Abstract
Sensitization is a form of non-associative conditioning in which amplification of behavioral responses can occur following presentation of an aversive or noxious stimulus. Understanding the cellular and molecular underpinnings of sensitization has been an overarching theme spanning the field of learning and memory as well as that of pain research. In this review we examine how sensitization, both in the context of learning as well as pain processing, shares evolutionarily conserved behavioral, cellular/synaptic, and epigenetic mechanisms across phyla. First, we characterize the behavioral phenomenon of sensitization both in invertebrates and vertebrates. Particular emphasis is placed on long-term sensitization (LTS) of withdrawal reflexes in Aplysia following aversive stimulation or injury, although additional invertebrate models are also covered. In the context of vertebrates, sensitization of mammalian hyperarousal in a model of post-traumatic stress disorder (PTSD), as well as mammalian models of inflammatory and neuropathic pain is characterized. Second, we investigate the cellular and synaptic mechanisms underlying these behaviors. We focus our discussion on serotonin-mediated long-term facilitation (LTF) and axotomy-mediated long-term hyperexcitability (LTH) in reduced Aplysia systems, as well as mammalian spinal plasticity mechanisms of central sensitization. Third, we explore recent evidence implicating epigenetic mechanisms in learning-and pain- related sensitization. This review illustrates the fundamental and functional overlay of the learning and memory field with the pain field which argues for homologous persistent plasticity mechanisms in response to sensitizing stimuli or injury across phyla.
Keywords: Aplysia, central sensitization, epigenetic, histone, methylation, sensitization
1. Introduction
Memory and pain, though two richly diverse fields, have many underlying commonalities. Both for example contain conscious and unconscious processes that allow for the acquisition of altered behavior in response to environmental stimuli. In popular culture, descriptive terms are often ascribed to memories emphasizing the pain experienced when recalling them. Colloquially it is not uncommon to see or hear the phrase “painful memory”. While these recalled memories are declarative or explicit memories, implicit memories, or memories that involve reflexive responses, can also involve pain. Though the philosopher John Locke did not attempt to understand how memory functions at a physiological level, he did recognize that, “those [memories] which naturally at first make the deepest and most lasting impressions, are those which are accompanied with pleasure or pain” (Locke, 1836).
A form of implicit learning that is evolutionarily vital to species survival is non-associative conditioning, in which behavior is modified in response to a single event. Sensitization is a form of non-associative conditioning where amplification of defensive behavioral responses is observed in response to aversive or noxious stimuli that cause or can lead to pain. Pain, according to the International Association for Pain 2011 taxonomy, is defined as: “An unpleasant sensory and emotional experience associated with actual or potential tissue damage”. Nociception is the sensory process that encodes noxious stimulation and presumably occurs in all animals that possess even the most primitive of sensory systems. In contrast, the emotional component of pain is a complex process that most requires a more complex brain. Long-lasting sensitization mechanisms in nociceptive pathways are the driving force behind numerous biological responses to injury and inflammation in both simple and complex animals. In some complex animals these sensitization mechanisms, a number of which appear to be highly conserved, enhance the activation of pathways that can in turn activate higher order responses such as the emotion of pain. Interestingly, there is evidence to suggest that many of the cellular and molecular mechanisms involved in sensitization are not only shared between pain and memory pathways but also conserved across phyla. Hence, as others have suggested, the evolutionary overlap of behavioral, cellular, and molecular mechanisms in long-term sensitization of both nociceptor and memory circuits argues for homologous plasticity mechanisms necessary for persistent experience-dependent behavioral change (Walters, 2009).
This review focuses on the behavioral, cellular, synaptic, and epigenetic alterations observed in sensitization, both in the context of learning as well as pain processing. Specifically, we will explore the behavioral phenomenon of sensitization in response to aversive and painful stimulation both in invertebrate and mammalian species. Then we will investigate the cellular and synaptic mechanisms underlying sensitization in both memory and pain pathways. Finally, we will explore the role of epigenetic mechanisms, such as DNA methylation and chromatin remodeling, in learning- and pain- related sensitization.
2. Behavioral Models
In almost every known species, noxious stimulation will result in defensive behavioral responses. These responses can include withdrawal responses, escape behavior, freezing, or immobility (Walters, 1994). Evolutionarily, this functions to prevent further or future harm to the organism. Sensitization behaviorally manifests as prolonged defensive behavioral responses, generalized defensive responsiveness to stimuli which would previously fail to evoke a behavioral response, or both. Here we will explore the behavioral phenotype observed in a number of invertebrate and vertebrate systems following sensitizing stimulation. Of the 15 million known species on Earth, 95% are invertebrates and less than 1% are mammalian (Myers, Mittermeier, Mittermeier, da Fonseca, and Kent, 2000). The readily identifiable neurons present in many invertebrate species provide ideal systems for the study of neuronal circuitry and the behavior it mediates. Studies in invertebrate models have informed, and in many cases, precipitated studies of non-associative conditioning in vertebrate systems. Our description of vertebrate studies will be confined to mammalian rodent models of non-associative conditioning as findings in these model systems comprise the bulk of present sensitization research.
2.1. Invertebrate
2.1.1. Learning and memory
No marine invertebrate system has been studied more in the learning and memory field than Aplysia californica. Eric Kandel’s Nobel Prize winning research focused on the responsiveness of this benthic sea hare to aversive stimulation. Aplysia is a large, soft bodied invertebrate without a shell that has a simple nervous system consisting of a mere 20,000 neurons, compared to 1012 found in many higher order mammals. These neurons are comparatively large in size, consistent in anatomical position, and readily identifiable; thus the nervous system of this sea hare lends itself to investigation of plasticity mechanisms and function following non-associative conditioning.
Aplysia has a gill-siphon complex that in a resting state is extended; aversive stimulation of the complex elicits a defensive response known as the siphon-gill withdrawal reflex (SGWR) from the animal. A similar response is also observable in the Aplysia tail. In pioneering work, Eric Kandel’s group observed prolonged withdrawal of the gill-siphon in response to non-noxious stimulation when preceded by shock to the anterior mantle region (Pinsker, Hening, Carew, and Kandel, 1973). The sensitization of this defensive withdrawal response lasts for a short period of time (< 1 hr), however if multiple shocks are applied over a number of days, the SGWR is both longer in duration and the ability to elicit the reflex persists for a longer duration of time (Pinsker et al., 1973). The former is an example of short-term memory, while the latter is an example of long-term memory. Behaviorally the increased SGWR observed following multiple trains of electric shock is referred to as long-term sensitization (LTS).
These initial non-associative conditioning behavioral paradigms precipitated studies of dissociated sensory-motor Aplysia neurons that revealed many previously unknown mechanisms of synaptic plasticity. One of the hallmarks of this plasticity is an increase in modulatory neurotransmitter release; specifically serotonin (5-HT) plays a significant role in these responses (Marinesco and Carew, 2002; Marinesco, Wickremasinghe, and Carew, 2006). Following 5-HT application, dissociated sensory-motor neuronal cultures manifest plasticity known as facilitation, which is discussed in further detail in the cellular and synaptic mechanisms section (3.1.1). This simple sea slug’s SGWR laid the learning and memory field’s foundations of behavioral plasticity and the associated cellular and molecular mechanisms. The far-reaching implications of these findings extend not only to other invertebrate models, but also to mammalian models of non-associative conditioning.
The relatively simple design of invertebrate CNS has encouraged the investigation of the neural basis of learning in a number of species ranging from arthropods to annelid worms. Arthropods comprise approximately 84% of all known species making it the largest phylum in the animal kingdom. It is therefore no surprise that researchers have turned their sights to members of this phylum for examples of non-associative conditioning. We look first to the larval tobacco hornworm (Manduca sexta) which demonstrates the defensive behavior of “striking” that can be sensitized in non-stimulated prolegs following noxious pinch stimulation - this behavior is robust, albeit of short duration (< 1 day) (Walters, Illich, Weeks, and Lewin, 2001). Arguably the fresh water red swamp crayfish (Procambarus clarkii), also known as a crawfish, provides one of the tastier invertebrate species studied for its sensitizable escape behavior. Non-associative sensitization of the crayfish lateral giant neuron (LG)-mediated tailflip escape response was observed in vivo following aversive shock applied to the head or tail of the crustacean (Krasne and Glanzman, 1986). Similar to the Aplysia system, this response was mediated by modulatory release of a monoamine neurotransmitter, in this case octapamine. Octapamine applied to the crayfish CNS produces enhancement of escape behaviors that mimic those observed following non-associative sensitization (Glanzman and Krasne, 1983). As its common name would suggest, the medicinal leech (Hirudo medicinalis) has properties that have long been extolled by physicians. Leech bloodletting was a popular cure for balancing humors in the Middle Ages and the first written record of the medicinal leech dates back to the Indian physician Sushruta in 800 BC. These annelid worms demonstrate the defensive behaviors of reflexive shortening as well as swimming induction that are sensitized in a 5-HT-dependent fashion following noxious stimulation (Ehrlich, Boulis, Karrer, and Sahley, 1992; for review see Sahley, 1995; Zaccardi, Traina, Cataldo, and Brunelli, 2001). The shortening reflex is mediated by mechanosensory neurons connected to electrically coupled S neurons; together they comprise the fast conducting system (FCS). As will be discussed further in the synaptic and cellular mechanisms section (3.1.2), FCS integrity is essential for leech shortening reflex sensitization. In contrast, swimming induction is linked to a central pattern generator or oscillator comprised of a network of cells. In particular the unpaired intersegmental neuron, cell 204, has been ascribed “command-like properties” in the swimming induction response (Brodfuehrer and Thorogood, 2001). Modulation of cell 204 by endogenous glutamate contributes to sensitization of the swimming induction reflex (Brodfuehrer and Thorogood, 2001; Thorogood and Brodfuehrer, 1995). The wide-range of complex defensive behavioral responses that are related to well-studied, identifiable neural networks in the leech provide a useful paradigm for further investigation of the neural mechanisms underlying sensitization.
2.1.2. Injury-related sensitization models
Non-associative conditioning in the invertebrate Aplysia system has also been demonstrated in response to injury. It has been argued that the mechanosensory neurons innervating both the siphon and tail, specifically those with somata in the left E cluster (LE) of the abdominal ganglion and the ventrocaudal clusters (VC) of the pleural ganglia, are more appropriately classified as nociceptors because only high threshold responsivity is observed when Aplysia are unrestrained (Illich and Walters, 1997; Walters, 2009).The “nociceptive memory” manifests as sensitization measurable by a behavioral response that is both exaggerated and prolonged with even low threshold stimulation. Several in vivo and in vitro preparations that produce “nociceptive memory” of the underlying sensitization event have been reported in the literature (Walters, 2009). The first explicit models of Aplysia injury were reported by Walters, who demonstrated that electrical stimulation (shock) as well as injury (“bite injury” or nerve transection) evoked site-specific sensitization of defensive withdrawal reflexes that have a number of similarities to the hyperalgesic response observed in mammalian injury models (1987). Commonly used Aplysia preparations involve axotomy (Bedi, Salim, Chen, and Glanzman, 1998), inflammation (Farr, Mathews, Zhu, and Ambron, 1999), strong electric shock (20 trains of 10–40 milliamp electric shock with 5 sec inter-trial interval), incisions (Billy and Walters, 1989), or nerve crush of various tail-innervating pedal nerve/s (Dulin, Steffensen, Morris, and Walters, 1995; Weragoda and Walters, 2007). Unlike LTS, injury models in Aplysia are associated with local inflammatory responses and axonal regeneration which contribute to the observed sensitization behavior observed. The contribution of inflammatory response to sensory hyperexcitability was elegantly demonstrated in an Aplysia injury model analogous to the mammalian chronic constriction injury model (detailed in section 2.2.2). Within this model, loose ligation of Aplysia pedal nerves results in sensory excitability believed to be mediated by amebocyte release of inflammatory factors (Clatworthy, Castro, Budelmann, and Walters, 1994).
Strong electrical stimulation or deep transverse incisions from the tail to the midline of Aplysia results in decreased mechanical thresholds and expansion of mechanosensory receptive fields near the site of injury that is long-lasting (weeks). Of particular interest was the observation that noxious stimulation increased receptive field crossover of the tail midline in Aplysia. This finding argues for potential heterosynaptic facilitation (recruitment of non-activated synapses) between activated sensory neurons and their surrounding non-activated cells in addition to collateral sprouting of surrounding sensory neurons across the tail midline to compensate for the loss of damaged sensory tail neurons. (Billy and Walters, 1989). Following in vivo unilateral nerve crush, Aplysia demonstrate weakened or absent centrally-mediated defensive responses, including escape locomotion and siphon withdrawal reflex, in response to tail stimulation on the injured side. Recovery of behavioral defensive responses occurs 1–2 weeks following pedal nerve crush (Dulin et al., 1995). Nerve crush in Aplysia results in enhanced VC neuronal output that recruits uninjured central and peripheral nerves lacking sensory neuronal axons (Dulin et al., 1995).
The sensory neurons whose somata are located in the VC of the pleural ganglia innervate the ipsilateral surface of the Aplysia by projecting via the pedal ganglia (Walters, Byrne, Carew, and Kandel, 1983). Dissociated pedal-pleural ganglia preparations have been utilized for characterization of long-term hyperexcitability (LTH) in sensory neuronal axons following axotomy achieved by cell dissociation, neurite transection, and nerve crush of pedal nerve/s (Liao, Gunstream, Lewin, Ambron, and Walters, 1999). Aplysia injury models extend the previously detailed findings of LTS to encompass mechanisms contributing to nociceptive memory, a process associated with sensitization, axonal regeneration, and its own unique plasticity mechanisms (see section 2.1.3).
While Aplysia have been critical players in the study of non-associative conditioning, another well studied marine invertebrate, the Atlantic squid, has recently emerged in the field. It was in 1952 that Hodgkin and Huxley published a series of five papers detailing their investigation of axonal impulses observed in the giant axons of the Atlantic squid (Loligo pealeii), which provided the foundations of modern neurophysiology (Hodgkin and Huxley, 1952a; b; c; d; Hodgkin, Huxley, and Katz, 1952). More recently, investigation of sensory sensitization resulting from minor injury to one arm yielded the interesting finding that injured squid demonstrate tactile hypersensitivity near the site of injury, as well as general sensitization to tactile stimulation that extends over the entire dorsal surface of the body. This sensitization persists for at least 24 hours post-injury (Crook, Lewis, Hanlon, and Walters, 2011). Injured squid demonstrate increased responsiveness to visual stimuli as evaluated by augmented defensive behaviors of crypsis, inking, and jetting. Specifically, in response to visual stimuli injured squid employed crypsis more quickly than their sham controls and engaged in escape behaviors for longer durations of time.
Sensitization to injury, while evoking alterations in species-specific defensive behavior, can also induce the more ubiquitous reflexive pain-like behaviors of allodynia and hyperalgesia. Increased responsiveness to previously non-noxious levels of stimulation is defined as allodynia, while increased responsiveness to noxious stimulation is defined as hyperalgesia. Ultraviolet radiation treatment has been shown to induce allodynic and hyperalgesic responses to thermal stimulation in third-instar Drosophila larvae (Babcock, Landry, and Galko, 2009). Similar to the findings with moth larval sensitization, the effect in Drosophila larvae was short lived (< 24 hr). The short-lasting sensitization observed in both holometabolous insects should not belie the fact that observed behavioral responses are significant relative to the limited time spans these larvae spend in their respective developmental stages.
The CNS of the invertebrate in general and the leech in particular, provides a fascinating model within which to characterize sensitization in a framework with injury-induced axonal regeneration and circuitry rewiring. While medicinal leeches have long been on the opposite side of the operating scalpel, recent studies have placed them on the metaphorical operating table. Laser axotomy of a single S-cell interneuron axon in the medicinal leech eliminates sensitization of the whole-body shortening response prior to S-cell synaptic regeneration (evaluated 7 – 10 days post axotomy). Interestingly, S-cell chain regeneration was insufficient for sensitization in an intermediate recovery group (14 – 22 days post axotomy), whereas the long-term recovery group (30 – 126 days post axotomy) showed similar sensitization to sham controls (Burrell, Sahley, and Muller, 2003). The authors postulate that since synaptic regeneration alone is insufficient for the expression of behavioral sensitization, modulatory neurotransmitter regulation – specifically 5-HT which excites S-cells and is required for sensitization of the leech shortening reflex (Ehrlich et al., 1992) – may be impacted by axotomy.
2.2. Mammalian
2.2.1. Hyperarousal and sensitization
Mammalian models of learning and memory are by-and-large associative or hybrid models that contain both associative and non-associative components. For a mammalian model of non-associative conditioning we first turn to the state of hyperarousal and sensitization observed in response to neutral cues in murine models of post-traumatic stress disorder (PTSD). Siegmund and Wotjak have developed a mouse model of PTSD that they claim can differentiate between the associative fear memory and non-associative hyperarousal/sensitization, two distinct classes of symptoms observed in clinical PTSD patients. Their paradigm involves presentation of a shock in a specific context and subsequently re-exposing the animal to the same context with no shock (CS+) or testing the response to a novel tone in a novel context (CSn). Interestingly, the amount of incubation time following the initial shock was positively correlated with increased freezing to the CSn; a longer duration of time from initial shock exposure (up to 28 days) equated to longer freezing durations to CSn (Siegmund and Wotjak, 2007b). Importantly, the authors demonstrated significantly higher freezing during CSn presentation than during the pre-tone period, which supports the conclusion that the protocol reflects measurement of non-associative hyperarousal and sensitization rather than a generalization of the contextual fear response. Injection of an NMDA receptor antagonist bilaterally into the hippocampus reduced conditioned, but not sensitized fear response when animals were evaluated one month after initial shock exposure (Siegmund and Wotjak, 2007a). The findings suggest the mechanisms underlying sensitized hyperarousal are distinct from those typically linked to associative fear memories in this model of PTSD. Further studies are necessary to elucidate shared and differential mechanisms of sensitization observed in mammalian associative vs. non-associative conditioning. Unfortunately, current studies of PTSD-associated cellular and molecular changes do not differentiate effects which are clearly non-associative; therefore our discussion of this model is limited to the behavioral phenotype.
2.2.2. Pain injury models
Injury provides another clear example of mammalian non-associative sensitization. Injury in both the invertebrate and mammalian vertebrate systems shares a number of common features including alterations in synaptic plasticity, increases in neuronal excitability, and growth cone formation. Where they differ is that while many invertebrates have been shown to regrow injured axons, mammalian species generally lack this ability within the central nervous system. A plethora of rodent pain models are currently used to evaluate pain-like symptoms. Heuristic division of pain models and symptomology yields three principle categories with related underlying mechanisms: acute, post injury/inflammation, and post nerve injury (for review see Xu and Yaksh, 2011). Here we will focus on behavioral nociceptive responses to stimulation (allodynia or hyperalgesia) observed following injection of inflammatory noxious agents or nerve injury.
Behaviorally the pain field has traditionally concentrated on either unevoked nocifensive behaviors (e.g., biting, licking, flinching) or evoked reflexive responses to tactile and thermal stimulation, although a recent shift towards the study of affective and emotion-like behaviors is apparent (Andrews et al., 2011; King et al., 2009; Langford et al., 2010). As with invertebrate injury models, we should note the important caveat that mammalian pain models will induce local inflammatory responses that contribute to behavioral pain phenotypes. These changes can contribute both to the nociceptive memory response (e.g., hyperalgesic priming) and persistent changes in local sensory function. Importantly, central synaptic plasticity (3.2) also contributes to behavioral pain sensitization. Our review will highlight only a fraction of the currently characterized behavioral pain models (for comprehensive review of these models see: Jaggi, Jain, and Singh, 2011; Ren and Dubner, 1999). We focus on those models which are further characterized for epigenetic modifications discussed in section 4.2.
Unilateral injection of noxious inflammatory agents produces locally-mediated inflammatory responses, in addition to centrally-mediated synaptic alterations. Two well-characterized inflammatory models relevant for our review are the formalin and complete Freund’s adjuvant (CFA) models. Various dilutions of formalin (itself a 37% dilution of formaldehyde) can be unilaterally injected into the plantar surface of the hindpaw to induce nocifensive behaviors (Dubuisson and Dennis, 1977; for review see Tjolsen, Berge, Hunskaar, Rosland, and Hole, 1992). Formalin injection produces two temporally distinct phases, the early C-fiber mediated phase (5–10 min post-formalin) (McCall, Tanner, and Levine, 1996) and the late phase, which is associated with a combination of inflammatory changes and transcription-dependent spinal synaptic plasticity (20 – 60 min post-formalin) (Abbott, Franklin, and Westbrook, 1995; Asante, Wallace, and Dickenson, 2009; McNamara et al., 2007; Tjolsen et al., 1992). CFA contains inactivated Mycobacterium tuberculosis that produces arthritis-like symptomology and local inflammation when injected into the ankle joint or plantar surface of the hindpaw (Larson, Brown, el-Atrash, and Walser, 1986; Vikman, Duggan, and Siddall, 2003). CFA injection results in well-documented spinal plasticity changes, mechanical allodynia, as well as thermal and mechanical hyperalgesia (for review see Sandkuhler, 2009).
Neuropathic pain is a debilitating form of chronic pain that can be associated with peripheral nerve injury, diseases (e.g., HIV, shingles), or toxic insults (e.g., chemotherapy) (for review see Rahn and Hohmann, 2009). A number of traumatic peripheral nerve injury models have been detailed in the literature (for review see Sorkin and Yaksh, 2009). Many of these models target nerves innervating the hindpaw. Of particular interest for this review are the chronic constriction injury (CCI), spared nerve injury (SNI), partial sciatic nerve ligation (PSNL), and spinal nerve ligation (SNL) models, all of which produce lasting alterations of neuronal plasticity in the spinal dorsal horn.
The landscape of pain research was forever altered by Bennett and Xie’s introduction of the CCI model in 1988. The CCI model involves unilaterally tying loose ligatures around the common sciatic nerve. As early as two days post-CCI, animals show evidence of mechanical allodynia, as well as thermal hyperalgesia and allodynia in the injured paw (Bennett and Xie, 1988). Spared nerve injury was first described in 2000 by Decosterd and Woolf who argued their model to be both more reproducible and less surgically challenging. SNI is achieved by axotomy of the common peroneal and tibial nerves while “sparing” the sural nerve. Unlike the CCI model, the SNI denervation model allows for investigation of adjacent injured and intact primary sensory neurons. The SNI model reliably produces allodynia and hyperalgesia in response to either mechanical or thermal stimulation (cold) of the injured hindpaw, additionally hyper-responsiveness to suprathreshold heat stimulation is observed (Decosterd and Woolf, 2000). The partial sciatic nerve ligation model (PSNL; Seltzer model), described by Seltzer and colleagues in 1990, involves the tight ligation of one-third to one-half of the sciatic nerve. Hyperalgesia and allodynia in response to both mechanical and thermal stimuli are consistently reported following this injury (Seltzer, Dubner, and Shir, 1990). Kim and Chung developed the spinal nerve ligation (SNL; Chung model) model shortly thereafter which, in its unmodified form, involves the unilateral ligation of either the L5 or both the L5 and L6 spinal nerves (Kim and Chung, 1992). SNL results in several distinct forms of neuropathic nociception including mechanical allodynia and thermal hyperalgesia. Although fewer SNL studies have evaluated cold allodynia, its presence has also been documented.
One distinction between inflammatory and nerve injury models is that cessation of painlike behaviors in the CFA and formalin models parallel resolution of local inflammation (i.e., wound-healing is complete). This is in contrast to nerve injury, which does not demonstrate similar reversal of allodynic and hyperalgesic responses despite injury resolution (Xu and Yaksh, 2011). However, despite apparent resolution of local inflammation in models of acute inflammatory pain, work by Levine and colleagues would suggest changes occur at both the peripheral and central level that leave the animal “primed” for hyper-responsiveness to subsequent injuries or stressors (Reichling and Levine, 2009).
3. Cellular and Synaptic Mechanisms
Behavioral sensitization phenotypes observed in both invertebrate and mammalian species set the stage for investigation of associated functional cellular and synaptic changes. This section will first focus on Aplysia synaptic sensitization induced by 5-HT exposure, termed facilitation. This will be followed by a brief review of the cellular and synaptic mechanisms involved in other invertebrate models of sensitization. We will detail mechanistic alterations associated with each of these distinct non-associative conditioning paradigms highlighting similarities and differences. Limited investigation of injury-induced synaptic and cellular changes in invertebrate models will confine our discussion to injury models in the Aplysia system, changes which produce a phenomenon termed hyperexcitability. Axotomy-induced hyperexcitability in the Aplysia system results in a number of cellular and synaptic alterations that are both unique to this injury model and share characteristics of Aplysia facilitation. Finally, we will examine and discuss central sensitization, a phenomenon critical for the induction and maintenance of pain hypersensitivity in the mammalian system. Throughout this section, signaling cascades, neuromodulatory signals, and proteins that have been implicated in both memory and pain will be compared and contrasted across differential sensitization models.
3.1. Sensitization Mechanisms in Invertebrates
3.1.1. Sensory facilitation in the Aplysia system
Dissociated cultures of sensory-motor neurons from Aplysia allow for detailed characterization of learning-related plasticity changes (see Figure 1). Activity-dependent presynaptic mechanisms of facilitation and depression were once thought to exclusively underlie the learning-related plasticity changes observed in the Aplysia system. More recent studies (e.g., Jin et al., 2012b) have demonstrated that postsynaptic mechanisms also play an important role. Whereas short-term facilitation (minutes) is believed to involve only presynaptic covalent modifications of existing proteins, long-term facilitation ( > 24 hrs) within the Aplysia sensorimotor synapse requires coordinated pre- and post- synaptic modifications (Bailey, Bartsch, and Kandel, 1996). Short- and long- term facilitation (STF and LTF, respectively) have historically been induced with dissociated Aplysia sensorimotor synapses exposed to serotonin (5-HT) (Montarolo et al., 1986). 5-HT is normally released by interneurons in response to sensitizing tail stimuli (Marinesco and Carew, 2002; Marinesco et al., 2006). One 5-HT application results in STF, whereas repeated or long lasting exposure to 5-HT results in LTF. LTF has been linked to changes in gene expression, protein synthesis, and synaptic plasticity (Jin et al., 2012b). Intermediate-term facilitation (ITF), which integrates elements of both STF and LTF, has also been recognized (Ghirardi, Montarolo, and Kandel, 1995). A key component of ITF is the release of calcium (Ca2+) from inositol 1,4,5-trisphosphate (IP3)-sensitive stores, an effect necessary for LTF (Li, Roberts, and Glanzman, 2005).
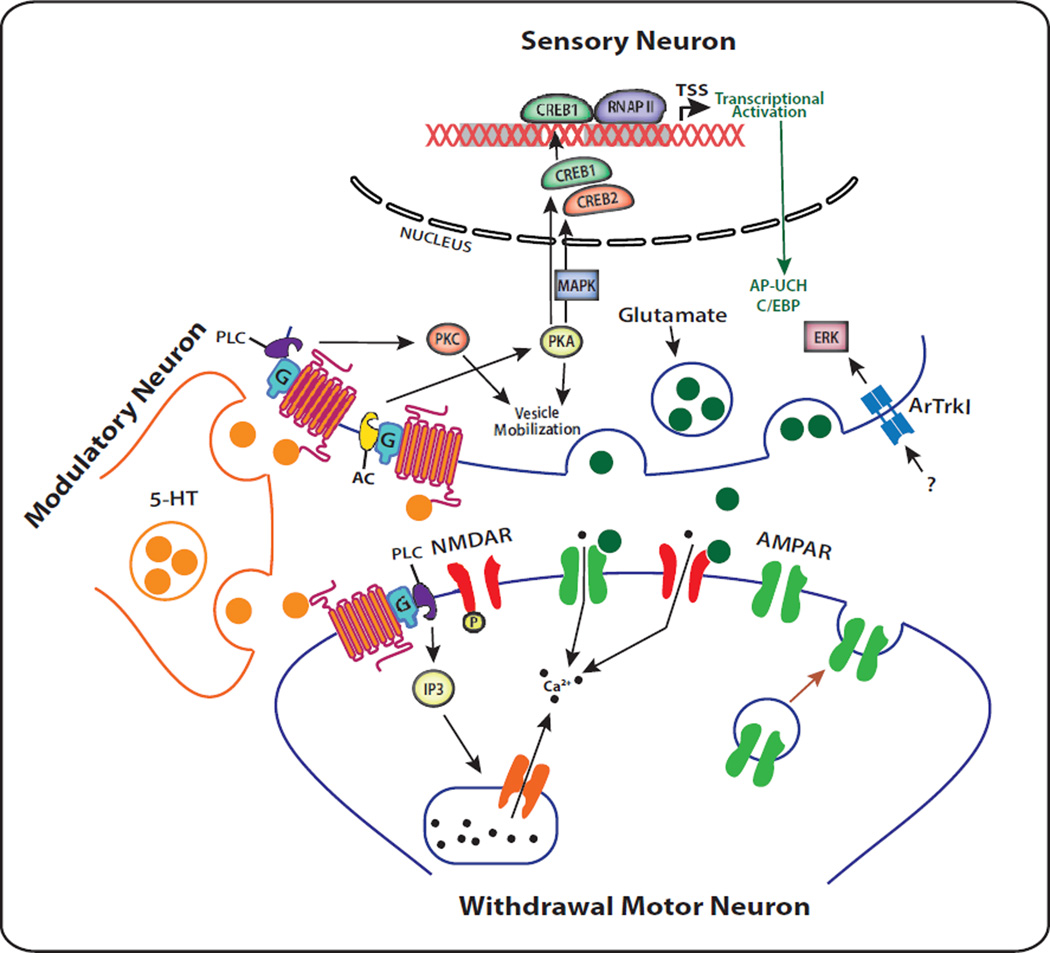
Figure 1. Molecular Mechanisms and Signal Transduction Cascades of Aplysia Long-term Facilitation (LTF).
Long-term facilitation in Aplysia sensorimotor neurons in response to serotonin (5-HT). 5-HT-induced Short-Term Facilitation (STF) of Aplysia sensorimotor synapse results in cAMP-dependent protein kinase (PKA)-mediated presynaptic covalent modifications of existing proteins thereby producing short-term enhancement of neuronal excitability. Intermediate-Term Facilitation (ITF) is associated with spontaneous release of presynaptic neurotransmitters, including glutamate, and trafficking of AMPA GluR1-type receptors to the postsynaptic membrane. ITF is marked by long-term hyperexcitability and postsynaptic modifications associated with increased levels of intracellular Ca2+ through NMDA-mediated influx and release from IP3-meditated intracellular stores. Release of an as yet, uncharacterized brain derived neurotrophic factor (BDNF)-like ligand activates presynaptic tyrosine kinase-like receptors (ApTrkl) that contribute to extracellular receptor kinase (ERK) activation. Long-Term Facilitation is marked by the growth of new synaptic connections and results in translocation of PKA and mitogen activated protein kinase (MAPK) to the nucleus where cAMP-response element-1 (CREB-1) is activated and CREB-2 is repressed. This results in the induction of several immediate early genes (e.g., ubiquitin C-terminal hydrolase (Ap-UCH) and CCAAT enhancer binding protein (C/EBP)).
Sensitization in the Aplysia system is subject to a number of unique plasticity mechanisms that allow for the temporal and spatial summation of signals. The processes induced by STF are crucial to the maintenance and propagation of long-lasting LTF. Interestingly, the order in which short- and long- term facilitation occur, while functioning under certain temporal restrictions, is not static. Multiple applications of 5-HT can induce LTF machinery which in turn is stabilized following STF-induced changes resulting from a single pulse of 5-HT and vice versa (Casadio et al., 1999). STF induction facilitates cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PKA)-mediated covalent modifications that subserve the important function of marking synapses where subsequent synthesis of local proteins stabilizes synaptic growth and maintains synapse-specific rapamycin-sensitive LTF (Casadio et al., 1999).
LTF induces translocation of PKA and mitogen activated protein kinase (MAPK) to the nucleus (Bacskai et al., 1993; Martin et al., 1997). Following translocation, cAMP response element binding protein 1 (CREB-1) is activated and CREB-2 is depressed thereby resulting in induction of several immediate early genes (Alberini, Ghirardi, Metz, and Kandel, 1994; Bartsch, Casadio, Karl, Serodio, and Kandel, 1998; Bartsch et al., 1995; Dash, Hochner, and Kandel, 1990; Hegde et al., 1997). Both cell-wide and synaptic-specific CREB-dependent facilitation is possible in this system (Casadio et al., 1999). However, Kandel and colleagues demonstrated that LTF requires more than cAMP response element (CRE)-driven gene products in the nucleus. Exposure to CREB-1 results in LTF at all synapses, which is only maintained for 24–48 hrs in the absence of PKA-mediated covalent signaling and protein synthesis to stabilize the mark (Casadio et al., 1999; Martin et al., 1997). Stabilization of this activity-induced protein synthesis in the early phase of LTF may occur through the self-sustained propagation of cytoplasmic polyadenylation element binding protein (CPEB). ApCPEB is a prion-like protein homolog found in Aplysia that can undergo a change from an inactive monomer to a self-perpetuating active multimer (Miniaci et al., 2008; Si, Choi, White-Grindley, Majumdar, and Kandel, 2010; Si et al., 2003a; Si, Lindquist, and Kandel, 2003b).
LTF is accompanied by new synaptic growth between sensory and motor neurons orchestrated by a number of molecular events and signaling cascades. One potential contributory mechanism is the evolutionarily conserved target of rapamycin (TOR) pathway. LTF requires activation of TOR pathway-dependent translation that is blocked with rapamycin application (Casadio et al., 1999; Hu, Wu, and Schacher, 2006). As the name would imply, rapamycin acts on the TOR pathway (Heitman, Movva, and Hall, 1991), an important pathway for growth regulation and proliferation through its regulation of both translational initiation and elongation factors. TOR controls translation by regulating phosphorylation of two major downstream targets, S6 kinase (S6K) and eukaryotic translation initiation factor 4E (EIF4E) binding protein (4EBP). 5-HT has been shown to increase phosphorylation of S6K which leads to the decreased phosphorylation of eukaryotic elongation factor 2 (eEF2) through rapamycin-sensitive pathways (Carroll, Warren, Fan, and Sossin, 2004; Khan, Pepio, and Sossin, 2001; Weatherill et al., 2011), whereas effects on 4EBP do not appear to contribute to LTF (Weatherill, Dyer, and Sossin, 2010).
While the modulatory role of serotonin transmission traditionally takes the spotlight in LTF, glutamatergic transmission is also vital for the sensitization behavioral response and associated plasticity (Conrad, Wu, and Schacher, 1999; Dale and Kandel, 1993). Exposure of dissociated Aplysia neurons in cell culture to 5-HT results in long-term increased sensitivity of motor neuron AMPA-type receptors (Trudeau and Castellucci, 1995; Zhu, Wu, and Schacher, 1997), in addition to enhancement of motor neuron responsivity to glutamate exposure (Dale and Kandel, 1993; Levenson et al., 2000). It has been proposed that STF-induced spontaneous presynaptic transmitter release (Jin et al., 2012a) results in increased postsynaptic Ca2+ levels, which lead to exocytosis of AMPA-type receptors (Li et al., 2005; Roberts and Glanzman, 2003). Recently, it was reported that 10 min exposure to 5-HT in dissociated cultures induced ITF marked by increased trafficking of GluR1 AMPA receptors subunits to the postsynaptic membrane; interestingly, ApGluR1 share a high level of homology with mammalian GluR1 AMPA receptors (Jin et al., 2012b).
In addition to glutamatergic receptor trafficking, signaling of neurotrophic factors has also been implicated in Aplysia LTF. While the mechanisms of Aplysia LTF both antedate and galvanized investigation of synaptic plasticity in mammalian systems, research in mammals has now begun to guide work in the Aplysia model. The signaling molecule brain derived neurotrophic factor (BDNF) has long been recognized for its role in hippocampally-mediated long-term potentiation (LTP) of glutamatergic synapses (for review see Cowansage, LeDoux, and Monfils, 2010). In mammals, BDNF activates the tyrosine kinase receptor B (TrkB) which leads to activation of the extracellular receptor kinase (ERK) signaling pathway (for review see Santos, Comprido, and Duarte, 2010). The ERK pathway is a member of the MAPK-superfamily critical for cell proliferation, differentiation, and neuronal synaptic plasticity. 5-HT-induced LTF and ERK signaling are suppressed following administration of the sequestering agent, TrkB-Fc chimera (Sharma, Sherff, Stough, Hsuan, and Carew, 2006). Additional evidence for the importance of an endogenous Aplysia TrkB-like ligand in the induction of LTF comes from a study where human recombinant BDNF enhanced induction of LTF through MAPK signaling (Purcell, Sharma, Bagnall, Sutton, and Carew, 2003). Furthermore it has been shown that phosphorylation of ERK requires activation of a tyrosine kinase-like receptor (Ap-Trkl) (Ormond et al., 2004).
Mammalian studies have also implicated the protein kinase C (PKC) isoform, protein kinase M zeta (PKMζ), as a critical constituent in long-term memory formation (Ling et al., 2002; Shema, Sacktor, and Dudai, 2007; but see also Volk, Bachman, Johnson, Yu, and Huganir, 2013). These mammalian studies prompted the exploration for a PKMζ ortholog in the Aplysia system where protein PKM AplIII, an isoform of protein kinase M resulting from calpain-dependent cleavage of PKC AplIII, was recently discovered (Bougie et al., 2009). Cai and colleagues demonstrated that intrahemocoel injection (delivered to entire CNS) of the purported PKMζ inhibitor (but see Volk et al., 2013), zeta inhibitory peptide (ZIP), or the PKC inhibitor, chelerythrine, was sufficient to erase long-term sensitization of the siphon-gill withdrawal reflex up to 7 days post-training (2011). Both inhibitors disrupted LTF at dissociated sensorimotor synapses. Neither spontaneous recovery nor reinstatement of LTS and LTF were observed. A subsequent study demonstrated that 5-HT-induced ITF processes result in calpain- and protein synthesis- dependent cleavage of PKC AplIII to the active PKM AplIII in motor neurons (Bougie et al., 2012).
3.1.2. Synaptic plasticity in other invertebrate systems
A common theme throughout invertebrate sensitization is that this behavior is mediated by release of modulatory neurotransmitters. For example, the crayfish LG-mediated tailflip escape behavior is augmented through release of octapamine, which is dependent on IP3 (Araki and Nagayama, 2012), but not cAMP (Araki, Nagayama, and Sprayberry, 2005) signaling cascades. Mechanosensory afferents from the crayfish tailfan excite behavior primarily through electrical synapses on the LG (Zucker, 1972); however they also impact cholinergic signaling on sensory interneurons (Miller, Vu, and Krasne, 1992). Heterosynaptic facilitation in response to current-mediated LG depolarization occurs through sensitization of a lateral excitatory network of mechanosensory afferents in the last abdominal ganglia via antidromic depolarizing current (Antonsen and Edwards, 2003; Herberholz, Antonsen, and Edwards, 2002). Interestingly, the LG-mediated escape behavior shows Hebbian-like LTP neuronal excitability in response to repetitive suprathreshold stimulation (Miller, Lee, and Krasne, 1987).
Like the crayfish, the medicinal leech behavioral sensitization is both linked to potentiation of electrical synapses and release of moludatory neurotransmitters. The leech nervous system is organized into segmental ganglia. Sensitization of the shortening reflex in the leech is linked to mechanosensory neurons that are connected to the electrically coupled S neurons. Ablation of a single S-cell interrupts the FCS chain, which eliminates sensitization (Bowling, Nicholls, and Parnas, 1978; Sahley, Modney, Boulis, and Muller, 1994). In contrast to the shortening reflex, the defensive behavior of swimming induction involves a complex pattern produced in part by the actions of cell 204 on the central pattern generator. Research has shown that swimming induction requires glutamatergic-mediated depolarization of cell 204 (Brodfuehrer and Cohen, 1990; Thorogood and Brodfuehrer, 1995). As observed with Aplysia LTF, serotonin is a principle neurotransmitter that modulates sensitization (both shortening reflex and swimming induction) in the leech through increases in cAMP. (Belardetti, Biondi, Colombaioni, Brunelli, and Trevisani, 1982; Zaccardi, Traina, Cataldo, and Brunelli, 2004).
3.1.3. Hyperexcitability in the Aplysia system
Dissociated nociceptive sensory and motor neuron cultures have been utilized to study Aplysia nociceptive-specific excitability plasticity in response to axotomy. Dissociated pedal-pleural ganglia demonstrate a long-lasting (persisting for weeks) form of excitability plasticity marked by increases in repetitive firing and decreases in threshold of nociceptive somata, termed long-term hyperexcitability (LTH) (for review see Walters, 2009). LTH, like LTF, is dependent upon serotonergic activation and subsequent rapamycin-dependent protein synthesis at local sites (Weragoda, Ferrer, and Walters, 2004; Weragoda and Walters, 2007). Interestingly, purported unpublished observations (Klaassen and Walters) demonstrate that local application of a transcription inhibitor, actinomycin D, does not inhibit 5-HT-dependent LTH (Weragoda and Walters, 2007).
Activation of the TOR pathway is known to enhance processes related to axonal growth and regeneration (Verma et al., 2005). The TOR pathway has been proposed to mediate synapse-specific growth observed in association with LTF through local protein synthesis (Casadio et al., 1999; Si et al., 2003a), whereas similar mechanisms likely promote regenerative growth in models of axonal injury (Weragoda and Walters, 2007). Aplysia axotomy results in neurite outgrowth (Bedi et al., 1998); interestingly, similar neurite outgrowth is observed in a 4 day LTS protocol (Wainwright, Zhang, Byrne, and Cleary, 2002). A Ca2+-mediated increase in neurite eEF2 phosphorylation following axotomy has been reported and these observed effects were decreased following a 10-min application of 5-HT as would be predicted based on LTF findings (Carroll et al., 2004).
Following axotomy, Aplysia sensory axons display sprouting, also referred to as hypermorphogenesis that is dependent on activation of the cAMP/PKA pathway (Bedi et al., 1998; Steffensen, Dulin, Walters, and Morris, 1995). Activation of this pathway is critical for synthesis of the transcription factor Aplysia CCAAT enhancer binding protein (ApC/EBP) in response to either injury (Sung, Povelones, and Ambron, 2001) or 5-HT (Alberini et al., 1994). Phosphorylation of C/EBP following axotomy is catalyzed by retrogradely-transported kinase (RISK-1) (Sung et al., 2001).
In contrast to LTF, Aplysia axotomy models have demonstrated that PKA, while necessary for expression of LTH, is neither sufficient nor necessary for induction of this response (Liao et al., 1999). LTH does however appear to require contribution from both PKC (Manseau, Sossin, and Castellucci, 1998) and the nitric oxide (NO)-cyclic GMP-protein kinase G (PKG) pathways (Lewin and Walters, 1999). It has been suggested that multiple pathways may be involved in the induction and maintenance of LTH due to the high relative cost of injury response and axonal regeneration (Liao et al., 1999).
3.2. Central Sensitization Mechanisms in Mammals
Canonical central sensitization refers to an activity-induced increase in mammalian spinal dorsal horn neuronal excitability in response to peripheral nociceptive input (see Figure 2). Central sensitization does occur at supraspinal sites (e.g., thalamus (Dostrovsky and Guilbaud, 1990), amygdala (Neugebauer and Li, 2003), and anterior cingulate cortex (Seifert et al., 2009)), however for the purposes of this review we will only explore central sensitization at the level of the spinal cord. This section will highlight only a small fraction of the mechanisms that contribute to the initiation and maintenance of central sensitization as this complex phenomenon has been reviewed extensively elsewhere (see Latremoliere and Woolf, 2009). Importantly, emphasis in this section is placed primarily on post-synaptic mechanisms that contribute to central sensitization, however nociceptive sensitization results in a number of presynaptic alterations (e.g., increases in modulatory neurotransmitter release) that contribute to the initiation and maintenance of this phenomenon.
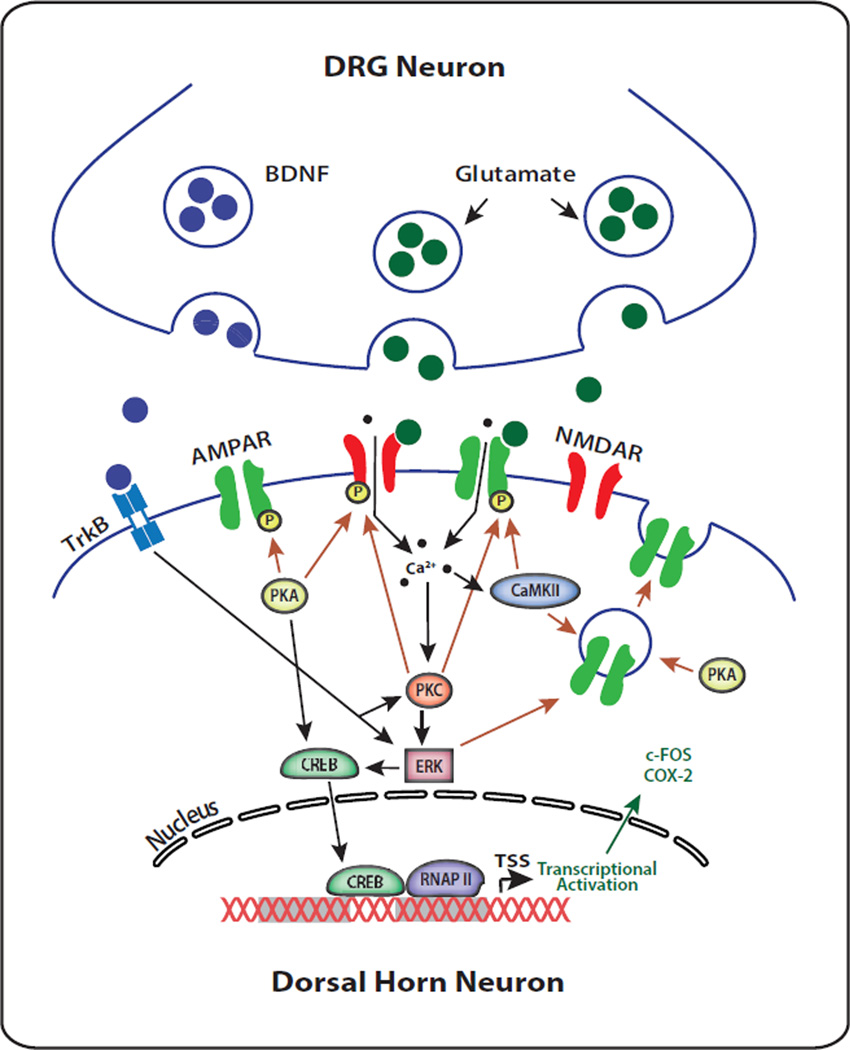
Figure 2. Spinal Cord Dorsal Horn Central Sensitization.
Mechanisms underlying Central Sensitization in dorsal horn spinal neurons. Nociceptive input from peripheral dorsal root ganglia (DRG) synapse in the dorsal horn of the spinal cord. DRG inputs release the neurotransmitter glutamate and the neurotrophic factor BDNF. Glutamate and BDNF bind to postsynpatic AMPA/NMDA ionotropic receptors and tyrosine kinase B (TrkB) receptors, respectively. Activation of AMPA and NMDA receptors results in increased intracellular Ca2+ thereby augmenting synaptic efficacy. Postsynaptic Ca2+ influx triggers protein kinase C (PKC), and Ca2+/calmodulin-dependent kinase II (CaMKII), both of which have the ability to phosphorylate AMPA receptor subunits. In addition, increased intracellular Ca2+ indirectly activates cAMP-dependent protein kinase (PKA) through increased calmodulin and cAMP. PKA and PKC phosphorylate NMDA receptors. PKA, CaMKII, and extracellular receptor kinase (ERK) contribute to recruitment of intracellular GluR1-type AMPA receptors to the membrane from intracellular vesicular stores, ultimately increasing calcium conductance and synaptic efficacy. ERK and PKA contribute to cAMP-response element (CREB)-mediated induction of gene transcription (e.g., c-FOS and cyclooxygenase-2 (COX-2)). Binding of BDNF to TrkB activates PKC- and ERK- mediated signaling cascades.
Increased dorsal spinal cord neuronal excitability is characterized by decreased thresholds, increased receptive field size, and heterosynaptic facilitation (Dougherty and Willis, 1992; for review see Latremoliere and Woolf, 2009; Mantyh et al., 1997; Woolf, 1983). Nociceptive fiber input from C and Aδ fibers can lead to the recruitment of Aβ fibers, fibers typically reserved for non-noxious mechanical stimulation (Koerber, Mirnics, Kavookjian, and Light, 1999; Kohama, Ishikawa, and Kocsis, 2000; for review see Woolf and Salter, 2000; Woolf, Shortland, and Coggeshall, 1992). Noxious input can also transform nociceptive-specific neurons into wide-dynamic range neurons, making them receptive to both low- and high-intensity stimulation (for review see Latremoliere and Woolf, 2009). Central sensitization represents a gain of function for the nociceptive sensory system which is no longer yoked to the intensity, interval, or incidence of peripheral stimulation. Similar to Aplysia LTF, central sensitization has been partitioned into phases with differential mechanistic hallmarks. The first, more rapid temporal phase is dependent upon phosphorylation primarily associated with glutamatergic receptors and ion channel properties (for review see Woolf and Salter, 2000). The second, longer duration phase requires gene transcription ultimately resulting in new protein translation which contributes to the maintenance of central sensitization (for review see Woolf and Salter, 2000).
It is important to note, central sensitization is a normal, evolutionary adaptive mechanism that promotes healing. Both the shorter, transcription-independent and longer, transcription- and translation- dependent phases are reversible. The shorter, transcription-independent phase is typically caused by a priming event such as extreme noxious stimulation. When continued nociceptor activation and peripheral injury persists, it results in induction of the longer maintenance phase. This process becomes maladaptive when it is maintained in the absence of nociceptive input and peripheral pathology (for review see Latremoliere and Woolf, 2009).
Similar to recent findings with intermediate- and long- term facilitation in the Aplysia system, central sensitization is associated with an increase in GluR1-containing AMPA receptors at the postsynaptic membrane. GluR1- and GluR2- containing AMPA receptors are the predominant subtypes expressed in the superficial dorsal horn of the spinal cord (Kerr, Maxwell, and Todd, 1998; Nagy et al., 2004). Inflammation, post-surgical injury, or strong noxious stimulation in the periphery has been shown to produce post-translational modification of AMPA receptors through intracellular protein kinase cascades mediated by PKA, PKC, and calmodulin-dependent protein kinase II (CAMKII). These kinase cascades result in phosphorylation of spinal Glur-1 containing AMPA receptors at the Ser-831 and 845 sites (Fang, Wu, Lin, and Willis, 2002; 2003; Fang, Wu, Zhang, Lin, and Willis, 2003; Wang et al., 2011b; for review see Wang et al., 2010a). Phosphorylation of GluR-1 is associated with enhanced responsiveness to noxious stimulation and central sensitization. Recent reports indicate that NMDA receptor activation results in rapid internalization of Glur2-containing AMPA receptors in various pain models via PKC-induced phosphorylation of Glur2-Ser-880 (Katano et al., 2008; Park et al., 2009; Park et al., 2008). These combined effects lead to decreased affinity of Glur2 for the synaptic anchoring protein glutamate receptor-interacting protein 1 (GRIP-1) (Matsuda, Mikawa, and Hirai, 1999; Park et al., 2009; for review see Wang et al., 2010a). The absence of GluR2-containg AMPAR results in increased synaptic permeability of Ca2+, which leads to a contingent of intracellular cascades contributing to the induction and maintenance of central sensitization. Collectively this results in a shift of AMPA receptor subtypes at the postsynaptic membrane with increased GluR1 representation. The postsynaptic accumulation of GluR1-containing subunits is further enhanced by decreased endocytosis and “diffusional trapping” of these AMPA receptors (Ehlers, Heine, Groc, Lee, and Choquet, 2007; Man, Sekine-Aizawa, and Huganir, 2007).
Increased levels of intracellular Ca2+ directly activate spinal PKC and indirectly lead to activation of PKA through increased calmodulin and cAMP (Kawasaki et al., 2004). Activation of spinal PKA and PKC contributes to ERK activation, which is coupled to phosphorylation of CREB at the serine 133 site (Ji and Rupp, 1997) and expression of c-Fos and cyclooxygenase-2 (COX-2) in pain models (for review see Ji, Gereau, Malcangio, and Strichartz, 2009; Kawasaki et al., 2004). pCREB and c-Fos are known to be temporally associated with the presence of painlike behavior in rodent models (Miletic, Pankratz, and Miletic, 2002; Song et al., 2005). COX-2 is one of the rate-limiting enzymes for prostaglandin E2 (PGE2) production in the dorsal horn; increases in PGE2 synthesis have been observed in response to both inflammatory mediators (Beiche, Brune, Geisslinger, and Goppelt-Struebe, 1998; Yamamoto and Nozaki-Taguchi, 1996) and nerve injury (Durrenberger et al., 2004; Schafers, Marziniak, Sorkin, Yaksh, and Sommer, 2004; Wang et al., 2010b). PGE2 acts to potentiate and disinhibit cation conductance thereby contributing to the maintenance of neuronal states of hyperexcitability (for review see Latremoliere and Woolf, 2009).
PGE2 contributes to the synthesis of the neurotrophic factor BDNF, an important mediator of synaptic plasticity released by first order nociceptive neurons projecting from the periphery and activated spinal microglia in response to injury or noxious stimulation (for review see: Merighi et al., 2008; Scholz and Woolf, 2007; Trang, Beggs, and Salter, 2011). Binding of BDNF to postsynaptic TrkB induces increases in C-fiber activity and activation of PKC- and ERK- mediated signaling cascades (Kerr et al., 1999; Pezet et al., 2002; Yajima et al., 2005). Nerve injury can induce an ERK-mediated shift in BDNF expression from small/medium sized dorsal root ganglion (DRG) neurons to large DRG neurons thereby contributing to central sensitization at this first order synapse in the spinothalamaic tract (Cruz Duarte, St-Jacques, and Ma, 2012). Injury-associated activation of spinal microglia leads to the release of BDNF stores from microglia which disinhibits spinal projection neurons through GABAA and glycine receptors (Coull et al., 2005). More recent work has demonstrated an injury-related upregulation of microglial purigenic receptor P2×4 which leads to increased synthesis and release of microglial BDNF (Trang, Beggs, Wan, and Salter, 2009; Ulmann et al., 2008).
In light of the role it plays in invertebrate and mammalian learning and memory, pain researchers have begun to explore the role that PKMζ may play in central sensitization. The recent findings indicate a potential role for PKMζ in regulation of a spinal engram that may contribute to the induction of pathological central sensitization. Spinal inhibition of PKMζ suppressed nociceptive responses in two inflammatory models (CFA and formalin) while failing to alter levels of increased phosphorylated PKCζ/PKMζ, observed post-injury (Marchand et al., 2011). Inhibition of constitutively active spinal PKMζ suppressed both the initiation and maintenance of nociception in a modified “hyperalgesic priming” model (Asiedu et al., 2011; Reichling and Levine, 2009). This model involves the local injection of interleukin 6 (IL-6) into the hindpaw which results in transient mechanical allodynia (< 3 days) that has been shown to enhance protein translation through its actions on the eIF4E complex (Melemedjian et al., 2010). IL-6 injection leaves the dorsal spinal horn poised to induce a hyperalgesic response to either local (PGE2 intraplantar injection) or central challenges (intrathecal injection of the metabotropic glutamate receptor 1/5 (mGLUR1/5) agonist, dihydroxyphenylglycine (DHPG)) introduced following resolution of IL-6-induced pain. Intrathecal administration of ZIP, the purported PKMζ inhibitor (see below), at multiple timepoints following IL-6-induced mechanical allodynia resolution suppressed the hyperalgesic response observed with either local or central challenges. While PKMζ inactivation is effective at suppressing evoked reflexive responses, it produced no effect on spontaneous pain (King et al., 2012).
One caveat of note is a very recent publication that reported the ability of ZIP to inhibit hippocampally-mediated LTP in mice lacking PKCζ/PKMζ, indicating that this drug likely produces effects via an alternative target (Volk et al., 2013). It is therefore of note that in the aforementioned study, authors intrathecally injected a lentivirus containing a PKCζ construct that is constitutively active which was preferentially transduced in neurons of the dorsal horn substantia gelatinosa (Melemedjian et al., 2010). Administration of the PKCζ virus produced mechanical allodynia relative to animals receiving control virus and enhanced nocifensive responses to intrathecal DHPG challenge 31 days following virus injection (Asiedu et al., 2011). In this hyperalgesic priming model protein synthesis appears to only be involved in the induction of spinal sensitization. Intrathecal injection of the mTOR inhibitor, temsirolimus, and the eIF4F complex inhibitor, 4EGI-1, both independently blocked IL-6-induced allodynia. Additionally, both inhibitors independently blocked PGE2-induced hyperalgesic response when administered immediately following IL-6, but not when administered after resolution of IL-6-induced allodynia.
Researchers have long extolled the similar synaptic machinery underlying hippocampal LTP (Arancio, Kandel, and Hawkins, 1995) and central sensitization (Ji, Kohno, Moore, and Woolf, 2003). However despite the commonalities, there are several very important distinctions between these two forms of synaptic plasticity. While hippocampal LTP is generally considered to be permanent, central sensitization is reversible. As with statements of this nature, there are important caveats to consider. The phenomena of reconsolidation and erasure do suggest that hippocampal LTP, while an enduring form of synaptic plasticity, can, under certain pressures, be reversed. Hippocompal LTP is primarily associated with homosynaptic plasticity (sensitization of only those synapses directly activated); central sensitization, while possessing forms of homosynaptic plasticity (e.g., wind-up phenomenon, long-term potentiation), is defined by the property of heterosynaptic plasticity. Heterosynaptic plasticity is also a hallmark of long-term facilitation in Aplysia (Glanzman et al., 1989). Important overlap in cellular and plasticity mechanisms include AMPA GluR1 receptor trafficking and activation of similar signaling cascades. For example, evidence suggests the importance of the cyclic guanosine monophosphate (cGMP) pathway contributions in spinal LTP (Luo et al., 2012), hippocampal LTP (Arancio et al., 1995; Taqatqeh et al., 2009), and LTH (Lewin and Walters, 1999). The similarities and distinctions between these phenomena may reflect how different experimental stimuli in a number of preparations engage homo- and/or hetero- synaptic plasticity mechanisms and ultimately argue for evolutionarily conserved homologous mechanisms to promote persistent plasticity in the face of aversive and noxious stimulation.
4. Epigenetics
The realization that learning and pain-related LTS rely on persistent cellular and behavioral changes prompted researchers to further investigate potentially self-perpetuating mechanisms involved in controlling gene transcription and subsequent protein synthesis. Epigenetic regulation of gene expression has long been lauded by developmental biologists and cancer researchers for its ability to maintain cellular phenotype across cell divisions. However, only recently has the neuroscience community appreciated the capacity of epigenetic mechanisms to regulate gene expression dynamically in response to neuronal activity. More importantly, evidence accrued within the last ten years further pinpoints epigenetic mechanisms as critical for the gene expression profile necessary to induce and maintain long-lasting neuronal plasticity and behavior (for review see: Haggarty and Tsai, 2011; Zovkic, Guzman-Karlsson, and Sweatt, 2013). Defined broadly, epigenetics mechanisms are a set of processes and modifications that influence gene function without alteration of the primary DNA sequence. Canonical epigenetic mechanisms include histone post-translational modifications (PTMs) and DNA methylation, although recent research has also identified a number of other processes involved in epigenetic regulation including non-coding RNAs, prions, chromosome position effects, and Polycomb repressors (Tollefsbol, 2011). Within this section we will focus exclusively on the role of histone PTMs and DNA methylation in non-associative conditioning (see Figure 3). Given the field’s infancy, the role of epigenetic mechanisms in sensitization at large is limited and as such we will focus our discussion on Aplysia for non-associative learning and mammalian injury models for pain hypersensitivity.
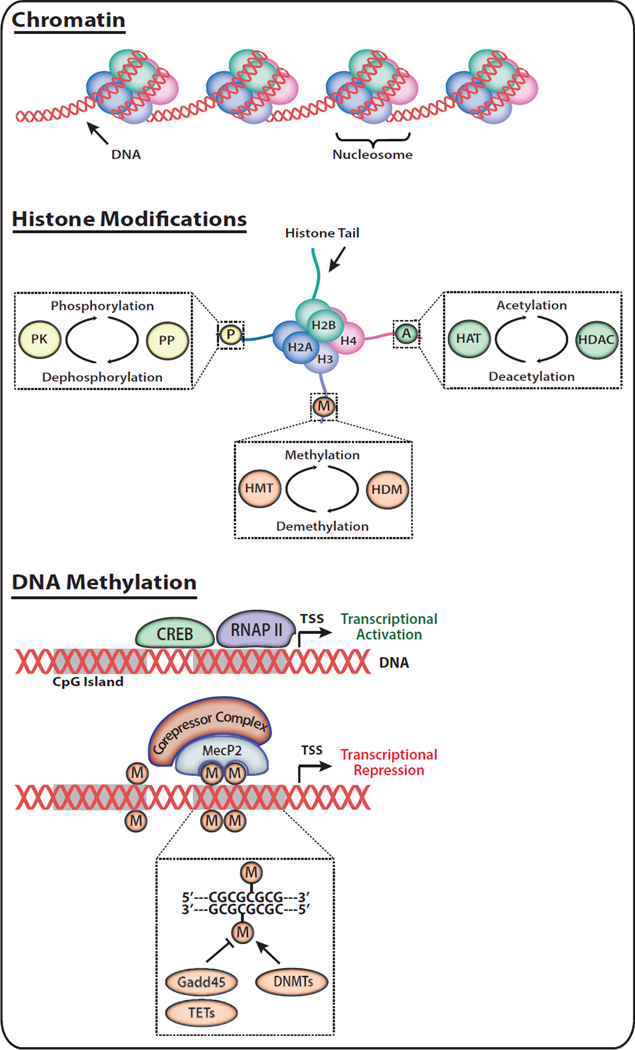
Figure 3. Epigenetic Mechanisms and Modifications.
The DNA-protein complex of chromatin is achieved by packaging DNA into nucleosomes comprised of 147 nucleotide base pairs wrapped around octamers of histone proteins. Histone Modifications, including acetylation, methylation, and phosphorylation dictate chromatin conformation (euchromatin vs. heterochromatin); these modifications contribute to the accessibility of genes for transcription. Enzymatic machinery for histone post-translational modifications include histone acetyltransferases (HAT; acetylate histones), histone deacetylases (HDAC; remove histone acetyl groups), histone methyltransferases (HMT; methylate histones), histone demethylases (HDM; remove histone methyl groups), protein kinases (PK; phosphorylate histones), and protein phosphatases (PP; remove histone phosphate groups). DNA Methylation occurs when DNA methyltransferases (DNMTs) catalyze the addition of a methyl group to the 5’ position of the cytosine pyrimidine ring. In the absence of DNA methylation, transcription factors and RNA polymerase II (RNAP II) bind DNA, resulting in gene expression. Recent evidence suggests the potential for active DNA demethylation through members of the GADD45 and TET families. DNA methylation results in transcriptional repression via recruitment of proteins with methyl-binding domains such as methyl CpG binding protein 2 (MeCP2), that can further recruit corepressor complexes containing HDACs. TSS, transcription start site.
Chromatin remodeling via histone PTMs influence the degree to which the DNA associates with the histones. Chromatin consists of 147 base pairs of DNA wrapped around an octamer of histone proteins that consists of an H3-H4 tetramer as well as two pairs of H2A-H2B dimers. Euchromatin is characterized by an open and relaxed state that encourages transcription whereas the condensed state of heterochromatin impedes gene expression. The N-terminal lysine residues of histones can undergo an array of modifications including, but not limited to, acetylation, methylation, and phosphorylation. Conventionally, acetylation and phosphorylation of histones promote gene transcription whereas histone methylation has been shown to both repress and activate gene transcription (for review see: Jiang et al., 2008; Meaney and Ferguson-Smith, 2010). Whether gene expression is up- or down- regulated depends on the exact amino residue methylated as well as the number of methyl groups being added. Catalytic addition and removal of each type of modification is mediated by specific enzymes. For example, the addition of acetyl groups to histones is catalyzed by histone acetyltransfereases (HATs) whereas the removal of acetyl groups is catalyzed by histone deacetylases (HDACs).
DNA methylation refers to the process by which a methyl group from S-adenosyl-methionine (SAM) is covalently added to the 5′ position on a cytosine pyrimidine ring. DNA methyltransferases (DNMTs) catalyze DNA methylation by preferentially targeting cytosines positioned adjacent to guanine nucleobases (CpG). DNA methylation is associated with repression of gene transcription via steric hindrance of transcription factor binding or through recruitment of proteins with methyl-binding domains (MBDs) that can further engage corepressor complexes (for review see: Day and Sweatt, 2010; Moore, Le, and Fan, 2013). In addition to de novo DNA methylation, recent evidence suggests that active DNA demethylation is equally important for neuronal plasticity and cognition and can occur via several mechanisms involving members of the GADD45 (Leach et al., 2012; Ma, Guo, Ming, and Song, 2009; Sultan, Wang, Tront, Liebermann, and Sweatt, 2012) and TET (Guo, Su, Zhong, Ming, and Song, 2011) families.
4.1. Non-associative Learning and Memory in Aplysia
4.1.1. Histone modifications
The discovery of chromatin regulation in non-associative learning and memory was first reported by Kandel and colleagues using dissociated Aplysia cultures in which sensory neurons with bifurcated axons were cultured with two spatially distinct motor neurons (Guan et al., 2002). This study elegantly demonstrated bidirectional regulation of gene expression and chromatin structure in response to both LTF (induced by 5-HT application) and long-term depression (LTD; induced by application of the endogenous tetrapetide Pre-Met-ARg-Phe-NH2 – also known as FMRFa or FMRFamide). LTD is a cellular correlate of habituation, another form of non-associative conditioning where response decrement is observed following repeated presentations of a given stimulus (for review see Glanzman, 2009). Due to the limited landscape of epigenetic non-associative conditioning literature, inclusion of LTD in our discussion of synaptic facilitation creates a better framework with which to understand changes occurring during 5-HT-mediated LTF.
The study by the Kandel group found that treatment with 5-HT induced C/EBP mRNA expression and was further correlated with CREB-1 and CREB-binding protein (CBP) enrichment at the C/EBP promoter (Guan et al., 2002). As would be expected with the intrinsic HAT activity of CBP, acetyl H4 at lysine 8 and acetyl H3 at lysine 14 were also enriched at the C/EBP promoter. In contrast, treatment with FMRFa failed to produce similar alterations and was instead associated with CREB-2 and HDAC5 enrichment of the C/EBP promoter. Association of CREB-1 and acetyl H4 with the C/EBP promoter was in fact reduced from baseline levels, suggesting that FMRFa-induced LTD is more than just a mirror image of LTF and instead relies on a set of distinct molecular mechanisms.
Even more surprising was the fact that these distinct molecular mechanisms appear to converge in the nucleus and compete at the level of the C/EBP promoter (Guan et al., 2002). When 5-HT and FMRFa were applied to distinct synapses, LTD was expressed as expected in the FMRFa-treated synapse whereas the expression of LTF in the 5-HT-treated synapse was completely blocked. This phenotype was further recapitulated at the genome level in which CREB-2 and HDAC5 occupancy of the C/EBP promoter dominated. Interestingly, the balance between facilitation and depression at the level of the C/EBP promoter was further modifiable utilizing pharmacological tools. Inhibition of deacetylation with the HDAC inhibitor (HDACi), Trichostatin A (TSA), reversed the inhibitory override of 5-HT by FMRFa and produced a low level of facilitation in the FMRFa-treated synapse. In addition, the presence of TSA was sufficient to produce LTF with a single puff of 5-HT.
These data were the first to introduce the notion of an “epigenetic threshold,” which can be defined as a level of epigenetic modifications that must be exceeded to elicit gene expression necessary for learning. With this concept in mind, it appears that the degree of histone acetylation at the C/EBP promoter regulates how readily gene expression and synaptic plasticity are induced in Aplysia.
Subsequent research has revealed other genes whose expression and epigenetic regulation are important for synaptic plasticity of Aplysia sensorimotor synapse. LTF also relies on expression of synapsin, a synaptic vesicle-associated protein involved in the regulation of neutrotransmitter release and short-term synaptic plasticity (Hart et al., 2011). Treatment with 5-HT induced expression of synapsin mRNA and protein expression. These changes were further correlated with increased CREB-1 and acetyl H3 and acetyl H4 association at the CRE site of the synapsin promoter. The increase in histone acetylation was also associated with decreased enrichment of HDAC5 at the same site.
Although Kandel and colleagues showed that LTD induced by the neuropeptide FMRFa was associated with CREB-2-mediated repression of C/EBP expression (Guan et al., 2002), a more recent study revealed that LTD was also associated with CREB-2-mediated expression of the ubiquitin C-terminal hydrolase (Ap-UCH) (Fioravante, Liu, and Byrne, 2008). As its name implies, Ap-UCH is a deubiquinating enzyme that serves a key role in the ubiquitin-proteasome system. The proteasome system has been implicated in producing persistent PKA activity in the early phases of LTF via degradation of the regulatory subunit of PKA (Hegde, Goldberg, and Schwartz, 1993). In contrast with C/EBP epigenetic regulation, the Ap-UCH promoter was enriched with both CREB-2 and hyperacetylation (reflected by increased H3 and H4 acetylation as well as decreased HDAC5 promoter association), suggesting that under certain conditions CREB-2 may act as a transcriptional activator as well as transcriptional repressor.
In addition to histone acetylation, recent papers have also shown a role for Poly-ADP ribosylation of histones in LTF (Cohen-Armon et al., 2004; Hernandez et al., 2009). Poly-ADP ribosylation of histones is mediated via poly-(ADP-ribose) polymerases (PARP) such as PARP1 and PARP2 (Messner et al., 2010; Quenet, El Ramy, Schreiber, and Dantzer, 2009). Serotonin application to isolated Aplysia ganglia in vitro as well as behavioral GSWR sensitization in the intact animal activated PARP1, which itself is poly-ADP-ribosylated (Cohen-Armon et al., 2004). This increase in activated PARP1 was also correlated with an increase in linker histone H1 poly-ADP-ribosylation. Furthermore, inhibition of PARP1 expression and activity blocked 5-HT induced LTF. These findings, in addition to the mechanisms reviewed above, highlight that histone modifications and the responsible chromatin enzymatic machinery are critical for enabling the transcriptional program necessary for LTF.
4.1.2. DNA methylation
In contrast to chromatin regulation, research on the role of DNA methylation in synaptic plasticity of Aplysia sensorimotor synapse is currently limited. Only recently was the presence of DNMT1 discovered in Aplysia (Moroz and Kohn, 2010) which is peculiar considering that both Drosophila and C. elegans lack DNMTs in general (Suzuki and Bird, 2008) and mammals have three major DNMTs (Cheng X, 2010). A very recent study by Kandel and colleagues revealed that bath application of the DMNT inhibitor RG108, an inhibitor that blocks the enzyme catalytic site, fully abolished 24 and 48 hr LTF induced by 5-HT application, suggesting that DNA methylation is necessary for 5-HT-dependent LTF (Rajasethupathy et al., 2012). 5-HT application was also correlated with increased methylation in the proximal CpG island of the CREB2 promoter, as well as decreased CREB2 mRNA and protein expression. As expected, these effects were also eliminated when neurons were concomitantly treated with RG108.
Arguably one of the most interesting aspects of this recent publication (Rajasethupathy et al., 2012) was the linkage of Piwi-interacting RNAs (Pi-RNAs) to DNA methylation of CREB2 and LTF. Although it is known that in mice the Piwi/piRNA complexes act to silence transcription of transposable elements via DNA methylation (Aravin et al., 2008; Carmell et al., 2007; Kuramochi-Miyagawa et al., 2008), this study demonstrated that piRNAs are surprisingly expressed in the Aplysia CNS and can also regulate genes involved in synaptic plasticity (Rajasethupathy et al., 2012). Knockdown of Piwi resulted in both significantly impaired LTF, whereas overexpression of Piwi potentiated LTF. Piwi knockdown produced demethylation of the CREB2 proximal promoter and subsequent upregulation of CREB2 mRNA. Additionally, knockdown of a candidate piRNA with shared complementarity to the CREB2 promoter, aca-piR-F, resulted in increased CREB2 mRNA and protein expression. It is of note that while this study produced many significant findings; it did not demonstrate that aca-piR-F knockdown leads to demethylation of the CREB2 CpG island as proposed by their model.
Together, the studies in LTF and LTD implicate epigenetic mechanisms in the gene expression profile necessary for synaptic plasticity in Aplysia. More importantly, these studies further highlight the importance of concerted modulation of both positive and negative regulators of memory formation. In addition to CREB-1-mediated activation of gene expression, LTF in Aplysia relies on the removal of inhibitory constraints such as CREB-2 and the regulatory subunit of PKA. The data suggests that chromatin regulation and DNA methylation may be involved in modulating the levels of these different positive and negative regulators (e.g. DNA methylation of the CREB2 promoter; hyperacetylation of the Ap-UCH and synapsin promoter; hyper-/hypoacetlaytion of the C/EBP promoter). Furthermore, a similar balance between suppressive and activating modifications may be required at the epigenetic level as suggested by the ability of histone acetylation to regulate the threshold for learning and memory (Guan et al., 2002).
4.2. Pain Injury Models
Like the synaptic plasticity that underlies non-associative learning and memory, pain processing has been correlated with changes in gene expression (LaCroix-Fralish, Austin, Zheng, Levitin, and Mogil, 2011; Lacroix-Fralish, Tawfik, Tanga, Spratt, and DeLeo, 2006). These changes allow for adaptation in multiple sites of nociceptive pathways including the DRG, spinal cord, and supra-spinal sites. Recent studies suggest that epigenetic mechanisms may also be engaged in the regulation of gene expression necessary for the induction and maintenance of chronic pain states. As previously mentioned, we will emphasize studies focusing on epigenetic regulation at the level of the spinal cord; however we will also briefly discuss studies implicating epigenetic regulation of pain in murine DRG (Chiechio et al., 2009; Qi et al., 2013; Uchida, Ma, and Ueda, 2010a; Uchida, Sasaki, Ma, and Ueda, 2010b) and brain stem (Zhang, Cai, Zou, Bie, and Pan, 2011). A single study has reported epigenetic modifications associated with pain in humans. Within this study, increases in methylation of the extracellular matrix protein SPARC (Secreted Protein, Acidic, Rich in Cysteine) promoter were observed in patients experiencing chronic low back pain associated with disc degeneration (Tajerian et al., 2011). Early evidence for a role of epigenetic mechanisms in pain processing consists of studies correlating epigenetic modifications with pain-related changes in gene expression, as well as causal evidence obtained by genetic and pharmacological targeting of epigenetic machinery enzymes (for review see: Denk and McMahon, 2012; Doehring, Geisslinger, and Lotsch, 2011; Geranton, 2012).
4.2.1. Correlating gene expression with epigenetic mechanisms
Géranton and colleagues were the first to implicate epigenetic mechanisms in pain processing in vivo using an inflammatory CFA pain model (Geranton, Morenilla-Palao, and Hunt, 2007). CFA injection was associated with methyl-CpG-binding protein 2 (MeCP2) phosphorylation in the superficial dorsal horn (including lamina I projection neurons) as well as downregulation of Sin3a mRNA at early time points following induction. As its name implies, MeCP2 is a methyl-CpG binding protein that inhibits gene transcription by binding to methylated DNA of specific genes (Nan, Cross, and Bird, 1998a), although it can also activate transcription via its interaction with CREB (Chahrour et al., 2008). When bound to DNA, MeCP2 can not only block the binding of transcription factors, but also actively recruit corepressor complexes to remodel chromatin and inhibit gene transcription (Fuks et al., 2003; Jones et al., 1998; Nan et al., 1998b). SIN3A comprises part of the silencing complex with MeCP2 (Cheng et al., 2011; Martinowich et al., 2003). When phosphorylated, MeCP2 can be dissociated from the promoter regions of genes, thereby facilitating gene expression (Chen et al., 2003). Hence as expected, the increase in MeCP2 phosphorylation and decrease in Sin3a mRNA was correlated with the upregulation of transcripts that had previously been repressed by MeCP2, including serum- and glucocorticoid-inducible kinase (Sgk1), FK 506 binding protein 5 (Fkbp5), sulfotransferase family 1A, phenol-preferring, member 1 (Sult1a1). Additionally, antisense knock-down of SGK1 further delayed the induction of inflammatory hyperalgesia, providing direct evidence for the role of MeCP2-regulated gene expression in controlling pain behavior. Subsequent studies have indicated MeCP2 phosphorylation of superficial dorsal horn neurons following CFA intraplantar injection is dependent on descending modulatory serotonergic pathways. Pharmacological depletion of 5-HT in this model prevents both MeCP2 phosphorylation as well as the mechanical hypersensitivity observed following CFA injection (Geranton, Fratto, Tochiki, and Hunt, 2008).
MeCP2 phosphorylation appears to be involved in induction of CFA-induced mechanical hypersensitivity, whereas maintenance of this behavior is associated with regulation of Mecp2 mRNA and protein levels. CFA injection in the rat ankle joint resulted in increased Mecp2 mRNA expression in the superficial dorsal horn when measured 7 days post-injection (Tochiki, Cunningham, Hunt, and Geranton, 2012). Interestingly, there were no observed mRNA changes in the MeCP2 target genes (Sgk1, Fkbp5, and Sult1a1) during the maintenance phase, whereas expression of these targets was altered during the induction phase. Additionally, global expression of DNMTs as well as certain HDACs (HDAC1 and HDAC5 in particular) was also increased 7 days post-CFA injection (Tochiki et al., 2012), which correlates well with microarray data that revealed predominate downregulation of gene expression at this time point (Geranton et al., 2007). Additional data supporting Mecp2 mRNA levels in pain processing comes from studies showing altered pain sensitivity in patients with Rett Syndrome, a neurodevelopmental disorder primarily caused by mutations in the Mecp2 locus, as well as mice with a hypomorphic allele of Mecp2 (Downs et al., 2010; Samaco et al., 2008). Together these data suggest that in the dorsal spinal cord the maintenance of inflammatory pain is reliant on chromatin compaction and consequently, an overall decrease in gene expression.
However, in addition to changes observed in the dorsal spinal cord, several groups have also measured changes in epigenetic modifying enzymes, epigenetic modifications, and gene expression in the DRG and brain stem in animal models of inflammatory pain. CFA-induced painful inflammation was associated with demethylation of the cystathionine-β-synthase (Cbs) gene as well as elevated levels of Cbs mRNA and protein in the DRG (Qi et al., 2013). CBS synthesizes hydrogen sulfide (H2S), an endogenous gas molecule whose activity was also shown to be necessary and sufficient to elicit mechanical hyperalgesia and enhanced excitability of DRG neurons. Furthermore, the fact that peripheral inflammation did not alter DNMT expression, but instead upregulated methyl-binding domain protein 4 (MBD4) and GADD45a, suggests that the observed demethylation of the Cbs promoter may have occurred due to active and not passive demethylation.
In contrast to the epigenetic activation of Cbs in the DRG, CFA intraplantar injection epigenetically suppressed Gad2 (encoding glutamic acid decarboxylase 65 (GAD65)) transcription in the brain stem nucleus raphe magnus (NRM) (Zhang et al., 2011). Persistent pain (lasting for 3 days), but not acute pain (lasting for 4 hours), involved decreased histone H3 acetylation and concomitant HDAC1, HDAC2, and HDAC4 enrichment at the Gad2 promoter. This gene-specific decrease in histone acetylation occurred in a backdrop of global hyperacetylation of histone H3 and H4 suggesting that the degree of facilitating or repressive epigenetic modifications is specific to both the genes and brain regions being investigated. The decrease in GAD65 mRNA and protein was further shown to directly contribute to impaired GABA synaptic inhibition as well as the development of persistent inflammatory pain (Zhang et al., 2011).
Investigations of nerve injury pain models such as chronic constriction injury (CCI), partial sciatic nerve ligation (PSNL/Seltzer model), spinal nerve ligation (SNL/Chung Model), and spared never injury (SNI) reveal a more varied epigenetic regulatory landscape involved in the maintenance of neuropathic pain. Similar to what was observed with inflammatory pain models, neuropathic pain induced by CCI increased Mecp2 mRNA and nuclear MeCP2 protein localization in the lumbar spinal cord 14 days post-CCI (Wang et al., 2011a). Increased levels of global DNA methylation accompanied these changes, suggesting that repression of gene transcription in the dorsal spinal cord might also underlie chronic pain induced by CCI (Wang et al., 2011a). Such an interpretation would appear to conflict with findings that the histone acetyltransferase E1A binding protein p300 (p300) and COX-2 are upregulated in the lumbar spinal cord 14 days following CCI and that the degree of p300 binding to the C2 promoter regulates the level of Cox2 mRNA expression (Zhu et al., 2012). COX-2 is a well studied proinflammatory mediator whose expression is associated with the induction and maintenance of neuropathic pain through its effects on PGE2 synthesis (for review see Latremoliere and Woolf, 2009). A possible resolution between the apparent discrepancies would be that the maintenance of chronic pain states involves the concerted epigenetic regulation of both pro- and anti-nociceptive genes, with concurrent upregulation of pro-nociceptive genes and silencing of antinociceptive genes. In contrast to CCI, neuropathic pain induced by SNI results in downregulation of MeCP2, DNMTs, and a subset of HDACs (HDAC2 and HDAC5 in particular) in the superficial dorsal horn 7 days post-injury (Tochiki et al., 2012). Again, there were no observed mRNA changes in the MeCP2 target genes (Sgk1, Fkbp5, and Sult1a1). Presumably, a decrease in global DNA methylation following SNI would be anticipated but this has yet to be tested. The differing result between CCI and SNI (especially in regards to the directionality of MeCP2 expression and the specificity of the HDAC isoforms regulated) highlight the mechanistic differences underlying distinct pain models (for review see Sorkin and Yaksh, 2009). Differing epigenetic modulation most likely reflects differential regulation in response to specific signaling cascades set off by initiating stimuli.
As with inflammatory pain models, nerve injury pain models have also been shown to elicit epigenetic changes in the DRG and NRM. Similar to what was observed in the dorsal spinal cord, neuropathic pain induced by SNI also resulted in downregulation of MeCP2 in DRG neurons 7 days following nerve injury (Tochiki et al., 2012). In the case for SNL, the maintenance (lasting for 21 days), but not induction (lasting for 1 day) phase, involved decreased histone H3 acetylation at the Gad2 promoter with a concomitant decrease in GAD65 mRNA and protein levels in the NRM (Zhang et al., 2011). In contrast to SNI and SNL, a series of studies (Uchida et al., 2010a; Uchida et al., 2010b) revealed that PSNL results in the upregulation of neuron-restrictive silence factor (NRSF, also known as REST) mRNA and protein in the DRG for several weeks following surgery. NRSF is a transcription factor known to silence genes containing neuron-restrictive silencer element (NRSE, also known as RE1). NSRF represses gene transcription by actively recruiting corepressor complexes that can include MeCP2, Co-REST, Sin3a, HDACs, and histone methyltransferases and histone demethylases (Ballas and Mandel, 2005; Buckley, Johnson, Zuccato, Bithell, and Cattaneo, 2010). This sustained elevation in NSRF expression was also correlated with increased acetyl H4 enrichment at the NSRF promoter II. Interestingly, enhanced binding of NSRF at NRSEs was further shown to be responsible for the chronic downregulation of the sodium channel Nav1.8, the μ-opiod receptor, and the potassium channel Kv4.3, all of which are genes involved in nociceptive processing. Unlike the NSRF promoter II, the NRSEs of these genes were instead characterized by hypoacetylation of histone H3 and H4 (Uchida et al., 2010a; Uchida et al., 2010b).
4.2.2. Manipulation of HDACs and DNMTs
In addition to correlating epigenetic modifications with pain-related changes in gene expression, several studies have also provided causal evidence for epigenetic regulation of pain states via pharmacological and genetic targeting of HDACs and DNMTs. It should be noted that approximately half of the outlined studies targeted their manipulation of HDACs or DNMTs to the nervous system via intrathecal or brain-specific (i.e., NRM) infusions, whereas the other half employed more systemic approaches with either subcutaneous or intraperitoneal injections. Even with site-specific intrathecal administration, the observed effects that these inhibitors have on pain states could be attributed to changes in the central (ie. spinal or supra-spinal) and/or peripheral (i.e., DRG) nervous system. Future studies with spatially targeted manipulations of these enzymes will undoubtedly reveal the mechanism of action by which these drugs mitigate pain.
Most of the studies performed have focused on the use of HDAC inhibitors as therapeutic agents in the treatment of inflammatory pain. One study found that a 5-day subcutaneous treatment with MS-275 (inhibitor of Class I HDACs) or suberoylanilide hydroxamic acid (SAHA; inhibitor of Class I and II HDACs) prior to formalin administration produced analgesia in mice during the second phase of the formalin test. This effect was further shown to rely on upregulation of metabotropic glutamate 2 (mGLu2) receptors in both the DRG and the spinal cord via increased acetylation of p65/RelA on lysine 310, a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) family member (Chiechio et al., 2009). Whether or not HDACi treatment also modulated histone acetylation, in addition to augmenting NF-κB transcriptional activity, was not investigated in this particular pain model and hence requires further study.
Similarly, another study found that intrathecal administration of inhibitors that target class I and II HDACs (SAHA, TSA, LAQ824) or class IIa alone (valproic acid, 4-PB) significantly attenuated CFA-induced thermal hyperalgesia (Bai, Wei, Zou, Ren, and Dubner, 2010). HDACi infusion prior to CFA delayed the development of CFA-induced thermal hyperalgesia, whereas infusion post-CFA attenuated established thermal hyperalgesia. Further investigation revealed that SAHA treatment increased acetylation of H3 lysine 9 and 18 (H3K9/18). Interestingly, when the class I HDACi MS-275 was tested, it failed to alter CFA-induced hyperalgesia, despite increased acetylation at those residues. These results suggest that the antinociceptive properties of HDAC inhibition in the CFA model might instead be mediated by posttranslational histone modifications other than H3K9/18 or by the targeting of specific genes by particular HDACs. It is also possible that the antinociceptive properties of Class II HDAC inhibition are mediated by increasing acetylation of non-histone proteins. It should also be noted that whether or not these effects are sustained is unresolved, this is especially important given that the attenuation of thermal hyperalgesia with pre-injected HDAC inhibitors is shortlived. It is possible that a chronic HDACi treatment would be necessary to completely block induction and maintenance of inflammatory pain.
A role for HDACs in pain processing is also exemplified by the fact that valproic acid, a class I and class IIa HDACi, has analgesic properties in animal models of neuropathic pain (administered via intraperitoneal injections) (Winkler et al., 2005), as well as patients with type II diabetic neuropathy (Agrawal, Goswami, Jain, and Kochar, 2009). However, unlike other HDAC inhibitors, valproic acid is also known to inhibit sodium channels and therefore its effects on nociception may be through direct modulation of GABAergic signaling, excitatory transmission, and monoamine quantity (Johannessen and Johannessen, 2003). Potential nonspecific effects of HDAC inhibitors such as these argue for targeted genetic inhibition of specific HDAC isoforms to more accurately evaluate HDAC function in pain processing. One study employed such an approach using mutant mice with a loss of the catalytic domain of HDAC4, a class IIa HDAC (Rajan et al., 2009). These mice exhibited reduced thermal nociception but surprisingly did not respond differently to the formalin test when compared to their wild-type littermates, suggesting that HDAC4 may specifically regulate thermal nociceptors as opposed to chemical nociceptors. Future studies will undoubtedly need to distinguish how specific HDAC isoforms may be differentially regulated in persistent pain models.
Currently, there is only one study from Zhang and colleagues that successfully attenuates sensitized pain behavior via targeted infusion of HDAC inhibitors into a specific brain region (Zhang et al., 2011). In their study, repeated administration of TSA or SAHA (once a day for 4 days) into the NRM attenuated CFA-induced thermal hyperalgesia, similar to observations following systemic HDACi administration. TSA and SAHA treatment also restored the CFA-induced decrease in H3 acetylation at the Gad2 promoter as well as increased global H3 acetylation, although these observations were made following a systemic administration of TSA and SAHA, and not a targeted one. Finally, systemic TSA administration was also shown to restore both GAD65 mRNA and protein abundance, as well as GABA neurotransmission.
In contrast to the work performed with HDAC inhibitors, there is only one published study that uses pharmacological inhibition of DNMTs in vivo in the context of pain states. Wang and colleagues demonstrated attenuation of CCI-induced mechanical allodynia and thermal hyperalgesia with chronic intrathecal infusion of the DNMT inhibitor (DNMTi) 5-azacytidine (5-aza), a cytidine analog (Wang et al., 2011a). Furthermore, 5-aza was shown to reduce the upregulation of global DNA methylation and Mecp2 mRNA and protein expression that typically occurs 14 days post-CCI. Although this work highlights the exciting therapeutic value of DNMT inhibition, much work remains to mechanistically understand how DNA methylation (and active demethylation) is involved in regulating pain states.
Future studies are needed to determine if DNA methylation has a role in other pain models through the use of both genetic manipulations that target specific DNMT isoforms as well as different pharmacological DNMT inhibitors such as zebularine, another cytidine analog, and RG108. The important question remains whether or not inhibition of DNA methylation or histone deacetylation can also reverse the neuronal hyperexcitability observed in pain states. The work by Zhang and colleagues (2011) suggest that this may be the case given that the ability of HDAC inhibitors to normalize disrupted GABA synaptic inhibition in the NRM. The restoration of GABA neurotransmission presumably dampens the action of descending pain-facilitatory pathways which then results in the alleviation of persistent pain sensitization. However, whether or not modulation of functional plasticity by HDAC and DNMT inhibitors occurs at other sites like the dorsal spinal cord and the DRG remain to be explored.
Taken together, the findings using Aplysia and mammalian injury models implicate epigenetic regulation of gene expression as critical for the experience-driven behavioral change that occurs during sensitization. It should be noted though that amongst the many similarities driving the observed behavioral change, there are potential stark differences in fundamental mechanisms. Of special note is the fact that DNMT and HDAC inhibitors appear to differentially modulate pain hypersensitivity and non-associative learning. In pain hypersentivity, DNMT and HDAC inhibitors appear to work synergistically with both inhibitors displaying analgesic properties. By contrast, in non-associative learning (as well as associative learning; for review see Zovkic et al., 2013) HDAC inhibition facilitates plasticity and learning whereas DNMT inhibition plays a more repressive role. Mechanistic understanding of how these inhibitors act differentially will undoubtedly require a more holistic understanding of the kind of genes, cells, and circuits that are particularly affected by non-associative learning and pain hypersensitivity.
5. Conclusions and Future Directions
The popular author, Chuck Palahniuk wrote in his novel Diary, “It’s so hard to forget pain, but it's even harder to remember sweetness. We have no scar to show for happiness (Palahniuk, 2003).” While not all aversive stimulation or injury will leave a scar, here we demonstrate that each makes its own unique mark. We have reviewed behavioral evidence in non-associative conditioning paradigms spanning arthropods to mammals, which highlight sensitization in response to noxious stimulation or injury. Furthermore, we have examined the functional, cellular, and synaptic modifications that accompany this sensitization. In our final section, we explored epigenetic modifications in the sensitization framework of Aplysia LTS/LTF and mammalian injury models of pain.
Collectively our review argues for homologous functional mechanisms responsible for behavioral sensitization phenotypes across phyla. Studies reviewed demonstrate similar behavioral plasticity in response to sensitizing stimuli across phyla. Aversive stimuli and injury both provide strong selection pressures that enhance synaptic output by inducing functional synaptic plasticity, disinhibition, and enhanced output. These functional mechanisms could be evolutionarily conserved across species or could have developed in parallel (Walters, 2009). Arguably primitive cell signaling mechanisms spanning the plasma membrane to the nucleus are evolutionarily conserved in the animal kingdom. The presence of epigenetic mechanisms in both mammalian and invertebrate models of sensitization highlights a fundamental framework that regulates gene expression necessary for plasticity.
Non-associative learning and in particular the sensitization paradigm framework, offers the unique opportunity to relate two diverse fields that share homologous mechanisms to contend with aversive and noxious stimulation. The shared homology between learning and pain argues for an intimate dialogue between the two fields, where novel findings in one are explored in the other. In addition to cross-talk between fields that promotes investigation of shared novel cellular and molecular mechanisms, findings in both fields are poised to influence ongoing research in mammalian models of learning and memory. Within our review we note the lack of research on non-associative mammalian conditioning in the absence of pain states. Models of mammalian learning and memory are dominated by paradigms that are associative or hybrid (associative and non-associative) in nature. The advent of a mammalian model of PTSD (Siegmund and Wotjak, 2007b) that differentiates between associative and non-associative processes opens up new possibilities for exploring the cellular and molecular mechanisms underlying mammalian sensitization in non-pain states. This line of research is particularly relevant for all anxiety-related disorders (e.g. phobias, general anxiety disorder, panic disorder), which are characterized by hyperarousal and sensitized states. Our review outlines significant progress in the study of invertebrate sensitization and mammalian pain models, progress that has the potential to inform future investigation of non-pain related mammalian sensitization. A more thorough mechanistic understanding of hyperarousal and sensitization could yield novel therapeutic strategies targeting non-associative components of these disorders, an important advance given the limited therapeutic efficacy of currently available pharmacologic and behavioral interventions.
Review behavioral sensitization in response to noxious stimulation or injury
Review functional, synaptic, and cellular mechanisms of sensitization
Review epigenetic mechanisms in learning- and pain- related sensitization
Acknowledgements
This work was supported by NIMH 57014, NIMH 091122, NIA 031722, NR012686, Ellison Senior Scholar Foundation, Evelyn F. McKnight Brain Research Foundation, and Civitan International (JDS). MCGK is supported by NINDS 3T32NS061788.
Abbreviations
- BDNF
brain derived neurotrophic factor
- CAMKII
calmodulin-dependent protein kinase II
- Ca2+
calcium
- cAMP
cyclic adenosine monophosphate
- CBS
cystathionine-β-synthase
- CCI
chronic constriction injury
- CFA
complete Freund’s adjuvant
- cGMP
cyclic guanosine monophosphate
- COX-2
cyclooxygenase-2
- CPEB
cytoplasmic polyadenylation element binding protein
- CPB
CREB-binding protein
- C/EBP
CCAAT enhancer binding protein
- CRE
cAMP response element
- CREB
cAMP response element binding protein
- DHPG
dihydroxyphenylglycine
- DNMT
DNA methyltransferase
- DNMTi
DNA methyltransferase inhibitor
- DRG
dorsal root ganglion
- eEF2
eukaryotic elongation factor 2
- EIF4E
eukaryotic translation initiation factor 4E
- ERK
extracellular receptor kinase
- FCS
fast conducting system
- FKBP5
FK 506 binding protein
- FMRFa
Pre-Met-ARg-Phe-NH2
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- IL-6
interleukin 6
- IP3
inositol 1,4,5-trisphosphate
- ITF
intermediate-term facilitation
- LE
Left E cluster
- LG
lateral giant neuron
- LTD
long-term depression
- LTF
long-term facilitation
- LTH
long-term hyperexcitability
- LTP
long-term potentiation
- LTS
long-term sensitization
- MAPK
mitogen activated protein kinase
- MeCP2
methyl-CpG-binding protein 2
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- NRM
nucleus raphe magnus
- NRSE
neuron-restrictive silencer element
- NRSF
neuron-restrictive silence factor
- PARP
poly-(ADP-ribose) polymerase
- PSNL
partial sciatic nerve ligation
- Pi-RNAs
Piwi-interacting RNAs
- PGE2
prostaglandin E2
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PKG
protein kinase G
- PKM
protein kinase M
- PTM
post-translational modifications
- PTSD
post-traumatic stress disorder
- p300
histone acetyltransferase E1A binding protein p300
- SAM
S-adenosyl-methionine
- SAHA
suberoylanilide hydroxamic acid
- SGK1
serum- and glucocorticoid-inducible kinase
- SGWR
siphon-gill withdrawal reflex
- SNI
spared nerve injury
- SNL
spinal nerve ligation
- STF
short-term facilitation
- SULT1A1
sulfotransferase family 1A, phenol-preferring, member 1
- S6K
S6 kinase
- TOR
target of rapamycin
- TrkB
tyrosine kinase receptor B
- TSA
trichostatin A
- UCH
ubiquitin C-terminal hydrolase
- VC
ventrocaudal clusters
- ZIP
zeta inhibitory peptide
- 4EBP
EIF4E binding protein
- 5-aza
5-azacytidine
- 5-HT
serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott FV, Franklin KB, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. doi: 10.1016/0304-3959(94)00095-V. [DOI] [PubMed] [Google Scholar]
- Agrawal RP, Goswami J, Jain S, Kochar DK. Management of diabetic neuropathy by sodium valproate and glyceryl trinitrate spray: a prospective double-blind randomized placebo-controlled study. Diabetes Res Clin Pract. 2009;83:371–378. doi: 10.1016/j.diabres.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Huang W, Burgess G, Machin I, Rice AS. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain. 2011 doi: 10.1016/j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Antonsen BL, Edwards DH. Differential dye coupling reveals lateral giant escape circuit in crayfish. J Comp Neurol. 2003;466:1–13. doi: 10.1002/cne.10802. [DOI] [PubMed] [Google Scholar]
- Araki M, Nagayama T. IP3-mediated octopamine-induced synaptic enhancement of crayfish LG neurons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198:607–615. doi: 10.1007/s00359-012-0733-2. [DOI] [PubMed] [Google Scholar]
- Araki M, Nagayama T, Sprayberry J. Cyclic AMP mediates serotonin-induced synaptic enhancement of lateral giant interneuron of the crayfish. J Neurophysiol. 2005;94:2644–2652. doi: 10.1152/jn.00502.2005. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3',5'-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, Bestor T, Hannon GJ. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell. 2008;31:785–799. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante CO, Wallace VC, Dickenson AH. Formalin-induced behavioural hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol Pain. 2009;5:27. doi: 10.1186/1744-8069-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- Bai G, Wei D, Zou S, Ren K, Dubner R. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain. 2010;6:51. doi: 10.1186/1744-8069-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci U S A. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15:500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Bedi SS, Salim A, Chen S, Glanzman DL. Long-term effects of axotomy on excitability and growth of isolated Aplysia sensory neurons in cell culture: potential role of cAMP. J Neurophysiol. 1998;79:1371–1383. doi: 10.1152/jn.1998.79.3.1371. [DOI] [PubMed] [Google Scholar]
- Beiche F, Brune K, Geisslinger G, Goppelt-Struebe M. Expression of cyclooxygenase isoforms in the rat spinal cord and their regulation during adjuvant-induced arthritis. Inflamm Res. 1998;47:482–487. doi: 10.1007/s000110050362. [DOI] [PubMed] [Google Scholar]
- Belardetti F, Biondi C, Colombaioni L, Brunelli M, Trevisani A. Role of serotonin and cyclic AMP on facilitation of the fast conducting system activity in the leech Hirudo medicinalis. Brain Res. 1982;246:89–103. doi: 10.1016/0006-8993(82)90145-7. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Billy AJ, Walters ET. Long-term expansion and sensitization of mechanosensory receptive fields in Aplysia support an activity-dependent model of whole-cell sensory plasticity. J Neurosci. 1989;9:1254–1262. doi: 10.1523/JNEUROSCI.09-04-01254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougie JK, Cai D, Hastings M, Farah CA, Chen S, Fan X, McCamphill PK, Glanzman DL, Sossin WS. Serotonin-induced cleavage of the atypical protein kinase C Apl III in Aplysia. J Neurosci. 2012;32:14630–14640. doi: 10.1523/JNEUROSCI.3026-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougie JK, Lim T, Farah CA, Manjunath V, Nagakura I, Ferraro GB, Sossin WS. The atypical protein kinase C in Aplysia can form a protein kinase M by cleavage. J Neurochem. 2009;109:1129–1143. doi: 10.1111/j.1471-4159.2009.06045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling D, Nicholls J, Parnas I. Destruction of a single cell in the central nervous system of the leech as a means of analysing its connexions and functional role. J Physiol. 1978;282:169–180. doi: 10.1113/jphysiol.1978.sp012455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodfuehrer PD, Cohen AH. Initiation of swimming activity in the medicinal leech by glutamate, quisqualate and kainate. J Exp Biol. 1990;154:567–572. doi: 10.1242/jeb.154.1.567. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Thorogood MS. Identified neurons and leech swimming behavior. Prog Neurobiol. 2001;63:371–381. doi: 10.1016/s0301-0082(00)00048-4. [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Johnson R, Zuccato C, Bithell A, Cattaneo E. The role of REST in transcriptional and epigenetic dysregulation in Huntington's disease. Neurobiol Dis. 2010;39:28–39. doi: 10.1016/j.nbd.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL, Muller KJ. Progressive recovery of learning during regeneration of a single synapse in the medicinal leech. J Comp Neurol. 2003;457:67–74. doi: 10.1002/cne.10530. [DOI] [PubMed] [Google Scholar]
- Cai D, Pearce K, Chen S, Glanzman DL. Protein kinase M maintains long-term sensitization and long-term facilitation in aplysia. J Neurosci. 2011;31:6421–6431. doi: 10.1523/JNEUROSCI.4744-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmell MA, Girard A, van de Kant HJ, Bourc'his D, Bestor TH, de Rooij DG, Hannon GJ. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell. 2007;12:503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Carroll M, Warren O, Fan X, Sossin WS. 5-HT stimulates eEF2 dephosphorylation in a rapamycin-sensitive manner in Aplysia neurites. J Neurochem. 2004;90:1464–1476. doi: 10.1111/j.1471-4159.2004.02634.x. [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Lin YP, Chen YL, Lee YC, Tai CC, Wang YT, Chen YJ, Kao CF, Yu J. Interplay between SIN3A and STAT3 mediates chromatin conformational changes and GFAP expression during cellular differentiation. PLoS One. 2011;6:e22018. doi: 10.1371/journal.pone.0022018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X HH, Horton JR, Zhang X. Mechanisms of DNA methylation, methyl-CpG recognition,and demethylation in mammals. In: Tollefsbol T, editor. Handbook of epigenetics. Oxford, UK: Academic Press; 2010. pp. 9–24. [Google Scholar]
- Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RWt, Copani A, Nicoletti F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol. 2009;75:1014–1020. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- Clatworthy AL, Castro GA, Budelmann BU, Walters ET. Induction of a cellular defense reaction is accompanied by an increase in sensory neuron excitability in Aplysia. J Neurosci. 1994;14:3263–3270. doi: 10.1523/JNEUROSCI.14-05-03263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Armon M, Visochek L, Katzoff A, Levitan D, Susswein AJ, Klein R, Valbrun M, Schwartz JH. Long-term memory requires polyADP-ribosylation. Science. 2004;304:1820–1822. doi: 10.1126/science.1096775. [DOI] [PubMed] [Google Scholar]
- Conrad P, Wu F, Schacher S. Changes in functional glutamate receptors on a postsynaptic neuron accompany formation and maturation of an identified synapse. J Neurobiol. 1999;39:237–248. [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Crook RJ, Lewis T, Hanlon RT, Walters ET. Peripheral injury induces long-term sensitization of defensive responses to visual and tactile stimuli in the squid Loligo pealeii, Lesueur 1821. J Exp Biol. 2011;214:3173–3185. doi: 10.1242/jeb.058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Duarte P, St-Jacques B, Ma W. Prostaglandin E2 contributes to the synthesis of brain-derived neurotrophic factor in primary sensory neuron in ganglion explant cultures and in a neuropathic pain model. Exp Neurol. 2012;234:466–481. doi: 10.1016/j.expneurol.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Dale N, Kandel ER. L-glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1993;90:7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;13:1319–1323. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Denk F, McMahon SB. Chronic pain: emerging evidence for the involvement of epigenetics. Neuron. 2012;73:435–444. doi: 10.1016/j.neuron.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehring A, Geisslinger G, Lotsch J. Epigenetics in pain and analgesia: an imminent research field. Eur J Pain. 2011;15:11–16. doi: 10.1016/j.ejpain.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain. 1990;40:93–104. doi: 10.1016/0304-3959(90)91056-O. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs J, Geranton SM, Bebbington A, Jacoby P, Bahi-Buisson N, Ravine D, Leonard H. Linking MECP2 and pain sensitivity: the example of Rett syndrome. Am J Med Genet A. 2010;152A:1197–1205. doi: 10.1002/ajmg.a.33314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Dulin MF, Steffensen I, Morris CE, Walters ET. Recovery of function, peripheral sensitization and sensory neurone activation by novel pathways following axonal injury in Aplysia californica. J Exp Biol. 1995;198:2055–2066. doi: 10.1242/jeb.198.10.2055. [DOI] [PubMed] [Google Scholar]
- Durrenberger PF, Facer P, Gray RA, Chessell IP, Naylor A, Bountra C, Banati RB, Birch R, Anand P. Cyclooxygenase-2 (Cox-2) in injured human nerve and a rat model of nerve injury. J Peripher Nerv Syst. 2004;9:15–25. doi: 10.1111/j.1085-9489.2004.09104.x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich JS, Boulis NM, Karrer T, Sahley CL. Differential effects of serotonin depletion on sensitization and dishabituation in the leech, Hirudo medicinalis. J Neurobiol. 1992;23:270–279. doi: 10.1002/neu.480230306. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Brain Res Mol Brain Res. 2003;118:160–165. doi: 10.1016/j.molbrainres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Zhang X, Lin Q, Willis WD. Increased phosphorylation of the GluR1 subunit of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor in rats following intradermal injection of capsaicin. Neuroscience. 2003;122:237–245. doi: 10.1016/s0306-4522(03)00526-8. [DOI] [PubMed] [Google Scholar]
- Farr M, Mathews J, Zhu DF, Ambron RT. Inflammation causes a long-term hyperexcitability in the nociceptive sensory neurons of Aplysia. Learn Mem. 6:331–340. [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Liu RY, Byrne JH. The ubiquitin-proteasome system is necessary for long-term synaptic depression in Aplysia. J Neurosci. 2008;2008;28:10245–10256. doi: 10.1523/JNEUROSCI.2139-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278:4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- Geranton SM. Targeting epigenetic mechanisms for pain relief. Curr Opin Pharmacol. 2012;12:35–41. doi: 10.1016/j.coph.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Geranton SM, Fratto V, Tochiki KK, Hunt SP. Descending serotonergic controls regulate inflammation-induced mechanical sensitivity and methyl-CpG-binding protein 2 phosphorylation in the rat superficial dorsal horn. Mol Pain. 2008;4:35. doi: 10.1186/1744-8069-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranton SM, Morenilla-Palao C, Hunt SP. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J Neurosci. 2007;27:6163–6173. doi: 10.1523/JNEUROSCI.1306-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Glanzman DL. Habituation in Aplysia: the Cheshire cat of neurobiology. Neurobiol Learn Mem. 2009;92:147–154. doi: 10.1016/j.nlm.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish's lateral giant escape reaction. J Neurosci. 1983;3:2263–2269. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell Cycle. 2011;10:2662–2668. doi: 10.4161/cc.10.16.17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Tsai LH. Probing the role of HDACs and mechanisms of chromatin-mediated neuroplasticity. Neurobiol Learn Mem. 2011;96:41–52. doi: 10.1016/j.nlm.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AK, Fioravante D, Liu RY, Phares GA, Cleary LJ, Byrne JH. Serotonin-mediated synapsin expression is necessary for long-term facilitation of the Aplysia sensorimotor synapse. J Neurosci. 2011;31:18401–18411. doi: 10.1523/JNEUROSCI.2816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN, Goldberg AL, Schwartz JH. Regulatory subunits of cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: a molecular mechanism underlying long-term synaptic plasticity. Proc Natl Acad Sci U S A. 1993;90:7436–7440. doi: 10.1073/pnas.90.16.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain DG, Martin KC, Kandel ER, Schwartz JH. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 1997;89:115–126. doi: 10.1016/s0092-8674(00)80188-9. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Herberholz J, Antonsen BL, Edwards DH. A lateral excitatory network in the escape circuit of crayfish. J Neurosci. 2002;22:9078–9085. doi: 10.1523/JNEUROSCI.22-20-09078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Wolk J, Hu JY, Liu J, Kurosu T, Schwartz JH, Schacher S. Poly-(ADP-ribose) polymerase-1 is necessary for long-term facilitation in Aplysia. J Neurosci. 2009;29:9553–9562. doi: 10.1523/JNEUROSCI.1512-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952a;116:473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952b;116:449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952c;116:497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952d;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, Katz B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952;116:424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JY, Wu F, Schacher S. Two signaling pathways regulate the expression and secretion of a neuropeptide required for long-term facilitation in Aplysia. J Neurosci. 2006;26:1026–1035. doi: 10.1523/JNEUROSCI.4258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illich PA, Walters ET. Mechanosensory neurons innervating Aplysia siphon encode noxious stimuli and display nociceptive sensitization. J Neurosci. 1997;17:459–469. doi: 10.1523/JNEUROSCI.17-01-00459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol. 2011;25:1–28. doi: 10.1111/j.1472-8206.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 1997;17:1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, Kumar A, Nestler EJ, Akbarian S, Beckel-Mitchener AC. Epigenetics in the nervous system. J Neurosci. 2008;28:11753–11759. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin I, Puthanveettil S, Udo H, Karl K, Kandel ER, Hawkins RD. Spontaneous transmitter release is critical for the induction of long-term and intermediate-term facilitation in Aplysia. Proc Natl Acad Sci U S A. 2012a;109:9131–9136. doi: 10.1073/pnas.1206914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin I, Udo H, Rayman JB, Puthanveettil S, Kandel ER, Hawkins RD. Spontaneous transmitter release recruits postsynaptic mechanisms of long-term and intermediate-term facilitation in Aplysia. Proc Natl Acad Sci U S A. 2012b;109:9137–9142. doi: 10.1073/pnas.1206846109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CU, Johannessen SI. Valproate: past, present, and future. CNS Drug Rev. 2003;9:199–216. doi: 10.1111/j.1527-3458.2003.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, Yoshimura M, Ito S. N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci. 2008;27:3161–3170. doi: 10.1111/j.1460-9568.2008.06293.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr RC, Maxwell DJ, Todd AJ. GluR1 and GluR2/3 subunits of the AMPA-type glutamate receptor are associated with particular types of neurone in laminae I-III of the spinal dorsal horn of the rat. Eur J Neurosci. 1998;10:324–333. doi: 10.1046/j.1460-9568.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- Khan A, Pepio AM, Sossin WS. Serotonin activates S6 kinase in a rapamycin-sensitive manner in Aplysia synaptosomes. J Neurosci. 2001;21:382–391. doi: 10.1523/JNEUROSCI.21-02-00382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- King T, Qu C, Okun A, Melemedjian OK, Mandell EK, Maskaykina IY, Navratilova E, Dussor GO, Ghosh S, Price TJ, Porreca F. Contribution of PKMzeta-dependent and independent amplification to components of experimental neuropathic pain. Pain. 2012;153:1263–1273. doi: 10.1016/j.pain.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Mirnics K, Kavookjian AM, Light AR. Ultrastructural analysis of ectopic synaptic boutons arising from peripherally regenerated primary afferent fibers. J Neurophysiol. 1999;81:1636–1644. doi: 10.1152/jn.1999.81.4.1636. [DOI] [PubMed] [Google Scholar]
- Kohama I, Ishikawa K, Kocsis JD. Synaptic reorganization in the substantia gelatinosa after peripheral nerve neuroma formation: aberrant innervation of lamina II neurons by Abeta afferents. J Neurosci. 2000;20:1538–1549. doi: 10.1523/JNEUROSCI.20-04-01538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasne FB, Glanzman DL. Sensitization of the crayfish lateral giant escape reaction. J Neurosci. 1986;6:1013–1020. doi: 10.1523/JNEUROSCI.06-04-01013.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, Hata K, Li E, Matsuda Y, Kimura T, Okabe M, Sakaki Y, Sasaki H, Nakano T. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–917. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix-Fralish ML, Austin JS, Zheng FY, Levitin DJ, Mogil JS. Patterns of pain: metaanalysis of microarray studies of pain. Pain. 2011;152:1888–1898. doi: 10.1016/j.pain.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Lacroix-Fralish ML, Tawfik VL, Tanga FY, Spratt KF, DeLeo JA. Differential spinal cord gene expression in rodent models of radicular and neuropathic pain. Anesthesiology. 2006;104:1283–1292. doi: 10.1097/00000542-200606000-00025. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Tuttle AH, Brown K, Deschenes S, Fischer DB, Mutso A, Root KC, Sotocinal SG, Stern MA, Mogil JS, Sternberg WF. Social approach to pain in laboratory mice. Soc Neurosci. 2010;5:163–170. doi: 10.1080/17470910903216609. [DOI] [PubMed] [Google Scholar]
- Larson AA, Brown DR, el-Atrash S, Walser MM. Pain threshold changes in adjuvant-induced inflammation: a possible model of chronic pain in the mouse. Pharmacol Biochem Behav. 1986;24:49–53. doi: 10.1016/0091-3057(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach PT, Poplawski SG, Kenney JW, Hoffman B, Liebermann DA, Abel T, Gould TJ. Gadd45b knockout mice exhibit selective deficits in hippocampus-dependent long-term memory. Learn Mem. 2012;19:319–324. doi: 10.1101/lm.024984.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Sherry DM, Dryer L, Chin J, Byrne JH, Eskin A. Localization of glutamate and glutamate transporters in the sensory neurons of Aplysia. J Comp Neurol. 2000;423:121–131. doi: 10.1002/1096-9861(20000717)423:1<121::aid-cne10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Lewin MR, Walters ET. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci. 1999;2:18–23. doi: 10.1038/4520. [DOI] [PubMed] [Google Scholar]
- Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: dependence on release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis, and modulation of postsynaptic AMPA receptor efficacy. J Neurosci. 2005;25:5623–5637. doi: 10.1523/JNEUROSCI.5305-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Gunstream JD, Lewin MR, Ambron RT, Walters ET. Activation of protein kinase A contributes to the expression but not the induction of long-term hyperexcitability caused by axotomy of Aplysia sensory neurons. J Neurosci. 1999;19:1247–1256. doi: 10.1523/JNEUROSCI.19-04-01247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Locke J. An essay concerning human understanding. London: Tegg; 1836. [Google Scholar]
- Luo C, Gangadharan V, Bali KK, Xie RG, Agarwal N, Kurejova M, Tappe-Theodor A, Tegeder I, Feil S, Lewin G, Polgar E, Todd AJ, Schlossmann J, Hofmann F, Liu DL, Hu SJ, Feil R, Kuner T, Kuner R. Presynaptically localized cyclic GMP-dependent protein kinase 1 is a key determinant of spinal synaptic potentiation and pain hypersensitivity. PLoS Biol. 2012;10:e1001283. doi: 10.1371/journal.pbio.1001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Guo JU, Ming GL, Song H. DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle. 2009;8:1526–1531. doi: 10.4161/cc.8.10.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manseau F, Sossin WS, Castellucci VF. Long-term changes in excitability induced by protein kinase C activation in Aplysia sensory neurons. J Neurophysiol. 1998;79:1210–1218. doi: 10.1152/jn.1998.79.3.1210. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Marchand F, D'Mello R, Yip PK, Calvo M, Muller E, Pezet S, Dickenson AH, McMahon SB. Specific involvement of atypical PKCzeta/PKMzeta in spinal persistent nociceptive processing following peripheral inflammation in rat. Mol Pain. 2011;7:86. doi: 10.1186/1744-8069-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Carew TJ. Serotonin release evoked by tail nerve stimulation in the CNS of aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci. 2002;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Wickremasinghe N, Carew TJ. Regulation of behavioral and synaptic plasticity by serotonin release within local modulatory fields in the CNS of Aplysia. J Neurosci. 2006;26:12682–12693. doi: 10.1523/JNEUROSCI.3309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- McCall WD, Tanner KD, Levine JD. Formalin induces biphasic activity in C-fibers in the rat. Neurosci Lett. 1996;208:45–48. doi: 10.1016/0304-3940(96)12552-0. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Ferguson-Smith AC. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat Neurosci. 2010;13:1313–1318. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Messner S, Altmeyer M, Zhao H, Pozivil A, Roschitzki B, Gehrig P, Rutishauser D, Huang D, Caflisch A, Hottiger MO. PARP1 ADP-ribosylates lysine residues of the core histone tails. Nucleic Acids Res. 2010;38:6350–6362. doi: 10.1093/nar/gkq463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletic G, Pankratz MT, Miletic V. Increases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany thermal hyperalgesia following chronic constriction injury in rats. Pain. 2002;99:493–500. doi: 10.1016/S0304-3959(02)00242-7. [DOI] [PubMed] [Google Scholar]
- Miller MW, Lee SC, Krasne FB. Cooperativity-dependent long-lasting potentiation in the crayfish lateral giant escape reaction circuit. J Neurosci. 1987;7:1081–1092. doi: 10.1523/JNEUROSCI.07-04-01081.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Vu ET, Krasne FB. Cholinergic transmission at the first synapse of the circuit mediating the crayfish lateral giant escape reaction. J Neurophysiol. 1992;68:2174–2184. doi: 10.1152/jn.1992.68.6.2174. [DOI] [PubMed] [Google Scholar]
- Miniaci MC, Kim JH, Puthanveettil SV, Si K, Zhu H, Kandel ER, Bailey CH. Sustained CPEB-dependent local protein synthesis is required to stabilize synaptic growth for persistence of long-term facilitation in Aplysia. Neuron. 2008;59:1024–1036. doi: 10.1016/j.neuron.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Kohn AB. Do different neurons age differently? Direct genome-wide analysis of aging in single identified cholinergic neurons. Front Aging Neurosci. 2010;2 doi: 10.3389/neuro.24.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nagy GG, Al-Ayyan M, Andrew D, Fukaya M, Watanabe M, Todd AJ. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J Neurosci. 2004;24:5766–5777. doi: 10.1523/JNEUROSCI.1237-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Cross S, Bird A. Gene silencing by methyl-CpG-binding proteins. Novartis Found Symp. 1998a;214:6–16. doi: 10.1002/9780470515501.ch2. discussion 16–21, 46–50. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998b;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Ormond J, Hislop J, Zhao Y, Webb N, Vaillaincourt F, Dyer JR, Ferraro G, Barker P, Martin KC, Sossin WS. ApTrkl, a Trk-like receptor, mediates serotonin- dependent ERK activation and long-term facilitation in Aplysia sensory neurons. Neuron. 2004;44:715–728. doi: 10.1016/j.neuron.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Palahniuk C. Diary : a novel. 1st ed. New York: Doubleday; 2003. [Google Scholar]
- Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, Raja SN, Tao YX. Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain. Mol Pain. 2008;4:67. doi: 10.1186/1744-8069-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, Malcangio M, Lever IJ, Perkinton MS, Thompson SW, Williams RJ, McMahon SB. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol Cell Neurosci. 2002;21:684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- Pinsker HM, Hening WA, Carew TJ, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron. 2003;37:473–484. doi: 10.1016/s0896-6273(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Qi F, Zhou Y, Xiao Y, Tao J, Gu J, Jiang X, Xu GY. Promoter demethylation of cystathionine-beta-synthetase gene contributes to inflammatory pain in rats. Pain. 2013;154:34–45. doi: 10.1016/j.pain.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Quenet D, El Ramy R, Schreiber V, Dantzer F. The role of poly(ADP-ribosyl)ation in epigenetic events. Int J Biochem Cell Biol. 2009;41:60–65. doi: 10.1016/j.biocel.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Savelieva KV, Ye GL, Wang CY, Malbari MM, Friddle C, Lanthorn TH, Zhang W. Loss of the putative catalytic domain of HDAC4 leads to reduced thermal nociception and seizures while allowing normal bone development. PLoS One. 2009;4:e6612. doi: 10.1371/journal.pone.0006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Antonov I, Sheridan R, Frey S, Sander C, Tuschl T, Kandel ER. A role t for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell. 2012;149:693–707. doi: 10.1016/j.cell.2012.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999;40:111–118. doi: 10.1093/ilar.40.3.111. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Glanzman DL. Learning in Aplysia: looking at synaptic plasticity from both sides. Trends Neurosci. 2003;26:662–670. doi: 10.1016/j.tins.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Sahley CL. What we have learned from the study of learning in the leech. J Neurobiol. 1995;27:434–445. doi: 10.1002/neu.480270314. [DOI] [PubMed] [Google Scholar]
- Sahley CL, Modney BK, Boulis NM, Muller KJ. The S cell: an interneuron essential for sensitization and full dishabituation of leech shortening. J Neurosci. 1994;14:6715–6721. doi: 10.1523/JNEUROSCI.14-11-06715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, Greer JJ, Zoghbi HY, Neul JL. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Santos AR, Comprido D, Duarte CB. Regulation of local translation at the synapse by BDNF. Prog Neurobiol. 2010;92:505–516. doi: 10.1016/j.pneurobio.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Schafers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C. Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Exp Neurol. 2004;185:160–168. doi: 10.1016/j.expneurol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Seifert F, Bschorer K, De Col R, Filitz J, Peltz E, Koppert W, Maihofner C. Medial prefrontal cortex activity is predictive for hyperalgesia and pharmacological antihyperalgesia. J Neurosci. 2009;29:6167–6175. doi: 10.1523/JNEUROSCI.4654-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Sherff CM, Stough S, Hsuan V, Carew TJ. A tropomyosin-related kinase B ligand is required for ERK activation, long-term synaptic facilitation, and long-term memory in aplysia. Proc Natl Acad Sci U S A. 2006;103:14206–14210. doi: 10.1073/pnas.0603412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140:421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell. 2003a;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003b;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. Hyperarousal does not depend on trauma-related contextual memory in an animal model of Posttraumatic Stress Disorder. Physiol Behav. 2007a;90:103–107. doi: 10.1016/j.physbeh.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007b;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Song XS, Cao JL, Xu YB, He JH, Zhang LC, Zeng YM. Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol Sin. 2005;26:789–798. doi: 10.1111/j.1745-7254.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Yaksh TL. Behavioral models of pain states evoked by physical injury to the peripheral nerve. Neurotherapeutics. 2009;6:609–619. doi: 10.1016/j.nurt.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen I, Dulin MF, Walters ET, Morris CE. Peripheral regeneration and central sprouting of sensory neurone axons in Aplysia californica following nerve injury. J Exp Biol. 1995;198:2067–2078. doi: 10.1242/jeb.198.10.2067. [DOI] [PubMed] [Google Scholar]
- Sultan FA, Wang J, Tront J, Liebermann DA, Sweatt JD. Genetic deletion of Gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity. J Neurosci. 2012;32:17059–17066. doi: 10.1523/JNEUROSCI.1747-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YJ, Povelones M, Ambron RT. RISK-1: a novel MAPK homologue in axoplasm that is activated and retrogradely transported after nerve injury. J Neurobiol. 2001;47:67–79. doi: 10.1002/neu.1016. [DOI] [PubMed] [Google Scholar]
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Tajerian M, Alvarado S, Millecamps M, Dashwood T, Anderson KM, Haglund L, Ouellet J, Szyf M, Stone LS. DNA methylation of SPARC and chronic low back pain. Mol Pain. 2011;7:65. doi: 10.1186/1744-8069-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taqatqeh F, Mergia E, Neitz A, Eysel UT, Koesling D, Mittmann T. More than a retrograde messenger: nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation. J Neurosci. 2009;29:9344–9350. doi: 10.1523/JNEUROSCI.1902-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood MS, Brodfuehrer PD. The role of glutamate in swim initiation in the medicinal leech. Invert Neurosci. 1995;1:223–233. doi: 10.1007/BF02211024. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Tochiki KK, Cunningham J, Hunt SP, Geranton SM. The expression of spinal methyl-CpG-binding protein 2, DNA methyltransferases and histone deacetylases is modulated in persistent pain states. Mol Pain. 2012;8:14. doi: 10.1186/1744-8069-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsbol T, editor. Handbook of Epigenetics: The New Molecular and Medical Genetics. San Diego, CA: Elsevier; 2011. [Google Scholar]
- Trang T, Beggs S, Salter MW. Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol. 2011;7:99–108. doi: 10.1017/S1740925X12000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW. P2×4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci VF. Postsynaptic modifications in long-term facilitation in Aplysia: upregulation of excitatory amino acid receptors. J Neurosci. 1995;15:1275–1284. doi: 10.1523/JNEUROSCI.15-02-01275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Ma L, Ueda H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J Neurosci. 2010a;30:4806–4814. doi: 10.1523/JNEUROSCI.5541-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Sasaki K, Ma L, Ueda H. Neuron-restrictive silencer factor causes epigenetic silencing of Kv4.3 gene after peripheral nerve injury. Neuroscience. 2010b;166:1–4. doi: 10.1016/j.neuroscience.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2×4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman KS, Duggan AW, Siddall PJ. Increased ability to induce long-term potentiation of spinal dorsal horn neurones in monoarthritic rats. Brain Res. 2003;990:51–57. doi: 10.1016/s0006-8993(03)03385-7. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493:420–423. doi: 10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright ML, Zhang H, Byrne JH, Cleary LJ. Localized neuronal outgrowth induced by long-term sensitization training in aplysia. J Neurosci. 2002;22:4132–4141. doi: 10.1523/JNEUROSCI.22-10-04132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET. Site-specific sensitization of defensive reflexes in Aplysia: a simple model of long-term hyperalgesia. J Neurosci. 1987;7:400–407. doi: 10.1523/JNEUROSCI.07-02-00400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET. Injury-related behavior and neuronal plasticity: an evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int Rev Neurobiol. 1994;36:325–427. doi: 10.1016/s0074-7742(08)60307-4. [DOI] [PubMed] [Google Scholar]
- Walters ET. Chronic pain, memory, and injury: Evolutionary clues from snail and rat nociceptors. International Journal of Comparative Psychology. 2009;22:127–140. [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol. 1983;50:1522–1542. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- Walters ET, Illich PA, Weeks JC, Lewin MR. Defensive responses of larval Manduca sexta and their sensitization by noxious stimuli in the laboratory and field. J Exp Biol. 2001;204:457–469. doi: 10.1242/jeb.204.3.457. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu C, Guo QL, Yan JQ, Zhu XY, Huang CS, Zou WY. Intrathecal 5-azacytidine inhibits global DNA methylation and methyl- CpG-binding protein 2 expression and alleviates neuropathic pain in rats following chronic constriction injury. Brain Res. 2011a;1418:64–69. doi: 10.1016/j.brainres.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mu X, Wu J, Wu A, Fang L, Li J, Yue Y. Differential roles of phosphorylated AMPA receptor GluR1 subunits at Serine-831 and Serine-845 sites in spinal cord dorsal horn in a rat model of post-operative pain. Neurochem Res. 2011b;36:170–176. doi: 10.1007/s11064-010-0288-y. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu J, Wu Z, Lin Q, Yue Y, Fang L. Regulation of AMPA receptors in spinal nociception. Mol Pain. 2010a;6:5. doi: 10.1186/1744-8069-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang X, Guo QL, Zou WY, Huang CS, Yan JQ. Cyclooxygenase inhibitors suppress the expression of P2X(3) receptors in the DRG and attenuate hyperalgesia following chronic constriction injury in rats. Neurosci Lett. 2010b;478:77–81. doi: 10.1016/j.neulet.2010.04.069. [DOI] [PubMed] [Google Scholar]
- Weatherill DB, Dyer J, Sossin WS. Ribosomal protein S6 kinase is a critical downstream effector of the target of rapamycin complex 1 for long-term facilitation in Aplysia. J Biol Chem. 2010;285:12255–12267. doi: 10.1074/jbc.M109.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill DB, McCamphill PK, Pethoukov E, Dunn TW, Fan X, Sossin WS. Compartment-specific, differential regulation of eukaryotic elongation factor 2 and its kinase within Aplysia sensory neurons. J Neurochem. 2011;117:841–855. doi: 10.1111/j.1471-4159.2011.07251.x. [DOI] [PubMed] [Google Scholar]
- Weragoda RM, Ferrer E, Walters ET. Memory-like alterations in Aplysia axons after nerve injury or localized depolarization. J Neurosci. 2004;24:10393–10401. doi: 10.1523/JNEUROSCI.2329-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weragoda RM, Walters ET. Serotonin induces memory-like, rapamycin-sensitive hyperexcitability in sensory axons of aplysia that contributes to injury responses. J Neurophysiol. 2007;98:1231–1239. doi: 10.1152/jn.01189.2006. [DOI] [PubMed] [Google Scholar]
- Winkler I, Blotnik S, Shimshoni J, Yagen B, Devor M, Bialer M. Efficacy of antiepileptic isomers of valproic acid and valpromide in a rat model of neuropathic pain. Br J Pharmacol. 2005;146:198–208. doi: 10.1038/sj.bjp.0706310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol. 2011;24:400–407. doi: 10.1097/ACO.0b013e32834871df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93:584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N. Analysis of the effects of cyclooxygenase (COX)-1 and COX-2 in spinal nociceptive transmission using indomethacin, a non-selective COX inhibitor, and NS-398, a COX-2 selective inhibitor. Brain Res. 1996;739:104–110. doi: 10.1016/s0006-8993(96)00817-7. [DOI] [PubMed] [Google Scholar]
- Zaccardi ML, Traina G, Cataldo E, Brunelli M. Nonassociative learning in the leech Hirudo medicinalis. Behav Brain Res. 2001;126:81–92. doi: 10.1016/s0166-4328(01)00247-9. [DOI] [PubMed] [Google Scholar]
- Zaccardi ML, Traina G, Cataldo E, Brunelli M. Sensitization and dishabituation of swim induction in the leech Hirudo medicinalis: role of serotonin and cyclic AMP. Behav Brain Res. 2004;153:317–326. doi: 10.1016/j.bbr.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med. 2011;17:1448–1455. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wu F, Schacher S. Site-specific and sensory neuron-dependent increases in postsynaptic glutamate sensitivity accompany serotonin-induced long-term facilitation at Aplysia sensorimotor synapses. J Neurosci. 1997;17:4976–4986. doi: 10.1523/JNEUROSCI.17-13-04976.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XY, Huang CS, Li Q, Chang RM, Song ZB, Zou WY, Guo QL. p300 exerts an epigenetic role in chronic neuropathic pain through its acetyltransferase activity in rats following chronic constriction injury (CCI) Mol Pain. 2012;8:84. doi: 10.1186/1744-8069-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovkic IB, Guzman-Karlsson MC, Sweatt JD. Epigenetic regulation of memory formation and maintenance. Learn Mem. 2013;20:61–74. doi: 10.1101/lm.026575.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Crayfish escape behavior and central synapses. I. Neural circuit exciting lateral giant fiber. J Neurophysiol. 1972;35:599–620. doi: 10.1152/jn.1972.35.5.599. [DOI] [PubMed] [Google Scholar]