Abstract
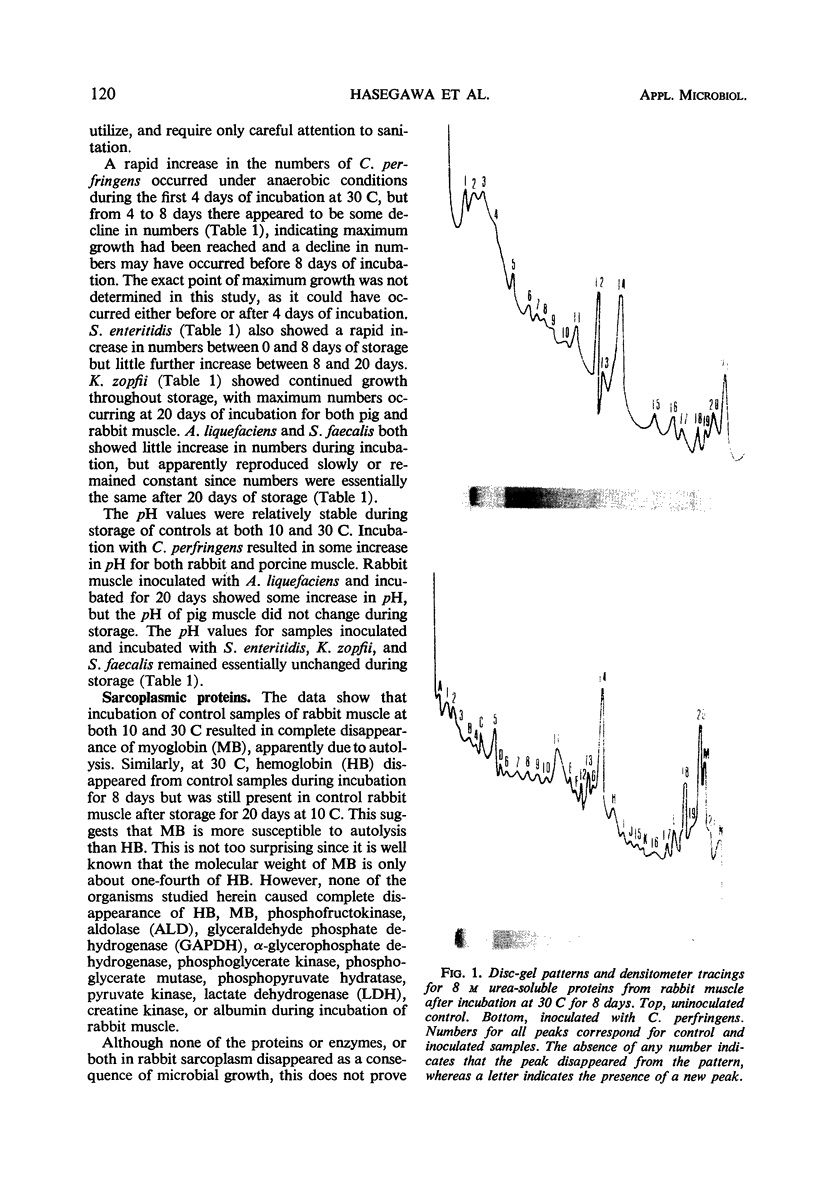
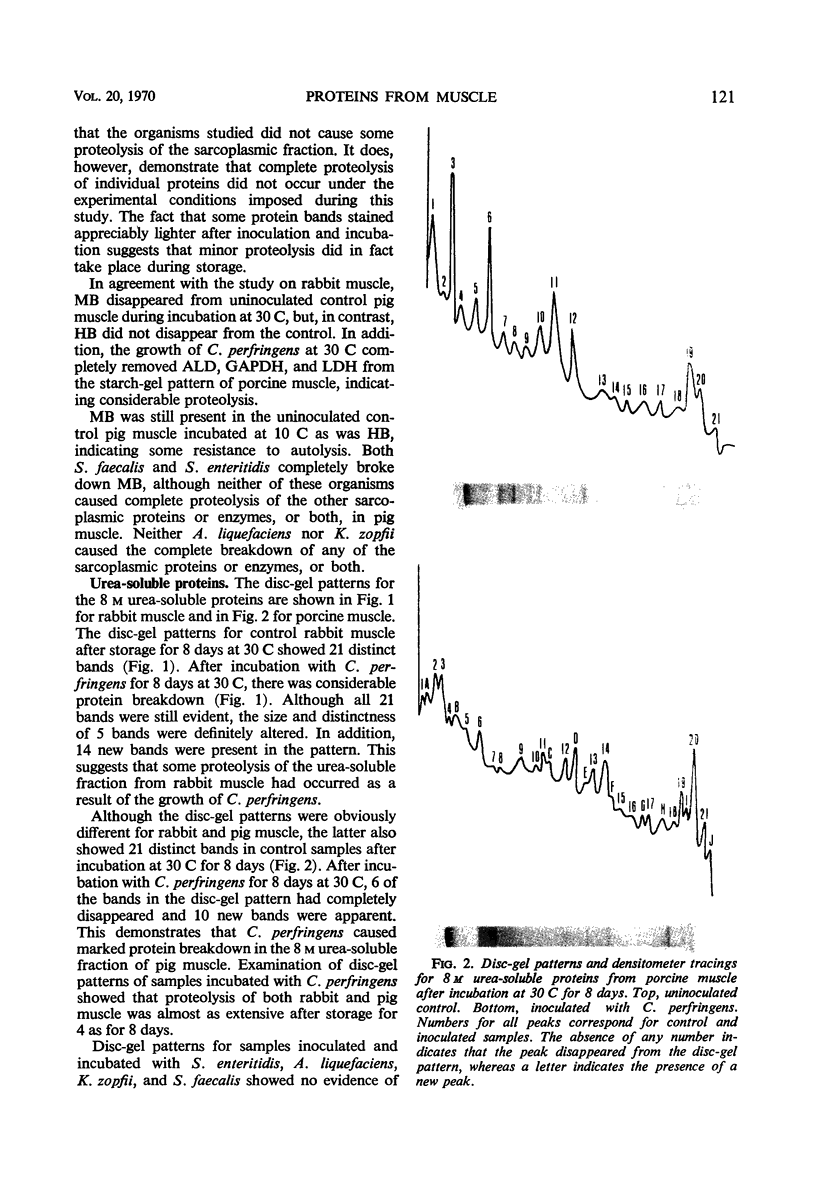
Comparisons of the starch-gel patterns of uninoculated aseptic control samples from rabbit and pig muscle with similar samples inoculated and incubated with Clostridium perfringens, Salmonella enteritidis, Achromobacter liquefaciens, and Kurthia zopfii were made. Results indicated that C. perfringens caused extensive alteration in the proteins or enzymes, or both, of the sarcoplasmic fraction of porcine muscle, whereas S. enteritidis and S. faecalis caused complete breakdown of only myoglobin. Neither A. liquefaciens nor K. zopfii showed any measurable amount of proteolysis in the sarcoplasmic fraction from pig muscle. Although some of the bands in the starch-gel pattern of rabbit muscle decreased in size and intensity of staining, complete proteolysis of any protein fraction was absent for all test organisms. The disc-gel patterns of the 8 m urea-soluble proteins showed that C. perfringens caused extensive proteolysis in pig muscle and a lesser extent of proteolysis in rabbit muscle. None of the other organisms utilized in this study had any measurable effect upon the urea-soluble proteins. In addition, a simple procedure for aseptic isolation of muscle samples for studying meat spoilage is outlined. Results indicate that careful sanitation and cleanliness will give suitable samples for meat spoilage investigations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- JACOBS S. Determination of nitrogen in proteins by means of idanetrione hydrate. Nature. 1959 Jan 24;183(4656):262–262. doi: 10.1038/183262a0. [DOI] [PubMed] [Google Scholar]
- Jay J. M., Kontou K. S. Fate of free amino acids and nucleotides in spoiling beef. Appl Microbiol. 1967 Jul;15(4):759–764. doi: 10.1128/am.15.4.759-764.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerke P., Farber L., Adams R. Bacteriology of spoilage of fish muscle. IV. Role of protein. Appl Microbiol. 1967 Jul;15(4):770–776. doi: 10.1128/am.15.4.770-776.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. Isolation and properties of a basic protein from skeletal-muscle sarcoplasm. Biochem J. 1966 Jan;98(1):193–197. doi: 10.1042/bj0980193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. Methods for starch-gel electrophoresis of sarcoplasmic proteins. An investigation of the relative mobilities of the glycolytic enzymes from the muscles of a variety of species. Biochem J. 1968 Mar;107(2):139–150. doi: 10.1042/bj1070139. [DOI] [PMC free article] [PubMed] [Google Scholar]




