Abstract
We recently created a cystic fibrosis ferret model that acquires neonatal lung infection. To develop lung gene therapies for this model, we evaluated recombinant adeno-associated virus (rAAV)-mediated gene transfer to the neonatal ferret lung. Unlike in vitro ferret airway epithelial (FAE) cells, in vivo infection of the ferret lung with rAAV1 required proteasome inhibitors to achieve efficient airway transduction. We hypothesized that differences in transduction between these two systems were because of an in vivo secreted factor that alter the transduction biology of rAAV1. Indeed, treatment of rAAV1 with ferret airway secretory fluid (ASF) strongly inhibited rAAV1, but not rAAV2, transduction of primary FAE and HeLa cells. Properties of the ASF inhibitory factor included a strong affinity for the AAV1 capsid, heat-stability, negative charge, and sensitivity to endoproteinase Glu-C. ASF-treated rAAV1 dramatically inhibited apical transduction of FAE ALI cultures (512-fold), while only reducing viral entry by 55-fold, suggesting that postentry processing of virus was influenced by the inhibitor factor. Proteasome inhibitors rescued transduction in the presence of ASF (∼1600-fold) without effecting virus internalization, while proteasome inhibitors only enhanced transduction 45-fold in the absence of ASF. These findings demonstrate that a factor in lung secretions can influence intracellular processing of rAAV1 in a proteasome-dependent fashion.
Yan and colleagues identify a protease-sensitive factor in ferret airway secretions that profoundly reduces transduction by AAV1. The unidentified factor inhibits both vector entry and postentry processing in polarized ferret airway epithelial (FAE) cell cultures. Transduction can be rescued both in vitro and in vivo by a proteasome inhibitor, suggesting this novel factor interacts with the ubiquitin–proteasome system during intracellular virus processing.
Introduction
Cystic fibrosis (CF) is the most common life-threatening autosomal recessive disorder in Caucasians and is caused by defects in the cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial dysfunction in multiple organs, including the lung, gastrointestinal tract, pancreas, liver, and gallbladder (Welsh et al., 2001; Rowe et al., 2005). The primary target for gene therapy of CF is the lung, given that chronic bacterial infections in the airways are the most life-threatening aspects of the disease (Driskell and Engelhardt, 2003; Griesenbach et al., 2010). Preclinical studies to test the efficacy of gene therapies to the CF lung have been hindered by the fact that CF mice do not acquire spontaneous lung disease (Grubb and Boucher, 1999; Wilke et al., 2011), likely because of an alternative Ca+2-activated chloride channel that compensates for the lack of CFTR in the airways (Grubb and Boucher, 1999). Interestingly, this alternative chloride channel in mouse airway epithelium is responsive to cAMP induction, making it difficult to functionally evaluate CFTR complementation electrophysiologically in CF mouse airway epithelium using viral vectors (Liu et al., 2006). However, the recent creation of a new CF ferret model that spontaneously acquires rapid lung infections (Sun et al., 2008, 2010) provides new opportunities to develop and test gene therapies for CF lung disease. In the current study, we sought to evaluate recombinant adeno-associated viral (rAAV) vectors for gene therapy of the neonatal ferret lung as a first step in developing this model.
rAAV vectors have been extensively studied in the field of gene therapy and has been evaluated in CF clinical trials (Flotte, 2001; Carter, 2005; Griesenbach and Alton, 2009). Although phase I and II trials for CF lung disease with rAAV2 have demonstrated a promising safety profile, CFTR expression in these trials was sufficiently low to preclude the detection of transgene-derived CFTR mRNA (Aitken et al., 2001; Wagner et al., 2002; Moss et al., 2007). In vitro studies using polarized human airway epithelia (HAE) cultured at an air–liquid interface (ALI) led to the finding that postentry barriers are primarily responsible for low efficiency of rAAV2 transduction from the apical surface (Duan et al., 2000). While rAAV2 is efficiently internalized from both the apical and basolateral membranes of HAE, entry through the basolateral membrane is 200-fold more effective at expressing transgenes as compared with apical entry (Duan et al., 1998, 2000). The apical block of rAAV transduction appears to be linked to proteasome function and ubiquitination of the capsid, since transient inhibition of the proteasome at the time of infection dramatically enhances capsid ubiquitination and transduction by improving nuclear translocation of the virus (Duan et al., 2000; Yan et al., 2002). Various rAAV serotypes have differences in their susceptibility to proteasome-dependent blocks in apical transduction of HAE, with rAAV1 being the least sensitive, and rAAV2 and rAAV5 being the most sensitive (Yan et al., 2004, 2006).
While the HAE ALI culture model has been very useful for sorting through rAAV serotypes with the highest tropism for the apical surface (Yan et al., 2013), this model cannot completely reproduce the in vivo extracellular environment of the airway that contains mucins and other secreted components of innate immunity (Knowles and Boucher, 2002). Although certain rAAV serotypes have demonstrated effective in vivo airway gene transfers in the mouse (Halbert et al., 2001; Auricchio et al., 2002; Bell et al., 2011; Li et al., 2011), the utility of the CF mouse for testing CFTR gene transfer is limited because they failed to develop the spontaneous lung infection seen in CF patients (Guilbault et al., 2007; Fisher et al., 2011; Keiser and Engelhardt, 2011). Thus, there is a need to evaluate rAAV vectors in less studied species, like the ferret, so that they can eventually be applied in the context of CFTR gene therapy for CF lung disease.
Previously, we compared the transduction efficiency of different rAAV serotypes in polarized ferret airway epithelial (FAE) cultures grown at an ALI and discovered that the rAAV1 most effectively expressed its transgenes after apical infection compared with rAAV2 and rAAV5 (Liu et al., 2007a). Therefore, in the current study, we sought to evaluate the efficiency of rAAV1 to transduce the neonatal ferret airway in vivo. We found that rAAV1 was efficient at transducing the trachea and intralobar airways of newborn ferrets only when a proteasome inhibitor was applied in the vector inoculum. Interestingly, the adult ferret lung was considerably resistant to rAAV1 infection even in the presence of a proteasome inhibitor. These findings were in stark contrast to the high level of rAAV1 transduction seen in vitro after infection of primary FAE grown at an ALI. Seeking to better understand why the in vivo, but not in vitro, FAE is so resistant to transduction by rAAV1, we evaluated whether the airway secretory fluid (ASF) of the ferret contained an inhibitory factor that altered the biology of rAAV1 transduction. Using in vitro reconstitution experiments, we demonstrate that ferret tracheal and whole-lung ASF contains factor that alters both the uptake and the postentry intracellular processing of rAAV1 in a proteasome-dependent fashion after apical infection of airway epithelia. These studies demonstrate for the first time that an extracellular factor can influence intracellular processing of rAAV and shed new insights into potential innate immunity barriers that can limit in vivo gene delivery to the lung. Further characterization of such an extracellular inhibitor factor may lead to improvements in rAAV vectors through the further engineering of capsid-interacting domains.
Materials and Methods
Recombinant AAV vector production
All rAAV vector stocks were generated by triple-plasmid cotransfection using an adenovirus-free system and purified with an identical procedure involving iodixanol ultracentrifugation following with HPLC, as previously described (Yan et al., 2006). Constructs for the triple-plasmid cotransfection were previously described (Yan et al., 2006) and included: (1) adenovirus helper plasmid, pAD4.1; (2) AAV trans helper plasmid, either pBS-HSP-RC2.3 for rAAV2 or p5E18RXC1 for rAAV1; and (3) an rAAV proviral plasmid designated below. rAAV2/2.CMVluc and rAAV2/1.CMVluc were generated by packaging the same rAAV2 proviral plasmid [pAV2-CMVluc-flag, harboring a CMV-promoter-driven firefly luciferase expression cassette (Yan et al., 2006)] into AAV2 and AAV1 capsid, respectively. The rAAV1 vector rAAV2/1.RSV-AP contained an RSV-LTR-driven alkaline phosphatase expression cassette and rAAV2/1.CBA-RL contained a CMV enhancer and chicken actin promoter (CBA)-driven renilla luciferase expression cassette. rAAV2/1.RSV-AP and rAAV2/1.CBA-RL were generated from rAAV2 proviral plasmids pCWRAPSP (Yang et al., 1999) and pAV2-CBA-RL (Flotte et al., 2010), respectively. Viral titers of purified stocks were calculated as DNase-resistant particles (DRP) using slot blot assays with P32-labeled probes as previously described (Yan et al., 2006).
AAV infection of ferret lungs
All animal experimentation was performed according to protocols approved by the Institutional Animal Care and Use Committees of the University of Iowa. rAAV1 in vivo infection of ferret lungs was performed by intratracheal instillation. The administration volume and virus load varied depending on the age of the ferret and is indicated in the figure legends. Proteasome inhibitor doxorubicin (Dox) was included in the vector inoculum at a final concentration of 250 μM, unless otherwise stated. Mock infections were performed by intratracheal instillation of phosphate buffered saline (PBS) lacking virus. Ferret kits were anesthetized by inhalation of a mixture of isofluorane and oxygen, and the installation of inoculum was performed over 10–20 min (vector titers and volumes for each age group are giving in the figure legends).
Detection of reporter gene expression in ferret lung
Animals were euthanized at desired time points postinfection with an overdose sodium pentobarbital injection (i.p.). For renilla luciferase expression assay, the ferret trachea/lung cassette was immediately frozen in liquid nitrogen and then pulverized using a cryogenic tissue pulverizer. About 1 ml of passive lysis buffer (Promega, Madison, WI) was added to the pulverized tissue to extract protein. After freeze–thaw cycles, the tissue extract was then centrifuged at 15,000 rpm for 5 min, and the clarified tissue extract was used for renilla luciferase assays with a renilla luciferase assay kit from Promega. For detection of alkaline phosphatase expression, the in situ trachea/lung cassette was washed by inflation with ∼5–10 ml PBS, followed by instillation of 10 ml fixation solution (0.5% glutaraldehyde in PBS). After dissecting from the chest cavity, the trachea and lung were submerged in ∼40 ml fixation solution for 1 hr and then washed three times with PBS over the course of 1 hr. After the last PBS wash, the tissues (in fresh PBS) were heated at 70°C in a water bath for 2 hr to inactivate the endogenous alkaline phosphatase activity. Alkaline phosphatase staining was then performed with a 1:50 dilution of the NBT/BCIP stock solution (Roche, Indianapolis, IN) in 0.1 M Tris-HCl, 0.1 M NaCl, and pH 9.5 buffer containing 1:100 dilution of levamisole (Vector Laboratory, Burlingame, CA). After staining at 37°C for 4 hr, tissues were rinsed three times with PBS over the course of 1 hr and then postfixed in 10% buffered formalin.
Chemical and enzymes
Proteasome inhibitor Dox was from Sigma (St. Louis, MO), and LLnL (N-Acetyl-L-leucine-L-leucine-L-norleucine) was from Boston Biochem (Cambridge, MA). The Dynabeads Antibody Coupling kit was from Invitrogen (Carlsbad, CA). Anion (Poros 20 PI) and cation (Poros 20 HS) exchange resin were from Applied Biosystems (Foster City, CA). Protenase K was from Roche, and sequencing-grade endoproteinases (trypsin, Arg-C, and Asp-N) were from Promega. N- and O-deglycosidase mix (PNGase F; Endo-α-N-Acetylgalactosaminidase; Neuraminidase and β-1-4 Galactosidase; and N-acetylglucosaminidase) was from New England Biolabs (Ipswich, MA). Heparinase, chondroitinase, and hyaluronidase were from Sigma. Methods of digestion with the various enzymes were performed under conditions provided by the manufacturers.
Collection of ASF from ferret
ASF from ferrets of different ages was collected after euthanasia. For lung lavages, the trachea and lung cassette was dissected from the chest cavity with the proximal end of the trachea held closed with hemostat. For adult ferrets, 4 ml of sterile PBS was injected into the cannulated trachea, and the lung was lavaged. The procedure was repeated, and the lavages were pooled and briefly spun at 1000 rpm for 5 min. The supernatant was filtered through 0.22 μM filter and adjusted to OD280=1 with sterile PBS. Typically ∼10–12 ml PBS-diluted ASF of OD280=1 could be collected from one adult ferret. Adult tracheal washes harvested into 500 μl PBS gave similar results to the whole lung lavages. All the data presented in the article used ASF harvested from lung lavages.
Cell culture and virus infection conditions
HeLa cells were cultured as monolayers in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum and penicillin–streptomycin, and maintained in a 37°C incubator at 5% CO2. FAE cultures grown at an ALI were generated as previously described (Liu et al., 2007a,b). Briefly, primary epithelial cells obtained from adult ferret trachea were seeded onto 12 mm Millicell cell culture inserts (Millipore, Billerica, MA) and differentiated with 2% Ultroser G (USG; Pall Corporation, Port Washington, NY) medium at an ALI for 3 weeks before use. Before infection, AAV vectors were premixed with PBS or ASF at room temperature for 1 hr. AAV infections of HeLa cells were performed by directly adding the viruses at a multiplicity of infection of 1000 DRP/cell (108 DRP) to cell cultures in a 48-well plate at ∼70% confluence. Viruses were typically left in the culture medium over a 24 hr infection period, unless otherwise stated. Infections of polarized FAE ALI cultures were performed by apical application of 4×109 DRP diluted in PBS or premixing with ASF in the final volume of 50 μl to the upper chamber of the Millicell insert. Viruses were exposed to epithelia for 16 hr and then removed. At this time, the Millicell inserts were briefly washed with a small amount USG medium and fed with fresh USG medium to the bottom chamber only. Approximately 106 cells were in each Millicell insert and thus the multiplicity of infection was ∼4000 DRP/cell.
Measurement of firefly luciferase reporter expression
At the time points of 24 hr postinfection for HeLa cells and 72 hr postinfection for FAE ALI cultures, cells were lysed with luciferase cell lysis buffer, and luciferase enzyme activity in cell lysates was determined using the Luciferase Assay System (Promega) in a 20/20 luminometer equipped with an automatic injector (Turner Biosystems, Sunnyvale, CA).
Analysis of internalized viral genomes
At the time point of 4 hr postinfection for HeLa cells or 16 hr postinfection for FAE ALI cultures, virus was removed by extensive washing with PBS. Cells were detached from the culture support by trypsin digestion, and the cell pellets were washed twice with PBS before lysing with a digestion buffer containing 50 mM KCl, 2.5 mM MgCl2, 10 mM Tris pH 8.0, 0.5% NP40, 0.5% Tween-20, and 400 μg/ml proteinase K. After digestion at 56°C for 45 min and heat inactivation at 95°C for 15 min, 1 μl of the digestion mixture was used for TaqMan polymerase chain reaction (PCR).
Quantitative analysis of rAAV genome by TaqMan PCR
TaqMan real-time PCR was used to quantify the physical titer of the viral stocks and copies of viral genome in cell lysates from AAV-infected cells as previously described (Ding et al., 2006; Yan et al., 2006). The PCR primers used were 5′-TTTTTGAAGCGAAGGTTGTGG-3′ (forward) and 5′-CACACACAGTTCGCCTCTTTG-3′ (reverse), and amplified a 73 bp fragment of the rAAV2.Luc genome. The TaqMan probe (5′-ATCTGGATACCGGGAAAACGCTGGGCG TTAAT-3′) was synthesized by IDT (Coralville, IA). This probe was tagged with 6-carboxy fluorescein (FAM) at the 5′-end as the reporter and Dark Hole Quencher 1 (BHQ1) at the 3′-end as the quencher. The PCR was performed and analyzed using Bio-Rad My IQ Real-time PCR detection system and software (Ding et al., 2006; Yan et al., 2006).
Purification of ASF inhibitor factor bound to rAAV1
About 6×1010 DPR of AAV2/1.CMVluc was diluted in 3 ml PBS or ASF and incubated at room temperature for 2 hr. After incubation, the virus suspensions were loaded on the top of a two-layer (0.5 ml) 30% and 50% sucrose-PBS solution. The AAV particles were then pelleted through the sucrose cushions by ultracentrifugation at 45,000 rpm for 2.5 hr in an SW55 Ti rotor. Virus pellets were resuspended in 100 μl PBS and used for transduction assays on HeLa cells and TaqMan PCR for viral genome quantification. A portion of the original sample loaded onto sucrose was also retained for both transduction and PCR.
Results
rAAV1 highly transduces neonatal ferret airways only when proteasome function is inhibited
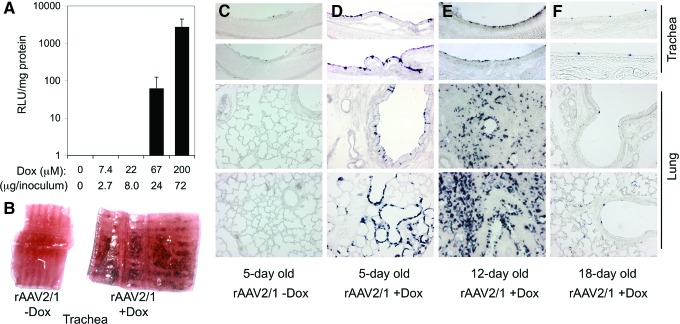
Our previous comparative studies of rAAV2/1, rAAV2/2, and rAAV2/5 transduction of polarized FAE ALI cultures demonstrated that rAAV2/1 affords ∼50-fold greater transgene expression than rAAV2/2 and rAAV2/5 vectors after apical infection (Liu et al., 2007a). This study also demonstrated that coadministration of proteasome inhibitors at the time of infection could enhance apical transduction of FAE ALI cultures with these serotypes in a similar manner to that observed in HAE ALI cultures (Yan et al., 2006). However, rAAV1 transduction of both HAE and FAE ALI cultures was ∼10-fold less responsive to proteasome inhibitor induction than rAAV2/2 and rAAV2/5, suggesting that rAAV1 is less susceptible to ubiquitin/proteasome-dependent barriers after apical infection. Our initial studies evaluating in vivo intratracheal rAAV2/1 infection to the neonatal ferret lung without coadministration of proteasome inhibitor failed to detect transgene expression (Fig. 1), suggesting that the biology of rAAV2/1 transduction in vivo differed from that in polarized FAE ALI cultures. To this end, we sought to determine whether coadministration of the proteasome inhibitor Dox could enhance in vivo transduction by performing a dose response of Dox administration with an rAAV2/1 CBA-driven rellina luciferase reporter (rAAV2/1.CBA-RL) (Flotte et al., 2010). To our surprise, rAAV2/1.CBA-RL transduction of neonatal ferret lung after intratracheal delivery was remarkably augmented by the inclusion of Dox in the vector inoculum. At a dose of 67 and 200 μM Dox, transgene expression in lung lysates was induced by ∼60- and 2700-fold, respectively (Fig. 1A).
FIG. 1.
Proteasome inhibitors dramatically induce rAAV2/1 transduction of the ferret lung. (A) Fourteen-day-old ferrets were infected with 5×1010 DRP of rAAV2/1.CBA-RL in 600 μl inoculum containing the indicated concentrations of Dox through intratracheal instillation. Graph shows the mean±SEM of luciferase activity (RLU/mg protein) measured at 8 days postinfection (n=4 animals for each condition). (B–D) Five-day-old ferrets were infected with 2.5×1011 DRP of rAAV2/1.RSV-AP in the presence or absence of 250 μM Dox (21.6 μg in 150 μl inoculum). (E) Twelve-day-old ferrets were infected with 1×1012 DRP of rAAV2/1.RSV-AP in the presence of 250 μM Dox (72 μg in 500 μl inoculum); this embedded lung sample was not inflated. (F) Eighteen-day-old ferrets were infected with 1×1012 DRP of rAAV2/1.RSV-AP in the presence of 250 μM Dox (86.4 μg in 600 μl inoculum). Dark purple staining demonstrates the expression of the alkaline phosphatase reporter at 8 days postinfection. Images in (C–F) were acquired at the 100× magnification. Dox, doxorubicin; DRP, DNase-resistant particles; rAAV, recombinant adeno-associated virus; SEM, standard error of the mean. Color images available online at www.liebertpub.com/hum
To confirm these findings with an alternative promoter-driven reporter, we performed similar experiments using an rAAV2/1 RSV LTR/promoter-driven alkaline phosphatase vector (rAAV2/1.RSV-AP) (Yang et al., 1999) and used histochemical staining to determine the cellular targets of transduction. Similar to the findings with rAAV2/1.CBA-RL, transduction with rAAV2/1.RSV-AP was significantly enhanced by the addition of Dox in the vector inoculum, and no transgene expression was observed in the absence of Dox (Fig. 1B–D). After infection of 5- and 12-day-old kits, transgene expression was observed in the tracheobronchial epithelium, bronchioles, and scatter alveolar cells (Fig. 1D and E). When the rAAV2/1 infections were performed with 18-day-old and adult ferrets in the presence of Dox, transgene expression in airways was significantly lower in 18-day-old animals (Fig. 1F) and undetectable in adult animals (data not shown).
Adult ferret tracheal ASF contains an inhibitory factor that selectively block rAAV1 transduction
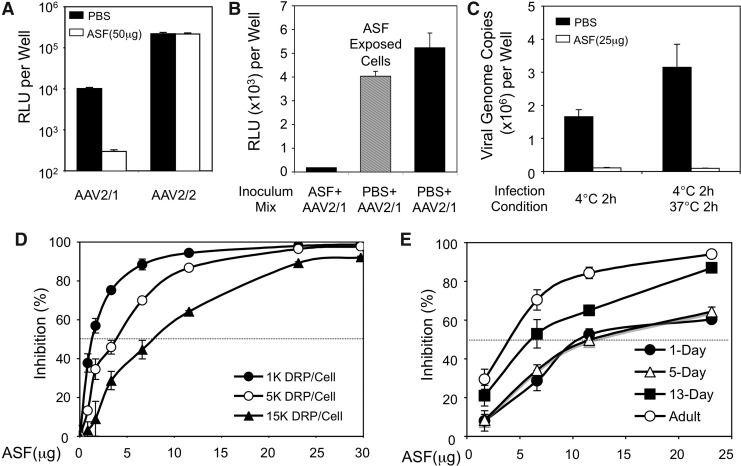
rAAV2/1 is relatively efficient at transducing polarized FAE ALI culture in vitro in the absence of proteasome inhibitors. By contrast, in vivo rAAV2/1 transduction to the ferret airway required a high concentration of Dox to achieve efficient transgene expression. Furthermore, the adult ferret lung is refractory to rAAV2/1 transduction even in the presence of Dox. Thus, it appears that the ferret lung develops a host defense mechanism with age that compromises rAAV2/1 infection in vivo. We hypothesized that a specific factor within the ASF might act as extracellular barriers to block in vivo access of rAAV2/1 to the airway epithelium. To test this hypothesis, we investigated whether the ASF harvested from adult ferret tracheal washes and bronchoalveolar lavage contained substances that are capable of inhibiting rAAV2/1 infection. To evaluate the specificity toward the rAAV1 serotype, we performed comparative experiments in which rAAV2/1 or rAAV2/2 was incubated with ferret ASF or PBS at room temperature for 1 hr and then applied to HeLa cells. Results from this experiment demonstrated that ferret ASF only inhibited rAAV2/1 transduction, but not transduction by rAAV2/2. The level of transgene expression from HeLa cells infected with the ASF-exposed rAAV2/1 was about 3% of the PBS-treated (control) virus (Fig. 2A). This inhibitory effect was rAAV1 specific, since the transgene expression after infection with ASF- or PBS-treated rAAV2 was unchanged (Fig. 2A). The inhibitory effect of ASF on rAAV1 transduction of HeLa cells was mediated through virion–ASF interactions, since pre-exposure of ASF to cells in the absence of virus, followed by viral infection in the absence of ASF, did not alter the transduction (Fig. 2B). Furthermore, premixing of rAAV1 with ASF before infection blocked binding of the virus to HeLa cells at 4°C and thus also internalization after a shift to 37°C (Fig. 2C). The extent of ASF-mediated inhibition of rAAV1 transduction was dependent on the concentrations of both ASF and virus (Fig. 2D). While the transduction activity of 103 DRP of rAAV2/1 was inhibited by 50% after incubating with ∼2 μg ASF, at 5- and 15-fold higher viral inoculums, 50% inhibition required only ∼4 and ∼7.5 μg of ASF, respectively (Fig. 2D). This nonlinear relationship between ASF concentration and viral inoculum titer suggests that the ASF inhibitory factor might cooperatively act to inhibit virion binding to the cell surface.
FIG. 2.
Inhibition of rAAV2/1 transduction by ferret ASF. (A) 1×108 DRP of rAAV2/1.CMVluc or rAAV2/2.CMVluc was incubated with 50 μl ferret ASF (1 μg/μl) or PBS at room temperature for 1 hr before applying to HeLa cells cultured on 48-well plates (∼105 cell/well, MOI=103 DRP/cell). Luciferase assays were performed at 24 hr postinfection. Data represent the mean (±SEM) relative luciferase activity per well (n=3 independent wells). (B) HeLa cells in 48-well plates were overlaid with 200 μl PBS (black column) or ASF (gray column) and incubated at 37°C for 1 hr. After the removal of PBS and ASF, cells were fed with 450 μl complete culture medium and infected with rAAV2/1.CMVluc at an MOI of 103 DRP/cell. rAAV2/1.CMVluc was premixed in 50 μl ferret ASF (1 μg/μl) or PBS at room temperature for 1 hr before infection. Luciferase assay was performed at 24 hr postinfection. Data represent the mean (±SEM) relative luciferase activity per well (n=3 independent wells). (C) Prechilled HeLa cells were incubated with 1×108 DRP of rAAV2/1.CMVluc (premixed with 25 μl PBS or ASF) at 4°C for 2 hr. The virus bound to the cell surface (4°C for 2 hr) or internalized into cells (after an additional 2 hr incubation at 37°C) were accessed by TaqMan PCR for viral genome copies. Data represent mean±SEM of n=6 infections. (D) 1×108, 5×108, or 1.5×109 DRP of rAAV2/1.CMVluc were incubated with different amount of ferret ASF at room temperature for 1 hr before applying to HeLa cells cultured on a 48-well plate (∼105 cell/well). Luciferase assay was performed at 24 hr postinfection. Data represent the mean (±SEM) relative luciferase activity per well (n=3 independent wells). (E) 5×108 DRP of rAAV2/1.CMVluc was incubated with ferret ASF of different ages at room temperature for 1 hr before applying to HeLa cells cultured on a 48-well plate (∼105 cell/well). Luciferase assay was performed at 24 hr postinfection. Data represent the mean (±SEM) relative luciferase activity per well (n=3 independent wells). ASF, airway secretory fluid; PBS, phosphate buffered saline; MOI, multiplicity of infection.
Given the differences in transduction observed after in vivo infection of ferrets of various ages (Fig. 1C–F), we hypothesized that the rAAV1 ASF inhibitory factor increased in abundance (or became more active) as ferrets matured. To test this hypothesis, ASF was collected from ferrets of various ages ranging from newborn to adult. This ASF was then used to titrate dose-dependent inhibition of rAAV1 transduction in HeLa cells. Results from this analysis (Fig. 2E) demonstrated that the ability of ASF to inhibit rAAV1 transduction indeed increased with age. No differences in the specific activity of ASF was seen between 1- and 5-day-old ferrets—both ages retained inhibitory activity with 50% inhibition of transduction observed at ∼12 μg ASF. By contrast, ASF from 13-day-old and adult ferrets inhibited rAAV1 transduction more significantly, giving rise to 50% inhibition of transduction at ∼7 and ∼4 μg ASF, respectively. These findings correlated with the decreasing efficiency of rAAV2/1 to transduce the ferret airways as animals aged (Fig. 1C–F) and suggest that either the inhibitory activity or the abundance of the rAAV1 inhibitory factor increased in the ASF with age.
The inhibitor factor in ASF reduces rAAV1 entry into HeLa cells and transduction of HeLa cells in a proteasome-independent manner
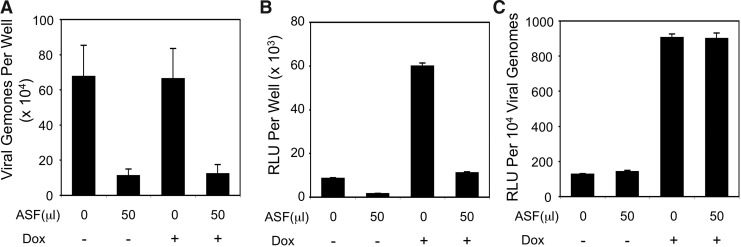
We further investigated whether ASF-mediated inhibition of rAAV1 transduction was the result of reduced viral entry. To this end, rAAV2/1 was incubated with ferret ASF or PBS (control) at room temperature for 1 hr and then applied to HeLa cells. The cells were exposed to vector inoculum at 37°C for 4 hr in the absence or presence of 1 μM Dox, and then they were re-fed with fresh medium after the inoculum was removed. At 24 hr postinfection, cells were assessed for both internalized viral genomes and luciferase reporter expression (Fig. 3A and B). Results from these analyses demonstrated significantly reduced internalization of ASF pre-exposed rAAV2/1 and also revealed a direct correlation between internalized viral genomes and transgene expression. ASF inhibition led to a 5.4-fold decrease in transgene expression regardless of Dox treatment, and this was similar to the 5.4–5.9-fold reduction in vector uptake. Inhibition of the proteasome has been shown to enhance rAAV transduction through postentry virion processing and trafficking with minimal effect on viral uptake in HeLa cells (Yan et al., 2002). Thus, our results (Fig. 3A and B) are consistent with these observations. Given that transgene expression per viral genome was similarly enhanced by the presence of Dox regardless of ASF inhibition (Fig. 3C), these findings suggest that ASF does not alter the proteasome-dependent processing of the internalized rAAV2/1 virions in HeLa cells. Thus, the primary mechanism by which ASF inhibits rAAV1 transduction of HeLa cells is through reduced virus binding and internalization.
FIG. 3.
Ferret ASF inhibits rAAV2/1 virus uptake and transduction in HeLa cells. HeLa cells cultured on a 48-well plate (∼105 cell/well) were infected with rAAV2/1.CMVluc preincubated with 50 μl ferret ASF or 50 μl PBS at an MOI of 103 DRP/cell. Infections were performed for 4 hr in the absence or presence of 1 μM Dox, and then the inoculums were removed and the cells were replenished with fresh medium. (A, B) Quantification of internalized viral genomes (A) and luciferase transgene expression (B) in cell lysates were performed at 24 hr postinfection. (C) An index for rAAV2/1 vector efficiencies after the indicated infection conditions was calculated as the ratio of luciferase expression divided by the number of viral genome. Data represent the mean (±SEM) values from n=4 independent infections.
Characterization of the rAAV1 inhibitory factor in ferret ASF
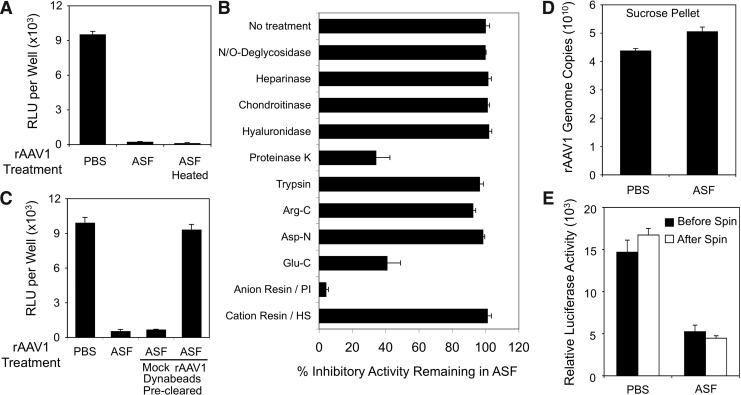
To evaluate whether the ASF inhibitory factor was an enzyme that might modify the virus capsid to prevent binding/endocytosis, we first investigated whether inhibition could be reversed by heat inactivation (85°C for 45 min) of ASF before mixing with rAAV1 and infection of HeLa cells. Results demonstrated that the inhibitor in the ASF was resistant to heat inactivation and thus likely not an enzyme (Fig. 4A). We next performed studies to determine if the ASF inhibitory factor was a protein and define its biochemical properties. As summarized in Fig. 4B, results from these experiments suggested that the inhibitory factor was a heat-stable protein with a net negative charge. Both predigestion of ASF with proteinase K (followed by heat inactivation of proteinase K) and preadsorption of ASF using an anion exchange resin significantly depleted ASF inhibitory activity in the HeLa cell transduction assay. The inhibitory factor was also sensitive to endoproteinase Glu-C digestion, but resistant to other endoproteinases, such as trypsin, Arg-C, and Asp-N. The inhibitory factor was also resistant to digestion with N- and O-deglycosidase, heparinase, chondroitinase, and hyaluronidase, suggesting that these glycan modifications were not responsible for the inhibitory effect on transduction.
FIG. 4.
Biochemical properties of the rAAV1 inhibitory factor in ferret ASF. (A–C) All infections were performed in HeLa cells cultured on a 48-well plate (∼105 cell/well) with 108 DRP of rAAV2/1.CMVluc incubated with 50 μl ASF or PBS for 1 hr before infection. When indicated, the ASF was pretreated before incubation with virus. Luciferase activities in cell lysates were determined 24 hr postinfection. (A) Ferret ASF preheated at 85°C for 45 min before the infection assay retains inhibitory activity on rAAV1 transduction. Data represent the mean (±SEM) values from n=4 independent infections. (B) Ferret ASF was treated with the agents shown on the y-axis. After the enzymatic treatments, ASF was heated at 85°C for 45 min to inactivate enzymes before mixing with rAAV2/1.CMVluc. The N/O-deglycosidase mixture contains five enzymes: PNGase F, endo-α-N-acetylgalactosaminidase, neuraminidase, β-1-4 galactosidase, and N-acetylglucosaminidase. Anion or cation exchange resins were used to preclear the ASF by mixing 450 μl of the ASF and 25 μl (dry volume) of resin slurry pre-equilibrated with PBS. Results indicated the mean (±SEM)% inhibitory activity (with respect to rAAV1 transduction assays, n=3–4 independent infections) remaining in the ASF as compared with the untreated control ASF (as 100%). (C) rAAV2/1-RSV.AP was covalently conjugated to Dynabeads using the Dynabeads antibody coupling kit (Invitrogen) and used to preclear ASF before incubation with rAAV2/1.CMVluc and infection of HeLa cells. Mock control beads were run through the coupling reaction in the absence of rAAV1. Data represent the mean (±SEM) values from n=3 independent infections. (D, E) 6×1010 DPR of rAAV2/1.CMVluc were mixed with 3 ml PBS or ASF. After 2 hr of incubation at room temperature, the virus solutions were loaded onto the top of two layers of 0.5 ml sucrose-PBS solution of 30% and 50% (w/v) and spun at 45,000 rpm for 2.5 hr in an SW55 Ti rotor. After spinning, the virus pellets were resuspended in 100 μl PBS and used for (D) quantification of viral genomes by TaqMan PCR and (E) quantification of viral transduction. Recovery of virus pelleted was 73% from the PBS condition and 86% from the ASF condition. Approximately 2×108 DRP of rAAV2/1.CMVluc before spinning (but after mixing with PBS or ASF) and after spinning (from the sucrose pellets) were used to infect HeLa cells. Luciferase reporter expression assays were conducted at 24 hr postinfection. Data represent the mean (±SEM) for n=3 independent experiments.
To evaluate the specificity of binding of the inhibitor factor to AAV1 capsids, the binding characteristics of the ASF factor was examined using Dynabeads covalently coupling to rAAV1. ASF precleared with rAAV1-Dynabeads failed to block rAAV1 transduction of HeLa cells in comparison to the significant inhibition observed with mock-cleared ASF (i.e., using Dynabeads alone) (Fig. 4C). Notably, the inhibitory factor bound extremely tightly to rAAV1-conjugated Dynabeads and could not be eluted with high salt (5 M NaCl) (data not shown). Furthermore, ASF-bound virus retained reduced transduction activity when pelleted through a sucrose step gradient (Fig. 4D and E), emphasizing the strong binding interactions between the inhibitory factor and the rAAV1 capsid. Results from this analysis demonstrated that >75% of rAAV1 DNA-containing particles were recovered in the sucrose pellet after either PBS or ASF incubation (Fig. 4D) and that ASF-bound virus demonstrated a similar % inhibition of transduction on HeLa cells before and after sucrose purification (Fig. 4E).
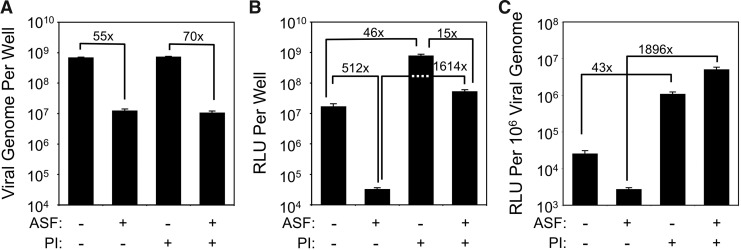
Proteasome inhibitor treatment rescues rAAV1 transduction in polarized FAE from inhibition by ASF
We next sought to determine the manner in which ferret ASF might alter rAAV2/1 transduction of polarized FAE in ALI culture. On the basis of the findings in HeLa cells, we hypothesized that the factor might inhibit viral uptake. Indeed, preincubation of rAAV2/1 with ASF significantly reduced viral uptake (55-fold) from the apical surface (Fig. 5A). Similar to HeLa cell studies, viral uptake in the presence or absence of ASF was unaffected by the treatment with proteasome inhibitors at the time of infection (Fig. 5A). Surprisingly, however, ASF more significantly inhibited rAAV2/1 transduction (512-fold) of ALI cultures (Fig. 5B) than viral uptake (55-fold), unlike the findings from HeLa cells. Furthermore, proteasome inhibition at the time of infection significantly rescued transduction in the presence of ASF (∼1600-fold) (Fig. 5B), while proteasome inhibition increased transduction in the absence of ASF by only 46-fold. These findings suggest that although the inhibitory factor in ASF reduces AAV1 endocytosis in FAE, it also redirects AAV1 that does get internalized to a proteasome-sensitive pathway of transduction. In light of changes in viral uptake in the presence of ASF, this can more easily be appreciated by evaluating the functionality of internalized vector genomes to express their transgene (Fig. 5C). Proteasome inhibitors increased the functionality of the rAAV1 vector genome (RLU/vg) by 44-fold in the presence of ASF (i.e., 1896-fold increase in the presence of ASF vs. 43-fold increase in the absence of ASF). This is in contrast to the findings from HeLa cells, where proteasome inhibition similarly increased the functionality of rAAV1 genomes by 7.1-fold and 6.4-fold in the presence and absence of ASF, respectively. These findings provide insights into why the in vivo ferret airway is highly resistant to rAAV1 transduction in the absence of proteasome inhibitors.
FIG. 5.
Ferret ASF influences transduction of ferret airway epithelia by altering uptake and proteasome-dependent intracellular processing of rAAV1. (A–C) 4×109 DRP of rAAV2/1.CMVluc were mixed with 45 μl ferret ASF or PBS and incubated for 1 hr at room temperature. The inoculum was then apically applied to FAE ALI cultures (at an MOI of ∼4×103 DRP/cell) for 16 hr. When proteasome inhibitors (PI) were applied, they were added to the medium in the basolateral chamber to a final concentration of 1 μM Dox and 10 nM N-Acetyl-L-leucine-L-leucine-L-norleucine (LLnL) for the entire infection period. At 16 hr postinfection, the apical inoculum was removed. After washing, the cultures were either (A) lysed for the analyses of internalized viral genomes by TaqMan PCR or (B) fed with fresh medium in the basolateral chamber and cultured till 3 days postinfection for luciferase expression assays. (C) The vector efficiencies under the different infection conditions were calculated as the ratio of luciferase expression to the number of viral genome in the sample at 16 hr postinfection. Data represent the mean (±SEM) values for n=3–4 independent infections.
Discussion
Our studies demonstrate that rAAV2/1 may be a suitable viral vector to test gene therapy to the lung of neonatal CF ferrets. Interestingly, the airways of adult ferrets were extremely resistant to rAAV2/1 transduction, and this appears to be caused, at least in part, by the increased abundance of the secreted inhibitory factor in the adult ferret airway. The identity of the secreted inhibitory factor remains unknown; however, resistance of the airway to rAAV1 infection appears to develop at 18 days after birth, a time point when submucosal glands are nearing a mature state in ferrets (Leigh et al., 1986; Liu et al., 2004). Thus, the inhibitor factor may originate from airway glands. One other group has previously noted an inhibitory factor specific to CF bronchioalveolar lavage, but not non-CF, that reduces rAAV2 infection in cell lines ∼6-fold (Virella-Lowell et al., 2000). These authors concluded that the likely inhibitor factor was a human neutrophil peptide. Our studies shed new insights on how inhibitory factors found in the lung can impact rAAV transduction through the altered intracellular processing of virions.
The specificity of the ASF inhibitory factor toward blocking rAAV1, but not rAAV2, transduction is intriguing and suggests that interactions with the AAV1 capsid are very specific. The ability of the inhibitory factor to bind very tightly to the AAV1 capsid is emphasized by the fact that ASF prebound rAAV1 retains its inhibitory activity on transduction after sucrose gradient purification (Fig. 4D and E) and the finding that 5 M NaCl failed to elute the inhibitory factor from virus. Given the sensitivity of the inhibitory factor to proteinase K and endoproteinase Glu-C, we conclude that it is a protein. However, if it is a protein, it is highly resistant to heat denaturation and other endoproteinases. Given the negative charge of the ASF inhibitory factor, we conclude that defensins are unlikely candidates since they are highly positively charged.
Although the ASF inhibitory factor reduced rAAV1 uptake in both HeLa cells and primary FAE, there were interesting differences that likely account for the ability of proteasome inhibitors to rescue transduction in the presence of ASF only in airway epithelium in vitro and in vivo. In HeLa cell, the ASF inhibited rAAV1 endocytosis and transduction to similar extent (5.4–6-fold). Thus, in HeLa cells, the functionality of each internalized vector genome remained the same in the presence and absence of ASF inhibition (i.e., as indexed by the ratio of transgene expression/internalized viral genome; Fig. 3C). Although proteasome inhibitors augmented rAAV1 transduction in HeLa cells, the functionality of endocytosed viral genomes remained the same in the presence and absence of ASF when Dox was present. Thus, in Hela cells, the pathway of ASF inhibition appears to be primarily at the level of blocking viral binding/endocytosis and does not alter the intracellular proteasome-dependent processing. In contrast to studies in HeLa cells, the reconstitution experiments with FAE showed that ASF strongly inhibited functionality of rAAV1 genomes by ∼10-fold, decreasing viral uptake by 55-fold and transduction by 512-fold (Fig. 5). These findings suggested that intracellular processing of internalized virions were also inhibited by the ASF inhibitory factor. The ability of proteasome inhibitors to rescue ASF-inhibited rAAV1 transduction (∼1600-fold) supports this hypothesis. Even more striking was the fact that ASF-treated rAAV1 in the presence of proteasome inhibitor gave rise to a 5-fold greater functionality of internalized viral genomes than untreated rAAV1 in the presence of proteasome inhibitor (Fig. 5C). These findings suggest that the ASF inhibitor factor routes internalized virus to a highly proteasome-sensitive compartment within airway epithelia, thus rendering transduction to greater modulation by proteasome inhibitors. Given the large body of work on the ability of proteasome inhibitors to improve trafficking of several AAV serotypes to the nucleus, this is the likely mechanism of improved transduction in the presence of the ASF inhibitory factor. However, the mechanism by which ASF enhances virus trafficking to a proteasome-sensitive compartment remains unclear. This could occur by changing the primary receptor–co-receptor pair through binding of the ASF factor to the capsid sites that normally bind a more abundant primary receptor–co-receptor on the cell surface of airway epithelia. Alternatively, the ASF factor may enter with the virion through its normal receptor–co-receptor and alter postendocytic virion processing.
It is a very intriguing finding that neonatal ferrets less than 2 weeks of age can be highly transduced with rAAV1 in the presence of proteasome inhibitors, but ferrets older than 18 days of age lose this sensitivity. We hypothesize that at lower abundance, the ASF inhibitory factor routes virus to an intracellular compartment that can be highly modulated by proteasome inhibitors. Such findings are consistent with our in vitro FAE data. However, given that the ASF inhibitory factor also blocks binding and uptake of rAAV1 in FAE, we hypothesize that when the factor reaches a critical concentration in the ferret lung, this mechanism of inhibition predominates and that there is little internalized virus to be rerouted to the nucleus after proteasome inhibitor treatment.
The innate immune system provides immediate protections against viral infections and plays an important role in stimulating adaptive immune responses. Such innate immune mechanisms against enveloped and nonenveloped viruses can attack nearly all stages of the infectious process, including binding, endocytosis, endosomal escape, and virus processing in the cytoplasm (Gaiha et al., 2008; See and Wark, 2008; Ding et al., 2009; Brennan and Bowie, 2010; Faure and Rabourdin-Combe, 2011)—secreted defensins and the cytosolic and membrane-bound pattern recognition receptors such as toll-like receptors are two examples (Ding et al., 2009; Brennan and Bowie, 2010; Faure and Rabourdin-Combe, 2011). The airway epithelium has multiple modalities of innate immunity, including passive (i.e., mechanical clearance), resident active biomolecules in the airway secretions (i.e., defensins and surfactant proteins), and reactive systems (i.e., pattern recognition receptors) (Diamond et al., 2000; See and Wark, 2008). Viruses have evolved different entry and intracellular trafficking mechanisms to evade the innate host surveillance. For example, while the lung surfactant proteins commonly inhibit infections from many respiratory viruses (Matalon and Wright, 2004), the binding of surfactant protein A to cytomegalovirus enhances virus entry into lung cells (Weyer et al., 2000). The cellular functions of the ubiquitin-proteasome system (UPS) also affect virus infections at each stage of their life cycle (Yu and Lai, 2005; Gao and Luo, 2006); however, viruses have also adapted to counteract immune defenses by inhibiting or redirecting the host UPS (Viswanathan et al., 2010). For example, certain wild-type parvoviruses require an active UPS to translocate to the nucleus (Ros and Kempf, 2004), and in the case of many rAAV2 and rAAV5, enhanced ubiquitination of the capsid in the presence of proteasome inhibitors correlates with enhanced nuclear translocation (Duan et al., 2000; Yan et al., 2002). Although the mechanism by which proteasome inhibitors counteract the rAAV1 innate immune factor found in ferret ASF remains unclear, our findings suggest that factors found in biologic fluids that passively bind to AAV can dramatically influence both entry and postentry processing of the virion.
In summary, this study describes the existence of protein inhibitors in airway fluid that compromise rAAV1 transduction to the ferret airway in vivo by inhibiting both uptake of virus and postentry processing. Interestingly, binding of ASF to rAAV1 reroutes the internalized virions to a nonproductive pathway that can be rescued by the application of a proteasome inhibitor. A better understanding of how this inhibitory factor influences rAAV1 transduction biology and how proteasome inhibitors rescue virus will be important for improving the design of rAAV-based gene therapies for CF. Whether similar innate immune factors exist in other biologic fluids and/or act on other serotypes of rAAV remains unclear, but warrants further investigation. Given that proteasome inhibitors appear to enhance rAAV transduction of several rAAV serotypes after in vivo delivery to several organs, including vasculature, liver, and joints (Denby et al., 2005; Jennings et al., 2005; Finn et al., 2010; Monahan et al., 2010), the mechanism by which proteasome inhibitors enhance rAAV transduction in vivo to these organs may have a molecular basis that is more complex than previously thought. Despite the finding that ferret ASF strongly inhibits in vivo rAAV1 transduction of the ferret airways, reasonable transgene expression was achieved in younger ferret kits infected at the age of 5–12 days old when proteasome inhibitors were administered at the time of infection. Thus, rAAV1 may be useful in testing gene therapies for the neonatal CF ferret lung.
Acknowledgments
This work was supported by NIH Grant HL108902 (to J.F.E.), the Roy J. Carver Chair in Molecular Medicine (to J.F.E.), and the University of Iowa Center for Gene Therapy (DK54759).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- Aitken M.L. Moss R.B. Waltz D.A., et al. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 2001;12:1907–1916. doi: 10.1089/104303401753153956. [DOI] [PubMed] [Google Scholar]
- Auricchio A. O'Connor E. Weiner D., et al. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Invest. 2002;110:499–504. doi: 10.1172/JCI15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.L. Vandenberghe L.H. Bell P., et al. The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J. Clin. Invest. 2011;121:2427–2435. doi: 10.1172/JCI57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan K. Bowie A.G. Activation of host pattern recognition receptors by viruses. Curr. Opin. Microbiol. 2010;13:503–507. doi: 10.1016/j.mib.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Carter B.J. Adeno-associated virus vectors in clinical trials. Hum. Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- Denby L. Nicklin S.A. Baker A.H. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Ther. 2005;12:1534–1538. doi: 10.1038/sj.gt.3302564. [DOI] [PubMed] [Google Scholar]
- Diamond G. Legarda D. Ryan L.K. The innate immune response of the respiratory epithelium. Immunol. Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- Ding W. Zhang L.N. Yeaman C. Engelhardt J.F. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol. Ther. 2006;13:671–682. doi: 10.1016/j.ymthe.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J. Chou Y.Y. Chang T.L. Defensins in viral infections. J. Innate Immun. 2009;1:413–420. doi: 10.1159/000226256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell R.A. Engelhardt J.F. Current status of gene therapy for inherited lung diseases. Annu. Rev. Physiol. 2003;65:585–612. doi: 10.1146/annurev.physiol.65.092101.142426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D. Yue Y. Yan Z., et al. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum. Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- Duan D. Yue Y. Yan Z., et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure M. Rabourdin-Combe C. Innate immunity modulation in virus entry. Curr. Opin. Virol. 2011;1:6–12. doi: 10.1016/j.coviro.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn J.D. Hui D. Downey H.D., et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol. Ther. 2010;18:135–142. doi: 10.1038/mt.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J.T. Zhang Y. Engelhardt J.F. Comparative biology of cystic fibrosis animal models. Methods Mol. Biol. 2011;742:311–334. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T.R. Recombinant adeno-associated virus vectors for cystic fibrosis gene therapy. Curr. Opin. Mol. Ther. 2001;3:497–502. [PubMed] [Google Scholar]
- Flotte T.R. Fischer A.C. Goetzmann J., et al. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol. Ther. 2010;18:594–600. doi: 10.1038/mt.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiha G.D. Dong T. Palaniyar N., et al. Surfactant protein a binds to HIV and inhibits direct infection of CD4(+) cells, but enhances dendritic cell-mediated viral transfer. J. Immunol. 2008;181:601–609. doi: 10.4049/jimmunol.181.1.601. [DOI] [PubMed] [Google Scholar]
- Gao G. Luo H. The ubiquitin-proteasome pathway in viral infections. Can. J. Physiol. Pharmacol. 2006;84:5–14. doi: 10.1139/y05-144. [DOI] [PubMed] [Google Scholar]
- Griesenbach U. Alton E.W. Gene transfer to the lung: lessons learned from more than 2 decades of CF gene therapy. Adv. Drug Deliv. Rev. 2009;61:128–139. doi: 10.1016/j.addr.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Griesenbach U. Inoue M. Hasegawa M. Alton E.W. Viral vectors for cystic fibrosis gene therapy: what does the future hold? Virus Adaptation Treat. 2010;2:159–171. [Google Scholar]
- Grubb B.R. Boucher R.C. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol. Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Guilbault C. Saeed Z. Downey G.P. Radzioch D. Cystic fibrosis mouse models. Am. J. Respir. Cell Mol. Biol. 2007;36:1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- Halbert C.L. Allen J.M. Miller A.D. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J. Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings K. Miyamae T. Traister R., et al. Proteasome inhibition enhances AAV-mediated transgene expression in human synoviocytes in vitro and in vivo. Mol. Ther. 2005;11:600–607. doi: 10.1016/j.ymthe.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Keiser N.W. Engelhardt J.F. New animal models of cystic fibrosis: what are they teaching us? Curr. Opin. Pulm. Med. 2011;17:478–483. doi: 10.1097/MCP.0b013e32834b14c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M.R. Boucher R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh M.W. Gambling T.M. Carson J.L., et al. Postnatal development of tracheal surface epithelium and submucosal glands in the ferret. Exp. Lung Res. 1986;10:153–169. doi: 10.3109/01902148609061490. [DOI] [PubMed] [Google Scholar]
- Li W. Zhang L. Wu Z., et al. AAV-6 mediated efficient transduction of mouse lower airways. Virology. 2011;417:327–333. doi: 10.1016/j.virol.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Driskell R.R. Engelhardt J.F. Airway glandular development and stem cells. Curr. Top. Dev. Biol. 2004;64:33–56. doi: 10.1016/S0070-2153(04)64003-8. [DOI] [PubMed] [Google Scholar]
- Liu X. Yan Z. Luo M. Engelhardt J.F. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am. J. Respir. Cell Mol. Biol. 2006;34:56–64. doi: 10.1165/rcmb.2005-0189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Luo M. Guo C., et al. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther. 2007a;14:1543–1548. doi: 10.1038/sj.gt.3303014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Luo M. Zhang L., et al. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am. J. Respir. Cell Mol. Biol. 2007b;36:313–323. doi: 10.1165/rcmb.2006-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S. Wright J.R. Surfactant proteins and inflammation—The Yin and Yang. Am. J. Respir. Cell Mol. Biol. 2004;31:585–586. doi: 10.1165/rcmb.F286. [DOI] [PubMed] [Google Scholar]
- Monahan P.E. Lothrop C.D. Sun J., et al. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol. Ther. 2010;18:1907–1916. doi: 10.1038/mt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R.B. Milla C. Colombo J., et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 2007;18:726–732. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- Ros C. Kempf C. The ubiquitin-proteasome machinery is essential for nuclear translocation of incoming minute virus of mice. Virology. 2004;324:350–360. doi: 10.1016/j.virol.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Rowe S.M. Miller S. Sorscher E.J. Cystic fibrosis. N. Engl. J. Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- See H. Wark P. Innate immune response to viral infection of the lungs. Paediatr. Respir. Rev. 2008;9:243–250. doi: 10.1016/j.prrv.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. Yan Z. Yi Y., et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J. Clin. Invest. 2008;118:1578–1583. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. Sui H. Fisher J.T., et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virella-Lowell I. Poirier A. Chesnut K.A., et al. Inhibition of recombinant adeno-associated virus (rAAV) transduction by bronchial secretions from cystic fibrosis patients. Gene Ther. 2000;7:1783–1789. doi: 10.1038/sj.gt.3301268. [DOI] [PubMed] [Google Scholar]
- Viswanathan K. Fruh K. Defilippis V. Viral hijacking of the host ubiquitin system to evade interferon responses. Curr. Opin. Microbiol. 2010;13:517–523. doi: 10.1016/j.mib.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J.A. Nepomuceno I.B. Messner A.H., et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum. Gene Ther. 2002;13:1349–1359. doi: 10.1089/104303402760128577. [DOI] [PubMed] [Google Scholar]
- Welsh M.J. Ramsey B.W. Accurso F. Cutting G.R. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 2001. [Google Scholar]
- Weyer C. Sabat R. Wissel H., et al. Surfactant protein A binding to cytomegalovirus proteins enhances virus entry into rat lung cells. Am. J. Respir. Cell Mol. Biol. 2000;23:71–78. doi: 10.1165/ajrcmb.23.1.3859. [DOI] [PubMed] [Google Scholar]
- Wilke M. Buijs-Offerman R.M. Aarbiou J., et al. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J. Cyst. Fibros. 2011;10(Suppl 2):S152–S171. doi: 10.1016/S1569-1993(11)60020-9. [DOI] [PubMed] [Google Scholar]
- Yan Z. Zak R. Luxton G.W., et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z. Zak R. Zhang Y., et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J. Virol. 2004;78:2863–2874. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z. Lei-Butters D.C. Liu X., et al. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J. Biol. Chem. 2006;281:29684–29692. doi: 10.1074/jbc.M604099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z. Lei-Butters D.C. Keiser N.W. Engelhardt J.F. Distinct transduction difference between adeno-associated virus type 1 and type 6 vectors in human polarized airway epithelia. Gene Ther. 2013;20:328–337. doi: 10.1038/gt.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Zhou W. Zhang Y., et al. Concatamerization of adeno-associated virus circular genomes occurs through intermolecular recombination. J. Virol. 1999;73:9468–9477. doi: 10.1128/jvi.73.11.9468-9477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G.Y. Lai M.M. The ubiquitin-proteasome system facilitates the transfer of murine coronavirus from endosome to cytoplasm during virus entry. J. Virol. 2005;79:644–648. doi: 10.1128/JVI.79.1.644-648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]