Abstract
Background & Aims
Nuclear factor (NF)-κB is activated during early stages of pancreatitis. This transcription factor regulates genes that control many cell activities, including inflammation and survival. There is evidence that activation of NF-κB protects against pancreatitis, and in other cases, that it promotes this disease. We compared the effects NF-κB in different mouse models of pancreatitis to understand these complications.
Methods
To model constitutive activation of NF-κB, we expressed a transgene that encodes its p65 subunit or the inhibitor of κB kinase (IKK) 2 in pancreatic acinar cells of mice. We analyzed effects on pancreatic tissues and levels of NF-κB target genes in these mice and compared them to mice that did not express transgenic p65 or IKK2 (controls).
Results
Transgenic expression of p65 led to compensatory expression of the inhibitory subunit IKB-α and therefore, no clear phenotype. However, p65 transgenic mice given injections of caerulein, to induce acute pancreatitis, had higher levels of NF-κB activity in acinar cells, greater levels of inflammation, and more severe outcomes than control mice. In contrast, constitutive expression of IKK2 directly increased the activity of NF-κB in acinar cells and induced pancreatitis. Prolonged activity of IKK2 (3 months) resulted in activation of stellate cells, loss of acinar cells, and fibrosis, which are characteristics of chronic pancreatitis. Co-expression of IKK2 and p65 greatly increased the expression of inflammatory mediators and the severity of pancreatitis, compared with control mice.
Conclusions
The level of NF-κB activation correlates with the severity of acute pancreatitis in mice. Longer periods of activation (3 months) lead to chronic pancreatitis. These findings indicate that strategies to inactivate NF-κB might be used to treat patients with acute or chronic pancreatitis.
Keywords: immune regulation, cytokines, RelA, gene regulation
Background
Pancreatitis is an inflammatory disease of the pancreas that causes severe morbidity and mortality. Specific and effective interventions for this disease are not available largely because of a lack of understanding of the early cellular events in its pathophysiology. The transcription factor NF-κB is activated early in acinar cells during acute pancreatitis and increases expression of multiple proinflammatory genes1–6. NF-κB is composed of a group of structurally related transcriptional proteins7. These proteins belong to two classes, both of which contain Rel homology domains (RHD) but which are distinguishable by their modes of synthesis and transactivation properties. One class consists of RelA (also known as p65), RelB and c-Rel. These proteins are synthesized in their mature forms and contain transcription-modulating domains which initiate gene transcription. The second class consists of NF-κB1 (also known as p105) and NF-κB2 (also known as p100), which lack transcription-modulating domains. The subunits function as either homo- or hetero-dimers. In the pancreas, the predominant form of NF-κB is a p65/p50 heterodimer8. Under control conditions, NF-κB dimers are bound to inhibitory proteins, IκBs, which block nuclear localization sequences and thus trap the dimers within the cytoplasm where they are inactive. In the classical inflammatory response, the inhibitory proteins are phosphorylated by upstream IκB kinase (IKK), which targets them for ubiquitination by ubiquitin ligase and subsequent degradation in the 26S proteasome system. IκB degradation allows the NF-κB dimer to translocate into the nucleus, where it interacts with other transcription factors and binds to its consensus sequence on the promoters of NF-κB target genes 9. Activated NF-κB induces the transcription of many genes involved in inflammatory and apoptotic responses 10. Inflammatory targets of NF-κB include cytokines, chemokines, immune receptors, and adhesion molecules. Apoptosis related targets of NF-κB consist of several anti-apoptotic molecules including BCL-XL and cIAPs (cellular inhibitors of apoptosis). In some more complex systems, NF-κB can actually be pro-apoptotic, but that is the exception rather than the rule 11. NF-κB activity also induces feedback inhibition of its own signaling through up-regulation of the inhibitory IκB-α subunit 8. Therefore, the cellular response to activation of NF-κB is complex and involves a balance of opposing cellular responses.
With these complexities in the NF-κB signaling pathway, it is not surprising that controversy has arisen in the literature concerning the role of this pathway in pancreatitis. Most early studies suggested that NF-κB was critical for the initiation of pancreatitis1, 5. For example, pharmacological inhibition of NF-κB resulted in an amelioration of the disease1, 5. However, because the inhibitors utilized were not highly specific, no definitive conclusion could be made. However further supporting data came from the observations that pancreatitis was induced by pancreatic expression of p65 using adenoviral-mediated gene transfer 12 and by transgenic expression of active IKK2 in the pancreas 13. Thus, several studies supported that NF-κB increased pancreatitis severity, as might be expected7.
However, this was not universally the case. In contrast to the other studies of its type, one early study involving pharmacological inhibition of NF-κB during caerulein-induced pancreatitis suggested that NF-κB inhibition actually worsened acute pancreatitis6. Also, in contrast to the study showing active IKK2 expression in the pancreas causing pancreatitis13, in a different study the same investigators reported that transgenic expression of active IKK2 had no ability to induce pancreatic damage14. More recently, it has been reported that mice with genetic elimination of NF-κB signaling in pancreatic cells when challenged with caerulein developed more severe pancreatitis compared to those with wild-type NF-κB 15. This body of studies has been interpreted to indicate that NF-κB activity, rather than contributing to increased severity, is in fact protective against pancreatitis. These apparently conflicting and contradictory outcomes have rendered the inhibition of NF-κB during pancreatitis controversial.
To help sort out these issues, in the current study we analyzed three novel genetic mouse models. We found that increased acinar cell NF-κB activity correlated with higher cytokine expression and greater severity of acute pancreatitis. We also observed that persistent NF-κB activity led to the development of chronic pancreatitis. These data support that NF-κB activity worsens pancreatitis through its effects on inflammation. Therefore, the data indicate that reducing the increased NF-κB activity that occurs during acute and chronic pancreatitis would likely be beneficial for patients.
MATERIALS AND METHODS
Generation of conditional NF-κB overexpression transgenic mice
A full-length cDNA encoding p65 was excised from a pBluescript SK+ plasmid as an XhoI/XbaI restriction fragment (from Dr. G. Nabel, University of Michigan). This was ligated behind a loxp-GFP-STOP-loxp (LSL) fragment and cloned into the EcoR I site of the pCAGGS vector (provided by Dr. Miyazaki, Kumamoto University Medical School, Japan). The pCAGGS vector contains a CMV promoter and a chicken beta actin intron which has been proven to increase the level of expression16. The CMV-loxp-GFP-STOP-loxp-p65 (hereafter referred to as LSL-p65) cassette was isolated by SpeI and Hind III digestion and submitted for pronuclear injection to generate LSL-p65 mice. Transgenic mice with expression of HA tagged constitutive active IKK2, produced by conversion of two conserved serines in the T loop of IKK2 with glutamates 17, were developed using the same strategy and referred to as LSL-IKK2. The active IKK2 construct was a gift of Dr. Michael Karin at the University of California, San Diego. Transgenic GFP expression in pups was visualized under a UV lamp. All experiments were conducted with the consent of the Institutional Animal Care and Use Committee at the University of Texas MD Anderson Cancer Center.
Cross-breeding and induction of transgenes expression
To specifically express transgenes in pancreas, the LSL-p65 and LSL-IKK2 mice were crossed with mice expressing tamoxifen regulated CreERT driven by a full length pancreatic acinar specific elastase I promoter to create double or triple transgenic animals 18. In these mice, transgene expression was induced with daily oral tamoxifen (TM) administration (3mg/40g body weight for 5 days). In this study, TM treatment of transgenic mice without CreERT, wild-type mice or mice bearing CreERT alone were used as controls and no significant changes were observed in pancreatic histology in these mice.
Caerulein pancreatitis induction
To induce pancreatitis with caerulein, mice were treated by hourly intraperitoneal injections of caerulein (50 µg/kg/h) 19. Control animals received an equal amount of saline. Animals were killed by CO2 asphyxiation 1hr after the final caerulein injection and tissue samples were collected for studies.
Histology
For routine histology, tissues were fixed with 10% formaldehyde in phosphate-buffered saline (pH 7.4), embedded in paraffin, sectioned, and stained with H&E. A pathologist (H.W.) blinded to experimental treatments scored the degree of pancreatic injury by light microscopy based on severity of edema, inflammatory cell infiltration and cell death. Pathological scores of pancreatic injury were evaluated as previously described (see criteria in Supplemental Table 1) 20.
Measurement of pancreatitis parameters
Serum amylase was measured in an aliquot of 10 µl serum from each animal using the Phadebas amylase test and edema was evaluated by measuring the wet-to-dry weight ratio as previously described 12. The results were calculated and expressed as a water index (wet wt/dry wt).
Immunohistochemistry
Mice pancreata were either frozen in optimal cutting temperature compound (OCT, Tissue-Tek) or fixed in 10% formalin (24–48h) and embedded in paraffin. Immunohistochemical (IHC) staining for cleaved caspase-3 (1:200, Cell Signaling) and p65 (1:50, Cell Signaling) was performed in pancreatic paraffin sections. Briefly, after deparaffinization and antigen retrieval, nonspecific bindings were blocked and primary antibodies were applied (4°C, overnight). After washing incubation with the appropriate HRP-labeled polymers (BioCare) was performed. Positive labeling was detected by exposing the sample to substrate 3,3‘-diaminobenzidine (Phoenix BioTechnologies). Samples were then counterstained with hematoxylin solution. IHC for CD 45 (1:50, BD Pharmingen) was performed in frozen pancreatic sections. Briefly, frozen sections were fixed in pure acetone at −20°C for 10 min and blocked with 10% fetal bovine serum/2% normal horse serum for 1 h at room temperature. Primary antibodies were applied for 1 h at room temperature. After brief washing, incubation with biotinylated secondary antibodies and streptavidin-labeled horseradish peroxidase were performed. Finally, positive reactions in the sections were detected using the NovaRED substrate for peroxidase (Vector Laboratories). Counterstaining was performed with hematoxylin.
Western blotting
Pancreas tissues were homogenized in lysis buffer and immunoblot analysis was performed with antibodies against NF-κB p65 subunit, IκB-α (Santa Cruz Biotechnology) and, as a loading control, an anti–GAPDH antibody (Sigma). Appropriate fluorescent dye-labeled secondary antibodies were used to allow detection with the Odyssey Infrared Imaging System (LI-COR Biosciences) as previously described16.
RNA extraction and real time RT-PCR
Total RNA was prepared from mouse pancreas using TriZol reagent (Invitorgen). RNA was further purified by digestion for 15 minutes with DNase and recovery of RNA using an RNeasy kit (Qiagen). Reverse transcription was conducted for 45 minutes at 48°C from 1µg of purified total RNA in a 25µL volume of RT reaction mixture (Promega). Quantitative PCR was conducted using SYBR green I to monitor the PCR products on the I-Cycler thermal cycler and IQ real-time PCR detection system (BioRad). Primers were designed to span at least one intron to further eliminate the influence of genomic DNA contamination in the RNA preparation. RPS6 gene was used as an internal RNA loading control.
Primers sequences were:
IL-1β (Genebank accession 16176, Forward: 5’- AGA GCA TCC AGC TTC AAA TCT C-3’; Reverse: 5’-CAG TTG TCT AAT GGG AAC GTC A-3’).
IL-6 (Genebank accession 16193, Forward: 5’-GTT GCC TTC TTG GGA CTG ATG-3’; Reverse: 5’-ATT GCC ATT GCA CAA CTC TTT-3’).
TNF-α (Genebank accession 21926, Forward: 5’-TTA GAA AGG GGA TTA TGG CTC A-3’; Reverse: 5’-ACT CTC CCT TTG CAG AAC TCA G-3’)
TGF-β (Genebank accession 21803, Forward: 5’-GAA CCA AGG AGA CGG AAT ACA G-3’; Reverse: 5’-AAC CCA GGT CCT TCC TAA AGT C-3’)
Fibronectin (Genebank accession 14268, Forward: 5’-TGT GGT CTA CTC TGT GGG AAT G-3’; Reverse: 5’-TTG AAT TGC CAC CAT AAG TCT G-3’)
alpha-SMA (Genebank accession 11475, Forward: 5’-ATG ACC CAG ATT ATG TTT GAG ACC-3’; Reverse: 5’-CCA GAG TCC AGC ACA ATA CCA-3’
RPS6 (Genebank accession 20104, Forward: 5’-GAT GAT GTC CGC CAG TAT GTT-3’; Reverse: 5’-TTG TTC TTC TTA GTG CGT TGC-3’)
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared using nuclear protein extraction kit (Pierce) according to manufacturer’s instructions. Aliquots of nuclear extract with equal amounts of protein (10 µg) were incubated in 20-mL reactions in a buffer containing 10 mmol/L HEPES (pH 7.9), 10% glycerol (vol/vol), 1 mmol/L dithiothreitol, 1 mg poly(dIdC), and 5 mg nuclease-free bovine serum albumin. The binding reaction was started by adding 10,000 cpm of the 22–base pair oligonucleotide AGT TGA GGG GAC TTT CCC AGG C containing the NF-κB consensus sequence (Promega, Madison, WI) that had been labeled with 32P-adenosine triphosphate (10 mCi/mmol) by T4 polynucleotide kinase. All reaction mixtures were subjected to polyacrylamide gel electrophoresis on 4.5% gel in 0.53 Tris-borate-EDTA buffer (44.5 mmol/L Tris base, 44.5 mmol/L boric acid, and 1 mmol/L disodium EDTA, pH 8.3) at 100 V. Gels were dried and directly exposed to a X-ray film 8. All images were converted to grayscale. Densitometry was measured using ImageJ software (http://rsbweb.nih.gov/ij/).
Statistical analysis
Results are expressed as means ± SEM. Data were analyzed using one-way ANOVA with Tukey–Kramer post hoc test. p values <0.05 were considered significant.
Results
Transgenic expression of p65 in pancreatic acinar cells leads to compensatory changes with no obvious phenotype
The NF-κB DNA binding subunit p65(RelA), which is fully functional as a homodimer, was developed in a conditional over-expression vector for transgenic mouse production (LSL-p65) (Supplemental Figure 1A). In this vector, a CAG promoter 21 drove a cassette flanked by two loxP sites consisting of the coding region for enhanced green fluorescent protein (GFP) followed by a transcriptional stop signal. This cassette was followed immediately by the sequence coding for p65. The CAG promoter utilized in this construct has strong transcriptional activity in pancreas22. The presence of EGFP and its translation stop code between the CAG promoter and p65 prevent NF-κB p65 expression. As expected, before Cre recombination, GFP was expressed throughout the whole animal (Supplemental Figure 1B) as well as in pancreatic acinar cells (Supplemental Figure 1C).
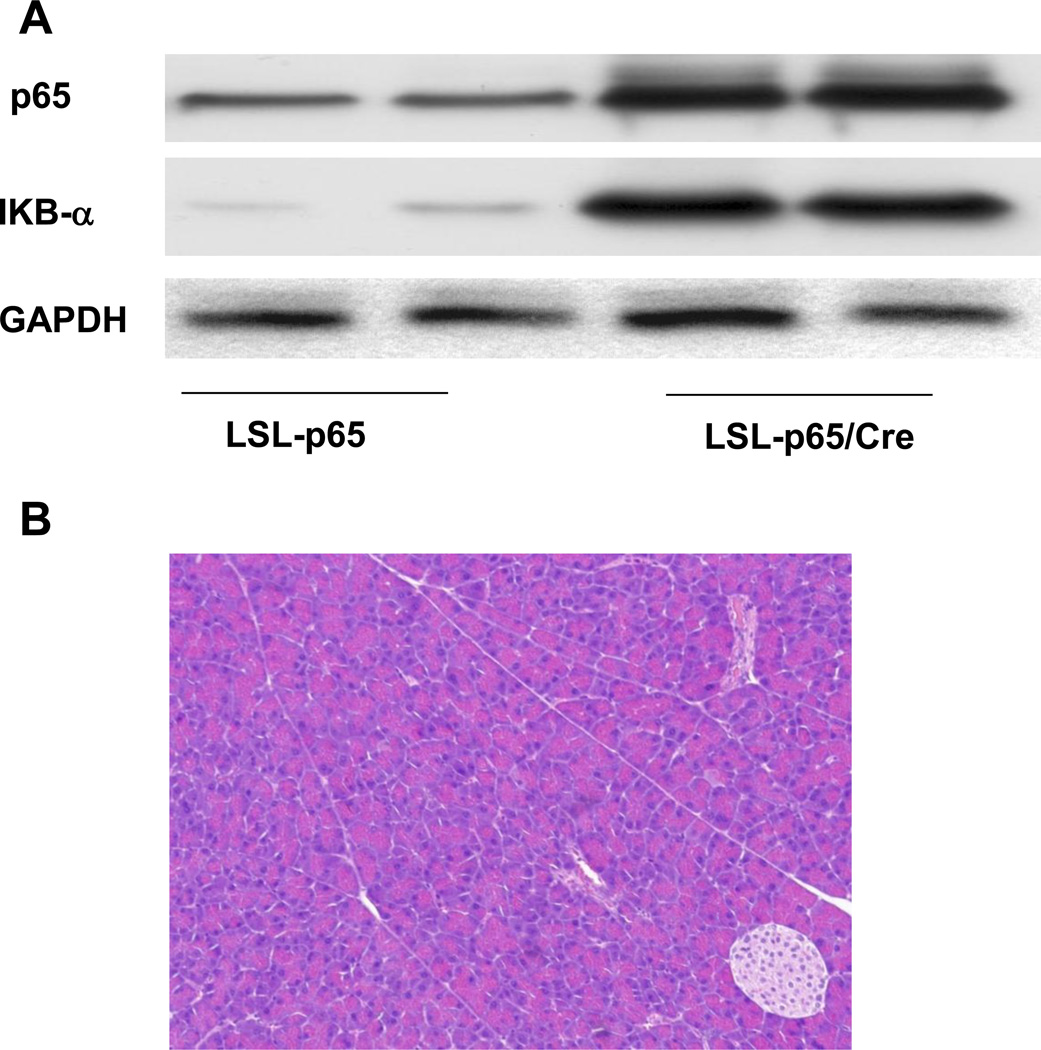
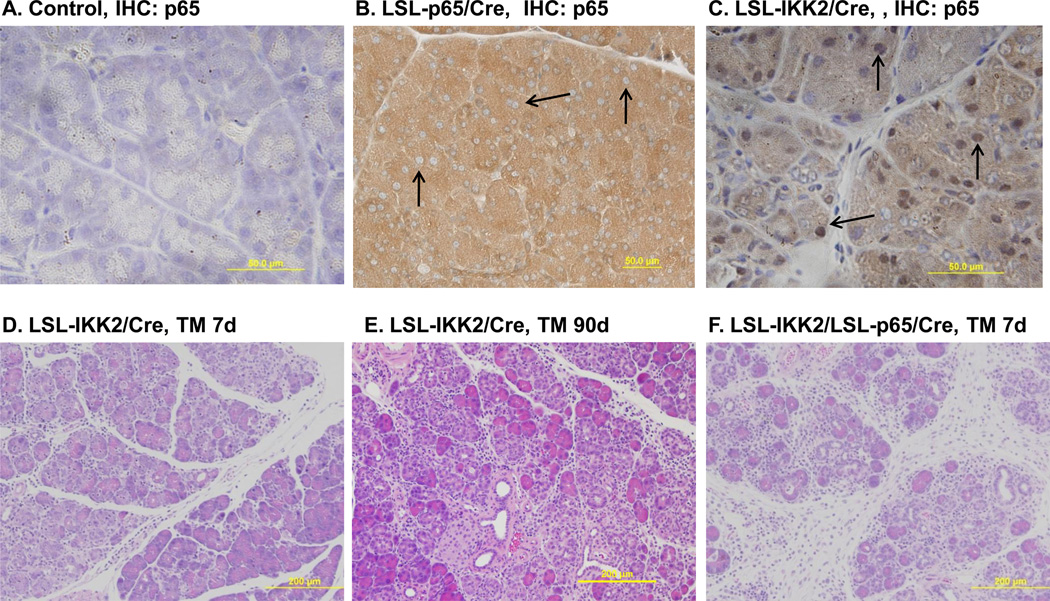
To express p65 specifically in the pancreatic acinar cells, the LSL-p65 mice were crossed with Ela-CreERT mice which have been previously described18. Ela-CreERT mice express tamoxifen regulated CreERT driven by a full-length pancreatic acinar specific elastase I promoter. Double transgenic Ela-CreERT × LSL-p65 (LSL-p65/Cre) mice dramatically elevated expression of p65 in pancreatic acinar cells once induced with tamoxifen (TM) (Figure 1A). However, no obvious phenotypic changes in the pancreas of double transgenic Ela-CreERT × LSL-p65 mice were observed at any time points after TM (Figure 1B). This lack of phenotype was likely due to the negative feedback mechanism since the NF-κB inhibitory subunit IκB-α is also a major target gene of NF-κB. Indeed, we observed increased IκB-α expression in Ela-CreERT × LSL-p65 (LSL-p65/Cre) mice (Figure 1A). The compensation was further illustrated by the lack of NF-κB DNA binding activity in the double transgenic Ela-CreERT × LSL-p65 (p65) mice compared with controls (Figure 2A).
Figure 1.
Transgenic expression of p65 led to a compensatory response. A. Pancreatic p65 expression increased after conditional transgenic mice (LSL-p65) were crossed with mice harboring pancreatic Ela-CreERT (LSL-p65/Cre, 3 days after last TM induction). NF-κB inhibitory subunit IκB-α was unregulated in LSL-p65/Cre mice. B. No obvious phenotypic changes in the pancreata of LSL-p65/Cre mice were observed (3 days after last TM induction).
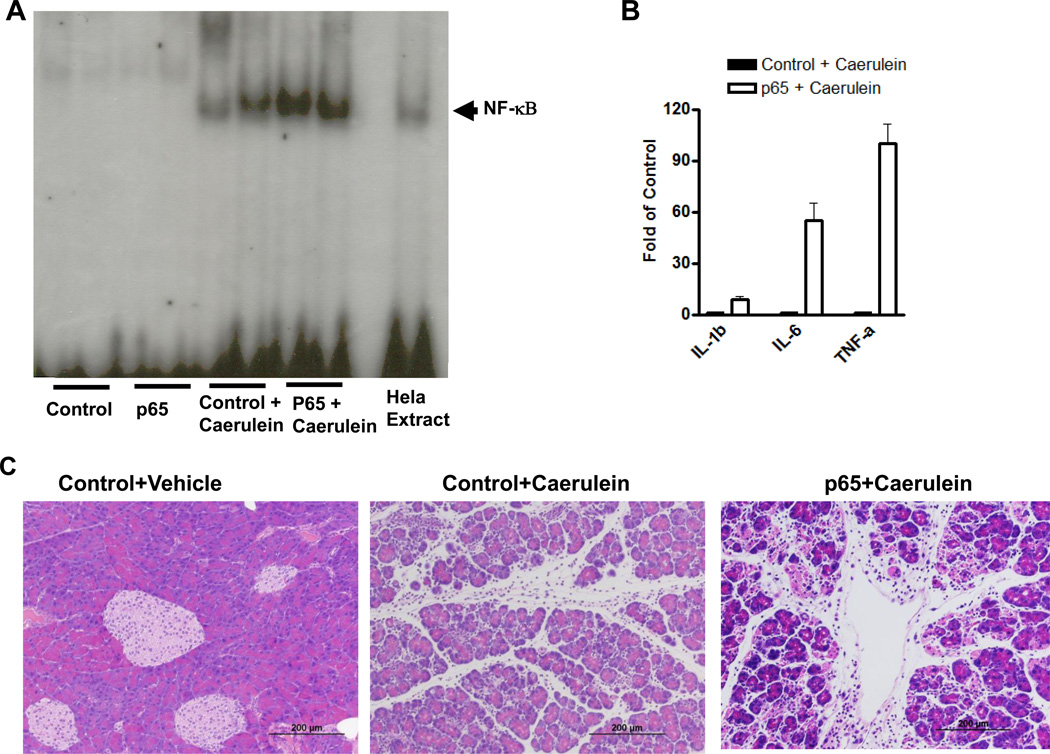
Figure 2.
High-dose caerulein caused enhanced NF-κB activity and increased severity of pancreatitis in mice with high basal p65 expression. A. In an EMSA assay, no NF-κB DNA binding changes were observed in the pancreata of control and LSL-p65/Cre (p65) mice. 1 hour after caerulein (50µg/kg, i.p.) treatment, NF-κB DNA binding activity was increased in control mice (Control+Caerulein) and further upregulated in LSL-p65/Cre mice (p65+Caerulein). B. Real time RT-PCR revealed that cytokines IL-1β, IL-6 and TNF-α dramatically increased in the pancreas of mice with transgenic p65 expression (p65) compared to control mice 1 hour after a single dose of caerulein administration (50µg/kg, i.p.). C. Pancreata were removed 1 hour after 12 hourly repeated caerulein treatments. More severe pancreatic damages were observed in Ela-CreERT × LSL-p65 mice with transgenic p65 overexpression (p65+Caerulein) than controls (Control+Caerulein).
High concentrations of caerulein activate NF-κB in pancreatic acinar cells 8. Therefore, we investigated if elevated levels of NF-κB complexes generated in double transgenic Ela-CreERT × LSL-p65 mice would affect NF-κB activity after caerulein stimulation. Caerulein increased NF-κB DNA binding activity in control animals (control+caerulein) as expected (Figure 2A). In comparison, the level of NF-κB DNA binding activity induced by caerulein in the double transgenic Ela-CreERT × LSL-p65 mice (p65+caerulein) was dramatically higher (Figure 2A, Supplemental Figure 2). The increased NF-κB activity in the double transgenic Ela-CreERT × LSL-p65 mice was also associated with increased transcription of its target genes IL-1β, IL-6 and TNF-α (Figure 2B).
Transgenic expression of p65 increased NF-κB activity and the severity of caerulein-induced acute and chronic pancreatitis
We previously reported that elevated expression of p65 using adenoviral delivery induced pancreatitis 12. To determine whether elevated p65 levels generated by genetic engineering had a similar effect, we measured pancreatitis parameters after caerulein injections in control mice and in double transgenic Ela-CreERT × LSL-p65 animals with elevated expression of NF-κB. Caerulein administration to control animals induced a typical mild pancreatitis (Figure 2C), with increased serum amylase (Figure 3A), pancreatic edema (Figure 3B) and inflammatory cell infiltrations (Figure 3C, Supplemental Figure 3). In contrast, each of these inflammatory parameters was significantly increased in the mice with increased levels of NF-κB (Figure 3, Supplemental Figure 3). In all of the experiments, we used WT and mice harboring either Cre or LSL-p65 alone as controls. Additionally, to exclude the potential complications of TM administration, LSL-p65 mice were also crossed with Pdx1-Cre mice to activate p65 expression without the need for TM. The same effects on pancreatitis were observed in all of these models.
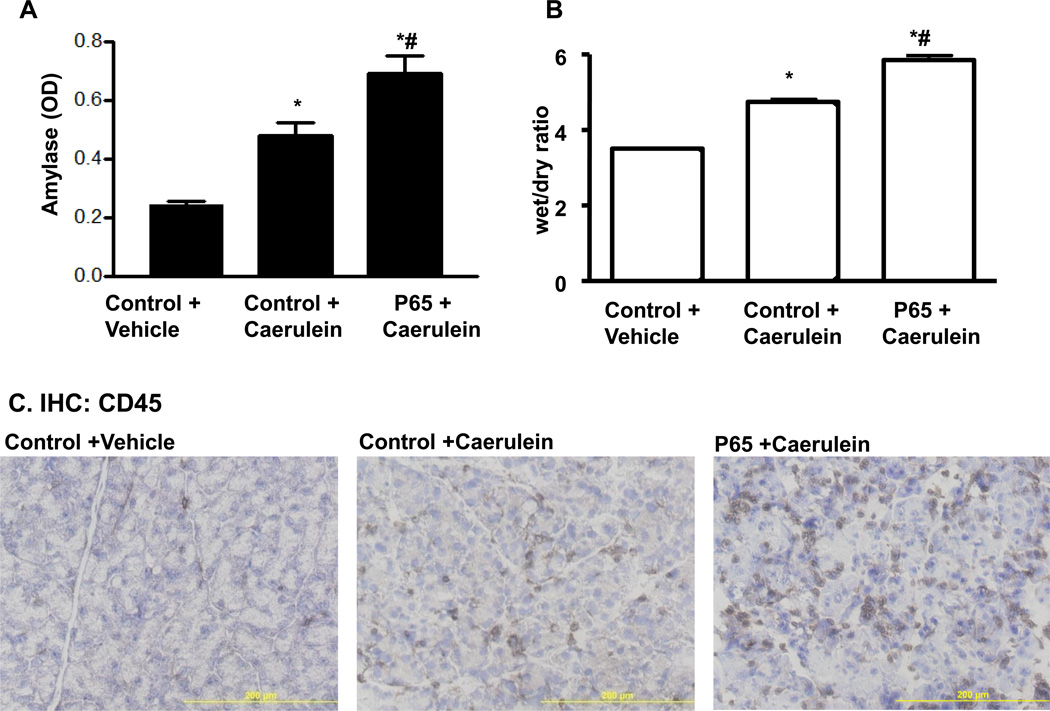
Figure 3.
Caerulein treatment increased severity of pancreatitis parameters. A. After 12 repeated caerulein treatments, serum amylase was higher in Ela-CreERT × LSL-p65 mice with pancreatic expression of p65 (p65+Caerulein). B. Water content of the pancreata with high basal p65 expression was much higher. C. In pancreata with transgenic expression of p65 (p65+Caerulein), there were more CD45 inflammatory cell infiltration. (*p<0.05 vs control, #p<0.05 vs control+caerulein).
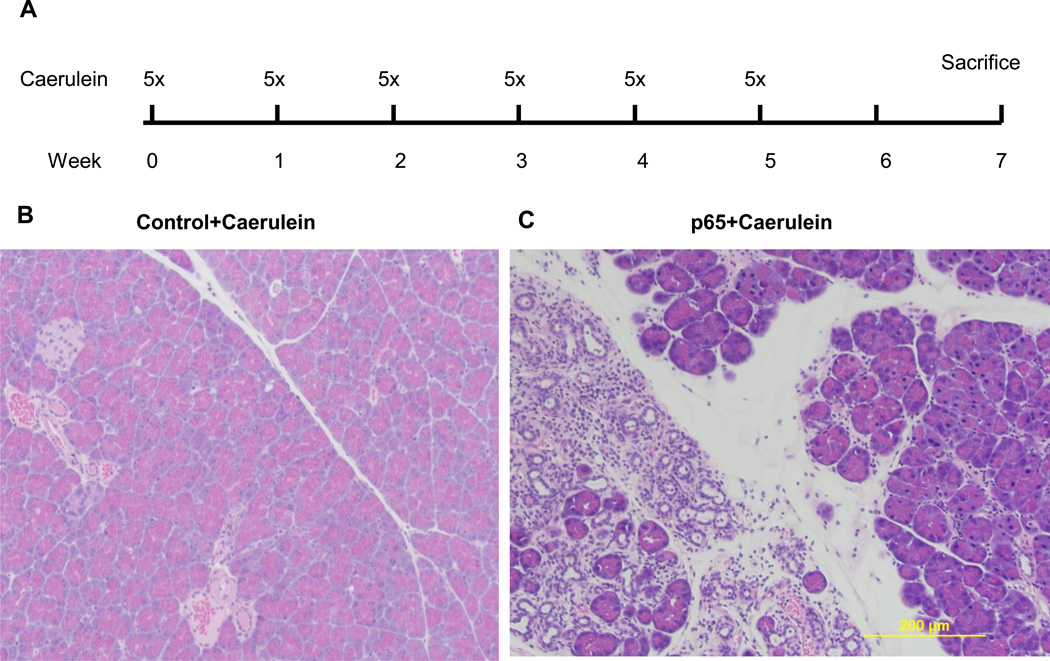
Repeated injections of caerulein are sometimes used to model chronic pancreatitis23. We expected that with increased NF-κB activity and severity of acute pancreatitis in p65 transgenic mice repeated caerulein injection would lead to more severe chronic pancreatitis. Thus, we used a much milder caerulein treatment strategy in the current study (5 hourly injections, once a week for 5 weeks) than that used by others (6 hourly injections, twice a week for 10 weeks)24. In our study, multiple caerulein injections in control mice (Figure 4A) did not lead to any obvious chronic damages to the pancreas (Figure 4B). In contrast, when double transgenic Ela-CreERT × LSL-p65 mice with high levels of NF-κB were treated identically, focal chronic damage including acinar atrophy, persistent inflammatory cell infiltration and fibrosis were observed, suggesting much greater severity of chronic pancreatitis (Figure 4C).
Figure 4.
Repeated caerulein treatment causes chronic pancreatitis in mice with high basal p65 expression. A. Control mice and Ela-CreERT × LSL-p65mice were treated with 5 hourly ip injection of caerulein, once a week for 5 consecutive weeks. Pancreata were harvested 2 weeks after last injection. B. Pancreata of control mice (control+caerulein) showed no obvious damages while focal chronic lesions were observed in most (5/6) of the Ela-CreERT × LSL-p65 mice (p65+caerulein).
Pancreatic specific expression of active IKK2 induces pancreatitis
As an independent approach to increase acinar cell NF-κB activity levels, we developed LSL-IKK2 transgenic mice with conditional expression of HA-tagged constitutively active IKK2, an upstream activator of NF-κB pathway, using the same strategy as for p65 expression. LSL-IKK2 mice were crossed with Ela-CreERT mice to allow pancreatic acinar specific expression of active IKK2. Treatment of these Ela-CreERT × LSL-IKK2 mice with TM induced expression of HA-IKK2 in acinar cells as expected (Supplemental Figure 4). However, unlike ectopic expression of p65, expression of active IKK2 did not lead to successful compensation and rather led directly to increased nuclear localization of NF-κB p65, an indicator of NF-κB activation (Figure 5A–C, Supplemental Figure 5). This may be because active IKK2 continuously phosphorylates and subsequently leads to the degradation of inhibitory IKBs. Consequently, acute pancreatitis with acinar cell damage, edema and inflammatory cell infiltration developed spontaneously after TM-induced IKK2 expression (Figure 5D). Over longer periods, the observed pancreatic damage was more severe. Persistent IKK2 expression for 3 months led to obvious atrophy of the pancreas in all of the mice with at least 50% weight reduction. Histological signs including inflammatory cell infiltration, acinar cell atrophy and fibrosis were always present indicating the development of chronic pancreatitis (Figure 5E).
Figure 5.
Acinar specific expression of constitutively active IKK2 led to NF-κB p65 nuclear translocation and pancreatitis. A. Immunohistochemical staining with antibody against p65 did not detect p65 nuclear translocation in control mice. B. Transgenic p65 expression in Ela-CreERT × LSL-p65 (LSL-p65/Cre) mice led to higher basal p65 level (brown) without nuclear translocation (arrow). C. Pancreatic specific expression of constitutively active IKK2 in Ela-CreERT × LSLIKK2 (LSL-IKK2/Cre) mice caused p65 nuclear translocation (arrow). D. Pancreatic acinar damage and inflammatory cell infiltration were obvious seven day after TM induction of IKK2 expression. E. Pancreatic acinar damage, inflammatory cell infiltration and fibrosis were evident 3 months after TM induction of IKK2 expression. E. Co-expression of IKK2 and p65 further increased the severity of pancreatitis in Ela-CreERT × LSL-IKK2 × LSL-p65 (LSL-IKK2/LSL-p65/Cre) mice (7 days after TM).
Co-expression of IKK2 and p65 generates a severe form of pancreatitis
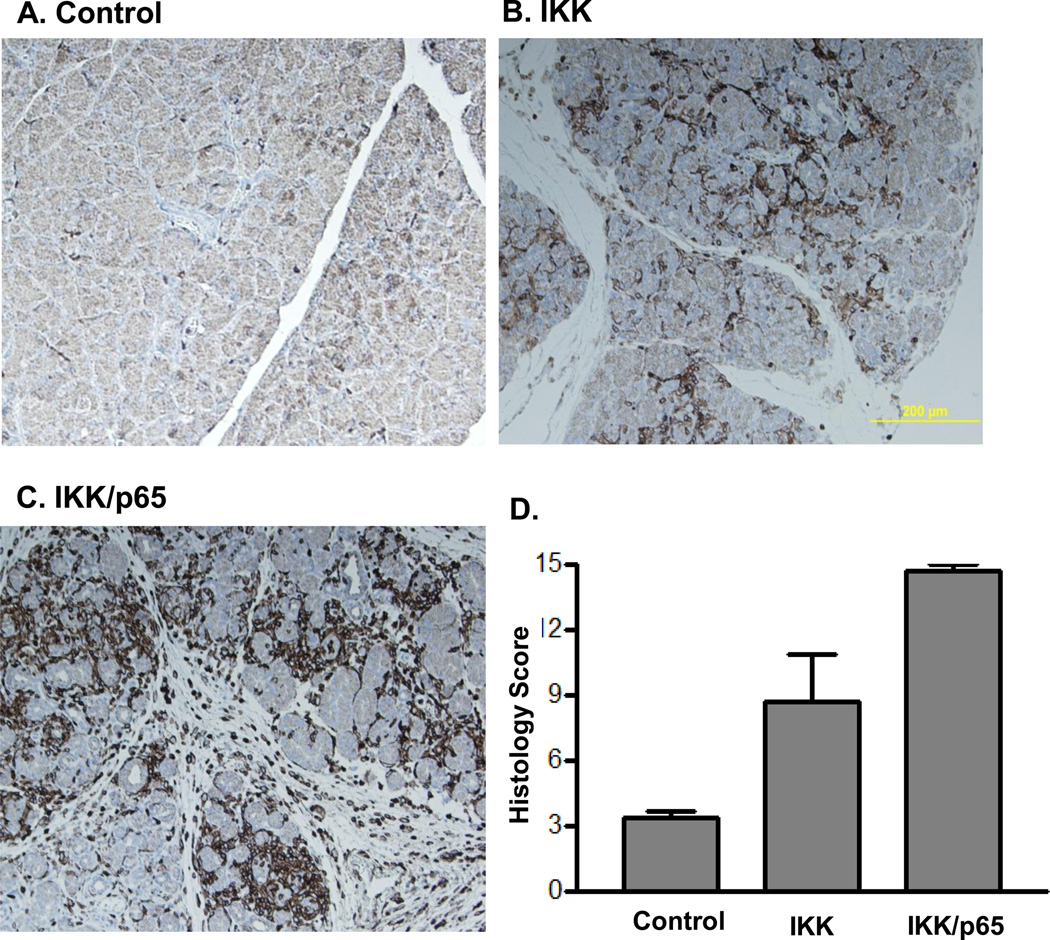
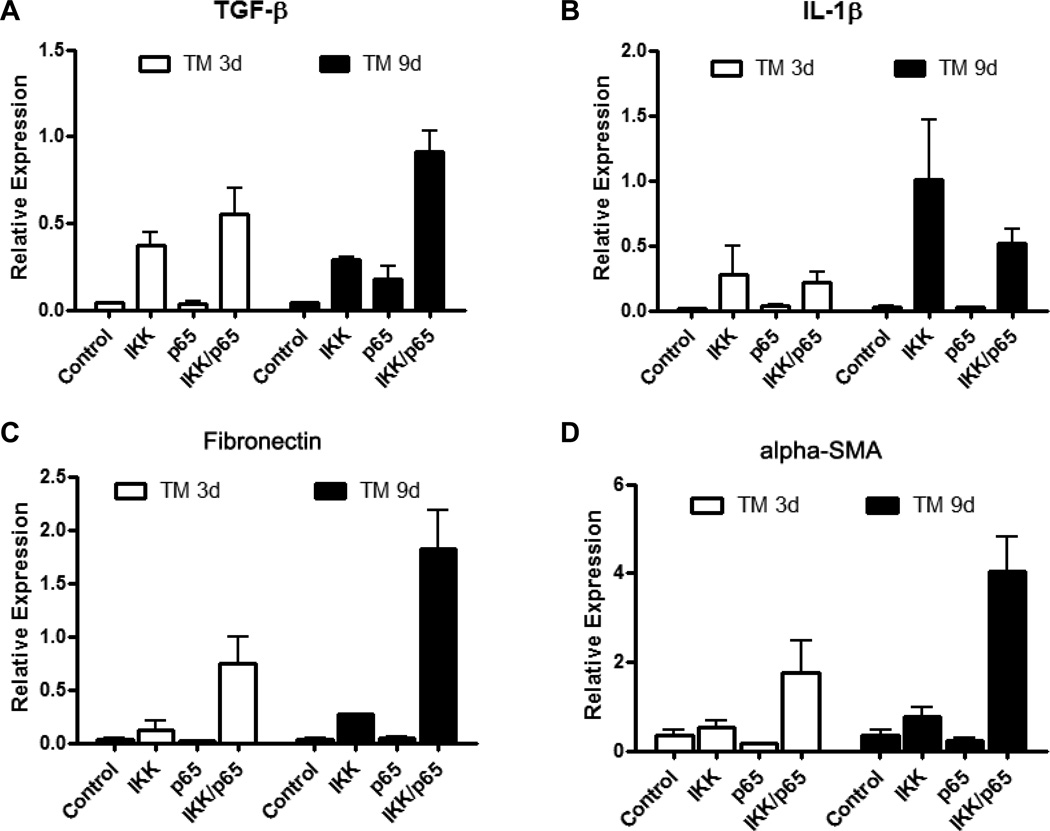
We further crossed LSL-IKK2 and LSL-p65 mice with Ela-CreERT mice to generate Ela-CreERT × LSL-IKK2 × LSL-p65 triple transgenic mice. Induction of these mice with TM led to elevated expression of both IKK2 and p65 (data not shown). TM treatment of these mice generated a more severe pancreatitis with highly elevated levels of acinar cell damage (Figure 5F) and inflammatory cell infiltration (Figure 6A–C). Semi-quantitative evaluation of pancreatitis including edema, cell death and inflammatory cells infiltration further indicated increased severity of pancreatitis in Ela-CreERT × LSL-IKK2 × LSL-p65 triple transgenic mice (Figure 6D). The damage was associated with a large increase in the expression of several cytokines and profibrogenic factors including TGF-β (Figure 7A), IL-1β(Figure 7B), fibronectin (Figure 7C) and alpha smooth muscle actin (alpha-SMA, Figure 7D).
Figure 6.
Pancreatic specific expression of active IKK2 or combination of active IKK2 and p65 increased pancreatitis parameters. A. pancreata from control mice (Control), B. pancreata from Ela-CreERT × LSL-IKK2 mice with pancreatic acinar specific expression of IKK2(IKK) and C. pancreata from Ela-CreERT × LSL-IKK2 × LSL-p65 mice with pancreatic acinar specific expression of both IKK2 and p65 (IKK/p65) were stained with CD45 antibody by immunohistochemistry (7 days after TM). D. Semi-quantitative pathological scores evaluation including edema, inflammatory cells infiltration and cell death were compared (note: normal tissues were assigned a total score of 3).
Figure 7.
Elevated NF-κB led to increased mRNAs expression of cytokines and profibrogenic factors. A. TGF-β, B. IL-1β, C. fibronectin and D. alpha smooth muscle actin (alpha-SMA) were upregulated in the pancreata of mice expressing IKK2 (IKK) or in combination of IKK2 and p65 (IKK/p65) but not in control mice (control) and mice expressing p65 (p65) 3 days and 9 days after last TM induction.
Discussion
In this study, we used conditional transgenic mouse lines bearing the p65 NF-κB subunit and/or an active IKK2 subunit to increase NF-κB expression and activity specifically in pancreatic acinar cells. Overall our data indicated doing so greatly increased the severity of acute pancreatitis mostly likely by up-regulating the expression of proinflammatory cytokines. We also found that high persistent levels of NF-κB led to increased fibrosis and loss of parenchymal cells causing a condition resembling chronic pancreatitis. Therefore, elevated NF-κB activity in pancreatic acinar cells increases pancreatitis severity.
Increased NF-κB activity has long been known to increase cytokine gene expression, leukocytes infiltration, and inflammation. The current data are similar to what was observed with adenoviral expression of p6512 and at least one previous study on the transgenic expression of IKK213. The inflammatory cascade initiated during acute pancreatitis is the most deadly aspect of the disease, as the ultimate cause of death in patients with severe acute pancreatitis is either acute respiratory distress syndrome or multiple organ failure 25. Thus, reducing NF-κB activity and the associated inflammation are likely to be useful approaches to therapy.
These data stand in apparent contrast to studies indicating that inhibition of NF-κB by p65 truncation also increased the severity of pancreatitis15. However, this effect is readily explained by the known importance of the NF-κB pathway in anti-apoptosis. A basal level of NF-κB activity is required for cell survival. Therefore, under the artificial situation in which NF-κB is completely eliminated by genetic manipulation, cellular apoptosis results. While low levels of apoptosis of a few damaged cells is considered protective in acute pancreatitis 26, overwhelming apoptosis has been shown to lead to necrosis and increased inflammation 22. This may be caused by overwhelming immune mechanisms responsible for clearing apoptotic cells and therefore provide an alternative mechanism of initiating pancreatitis related inflammation. Therefore, although the scientific insights developed by genetic elimination of NF-κB are highly instructive about basic mechanisms, there is no probability of patients presenting with this problem. Therefore, under physiologically realistic circumstances, NF-κB activity increases the severity of pancreatitis and efforts to reduce its activity are likely to be clinically beneficial.
The compensatory changes observed in IκB-α when p65 levels are raised during development tell a cautionary tale. This pathway, like most complex biological pathways, has a number of checks and balances. Traditional transgenes which alter one part of the system during development are very likely to lead to compensatory changes in other parts of the pathway. Thus, results obtained with traditional genetic approaches need to be interpreted carefully.
Taken together, the data from these novel models indicate that the NF-κB pathways directly increase the severity of pancreatitis and are sufficient to produce chronic pancreatitis. Thus, while the complete absence of NF-κB in experimental models can also lead to more severe pancreatitis, increased NF-κB activity is much more likely to be observed in patients and is therefore more clinically relevant. For typical patients the inhibition of NF-κB activity would be appropriate and should be beneficial for pancreatitis prevention and treatment.
Supplementary Material
Acknowledgments
Grant Support: This research was supported by funds from DK052067, Cancer Center Support Core grant CA016672, Pancreatic Specialized Programs of Research Excellence grant P20 CA101936 and P50 CA102701, and by the Lockton Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors have no conflict of interest to disclose.
Author Contributions- HH contributed to acquisition and interpretation of data, statistical analysis and drafting of the manuscript. YL, JD, SG and JC involved acquisition of data and technical support. HW provided histology support. CDL and BJ governed study concept and design, interpretation of data, drafting of the manuscript and obtaining funding.
References
- 1.Dunn JA, Li C, Ha T, Kao RL, Browder W. Therapeutic modification of nuclear factor kappa B binding activity and tumor necrosis factor-alpha gene expression during acute biliary pancreatitis. Am Surg. 1997;63:1036–1043. discussion 1043-4. [PubMed] [Google Scholar]
- 2.Grady T, Liang P, Ernst SA, Logsdon CD. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;113:1966–1975. doi: 10.1016/s0016-5085(97)70017-9. [DOI] [PubMed] [Google Scholar]
- 3.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 4.Saluja AK, Steer MLP. Pathophysiology of pancreatitis. Role of cytokines and other mediators of inflammation. Digestion. 1999;60(Suppl 1):27–33. doi: 10.1159/000051450. [DOI] [PubMed] [Google Scholar]
- 5.Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253–258. doi: 10.1136/gut.44.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420–430. doi: 10.1016/s0016-5085(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 7.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 8.Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NFkappaB in rat pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;280:C465–C472. doi: 10.1152/ajpcell.2001.280.3.C465. [DOI] [PubMed] [Google Scholar]
- 9.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karin M. The NF-kappa B activation pathway: its regulation and role in inflammation and cell survival. Cancer J Sci Am. 1998;4(Suppl 1):S92–S99. [PubMed] [Google Scholar]
- 11.Radhakrishnan SK, Kamalakaran S. Pro-apoptotic role of NF-kappaB: implications for cancer therapy. Biochim Biophys Acta. 2006;1766:53–62. doi: 10.1016/j.bbcan.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 13.Baumann B, Wagner M, Aleksic T, von Wichert G, Weber CK, Adler G, Wirth T. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest. 2007;117:1502–1513. doi: 10.1172/JCI30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aleksic T, Baumann B, Wagner M, Adler G, Wirth T, Weber CK. Cellular immune reaction in the pancreas is induced by constitutively active IkappaB kinase-2. Gut. 2007;56:227–236. doi: 10.1136/gut.2005.084665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algul H, Treiber M, Lesina M, Nakhai H, Saur D, Geisler F, Pfeifer A, Paxian S, Schmid RM. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J Clin Invest. 2007;117:1490–1501. doi: 10.1172/JCI29882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji B, Tsou L, Wang H, Gaiser S, Chang DZ, Daniluk J, Bi Y, Grote T, Longnecker DS, Logsdon CD. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. doi: 10.1053/j.gastro.2009.05.052. 1082 e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 18.Ji B, Song J, Tsou L, Bi Y, Gaiser S, Mortensen R, Logsdon C. Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis. 2008;46:390–395. doi: 10.1002/dvg.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan JD, Romac J, Peng RY, Peyton M, Macdonald RJ, Liddle RA. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–727. doi: 10.1053/j.gastro.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Sternfeld L, Yang F, Rodriguez JA, Ross C, Hayden MR, Carriere F, Liu G, Hofer W, Schulz I. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 2009;58:422–430. doi: 10.1136/gut.2007.146258. [DOI] [PubMed] [Google Scholar]
- 21.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 22.Gaiser S, Daniluk J, Liu Y, Tsou L, Chu J, Lee W, Longnecker DS, Logsdon CD, Ji B. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 60:1379–1388. doi: 10.1136/gut.2010.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuschwander-Tetri BA, Burton FR, Presti ME, Britton RS, Janney CG, Garvin PR, Brunt EM, Galvin NJ, Poulos JE. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci. 2000;45:665–674. doi: 10.1023/a:1005423122127. [DOI] [PubMed] [Google Scholar]
- 24.Neuschwander-Tetri BA, Burton FR, Presti ME, Britton RS, Janney CG, Garvin PR, Brunt EM, Galvin NJ, Poulos JE. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Digestive diseases and sciences. 2000;45:665–674. doi: 10.1023/a:1005423122127. [DOI] [PubMed] [Google Scholar]
- 25.Zhou MT, Chen CS, Chen BC, Zhang QY, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 16:2094–2099. doi: 10.3748/wjg.v16.i17.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.