Abstract
Growth hormone (GH) is a key determinant of postnatal growth and plays an important role in the control of metabolism and body composition. Surprisingly, deficiency in GH signaling delays aging and remarkably extends longevity in laboratory mice. In GH-deficient and GH-resistant animals, the “healthspan” is also extended with delays in cognitive decline and in the onset of age-related disease. The role of hormones homologous to insulin-like growth factor (IGF, an important mediator of GH actions) in the control of aging and lifespan is evolutionarily conserved from worms to mammals with some homologies extending to unicellular yeast. The combination of reduced GH, IGF-I, and insulin signaling likely contributes to extended longevity in GH or GH receptor-deficient organisms. Diminutive body size and reduced fecundity of GH-deficient and GH-resistant mice can be viewed as trade-offs for extended longevity. Mechanisms responsible for delayed aging of GH-related mutants include enhanced stress resistance and xenobiotic metabolism, reduced inflammation, improved insulin signaling, and various metabolic adjustments. Pathological excess of GH reduces life expectancy in men as well as in mice, and GH resistance or deficiency provides protection from major age-related diseases, including diabetes and cancer, in both species. However, there is yet no evidence of increased longevity in GH-resistant or GH-deficient humans, possibly due to non-age-related deaths. Results obtained in GH-related mutant mice provide striking examples of mutations of a single gene delaying aging, reducing age-related disease, and extending lifespan in a mammal and providing novel experimental systems for the study of mechanisms of aging.
I. INTRODUCTION
Phenotypic characteristics of all living organisms are determined by the interplay of genetic and environmental influences. This applies to the control of aging as well. There is also increasing evidence that the endocrine system plays a central role in mediating the impact of genetic and environmental factors on aging and longevity. Mice with mutations causing a deficiency of growth hormone (GH) or resistance to its actions are remarkably long-lived (50, 70, 100), and humans with similar mutations are protected from major age-related diseases (115, 207). The involvement of somatotropic (GH-related) signaling as well as secondary alterations in insulin signaling in the control of mammalian aging represents a fundamental and evolutionarily conserved mechanism. Insulin-like growth factor I (IGF-I), insulin, and their receptors are homologous to signaling molecules and receptors that have major impact on aging and longevity in yeast, worms, and insects (114, 153, 206, 299). Since life-extending GH and IGF-I-related mutations reduce or block the corresponding endocrine signals, it is pertinent to ask what trade-offs may be involved in balancing the detrimental, “pro-aging” actions of the somatotropic axis with its beneficial effects on other characteristics.
Against this background, the objectives of this article are to present evidence that reduced somatotropic signaling can delay and/or slow down the aging process and promote a remarkable optimization of the healthy lifespan, to discuss the mechanisms believed to be responsible for these effects, and to relate the findings in mammals to the fundamental genetic control of aging in yeast and invertebrates. We will also describe age-related changes in the GH-IGF-I axis in mammals and discuss the controversial topic of potential benefits of GH therapy in the elderly.
II. OVERVIEW OF THE SOMATOTROPIC AXIS
A. Growth Hormone
Growth hormone (GH), also called somatotropin, is a 22-kDa protein hormone composed of 191 amino acid residues forming a single chain with four helical regions and two disulfide bridges. It is produced and secreted by specialized cells in the anterior lobe of the pituitary gland, the somatotrophs (also referred to as somatotropes). Its secretion is controlled primarily by two hypothalamic peptides: growth hormone releasing hormone (GHRH) and somatostatin (SST, also called somatotropin release inhibiting factor, SRIF). The interplay of GHRH and SST actions and GH/IGF-I feedback on their release produce a pulsatile pattern of GH release with brief secretory episodes followed by gradual clearance from the circulation during interpulse intervals (121). In the human, these pulses occur approximately every 2–2.5 h (307).
Release of GH is strongly associated with sleep, stimulated by hypoglycemia and inhibited by overeating, hyperglycemia, and obesity (68, 121). Many aspects of the regulation of GH secretion are species specific. For example, exercise, starvation, and stress stimulate GH release in different mammalian species including humans (128, 318) and ruminants, but some of the same stimuli inhibit GH release in the rat (298) and may have different effects on GH release at different time points. Interestingly, prolonged calorie restriction preserved pulsatile GH release in the latter species, presumably by attenuating the suppressive effects of aging (277). In mice, fasting was reported to both increase and suppress circulating GH levels with discrepancies between results obtained in different laboratories being possibly related to differences in protocols for blood collection and associated handling of the animals or to time of treatment (191, 281). Key gonadal steroid hormones, testosterone and estradiol, stimulate GH release, and these actions likely account for the prepubertal elevation of GH levels and contribute to the well-known growth spurt during this period.
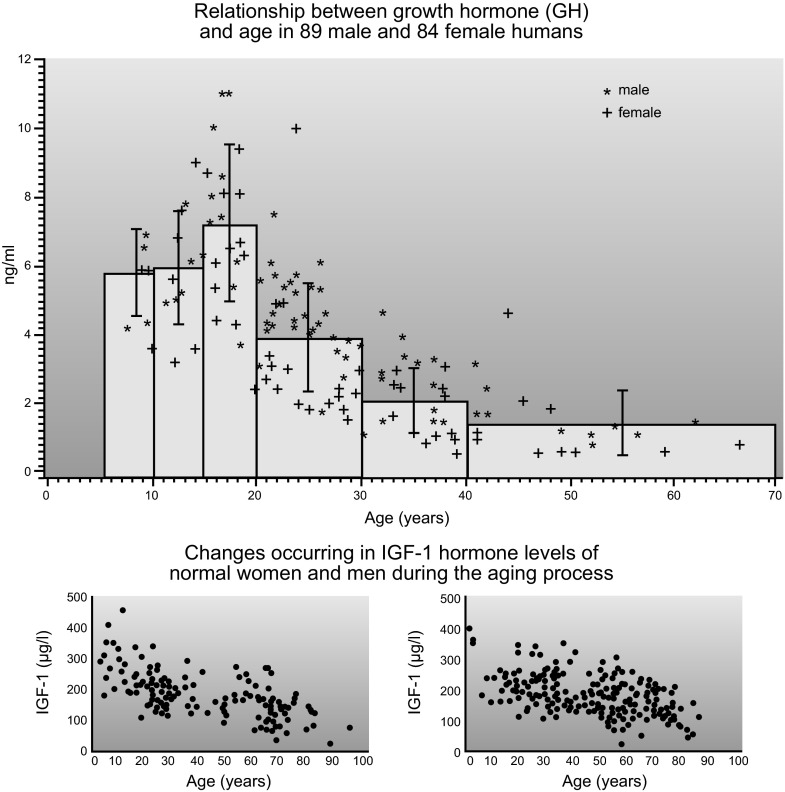
Aging is associated with a pronounced, progressive decline in circulating GH levels which, in the human, is due primarily to reduction in the amount of the hormone released in each secretory burst (309). After age 20, GH secretion declines by approximately one-half every 7–12 yr (107, 215) (FIGURE 1). The age-related decline in GH secretion and average serum levels is accompanied by a less steep but statistically significant decline in serum IGF-I (195) (FIGURE 1). These changes are often referred to as “somatopause,” by analogy with the age-related decline in gonadal function, the menopause and the andropause. Declining GH levels are believed to contribute to concomitant changes in body composition (including increased adiposity and reduced muscle mass) and to other physiological changes that accompany aging. Secretion of GH also declines with age in other species, including the domestic dog (214) and laboratory rats and mice (162, 276). However, recent data from the Jackson Laboratory indicate that in some inbred mouse strains IGF-I levels in old age resemble or exceed those measured in early adulthood (R. Yuan, personal communication). The age-related reduction in GH release led to an interest in using GH to reduce the symptoms of aging. This important and highly controversial issue will be discussed later in this article.
Figure 1.
Age-related decline in circulating levels of human growth hormone (GH) and insulin-like growth factor I (IGF-I).[From Corpas et al. (69) and Zadik et al. (335), with permission from The Endocrine Society.]
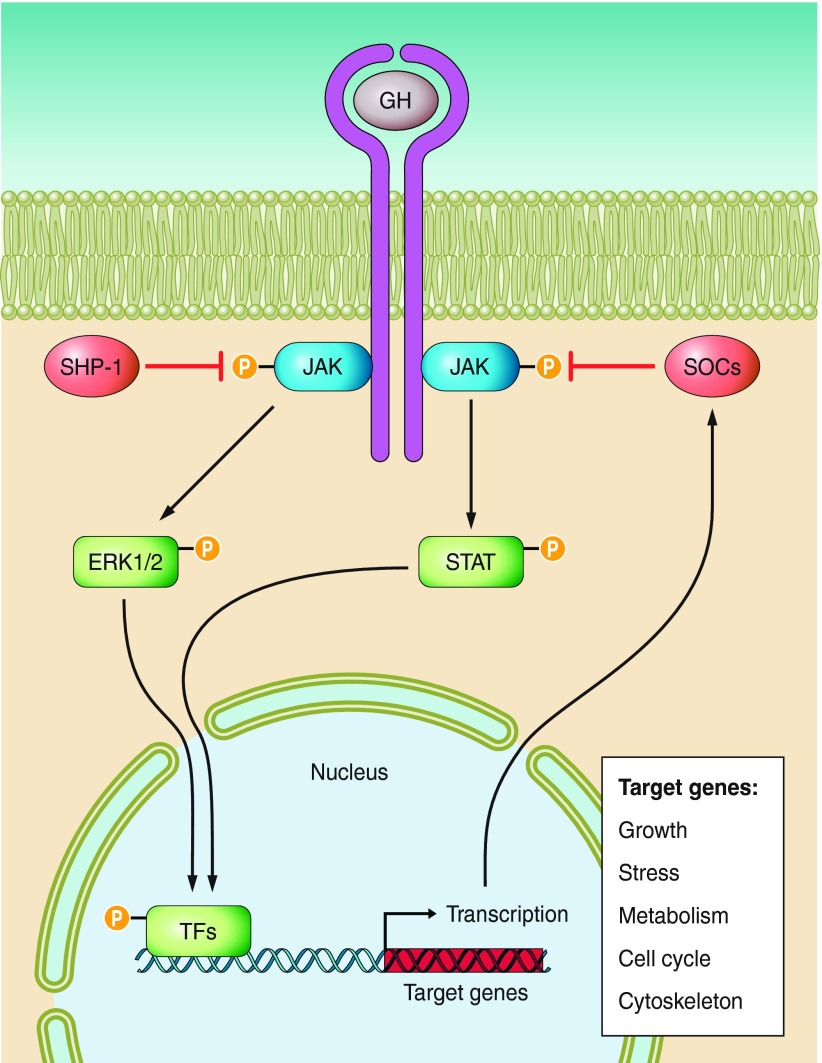
Growth hormone acts by binding to specific cytokine family receptors located in the membrane of target cells (FIGURE 2). GH receptors are present in multiple cell types including hepatocytes, adipocytes, muscle fibers, bone cells, and cells of the immune system (156, 208). Each GH molecule has two receptor binding sites allowing it to interact simultaneously with two GH receptors. GH binding leads to dimerization of GH receptors and activation of the associated JAK2, a member of the Janus family of tyrosine kinases (FIGURE 2). JAK2 activation leads to tyrosine phosphorylation of both JAK2 and the GH receptor. This initiates signaling via several pathways and, in particular, includes signaling via phosphorylation of signal transducers and activators of transcription (STATs). Phosphorylated STATs disassociate from the receptor-JAK2 complex, move to the nucleus, and bind to promoters of GH-regulated genes. In addition, this pathway creates a negative-feedback loop that terminates the GH signal by stimulating the expression of suppressors of cytokine signaling (SOCS) proteins that inhibit JAK activity and STAT activation and promote internalization of the GH receptors.
Figure 2.
Schematic representation of key mechanisms of growth hormone (GH) signaling. GH binds to two transmembrane GH receptors and alters expression of numerous genes via activation of STAT and ERK; details and references are in the text.
GH signaling pathways also include mitogen-activated protein kinase (MAPK) as well as protein kinase B (Akt) and mammalian target of rapamycin (mTOR), one of the Akt targets. For more information on GH signaling, the reader is referred to review articles (45, 157, 166, 316).
Mutations of the gene encoding the GH receptor in humans or targeted disruption of the same gene in mice lead to the loss of receptor function and consequently to GH resistance and severe retardation of postnatal growth. This endocrine syndrome was first described by Laron et al. (172) in 1966 in a patient exhibiting dwarfism and elevated GH levels and became known as Laron dwarfism. In the 1990s, it was produced in mice by Zhou et al. in the Kopchick laboratory (340). Studies in Laron dwarfs and in GH receptor-deleted, Ghr−/− (Laron dwarf) mice provided a wealth of information on the role of GH in aging and will be discussed later in this article.
In the circulation, GH is present in a free form or bound to a binding protein. Growth hormone binding protein (GHBP) in the human and some other species consists of cleaved extracellular fragments of the GH receptor and thus provides a clinically useful estimate of GH receptor abundance. In mice, GHBP is produced separately from the GH receptor by alternate splicing of the same gene (269, 339).
In contrast to steroid as well as most polypeptide hormones, the biological activity of GH exhibits peculiar species specificity. Thus fish, amphibians, and reptiles respond to mammalian GHs, and “lower” mammals respond to primate GH while the opposite is generally not the case (108). In rodents, human GH binds to both GH and prolactin (PRL) receptors and produces a full spectrum of both GH and PRL effects (102, 122, 279). Lactogenic (PRL) activity of human GH in rats and mice has been documented for over 40 years (102, 122) but is overlooked in many recent publications. Very often, the authors focus on the somatotropic (GH) effects of human GH administration to rodents, ignoring the possible contribution of the concomitant activation of PRL receptors and eliciting effects unrelated to somatotropic signaling.
B. Insulin-Like Growth Factor
One of the key actions of GH is stimulation of the hepatic expression of the IGF-I gene. IGF-I, formerly known as somatomedin C, mediates many, although not all, GH actions. Most of the circulating IGF-I is of hepatic origin, but some is derived from adipose tissue and from other sources. Since production of circulating (“endocrine”) IGF-I depends on GH, serum (or plasma) IGF-I levels are used as an index of GH secretion and its biological actions in both research and clinical (diagnostic) settings. This allows evaluation of the activity of the somatotropic axis without collecting serial blood samples that would be needed for precise evaluation of GH secretion. Actions of IGF-I include inhibition of GH release, primarily via actions within the hypothalamus, thus creating a classical negative-feedback relationship between these two hormones. As expected from this relationship, GH levels are elevated in animals in which circulating IGF-I is reduced by genetic GH resistance or by deletion of the IGF-I gene in the liver (327, 340).
In contrast to the levels of circulating (endocrine) IGF-I, which are determined primarily by GH stimulation, local expression of IGF-I in different tissues and its local (paracrine and autocrine) actions can be relatively or completely independent of GH. For example, expression of IGF-I in the somatic cells of the ovary and the testis is regulated primarily by pituitary gonadotropins rather than by GH (51, 131). Lupu et al. (190) in the Efstratiadis laboratory reported that blocking GH signaling by deletion of GH receptors led to the expected profound suppression of IGF-I message levels in the liver, while IGF-I expression in the kidneys was only partially suppressed and its expression in the heart and in the brain was not affected. Unaltered expression of IGF-I in the heart of GH-resistant mice may be physiologically important because IGF-I is viewed as a cardioprotective agent (54). In fact, cardiac specific overexpression of IGF-I in male mice increased their median lifespan (176). However, interpretation of this very intriguing finding needs to be cautious, because plasma IGF-I levels in these transgenics were increased and thus GH secretion may have been suppressed. Preserved expression of IGF-I in the hippocampus of mice with complete GH deficiency will be discussed later in this article. Growth hormone-dependent and GH-independent IGF-I biosynthesis and its targets are schematically represented in FIGURE 3.
Figure 3.
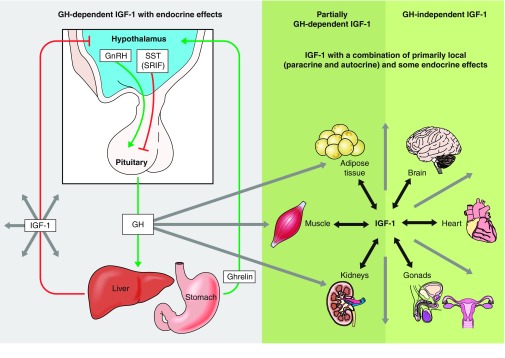
The somatotropic axis. This diagram contrasts endocrine, primarily hepatic IGF-I that acts as a mediator of GH actions with partially or completely GH-independent IGF-1 produced by other organs that can act locally in paracrine or autocrine fashion.
IGF-I receptors belong to the tyrosine kinase receptor family, are composed of α and β subunits, and resemble insulin receptors. IGF-I receptors are present in most cell types but, somewhat surprisingly, not in the hepatocytes, which are an important source of IGF-I. Consequently, IGF-I affects virtually every tissue and organ. Multiple actions of IGF-I include stimulation of cell proliferation and growth, inhibition of apoptosis, and mimicking some of the insulin effects. IGF-I receptors are present in the cell membrane and signal primarily via activation (phosphorylation) of insulin receptor substrates (IRS-1 and IRS-2) and Akt leading to maintenance of cytoplasmic localization of a transcription factor, FOXO, and inhibition of its various effects (including effects on antioxidant defenses). Signaling pathways and biological effects of IGF-I are described in far more detail in recent reviews (39, 112, 235).
In the circulation, IGF-I is present mainly as a part of a ternary complex with one of the IGF binding proteins (IGFBPs) and acid-labile subunit (ALS). Biological activity of IGF-I is believed to reflect primarily the amount that is free rather than complexed. There are six different IGFBPs of which IGFBP3, produced by the liver under influence of GH, is quantitatively most important (143). The physiological role of IGFBPs is very complex and includes 1) regulating the amount of free (bioavailable) IGF-I in circulation and locally, within the target tissues; 2) protecting IGF-I from metabolic degradation and clearance and thus maintaining an IGF-I reservoir; and 3) exerting IGF-independent actions mediated mostly by separate receptors (65).
III. SOMATOTROPIC SIGNALING IN THE CENTRAL NERVOUS SYSTEM: ROLE IN AGING
Results obtained in Caenorhabditis elegans and Drosophila indicate that insulin/IGF-I-like signaling in neurons and brain is involved in the control of aging and longevity in these animals (6, 325). The impact of deleting IGF-I receptors or IRS-2 selectively in the brain of mice (297) on age-related decline of cognitive function and on longevity will be discussed later in this article. Long-lived mutant mice with GH resistance or deficiency maintain youthful measures of learning and memory into advanced age (154, 155). Against this background, the role of somatotropic signaling in the mammalian brain in the control of aging and particularly cognitive aging is of considerable interest and will be discussed below.
A. Brain GH
Growth hormone affects multiple targets, but its role in the control of cognitive function and brain development is largely unknown. Although pituitary somatotrophs are the primary site of GH production, extrapituitary tissues have been reported to express GH genes (123, 124) and neural tissues were shown to produce and to respond to GH (123). Immunoreactive GH has been shown to be present in the rat midbrain, cortex, hippocampus, striatum, olfactory bulb, and cerebellum at different concentrations (219, 224), and the level of GH signal density in the various brain regions was found to decline during aging (219, 224). Recent studies (79, 80, 287–289, 291) indicate that in murine GH is produced endogenously within the hippocampal formation, a brain structure associated with learning and aspects of emotional experience. Interestingly, both GH mRNA and protein can be detected in the hippocampus of Ames dwarf mice (288, 289, 291), in which pituitary GH is absent because of the loss-of-function mutation at the Prop-1 locus and the resulting failure of somatotroph differentiation. Since the circulating level of GH is very low in these animals, GH present in the dwarf brain is mostly produced locally within the central nervous system (CNS). Furthermore, these studies suggest that the local synthesis of GH in the hippocampus is Pit1 independent.
Despite controversy in the field, there is growing evidence that GH can cross the blood-brain barrier (BBB) from circulation into the brain (1, 63). For example, Johansson et al. (139) reported increased GH concentration in the cerebrospinal fluid in a GH-deficient patient after recombinant human GH treatment. GH receptors are present in the choroid plexus at much higher levels than in any other brain region and may function as GH transporters across the BBB leading to accumulation in the cerebrospinal fluid (CSF) (164). GH can also cross the BBB into the brain through simple diffusion independent of a specific transport system (232). In certain pathological conditions, including hypoxic-ischemic brain injury, the BBB is often compromised, so increasing access of GH to the CSF (258). Taken together, there is growing support for the passage of GH across the BBB, although the exact mechanisms remain to be further elucidated.
B. Function
Several studies suggest that GH may play a role in influencing aspects of mood and cognition (275). GH-binding sites have been identified in several areas of the brain, suggesting the local physiological function of GH signaling in these tissues. Furthermore, in a transcriptional profiling study aimed at identifying genes that were altered in the course of learning and memory formation, GH expression in the hippocampus was significantly increased with acquisition of a hippocampal-dependent learning task (79). This increase occurred in animals that had learned, whereas naive animals and those exposed to other types of training experiences had very low levels of GH expression. GH has been shown to enhance both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)- and NMDA receptor-mediated excitatory postsynaptic potentials (EPSPs) partially through the JAK-Stat and MAPK signaling pathways (196). These findings suggest that hippocampal GH acts in an autocrine or paracrine manner to contribute to enhanced synaptic function during memory formation. Moreover, studies in CPEB-1 (cytoplasmic polyadenylation element binding protein 1) knockout mice (336), which have defects in synaptic plasticity and hippocampal-dependent memory, support a role of GH in certain types of synaptic plasticity and in learning and memory. With the use of two-dimensional gel electrophoresis and mass spectrometry, GH was found to be the most dramatically changed of the proteins with altered abundance levels in the hippocampus of CPEB-1 knockout. GH mRNA and premRNA were also reduced, suggesting that GH transcription may be directly regulated by CPEB-1 (336).
C. Brain IGF-I
IGF-I plays an essential role in regulating somatic growth and development and is also important in neuronal function (332). As discussed earlier, many types of cells, including all types of cells in the brain, are capable of IGF-I production (71). IGF-I expression in the CNS is particularly high during fetal development and in the mouse reaches its peak expression during the first 2 wk of postnatal life, predominantly in neurons, but also in glial cells, suggesting that it may play a critical role in the development of the nervous system (71). IGF-I has indeed been shown to act as a prominent neurotrophic factor during development of the nervous system, stimulating differentiation and survival of specific neuronal populations (71, 313, 321). Several studies have demonstrated neurotrophic actions of IGF-I and IGF-II in both neurons and glia including stimulation of DNA and RNA synthesis, induction of neurite outgrowth, regulation of neurotransmitter release and synaptogenesis, and protection against neurotoxic insults (82, 118, 135, 145, 247). In the adult CNS, IGF-I is a neuromodulator involved in regulation of synaptic plasticity (272, 302, 303).
Altered IGF-I signaling in the neuroendocrine hypothalamus may contribute to reproductive aging (210, 294). It was suggested that the decline in circulating IGF-I levels during aging plays a direct role in age-associated brain deterioration (242). Restoring IGF-I levels was reported to enhance neurogenesis and ameliorate the age-related cognitive malfunction in aged brain (197). In transgenic mice with increased expression of IGF-I in the brain, the weight and volume of the brain are increased substantially due to increases in neuron number and total myelin (72, 174). In contrast, transgenic mice with ectopic brain expression of IGFBP-1, an inhibitor of IGF action, and mice with ablated IGF-I gene expression have brain growth retardation with impaired neuronal somatic and dendritic growth (60). Levels of the synaptic proteins were significantly reduced in the brain of IgfI−/− mice, suggesting a reduction in synapses (60, 184). Furthermore, IGF-I gene deletion in humans has been reported to be associated with mental retardation (326).
In the studies of Ames dwarf mice, circulating IGF-I is very low (55) and the IGF-I immunoreactivity detected in the hippocampus of these mice seems unlikely to be from peripheral circulation and transport across the BBB. Therefore, IGF-I is hypothesized to be produced locally in the hippocampus and act directly on the progenitor cells in the hippocampus (291). Phosphorylation of Akt and cAMP responsive element-binding protein (CREB) were increased in the hippocampus of Ames dwarf mice (287) along with activation of the anti-apoptosis signal transduction cascade. These data support the suggestion that increase in hippocampal GH and IGF-I expression and subsequent activation of PI3K/Akt-CREB and anti-apoptotic signals might be responsible for the maintenance of the integrity of neuronal structure and cognitive function (154, 155) in these long-lived mice.
D. Aging, Hippocampal Neurogenesis, and GH-IGF-I
In the adult mammalian brain, neurogenesis occurs in the olfactory bulb and hippocampus (283). Physiological and behavioral events, such as aging, stress, diseases, seizures, learning, and exercise, can modulate hippocampal neurogenesis (103, 283). These newborn neurons go through an extended maturation process and eventually functionally integrate themselves into the existing DG circuitry (103, 283). The significance of the production of new neurons during adulthood is unknown, although studies in rodents suggest that neurogenesis plays an important role in learning and memory (88, 109, 111).
Neurogenesis in the DG decreases with age in rats, mice, monkeys and humans (53, 110, 341). Several studies have suggested that cognitive decline in aging may be attributable to decreased DG neurogenesis (83, 267). Conversely, hippocampal neurogenesis was reported to increase in aged mice living in an enriched environment associated with enhanced synaptic plasticity (by increasing long-term potentiation), dendritic spine density, and expression of synaptic proteins, receptors and neurotrophins (149, 222, 227). Moreover, training in a task that requires hippocampal function stimulates granule cell proliferation in the hippocampus (103, 109, 111).
Aging has a major impact on the activity and differentiation capacity of stem cells within the DG in the adult hippocampus, leading to a marked reduction of the number of newborn neurons within this area in rodents, non-human primates, and potentially in humans (103, 148, 160). The progressive decline of neuronal stem cells during aging indicates that the progenitor cells become unresponsive to environmental cues in the aged hippocampus and may also suggest that the newly born stem cells lose their appropriate signaling mechanism in response to the mitotic stimulus (103, 160). However, there is also evidence suggesting that alteration in the local environment in the aged brain does not provide sufficient support or mitotic stimuli for these newborn cells. Several factors that regulate neuronal birth in the adult DG were recently studied. Blockade of adrenal steroids, blockade of excitatory input into the DG, and NMDA receptor inactivation all lead to an increased birth of granule cells (110, 213).
Some cell proliferation factors, such as fibroblast growth factor-2 (FGF-2), IGF-I, and vascular endothelial growth factor (VEGF), have been shown to be responsible for the regulation of hippocampal neurogenesis (103). Intracerebroventricular (ICV) or subcutaneous injections of FGF-2 enhance dentate neurogenesis in neonatal, adult, and aged brain (138, 314, 337). IGF-I levels have been closely associated with increased neurogenesis in the adult hippocampus under different conditions (61, 185, 241, 304, 338). VEGF is another factor that considerably increases DG neurogenesis in both the intact and the injured adult brain following ICV administration (138). Moreover, it has been hypothesized that age-related decreases in some of these proliferation factors are a consequence of age-related impairments in the neurotrophic factors (103, 271), such as IGF-I.
Interestingly, increased hippocampal neurogenesis has been reported in the adult Ames dwarf mice (292). BrdU labeling studies showed an increase in numbers of newly generated cells (BrdU positive) and newborn neurons (neuronal nuclear antigen and BrdU positive) in the DG of dwarfs compared with normal mice at 3 mo of age (292). Furthermore, there was a dramatic reduction of BrdU-positive cells in the DG during aging in both dwarf and normal mice, indicating an age-related decline of hippocampal neurogenesis. Although there was no significant difference in the total number of BrdU-labeled cells between aged dwarf and normal mice, the total number of newly generated neurons (BrdU and NeuN double positive) in old dwarf mice was significantly greater than in old normal mice. Considering the evidence for a significant increase in hippocampal IGF-I protein expression and activation of an anti-apoptosis signal transduction cascade, it is likely that the increase in the fraction of newborn neurons in aged dwarf mice was due, at least in part, to an effect of local IGF-I on cell survival.
V. EXTENDED LONGEVITY OF MICE WITH CONGENITAL GH DEFICIENCY OR DELETION OF GH RECEPTORS
In 1996, Brown-Borg et al. (50) reported that Ames dwarf mice live ∼50% longer than their normal siblings and suggested that extension of their longevity is most likely due to deficiency of GH. Ames dwarf mice (257) are homozygous for a recessive mutation of the Prophet of pituitary factor-1 (Prop1) gene, which is normally expressed in the anterior pituitary and required for differentiation of cells that express Pituitary factor 1 (Pit1) and develop into somatotrophs, lactototrophs, and thyrotrophs, that is cell producing GH, PRL, and thyroid-stimulating hormone (TSH) (18, 20, 278). The causative role of GH deficiency in the remarkable extension of longevity of these mutants was supported by the demonstration that little mice with isolated GH deficiency (85) and “Laron dwarf” mice with complete GH resistance due to deletion of GH receptors (340) are also long-lived (70, 100). Subsequent work established that the extension of longevity of these mutants is reproducible (23, 24, 233) and that the remarkable longevity of Laron dwarf (Ghr−/−) mice is not limited to a particular laboratory, genetic background, or diet composition (21, 23, 42, 70). The possibility that effects of Ames dwarfism on longevity may not be due to their endocrine phenotype but instead to some other, unknown effect of the Prop1df mutation was eliminated (or at the very least made exceedingly unlikely) by the report of comparable longevity extension in Snell dwarf mice (100) which have the same endocrine defects as Ames dwarf mice (deficiency of GH, PRL, and TSH) due to mutation of Pit1, a gene distinct from Prop1 and located on a different chromosome (17, 19, 20, 177, 270).
Major longevity benefits in animals with severe endocrine defects, growth retardation, and dwarf phenotype appeared counterintuitive and were initially received with considerable skepticism. However, these findings were not unprecedented. Everitt (91) reported in 1980 that rats deprived of all anterior pituitary hormones by hypophysectomy (surgical removal of the pituitary gland) lived longer than normal animals, as long as they were provided adequate glucocorticoid replacement. Powers et al. (243) demonstrated that hypophysectomy can also extend longevity in mice, although in this species the effect on longevity was dependent on the age of the animals at the time of surgery. In a work that appears to have been overlooked by gerontologists until recently, Silberberg (268) reported histopathological findings in Snell dwarf mice up to the age of 41 mo (mice normally live ∼24–30 mo) and mentioned “unusual longevity of the dwarf animals” in the discussion section of her paper. In further support of the role of GH in the control of aging, Banks et al. (11) recently reported that treatment of short-lived “senescence accelerated” (SAMP8) mice with an antagonist of GHRH receptor increased telomerase activity, improved cognition, reduced tumor incidence, and increased mean (although not maximal) life expectancy. Evidence linking somatotropic signaling and longevity in genetically normal (“wild-type”) rather than mutant or gene knockout mice and in other species will be discussed later in this article.
VI. LONGEVITY OF MICE WITH DELETION OF GENES AFFECTING IGF-I SIGNALING AND PATHWAYS DOWNSTREAM OF THE IGF-I RECEPTOR
One of the phenotypic characteristics shared by Ames dwarf, Snell dwarf, little, and Ghr−/− mice is profound suppression of circulating levels of IGF-I (55, 81, 306, 340). Since IGF-I mediates many of the GH effects, it seems likely that extension of longevity in these mutants may be due to reduced IGF-I signaling. Consistent with this possibility, heterozygous deletion of the IGF-I receptor gene reduces the number of IGF-I receptors by ∼50% and extends longevity of female (although not male) mice (130). Results of subsequent studies indicate that the magnitude of lifespan extension in Igf1r+/− mice with partial IGF-I resistance is strongly dependent on the genetic background (36). Partial inactivation of the IGF-I receptor gene only in the brain impacts development of GH-secreting cells in the pituitary leading to reduced growth and adult body size and significant extension of longevity (144). Interestingly, in amyloid precursor protein plus presenilin transgenic mice with phenotypic characteristics resembling various features of Alzheimer's disease, heterozygous deletion of IGF-I receptor gene resulted in significant improvements of special memory and motor skills and reduced neuroinflammation and neuronal loss (64). Hypomorphic mutation of the IGF-I gene leads to a reduction of circulating levels of IGF-I and extends female longevity similarly to the findings in the Igf1r+/− mice (260).
Deletion of IGF-I gene expression selectively in the liver led to a marked (∼70%) decline in circulating IGF-I levels and a modest decrease in adult body size (328), but median lifespan was not affected in females and somewhat reduced in males (M. Adamo, personal communication). This may have been due to reduced negative feedback of IGF-I on GH secretion and the resulting increase in plasma GH levels (328, 329). Elevated GH levels could impact longevity by several mechanisms including induction of insulin resistance (329), shifting adipocytes secretory profile to proinflammatory, and enhancing mTOR signaling.
Svensson et al. (296) recently described effects of inactivating the IGF-I gene in mice selectively in the liver at 1 mo of age. As expected from studies in hepatic IGF-I deleted (LID) mice (328), IGF-I levels were reduced by ∼80% and body weight was reduced, with the greatest differences in body weight noted in middle-aged (12–24 mo old) males. Mean longevity of females was significantly increased, while a similar trend in males and a suggestive increase of maximal longevity in females were not statistically significant. Fertility did not appear affected in either sex.
The important role of IGF-I in the control of mouse longevity is strongly supported by findings in animals with deletion of pregnancy-associated plasma protein A (PAPP-A), a protease involved in degradation of IGF-I binding proteins and particularly IGFBP4. Deletion of PAPP-A thus leads to increased levels of IGFBP with the consequent reduction of free (bioavailable) IGF-I at the tissue levels. Both average and maximal lifespan are increased in both sexes of PAPP-A-1- mice (66, 67).
Extension of mouse longevity was produced also by deletion of insulin receptor substrate 1 (IRS1) (261) or IRS2 (297) as well as by heterozygous or homozygous deletion of IRS2 selectively in the brain (297). The effect of heterozygous deletion of IRS2 in all tissues on longevity reported by Taguchi et al. (297) were not seen in the studies of Selman and co-workers (261, 262) possibly due to the use of diets with a different percentage of fat. It is difficult to know whether the impact of reducing IRS expression on longevity is due to suppression of IGF-I signaling, insulin signaling, or perhaps both. Although effects of IGF-I and insulin on cell function are distinct with relatively little overlap, signaling by both of these hormones involves activation of IRSs. Alterations in insulin signaling are involved in mediating the effects of GH on aging and longevity (details later in this article), and deletion of insulin receptors in adipose tissue increased longevity in mice (34).
In the context of discussing the effects of somatotropic axis on aging, it is of considerable interest that mice with deletion of S6 kinase (S6K) are long-lived (263). S6K is regulated by the mTOR, and mTor signaling is reduced in long-lived GH-resistant and GH-deficient mice (40, 265). Pharmacological suppression of mTOR increases longevity of mice (120), and involvement of TOR pathway in the control of aging is highly conserved (205).
Key characteristics of long-lived GH and/or IGF-I related mouse mutants are listed in TABLE 1.
Table 1.
Phenotypic characteristics of long-lived GH- and IGF-I-related mouse mutants
| Name | Gene | Lifespan (Male) | Lifespan (Female) | Strain | BW | Blood GH | Blood IGF-I | Insulin Sensitivity | Cellular Stress Resistance | In Vivo Stress Resistance |
|---|---|---|---|---|---|---|---|---|---|---|
| Ames Dwarf [150] | Prop-1 | 49% | 68% | Heterogeneous | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ |
| Snell Dwarf [100] | Pit-1 | >40% | >40% | DW/J × C3H/HeJ | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ |
| Little [100] | Ghrhr | >20% | >25% | C57BL/6J | ↓ | ↓ | ↓ | ↑ | ? | ? |
| GHR KO [70] | Ghr/bp | >35% | >35% | 129Ola × BalbC | ↓ | ↑ | ↓ | ↑ | ↑ | ? |
| PAPPA KO [67] | Pappa | >25% | >25% | C57BL6 × 129SV/E | ↓ | NC | NC | ? | ? | ? |
| AC5 KO [331] | Ac5 | 32% | 32% | 129/SvJ-C57BL/6 | NC | ↓ | ? | ? | ↑ | ? |
| MIF KO [119] | Mif | ? | 16% | C57BL/6J 129/SvJ | ↓ | ? | NC | NC | NC | ? |
| Klotho Tg [161] | Klotho | >20% | >18% | C57BL/6 × C3H | NC | ? | NC | ↓ | ↑ | ? |
| Surf1 KO [76] | Surf1 | 18% | 18% | 129S1/SvJxC57 BL6J × DBA2 | NC | ? | ? | ? | ↑ | ? |
| P66 shc KO [209] | p66 shc | 30% | 30% | C57Bl/6Sv/129 | NC | ? | ? | ↑ | ↑ | ↑ |
| Igf-I Tg Heart [176] | IGF-I | 18% | ? | FVB | NC | ? | ↑ | ? | ? | ? |
| Irs1 KO [261] | Irs-1 | 16% | 30% | C57BL/6 | ↓ | NC | NC | ↓ | ? | ? |
| Irs2 KO [297] | Irs-2 | ? | 18% | C57BI/6 | NC | ? | ? | ↑ | ? | ? |
| FIR KO [34] | Ir | 18% | 18% | 129Sv × C57BI/6 | ↓ | ? | ? | NC | ? | ? |
| Igf-IR ± KO [130] | Igfr | 16% | 33% | 129/Sv | ↓ | ? | ↑ | ? | ↑ | ↑ |
| Igf-IR ± KO [36] | Igfr | 0% | <5% | 129/SvJ-C57BL/6 | ↓ | ? | NC | ↓ | ? | ↑ (female) |
| S6KO [263] | S6k | NC | 19% | C57BL/6 | ↓ | NC | NC | ↑ | ? | ? |
VII. EXTENSION OF LONGEVITY IN GH-DEFICIENT AND GH-RESISTANT MUTANTS INVOLVES VARIOUS TRADE-OFFS
In organisms ranging from yeast to mice, there are numerous examples of extension of longevity by targeted deletion of individual genes or by loss-of-function or hypomorphic mutations. These findings imply that the normal physiological levels of expression of these genes are not optimal for life expectancy and could be viewed as factors that promote aging and reduce longevity. Failure of natural selection mechanisms to eliminate genes with such detrimental effects fits the concept of antagonistic pleiotropy, first formulated by Williams (323). This concept is based on the appreciation that the force of natural selection declines after the age of maximal reproductive effort and posits that genes with positive effects on attainment and maintenance of reproductive fitness early in life will not be selected against even if they have detrimental effects later on. Genes involved in GH signaling would appear to fit this concept. Growth hormone actions early in life promote somatic growth and sexual maturation and may also enhance gonadal function and fecundity, thus clearly increasing reproductive fitness, while its effects in adult life include increased susceptibility to cancer and promoting insulin resistance and hyperinsulinemia, that is, effects that may accelerate aging and be detrimental to long-term survival. When viewed in light of antagonistic pleiotropy, the anti-aging and life-extending effects of reduced GH signaling in mutant mice would be expected to be associated with trade-offs reflecting loss of beneficial effects of GH early in life. Phenotypes of Ames dwarf, Snell dwarfs, and Ghr−/− mice in fact do provide examples of such trade-offs.
The most obvious phenotypic effect of GH deficiency or resistance is profound suppression of postnatal (and particularly postweaning) growth and reduction of adult body size [in Ames, Snell. and Ghr −/− (Laron) dwarf mice, by >50%]. Although the impact of small body size of GH-related mouse mutants on reproductive success or survival under natural conditions has not been examined, it can be assumed that in competition with normal males, male dwarfs would be unlikely to establish and defend territories and thus would have reduced mating opportunities. It is also likely that in temperate or northern climates, these diminutive animals would have reduced winter survival due to unfavorable body surface-to-mass ratio.
Sexual maturation, as measured by the age of establishment of vaginal introitus (“vaginal opening”) in females and balano-preputial separation in males is delayed in Ghr−/− mice by ∼1 wk (73, 147). Although both sexes are fertile, various parameters related to fecundity including interval from pairing until conception, litter size, interval between litters, and percentage of animals that reproduce are reduced in Ghr−/− mice compared with normal animals from the same strain (56, 58, 334). Humans with Laron dwarfism, i.e., analogous endocrine syndrome of GH resistance, exhibit reproductive phenotype closely resembling Ghr−/− mice. Both sexes are fertile, but puberty is delayed (167). In the author's laboratory, sexual maturation in Ames dwarfs is delayed by 3–4 wk (52 ± 8 versus 23 ± 2 days in females and 48 ± 6 versus 25 ± 2 days in males; P < 0.0001). This characteristic is strongly dependent on genetic background. In some stocks segregating for Ames or Snell dwarfism, sexual maturation of the mutants is delayed much more, and in some cases, indefinitely (17). In our breeding colony of Ames dwarf mice, nearly all males are fertile and we use them routinely for breeding. Female Ames dwarf and Snell dwarf mice can ovulate and mate, but their fertilized eggs fail to implant and develop due to prolactin deficiency and the resulting luteal failure (15). In many mammalian species, including mice, activation of corpora lutea and secretion of progesterone in amounts needed for implantation and maintenance of pregnancy absolutely require the action of PRL, which is lacking in Snell and Ames dwarf mice due to the absence of lactotrophes (18, 177, 278). Female dwarf mice treated with PRL can get pregnant, deliver live young, and raise them to weaning (14).
It is interesting that delayed sexual maturation and reduced fecundity of GH-resistant (and likely also GH-deficient) mice appear to be counterbalanced by a delay in the age-related decline in fertility (10; Westbrook & Bartke, unpublished data). This echoes the findings in genetically normal animals subjected to calorie restriction in which fertility may be partially or completely suppressed, but a potential to reproduce upon shift to ad libitum feeding is maintained beyond the age at which reproductive function normally ceases (129). Moreover, age-related alterations in neuroendocrine function related to male reproduction are attenuated in Ghr −/− mice. Decline in plasma testosterone and increases in prolactin and in luteinizing hormone responses to gonadotropin-releasing hormone stimulation accompanied aging in normal mice but were absent in 2-yr-old Ghr−/− animals (57).
Alterations in immune function may provide another example of trade-offs between longevity and various aspects of physiological functioning in GH-deficient and GH-resistant mutants. Snell dwarf mice were described in 1972 as being grossly immunodeficient (92). These findings are somewhat difficult to interpret, because early mortality and extremely short life expectancy of Snell dwarf mice reported in the same publication (92) were not seen in other laboratories working with these mutants (259, 266, 268), and thus may have been peculiar to environmental conditions and/or husbandry practices in this particular study. However, findings of Fabris et al. (92) in Snell dwarf mice were very important by providing some of the earliest evidence that function of the immune system is subject to endocrine control. More recent work clearly established that under modern husbandry conditions, Snell dwarf mice are long rather than short-lived and provided evidence that aging of their immune systems is significantly delayed as assessed by the ratio of naive to memory T cells (100).
In Ames dwarf mice, activity of the immune system is lower than in normal mice as indicated by reductions in cellularity of the thymus and spleen (90) in the responses of lymphocytes to mitogens (89) and in the activity of natural killer cells (90) However, the ability to produce antibodies in response to a challenge with tetanus toxoid was not compromised in Ames dwarf mice (116), and these animals can thrive under standard conditions, as opposed to specific pathogen-free or “barrier” conditions, which implies resistance to various pathogens. Similarly, we are not aware of any reports of immunodeficiency or increased susceptibility to infectious disease in GH-resistant or GH-deficient humans.
Maintenance of the immune system is energetically costly and activation of a “survival program” which is believed to occur in response to calorie restriction includes partial suppression of immune functions. This does not interfere with health or survival under modern laboratory conditions but can increase the susceptibility of the animals to viral, microbial, and helmitic pathogens or parasites (105, 158, 285). Interesting similarities between the effects of CR and reduced somatotropic signaling on immune function also include a delay in immune aging in animals subjected to CR (231).
Thus, in laboratory rodents, both reproductive and immune functions tend to be reduced by either dietary or genetic interventions that produce a major extension of longevity. However, these suppressive effects are counterbalanced by attenuating the age-related decline in reproductive and immune functions by the same interventions.
VII. GROWTH SIGNALING, CELLULAR PROTECTION, AND AGING IN SIMPLE ORGANISMS
The trade-off between investment in either growth or cellular protection is likely to be a fundamental property of all living organisms. This trade-off also provides the foundation for several evolutionary theories of aging including antagonistic pleiotropy and the disposable soma. Not surprisingly, the great majority of long-lived mutants in organisms ranging from bacteria to mice are stress resistant but also display defects in either growth rate, fertility, or size (187). Notably, the trade-off between cellular protection and longevity and growth is not necessarily observed under standard laboratory conditions, since the trade-off may be subtle but detrimental for fitness. Therefore, to understand somatotropic signaling and its effect on aging and disease in mammals, it is important to introduce the fundamental relationship between growth-stimulating nutrient signaling pathways, cellular protection, and aging in yeast, worms, and flies. In yeast, the Tor/Sch9 (Tor/S6K) pathway is perhaps the most closely associated with the mammalian IGF-I/AKT and Tor/S6K pathways since all three 1) are affected by amino acid/protein levels, 2) play central roles in cellular division and organismal growth, and 3) promote aging and cellular sensitization to stress. In the yeast, Saccharomyces cerevisiae, mutations in either the Tor/Sch9 and glucose-response Ras/adenylate cyclase/PKA (Ras/AC/PKA) pathways extend lifespan and also increase cellular resistance to a variety of stresses including oxidative and heat stress (94, 96, 101, 186). Whereas Tor/S6K-deficient yeast cells display slow growth and generate both cells and colonies of reduced size, cells deficient in Ras/AC/PKA signaling grow normally and generate cells and colonies of normal size. Notably, in many mammalian cell types, Tor, S6K, and Ras can all be activated in response to GH and/or IGF-I signaling, indicating that downregulation of both the Tor/S6K and Ras/AC/PKA pathways in yeast can model many of the molecular changes that occur in GH, GHR, and IGF-I-deficient mice. As discussed elsewhere in this review, it is not clear what portion of the effects of GH and GHR deficiencies on protection and lifespan are mediated by reduced circulating IGF-I levels. In fact, GH/GHR can affect Tor/S6K and AKT signaling independently of its effects on IGF-I levels and also affect insulin levels, raising the possibility that multiple factors are responsible for the effects of GH on longevity (126).
One of the consistent effects of the inactivation of the Tor/Sch9 and Ras/AC/PKA pathways in yeast is a major reduction in age-dependent DNA damage including point mutations, gross chromosomal rearrangements, and small DNA insertions/deletions, all damage important for cancer development in mammals (93, 95, 192, 193). These effects on genomic instability but also those on cellular protection and longevity extension are mediated in large part by stress resistance transcription factors Gis1 and Msn2/Msn4 (94, 96, 101), and require expression of the mitochondrial superoxide scavenger SOD2, which is positively regulated by all three transcription factors (94, 96, 101).
In worms, the relationship between the pro-aging signaling daf-2 insulin/IGF-I-like pathway and body size is not clear, since many long-lived mutant alleles are of normal size. However, both the daf-2 and the Tor pathways converge onto a set of genes that affect growth of the worm into a reproductive adult but also limit its longevity (137), suggesting that the effect of these pathways on growth and longevity is conserved. Similarly to the effects observed in yeast, the downregulation of the PI3K age-1/AKT pathway downstream of the daf-2 insulin/IGF-I-like receptor promotes cellular protection and longevity by activation of stress resistance transcription factor DAF-16 (140, 151).
In fruitflies, the insulin-like receptor (InR) and its downstream substrate chico also regulate the size of the organism as well as metabolism and longevity. Flies deficient in Chico, the insulin receptor substrate, are small, display a smaller cell size (35), and have an increased lifespan (300). Deficiencies in the genes encoding for insulin-like ligands (Drosophila insulin-like peptides; DILP 1–7) also increase lifespan and resistance to oxidative stress and heat (48).
In summary, in yeast, worms, and flies, there is a strong inverse association between lifespan extension and growth, determined by the activities of conserved proteins including AKT, Tor, and S6K, which are regulated by GH, IGF-I, or both.
VIII. GROWTH SIGNALING, ADIPOSITY, AND AGE-RELATED DISEASE: FROM SIMPLE ORGANISMS TO MAMMALS
A. Growth Signaling and Reserve Carbon Sources in Simple Organisms
One of the phenotypes consistently associated with mutations that extend lifespan in organisms ranging from yeast to mice is the increased storage of reserve carbon sources. In yeast, the Ras/AC/PKA and the Tor/Sch9 pathways block the accumulation of glycogen, the major reserve carbon source stored during aging (97, 101, 240). As for stress resistance, the glycogen accumulation effects of reduced activity of the Ras/AC/PKA and the Tor/Sch9 pathways are also mediated in large part by stress resistance kinase Rim15 and the downstream transcription factors Msn2/Msn4 and Gis1 (96, 240, 317). The effect of analogous nutrient signaling pathways on the storage of reserve carbon sources is also observed in worms. For example, worms with reduced activity in components of the insulin-like/AKT and TOR/S6K pathways are long-lived and accumulate both fat and glycogen (151). Analogously to the effects observed in yeast, the stress resistance transcription factor DAF-16 promotes the accumulation of fat in worms (218). Defects in insulin-like signaling also increase fat storage in Drosophila (300).
B. Growth Signaling and Adiposity in Rodents
In agreement with the phenotype of lower eukaryotes with deficiencies in growth pathways, mice with deficiencies in the growth hormone-IGF-I axis, including mice lacking GH and the GH receptor (GHR−/−), are long-lived and obese (19, 20, 179). GHR−/− mice, which lack the GH receptor and binding protein and which, as a result, have severe IGF-I deficiency and growth defects, display a major increase in fat mass (29–31, 41). Surprisingly, these mice are not insulin resistant and, in fact, are insulin sensitive (40, 78, 183). These studies are in contrast to those of mice and humans with mutations that reduce IGF-I gene expression directly, which instead are associated with insulin resistance (52). When placed on a high-fat diet, the GHR−/− mice display an approximately twofold increase in fat mass versus the threefold increase observed in wild-type mice, suggesting that the increased fat deposition on the standard diet is not due to an altered response to high-fat nutrition. When the sites of fat deposition were examined, GHR−/− mice displayed lower levels of intra-abdominal fat and higher levels of subcutaneous fat. Remarkably, the insulin level on the low-fat diet was over 65% lower and that on the high-fat diet 80% lower in GHR−/− compared with that in controls (30). Glucose levels were also more than 40% lower in GHR−/− compared with those in wild-type mice on both the low- and high-fat diets. In contrast, insulin but not glucose levels were elevated in the GH overexpressor mice, particularly on the low-fat diet (30). Not surprisingly, these mice placed on a high-fat diet develop diabetes (228), whereas GHR−/− mice are protected from glomerulosclerosis and other markers for diabetes induced with the drug streptozotocin (28). Notably, mice with excess GH function, which are larger than control mice, also displayed a 2.1-fold increase in fat mass; however, the percent body fat for these mice was greatly reduced compared with wild-type mice and especially compared with GHR−/− mice (body fat: wild-type low-fat diet = 10%, GHR−/− low-fat diet = 20%, GH overexpressor low-fat diet = 6%). This increase in percent body fat in GHR−/− begins at ∼13 wk and continues for at least 2 yr (31). Notably, no significant difference in percent lean mass was observed in either female or male GHR−/− (31). In contrast, in 9- to 13-wk and 1-yr-old GH deficient rats, visceral fat was decreased, although marrow adiposity was generally increased in GH-deficient compared with wild-type rats (74). This is particularly surprising, considering the increased storage of either glycogen or fat in organisms lacking pro-growth genes ranging from yeast to humans (see next section).
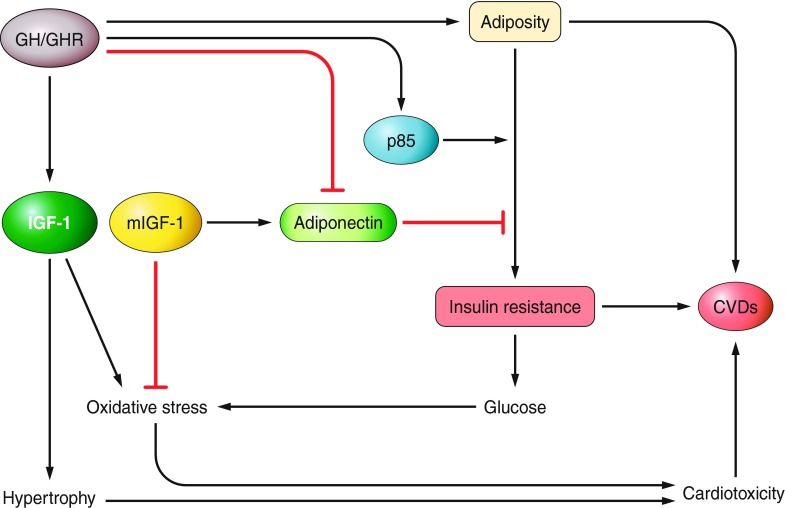
These studies indicate that reduced GH and GHR levels promote obesity (FIGURE 4). However, mutations that directly cause reduced expression of IGF-I are associated with insulin resistance, suggesting that GH may act independently of IGF-I to promote insulin resistance (FIGURE 4).
Figure 4.
Key mechanisms linking GH signaling with adiposity, insulin resistance, and cardiovascular disease (CVD). Details and additional mechanisms are in the text.
C. Growth Signaling and Adiposity in Humans
Much of the understanding of the relationship between human GH, IGF-I, and obesity comes from studies of Laron syndrome (LS, or GHR-deficient, GHRD) subjects, who display severe dwarfism, high GH levels, and low serum IGF-I levels (171). A study of 25 GHRD subjects showed that they develop marked subcutaneous and visceral adiposity and tend to have elevated cholesterol (170). Long-term treatment of GHRD adults with IGF-I decreased subcutaneous fat as well as serum cholesterol and triglycerides (170). Because GHRDs cannot respond properly to GH since the receptors are mostly inactive, the authors hypothesized that IGF-I acts directly on adipose tissue storage. However, another preliminary study with just three children suggested that long-term IGF-I treatment may actually increase adiposity, raising the possibility that part of the effect of the GH and GHR on obesity may be independent of IGF-I levels (168). Another adiposity-related characteristic of GHR-deficient subjects is nonalcoholic fatty liver, which was detected in the majority of GHRD males but also in many GHRD females (169).
Remarkably, the obese GHRD subjects display a major reduction in diabetes prevalence. Whereas a few cases of diabetes have been reported for the GHRD subjects in the Middle East (169), diabetes has not been diagnosed in any of the 99 GHR-deficient subjects in Ecuador (115). In 13 relatives and 16 GHRD male and female subjects ranging from 20 to 50 years of age, no significant difference in fasting glucose concentrations was observed, but the insulin concentration in the GHRD group was about one-third compared with that in the relatives (115). Consequently, based on the HOMA-IR (homeostatic model assessment-insulin resistance) index, the GHRD subjects (HOMA-IR = 0.34) appeared to be much more insulin sensitive than their relatives (HOMA-IR = 0.96). These human results are consistent with those for GHRD mice (see above). In agreement with these findings, despite the marked obesity, HOMA-IR was elevated in only one of the GHRD subjects tested in the Middle East by Laron et al. (142). One of the changes that may explain part of the uncoupling between obesity and insulin resistance/diabetes in GHRD subjects is the increase in adiponectin, which is one of the major hormones produced by fat cells and acts to increase insulin sensitivity (142). An increase in adiponectin levels along with improved insulin signaling were also reported in subjects lacking the growth hormone releasing hormone receptor (GHRHR) (225).
D. Mechanisms of GH/IGF-I-Dependent Insulin Resistance
Adiponectin levels have been consistently shown to be reduced in insulin-resistant and diabetic mice and humans and in patients with cardiovascular diseases (141). The well-established insulin-sensitizing effect of adiponectin indicate that its low levels contribute to insulin resistance and the consequent metabolic diseases. Thus the elevated adiponectin in GHRD could explain in part why a syndrome that promotes obesity does not cause diabetes. In fact, replenishment of physiological levels of adiponectin in a diabetic mouse model reduced insulin resistance and the replenishment of the combination of adiponectin and leptin completely reversed the insulin resistance in this diabetic lipoatrophic animal (330). Moreover, transgenic mice overexpressing adiponectin share many phenotypic characteristics with long-lived Ghr−/− and Ames dwarf mice (26, 189) and an increase in median lifespan was associated with hepatic expression of human adiponectin (230). One of the mechanisms by which adiponectin may affect insulin resistance is by decreasing plasma triglycerides through fatty acid oxidation and transport (141). These triglycerides have been reported to interfere with PI3K activation and the subsequent translocation/activation of the glucose transporter 4 (141).
Growth hormone has also been linked directly to insulin resistance using mice overexpressing or lacking GH and 3T3-F442A adipocytes. In mice, elevated GH expression increased the expression of p85a, the regulatory subunit of PI3K, and also caused a major reduction in adiponectin (75), whereas GH deficiency increases adiponectin and reduces p85a protein levels in epididymal fat, changes accompanied by a major decrease in insulin and insulin resistance index (75). The authors proposed a direct effect for GH in the regulation of p85a levels resulting in decreased PI3K activity and increased insulin resistance (FIGURE 4). Because IGF-I levels were reduced and adiponectin levels were increased in the fat cells of GH-deficient mice (75), the regulation of p85a by GH could be mediated indirectly by modulation of IGF-I and/or adiponectin. However, the studies indicating that mutations that directly affect the IGF-I gene increase and not decrease insulin resistance (52, 117, 327) support the direct effect of GH on insulin resistance independently of IGF-I (FIGURE 4). Another mechanism linking the absence of GH signals with improved sensitivity to insulin is reduced inhibitory (serine) phosphorylation of insulin receptor substrate 1 (IRS-1), as discussed later in this article.
E. Growth Factors, Cardiovascular Disease, and Cancer in Humans
Obesity is one of the major risk factors for diabetes but also for coronary artery disease and stroke. Thus the adiposity associated with GH and IGF-I deficiencies, in mice and humans, would be expected to also promote cardiovascular diseases. However, subjects from Brazil with deficiency in the growth hormone releasing hormone receptor (GHRHRD) gene, and consequently in GH, are obese, display higher levels for LDL and an inflammatory marker for CVD, but do not develop premature atherosclerosis (5, 226). Also, prolonged treatment of these GH-deficient subjects with GH caused weight loss but also an increase in the intima-media thickness and atherosclerotic plaques (226) as well as elevated diastolic and systolic blood pressure (226). Results consistent with those from GHRHRD subjects were obtained for the GHRD cohort of Ecuador. About 30% of the deaths for both GHRDs and their normal relatives were from cardiac disease and stroke, providing evidence in support of normal cardiovascular disease incidence in severely IGF-I-deficient subjects (115).
As discussed in the rodent section, both GH and GHRD mice either display a reduced incidence of tumors and an increase in benign tumors or a delay in the age at which tumors develop (133, 134, 312). In GHRD humans, none of the 30 deaths that occurred in the Ecuador cohort were reported to be from cancer despite a 20–22% portion of the deaths of their relatives caused by cancer (115). The exposure of human epithelial cells to IGF-I-deficient serum obtained from GHRD subjects caused a decrease in the DNA damage but also an increase in the apoptosis caused by treatment with hydrogen peroxide, indicating that very low IGF-I may both protect cells from oxidative DNA damage and promote cell death in highly damaged cells (115). These results in GHRD mice and humans are in agreement with the effect of deficiencies in Tor/S6K, Ras/AC/PKA, or both in reducing genomic instability in yeast (see model organisms section) (188). In support of a progenomic instability effect of growth factors during aging conserved from yeast to humans is also the finding that in the human epithelial cells incubated with serum obtained from GHRD subjects the expression of TOR was reduced and both N-Ras and PKA were among the most downregulated genes compared with cells incubated with serum from normal subjects (115). Taken together, these results provide additional evidence for a fundamental and conserved trade-off between cellular protection and growth.
Although a range of phenotypes including increased adiposity, reduced age-dependent cancer, reduced insulin resistance, or diabetes were found in both GHRD mice and humans, survival analyses of both GHRHRD and GHRD subjects revealed an increased mortality in young subjects but normal longevity (115, 225), suggesting that severe IGF-I deficiency in humans promotes some side effects that counterbalance its antiaging effects. Considering the unusually high (50%) portion of deaths from accidents, alcohol-related causes, and unknown causes in GHRDs, an alternative possibility is that the very short stature of these subjects may increase their risk of accidents or cause behavioral changes that promote early deaths (115). Although subjects with GHRD (GHRHR deficiency) or hypopituitarism including GH deficiency (159) have essentially normal life expectancies and can reach very advanced age, isolated GH deficiency was reported to reduce longevity (32).
IX. SOMATOTROPIC AXIS-RELATED GENETIC VARIANTS ASSOCIATED WITH EXCEPTIONAL HUMAN LONGEVITY
As discussed elsewhere in this review, the inhibition of components of the GH/IGF-I axis but particularly very low levels of GH or GHR have a consistent effect on protection against aging and age-related diseases in laboratory mice, resulting in a 40–60% lifespan extension. A number of studies are now suggesting that either mutations or polymorphisms that affect the GH or IGF-I levels or signaling are associated with human longevity. For example, Bonafe et al. (38) found that subjects carrying a specific allele of the IGF-I receptor have low levels of plasma IGF-I and are more represented among long-lived people. Another study of a cohort of Ashkenazi Jewish centenarians, their offspring, and matched controls showed overrepresentation of mutations in the IGF-IR gene among centenarians relative to controls (284). In agreement with the study by Bonafe et al. (38), these mutations were associated with reduced activity of the IGF-IR in lymphocytes, suggesting a state of IGF-I resistance (284). Another study identified additional potentially protective polymorphisms in two additional IGF-I pathway genes: a single nucleotide polymorphism (SNP) in AKT1 was significantly associated with increased longevity across three cohorts including the Ashkenazi Jewish centenarians, and a polymorphism in the FOXO3A gene was associated with longevity in females only (239). FOXO3A and other FOXO forkhead transcription factors are human homologs of the DAF-16 transcription factor associated with longevity extension in worms. Both in worms and mammals, these forkhead transcription factors are phosphorylated and negatively regulated by IGF-I signaling proteins including AKT (112, 152). The link between FOXO3a SNPs and longevity was previously described in several studies including one of a genetic variation within the FOXO3A gene associated with longevity in Japanese (322) and German centenarians (99). As discussed in the previous section, neither GHR- nor GHRHR-deficient subjects appear to be long-lived, as observed consistently for GH- and GHR-deficient mice. However, the major reduction in both diabetes and cancer provides solid evidence to support a conserved role for growth factors in promoting both aging and age-related diseases. It will be important to determine whether other factors such as EGF are also promoters of aging.
In comparisons of middle-aged offspring of exceptionally long-lived families with their partners in the Leiden Longevity Study, no significant differences were detected in the levels of IGF1 or IGFBP3 in the circulation (250). However, the levels of adiponectin that are negatively regulated by GH were higher in the offspring (Westendorp and VanHeemst, personal communication) and the offspring were more insulin sensitive (250, 320), thus resembling the findings in long-lived GH-deficient and GH-resistant mice.
X. MECHANISMS LINKING REDUCED SOMATOTROPIC SIGNALING WITH EXTENDED LONGEVITY
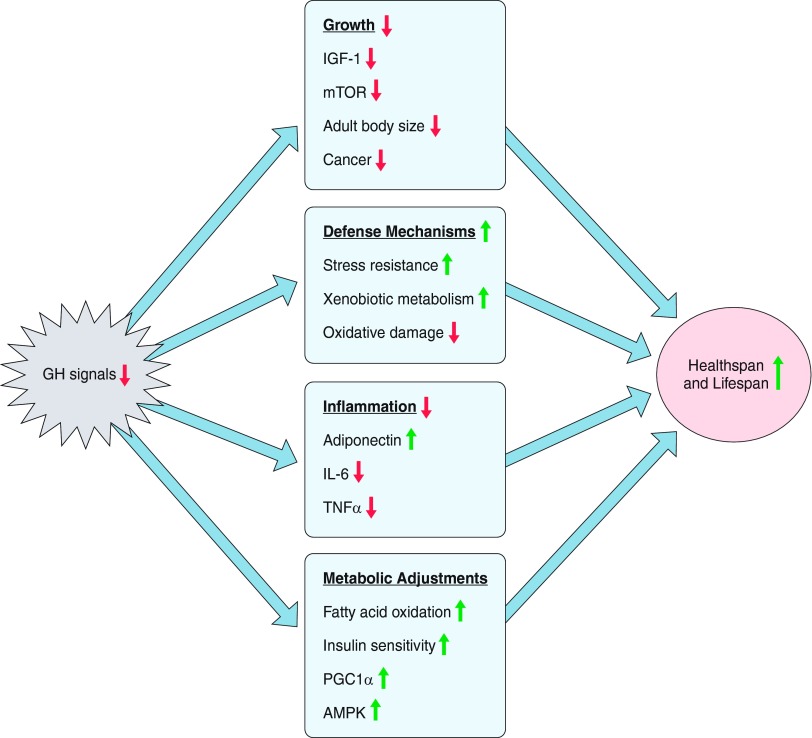
Delayed aging and remarkably increased longevity of GH-deficient and GH-resistant mutant mice prompted a search for mechanisms that may be involved. Studies in several laboratories identified numerous characteristics of these animals that are likely to delay aging and prevent age-related disease. Many of these characteristics have been previously associated with extended longevity in animals subjected to calorie restriction and/or in epidemiological studies of exceptionally long-lived people and their relatives. The list of candidate mechanisms of extended longevity in Ames dwarf, Snell dwarf, Ghr−/−, and/or little (Ghrhrlit) mice includes increased stress resistance, reduced oxidative damage, enhanced capacity to metabolize xenobiotics, increased ratio of anti- to proinflammatory cytokines, improved insulin signaling combined with reduced insulin levels, reduced hepatic cholesterol biosynthesis and serum lipid levels, metabolic shift toward greater fatty acid utilization, reduced body temperature, reduced depletion of very small embryonic-like stem cells in the bone marrow, and improved genome maintenance. Additional mechanisms of extended longevity of GH-related mutants can be deduced from changes in gene expression in different organs of these animals. The reader is referred to recent reviews (19, 20, 22) and to TABLE 2 for details, references, and additional putative mechanisms of extended longevity in these animals. However, what may best explain the protective changes listed above, and the longevity phenotype of these dwarf mice and also of other dwarf organisms such as the Tor/S6K deficient yeast, is the previously discussed ability of most, if not all, organisms to either remain in a progrowth and proreproductive state associated with a higher rate of aging or in a standby state in which the protection of the germline and soma are the most important tasks.
Table 2.
Characteristics of GH-resistant and/or GH-deficient mice believed to contribute to their extended longevity
| Characteristics of GH-Resistant and/or GH-Deficient Mice |
|---|
| Energy and fat metabolism |
| Reduced hepatic sterol biosynthesis (44) |
| Reduced lipolysis and serum lipids (315) |
| Increased expression of PPARγ, PPARα, and PGCIα (198, 199) |
| Altered mitochondrial function and reduced respiratory quotient (62, 319) |
| Hypothyroidism (severe in some mutants; mild or absent in others) (20, 319) |
| Reduced body temperature (in spite of increased mass of brown adipose tissue) (125, 132) |
| Increased food intake and O2 consumption per unit body weight at standard animal room temperature (319) |
| Shift of energy utilization from growth and fecundity to maintenance and repair (19) |
| Insulin signaling and glucose homeostasis |
| Reduced mass and secretory activity of pancreatic β cells; hypoinsulinemia (43, 70, 183, 237, 340) |
| Shift from pro- to anti-inflammatory adipokine profile: increased adiponectin, reduced TNF-α and IL-6 (3, 201, 315) |
| Increased whole animal insulin sensitivity (40, 202) |
| Mild hypoglycemia (43, 125) |
| Stress resistance and oxidative damage |
| Cellular resistance to a variety of oxidative, cytotoxic and metabolic stresses (175, 253) |
| Improved antioxidant defense mechanisms; reduced ROS production (49, 235) |
| Greater capacity for xenobiotic metabolism (7) |
| Reduced oxidative damage to macromolecules (49, 256) |
| Reduced mutation rate (104) |
| Growth, tissue maintenance, and cancer |
| Reduced hepatic IGF-I expression and circulating IGF-I levels (20, 340) |
| Preserved local IGF-I signaling (including the brain and the heart) (200, 288) |
| Reduced mTOR-mediated translation and cell size (40, 127, 265) |
| Delayed onset and reduced severity of neoplastic disease (133, 134, 312) |
| Delayed maturation; reduced adult body size (20, 147, 334) |
| Improved maintenance of stem cells (246) |
| Organ-specific alterations in apoptosis (106, 150) |
| Cardiovascular function |
| Reduced blood pressure (179) |
| Reduced cardiac extracellular collagen (127) |
| Rheological advantages of reduced body size (254) |
| Other potential mechanisms |
| Delayed immune system aging (100) |
| Altered N-glycosylation (308) |
| Altered profile of expression of multiple genes and micro RNAs (8, 25, 77, 211, 305) |
The picture that emerges from these studies is a network of interacting phenotypic characteristics that collectively protect from age-related disease and improve life expectancy (FIGURE 5). This “longevous” phenotype resembles many characteristics of genetically normal animals subject to calorie restriction (CR) that are also long-lived and partially protected from age-related disease. Thus both dwarf mice and CR animals are small, hypoinsulinemic, and insulin sensitive with reduced body size, body temperature, and fecundity, as well as a reduced incidence of cancer. However, important differences also exist. For example, CR animals are lean but long-lived dwarf mice are not. In fact, Ghr−/− males are clearly obese with percentage of body fat reaching ∼40%, i.e., values roughly double those measured in their normal siblings (31, 41). The paradox of obesity coexisting with improved insulin signaling, delayed onset of cancer, and extended longevity appears to be due to striking differences in the secretory activity of adipose tissue exemplified by increased rather than reduced adiponectin levels in Ghr−/−, Ames, and Snell dwarfs (29, 46, 315). In addition, adipocyte senescence is delayed (J. Kirkland, personal communication), and the accretion of white adipose tissue is predominantly subcutaneous (29, 41) in these mutants.
Figure 5.
Mechanisms believed to be involved in linking GH signaling with healthspan and lifespan. Details and references are in the text and in TABLE 2.
Recent studies involving surgical removal of most of the epididymal and perinephric fat pads provided evidence that visceral adipose tissue of Ghr−/− males is an important source of adiponectin and promotes enhanced insulin sensitivity (201). This is in sharp contrast to the findings in control (normal) animals in which visceral fat secretes less adiponectin and more proinflammatory cytokines and promotes insulin resistance (201).
It is very difficult to know which of the characteristics of dwarf mice believed to represent candidate mechanisms of delayed aging can be directly related to suppression of somatotropic signaling and which are secondary or tertiary. For example, deficiency of GH and IGF-I interferes with development of insulin-producing β cells in the pancreas. However, reduction of insulin levels in dwarf mice can also be a response to the improved insulin sensitivity caused by the absence of GH signaling. Increased accumulation of subcutaneous fat in these mutants likely reflects the absence of lipolytic effects of GH but could also be due to improved sensitivity to insulin. Delayed onset and reduced incidence of age-related disease can be related to increased resistance to oxidative stress, improved insulin signaling, altered stem cell maintenance, delayed senescence, and altered secretory profile of adipocytes and associated macrophages, as well as reduced somatotropic support of cancer growth.
Of the numerous mechanisms likely to contribute to delayed aging of the GH-related mutants, we are particularly interested in improved insulin sensitivity combined with reduced insulin levels because these characteristics are consistent with highly conserved impact of insulin-like and insulin signaling on aging in other organisms and have been specifically associated with human longevity (234, 250, 320). Moreover, opposite phenotypic characteristics, hyperinsulinemia, and insulin resistance figure prominently in the definition of metabolic syndrome which is associated with increased risk of various age-related diseases and thus with reduced human life expectancy (223, 280).
Our studies revealed that improved whole animal insulin sensitivity of Ghr−/− mice is associated, most likely causally, with alterations in the insulin signaling pathway and in acute responses to insulin stimulation in different insulin target tissues (40, 42, 78). In the liver, the levels and activation of insulin receptors were increased in Ghr−/− mice. In the skeletal muscle of these animals, there were increases in the levels and activation of Akt1 and Akt2 leading to increased levels of a glucose transporter, Glut4. Improved muscle insulin sensitivity in Ghr−/− mice was also associated with reduced inhibitory (serine 307) phosphorylation of insulin receptor substrate1 (IRS-1). This, in turn, was likely due to reductions in mTOR signaling (40) and in activation of c-Jun NH2-terminal kinase, JNK1 (4). The role of inhibitory (serine) phosphorylation of IRS-1 in the control of insulin signaling is well documented in rodents as well as in the human (2, 84). Studies of the interactive effects of GH receptor deletion with 30% calorie restriction resulted in identification of characteristics and responses related to insulin signaling which were consistently associated with alterations of longevity. These were present in animals in which longevity was extended by GH resistance or by CR but, importantly, were not present in animals in which CR failed to produce a further increase in lifespan (16, 40, 202).
XI. STRESS RESISTANCE AND RESPONSES IN THE CELLS AND TISSUES OF MICE WITH ALTERATION IN SOMATOTROPIC SIGNALING
Long-lived yeast with mutations in the Tor/S6K and Ras pathways, both major IGF-I signaling components, are remarkably resistant to a variety of stresses including paraquat, hydrogen peroxide, heat, and chemotherapy drugs (96, 245). Long-lived nematodes C. elegans with mutations in the insulin/IGF-I pathway also show an increased resistance to multiple forms of stress, including oxidative stress, heat, UV stress, and the heavy metals cadmium and copper (12, 173, 180, 216). It has been hypothesized that resistance to multiple forms of stress leads to delayed aging and extended longevity (98). Interestingly, the mean lifespan of worms can also be extended by transient exposure to nonlethal thermal stress, which induces a temporary increase in stress resistance (181). Similar observations have previously been reported in Drosophila after transient exposure to thermal stress (203). These results suggest that the relationship between stress resistance and lifespan found in C. elegans could also extend to mammals and that mutations that extend lifespan in mice may also render them resistant to oxidative damage and death caused by agents that induce oxidative stress. It was found that mouse embryonic fibroblast (MEF) cultures derived from hemizygous IGF-IR(+/−) mice are significantly more resistant to death induced by hydrogen peroxide than those derived from control mice (130). Female mice carrying a heterozygous deletion of this gene live longer than normal controls (130). Interestingly, hemizygous IGF-IR(+/−) mice survive significantly longer than controls following in vivo injection of paraquat, which causes massive oxidative damage. The increase in the in vivo stress resistance of these mutants is greater in females than in males, thus corresponding to their longevity (130). Another early example of increased stress resistance of long-lived mice involved animals with targeted mutation of the p66shc gene, a downstream effector of IGF-I receptor signaling, which were reported to live 28% longer than their controls (209). p66shc (molecular mass 66 kDa) is encoded by the protooncogene SHC locus and is a splice variant of p52shc and p46shc. p66shc was found to inhibit c-fos promoter activation, which occurs in response to environmental stress such as UV light and oxidative stress (209). Resembling findings in hemizygous IGF-IR(+/−) mice, MEFs from the p66shc mutant mice show resistance to apoptosis induced by H2O2 and ultraviolet light. Moreover, the p66shc mice also show increased survival after paraquat injections in vivo (209). However, interpretation of these results requires caution because of the relatively short lifespan of control mice in both of these studies. Thus the differences in lifespan, and perhaps also in resistance to the oxidative stress in these animals, may have in part reflected differences that might be unrelated to aging (163). Bokov and colleagues recently reported that in C57BL/6 mice, heterozygous deletion of IGF-I receptor caused a very modest (less than 5%) extension of longevity in females and no extension of longevity in males (36).
A series of in vitro experiments have shown that dermal fibroblast cell lines derived from adult long-lived Ames dwarf, Snell dwarf, and GHRKO mice are resistant, in culture, to the cytotoxic effects of multiple forms of stress including hydrogen peroxide, cadmium, ultraviolet light, paraquat, and heat (175, 204, 217, 253). Stress resistance of these fibroblasts was demonstrated in defined media in the absence of continued hormonal alterations and therefore might be due to epigenetic effects during early postnatal development (217, 253). Intriguingly, GH replacement sufficient to restore normal lifespan in Ames dwarf mice also led to a reversal of the resistance of skin-derived cells to multiple forms of stress (233). Thus cellular stress resistance appears to be determined by GH exposure during critical developmental windows (233). Ames dwarf mice have been shown to be very resistant to the lethal effects of exposure to the oxidative toxins diquat and paraquat in vivo (37, 290). Paraquat induces oxidative stress by catalyzing the generation of the superoxide anion and the oxidation of cellular NADPH. Diquat is a potent redox cycler and is readily converted to a free radical that, in reaction with molecular oxygen, generates superoxide anions and subsequently other redox products. These products can induce lipid peroxidation in cell membranes and potentially cause cell death. As measured by overall survival after a lethal dose of diquat or paraquat, Ames dwarf mice, both males and females, were much more resistant to both stressors than were normal littermates, and this resistance to paraquat toxicity was maintained with age in the Ames dwarf mice (37, 290).
Biochemical studies have begun to delineate stress response pathways that might contribute to the phenotype in long-lived mutant mice. The MAPKs comprise a ubiquitous group of signaling proteins that play a prominent role in regulating cell proliferation, differentiation, and adaptation, emerging as important regulators of cellular responses to various stimuli (59, 146). There are three major MAPK subfamilies, namely, the extracellular signal-regulated protein kinases (ERK), the c-Jun NH2-terminal kinases (JNK), and p38 MAPK. Each of these sets of kinases has been implicated in cell injury and disease pathogenesis (59, 146). Exposure of primary skin-derived fibroblasts to peroxide, cadmium, or paraquat leads to phosphorylation of the stress-activated kinases ERK1 and ERK2. Surprisingly, the level of ERK phosphorylation induced by these stresses is significantly lower in cells from dwarf mice. This suggests defects in the pathways that sense cellular injury and lead to activation of the kinase MEK, responsible for phosphorylation of ERK (292). Moreover, p38 phosphorylation response to the mitochondrial inhibitor rotenone was also attenuated in fibroblasts from Ames dwarf compared with normal mice (194).
Stress resistance of Snell dwarf mice was also studied by using 3-nitropropionic acid (3-NPA) challenging. 3-NPA induces mitochondrial free radical generation in liver tissue and activates MAPK signaling cascade. After treatment with 3-NPA, phosphorylation of ERK signaling is lower in the liver of Snell dwarf mice, suggesting that this tissue is protected, in vivo, from oxidative damage (194). Analysis of the responses of hepatic tissues to oxidative stress revealed dramatic differences in the pattern of stress-induced MAPK activation in Ames dwarf mice compared with controls (290, 292). While normal animals responded by increases in ERK and p38 phosphorylation, dwarf mice responded by increases in p38 and JNK activation. It is suggested that the lower levels of diquat-induced P-ERK and P-p38 or the higher levels of JNK phosphorylation may be part of the mechanism of resistance of dwarf mice to oxidative stress (290, 292).
XII. SOMATOTROPIC SIGNALING AT DIFFERENT STAGES OF LIFE HISTORY
Studies of GH-deficient, GH-resistant, and IGF-I-resistant mutant mice provided strong evidence that reducing somatotropic signaling can extend longevity. However, results of these studies did not allow for separating the importance of adult as opposed to developmental effects of GH and IGF-I on lifespan. The potential significance of early “developmental” events is emphasized by the recent appreciation of the impact of prenatal and early postnatal events on adult phenotype, presumably via epigenetic mechanisms. This includes rapidly accumulating evidence that prenatal events and birth weight can determine susceptibility to chronic disease in adulthood (9, 249, 286).
Several studies addressed the role of early versus adult actions of hormones of the somatotropic axis on rodent aging. Sonntag et al. (273) utilized genetically dwarf rats in which GH signaling is reduced but longevity is not altered and demonstrated that the treatment of these animals with exogenous GH between the ages of 4 and 14 wk produced a significant extension of longevity. The authors suggested that early GH treatment corrected developmental defects that may have been present in these animals, while adult GH deficiency (after GH treatment was stopped) protected them from cancer (273). In contrast with these observations, we have reported that treatment of Ames dwarf mice with GH limited to the period between 2 and 8 wk of age can be sufficient to drastically shorten longevity of these normally long-lived mutants (233). Further work will be needed to determine whether these results represent species differences or developmental differences between GH-deficient dwarf rats and hypopituitary Ames dwarf mice.
As was previously mentioned, Svensson et al. (296) recently described the effects of suppressing hepatic IGF-I expression in mice at the age of 1 mo using pharmacological induction of Cre-Lox system. This treatment reduced circulating IGF-I levels to approximately one-fifth of the levels measured in control animals, reduced growth, and extended mean lifespan of females (296). Extension of mouse longevity in response to reduced availability of nutrients between birth and weaning (286) likely involves suppression of IGF-I signaling during this period. Collectively, results of these studies suggest that alterations in the somatotropic signaling limited to either “early” or “adult” stages of life history can have significant impact on longevity. Precise delineation of the impact of GH and IGF-I at different stages of ontogency on aging, age-related disease, and lifespan in different species will be important for devising interventions that could produce health benefits.
XIII. IMPACT OF DIFFERENCES IN SOMATOTROPIC SIGNALING ON LONGEVITY OF GENETICALLY NORMAL ANIMALS
There is persuasive, although largely indirect, evidence that differences in somatotropic signaling lead to differences in longevity not only in mutants with complete absence of GH or GH receptors or in transgenic animals with pathologic GH excess, but also in genetically normal individuals. Much of this evidence is derived from studies relating lifespan to body size because somatic growth and adult body size strongly depend on the actions of GH and IGF-I. It has been known for over 50 years that in comparisons of mice from inbred strains, outbred stocks, or selected lines differing in body size, smaller animals live longer (22, 254, 324). Similar negative association of body size and lifespan is very robust and well documented in comparisons of different breeds of domestic dogs and in large cohorts of mixed breed dogs (86, 113, 238). In dogs, these differences have been related to circulating IGF-I levels (295). Negative correlation of body size and lifespan was described also in rats (248) and horses (47) as well as in various human data sets analyzed by Samaras (255). His important work on this relationship was summarized in a recent book (254).
In 2002, Miller et al. (212) reported that in a genetically heterogeneous population of mice derived from crossing four inbred strains, longevity of individual animals was negatively correlated with body weight measured in early adulthood. More recently, Yuan et al. (333) reported a similar negative correlation of adult plasma IGF-I levels and median lifespan in comparison of 31 inbred mouse strains. Sun et al. (286) reported that mice with mild nutritional restriction during lactation (produced by adding pups to a litter) were smaller than mice raised in smaller litters and lived significantly longer.
It should be mentioned that these results contrast sharply with the well-known positive correlation of body size and longevity in comparisons of animals (mammals or birds) from different species. In this context, it is intriguing that circulating IGF-I levels were recently reported to be generally lower in larger species (282). Discussion of the possible physiological significance of differences in body size comparisons “within species” versus “between species” and likely reasons for numerous exceptions from the overall relationships between body size and longevity are outside the scope of this article.
XIV. IS AGE-RELATED DECLINE IN GROWTH HORMONE LEVELS A RESULT OR A CAUSE OF AGING? CAN TREATMENT WITH GH SLOW DOWN OR REVERSE AGING?
As was mentioned earlier in this article, levels of circulating GH reach their maximum around the time of attaining adulthood and thereafter progressively decline. In the rat, age-related decrease of pulsatile GH release has been linked to altered secretion of somatostatin into portal circulation (274). Deconvolution analysis of data derived from frequent blood sampling in the human provided evidence for age-related alterations in the hypothalamic control, of GH secretion, its modulation by gonadal steroids, and in GH autofeedback leading to reduced GH secretion (310, 311). Reduced levels of GH that are a consistent finding in middle-aged and especially in elderly individuals (107, 215, 251, 309) can be viewed as a result (a symptom) and perhaps also as a “biomarker” of aging. Thus alterations in GH levels could be added to a long list of the effects of aging on various physiological parameters including circulating levels of gonadal sex steroids, and, in primates, adrenal dehydroepiandrosterone (DHEA) and its sulfate, which also decline with age.
However, it has been suggested that in addition to being the normal accompaniment of aging, decline in GH levels is also a cause of aging, or more precisely, a cause of many of its symptoms. This interpretation of the functional significance of age-related decline in GH release is based primarily on two sets of observations: 1) some of the symptoms of aging, including loss of muscle mass and bone mineral density (BMD), increased adiposity, reduced energy, decline in sexual activity and increased risk of developing psychological depression resemble symptoms of adult GHD; and 2) in young and middle-aged adults, most of the symptoms of GHD markedly improve in response to GH therapy (27, 264). This line of reasoning gained powerful support from a widely quoted landmark study of Rudman et al. (252) who reported in 1990 that GH treatment of elderly men reduced adiposity, increased lean body mass, increased BMD in some of the examined sites of the skeleton, and tended to increase skin thickness, i.e., succeeded in reversing some of the symptoms of aging. Not surprisingly, this report led to considerable interest in using GH treatment as an antiaging therapy. However, subsequent studies involving larger numbers of subjects and more rigorous design (double blind, placebo controlled rather than open label) were less successful in producing the desired outcomes and identified various side effects including troublesome evidence for producing insulin resistance (33, 165, 236). Meta-analysis of available data confirmed the ability of injected GH to cause beneficial although generally modest changes in body composition (reduced adiposity and increased muscle mass) but reiterated the evidence for side effects such as arthralgias and carpal tunnel syndrome and raised the issue of the potential risk of diabetes and cancer (182). Thus these analyses supported the currently prevailing opinion of clinical endocrinologists and guidelines from the Endocrine Society and the American Association of Clinical Endocrinologists that GH therapy in endocrinologically normal elderly must be considered as experimental treatment and cannot be recommended for routine use (182, 301).
It is interesting that in spite of these publications and recommendations and the fact that use of recombinant human GH as an antiaging treatment is specifically prohibited in the United States, GH along with various “GH-related products” continues to be aggressively promoted. Advertisements promise the users many appealing benefits including improvements in energy level and sex life, reversal of balding, and return of original hair color with assurances of looking and feeling younger. Although many of these claims appear to be grossly exaggerated (to say the least), the beneficial potential of administering GH or increasing its release in selected groups of elderly individuals deserves attention and continues to be explored. Of particular interest in this context is the evidence that treatment with ghrelin analogs can produce near-normal patterns of GH release and thus reduce the incidence and severity of untoward side effects (220, 221). However, ghrelin levels normally increase with age (293), perhaps as a result of reduced GH negative feedback (244), and in mice this has been linked to increased adiposity, reduced thermogenesis and development of insulin resistance (178). Moreover, the ability of GH to increase muscle mass suggests that combining GH treatment with a program of exercise could offer a therapeutic approach to treatment of sarcopenia and frailty, particularly in women in whom androgen therapy would be generally contraindicated. However, the benefits of such interventions remain to be conclusively shown (165, 301), and the risks of cancer and diabetes would have to be considered before recommending GH therapy.
It should be pointed out that the controversial issues concerning the relationship of declining GH levels to aging (whether a symptom or a cause), the possibility of using GH as an anti-aging agent and the risk-benefit considerations of such use mirror the corresponding and generally better understood issues concerning estrogen replacement in peri- and postmenopausal women and androgen therapy in elderly men. It should also be pointed out that while GH therapy may eventually find its place in the management of individual geriatric patients, there is considerable evidence (described earlier in this article) that action of GH during lifespan tends to accelerate rather than to slow or prevent aging and age-related diseases. This would suggest that age-related decline in GH-release can be viewed not only as a symptom or one of the mechanisms of aging but rather as an evolutionarily selected protective mechanism that reduces the risk of age-related disease, primarily cancer and type 2 diabetes. The latter interpretation would seem consistent with reduced lifespan and various indices of premature or accelerated aging in patients with GH secreting tumors (136, 229) and in transgenic mice overexpressing various GH genes (13). Moreover, increased serum IGF-I levels in hepatocyte-specific IGF-transgenic mice are associated with increased mortality (87).
GRANTS
Preparation of this article was supported by The National Institute on Aging via Grants P01 AG031736, R01 AG019899, and R21 AG038850 (to A. Bartke); AG20642, AG025135, P01 AG034906, Ted Bakewell (The Bakewell Foundation), and the V Foundation for Cancer Research (to V. Longo); and a University of Michigan Nathan Shock Center Pilot Grant (to L. Sun).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of P. Schafer with typing, formatting, and editing. We also thank Min Wei for assistance with the manuscript preparation. We apologize to those whose work pertinent to this topic was not cited or discussed due to limitations of space or inadvertent omissions.
Address for reprint requests and other correspondence: A. Bartke, Dept. of Internal Medicine, Geriatric Research, Southern Illinois University School of Medicine, Springfield, IL 62703 (e-mail: abartke@siumed.edu).
REFERENCES
- 1. Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Scientific World J 6: 53–80, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor I/insulin signaling and caloric restriction. Endocrinology 146: 851–860, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Al-Regaiey KA, Masternak MM, Bonkowski MS, Panici JA, Kopchick JJ, Bartke A. Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J Gerontol A Biol Sci Med Sci 62: 18–26, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Alcantara MR, Salvatori R, Alcantara PR, Nobrega LM, Campos VS, Oliveira EC, Oliveira MH, Souza AH, Aguiar-Oliveira MH. Thyroid morphology and function in adults with untreated isolated growth hormone deficiency. J Clin Endocrinol Metab 91: 860–864, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41: 45–55, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Amador-Noguez D, Dean A, Huang W, Setchell K, Moore D, Darlington G. Alterations in xenobiotic metabolism in the long-lived Little mice. Aging Cell 6: 453–470, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell 3: 423–441, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol 561: 355–377, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bachelot A, Monget P, Imbert-Bollore P, Coshigano K, Kopchick JJ, Kelly PA, Binart N. Growth hormone is required for ovarian follicular growth. Endocrinology 143: 4104–4112, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Banks WA, Morley JE, Farr SA, Price TO, Ercal N, Vidaurre I, Schally AV. Effects of a growth hormone-releasing hormone antagonist on telomerase activity, oxidative stress, longevity, and aging in mice. Proc Natl Acad Sci USA 107: 22272–22277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J 15: 627–634, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology 78: 210–216, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Bartke A. Differential requirement for prolactin during pregnancy in the mouse. Biol Reprod 9: 379–383, 1973 [DOI] [PubMed] [Google Scholar]
- 15. Bartke A. Influence of luteotrophin on fertility of dwarf mice. J Reprod Fertil 10: 93–103, 1965 [DOI] [PubMed] [Google Scholar]
- 16. Bartke A. Insulin and aging. Cell Cycle 7: 3338–3343, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Bartke A. Life extension in the dwarf mouse. In: Handbook of Models for the Study of Human Aging, edited by Conn PM. New York: Elsevier Academic, 2006, p. 403–414 [Google Scholar]
- 18. Bartke A. Prolactin-deficient mice. In: Animal Models for Research on Contraception and Fertility, edited by Alexander NJ. Hagerstown, MD: Harper & Row, 1979, p. 360–365 [Google Scholar]
- 19. Bartke A. Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci 366: 28–34, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol 63: 189–225, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides 36: 201–208, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Bartke A, Heiman M, Turyn D, Dominici F, Kopchick JJ. The role of growth hormone signaling in the control of ageing. In: The Neuroendocrine Immune Network in Aging, edited by Straub RH, Mocchegiani E. Berlin: Elsevier, 2004, p. 123–137 [Google Scholar]
- 23. Bartke A, Peluso MR, Moretz N, Wright C, Bonkowski M, Winters TA, Shanahan MF, Kopchick JJ, Banz WJ. Effects of Soy-derived diets on plasma and liver lipids, glucose tolerance, and longevity in normal, long-lived and short-lived mice. Horm Metab Res 36: 550–558, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the life span of long-lived mice. Nature 414: 412, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Bates DJ, Li N, Liang R, Sarojini H, An J, Masternak MM, Bartke A, Wang E. MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging. Aging Cell 9: 1–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauche IB, El Mkadem SA, Pottier AM, Senou M, Many MC, Rezsohazy R, Penicaud L, Maeda N, Funahashi T, Brichard SM. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology 148: 1539–1549, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Baum HB, Katznelson L, Sherman JC, Biller BM, Hayden DL, Schoenfeld DA, Cannistraro KE, Klibanski A. Effects of physiological growth hormone (GH) therapy on cognition and quality of life in patients with adult-onset GH deficiency. J Clin Endocrinol Metab 83: 3184–3189, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Bellush LL, Doublier S, Holland AN, Striker LJ, Striker GE, Kopchick JJ. Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology 141: 163–168, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res 14: 309–318, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology 147: 2801–2808, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O'Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci 65: 31–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Besson A, Salemi S, Gallati S, Jenal A, Horn R, Mullis PS, Mullis PE. Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab 88: 3664–3667, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O'Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288: 2282–2292, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572–574, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell 97: 865–875, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Bokov AF, Garg N, Ikeno Y, Thakur S, Musi N, DeFronzo RA, Zhang N, Erickson RC, Gelfond J, Hubbard GB, Adamo ML, Richardson A. Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS One 6: e26891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci 64: 819–827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonafè M, Olivieri F. Genetic polymorphism in long-lived people: cues for the presence of an insulin/IGF-pathway-dependent network affecting human longevity. Mol Cell Endocrinol 299: 118–123, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol 490: 25–31, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One 4: e4567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41. Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci 61: 562–567, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA 103: 7901–7905, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borg KE, Brown-Borg HM, Bartke A. Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse. Proc Soc Exp Biol Med 210: 126–133, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Boylston WH, Gerstner A, DeFord JH, Madsen M, Flurkey K, Harrison DE, Papaconstantinou J. Altered cholesterologenic and lipogenic transcriptional profile in livers of aging Snell dwarf (Pit1dw/dwJ) mice. Aging Cell 3: 283–296, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol 6: 515–525, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Brooks NL, Trent CM, Raetzsch CF, Flurkey K, Boysen G, Perfetti MT, Jeong YC, Klebanov S, Patel KB, Khodush VR, Kupper LL, Carling D, Swenberg JA, Harrison DE, Combs TP. Low utilization of circulating glucose after food withdrawal in Snell dwarf mice. J Biol Chem 282: 35069–35077, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Brosnahan MM, Paradis MR. Demographic and clinical characteristics of geriatric horses: 467 cases (1989–1999). J Am Vet Med Assoc 223: 93–98, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer life span, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA 102: 3105–3110, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown-Borg H, Johnson W, Rakoczy S, Romanick M. Mitochondrial oxidant generation and oxidative damage in Ames dwarf and GH transgenic mice. J Am Aging Assoc 24: 85–96, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the aging process. Nature 384: 33, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Cailleau J, Vermeire S, Verhoeven G. Independent control of the production of insulin-like growth factor I and its binding protein by cultured testicular cells. Mol Cell Endocrinol 69: 79–89, 1990 [DOI] [PubMed] [Google Scholar]
- 52. Camacho-Hubner C, Woods KA, Miraki-Moud F, Hindmarsh PC, Clark AJ, Hansson Y, Johnston A, Baxter RC, Savage MO. Effects of recombinant human insulin-like growth factor I (IGF-I) therapy on the growth hormone-IGF system of a patient with a partial IGF-I gene deletion. J Clin Endocrinol Metab 84: 1611–1616, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci 2: 894–897, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Ceda GP, Dall'Aglio E, Maggio M, Lauretani F, Bandinelli S, Falzoi C, Grimaldi W, Ceresini G, Corradi F, Ferrucci L, Valenti G, Hoffman AR. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest 28: 96–100, 2005 [PubMed] [Google Scholar]
- 55. Chandrashekar V, Bartke A. Induction of endogenous insulin-like growth factor-I secretion alters the hypothalamic-pituitary-testicular function in growth hormone-deficient adult dwarf mice. Biol Reprod 48: 544–551, 1993 [DOI] [PubMed] [Google Scholar]
- 56. Chandrashekar V, Bartke A, Coschigano KT, Kopchick JJ. Pituitary and testicular function in growth hormone receptor gene knockout mice. Endocrinology 140: 1082–1088, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Chandrashekar V, Dawson CR, Martin ER, Rocha JS, Bartke A, Kopchick JJ. Age-related alterations in pituitary and testicular functions in long-lived growth hormone receptor gene-disrupted mice. Endocrinology 148: 6019–6025, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biol Reprod 71: 17–27, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37–40, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Cheng CM, Mervis RF, Niu SL, Salem N, Jr, Witters LA, Tseng V, Reinhardt R, Bondy CA. Insulin-like growth factor 1 is essential for normal dendritic growth. J Neurosci Res 73: 1–9, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia 56: 791–800, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Choksi KB, Nuss JE, DeFord JH, Papaconstantinou J. Mitochondrial electron transport chain functions in long-lived Ames dwarf mice. Aging 3: 754–767, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coculescu M. Blood-brain barrier for human growth hormone and insulin-like growth factor-I. J Pediatr Endocrinol Metab 12: 113–124, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 139: 1157–1169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cohen P. Insulin-like growth factor binding protein-3: insulin-like growth factor independence comes of age. Endocrinology 147: 2109–2111, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Conover CA. PAPP-A: a new anti-aging target? Aging Cell 9: 942–946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends life span in mice. Aging Cell 6: 727–729, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Cornford AS, Barkan AL, Horowitz JF. Rapid suppression of growth hormone concentration by overeating: potential mediation by hyperinsulinemia. J Clin Endocrinol Metab 96: 824–830, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev 14: 20–39, 1993 [DOI] [PubMed] [Google Scholar]
- 70. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144: 3799–3810, 2003 [DOI] [PubMed] [Google Scholar]
- 71. D'Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol 13: 227–255, 1996 [DOI] [PubMed] [Google Scholar]
- 72. D'Ercole AJ, Ye P, O'Kusky JR. Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides 36: 209–220, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice: role of IGF-I. Endocrinology 140: 2637–2640, 1999 [DOI] [PubMed] [Google Scholar]
- 74. Davies JS, Gevers EF, Stevenson AE, Coschigano KT, El-Kasti MM, Bull MJ, Elford C, Evans BA, Kopchick JJ, Wells T. Adiposity profile in the dwarf rat: an unusually lean model of profound growth hormone deficiency. Am J Physiol Endocrinol Metab 292: E1483–E1494, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, Kopchick JJ, Friedman JE, Draznin B, Thorner MO. Growth hormone regulation of p85α expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes 56: 1638–1646, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Dell'Agnello C, Leo S, Agostino A, Szabadkai G, Tiveron C, Zulian A, Prelle A, Roubertoux P, Rizzuto R, Zeviani M. Increased longevity and refractoriness to Ca2+-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet 16: 431–444, 2007 [DOI] [PubMed] [Google Scholar]
- 77. Dhahbi J, Li X, Tran T, Masternak MM, Bartke A. Circulating blood leukocyte gene expression profiles: effects of the Ames dwarf mutation on pathways related to immunity and inflammation. Exp Gerontol 42: 772–788, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dominici FP, Arostegui Diaz G, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol 166: 579–590, 2000 [DOI] [PubMed] [Google Scholar]
- 79. Donahue CP, Jensen RV, Ochiishi T, Eisenstein I, Zhao M, Shors T, Kosik KS. Transcriptional profiling reveals regulated genes in the hippocampus during memory formation. Hippocampus 12: 821–833, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Donahue CP, Kosik KS, Shors TJ. Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proc Natl Acad Sci USA 103: 6031–6036, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Donahue LR, Beamer WG. Growth hormone deficiency in “little” mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J Endocrinol 136: 91–104, 1993 [DOI] [PubMed] [Google Scholar]
- 82. Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci USA 94: 4772–4777, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA 100: 14385–14390, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes 55: 2392–2397, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Eicher EM, Beamer WG. Inherited ateliotic dwarfism in mice. Characteristics of the mutation, little, on chromosome 6. J Hered 67: 87–91, 1976 [DOI] [PubMed] [Google Scholar]
- 86. Eigenmann JE, Amador A, Patterson DF. Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs and in giant dogs. Acta Endocrinol 118: 105–108, 1988 [DOI] [PubMed] [Google Scholar]
- 87. Elis S, Wu Y, Courtland HW, Sun H, Rosen CJ, Adamo ML, Yakar S. Increased serum IGF-1 levels protect the musculoskeletal system but are associated with elevated oxidative stress markers and increased mortality independent of tissue igf1 gene expression. Aging Cell 10: 547–550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317, 1998 [DOI] [PubMed] [Google Scholar]
- 89. Esquifino AI, Szary A, Brown-Borg HM, Bartke A. Age-related effects of ectopic pituitary transplants on the activation of Ames dwarf mouse lymphocytes in vitro. Proc Soc Exp Biol Med 211: 87–93, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Esquifino AI, Villanua MA, Szary A, Yau J, Bartke A. Ectopic pituitary transplants restore immunocompetence in Ames dwarf mice. Acta Endocrinol 125: 67–72, 1991 [DOI] [PubMed] [Google Scholar]
- 91. Everitt AV, Seedsman NJ, Jones F. The effects of hypophysectomy and continuous food restriction, begun at ages 70 and 400 days, on collagen aging, proteinuria, and incidence of pathology and longevity in the male rat. Mech Ageing Dev 12: 161–172, 1980 [DOI] [PubMed] [Google Scholar]
- 92. Fabris N, Pierpaoli W, Sorkin E. Lymphocytes, hormones and ageing. Nature 240: 557–559, 1972 [DOI] [PubMed] [Google Scholar]
- 93. Fabrizio P, Li L, Longo VD. Analysis of gene expression profile in yeast aging chronologically. Mech Ageing Dev 126: 11–16, 2005 [DOI] [PubMed] [Google Scholar]
- 94. Fabrizio P, Liou LL, Moy VN, Diaspro A, Valentine JS, Gralla EB, Longo VD. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics 163: 35–46, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fabrizio P, Pletcher SD, Minois N, Vaupel JW, Longo VD. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett 557: 136–142, 2004 [DOI] [PubMed] [Google Scholar]
- 96. Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science 292: 288–290, 2001 [DOI] [PubMed] [Google Scholar]
- 97. Fernandez-Banares I, Clotet J, Arino J, Guinovart JJ. Glycogen hyperaccumulation in Saccharomyces cerevisiae ras2 mutant. A biochemical study. FEBS Lett 290: 38–42, 1991 [DOI] [PubMed] [Google Scholar]
- 98. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 99. Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci USA 106: 2700–2705, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Life span extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA 98: 6736–6741, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science 328: 321–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Forsyth IA, Folley SJ, Chadwick A. Lactogenic and pigeon crop-stimulating activities of human pituitary growth hormone preparation. J Endocrinol 31: 115–126, 1965 [DOI] [PubMed] [Google Scholar]
- 103. Gage FH. Neurogenesis in the adult brain. J Neurosci 22: 612–613, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Garcia AM, Busuttil RA, Calder RB, Dollé MET, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev 129: 528–533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci 60: 688–694, 2005 [DOI] [PubMed] [Google Scholar]
- 106. Gesing A, Bartke A, Wang F, Karbownik-Lewinska M, Masternak MM. Renal pro-apoptotic proteins are reduced by growth hormone resistance but not by visceral fat removal. Biol Chem 392: 475–481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19: 717–797, 1998 [DOI] [PubMed] [Google Scholar]
- 108. Golde DW, Bersch N, Li CH. Growth hormone: species-specific stimulation of erythropoiesis in vitro. Science 196: 1112–1113, 1977 [DOI] [PubMed] [Google Scholar]
- 109. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260–265, 1999 [DOI] [PubMed] [Google Scholar]
- 110. Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA 96: 5263–5267, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry 48: 715–720, 2000 [DOI] [PubMed] [Google Scholar]
- 112. Greer EL, Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol 192: 19–28, 2008 [DOI] [PubMed] [Google Scholar]
- 113. Greer KA, Canterberry SC, Murphy KE. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res Vet Sci 82: 208–214, 2007 [DOI] [PubMed] [Google Scholar]
- 114. Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature 408: 255–262, 2000 [DOI] [PubMed] [Google Scholar]
- 115. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3: 70ra13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hall MA, Bartke A, Martinko JM. Humoral immune response in mice over-expressing or deficient in growth hormone. Exp Biol Med 227: 535–544, 2002 [DOI] [PubMed] [Google Scholar]
- 117. Haluzik M, Yakar S, Gavrilova O, Setser J, Boisclair Y, LeRoith D. Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: relative roles of growth hormone and IGF-1 in insulin resistance. Diabetes 52: 2483–2489, 2003 [DOI] [PubMed] [Google Scholar]
- 118. Han VK, Smith A, Myint W, Nygard K, Bradshaw S. Mitogenic activity of epidermal growth factor on newborn rat astroglia: interaction with insulin-like growth factors. Endocrinology 131: 1134–1142, 1992 [DOI] [PubMed] [Google Scholar]
- 119. Harper JM, Wilkinson JE, Miller RA. Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction. FASEB J 24: 2436–2442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends life span in genetically heterogeneous mice. Nature 460: 392–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hartman ML, Veldhuis JD, Thorner MO. Normal control of growth hormone secretion. Horm Res 40: 37–47, 1993 [DOI] [PubMed] [Google Scholar]
- 122. Hartree AS, Kovacic N, Thomas M. Growth-promoting and luteotrophic activities of human growth hormone. J Endocrinol 33: 249–258, 1965 [DOI] [PubMed] [Google Scholar]
- 123. Harvey S, Hull K. Neural growth hormone: an update. J Mol Neurosci 20: 1–14, 2003 [DOI] [PubMed] [Google Scholar]
- 124. Harvey S, Hull KL. Growth hormone. A paracrine growth factor? Endocrine 7: 267–279, 1997 [DOI] [PubMed] [Google Scholar]
- 125. Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med 226: 552–558, 2001 [DOI] [PubMed] [Google Scholar]
- 126. Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab 292: E1647–E1655, 2007 [DOI] [PubMed] [Google Scholar]
- 127. Helms SA, Azhar G, Zuo C, Theus SA, Bartke A, Wei JY. Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of Ames dwarf mice. Int J Biol Sci 6: 475–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, Thorner MO. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest 81: 968–975, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Holehan AM, Merry BJ. Lifetime breeding studies in fully fed and dietary restricted female CFY Sprague-Dawley rats. 1. Effect of age, housing conditions and diet on fecundity. Mech Ageing Dev 33: 19–28, 1985 [DOI] [PubMed] [Google Scholar]
- 130. Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates life span and resistance to oxidative stress in mice. Nature 421: 182–187, 2003 [DOI] [PubMed] [Google Scholar]
- 131. Hsu CJ, Hammond JM. Gonadotropins and estradiol stimulate immunoreactive insulin-like growth factor-I production by porcine granulosa cells in vitro. Endocrinology 120: 198–207, 1987 [DOI] [PubMed] [Google Scholar]
- 132. Hunter WS, Croson WB, Bartke A, Gentry MV, Meliska CJ. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol Behav 67: 433–437, 1999 [DOI] [PubMed] [Google Scholar]
- 133. Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci 58: 291–296, 2003 [DOI] [PubMed] [Google Scholar]
- 134. Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci 64: 522–529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ishii DN. Relationship of insulin-like growth factor II gene expression in muscle to synaptogenesis. Proc Natl Acad Sci USA 86: 2898–2902, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jadresic A, Banks LM, Child DF, Diamant L, Doyle FH, Fraser TR, Joplin GF. The acromegaly syndrome. Relation between clinical features, growth hormone values and radiological characteristics of the pituitary tumours. Q J Med 51: 189–204, 1982 [PubMed] [Google Scholar]
- 137. Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131: 3897–3906, 2004 [DOI] [PubMed] [Google Scholar]
- 138. Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2: 175–183, 2003 [DOI] [PubMed] [Google Scholar]
- 139. Johansson JO, Larson G, Andersson M, Elmgren A, Hynsjo L, Lindahl A, Lundberg PA, Isaksson OG, Lindstedt S, Bengtsson BA. Treatment of growth hormone-deficient adults with recombinant human growth hormone increases the concentration of growth hormone in the cerebrospinal fluid and affects neurotransmitters. Neuroendocrinology 61: 57–66, 1995 [DOI] [PubMed] [Google Scholar]
- 140. Johnson TE, Lithgow GJ, Murakami S. Hypothesis: interventions that increase the response to stress offer the potential for effective life prolongation and increased health. J Gerontol A Biol Sci Med Sci 51: B392–395, 1996 [DOI] [PubMed] [Google Scholar]
- 141. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 26: 439–451, 2005 [DOI] [PubMed] [Google Scholar]
- 142. Kanety H, Hemi R, Ginsberg S, Pariente C, Yissachar E, Barhod E, Funahashi T, Laron Z. Total and high molecular weight adiponectin are elevated in patients with Laron syndrome despite marked obesity. Eur J Endocrinol 161: 837–844, 2009 [DOI] [PubMed] [Google Scholar]
- 143. Kaplan SA, Cohen P. The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab 92: 4529–4535, 2007 [DOI] [PubMed] [Google Scholar]
- 144. Kappeler L, De Magalhaes Filho C, Dupont J, Leneuve P, Cervera P, Perin L, Loudes C, Blaise A, Klein R, Epelbaum J, Le Bouc Y, Holzenberger M. Brain IGF-1 receptors control mammalian growth and life span through a neuroendocrine mechanism. PLoS Biol 6: e254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kar S, Seto D, Dore S, Hanisch U, Quirion R. Insulin-like growth factors-I and -II differentially regulate endogenous acetylcholine release from the rat hippocampal formation. Proc Natl Acad Sci USA 94: 14054–14059, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Karin M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann NY Acad Sci 851: 139–146, 1998 [DOI] [PubMed] [Google Scholar]
- 147. Keene DE, Suescun MO, Bostwick MG, Chandrashekar V, Bartke A, Kopchick JJ. Puberty is delayed in male growth hormone receptor gene-disrupted mice. J Androl 23: 661–668, 2002 [PubMed] [Google Scholar]
- 148. Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 52: 135–143, 2002 [DOI] [PubMed] [Google Scholar]
- 149. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature 386: 493–495, 1997 [DOI] [PubMed] [Google Scholar]
- 150. Kennedy MA, Rakoczy SG, Brown-Borg HM. Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp Gerontol 38: 997–1008, 2003 [DOI] [PubMed] [Google Scholar]
- 151. Kenyon C. A pathway that links reproductive status to life span in Caenorhabditis elegans. Ann NY Acad Sci 1204: 156–162, 2010 [DOI] [PubMed] [Google Scholar]
- 152. Kenyon CJ. The genetics of ageing. Nature 464: 504–512, 2010 [DOI] [PubMed] [Google Scholar]
- 153. Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946, 1997 [DOI] [PubMed] [Google Scholar]
- 154. Kinney BA, Coschigano KT, Kopchick JJ, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav 72: 653–660, 2001 [DOI] [PubMed] [Google Scholar]
- 155. Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav 39: 277–284, 2001 [DOI] [PubMed] [Google Scholar]
- 156. Kopchick JJ. History and future of growth hormone research. Horm Res 60 Suppl 3: 103–112, 2003 [DOI] [PubMed] [Google Scholar]
- 157. Kopchick JJ, Andry JM. Growth hormone (GH), GH receptor, and signal transduction. Mol Genet Metab 71: 293–314, 2000 [DOI] [PubMed] [Google Scholar]
- 158. Kristan DM. Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell 6: 817–825, 2007 [DOI] [PubMed] [Google Scholar]
- 159. Krzisnik C, Kolacio Z, Battelino T, Brown M, Parks JS, Laron Z. The “Little People” of the island of Krk–revisited. Etiology of hypopituitarism revealed. J Endocr Genet 1: 9–19, 1999 [Google Scholar]
- 160. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027–2033, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomur I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kuwahara S, Kesuma Sari D, Tsukamoto Y, Tanaka S, Sasaki F. Age-related changes in growth hormone (GH)-releasing hormone and somatostatin neurons in the hypothalamus and in GH cells in the anterior pituitary of female mice. Brain Res 1025: 113–122, 2004 [DOI] [PubMed] [Google Scholar]
- 163. Ladiges W, Van Remmen H, Strong R, Ikeno Y, Treuting P, Rabinovitch P, Richardson A. Life span extension in genetically modified mice. Aging Cell 8: 346–352, 2009 [DOI] [PubMed] [Google Scholar]
- 164. Lai ZN, Emtner M, Roos P, Nyberg F. Characterization of putative growth hormone receptors in human choroid plexus. Brain Res 546: 222–226, 1991 [DOI] [PubMed] [Google Scholar]
- 165. Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, Juul A, Bulow J, Kjaer M. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab 87: 513–523, 2002 [DOI] [PubMed] [Google Scholar]
- 166. Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 7: 225–235, 2006 [DOI] [PubMed] [Google Scholar]
- 167. Laron Z. Development and biological function of the female gonads and genitalia in IGF-I deficiency: Laron syndrome as a model. Pediatr Endocrinol Rev 3 Suppl 1: 188–191, 2006 [PubMed] [Google Scholar]
- 168. Laron Z, Ginsberg S, Lilos P, Arbiv M, Vaisman N. Long-term IGF-I treatment of children with Laron syndrome increases adiposity. Growth Horm IGF Res 16: 61–64, 2006 [DOI] [PubMed] [Google Scholar]
- 169. Laron Z, Ginsberg S, Webb M. Nonalcoholic fatty liver in patients with Laron syndrome and GH gene deletion: preliminary report. Growth Horm IGF Res 18: 434–438, 2008 [DOI] [PubMed] [Google Scholar]
- 170. Laron Z, Klinger B. Body fat in Laron syndrome patients: effect of insulin-like growth factor I treatment. Horm Res 40: 16–22, 1993 [DOI] [PubMed] [Google Scholar]
- 171. Laron Z, Pertzelan A, Karp M. Pituitary dwarfism with high serum levels of growth hormone. Isr J Med Sci 4: 883–894, 1968 [PubMed] [Google Scholar]
- 172. Laron Z, Pertzelan A, Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone: a new inborn error of metabolism? Isr J Med Sci 2: 152–155, 1966 [PubMed] [Google Scholar]
- 173. Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci USA 90: 8905–8909, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lee KH, Calikoglu AS, Ye P, D'Ercole AJ. Insulin-like growth factor-I (IGF-I) ameliorates and IGF binding protein-1 (IGFBP-1) exacerbates the effects of undernutrition on brain growth during early postnatal life: studies in IGF-I and IGFBP-1 transgenic mice. Pediatr Res 45: 331–336, 1999 [DOI] [PubMed] [Google Scholar]
- 175. Leiser SF, Salmon AB, Miller RA. Correlated resistance to glucose deprivation and cytotoxic agents in fibroblast cell lines from long-lived pituitary dwarf mice. Mech Ageing Dev 127: 821–829, 2006 [DOI] [PubMed] [Google Scholar]
- 176. Li Q, Ren J. Influence of cardiac-specific overexpression of insulin-like growth factor 1 on life span and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell 6: 799–806, 2007 [DOI] [PubMed] [Google Scholar]
- 177. Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347: 528–533, 1990 [DOI] [PubMed] [Google Scholar]
- 178. Lin L, Saha PK, Ma X, Henshaw IO, Shao L, Chang BH, Buras ED, Tong Q, Chan L, McGuinness OP, Sun Y. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell 10: 996–1010, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J, Cruz-Topete D, Kopchick JJ. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr Rev 32: 356–386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol 49: B270–276, 1994 [DOI] [PubMed] [Google Scholar]
- 181. Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA 92: 7540–7544, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med 146: 104–115, 2007 [DOI] [PubMed] [Google Scholar]
- 183. Liu JL, Coschigano KT, Robertson K, Lipsett M, Guo Y, Kopchick JJ, Kumar U, Liu YL. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab 287: E405–E413, 2004 [DOI] [PubMed] [Google Scholar]
- 184. Liu W, Ye P, O'Kusky JR, D'Ercole AJ. Type 1 insulin-like growth factor receptor signaling is essential for the development of the hippocampal formation and dentate gyrus. J Neurosci Res 87: 2821–2832, 2009 [DOI] [PubMed] [Google Scholar]
- 185. Llorens-Martin M, Torres-Aleman I, Trejo JL. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist 15: 134–148, 2009 [DOI] [PubMed] [Google Scholar]
- 186. Longo VD. The Chronological Life Span of Saccharomyces cerevisiae: Studies of Superoxide Dismutases, Ras, and Bci-2 (thesis) Los Angeles: Univ. of California, Los Angeles, 1997 [Google Scholar]
- 187. Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299: 1342–1346, 2003 [DOI] [PubMed] [Google Scholar]
- 188. Longo VD, Lieber MR, Vijg J. Turning anti-ageing genes against cancer. Nat Rev Mol Cell Biol 9: 903–910, 2008 [DOI] [PubMed] [Google Scholar]
- 189. Luo N, Wang X, Chung BH, Lee MH, Klein RL, Garvey WT, Fu Y. Effects of macrophage-specific adiponectin expression on lipid metabolism in vivo. Am J Physiol Endocrinol Metab 301: E180–E186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor I in mouse postnatal growth. Dev Biol 229: 141–162, 2001 [DOI] [PubMed] [Google Scholar]
- 191. Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology 148: 300–309, 2007 [DOI] [PubMed] [Google Scholar]
- 192. Madia F, Frisullo G, Nociti V, Conte A, Luigetti M, Del Grande A, Patanella AK, Iorio R, Tonali PA, Batocchi AP, Sabatelli M. pSTAT1, pSTAT3, and T-bet as markers of disease activity in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst 14: 107–117, 2009 [DOI] [PubMed] [Google Scholar]
- 193. Madia F, Gattazzo C, Wei M, Fabrizio P, Burhans WC, Weinberger M, Galbani A, Smith JR, Nguyen C, Huey S, Comai L, Longo VD. Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J Cell Biol 180: 67–81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Madsen MA, Hsieh CC, Boylston WH, Flurkey K, Harrison D, Papaconstantinou J. Altered oxidative stress response of the long-lived Snell dwarf mouse. Biochem Biophys Res Commun 318: 998–1005, 2004 [DOI] [PubMed] [Google Scholar]
- 195. Maggio M, Ble A, Ceda GP, Metter EJ. Decline in insulin-like growth factor-1 levels across adult life span in two large population studies. J Gerontol A Biol Sci Med Sci 61: 182–183, 2006 [DOI] [PubMed] [Google Scholar]
- 196. Mahmoud GS, Grover LM. Growth hormone enhances excitatory synaptic transmission in area CA1 of rat hippocampus. J Neurophysiol 95: 2962–2974, 2006 [DOI] [PubMed] [Google Scholar]
- 197. Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience 87: 559–569, 1998 [DOI] [PubMed] [Google Scholar]
- 198. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Bonkowski MS, Panici JA, Przybylski GK, Bartke A. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci 60: 1238–1245, 2005 [DOI] [PubMed] [Google Scholar]
- 199. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Kopchick JJ, Bartke A. Effects of caloric restriction and growth hormone resistance on the expression level of peroxisome proliferator-activated receptors superfamily in liver of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci 60: 1394–1398, 2005 [DOI] [PubMed] [Google Scholar]
- 200. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Kopchick JJ, Wang Z, Bartke A. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol 41: 417–429, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Masternak MM, Bartke A, Wang F, Spong A, Gesing A, Fang Y, Salmon AB, Hughes LF, Liberati T, Boparai R, Kopchick JJ, Westbrook R. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell 11: 73–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci 64: 516–521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Maynard Smith J. Acclimatization to high temperatures in inbred and outbred Drosophila subobscura. J Genet 84: 37–45, 2005 [DOI] [PubMed] [Google Scholar]
- 204. Maynard SP, Miller RA. Fibroblasts from long-lived Snell dwarf mice are resistant to oxygen-induced in vitro growth arrest. Aging Cell 5: 89–96, 2006 [DOI] [PubMed] [Google Scholar]
- 205. McCormick MA, Tsai SY, Kennedy BK. TOR and ageing: a complex pathway for a complex process. Philos Trans R Soc Lond B Biol Sci 366: 17–27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. McElwee JJ, Schuster E, Blanc E, Piper MD, Thomas JH, Patel DS, Selman C, Withers DJ, Thornton JM, Partridge L, Gems D. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol 8: R132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Menezes Oliveira JL, Marques-Santos C, Barreto-Filho JA, Ximenes Filho R, de Oliveira Britto AV, Oliveira Souza AH, Prado CM, Pereira Oliveira CR, Pereira RM, Ribeiro Vicente Tde A, Farias CT, Aguiar-Oliveira MH, Salvatori R. Lack of evidence of premature atherosclerosis in untreated severe isolated growth hormone (GH) deficiency due to a GH-releasing hormone receptor mutation. J Clin Endocrinol Metab 91: 2093–2099, 2006 [DOI] [PubMed] [Google Scholar]
- 208. Mertani HC, Delehaye-Zervas MC, Martini JF, Postel-Vinay MC, Morel G. Localization of growth hormone receptor messenger RNA in human tissues. Endocrine 3: 135–142, 1995 [DOI] [PubMed] [Google Scholar]
- 209. Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402: 309–313, 1999 [DOI] [PubMed] [Google Scholar]
- 210. Miller BH, Gore AC. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and ageing. J Neuroendocrinol 13: 728–736, 2001 [DOI] [PubMed] [Google Scholar]
- 211. Miller RA, Chang Y, Galecki AT, Al-Regaiey K, Kopchick JJ, Bartke A. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol Endocrinol 16: 2657–2666, 2002 [DOI] [PubMed] [Google Scholar]
- 212. Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell 1: 22–29, 2002 [DOI] [PubMed] [Google Scholar]
- 213. Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus 16: 233–238, 2006 [DOI] [PubMed] [Google Scholar]
- 214. Muller E, Cella S, DeGennaro Colonna V, Parenti M, Cocchi D, Locatelli V. Aspects of the neuroendocrine control of growth hormone secretion in ageing mammals. J Reprod Fertil Suppl 46: 99–114, 1993 [PubMed] [Google Scholar]
- 215. Muniyappa R, Sorkin JD, Veldhuis JD, Harman SM, Munzer T, Bhasin S, Blackman MR. Long-term testosterone supplementation augments overnight growth hormone secretion in healthy older men. Am J Physiol Endocrinol Metab 293: E769–E775, 2007 [DOI] [PubMed] [Google Scholar]
- 216. Murakami S, Johnson TE. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics 143: 1207–1218, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J 17: 1565–1566, 2003 [DOI] [PubMed] [Google Scholar]
- 218. Murphy CT. The search for DAF-16/FOXO transcriptional targets: approaches and discoveries. Exp Gerontol 41: 910–921, 2006 [DOI] [PubMed] [Google Scholar]
- 219. Mustafa A, Adem A, Roos P, Nyberg F. Sex differences in binding of human growth hormone to rat brain. Neurosci Res 19: 93–99, 1994 [DOI] [PubMed] [Google Scholar]
- 220. Nass R, Gaylinn BD, Thorner MO. The role of ghrelin in GH secretion and GH disorders. Mol Cell Endocrinol 340: 10–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FE, Jr, Clasey JL, Heymsfield SB, Bach MA, Vance ML, Thorner MO. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med 149: 601–611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 39: 569–578, 1999 [DOI] [PubMed] [Google Scholar]
- 223. Noale M, Maggi S, Zanoni S, Limongi F, Zambon S, Crepaldi G. The metabolic syndrome, incidence of diabetes and mortality among the elderly: The Italian Longitudinal Study of Ageing. Diabetes Metab 2011 [Google Scholar]
- 224. Nyberg F. Growth hormone in the brain: characteristics of specific brain targets for the hormone and their functional significance. Front Neuroendocrinol 21: 330–348, 2000 [DOI] [PubMed] [Google Scholar]
- 225. Oliveira CR, Salvatori R, Meneguz-Moreno RA, Aguiar-Oliveira MH, Pereira RM, Valenca EH, Araujo VP, Farias NT, Silveira DC, Vieira JG, Barreto-Filho J. Adipokine profile and urinary albumin excretion in isolated growth hormone deficiency. J Clin Endocrinol Metab 95: 693–698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Oliveira JL, Aguiar-Oliveira MH, D'Oliveira A, Jr, Pereira RM, Oliveira CR, Farias CT, Barreto-Filho JA, Anjos-Andrade FD, Marques-Santos C, Nascimento-Junior AC, Alves EO, Oliveira FT, Campos VC, Ximenes R, Blackford A, Parmigiani G, Salvatori R. Congenital growth hormone (GH) deficiency and atherosclerosis: effects of GH replacement in GH-naive adults. J Clin Endocrinol Metab 92: 4664–4670, 2007 [DOI] [PubMed] [Google Scholar]
- 227. Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus 16: 250–260, 2006 [DOI] [PubMed] [Google Scholar]
- 228. Olsson B, Bohlooly YM, Fitzgerald SM, Frick F, Ljungberg A, Ahren B, Tornell J, Bergstrom G, Oscarsson J. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology 146: 920–930, 2005 [DOI] [PubMed] [Google Scholar]
- 229. Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab 83: 2730–2734, 1998 [DOI] [PubMed] [Google Scholar]
- 230. Otabe S, Yuan X, Fukutani T, Wada N, Hashinaga T, Nakayama H, Hirota N, Kojima M, Yamada K. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am J Physiol Endocrinol Metab 293: E210–E218, 2007 [DOI] [PubMed] [Google Scholar]
- 231. Pahlavani MA. Influence of caloric restriction on aging immune system. J Nutr Health Aging 8: 38–47, 2004 [PubMed] [Google Scholar]
- 232. Pan W, Yu Y, Cain CM, Nyberg F, Couraud PO, Kastin AJ. Permeation of growth hormone across the blood-brain barrier. Endocrinology 146: 4898–4904, 2005 [DOI] [PubMed] [Google Scholar]
- 233. Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J 24: 5073–5079, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Paolisso G, Gambardella A, Ammendola S, D'Amore A, Balbi V, Varricchio M, D'Onofrio F. Glucose tolerance and insulin action in healty centenarians. Am J Physiol Endocrinol Metab 270: E890–E894, 1996 [DOI] [PubMed] [Google Scholar]
- 235. Papaconstantinou J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol Cell Endocrinol 299: 89–100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Papadakis MA, Hamon G, Stotts N, Tierney MJ, Martin Spencer E, Scheuenstuhl H, Hunt TK. Effect of growth hormone replacement on wound healing in healthy older men. Wound Repair Regen 4: 421–425, 1996 [DOI] [PubMed] [Google Scholar]
- 237. Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology 136: 2013–2021, 1995 [DOI] [PubMed] [Google Scholar]
- 238. Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci 52: B171–178, 1997 [DOI] [PubMed] [Google Scholar]
- 239. Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 8: 460–472, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Pedruzzi I, Dubouloz F, Cameroni E, Wanke V, Roosen J, Winderickx J, De Virgilio C. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol Cell 12: 1607–1613, 2003 [DOI] [PubMed] [Google Scholar]
- 241. Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM, Fernandez-Llebrez P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci 31: 1533–1548, 2010 [DOI] [PubMed] [Google Scholar]
- 242. Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol 46: 96–99, 2011 [DOI] [PubMed] [Google Scholar]
- 243. Powers RW, 3rd, Harrison DE, Flurkey K. Pituitary removal in adult mice increases life span. Mech Ageing Dev 127: 658–659, 2006 [DOI] [PubMed] [Google Scholar]
- 244. Qi X, Reed J, Englander EW, Chandrashekar V, Bartke A, Greeley GH., Jr Evidence that growth hormone exerts a feedback effect on stomach ghrelin production and secretion. Exp Biol Med 228: 1028–1032, 2003 [DOI] [PubMed] [Google Scholar]
- 245. Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA 105: 8215–8220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Ratajczak J, Shin DM, Wan W, Liu R, Masternak MM, Piotrowska K, Wiszniewska B, Kucia M, Bartke A, Ratajczak MZ. Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice: novel view on Igf-1, stem cells and aging. Leukemia 25: 729–733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Recio-Pinto E, Rechler MM, Ishii DN. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci 6: 1211–1219, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev 4: 55–61, 2002 [DOI] [PubMed] [Google Scholar]
- 249. Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Adult survival after prenatal exposure to the Dutch famine 1944–45. Paediatr Perinat Epidemiol 15: 220–225, 2001 [DOI] [PubMed] [Google Scholar]
- 250. Rozing MP, Westendorp RG, de Craen AJ, Frolich M, de Goeij MC, Heijmans BT, Beekman M, Wijsman CA, Mooijaart SP, Blauw GJ, Slagboom PE, van Heemst D. Favorable glucose tolerance and lower prevalence of metabolic syndrome in offspring without diabetes mellitus of nonagenarian siblings: the Leiden longevity study. J Am Geriatr Soc 58: 564–569, 2010 [DOI] [PubMed] [Google Scholar]
- 251. Rudman D. Growth hormone, body composition, and aging. J Am Geriatr Soc 33: 800–807, 1985 [DOI] [PubMed] [Google Scholar]
- 252. Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med 323: 1–6, 1990 [DOI] [PubMed] [Google Scholar]
- 253. Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab 289: E23–E29, 2005 [DOI] [PubMed] [Google Scholar]
- 254. Samaras TT. Human Body Size and the Laws of Scaling: Physiological, Performance, Growth, Longevity and Ecological Ramifications. New York: Nova Science, 2007 [Google Scholar]
- 255. Samaras TT, Elrick H, Storms LH. Is height related to longevity? Life Sci 72: 1781–1802, 2003 [DOI] [PubMed] [Google Scholar]
- 256. Sanz A, Bartke A, Barja G. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. J Am Aging Assoc 25: 119–122, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Schaible R, Gowen JW. A new dwarf mouse. Genetics 46: 896, 1961 [Google Scholar]
- 258. Scheepens A, Sirimanne E, Beilharz E, Breier BH, Waters MJ, Gluckman PD, Williams CE. Alterations in the neural growth hormone axis following hypoxic-ischemic brain injury. Brain Res 68: 88–100, 1999 [DOI] [PubMed] [Google Scholar]
- 259. Schneider GB. Immunological competence in Snell-Bagg pituitary dwarf mice: response to the contact-sensitizing agent oxazolone. Am J Anat 145: 371–393, 1976 [DOI] [PubMed] [Google Scholar]
- 260. Sell C, Lorenzini S. Aging in IGF-1 hypomorphic mice. In: The American Aging Association 36th Annual Meeting San Antonio, TX: American Aging Association, 2007 [Google Scholar]
- 261. Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for life span extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J 22: 807–818, 2008 [DOI] [PubMed] [Google Scholar]
- 262. Selman C, Lingard S, Gems D, Partridge L, Withers DJ. Comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis”. Science 320: 1012, 2008 [DOI] [PubMed] [Google Scholar]
- 263. Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326: 140–144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Shalet SM, Toogood A, Rahim A, Brennan BM. The diagnosis of growth hormone deficiency in children and adults. Endocr Rev 19: 203–223, 1998 [DOI] [PubMed] [Google Scholar]
- 265. Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med Sci 60: 293–300, 2005 [DOI] [PubMed] [Google Scholar]
- 266. Shire JG. Growth hormone and premature ageing. Nature 245: 215–216, 1973 [DOI] [PubMed] [Google Scholar]
- 267. Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372–376, 2001 [DOI] [PubMed] [Google Scholar]
- 268. Silberberg R. Articular aging and osteoarthrosis in dwarf mice. Pathol Microbiol 38: 417–430, 1972 [DOI] [PubMed] [Google Scholar]
- 269. Smith WC, Linzer DI, Talamantes F. Detection of two growth hormone receptor mRNAs and primary translation products in the mouse. Proc Natl Acad Sci USA 85: 9576–9579, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Snell GD. Dwarf, a New Mendelian Recessive Character of the House Mouse. Proc Natl Acad Sci USA 15: 733–734, 1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature 417: 39–44, 2002 [DOI] [PubMed] [Google Scholar]
- 272. Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-d-aspartate receptor subtype expression in rats. Brain Res Bull 51: 331–338, 2000 [DOI] [PubMed] [Google Scholar]
- 273. Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology 146: 2920–2932, 2005 [DOI] [PubMed] [Google Scholar]
- 274. Sonntag WE, Gottschall PE, Meites J. Increased secretion of somatostatin-28 from hypothalamic neurons of aged rats in vitro. Brain Res 380: 229–234, 1986 [DOI] [PubMed] [Google Scholar]
- 275. Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev 4: 195–212, 2005 [DOI] [PubMed] [Google Scholar]
- 276. Sonntag WE, Steger RW, Forman LJ, Meites J. Decreased pulsatile release of growth hormone in old male rats. Endocrinology 107: 1875–1879, 1980 [DOI] [PubMed] [Google Scholar]
- 277. Sonntag WE, Xu X, Ingram RL, D'Costa A. Moderate caloric restriction alters the subcellular distribution of somatostatin mRNA and increases growth hormone pulse amplitude in aged animals. Neuroendocrinology 61: 601–608, 1995 [DOI] [PubMed] [Google Scholar]
- 278. Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384: 327–333, 1996 [DOI] [PubMed] [Google Scholar]
- 279. Steger RW, Bartke A, Parkening TA, Collins T, Buonomo F, Tang K, Wagner TE, Yun JS. Effects of heterologous growth hormones on hypothalamic and pituitary function in transgenic mice. Neuroendocrinology 53: 365–372, 1991 [DOI] [PubMed] [Google Scholar]
- 280. Stensvold D, Nauman J, Nilsen TI, Wisloff U, Slordahl SA, Vatten L. Even low level of physical activity is associated with reduced mortality among people with metabolic syndrome, a population based study (the HUNT 2 study, Norway). BMC Med 9: 109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology 152: 3165–3171, 2011 [DOI] [PubMed] [Google Scholar]
- 282. Stuart JA, Page MM. Plasma IGF-1 is negatively correlated with body mass in a comparison of 36 mammalian species. Mech Ageing Dev 131: 591–598, 2010 [DOI] [PubMed] [Google Scholar]
- 283. Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol 25: 253–275, 2009 [DOI] [PubMed] [Google Scholar]
- 284. Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA 105: 3438–3442, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Sun D, Muthukumar AR, Lawrence RA, Fernandes G. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin Diagn Lab Immunol 8: 1003–1011, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci 64: 711–722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Sun LY. Hippocampal IGF-1 expression, neurogenesis and slowed aging: clues to longevity from mutant mice. Age 28: 181–189, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging 26: 929–937, 2005 [DOI] [PubMed] [Google Scholar]
- 289. Sun LY, Bartke A. Adult neurogenesis in the hippocampus of long-lived mice during aging. J Gerontol A Biol Sci Med Sci 62: 117–125, 2007 [DOI] [PubMed] [Google Scholar]
- 290. Sun LY, Bokov AF, Richardson A, Miller RA. Hepatic response to oxidative injury in long-lived Ames dwarf mice. FASEB J 25: 398–408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Sun LY, Evans MS, Hsieh J, Panici J, Bartke A. Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice. Endocrinology 146: 1138–1144, 2005 [DOI] [PubMed] [Google Scholar]
- 292. Sun LY, Steinbaugh MJ, Masternak MM, Bartke A, Miller RA. Fibroblasts from long-lived mutant mice show diminished ERK1/2 phosphorylation but exaggerated induction of immediate early genes. Free Radic Biol Med 47: 1753–1761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Sun Y, Garcia JM, Smith RG. Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology 148: 1323–1329, 2007 [DOI] [PubMed] [Google Scholar]
- 294. Sun Y, Todd BJ, Thornton K, Etgen AM, Neal-Perry G. Differential effects of hypothalamic IGF-I on gonadotropin releasing hormone neuronal activation during steroid-induced LH surges in young and middle-aged female rats. Endocrinology 152: 4276–4287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA. A single IGF1 allele is a major determinant of small size in dogs. Science 316: 112–115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Svensson J, Sjogren K, Faldt J, Andersson N, Isaksson O, Jansson JO, Ohlsson C. Liver-derived IGF-I regulates mean life span in mice. PLoS One 6: e22640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317: 369–372, 2007 [DOI] [PubMed] [Google Scholar]
- 298. Tannenbaum GS, Epelbaum J, Colle E, Brazeau P, Martin JB. Antiserum to somatostatin reverses starvation-induced inhibition of growth hormone but not insulin secretion. Endocrinology 102: 1909–1914, 1978 [DOI] [PubMed] [Google Scholar]
- 299. Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351, 2003 [DOI] [PubMed] [Google Scholar]
- 300. Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292: 107–110, 2001 [DOI] [PubMed] [Google Scholar]
- 301. Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci 64: 1039–1044, 2009 [DOI] [PubMed] [Google Scholar]
- 302. Torres-Aleman I. Insulin-like growth factors as mediators of functional plasticity in the adult brain. Horm Metab Res 31: 114–119, 1999 [DOI] [PubMed] [Google Scholar]
- 303. Torres-Aleman I. Serum growth factors and neuroprotective surveillance: focus on IGF-1. Mol Neurobiol 21: 153–160, 2000 [DOI] [PubMed] [Google Scholar]
- 304. Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci 37: 402–411, 2008 [DOI] [PubMed] [Google Scholar]
- 305. Tsuchiya T, Dhahbi JM, Cui X, Mote PL, Bartke A, Spindler SR. Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol Genomics 17: 307–315, 2004 [DOI] [PubMed] [Google Scholar]
- 306. Van Buul-Offers SC, Bloemen RJ, Reijnen-Gresnigt MG, van Leiden HA, Hoogerbrugge CM, Van den Brande JL. Insulin-like growth factors-I and -II and their binding proteins during postnatal development of dwarf Snell mice before and during growth hormone and thyroxine therapy. J Endocrinol 143: 191–198, 1994 [DOI] [PubMed] [Google Scholar]
- 307. Van den Berg G, Veldhuis JD, Frolich M, Roelfsema F. An amplitude-specific divergence in the pulsatile mode of growth hormone (GH) secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab 81: 2460–2467, 1996 [DOI] [PubMed] [Google Scholar]
- 308. Vanhooren V, Dewaele S, Kuro OM, Taniguchi N, Dolle L, van Grunsven LA, Makrantonaki E, Zouboulis CC, Chen CC, Libert C. Alteration in N-glycomics during mouse aging: a role for FUT8. Aging Cell 10: 1056–1066, 2011 [DOI] [PubMed] [Google Scholar]
- 309. Veldhuis JD. Aging and hormones of the hypothalamo-pituitary axis: gonadotropic axis in men and somatotropic axes in men and women. Ageing Res Rev 7: 189–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Veldhuis JD, Bowers CY. Human GH pulsatility: an ensemble property regulated by age and gender. J Endocrinol Invest 26: 799–813, 2003 [DOI] [PubMed] [Google Scholar]
- 311. Veldhuis JD, Iranmanesh A, Bowers CY. Joint mechanisms of impaired growth-hormone pulse renewal in aging men. J Clin Endocrinol Metab 90: 4177–4183, 2005 [DOI] [PubMed] [Google Scholar]
- 312. Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci 59: 1244–1250, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Vicario-Abejon C, Yusta-Boyo MJ, Fernandez-Moreno C, de Pablo F. Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia. J Neurosci 23: 895–906, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Wagner JP, Black IB,., DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor J Neurosci 19: 6006–6016, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci 61: 323–331, 2006 [DOI] [PubMed] [Google Scholar]
- 316. Waters MJ, Brooks AJ. Growth hormone receptor: structure function relationships. Horm Res Paediatr 76 Suppl 1: 12–16, 2011 [DOI] [PubMed] [Google Scholar]
- 317. Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet 4: e13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Weltman A, Weltman JY, Roy CP, Wideman L, Patrie J, Evans WS, Veldhuis JD. Growth hormone response to graded exercise intensities is attenuated and the gender difference abolished in older adults. J Appl Physiol 100: 1623–1629, 2006 [DOI] [PubMed] [Google Scholar]
- 319. Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci 64: 443–451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Wijsman CA, Rozing MP, Streefland TC, le Cessie S, Mooijaart SP, Slagboom PE, Westendorp RG, Pijl H, van Heemst D. Familial longevity is marked by enhanced insulin sensitivity. Aging Cell 10: 114–121, 2011 [DOI] [PubMed] [Google Scholar]
- 321. Wilkins A, Chandran S, Compston A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia 36: 48–57, 2001 [DOI] [PubMed] [Google Scholar]
- 322. Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA 105: 13987–13992, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Williams G. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11: 398–411, 1957 [Google Scholar]
- 324. Wirth-Dzieciolowska E, Czuminska K, Reklewska B, Katkiewicz M. Life time reproductive performance and functional changes in reproductive organs of mice selected divergently for body weight over 90 generations. Anim Sci Papers Reports 14: 187–198, 1996 [Google Scholar]
- 325. Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290: 147–150, 2000 [DOI] [PubMed] [Google Scholar]
- 326. Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335: 1363–1367, 1996 [DOI] [PubMed] [Google Scholar]
- 327. Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, Chernausek SD, Mejia W, LeRoith D. Liver-specific IGF-I gene deletion leads to muscle insulin insensitivity. Diabetes 50: 1110–1118, 2001 [DOI] [PubMed] [Google Scholar]
- 328. Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96: 7324–7329, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, Glatt V, Bouxsein ML, Kopchick JJ, LeRoith D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest 113: 96–105, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001 [DOI] [PubMed] [Google Scholar]
- 331. Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130: 247–258, 2007 [DOI] [PubMed] [Google Scholar]
- 332. Ye P, D'Ercole AJ. Insulin-like growth factor actions during development of neural stem cells and progenitors in the central nervous system. J Neurosci Res 83: 1–6, 2006 [DOI] [PubMed] [Google Scholar]
- 333. Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median life spans and circulating IGF1 levels. Aging Cell 8: 277–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Zaczek D, Hammond J, Suen L, Wandji S, Service D, Bartke A, Chandrashekar V, Coschigano K, Kopchick J. Impact of growth hormone resistance on female reproductive function: new insights from growth hormone receptor knockout mice. Biol Reprod 67: 1115–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 335. Zadik Z, Chalew SA, McCarter RJ, Meistas M, Kowarski AA. The influence of age on the twenty-four hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab 60: 523–516, 1985 [DOI] [PubMed] [Google Scholar]
- 336. Zearfoss NR, Alarcon JM, Trifilieff P, Kandel E, Richter JD. A molecular circuit composed of CPEB-1 and c-Jun controls growth hormone-mediated synaptic plasticity in the mouse hippocampus. J Neurosci 28: 8502–8509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Zechel S, Werner S, Unsicker K, von Bohlen, Halbach O. Expression and functions of fibroblast growth factor 2 (FGF-2) in hippocampal formation. Neuroscientist 16: 357–373, 2010 [DOI] [PubMed] [Google Scholar]
- 338. Zhao J, Harada N, Kurihara H, Nakagata N, Okajima K. Cilostazol improves cognitive function in mice by increasing the production of insulin-like growth factor-I in the hippocampus. Neuropharmacology 58: 774–783, 2010 [DOI] [PubMed] [Google Scholar]
- 339. Zhou Y, He L, Kopchick JJ. An exon encoding the mouse growth hormone binding protein (mGHBP) carboxy terminus is located between exon 7 and 8 of the mouse growth hormone receptor gene. Receptor 4: 223–227, 1994 [PubMed] [Google Scholar]
- 340. Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA 94: 13215–13220, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Zitnik G, Martin GM. Age-related decline in neurogenesis: old cells or old environment? J Neurosci Res 70: 258–263, 2002 [DOI] [PubMed] [Google Scholar]