoclastic bone resorption and osteoblastic bone formation (FIGURE 1) (208). Osteoclasts resorb old bone matrix and make resorbed cavities, termed “Howship lacunae.” In this concave surface, osteoblasts produce bone matrix composed of type I collagen, osteocalcin, osteopontin, and some other proteins to fill the lacunae. This unmineralized bone matrix is called an osteoid. On the surface of the osteoid, osteoblasts mineralize the bone matrix with hydroxyapatite to complete the remodeling of the bone. In this remodeling process, it is critical that bone resorption and bone formation are balanced to maintain the homeostasis of bone tissue. If this balance equilibrium is altered, a pathological condition may develop.
Figure 1.
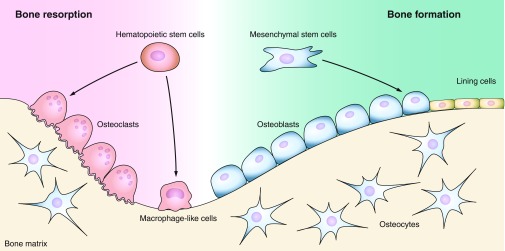
Bone cellular structure. Three major types of cells constitute bone tissue: osteoclasts are bone resorbing cells derived from a monocyte lineage of the hematopoietic stem cells, osteoblasts are bone forming cells descended from the mesenchymal stem cells, and osteocytes are bone matrix embedded cells originated from osteoblasts. Mature quiescent osteoblasts on the bone surfaces are distinguished as bone lining cells.
If osteoblastic bone formation overtakes osteoclastic bone resorption, it would lead to an increase in the bone mass. Elevated bone mass can be attributed either to a decrease in the bone resorption [due to the failures of osteoclastogenesis and/or osteoclast functions (390)] or an increase in the bone formation due to abnormally high activation of osteoblasts (77, 502). Deficiencies of osteoclastogenesis and/or osteoclast functions cause osteopetrosis or pycnodysostosis that may develop as a result of functional deficiency of some of the key molecules, such as M-CSF (555), RANKL (449), RANK (148, 269), c-Fos (315), NF-κB (180), Ctsk (133, 268, 414), carbonic anhydrase (445), and H+-ATPase (127, 239, 450), as observed in humans with genetic diseases and knockout mice. A hyperactivation of bone formation causes osteosclerosis or hyperostosis due to constitutively active mutations in mediators of the TGF-β and Wnt signaling, as it has been revealed by genetic analyses in patients (41, 502, 533) and genetically engineered mutant mice (77).
A prevalence of osteoclastic bone resorption over osteoblastic bone formation increases a risk of development of osteopenia or osteoporosis. Osteoporosis is well known as a bone metabolic disease associated with high risks for bone fractures that often lead to prolonged incapacitation, although patients may be unaware of the disease because of a lack of clear symptoms (408). A large population of postmenopausal women suffer from high turnover osteoporosis due to estrogen deficiency (451). Although more prevalent among women, elderly individuals of both sexes are at risk of developing osteoporosis (222). Osteoporosis may be also caused by a side effect of glucocorticoid treatment of autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) (520). Furthermore, astronauts often suffer from osteoporosis after prolonged space flight (220, 250, 433). Long-term bed rest also reduces bone mass, and malnutrition may worsen long-term immobility-induced osteoporosis (518). Osteoporosis is a more significant and frequent clinical problem than high bone mass pathologies. To treat osteoporosis, many kinds of drugs have been tested and applied in clinical practice. Almost all currently used antiresorptive pharmaceuticals, such as bisphosphonates (116), selective estrogen receptor modulators (SERMs) (400), vitamin D analogs (310, 312) and anti-RANKL antibody (78, 448) act as anticatabolic agents (408). In contrast, anabolic therapeutic agents enhance bone formation. Currently, PTH is the only anabolic agent approved for the clinical use. However, several kinds of anabolic compounds, such as Ca sensing receptor antagonists, anti-sclerostin antibody and anti-Dkk1 antibody are undergoing preclinical or clinical trials (86). In addition, strontium ranelate may potentially produce a dual therapeutic effect owing to its anti-resorptive and anabolic properties (121).
As described above, the relationship between osteoblasts and osteoclasts, termed “coupling,” is particularly important in the maintenance of bone homeostasis in remodeling. Differentiation of osteoclasts from monocytes/macrophages is regulated by M-CSF, RANKL, and OPG. These pivotal factors for osteoclastic differentiation are mainly derived from osteoblastic lineage cells (42). In studies on the osteoblastic-osteoclastic coupling, regulatory signals from osteoblasts to control osteoclasts as well as signals from osteoclasts to osteoblasts have been investigated. There are at least three possible mechanisms of the coupling. First, the factors released from the bone matrix by osteoclastic resorption might stimulate osteoblastic bone formation. Second, secreted factors from osteoclasts might control osteoblastic bone formation. Third, direct contact between osteoclast and osteoblast might regulate the differentiation and/or functions of both cell lineages.
Recent studies have clarified some aspects of the coupling. Active TGF-β1 released from bone matrices by osteoclastic bone resorption facilitates bone formation by recruitment of bone marrow mesenchymal stem cells to the bone resorptive sites. This recruitment is mediated by the SMAD signaling pathway (481) and can be modulated by PTH signaling (385, 534). It has been reported that PTH signals through G(α)s require LRP6, Wnt ligand coreceptor, to accumulate intracellular cAMP (508). Thus the factors released from the bone matrix may regulate the coupling. On the other hand, osteoclast conditioned medium can enhance mesenchymal cell migration and differentiation, suggesting that osteoclasts may secrete some factors to recruit osteoprogenitors to the site of bone remodeling, possibly through S1P and BMP6, and stimulate bone formation by activation of Wnt/BMP pathways (364). In addition, Zhao et al. (565) revealed that signaling through Ephrin B2 (Efnb2, an NFATc1 target gene) in osteoclast precursors represses osteoclastogenesis by inhibiting the c-Fos-NFATc1 cascade, whereas signaling through Eph receptor B4 (Ephb4) in osteoblasts facilitates osteoblastic differentiation. Overexpression of Ephb4 in osteoblasts increases bone mass in transgenic mice (314, 565). These and similar recent reports represent significant progress in our understanding of the mechanisms of tightly coupled processes of osteoclast-mediated bone resorption and osteoblast-mediated bone formation.
III. NUCLEAR RECEPTORS
A. Nuclear Receptor Gene Superfamily
NRs are DNA-binding transcription factors and form the NR gene superfamily that is well conserved among metazoans from C. elegans to human (299). In human, 48 members of the superfamily have been identified, and each of the known NR gene loci is precisely mapped on the chromosomes (FIGURE 2) (224). NR family members are classified into two groups. One group consists of NRs for which the endogenous ligands have been identified. NRs of the other group are referred to as orphan NRs, as their endogenous ligands remain unknown, if exist at all. Certain NRs were originally designated as orphan NRs, but later were renamed as “adopted” NRs when their potential endogenous ligands had emerged (137). It is still unclear if all of the known mammalian orphan NRs require an endogenous ligand for the activation. Since most of the known insect NRs appear to be “orphan,” and NRs in metazoan are assumed to evolutionarily derive from the same ancestral orphan NR, the presence of orphan NRs without functional endogenous ligands seems very likely in humans. However, synthetic ligands capable to modulate the function of some of these “ligandless” human orphan NRs have been recently identified (172, 452, 453). These findings raise a possibility that all NRs may be potential pharmacological targets, irrespective of the absence of endogenous ligands.
Figure 2.
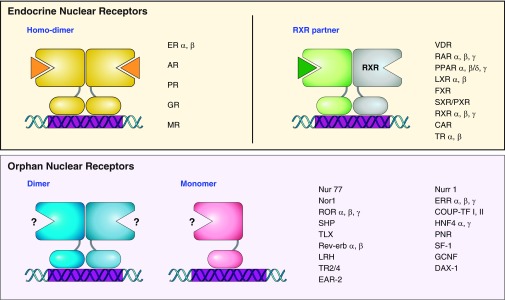
Nuclear receptor (NR) superfamily. NR superfamily members are divided into two main groups depending on the identification of endogenous ligands. NRs for which specific cognate ligands have been identified are known as endocrine NRs (top panels). NRs of this group bind to specific DNA elements as homodimers (top left) or heterodimers with RXR (top right). The other group is referred to as orphan NRs, for which endogenous ligands remain unknown (bottom panels). The orphan NRs bind to specific DNA elements as monomers or homo- and heterodimers. ER, estrogen receptor; AR, androgen receptor; PR, progesterone receptor; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; VDR, vitamin D receptor; RAR, retinoic acid receptor; PPAR, peroxisome proliferator-activated receptor; LXR, liver X receptor; FXR, farnesoid X receptor; SXR/PXR, steroid and xenobiotic receptor/pregnane X receptor; RXR, retinoid X receptor; CAR, constitutive androstane receptor; TR, thyroid hormone receptor; Nurr1, nuclear receptor related 1; Nor1, neuron-derived orphan receptor 1; ERR, estrogen-related receptor; COUP-TF, chicken ovalbumin upstream promoter-transcription factor; SHP, small heterodimer partner; HNF4, hepatocyte nuclear factor-4; TLX, homolog of the Drosophila tailless gene; PNR, photoreceptor cell-specific nuclear receptor; SF-1, steroidogenic factor 1; LRH, liver receptor homolog; GCNF, germ cell nuclear factor; TR2/4, testicular receptor 2/4; DAX-1, dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1, EAR-2, V-erbA-related protein 2.
B. NR as a Gene Regulator
As DNA-binding transcription factors, NRs recognize and bind specific DNA element sequences in the target gene loci to transcriptionally control expression of particular sets of target genes (FIGURE 3) (225, 299). However, presence of cis response elements might be insufficient for NR binding to occur, since recent ChIP-sequencing analysis revealed that the numbers of consensus or close to consensus NR binding genomic sequences were consistently greater than the numbers of actual NR binding sites. Significantly, most of the NR binding sites have been mapped in the vicinity of DNA binding sites for other transcription factors (46, 286, 510). Thus it appears that recruitment of NRs to chromatin for gene regulation may also require their interaction with other classes of DNA binding transcription factors that presumably serve as anchors for NR association with chromatin. NR bound to chromatin may regulate gene expression as a transcriptional activator or repressor, depending on the presence of ligand and/or other transcription factors, and context of a given target gene promoter and/or enhancer elements (215, 553). Consistently, the mode of NR action may diverge in a promoter-, enhancer-, and/or cell-context-dependent manner. In general, NRs mediate most of the biological actions of their cognate ligands through positive and negative transcriptional regulation of target genes in response to the ligand binding to NRs. On the other hand, orphan NRs act as constitutive transcriptional regulators, either activators or repressors (215, 553). DNA-binding transcription factors bound to chromatin alongside of NR may be effective to modulate transcriptional activity of this NR. The products (mRNA or miRNA) of NR target genes exert biological action as downstream factors of the NR-mediated signaling pathway (20, 74, 544) (FIGURE 3).
Figure 3.
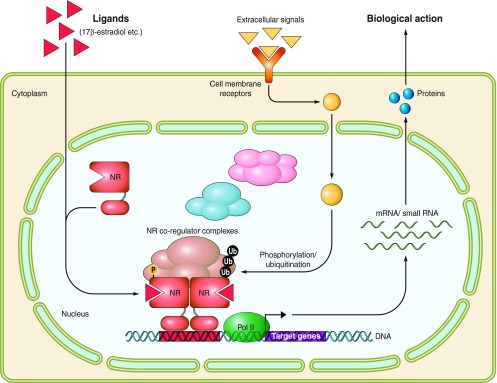
Schematic illustration of ligand-dependent transcriptional controls by nuclear receptors. Ligand binding induces dimerization and nuclear translocation of NR dimers. NRs recognize and bind specific DNA element sequences in the target gene regulatory regions to control the transcription of targets genes. Liganded NRs associate with transcriptional coregulator complexes. The transcriptional activity of NRs is modulated through various posttranslational protein modifications (for example, shown phosphorylation and ubiquitination) in response to the signals from activated cell membrane receptors. The transcripts (mRNA or miRNA) of target genes exert biological actions as downstream factors of the NR-mediated signaling pathway.
C. NR Structure and Function
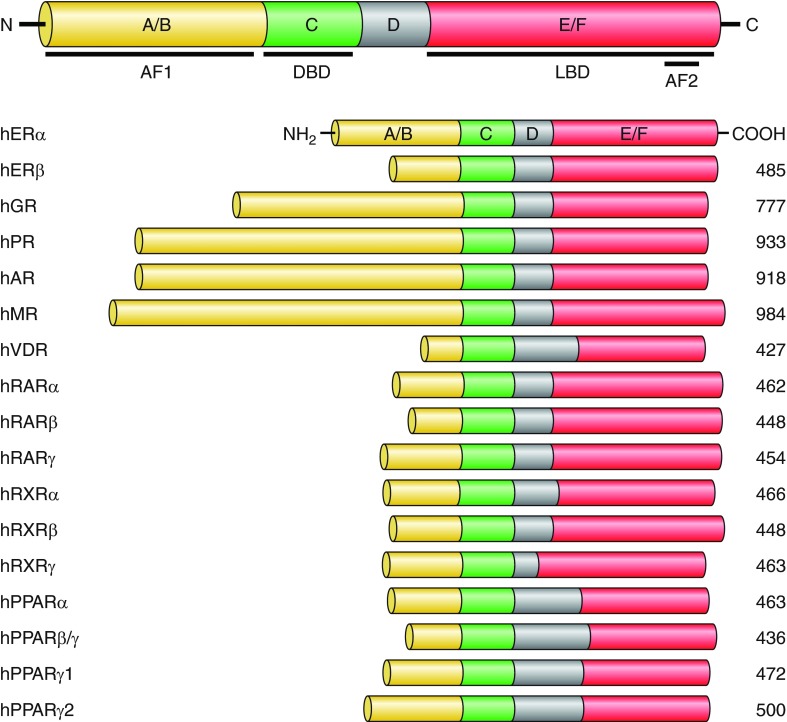
As the members of the same gene superfamily, all NRs bear significant structural and functional similarities. In NR protein primary structure, several distinct functional domains have been identified and designated A to E from the NH2-terminal end (FIGURE 4) (299). The hallmark of the NR gene superfamily members from different species is a presence in the protein middle region, or C domain of two zinc fingers formed by four cysteine residues. This C domain is evolutionary highly conserved and serves as a DNA binding domain (DBD). Ligand binding domain (LBD) localizes in a less conserved E domain that is composed of 12 α-helixes forming a hydrophobic cave in the center to accommodate the ligand. Based on X-ray structural analysis, the LBD domain is depicted as a globular structure. It has been established that the ligand binding triggers drastic shift in the positioning of the most COOH-terminal α-helix 12 (H12), resulting in the coregulator switching (106, 205, 428). Ligand binding also induces dimerization followed by nuclear translocation of NR dimers. Both the E and A/B domains function as docking sites for transcriptional coregulators, and thereby the activities of these domains in transcriptional controls are cell type specific, presumably owing to a variety of coregulator species expressed in a given cell type (482, 492). The transcriptional activity of E and A/B domains can be also modulated through posttranslational protein modifications (PTMs) (usually acetylation, phosphorylation or ubiquitination) in response to signals from activated cell membrane receptors (FIGURE 3) (166, 213, 279).
Figure 4.
Structures and functional domains of nuclear receptors. The members of NR superfamily bear significant structural and functional similarities. Several distinct functional domains in NR proteins have been identified and designated A to E/F from the NH2 to COOH terminus. The A/B domain is the least conserved NR region that contains a ligand-independent transcriptional activation function 1 (AF1). The evolutionary highly conserved C domain contains two zinc fingers formed by four cysteine residues and serves as a DNA binding domain (DBD). The COOH-terminal E/F domain harbors a ligand binding domain (LBD) that is composed of 12 α-helixes forming a hydrophobic cave in the center to accommodate the ligand. The LBD contains a ligand-dependent transcriptional activation function 2 (AF2) domain.
D. Mechanism of Transcriptional Controls by NRs
Expression of NR target genes is regulated at the transcriptional level. The principles of transcriptional activation of target genes by NRs are comparatively well established in terms of transcriptional events with the characterization of transcriptional NR coregulators (215, 553), whereas the molecular basis underlying NR-mediated transcriptional repression is far from being clear (334, 373).
Ligand binding to NR causes dissociation of transcriptional corepressors and association of coactivators through ligand binding-induced conformational alterations of NR, including drastic shifting of the α-helix 12 position in the LBD (106, 428). NR corepressors form multisubunit complexes, in which histone deacetylases (HDACs) represent functional key subunits (156, 215, 553). Since HDACs catalyze histone deacetylation, HDAC-associated unliganded NRs are potent to inactivate the chromatin in the vicinity of NR binding sites. Consistently, histone acetyltranferases (HATs) are known to coactivate NR-induced transcriptions through histone acetylation and also to form multisubunit complexes (206, 215, 553). In addition, general NR coactivators represent physical mediators bridging NR with basic transcription machinery for promoting efficient transcription by RNA polymerase II (389, 558).
IV. NUCLEAR RECEPTORS AND BONE METABOLISM: STEROID HORMONE RECEPTORS
A. Estrogen Receptors
Two subtypes of Estrogen Receptors (ERs), α and β, exert biological effects of estrogens (74, 243, 299). Nuclear estrogen-bound ERs mediate the genomic actions of estrogens through estrogen response element (ERE)-dependent transcription of target genes (46, 47, 213, 214). In contrast, immediate estrogen responses, so-called nongenomic actions, probably involve cytoplasmic ERs and/or uncharacterized estrogen receptors on the cell membrane (329).
Ligand-activated ERα and ERβ specifically bind to EREs in the regulatory regions of the target genes as homo- (α-α, β-β) and/or hetero- (α-β) dimers (243, 299, 377). There is no significant difference between ERα and ERβ in the binding affinity for endogenous estrogens. However, ER subtypes seem to have different affinities for SERMs (149). Compared with ERα, ERβ displays a significantly lower ability of hormone-induced transcriptional activation when bound with SERMs (275). Therefore, ERβ can be considered as a possible dominant negative equivalent of ERα that modifies the induction of endogenous estrogen target genes. The molar or quantitative ratio of ERβ to ERα in a cell may indicate a cell's sensitivity and the extent of its biological responses to estrogens (177). Expression patterns of ERα and ERβ overlap in various types of cells, organs, and tissues.
1. Estrogen functions in bone
Estrogens are female steroid hormones and crucial regulators in various biological processes such as the development and maintenance of female reproductive organs and the maintenance of bone. Postmenopausal osteoporosis caused by estrogen deficiency is one of the most widely recognized locomotive diseases, especially in developed countries. Osteoporosis in both genders is now considered a serious pathological condition of middle age and elderly people because of the high risk of fracture, often leading to high morbidity and mortality (105, 343).
In women with impaired estrogen signaling or estrogen deficiency, there is a noticeable reduction of bone mass due to increased or imbalanced bone resorption versus bone formation. The postmenopausal osteoporotic bone phenotype can be experimentally induced in female rodents by ovariectomy (OVX) followed by estrogen depletion (152, 153). Clinically available estrogens and SERMs are effective in normalizing increased bone resorption and can rescue bone loss in osteoporotic patients and ovariectomized animals (96, 153, 344). Multiple genetic studies and clinical observations indicate that male patients lacking either estrogen biosynthesis or ERα function exhibit the symptoms of osteoporosis (200, 447). Thus it is obvious that estrogens exert osteoprotective effects and play a pivotal role in the maintenance of skeletal homeostasis in both genders.
To clarify the physiological functions of ERs in vivo, the first mice lacking ERs were generated in the early 1990s (74). However, neither male nor female of these ER-deficient mice displayed typical osteoporotic phenotypes (74, 469, 531). Consequently, the roles of ERs in bone health remained unclear, and indirect mechanisms through extraskeletal tissues have been assumed to account for the osteoprotective actions of estrogens (357, 560).
2. Conventional ER-deficient mice
The first generated ERα knockout mice (ERαKO) displayed various phenotypic abnormalities. In accord with previous in vitro and in vivo evidences, ERαKO exhibited poorly developed female reproductive organs, and their phenotypes were similar to ovariectomized mice, except bone phenotypes (74). However, the first ERαKO expressed shortened ER transcripts, and residual ERα activity was postulated to exert the endothelial effects of estrogens (368). Later, complete ERα knockout mice (ERα−/−) were generated without detectable ERα transcripts (103). Phenotypes of these ERα−/− mice substantiated the significance of ERα for female reproductive organs reported earlier in the ERαKO mice (103). Several groups have generated mice with a disrupted ERβ gene. However, the reported phenotypes of differently generated ERβ-deficient mice have been inconsistent with the abnormalities of estrogen target tissues (177, 531). The inconsistencies between these initial studies might be due to incomplete knockouts of the ERβ (ERβKO), which would permit expression of partial transcripts. Although the phenotypic differences reported by these studies could be based on residual ERβ activity, the molecular mechanisms of the differences in observed phenotypes remain unclear. Interestingly, recently generated complete ERβ knockout (ERβ−/−) mice exhibited significantly fewer defects (11, 103). Since ERα−/− mice display much more severe abnormalities in estrogen target tissues than ERβ−/− mice (11, 74, 90, 469, 531), it has been postulated that the major physiological actions of estrogens are mediated through ERα, which appears to be the primary estrogen receptor.
Unlike other estrogen target tissues or organs, the osteophenotype of female mice was not significantly altered by disruption of ERα (ERα−/−), ERβ (ERβ−/−), or both (ERαβ−/−) ER genes (274, 437, 532). These results were unexpected, because OVX in the same mouse strains decreased bone mass due to higher bone turnover, resembling that seen in estrogen deficiency-induced postmenopausal osteoporotic women (152, 343, 401, 560).
Unexpectedly, female ERα−/− mice displayed increased bone mass with low bone turnover, while ovariectomized females in the same strain exhibited decreased bone mass due to increased bone resorption (274, 437). The absence of the negative feedback loop in estrogen synthesis due to the lack of ERα in the hypothalamus in ERα−/− mice leads to a remarkably high level of serum testosterone, a major estrogen precursor. The absence of osteoporotic phenotypes in mice lacking ERα might be due to an excessive amount of circulating testosterone that results in enhanced androgen signaling and osteoprotective effects (401). Supporting this possibility, OVX decreases bone mass in ERα−/− mice, whereas androgen administration increases bone mass in ovariectomized ERα−/− (73, 436).
ERβ−/− female mice exhibit slightly decreased bone resorption and increased trabecular bone mass without changes of serum levels of sex steroid hormones (437). Since bone loss in ovariectomized ERα−/− females can be partially rescued by estrogen treatment (436), it is possible that ERβ partially mediates the osteoprotective actions of estrogens (436, 531). The levels of circulating sex steroid hormones were also elevated in double knockout (ERαβ−/−) mice, similar to that observed in ERα−/−. In contrast to ERα−/− or ERβ−/− mice, ERαβ−/− mice exhibited a significant reduction in the bone mass, but it was less severe than that observed in OVX wild-type females (73, 274, 437). Although these findings suggest coordination between ERα and ERβ in the osteoprotective action of estrogens, female mice lacking both ERα and ERβ fail to recapitulate the bone phenotype of estrogen-deficient postmenopausal osteoporosis. It can also be postulated that androgen receptor (AR) may partially compensate for the absence of ERs in these mice at high levels of serum sex steroid hormones.
Interestingly, male mice disrupted in one (ERα−/− or ERβ−/−) or both (ERαβ−/−) of the ER receptors displayed no clear bone mass alteration. Furthermore, male ERα−/− mice exhibited increased trabecular bone mass due to decreased bone resorption with elevated serum level of testosterone (73, 274, 437). This indicated that the high level of serum testosterone in male ERα−/− mice counteracts diminished estrogen action through facilitated AR signaling in the bone (217, 436). However, these observations are incompatible with severe osteoporosis and unfused epiphyses observed in male patients harboring nonfunctional ERα due to hereditary mutations in spite of high level of circulating testosterone (74, 200, 503). Estrogen treatment could not rescue the phenotypes of these male patients.
Aromatase (CYP19) is an enzyme that catalyzes conversion of androgens into estrogens. Clinical observations of reduced bone mass in aromatase-deficient male patients support the idea that ERα-mediated estrogen signaling plays a pivotal role in the maintenance of the male skeleton (38, 294, 447). As serum estrogen levels are apparently lower in males than in females, the local synthesis of estrogens from testosterone by aromatase appears to maintain physiological osteoprotective estrogen action in male bones. Aromatase-deficient male patients displayed an osteoporotic bone phenotype and unclosed epiphyses as do ERα-deficient men (200). As expected, such skeletal defects could be compensated by estrogen treatment only in aromatase-deficient, but not in ERα-deficient patients (356). Bone loss phenotype was experimentally recapitulated in male aromatase-deficient mice (18, 124); however, increased bone length due to unfused epiphyses in human male patients was not observed in male aromatase-deficient mice. This contradiction is presumably explained by the innate absence of epiphyseal plate closure in mice (18, 503). Thus the osteoporotic bone phenotypes observed in inherited aromatase-deficiency male patients and in experimentally generated aromatase-deficient male mice indicate that aromatase-mediated locally synthesized estrogens are critical for the maintenance of bone health in males.
3. Osteoclast-specific ERα-deficient mice
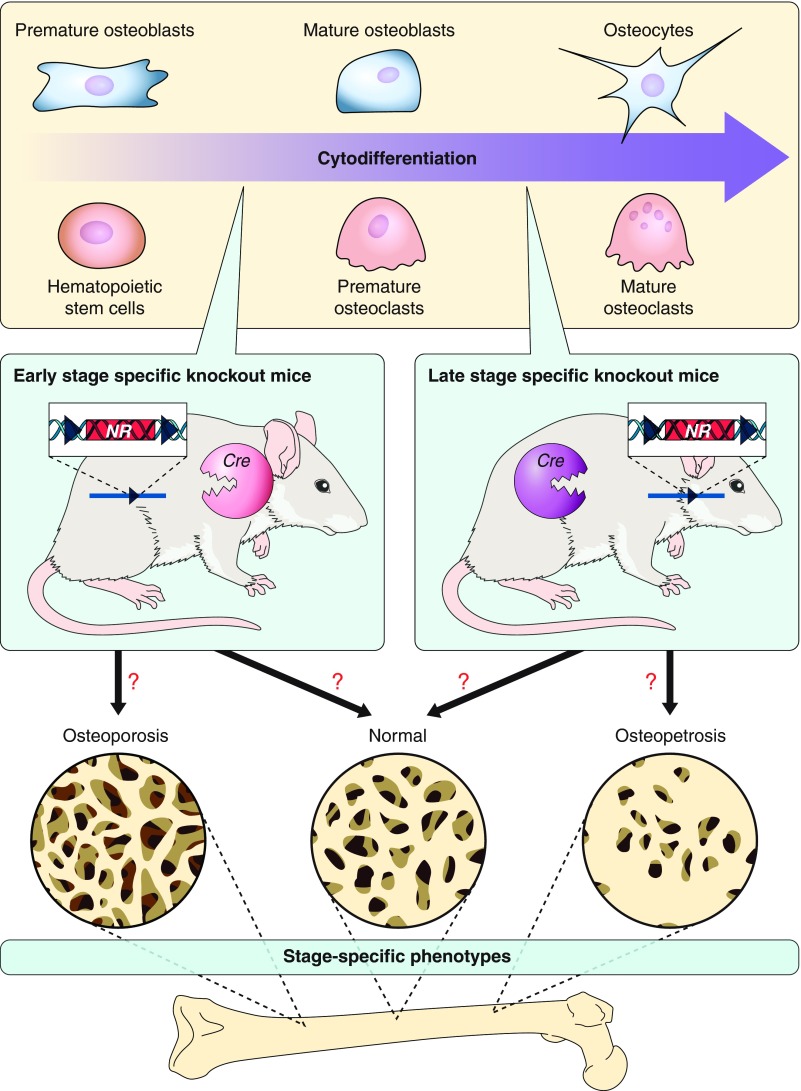
As described above, studies of systemic estrogen deficiency and impaired estrogen signaling in experimental animal models have not identified the osteoprotective mechanisms of estrogens. In fact, systemic disruption of estrogen signaling in whole animals by conventional knockout of the ER genes disturbs the endocrine system and can lead to potential pathological outcomes such as inflammatory cytokine production (357, 560). Thus it appeared that a bone cell-specific knockout of the ER genes was essential to directly evaluate the role of estrogen signaling in the bone without influences from its systemic actions or possible secondary effects.
The osteoprotective action of estrogens is associated with a regulation of bone resorption and maintenance of normal bone remodeling. Therefore, we selectively disrupted the ERα gene in mature osteoclasts (339). As cathepsin K is abundantly expressed only in the mature osteoclasts (268), the Cre recombinase gene was knocked into the gene locus of cathepsin K, and the resulting Cre knock-in mouse line (Ctsk-Cre) was shown to express Cre recombinase at detectable and effective levels only in sufficiently developed osteoclasts, thus Ctsk-Cre/+ mice seems to be heterozygous Ctsk knockout mice. Osteoclast-specific ERα knockout mice (ERαΔOc/ΔOc) were generated by crossing the Ctsk-Cre mice with floxed ERα gene mice that were previously used to obtain complete ERα knockout mice (ERα−/−) (103). As expected, ERαΔOc/ΔOc mice displayed neither clear alterations of circulating sex steroids, nor follicle stimulating hormone (FSH), nor phenotypic abnormalities in growth and reproduction regardless of gender. Significant bone mass reduction in trabecular bone with high turnover bone metabolism was observed only in the ERαΔOc/ΔOc females (and not males) at 8 wk of age. OVX induced only a slight bone loss in the ERαΔOc/ΔOc mice when compared with the bone loss in the ovariectomized control mice. Moreover, 17β-estradiol failed to recover bone mass in the ovariectomized ERαΔOc/ΔOc females.
Note that bone mass reduction is evident primarily in trabecular areas in estrogen-deficient women and female rodents. Thus the osteoporotic phenotypes observed in ERαΔOc/ΔOc females seem to substantiate the idea that the osteoprotective action of estrogens is mediated, at least in part, by osteoclastic ERα in females (339). We found that the pro-apoptotic actions of estrogens in mature osteoclasts underlie the antiresorptive effects of estrogens in bone since estrogens can initiate osteoclastic apoptosis through induction of the Fas ligand gene (Fasl) (339). Furthermore, bone phenotypes of osteoclastic ERα knockout mice produced by using different Cre expression mice lines have confirmed that estrogens regulate the life span of osteoclasts and exert their osteoprotective action through the osteoclastic ERα (305). These observations agree with previous evidences that estrogen deficiency in mice induced by OVX leads to reduced apoptosis and extended life span of the osteoclasts (171).
Osteoporotic phenotypes observed in male patients harboring loss-of-function ERα mutation (18, 124, 447) would suggest that the osteoprotective action of estrogens should be also crucial in male bone physiology. However, no detectable abnormality has been observed in bone tissue in ERαΔOc/ΔOc male mice (339). It is not clear why ERαΔOc/ΔOc males do not display abnormal bone phenotypes, and we can only speculate about possible mechanisms of osteoprotective estrogen actions in males. First, high levels of circulating androgens and activated AR may play pivotal roles in male bone formation and resorption, even in the absence of ERα. Second, in Ctsk-Cre mice, ERα is likely disrupted only in mature osteoclasts, whereas activated ERα may fulfill crucial functions at earlier stages of osteoclastogenesis regardless of sex. There may be stage-specific roles of ERα action during osteoclast differentiation and maturation that differ between males and females. Besides controlling the life span of osteoclasts, estrogens may also have inhibitory effects on osteoclastogenesis and/or osteoclastic function in vitro (426, 460). Generation and analyses of differentiation stage-specific osteoclastic ERα knockout mice may resolve these issues. Third, ERα in osteoblasts might mediate the osteoprotective actions of estrogens, and the osteoblastic ERα might have a dominant function in estrogen osteoprotective action in males. Estrogens have been shown to stimulate osteoblastogenesis in vitro (351). Furthermore, Krum et al. (246) reported strong evidence that Fasl is a direct ER target gene in cultured osteoblasts, as determined by genome wide ChIP on chip analysis. Thus it is possible that estrogens could facilitate apoptosis of mature osteoclasts via induction of Fasl expression in both osteoclasts and osteoblasts (245). Previous reports demonstrating antiapoptotic action of estrogens on osteoblasts (240, 241) suggest the existence of different pathways or mechanisms of estrogen action in these types of bone cells. The existence of cell- and tissue-specific regulation of ER transcriptional activity has been recently supported by demonstration that different pioneer factors facilitate ER chromatin binding in different types of cells. While FOXA1 has been shown to be critical for ERα transcriptional action in breast cancer and liver cells (173, 202), GATA4 appears to act as a primary pioneer factor for ER recruitment to target chromatin loci in osteoblasts (327). Generation of osteoblast-specific ER knockout mice is, therefore, essential to elucidate the impact of osteoblastic ER on bone metabolism and cell specificity of ER signaling pathways in osteoblasts and osteoclasts. In addition, there are other types of cells in bone, such as bone marrow cells and osteocytes that remain to be explored for the involvement of their ERs in the osteoprotective action of estrogens, especially in males.
Thus estrogens exert osteoprotective actions in both sexes. Important features observed in postmenopausal osteoporosis, such as bone loss with high bone turnover, have been recapitulated in osteoclast-specific ERα knockout female mice (339). This observation is in apparent contrast to the phenotypes of mice with conventional or systemic ER knockouts that displayed increased bone mass. In addition, it seems that osteoclastic ERα mediates estrogen-dependent reduction of bone resorption through induction of osteoclastic apoptosis in females. Primary cultured osteoclasts derived from bone marrow cells of males and females respond in the same way to estrogen. However, osteoclast-selective ablation of ERα in male mice failed to recapitulate bone defects or elongated long bones due to unfused epiphyses (339) that were observed in male patients with dysfunctional estrogen signaling (200, 447). This contradiction indicates gender-specific mechanisms in the osteoprotective actions of estrogens in vivo. For example, in male bones, positive effects of estrogens on bone mass might be preferentially mediated by ERα in other types of cells rather than through the antiresorptive action of osteoclastic ERα, which is pivotal in females. This hypothesis can be examined by comparison between various cell type-specific ERα knockout phenotypes in males and females to clarify sex-specific molecular and cellular mechanisms of anabolic (not anticatabolic) action of estrogens in the skeleton. The compensatory effects of AR with high concentrations of circulating androgens may elucidate different physiological results of osteoclast-specific ERα knockouts in male mice. This possibility may be clarified with generation of double osteoclast-specific AR and ERα knockout.
4. Indirect osteoprotective action of estrogen
Systemically circulating estrogens affect the function of many types of cells and tissues. Thus it is possible that bone mass regulation by estrogens could be influenced by secondary effects through other estrogen target organs that in response to estrogens may secrete bone remodeling factors in an endocrine or paracrine manner. It has been postulated that the osteoprotective effects of estrogens might be mediated by such estrogen-induced factors. For example, insulin-like growth factors (IGFs) that are known to stimulate osteoblastogenesis, are secreted mainly by the liver. Their effects on the skeletal development are influenced by the growth hormone (GH), especially during longitudinal growth stages (435, 441, 503). Thus, IGFs production induced by estrogens might be a part of the osteoprotective effects of estrogen. However, the molecular mechanism of estrogen-induced IGF regulation in bone is largely unknown.
FSH can mediate indirect estrogen action on maintenance of bone mass (560). FSH is an upstream pituitary hormone that stimulates estrogen synthesis in the ovary. FSH secretion is under negative-feedback regulation of circulating estrogens that activate ERs in the hypothalamus, consequently suppressing FSH production. Estrogen deficiency in postmenopausal women is usually associated with increased levels of circulating FSH. It has been reported by Sun et al. (468) that FSH signaling-defective female mice displayed increased bone mass with low bone turnover and without significant alteration of serum estrogen levels. This group also showed that FSH positively affected osteoclastogenesis. Thus they hypothesized that increased FSH levels caused by estrogen deficiency could reduce bone mass via acceleration of the osteoclastogenesis (468). However, there is some inconsistency in clinical reports on the relationship between levels of FSH and bone mass and mineral density in postmenopausal women (92, 147, 457). Therefore, it is necessary to determine the effects of FSH on the bone metabolism under normal physiological concentrations of circulating sex steroids.
Estrogen deficiency-induced osteoporosis is associated with persistently high turnover bone metabolism, which causes progressive bone loss. This indicates that osteoclastogenesis and/or osteoclastic activities are enhanced under decreased estrogen concentrations. Since it is well known that inflammatory cytokines are able to induce osteoclastogenesis in vitro, various cytokines expressed by cells other than osteoblasts or osteoclasts have been examined for a possible contribution to osteoclastogenesis induced by estrogen deficiency (357, 405). From this point of view, immune cells in the bone marrow could mediate bone remodeling via paracrine secretion of various pro- and/or antiosteoclastogenic cytokines. Among immune cells, T cells are considered as the most likely regulators of bone resorption. Adaptive immune responses are activated by estrogen deficiency, leading to stimulated production of interleukin-7 and IGF-I by activated T cells residing in bone followed by increased secretion of interferon-γ (IFN-γ) (406, 412). IFN-γ is one of the factors related to osteoclastogenesis in concert with locally produced RANKL and TNF (477, 483). In cell culture systems, it was shown that these cytokines activated transcription factors, such as AP-1 and NF-κB, known to promote osteoclastogenesis. Recent study suggests that RANKL expressed in B cells may play a role in inflammation-induced bone loss. Thus loss of bone mass under conditions of estrogen deficiency can be ascribed, at least in part, to a resultant increase of pro-osteoclastogenic inflammatory cytokine production (357). However, this cytokine-dependent acceleration of differentiation and increased activity of osteoclasts is secondary to the estrogen deficiency and, therefore, cannot clearly explain the molecular mechanisms of beneficial effects of estrogens on bone metabolisms.
B. Androgen Receptor
Androgen receptor (AR) acts as a ligand-dependent transcription factor by homodimerizing and binding to a specific DNA sequence, the androgen response element (ARE). Males have a higher bone mineral density and lower risk for fracture and osteoporosis than females (503). Reduction of circulating androgen is closely related to various symptoms and features of aging, such as a cognitive decline, reduction of muscle mass and muscle strength, decrease in bone mass, and increase in abdominal fat mass (108). The elevated strength of male bone is likely caused by the anabolic effects of androgens. Supporting that view, AR knockout (ARKO) mice display high bone turnover with increased bone resorption and associated reduction in trabecular and cortical bone mass, but without change in the bone morphology. Bone mass reduction induced by orchiectomy in male ARKO mice was partially reversed by treatment with testosterone, which can be converted into estradiol. In primary cultured osteoblasts and osteoclasts of ARKO mice, it was shown that AR function was needed for androgen-mediated suppression of osteoclastogenesis (217). However, it has not been determined whether androgen-AR signaling directly targets bone forming osteoblasts or bone resorptive osteoclasts.
It remains unclear, however, whether bone loss in ARKO mice is caused by a systemic endocrine disturbance or represents a direct consequence of the lack of AR in the bone tissue. Notini et al. (349) analyzed the role of AR in osteoblasts using osteoblast-specific AR deficient mice (ObARKO) generated by mating 2.3 kb-Col1a1-Cre with AR flox mice with targeted exon 3 (DNA-binding domain). They concluded that disruption of the DNA binding-dependent functions of the AR, specifically in osteoblasts in male mice, led to increased bone resorption and decreased structural strength of the long bones, resulting in a reduction in trabecular bone volume at 32 wk of age. This group also described bone phenotypes of another osteoblast-specific ARKO, using osteocalcin promoter-driven Cre recombinase. They concluded that androgens exert their functions via AR in osteoblasts to preserve bone mass by controlling bone resorption and bone formation coordinated with matrix synthesis and mineralization (60). Taking these observations together, osteoblastic AR has direct functions in the maintenance of bone metabolism as well as influence on secondary effects of endocrine systems. More detailed study is required to clarify the molecular basis of androgen anabolic action in bone.
C. Progesterone Receptors
The progesterone receptor (PR) exists in two isoforms, A (PR-A) and B (PR-B), that are generated from the same gene through transcription from alternative promoters. The expressions of both isoforms are regulated by the estrogen/ER signaling (211). Progesterone reportedly exhibits anti-estrogen effects in uterine epithelium in mice so that ligand-bounded PR-A suppresses the transcriptional activities of ER and PR-B. PR-A knockout (PRAKO) mice generated by selective ablation of the PR-A isoform exhibited enhanced responsiveness of uterine epithelial growth to progesterone (331).
The function of PR in the bone tissue is less understood than that in reproductive organs. Osteoblasts (292) and osteoclasts (369) express both PR-A and PR-B (550), suggesting that progesterone may exert its actions on both types of bone cells. Some in vitro studies showed that progesterone can be metabolized by osteoblasts (388) and that progesterone bounded PR exerts its anti-apoptotic action on osteoblast through inhibiting the activations of caspase-9 and -3 (514). Recent studies using conventional PR knockout (PRKO) mice (289) reported that PRKO mutants exhibited high bone mass with increased bone formation and reduced osteoclast surfaces (398). These findings agree with clinical observations that oral contraceptives containing progesterone can modestly reduce bone mineral density (76, 79). An in vitro study indicated that osteogenic differentiation is accelerated in primary cultured bone marrow cells derived from PRKO mice compared with that of WT mice (550). Taken together, these findings suggested that progesterone might act negatively on bone homeostasis by inhibiting osteoblastic differentiation and bone formation. However, a number of important issues remain unanswered, including whether progesterone directly affects bone tissue? Interestingly, recent studies of progesterone effects on breast cancer have shown that progesterone/PR stimulation can induce RANKL expression in mammary gland epithelial cells that is believed to associate with an onset and development of breast cancers (14, 146, 201, 288, 421). These reports suggest that progesterone/PR signaling might play a regulatory role in bone metabolism through effects on the RANK/RANKL-induced osteoclastogenesis.
D. Glucocorticoid Receptors
Similar to other NRs, the glucocorticoid receptor (GR) acts as a ligand-dependent transcription factor to mediate genomic actions of glucocorticoids. GR forms homodimers or heterodimers with the other transcription factor to regulate transcription in targeted cells. Glucocorticoids (GCs) can accelerate bone resorption and reduce bone formation (44, 181). Excessive exposure to glucocorticoids (resulting from Cushing's syndrome or prolonged steroid therapy) decreases bone mineral density (BMD) and may lead to development of glucocorticoid-induced osteoporosis (GIO) (157, 283, 284, 355, 363). In GIO, bone mass reduction, which is more apparent in trabecular than in cortical bones, appears to be closely related to both the dose and duration of the treatment with glucocorticoids (44).
GCs suppress bone-forming osteoblasts in vivo through facilitating the apoptosis (520–522) and suppression of osteoblast-related differentiation marker genes such as Runx2 and type I collagen (372). Reportedly, these steroids have the potential to induce the expression of adipocytic genes in the bone marrow mesenchymal stem cells (371). Inconsistent with these observations, however, the ability of cultured osteoprogenitor cells to form mineralized bone nodules was apparently accelerated by treatment with GCs (15, 384). This paradox suggests that suppressive effects of GCs on bone formation in vivo may reflect GCs action in nonosteoblastic bone cells that then release secondary paracrine factors directly affecting the osteoblastic bone formation.
GR function is critical at least during the postnatal development, as GR knockout (GRKO) mice die within a few hours of delivery due to respiratory failure (67). Due to the neonatal lethality, the physiological functions of GR in the tissue and organs of mature adults have not been clarified. However, Kim et al. (223) reported the phenotypes of conditional GR knockout mice in osteoclastic lineage cells (OcGRKO) using mice with the lysozyme promoter-driven Cre recombinase expression. Using this mouse line, they compared the effects of dexamethasone (DEX) in primary cultured osteoclasts derived from bone marrow cells of the control and OcGRKO mice to determine direct impact of GR action in the bone-resorptive cells. Although DEX enhanced the life span of osteoclasts, their bone resorptive activity in vitro was suppressed by DEX. OcGRKO mice are resistant to the inhibitory effects of GCs on bone formation. Consistently, GCs fail to decrease bone formation in osteoblast-specific GR knockout mice produced by using the Runx2-Cre mouse line (393). These results may indicate that the negative effects of GCs on bone formation are mediated through osteoblastic GR. However, in contrast to the effects of GCs in OcGRKO mice, in mice with mutation disrupting GR homodimerization treatment with GCs resulted in reduction of bone mass in vivo, or enhanced apoptosis, and suppressed osteoblast differentiation in vitro. Interestingly, in this mouse line, the expression levels of AP-1-inducible inflammatory cytokines is increased, and GRs retain the ability to form heterodimers with Jun. These results suggest that some of GC effects on osteoblasts may be due to suppression of cytokines, such as interleukin-11, through interaction between monomeric GR and AP-1 (393). Taken together, direct glucocorticoid-GR signaling in bone can reduce bone mass though complex intra- and intercellular regulatory interactions between different types of bone cells.
E. Mineralocorticoid Receptor
Mineralocorticoid receptor (MR) mediates genomic actions of mineralocorticoid hormones, such as aldosterone. It is well known that MR is expressed in distal renal tubules, blood vessels, and the heart and has been shown to control renal functions and blood pressure. The structure of MR is similar to GR, and MR shows functional homology to GR including binding to identical response elements (13). Moreover, in vitro binding studies show that glucocorticoids and aldosterone bind to MR with similar affinities and induce gene transcription of the MR target genes (12). Consequently, MR is also able to mediate the action of glucocorticoids, and effects of glucocorticoids should be considered when analyzing MR function. Although patients with high concentration of mineralocorticoids exhibited osteopenia, high risk of fractures, and short stature (326), the function of MR in bone tissue is largely unknown.
One study reported that MR is expressed in bone-resorbing osteoclasts and bone-forming osteoblasts (25). Another study showed that vascular smooth muscle cells express functional MR and that ligand-activated MR facilitated osteoblastic differentiation and mineralization (187). These reports, however, cannot explain clinical observations that high concentration of mineralocorticoids may induce osteopenia, since it remains unclear whether this osteopenia is caused by the increase of osteoclastic bone resorption or reduction of osteoblastic bone formation. Obviously, further analysis of the biological effects of mineralocorticoids and glucocorticoids through MR is required to clarify MR functions in vivo.
V. NUCLEAR RECEPTORS AND BONE METABOLISM: FAT-SOLUBLE VITAMIN RECEPTORS
Fat-soluble vitamins A and D are considered as pro-hormones. Strictly speaking however, both are neither vitamins nor hormones. They are normally obtained from dietary sources or, in the case of vitamin D, synthesized in the skin in response to ultraviolet light exposure and require further enzymatic modifications in specialized cells and tissues to produce physiologically active derivates capable of activating corresponding intracellular receptors (101, 380).
A. Vitamin D Receptor
The vitamin D receptor (VDR) is a member of the thyroid hormone and retinoic acid receptor subfamily of nuclear hormone receptors that mediates physiological actions of the biologically active form of vitamin D, 1,25-dihydroxyvitamin D3, or 1,25(OH)2D3. Following activation by the ligand, VDR heterodimerizes with retinoid X receptor (RXR), and VDR/RXR complexes bind to specific genomic DNA sequences (vitamin D response elements, VDREs) in the proximal promoter and distal regulatory regions of target genes in a wide variety of cells to regulate proliferation, differentiation, apoptosis, mineral homeostasis, immune response, metabolism, and some other functions (39, 155, 212, 321). Ligand-bound VDR/RXR heterodimers trans-activate target genes through binding at two direct hexameric repeats (A/G)G(G/T)TCA spaced by three nonspecific nucleotides, or so-called DR3 (direct repeat with a 3 bp spacer) VDRE. VDR also mediates 1,25(OH)2D3-dependent trans-repression through binding to negative VDREs (nVDREs) that contain a single copy of a consensus DNA repeat of the positive DR3 VDRE. Mechanisms of negative regulation by VDR appear to be more diverse and are less understood. They involve VDR action as a cofactor that represses gene expression via interaction with other transcription factors and without direct binding to DNA (128, 334).
It has been long established that VDR is an important regulator of bone and cartilage functions. The significance of VDR in human bone physiology can be illustrated by the abnormalities observed in patients with hereditary hypocalcemic vitamin D-resistant rickets, also known as vitamin D-dependent hereditary rickets type II (VDDR-II). VDDR-II is an autosomal recessive disease that develops as a consequence of loss-of-function mutations in the human VDR gene. Clinical manifestations of VDDR-II include low bone mineral density, rachitic malformation, growth retardation and short stature, hypocalcemia, hypophosphatemia, hyperparathyroidism, and increased serum levels of alkaline phosphatase and 1,25(OH)2D3 (128, 297, 334). Similar defects, except elevated serum 1,25(OH)2D3, are typically observed in patients with pseudovitamin D deficiency, or vitamin D-dependent hereditary rickets type I (VDDR-I) that develop as a result of inactivating mutations in the 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) gene affecting the biosynthesis of 1,25(OH)2D3 (216, 226).
Several research groups generated independent lines of VDR knockout mice (272, 500, 556), and phenotypes of the VDR−/− animals displayed arrays of abnormalities commonly observed in VDDR-II patients (TABLE 1). Considering that classical functions of the VDR had been long associated with bone growth and maintenance, and that important VDR target genes were known to express in bone cells, it was rather surprising that the bone phenotype of homozygous VDR-deficient mice was rescued when the blood calcium and phosphate levels in the mice were normalized by a special diet (271, 306, 307). The successful rescue of bone phenotype in VDR−/− mice implies that the fundamental biological role of VDR is to promote intestinal calcium and phosphate absorption, particularly under conditions of low calcium availability. Therefore, these and earlier studies on vitamin D-deficient rats (150, 523) suggest that vitamin D and its biologically active metabolites are not required for reproduction and development, and that VDR signaling is redundant for the promotion and sustainment of bone growth and mineralization if serum calcium and phosphate are maintained at physiological levels. However, further studies on VDR−/− animals identified novel functions of VDR in the bone that could not be deduced as merely consequential effects of the systemic VDR action of maintaining serum calcium and phosphate homeostasis.
Table 1.
Phenotypic abnormalities in Rickets
| Gene | General Phenotypes | Skeletal Phenotypes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VDR knockout mice | VDR | Growth retardation | Hypocalcemia | Hypophosphatemia | Hyperparathyroidism | Increased serum levels of D3 | Alopecia | Low BMD | Osteomalacia | |
| VDDR I | 1α-Hydroxylase | Growth retardation | Hypocalcemia | Hypoparathyroidism | Decreased serum levels of D3 | Low BMD | Osteomalacia | |||
| VDDR II | VDR | Growth retardation | Hypocalcemia | Hypophosphatemia | Hyperparathyroidism | Increased serum levels of D3 | Alopecia | Low BMD | Osteomalacia | |
| XLH | PHEX | Growth retardation | Hypophosphatemia | Hyperparathyroidism | Low BMD | Osteomalacia | Craniosynostosis | |||
1. Role of VDR signaling in bone cell differentiation
Prior to ossification, the expression of VDR in mammalian embryonic development is already detectable during formation of the mesenchymal condensations in bone primordia and then throughout the osteoblast/osteocyte and chondrocyte lineages (196). Such an early expression of VDR in the developing fetal skeleton suggests that it may regulate the differentiation of mesenchymal progenitors into bone cells. Although VDR-deficient mice display normal embryonic bone formation and the rescue diet is able to normalize postnatal bone growth and mineralization, more detailed examination of VDR- and 1,25(OH)2D3-deficient animals have identified direct effects of VDR signaling on bone formation and osteoblast differentiation (for review, see Refs. 144, 158). While osteoclast numbers appeared to be normal in the VDR−/− mice (9), osteoblast numbers and osteoblast colony forming units were reduced in bones and bone marrow stromal cells, respectively, from singly, VDR−/− or CYP27B1−/− and doubly, VDR−/−CYP27B1−/− knockout animals regardless of the type of diet (358). Osteocalcin promoter-driven transgenic overexpression of VDR in mature osteoblasts (cuboidal osteoblasts, osteocytes, and lining cells) increased both trabecular and cortical bone mass that was associated with decreased bone resorption through suppression of osteoclastogenesis (131). Unexpectedly, heterozygous VDR+/− mice displayed higher bone mineral density and formation compared with the wild-type littermates, whereas ablation of the VDR in differentiating osteoblasts in VDRf/f mice by the 2.3kb α1(I) collagen promoter-driven Cre recombinase led to increased mass and mineral density in bones of the transgenic animals associated with decreased rates of the osteoclastogenesis and, consequently, bone resorption (Yamamoto et al. Endocrinology. In press). This is consistent with observation that the volume and mineral density of VDR−/− bone engrafts transplanted into wild-type recipients were higher than those of wild-type bone engrafts under the same conditions (479). These data suggest that mechanisms and effects of the osteoblastic VDR signaling on osteoclastogenesis are differentiation stage-specific: stimulatory from the immature osteoblasts (478) and inhibitory from the mature osteoblasts and osteocytes (19). Interestingly, chondrocyte-specific VDR knockout did not affect chondrocyte development, but resulted in increased trabecular bone mass due to reduced osteoclasts numbers (308), implying that, in osteoblasts and chondrocytes, VDR acts as a regulator of osteoclastogenesis.
Thus VDR signaling exerts direct physiological effects in cells of the chondrocytic and osteoblastic lineages, thereby affecting bone mass and mineral density independently from its systemic effects through regulation of calcium and phosphate homeostasis.
2. VDR target genes in bone cells
It has been estimated in various studies on gene expression profiling that VDR signaling is involved directly or indirectly in the regulation of up to 5% of human and mouse genes (reviewed in Ref. 39), implying a wide range of VDR biological actions and effects. In the skeleton, VDR is expressed in the bone osteoblasts and osteocytes and in cartilage chondrocytes (461).
The osteocalcin gene was the first gene shown to be directly controlled by the VDR and became a classical model for studies on 1,25(OH)2D3-dependent transactivation. It is induced during late stages of osteoblastic differentiation with the onset of mineralization and remains expressed at high levels in surviving osteocytes and lining cells (463). Although osteocalcin represents the most abundant noncollagenous osteoblast-specific protein and may contribute to the density and structural integrity of bone tissue, its exact role in the skeleton is not completely understood. Osteocalcin-deficient mice develop bones of higher mass and improved functional quality owing to an increase in bone formation without apparent defects in bone resorption (99). It was noticed, however, that osteocalcin-deficient mice were glucose intolerant and accumulated high amounts of visceral fat. This was consistent with earlier observations that 1,25(OH)2D3 deficiency impaired both insulin secretion and glucose tolerance in humans and experimental animals (59, 132, 348). Similar defects in insulin production, insulin sensitivity in target tissues, and glucose tolerance have been observed in VDR−/− mice (561). Further studies have identified bone-secreted osteocalcin as a hormone that stimulates both proliferation of pancreatic β-cells and insulin production, and improves insulin metabolic responsiveness in target tissues (260). Thus VDR signaling in osteoblasts can influence systemic energy metabolism through regulation of the osteocalcin gene expression in the bone (130, 259).
Besides osteocalcin, VDR controls bone expression of several genes encoding important effectors of both bone and systemic homeostasis, such as osteopontin (OPN), OPG, RANKL, Runt-related transcription factor X 2 (Runx2), and fibroblast growth factor 23 (FGF23).
OPN, also known as early T-cell activation gene-1 (ETA-1) or secreted phosphoprotein 1 (SPP1), is a multifunctional secreted phosphoglycoprotein that was initially isolated from the bone extracellular matrix (353, 395). OPN is expressed in various types of cells, including osteoclasts, osteoblasts, chondrocytes, macrophages, activated T cells, smooth muscle cells, and epithelial cells. Outside the skeleton, comparatively high levels of OPN have been found in kidneys, placenta, smooth muscles, and secretory epithelia (317, 512). Osteoblastic OPN is highly responsive to 1,25(OH)2D3 and is thought to control bone mineralization and remodeling. OPN was proposed to anchor bone-resorbing osteoclasts to the mineral matrix of bone surfaces (395). OPN-deficient bones are hypermineralized and more fragile than normal bones, largely due to impaired bone resorption (182, 330). Nonskeletal OPN appear to regulate ectopic calcification acting as a natural inhibitor of soft tissue mineralization (458, 464).
RANKL represents one of the genes that is most responsive to induction by 1,25(OH)2D3 and is highly expressed in osteoblasts and stromal cells, especially in areas undergoing active bone remodeling or inflammatory osteolysis (154). RANKL, a cytokine from the tumor necrosis factor (TNF) superfamily, acts through activation of its receptor RANK. RANKL/RANK signaling is indispensable for the development of multinucleated bone-resorptive osteoclasts from monocytic progenitors and promotes survival and activity of mature osteoclasts (219). OPG is a soluble glycoprotein widely expressed in most tissues, including bone osteoblasts. OPG is a decoy receptor that protects bone from excessive resorption by binding to RANKL, thereby inhibiting RANKL/RANK signaling and preventing differentiation of osteoclasts. Therefore, the ratio of RANKL and OPG concentrations in the bone represents a major determinant of bone mass and strength (110, 219). Thus, through direct regulation of skeletal production of RANKL and OPG, osteoblastic VDR signaling couples bone formation to bone resorption and controls normal adult bone remodeling (154).
Runx2 is a key regulator of hypertrophic chondrocyte and osteoblast differentiation (129, 554). It acts as both a DNA binding transcription factor and a coregulatory component of nuclear complexes with other factors, including VDR, to regulate the stage-specific expression of genes controlling chondrogenesis and osteoclastogenesis (83, 303, 359, 425). VDR suppresses Runx2 gene transcription through interaction with VDREs in the proximal promoter and distal regulatory regions of the gene (98, 456).
1,25(OH)2D3/VDR signaling induces production of FGF23 in osteoblasts and osteocytes (23, 416, 546). FGF23 is a circulating glycosylated peptide that suppresses renal phosphate reabsorption and production of 1,25(OH)2D3, thus functioning as a phosphaturic factor and counter-regulatory hormone to 1,25(OH)2D3 (28, 115, 165, 179, 557). Excess of circulating 1,25(OH)2D3 can cause symptoms similar to the phenotype of FGF23 knockout mice that is characterized by ectopic calcification, osteoporosis, skin atrophy, arteriosclerosis, and chronic obstructive pulmonary disease (429, 430). Disruption of the vitamin D signaling by ablation of either VDR or CYP27B1 rescues the FGF23−/− phenotype (160, 397), supporting a view that FGF23 functions as a negative feedback regulator that restrains the mineralotropic and osteotrophic actions of 1,25(OH)2D3 (28, 165).
3. Osteogenic VDR agonists
Besides its fundamental physiological function of regulating calcium homeostasis, VDR signaling is known to exert noncalcemic effects on immunity, cell growth, and differentiation. Hence, VDR agonists are thought to be potentially beneficial in treatment of VDDR-I, renal failure, psoriasis, autoimmune disorders, and certain cancers (39, 336). Calcitriol and its synthetic analog alfacalcidol have been used in combination with other osteogenic compounds for the treatment of osteoporosis and osteomalacia (85, 117, 186). However, the therapeutic application of 1,25(OH)2D3 is limited due to deleterious side effects such as hypercalcemia and heterotopic ossification. Therefore, significant efforts have been made to develop novel noncalcemic VDR ligands with improved therapeutic properties (48, 85, 178, 291, 443, 444).
A number of vitamin D analogs have been synthesized that exhibit a separation between bone efficacy and hypercalcemia. Several VDR ligands with prominent osteogenic effects are currently undergoing various stages of preclinical development or clinical trials. These include 2-methylene-19-nor-(20S)-1a,25(OH)2D3, designated 2MD (218, 427) and 2MD derivates (24, 432); 1a-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25(OH)D3, or Ro-26–9228 (183, 367); and 1a,25(OH)2–2b-(3-hydroxypropoxy)D3, or ED-71 (310, 312).
The phase III of clinical trials has been completed for ED-71, also known as eldecalcitol. Longitudinal clinical studies have shown that ED-71 can effectively increase lumbar spine bone mineral density, decrease bone turnover, and lower risk of vertebral and wrist fractures in osteoporotic patience regardless on their vitamin D status. ED-71 at a clinically effective dose was well tolerated in these patients without causing sustained hypercalcemia or other apparent side effects (185, 309).
B. Retinoid Acid Receptors
Retinoic acid (RA), a biologically active form of the vitamin A or retinol, plays a crucial role in regulation of vertebrate embryonic development, postnatal growth, and physiology of adult organs and tissues. Retinoids may exert both beneficial and detrimental effects. RA deficiency dramatically alters fundamental physiological and developmental processes. It is also well documented that RA is teratogenic and treatments with retinoids are contraindicated during pregnancy (524, 526). Thus appropriate physiological effects of RA require a very narrow range of RA concentrations to avoid negative consequences of both deficiency and toxicity (101). Biological effects of RA are mediated by the action of two types of retinoic acid receptors: RA receptors (RAR) and retinoid X receptors (RXR). Each of these receptor types consists of three subtypes (RARα, RARβ, RARγ and RXRα, RXRβ, RXRγ, respectively) that originate from distinct genes. Alternative promoter usage and splicing generate several isoforms of each of the receptor subtypes, further adding to the complexity of RA signaling (52).
All-trans-RA (ATRA), the abundant and most potent biologically active derivate of vitamin A, is a high-affinity ligand for RARs, whereas 9-cis-RA can activate both types of the receptors, RARs and RXRs. The physiological role of 9-cis-RA remains unconfirmed, however, since this isomer is detectable in vivo only under conditions of excess of vitamin A or ATRA (101, 323).
RARs and RXRs transduce RA signaling as RAR/RXR heterodimers. Loss-of-function studies have demonstrated requirements for distinct RAR and, probably RXR subtype combinations at different stages of mouse embryonic development and postnatal growth (304). In addition, RXRs function as common heterodimerization partners for several members of the nuclear receptor superfamily, such as VDR, TRs, PPARs, LXRs, FXR, and other so-called subfamily 1 nuclear receptors (251). The RXR heterodimerization partners do not exhibit a marked preference for any of the three RXR subtypes. It has been suggested that RXR primarily functions as a scaffold protein to facilitate DNA binding for these receptors (55).
RAR/RXR heterodimers act as transcription factors through binding to the specific RA response element (RARE) DNA sequences that correspond to direct repeats of the canonical hexameric motif (A/G)G(G/C)TCA separated by five, one, or two nucleotides and referred to as DR5, DR1, or DR2 elements, respectively (298). In the absence of the ligand, RAR/RXR heterodimers act as transcriptional suppressors via recruitment to the RAREs of corepressor complexes. Binding of RAR agonist triggers the dismissal of corepressors and recruitment of coactivator complexes at DR5 and DR2 RAREs to stimulate the transcription (141, 254, 373, 374). In contrast, RXR agonist binding is unable to induce corepressor-coactivator complex exchange and activate the RARE-dependent transcription in the absence of RAR agonist (135). This phenomenon is referred to as RXR subordination (52, 304). Although formation of RXR homodimers and their binding to DR1 elements has been demonstrated in vitro, the existence of autonomous physiological function of RXR/RXR homodimers remains elusive (176, 506). Interestingly, RAR ligand binding does not cause the dissociation of corepressors from DR1-bound RAR/RXR heterodimers, suggesting that the DR1 RARE might be universally repressive (247).
1. Role of RARs in the skeleton
It has been established that in early embryonic development, RA/RARs provide instructive signaling for posterior neuroectoderm and posterior foregut endoderm, and permissive signaling for trunk mesoderm. At later stages, RA/RARs are involved in the development of the heart, eye, lung, pancreas, head structures and skeleton, genitourinary tract, and other organs (reviewed in Refs. 94, 101, 347). Such a profound influence of RA signaling on a wide range of the critical events in the morphogenesis and organogenesis creates significant methodological difficulties for dissecting its specific functions in the physiology of adult organs and tissues, including the skeleton.
High alimentary intake and serum levels of retinol have been associated with increased risk of bone fractures in humans (122, 324) and in laboratory animals (195, 273). However, RA appears to stimulate the regeneration of deer antlers (8), whereas vitamin A deficiency was reported to delay bone healing/repair in mice (480). These and similar observations might reflect both a wide spectrum of RA biological effects in bone and narrow range of physiologically optimal RA concentrations (101).
Studies on cultured bone cells and tissues have shown that effects of retinoids depend on the cell type and context. RA has been shown to stimulate bone resorption through activation of differentiated osteoclasts (233, 407, 499). However, RA suppressed the resorption in the presence of 1,25(OH)2D3 (499, 515). Further studies demonstrated differential effects of retinoids in mature osteoclasts and their progenitors. RA has been shown to stimulate osteoclast precursor proliferation and inhibit RANKL-induced osteoclast differentiation in both human and murine cells (70, 71, 168).
RA has been reported to promote the differentiation of primary osteoblasts (402, 454, 547) and to synergize with the BMP signaling to promote the osteogenic differentiation of mesenchymal cells (93, 188, 442) and adipose-derived adult stromal cells (266, 352, 507) at the expense of adipogenesis. In other experimental settings, RA produced inhibitory effects on the expression of osteoblastogenesis marker genes in vitro (2, 63).
Studies on developing zebrafish identified distinct effects of RA in the cells of osteoblastic lineage: blocking the recruitment of precursor cells to the osteoblastic lineage and promotion of bone matrix synthesis in mature osteoblasts (270). This dual action of RA results in inhibitory effects on the induction of ossification in early development, and in the stimulation of bone formation after skeleton ossification has been initiated (252, 270, 459, 566). These findings supported previous reports on the crucial role of unliganded RAR-mediated gene repression in skeletal progenitor cell differentiation (95, 162, 524). Aberrant localized RA signaling has been implicated in the development of multiple congenital skeletal abnormalities in humans and experimental animals (253).
Although a large body of evidence has been accumulated on the effects of RA in differentiating and mature bone cells, our understanding of the roles of specific RAR subtypes in mediation of these effects, particularly in vivo, remains fragmented. Earlier experiments with knockout of individual RARs in mice suggested a high degree of functional redundancy among the RAR subtypes, since single RAR-KO mice were found to be viable and displaying few abnormalities (reviewed in Ref. 134). However, double RAR subtype KO is lethal owing to numerous developmental abnormalities (276, 320). Compound ablation of the RARγ (RARα−/RARγ− or RARβ−/RARγ−) has been shown to cause severe skeletal defects including homeotic transformations, growth retardation, and limb malformations (276). Further studies confirmed the pivotal role of RARγ in the skeletal development and maintenance. RARγ was found to be the highest expressed RAR subtype in mouse growth plates (528). Conditional double KO of RARγ with either RARα or RARβ in the cartilage has been shown to lead to significant skeletal growth retardation and growth plate abnormalities, whereas cartilage deficiency in both RARα and RARβ had no apparent effect on the bone formation and growth (528). Furthermore, cartilage is an avascular tissue, and the upper zones of mouse limb growth plates are devoid of active retinoids (527, 528), suggesting that in the growth plate chondrocytes, RARγ operates as an unliganded transcriptional repressor. Recent report on inhibition of heterotopic ossification by selective RARγ agonists (431) further supported conclusion on the central role of the RARγ in the skeletal development, growth, and maintenance.
VI. NUCLEAR RECEPTORS AND BONE METABOLISM: FATTY ACID-RELATED RECEPTORS
A. Peroxisome Proliferator-Activated Receptors
The first peroxisome proliferator-activated receptor (PPAR) was originally identified as an orphan receptor (later designated PPARα) activated by various synthetic compounds including hypolipidemic drugs known to cause proliferation of peroxisomes and activation of the peroxisomal β-oxidation of fatty acids (184). Subsequently, three PPAR subtypes have been identified: α, β/δ, and γ (97, 325). PPARs of all the subtypes form heterodimers with RXRs and bind to consensus peroxisome proliferator response element (PPRE), composed of two core motifs separated by a single base pair (DR1 element: 5′-AGGTCA-N-AGGTCA-3′) (325).
PPARs are pivotal regulators of the systemic energy metabolism and are highly expressed in liver, adipose tissue, and skeletal muscles. Activated PPARα and PPARβ(δ) promote energy expenditure, whereas energy storage is associated with the activation of PPARγ. Although the profound roles of PPARs in energy metabolisms have been intensively illustrated over the last two decades in adipose, liver, and muscle tissues, bone was not considered as a significant target for the PPAR action. However, recently, bone has emerged as an endocrine organ that is actively involved in systemic regulation of the energy metabolism, and physiological roles of PPARs in bone cells are now drawing much attention.
PPARα is expressed predominantly in the liver, and to a lesser extent in the heart, skeletal muscles, and bone. Hepatic PPARα plays a crucial role in regulation of fatty acid oxidation, which provides energy for peripheral tissues. Expression of PPARδ (also known as PPARβ) is detectable in many tissues including bone (325), and genetic mutations of PPARδ have revealed its primary function as a fat burner. PPARγ has two major isoforms (γ1 and γ2) generated by alternative promoter usage and primary transcript splicing. PPARγ1 is expressed at low levels in many cell types and tissues including macrophages, kidney, uterus, breast, prostate, and adipocyte (265). PPARγ2 is a predominant isoform expressing in adipose tissue (491). The expressions of both PPARγ1 and PPARγ2 are significantly induced during the adipogenesis, and both isoforms are potent adipogenic factors that shift the differentiation of bone marrow mesenchymal stem cells (MSCs) into adipocytes and inhibit MSCs differentiation into osteoblasts, chondrocytes, or muscle cells. A balanced MSC differentiation into osteoblasts and adipocytes is an important factor in the maintanance of bone mass and strength. As patients with osteoporosis often display increased fat content in the bone marrow, regulation of pro-adipogenic function of PPARγ represents an important factor supporting bone homeostasis.
Arachidonic acid metabolites, such as leukotriene B4 and 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC), are thought to be endogenous ligands for PPARα (51, 91). Prostaglandin I2 has been reported as an endogenous ligand for PPARδ (159), while 15-deoxy-delta (12,14)-PGJ2 (15d-PGJ2), 9-HODE, 13-HODE, and nitrolinoleic-acid are known as PPARγ ligands (228, 337, 419). In addition to eicosanoid derivatives, metabolites of polyunsaturated fatty acids have been found to activate PPARγ function. A number of synthetic compounds are registered as selective activators of a particular PPAR subtype or as pan-ligands. Fibrate derivatives [Wy14643 (184), bezafibrate, GW409544 (539)] are PPARα-specific agonists, whereas GW6471 is a PPARα-specific antagonist (540). L165041 (27) and GW501516 (354) are specific agonists for PPARδ. A class of thiazolidinediones (TZDs) (264), GW9662 (170), THR0921 (489), and CLX-090717 (467), are known to act as specific PPARγ agonists. Two TZD derivates, rosiglitazone and pioglitazone, have been clinically successful as anti-diabetic agents with antihyperglycemic profile.
1. Role of PPARα in bone cells
PPARα−/− mice are impaired to meet energy demands during fasting and suffer from hypoglycemia, hyperlipidemia, and hypoketonemia (261). In the fasting state, whole body use of glucose was aberrantly increased in adipose tissues of PPARα−/− mice, apparently due to upregulated expression of the neuropeptide Y (NPY) and proopiomelanocortin (POMC) in the hypothalamus (232). Conversely, PPARα activation with specific agonists reduces adiposity and improves hepatic and muscle steatosis, leading to improvement in insulin sensitivity (263). Fibrates are currently on the market and are clinically used to lower plasma triglyceride levels in patients with hypertriglyceridemia (396).
A) ROLES OF PPARα IN OSTEOBLASTIC CELLS.
Although expression of PPARα is detectable in the bone cells as well as in the cells of hematopoietic and mesenchymal lineages (136), PPARα−/− mice do not exhibit an obvious bone phenotype (535). Bone mass of PPARα−/− mice was not altered, and bone cortical area did not differ from that in control animals. In addition, the differentiation potential of the bone marrow MSCs toward osteoblasts and adipocytes appeared normal in PPARα−/− mice (535). Nevertheless, PPARα deficiency may affect bone morphology, as a significantly larger bone marrow space was observed in male PPARα−/− mice (535). In a cell culture system, PPARα was shown to shift bone marrow myeloid cell commitment toward the B cell lineage (548), suggesting a regulatory role of PPARα in the bone marrow environment.
In vitro studies demonstrated that bezafibrate [PPARα-specific agonists (474)] stimulated osteoblast differentiation (465) and inhibited osteoclastogenesis (53). In intact rats, bezafibrate stimulated periosteal bone formation (465) and increased femoral BMD, while decreasing the bone marrow space (471). These studies suggest that bezafibrate may have anabolic effects in bone; however, clinical assessment of bone health showed that there was no significant reduction in the incidence of bone fractures in the patients treated with fibates (319).
B) ROLES OF PPARα IN CARTILAGE.
Recent studies have shown that activated PPARs are potent to attenuate TGF-β-dependent proteoglycan synthesis in rat chondrocytes, whereas the matrix metalloproteinase (MMP) activity was suppressed only by PPARα agonists (381). In cartilage explants from osteoarthritic patients, treatment with PPARα agonist (Wy14643) was effective to suppress mRNA expression levels of MMP1, MMP3, and MMP13 (65). Since activation of TGF-β signaling and elevated MMP activities have been established to associate with the development of osteoarthritis (OA), these results imply that PPARα agonists may be beneficial for the treatment of OA.
2. PPARδ (β)
The body sizes of PPARδ−/− mice were significantly smaller than those of control animals at every stage of the development, from fetus to adults, but no phenotypic abnormality was observed in their tissues including bone (376). Animal models with genetically altered PPARδ expression and application of selective PPARδ agonists in intact animals revealed a critical role of this nuclear receptor in the regulation of fat-burning processes. Transgenic expression of PPARδ in adipose tissue made the transformed mice resistant to high-fat diet-induced obesity, hyperlipidemia, and tissue steatosis. In contrast, when fed with high-fat diet, PPARδ−/− mice displayed reduced expression of the uncoupling protein 1 (Ucp1) and were prone to obesity (516). In the skeletal muscles, activated PPARδ upregulated fatty acid oxidation and energy expenditure to a far greater extent than activated PPARα (21). Thus PPARδ appears to perform a unique function as a fat burner, as opposite to a fat-storing function of PPARγ.
A) PPARδ FUNCTION IN OSTEOCLASTS.
In rabbits, PPARδ is highly expressed in mature osteoclasts, and its agonist was potent to induce bone-resorbing activity and upregulation of cathepsin K gene expression in isolated mature rabbit osteoclasts (300). Likewise, in cultured human osteoclasts derived from peripheral blood mononuclear cell, selective PPARδ agonist L165041 (27) stimulated the resorptive activity of mature osteoclasts. However, this compound was inhibitory for human osteoclast maturation (53). Thus, at the present stage, adverse actions of activated PPARδ(β) obscure our understanding of the physiological roles of osteoclastic PPARδ(β).
3. PPARγ
Initially, PPARγ was identified as a transcription factor regulating adipocyte differentiation (491), and this adipogenic function of PPARγ was further confirmed by various experimental approaches. Synthetic agonists of PPARγ have been successfully used for the treatment of type II diabetes mellitus (DM), although the molecular basis of PPARγ beneficial actions in type II DM development and treatment still remains to be determined. Recently, clinical studies of the type II DM patients treated with TDZs showed an increased risk of bone fracture incidence with decreased BMD compared with patients treated with other classes of glucose-lowering medications (203, 204, 423, 552). It remains unclear whether such side effects can be attributed to a generally higher risk of bone fracture incidence in type II DM patients compared with healthy individuals irrespective of the BMD (1, 346, 422). However, it is likely that induced by activated PPARγ adipogenesis in the bone marrow is causal for the skeletal side effects. Thus there is a caveat that synthetic PPARγ ligands may exhibit side effects detrimental to the bone health.
A) EFFECTS OF PPARδ IN BONE MARROW MSC.
PPARγ is commonly accepted as a master adipogenic factor, since activation of PPARγ in precursors of nonadipogenic lineage cells triggers their transdifferentiation into adipocytes (167, 491). For example, endogenous and synthetic PPARγ agonists promote adipogenesis and inhibit osteoblastogenesis in primary bone marrow MSC culture (256). Moreover, treatment of mice with rosiglitazone resulted in increased bone marrow adiposity and decreased BMD (or bone mass) apparently through suppression of the pro-osteoblastic transcription factors, Runx2, Osterix, and Dlx5 (7, 413). Consistently, haploinsufficiency of PPARγ in mice resulted in enhanced osteoblastogenesis and decreased bone marrow adipogenesis with increased trabecular bone volume (4). Similarly, an abnormally increased bone formation leading to insufficient space in the marrow cavity to maintain normal hematopoiesis has been observed in PPARγhyp/hyp mice that carry a hypomorphic mutation in the PPARγ locus and show reduced expression of both PPARγ isoforms in the white adipose (66). Collectively, these in vivo and in vitro studies suggest that in the bone marrow, PPARγ functions as a differention switch between osteoblastogenesis and adipogenesis, acting as an inhibitor of osteoblastogenesis. However, the molecular basis of such inhibitory action of PPARγ in osteoblastogenesis is still poorly understood.
In addition to the ligand-dependent activation, various signaling pathways are known to modulate the transactivation function of PPARγ in the MSCs. For example, several cytokines (TGF-β1, and BMP2) inhibited adipocyte differentiation from bone marrow MSCs through suppression of the PPARγ transactivation function (163, 345).
Wnt ligands regulate differentiation of MSCs into osteoblasts and adipocytes (410). The canonical Wnt ligands (Wnt1, Wnt3a, Wnt5b, Wnt7a, Wnt10b, etc.) bind to cell membrane heterodimers between frizzled receptor (Fzd) and low-density lipoprotein-related protein (LRP5/6) and transmit their signals by inducing intracellular translocation of β-catenin into the nucleus and subsequent activation the T-cell factor/lymphoid-enhancing factor (TCF/LEF) family of transcription factors. The canonical Wnt signal promotes bone formation, as it was initially illustrated in clinical studies that gain-of-function mutation in the Wnt receptor subunit gene, LRP5 stimulated osteoblastogenesis in humans (145). Further in vitro studies showed that bone formation is stimulated by canonical Wnt signaling at the stages of maturation of osteoblastic precursors into mature osteoblasts rather than by determining osteoblastic cell fate in the bone marrow MSCs (22, 142, 244, 302). The other group of Wnt ligands (Wnt4, Wnt5a, and Wnt11, etc.) activates the noncanonical Wnt signaling through cell membrane heterodimers of Fzd and pathway-specific coreceptors such as knypek associated with membrane receptor-type tyrosine kinases Ror1 and Ror2 (504). As mice deficient of noncanonical Wnt signal transducers exhibit severe developmental defects including impaired bone formation (350, 545), it is likely that the noncanonical Wnt signaling supports osteoblastogenesis.
Cross-talks between Wnt signals and various nuclear receptor-mediated signaling pathways have been described in many cell types (332). A noncanonical Wnt ligand, Wnt5a, has been shown to induce osteoblastogenesis and suppress PPARγ-dependent adipogenesis in cultured MSCs. Consistently, Wnt5a+/− mice exhibited an accumulation of adipocytes in the bone marrow and reduced BMD (473). Cytogenetic and biochemical characterization of the downstream factors in the signaling has revealed that Wnt5a activates a MAP kinase-like, Nemo-like kinase (NLK). Next, activated NLK forms a complex with PPARγ and SET domain, bifurcated 1 (SETDB1). Since SETDB1 is known as a histone H3 lysine 9 (H3K9), methyltransferase that renders chromatin transcriptionaly inactive, Wnt5a, appears to serve as a switcher of MSC differention fate from adipogenesis into osteoblastogenesis via suppression of PPARγ function (473).
B) ROLE OF PPARγ IN OSTEOCLASTOGENESIS.
Recently, a regulatory role of PPARγ has been proposed during the osteoclastogenesis (509). Mice with selectively knockout PPARγ gene in osteoclastic precursor cells exhibited increased bone mass and density with extramedullary hematopoiesis. Conversely, administration of PPARγ agonist rosiglitazone to intact mice led to acceleration of osteoclast differentiation and increased bone resorption that were apparently mediated through the action of osteoclastic PPARγ. Furthermore, in this study, c-fos, a key regulator of the macrophage/osteoclast lineage and bone remodeling, has been identified as a direct PPARγ target gene, and PPARγ antagonists reduced c-fos mRNA expression in primary cultured osteoclasts (509). From these findings, PPARγ emerged as a significant osteoclastogenic factor.
C) ROLE OF PPARγ IN DEVELOPMENT OF JOINT DISORDERS.
A possible role of PPARγ in the development of OA has been recently suggested (112). In cultured chondrocytes from OA patients, PPARγ activation by 15d-PGJ2 or Tro was shown to suppress IL-1β-induced production of NO and PGE2 through attenuation of expression of their respective synthases, iNOS and COX-2 (40, 111, 113). Such suppressive action of PPARγ agonists is inferred to occur at the transcriptional level, since it was reported that activated PPARγ attenuated the transactivation function of pro-inflammatory transcription factors, AP-1 and NF-κB (399). Protective anti-inflamatory action of PPARγ agonists have been also implied from observations in rheumatoid arthritis (RA) experimental model systems. In a mouse model of type II collagen-induced arthritis (CIA), a treatment with rosiglitazone (81), or THR0921 (489), or CLX-090717(467) ameliorated clinical scores of CIA and reduced histological scores of joint destruction. Although the development of OA and RA may be modulated by PPARγ action, there was no association found between well-known PPARγ gene variants (missense mutation Pro12Ala polymorphism and silent C to T substitution in the exon 6 at the nucleotide 161, or C161T polymorphism at the His477 codon) and the incidence of OA (58). Recently, Wnt5a-Ror2 (receptor tyrosine kinase-like orphan receptor) signaling has been shown to regulate balanced osteoblast-induced osteoclastogenesis that is critical for normal bone physiology (293). Aberrant activation of PPARγ may perturb this regulation through the crosstalk with noncanonical Wnt5a signaling and cause pathophysiological conditions potentially leading to RA and other bone diseases.
B. Liver X Receptor α and β
Liver X receptors (LXRs) α and β are thought to function as cholesterol sensors and regulate the expression of genes involved in cholesterol metabolism. Oxidized derivatives of cholesterol and oxysterols are known as endogenous ligands of the LXRs (190, 529). d-Glucose and d-glucose-6-phosphate are also reported to activate LXRs and, expectedly, both of them were effective to regulate LXR-mediated biological events in hepatic glucose metabolism and fatty acid synthesis (328). LXRs heterodimerize with RXRs and recognize DR4-type DNA elements (5′-AGGTCA-NNNN -AGGTCA-3′) as binding sites in the target genes (529).
When fed a cholesterol-rich diet, LXRα−/− mice develop hepatic steatosis associated with cholesteryl-ester accumulation. This has been attributed to the lack of LXRα-mediated upregulation of the Cyp7a1 that encodes a rate-limiting enzyme in the conversion of cholesterol into bile acid (365). In contrast to LXRα−/− mice, LXRβ−/− mice maintained their resistance to dietary cholesterol and exhibited no apparent aberrant phenotype (5).
1. Roles of LXRs in bone
A detailed analysis of bone phenotypes of LXR-deficient mice has been reported (403). A significant increase in BMD attributed to cortical bone areas with less bone resorptive activity was observed in female LXRα−/− mice at the age of 4 mo, but not in male LXRα−/− mice. The LXRα−/− mice displayed no difference in trabecular areas, whereas osteoclastic differentiation in trabecular areas was affected in female LXRα−/− mice without alteration in BMD. Although it remains to be studied if LXRs are activated by endogenous ligands or are constitutively functional without ligand binding in osteoclasts, these findings imply that LXRs are regulators of osteoclastic differentiation and function. Interestingly, LXRα deficiency affected bone phenotype only in the mutant females, suggesting an interaction between LXR and sex steroid signaling pathways.
2. Effects of LXR agonists in bone cells
A short-term (48 h) treatment with LXR agonist reduced mRNA levels of osteocalcin and alkaline phosphatase in primary cultured murine osteoblasts, while oral administration of LXR agonists for 6 days to mice did not affect bone morphology (383).
The expressions of both LXRs and cholesterol efflux-related genes (APOA1, ABCA1) were significantly lower in osteoarthritic cartilages compared with normal human cartilage samples. Consistently, aberrant intracellular lipid accumulation was found in primary cultured chondrocytes from OA patients, whereas treatment with LXR agonist induced APOA1 and ABCA1 genes and decreased lipid accumulation in chondrocytes (495). Likewise, in human articular cartilage explants, an LXR agonist was shown to inhibit cytokine-induced proteoglycan degradation (68). Thus these findings imply that LXR-activating compounds may be effective in prevention of pathological development of OA through stimulation in chondrocytes of cholesterol efflux.
C. Farnesoid X Receptor
Farnesoid X receptor (FXR) was originally identified as an orphan receptor that could be activated by farnesoids (125). However, after the identification of bile acids (BAs) as its endogenous ligands, FXR was also referred to as BAR (296, 361, 511). BAs are amphipathic molecules with a steroid backbone that are synthesized from cholesterol exclusively in parenchymal cells of the liver. Chenodeoxycholic acid (CDCA), cholic acid (CA), deoxycholic acid (DCA), and lithocholic acid (LCA) are potent to activate FXR at their physiological concentrations (∼100 μM). FXR heterodimerizes with RXR subtypes, and FXR/RXR heterodimers bind to multiple direct repeat elements (DR2, DR4, and DR5). Inverted repeats, IR0 (5′-AGGTCA-TGACCT-3′) and IR1 (5′-AGGTCA-N-TGACCT-3′), were also found to bind FXR/RXR (126).
FXR−/− mice show elevated serum and hepatic levels of bile acid, cholesterol, and triglycerides, with a pro-atherogenic serum lipoprotein profile. Under standard housing and dietary conditions, FXR−/− mice had no overt bone abnormality and appeared indistinguishable from their wild-type littermates (438). However, in human osteosarcoma cell line (Saos-2) and bone marrow stroma cells, agonist-activated FXR was shown to bind to the Runx2 promoter, and stimulated the expression of osteoblastic marker genes, such as bone sialoprotein (BSP), osteocalcin (OC), osteopontin (OPN), and alkaline phosphatase (ALP) (175). Together with the report characterizing FXR endogenous ligands in human MG63 cells and bone tissues (434), these data suggest that activated FXR might potentiate bone formation.
D. Steroid and Xenobiotic Receptor/Pregnane X Receptor
The human steroid and xenobiotic receptor (SXR) and its murine ortholog, pregnane X receptor (PXR) (also known as PAR and NR1I2), were identified as orphan receptors regulating gene expression of cytochrome P-450 monooxygenase (CYP3A), a pivotal enzyme in the initial catabolism of a wide range of xenobiotics (29, 30, 229). Besides the regulation of CYP enzymes such as CYP3A4, CYP2B6, and CYP2C8, SXR was shown to regulate the expression of conjugation enzymes such as UDP-glucuronosyltransferase (UGT1A1) and sulfotransferase (SULT1A1), and ABC family transporters, ABCB1 and ABCC2 (104, 470). SXR can be activated by various pharmaceutical agents such as taxol, rifampicin (RIF), SR12813, clotrimazole, phenobarbital, an herbal antidepressant St. John's wort (Hypericum perforatum), as well as peptide mimetic HIV protease inhibitors such as ritonavir (227). However, owing to species specificity in ligand recognition, some human SXR agonists (RIF, clotrimazole, and phenobarbital) were reported unable to activate the murine SXR ortholog PXR (537). DNA binding sites for SXR in the target genes consist of directly repeated two 5′-AGGTCA-3′ core motifs separated by 3 bps, or so called DR3 elements.
PXR−/− mice are viable, fertile, breed with normal Mendelian distribution, and do not exhibit any overt phenotypic abnormality including serum parameters (462, 537). However, PXR-deficient mice do not respond with Cyp3a gene induction to the administration of its prototypic inducers such as prognenolone-16a-carbonitrile (PCN) and dexamethasone, confirming the key role of PXR/SXR in activation of response to xenobiotics.
1. SXR function in bone
Reported action of the vitamin K2, or menaquinone-4 (MK4), as an SXR agonist suggested a possibility of SXR function in the bone homeostasis (472), since MK4 has been clinically demonstrated to be effective as anti-osteoporotic agent reducing the incidence of bone fractures (5, 6). Supporting the osteoprotective activity of MK4 in humans, SXR-mediated MK4 signaling has been shown to induce the expression of key osteoblastic marker genes like ALP, OPG, OPN, and OC in human osteosarcoma cells (472). In addition, several genes of secreted extracellular matrix proteins, such as Tsukushi and matrilin-2, have been identified as SXR/MK4 target genes in human osteoblastic cells (174). As Tsukushi has been shown to mediate the MK4-induced collagen accumulation (174), MK4-activated SXR is likely to stimulate bone formation and maintenance. These suggest that SXR signaling might promote the osteoblastogenesis.
Data from in vitro cell culture systems have been further experimentally recapitulated in vivo, by disruption of the PXR gene in mice (16). Four-month-old PXR−/− female mice displayed osteopenia with low bone turnover, reduced stiffness in cortical bones, and bone formation. These implicate PXR/SXR as a significant factor that supports bone remodeling and formation. In this respect, an identification of endogenous SXR ligands in bone would be quite a promising project.
VII. NUCLEAR RECEPTORS AND BONE METABOLISM: OTHER RECEPTORS
A. NR4A Receptors
The NR4A subgroup of orphan nuclear receptors consists of NR4A1 (also called Nur77 or NGFI-B), NR4A2 (also called Nurr1), and NR4A3 (also called NOR1) (69). Because of the absence of structural cavities for ligand binding in these nuclear receptors, they can be considered as ligand-independent NRs (17, 394, 517). Generally, these receptors exert their transcriptional activities by binding as monomers or homodimers to NGFI-B response element (NBRE; AAAGGTCA) and Nur-response element (NuRE), respectively (335, 530). Additionally, NR4A1 and NR4A2 form transcriptionally active heterodimers with RXR that bind DR5 motifs (direct repeats separated by five nucleotides), and these heterodimers are efficiently activated by RXR ligands (126, 375).
NR4A receptors are abundantly expressed in the brain and neuroendocrine tissues (137). NR4A2 is expressed at high levels particularly in dopaminergic neurons during development (536, 564). Therefore, most studies of NR4A receptors have focused on the nervous system. However, it was recently reported that NR4A receptors are also expressed in other tissues including the liver, arterial wall smooth muscle cells, and macrophages (295, 316, 366, 563). The expression of NR4A receptors in these tissues can be induced in response to various stimuli, whereas NR4A receptors are constitutively expressed in the brain (316).
NR4A receptors are also expressed in bone tissue, especially in osteoblasts (249, 484), implying their functional activity in bone formation. Three groups have reported that NR4A receptors regulate the expression of several osteoblastic marker genes such as osteopontin (OPN), osteocalcin (OCN), collagen type I alpha 1 (COL1A1), and alkaline phosphatase (ALP), suggesting the presence of potential NBREs in the promoter region of these genes (249, 258, 379). NR4A2 overexpression led to increased expression of ALP, COL1A1, and OCN genes. In contrast, the expression of these genes was decreased in MC3T3-E1 cells with NR4A2-knockdown and in primary cultured mouse calvarial osteoblasts derived from Nurr1 null mice (249, 258, 379). These reports suggested that NR4A2 might play a regulatory role in both early (COL1A1 and ALP) and late (OCN) stages of osteoblast differentiation.
In the bone, the expression of NR4A receptors can be induced by two key regulators, PTH and fibroblast growth factor 8b (FGF-8b) (248, 378, 484, 485). Tetradis and co-workers (378, 484, 485) reported that PTH can induce the expression of NR4A receptors in primary cultured osteoblasts and in organ cultured calvariae. Similarly, Lammi et al. (248) showed that the expression of NR4A receptors was increased in MC3T3-E1 cells or mouse bone marrow mesenchymal cells treated with FGF-8b, resulting in increased proliferation of preosteoblasts.
Although the functions of NR4A receptors in osteoblasts have been demonstrated, the role of NR4A receptors in other types of bone cells is unclear. Similarly, while NR4A receptors are expressed in the bone marrow mesenchymal stem cells (242), their functions in chondrocytes and adipocytes differentiated from mesenchymal stem cells remain to be elucidated.
NR4A2 is essential for differentiation of dopaminergic neurons because NR4A2 knockout mice die soon after birth due to their failure to generate midbrain dopaminergic neurons (49, 50, 562). Moreover, mutations of NR4A2 may predispose to the development of Parkinson's disease (255). Interestingly, Sato et al. (418) reported that Parkinson's disease patients showed a high incidence of hip fractures (418). A decreased activity of NR4A2 during osteoblast differentiation might underlie a high incidence of bone fractures in the patients with Parkinson's disease. However, because of neonatal lethality of conventional NR4A2 knockout mice, the physiological roles of NR4A2 in skeletal modeling during growth and bone remodeling after skeletal maturation has not been examined. Generation and analyses of bone specific NR4A2 conditional knockout mice would be essential to clarify this question.
B. Estrogen Receptor-Related Receptors
The estrogen receptor-related receptors (ERRs), a subgroup of the orphan nuclear receptors, include three subtypes: ERRα, ERRβ, and ERRγ (493). These receptors are closely related to the ERs, sharing high homology in their DNA binding domains. However, they do not bind the classical ER ligands, and no natural ERR ligand has been identified so far (138, 164, 493). Additionally, ERRs activate transcription of the target genes through binding to a specific ERRE (TNAAGGTCA), distinct from estrogen response element (ERE) DNA sequences (57, 87, 102).
ERRs are expressed in several tissues during embryonic development and in the adult organism (37). Consistent with their expression patterns, a number of physiological functions for ERRs have been suggested. For example, ERRβ appears to regulate placental formation (285), maintenance of pluripotency in embryonic stem cells (501), and the fate of certain epithelial cells of the inner ear (56). ERRα and -β are involved in several aspects of metabolic control with PPARγ and peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) (139, 505). ERRα transcripts have been identified in the ossification zones of the long and short bones during mouse embryonic development (36). Similarly, ERRα is continuously expressed during ex vivo differentiation of rat osteoblast progenitors originating from calvaria or bone marrow (33, 35). Therefore, it is expected that ERRα play an important role in bone metabolism.
The functional importance of ERRs in bone was first investigated using ex vivo approaches with rat calvarial cells. ERRα knockdown by anti-sense oligonucleotides reduced the proliferation and differentiation of calvarial cells and decreased the number of mineralized colonies, while ERRα overexpression led to the opposite effects (35). Additionally, when calvarial cells were induced to differentiate into adipocytes, the inhibition of ERRα function resulted in an increased number of adipocyte colonies (34). Therefore, it was suggested that ERRα could act as a switching factor, inducing differentiation of precursor cells into the osteoblastic pathway and repressing the adipocytic pathway.
However, recent in vivo studies using ERRα knockout (ERRα−/−) mice suggested the opposite conclusion against the results mentioned above. Two independently established lines of ERRα−/− mice showed increased trabecular BMD compared with those in wild-type mice (89, 486). In both cases, increased BMD appeared to be caused by enhanced osteoblast activities. Osteoblast surface and bone formation rates were increased in ERRα−/−, while the volume of marrow fat cells was reduced (89). Thus, in vivo, ERRα can be considered as a switching factor in mesenchymal cell fate decision, inducing commitment to the adipocyte lineage. Moreover, the function of ERRα in osteoclasts was suggested from the phenotype of aged ERRα−/− mice that displayed a significantly reduced number of osteoclasts and reduced bone resorption (519), although it remains elusive whether this phenotype was caused by the osteoclastic ERRα or the secondary effects from other cell types in bone.
In addition to ERRα, it was reported that ERRγ has an important role in regulation of bone metabolism. Jeong et al. (192) showed that ERRγ negatively affected BMP2-mediated osteoblast differentiation of MC3T3 and C2C12 cell lines. ERRγ interacted with Runx2, a master transcription factor for osteoblast differentiation, thereby preventing physical interaction between Runx2 and p300 coactivator and impairing transcriptional activity of Runx2. This idea was further confirmed by the BMP2-induced ectopic bone formation assay (192).
Thus there are divergent interpretations of the roles of ERRs in bone. This might result from differences between in vitro cell culture and in vivo animal models. Although the clinical significance of ERRs in human bone tissue remains to be uncovered, ERRs therapeutic targeting may offer a possibility of increasing bone formation by enhancing osteoblast differentiation and activity.
C. Retinoid-Related Orphan Receptors
Retinoid-related orphan receptors (RORs) constitute a subfamily that includes three members: RORα that is present in four splicing isoforms (RORα1, RORα2, RORα3, and RORα4), RORβ, and RORγ (109, 193, 420). Natural ligands for these receptors remain unknown. Each of these receptors appears to act as a constitutive transcriptional activator by binding to a ROR response element, RORE [(A/T)6AGGTCA] as a monomer (140, 318).
RORα is expressed in various tissues, including the brain, skeletal muscles, kidney, testis, and hair follicles (45, 140, 193, 420). Among the four known splicing isoforms, the expression of RORα2 and RORα3 is limited to immune cells and testis, respectively (193, 382). Additionally, RORβ is brain specific and is highly expressed in the pineal gland. RORγ is expressed most highly in the thymus (137, 193).
RORs are also expressed in the osteoblastic lineage cells, suggesting their functions in the bone. Specifically, RORα and RORγ (but not RORβ) are expressed in mesenchymal stem cells derived from bone marrow (322). Furthermore, mRNA level of RORα is significantly increased during osteoblast differentiation of mesenchymal stem cells, implying its positive regulatory functions in osteoblasts.
The role of RORs in bone metabolism was analyzed in in vitro experiments. Benderdour et al. (26) showed that RORα1 modulates the expression of osteoblast marker genes. Overexpressed RORα1 increased the expression of early- and late-stage osteoblastic differentiation marker genes (ALP, COL1A1, and OCN), and consistently, knockdown of RORα1 inhibited the expression of these genes. Additionally, RORα1 has the inhibitory effect for TNF-α-induced NF-κB activation (26, 88). NF-κB signaling is critical in bone resorption through stimulation of osteoclast differentiation (3, 194) and induction of inflammatory cytokines and matrix metalloproteinases (MMPs) that are involved in extracellular matrix degradation (80, 207, 281, 392). Therefore, it is suggested that RORα1 may play an important protective role in bone metabolism.
In vivo evidence for the function of RORα in bone metabolism was first obtained by studying the staggerer (sg) mutant mice, a mouse line that carries a deletion within the RORα gene (151). Homozygote sg mutants (sg/sg) have thin long bones compared with the heterozygote and wild-type animals (322). Also, the bones of these mice are osteopenic. RORα-deficient mice consistently exhibit abnormalities in bone formation and maintenance (287), suggesting that RORα functions as a positive regulator of the osteoblastogenesis.
D. Chick Ovalbumin Upstream Promoter-Transcription Factors
Chick ovalbumin upstream promoter-transcription factors (COUP-TFs) include two members: COUP-TFI and COUP-TFII (513). COUP-TFs exert their transcriptional activities by binding to an imperfect repeat element (AGGTCA) (362, 415, 494). COUP-TFs bind as monomers to its target promoters but also may bind as heterodimers with RXRs. Because RXRs also form a heterodimer with various nuclear receptors including VDR, TR, and RAR, it is anticipated that COUP-TFs can inhibit the ligand-dependent activities of VDR, TR, and RAR by competition for heterodimerization with RXRs (72, 230, 231, 375). Thus the ratio of COUP-TFs levels to RXR levels in a given cell may allow the balancing of diverse hormonal and growth signals (282).
Although there is some overlapping expression patterns of COUP-TFs during mouse embryogenesis, COUP-TFI is more highly expressed in nervous tissues, while COUP-TFII is preferentially expressed in mesenchymal stem cells in developing tissues and organs, such as stomach, salivary gland, pancreas primordium, lung, kidney, and prostate (370, 386). Expression pattern of COUP-TFI correlates with that of BMP-4, implying potential significance in bone metabolism. In E10.5 embryos, there is a distal-to-proximal direction gradient of BMP-4 expression, while COUP-TFI expression shows an opposite proximal-to-distal gradient (197, 282). In the limbs of E14.5 embryos, localization of BMP-4 expression overlaps with that of COUP-TFI expression in the apoptotic regions between the developing digits and in the mesenchymal cells of perichondrium (197, 282). Feng et al. (118) reported that COUP-TFI could modulate BMP-4 gene transcription in osteoblasts. They found a potential regulatory response region of COUP-TFI in the BMP-4 promoter and that COUP-TFI was specifically bound to this region DNA. Additionally, they showed that COUP-TFI inhibited BMP-4 promoter activity in fetal rat calvarial osteoblasts by overexpression of COUP-TFI. These results suggest that COUP-TFI controls the BMP expression level.
Targeted disruption of mouse COUP-TFI resulted in perinatal lethality owing to a defective nervous system development (387). Thus it is necessary to generate a conditional, bone-cell type specific COUP-TFI knockout mouse line to examine its functions in the bone metabolism.
E. Small Heterodimer Partner
Small heterodimer partner (SHP) is an atypical orphan nuclear receptor that has only a putative LBD and two LXXLL-like motifs (424). Because SHP does not possess a DNA-binding domain, it exerts its activity through interaction with various nuclear receptors and transcription factors including ERs, PPARs, RARs, LXRs, c-Jun, Smads, and HDACs. Through these interactions, SHP negatively regulates transcriptional activities of these transcription factors, with exception of PPARs (54, 210, 262).
SHP participates in various cellular functions such as cell proliferation, differentiation, and metabolism depending on the cell types (161, 169, 360, 455). For example, using SHP null or transgenic mice, it was found that hepatic SHP has multiple regulatory roles in metabolic pathways including hepatic bile acids, lipids, and glucose homeostasis (54, 262).
In bone tissue, SHP has been shown to function as a positive regulator of osteoblastic bone formation (191). SHP expression is significantly increased during the osteoblastogenesis, and it is also induced by BMP-2, a strong positive regulator of bone formation. SHP physically interacts with Runx2 on the promoter region of osteocalcin gene. Overexpression of SHP increases the transcriptional activity of Runx2 via a competition for association with HDAC4 (191). These results indicate that SHP is involved in the regulation of osteoblastogenesis. Consistent with the in vitro results, trabecular BMDs of long bones were significantly decreased in SHP knockout mice with decreased osteoblast and osteoclast numbers (191). Since loss of SHP expression had no significant effect on the expression of osteoclast-specific marker genes, it was thought that osteoclast numbers were reduced by the indirect effects mediated by osteoblasts. Furthermore, SHP knockout mice showed considerably fewer chondrocytes than WT mice (191).
Natural ligands of SHP have not been identified. However, for therapeutic drug development, the efforts are underway to discover the compounds that may regulate activities of SHP (114, 189). For example, Farnaha et al. (115) reported that adamantyl-substituted retinoid-related compounds (CD437/AHPN and 3-Cl-AHPC/MM002) promote interaction between SHP and a corepressor complex (115). Jang et al. (188) showed that metformin that is already used as an anti-diabetic drug induces osteoblast differentiation via SHP-mediated activation of Runx2 (188). Therefore, therapies based on the manipulation of SHP activity might offer new possibilities for treating bone diseases.
VIII. CONCLUSION AND FUTURE PERSPECTIVES
NRs mediate systemic action of lipophilic hormones and play a crucial role in the bone physiology and pathophysiology. Recent studies delineated many functions of NRs in the bone development and maintenance, and uncovered molecular mechanisms of NR specific actions in bone cells, still leaving many questions to be further addressed. Particularly, clinical applications of NR synthetic ligands for treatment of skeletal disorders remain largely at initial stages and require comprehensive understanding of bone cell-type specific function of NRs as well as NR coregulators.
A. Spatiotemporal Functions of NRs in Bone Tissue
The cellular functions of osteoblasts and osteoclasts have been well documented, leading us to understand how these cells support bone growth and remodeling (32, 152, 208, 391, 475), whereas the role of other bone cells remained not as clear. Recently, using a variety of modern experimental approaches, unexpected roles of osteocytes as endocrine cells and conductor cells for bone remodeling have been uncovered (217, 339, 556). Although the significance of osteocytes in bone maintenance and serum mineral control has become evident, little information of NR function in osteocytes is still available. Nevertheless, detectable levels of the expression of certain NRs in these cells indicate that NRs are likely to play an important regulatory role in osteocytes, and possible actions of vitamin D and sex hormones in osteocytes have already been suggested in some recent studies (217, 339, 556). In this respect, characterization of effects of osteocytic VDR, ERs, and AR in the bone by a cell type-specific conditional gene disruption in osteocytes would verify such an idea.
Osteocytes represent the terminal stage of osteoblastic cell lineage differentiation. It appears that the roles of osteoblasts in bone remodeling vary at different stages of their differentiation (31, 208, 236, 340, 549). This suggests that functions of NRs in the osteoblastic lineage cells may change during the cell differentiation. Although osteoblast-specific KO of NR genes has successfully illustrated the involvement of NRs in regulation of bone physiology (60, 349), we cannot exclude a possibility that NRs may exert different regulatory action at different stages of the osteoblastic differentiation (FIGURE 1, 5). Similarly, cytodifferentiation stage-specific KO of NRs (305, 339) in osteoclasts (FIGURE 5) would be of a great interest, since the osteoclastic differentiation includes several distinct stages leading to formation of mature multi-nucleated bone-resorptive cells.
Figure 5.
Strategy for identification of NR cell- and differentiation stage-specific functions. Conventional NR gene knockout mice usually exhibit various systemic abnormalities that obscure cell- and tissue-specific effects of the knockout. To overcome this problem, cell type- and cytodifferentiation stage-specific NR gene knockout mice are generated and analyzed to dissect physiological functions of NRs.
B. Synthetic NR Ligands for Treatment of Skeletal Disorders
A group of synthetic ER ligands, named as SERMs, have been successfully used to ameliorate osteoporotic bone loss or aberrantly enhanced bone resorption and without increased incidence of estrogen-dependent tumor development in female reproductive organs (343, 344, 408). Thus SERMs represent NR synthetic ligands with beneficial properties for treatment of skeletal disorders. Owing to its anabolic actions in bone and muscle tissues, androgens are expected to be a promising lead hormone for elderly male patients suffering from age-related bone loss and sarcopenia (43, 123). Synthetic AR ligands are now under intensive development to generate SARMs that would be effective in skeletal organs, but without side effects, such as development of prostate cancer. Vitamin D and its synthetic analog ED-71 are approved and widely used in Japan to potentiate bone strength and prevent bone fractures, presumably by inducing mineral accumulation in bones (185, 309, 313). As beneficial effects of vitamin D are still debated by North American and European practitioners (409), vitamin D supplements are shown to be beneficial for bone health in Japanese population under conditions of deficient dietary calcium intake. In contrast, synthetic glucocorticoids are drawing clinical concerns when chronically administered as anti-inflammatory agents, since GR ligands are causative for bone loss, known as secondary osteoporosis (10). Therefore, significant efforts have been made to develop novel anti-inflammatory GR ligands lacking negative side effects on bone mass and mineral density, and some promising lead compounds are currently undergoing clinical trials.
Therapeutically, NRs are considered as important therapeutic targets for treatment of various skeletal diseases. These might also include joint disorders, even though NR involvement at least in some of them still remains to be addressed. To accelerate NR-targeted drug development, a variety of modern approaches have been applied, such as structural analysis of liganded NR proteins and pharmacological tests using genetically engineered animals. In this respect, pharmacological lessons from SERMs are particularly instructive, since protein conformation of the raloxifene-bound ERα was shown to be markedly different from that of the estrogen-bound ERα (428), and resulted in association with different sets of transcriptional coregulators (446). These observations were further supported by DNA microarray studies which showed that estrogen- and raloxifene-induced gene expression patterns were not identical. Thus these finding have formulated a concept that some synthetic ligands may induce NR protein conformation different from that of a natural ligand-activated NR to trigger atypical coregulator complex association and, consequently, different pattern of NR target gene expression, thereby exerting tissue-specific and desirable therapeutic effects (61, 62, 84). Since ligand-induced interactions of coregulators with NR reflect ligand-specific conformational alteration of NR protein structure (FIGURE 6), identification of coregulator complexes bound by a synthetic compound-activated NR may provide a promising strategy for design and screening of synthetic NR ligands with desirable tissue specificity and/or pharmacological effects (278, 290). This approach may also link specific coregulator-NR interactions to particular desirable therapeutic outcomes and, thus, identify transcriptional coregulators as novel pharmaceutical targets in treatment of skeletal disorders.
Figure 6.
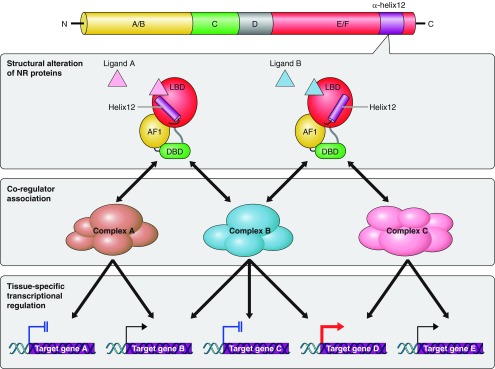
Ligand-specific conformational alterations of nuclear receptor protein define ligand type-specific coregulator recruitment and subsequent physiological effects. Ligand binding triggers a shift in the positioning of the most COOH-terminal α-helix 12 (top panel). Certain synthetic ligands may induce NR protein conformation different from that of a natural ligand-activated NR, resulting in the coregulator switching (middle panel). As a result of different complex association, a different pattern of NR target gene expression is induced, potentially exerting tissue-specific and desirable therapeutic effects (bottom panel).
GRANTS
This work was supported by Ministry of Education, Culture, Sports, Science and Technology of Japan Grants-in-Aid 22000012 (to S. Kato) as well as 23689066 and 23659712 (to Y. Imai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Atsushi Yokoyama and Mihoko Shibata for assistance with manuscript preparation.
Address for reprint requests and other correspondence: Y. Imai, Laboratory of Epigenetic Skeletal Diseases, Institute of Molecular and Cellular Biosciences, The University of Tokyo, 1-1-1, Yayoi, Bunkyo-ku, Tokyo 113-0032, Japan (e-mail: yimai@iam.u-tokyo.ac.jp).
REFERENCES
- 1. Ahmed LA, Joakimsen RM, Berntsen GK, Fonnebo V, Schirmer H. Diabetes mellitus and the risk of non-vertebral fractures: the Tromso study. Osteoporos Int 17: 495–500, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Ahmed N, Sammons J, Khokher MA, Hassan HT. Retinoic acid suppresses interleukin 6 production in normal human osteoblasts. Cytokine 12: 289–293, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann NY Acad Sci 1056: 218–233, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113: 846–855, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alberti S, Schuster G, Parini P, Feltkamp D, Diczfalusy U, Rudling M, Angelin B, Bjorkhem I, Pettersson S, Gustafsson JA. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J Clin Invest 107: 565–573, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander T, Nolte C, Krumlauf R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu Rev Cell Dev Biol 25: 431–456, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 146: 1226–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Allen SP, Maden M, Price JS. A role for retinoic acid in regulating the regeneration of deer antlers. Dev Biol 251: 409–423, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140: 4982–4987, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Angeli A, Dovio A, Sartori ML, Masera RG, Ceoloni B, Prolo P, Racca S, Chiappelli F. Interactions between glucocorticoids and cytokines in the bone microenvironment. Ann NY Acad Sci USA 966: 97–107, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci USA 105: 2433–2438, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arriza JL, Simerly RB, Swanson LW, Evans RM. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron 1: 887–900, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237: 268–275, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. Control of mammary stem cell function by steroid hormone signalling. Nature 465: 798–802, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem 72: 396–410, 1999 [PubMed] [Google Scholar]
- 16. Azuma K, Casey SC, Ito M, Urano T, Horie K, Ouchi Y, Kirchner S, Blumberg B, Inoue S. Pregnane X receptor knockout mice display osteopenia with reduced bone formation and enhanced bone resorption. J Endocrinol 207: 257–263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker KD, Shewchuk LM, Kozlova T, Makishima M, Hassell A, Wisely B, Caravella JA, Lambert MH, Reinking JL, Krause H, Thummel CS, Willson TM, Mangelsdorf DJ. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell 113: 731–742, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Balasch J. Sex steroids and bone: current perspectives. Hum Reprod Update 9: 207–222, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Baldock PA, Thomas GP, Hodge JM, Baker SU, Dressel U, O'Loughlin PD, Nicholson GC, Briffa KH, Eisman JA, Gardiner EM. Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res 21: 1618–1626, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 17: 245–261, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest 116: 590–597, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol 76: 103–127, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HAt Hsieh JC, Slater SA, Hsieh G, Kaczmarska M, Jurutka PW, Kolek OI, Ghishan FK, Haussler MR. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol 103: 381–388, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Barycki R, Sicinski RR, Plum LA, Grzywacz P, Clagett-Dame M, Deluca HF. Removal of the 20-methyl group from 2-methylene-19-nor-(20S)-1alpha,25-dihydroxyvitamin D(3) (2MD) selectively eliminates bone calcium mobilization activity. Bioorg Med Chem 17: 7658–7669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beavan S, Horner A, Bord S, Ireland D, Compston J. Colocalization of glucocorticoid and mineralocorticoid receptors in human bone. J Bone Miner Res 16: 1496–1504, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Benderdour M, Fahmi H, Beaudet F, Fernandes JC, Shi Q. Nuclear receptor retinoid-related orphan receptor alpha1 modulates the metabolic activity of human osteoblasts. J Cell Biochem 112: 2160–2169 [DOI] [PubMed] [Google Scholar]
- 27. Berger J, Leibowitz MD, Doebber TW, Elbrecht A, Zhang B, Zhou G, Biswas C, Cullinan CA, Hayes NS, Li Y, Tanen M, Ventre J, Wu MS, Berger GD, Mosley R, Marquis R, Santini C, Sahoo SP, Tolman RL, Smith RG, Moller DE. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J Biol Chem 274: 6718–6725, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 61: 91–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA 95: 12208–12213, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 12: 3195–3205, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonewald LF. The amazing osteocyte. J Bone Miner Res 26: 229–238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann NY Acad Sci 1116: 281–290, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Bonnelye E, Aubin JE. Differential expression of estrogen receptor-related receptor alpha and estrogen receptors alpha and beta in osteoblasts in vivo and in vitro. J Bone Miner Res 17: 1392–1400, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Bonnelye E, Kung V, Laplace C, Galson DL, Aubin JE. Estrogen receptor-related receptor alpha impinges on the estrogen axis in bone: potential function in osteoporosis. Endocrinology 143: 3658–3670, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Bonnelye E, Merdad L, Kung V, Aubin JE. The orphan nuclear estrogen receptor-related receptor alpha (ERRalpha) is expressed throughout osteoblast differentiation and regulates bone formation in vitro. J Cell Biol 153: 971–984, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonnelye E, Vanacker JM, Dittmar T, Begue A, Desbiens X, Denhardt DT, Aubin JE, Laudet V, Fournier B. The ERR-1 orphan receptor is a transcriptional activator expressed during bone development. Mol Endocrinol 11: 905–916, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Bonnelye E, Vanacker JM, Spruyt N, Alric S, Fournier B, Desbiens X, Laudet V. Expression of the estrogen-related receptor 1 (ERR-1) orphan receptor during mouse development. Mech Dev 65: 71–85, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 89: 6025–6029, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29: 726–776, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyault S, Simonin MA, Bianchi A, Compe E, Liagre B, Mainard D, Becuwe P, Dauca M, Netter P, Terlain B, Bordji K. 15-Deoxy-delta12,14-PGJ2, but not troglitazone, modulates IL-1beta effects in human chondrocytes by inhibiting NF-kappaB and AP-1 activation pathways. FEBS Lett 501: 24–30, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346: 1513–1521, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 423: 337–342, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Bross R, Storer T, Bhasin S. Aging and muscle loss. Trends Endocrinol Metab 10: 194–198, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Canalis E. Clinical review 83: mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab 81: 3441–3447, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Carlberg C, Hooft van Huijsduijnen R, Staple JK, DeLamarter JF, Becker-Andre M. RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol Endocrinol 8: 757–770, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122: 33–43, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38: 1289–1297, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Castillo AI, Sanchez-Martinez R, Jimenez-Lara AM, Steinmeyer A, Zugel U, Aranda A. Characterization of vitamin D receptor ligands with cell-specific and dissociated activity. Mol Endocrinol 20: 3093–3104, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Castillo SO, Baffi JS, Palkovits M, Goldstein DS, Kopin IJ, Witta J, Magnuson MA, Nikodem VM. Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci 11: 36–46, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Castro DS, Hermanson E, Joseph B, Wallen A, Aarnisalo P, Heller A, Perlmann T. Induction of cell cycle arrest and morphological differentiation by Nurr1 and retinoids in dopamine MN9D cells. J Biol Chem 276: 43277–43284, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell 138: 476–488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J 10: 940–954, 1996 [PubMed] [Google Scholar]
- 53. Chan BY, Gartland A, Wilson PJ, Buckley KA, Dillon JP, Fraser WD, Gallagher JA. PPAR agonists modulate human osteoclast formation and activity in vitro. Bone 40: 149–159, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Chanda D, Park JH, Choi HS. Molecular basis of endocrine regulation by orphan nuclear receptor Small Heterodimer Partner. Endocr J 55: 253–268, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science 294: 1866–1870, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Chen J, Nathans J. Estrogen-related receptor beta/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell 13: 325–337, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh YH, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung WK, Clarke ND, Wei CL, Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133: 1106–1117, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Cheng S, Afif H, Martel-Pelletier J, Benderdour M, Pelletier JP, Hilal G, Haraoui P, Raynauld JP, Choquette D, Fahmi H. Association of polymorphisms in the peroxisome proliferator-activated receptor gamma gene and osteoarthritis of the knee. Ann Rheum Dis 65: 1394–1397, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chertow BS, Sivitz WI, Baranetsky NG, Clark SA, Waite A, Deluca HF. Cellular mechanisms of insulin release: the effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology 113: 1511–1518, 1983 [DOI] [PubMed] [Google Scholar]
- 60. Chiang C, Chiu M, Moore AJ, Anderson PH, Ghasem-Zadeh A, McManus JF, Ma C, Seeman E, Clemens TL, Morris HA, Zajac JD, Davey RA. Mineralization and bone resorption are regulated by the androgen receptor in male mice. J Bone Miner Res 24: 621–631, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 466: 451–456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, Marciano DP, Cameron MD, Laznik D, Jurczak MJ, Schurer SC, Vidovic D, Shulman GI, Spiegelman BM, Griffin PR. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature 477: 477–481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Choong PF, Martin TJ, Ng KW. Effects of ascorbic acid, calcitriol, and retinoic acid on the differentiation of preosteoblasts. J Orthop Res 11: 638–647, 1993 [DOI] [PubMed] [Google Scholar]
- 64. Chu EY, Fong H, Blethen FA, Tompkins KA, Foster BL, Yeh KD, Nagatomo KJ, Matsa-Dunn D, Sitara D, Lanske B, Rutherford RB, Somerman MJ. Ablation of systemic phosphate-regulating gene fibroblast growth factor 23 (Fgf23) compromises the dentoalveolar complex. Anat Rec 293: 1214–1226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clockaerts S, Bastiaansen-Jenniskens YM, Feijt C, Verhaar JA, Somville J, De Clerck LS, Van Osch GJ. Peroxisome proliferator activated receptor alpha activation decreases inflammatory and destructive responses in osteoarthritic cartilage. Osteoarthritis Cartilage 19: 895–902, 2011 [DOI] [PubMed] [Google Scholar]
- 66. Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J. Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep 5: 1007–1012, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9: 1608–1621, 1995 [DOI] [PubMed] [Google Scholar]
- 68. Collins-Racie LA, Yang Z, Arai M, Li N, Majumdar MK, Nagpal S, Mounts WM, Dorner AJ, Morris E, LaVallie ER. Global analysis of nuclear receptor expression and dysregulation in human osteoarthritic articular cartilage: reduced LXR signaling contributes to catabolic metabolism typical of osteoarthritis. Osteoarthritis Cartilage 17: 832–842, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Committee NRN. A unified nomenclature system for the nuclear receptor superfamily. Cell 97: 161–163, 1999 [DOI] [PubMed] [Google Scholar]
- 70. Conaway HH, Persson E, Halen M, Granholm S, Svensson O, Pettersson U, Lie A, Lerner UH. Retinoids inhibit differentiation of hematopoietic osteoclast progenitors. FASEB J 23: 3526–3538, 2009 [DOI] [PubMed] [Google Scholar]
- 71. Conaway HH, Pirhayati A, Persson E, Pettersson U, Svensson O, Lindholm C, Henning P, Tuckermann J, Lerner UH. Retinoids stimulate periosteal bone resorption by enhancing the protein RANKL, a response inhibited by monomeric glucocorticoid receptor. J Biol Chem 286: 31425–31436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cooney AJ, Tsai SY, O'Malley BW, Tsai MJ. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol 12: 4153–4163, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol 9: 1441–1454, 1995 [DOI] [PubMed] [Google Scholar]
- 74. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20: 358–417, 1999 [DOI] [PubMed] [Google Scholar]
- 75. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 12: 722–734, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cromer BA, Scholes D, Berenson A, Cundy T, Clark MK, Kaunitz AM. Depot medroxyprogesterone acetate and bone mineral density in adolescents–the Black Box Warning: a Position Paper of the Society for Adolescent Medicine. J Adolesc Health 39: 296–301, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG. Lrp5 functions in bone to regulate bone mass. Nat Med 17: 684–691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361: 756–765, 2009 [DOI] [PubMed] [Google Scholar]
- 79. Cundy T, Farquhar CM, Cornish J, Reid IR. Short-term effects of high dose oral medroxyprogesterone acetate on bone density in premenopausal women. J Clin Endocrinol Metab 81: 1014–1017, 1996 [DOI] [PubMed] [Google Scholar]
- 80. Cunnane G, FitzGerald O, Hummel KM, Youssef PP, Gay RE, Gay S, Bresnihan B. Synovial tissue protease gene expression and joint erosions in early rheumatoid arthritis. Arthritis Rheum 44: 1744–1753, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Cuzzocrea S, Mazzon E, Dugo L, Patel NS, Serraino I, Di Paola R, Genovese T, Britti D, De Maio M, Caputi AP, Thiemermann C. Reduction in the evolution of murine type II collagen-induced arthritis by treatment with rosiglitazone, a ligand of the peroxisome proliferator-activated receptor gamma. Arthritis Rheum 48: 3544–3556, 2003 [DOI] [PubMed] [Google Scholar]
- 82. Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, Morton RA, Steiner MS. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle 2: 153–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. De Frutos CA, Dacquin R, Vega S, Jurdic P, Machuca-Gayet I, Nieto MA. Snail1 controls bone mass by regulating Runx2 and VDR expression during osteoblast differentiation. EMBO J 28: 686–696, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. De Lera AR, Bourguet W, Altucci L, Gronemeyer H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nature Rev Drug Discovery 6: 811–820, 2007 [DOI] [PubMed] [Google Scholar]
- 85. De Nijs RN, Jacobs JW, Lems WF, Laan RF, Algra A, Huisman AM, Buskens E, de Laet CE, Oostveen AC, Geusens PP, Bruyn GA, Dijkmans BA, Bijlsma JW. Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med 355: 675–684, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Deal C. Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol 5: 20–27, 2009 [DOI] [PubMed] [Google Scholar]
- 87. Deblois G, Hall JA, Perry MC, Laganiere J, Ghahremani M, Park M, Hallett M, Giguere V. Genome-wide identification of direct target genes implicates estrogen-related receptor alpha as a determinant of breast cancer heterogeneity. Cancer Res 69: 6149–6157, 2009 [DOI] [PubMed] [Google Scholar]
- 88. Delerive P, Monte D, Dubois G, Trottein F, Fruchart-Najib J, Mariani J, Fruchart JC, Staels B. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep 2: 42–48, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Delhon I, Gutzwiller S, Morvan F, Rangwala S, Wyder L, Evans G, Studer A, Kneissel M, Fournier B. Absence of estrogen receptor-related-alpha increases osteoblastic differentiation and cancellous bone mineral density. Endocrinology 150: 4463–4472, 2009 [DOI] [PubMed] [Google Scholar]
- 90. Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest 116: 561–570, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature 384: 39–43, 1996 [DOI] [PubMed] [Google Scholar]
- 92. Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J Bone Miner Metabo 22: 360–364, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Dingwall M, Marchildon F, Gunanayagam A, Louis CS, Wiper-Bergeron N. Retinoic acid-induced Smad3 expression is required for the induction of osteoblastogenesis of mesenchymal stem cells. Differentiation res biol diversity 82: 57–65, 2011 [DOI] [PubMed] [Google Scholar]
- 94. Dolle P, Fraulob V, Gallego-Llamas J, Vermot J, Niederreither K. Fate of retinoic acid-activated embryonic cell lineages. Dev Dyn 239: 3260–3274, 2010 [DOI] [PubMed] [Google Scholar]
- 95. Dranse HJ, Sampaio AV, Petkovich M, Underhill TM. Genetic deletion of Cyp26b1 negatively impacts limb skeletogenesis by inhibiting chondrogenesis. J Cell Sci 124: 2723–2734, 2011 [DOI] [PubMed] [Google Scholar]
- 96. Draper MW. The role of selective estrogen receptor modulators (SERMs) in postmenopausal health. Ann NY Acad Sci 997: 373–377, 2003 [DOI] [PubMed] [Google Scholar]
- 97. Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68: 879–887, 1992 [DOI] [PubMed] [Google Scholar]
- 98. Drissi H, Pouliot A, Koolloos C, Stein JL, Lian JB, Stein GS, van Wijnen AJ. 1,25-(OH)2-vitamin D3 suppresses the bone-related Runx2/Cbfa1 gene promoter. Exp Cell Res 274: 323–333, 2002 [DOI] [PubMed] [Google Scholar]
- 99. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature 382: 448–452, 1996 [DOI] [PubMed] [Google Scholar]
- 100. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89: 747–754, 1997 [DOI] [PubMed] [Google Scholar]
- 101. Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell 134: 921–931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab 5: 345–356, 2007 [DOI] [PubMed] [Google Scholar]
- 103. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127: 4277–4291, 2000 [DOI] [PubMed] [Google Scholar]
- 104. Dussault I, Lin M, Hollister K, Wang EH, Synold TW, Forman BM. Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J Biol Chem 276: 33309–33312, 2001 [DOI] [PubMed] [Google Scholar]
- 105. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med 358: 1474–1482, 2008 [DOI] [PubMed] [Google Scholar]
- 106. Egea PF, Mitschler A, Rochel N, Ruff M, Chambon P, Moras D. Crystal structure of the human RXRalpha ligand-binding domain bound to its natural ligand: 9-cis retinoic acid. EMBO J 19: 2592–2601, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D, Parada LF, Karsenty G. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab 4: 441–451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 299: 39–52, 2008 [DOI] [PubMed] [Google Scholar]
- 109. Enmark E, Gustafsson JA. Orphan nuclear receptors–the first eight years. Mol Endocrinol 10: 1293–1307, 1996 [DOI] [PubMed] [Google Scholar]
- 110. Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disorders 11: 219–227, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fahmi H, Di Battista JA, Pelletier JP, Mineau F, Ranger P, Martel-Pelletier J. Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum 44: 595–607, 2001 [DOI] [PubMed] [Google Scholar]
- 112. Fahmi H, Martel-Pelletier J, Pelletier JP, Kapoor M. Peroxisome proliferator-activated receptor gamma in osteoarthritis. Mod Rheumatol 21: 1–9, 2011 [DOI] [PubMed] [Google Scholar]
- 113. Fahmi H, Pelletier JP, Mineau F, Martel-Pelletier J. 15d-PGJ2 is acting as a “dual agent” on the regulation of COX-2 expression in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 10: 845–848, 2002 [DOI] [PubMed] [Google Scholar]
- 114. Farhana L, Dawson MI, Leid M, Wang L, Moore DD, Liu G, Xia Z, Fontana JA. Adamantyl-substituted retinoid-related molecules bind small heterodimer partner and modulate the Sin3A repressor. Cancer Res 67: 318–325, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Farrow EG, White KE. Recent advances in renal phosphate handling. Nature Rev Nephrol 6: 207–217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med 363: 2027–2035, 2010 [DOI] [PubMed] [Google Scholar]
- 117. Felsenberg D, Bock O, Borst H, Armbrecht G, Beller G, Degner C, Stephan-Oelkers M, Schacht E, Mazor Z, Hashimoto J, Roth HJ, Martus P, Runge M. Additive impact of alfacalcidol on bone mineral density and bone strength in alendronate treated postmenopausal women with reduced bone mass. J Musculoskeletal Neuronal Interact 11: 34–45, 2011 [PubMed] [Google Scholar]
- 118. Feng JQ, Chen D, Cooney AJ, Tsai MJ, Harris MA, Tsai SY, Feng M, Mundy GR, Harris SE. The mouse bone morphogenetic protein-4 gene. Analysis of promoter utilization in fetal rat calvarial osteoblasts and regulation by COUP-TFI orphan receptor. J Biol Chem 270: 28364–28373, 1995 [DOI] [PubMed] [Google Scholar]
- 119. Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38: 1310–1315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol 6: 121–145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ferrari S. Comparing and contrasting the effects of strontium ranelate and other osteoporosis drugs on microarchitecture. Osteoporos Int 21 Suppl 2: S437–442, 2010 [DOI] [PubMed] [Google Scholar]
- 122. Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA 287: 47–54, 2002 [DOI] [PubMed] [Google Scholar]
- 123. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences International working group on sarcopenia. J Am Med Directors Assoc 12: 249–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95: 6965–6970, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81: 687–693, 1995 [DOI] [PubMed] [Google Scholar]
- 126. Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell 81: 541–550, 1995 [DOI] [PubMed] [Google Scholar]
- 127. Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25: 343–346, 2000 [DOI] [PubMed] [Google Scholar]
- 128. Fujiki R, Kim MS, Sasaki Y, Yoshimura K, Kitagawa H, Kato S. Ligand-induced transrepression by VDR through association of WSTF with acetylated histones. EMBO J 24: 3881–3894, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129. Fujita T, Azuma Y, Fukuyama R, Hattori Y, Yoshida C, Koida M, Ogita K, Komori T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol 166: 85–95, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab 20: 230–236, 2009 [DOI] [PubMed] [Google Scholar]
- 131. Gardiner EM, Baldock PA, Thomas GP, Sims NA, Henderson NK, Hollis B, White CP, Sunn KL, Morrison NA, Walsh WR, Eisman JA. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J 14: 1908–1916, 2000 [DOI] [PubMed] [Google Scholar]
- 132. Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia 29: 142–145, 1986 [DOI] [PubMed] [Google Scholar]
- 133. Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273: 1236–1238, 1996 [DOI] [PubMed] [Google Scholar]
- 134. Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev 58: 712–725, 2006 [DOI] [PubMed] [Google Scholar]
- 135. Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415: 187–192, 2002 [DOI] [PubMed] [Google Scholar]
- 136. Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundam Clin Pharmacol 21: 231–244, 2007 [DOI] [PubMed] [Google Scholar]
- 137. Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev 20: 689–725, 1999 [DOI] [PubMed] [Google Scholar]
- 138. Giguere V. To ERR in the estrogen pathway. Trends Endocrinol Metab 13: 220–225, 2002 [DOI] [PubMed] [Google Scholar]
- 139. Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29: 677–696, 2008 [DOI] [PubMed] [Google Scholar]
- 140. Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev 8: 538–553, 1994 [DOI] [PubMed] [Google Scholar]
- 141. Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14: 121–141, 2000 [PubMed] [Google Scholar]
- 142. Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764, 2005 [DOI] [PubMed] [Google Scholar]
- 143. Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res 18: 807–817, 2003 [DOI] [PubMed] [Google Scholar]
- 144. Goltzman D. Vitamin D action: lessons learned from genetic mouse models. Ann NY Acad Sci 1192: 145–152, 2010 [DOI] [PubMed] [Google Scholar]
- 145. Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107: 513–523, 2001 [DOI] [PubMed] [Google Scholar]
- 146. Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468: 103–107, 2010 [DOI] [PubMed] [Google Scholar]
- 147. Gourlay ML, Specker BL, Li C, Hammett-Stabler CA, Renner JB, Rubin JE. Follicle-stimulating hormone is independently associated with lean mass but not BMD in younger postmenopausal women. Bone 50: 311–316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Guerrini MM, Sobacchi C, Cassani B, Abinun M, Kilic SS, Pangrazio A, Moratto D, Mazzolari E, Clayton-Smith J, Orchard P, Coxon FP, Helfrich MH, Crockett JC, Mellis D, Vellodi A, Tezcan I, Notarangelo LD, Rogers MJ, Vezzoni P, Villa A, Frattini A. Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet 83: 64–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140: 5566–5578, 1999 [DOI] [PubMed] [Google Scholar]
- 150. Halloran BP, Barthell EN, DeLuca HF. Vitamin D metabolism during pregnancy and lactation in the rat. Proc Natl Acad Sci USA 76: 5549–5553, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, van Berkel V, Birren BW, Kruglyak L, Lander ES. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature 379: 736–739, 1996 [DOI] [PubMed] [Google Scholar]
- 152. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature 423: 349–355, 2003 [DOI] [PubMed] [Google Scholar]
- 153. Harman SM. Estrogen replacement in menopausal women: recent and current prospective studies, the WHI and the KEEPS. Gend Med 3: 254–269, 2006 [DOI] [PubMed] [Google Scholar]
- 154. Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, Bartik L, Egan JB, Wu Y, Kubicek JL, Lowmiller CL, Moffet EW, Forster RE, Jurutka PW. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol 121: 88–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Heikkinen S, Vaisanen S, Pehkonen P, Seuter S, Benes V, Carlberg C. Nuclear hormone 1alpha,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res 39: 9181–9193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387: 43–48, 1997 [DOI] [PubMed] [Google Scholar]
- 157. Henderson NK, Sambrook PN. Relationship between osteoporosis and arthritis and effect of corticosteroids and other drugs on bone. Curr Opin Rheumatol 8: 365–369, 1996 [DOI] [PubMed] [Google Scholar]
- 158. Hendy GN, Hruska KA, Mathew S, Goltzman D. New insights into mineral and skeletal regulation by active forms of vitamin D. Kidney Int 69: 218–223, 2006 [DOI] [PubMed] [Google Scholar]
- 159. Hertz R, Berman I, Keppler D, Bar-Tana J. Activation of gene transcription by prostacyclin analogues is mediated by the peroxisome-proliferators-activated receptor (PPAR). Eur J Biochem 235: 242–247, 1996 [DOI] [PubMed] [Google Scholar]
- 160. Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol 26: 75–84, 2007 [DOI] [PubMed] [Google Scholar]
- 161. Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 58: 30–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hoffman LM, Garcha K, Karamboulas K, Cowan MF, Drysdale LM, Horton WA, Underhill TM. BMP action in skeletogenesis involves attenuation of retinoid signaling. J Cell Biol 174: 101–113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309: 1074–1078, 2005 [DOI] [PubMed] [Google Scholar]
- 164. Horard B, Vanacker JM. Estrogen receptor-related receptors: orphan receptors desperately seeking a ligand. J Mol Endocrinol 31: 349–357, 2003 [DOI] [PubMed] [Google Scholar]
- 165. Hori M, Shimizu Y, Fukumoto S. Minireview: fibroblast growth factor 23 in phosphate homeostasis and bone metabolism. Endocrinology 152: 4–10, 2011 [DOI] [PubMed] [Google Scholar]
- 166. Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 274: 2100–2103, 1996 [DOI] [PubMed] [Google Scholar]
- 167. Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA 92: 9856–9860, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hu L, Lind T, Sundqvist A, Jacobson A, Melhus H. Retinoic acid increases proliferation of human osteoclast progenitors and inhibits RANKL-stimulated osteoclast differentiation by suppressing RANK. PLoS One 5: e13305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, Moore DD, Wang L. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology 46: 147–157, 2007 [DOI] [PubMed] [Google Scholar]
- 170. Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 400: 378–382, 1999 [DOI] [PubMed] [Google Scholar]
- 171. Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med 2: 1132–1136, 1996 [DOI] [PubMed] [Google Scholar]
- 172. Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, Littman DR. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature 472: 486–490, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 43: 27–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem 281: 16927–16934, 2006 [DOI] [PubMed] [Google Scholar]
- 175. Id Boufker H, Lagneaux L, Fayyad-Kazan H, Badran B, Najar M, Wiedig M, Ghanem G, Laurent G, Body JJ, Journe F. Role of farnesoid X receptor (FXR) in the process of differentiation of bone marrow stromal cells into osteoblasts. Bone 49: 1219–1231, 2011 [DOI] [PubMed] [Google Scholar]
- 176. Ijpenberg A, Tan NS, Gelman L, Kersten S, Seydoux J, Xu J, Metzger D, Canaple L, Chambon P, Wahli W, Desvergne B. In vivo activation of PPAR target genes by RXR homodimers. EMBO J 23: 2083–2091, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Imamov O, Shim GJ, Warner M, Gustafsson JA. Estrogen receptor beta in health and disease. Biol Reprod 73: 866–871, 2005 [DOI] [PubMed] [Google Scholar]
- 178. Inaba Y, Yamamoto K, Yoshimoto N, Matsunawa M, Uno S, Yamada S, Makishima M. Vitamin D3 derivatives with adamantane or lactone ring side chains are cell type-selective vitamin D receptor modulators. Mol Pharmacol 71: 1298–1311, 2007 [DOI] [PubMed] [Google Scholar]
- 179. Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, Fukushima N, Kato S, Kanayama HO, Miyamoto K. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J 390: 325–331, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med 3: 1285–1289, 1997 [DOI] [PubMed] [Google Scholar]
- 181. Ishida Y, Heersche JN. Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation. J Bone Miner Res 13: 1822–1826, 1998 [DOI] [PubMed] [Google Scholar]
- 182. Ishijima M, Rittling SR, Yamashita T, Tsuji K, Kurosawa H, Nifuji A, Denhardt DT, Noda M. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J Exp Med 193: 399–404, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ismail A, Nguyen CV, Ahene A, Fleet JC, Uskokovic MR, Peleg S. Effect of cellular environment on the selective activation of the vitamin D receptor by 1alpha,25-dihydroxyvitamin D3 and its analog 1alpha-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3 (Ro-26–9228). Mol Endocrinol 18: 874–887, 2004 [DOI] [PubMed] [Google Scholar]
- 184. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645–650, 1990 [DOI] [PubMed] [Google Scholar]
- 185. Ito M, Nakamura T, Fukunaga M, Shiraki M, Matsumoto T. Effect of eldecalcitol, an active vitamin D analog, on hip structure and biomechanical properties: 3D assessment by clinical CT. Bone 49: 328–334, 2011 [DOI] [PubMed] [Google Scholar]
- 186. Iwamoto N, Inaba Y, Kobayashi N, Ishida T, Yukizawa Y, Saito T. A comparison of the effects of alendronate and alfacalcidol on bone mineral density around the femoral implant and in the lumbar spine after total hip arthroplasty. J Bone Joint Surgery 93: 1203–1209, 2011 [DOI] [PubMed] [Google Scholar]
- 187. Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol 27: 799–805, 2007 [DOI] [PubMed] [Google Scholar]
- 188. James AW, Levi B, Xu Y, Carre AL, Longaker MT. Retinoic acid enhances osteogenesis in cranial suture-derived mesenchymal cells: potential mechanisms of retinoid-induced craniosynostosis. Plastic Reconstructive Surg 125: 1352–1361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK, Kim SH, Lee CH, Franceschi RT, Choi HS, Koh JT. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone 48: 885–893 [DOI] [PubMed] [Google Scholar]
- 190. Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383: 728–731, 1996 [DOI] [PubMed] [Google Scholar]
- 191. Jeong BC, Lee YS, Bae IH, Lee CH, Shin HI, Ha HJ, Franceschi RT, Choi HS, Koh JT. The orphan nuclear receptor SHP is a positive regulator of osteoblastic bone formation. J Bone Miner Res 25: 262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Jeong BC, Lee YS, Park YY, Bae IH, Kim DK, Koo SH, Choi HR, Kim SH, Franceschi RT, Koh JT, Choi HS. The orphan nuclear receptor estrogen receptor-related receptor gamma negatively regulates BMP2-induced osteoblast differentiation and bone formation. J Biol Chem 284: 14211–14218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Jetten AM, Ueda E. Retinoid-related orphan receptors (RORs): roles in cell survival, differentiation and disease. Cell Death Differ 9: 1167–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 194. Jimi E, Ghosh S. Role of nuclear factor-kappaB in the immune system and bone. Immunol Rev 208: 80–87, 2005 [DOI] [PubMed] [Google Scholar]
- 195. Johansson S, Lind PM, Hakansson H, Oxlund H, Orberg J, Melhus H. Subclinical hypervitaminosis A causes fragile bones in rats. Bone 31: 685–689, 2002 [DOI] [PubMed] [Google Scholar]
- 196. Johnson JA, Grande JP, Roche PC, Kumar R. Ontogeny of the 1,25-dihydroxyvitamin D3 receptor in fetal rat bone. J Bone Miner Res 11: 56–61, 1996 [DOI] [PubMed] [Google Scholar]
- 197. Jones CM, Lyons KM, Hogan BL. Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development 111: 531–542, 1991 [DOI] [PubMed] [Google Scholar]
- 198. Jones DC, Wein MN, Oukka M, Hofstaetter JG, Glimcher MJ, Glimcher LH. Regulation of adult bone mass by the zinc finger adapter protein Schnurri-3. Science 312: 1223–1227, 2006 [DOI] [PubMed] [Google Scholar]
- 200. Jones ME, Boon WC, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 17: 55–64, 2006 [DOI] [PubMed] [Google Scholar]
- 201. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature 465: 803–807, 2010 [DOI] [PubMed] [Google Scholar]
- 202. Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nature Rev Cancer 12: 381–385, 2012 [DOI] [PubMed] [Google Scholar]
- 203. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355: 2427–2443, 2006 [DOI] [PubMed] [Google Scholar]
- 204. Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 31: 845–851, 2008 [DOI] [PubMed] [Google Scholar]
- 205. Kallenberger BC, Love JD, Chatterjee VK, Schwabe JW. A dynamic mechanism of nuclear receptor activation and its perturbation in a human disease. Nat Struct Biol 10: 136–140, 2003 [DOI] [PubMed] [Google Scholar]
- 206. Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85: 403–414, 1996 [DOI] [PubMed] [Google Scholar]
- 207. Kaneko M, Tomita T, Nakase T, Ohsawa Y, Seki H, Takeuchi E, Takano H, Shi K, Takahi K, Kominami E, Uchiyama Y, Yoshikawa H, Ochi T. Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology 40: 247–255, 2001 [DOI] [PubMed] [Google Scholar]
- 208. Karsenty G. How many factors are required to remodel bone? Nat Med 6: 970–971, 2000 [DOI] [PubMed] [Google Scholar]
- 209. Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol 25: 629–648, 2009 [DOI] [PubMed] [Google Scholar]
- 210. Kassam A, Capone JP, Rachubinski RA. The short heterodimer partner receptor differentially modulates peroxisome proliferator-activated receptor alpha-mediated transcription from the peroxisome proliferator-response elements of the genes encoding the peroxisomal beta-oxidation enzymes acyl-CoA oxidase and hydratase-dehydrogenase. Mol Cell Endocrinol 176: 49–56, 2001 [DOI] [PubMed] [Google Scholar]
- 211. Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9: 1603–1614, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Kato S. The function of vitamin D receptor in vitamin D action. J Biochem 127: 717–722, 2000 [DOI] [PubMed] [Google Scholar]
- 213. Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270: 1491–1494, 1995 [DOI] [PubMed] [Google Scholar]
- 214. Kato S, Tora L, Yamauchi J, Masushige S, Bellard M, Chambon P. A far upstream estrogen response element of the ovalbumin gene contains several half-palindromic 5′-TGACC-3′ motifs acting synergistically. Cell 68: 731–742, 1992 [DOI] [PubMed] [Google Scholar]
- 215. Kato S, Yokoyama A, Fujiki R. Nuclear receptor coregulators merge transcriptional coregulation with epigenetic regulation. Trends Biochem Sci 36: 272–281, 2011 [DOI] [PubMed] [Google Scholar]
- 216. Kato S, Yoshizazawa T, Kitanaka S, Murayama A, Takeyama K. Molecular genetics of vitamin D- dependent hereditary rickets. Horm Res 57: 73–78, 2002 [DOI] [PubMed] [Google Scholar]
- 217. Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci USA 100: 9416–9421, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ke HZ, Qi H, Crawford DT, Simmons HA, Xu G, Li M, Plum L, Clagett-Dame M, DeLuca HF, Thompson DD, Brown TA. A new vitamin D analog, 2MD, restores trabecular and cortical bone mass and strength in ovariectomized rats with established osteopenia. J Bone Miner Res 20: 1742–1755, 2005 [DOI] [PubMed] [Google Scholar]
- 219. Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 29: 155–192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF. Reduction in proximal femoral strength due to long-duration spaceflight. Bone 44: 449–453, 2009 [DOI] [PubMed] [Google Scholar]
- 221. Khosla S. Increasing options for the treatment of osteoporosis. N Engl J Med 361: 818–820, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev 29: 441–464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kim HJ, Zhao H, Kitaura H, Bhattacharyya S, Brewer JA, Muglia LJ, Ross FP, Teitelbaum SL. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest 116: 2152–2160, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. King-Jones K, Thummel CS. Nuclear receptors: a perspective from Drosophila. Nat Rev Genet 6: 311–323, 2005 [DOI] [PubMed] [Google Scholar]
- 225. Kishimoto M, Fujiki R, Takezawa S, Sasaki Y, Nakamura T, Yamaoka K, Kitagawa H, Kato S. Nuclear receptor mediated gene regulation through chromatin remodeling and histone modifications. Endocr J 53: 157–172, 2006 [DOI] [PubMed] [Google Scholar]
- 226. Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S. Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med 338: 653–661, 1998 [DOI] [PubMed] [Google Scholar]
- 227. Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 23: 687–702, 2002 [DOI] [PubMed] [Google Scholar]
- 228. Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 83: 813–819, 1995 [DOI] [PubMed] [Google Scholar]
- 229. Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92: 73–82, 1998 [DOI] [PubMed] [Google Scholar]
- 230. Kliewer SA, Umesono K, Heyman RA, Mangelsdorf DJ, Dyck JA, Evans RM. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci USA 89: 1448–1452, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 358: 771–774, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Knauf C, Rieusset J, Foretz M, Cani PD, Uldry M, Hosokawa M, Martinez E, Bringart M, Waget A, Kersten S, Desvergne B, Gremlich S, Wahli W, Seydoux J, Delzenne NM, Thorens B, Burcelin R. Peroxisome proliferator-activated receptor-alpha-null mice have increased white adipose tissue glucose utilization, GLUT4, and fat mass: Role in liver and brain. Endocrinology 147: 4067–4078, 2006 [DOI] [PubMed] [Google Scholar]
- 233. Kneissel M, Studer A, Cortesi R, Susa M. Retinoid-induced bone thinning is caused by subperiosteal osteoclast activity in adult rodents. Bone 36: 202–214, 2005 [DOI] [PubMed] [Google Scholar]
- 234. Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428: 758–763, 2004 [DOI] [PubMed] [Google Scholar]
- 235. Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, Nakashima K, Takayanagi H. NFAT and Osterix cooperatively regulate bone formation. Nature Med 11: 880–885, 2005 [DOI] [PubMed] [Google Scholar]
- 236. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89: 755–764, 1997 [DOI] [PubMed] [Google Scholar]
- 237. Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304–309, 1999 [DOI] [PubMed] [Google Scholar]
- 238. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397: 315–323, 1999 [DOI] [PubMed] [Google Scholar]
- 239. Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104: 205–215, 2001 [DOI] [PubMed] [Google Scholar]
- 240. Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104: 719–730, 2001 [PubMed] [Google Scholar]
- 241. Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298: 843–846, 2002 [DOI] [PubMed] [Google Scholar]
- 242. Kramer BC, Woodbury D, Black IB. Adult rat bone marrow stromal cells express genes associated with dopamine neurons. Biochem Biophys Res Commun 343: 1045–1052, 2006 [DOI] [PubMed] [Google Scholar]
- 243. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95: 15677–15682, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest 116: 1202–1209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Krum SA, Brown M. Unraveling estrogen action in osteoporosis. Cell Cycle 7: 1348–1352, 2008 [DOI] [PubMed] [Google Scholar]
- 246. Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 27: 535–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Kurokawa R, Soderstrom M, Horlein A, Halachmi S, Brown M, Rosenfeld MG, Glass CK. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature 377: 451–454, 1995 [DOI] [PubMed] [Google Scholar]
- 248. Lammi J, Aarnisalo P. FGF-8 stimulates the expression of NR4A orphan nuclear receptors in osteoblasts. Mol Cell Endocrinol 295: 87–93, 2008 [DOI] [PubMed] [Google Scholar]
- 249. Lammi J, Huppunen J, Aarnisalo P. Regulation of the osteopontin gene by the orphan nuclear receptor NURR1 in osteoblasts. Mol Endocrinol 18: 1546–1557, 2004 [DOI] [PubMed] [Google Scholar]
- 250. Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19: 1006–1012, 2004 [DOI] [PubMed] [Google Scholar]
- 251. Laudet aGH V. The Nuclear Receptor Facts Book. San Diego, CA: Academic, 2002 [Google Scholar]
- 252. Laue K, Janicke M, Plaster N, Sonntag C, Hammerschmidt M. Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development. Development 135: 3775–3787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Laue K, Pogoda HM, Daniel PB, van Haeringen A, Alanay Y, von Ameln S, Rachwalski M, Morgan T, Gray MJ, Breuning MH, Sawyer GM, Sutherland-Smith AJ, Nikkels PG, Kubisch C, Bloch W, Wollnik B, Hammerschmidt M, Robertson SP. Craniosynostosis and multiple skeletal anomalies in humans and zebrafish result from a defect in the localized degradation of retinoic Acid. Am J Hum Genet 89: 595–606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Le Maire A, Teyssier C, Erb C, Grimaldi M, Alvarez S, de Lera AR, Balaguer P, Gronemeyer H, Royer CA, Germain P, Bourguet W. A unique secondary-structure switch controls constitutive gene repression by retinoic acid receptor. Nature Struct Mol Biol 17: 801–807, 2010 [DOI] [PubMed] [Google Scholar]
- 255. Le WD, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet 33: 85–89, 2003 [DOI] [PubMed] [Google Scholar]
- 256. Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143: 2376–2384, 2002 [DOI] [PubMed] [Google Scholar]
- 257. Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffroy V, Ducy P, Karsenty G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet 16: 307–310, 1997 [DOI] [PubMed] [Google Scholar]
- 258. Lee MK, Choi H, Gil M, Nikodem VM. Regulation of osteoblast differentiation by Nurr1 in MC3T3–E1 cell line and mouse calvarial osteoblasts. J Cell Biochem 99: 986–994, 2006 [DOI] [PubMed] [Google Scholar]
- 259. Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab 19: 161–166, 2008 [DOI] [PubMed] [Google Scholar]
- 260. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell 130: 456–469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15: 3012–3022, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Lee YS, Chanda D, Sim J, Park YY, Choi HS. Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol 261: 117–158, 2007 [DOI] [PubMed] [Google Scholar]
- 263. Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116: 571–580, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 270: 12953–12956, 1995 [DOI] [PubMed] [Google Scholar]
- 265. Lehrke M, Lazar MA. The many faces of PPARgamma. Cell 123: 993–999, 2005 [DOI] [PubMed] [Google Scholar]
- 266. Levi B, Nelson ER, Brown K, James AW, Xu D, Dunlevie R, Wu JC, Lee M, Wu B, Commons GW, Vistnes D, Longaker MT. Differences in osteogenic differentiation of adipose-derived stromal cells from murine, canine, and human sources in vitro and in vivo. Plastic Reconstructive Surgery 128: 373–386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Li CY, Jepsen KJ, Majeska RJ, Zhang J, Ni R, Gelb BD, Schaffler MB. Mice lacking cathepsin K maintain bone remodeling but develop bone fragility despite high bone mass. J Bone Miner Res 21: 865–875, 2006 [DOI] [PubMed] [Google Scholar]
- 269. Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 97: 1566–1571, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Li N, Kelsh RN, Croucher P, Roehl HH. Regulation of neural crest cell fate by the retinoic acid and Pparg signalling pathways. Development 137: 389–394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 139: 4391–4396, 1998 [DOI] [PubMed] [Google Scholar]
- 272. Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94: 9831–9835, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Lind T, Lind PM, Jacobson A, Hu L, Sundqvist A, Risteli J, Yebra-Rodriguez A, Rodriguez-Navarro A, Andersson G, Melhus H. High dietary intake of retinol leads to bone marrow hypoxia and diaphyseal endosteal mineralization in rats. Bone 48: 496–506, 2011 [DOI] [PubMed] [Google Scholar]
- 274. Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol 171: 229–236, 2001 [DOI] [PubMed] [Google Scholar]
- 275. Lindberg MK, Erlandsson M, Alatalo SL, Windahl S, Andersson G, Halleen JM, Carlsten H, Gustafsson JA, Ohlsson C. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the OPG/RANKL (osteoprotegerin/receptor activator of NF-kappa B ligand) ratio and serum interleukin-6 in male mice. J Endocrinol 171: 425–433, 2001 [DOI] [PubMed] [Google Scholar]
- 276. Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development 120: 2723–2748, 1994 [DOI] [PubMed] [Google Scholar]
- 277. Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13: 1015–1024, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Lonard DM, Kumar R, O'Malley BW. Minireview: the SRC family of coactivators: an entree to understanding a subset of polygenic diseases? Mol Endocrinol 24: 279–285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Lonard DM, Nawaz Z, Smith CL, O'Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell 5: 939–948, 2000 [DOI] [PubMed] [Google Scholar]
- 280. Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38: 1248–1250, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Lu M, Rabie AB. Matrix metalloproteinase-9 regulates graft bone resorption. Angle Orthod 76: 598–604, 2006 [DOI] [PubMed] [Google Scholar]
- 282. Lu XP, Salbert G, Pfahl M. An evolutionary conserved COUP-TF binding element in a neural-specific gene and COUP-TF expression patterns support a major role for COUP-TF in neural development. Mol Endocrinol 8: 1774–1788, 1994 [DOI] [PubMed] [Google Scholar]
- 283. Lukert BP. Osteoporosis: prevention and treatment. Comprehensive Ther 16: 36–42, 1990 [PubMed] [Google Scholar]
- 284. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Internal Med 112: 352–364, 1990 [DOI] [PubMed] [Google Scholar]
- 285. Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature 388: 778–782, 1997 [DOI] [PubMed] [Google Scholar]
- 286. Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132: 958–970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Lyashenko N, Weissenbock M, Sharir A, Erben RG, Minami Y, Hartmann C. Mice lacking the orphan receptor ror1 have distinct skeletal abnormalities and are growth retarded. Dev Dyn 239: 2266–2277 [DOI] [PubMed] [Google Scholar]
- 288. Lydon JP. Stem cells: cues from steroid hormones. Nature 465: 695–696, 2010 [DOI] [PubMed] [Google Scholar]
- 289. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9: 2266–2278, 1995 [DOI] [PubMed] [Google Scholar]
- 290. Lydon JP, O'Malley BW. Minireview: steroid receptor coactivator-3: a multifarious coregulator in mammary gland metastasis. Endocrinology 152: 19–25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Ma Y, Khalifa B, Yee YK, Lu J, Memezawa A, Savkur RS, Yamamoto Y, Chintalacharuvu SR, Yamaoka K, Stayrook KR, Bramlett KS, Zeng QQ, Chandrasekhar S, Yu XP, Linebarger JH, Iturria SJ, Burris TP, Kato S, Chin WW, Nagpal S. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J Clin Invest 116: 892–904, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. MacNamara P, O'Shaughnessy C, Manduca P, Loughrey HC. Progesterone receptors are expressed in human osteoblast-like cell lines and in primary human osteoblast cultures. Calcif Tissue Int 57: 436–441, 1995 [DOI] [PubMed] [Google Scholar]
- 293. Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, Takahashi N. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 18: 405–412, 2012 [DOI] [PubMed] [Google Scholar]
- 294. Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab 89: 61–70, 2004 [DOI] [PubMed] [Google Scholar]
- 295. Mages HW, Rilke O, Bravo R, Senger G, Kroczek RA. NOT, a human immediate-early response gene closely related to the steroid/thyroid hormone receptor NAK1/TR3. Mol Endocrinol 8: 1583–1591, 1994 [DOI] [PubMed] [Google Scholar]
- 296. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 297. Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev 20: 156–188, 1999 [DOI] [PubMed] [Google Scholar]
- 298. Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell 83: 841–850, 1995 [DOI] [PubMed] [Google Scholar]
- 299. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell 83: 835–839, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Mano H, Kimura C, Fujisawa Y, Kameda T, Watanabe-Mano M, Kaneko H, Kaneda T, Hakeda Y, Kumegawa M. Cloning and function of rabbit peroxisome proliferator-activated receptor delta/beta in mature osteoclasts. J Biol Chem 275: 8126–8132, 2000 [DOI] [PubMed] [Google Scholar]
- 301. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115–137, 2000 [DOI] [PubMed] [Google Scholar]
- 302. Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol 21: 2605–2614, 2007 [DOI] [PubMed] [Google Scholar]
- 303. Marcellini S, Bruna C, Henriquez JP, Albistur M, Reyes AE, Barriga EH, Henriquez B, Montecino M. Evolution of the interaction between Runx2 and VDR, two transcription factors involved in osteoblastogenesis. BMC Evolutionary Biol 10: 78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 46: 451–480, 2006 [DOI] [PubMed] [Google Scholar]
- 305. Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 24: 323–334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Masuyama R, Nakaya Y, Katsumata S, Kajita Y, Uehara M, Tanaka S, Sakai A, Kato S, Nakamura T, Suzuki K. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J Bone Miner Res 18: 1217–1226, 2003 [DOI] [PubMed] [Google Scholar]
- 307. Masuyama R, Nakaya Y, Tanaka S, Tsurukami H, Nakamura T, Watanabe S, Yoshizawa T, Kato S, Suzuki K. Dietary phosphorus restriction reverses the impaired bone mineralization in vitamin D receptor knockout mice. Endocrinology 142: 494–497, 2001 [DOI] [PubMed] [Google Scholar]
- 308. Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest 116: 3150–3159, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures–a randomized, active comparator, double-blind study. Bone 49: 605–612, 2011 [DOI] [PubMed] [Google Scholar]
- 310. Matsumoto T, Kubodera N. ED-71, a new active vitamin D3, increases bone mineral density regardless of serum 25(OH)D levels in osteoporotic subjects. J Steroid Biochem Mol Biol 103: 584–586, 2007 [DOI] [PubMed] [Google Scholar]
- 312. Matsumoto T, Miki T, Hagino H, Sugimoto T, Okamoto S, Hirota T, Tanigawara Y, Hayashi Y, Fukunaga M, Shiraki M, Nakamura T. A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 90: 5031–5036, 2005 [DOI] [PubMed] [Google Scholar]
- 313. Matsumoto T, Takano T, Yamakido S, Takahashi F, Tsuji N. Comparison of the effects of eldecalcitol and alfacalcidol on bone and calcium metabolism. J Steroid Biochem Mol Biol 121: 261–264, 2010 [DOI] [PubMed] [Google Scholar]
- 314. Matsuo K. Eph and ephrin interactions in bone. Adv Exp Med Biol 658: 95–103, 2010 [DOI] [PubMed] [Google Scholar]
- 315. Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet 24: 184–187, 2000 [DOI] [PubMed] [Google Scholar]
- 316. Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal 4: e002, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin–a molecule for all seasons. Qjm 95: 3–13, 2002 [DOI] [PubMed] [Google Scholar]
- 318. McBroom LD, Flock G, Giguere V. The nonconserved hinge region and distinct amino-terminal domains of the ROR alpha orphan nuclear receptor isoforms are required for proper DNA bending and ROR alpha-DNA interactions. Mol Cell Biol 15: 796–808, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA 283: 3205–3210, 2000 [DOI] [PubMed] [Google Scholar]
- 320. Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 120: 2749–2771, 1994 [DOI] [PubMed] [Google Scholar]
- 321. Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J Biol Chem 285: 15599–15610, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Meyer T, Kneissel M, Mariani J, Fournier B. In vitro and in vivo evidence for orphan nuclear receptor RORalpha function in bone metabolism. Proc Natl Acad Sci USA 97: 9197–9202, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci USA 100: 7135–7140, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med 348: 287–294, 2003 [DOI] [PubMed] [Google Scholar]
- 325. Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58: 726–741, 2006 [DOI] [PubMed] [Google Scholar]
- 326. Milford DV, Shackleton CH, Stewart PM. Mineralocorticoid hypertension and congenital deficiency of 11 beta-hydroxysteroid dehydrogenase in a family with the syndrome of “apparent” mineralocorticoid excess. Clin Endocrinol 43: 241–246, 1995 [DOI] [PubMed] [Google Scholar]
- 327. Miranda-Carboni GA, Guemes M, Bailey S, Anaya E, Corselli M, Peault B, Krum SA. GATA4 regulates estrogen receptor-alpha-mediated osteoblast transcription. Mol Endocrinol 25: 1126–1136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. The nuclear receptor LXR is a glucose sensor. Nature 445: 219–223, 2007 [DOI] [PubMed] [Google Scholar]
- 329. Moggs JG, Orphanides G. Estrogen receptors: orchestrators of pleiotropic cellular responses. EMBO Rep 2: 775–781, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Morinobu M, Ishijima M, Rittling SR, Tsuji K, Yamamoto H, Nifuji A, Denhardt DT, Noda M. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J Bone Miner Res 18: 1706–1715, 2003 [DOI] [PubMed] [Google Scholar]
- 331. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289: 1751–1754, 2000 [DOI] [PubMed] [Google Scholar]
- 332. Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev 26: 898–915, 2005 [DOI] [PubMed] [Google Scholar]
- 333. Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, Owen MJ, Mertelsmann R, Zabel BU, Olsen BR. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89: 773–779, 1997 [DOI] [PubMed] [Google Scholar]
- 334. Murayama A, Kim MS, Yanagisawa J, Takeyama K, Kato S. Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching. EMBO J 23: 1598–1608, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 335. Murphy EP, Dobson AD, Keller C, Conneely OM. Differential regulation of transcription by the NURR1/NUR77 subfamily of nuclear transcription factors. Gene Expr 5: 169–179, 1996 [PMC free article] [PubMed] [Google Scholar]
- 336. Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26: 662–687, 2005 [DOI] [PubMed] [Google Scholar]
- 337. Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93: 229–240, 1998 [DOI] [PubMed] [Google Scholar]
- 338. Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4: 353–362, 1999 [DOI] [PubMed] [Google Scholar]
- 339. Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130: 811–823, 2007 [DOI] [PubMed] [Google Scholar]
- 340. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29, 2002 [DOI] [PubMed] [Google Scholar]
- 341. Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17: 1231–1234, 2011 [DOI] [PubMed] [Google Scholar]
- 342. Nampei A, Hashimoto J, Hayashida K, Tsuboi H, Shi K, Tsuji I, Miyashita H, Yamada T, Matsukawa N, Matsumoto M, Morimoto S, Ogihara T, Ochi T, Yoshikawa H. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J Bone Miner Metab 22: 176–184, 2004 [DOI] [PubMed] [Google Scholar]
- 343. Nelson HD. Menopause. Lancet 371: 760–770, 2008 [DOI] [PubMed] [Google Scholar]
- 344. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 288: 872–881, 2002 [DOI] [PubMed] [Google Scholar]
- 345. Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112: 295–307, 2008 [DOI] [PubMed] [Google Scholar]
- 346. Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 24: 1192–1197, 2001 [DOI] [PubMed] [Google Scholar]
- 347. Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet 9: 541–553, 2008 [DOI] [PubMed] [Google Scholar]
- 348. Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 209: 823–825, 1980 [DOI] [PubMed] [Google Scholar]
- 349. Notini AJ, McManus JF, Moore A, Bouxsein M, Jimenez M, Chiu WS, Glatt V, Kream BE, Handelsman DJ, Morris HA, Zajac JD, Davey RA. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res 22: 347–356, 2007 [DOI] [PubMed] [Google Scholar]
- 350. Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8: 645–654, 2003 [DOI] [PubMed] [Google Scholar]
- 351. Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 143: 2349–2356, 2002 [DOI] [PubMed] [Google Scholar]
- 352. Oki Y, Watanabe S, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct 33: 211–222, 2008 [DOI] [PubMed] [Google Scholar]
- 353. Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci USA 83: 8819–8823, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA 98: 5306–5311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Osella G, Terzolo M, Reimondo G, Piovesan A, Pia A, Termine A, Paccotti P, Angeli A. Serum markers of bone and collagen turnover in patients with Cushing's syndrome and in subjects with adrenal incidentalomas. J Clin Endocrinol Metab 82: 3303–3307, 1997 [DOI] [PubMed] [Google Scholar]
- 356. Oz OK, Zerwekh JE, Fisher C, Graves K, Nanu L, Millsaps R, Simpson ER. Bone has a sexually dimorphic response to aromatase deficiency. J Bone Miner Res 15: 507–514, 2000 [DOI] [PubMed] [Google Scholar]
- 357. Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol 2007 [DOI] [PubMed] [Google Scholar]
- 358. Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 279: 16754–16766, 2004 [DOI] [PubMed] [Google Scholar]
- 359. Paredes R, Arriagada G, Cruzat F, Villagra A, Olate J, Zaidi K, van Wijnen A, Lian JB, Stein GS, Stein JL, Montecino M. Bone-specific transcription factor Runx2 interacts with the 1alpha,25-dihydroxyvitamin D3 receptor to up-regulate rat osteocalcin gene expression in osteoblastic cells. Mol Cell Biol 24: 8847–8861, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Park KG, Lee KM, Seo HY, Suh JH, Kim HS, Wang L, Won KC, Lee HW, Park JY, Lee KU, Kim JG, Kim BW, Choi HS, Lee IK. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes 56: 431–437, 2007 [DOI] [PubMed] [Google Scholar]
- 361. Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368, 1999 [DOI] [PubMed] [Google Scholar]
- 362. Pastorcic M, Wang H, Elbrecht A, Tsai SY, Tsai MJ, O'Malley BW. Control of transcription initiation in vitro requires binding of a transcription factor to the distal promoter of the ovalbumin gene. Mol Cell Biol 6: 2784–2791, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Pearce G, Tabensky DA, Delmas PD, Baker HW, Seeman E. Corticosteroid-induced bone loss in men. J Clin Endocrinol Metab 83: 801–806, 1998 [DOI] [PubMed] [Google Scholar]
- 364. Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 105: 20764–20769, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 93: 693–704, 1998 [DOI] [PubMed] [Google Scholar]
- 366. Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 12: 1048–1055, 2006 [DOI] [PubMed] [Google Scholar]
- 367. Peleg S, Uskokovic M, Ahene A, Vickery B, Avnur Z. Cellular and molecular events associated with the bone-protecting activity of the noncalcemic vitamin D analog Ro-26–9228 in osteopenic rats. Endocrinology 143: 1625–1636, 2002 [DOI] [PubMed] [Google Scholar]
- 368. Pendaries C, Darblade B, Rochaix P, Krust A, Chambon P, Korach KS, Bayard F, Arnal JF. The AF-1 activation-function of ERalpha may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci USA 99: 2205–2210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Pensler JM, Radosevich JA, Higbee R, Langman CB. Osteoclasts isolated from membranous bone in children exhibit nuclear estrogen and progesterone receptors. J Bone Miner Res 5: 797–802, 1990 [DOI] [PubMed] [Google Scholar]
- 370. Pereira FA, Qiu Y, Tsai MJ, Tsai SY. Chicken ovalbumin upstream promoter transcription factor (COUP-TF): expression during mouse embryogenesis. J Steroid Biochem Mol Biol 53: 503–508, 1995 [DOI] [PubMed] [Google Scholar]
- 371. Pereira RC, Delany AM, Canalis E. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone 30: 685–691, 2002 [DOI] [PubMed] [Google Scholar]
- 372. Pereira RM, Delany AM, Canalis E. Cortisol inhibits the differentiation and apoptosis of osteoblasts in culture. Bone 28: 484–490, 2001 [DOI] [PubMed] [Google Scholar]
- 373. Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: evolving models of co-repressor action. Nat Rev Genet 11: 109–123, 2010 [DOI] [PubMed] [Google Scholar]
- 374. Perissi V, Rosenfeld MG. Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol 6: 542–554, 2005 [DOI] [PubMed] [Google Scholar]
- 375. Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev 9: 769–782, 1995 [DOI] [PubMed] [Google Scholar]
- 376. Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol 20: 5119–5128, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene 19: 4970–4978, 2000 [DOI] [PubMed] [Google Scholar]
- 378. Pirih FQ, Nervina JM, Pham L, Aghaloo T, Tetradis S. Parathyroid hormone induces the nuclear orphan receptor NOR-1 in osteoblasts. Biochem Biophys Res Commun 306: 144–150, 2003 [DOI] [PubMed] [Google Scholar]
- 379. Pirih FQ, Tang A, Ozkurt IC, Nervina JM, Tetradis S. Nuclear orphan receptor Nurr1 directly transactivates the osteocalcin gene in osteoblasts. J Biol Chem 279: 53167–53174, 2004 [DOI] [PubMed] [Google Scholar]
- 380. Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nature Rev Drug Discovery 9: 941–955, 2010 [DOI] [PubMed] [Google Scholar]
- 381. Poleni PE, Bianchi A, Etienne S, Koufany M, Sebillaud S, Netter P, Terlain B, Jouzeau JY. Agonists of peroxisome proliferators-activated receptors (PPAR) alpha, beta/delta or gamma reduce transforming growth factor (TGF)-beta-induced proteoglycans' production in chondrocytes. Osteoarthritis Cartilage 15: 493–505, 2007 [DOI] [PubMed] [Google Scholar]
- 382. Pozo D, Garcia-Maurino S, Guerrero JM, Calvo JR. mRNA expression of nuclear receptor RZR/RORalpha, melatonin membrane receptor MT, and hydroxindole-O-methyltransferase in different populations of human immune cells. J Pineal Res 37: 48–54, 2004 [DOI] [PubMed] [Google Scholar]
- 383. Prawitt J, Beil FT, Marshall RP, Bartelt A, Ruether W, Heeren J, Amling M, Staels B, Niemeier A. Short-term activation of liver X receptors inhibits osteoblasts but long-term activation does not have an impact on murine bone in vivo. Bone 48: 339–346, 2011 [DOI] [PubMed] [Google Scholar]
- 384. Purpura KA, Aubin JE, Zandstra PW. Sustained in vitro expansion of bone progenitors is cell density dependent. Stem Cells 22: 39–50, 2004 [DOI] [PubMed] [Google Scholar]
- 385. Qiu T, Wu X, Zhang F, Clemens TL, Wan M, Cao X. TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nature Cell Biol 12: 224–234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Qiu Y, Cooney AJ, Kuratani S, DeMayo FJ, Tsai SY, Tsai MJ. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: evidence for a role in segmental patterning of the diencephalon. Proc Natl Acad Sci USA 91: 4451–4455, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Qiu Y, Pereira FA, DeMayo FJ, Lydon JP, Tsai SY, Tsai MJ. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev 11: 1925–1937, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Quinkler M, Kaur K, Hewison M, Stewart PM, Cooper MS. Progesterone is extensively metabolized in osteoblasts: implications for progesterone action on bone. Horm Metab Res 40: 679–684, 2008 [DOI] [PubMed] [Google Scholar]
- 389. Rachez C, Lemon BD, Suldan Z, Bromleigh V, Gamble M, Naar AM, Erdjument-Bromage H, Tempst P, Freedman LP. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 398: 824–828, 1999 [DOI] [PubMed] [Google Scholar]
- 390. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 377: 1276–1287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115: 3318–3325, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Raisz LG, Pilbeam CC, Fall PM. Prostaglandins: mechanisms of action and regulation of production in bone. Osteoporos Int 3 Suppl 1: 136–140, 1993 [DOI] [PubMed] [Google Scholar]
- 393. Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schutz G, Tuckermann JP. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 11: 517–531, 2010 [DOI] [PubMed] [Google Scholar]
- 394. Razzera G, Vernal J, Portugal RV, Calgaro MR, Fernandez P, Zakin MM, Polikarpov I, Terenzi H. Expression, purification, and initial structural characterization of rat orphan nuclear receptor NOR-1 LBD domain. Protein Expr Purif 37: 443–449, 2004 [DOI] [PubMed] [Google Scholar]
- 395. Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin–a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA 87: 4473–4475, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Remick J, Weintraub H, Setton R, Offenbacher J, Fisher E, Schwartzbard A. Fibrate therapy: an update. Cardiol Rev 16: 129–141, 2008 [DOI] [PubMed] [Google Scholar]
- 397. Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 40: 82–91, 2008 [DOI] [PubMed] [Google Scholar]
- 398. Rickard DJ, Iwaniec UT, Evans G, Hefferan TE, Hunter JC, Waters KM, Lydon JP, O'Malley BW, Khosla S, Spelsberg TC, Turner RT. Bone growth and turnover in progesterone receptor knockout mice. Endocrinology 149: 2383–2390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391: 79–82, 1998 [DOI] [PubMed] [Google Scholar]
- 400. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators–mechanisms of action and application to clinical practice. N Engl J Med 348: 618–629, 2003 [DOI] [PubMed] [Google Scholar]
- 401. Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23: 279–302, 2002 [DOI] [PubMed] [Google Scholar]
- 402. Roberts SJ, Chen Y, Moesen M, Schrooten J, Luyten FP. Enhancement of osteogenic gene expression for the differentiation of human periosteal derived cells. Stem Cell Res 7: 137–144, 2011 [DOI] [PubMed] [Google Scholar]
- 403. Robertson KM, Norgard M, Windahl SH, Hultenby K, Ohlsson C, Andersson G, Gustafsson JA. Cholesterol-sensing receptors, liver X receptor alpha and beta, have novel and distinct roles in osteoclast differentiation and activation. J Bone Miner Res 21: 1276–1287, 2006 [DOI] [PubMed] [Google Scholar]
- 404. Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Med Sci Sports Exercise 34: 196–202, 2002 [DOI] [PubMed] [Google Scholar]
- 405. Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science 289: 1508–1514, 2000 [DOI] [PubMed] [Google Scholar]
- 406. Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98: 13960–13965, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Rohde CM, DeLuca H. Bone resorption activity of all-trans retinoic acid is independent of vitamin D in rats. J Nutr 133: 777–783, 2003 [DOI] [PubMed] [Google Scholar]
- 408. Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med 353: 595–603, 2005 [DOI] [PubMed] [Google Scholar]
- 409. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med 364: 248–254, 2011 [DOI] [PubMed] [Google Scholar]
- 410. Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science 289: 950–953, 2000 [DOI] [PubMed] [Google Scholar]
- 411. Rubin CT. Skeletal strain and the functional significance of bone architecture. Calcif Tissue Int 36 Suppl 1: S11–18, 1984 [DOI] [PubMed] [Google Scholar]
- 412. Ryan MR, Shepherd R, Leavey JK, Gao Y, Grassi F, Schnell FJ, Qian WP, Kersh GJ, Weitzmann MN, Pacifici R. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci USA 102: 16735–16740, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145: 401–406, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci USA 95: 13453–13458, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Sagami I, Tsai SY, Wang H, Tsai MJ, O'Malley BW. Identification of two factors required for transcription of the ovalbumin gene. Mol Cell Biol 6: 4259–4267, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Saji F, Shigematsu T, Sakaguchi T, Ohya M, Orita H, Maeda Y, Ooura M, Mima T, Negi S. Fibroblast growth factor 23 production in bone is directly regulated by 1α,25-dihydroxyvitamin D, but not PTH. Am J Physiol Renal Physiol 299: F1212–F1217, 2010 [DOI] [PubMed] [Google Scholar]
- 417. Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T, Kodama T, Chatila TA, Bito H, Takayanagi H. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med 12: 1410–1416, 2006 [DOI] [PubMed] [Google Scholar]
- 418. Sato Y, Kaji M, Tsuru T, Oizumi K. Risk factors for hip fracture among elderly patients with Parkinson's disease. J Neurol Sci 182: 89–93, 2001 [DOI] [PubMed] [Google Scholar]
- 419. Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci USA 102: 2340–2345, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Schrader M, Danielsson C, Wiesenberg I, Carlberg C. Identification of natural monomeric response elements of the nuclear receptor RZR/ROR. They also bind COUP-TF homodimers. J Biol Chem 271: 19732–19736, 1996 [DOI] [PubMed] [Google Scholar]
- 421. Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, Pasparakis M, Khokha R, Ormandy CJ, Widschwendter M, Schett G, Penninger JM. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468: 98–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 86: 32–38, 2001 [DOI] [PubMed] [Google Scholar]
- 423. Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA, Park SW, Lane NE, Harris TB, Cummings SR. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 91: 3349–3354, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Seol W, Choi HS, Moore DD. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272: 1336–1339, 1996 [DOI] [PubMed] [Google Scholar]
- 425. Shen Q, Christakos S. The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol Chem 280: 40589–40598, 2005 [DOI] [PubMed] [Google Scholar]
- 426. Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA 97: 7829–7834, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Shevde NK, Plum LA, Clagett-Dame M, Yamamoto H, Pike JW, DeLuca HF. A potent analog of 1alpha,25-dihydroxyvitamin D3 selectively induces bone formation. Proc Natl Acad Sci USA 99: 13487–13491, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95: 927–937, 1998 [DOI] [PubMed] [Google Scholar]
- 429. Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289: F1088–F1095, 2005 [DOI] [PubMed] [Google Scholar]
- 431. Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto M, Pacifici M, Iwamoto M. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med 17: 454–460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Sibilska I, Barycka KM, Sicinski RR, Plum LA, Deluca HF. 1-Desoxy analog of 2MD: synthesis and biological activity of (20S)-25-hydroxy-2-methylene-19-norvitamin D3. J Steroid Biochem Mol Biol 121: 51–55, 2010 [DOI] [PubMed] [Google Scholar]
- 433. Sibonga JD, Evans HJ, Sung HG, Spector ER, Lang TF, Oganov VS, Bakulin AV, Shackelford LC, LeBlanc AD. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone 41: 973–978, 2007 [DOI] [PubMed] [Google Scholar]
- 434. Silva J, Dasgupta S, Wang G, Krishnamurthy K, Ritter E, Bieberich E. Lipids isolated from bone induce the migration of human breast cancer cells. J Lipid Res 47: 724–733, 2006 [DOI] [PubMed] [Google Scholar]
- 435. Sims NA, Clement-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest 106: 1095–1103, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Sims NA, Clement-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M, Baron R. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest 111: 1319–1327, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 30: 18–25, 2002 [DOI] [PubMed] [Google Scholar]
- 438. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731–744, 2000 [DOI] [PubMed] [Google Scholar]
- 439. Sinha KM, Yasuda H, Coombes MM, Dent SY, de Crombrugghe B. Regulation of the osteoblast-specific transcription factor Osterix by NO66, a Jumonji family histone demethylase. EMBO J 29: 68–79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Sitara D, Kim S, Razzaque MS, Bergwitz C, Taguchi T, Schuler C, Erben RG, Lanske B. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet 4: e1000154, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Sjogren K, Bohlooly YM, Olsson B, Coschigano K, Tornell J, Mohan S, Isaksson OG, Baumann G, Kopchick J, Ohlsson C. Disproportional skeletal growth and markedly decreased bone mineral content in growth hormone receptor −/− mice. Biochem Biophys Res Commun 267: 603–608, 2000 [DOI] [PubMed] [Google Scholar]
- 442. Skillington J, Choy L, Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol 159: 135–146, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol 300: C526–C541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, Nelson KE, Tuckey RC, Miller D, Jiao Y, Gu W, Postlethwaite AE. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol 131: 1167–1169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci USA 80: 2752–2756, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Smith CL, O'Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25: 45–71, 2004 [DOI] [PubMed] [Google Scholar]
- 447. Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331: 1056–1061, 1994 [DOI] [PubMed] [Google Scholar]
- 448. Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A, Leder BZ, Goessl C. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361: 745–755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop N, Grabowski P, Del Fattore A, Messina C, Errigo G, Coxon FP, Scott DI, Teti A, Rogers MJ, Vezzoni P, Villa A, Helfrich MH. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 39: 960–962, 2007 [DOI] [PubMed] [Google Scholar]
- 450. Sobacchi C, Frattini A, Orchard P, Porras O, Tezcan I, Andolina M, Babul-Hirji R, Baric I, Canham N, Chitayat D, Dupuis-Girod S, Ellis I, Etzioni A, Fasth A, Fisher A, Gerritsen B, Gulino V, Horwitz E, Klamroth V, Lanino E, Mirolo M, Musio A, Matthijs G, Nonomaya S, Notarangelo LD, Ochs HD, Superti Furga A, Valiaho J, van Hove JL, Vihinen M, Vujic D, Vezzoni P, Villa A. The mutational spectrum of human malignant autosomal recessive osteopetrosis. Hum Mol Genet 10: 1767–1773, 2001 [DOI] [PubMed] [Google Scholar]
- 451. Society NAM. Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause 13: 340–367, 2006 [DOI] [PubMed] [Google Scholar]
- 452. Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, Schurer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472: 491–494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485: 62–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Song HM, Nacamuli RP, Xia W, Bari AS, Shi YY, Fang TD, Longaker MT. High-dose retinoic acid modulates rat calvarial osteoblast biology. J Cell Physiol 202: 255–262, 2005 [DOI] [PubMed] [Google Scholar]
- 455. Song KH, Ellis E, Strom S, Chiang JY. Hepatocyte growth factor signaling pathway inhibits cholesterol 7alpha-hydroxylase and bile acid synthesis in human hepatocytes. Hepatology 46: 1993–2002, 2007 [DOI] [PubMed] [Google Scholar]
- 456. Sooy K, Sabbagh Y, Demay MB. Osteoblasts lacking the vitamin D receptor display enhanced osteogenic potential in vitro. J Cell Biochem 94: 81–87, 2005 [DOI] [PubMed] [Google Scholar]
- 457. Sowers MR, Zheng H, Jannausch ML, McConnell D, Nan B, Harlow S, Randolph JF., Jr Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab 95: 2155–2162, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med 196: 1047–1055, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Spoorendonk KM, Peterson-Maduro J, Renn J, Trowe T, Kranenbarg S, Winkler C, Schulte-Merker S. Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development 135: 3765–3774, 2008 [DOI] [PubMed] [Google Scholar]
- 460. Srivastava S, Toraldo G, Weitzmann MN, Cenci S, Ross FP, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-kappa B ligand (RANKL)-induced JNK activation. J Biol Chem 276: 8836–8840, 2001 [DOI] [PubMed] [Google Scholar]
- 461. St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys 473: 225–230, 2008 [DOI] [PubMed] [Google Scholar]
- 462. Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98: 3369–3374, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Stein GS, Lian JB, Stein JL, Van Wijnen AJ, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol Rev 76: 593–629, 1996 [DOI] [PubMed] [Google Scholar]
- 464. Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol 161: 2035–2046, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Still K, Grabowski P, Mackie I, Perry M, Bishop N. The peroxisome proliferator activator receptor alpha/delta agonists linoleic acid and bezafibrate upregulate osteoblast differentiation and induce periosteal bone formation in vivo. Calcif Tissue Int 83: 285–292, 2008 [DOI] [PubMed] [Google Scholar]
- 466. Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20: 345–357, 1999 [DOI] [PubMed] [Google Scholar]
- 467. Sumariwalla PF, Palmer CD, Pickford LB, Feldmann M, Foxwell BM, Brennan FM. Suppression of tumour necrosis factor production from mononuclear cells by a novel synthetic compound, CLX-090717. Rheumatology 48: 32–38, 2009 [DOI] [PubMed] [Google Scholar]
- 468. Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell 125: 247–260, 2006 [DOI] [PubMed] [Google Scholar]
- 469. Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328: 688–696, 2005 [DOI] [PubMed] [Google Scholar]
- 470. Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med 7: 584–590, 2001 [DOI] [PubMed] [Google Scholar]
- 471. Syversen U, Stunes AK, Gustafsson BI, Obrant KJ, Nordsletten L, Berge R, Thommesen L, Reseland JE. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord 9: 10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem 278: 43919–43927, 2003 [DOI] [PubMed] [Google Scholar]
- 473. Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nature Cell Biol 9: 1273–1285, 2007 [DOI] [PubMed] [Google Scholar]
- 474. Takada I, Yu RT, Xu HE, Lambert MH, Montana VG, Kliewer SA, Evans RM, Umesono K. Alteration of a single amino acid in peroxisome proliferator-activated receptor-alpha (PPAR alpha) generates a PPAR delta phenotype. Mol Endocrinol 14: 733–740, 2000 [DOI] [PubMed] [Google Scholar]
- 475. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7: 292–304, 2007 [DOI] [PubMed] [Google Scholar]
- 476. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889–901, 2002 [DOI] [PubMed] [Google Scholar]
- 477. Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature 416: 744–749, 2002 [DOI] [PubMed] [Google Scholar]
- 478. Takeda S, Yoshizawa T, Nagai Y, Yamato H, Fukumoto S, Sekine K, Kato S, Matsumoto T, Fujita T. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: studies using VDR knockout mice. Endocrinology 140: 1005–1008, 1999 [DOI] [PubMed] [Google Scholar]
- 479. Tanaka H, Seino Y. Direct action of 1,25-dihydroxyvitamin D on bone: VDRKO bone shows excessive bone formation in normal mineral condition. J Steroid Biochem Mol Biol 89–90: 343–345, 2004 [DOI] [PubMed] [Google Scholar]
- 480. Tanaka K, Tanaka S, Sakai A, Ninomiya T, Arai Y, Nakamura T. Deficiency of vitamin A delays bone healing process in association with reduced BMP2 expression after drill-hole injury in mice. Bone 47: 1006–1012, 2010 [DOI] [PubMed] [Google Scholar]
- 481. Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15: 757–765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Tasset D, Tora L, Fromental C, Scheer E, Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell 62: 1177–1187, 1990 [DOI] [PubMed] [Google Scholar]
- 483. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet 4: 638–649, 2003 [DOI] [PubMed] [Google Scholar]
- 484. Tetradis S, Bezouglaia O, Tsingotjidou A. Parathyroid hormone induces expression of the nuclear orphan receptor Nurr1 in bone cells. Endocrinology 142: 663–670, 2001 [DOI] [PubMed] [Google Scholar]
- 485. Tetradis S, Bezouglaia O, Tsingotjidou A, Vila A. Regulation of the nuclear orphan receptor Nur77 in bone by parathyroid hormone. Biochem Biophys Res Commun 281: 913–916, 2001 [DOI] [PubMed] [Google Scholar]
- 486. Teyssier C, Gallet M, Rabier B, Monfoulet L, Dine J, Macari C, Espallergues J, Horard B, Giguere V, Cohen-Solal M, Chassande O, Vanacker JM. Absence of ERRalpha in female mice confers resistance to bone loss induced by age or estrogen-deficiency. PLoS One 4: e7942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Thevis M, Gerace E, Thomas A, Beuck S, Geyer H, Schlorer N, Kearbey JD, Dalton JT, Schanzer W. Characterization of in vitro generated metabolites of the selective androgen receptor modulators S-22 and S-23 and in vivo comparison to post-administration canine urine specimens. Drug Testing Analysis 2: 589–598, 2010 [DOI] [PubMed] [Google Scholar]
- 488. Thompson DL, Sabbagh Y, Tenenhouse HS, Roche PC, Drezner MK, Salisbury JL, Grande JP, Poeschla EM, Kumar R. Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J Bone Miner Res 17: 311–320, 2002 [DOI] [PubMed] [Google Scholar]
- 489. Tomita T, Kakiuchi Y, Tsao PS. THR0921, a novel peroxisome proliferator-activated receptor gamma agonist, reduces the severity of collagen-induced arthritis. Arthritis Res Ther 8: R7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, Teitelbaum SL. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386: 81–84, 1997 [DOI] [PubMed] [Google Scholar]
- 491. Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8: 1224–1234, 1994 [DOI] [PubMed] [Google Scholar]
- 492. Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 59: 477–487, 1989 [DOI] [PubMed] [Google Scholar]
- 493. Tremblay AM, Giguere V. The NR3B subgroup: an ovERRview. Nucl Recept Signal 5: e009, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Tsai SY, Sagami I, Wang H, Tsai MJ, O'Malley BW. Interactions between a DNA-binding transcription factor (COUP) and a non-DNA binding factor (S300-II). Cell 50: 701–709, 1987 [DOI] [PubMed] [Google Scholar]
- 495. Tsezou A, Iliopoulos D, Malizos KN, Simopoulou T. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J Orthop Res 28: 1033–1039, 2010 [DOI] [PubMed] [Google Scholar]
- 496. Turner CH, Forwood MR, Otter MW. Mechanotransduction in bone: do bone cells act as sensors of fluid flow? FASEB J 8: 875–878, 1994 [DOI] [PubMed] [Google Scholar]
- 497. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736, 2008 [DOI] [PubMed] [Google Scholar]
- 498. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 499. Van Beek E, Lowik C, Karperien M, Papapoulos S. Independent pathways in the modulation of osteoclastic resorption by intermediates of the mevalonate biosynthetic pathway: the role of the retinoic acid receptor. Bone 38: 167–171, 2006 [DOI] [PubMed] [Google Scholar]
- 500. Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98: 13324–13329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot RA. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol 28: 5986–5995, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, Warman ML, De Vernejoul MC, Bollerslev J, Van Hul W. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 72: 763–771, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev 25: 389–425, 2004 [DOI] [PubMed] [Google Scholar]
- 504. Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5: 367–377, 2003 [DOI] [PubMed] [Google Scholar]
- 505. Villena JA, Kralli A. ERRalpha: a metabolic function for the oldest orphan. Trends Endocrinol Metab 19: 269–276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Vivat-Hannah V, Bourguet W, Gottardis M, Gronemeyer H. Separation of retinoid X receptor homo- and heterodimerization functions. Mol Cell Biol 23: 7678–7688, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. Wan DC, Shi YY, Nacamuli RP, Quarto N, Lyons KM, Longaker MT. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci USA 103: 12335–12340, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Wan M, Li J, Herbst K, Zhang J, Yu B, Wu X, Qiu T, Lei W, Lindvall C, Williams BO, Ma H, Zhang F, Cao X. LRP6 mediates cAMP generation by G protein-coupled receptors through regulating the membrane targeting of Galpha(s). Sci Signal 4: ra15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med 13: 1496–1503, 2007 [DOI] [PubMed] [Google Scholar]
- 510. Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, Qiu J, Liu W, Kaikkonen MU, Ohgi KA, Glass CK, Rosenfeld MG, Fu XD. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474: 390–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3: 543–553, 1999 [DOI] [PubMed] [Google Scholar]
- 512. Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev 19: 333–345, 2008 [DOI] [PubMed] [Google Scholar]
- 513. Wang LH, Tsai SY, Cook RG, Beattie WG, Tsai MJ, O'Malley BW. COUP transcription factor is a member of the steroid receptor superfamily. Nature 340: 163–166, 1989 [DOI] [PubMed] [Google Scholar]
- 514. Wang QP, Xie H, Yuan LQ, Luo XH, Li H, Wang D, Meng P, Liao EY. Effect of progesterone on apoptosis of murine MC3T3–E1 osteoblastic cells. Amino Acids 36: 57–63, 2009 [DOI] [PubMed] [Google Scholar]
- 515. Wang X, Wu J, Shidoji Y, Muto Y, Ohishi N, Yagi K, Ikegami S, Shinki T, Udagawa N, Suda T, Ishimi Y. Effects of geranylgeranoic acid in bone: induction of osteoblast differentiation and inhibition of osteoclast formation. J Bone Miner Res 17: 91–100, 2002 [DOI] [PubMed] [Google Scholar]
- 516. Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 113: 159–170, 2003 [DOI] [PubMed] [Google Scholar]
- 517. Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NP, Perlmann T. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423: 555–560, 2003 [DOI] [PubMed] [Google Scholar]
- 518. Watanabe Y, Ohshima H, Mizuno K, Sekiguchi C, Fukunaga M, Kohri K, Rittweger J, Felsenberg D, Matsumoto T, Nakamura T. Intravenous pamidronate prevents femoral bone loss and renal stone formation during 90-day bed rest. J Bone Miner Res 19: 1771–1778, 2004 [DOI] [PubMed] [Google Scholar]
- 519. Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab 11: 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 365: 62–70, 2011 [DOI] [PubMed] [Google Scholar]
- 521. Weinstein RS. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disorders 2: 65–73, 2001 [DOI] [PubMed] [Google Scholar]
- 522. Weinstein RS, Manolagas SC. Apoptosis and osteoporosis. Am J Med 108: 153–164, 2000 [DOI] [PubMed] [Google Scholar]
- 523. Weinstein RS, Underwood JL, Hutson MS, DeLuca HF. Bone histomorphometry in vitamin D-deficient rats infused with calcium and phosphorus. Am J Physiol Endocrinol Metab 246: E499–E505, 1984 [DOI] [PubMed] [Google Scholar]
- 524. Weston AD, Blumberg B, Underhill TM. Active repression by unliganded retinoid receptors in development: less is sometimes more. J Cell Biol 161: 223–228, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Weston AD, Hoffman LM, Underhill TM. Revisiting the role of retinoid signaling in skeletal development. Birth defects research Part C. Embryo Today Reviews 69: 156–173, 2003 [DOI] [PubMed] [Google Scholar]
- 527. Williams JA, Kane M, Okabe T, Enomoto-Iwamoto M, Napoli JL, Pacifici M, Iwamoto M. Endogenous retinoids in mammalian growth plate cartilage: analysis and roles in matrix homeostasis and turnover. J Biol Chem 285: 36674–36681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Williams JA, Kondo N, Okabe T, Takeshita N, Pilchak DM, Koyama E, Ochiai T, Jensen D, Chu ML, Kane MA, Napoli JL, Enomoto-Iwamoto M, Ghyselinck N, Chambon P, Pacifici M, Iwamoto M. Retinoic acid receptors are required for skeletal growth, matrix homeostasis and growth plate function in postnatal mouse. Dev Biol 328: 315–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev 9: 1033–1045, 1995 [DOI] [PubMed] [Google Scholar]
- 530. Wilson TE, Paulsen RE, Padgett KA, Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science 256: 107–110, 1992 [DOI] [PubMed] [Google Scholar]
- 531. Windahl SH, Andersson G, Gustafsson JA. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab 13: 195–200, 2002 [DOI] [PubMed] [Google Scholar]
- 532. Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. J Clin Invest 104: 895–901, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Wu S, Liang S, Yan Y, Wang Y, Li F, Deng Y, Huang W, Yuan W, Luo N, Zhu C, Wang Y, Li Y, Liu M, Wu X. A novel mutation of TGF beta1 in a Chinese family with Camurati-Engelmann disease. Bone 40: 1630–1634, 2007 [DOI] [PubMed] [Google Scholar]
- 534. Wu X, Pang L, Lei W, Lu W, Li J, Li Z, Frassica FJ, Chen X, Wan M, Cao X. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell 7: 571–580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Wu X, Peters JM, Gonzalez FJ, Prasad HS, Rohrer MD, Gimble JM. Frequency of stromal lineage colony forming units in bone marrow of peroxisome proliferator-activated receptor-alpha-null mice. Bone 26: 21–26, 2000 [DOI] [PubMed] [Google Scholar]
- 536. Xiao Q, Castillo SO, Nikodem VM. Distribution of messenger RNAs for the orphan nuclear receptors Nurr1 and Nur77 (NGFI-B) in adult rat brain using in situ hybridization. Neuroscience 75: 221–230, 1996 [DOI] [PubMed] [Google Scholar]
- 537. Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406: 435–439, 2000 [DOI] [PubMed] [Google Scholar]
- 538. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 17: 1235–1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Xu HE, Lambert MH, Montana VG, Plunket KD, Moore LB, Collins JL, Oplinger JA, Kliewer SA, Gampe RT, Jr, McKee DD, Moore JT, Willson TM. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA 98: 13919–13924, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Xu HE, Stanley TB, Montana VG, Lambert MH, Shearer BG, Cobb JE, McKee DD, Galardi CM, Plunket KD, Nolte RT, Parks DJ, Moore JT, Kliewer SA, Willson TM, Stimmel JB. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature 415: 813–817, 2002 [DOI] [PubMed] [Google Scholar]
- 541. Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135: 825–837, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202: 345–351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Yamagata K, Fujiyama S, Ito S, Ueda T, Murata T, Naitou M, Takeyama K, Minami Y, O'Malley BW, Kato S. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell 36: 340–347, 2009 [DOI] [PubMed] [Google Scholar]
- 545. Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126: 1211–1223, 1999 [DOI] [PubMed] [Google Scholar]
- 546. Yamamoto R, Minamizaki T, Yoshiko Y, Yoshioka H, Tanne K, Aubin JE, Maeda N. 1Alpha,25-dihydroxyvitamin D3 acts predominately in mature osteoblasts under conditions of high extracellular phosphate to increase fibroblast growth factor 23 production in vitro. J Endocrinol 206: 279–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547. Yamashita A, Takada T, Narita J, Yamamoto G, Torii R. Osteoblastic differentiation of monkey embryonic stem cells in vitro. Cloning Stem Cells 7: 232–237, 2005 [DOI] [PubMed] [Google Scholar]
- 548. Yang Q, Gonzalez FJ. Peroxisome proliferator-activated receptor alpha regulates B lymphocyte development via an indirect pathway in mice. Biochem Pharmacol 68: 2143–2150, 2004 [DOI] [PubMed] [Google Scholar]
- 549. Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology: implication for Coffin-Lowry Syndrome. Cell 117: 387–398, 2004 [DOI] [PubMed] [Google Scholar]
- 550. Yao W, Dai W, Shahnazari M, Pham A, Chen Z, Chen H, Guan M, Lane NE. Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice. PLoS One 5: e11410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95: 3597–3602, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552. Yaturu S, Bryant B, Jain SK. Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care 30: 1574–1576, 2007 [DOI] [PubMed] [Google Scholar]
- 553. Yokoyama A, Fujiki R, Ohtake F, Kato S. Regulated histone methyltransferase and demethylase complexes in the control of genes by nuclear receptors. Cold Spring Harb Symp Quant Biol 2011 [DOI] [PubMed] [Google Scholar]
- 554. Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y, Komori T. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18: 952–963, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555. Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345: 442–444, 1990 [DOI] [PubMed] [Google Scholar]
- 556. Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16: 391–396, 1997 [DOI] [PubMed] [Google Scholar]
- 557. Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone 36: 971–977, 2005 [DOI] [PubMed] [Google Scholar]
- 558. Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci USA 95: 7939–7944, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559. Yuan Q, Sitara D, Sato T, Densmore M, Saito H, Schuler C, Erben RG, Lanske B. PTH ablation ameliorates the anomalies of Fgf23-deficient mice by suppressing the elevated vitamin D and calcium levels. Endocrinology 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Zaidi M. Skeletal remodeling in health and disease. Nat Med 13: 791–801, 2007 [DOI] [PubMed] [Google Scholar]
- 561. Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 17: 509–511, 2003 [DOI] [PubMed] [Google Scholar]
- 562. Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science 276: 248–250, 1997 [DOI] [PubMed] [Google Scholar]
- 563. Zetterstrom RH, Solomin L, Mitsiadis T, Olson L, Perlmann T. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol 10: 1656–1666, 1996 [DOI] [PubMed] [Google Scholar]
- 564. Zetterstrom RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res 41: 111–120, 1996 [DOI] [PubMed] [Google Scholar]
- 565. Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4: 111–121, 2006 [DOI] [PubMed] [Google Scholar]
- 566. Zhao X, Sirbu IO, Mic FA, Molotkova N, Molotkov A, Kumar S, Duester G. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr Biol 19: 1050–1057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]