Abstract
The mammalian Slc4 (Solute carrier 4) family of transporters is a functionally diverse group of 10 multi-spanning membrane proteins that includes three Cl-HCO3 exchangers (AE1–3), five Na+-coupled HCO3− transporters (NCBTs), and two other unusual members (AE4, BTR1). In this review, we mainly focus on the five mammalian NCBTs-NBCe1, NBCe2, NBCn1, NDCBE, and NBCn2. Each plays a specialized role in maintaining intracellular pH and, by contributing to the movement of HCO3− across epithelia, in maintaining whole-body pH and otherwise contributing to epithelial transport. Disruptions involving NCBT genes are linked to blindness, deafness, proximal renal tubular acidosis, mental retardation, and epilepsy. We also review AE1–3, AE4, and BTR1, addressing their relevance to the study of NCBTs. This review draws together recent advances in our understanding of the phylogenetic origins and physiological relevance of NCBTs and their progenitors. Underlying these advances is progress in such diverse disciplines as physiology, molecular biology, genetics, immunocytochemistry, proteomics, and structural biology. This review highlights the key similarities and differences between individual NCBTs and the genes that encode them and also clarifies the sometimes confusing NCBT nomenclature.
I. INTRODUCTION
A. Regulation of pH
pH is one of the most important parameters for life. Virtually every biological process is sensitive to changes in pH, and some are exquisitely sensitive. Thus transporters have evolved to regulate pH in organelles, the cytosol, and the extracellular fluid. Not surprisingly, dysregulation of pH is associated with a wide array of pathologies (TABLE 1), including cancer, hypertension, reperfusion injury, amyloid deposition (e.g., in Alzheimer's disease), and aging.
Table 1.
The importance of pH regulation
| Process | pH-Dependent Physiology | Pathological Associations |
|---|---|---|
| Cell survival | Acid-extruding mechanisms defend intracellular pH from catastrophic, pro-apoptotic acidosis (e.g., Ref. 112). However, acidosis is anti-apoptotic for some cells (e.g., Refs. 978, 1057). Telomere structure is pH sensitive (417). | Tumor proliferation: In cancer cells, enhanced acid-extrusion ability and a lowering of local extracellular pH, contributing to an acidic, tumor-permissive environment while defending tumor pHi (546, 935). Autophagy is reduced at acidic extracellular pH (1058). Heart failure: Hypoxia in combination with acidosis is pro-apoptotic in cardiac myocytes (519). |
| Na+ homeostasis | NCBTs and NHEs are secondary active transporters that couple acid extrusion with Na+ influx, thereby contributing to regulation of [Na+]i and plasma [Na+]. ENaC activity is modulated by pH and [HCO3−] (163, 196, 730). | Reperfusion injury: The influx of Na+ that accompanies enhanced acid extrusion following ischemia can tend to reverse Na+-Ca2+ exchangers, causing a pathological increase in [Ca2+]i (956, 993, 997). Hypertension: Dysregulation of H+ and HCO3− transporters is associated with hypertension (89, 92, 1020). |
| Cell migration and Wound healing | Acid-extruders act as plasma membrane anchors for cytoskeletal components (e.g., Ref. 243) and can promote an isosmotic volume increase at the leading edge of migrating cells (910). Acid extrusion promotes wound healing (1062) as well as dendritic spine growth (249). | Tumor metastasis: Acidosis, by stimulating the acid-extruding activity of NHE1, can promote metastasis of tumor cells (151, 547). HCO3−, in its capacity as a buffer, is inhibitory to metastasis (410, 801). |
| Solute transport | Many solute carriers such as H+-coupled amino acid transporters (95) influence or are influenced by pH. Furthermore, acid-base status influences the expression of other, nominally pH-independent carriers (660, 688). | Drug sensitivity: Acid-base status can influence the efficacy and toxicity of drugs (647, 705) and acidosis induces drug resistance in tumors via activation of P-glycoprotein (963). |
| Protein folding/assembly | The stability and conformation of almost all proteins is pH dependent, due to electrostatic effects (946). Consequently, the oligomeric state of diverse proteins (e.g., Refs. 145, 838, 1084) as well as interactions between protein binding partners (e.g., Refs. 661, 687) can be pH dependent. | Amyloidosis: Acidosis promotes amyloid formation (294, 395, 784, 815, 936), potentially impacting the severity of Alzheimer's Disease and scrapie. Carcinogenesis: The stability of the tumor-suppressing tetrameric form of a mutant p53 is readily destabilized by mild alkalosis, a mechanism suggested to underlie the increased incidence of carcinomas in individuals who carry this mutation (250). |
| Protein glycosylation | An acidic environment in the Golgi is crucial for appropriate localization of glycosyltransferases and therefore for N-glycosylation of proteins (799). | |
| Interactions at the cell surface | Some interactions between proteins and the plasma membrane or between proteins and cell-surface receptors are pH dependent (e.g., Refs. 255, 370). | Amyloid deposition: Deposition of amyloids is enhanced at acidic pH (131, 171, 513, 785). Viral infection: The fusion of viral particles with the host plasma membrane is pH dependent, although the direction of the dependence may vary between viruses (e.g., Refs. 363, 548, 751, 803, 1046). Bacterial colonization: The colonization of H. pylori on the surface of gastric mucosa is enhanced at acidic pH (787). Moreover, in a porcine model of cystic fibrosis, the acidity of airway surface liquid diminishes its antimicrobial properties (745). |
| Cell signaling | Sensors for acid, alkali, and CO2/HCO3− (129, 181, 1105, 1107) are expressed in multiple cell types, mediating the cellular effects of acid-base status. Furthermore, numerous receptor/ligand interactions are influenced by pH (e.g., Refs. 227, 295, and 691). | Type 2 diabetes mellitus: Elevated serum HCO3− was associated with a reduced risk of developing type 2 diabetes in a study of 650 women (625). Tumor proliferation: Expression of the acid sensor TDAG8 in tumor cells enables the cells to adapt to the extracellular acidic environment (415). Anxiety disorders: Acidosis and detection of H+ by the acid sensor ASIC-1a elicits acquired fear behavior. Overexpression of ASIC-1a in mice is a model of anxiety (204, 205, 1032, 1117). |
| DNA and protein synthesis and stability | Incorporation of amino acids into polypeptides is reduced under acidic conditions (451, 736). pH-responsive elements in certain RNAs confer increased lifetime to those transcripts in acidosis (409). | |
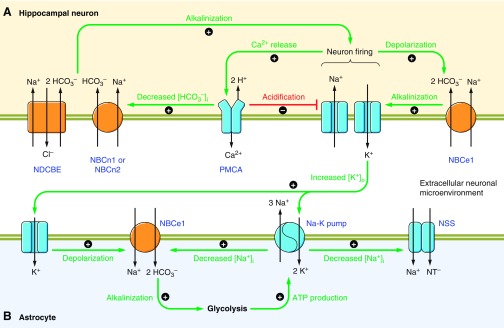
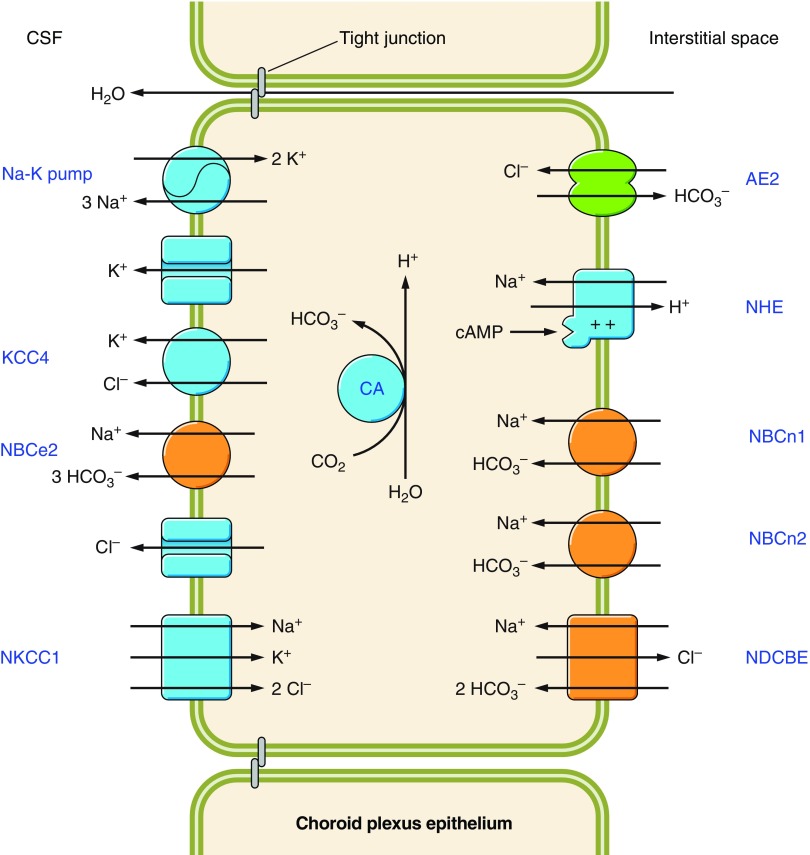
| Neuronal excitability | Excessive neuronal firing can reduce neuronal pH and in turn, neuronal excitability is reduced in response to lowering extracellular and intracellular pH (186, 187, 783). Most K+ channels are pH dependent (e.g., Refs. 67, 424, 1053). NCBTs play critical roles in defending neuronal pHi and regulating the pH of the neuronal microenvironment (via their action in astrocytes and choroid plexus epithelia). | Altered neuronal excitability: Disruption of NCBT genes is associated with autism, epilepsy, mental retardation, and migraine (360, 411, 516, 830, 930). |
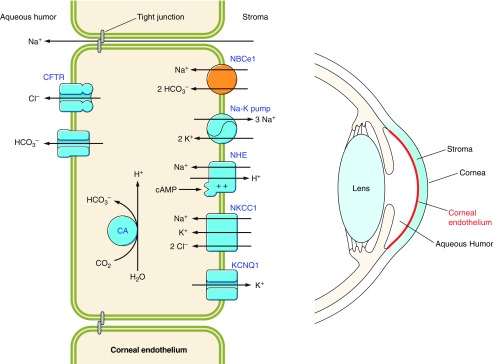
| Special senses | The fluid movement that follows HCO3− transport maintains the clarity of the cornea (96) and lens (65) and also maintains retinal attachment (400, 534). In the inner ear, low endolymph pH can reduce response of hair cells to auditory stimuli (150). | Loss of vision: Mutations in acid-base transporters are associated with cataracts, glaucoma, and retinopathy (e.g., Refs. 30, 93, 411). Acidosis induces retinopathy in neonatal rats (391, 392). Loss of hearing: Mutations in acid-base transporters are associated with hearing loss (e.g., Refs. 93, 473). |
| Muscle contraction | Multiple elements of excitation-contraction coupling in cardiac, smooth, and skeletal muscle are inhibited at low pH including neurotransmitter release (586), gap junction conductivity (379, 707), as well as the action of the contractile apparatus (e.g., Refs. 286, 497, 892, 1045). | Paralysis: Lactic acidosis (e.g., Ref. 85) and renal tubular acidosis (e.g., Ref. 119) result in muscle weakness. |
| Bone remodeling | Bone remodeling requires H+ secretion (62) and HCO3− resorption (797), thus bone maintenance is exquisitely pH sensitive. Furthermore, osteoclast survival is reduced by acidosis (e.g., Ref. 112). | Bone remodeling defects: H+ secretion defects in osteoclasts are associated with osteopetrosis (e.g., Refs. 455, 866), whereas whole-body acidosis can be associated with bone dysplasia (e.g., Refs. 313, 602). |
| Digestion | Enamel formation (456), saliva secretion (555), enzymatic digestion, and mucosal protection (17) are all pH/HCO3−-dependent processes. | Poor dentition: Defects in acid-base transporters result in defective enamel deposition (540, 617). Ulceration: Metabolic and respiratory acidoses increase the incidence of gastric lesions (142, 507). Gut lumen pH is unusually acidic in some individuals with ulcerative colitis (690). Diarrhea: Dysregulation of acid-base transport can result in decreased nutrient absorption, increased fluid secretion, and diarrhea (388, 938, 1092). |
| Immune response (544) | Extracellular acidosis activates neutrophils (978) but reduces TNF-α secretion by alveolar macrophages (82). Superoxide production by NADPH oxidase during the respiratory burst is accompanied by a decrease in pHi that is countered by the action of H+ channels (230). | Tumor proliferation: The reduction of macrophage cytotoxicity in the acidic tumor microenvironment would promote tumor survival (82). Immunodeficiency: Inability to defend macrophage pHi during respiratory burst might reduce the ability of macrophages to counter bacterial infection (discussed in Ref. 230). |
| Fertility | Multiple aspects of male and female fertility, including sperm maturation and cervical mucus release are influenced by pH and HCO3− (597, 665). | Reduced fertility: Mice with disrupted HCO3− transporters are sub- or infertile (e.g., Refs. 165, 389, 638). |
Proteins, processes, and pathologies in mammals that are influenced by or that influence pH. Processes and diseases that are specifically related to NCBT function and dysfunction are discussed in detail in later sections of the review.
The transporters responsible for pH regulation in various compartments include vacuolar-type ATPases or H+ pumps, gastric-type H+-K+-ATPases or pumps, Na-H exchangers, and bicarbonate (HCO3−) transporters. Physiological Reviews last appraised the general subject of intracellular pH (pHi) regulation in 1981, with the review by Roos and Boron (811). In 2003, Chesler (186) focused on pH regulation in the brain. This journal reviewed vacuolar H+ pumps in the contributions by Forgac in 1989 (292), by Nelson and Harvey in 1999 (678), and by Wagner et al. in 2004 (1015). The journal considered H-K pumps in the effort of Hersey and Sachs in 1995 (380). Na-H exchange was the subject of the 1997 review by Wakabayashi and co-workers (1017). Recently, Lee et al. (555) have examined HCO3− secretion by the pancreas and salivary glands (555). However, Physiological Reviews has not examined HCO3− transporters per se.
B. Scope of This Review
The movement of bicarbonate equivalents, HCO3− itself, CO32−, or the NaCO3− ion pair, across the plasma membrane is an integral part of the regulation of pHi and the transepithelial transport of solutes and fluid. Disturbances in HCO3− transporter genes are associated with a variety of pathologies and can potentially impact any of the vast array of pH-sensitive proteins and processes summarized in TABLE 1.
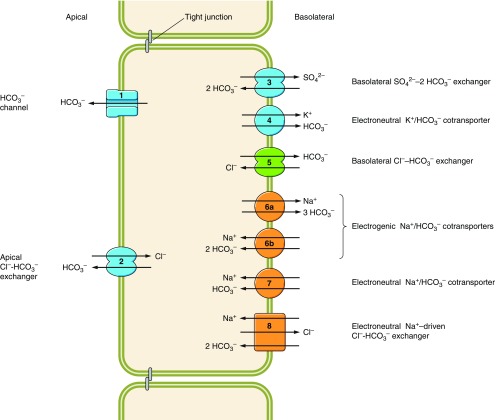
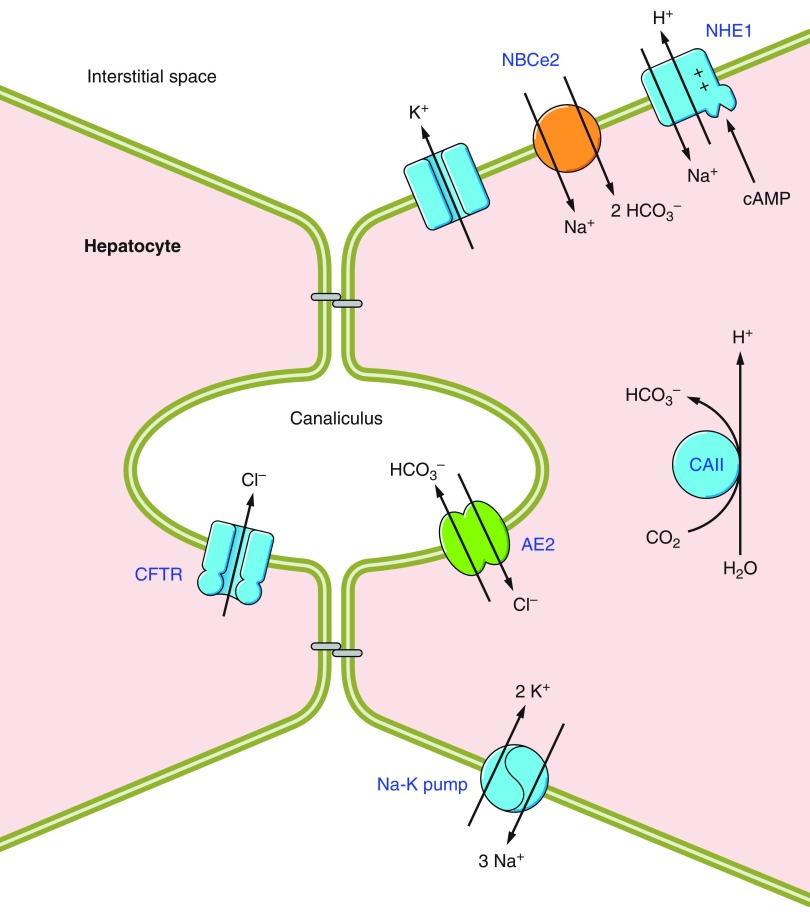
Bicarbonate transport in animals is effected by the eight physiologically distinct mechanisms numbered 1–8 in the generic epithelial cell in FIGURE 1.
Figure 1.
Functional classifications of bicarbonate transporters. Diagram of a generic epithelial cell showing the typical subcellular distribution of the 8 classes of HCO3− transporters. Anion channels (1) and anion exchangers of the Slc26 family (2) perform HCO3− secretion across the apical membrane. Basolateral Slc26a1 functions as SO42--2HCO3− exchanger (3). An as yet unknown transporter (4) is presumed to be responsible for a basolateral K/HCO3 cotransport activity in the inner medulla. Members of the Slc4 family (5–8) are usually located in the basolateral membranes of polarized epithelia. Cl-HCO3 exchangers (5) and electrogenic Na/HCO3 cotransporters with a calculated 1:3 stoichiometry (6a) act as acid-loaders, supporting HCO3− absorption into the blood. Electrogenic Na/HCO3 cotransporters with a 1:2 stoichiometry (6b), electroneutral Na/HCO3 cotransporters (7), and Na+-driven Cl-HCO3 exchangers (8) act as acid-extruders, supporting HCO3− secretion across the apical membrane by transporter classes 1–2.
1) Conductive HCO3− transport mediated by anion permeable channels such as GABA- and glycine-gated anion channels (98, 460), the cystic fibrosis transmembrane conductance regulator CFTR (752), ClC channels (827), and Ca2+-activated chloride channels (776, 777).1
2) Apical Na+-independent Cl-HCO3 exchange, effected by anion exchangers encoded by members of the solute carrier 26 (Slc26) gene family (Slc26a3, Slc26a4, Slc26a6, and Slc26a9), reviewed in References 153, 259, and 888.2
3) A basolateral Na+-independent SO4-2HCO3 exchanger, or oxalate-2HCO3 exchange encoded by Slc26a1 (474, 517, 525).
4) Electroneutral K/HCO3 cotransport. The molecular identity of the responsible protein(s) has yet to be established (386, 387, 570, 1097).
5) Basolateral Na+-independent Cl-HCO3 exchange, mediated mainly by the electroneutral anion exchangers AE1 (Slc4a1),3 AE2 (Slc4a2), and AE3 (Slc4a3) and perhaps some members of the Slc26 family (e.g., Slc26a7).
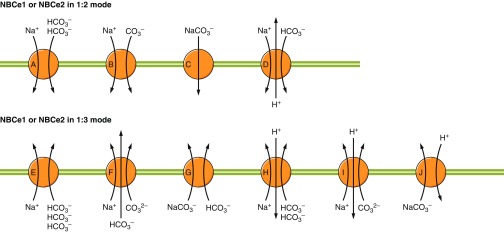
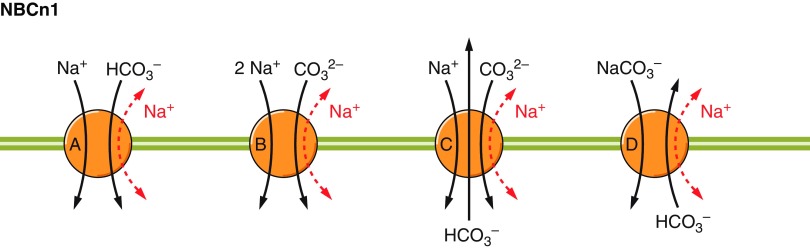
6) Electrogenic Na/HCO3 cotransport, mediated by NBCe1 (Slc4a4) and NBCe2 (Slc4a5), which are predicted to operate with varying stoichiometry in different cell-types (6a versus 6b in FIGURE 1).
7) Electroneutral Na/HCO3 cotransport, mediated by NBCn1 (Slc4a7) and NBCn2 (Slc4a10).
8) Na+-driven Cl-HCO3 exchange, mediated by NDCBE (Slc4a8).
Groups 5–8 include members of the Slc4 family that, in vertebrates, are normally located in the basolateral (or equivalent) membranes of polarized cells, in some instances complementing the usually apical (or equivalent) distribution of certain HCO3−-transporting Slc26 family members. Groups 6–8 are collectively referred to as Na+-coupled bicarbonate transporters (NCBTs) and are the major focus of the present review.
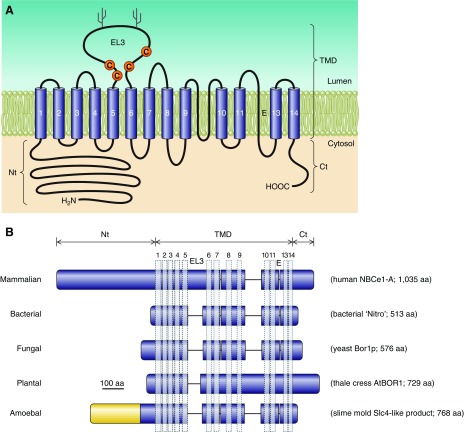
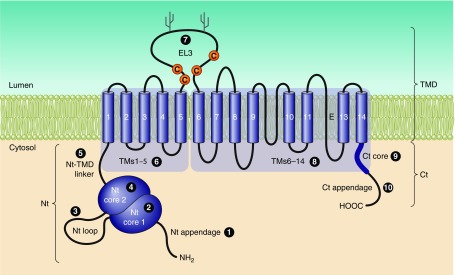
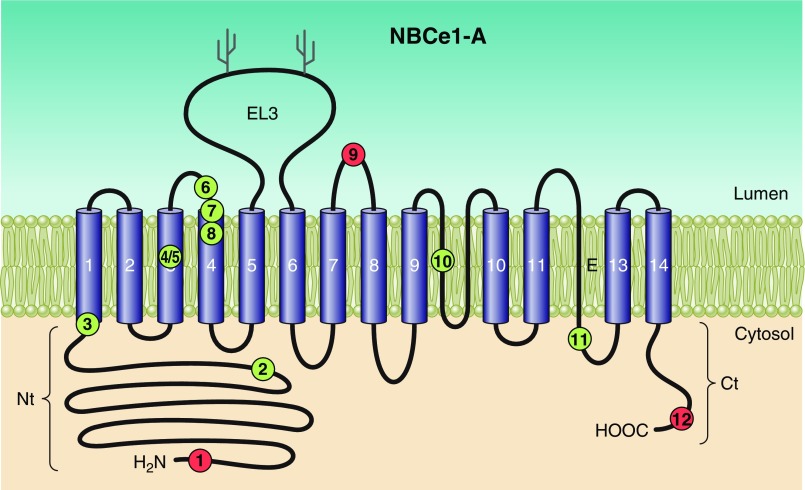
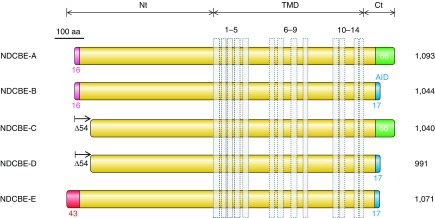
The general predicted topology of mammalian, and likely all vertebrate, Slc4s is exemplified by the depiction of human NBCe1 in FIGURE 2A. Typically, each Slc4 protein has a large NH2-terminal (Nt) cytoplasmic domain, followed by a large multi-spanning transmembrane domain (TMD) that includes one glycosylated extracellular loop, and concludes with a shorter COOH-terminal cytoplasmic domain (Ct). As depicted in FIGURE 2B, nonvertebrate Slc4-like (see footnote 3) products, such as those from bacteria, fungi, amoebas, and plants, are predicted to retain the same general topology but to have shorter Nts and to have extracellular loops of varying lengths.
Figure 2.
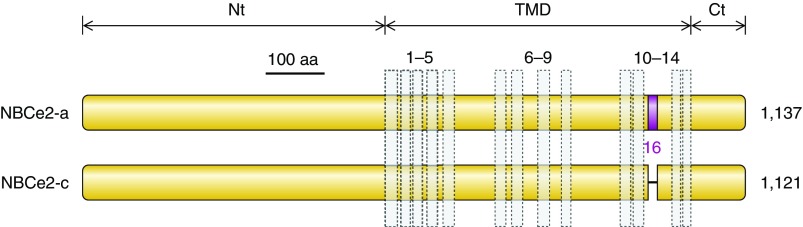
Presumed topology of NCBTs and Slc4-like transporters. Presumed topology of the electrogenic Na/HCO3 cotransporter NBCe1 (A), representing a probable common structure for all five mammalian NCBTs and most nonmammalian NCBTs. The model shows the extended cytosolic amino- and carboxy-terminal domains (Nt and Ct) linked via a transmembrane domain (TMD) that includes 14 transmembrane spans (TMs), one of which is thought to be an extended region (E) rather than an α-helix. In mammalian NCBTs, the third extracellular loop (EL3) between TMs 5 and 6 usually includes multiple cysteine residues (C) and multiple putative glycosylation sites (Y). A sequence alignment displaying these features for human NCBTs is provided in Appendix I. Pictorial depiction of NBCe1 domain sequences aligned against homologous regions of nonvertebrate Slc4-like transporters (B). Horizontal purple bars represent protein sequence laid out from Nt to Ct. Gaps in sequence alignment are depicted as horizontal lines. Vertical bars represent position of α-helical TMs. Note the shorter Nt and EL3 in nonvertebrates. The amoebal Slc4-like transporter includes an extended Nt (yellow region), but it shares no significant sequence identity with the extended Nt of vertebrate Slc4s. The sequence of nonmammalian Slc4-like protein is provided in Appendix II.
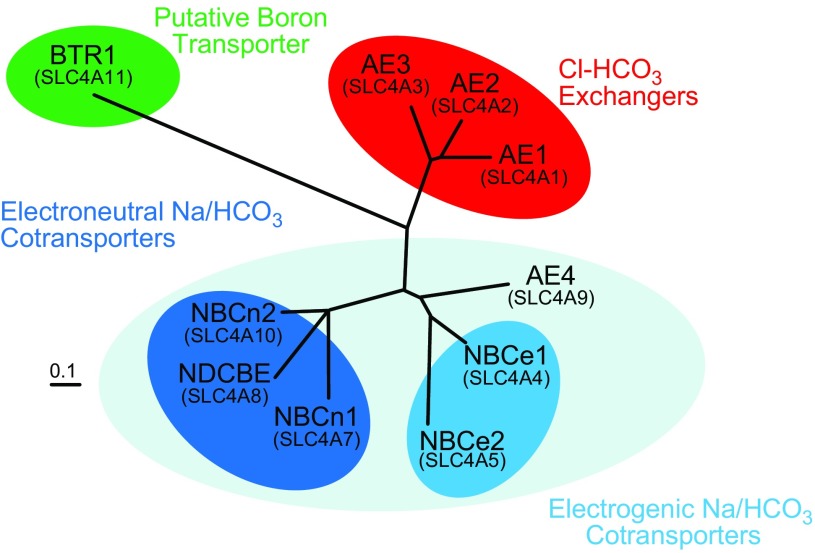
The molecular identity of the Na+-independent Cl-HCO3 exchangers AE1-AE3 (included in group 5, above) has been known for some time. It is more than 30 years since AE1 was first demonstrated to be the erythrocyte anion transporter (1040). The cloning of the murine Slc4a1 cDNA that encodes AE1 was reported in 1985 (510) and was soon followed by the discovery and cloning of Slc4a2 (23, 242) and Slc4a3 (509) products. These three genes appeared to be the extent of the gene family until 1997, when Romero et al. (809) published the cDNA and the elucidated protein sequence of an electrogenic Na/HCO3 cotransporter from the tiger salamander, Ambystoma tigrinum. Electrogenic Na/HCO3 cotransport had first been described in the salamander proximal tubule (PT) by Boron and Boulpaep 14 years earlier (103), and the cloning of the responsible gene product allowed sequence comparisons that importantly demonstrated that NCBTs were members of the same Slc4 family as AE1–3. The salamander cDNA reported by Romero et al. is now recognized as the archetypal Slc4a4 gene product. Work from several groups then revealed the existence of six further members of the vertebrate Slc4 gene family (337, 720, 765, 767, 982, 1021), bringing the total number to 10. These novel genes were designated Slc4a5 and 7–11 (Slc4a6 was rescinded, see below). The products of 5 of these 10 Slc4 genes (NBCe1, NBCe2, NBCn1, NBCn2, and NDCBE) have demonstrated NCBT activity. The function of AE4, the product of Slc4a9, is controversial, but it is reported to mediate Cl-HCO3 exchange in some heterologous systems. Bicarbonate transporter related protein 1 (BTR1), the product of Slc4a11, likely mediates borate transport, a function common to the Slc4-like transporters (the BORs) of some fungi and higher plants. The values in TABLE 2 and the unrooted phylogenetic tree in FIGURE 3 summarize the relatedness, at the level of protein sequence, of the TMDs of human SLC4s. Note that SLC4 function follows sequence relatedness. The first major sequence classification corresponds to AEs (red group) versus NCBTs/AE4 (gray group) versus BTR1. The second major sequence classification corresponds to electrogenic NCBTs (blue group) versus electroneutral NCBTs (red group).
Table 2.
Relatedness among human SLC4 protein sequences
| NBCe1 | NBCe2 | NBCn1 | NBCn2 | NDCBE | AE4 | BTR1 | |
|---|---|---|---|---|---|---|---|
| AE1 | 39 | 38 | 38 | 38 | 39 | 38 | 28 |
| AE2 | 42 | 39 | 41 | 40 | 41 | 43 | 30 |
| AE3 | 42 | 39 | 42 | 42 | 41 | 43 | 30 |
| NBCe1 | 100 | 71 | 57 | 58 | 58 | 62 | 28 |
| NBCe2 | 100 | 50 | 55 | 54 | 58 | 28 | |
| NBCn1 | 100 | 81 | 81 | 52 | 28 | ||
| NBCn2 | 100 | 84 | 52 | 29 | |||
| NDCBE | 100 | 52 | 30 | ||||
| AE4 | 100 | 29 | |||||
| BTR1 | 100 |
Percentage identities among the protein sequences of human SLC4s transmembrane domains. Identities were computed by pairwise BLAST (951). AE1–AE3 share 50–60% identity within their transmembrane domains. Alignments of human NCBT protein sequences are provided in Appendix I, and GenBank protein accession numbers are provided in Appendix IV.
Figure 3.
Relatedness among human SLC4s. The unrooted phylogram displays the relatedness at the level of protein sequence among the transmembrane domains of the 10 human SLC4 proteins. Note how transporter function correlates with protein sequence similarity. The phylogram was generated using ClustalW (183) and TreeView (704). A sequence alignment of the 10 human SLC4s is provided in Appendix I, and the protein sequence identity among the transmembrane domains of human SLC4s is provided in TABLE 2.
C. Review Outline
In the first major part of our review, section II, we examine the NCBTs and Slc4-like genes from bacteria, fungi, plants, and animals and consider how the Slc4 gene family has diverged from a single common ancestor into the 10 members that we recognize today, including the 5 mammalian NCBTs. In addition, we examine the genealogy of extant vertebrate NCBT genes based on an analysis of conserved exon boundaries. Section II should be valuable to those interested in any Slc4 protein.
In section III we review the actions and roles of NCBTs and Slc4-like transporters in nonmammalian species. In addition to being of interest to comparative physiologists, this discussion brings together, for the first time, data that provide insight into how the actions and roles of Slc4-like proteins have evolved to their present status in mammals.
In section IV, we look at the structural features/domains of a typical mammalian NCBT. Here we present a second way to consider the structural relation between NCBTs: an analysis of conserved and variable protein regions. We also present a summary of maneuvers known to inhibit or stimulate mammalian NCBTs.
In section V we then consider, in turn, each of the 5 mammalian NCBTs and, for each, 10 categories of key characteristics. The italicized terms below correspond to the titles of the headings in section V.
A) Summary. A précis of the key characteristics, actions, and roles for each NCBT, serving as a quick reference guide for the casual reader.
B) Nomenclature. A definitive guide to the naming of each NCBT, necessary because nonstandard and redundant nomenclatures have made collation and interpretation of the literature confusing. In each case we link the nomenclature used in this review with a GenBank sequence accession number.
C) Molecular action. A detailed account of the substrates and transport modes of each NCBT.
D) Genome. A summary of the key features of the genes encoding each NCBT.
E) Structural features and variants. A definitive guide to the known products created from each NCBT gene.
F) Distribution. A comprehensive detailing of the localization of NCBT transcripts and proteins from the intracellular to the whole organ level.
G) Physiological roles. A review of the known and speculated physiological roles of each NCBT in specific tissues.
H) Causes of upregulation. A consideration of the perturbations that result in upregulation of NCBT at the level of transcript/protein abundance or activity.
I) Causes of downregulation. A consideration of the perturbations that result in downregulation of NCBT at the level of transcript/protein abundance or activity.
J) Consequences of dysfunction. A review of the diverse pathological states associated with defects and variations in NCBT genes and products.
Characteristics G–J, taken together, provide an integrated picture of the importance of each NCBT.
In section VI we provide a similar, though abbreviated, consideration for the three AEs (AE1–3) and the related products AE4 and BTR1. Section VI, A and B, with their organization of the NCBT literature in light of the wealth of AE data, will be of particular value to those new to the larger Slc4 field. Our consideration of AE4 and BTR1, which are of interest to the NCBT community, are the first detailed reviews of these unusual family members.
In section VII, our final section entitled “Concluding Remarks,” we draw together from Section V several recurring themes, unresolved issues, and emerging topics in the NCBT field.
Throughout the review we summarize critical information, for quick reference, in the form of Tables. Here the reader will find guides to the importance of pH regulation, the relatedness among Slc4 and Slc4-like gene products, NCBT inhibitors, NCBT distribution, and pathological mutations in the SLC4A4 gene.
In our Appendices, we complement the content of the review with detailed information, such as complete sequence alignments of the NCBT proteins and their splice variants. We also present tables of GenBank protein accession numbers of all of the Slc4 and Slc4-like gene products and variants discussed in this review to allay confusion about nomenclature. The accession numbers are hyperlinked to the National Center for Biotechnology Information (NCBI) database for ease of reference. We also present some additional data about NCBT distribution, namely: 1) an NCBT expression pattern in humans and mice inferred from a tabulation of the origins of NCBT expressed-sequence tags deposited on a public database; 2) a discussion of “anti-NBC3” immunoreactivity, which discloses a distribution pattern for NBCn1 (Slc4a7) that is different from that suggested by other probes; and 3) a discussion of several apparently conflicting reports of AE4 (Slc4a9) localization within the mammalian kidney. These last two appendices will be useful for those who seek to make sense of the often conflicting data concerning the distribution of these proteins.
This review is not intended to focus on the regulation of pHi per se, although the NCBTs play key roles in this task. Rather, the reader is referred to the review by Roos and Boron (811), the more recent chapter by Bevensee et al. (77), or the analysis of Boron (101). Likewise, the present review does not focus on the kinetics or thermodynamics of HCO3− transport, for which we would recommend References 339 and 529. For a more concise overview of NCBTs, we direct the reader to recent reviews in 2004 by Romero et al. (807), in 2006 by Pushkin and Kurtz (772), in 2007 by Parker and Boron (714), and in 2009 by Casey and Cordat (153), Romero et al. (805), and Boron et al. (104). We intend this document to provide a clear review of NCBT genes and proteins for those new to the field, as well as an up-to-date and comprehensive reference resource for Slc4 researchers. Note that meta-analyses and reinterpretations of published data that do not include a link to a published article are the opinions of the authors.
II. NCBT EMERGENCE AND DIVERGENCE
A. Summary
In this section we consider how the five mammalian NCBT genes emerged from a single primordial Slc4-like gene. As we shall see, gene and genome duplications as well as gene losses have resulted in the inclusion of a diverse number of Slc4-like genes in the genomes of diverse organisms. Fungal and plantal Slc4-like genes predominantly encode boron transporters. In the animal lineage, distinct NCBT-like genes appeared no later than the emergence of Eumetazoa such as sea anemones. The most primordial Slc4-like gene-product with NCBT activity is the Na+-driven anion/bicarbonate exchanger ABTS-1 from the nematode worm, Caenorhabditis elegans. The genome of the chordate sea squirt Ciona intestinalis includes three Slc4-like genes, one of which shares a single common ancestor with the five mammalian NCBTs and “AE4.” The emergence of individual NCBTs was initiated by the divergence of an NBCe1/NBCe2/“AE4” ancestor from an NBCn1/NDCBE/NBCn2 ancestor. The five mammalian NCBTs were probably distinct entities no later than the emergence of primordial vertebrates such as lampreys.
B. Emergence, From an Ancestral Prokaryote to Early Chordates, of AE-like, NCBT-like, and BOR-like Genes
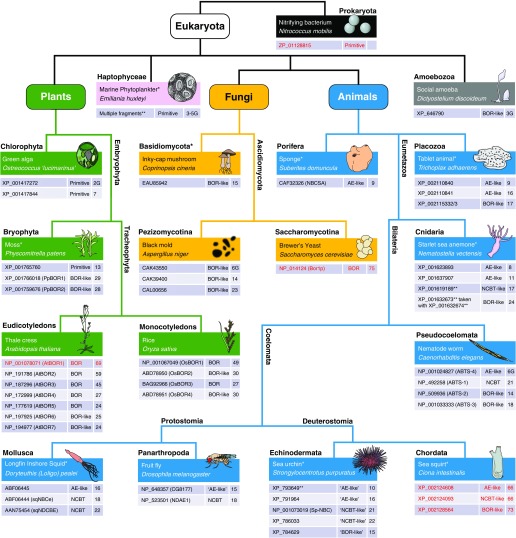
The recent proliferation of genome sequence data, backed up by the physiological characterization of certain products, allows us to begin to appreciate the diversity of Slc4 and Slc4-like genes and products. In FIGURE 4, which gives examples of the major taxonomic divisions, we represent the taxonomic relationship of diverse organisms along with their known complement of Slc4-like genes. Some of these products have demonstrated Cl-HCO3 exchanger (AE), Na-coupled HCO3 transporter (NCBT), or borate transporter (BOR) function and thus we have assigned them as being AEs, NCBTs, and BORs. However, the function of many of the products is currently unknown. The phylogenetic relationships between the proteins in these groups are shown in FIGURE 5 (plants) and FIGURE 6 (animals, i.e., metazoa). We are not showing dendrograms for the other major taxonomic divisions that contain identified Slc4-like genes because: 1) bacteria have only two such genes, 2) the only known Slc4 sequences from phytoplankton are fragments from an unknown number of distinct Slc4-like products that cannot be meaningfully grouped, 3) the only two known amoebal genomes each has only one such gene, and 4) fungal Slc4-like genes are all BOR-like and differences among them appear to reflect mainly species divergence. We have attributed presently uncharacterized Slc4-like transporters to one of four groups, according to their relatedness at the protein level (within their transmembrane domains) to selected reference proteins.
Figure 4.
Diversity of nonvertebrate Slc4-like transporters. The cladogram represents the evolutionary relationships of a variety of organisms, based on the taxonomy defined by The Tree of Life Web Project (http://tolweb.org) and the taxonomy database at NCBI (839). Each organism is represented by a boxed table that includes, in the top row, the common and scientific name of the organism. If the full genome sequence of the organism has not been published, the organism's name is noted with an asterisk. The remaining rows of each table provide information about the Slc4-like proteins that are encoded by the genome of each organism. These rows are divided into three columns: Column 1 lists the GenBank protein accession number and, where appropriate, common name for each Slc4-like protein. Partial sequences are marked with a double asterisk. Column 2 lists the function of each transporter (AE, NCBT, or BOR) or, if unknown, the assignment of each protein to one of the four groups (AE-like, NCBT-like BOR-like, or Primitive) defined in text that indicates their relatedness to their reference sequences for each kingdom (red text). Column 3 shows a numerical “divergence score” (DS, see text) denoting the extent of similarity to the reference protein. Proteins that do not bear a strong similarity to any one of the reference proteins are marked with a “G” for “generic.” A hyperlink to the protein sequence of each transporter is provided in Appendix II.
Figure 5.
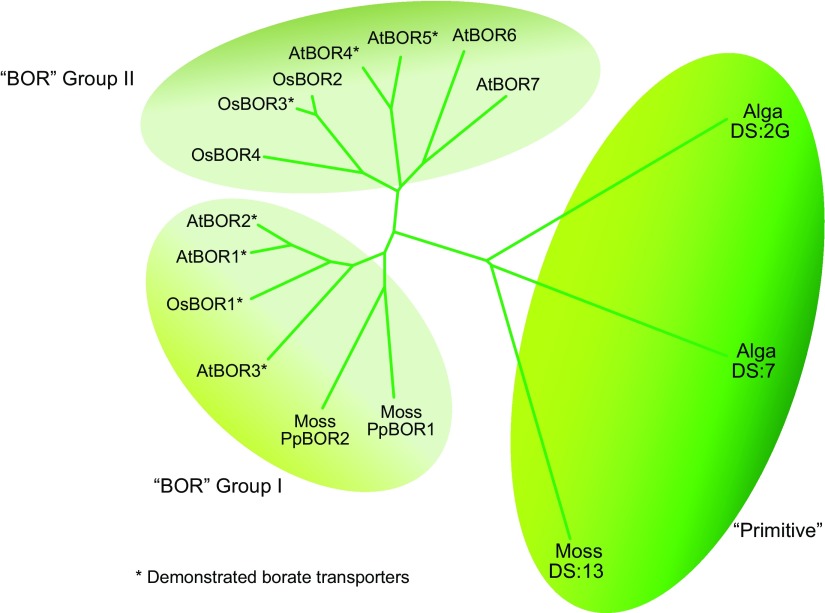
Relatedness among plantal Slc4-like proteins. The unrooted phylogram displays the relatedness, at the level of protein sequence, among the transmembrane domains of the 16 plantal Slc4-like proteins shown in FIGURE 4. Proteins are identified by the name of parent organism and the common name or the divergence score (DS), of each transporter, as listed in FIGURE 4. Note how the BOR and/or BOR-like transporters fall into two groups. It is unknown if members of Group I versus Group II are functionally distinct, like members of animal Slc4 groups in FIGURES 3 AND 6. The phylogram was generated using ClustalW (183) and TreeView (704).
Figure 6.
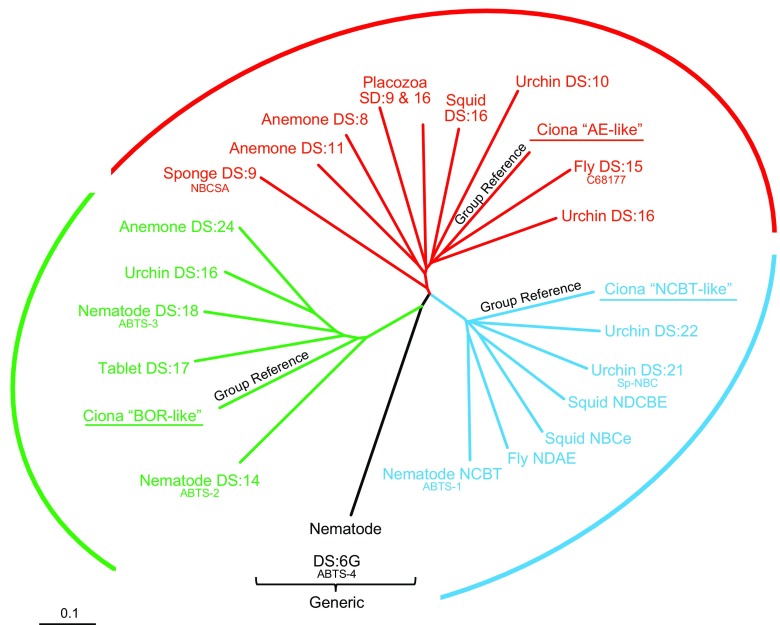
Relatedness among invertebrate Slc4-like proteins. The unrooted phylogram displays the relatedness, at the level of protein sequence, among the transmembrane domains of the 23 invertebrate Slc4-like proteins shown in FIGURE 4. Proteins are identified by the name of parent organism and the common name or the divergence score (DS), of each transporter, as listed in FIGURE 4. Note how the transporters fall into four groups: 1) including AEs and AE-like transporters (red), 2) including NCBTs and NCBT-like transporters (blue), 3) including BORs and BOR-like transporters (green), and 4) a “Generic” outlier (black) that does not fall into any of the three previous groups. The phylogram was generated using ClustalW (183) and TreeView (704).
1) “Primitive” (present only in bacteria and plants): most resembling the bacterial Slc4-like transporter that we have provisionally termed “Nitro” (see “Primitive” in FIGURE 4/Plants and FIGURE 5).
2) AE-like (present only in animals): most resembling the sea-squirt protein that shares a common ancestor with all vertebrate Na+-independent Cl-HCO3 exchangers (see Ciona “AE-like” in FIGURE 4/Sea Squirt). AE-like transporters cluster with the Ciona AE-like reference protein on an unrooted phylogenetic tree (FIGURE 6) and exhibit a characteristic “fingerprint” of sequence identity inasmuch as they are more similar to NCBTs than BORs.
3) NCBT-like (present only in animals): most resembling the sea-squirt protein that shares a common ancestor with all vertebrate Na+-coupled HCO3− transporters (see “NCBT-like” in FIGURE 4/Sea Squirt). NCBT-like transporters cluster with the Ciona NCBT-like reference protein on an unrooted phylogenetic tree (FIGURE 6) and exhibit a characteristic “fingerprint” of sequence identity inasmuch as they are more similar to AEs than BORs. Of course, all invertebrate Slc4-like transporters with demonstrated Na+-coupled HCO3− transport function fall into this category.
4) BOR-like: because borate transporter proteins share little identity across kingdoms (22–27%; see TABLE 3), we define “BOR-like” as follows. For plants, most resembling the established borate transporter of thale cress (see “AtBOR1” in FIGURE 4/Thale Cress) than our bacterial Slc4-like reference protein “Nitro.” Of course, all plantal Slc4-like transporters with demonstrated borate transport function fall into this category. For fungi, most resembling the established borate transporter of brewer's yeast (see “Bor1p” in FIGURE 4/Brewer's Yeast) than “Nitro”. For animals, most resembling the sea-squirt protein that shares a common ancestor with the vertebrate boron transporter BTR1 (see “BOR-like” in FIGURE 4/Sea Squirt). Thus an assignment of BOR-like character is kingdom-specific. For example, a BOR-like transporter from worms is really “Ciona-BOR-like,” and not particularly “Bor1p-like” or “AtBOR1-like.” Within a kingdom, BOR-like transporters cluster with their BOR-like reference protein on an unrooted phylogenetic tree (FIGURES 5 AND 6) and in the majority of cases are more similar to NCBTs than AEs.
Table 3.
Relatedness among representative Slc4-like transporters from bacteria and eukaryotes
| Domain: |
Bacteria |
Eukaryota |
|||||
|---|---|---|---|---|---|---|---|
| Kingdom: |
Amoebozoa | Plantae | Fungi/Metazoa |
||||
| Genus: | Nitrococcus | Dictostelium | Arabidopsis | Saccharomyces | Ciona | ||
| Gene product: | “Nitro” | “Dicty” | AtBOR1 | Bor1p | AE-like | NCBT-like | BOR-like |
| Nitrococcus “Nitro” | 100 | 33 | 31 | 25 | 36 | 35 | 32 |
| Dictyostelium | 100 | 29 | 26 | 27 | 28 | 36 | |
| Arabidopsis AtBOR1 | 100 | 33 | 28 | 27 | 26 | ||
| Saccharomyces Bor1p | 100 | 25 | 27 | 22 | |||
| Ciona AE-like | 100 | 41 | 27 | ||||
| Ciona NCBT-like | 100 | 28 | |||||
| Ciona BOR-like | 100 | ||||||
Percentage identities among the transmembrane domain sequences of Slc4-like proteins. Identities were computed by pairwise BLAST (951). Accession numbers are provided in Figure 4. Note that gaps in protein sequence alignments (represented in Figure 2) reduce the computed percentage identity between Slc4-like proteins from different genera. GenBank protein accession numbers are provided in Appendix II.
We chose the sea squirt (Ciona intestinalis) as our animal reference point for items 2–4 in the list immediately above because the sea squirt is the most primordial animal with three genes, each of which, on the basis of deduced amino acid sequence and conserved exon boundaries, shares a single common ancestor with the three mammalian AEs (Ciona AE-like), or the five mammalian NCBTs (Ciona NCBT-like), or the singleton mammalian BTR1 (Ciona BOR-like).
Because the assignments to the AE-like, NCBT-like, and BOR-like groups are not always clear cut, FIGURE 4 includes-for each accession number-a “divergence score” (DS)4 that is a quantitative index of the protein's divergence from a hypothetical “generic state.” A perfectly generic transporter, one that bears no greater resemblance of any one of the reference proteins to which it is compared, has a DS of zero. In the example case of “NCBT-like” transporters, the maximum DS is 66 because our reference NCBT-like transporter from Ciona exhibits an average 34% identity (i.e., 100% - 34% = 66%) with our AE-like and BOR-like reference genes (TABLE 3). These scores provide an index of how “AE-like,” “NCBT-like,” or “BOR-like” any particular transporter is. We note that one nematode transporter with a DS of 6, which is more like the Ciona AE-like gene-product than either the NCBT- or BOR-like products, does not group with its assigned reference proteins in the phylogenetic tree in FIGURE 6. Thus we have assigned it as generic (noted by a “G” following the “DS” in FIGURE 4). Based on this assessment, we have also marked with a “G” plant and yeast transporters that have a DS of 6 or lower, indicating their possible generic nature. Our bacterial reference protein “Nitro” is the most generic of all of the protein considered here (DS of 3) compared with animal reference proteins, as befits its primitive nature.
For amoebae, we lack an amoebozoan reference protein. Therefore, in this special case, we compared the Slc4-like protein from social amoeba Dictyostelium to all of our Slc4-like reference sequences. It is revealed to share most identity with the Ciona BOR-like transporter (36%) and “Nitro” (33%; see TABLE 3). Thus we assign it as BOR-like with a DS of 3G compared with “Nitro” (FIGURE 4/Social Amoeba).
For phytoplantkon, we also lack a reference protein. Compared against all other reference proteins, the four fragmented Slc4-like sequences appear to be primitive with DS of 3–5G.
1. Copy number of Slc4-like genes in diverse genomes
Although our analysis is limited by the availability of complete genome sequences for key organisms, we find that the number of Slc4-like genes varies on a genome-to-genome basis. Notable findings are as follows.
1) Of the many hundreds of complete bacterial genome sequences presently available, only two, those of the marine bacterium Nitrococcus mobilis and the opportunistic pathogen Segniliparus rugosus, contain an Slc4-like gene.5
2) In a sampling of 34 fungal genomes (not shown), each includes between one and three Slc4-like genes (all BOR-like). About one-third of these genomes (predominantly of the classes Eurotiomycetes and Sordariomycetes) contain more than one Slc4-like gene.
3) A number of overlapping and nonoverlapping Slc4-like sequence fragments have been identified in the genome of the phytoplankter Emilyiana huxleyi (621, 795). Analysis of these fragments suggests that they might be derived from more than two, and perhaps as many as four, Slc4-like genes. Fragments of sufficient length to be reliably analyzed appear to be technically BOR-like, although generic.
4) Only two amoebal genomes are known, each from a different species of Dictyostelium. Each genome includes one Slc4-like gene, both are technically BOR-like, but generic.
5) Plant genomes, which predominantly encode BOR-like products, include between two and seven Slc4-like genes (FIGURE 4/Plants), the number of genes being greatest in more recently emerged clades. This trend likely reflects a gradual accumulation of Slc4-like paralogs, resulting from gene/genome duplication. None of these products is more AE-like or NCBT-like than BOR-like. Some plant strains have multiple copies of the same BOR gene. For example, the boron tolerant “Sahara” cultivar of barley may have four times as many copies of the BOR1 gene as the boron-sensitive cultivar “Clipper” (927).
6) Animal genomes include 2 or more Slc4-like genes, and most vertebrate genomes include at least 10. The number of Slc4-like genes is not always greater in more recently emerged clades, demonstrating that some Slc4-like genes have been lost following the emergence of certain clades (e.g., the fruit fly has fewer Slc4-like genes than most other animals). Animal Slc4-like products predominantly fall into the three categories: AE-like, NCBT-like, and BOR-like (which are underrepresented). Some fish genomes include two similar copies of each Slc4 gene, reflecting a recent genome duplication event.
In the following paragraphs we discuss in further detail the divergence of present day AE-like, NCBT-like, and BOR-like genes from a single common Slc4-like progenitor. The actions and roles of nonvertebrate Slc4-like products are discussed in section III.
2. Archetypal and bacterial Slc4-like genes and products
We have tentatively dubbed “Nitro” the gene-product from Nitrococcus (FIGURES 2B AND 4/Bacteria). A comparison of overall protein sequence identity shared between the transmembrane domains of “Nitro” and sea squirt Slc4s shows that “Nitro” itself is almost equally similar to AEs, NCBTs, and BORs (TABLE 3). It is interesting to note that the cytosolic C terminus (Ct) of “Nitro” and the Ct the Slc4-like gene product from Segniliparus both contain a sequence “LDA[D/E]E” that is similar to the proposed binding site, in the Ct of mammalian Slc4 proteins, for carbonic anhydrase (CA) II (1007). Also notable, although perhaps coincidental, is that the Ct of the Segniliparus Slc4-like transporter and the Ct of human BTR1 both terminate with the sequence [D/E]xRP, although the significance of that motif has not been described for either protein.
A curiosity is that “Nitro” has ∼20% sequence identity at the amino-acid level with certain prokaryotic sulfate permeases6 that share common ancestry with the Slc26 family of vertebrate anion exchangers. Thus, archetypal Slc4-like and Slc26-like genes may have been preceded by a single common ancestral gene. Furthermore, the genome of the archaebacterium Methanococcus maripaludis includes a sequence (YP_001548276) predicted to encode a multi-spanning membrane protein that is equally similar to “Nitro” and the prokaryotic Slc26-like paralog BicA (p. 820).
3. Emergence of BOR-like genes and products in fungi
The known Slc4-like products of fungi are all BOR-like. The best known of these is Bor1p, the sole Slc4-like gene-product from the brewer's yeast Saccharomyces cerevisiae, encoded by the BOR1 gene (FIGURES 2B AND 4/Brewer's Yeast). Similar, singleton Slc4-like genes are found in the genomes of many other model, commercial and pathogenic species of fungi. The genomes of yet other fungal species, representing nearly one-third of fungal species whose Slc4-like genes have been reported, contain multiple slc4-like genes. For example, Aspergillus niger (FIGURE 4/Black Mold) has three. As with the BOR-like transporters of plants, those from fungi are more similar to each other than to those of other kingdoms, suggesting divergence from a single fungal BOR-like ancestor, but not necessarily indicating a common function. We discuss the action and role of Bor1p below.
4. Emergence of BOR-like genes and products in true plants
The known Slc4-like products of plants are all either BOR-like or “Primitive.” Slc4-like transporters in the plant kingdom are represented in FIGURE 4 by the genomes of one species each of green alga, moss, a monocotyledonous flowering plant, and a dicotyledonous flowering plant. The relatedness at the amino acid level of these transporters is represented in FIGURE 5.
The green alga Ostreococcus is a unicellular organism and one of the smallest known eukaryotes, having only a single mitochondrion and a single plastid (206). The Ostreococcus “lucimarinus” genome includes two Slc4-like genes that appear to have diverged from a common ancestor. Both Ostreococcus transporters retain most similarity to the bacterial ortholog7 “Nitro,” and we thus consider them “Primitive.” However, because their divergence scores are small, they are also “Generic” or nearly “Generic.”
Representing the Slc4 complement of an early land colonizing plant, the moss Physcomitrella retains a single “Primitive” Slc4-like gene along with two BOR-like genes. The two BOR-like genes appear to have diverged in the bryophyte lineage from a single common archetypal BOR-like ancestor that probably also gave rise to the rice and thale cress BORs in the “higher” plant/tracheophyte lineage. Thus the presence of recognizable BOR-like genes in plants appears to be contemporary with land colonization.
During the emergence of “higher plants,” the archetypal plantal BOR-like ancestor appears to have diverged many times. The first duplication of the BOR-like ancestral gene appears to postdate the emergence of moss, but predate the divergence of monocotyledons (such as rice) and eudicotyledons (such as thale cress). One copy of the archetype retained a high degree of similarity to the original ancestral protein and gave rise to the precursor of AtBOR1–3 as well as OsBOR1 (BOR Group I, FIGURE 5). The second copy of the archetype gave rise to the precursors of AtBOR4–7 as well as OsBOR2–4 (BOR Group II, FIGURE 5). We discuss the actions and roles of plantal BOR products below.
5. Emergence of AE-like, NCBT-like, and BOR-like genes and products in animals
The emergence of animals was more or less accompanied by the duplication of an archetypal Slc4-like gene, the inclusion of sequence that encodes a substantial amino-terminal domain, and the subsequent evolution of distinct AE-, NCBT-, and BOR-like genes in animals. The overrepresentation of BORs and BOR-like transporters in the genomes of plants, amoebozoa, and fungi is complemented by their comparative underrepresentation in the genomes of animals (FIGURE 4/Animals). Most animals retain multiple AE-like and NCBT-like genes but only a single BOR-like gene, with two exceptions: the nematode worm has two BOR-like paralogs and the fruit fly has none.
The most primordial, animal Slc4-like transporters presently identified may be in the “tablet animal” Trichoplax adhaerens (FIGURE 4/Tablet Animal). An early draft of the Trichoplax genome sequence indicates the presence of at least three Slc4-like genes; two AE-like and one BOR-like.
The most primordial, animal Slc4-like sequence that represents a complete cDNA is the AE-like transporter known as “NBCSA” (847) from a sponge (FIGURE 4/Sponge). Notably, these placozoan and sponge Slc4-like proteins are predicted to have in place two features that are absent from Slc4-like proteins of plants and fungi, but that are found in vertebrate Slc4s: 1) a large cytosolic Nt and 2) an extended third extracellular loop-between the fifth and sixth transmembrane spans (TMs), which includes cysteine residues and multiple, putative N-glycosylation sites (FIGURE 2A).
In Slc4 evolution, the large Nt appears for the first time in animals (e.g., tablet animal in FIGURE 4).8 The origin of the Nt is unknown, but it is likely to be derived from a preexisting open-reading frame that became appended to the transporter gene. An Nt-precursor gene is not identifiable as an isolated entity in any presently available genome sequence, nor is an Slc4-independent function for the Nt-precursor protein suggested by sequence homology to other soluble proteins. However, some mammalian Slc4 genes express variant transcripts that encode an isolated Nt (see below), which may be a vestige of the original genetic independence the of Nt-encoding sequence. It is noteworthy that a region of the crystal structures of the Nt of AE1 and NBCe1 shares substantial structural homology with some EIIA proteins, which are components of bacterial phosphotransferase systems that can act as soluble regulators of certain K+ channels and sugar transporters (245, 552).9
Clues to the divergence of AE-like, NCBT-like, and BOR-like transporters in animals are provided by the visual guides to protein identity shown in FIGURES 4 AND 6. In tablet animals, two AE-like proteins are already distinct from a BOR-like transporter. In our analysis, the most primordial organism with at least one gene each that is distinctly AE-like, NCBT-like, and BOR-like is the sea anemone (FIGURE 4/Starlet Sea Anemone). However, because the partial sequence that we have assigned as NCBT-like is only ∼100 amino acids long, our assignment may not accurately reflect the nature of the full-length gene-product. Nevertheless, the divergence of NCBT-like genes must have occurred no later than the appearance of the Bilateria because NCBT-like genes appear in Coelomates and Pseudocoelomates (FIGURE 4).
6. Emergence of AE, NCBT, and BOR activity in animals
Borate transport is likely a primitive function of Slc4-like transporters as evidenced by the presence of Slc4-like products with borate transport function in plants, fungi, and animals. Bicarbonate transport function appears to be a more recent specialization.
To date, the only nonvertebrate Slc4-like gene-product with demonstrated Na+-independent Cl-HCO3 exchange activity is the AeAE from mosquitos (747), an ortholog of the AE-like transporter from Drosophila (FIGURE 4/Fruit Fly). Thus AE activity presumably arose prior to the divergence of protostomes (e.g., flies) from deuterostomes (e.g., mammals).
Many nonvertebrate NCBT-like products have demonstrated NCBT function. NCBT-like proteins that perform Na/HCO3 cotransport are common to both coelomates (e.g., humans) and pseudocoelomates (e.g., ABTS-1 from C. elegans, see below). Thus it seems likely that NCBT-like products had, at the latest, acquired the ability to perform Na+-coupled HCO3− transport soon after the emergence of the Bilateria, over 900 million years ago (368).
C. Divergence of Vertebrate Slc4 Genes From an Early Chordate Slc4-like Gene
As far as we can discern from the presently available genome data, mammals, and likely most extant vertebrates, have at least one copy of each of the five distinct, known NCBT paralogs: Slc4a4, Slc4a5, Slc4a7, Slc4a8, and Slc4a10 (FIGURE 3). To investigate the genetic origins of these five genes, we look again to our reference genome from the sea squirt Ciona intestinalis, the genome of which includes one known NCBT-like gene. Ciona is a primordial chordate and shares a single common ancestor with all vertebrates. Thus the singleton Ciona NCBT-like gene is likely very similar to the archetypal vertebrate NCBT.
1. Analysis of exon-exon boundaries
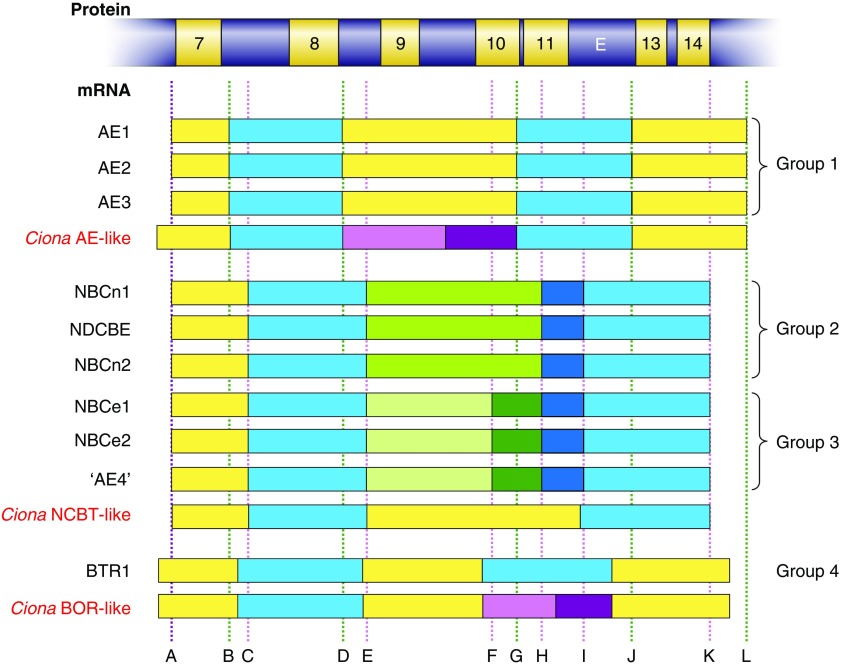
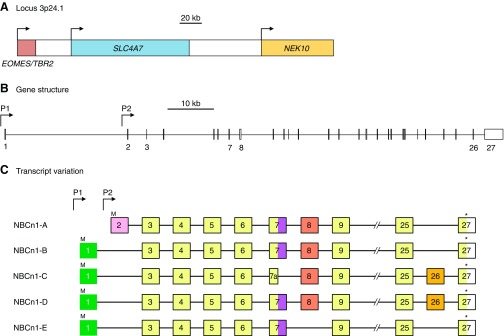
We can make some inferences about the emergence of chordate and vertebrate Slc4s from their common ancestor by analyzing the exon boundaries of Ciona and vertebrate paralogs. FIGURE 7 shows a representative region of an Slc4 protein, from presumptive TM7 to TM14, aligned against the mRNAs that encode this region for all ten human SLC4 genes and all three Ciona Slc4-like genes.10 In the horizontal bars that represent mRNAs, different colored blocks represent sequences that are encoded by different exons. The analysis in FIGURE 7 shows that human SLC4s can be grouped into four categories by virtue of their gene structure. Group 1 is composed of the genes that encode the human Na+-independent Cl-HCO3 exchangers, AE1, AE2, and AE3. Group 2 is composed of electroneutral NCBT genes NBCn1, NDCBE, and NBCn2. Group 3 is composed of the electrogenic NCBT genes NBCe1, NBCe2, and the functionally controversial gene AE4. Group 4 is composed solely of the BTR1 gene that encodes the putative borate transporter.
Figure 7.
Analysis of common and unique exon boundaries among human SLC4 and Ciona Slc4-like genes. The gray horizontal protein bar represents a region of a typical Slc4 protein, between putative TMs 7–14. White numbered boxes within the protein bar mark the positions of α-helical TMs, similar to the representations in FIGURE 2B. Aligned below the protein bar are the corresponding regions of mRNAs that encode the TMs 7–14 region for all 10 human SLC4 genes and all 3 Ciona Slc4-like genes. Each mRNA bar is divided into colored boxes that represent the individual exons that comprise each sequence: thus a change in color on the horizontal axis marks the position of an exon boundary. Exons that appear to have been derived by splitting of a larger ancestral exon are colored green in the human lineage and purple in the Ciona lineage. Common exon boundaries that are discussed in the text are labeled A–L. The position of the exon boundaries for each gene are provided by NCBI Evidence Viewer (839). The sequence alignment from which this analysis is derived is presented in Appendix III.
The number of shared and unique exon boundaries among groups provides an indication of their relatedness. For gene regions encoding TM7 to TM14, the AEs (group 1) and NCBTs (groups 2+3), which share a single exon boundary (FIGURE 7A), are more closely related to one another than to BTR1.
Most closely related to the Ciona AE-like gene is group 1, which shares five exon boundaries (FIGURE 7, B, D, G, J, AND L) with the Ciona AE-like gene, indicating that the Ciona AE-like gene shares a single common ancestor with all vertebrate AEs.
Most closely related to the Ciona NCBT-like gene structure are groups 2 and 3, which share four exon boundaries (FIGURE 7, A, C, E, AND K) with the Ciona NCBT-like indicating that the Ciona NCBT-like shares a single common ancestor with all vertebrate NCBTs. Exon boundaries “F,” “H,” and “I” (FIGURE 7), none of which is found in the Ciona NCBT gene, mark the divergence of group 2 genes (containing only exon boundary “H” and “I”) and group 3 genes (containing exon boundaries “F,” “H,” and “I”). It is not necessarily the case that the gain of exon boundaries “H” and “I” predate the gain of boundary “F,” as introns may also be lost during the course of evolution (53), but the simplest explanation is that the group 2 (electroneutral NCBTs) archetype structure arose earlier and is the parent of the group 3 (electrogenic NCBTs plus “AE4”) archetype structure. Finally, the most recent NCBT gene divergence created the individual members of groups 2 and 3.
Most closely related to the Ciona BOR-like gene is group 4 (i.e., BTR1). BTR1 shares no exon boundaries with the AEs and NCBTs in groups 2–4, but shares six exon boundaries with the Ciona BOR-like gene (FIGURE 7).
To trace the divergence of individual genes within these groups, we must rely on assessments of overall protein sequence relatedness, such as those presented in TABLE 3, depicted in FIGURE 3, and discussed in the following section.
2. Analysis of deduced amino acid sequences
A) EMERGENCE OF THE FIVE NCBTS.
Among vertebrates, the earliest indicators of NCBT divergence are 41 NCBT-like gene fragments in the draft genome sequence of the sea lamprey Petromyzon marinus. By comparing fragments that have overlapping sequence homology, we estimate that there are at least two and perhaps as many as three NBCe1/NBCe2-like genes (predominantly NBCe1-like) and at least two NBCn1/NBCn2/NDCBE-like genes (predominantly NBCn1/NDCBE-like). Thus it seems that the split between electroneutral NCBT-like and electrogenic NCBT-like genes predates the divergence of lampreys and jawed vertebrates. Although the fragmented and incomplete nature of the sequence information makes direct correlation of fragments to specific mammalian orthologs impossible, all five NCBT genes may already have been distinct entities by the time that lampreys appeared.
The most primordial vertebrate NCBT cDNA sequence described may be a fragment cloned from a cartilaginous fish, the Atlantic stingray Dasyatis sabina (GenBank protein accession no. AAU29553). This cDNA fragment is most similar to mammalian Slc4a4 (NBCe1). The most primordial organism with a documented set of orthologs of the five mammalian NCBTs is the zebrafish Danio rerio, a bony fish. The zebrafish genome contains orthologs of all Slc4 genes, with the exception of Slc4a9, indicating that the five NCBTs were distinct entities at the point at which a common ancestral organism diverged to give rise to 1) ray-finned fishes (i.e., most modern bony fish, including zebrafish) and 2) lobe-finned fishes and tetrapods.
B) EMERGENCE OF DUPLICATE NCBT-LIKE GENES IN BONY FISHES.
All vertebrates likely have a full complement of five NCBT genes, as evidenced by the presence of Slc4a4, Slc4a5, Slc4a7, Slc4a8, and Slc4a10 in the genomes of zebrafish and African clawed frogs (i.e., Xenopus). However, the complement of Slc4 genes may vary between genera. For example, due to a whole-genome duplication, zebrafish and many other fishes have two copies of at least Slc4a1 (561), Slc4a2 (882), Slc4a4 (167, 561), Slc4a5, and Slc4a10 (1101).
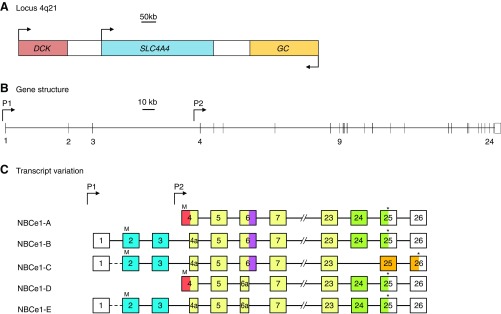
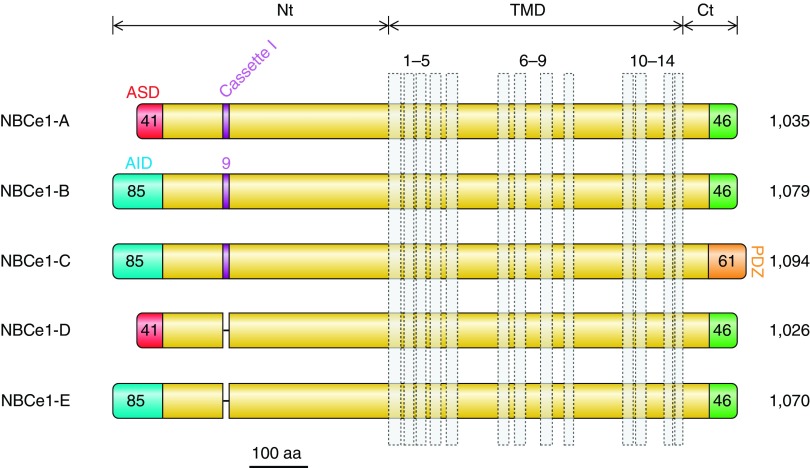
Let us consider the NCBT complement of zebrafish. For clarity we will provisionally refer to duplicate Slc4 genes as Slc4aX.1 and Slc4aX.2. In the case of Slc4a4, the sole reported Slc4a4.1 product (aka zNBCe1a aka zNBCe1-B aka NBCe1.1) most resembles the mammalian NBCe1-B variant (see below for a discussion of NBCe1 variants) inasmuch as it includes Nt sequence similar to the auto inhibitory and IRBIT (IP3 receptor binding protein released with inositol 1,4,5 trisphosphate)-binding determinants of NBCe1-B/C and terminates with an NBCe1-A/B-like Ct. An analysis of the Slc4a4.1 gene suggests that it would be unable to produce an NBCe1-A-like or an NBCe1-D-like transcript (926): specifically, 5′ extension of exon 4a (see FIGURE 17) would not append a sequence to zebrafish NBCe1 that has obvious sequence similarity with the autostimulatory domain of mammalian NBCe1-A and NBCe1-D. Although Slc4a4.1 does have the capacity to encode a NBCe1-C like variant, the corresponding transcript has not been isolated. However, the presence of Slc4a4.1 variant products that lacks splice cassette I sequence (599), i.e., an NBCe1-E-like sequence indicates that posttranscriptional processing of NBCe1 does occur in these fish. Thus Slc4a4.1 is demonstrated to encode NBCe1-B and NBCe1-E-like sequences, but also has the potential to encode an NBCe1-C-like sequence.
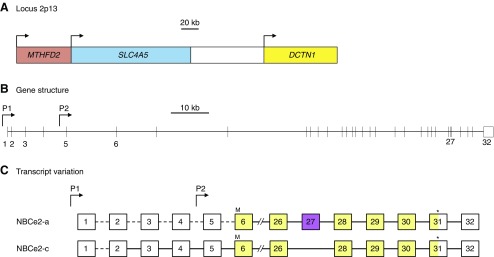
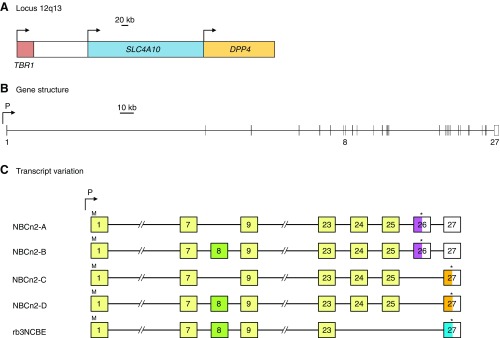
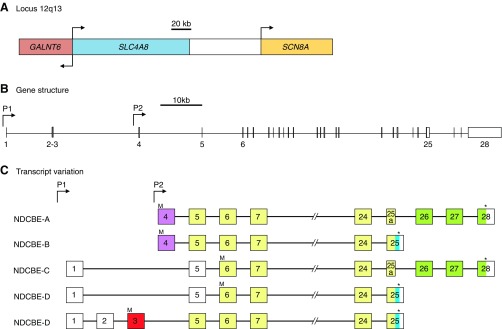
Figure 17.
SLC4A4 gene structure and NBCe1 transcript variants. Scale diagrams showing the human SLC4A4 gene locus together with the position of neighboring genes (A), the position of promoters (P1 and P2), and the position of exons within SLC4A4 (B). Transcript variants are represented, not to scale, as numbered boxes joined by a horizontal line (C). Each numbered box represents the inclusion of that exon in the mature transcript. “//” denotes that all five transcripts include exons 7–23. Exons that include the initiator ATG codon (“M”) and termination codon (“*”) are marked for each transcript. Sequences that are derived from part of a larger exon sequence are labeled with an “a” (e.g., exon 4a is a subdivision of exon 4). Colored exons, or parts of exons, correspond to the protein regions that each encodes, which are identically colored in FIGURE 18. Uncolored exons, or parts of exons, denote untranslated 5′ and 3′ sequence. Exons that are connected with a dashed line are predicted, but not demonstrated, to be included in the mRNA.
The sole reported Slc4a4.2 product (aka zNBCe1b aka NBCe1.2) is a partial clone that includes an NBCe1-B/C-like Nt, but lacks Ct sequence. A predicted complete open reading frame terminates with an NBCe1-C-like Ct that includes a PDZ-domain binding sequence. If each of the duplicate Slc4a4 genes has permanently taken on the character of a specific mammalian-like Slc4a4 splice variant, the distribution of “NBCe1-B versus NBCe1-C” in zebrafish could be controlled at the transcriptional level rather than, as in mammals, at the posttranscriptional level. Presumably these duplicate NCBT-like genes could serve as genetic back up for each other, although preliminary studies that reveal distinct distribution patterns (561) suggest that each may have carved out its own physiological niche.
C) EMERGENCE OF Slc4a9.
The unusual Slc4a9 gene-product was initially described as NBC5 due to its relatedness to NCBT sequences, but was subsequently redesignated as AE4 following reports that the rabbit and rat orthologs are capable of Cl-HCO3 exchange activity. Slc4a9 clearly shares a common genetic origin with electrogenic NCBTs (FIGURES 3 AND 7), yet an Slc4a9 gene is notably absent from the draft genome of Danio rerio. Indeed, no Slc4a9 genes or products have been reported from any non-tetrapodan species. These observations suggest that Slc4a9 is a tetrapod-specific gene.
The deduced amino-acid sequence of Slc4a9 orthologs is not as well conserved as those of its closest paralog, NBCe1. Human, rabbit, rat, and mouse orthologs of NBCe1 are 96–99% identical to each other, whereas Slc4a9 orthologs only share 79–91% identity among those same species. The Nt sequence of Slc4a9 orthologs are more divergent (70–89% identity) than their TMD sequence (85–93% identity). The greater divergence of Slc4a9 compared with NBCe1 may reflect a reduced selective pressure to retain electrogenic NCBT function.
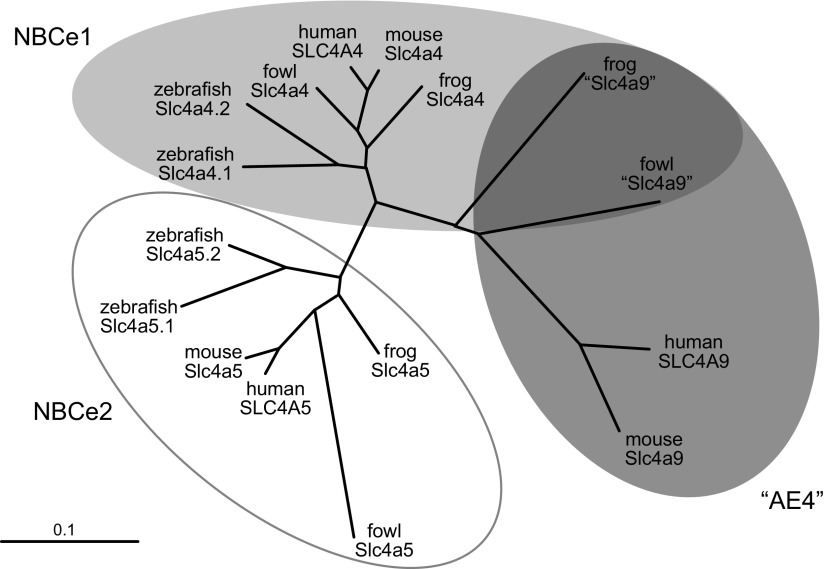
A comparison of vertebrate NBCe1, NBCe2, and Slc4a9 deduced protein sequences (FIGURE 8) provides important information about the origin of Slc4a9. The fish Danio rerio has no Slc4a9 gene, but two copies of the Slc4a4 gene (Slc4a4.1 and Slc4a4.2). The amphibian Xenopus tropicalis has an AE4-like gene, but Xenopus “AE4” is actually more identical at the amino acid level to human NBCe1 (66% identity between TMDs) than to human “AE4” (63% in the TMD). In the fowl Gallus gallus, “AE4” shares an equal degree of identity with human NBCe1 and human “AE4” (66% between the TMDs). Only in the mammalian lineage has the Slc4a9 gene diverged sufficiently to be clearly distinguishable from Slc4a4. The divergence of Danio Slc4a4.1 and Slc4a4.2, likely contemporary with a whole-genome duplication event (40), postdates the divergence of ray-finned fishes and lobe-finned fishes/tetrapods. Thus it is unlikely that either Slc4a4.1 or Slc4a4.2 is an ortholog of tetrapodan Slc4a9. It is more likely that a later Slc4a4 gene duplication in the tetrapod lineage gave rise to the precursors of mammalian Slc4a4 and slc4a9. Such interspecific divergence between Slc4a9 sequences could result in Slc4a9 products from different animals having different function and distribution, a subject that we discuss later in this review. Therefore, it may be helpful to think of Slc4a9 products not as a singular entity but as a group of related proteins, the genes for which diverged following a “recent” Slc4a4 duplication.
Figure 8.
Divergence of NBCe1, NBCe2, and “AE4” in vertebrates. The unrooted phylogram displays the relatedness, at the level of protein sequence, among the transmembrane domains of NBCe1, NBCe2, and “AE4” for zebrafish (Danio rerio), frogs (Xenopus tropicalis), fowl (Gallus gallus), mice (Mus musculus), and humans. The phylogram was generated using ClustalW (183) and TreeView (704). The GenBank protein accession numbers for each transporter are provided in Appendix IV.
III. NCBTs AND RELATIVES IN NONMAMMALS
A. Bacteria
1. Cation-coupled bicarbonate transport in bacteria
CO2 sequestration by photosynthetic cyanobacteria makes a significant contribution to the global carbon cycle (284). The efficiency of carbon fixing by cyanobacteria is enhanced by a CO2-concentrating mechanism, of which cation-coupled HCO3− transport is a vital component (recently reviewed by Price in Ref. 759). In animals, all Na+-coupled HCO3− transport performed by Slc4 proteins. However, none of the prokaryotic cation-coupled HCO3− transporters identified to date are Slc4-like.
In the freshwater cyanobacterium Synechococcus sp. strain PCC 7942, HCO3− transport is a high-affinity, primary active process that is induced under CO2-limiting conditions (701). HCO3− transport is effected by an ABC (ATP binding cassette) protein assembly, called BCT1, encoded by the products of cmpABCD gene cluster (701). Together these four components create a complex equivalent to a mammalian ABC-type transporter that, in mammals, would be encoded by a single gene. The components of this transport complex are highly similar to those of a nitrate/nitrite transporter assembly from the same species, encoded by the nitrate assimilation (nirA) operon (701). Within BCT1, CmpA is an extracellular membrane-anchored HCO3− binding lipoprotein that confers high affinity to the transport process. CmpB is the membrane-multispanning HCO3− permease. CmpC and CmpD are intracellular ATPase subunits (511). CmpD is also predicted to have a solute-binding/transport modulatory role, based on its homology to the nitrite/nitrate transporter component NrtD (501). Structural data indicate that HCO3− binding to the extracellular subunit CmpA is strongly Ca2+, but not Na+ dependent, although it is presently unclear whether Ca2+ is cotransported with HCO3− (511).
In another freshwater photosynthetic cyanobacterium, Synechococcus sp. strain PCC 6803, deletion of the cmp gene cluster has little effect on HCO3− transport (877). Another transporter called BicA, a paralog of the vertebrate Slc26 family of anion exchangers, has been suggested to be responsible for HCO3− uptake by Synechocystis PCC6803 under normal conditions (760). Furthermore, in this strain, CO2 limitation induces expression of a Na+-dependent HCO3− transporter, called SbtA, that appears to have no eukaryotic equivalent. It is not known whether SbtA cotransports Na+ with HCO3− and if so, in what ratio. However, it has been suggested that the process is driven by an inwardly directed Na+ gradient established by an active Na+-extrusion pump (877).
2. Bacterial Slc4-like transporters
To date, the only reported occurrences of Slc4 orthologs in identifiable prokaryotic genomes are singular examples from the marine nitrifying bacterium Nitrococcus mobilis (FIGURES 2B AND 4/Bacteria) and the pathogenic bacterium Segniliparus rugosus. At 513 amino acids in length, the Nitrococcus clone, that we have provisionally termed “Nitro,” is the most compact of all known Slc4-like transporters. “Nitro” lacks many of the extended extramembranous regions of its vertebrate SLC4 counterparts, but is predicted to retain their topology in the transmembrane domain (FIGURE 2B). The codon usage pattern of the “Nitro” gene is more similar to that of eukaryotes than of bacteria (e.g., E. coli). The function of “Nitro” has yet to be fully characterized, but when heterologously expressed in Xenopus oocytes, Nitro does not mediate detectable HCO3− transport but does permit the electroneutral and Na+-independent influx of 36Cl (713). It is intriguing to speculate that its retention in a nitrifying bacterium, which imports toxic NO2− and exports NO3−, may indicate a role for “Nitro” in NO2-NO3 exchange. This hypothesis is especially tempting in light of the homology between NO3− and HCO3− transporters in cyanobacteria (701), the role of an Arabidopsis ClC ortholog as a H/NO3 cotransporter (222), and the penchant of mammalian AE2 for NO3− as a nonphysiological substrate (401). A possible BOR-like action of the archetypal Slc4-like gene-product is suggested by a consideration of Slc4-like transporters encoded in the eukaryotic domain. Only Slc4-like products with borate transport function are present across the kingdoms of plants (e.g., AtBOR1), fungi (e.g., Bor1), and animals (e.g., BTR1). Moreover, among plants, amoebozoa, and fungi, no HCO3− transporters are known.
B. Fungi
In eukaryotic organisms of primordial origin, such as yeast, no Slc4-like proteins with NCBT function have yet been identified. The best characterized example of a fungal Slc4-like gene is BOR1 (aka YNL275W), the singular example from the baker's yeast Saccharomyces cerevisiae. Its product Bor1p is a 576-amino acid nonglycosylated transporter that is similar in predicted secondary structure to its bacterial ortholog “Nitro,” except that the predicted Nt and Ct are slightly longer (FIGURE 2B).
In Saccharomyces, Bor1p is localized to the plasma membrane (941, 1098), where it functions as a boron, or borate, efflux pathway, allowing cells to survive in media containing high levels of boric acid (689, 941, 943). A phosphate transporter, Pho88p, has been identified as a partner of Bor1p in a split-ubiquitin-based yeast two-hybrid screen (646), suggesting that Bor1p may be part of a larger integral membrane protein complex. In light of the observations that Bor1p is 1) not downregulated under boron-limiting conditions (442), 2) not strongly upregulated by high borate levels (442), 3) not the sole candidate borate transporter in this organism (116, 476, 689), and 4) does not compensate for boron efflux defects in an ATR1-deletion strain (476),11 it has been suggested that Bor1p may have another as yet uncharacterized function (442).
A report that an overexpressed Bor1p-GFP fusion protein is enriched in vacuolar preparations compared with total cell homogenate (229) has been cited as evidence that the transporter is primarily localized to an internal compartment. However, this report should be interpreted carefully for three reasons. First, it conflicts with the earlier work on endogenously expressed Bor1p, noted above, that supports the plasma membrane localization of Bor1p protein and action (689, 941, 943, 1098). Second, overexpression of GFP-tagged Bor1p could swamp the trafficking machinery and lead to aberrant protein localization. Third, this report also describes increased vacuolar fragmentation in a Bor1p deletant strain (229), which conflicts with the findings of a later study (442), thereby weakening the association between Bor1p and the vacuole.
Bor1p binds to stilbene derivatives such as DIDS and SITS (inhibitors of mammalian HCO3− transporter), but there is no indication that Bor1p can transport HCO3−. Moreover, reports differ as to whether Bor1p-mediated boron efflux is inhibited by the presence of NaHCO3 in the growth medium (442, 943). Furthermore, as Na+ and Cl− accumulation in yeast is unaffected by genetic ablation or overexpression of Bor1p (441, 442), it seems unlikely that Bor1p shares any common substrates with mammalian NCBTs. However, other anions may at least interact with Bor1p, as evidenced by the displacement of Bor1p from a SITS-affinity column by high concentrations of Br−, Cl−, HCO3−, I−, or NO3− (1098). Borate efflux by yeast is faster at more acidic extracellular pH, which is consistent with the hypothesis that uphill borate efflux is driven by an inward H+ gradient (442), i.e., H/borate exchange. Inasmuch as the genetic diversity among Slc4-like products in fungi is at least as great as among their animal paralogs (e.g., human AE1 versus BTR1), it is possible that not all fungal Slc4-like transporters share the same molecular action as Saccharomyces Bor1p.
C. Phytoplankton
The phytoplankter Emilyiana huxleyi is surrounded by a shell (a coccosphere) composed of CaCO3 plates (coccoliths). Coccoliths are formed from Ca2+ and CO32−/HCO3− in internal compartments (coccolith vesicles) and are exocytosed onto the cell surface. Coccoliths are an important sink of carbon in the global carbon cycle (e.g., the White Cliffs of Dover are composed of coccoliths), but the physiological role of coccoliths is unknown. One possibility is that coccoliths are a store of carbon for photosynthesis (860). Alternatively, coccoliths may be a sink for excess Ca2+, a desalting mechanism that would parallel the deposition of CaCO3 in the intestines of marine fishes. The molecular action of Slc4-like transporters in phytoplankton is unknown, but the abundance of one of these Slc4-like products in Emilyiana huxleyi increases in the presence of extracellular Ca2+. One possibility is that Slc4-like products might be responsible for HCO3− influx across the plasma membrane (621). Another is that cytoplasmic carbonic anhydrases produce HCO3− from CO2 when CO2 is abundant. In either situation, a transporter, conceivably an Slc4-like protein, would move HCO3− from the cytoplasm, across the vesicle membrane, and into coccolith vesicles. However, the molecular actions and the subcellular locations of any Slc4-like gene-product from phytoplankton are presently unknown.
Considering the primordial boron transport function of Slc4-like proteins, it is also possible that some phytoplankton Slc4-like transporters might transport boron. Indeed, coccoliths do contain boron. However, the influx of uncharged H2BO3 across phytoplankton membranes is thought sufficient to account for the observed coccolith boron content (911). Even if this hypothesis were true, it would not preclude a role of an Slc4-like transporter as a borate efflux pathway in the plasma membrane, analogous to the role of Bor1p in yeast.
D. Amoebae
Valproic acid (VPA; 2-propylpentanoic acid) is a commonly prescribed anticonvulsant that acts upon ion channels as well as intracellular targets such as histone deacetylases (173). At doses above 1 mM, VPA is toxic to the model unicellular slime mold Dictyostelium discoideum; VPA-resistant strains have their singleton Slc4-like gene disrupted (960). The link between the Slc4-like gene and VPA transport in slime mold is strengthened by the inhibition of VPA uptake by the Slc4-blockers DIDS and tenidap and by inhibition of VPA uptake by extracellular HCO3− (960). VPA uptake is independent of extracellular Na+ but stimulated by acidic extracellular pH (960). Thus it is possible that the protonated form of VPA moves into the slime mold, perhaps via an Slc4-like protein. Heterologous expression studies would be helpful to determine whether the Slc4-like protein is capable of such activity. It is unlikely that VPA is the physiological substrate of this transporter, and it is unknown if HCO3− is carried by the Slc4-like transporter. An intriguing possibility is that VPA is a substrate or inhibitor of mammalian Slc4s. If VPA is a substrate of mammalian Slc4s, these transporters could promote VPA action upon intracellular targets. On the other hand, if VPA blocks neuronal NCBTs, the resulting fall in pHi could dampen neuronal excitability, contributing to the anticonvulsive properties of the drug.
E. Plants
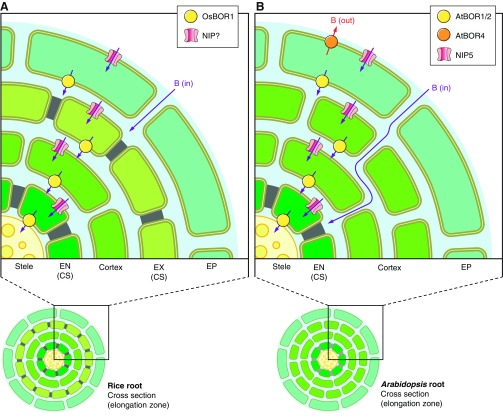
Algae, moss, and both mono- and dicotyledonous flowering plants each have their own unique complement of Slc4-like genes (FIGURE 4/Plants) that bear more sequence similarity among themselves than to any of their animal orthologs. These plantal Slc4-like genes are structurally similar to their yeast homologs, having short Nt and Ct cytosolic domains, but appear to always include an extended extracellular loop between TMs 9 and 10 (FIGURE 2B). The only plantal Slc4-like transporters characterized to date are those from flowering plants. The founder member BOR1 (see next section) is a boron-efflux transporter from the thale cress Arabidopsis thaliana (943) and shares many properties with the yeast Slc4-like protein Bor1p. Boron is a highly significant element for plants: boron cross-linked rhamnogalacturonan II dimers are important cell-wall components (reviewed in Ref. 692). Too little boron can cause reproductive and growth problems in plants, whereas excessive boron can be toxic (reviewed in Ref. 86). Boron transport is likely a complex process, aside from Slc4-like transporters, other plant proteins such as the aquaporin-like NIP5 (945, 1089) and NIP6 (947) are necessary for efficient boron transport throughout the plant (FIGURE 9).
Figure 9.
Roles of Slc4-like boron transporters in boron uptake and boron tolerance in the roots of plants. Radial cross sections through the roots of rice (A) and thale cress (B). Boron can enter the roots from the soil via the intercellular apoplast (blue shaded area), but is blocked from the root vasculature (in the stele) by casparian strips (CS). NIP boron channels, BOR1, and BOR2 transporters in root cells, provide a transcellular pathway through the epidermis (EP), exodermis (EX), cortex, and endodermis (EN) directing boron towards the stele and past the CS allowing boron to access the xylem. In thale cress, AtBOR4 directs excess boron into the soil.
For at least two reasons, the nomenclature for plantal Slc4-like transporters requires careful interpretation: 1) Not all products named “BOR” have demonstrated boron transport function. 2) BOR genes from dicotyledonous genomes do not have exact orthologs in monocotyledonous genomes. Thus, although a common ancestor presumably gave rise to four BOR genes in rice (monocotyledonous) and to seven BOR genes in thale cress (dicotyledonous), most paralogs within each group arose independently and the numbering is arbitrary. For example, although OsBOR1 in rice and AtBOR1 in thale cress are truly orthologous, OsBOR2 in rice is not the direct ortholog of AtBOR2 in thale cress. The relatedness of some plantal Slc4 products is as shown in the dendrogram in FIGURE 5.
It is interesting to note that the plantal BOR-like transporters can be separated, according to protein sequence relatedness, into three distinct groups. The first group, “Primitive,” includes plantal Slc4-like products of unknown function. The second and third groups, “BOR group 1” and “BOR group II,” both include demonstrated borate transporters (marked with an asterisk in FIGURE 5, and discussed in the following sections). By analogy to similar groupings observed for mammalian Slc4s (e.g., FIGURE 3), it is possible that the molecular action of borate transport is different between groups I and II. However, nothing is presently known about the borate transport mode of any plantal transporter. The sole feature by which BOR-like transporters currently can be categorized is the ability to confer tolerance to low-boron stress versus high-boron stress. This distinction may follow the polarity of BOR expression within plants cells: a boron-efflux transporter in the apical membrane of plant cells will move boron in the direction of the shoots, whereas a boron-efflux transporter in the basal membrane will move boron in the direction of the soil (FIGURE 9). Note that both groups I and II contain members that confer tolerance to low-boron stress (e.g., AtBOR1 and OsBOR3).
As boron availability and toxicity are critical determinants of crop growth, and boron in plants acts as an antimicrobial agent, the function of BOR transporters and the linkage of BOR gene variation to enhanced boron tolerance is currently of considerable interest in plant physiology. Of special importance to animal physiologists is the growing number of reports of BOR-gene mutations, which may reveal much about the structure/function relationships of Slc4s. Notable is the coincidence, discussed below, that a mutation identified in the Arabidopsis AtBOR1 gene also occurs in human AE1, where the mutation is associated with hereditary spherocytosis. In the following two sections, we summarize the current knowledge concerning the physiological roles of BOR transporters in monocotyledonous and dicotyledonous plants.
1. Boron transport in monocotyledonous plants
A) RICE.
The genome of rice (Oryza sativa) contains four Slc4-like genes named OsBOR1–4 (FIGURE 4/Rice). OsBOR1 and OsBOR3 both mediate boron efflux. At present very little is known about the physiological roles of OsBOR2 and OsBOR4.
I) OsBOR1. The heterologously expressed OsBOR1-GFP fusion protein localizes at/near the plasma membrane of onion epidermal cells (674). In terms of the amino acid sequence, of the seven Arabidopsis AtBORs, OsBOR1 is most, and equally, similar to AtBOR1 and AtBOR2 (FIGURE 5). In terms of function, OsBOR1 is also similar to AtBOR1 and -2, mediating boron efflux at the level of individual cells, and mediating boron uptake (i.e., root to shoot) at the level of the whole plant (FIGURE 9A). Solutes and water can travel freely throughout the root apoplast (an extracellular space that includes cell walls) but are barred from the xylem-surrounding apoplast by two corky casparian strips. By analogy with boron uptake pathways in Arabidopsis, it is likely that boron crosses the basal membrane of rice root cells via aquaporin-like NIP5 transporters and exits root cells across the apical membrane via BORs (256, 674, 944).12 The presence of OsBOR1 in root cells that span the exodermal casparian strip likely provides a transcellular efflux pathway by which boron is directed towards the endodermis where OsBOR1 in endodermal root cells would finally transport boron into the xylem-surrounding apoplast of the stele on the other side of the endodermal strip (674).
In the root cells surrounding the xylem, the expression of OsBOR1 is constitutive under normal-boron conditions and is only modestly increased by boron starvation (674). On the other hand, in the exodermis, prolonged boron deficiency massively increases the expression of OsBOR1 (674), thereby enhancing boron extraction from the soil.
II) OsBOR3. OsBOR3 functions as a boron-efflux transporter necessary for normal growth under boron-limited conditions (675) and probably plays a similar role to OsBOR1. The expression of OsBOR3 is regulated such that the OsBOR3 promoter drives exodermal expression in root tips, but endodermal expression in the root elongation zone (675).
B) BARLEY.
Variations in the sensitivity of barley cultivars to high boron levels have been linked to the Slc4-like HvBOR2 (aka bot1) gene locus (788, 927). Of the transporters displayed in FIGURE 5, HvBOR2 is most like OsBOR2. HvBOR2 transcripts are expressed in roots and leaf blade tips. In the latter, the transporter may contribute to the excretion of boron in guttation fluid (927), the liquid that some vascular plants secrete onto the leaf surface.
Four observations underlie the boron-tolerance of the hardy “Sahara” cultivar, which can withstand high boron levels, compared with boron-sensitive cultivars such as “Clipper” and “Schooner”: 1) Southern blotting suggests that the genome of “Sahara” may have four times as many copies of the HvBOR2 gene than “Clipper” (927). 2) Real-time quantitative PCR (qPCR) suggests that HvBOR2 transcripts may be many hundred-fold more plentiful in “Sahara” than in “Clipper” and “Schooner” (788, 927). 3) In conditions of elevated boron levels, “Sahara” maintains an abundance of HvBOR2 transcripts, whereas “Schooner” is unable to substantially increase HvBOR2 transcript abundance from its constitutively low level (788). 4) Heterologously expressed “Sahara” HvBOR2 is superior to “Clipper” HvBOR2 at enhancing the boron tolerance of yeast (927). Of 11 differences in nucleotide sequence between the 2 cDNAs, 2 are predicted to change the protein sequence. The first is the “Clipper” L305S polymorphism, which would disrupt a conserved Leu residue in putative TM8. The orthologous mutant L750C in rat NBCe1 causes a 50% loss of wild-type activity and is predicted to be located in a very conformationally sensitive part of the ion-translocation pathway (633). Thus, if borate transport via HvBOR2 and bicarbonate transport via NBCe1 share commonality in their translocation pathways, one explanation for the relatively poor ability of the “Clipper” versus “Sahara” gene to confer boron tolerance to yeast is a disrupted boron-efflux pathway. The second polymorphism is D592G, in a poorly conserved region of the cytosolic COOH terminus, close to the last putative transmembrane segment. What effect, if any, this amino-acid substitution would have on the HvBOR2 transporter has yet to be elucidated.
C) WHEAT.
In wheat (Triticum aestivum), TaBOR2 is an OsBOR2- and bot1/HvBOR2-like gene-product associated with increased high-boron tolerance in certain cultivars (788), consistent with a role in root to soil boron efflux. The TaBOR2 gene is expressed at a higher level in the boron-tolerant “India” cultivar over the boron-sensitive “WIMMC*10” cultivar (788). Furthermore, in response to boron-excess, boron-tolerant strains maintain substantial TaBOR2 transcript levels, whereas boron-sensitive strains are unable to substantially upregulate TaBOR2 transcript abundance from its constitutively low level (788).
2. Boron transport in dicotyledonous plants
A) THALE CRESS.
Arabidopsis thaliana is a popular model organism used for the study of flowering plants. The Arabidopsis genome includes seven Slc4-like genes (FIGURE 4/Thale cress and FIGURE 5).
I) AtBOR1. In 1997, Noguchi and co-workers (686) reported the creation, by tilling, of a mutant Arabidopsis strain called bor1–1. Unlike wild-type plants, bor1–1 failed to thrive in boron-limiting conditions and produced fewer seeds. Linkage analysis showed that the reduced boron content of this and a similar mutant cultivar is due to mutation of the AtBOR1 gene (943). This study provided the first evidence, in any species, of an Slc4-like gene-product being involved in boron transport. One of the mutations in AtBOR1, G86E, is located in the short intracelullar sequence linking putative TM segments 2 and 3 (943) (see FIGURE 2B for putative BOR1 topology) and is orthologous to the naturally occurring human AE1 mutation G455E, which is associated with hereditary spherocytosis (433). It is not yet clear for either AE1 or AtBOR1 whether the G to E mutation causes a functional or trafficking defect in the transporter. A second mutant Arabidopsis strain bor1–2 is associated with an S74P mutation in AtBOR1, at a position midway through putative TM2 (943). The inappropriate positioning of a Pro residue in a helical region is likely to be very disruptive. For example, the L522P mutation in TM4 of human NBCe1-A leads to rapid protein degradation (241).
The evidence that AtBOR1 is a plasma-membrane protein is the localization of heterologously expressed AtBOR1-GFP fusion protein at/near the plasma membrane of tobacco leaf cells (943). Five lines of evidence indicate that AtBOR1 mediates boron uptake at the organismal level (i.e., boron efflux from root cell into the xylem; see FIGURE 9B) and makes an important contribution towards “root-to-shoot” boron transport. 1) The AtBOR1 gene locus is associated with tolerance to boron-limiting conditions (1089). 2) In a transgenic Arabidopsis strain, boron-limiting conditions upregulate the expression of AtBOR1-GFP in roots, whereas the protein is mainly expressed in shoots when boron is in ready supply (942). 3) The restoration of high levels of boron after a period of boron limitation triggers the endocytosis and degradation of AtBOR1-GFP (942). 4) bor1–1 mutant plants, with mutant AtBOR1 genes, have a reduced boron content (650, 943) and exhibit reduced dimerization of rhamnogalacturonan II (see above and Ref. 685). Conversely, 5) the overexpression of AtBOR1-GFP in transgenic plants enhances boron accumulation in the shoot and shoot apices by about five times (650).
With regard to AtBOR1 localization at the tissue level, AtBOR1-GFP is expressed throughout the roots, but only under boron-limiting conditions, and mainly in the apical membranes of cells (see footnote 12), opposing the basal distribution of the boron-uptake transporter NIP5 (944). AtBOR1 expression in the roots is also enriched in the membranes of endodermal (EN) cells surrounding the stele that contains xylem vessels (650), which transport water and solutes from the roots to the rest of the plant (FIGURE 9B).
The cartoon in FIGURE 9B outlines a proposal for how the thale cress root transports boron from the soil to the xylem (568, 789). Solutes and water can travel freely throughout the root apoplast (extracellular space) but are barred by a single casparian strip from the apoplast surrounding the xylem. Boron enters root cells via aquaporin-like NIP5 transporters on the basal membrane (256, 944). The presence of AtBOR1 in the apical membrane of root cells provides a transcellular efflux pathway that directs boron in stepwise fashion through the cortex towards the endodermis, where AtBOR1 would finally transport boron across the casparian strip into the xylem-surrounding apoplast of the stele. Indeed, bor1–1 mutants have reduced boron content in the xylem exudate (943) as well as in shoots, shoot apices, and rosette leaves (650), but not the roots (943). Thus the critical AtBOR1-dependent step in the “root to shoot” boron-transport pathway is presumably the endodermal step responsible for xylem loading (943). This hypothesis is consistent with the observation that AtBOR1 enhances plant tolerance to boron-limiting conditions, and also explains why, when AtBOR1-GFP is overexpressed in a transgenic Arabidopsis strain, the protein does not afford increased protection from toxic levels of boron (650). However, in the context of a unicellular organism, heterologous expression of AtBOR1-GFP in a Bor1p-deficient yeast strain promotes boron efflux and increases tolerance to boric acid (650, 943).
II) AtBOR2. This transporter shares many properties with AtBOR1, to which it is 90% identical. Like AtBOR1, AtBOR2 mediates boron efflux when heterologously expressed in yeast (652). Disruption of the AtBOR2 gene in Arabidopsis is associated, under boron-limiting conditions, with retardation of both overall plant growth and elongation of cells at the root tip. Indeed, the AtBOR2 promoter is active in root tips (652). In seeds, a 24-h imbibing period induces AtBOR2 gene expression (1038). AtBOR2 likely contributes, in parallel with AtBOR1, to the directed “root-to-shoot” movement of boron depicted in FIGURE 9B.
III) AtBOR3. Transcripts have been detected in shoot guard cells, trichomes, and root cortex (653) as well as stigma and ovaries (1038). Although AtBOR3 mediates boron efflux when expressed in yeast, disruption of the AtBOR3 gene in Arabidopsis has no obvious phenotype. However, a bor1/bor2/bor3 triple mutant suffers more root-growth retardation than a bor1/bor2 double mutant, consistent with the hypothesis that AtBOR3 activity can compensate for defects in AtBOR1 and AtBOR2 expression (653). Thus AtBOR3 likely contributes to the directed “root-to-shoot” movement of boron depicted in FIGURE 9B.
IV) AtBOR4. The distribution of a GFP-tagged AtBOR4 construct in Arabidopsis plants indicates that this transporter is normally expressed in the basal (soil facing) membranes of root epidermal cells (651) and transcripts are additionally detected in stamens (1038). In the root cells of transgenic plants, elevated boron levels result in increased AtBOR4-GFP expression (651). Furthermore, transgenic Arabidopsis and Sativa (rice) that are overexpressing AtBOR4-GFP, from a non-native promoter, exhibit enhanced tolerance to boric acid (461, 651). These observations are consistent with the idea that AtBOR4 normally exports excess boron from root to soil (651), as depicted in FIGURE 9B. An unexpected observation is that transgenic rice plants that heterologously express exceptionally large quantities of AtBOR4-GFP RNA (∼100-fold more than the aforementioned transgenic rice plants tolerant to boric acid) exhibit a paradoxical diminution in tolerance to boric acid (461), as if excessive expression of AtBOR4 creates a root-to-shoot boron transport pathway. In principle, this could result from errant accumulation of AtBOR4 in the apical membranes of root epidermal cells, where AtBOR4 activity would parallel that of AtBOR1 and AtBOR2.
V) AtBOR5. AtBOR5 mediates boron efflux when expressed in yeast (653). The AtBOR5 gene is situated in a genetic locus associated with tolerance to low-boron stress (1089), although disruption of the AtBOR5 gene in Arabidopsis has no reported boron-related phenotype (653). AtBOR5 transcripts are enriched in guard cells and sepals (1038) and are upregulated approximately eightfold in response to nitrate starvation of seedlings (1118).
VI) AtBOR6 and -7. Very little information is available on the expression and function of AtBOR6 and AtBOR7. AtBOR6 transcripts are enriched in mature versus immature pollen (1118).
B) GRAPEVINE.
Analysis of the Vitis vinifera genome indicates that grapevine plants have six Slc4-like genes, all of which are BOR-like (735).13 Three are most similar to AtBOR1 and -2 and are members of group 1 in FIGURE 5. Three are most similar to AtBOR4 and -5 and are members of group II in FIGURE 5. Interestingly, none of the six Vitis BOR-like genes appear to be direct orthologs of any of the seven Arabidopsis BORs (735). The lack of orthology between Arabidopsis and Vitis BORs is likely a complication of independent gene-duplication and gene-loss events following the divergence of the two organisms from a common ancestor.
I) VvBOR1. Although not a direct ortholog of AtBOR1, VvBOR1 is at least the most closely related paralog of AtBOR1 in the grapevine genome. Heterologous expression of VvBOR1 compensates for loss of boron efflux pathways in Bor1p-deficient Saccharomyces as well as in AtBOR1-deficient Arabidopsis (735), indicating that VvBOR1 is a boron efflux transporter and normally plays a role in “root-to-shoot” boron transport (mimicking the action of AtBOR1 in FIGURE 9B). It has been suggested, in light of the reduced fertility of bor1–1 mutant plants, that VvBOR1 action promotes the fertility of individual Vitis flowers and thereby reduces the incidence of formation of small, seedless “shot” berries that can be formed parthenocarpically from unfertilized flowers (735). Consistent with this hypothesis, VvBOR1 transcript abundance and boron content are both lower in shot versus seeded grapes, although a causal link has yet to be established (735).
F. Invertebrate Animals
The first NCBT to be described, the Na+-driven Cl-HCO3 exchanger, was originally detected in squid axons and snail neurons (105, 106, 111, 826). Moreover, experiments on these preparations, as well as barnacle muscle fibers (110) and crayfish neurons (658), first elucidated the importance of these transporter activities for pHi regulation. In the post-genomic era, many invertebrate HCO3− transport activities have been specifically attributed to Slc4-like products. It is possible to see structural features, actions, and physiological roles of these transporters that are shared with some mammalian Slc4s. The invertebrate Slc4-like proteins are structurally diverse, but have a similar inferred topology to their vertebrate counterparts, including extended Nt and Ct (e.g., FIGURE 2A).
1. Sponge
In the demosponge Suberites domuncula, cells associated with the siliceous endoskeleton contain transcripts encoding an Slc4-like transporter “NBCSA” (847). Three lines of evidence suggest that NBCSA may be a silicate transporter. 1) Many Slc4 transporters are stilbene-sensitive, and sponge cells have a DIDS-inhibitable silicate uptake activity; 2) NBCSA is the only Slc4-like transporter identified so far in this organism; and 3) NBCSA transcripts are upregulated in sponge cells by the presence of silicic acid in the bathing medium (847). No data address the issue of whether the transporter is Na+-coupled or has the ability to transport HCO3−.
2. Nematode worms
The C. elegans genome contains four Slc4-like genes, abts-1 (anion bicarbonate transporter-1 aka CeNBC) through abts-4 (FIGURE 4/Pseuodocoelomata). The abts-1 gene has two alternative promoters that are active in different cell types (71), and abts-4 has at least two splice variants (876). Yet more predicted transcript variants are represented on Wormbase.14
A) DISTRIBUTION.
In transgenic worms, the promoters of these four abts genes drive GFP expression in neurons (abt-1–4), hypodermal cells (abts-1 and -3), body wall, pharynx, and vulval muscle cells (abts-1) as well as the intestine (abts-1 and -4) (71, 584, 876). Additional sites of expression for ABTS-2 and ABTS-4 protein are revealed in transgenic worms in which the natural termination codons of the genes are replaced by an in-frame GFP open-reading frame. In these animals, ABTS-2-GFP is expressed in the excretory cell of larvae and the ovaries of adults, whereas ABTS-4-GFP is expressed in gut cells (876). Both transporter fusions exhibited a basolateral distribution.
B) MOLECULAR ACTION.
To date, only the function of ABTS-1 (aka ceNBC), which, of the four ABTS proteins bears most sequence similarity to vertebrate Slc4s, has been characterized in detail. When heterologously expressed in Xenopus oocytes, ABTS-1 mediates a robust 36Cl influx and also mediates a detectable Cl-HCO3 exchange activity (71, 876). Furthermore ABTS-1 mediates electroneutral Na/HCO3 cotransport (71, 804). Taken together, these actions could be consistent with Na+-driven Cl-HCO3 exchange, although the hallmarks of classical NDCBE activity (i.e., Na+- and HCO3−-dependent Cl− efflux and an absolute trans-side requirement for Cl− to mediate Na+ and HCO3− efflux) have not been formally demonstrated. At least the molecular action of ABTS-1 is qualitatively indistinguishable from that of the Na+-driven anion exchanger NDAE1 from Drosophila (see Ref. 71).
ABTS-1 also transports iodide (71). Of additional interest are the observations that abts-1 transcript and protein abundance doubled during arsenite exposure and that abts-1-null worms are hypersensitive to arsenite toxicity (584). Arsenite causes intracellular acidification in a human cell line (425), leading Liao and co-workers to suggest that, if arsenite also lowers pHi in worms, abts-1-null worms may be unable to adequately counter the drop in pHi, leading to apoptosis (584). Although untested, another intriguing possibility is that ABTS-1 itself might counter arsenite toxicity by providing an arsenite-efflux pathway, paralleling the borate tolerance conferred by yeast and plantal Slc4-like products.
The substrates of ABTS-2, -3, and -4 are unknown. ABTS-2 does not mediate substantial Cl− or oxalate2− uptake when expressed in Xenopus oocytes (876).
C) PHYSIOLOGICAL ROLE OF ABTS-1.
In mammals, the concerted efforts of a Na+ driven Cl-HCO3 exchanger (NDCBE) and a K/Cl cotransporter (KCC-2) are hypothesized to play a role in nervous system maturation by lowering intracellular [Cl−] and potentiating the inhibitory effect of GABAergic and glycinergic signaling. In the case of C. elegans, ABTS-1 (together with KCC-2) is thought to play a similar role in the maturation of GABAergic signaling (see Refs. 71 and 948 as well as FIGURE 10) because of the following observations of neuronal hyperexcitability in abts-1-null worms.
Figure 10.
Role of the Na+-driven anion exchanger ABTS-1 in the neurons of nematode worms. In wild-type C. elegans worms, ABTS-1 and the K/Cl cotransporter KCC-2 lower intracellular [Cl−] rendering GABAergic, glutamatergic, and 5-HT-ergic signals inhibitory to neurotransmitter release (A). In abts-1—null worms, the reduced ability to lower intracellular [Cl−] reduces the potency of inhibitory signals (B).
1) abts-1-null worms are hypersensitive to the postsynaptic acetylcholinesterase inhibitor aldicarb as well as to the nicotinic acetylcholine receptor agonist levamisole (361, 584), indicating excessive ACh release.
2) Worms with a defective 5-HT reuptake transporter are typically hypersensitive to the inhibitory (i.e., hyperpolarizing) effects of 5-HT, causing the worms to slow down more than normal. Worms with mutations in the abts-1 gene (which would lead instead to depolarization) lose this hypersensitivity (361).
3) Hermaphrodite specific neurons (HSNs), which innervate the vulval muscles, express ABTS-1. In wild-type worms, the GABA receptor agonist muscimol inhibits egg laying via an inhibitory effect on the GABAergic HSNs. In worms carrying a mutation in abts-1, muscimol has no effect on egg laying, that is, GABA no longer elicits an inhibitory response.
4) Worms with a mutation in the G protein-coupled receptor EGL-47 (659) typically exhibit a reduction in egg laying. Egg laying is restored in worms with an additional mutation in abts-1 (71).
5) Muscimol causes body-wall muscles to hyperpolarize, resulting in an increase in body length. However, in worms with a disrupted abts-1 allele, muscimol causes a decrease in body length (71).
3. Annelid worms
Although the molecular identity of an annelid NCBT has yet to be elucidated, multiple studies have demonstrated the presence of NCBT activity in these organisms. NCBT activity in annelidan cells was first demonstrated in 1985 by Schlue and Thomas, who studied the medicinal leech Hirudo medicinalis (841). In leech Retzius neurons, Schlue and Thomas showed that pHi recovery from an acid-load in the presence of CO2/HCO3− is mediated by the dual action of an amiloride-sensitive Na-H exchanger (NHE) activity as well as a SITS-sensitive electroneutral Na/HCO3 cotransport activity that was proposed, although not demonstrated, to be due to a Na+-driven Cl-HCO3 exchanger (841). A third pHi regulatory mechanism was described in leech neuropile glial cells, a SITS-insensitive NCBT, the activity of which was accompanied by a small membrane hyperpolarization (235). This electrogenic glial transporter, suggested to be an electrogenic NBC operating with a Na+:HCO3− stoichiometry of 1:2, was later demonstrated to be blocked by DIDS (236) and to readily perform electrogenic Li/HCO3 cotransport (671). The DIDS-sensitive current carried by the transporter has a reversal potential close to the resting potential of the glial membrane (671) and thus the net direction of HCO3− transport mediated by the leech glial NCBT may either be inwards or outwards, depending on the membrane potential (Vm) of the cell (239) and the extracellular pH (233). In this way, HCO3− transport across the glial membrane can substantially modulate pHo to counter changes induced by neuronal activity (234, 812). The high-affinity of the transporter (Km <1 mM) for HCO3− means that the system contributes to pHi regulation in these cells even in the nominal absence of HCO3− (237). Further experiments on leech giant glia indicate that the action of electrogenic NCBT in these cells could influence the rate of glutamate uptake through excitatory amino acid transporters, contributing towards termination of synaptic transmission (238). In summary, functional data would suggest that the genome of the leech includes at least two NCBT-like genes.
4. Mollusks
Snail neurons and squid giant axons are classic systems for the study of intracellular pH regulatory mechanisms, and it was in these cell types that the first Na+-driven Cl-HCO3 exchanger (105, 106, 111, 826, 965, 966) and K/HCO3 cotransporter (220, 386, 387, 1097) activities were identified. To date, three Slc4-like genes have been cloned from squid (i.e., Loligo pealei) giant fiber lobe: sqNBCe, sqNDCBE, and the AE-like “SF4” (FIGURE 4/Mollusca). It is unknown whether squid genomes include a BOR-like gene.
A) sqNDCBe.
Characterized as an electroneutral Na+-driven Cl-HCO3 exchanger, this gene-product is also known as “SF1” (1008). This is not an Slc4a8 gene product, but sqNDCBE at least shares a common ancestor with mammalian electroneutral NCBTs. Transcripts of sqNDCBE are detected by northern blot in the giant fiber lobe, optic lobe, heart, and stellate ganglion (1008). The physiological characteristics of sqNDCBE expressed in Xenopus oocytes differs in two respects from the Na+-driven Cl-HCO3 exchange activity reported from squid axons. 1) In oocytes but not axons, Li+ can support sqNDCBE-mediated HCO3− transport ∼75% as well as Na+. 2) In oocytes but not axons, sqNDCBE-mediated HCO3− transport can be readily driven in the efflux direction by removal of bath Na+. The precise reasons for these discrepancies have yet to be resolved (1008).
In situ, squid-axon NDCBE activity requires ATP, possibly for the phosphorylation of the transporter or an essential activator (107, 221). In addition, three lines of kinetic evidence are consistent with the hypothesis that the squid-axon transporter, in situ, actually transports the NaCO3− ion pair. 1) Reciprocal changes in [Na+]o and [HCO3−]o have no effect on the flux as long as the product [Na+]o × [HCO3−]o is maintained fixed at constant extracellular pH (pHo) (111). Indeed, at a fixed pHo, this product is proportional to [NaCO3−]o. 2) Changes in pHo have no effect on the flux as long as [NaCO3−]o is fixed (109). 3) The reversible stilbene derivative DNDS (a divalent anion) appears to be a competitive inhibitor not only with extracellular HCO3− but also with extracellular Na+. A kinetic analysis is consistent with the hypothesis that DNDS in fact competes with the NaCO3− ion pair (108). Studies in frogs (534) and rabbits (see p. 847) indicate that vertebrate NCBTs do not transport the NaCO3− ion pair.
B) sqNBCe.
The predicted amino acid sequence of sqNBCe (aka “SF3”) has a higher overall identity to the electroneutral rather than electrogenic vertebrate Slc4s. Thus it was surprising that SF3, when heterologously expressed in Xenopus oocytes, proved to be an electrogenic Na/HCO3 cotransporter, named sqNBCe, the first electrogenic NCBT to be cloned from an invertebrate (746). This observation highlights a potential problem with making sequence-based predictions of transporter function. sqNBCe transcripts have a very different distribution to those of sqNDCBE and are predominantly detected by Northern blot in the gill and heart with additional expression in the giant fiber lobe (746). As expressed in oocytes, sqNBCe is unable to support Li+-stimulated HCO3− transport, unlike the situation for the electrogenic NBC from leech glia, studied in situ. An intriguing observation, again for sqNBCe expressed in oocytes, is that removal of extracellular Na+ causes a prolonged inhibition of the transporter that is not reversed by restoring Na+ to the bath (746).
C) SF4.
Its deduced amino acid sequence suggests that SF4, the third Slc4-like transporter to be cloned from giant fiber lobe, is an AE-like gene-product. The function of SF4 has yet to be reported.
D) PHYSIOLOGICAL ROLE.
Clues as to the role of NCBTs in mollusks come from a recent study of the cuttlefish Sepia officinalis. Adult cuttlefish counter acidosis under conditions of chronic hypercapnia by elevating plasma [HCO3−] (396). Cuttlefish express two NCBTs in their gill epithelia: “soNBC” and “soNDCBE” (362), orthologs of sqNBCe and sqNDCBE. If soNBC is an electrogenic NCBT, it would be positioned to mediate an increased branchial HCO3− reabsorption in these animals under hypercapnic conditions, per the role of branchial NBCe1 in fish. However, soNBC transcript abundance is not specifically altered by chronic elevations of Pco2 in juveniles and is paradoxically decreased in embryos and hatchlings (362). It is possible in juveniles that upregulation of soNBCe occurs at the post transcriptional level, or that pH regulation is effected via an alternative mechanism.
In snail neurons, an NDCBE-like activity contributes to pHi regulation (966) and therefore likely maintains neuronal excitability, per the action of NCBTs in mammalian neurons. However, it has not been formally established whether the activity described in snail neurons is mediated by a Na+-driven Cl-HCO3 exchanger or the tightly-coupled action of an AE and an NHE (966).
5. Insects
No insect genome reported to date appears to contain more than three Slc4-like genes, and none is known to include a BOR-like gene. The best-studied insect genome, that of the fruit fly Drosophila melanogaster, encodes two Slc4-like proteins (FIGURE 4/Panarthropoda): NDAE1 and CG8177. Multiple splice variants have been reported for each gene-product.
A) NDAE1.
Drosophila NDAE1 (“Na+-driven anion exchanger”) has been characterized as a Na+-driven Cl-HCO3 (or Na+-driven Cl-OH) exchanger with a small associated anion leak (71, 810). Drosophila NDAE1 is also capable of mediating a substantial DIDS-sensitive NO3− influx when heterologously expressed in Xenopus oocytes (852). An ortholog of NDAE1, AgNDAE1, from the mosquito Anopheles gambiae, mediates a similar Na+-driven anion exchange activity and is also capable of mediating some I− influx (589).
In Drosophila, NDAE1 is widely expressed throughout the gut, Malpighian tubules, nervous system, and sensilla, with the majority of protein being basolaterally distributed (589, 854). NDAE1 transcripts have also been demonstrated in a specific subset of myocytes15 in the heart region of the cardiac tube of Drosophila (737). In the mosquito Aedes aegypti, multiple NDAE1 transcript splice variants have been detected in the Malpighian tubules of adults (1070), in which NDAE1 protein is localized to the basolateral membranes of principal cells (589). A preliminary immunohistochemical study also localized NDAE1 to the basolateral membrane of the anterior stomach epithelia of Aedes aegypti larvae (654). An ortholog of NDAE1 likely mediates the stilbene-sensitive Na+-driven Cl-HCO3 exchange activity that has been detected in locust neurons (851). Also like NDAE1, this locust transporter is active in the absence of HCO3−. The importance of NDAE1 for insect health is underscored by the lethal nature of a P-element insertion in the Drosophila ndae1 5′ untranslated region (UTR) (810).
B) CG8177 AND AeAE.
CG8177 is an AE-like gene-product, the distribution and molecular action of which has yet to be fully elucidated in Drosophila. The name CG8177 refers to “computed gene.” However, a preliminary study suggests that the protein mediates a 36Cl influx when expressed in Xenopus oocytes (454). In Drosophila larvae, CG8177, also termed DAE (Drosophila anion exchanger), is located in the basal membranes of interstitial cells of the midgut (263). Although RNAi knockdown of CG8177 in the midgut did not result in a phenotypic change in one study, global knockdown of CG8177 is lethal (263). A study of one of the two CG8177 orthologs from the mosquito Aedes aegypti (termed AeAE or sometimes AaAE1),16 localized the transporter to the basal membrane of stellate cells in the Malpighian tubules (589, 747). The same distribution is also observed for the Anopheles gambiae ortholog AgAE1 (589). Besides the Malpighian tubules, “AE1” is abundant in the gastric cecae and anterior midgut of larval Aedes and Anopheles, the lumen of which maintains an extremely alkaline pH to aid digestion (589, 590). When heterologously expressed in Xenopus oocytes, AeAE mediates stilbene-sensitive, Na+-independent Cl-HCO3 exchange (747). AeAE could be responsible for the DIDS-sensitive basolateral Cl-HCO3 exchange activity detected in the anterior segment of mosquito rectal saltglands (912). Little is known about the second AE-like gene-product from mosquitos (AgAE2) except for a report that it is widely expressed thought the gut of mosquito larvae (589).
6. Echinoderms
A single Slc4-like gene has been cloned from the testes of the sea urchin Strongylocentrotus purpuratus (358), although genome analysis suggests that sea urchins may have four other Slc4-like genes (FIGURE 4/Echinoderms). The NCBT-like protein product of the cloned gene-“Sp-NBC”-is concentrated in the flagellar membrane of sea urchin sperm, where, as suggested by the authors of that study, it may play a role in sperm capacitation and regulation of sperm motility (358).
7. Urochordates
The draft genome of the sea squirt Ciona intestinalis predicts the existence of three Slc4-like genes, one each that can be described as AE-like, NCBT-like, and BOR-like (FIGURE 4/Chordata). An in situ hybridization study shows that the NCBT-like gene is transcribed in the brain and visceral ganglion of tailbud embryos (837). These data are reinforced by the distribution of Ciona expressed sequence tags (ESTs), which suggests an exclusively neuronal expression of the NBC-like gene (837). EST data further indicate that the AE-like gene is expressed in the digestive gland, heart, and hemocytes, whereas the BOR-like gene is expressed in the heart, hemocytes, and neural complex (837). At present, nothing is known of the molecular action or physiological role of the Ciona Slc4-like transporters.
G. Nonmammalian Vertebrates
It is likely that most vertebrate genomes encode orthologs of the three AEs, five NCBTs, and the singular BOR encoded by the human genome. However, not all vertebrate genomes include an Slc4a9 ortholog. The following sections summarize our current knowledge of the five NCBTs, as well as the BOR, in nonmammalian vertebrates. Note that the overwhelming majority of the published work in this area concerns electrogenic NCBTs of fishes and amphibians.
1. Cartilaginous fishes
As far as we are aware, only one study has addressed the role of NCBTs in cartilaginous fishes. From the spiny dogfish (Squalus acanthias), Bleich et al. (84) studied isolated perfused rectal gland tubules, which contribute to whole animal osmoregulation by secreting a hyperosmotic NaCl solution (137). The molecular mechanism of NaCl secretion by tubule cells is represented in FIGURE 11. In cells from these tubules, pHi recovery from an acid load (imposed by an NH4+ prepulse; Ref. 106) requires basolateral Na+, is slowed by removing CO2/HCO3−, and is inhibited by DIDS. Thus these cells probably have an NCBT at the basolateral membrane. In separate experiments, the authors also observed that either reducing basolateral [Cl−] or depolarizing the cell causes a slow rise in pHi. They proposed a basolateral Na+-driven Cl-HCO3 exchanger with an unexpected voltage dependence, which they explained by suggesting that depolarization leads to a rise in [Cl−]i, which in turn enhances Na+-driven Cl-HCO3 exchange. Other possibilities include the following: 1) basolateral Na+-driven Cl-HCO3 exchanger in parallel with a depolarization-induced alkalinization (DIA; see Refs. 884 and 885) that is totally independent of the HCO3− transporter; 2) basolateral electroneutral NBC in parallel with a Cl-HCO3 exchanger, plus an independent DIA; and 3) basolateral electrogenic NBC in parallel with a basolateral Cl-HCO3 exchanger or Cl− channel. The presence of a basolateral NCBT is consistent with the stimulation of rectal-gland NaCl secretion by the infusion of NaHCO3 into the blood, a maneuver that mimics the post-prandial metabolic alkalosis known as the “alkaline tide” (1042). Note that, in mammals, it is typically basolateral NBCe1 and/or NBCn1 that supports fluid and salt secretion across epithelia by maintaining pHi and, under stimulated conditions, supplying HCO3− for secretion (e.g., see below).
Figure 11.
Role of an NCBT in the rectal gland of cartilaginous fish. The rectal gland duct joins the intestine at a point upstream of the rectum (see cartoon dogfish). In rectal gland epithelia, the Na-K pump maintains a low intracellular [Na+] driving basolateral Na/K/Cl cotransporter (NKCC) activity that supplies Cl− for secretion across the apical membrane by CFTR. Anion secretion draws Na+ and H2O through a paracellular pathway, resulting in the secretion of a NaCl-rich solution from the interstitial fluid/blood. CO2 accumulation is dissipated by intracellular carbonic anhydrase (CA). Respiratory acidosis is prevented by NHE and NCBT action. NHE and NCBT could also support HCO3− secretion via the unidentified apical anion exchanger, that is likely a member of the Slc26 family (84). KCNQ1 is a voltage-sensitive K+ channel (1018), also known as Kv7.1.
2. Bony fishes
In bony fishes, NCBTs are vitally important to pHi and salt homeostasis, processes that are mainly associated with the mitochondrion-rich (MR) pavement cells17 of the gills as well as other cells in the intestines.
A) MOLECULAR ACTION OF BONY FISH NBCe1.
Currently, of the NCBT orthologs expressed by bony fishes, only NBCe1 has been cloned and functionally characterized. NBCe1 clones from Osorezan dace (Tribolodon hakonensis; Ref. 382), pufferfish (Takifugu obscurus; Ref. 526), and zebrafish (Danio rerio; Ref. 926) all mediate electrogenic Na/HCO3 cotransport activity when expressed in Xenopus oocytes. In addition, pufferfish NBCe1 is capable of electrogenic Li/HCO3 cotransport (167). The electrogenicity of gulf toadfish (Opsanus beta) NBCe1 has not been demonstrated, but the clone mediates a HCO3−-dependent Na-influx into Xenopus oocytes with a Km for HCO3− of ∼8.5 mM (952).
Pufferfish NBCe1, as expressed in Xenopus oocytes, exhibits a number of unique features not described for other NBCe1 orthologs: 1) oocytes expressing pufferfish NBCe1 are unusually loaded with Na+; 2) pufferfish NBCe1 exhibits an inwardly rectifying, HCO3−-independent, ion conductance; 3) pufferfish NBCe1 exhibits an inwardly rectifying CO2/HCO3−-dependent conductance; 4) the apparent Km of the cotransporter for extracellular Na+ is voltage dependent (the apparent affinity being lower in the negative voltage range); and 5) the reversal potential (Erev) for the cotransporter is not substantially altered by a reduction in [Na+]o, as if the stoichiometry of the transporter is variable (167).
Many of these observations could be explained if the HCO3−-independent conductance associated with pufferfish NBCe1 (observation 2 above) persists in the presence of CO2/HCO3− and makes a substantial contribution towards Vm. Such a conductance could interfere with measurements of true Na/HCO3 cotransport activity. A similar phenomenon has been described in the case of the HCO3−-independent conductance associated with the human NBCe1 mutant A799V (721). The HCO3−-independent conductance associated with pufferfish NBCe1 is also reminiscent of the conductive features of trout AE1 (663) and mammalian NBCn1 (189).
B) DISTRIBUTION OF NCBTs IN BONY FISHES.
In the gill lamellae of Osorezan dace, trout (Oncorhynchus mykiss), and zebrafish, immunocytochemistry confirms the basolateral distribution of NBCe1 protein in a subpopulation of MR cells (382, 726, 925). Here NBCe1 is located in a “cytoplasmic” compartment, which the authors of the dace study attribute to an extensive basolateral system of infoldings (382), similar to those of salamander and mammalian PTs (632). Indeed, these MR cells express many transporters common to mammalian renal epithelia (reviewed in Refs. 278, 406). In zebrafish gills, NBCe1 colocalizes with NCC in a subpopulation of MR cells that do not express AE1 or the H pump (561).
Outside the gill, NBCe1 mRNAs are detected in trout heart, liver, stomach, white muscle (741, 742), toadfish brain (952) and zebrafish brain, intestine, skin, and eye (561), specifically in the corneal endothelium,18 ganglion cell layer, rods, and cones (925, 926). In zebrafish embryos, NBCe1 is localized to the pronephros, specifically the anterior tubules and ducts, optic cup, and the ependymal cells that line the brain ventricles (926). NBCn1 and BTR1 expression appears to be widespread in zebrafish, whereas transcripts of NBCe2, NDCBE, and NBCn2 appear particularly abundant in zebrafish brain and eye (561). Furthermore, in zebrafish, an abundance of NDCBE transcripts is notable in the heart and NBCn2 expression is notable in the spleen (561).
C) ROLE OF NCBTs IN BONY FISHES.
I) pH homeostasis. In primary cultures of gill epithelia from the freshwater rainbow trout, a stilbene-sensitive Na+-dependent HCO3− transport process is a major contributor to pHi homeostasis (1043). A striking example of how NCBTs can contribute to the pHi homeostasis of teleost fish is provided by the Osorezan dace, which, aside from spawning season, lives in a lake that has a pH of ∼3.5. However, the MR cells in the gills of Osorezan dace are uniquely able to adapt to the acidic environment by the coordinated transcriptional upregulation of NHE3, CA II, the Na-K pump, and NBCe1 (382), a complete branchial Na/HCO3 uptake system (FIGURE 12A) that closely resembles that responsible for HCO3− reabsorption in the mammalian proximal tubule (PT). Other, unadaptable fish species cannot survive in these acidic conditions due to a fatal combination of acidosis and an inability to accumulate salts against the osmotic gradient. Indeed, in the gills of zebrafish, NBCe1 transcript abundance is reduced in response to water acidification (561).
Figure 12.
Role of NCBTs in the gills and intestines of bony fishes. In the gill epithelia of freshwater fish such as trout (A), NBCe1 contributes to Na+ and HCO3− absorption from the water. In the gut epithelia of marine fishes such as pufferfish (B), NBCe1 contributes to HCO3− secretion into the gut lumen. ENaC is an epithelial Na+ channel. The identities of the non-Slc4 transporters involved in these pathways have not been determined for all species in which these systems have been identified.
The molecular response of fishes to hypercapnia appears to vary among species, but generally hypercapnia results in a compensatory increase in HCO3− reabsorption to counter acidosis. In trout, a hypercapnic challenge leads to a transient upregulation of branchial NBCe1 mRNA at 1–4 h, and a delayed rise in renal NBCe1 mRNA levels from 6 to 24 h (741). In the marine eelpout (Zoarces viviparous), a 24-h exposure to hypercapnia causes a paradoxical decrease in NBCe1 mRNA abundance in the gills, whereas NBCe1 transcript abundance gradually increases during a 6-wk period of chronic hypercapnia (232). In eels, which reportedly have no branchial NBCe1, hypercapnia elicits a rapid upregulation of renal NBCe1 mRNA that can reach levels 300-fold greater than basal after 12 h (741). If these changes correlate with increased HCO3− reabsorption by the gills and kidney, these would tend to protect the fish from acidosis. In the African lungfish (Protopterus annectens), on the other hand, the abundance of branchial and renal NBCe1, at least at the level of mRNA, are unaltered by acid-base disturbances. Instead, these animals use ventilatory control to blow off excess CO2 and thereby achieve whole body pH homeostasis (322).
Regarding the liver, evidence suggests that an electrogenic Na/HCO3 cotransport activity is important for pHi homeostasis in trout hepatocytes (305), although, as in mammals (8), this activity may be attributable to NBCe2.
Regarding the stomach, trout NBCe1 transcripts are detected in both the antrum and the corpus, where the transporter is hypothesized to support a protective secretion of HCO3− onto the apical surface of the cells. However, contrary to those authors expectations, NBCe1 mRNA levels fell with dietary acidification (918).
II) Salt homeostasis. Killifish (Fundus heteroclitis) are vulnerable to Na+ loss by fluctuation in salinity in their environment, as reviewed briefly by Scott et al. (856). In these fish (856) and in Japanese eels (981), NBCe1 is a constitutive player in a freshwater inducible branchial Na+ uptake system, similar to that shown in FIGURE 12A. Following the same theme, the MR cells of Mozambique tilapia (Oreochromis mossambicus) tend to exhibit reduced expression of NHE3 and NBCe1 when the salinity of their environment is increased, although the data do not achieve statistical significance (306).
Marine fish desalt seawater as it passes along the gut. HCO3− secretion into the gut lumen plays a key role in one aspect of this desalting, the removal of Ca2+ and Mg2+ from the gut lumen as the secreted HCO3− precipitates concentrated divalent cations as an excretable carbonate deposit (reviewed in Ref. 1037). A role for NBCe1 in this process is suggested by the presence of NBCe1 transcripts in the intestines of the gulf toadfish (952), trout (343, 741), and pufferfish (526). Moreover, deposit formation by isolated mucosal layers from the intestines of the sea bass (Cicentrarchus labrax) depends on basolateral Na+ and HCO3− (281). Immunocytochemistry demonstrates a basolateral distribution for NBCe1 in intestinal epithelium (526), where the cotransporter would presumably operate with a 2:1 stoichiometry (FIGURE 12B). The apical step of HCO3− secretion is likely effected by Slc26a6 (526). Transferring these fishes from freshwater into seawater, or in the case of the toadfish from less saline to more saline seawater, causes the upregulation of NBCe1 transcripts in their intestine (343, 526, 952). This would presumably support the increased base excretion observed in these animals during seawater acclimatization (741).
III) Development. Inhibition of NBCe1 translation in zebrafish embryos produces developmental defects, including hydrocephalus, retinal distention, and the presence of unidentified particulate matter in the ventricular spaces (926).
3. Amphibians
The first description of a Na-coupled HCO3− transporter that is independent of Cl, the electrogenic Na/HCO3 cotransporter, came from Boron and Boulpaep's 1983 study of pHi regulation in the proximal tubule of the tiger salamander Ambystoma tigrinum (103). They demonstrated NCBT activity at the basolateral membrane of the PT epithelia (103). A similar activity is present in the PT of the mudpuppy Necturus maculosus (606). The injection of poly(A) mRNA from salamander PT into Xenopus oocytes led to the cloning of the first NCBT cDNA, which encodes a protein termed aNBC (Ambystoma Na bicarbonate cotransporter; Ref. 809). We now recognize aNBC19 as Ambystoma NBCe1-A; a product of the Ambystoma Slc4a4 gene.20
A) MOLECULAR ACTION OF AMPHIBIAN NBCe1.
Currently, of the NCBT orthologs expressed by amphibians, only an NBCe1-A ortholog from the salamander Ambystoma has been cloned and functionally characterized. This protein is an electrogenic Na/HCO3 cotransporter with kinetic properties that are very similar to mammalian (i.e., rat) NBCe1-A (339). Like mammalian NBCe1, salamander NBCe1 is blocked by DIDS (339). Kinetic data suggest that an NBCe1/NBCe2-like activity in frog retinal pigment epithelium does not, unlike the NCBT activity in squid giant axons, transport the ion pair NaCO3− inasmuch as the Km for Na+ of this activity appears to be independent of [CO32−] (534).
B) DISTRIBUTION OF NCBTs IN AMPHIBIANS.
I) NBCe1. The majority of renal Ambystoma NBCe1 protein is localized to the basal folds of the late distal tubule of this mesonephric kidney, with a smaller basolateral presence in the PT (632, 844). A developmental expression pattern of NBCe1 in Xenopus laevis pronephroi is revealed by in situ hybridization experiments (1102). Low abundance of Xenopus NBCe1 (“XNBC1”) mRNA occurs in the early and late PTs at developmental stage NF29,21 but a greater abundance of XNBC1 is present in the late distal segment by stage NF33 (1102), where its distribution overlaps with that of Ca2 (1103). A more recent model of the Xenopus pronephros, based on a large-scale in situ hybridization mapping of transcripts, localizes a significant population of NBCe1 transcripts to an early distal tubule region “DT(1)” that is homologous to the mammalian thick ascending limb (581, 779). Aside from the pronephric presence, NBCe1 transcripts are detected in the cranial ganglia, nasal pit, otic vesicle, somites, hatching gland, and cement gland of Xenopus embryos as well as in the bladder, brain, and small intestine of Ambystoma (809).
II) NBCe2. Slc4a5 transcripts that encode the second electrogenic NCBT, NBCe2, are detected by in situ hybridization of Xenopus oocytes and appear to persist in most tissues throughout embryonic development.22 Note that isolated, defollicated oocytes exhibit no detectable electrogenic NCBT activity (1009).
III) NBCn1. Slc4a7 transcripts that encode the electroneutral Na/HCO3 cotransporter NBCn1 are detected by in situ hybridization mainly in the central nervous system of Xenopus embyros, with an additional presence in the PT, epidermis and external gills. Slc4a7 is expressed at an earlier developmental stage than slc4a10.
IV) NDCBE. We are unaware of any reports concerning the distribution of Slc4a8 products in amphibians, although if it exhibits a similar expression pattern to its mammalian ortholog, we might expect Slc4a8 to be abundantly expressed in neurons.
V) NBCn2. Slc4a10 transcripts are detected by in situ hybridization mainly in the central nervous system (brain, retina, and spinal cord) and the pineal gland of Xenopus embryos and at a later developmental stage than Slc4a7.
VI) Slc4a9. We are unaware of any reports concerning the distribution of Slc4a9 products in amphibians.
VII) BTR1. Transcripts for another Slc4 family member Slc4a11 (referred to as “XNBC2”) are evident in the early PT at developmental stage NF26. Some transient expression also occurs in the early distal segment, but is much diminished by stage NF38 (1102).
C) ROLE OF NCBTs IN AMPHIBIANS.
I) Vision. In the amphibian eye, electrogenic Na/HCO3 cotransport activity, presently unattributed to a specific NCBT, has been detected in 1) lens epithelia of the cane toad Bufo marinus (1039), 2) optic nerve glial cells of Necturus (50, 51), and 3) retinal glial cells of both Necturus (51) and Ambystoma (680). In retinal glial cells from Ambystoma, electrogenic NCBT activity is calculated to operate with a Na+:HCO3− stoichiometry of 1:3 and is preferentially localized at the glial end-feet (681, 682). In glial cells from both the retina and optic nerve, the electrogenic NCBT activity contributes towards maintaining a slightly alkaline resting pHi (49, 681), implying that the electrogenic NBC mediates a net HCO3− uptake even when operating with a stoichiometry of 1:3 (see below). When retinal glial cells from Ambystoma are depolarized, the resulting HCO3− influx mediated by the electrogenic NCBT causes a drop in pHo that could serve to balance the extracellular alkalinizations resulting from neuronal activity or light stimulation of the retina (681). Because the NCBT-mediated drop in pHo is localized at the glial endfeet that contact blood vessels, and because blood vessels dilate in response to a fall in pHo, it has been suggested that NCBT activity may also contribute to a mechanism that increases blood flow during neuronal activity (681).
The apical membrane of bullfrog retinal pigment epithelia (RPE) has stilbene-sensitive electrogenic Na/HCO3 cotransport activity (400, 532) that is a major contributor to the transepithelial HCO3− absorption from retina to blood. This HCO3− absorption helps to drive fluid absorption across the RPE, preventing subretinal edema and promoting retinal attachment (533, 536). The absorption of HCO3− per se lowers subretinal pHo (535). It has been suggested that the NBC activity may be responsible for the lowering of subretinal pHo that occurs in response to a light-induced reduction in [K+]o (585). FIGURE 13A shows a model of the role of electrogenic NCBT activity in the transepithelial ion transport across the amphibian RPE. In RPE, the apical polarity of normally basolaterally distributed transporters such as NCBTs and the Na pump is related to an unusual, partial reversal of polarized protein distribution in these cells (reviewed in Ref. 627). By analogy to the distribution of NCBTs in rats and humans, the apical NCBT in the RPE of bullfrogs is probably an Slc4a4 product (11, 94), although Slc4a5 transcripts are expressed in human RPE and, in mice, retinal detachment is a phenotype of Slc4a5 gene disruption (see p. 880).
Figure 13.
Role of NCBTs in the retinas and stomachs of amphibia. In the retinal pigment epithelia of frogs (A), an electrogenic NCBT exhibits an unusual apical distribution and contributes towards fluid absorption from the subretinal space, promoting retinal attachment. Although undemonstrated in frogs, study of mammalian RPE indicates that the apical NCBT could be either NBCe1 or NBCe2 and the basolateral AE could be AE2 (11). In gastric epithelia of frogs (B), an electrogenic NCBT contributes towards HCO3− secretion onto the cell surface that protects cells from acid attack. The transporter responsible for moving HCO3− across the luminal membrane of these cells has not been identified, although both Slc26a6 and Slc26a9 have been suggested to perform this function in mammalian gastric mucosa (744, 1055).
II) Mucosal protection. In the stomach of the edible frog Rana esculenta, NCBT activity is present at the basolateral membranes of the oxynt(ic)opeptic cells that alternately secrete both HCl and HCO3− at the surface of the gastric fundus (Ref. 209 and FIGURE 13B). In the edible frog, the NCBT activity is clearly mediated by an electrogenic Na/HCO3 cotransporter, whereas in the in the North American bullfrog Rana castesbiana, it is not clear whether the transporter is electrogenic or electroneutral (1074). In both cases, NCBT activity, the basolateral step in the secretion of HCO3− into the mucus that covers the stomach surface, would play a protective role by helping to counter the acidifying effect of HCl back-diffusion from the gastric lumen, and in the process would keep pHi relatively high (208, 1074). Electrogenic NCBT activity in the oxynt(ic)opeptic cells of Rana esculenta is stimulated by carbachol but inhibited by histamine (228).23
III) Renal HCO3− reabsorption. aNBC has a similar molecular physiology to mammalian NBCe1-A (338) and likely plays the same important role in HCO3− reabsorption from the glomerular filtrate. However, the acidification of amphibian tubule fluid is predominantly achieved in the late distal tubule, by an electrogenic Na+-dependent process (749), as demonstrated by early experiments on Necturus and leopard frog (Rana pipiens) renal tubules (657) and later measurements of bicarbonate reabsorption in Ambystoma maculatum renal tubules (1088). FIGURE 14 shows models of the role of NBCe1 in bicarbonate reabsorption by amphibian pronephric epithelia in the proximal and distal tubules. Norepinephrine has an inhibitory effect upon NBCe1-mediated HCO3− reabsorption in the Ambystoma PT, perhaps by elevating cAMP levels (2), a factor that is also inhibitory to electrogenic NCBT activity in rabbit renal tubule preparations (821).
Figure 14.
Role of NCBTs in the renal tubules of amphibia. In proximal and distal renal tubule epithelia of amphibia, NBCe1 contributes towards a H+ secretion/HCO3− reabsorption pathway, which maintains whole body pH within a narrow physiological range.
In the Necturus PT, the movement of Cl− across the basolateral membrane has a strong trans-side dependence on Na+ and HCO3−, consistent with the presence of a Na+-driven Cl-HCO3 exchanger (356). On the other hand, the data are also consistent with the presence of a basolateral Cl− channel in parallel with the subsequently identified electrogenic NBC activity (606), with voltage changes providing indirect coupling of Cl− to Na+ and HCO3−. Viewed somewhat differently, if a cell has a Cl− conductance and an electrogenic NBC in the same membrane, it would be very difficult, using only classical electrophysiological approaches, to resolve the presence of a Na+-driven Cl-HCO3 exchanger (see also our discussion of the dogfish NDCBE with unusual voltage dependence on p. 829).
4. Reptiles
Orthologs of all mammalian Slc4s, with the exception of NBCe2, are identifiable in the draft genome of the green anole lizard Anolis carolinensis. However, we know of no definitive demonstration of NCBT activity in any reptilian cell or tissue. The lack of reports concerning reptilian NCBT activity is likely related to 1) the underrepresentation of reptiles among physiological model organisms and 2) the unusual acid-base physiology of the reptiles that have been studied. For example, Alligator mississippiensis excretes an unusually alkaline urine and has a low plasma [HCO3−] (566). The PT epithelia of these animals apparently do not express NHE or CAII (1000), proteins that are considered necessary for substantial HCO3− reabsorption in mammals. Furthermore, the distal renal epithelia of alligators actually mediate a net secretion of HCO3− under normal conditions (565, 1000), reminiscent of collecting ducts of mammals fed an alkaline diet (e.g., see Refs. 271 and 319). However, the working of an as-yet unidentified HCO3− reabsorbtive mechanism is disclosed in alligator distal tubules when tubular HCO3− secretion is blocked by acetazolamide (565). In alligators, HCO3− secretion may serve to balance the renal excretion of NH4+ (due to the high pH of the urine, pNH3 in alligator urine is ∼0.1 mmHg; Ref. 566) that is necessary due to their inability to synthesize urea (565). Even from studies of snakes, which are capable of acidifying their urine, there are no reports of NCBT activity in isolated proximal or distal renal tubules (217, 494).
5. Birds
Orthologs of all 10 mammalian Slc4s are identifiable in the draft genome of the fowl Gallus gallus. However, published studies of avian NCBT activity are few in number. Nephrons in avian kidneys are graded into three categories, according to differences in the length of their loops of Henle (briefly reviewed in Ref. 122): 1) long-looped “mammalian-like” nephrons; 2) short-looped “reptilian-like” nephrons; and, falling between the two extreme forms, 3) a population of “transitional” nephrons. A stilbene-sensitive NCBT activity, most consistent with the presence of NDCBE, is detected in isolated nonperfused PT from chicken transitional (493) and long-looped nephrons (122, 123), but not in short-looped nephrons (122, 629).
NCBT activity has also been detected in studies of nonrenal avian cells. The steady-state pHi of cultured chick embryonic heart cells is maintained at a level higher than electrochemical equilibrium by a combination of NHE and SITS-sensitive NDCBE-like activities, both of which play a role in recovery from an acid load imposed by an NH4+ prepulse (594). In chicken chondrocytes, an NDCBE-like activity is expected to contribute to the recovery from intracellular acidosis that would accompany a mechanical load (218). Stilbene-sensitive NCBT activity has also been reported in chicken enterocytes (734) and colonocytes (146).
IV. GENERAL FEATURES OF NCBTs
The human genome, and likely every mammalian genome, includes 10 SLC4 genes. Five of these have been unequivocally characterized as encoding NCBTs: Slc4a4 (NBCe1), Slc4a5 (NBCe2), Slc4a7 (NBCn1), Slc4a8 (NDCBE), and Slc4a10 (NBCn2) as displayed in FIGURE 3. Each has a distinct molecular action, distribution, and role, considered in section V. In the present section, we consider features that are common among NCBTs, including oligomeric state, domain structure (e.g., predicted topology, conserved sequence motifs), and maneuvers that inhibit or stimulate transport.
A. General Structural Features of Mammalian NCBTs
Because the Na+-independent Cl-HCO3 exchangers (i.e., AE1–3) in the Slc4 family are 28–34% identical to NCBTs at the amino acid level (807), it is likely that NCBTs share many common structural features with AEs. Studies concerning the structure of NCBTs are therefore heavily supplemented by reference to the wealth of data produced by ongoing studies into the structure of AE1. However, because of their differing functions, crucial structural differences are likely to exist between the AEs and NCBTs (1112), and perhaps even among NCBTs. Here, using as our template a model of AE1 structure, refined with new data from recent studies of NBCe1 topology (see Refs. 1112, 1113 as well as FIGURE 2A), we consider the common structural features of mammalian NCBTs and note some key differences 1) between NCBTs and AE1 and 2) among individual NCBTs. Although this section is intended to refer specifically to mammalian NCBTs, many conclusions likely hold true for most vertebrate NCBT-like transporters, and even some invertebrate NCBTs, which are predicted to have a similar topology to mammalian NCBTs.
The NCBTs are glycosylated membrane proteins with predicted nonglycosylated molecular weights of between 116 and 140 kDa. As shown in FIGURES 2A AND 15, each transporter has three major domains: a large 46–66 kDa cytosolic Nt, a ∼60 kDa transmembrane domain (TMD) encompassing 12–14 TMs, and a smaller ∼10–14 kDa cytosolic Ct.
Figure 15.
Domains and subdomains of NCBTs. Representation of a typical NCBT showing the three domains (Nt, TMD, and Ct) together with the 10 numbered subdomains that comprise them. The common and unique features among NCBTs in each subdomain are discussed in the text. An annotated alignment of human NCBTs showing the division of subdomains is provided in Appendix I.
FIGURE 15 shows the TMD with 13 α-helical spans plus an “extended structure” linking TM11 and TM13, as well as an extended, glycosylated extracellular loop (EL3) between TMs 5 and 6.
1. Oligomerization
The detection of NBCe1 dimers in rat kidney sections (865) and NBCe1, as well as NBCe2, tetramers in the human embryonic kidney cell line HEK-293 (773) indicates that NCBTs, like AE1, form higher oligomers. Like AE1, NBCe1 molecules, and likely all NCBTs, form oligomers stabilized at multiple contact points. Oligomerization is presumed to be a prerequisite for functional expression of the transporter.24 In the case of AE1, homodimers are stabilized by Nt-Nt interactions (1091) as well as TMD-TMD interactions (791, 1023). AE1 tetramers are dimers of homodimers that are linked, at contact points in their Nt, by cytoskeletal proteins such as ankyrin (156). It is unknown whether NBCe1 tetramerization requires an accessory protein.
Size-exclusion chromatography indicates that the isolated Nt of human NBCe1, human NBCe2, and rat NBCn1 all form homodimers (320), and preliminary X-ray diffraction data demonstrates that the NBCe1-Nt dimer is stabilized by interlocking arms (321), homologous to those that stabilize AE1-Nt dimers (1091). Evidence of TMD-TMD interactions (or perhaps even Nt-Ct interactions) within an NBCe1 dimer is provided by experiments in which an NBCe1 construct that lacks a Ct coimmunoprecipitates with an NBCe1 molecule that lacks an Nt (276). Unlike AE1 dimers, NBCe1 dimers are further stabilized by disulfide bridges between cysteine residues in the third extracellular loops (EL3 in FIGURE 15) of opposing monomers (471, 632, 834).
Some evidence suggests that NBCe1 monomers within a dimer are capable of functioning independently (471). A concatameric NBCe1 molecule was created in which a mutant NBCe1 monomer [T442C, which can be selectively blocked with (2-sulfonatoethyl) methanethiosulfonate, also known as MTSES, a cysteine-reactive reagent] was joined to a wild-type monomer (WT, which is unaffected by MTSES). Unlike a WT-WT concatamer that is not inhibited by MTSES and a T442C-T442C concatamer that is 100% blocked by MTSES, hybrid concatamers are only 50% blocked, as if the WT monomer within the dimer operates independently of the blocked T442C monomer (471). Another possibility is that MTSES binding to the WT-T442C heterodimer produces a 50% blockade of each monomer within the dimer.
2. NCBT domain structure
Based on the presence of alternating variable and conserved regions of protein sequence, we can consider each of the three major NCBT domains as being divided into a total of 10 subdomains (see diagram in FIGURE 15 and sequence alignments in Appendix I). The first five subdomains are all part of the Nt. The TMD includes the sixth (transmembrane spans 1–5, TMs1–5), seventh (third extracellular loop, EL3), and eighth (TMs 6–14) subdomains. Finally, the Ct consists of two subdomains (a conserved core and a variable region). We now will discuss each domain and subdomain individually. Unless stated otherwise, the amino acid residue numbers, provided as a guide, refer to the human renal variant of NBCe1 (NBCe1-A; GenBank protein accession no. NP_003750; see guide to NBCe1 nomenclature below).
A) THE CYTOSOLIC Nt.
The Nt can be divided into five subdomains (Nt appendage, Nt core 1, Nt loop, Nt core 2, and Nt-TMD linker; FIGURE 15). The protein sequences of the appendage, loop, and linker subdomains of the Nt differ greatly among NCBTs, and often include splice cassettes (reviewed in Ref. 104). On the other hand, the protein sequences of the two core subdomains are well conserved among NCBTs and, according to the preliminary crystal-structure data (321) and by comparison to the crystal structure of the AE1 Nt (1091), form the structural core of the Nt. The core of the Nt exhibits considerable structural homology to certain bacterial EIIA proteins, a class that function as cytosolic regulators of membrane proteins.
In the case of the AEs, the Nt is not vital for either cell-surface presentation or transporter activity, but rather includes binding sites for protein partners and determinants that direct the trafficking of the transporter. Studies of NBCe1 suggest that the Nt of NCBTs is not vital for cell surface presentation of the rest of the molecule (276, 575, 634) but that it is required for NCBT activity (276, 634). Preliminary studies show that coexpression of an isolated NBCe1-A Nt enables the otherwise inactive NBCe1 TMD to perform electrogenic Na/HCO3 cotransport in Xenopus oocytes, indicating that the Nt is an activating binding partner of the TMD (724). Similarly the TMD of the human chloride channel ClC-1 is activated by its cytosolic domain (842, 1050). The mode of action by which the NBCe1 Nt activates the TMD is unknown. In the case of ClCs, the cytosolic domain is thought to act as a scaffold that influences the alignment of transmembrane spans within the TMD (283) as well as sensor that conveys information to the TMD (reviewed in Ref. 66). Structural studies of a ClC homolog from a red alga reveal an extensive interface between the transmembrane and cytosolic domains (283).
I) Nt appendage (subdomain 1). The protein sequence of the Nt appendage is poorly conserved among NCBTs. Transcription from alternative promoters leads to great divergence in sequence and size of this subdomain (41–92 amino acids). The result may be protein variants with little or no homology in the affected region (e.g., NBCe1-A versus NBCe1-B) or variants with an effectively truncated Nt in which translation initiates at an otherwise “internal” Met residue (e.g., NDCBE-A versus NDCBE-C). Inasmuch as the electron density corresponding to sequence encoded by residues 1–62 is sparse in X-ray diffraction data gathered from crystals of NBCe1-A Nt, it is likely that the Nt appendage is either loosely structured or is structured but tethered to the Nt core 1 subdomain by a flexible linker.
Reflecting its variable nature, most of the sequence within the Nt appendage is nonessential for NCBT activity (634, 718). However, this region can include such elements as: 1) the autostimulatory domain (ASD) of NBCe1-A, the inclusion of which stimulates NBCe1 activity; 2) the autoinhibitory domain (AID) of at least NBCe1-B and NBCn2, the inclusion of which inhibits NCBT activity; and 3) the IRBIT binding determinants (IBD) of at least NBCe1-B and NBCn2-B, and presumably also the IBD of NBCn1-B and NDCBE-B, the inclusion of which confers sensitivity of the NCBT to stimulation by the cytosolic protein IRBIT. Sequestration of the Nt AID may be one of the mechanisms by which IRBIT activates NCBTs (559, 718, 859).
It is unknown how the ASD and AID exert their effects upon the NCBT TMD. The Nt appendage of NBCe2 is different from that of other NCBTs both in primary sequence and charge distribution, suggesting that NBCe2 may not contain a typical Nt AID or ASD.
The Nt appendage also includes a number of potential phosphorylation sites, four of which–Ser89, Ser91, and Tyr92 of NBCn1-B (210, 385, 700) and Thr49 of NBCe1-B (345)–have been demonstrated to be phosphorylated in vivo. Thr49 is required for cAMP-induced activation of NBCe1-B, although phosphorylation of Thr49 is not (345).
II) Nt core 1 (subdomain 2). This ∼65-amino-acid-long region is intertwined in three dimensions with Nt core 2 and together the two subdomains form the core structure of the Nt. Over a quarter of the sequence in Nt core 1 consists of Glu/Asp residues. In AE2, the Nt residues that confer pH sensitivity to Cl− transport are particularly concentrated in Nt core 1 (summarized in Ref. 907).
Centrally positioned in Nt core 1 is the well-conserved “ETARWIKFEE” signature sequence, more precisely for NCBTs “W87[K/R]E[S/T]ARW[I/L]KFEE92”, that marks the start of conservation between NCBTs and AEs. By structural homology with the AE1 Nt, residues within this motif are predicted to form charge interactions with each other (Arg86 interacts with Glu92) and residues in Nt core 2 (Arg86 interacts with Lys227; Glu91 interacts with Arg298; see Refs. 166 and 577). Together, these residues are situated at one end of a “tunnel” of polar residues within the Nt that has been proposed to be part of an ion-permeation pathway (166). Speaking to the proposed importance of and interaction between Glu91 and Arg298 is the severe phenotype (discussed below) of the naturally occurring human mutation R298S (411). Mutation of either Glu91 or Arg298 can cause trafficking and per-molecule transport defects25 in NBCe1 (166, 411, 577), whereas the complementary compound mutant E91R/R298E has near-normal activity (166).
One preliminary report suggests that a conserved Cys120 towards the end of Nt core 1 is important for the functional expression and oligomerization of NBCe1 (52). In all NCBTs, this cysteine falls within a region homologous to the first α-helix in the AE1 Nt, which in the three-dimensional structure is near but not at the Nt dimer interface (1091). In NBCe2 and AE2, this region contains a leucine-zipper motif (542).
III) Nt loop (subdomain 3). In the AE1 Nt, this variable region corresponds to a flexible “hinge,” including 10 residues that are not defined in the crystal structure, that is likely to be a loop that extends from the core structure of the Nt, linking the two core subdomains. In AE1, the Nt loop contains determinants of protein 4.2 binding (469) and ankyrin binding (169, 252). The Nt loop exhibits strong sequence conservation between NBCe1 and NBCe2 and among NBCn1, NBCn2, and NDCBE, but not between these two sets.
In NCBTs, the Nt loop can vary in length due to the inclusion/exclusion of splice cassettes (e.g., cassette I of NBCe1, cassette II of NBCn1, and cassette A of NBCn2). As with the variable sequence in the Nt appendage, the splice cassettes within the Nt loop are nonessential to transporter function (201, 317), suggestive of a regulatory or protein-binding role for the Nt loop. Concordantly, a preliminary report suggests that cassette II of NBCn1 interacts with calcineurin A (715).
IV) Nt core 2 (subdomain 4). This region, the second and longer conserved region in the Nt (encompassing ∼132 amino acids), includes the interlocking arms that are critical for dimerization of the Nt (320, 1091). An AE1-Nt based homology model of the NBCe1 Nt predicts that Arg298 can form charge interactions with either of two residues that are adjacent in the three-dimensional structure, Glu91 in Nt core 1 and Glu295 in Nt core 2 (166). Finally, at least in the case of NBCe1, Lys227 is predicted to interact with residue Glu92 in Nt core 1 (166). As mentioned earlier, these polar residues line a tunnel within the Nt that is important for normal functioning of NBCe1.
V) Nt-TMD linker (subdomain 5). A flexible linker joins the core structure of the Nt and the TMD. The majority of this region has a disordered structure in AE1 (1091) and is poorly conserved among Slc4s. In NBCe1 and NBCe2, this region contains an additional glycine-rich sequence that may confer some extra flexibility between the Nt and TMD, although a precise role for this region has not yet been described. The length of the Gly-rich region in NBCe2 (23 Gly in a stretch of 30 residues in human NBCe2) varies among mammalian species, is reduced to three or four Gly residues in zebrafish NBCe2 isoforms, and is absent altogether in the predicted protein sequence of Xenopus tropicalis NBCe2. Conservation among NCBTs returns close to the start of TM1 at the D405IKRK409 motif, which is homologous to the protein-4.1–interaction motif in AE1 (452). Indeed, 4.1B and NBCe1 are colocalized in, and can be coimmunoprecipitated from, murine PT epithelia (957, 958). The NBCe1 protein complex also includes the membrane-associated guanylate kinase homolog p55 (957), which can act as cytoskeletal anchor (reviewed in Ref. 41). Asp405 as well as Asp416 in the conserved portion of the linker are critical for plasma membrane targeting of NBCe1 (574).
B) THE TMD.
This region comprises three subdomains: transmembrane spans (TMs) 1–5, the large extracellular loop (EL3), and TMs6–14 (FIGURE 15). Although neither TMs 1–5 nor TMs 6–14 of AE1 or NBCe1 are capable of HCO3− transport by themselves, when coexpressed as two separate fragments in Xenopus oocytes, TMs 1–5 and TMs 6–14 are capable of self-associating to recreate the transport activity of the full-length protein (AE1 or NBCe1, see Refs. 353 and 723). The TMDs of NCBTs have a high degree of sequence identity. A high-resolution crystal structure has yet to be reported for the transmembrane domain of any Slc4 family member, but topology models predict a 10–14 TMs together with the hydrophilic loops that link them (48, 302, 1116). Although one group had proposed 10-TM model (950), new preliminary data generated by probing the chemical accessibility of introduced cysteine residues (1114, 1115) are consistent, between TM1–TM8, with the model in FIGURE 2. For the results of extensive mutagenesis studies that highlight residues within this domain important to NBCe1 folding and function, we refer the reader to studies from the Kurtz laboratory (e.g., Refs. 5 and 1112).
As noted above, the TMD of NBCe1, plus its Ct, is capable of trafficking to the cell membrane without the Nt, yet it is nonfunctional (276, 634). The TMD also includes determinants for the electrogenicity/electroneutrality of transport cycles (178, 179, 193).
I) TMs 1–5 (subdomain 6). Sequence conservation between NCBTs and AE1 extends throughout the first five putative TM spans, which are linked by short, hydrophilic loops. TM1 contains residues that appear to lie in the ion-translocation pathway. Indeed, in a study employing cysteine-scanning mutagenesis, residues predicted to map along one edge of a TM1 helix were targets of Cys-reactive agents that blocked transport activity (1110).
By homology to AE1, TM2 and TM3 may form a re-entrant loop that is stabilized in the membrane more by interactions with surrounding TMs than by protein-lipid interactions (188). Thus TM2 and TM3 may not be topogenic without TM1 and TM4 in place (188, 703). The observation that two neighboring mutations in TM3–G485S and G486R, both associated with proximal renal tubular acidosis (pRTA)–cause per-molecule defects in NBCe1 without apparently affecting protein delivery to the plasma membrane (393, 576, 929, 930) suggests that TM3 residues are important for ion translocation.
The extracellular end of TM5 leading into the third extracellular loop contains a conserved lysine (807), Lys559 in NBCe1. In NBCe1, this Lys residue is the second K in the motif “KKMIK,” which plays a critical role in both the reversible and irreversible interaction of disulfonic stilbenes that inhibit anion transport (611). The determinants of stilbene inhibition are discussed in greater detail below; other compounds known to inhibit NCBT activity are considered. Although all five NCBTs retain this Lys, the Na/HCO3 cotransport activity of NBCn1 is relatively insensitive to blockade by DIDS.
II) EL3 (subdomain 7). The third extracellular loop of NCBTs is an extended region (∼86 amino acids) that links TMs 5 and 6. The integrity of this loop is not vital for NCBT activity (Boron lab, unpublished data; see Ref. 723). Moreover, a 9-amino acid hemagglutinin epitope-tag can be introduced into EL3 of NBCe1 without disruption of NBCe1 activity (634). Despite a high degree of sequence conservation between NBCe1 and NBCe2 and among NBCn1, NBCn2, and NDCBE in this region, the only globally conserved motifs are a series of consensus N-linked glycosylation sites and four cysteine residues. We will consider these two features in the following paragraphs.
NBCe1 (190, 515), NBCn1 (174), NBCn2 (177, 755), and NDCBE (176) are all glycosylated in vivo, as evidenced by an increase in gel mobility upon PNGase F treatment. Human NBCe1 and NBCn2 have three sites, human NBCn1 and NBCe2 have four, and human NDCBE has only two. All are within EL3; however, not all of the N-glycosylation sites in an NCBT may actually be glycosylated. In the case of NBCe1, which has three putative sites, only the distal two sites of the form “Asn-Xaa-Thr,” but not the proximal “Asn-Xaa-Ser” site, are normally glycosylated in Xenopus oocytes (190) (Xaa is a 3-letter placeholder for any amino acid). Glycosylation does not appear to be vital for the NCBT function of NBCe1 (190), but a mutant NBCn2 in which all three glycosylatable Asn residues are replaced by Gln, exhibits poor protein expression compared with the wild-type transporter (177).
Within an NBCe1 dimer, the four conserved cysteine residues (Cys583, Cys585, Cys630, and Cys642) form disulfide bonds. Cys583 and Cys585 form an intramolecular disulfide bond with each other, and Cys630 and Cys642 intermolecularly bond to their counterparts within an NBCe1 dimer (1111).
III) TMs 6–14 (subdomain 8). Global conservation of protein sequence among Slc4s continues throughout the remainder of the TMD. Gly723 in the fourth extracellular loop (EL4) of NBCe1 is reported to be necessary for interaction with CA IV (32). This loop also contains determinants of transporter action, inasmuch as the electrogenicity versus electroneutrality of chimeric NBCe1/NBCn1 transporters depends on the origin (NBCe1 versus NBCn1) of EL4 (178). A cysteine residue (Cys916) at the putative intracellular end of TM12 is palmitoylated in the related transporter AE1 (698). However, this Cys residue is not necessary for the function or surface expression in heterologous systems of either AE1 (154, 465) or NBCe1 (471). Cysteine-scanning mutagenesis studies suggest structural differences between AE1 and NBCe1 in this region (1112) and that residues in TM8 of NBCe1 lie along the ion-translocation pathway (633). Of particular interest in this region are Leu750 in TM8 that appears to lie in a conformationally sensitive part of the transporter (633) and the pRTA-associated residue Ala799 in the vicinity of TM9/TM10, mutation of which causes per-molecule transport defects and elicits an unusual DIDS-stimulated, HCO3−-independent conductance in NBCe1 (721) that is similar to that observed for NBCn1.
C) THE CYTOSOLIC Ct.
The Ct (90–105 amino acids) consists of two subdomains: a conserved region (Ct core) and a variable region (Ct appendage). The variable region is the site of extensive variation in splicing, which potentially enables each transporter to interact with a variety of protein binding partners. As evidenced by studies on NBCe1-A and NBCn1, determinants within the Ct are vital for the stable plasma membrane expression of NCBTs (e.g., Refs. 276, 578, 603, and 930). A study of the isolated Ct domain of NBCn1 (603) indicates that this domain is relatively unstructured, although a subsequent study of a smaller peptide corresponding to sequence within the NBCe1 Ct reveals some α-helical content (573).
I) Ct core (subdomain 9). The protein sequence of the first subdomain of the Ct is well conserved among NCBTs and includes three notable motifs that are discussed below: 1) a dihydrophobic trafficking signal, 2) aspartate clusters, and 3) lysine clusters.
II) FL targeting motif. An “FL” sequence in the Ct of NBCe1 is necessary for the basolateral presentation of the transporter, and is located in a region determined by CD spectroscopy to have some α-helical content (573). Deletion of the last 92 amino acids (thereby deleting the “FL” motif) of NBCe1-A causes the protein to be destabilized in the basolateral membrane and to be mistargeted to the apical membrane of an opossum kidney cell line (276). A study in Xenopus oocytes demonstrates that deletion of the last 41 amino acids (deleting the “FL” motif) from NBCe1-A is sufficient to cause near-total intracellular retention of the transporter (634).
III) Asp clusters. A motif similar to the “LDADD” sequence in AE1 has been reported to be important for CA II binding and consequently NBCe1 activation (68, 350, 604, 764), the CAII metabolon hypothesis. Furthermore, expression of NBCn1 is reported to cause a redistribution of CAII to the plasma membrane of HEK cells (604). However, in subsequent studies by others, peptides corresponding to human AE1, NBCe1, or NDCBE Ct do not bind CA II in vitro (748) and a CA II-dependent activation of NBCe1 cannot be demonstrated by co-expressing NBCe1 and CA II in Xenopus oocytes (1063), by coinjecting purified CA II into NBCe1-expressing oocytes (613), or by expression of an NBCe1-CA II fusion protein (613). The evidence presented in favor and against a physiologically relevant interaction between NCBTs and CA II was recently evaluated in Reference 102.
IV) Lys clusters. Following the Asp cluster is a long stretch of charged residues that are characteristic of the NCBTs but not the AEs. The most striking example is in NBCe1, which has a string of 17 consecutive charged residues, 12 of which are lysines.
V) Ct appendage (subdomain 10). The terminal subdomain of the Ct can vary greatly among NCBT isoforms. Some of the features that can be included or excluded by alternative splicing in this region are listed below.
A) Arg-based ER localization signals. The Ct of both NBCe1-A and NBCe2c contain “R-X-R” sequences that, in many other transporters, prevents forward trafficking of transporter molecules until the signal is masked by oligomer formation, or interaction with a binding partner (recently reviewed in Ref. 644). The relevance of this motif in NCBTs has yet to be tested, but it is notable that a truncated NBCe1-A that lacks the last 65 amino acids of the Ct (including an “RER” sequence) has a dominant-negative effect on the forward trafficking of full-length NBCe1-A molecules (930).
B) Motifs for binding PDZ domains. A class I PDZ-domain–binding sequence, conforming to an “-ET[T/S/C]L” consensus (874, 992), is common to NBCe1-C (79), NBCn1 (769), and NBCn2-C/D (317) and mediates interactions between the transporter and cytoskeletal scaffolding proteins such as NHERF1 (562, 711, 769), harmonin (790), and PSD-95 (780). These binding partners serve as foci for clustering of membrane proteins, such that NCBTs may associate with the vacuolar-type H+-pump (769), the N-methyl-d-aspartic acid (NMDA) receptor (780), and pertinent to the infrequent examples of apical NCBT localization, CFTR (711). The extreme Ct sequence of NBCe2c “-SYSL” has been suggested to resemble a class II PDZ-domain ligand (768), conforming to a “-X-φ-X-φ” consensus (874). No currently identified NDCBE variant terminates with a consensus PDZ-binding domain. A truncated NBCn1 that lacks only the PDZ-domain binding sequence traffics to the plasma membrane of HEK cells and mediates a similar transport activity to full-length NBCn1 (711). Thus, at least in heterologous systems, the PDZ-binding motif is not critical for functional expression of NCBTs.
C) Autoinhibitory sequence. Alternative splicing of NBCe1 at its extreme Ct can result in the inclusion of a 46-amino-acid appendage (in NBCe1-A/B) or a 61-amino-acid appendage (in NBCe1-C). Although NBCe1-B and NBCe1-C have similar intrinsic activities (634), NBCe1-C has a greater activity than NBCe1-B when the Nt auto inhibitory domain of both is neutralized by Nt truncation (634) or by IRBIT coexpression (967). It is unknown to what extent the 61-amino acid appendage exerts a stimulatory effect or the 43-amino acid appendage exerts an inhibitory effect. Similarly, alternative splicing of NDCBE at its extreme Ct can result in the inclusion of a 17-amino acid Ct sequence (see NDCBE-B/D below) that is inhibitory to the functional expression of the transporter (717).
B. Inhibition and Stimulation of NCBTs
1. NCBT inhibition
NCBTs are amenable to blockade although chemical inhibitors specific to any one native NCBT has yet to be reported. Thus the pharmacological tools for distinguishing NCBTs from each other are currently lacking. On the other side of the coin, an NCBT activity that is not stilbene sensitive typically correlates with the presence of a single NCBT, namely NBCn1. Furthermore, blockade of selected NCBT molecules can be achieved by mutagenic introduction of inhibitor binding sites (e.g., Ref. 471) and the use of specific antibodies or antisense probes appear to be promising methods for effective knockdown of specific NCBTs (see below).
Demonstrated NCBT inhibitors and methods of NCBT inhibition are listed below and the chemical structures of pharmacological agents mentioned are presented in TABLE 4.
Table 4.
NCBT inhibitors
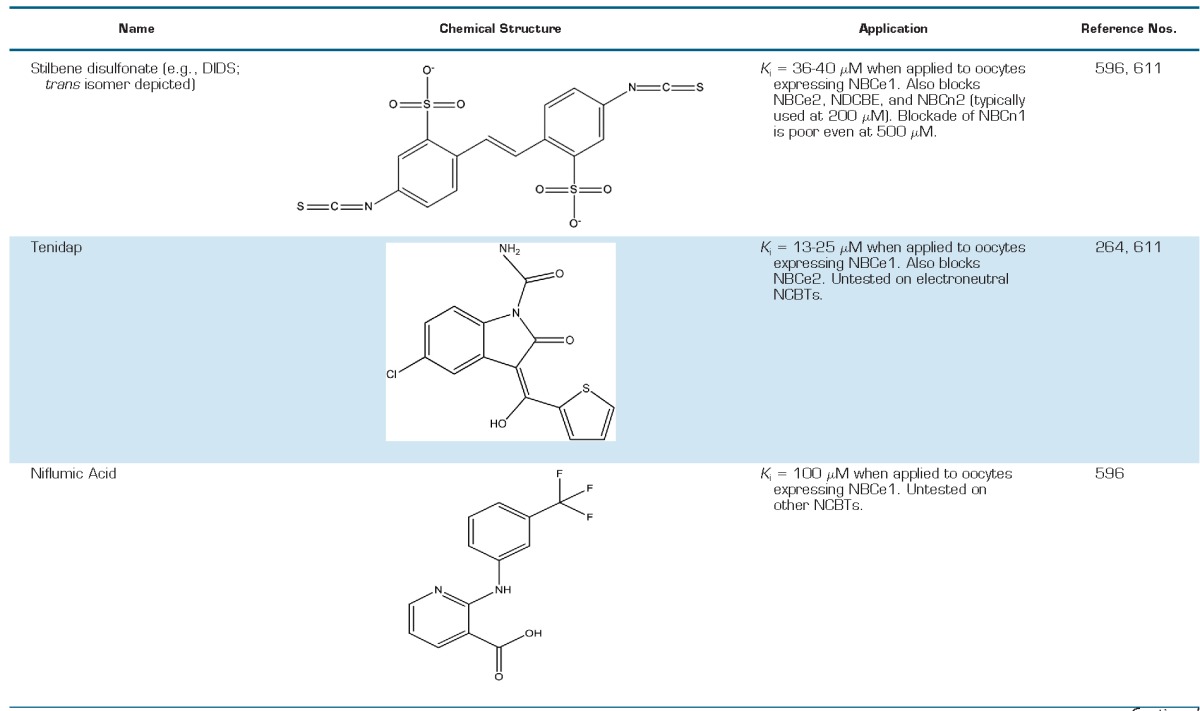
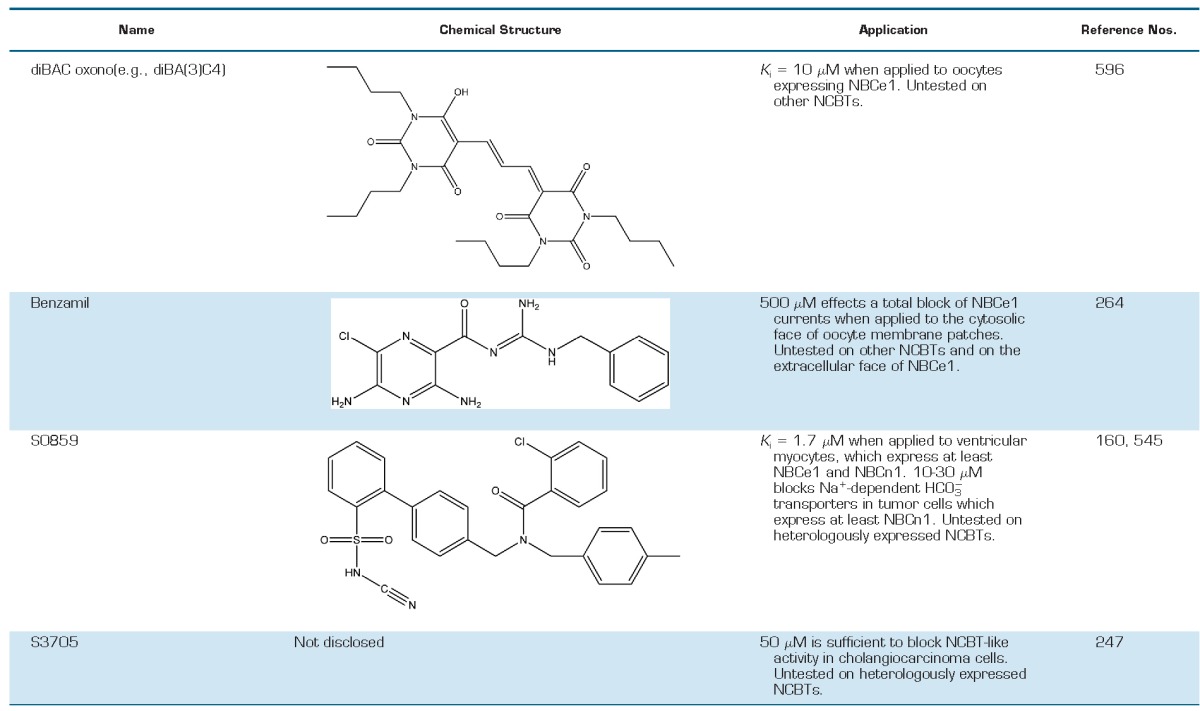
Detailed information concerning the use of the NCBT inhibitors listed in this table, together with a list of drugs that have been suggested but not demonstrated to act on NCBTs, is provided in the text. Chemical structures were drawn using ChemBioDraw Ultra version 12.0 (Perkin Elmer, Akron, OH).
Interventions that downregulate NCBT transcription, translation, and activity in vivo are discussed for each NCBT in section V.
A) STILBENE DISULFONATES.
All NCBTs, with the exception of NBCn1, are inhibited by stilbene derivatives such as DIDS (337, 835, 1009, 1021) and DNDS (634).26 DIDS is a disulfonic stilbene (i.e., it has 2 negative charges). DIDS blocks the HCO3−-dependent conductance mediated by NBCe1 (611) in Xenopus oocytes with an apparent Ki of ∼40 μM (596, 611), which is less potent than its blockade of AE1 in oocytes (Ki ∼6 μM; Ref. 1044). In an exploratory setting, we consider 200 μM DIDS to be an appropriate experimental concentration to achieve a substantial block of the activities of NBCe1, NBCe2, NDCBE, and NBCn2. NBCn1 is unique inasmuch as it is poorly inhibited by even 500μM DIDS.27
As is the case for AE1 (144, 569), inhibition of NBCe1 by DIDS is temporally biphasic (611). The first phase is a rapid, reversible component of inhibition that presumably reflects an ionic interaction with the protein. In the case of NBCe1-A, this inhibition depends to a large extent on three lysine residues, in the motif KKMIK—located at the putative extracellular end of TM5. Replacing all three Lys residues with either Asn or Asp results in a 10-fold or more increase in Ki, and replacing with three Glu residues results in a 20-fold increase (611). However, it is the second lysine (K559 for human NBCe1-A) that is the most important of the three for DIDS inhibition and, indeed, only K559 that is conserved among all five human NCBTs. However, the influence of this lysine must be context dependent, inasmuch as the poorly DIDS-sensitive NBCn1 includes the TM5 motif “EKLFD”.28 A preliminary report suggests that mutating this motif to “EKLFK” renders the Na/HCO3 cotransport activity of NBCn1 readily inhibitable by 500 μM DIDS (191).
The second, slower phase of inhibition by extracellular DIDS is irreversible and presumably reflects the covalent reaction of the bifunctional DIDS molecule with the –NH2 moiety of one or more Lys residues, although in principle the electrophilic isothiocyanate groups of DIDS could derivatize the nucleophilic side chains of other amino acid residues, such as Cys, His, Ser, or Tyr (622). Because irreversible DIDS inhibition still occurs with the mutants NNMIN and RRMIR, the covalent reaction requires additional determinants elsewhere in the molecule that have yet to be identified (611).
DIDS is also capable of inhibiting NBCe1 when applied to the intracellular side of the protein in cell-detached plasma membrane patches (264, 381, 634).
B) NONSTEROIDAL ANTI-INFLAMMATORY DRUGS.
Tenidap29 blocks, but with only partial reversibility, at least NBCe1 (Ki ∼15–25 μM; Refs. 264, 611) and NBCe2 (869). Niflumic acid, an NSAID often used to inhibit anion channels, blocks at least NBCe1 (Ki ∼100 μM; Ref. 596).
C) DIBAC OXONOL DYES.
These fluorescent, voltage-sensitive dyes block at least NBCe1 (Ki ∼10 μM; Ref. 596).
D) BENZAMIL.
This analog of amiloride effects a complete, yet reversible block of rat NBCe1 when applied to the cytosolic surface of oocyte membrane patches at 500 μM (264).
E) S0859.
This drug30 blocks at least the NCBT activity present in 1) colonic crypt cells (likely a combination of NBCe1 and NBCn1 action, see Ref. 55),31 2) ventricular myocytes (likely a combination of NBCe1, NBCe2, and NBCn1 action, see Ref. 160), 3) coronary endothelial cells (likely a combination of NBCe1 and NBCn1 action, see Ref. 523), and 4) mammalian tumor cell lines (likely NBCn1, see Ref. 545). Thus S0859 may be the only reported potent inhibitor of NBCn1. S0859 may be more specific than stilbene derivatives, inasmuch as it is reported to be ineffective at blocking Na+-independent Cl-HCO3 exchanger activity (160). There are presently no reports of S0859 action on any of the five NCBTs expressed in isolation.32
F) S3705.
This agent, when applied at a concentration of 40 μM, blocks at least the NCBT activity present in breast carcinoma (1041) and cholangiocarcinoma (247) cell lines, slowing tumor growth and, in the latter case, promoting apoptosis. Although both studies report blockade of NDCBE-like activity, the molecular identities of the NCBT responsible are not demonstrated. These cell lines likely express at least NBCn1 (546) as well as a stilbene-sensitive NCBT.
G) ANTIBODIES.
An alternative approach to target specific NCBTs involves the use of inhibitory antibodies directed against extracellular epitopes, or to reduce NCBT transcript, and consequently protein, abundance via antisense technology. In two studies, the action of NBCe1 was inhibited using antibodies raised against an epitope in the third (i.e., longest) extracellular loop of NBCe1 (225, 481). In a third study, conducted prior to the cloning of NBCe1, rabbit proximal tubule vesicles enriched for Na/HCO3 cotransporter activity were used to raise an anti-Na/HCO3 cotransporter antibody (74). The antibody reacted on western blots with a 56-kDa protein and apparently blocked NBCe1 activity (74). However, after the cloning of NBCe1, we now appreciate that the immunoreactive protein is too small to have been NBCe1-A. Thus the blockade must have been indirect. The identity of the 56-kDa protein is unknown.
H) ANTISENSE PROBES.
shRNAs, siRNAs, and hammerhead ribozymes have been used to reduce the abundance of specific NCBTs (90, 546, 593, 642, 989).
I) PHOSPHATASES.
The time-dependent rundown of NBCe1-A activity in excised Xenopus oocyte macropatches can be slowed by maneuvers that inhibit protein phosphatase activity, indicating that at least NBCe1-A can be inhibited by dephosphorylation (1049).
J) INTRACELLULAR MAGNESIUM.
In bovine parotic acinar cells, as well as in mammalian cell lines overexpressing NBCe1-B, NBCe1 activity is inhibited by intracellular Mg2+ (1066). Although this effect has not been demonstrated to be direct, a mutant NBCe1 construct that lacks the Nt sequence specific to NBCe1-B (a region that includes the AID as well as IRBIT binding determinants) exhibits a substantially reduced sensitivity to Mg2+ (1066). In HEK cells, the coexpression of IRBIT also reduces the Mg2+ sensitivity of NBCe1-B (1067).
K) MOLECULAR BIOLOGICAL APPROACHES.
The per-molecule activity of NBCe1 is reduced by the removal of the Nt ASD, or the inclusion of the Nt AID (634). The per-molecule activity of NDCBE is reduced by the inclusion of the Ct AID (717).
L) AGENTS SUGGESTED, BUT NOT PROVEN, TO BLOCK NCBTs.
The anticonvulsant levetiracetam (aka Keppra) and the diuretic hydrochlorothiazide (HCTZ) have both been reported to inhibit NCBT activities in isolated tissues (567, 571), but evidence of direct interaction of these drugs with NCBTs is presently lacking. Although direct blockade of NCBTs would indeed be anticonvulsive, the properties of levetiracetam instead appear to be a consequence of its interaction with synaptic vesicle protein 2 (SV2A; see Ref. 618). The possibility that levetiracetam exerts an indirect effect on the functional expression of NCBTs cannot be excluded.
The psychoactive alkaloid harmaline blocks Na+-coupled transporters, perhaps by interaction with Na+-binding determinants (47, 864). When applied at 200 μM, harmaline is reported to effect a near-total, yet reversible block of at least 1) the Na+ and HCO3− dependent pHi recovery in HEK cells expressing human NBCe1 (39), 2) the electrogenic NCBT activity in salamander Müller cells (680), and 3) the HCO3−-dependent Na+ flux in basolateral membrane vesicle preparations from rat (332) and rabbit (893, 897) renal cortex. However, 200 μM harmaline does not substantially inhibit human NBCe1-A expressed in Xenopus oocytes (Lee, Boron, and Parker, unpublished data), and thus blockade of NCBT activity by harmaline in renal membranes may be an indirect effect of blockade of other Na+-dependent transporters.
2. NCBT stimulation
Physiological stimuli that enhance transcription, translation, and activity of individual NCBTs in vivo are discussed, for each NCBT, in section V. Other maneuvers that enhance the activity of NCBTs are listed below.
A) PIP2.
Application of PIP2 to the intracellular face of excised Xenopus oocytes patches containing NBCe1-A stimulates transport (1049). However, PIP2 is rapidly hydrolyzed when injected into intact oocytes, an observation that may explain why injection of PIP2 does not result in the stimulation of NBCe1-A in whole cells (968).
Injection of PIP2 doubles the functional expression of NBCe1-B and NBCe1-C in intact oocytes, via a pathway that can be mimicked by IP3 injection and/or elevation of cytoplasmic [Ca2+] (968). The stimulation of NBCe1-B/C by IP3 injection is blocked by the kinase inhibitor staurosporine, consistent with the involvement of endogenous kinase activity (968).
B) G PROTEIN–COUPLED RECEPTOR AGONISTS.
Exposing oocytes to lysophosphatidic acid (LPA), which binds to endogenous LPA receptors and presumably acts via a pathway mimicked by PIP2/IP3 injection (462), increases the per-molecule activity of exogenously expressed NBCe1-C (968). A nuance is that LPA application, but not PIP2/IP3 injection, also increases the plasma membrane abundance of NBCe1-B (but not NBCe1-C) via a Ca2+-independent mechanism (968).
C) ANTI-NBCe1 ANTIBODIES.
Application of an antibody raised against EL4 of NBCe1 stimulates NBCe1-like activity in myocytes (225).
D) INCLUSION OF AUTOSTIMULATORY SEQUENCE.
Full-length NBCe1-A exhibits a twofold greater activity than an NBCe1 construct that lacks the Nt ASD (559, 634). It is possible, although untested, that all NCBTs would also be stimulated by the replacement of their Nt appendages with NBCe1 autostimulatory sequence. Note that autostimulatory sequences specific to NBCe2, NBCn1, NDCBE, or NBCn2 have yet to be reported.
E) REMOVAL OF AUTOINHIBITORY SEQUENCE.
Truncation of the Nt AID from NBCe1-B/C and NBCn2 (634, 718) or truncation of the Ct AID from NDCBE-B/D (717) increases NCBT activity.
F) IRBIT.
This soluble, 60-kDa protein is an important activator of certain NCBTs. Coexpression of IRBIT with NBCe1-B, NBCn1-B, NBCn2-B, or NDCBE-B (722, 881, 1067), nNCBTs with defined autoinhibitory domains in the Nt, stimulates NCBT activity, in part by binding to the Nt and relieving transporter autoinhibition (559, 881). IRBIT must undergo a series of phosphorylations to become active, although the optimal phosphorylation state remains undefined (246, 881). Maximal stimulation of NBCe1-B activity by IRBIT, greater than that achieved by removal of the Nt AID, can be accomplished using a potent mutant IRBIT that lacks a PP-1 docking site and thus presumably becomes suitably phosphorylated (559). IRBIT has no effect on NBCe1-A because this variant has an autostimulatory domain but neither an IRBIT binding site or autoinhibitory domain (559, 634, 881).
In mammalian cells, the effect of IRBIT upon NBCe1-B is twofold. In addition to stimulating the per-molecule activity of NBCe1-B, IRBIT, by antagonizing the WNK/SPAK signaling pathway, also causes an increase in plasma membrane abundance of NBCe1-B (1075).
Other IRBIT binding partners include CFTR (1076), the IP3 receptor (43), NHE3 (371), and the cleavage and polyadenylation specificity factor CPSF (483).
V. NCBTs IN MAMMALS
Each of the five mammalian NCBTs–Slc4a4 (NBCe1), Slc4a5 (NBCe2), Slc4a7 (NBCn1), Slc4a8 (NDCBE), and Slc4a10 (NBCn2)–plays a vital and unique role in acid-base homeostasis, and each has been the subject of much investigation. The two major roles played by NCBTs, reviewed here in section V, are 1) maintenance of pHi, local extracellular pH, interstitial pH, and plasma pH within a normal range (all NCBTs); and 2) support of transepithelial anion and fluid movement (e.g., NBCe1 and NBCn1 in salivary gland epithelia).
We will detail crucial similarities and differences in the action, distribution, and role of each transporter, with the caveat that not all information on a particular NCBT may be transferrable among all mammalian species. For example, in one recent comparative analysis, NBCe1 transcripts were noticeably more abundant in preparations from human duodenum, than in equivalent samples isolated from mice and rats (485).
In this section, we consider first the two electrogenic NCBTs (NBCe1 and NBCe2), and then the three electroneutral NCBTs (NBCn1, NDCBE, and NBCn2), these groupings reflecting the relatedness of the two major groups of NCBTs in FIGURE 3 AND TABLE 2. In section VI, we discuss the AEs (encoded by Slc4a1–3) and two other related Slc4-family members, Slc4a9 and Slc4a11. Also noteworthy, although not discussed further in this review, is the presence, in rat medullary thick ascending limb (mTAL) cells, of a stilbene-sensitive, electroneutral K/HCO3 cotransport mechanism (570), which has yet to be attributed to the activity of a specific transporter.
A. Mammalian Electrogenic NCBTs: NBCe1 and NBCe2
Of the five mammalian NCBTs, only NBCe1 and NBCe2 perform electrogenic Na+/HCO3− cotransport. The molecular actions of NBCe1 and NBCe2 appear to be virtually indistinguishable; the major differences between NBCe1 and NBCe2 reside in their distribution and perhaps in their means of regulation.
1. NBCe1 (Slc4a4)
A) SUMMARY.
The electrogenic Na/HCO3 cotransporter NBCe1 (encoded by the Slc4a4 gene) is present in many organ systems throughout the body but is notably abundant in the following: 1) plasma membranes of neurons and glia in the central nervous system, where changes in pHi and pHo modulate neuronal excitability; 2) basolateral membranes of secretory epithelia, where NBCe1 mediates a HCO3− influx that supports luminal HCO3− secretion; and 3) basolateral membranes of renal PT cells, where NBCe1 mediates a HCO3− efflux that is critical for the secretion of H+ into the tubule lumen, one consequence of which is HCO3− reabsorption. This activity helps maintain a normal plasma [HCO3−].
In keeping with these roles, NBCe1 dysfunction is associated with alterations in neuronal excitability (e.g., epilepsy), fluid-movement defects (e.g., corneal edema), and acid-base disturbances (e.g., proximal renal tubular acidosis or pRTA).
NBCe1 has five known variants (-A through -E). NBCe1-A is the constitutively active renal variant. NBCe1-B, -C, and probably also -E are stimulated by the soluble protein IRBIT. NBCe1 is upregulated in acidosis and hypercapnia, conditions in which the action of NBCe1 would raise [HCO3−] in the blood plasma and/or intracellular fluid, thereby stabilizing pH. However, in some instances, the obligatory influx of Na+ that is coupled to the movement of HCO3− into cells can contribute towards ischemic damage. NBCe1 is downregulated in conditions such as alkalosis and Na+ loading, when the requirement for NBCe1 action is reduced.
B) NOMENCLATURE OF Slc4a4 PRODUCTS.
The nomenclature of Slc4a4 products has gradually evolved over the last 15 years. The original Slc4a4 gene-product, cloned from salamander kidney, was termed simply NBC for Na bicarbonate cotransporter (809). Prefixes have been variously added to this acronym to reflect either the genus of animal from which the NBC was cloned (e.g., rNBC was used to refer to rat Slc4a4 products), the organ from which the NBC was cloned (e.g., kNBC was used to refer to kidney Slc4a4 products), or both (e.g., rkNBC was used to refer to rat kidney Slc4a4 products). In a case where more than one Slc4a4 splice variant was identified in an organ from a particular species, some authors added a number to the prefix (e.g., rb1NBC and rb2NBC were used to refer to two distinct products of the Slc4a4 gene from rat brain). Following the cloning of a cDNA from a second NBC-encoding gene, the original NBC was referred to as NBC1 (e.g., kNBC1 and pNBC1 distinguished kidney and pancreas Slc4a4 products) and the new ones given higher numbers (and not always different ones). Finally, with the cloning of electroneutral NBCs, a lowercase “e” for electrogenic was inserted into the acronym (e.g., NBCe1 and NBCn1 distinguish the electrogenic Slc4a4 gene-product from the electroneutral Slc4a7 gene-product) and thus the original, electrogenic Na/HCO3 cotransporter was finally renamed NBCe1 (100).
Despite the multiplicity of acronyms, only five mammalian NBCe1 variants have been described to date. The current nomenclature defines Slc4a4 products as follows: NBCe1-A (previously known as rNBC, aNBC, kNBC, kNBC1, hkNBCe1), NBCe1-B (previously known as pNBC, pNBC1, rpNBC, hhNBC, hcNBC, rb1NBC), NBCe1-C (previously known as bNBC1, rb2NBC), NBCe1-D, or NBCe1-E.
The unique and common features of each of these variants are discussed below.
C) MOLECULAR ACTION OF NBCe.
I) Physiological substrates. NBCe1 was the first NCBT to be cloned from mammals (138, 140, 806, 808) and, like its amphibian ortholog, catalyzes the cotransport of 1 Na+ with 2 or 3 HCO3− equivalents (FIGURE 16), resulting in the net movement of negative charge in the direction of net transport. There are a number of potential mechanisms by which electrogenic Na/HCO3 cotransport could occur. Preliminary reports suggests that it is CO32−, rather than HCO3−, that is the transported anion when the transporter is working with a 1:2 stoichiometry (336, 560), as shown in FIGURE 16B. It cannot be ruled out that the NaCO3− ion pair is the transported anion (FIGURE 16C as well as Refs. 16 and 448), although potential evidence against this possibility are as follows: 1) the poor Li/HCO3 cotransport activity of NBCe1 that indicates a stronger cation selectivity than might be achieved in the case of NaCO3– versus LiCO3− (39) and 2) the reported inhibition of NBCe1 by harmaline and inhibition by benzamil, drugs that interact with Na+ binding sites. Kinetic studies in other animals indicate that squid NDCBE, but not frog NBCe1, can transport the NaCO3− ion pair.
Figure 16.
Molecular action of electrogenic NCBTs. Possible molecular mechanisms by which an electrogenic NCBT could operate with an apparent Na+:HCO3− stoichiometry of 1:2 (A–D in top panel) and 1:3 (E–J in bottom panel). Note that we have not considered any models that are based on CO32−-HCO3− exchange (considered for NBCn1 in FIGURE 30), or models of HCO3−-stimulated electrogenic Na-2H exchange. We have also omitted mechanisms of 1:3 stoichiometry that could result from modification of model D.
II) Apparent stoichiometry shift. NBCe1 certainly can function with a Na+:HCO3− stoichiometry of 1:2 and appears capable of operating with a stoichiometry of 1:3. Stoichiometry plays a pivotal role in determining the direction of net transport. With an NBCe1 stoichiometry of 1:2, and typical ion concentration and voltage profiles across the membrane, Vm is more positive than the reversal potential and thus NBCe1 mediates a net influx of HCO3− equivalents (for thermodynamic calculations, see examples in Refs. 103, 339, and 349). With a stoichiometry of 1:3, however, Vm would be more negative than Erev so that NBCe1 would mediate a net efflux of HCO3−.
In astrocytes (75), parotid acinar cells (1065), corneal endothelial vesicles (543), pancreatic duct cells (344), ventricular myocytes (14, 1006) or when overexpressed in Xenopus oocytes (381, 853) and HEK cells (870), NBCe1 operates with a 1:2 stoichiometry, mediating net Na+ and HCO3− influx (FIGURE 16, A–D, most likely FIGURE 16B). One study reports that NBCe1 also operates with a 1:2 stoichiometry in rabbit PTs (858) (where NBCe1 mediates net Na+ and HCO3− efflux) but in other studies, renal NBCe1, including that of rabbit, is calculated to operate with an apparent 1:3 Na:HCO3 stoichiometry (348, 896, 1085). Indeed, some studies suggest that NBCe1 could fulfill its physiological mission of HCO3− reabsorption only if it operated with a stoichiometry of greater than 1:2 in the PT (381, 1086) (see above). Such a shift in stoichiometry could be achieved by unveiling a cryptic HCO3− cotransport site (e.g., FIGURE 16, A–C versus E–G), or a cryptic H+ exchange site (e.g., FIGURE 16, A–C versus H–J). The mechanism(s) that control the apparent change in stoichiometry from 1:2 to 1:3 and vice versa (reviewed in Ref. 349) are unclear but have been suggested to involve a number of factors, such as changes in [Ca2+]i (667), changes in the phosphorylation state of the transporter (347), changes in the direction of transport (750), the presence of an as-yet-unidentified binding partner in PT epithelia (344), differences in cell type in which the transporter is being expressed (346), and/or primary culture conditions in the case of proximal tubules (666, 668).
III) Substrate specificity. When expressed heterologously, NBCe1 does not require extracellular Cl− to function (138, 339) and, as described above, is inhibited by stilbene disulfonates (140, 611, 853). The Km of the transporter for Na+ is ∼20–30 mM (634, 822, 853). Rat NBCe1 expressed in Xenopus oocytes mediates a small amount of Li/HCO3 cotransport (estimated at ∼3% of Na/HCO3 cotransport) but does not mediate K/HCO3 cotransport (853). We estimate that human NBCe1, as expressed in oocytes, can support ∼10% Li/HCO3 cotransport compared with Na/HCO3 cotransport (Lee, Boron, and Parker, unpublished data) but ∼25% when expressed in a kidney cell line (39). Similarly, NBCe1 assessed in basolateral membrane vesicles from rabbit PT does not exhibit a strong Na+/Li+ selectivity (897). It is unclear whether the poorer Na+/Li+ selectivity in renal membranes versus oocyte membranes reflects differences in NBCe1 behavior, assay method, or contributions from other endogenous kidney transporters.
The Km of NBCe1 for HCO3− is ∼4–10 mM (339, 543, 634). Preliminary studies indicate that at least rat NBCe1 expressed in Xenopus oocytes can also transport select anions other than HCO3−/CO32−, such as NO3– (852). Na+-coupled, DIDS-sensitive HSO3−/SO32− cotransport attributed to NBCe1 has been reported in rabbit PT vesicles (893) and in oocytes injected with rabbit kidney RNA (822), but not in oocytes expressing human (339) or rabbit (Lee, Boron, and Parker, unpublished data) NBCe1, suggesting that the observations from renal preparations could be complicated by the presence of other anion transporters, such as Slc26a1 (FIGURE 1). Finally, according to one report, NBCe1-A, at least at high extracellular pH, might mediate a small degree of OH− transport (39).
D) THE SLC4A4 GENE.
The human NBCe1 gene maps to chromosomal locus 4q21 (6) and has at least 26 exons that encompass ∼390 kb of genomic DNA. As shown in FIGURE 17A, the upstream neighbor of SLC4A4 is DCK (deoxycytidine kinase) and the downstream neighbor of SLC4A4, transcribed from the opposite DNA strand, is GC (group-specific complement, vitamin D binding protein).
The SLC4A4 gene has two distinct promoters (P1 and P2 in FIGURE 17B, see Ref. 9). The first promoter, P1, is located upstream of noncoding exon 1 and promotes transcription of NBCe1-B, -C, and -E in diverse cell types (FIGURE 17C).33 Translation of these variants begins at exon 2 (initiator methionines are marked “M” in FIGURE 17C). The transcription of NBCe1-B from promoter P1 in mouse ameloblast-like LS8 cells is pH-dependent: transcript abundance is increased in acid-incubated cells and decreased in alkali-incubated cells (706, 891). The human P1 region includes a 284 bp, “pH-responsive” sequence that ends 8 bp upstream of the transcriptional start site (891). If this sequence is placed upstream of a reporter gene that has a minimal promoter, the transcription of the reporter in LS8 cells is enhanced when the cells are maintained in media with an acidic pH (pH 6.8 versus pH 7.4; Ref. 891). The action of the “pH-responsive” enhancer requires DNA elements that contain consensus binding sites for NF-κB and p53 (891).
The second promoter, P2, is located upstream of exon 4 (FIGURE 17B) and promotes transcription of NBCe1-A, and likely also NBCe1-D (FIGURE 17C). Translation of NBCe1-A/D begins in exon 4 (initiator methionines are marked “M” in FIGURE 17C). The P2 promoter is very active in renal PT cells.
E) STRUCTURAL FEATURES AND VARIANTS OF NBCe1.
The five distinct Slc4a4-encoded transcripts (FIGURE 17C) encode protein products NBCe1-A through NBCe1-E (FIGURE 18). Variants differ in the inclusion of one of two distinct Nt appendages, the exclusion of a 9-amino acid cassette I within the Nt domain, and the choice of one of two distinct Ct appendages. Below, we consider in detail the mechanisms that generate this diversity, the similarities and differences among the variants and, anticipating the next section of this review, briefly outline the distribution of each variant. The splicing of NBCe1 along with that of other renal transporters has been reviewed in Ref. 310.
Figure 18.
NBCe1 protein variants. Scale diagram of protein variants that are encoded by the transcripts represented in FIGURE 17C. Horizontal bars represent protein sequence laid out from Nt to Ct. Vertical bars represent position of α-helical TMs. Protein cassettes are labeled with a number denoting their size in amino acids and colored to denote their genetic origin as shown in FIGURE 17C. NBCe1-A and NBCe1-D include an autostimulatory domain (ASD), whereas all other variants include an autoinhibitory domain (AID). NBCe1-C terminates with a PDZ-domain binding sequence. A color-matched protein sequence alignment of the variants is provided in Appendix V.
I) Sources of variation in coding sequence among NBCe1 variants. A) Alternative Nt appendages (“MSTE-” versus “MEDE-”). The mechanisms that result in the production of two alternative NBCe1 Nt appendages (FIGURE 18/blue versus red modules) are shown in FIGURE 17C. The 41-amino acid Nt appendage (FIGURE 18/red module) common to NBCe1-A and NBCe1-D (encoded by exon 4 and beginning with the amino acid sequence “MSTE-”) includes an autostimulatory domain (ASD) that enhances NBCe1 activity (634).
The 85-amino acid Nt appendage (FIGURE 18/blue module) common to NBCe1-B, -C, and -E (encoded by exons 2 and 3 and beginning with the amino acid sequence “MEDE-”) includes an autoinhibitory domain (AID) that inhibits NBCe1 activity (634). The AID also includes binding determinants (IBD) for the NBCe1 activating protein IRBIT (881).
B) Cassette I. In NBCe1-D/E, the excision of a 27 nt region, homologous to cassette I of NBCn1, arises due to the use of a cryptic splice site within exon 6 of the gene (see FIGURE 17C and Ref. 599). Omission of cassette I (FIGURE 18/purple module) is predicted to shorten the Nt loop region (see FIGURE 15) by nine residues (loss of “RMFSNPDNG” in mouse NBCe1). The effect of losing cassette I is unknown, although cassette I does contain a consensus casein kinase II phosphorylation site (599), indicating a regulatory role. Transcripts lacking cassette I appear to be widely distributed but only account for a small fraction of the pool of total NBCe1 transcripts that had previously been identified as NBCe1-A/B in any given organ (599). Omission of cassette I from NBCe1-C-like transcripts has not been reported.
C) Alternative Ct (“-HTSC” versus “-ETTL”). Alternative splicing of exon 24 (the length of which is not a multiple of 3 nt) in NBCe1 transcripts determines the reading frame in which exon 25 is translated, impacting the remainder of the Ct sequence. In NBCe1-A/B/D/E transcripts, exon 24 (which encodes a 32-amino acid sequence) is spliced to exon 25 (which encodes a 14-amino acid sequence, followed by a termination codon) producing a 46-amino acid Ct appendage (FIGURE 18/green module) that terminates with the sequence “-HTSC”. The remainder of exon 25 and all of terminal-exon 26 of NBCe1-A/B/D/E comprise the 3′-UTR (FIGURE 17C).
In NBCe1-C transcripts, exon 24 is omitted (FIGURE 17C). Due to the resulting frame shift, exon 25 now encodes a 27-amino acid sequence and the terminal exon 26 encodes a 34-amino acid sequence followed by a termination codon and the 3′-UTR. Thus it is that, in NBCe1-C, exons 25–26 encode a 61-amino acid Ct appendage (FIGURE 18/orange module) that terminates with the sequence “-ETTL”. The consequences of alternative Ct choice are unclear, but “-ETTL” is a PDZ-binding domain interacting sequence (79) and deletion of either the 46-amino acid or the 61-amino acid Ct sequences results in reduced NBCe1 accumulation in the plasma membrane (276, 634). In the absence of the Nt autoinhibitory domain, NBCe1-C has a greater activity than NBCe1-B, as if the 61-amino acid Ct appendage is stimulatory, or the 46-amino acid appendage is inhibitory, in the absence of the Nt AID (634).
II) Cloned NBCe1 variants that are demonstrated or likely to exhibit NCBT activity. A representation of the five variants NBCe1-A through NBCe1-E is shown in FIGURE 18, and the composition of each is described below. Also listed here are the major anatomical locations from which each variant has been cloned as a full-length cDNA (the only reliable demonstration of the presence of each in any preparation). Distribution of subsets of NBCe1 variants (such as might be determined using an antibody that recognizes the common Ct of NBCe1-A/B/D/E) are discussed separately in section “Distribution of NBCe1” below. GenBank protein accession numbers for the variants discussed in this section are provided in Appendix IV.
A) NBCe1-A (NCBT activity demonstrated). This predominantly renal variant of NBCe1 (64, 138, 806) has a predicted nonglycosylated molecular mass of 116 kDa (190). NBCe1-A includes 1) the 41-amino acid “MSTE-” Nt sequence that includes an ASD, 2) cassette I, and 3) the 46-amino acid “-HTSC” Ct sequence. Due to the presence of the ASD, NBCe1-A has a greater per-molecule activity than either NBCe1-B or NBCe1-C (634). NBCe1-A has also been cloned from testis, epididymis, and ovary (599).
B) NBCe1-B (NCBT activity demonstrated). This widely expressed splice form of NBCe1 (6, 192) includes 1) the 85-amino acid “MEDE-” Nt sequence that contains an AID and an IRBIT-binding sequence, 2) cassette I, and 3) the 46-amino acid “-HTSC” Ct sequence. Due to the presence of the AID, NBCe1-B has a lower per-molecule activity than NBCe1-A and a similar per-molecule activity to NBCe1-C (634). Apart from the pancreas, where NBCe1-B transcripts are most abundant, NBCe1-B has been cloned from the brain (79), cornea (922), heart (192), parotid salivary gland (508, 710), ileum (64), and from diverse tissues within the male and female reproductive tracts (599).
C) NBCe1-C (NCBT activity demonstrated). This predominantly brain-expressed variant (79) includes 1) the 85-amino acid “MEDE-” Nt sequence that constitutes an AID and an IRBIT-binding sequence, 2) cassette I, and 3) the 61-amino acid “-ETTL” Ct sequence. NBCe1-C is uniquely distinguished by the presence of the 61-amino acid Ct as it is the sole variant that includes this Ct. NBCe1-C has also been cloned from murine epididymis and testis (599) and human heart. Due to the presence of the AID, NBCe1-C has a lower per-molecule activity than NBCe1-A and a similar per-molecule activity to NBCe1-B (634).
D) NBCe1-D (NCBT activity untested). NBCe1-D is identical to NBCe1-A except for the absence of cassette I. Transcripts lacking cassette I appear to be widely distributed but only account for a small fraction of the pool of total NBCe1 transcripts that, until now, had been identified as NBCe1-A in any given preparation (599). Full-length NBCe1-D cDNA has been cloned from murine epididymis (599). We regard NBCe1-D as likely to have NCBT activity because NBCn1-H, which lacks the homologous cassette I, has NBCn1 activity.
E) NBCe1-E (NCBT activity untested). This variant is identical to NBCe1-B in its coding sequence except for the absence of cassette I (599). NBCe1-E transcripts only account for a small fraction of the pool of total NBCe1 transcripts that until now had been identified as NBCe1-B in any given preparation (599). Full-length NBCe1-E cDNA has been cloned from murine ovary, uterus, and epididymis (599). We regard NBCe1-E as likely to have NCBT activity because NBCn1-H, which lacks the homologous cassette I, has NBCn1 activity.
III) Predicted NBCe1 variants. NBCe1 variants that include the 61-amino acid Ct of NBCe1-C and that also 1) include the ASD of NBCe1-A or 2) lack cassette I have not been reported. Possibly the splice machinery that excises exon 24 is absent from the pool of cell types that promote NBCe1-A transcription. A lab-created, chimeric NBCe1 that includes the Nt of NBCe1-A and the Ct of NBCe1-C is reported to exhibit an activity that is slightly greater than NBCe1-A (634), consistent with the mildly stimulatory effect of the NBCe1-C Ct in the absence of an Nt AID (634).
IV) Other NBCe1 variants. We are not aware of any cloned or predicted NBCe1 variants besides those mentioned above.
F) DISTRIBUTION OF NBCe1.
The major organs most often associated with NBCe1 expression are the pancreas and kidney, although NBCe1 is also abundant in many other organs. The distribution of NBCe134 in specific organ systems is discussed below. The distribution of NBCe1 is summarized and compared with that of other NCBTs in TABLE 5. In instances where a detection method would not distinguish between two variants, for example, use of an antibody against the common Nt of NBCe1-B and NBCe1-C, we refer to NBCe1-B/C. If it is unknown which variant is being discussed, or in instance of organs in which there appears to be no obvious bias in variant expression, we refer to NBCe1 without a variant designation.
Table 5.
Sites of NCBT expression
| NBCe1 | NBCe2 | NBCn1 | NDCBE | NBCn2 | |
|---|---|---|---|---|---|
| Central nervous system | Widespread, neurons and astrocytes | Blood-brain barrier and elsewhere | Widespread, neurons | Widespread, neurons | Widespread, mainly neurons |
| Sensory organs | Eye | Eye | Ear | Eye, ear | |
| Peripheral nervous system | Trigeminal ganglion | Trigeminal ganglion | Trigeminal ganglion | ||
| Respiratory system | Nose and elsewhere | Lung | Trachea and lung | Trachea and lung | |
| Circulatory system | Cardiac myocytes and elsewhere | Heart | Vasculature | Heart | Heart |
| Musculoskeletal system | Skeletal muscle | Skeletal muscle | Osteoclasts, skeletal muscle | Skeletal muscle | Skeletal muscle |
| Upper digestive system | Widespread | Stomach | Widespread | Stomach | |
| Lower digestive system | Widespread, abundant in pancreas | Widespread, abundant in liver | Widespread | Widespread | Widespread |
| Lymphatic system | Spleen and leukocytes | Spleen and macrophages | Widespread | Spleen | |
| Endocrine system | Thyroid and pancreas | Thyroid | Widespread | Pituitary gland | |
| Urinary system | Kidney | Kidney | Bladder and kidney | Kidney | Kidney |
| Reproductive system | Widespread | Placenta and testes | Widespread | Testes and elsewhere | Testes |
A more complete and detailed examination of each NCBT distribution is provided in text. A distribution of NCBT expression based on the origins of corresponding expressed-sequence-tags is provided in Appendix VI.
I) Central nervous system. A) General. The distribution of NBCe1 variants in the central nervous system was recently reviewed by Majumdar and Bevensee (623). Assessed by PCR, NBCe1-B/C transcripts are abundant in rat brain, whereas NBCe1-A is much less abundant (318). By immunoblot, NBCe1-C is abundant in rat brain versus kidney, whereas NBCe1-A/B expression in brain is negligible compared with kidney (79). In mouse brain (796), NBCe1-A can be identified by quantitative PCR,35 and the protein is reported to be widespread by immunohistochemistry.36 However, in rat and human brain, northern blot and in situ hybridization studies using NBCe1 variant-specific probes indicate that expression of NBCe1-A transcripts is insubstantial (6, 318, 624). Thus it would appear that the predominant NBCe1 variants in mammalian brain are NBCe1-B and NBCe1-C.
As determined by in situ hybridization or immunohistochemistry, NBCe1-B and NBCe1-C are present throughout the rat brain but exhibit particularly robust expression in the dentate gyrus of the hippocampus, cerebellum, olfactory bulb, and piriform cortex (318, 624, 843), and in the brain stem/diencephalon region (260, 1060). In general, NBCe1-C transcripts appear to outnumber those encoding NBCe1-B (624), although the ratio of NBCe1-A/B (likely NBCe1-B) to NBCe1-C protein is greater in cerebellum compared with other brain regions (261, 1060). In rats, expression of NBCe1 transcripts (318) and protein (260) is not detected in the brain until birth, whereupon NBCe1 levels increase gradually until an age of 4 wk (260).
B) Neurons versus glia. In primary cultures from cerebral cortex, NBCe1-B protein is expressed mainly in astrocytes, whereas NBCe1-C is expressed mostly in neurons (79). However, the expression pattern appears to be just the opposite in situ (624), where immunohistochemistry and immuno-gold labeling reveals NBCe1-A/B (likely NBCe1-B) inside neurons, and reveals NBCe1-C on the plasma membrane of astrocytes. A study on mouse or rat brain oligodendrocytes demonstrates NBCe1-A/B (likely NBCe1-B) immunoreactivity in the dendrites of these cells (800).
C) Spinal cord. NBCe1 transcripts are detected in the developing rat spinal cord from embryonic day 19 (318), and NBCe1 protein has been noted in the spinal cord (both white and gray matter; Ref. 843), according with a substantial presence of NBCe1-B/C transcripts in spinal cord mRNA (6).
D) Blood-brain barrier. NBCe1 protein has been detected in basolateral membranes of choroid plexus epithelia (843). Transcripts also are detected in the outer meningeal layer (843).
II) Sensory Organs. A) Eye. NBCe1-B is often described as the major NBCe1 variant expressed in the eye, although most evidence relies on molecular tools that do not discriminate between NBCe1-B and NBCe1-C. To our knowledge, an antibody specific for NBCe1-C has never been used to examine the distribution of NBCe1 in the eye. In one study on human corneal endothelium, a primer pair that should amplify both NBCe1-B and NBCe1-C yielded one full-length cDNA clone, which corresponds to full-length NBCe1-B (922), but this does not exclude the presence of NBCe1-C. In ciliary body, an antibody that recognizes both NBCe1-A and NBCe1-B exhibits robust immunoreactivity (1013), even though NBCe1-A is not abundantly expressed in the eye (see below). Taken together, these data are consistent with the hypothesis that NBCe1-B is the dominant NBCe1 variant in the eye.
NBCe1-B/C cDNA and protein are detected in a variety of ocular tissues, namely the surface and wing cells, but not the stroma, of the conjunctiva (94);37 the keratocytes of the corneal stroma (94); the endothelial cells of the cornea (94, 248, 593, 922, 923, 989, 990), predominantly at the basolateral membrane (94, 248, 922, 923, 989; see cartoon in FIGURE 19); the trabecular meshwork in the anterior chamber (989), responsible for draining aqueous humor; the pigmented epithelium of the ciliary body (94, 989) in the posterior chamber, specifically at the basolateral membrane of the pigmented cells (94). Data conflict concerning the presence of NBCe1 in the non-pigmented epithelia of the ciliary body (94, 868, 989), which is responsible for secretion of the aqueous humor; the epithelium of the lens, in both apical and basolateral membranes, and a human lens anterior epithelium cell line (94, 875, 989); and the retina (30) including specifically the apical microvilli and end feet of Müller glial cells (94), the apical membrane of retinal pigment epithelial cells (11, 94), and the choriocapillaris (1079).
Figure 19.
Role of NBCe1 in the cornea. The corneal endothelium reabsorbs fluid from the collagen matrix that constitutes the stroma, preventing corneal edema (see cartoon of eye). NBCe1-B in the basolateral membrane supports transepithelial anion secretion. Note the similarities between this pathway and the mechanism of NaCl secretion in dogfish salt glands (FIGURE 11).
A small amount of NBCe1-A cDNA has been detected by PCR in a human corneal endothelial cell line, but this is swamped by a greater population of NBCe1-B/C cDNAs (990). On the other hand, the cornea per se is reported to be negative for NBCe1-A cDNA in cattle (923) and humans (922), and corneal endothelium is negative for NBCe1-A protein in rat (94). A report of NBCe1-A expression in rat ciliary body was based on an antibody raised against an epitope common to NBCe1-A and NBCe1-B (1013), and a report of NBCe1-A expression in porcine nonpigmented ciliary epithelial cDNA depended on a primer pair that does not distinguish among NBCe1 variants (868). Indeed, an antibody study by Bok et al. (94) found only NBCe1-B, and not NBCe1-A, expression in the ciliary body. An immunohistochemical study by the same workers did detect NBCe1-A in the basal epithelium of rat conjunctiva (94). Only one study reports appreciable NBCe1-A protein expression elsewhere in the rat eye: using immunohistochemistry, Usui and co-workers found NBCe1-A protein together with NBCe1-B protein in the ciliary body, lens, and cornea of the rat eye (990). However, the NBCe1-A immunoreactivity was diffuse, in contrast to the clear membrane localization of NBCe1-B immunoreactivity in the study of Bok and co-workers.
Despite the relatively low abundance of NBCe1-A in the eye, it is interesting to note that an individual with the mutation Q29X, predicted to specifically eliminate NBCe1-A (FIGURE 25 AND TABLE 6), has bilateral glaucoma (412). Thus either NBCe1-A is expressed in tissues involved in regulating anterior-chamber volume (e.g., nonpigmented epithelium of the ciliary body, trabecular meshwork), presumably early in development, or the ocular phenotype is secondary to the whole body acidosis caused by NBCe1-A deficit in the kidney.
Figure 25.
Location of disease-associated mutations in NBCe1-A. Representation of human NBCe1-A topology from FIGURE 2A. Numbered circles show the positions of SLC4A4 mutations (numbers 1–12 match the numbered mutant descriptions in TABLE 6) that cause proximal renal tubular acidosis. Nonsense mutations that result in premature translational termination are colored red, and missense mutations are colored green.
Table 6.
SLC4A4 mutations in individuals with proximal renal tubular acidosis
| Label in Figure 25 | Trivial Name and Original Report | Predicted Protein Producta | DNAb | Likely Molecular Basis for pRTA | Pathological Features From Original Report (Other Than pRTA)c |
|---|---|---|---|---|---|
| 1 | Q29X (412) | p.Gln29X | c.85C>T | Protein not translated (929) | Mental retardation, growth retardation, glaucoma. No evidence of cataracts or band keratopathy. |
| 2 | R298S (411) | p.Arg298Ser | c.894A>C | Partial mistargeting to apical membrane with some cytosolic retention (929)d combined with a approximately 25% reduction in per-molecule function (166). | Mental retardation, growth retardation, glaucoma, cataracts, and band keratopathy. Elevated serum amylase. Calcification of basal ganglia (414). |
| 3 | S427L (253) | p.Ser427Leu | c.1280C>T | Partial mistargeting to apical membrane (577) combined with a likely reduction in per-molecule function (253, 577). | Growth retardation, glaucoma, and cataracts. Poor dentition. Normal intelligence. No specific mention of band keratopathy, but corneal clouding was evident. Also some evidence of respiratory acidosis (i.e., elevated Pco2). |
| 4 | T485S (393) | p.Thr485Ser | c.1453A>T | Mutant traffics normally thus loss of function is likely explained by impaired per-molecule activity (393, 576, 929, 930). | Growth retardation, cataracts, and band keratopathy. No specific mention of mental retardation or glaucoma. |
| 5 | G486R (929) | p.Gly486Arg | c.1456G>A | Mutant traffics normally;e thus loss of function is likely explained by impaired per-molecule activity (929). | Growth retardation, cataracts, and band keratopathy. Normal intelligence and no glaucoma. |
| 6 | R510H (411, 879) | p.Arg510His | c.1529G>A | Intracellular retention of mutant (577, 930). Additional per-molecule activity defects not reported.f | Growth retardation, glaucoma, cataracts, and band keratopathy. Delayed neurological and motor development. No mention of mental retardation. Migraines (930). |
| 7 | W516X (602) | p.Trp516X | Not reportedg | Complete loss of protein (602) | Growth retardation, glaucoma, cataracts, and band keratopathy. Calcification of basal ganglia. No mention of mental retardation. |
| 8 | L522P (241) | p.Leu522Pro | c.1565T>C | Intracellular retention of mutant (241, 929, 930) | Motor and mental retardation, growth retardation, glaucoma, cataracts, and band keratopathy. Dental abnormalities. Migraines. |
| 9 | nt2311h (416) | p.Asn721ThrfsX30i | c.2162delA | Complete loss of protein (416) | Growth retardation, cataracts, band keratopathy. Dental abnormalities. Calcification of basal ganglia. Normal intelligence. No glaucoma, but elevated ocular pressure. Elevated serum lipase and amylase.j |
| 10 | A799V (393) | p.Ala799Val | c.2396C>T | Intracellular accumulation of mutant, reduced per-molecule function, and a HCO3− independent conductance (721). | Motor and mental retardation, growth retardation, low weight, glaucoma, cataracts, band keratopathy, and calcification of basal ganglia (231). |
| 11 | R881C (393) | p.Arg881Cys | c.2641C>T | Intracellular retention of protein (576, 930, 977, 1113). Protein has close to normal per-molecule activity (977). | Growth retardation, glaucoma, cataracts. No specific mention of mental retardation or band keratopathy. Elevated serum amylase. Migraines (930). |
| 12 | Δ65bp (413, 930) | p.Ser982AsnfsX4k | c.2944_2967 + 42dell | Intracellular retention of mutant (930), but mutant protein functions normally when expressed in oocytes (930). | Glaucoma, cataracts, and band keratopathy. Migraines. Normal intelligence and stature. Epilepsy. Nausea. |
A database of human SLC4A4 mutations is curated at the Leiden Open Variation Database (https://grenada.lumc.nl/LOVD2/shared1/home.php?select_db=SLC4A4). The position of the mutated residues within NBCe1 is depicted in Figure 25 and shown on sequence alignments in Appendix I.
Based on NP_003750.
Based on NM_003759.3 (“A” of initiating ATG codon is counted as nucleotide 1).
Features not described in the original report are provided together with a reference to the paper in which the feature was described.
Reports conflict as to whether the equivalent mutation in NBCe1-B (p.Arg324Ser) causes the protein to be mistargeted. The mutant is reported to accumulate normally in the plasma membrane of a human-bladder-endothelium cell line (836) and a rat glioma cell line (930) but to be retained in the cytosol of a canine-kidney-epithelium cell line (577). Interestingly, an artifical mutant—R298C—created in a version of NBCe1-A that lacks the five endogenous, cytoplasmic cysteine residues traffics efficiently to the plasma membrane in a human-kidney-epithelium cell line (1113).
In a rat glioma cell line, the equivalent mutant of NBCe1-B (p.G530R) appears to have an increased intracellular presence compared with the wild-type transporter (930).
One study finds that the equivalent mutation in NBCe1-B (p.Arg554His) exhibits a loss of function but not reduced accumulation of transport protein in the plasma membrane of human endothelial cell line (836).
Likely c.1547G>A or c.1548G>A.
This designation counts the first base of the 5′-UTR as nucleotide 1.
First affected amino acid is Asn721, which changed to Thr. Thr becomes residue #1 of the frame shifted reading frame (fs) that has a termination codon at position #30. The unique 29-amino acid appendage is predicted to have the sequence TEVGSFHRLEKTPGGCALLLLSRLCWSLY.
The authors mention only elevated lipase in their clinical description of the patient, but refer also to elevated amylase in the discussion of their findings.
The unique 3-amino acid appendage is NKF (930).
2944–2967 of the exon 23 are missing plus 42 of the following intron.
III) Peripheral nervous system. A) Trigeminal ganglion. NBCe1-B/C, but not NBCe1-A, transcripts are detected by reverse transcription polymerase chain reaction (RT-PCR) in preparations of rat trigeminal ganglion neurons (408).
IV) Respiratory system. A) Nose. In human nasal mucosa, NBCe1-A, but not NBCe1-B/C, transcripts are detected in the epithelia and submuscosal gland cells of the inferior turbinate mucosa, and in the superficial epithelia of nasal polyps (558).
B) Lungs. NBCe1-B/C immunoreactivity is detected in preparations of basolateral membrane proteins of the Calu cell line, which is derived from pulmonary airway submucosal-gland serous cells (515).
V) Circulatory system. A) Heart. Most northern blot analyses of human mRNAs and qPCR experiments indicate that NBCe1 is expressed in the heart, although at a lower abundance than in kidney or pancreas (6, 31, 140, 192, 480, 684, 831). Full-length NBCe1-B has been cloned from human heart cDNA (192), and an antibody directed against the third extracellular loop of NBCe1 immunoreacts with protein in rat and human ventricular myocardial cells (481). NBCe1-A/B immunoreactivity, likely representing NBCe1-B, is present in the left and right ventricles as well as in the interventricular septum of rat heart (831). A preliminary immunocytochemical study of rat ventricular myocytes suggests that NBCe1 protein is located in the traverse (T) tubules, in contrast to the predominantly surface-sarcolemmal distribution of NHE1 (311).
B) Capillaries. In the testes of rats, NBCe1 immunoreactivity is detected in capillary-lining endothelial cells (445).
VI) Musculoskeletal system. A) Skeletal muscle. NBCe1 immunoreactivity is detected in skeletal muscle homogenates from humans and rats (518) and has been detected in soleus and extensor digitorum longus (i.e., calf) muscles of rats (964). Immunohistochemistry of rat muscle suggests that NBCe1 is located in the sarcolemmal membrane and perhaps also, the authors of the study suggest, in T tubules (518).
VII) Upper digestive system. A) Enamel organ. Ameloblasts promote enamel deposition on developing teeth and NBCe1-B, but not NBCe1-A, transcripts are detected in preparations of microdissected ameloblasts from mice and humans (538, 1099).38 NBCe1 transcripts are more abundant in mature than secretory ameloblasts (539, 540, 1099). Immunohistochemistry appears to demonstrate a basolateral distribution of NBCe1-A/B protein in mouse ameloblasts (538, 706), with an additional presence in the adjoining stratum intermedium of the papillary cell layer (538). However, high-resolution images presented in a study of mouse dentition disclose NBCe1-A/B immunoreactivity only in the stratum intermedium, with no NBCe1 expression in the ameloblasts themselves (456), as depicted in the cartoon in FIGURE 20.
Figure 20.
Role of NBCe1 in the enamel organ. Apatite formation in the enamel compartment generates H+ that are neutralized by HCO3− secreted from mature ameloblasts. NBCe1 mediates HCO3− influx in papillary cells, and the HCO3− is transferred to ameloblasts via connecting gap junctions. Transported HCO3− and HCO3− generated within ameloblasts by CA is secreting into the enamel compartment via lateral AE2 and perhaps apical pendrin (Slc26a4). The presence of pendrin in the apical membrane of ameloblasts is controversial (124, 125, 297). The extracellular face of AE2 is exposed to the enamel compartment by a rearrangement of tight junctions (456).
Three factors could underlie the apparent discrepancy among the above studies: 1) it is difficult to resolve the ameloblast basolateral membrane from the membranes of abutting papillary cells in the stratum intermedium, 2) the studies were performed in different species, and 3) the studies employed different antibodies.
B) Salivary gland. In parotid salivary glands, the acinar cells are a site of NBCe1 expression (see cartoon in FIGURE 21A). Only NBCe1-B/C, and not NBCe1-A, is detected by PCR of mouse and bovine parotid cDNA (490, 1065). NBCe1-B was cloned from these cells in guinea pigs (508). Moreover, strong basolateral NBCe1 immunoreactivity is present in the parotid acini of humans (using an anti-NBCe1-B/C antibody; Ref. 710) and rats (anti-NBCe1; Ref. 818) as well as in a rat parotid acinar cell line (anti-NBCe1-A/B; Ref. 740). Taken together, these data suggest that NBCe1-B is the major NBCe1 variant expressed in parotid acini. To our knowledge, the presence of NBCe1-C in salivary glands has not been examined.
Figure 21.
Role of NCBTs in exocrine glands. The inset in A displays a generic acinus (acinar epithelia, blue) and duct (duct epithelia, yellow) for an exocrine gland such as the salivary gland or pancreas. NBCe1 activity regulates intracellular pH and could support transepithelial fluid and ion secretion by salivary gland acinar cells (A). NBCe1 and NBCn1 support transepithelial HCO3− secretion by salivary gland duct cells (B), contributing to formation of a HCO3−-rich saliva. The presence of AE2 in the duct cells of salivary glands may be species specific [present in humans (998) but not in rats (818)]. Similarly, the presence of NKCC1 in duct cells is not reported in all species (e.g., absent from mice in Ref. 279). The Na pump has been omitted from both cell types for clarity. A similar mechanism for fluid secretion operates in pancreatic acini and ducts.
Apart from acinar cells, NBCe1 immunoreactivity is also evident in the basolateral membranes of striated and main duct cells of rat parotid glands (818). In duct cells, NBCe1 would act in parallel with NBCn1 (see cartoon in FIGURE 21B).
Concerning the sublingual and submandibular glands, NBCe1 transcripts are absent from cDNA prepared from the sublingual salivary glands of mice (490), but are detected in the submandibular glands of guinea pigs (508). Furthermore, two studies describe NBCe1 immunoreactivity in the basolateral membranes of rodent submandibular gland duct cells (615, 818).
C) Esophagus. NBCe1-A/B immunoreactivity is present in the basolateral membranes of acinar and duct cells of esophageal submucosal glands (3, 4). NBCe1 is also detected in enzyme-secreting serous cells, but here the polarity of NBCe1 distribution is not evident (3, 4).
D) Stomach. NBCe1-B/C transcripts are present in stomach preparations from rabbits (427), guinea pigs (508), and humans (6). Northern blots and qPCR of rabbit gastric mucosal cell preparations suggest that NBCe1 is more abundant in mucous cells than chief or parietal cells (814). NBCe1 transcripts are also present in a cell line derived from rat gastric mucosa (369).
VIII) Lower digestive system. A) Intestines. At the level of mRNA or cDNA, NBCe1-B (or NBCe1-B/C) is widely expressed in the lower digestive tract. Full-length NBCe1-B has been cloned from rabbit duodenum (427). Intestinal expression of NBCe1-B/C transcripts has also been demonstrated in 1) rabbit colonic mucosa, with lower levels of expression in the ileum (427); 2) mouse duodenum (753) and colon (1087) [in the mouse proximal colon, in situ hybridization detects NBCe1 transcripts only in crypt epithelia (55)]; 3) rat small intestine and colon (318) [along the rat distal colon, NBCe1 transcripts are more numerous in the last quarter (furthest from the lymph node), than the first quarter (closest to the lymph node; see Ref. 1059)]; 4) guinea pig small intestine and proximal colon (508); 5) opossum ileum (64); and 6) human colon (6).
Aside from NBCe1-B/C, a small population of NBCe1-A transcripts is detected in the ileum and colon of rabbits (427) as well as in the duodenum (753) and colon (482) of mice, but NBCe1-A is not present at appreciable levels in the ileum of opossums (64). NBCe1-A is reportedly the predominant form of NBCe1 expressed in the human cancer cell line HT29, although evidence for the assignment is not provided (642).
At the level of protein, NBCe1 immunoreactivity is detected in mouse duodenum in the basolateral membranes of enterocytes (see FIGURE 22 and Ref. 753). In the enterocytes of rat proximal duodenum, NBCe1 immunoreactivity is strongest in villar enterocytes and decreased in abundance closer to the crypts, such that NBCe1 immunoreactivity is not detectable in goblet cells (430). A similar distribution is detected in opossum ileum (64). NBCe1 immunoreactivity is also evident in the enterocytes of the proximal jejunum (villar and crypt enterocytes), ileum and proximal, but not distal, colon (430). NBCe1 immunoreactivity has also been detected in the basolateral membranes of brush cells from rat cecum (696).
Figure 22.
Role of NCBTs in intestine. Shown is a duodenal villus enterocyte. The mechanism of transepithelial fluid and HCO3− secretion is very similar to that shown in FIGURE 21 for salivary glands. The Na pump is omitted for clarity.
B) Liver. Northern blots indicate that the liver may be an additional, albeit minor, site of NBCe1 expression (6, 806). In rat bile duct, basolateral NBCe1 immunoreactivity is detected in brush cells that are hypothesized to secrete HCO3− (695).
C) Pancreas. At the mRNA level, the pancreas is the single most abundant site of expression of NBCe1, specifically NBCe1-B (6). The role of NBCe1 in this organ is also reviewed in Reference 906. NBCe1-B has been cloned from human pancreatic cDNA (6). Taken together, in situ hybridization and immunohistochemical data demonstrate that pancreatic NBCe1 is expressed in the acinar and duct cells. NBCe1 is also expressed in insulin-secreting β cells in the islets.
In the acinar cells of mice, NBCe1-B transcripts have been detected by in situ hybridization (6) and NBCe1-B protein is located in the basolateral membrane of rat acinar cells (817, 836, 962). However, no NBCe1-B immunoreactivity is detected in human pancreatic acini (626, 836).
In the duct cells of the human pancreas, one study, using antibodies to an Nt epitope or a Ct epitope that are common to all NBCe1 variants, demonstrated that NBCe1 colocalizes with Na-K pump (basolateral) but not with CFTR (apical) (626). In other studies investigators variously report NBCe1-B immunoreactivity in the duct cells of both human and rats as apical and/or basolateral, or as different among duct types and even among cells in the same duct (94, 817, 836, 962). In some cases this confusion may arise from quality-control issues surrounding the use of antibodies raised to be specific to NBCe1-B.39 NBCe1-B is also expressed in the pancreatic duct cell lines CAPAN-1 (883) and mPEC1 (344), as well as in the cystic fibrotic pancreatic duct cell line CFPAN-1 (883). Physiological data support an exclusive presence of NCBT activity in the basolateral membranes of duct cells (422, 883, 1096).
Although, as we have just seen, NBCe1-B is undoubtedly the major, pancreatic NBCe1 variant, it is perhaps not the only one. A pool of NBCe1-A transcripts is detected by PCR from pancreatic cDNA (135, 817, 836, 901) and antibodies raised against an epitope in NBCe1-A immunoreact with a diffuse population of protein in pancreatic duct (817, 836). Considering their relative transcript levels (6), the functional significance of NBCe1-A is likely trivial compared with that of NBCe1-B under basal conditions. To our knowledge, the presence of NBCe1-C in pancreas has not been examined.
IX) Lymphatic and immune systems. As far as we are aware, there are no reports of substantial NBCe1 expression in the lymphatic or immune systems. An NCBI-curated database reports a small number of human NBCe1 ESTs derived from bone marrow and spleen (Appendix VI). However, NBCe1 transcripts are noted as undetectable by northern blot of mouse spleen RNA (313).
X) Endocrine system. A) Thyroid. NBCe1 transcripts are detected in extracts prepared from human thyroid (309, 486).
B) Pancreas. NBCe1-B transcripts are not detected in pancreatic islet cells of mice (6). However, NBCe1-A and NBCe1-B transcripts are detected in the pancreatic islet cells of rats, although the immunoreactivity of anti-NBCe1-A and -B/C antibodies are not robust at the level of western blots (901). In immunohistochemical studies on rat using those same antibodies, NBCe1-B immunoreactivity is detected in the β cells that secrete insulin but not in the α cells that secrete glucagon (901). Moreover, NBCe1-A/B immunoreactivity is detected in insulin-positive cells of pancreatic samples isolated from human cadavers (365). NBCe1-B immunoreactivity is also expressed in the insulin-secreting cell line BRIN-BD11 (135). A diffuse staining of NBCe1-A is detected in islet cells (901). In the exocrine pancreas, NBCe1 is also present in acinar and duct cells.
XI) Urinary system. A) Kidney. The kidney is the major site of expression for the NBCe1-A transcript (6). Renal NBCe1-A transcripts have been cloned from many species including humans (138) and rats (140, 806). NBCe1-A protein is expressed in the kidney cortex, specifically in the basolateral membranes of the S1 (i.e., just distal to Bowman's capsule) and early S2 PT segments in humans, rabbits, and rats (7, 273, 632, 829, 844, 1024, 1064), as depicted in the cartoon in FIGURE 23. The segmental distribution of NBCe1 along the nephron significantly overlaps with that of protein 4.1B in the S1 and S2 tubules (958), and with the Na/glucose cotransporter SGLT1 in the S2 tubule (806).
Figure 23.
Role of NBCe1 in the proximal tubule. H+ secreted by proximal tubule (PT) epithelia into the PT lumen can either be titrated by buffers such as phosphate or NH3, in which case they are excreted in the urine or, catalyzed by extracellular CA, they can be titrated by HCO3−. CO2 that enters PT epithelia from the lumen, and CO2 that is generated by PT metabolism, is hydrated by CAII into H+ and HCO3−. Reabsorption of HCO3− via NBCe1 drives H+ secretion and supplies the blood with HCO3−, regulating whole body pH.
A lesser amount of NBCe1 mRNA expression is detected in the S3 proximal tubule segments of rabbits (7), as expected from a tubule segment in which HCO3− reabsorption is less than for the S1 and S2 segments (7). Indeed, NBCe1 protein is totally absent from the S3 segment of rats (632). Traces of NBCe1 transcript expression have also been detected in the renal medulla of rats (140) and in a mouse cell line from the inner medullary collecting duct (35).
NBCe1-A is undoubtedly the major, but perhaps not the only renally expressed NBCe1 variant. A small fraction of NBCe1-B/C transcripts are detected by PCR from renal cDNA (135, 318, 427, 817, 901), and antibodies raised against an epitope in NBCe1-B/C immunoreact with a diffuse subapical population of protein in the rat PT (273, 817). Furthermore, on western blots, an NBCe1-C specific antibody exhibits some immunoreactivity with a rat renal protein extract (79). The expression level of these alternative variants is trivial compared with that of NBCe1-A under basal conditions, but their presence may be of importance during stressed conditions (117).
XII) Reproductive system. A) Female. NBCe1 transcripts are detected in mouse ovarian, uterine, and vaginal preparations (599, 1027).
B) Male. NBCe1 expression is detected in testis (599), epididymis (445, 453, 599, 729), prostate (6, 684), sperm (445), and vas deferens (152, 599). A combination of northern blotting and qPCR data indicate that the prostate is a major site of NBCe1-B/C transcript expression in human males (6, 684). Full-length NBCe1-B has been cloned from human prostate cDNA (GenBank protein accession no. AF053753). NBCe1-A/B immunoreactivity is present in sperm extracts and in the basolateral membranes of apical and principal cells of the epididymis (445).40 In the epididymis, NBCe1-A/B immunoreactivity is most pronounced in the initial segments, growing progressively weaker towards the cauda, a pattern matched by in situ hybridization results using an anti-NBCe1 probe (445). In the testes of rats, NBCe1 immunoreactivity is detected in smooth muscle cells and in capillary-lining endothelial cells (445). The relative abundance of the five NBCe1 variants throughout the mouse reproductive tract is examined in Reference 599.
G) PHYSIOLOGICAL ROLES OF NBCe1.
Its ability to transport HCO3− across membranes enables NBCe1 to play diverse roles according to its location. In all of the cell types in which NBCe1 is expressed, its action influences pH-sensitive processes within the cell and at the extracellular surface. In polarized epithelia, HCO3− transport can also support HCO3− secretion (i.e., away from the blood) or HCO3− absorption (i.e., toward the blood). Here we first discuss those processes that have general relevance to the function of a number of systems, and then we discuss specialized processes that are specific to certain organs and tissues.
I) General. A) Intracellular pHi regulation. NBCe1 presumably contributes to pHi regulation in every cell in which it is expressed. However, the role played by NBCe1 would depend critically on its stoichiometry. In cultured rat cerebellar (132) and hippocampal (75) astrocytes, an electrogenic Na/HCO3 cotransporter enhances the pHi recovery from an acute intracellular acid load (i.e., the transporter functions as an acid extruder, mediating the uptake of HCO3− equivalents). Thus this transporter, subsequently identified as NBCe1-B in cultured hippocampal astrocytes, must operate with a 1:2 stoichiometry. In renal PTs, NBCe1-A operates with an apparent stoichiometry of 1:3 and thus mediates a net efflux of HCO3− equivalents (i.e., it functions as an acid loader, mediating the efflux of HCO3− equivalents). In these cells, we would expect that NBCe1-A would contribute to the pHi decrease following an acute intracellular alkaline load, although, to our knowledge, this experiment has not been done.
A word of caution is that one could easily be fooled by an unanticipated combination of 1) an electroneutral acid-base transporter that requires Na+ and HCO3 (e.g., an electroneutral NCBT, or a Na-H exchanger activated by a CO2/HCO3− receptor) and 2) a parallel though unlinked electrogenic process (e.g., a pH-sensitive ion channel). Thus, in reaching the conclusion that an electrogenic Na/HCO3 cotransporter is responsible for a pHi change, it is important that the investigator verify that the cells do indeed express the transporter, that the rate of pHi change quantitatively matches some measure of electrogenic transport (e.g., a change in Vm but preferably a membrane current), and that the indexes of both transport and electrogenicity have the same ionic and pharmacological properties. A case in point is a report that concluded that, in spinal cord neurons of embryonic rats, an electrogenic NBC contributed to the observed, robust pHi recovery from an acid load (118). The electrical link was a demonstration that an increase in [K+]o caused an abrupt pHi increase. However, in salamander PTs, where such a depolarization-induced alkalinization (DIA) was first described, the DIA occurs in the nominal absence of CO2/HCO3− and, in fact, is mediated by electroneutral Na/lactate cotransport across the apical membrane, followed by H/lactate cotransport across the basolateral membrane (884, 885). The spinal cord neuron study did not include an analysis of the Na+ or HCO3− dependence of the DIA, nor of its sensitivity to DIDS. Thus one must exercise prudence in interpreting these data.
B) Possible role in cell migration. A localized regulatory volume increase (RVI) at the leading edge of lamellipodia in migrating cells is mainly mediated by NHE1 (910). However, on the basis of a residual migratory capability of NHE-deficient MDCK-F cells that is sensitive to the NCBT inhibitor S0859, NBCe1 has been suggested to be capable of making a minor contribution to migration (849). Because 1) S0859 has an untested specificity, 2) NBCe1 transcripts are scarce in these cells, 3) NBCe1 protein expression is undemonstrated in these cells, and 4) other NCBTs aside from NBCe1 may be expressed in these cells, the authors were not able to definitively link NBCe1 activity with cell migration (849).
Overexpression of CA IX on the extracellular surface promotes cell migration in MDCK cells (931). Furthermore, NBCe1 and CA IX immunoreactivity colocalize in a hypoxic A549 lung-tumor cell line (254). Thus it has been proposed that CA IX and NBCe1 form a “metabolon” in which CA IX activity (CO2 + H2O ↔ HCO3− + H+) is promoted by the action of NBCe1 that removes HCO3− from the cell surface (254, 931). The importance of CA IX in tumor pH regulation is reviewed in Ref. 934.
A study of wound repair in monolayers of a rat gastric epithelial cell line showed that the wound-healing process (i.e., cell migration) could be inhibited by DIDS or by the removal of Na+, Cl−, and/or HCO3− (369). Although the transport processes responsible for these phenomena remain unidentified, the authors detect both NBCe1 and AE2 transcripts in these cells (369).
In summary, although data are consistent with the appealing hypothesis that NBCe1 could support cell migration/tumor metastasis, the data are not conclusive and the role of NBCe1 in this regard could be minor compared with that of NHE1.
II) Central nervous system. A) Enhancement of neuronal excitability. Neuronal firing results in an intracellular acidification of neurons that tends to dampen neuronal excitability (reviewed in Refs. 76, 186, 187, and 898). The action of NCBTs in neurons, that as a population express at least NBCe1, NBCn1, NBCn2, and NDCBE, re-alkalinizes cells following firing events and thereby enhances the rate at which excitability recovers (FIGURE 24A). Indeed, mice lacking either of two other NCBTs, NDCBE and NBCn2, exhibit signs of reduced neuronal excitability.41 In hippocampal neurons under high-[K+]o conditions (a mimic of intense firing), the activity of NBCe1 is sufficiently strong that NBCe1 (and other factors) produce a depolarization-induced alkalinization that overwhelms the natural tendency toward intracellular acidification (932).
Figure 24.
Role of NCBTs in excitable cells. Neuronal firing causes a depolarization induced alkalinization (DIA), via NBCe1, that anticipates and counters the dampening of neuronal excitability by Ca2+ pump-mediated H+ influx. The three electroneutral NCBTs also play critical roles in restoring neuronal pHi after a firing event (A). K+ released by firing neurons is absorbed by astrocytes causing a DIA, via NBCe1, that stimulates glycolytic ATP production (B), anticipating the increased energetic demand of the astrocyte for secondary active neurotransmitter (NT) uptake via neurotransmitter/sodium symporters (NSS).
B) Dampening of neuronal excitability by astrocytes. As neurons fire action potentials, they release K+ into the extracellular microenvironment (FIGURE 24A). One effect of the resulting elevated [K+]o would be to enhance neuronal excitability. However, the action of the Na-K pump in astrocytes tends to remove this accumulated extracellular K+, thereby dampening neuronal firing. The Na-K pump also maintains a low astrocytic [Na+]i, thereby promoting Na+-coupled neurotransmitter uptake. A second effect of the elevated [K+]o is the stimulation of glycolysis in astrocytes via a feed-forward mechanism that anticipates the energy requirements of the astrocyte Na-K pump (83). The link between elevated [K+]o and the stimulation of astrocyte glycolysis appears to be NBCe1. Under conditions of intense neuronal activity, substantial K+ release would cause an NBCe1-dependent DIA in astrocytes (825). The consequent pH-dependent increase in the activity of glycolytic enzymes stimulates ATP production (FIGURE 24B). The importance of NBCe1 for this pathway is demonstrated by 1) the blockade of the pathway by S0859 and 2) the absence of this pathway from astrocytes cultured from neonatal NBCe1-null mice (825). As the action of NBCe1 produces the DIA in astrocytes, the concomitant decrease in extracellular pH would dampen neuronal excitability and decrease the ability of neuronal NCBTs to counter intracellular acidification, thereby preventing excessive firing (as in FIGURE 24A).
III) Sensory organs. A) Transepithelial HCO3− secretion across corneal endothelium. Working with a 1:2 stoichiometry (543) and importing HCO3− from the stroma (FIGURE 19) into the cell, NBCe1 in the corneal endothelium is in a position to make a substantial contribution to the basolateral step of transepithelial HCO3− secretion into the anterior chamber (i.e., aqueous humor). It is thought that this transcellular HCO3− movement drives fluid reabsorption from the stroma into the anterior chamber, thereby maintaining appropriate corneal hydration and transparency (579, 922, 923, 1035).42 The molecular mechanisms underlying this process (shown in FIGURE 19) are reviewed in Reference 96. Briefly, cytosolic HCO3− accumulates either as HCO3− enters the cell directly across the basolateral membrane via NBCe1-B, or as HCO3− forms from cytosolic CO2 (catalyzed by CA II) as Na-H exchangers extrude H+ across the basolateral membrane. Apical anion channels secrete HCO3− into the anterior chamber. Thus the action of NBCe1-B helps provide cytosolic HCO3− for secretion and also regulates pHi. Consistent with a contribution to fluid secretion by NBCe1, NBCe1 knockdown by siRNA reduces the transepithelial HCO3− flux in cultured bovine corneal endothelium (579). In one study, a partial in-vivo knockdown (i.e., 25%) of NBCe1 by shRNA in rabbit eyes was not sufficient to produce the expected corneal thickening without the additional pharmaceutical inhibition of carbonic anhydrases (593). Given the mild knockdown, perhaps this result is not surprising. However, even in NBCe1-null mice, the effect of NBCe1 deficiency on HCO3− secretion by colonic mucosa is detectable only following CA inhibition, even under secretagogue stimulated conditions.
B) Potential to promote retinal attachment. In the retinal pigment epithelium, NBCe1-B is present in the apical membrane (94). NBCe1-mediated uptake of HCO3− across the apical membrane would contribute to fluid absorption from the subretinal space to blood, presumably minimizing subretinal edema, as has been proposed for an apical electrogenic NCBT activity in bullfrogs (FIGURE 13A). Subretinal edema has not been described in Slc4a4-null mice nor in patients with NBCe1-associated pRTA, although it is possible that the presence of edema is masked by other ocular defects present in these individuals.
IV) Peripheral nervous system. A) Neuronal excitability. In primary cultures of neurons from the rat trigeminal ganglion, application of anti-NBCe1-B/C siRNA results in a ∼50% reduction of NBCe1-B/C protein abundance and, following an NH4+ prepulse, causes a near total elimination of HCO3−-dependent acid-extrusion in these cells (408). Thus NBCe1-B/C is likely to be the major NCBT in these cells. Furthermore, the frequency of action potential firing in response to current-injection in these cells is reduced by intracellular acidification and by DIDS treatment (408). Taken together, these data indicate that NBCe1-B/C mediates an uptake of HCO3− that counters the dampening effect of intracellular acidification (408), FIGURE 24A pe and thereby plays an important role in maintaining excitability in these neurons.
V) Circulatory system. A) Myocardial contractility and excitability. An NCBT-mediated increase in pHi enhances the contractility of myocardium in mammals (160, 702, 972). Moreover, HCO3−-dependent alkalinization is enhanced by repeated depolarizations of cat papillary muscle (147), consistent with the involvement of electrogenic NBCs. The current carried by electrogenic NCBT activity modulates the shape of myocyte action potentials, shortening action-potential duration and contributing towards a hyperpolarized resting membrane potential (14, 1006). Rat cardiac myocytes transfected with an adenoviral vector designed to overexpress NBCe1 are reported to exhibit an altered beat rate compared with nontransfected cells, although the direction of the rate change is not reported, and overexpression of NBCe1 transcripts or protein is not demonstrated (649). The relative contributions of NBCe1 and NBCe2 to these processes are unresolved. The influence of the action of NBCe1 and other acid-base transporters on cardiac myocyte function is reviewed in Reference 996.
VI) Musculoskeletal system. A) Myocyte contractility. By contributing towards pHi regulation in myocytes, NBCe1 likely contributes towards maintenance of contractility and excitability, as it does in cardiac myocytes (see above).
VII) Upper digestive system. A) Role in enamel deposition. The role of enamel organ epithelia in the formation of enamel in the enamel-surface compartment is still largely unknown. The apical membranes of ameloblasts face the enamel-surface compartment and, when mature, form alternating zones of ruffle-ended cells (facing enamel fluid that is acidic) and smooth-ended cells (facing enamel fluid that has a neutral pH).
NBCe1 dysfunction is associated with enamel defects. Two alternative models have been proposed to explain how NBCe1 contributes to enamel formation. Both models posit that NBCe1 supports AE2-mediated HCO3− secretion into the enamel surface compartment to buffer the H+ formed by apatite formation (706). However, the models differ in the location of NBCe1 and AE2, as well as in the consideration of how the enamel organ cells interact.
In the first model (not shown), ameloblasts express NBCe1 in their basolateral membrane and AE2 (unusually for an Slc4) in their apical membrane. The concerted action of NBCe1-mediated HCO3− influx and AE2-mediated HCO3− efflux are proposed to form a pathway that secretes HCO3− into the enamel-surface compartment (706).
The second model (shown in FIGURE 20) is based on an alternative distribution of NBCe1 and AE2, and considers that the papillary cells and ameloblasts form a syncytium (456). In this model, NBCe1 is present in the membranes of papillary cells that abut the basal surface of ameloblasts, and AE2 is located in the basolateral membranes of ameloblasts (126, 456, 617). During the morphological switch of ruffle-ended ameloblasts to smooth-ended ameloblasts (associated with neutralization of the enamel-surface compartment acidity) a rearrangement of tight junctions exposes the lateral, but not the basal, surface of these cells to the enamel-surface compartment. Thus the action of papillary cell NBCe1, translated via gap junctions to the cytoplasm of the ameloblasts, is still in a position to support ameloblast HCO3− secretion into the enamel fluid via AE2 (456). In an update to the second model, pendrin (Slc26a4) has been immunolocalized to the apical membranes of ameloblasts, providing an apical exit route for HCO3− (125). However, unlike mice with AE2 or NBCe1 dysfunction, mice with a pendrin deficiency do not exhibit obvious defects in enamel deposition (125).
B) Transepithelial fluid and HCO3− secretion in the parotid salivary gland. Acinar cells in the parotid glands secrete an isotonic fluid. The composition of the fluid is modified by duct cells that, among other functions, secrete proteins and HCO3− to produce the HCO3−-rich saliva that acts to optimize amylase activity and to buffer gastric juices. Working with a 1:2 stoichiometry and importing HCO3− into a cell, basolateral NBCe1 in parotid acinar cells is in a position to regulate pHi. Moreover, in concert with AE2, which would recycle HCO3− back into the interstitium, the NBCe1 could make a contribution to the basolateral step of transepithelial NaCl and fluid secretion (Ref. 710, as shown in FIGURE 21A).
In the duct cells, where basolateral NBCn1 is abundant (FIGURE 21B), ductal NBCe1 could contribute to the support of transepithelial secretion of HCO3−, via a mechanism similar to that described for NBCe1 in corneal endothelia (FIGURE 19). The role of basolateral NCBT activity in HCO3− and fluid secretion in salivary glands is reviewed in Reference 555.
C) Protection of gastric mucosa from acid attack. The presence of NBCe1 transcripts in mammalian stomach preparations suggests that NBCe1 could, as has been proposed for an electrogenic NCBT in amphibian gastric mucosa (FIGURE 13B), support HCO3− secretion into the mucus layer that covers the stomach lining, thereby protecting gastric epithelia from acid attack.
VIII) Lower digestive system. A) Transepithelial HCO3− secretion across pancreatic duct cells. Working with a 1:2 stoichiometry and importing HCO3− into a cell, NBCe1 in pancreatic duct cells can make a substantial contribution to the basolateral step of transepithelial HCO3− secretion, and thus fluid secretion (422, 883, 1096). The contribution of NBCe1 towards the formation of pancreatic juice by acinar and duct cells is likely identical to that shown in FIGURE 21 for salivary glands. Cytosolic HCO3− enters the cell either through NBCe1-B or is generated de novo by CA II action upon CO2. Apical Slc2643 proteins secrete HCO3− into the duct lumen in exchange for Cl−. When fully stimulated by secretagogues (e.g., secretin), the luminal fluid in humans can be near-isotonic NaHCO3. The alkaline duct fluid keeps the pancreatic digestive enzymes in an inactive state and flushes them from the ducts, both of which protect from pancreatitis. In addition, pancreatic juice neutralizes acidic gastric chyme. NBCe1 has also been suggested to contribute towards the endocrine function of the pancreatic islets. The role of basolateral NCBT activity in ductal fluid and HCO3− secretion is reviewed in Ref. 555.
B) Transepithelial HCO3− secretion across intestinal enterocytes. HCO3− secretion across duodenal enterocytes plays a major role in protecting mucosa from acid attack (17). Consistent with a role for NBCe1-B in HCO3− secretion throughout the gut (via a mechanism such as that shown for a duodenal villar enterocyte in FIGURE 22), the secretagogues carbachol and forskolin increase the basolateral abundance of NBCe1 protein in rat proximal jejunum enterocytes (430) and in murine colonic crypts (1087). Furthermore, both DIDS and siRNA knockdown of NBCe1 inhibit parathyroid-hormone–stimulated short-circuit currents, a measure of transepithelial anion secretion, across monolayers of a human intestinal epithelial-like cell line (172). On the other hand, compared with tissues from wild-type mice, proximal colons from NBCe1-null mice exhibit a reduced cAMP-stimulated HCO3− secretion only under conditions in which blockade of CA II severely curtails the generation of intracellular HCO3− from CO2 (313). At face value, these data are inconsistent with the idea that NBCe1 plays a major role in HCO3− secretion under physiological conditions. On the other hand, it is possible that the NBCe1-null mice may have upregulated the CA-dependent pathway by enhancing basolateral H+ extrusion via NHEs or NBCn1. At least in the duodena of mice, NBCn1 makes a more substantial contribution to HCO3− secretion than NBCe1.
C) Potential role in drug resistance of colon cancer cells. siRNA suppression of NBCe1 expression in a human colon carcinoma cell line increases sensitivity of the cells to the anti-cancer agent methotrexate (642), a phenomenon that the authors of the study hypothesize to be due to pH dependence of methotrexate uptake transporters.
D) Potential role in transepithelial HCO3− secretion across cholangiocytes in the liver. In cholangiocytes, immunolocalization studies seem to indicate that AE2 has an unusual apical disposition (46, 631, 855, 902, 971). Moreover, it has been proposed that this apical AE2 mediates HCO3− secretion into the bile duct lumen (46, 631, 902), thereby protecting the liver from bile acid attack (390). In the cholangiocytes of mice that are unable to express the a and b variants of AE2, NBCe1 transcript and protein abundance are increased compared with control cells from wild-type mice, as is an electrogenic NBC activity (987). As cholangiocytes of AE2a,b-null mice are able to compensate for their HCO3− secretion deficit via a Na+-dependent mechanism, it is suggested that NBCe1, again targeted to the apical rather than the basolateral membrane, might be able to compensate for a HCO3− secretion defect by operating with a 1:3 stoichiometry (987).44 Neither the apical presence of NBCe1 protein in mouse cholangiocytes nor the stoichiometry of the transport process in these cells has yet been demonstrated, although NBCe2 immunoreactivity has been detected in the apical membrane of rat cholangiocytes (8).
IX) Lymphatic and immune systems. The lymphatic and immune systems are not major sites of NBCe1 expression. We are unaware of any reports that assign a physiological role to NBCe1 in these systems.
X) Endocrine system. A) Possible role in HCO3− exit from pancreatic islet cells. Insulin-producing cells generate a substantial amount of CO2 that is linked to the production of insulin. One group suggests that the CO2 generated from nutrient insulin secretagogues (e.g., glucose) first is converted to HCO3− for exit across the plasma membrane (863). NBCe1 is expressed in both pancreatic islet cells and a related tumor cell line (135, 901). Although both NBCe1-A and NBCe1-B are present in islets, NBCe1-B predominates in the insulin-producing β cells. Treatment with tenidap (an inhibitor of NBCe1) reduces glucose metabolism, reduces glucose-stimulated insulin secretion, and also lowers pHi. The last observation is consistent with the hypothesis that NBCe1-B normally functions as an acid extruder (i.e., mediates HCO3− uptake) in these cells. However, tenidap also increased 22Na uptake and hyperpolarized the cells, which would be consistent with the opposite hypothesis: that NBCe1-B normally operates as an acid loader (i.e., mediating HCO3− efflux). It seems clear that NBCe1 is important for maintaining insulin secretion from pancreatic tissue, by promoting fluid secretion. However, the tenidap (which was developed by Pfizer as a nonsteroidal anti-inflammatory drug) probably has complex actions in these cells, witness the effects on 22Na fluxes and Vm. In any case, we would not expect the CO2 generated from the metabolism of nutrient secretagogues to exit the cell via NBCe1, which is presumably mediating the net uptake of HCO3−. Even in the renal PT, which generates large amounts of CO2 and in which NBCe1-A mediates HCO3− efflux, the most straightforward mechanism for the disposal of metabolically generated CO2 is the same as for other cells in the body: CO2 in the steady state moves passively across the cell membrane, perhaps via gas channels, and diffuses into systemic capillaries for disposal in the exhaled air. For a discussion of the contribution of NBCe1 to the digestive role of the pancreas, see above.
XI) Urinary system. A) HCO3− reabsorption across proximal tubule epithelia. As illustrated in FIGURE 23, NBCe1-A plays a central role in the transepithelial secretion of H+ by the renal PT. H+ extruded across the apical membrane has three fates, titrating: 1) HCO3− (filtered from blood in the glomerulus) to CO2 + H2O, 2) NH3 to NH4+, and 3) HPO42– (and weak bases other than HCO3− and NH3) to H2PO4– (and the conjugate weak acids of the other weak bases), the so-called titratable acidity. In the case of HCO3− reabsorption, the newly formed CO2 and H2O enter the PT and form HCO3−. In the case of NH4+ excretion and formation of titratable acidity, the intracellular HCO3− forms from CO2 that originates either from PT oxidative metabolism or from the blood. The common denominator is that NBCe1-A exports the HCO3− across the basolateral membrane.
The role of an electrogenic NCBT as the basolateral step in the pathway that reabsorbs HCO3− from the PT lumen was first demonstrated in salamanders in 1983 (103). Demonstration of the equivalent activity in mammals, namely, rabbits and rats, followed in an array of papers published between 1985 and 1987 (28, 80, 81, 331, 514, 832, 896, 1085). The subsequent cloning and characterization (809) as well as the immunolocalization of NBCe1 to the basolateral membranes of PT epithelia (632, 844) demonstrated that NBCe1 is indeed the transporter responsible for this activity.
XII) Reproductive system. A) Possible role in HCO3− reabsorption and/or secretion in the epididymis. The lumen of the epididymis is a site of Na+ reabsorption and H+ secretion (572), with low luminal pH being a requirement for storage of viable sperm. NBCe1-B is present in the cells of the epididymis, and cultured epididymal cells exhibit a DIDS-sensitive, Na+- and HCO3−-dependent pHi recovery from an acid load, leading several groups to suggest that NBCe1 might be involved in H+ secretion/HCO3− reabsorption by these cells (164, 445, 729, 1119). Mice deficient in the estrogen receptor ESRα (or ESR1) are defective in their ability to acidify the epididymal lumen. In the initial segment of the epididymis, these mice exhibit a ∼50% reduction in protein abundance of apical NHE3 and CA XIV, as well as basolateral NBCe1 (453). At present there is no direct evidence that NBCe1 plays a substantial role in epididymal HCO3− reabsorption in these cells. Relevant issues include: 1) the direction of NBCe1-mediated transport can vary in a tissue-specific manner (346) and physiological data to support an outwardly directed basolateral NCBT activity in these cells is presently lacking. 2) In order for a basolateral NBCe1-B to contribute to HCO3− reabsorption, it would presumably have to operate with a 1:3 stoichiometry, rather than the 1:2 stoichiometry that it has in pancreatic ducts (344). On the other hand, one report suggests that the stoichiometry of NBCe1-B might depend on the cell-type in which it is expressed (346). 3) NBCe1 immunoreactivity in the epididymis is not restricted to the acid-secreting narrow or clear cells (729). 4) AE2, a related acid-loading transporter, is also expressed in the basolateral membranes of epididymal epithelia (446). Mice that are unable to express the a, b1, and b2 variants of AE2 are infertile (638). Thus NBCe1-B is unable to compensate sufficiently in these knockouts.45 On the other hand, luminal H+ secretion from epididymal epithelia is stilbene-sensitive and independent of Cl− (120, 164, 1119). We conclude that the physiological role of NBCe1-B in epididymal H+ secretion/HCO3− reabsorption remains open. One possibility is that NBCe1-B plays a role in pHi regulation in epididymal epithelia. Another possibility is that NBCe1-B supports regulated HCO3− secretion in epididymal epithelia (152, 164), which could activate sperm mobility prior to ejaculation (697, 937).
B) Transepithelial HCO3− secretion across uterine epithelia. Basolateral NBCe1 is in a position to support HCO3− secretion across endometrium epithelia (1027) that secrete a HCO3−-rich uterine fluid, which is important for sperm capacitation and egg fertilization (e.g., see Ref. 554).
H) CAUSES OF NBCe1 UPREGULATION.
In this section we consider disturbances that result in upregulation of NBCe1 at the level of transcript abundance, protein abundance, translocation to the plasma membrane, or transporter activity. Note that an increase in any one of these factors need not necessarily correlate with an increase in the others.
The plasma-membrane abundance, as well as per-molecule activity, of NBCe1-B/C can be increased by activation of the soluble binding partner IRBIT. However, the physiological cues that activate IRBIT have not been described.
In the following discussion, we have omitted cellular studies that report only indirect evidence of NBCe1 upregulation (e.g., upregulation of HCO3− reabsorption) because such observations might at least in part be explained by effects on other proteins. We have arranged the reports in the order of the organ in which each observation was made and then in order of disturbances that are shown to increase NBCe1 transcript abundance, increase NBCe1 protein abundance, increase NBCe1 abundance in the plasma membrane, and stimulate NBCe1 activity.
I) Central nervous system. A) Increased transcript abundance following cerebral arterial occlusion. In rats subjected to permanent cerebral-artery occlusion, the abundance of NBCe1 protein in the ischemic penumbra is more than twice as great as in sham-operated controls (458). It is reasonable to suggest that this upregulation of NBCe1 leads to an increase in [Na+]i that could contribute to edema as well as other secondary brain injuries. Thus NBCe1 inhibitors have the potential to limit such ischemic damage (458).
B) Increased protein abundance following seizure induction. Seizure-sensitive and seizure-resistant gerbils exhibit similar expression patterns for NHE1 and NBCe1 immunoreactivity. However, 30 min and 180 min after the induction of seizures in the SS gerbils, the expression of both proteins increased markedly in the hippocampal CA1–3 regions and granule layer of the dentate gyrus (467). Also, NBCe1 protein levels are elevated in the hippocampi of gerbils 4 h after administration of the GABAB receptor agonist baclofen but not after administration of the GABAA receptor agonist muscimol (466). An elevation of NBCe1 protein abundance also occurs in gerbils treated with the GABA degradation inhibitor vigabatrin (466). Two critical issues not addressed in the aforementioned studies are the identity of the upregulated NBCe1 splice variant (i.e., NBCe1-B versus -C) and the identity of the cells in which it was upregulated. For example, in rat hippocampus, NBCe1-C is abundant in the astrocytes that surround the neuronal cell bodies in the pyramidal cell layer (624). If the seizure activity leads to an increase in NHE1 and NBCe1-C activity in astrocytes, that would lower extracellular pH and reduce neuronal excitability (reviewed in Refs. 186, 187, and 898). However, if the seizure activity leads to an increase in NHE1 and NBCe1-B in neurons, that would tend to increase neuronal excitability, which would be a maladaptive consequence of an attempt to protect neurons from acidosis.
II) Circulatory system. A) Increased transcript abundance and activity in heart following abdominal aortal constriction. NBCe1 (and NBCn1) transcript abundance increases in a rat model of ventricular hypertrophy (1071), generated by constriction of the abdominal aorta, and is accompanied by an increase in HCO3−-dependent acid extrusion in myocytes isolated from the hypertrophic ventricles. The authors suggest that NBCe1 contributes to an increased Na+ load in hypertrophic myocytes, promoting arrhythmia and reperfusion injury via activation of the Na-Ca exchanger (1071). The action of NBCe1 also could contribute towards the severity of the hypertrophy in myocytes, as described for NHE1 (676, 1061). Indeed, overexpression of cardiac NBCe1 may exacerbate reperfusion injury by contributing to ischemic [Na+]i overload (956).
B) Increased transcript and protein abundance in heart following terminal heart failure. Cardiac NBCe1 transcript and protein levels are both elevated in preparations from individuals that suffered terminal heart failure, although whether this is a cause or consequence of heart failure has yet to be established (481).46
C) Increased protein abundance in heart by chronic hypercapnia. In neonatal, but not adult mice, chronic (2 wk) exposure to 12% CO2 causes NBCe1 protein abundance to increase by ∼40% in heart, reflecting a general pattern of increased abundance of acid extruders (e.g., NBCn1 and NHE1), which may help to counter the acidifying effects of hypercapnia (463). Also in the kidney, hypercapnia increases NBCe1 protein abundance.
D) Potential stimulation of NBCe1 in response to ethanol-induced acidosis. The application of 30–1,000 mM ethanol to human atrial cardiac myocytes causes a graded fall in pHi and a modest stimulation of an unidentified HCO3−-dependent acid extruder (979), likely NBCe1. Note that even 30 mM ethanol is about twice the legal limit for alcohol intoxication in many jurisdictions. Moreover, the study did not take into consideration either the osmolality or reflection coefficient of ethanol.
E) Increased activity in cardiac myocytes in response to acidosis and/or angiotensin II. In cardiac myocytes (1072), acute intracellular acidosis stimulates an unidentified electrogenic NCBT, likely NBCe1, that contributes to pHi recovery. In infarcted rat hearts, acidosis increases the abundance of NBCe1 transcripts and protein in the left ventricular free wall via a pathway that involves a local renin-angiotensin system, including angiotensin converting enzyme, angiotensin II (ANG II), and stimulation of AT2 receptors (831). Stimulation of NCBT activity by 10−7 M ANG II via an AT2-dependent pathway has also been demonstrated in neonatal rat cardiac myocytes in which the stimulation can be mimicked by application of arachadonic acid (503).
Other studies report that the stimulatory effect of 10−7 M ANG II upon NCBT activity in rat (313)and cat cardiac myocytes is mediated by the AT1 receptor (58, 224), similar to the stimulation of NBCe1 functional expression in the proximal tubule. However, in cat cardiac myocytes, the phenomenon stimulated by ANG II is reported to represent stimulation of NBCn1 and inhibition of NBCe1.
We note in summary that the study of Sandmann and co-workers (831), in which ANG II stimulates NBCe1, is corroborated by molecular evidence of the increased NBCe1 abundance. On the other hand, the presence of NBCn1 in cardiac myocytes is not well demonstrated. Without invoking species differences, these studies are not readily reconciled.
III) Musculoskeletal system. A) Increased protein abundance in skeletal muscle following training. NBCe1 protein abundance is doubled in the soleus muscle (a predominantly oxidative organ), but not the extensor digitorum longus muscle (a predominantly glycolytic organ), of rats after 5 wk of interval training on a treadmill (964).
B) Increased protein abundance in skeletal muscle at high altitude. Human subjects that live at high altitude, or those who normally live at low altitude but move to high altitude for 8 wk, have double the abundance of NBCe147 protein in their skeletal muscle compared with individuals who live at sea level (457). The authors of the study suggest that this upregulation of NBCe1 as an acid extruder may reflect a mechanism that compensates for lower-than-normal arterial HCO3− (at rest), itself a compensation for the respiratory alkalosis produced by hyperventilation, measured in these individuals at high altitudes (457). If it turns out that CO32− is the substrate of NBCe1 (p. 56), then a critical question is whether the combination of the uncompensated alkalosis (i.e., high pHo) and compensatory low [HCO3−]o results in a depressed [CO32−]o.
IV) Upper digestive system. A) Increased transcript abundance in ameloblast-like cells maintained at acidic pH. LS8 cells are derived from the enamel organ of embryonic mice. In LS8 cells maintained for 24 h in acidic medium, NBCe1 transcripts are more abundant than in LS8 cells maintained in an alkaline medium (706, 891). This phenomenon is controlled by a pH-responsive enhancer region in the NBCe1-B/C promoter. If the increased abundance of NBCe1-B transcripts in LS8 cells translates to an increase in NBCe1 acid-extruding activity (see FIGURE 20), enamel-organ cells exposed to acid should be adapted to 1) defend pHi from acidosis and 2) secrete HCO3− into acidic enamel fluid.
B) Increased transcript abundance during dark periods. Investigators studying the circadian rhythm of transcript abundance in mouse molars determined that NBCe1 transcript abundance is nearly doubled during dark periods compared with light periods (537). The authors suggest that this observation correlates with periods of enhanced enamel deposition by ameloblasts (537).
V) Lower digestive system. A) Increased protein abundance in the plasma membrane of colonic mucosa by secretagogues. The application of forskolin, which raises [cAMP]i, stimulates colonic HCO3− secretion in vivo, without increasing NBCe1 transcript abundance (55), by enhancing the accumulation of NBCe1-B protein in the plasma membrane (1087). In contrast, forskolin inhibits heterologously expressed NBCe1-B activity in a renal cell line (54).
VI) Urinary system. A) Increased transcript abundance by chronic dexamethasone treatment. Glucocorticoid excess causes a metabolic alkalosis associated with enhanced renal HCO3− reabsorption. Consistent with this phenomenon, a 4-day period of dexamethasone treatment results in a doubling of NBCe1 transcript abundance in the renal cortex of rats (21).
B) Increased transcript and protein abundance following birth. In mice, NBCe1 transcript and protein abundance increases in the PT following birth (in this study, day 3 to day 18), coordinated with the upregulation of other renal ion transporters, such as NHE3, and concomitant with a drop in urinary pH over the same time period (97).
C) Increased transcript and protein abundance following renal transplant rejection. NBCe1 transcripts and protein levels are increased in PTs of transplanted rat kidneys following acute rejection (999). The significance of these findings is presently unclear.
D) Increased transcript abundance in Aadc-null mice. Mice with a PT-specific deletion of aromatic amino acid decarboxylase (AADC) exhibit elevated NBCe1 mRNA abundance (1094). Because AADC catalyzes the final step in dopamine synthesis, and because dopamine reduces Na/HCO3 cotransport activity, these observations suggest that it is intrarenal dopamine-signaling pathways that normally limit NBCe1 abundance.
E) Increased transcript abundance and stimulation of activity in K+-deprived rats. Increased NBCe1 transcript abundance and elevated NCBT activity has been described in the PT and medullary thick ascending limb (mTAL) of K+-deprived (KD) rats. Upregulation of HCO3− reabsorption by these tubule segments has been implicated in the pathogenesis of whole-body alkalosis in KD animals (38, 894). For the mTAL, this hypothesis requires that the NBCe1, the splice variant of which is unknown, be basolateral and operate in the HCO3−-outward direction.
The medulla is not usually associated with NBCe1 expression (38); mTAL epithelia normally only express the relatively DIDS-insensitive NBCn1. In the renal medulla of KD rats, NBCe1 transcript abundance is increased approximately fourfold, and an unusual DIDS-sensitive NCBT activity is detected in mTAL epithelia (38). A whole-kidney intracellular acidosis, measured by 31P-NMR, has also been noted in KD rats (10). To the extent that this “renal” pHi decrease reflects a fall in the pHi of PT cells, it would be consistent with an increase in basolateral NBCe1-A activity, which would in turn tend to alkalinize the blood.
F) Increased protein abundance by chronic norepinephrine treatment. Consistent with a potentially causative role in norepinephrine-promoted Na+ retention, NBCe1 protein abundance is doubled in the renal cortex of norepinephrine-infused rats, as is the abundance of two other Na+ transporters, namely, the Na/K/Cl cotransporter 2 (NKCC2) and NHE3 (899).
G) Increased protein abundance in hypovolemic rats. The observed increase in NBCe1 and NHE3 protein abundance in the PT epithelia of hypovolemic (i.e., volume depleted) rats might contribute to the increased urine acidity in these animals (620). These changes would promote fluid reabsorption and thus be a reasonable adaptation to hypovolemia.
H) Increased protein abundance in some spontaneously hypertensive rats. Dopamine normally reduces Na/HCO3 cotransport activity in the proximal tubules of rabbits and rats (524). In a particular strain of spontaneously hypertensive rats (SHRs), an undetermined defect in the DA1 dopamine receptor leaves presumed NBCe1 activity unresponsive to downregulation by dopamine (524), thereby limiting the ability of the PT to reduce Na+ reabsorption (405). Two other reports that directly address perturbation of NBCe1 in SHRs appear to differ in their findings.
According to the first report, NBCe1 protein abundance is doubled in the renal cortex of SHR compared with control rats (900). With the assumption that this change corresponds to an increase in functional NBCe1 activity, the result would be increased Na+ reabsorption, which would be expected to contribute toward a hypertensive phenotype.
According to the second report, a study of immortalized PT epithelia from SHRs, NBCe1 transcript abundance and NCBT activity are reduced (731).48 These results may seem counterintuitive because downregulation of NBCe1 would tend to reduce, not increase, Na+ reabsorption. However, reduction of NBCe1 in these immortalized cells may reflect an adaptation to hypertension that occurred in the donor SHR rat.
Possible explanations for the apparent discrepancy between the two studies include 1) differences between rat tissue and immortalized cell lines, 2) variability in the genetic basis of hypertension (e.g., in one case NBCe1 contributes to hypertension whereas in the other it opposes it) between populations of SHR rats (672), and 3) an increase in NBCe1 protein abundance may not result in an increase in NCBT activity.
I) Increased protein abundance in the kidney during chronic hypercapnia. In adult rats, chronic (10 day) exposure to 8% CO2, 13% O2 (i.e., hypoxic hypercapnia) results in a near doubling of NBCe1 protein abundance in the PT (226). This observation is consistent with the findings of an earlier study of cultured rat proximal tubules cells in which stimulation of Na/HCO3 cotransport activity by respiratory acidosis was prevented by treatment with inhibitors of protein synthesis (824). In neonatal, but not adult mice, chronic (2 wk) exposure to 12% CO2 causes NBCe1 protein abundance to increase by ∼20% in kidney (463). These responses reflect a general pattern of increased abundance of acid extruders (e.g., NBCn1 and NHE1), which may help to counter the acidifying effects of hypercapnia (463). Hypercapnia also increases NBCe1 protein abundance in the heart (p. 877).
J) Increased plasma membrane abundance in the kidney in response to ANG II. In the proximal tubule, low doses of ANG II stimulate reabsorption of HCO3− (JHCO3) and Na+ (e.g., see Refs. 367, 394, and 1106). A 15-min application of 10−10 M ANG II to a polarized monolayer of immortalized renal epithelial cells from opossums (OK cells) increases the basolateral plasma membrane abundance of NBCe1 (802). The enhancement of NBCe1 functional expression by ANG II in these cells is blocked by antagonists of the AT1 receptor, blockers of Src family tyrosine kinases, and blockers of the mitogen-associated protein kinase (MAPK) signaling pathway (802).49 Furthermore, the ANG II–induced increase in JHCO3 is absent in AT1A receptor-null mice (394, 1100). As shown in studies of perfused tubules, and as modeled in Xenopus oocytes, the effects of ANG II are biphasic; low concentrations (10−10 and 10−11 M) of ANG II are stimulatory to NBCe1 functional expression, whereas higher concentrations are inhibitory (367, 394, 738, 739).
In isolated perfused rabbit proximal tubules, acute isolated increases in basolateral [CO2] or isolated decreases in basolateral [HCO3−] cause an increase in JHCO3 (1108). This response requires the secretion of local ANG II into the tubule lumen (1104) and is blocked by inhibition or knockout of luminal AT1 receptors (1107).
Note that agonism of the M1 muscarinic receptor, another G protein-coupled receptor, is associated with an increase in Na+ and HCO3− reabsorption by the PT (823). The action of the non-receptor tyrosine kinase Pyk2 appears to be a common factor in the costimulation of NBCe1 and NHE3 activity by GPCR agonists and by acidosis (277, 582).
ANG II also enhances functional expression of NBCe1 in the heart via an AT2-dependent pathway that appears to share commonality with the pathway that upregulates NBCe1 in response to acidosis (see p. 866).
K) Stimulation of activity in response to acidosis. In the renal cortex of rats, NBCe1-A transcript (140) and protein (36, 484, 530) levels are unperturbed by NH4Cl-induced acidosis. However, consistent with a model in which existing NBCe1 protein is activated, NBCe1 activity is increased in acidotic rats (757) and rabbits (15), isolated basolateral membrane vesicles prepared from suspensions of rabbit PTs subjected to metabolic acidosis (895), and immortalized rat PT epithelia treated with NH4Cl (731).
Transcript abundance of an unidentified NBCe1 variant in an inner medullary collecting duct (IMCD) cell line is decreased by acid stress (1029); however, the IMCD is not a site of substantial NBCe1 expression. Acidosis also stimulates NBCe1 activity in the heart.
L) Stimulation of activity in PT by chronic hyperfiltration. In rats, following removal of a kidney, the remaining kidney experiences a ∼50% increase in glomerular filtration rate. In addition, the PTs from the remnant kidney adapt (2 wk after surgery) by doubling the functional expression of both apical Na-H exchange and basolateral Na/HCO3 cotransport (758).50 This adaptation does not appear to be an acute effect of acidosis inasmuch as blood pH was not different from that of control rats (758). Whether this upregulation of NBCe1 activity is accompanied by an increase in the abundance of NBCe1 transcripts and/or protein is not documented in this study. However, in rats in which one ureter is partially obstructed within 48 h of birth, NBCe1 protein abundance is doubled after 7 wk in both the obstructed kidney and unobstructed kidney (1025).
XIII) Stimulation of functional expression in PT by thiazolidinediones. Drugs such as pioglitazone (PGZ) and rosiglitazone (RGZ), agonists of peroxisome proliferator-activated receptor gamma (PPARγ), are used to increase insulin sensitivity in patients with type II diabetes. Thiazolidinedione (TZD) use is associated with an expansion of plasma volume that is hypothesized to be due to increased renal solute reabsorption (272). Endo and co-workers (272) report that PGZ and RGZ stimulate basolateral HCO3− transport in rabbit PTs via a PPARγ-dependent pathway that also increases the phosphorylation of Src family kinases and MAPK. Although the mechanism of increased basolateral HCO3− transport in response to TZD treatment is untested in this instance, other studies link the activation of Src and MAPK pathways with an increase in the plasma membrane abundance of NBCe1 in the PT (see p. 868).
I) CAUSES OF NBCe1 DOWNREGULATION.
In this section we consider disturbances that result in downregulation of NBCe1 either at the level of transcript abundance, protein abundance, translocation to the plasma membrane, or transporter activity. Note that a decrease in any one of these factors need not necessarily correlate with a decrease in the others. We have omitted cellular studies that report only indirect evidence of NBCe1 downregulation (e.g., inhibition of HCO3− reabsorption) because such observations might at least in part be explained by effects on other proteins. We have arranged the reports in the order of the organ in which each observation was made and then in order of disturbances that are shown to reduce NBCe1 transcript abundance, reduce NBCe1 protein abundance, reduce translocation to the plasma membrane, and reduce NBCe1 activity.
I) Central nervous system. A) Reduced protein abundance in brain in response to intermittent hypoxia. Chronic intermittent hypoxia (CIH), a model for sleep apnea, appeared to decrease NBCe1 protein abundance in the cerebellum as assessed with an antibody that should not discriminate among NBCe1 variants (261). On the other hand, antibodies specific for NBCe1-A/B and NBCe1-C did not reveal statistically significant changes in the cerebellum.
II) Circulatory system. A) Potentially reduced activity in cardiac myocytes in response to angiotensin II. Although studies from two groups of investigators are consistent with upregulation of NBCe1 in rat cardiac myocytes by ANG II (via an AT2-dependent pathway), studies by a third group are consistent with downregulation of an NBCe1-like activity by the same concentration of ANG II in cat cardiac myocytes (via an AT1-dependent pathway). These observations are not readily reconciled, but could be explained by species differences.
III) Upper digestive system. A) Reduced transcript abundance in ameloblast-like cells maintained at alkaline pH. NBCe1 transcripts are less abundant in LS8 cells (derived from the enamel organ of embryonic mice) that are maintained for 24 h in alkaline medium (891). This adaptation is the counterpart to the upregulation of NBCe1 in response to decreased extracellular pH, which is proposed to support ameloblast function.
IV) Lower digestive system. A) Downregulation in jejunum by chronic gamma-irradiation. Diarrhea such as that associated with radiation exposure follows a decrease in anion (and thus fluid) absorption and/or an increase in anion/fluid secretion. In the case of secretagogue-induced diarrhea, anion secretions are rich in Cl− and HCO3−, supported by upregulation in the gut of NKCC1 (211) and NBCe1. However, in gamma-irradiated mice, increased anion secretion is effected with a seemingly counterintuitive reduction in HCO3− secretion (1093). In the jejuna of these mice, NBCe1-A/B immunoreactivity is lost, reducing support for HCO3− secretion from jejunal enterocytes (1093). An accompanying increase in NKCC1 immunoreactivity in the jejuna of irradiated mice suggests that increased Cl− uptake across the basolateral membrane may compensate for decreased anion secretion support from NBCe1 insufficiency, producing Cl−–rich secretions (1093).
B) Suggested reduction of activity in pancreas by elevated glucose levels. In isolated, perfused pancreatic ducts, high levels of luminal glucose lead to an accumulation of Na+ and membrane depolarization, via the action of the apical Na/glucose cotransporter SGLT1, in duct cells (307). The authors of the study suggest that elevated [Na+]i inhibits NBCe1-mediated influx of Na+ and HCO3− at the basolateral membrane, thereby contributing to the decreased pancreatic HCO3− secretion associated with diabetes. On the other hand, the authors suggest that the depolarization, via an inhibitory effect upon CFTR, could be a more important factor in reducing HCO3− secretion. Depolarization would be expected to enhance NBCe1 activity. This model requires that elevated serum [glucose] results in an elevated luminal [glucose] via an undetermined transcellular glucose transport pathway.
C) Downregulation by microRNAs in colon carcinoma cells. In the human colon carcinoma cell line HT29 (642), the microRNA miR-224 is under-represented. The 3′-UTR of NBCe1 transcripts is a potential target of miR-224 (642). Consistent with this hypothesis, NBCe1 transcript abundance are unusually abundant in HT29 cells.
V) Endocrine system. A) Decreased transcript abundance in thyroid cancer. Two studies report a greater than threefold reduction in NBCe1 transcript abundance in papillary thyroid carcinoma compared with normal thyroid tissue isolated from the same patients (309, 486). With the assumption that this downregulation correlates with a decrease in functional expression, and that in these cancer cells NBCe1 operates in the HCO3−-inward direction, these changes, which would tend to lower pHi, might be predicted to harm the cancer cell rather than encourage tumor proliferation (see below for a discussion of the reported downregulation of NBCn1 in breast cancer).
VI) Urinary system. A) Decreased transcript and protein abundance and reduction of activity in kidneys of Na+-loaded and alkalotic animals. In the PT epithelia of rats whose drinking water is spiked with NaHCO3 or NaCl (thereby producing a Na+ load that could make the animal hypervolemic), NBCe1 transcript (140) and protein (36, 620) abundance is reduced. These adaptations would tend to reduce Na+ reabsorption and thus tend to oppose the development of hypertension. The adaptations would also tend to reduce HCO3− reabsorption, which in the case of NaCl feeding might render the animals less able to respond to an acute acid load. In the case of NaHCO3 feeding, the adaption would oppose whole body alkalosis (37). In rabbits, Cl−-depletion alkalosis (CDA), which presumably results in volume contraction, causes a fall of NBCe1 activity in basolateral renal cortical membrane preparations (15). On the other hand, in rats, a similar stress has no effect on NBCe1 transcript levels in the PT (140). If these two observations can be taken together, they are consistent with the hypotheses that 1) the regulation of NBCe1 activity by alkalosis is purely posttranslational or 2) the response to CDA is species specific.
B) Decreased transcript abundance and reduction of activity in PT of some spontaneously hypertensive rats. As discussed and contrasted above, although some spontaneously hypertensive rats exhibit increased NBCe1 protein abundance, authors of a separate study report reduced NBCe1 transcript abundance and reduced NBCe1 activity in another population of SHRs. Inasmuch as reduced NBCe1 activity would tend to counter hypertension by reducing Na+ reabsorption, these reductions are more likely to be a consequence than a cause of hypertension in these animals.
C) Reduced protein abundance in response to hypoxia. An immunohistochemical study of mouse kidney slices appears to show, although the authors of that study do not specifically comment on this phenomenon, a reduced abundance of NBCe1 protein in the basolateral membranes of PT epithelia in slices that are briefly (10 s to 2 min) exposed to hypoxia prior to cryofixation (829).
D) Decreased protein abundance in kidney following ureteral obstruction. Following a 24-h bilateral ureteral occlusion in rats, NBCe1 and NHE3 protein abundance is substantially decreased in the PTs (1024). This downregulation may partly explain the phenomenon of obstruction-induced renal tubular acidosis. In rats in which only one ureter is partially obstructed within 48 h of birth, NBCe1 protein abundance is doubled after 7 wk in both the obstructed kidney and unobstructed kidney (1025). On the other hand, after 14 wk, the obstructed kidney exhibits a ∼40% decrease in NBCe1 protein abundance, whereas the abundance of NBCe1 in the unobstructed kidney appears to be close to normal (1025).
E) Decreased protein abundance in kidney during gentamycin-induced nephropathy. Treatment of bacterial infections with gentamycin can cause PT dysfunction in humans (reviewed in Ref. 308). In rats treated with gentamycin for 7 days, renal NBCe1 protein abundance is decreased by half (57). The abundance of other renal Na+ transporters, namely, NHE3 and the Na-K pump, and the water/carbon dioxide channel AQP1 are also reduced under these conditions (57).
J) CONSEQUENCES OF NBCe1 DYSFUNCTION.
In this section we consider the pathologies that are associated with genetic ablation of, genetic alterations in, and dysfunction of NBCe1 in the brain, eye, heart, enamel organ, lower digestive system, and kidney. The primary syndrome associated with NBCe1 dysfunction is proximal renal tubular acidosis. These data are mostly obtained from clinical studies of individuals with defects in the SLC4A4 gene, genetic linkage studies, and studies of genetically altered mice.
I) Central nervous system. A) Mental retardation, migraine, and epilepsy. Many individuals with defects in the SLC4A4 gene present with mental retardation (see TABLE 6). The presence of this trait in individuals with the Q29X mutation, which is predicted to abrogate renal (i.e., NBCe1-A) but not neuronal or glial NBCe1 expression (i.e., NBCe1-B and -C), suggests that mental retardation can be secondary to whole body acidosis that is the signature of pRTA.
Migraine is a symptom associated with many different NBCe1 mutations that cause pRTA (930), although the precise molecular mechanism underlying this pathology is unknown. At least in one case of two sisters, it appears that a 65-bp deletion affecting the Ct of all NBCe1 variants is also associated with epilepsy (72, 930).
In a separate study, genetic-linkage analysis mapped a familial temporal lobe epilepsy (including “déjà vu” auras) to a chromosomal locus that includes SLC4A4, although sequencing of SLC4A4 exons in two affected individuals did not reveal any genetic abnormalities (374). However, the possibility of a linkage remains open as the promoter regions of SLC4A4 were not included in this analysis and it is not disclosed whether the exons of all NBCe1 variants were sequenced.
II) Sensory organs. A) Glaucoma, band keratopathy, cataracts, and corneal edema. Many individuals presenting with pRTA have ocular defects ranging from glaucoma and band keratopathy to total blindness (TABLE 6). The widespread expression of NBCe1 throughout the eye as well as the absence of certain ocular phenotypes in some individuals with pRTA indicate that ocular phenotypes need not necessarily be secondary to whole body acidosis.
In these patients, it is not clear what causes the high-tension glaucoma, a buildup of aqueous humor that causes an increase in intraocular pressure. The two major forms of glaucoma in the general population are due to decreased disposal of the aqueous humor, ultimately via the trabecular meshwork in the anterior chamber. As noted earlier, NBCe1 has been detected in the trabecular meshwork (989), but the role of NBCe1 in this tissue is untested. Glaucoma is not a feature associated with pRTA in individuals with either the T485S or G486R mutations (TABLE 6).
The pathogenesis of cataracts in some individuals with SLC4A4 defects could be caused by defective Na+ transport or pHi regulation in lens epithelial cells. Perturbations of either process are associated with increased lens opacity (65, 240, 732). Cataracts are not a feature associated with pRTA in the individual with the Q29X mutation that is specific to NBCe1-A (TABLE 6).
Band keratopathy, corneal opacity caused by the deposition of Ca2+ salt can be secondary to renal failure (briefly reviewed in Ref. 149). In one case, a 12-yr-old girl with a defect only in (renal) NBCe1-A variant did not have band keratopathy (412), consistent with variable penetrance or with the hypothesis that it is specifically a defect in NBCe1-B and -C in the corneal endothelium that causes the band keratopathy. If HCO3− secretion across the corneal endothelium and into the aqueous humor were compromised, a localized elevation of [HCO3−] in the subepithelial region of the corneal stroma might be expected to enhance the deposition of Ca2+ salts (928, 989). Band keratopathy is not a feature associated with pRTA in individuals with the Q29X or R881C mutations (TABLE 6).
The eyes of NBCe1-null mice appear normal. These mice die at 4 wk (313) before any ocular defects are evident. However, in a line of transgenic mice that express the mouse ortholog of the human NBCe1/W516X pRTA mutant (FIGURE 25 and TABLE 6), corneal opacity and edema are evident when these mice are kept alive beyond week 7 by administration of HCO3− (602).
III) Peripheral nervous system. We are unaware of any reports of peripheral nervous system dysfunction associated with NBCe1 mutations.
IV) Circulatory system. A) Possible contribution to ischemic and reperfusion injury in the heart. As discussed earlier, humans with cerebral artery occlusion, abdominal aorta constriction, or heart failure all appear to have elevated NBCe1 abundance. It is undemonstrated whether ischemic and reperfusion injuries are a cause or consequence of NBCe1 upregulation, but it is possible that elevated NBCe1 levels could increase the risk factor for ischemic injury and heart failure. Indeed, Giffard and co-workers (318) observed that overexpressing NBCe1-B in the 3T3 fibroblast cell line renders these cells susceptible to acid injury in the presence of HCO3−, perhaps via Na+-loading and thence Ca2+-loading via a Na-Ca exchanger (318). Many studies have suggested that NBCe1 inhibitors could be cardioprotective (225, 481, 840).
V) Musculoskeletal system. A) Growth retardation. Individuals with defects in NBCe1 typically exhibit below average height and weight (see TABLE 6), and mouse models of NBCe1-associated pRTA exhibit bone dysplasia (313, 602) and reduced muscle mass (602). NBCe1 is not known to be directly involved in bone remodeling, and it is possible that these signs are secondary to the whole body acidosis that accompanies pRTA.
B) Hypokalemic paralysis. An individual with the A799V mutation in NBCe1 exhibited hypokalemic paraplegia (231). It is notable that the mutant NBCe1, when expressed in Xenopus oocytes exhibits a HCO3−-independent ion leak (721). Such a leak could in principle contribute towards the observed paralysis, as has been suggested in the case of unusual ion leaks though Na+ and Ca2+ channels (293, 1047). Under hypokalemic conditions, the conductance of inward-rectifier K+ channels in muscle cells is reduced, destabilizing their membrane potential. In this situation, small pathological currents can make a disproportionately large contribution to resting Vm, and the prolonged depolarization of the cells that results, inactivates voltage-gated Na+ channels (820).
VI) Upper digestive system. A) Compromised enamel deposition. The teeth of humans with a mutant SLC4A4 gene (TABLE 6), and of mice with a disrupted Slc4a4 gene (313, 538), exhibit signs of defective enamel deposition. NBCe1 is expressed in the enamel organ (456, 538, 706), and CO32− is an important constituent of enamel (reviewed in Ref. 564). However, the precise role of NBCe1 in the process of enamel remodeling (see FIGURE 20) has yet to be elucidated. It is possible that the defect is not secondary to whole body acidosis, inasmuch as unusual dentition was not noted in the description of a 12-yr-old girl who was predicted to lack only the renal variant of NBCe1 and unaffected ameloblastic NBCe1 (412). Furthermore, the defect is unlikely to be secondary to a reduced salivary pH because, in mice, the defect is noted in preerupted teeth (538). The likely importance of NBCe1 for correct enamel deposition was recently reviewed by Urzúa et al. (988).
VII) Lower digestive system. A) Intestinal obstruction. Although individuals with SLC4A4 defects are not reported to have intestinal problems, genetic linkage analysis has indicated that NBCe1 could contribute to the severity of ileal obstruction in newborns with cystic fibrosis (257). Indeed, NBCe1-null mice have small ceca and those that survive beyond 20 days have impacted terminal ilea, ceca, and colons (313), a phenomena also reported in the W516X mouse model of pRTA (602). However, the defective net ion secretion that is observed in isolated colons from NBCe1-null mice could be explained by increased fluid absorption due to dysregulation of other ion transporters such as ENaC and NKCC, rather than by decreased fluid secretion due to a lack of NBCe1 per se (313).
B) Possible signs of pancreatitis. The abundance of NBCe1-B in the pancreas suggests that this protein plays a major role in HCO3−, and consequently fluid, secretion by this organ. Dysfunction of NBCe1-B, like dysfunction of CFTR (e.g., Ref. 845), might therefore result in pancreatitis. Although some individuals with NBCe1-associated pRTA exhibit molecular signs of pancreas dysfunction, such as elevated serum amylase and lipase (TABLE 6), clinical signs of pancreatitis have not been reported. NBCe1-null mice have a normal pancreatic histopathology, although, as the authors of that study note, these young mice51 may have been examined prior to development of pancreatic pathology. In addition, the mouse pancreas is known to be a poor model for human pancreatic insufficiency (313). Taken together, these observations suggest that development of pancreatitis due to NBCe1 dysfunction may be age-dependent, or prevented by an as-yet-unidentified mechanism. For example, NBCn1 could compensate for NBCe1 loss in the basolateral membranes of exocrine duct cells (FIGURE 21).
C) Potential contribution to diarrhea. In the colon, NBCe1 action could support HCO3− secretion and thereby promote Cl− absorption via the apical Cl-HCO3 exchanger Slc26a3 (DRA, see Ref. 388) and the basolateral chloride channel ClC-2 (see Ref. 159). If this hypothesis is correct, then NBCe1 dysfunction is expected to reduce fluid absorption and promote diarrhea. However, the colons of NBCe1-null mice do not exhibit fluid absorption defects perhaps due to the documented dysregulation of other ion transporters in the colons of these mice (313).
VIII) Lymphatic and immune systems. Mice with a disrupted Slc4a4 gene exhibit severe splenomegaly with an increased count of nucleated red blood cells (313, 602). However, the spleen is not a major site of NBCe1 expression, and both phenomena are considered to be secondary to whole body acidosis (313, 602).
IX) Urinary system. A) Proximal renal tubular acidosis. Twelve homozygous mutations have been described in the SLC4A4 gene in individuals with proximal renal tubular acidosis (pRTA; see TABLE 6 AND FIGURE 25). As expected, considering the role of NBCe1 in the kidney, pRTA is characterized by an impaired ability of the PT epithelium to reabsorb HCO3−, leading to a whole body metabolic acidosis. In individuals with defective NBCe1, plasma pH (7.08–7.23) and plasma [HCO3−] (5.6–15 mM) are both below the normal range. A genetic defect specific to NBCe1-A “Q29X” (FIGURE 25 AND TABLE 6) is predicted to produce no functional NBCe1-A transporter, but not to affect the production or activity of either the NBCe1-B or -C variants (412). Two further nonsense and nine missense mutations have also been identified in the SLC4A4 gene in individuals with pRTA (see TABLE 6). The genetic linkage between defective NBCe1 and pRTA is further strengthened by the demonstration of whole body acidosis in Slc4a4-null mice, which die shortly after weaning (313), and in a transgenic mouse model of pRTA that carries the human NBCe1/W516X pRTA-associated mutation (602). It is likely that at least some of the nonrenal sequelae associated with pRTA, such as retarded growth and mental retardation, may in part be secondary to the whole-body metabolic acidosis.
B) Hypertension. Two independent genome-wide association studies reported in Reference 1077 link the SLC4A4 gene locus with hypertension in individuals from China. However, the precise location of hypertension-associated markers within SLC4A4 is not stated.
Mice that lack aromatic amino acid decarboxylase (AADC) in their PTs develop salt-sensitive hypertension. The kidneys of these mice exhibit elevated mRNA abundance of a number of transporters involved in Na+ reabsorption, including NBCe1 mRNA (1094). However, because of the complex effects of AADC disruption, the specific contribution of NBCe1 to hypertension in these mice is difficult to assess. It is noteworthy that one strain of spontaneously hypertensive rat exhibits elevated renal NBCe1 abundance (900).
X) Reproductive system. A) Expected, but not demonstrated, reduction in fertility. Based on the putative role of NBCe1 in the reproductive tracts of males (i.e., maintenance and activation of sperm) and females (i.e., enhancing sperm fertilizing capacity), it is possible that NBCe1 defects could be associated with a loss of fertility. However, this remains unverified as Slc4a4-null mice do not survive to breeding age (313) and the fertility of humans with NBCe1-associated pRTA remains unreported.
2. NBCe2 (Slc4a5)
A) SUMMARY.
The electrogenic Na/HCO3 cotransporter NBCe2 (encoded by the Slc4a5 gene) is present in many organ systems throughout the body but is notably abundant in the choroid plexus, where NBCe2 contributes towards HCO3−/fluid (i.e., CSF) secretion into the brain ventricles, and the liver, where robust expression of NBCe2 likely maintains hepatocyte pHi.
Consistent with its proposed role in the choroid plexus, one strain of Slc4a5-null mouse exhibits a CSF secretion defect that likely contributes to the reduced neuronal excitability observed in these animals. In humans, multiple studies report genetic linkage between the SLC4A5 locus and blood pressure traits. Although the physiopathology underlying this linkage is presently unclear, Slc4a5-null mice exhibit elevated blood pressure. Little is known about the regulation of the Slc4a5 gene or products and is the only one of the five NCBTs that has not been demonstrated to be stimulated by the cytosolic protein IRBIT. NBCe2 has only one variant, NBCe2-c, that is known to be functional.
B) NOMENCLATURE OF Slc4A5 PRODUCTS.
The Slc4a5 gene product was initially called NBC4 (767), being the fourth member of the gene family to be identified at the molecular level. The gene product has since been renamed NBCe2 (1009) to reflect its characterization as the second electrogenic member of the family (835, 1009).
Six variant products have been reported (NBC4a-f), but only two (NBC4a and NBC4c) seem likely to produce a functional transporter. We provisionally refer to these as NBCe2-a and NBCe2-c (note the lowercase “a” and “c”).
C) MOLECULAR ACTION OF NBCE2.
Overexpressed in HEK-293T or mPCT renal cells (835) and Xenopus oocytes (1009), NBCe2-c mediates a reversible, DIDS-sensitive, and Na+-dependent HCO3− transport that is accompanied by a Na+- and HCO3−-dependent conductance. Thus NBCe2 is an electrogenic Na/HCO3 cotransporter (FIGURE 16). Furthermore, in oocytes, NBCe2-mediated HCO3− efflux does not require extracellular Cl− and thus NBCe2 is not a Na+-driven Cl-HCO3 exchanger (1009). In mPCT cells (a mouse PT cell line) that are overexpressing NBCe2 (835), and in mouse choroid plexus epithelia (645), NBCe2 operates with a Na+:HCO3− stoichiometry of 1:3. However, when heterologously expressed in oocytes (1009) or HEK-293 cells (869), NBCe2 operates with a 1:2 stoichiometry. This cell-dependent stoichiometry is also characteristic of NBCe1. As well as being blocked by DIDS, NBCe2 is inhibited by the NBCe1 blocker tenidap, as demonstrated for NBCe2 expressed in HEK-293 cells (869).
D) THE SLC4A5 GENE.
The human NBCe2 gene maps to chromosomal locus 2p13(FIGURE 26A and Ref. 767) and has at least 31 exons that encompass ∼127 kb of genomic DNA (FIGURE 26B). Reports that the 5′-UTR of NBCe2 includes sequence from exons shared with its neighboring gene DCTN1 (770), which encodes the p150GLUED subunit of dynactin, appear to have been premature. The presently assigned SLC4A5 and DCTN1 gene boundaries are separated by ∼15 kb (NCBI human genome assembly 37 version 1) and at least two promoters for SLC4A5 transcription are located within this intergenic region (916). The initiator codon for all presently known variants of NBCe2 is located in exon 6 of SLC4A5. One SLC4A5 promoter regions is located upstream of exon 1, but transcripts most often exclude exon 1 and start at exon 2, and promotes robust expression of a reporter gene in a human-lung and a mouse-myoblastoma cell line, but not in a human embryonic kidney cell line. The second SLC4A5 promoter is upstream of exon 5 and promotes robust expression of a reporter gene in the lung and kidney cell lines, but not in the myoblastoma cell line (916).
Figure 26.
SLC4A5 gene structure and NBCe2 transcript variants. Scale diagrams showing the human SLC4A5 gene locus together with the position of neighboring genes (A), the position of promoters (P1, P2, and P3), and the position of exons within SLC4A5 (B). Transcript variants are represented, not to scale, as numbered boxes joined by a horizontal line (C). Each numbered box represents the inclusion of that exon in the mature transcript. “//” denotes that both transcripts include exons 6–26. Exons that include the initiator ATG codon (“M”) and termination codon (“*”) are marked for each transcript. Colored exons, or parts of exons, correspond to the protein regions that each encodes, which are identically colored in FIGURE 27. Uncolored exons, or parts of exons, denote untranslated 5′ and 3′ sequence. Exons that are connected with a dashed line are predicted, but not demonstrated, to be included in the mRNA. Not shown are NBC4b and NBC4d that are unlikely to encode stable/functional transporters, or NBC4e and NBC4f that are cloning artifacts (see text).
E) STRUCTURAL FEATURES AND VARIANTS OF NBCe2.
Of the six originally reported splice variants of NBCe2 (NBC4a-f) (768, 770, 835, 1009, 1056), the cDNA sequences of only four (NBC4a-d) match the human genome. Only NBCe2-a/NBC4a and NBCe2-c/NBC4c are predicted to encode a functional transporter, NBCe2-c being the more abundant of the two. GenBank protein accession numbers for the variants discussed in this section are provided in Appendix IV.
I) Sources of variation in coding sequence among NBCe2 variants. Unusually for an NCBT, the Nt and Ct sequences of NBCe2 are not known to be variant, although in silico analysis suggests the existence of yet-to-be-reported gene products. As there are only two validated variants of NBCe2, there is only one validated source of variation that distinguishes NBCe2-a from NBCe2-c.
A) Extension between putative TMs 11 and 13. Transcripts that encode NBCe2-a include exon 27 (FIGURE 26C). The inclusion of the novel exon is predicted to lengthen, by the 16-amino acid sequence “MGTGGSEFKIQKKLTP,” the predicted extended structure between putative TMs 11 and 13, which includes an intracellular loop (FIGURE 27). This extension, also predicted to be included in NBCe2 variants from various primates,52 includes a consensus PKA phosphorylation site. The functional consequences of the splicing of this cassette are unknown.
Figure 27.
NBCe2 protein variants. Scale diagram of protein variants that are encoded by the transcripts represented in FIGURE 26C. Horizontal bars represent protein sequence laid out from Nt to Ct. Vertical bars represent position of α-helical TMs. Protein cassettes are labeled with a number denoting their size in amino acids and colored to denote their genetic origin as shown in FIGURE 26C. A color-matched protein sequence alignment of the variants is provided in Appendix V.
II) Cloned NBCe2 variants that are demonstrated or likely to exhibit NCBT activity. A) NBCe2-a/NBC4a (NCBT activity untested). NBCe2-a is the longest NBCe2 variant, as it includes the 16-amino acid splice cassette mentioned above (FIGURE 27). We regard NBCe2-a as likely to exhibit NCBT activity because we have no evidence that the 16-amino acid insertion would disrupt function.
B) NBCe2-c/NBC4c (NCBT activity demonstrated). This remains the only variant that has been functionally characterized (835, 1009). NBCe2-c lacks the 16-amino acid insertion found in NBCe2-a (FIGURE 27) and is therefore structurally most similar to other NCBTs within its TMD.
III) Predicted NBCe2 variants. Based on our in silico analysis, the alternative splicing of NBCe2 RNA has the potential to generate a novel protein variant (i.e., not NBC4a–f). Excision of exon 30 would produce an NBCe2 variant in which the last 39 amino acid of NBCe2-c are replaced with an alternative 77-amino acid Ct appendage that, like the 61-amino acid Ct appendage of NBCe1-C, is predicted to terminate with the class I PDZ-binding domain motif “ETTL.” However, such transcripts have yet to be amplified from mammalian cDNA.
IV) Other NBCe2 variants. A) NBC4b (potentially legitimate transcript, NCBT activity unlikely). NBC4b is identical to NBCe2-a except for the presence of a 16-nt exon (768) that produces a frame-shift, causing the last 8 amino acids of putative TM14 as well as the entirety of the 83 amino acids in the Ct to be replaced by 28 novel amino acids. Especially with the changes to TM14, it is not clear that the protein would be stable or functional.
B) NBC4d (potentially legitimate transcript, NCBT activity unlikely). NBC4d (770) lacks sequence encoding TMs 11–13 and is thus unlikely to encode a functional transporter.
C) NBC4e and NBC4f (probable cloning artifacts). The nucleotide sequences of NBC4e and NBC4f (1056) poorly match the human genome and contain frame-shifts and nonsense mutations (producing premature termination) that distinguish them as being artifactual. At least as expressed in oocytes, NBC4e, which has a truncated Ct, was reported to mediate a substantial pHi recovery from a CO2/HCO3−–induced acid-load. In separate experiments on the same cell, Vm was unperturbed by the application of CO2/HCO3− (1056). Taken at face value, these data suggest that the peculiarities of the NBC4e construct compromise its electrogenicity. DIDS sensitivity, Na+ dependence, and HCO3− dependence of the pHi recovery mechanism were not examined in these experiments.
F) DISTRIBUTION OF NBCe2.
The major organ most often associated with NBCe2 expression is the liver, although NBCe2 is expressed in many other organs. The distribution of NBCe2 in specific organ systems is discussed below. The distribution of NBCe2 is summarized and compared with that of other NCBTs in TABLE 5.
I) Central nervous system. A) Blood-brain barrier and elsewhere. RT-PCR analysis reveals the presence of NBCe2 transcripts in human brain (214, 835), specifically in the choroid plexus epithelium (CPE), hippocampus, cerebrum, and cerebellum (214) and in the hippocampi of mice (73). Western blotting and immunohistochemical studies localize NBCe2 protein to the apical membranes of rat and mouse CPE (113, 470), see cartoon in FIGURE 28. Immunogold staining confirms the presence of NBCe2 protein in the membranes of apical microvilli of mouse CPE (113). Interestingly, human CPE is not labeled by existing anti-NBCe2 antibodies (113), despite the presence of NBCe2 transcripts in this choroid-plexus preparation.
Figure 28.
Role of NCBTs in the choroid plexus. The secretion of cerebrospinal fluid by the choroid plexus (CP) and intracellular pH regulation in CP epithelia is achieved by a combination of NCBTs. NBCe2 is the major NCBT that is responsible for HCO3− secretion across the apical membrane. NBCn1, NDCBE, and NBCn2 have all been detected in the basolateral membrane of CP epithelia, mediating HCO3− influx. NBCn1 has also been detected in the apical membrane of the CP in some strains of mice. Note that NDCBE is not present in the CP of adults. Reported, but not shown, is the presence of NBCe1 in the basolateral membrane.
II) Sensory organs. A) Eye. Analysis of EST abundance suggests that NBCe2 is expressed in the human and mouse eye (Appendix VI). In the retina, NBCe2 protein is located in the outer plexiform layer (470).
III) Peripheral nervous system. A) Trigeminal ganglion. NBCe2 transcripts are detected by RT-PCR in preparations of rat trigeminal ganglion neurons (408).
IV) Respiratory system. A) Lungs. Northern blot analysis reveals the presence of NBCe2 transcripts in a human lung preparation (767, 768). Western blotting and immunohistochemical studies localize NBCe2 protein to the basolateral membranes of a human airway epithelial cell line (515).
V) Circulatory system. A) Heart. NBCe2 transcripts have been detected in heart preparations from humans (767, 768), mice (31), and rats (1056). Indeed, the archetypa human NBC4a transcript was cloned from heart cDNA (768). Analysis by qPCR reveals NBCe2 transcripts in mouse ventricles at a similar abundance to other cardiac HCO3− transporters such as AE3 and NBCe1 (31). A preliminary immunocytochemical study on rat ventricular myocytes suggests that NBCe1 protein is more abundant than NBCe2 in these cells (311).
VI) Musculoskeletal system. A) Muscles. RT-PCR reveals the presence of NBCe2 transcripts in a human muscle preparation (835). NBCe2 protein is detected in rat and human muscle homogenates (518). In rat muscle, NBCe2 is predominantly localized to the sarcolemma with an additional presence in T tubules (518).
VII) Upper digestive system. A) Stomach. Northern blot analysis reveals the presence of NBCe2 cRNA in a human stomach preparation (767, 768).
VIII) Lower digestive system. A) Widespread, abundant in liver. The liver appears to be one of the major sites of expression of NBCe2 transcripts (767, 768, 1056). A western blot and immunohistochemical study have localized NBCe2 protein to the basolateral (i.e., sinusoidal) membrane of rat hepatocytes (FIGURE 29) and to the apical membrane of cholangiocytes in rat intrahepatic bile ducts (8). Northern blots reveal the presence of NBCe2 transcripts in human small intestines (767, 768), a distribution that was determined by RT-PCR to correspond to NBCe2 expression in at least ileum, jejunum, and duodenum (214). Elsewhere, NBCe2 transcripts have also been detected in preparations of human pancreas (835) and proximal and distal colon of rodents (512, 1056).53 However, in mouse duodenum, jejunum, ileum, and colon, the abundance of NBCe2 mRNA is trivial compared with the abundance of NBCe1 or NBCn1 mRNAs (180). Accordingly, the duodena of NBCe2-null mice exhibit no detectable HCO3− secretion defects (180).
Figure 29.
Role of NBCe2 in hepatocytes. NBCe2 mediates HCO3− influx across the basolateral membrane of hepatocytes. This activity regulates intracellular pH and supports AE2-mediated HCO3− secretion into the bile ducts.
IX) Lymphatic and immune systems. A) Spleen and leukocytes. NBCe2 transcripts are have been detected in preparations of human spleen (767, 768) and peripheral blood leukocytes (835).
X) Endocrine system. A) Thyroid. In situ hybridization experiments reveal the presence of NBCe1 transcripts in the thyroid glands of 1-day-old and adult mice (341).54 Analysis of the abundance of ESTs suggests that NBCe2 is expressed in the human thyroid and parathyroid glands (Appendix VI).
XI) Urinary system. A) Kidney. NBCe2 transcripts have been detected in human kidney preparations (767, 768), corresponding to the presence of NBCe2 transcripts in both the kidney cortex and medulla (214, 341, 1056). Within the medulla, NBCe2 transcripts have been detected in the outer medullary segments of the TAL of rats (1056), and in IMCD of humans (986) and mice (341). Immunohistochemical studies suggest an apical localization of NBCe2 protein in outer medullary collecting duct cells of humans (214), and in uroepithelial cells of the renal pelvis of rats (8).
XII) Reproductive system. A) Male. NBCe2 transcripts have been detected in preparations of human testis (767, 768, 835) and in mouse testis, epididymis, and vas deferens (599). The archetypa clones of NBC4b (767, 768), NBC4c (835), and NBC4d (835) originate from human testicular cDNA.
B) Female. NBCe2 transcripts have been detected by RT-PCR of mouse ovary, uterus, and vagina (599).
C) Placenta. NBCe2 transcripts have been detected by northern blot of human placenta RNA (767, 768).
G) PHYSIOLOGICAL ROLES OF NBCe2.
Despite the broad distribution of NBCe2 throughout the body, few physiological roles have been ascribed to NBCe2 action. Aside from its roles in the choroid plexus and liver, described below, NBCe2 likely contributes to pHi regulation in all of the cell types in which it is located. Further physiological roles are suggested by the signs exhibited by NBCe2-null mice, although primary and secondary effects of NBCe2 loss have yet to be distinguished.
I) Central nervous system. A) Support of CSF secretion. In the choroid plexus epithelium, which is basically a backwards proximal tubule, the apical membrane faces the CSF. Thus apical NBCe2 (113), which appears to operate with a 1:3 stoichiometry (645), would be in a position to mediate the apical step (i.e., HCO3− efflux) of HCO3− secretion into the CSF (FIGURE 28). Other NCBTs such as NBCn2, and in some instances NBCn1 and NDCBE, are present at the basolateral (blood-side) membrane of CPE, and presumably mediate the basolateral (i.e., HCO3− uptake) step of HCO3− secretion. The role of NBCe2 in proper CSF secretion is supported by the exhibition of defective CSF secretion in NBCe2-gene-trapped mice, although the CPE in these mice exhibit other defects. Furthermore, ventricle size is normal in a different strain of NBCe2-null mice (341).
II) Peripheral nervous system. A) Potential contribution to neuronal excitability. NBCe2 in cultured rat trigeminal ganglion neurons could contribute towards countering the excitability-dampening effects of intracellular acidification, although NBCe1-B/C appears to be the dominant DIDS-sensitive Na/HCO3 transporter in these cells (408).
III) Lower digestive system. A) pHi regulation in hepatocytes. The basolateral location of hepatocellular NBCe2 (FIGURE 29) is consistent with the previously demonstrated presence of an electrogenic NCBT activity in the basolateral membranes of hepatocytes (291, 792). The influx of Na+ and HCO3−, presumably in a 1:2 stoichiometry, mediated by the transporter under basal conditions in vivo (288, 291) plays a major role in regulating hepatocellular pHi (266, 289, 326) and, as discerned by studies of perfused rat liver, is the primary mechanism of pHi regulation in the intact liver under physiological conditions (266). The maintenance of hepatocyte pHi within a narrow range is crucial for the functioning of the diverse cellular processes such as gluconeogenesis, biotransformation of xenobiotics, and mitogenesis (reviewed in Ref. 287). NBCe2 is cooperatively regulated by the activity of a pH-dependent K+ conductance pathway (gK), such that 1) a decrease in pHi causes a downregulation of gK and depolarizes the plasma membrane (presumably reflecting the decrease in gK) and 2) the depolarization stimulates NBCe2 activity and causes a compensatory increase in pHi (290).
B) [Na+]i regulation in hepatocytes. The addition of CO2/HCO3− to primary cultures of rat hepatocytes causes a substantial increase in hepatocellular [Na+]i. The increase in [Na+]i accompanying NBCe2-mediated HCO3− influx has a substantial stimulatory effect on the activity of the Na-K pump (288). Moreover, in perfused livers, the addition of CO2/HCO3− increases O2 consumption. These data are consistent with the hypothesis that a Na/HCO3 cotransporter, most likely NBCe2, mediates a substantial Na+ influx, which in turn increases the demand on the Na pump, and thus on oxidative metabolism.
C) Choliangiocyte viability. The application of the NCBT inhibitor S3705 inhibits the growth of and promotes apoptosis in cholangiocarcinoma cells (247), perhaps in part by inhibition of cholangiocyte-expressed NBCe2.
D) Potential contribution to transepithelial HCO3– transport in cholangiocytes. Na/HCO3 cotransport, most likely mediated by NBCe2, may constitute a basolateral step (i.e., HCO3− uptake) in secretion of HCO3− into the hepatic bile canaliculi (60, 88). However, in cholangiocytes lining the bile ducts of rats, NBCe2 apparently has an apical distribution (8). Considering that a major role of the bile duct is to secrete HCO3− into the lumen, and that the accepted major pathway for apical HCO3− exit from rat cholangiocytes is Cl-HCO3 exchange mediated by AE2 (46, 59, 631, 902, 987), the role of an apical NBCe2 in these cells is unclear. The cholangiocytes of mice express NBCe1 (987). In these animals, NBCe1 may compensate for AE2 insufficiency (987). It is possible that NBCe2 plays a similar support role in the cholangiocytes of rats.
IV) Urinary system. A) Possible role in HCO3– reabsorption. Because NBCe2-null mice exhibit signs of urinary HCO3− wasting, it has been suggested that NBCe2 might normally contribute towards renal HCO3− reabsorption (341). However, as noted below, this phenotype could reflect enhanced HCO3− secretion.
H) CAUSES OF NBCe2 UPREGULATION.
To date, only two groups have reported a maneuver that upregulates NBCe2.
I) General. Increased transcript abundance in response to HDAC and methyltransferase inhibitors. NBCe2 transcript levels are increased in an adenocarcinoma cell line after treatment with inhibitors of histone deacetylase and DNA methyltransferase, evidence for the specific regulation of NBCe2 gene expression by epigenetic factors (478).
II) Lower digestive system. A) Increased transcript abundance in colon of mice treated with probiotics. Probiotic treatment of mice is a model used to investigate the molecular mechanisms that underlie the pathogenesis of inflammatory bowel disorder, ulcerative colitis, and the health benefits associated with probiotic treatment. NBCe2 transcript abundance is doubled in the mouse colon 20 days after a probiotic treatment (512).
I) CAUSES OF NBCe2 DOWNREGULATION.
I) Central nervous system. A) Decreased transcript abundance in brain of a mouse model of drug-responsive depression. Mice that become indifferent to rewards after exposure to chronic mild stress are a model for anhedonia, a symptom of depression. A subset of those mice in which such behavior is induced are responsive to treatment with the antidepressant escitalopram. NBCe2 transcripts are twofold lower in the hippocampi of mice that are responsive to escitalopram treatment compared with NBCe2 transcript levels in the hippocampi of nonresponsive mice (73). The physiological relevance of this finding is unclear, although the finding is consistent with a possible link between NBCe2 and drug resistance.
J) CONSEQUENCES OF NBCe2 DYSFUNCTION.
Despite its abundance in the liver, and presumed importance for hepatic function, NBCe2-null mice have no reported hepatic phenotype. In an early report, NBCe2 was considered as a candidate gene for the neurodegenerative and metabolic disease Alström syndrome (767), which maps to the same genetic locus as NBCe2 (i.e., 2p13). However, subsequent work has shown that mutations in the ALMS1 gene on 2p13 cause this syndrome (197, 373).
I) Central nervous system. A) Defective CSF secretion in mice with a disrupted Slc4a5 gene.55 As expected by comparison with mice that lack NBCn2, another NCBT that is expressed in CPE and that contributes to CSF secretion, one strain of NBCe2-deficient mice exhibits defects in CSF secretion and thus a decreased brain ventricle volume and intracranial pressure (470). Unexpectedly however, these Slc4a5–gene-trapped mice also exhibit in their CPE, among other defects, a partial redistribution of NBCn2 into the apical membrane, a partial redistribution of the Na/K pump α1 subunit into the basolateral membrane, and a complete loss of the Na pump β2 subunit (470). Because all of these changes are predicted to exacerbate the CSF secretion defect, we cannot ascribe the decreased CSF secretion solely to the loss of NBCe2 activity per se. CSF composition is also perturbed in these mice inasmuch as it is deficient in HCO3− (but not Na+) but contains an overabundance of K+ (470). In a second strain of NBCe2-deficient mouse, no decrease in brain ventricle size was observed, although the presence of compensatory mechanisms was not examined in these mice (341).
B) Decreased neuronal excitability in mice with a disrupted Slc4a5 gene. Slc4a5–gene-trapped mice have a reduced sensitivity to the proconvulsive drug pentylenetetrazol, a finding that the authors of that study interpret as either a consequence of the reduced intracranial pressure of these mice or of the increased [K+]/decreased [HCO3−] that is characteristic of the CSF of these mice (470). As NBCe2 has not been demonstrated to be expressed in neurons or glia, it is unlikely that defective pHi regulation in these cells could explain the decreased neuronal excitability, as is thought to underlie a similar resistance in NBCn2-null mice.
C) Retinal abnormalities and detachment in mice with a disrupted Slc4a5 gene. Among the diverse morphological abnormalities in Slc4a5–gene-trapped mice, these mice have impaired vision and detached retinas (470). The authors of that study suggest that at least some of these signs may be related to the decreased intracranial pressure in these mice.
II) Circulatory system. A) Blood pressure-related traits. Genetic analysis links single nucleotide polymorphisms (SNPs) in the SLC4A5 gene locus with hypertension and other blood pressure-related traits (61, 404, 619, 915, 953, 954), including peripheral artery disease (472). One study reports that the contribution of a particular SLC4A5 SNP to elevated systolic blood pressure in African-American women is greater in individuals with dark versus medium skin color (955). A preliminary report suggests that (SNPs) in the SLC4A5 gene locus are also associated with the relative thickness of the left ventricular wall in hypertensive African-Americans (924). NBCe2-null-mice exhibit elevated blood pressure compared with wild-type littermates (341), although the expression of other hypertension-linked genes (e.g., Slc4a7; see p. 113) is upregulated in these mice (341). The molecular basis for the observed hypertension in NBCe2-null mice is unknown.
III) Urinary system. A) Metabolic acidosis. NBCe2-null mice exhibit a compensated metabolic acidosis that is evidenced by normal blood pH, reduced plasma CO2 and [HCO3−], and elevated urinary HCO3− excretion (341). These observations are consistent for a role for NBCe2 in HCO3− reabsorption. However, the NBCe2-null mice also exhibit an increased abundance of pendrin (341), which secretes HCO3− in the collecting duct (reviewed in Ref. 1016). Thus the urinary phenotype might not be solely related to loss of NBCe2 function. Another, untested, possibility is that the acidosis is secondary to disrupted hepatobiliary interactions (e.g., reduced glutamine synthesis).
B) Polyuria. NBCe2-null mice exhibit polyuria and polydipsia, although urine osmolality is normal (341).
B. Mammalian Electroneutral NCBTs: NBCn1, NDCBE, and NBCn2
Three of the five mammalian NCBTs perform electroneutral Na+-coupled HCO3− transport: NBCn1, NDCBE, and NBCn2. These three transporters are encoded by a group of three closely related Slc4 genes (Slc4a7, -a8, and -a10) that is distinct from two gene groups that encode electrogenic NCBTs or AEs. The three electroneutral transporters appear to have somewhat overlapping distributions, all being abundantly expressed in the CNS where they contribute to enhancing neuronal excitability. As we shall see, the three transporters differ most in their molecular actions. The physiological relevance of these differences is unknown, but these transporters all have the ability to regulate pHi without affecting or being influenced by Vm.
1. NBCn1 (Slc4a7)
A) SUMMARY.
The electroneutral Na/HCO3 cotransporter NBCn1 (encoded by the Slc4a7 gene) is unique among NCBTs in its low sensitivity to blockade by DIDS as well as in having a HCO3−-independent conductance. NBCn1 is unique among electroneutral NCBTs because it does not transport Cl−. NBCn1 has the greatest known multiplicity of products for an Slc4 family member (at least 12 known variants, -A through -L) and is present in many organs/organ systems throughout the body. In common with NBCe1-B/C, NDCBE, and NBCn2, NBCn1 is stimulated by the soluble protein IRBIT. NBCn1 is notably abundant in 1) the central nervous system, where NBCn1 contributes towards neuronal excitability; 2) the eye and the ear; 3) secretory epithelia, where NBCn1 contributes towards transepithelial HCO3−/fluid movement (e.g., in the intestines); and 4) the kidney, where NBCn1 promotes NH4+ excretion. In keeping with its presence in the eye and ear, mice that lack NBCn1 are both blind and deaf. Multiple studies report genetic linkage between the SLC4A7/NEK10 gene locus and breast cancer in humans. Although the genetic basis underlying the linkage has not been elucidated, the association between pHi regulation and tumor viability is clear.
B) NOMENCLATURE OF Slc4a7 PRODUCTS.
The first report of what we now know as NBCn1 was a partial cDNA, called SBC2 or hNBC2, from a human retinal cDNA library (420). The cDNA was interpreted as the product of a novel gene, which was assigned the name SLC4A6. Subsequent to this study was a report of a full-length NBCn1 cDNA, called mNBC3, from human skeletal muscle (765). Unfortunately, because of differences between the SBC2/hNBC2 and mNBC3 cDNA sequences,56 mNBC3 was interpreted as the product of a novel gene, which was assigned the name SLC4A7 (765).57 By the time NBC2 and NBC3 had been rationalized as alternative products of the same gene (139, 189), the designation SLC4A7 had already been introduced to the literature. As a consequence, the designation SLC4A6, which had never been mentioned in the literature, was withdrawn. Following the characterization of a rat Slc4a7 product as an electroneutral Na/HCO3 transporter, the gene product was renamed NBCn1 (189). The confusion caused by this changeable nomenclature was fortunately minimal. Few papers refer to NBC2 or SBC2 (344, 426, 427, 457, 485, 814, 989) and fewer still refer to NBCn1, NBC2, SBC2, and NBC3 as though they are the products of distinct genes (344, 426, 457, 485, 530). We further note that NBC2 has been used on one occasion to refer to NBCe1-B (485) and XNBC2 to refer to a Xenopus Slc4a11 product (1102). Due to the withdrawal of the name “NBC2” and because the term “NBC3” is degenerate (i.e., it has been used to refer to both Slc4a7 and Slc4a8 products), we consider NBCn1 as the preferred nomenclature for Slc4a7 products.
C) MOLECULAR ACTION OF NBCn1.
NBCn1 is an electroneutral Na/HCO3 cotransporter with an associated HCO3−-independent conductance (FIGURE 30; 30; Ref. 189), conclusions supported by the following observations.
Figure 30.
Molecular action of NBCn1. Possible molecular mechanisms by which an electroneutral NCBT could operate with an apparent Na+:HCO3− stoichiometry of 1:1. NBCn1 also exhibits a HCO3−-independent conductance that is represented by the red dashed arrow. Note that we have not included any models that are based on CO3/H cotransport, such as those represented for NBCe1 in FIGURE 16.
I) NBCn1 is an electroneutral NCBT with poor DIDS-sensitivity. In Xenopus oocytes subjected to a CO2/HCO3−-induced acid load, rat NBCn1 mediates a pHi recovery that is electroneutral, insensitive to 5-(N-ethyl-N-isopropyl)amiloride (EIPA),58 and dependent on both Na+ and HCO3−, consistent with the activity of an electroneutral NBC (189). Human NBCn1 also mediates a Na+-dependent pHi recovery when overexpressed in HEK-293 cells (711). Unusually for an NCBT, NBCn1 cotransport activity is not greatly sensitive to DIDS; in oocytes, 500 μM inhibits only 25% of the NBCn1-mediated pHi recovery (189), whereas the Ki for DIDS of the related transporter NBCe1 is ∼40 μM (612).
II) NBCn1 operates independently of Cl−. In Xenopus oocytes, the HCO3− efflux mediated by NBCn1, when operating in “reverse” (i.e., initiated by the removal of extracellular Na+), does not depend on extracellular Cl− (189). Nor does the HCO3− influx mediated by NBCn1, when operating in the “forward” direction, result in the net efflux of Cl− from the cell (719). Thus NBCn1 is not a Na+-driven Cl-HCO3 exchanger.
III) NBCn1 has an associated cation leak. After several days incubation, Xenopus oocytes expressing rat NBCn1 are unusually depolarized and loaded with nearly 40 mM [Na+]i, more than six times the [Na+]i of H2O-injected control oocytes from the same batch (189). Furthermore, NBCn1-expressing oocytes, even in the nominal absence of CO2/HCO3−, are substantially hyperpolarized by the removal of extracellular Na+, indicating the presence of an associated Na+-conductance pathway (189), that is also a feature of NBCn1 expressed in HEK 293 cells (201). About 50% of the current through the conductive pathway is carried by Na+ (189, 201), and Na+ conduction is estimated to be responsible for 1/300 of the total Na+ movement though the transporter (201). The reversal potential of the conductance varies in a less than Nernstian manner with respect to extracellular [Na+], which indicates that the conductance cannot be explained by a simple Na+-channel model (201). It is not clear what carries the remainder of the current. Indeed, the Na+-independent component of the current remains even in the absence of all extracellular ions except Mg2+, Cl−, and HEPES, perhaps indicative of an anion-efflux component. The magnitude of the conductance exhibited by membranes of NBCn1-expressing oocytes is not reduced in cells preincubated in Cl−-free media, although the Cl− depletion of these cells was not demonstrated (201). The Na+-conductive pathway neither depends on nor carries HCO3− and is paradoxically stimulated by DIDS exposure (189). A preliminary report shows that both the HCO3−-transport and Na+-conductive elements of NBCn1 function are upregulated by coexpression of NBCn1 with the NCBT binding partner IRBIT (722), further evidence that the two components may be mediated by the same protein. Finally, a study of NBCe1/NBCn1 chimeras indicates that the Na+ conductance requires elements in the back half of the TMD (i.e., TM6-TM14, inclusive: subdomain 8 in FIGURE 15) of NBCn1 (193).
D) THE SLC4A7 GENE.
The human SLC4A7 gene was originally mapped to chromosome 3p22 (766), although more recent genomic assembly suggests that 3p24.1 is a more accurate assignment.59 SLC4A7 occupies at least 27 exons spread over ∼100 kb (FIGURE 31A). The upstream neighbor of SLC4A7 is the T-box region (TBR) gene EOMES, aka TBR2, which encodes the neuronal transcription factor eomesodermin (495). The SLC4A10 gene, which encodes a second electroneutral Na/HCO3 cotransporter, also has a TBR gene as its upstream neighbor (FIGURE 39A), suggesting a longstanding association between the two gene families, and one that predates the duplication of this gene region.
Figure 31.
SLC4A7 gene structure and NBCn1 transcript variants. Scale diagrams showing the human SLC4A7 gene locus together with the position of neighboring genes (A), the position of promoters (P1 and P2), and the position of exons within SLC4A7 (B). Transcript variants NBCn1-A to NBCn1-E, which among themselves display the diversity of NBCn1-A to NBCn1-E, are represented, not to scale, as numbered boxes joined by a horizontal line (C). Each numbered box represents the inclusion of that exon in the mature transcript. “//” denotes that all transcripts include exons 9–25. Exons that include the initiator ATG codon (“M”) and termination codon (“*”) are marked for each transcript. Sequence that is derived from part of a larger exon sequence are labeled with an “a” (e.g., exon 7a is a subdivision of exon 7). Colored exons, or parts of exons, correspond to the protein regions that each encodes, which are identically colored in FIGURE 32. Uncolored exons, or parts of exons, denote untranslated 5′ and 3′ sequence. Exons that are connected with a dashed line are predicted, but not demonstrated, to be included in the mRNA.
Figure 39.
SLC4A10 gene structure and NBCn2 transcript variants. Scale diagrams showing the human SLC4A10 gene locus together with the position of neighboring genes (A), the position of the promoters (P), and the position of exons within SLC4A10 (B). Transcript variants are represented, not to scale, as numbered boxes joined by a horizontal line (C). Each numbered box represents the inclusion of that exon in the mature transcript. “//” denotes that all four transcripts include exons 1–7 and 9–23. Exons that include the initiator ATG codon (“M”) and termination codon (“*”) are marked for each transcript. Colored exons, or parts of exons, correspond to the protein regions that each encodes, which are identically colored in FIGURE 40. Uncolored exons, or parts of exons, denote untranslated 5′ and 3′ sequence. Exons that are connected with a dashed line are predicted, but not demonstrated, to be included in the mRNA. Note that rb3NCBE has only been isolated from rat cDNA.
E) STRUCTURAL FEATURES AND VARIANTS OF NBCn1.
Slc4a7 products that encode full-length transporters are currently named NBCn1-A through -L and are the most diverse in terms of number of reported and predicted variant transcripts. Here we describe the nature of the variant features (i.e., alternative promoters, splice cassettes), followed by a description of NBCn1-A through -L. A diagrammatic representation of the sources of transcript variation is provided in FIGURE 31C. A representation of each NBCn1 protein variant is provided in FIGURE 32.
Figure 32.
NBCn1 protein variants. Scale diagram of protein variants that are encoded by the transcripts represented in FIGURE 31C. Horizontal bars represent protein sequence laid out from Nt to Ct. Vertical bars represent position of α-helical TMs. Protein cassettes are labeled with a number denoting their size in amino acids and colored to denote their genetic origin as shown in FIGURE 31C. All NBCn1 variants are presumed to include an autoinhibitory domain and IRBIT-binding determinants in their Nt. All NBCn1 variants terminate with a PDZ binding motif. A color-matched protein sequence alignment of the variants is provided in Appendix V.
I) Sources of variation in coding sequence among NBCn1 variants. Alternative promoter choice and Nt (“MERF”- versus “MEAD”-). Mammalian NBCn1 transcription can initiate at either exon 1 or exon 2, presumably dictated by a choice of alternative promoter regions (FIGURE 31B). The result is variant protein products whose Nt begins either with an 11-amino acid or a 16-amino acid protein cassette. In humans, exon 2, which encodes the 11-amino sequence (beginning with the sequence MERF), is located 4 kb upstream of exon 3 in the SLC4A7 gene, whereas exon 1, which encodes the 16-amino acid sequence (beginning with the sequence MEAD), is 32 kb upstream of exon 3. The consequences of this promoter choice for NBCn1 function and/or regulation are unclear.
The full-length NBCn1 protein variants that begin with “MERF” are NBCn1-A, -F, -J, -K, and -L, whereas the variants that begin with “MEAD” are NBCn1-B, -C, -D, -E, G, -H, and -I.
A) Variation in the length of “MEAD”-encoding exon 1 (NBCn1-X′). In some variants, the 3′ boundary of exon 1 is extended by the use of an alternative splice site such that the 16-amino acid sequence is lengthened at its carboxy-terminal end by the 4-amino acid sequence “VTSR”. The terminology for such variants has not been settled. Some have been designated either with a prime (e.g., NBCn1-D′ is identical to NBCn1-D except for the inclusion of “VTSR”; Appendix IV and Appendix V) and others with a lowercase “a” and “b” (e.g., NBCn1-Hb is identical to NBC1-Ha except for the inclusion of “VTSR”). The consequences of the inclusion of “VTSR” are unknown.
B) Cassette I (aka “cassette A”). A 13-amino acid “cassette I” (originally termed “cassette A” in Ref. 189)60 is encoded by a 3′ extension of exon 7 (FIGURE 31C). The protein sequence encoded by cassette I is located in the Nt loop subdomain (FIGURE 15) and is absent from some variants of NBCn1 (FIGURE 32). The consequences of this splice for NBCn1 function and/or regulation are unclear.
Several studies have used RT-PCR to address the spatial distribution of splice cassette I, as well as II and III (discussed below). The most exhaustive to date is presented in Reference 213. In preparations from various organs/tissues of adult mice, NBCn1 splicing generally does not appear to favor the omission or inclusion of cassette I (55, 213). However, a preference for cassette I inclusion seems to exist in kidney cortex, submandibular gland, parotid gland, and liver. Conversely, a preference for cassette I exclusion appears to exist in the lung.>
The full-length NBCn1 protein variants that include cassette I are NBCn1-A, -B, -D, -E, -F, -G, and -J. Variants lacking cassette I are NBCn1-C, -H, -I, -K, and -L. Among those variants lacking cassette I, at least NBCn1-H is functional.
C) Cassette II (aka “exon 7”).61 Abutting cassette I, and also within the Nt loop subdomain (FIGURE 15), is the 124-amino acid cassette II (123 amino acids in rodents due to the lack of an Ala residue close to the Ct end of the cassette), encompassing the entirety of exon-8–encoded sequence (FIGURES 31C AND 32). Cassette II sequence is unlike that encoded by any other mammalian gene, but contains a number of consensus PKA and PKC phosphorylation sites and, as an extension of the Nt loop subdomain, is likely accessible to a number of cytosolic binding partners. A preliminary report demonstrates that an isolated cassette II is able to interact with calcineurin-Aβ in vitro (715). Indeed, cassette II includes a motif “PTVVIH” that is similar to a consensus calcineurin-binding motif (45). Moreover, perturbation of this sequence disrupts the in vitro interaction between isolated cassette II and calcineurin (715). The physiological relevance of this interaction remains untested, although perhaps pertinent is the observation that NBCn1 protein abundance is decreased in the renal medulla of rats treated with the calcineurin inhibitor FK506 (655).
Comparisons of Xenopus-oocyte–expressed NBCn1 variants, with or without cassette II (specifically NBCn1-B versus NBCn1-E), indicate that NBCn1 + cassette II accumulates in the plasma membrane more slowly than NBCn1 − cassette II (1078). However, after 72 h of expression, both variants accumulate to a similar extent in the oocyte membrane, and both mediate a Na+ conductance of equivalent magnitude (1078). Some differences are evident between the two variants at low [Na+]o: 1) NBCn1 + cassette II appears to have a lower Na+ affinity, and 2) the variants exhibit differences in the appearance of a conductive leak of an as-yet-unidentified nature (1078).
The reduced functional expression of NBCn1 + cassette II is also indirectly evident in an opossum kidney cell line, inasmuch as the endogenous Na pump in these cells is less active in cells coexpressing NBCn1 + cassette II than in cells coexpressing NBCn1 − cassette II, presumably because the less active variant of NBCn1 imposes a lesser Na+ load on the cells (1078).
Thus cassette II appears to be inhibitory in at least certain contexts, perhaps because it serves as a binding site for calcineurin or other proteins with an inhibitory effect.
In mice (55, 213) and in rats (200), most NBCn1 transcripts lack cassette II, as assessed by PCR across a region including cassette II. However, the inclusion of cassette II appears to be favored in rodent aorta (55, 200), rat heart (fetal > adult; interventricular septum > ventricles > auricles > atria > AV node; Ref. 200), human heart and muscle (200, 765), and in fetal rat hippocampal neurons (201).
The full-length NBCn1 protein variants that include cassette II are NBCn1-A, -B, -C, -D, -H, and -K. Variants lacking cassette II are NBCn1-E, -F, -G, -I, -J, and -L.
Variants that include both cassettes I and II are NBCn1-A, -B, and -D. The only two variants that lack both cassettes I and II are NBCn1-I and -L.
D) Cassette III (aka “cassette B”). The final published source of NBCn1 variation lies in the Ct of the protein (FIGURE 32). NBCn1 variants are unique among NCBTs in that the sequence of the extreme Ct is invariant: all variants terminate with the PDZ-binding domain ligand -ETSL. Instead, Ct variation among NBCn1 forms is achieved by the optional inclusion of a 36-amino acid “cassette III” (originally termed “cassette B” in Ref. 189). As far as other NCBTs are concerned, cassette III–like sequence is also present in the Ct of NDCBE-A and NDCBE-C, and inclusion of cassette III–like sequence is obligatory in the Ct of all NBCn2 variants (see extended domain alignment in Appendix V).
Experiments on oocytes expressing NBCn1 variants indicate that cassette III stimulates overall functional expression in the absence (but not in the presence) of cassette II (compare NBCn1-G versus -E and -D versus -B).
In preparations from various organs/tissues of adult mice, NBCn1 splicing generally does not appear to favor the omission or inclusion of cassette III (55, 213). However, in rodents, a preference for cassette III inclusion seems to exist in kidney cortex (213); renal mTAL (694); submandibular gland (213), specifically the acini, see Reference 615; sublingual gland (213); pylorus (213); colon (213); pancreas (213); lung (213); cerebrum (213) and certain other areas of the brain (755); and epididymis (213). On the other hand, a preference for cassette III exclusion appears to exist in cardiac ventricles (213) and in the ducts of submandibular glands (615).
The full-length NBCn1 protein variants that include cassette III are NBCn1-C, -D, -G, I-, -J, and -L. Variants lacking cassette III are NBCn1-A, -B, -E, -F, -H, and -K.
Of the 12 confirmed transcripts, only NBCn1-D includes all three cassettes I, II, and III.
II) Cloned NBCn1 variants that are demonstrated or likely to exhibit NCBT activity. The known NBCn1 variants are listed below along with their features and their demonstrated locations. Information about clones that have not yet been reported in a full manuscript is derived from their GenBank entries (accession numbers are provided in Appendix IV).
A) NBCn1-A (NCBT activity demonstrated). NBCn1-A is the archetypal human NBCn1 clone (765). NBCn1-A initiates with the “MERF” Nt, includes cassette I and II, but omits cassette III. A full-length NBCn1-A clone has been isolated from a human skeletal muscle cDNA preparation (765).
B) NBCn1-B (NCBT activity demonstrated). NBCn1-B is the archetypal rat NBCn1 clone (189). NBCn1-B initiates with the “MEAD” Nt, includes cassette I and II, but omits cassette III. A full-length NBCn1-B clone has been isolated from rat aorta (189) and embryonic rat hippocampal neuron cDNA preparations (201).
C) NBCn1-C and C′ (NCBT activities untested). NBCn1-C initiates with the “MEAD” Nt sequence, includes cassette II, but omits cassettes I and III. NBCn1-C′ is the same but initiates with the extended 20-amino acid “MEAD” Nt sequence. A full-length NBCn1-C clone has been isolated from rat aorta cDNA preparations (189). A full-length NBCn1-C′ clone has been isolated from human skeletal muscle cDNA preparations.
D) NBCn1-D and D′ (NCBT activity demonstrated). NBCn1-D initiates with the “MEAD” Nt sequence, and includes cassettes I, II, and III. NBCn1-D′ is the most complete “MEAD”-initiated NBCn1 clone as it initiates with the extended 20-amino acid “MEAD” Nt sequence and also includes all three splice cassettes. Full-length NBCn1-D has been isolated from rat aorta cDNA preparations (189). Full-length NBCn1-D′ has been isolated from human liver cDNA preparations.
E) NBCn1-E (NCBT activity demonstrated). NBCn1-E initiates with the “MEAD” Nt, includes cassette I, but omits cassettes II and III. Full-length NBCn1-E clones have been isolated from human skeletal muscle, adult-rat hippocampal neurons (201), and mouse reproductive tract cDNA preparations.
F) NBCn1-F (NCBT activity untested). NBCn1-F initiates with the “MERF” Nt, includes cassette I, but omits cassettes II and III. Full-length NBCn1-F clones have been isolated from human kidney cDNA preparations.
G) NBCn1-G (NCBT activity demonstrated). NBCn1-G initiates with the “MEAD” Nt, includes cassettes I and III, but omits cassette II. Full-length NBCn1-G clones have been isolated from human skeletal muscle cDNA preparations.
H) NBCn1-H and H′ (NCBT activity demonstrated). NBCn1-H initiates with the “MEAD” Nt, includes cassette II, but omits cassettes I and III. NBCn1-H′ is the same but initiates with the extended 20-amino acid “MEAD” Nt sequence. Full-length NBCn1-H and -H′ clones have both been isolated from human skeletal muscle cDNA preparations.
I) NBCn1-I (NCBT activity untested). NBCn1-I initiates with the “MEAD” Nt, includes cassette III, but omits cassettes I and II. Full-length NBCn1-I clones have been isolated from mouse reproductive tract cDNA preparations.
J) NBCn1-J (NCBT activity untested). NBCn1-J initiates with the “MERF” Nt, includes cassettes I and III, but omits cassette II. Full-length NBCn1-I clones have been isolated from mouse ovary and testis cDNA preparations.
K) NBCn1-K (NCBT activity untested). NBCn1-K initiates with the “MERF” Nt, includes cassette II, but omits cassettes I and III. Full-length NBCn1-I clones have been isolated from mouse skeletal muscle cDNA preparations.
L) NBCn1-L (NCBT activity untested). NBCn1-L initiates with the “MEAD” Nt, includes cassette III, but omits cassettes I and II. Full-length NBCn1-L clones have been isolated from mouse reproductive tract cDNA preparations.
III) Predicted NBCn1 variants. The choice of three alternative first exons and the omission or inclusion of any of the three splice cassettes I, II, or III could, together, produce as many as 24 variants, although presently only 15 of the 24 have been cloned as full-length cDNAs.62 No pattern of association between promoter/cassette usages has emerged that suggests any of the “missing” combinations63 are unfavored; thus it is likely that their existence will be documented in due course.
IV) Other NBCn1 variants. A) Unusual variants that represent only the isolated Nt. Six unusual cDNA species from brain, heart, and skeletal muscle cDNA are identical to full-length NBCn1 transcripts except for the omission of exon 13 (GenBank DNA accessions nos. FJ178574, FJ178575, FJ178576, GU354307, GU354309, and GU354310).64 If translated, each of these cDNAs is predicted to produce a soluble protein that would include almost the entire “MEAD” Nt (i.e., the sequence encoded by exons 1 and 3–12, per normal) plus two residues “VQ” followed by a termination codon (encoded by an out-of-frame exon 14). The protein would be truncated at a point 25 amino acids upstream of TM1, and thus would precisely terminate in the region that, in AE1, is predicted to be an unstructured linker that join the Nt to the TMD. The physiological relevance of these clones remains obscure, and the cognate protein has yet to be identified. It is possible that the premature termination codons included in these unusual mRNAs would make them targets for nonsense-mediated decay (170). These clones are reminiscent of isolated Nt variants of NDCBE and NBCn2.
B) SBC2/hNBC2 (probable cloning artifact). The SBC2/hNBC2 cDNA sequence (GenBank protein accession no. BAA25898) was the first reported SLC4A7 product (420). It differs from subsequently reported SLC4A7 products in three respects: 1) hNBC2 lacks exons 0–4 of verified SLC4A7 transcripts. 2) The 5′-UTR and the initial portion of the purported open reading frame of hNBC2 is derived from the exons 5 and 6 of the SLC4A7 gene, but this sequence has an inverted orientation with respect to the remainder of the transcript. 3) The 3′ end of the hNBC2 transcript is not predicted by the genomic sequence, presumably due to low fidelity of the amplified cDNA: because of this the ORF contains three missense mutations and reaches a premature stop, 18 amino acid short of the Ct end of verified Slc4a7 products.
F) DISTRIBUTION OF NBCn1.
The distribution of NBCn1 is broad, as summarized in TABLE 5. Its location in specific organ systems is discussed below. Note that the tissue distributions of the alternate promoters and three splice cassettes were discussed above in section VB5 on p. 882.
NBCn1 distribution has been the focus of much investigation, revealing some discrepancies between studies. That is to say, certain NBCn1-directed polyclonal antibodies suggest a different transporter distribution than others. Some studies report that the localization of NBCn1 in a tissue may vary among species (485) and even among mouse strains (216). Data concerning NBCn1 protein distribution come from the use of the three types of antibodies, those that are generated against: 1) epitopes in the Ct of rat NBCn1 (1014), 2) epitopes in the Ct of human NBCn1 (“anti-NBC3”; Ref. 774), and 3) an epitope in the Nt of rat NBCn1 (213). The studies using antibodies 1 and 3 reinforce each other and are confirmed by β-galactosidase staining in mice heterozygous for the insertion of lacZ into the Slc4a7 gene (91). It is therefore the results of these studies that are cited here for NBCn1 protein localization.
The distribution of NBCn1 as reported by antibody 2, the “anti-NBC3” antibody, is unusual in many respects (discussed in Ref. 334) and is considered separately in Appendix VII (see section V).
I) Central nervous system. A) Brain. At the level of subregions of the central nervous system, NBCn1 transcripts (755) and protein (174, 709) are widespread, being present in the cerebral cortex, hippocampus, subcortex, cerebellum, and olfactory bulb of rodents. NBCn1 immunoreactivity in wild-type rats and β-galactosidase staining of heterozygous mice with a lacZ insertion in the Slc4a7 gene shows that the highest level of NBCn1 promoter activity, at the tissue level, is in the pyramidal cell layers of the hippocampus, appearing equally robust in the CA1, CA2, and CA3 regions, and in the granule cells layer of the dentate gyrus (91, 709). In these “lacZ” mice, NBCn1 promoter activity is also evident in certain regions of the cortex and the dentate nucleus of the cerebellum (91).
At the cellular level, NBCn1 expression is detected in hippocampal neurons of embryonic rats (201) and adult mice (91). In primary cultures of hippocampal neurons from embryonic rats, most cells, both GABAergic and non-GABAergic, express NBCn1 transcripts (201). NBCn1-like activity is detected in locus coeruleus neurons (479), although the presence of NBCn1 transcripts and protein in these cells has yet to be demonstrated.
At the subcellular level in embryonic rat neurons, NBCn1 protein is detected in the plasma membrane of the cell soma as well as in the dendrites, with a punctuate distribution that is consistent with a presence in the dendritic spines (201, 709).
B) Choroid plexus. NBCn1 protein has a basolateral presence (see FIGURE 28) in the choroid plexus of rats (213, 709, 755), some strains of mice (755), and humans (756). NBCn1 also has a small apical presence in the choroid plexus of human ventricle IV (756) and is predominantly apically expressed in some strains of mice (470, 755).
II) Sensory organs. A) Eye. NBCn1 promoter activity is evident in the photoreceptor and ganglion cells of mouse retina (91) and NBCn1 transcripts have been detected in Northern blots of rabbit eyes (814). Microarray analysis demonstrates that cultured mouse keratocytes express NBCn1 transcripts (see Gene Expression Omnibus database entry GDS85765 that accompanies Ref. 162). See Appendix VII for subretinal distribution based on the “anti-NBC3” antibody.
B) Ear. See Appendix VII for distribution of NBCn1 within the inner ear according to the “anti-NBC3” antibody.
III) Peripheral nervous system. A) Trigeminal ganglion. In rats, NBCn1 transcripts are detected by RT-PCR in trigeminal ganglion preparations (408).
IV) Respiratory system. A) Trachea and lung. NBCn1 transcripts and protein are present in preparations of rat lung (189, 213), and NBCn1 transcripts are present in the Calu-3 airway cell line (see “NBC2” in Ref. 515). NBCn1 promoter activity is evident in nonvascular smooth muscle cells of mouse trachea (91).
V) Circulatory system. A) Heart. In human heart, NBCn1 cDNAs have been amplified from tissue dissected from aorta, apex, atria, auricles, ventricles, interventricular septum, and atrioventricular node (200). In rat heart, NBCn1 protein66 is found in myocardial capillaries, the endothelium and vasa vasorum of aorta, and in the endothelia of atria and ventricles. In ventricular endothelia, immunogold staining reveals NBCn1 protein in both the luminal and abluminal (i.e., basal) membranes (213). β-Galactosidase staining of mice with a lacZ insertion in Slc4a7 provides evidence for NBCn1 promoter activity in the aorta and in cardiac myocytes of the atria, but not the ventricles (91). A preliminary study reports a diffuse NBCn1 immunoreactivity in rat ventricular myocytes (311).
B) Vasculature. As noted in the previous paragraph, NBCn1 is present in cardiac endothelial cells. In vascular smooth muscle, NBCn1 cDNA has been amplified from pulmonary artery and aorta (189), and NBCn1 protein is detected in the portal vein, and in the hepatic, mesenteric, and intrarenal cortical arteries of rats. NBCn1 protein is also present in rat skeletal muscle vasculature. In the arteries, it is the endothelial cells and the smooth muscle cells of the tunica media that are NBCn1 positive (213). LacZ/β-galactosidase staining provides evidence for NBCn1 promoter activity in mouse cerebral arteries and veins, mesenteric arteries, and renal arteries of mice (91). In the vascular smooth muscle of a mouse mesenteric small artery, immunogold staining detects NBCn1 expression in the sarcolemmal membrane (90).
VI) Musculoskeletal system. A) Skeletal muscle. The archetypal human NBCn1 clone was amplified from skeletal muscle cDNA. Within skeletal muscle of rats, NBCn1 protein localizes to vasculature as well as to the vicinity of neuromuscular junctions. However, NBCn1 does not colocalize with α-bungarotoxin, suggesting that NBCn1 is present in motor neuron terminals or sarcolemmal areas that lack the nicotinic acetylcholine receptor (213).
B) Osteoblasts. A proteomic study reveals NBCn1 protein to be present in the hydroxyapatite-releasing microvesicles that bud from the apical membranes of osteoblasts (969).
C) Osteoclasts. Osteoclasts express NBCn1 protein (112, 797), specifically in the ruffled membrane that faces the bone resorption lacuna (see cartoon in FIGURE 33 and ref. 797).67
Figure 33.
Role of NBCn1 in osteoclasts. Intracellular carbonic anhydrase generates H+ that are secreted by the H-pump into the resorption lacuna to dissolve bone minerals. H+ secretion is supported by AE2 in the contra-lacunar membrane. Liberated HCO3− is absorbed across the lacunar membrane by NBCn1 and across the contra-lacunar membrane by AE2. Liberated Ca2+ is absorbed by the combined actions of NCX and TRPV5 channels (580, 994).
VII) Upper digestive system. A) Enamel organ. NBCn1 immunoreactivity is reported in the papillary cell layer (456) of the enamel organ (FIGURE 20).
B) Salivary gland. In human, but not rat, parotid and submandibular salivary glands, NBCn1 protein is enriched in the basolateral membranes of the striated duct cell (FIGURE 21B). However, NBCn1 is not detected in acinar cells (334), which are a site of basolateral NBCe1 expression (FIGURE 21A). NBCn1 is expressed in the basolateral membranes of an immortalized cell line from rat parotid acini (740). The evidence for the presence of NBCn1 in the apical membrane of salivary gland duct cells is indirect, being based on Co-IP of NBCn1 and CFTR in isolated tissues, rather than immunohistochemical data (711).
See Appendix VII for distribution of NBCn1 within the salivary glands according to the “anti-NBC3” antibody.
C) Stomach. Results of qPCR show that rabbit NBCn1 transcripts are expressed in gastric mucosa, most prominently in the chief cells and mucous cells, with lesser expression in parietal cells (814). LacZ/β-galactosidase staining is negative in mouse gastric mucosa (91).
VIII) Lower digestive system. A) Intestines. In rabbits, NBCn1 transcripts are more abundant in the duodenal and ileal mucosa than in either gastric or colonic mucosa (427). In mice, NBCn1 transcripts are detected in duodenal and colonic epithelia (55, 180, 213). In mice, anti-NBCn1 antibodies localize NBCn1 protein to the basolateral membrane of the enterocytes of duodenal villi (see FIGURE 22 as well as Refs. 180, 213, and 753). The presence of NBCn1 in the enterocytes of mouse duodenal villi, but not of crypts, is confirmed by lacZ/β-galactosidase staining of NBCn1/lacZ transgenic mice (91). In the colons of NBCn1/lacZ mice, β-galactosidase staining reveals the presence of Slc4a7 products in villar epithelial cells, but not in crypt epithelial cells (91). In colonic crypts, qPCR indicates a low level of NBCn1 transcript expression that is swamped by an ∼80-fold greater abundance of NBCe1-B transcripts (1087).
LacZ/β-galactosidase staining of NBCn1/lacZ mice reveals that some of the NBCn1 transcripts detected by qPCR in the duodenum and colon, and the majority of the NBCn1 transcripts detected in the jejunum and ileum (180), represent NBCn1 expression in the nonvascular smooth muscle cell layers, rather than the epithelium (91).
B) Liver. Slc4a7 products have been amplified from cDNA preparations of rat and mouse liver (189, 213). See Appendix VII for hepatic distribution of NBCn1 according to the “anti-NBC3” antibody.
C) Pancreas. The detection of NBCn1 transcripts in preparation of mouse pancreas are reported as unpublished data by Xuo and Muallem (711). Furthermore, ESTs appear to be common in preparations from mouse pancreas (Appendix VI).
See Appendix VII for pancreatic distribution of NBCn1 according to the “anti-NBC3” antibody.
IX) Lymphatic and immune systems. A) Spleen and macrophages. Slc4a7 products have been amplified from cDNA preparations of macrophages (630) and spleen (189, 213).
X) Endocrine system. A) Thyroid. According to an NCBI-curated database of ESTs, the human thyroid gland is a site of NBCn1 transcription (Appendix VI).
XI) Urinary system. A) Kidney. NBCn1 transcripts are amplified from cortical preparations from rabbits (427), as well as the inner stripe of the outer medulla (754), IMCD (986), and mTAL (694) preparations from rats. Anti-NBCn1 antibodies detect an 180-kDa protein in western blots of rat preparations of the inner medulla as well as the inner and outer stripes of the outer medulla (213, 1014). An anti-NBCn1-Nt antibody, but not an anti-NBCn1-Ct antibody, detects NBCn1 protein in the renal cortex (213), where NBCn1 expression appears to be less than in the medulla (213). LacZ/β-galactosidase staining indicates that NBCn1 expression in mouse cortex may predominantly represent vascular expression in the afferent arterioles and renal corpuscles (91). No evidence of NBCn1 promoter activity is detected in cortical collecting ducts (CCDs, Ref. 91).
At the cellular level, anti-NBCn1 antibodies detect a basolaterally located protein in mTAL epithelial cells (334, 431, 491, 530, 754, 797, 1014, 1026), intercalated cells in the inner stripe of the outer medulla (1014), α-intercalated cells in the IMCD (694, 1014), and renal papilla epithelial cells (754) of rats. Only an anti-NBCn1-Nt antibody detects NBCn1 protein at the basolateral membrane of a subset of outer medullary collecting ducts (OMCD) intercalated cells (213). This presence of NBCn1 in rat mTAL, IMCD, and OMCD matches the pattern of Slc4a7 promoter activity disclosed by lacZ/β-galactosidase staining (91). In these mice, renal Slc4a7 promoter activity is particularly robust in the epithelium lining the renal pelvis.
See Appendix VII for renal distribution of NBCn1 according to the “anti-NBC3” antibody.
B) Bladder. Slc4a7 promoter activity has been detected in nonvascular smooth muscle cells from mouse bladder (91).
XII) Reproductive system. A) Male. NBCn1 transcripts have been detected by RT-PCR of mouse testis, epididymis, and vas deferens (599) as well as by Northern blot of human testis (420). NBCn1 protein (213) has been detected in preparations of rat epididymis (213).
See Appendix VII for distribution of NBCn1 in the epididymis according to the “anti-NBC3” antibody.
B) Female. NBCn1 transcripts have been detected by RT-PCR of mouse ovary, uterus, and vagina (599), and ESTs are abundant in mouse mammary gland preparations (Appendix VI). NBCn1 protein is present in the lobular acini of the human breast (182). Slc4a7 promoter activity has been detected in the myometrium of the uterus of mice (91).
G) PHYSIOLOGICAL ROLES OF NBCn1.
We have seen that NBCn1 has a broad distribution throughout the body and likely supports HCO3− secretion across a number of epithelia and contributes to pHi regulation in all of the cell types in which it is located. Further physiological roles are suggested by characteristics exhibited by NBCn1-null mice, although primary versus secondary effects of NBCn1 loss have yet to be distinguished.
I) General. A) pHi regulation. DIDS-insensitive (or poorly DIDS-sensitive) Na/HCO3 cotransport, a strong indicator of NBCn1 activity, contributes to pHi regulation in many tissues, including the choroid plexus (113), duodenum (427), renal mTAL (530, 694) and IMCD (754), as well as ureter (13). NBCn1 is also strongly implicated as a contributor to pHi regulation of mouse vascular smooth muscle cells, although, as discussed below, overall Na/HCO3 transport in these cells is partly sensitive to DIDS (90). As discussed in footnote 54, NBCn1 is unlikely to be responsible for the DIDS-insensitive, EIPA-sensitive Na/base transport that has been detected at the apical membrane of OMCD α intercalated cells.
II) Central nervous system. A) Potential contribution to neuronal excitability. Being widely expressed in neurons throughout the brain, NBCn1 likely contributes towards control of neuronal excitability, as has been demonstrated for other NCBTs, such as NBCe1 (FIGURE 24).
B) Potential contribution to CSF secretion. In choroid plexus epithelia (FIGURE 28), the basolateral presence of NBCn1 protein (213, 755, 756) parallels the distribution of NBCn2. In light of the prominent role played by NBCn2 in CSF secretion (429), it has been suggested that the role played by NBCn1 in CSF formation may be less significant. Indeed, in some strains of mice, NBCn1 in the CPE is predominantly at the apical membrane (216, 470), where the protein would not be in a position to contribute to CSF secretion. Moreover, in NBCn2 and NBCe2 knockout mice, endogenous NBCn1 does not compensate for defective CSF secretion (216, 470).
III) Peripheral nervous system. A) Potential contribution to neuronal excitability. Although NBCn1 transcripts are present in neurons cultured from trigeminal ganglions of rats, the NCBT activity in these cells is fully blocked by DIDS, an observation that is inconsistent with the relative DIDS insensitivity of NBCn1 in oocytes (see Ref. 408).
IV) Circulatory system. A) Tone and contractility of vascular smooth muscle. In the vascular smooth muscle cells of mice, Na+-dependent HCO3− transport makes a major contribution to the recovery of pHi from an acid-load (90). The presence of CO2/HCO3− in the extracellular fluid contributes to enhanced myogenic tone and the ability to maintain contractile ability during sustained agonist exposure, presumably due to transporter-mediated HCO3− uptake. Two pieces of data speak to the importance of NBCn1 in mediating this HCO3− uptake. 1) At the transcript level, NBCn1 is the only Na+-dependent Slc4 family member detectable in mesenteric, coronary, and cerebral arteries, and 2) siRNA-directed knockdown of NBCn1 (to ∼50% normal levels) reduces both the steady-state pHi of these cells and the rate at which their pHi recovers from an acid-load (90). One usual aspect of these studies is that the pHi recovery in mesenteric arteries is unusually DIDS sensitive (90) whereas, as mentioned above, NBCn1 activity is relatively DIDS insensitive in other tissues and heterologous expression systems.
V) Musculoskeletal system. A) Osteoclast survival and function. Treatment of osteoclasts with CSF-1 results in an increase in pHi via a mechanism that depends on Na+ and HCO3−, but that is not sensitive to DIDS or EIPA—the hallmarks of NBCn1 activity. Because treatment of osteoclasts with CO2/HCO3− reduces apoptosis in osteoclasts, an effect further promoted by the addition of CSF-1, it has been proposed that NBCn1, by raising pHi and/or [HCO3−]i, inhibits caspase activity and thereby promotes osteoclast survival (112). More recently, NBCn1 has been suggested to play a direct role in reabsorbing the HCO3− liberated from the hydroxyapatite matrix during bone remodeling (797). This observation is supported by the presence of NBCn1 protein in the ruffled membrane that faces the resorption space (lacuna, see FIGURE 33) and the decreased bone absorptive capabilities of osteoclasts when NBCn1 abundance is reduced in these cells by shRNA (797).
VI) Upper digestive system. A) Transepithelial HCO3− secretion in salivary gland. The concerted action of NBCe1 and NBCn1 in the basolateral membranes of striated duct epithelia could support transepithelial HCO3− secretion into the saliva (FIGURE 21B). A suggested role for NBCn1 in apical HCO3− salvage in salivary gland duct cells lacks evidence that NBCn1 resides in the apical membrane of these cells.
VII) Lower digestive system. A) Transepithelial HCO3− secretion in intestines. NBCn1 is present in the basolateral membranes of epithelia in the lower digestive system (FIGURE 22), enabling the transporter to contribute to the basolateral step of transepithelial HCO3− secretion into the gut lumen and thereby protect the mucosa from gastric acid. The importance of NBCn1 for this process is demonstrated by a substantial reduction in the basal and forskolin-stimulated rates of HCO3− secretion by the duodena of NBCn1-null mice (180).
VIII) Urinary system. A) Enhancement of renal NH4+ excretion. The renal medullary thick ascending limb is a major site of NH4+ reabsorption, which occurs as NH4+ enters the cell across the apical membrane via NKCC2 and the renal outer medullary K+ channel (ROMK) and then sheds a proton—thereby acidifying the cell—to form NH3 (FIGURE 34). This NH3 exits across the basolateral membrane and then enters the medullary collecting duct, where it is trapped as NH4+ which appears in the urine and thereby plays a major role in urinary acid secretion (316). One would expect that NBCn1, present at the basolateral membrane of mTAL cells, would tend to neutralize pHi during NH4+ reabsorption. Indeed, NBCn1 protein is upregulated during metabolic acidosis and downregulated during metabolic alkalosis. Furthermore, the influx of ammonium and methylammonium in NBCn1-expressing Xenopus oocytes is stimulated in the joint presence of Na+ and HCO3− (556, 557). However, in the absence of an acid load, NBCn1-knockout mice lack an obvious renal phenotype (93).
Figure 34.
Role of NBCn1 in the renal medulla. To avoid absorption of ammonia into the blood, NH4 traveling along the nephron bypasses the renal cortex by passing through the medullary interstitium to the collecting tubules. NH4+ enters thick ascending limb epithelia via K+ channels (ROMK) and the Na/K/Cl cotransporter. NH3 is absorbed across the basolateral membrane into the medullary interstitium, perhaps via a channel. Residual H+ is extruded by NHE and titrated by HCO3− that enters the cell via NBCn1. The basolateral Na-pump has been omitted for clarity.
IX) Reproductive system. A) Possible role in HCO3− reabsorption and/or secretion in the epididymis. An apical distribution of NBCn1 protein in certain cells along the rat epididymis is indicated by the use of the “anti-NBC3” antibody discussed in Appendix VII. At the apical membrane, NBCn1 would be positioned to reabsorb HCO3− from the epididymal fluid, contributing to the luminal acidification that maintains sperm in a quiescent state (771). On the other hand, as discussed above and in Appendix VII, the anti-NBC3 antibody has not been a reliable tool. Independent verification of this apical polarity of NBCn1 distribution is presently lacking. If NBCn1 were instead basolaterally disposed, it could contribute towards HCO3− secretion, and thence fertility, as proposed for NBCe1 (p. 73).
H) CAUSES OF NBCn1 UPREGULATION.
NBCn1 transcript and protein abundance are typically increased by maneuvers that elicit an acidosis, reflecting a general pattern of increased abundance of acid extruders (e.g., NBCe1 and NHE1) and decreased abundance of acid loaders (e.g., AE3) under these conditions.
I) Central nervous system. A) Increased transcript and protein abundance in brain in response to acidosis. In primary cultures of rat hippocampal neurons, lowering extracellular pH below 6.8, a maneuver that presumably lowers pHi to some extent, results in an increase in NBCn1 protein levels (202). The abundance of NBCn1 transcripts and protein in the brain is increased in a rat model of chronic metabolic acidosis (709).
B) Increased protein abundance in response to hypercapnia. Chronic hypercapnia generally increases NBCn1 protein abundance in the neonatal, but not adult, mouse cerebral cortex (463), which may help to counter the acidifying effects of hypercapnia.
II) Circulatory system. A) Increased transcript abundance and transporter activity in heart during pressure-overload hypertrophy. Hypertrophy of ventricles in rats with constricted aortas is accompanied by an increase in ventricular NBCe1 and NBCn1 transcript abundance (1071) and an increase in HCO3−-dependent acid extrusion in myocytes isolated from the hypertrophic ventricles. The presence of NBCn1 protein has yet to be demonstrated in ventricular myocytes. Indeed, the authors do not exclude the possibility that the ventricular NBCn1 transcripts may originate from nonmyocytes. Nevertheless, they suggest that NBCn1 contributes to an increased intracellular Na+ load in hypertrophic myocytes, which would tend to reverse the Na-Ca exchanger, and thereby promote arrhythmia and reperfusion injury (1071).
B) Increased protein abundance in response to hypercapnia. Chronic hypercapnia generally increases NBCn1 protein abundance in the neonatal, but not adult, mouse heart (463).
C) Upregulation of NBCn1-like activity in cardiac myocytes by ANG II. In cat cardiac myocytes, 10−7 M ANG II stimulates HOE64268-insensitive pHi recovery from an acid-load (presumed to represent the sum of NBCe1 and NBCn1 action, Ref. 223). However, this same dose of ANG II inhibits a DIA that is blocked by S085969 (presumed to represent isolated NBCe1 action, Ref. 224). Therefore, the authors of the study conclude that the stimulatory effect of ANG II upon NCBT activity in cat cardiac myocytes represents activation of NBCn1, rather than of NBCe1. However, although some electroneutral NCBT activity is evident in cardiac myocytes (1072), compelling evidence for NBCn1 expression in cardiac myocytes, as opposed to endothelia, is presently lacking.
A pharmacological dissection of the pathway of NCBT activation in cat cardiac myocytes by De Giusti and co-workers led the authors of the study to propose that the stimulatory effect of ANG II upon NBCn1 involves activation of NADPH oxidase, generation of reactive oxygen species (ROS), ROS-induced release of mitochondrial ROS, and stimulation of the extracellular-signal regulated kinase (ERK) signaling pathway (223, 224).
III) Urinary system. A) Increased transcript and protein abundance in kidney in response to acidosis. Multiple reports demonstrate that chronic metabolic acidosis upregulates NBCn1. NBCn1 transcript abundance is increased in the rodent kidney by the oral administration of NH4Cl (207, 688),70 oral administration of HCl (207), or by the acidosis that accompanies hyperkalemia (664). Compared with wild-type controls, NBCe2-null mice exhibit a slightly greater abundance of NBCn1 transcripts and a compensated metabolic acidosis (341). At the level of NBCn1 protein, the acidosis that follows oral administration of NH4+ increases the levels of NBCn1 protein (530, 664), consistent with the increase of NBCn1-like activity in the isolated mTAL of these animals (694), as discussed above. NBCn1 protein levels in the mTAL also increase during the acidosis that accompanies Li+-induced nephrogenic diabetes insipidus (491) and hyperkalemia (431). Finally, NBCn1 protein abundance in the ST-1 mTAL cell line rises following a 24-h exposure to a medium that is acidic (pH 6.8) or that contains 10 mM NH4Cl (557).
B) Increased protein abundance in response to hypercapnia. Chronic hypercapnia generally increases NBCn1 protein abundance in the neonatal, but not adult, mouse kidney (463).
I) CAUSES OF NBCn1 DOWNREGULATION.
Maneuvers that downregulate NBCn1 have only been reported in the brain and kidney.
I) Central nervous system. A) Decreased protein abundance in brain in response to hypoxia. As is usually the case for NDCBE and NBCn2, NBCn1 protein levels generally fall in response to chronic continuous hypoxia in the hippocampus, cerebral cortex, subcortex, and cerebellum of neonatal and adult mice (174). One possibility for these effects is that the hypoxia downregulates a range of energy-requiring systems, including NCBTs. Another is that the hypoxia triggers hyperventilation and thus respiratory alkalosis, which indirectly causes a downregulation of NCBTs.
B) Apparent lack of decreased protein abundance in response to alkalosis. Note that in cultured rat hippocampal neurons, raising extracellular solution to pH 8.3 does not significantly change NBCn1 protein levels, although it might be noted that the high pHo does not cause these neurons to acquire a substantially higher pHi (202).
II) Urinary system. A) Decrease in protein abundance in response to ureteral obstruction. In the renal mTAL of rats in which both ureters are occluded for 24 h by tying with a silk ligature, NBCn1, together with NKCC2 (previous topic), protein abundance is substantially decreased four days after ligature release (1024).
In rats in which only one ureter is obstructed within 48 h of birth, NBCn1 protein abundance is unchanged after 7 wk in the continuously obstructed kidney but is increased ∼30% in the contralateral unobstructed kidney (1025), a pattern consistent with a compensation to the metabolic acidosis that accompanies ureteral obstruction. After 14 wk of continuous unilateral obstruction, both kidneys exhibit a ∼20% decrease in NBCn1 protein abundance (1025), a pattern consistent with a contribution to the metabolic acidosis.
B) Apparently decreased protein abundance in pendrin knockouts. Pendrin/Slc26a4 is a Cl-HCO3 exchanger that mediates the secretion of HCO3− across the apical membrane of renal β- and non-α/non-β-intercalated cells (819). The physiological importance of pendrin is underlined by the observation that perfused collecting ducts from pendrin-null mice absorb rather than secrete HCO3− (819). One immunohistochemical study suggests that NBCn1 protein levels are decreased in the cortical collecting ducts of pendrin-knockout mice, particularly in those cells that usually express pendrin (492). However, as discussed above and in Appendix VII, the anti-NBC3 antibody used in this study yields results that conflict with those obtained using other methods of detecting NBCn1 protein.
C) Decreased protein abundance in response to FK506 administration. NBCn1 protein abundance falls by ∼20% during and following the renal tubular acidosis that accompanies administration of the calcineurin inhibitor FK506 (655). Inasmuch as acidosis per se appears to increase NBCn1 protein abundance, this seemingly counterintuitive observation in FK506-treated mice probably reflects an effect of calcineurin blockade in these animals.
D) Decreased protein abundance in response to alkalosis. Hypercalcemia caused by infusion of parathyroid hormone (PTH) inhibits acid secretion by the proximal tubule but causes a mild, paradoxical metabolic alkalosis and decreased urine pH. The paradox is at least in part due to an increased expression of the B1 subunit of the V-type H+ pump in the inner medullary collecting duct (1026). The challenge also causes a reduction in ammonium excretion. Indeed, rats treated with PTH have a ∼60% decrease in NBCn1 protein abundance in the basolateral membranes of their mTAL and IMCD epithelia (1026). The downregulation of NBCn1 expression in the mTAL would presumably reduce urinary NH4+ (i.e., acid) excretion by the mechanism above. Furosemide-induced alkalosis also reduces NBCn1 protein abundance in the renal medulla of rats (754).
J) CONSEQUENCES OF NBCn1 DYSFUNCTION.
Much of what we know about the pathology of NBCn1 dysfunction comes from NBCn1-null mice that, exhibiting hearing and vision loss, are a potential model of human Usher 2B syndrome. Human genetic studies have linked the SLC4A7 locus with substance abuse, neuropathy, lead accumulation, and breast cancer. These studies are considered below.
I) Central nervous system. A) Neuroprotection from glutamate cytotoxicity in a model of stroke-induced epilepsy. Ischemic injury, such as might follow a stroke, causes the release of glutamate (134) and can be associated with lowering of extracellular magnesium levels (reviewed in Ref. 635). Both glutamate addition (920) and magnesium depletion (42, 933)71 induce seizure-like activity in hippocampal neurons and are models used to study the etiology of stroke-induced epilepsy. Furthermore, glutamate can cause long-term changes in neuronal excitability and cell death (920).
In cultures of mouse hippocampal neurons, NBCn1 knockdown is neuroprotective inasmuch as fewer NBCn1-null than wild-type neurons die when glutamate is applied in the nominal absence of extracellular Mg2+ (202). The neuroprotective role of NBCn1 knockdown in these experiments is consistent with the hypothesis that a reduction in pHi reduces neuronal excitability and is also in accordance with the higher seizure threshold of brain slices from NDCBE and NBCn2 knockout mice (429, 889), the enhanced neuronal survival in NHE1-null mice following ischemic injury (614), and the proposed enhancement of neuronal excitability by NBCe1.
B) Possible contribution to enhanced NMDA-associated neurotoxicity in acidosis. NMDA, an agonist for the NMDA class of ionotropic glutamate receptors, causes cell death through excitotoxicity. As judged by caspase-3 activation, NMDA-induced neuronal death is greater in acidotic rats than in wild-type rats (709). Although upregulation of NBCn1 in acidotic rats normally counters intracellular acidosis, NBCn1 is not upregulated by acidosis in NMDA-treated rats (709). The authors suggest that the lack of enhanced NBCn1 activity might render neurons more susceptible to acid injury in NMDA-treated rats, resulting in increased cell death (709). This hypothesis is consistent with the anti-apoptotic effect of NBCn1, apparently mediated by a rise in pHi, in CSF1-stimulated osteoclasts. An alternative explanation provided by those authors is that NMDA is killing the cells before they have a change to upregulate NBCn1 (709). It is unknown how other neuronally expressed NCBTs are affected by NMDA treatment in this model.
C) Genetic linkage with propensity towards substance abuse. A chromosomal locus associated with a high degree of allelic variation in substance abusers includes the SLC4A7 gene. Ishiguro and co-workers (423) studied the association of addictive behavior with the frequency of occurrence of 22 single-nucleotide polymorphisms (SNPs) in the SLC4A7 gene region. Of these 22 SNPs, 12 occurred with a significantly increased frequency in genomic DNA samples from substance abusers compared with control samples. Of these 12 SNPs, 5 are located in exons but only one, designated rs3755652, changes the predicted coding sequence of the NBCn1 protein, producing a Glu to Lys mutation midway through splice cassette II. The effect of any of these SNPs on the functional expression of NBCn1 activity is untested. Ishiguro et al. note that far more than 22 SNPs may need to be examined in the gene region,72 and they do not exclude the importance of SNPs in neighboring genes. Furthermore, SLC4A7 was not sequenced in its entirety for mutations. Thus the role, if any, of NBCn1 in the etiology of addictive behaviors is unknown. However, the association seems reasonable, given the potential contribution of NBCn1 toward control of neuronal excitability.
II) Sensory organs. A) Vision and hearing loss: a potential model of Usher syndrome. One strain of NBCn1 knockout mice develop blindness and auditory impairment due to the degeneration of photoreceptors in the retina (93) and of hair cells in the inner and outer ear (93, 607). The signs manifested in the knockout mouse are similar to those of Usher syndrome 2B, a disease once linked to the human chromosomal locus 3p23–3p24.2 (383), which is close to the location of the human SLC4A7 gene. However, the original assignment of an Usher locus at 3p23–24 has since been retracted (384). Ironically, molecular evidence gathered in the interim suggests that NBCn1 could be part of the Usher protein network (790), disruption of which is considered to be the molecular basis of Usher syndrome (789).
In the absence of a clear demonstration that mutations in human SLC4A7 gene are linked to Usher syndrome 2B, the Slc4a7-null mouse remains only a potential model of the human disease. In a screen of 172 individuals with Usher syndrome, no mutations localized within the exons that encode the NBCn1-A product (549). This observation does not exclude the possibility that disease-associated mutations could be located in the promoter, introns, or exons included in NBCn1 variants besides NBCn1-A (e.g., those that include cassette III). However, the majority of the 172 individuals in the study exhibited mutations in known Usher-associated genes (549).
B) Genetic linkage with central cornea thickness in mice. Strains of mice with thicker corneas tend to exhibit, in their corneas, greater abundance of certain transcripts, including those encoded by Slc4a7 (601). Corneal thickness in the studied mouse strains is mostly determined by the number of lamellae in the corneal stroma, an observation presumed to be due to altered keratocyte function (601). The role of NBCn1 in this likely complex phenotype, has not been determined.
III) Peripheral nervous system. A) Possible role in hereditary sensory neuropathy. The human SLC4A7 gene locus falls within the boundaries of a region (3p24) that has been linked to a mild sensory neuropathy associated with a chronic cough and gastroesophageal reflux (505). In their 2004 study, Kok et al. (504) sequenced the coding exons of SLC4A7 from genomic DNA amplified from the white blood cells of at least one affected individual and found no nonsynonymous mutations. On these grounds alone, the authors exclude SLC4A7 as a candidate gene for the neuropathy. However, this study does not identify nonsynonymous mutations in the coding exons of any other genes in this candidate region and does not consider the possibility that causal mutations may be located in regulatory regions of the SLC4A7 gene or in additional genes outside of 3p24. Therefore, it is premature to exclude a role for SLC4A7 in the physiopathology of this syndrome.
IV) Circulatory system. A) Genetic linkage with hypertension. A genome-wide association study (GWAS) links a single nucleotide polymorphism, rs13082711, in the SLC4A7 gene locus with slightly elevated systolic and diastolic blood pressure in individuals of European and African ancestry (961). Inasmuch as NBCn1 is a Na+ transporter that is expressed in the vasculature and kidney, the protein has the potential to influence blood pressure. However, the SNP is located ∼10 kb upstream of any known transcriptional start site for NBCn1, and the effect of this SNP, or of yet to be discovered SNPs in the linked region, upon NBCn1 expression has yet to be established.
NBCn1-null mice are mildly hypertensive at rest, an observation that accords with the finding that isolated, precontracted arteries from these mice exhibit a reduced ability to relax in response to acetylcholine application (92). Underlying this phenotype is reduced pHi in vascular cells. Moreover, endothelial nitric oxide synthase (eNOS) is inhibited by acidosis in endothelial cells, whereas rho-kinase signaling is inhibited in vascular smooth muscle cells, rendering contraction of isolated arteries less sensitive to Ca2+ (92).
B) Suggested linkage with blood lead accumulation. A genetic linkage study suggests a quantitative trait locus for erythrocyte lead accumulation, with a linkage peak near the gene locus of 62 genes or putative genes, including human SLC4A7 (1033, 1034). NBCn1 is the only transporter encoded by any of these 62 genes, leading the authors to conclude that NBCn1 affects lead transport. However, this conclusion must be regarded with caution because the authors present no evidence that 1) erythrocytes express NBCn1,73 2) NBCn1 mediates lead transport, or 3) mutations in SLC4A7 affect lead transport in any cell type. On a related note, the author of one study on red blood cells reports evidence consistent with AE1-mediated transport of PbCO3− (886).
V) Lower digestive system. A) Potential role in susceptibility to duodenal ulcers. Helicobacter pylori markedly inhibits the ability of the duodenal epithelium to increase HCO3− secretion in response to the appropriate stimuli (984), leading to duodenal ulceration (reviewed in Ref. 639). Because NBCn1 action supports duodenal HCO3− secretion, NBCn1 defects could increase susceptibility to duodenal ulceration.
VI) Reproductive system. A) Linkage to breast cancer. An association between NBCn1 and cancer was first broached in a 2003 review by Izumi et al. (426). Since then, 10 studies have been published concerning the link between susceptibility to breast cancer and a genetic locus marked by an SNP, rs4973768, that is located in the long terminal exon that encodes the 3′-UTR of NBCn1 (12, 44, 148, 182, 364, 605, 648, 670, 733, 917). It is important to note that it is the genetic locus marked by this SNP, rather than the SNP itself, that is linked to breast cancer susceptibility. Thus it is possible, as noted by the authors of these studies, that dysregulation of the neighboring NEK10 gene, a UV-stimulated kinase that is independently associated with cancers (656), could underlie the genetic susceptibility.
In the first study that linked cancer with SLC4A7, Chen and co-workers (182) identified NBCn1 as a tyrosine kinase substrate expressed in the lobular acini of the breast. The authors make two observations that link NBCn1 to cancer.
1) In the MCF10AT cell line model of breast cancer progression, NBCn1 tyrosine phosphorylation was increased threefold in premalignant and low-grade-lesion-like cells but was decreased twofold in high-grade-lesion-like cells (182). However, the effect of phosphorylation events on the functional expression of NBCn1 activity in tumor cells is untested.
2) NBCn1 protein abundance is decreased in MCF10AT cells and in most of the cancerous breast-tissue samples examined in their study. However, the downregulation of NBCn1 as a contributory factor in breast cancer seems counterintuitive: NBCn1 expression would help cancer cells to maintain a normal pHi in the acidic environment of a tumor and would enhance local extracellular acidity.
Other studies provide evidence that cancer is associated with upregulation of NBCn1. The breast-cancer cell line MCF-7, when overexpressing a truncated ErbB2 receptor, exhibits enhanced acid-extruding capability in part by increasing the abundance of NBCn1 protein in the plasma membrane (546). A report by Wong et al. (1041) demonstrates the importance of NCBT activity, including a DIDS-insensitive component, to pHi regulation in two human and one murine breast-cancer cell lines. Note that both groups discount a significant role for NBCn1 action in cancer cell migration (547, 849), although in theory NCBT activity could contribute to a regulated volume increase.
In summary, the role of NBCn1 in the etiology of cancer progression is presently speculative, although NBCn1 abundance and phosphorylation may be useful markers for cancer screening (182).
2. NDCBE (Slc4a8)
A) SUMMARY.
The electroneutral Na+-driven Cl-HCO3 exchanger NDCBE (encoded by the Slc4a8 gene) exchanges Na+ and two HCO3− equivalents for Cl−, a function shared by NBCn2 under certain conditions. NDCBE has four known variants (-A through -D). In common with several other NCBTs, NDCBE-B is stimulated by the soluble protein IRBIT. Although present in many organs, NDCBE is notably abundant in the brain. In neurons, NDCBE-mediated HCO3− influx enhances neuronal excitability, a role corroborated by a study of NDCBE-null mice. To date, no human pathologies have been linked to NDCBE dysfunction.
B) NOMENCLATURE OF SLC4A8 PRODUCTS.
Following the provisional assignment of NBC1 (now called NBCe1) to refer to Slc4a4 products, and the provisional assignment of NBC2 (now called NBCn1) to refer to Slc4a7 products, two groups simultaneously reported the cloning of two different Slc4 products to which they inadvertently assigned the degenerate name NBC3. We now appreciate that the “NBC3” reported by Pushkin et al. (765) is an Slc4a7 product (139), whereas the “NBC-3” reported Amlal et al. (35) is a partial human Slc4a8 product.74 In fact, the report of the partial sequence postdated the depositing of a full-length human SLC4A8 product by Grichtchenko et al.75 With the physiological characterization of the SLC4A8 product as a Na+-driven Cl-HCO3 exchanger, the product was renamed NDCBE1 (337). With the assumption that the SLC4A8 gene encodes the sole human Na+-driven Cl-HCO3 exchanger, we propose to drop the numerical suffix, and refer to the transporter as NDCBE.
C) MOLECULAR ACTION OF NDCBE.
When expressed in Xenopus oocytes, human NDCBE mediates electroneutral codependent Na+ and HCO3− influx (i.e., Na+ influx requires HCO3− and vice versa), accompanied by a Na+ and HCO3−-dependent Cl− efflux (337). When NDCBE operates in the “reverse” direction, that is to say, mediating the coefflux of Na+ and HCO3− from the oocyte, the transport process has an absolute dependence on extracellular Cl− (337). Thus NDCBE is a Na+-driven Cl-HCO3 exchanger (FIGURE 35A). A preliminary study suggests that it is CO32− and not HCO3− that is the transported base (Fig. 35, B and C; Ref. 335). An NCBT activity attributed to NDCBE in mouse IMCD cells is poorly selective for Na+ over Li+ (35). The approximate stoichiometry of transport is estimated to be 1Na+:2HCO3−, which would require the net countertransport of 1 Cl− for electroneutrality (337). Inasmuch as 1) the estimated unidirectional efflux of 36Cl is manyfold greater than the estimated fluxes of Na+ and HCO3− (337) and 2) the NDCBE-mediated efflux of Cl− has a trans-side Cl− dependence (719), it seems likely that the net movement of chloride by NDCBE is accompanied by a much larger component of futile Cl-Cl self-exchange (FIGURE 35D; Ref. 337). Moreover, this Cl-Cl self-exchange has an absolute requirement for extracellular Na+ and HCO3− (337, 719).
Figure 35.
Molecular action of NDCBE. Possible molecular mechanisms by which NDCBE could operate in an electroneutral mode to exchange Na+ and HCO3− equivalents for Cl−. Note that we have not considered any models that are based on CO32−/H+ cotransport. In the original characterization of NDCBE action, Cl− flux was estimated to be sixfold greater than Na+ flux.
I) Assignment of transport activity to NDCBE. It is straightforward to overexpress NDCBE in a cell such as a Xenopus oocyte and convincingly demonstrate NDCBE activity. However, because of the vagaries of Cl− transport, it can be a challenge to demonstrate that transport activity is indeed due to NDCBE in a setting where the transporter coexists with other NCBTs, Cl-HCO3 exchangers (including those from the Slc26 family), Na-H exchangers, and Cl− channels. Thus reports of NDCBE activity, hereafter referred to as NDCBE-like activity, can rarely be taken at face value. To illustrate, we will note the potential difficulties in using three common approaches to test for the presence of NDCBE activity.
A) 36Cl fluxes. An NDCBE should mediate an efflux of 36Cl that requires extracellular Na+ and HCO3−, and that is blocked by DIDS. As discussed in a later section, the human electroneutral Na/HCO3 cotransporter NBCn2, as expressed in oocytes, does not normally mediate net Cl− transport, but is nonetheless capable of futile Cl-Cl exchange (detected as 36Cl efflux) that requires HCO3− but not Na+, and that is blocked by DIDS. It is not known whether the closely related NBCn1 can also mediate futile Cl-Cl exchange. Thus a convincing demonstration of NDCBE activity requires evidence of net Cl− efflux, either from a direct and quantitative comparison 36Cl influx and 36Cl efflux, or from surface-[Cl−] transients as discussed below. Further complicating matters, in the absence of extracellular Cl−, NBCn2 appears capable of Na+-driven Cl-HCO3 exchange. Even the red cell anion exchanger AE1 has been reported to be capable of exchanging Cl− for either the NaCO3 or the LiCO3− ion pairs under certain conditions (303, 304).
B) Net Cl− fluxes. An NDCBE should mediate a net efflux of Cl−. However, in cells that express a Cl− channel plus NBCe1 or NBCe2, the coupled influx of Na+, HCO3−, and net negative charge would hyperpolarize the cell and thus drive the net efflux of Cl− through the Cl− channel. Voltage-clamp experiments could test this possibility.
C) Washout of intracellular Cl−. The net influx of Na+ and HCO3− mediated by an NDCBE should require intracellular Cl−. Unfortunately, it is notoriously difficult to wash Cl− out of cells (115, 850, 851). Moreover, human NDCBE appears to require extracellular Cl− for NDCBE activity (719). Finally, as noted above, NBCn2 appears to act as a Na+-driven Cl-HCO3 exchanger in the absence of extracellular Cl−. Thus removing extracellular Cl− for the purpose of Cl− washout could have unintended consequences for key NCBTs.
Thus a demonstration of a pHi recovery from an acid load, together with dependence on Na+ and HCO3− but blockade by DIDS, is only the beginning of a physiological assignment of NDCBE. The next critical step is to demonstrate net Cl− efflux under conditions in which Vm does not change or in a cell verified to be devoid of electrogenic NBCs or Cl− channels. Finally, it is advisable to demonstrate the presence of NDCBE protein at the plasma membrane, and show that knock-down of the protein eliminates the hypothesized NDCBE activity. With these caveats in mind, we summarize the reports of Na+-dependent Cl-HCO3 exchange activity and indicate, where possible, which reports are strongly linked to the accompanying presence of NDCBE itself and which are unlikely to involve NDCBE.
D) THE SLC4A8 GENE.
The human SLC4A8 gene maps to 12q13 (337, 673), locus 12q13.13 in version 36.3 of the NCBI human genome map, and includes at least 28 exons spread over 124 kb (FIGURE 36A; Ref. 717). SLC4A8 is located between GALTNT6 (that encodes UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase) and SCN8A. We discuss alternative splicing of SLC4A8 pre-mRNA in the following section. The intrinsic promoter activity of a virally derived long-terminal repeat sequence LTR129, present normally in intron 5 of the human SLC4A8 gene, is activated in testicular cancer. The transcript promoted by the LTR is a short antisense-SLC4A8 intronic sequence. However, this antisense pre-mRNA does not appear to affect NDCBE transcript abundance in seminoma versus normal testicular parenchyma (143). However, when overexpressed in a testicular carcinoma cell line, the antisense sequence does have the capability to reduce NDCBE transcript abundance, acting at the level of pre-mRNA (327).
Figure 36.
SLC4A8 gene structure and NDCBE transcript variants. Scale diagrams showing the human SLC4A8 gene locus together with the position of neighboring genes (A), the position of promoters (P1 and P2), and the position of exons within SLC4A8 (B). Transcript variants are represented, not to scale, as numbered boxes joined by a horizontal line (C). Each numbered box represents the inclusion of that exon in the mature transcript. “//” denotes that all five transcripts include exons 7–24. Exons that include the initiator ATG codon (“M”) and termination codon (“*”) are marked for each transcript. Sequences that are derived from part of a larger exon sequence are labeled with an “a” (e.g., exon 25a is a subdivision of exon 25). Colored exons, or parts of exons, correspond to the protein regions that each encodes, which are identically colored in FIGURE 37. Uncolored exons, or parts of exons, denote untranslated 5′ and 3′ sequence. Exons that are connected with a dashed line are predicted, but not demonstrated, to be included in the mRNA.
E) STRUCTURAL FEATURES AND VARIANTS OF NDCBE.
The human SLC4A8 gene has the capacity to encode at least five variant products named A–E (FIGURES 36, B and C, and 37). Evidence for other minor variants has also been reported, including NDCBE-D′, which has an alternative 5′-UTR. NDCBE-A and NDCBE-B share a common Nt, but NDCBE-A has a longer and different Ct. NDCBE-C and NDCBE-D are identical to “A” and “B,” respectively, but their Nt are truncated by 54 amino acid. NDCBE-E has a longer and different Nt appendage compared with NDCBE-B. Protein variants A–D exhibit NCBT function when expressed in Xenopus oocytes; NDCBE-E ought to be functional but has not been tested. The choice of Nt has no obvious bearing on basal functional expression of NDCBE. However, variants with the shorter Ct (i.e., NDCBE-B and -D) show reduced functional expression compared with variants with the longer Ct (i.e., NDCBE-A and -C). A comparison of the functional expression of NDCBE-A and NDCBE-B with an artificial construct that includes neither the 17-amino acid nor the 66-amino acid sequence (FIGURE 37) demonstrates that the 17-amino acid sequence is inhibitory to the functional expression of NDCBE. The 66-amino acid sequence has no effect on the basal functional expression of NDCBE (717).
Figure 37.
NDCBE protein variants. Scale diagram of protein variants that are encoded by the transcripts represented in FIGURE 36C. Horizontal bars represent protein sequence laid out from Nt to Ct. Vertical bars represent position of α-helical TMs. Protein cassettes are labeled with a number denoting their size in amino acids and colored to denote their genetic origin as shown in FIGURE 36C. A color-matched protein sequence alignment of the variants is provided in Appendix V.
I) Sources of variation in coding sequence among NDCBE variants. Known NDCBE variants differ only in the length of their Nt and the choice of one of two Ct appendages. Unique among NCBTs, transcripts that encode each Ct include mutually exclusive 3′-UTR regions.
A) Alternative promoter choice and truncated Nt. Some variants of NDCBE are truncated by 54 amino acid in their Nt as a result of alternative promoter choice (717). The SLC4A8 gene appears to have two promoters (FIGURE 36C). One promoter (P1) is located just upstream of exon 1 and promotes transcription from exon 1. Transcription initiated at exon 1 produces pre-mRNAs that can be processed to form either NDCBE-C, NDCBE-D, or NDCBE-E. Because exons 2–4 are omitted from NDCBE-C/D transcripts and because neither exon 1 nor exon 5 contains an initiator Met, translation of NDCBE-C/D is predicted to begin with an initiator Met located within exon 6 (that encodes internal Met55 of NDCBE-A/B). Thus in NDCBE-C/D the first 54 amino acids of NDCBE-A/B are absent. The consequence of the loss of this Nt sequence are currently unclear, but preliminary data suggest that unlike NDCBE-B (722), NDCBE-D may not be sensitive to stimulation by IRBIT due to the loss of sequence homologous to that which contains IRBIT binding determinants in NBCe1-B (Parker and Boron, unpublished data). Translation of NDCBE-E is predicted to begin with an initiator Met located within exon 3.
A second promoter (P2) is situated upstream of exon 4 and promotes transcription of NDCBE from exon 4, which encodes an initiator Met. Transcription from this promoter produces pre-mRNAs that can be processed to form either NDCBE-A or NDCBE-B.
B) Alternative Ct and 3′-UTR. NDCBE variants can have either a 17-amino acid or a 66-amino acid sequence appended to the ∼30 amino acid that is common to the Ct of all NDCBE variants. A 66-amino acid Ct appendage is produced when exons 25a-28 are spliced together, making exon 28 the terminal exon (FIGURE 36C). The 66-amino acid appendage (encoded by exons 26–28) is common to NDCBE-A and NDCBE-C. The role of the 66-amino acid Ct is presently unknown; this 66-amino acid Ct can be removed without any apparent consequence to functional expression of the transporter in Xenopus oocytes (717).
An alternative mRNA is produced when the splice machinery does not recognize the exon-25a/intron-25 splice boundary and a polyadenylation signal located ∼3 kb downstream of the start of exon 25 is used to produce a mature, polyadenylated mRNA. This “long” version of exon 25 is defined as a composite terminal exon (268). The 17-amino acid appendage (encoded by exon 25) is common to NDCBE-B, -D, and -E and constitutes an autoinhibitory domain, inasmuch as a mutant NDCBE that lacks the 17-amino acid sequence has a greater functional expression than an NDCBE that includes the 17-amino acid sequence (717).
The 3′-UTR of NDCBE-B/D/E (comprised of exon 25 sequence) is different from and shorter than that of NDCBE-A/C (comprised of exon 28 sequence), probably accounting for the two groups of transcript sizes (9.5–12 kb and 4.4–6.3 kb) observed in Northern blots (35, 337, 717). This choice of alternative 3′-UTRs is a mechanism of variation that is unique among SLC4 products.
C) Cloned NDCBE variants that are demonstrated or likely to exhibit NCBT activity. There are five NDCBE protein variants, the features of which are described below and depicted in FIGURE 37. GenBank protein accession numbers for the variants discussed in this section are provided in Appendix IV.
1) NDCBE-A (NCBT activity demonstrated). Human NDCBE-A is the counterpart of the archetypal mouse NDCBE variant that was reported in Reference 1029. NDCBE-A includes the full-length Nt and the 66-amino acid Ct appendage. NDCBE-A protein is the longest of the four variants. Full-length NDCBE-A has been cloned from a mouse renal cell line (1029) and human brain (717) cDNA preparations.
2) NDCBE-B (NCBT activity demonstrated). NDCBE-B is the archetypal human NDCBE clone reported in Reference 337. NDCBE-B includes the full-length Nt and the autoinhibitory 17-amino acid Ct appendage. As a consequence, NDCBE-B has a lower per-molecule activity than NDCBE-A when expressed in Xenopus oocytes. Full-length NDCBE-B has been cloned from human brain cDNA preparations (337, 717).
3) NDCBE-C (NCBT activity demonstrated). NDCBE-C is identical to NDCBE-A, except that it does not include the first 54 amino acids of the NDCBE-A Nt. NDCBE-C includes the 66-amino acid Ct appendage. Full-length NDCBE-C has been cloned from human brain, heart, and kidney cDNA preparations (717).
4) NDCBE-D and -D′ (NCBT activity demonstrated). NDCBE-D is identical to NDCBE-B, except that it does not include the first 54 amino acids of the NDCBE-B Nt. NDCBE-D includes the autoinhibitory 17-amino acid Ct appendage. NDCBE-D protein is the shortest of the four variants. Full-length NDCBE-A has been cloned from human brain and kidney cDNA preparations (717). NDCBE-D′ is identical to NDCBE-D, except for a 5′ extension to exon 6, which extends the 5′-UTR and is specifically expressed in the heart. The relevance of the 5′-UTR extension is presently unclear (717).
5) NDCBE-E (NCBT activity untested). A singleton cDNA from brain (GenBank DNA accession no. AB018282) that appears to represent a full-length mRNA would, if translated, produce a protein product in which the 16 amino acids encoded by exon 4 of NDCBE-A/B are replaced by 43 amino acids encoded by exon 3. Such modification of the Nt appendage is unlikely to eliminate NCBT activity and thus NDCBE-E is likely to be functional.
D) Predicted NDCBE variants. 1) Partial clones from human cDNA. A number of other human NDCBE cDNA sequences that have been deposited in GenBank have a structure similar to NDCBE-C/D, in that as their transcription begins at a position that is upstream of, but omits, exon 2. If a full-length NDCBE-C/D clone was modified to include any of these partial sequences (e.g., GenBank DNA accession nos. CN286464 and DB090766), the altered sequence of the Nt appendage ought not eliminate NCBT function.
E) Other NDCBE variants. 1) An unusual variant that represents only the isolated Nt. A singleton NDCBE cDNA amplified from thymus (GenBank DNA accession no. AK128321) includes exons 4–11, and exon 12, which becomes a composite terminal exon that includes intron 12 sequence and a polyadenylation signal therein. Such a transcript would encode residues 1–314 of NDCBE-C/D plus 15 novel amino acids encoded by the exon 12 extension, followed by a termination codon: that is, most of the soluble Nt of NDCBE-C/D. It is not clear whether this protein product, truncated within the Nt, would be stable. This cDNA is reminiscent of isolated Nt variants of NBCn1 and NBCn2.
A polyadenylation signal has been identified in intron 5 of SLC4A8 (see Supplemental Table 1 of Ref. 970). The existence of a transcript composed of exon 4 and a composite terminal exon 5 has not been demonstrated, but such an mRNA could, for example, encode amino acid residues 1–44 of NDCBE-B plus the 7-amino acid sequence “GKNCHAV” followed by a termination codon. The function, if any, of such a polypeptide is unknown.
2) Putative variants cloned from rodent cDNA (potentially legitimate transcripts, NCBT activity unlikely). Mouse Slc4a8 encodes NDCBE-A, but it is unknown whether the gene has the capability to encode orthologs of NDCBE-B, -C, or -D. The rat Slc4a8 gene has not been demonstrated to produce orthologs of any of the four human variants. The three reported transcripts from rat kidney are named “NDCBE1-A,” “NDCBE1-B,” and “NDCBE1-C” and appear to be unique to rat and are not the same as the human NDCBE-A/B/C variants. In fact, none of the three rat clones is predicted to encode a functional transporter. Rat “NDCBE1-A” lacks putative TM6 and part of putative TM7. Rat “NDCBE1-B” lacks 25 amino acids in the cytoplasmic Nt close to TM1; at least for NBCe1, this sequence is necessary for functional expression of NCBT activity (575). Rat “NDCBE1-C” lacks both of the regions missing from “NDCBE1-A” and “-B”. The rat Slc4a8 gene does have the potential to encode a complete ortholog of the human NDCBE-A variant, but cDNA representing such a transcript has yet to be cloned.
F) DISTRIBUTION OF NDCBE.
NDCBE expression is particularly abundant in brain, specifically in neurons, although NDCBE transcripts are also abundant in the testis and are expressed to a lesser extent in many other organs. The distribution of NDCBE in specific organ systems is discussed below. The distribution of NDCBE is summarized and compared with that of other NCBTs in TABLE 4.
I) Central nervous system. A) Brain. In Northern blots and RT-PCR analysis of mouse and human RNA preparations, of those organs tested, NDCBE transcripts are particularly abundant in the brain (35, 214, 337, 673, 684, 1029). In Northern blots of human brain shown in Reference 337, probed with oligonucleotide specific for NDCBE-A/B, a ∼12 kb transcript (likely NDCBE-A) predominates over a ∼6.3 kb transcript (likely NDCBE-B).
At the regional level, NDCBE-A/B transcripts are present in RNA preparations from amygdala, caudate nucleus, cerebellum, cerebral cortex, corpus callosum, hippocampus, medulla, substantia nigra, and thalamus (337, 1029). RT-PCR amplifies NDCBE, as well as NBCe1 and NBCn1, transcripts from basal ganglion, occipital cortex, hypothalamus, and frontal lobe (327). The widespread distribution of NDCBE throughout the CNS of rats and mice is confirmed by the use of anti-NDCBE antibodies (553, 889). The use of a pan-NDCBE antibody further extends the distribution of NDCBE to the entorhinnal cortex, midbrain, striatum, pons, thalamus, and olfactory bulb of rats (553). An anti-NDCBE-A/C antibody does not detect NDCBE in the corpus callosum of mice (889).
At the cellular level, and in brain slices, anti-NDCBE-A/B antibodies detect NDCBE in hippocampal pyramidal neurons of human (214), rat, and mouse (176; decreasing in abundance from CA1 to CA3) and in cerebellar Purkinje cells of rat (214) and mouse (176). In mouse brain slices, NDCBE-A/B is further detected in cerebellar granule cells, white matter, substantia nigra, and neurons of the brain stem (176) as well as in unipolar brush cells and cartwheel cells (interneurons) in the dorsal cochlear nucleus and in unipolar brush cells in the cerebellum (489).
NDCBE-A/B is mainly expressed in neurons, as evidenced by staining of brain slices, freshly dissociated neurons, and cultured neurons, and does not have a substantial astrocytic presence (176, 889). However, a Cl−-dependent NCBT activity detected in cultured rat cerebellar astrocytes (500) may be attributable to an alternative Slc4a8, or even an Slc4a10, gene-product. At the subcellular level, NDCBE is abundant throughout the cell body, and to a lesser extent the processes, of hippocampal pyramidal neurons of rats (553). NDCBE-A/C immunoreactivity is predominantly localized to the presynaptic nerve endings of glutamatergic neurons where it is colocalized with glutamate transporters (136, 889), with only a marginal presence in GABAergic neurons (136, 889).
B) Spinal cord. Northern blots detect the presence of NDCBE transcripts in human spinal cord preparations (35), a result not duplicated using an NDCBE-A/B specific probe (337) perhaps indicating that NDCBE-C/D are prevalent here. Indeed, an antibody that should recognize all NDCBE variants detects NDCBE protein in protein preparations from rat spinal cord (553).
C) Choroid plexus. RT-PCR amplifies NDCBE, as well as NBCe1 and NBCn1, cDNAs from human choroid plexus preparations (214) but not from adult mouse or rat CPE (755). NDCBE-A/B protein is however, expressed at the basolateral membrane of choroid plexus epithelia in fetal, but not adult, rats (176).
II) Sensory organs. We are not aware of any reports of NDCBE expression in the eye, ear, or olfactory system. However, NDCBE-like activity has been reported in mammalian lens cells (33, 265).
III) Peripheral nervous system. A) Possible presence in trigeminal ganglion neurons. Although a preliminary study suggested the presence of NDCBE transcripts in trigeminal ganglion neurons (407), a later single-cell PCR study by the same authors was negative for NDCBE in these cells (408).
IV) Respiratory system. A) Trachea. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the human trachea (35).
B) Lung. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the lungs of mice (1029) and humans (35). NDCBE transcripts are also detected in a Calu-3 human airway epithelia cell line (515).
V) Circulatory system. A) Heart. NDCBE-C and NDCBE-D′ transcripts can be amplified from human heart cDNA, and the significant presence of NDCBE transcripts in mouse ventricle preparations has been confirmed by qPCR (31).
B) Vasculature. NDCBE cDNAs have been amplified from preparations of mouse aorta (571).
VI) Musculoskeletal system. A) Skeletal muscle. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in human skeletal muscle (35).
VII) Upper digestive system. A) Stomach. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the human stomach (35).
VIII) Lower digestive system. A) Widespread. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the human pancreas and liver (35). RT-PCR analysis confirms the presence of NDCBE transcripts in the pancreas and extends this distribution to include the human duodenum, ileum, and colon (214).
IX) Lymphatic and immune systems. A) Widespread. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the bone marrow and lymph nodes of humans (35). PCR analysis extends this distribution to include a T-cell-derived cell line (983).
An NDCBE-like activity has been described in rat lymphocytes. Stakisaitis et al. (903) report that Na+-dependent Cl-HCO3 exchange is responsible for the net Cl− efflux, measured as a fall in [Cl−]i, observed when cells are bathed in a solution lacking Cl− (903). However, this result is not consistent with the phenotype of human NDCBE heterologously expressed in Xenopus oocytes: human NDCBE mediates a 36Cl efflux only in the presence of extracellular Cl− (719). The apparent discrepancy between the lymphocyte and oocyte data could represent systematic differences between rat versus human NDCBE, or between native lymphocytes versus heterologous expression in oocytes. However, it is possible that the Cl− efflux observed by Stakisaitis et al. in rat lymphocytes is mediated by NBCn2. Expression of NDCBE and/or NBCn2 in lymphocytes has, to our knowledge, never been formally demonstrated.
X) Endocrine system. A) Widespread. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the thyroid glands of mice (571) and in the thyroid and adrenal glands of humans (35).
XI) Urinary system. A) Kidney. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the kidneys of mice (1029) and humans (35). In rat kidney, Northern blotting reveals that NDCBE transcripts are enriched in the medulla compared with the cortex (1029), a result confirmed by RT-PCR from human RNA preparations (214). In rat kidney, NDCBE transcripts predominate in the inner medulla (986, 1029), with lower abundance in outer medulla (1029). RT-PCR also detects NDCBE transcripts in a mouse IMCD-3 cell line from the inner medullary collecting duct (1029).
Immunocytochemistry using an antibody directed against an epitope common to NDCBE-A and -B has not demonstrated the presence of NDCBE protein in any renal structure except endothelial cells (214), whereas an antibody directed against an epitope common to NDCBE-A and -C exhibits strong immunoreactivity in mouse renal cortical preparations, including isolated CCD preparations (571). Thus it is possible that renal epithelia predominantly express NDCBE-C and/or -D, or novel NDCBE variants.
XII) Reproductive system. A) Male. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the human testis (337). RT-PCR analysis confirms the presence of NDCBE transcripts in rat testis (214) and extends the distribution, in mice, to testis, epididymis, and vas deferens (599).
B) Female. NDCBE transcripts have been detected by RT-PCR of mouse oocytes, ovary, uterus, and vagina (275, 599). According to an NCBI-curated database of ESTs, NDCBE transcripts may be abundant in human mammary gland preparations (Appendix VI).
C) Placenta. Northern blots of RNA preparations reveal the presence of NDCBE transcripts in the human placenta (35).
G) PHYSIOLOGICAL ROLES OF NDCBE.
NDCBE likely contributes to pHi regulation in all of the cell types in which it is expressed. In neurons, pHi, and therefore NDCBE action, influences neuronal excitability. In epithelia, NDCBE has been suggested to contribute to HCO3− secretion and Cl− reabsorption.
I) General. A) pHi regulation. Investigators have proposed that NDCBE-like activity plays a key role in pHi regulation in many mammalian cell types, including pyramidal neurons from the CA1 region of hippocampi (850, 889), aortic endothelial cells (280), fibroblasts (157, 531), cultured vascular smooth muscle cells (459, 775), cultured lens cells (33), esophageal epithelia (973), glomerular mesangial cells (114, 115), thyrocytes (477), intrahepatic bile duct cells (354, 913), lymphocytes (786), a monocyte-lymphoma cell line (541), macrophages (949), and mouse oocytes (275). Note that the presence of NDCBE mRNA or protein is not documented for all of these cells types.
II) Central nervous system. A) Enhancement of neuronal excitability. In 1992, Church (194) found that the switch from a CO2/HCO3−-free HEPES buffer to a CO2/HCO3− buffer is associated with enhanced neuronal excitability in CA1 neurons of rat hippocampal slices. He suggested that excitability increases because CO2/HCO3− causes pHi to rise. Later Bevensee et al. (78) demonstrated that CA1 neurons in fact exist in two resting pHi states, those with a relatively low and those with a relatively high pHi (78). In those with a relatively low initial pHi in a CO2/HCO3−-free buffer, the switch to CO2/HCO3− causes a net increase in steady-state pHi (78, 121). This elevated pHi is most likely maintained at least in part by NDCBE and NBCn1, which are robustly expressed in pyramidal neurons from this region. In CA1 neurons with a relatively high initial pHi, the switch to CO2/HCO3− has no effect on steady-state pHi or causes it to fall (78, 121). The most straightforward explanation of Church's data is that he was mainly working with low-pHi neurons.
Three studies on genetically altered mice support Church's hypothesis: the NDCBE- and NBCn2-null mice (which lack a single acid extruder) exhibit signs of reduced neuronal excitability, whereas the AE3-null mouse (which lacks a single acid loader) has a reduced seizure threshold (378). The link between neuronal pHi regulation and excitability is reviewed in References 76, 186, 187, and 898.
B) Role in central nervous system plasticity. The switch of glycine evoked responses of cartwheel cells (glycinergic interneurons) in the dorsal cochlear nucleus of mice from excitatory to inhibitory follows the lowering of [Cl−]i, which shifts ECl from a value more positive to a value more negative than Vm (see review in Ref. 34). The shift in ECl is subsequent to cellular acidification which follows rapid spiking events (489). The shift requires CO2/HCO3− and is blocked by H2DIDS (489). Immunohistochemistry reveals that these cells express NDCBE and thus, taken together, these data are consistent with the hypothesis that NDCBE contributes to the manifestation of inhibitory signaling (489). Lowering of [Cl−]i by an NDCBE-like activity has also been implicated in the development of inhibitory GABA-evoked responses during central nervous system maturation. A similar role is shared by the NDCBE-like SLC4 homolog ABTS-1 in nematodes.
C) Potential contribution to CSF secretion. The basolateral presence of NDCBE protein in the choroid plexus epithelium of fetal, but not adult, rats suggests that NDCBE contributes to CSF secretion in early developmental stages (176). In adult rats, the basolateral step of transepithelial HCO3− transport across the CPE is likely mediated by NBCn2 and, to a lesser extent, by NBCn1. Recall that NDCBE transcripts are present in the choroid plexus of the adult human, where it is possible that NDCBE plays a functional role.
III) Urinary system. A) Unproven role in Cl− reabsorption in the PT. Na+-dependent Cl-HCO3 exchange has been proposed to contribute to the basolateral step of Cl− reabsorption by the renal proximal tubule (29, 419, 833). However, this hypothesized Na+-dependent Cl-HCO3 exchange activity has not been demonstrated to be directly coupled to Na+ flux in the proximal tubule, and is difficult to isolate experimentally due to the much larger HCO3− flux mediated by the electrogenic Na/HCO3 cotransporter NBCe1 in the same basolateral membrane (29, 419, 677, 833). Three observations speak directly to the issue of whether NDCBE contributes to proximal-tubule Cl– reabsorption: 1) anti-NDCBE immunoreactivity has not been observed in the PT;76 2) although removing peritubular Na+ does indeed reduce Cl− efflux across the basolateral membrane of the proximal tubule, removing peritubular Cl− does not lead to a change in intracellular Na+ activity; and 3) the 1:1 Cl−:HCO3− exchange stoichiometry estimated for the activity (506) is different from the expected 1:2 coupling ratio for NDCBE activity (337, 719).
The molecular identity of the transporter(s) responsible for this basolateral Na+-dependent Cl-HCO3 exchange phenomenon have yet to be determined.77 NDCBE is also suggested to contribute to NaCl reabsorption in the collecting ducts of Na+-depleted mice.
H) CAUSES OF NDCBE UPREGULATION.
I) General. A) Increased NDCBE-like activity in response to cell shrinkage. In Chinese hamster ovary cells, a rise in pHi upon exposure to hypertonic medium is dependent on extracellular Na+, Cl−, and HCO3−, consistent with the activity of NDCBE (793). It is unlikely that NHE1 contributes inasmuch as the pHi increase is insensitive to amiloride. A question that arises is whether Na+-driven Cl-HCO3 exchange would contribute to a net increase in intracellular osmotically active particles, and thereby to a regulatory volume increase. If the non-HCO3− buffering power of the cell were infinite (so that pHi did not change), then the Na+-driven Cl-HCO3 exchanger would be osmotically silent (219): the uptake of 1 Na+ would be balanced by the efflux of 1 Cl−, and the equivalent uptake of 2 HCO3− would have no net effect as intracellular buffers released H+ to titrate the HCO3− to CO2 + H2O, which would exit the cell. However, at finite non-HCO3− buffering powers, Na+-driven Cl-HCO3 exchange activity would cause pHi to rise, leading to a rise in [HCO3−]i, more so for lower buffering powers, which would in principle contribute to cell swelling. Finally, to the extent that a rise in pHi stimulates Cl-HCO3 exchange, the net effect would be the intracellular accumulation of NaCl, which would contribute to cell swelling.
II) Central nervous system. A) Increased protein abundance in some brain regions in response to metabolic acidosis. At the level of the whole brain, NDCBE protein abundance is unperturbed by metabolic acidosis in rats (553). However, at the regional level, the protein abundance in the hippocampal CA3 region, traditionally a region of lower NDCBE expression than other regions of the hippocampus, is 2.5-fold more abundant in acidotic than control rats (553). NDCBE abundance is also increased in some populations of cortical neurons (553). The presumed increase in acid extrusion in these cells would tend to counter decreases in pHi causes by acidosis.
B) Lack of increased protein abundance in response to hypercapnia. Different from the response of NBCn1 in the brain, but similar to the response of NBCn2, NDCBE protein abundance is not increased in the brain of mice exposed to chronic hypercapnia (463).
III) Lymphatic and immune systems. A) Increased transcript abundance in a model of systemic lupus erythematosus. Systemic lupus erythematosus (SLE) is an autoimmune disease causing inflammation in multiple organs. Two mutants of the T-cell receptor ζ chain have been linked to SLE, and overexpression of these unstable mutants in murine T-cells is associated with an eightfold increase in NDCBE transcripts in these cells (983). The cause and effect of this upregulation remains to be studied, although it has been noted that apoptosis of thymocytes, T-cell precursors, is increased by cellular alkalinization, yet is inhibited by stilbene derivatives, consistent with a proapoptotic action of NDCBE (980). Note that this proapoptotic effect of NDCBE contrasts with the antiapoptotic effect of NBCn1 in osteoclasts.
IV) Urinary system. A) Increased transcript abundance and activity in a renal cell line in response to metabolic acidosis. In a mouse collecting duct cell line, metabolic acidosis increases NDCBE transcript abundance and induces a robust DIDS-sensitive, Na+- and HCO3−-dependent pHi recovery attributed to NDCBE. These results are consistent with a protective role for NDCBE during metabolic acidosis (35).
B) Increased NDCBE-like activity in CCD of alkali-loaded rabbits. Although the renal CCD normally reabsorbs HCO3−, the CCD in alkali-loaded animals secretes HCO3− and thereby tends to restore a normal (i.e., less alkaline) blood pH (636). In alkali-loaded rabbits, the blood-to-lumen movement of HCO3− across the basolateral membrane of CCD β-intercalated cells involves a stilbene-sensitive, Na+ and Cl−-dependent mechanism consistent with the activity of NDCBE (271). However, the molecular identity of the transporter(s) responsible for this activity is presently unknown.
C) Increased NDCBE-like activity in the CCD of Na-deficient mice. Feeding mice a Na+-restricted diet leads to the upregulation of a novel thiazide-sensitive NaCl reabsorption pathway in cortical collecting ducts, contributing to an increase in Na+ reabsorption (959). Leviel and co-workers make three observations consistent with a contribution of NDCBE to the NaCl-reabsorption pathway (571): 1) NDCBE protein is expressed in mouse CCD preparations, 2) an apical thiazide-sensitive NDCBE-like activity is upregulated in the intercalated cells of Na+-depleted mice, and 3) the CCDs of NDCBE-null mice that have been fed a Na+-restricted diet are unable to reabsorb NaCl (571). According to the authors' model, the parallel action of apical pendrin would recycle HCO3− out of the cell and mediate the requisite uptake of Cl− (571). Not demonstrated are stilbene sensitivity of the NaCl reabsorption pathway and the presence of NDCBE protein in the apical membranes of CCD intercalated cells.
I) CAUSES OF NDCBE DOWNREGULATION.
I) Central nervous system. A) Decreased protein abundance in brain in response to hypoxia. Chronic continuous hypoxia (CCH) decreases the amount of NDCBE protein in the cortex, subcortex, hippocampus, and cerebellum of adult rat brains, but generally not in neonates (175). CCH also reduces the abundance of NBCn1 and NBCn2.
II) Reproductive system. A) Decreased transcript abundance in testes of feminized mice. NDCBE transcripts are abundant in testis, but virtually absent in the testes of feminized mice, that is to say, mice with a disrupted androgen receptor, or those whose testes cannot descend due to surgical intervention prior to puberty (693). The authors suggest that downregulation of this and other transporter activities may perturb the composition of seminiferous fluid, inhibiting germ cell maturation and contributing to the feminized phenotype.
J) CONSEQUENCES OF NDCBE DYSFUNCTION.
NDCBE null-mice exhibit reduced neuronal excitability and renal Na+-reabsorption defects. Human pathologies that are linked to NDCBE dysfunction have yet to be described.
I) Central nervous system. A) Reduced network excitability and increased presynaptic plasticity in NDCBE-null mice. As discussed, the action of NDCBE is hypothesized to enhance neuronal excitability. Four observations demonstrate that neuronal excitability is reduced in NDCBE-null mice.
1) In the pyramidal layer of the CA1 region of hippocampal slices prepared from NDCBE-null mice, the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs) is twofold less than in preparations from wild-type mice (889). A reduction in mEPSC frequency can be mimicked in wild-type preparations by simultaneously lowering both pHo and pHi. Moreover, an increase in mEPSC frequency occurs in preparations from NDCBE-null mice with an increase in either pHo and pHi together or pHi alone. These results are consistent with the idea that the reduced excitability in NDCBE-null mice results from a low pHi (889).
2) In the CA1 region, the amplitude of population spikes evoked by stimulating Schaffer collaterals is lower in NDCBE-null than in wild-type mice (889). Wild-type mice subjected to a second round of stimulation exhibited population spikes with a 1.5-fold greater amplitude than in the first round, whereas NDCBE-null mice exhibited population spikes with a 2-fold greater amplitude than in the first round, consistent with a greater presynaptic plasticity in the mutant mice (889).
3) During repetitive stimulation, the time constant for the release of vesicle contents from boutons of hippocampal slices was greater (i.e., the release was slower) in NDCBE-null versus wild-type mice (889).
4) NDCBE-null mice have an increased latency until onset of seizures/ictal activity in response to interperitoneal administration of the proconvulsive substances pentylenetetrazol and pilocarpine and to hyperthermia (889).
II) Urinary system. A) Defective regulation of NaCl reabsorption. NDCBE-null mice fed a Na+-deficient diet are unable to upregulate a thiazide-sensitive NaCl reabsorption pathway that would tend to enhance Na+ retention (see Ref. 571). Thus, under conditions of Na+ restriction, NDCBE dysfunction might be expected to be associated with volume depletion.
3. NBCn2/NCBE (Slc4a10)
A) SUMMARY.
The electroneutral Na/HCO3 cotransporter NBCn2 (encoded by the SLC4A10 gene) cotransports Na+ and HCO3− with accompanying futile cycles of Cl-Cl exchange. NBCn2 appears to undergo a mode switch into a Na+-driven Cl-HCO3 exchanger (“NCBE”) under certain assay conditions when extracellular Cl− is unavailable. Some investigators report that mouse and rat Slc4a10 products act in NCBE mode even under physiological conditions. In common with several other NCBTs, NBCn2 is stimulated by the soluble protein IRBIT. NBCn2 is present in many organs but is notably abundant in the central nervous system, where its action is predicted to enhance neuronal excitability. Such a role is corroborated by a study of NBCn2-null mice. Genetic disruption of the SLC4A10 gene locus in humans is linked with autism and epilepsy.
B) NOMENCLATURE OF Slc4a10 PRODUCTS.
Slc4a10 products were initially termed NCBE following a report that mouse Slc4a10 functions as a Na+-driven chloride/bicarbonate exchanger in both Xenopus oocytes and HEK-293 cells (1021). However, under near-physiological conditions, the isotopic Cl− efflux associated with human SLC4A10 activity in Xenopus oocytes does not require extracellular Na+ and does not represent a net movement Cl− but rather Cl-Cl exchange. Thus,the human SLC4A10 product normally functions as an electroneutral Na/HCO3 cotransporter (FIGURE 38A) that the authors propose to rename NBCn2 (719), an acronym that we will use in following sections in place of NCBE.
Figure 38.
Molecular action of NBCn2. Possible molecular mechanisms by which NBCn2 could operate in an electroneutral mode to cotransport Na+ and HCO3− with accompanying futile cycles of HCO3−-dependent Cl-Cl self-exchange (A). In the absence of extracellular Cl−, NBCn2 performs Na+-driven Cl-HCO3 exchange (B). The Slc4a10 gene product from mice and rats is reported to act like NDCBE (FIGURE 35) even in the presence of extracellular Cl−. Note that we have not considered any models that are based on CO32− or H+ cotransport.
C) MOLECULAR ACTION OF NBCN2.
Four functional studies demonstrate that NBCn2, whether it is human NBCn2 heterologously expressed in Xenopus oocytes, or mouse or rat NBCn2 in mammalian cells, mediates a Na+-dependent HCO3− uptake that can be blocked by DIDS (212, 317, 719, 1021). One of these studies further demonstrates that the transport mediated by human NBCn2 is electroneutral (719). Three groups provide evidence that the Na+ -dependent HCO3− influx mediated by NBCn2 is accompanied by the efflux of 36Cl (212, 719, 1021). However, controversy has arisen over whether this efflux represents a net movement of Cl− under physiological conditions. Two groups of investigators (212, 1021) interpret the Cl− efflux data as evidence that mouse and rat NBCn2 mediate Na+-driven Cl-HCO3 exchange (like NDCBE) under physiological conditions (FIGURE 38B). A third group (719) provides evidence that the 36Cl efflux that accompanies human NBCn2 action represents futile cycles of Cl-Cl exchange under physiological conditions (FIGURE 38A) and that NBCn2 is a second electroneutral Na/HCO3 cotransporter (the other being NBCn1). However, NBCn2 does behave as a Na+-driven Cl-HCO3 exchanger in the absence of extracellular Cl− (719). The evidence pertaining to NBCn2-mediated Cl− movement in these studies is considered below.
I) A study of mouse Slc4a10 expressed in Xenopus oocytes. When expressed in Xenopus oocytes, mouse Slc4a10 mediates HCO3−-dependent isotopic influxes of Na+ and Cl− and efflux of Cl−. This Cl− efflux is maximal in the collective presence of extracellular Na+, Cl−, and HCO3−. In their original description of “NCBE,” Wang and co-workers (1021) interpreted these data as evidence for Na+-driven Cl-HCO3 exchange. However, three additional observations in the same study are more consistent with Cl-Cl exchange in parallel with Na/HCO3 cotransport rather than a classical model of Na+-driven Cl-HCO3 exchange activity. 1) The transporter mediates an influx (in addition to an efflux) of 36Cl in the presence of extracellular Na+ and HCO3−, 2) Cl− influx does not require extracellular Na+ or HCO3−, and 3) Cl− efflux requires extracellular Cl− (i.e., trans-side dependence).
II) A study of human SLC4A10 expressed in Xenopus oocytes. To address the question of whether the transporter mediates a net efflux of Cl−, Parker et al. (719) in a later study expressed human NBCn2-B in oocytes and used a Cl−-sensitive microelectrode to monitor [Cl−] on the extracellular surface ([Cl−]S) of the oocyte. Bulk extracellular [Cl−] was maintained at 10 mM to enhance electrode sensitivity. The authors found that, when exposed to CO2/HCO3−, oocytes expressing either AE1, human NDCBE, or squid NDCBE exhibited a transient rise in [Cl−]S, indicating a HCO3−-stimulated net efflux of Cl−. However, oocytes expressing NBCn1 or NBCn2-B exhibited no [Cl−]S increase. Thus, under these conditions, SLC4A10 does not mediate a net efflux of Cl−. The authors also found that the NBCn2-mediated 36Cl efflux that is stimulated by application of HCO3−, is independent of the presence of extracellular Na+. Instead, the 36Cl fluxes must represent a futile Cl-Cl exchange that accompanies the true physiological function, the apparent 1:1 coupled influx of Na+ and HCO3− (FIGURE 38A).
Although the preceding study demonstrates that NBCn2 does not normally mediate Na+-driven Cl-HCO3 exchange, an interesting observation is that human NBCn2-B appears to be capable of Na+-driven Cl-HCO3 exchange under a particular nonphysiological condition, namely, the absence of extracellular Cl−. Removing extracellular Cl− reduces 36Cl efflux by half (presumably by eliminating Cl-Cl exchange), and the remaining 36Cl efflux now requires both extracellular Na+ and HCO3− (719). Moreover, in the absence of extracellular Cl−, NBCn2 mediates a robust, pHi recovery. Thus it appears that, with no extracellular Cl− to participate in Cl-Cl exchange, the transporter is now obligated to engage in Na+-driven Cl-HCO3 exchange (FIGURE 38B).
III) Studies of rodent Slc4a10 expressed in mammalian cells. In the case of mouse NBCn2-B heterologously expressed in HEK-293 cells (1021) or mouse NBCn2-A and rat NBCn2-C/D heterologously expressed in 3T3 cells (212, 317), removing extracellular Cl− blocks HCO3− influx. The most straightforward explanation for these data is that removing extracellular Cl− switches the activity of Slc4a10 from electroneutral Na/HCO3 cotransport to Na+-driven Cl-HCO3 exchange, as predicted by Parker et al. (719), but that the concomitant depletion of intracellular Cl− eliminates this activity. On the other hand, if extracellular Cl− removal fails to deplete intracellular Cl− over the time period examined, then an alternative explanation is that the rodent transporter expressed in mammalian cells behaves differently than the human transporter expressed in oocytes.
A study of rodent-Slc4a10-transfected 3T3 cells (212) includes four novel observations that are provided as evidence that Na+-driven Cl-HCO3 exchange is the normal mode of action for Slc4a10.
1) Slc4a10-transfected cells alkalinize at a faster rate than nontransfected cells in response to the acute removal of extracellular Cl− as if, in the absence of Cl−, the driving force for Cl− efflux and thence Na/HCO3 influx is increased (212). This observation agrees with the findings in Reference 719, namely, that NBCn2 can act as a Na+-driven Cl-HCO3 exchanger in the absence of bath Cl−. The 3T3-cell data are complicated by the presence of substantial endogenous anion exchange activity in these cells (212) that would tend to enhance the rate of alkalinization upon Cl removal in Slc4a10-transfected cells.
2) 36Cl efflux occurs at a greater rate from 3T3 cells transfected with rat Slc4a10 and bathed in a HCO3−-buffered solution compared with similar cells bathed in a HEPES-buffered solution, but only in the presence of Na+ (212). In this respect, the behavior of rat Slc4a10 in 3T3 cells appears to differ from the behavior of human NBCn2. When expressed in oocytes, human NBCn2-B mediates a Cl− efflux that is independent of the presence of extracellular Na+ (719).
3) Cl− efflux, but not Cl− influx, is enhanced in the presence of HCO3−, an observation offered as evidence that the Cl− efflux mediated by mouse Slc4a10 represents a net efflux (212). An alternative explanation is that the extent of 36Cl influx over the 2-min duration of the influx assay is underestimated due to the simultaneous 36Cl efflux mediated by NBCn2. With human NBCn2-B expressed in oocytes, 36Cl efflux also is enhanced by HCO3−, which simply appears to stimulate Cl-Cl exchange (719).
4) A comparison of the rates of Na+ and HCO3− influx into Slc4a10-transfected cells, calculated from measurements of fluorometric dyes, suggest that the Na:HCO3 cotransport ratio is 1:2 for rodent Slc4a10. Thus the Cl− efflux would have to be net to maintain electroneutrality (212). However, there are potential risks in comparing rates obtained by two different methods (Na+- versus pH-sensitive dyes) as well as concerns that the Na dye (CoroNa) might not be suitable for quantitative measurements at low [Na+] (see Ref. 641). Cited as validation for the Slc4a10 stoichiometry measurements, the stoichiometry of NBCn1 calculated by this method was, as expected, 1:1.
In summary, it is unknown whether human SLC4A10 and rodent Slc4a10 exhibit true functional differences, or whether the disparities between the studies are methodological in nature. Indeed, as human SLC4A10 is capable of shifting between “NCBE” and “NBCn” modes, it is not inconceivable that rodent Slc4a10 could behave differently from human SLC4A10.
D) THE Slc4a10 GENE.
The human SLC4A10 gene occupies 27 exons over ∼360 kb (FIGURE 39, A and B) on chromosome 2q24.2 (1081). A singleton EST (GenBank DNA accession no. BP229748) from fetal brain provides evidence that the SLC4A10 locus may extend 150 kb further upstream than presently thought, filling the apparent gap between SLC4A10 and its upstream neighbor TBR1. TBR1 encodes a transcription factor that is expressed in cortical neurons during development (133). The TBR1 paralog EOMES/TBR2 is the upstream neighbor of SLC4A7 (FIGURE 31A), indicating a longstanding relationship between these two gene families. The downstream neighbor of SLC4A10, DPP4, encodes the plasminogen receptor dipeptidyl peptidase IV (328). DPP4 protein interacts with and enhances the activity of NHE3 in the proximal tubule (324, 325) and also dampens stimulation of duodenal HCO3− secretion by degrading glucagon-like peptide (418). It is unknown whether DPP4 influences the activity of NCBTs.
Intron 1 of SLC4A10 contains a binding site for the transcription factor and tumor-suppressor p53 (1031). SLC4A10 transcription is downregulated in a colon carcinoma cell line by 5-fluorouracil induction of p53 expression (see supplemental data for Ref. 1031). It would be interesting to know whether p53 in fact decreases the expression of NBCn2.
E) STRUCTURAL FEATURES AND VARIANTS OF NBCn2.
Variation among mammalian NBCn2 transcripts arises by alternative splicing at any or all of the following three sites in Slc4a10 pre-mRNA (317).
I) Sources of variation in coding sequence among NBCn2 variants. The NBCn2 gene is only known to include a single promoter. As depicted in FIGURE 39C and FIGURE 40, there are two major sources of variation between NBCn2 transcripts: the optional inclusion of a 30-amino acid cassette in the Nt and the choice of one of two Ct appendages (a 22-amino acid appendage that ends “-ETCL” or a 4-amino acid appendage that ends “-SSPS”). A further, minor source of variation arises due to the optional inclusion of a single alanine residue due to an apparently degenerate splice boundary. All four NBCn2 clones reported to date are predicted to include an autoinhibitory domain and IRBIT binding determinants in their Nt (718, 722).
Figure 40.
NBCn2 protein variants. Scale diagram of protein variants that are encoded by the transcripts represented in FIGURE 39C. Horizontal bars represent protein sequence laid out from Nt to Ct. Vertical bars represent position of α-helical TMs. Protein cassettes are labeled with a number denoting their size in amino acids and colored to denote their genetic origin as shown in FIGURE 39C. All NBCn2 variants are presumed to include an autoinhibitory domain and IRBIT-binding determinants in their Nt. A color-matched protein sequence alignment of the variants is provided in Appendix V.
A) Optional Ala. The 5′ end of exon 7 which, at least in rat NBCn2, contains a cryptic splice-site that, when utilized by the splice machinery, shortens the transcript by three nts (CAG) resulting in the loss of a single Ala residue from the Nt of the transporter. Thus the Ala is optional in rats. In humans, the Ala is always present.
B) Cassette A. NBCn2 transcripts can differ in the inclusion or exclusion of exon 8, sometimes referred to as DNA cassette or insert “A,” the excision of which by splicing removes sequence that encodes a 30-amino acid protein “cassette A” (FIGURES 39C and 40). The inclusion of cassette A is predicted to extend the Nt loop (FIGURE 15).
C) Choice of alternative Ct (“-SSPS” or “-ETCL”). Exon 26, sometimes referred to as DNA cassette or insert “B,” encodes the 4-amino acid Ct appendage “-SSPS” (FIGURES 39C and 40). The excision by splicing of cassette B allows translational read-through to an alternative downstream termination codon in exon 27. Thus removal of cassette B produces NBCn2 variants with a longer and different Ct (21-amino acid of NBCn2-C/D replaces 4-amino acid of NBCn2-A/B). The 21-amino acid Ct terminates with a type I consensus PDZ-domain binding motif “ETCL” (317).
In an astrocytic cell line, the inclusion of the 21-amino acid Ct appendage in rat NBCn2 (rb2NCBE, see below) results in increased colocalization of NBCn2 with the actin cytoskeleton, compared with rb1NCBE, a variant with the 4-amino acid Ct appendage (317). The cytoskeletal attachment of the 21-amino acid appendage is mediated via EBP50 and ezrin (562). Consistent with the hypothesis that an enhanced cytoskeletal interaction is important for efficient trafficking of the transporter to the plasma membrane, cytoskeletal disruption by cytochalasin B treatment of fibroblasts expressing rb2NCBE results in the loss of transporter activity from the plasma membrane (562). Indeed, in a mouse fibroblast cell line, rb2NCBE variant traffics more efficiently to the plasma membrane than rb1NCBE (317). However, the opposite is observed when NBCn2 variants are expressed in MDCK cells (756) perhaps, the authors suggest, due to the lack of an accessory protein.
II) Cloned NBCn2 variants that are demonstrated or likely to exhibit NCBT activity. GenBank protein accession numbers for the variants discussed in this section are provided in Appendix IV.
A) NBCn2-A (NCBT activity demonstrated). NBCn2-A lacks the 30-amino acid cassette A and includes the 4-amino acid Ct appendage that ends with “-SSPS.” It is orthologous to the rat variant rb5NCBE. Full-length NBCn2-A transcripts have been isolated from a mouse pancreatic cell line cDNA library (1021), and from rat hippocampus and mouse brain cDNA preparations. In the brains of mice, NBCn2-A appears to be the most abundant NBCn2 variant in the subcortex (598).
B) NBCn2-B (NCBT activity demonstrated). NBCn2-B includes the 30-amino acid cassette A and the 4-amino acid Ct appendage that ends with “-SSPS.” It is most orthologous to the rat variant rb1NCBE, which does not include the optional Ala that is always present in humans. Full-length NBCn2-B transcripts have been cloned from human kidney cDNA (719) and from rat hippocampus and mouse brain cDNA preparations. In the brains of mice, NBCn2-B appears to be the most abundant NBCn2 variant in the medulla (598).
C) NBCn2-C (NCBT activity untested). NBCn2-C lacks the 30-amino acid Nt cassette A and includes the 21-amino acid Ct appendage that ends with “-ETCL.” The orthologous rat variant is rb4NCBE. Full-length NBCn2-C transcripts have been cloned from mouse brain cDNA preparations (598).
D) NBCn2-D (NCBT activity demonstrated). NBCn2-D includes the 30-amino acid Nt cassette A and the 21-amino acid Ct appendage that ends with “-ETCL.” The most similar rat variant is rb2NCBE, which does not include the optional Ala that is always present in humans. Full-length NBCn2-D transcripts have been cloned from mouse brain cDNA (598).
E) rb3NCBE (NCBT activity untested). This variant cloned from rat brain is similar to NBCn2-D except that, instead of lacking exon 26, it lacks exons 24–26. The now out-of-frame exon 27 encodes a singleton His residue that is immediately followed by a termination codon. Thus, in rb3NCBE, the most Ct 83 amino acids of NBCn2-D are replaced by a single His. By comparison with an artificially truncated version of human NDCBE that has a Ct of similar length (717), rb3NCBE ought to be functional.
III) Predicted NBCn2 variants. A) Predicted variants with an alternative Ct “-RS.” Although not yet demonstrated to be included in a full-length transcript, a novel fragment amplified from human brain cDNA includes a partial, out-of-frame exon 26 created by the utilization of a cryptic splice site with exon 26 (see supplemental material of Ref. 719). The internally spliced exon 26 includes the third base position of a codon hung-over from exon 25 (the triplet in the novel fragment now encodes an Arg rather than the usual Ser) and a singleton Ser codon followed by a termination codon. Thus the fragment is predicted to be part of a transcript that encodes a novel variant that terminates in a protein kinase C consensus phosphorylation site “KRS.” Such variants would be identical to NBCn2-A/B except that, in the novel variants, the 2-amino acid “-RS” replaces the 4-amino acid “-SSPS” in NBCn2-A/B.
IV) Other NBCn2 variants. A) An unusual variant that represents only the isolated Nt. One variant, rb7NCBE (GenBank DNA accession no. AY579377), which originates from rat brain, is identical to rb5NCBE at the transcript level save for the inclusion in rb7NCBE of sequence derived from a cryptic exon between exons 11 and 12. The novel sequence encodes 18 amino acids followed by a termination codon. Thus rb7NCBE encodes an isolated but near-complete cytoplasmic Nt (equivalent to residues 1–451 of human NBCn2-A) plus 18 novel residues. It is possible that the premature termination codon included in this mRNA would make it a target for nonsense-mediated decay (170). rb7NCBE is reminiscent of isolated Nt variants of NBCn1 and NDCBE.
B) rb6NCBE (potentially legitimate transcript, NCBT activity unlikely). This variant (GenBank DNA accession no. AY579376), which originates from rat brain cDNA, is identical to rb5NCBE at the transcript level (i.e., like NBCn2-A, it omits cassette A and includes cassette B) except for the alternative splicing, at nonconsensus splice sites, of exons 14 and 15. The effect is that the last two-thirds of exon 14 are discarded, together with the first half of exon 15. Furthermore, the remaining exon 15 sequence is out of frame and encodes only seven amino acids followed by a termination codon. The resulting rb6NCBE protein product encodes the entire Nt and TM1–3 of NBCn2. However, the frame shift and premature termination at a point within putative TM4 make it unlikely that this product is functional or even stable.
F) DISTRIBUTION OF NBCn2.
NBCn2 is predominantly expressed in the central nervous system. The distribution of NBCn2 in the CNS and in other organ systems is discussed below and compared with the distribution of other NCBTs in TABLE 5.
I) Central nervous system. A) Brain. NBCn2 transcripts are most abundant in and widely distributed throughout the CNS (214, 317, 399, 428, 684, 719, 1021) in the forebrain (frontal, temporal and occipital lobes and olfactory bulb), the limbic system (in the hypothalamus, geniculate nucleus, thalamic eminence, hippocampus, substantia nigra and in the amygdala, caudate nucleus, and putamen of the corpus striatum), and the hindbrain (cerebellum, medulla, spinal cord).
In mouse brains, an antibody directed against an epitope common to all known variants of NBCn2 has the highest level of immunoreactivity in the cerebral cortex, cerebellum and hippocampus, and the least in subcortex (174). This pattern is similar to the distribution of NBCn1 but different from that of NDCBE, which is most abundant in the subcortex compared with the other three tested regions (175). Within CA1-CA3 regions of the hippocampus, NBCn2 also exhibits an expression pattern complementary to that of NDCBE, inasmuch as NBCn2 expression is greatest in the CA3 region (429), whereas NDCBE expression appears to be strongest in the CA1 and CA2 regions (176). A study of the developmental expression of NBCn2 transcripts in rodent brains is presented in References 317 and 399. The abundance of NBCn2-A through -D appears to vary among brain regions in mice (317, 598, 600). At the cellular level, NBCn2-A and NBCn2-B transcripts are more abundant than NBCn2-C and NBCn2-D transcripts in neurons (317).
In prenatal rat hippocampal neurons, NBCn2 protein is detected by immunocytochemistry in the soma of freshly dissociated cells (177), as well as in the processes (177) and the plasma membrane of the soma (177, 199) of cultured cells (see cartoon in FIGURE 24A).
As far as astrocytes are concerned, one group detected NBCn2 transcripts in mouse cortical astrocytes (317), whereas another did not detect NBCn2 protein in rat hippocampal astrocytes (177). The difference may be explained by 1) phenotypic differences between astrocytes isolated from different brain regions (56), 2) astrocytes not maintaining substantial levels of NBCn2 protein despite the presence of mRNA, or 3) a species difference. As to the splice variants expressed, an analysis of transcripts indicates that astrocytes, in distinction to the neurons discussed above, lack NBCn2-A and NBCn2-B messages, but instead are enriched in NBCn2-D transcripts (317).
B) Choroid plexus and dura mater. In immunohistochemistry studies, the most striking anti-NBCn2 immunoreactivity is in the choroid plexus (see cartoon in FIGURE 28). Antibodies raised against the Nt common to all NBCn2 variants, or to one or the other alternative NBCn2 Ct78 (i.e., short Ct versus long PDZ-binding-motif containing Ct) all stain the basolateral membrane of the choroid plexus epithelium in human, mouse, and rat (113, 177, 216, 429, 755, 756). Immunogold staining of mouse choroid plexus epithelial cells from the third and fourth ventricles, using an anti-Ct NBCn2 antibody, confirms the basolateral distribution and shows the protein to be especially abundant in “highly folded membrane processes between neighboring epithelial cells.” This study also confirms the cytosolic disposition of the Ct (755). An analysis of rodent cDNAs indicates that NBCn2-A may be the predominant transcript in choroid plexus (755). NBCn2 transcripts are also present in the dura mater (399).
II) Sensory organs. A) Eye. In the retinas of mice, NBCn2 transcripts have been detected in the neuronal cell layer and pigment epithelium (399). An NDCBE-like activity, which could be mediated by NBCn2, has been reported in mammalian lens cells (33, 265).
B) Ear. NBCn2 transcripts have been detected in the cochlear ganglion (399).
III) Peripheral nervous system. A) We are not aware of any reports of NBCn2 expression in the peripheral nervous system.
IV) Respiratory system. A) Lung. A mutation in the SLC4A10 gene is associated with lung cancer, although the expression of NBCn2 in healthy or cancerous lung tissue has not been formally demonstrated.
V) Circulatory system. A) Heart. NBCn2 transcripts have been detected in preparations of heart ventricles from mice (31).
VI) Musculoskeletal system. A) Skeletal muscle. NBCn2 transcripts have been detected in human skeletal muscle preparations (see supplemental data in Ref. 719). Transcripts including the PDZ-binding domain (i.e., NBCn2-C and -D) appear to predominate over those lacking the PDZ binding domain (i.e., NBCn2-A and -B; see supplemental data of Ref. 719).
VII) Upper digestive system. A) Stomach. NBCn2 transcripts have been detected in preparations of human (214) and mouse (399) stomach.
VIII) Lower digestive system. A) Widespread. NBCn2 transcripts have been detected in preparations of human duodenum (214), rat ileum (1021), and human liver (719). In the liver, NBCn2-A appears to be more abundant that NBCn2-B-D (see supplemental data of Ref. 719).
IX) Lymphatic and immune systems. A) Lymph node and spleen. According to an NCBI-curated database of ESTs, the human lymph node is a potential site of NBCn2 transcription (Appendix VI). NBCn2 transcripts have also been detected in preparation of rat spleen (317).
X) Endocrine system. A) Pancreas. NBCn2-A was originally cloned from a mouse pancreatic beta-cell line (1021).
B) Pituitary gland. NBCn2 transcripts have been detected in a preparation of rat pituitary glands (1021).
XI) Urinary system. A) Kidney. The archetypal NBCn2-B variant was cloned from human kidney cDNA (719), and transcripts have also been detected in rat kidney preparations (1021). In humans, NBCn2 transcripts are present at least in the renal cortex (214).
XII) Reproductive system. A) Male. NBCn2 transcripts have been detected in preparation of rat and mouse testes (1021) and in preparations of mouse epididymis and vas deferens (599).
B) Female. NBCn2 transcripts have been detected in preparations of ovary, uterus, and vagina of mice (599).
G) PHYSIOLOGICAL ROLES OF NBCn2.
At present, studies of NBCn2-specific activity in situ are few in number. The major issues, to some extent common to all the NCBTs, are that 1) a cell (particularly neurons and choroid plexus epithelia) may express more than one NCBT, 2) specific blockers are not available, 3) physiological dissections of NCBTs are not straightforward because of the difficulty of performing a sufficiently wide range of assays on one cell, and 4) evidence from knockout mice is complicated by dysregulation of other transporters. Ideally, one might use immunocytochemistry or single-cell PCR to verify that an identifiable cell has a single NCBT, which could be approached with standard techniques for studying pHi regulation. Failing that, knockdown approaches are promising, although one must remain wary of secondary effects.
I) Central nervous system. A) Neuronal excitability. A comparison of average resting pHi values of cells in mouse brain slices shows no significant different between wild-type and NBCn2-knockout mice (429). Even so, the knockout of NBCn2 substantially slows the HCO3−-dependent pHi recovery from an intracellular acid load in a mouse brain slice from the hippocampal CA3 region and isolated cells from the mouse choroid plexus (429). As discussed earlier in this review, a faster recovery of pHi following neuronal firing leads to a faster recovery of neuronal excitability.
B) CSF secretion. Basolateral NBCn2, along with other basolateral NCBTs (FIGURE 28), is suitably positioned to mediate the basolateral step in the transepithelial movement of Na+ and HCO3− from the blood into the CSF, thereby contributing to CSF secretion. This role appears to be confirmed by exhibition of CSF secretion defects in an NBCn2-null mouse, although other transporters are perturbed in the CPE of these mice.
C) Possible role in central nervous system maturation. The detection of NBCn2 transcripts in the CNS of embryonic mice led to the hypothesis that the expression of Slc4a10 is a developmental switch in which the gene-product lowers [Cl−]i and thereby shifts ECl from a value more positive to a value more negative than Vm. Such a shift in ECl would causes the GABA-evoked response to change from excitatory to inhibitory (399). Indeed, the probable Na+-driven Cl-HCO3 exchanger ABTS-1 fulfills this role in nematodes. However, the underlying premise of this hypothesis in mice is that Slc4a10 mediates Na+-driven Cl-HCO3 exchange. Inasmuch as human NBCn2 is unable to effect net Cl− movements under physiological conditions (719), the original hypothesis is unlikely to be correct in humans. NDCBE action could theoretically fulfill this role in humans, given the correct temporal expression pattern.
II) Reproductive system. A) Possible role in sperm capacitation. In 1996, Zeng et al. (1090) reported that the recovery of pHi in sperm following an acid-load is stilbene-sensitive and requires Na+, Cl−, and HCO3− (1090). Subsequently, Wang et al. (1021) speculated that NBCn2 contributes to the alkalinization of sperm required for their capacitation. This speculation could be correct if 1) the removal of external Cl− converted the activity of NBCn2 to Na+-driven Cl-HCO3 exchange, 2) the depletion of intracellular Cl− blocked this Na+-driven Cl-HCO3 exchange activity, and 3) the NDCBE-like activity is not mediated by NDCBE. However, formal demonstration of NBCn2 expression in these cells is presently lacking.
H) CAUSES OF NBCn2 UPREGULATION.
To our knowledge there are no reports of maneuvers that increase the transcript abundance, protein abundance, or plasma membrane abundance of NBCn2. However, preliminary reports show that the functional expression of NBCn2 is enhanced by coexpression with IRBIT (718, 722).
I) CAUSES OF NBCn2 DOWNREGULATION.
We are not aware of any reports of maneuvers that decrease NBCn2 transcript abundance. Two studies have reported maneuvers that downregulate NBCn2 at other levels.
I) General. A) Inhibition of NBCn2 activity by PKA. As expressed in 3T3 cells, NBCn2 activity is regulated by phosphorylation, such that 1) the action of PKA is inhibitory to the functional expression of the transporter and 2) inhibition of PKA enhances functional expression of the transporter (562). It is unknown whether this phenomenon reflects a direct effect of PKA action on NBCn2.
II) Central nervous system. A) Decreased protein abundance in the brain in response to hypoxia. NBCn2 protein levels generally fall in response to chronic continuous hypoxia (CCH) in the hippocampus, cerebral cortex, subcortex, and cerebellum of neonatal and adult mice (174).
Downregulation in hypoxia is also characteristic of NBCn1 and NDCBE, except that the downregulation of NDCBE occurs in adults but generally not in neonates (175).
B) Lack of decreased protein abundance in response to hypercapnia. Neither NBCn2 nor NDCBE protein abundance is increased in the brains of mice exposed to chronic hypercapnia (463).
J) CONSEQUENCES OF NBCn2 DYSREGULATION.
As expected for a gene most abundantly expressed in the central nervous system, most reported signs of NBCn2 ablation in mice and pathologies linked to the SLC4A10 gene in humans relate to the brain and bear on changes in neuronal excitability (e.g., reduced sensitivity to proconvulsants, epilepsy, autism). Defective CSF secretion is also described in NBCn2-null mice, but the molecular basis of this pathology appears to be complex and may not be primarily due to loss of NBCn2 activity per se. A single report of a genetic linkage between SLC4A10 and lung cancer bears on the consequence of NBCn2 dysfunction outside of the brain.
I) General. A) Potential role in tumor growth. A report that Na+-dependent Cl-HCO3 exchange activity attributed to NBCn2 is important for pHi regulation, and therefore proliferation, in the breast cancer cell lines EMT6, MCF7, and MDA-MB231 (1041), must be interpreted with caution. The authors assumed that NBCn2 was the only HCO3− transporter that could mediate recovery of pHi from an acid load in a mammalian cell. In fact, any of the five NCBTs could mediate such a pHi recovery. Subsequent studies have identified NBCn1 as a major pH regulator in MCF7 cells (546), although the presence of NBCn2 in these cells and in other breast cancer cell lines cannot be discounted.
II) Central nervous system. A) Reduced neuronal excitability in mice with a disrupted Slc4a10 gene. As noted above, in the hippocampal CA3 region, the recovery of pHi from an acid load is slower with an NBCn2-null than with a WT mouse. Moreover, in both NBCn2-null and WT mice, the frequency of 4-aminopyridine–induced seizure-like events in the CA3 region is reduced by neuronal acidification (429). Thus it is not surprising that the subsequent recovery in the frequency of these seizure-like events is slower in the knockout than in the WT mice (429). Consistent with this indication of reduced neuronal excitability, NBCn2-knockout mice have an increased tolerance to seizure induction, both in terms of latency until onset and in survival rate (429).
B) Genetic linkage to epilepsy in humans. Gurnett et al. (360) described a 13-year-old girl presenting with cognitive dysfunction and complex partial epilepsy was determined to have a balanced chromosomal translocation t(2;13)(q24;q31) involving the SLC4A10 gene. The break point on chromosome 2q24 disrupted SLC4A10 at a point between exons 2 and 3, with the rest of the gene joined at a breakpoint in a gene desert on chromosome 13q31.79 If this translocation event resulted in reduced NBCn2 activity due to haploinsufficiency, then the neurological phenotype would be inconsistent with the described phenotype of Slc4a10 knockout mice, which have no behavioral abnormalities and a reduced, rather than increased, neuronal excitability (429). We consider five possible explanations for this apparent disparity:
1) Systematic difference. A haploinsufficient human is not a null mouse and chemically induced seizures are not complex partial epilepsy.
2) Creation of an uninhibited NBCn2. By real-time quantitative PCR (qPCR), Gurnett et al. (360) found that the level of NBCn2 mRNA (specifically that encoded by exons 1–3) was only about half of normal in lymphocytes from the patient. Thus, although loss of NBCn2 protein could contribute to the disease phenotype, protein levels remain untested in this patient. In addition, it is similarly unknown whether the relocated, telomeric end of SLC4A10 (i.e., exon 3 onwards), from its new locus, might be capable of producing a truncated or alternative gene-product with enhanced function. It is noteworthy that Xenopus oocytes expressing an Nt truncated NBCn2 (i.e., lacking sequence encoded by exons 1–3) exhibit a pHi recovery rate twofold greater than cells expressing the full-length transporter (718). However, the only start codon identified in the SLC4A10 gene to date is that in exon 1.
3) Overcompensation. In the choroid plexus of mice, the loss of NBCn2 is compensated by the redistribution of NHE1 in its place (216). It is conceivable that a loss of neuronal NBCn2 might be overcompensated in the proband by a more active population of Na+ and/or base transport mechanisms as is the case in NHE1-null mice. Although NHE1-null mice might be expected to be less sensitive to seizures because of their compromised neuronal pHi regulation (1082), they in fact exhibit seizures (70) due to an enhanced neuronal excitability, perhaps caused by the observed compensatory increase in the functional expression of Na+ channels (355, 1051). The demonstrated ∼60% decrease of the acid-loading AE3 protein in NHE1-null mice (1060) could also contribute towards a higher pHi and thus a lower seizure threshold (378).
4) Position effects. The chromosomal translocation might affect the transcription of genes other than SLC4A10, or of microRNAs. Although the report by Gurnett et al. focuses on the SLC4A10 locus on 2q24.2, it is worth noting that 13q22–13q31 is also a susceptibility locus associated with seizures (376). The translocation of the broken arm of chromosome 13 to chromosome 2, and vice versa, might result in altered expression of chromosome 2 or 13-translocated genes such as SCN1A. SCN1A is located at 2q24.3, encodes a voltage-gated Na+ channel, and is implicated in epilepsy (669).
5) The phenotype is a function of reduced inhibitory signaling. The loss of NBCn2 could result in a reduced excitability of inhibitory neurons, due to an inability to regulate pHi. Alternatively, if the loss of NBCn2, which can act in an NDCBE-like manner under some conditions, were either directly or indirectly to result in a rise in neuronal [Cl−]i, the result might be to convert glycine and GABA signals from the usual inhibitory, to excitatory postsynaptic potentials.
Two other epileptic individuals have since been identified as having an SLC4A10 haploinsufficiency. The first individual has a de novo chromosomal deletion of 6.6 Mb that encompasses SLC4A10 and numerous downstream genes in 2q24.2–2q24.3, including SCN1A, and is both epileptic and mentally retarded (516). The deletion of epilepsy-associated SCN genes in this individual confounds attempts to assess the contribution of NBCn2 loss to this pathology. The second individual has a de novo chromosomal deletion of 6.4 Mb that encompasses SLC4A10 and numerous upstream genes in 2q24.1–2q24.2. This individual is epileptic, autistic, and mentally retarded (516). Again, the deletion of other genes in this individual makes it difficult to assess the contribution of NBCn2 loss to this pathology. Nevertheless, NBCn2 remains a compelling candidate in the pathogenesis of epilepsy.
C) Genetic linkage to autism. A spontaneous deletion of exon 1 of the SLC4A10 gene has been identified in a pair of autistic twins (857). What is presently unclear is 1) whether the two phenomena are linked, 2) how perturbation of the SLC4A10 gene might result in autism, and 3) whether the deletion of exon 1 would result in a haploinsufficiency of functional NBCn2. As noted above, exon 1 includes the only reported initiation codon for NBCn2. It is interesting to note that the twins have not presented with epileptic symptoms.
A third individual (discussed two paragraphs above) has a de novo chromosomal deletion of 6.4 Mb that encompasses SLC4A10 and numerous upstream genes in 2q24.1–2q24.2 and is autistic, epileptic, and mentally retarded (516). The contribution of NBCn2 loss to the autistic pathology is difficult to assess inasmuch as autism, in some individuals, is associated with genetic deletions in 2q24.1–2q24.2 that do not encroach into the known extent of the SLC4A10 gene locus (679).
Another linkage to autism is to be found in the gene that is the upstream neighbor of SLC4A10: a recent study found that the TBR1 gene-product associates with the product of the AUTS2 autism-susceptibility candidate gene (69).
D) Defective CSF secretion in mice with a disrupted Slc4a10 gene. Mice with a targeted disruption of Slc4a10 exhibit a 78% decrease in brain ventricular volume (429). A subsequent study indicates that, in NBCn2-null mice, the deficit of basolateral Na/base cotransport is at least partly compensated by the relocation of normally apical NHE1 to the basolateral membrane (216). Apical NHE1 is in turn replaced by an as yet unidentified amiloride-insensitive NHE. Further preliminary work by Damkier and Praetorius indicates that AQP1 and the Na pump, transporters critical to CSF secretion, also have reduced abundance in the choroid plexus of NBCn2-null mice, reflecting a compensation that would favor cell survival (and thus the integrity of the blood-brain barrier) at the expense of CSF secretion (215). Thus, although the CSF secretion defect in NBCn2-null mice is appropriate, given the location and presumed role of NBCn2, the pathogenesis of this phenotype in NBCn2-null mice is likely more complex than the simple deletion of NBCn2.
E) Unproven genetic linkage to depression. Although 2 out of 16 SNPs examined in a study of the human SLC4A10 gene locus were initially linked to major depressive disorder, the linkage was found to be not significant upon further statistical analysis (846).
III) Sensory organs. A) Suggested genetic linkage to primary open-angle glaucoma. Defective CSF secretion results in an increased pressure differential between the CSF and intraocular compartment that may contribute to the development of glaucoma (briefly reviewed in Ref. 595). Because NBCn2-null mice have reduced ventricle volume, it was hypothesized that variations in the SLC4A10 gene locus might be associated with the incidence of glaucoma in humans. However, a genetic linkage study did not establish a link between affected individuals and seven common SNPs in SLC4A10 (595), and no gross ocular phenotype has been reported for Slc4a10-null mice (429). These data alone do not preclude the possibility that defects in Slc4a10 contribute to glaucoma for four reasons: 1) NBCn2 is a major contributor to CSF secretion and thus a link to glaucoma is sensible, 2) seven SNPs are likely a minor sampling of the true genetic variability among human SLC4A10 genes, 3) none of the 7 SNPs tested has been shown to affect the functional expression of NBCn2, and 4) no studies that describe the lack of ocular pathology in Slc4a10-null mice have been reported.
IV) Respiratory system. A) Genetic linkage to lung cancer. A genetic linkage study found somatic mutations in SLC4A10 in 2 of 11 lung carcinoma samples.80 One is a P690L substitution at the distal end of EL3, close to the extracellular end of TM6. What effect, if any, this alteration might have on NBCn2 function remains untested. The second polymorphism is not predicted to change the NBCn2 protein sequence, being a synonymous change within the codon for K901, a residue located at the intracellular end of TM11. To date, the expression of SLC4A10 products in healthy or cancerous lung tissue has not been demonstrated.
VI. RELATIVES OF NCBTs IN MAMMALS
Here we provide a brief overview of the anion exchangers AE1–3; the three Na+-independent, electroneutral Cl-HCO3 exchangers, which are closely related both structurally and functionally to the NCBTs. This brief analysis should assist in the interpretation of material presented above. Interested readers might consult comprehensive AE reviews, such as those by 1) Jennings (436), who provides an excellent evaluation of the physiological studies that first defined the molecular actions of AE1; 2) Alper (22), who provides an extensive survey of current knowledge concerning the structure, function, splice variants, distribution, physiological importance, and pathologies associated with the AEs; and 3) Cordat and Casey (153) and Romero et al. (805), who provide a thorough consideration of the physiological and pathological importance of HCO3− transporters in general, including the AEs and NCBTs of the Slc4 family, as well as the anion exchangers of the Slc26 family.
In the last part of this section, we summarize current knowledge concerning the two most recently described members of the Slc4 family, Slc4a9 and Slc4a11. Slc4a9 is unique inasmuch as it is like the electrogenic NCBTs in structure but was named as if it mediated Cl-HCO3 exchange. Slc4a11 is unique inasmuch as it is reported to have neither AE-like nor NCBT-like activity, but instead retains the borate-transport activity common to fungal and plantal Slc4-like products. However, the functions of both Slc4a9 and Slc4a11 remain controversial.
A. Anion Exchangers (AE1–3; Slc4a1–3)
1. Summary
The Cl-HCO3 exchangers of the Slc4 family act as acid-loaders (HCO3− export mechanisms) and are the basolateral counterparts of the apically distributed Slc26 family of anion exchangers (FIGURE 1). In erythrocytes, the HCO3− fluxes mediated by AE1 contributes towards the Bohr effect. In addition, red cell AE1 acts as a scaffold protein providing a linkage between the membrane and cytoskeleton, contributing towards maintenance of the structural integrity of the circulating cell. In the kidney, AE1 action contributes towards the maintenance of blood pH and supports urinary acidification. Thus AE1-related pathologies include red cell fragility and whole body acidosis. AE2 exhibits the widest distribution of the three AEs and contributes towards pH balance in a variety of cell types. In the kidney, AE2 contributes towards HCO3− reabsorption in the late PT81 and in the TAL. AE3 is expressed in the eye, brain, and heart. AE3 dysfunction is associated with blindness, epilepsy, and cardiac hypertrophy.
2. Nomenclature
AE1, AE2, and AE3 are named for their anion exchange function, which physiologically is the one-for-one exchange of Cl− for HCO3−. AE1 is the product of the Slc4a1 gene and is the founder member of the family. AE1 is also referred to as “band 3,” being the third largest protein band evident on coommassie-stained gels of red cell membrane preparations. In older literature, AE1 is sometimes referred to as capnophorin (literally “smoke carrier”). AE2 and AE3 are, respectively, the products of the Slc4a2 and Slc4a3 genes. AE4 refers to the Slc4a9 gene product, which is discussed separately below.
3. Molecular action
Under physiological conditions, all three AEs perform Na+-independent, electroneutral Cl-HCO3 exchange (509, 587, 728), a capability not shared with NCBTs. AEs can also perform futile cycles of HCO3−-independent Cl-Cl self-exchange, a mode often exploited in assays as a proxy for physiological AE function (i.e., Cl-HCO3 exchange). Other nonphysiological and minor transport modes described for AEs, but never for NCBTs, include the exchange of monovalent anions such as Br− and NO3− and divalent anions, which are cotransported with H+ thereby maintaining electroneutrality, such as SO42− and oxalate2− (e.g., Refs. 359, 401, 434, 439, and 861). In light of these observations, and the observations of Boron and co-workers, which suggest that at least NBCe1 and NDCBE are Na+-coupled CO32−, as opposed to HCO3−, transporters, it is intriguing to speculate that the AEs could be considered to be H+-coupled CO32− transporters. The divergence of transporters that perform H+ versus Na+ coupled cotransport of a particular substrate has been documented for other solute carrier orthologs. For example, members of the Slc23 protein family in mammals are Na+ coupled, whereas bacterial Slc23-like orthologs are H+ coupled (reviewed in Ref. 940). Due to the permissiveness of the AEs, it is possible that they could be capable of borate transport like their orthologs in plants and fungi. There is some indirect evidence that borohydride (BH4-) is a substrate of AE1 (435).
Some have suggested, based on studies of red blood cells, that AE1 is involved in the transport of alkali (i.e., Na and Li) and heavy metals (i.e., Cd, Cu, Mb, Pb, and Zn) carried in the form of anionic complexes (18, 303, 304, 323, 610, 886, 974). For example, Na+ is suggested to be transported by AE1 in the form of NaCO3−, the same substrate predicted by kinetic analysis to be carried by an NCBT from squid axons (99). At present we can only speculate on how the transport mechanisms of AEs and NCBTs are related, although at least NDCBE and NBCn2 are capable of performing anion exchange (i.e., consistent with NaCO3-Cl exchange) under certain conditions and in principle the Na/HCO3 cotransport mediated by NBCn1, NBCe1, and NBCe2 could be achieved by variations on a NaCO3-HCO3 exchange mechanism.
After correction for transporter abundance, all three full-length mammalian AE products mediate anion exchange at similar rates (301, 551, 862). Among the three, AE2 is uniquely pHi sensitive, becoming increasingly inactive as pH decreases over the range 9–6 (401, 905). Multiple groups have also reported variations in the relative efficacy of anion transport inhibitors between pairs of AEs (e.g., see Refs. 551, 862, 905), although conclusions appear to vary between expression systems. Further distinctions between the paralogs become apparent when we consider individual gene variants and their distribution.
4. Genome
AE-encoding genes are considerably more compact (∼14–20 kb) than NCBTs genes (∼100–360 kb), which is mainly a function of shorter introns and 3′-UTR regions. The human SLC4A1 gene that encodes AE1 covers ∼20 kb at chromosomal locus 17q21-q22. The SLC4A2 gene that encodes AE2 covers ∼17 kb at chromosomal locus 7q36.1. The SLC4A3 gene that encodes AE3 covers ∼14 kb at chromosomal locus 2q36. Only human chromosome 2 carries more than one SLC4 gene (SLC4A3 and SLC4A10).
As previously discussed above, genes that encode AEs and NCBTs share many common exon boundaries (FIGURE 7), which indicates their relatedness. On the other hand, some unique exon boundaries are shared only among AEs and are not shared with NCBTs, indicating that the three AEs diverged from a common ancestor after the divergence of the common NCBT ancestor.
5. Structural features and variants
AE proteins are predicted to have a similar structure to NCBTs, due to their sequence similarity at the amino acid level (see FIGURES 2 AND 3). AEs and NCBTs both have a large cytosolic Nt, multiple transmembrane spanning segments, and a relatively short Ct. The crystal structure of the AE1 Nt was first described in Reference 1091 and low-resolution structural reconstructions of the TMD have been reported in References 1022, 1023, 1068, and 1069. One group has suggested that the structure of the AE1 TMD may be similar to that of prokaryotic ClC Cl/H antiporters (1069). There are some key structural differences between AEs and NCBTs, among AEs and among variants of each AE.
A) AMINO TERMINUS.
In contrast to the Nt of NBCe1, which is absolutely required for Na/HCO3 cotransport activity (276, 634), the Nt of AE1 is not at all required for basal anion exchanger function (351, 353, 468, 568). However, the Nt of AE1 contains important trafficking determinants (975) and has many protein binding partners (recently reviewed in Ref. 141). The Nt of AE2 is not required for anion exchange activity (587, 1095) but contains determinants that influence the pH sensitivity of AE2 (527, 907, 1095). The necessity of the Nt of AE3 for anion exchange function is untested, but determinants in the Nt of AE3 contribute toward its relatively poor plasma membrane accumulation compared with AE1 and AE2 (301). The three-dimensional structure of the AE1 Nt dimer at pH 4.8 has been solved at 2.6-Å resolution by X-ray crystallography (1091). A subsequent study of the AE1 Nt dimer in solution at neutral and close-to-neutral pH indicates that the original, “low pH” crystal structure is a good representation of the native AE1 Nt structure at physiological pH (1109).
B) TRANSMEMBRANE DOMAIN.
The AEs have a short third extracellular loop compared with NCBTs. The shortest is that of AE1, which lacks the glycosylation sites that are a common feature of AE2, AE3, and NCBTs. AE1 is also unique in having a glycosylation site in its fourth extracellular loop. As is the case for the NCBTs, glycosylation of AE1 is not essential for transport (155, 352). The AEs lack the four conserved cysteines in EL3 common to NCBTs, although AE2 has a single Cys in this loop. A comparative study of the accessibility of substituted cysteines in the latter half of the TMD indicates structural differences between AE1 and NBCe1 in this region (1112). Lysine-rich motifs, also found in the NCBTS, at the extracellular ends of putative TMs 5 and 13 contribute to the stilbene sensitivity of the AEs (63, 435, 699). Finally, a glutamate in putative TM8 forms an important part of the transport gate of AE1 (437, 438, 440, 443). The three-dimensional structure of the AE1 TMD dimer has been solved at 7.5-Å resolution using cryo-electron microscopy (1068, 1069). However, this resolution is not sufficient to visualize all TMs nor to assign amino acid sequence to regions of electron density.
C) CARBOXY TERMINUS.
The sequence of the Ct is well conserved among AEs and is ∼40 amino acid in length, far shorter than that of the NCBTs. The AE Ct lacks the characteristic Lys-rich stretches common to all NCBT Cts and the class I PDZ binding motif characteristic of some NCBTs. As is the case with the NBCe1 Ct, the Ct of AE1 contains vital trafficking determinants (184, 203, 300, 976).
D) AE VARIANTS.
Each of the three AE genes produces one full-length product and one to three additional truncated variants, transcribed under the control of internal promoters. Thus all AE variants differ only in their extreme Nt sequences (the variants discussed below are depicted in Appendix V). Posttranscriptional processing of AE transcripts is not known to include the splicing that, for NCBTs, results in the optional inclusion of protein cassettes within the Nt and Ct, and variations in extreme Ct sequences.
SLC4A1 contains two alternative promoters. The first produces the full-length gene product erythrocyte AE1 (eAE1; 911 amino acid) and the second produces the truncated kidney AE1 (kAE1; 846 amino acid) that lacks the first 65 amino acid of eAE1 (127, 520, 521) and thereby loses the ability to bind ankyrin (251). A similar transcriptional mechanism produces truncated versions of the AE2 and NDCBE products (e.g., NDCBE-A versus NDCBE-C in FIGURES 36C AND 37).
SLC4A2 contains three alternative promoter regions: a, b, and c (1030). The first produce the full-length gene product AE2a (1,241 amino acid in humans, 1,237 amino acid in mice). The second can produces one of two shorter products, AE2b1 or AE2b2, depending on which of two closely positioned transcriptional start sites are utilized. In AE2b1, the first 17 amino acid of AE2a are replaced by a novel 3-amino acid sequence. In AE2b2, the first 17 amino acids of AE2a are replaced by a novel 8-amino acid sequence. In mice, the third promoter region can produce one of two even-shorter products, AE2c1 or AE2c2,82 again depending on which of two closely positioned transcriptional start sites are utilized. Mouse AE2c1 is the shortest of all AE2 products and is a truncated version of the other AE2 products, such that AE2c1 initiates at Met199 of AE2a. In mouse AE2c2, the first 193 amino acid of AE2a are replaced by a novel 27-amino acid sequence. The complex transcriptional and posttranscriptional mechanisms that produce each variant are depicted in detail in References 550 and 637. In side-by-side comparisons, AE2b variants have a greater functional expression than AE2a or AE2c1, whereas AE2c2 activity was undetectable (527). It is possible that these observations might at least in part be explained by differences in surface expression; however, AE2c1 clearly has an alkaline shifted pHo dependence (527) compared with the other forms. Alternative promoter choice, resulting in small, seemingly insignificant alterations in Nt sequence, are also common to NBCn1 (e.g., NBCn1-A versus NBCn1-B in FIGURES 31C AND 32).
SLC4A3 contains two alternative promoters. The first produces the full-length gene product brain AE3 (bAE3, aka AE3fl; 1,232 amino acids). The second produces the shorter cardiac AE3 (cAE3; 1,034 amino acids) from an alternative transcription site that includes a novel ATG codon. Thus, in cAE3, the first 271 amino acids of bAE3 are replaced by a novel 73-amino acid sequence (588, 1080). One study found that the intrinsic Cl-HCO3 activity of bAE3 is doubled compared with cAE3 and to a truncated AE3 that lacks the unique sequence of both (905), as though the unique longer sequence of bAE3 has a mildly autostimulatory effect. The transcriptional mechanism that produces bAE3 versus cAE3 is similar to that which produces NBCe1-B and NBCe1-A. In fact, the point at which bAE3 and cAE3 sequences converge is only four amino acids downstream of where NBCe1-A and NBCe1-B sequences converge.
6. Distribution
Whereas elsewhere we have considered the distribution of NCBTs by organ system, in this section we consider each AE in turn and consider whether its distribution overlaps with that reported for any NCBT. In common with NCBTs, the location of AEs in polarized cells is overwhelmingly basolateral.
A) AE1.
eAE1 is prominently expressed in the plasma membrane of red blood cells, whereas kAE1 is located in the basolateral membrane of α-intercalated cells in the renal collecting duct (24, 262). Red blood cells have not been demonstrated to express any NCBT. Some researchers describe NBCn1 (694, 1014) and AE4 (498) immunoreactivity in the basolateral membranes of α-intercalated cells in the medullary segments of the collecting duct. However, we are unaware of the direct colocalization, even of any two of these three Slc4 proteins, by the same authors in the basolateral membrane of the α-intercalated cell.
B) AE2.
Of the AEs, AE2 is by far the most widely distributed. Locations include the basolateral membranes of epithelia that line the gastrointestinal tract (e.g., see cartoons in FIGURES 21 AND 22 as well as Refs. 25 and 813) and also the basolateral membranes of some renal tubule segments, most prominently in the mTAL (see cartoon in FIGURE 34 as well as Refs. 27, 158, 298, and 914). The AE2a and AE2b variants have a similar broad distribution, whereas robust AE2c expression appears to be restricted to the stomach (25, 550, 1030).
In many cases the distribution of AE2 overlaps with that of an NCBT. For example, sites of AE2 expression that match those of NBCe1, presented in separate reports, include the basolateral membranes of salivary parotid acinar cells (372, 818), pancreatic acinar cells (816), duodenal enterocytes (25), proximal colon enterocytes (25), late PT, and epididymal epithelia (446). The distribution of AE2 overlaps with that of NBCn1 in the basolateral membrane of mTAL epithelia, and with NBCn2 in the basolateral membrane of choroid plexus epithelia (see cartoon in FIGURE 28 AND Ref. 587). In other cell types, AE2 is coexpressed with NBCe2 but in polar opposite membranes. For example, in choroid plexus epithelia (basolateral AE2, apical NBCe2, see cartoon in FIGURE 28) and in hepatocytes (apical AE2, basolateral NBCe2; see cartoon in FIGURE 29 AND Ref. 46). Reports conflict as to whether NBCe1 is present in ameloblasts, which also express AE2 (see cartoon in FIGURE 20 as well as Refs. 456 and 706). Despite these apparent overlaps in reported distribution, we are not aware of any publications in which the same set of authors has visualized NCBTs and AE2 protein in the same cell.
There is no evidence that AEs and NCBTs are capable of forming heterodimers. However, functional coupling of AEs, specifically AE2, and NCBTs has been proposed (571, 1019). For example, NBCn1 in the basolateral membrane of the mTAL presumably mediates the uptake of Na+ and HCO3− (571). In parallel, some of the HCO3− exits the cell in exchange for Cl− via AE2. To the extent that the HCO3− fluxes of the two transporters balance, the net effect is NaCl uptake.
C) AE3.
AE3 is expressed in neurons and glia throughout the central nervous system (378, 463, 502, 509) and in heart preparations (588, 761), specifically in the sarcolemma and T tubules of myocytes (31). Thus the expression of AE3 potentially overlaps with all NCBTs in the CNS and with NBCe1 and NBCn1 in cardiac myocytes. Again, despite the apparently overlapping distribution, NCBTs and AE3 have not, to our knowledge, been formally colocalized by immunocytochemistry in the same cell.
7. Physiological roles
Here we discuss the generally complementary, but sometimes similar, physiological roles of AEs and NCBTs, and how their different molecular actions impact these roles.
A) GENERAL: PHI REGULATION.
AEs typically operate as acid-loaders, exchanging intracellular HCO3− for extracellular Cl−, tending to restore pHi after an alkaline load (995). NCBTs on the other hand typically operate as acid-extruders, tending, as is the case with Na-H exchangers, to restore pHi after an acid load. The concerted action of these three transport mechanisms over a range of pHi values is nicely demonstrated in a study of ventricular myocytes by Leem and coworkers (563).
B) AE1: MAINTENANCE OF RED CELL MORPHOLOGY.
The cytosolic Nt of AE1 forms extensive interactions with cytoskeletal proteins, tethering the red cell cytoskeleton to the red cell membrane. Not only do these interactions help the red cell maintain its biconcave shape, thereby maximizing its surface area-to-volume ratio for gas exchange, but they also allow each cell to be temporarily deformed, rather than sheared, as it passes through the microcirculation. The importance of AE1 for red cell morphology is reviewed by Burton and Bruce (141).
C) AE1: PROMOTION OF GAS EXCHANGE ACROSS THE RED CELL MEMBRANE.
Carbon dioxide entering red blood cells in the systemic capillaries is hydrated to H+ plus HCO3− via the action of CA II. The HCO3− exits into the plasma via AE1, maintaining a driving force for CO2 entry into the red blood cell and maximizing the CO2-carrying ability of the blood. The H+ generated by the CA reaction is buffered by hemoglobin (Hb), which may be tethered to the cytoplasmic Nt domain of AE1 (475). The binding of H+ to Hb reduces the affinity of Hb for O2, thereby promoting O2 release (the Bohr effect). The Bohr effect also plays a role in the pulmonary capillaries, where HCO3− entry into the red cell plasma via AE1, maintains a driving force for CO2 exit from the red cell, and promotes H+ consumption thereby increasing the affinity of Hb for O2. The relationship between red cell pH and gas exchange is reviewed in Reference 444.
D) AE1 AND AE2: HCO3− REABSORPTION/H+ SECRETION.
In the basolateral membrane of mTAL epithelia (FIGURE 34), AE2 is predicted to move HCO3− from cell to the blood, thereby contributing towards reabsorption of residual HCO3− from the mTAL lumen into the blood (269). HCO3− exit across the basolateral membrane also promotes the generation of intracellular H+, which stimulates H+ secretion into the lumen. This secreted H+ either titrates HCO3− in the lumen (HCO3− reabsorption) or titrates NH3 and other non-HCO3− buffers (H+ excretion). Further along the nephron, in the basolateral membrane of collecting duct α-intercalated cells, AE1, rather than AE2, performs a similar function (282, 904). The renal actions of AE2 and AE1 both counter metabolic acidosis in the blood. In ameloblasts and osteoclasts, AE2-mediated HCO3− efflux across the basolateral membrane supports H+ secretion across the apical membrane, contributing to tooth (456) and bone (1048) remodeling (see FIGURES 20 AND 33).
E) AE2: HCO3− SECRETION/H+ REABSORPTION.
Although AEs typically support HCO3− reabsorption, in three instances, AE2 is positioned to support HCO3− secretion: 1) AE2 exhibits an apical distribution in cholangiocytes and 2) hepatocytes (46, 987) and 3) AE2 exhibits a lateral distribution in ameloblasts, becoming exposed to the apical compartment during ameloblast maturation by a rearrangement of tight junctions (456). In contrast to AEs, NCBTs typically support HCO3− secretion. Two notable exceptions in the case of NCBTs are NBCe1-A in the basolateral membrane of the proximal tubule and NBCe2 in the apical membrane of the choroid plexus, both of which are predicted to operate with the unusual apparent Na+:HCO3− stoichiometry of 1:3, thereby mediating HCO3− reabsorption.
F) AE2: SALT SECRETION.
A consequence of the HCO3− transport function of AEs and NCBTs is that AEs import Cl−, whereas NCBTs generally import Na+. As noted earlier, both of these consequences can contribute towards the vectoral transport of NaCl, together with osmotically obligated H2O, across epithelia. In airway, duodenal, and colonic epithelia, the functionally coupled action of AE2 and an NCBT (e.g., AE2 and NBCe1 in FIGURE 22) performs, along with NKCC1, Na+ and Cl− influx across the basolateral membrane that supports CFTR-mediated Cl− secretion across the apical membrane (312, 397, 1019). Knocking out murine AE2 or NBCe1 is associated with a presumably compensatory increase in NKCC activity in intestinal epithelia (312, 313).
G) AE2: VOLUME REGULATION.
The intracellular alkalinization that follows the shrinkage-induced activation of NHE1 subsequently activates AE2. The resulting net influx of Na+ and Cl−, followed by water, tends to restore cell volume (449, 873). One group has suggested that shrinkage-induced activation of NDCBE might also tend to restore cell volume. In the red blood cells of trout, swelling opens a cryptic solute channel within AE1 protein (285). The efflux of ions and uncharged solutes through trout AE1 would tend to restore cell volume. Regulated volume decrease is common to the red blood cells of many species, but the physiological involvement of AE1 in such a pathway is not well demonstrated in mammals (357).
H) AE3: CONTROL OF NEURONAL EXCITABILITY.
The acid-loading action of AE3 tends to dampen neuronal excitability, consistent with the association of AE3 dysfunction with epilepsy.
8. Causes of AE upregulation
Here as well as in the following section, we mainly consider the effect upon AEs of those perturbations that have been described elsewhere to affect NCBT functional expression. Typically, acid-extruding NCBTs are upregulated by metabolic and respiratory acidosis, the consequence of which is defense of pHi. Even NBCe1, acting as an acid-loader in the proximal tubule, is upregulated by acidosis, increasing HCO3− reabsorption, the consequence of which is defense of plasma pH.
A) AE1.
AE1 plays a role in support of renal HCO3− reabsorption/H+ secretion in the CCD. Indeed, AE1 transcript and protein abundance increase in response to metabolic acidosis (282, 398, 763, 828, 1002), which is the appropriate response. One study reports that the red blood cells of individuals permanently living at high altitude (e.g., Bolivians) contain 50% more AE1 protein than red blood cells of individuals permanently living at sea level (e.g., Danes, see Ref. 457).
B) AE2.
In the mTAL, AE2 protein abundance is increased in response to metabolic acidosis, a compensatory mechanism that could increase HCO3− reabsorption/H+ secretion by this nephron segment (778). AE2 protein abundance in the mTAL is also elevated by NaCl loading, consistent with its role in support of salt secretion (778), as discussed above.
9. Causes of AE downregulation
A) AE1.
In keeping with its upregulation during acidosis, AE1 protein abundance in the collecting duct is reduced during metabolic alkalosis (828).
B) AE3.
AE3 protein abundance is reduced in the brains of rats following a 2-wk exposure to 12% CO2 (463), a response that is consistent with the reduced usefulness of an acid-loading transporter under hypercapnic conditions.
10. Consequences of AE dysfunction
Interested readers might refer to the reviews of others for extensive discussions of the pathological consequences of AE1–3 dysfunction (e.g., see reviews in Refs. 22 and 1036). Here we discuss pathologies that are relevant to defects in both AEs and NCBTs and how differences between the transporters may impact the sequelae.
A) AE1: DISTAL RENAL TUBULAR ACIDOSIS AND HEMOLYTIC ANEMIA.
Individuals with mutations in the SLC4A1 gene (130) and mice with a disrupted Slc4a1 gene (904) have a lower-than-normal blood pH and [HCO3−] and excrete an unusually alkaline urine. Underlying these signs is loss of per-molecule function and/or reduced accumulation of AE1 in the basolateral membrane of collecting duct α-intercalated cells (130). The result is an impaired ability of the collecting duct to reabsorb HCO3−/secrete H+, processes that normally support H+ secretion into the duct lumen. Similarly, genetic defects in NBCe1 result in proximal renal tubular acidosis (pRTA), although underlying the acidosis in this case is a failure of HCO3− reabsorption/H+ secretion at the level of the PT. Individuals with AE1-associated distal renal tubular acidosis (dRTA) typically have a less severe deficit in plasma [HCO3−] because the PT, which is responsible for ∼80% of the HCO3− reabsorption, is intact. However, patients with an AE1 defect do relatively poorly in acidifying their urine. Individuals with NBCe1-associated pRTA typically have a severe deficit in plasma [HCO3−]. Thus the filtered load of HCO3− is low enough that the intact distal nephron can reabsorb the HCO3− and also lower urine pH. (e.g., see Ref. 253). AE1-associated dRTA also have a different set of extrarenal sequelae from NBCe1-associated pRTA because of the different sites of AE1 and NBCe1 expression. For example, dRTA is sometimes accompanied by loss of AE1 from red blood cells, resulting in hemolytic anemia (see review in Ref. 1036) and, secondary to the anemia, cardiac hypertrophy (31, 708).
B) AE2: BONE AND ENAMEL DEFECTS.
Mice and cattle with AE2 insufficiency exhibit signs of osteoclast dysfunction, such as growth retardation and osteopetrosis (432, 455, 643, 1048). On its free or contralacunar membrane surface (facing bone interstitium), the osteoclast expresses AE2 (FIGURE 33). The Cl-HCO3 exchange activity acidifies the cell, thereby promoting H+ secretion across the ruffled border by the vacuolar-type H+ pumps. As the H+ enters the resorption lacuna (the space between the ruffled border and calcified bone), the acidity promotes bone resorption (solubilization of bone mineral and hydrolysis of matrix proteins). Osteoclasts in which NBCn1 abundance has been reduced by antisense technology also exhibit reduced bone-resorption function. NBCn1 appears to be expressed at high levels in the ruffled-border membrane that faces the resorption lacuna, where the action of H+ on CaCO3 forms HCO3−. Presumably the NBCn1 would move this newly formed HCO3− from resorption lacuna to the cytosol of the osteoclast for removal by AE2 into the interstitium (797).
AE2-null mice are toothless, exhibit growth retardation, and die prematurely (314). Mice that are unable to express the a and b variants of AE2 also have defective tooth enamel (126, 616) because AE2 supports H+ secretion in ameloblasts. NBCe1 dysfunction is similarly associated with enamel defects, although the underlying mechanism in that case has yet to be resolved.
C) AE2: INFERTILITY.
AE2-null mice do not live to breeding age, but male mice that lack only the a and b variants of AE2 are infertile due to defects in spermiogenesis (638).
D) AE2: GASTRIC ACID SECRETION DEFECTS.
Gastric secretions in AE2-null mice are not acidic due to a combination of loss of AE2-mediated HCO3− efflux across the basolateral membrane of parietal cells (which normally supports H+ secretion), loss of parietal cells, and ultrastructural defects in remaining parietal cells (314).
E) AE3: EPILEPSY.
A mutation, A679D, in AE3 reduces the per-molecule activity of the transporter (1004) and is associated with idiopathic generalized epilepsy in humans (830). Furthermore, mice lacking AE3 have a reduced seizure threshold in response to proconvulsive agents (378). The neurons of mice lacking the acid-loading AE3 exhibit an elevated pHi that likely contributes to neuronal hyperexcitability (378). Note that these features of AE3-null mice are opposite to the phenotype of mice that lack the acid-extruding transporters NDCBE and NBCn2, which have an increased seizure threshold.
F) AE3: BLINDNESS.
AE3-null mice exhibit signs of reduced inner retina function and increased apoptosis of photoreceptor cells (30). A similar phenotype is observed in certain strains of NBCe2-null and NBCn1-null mice, the common denominator perhaps being an inability to regulate pHi in these cells, assuming that this is not a side effect of the expression of misfolded protein fragments expressed from the disrupted genes.
B. AE4 (Slc4a9)
1. Summary
Despite its reported function as a Cl-HCO3 exchanger in heterologous systems, Slc4a9 is more closely related, at the level of exon-boundary structure and deduced amino acid sequence, to the Na+-coupled members of the Slc4 gene family. The molecular action and subcellular distribution of Slc4a9 products remains controversial and may even be species-specific. AE4 expression appears to be mainly restricted to the kidney, most likely in the basolateral membranes of non-α-type intercalated cells of the collecting duct. As discussed earlier, the Slc4a9 gene seems to have arisen from a recent duplication (the most primordial Slc4a9 known appears in two frog genomes) of an electrogenic NCBT-encoding gene. Among the 10 vertebrate Slc4s, Slc4a9 orthologs have the most divergent sequences. It is possible that this recently duplicated gene is still in the process of diverging.
2. Nomenclature
Originally termed hSBC5 (human sodium bicarbonate cotransporter 5) in an early GenBank submission, the Slc4a9 product was renamed AE4 (anion exchanger 4) following a report that the rabbit Slc4a9 product mediates Cl-HCO3 exchange (982).
3. Molecular action
A cDNA encoding an Slc4a9 product was first reported by Tsuganezawa et al. (982). There are three reports of Cl-HCO3 exchange activity mediated by mammalian AE4.
1) Tsuganezawa et al. (982) report that 60% of COS-7 cells transiently transfected with rabbit AE4 cDNA rapidly and reversibly alkalinize in response to the removal of bath Cl− in the presence of CO2/HCO3−. The 60% figure is consistent with the 68% transfection efficiency calculated for these cells. Supposed evidence for electroneutrality is provided in experiments in which the authors used a whole cell patch to monitor Vm while applying CO2/HCO3− and then, in the continued presence of CO2/HCO3−, removing Na+. They observed no substantial Vm changes. It is not clear how this protocol could address the issue of whether the putative Cl-HCO3 exchanger is electroneutral; a better approach would have been to monitor an electrical parameter while removing Cl− in the presence versus the absence of CO2/HCO3−. Only the switch to 130 mM K+ produced a Vm change, although the depolarization was slow and poorly reversible.
The authors also reported that Xenopus oocytes expressing rabbit AE4 mediate a Na+-independent and DIDS-insensitive 36Cl uptake in the nominal absence of CO2/HCO3−. Because they did not examine oocytes in the presence of CO2/HCO3−, this result cannot be taken as evidence of Cl-HCO3 exchange.
2) Ko et al. (498) demonstrate that HEK-293 cells transiently transfected with rat AE4 cDNA alkalinize in response to the removal of bath Cl− in the presence of CO2/HCO3−. This pHi increase appears to be predominantly CO2/HCO3− dependent. The alkalinization is unaffected by lowering of bath Na+, but is strongly inhibited by the application of H2DIDS. Evidence of electroneutrality of the transport process is provided by the lack of effect of valinomycin and elevated [K+]o on the alkalinization.
3) Xu et al. (1054) expressed mouse AE4 in Xenopus oocytes, using the fluorescence of BCECF to monitor pHi. They reported that, following a 20–30 min equilibration in CO2/HCO3−-containing solution, oocytes expressing AE4 alkalinized upon removal of bath Cl−. These authors did not discuss whether pHi recovered from the CO2-induced acid load when the oocytes were exposed to Cl− or whether the alkalinization induced by Cl− removal was DIDS sensitive. An unusual aspect of the data was that the pHi of the control oocyte (i.e., the one not expressing AE4) in CO2/HCO3− was ∼7.2 (i.e., roughly the pHi expected in the absence of CO2/HCO3−). We would have expected the application of 5% CO2 to cause pHi to fall to ∼6.9 and not recover much from there (e.g., see Ref. 725).
In one preliminary study on Xenopus oocytes, Parker et al. (716) observed that Xenopus oocytes expressing a human AE4 splice variant exhibited a small but significant pHi recovery from a CO2-induced acid load. Moreover, the removal of extracellular Na+ produced a very small but significant pHi decrease, consistent with electroneutral Na/HCO3 cotransport activity (716).
We conclude that the functional data on AE4 are inconsistent, possibly due to the use of cDNAs from different species, the use of different heterologous expression systems, different and nonoverlapping protocols, and different experimental approaches. We cannot rule out the possibility that the pHi increases observed after removal of Cl− required a protein endogenous to the host cell.
4. Genome
The human SLC4A9 gene occupies at least 22 exons spread over 16 kb at the chromosomal locus 5q31 (591, 720), making it similar in size to the genes encoding AE1, AE2, AE3, and BTR1 but considerably more compact than genes encoding verified NCBTs, which are typically ≥100 kb. However, Slc4a9 shares more common exon boundaries with Slc4a4 and Slc4a5, genes that encode electrogenic NCBTs (FIGURE 7). Putative promoter elements are located upstream of the transcriptional starting position of human SLC4A9, including a CCAAT box, a GC box, and a TATA box (720). A region upstream of the first exon of mouse AE4 has basal promoter activity and contains a consensus motif for binding the transcription activator Foxi1. Indeed, AE4 transcription is enhanced 100-fold by Foxi1 (528), and mice lacking Foxi1 also lack AE4 (87) as well as subunits of the H+-pump (1003). Sequences with promoter activity are also found within the mouse Slc4a9 gene upstream of exon 3 and upstream of exon 6 (377).
5. Structural features and variants83
A 2001 survey of human ESTs describes 14 distinct AE4 transcripts, most of which are not predicted to encode a functional transporter/stable membrane protein, owing to the presence of stop codons or the absence of transmembrane spans (591). Compared with the most complete reported mammalian transcript, a 15th human transcript (720) lacks part of exon 9 (i.e., leading to the absence of the 11-amino acid “LFGGLIQDVRR” in the cytosolic Nt domain close to TM1) as well as part of exon 12 (i.e., leading to the absence of the 3 amino acid “VSM” in the 3rd extracellular loop). Northern blots of human, mouse, and rabbit kidney AE4 RNA demonstrate a multiplicity of bands in these organisms (377, 591, 982). Only two rabbit AE4 variants, AE4a and AE4b, have been cloned, and both have a full complement of transmembrane spans, AE4b differing from AE4a only in the absence of a 16-amino acid sequence within the cytosolic Nt domain of AE4b (982). In mice, Slc4a9 transcription can initiate at multiple points, resulting in the production of Nt truncated AE4 splice variants that are shorter than the longest reported AE4 protein by 157 and 251 amino acids (377). The effect of such truncation on the function and/or trafficking of AE4 remains untested.
The Nt domain of AE4 includes a leucine-zipper–consensus sequence that may contribute towards protein oligomerization or interactions with binding partners (498, 720). The third extracellular loop includes the four cysteines that are common to NCBTs, and includes multiple putative glycosylation sites, although an in vitro study failed to demonstrate N-glycosylation of human AE4 (720).
6. Distribution
AE4 is predominantly expressed in the kidney. The apparent AE4 distribution in specific organ systems is discussed below. Some reports of protein distribution must be regarded with caution due to inadequate characterization of the antibodies used.
A) CENTRAL NERVOUS SYSTEM.
AE4 immunoreactivity has been detected in the apical membranes of ciliated ependymal cells in the third ventricle of the choroid plexus of mice and rats (755).
B) SENSORY ORGANS.
As far as we are aware, there are currently no reports of AE4 expression in the eye, ear, or olfactory system.
C) PERIPHERAL NERVOUS SYSTEM.
As far as we are aware, there are currently no reports of AE4 expression in the peripheral nervous system.
D) RESPIRATORY SYSTEM.
AE4 transcripts are detected in cultured human nasal epithelial cells, increasing in abundance as the cells grow to confluence and project cilia (878). The authors of that study report that “immunofluorescent staining was seen along the whole cell membrane, which suggests that AE4 is localized in both the luminal and basolateral membranes.” However, the immunocytochemistry presented in the study, performed on permeabilized cells, does not support the stated conclusion for several reasons. 1) The cells are of undemonstrated confluence and polarity. 2) The confocal microscopic image, which is only in the x-y plane, shows predominantly perinuclear staining, with no evidence of a signal at the plasma membrane. 3) The specificity of the commercial anti-AE4 antibody is not demonstrated.
E) CIRCULATORY SYSTEM.
A study of laser-captured rat brain microvessels demonstrated the presence of AE4 protein by ICAT (isotope-coded affinity tagging) nanoLC-MS/MS (366).
F) MUSCULOSKELETAL SYSTEM.
We are not aware of any reports of AE4 expression in the musculoskeletal system.
G) UPPER DIGESTIVE SYSTEM.
I) Salivary gland. AE4 immunoreactivity is reported in the basolateral membranes of duct cells from the mouse submandibular gland (498).
II) Stomach. AE4 transcripts are detected in northern blots of stomach RNA preparations from mouse, rabbit, and rat (498, 1054). Specifically, rabbit AE4 transcripts were detected in preparations from gastric mucous and parietal cells (1054). A 2003 immunohistochemical study reports the presence of AE4 protein in the apical membranes of mouse and rabbit gastric surface mucous cells (1054).
H) LOWER DIGESTIVE SYSTEM.
I) Intestines. AE4 immunoreactivity is reported in the apical villus membranes of human, rabbit, and mouse duodenum (1054). However, the evidence presented for the presence of AE4 protein in duodenal cells hinges on the specificity of the antibody, which, in mouse preparations, does not immunoreact with a protein of the molecular weight expected for AE4 (see FIGURE 4B of Ref. 1054). Furthermore, others report that AE4 transcripts are absent from mouse duodenum preparations (482, 887). Thus the presence of AE4 in duodenum remains controversial.
AE4 transcripts have been reported in rat cecum (498).
II) Liver. An NCBI-curated database suggests that the mouse liver is a minor site of AE4 transcription (Appendix VI).
I) ENDOCRINE SYSTEM.
We are unaware of any reports of AE4 expression in the endocrine system.
J) LYMPHATIC AND IMMUNE SYSTEMS.
We are unaware of any reports of AE4 expression in the lymphatic or immune systems.
K) URINARY SYSTEM.
I) Kidney. Northern blotting studies demonstrate that AE4 transcripts are predominantly renal in humans (591, 720), rats (498), and rabbits (982). In mice, the kidney-specific transcription factor Foxi1 is responsible for the predominantly renal expression of AE4 (377). In microdissected preparations of rat kidneys, AE4 transcripts are most abundant in the cortical collecting duct (498). AE4 mRNA and immunoreactivity are also detected in the rat renal collecting duct cell line RCCD1 (798). Reports, individually considered in Appendix VIII, conflict as to the precise location of AE4 protein within the collecting duct. Although the consensus is that the AE4 protein is expressed predominantly in intercalated cells, it is not agreed whether AE4 is located in α- vs β-intercalated cells (see Ref. 498 versus Refs. 87, 982, and 1054). There is also controversy as to whether AE4 is localized to the apical versus basolateral membrane (see Refs. 762, 982, and 1054 versus Refs. 87 and 498), although we note that the data pointing to an apical location are all from rabbits. These disparate observations have been suggested to represent interspecific differences, although they might equally be explained by two other factors.
1) None of the anti-AE4 antibodies that are considered in Appendix VIII recognizes a single band of the appropriate molecular weight in western blots of kidney preparations. Thus the specificity of these antibodies is not demonstrated.
2) Putative sites of AE4 expression are designated as either α- or β-intercalated cell subtypes, but none of the studies considers the substantial subpopulation of intercalated cells in the collecting ducts of mice, rabbits, and rats that are non-α/non-β types (270, 487).84
The evidence discussed in Appendix VIII is consistent with the hypothesis that AE4 is expressed in the basolateral membranes of both β-intercalated cells and non-α/non-β intercalated cells (collectively known as non-α types) in rats and mice. The expression of AE4 in α-intercalated cells is not well demonstrated. It is unclear why anti-AE4 antibodies immunoreact with epitopes in the apical membranes of rabbit intercalated cells.
L) REPRODUCTIVE SYSTEM.
An NCBI-curated EST database suggests that human and mouse testes are a minor site of AE4 transcription (Appendix VI).
7. Physiological roles
Inasmuch as the expression of AE4 is mainly restricted to the kidney and inasmuch as AE4-null mice do not have an obvious renal phenotype, the physiological role(s) of AE4 is presently unknown. Four possible roles for AE4 have been proposed.
A) UPPER DIGESTIVE SYSTEM.
I) Suggested role in gastric HCO3− secretion. A 2003 study reported AE4 immunoreactivity in the apical membranes of mouse stomach epithelia, where HCO3− secretion mediated by an apical DIDS-sensitive Cl-HCO3 exchanger would contribute to mucosal protection (87). However, later work by the same group suggests that an Slc26a9 product, not an Slc4a9 product, is responsible for the apical Cl-HCO3 exchange activity in stomach epithelia (1055). Thus, if AE4 is indeed at the apical membrane of these cells, its role is unclear.
B) LOWER DIGESTIVE SYSTEM.
I) Suggested role in duodenal HCO3− secretion. A 2003 study detected AE4 immunoreactivity in the apical membranes of mouse and rabbit duodenal epithelia where HCO3− secretion mediated by an apical Cl-HCO3 exchanger might play a role in mucosal protection (87). However, 1) others do not detect AE4 transcripts in duodenal epithelial cells of mice (887), 2) Cl-HCO3 exchange is unperturbed in the apical membranes of duodenal villus cells from AE4-null mice (887), and 3) later work suggests that an Slc26a6 product is the apical Cl-HCO3 exchanger in these cells (887). Thus, if AE4 is indeed in the apical membranes of these cells, its role remains undemonstrated.
C) URINARY SYSTEM.
I) Suggested role in support of renal H+ secretion. The apparent localization of AE4 to the basolateral membranes of collecting-duct α-intercalated cells led to the suggestion that AE4 (acting as a Cl-HCO3 exchanger) could act in parallel with basolateral AE1 to support H+ secretion (498). However, experimental evidence for such a physiological role is lacking, inasmuch as the presence of AE4 in α-intercalated cells is not well demonstrated, and AE4 protein levels are not compensatorily increased in AE1 null-mice (904).85
II) Suggested role in support of renal HCO3− reabsorption in acidosis. A preliminary study suggests that, in collecting duct β-intercalated cells isolated from rabbits (see footnote 84), acidosis reduces the abundance not only of pendrin at the apical membrane but also the abundance of AE4 mRNA as well as the abundance of AE4 immunoreactivity in a subapical compartment (762). Inasmuch as β cells mediate transepithelial HCO3− secretion and pendrin mediates the apical step (HCO3− efflux into lumen), it is tempting to speculate that AE4 might normally contribute to HCO3− secretion in rabbits. If this speculation is correct, and if the AE4 protein is present in the apical membrane of these cells, then AE4 would have to be a Cl-HCO3 exchanger. On the other hand, if AE4 exhibits a basolateral distribution in rabbit β-intercalated cells, as is the case in other model animals, then AE4 would have to mediate HCO3− transport coupled to Na+ influx (i.e., Na/HCO3 cotransport), rather than to Cl− efflux (i.e., Cl-HCO3 exchange), to contribute to HCO3− secretion. We note that NCBT activity has not been reported in β-intercalated cells.
8. Causes of AE4 upregulation
A) CIRCULATORY SYSTEM.
I) Increased protein abundance in ischemia/reperfusion. Haqqani et al. (366) used a proteomic approach (ICAT-nanoLC-MS/MS) to identify proteins, among them AE4, whose expression level was altered in the microvasculature of rats following global cerebral ischemia and reperfusion (366). Microvascular AE4 protein abundance was transiently increased 1 h after ischemia/reperfusion but returned close to normal levels after 6 h. The physiological relevance of this phenomenon is unknown.
9. Causes of AE4 downregulation
A) URINARY SYSTEM.
I) Downregulation in Foxi1-deficient mice. Foxi1-deficient mice lack properly differentiated α- and β-intercalated cells, are afflicted with distal renal tubular acidosis, and not surprisingly lack renal AE4 (87). Due to the morphological abnormalities in the tubules, and because Foxi1-null mice also lack AE1 and the H+-pump subunit ATP6B1, defects in either of which alone is sufficient to cause dRTA (130, 473), the acidosis in these mice cannot be uniquely linked to the loss of AE4.
II) Decreased mRNA abundance and disturbance of AE4 protein distribution in acidosis. A preliminary study suggests that in collecting duct β-intercalated cells acidosis reduces the abundance not only of pendrin at the apical membrane but also the abundance of AE4 mRNA as well as the basolateral presence of AE4 protein (762).
10. Consequences of AE4 dysfunction
There are no reports of pathologies linked to the SLC4A9 locus in humans, nor are we aware of any reports of phenotypical consequences related to the loss of AE4 in mice.
C. BTR1 (Slc4a11)
1. Summary
In mammalian genomes, Slc4a11 is the singleton representative of a third subgroup of Slc4 genes. The exon boundaries of Slc4a11 are distinct from those of genes that encode NCBTs and AEs (FIGURE 7). The demonstration that Slc4-like family members from Arabidopsis thaliana (BOR1) and Saccharomyces cerevisiae (Bor1p) promote boron efflux from cells (943) led to the suggestion that BTR1, too, is a boron transporter (299). However, the apparent clustering of BOR1 and Bor1p with mammalian BTR1 protein sequences on a cladogram (299) owes more to their collective lack of identity to mammalian AEs and NBCs than any specific identity among BOR1, Bor1p, and BTR1 (TABLE 3). Nonetheless, a subsequent report provided indirect evidence that BTR1 is an electrogenic Na/borate cotransporter (712). Although BTR1 exhibits a wide distribution, genetic defects in BTR1 so far are associated only with a number of corneal dystrophies. Despite the uniqueness of BTR1, there is much to be learned from BTR1 about AEs and NCBTs. For example, pathological mutations occur at many of the same conserved sequence positions in BTR1, AE1, and NBCe1. Thus the numerous mutations that have been described in BTR1, but not (yet) in NBCe1, could point at critical residues for NBCe1 structure-function analysis.
2. Nomenclature
BTR1, bicarbonate transporter related protein 1 (720), is the most divergent member of the vertebrate Slc4 family and also the last member to be cloned. An alternative name, NaBC1, Na-coupled borate cotransporter 1 (712), was proposed following a report that BTR1 is a borate transporter. For the purposes of the present review, we will continue to refer to Slc4a11 products as BTR1 because NaBC1 is unfortunately similar in name to NABC1, a breast cancer-associated gene (198) that is located on the same human chromosome as SLC4A11, and the acronym NaBC1 does not usefully distinguish Na-borate cotransporters from Na-bicarbonate cotransporters.
3. Molecular action
The function of mammalian BTR1 as a borate transporter remains controversial. One group suggests that BTR1 has a dual action (712). In the absence of borate, BTR1 is proposed to function as an electrogenic Na/OH cotransporter, or Na-H exchanger, which is thermodynamically equivalent, that carries two or more OH− per Na+. However, in the presence of borate, BTR1 is proposed to function as an electrogenic Na/B(OH)4 cotransporter that carries two or more Na+ per borate, a Na/anion stoichiometry that is opposite to that for Na/OH cotransport.
Three main observations support the borate-independent action of BTR1.
1) BTR1-expressing HEK-293 cells acidify to a greater extent than control cells upon removal of extracellular Na+. This NHE-like activity in BTR1-expressing cells is not blocked by 10 μM EIPA nor by 500 μM DIDS. These cells also acidify in response to removal of extracellular K+, which could either be interpreted as K-H exchange or, because that maneuver would tend to hyperpolarize these cells, outward electrogenic Na/OH cotransport.
2) BTR1 expression reduces the ability of HEK-293 cells to defend pHi from increases or decreases in pHo, as if the cells have an increased flux of OH–/H+.
3) Elevating [K+]o causes a reduction of [Na+]i in BTR1-expressing cells. Assuming that the rise in [K+]o shifts Vm in the positive direction, we do not understand how this observation can be interpreted in light of the electrogenic Na/OH model (point 1), which should, by itself, have caused [Na+]i to rise.
Three main observations support the borate-dependent action of BTR1.
1) In BTR1-expressing HEK-293 cells, the presence of “borate, i.e., an equilibrated mixture of H2BO3 and the anion B(OH)4–, exaggerates the pHi decrease caused by removing extracellular Na+ or by lowering pHo. If we assume that H3BO3 is freely diffusible, this acidification would be expected if B(OH)4– export caused the intracellular reaction H3BO3 + H2O ⇄ B(OH)4– + H+ (pK ≅9.2) to shift to the right.
2) The presence of borate stimulates a small inward current in BTR1-expressing HEK-293 cells and oocytes.
3) In the presence of borate, the removal of extracellular Na+ elicits a larger outward current in BTR1-expressing HEK-293 cells and oocytes than in control cells. It is this result that requires a Na+:borate stoichiometry greater than 1:1.
It must be noted that the above study presents no direct evidence of borate flux. Moreover, the concentrations of borate (i.e., 5 mM) are two orders of magnitude greater than physiological concentrations of this trace element (plasma [B] values are reviewed in Ref. 403). Finally, we are surprised that that BTR1 would bind 1 Na+ plus 2 anions in the absence of borate but 2 Na+ plus 1 anion in the presence of borate.
Another group (1005), in a paper about BTR1 trafficking, reports (citing unpublished data) that they are unable to replicate the data of the previous group.
At present, no data are available concerning the ability of AEs or NCBTs to transport borate in place of bicarbonate or carbonate. Likewise, the ability of BTR1 to transport HCO3− has not been directly measured.
4. Genome
The human SLC4A11 gene, which contains 20 exons that occupy 12 kb on chromosome 20 (720), is the shortest of the 10 mammalian Slc4 genes. The gene locus was originally assigned to position 20p12 (720), but subsequent refinements of the human genome map now place SLC4A11 at 20p13. The absence of a TATA-box and presence of a downstream promoter-element–like sequence suggests SLC4A11 gene expression is under the control of a TATA-less promoter (720).
5. Structural features and variants
Three full-length transcripts are reported to be transcribed from the SLC4A11 gene. The three differ in the inclusion of alternative extreme Nt sequences. The archetypal BTR1 sequence, which we provisionally term BTR1-a, is a 3.1-kb transcript derived from exons 3–20 of SLC4A11. In BTR1-b, 30 amino acids encoded by part of exon 3 in BTR1-a are replaced by 57 amino acids encoded by exon 2. In BTR1-c, the 30 amino acids of BTR1-a are replaced by 14 amino acids encoded by exon 1.
In theory, any of the aforementioned transcripts could encode two proteins variants of ∼100 kDa, inasmuch as there are two initiating methionine codons, corresponding to Met1 and Met36 of BTR1-a. The second of the two start codons is preceded by the consensus Kozak sequence “CCACC.” Interestingly, the shorter variant in each case would begin Met-Ser-Gln-Xaa-Gly; the same sequence that initiates from Met1 in BTR1-a. It is unknown what fraction of protein product expressed from BTR1-a mRNA initiates with Met1 versus Met36.
Hydropathy analysis predicts that BTR1 has a similar topology to other Slc4 family members (720, 1005). The Nt domain is shorter than most other Slc4 members and contains a number of consensus PKA and PKC phosphorylation sites, together with an unusually high proportion, for an Slc4, of cysteine residues.
As noted above, BTR1 does not appear to be blocked by DIDS (712). On the other hand, the BTR1 protein binds to H2DIDS and SITS affinity columns (1005), and BTR1 does include a putative DIDS-interaction motif “KGTVK” at the extracellular end of TM5.
Cell-free translation in canine pancreatic microsomes (720) and western blots of BTR1 expressed in HEK-293 cells (1005, 1010) reveal that BTR1 is N-glycosylated on at least one of the two consensus glycosylation sites in its third extracellular loop. This loop lacks the four conserved cysteines characteristic of NCBTs.
6. Distribution
BTR1 expression is widespread and in many cases is expressed in cell types that also express NCBTs. The distribution of BTR1 in specific organ systems is discussed below.
A) CENTRAL NERVOUS SYSTEM.
I) Brain. BTR1 transcripts have been detected in mouse whole brain preparations (378) but not in rat cerebellum preparations (755). It is possible that the brain BTR1 transcripts are derived from choroid plexus, rather than neurons or glia (see next section).
II) Choroid plexus. BTR1 immunoreactivity is evident in the apical membranes of human choroid plexus epithelial cells, although BTR1 transcripts are reportedly absent from mouse choroid plexus (755).
B) SENSORY ORGANS.
I) Eye. BTR1 transcripts (1011) and protein (214, 608) are detected in corneal endothelium, as is a BTR1-lacZ fusion protein86 in a transgenic mouse (340). The polarity of BTR1 expression in these cells has not been described, and thus BTR1 is not shown in FIGURE 19. The absence of BTR1 transcripts has been reported in the human retina (see supplemental information in Ref. 1011). Except for a weak and occasional presence in the anterior corneal squamous epithelium (214, 608),87 the presence of BTR1 has not been reported in any other ocular structures.
II) Ear. BTR1 immunoreactivity is detected throughout the inner ear, specifically in the fibrocytes of the spiral ligament that underlie the stria vascularis of the cochlea (340, 608), together with a lesser presence in the spiral limbus of the cochlea and in the stroma that underlies the sensory epithelia of the macula of saccule in the vestibular system (608). According to an NCBI-curated database, more ESTs have been isolated from mouse inner ear preparations than from any other organ (Appendix VI).
C) RESPIRATORY SYSTEM.
BTR1 transcripts have been detected in RNA preparations from human trachea (720). According to an NCBI-curated database of ESTs, the olfactory mucosa of mice and lung of humans and mice are additional sites of BTR1 transcription (Appendix VI).
D) CIRCULATORY SYSTEM.
According to an NCBI-curated EST database, BTR1 transcripts are present in RNA preparations derived from human blood (Appendix VI). BTR1 immunoreactivity is present in blood vessels in rat submandibular salivary glands (712).
E) MUSCULOSKELETAL SYSTEM.
We are unaware of reports of BTR1 expression in the musculoskeletal system.
F) UPPER DIGESTIVE SYSTEM.
BTR1 transcripts have been detected in salivary gland extracts (720). BTR1 protein has been detected in both parotid and submandibular salivary gland preparations from rats and mice as well as in a rat submandibular cell line (712). In rat submandibular glands, BTR1 immunoreactivity is reported to be in the basolateral membranes of acinar cells (i.e., in the same membrane as NBCe1 in FIGURE 21A) but not duct cells (712).
G) LOWER DIGESTIVE SYSTEM.
I) Pancreas. BTR1 protein is detected in extracts from rat pancreas (712).
II) Liver. According to an NCBI-curated EST database, the mouse liver is a site of BTR1 transcription (Appendix VI).
III) Intestines. BTR1 transcripts have been detected in preparations of ileum and jejunum from pigs (583) and from colonic extracts from mice (512).
H) ENDOCRINE SYSTEM.
We are aware of no reports of BTR1 expression in the endocrine system.
I) LYMPHATIC AND IMMUNE SYSTEMS.
I) Bone marrow. According to an NCBI-curated EST database, the bone marrow of mice is a site of BTR1 transcription (Appendix VI).
II) Spleen. BTR1 protein has been detected in extracts from rat spleen (712).
J) URINARY SYSTEM.
BTR1 transcripts (720, 755, 1011) and protein (712) are abundant in whole kidney extracts and in the HEK-293 and MDCK renal cell lines (712).
In the renal cortex, BTR1 immunoreactivity is demonstrated in glomerular podocytes and in the basolateral membranes of proximal tubule epithelia (214). Immunoreactivity also is reported in the apical membranes of cortical collecting duct epithelia (214).
In the renal medulla, BTR1 transcripts are detected in the inner medulla, including preparations that are enriched in IMCDs (986) and in microdissected segments of the thin descending (754) and thick ascending (694) limbs of the loop of Henle. In outer medullary collecting ducts, BTR1 immunoreactivity is reported in the apical membranes of intercalated cells. However, in inner medullarly collecting ducts, BTR1 is in the basolateral membranes of intercalated cells (214).88
A different renal distribution of BTR1 gene expression is suggested by studies of transgenic mice that express a BTR1-lacZ fusion protein. In these mice, the β-galactosidase reporter activity is absent from renal cortex, but present in the renal papilla and also in structures that are reported, by process of elimination, to represent the thin descending limbs of Henle's loop (340). Thus the antibody and the lacZ data are consistently positive for BTR1 only in the case of the thin descending limb.
K) REPRODUCTIVE SYSTEM.
BTR1 protein has been detected in a human cervical cancer cell line (712), and ESTs have been detected in preparation of human ovary (Appendix VI).
7. Physiological roles
BTR1 has no demonstrated physiological role, although as discussed in the following sections, defective BTR1 expression is associated with a number of pathologies. Assigning a hypothetical role for BTR1 is difficult because 1) no consensus has yet been reached concerning the molecular action or polarized distribution of BTR1 and 2) it is unlikely that the human pathologies associated with BTR1 defects are solely the result of a functional deficit in BTR1. However, we can at least speculate on the role of borate transport, the suggested role of BTR1.
The United States Department of Agriculture does not currently classify boron as an essential nutrient,89 but several nutritional studies suggest that boron deficiency can have detrimental consequences for mammalian physiology. The underlying cause to most of the pathologies associated with boron deficiency (reviewed in Refs. 402 and 403) is the increased activity of enzymes that are normally inhibited in the presence of boric acid. Such enzymes include serine proteases (e.g., those released by activated leukocytes) and vitamin D-24-hydroxylase (the enzyme that catalyzes the first step in the inactivation of vitamin D3). Thus “boron” has anti-inflammatory action and also potentiates the effects of vitamin D3, promoting Ca2+ reabsorption and increasing insulin sensitivity (402, 403). Furthermore, boric acid is a ligand for molecules such as ribose, S-adenosylmethionine, ATP, ADP, cAMP, NAD+, and NADH, although the consequences of boric acid binding for the bioactivity of these molecules has not been investigated (402, 403).
Even given the usefulness of boron, it is reasonable to ask if a borate transporter would be useful in mammals. Dietary insufficiency of boron is rare. In fact, boron is so pervasive, and its normal dietary level so low, that it is technically challenging to reduce the boron content in animal feed to lower-than-normal levels. Although, boric acid is freely diffusible across artificial lipid bilayers (256), this observation does not address boric acid permeability of living cell membranes. In plants, aquaporins are responsible for boron uptake, whereas Slc4-like molecules are responsible for boron extrusion (FIGURE 9). Even if certain mammalian AQPs could provide a pathway for passive boric acid fluxes across membranes, BTR1 might still be useful for concentrating, or, alternatively, preventing toxic buildup of, boron inside cells.
Studies that address the ability of mammalian organs to accumulate, or defend themselves from overaccumulation, of boron are difficult to reconcile among themselves because of differences among species, and individuals of different maturity. However, these studies provide indications that boron is not passively distributed throughout the body but instead is selectively accumulated or eliminated by certain cell types (781). There is insufficient data to determine whether BTR1 is involved in these processes.
8. Causes of BTR1 upregulation
A) LOWER DIGESTIVE SYSTEM.
I) Increased transcript abundance following dietary boron supplementation. In a study of pigs, a doubling of dietary boron content, maintained over 18 days, resulted in a threefold increase in BTR1 transcript abundance in jejunal preparations but no increase in ileal preparations (583). A quadrupling of normal dietary boron intake had no greater effect on BTR1 transcript abundance in either the ileum or jejunum (583). The consequence of the upregulation is not known, but is consistent with a role of BTR1 in boron homeostasis. If BTR1 was normally involved in boron secretion, upregulation of BTR1 would enhance boron loss under conditions of excess boron intake.
9. Causes of BTR1 downregulation
A) SENSORY ORGANS.
I) Reduced transcript abundance in the cochlea in response to acoustic trauma. A preliminary qPCR study reveals that BTR1 transcript abundance is reduced in the cochlear lateral wall of mice in response to acoustic trauma, an observation that the authors of that study link to a consequence of hypoxia (1073).
B) LOWER DIGESTIVE SYSTEM.
II) Reduced transcript abundance following probiotic treatment. Probiotic treatment of mice is a model for investigating the molecular mechanism underlying the health benefits associated with probiotic treatment of inflammatory bowel disorder and ulcerative colitis. Kotka and co-workers (512) report a fourfold decrease in BTR1 transcript abundance in mouse colon 24 h after treatment with a probiotic mix (512).
C) URINARY SYSTEM.
I) Decreased transcript abundance following dietary boron supplementation. In the same study of pigs as was mentioned above, a doubling of dietary boron content, maintained over 18 days, resulted in a twofold decrease (rather than the increase observed in the jejunum) in renal BTR1 transcript abundance (583). A quadrupling of normal dietary boron intake had no greater effect on renal BTR1 transcript abundance (583). The consequence of the downregulation is not known, but is consistent with a role of renal BTR1 in boron homeostasis. If BTR1 was normally involved in boron reabsorption, downregulation of BTR1 would enhance urinary boron loss under conditions of excess boron intake.
10. Consequences of BTR1 dysfunction
A) GENERAL.
I) Cell proliferation. One study found that siRNA knockdown of BTR1 in HeLa cells resulted in a reduction in cell proliferation that could be rescued by increasing the concentration of extracellular boron (712). In individuals with SLC4A11 defects, a decreased proliferative ability may be expected to contribute towards the severity of dystrophies involving cell types in which BTR1 is normally expressed.
B) SENSORY ORGANS.
I) Vision: corneal dystrophy. To date, nearly 60 mutations90 have been identified, scattered across the length of the BTR1 molecule, among individuals with corneal dystrophies (19, 20, 161, 244, 375, 450, 522, 640, 782, 794, 867, 919, 1011, 1012). Pathological SLC4A11 mutations are most frequently inherited in a homozygous recessive manner, although numerous cases of compound heterozygous inheritance of SLC4A11 mutations have been described (20, 244, 375, 782, 919). There are three SLC4A11-associated corneal endothelial dystrophies: 1) congenital hereditary endothelial dystrophy (CHED2), first associated with SLC4A11 in Reference 1011; 2) Harboyan syndrome, also known as corneal dystrophy and perceptive deafness (CDPD), first associated with SLC4A11 in Reference 244; and 3) late-onset Fuchs' endothelial corneal dystrophy (FECD4), first associated with SLC4A11 in References 330 and 1012.
BTR1 is abundantly expressed in the corneal endothelium, a monolayer of squamous/low-cuboidal epithelial cells that forms the inner surface of the cornea. The endothelium plays a role in maintaining stromal deturgescence (i.e., corneal transparency) by reabsorbing fluid that moves by osmosis from the aqueous humor into the stroma. This reabsorption prevents disruption of the transparent crystalline array of collagen fibers and proteoglycans that constitute the stromal matrix (267). The manifestations of the dystrophy are a loss of endothelial cell density, stromal thickening, corneal clouding, and visual impairment (reviewed in Ref. 496).
Interestingly, gene-trap disruption of Slc4a11 in mice (caused by the random insertion of a neomycin-resistance cDNA) causes no major corneal phenotype, other than a slight thickening of the basal cell layer of the corneal anterior epithelium (608). A separate study of transgenic mice in which Slc4a11 was disrupted with β-galactosidase also revealed a slight thickening of the epithelium as well as a doubled thickness of the corneal endothelium, Descemet's membrane, and stroma (340). Furthermore, the endothelial layer was vacuolized and the stroma included Na- and Cl-enriched crystalline deposits (340). The clarity (or lack thereof) of the mouse cornea was not reported in this study. Thus neither of these mice are demonstrated to adequately model all of the features of human corneal dystrophy. Others have suggested that these gene-disrupted mice do not have a clear CHED phenotype due to factors such as the proliferative ability of mouse endothelial cells, functional redundancy with other transporters, incomplete inactivation of the gene, or other undefined mouse/human differences (340, 608, 1012).
An alternate possibility is that the absence of transport function (whatever the nature of that function) is not the main cause of the corneal endothelial dystrophy in humans with mutations in SLC4A11. With a few possible exceptions identified in individuals with late-onset dystrophy (794), all of the human mutant BTR1 proteins tested to date accumulate in the ER when overexpressed in mammalian cells (1005, 1011, 1012). It is possible, as has been demonstrated for other mutant proteins that misfold in the corneal endothelium (274), that the expression of large amounts of misfolded mutant protein causes ER stress (the “misfolded protein response”) and ultimately death of corneal endothelial cells. However, this hypothesis remains to be tested.
II) Hearing: loss. Slc4a11-null mice exhibit a reduced response to auditory stimuli, an observation that is consistent with the genetic link between mutations in SLC4A11 and the perceptive deafness associated with Harboyan syndrome (340, 608).
C) URINARY SYSTEM.
I) Polyuria. A study of mice in which Slc4a11 was disrupted with a lacZ gene revealed that these mutant mice excrete more urine per day than wild-type mice and that the urine of mutant mice has a lower osmolarity and [Ca2+] compared with that excreted by wild-type mice (340). Gröger and co-workers explain the polyuria by suggesting that BTR1, in the thin descending limb of the loop of Henle, normally mediates Na+ influx and thereby contributes towards the efficacy of the countercurrent multiplier. However, no direct evidence for such a role is provided in that study, and the role of BTR1 elsewhere in the kidney is not considered. Furthermore, the authors report a significant decrease in NKCC2 mRNA in one of two data sets from these mice (see Supplementary Table 2 that accompanies Ref. 340), an alteration that could potentially contribute to a polyuric phenotype (939).
VII. CONCLUDING REMARKS
A. Summary
In this section we consider the common themes that emerge as we revisit the structure, actions, and roles of the five NCBTs. Our consideration reveals a number of unresolved issues as well as several emerging topics that are understudied. In this section we summarize these points using the same subject areas that we used in organizing sections V and VI.
B. Nomenclature
1. Nonmammalian
Even within the vertebrate realm, where the homology among Slc4-like genes is sufficiently high to permit adherence to a consistent system of trivial nomenclature, some investigators have created their own nomenclature. An example is the frog ortholog of BTR1, which has been dubbed XNBC2 (1102). Outside the vertebrate realm, where direct orthologs of the 10 vertebrate Slc4 genes simply do not exist, no consistent system of trivial nomenclature is possible, a situation exacerbated by the lack of functional data that would normally inform the nomenclature. These nomenclature issues will likely cause confusion as the body of literature expands. We recommend that each study of an Slc4-like gene includes reference to GenBank or Ensembl DNA and protein accession numbers, as well as a list of any previous terminologies applied to the same gene/gene products in related organisms by others. Of course, wherever possible, the guidelines laid down by the nomenclature committees that oversee the genomes of those organisms should be followed.
2. Mammalian
In the mammalian realm, the initially confusing nomenclature is now generally settled both for the transporters themselves (NBCe1, NBCe2, NBCn1, and NDCBE) as well as for the variants of each (e.g., NBCe1-A, NBCe1-B). However, the nomenclature for Slc4a9, Slc4a10, and Slc4a11 products is controversial because the molecular actions of these transporters, which determines their acronyms, is not universally agreed upon. For example, AE4 does not mediate Cl-HCO3 exchange in the hands of all investigators, nor does BTR1/NaBC1 mediate boron transport in the hands of all investigators. Furthermore, the action of the Slc4a10 product is reportedly different for the human (“NBCn2”) versus the mouse and rat (“NCBE”) protein. Until these matters are resolved, caution must be exercised in the use of these acronyms. We recommend that papers on mammalian clones always refer at least once, preferably in a prominent way near the beginning of the manuscript, to the Slc4 designation. Investigators in doubt about the phenotype might use the Slc4 designation exclusively.
C. Molecular Action
The first two topics in this section deal with the diversity of NCBT gene products, either multiple NCBTs performing different actions, or multiple NCBTs performing the same action.
1. Benefits of having multiple NCBTs with distinct molecular actions
Among the five mammalian NCBTs, are engendered at least four distinct molecular actions: 1) electrogenic Na/HCO3 cotransport, 2) electroneutral Na/HCO3 cotransport, 3) electroneutral Na/HCO3 cotransport with a HCO3−-independent conductance, and 4) Na+-driven Cl-HCO3 exchange. Each action has its own physiological niche.
In the kidney, the basolateral step of HCO3− reabsorption could not be effected by an electroneutral NCBT, which, driven by prevailing ion gradients, would contribute to HCO3− secretion. Only a Cl-HCO3 exchanger (e.g., AE2 in the TAL, and AE1 in the α-intercalated cells) driven by an inwardly directed Cl− gradient, or an electrogenic NBC (i.e., proximal tubule) driven by Vm in addition to prevailing ion gradients, could contribute to HCO3− reabsorption.
Another example of the advantage of being electrogenic versus electroneutral is illustrated in the case of NBCe1 in neurons. Neuronal acidification, which could dampen neuronal excitability during repetitive firing, is avoided due to an NBCe1-mediated DIA because depolarization promotes electrogenic HCO3− import.
On the other hand, being electroneutral is sometimes advantageous. For example, electroneutral NCBTs can counter the effects of a whole body acidosis on neuronal pHi, and thereby maintain neuronal excitability, without influencing or being influenced by Vm.
Being coupled to Cl− transport also has important consequences. A study of NCBT action in nematode neurons indicates that Cl− efflux mediated by the action of an NDCBE plays an important role in the maturation of the CNS: by lowering [Cl−]i, it make ECl more negative than Vm, rendering GABAergic and glycinergic signaling inhibitory. On the other hand, NBCn1, which is not coupled to Cl−, regulates neuronal pHi without influencing [Cl−]i.
The only molecular action of NCBTs that has no currently demonstrated role is the HCO3−-independent conductance mediated by NBCn1. In theory, this conductance could make Vm more positive in neurons, influencing the action of ion channels and electrogenic transporters. For example, a more positive Vm in neurons would tend to inactivate voltage-gated Na+ channels, rendering these cells less excitable.
Benefits of having NCBTs with functional redundancy. There appears to be no discernible difference in the molecular actions of NBCe1 and NBCe2 or between the net transport activities of human NBCn1 and NBCn2. It is unclear, for example, why NBCe1 could not take the place of NBCe2 in hepatocytes, or why NBCe2 could not take the place of NBCe1 in proximal tubules. However, genetic redundancy has obvious potential benefits in providing backup in the case of haploinsufficiency. An example may be CNS neurons that contain both NBCn1 and NBCn2 (195). Moreover, the presence of both NBCe1 and NBCe2 (or NBCn1 and NBCn2) provides the opportunity for differential regulation during development or in response to stresses, or differential expression in different parts of the cell.
2. Transporters with controversial action
The molecular actions of Slc4a9 (AE4) and Slc4a11 (BTR1/NaBC1) remain controversial, as does the action of SLC4A10/Slc4a10 (NBCn2/NCBE). NCBTs may behave differently in diverse heterologous expression systems and when heterologously versus natively expressed. All systems include endogenous factors (e.g., ion channels) that can interfere with electrophysiological measurements. The benefits of performing transporter characterization in a heterologous system are many, but it is important, where possible, to reconcile the transport properties and inhibitor profile of the heterologously expressed transporter with the properties of the native protein.
Another aspect to bear in mind is that some transporters exhibit different molecular actions in difference species. For example, some Slc4 proteins from fishes exhibit conductive features that are not shared with their mammalian counterparts (e.g., trout versus human AE1, Ref. 285).
3. Novel substrates
Slc4-like proteins from invertebrate species have been suggested to transport non-HCO3− species such as silicate and valproic acid, although direct evidence is lacking. Cl-HCO3− versus CO32− versus NaCO3–: even for extensively characterized NCBTs, there remain many key questions concerning molecular action.
Do NCBTs carry HCO3− or CO32−? Preliminary studies indicate that, at least in the case of NBCe1 and NDCBE as expressed in oocytes, CO32− is the dominant, if not the only, carbon-containing substrate.
With the assumption that a transporter carries CO32−, does the CO32− move in the form of the NaCO3− ion pair, as may be the case for the Na+-driven Cl-HCO3 exchanger in the intact squid giant axon?
What is the mechanism by which electrogenic NCBTs appear to be able to switch between a 1:2 and a 1:3 stoichiometry?
What is the relationship between the molecular mechanisms of the exchangers/antiporters (e.g., AEs, NDCBE) and the presumed cotransporters/symporters (e.g., NBCe1, NBCn1)? Could all Slc4s be antiporters? For example, the electroneutral cotransport of one Na+ and one HCO3− would be thermodynamically equivalent to, and difficult to distinguish from, the cotransport of two Na+ and one CO32−, or the exchange of one Na+ and one CO32− (or 1 NaCO–3 ion pair) for one HCO3−.
How does the molecular action of an electrogenic NCBT compare with that of an electroneutral NCBT? So far, all that is known of the determinants of electrogenicity versus electroneutrality is that critical amino acid residues reside in EL4.
4. Unusual features of NBCn1
Three questions arise when we consider the action of NBCn1.
What is the relationship between the Na/HCO3 cotransport activity and the HCO3−-independent conductance of NBCn1?
Why is NBCn1 relatively insensitive to blockade by DIDS, despite retaining a seemingly intact DIDS-binding motif on TM5?
Furthermore, why does DIDS block the NCBT activity attributed to NBCn1 in mesenteric arteries and trigeminal ganglion neurons–is it possible that differences in posttranslational processing or local environment can impact DIDS sensitivity?
5. Undetermined inhibitor binding sites
Although the binding site for reversible DIDS inhibition is well described for NBCe1, the irreversible binding determinants are unknown as are the binding determinants for other drugs such as tenidap.
6. The K/HCO3 cotransporter
The protein(s) responsible for this activity are undescribed. Does an NCBT working in an unusual mode contribute? Intriguing in this regard is the observation that the Na+ versus Li+ specificity of certain NCBTs appears to be cell-type specific. An alternative explanation is that K/HCO3 cotransport could be mediated by another transporter family (e.g., SLC12, which includes the KCC K/Cl cotransporters).
7. Three-dimensional structure
Critical to understanding the molecular action of any NCBT will be a high-resolution three-dimensional structure together with molecular dynamic simulations. However, no high-resolution structure is available for any Slc4 or Slc4-like protein.
D. Genome
1. Diversity
Humans, mice, and rats have 10 Slc4 genes. Other mammals likely have the same number, but we have yet to truly appreciate the diversity of Slc4-like genes in nonmammalian species.
2. Gene clusters
SLC4A7 and SLC4A10 are both neighbored by T-box transcription factor genes (FIGURES 31 AND 39), indicating a long-standing relationship between the two gene families. Furthermore, growth factor genes are often located at similar chromosomal loci to Slc4 genes (e.g., TGFA and SLC4A5, see Ref. 591).
3. Promoter characterization
An emerging and underexplored area in the study of mammalian Slc4 genes is the mapping and characterization of Slc4 promoter regions, the understanding of which will impact our knowledge of the consequence of the use of alternative promoters, NCBT dysregulation in disease, and the factors that are responsible for altering NCBT abundance in response to diverse physiological and non-physiological stimuli.
E. Structural Features and Variants
1. Role of UTRs
At the level of Slc4 transcripts, we do not yet understand the consequences of alternative 5′- and 3′-UTR inclusion. These sequences likely include many determinants that impact the stability and efficiency of translation of the transcript, such as miRNA target sites. Because NCBT overabundance is linked to the poor outcomes in cancer, heart disease, and stroke, understanding how to manipulate NCBT transcript abundance would be valuable.
2. The diversity of NCBT protein variants
At the level of protein sequence, most NCBTs exhibit similar patterns of variation. For example, all NCBTs, with the current exception of NBCe2, have variants that include an autoinhibitory Nt appendage.
All NCBTs, with the possible exception of NBCe2, have variants that are stimulated by interaction with the soluble protein IRBIT.
NBCe1, NBCn1, and NBCn2 all exhibit variation in their Nt loop region. Note that this region is not known to be variable in the three AEs.
NBCe1, NBCn1, and NBCn2 all have the capacity to encode a Ct that terminates with a PDZ domain. Note that the Ct of the three AEs is not known to be variable.
The commonality between these gene variations is evident when we compare the primary structure of the variants side by side as illustrated in Appendix V. Despite the wealth of variants encoded by the NCBTs genes, we can be certain, based on variation between EST sequences, that more are yet to be described. In most cases, the physiological consequence of such variance is unknown, as are the mechanisms that dictate the presence or absence of the splice cassettes in specific tissues.
3. Influence of the Nt upon the TMD
An emerging theme is that many NCBT splice cassettes include docking sites for protein partners (e.g., IRBIT) that can influence NCBT activity. Furthermore, the Nt of NBCe1 appears to be a binding partner for the TMD of NBCe1, and this interaction is necessary for Na/HCO3 cotransport activity. There are a number of important mechanisms that have yet to be elucidated in this regard. For example, how do the Nt, the ASD, the AID, and IRBIT exert their effects on Na/HCO3 cotransport by the TMD? One possibility is that the cytosolic domain acts as a scaffold for the TMD and that structural rearrangements in the Nt influence the ability of the Nt to scaffold the TMD in a transport-competent conformation, as has been proposed for the cytosolic domain of a ClC from red alga (283).
4. Isolated Nt variants
Working with multiple cDNA libraries, investigators have amplified transcripts predicted to encode an isolated NCBT Nt domain (i.e., without a TMD). Such transcripts are produced by the Slc4a7, Slc4a8, and Slc4a10 genes. The abundance, stability, and relevance of protein expressed from these transcripts has yet to be described. Because the Nt does not appear in Slc4 evolution until the emergence of animals, the isolated Nt transcripts may represent the vestigial expression of the original “isolated Nt” open-reading frame that was appended to the “isolated TMD” transporter gene. The origin and original function of the ancestral isolated Nt is unknown. Note that the archetypal-Nt gene product need not have had the same open reading frame as the modern Nt.
F. Distribution
1. Overview
The distribution of NCBTs in the mammalian body is likely broader than is presently appreciated. In some instances, the location, but not the identity, of an NCBT is known as is the case with the DIDS-sensitive electroneutral NCBT activity in platelets (315). A thorough analysis of NCBT distribution would require the use of variant-specific and variant-independent primers and antibodies that are still in development, together with their application in normal and stressed tissues that have yet to be probed. In this respect, the study of transgenic animals that express reporter genes under the control of NCBT promoters could be useful.
2. Apparently overlapping distribution
Existing studies suggest that all five NCBTs are present in the central nervous system, the choroid plexus, and the kidney. The reason for such apparent redundancy is unknown. It is also unknown if two NCBTs coexpressed in the same cell type, such as NBCe1 and NBCn1 in duodenal villar cells (FIGURE 22), are capable of heterodimerizing to create a transporter with novel properties.
3. The polarity of NCBT expression
With few exceptions, the epithelial polarity of NCBTs, and indeed all Slc4s, is basolateral, complementing the usually apical distribution of Slc26 proteins (FIGURE 1). Some unusual epithelia express basolateral markers in their apical membranes, two examples that pertain to NCBT expression being the choroid plexus (with apical NBCe2) and the retinal pigment epithelium (with apical NBCe1). However, other reports of apical NCBT expression ought to be regarded with caution, pending independent confirmation. One example is the unusual apical NBCn1 immunoreactivity in renal tubules disclosed by the “anti-NBC3” antibody, the use of which is documented in Appendix VII. This distribution has not been confirmed by the use of any other anti-NBCn1 antibodies, which react with basolateral targets. The reason for this disparity remains unclear.
G. Physiological Roles
1. Overview
NCBTs fulfill three main roles: pHi regulation, HCO3− secretion, and Na/HCO3 reabsorption. In most cell types NCBTs mediate Na+ and HCO3− influx. Indeed, when expressed in the basolateral membranes of polarized cells (e.g., NBCn1 in salivary acinar cells in FIGURE 21), NCBTs support ion and fluid secretion. In several studies, this contribution becomes apparent only following inhibition of intracellular CAs, consistent with the idea that it is the CAs (e.g., in conjunction with Na-H exchangers) that are dominant in generating HCO3− for secretion under unstimulated conditions. In the CPE, apical NBCe2 could support HCO3− secretion by operating with an apparent 1:3 stoichiometry to support HCO3− secretion across the apical membrane into the CSF (FIGURE 28).
NBCe1, by operating with an apparent 1:3 stoichiometry in the basolateral membranes of PT epithelia(FIGURE 23), is the only NCBT demonstrated to support HCO3− reabsorption.
In neurons and glia, HCO3− transport mediated by electrogenic NCBTs (the direction of which appears to depend upon prevailing Vm and ion gradients) tends to maintain neuronal excitability.
2. Inferences from phenotypes of transgenic mice
Many of the physiological roles ascribed to NCBTs are inferred from the signs of mice and humans with disrupted NCBT genes. A potential complication is that, in some cases, the pathological signs may have more to do with the expression of misfolded protein (e.g., a partial transmembrane domain) rather than the absence of the physiological NCBT function per se. The observed signs may also depend on the nature of the NCBT disruption and the genetic background of the disrupted gene. In this regard, it will be informative to observe the phenotypes of multiple strains of mice in which the Slc4 has been disrupted in diverse ways (e.g., knockout versus gene-trap versus knocked-in mutation). The unintended consequences of dysregulation of other genes could also contribute to the observed phenotype, in some cases requiring that the investigators systematically examine the expression and activity of other transporters expected to contribute to the phenotype.
H. Causes of Upregulation
As depicted in Appendix V, NBCe1-B/C, NBCn1, NDCBE-A/B, and NBCn2 all include both autoinhibitory domains and modules in their Nt appendage that are predicted to render them sensitive to stimulation by IRBIT. However, although the physiological cues that activate IRBIT with respect to NCBTs are unknown, the abundance of NBCe1, NBCn1, and NDCBE transcripts are all increased during acidosis. In addition, NBCe1 and NBCn1 protein abundance is increased during hypercapnia. These observations are consistent with the role of these NCBTs in maintaining pH within a narrow physiological range.
Lacking from our current knowledge are the full details of the molecular mechanism(s) by which the NCBTs are upregulated by acidosis/hypercapnia. In principle, upregulation could occur at any or all of four levels: transcript abundance, total protein abundance, plasma-membrane protein abundance, and per-molecule protein activity. At least in the case of NBCe1-B, a pH-responsive element has been identified in the promoter region, and in the case of NBCe1-A, some of the signaling components that enhance HCO3− reabsorption in response to respiratory acidosis have been elucidated (e.g., Refs. 824, 890, 1104, and 1107). Characterization of promoter regions and the signaling cascades that enhance functional expression of NCBTs would be helpful towards the goal of understanding how the activity of multiple transporters are coordinated in a cell type. For example, an emerging theme is that IRBIT stimulates ion and fluid secretion by coordinated upregulation of multiple transporters.
On the topic of activating binding partners, CAs are proposed to bind to the Ct and EL4 of NBCe1 and to the Ct of NBCn1, forming a “metabolon.” The concept is that CAs supply HCO3− to the outer face of the transporter and remove HCO3− from the inner face of the transporter, or vice versa, depending on the direction of movement, thereby speeding transport. This theory remains controversial for four major reasons: 1) NBCe1 stimulation by CAs is observed only in some studies; 2) the binding of CAs to the Ct of NCBTs is observed only in some studies; 3) if, as preliminary data suggest, NCBTs transport CO32−, rather than HCO3−, it is not clear that CAs would enhance NCBT action substantially; and 4) modeling data suggest that CAs would have only a minor effect on HCO3− or CO32− transport rates (Ref. 342 and Rossana Occhipinti, personal communications).
Rather than stimulating transport per se, the action of CAs in the vicinity of NCBTs could minimize pH changes close to the plasma membrane, thereby minimizing adverse effects on the activities of other membrane proteins. The metabolon controversy is reviewed in Reference 102.
I. Causes of Downregulation
The abundance of NBCe1 and NBCn1 protein falls under alkalotic conditions, consistent with a reduced requirement for reabsorption and cellular influx of HCO3−. The abundance of NBCe1, NBCn1, NDCBE, and NBCn2 protein falls under hypoxic conditions, consistent with energy conservation and possibly also reflecting a response to respiratory alkalosis in these animals. The perturbation of NBCe2 abundance in response to hypoxia/alkalosis has not been reported. With the exception of the pH-responsive element located within the NBCe1-B/C promoter, the mechanisms that result in reduced NCBT abundance remain to be elucidated. An emerging and underexplored theme is that NCBT abundance can be reduced by miRNAs.
J. Consequences of Dysfunction
1. Transgenic mice
Recurring phenotypes observed in NCBT-null mice are acidosis (NBCe1 and NBCe2), hypertension (NBCn1 and NBCe2), reduced neuronal excitability (observed for NBCe2, NDCBE, NBCn2, and inferred for NBCe1), impaired CSF secretion (NBCe2 and NBCn2), and ocular defects (NBCe1, NBCe2, and NBCn1). In the majority of cases, these phenotypes accord well with the known distribution and inferred physiological roles of each NCBT.
Somewhat surprising, given the abundance of NBCe1 in the pancreas, NBCe2 in the liver, and NBCn1 in the mTAL, NBCe1-null mice lack an obvious pancreatic phenotype, NBCn1-null mice lack an obvious renal phenotype, and NBCe2-null mice lack an obvious hepatic phenotype. Presumably these mice have upregulated unknown compensatory pathways or have not been subjected to the appropriate challenges. In the case of NBCe1-null mice, another possibility is that the animals, which die shortly after weaning, have not lived long enough to exhibit a phenotype. In this respect, studies of conditional and inducible knockouts, which have not been reported for any NCBT, will be illuminating, as should be the application of antisense RNA technology in animals.
One potentially confounding aspect of existing NCBT knockout mice is the interpretation of their phenotypes. For example, as mentioned above, especially in the case of gene-trapped mice, some phenotypes may be consequences of the expression of partial, misfolded NCBT protein rather than consequences of the absence of the NCBT activity per se. Such mice may be better models for the pathological consequences of NCBT mutation in humans because some signs in affected individuals may be specifically due to misfolded protein response. Studies of targeted transgenic mice that carry orthologs of human mutations, the NBCe1-mutant W516X mouse that mimics a human pRTA is the only example to date, are the most appropriate in regard to modeling pathologies associated with human disease. In terms of investigating the results of the loss of NCBT transport activity, a transgenic mouse that expresses a nonfunctional, yet full-length NCBT would perhaps provide the clearest picture. Attempts to disrupt an NCBT gene close to its initiator Met include the potential hazard of permitting normal transcription of alternative gene products.
2. Linkage studies
Numerous GWAS studies implicate variation at NCBT gene loci with susceptibility to various traits and disorders, such as autism, substance abuse, hypertension, and cancer. These associations are consistent with known roles for NCBTs in control of neuronal excitability, Na+ reabsorption, and in countering the apoptotic effects of acidosis. These studies will need to be followed up with deep sequencing, the results of which would provide a statistically significant link (or lack thereof) of the trait to a specific SNP within an NCBT gene. The next step would be to study, in a heterologous system or transgenic animal, the effect of the SNP on the functional expression or regulation of that NCBT.
In conclusion, it seems likely, given the widespread abundance of NCBTs, and the effects of pH on almost all physiological processes, that NCBT action modifies a wide array of complex genetic traits. Our current knowledge concerning the diverse molecular actions, distribution, regulation, physiological roles, and pathophysiological roles of NCBTs provides only a glimpse of their potential importance.
For Appendices I–VIII, the online version of this article contains supplemental material.
GRANTS
This work was supported by National Institutes of Health Grants EY021646 (to Michael L. Jennings and M. D. Parker), DK30344 (to W. F. Boron), DK81567 (to W. F. Boron), NS18400 (to W. F. Boron), HD032573 (to Gabriel G. Haddad/project 2 and W. F. Boron), and HL090969 (to Alanna C. Morrison).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dennis Brown for his patient editorial oversight. We thank the two reviewers for their thorough reading of the manuscript and their helpful comments. We also thank members of the Boron lab past and present as well as all those investigators whose studies and personal communications have contributed to fabric of this review.
Address for reprint requests and other correspondence: M. D. Parker, Dept. of Physiology and Biophysics, Case Western Reserve University School of Medicine, 10900 Euclid Ave., Cleveland, OH 44106-4970 (e-mail: mark.d.parker@case.edu).
Appendix I: Annotated Protein Sequence Alignments of Human SLC4s (See Figures 3 and 15)
Appendix II: GenBank or Ensembl Protein Accession Numbers for Nonmammalian Slc4-like Transporters (See Figures 4 and 8)
Appendix III: Analysis of Conserved Exon Boundaries Among Human SLC4 and Ciona Slc4-like Genes (See Figure 7)
Appendix IV: GenBank Protein Accession Numbers for Mammalian Slc4 Variants (See Sects, V and VI)
Appendix V: Annotated Protein Sequence Alignments of Human NCBT Variants (See Sect. V)
Appendix VI: The Distribution of Expressed Sequence Tags for Humans and Mouse NCBTs, AE4, and BTR1 (See Sects. V and VI)
Appendix VII: Locations of “Anti-NBC3” Immunoreactivity (See Sect. V)
Appendix VIII: Locations of Renal Anti-AE4 Immunoreactivity (See Sect. VI)
Footnotes
In most cases, these channels are at best poorly permeable to HCO3− and, in most cases, the physiological significance of this permeability is not demonstrated.
For the purposes of this review, we depict Slc26a3, -4, -6, -7, and -9 as electroneutral Cl-HCO3 exchangers; in fact, the molecular action of these Slc26 transporters is controversial. Slc26a3 has been described as being capable of electroneutral Cl-HCO3 exchange by some (26, 908) but electrogenic 2Cl-HCO3 exchange by others (499, 871). Slc26a4 is capable of electroneutral Cl-HCO3 exchange (872), a description that is uncontested. Slc26a6 has been described as being capable of electroneutral Cl-HCO3 exchange by some (185) but electrogenic Cl-2HCO3 exchange by others (499, 871, 1052). Slc26a7 has been described as an electroneutral Cl-HCO3 exchanger (743), and also as a pH-sensitive Cl− channel with no anion exchange activity (488). Slc26a9 has been decribed as a Cl-HCO3 exchanger of undetermined electrogenicity/electroneutrality (1055), an electrogenic nCl-HCO3 exchanger with a HCO3−-independent Cl− conductance (168), a HCO3−-independent Cl− channel with no anion exchange activity (258), and also as a HCO3−-stimulated Cl− channel with no anion exchange activity (609). The reasons underlying the apparent disparities among studies have not been determined.
Following the recommendations of The HUGO (Human Genome Organization) Gene Nomenclature Committee (HGNC), we use the terms “SLC4” (gene) and “SLC4” (gene-product) only in instances when we are specifically and exclusively referring to human genes and products. The terms “Slc4” and “Slc4” are used in reference to vertebrate genes/products in general (even if that grouping includes humans), whereas “Slc4-like” and “Slc4-like” refer to related nonvertebrate genes. The common names AE, NBC, and NDCBE are capitalized throughout, independently of parent organism.
The DS of an Slc4-like transporter is the difference between 1) the percent identity (computed by a pairwise BLAST alignment at http://blast.ncbi.nlm.nih.gov; see Ref. 951) of each transporter with its most similar reference protein and 2) the averaged percent identity of the transporter with the other reference proteins to which it has been compared.
Many partial, unattributed “Nitro”-like DNA sequences are present in the environmental genome database, making it likely that many other Slc4-like transporters are encoded in the genomes of marine bacteria. For example, nucleotide accession numbers AACY01572225, AACY01408744, AACY01136643, and AACY01500593.
For example, ZP_02948162 from Clostridium butyricum 5521.
Following the recommendations of Jensen (447), we use the term ortholog to distinguish gene/proteins with a common genetic ancestry from different species (e.g., human SLC4A4 versus mouse Slc4a4) and the term paralog to distinguish gene/proteins that diverged from each other following gene duplication (e.g., human SLC4A4 versus human SLC4A5 or human SLC4A4 versus mouse Slc4a5). These terms are intended to refer to phylogenetic rather than functional relatedness.
Note that the extended Nt of the slime mold Slc4-like transporter bears no significant sequence identity to the Nt of animal NCBTs and likely evolved independently.
Precomputed structural alignments between the Nt of AE1 and certain bacterial EIIA proteins can be accessed via http://www.ncbi.nlm.nih.gov/Structure/vast/vastsrv.cgi?sdid=51159.
FIGURE 7 is based on the sequence alignments of Ciona and human Slc4 genes in Appendix III.
Atr1p is a member of the multidrug-resistant transporter protein family originally noted for its ability to confer aminotriazole tolerance (464).
According to the terminology recommended in Reference 296, apical membranes face the shoot apex and basal membranes face the root apex.
The GenBank accession numbers are provided in Appendix II.
Those that coexpress the cardiac homeotic products Tin and Abd-A. NDAE1 expression is dependent on the expression of Abd-A (737).
This terminology is not intended to infer that AaAE1 is a direct ortholog of mammalian Slc4a1, but instead refers to AaAE1 being the first of two Na+-independent AEs cloned from insects.
According to terminology of Perry et al. (741), these cells are referred to as MR cells in freshwater fish and chloride cells in saltwater fish.
aka the corneal posterior epithelium.
GenBank protein accession O13134.
Nomenclature guidelines for Xenopus genes are provided at http://www.xenbase.org/gene/static/geneNomenclature.jsp.
About 1.5 days post-fertilization. “Nieuwkoop Faber” developmental stages are defined in Ref. 1, and the images that accompany the defintions are reproduced online at the Xenbase: Xenopus laevis and Xenopus tropicalis biology and genomics resource (http://www.xenbase.org/anatomy/static/NF/NF-all.jsp).
Data from The Xenopus Gene Expression Database (http://www.euregene.org/xgebase/pages/entry_page.html).
Although carbachol and histamine both stimulate HCl secretion.
We define functional expression as the product of surface expression and the intrinsic transporter activity of individual molecules.
Defects that reduce the intrinsic transporter activity of individual molecules.
Substituted stilbenes undergo cis-trans photoisomerization, the cis isoforms being less potent than the trans isoforms as Slc4 inhibitors (848, 880, 1001).
In fact, the Na+ conductance mediated by NBCn1 is stimulated after prolonged exposure to 500 μM DIDS (189, 201).
This motif is “KLFH” in mouse and rat orthologs of NBCn1.
Developed by Pfizer Inc (New York, NY).
Developed by Sanofi-Aventis U.S. LLC (Bridgewater, NJ). A synthesis protocol based on commercially available compounds has been developed by Larsen and co-workers and is provided in Ref. 545.
Bachmann et al. cite unpublished observations from Aventis Laboratories that S0859 blocks NBCe1 with a Ki ∼6 μM, but does not block NBC2/3 (i.e., NDCBE) as expressed in CHO cells.
The use of S0859 as an “NBC1 blocker” (849) or an “NBCn1 inhibitor” (546) in cells in which these transporters are the dominant NCBT paralog does not constitute a demonstration of paralog specificity of the compound.
Only the ORFs, and not the 5′ UTRs, have not been reported for NBCe1-C, -D, and -E so the presence of exon 1 has not been demonstrated in these transcripts. We cannot rule out the possibility that another promoter is present between exons 1 and 2.
Antibodies and most PCR probes used in these studies would not be able to differentiate NBCe1-A from NBCe1-D, or NBCe1-B from NBCe1-E, but NBCe1-D and NBCe1-E appear to only account for a minor fraction of total NBCe1 product and are not considered here in the discussion of reports of NBCe1-A and NBCe1-B.
NBCe1-A transcripts in mouse brain are nearly 50% as abundant as those encoding NBCe1-B/C (E. Roussa, personal communication).
The immunostaining of NBCe1-A protein throughout the mouse brain must be interpreted with some caution: 1) the preimmune serum from the rabbit used to generate the anti-NBCe1-A antibody diffusely labeled tubules in the rat renal cortex (see Fig. 3C of Ref. 817); and 2) no preimmune controls are presented for the brain sections (796).
In a separate study, Turner and co-workers were unable to demonstrate NBCe1-A/B immunoreactivity in the conjunctival epithelium of rats and pigs because their immunohistochemical studies were hampered by “discernable nonspecific labeling” (985).
The report of human NBCe1-A, and not NBCe1-B, expression in microdissected human enamel organ (1099) is probably incorrect. The “human NBCe1-A” primer pair used in Ref. 1099 is actually specific to NBCe1-B/C, whereas the “human NBCe1-B” primer pair used in Ref. 1099 is actually specific for NBCe1-A.
Some caution must be exercised when interpreting these studies, as the antibodies raised against epitopes in the common Nt of NBCe1-B/C appear to be troublesome. For example, one NBCe1-B antibody (796, 817) 1) exhibits more robust immunoreactivity with renal protein extracts than with pancreatic protein extracts, opposite to the distribution of NBCe1-B transcripts; 2) immunoreacts with a number of other proteins in pancreatic extracts, such that full-length NBCe1-B is a minor target for this antibody in the pancreas; and 3) exhibits a staining pattern in renal sections that is not different from the nonspecific staining produced using preimmune serum from a rabbit used in the same study (See Fig. 3, C versus D, in Ref. 817). Another NBCe1-B antibody, originally reported in Ref. 989, as expected, does not immunoreact with protein in human kidney extracts but does immunoreact with a rat kidney protein (273).
A later review by the same group refers to epididymal NBCe1 as NBCe1-A, although the immunohistochemistry by itself is not sufficient to support this specific assignment.
Enhanced seizure resistance in a third, NBCe2-null, mouse strain may be an indirect effect of altered CSF composition because NBCe2 is not expressed in neurons.
Although one model proposed by Wiederholt et al. places an electrogenic NCBT in the apical membrane, the authors state that this is an assumption since their methods did not allow them to determine the localization of NCBT in their cells (1035).
Anion secretion across the apical membrane of pancreatic duct epithelia is likely mediated by the concerted actions of CFTR, Ca2+-activated chloride channels, and Slc26a6 (aka PAT1) with Slc26a3 (aka DRA1) playing a supporting role (333, 421, 628, 909, 1028).
Although rat cholangiocytes express NBCe2 in their apical membranes (8), mouse cholangiocytes are reported to lack NBCe2 (987).
Because NBCe1 knockout mice have not survived to a breeding age (313), it is not known whether NBCe1 is necessary for fertility.
The authors of another study report a significant difference in NBCe1 mRNA expression levels between normal and failing hearts based on microarray data, but do not report the direction of the change (649). Transfecting cultured rat cardiomyocytes with an adenoviral vector that is designed to overexpress NBCe1 “modified the beating rate” and “lowered the viability,” although no confirmation of NBCe1 overexpression or primary data were provided (649). Mice genetically modified with an NBCe1 transgene (to mimic NBCe1 overexpression) exhibited no cardiac detectable or blood-pressure phenotype, although neither the identity of the splice variant nor confirmation of NBCe1 overexpression was provided (649).
In this report, the authors state that the antibody used in this study (no. 3212; Chemicon) does not discriminate between NBCe1, NBCe2, or NBCn1. The antibody was raised against an 54-amino acid epitope in the soluble Nt domain of rat NBCe1 and appears, as evidenced by lack of immunoreactivity with the mTAL in rat kidney section (844), to be at least unreactive towards NBCn1. A later paper by the same group demonstrated that this antibody and an NBCe2-specific antibody recognize proteins of different molecular weights in rat muscle preparations, indicating that this antibody is likely to be specific for NBCe1.
Interestingly, the characteristics the NCBT activity in immortalized SHR cells differ from the NCBT activity in control cells in two ways (731). In SHR cells, NCBT activity is 1) more sensitive to stimulation by acidosis and 2) poorly DIDS-sensitive (50% blockade by 1 mM DIDS). These features are reminiscent of NBCn1 which is strongly upregulated by acidosis and poorly sensitive to DIDS, leaving open the possibility that NBCn1 is expressed in these immortalized SHR cells.
Src and MAPK phosphorylation are also implicated in a PPARγ-associated pathway that stimulates NBCe1 functional expression in response to thiazolidinedione treatment.
Conversely, NaCl feeding, a maneuver that would tend to lower the glomerular filtration rate, results in a reduction of NBCe1 protein abundance.
NBCe1 null-mice rarely survive beyond weaning age (313).
For example, baboon (XP_003908879), gibbon (XP_003268733), and orangutan (XP_003775944).
It has been noted by Odgaard et al. (694) that the NBCe2-specific primers reported by Xu et al. are not derived from NBCe2 sequence. This appears to have been an errant description of the primer sequences in the paper rather than in the design of the actual primers used by Xu et al. that were GCCAGCTATGCATGAAATTG (sense) and ATGGGTCCTGTGCTGCTGAG (antisense; J. Xu and M. Soleimani, personal communication).
Despite the abundance of NBCe2 transcripts in the mouse thyroid, the morphology and serum T4 abundance were normal in NBCe2-null mice (341).
As the authors of this study note, the Slc4a5 gene of these mice is disrupted in the third extracellular loop, and it cannot be discounted that the phenotypes observed in these mice are due to the expression of misfolded Slc4a5 product in Slc4a5-expressing cells. Thus these mice may be a better model of the effects of mutations that cause NBCe2 to misfold, rather than a model for drawing inferences about the physiological roles of NBCe2.
In addition to numerous artifacts in the hNBC2/SBC clone, the inclusion in mNBC3 versus hNBC2/SBC2 of a different complement of splice cassettes also misled investigators to believe that these two cDNAs were transcribed from different genes.
This report was concurrent with a report of a partial product of truly novel gene (Slc4a8), which was also then named NBC3 (35). This product has since been renamed NDCBE.
The first report of NBCn1 expression in oocytes characterized the protein as being DIDS-insensitive, EIPA-sensitive, and capable of substantial Na/OH cotransport or Na-H exchange (765). Subsequent work published with members of the same laboratory reports that HEK-293-expressed NBCn1 does not have these qualities (711). Thus the authors conclude that the EIPA sensitivity and Na/OH cotransport are a feature of NBCn1 expression in oocytes. It is therefore likely that the initial report was confounded by endogenous NHE activity, and possible that none of the reported acid-base transport in fact represented NBCn1. In the meantime, an apical EIPA-sensitive Na-base cotransport activity in α-intercalated cells from the rabbit collecting duct (from the inner stripe of the outer medullary) was attributed to NBCn1 in two studies (774, 1083), guided by the original report of EIPA sensitivity of NBCn1 and the apical distribution of NBCn1 suggested by the use of the “anti-NBC3” antibody discussed in Appendix VII. Finally, in aortic smooth muscle of rats (a site of NBCn1 expression), the authors report both an EIPA-sensitive NCBT activity and a distinct SITS-sensitive, and unusually EIPA-sensitive, NDCBE-like activity (592).
A 67-nt sequence with 87% identity to a portion of SLC4A7 (exon 10, encoding sequence in the Nt) is found on chromosome 1p36, 13 kb upstream of the RhD gene. There is currently no evidence to suggest that this sequence is ever transcribed, or is part of an miRNA sequence.
Analysis of the Slc4a7 gene structure leads us to deduce that cassette I encompasses the 13-amino acid sequence “GKKHSDPHLLERN” and not, as originally deduced in Ref. 189, the 14-amino acid sequence “GKKHSDPHLLERNG.”
“Exon 7” is a misnomer. Although cassette II is encoded by the seventh exon of individual NBCn1 transcripts, cassette II is encoded by exon 8 of the Slc4a7 gene (see FIGURE 31C).
In a personal communication, Drs. Liming Chen and Ying Liu report to us the existence of a new promoter and a new cassette that could increase the number of possible variants to 64.
Considering only “MEAD” versus “MERF” and the three cassettes, the “missing” combinations are MEAD/-I/-II/-III, MERF/+I/+II/+III, MERF/-I/-II/+III, MERF/-I/+II/+III, and MERF/-I/-II/-III.
If these clones included exon 13, FJ178574 would encode NBCn1-H, FJ178575 would encode NBCn1-G, FJ178576 would encode NBCn1-E, GU354307 would encode NBCn1-C, GU354309 would encode NBCn1-E′, and GU354310 would encode NBCn1-G′. Because some of these full-length NBCn1 clones differ only in the protein sequence of their Ct, the accession pair FJ178574/GU354307 is predicted to encode identical isolated-Nt polypeptides as are the pairs FJ178575/FJ178576 and GU354309/GU354310.
Gene Expression Omnibus Entry at (http://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS857).
The antibody in this study detected only NH2 termini beginning with “MEAD” (see green cassette in FIGURE 32).
The osteoclast membrane has a ruffled border (facing the resorption lacuna) and a free surface (not facing the lacuna). Although these domains are sometimes considered equivalent to the apical and basolateral membranes of epithelia, the free surface is composed of subdomains that contain both apical and basolateral markers of classic epithelia, whereas the ruffled border could be considered as a “giant extracellular lysosome” (662, 991).
An NHE1 blocker.
An NCBT inhibitor of undemonstrated specificity.
One preliminary report found that rat renal NBCn1 transcript abundance was not increased by NH4Cl feeding, but that NBCn1 protein levels were increased (664).
By analogy to the inhibitory effect of intracellular Mg2+ on NBCe1-B, one might expect Mg2+ depletion to activate other AID-including NCBTs, such as NBCn1, which could lower the seizure threshold of hippocampal.
Indeed, as of May 2012, the number of allelic SLC4A7 variations in the NCBI SNP database stands at 1449, 85 of which are located in exons and 46 of which alter the predicted NBCn1 coding sequence.
SLC4A7 products are not among the 340 red cell proteins identified in a proteomic study by Pasini and co-workers (727), nor among the 751 proteins reported in a review of the human red cell proteome (329).
This partial human clone (AF107099) is identical along its length to subsequently cloned human SLC4A8 products. A full-length mouse Slc4a8 product (now called NDCBE-A) was subsequently cloned in its entirety by a group that included the same authors (1029).
GenBank nucleotide accession number AF069512.
Immunohistochemistry using an antibody specific to NDCBE-A and -B does not detect NDCBE protein in the proximal tubule (J. Praetorius, personal communication), although this observation does not preclude the presence of NDCBE-C or -D.
The difficulty of resolving NDCBE and NDCBE-like activities are discussed on p. 895.
The antibody raised against the short Ct of NBCn2-A/B has been shown to cross-react with the long Ct of NBCn2-C/D (756), although RT-PCR results predict that only NBCn2 variants with a short Ct are expressed in mouse choroid plexus (755).
Build 37.1 of the human genome indicates that the FISH probes used in this study to identify the break point in chromosome 13 in fact bind to 13q22, rather than to the originally assigned 13q31.
The data were obtained from the Wellcome Trust Sanger Institute Cancer Genome Project website: http://www.sanger.ac.uk/genetics/CGP/cosmic.
A basolateral Cl-HCO3 exchanger, thought to be AE2, also contributes towards HCO3− reabsorption in the S3 segment of the proximal tubule (506, 677). Although AE2 mRNA has been detected in rat proximal tubule preparations (128), robust AE2 immunoreactivity has not been observed in any segment of the proximal tubules of mice, rats, or humans (27, 158, 914).
In rats, AE2c1 and AE2c2 transcripts both encode the AE2c1 polypeptide (1030). Genomic differences may mean that the rabbit and human AE2 genes lack the capacity to produce AE2c transcripts (527).
GenBank protein accession numbers for the variants discussed in this section are provided in Appendix IV.
α-Type intercalated cells reabsorb HCO3− (i.e., lumen to blood) and are characterized immunologically by an apical presence of a vacuolar-type H+ pump and a basolateral presence of AE1 (mediating HCO3− efflux). β-Type intercalated cells secrete HCO3− (i.e., blood to lumen) and are characterized immunologically by an apical presence of pendrin (Slc26a4, mediating HCO3− efflux), a basolateral presence of a vacuolar H+ pump, and a lack of basolateral AE1. Non-α/non-β-intercalated cells (sometimes refered to as γ-subtypes) have an apical presence of pendrin and H+ pump and lack basolateral AE1.
The defect in AE1-null mice may be partly compensated by upregulation of the AE1-colocalized transporter Slc26a7, which some investigators describe as a Cl-HCO3 exchanger (921) (see footnote 2).
A soluble, ∼320-amino acid fragment of the BTR1 NH2 terminus fused to β-galactosidase.
Groger and co-workers find no evidence of BTR1 expression in the anterior corneal epithelium in mice using an anti-BTR1 antibody (340), nor is BTR1 promoter activity disclosed in these cells by β-galactosidase assays of corneal sections from BTR1-lacZ transgenic mice (340).
BTR1 transcripts have been detected in inner medullary preparations, apparently decreasing in abundance in segments closest to the papilla, according to a semiquantitative study (754), but oddly were undetectable in microdissected inner medullary duct preparations (754).
Boron is one of 11 “ultratrace elements” that have a dietary requirement of <1 μg/g body wt, and for which pathological consequences of dietary insufficiency have not been adequately demonstrated (683). Boron is, however, an essential nutrient for plants.
A database of SLC4A11 mutations is curated at the Leiden Open Variation Database (https://grenada.lumc.nl/LOVD2/mendelian_genes/home.php?select_db=SLC4A11).
REFERENCES
- 1. Normal Table of Xenopus laevis (Daudin) (2nd ed.). New York: Garland, 1994. [Google Scholar]
- 2. Abdulnour-Nakhoul S, Khuri RN, Nakhoul NL. Effect of norepinephrine on intracellular pH in kidney proximal tubule: role of Na+-(HCO3−)n cotransport. Am J Physiol Renal Physiol 275: F33–F45, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Abdulnour-Nakhoul S, Nakhoul HN, Kalliny MI, Gyftopoulos A, Rabon E, Doetjes R, Brown K, Nakhoul NL. Ion transport mechanisms linked to bicarbonate secretion in the esophageal submucosal glands. Am J Physiol Regul Integr Comp Physiol 301: R83–R96, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdulnour-Nakhoul S, Nakhoul NL, Wheeler SA, Wang P, Swenson ER, Orlando RC. HCO3− secretion in the esophageal submucosal glands. Am J Physiol Gastrointest Liver Physiol 288: G736–G744, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Abuladze N, Azimov R, Newman D, Liu W, Tatishchev S, Pushkin A, Kurtz I. Critical amino acid residues involved in the electrogenic sodium bicarbonate cotransporter kNBC1-mediated transport. J Physiol 565: 717–730, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem 273: 17689–17695, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Abuladze N, Lee I, Newman D, Hwang J, Pushkin A, Kurtz I. Axial heterogeneity of sodium-bicarbonate cotransporter expression in the rabbit proximal tubule. Am J Physiol Renal Physiol 274: F628–F633, 1998. [DOI] [PubMed] [Google Scholar]
- 8. Abuladze N, Pushkin A, Tatishchev S, Newman D, Sassani P, Kurtz I. Expression and localization of rat NBC4c in liver and renal uroepithelium. Am J Physiol Cell Physiol 286: 2004. [DOI] [PubMed] [Google Scholar]
- 9. Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, Kurtz I. Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene 251: 109–122, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Adam WR, Koretsky AP, Weiner MW. 31P-NMR in vivo measurement of renal intracellular pH: effects of acidosis and K+ depletion in rats. Am J Physiol Renal Fluid Electrolyte Physiol 251: F904–F910, 1986. [DOI] [PubMed] [Google Scholar]
- 11. Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol 133: 603–622, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed S, Thomas G, Ghoussaini M, Healey CS, Humphreys MK, Platte R, Morrison J, Maranian M, Pooley KA, Luben R, Eccles D, Evans DG, Fletcher O, Johnson N, Dos SS, I, Peto J, Stratton MR, Rahman N, Jacobs K, Prentice R, Anderson GL, Rajkovic A, Curb JD, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Diver WR, Bojesen S, Nordestgaard BG, Flyger H, Dork T, Schurmann P, Hillemanns P, Karstens JH, Bogdanova NV, Antonenkova NN, Zalutsky IV, Bermisheva M, Fedorova S, Khusnutdinova E, Kang D, Yoo KY, Noh DY, Ahn SH, Devilee P, van Asperen CJ, Tollenaar RA, Seynaeve C, Garcia-Closas M, Lissowska J, Brinton L, Peplonska B, Nevanlinna H, Heikkinen T, Aittomaki K, Blomqvist C, Hopper JL, Southey MC, Smith L, Spurdle AB, Schmidt MK, Broeks A, van Hien RR, Cornelissen S, Milne RL, Ribas G, Gonzalez-Neira A, Benitez J, Schmutzler RK, Burwinkel B, Bartram CR, Meindl A, Brauch H, Justenhoven C, Hamann U, Chang-Claude J, Hein R, Wang-Gohrke S, Lindblom A, Margolin S, Mannermaa A, Kosma VM, Kataja V, Olson JE, Wang X, Fredericksen Z, Giles GG, Severi G, Baglietto L, English DR, Hankinson SE, Cox DG, Kraft P, Vatten LJ, Hveem K, Kumle M, Sigurdson A, Doody M, Bhatti P, Alexander BH, Hooning MJ, van den Ouweland AM, Oldenburg RA, Schutte M, Hall P, Czene K, Liu J, Li Y, Cox A, Elliott G, Brock I, Reed MW, Shen CY, Yu JC, Hsu GC, Chen ST, Anton-Culver H, Ziogas A, Andrulis IL, Knight JA, Beesley J, Goode EL, Couch F, Chenevix-Trench G, Hoover RN, Ponder BA, Hunter DJ, Pharoah PD, Dunning AM, Chanock SJ, Easton DF. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2 Nat Genet 41: 585–590, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aickin CC. Regulation of intracellular pH in the smooth muscle of guinea-pig ureter: HCO3− dependence. J Physiol 479: 317–329, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aiello EA, Petroff MG, Mattiazzi AR, Cingolani HE. Evidence for an electrogenic Na-HCO3 symport in rat cardiac myocytes. J Physiol 512: 137–148, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akiba T, Rocco VK, Warnock DG. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest 80: 308–315, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akiba T, Warnock DG. Evidence for Na+/carbonate cotransport in rabbit renal cortical basolateral membrane vesicles (BLMV) (Abstract). Clin Res 35: 633A, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akiba Y, Kaunitz JD. Duodenal chemosensing and mucosal defenses. Digestion 83 Suppl 1: 25–31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alda JO, Garay R. Chloride (or bicarbonate)-dependent copper uptake through the anion exchanger in human red blood cells. Am J Physiol Cell Physiol 259: C570–C576, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Aldahmesh MA, Khan AO, Meyer BF, Alkuraya FS. Mutational spectrum of SLC4A11 in autosomal recessive CHED in Saudi Arabia. Invest Ophthalmol Vis Sci 50: 4142–4145, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Aldave AJ, Yellore VS, Bourla N, Momi RS, Khan MA, Salem AK, Rayner SA, Glasgow BJ, Kurtz I. Autosomal recessive CHED associated with novel compound heterozygous mutations in SLC4A11. Cornea 26: 896–900, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Ali R, Amlal H, Burnham CE, Soleimani M. Glucocorticoids enhance the expression of the basolateral Na+:HCO3− cotransporter in renal proximal tubules. Kidney Int 57: 1063–1071, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol 212: 1672–1683, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alper SL, Kopito RR, Libresco SM, Lodish HF. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. J Biol Chem 263: 17092–17099, 1988. [PubMed] [Google Scholar]
- 24. Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Definition of intercalated cell subtypes in rat kidney collecting duct using antibodies against erythroid band 3 and renal vacuolar H+ ATPase. Proc Natl Acad Sci USA 86: 5429–5433, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am J Physiol Gastrointest Liver Physiol 277: G321–G322, 1999. [DOI] [PubMed] [Google Scholar]
- 26. Alper SL, Stewart AK, Vandorpe DH, Clark JS, Horack RZ, Simpson JE, Walker NM, Clarke LL. Native and recombinant Slc26a3 (downregulated in adenoma, Dra) do not exhibit properties of 2Cl−/1HCO3− exchange. Am J Physiol Cell Physiol 300: C276–C286, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alper SL, Stuart-Tilley AK, Biemesderfer D, Shmukler B, Brown D. Immunolocalization of AE2 anion exchanger in rat kidney. Am J Physiol Renal Physiol 273: F601–F614, 1997. [DOI] [PubMed] [Google Scholar]
- 28. Alpern RJ. Mechanism of basolateral membrane H+/OH−/HCO3− transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol 86: 613–636, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alpern RJ, Chambers M. Basolateral membrane Cl/HCO3 exchange in the rat proximal convoluted tubule. Na-dependent and independent modes. J Gen Physiol 89: 581–598, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alvarez BV, Gilmour GS, Mema SC, Martin BT, Shull GE, Casey JR, Sauve Y. Blindness caused by deficiency in AE3 chloride/bicarbonate exchanger. PLoS ONE 2: e839, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alvarez BV, Kieller DM, Quon AL, Robertson M, Casey JR. Cardiac hypertrophy in anion exchanger 1-null mutant mice with severe hemolytic anemia. Am J Physiol Heart Circ Physiol 292: H1301–H1312, 2007. [DOI] [PubMed] [Google Scholar]
- 32. Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR. Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry 42: 12321–12329, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Alvarez LJ, Candia OA, Wolosin JM. Evidence for parallel Na(+)-H+ and Na(+)-dependent Cl−-HCO3− exchangers in cultured bovine lens cells. Exp Eye Res 55: 747–755, 1992. [DOI] [PubMed] [Google Scholar]
- 34. Alvarez-Leefmans FJ, Delpire E. Thermodynamics and kinetics of chloride transport in neurons: an outline. In: Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases , edited by Alvarez-Leefmans FJ, Delpire E. New York: Academic, 2009, p. 82–105. [Google Scholar]
- 35. Amlal H, Burnham CE, Soleimani M. Characterization of the Na+:HCO3− cotransporter isoform NBC-3. Am J Physiol Renal Physiol 276: F903–F913, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int 60: 1824–1836, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Amlal H, Chen Q, Soleimani M. Coordinated regulation of basolateral Na+:HCO3− cotransporter NBC-1 and apical Na+/H+ exchanger in bicarbonate loading (Abstract). J Am Soc Nephrol 11: 1A, 2000.10616834 [Google Scholar]
- 38. Amlal H, Habo K, Soleimani M. Potassium deprivation upregulates expression of renal basolateral Na+-HCO3− cotransporter (NBC-1). Am J Physiol Renal Physiol 279: F532–F543, 2000. [DOI] [PubMed] [Google Scholar]
- 39. Amlal H, Wang Z, Burnham C, Soleimani M. Functional characterization of a cloned human kidney Na+:HCO3− cotransporter. J Biol Chem 273: 16810–16815, 1998. [DOI] [PubMed] [Google Scholar]
- 40. Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science 282: 1711–1714, 1998. [DOI] [PubMed] [Google Scholar]
- 41. Anderson JM. Cell signalling: MAGUK magic. Curr Biol 6: 382–384, 1996. [DOI] [PubMed] [Google Scholar]
- 42. Anderson WW, Lewis DV, Swartzwelder HS, Wilson WA. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res 398: 215–219, 1986. [DOI] [PubMed] [Google Scholar]
- 43. Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem 278: 10602–10612, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Antoniou AC, Beesley J, McGuffog L, Sinilnikova OM, Healey S, Neuhausen SL, Ding YC, Rebbeck TR, Weitzel JN, Lynch HT, Isaacs C, Ganz PA, Tomlinson G, Olopade OI, Couch FJ, Wang X, Lindor NM, Pankratz VS, Radice P, Manoukian S, Peissel B, Zaffaroni D, Barile M, Viel A, Allavena A, Dall'Olio V, Peterlongo P, Szabo CI, Zikan M, Claes K, Poppe B, Foretova L, Mai PL, Greene MH, Rennert G, Lejbkowicz F, Glendon G, Ozcelik H, Andrulis IL, Thomassen M, Gerdes AM, Sunde L, Cruger D, Birk JU, Caligo M, Friedman E, Kaufman B, Laitman Y, Milgrom R, Dubrovsky M, Cohen S, Borg A, Jernstrom H, Lindblom A, Rantala J, Stenmark-Askmalm M, Melin B, Nathanson K, Domchek S, Jakubowska A, Lubinski J, Huzarski T, Osorio A, Lasa A, Duran M, Tejada MI, Godino J, Benitez J, Hamann U, Kriege M, Hoogerbrugge N, van der Luijt RB, van Asperen CJ, Devilee P, Meijers-Heijboer EJ, Blok MJ, Aalfs CM, Hogervorst F, Rookus M, Cook M, Oliver C, Frost D, Conroy D, Evans DG, Lalloo F, Pichert G, Davidson R, Cole T, Cook J, Paterson J, Hodgson S, Morrison PJ, Porteous ME, Walker L, Kennedy MJ, Dorkins H, Peock S, Godwin AK, Stoppa-Lyonnet D, de Pauw A, Mazoyer S, Bonadona V, Lasset C, Dreyfus H, Leroux D, Hardouin A, Berthet P, Faivre L, Loustalot C, Noguchi T, Sobol H, Rouleau E, Nogues C, Frenay M, Venat-Bouvet L, Hopper JL, Daly MB, Terry MB, John EM, Buys SS, Yassin Y, Miron A, Goldgar D, Singer CF, Dressler AC, Gschwantler-Kaulich D, Pfeiler G, Hansen TV, Jonson L, Agnarsson BA, Kirchhoff T, Offit K, Devlin V, Dutra-Clarke A, Piedmonte M, Rodriguez GC, Wakeley K, Boggess JF, Basil J, Schwartz PE, Blank SV, Toland AE, Montagna M, Casella C, Imyanitov E, Tihomirova L, Blanco I, Lazaro C, Ramus SJ, Sucheston L, Karlan BY, Gross J, Schmutzler R, Wappenschmidt B, Engel C, Meindl A, Lochmann M, Arnold N, Heidemann S, Varon-Mateeva R, Niederacher D, Sutter C, Deissler H, Gadzicki D, Preisler-Adams S, Kast K, Schonbuchner I, Caldes T, de la HM, Aittomaki K, Nevanlinna H, Simard J, Spurdle AB, Holland H, Chen X, Platte R, Chenevix-Trench G, Easton DF. Common breast cancer susceptibility alleles and the risk of breast cancer for BRCA1 and BRCA2 mutation carriers: implications for risk prediction. Cancer Res 70: 9742–9754, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285: 2129–2133, 1999. [DOI] [PubMed] [Google Scholar]
- 46. Aranda V, Martinez I, Melero S, Lecanda J, Banales JM, Prieto J, Medina JF. Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun 319: 1040–1046, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Aronson PS, Bounds SE. Harmaline inhibition of Na-dependent transport in renal microvillus membrane vesicles. Am J Physiol Renal Fluid Electrolyte Physiol 238: F210–F217, 1980. [DOI] [PubMed] [Google Scholar]
- 48. Askin D, Bloomberg GB, Chambers EJ, Tanner MJ. NMR solution structure of a cytoplasmic surface loop of the human red cell anion transporter, band 3. Biochemistry 37: 11670–11678, 1998. [DOI] [PubMed] [Google Scholar]
- 49. Astion ML, Chvatal A, Orkand RK. Further studies of electrogenic Na+/HCO3− cotransport in glial cells of Necturus optic nerve: regulation of pHi. Glia 4: 461–468, 1991. [DOI] [PubMed] [Google Scholar]
- 50. Astion ML, Obaid AL, Orkand RK. Effects of barium and bicarbonate on glial cells of Necturus optic nerve. Studies with microelectrodes and voltage-sensitive dyes. J Gen Physiol 93: 731–744, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Astion ML, Orkand RK. Electrogenic Na+/HCO3− cotransport in neuroglia. Glia 1: 355–357, 1988. [DOI] [PubMed] [Google Scholar]
- 52. Azimov R, Abuladze N, Pushkin A, Newman D, Sassani P, Kurtz I. Role of cysteine residues in kNBC1 oligomeric structure and function (Abstract). FASEB J 20: A1232, 2006. [Google Scholar]
- 53. Babenko VN, Rogozin IB, Mekhedov SL, Koonin EV. Prevalence of intron gain over intron loss in the evolution of paralogous gene families. Nucleic Acids Res 32: 3724–3733, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bachmann O, Franke K, Yu H, Riederer B, Li HC, Soleimani M, Manns MP, Seidler U. cAMP-dependent and cholinergic regulation of the electrogenic intestinal/pancreatic Na+/HCO3− cotransporter pNBC1 in human embryonic kidney (HEK293) cells. BMC Cell Biol 9: 70–81, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol 284: G37–G45, 2003. [DOI] [PubMed] [Google Scholar]
- 56. Bachoo RM, Kim RS, Ligon KL, Maher EA, Brennan C, Billings N, Chan S, Li C, Rowitch DH, Wong WH, DePinho RA. Molecular diversity of astrocytes with implications for neurological disorders. Proc Natl Acad Sci USA 101: 8384–8389, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bae WK, Lee J, Park JW, Bae EH, Ma SK, Kim SH, Kim SW. Decreased Expression of Na/K-ATPase, NHE3, NBC1, AQP1 and OAT in Gentamicin-induced Nephropathy. Korean J Physiol Pharmacol 12: 331–336, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baetz D, Haworth RS, Avkiran M, Feuvray D. The ERK pathway regulates Na+-HCO3− cotransport activity in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol 283: H2102–H2109, 2002. [DOI] [PubMed] [Google Scholar]
- 59. Banales JM, Arenas F, Rodríguez-Ortigosa CM, Sáez E, Uriarte I, Doctor RB, Prieto J, Medina JF. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology 43: 266–275, 2006. [DOI] [PubMed] [Google Scholar]
- 60. Banales JM, Prieto J, Medina JF. Cholangiocyte anion exchange and biliary bicarbonate excretion. World J Gastroenterol 12: 3496–3511, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, Turner ST, Weder AB, Boerwinkle E. Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension 43: 477–482, 2004. [DOI] [PubMed] [Google Scholar]
- 62. Baron R, Neff L, Louvard D, Courtoy PJ. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol 101: 2210–2222, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bartel D, Lepke S, Layh-Schmitt G, Legrum B, Passow H. Anion transport in oocytes of Xenopus laevis induced by expression of mouse erythroid band 3 protein-encoding cRNA and of a cRNA derivative obtained by site-directed mutagenesis at the stilbene disulfonate binding site. EMBO J 8: 3601–3609, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bartolo RC, Harfoot N, Gill M, McLeod BJ, Butt AG. Secretagogues stimulate electrogenic HCO3− secretion in the ileum of the brushtail possum, Trichosurus vulpecula: evidence for the role of a Na+/HCO3− cotransporter. J Exp Biol 212: 2645–2655, 2009. [DOI] [PubMed] [Google Scholar]
- 65. Bassnett S, Duncan G. Direct measurement of pH in the rat lens by ion-sensitive microelectrodes. Exp Eye Res 40: 585–590, 1985. [DOI] [PubMed] [Google Scholar]
- 66. Baykov AA, Tuominen HK, Lahti R. The CBS domain: a protein module with an emerging prominent role in regulation. ACS Chem Biol 6: 1156–1163, 2011. [DOI] [PubMed] [Google Scholar]
- 67. Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive “leak” K+ channel expressed in brainstem respiratory neurons. Respir Physiol 129: 159–174, 2001. [DOI] [PubMed] [Google Scholar]
- 68. Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3− cotransporter. J Biol Chem 282: 13508–13521, 2007. [DOI] [PubMed] [Google Scholar]
- 69. Bedogni F, Hodge RD, Nelson BR, Frederick EA, Shiba N, Daza RA, Hevner RF. Autism susceptibility candidate 2 (Auts2) encodes a nuclear protein expressed in developing brain regions implicated in autism neuropathology. Gene Expr Patterns 10: 9–15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276: C788–C795, 1999. [DOI] [PubMed] [Google Scholar]
- 71. Bellemer A, Hirata T, Romero MF, Koelle MR. Two types of chloride transporters are required for GABA(A) receptor-mediated inhibition in C. elegans EMBO J 30: 1852–1863, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bergmans BA, Van Paesschen W, Legius E, Igarashi T. Familiar migraine with aura and partial epilepsy involving posterior brain regions caused by a mutation in SLC4A4, a sodium bicarbonate co-transporter gene. Epilepsia 46: 365–366, 2005. [Google Scholar]
- 73. Bergstrom A, Jayatissa MN, Thykjaer T, Wiborg O. Molecular pathways associated with stress resilience and drug resistance in the chronic mild stress rat model of depression-a gene expression study. J Mol Neurosci 33: 201–215, 2007. [DOI] [PubMed] [Google Scholar]
- 74. Bernardo AA, Kear FT, Stim JA, Ruiz OS, Arruda JA. Renal cortical basolateral Na+/HCO3− cotransporter. IV. Characterization and localization with polyclonal antibodies. J Membr Biol 154: 155–162, 1996. [DOI] [PubMed] [Google Scholar]
- 75. Bevensee MO, Apkon M, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. J Gen Physiol 110: 467–483, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bevensee MO, Boron WF. pH regulation in mammalian neurons. In: pH and Brain Function , edited by Kaila K., Ransom BR. New York: Wiley-Liss, 1998, p. 211–231. [Google Scholar]
- 77. Bevensee MO, Boron WF. Regulation of intracellular pH. In: Seldin and Giebisch's The Kidney: Physiology and Pathophysiology , edited by Alpern RJ, Hebert SC. Burlington, MA: Academic, 2007, p. 1429–1480. [Google Scholar]
- 78. Bevensee MO, Cummins TR, Haddad GG, Boron WF, Boyarsky G. pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J Physiol 494: 315–328, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na/HCO3 cotransporter (NBC) with a novel C terminus, cloned from rat brain. Am J Physiol Cell Physiol 278: C1200–C1211, 2000. [DOI] [PubMed] [Google Scholar]
- 80. Biagi BA. Effects of the anion transport inhibitor, SITS, on the proximal straight tubule of the rabbit perfused in vitro. J Membr Biol 88: 25–31, 1985. [DOI] [PubMed] [Google Scholar]
- 81. Biagi BA, Sohtell M. Electrophysiology of basolateral bicarbonate transport in the rabbit proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 250: F267–F272, 1986. [DOI] [PubMed] [Google Scholar]
- 82. Bidani A, Wang CZ, Saggi SJ, Heming TA. Evidence for pH sensitivity of tumor necrosis factor-alpha release by alveolar macrophages. Lung 176: 111–121, 1998. [DOI] [PubMed] [Google Scholar]
- 83. Bittner CX, Valdebenito R, Ruminot I, Loaiza A, Larenas V, Sotelo-Hitschfeld T, Moldenhauer H, San Martín A, Gutiérrez R, Zambrano M, Barros LF. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci 31: 4709–4713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bleich M, Warth R, Thiele I, Greger R. pH-regulatory mechanisms in in vitro perfused rectal gland tubules of Squalus acanthias. Pflügers Arch 436: 248–254, 1998. [DOI] [PubMed] [Google Scholar]
- 85. Bleul U, Schwantag S, Stocker H, Corboz L, Grimm F, Engels M, Borel N, Lutz H, Schonmann M, Kahn W. Floppy kid syndrome caused by d-lactic acidosis in goat kids. J Vet Intern Med 20: 1003–1008, 2006. [DOI] [PubMed] [Google Scholar]
- 86. Blevins DG, Lukaszewski KM. Boron in plant structure and function. Annu Rev Plant Physiol Plant Mol Biol 49: 481–500, 1998. [DOI] [PubMed] [Google Scholar]
- 87. Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom GG, Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest 113: 1560–1570, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Blot-Chabaud M, Dumont M, Corbic M, Erlinger S. Effect of acid-base balance on biliary bicarbonate secretion in the isolated perfused guinea pig liver. Am J Physiol Gastrointest Liver Physiol 258: G863–G872, 1990. [DOI] [PubMed] [Google Scholar]
- 89. Bobulescu IA, Di Sole F, Moe OW. Na+/H+ exchangers: physiology and link to hypertension and organ ischemia. Curr Opin Nephrol Hypertens 14: 485–494, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Boedtkjer E, Praetorius J, Aalkjær C. NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circ Res 98: 515–523, 2006. [DOI] [PubMed] [Google Scholar]
- 91. Boedtkjer E, Praetorius J, Fuchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+,HCO3− cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol 294: C591–C603, 2008. [DOI] [PubMed] [Google Scholar]
- 92. Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Füchtbauer AC, Simonsen U, Füchtbauer EM, Aalkjaer C. Disruption of Na+,HCO3− cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation 124: 1819–1829, 2011. [DOI] [PubMed] [Google Scholar]
- 93. Bok D, Galbraith G, Lopez I, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang WH, Zhao SL, Geske R, Montgomery C, Van Sligtenhorst I, Friddle C, Platt K, Sparks MJ, Pushkin A, Abuladze N, Ishiyama A, Dukkipati R, Liu WX, Kurtz I. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat Genet 34: 313–319, 2003. [DOI] [PubMed] [Google Scholar]
- 94. Bok D, Schibler MJ, Pushkin A, Sassani P, Abuladze N, Naser Z, Kurtz I. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. Am J Physiol Renal Physiol 281: F920–F935, 2001. [DOI] [PubMed] [Google Scholar]
- 95. Boll M, Daniel H, Gasnier B. The SLC36 family: proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Arch 447: 776–779, 2004. [DOI] [PubMed] [Google Scholar]
- 96. Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res 95: 2–7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bonnici B, Wagner CA. Postnatal expression of transport proteins involved in acid-base transport in mouse kidney. Pflügers Arch 448: 16–28, 2004. [DOI] [PubMed] [Google Scholar]
- 98. Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurones. J Physiol 385: 243–286, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Boron WF. Intracellular-pH-regulating mechanism of the squid axon: relation between the external Na+ and HCO3− dependences. J Gen Physiol 85: 325–345, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Boron WF. Sodium-coupled bicarbonate transporters. JOP 2: 176–181, 2001. [PubMed] [Google Scholar]
- 101. Boron WF. Regulation of intracellular pH. Adv Physiol Educ 28: 160–179, 2004. [DOI] [PubMed] [Google Scholar]
- 102. Boron WF. Evaluating the role of carbonic anhydrases in the transport of HCO3−-related species. Biochim Biophys Acta 1804: 410–421, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO3− transport. J Gen Physiol 81: 53–94, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Boron WF, Chen LM, Parker MD. Modular structure of sodium-coupled bicabonate transporters. J Exp Biol 212: 1697–1706, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Boron WF, De Weer P. Active proton transport stimulated by CO2/HCO3− blocked by cyanide. Nature 259: 240–241, 1976. [DOI] [PubMed] [Google Scholar]
- 106. Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3 and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Boron WF, Hogan E, Russell JM. pH-sensitive activation of the intracellular-pH regulation system in squid axons by ATP-γ-S. Nature 332: 262–265, 1988. [DOI] [PubMed] [Google Scholar]
- 108. Boron WF, Knakal RC. Intracellular pH-regulating mechanism of the squid axon: interaction between DNDS and extracellular Na+ and HCO3−. J Gen Physiol 93: 123–150, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Boron WF, Knakal RC. Na+-dependent Cl-HCO3 exchange in the squid axon. Dependence on extracellular pH. J Gen Physiol 99: 817–837, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Boron WF, McCormick WC, Roos A. pH regulation in barnacle muscle fibers: dependence on extracellular sodium and bicarbonate. Am J Physiol Cell Physiol 240: C80–C89, 1981. [DOI] [PubMed] [Google Scholar]
- 111. Boron WF, Russell JM. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol 81: 373–399, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bouyer P, Sakai H, Itokawa T, Kawano T, Fulton CM, Boron WF, Insogna KL. Colony-stimulating factor-1 increases osteoclast intracellular pH and promotes survival via the electroneutral Na/HCO3 cotransporter NBCn1. Endocrinology 148: 831–840, 2007. [DOI] [PubMed] [Google Scholar]
- 113. Bouzinova EV, Praetorius J, Virkki LV, Nielsen S, Boron WF, Aalkjær C. Na+-dependent HCO3− uptake into the rat choroid plexus epithelium is partially DIDS sensitive. Am J Physiol Cell Physiol 289: C1448–C1456, 2005. [DOI] [PubMed] [Google Scholar]
- 114. Boyarsky G, Ganz MB, Cragoe EJ, Jr, Boron WF. Intracellular pH dependence of Na-H exchange and acid loading in quiescent and arginine vasopressin-activated mesangial cells. Proc Natl Acad Sci USA 87: 5921–5924, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Boyarsky G, Ganz MB, Sterzel B, Boron WF. pH regulation in single glomerular mesangial cells. II. Na-dependent and -independent Cl-HCO3 exchangers. Am J Physiol Cell Physiol 255: C857–C869, 1988. [DOI] [PubMed] [Google Scholar]
- 116. Bozdag GO, Uluisik I, Gulculer GS, Karakaya HC, Koc A. Roles of ATR1 paralogs YMR279c and YOR378w in boron stress tolerance. Biochem Biophys Res Commun 409: 748–751, 2011. [DOI] [PubMed] [Google Scholar]
- 117. Brandes A, Oehlke O, Schumann A, Heidrich S, Thevenod F, Roussa E. Adaptive redistribution of NBCe1-A and NBCe1-B in rat kidney proximal tubule and striated ducts of salivary glands during acid-base disturbances. Am J Physiol Regul Integr Comp Physiol 292: 2007. [DOI] [PubMed] [Google Scholar]
- 118. Brechenmacher C, Rodeau JL. Intracellular pH regulation in ventral horn neurones cultured from embryonic rat spinal cord. Mol Membr Biol 17: 101–108, 2000. [DOI] [PubMed] [Google Scholar]
- 119. Bresolin NL, Grillo E, Fernandes VR, Carvalho FL, Goes JE, da Silva RJ. A case report and review of hypokalemic paralysis secondary to renal tubular acidosis. Pediatr Nephrol 20: 818–820, 2005. [DOI] [PubMed] [Google Scholar]
- 120. Breton S, Hammar K, Smith PJ, Brown D. Proton secretion in the male reproductive tract: involvement of Cl−-independent HCO3− transport. Am J Physiol Cell Physiol 275: C1134–C1142, 1998. [DOI] [PubMed] [Google Scholar]
- 121. Brett CL, Kelly T, Sheldon C, Church J. Regulation of Cl−-HCO3− exchanger by cAMP-dependent protein kinase in adult rat hippocampal CA1 neurons. J Physiol 545: 837–853, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Brokl OH, Martinez CL, Kim YK, Abbott DE, Dantzler WH. Basolateral regulation of pHi in proximal tubules of avian loopless and long-looped nephrons in bicarbonate. J Exp Zool 284: 174–187, 1999. [DOI] [PubMed] [Google Scholar]
- 123. Brokl OH, Martinez CL, Shuprisha A, Abbott DE, Dantzler WH. Regulation of intracellular pH in proximal tubules of avian long-looped mammalian-type nephrons. Am J Physiol Regul Integr Comp Physiol 274: R1526–R1535, 1998. [DOI] [PubMed] [Google Scholar]
- 124. Bronckers A, Guo J, Lyaruu D, Denbesten P, Zandieh-Doulabi B. Immunolocalization and western blotting of the anion exchanger pendrin in ameloblasts. Eur J Oral Sci 120: 369–372, 2012. [Google Scholar]
- 125. Bronckers AL, Guo J, Zandieh-Doulabi B, Bervoets TJ, Lyaruu DM, Li X, Wangemann P, Denbesten P. Developmental expression of solute carrier family 26A member 4 (SLC26A4/pendrin) during amelogenesis in developing rodent teeth. Eur J Oral Sci 119: 185–192, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bronckers AL, Lyaruu DM, Jansen ID, Medina JF, Kellokumpu S, Hoeben KA, Gawenis LR, Oude Elferink RP, Everts V. Localization and function of the anion exchanger Ae2 in developing teeth and orofacial bone in rodents. J Exp Zool B Mol Dev Evol 312B: 375–387, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Brosius FC, III, Alper SL, Garcia AM, Lodish HF. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem 264: 7784–7787, 1989. [PubMed] [Google Scholar]
- 128. Brosius FC, Nguyen K, Stuart-Tilley AK, Haller C, Briggs JP, Alper SL. Regional and segmental localization of AE2 anion exchanger mRNA and protein in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F461–F468, 1995. [DOI] [PubMed] [Google Scholar]
- 129. Brown D, Wagner CA. Molecular mechanisms of acid-base sensing by the kidney. J Am Soc Nephrol 23: 774–780, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ. Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest 100: 1693–1707, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Brunden KR, Richter-Cook NJ, Chaturvedi N, Frederickson RC. pH-dependent binding of synthetic beta-amyloid peptides to glycosaminoglycans. J Neurochem 61: 2147–2154, 1993. [DOI] [PubMed] [Google Scholar]
- 132. Brune T, Fetzer S, Backus KH, Deitmer JW. Evidence for electrogenic sodium-bicarbonate cotransport in cultured rat cerebellar astrocytes. Pflügers Arch 429: 64–71, 1994. [DOI] [PubMed] [Google Scholar]
- 133. Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JL. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron 15: 63–78, 1995. [DOI] [PubMed] [Google Scholar]
- 134. Bullock R, Zauner A, Woodward J, Young HF. Massive persistent release of excitatory amino acids following human occlusive stroke. Stroke 26: 2187–2189, 1995. [DOI] [PubMed] [Google Scholar]
- 135. Bulur N, Virreira M, Soyfoo MS, Louchami K, Delporte C, Perret J, Beauwens R, Malaisse WJ, Sener A. Expression of the electrogenic Na+-HCO3−-cotransporter NBCe1 in tumoral insulin-producing BRIN-BD11 cells. Cell Physiol Biochem 24: 187–192, 2009. [DOI] [PubMed] [Google Scholar]
- 136. Burette AC, Weinberg RJ, Sassani P, Abuladze N, Kao L, Kurtz I. The sodium-driven chloride/bicarbonate exchanger in presynaptic terminals. J Comp Neurol 520: 1481–1492, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Burger JW, Hess WN. Function of the rectal gland in the spiny dogfish. Science 131: 670–671, 1960. [DOI] [PubMed] [Google Scholar]
- 138. Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3− cotransporter. J Biol Chem 272: 19111–19114, 1997. [DOI] [PubMed] [Google Scholar]
- 139. Burnham CE, Conforti L, Petrovic S, Soleimani M. The Na+/HCO3− cotransporter mNBC3 is a variant of NBC2 and is not a distinct isoform (Abstract). J Am Soc Nephrol 11: 2A, 2000.11182927 [Google Scholar]
- 140. Burnham CE, Flagella M, Wang Z, Amlal H, Shull GE, Soleimani M. Cloning, renal distribution, and regulation of the rat Na+-HCO3− cotransporter. Am J Physiol Renal Physiol 274: F1119–F1126, 1998. [DOI] [PubMed] [Google Scholar]
- 141. Burton NM, Bruce LJ. Modelling the structure of the red cell membrane. Biochem Cell Biol 89: 200–215, 2011. [DOI] [PubMed] [Google Scholar]
- 142. Bushell M, O'Brien P. Acid-base imbalance and ulceration in the cold restrained rat. Surgery 91: 318–321, 1982. [PubMed] [Google Scholar]
- 143. Buzdin A, Kovalskaya-Alexandrova E, Gogvadze E, Sverdlov E. At least 50% of human-specific HERV-K (HML-2) long terminal repeats serve in vivo as active promoters for host nonrepetitive DNA transcription. J Virol 80: 10752–10762, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cabantchik ZI, Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol 10: 311–328, 1972. [DOI] [PubMed] [Google Scholar]
- 145. Cabezon E, Butler PJ, Runswick MJ, Walker JE. Modulation of the oligomerization state of the bovine F1-ATPase inhibitor protein, IF1, by pH. J Biol Chem 275: 25460–25464, 2000. [DOI] [PubMed] [Google Scholar]
- 146. Calonge ML, Ilundain AA. HCO3−-dependent ion transport systems and intracellular pH regulation in colonocytes from the chick. Biochim Biophys Acta 1371: 232–240, 1998. [DOI] [PubMed] [Google Scholar]
- 147. Camilion de Hurtado MC, Alvarez BV, Perez NG, Cingolani HE. Role of an electrogenic Na+-HCO3− cotransport in determining myocardial pHi after an increase in heart rate. Circ Res 79: 698–704, 1996. [DOI] [PubMed] [Google Scholar]
- 148. Campa D, Kaaks R, Le Marchand L, Haiman CA, Travis RC, Berg CD, Buring JE, Chanock SJ, Diver WR, Dostal L, Fournier A, Hankinson SE, Henderson BE, Hoover RN, Isaacs C, Johansson M, Kolonel LN, Kraft P, Lee IM, McCarty CA, Overvad K, Panico S, Peeters PH, Riboli E, Sanchez MJ, Schumacher FR, Skeie G, Stram DO, Thun MJ, Trichopoulos D, Zhang S, Ziegler RG, Hunter DJ, Lindstrom S, Canzian F. Interactions between genetic variants and breast cancer risk factors in the breast and prostate cancer cohort consortium. J Natl Cancer Inst 103: 1252–1263, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Canellos HM, Cooper J, Paek A, Chien J. Multiple calcified deposits along the eyelid margins secondary to chronic renal failure and hyperparathyroidism. Optometry 76: 181–184, 2005. [DOI] [PubMed] [Google Scholar]
- 150. Canlon B, Brundin L. Mechanically induced length changes of isolated outer hair cells are metabolically dependent. Hear Res 53: 7–16, 1991. [DOI] [PubMed] [Google Scholar]
- 151. Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 5: 786–795, 2005. [DOI] [PubMed] [Google Scholar]
- 152. Carlin RW, Quesnell RR, Zheng L, Mitchell KE, Schultz BD. Functional and molecular evidence for Na+-HCO3− cotransporter in porcine vas deferens epithelia. Am J Physiol Cell Physiol 283: C1033–C1044, 2002. [DOI] [PubMed] [Google Scholar]
- 153. Casey JR, Cordat E. Bicarbonate transport in cell physiology an disease. Biochem J 417: 423–439, 2009. [DOI] [PubMed] [Google Scholar]
- 154. Casey JR, Ding Y, Kopito RR. The role of cysteine residues in the erythrocyte plasma membrane anion exchange, AE1. J Biol Chem 270: 8521–8527, 1995. [DOI] [PubMed] [Google Scholar]
- 155. Casey JR, Pirraglia CA, Reithmeier RA. Enzymatic deglycosylation of human Band 3, the anion transport protein of the erythrocyte membrane. Effect on protein structure and transport properties. J Biol Chem 267: 11940–11948, 1992. [PubMed] [Google Scholar]
- 156. Casey JR, Reithmeier RA. Analysis of the oligomeric state of Band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. J Biol Chem 266: 15726–15737, 1991. [PubMed] [Google Scholar]
- 157. Cassel D, Scharf O, Rotman M, Cragoe EJ, Jr, Katz M. Characterization of Na+-linked and Na+-independent Cl−/HCO3− exchange systems in Chinese hamster lung fibroblasts. J Biol Chem 263: 6122–6127, 1988. [PubMed] [Google Scholar]
- 158. Castillo JE, Martinez-Anso E, Malumbres R, De Alava E, Garcia C, Medina JF, Prieto J. In situ localization of anion exchanger-2 in the human kidney. Cell Tissue Res 299: 281–287, 2000. [DOI] [PubMed] [Google Scholar]
- 159. Catalan M, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology 126: 1104–1114, 2004. [DOI] [PubMed] [Google Scholar]
- 160. Ch'en FF, Villafuerte FC, Swietach P, Cobden PM, Vaughan-Jones RD. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br J Pharmacol 153: 844–845, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Chai SM, Vithana EN, Venkataraman D, Saleh H, Chekkalichintavida NP, al-Sayyed F, Aung T. Novel human pathological mutations. Gene symbol: SLC4A11. Disease: corneal endothelial dystrophy 2. Hum Genet 127: 110, 2010. [PubMed] [Google Scholar]
- 162. Chakravarti S, Wu F, Vij N, Roberts L, Joyce S. Microarray studies reveal macrophage-like function of stromal keratocytes in the cornea. Invest Ophthalmol Vis Sci 45: 3475–3484, 2004. [DOI] [PubMed] [Google Scholar]
- 163. Chalfant ML, Denton JS, Berdiev BK, Ismailov II, Benos DJ, Stanton BA. Intracellular H+ regulates the alpha-subunit of ENaC, the epithelial Na+ channel. Am J Physiol Cell Physiol 276: C477–C486, 1999. [DOI] [PubMed] [Google Scholar]
- 164. Chan HC, Ko WH, Zhao W, Fu WO, Wong PY. Evidence for independent Cl− and HCO3− secretion and involvement of an apical Na+-HCO3− cotransporter in cultured rat epididymal epithelia. Exp Physiol 81: 515–524, 1996. [DOI] [PubMed] [Google Scholar]
- 165. Chan HC, Shi QX, Zhou CX, Wang XF, Xu WM, Chen WY, Chen AJ, Ni Y, Yuan YY. Critical role of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Mol Cell Endocrinol 250: 106–113, 2006. [DOI] [PubMed] [Google Scholar]
- 166. Chang MH, DiPiero J, Sonnichsen FD, Romero MF. Entry to “HCO3 tunnel” revealed by SLC4A4 human mutation and structural model. J Biol Chem 283: 18402–18410, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Chang MH, Plata C, Kurita Y, Kato A, Hirose S, Romero MF. Euryhaline pufferfish NBCe1 differs from non-marine species NBCe1 physiology. Am J Physiol Cell Physiol 302: C1083–C1095, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Chang MH, Plata C, Zandi-Nejad K, Sindic A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF. Slc26a9–anion exchanger, channel and Na+ transporter. J Membr Biol 228: 125–140, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Chang SH, Low PS. Identification of a critical ankyrin-binding loop on the cytoplasmic domain of erythrocyte membrane band 3 by crystal structure analysis and site-directed mutagenesis. J Biol Chem 278: 6879–6884, 2003. [DOI] [PubMed] [Google Scholar]
- 170. Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 76: 51–74, 2007. [DOI] [PubMed] [Google Scholar]
- 171. Charge SB, de Koning EJ, Clark A. Effect of pH and insulin on fibrillogenesis of islet amyloid polypeptide in vitro. Biochemistry 34: 14588–14593, 1995. [DOI] [PubMed] [Google Scholar]
- 172. Charoenphandhu N, Laohapitakworn S, Kraidith K, Nakkrasae LI, Jongwattanapisan P, Tharabenjasin P, Krishnamra N. Electrogenic Na+/HCO3− co-transporter-1 is essential for the parathyroid hormone-stimulated intestinal HCO3− secretion. Biochem Biophys Res Commun 409: 775–779, 2011. [DOI] [PubMed] [Google Scholar]
- 173. Chateauvieux S, Morceau F, Dicato M, Diederich M. Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol 2010: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Chen LM, Choi I, Haddad GG, Boron WF. Chronic continuous hypoxia decreases the expression of SLC4A7 (NBCn1) and SLC4A10 (NCBE) in mouse brain. Am J Physiol Regul Integr Comp Physiol 293: R2412–R2420, 2007. [DOI] [PubMed] [Google Scholar]
- 175. Chen LM, Haddad GG, Boron WF. Effects of chronic continuous hypoxia on the expression of SLC4A8 (NDCBE) in neonatal vs adult mouse brain. Brain Res 1238: 85–92, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Chen LM, Kelly ML, Parker MD, Bouyer P, Gill HS, Felie JM, Davis BA, Boron WF. Expression and localization of Na-drivenHCO3− exchanger (SLC4A8) in rodent CNS. Neuroscience 153: 162–174, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Chen LM, Kelly ML, Rojas JD, Parker MD, Gill HS, Davis BA, Boron WF. Use of a new polyclonal antibody to study the distribution and glycosylation of the sodium-coupled bicarbonate transporter NCBE in rodent brain. Neuroscience 151: 374–385, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Chen LM, Liu Y, Boron WF. Role of an extracellular loop in determining the stoichiometry of Na+-HCO3− cotransporters. J Physiol 589: 877–890, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Chen LM, Qin X, Moss FJ, Liu Y, Boron WF. Effect of simultaneously replacing putative TM6 and TM12 of human NBCe1-A with those from NBCn1 on surface abundance in Xenopus oocytes. J Membr Biol 245: 131–140, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chen M, Praetorius J, Zheng W, Xiao F, Riederer B, Singh AK, Stieger N, Wang J, Shull GE, Aalkjaer C, Seidler U. The electroneutral Na+:HCO3− cotransporter NBCn1 is a major pHi regulator in murine duodenum. J Physiol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000. [DOI] [PubMed] [Google Scholar]
- 182. Chen Y, Choong LY, Lin Q, Philp R, Wong CH, Ang BK, Tan YL, Loh MC, Hew CL, Shah N, Druker BJ, Chong PK, Lim YP. Differential expression of novel tyrosine kinase substrates during breast cancer development. Mol Cell Proteomics 6: 2072–2087, 2007. [DOI] [PubMed] [Google Scholar]
- 183. Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31: 3497–3500, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Chernova MN, Humphreys BD, Robinson DH, Stuart-Tilley AK, Garcia AM, Brosius FC, Alper SL. Functional consequences of mutations in the transmembrane domain and the carboxy-terminus of the murine AE1 anion exchanger. Biochim Biophys Acta 1329: 111–123, 1997. [DOI] [PubMed] [Google Scholar]
- 185. Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280: 8564–8580, 2005. [DOI] [PubMed] [Google Scholar]
- 186. Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003. [DOI] [PubMed] [Google Scholar]
- 187. Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci 15: 396–402, 1992. [DOI] [PubMed] [Google Scholar]
- 188. Cheung JC, Li J, Reithmeier RA. Topology of transmembrane segments 1–4 in the human chloride/bicarbonate anion exchanger 1 (AE1) by scanning N-glycosylation mutagenesis. Biochem J 390: 137–144, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Choi I, Aalkjær C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405: 571–575, 2000. [DOI] [PubMed] [Google Scholar]
- 190. Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+-HCO3− cotransporter (NBCe1). Am J Physiol Renal Physiol 284: F1199–F1206, 2003. [DOI] [PubMed] [Google Scholar]
- 191. Choi I, Kobayashi C, Jacovich M, Boron WF. Structure/function analysis of an electroneutral Na/HCO3 cotransporter (NBCn1) (Abstract). FASEB J 15: A446, 2001. [Google Scholar]
- 192. Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC). Am J Physiol Cell Physiol 276: C576–C584, 1999. [DOI] [PubMed] [Google Scholar]
- 193. Choi I, Yang HS, Boron WF. The electrogenicity of the rat sodium-bicarbonate cotransporter NBCe1 requires interactions among transmembrane segments of the transporter. J Physiol 578: 131–142, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Church J. A change from HCO3−-CO2- to HEPES-buffered medium modifies membrane properties of rat CA1 pyramidal neurones in vitro. J Physiol 455: 51–71, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Coley AA, Ruffin VA, Hopfer U, Boron WF. Immunocytochemical techniques identify Na+-coupled HCO3− transporters (NCBTs) in chemosensitive neurons of the Medullary Raphé. FASEB J 26: 882.51–7, 2012. [Google Scholar]
- 196. Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 284: 792–798, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, Nishina PM, Naggert JK. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nat Genet 31: 74–78, 2002. [DOI] [PubMed] [Google Scholar]
- 198. Collins C, Rommens JM, Kowbel D, Godfrey T, Tanner M, Hwang SI, Polikoff D, Nonet G, Cochran J, Myambo K, Jay KE, Froula J, Cloutier T, Kuo WL, Yaswen P, Dairkee S, Giovanola J, Hutchinson GB, Isola J, Kallioniemi OP, Palazzolo M, Martin C, Ericsson C, Pinkel D, Albertson D, Li WB, Gray JW. Positional cloning of ZNF217 and NABC1: genes amplified at 20q13.2 and overexpressed in breast carcinoma. Proc Natl Acad Sci USA 95: 8703–8708, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Cooper DS, Cucoranu D, Choi I. Role of the 123 amino acids deleted in the electroneutral sodium bicarbonate cotransporter (NBCn1). Neuroscience Abstr 2004. [Google Scholar]
- 200. Cooper DS, Lee HJ, Yang HS, Kippen J, Yun CC, Choi I. The electroneutral sodium/bicarbonate cotransporter containing an amino terminal 123-amino-acid cassette is expressed predominantly in the heart. J Biomed Sci 13: 593–595, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem 280: 17823–17830, 2005. [DOI] [PubMed] [Google Scholar]
- 202. Cooper DS, Yang HS, He P, Kim E, Rajbhandari I, Yun CC, Choi I. Sodium/bicarbonate cotransporter NBCn1/slc4a7 increases cytotoxicity in magnesium depletion in primary cultures of hippocampal neurons. Eur J Neurosci 29: 437–446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Cordat E, Li J, Reithmeier RA. Carboxyl-terminal truncations of human anion exchanger impair its trafficking to the plasma membrane. Traffic 4: 642–651, 2003. [DOI] [PubMed] [Google Scholar]
- 204. Coryell MW, Wunsch AM, Haenfler JM, Allen JE, McBride JL, Davidson BL, Wemmie JA. Restoring acid-sensing ion channel-1a in the amygdala of knock-out mice rescues fear memory but not unconditioned fear responses. J Neurosci 28: 13738–13741, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry 62: 1140–1148, 2007. [DOI] [PubMed] [Google Scholar]
- 206. Courties C, Vaquer A, Troussellier M, Lautier J, Chretiennot-Diner MJ, Neveux J, Machado C, Claustre H. Smallest eukaryotic organism. Nature 370: 255, 1994. [Google Scholar]
- 207. Cucoranu D, Cooper DS, Shyntum DY, Chen GP, Klein JD, Sands JM, Choi I. Quantitative measurements of the electroneutral Na/bicarbonate cotransporter (NBCn1) expression in chronic metabolic acidosis (Abstract). FASEB J 18: A1018, 2004. [Google Scholar]
- 208. Curci S, Debellis L, Caroppo R, Fromter E. Model of bicarbonate secretion by resting frog stomach fundus mucosa. I. Transepithelial measurements. Pflügers Arch 428: 648–654, 1994. [DOI] [PubMed] [Google Scholar]
- 209. Curci S, Debellis L, Frömter E. Evidence for rheogenic sodium bicarbonate cotransport in the basolateral membrane of oxyntic cells of frog gastric fundus. Pflügers Arch 408: 497–504, 1987. [DOI] [PubMed] [Google Scholar]
- 210. Curthoys NP, Taylor L, Hoffert JD, Knepper MA. Proteomic analysis of the adaptive response of rat renal proximal tubules to metabolic acidosis. Am J Physiol Renal Physiol 292: F140–F147, 2007. [DOI] [PubMed] [Google Scholar]
- 211. D'Andrea L, Lytle C, Matthews JB, Hofman P, Forbush B3, Madara JL. Na:K:2Cl cotransporter (NKCC) of intestinal epithelial cells. Surface expression in response to cAMP. J Biol Chem 271: 28969–28976, 1996. [DOI] [PubMed] [Google Scholar]
- 212. Damkier HH, Aalkjaer C, Praetorius J. Na+-dependent HCO3− import by the slc4a10 gene product involves Cl− export. J Biol Chem 285: 26998–27007, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Damkier HH, Nielsen S, Praetorius J. An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 290: H172–H180, 2006. [DOI] [PubMed] [Google Scholar]
- 214. Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4 derived Na+ dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol 293: R2136–R2146, 2007. [DOI] [PubMed] [Google Scholar]
- 215. Damkier HH, Praetorius J. Decreased abundance of proteins involved in cerebrospinal fluid production in slc4a10 knockout mice. FASEB J 25: 1038.R2136–3, 2011. [Google Scholar]
- 216. Damkier HH, Prasad V, Hubner CA, Praetorius J. Nhe1 is a luminal Na+/H+ exchanger in mouse choroid plexus and is targeted to the basolateral membrane in Ncbe/Nbcn2-null mice. Am J Physiol Cell Physiol 296: C1291–C1300, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Dantzler WH, Serrano OK, Abbott DE, Kim YK, Brokl OH. Basolateral regulation of pHi in isolated snake renal proximal tubules in presence and absence of bicarbonate. Am J Physiol Regul Integr Comp Physiol 276: R1673–R1681, 1999. [DOI] [PubMed] [Google Scholar]
- 218. Dascalu A, Nevo Z, Korenstein R. The control of intracellular pH in cultured avian chondrocytes. J Physiol 461: 583–599, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Davis BA, Hogan EM, Boron WF. Activation of Na-H exchange by intracellular lithium in barnacle muscle fibers. Am J Physiol Cell Physiol 263: C246–C256, 1992. [DOI] [PubMed] [Google Scholar]
- 220. Davis BA, Hogan EM, Cooper GJ, Bashi E, Zhao J, Boron WF. Inhibition of K/HCO3 cotransport in squid axons by quaternary ammonium ions. J Membr Biol 183: 25–32, 2001. [DOI] [PubMed] [Google Scholar]
- 221. Davis BA, Hogan EM, Russell JM, Boron WF. ATP dependence of Na+-driven Cl-HCO3 exchange in squid axons. J Membr Biol 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier-Brygoo H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442: 939–942, 2006. [DOI] [PubMed] [Google Scholar]
- 223. De Giusti VC, Garciarena CD, Aiello EA. Role of reactive oxygen species (ROS) in angiotensin II-induced stimulation of the cardiac Na+/HCO3− cotransport. J Mol Cell Cardiol 47: 716–722, 2009. [DOI] [PubMed] [Google Scholar]
- 224. De Giusti VC, Orlowski A, Aiello EA. Angiotensin II inhibits the electrogenic Na+/HCO3− cotransport of cat cardiac myocytes. J Mol Cell Cardiol 49: 812–818, 2010. [DOI] [PubMed] [Google Scholar]
- 225. De Giusti VC, Orlowski A, Villa-Abrille MC, de Cingolani GE, Casey JR, Alvarez BV, Aiello EA. Antibodies against the cardiac sodium/bicarbonate cotransporter (NBCe1) as a pharmacological tool. Br J Pharmacol 164: 1976–1989, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. De Seigneux S, Malte H, Dimke H, Frøkiaer J, Nielsen S, Frische S. Renal compensation to chronic hypoxic hypercapnia: downregulation of pendrin and adaptation of the proximal tubule. Am J Physiol Renal Physiol 292: F1256–F1266, 2007. [DOI] [PubMed] [Google Scholar]
- 227. De Smet P, Parys JB, Vanlingen S, Bultynck G, Callewaert G, Galione A, De Smedt H, Missiaen L. The relative order of IP3 sensitivity of types 1 and 3 IP3 receptors is pH dependent. Pflügers Arch 438: 154–158, 1999. [DOI] [PubMed] [Google Scholar]
- 228. Debellis L, Iacovelli C, Fromter E, Curci S. Model of bicarbonate secretion by resting frog stomach fundus mucosa. II. Role of the oxyntopeptic cells. Pflügers Arch 428: 655–663, 1994. [DOI] [PubMed] [Google Scholar]
- 229. Decker BL, Wickner WT. Enolase activates homotypic vacuole fusion and protein transport to the vacuole in yeast. J Biol Chem 281: 14523–14528, 2006. [DOI] [PubMed] [Google Scholar]
- 230. DeCoursey TE. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology 25: 27–40, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Deda G, Ekim M, Guven A, Karagol U, Tumer N. Hypopotassemic paralysis: a rare presentation of proximal renal tubular acidosis. J Child Neurol 16: 770–771, 2001. [DOI] [PubMed] [Google Scholar]
- 232. Deigweiher K, Koschnick N, Pörtner HO, Lucassen M. Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol Regul Integr Comp Physiol 295: R1660–R1670, 2008. [DOI] [PubMed] [Google Scholar]
- 233. Deitmer JW. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J Gen Physiol 98: 637–655, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Deitmer JW. Bicarbonate-dependent changes of intracellular sodium and pH in identified leech glial cells. Pflügers Arch 420: 584–589, 1992. [DOI] [PubMed] [Google Scholar]
- 235. Deitmer JW, Schlue WR. The regulation of intracellular pH by identified glial cells and neurones in the central nervous system of the leech. J Physiol 388: 261–283, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Deitmer JW, Schlue WR. An inwardly directed electrogenic sodium-bicarbonate cotransport in leech glial cells. J Physiol 411: 179–194, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Deitmer JW, Schneider HP. Acid/base transport across the leech giant glial cell membrane at low external bicarbonate concentration. J Physiol 512: 459–469, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Deitmer JW, Schneider HP. Enhancement of glutamate uptake transport by CO(2)/bicarbonate in the leech giant glial cell. Glia 30: 392–400, 2000. [PubMed] [Google Scholar]
- 239. Deitmer JW, Szatkowski M. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J Physiol 421: e-dependent, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Delamere NA, Tamiya S. Expression, regulation and function of Na,K-ATPase in the lens. Prog Retin Eye Res 23: 593–615, 2004. [DOI] [PubMed] [Google Scholar]
- 241. Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: a novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1). Mol Vis 12: 324–330, 2006. [PubMed] [Google Scholar]
- 242. Demuth DR, Showe LC, Ballantine M, Palumbo A, Frzser PJ, Cioe L, Rovera G, Curtis PJ. Cloning and structural characterization of human non-erythroid band 3-like protein. EMBO J 5: 1205–1214, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol 159: 1087–1096, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Desir J, Moya G, Reish O, Van Regemorter N, Deconinck H, David KL, Meire FM, Abramowicz MJ. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet 44: 322–326, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70: 939–1031, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Devogelaere B, Beullens M, Sammels E, Derua R, Waelkens E, van Lint J, Parys JB, Missiaen L, Bollen M, De Smedt H. Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1,4,5-trisphosphate receptor. Biochem J 407: 303–311, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Di Sario A, Bendia E, Omenetti A, De Minicis S, Marzioni M, Kleeman HW, Candelaresi C, Saccomanno S, Alpini G, Benedetti A. Selective inhibition of ion transport mechanisms regulating intracellular pH reduces proliferation and induces apoptosis in cholangiocarcinoma cells. Dig Liver Dis 39: 60–69, 2007. [DOI] [PubMed] [Google Scholar]
- 248. Diecke FP, Wen Q, Sanchez JM, Kuang K, Fischbarg J. Immunocytochemical localization of Na+-HCO3− cotransporters and carbonic anhydrase dependence of fluid transport in corneal endothelial cells. Am J Physiol Cell Physiol 286: C1434–C1442, 2004. [DOI] [PubMed] [Google Scholar]
- 249. Diering GH, Mills F, Bamji SX, Numata M. Regulation of dendritic spine growth through activity-dependent recruitment of the brain-enriched Na+/H+ exchanger NHE5. Mol Biol Cell 22: 2246–2257, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. DiGiammarino EL, Lee AS, Cadwell C, Zhang W, Bothner B, Ribeiro RC, Zambetti G, Kriwacki RW. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat Struct Biol 9: 12–16, 2002. [DOI] [PubMed] [Google Scholar]
- 251. Ding Y, Casey JR, Kopito RR. The major kidney AE1 isoform does not bind ankyrin (Ank1) in vitro. An essential role for the 79 NH2-terminal amino acid residues of band 3. J Biol Chem 269: 32201–32208, 1994. [PubMed] [Google Scholar]
- 252. Ding Y, Kobayashi S, Kopito R. Mapping of ankyrin binding determinants on the erythroid anion exchanger, AE1. J Biol Chem 271: 22494–22498, 1996. [DOI] [PubMed] [Google Scholar]
- 253. Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279: 52238–52246, 2004. [DOI] [PubMed] [Google Scholar]
- 254. Ditte P, Dequiedt F, Svastova E, Hulikova A, Ohradanova-Repic A, Zatovicova M, Csaderova L, Kopacek J, Supuran CT, Pastorekova S, Pastorek J. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res 71: 7558–7567, 2011. [DOI] [PubMed] [Google Scholar]
- 255. Domon MM, Matar G, Strzelecka-Kiliszek A, Bandorowicz-Pikula J, Pikula S, Besson F. Interaction of annexin A6 with cholesterol rich membranes is pH-dependent and mediated by the sterol OH. J Colloid Interface Sci 346: 436–441, 2010. [DOI] [PubMed] [Google Scholar]
- 256. Dordas C, Chrispeels MJ, Brown PH. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol 124: 1349–1362, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Dorfman R, Li W, Sun L, Lin F, Wang Y, Sandford A, Pare PD, McKay K, Kayserova H, Piskackova T, Macek M, Czerska K, Sands D, Tiddens H, Margarit S, Repetto G, Sontag MK, Accurso FJ, Blackman S, Cutting GR, Tsui LC, Corey M, Durie P, Zielenski J, Strug LJ. Modifier gene study of meconium ileus in cystic fibrosis: statistical considerations and gene mapping results. Hum Genet 126: 763–778, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl− channel regulated by the WNK kinases. J Physiol 584: 333–345, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology 23: 104–114, 2008. [DOI] [PubMed] [Google Scholar]
- 260. Douglas RM, Schmitt BM, Xia Y, Bevensee MO, Biemesderfer D, Boron WF, Haddad GG. Sodium-hydrogen exchangers and sodium-bicarbonate co-transporters: ontogeny of protein expression in the rat brain. Neuroscience 102: 217–228, 2001. [DOI] [PubMed] [Google Scholar]
- 261. Douglas RM, Xue J, Chen JY, Haddad CG, Alper SL, Haddad GG. Chronic intermittent hypoxia decreases the expression of Na+/H+ exchangers and HCO3− dependent transporters in mouse CNS. J Appl Physiol 95: 292–299, 2003. [DOI] [PubMed] [Google Scholar]
- 262. Drenckhahn D, Schlüter K, Allen DP, Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science 230: 1287–1289, 1985. [DOI] [PubMed] [Google Scholar]
- 263. Dubreuil RR, Das A, Base C, Mazock GH. The Drosophila Anion Exchanger (DAE) lacks a detectable interaction with the spectrin cytoskeleton. J Negat Results Biomed 9: 5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Ducoudret O, Diakov A, Muller-Berger S, Romero MF, Frömter E. The renal Na-HCO3−cotransporter expressed in Xenopus laevis oocytes: inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflügers Arch 442: 709–717, 2001. [DOI] [PubMed] [Google Scholar]
- 265. Duncan G, Dart C, Croghan PC, Gandolfi SA. Evidence for a Na+-Cl−-H+-HCO3− exchange system in the mammalian lens. Exp Eye Res 54: 941–946, 1992. [DOI] [PubMed] [Google Scholar]
- 266. Durand T, Gallis JL, Masson S, Cozzone PJ, Canione P. pH regulation in perfused rat liver: respective role of Na+-H+ exchanger and Na+-HCO3− cotransport. Am J Physiol Gastrointest Liver Physiol 265: G43–G50, 1993. [DOI] [PubMed] [Google Scholar]
- 267. Edelhauser HF, Ubels JL. The cornea and the sclera. In: Adler's Physiology of the Eye: Clinical Application, edited by Kaufman PL, Alm A. St. Louis: Mosby, 2002, p. 47–114. [Google Scholar]
- 268. Edwalds-Gilbert G, Veraldi KL, Milcarek C. Alternative poly(A) site selection in complex transcription units: means to an end? Nucleic Acids Res 25: 2547–2561, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Eladari D, Blanchard A, Leviel F, Paillard M, Stuart-Tilley AK, Alper SL, Podevin RA. Functional and molecular characterization of luminal and basolateral Cl−/HCO3− exchangers of rat thick limbs. Am J Physiol Renal Physiol 275: F334–F342, 1998. [DOI] [PubMed] [Google Scholar]
- 270. Emmons C, Kurtz I. Functional characterization of three intercalated cell subtypes in the rabbit outer cortical collecting duct. J Clin Invest 93: 417–423, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Emmons C, Stokes JB. Cellular actions of cAMP on HCO3−-secreting cells of rabbit CCD: dependence on in vivo acid-base status. Am J Physiol Renal Fluid Electrolyte Physiol 266: F528–F535, 1994. [DOI] [PubMed] [Google Scholar]
- 272. Endo Y, Suzuki M, Yamada H, Horita S, Kunimi M, Yamazaki O, Shirai A, Nakamura M, Iso O, Li Y, Hara M, Tsukamoto K, Moriyama N, Kudo A, Kawakami H, Yamauchi T, Kubota N, Kadowaki T, Kume H, Enomoto Y, Homma Y, Seki G, Fujita T. Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPARgamma-dependent nongenomic signaling. Cell Metab 13: 550–561, 2011. [DOI] [PubMed] [Google Scholar]
- 273. Endo Y, Yamazaki S, Moriyama N, Li Y, Ariizumi T, Kudo A, Kawakami H, Tanaka Y, Horita S, Yamada H, Seki G, Fujita T. Localization of NBC1 variants in rat kidney. Nephron Physiol 104: 87–94, 2006. [DOI] [PubMed] [Google Scholar]
- 274. Engler C, Kelliher C, Spitze AR, Speck CL, Eberhart CG, Jun AS. Unfolded protein response in fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol 149: 194–202, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Erdogan S, Cetinyaka A, Tuli A, Yilmaz ED, Dogan A. Changes in the activity of defense mechanisms against induced acidosis during meiotic maturation in mouse oocytes. Theriogenology 75: 1057–1066, 2011. [DOI] [PubMed] [Google Scholar]
- 276. Espiritu DJ, Bernardo AA, Arruda JA. Role of NH2 and COOH termini in targeting, stability, and activity of sodium bicarbonate cotransporter 1. Am J Physiol Renal Physiol 291: F588–F596, 2006. [DOI] [PubMed] [Google Scholar]
- 277. Espiritu DJ, Bernardo AA, Robey RB, Arruda JA. A central role for Pyk2-Src interaction in coupling diverse stimuli to increased epithelial NBC activity. Am J Physiol Renal Physiol 283: F663–F670, 2002. [DOI] [PubMed] [Google Scholar]
- 278. Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85: 97–177, 2005. [DOI] [PubMed] [Google Scholar]
- 279. Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem 275: 26720–26726, 2000. [DOI] [PubMed] [Google Scholar]
- 280. Faber S, Lang HJ, Hock FJ, Scholkens BA, Mutschler E. Intracellular pH regulation in bovine aortic endothelial cells: evidence of both Na+/H+ exchange and Na+-dependent Cl−/HCO3− exchange. Cell Physiol Biochem 8: 202–211, 1998. [DOI] [PubMed] [Google Scholar]
- 281. Faggio C, Torre A, Lando G, Sabatino G, Trischitta F. Carbonate precipitates and bicarbonate secretion in the intestine of sea bass, Dicentrarchus labrax. J Comp Physiol B 181: 517–525, 2011. [DOI] [PubMed] [Google Scholar]
- 282. Fejes-Tóth G, Chen WR, Rusvai E, Moser T, Náray-Fejes-Tóth A. Differential expression of AE1 in renal HCO3−secreting and -reabsorbing intercalated cells. J Biol Chem 269: 26717–26721, 1994. [PubMed] [Google Scholar]
- 283. Feng L, Campbell EB, Hsiung Y, MacKinnon R. Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science 330: 635–641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281: 237–240, 1998. [DOI] [PubMed] [Google Scholar]
- 285. Fievet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO J 14: 5158–5169, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994. [DOI] [PubMed] [Google Scholar]
- 287. Fitz JG, Lidofsky SD, Scharschmidt BF. Regulation of hepatic Na+-HCO3− cotransport and pH by membrane potential difference. Am J Physiol Gastrointest Liver Physiol 265: G1–G8, 1993. [DOI] [PubMed] [Google Scholar]
- 288. Fitz JG, Lidofsky SD, Weisiger RA, Xie MH, Cochran M, Grotmol T, Scharschmidt BF. HCO3−-coupled Na+ influx is a major determinant of Na+ turnover and Na+/K+ pump activity in rat hepatocytes. J Membr Biol 122: 1–10, 1991. [DOI] [PubMed] [Google Scholar]
- 289. Fitz JG, Lidofsky SD, Xie MH, Cochran M, Scharschmidt BF. Plasma membrane H+-HCO3− transport in rat hepatocytes: a principal role for Na+-coupled HCO3− transport. Am J Physiol Gastrointest Liver Physiol 261: G803–G809, 1991. [DOI] [PubMed] [Google Scholar]
- 290. Fitz JG, Lidofsky SD, Xie MH, Scharschmidt BF. Transmembrane electrical potential difference regulates Na+/HCO3− cotransport and intracellular pH in hepatocytes. Proc Natl Acad Sci USA 89: 4197–4201, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Fitz JG, Persico M, Scharschmidt BF. Electrophysiological evidence for Na+-coupled bicarbonate transport in cultured rat hepatocytes. Am J Physiol Gastrointest Liver Physiol 256: G491–G500, 1989. [DOI] [PubMed] [Google Scholar]
- 292. Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev 69: 765–796, 1989. [DOI] [PubMed] [Google Scholar]
- 293. Francis DG, Rybalchenko V, Struyk A, Cannon SC. Leaky sodium channels from voltage sensor mutations in periodic paralysis, but not paramyotonia. Neurology 76: 1635–1641, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Fraser PE, Nguyen JT, Surewicz WK, Kirschner DA. pH-dependent structural transitions of Alzheimer amyloid peptides. Biophys J 60: 1190–1201, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J Biol Chem 270: 4334–4340, 1995. [DOI] [PubMed] [Google Scholar]
- 296. Friml J, Benfey P, Benkova E, Bennett M, Berleth T, Geldner N, Grebe M, Heisler M, Hejatko J, Jurgens G, Laux T, Lindsey K, Lukowitz W, Luschnig C, Offringa R, Scheres B, Swarup R, Torres-Ruiz R, Weijers D, Zazimalova E. Apical–basal polarity: why plant cells don't stand on their heads. Trends Plant Sci 11: 12–14, 2006. [DOI] [PubMed] [Google Scholar]
- 297. Frische S. Expression of SLC26A4/pendrin in ameloblasts. Eur J Oral Sci 120: 368–369, 2012. [DOI] [PubMed] [Google Scholar]
- 298. Frische S, Zolotarev AS, Kim YH, Praetorius J, Alper S, Nielsen S, Wall SM. AE2 isoforms in rat kidney: immunohistochemical localization and regulation in response to chronic NH4Cl loading. Am J Physiol Renal Physiol 286: F1163–F1170, 2004. [DOI] [PubMed] [Google Scholar]
- 299. Frommer WB, von Wirén N. Plant biology: Ping-pong with boron. Nature 420: 282–283, 2002. [DOI] [PubMed] [Google Scholar]
- 300. Fry AC, Su Y, Yiu V, Cuthbert AW, Trachtman H, Frankl FE. Mutation conferring apical-targeting motif on AE1 exchanger causes autosomal dominant distal RTA. J Am Soc Nephrol 23: 1238–1249, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Fujinaga J, Loiselle FB, Casey JR. Transport activity of chimaeric AE2-AE3 chloride/bicarbonate anion exchange proteins. Biochem J 371: 687–696, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Fujinaga J, Tang XB, Casey JR. Topology of the membrane domain of human erythrocyte anion exchange protein, AE1. J Biol Chem 274: 6626–6633, 1999. [DOI] [PubMed] [Google Scholar]
- 303. Funder J. Alkali metal cation transport through the human erythrocyte membrane by the anion exchange mechanism. Acta Physiol Scand 108: 31–37, 1980. [DOI] [PubMed] [Google Scholar]
- 304. Funder J, Tosteson DC, Wieth JO. Effects of bicarbonate on lithium transport in human red cells. J Gen Physiol 71: 721–746, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Furimsky M, Moon TW, Perry SF. Evidence for the role of a Na+/HCO3− cotransporter in trout hepatocyte pHi regulation. J Exp Biol 203: 2201–2208, 2000. [DOI] [PubMed] [Google Scholar]
- 306. Furukawa F, Watanabe S, Inokuchi M, Kaneko T. Responses of gill mitochondria-rich cells in Mozambique tilapia exposed to acidic environments (pH 4.0) in combination with different salinities. Comp Biochem Physiol A 158: 468–476, 2011. [DOI] [PubMed] [Google Scholar]
- 307. Futakutchi S, Ishiguro H, Naruse S, Ko SB, Fujiki K, Yamamoto A, Nakakuki M, Song Y, Steward MC, Kondo T, Goto H. High glucose inhibits HCO3− and fluid secretion in rat pancreatic ducts. Pflügers Arch 459: 215–226, 2009. [DOI] [PubMed] [Google Scholar]
- 308. Gainza FJ, Minguela JI, Lampreabe I. Aminoglycoside-associated Fanconi's syndrome: an underrecognized entity. Nephron 77: 205–211, 1997. [DOI] [PubMed] [Google Scholar]
- 309. Galeza-Kulik M, Zebracka J, Szpak-Ulczok S, Czarniecka AK, Kukulska A, Gubala E, Stojcev Z, Wiench M. Expression of selected genes involved in transport of ions in papillary thyroid carcinoma. Endokrynol Pol 57: 26–31, 2006. [PubMed] [Google Scholar]
- 310. Gamba G. Alternative splicing and diversity of renal transporters. Am J Physiol Renal Physiol 281: F781–F794, 2001. [DOI] [PubMed] [Google Scholar]
- 311. Garciarena CD, Lim G, Ma Y, Huc L, Swietach P, Vaughan-Jones RD. Spatial localisation of pH-regulatory transporters in the rat ventricular myocyte. Proc Physiol Soc 19: C25, 2010. [Google Scholar]
- 312. Gawenis LR, Bradford EM, Alper SL, Prasad V, Shull GE. AE2 Cl−/HCO3− exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am J Physiol Gastrointest Liver Physiol 299: G493–G503, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem 282: 9042–9052, 2007. [DOI] [PubMed] [Google Scholar]
- 314. Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a targeted disruption of the AE2 Cl−/HCO3− exchanger are achlorhydric. J Biol Chem 279: 30531–30539, 2004. [DOI] [PubMed] [Google Scholar]
- 315. Gende OA, Cingolani HE. Identification of a sodium-bicarbonate symport in human platelets. Biochim Biophys Acta 1278: 119–124, 1996. [DOI] [PubMed] [Google Scholar]
- 316. Giebisch G, Windhager E. Transport of Acids and Bases. In: Medical Physiology. A Cellular and Molecular Approach, edited by Boron WF, Boulpaep EL. Philadelphia, PA: Saunders Elsevier, 2009, p. 851–865. [Google Scholar]
- 317. Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H. Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. Eur J Neurosci 18: 2935–2945, 2003. [DOI] [PubMed] [Google Scholar]
- 318. Giffard RG, Papadopoulos MC, van Hooft JA, Xu L, Giuffrida R, Monyer H. The electrogenic sodium bicarbonate cotransporter: developmental expression in rat brain and possible role in acid vulnerability. J Neurosci 20: 1001–1008, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Gifford JD, Sharkins K, Work J, Luke RG, Galla JH. Total CO2 transport in rat cortical collecting duct in chloride-depletion alkalosis. Am J Physiol Renal Fluid Electrolyte Physiol 258: F848–F853, 1990. [DOI] [PubMed] [Google Scholar]
- 320. Gill HS, Boron WF. Expression and purification of the cytoplasmic N-terminal domain of the Na/HCO3 cotransporter NBCe1-A: structural insights from a generalized approach. Protein Expr Purif 49: 228–234, 2006. [DOI] [PubMed] [Google Scholar]
- 321. Gill HS, Boron WF. Preliminary X-ray diffraction analysis of the cytoplasmic N-terminal domain of the Na/HCO3 cotransporter NBCe1-A. Acta Crystallograph Sect F Struct Biol Cryst Commun 62: 534–537, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Gilmour KM, Euverman RM, Esbaugh AJ, Kenney L, Chew SF, Ip YK, Perry SF. Mechanisms of acid-base regulation in the African lungfish Protopterus annectens. J Exp Biol 210: 1944–1959, 2007. [DOI] [PubMed] [Google Scholar]
- 323. Giminez I, Garay R, Alda JO. Molybdenum uptake through the anion exchanger in human erythrocytes. Pflügers Arch 424: 245–249, 1993. [DOI] [PubMed] [Google Scholar]
- 324. Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. Association of Na+-H+ exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem 276: 46671–46677, 2001. [DOI] [PubMed] [Google Scholar]
- 325. Girardi AC, Knauf F, Demuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245, 2004. [DOI] [PubMed] [Google Scholar]
- 326. Gleeson D, Smith ND, Boyer JL. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. J Clin Invest 84: 312–321, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Gogvadze E, Stukacheva E, Buzdin A, Sverdlov E. Human specific modulation of transcriptional activity provided by endogenous retroviral inserts. J Virol 83: 6098–6105, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Gonzalez-Gronow M, Kaczowka S, Gawdi G, Pizzo SV. Dipeptidyl peptidase IV (DPP IV/CD26) is a cell-surface plasminogen receptor. Front Biosci 13: 1610–1618, 2008. [DOI] [PubMed] [Google Scholar]
- 329. Goodman SR, Kurdia A, Ammann L, Kakhniashvili D, Daescu O. The human red blood cell proteome and interactome. Exp Biol Med 232: 1391–1408, 2007. [DOI] [PubMed] [Google Scholar]
- 330. Gottsch JD, Bowers AL, Margulies EH, Seitzman GD, Kim SW, Saha S, Jun AS, Stark WJ, Liu SH. Serial analysis of gene expression in the corneal endothelium of Fuchs' dystrophy. Invest Ophthalmol Vis Sci 44: 594–599, 2003. [DOI] [PubMed] [Google Scholar]
- 331. Grassl SM, Aronson PS. Na+/HCO3− co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J Biol Chem 261: 8778–8783, 1986. [PubMed] [Google Scholar]
- 332. Grassl SM, Holohan PD, Ross CR. HCO3− transport in basolateral membrane vesicles isolated from rat renal cortex. J Biol Chem 262: 2682–2687, 1987. [PubMed] [Google Scholar]
- 333. Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol 281: G1301–G1308, 2001. [DOI] [PubMed] [Google Scholar]
- 334. Gresz V, Kwon TH, Vorum H, Zelles T, Kurtz I, Steward MC, Aalkjær C, Nielsen S. Immunolocalization of electroneutral Na+-HCO3− cotransporters in human and rat salivary glands. Am J Physiol Gastrointest Liver Physiol 283: G473–G480, 2002. [DOI] [PubMed] [Google Scholar]
- 335. Grichtchenko II, Boron WF. Surface-pH gradient measurements in Xenopus oocytes co-expressing the Na+-driven Cl-HCO3 exchanger (NDCBE1) and CAIV: evidence for CO3= transport (Abstract). FASEB J 16: A797 2002. [Google Scholar]
- 336. Grichtchenko II, Boron WF. Surface-pH measurements in voltage-clamped Xenopus oocytes co-expressing NBCe1 and CAIV: evidence for CO32− transport (Abstract). FASEB Journal 16: A795, 2002. [Google Scholar]
- 337. Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem 276: 8358–8363, 2001. [DOI] [PubMed] [Google Scholar]
- 338. Grichtchenko II, Romero MF, Boron WF. Electrogenic Na/HCO3 cotransporters from rat and salamander kidney have similar HCO3 dependence (Abstract). FASEB J 12: A638 1998. [Google Scholar]
- 339. Grichtchenko II, Romero MF, Boron WF. Extracellular HCO3− dependence of electrogenic Na/HCO3 cotransporters cloned from salamander and rat kidney. J Gen Physiol 115: 533–545, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Groger N, Frolich H, Maier H, Olbrich A, Kostin S, Braun T, Boettger T. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. J Biol Chem 285: 14467–14474, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Groger N, Vitzthum H, Frohlich H, Kruger M, Ehmke H, Braun T, Boettger T. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet 21: 1025–1036, 2011. [DOI] [PubMed] [Google Scholar]
- 342. Gros G, Al-Samir S, Sly WS, Papadopoulos S, Endeward V. Does direct interaction of the anion exchanger AE1 and carbonic anhydrase II facilitate HCO3− transport? Acta Physiol 198 Suppl: S-SUN-3–4, 2010. [Google Scholar]
- 343. Grosell M, Gilmour KM, Perry SF. Intestinal carbonic anhydrase, bicarbonate, and proton carriers play a role in the acclimation of rainbow trout to seawater. Am J Physiol Regul Integr Comp Physiol 293: R2099–R2111, 2007. [DOI] [PubMed] [Google Scholar]
- 344. Gross E, Abuladze N, Pushkin A, Kurtz I, Cotton CU. The stoichiometry of the electrogenic sodium bicarbonate cotransporter pNBC1 in mouse pancreatic duct cells is 2 HCO3−:1 Na+. J Physiol 531: 375–382, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Gross E, Fedotoff O, Pushkin A, Abuladze N, Newman D, Kurtz I. Phosphorylation-induced modulation of pNBC1 function: distinct roles for the amino- and carboxy-termini. J Physiol 549: 673–682, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J Physiol 531: 597–603, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Gross E, Hawkins K, Pushkin A, Sassani P, Dukkipati R, Abuladze N, Hopfer U, Kurtz I. Phosphorylation of Ser982 in the sodium bicarbonate cotransporter kNBC1 shifts the HCO3−:Na+ stoichiometry from 3:1 to 2:1 in murine proximal tubule cells. J Physiol 537: 659–665, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Gross E, Hopfer U. Activity and stoichiometry of Na+:HCO3− cotransport in immortalized renal proximal tubule cells. J Membr Biol 152: 245–252, 1996. [DOI] [PubMed] [Google Scholar]
- 349. Gross E, Kurtz I. Structural determinants and significance of regulation of electrogenic Na+-HCO3− cotransporter stoichiometry. Am J Physiol Renal Physiol 283: F876–F887, 2002. [DOI] [PubMed] [Google Scholar]
- 350. Gross E, Pushkin A, Abuladze N, Fedotoff O, Kurtz I. Regulation of the sodium bicarbonate cotransporter kNBC1 function: role of Asp986, Asp988 and kNBC1-carbonic anhydrase II binding. J Physiol 544: 679–685, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Groves JD, Falson P, le Maire M, Tanner MJ. Functional cell surface expression of the anion transport domain of human red cell band 3 (AE1) in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA 93: 12245–12250, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Groves JD, Tanner MJ. Role of N-glycosylation in the expression of human band 3-mediated anion transport. Mol Membr Biol 11: 31–38, 1994. [DOI] [PubMed] [Google Scholar]
- 353. Groves JD, Tanner MJ. Co-expressed complementary fragments of the human red cell anion exchanger (band 3, AE1) generate stilbene disulfonate-sensitive anion transport. J Biol Chem 270: 9097–9105, 1995. [DOI] [PubMed] [Google Scholar]
- 354. Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol Gastrointest Liver Physiol 266: G1060–G1070, 1994. [DOI] [PubMed] [Google Scholar]
- 355. Gu XQ, Yao H, Haddad GG. Increased neuronal excitability and seizures in the Na+/H+ exchanger null mutant mouse. Am J Physiol Cell Physiol 281: C496–C503, 2001. [DOI] [PubMed] [Google Scholar]
- 356. Guggino WB, London R, Boulpaep EL, Giebisch G. Chloride transport across the basolateral cell membrane of the Necturus proximal tubule. Dependence on bicarbonate and sodium. J Membr Biol 71: 227–240, 1983. [DOI] [PubMed] [Google Scholar]
- 357. Guizouarn H, Christen R, Borgese F. Phylogeny of anion exchangers: could trout AE1 conductive properties be shared by other members of the gene family? Biochim Biophys Acta 1726: 244–250, 2005. [DOI] [PubMed] [Google Scholar]
- 358. Gunaratne HJ, Nomura M, Moy GW, Vacquier VD. A sodium bicarbonate transporter from sea urchin spermatozoa. Gene 375: 37–43, 2006. [DOI] [PubMed] [Google Scholar]
- 359. Gunn RB, Wieth JO, Tosteson DC. Some effects of low pH on chloride exchange in human red blood cells. J Gen Physiol 65: 731–749, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Gurnett CA, Veile R, Zempel J, Blackburn L, Lovett M, Bowcock A. Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Arch Neurol 65: 550–553, 2008. [DOI] [PubMed] [Google Scholar]
- 361. Gustafson MA. Serotonin Signaling in C. elegans (PhD thesis) Cambridge, MA: MIT, 2007. [Google Scholar]
- 362. Gutowska MA, Melzner F, Langenbuch M, Bock C, Claireaux G, Pörtner HO. Acid-base regulatory ability of the cephalopod (Sepia officinalis) in response to environmental hypercapnia. J Comp Physiol B 180: 323–335, 2010. [DOI] [PubMed] [Google Scholar]
- 363. Haid S, Pietschmann T, Pecheur EI. Low pH-dependent hepatitis C virus membrane fusion depends on E2 integrity, target lipid composition, and density of virus particles. J Biol Chem 284: 17657–17667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Han W, Woo JH, Yu JH, Lee MJ, Moon HG, Kang D, Noh DY. Common genetic variants associated with breast cancer in Korean women and differential susceptibility according to intrinsic subtype. Cancer Epidemiol Biomarkers Prev 20: 793–798, 2011. [DOI] [PubMed] [Google Scholar]
- 365. Hanzu FA, Gasa R, Bulur N, Lybaert P, Gomis R, Malaisse WJ, Beauwens R, Sener A. Expression of TMEM16A and SLC4A4 in human pancreatic islets. Cell Physiol Biochem 29: 61–64, 2012. [DOI] [PubMed] [Google Scholar]
- 366. Haqqani AS, Nesic M, Preston E, Baumann E, Kelly J, Stanimirovic D. Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLC-MS/MS. FASEB J 19: 1809–1821, 2005. [DOI] [PubMed] [Google Scholar]
- 367. Harris PJ, Young JA. Dose-dependent stimulation and inhibition of proximal tubule sodium reabsorption by angiotensin II in the rat kidney. Pflügers Arch 367: 295–297, 1977. [DOI] [PubMed] [Google Scholar]
- 368. Hausdorf B. Early evolution of the bilateria. Syst Biol 49: 130–142, 2000. [DOI] [PubMed] [Google Scholar]
- 369. Hayashi S, Nakamura E, Kubo Y, Takahashi N, Yamaguchi A, Matsui H, Hagen SJ, Takeuchi K. Impairment by allyl isothiocyanate of gastric epithelial wound repair through inhibition of ion transporters. J Physiol Pharmacol 59: 691–706, 2008. [PubMed] [Google Scholar]
- 370. He J, Vora M, Haney RM, Filonov GS, Musselman CA, Burd CG, Kutateladze AG, Verkhusha VV, Stahelin RV, Kutateladze TG. Membrane insertion of the FYVE domain is modulated by pH. Proteins 76: 852–860, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem 283: 33544–33553, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. He X, Tse CM, Donowitz M, Alper SL, Gabriel SE, Baum BJ. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflügers Arch 433: 260–268, 1997. [DOI] [PubMed] [Google Scholar]
- 373. Hearn T, Renforth GL, Spalluto C, Hanley NA, Piper K, Brickwood S, White C, Connolly V, Taylor JF, Russell-Eggitt I, Bonneau D, Walker M, Wilson DI. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alstrom syndrome. Nat Genet 31: 79–83, 2002. [DOI] [PubMed] [Google Scholar]
- 374. Hedera P, Blair MA, Andermann E, Andermann F, D'Agostino D, Taylor KA, Chahine L, Pandolfo M, Bradford Y, Haines JL, Abou-Khalil B. Familial mesial temporal lobe epilepsy maps to chromosome 4q132-q213. Neurology 68: 2107–2112, 2007. [DOI] [PubMed] [Google Scholar]
- 375. Hemadevi B, Veitia RA, Srinivasan M, Arunkumar J, Prajna NV, Lesaffre C, Sundaresan P. Identification of mutations in the SLC4A11 gene in patients with recessive congenital hereditary endothelial dystrophy. Arch Ophthalmol 126: 700–708, 2008. [DOI] [PubMed] [Google Scholar]
- 376. Hempelmann A, Taylor KP, Heils A, Lorenz S, Prud'homme JF, Nabbout R, Dulac O, Rudolf G, Zara F, Bianchi A, Robinson R, Gardiner RM, Covanis A, Lindhout D, Stephani U, Elger CE, Weber YG, Lerche H, Nürnberg P, Kron KL, Scheffer IE, Mulley JC, Berkovic SF, Sander T. Exploration of the genetic architecture of idiopathic generalized epilepsies. Epilepsia 47: 1682–1690, 2006. [DOI] [PubMed] [Google Scholar]
- 377. Hentschke M, Hentschke S, Borgmeyer U, Hubner CA, Kurth I. The murine AE4 promoter predominantly drives type B intercalated cell specific transcription. Histochem Cell Biol 132: 405–412, 2009. [DOI] [PubMed] [Google Scholar]
- 378. Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl−/HCO3− exchanger AE3 display a reduced seizure threshold. Mol Cell Biol 26: 182–191, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Hermans MM, Kortekaas P, Jongsma HJ, Rook MB. pH sensitivity of the cardiac gap junction proteins, connexin 45 and 43. Pflügers Arch 431: 138–140, 1995. [DOI] [PubMed] [Google Scholar]
- 380. Hersey SJ, Sachs G. Gastric acid secretion. Physiol Rev 75: 155–189, 1995. [DOI] [PubMed] [Google Scholar]
- 381. Heyer M, Muller-Berger S, Romero MF, Boron WF, Frömter E. Stoichiometry of the rat kidney Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes. Pflügers Arch 438: 322–329, 1999. [DOI] [PubMed] [Google Scholar]
- 382. Hirata T, Kaneko T, Ono T, Nakazato T, Furukawa N, Hasegawa S, Wakabayashi S, Shigekawa M, Chang MH, Romero MF, Hirose S. Mechanism of acid adaptation of a fish living in a pH 3.5 lake. Am J Physiol Regul Integr Comp Physiol 284: R1199–R1212, 2003. [DOI] [PubMed] [Google Scholar]
- 383. Hmani M, Ghorbel A, Boulila-Elgaied A, Ben Zina Z, Kammoun W, Drira M, Chaabouni M, Petit C, Ayadi H. A novel locus for Usher syndrome type II, USH2B, maps to chromosome 3 at p23–24.2. Eur J Hum Genet 7: 363–367, 1999. [DOI] [PubMed] [Google Scholar]
- 384. Hmani-Aifa M, Benzina Z, Zulfigar F, Dhouib H, Shahzadi A, Ghorbel A, Rebaï A, Söderkvist P, Riazuddin S, Kimberling WJ, Ayadi H. Identification of two new mutations in the GPR98 and the PDE6B genes segregating in a Tunisian family. Eur J Hum Genet 17: 474–482, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Hoffert JD, Wang G, Pisitkun T, Shen RF, Knepper MA. An automated platform for analysis of phosphoproteomic datasets: application to kidney collecting duct phosphoproteins. J Proteome Res 6: 3501–3508, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Hogan EM, Cohen MA, Boron WF. K+- and HCO3−-dependent acid-base transport in squid giant axons. I. Base efflux. J Gen Physiol 106: 821–844, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Hogan EM, Cohen MA, Boron WF. K+- and HCO3−-dependent acid-base transport in squid giant axons. II. Base influx. J Gen Physiol 106: 845–862, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la CA, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14: 316–319, 1996. [DOI] [PubMed] [Google Scholar]
- 389. Hoglund P, Hihnala S, Kujala M, Tiitinen A, Dunkel L, Holmberg C. Disruption of the SLC26A3-mediated anion transport is associated with male subfertility. Fertil Steril 85: 232–235, 2006. [DOI] [PubMed] [Google Scholar]
- 390. Hohenester S, Maillette de Buy WL, Jefferson DM, Oude Elferink RP, Beuers U. Biliary bicarbonate secretion constitutes a protective mechanism against bile acid-induced injury in man. Dig Dis 29: 62–65, 2011. [DOI] [PubMed] [Google Scholar]
- 391. Holmes JM, Zhang S, Leske DA, Lanier WL. Carbon dioxide-induced retinopathy in the neonatal rat. Curr Eye Res 17: 608–616, 1998. [PubMed] [Google Scholar]
- 392. Holmes JM, Zhang S, Leske DA, Lanier WL. Metabolic acidosis-induced retinopathy in the neonatal rat. Invest Ophthalmol Vis Sci 40: 804–809, 1999. [PubMed] [Google Scholar]
- 393. Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al Gazali L, Shimadzu M, Seki G, Fujita T. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol 16: 2270–2278, 2005. [DOI] [PubMed] [Google Scholar]
- 394. Horita S, Zheng Y, Hara C, Yamada H, Kunimi M, Taniguchi S, Uwatoko S, Sugaya T, Goto A, Fujita T, Seki G. Biphasic regulation of Na+-HCO3− cotransporter by angiotensin II type 1A receptor. Hypertension 40: 707–712, 2002. [DOI] [PubMed] [Google Scholar]
- 395. Hornemann S, Glockshuber R. A scrapie-like unfolding intermediate of the prion protein domain PrP(121–231) induced by acidic pH. Proc Natl Acad Sci USA 95: 6010–6014, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Hu MY, Tseng YC, Stumpp M, Kiko R, Lucassen M, Melzner F. Elevated seawater PCO2 differentially affects branchial acid-base transporters over the course of development in the cephalopod Sepia officinalis. Am J Physiol Regul Integr Comp Physiol 300: R1100–R1114, 2011. [DOI] [PubMed] [Google Scholar]
- 397. Huang J, Shan J, Kim D, Liao J, Evagelidis A, Alper SL, Hanrahan JW. Basolateral chloride loading by AE2: role in fluid secretion by the human airway epithelial cell line Calu-3. J Physiol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Huber S, Asan E, Jons T, Kerscher C, Puschel B, Drenckhahn D. Expression of rat kidney anion exchanger 1 in type A intercalated cells in metabolic acidosis and alkalosis. Am J Physiol Renal Physiol 277: F841–F849, 1999. [DOI] [PubMed] [Google Scholar]
- 399. Hübner CA, Hentschke M, Jacobs S, Hermans-Borgmeyer I. Expression of the sodium-driven chloride bicarbonate exchanger NCBE during prenatal mouse development. Gene Expression Patterns 5: 219–223, 2004. [DOI] [PubMed] [Google Scholar]
- 400. Hughes BA, Adorante JS, Miller SS, Lin H. Apical electrogenic Na/HCO3 cotransport. A mechanism for HCO3 absorption across the retinal pigment epithelium. J Gen Physiol 94: 125–150, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol Cell Physiol 267: C1295–C1307, 1994. [DOI] [PubMed] [Google Scholar]
- 402. Hunt CD. Boron. In: Encyclopedia of Dietary Supplements, edited by Coates PM, Blackman M, Cragg GM, Levine MA, Moss J, White JD. New York: Dekker, 2005, p. 55–63. [Google Scholar]
- 403. Hunt CD. Dietary boron: evidence for essentiality and homeostatic control in humans and animals. In: Advances in Plant and Animal Boron Nutrition. Berlin: Springer, 2007, p. 251–267. [Google Scholar]
- 404. Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, Hopkins PN. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10-year follow-up blood pressures. Hypertension 47: 532–536, 2006. [DOI] [PubMed] [Google Scholar]
- 405. Hussain T, Lokhandwala MF. Renal dopamine receptor function in hypertension. Hypertension 32: 187–197, 1998. [DOI] [PubMed] [Google Scholar]
- 406. Hwang PP. Ion uptake and acid secretion in zebrafish (Danio rerio). J Exp Biol 212: 1745–1752, 2009. [DOI] [PubMed] [Google Scholar]
- 407. Hwang S, Park K. Membrane transporters involved in pHi regulation in trigeminal ganglion. Soc Neurosci Abstr 401.1745–17, 2004. [Google Scholar]
- 408. Hwang SM, Koo NY, Jin M, Davies AJ, Chun GS, Choi SY, Kim JS, Park K. Intracellular acidification is associated with changes in free cytosolic calcium and inhibition of action potentials in rat trigeminal ganglion. J Biol Chem 286: 1719–1729, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Ibrahim H, Lee YJ, Curthoys NP. Renal response to metabolic acidosis: role of mRNA stabilization. Kidney Int 73: 11–18, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Ibrahim HA, Cornnell HH, Coelho Ribeiro ML, Abrahams D, Cunningham J, Lloyd M, Martinez GV, Gatenby RA, Gillies RJ. Reduction of metastasis using a non-volatile buffer. Clin Exp Metastasis 28: 841–849, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23: 264–266, 1999. [DOI] [PubMed] [Google Scholar]
- 412. Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H. Novel nonsense mutation in the Na+/HCO3− cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol 12: 713–718, 2001. [DOI] [PubMed] [Google Scholar]
- 413. Igarashi T, Inatomi J, Sekine T, Seki G, Yamada H, Horita S, Fujita T. Mutational and functional analysis of the Na+/HCO3− cotransporter gene (SLC4AC) in patients with permanent isolated proximal renal tubular acidosis and ocular abnormalities (Abstract). J Am Soc Nephrol 14: 302A, 2003. [Google Scholar]
- 414. Igarashi T, Sekine T, Inatomi J, Seki G. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol 13: 2171–2177, 2002. [DOI] [PubMed] [Google Scholar]
- 415. Ihara Y, Kihara Y, Hamano F, Yanagida K, Morishita Y, Kunita A, Yamori T, Fukayama M, Aburatani H, Shimizu T, Ishii S. The G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) facilitates tumor development by serving as an extracellular pH sensor. Proc Natl Acad Sci USA 107: 17309–17314, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, Kawahara K, Moriyama N, Kudo A, Kawakami H, Shimadzu M, Endou H, Fujita T, Seki G, Igarashi T. Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflügers Arch 448: 438–444, 2004. [DOI] [PubMed] [Google Scholar]
- 417. Inoue M, Miyoshi D, Sugimoto N. Structural switch of telomere DNA by pH and monovalent cation. Nucleic Acids Symp Ser 243–244, 2005. [DOI] [PubMed] [Google Scholar]
- 418. Inoue T, Wang JH, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD, Akiba Y. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol 302: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Ishibashi K, Rector FC, Jr, Berry CA. Role of Na-dependent Cl/HCO3 exchange in basolateral Cl transport of rabbit proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 264: F251–F258, 1993. [DOI] [PubMed] [Google Scholar]
- 420. Ishibashi K, Sasaki S, Marumo F. Molecular cloning of a new sodium bicarbonate cotransporter cDNA from human retina. Biochem Biophys Res Commun 246: 535–538, 1998. [DOI] [PubMed] [Google Scholar]
- 421. Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J, Lee MG, Soleimani M. Effect of Slc26a6 deletion on apical Cl−/HCO3− exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am J Physiol Gastrointest Liver Physiol 292: G447–G455, 2007. [DOI] [PubMed] [Google Scholar]
- 422. Ishiguro H, Steward MC, Lindsay ARG, Case RM. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. J Physiol 495: 169–178, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Ishiguro H, Walther D, Arinami T, Uhl GR. Variation in a bicarbonate co-transporter gene family member SLC4A7 is associated with propensity to addictions: a study using fine-mapping and three samples. Addiction 102: 1320–1325, 2007. [DOI] [PubMed] [Google Scholar]
- 424. Ishii K, Nunoki K, Yamagishi T, Okada H, Taira N. Differential sensitivity of Kv1.4, Kv12, and their tandem channel to acidic pH: involvement of a histidine residue in high sensitivity to acidic pH. J Pharmacol Exp Ther 296: 405–411, 2001. [PubMed] [Google Scholar]
- 425. Iwama K, Nakajo S, Aiuchi T, Nakaya K. Apoptosis induced by arsenic trioxide in leukemia U937 cells is dependent on activation of p38, inactivation of ERK and the Ca2+-dependent production of superoxide. Int J Cancer 92: 518–526, 2001. [DOI] [PubMed] [Google Scholar]
- 426. Izumi H, Torigoe T, Ishiguchi H, Uramoto H, Yoshida Y, Tanabe M, Ise T, Murakami T, Yoshida T, Nomoto M, Kohno K. Cellular pH regulators: potentially promising molecular targets for cancer chemotherapy. Cancer Treat Rev 29: 541–549, 2003. [DOI] [PubMed] [Google Scholar]
- 427. Jacob P, Christiani S, Rossman H, Lamprecht G, Viellard-Baron D, Muller R, Gregor M, Seidler U. Role of Na+-HCO3− cotransporter NBC1, Na+/H+ exchanger NHE1, and carbonic anhydrase in rabbit duodenal bicarbonate secretion. Gastroenterology 119: 406–419, 2000. [DOI] [PubMed] [Google Scholar]
- 428. Jacobs S. Funktionelle Analyse des Slc4a10-Gens an transgenen Mausmodellen (Mus musculus, Linné 1758). Hamburg, Germany: Univ. of Hamburg, 2007. [Google Scholar]
- 429. Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hubner CA. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci USA 105: 311–316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300: G82–G98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Jakobsen JK, Odgaard E, Wang W, Elkjaer ML, Nielsen S, Leipziger J, Aalkjær C. Functional up-regulation of basolateral Na+-dependent HCO3− transporter NBCn1 in medullary thick ascending limb of K+-depleted rats. Pflügers Arch 448: 571–578, 2004. [DOI] [PubMed] [Google Scholar]
- 432. Jansen ID, Mardones P, Lecanda F, de Vries TJ, Recalde S, Hoeben KA, Schoenmaker T, Ravesloot JH, van Borren MM, van Eijden TM, Bronckers AL, Kellokumpu S, Medina JF, Everts V, Oude Elferink RP. Ae2(a,b)-deficient mice exhibit osteopetrosis of long bones but not of calvaria. FASEB J 23: 3470–3481, 2009. [DOI] [PubMed] [Google Scholar]
- 433. Jarolim P, Murray JL, Rubin HL, Taylor WM, Prchal JT, Ballas SK, Snyder LM, Chrobak L, Melrose WD, Brabec V, Palek J. Characterization of 13 novel band 3 gene defects in hereditary spherocytosis with band 3 deficiency. Blood 88: 4366–4374, 1996. [PubMed] [Google Scholar]
- 434. Jennings ML. Proton fluxes associated with erythrocyte-membrane anion-exchange. J Membr Biol 28: 187–205, 1976. [DOI] [PubMed] [Google Scholar]
- 435. Jennings ML. Reductive methylation of the two 4,4′-diisothiocyanodihydrostilbene- 2,2′-disulfonate-binding lysine residues of band 3, the human erythrocyte anion transport protein. J Biol Chem 257: 7554–7559, 1982. [PubMed] [Google Scholar]
- 436. Jennings ML. Structure and function of the red blood cell anion transport protein. Annu Rev Biophys Biophys Chem 18: 397–430, 1989. [DOI] [PubMed] [Google Scholar]
- 437. Jennings ML. Rapid electrogenic sulfate-chloride exchange mediated by chemically modified band 3 in human erythrocytes. J Gen Physiol 105: 21–47, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Jennings ML. Evidence for a second binding/transport site for chloride in erythrocyte anion transporter AE1 modified at glutamate 681. Biophys J 88: 2681–2691, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Jennings ML, Adame MF. Characterization of oxalate transport by the human erythrocyte band 3 protein. J Gen Physiol 107: 145–159, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Jennings ML, Al Rhaiyel S. Modification of a carboxyl group that appears to cross the permeability barrier inthe red blood cell anion transporter. J Gen Physiol 92: 161–178, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Jennings ML, Cui J. Chloride homeostasis in Saccharomyces cerevisiae: high affinity influx, V-ATPase-dependent sequestration, and identification of a candidate Cl− sensor. J Gen Physiol 131: 379–391, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Jennings ML, Howren TR, Cui J, Winters M, Hannigan R. Transport and regulatory characteristics of the yeast bicarbonate transporter homolog Bor1p. Am J Physiol Cell Physiol 293: C468–C476, 2007. [DOI] [PubMed] [Google Scholar]
- 443. Jennings ML, Smith JS. Anion-proton cotransport through the human red blood cell band 3 protein. J Biol Chem 267: 13964–13971, 1992. [PubMed] [Google Scholar]
- 444. Jensen FB. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol Scand 182: 215–227, 2004. [DOI] [PubMed] [Google Scholar]
- 445. Jensen L, Schmitt BM, Brown D, Berger UV, Hediger MA, Boron WF, Breton S. Localization of sodium bicarbonate co-transporter (NBC) protein and mRNA in rat epididymis. Biol Reprod 60: 573–579, 1999. [DOI] [PubMed] [Google Scholar]
- 446. Jensen L, Stuart-Tilley AK, Peters LL, Lux SE, Alper SL, Breton S. Immunolocalization of AE2 anion exchanger in rat and mouse epididymis. Biol Reprod 61: 973–980, 1999. [DOI] [PubMed] [Google Scholar]
- 447. Jensen RA. Orthologs and paralogs–we need to get it right. Genome Biol 2: 1002, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Jentsch TJ, Schwartz P, Schill BS, Langner B, Lepple AP, Keller SK, Wiederholt M. Kinetic properties of the sodium bicarbonate (carbonate) symport in monkey kidney epithelial cells (BSC-1). J Biol Chem 261: 10673–10679, 1986. [PubMed] [Google Scholar]
- 449. Jiang L, Chernova MN, Alper SL. Secondary regulatory volume increase conferred on Xenopus oocytes by expression of AE2 anion exchanger. Am J Physiol Cell Physiol 272: C191–C202, 1997. [DOI] [PubMed] [Google Scholar]
- 450. Jiao X, Sultana A, Garg P, Ramamurthy B, Vemuganti GK, Gangopadhyay N, Hejtmancik JF, Kannabiran C. Autosomal recessive corneal endothelial dystrophy (CHED2) is associated with mutations in SLC4A11. J Med Genet 44: 64–68, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Johansson M, Ieong KW, Trobro S, Strazewski P, Aqvist J, Pavlov MY, Ehrenberg M. pH-sensitivity of the ribosomal peptidyl transfer reaction dependent on the identity of the A-site aminoacyl-tRNA. Proc Natl Acad Sci USA 108: 79–84, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Jons T, Drenckhahn D. Identification of the binding interface involved in linkage of cytoskeletal protein 4.1 to the erythrocyte anion exchanger. EMBO J 11: 2863–2867, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Joseph A, Hess R, Schaeffer DJ, Ko C, Hudgin-Spivey S, Chambon P, Shur BD. Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod 82: 948–957, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Josephs Z, Satoh J, Chang MH, Mercado A, Zandi-Nejad K, Mount DB, Romero MF. Cloning and expression of Drosophila Slc4 and Slc26 homologs (Abstract). FASEB J 17: A462 2003. [Google Scholar]
- 455. Josephsen K, Praetorius J, Frische S, Gawenis LR, Kwon TH, Agre P, Nielsen S, Fejerskov O. Targeted disruption of the Cl−/HCO3− exchanger Ae2 results in osteopetrosis in mice. Proc Natl Acad Sci USA 106: 1638–1641, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Josephsen K, Takano Y, Frische S, Praetorius J, Nielsen S, Aoba T, Fejerskov O. Ion transporters in secretory and cyclically modulating ameoblasts. A new hypothesis for cellular control of preeruptive enamel maturation. Am J Physiol Cell Physiol 299: C1299–C1307, 2010. [DOI] [PubMed] [Google Scholar]
- 457. Juel C, Lundby C, Sander M, Calbet JA, Hall G. Human skeletal muscle and erythrocyte proteins involved in acid-base homeostasis: adaptations to chronic hypoxia. J Physiol 548: 639–648, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Jung YW, Choi IJ, Kwon TH. Altered expression of sodium transporters in ischemic penumbra after focal cerebral ischemia in rats. Neurosci Res 59: 152–159, 2007. [DOI] [PubMed] [Google Scholar]
- 459. Kahn AM, Cragoe EJ, Jr, Allen JC, Halligan RD, Shelat H. Na-H and Na-dependent Cl-HCO3 exchange control pHi in vascular smooth muscle. Am J Physiol Cell Physiol 259: C134–C143, 1990. [DOI] [PubMed] [Google Scholar]
- 460. Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature 330: 163–165, 1987. [DOI] [PubMed] [Google Scholar]
- 461. Kajikawa M, Fujibe T, Uraguchi S, Miwa K, Fujiwara T. Expression of the Arabidopsis borate efflux transporter gene, AtBOR4, in rice affects the xylem loading of boron and tolerance to excess boron. Biosci Biotechnol Biochem 75: 2421–2423, 2011. [DOI] [PubMed] [Google Scholar]
- 462. Kakizawa K, Nomura H, Yoshida A, Ueda H. Signaling of lysophosphatidic acid-evoked chloride current: calcium release from inositol trisphosphate-sensitive store. Brain Res 61: 232–237, 1998. [DOI] [PubMed] [Google Scholar]
- 463. Kanaan A, Douglas RM, Alper SL, Boron WF, Haddad GG. Effect of chronic elevated carbon dioxide on the expression of acid-base transporters in the neonatal and adult mouse. Am J Physiol Regul Integr Comp Physiol 293: R1294–R1302, 2007. [DOI] [PubMed] [Google Scholar]
- 464. Kanazawa S, Driscoll M, Struhl K. ATR1, a Saccharomyces cerevisiae gene encoding a transmembrane protein required for aminotriazole resistance. Mol Cell Biol 8: 664–673, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Kang D, Karbach D, Passow H. Anion transport function of mouse erythroid band 3 protein (AE1) does not require acylation of cysteine residue 861. Biochim Biophys Acta 1194: 341–344, 1994. [DOI] [PubMed] [Google Scholar]
- 466. Kang TC, An SJ, Park SK, Hwang IK, Bae JC, Won MH. The evidence for GABAB receptor-mediated regulation of acid-base balance: involvement of Na+/H+ exchanger and Na+/HCO3− cotransporter. Brain Res 114: 86–90, 2003. [DOI] [PubMed] [Google Scholar]
- 467. Kang TC, An SJ, Park SK, Hwang IK, Suh JG, Oh YS, Bae JC, Won MH. Alterations in Na+/H+ exchanger and Na+/HCO3− cotransporter immunoreactivities within the gerbil hippocampus following seizure. Brain Res 109: 226–232, 2002. [DOI] [PubMed] [Google Scholar]
- 468. Kanki T, Young MT, Sakaguchi M, Hamasaki N, Tanner MJ. The N-terminal region of the transmembrane domain of human erythrocyte band 3. Residues critical for membrane insertion and transport activity. J Biol Chem 278: 5564–5573, 2003. [DOI] [PubMed] [Google Scholar]
- 469. Kanzaki A, Hayette S, Morle L, Inoue F, Matsuyama R, Inoue T, Yawata A, Wada H, Vallier A, Alloisio N, Yawata Y, Delaunay J. Total absence of protein 4.2 and partial deficiency of band 3 in hereditary spherocytosis. Br J Haematol 99: 522–530, 1997. [DOI] [PubMed] [Google Scholar]
- 470. Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, Weinreb J, Luong SS, Piri N, Kwong JM, Newman D, Kurtz I. Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5). J Biol Chem 286: 32563–32574, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Kao L, Sassani P, Azimov R, Pushkin A, Abuladze N, Peti-Peterdi J, Liu W, Newman D, Kurtz I. Oligomeric structure and minimal functional unit of the electrogenic sodium bicarbonate cotransporter NBCe1-A. J Biol Chem 283: 26782–26794, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Kardia SL, Greene MT, Boerwinkle E, Turner ST, Kullo IJ. Investigating the complex genetic architecture of ankle-brachial index, a measure of peripheral arterial disease, in non-Hispanic whites. BMC Med Genomics 1: 16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21: 84–90, 1999. [DOI] [PubMed] [Google Scholar]
- 474. Karniski LP, Lotscher M, Fucentese M, Hilfiker H, Biber H, Murer H. Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol Renal Physiol 275: F79–F87, 1998. [DOI] [PubMed] [Google Scholar]
- 475. Kaul RK, Kohler H. Interaction of hemoglobin with band 3: a review. Klin Wochenschr 61: 831–837, 1983. [DOI] [PubMed] [Google Scholar]
- 476. Kaya A, Karakaya HC, Fomenko DE, Gladyshev VN, Koc A. Identification of a novel system for boron transport: Atr1 is a main boron exporter in yeast. Mol Cell Biol 29: 3665–3674, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477. Kayser L, Hoyer PE, Perrild H, Wood AM, Robertson WR. Intracellular pH regulation in human thyrocytes: evidence of both Na+/H+ exchange and Na+-dependent Cl−/HCO3− exchange. J Endocrinol 135: 391–401, 1992. [DOI] [PubMed] [Google Scholar]
- 478. Keen JC, Garrett-Mayer E, Pettit C, Mack KM, Manning J, Herman JG, Davidson NE. Epigenetic regulation of protein phosphatase 2A (PP2A), lymphotactin (XCL1) and estrogen receptor alpha (ER) expression in human breast cancer cells. Cancer Biol Ther 3: 1304–1312, 2004. [DOI] [PubMed] [Google Scholar]
- 479. Kersh AE, Hartzler LK, Havlin K, Hubbell BB, Nanagas V, Kalra A, Chua J, Whitesell R, Ritucci NA, Dean JB, Putnam RW. pH regulating transporters in neurons from various chemosensitive brainstem regions in neonatal rats. Am J Physiol Regul Integr Comp Physiol 297: R1409–R1420, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Khandoudi N, Albadine J, Robert P, Bertrand I, Krief S, Bevensee MO, Boron WF, Bril A. The electrogenic sodium bicarbonate cotransporter, hhNBC, plays a crucial role in ischemic heart diseases. Circulation 102: S330, 2000. [Google Scholar]
- 481. Khandoudi N, Albadine J, Robert P, Krief S, Berrebi-Bertrand I, Martin X, Bevensee MO, Boron WF, Bril A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc Res 52: 387–396, 2001. [DOI] [PubMed] [Google Scholar]
- 482. Khorrami Borozadi M, Nowik M, Wagner CA. The distribution of intestinal and acid-base transporters of the Slc4, Slc9 and Slc26 gene families. Acta Physiol 189: P11–L1–05, 2007. [Google Scholar]
- 483. Kiefer H, Mizutani A, Iemura S, Natsume T, Ando H, Kuroda Y, Mikoshiba K. Inositol 1,4,5-triphosphate receptor-binding protein released with inositol 1,4,5-triphosphate (IRBIT) associates with components of the mRNA 3′ processing machinery in a phosphorylation-dependent manner and inhibits polyadenylation. J Biol Chem 284: 10694–10705, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Kim EY, Choi JS, Lee KE, Kim CS, Bae EH, Ma SK, Kim SH, Lee JU, Kim SW. Altered regulation of renal Acid base transporters in response to ammonium chloride loading in rats. Korean J Physiol Pharmacol 16: 91–95, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Kim HR, Park SW, Cho HJ, Chae KA, Sung JM, Kim JS, Landowski CP, Sun D, Abd El-Aty AM, Amidon GL, Shin HC. Comparative gene expression profiles of intestinal transporters in mice, rats and humans. Pharmacol Res 56: 224–236, 2007. [DOI] [PubMed] [Google Scholar]
- 486. Kim HS, Kim DH, Kim JY, Jeoung NH, Lee IK, Bong JG, Jung ED. Microarray analysis of papillary thyroid cancers in korean. Korean J Intern Med 25: 399–407, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol 10: 1–12, 1999. [DOI] [PubMed] [Google Scholar]
- 488. Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem 280: 6463–6470, 2005. [DOI] [PubMed] [Google Scholar]
- 489. Kim Y, Trussell LO. Negative shift in the glycine reversal potential mediated by a Ca2+- and pH-dependent mechanism in interneurons. J Neurosci 29: 11495–11510, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Kim YB, Yang BH, Piao ZG, Oh SB, Kim JS, Park K. Expression of Na+/HCO3− cotransporter and its role in pH regulation in mouse parotid acinar cells. Biochem Biophys Res Commun 304: 593–598, 2003. [DOI] [PubMed] [Google Scholar]
- 491. Kim YH, Kwon TH, Christensen BM, Nielsen J, Wall SM, Madsen KM, Frokiær J, Nielsen S. Altered expression of renal acid-base transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 285: F1244–F1257, 2003. [DOI] [PubMed] [Google Scholar]
- 492. Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin W, Weiner ID, Everett LA, Green ED, Nielsen S, Wall SM. Intercalated cell H+/OH− transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol 289: F1262–F1272, 2005. [DOI] [PubMed] [Google Scholar]
- 493. Kim YK, Brokl OH, Dantzler WH. Regulation of intracellular pH in avian renal proximal tubules. Am J Physiol Regul Integr Comp Physiol 272: R341–R349, 1997. [DOI] [PubMed] [Google Scholar]
- 494. Kim YK, Dantzler WH. Intracellular pH in snake renal proximal tubules. Am J Physiol Regul Integr Comp Physiol 269: R822–R829, 1995. [DOI] [PubMed] [Google Scholar]
- 495. Kimura N, Nakashima K, Ueno M, Kiyama H, Taga T. A novel mammalian T-box-containing gene, Tbr2, expressed in mouse developing brain. Brain Res 115: 183–193, 1999. [DOI] [PubMed] [Google Scholar]
- 496. Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis 23: 7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Knuth ST, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 degrees C and 15 degrees C: implications for muscle fatigue. J Physiol 575: 887–899, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl−/HCO3− exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol 283: C1206–C1218, 2002. [DOI] [PubMed] [Google Scholar]
- 499. Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J 21: 5662–5672, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Ko YP, Lang HJ, Loh SH, Chu KC, Wu ML. Cl−-dependent and Cl−-independent Na+/HCO3− acid extrusion in cultured rat cerebellar astrocytes. Chin J Physiol 42: 237–248, 1999. [PubMed] [Google Scholar]
- 501. Kobayashi M, Rodriguez R, Lara C, Omata T. Involvement of the C-terminal domain of an ATP-binding subunit in the regulation of the ABC-type nitrate/nitrite transporter of the Cyanobacterium synechococcus sp. strain PCC 7942. J Biol Chem 272: 27197–27201, 1997. [DOI] [PubMed] [Google Scholar]
- 502. Kobayashi S, Morgans CW, Casey JR, Kopito RR. AE3 anion exchanger isoforms in the vertebrate retina: Developmental regulation and differential expression in neurons and glia. J Neurosci 14: 6266–6279, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Kohout TA, Rogers TB. Angiotensin II activates the Na+/HCO3− symport through a phosphoinositide-independent mechanism in cardiac cells. J Biol Chem 270: 20432–20438, 1995. [DOI] [PubMed] [Google Scholar]
- 504. Kok C, Kennerson ML, Myer SJ, Nicholson GA. Transcript map of the candidate region for HSNI with cough and gastroesophageal reflux on chromosome 3p and exclusion of candidate genes. Neurogenetics 5: 197–200, 2004. [DOI] [PubMed] [Google Scholar]
- 505. Kok C, Kennerson ML, Spring PJ, Ing AJ, Pollard JD, Nicholson GA. A locus for hereditary sensory neuropathy with cough and gastroesophageal reflux on chromosome 3p22-p24. Am J Hum Genet 73: 632–637, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Kondo Y, Frömter E. Evidence of chloride/bicarbonate exchange mediating bicarbonate efflux from S3 segments of rabbit renal proximal tubule. Pflügers Arch 415: 726–733, 1990. [DOI] [PubMed] [Google Scholar]
- 507. Koo MW, Cho CH, Ogle CW. Does acidosis contribute to stress-induced ulceration in rat stomachs? Pharmacol Biochem Behav 33: 563–566, 1989. [DOI] [PubMed] [Google Scholar]
- 508. Koo NY, Li J, Hwang SM, Choi SY, Lee SJ, Oh SB, Kim JS, Lee JH, Park K. Molecular cloning and functional expression of a sodium bicarbonate cotransporter from guinea-pig parotid glands. Biochem Biophys Res Commun 342: 1114–1122, 2006. [DOI] [PubMed] [Google Scholar]
- 509. Kopito RR, Lee BS, Simmons DM, Lindsey AE, Morgans CW, Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell 59: 927–937, 1989. [DOI] [PubMed] [Google Scholar]
- 510. Kopito RR, Lodish HF. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature 316: 234–238, 1985. [DOI] [PubMed] [Google Scholar]
- 511. Koropatkin NM, Koppenaal DW, Pakrasi HB, Smith TJ. The structure of a cyanobacterial bicarbonate transport protein, CmpA. J Biol Chem 282: 2606–2614, 2007. [DOI] [PubMed] [Google Scholar]
- 512. Kotka M, Lieden A, Pettersson S, Trinchieri V, Masci A, D'Amato M. Solute carriers (SLC) in inflammatory bowel disease: a potential target of probiotics? J Clin Gastroenterol 42: S133–S135, 2008. [DOI] [PubMed] [Google Scholar]
- 513. Kozlowski MR, Spanoyannis A, Manly SP, Fidel SA, Neve RL. The neurotoxic carboxy-terminal fragment of the Alzheimer amyloid precursor binds specifically to a neuronal cell surface molecule: pH dependence of the neurotoxicity and the binding. J Neurosci 12: 1679–1687, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Krapf R, Alpern RJ, Rector FCJ, Berry CA. Basolateral membrane Na/base cotransport is dependent on CO2/HCO3 in the proximal convoluted tubule. J Gen Physiol 90: 833–853, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Kreindler JL, Peters KW, Frizzell RA, Bridges RJ. Identification and membrane localization of electrogenic sodium bicarbonate cotransporters in Calu-3 cells. Biochim Biophys Acta 1762: 704–710, 2006. [DOI] [PubMed] [Google Scholar]
- 516. Krepischi AC, Knijnenburg J, Bertola DR, Kim CA, Pearson PL, Bijlsma E, Szuhai K, Kok F, Vianna-Morgante AM, Rosenberg C. Two distinct regions in 2q24.2-q243 associated with idiopathic epilepsy. Epilepsia 51: 2457–2460, 2010. [DOI] [PubMed] [Google Scholar]
- 517. Krick W, Schnedler N, Burckhardt G, Burckhardt BC. Ability of sat-1 to transport sulfate, bicarbonate, or oxalate under physiological conditions. Am J Physiol Renal Physiol 297: F145–F154, 2009. [DOI] [PubMed] [Google Scholar]
- 518. Kristensen JM, Kristensen M, Juel C. Expression of Na+/HCO3− co-transporter proteins (NBCs) in rat and human skeletal muscle. Acta Physiol Scand 182: 69–76, 2004. [DOI] [PubMed] [Google Scholar]
- 519. Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA 99: 12825–12830, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Kudrycki KE, Shull GE. Primary structure of the rat kidney band 3 anion exchange protein deduced from a cDNA. J Biol Chem 264: 8185–8192, 1989. [PubMed] [Google Scholar]
- 521. Kudrycki KE, Shull GE. Rat kidney band 3 Cl−/HCO3− exchanger mRNA is transcribed from an alternative promoter. Am J Physiol Renal Fluid Electrolyte Physiol 264: F540–F547, 1993. [DOI] [PubMed] [Google Scholar]
- 522. Kumar A, Bhattacharjee S, Prakash DR, Sadanand CS. Genetic analysis of two Indian families affected with congenital hereditary endothelial dystrophy: two novel mutations in SLC4A11. Mol Vis 13: 39–46, 2007. [PMC free article] [PubMed] [Google Scholar]
- 523. Kumar S, Flacke JP, Kostin S, Appukuttan A, Reusch HP, Ladilov Y. SLC4A7 sodium-bicarbonate co-transporter controls mitochondrial apoptosis in ischemic coronary endothelial cells. Cardiovasc Res 89: 392–400, 2010. [DOI] [PubMed] [Google Scholar]
- 524. Kunimi M, Seki G, Hara C, Taniguchi S, Uwatoko S, Goto A, Kimura S, Fujita T. Dopamine inhibits renal Na+:HCO3− cotransporter in rabbits and normotensive rats but not in spontaneously hypertensive rats. Kidney Int 57: 534–543, 2000. [DOI] [PubMed] [Google Scholar]
- 525. Kuo SM, Aronson PS. Oxalate transport via the sulfate/HCO3 exchanger in rabbit renal basolateral membrane vesicles. J Biol Chem 263: 9710–9717, 1988. [PubMed] [Google Scholar]
- 526. Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 295: 2008. [DOI] [PubMed] [Google Scholar]
- 527. Kurschat CE, Shmukler BE, Jiang L, Wilhelm S, Kim EH, Chernova MN, Kinne RK, Stewart AK, Alper SL. Alkaline-shifted pHo sensitivity of AE2c1-mediated anion exchange reveals novel regulatory determinants in the AE2 N-terminal cytoplasmic domain. J Biol Chem 281: 1885–1896, 2006. [DOI] [PubMed] [Google Scholar]
- 528. Kurth I, Hentschke M, Hentschke S, Borgmeyer U, Gal A, Hubner CA. The forkhead transcription factor Foxi1 directly activates the AE4 promoter. Biochem J 393: 277–283, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Kurtz I, Petrasek D, Tatishchev S. Molecular mechanisms of electrogenic sodium bicarbonate cotransport: structural and equilibrium thermodynamic considerations. J Membr Biol 197: 77–90, 2004. [DOI] [PubMed] [Google Scholar]
- 530. Kwon TH, Fulton C, Wang W, Kurtz I, Frokiær J, Aalkjær C, Nielsen S. Chronic metabolic acidosis upregulates rat kidney Na-HCO cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol 282: F341–F351, 2002. [DOI] [PubMed] [Google Scholar]
- 531. L'Allemain G, Paris S, Pouysségur J. Role of a Na+-dependent Cl−/HCO3− exchange in regulation of intracellular pH in fibroblasts. J Biol Chem 260: 4877–4883, 1985. [PubMed] [Google Scholar]
- 532. La Cour M. Rheogenic sodium-bicarbonate co-transport across the retinal membrane of the frog retinal pigment epithelium. J Physiol 419: 539–553, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. La Cour M. Kinetic properties and Na+ dependence of rheogenic Na+-HCO3− co-transport in frog retinal pigment epithelium. J Physiol 439: 59–72, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. La Cour M. Kinetic properties and Na+ dependence of rheogenic Na+-HCO3− co-transport in frog retinal pigment epithelium. J Physiol 439: 59–72, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. La Cour M. pH homeostasis in the frog retina: the role of Na+. Acta Ophthalmol 69: 496–504, 1991. [DOI] [PubMed] [Google Scholar]
- 536. La Cour M. Rheogenic sodium-bicarbonate co-transport across the retinal membrane of the frog retinal pigment epithelium. J Physiol 419: 539–553, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Lacruz RS, Hacia JG, Bromage TG, Boyde A, Lei Y, Xu Y, Miller JD, Paine ML, Snead ML. The circadian clock modulates enamel development. J Biol Rhythms 27: 237–245, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538. Lacruz RS, Nanci A, White SN, Wen X, Wang H, Zalzal SF, Luong VQ, Schuetter VL, Conti PS, Kurtz I, Paine ML. The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem 285: 24432–24438, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Lacruz RS, Smith CE, Bringasjr P, Chen YB, Smith SM, Snead ML, Kurtz I, Hacia JG, Hubbard MJ, Paine ML. Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J Cell Physiol 227: 2264–2275, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Lacruz RS, Smith CE, Moffatt P, Chang EH, Bromage TG, Bringas P, Jr, Nanci A, Baniwal SK, Zabner J, Welsh MJ, Kurtz I, Paine ML. Requirements for ion and solute transport, and pH regulation, during enamel maturation. J Cell Physiol 227: 1776–1785, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Ladoux A, Krawice I, Cragoe EJ, Jr, Abita JP, Frelin C. Properties of the Na-dependent Cl-HCO3 exchange system in U937 human leukemic cells. Eur J Biochem 170: 43–49, 1987. [DOI] [PubMed] [Google Scholar]
- 542. Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240: 1759–1764, 1988. [DOI] [PubMed] [Google Scholar]
- 543. Lane J, Wigham CG, Hodson SA. A chloride-activated Na+/HCO3−-coupled transport activity in corneal endothelial membranes. Biophys J 78: 2493–2498, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol 69: 522–530, 2001. [PubMed] [Google Scholar]
- 545. Larsen AM, Krogsgaard-Larsen N, Lauritzen G, Olesen CW, Honore HS, Boedtkjer E, Pedersen SF, Bunch L. Gram-scale solution-phase synthesis of selective sodium bicarbonate co-transport inhibitor s0859: in vitro efficacy studies in breast cancer cells. Chem Med Chem 2012. [DOI] [PubMed] [Google Scholar]
- 546. Lauritzen G, Jensen MB, Boedtkjer E, Dybboe R, Aalkjaer C, Nylandsted J, Pedersen SF. NBCn1 and NHE1 expression and activity in DeltaNErbB2 receptor-expressing MCF-7 breast cancer cells: contributions to pHi regulation and chemotherapy resistance. Exp Cell Res 316: 2538–2553, 2010. [DOI] [PubMed] [Google Scholar]
- 547. Lauritzen G, Stock CM, Lemaire J, Lund SF, Jensen MF, Damsgaard B, Petersen KS, Wiwel M, Rønnov-Jessen L, Schwab A, Pedersen SF. The Na+/H+ exchanger NHE1, but not the Na+, HCO3− cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett 317: 173–183, 2011. [DOI] [PubMed] [Google Scholar]
- 548. Lavillette D, Bartosch B, Nourrisson D, Verney G, Cosset FL, Penin F, Pecheur EI. Hepatitis C virus glycoproteins mediate low pH-dependent membrane fusion with liposomes. J Biol Chem 281: 3909–3917, 2006. [DOI] [PubMed] [Google Scholar]
- 549. Le Quesne Stabej P, Saihan Z, Rangesh N, Steele-Stallard HB, Ambrose J, Coffey A, Emmerson J, Haralambous E, Hughes Y, Steel KP, Luxon LM, Webster AR, Bitner-Glindzicz M. Comprehensive sequence analysis of nine Usher syndrome genes in the UK National Collaborative Usher Study. J Med Genet 49: 27–36, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550. Lecanda J, Urtasun R, Medina JF. Molecular cloning and genomic organization of the mouse AE2 anion exchanger gene. Biochem Biophys Res Commun 276: 117–124, 2000. [DOI] [PubMed] [Google Scholar]
- 551. Lee BS, Gunn RB, Kopito RR. Functional differences among nonerythroid anion exchangers expressed in a transfected human cell line. J Biol Chem 266: 11448–11454, 1991. [PubMed] [Google Scholar]
- 552. Lee CR, Cho SH, Yoon MJ, Peterkofsky A, Seok YJ. Escherichia coli enzyme IIANtr regulates the K+ transporter TrkA. Proc Natl Acad Sci USA 104: 4124–4129, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553. Lee HJ, Park HJ, Lee S, Kim YH, Choi I. The sodium-driven chloride/bicarbonate exchanger NDCBE in rat brain is upregulated by chronic metabolic acidosis. Brain Res 1377: 13–20, 2011. [DOI] [PubMed] [Google Scholar]
- 554. Lee MA, Storey BT. Bicarbonate is essential for fertilization of mouse eggs: mouse sperm require it to undergo the acrosome reaction. Biol Reprod 34: 349–356, 1986. [DOI] [PubMed] [Google Scholar]
- 555. Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev 92: 39–74, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556. Lee S, Choi I. Sodium-bicarbonate cotransporter NBCn1/Slc4a7 inhibits NH4mediated inward current in Xenopus oocytes. Exp Physiol 96: 745–755, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557. Lee S, Lee HJ, Yang HS, Thornell IM, Bevensee MO, Choi I. Sodium-bicarbonate cotransporter NBCn1 in the kidney medullary thick ascending limb cell line is upregulated under acidic conditions and enhances ammonium transport. Exp Physiol 95: 926–937, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558. Lee SH, Park JH, Jung HH, Lee SH, Oh JW, Lee HM, Jun HS, Cho WJ, Lee JY. Expression and distribution of ion transport mRNAs in human nasal mucosa and nasal polyps. Acta Otolaryngol 125: 745–752, 2005. [DOI] [PubMed] [Google Scholar]
- 559. Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na/HCO3 cotransporter NBCe1-B: role of IRBIT versus amino-terminal truncation. Am J Physiol Cell Physiol 302: C518–C526, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Lee S-K, Grichtchenko II, Boron WF. Distinguishing HCO3− from CO32− transport by NBCe1-A. FASEB J 25: 656.9, 2011. [Google Scholar]
- 561. Lee YC, Yan JJ, Cruz SA, Horng JL, Hwang PP. Anion exchanger 1b, but not sodium-bicarbonate cotransporter 1b, plays a role in transport functions of zebrafish H+-ATPase-rich cells. Am J Physiol Cell Physiol 300: C295–C307, 2011. [DOI] [PubMed] [Google Scholar]
- 562. Lee YS, Ouyang YB, Giffard RG. Regulation of the rat brain Na+-driven Cl−/HCO3− exchanger involves protein kinase A and a multiprotein signaling complex. FEBS Lett 580: 4865–4871, 2006. [DOI] [PubMed] [Google Scholar]
- 563. Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol 517: 159–180, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. LeGeros RZ, Sakae T, Bautista C, Retino M, LeGeros JP. Magnesium and carbonate in enamel and synthetic apatites. Adv Dent Res 10: 225–231, 1996. [DOI] [PubMed] [Google Scholar]
- 565. Lemieux G, Berkofsky J, Quenneville A, Lemieux C. Net tubular secretion of bicarbonate by the alligator kidney. Antimammalian response to acetazolamide. Kidney Int 28: 760–766, 1985. [DOI] [PubMed] [Google Scholar]
- 566. Lemieux G, Craan AG, Quenneville A, Lemieux C, Berkofsky J, Lewis V. Metabolic machinery of the alligator kidney. Am J Physiol Renal Fluid Electrolyte Physiol 247: F686–F693, 1984. [DOI] [PubMed] [Google Scholar]
- 567. Leniger T, Thone J, Bonnet U, Hufnagel A, Bingmann D, Wiemann M. Levetiracetam inhibits Na+-dependent Cl−/HCO3− exchange of adult hippocampal CA3 neurons from guinea-pigs. Br J Pharmacol 142: 1073–1080, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568. Lepke S, Becker A, Passow H. Mediation of inorganic anion transport by the hydrophobic domain of mouse erythroid band 3 protein expressed in oocytes of Xenopus laevis. Biochim Biophys Acta 1106: 13–16, 1992. [DOI] [PubMed] [Google Scholar]
- 569. Lepke S, Fasold H, Pring M, Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4′-diisothiocyano stilbene- 2,2′-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol 29: 147–177, 1976. [DOI] [PubMed] [Google Scholar]
- 570. Leviel F, Borensztein P, Houillier P, Paillard M, Bichara M. Electroneutral K+/HCO3− cotransport in cells of medullary thick ascending limb of rat kidney. J Clin Invest 90: 869–878, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571. Leviel F, Hubner CA, Houllier P, Morla L, El Moghrabi S, Brideau G, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572. Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol 213: 557–570, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573. Li HC, Collier JH, Shawki A, Rudra JS, Li EY, Mackenzie B, Soleimani M. Sequence- or position-specific mutations in the carboxyl-terminal FL motif of the kidney sodium bicarbonate cotransporter (NBC1) disrupt its basolateral targeting and alpha-helical structure. J Membr Biol 228: 111–124, 2009. [DOI] [PubMed] [Google Scholar]
- 574. Li HC, Kucher V, Li EY, Conforti L, Zahedi KA, Soleimani M. The role of aspartic acid residues 405 and 416 of the kidney isotype of sodium-bicarbonate co-transporter 1 in its targeting to the plasma membrane. Am J Physiol Cell Physiol 302: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. Li HC, Kutcher V, Soleimani M. The role of two aspartic acid residues immediately prior to the first transmembrane domain on nbc1 membrane targeting and function. J Am Soc Nephrol 19: 592, 2008. [Google Scholar]
- 576. Li HC, Peng Y, Conforti L, Soleimani M. Trafficking and functional properties of missense mutations T485S and R881C in Na+:HCO3− cotransporter NBC1 in polarized epithelial cells (Abstract). J Am Soc Nephrol 17: 773A, 2007. [Google Scholar]
- 577. Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L, Soleimani M. Missense mutations in Na+:HCO3− cotransporter NBC1 show abnormal trafficking in polarized kidney cells: a basis of proximal renal tubular acidosis. Am J Physiol Renal Physiol 289: F61–F71, 2005. [DOI] [PubMed] [Google Scholar]
- 578. Li HC, Worrell RT, Matthews JB, Husseinzadeh H, Neumeier L, Petrovic S, Conforti L, Soleimani M. Identification of a carboxyl-terminal motif essential for the targeting of Na+-HCO3− cotransporter NBC1 to the basolateral membrane. J Biol Chem 279: 43190–43197, 2004. [DOI] [PubMed] [Google Scholar]
- 579. Li J, Sun XC, Bonanno JA. Role of NBC1 in apical and basolateral HCO3− permeabilities and transendothelial HCO3− fluxes in bovine corneal endothelium. Am J Physiol Cell Physiol 288: C739–C746, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580. Li JP, Kajiya H, Okamoto F, Nakao A, Iwamoto T, Okabe K. Three Na+/Ca2+ exchanger (NCX) variants are expressed in mouse osteoclasts and mediate calcium transport during bone resorption. Endocrinology 148: 2116–2125, 2007. [DOI] [PubMed] [Google Scholar]
- 581. Li L, Wen L, Gong Y, Mei G, Liu J, Chen Y, Peng T. Xenopus as a model system for the study of GOLPH2/GP73 function: Xenopus GOLPH2 is required for pronephros development. PLoS ONE 7: e38939, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582. Li S, Sato S, Yang X, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114: 1782–1789, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583. Liao SF, Monegue JS, Lindemann MD, Cromwell GL, Matthews JC. Dietary supplementation of boron differentially alters expression of borate transporter (NaBCl) mRNA by jejunum and kidney of growing pigs. Biol Trace Elem Res 143: 901–912, 2011. [DOI] [PubMed] [Google Scholar]
- 584. Liao VH, Liu JT, Li WH, Yu CW, Hsieh YC. Caenorhabditis elegans bicarbonate transporter ABTS-1 is involved in arsenite toxicity and cholinergic signaling. Chem Res Toxicol 23: 926–932, 2010. [DOI] [PubMed] [Google Scholar]
- 585. Lin H, Miller SS. pHi regulation in frog retinal pigment epithelium: two apical membrane mechanisms. Am J Physiol Cell Physiol 261: C132–C142, 1991. [DOI] [PubMed] [Google Scholar]
- 586. Lindgren CA, Emery DG, Haydon PG. Intracellular acidification reversibly reduces endocytosis at the neuromuscular junction. J Neurosci 17: 3074–3084, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587. Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci USA 87: 5278–5282, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Linn SC, Kudrycki KE, Shull GE. The predicted translation product of a cardiac AE3 mRNA contains an N terminus distinct from that of the brain AE3 Cl−/HCO3− exchanger. Cloning of a cardiac AE3 cDNA, organization of the AE3 gene, and identification of an alternative transcription initiation site. J Biol Chem 267: 7927–7935, 1992. [PubMed] [Google Scholar]
- 589. Linser PJ, Neira OM, Hirata T, Seron TJ, Smith KE, Piermarini PM, Romero MF. Slc4-like anion transporters of the larval mosquito alimentary canal. J Insect Physiol 58: 551–562, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590. Linser PJ, Smith KE, Seron TJ, Neira OM. Carbonic anhydrases and anion transport in mosquito midgut pH regulation. J Exp Biol 212: 1662–1671, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591. Lipovich L, Lynch ED, Lee MK, King MC. A novel sodium bicarbonate cotransporter-like gene in an ancient duplicated region: SLC4A9 at 5q31. Genome Biol 2: 11.1–11.13, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592. Little PJ, Neylon CB, Farrelly CA, Weissberg PL, Cragoe EJ, Jr, Bobik A. Intracellular pH in vascular smooth muscle: regulation by sodium-hydrogen exchange and multiple sodium dependent HCO3− mechanisms. Cardiovasc Res 29: 239–246, 1995. [PubMed] [Google Scholar]
- 593. Liu C, Cheng Q, Nguyen T, Bonanno JA. Knockdown of NBCe1 in vivo compromises the corneal endothelial pump. Invest Ophthalmol Vis Sci 51: 5190–5197, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594. Liu S, Piwnica-Worms D, Lieberman M. Intracellular pH regulation in cultured embryonic chick heart cells. Na+-dependent Cl−/HCO3− exchange. J Gen Physiol 96: 1247–1269, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595. Liu W, Liu Y, Qin XJ, Schmidt S, Hauser MA, Allingham RR. AQP1 and SLC4A10 as candidate genes for primary open-angle glaucoma. Mol Vis 16: 93–97, 2010. [PMC free article] [PubMed] [Google Scholar]
- 596. Liu X, Williams JB, Sumpter BR, Bevensee MO. Inhibition of the Na/bicarbonate cotransporter NBCe1-A by diBAC oxonol dyes relative to niflumic acid and a stilbene. J Membr Biol 215: 195–204, 2007. [DOI] [PubMed] [Google Scholar]
- 597. Liu Y, Wang DK, Chen LM. The physiology of bicarbonate transporters in mammalian reproduction. Biol Reprod 86: 99, 2012. [DOI] [PubMed] [Google Scholar]
- 598. Liu Y, Xu JY, Wang DK, Boron WF, Chen LM. Expression and distribution of NBCn2 (Slc4a10) splice variants in mouse brain: cloning of novel variant NBCn2-D. Brain Res 1390: 33–40, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599. Liu Y, Xu JY, Wang DK, Wang L, Chen LM. Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: a comparative study of NCBT genes. Genomics 98: 112–119, 2011. [DOI] [PubMed] [Google Scholar]
- 600. Liu Y, Xu K, Chen LM, Sun X, Parker MD, Kelly ML, LaManna JC, Boron WF. Distribution of NBCn2 (SLC4A10) splice variants in mouse brain. Neuroscience 169: 951–964, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601. Lively GD, Jiang B, Hedberg-Buenz A, Chang B, Petersen GE, Wang K, Kuehn MH, Anderson MG. Genetic dependence of central corneal thickness among inbred strains of mice. Invest Ophthalmol Vis Sci 51: 160–171, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602. Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS, Lin SW, Lin SH. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney Int 79: 730–741, 2011. [DOI] [PubMed] [Google Scholar]
- 603. Loiselle FB, Jaschke P, Casey JR. Structural and functional characterization of the human NBC3 sodium/bicarbonate co-transporter carboxyl-terminal cytoplasmic domain. Mol Membr Biol 20: 307–317, 2003. [DOI] [PubMed] [Google Scholar]
- 604. Loiselle FB, Morgan PE, Alvarez BV, Casey JR. Regulation of the human NBC3 Na+/HCO3− cotransporter by carbonic anhydrase II and PKA. Am J Physiol Cell Physiol 286: C1423–C1433, 2004. [DOI] [PubMed] [Google Scholar]
- 605. Long J, Shu XO, Cai Q, Gao YT, Zheng Y, Li G, Li C, Gu K, Wen W, Xiang YB, Lu W, Zheng W. Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol Biomarkers Prev 19: 2357–2365, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606. Lopes AG, Siebens AW, Giebisch G, Boron WF. Electrogenic Na/HCO3 cotransport across the basolateral membrane of the isolated perfused Necturus proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 253: F340–F350, 1987. [DOI] [PubMed] [Google Scholar]
- 607. Lopez IA, Acuna D, Galbraith G, Bok D, Ishiyama A, Liu W, Kurtz I. Time course of auditory impairment in mice lacking the electroneutral sodium bicarbonate cotransporter NBC3 (slc4a7). Brain Res 160: 63–77, 2005. [DOI] [PubMed] [Google Scholar]
- 608. Lopez IA, Rosenblatt MI, Kim C, Galbraith GC, Jones SM, Kao L, Newman D, Liu W, Yeh S, Pushkin A, Abuladze N, Kurtz I. Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J Biol Chem 284: 26882–26892, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609. Loriol C, Dulong S, Avella M, Gabillat N, Boulukos K, Borgese F, Ehrenfeld J. Characterization of SLC26A9, facilitation of Cl− transport by bicarbonate. Cell Physiol Biochem 22: 15–30, 2008. [DOI] [PubMed] [Google Scholar]
- 610. Lou M, Garay R, Alda JO. Cadmium uptake through the anion exchanger in human red blood cells. J Physiol 443: 123–136, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611. Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3 cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol 292: C1787–C1798, 2007. [DOI] [PubMed] [Google Scholar]
- 612. Lu J, Boron WF. The reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3 cotransporter (hNBCe1-A): importance of K558, K559 and K562 within the KKMIK motif of transmembrane segment 5. Am J Physiol Cell Physiol 292: C1787–C1798, 2007. [DOI] [PubMed] [Google Scholar]
- 613. Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem 281: 19241–19250, 2006. [DOI] [PubMed] [Google Scholar]
- 614. Luo J, Chen H, Kintner DB, Shull GE, Sun D. Decreased neuronal death in Na+/H+ exchanger isoform 1-null mice after in vitro and in vivo ischemia. J Neurosci 25: 11256–11268, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615. Luo X, Choi JY, Ko SB, Pushkin A, Kurtz I, Ahn W, Lee MG, Muallem S. HCO3− salvage mechanisms in the submandibular gland acinar and duct cells. J Biol Chem 276: 9808–9816, 2001. [DOI] [PubMed] [Google Scholar]
- 616. Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, Oude Elferink RP, Everts V. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol 27: 119–127, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617. Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, Oude Elferink RP, Everts V. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol 27: 119–127, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618. Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA 101: 9861–9866, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619. Lynn KS, Hsu WL, Li LL, Lin YJ, Wang CH, Sheng SH, Lin JH, Liao W, Pan WH. A neural network model for constructing endophenotypes of common complex diseases: an application to male young-onset hypertension microarray data. Bioinformatics 25: 981–988, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620. Ma SK, Kang JS, Bae EH, Choi C, Lee J, Kim SH, Choi KC, Kim SW. Effects of volume depletion and NaHCO3 loading on the expression of Na+/H+ exchanger isoform 3, Na+: HCO3− cotransporter type 1 and nitric oxide synthase in rat kidney. Clin Exp Pharmacol Physiol 35: 262–267, 2008. [DOI] [PubMed] [Google Scholar]
- 621. Mackinder L, Wheeler G, Schroeder D, von Dassow P, Riebesell U, Brownlee C. Expression of biomineralization-related ion transport genes in Emiliania huxleyi. Environ Microbiol 13: 3250–3265, 2011. [DOI] [PubMed] [Google Scholar]
- 622. Maddy AH. A fluorescent label for the outer components of the plasma membrane. Biochim Biophys Acta 88: 390–399, 1964. [DOI] [PubMed] [Google Scholar]
- 623. Majumdar D, Bevensee MO. Na-coupled bicarbonate transporters (NCBTs) in the nervous system: function, localization, and relevance to neurologic function. Neuroscience 171: 951–972, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624. Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, Harkins LE, Boron WF, Roth KA, Bevensee MO. Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience 155: 818–832, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625. Mandel EI, Curhan GC, Hu FB, Taylor EN. Plasma bicarbonate and risk of type 2 diabetes mellitus. CMAJ 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626. Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na+-HCO3− cotransporter in human pancreas. Am J Physiol Gastrointest Liver Physiol 277: G487–G494, 1999. [DOI] [PubMed] [Google Scholar]
- 627. Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic 2: 867–872, 2001. [DOI] [PubMed] [Google Scholar]
- 628. Marsey LL, Winpenny JP. Bestrophin expression and function in the human pancreatic duct cell line, CFPAC-1. J Physiol 587: 2211–2224, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629. Martinez CL, Brokl OH, Shuprisha A, Abbott DE, Dantzler WH. Regulation of intracellular pH in proximal tubules of avian loopless reptilian-type nephrons. Am J Physiol Regul Integr Comp Physiol 273: R1845–R1854, 1997. [DOI] [PubMed] [Google Scholar]
- 630. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177: 7303–7311, 2006. [DOI] [PubMed] [Google Scholar]
- 631. Martinez-Anso E, Castillo JE, Diez J, Medina JF, Preito J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology 1994: 1400–1406, 1994. [PubMed] [Google Scholar]
- 632. Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjær C. Immunoelectron microscopic localization of the electrogenic Na/HCO3 cotransporter in rat and Ambystoma kidney. J Am Soc Nephrol 11: 2179–2189, 2000. [DOI] [PubMed] [Google Scholar]
- 633. McAlear SD, Bevensee MO. A cysteine-scanning mutagenesis study of transmembrane domain 8 of the electrogenic sodium/bicarbonate cotransporter NBCe1. J Biol Chem 281: 32417–32427, 2006. [DOI] [PubMed] [Google Scholar]
- 634. McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol 127: 639–658, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635. McKee JA, Brewer RP, Macy GE, Borel CO, Reynolds JD, Warner DS. Magnesium neuroprotection is limited in humans with acute brain injury. Neurocrit Care 2: 342–351, 2005. [DOI] [PubMed] [Google Scholar]
- 636. McKinney TD, Burg MB. Bicarbonate transport by rabbit cortical collecting tubules. Effect of acid and alkali loads in vivo on transport in vitro. J Clin Invest 60: 766–768, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637. Medina JF, Lecanda J, Acin A, Ciesielczyk P, Prieto J. Tissue-specific N-terminal isoforms from overlapping alternate promoters of the human AE2 anion exchanger gene. Biochem Biophys Res Commun 267: 228–235, 2000. [DOI] [PubMed] [Google Scholar]
- 638. Medina JF, Recalde S, Prieto J, Lecanda J, Saez E, Funk CD, Vecino P, van Roon MA, Ottenhoff R, Bosma PJ, Bakker CT, Elferink RP. Anion exchanger 2 is essential for spermiogenesis in mice. Proc Natl Acad Sci USA 100: 15847–15852, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639. Mégraud F. A humble bacterium sweeps this year's Nobel Prize. Cell 123: 975–976, 2005. [DOI] [PubMed] [Google Scholar]
- 640. Mehta S, Henadevi B, Vithana EN, Arunkumar J, Srinivasan M, Prajna V, Tan DT, Aung T, Sundaresan P. Absence of phenotype-genotype correlation of patients expressing mutations in the SLC4A11 gene. Cornea 29: 302–306. 2010. [DOI] [PubMed] [Google Scholar]
- 641. Meier SD, Kovalchuk Y, Rose CR. Properties of the new fluorescent Na+ indicator CoroNa Green: comparison with SBFI and confocal Na+ imaging. J Neurosci Methods 155: 251–259, 2006. [DOI] [PubMed] [Google Scholar]
- 642. Mencia N, Selga E, Noé V, Ciudad CJ. Underexpression of miR-224 in methotrexate resistant human colon cancer cells. Biochem Pharmacol 82: 1572–1582, 2011. [DOI] [PubMed] [Google Scholar]
- 643. Meyers SN, McDaneld TG, Swist SL, Marron BM, Steffen DJ, O'Toole D, O'Connell JR, Beever JE, Sonstegard TS, Smith TP. A deletion mutation in bovine SLC4A2 is associated with osteopetrosis in Red Angus cattle. BMC Genomics 11: 337, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644. Michelsen K, Yuan H, Schwappach B. Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep 6: 717–722, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645. Millar ID, Brown PD. NBCe2 exhibits a 3 HCO3−:1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun 373: 550–554, 2008. [DOI] [PubMed] [Google Scholar]
- 646. Miller JP, Lo RS, Ben Hur A, Desmarais C, Stagljar I, Noble WS, Fields S. Large-scale identification of yeast integral membrane protein interactions. Proc Natl Acad Sci USA 102: 12123–12128, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647. Milne MD. Influence of acid-base balance on efficacy and toxicity of drugs. Proc R Soc Med 58: 961–963, 1965. [PMC free article] [PubMed] [Google Scholar]
- 648. Milne RL, Gaudet MM, Spurdle AB, Fasching PA, Couch FJ, Benitez J, Arias Perez JI, Zamora MP, Malats N, Dos SS, I, Gibson LJ, Fletcher O, Johnson N, Anton-Culver H, Ziogas A, Figueroa J, Brinton L, Sherman ME, Lissowska J, Hopper JL, Dite GS, Apicella C, Southey MC, Sigurdson AJ, Linet MS, Schonfeld SJ, Freedman DM, Mannermaa A, Kosma VM, Kataja V, Auvinen P, Andrulis IL, Glendon G, Knight JA, Weerasooriya N, Cox A, Reed MW, Cross SS, Dunning AM, Ahmed S, Shah M, Brauch H, Ko YD, Bruning T, Lambrechts D, Reumers J, Smeets A, Wang-Gohrke S, Hall P, Czene K, Liu J, Irwanto AK, Chenevix-Trench G, Holland H, Giles GG, Baglietto L, Severi G, Bojensen SE, Nordestgaard BG, Flyger H, John EM, West DW, Whittemore AS, Vachon C, Olson JE, Fredericksen Z, Kosel M, Hein R, Vrieling A, Flesch-Janys D, Heinz J, Beckmann MW, Heusinger K, Ekici AB, Haeberle L, Humphreys MK, Morrison J, Easton DF, Pharoah PD, Garcia-Closas M, Goode EL, Chang-Claude J. Assessing interactions between the associations of common genetic susceptibility variants, reproductive history and body mass index with breast cancer risk in the breast cancer association consortium: a combined case-control study. Breast Cancer Res 12: R110, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649. Min KD, Asakura M, Liao Y, Nakamaru K, Okazaki H, Takahashi T, Fujimoto K, Ito S, Takahashi A, Asanuma H, Yamazaki S, Minamino T, Sanada S, Seguchi O, Nakano A, Ando Y, Otsuka T, Furukawa H, Isomura T, Takashima S, Mochizuki N, Kitakaze M. Identification of genes related to heart failure using global gene expression profiling of human failing myocardium. Biochem Biophys Res Commun 393: 55–60, 2010. [DOI] [PubMed] [Google Scholar]
- 650. Miwa K, Takano J, Fujiwara T. Improvement of seed yields under boron-limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. Plant J 46: 1084–1091, 2006. [DOI] [PubMed] [Google Scholar]
- 651. Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. Plants tolerant of high boron levels. Science 318: 1417, 2007. [DOI] [PubMed] [Google Scholar]
- 652. Miwa K, Takano J, Seki M, Shinozaki K, Fujiwara T. Arabidopsis BOR2, an efflux-type boron transporter, is essential for root elongation under boron deficiency (Abstract). Plant Cell Physiol 46: S235, 2005. [Google Scholar]
- 653. Miwa K, Takano J, Seki M, Shinozaki K, Fujiwara T. Roles of BOR3–5 in boron transport in Arabidopsis thaliana (Abstract). Plant Cell Physiol 47: S154 2006. [Google Scholar]
- 654. Moffett DF, Moffett SB, Romero MF. Immunolocalization of Na+-dependent anion exchanger (NDAE1) in anterior stomach of mosquito larvae (Aedes aegypti): relevance to gut alkalinization (Abstract). Am Zoologist 40: 1134 2000. [Google Scholar]
- 655. Mohebbi N, Mihailova M, Wagner CA. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am J Physiol Renal Physiol 297: F499–F509, 2009. [DOI] [PubMed] [Google Scholar]
- 656. Moniz LS, Stambolic V. Nek10 mediates G2/M cell cycle arrest and MEK autoactivation in response to UV irradiation. Mol Cell Biol 31: 30–42, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657. Montgomery H, Pierce JA. The site of acidification of the urine within the renal tubule in amphibia. Am J Physiol 118: 144–152, 1936. [Google Scholar]
- 658. Moody WJ., Jr The ionic mechanism of intracellular pH regulation in crayfish neurones. J Physiol 316: 293–308, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659. Moresco JJ, Koelle MR. Activation of EGL-47, a Galpha(o)-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior. J Neurosci 24: 8522–8530, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660. Moret C, Dave MH, Schulz N, Jiang JX, Verrey F, Wagner CA. Regulation of renal amino acid transporters during metabolic acidosis. Am J Physiol Renal Physiol 292: F555–F566, 2007. [DOI] [PubMed] [Google Scholar]
- 661. Moss T, Cary PD, Abercrombie BD, Crane-Robinson C, Bradbury EM. A pH-dependent interaction between histones H2A and H2B involving secondary and tertiary folding. Eur J Biochem 71: 337–350, 1976. [DOI] [PubMed] [Google Scholar]
- 662. Mostov K, Werb Z. Journey across the osteoclast. Science 276: 219–220, 1997. [DOI] [PubMed] [Google Scholar]
- 663. Motais R, Fievet B, Borgese F, Garcia-Romeu F. Association of the band 3 protein with a volume-activated, anion and amino acid channel: a molecular approach. J Exp Biol 200: 361–367, 1997. [DOI] [PubMed] [Google Scholar]
- 664. Mrowiec A, Jensen BL, Praetorius J, Buus CL, Aalkjaer C. The NBCn1 protein expression is increased through different mechanisms in two models of enhanced renal distal tubular NH4+ delivery (Abstract). FASEB J 19: A141, 2005. [Google Scholar]
- 665. Muchekehu RW, Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol 588: 2329–2342, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666. Müller-Berger S, Coppola S, Samarzija I, Seki G, Frömter E. Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule. I. Change of amiloride-inhibitable K+ conductance. Pflügers Arch 434: 373–382, 1997. [DOI] [PubMed] [Google Scholar]
- 667. Muller-Berger S, Ducoudret O, Diakov A, Frömter E. The renal Na-HCO3−cotransporter expressed in Xenopus laevis oocytes: change in stoichiometry in response to elevation of cytosolic Ca2+ concentration. Pflügers Arch 442: 718–728, 2001. [DOI] [PubMed] [Google Scholar]
- 668. Muller-Berger S, Nesterov VV, Frömter E. Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule II change of Na-HCO3 cotransport stoichiometry and of response to acetazolamide. Pflügers Arch 434: 383–391, 1997. [DOI] [PubMed] [Google Scholar]
- 669. Mulley JC, Scheffer IE, Petrou S, Dibbens LM, Berkovic SF, Harkin LA. SCN1A mutations and epilepsy. Hum Mutat 25: 535–542, 2005. [DOI] [PubMed] [Google Scholar]
- 670. Mulligan AM, Couch FJ, Barrowdale D, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Robson M, Sherman M, Spurdle AB, Wappenschmidt B, Lee A, McGuffog L, Healey S, Sinilnikova OM, Janavicius R, Hansen TV, Nielsen FC, Ejlertsen B, Osorio A, Munoz-Repeto I, Duran M, Godino J, Pertesi M, Benitez J, Peterlongo P, Manoukian S, Peissel B, Zaffaroni D, Cattaneo E, Bonanni B, Viel A, Pasini B, Papi L, Ottini L, Savarese A, Bernard L, Radice P, Hamann U, Verheus M, Meijers-Heijboer HE, Wijnen J, Gomez Garcia EB, Nelen MR, Kets CM, Seynaeve C, Tilanus-Linthorst MM, van der Luijt RB, van Os T, Rookus M, Frost D, Jones JL, Evans DG, Lalloo F, Eeles R, Izatt L, Adlard J, Davidson R, Cook J, Donaldson A, Dorkins H, Gregory H, Eason J, Houghton C, Barwell J, Side LE, McCann E, Murray A, Peock S, Godwin A, Schmutzler RK, Rhiem K, Engel C, Meindl A, Ruehl I, Arnold N, Niederacher D, Sutter C, Deissler H, Gadzicki D, Kast K, Preisler-Adams S, Varon-Mateeva R, Schoenbuchner I, Fiebig B, Heinritz W, Schafer D, Gevensleben H, Caux-Moncoutier V, Fassy-Colcombet M, Cornelis F, Mazoyer S, Leone M, Boutry-Kryza N, Hardouin A, Berthet P, Muller D, Fricker JP, Mortemousque I, Pujol P, Coupier I, Lebrun M, Kientz C, Longy M, Sevenet N, Stoppa-Lyonnet D, Isaacs C, Caldes T, de Al HM, Heikkinen T, Aittomaki K, Blanco I, Lazaro C, Barkardottir RB, Soucy P, Dumont M, Simard J, Montagna M, Tognazzo S, D'Andrea E, Fox S, Yan M, Rebbeck TR, Olopade OI, Weitzel JN, Lynch HT, Ganz PA, Tomlinson GE, Wang X, Fredericksen Z, Pankratz VS, Lindor NM, Szabo C, Offit K, Sakr R, Gaudet M, Bhatia J, Kauff N, Singer CF, Tea MK, Gschwantler-Kaulich D, Fink-Retter A, Mai PL, Greene MH, Imyanitov E, O'Malley FP, Ozcelik H, Glendon G, Toland AE, Gerdes AM, Thomassen M, Kruse TA, Birk JU, Skytte AB, Caligo MA, Soller M, Henriksson K, von Wachenfeldt A, Arver B, Stenmark-Askmalm M, Karlsson P, Ding YC, Neuhausen SL, Beattie M, Pharoah PD, Moysich KB, Nathanson KL, Karlan BY, Gross J, John EM, Daly MB, Buys SM, Southey MC, Hopper JL, Terry MB, Chung W, Miron AF, Goldgar D, Chenevix-Trench G, Easton DF, Andrulis IL, Antoniou AC, Family Registry BC, Embrace Collaborators GS, Hebon Network OC, Swe B, Cimba Common breast cancer susceptibility alleles are associated with tumor subtypes in BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res 13: R110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671. Munsch T, Deitmer JW. Sodium-bicarbonate cotransport current in identified leech glial cells. J Physiol 474: 43–53, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672. Nabika T, Nara Y, Ikeda K, Endo J, Yamori Y. Genetic heterogeneity of the spontaneously hypertensive rat. Hypertension 18: 12–16, 1991. [DOI] [PubMed] [Google Scholar]
- 673. Nagase T, Ishikawa K, Suyama M, Kikuno R, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. XI. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res 5: 277–286, 1998. [DOI] [PubMed] [Google Scholar]
- 674. Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T. Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 19: 2624–2635, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675. Nakagawa-Yokoi Y, Kobayashi M, Aizawa K, Fujiwara T. Tissue specificity of OsBOR3 expression and its role for B transport (Abstract). Plant Cell Physiol 47: S155 2006. [Google Scholar]
- 676. Nakamura TY, Iwata Y, Arai Y, Komamura K, Wakabayashi S. Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ Res 103: 891–899, 2008. [DOI] [PubMed] [Google Scholar]
- 677. Nakhoul NL, Chen LK, Boron WF. Intracellular pH regulation in rabbit S3 proximal tubule: basolateral Cl-HCO3 exchange and Na-HCO3 cotransport. Am J Physiol Renal Fluid Electrolyte Physiol 258: F371–F381, 1990. [DOI] [PubMed] [Google Scholar]
- 678. Nelson N, Harvey WR. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev 79: 361–385, 1999. [DOI] [PubMed] [Google Scholar]
- 679. Newbury DF, Warburton PC, Wilson N, Bacchelli E, Carone S, International Molecular Genetic Study of Autism Consortium, Lamb JA, Maestrini E, Volpi EV, Mohammed S, Baird G, Monaco AP. Mapping of partially overlapping de novo deletions across an autism susceptibility region (AUTS5) in two unrelated individuals affected by developmental delays with communication impairment. Am J Med Genet A 149A: 588–597, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680. Newman EA. Sodium-bicarbonate cotransport in retinal Muller (glial) cells of the salamander. J Neurosci 11: 3972–3983, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681. Newman EA. Acid efflux from retinal glial cells generated by sodium bicarbonate cotransport. J Neurosci 16: 159–168, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682. Newman EA, Astion ML. Localization and stoichiometry of electrogenic sodium bicarbonate cotransport in retinal glial cells. Glia 4: 424–428, 1991. [DOI] [PubMed] [Google Scholar]
- 683. Nielsen FH. Ultratrace elements in nutrition. Annu Rev Nutr 4: 21–41, 1984. [DOI] [PubMed] [Google Scholar]
- 684. Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamiles. Drug Metab Pharmacokinet 20: 452–477, 2005. [DOI] [PubMed] [Google Scholar]
- 685. Noguchi K, Ishii T, Matsunaga T, Kakegawa K, Hayashi H, Fujiwara T. Biochemical properties of the cell wall in the Arabidopsis mutant bor1–1 in relation to boron nutrition. J Plant Nutr Soil Sci 166: 175–178, 2003. [Google Scholar]
- 686. Noguchi K, Yasumori M, Imai T, Naito S, Matsunaga T, Oda H, Hayashi H, Chino M, Fujiwara T. bor1–1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol 115: 901–906, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687. Nordenman B, Bjork I. Influence of ionic strength and pH on the interaction between high-affinity heparin and antithrombin. Biochim Biophys Acta 672: 227–238, 1981. [DOI] [PubMed] [Google Scholar]
- 688. Nowik M, Lecca MR, Velic A, Rehrauer H, Brandli AW, Wagner CA. Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol Genomics 32: 322–334, 2008. [DOI] [PubMed] [Google Scholar]
- 689. Nozawa A, Takano J, Kobayashi M, von Wiren N, Fujiwara T. Roles of BOR1, DUR3, and FPS1 in boron transport and tolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett 262: 216–222, 2006. [DOI] [PubMed] [Google Scholar]
- 690. Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 48: 571–577, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691. Nunez M, Mayo KH, Starbuck C, Lauffenburger D. pH sensitivity of epidermal growth factor receptor complexes. J Cell Biochem 51: 312–321, 1993. [DOI] [PubMed] [Google Scholar]
- 692. O'Neill MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 55: 109–139, 2004. [DOI] [PubMed] [Google Scholar]
- 693. O'Shaughnessy PJ, Abel M, Charlton HM, Hu B, Johnston H, Baker PJ. Altered expression of genes involved in regulation of vitamin A metabolism, solute transportation, and cytoskeletal function in the androgen-insensitive tfm mouse testis. Endocrinology 148: 2914–2924, 2007. [DOI] [PubMed] [Google Scholar]
- 694. Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjær C, Leipziger J. Basolateral Na+-dependent HCO3− transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J Physiol 555: 205–218, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695. Ogata T. Bicarbonate secretion by rat bile duct brush cells indicated by immunohistochemical localization of CFTR, anion exchanger AE2, Na+/HCO3−-cotransporter, carbonic anhydrase II, Na+/H+ exchangers NHE1 and NHE3, H+/K+-ATPase, and Na+/K+-ATPase. Med Mol Morphol 39: 44–48, 2006. [DOI] [PubMed] [Google Scholar]
- 696. Okamoto K, Hanazaki K, Akimori T, Okabayahi T, Okada T, Kobayashi M, Ogata T. Immunohistochemical and electron microscopic characterization of brush cells of the rat cecum. Med Mol Morphol 41: 145–150, 2008. [DOI] [PubMed] [Google Scholar]
- 697. Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem 260: 9699–9705, 1985. [PubMed] [Google Scholar]
- 698. Okubo K, Hamasaki N, Hara K, Kageura M. Palmitoylation of cysteine 69 from the COOH-terminal of band 3 protein in the human erythrocyte membrane. Acylation occurs in the middle of the consensus sequence of F–I-IICLAVL found in band 3 protein and G2 protein of Rift Valley fever virus. J Biol Chem 266: 16420–16424, 1991. [PubMed] [Google Scholar]
- 699. Okubo K, Kang D, Hamasaki N, Jennings ML. Red blood cell band 3. Lysine 539 and lysine 851 react with the same H2DIDS (4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonic acid) molecule. J Biol Chem 269: 1918–1926, 1994. [PubMed] [Google Scholar]
- 700. Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127: 635–648, 2006. [DOI] [PubMed] [Google Scholar]
- 701. Omata T, Price GD, Badger MR, Okamura M, Gohta S, Ogawa T. Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc Natl Acad Sci USA 96: 13571–13576, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702. Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol Cell Physiol 258: C967–C981, 1990. [DOI] [PubMed] [Google Scholar]
- 703. Ota K, Sakaguchi M, Hamasaki N, Mihara K. Assessment of topogenic functions of anticipated transmembrane segments of human band 3. J Biol Chem 273: 28286–28291, 1998. [DOI] [PubMed] [Google Scholar]
- 704. Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12: 357–358, 1996. [DOI] [PubMed] [Google Scholar]
- 705. Pahk AJ, Williams K. Influence of extracellular pH on inhibition by ifenprodil at N-methyl-d-aspartate receptors in Xenopus oocytes. Neurosci Lett 225: 29–32, 1997. [DOI] [PubMed] [Google Scholar]
- 706. Paine ML, Snead ML, Wang HJ, Abuladze N, Pushkin A, Liu W, Kao LY, Wall SM, Kim YH, Kurtz I. Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res 87: 391–395, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707. Palacios-Prado N, Briggs SW, Skeberdis VA, Pranevicius M, Bennett MV, Bukauskas FF. pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions. Proc Natl Acad Sci USA 107: 9897–9902, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708. Paplanus SH, Zbar MJ, Hays JW. Cardiac hypertrophy as a manifestation of chronic anemia. Am J Pathol 34: 149–159, 1958. [PMC free article] [PubMed] [Google Scholar]
- 709. Park HJ, Rajbhandari I, Yang HS, Lee S, Cucoranu D, Cooper DS, Klein JD, Sands JM, Choi I. Neuronal expression of sodium/bicarbonate cotransporter NBCn1 (SLC4A7) and its response to chronic metabolic acidosis. Am J Physiol Cell Physiol 298: C1018–C1028, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710. Park K, Hurley PT, Roussa E, Cooper GJ, Smith CP, Thevenod F, Steward MC, Case RM. Expression of a sodium bicarbonate cotransporter in human parotid salivary glands. Arch Oral Biol 47: 1–9, 2002. [DOI] [PubMed] [Google Scholar]
- 711. Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, Kurtz I. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporter human Na+-HCO3− cotransport isoform 3. J Biol Chem 277: 50503–50509, 2002. [DOI] [PubMed] [Google Scholar]
- 712. Park M, Li Q, Shcheynikov N, Zeng WZ, Muallem S. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell 16: 331–341, 2004. [DOI] [PubMed] [Google Scholar]
- 713. Parker MD, Boron WF. Expression and characterization of a prokaryotic SLC4-like anion transporter from Nitrococcus mobilis (Abstract). J Am Soc Nephrol 18: 592A, 2007. [Google Scholar]
- 714. Parker MD, Boron WF. Sodium-coupled bicarbonate transporters. In: Seldin and Giebisch's The Kidney: Physiology and Pathophysiology , edited by Alpern RJ, Hebert SC. Burlington, MA: Academic, 2008, p. 1481–1497. [Google Scholar]
- 715. Parker MD, Boron WF. Splice cassette II within the N terminus of the electroneutral Na+ coupled bicarbonate transporter NBCn1 includes a functional calcineurin A binding site. FASEB J 22: 759.331–12, 2008. [Google Scholar]
- 716. Parker MD, Boron WF, Tanner MJA. Characterization of human “AE4” as an electroneutral, sodium-dependent bicarbonate transporter (Abstract). FASEB J 16: A796, 2002. [Google Scholar]
- 717. Parker MD, Bouyer P, Daly CM, Boron WF. Cloning and characterization of novel human SLC4A8 gene products encoding Na+-driven Cl-HCO3 exchanger variants -A, -C and -D. Physiol Genomics 34: 265–276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718. Parker MD, Daly CM, Skelton LA, Boron WF. IRBIT functionally enhances the electroneutral Na+-coupled bicarbonate transporter NCBE by sequestering an N-terminal autoinhibitory domain (Abstract). FASEB J 21: A1285, 2007. [Google Scholar]
- 719. Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) withself-exchange activity. J Biol Chem 283: 12777–12788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720. Parker MD, Ourmozdi EP, Tanner MJ. Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun 282: 1103–1109, 2001. [DOI] [PubMed] [Google Scholar]
- 721. Parker MD, Qin X, Williamson RC, Toye AM, Boron WF. HCO3−-independent conductance with a mutant Na/HCO3 cotransporter (SLC4A4) in a case of proximal renal tubular acidosis with hypokalemic paralysis. J Physiol 590: 2009–2034, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722. Parker MD, Skelton LA, Daly CM, Boron WF. IRBIT binds to and functionally enhances the electroneutral Na+-coupled bicarbonate transporters NBCn1, NDCBE and NCBE (Abstract). FASEB J 21: A1285, 2007. [Google Scholar]
- 723. Parker MD, Tanner MJ. The disruption of the third extracellular loop of the red cell anion exchanger AE1 does not affect electroneutral Cl−/HCO3− exchange activity. Blood Cells Mol Dis 32: 379–383, 2004. [DOI] [PubMed] [Google Scholar]
- 724. Parker MD, Wass AB, Lee SK, Rahman F, Grant C, Boron WF. Functional reassembly of NBCe1-A from co-expressed cytosolic and transmembrane domains. FASEB J 26: 882.379–2, 2012. [Google Scholar]
- 725. Parker MD, Young MT, Daly CM, Meech RW, Boron WF, Tanner MJ. A conductive pathway generated from fragments of the human red cell anion exchanger AE1. J Physiol 581: 33–50, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726. Parks SK, Tresguerres M, Goss GG. Interactions between Na+ channels and Na+-HCO3− cotransporters in the freshwater fish gill MR cell: a model for transepithelial Na+ uptake. Am J Physiol Cell Physiol 292: C935–C944, 2007. [DOI] [PubMed] [Google Scholar]
- 727. Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood 108: 791–801, 2006. [DOI] [PubMed] [Google Scholar]
- 728. Passow H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol 103: 61–223, 1986. [DOI] [PubMed] [Google Scholar]
- 729. Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology 20: 417–428, 2005. [DOI] [PubMed] [Google Scholar]
- 730. Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM. Pendrin modulates ENaC function by changing luminal HCO3−. J Am Soc Nephrol 21: 1928–1941, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731. Pedrosa R, Goncalves N, Hopfer U, Jose PA, Soares-Da-Silva P. Activity and regulation of Na+-HCO3− cotransporter in immortalized spontaneously hypertensive rat and Wistar-Kyoto rat proximal tubular epithelial cells. Hypertension 49: 1186–1193, 2007. [DOI] [PubMed] [Google Scholar]
- 732. Pellegrino de Iraldi A, Pena C, Rodriguez de Lores Arnaiz G. The effect of an endogenous Na+,K+-ATPase inhibitor on rat lens transparency and ultrastructure. Cell Mol Neurobiol 23: 131–141, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733. Peng S, Lu B, Ruan W, Zhu Y, Sheng H, Lai M. Genetic polymorphisms and breast cancer risk: evidence from meta-analyses, pooled analyses, and genome-wide association studies. Breast Cancer Res Treat 127: 309–324, 2011. [DOI] [PubMed] [Google Scholar]
- 734. Peral MJ, Calonge ML, Ilundain AA. Na+-HCO3− cotransporter and intracellular pH regulation in chicken enterocytes. Pflügers Arch 430: 612–616, 1995. [DOI] [PubMed] [Google Scholar]
- 735. Perez-Castro R, Kasai K, Gainza-Cortes F, Ruiz-Lara S, Casaretto JA, Pena-Cortes H, Tapia J, Fujiwara T, Gonzalez E. VvBOR1, the grapevine ortholog of AtBOR1, encodes an efflux boron transporter that is differentially expressed throughout reproductive development of Vitis vinifera L. Plant Cell Physiol 53: 485–494, 2012. [DOI] [PubMed] [Google Scholar]
- 736. Perlin M, Hallum JV. Effect of acid pH on macromolecular synthesis in L cells. J Cell Biol 49: 66–74, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737. Perrin L, Monier B, Ponzielli R, Astier M, Semeriva M. Drosophila cardiac tube organogenesis requires multiple phases of Hox activity. Dev Biol 272: 419–431, 2004. [DOI] [PubMed] [Google Scholar]
- 738. Perry C, Blaine J, Le H, Grichtchenko II. PMA- and ANGII-induced PKC regulation of the renal Na+-HCO3− cotransporter (hkNBCe1). Am J Physiol Renal Physiol 290: F417–F427, 2006. [DOI] [PubMed] [Google Scholar]
- 739. Perry C, Le H, Grichtchenko II. ANG II and calmodulin/CaMKII regulate surface expression and functional activity of NBCe1 via separate means. Am J Physiol Renal Physiol 293: F68–F77, 2007. [DOI] [PubMed] [Google Scholar]
- 740. Perry C, Quissell DO, Reyland ME, Grichtchenko II. Electrogenic NBCe1 (SLC4A4), but not eletroneutral NBCn1 (SLC4A7), cotransporter undergoes cholinergic-stimulated endocytosis in salivary ParC5 cells. Am J Physiol Cell Physiol 295: C1385–C1398, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741. Perry SF, Furimsky M, Bayaa M, Georgalis T, Shahsavarani A, Nickerson JG, Moon TW. Integrated responses of Na+/HCO3− cotransporters and V-type H+-ATPases in the fish gill and kidney during respiratory acidosis. Biochim Biophys Acta 1618: 175–184, 2003. [DOI] [PubMed] [Google Scholar]
- 742. Perry SF, Shahsavarani A, Georgalis T, Bayaa M, Furimsky M, Thomas SL. Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid-base regulation. J Exp Zool A Comp Exp Biol 300: 53–62, 2003. [DOI] [PubMed] [Google Scholar]
- 743. Petrovic S, Ju X, Barone S, Seidler U, Alper SL, Lohi H, Kere J, Soleimani M. Identification of a basolateral Cl−/HCO3− exchanger specific to gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 284: G1093–G1103, 2003. [DOI] [PubMed] [Google Scholar]
- 744. Petrovic S, Wang Z, Ma L, Seidler U, Forte JG, Shull GE, Soleimani M. Colocalization of the apical Cl−/HCO3− exchanger PAT1 and gastric H-K-ATPase in stomach parietal cells. Am J Physiol Gastrointest Liver Physiol 283: G1207–G1216, 2002. [DOI] [PubMed] [Google Scholar]
- 745. Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Banfi B, Horswill AR, Stoltz DA, McCray PB, Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746. Piermarini PM, Choi I, Boron WF. Cloning and characterization of an electrogenic Na/HCO3 cotransporter from the squid giant fiber lobe. Am J Physiol Cell Physiol 292: C2032–C2045, 2007. [DOI] [PubMed] [Google Scholar]
- 747. Piermarini PM, Grogan LF, Lau K, Wang L, Beyenbach KW. A SLC4-like anion exchanger from renal tubules of the mosquito (Aedes aegypti): evidence for a novel role of stellate cells in diuretic fluid secretion. Am J Physiol Regul Integr Comp Physiol 298: R642–R660, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748. Piermarini PM, Kim EY, Boron WF. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J Biol Chem 282: 1409–1421, 2007. [DOI] [PubMed] [Google Scholar]
- 749. Planelles G, Anagnostopoulos T. Basolateral electrogenic Na/HCO3 symport in the amphibian distal tubule. Pflügers Arch 417: 582–590, 1991. [DOI] [PubMed] [Google Scholar]
- 750. Planelles G, Thomas SR, Anagnostopoulos T. Change of apparent stoichiometry of proximal-tubule Na+-HCO3− cotransport upon experimental reversal of its orientation. Proc Natl Acad Sci USA 90: 7406–7410, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751. Portis JL, McAtee FJ, Evans LH. Infectious entry of murine retroviruses into mouse cells: evidence of a postadsorption step inhibited by acidic pH. J Virol 55: 806–812, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752. Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 91: 5340–5344, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753. Praetorius J, Hager H, Nielsen S, Aalkjær C, Friis UG, Ainsworth MA, Johansen T. Molecular and functional evidence for electrogenic and electroneutral Na+-HCO3− cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol 280: G332–G343, 2001. [DOI] [PubMed] [Google Scholar]
- 754. Praetorius J, Kim YH, Bouzinova EV, Frische S, Rojek A, Aalkjær C, Nielsen S. NBCn1 is a basolateral Na+-HCO3− cotransporter in rat kidney inner medullary collecting ducts. Am J Physiol Renal Physiol 286: F903–F912, 2004. [DOI] [PubMed] [Google Scholar]
- 755. Praetorius J, Nejsum LN, Nielsen S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol 286: C601–C610, 2004. [DOI] [PubMed] [Google Scholar]
- 756. Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol 291: C59–C67, 2006. [DOI] [PubMed] [Google Scholar]
- 757. Preisig PA, Alpern RJ. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na/3HCO3 symporter in the rat proximal convoluted tubule. J Clin Invest 82: 1445–1453, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758. Preisig PA, Alpern RJ. Increased Na/H antiporter and Na/3HCO3 symporter activities in chronic hyperfiltration. J Gen Physiol 97: 195–217, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759. Price GD. Inorganic carbon transporters of the cyanobacterial CO2 concentrating mechanism. Photosynth Res 109: 47–57, 2011. [DOI] [PubMed] [Google Scholar]
- 760. Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci USA 101: 18228–18233, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761. Puceat M, Korichneva I, Cassoly R, Vassort G. Identification of band 3-like proteins and Cl−/HCO3− exchange in isolated cardiomyocytes. J Biol Chem 270: 1315–1322, 1995. [DOI] [PubMed] [Google Scholar]
- 762. Purkerson JM, Schwartz GJ. Anion exchanger 4 expression in β:intercalated cells is characterized by a unique subapical:lateral pattern and regulated by acid:base status (Abstract). J Am Soc Nephrol 22: 267A, 2011. [Google Scholar]
- 763. Purkerson JM, Tsuruoka S, Suter DZ, Nakamori A, Schwartz GJ. Adaptation to metabolic acidosis and its recovery are associated with changes in anion exchanger distribution and expression in the cortical collecting duct. Kidney Int 78: 993–1005, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764. Pushkin A, Abuladze N, Gross E, Newman D, Tatishchev S, Lee I, Fedotoff O, Bondar G, Azimov R, Ngyuen M, Kurtz I. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. J Physiol 559: 55–65, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765. Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem 274: 16569–16575, 1999. [DOI] [PubMed] [Google Scholar]
- 766. Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Mapping of the human NBC3 (SLC4A7) gene to chromosome 3p22. Genomics 58: 321–322, 1999. [PubMed] [Google Scholar]
- 767. Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta 1493: 215–218, 2000. [DOI] [PubMed] [Google Scholar]
- 768. Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Two C-terminal variants of NBC4, a new member of the sodium bicarbonate cotransporter family: cloning, characterization, and localization. IUBMB Life 50: 13–19, 2000. [DOI] [PubMed] [Google Scholar]
- 769. Pushkin A, Abuladze N, Newman D, Muronets V, Sassani P, Tatishchev S, Kurtz I. The COOH termini of NBC3 and the 56-kDa H+-ATPase subunit are PDZ motifs involved in their interaction. Am J Physiol Cell Physiol 284: C667–C673, 2003. [DOI] [PubMed] [Google Scholar]
- 770. Pushkin A, Abuladze N, Newman D, Tatishchev S, Kurtz I. Genomic organization of the DCTN1-SLC4A5 locus encoding both NBC4 and p150(Glued). Cytogenet Cell Genet 95: 163–168, 2001. [DOI] [PubMed] [Google Scholar]
- 771. Pushkin A, Clark I, Kwon TH, Nielsen S, Kurtz I. Immunolocalization of NBC3 and NHE3 in the rat epididymis: colocalization of NBC3 and the vacuolar H+-ATPase. J Androl 21: 708–720, 2000. [PubMed] [Google Scholar]
- 772. Pushkin A, Kurtz I. SLC4 base (HCO3−, CO32−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol 290: F580–F599, 2006. [DOI] [PubMed] [Google Scholar]
- 773. Pushkin A, Sassani P, Abuladze N, Newman D, Tatishchev S, Kurtz I. Oligomeric structure of electrogenic sodium bicarbonate cotransporters (Abstract). J Am Soc Nephrol 12: 8A, 2001. [Google Scholar]
- 774. Pushkin A, Yip KP, Clark I, Abuladze N, Kwon TH, Tsuruoka S, Schwartz GJ, Nielsen S, Kurtz I. NBC3 expression in rabbit collecting duct: colocalization with vacuolar H+-ATPase. Am J Physiol Renal Physiol 277: F974–F981, 1999. [DOI] [PubMed] [Google Scholar]
- 775. Putnam RW. pH regulatory transport systems in a smooth muscle-like cell line. Am J Physiol Cell Physiol 258: C470–C479, 1990. [DOI] [PubMed] [Google Scholar]
- 776. Qu Z, Hartzell HC. Anion permeation in Ca2+-activated Cl− channels. J Gen Physiol 116: 825–844, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777. Qu Z, Hartzell HC. Bestrophin Cl− channels are highly permeable to HCO3−. Am J Physiol Cell Physiol 295: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778. Quentin F, Eladari D, Frische S, Cambillau M, Nielsen S, Alper SL, Paillard M, Chambrey R. Regulation of the Cl−/HCO3− exchanger AE2 in rat thick ascending limb of Henle's loop in response to changes in acid-base and sodium balance. J Am Soc Nephrol 15: 2988–2997, 2004. [DOI] [PubMed] [Google Scholar]
- 779. Raciti D, Reggiani L, Geffers L, Jiang Q, Bacchion F, Subrizi AE, Clements D, Tindal C, Davidson DR, Kaissling B, Brandli AW. Organization of the pronephric kidney revealed by large-scale gene expression mapping. Genome Biol 9: R84, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780. Rajbhandari I, Kim E, Choi I. Interaction of the Na/HCO3 cotransporter SLC4A7 (NBCn1) and the N-methyl-d-aspartate (NMDA) receptor subunit NR2A. FASEB J 22: 759.8, 2008. [Google Scholar]
- 781. Ralston NV, Hunt CD. Transmembrane partitioning of boron and other elements in RAW 264.7 and HL60 cell cultures. Biol Trace Elem Res 98: 181–191, 2004. [DOI] [PubMed] [Google Scholar]
- 782. Ramprasad VL, Ebenezer ND, Aung T, Rajagopal R, Yong VH, Tuft SJ, Viswanathan D, El Ashry MF, Liskova P, Tan DT, Bhattacharya SS, Kumaramanickavel G, Vithana EN. Novel SLC4A11 mutations in patients with recessive congenital hereditary endothelial dystrophy (CHED2). Mutation in brief #958. Online Hum Mutat 28: 522–523, 2007. [DOI] [PubMed] [Google Scholar]
- 783. Ransom BR. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis. Prog Brain Res 94: 37–46, 1992. [DOI] [PubMed] [Google Scholar]
- 784. Ratnaswamy G, Koepf E, Bekele H, Yin H, Kelly JW. The amyloidogenicity of gelsolin is controlled by proteolysis and pH. Chem Biol 6: 293–304, 1999. [DOI] [PubMed] [Google Scholar]
- 785. Re F, Sesana S, Barbiroli A, Bonomi F, Cazzaniga E, Lonati E, Bulbarelli A, Masserini M. Prion protein structure is affected by pH-dependent interaction with membranes: a study in a model system. FEBS Lett 582: 215–220, 2008. [DOI] [PubMed] [Google Scholar]
- 786. Redon J, Batlle D. Regulation of intracellular pH in the spontaneously hypertensive rat. Role of bicarbonate-dependent transporters. Hypertension 23: 503–512, 1994. [DOI] [PubMed] [Google Scholar]
- 787. Reeves EP, Ali T, Leonard P, Hearty S, O'Kennedy R, May FE, Westley BR, Josenhans C, Rust M, Suerbaum S, Smith A, Drumm B, Clyne M. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology 135: 2043–2054, 2054, 2008. [DOI] [PubMed] [Google Scholar]
- 788. Reid R. Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol 48: 1673–1678, 2007. [DOI] [PubMed] [Google Scholar]
- 789. Reiners J, Nagel-Wolfrum K, Jürgens K, Märker T, Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res 83: 97–119, 2006. [DOI] [PubMed] [Google Scholar]
- 790. Reiners J, van Wijk E, Marker T, Zimmermann U, Jurgens K, te Brinke H, Overlack N, Roepman R, Knipper M, Kremer H, Wolfrum U. Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2. Hum Mol Genet 14: 3933–3943, 2005. [DOI] [PubMed] [Google Scholar]
- 791. Reithmeier RA. Fragmentation of the band 3 polypeptide from human erythrocyte membranes. Size and detergent binding of the membrane-associated domain. J Biol Chem 254: 3054–3060, 1979. [PubMed] [Google Scholar]
- 792. Renner EL, Lake JR, Scharschmidt BF, Zimmerli B, Meier PJ. Rat hepatocytes exhibit basolateral Na+/HCO3− cotransport. J Clin Invest 83: 1225–1235, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793. Reusch HP, Lowe J, Ives HE. Osmotic activation of a Na+-dependent Cl−/HCO3− exchanger. Am J Physiol Cell Physiol 268: C147–C153, 1995. [DOI] [PubMed] [Google Scholar]
- 794. Riazuddin SA, Vithana EN, Seet LF, Liu Y, Al-Saif A, Koh LW, Heng YM, Aung T, Meadows DN, Eghrari AO, Gottsch JD, Katsanis N. Missense mutations in the sodium borate cotransporter SLC4A11 cause late-onset Fuchs corneal dystrophya. Hum Mutat 31: 1261–1268. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795. Richier S, Kerros ME, de Vargas C, Haramaty L, Falkowski PG, Gattuso JP. Light-dependent transcriptional regulation of genes of biogeochemical interest in the diploid and haploid life cycle stages of Emiliania huxleyi. Appl Environ Microbiol 75: 3366–3369, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796. Rickmann M, Orlowski B, Heupel K, Roussa E. Distinct expression and subcellular localization patterns of Na+/HCO3− cotransporter (SLC4A4) variants NBCe1-A and NBCe1-B in mouse brain. Neuroscience 146: 1220–1231, 2007. [DOI] [PubMed] [Google Scholar]
- 797. Riihonen R, Nielsen S, Vaananen HK, Laitala-Leinonen T, Kwon TH. Degradation of hydroxyapatite in vivo and in vitro requires osteoclastic sodium-bicarbonate co-transporter NBCn1. Matrix Biol 29: 287–294, 2010. [DOI] [PubMed] [Google Scholar]
- 798. Rivarola V, Ford P, Chara O, Parisi M, Capurro C. Functional and molecular adaptation of Cl/HCO3− exchanger to chronic alkaline media in renal cells. Cell Physiol Biochem 16: 271–280, 2005. [DOI] [PubMed] [Google Scholar]
- 799. Rivinoja A, Hassinen A, Kokkonen N, Kauppila A, Kellokumpu S. Elevated Golgi pH impairs terminal N-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J Cell Physiol 220: 144–154, 2009. [DOI] [PubMed] [Google Scholar]
- 800. Ro HA, Carson JH. pH microdomains in oligodendrocytes. J Biol Chem 279: 37115–37123, 2004. [DOI] [PubMed] [Google Scholar]
- 801. Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, Gillies RJ. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 69: 2260–2268, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802. Robey RB, Ruiz OS, Espiritu DJ, Ibanez VC, Kear FT, Noboa OA, Bernardo AA, Arruda JA. Angiotensin II stimulation of renal epithelial cell Na/HCO3 cotransport activity: a central role for Src family kinase/classic MAPK pathway coupling. J Membr Biol 187: 135–145, 2002. [DOI] [PubMed] [Google Scholar]
- 803. Roche S, Gaudin Y. Evidence that rabies virus forms different kinds of fusion machines with different pH thresholds for fusion. J Virol 78: 8746–8752, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804. Romero MF, Boron WF. Identification and expression of an electroneutral Na/HCO3 cotransporter from C. elegans (ceNBC). J Am Soc Nephrol 9: 11A, 1998. [Google Scholar]
- 805. Romero MF, Chang MH, Mount DB. From cloning to structure, function, and regulation of chloride-dependent and independent bicarbonate transporters. In: Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases, edited by Alvarez-Leefmans FJ, Delpire E. New York: Academic, 2009, p. 43–79. [Google Scholar]
- 806. Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998. [DOI] [PubMed] [Google Scholar]
- 807. Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch 447: 495–509, 2004. [DOI] [PubMed] [Google Scholar]
- 808. Romero MF, Hediger MA, Boulpaep EL, Boron WF. Cloning and functional expression of the rat renal electrogenic Na/HCO3 cotransporter (rNBC). J Am Soc Nephrol 7: 1259, 1996. [Google Scholar]
- 809. Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 387: 409–413, 1997. [DOI] [PubMed] [Google Scholar]
- 810. Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. J Biol Chem 275: 24552–24559, 2000. [DOI] [PubMed] [Google Scholar]
- 811. Roos A, Boron WF. Intracellular pH. Physiol Rev 61: 296–434, 1981. [DOI] [PubMed] [Google Scholar]
- 812. Rose CR, Deitmer JW. Evidence that glial cells modulate extracellular pH transients induced by neuronal activity in the leech central nervous system. J Physiol 481: 1–5, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813. Rossmann H, Alper SL, Nader M, Wang Z, Gregor M, Seidler U. Three 5′-variant mRNAs of anion exchanger AE2 in stomach and intestine of mouse, rabbit, and rat. Ann NY Acad Sci 915: 81–91, 2000. [DOI] [PubMed] [Google Scholar]
- 814. Rossmann H, Bachmann O, Vieillard-Baron D, Gregor M, Seidler U. Na+/HCO3− cotransport and expression of NBC1 and NBC2 in rabbit gastric parietal and mucous cells. Gastroenterology 116: 1389–1398, 1999. [DOI] [PubMed] [Google Scholar]
- 815. Rostagno A, Vidal R, Kaplan B, Chuba J, Kumar A, Elliott JI, Frangione B, Gallo G, Ghiso J. pH-dependent fibrillogenesis of a VkappaIII Bence Jones protein. Br J Haematol 107: 835–843, 1999. [DOI] [PubMed] [Google Scholar]
- 816. Roussa E, Alper SL, Thévenod F. Immunolocalization of anion exchanger AE2, Na+/H+ exchangers NHE1 and NHE4, and vacuolar type H+-ATPase in rat pancreas. J Histochem Cytochem 49: 463–474, 2001. [DOI] [PubMed] [Google Scholar]
- 817. Roussa E, Nastainczyk W, Thevenod F. Differential expression of electrogenic NBC1 (SLC4A4) variants in rat kidney and pancreas. Biochem Biophys Res Commun 314: 382–389, 2004. [DOI] [PubMed] [Google Scholar]
- 818. Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thevenod F. Immunolocalization of anion exchanger AE2 and Na+-HCO3− cotransporter in rat parotid and submandibular glands. Am J Physiol Gastrointest Liver Physiol 277: G1288–G1296, 1999. [DOI] [PubMed] [Google Scholar]
- 819. Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820. Ruff RL. An important piece has been placed in the puzzle of hypokalemic periodic paralysis. Neurology 76: 1614–1615, 2011. [DOI] [PubMed] [Google Scholar]
- 821. Ruiz OS, Arruda JA. Regulation of the renal Na-HCO3 cotransporter by cAMP and Ca-dependent protein kinases. Am J Physiol Renal Fluid Electrolyte Physiol 262: F560–F565, 1992. [DOI] [PubMed] [Google Scholar]
- 822. Ruiz OS, Qiu YY, Arruda JA. The renal cortical Na-HCO3 cotransporter. V. Expression in Xenopus oocytes. Proc Soc Exp Biol Med 211: 199–204, 1996. [DOI] [PubMed] [Google Scholar]
- 823. Ruiz OS, Qiu YY, Cardoso LR, Arruda JA. Regulation of the renal Na-HCO3 cotransporter. VII. Mechanism of the cholinergic stimulation. Kidney Int 51: 1069–1077, 1997. [DOI] [PubMed] [Google Scholar]
- 824. Ruiz OS, Qiu YY, Wang LJ, Cardoso LR, Arruda JA. Regulation of renal Na-HCO3 cotransporter. VIII. Mechanism of stimulatory effect of respiratory acidosis. J Membrane Biol 162: 201–208, 1998. [DOI] [PubMed] [Google Scholar]
- 825. Ruminot I, Gutiérrez R, Peña-Münzenmayer G, Añazco C, Sotelo-Hitschfeld T, Lerchundi R, Niemeyer MI, Shull GE, Barros LF. NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K+. J Neurosci 31: 14264–14271, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826. Russell JM, Boron WF. Role of chloride transport in regulation of intracellular pH. Nature 264: 73–74, 1976. [DOI] [PubMed] [Google Scholar]
- 827. Rychkov GY, Pusch M, Roberts ML, Jentsch TJ, Bretag AH. Permeation and block of the skeletal muscle chloride channel, ClC-1, by foreign anions. J Gen Physiol 111: 653–665, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828. Sabolic I, Brown D, Gluck SL, Alper SL. Regulation of AE1 anion exchanger and H+-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int 51: 125–137, 1997. [DOI] [PubMed] [Google Scholar]
- 829. Saitoh S, Terada N, Ohno N, Saitoh Y, Soleimani M, Ohno S. Immunolocalization of phospho-Arg-directed protein kinase-substrate in hypoxic kidneys using in vivo cryotechnique. Med Mol Morphol 42: 24–31, 2009. [DOI] [PubMed] [Google Scholar]
- 830. Sander T, Toliat MR, Heils A, Leschik G, Becker C, Ruschendorf F, Rohde K, Mundlos S, Nurnberg P. Association of the 867Asp variant of the human anion exchanger 3 gene with common subtypes of idiopathic generalized epilepsy. Epilepsy Res 51: 249–255, 2002. [DOI] [PubMed] [Google Scholar]
- 831. Sandmann S, Yu M, Kaschina E, Blume A, Bouzinova E, Aalkjær C, Unger T. Differential effects of angiotensin AT1 and AT2 receptors on the expression, translation and function of the Na+-H+ exchanger and Na+-HCO3− symporter in the rat heart after myocardial infarction. J Am Coll Cardiol 37: 2154–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 832. Sasaki S, Shiigai T, Yoshiyama N, Takeuchi J. Mechanism of bicarbonate exit across basolateral membrane of rabbit proximal straight tubule. Am J Physiol Renal Fluid Electrolyte Physiol 252: F11–F18, 1987. [DOI] [PubMed] [Google Scholar]
- 833. Sasaki S, Yoshiyama N. Interaction of chloride and bicarbonate transport across the basolateral membrane of rabbit proximal straight tubule. J Clin Invest 81: 1004–1011, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834. Sassani P, Pushkin A, Abuladze N, Azimov R, Kao L, Peti-Peterdi J, Liu W, Newman D, Kurtz I. Role of S-S bond formation in the oligomerization of kNBC1 (NBCe1-A) (Abstract). FASEB J 21: 916.3 2007. [Google Scholar]
- 835. Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4: a new electrogenic sodium- bicarbonate cotransporter. Am J Physiol Cell Physiol 282: C408–C416, 2002. [DOI] [PubMed] [Google Scholar]
- 836. Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso O, Inatomi J, Kawakami H, Kudo A, Endou H, Igarashi T, Goto A, Fujita T, Seki G. Localization of Na+-HCO3− cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol 284: C729–C737, 2003. [DOI] [PubMed] [Google Scholar]
- 837. Satou Y, Takatori N, Yamada L, Mochizuki Y, Hamaguchi M, Ishikawa H, Chiba S, Imai K, Kano S, Murakami SD, Nakayama A, Nishino A, Sasakura Y, Satoh G, Shimotori T, Shin I, Shoguchi E, Suzuki MM, Takada N, Utsumi N, Yoshida N, Saiga H, Kohara Y, Satoh N. Gene expression profiles in Ciona intestinalis tailbud embryos. Development 128: 2893–2904, 2001. [DOI] [PubMed] [Google Scholar]
- 838. Saxena A, Hensley P, Osborne JC, Jr, Fleming PJ. The pH-dependent subunit dissociation and catalytic activity of bovine dopamine beta-hydroxylase. J Biol Chem 260: 3386–3392, 1985. [PubMed] [Google Scholar]
- 839. Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, Canese K, Chetvernin V, Church DM, Dicuccio M, Federhen S, Feolo M, Fingerman IM, Geer LY, Helmberg W, Kapustin Y, Krasnov S, Landsman D, Lipman DJ, Lu Z, Madden TL, Madej T, Maglott DR, Marchler-Bauer A, Miller V, Karsch-Mizrachi I, Ostell J, Panchenko A, Phan L, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Slotta D, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Wang Y, Wilbur WJ, Yaschenko E, Ye J. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 40: D13–D25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840. Schafer C, Ladilov YV, Siegmund B, Piper HM. Importance of bicarbonate transport for protection of cardiomyocytes against reoxygenation injury. Am J Physiol Heart Circ Physiol 278: H1457–H1463, 2000. [DOI] [PubMed] [Google Scholar]
- 841. Schlue WR, Thomas RC. A dual mechanism for intracellular pH regulation by leech neurones. J Physiol 364: 327–338, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 842. Schmidt-Rose T, Jentsch TJ. Reconstitution of functional voltage-gated chloride channels from complementary fragments of CLC-1. J Biol Chem 272: 20515–20521, 1997. [DOI] [PubMed] [Google Scholar]
- 843. Schmitt BM, Berger UV, Douglas RM, Bevensee MO, Hediger MA, Haddad GG, Boron WF. Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. J Neurosci 20: 6839–6848, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844. Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+/HCO3− cotransporter in mammalian and amphibian kidney. Am J Physiol Renal Physiol 276: F27–F36, 1999. [DOI] [PubMed] [Google Scholar]
- 845. Schneider A, Larusch J, Sun X, Aloe A, Lamb J, Hawes R, Cotton P, Brand RE, Anderson MA, Money ME, Banks PA, Lewis MD, Baillie J, Sherman S, Disario J, Burton FR, Gardner TB, Amann ST, Gelrud A, George R, Rockacy MJ, Kassabian S, Martinson J, Slivka A, Yadav D, Oruc N, Barmada MM, Frizzell R, Whitcomb DC. Combined bicarbonate conductance-impairing variants in CFTR and SPINK1 variants are associated with chronic pancreatitis in patients without cystic fibrosis. Gastroenterology 140: 162–171, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846. Schosser A, Gaysina D, Cohen-Woods S, Domenici E, Perry J, Tozzi F, Korszun A, Gunasinghe C, Gray J, Jones L, Binder EB, Holsboer F, Craddock N, Owen MJ, Craig IW, Farmer AE, Muglia P, McGuffin P. A follow-up case-control association study of tractable (druggable) genes in recurrent major depression. Am J Med Genet B Neuropsychiatr Genet 156B: 640–650, 2011. [DOI] [PubMed] [Google Scholar]
- 847. Schröder HC, Perovic-Ottstadt S, Rothenberger M, Wiens M, Schwertner H, Batel R, Korzhev M, Müller IM, Müller WE. Silica transport in the demosponge Suberites domuncula: fluorescence emission analysis using the PDMPO probe and cloning of a potential transporter. Biochem J 381: 665–673, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848. Schulte-Frohlinde D, Blume H, Güsten H. Photochemical cis-trans-isomerization of substituted stilbenes. J Phys Chem 66: 2486–2491, 1962. [Google Scholar]
- 849. Schwab A, Rossmann H, Klein M, Dieterich P, Gassner B, Neff C, Stock C, Seidler U. Functional role of Na+-HCO3− cotransport in migration of transformed renal epithelial cells. J Physiol 568: 445–458, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850. Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurons from the rat hippocampus by Na+-dependent Cl−-HCO3− exchange. J Physiol 475: 59–67, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851. Schwiening CJ, Thomas RC. Mechanism of pHi regulation by locust neurones in isolated ganglia: a microelectrode study. J Physiol 447: 693–709, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852. Sciortino CM. Characterization and Localization of the Sodium Mediated Bicarbonate Transporters NBC and NDAE1. (PhD thesis) Cleveland, OH: Case Western Reserve Univ., 2001. [Google Scholar]
- 853. Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+-HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol 277: F611–F623, 1999. [DOI] [PubMed] [Google Scholar]
- 854. Sciortino CM, Shrode LD, Fletcher BR, Harte PJ, Romero MF. Localization of endogenous and recombinant Na(+)-driven anion exchanger protein NDAE1 from Drosophila melanogaster. Am J Physiol Cell Physiol 281: C449–C463, 2001. [DOI] [PubMed] [Google Scholar]
- 855. Scoazec JY, Bringuier AF, Medina JF, Martinez-Anso E, Veissiere D, Feldmann G, Housset C. The plasma membrane polarity of human biliary epithelial cells: in situ immunohistochemical analysis and functional implications. J Hepatol 26: 543–553, 1997. [DOI] [PubMed] [Google Scholar]
- 856. Scott GR, Claiborne JB, Edwards SL, Schulte PM, Wood CM. Gene expression after freshwater transfer in gills and opercular epithelia of killifish: insight into divergent mechanisms of ion transport. J Exp Biol 208: 2719–2729, 2005. [DOI] [PubMed] [Google Scholar]
- 857. Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science 316: 445–449, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858. Seki G, Coppola S, Frömter E. The Na+-HCO3− cotransporter operates with a coupling ratio of 2 HCO3− to 1 Na+ in isolated rabbit renal proximal tubule. Pflügers Arch 425: 409–416, 1993. [DOI] [PubMed] [Google Scholar]
- 859. Seki G, Yamada H, Horita S, Suzuki M, Sekine T, Igarashi T, Fujita T. Activation and inactivation mechanisms of Na-HCO3 cotransporter NBC1. J Epithel Biol Pharmacol 1: 35–39, 2008. [Google Scholar]
- 860. Sekino K, Kobayashi H, Shiraiwa Y. Role of coccoliths in the utilization of inorganic carbon by a marine unicellular coccolithophorid, Emiliania huxleyi: a survey using intact cells and protoplasts. Plant Cell Physiol 37: 123–127, 1996. [Google Scholar]
- 861. Sekler I, Lo RS, Kopito RR. A conserved glutamate is responsible for ion selectivity and pH dependence of the mammalian anion exchangers AE1 and AE2. J Biol Chem 270: 28751–28758, 1995. [DOI] [PubMed] [Google Scholar]
- 862. Sekler I, Lo RS, Mastrocola T, Kopito RR. Sulfate transport mediated by the mammalian anion exchangers in reconstituted proteoliposomes. J Biol Chem 270: 11251–11256, 1995. [DOI] [PubMed] [Google Scholar]
- 863. Sener A, Jijakli H, Zahedi Asl S, Cortois P, Yates AP, Meuris S, Best LC, Malaisse WJ. Possible role of carbonic anhydrase in rat pancreatic islets: enzymatic, secretory, metabolic, ionic, and electrical aspects. Am J Physiol Endocrinol Metab 292: E1624–E1630, 2007. [DOI] [PubMed] [Google Scholar]
- 864. Sepulveda FV, Robinson JW. Harmaline, a potent inhibitor of sodium-dependent transport. Biochim Biophys Acta 373: 527–531, 1974. [DOI] [PubMed] [Google Scholar]
- 865. Sergeev M, Godin AG, Kao L, Abuladze N, Wiseman PW, Kurtz I. Determination of membrane protein transporter oligomerization in native tissue using spatial fluorescence intensity fluctuation analysis. PLoS ONE 7: e36215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866. Shah GN, Bonapace G, Hu PY, Strisciuglio P, Sly WS. Carbonic anhydrase II deficiency syndrome (osteopetrosis with renal tubular acidosis and brain calcification): novel mutations in CA2 identified by direct sequencing expand the opportunity for genotype-phenotype correlation. Hum Mutat 24: 272, 2004. [DOI] [PubMed] [Google Scholar]
- 867. Shah SS, Al Rajhi A, Brandt JD, Mannis MJ, Roos B, Sheffield VC, Syed NA, Stone EM, Fingert JH. Mutation in the SLC4A11 gene associated with autosomal recessive congenital hereditary endothelial dystrophy in a large Saudi family. Ophthalmic Genet 29: 41–45, 2008. [DOI] [PubMed] [Google Scholar]
- 868. Shahidullah M, To CH, Pelis RM, Delamere NA. Studies on bicarbonate transporters and carbonic anhydrase in porcine nonpigmented ciliary epithelium. Invest Ophthal Vis Sci 50: 1791–1800, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869. Shao X, Kao L, Abuladze N, Kurtz I. Stoichiometry and inhibitory pharmacology of electrogenic sodium bicarbonate cotransporter NBC4c (NBCe2-C) expressed in HEK-293 cells. FASEB J 23: 800.1791–2, 2009. [Google Scholar]
- 870. Shao XM, Kao L, Abuladze N, Kurtz I. Stoichiometry of the renal electrogenic sodium bicarbonate cotransporter NBCe1-A expressed in HEK-293 cells (Abstract). J Am Soc Nephrol 19: 349A, 2008.18094366 [Google Scholar]
- 871. Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol 127: 511–524, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872. Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J Physiol 586: 3813–3824, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873. Shen MR, Wilkins RJ, Chou CY, Ellory JC. Anion exchanger isoform 2 operates in parallel with Na+/H+ exchanger isoform 1 during regulatory volume decrease of human cervical cancer cells. FEBS Lett 512: 52–58, 2002. [DOI] [PubMed] [Google Scholar]
- 874. Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci 24: 1–29, 2001. [DOI] [PubMed] [Google Scholar]
- 875. Shepard AR, Rae JL. Ion transporters and receptors in cDNA libraries from lens and cornea epithelia. Curr Eye Res 17: 708–719, 1998. [PubMed] [Google Scholar]
- 876. Sherman T, Chernova MN, Clark JS, Jiang L, Alper SL, Nehrke K. The abts and sulp families of anion transporters from Caenorhabditis elegans. Am J Physiol Cell Physiol 289: C341–C351, 2005. [DOI] [PubMed] [Google Scholar]
- 877. Shibata M, Katoh H, Sonoda M, Ohkawa H, Shimoyama M, Fukuzawa H, Kaplan A, Ogawa T. Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: function and phylogenetic analysis. J Biol Chem 277: 18658–18664, 2002. [DOI] [PubMed] [Google Scholar]
- 878. Shin JH, Son EJ, Lee HS, Kim SJ, Kim K, Choi JY, Lee MG, Yoon JH. Molecular and functional expression of anion exchangers in cultured normal human nasal epithelial cells. Acta Physiol 191: 99–110, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879. Shiohara M, Igarashi T, Mori T, Komiyama A. Genetic and long-term data on a patient with permanent isolated proximal renal tubular acidosis. Eur J Pediatr 159: 892–894, 2000. [DOI] [PubMed] [Google Scholar]
- 880. Ship S, Shami Y, Breuer W, Rothstein A. Synthesis of tritiated 4,4′-diisothiocyano-2,2′-stilbene disulfonic acid ([3H]DIDS) and its covalent reaction with sites related to anion transport in human red blood cells. J Membr Biol 33: 311–323, 1977. [DOI] [PubMed] [Google Scholar]
- 881. Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc Natl Acad Sci USA 103: 9542–9547, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882. Shmukler BE, Clark JS, Hsu A, Vandorpe DH, Stewart AK, Kurschat CE, Choe SK, Zhou Y, Amigo J, Paw BH, Alper SL. Zebrafish ae2.2 encodes a second slc4a2 anion exchanger. Am J Physiol Regul Integr Comp Physiol 294: R1081–R1091, 2008. [DOI] [PubMed] [Google Scholar]
- 883. Shumaker H, Amlal H, Frizzell R, Ulrich CD, Soleimani M. CFTR drives Na+-nHCO3− cotransport in pancreatic duct cells: a basis for defective HCO3− secretion in CF. Am J Physiol Cell Physiol 276: C16–C25, 1999. [DOI] [PubMed] [Google Scholar]
- 884. Siebens AW, Boron WF. Depolarization-induced alkalinization in proximal tubules. I. Characteristics and dependence on Na+. Am J Physiol Renal Fluid Electrolyte Physiol 256: F342–F353, 1989. [DOI] [PubMed] [Google Scholar]
- 885. Siebens AW, Boron WF. Depolarization-induced alkalinization in proximal tubules. II. Effects of lactate and SITS. Am J Physiol Renal Fluid Electrolyte Physiol 256: F354–F365, 1989. [DOI] [PubMed] [Google Scholar]
- 886. Simons TJ. The role of anion transport in the passive movement of lead across the human red cell membrane. J Physiol 378: 287–312, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887. Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007. [DOI] [PubMed] [Google Scholar]
- 888. Sindic A, Chang MH, Mount DB, Romero MF. Renal physiology of SLC26 anion exchangers. Curr Opin Nephrol Hypertens 16: 484–490, 2007. [DOI] [PubMed] [Google Scholar]
- 889. Sinning A, Liebmann L, Kougioumtzes A, Westermann M, Bruehl C, Hübner CA. Synaptic glutamate release is modulated by the Na+-driven Cl−/HCO3− exchanger Slc4a8. J Neurosci 31: 7300–7311, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890. Skelton LA, Boron WF. ErbB1-ErbB2 heterodimer activation in rabbit renal proximal tubules exposed to acute respiratory acidosis (Abstract). J Am Soc Nephrol 22: 80A, 2011. [Google Scholar]
- 891. Snead CM, Smith SM, Sadeghein N, Lacruz RS, Hu P, Kurtz I, Paine ML. Identification of a pH-responsive DNA region upstream of the transcription start site of human NBCe1-B. Eur J Oral Sci 119: 136–141, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892. Solaro RJ, el Saleh SC, Kentish JC. Ca2+, pH and the regulation of cardiac myofilament force and ATPase activity. Mol Cell Biochem 89: 163–167, 1989. [DOI] [PubMed] [Google Scholar]
- 893. Soleimani M, Aronson PS. Ionic mechanism of Na+:HCO3− cotransport in renal basolateral membrane vesicles. Kidney Int 32: 407, 1988. [PubMed] [Google Scholar]
- 894. Soleimani M, Bergman JA, Hosford MA, McKinney TD. Potassium depletion increases luminal Na+/H+ exchange and basolateral Na+:CO32−:HCO3− cotransport in rat renal cortex. J Clin Invest 86: 1076–1083, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895. Soleimani M, Bizal GL, McKinney TD, Hattabaugh YJ. Effect of in vitro metabolic acidosis on luminal Na+/H+ exchange and basolateral Na+:HCO3− cotransport in rabbit kidney proximal tubules. J Clin Invest 90: 211–218, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896. Soleimani M, Grassl SM, Aronson PS. Stoichiometry of Na+-HCO3− cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest 79: 1276–1280, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897. Soleimani M, Lesoine GA, Bergman JA, Aronson PS. Cation specificity and modes of the Na+:CO32−:HCO3− cotransporter in renal basolateral membrane vesicles. J Biol Chem 266: 8706–8710, 1991. [PubMed] [Google Scholar]
- 898. Somjen GG, Tombaugh GC. pH modulation of neuronal excitability and central nervous system functions. In: pH and Brain Function, edited by Kaila K, Ransom BR. New York: Wiley-Liss, 1998, p. 373–393. [Google Scholar]
- 899. Sonalker PA, Tofovic SP, Bastacky SI, Jackson EK. Chronic noradrenaline increases renal expression of NHE-3, NBC-1, BSC-1 and aquaporin-2. Clin Exp Pharmacol Physiol 35: 594–600, 2008. [DOI] [PubMed] [Google Scholar]
- 900. Sonalker PA, Tofovic SP, Jackson EK. Increased expression of the sodium transporter BSC-1 in spontaneously hypertensive rats. J Pharmacol Exp Ther 311: 1052–1061, 2004. [DOI] [PubMed] [Google Scholar]
- 901. Soyfoo MS, Bulur N, Virreira M, Louchami K, Lybaert P, Crutzen R, Perret J, Delporte C, Roussa E, Thevenod F, Best L, Yates AP, Malaisse WJ, Sener A, Beauwens R. Expression of the electrogenic Na+-HCO3−-cotransporters NBCe1-A and NBCe1-B in rat pancreatic islet cells. Endocrine 35: 449–458, 2009. [DOI] [PubMed] [Google Scholar]
- 902. Spirlμ C, Granato A, Zsembery K, Anglani F, Okolicsányi L, LaRusso NF, Crepaldi G, Strazzabosco M. Functional polarity of Na+/H+ and Cl−/HCO3− exchangers in a rat cholangiocyte cell line. Am J Physiol Gastrointest Liver Physiol 275: G1236–G1245, 1998. [DOI] [PubMed] [Google Scholar]
- 903. Stakisaitis D, LaPointe MS, Batlle D. Mechanisms of chloride transport in thymic lymphocytes. Am J Physiol Renal Physiol 280: F314–F324, 2001. [DOI] [PubMed] [Google Scholar]
- 904. Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl−/HCO3− exchanger (slc4a1). J Am Soc Nephrol 18: 1408–1418, 2007. [DOI] [PubMed] [Google Scholar]
- 905. Sterling D, Casey JR. Transport activity of AE3 chloride/bicarbonate anion-exchange proteins and their regulation by intracellular pH. Biochem J 344: 221–229, 1999. [PMC free article] [PubMed] [Google Scholar]
- 906. Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol 67: 377–409, 2005. [DOI] [PubMed] [Google Scholar]
- 907. Stewart AK, Kerr N, Chernova MN, Alper SL, Vaughan-Jones RD. Acute pH-dependent regulation of AE2-mediated anion exchange involves discrete local surfaces of the NH2-terminal cytoplasmic domain. J Biol Chem 279: 52664–52676, 2004. [DOI] [PubMed] [Google Scholar]
- 908. Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol 301: C289–C303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909. Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H. Functional coupling of apical Cl−/HCO3− exchange with CFTR in stimulated HCO3− secretion by guinea pig interlobular pancreatic duct. Am J Physiol Gastrointest Liver Physiol 296: G1307–G1317, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 910. Stock C, Schwab A. Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol 187: 149–157, 2006. [DOI] [PubMed] [Google Scholar]
- 911. Stoll H, Langer G, Shimizu N, Kanamaru K. B/Ca in coccoliths and relationship to calcification vesicle pH and dissolved inorganic carbon concentrations. Geochim Cosmochim Acta 80: 143–157, 2012. [Google Scholar]
- 912. Strange K, Phillips JE. Cellular mechanism of HCO3− and Cl− transport in insect salt gland. J Membr Biol 83: 25–37, 1985. [DOI] [PubMed] [Google Scholar]
- 913. Strazzabosco M, Joplin R, Zsembery A, Wallace L, Spirli C, Fabris L, Granato A, Rossanese A, Poci C, Neuberger JM, Okolicsanyi L, Crepaldi G. Na+-dependent and -independent Cl−/HCO3− exchange mediate cellular HCO3− transport in cultured human intrahepatic bile duct cells. Hepatology 25: 976–985, 1997. [DOI] [PubMed] [Google Scholar]
- 914. Stuart-Tilley AK, Shmukler BE, Brown D, Alper SL. Immunolocalization and tissue-specific splicing of AE2 anion exchanger in mouse kidney. J Am Soc Nephrol 9: 946–959, 1998. [DOI] [PubMed] [Google Scholar]
- 915. Stuetz AM, Rao DC, Rice T, Bouchard C, Rankinen T. SLC4A5 gene polymorphisms are associated with cardiovascular and metabolic phenotypes in the HERITAGE Family study (Abstract). FASEB J 21: A571, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916. Stütz AM, Teran-Garcia M, Rao DC, Rice T, Bouchard C, Rankinen T. Functional identification of the promoter of SLC4A5, a gene associated with cardiovascular and metabolic phenotypes in the HERITAGE Family Study. Eur J Hum Genet 17: 1481–1489, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917. Sueta A, Ito H, Kawase T, Hirose K, Hosono S, Yatabe Y, Tajima K, Tanaka H, Iwata H, Iwase H, Matsuo K. A genetic risk predictor for breast cancer using a combination of low-penetrance polymorphisms in a Japanese population. Breast Cancer Res Treat 2011. [DOI] [PubMed] [Google Scholar]
- 918. Sugiura SH, Roy PK, Ferraris RP. Dietary acidification enhances phosphorus digestibility but decreases H+/K+-ATPase expression in rainbow trout. J Exp Biol 209: 3719–3728, 2006. [DOI] [PubMed] [Google Scholar]
- 919. Sultana A, Garg P, Ramamurthy B, Vemuganti GK, Kannabiran C. Mutational spectrum of the SLC4A11 gene in autosomal recessive congenital hereditary endothelial dystrophy. Mol Vis 13: 1327–1332, 2007. [PubMed] [Google Scholar]
- 920. Sun DA, Sombati S, DeLorenzo RJ. Glutamate injury-induced epileptogenesis in hippocampal neurons: an in vitro model of stroke-induced “epilepsy.” Stroke 32: 2344–2350, 2001. [DOI] [PubMed] [Google Scholar]
- 921. Sun X, Petrovic S. Increased acid load and deletion of AE1 increase Slc26a7 expression. Nephron Physiol 109: 29–35, 2008. [DOI] [PubMed] [Google Scholar]
- 922. Sun XC, Bonanno JA. Identification and cloning of the Na/HCO3− cotransporter (NBC) in human corneal endothelium. Exp Eye Res 77: 287–295, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923. Sun XC, Bonanno JA, Jelamskii S, Xie Q. Expression and localization of Na+-HCO3− cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol 279: C1648–C1655, 2000. [DOI] [PubMed] [Google Scholar]
- 924. Sun YV, Meyers KJ, Mosley TH, Boerwinkle E, Kullo IJ, Turner ST, Kardia SLR. Identification of disease-associated SNP clusters using a scan statistic. Genet Epidemiol 29: 278–279, 2005. [Google Scholar]
- 925. Sussman CR, Chang MH, Plata C, Angle N, Romero MF. Localization, function and gene structure of a zebrafish electrogenic Na+ bicarbonate cotransporter, zNBCe1 (Abstract). FASEB J 19: A140–A141, 2005. [Google Scholar]
- 926. Sussman CR, Zhao J, Plata C, Lu J, Daly C, Angle N, DiPiero J, Drummond IA, Liang JO, Boron WF, Romero MF, Chang MH. Cloning, localization, and functional expression of the electrogenic Na+ bicarbonate cotransporter (NBCe1) from zebrafish. Am J Physiol Cell Physiol 297: C865–C875, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927. Sutton T, Baumann U, Hayes J, Collins NC, Shi BJ, Schnurbusch T, Hay A, Mayo G, Pallotta M, Tester M, Langridge P. Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318: 1446–1449, 2007. [DOI] [PubMed] [Google Scholar]
- 928. Suzuki M, Seki G, Yamada H, Horita S, Fujita T. Functional roles of electrogenic sodium bicarbonate cotransporter NBCe1 in ocular tissues. Open Ophthalmol J 6: 36–41, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929. Suzuki M, Vaisbich MH, Yamada H, Horita S, Li Y, Sekine T, Moriyama N, Igarashi T, Endo Y, Cardoso TP, de Sa LC, Koch VH, Seki G, Fujita T. Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflügers Arch 455: 583–593, 2008. [DOI] [PubMed] [Google Scholar]
- 930. Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De Jonghe P, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G. Defective membrane expression of the Na+-HCO3− cotransporter NBCe1 is associated with familial migraine. Proc Natl Acad Sci USA 107: 15963–15968, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 931. Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A, Zatovicova M, Barathova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem 287: 3392–3402, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 932. Svichar N, Esquenazi S, Chen HY, Chesler M. Preemptive regulation of intracellular pH in hippocampal neurons by a dual mechanism of depolarization-induced alkalinization. J Neurosci 31: 6997–7004, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 933. Swartzwelder HS, Anderson WW, Wilson WA. Mechanism of electrographic seizure generation in the hippocampal slice in Mg2+-free medium: the role of GABAa inhibition. Epilepsy Res 2: 239–245, 1988. [DOI] [PubMed] [Google Scholar]
- 934. Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 29: 6509–6521, 2010. [DOI] [PubMed] [Google Scholar]
- 935. Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev 26: 299–310, 2007. [DOI] [PubMed] [Google Scholar]
- 936. Swietnicki W, Petersen R, Gambetti P, Surewicz WK. pH-dependent stability and conformation of the recombinant human prion protein PrP(90–231). J Biol Chem 272: 27517–27520, 1997. [DOI] [PubMed] [Google Scholar]
- 937. Tajima Y, Okamura N, Sugita Y. The activating effects of bicarbonate on sperm motility and respiration at ejaculation. Biochim Biophys Acta 924: 519–529, 1987. [DOI] [PubMed] [Google Scholar]
- 938. Takahashi A, Nakano M, Okamoto K, Fujii Y, Mawatari K, Harada N, Nakaya Y. Aeromonas sobria hemolysin causes diarrhea by increasing secretion of HCO3−. FEMS Microbiol Lett 258: 92–95, 2006. [DOI] [PubMed] [Google Scholar]
- 939. Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc Natl Acad Sci USA 97: 5434–5439, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940. Takanaga H, Mackenzie B, Hediger MA. Sodium-dependent ascorbic acid transporter family SLC23. Pflügers Arch 447: 677–682, 2004. [DOI] [PubMed] [Google Scholar]
- 941. Takano J, Kobayashi M, Noda Y, Fujiwara T. Saccharomyces cerevisiae Bor1p is a boron exporter and a key determinant of boron tolerance. FEMS Microbiol Lett 267: 230–235, 2007. [DOI] [PubMed] [Google Scholar]
- 942. Takano J, Miwa K, Yuan L, von Wiren N, Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci USA 102: 12276–12281, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943. Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos J, Miwa K, Hayashi H, Yoneyama T, Fujiwara T. Arabidopsis boron transporter for xylem loading. Nature 420: 337–340, 2002. [DOI] [PubMed] [Google Scholar]
- 944. Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA 107: 5220–5225, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 945. Takano J, Wada M, Ludewig U, Schaaf G, von Wiren N, Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18: 1498–1509, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946. Talley K, Alexov E. On the pH-optimum of activity and stability of proteins. Proteins 78: 2699–2706, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947. Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20: 2860–2875, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 948. Tanis JE, Bellemer A, Moresco JJ, Forbush B, Koelle MR. The potassium chloride cotransporter KCC-2 coordinates development of inhibitory neurotransmission and synapse structure in Caenorhabditis elegans. J Neurosci 29: 9943–9954, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 949. Tapper H, Sundler R. Cytosolic pH regulation in mouse macrophages. Characteristics of HCO3−-dependent mechanisms. Biochem J 281: 239–244, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 950. Tatishchev S, Abuladze N, Pushkin A, Newman D, Liu W, Weeks D, Sachs G, Kurtz I. Identification of membrane topography of the electrogenic sodium bicarbonate cotransporter pNBC1 by in vitro transcription/translation. Biochemistry 42: 755–765, 2003. [DOI] [PubMed] [Google Scholar]
- 951. Tatusova TA, Madden TL. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett 174: 247–250, 1999. [DOI] [PubMed] [Google Scholar]
- 952. Taylor JR, Mager EM, Grosell M. Basolateral NBCe1 plays a rate-limiting role in transepithelial intestinal HCO3− secretion, contributing to marine fish osmoregulation. J Exp Biol 213: 459–468, 2010. [DOI] [PubMed] [Google Scholar]
- 953. Taylor JY, Maddox R, Wu CY. Genetic and environmental risks for high blood pressure among African American mothers and daughters. Biol Res Nurs 11: 53–65, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954. Taylor JY, Sampson D, Taylor AD, Caldwell D, Sun YV. Genetic and BMI risks for predicting blood pressure in three generations of West African dogon women. Biol Res Nurs. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 955. Taylor JY, Wu CY, Darling D, Sun YV, Kardia SL, Jackson JS. Gene-environment effects of SLC4A5 and skin color on blood pressure among African American women. Ethn Dis 22: 155–161, 2012. [PMC free article] [PubMed] [Google Scholar]
- 956. Ten Hove M, Nederhoff MG, Van Echteld CJ. Relative contributions of Na+/H+ exchange and Na+/HCO3− cotransport to ischemic Na+i overload in isolated rat hearts. Am J Physiol Heart Circ Physiol 288: H287–H292, 2005. [DOI] [PubMed] [Google Scholar]
- 957. Terada N, Ohno N, Saitoh S, Seki G, Komada M, Suzuki T, Yamakawa H, Soleimani M. Interaction of membrane skeletal protein, protein 4.1B and p55, and sodium bicarbonate cotransporter1 in mouse renal S1–S2 proximal tubules. J Histochem Cytochem 55: 1199–1206, 2007. [DOI] [PubMed] [Google Scholar]
- 958. Terada N, Ohno N, Yamakawa H, Seki G, Fujii Y, Baba T, Ohara O, Ohno S. Immunoelectron microscopic localization of protein 4.1B in proximal S1 and S2 tubules of rodent kidneys. Med Electron Microsc 37: 45–51, 2004. [DOI] [PubMed] [Google Scholar]
- 959. Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 259: F519–F528, 1990. [DOI] [PubMed] [Google Scholar]
- 960. Terbach N, Shah R, Kelemen R, Klein PS, Gordienko D, Brown NA, Wilkinson CJ, Williams RS. Identifying an uptake mechanism for the antiepileptic and bipolar disorder treatment valproic acid using the simple biomedical model Dictyostelium. J Cell Sci 124: 2267–2276, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961.The International Consortium for Blood Pressure Genome-Wide Association Studies. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962. Thevenod F, Roussa E, Schmitt BM, Romero MF. Cloning and immunolocalization of a rat pancreatic Na+ bicarbonate cotransporter. Biochem Biophys Res Commun 264: 291–298, 1999. [DOI] [PubMed] [Google Scholar]
- 963. Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia 8: 143–152, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 964. Thomas C, Bishop D, Moore-Morris T, Mercier J. Effects of high-intensity training on MCT1, MCT4, and NBC expressions in rat skeletal muscles: influence of chronic metabolic alkalosis. Am J Physiol Endocrinol Metab 293: E916–E922, 2007. [DOI] [PubMed] [Google Scholar]
- 965. Thomas RC. Ionic mechanism of the H+ pump in a snail neurone. Nature 262: 54–55, 1976. [DOI] [PubMed] [Google Scholar]
- 966. Thomas RC. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol 273: 317–338, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 967. Thornell IM, Wu J, Bevensee MO. The IP3 receptor-binding protein IRBIT reduces phosphatidylinositol 4,5-bisphosphate (PIP2) stimulation of Na/bicarbonate cotransporter NBCe1 variants expressed in Xenopus laevis oocytes. FASEB J 24: 815.6, 2010. [Google Scholar]
- 968. Thornell IM, Wu J, Liu X, Bevensee MO. PIP2 hydrolysis stimulates electrogenic Na/bicarbonate cotransporter NBCe1-B and -C variants expressed in Xenopus laevis oocytes. J Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969. Thouverey C, Malinowska A, Balcerzak M, Strzelecka-Kiliszek A, Buchet R, Dadlez M, Pikula S. Proteomic characterization of biogenesis and functions of matrix vesicles released from mineralizing human osteoblast-like cells. J Proteomics 74: 1123–1134, 2011. [DOI] [PubMed] [Google Scholar]
- 970. Tian B, Pan Z, Lee JY. Widespread mRNA polyadenylation events in introns indicate dynamic interplay between polyadenylation and splicing. Genome Res 17: 156–165, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971. Tietz PS, Marinelli RA, Chen XM, Huang B, Cohn J, Kole J, McNiven MA, Alper SL, LaRusso NF. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem 278: 20413–20419, 2003. [DOI] [PubMed] [Google Scholar]
- 972. Tillisch JH, Langer GA. Myocardial mechanical responses and ionic exchange in high-sodium perfusate. Circ Res 40: 40–50, 1974. [DOI] [PubMed] [Google Scholar]
- 973. Tobey NA, Reddy SP, Khalbuss WE, Silvers SM, Cragoe EJ, Jr, Orlando RC. Na+-dependent and -independent Cl−/HCO3− exchangers in cultured rabbit esophageal epithelial cells. Gastroenterology 104: 185–195, 1993. [DOI] [PubMed] [Google Scholar]
- 974. Torrubia JO, Garay R. Evidence for a major route for zinc uptake in human red blood cells: [Zn(HCO3)2Cl]− influx through the [Cl−/HCO3−] anion exchanger. J Cell Physiol 138: 316–322, 1989. [DOI] [PubMed] [Google Scholar]
- 975. Toye AM, Banting G, Tanner MJ. Regions of human kidney anion exchanger 1 (kAE1) required for basolateral targeting of kAE1 in polarised kidney cells: mis-targeting explains dominant renal tubular acidosis (dRTA). J Cell Sci 117: 1399–1410, 2004. [DOI] [PubMed] [Google Scholar]
- 976. Toye AM, Bruce LJ, Unwin RJ, Wrong O, Tanner MJ. Band 3 Walton, a C-terminal deletion associated with distal renal tubular acidosis, is expressed in the red cell membrane but retained internally in kidney cells. Blood 99: 342–347, 2002. [DOI] [PubMed] [Google Scholar]
- 977. Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol 291: C788–C801, 2006. [DOI] [PubMed] [Google Scholar]
- 978. Trevani AS, Andonegui G, Giordano M, Lopez DH, Gamberale R, Minucci F, Geffner JR. Extracellular acidification induces human neutrophil activation. J Immunol 162: 4849–4857, 1999. [PubMed] [Google Scholar]
- 979. Tsai CS, Loh SH, Jin JS, Hong GJ, Lin HT, Chiung CS, Chang CY. Effects of alcohol on intracellular pH regulators and electromechanical parameters in human myocardium. Alcohol Clin Exp Res 29: 1787–1795, 2005. [DOI] [PubMed] [Google Scholar]
- 980. Tsao N, Lei HY. Activation of the Na+/H+ antiporter, Na+/HCO3−/CO32− cotransporter, or Cl−/HCO3− exchanger in spontaneous thymocyte apoptosis. J Immunol 157: 1107–1116, 1996. [PubMed] [Google Scholar]
- 981. Tse WK, Chow SC, Lai KP, Au DW, Wong CK. Modulation of ion transporter expression in gill mitochondrion-rich cells of eels acclimated to low-Na+ or-Cl− freshwater. J Exp Zool A 315: 385–393, 2011. [DOI] [PubMed] [Google Scholar]
- 982. Tsuganezawa H, Kobayashi K, Iyori M, Araki T, Koizumi A, Watanabe SI, Kaneko A, Fukao T, Monkawa T, Yoshida T, Kim DK, Kanai Y, Endou H, Hayashi M, Saruta T. A new member of the HCO3− transporter superfamily is an apical anion exchanger of β-intercalated cells in the kidney. J Biol Chem 276: 8180–8189, 2000. [DOI] [PubMed] [Google Scholar]
- 983. Tsuzaka K, Nozaki K, Kumazawa C, Shiraishi K, Setoyama Y, Yoshimoto K, Suzuki K, Abe T, Takeuchi T. DNA microarray gene expression profile of T cells with the splice variants of TCRzeta mRNA observed in systemic lupus erythematosus. J Immunol 176: 949–956, 2006. [DOI] [PubMed] [Google Scholar]
- 984. Tuo BG, Sellers ZM, Smith AJ, Barrett KE, Isenberg JI, Dong H. A role for CagA/VacA in Helicobacter pylori inhibition of murine duodenal mucosal bicarbonate secretion. Dig Dis Sci 49: 1845–1852, 2004. [DOI] [PubMed] [Google Scholar]
- 985. Turner HC, Alvarez LJ, Candia OA. Identification and localization of acid-base transporters in the conjunctival epithelium. Exp Eye Res 72: 519–531, 2001. [DOI] [PubMed] [Google Scholar]
- 986. Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987. Uriarte I, Banales JM, Saez E, Arenas F, Oude Elferink RP, Prieto J, Medina JF. Bicarbonate secretion of mouse cholangiocytes involves Na+-HCO3− cotransport in addition to Na+-independent Cl−/HCO3− exchange. Hepatology 51: 891–902, 2009. [DOI] [PubMed] [Google Scholar]
- 988. Urzúa B, Ortega-Pinto A, Morales-Bozo I, Rojas-Alcayaga G, Cifuentes V. Defining a new candidate gene for amelogenesis imperfecta: from molecular genetics to biochemistry. Biochem Genet 49: 104–121, 2010. [DOI] [PubMed] [Google Scholar]
- 989. Usui T, Hara M, Satoh H, Moriyama N, Kagaya H, Amano S, Oshika T, Ishii Y, Ibaraki N, Hara C, Kunimi M, Noiri E, Tsukamoto K, Inatomi J, Kawakami H, Endou H, Igarashi T, Goto A, Fujita T, Araie M, Seki G. Molecular basis of ocular abnormalities associated with proximal renal tubular acidosis. J Clin Invest 108: 107–115, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990. Usui T, Seki G, Amano S, Oshika T, Miyata K, Kunimi M, Taniguchi S, Uwatoko S, Fujita T, Araie M. Functional and molecular evidence for Na+-HCO3− cotransporter in human corneal endothelial cells. Pflügers Arch 438: 458–462, 1999. [DOI] [PubMed] [Google Scholar]
- 991. Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci 113: 377–381, 2000. [DOI] [PubMed] [Google Scholar]
- 992. Vaccaro P, Dente L. PDZ domains: troubles in classification. FEBS Lett 512: 345–346, 2002. [DOI] [PubMed] [Google Scholar]
- 993. Van Borren MM, Baartscheer A, Wilders R, Ravesloot JH. NHE-1 and NBC during pseudo-ischemia/reperfusion in rabbit ventricular myocytes. J Mol Cell Cardiol 37: 567–577, 2004. [DOI] [PubMed] [Google Scholar]
- 994. Van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP. The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci USA 102: 17507–17512, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 995. Vaughan-Jones RD. Chloride activity and its control in skeletal and cardiac muscle. Philos Trans R Soc Lond B Biol Sci 299: 537–548, 1982. [DOI] [PubMed] [Google Scholar]
- 996. Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol 46: 318–331, 2009. [DOI] [PubMed] [Google Scholar]
- 997. Vaughan-Jones RD, Villafuerte FC, Swietach P, Yamamoto T, Rossini A, Spitzer KW. pH-regulated Na+ influx into the mammalian ventricular myocyte: the relative role of Na+-H+ exchange and Na+-HCO3− co-transport. J Cardiovasc Electrophysiol 17 Suppl 1: S134–S140, 2006. [DOI] [PubMed] [Google Scholar]
- 998. Vazquez JJ, Vazquez M, Idoate MA, Montuenga L, Martinez-Anso E, Castillo JE, Garcia N, Medina JF, Prieto J. Anion exchanger immunoreactivity in human salivary glands in health and Sjogren's syndrome. Am J Pathol 146: 1422–1432, 1995. [PMC free article] [PubMed] [Google Scholar]
- 999. Velic A, Hirsch JR, Bartel J, Thomas R, Schroter R, Stegemann H, Edemir B, August C, Schlatter E, Gabriels G. Renal transplantation modulates expression and function of receptors and transporters of rat proximal tubules. J Am Soc Nephrol 15: 967–977, 2004. [DOI] [PubMed] [Google Scholar]
- 1000. Ventura SC, Northrup TE, Schneider G, Cohen JJ, Garella S. Transport and histochemical studies of bicarbonate handling by the alligator kidney. Am J Physiol Renal Fluid Electrolyte Physiol 256: F239–F245, 1989. [DOI] [PubMed] [Google Scholar]
- 1001. Verkman AS, Dix JA, Solomon AK. Anion transport inhibitor binding to band 3 in red blood cell membranes. J Gen Physiol 81: 421–449, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1002. Verlander JW, Madsen KM, Cannon JK, Tisher CC. Activation of acid-secreting intercalated cells in rabbit collecting duct with ammonium chloride loading. Am J Physiol Renal Fluid Electrolyte Physiol 266: F633–F645, 1994. [DOI] [PubMed] [Google Scholar]
- 1003. Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, Enerback S. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS ONE 4: e4471, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1004. Vilas GL, Johnson DE, Freund P, Casey JR. Characterization of an epilepsy-associated variant of the human Cl−/HCO3− exchanger AE3. Am J Physiol Cell Physiol 297: C526–C536, 2009. [DOI] [PubMed] [Google Scholar]
- 1005. Vilas GL, Morgan PE, Loganathan SK, Quon A, Casey JR. A biochemical framework for SLC4A11, the plasma membrane protein defective in corneal dystrophies. Biochemistry 50: 2157–2169, 2011. [DOI] [PubMed] [Google Scholar]
- 1006. Villa-Abrille MC, Petroff MG, Aiello EA. The electrogenic Na+/HCO3− cotransport modulates resting membrane potential and action potential duration in cat ventricular myocytes. J Physiol 578: 819–829, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1007. Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl−/HCO3− anion exchanger AE1. Biochemistry 39: 5527–5533, 2000. [DOI] [PubMed] [Google Scholar]
- 1008. Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol 285: C771–C780, 2003. [DOI] [PubMed] [Google Scholar]
- 1009. Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3− cotransporter (NBCe2). Am J Physiol Cell Physiol 282: C1278–C1289, 2002. [DOI] [PubMed] [Google Scholar]
- 1010. Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DTH, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VHK, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystorphy (CHED2). Nat Genet 2006. [DOI] [PubMed] [Google Scholar]
- 1011. Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DTH, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VHK, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet 38: 755–757, 2006. [DOI] [PubMed] [Google Scholar]
- 1012. Vithana EN, Morgan PE, Ramprasad V, Tan DT, Yong VH, Venkataraman D, Venkatraman A, Yam GH, Nagasamy S, Law RW, Rajagopal R, Pang CP, Kumaramanickevel G, Casey JR, Aung T. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet 17: 656–666, 2008. [DOI] [PubMed] [Google Scholar]
- 1013. Vorum H, Aalkjaer C, Hager H, Nielsen S, Maunsbach AB. Electrogenic Na+/HCO3− cotransporter rkNBC1 immunolocalized in rat eye. Ann NY Acad Sci 986: 646–648, 2003. [DOI] [PubMed] [Google Scholar]
- 1014. Vorum H, Kwon TH, Fulton C, Simonsen B, Choi I, Boron W, Maunsbach AB, Nielsen S, Aalkjær C. Immunolocalization of electroneutral Na-HCO3− cotransporter in rat kidney. Am J Physiol Renal Physiol 279: F901–F909, 2000. [DOI] [PubMed] [Google Scholar]
- 1015. Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar-ATPase. Physiol Rev 84: 1263–1314, 2004. [DOI] [PubMed] [Google Scholar]
- 1016. Wagner CA, Mohebbi N, Capasso G, Geibel JP. The anion exchanger pendrin (SLC26A4) and renal acid-base homeostasis. Cell Physiol Biochem 497–504, 2011. [DOI] [PubMed] [Google Scholar]
- 1017. Wakabayashi S, Shigekawa M, Pouysségur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev 77: 51–74, 1997. [DOI] [PubMed] [Google Scholar]
- 1018. Waldegger S, Fakler B, Bleich M, Barth P, Hopf A, Schulte U, Busch AE, Aller SG, Forrest JN, Jr, Greger R, Lang F. Molecular and functional characterization of s-KCNQ1 potassium channel from rectal gland of Squalus acanthias. Pflügers Arch 437: 298–304, 1999. [DOI] [PubMed] [Google Scholar]
- 1019. Walker MN, Flagella M, Gawenis LR, Shull GE, Clarke LL. An alternate pathway of cAMP-stimulated Cl secretion across the NKCC1-null murine duodenum. Gastroenterology 123: 531–541, 2002. [DOI] [PubMed] [Google Scholar]
- 1020. Wall SM, Pech V. Pendrin and sodium channels: relevance to hypertension. J Nephrol 23 Suppl 16: S118–S123, 2010. [PubMed] [Google Scholar]
- 1021. Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl−/HCO3− exchanger: cloning, tissue distribution, and functional characterization. J Biol Chem 275: 35486–35490, 2000. [DOI] [PubMed] [Google Scholar]
- 1022. Wang DN, Kuhlbrandt W, Sarabia VE, Reithmeier RA. Two-dimensional structure of the membrane domain of human band 3, the anion transport protein of the erythrocyte membrane. EMBO J 12: 2233–2239, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1023. Wang DN, Sarabia VE, Reithmeier RA, Kuhlbrandt W. Three-dimensional map of the dimeric membrane domain of the human erythrocyte anion exchanger, Band 3. EMBO J 13: 3230–3235, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1024. Wang G, Li C, Kim SW, Ring T, Wen J, Djurhuus JC, Wang W, Nielsen S, Frokiær J. Ureter obstruction alters expression of renal acid-base transport proteins in rat kidney. Am J Physiol Renal Physiol 295: F497–F506, 2008. [DOI] [PubMed] [Google Scholar]
- 1025. Wang G, Topcu SO, Ring T, Wen J, Djurhuus JC, Kwon TH, Nielsen S, Frokiær J. Age-dependent renal expression of acid-base transporters in neonatal ureter obstruction. Pediatr Nephrol 24: 1487–1500, 2009. [DOI] [PubMed] [Google Scholar]
- 1026. Wang W, Praetorius J, Li C, Praetorius HA, Kwon TH, Frokiær J, Nielsen S. Vacuolar H+-ATPase expression is increased in acid-secreting intercalated cells in kidneys of rats with hypercalcaemia-induced alkalosis. Acta Physiol 189: 359–368, 2007. [DOI] [PubMed] [Google Scholar]
- 1027. Wang XF, Yu MK, Leung KM, Yip CY, Ko WH, Liu CQ, Chan HC. Involvement of Na+-HCO3− cotransporter in mediating cyclic adenosine 3′,5′-monophosphate-dependent HCO3− secretion by mouse endometrial epithelium. Biol Reprod 66: 1846–1852, 2002. [DOI] [PubMed] [Google Scholar]
- 1028. Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J 25: 5049–5057, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029. Wang Z, Conforti L, Petrovic S, Amlal H, Burnham CE, Soleimani M. Mouse Na+:HCO3− cotransporter isoform NBC-3 (kNBC-3): cloning, expression, and renal distribution. Kidney Int 59: 1405–1414, 2001. [DOI] [PubMed] [Google Scholar]
- 1030. Wang Z, Schultheis PJ, Shull GE. Three N-terminal variants of the AE2 Cl−/HCO3− exchanger are encoded by mRNAs transcribed from alternative promoters. J Biol Chem 271: 7835–7843, 1996. [DOI] [PubMed] [Google Scholar]
- 1031. Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, Liu J, Zhao XD, Chew JL, Lee YL, Kuznetsov VA, Sung WK, Miller LD, Lim B, Liu ET, Yu Q, Ng HH, Ruan Y. A global map of p53 transcription-factor binding sites in the human genome. Cell 124: 207–219, 2006. [DOI] [PubMed] [Google Scholar]
- 1032. Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci USA 101: 3621–3626, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1033. Whitfield JB, Dy V, McQuilty R, Zhu G, Heath AC, Montgomery GW, Martin NG. Genetic effects on toxic and essential elements in humans: arsenic, cadmium, copper, lead, mercury, selenium and zinc in erythrocytes. Environ Health Perspect 118: 776–782, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1034. Whitfield JB, Dy V, McQuilty R, Zhu G, Montgomery GW, Ferreira MA, Duffy DL, Neale MC, Heijmans BT, Heath AC, Martin NG. Evidence of genetic effects on blood lead accumulation. Environ Health Perspect 115: 1224–1230, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1035. Wiederholt M, Jentsch TJ, Keller SK. Electrogenic sodium-bicarbonate symport in cultured corneal endothelial cells. Pflügers Arch 405: S167–S171, 1985. [DOI] [PubMed] [Google Scholar]
- 1036. Williamson RC, Toye AM. Glycophorin A: band 3 aid. Blood Cells Mol Dis 41: 35–43, 2008. [DOI] [PubMed] [Google Scholar]
- 1037. Wilson RW, Wilson JM, Grosell M. Intestinal bicarbonate secretion by marine teleost fish–why and how? Biochim Biophys Acta 1566: 182–193, 2002. [DOI] [PubMed] [Google Scholar]
- 1038. Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1039. Wolosin JM, Alvarez LJ, Candia OA. HCO3− transport in the toad lens epithelium is mediated by an electronegative Na+-dependent symport. Am J Physiol Cell Physiol 258: C855–C861, 1990. [DOI] [PubMed] [Google Scholar]
- 1040. Wolosin JM, Ginsburg H, Cabantchik ZI. Functional characterization of anion transport system isolated from human erythrocyte membranes. J Biol Chem 252: 2419–2427, 1977. [PubMed] [Google Scholar]
- 1041. Wong P, Kleemann HW, Tannock IF. Cytostatic potential of novel agents that inhibit the regulation of intracellular pH. Br J Cancer 87: 238–245, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1042. Wood CM, Munger RS, Thompson J, Shuttleworth TJ. Control of rectal gland secretion by blood acid-base status in the intact dogfish shark (Squalus acanthias). Respir Physiol Neurobiol 156: 220–228, 2007. [DOI] [PubMed] [Google Scholar]
- 1043. Wood CM, Part P. Intracellular pH regulation and buffer capacity in CO2/HCO3− buffered media in cultured epithelial cells from rainbow trout gills. J Comp Physiol B 170: 175–184, 2000. [DOI] [PubMed] [Google Scholar]
- 1044. Wood PG, Muller H, Sovak M, Passow H. Role of Lys 558 and Lys 869 in substrate and inhibitor binding to the murine band 3 protein: a study of the effects of site-directed mutagenesis of the band 3 protein expressed in the oocytes of Xenopus laevis. J Membr Biol 127: 139–148, 1992. [DOI] [PubMed] [Google Scholar]
- 1045. Wray S, Smith RD. Mechanisms of action of pH-induced effects on vascular smooth muscle. Mol Cell Biochem 263: 163–172, 2004. [DOI] [PubMed] [Google Scholar]
- 1046. Wu C, Wang S. A pH-sensitive heparin-binding sequence from Baculovirus gp64 protein is important for binding to mammalian cells but not to Sf9 insect cells. J Virol 86: 484–491, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1047. Wu F, Mi W, Burns DK, Fu Y, Gray HF, Struyk AF, Cannon SC. A sodium channel knockin mutant (NaV1.4–R669H) mouse model of hypokalemic periodic paralysis. J Clin Invest 121: 4082–4094, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1048. Wu J, Glimcher LH, Aliprantis AO. HCO3−/Cl− anion exchanger SLC4A2 is required for proper osteoclast differentiation and function. Proc Natl Acad Sci USA 105: 16934–16939, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1049. Wu J, McNicholas CM, Bevensee MO. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates the electrogenic Na/HCO3 cotransporter NBCe1-A expressed in Xenopus oocytes. Proc Natl Acad Sci USA 106: 14150–14155, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050. Wu W, Rychkov GY, Hughes BP, Bretag AH. Functional complementation of truncated human skeletal-muscle chloride channel (hClC-1) using carboxyl tail fragments. Biochem J 395: 89–97, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1051. Xia Y, Zhao P, Xue J, Gu XQ, Sun X, Yao H, Haddad GG. Na+ channel expression and neuronal function in the Na+/H+ exchanger 1 null mutant mouse. J Neurophysiol 89: 229–236, 2003. [DOI] [PubMed] [Google Scholar]
- 1052. Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002. [DOI] [PubMed] [Google Scholar]
- 1053. Xu H, Cui N, Yang Z, Wu J, Giwa LR, Abdulkadir L, Sharma P, Jiang C. Direct activation of cloned K(atp) channels by intracellular acidosis. J Biol Chem 276: 12898–12902, 2001. [DOI] [PubMed] [Google Scholar]
- 1054. Xu J, Barone S, Petrovic S, Wang Z, Seidler U, Riederer B, Ramaswamy K, Dudeja PK, Shull GE, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in gastric surface mucous and duodenal villus cells. Am J Physiol Gastrointest Liver Physiol 285: G1225–G1234, 2003. [DOI] [PubMed] [Google Scholar]
- 1055. Xu J, Henriksnäs J, Barone S, Witte D, Shull GE, Forte JG, Holm L, Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl−/HCO3− exchange, and is inhibited by NH4+. Am J Physiol Cell Physiol 289: C493–C505, 2005. [DOI] [PubMed] [Google Scholar]
- 1056. Xu J, Wang Z, Barone S, Petrovic M, Amlal H, Conforti L, Petrovic S, Soleimani M. Expression of the Na+-HCO3− cotransporter NBC4 in rat kidney and characterization of a novel NBC4 variant. Am J Physiol Renal Physiol 284: F41–F50, 2003. [DOI] [PubMed] [Google Scholar]
- 1057. Xu L, Glassford AJ, Giaccia AJ, Giffard RG. Acidosis reduces neuronal apoptosis. NeuroReport 9: 875–879, 1998. [DOI] [PubMed] [Google Scholar]
- 1058. Xu T, Su H, Ganapathy S, Yuan ZM. Modulation of autophagic activity by extracellular pH. Autophagy 7: 1316–1322, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059. Xue H, Tian YM, Yan M, Yang N, Chen X, Xing Y, Zhu JX. Appearance of segmental discrepancy of anion transport in rat distal colon. Biol Pharm Bull 30: 1407–1411, 2007. [DOI] [PubMed] [Google Scholar]
- 1060. Xue J, Douglas RM, Zhou D, Lim JY, Boron WF, Haddad GG. Expression of Na+/H+ and HCO3−-dependent transporters in Na+/H+ exchanger isoform 1 null mutant mouse brain. Neuroscience 122: 37–46, 2003. [DOI] [PubMed] [Google Scholar]
- 1061. Xue J, Mraiche F, Zhou D, Karmazyn M, Oka T, Fliegel L, Haddad GG. Elevated myocardial Na+/H+ exchanger isoform 1 activity elicits gene expression that leads to cardiac hypertrophy. Physiol Genomics 42: 374–383, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1062. Xue L, Aihara E, Wang TC, Montrose MH. Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair. J Biol Chem 286: 38375–38382, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1063. Yamada H, Horita S, Suzuki M, Fujita T, Seki G. Functional role of a putative carbonic anhydrase II-binding domain in the electrogenic Na+-HCO3− cotransporter NBCe1 expressed in Xenopus oocytes. Channels 5: 106–109, 2011. [DOI] [PubMed] [Google Scholar]
- 1064. Yamada H, Yamazaki S, Moriyama N, Hara C, Horita S, Enomoto Y, Kudo A, Kawakami H, Tanaka Y, Fujita T, Seki G. Localization of NBC-1 variants in human kidney and renal cell carcinoma. Biochem Biophys Res Commun 310: 1213–1218, 2003. [DOI] [PubMed] [Google Scholar]
- 1065. Yamaguchi S, Ishikawa T. Electrophysiological characterization of native Na+-HCO3− cotransporter in bovine parotid acinar cells. J Physiol 568: 181–197, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1066. Yamaguchi S, Ishikawa T. The electrogenic Na+-HCO3− cotransporter is regulated by intracellular Mg2+. Biochem Biophys Res Commun 376: 100–104, 2008. [DOI] [PubMed] [Google Scholar]
- 1067. Yamaguchi S, Ishikawa T. IRBIT reduces the apparent affinity for intracellular Mg2+ in inhibition of the electrogenic Na+-HCO3− cotransporter NBCe1-B. Biochem Biophys Res Commun 424: 433–438, 2012. [DOI] [PubMed] [Google Scholar]
- 1068. Yamaguchi T, Fujii T, Abe Y, Hirai T, Kang D, Namba K, Hamasaki N, Mitsuoka K. Helical image reconstruction of the outward-open human erythrocyte band 3 membrane domain in tubular crystals. J Struct Biol 169: 406–412, 2010. [DOI] [PubMed] [Google Scholar]
- 1069. Yamaguchi T, Ikeda Y, Abe Y, Kuma H, Kang D, Hamasaki N, Hirai T. Structure of the membrane domain of human erythrocyte anion exchanger 1 revealed by electron crystallography. J Mol Biol 397: 179–189, 2010. [DOI] [PubMed] [Google Scholar]
- 1070. Yamahiro A, Piermarini PM, Beyenbach KW. Identification of Na-driven anion exchanger (NDAE) splice variants from Malphigian (renal) tubules of the adult yellow-fever mosquito (Abstract). FASEB J 22: 757.22, 2008. [Google Scholar]
- 1071. Yamamoto T, Shirayama T, Sakatani T, Takahashi T, Tanaka H, Takamatsu T, Spitzer KW, Matsubara H. Enhanced activity of ventricular Na+-HCO3− cotransport in pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 293: H1254–H1264, 2007. [DOI] [PubMed] [Google Scholar]
- 1072. Yamamoto T, Swietach P, Rossini A, Loh SH, Vaughan-Jones RD, Spitzer KW. Functional diversity of electrogenic Na+-HCO3− cotransport in ventricular myocytes from rat, rabbit and guinea pig. J Physiol 562: 455–475, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1073. Yamomoto H, Wilson T, Omelchenko I, Zhang Y, Nakashima T, Shi X, Nuttall AL. Acoustic trauma reduces SLC4A11 expression in the mouse cochlea. FASEB J 26: 694.7, 2012. [Google Scholar]
- 1074. Yanaka A, Carter KJ, Goddard PJ, Silen W. Effect of luminal acid on intracellular pH in oxynticopeptic cells in intact frog gastric mucosa. Gastroenterology 100: 606–618, 1991. [DOI] [PubMed] [Google Scholar]
- 1075. Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 121: 956–965, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1076. Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest 119: 193–202, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1077. Yang HC, Liang YJ, Chen JW, Chiang KM, Chung CM, Ho HY, Ting CT, Lin TH, Sheu SH, Tsai WC, Chen JH, Leu HB, Yin WH, Chiu TY, Chern CI, Lin SJ, Tomlinson B, Guo Y, Sham PC, Cherny SS, Lam TH, Thomas GN, Pan WH. Identification of IGF1, SLC4A4, WWOX, and SFMBT1 as hypertension susceptibility genes in Han Chinese with a genome-wide gene-based association study. PLoS ONE 7: e32907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1078. Yang HS, Cooper DS, Rajbhandari I, Park HJ, Lee S, Choi I. Inhibition of rat Na+/HCO3− cotransporter (NBCn1) function and expression by the alternative splice domain. Exp Physiol 94: 1114–1123, 2009. [DOI] [PubMed] [Google Scholar]
- 1079. Yang Z, Alvarez BV, Chakarova C, Jiang L, Karan G, Frederick JM, Zhao Y, Sauvé Y, Li X, Zrenner E, Wissinger B, Hollander AI, Katz B, Baehr W, Cremers FP, Casey JR, Bhattacharya SS, Zhang K. Mutant carbonic anhydrase 4 impairs pH regulation and causes retinal photoreceptor degeneration. Nat Genet 14: 255–265, 2005. [DOI] [PubMed] [Google Scholar]
- 1080. Yannoukakos D, Stuart-Tilley A, Fernandez HA, Fey P, Duyk G, Alper SL. Molecular cloning, expression and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res 75: 603–614, 1994. [DOI] [PubMed] [Google Scholar]
- 1081. Yano H, Wang C, Yamashita S, Yokoyama Y, Yokoi N, Seino S. Assignment of the human solute carrier family 4, sodium bicarbonate cotransporter-like, member 10 gene (SLC4A10) to 2q23-q24 by in situ hybridization and radiation hybrid mapping. Cytogenet Cell Genet 89: 276–277, 2000. [DOI] [PubMed] [Google Scholar]
- 1082. Yao H, Ma E, Gu XQ, Haddad GG. Intracellular pH regulation of CA1 neurons in Na+/H+ isoform 1 mutant mice. J Clin Invest 104: 637–645, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1083. Yip KP, Tsuruoka S, Schwartz GJ, Kurtz I. Apical H+/base transporters mediating bicarbonate absorption and pHi regulation in the OMCD. Am J Physiol Renal Physiol 283: F1098–F1104, 2002. [DOI] [PubMed] [Google Scholar]
- 1084. Yoo SH, Lewis MS. Effects of pH and Ca2+ on monomer-dimer and monomer-tetramer equilibria of chromogranin A. J Biol Chem 267: 11236–11241, 1992. [PubMed] [Google Scholar]
- 1085. Yoshitomi K, Burckhardt BC, Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflügers Arch 405: 360–366, 1985. [DOI] [PubMed] [Google Scholar]
- 1086. Yoshitomi K, Frömter E. How big is the electrochemical potential difference of Na+ across rat renal proximal tubular cell membranes in vivo? Pflügers Arch 405: S121–S126, 1985. [DOI] [PubMed] [Google Scholar]
- 1087. Yu H, Riederer B, Stieger N, Boron WF, Shull GE, Manns MP, Seidler UE, Bachmann O. Secretagogue stimulation enhances NBCe1 (electrogenic Na+/HCO3− cotransporter) surface expression in murine colonic crypts. Am J Physiol Gastrointest Liver Physiol 297: G1223–G1231, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1088. Yucha CB, Stoner LC. Bicarbonate transport by amphibian nephron. Am J Physiol Renal Fluid Electrolyte Physiol 251: F865–F872, 1986. [DOI] [PubMed] [Google Scholar]
- 1089. Zeng C, Han Y, Shi L, Peng L, Wang Y, Xu F, Meng J. Genetic analysis of the physiological responses to low boron stress in Arabidopsis thaliana. Plant Cell Environ 31: 112–122, 2008. [DOI] [PubMed] [Google Scholar]
- 1090. Zeng Y, Oberdorf JA, Florman HM. pH regulation in mouse sperm: identification of Na+-, Cl−-, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol 173: 510–520, 1996. [DOI] [PubMed] [Google Scholar]
- 1091. Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood 96: 2925–2933, 2000. [PubMed] [Google Scholar]
- 1092. Zhang H, Ameen N, Melvin JE, Vidyasagar S. Acute inflammation alters bicarbonate transport in mouse ileum. J Physiol 581: 1221–1233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1093. Zhang K, Yin L, Zhang M, Parker MD, Binder HJ, Salzman P, Zhang L, Okunieff P, Vidyasagar S. Radiation decreases murine small intestinal HCO3− secretion. Int J Radiat Biol 87: 878–888, 2011. [DOI] [PubMed] [Google Scholar]
- 1094. Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 121: 2845–2854, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1095. Zhang Y, Chernova MN, Stuart-Tilley AK, Jiang L, Alper SL. The cytoplasmic and transmembrane domains of AE2 both contribute to regulation of anion exchange by pH. J Biol Chem 271: 5741–5749, 1996. [DOI] [PubMed] [Google Scholar]
- 1096. Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. J Gen Physiol 104: 57–85, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1097. Zhao J, Hogan EM, Bevensee MO, Boron WF. Out-of-equilibrium CO2/HCO3− solutions and their use in characterizing a new K/HCO3 cotransporter. Nature 374: 636–639, 1995. [DOI] [PubMed] [Google Scholar]
- 1098. Zhao R, Reithmeier RA. Expression and characterization of the anion transporter homologue YNL275w in Saccharomyces cerevisiae. Am J Physiol Cell Physiol 281: C33–C45, 2001. [DOI] [PubMed] [Google Scholar]
- 1099. Zheng L, Zhang Y, He P, Kim J, Schneider R, Bronckers AL, Lyaruu DM, Denbesten PK. NBCe1 in mouse and human ameloblasts may be indirectly regulated by fluoride. J Dent Res 90: 782–787, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1100. Zheng Y, Horita S, Hara C, Kunimi M, Yamada H, Sugaya T, Goto A, Fujita T, Seki G. Biphasic regulation of renal proximal bicarbonate absorption by luminal AT(1A) receptor. J Am Soc Nephrol 14: 1116–1122, 2003. [DOI] [PubMed] [Google Scholar]
- 1101. Zhou L, Irwin DM. Fish proglucagon genes have differing coding potential. Comp Biochem Physiol B Biochem Mol Biol 137: 255–264, 2004. [DOI] [PubMed] [Google Scholar]
- 1102. Zhou X, Vize PD. Proximo-distal specialization of epithelial transport processes within the Xenopus pronephric kidney tubules. Dev Biol 271: 322–338, 2004. [DOI] [PubMed] [Google Scholar]
- 1103. Zhou X, Vize PD. Pronephric regulation of acid-base balance; coexpression of carbonic anhydrase type 2 and sodium-bicarbonate cotransporter-1 in the late distal segment. Dev Dyn 233: 142–144, 2005. [DOI] [PubMed] [Google Scholar]
- 1104. Zhou Y, Boron WF. Role of endogenously secreted angiotensin II in the CO2-induced stimulation of HCO3 reabsorption by renal proximal tubules. Am J Physiol Renal Physiol 294: F245–F252, 2008. [DOI] [PubMed] [Google Scholar]
- 1105. Zhou Y, Boron WF. Effect of knocking out receptor protein tyrosine phosphatase g (RPTP g) in the CO2-induced stimulation of HCO3 reabsorption by mouse renal proximal tubule (Abstract). FASEB J 24: 1024.7 2010. [Google Scholar]
- 1106. Zhou Y, Bouyer P, Boron WF. Effects of angiotensin II on the CO2 dependence of HCO3− reabsorption by the rabbit S2 renal proximal tubule. Am J Physiol Renal Physiol 290: F666–F673, 2006. [DOI] [PubMed] [Google Scholar]
- 1107. Zhou Y, Bouyer P, Boron WF. Role of the AT1A receptor in the CO2-induced stimulation of HCO3 reabsorption by renal proximal tubules. Am J Physiol Renal Physiol 293: F110–F120, 2007. [DOI] [PubMed] [Google Scholar]
- 1108. Zhou Y, Zhao J, Bouyer P, Boron WF. Evidence from renal proximal tubules that HCO3− and solute reabsorption are acutely regulated not by pH but by basolateral HCO3− and CO2. Proc Natl Acad Sci USA 102: 3875–3880, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1109. Zhou Z, DeSensi SC, Stein RA, Brandon S, Dixit M, McArdle EJ, Warren EM, Kroh HK, Song L, Cobb CE, Hustedt EJ, Beth AH. Solution structure of the cytoplasmic domain of erythrocyte membrane band 3 determined by site-directed spin labeling. Biochemistry 44: 15115–15128, 2005. [DOI] [PubMed] [Google Scholar]
- 1110. Zhu Q, Azimov R, Kao L, Newman D, Liu W, Abuladze N, Pushkin A, Kurtz I. NBCe1-A transmembrane segment 1 lines the ion translocation pathway. J Biol Chem 284: 8918–8929, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1111. Zhu Q, Azimov R, Kao L, Pushkin A, Kurtz I. Cysteines in extracellular loop 3 of NBCe1-A form intra- and inter-molecular disulfide bonds. FASEB J 23: 800.10, 2009. [Google Scholar]
- 1112. Zhu Q, Kao L, Azimov R, Abuladze N, Newman D, Pushkin A, Liu W, Chang C, Kurtz I. Structural and functional characterization of the C-terminal transmembrane region of NBCe1-A. J Biol Chem 285: 37178–37187, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1113. Zhu Q, Kao L, Azimov R, Newman D, Liu W, Pushkin A, Abuladze N, Kurtz I. Topological location and structural importance of the NBCe1-A residues mutated in proximal renal tubular acidosis. J Biol Chem 285: 13416–13426, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1114. Zhu Q, Kao L, Liu W, Newman D, Kurtz I. Topology determination of the transmembrane domain of the electrogenic sodium bicarbonate cotransporter NBCe1-A. FASEB J 23: 800.7, 2009. [Google Scholar]
- 1115. Zhu Q, Kao L, Liu W, Newman D, Orozco N, Azimov R, Pushkin A, Abuladze N, Kurtz I. Topology of the C-terminal transmembrane region of NBCe1-A differs from AE1. FASEB J 23: 800.8, 2009. [Google Scholar]
- 1116. Zhu Q, Lee DW, Casey JR. Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. J Biol Chem 278: 3112–3120, 2003. [DOI] [PubMed] [Google Scholar]
- 1117. Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, III, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139: 1012–1021, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1118. Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1119. Zuo WL, Li S, Huang JH, Yang DL, Zhang G, Chen SL, Ruan YC, Ye KN, Cheng CH, Zhou WL. Sodium coupled bicarbonate influx regulates intracellular and apical pH in cultured rat caput epididymal epithelium. PLoS ONE 6: e22283, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.