Abstract
Cardiac fibrosis is characterized by net accumulation of extracellular matrix proteins in the cardiac interstitium, and contributes to both systolic and diastolic dysfunction in many cardiac pathophysiologic conditions. This review discusses the cellular effectors and molecular pathways implicated in the pathogenesis of cardiac fibrosis. Although activated myofibroblasts are the main effector cells in the fibrotic heart, monocytes/macrophages, lymphocytes, mast cells, vascular cells and cardiomyocytes may also contribute to the fibrotic response by secreting key fibrogenic mediators. Inflammatory cytokines and chemokines, reactive oxygen species, mast cell-derived proteases, endothelin-1, the renin/angiotensin/aldosterone system, matricellular proteins, and growth factors (such as TGF-β and PDGF) are some of the best-studied mediators implicated in cardiac fibrosis. Both experimental and clinical evidence suggests that cardiac fibrotic alterations may be reversible. Understanding the mechanisms responsible for initiation, progression, and resolution of cardiac fibrosis is crucial to design anti-fibrotic treatment strategies for patients with heart disease.
Keywords: Myofibroblast, Cardiac remodeling, Macrophage, Mast cell, Chemokine, TGF-β, Angiotensin, Extracellular matrix
Introduction
Cardiac fibrosis is characterized by net accumulation of extracellular matrix in the myocardium and is an integral component of most cardiac pathologic conditions [1]. Because the adult mammalian myocardium has negligible regenerative capacity, the most extensive fibrotic remodeling of the ventricle is found in diseases associated with acute cardiomyocyte death. Following acute myocardial infarction, sudden loss of a large number of cardiomyocytes triggers an inflammatory reaction, ultimately leading to replacement of dead myocardium with a collagen-based scar (Fig. 1) [2]. Several other pathophysiologic conditions induce more insidious interstitial and perivascular deposition of collagen, in the absence of completed infarction. Aging is associated with progressive fibrosis that may contribute to the development of diastolic heart failure in elderly patients. Pressure overload, induced by hypertension or aortic stenosis, results in extensive cardiac fibrosis that is initially associated with increased stiffness and diastolic dysfunction; a persistent pressure load may eventually lead to ventricular dilation and combined diastolic and systolic heart failure [1]. Volume overload due to valvular regurgitant lesions may also result in cardiac fibrosis, characterized by disproportionately large amounts of non-collagenous matrix [3]. Hypertrophic cardiomyopathy and post-viral dilated cardiomyopathy are also often associated with the development of significant cardiac fibrosis [4, 5]. Moreover, a variety of toxic insults (such as alcohol or anthracyclines) [6] and metabolic disturbances (such as diabetes [7] and obesity [8]) induce progressive fibrotic changes in the myocardium in both human patients and experimental models.
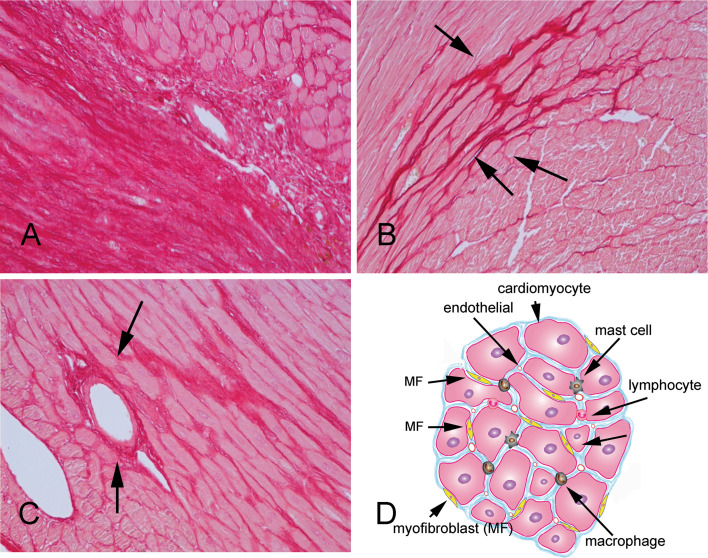
Fig. 1.
Types of cardiac fibrosis (histopathologic images show Sirius-red stained sections of samples from mouse models of fibrosis to identify the collagen network). a Myocardial infarction results in sudden loss of a large number of cardiomyocytes leading to replacement fibrosis. b Interstitial fibrosis is associated with increased deposition of collagen in the cardiac interstitial space in the absence of significant cardiomyocyte loss. c Perivascular fibrosis is characterized by expansion of the vascular adventitial matrix. d The fibrotic heart exhibits expansion of the interstitial space associated with deposition of collagens and other matrix proteins. Myofibroblasts (MF) are the main effector cells in cardiac fibrosis; however, macrophages, lymphocytes, mast cells, vascular endothelial cells, and cardiomyocytes may also participate in the process
Although the pathophysiologic mechanisms leading to fibrotic remodeling of the ventricle differ in patients with various cardiac diseases, the cellular effectors of fibrotic remodeling are common, and similar networks of molecular signals are involved. Our manuscript reviews the cellular and molecular mechanisms involved in the pathogenesis of cardiac fibrosis. After a general discussion on the cellular and molecular biology of the cardiac fibrotic response, we will summarize the current understanding of the pathogenesis of fibrotic myocardial remodeling in the most important cardiac pathophysiologic conditions. Because cardiac fibrosis has profound consequences on myocardial function, understanding its pathogenesis may identify promising targets for the treatment of patients with heart failure.
The normal cardiac interstitium
In the adult mammalian heart, ventricular myocytes are arranged in layers of tightly coupled cardiomyocytes [9]; adjacent layers are separated by clefts. The laminar architecture of the myocardium is defined by an intricate network of extracellular matrix proteins, comprised primarily of fibrillar collagen. Based on morphological characteristics, the cardiac matrix network can be subdivided into three constituents: the epi-, peri-, and endomysium [10]. The epimysium is located on the endocardial and epicardial surfaces providing support for endothelial and mesothelial cells. The perimysium surrounds muscle fibers, and perimysial strands connect groups of muscle fibers. The endomysium arises from the perimysium and surrounds individual muscle fibers. Endomysial struts tether muscle fibers together and to their nutrient microvasculature and function as the sites for connections to cardiomyocyte cytoskeletal proteins across the plasma membrane [1, 11]. The collagen-based cardiac matrix network does not only serve as a scaffold for the cellular components but is also important for transmission of the contractile force. Approximately 85 % of total myocardial collagen is type I, primarily associated with thick fibers that confer tensile strength. Type III collagen, on the other hand, represents 11 % of the total collagen protein in the heart, typically forms thin fibers, and maintains the elasticity of the matrix network [10, 12]. In addition to collagens, the cardiac extracellular matrix also contains glycosaminoglycans (such as hyaluronan), glycoproteins, and proteoglycans. Significant stores of latent growth factors and proteases are also present in the cardiac extracellular matrix; their activation following injury may trigger the fibrotic response.
The cardiac interstitium contains several distinct cell types. Cardiac fibroblasts are enmeshed in the endomysial interstitial matrix that surrounds cardiomyocytes and represent the most abundant interstitial cells in the adult mammalian heart. In the developing heart, cardiac fibroblasts regulate cardiomyocyte proliferation through a fibronectin/β1–integrin-mediated pathway [13]. As the predominant matrix-producing cells in the myocardium [14], fibroblasts play an important role in preserving the integrity of the matrix network. The cardiac fibroblast population undergoes a dramatic change during the neonatal period [15]. As the fetal circulation transitions to the neonatal circulation, elevated left ventricular pressures trigger a marked expansion of the cardiac fibroblast population within the first two neonatal weeks [15]. In the young adult heart, cardiac fibroblasts remain quiescent and do not exhibit significant inflammatory or proliferative activity. Vascular cells (smooth muscle cells, endothelial cells, and pericytes) are also abundant in the cardiac interstitium; relatively small numbers of mast cells and macrophages [16] also reside in the mammalian heart, usually localized around vessels.
The role of cardiac fibrosis in the pathogenesis of heart failure
Mature fibrillar collagen is highly stable with a half-life of 80–120 days. Collagen turnover in the normal heart is primarily regulated by resident cardiac fibroblasts. Homeostatic control of the cardiac extracellular matrix involves ongoing synthesis and degradation of matrix proteins [17, 18]. Disturbance of the tightly regulated balance between the synthetic and degradative aspects of collagen metabolism results in profound structural and functional abnormalities of the heart. Fibrosis disrupts the coordination of myocardial excitation–contraction coupling in both systole and diastole and may result in profound impairment of systolic and diastolic function [19]. Increased deposition of interstitial collagen in the perimysial space is initially associated with a stiffer ventricle and diastolic dysfunction. However, active fibrotic remodeling of the cardiac interstitium is also associated with matrix degradation leading to the development of ventricular dilation and systolic failure [20]. Disturbance of the collagen network in the fibrotic heart may cause systolic dysfunction through several distinct mechanisms. First, loss of fibrillar collagen may impair transduction of cardiomyocyte contraction into myocardial force development resulting in uncoordinated contraction of cardiomyocyte bundles [21]. Second, interactions between endomysial components (such as laminin and collagen) and their receptors may play an important role in cardiomyocyte homeostasis. Laminin α4 chain-deficient mice exhibit microvascular abnormalities leading to systolic ventricular dysfunction, suggesting a link between defects in the matrix network and the structural integrity of the myocardium [22]. Finally, fibrosis may result in sliding displacement (slippage) of cardiomyocytes leading to a decrease in the number of muscular layers in the ventricular wall and subsequent left ventricular dilation [23]. Beyond its profound effects on cardiac function, fibrotic ventricular remodeling also promotes arrhythmogenesis through impaired conduction and subsequent generation of re-entry circuits [24].
The cellular effectors of cardiac fibrosis
Regardless of the pathophysiologic mechanisms responsible for development of the fibrotic response, cardiomyocyte death is often the initial event responsible for activation of fibrogenic signals in the myocardium. In other cases, injurious stimuli (such as pressure overload or myocardial inflammation) may activate pro-fibrotic pathways in the absence of cell death. Several cell types are implicated in fibrotic remodeling of the heart, either directly by producing matrix proteins (fibroblasts) or indirectly by secreting fibrogenic mediators (macrophages, mast cells, lymphocytes, cardiomyocytes, and vascular cells). The relative contribution of the various cell types is often dependent on the underlying cause of fibrosis. However, in all conditions associated with cardiac fibrosis, fibroblast transdifferentiation into secretory and contractile cells, termed myofibroblasts, is the key cellular event that drives the fibrotic response.
The myofibroblasts
Definition
Myofibroblasts are phenotypically modulated fibroblasts that accumulate in sites of injury and combine ultrastructural and phenotypic characteristics of smooth muscle cells, acquired through formation of contractile stress fibers, with an extensive endoplasmic reticulum, a feature of synthetically active fibroblasts [25, 26]. Expression of α-smooth muscle actin (α-SMA) identifies differentiated myofibroblasts in injured tissues, but is not a requirement for the myofibroblast phenotype. At the earliest stages of reparative or fibrotic responses, myofibroblasts may lack α-SMA expression, but exhibit stress fibers composed of cytoplasmic actins; these cells are termed proto-myofibroblasts [27].
The origin of myofibroblasts in cardiac fibrosis
Regardless of the etiology of cardiac injury, myofibroblasts are prominently involved in both reparative and fibrotic processes. Increased myofibroblast accumulation in the cardiac interstitium has been reported, not only in myocardial infarction [28] but also in the pressure and volume overloaded myocardium [29, 30], in the aging heart [31], and in alcoholic cardiomyopathy [32]. The origin of myofibroblasts in the fibrotic heart remains controversial (Fig. 2). The abundance of fibroblasts in the normal myocardium, and the marked induction of mediators that promote myofibroblast transdifferentiation following cardiac injury (such as TGF-β1 and ED-A fibronectin), suggest that activation of resident cardiac fibroblasts may represent the most important source of myofibroblasts in the fibrotic heart. Moreover, proliferating myofibroblasts are commonly found in large numbers in infarcted hearts [33, 34]. Studies in human patients with cardiac fibrosis due to chronic transplant rejection have demonstrated that most of the collagen deposited in fibrotic human hearts is derived from cells of intracardiac origin [35].
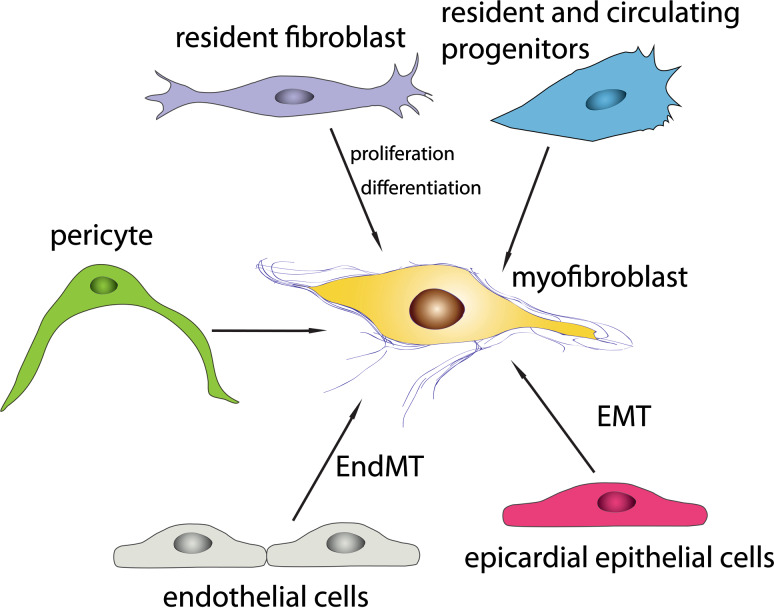
Fig. 2.
Origin of the myofibroblast in fibrotic hearts. Resident cardiac fibroblasts (abundant in adult mammalian hearts), circulating and resident fibroblast progenitors (including fibrocytes), epicardial epithelial cells undergoing epithelial to mesenchymal transition (EMT), and endothelial cells undergoing endothelial to mesenchymal transdifferentiation (EndMT) are documented sources of myofibroblasts in fibrotic hearts. Their relative contribution to the myofibroblast population likely depends on the underlying cause of fibrosis. Pericytes may represent an additional source of myofibroblasts in the fibrotic myocardium; however, their role in fibrotic remodeling of the ventricle has not been elucidated
A growing body of evidence suggests that bone marrow-derived circulating fibrocytes may represent an additional source of myofibroblasts in cardiac injury. In a model of non-infarctive cardiac fibrosis due to brief repetitive myocardial ischemia and reperfusion [36], we have documented recruitment of blood-derived fibroblast progenitors in the cardiac interstitium [37]. Moreover, studies using bone marrow transplantation with enhanced green fluorescent protein (eGFP)-labeled cells demonstrated numerous myofibroblasts of hematopoietic origin in the infarcted myocardium [38] and in the aging mouse heart [31]. Experiments in experimental mouse models have suggested that endothelial to mesenchymal transition may contribute to cardiac fibrosis in models of pressure overload and chronic allograft rejection [39]. Using Tie1Cre;R26RstoplacZ mice, in which endothelial cells and their descendants are marked by LacZ, and FSP-1-GFP transgenic mice, Zeisberg et al. [39] identified a substantial fraction of activated fibroblasts in the fibrotic heart as cells of endothelial origin. Although the poor specificity of FSP-1 as a fibroblast marker is a major limitation for interpretation of the findings, the possibility of an endothelial source of myofibroblasts in cardiac fibrosis should be strongly considered, considering the perivascular location of activated fibroblasts in many fibrotic conditions. The relative contribution of resident cardiac, hematopoietic, and endothelial sources of myofibroblasts in the fibrotic heart has not been systematically studied. In a transgenic model of dilated cardiomyopathy, a significant fraction (almost 17 %) of collagen-producing fibroblasts were of hematopoietic origin [40]. On the other hand, an investigation using bone marrow transplantation from GFP-transgenic mice into nude rats suggested that proliferation of resident cardiac fibroblasts is the main source of myofibroblasts in the healing infarct [41]. The cellular origin of cardiac myofibroblasts may be dependent on the pathophysiologic context; the contribution of blood-derived progenitors may be more significant in conditions associated with more intense inflammatory responses and chemokine upregulation [42, 43].
The molecular signals mediating myofibroblast transdifferentiation and activation
Quiescent cardiac fibroblasts exhibit no actin-associated cell–cell and cell–matrix contacts [44], and do not secrete significant amounts of matrix proteins [26, 27]. Following cardiac injury, alterations in the matrix environment, induction, and release of growth factors and cytokines and increased mechanical stress dynamically modulate fibroblast phenotype. Regardless of the etiology of fibrosis, myofibroblast transdifferentiation is a hallmark of the cardiac fibrotic response. Incorporation of α-SMA into the stress fibers is a characteristic of differentiated myofibroblasts and significantly increases fibroblast contractile activity. Several key factors are required for myofibroblast transdifferentiation in the injured heart. First activation of TGF-β in the cardiac interstitium promotes α-SMA transcription in fibroblasts through activation of the Smad3 signaling cascade [45]. Second, alterations in the composition and mechanical properties of the extracellular matrix facilitate myofibroblast transdifferentiation by altering responses to mechanical stress or by modulating transduction of growth factor signals. Induction of specialized matrix proteins (such as ED-A fibronectin), increased deposition of non-fibrillar collagens (such as collagen VI), and incorporation of matricellular proteins in the cardiac matrix (such as the potent TGF-β activator Thrombospondin-1/TSP-1) are implicated in differentiation of α-SMA-positive myofibroblasts in the injured heart. The splice variant ED-A of cellular fibronectin is upregulated in the infarcted heart [46] and mediates acquisition of the myofibroblast phenotype [47, 48]. Type VI collagen also potently induces myofibroblast differentiation in vitro [49]; in vivo, collagen VI disruption attenuates fibrosis and improves cardiac function following myocardial infarction. Third, expression of cell surface receptors, such as the integrins and syndecans, may be important for transduction of growth factor-mediated signals in cardiac fibroblasts leading to myofibroblast transdifferentiation. Mechanosensitive or cytokine-induced upregulation of cell surface integrins [50, 51] and syndecans [52, 53] may accentuate growth factor signaling, leading to myofibroblast transdifferentiation and promoting fibrotic cardiac remodeling. Finally, mechanical stress directly stimulates α-SMA mRNA synthesis in fibroblasts through Rho/Rho kinase signaling [54], but may not be sufficient to trigger myofibroblast transdifferentiation in the absence of TGF-β. As cardiac injury is often associated with disruption of the structural integrity of the myocardium, exposure of cardiac fibroblasts to increased mechanical stress may significantly contribute to proto-myofibroblast transdifferentiation [27].
The monocyte/macrophage system in cardiac fibrosis
A growing body of evidence implicates monocytes and macrophages in the regulation of the fibrotic response. Monocytes and macrophages not only play important roles in initiation and progression of fibrotic responses, but may also mediate resolution of fibrosis [55]. Both monocytes and macrophages are highly heterogeneous cells; their functional and phenotypic versatility enables them to exert a wide range of pro-fibrotic and anti-fibrotic actions, which are dependent on the relative activity of specific subpopulations and on the effects of microenvironmental factors. Thus, subsets of monocytes and macrophages may regulate fibrosis by differentiating into myofibroblasts, by serving as sources of cytokines and growth factors with fibrogenic properties, and by secreting proteases that participate in matrix remodeling. Moreover, through their phagocytotic properties, macrophages may contribute to the fibrotic process by removing dead cells (thus facilitating growth of reparative fibroblasts), or may negatively regulate fibrosis by clearing apoptotic myofibroblasts and cellular and matrix debris (thus eliminating key pro-fibrotic stimuli). Although large numbers of macrophages accumulate in injured hearts and are located in close proximity to matrix-producing myofibroblasts [56, 57], their role in regulation of the fibrotic response remains unknown. Characterization of subpopulations of “fibrogenic” and “matrix-degrading” macrophages in the injured myocardium and dissection of their role in cardiac fibrosis is urgently needed to understand the cell biology of the fibrotic response.
Monocytic cells as sources of myofibroblasts in the injured heart
Studies using bone marrow transplantation strategies to generate chimeric mice have suggested that at least some of the fibroblasts infiltrating the injured or failing heart may be of hematopoietic origin [31, 38, 40]. The identity of these cells remains obscure; they may represent monocyte subsets capable of fibroblast differentiation with similarities to the CD14+ “fibrocytes” identified in human subjects [58]. Because chemokine upregulation is a consistent feature of cardiac injury regardless of etiology [36, 42, 43, 59, 60], it is attractive to hypothesize that specific chemokine/chemokine receptor pairs may be involved in recruitment of monocyte subsets that differentiate into fibroblasts, thus contributing to the development of fibrosis. Differentiation of progenitor cells into fibroblasts appears to be dynamically regulated by cytokines [61] and growth factors [62].
Macrophage subpopulations as sources of inflammatory and fibrogenic mediators
Monocytes and macrophages are capable of producing and secreting large amounts of pro-inflammatory mediators (such as the cytokines interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6) and pro-fibrotic growth factors (such as TGF-β, PDGFs, and FGFs). In response to signals induced by ischemic cardiac injury, sequential recruitment of monocytes with distinct properties regulates the inflammatory and reparative response following myocardial infarction [63]. During the early inflammatory phase of infarct healing [2], monocytes with pro-inflammatory, phagocytic, and proteolytic properties are recruited; these cells express the chemokine receptor CCR2 and infiltrate the infarcted myocardium in response to the marked upregulation of the CC chemokine monocyte chemoattractant protein (MCP)-1 [59]. In contrast, during the reparative phase, monocytes with attenuated inflammatory activity and predominant expression of angiogenic mediators are predominantly recruited [63]; whether some of these cells exhibit fibrogenic properties remains unknown. In addition to the time-dependent chemokine-responsive recruitment of monocytes subsets with distinct properties, the contribution of the monocytes/macrophage system in the injured and fibrotic myocardium can be regulated through effects of microenvironmental signals on macrophage phenotype. Macrophages are known to exhibit considerable functional plasticity and to respond to changes in their microenvironment by modulating their cytokine and growth factor expression profile [64]. Traditionally, two distinct macrophage polarization states are recognized: classically activated M1 macrophages, induced by IFN-γ, either by itself or in combination with TNF-α, or GM-CSF, express pro-inflammatory cytokines and reactive oxygen species, whereas alternatively-activated M2 macrophages, induced by IL-4 or IL-13, express high levels of IL-10 and participate in the resolution of inflammation and angiogenesis [65]. Following cardiac injury, the complexity of environmental conditions may result in generation of multiple macrophage subpopulations with distinct properties that mediate pro-inflammatory, anti-inflammatory, or fibrogenic actions. Although differentiation of M2 macrophages in the myocardium has been associated with the development of cardiac fibrosis [66], “fibrogenic” macrophage subsets in the fibrotic myocardium have not been systematically characterized, and their role in the fibrotic response has not been investigated.
Are monocytes/macrophages essential for cardiac fibrotic responses?
The relative contribution of monocytes and macrophages in the cardiac fibrotic response is likely dependent on the pathophysiologic basis of cardiac fibrosis. In a model of ischemic non-infarctive cardiac fibrosis due to brief repetitive ischemic insults followed by reperfusion, chemokine-mediated recruitment of macrophages was crucial for the development of interstitial fibrosis [36]. Moreover, in experimental models of hypertensive fibrotic remodeling, angiotensin II and mineralocorticoids may mediate their pro-fibrotic actions, at least in part, through activation of macrophage responses [67, 68]. Deletion of mineralocorticoid receptors from lysozyme M-positive myeloid cells attenuated cardiac fibrosis in deoxycorticosterone/salt-induced hypertension [69], and in a model of hypertensive cardiac remodeling upon infusion of L-NAME/angiotensin II [70], suggesting that the fibrogenic effects of aldosterone signaling are mediated through modulation of macrophage phenotype [70].
The potential role of macrophages in inhibition and resolution of cardiac fibrosis
Most studies on the role of macrophages in tissue fibrosis have focused on identification of pro-fibrotic actions; however, a growing body of evidence suggests that distinct macrophage subpopulations may be involved in resolution of the fibrotic response. In a model of hepatic fibrosis, Ly6Clo macrophages expressed high levels of MMPs and were suggested to play a role in regression of the fibrotic response [71, 72]. Macrophage subsets with anti-inflammatory properties may have indirect anti-fibrotic effects by suppressing fibroblast activation [2]. Moreover, phagocytotic macrophages may contribute to resolution of the fibrotic response by removing apoptotic myofibroblasts. However, subpopulations of anti-fibrotic macrophages in the injured myocardium have not been characterized, and the role of monocytes/macrophage subsets in negative regulation and resolution of cardiac fibrosis remains unknown.
The mast cell in cardiac fibrosis
Mast cells are capable of releasing large amounts of fibrogenic mediators, including histamine, the mast cell-specific proteases, tryptase and chymase, and a wide range of cytokines and growth factors (Fig. 3). Both associative studies and experiments using mast cell-deficient rodent models suggest an important role for mast cells in tissue fibrosis and extracellular matrix remodeling [73, 74]. The normal myocardium is populated by chymase and tryptase-positive mast cells that exhibit a distinct immunologic and biochemical phenotype [75]. Cardiac fibrosis is associated with increased accumulation of mast cells that store a wide variety of pro-inflammatory and fibrogenic mediators in their granules. Increased mast cell density has been demonstrated in myocardial infarcts [33, 76] in patients with dilated and ischemic cardiomyopathy [77], as well as in the pressure- [78] and volume-overloaded [79] heart. The factors responsible for mast cell accumulation in areas of fibrosis are poorly understood. Several lines of evidence suggest that stem cell factor (SCF) is critically involved in recruitment of mast cell progenitors and in differentiation and growth of mature mast cells. Although, an association between SCF expression and increased mast cell density has been shown in failing hearts [77] and following myocardial infarction [33], direct evidence that SCF may stimulate mast cell growth in these conditions is lacking.
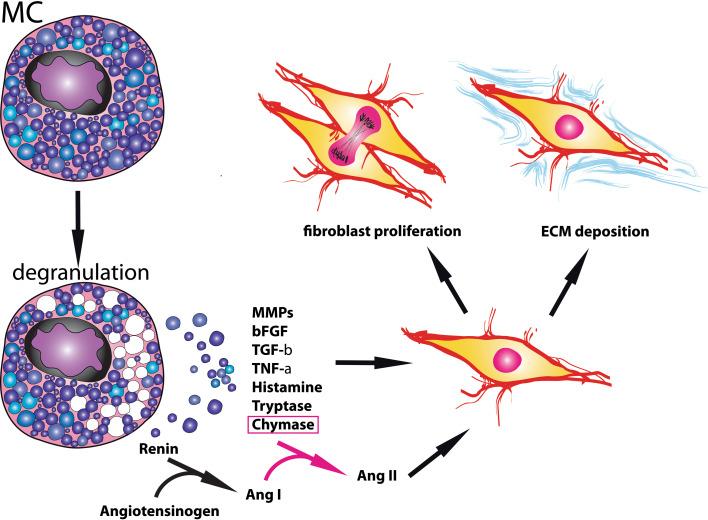
Fig. 3.
Mast cells in cardiac fibrosis. Cardiac mast cell numbers increase in failing and remodeling hearts. Mast cell degranulation results in release of a wide range of fibrogenic mediators, leading to activation, proliferation, and differentiation of cardiac fibroblasts
Several recent investigations provide compelling evidence that mast cells play an essential role in the pathogenesis of cardiac fibrosis. In mouse models of pressure overload, mast cell-deficient mice were protected from the development of perivascular fibrosis and the progression of compensated hypertrophy to heart failure [80]. Moreover, accumulation of mast cells in the atria of pressure-overloaded hearts was implicated in the pathogenesis of atrial fibrosis through expression of PDGF-A and caused atrial fibrillation [81]. In a rat model of hypertension, administration of a mast cell stabilizer prevented myocardial macrophage infiltration and attenuated fibrotic ventricular remodeling [82]. In a model of fibrotic cardiomyopathy due to TNF-α overexpression, mast cell–fibroblast interactions were required for the development of cardiac fibrosis [83]. Mast cells were also implicated in cardiac remodeling induced by chronic volume overload; stabilization of mast cells prevented ventricular dilation and attenuated systolic and diastolic dysfunction [84]. The potent pro-fibrotic actions of mast cell-derived mediators on the heart are supported by findings demonstrating diastolic left ventricular dysfunction in many patients with systemic mast cell activation disorders [85].
Which mediators are responsible for the pro-fibrotic actions of mast cells in the failing heart? Activated mast cells release a wide variety of granule-stored bioactive mediators, cytokines, and growth factors which have been demonstrated to stimulate cardiac fibroblast proliferation and collagen synthesis [86]. Mast cell granules contain large amounts of TNF-α [87], TGF-β, IL-4 [88], PDGFs, and FGFs; however, these fibrogenic mediators are also synthesized by many other cell types involved in cardiac fibrosis, including macrophages and lymphocytes. Thus, their contribution to the cardiac fibrotic response remains unknown. Mast cell-derived histamine also stimulates fibroblast proliferation [89] and collagen synthesis [90]; its actions may be mediated at least in part through increased connective tissue growth factor (CTGF) synthesis [91]. Administration of a histamine H2 receptor inhibitor in a small prospective study improved both cardiac symptoms and ventricular remodeling in patients with heart failure [92]; these findings may reflect, at least in part, inhibition of the pro-fibrotic effects of histamine in the failing heart.
On the other hand, the release of mast cell-specific products such as the proteases chymase and tryptase may represent a unique contribution of the mast cell in the fibrotic response and in extracellular matrix remodeling. Mast cell chymase exerts fibrogenic actions through generation of angiotensin II [93] or through activation of TGF-β-induced Smad-dependent pathways [94]. In failing hearts, over 75 % of cardiac-specific highly fibrogenic angiotensin II is derived from the angiotensin converting enzyme (ACE)-independent chymase pathway [95]. This pathway is not affected by ACE inhibitors and thus may constitute a potential mechanism for the progression of cardiac fibrosis despite ACE inhibition. Beyond its effects on extracellular matrix protein synthesis, chymase also modulates matrix metabolism through activation of MMPs [96, 97]. Studies in both rodent and large animal models of cardiac fibrosis have demonstrated the importance of chymase signaling in fibrotic remodeling of the ventricle, and suggest a potentially important opportunity for treatment. Matsumoto and co-workers [98] showed that chymase inhibition decreases fibrosis and attenuates diastolic but not systolic dysfunction in a dog model of tachycardia-induced heart failure. In a porcine model of ischemia/reperfusion, chymase antagonism reduced cardiac fibrosis and attenuated MMP expression [99]. In addition, chymase inhibition in a rat model of non-reperfused myocardial infarction attenuated left ventricular interstitial fibrosis and diastolic dysfunction without affecting the dilative pattern of cardiac remodeling [100]. Tryptase, the most abundant secretory product of the human mast cell, is also a fibrogenic mediator and potently stimulates proliferation [101] and collagen I synthesis [102] in dermal and pulmonary fibroblasts. In cardiac fibroblasts, tryptase activates Erk MAPK signaling and promotes collagen expression through activation of the protease-activated receptor (PAR)-2 [103]. Despite convincing in vitro evidence on the fibrogenic effects of tryptase and the abundance of tryptase-positive mast cells in experimental models of cardiac fibrosis [33, 76], the potential in vivo role of tryptase in the pathogenesis of fibrotic cardiac remodeling has not been documented.
Although the bulk of the evidence suggests that mast cell-derived mediators promote fibrous tissue deposition, a recent investigation demonstrated an antifibrotic role for mast cells in a rat model of homocysteine-induced cardiac fibrosis and remodeling [104]. These protective actions may be mediated through effects of mast cell products on the MMP:TIMP balance. Much like macrophages, mast cells may respond to microenvironmental cues by altering their growth factor and protease expression profile, thus transitioning from pro-fibrotic to anti-fibrotic phenotypes.
The role of lymphocytes in cardiac fibrosis
CD4+ T helper cells have been implicated in the pathogenesis of a wide range of fibrotic conditions [105]. Th2 cell differentiation is associated with marked upregulation of the pro-fibrotic cytokines IL-4 and IL-13, both potent stimulators of fibroblast-derived collagen synthesis. Moreover, Th2 cytokines drive macrophage differentiation towards an M2 phenotype, further enhancing fibrogenic responses. Although the role of Th2 cells in the pathogenesis of hepatic [106] and pulmonary fibrosis [107] is well established, direct evidence on the involvement of Th2 cells in cardiac fibrosis is lacking. Descriptive studies have suggested that increased expression of IL-4 and IL-13 in the aging heart was associated with cardiac fibrosis [108]; however, the hypothesis that Th2 cells may play a direct role in fibrotic cardiac remodeling has not been tested.
Other T cell subpopulations may also be involved in the pathogenesis of cardiac fibrosis, especially in conditions associated with intense T cell-mediated inflammation. In an experimental model of fibrosis due to autoimmune inflammatory myocarditis, Th17 cells have been implicated as important effectors of the cardiac fibrotic response [109]. Whether certain T cell subsets are also involved in inhibition of the fibrotic response remains unknown. However, a growing body of evidence suggests that cell therapy with regulatory T cells (Tregs) attenuates cardiac fibrosis in experimental models of hypertensive heart disease [110], angiotensin-induced cardiomyopathy [111], and myocardial infarction [112]. Whether Tregs reduce the fibrogenic potential of macrophages or fibroblasts through direct contact-mediated interactions, or express and release anti-fibrotic signals remains unknown. Considering the high level expression of the fibrogenic growth factor TGF-β by Tregs, their anti-fibrotic actions may reflect cardiomyocyte protection rather than attenuation of the fibrogenic cascade.
The endothelium
The involvement of endothelial cells in cardiac fibrosis is suggested by the frequent co-existence of fibrotic and angiogenic responses and by the common occurrence of perivascular fibrosis in pathophysiologic conditions associated with pressure overload. Endothelial cells may promote fibrotic cardiac remodeling through three distinct mechanisms:
By expressing pro-fibrotic mediators, such as TGF-β1, FGFs or endothelin (ET)-1. ET-1 derived from vascular endothelial cells was implicated in the pathogenesis of cardiac fibrosis in a model of angiotensin-induced cardiomyopathy [113] and in diabetic cardiac fibrosis [114]. However, the role of endothelial cells as a source of fibrogenic growth factors is less well established.
Endothelial cells may contribute to fibrosis through the release of pro-inflammatory cytokines and chemokines, thus promoting recruitment of macrophages and lymphocytes with fibrogenic actions.
Endothelial cells may undergo endothelial to mesenchymal transition [39], thus directly contributing to expansion of the fibroblast pool in the fibrotic heart.
In addition to their pro-fibrotic actions, endothelial cells may also produce anti-fibrotic mediators. The anti-fibrotic chemokine interferon-γ-inducible protein (IP)-10/CXCL10 exerts inhibitory actions on cardiac fibroblasts and is produced and secreted by endothelial cells following cardiac injury [115, 116]. Moreover, endothelial expression of hypoxia inducible factor (HIF)-1 has been shown to protect the pressure-overloaded myocardium from fibrosis; these anti-fibrotic actions may be mediated at least in part through suppression of TGF-β signaling [117].
The cardiomyocytes
Cardiomyocyte death triggers an inflammatory response that ultimately results in fibroblast activation and in replacement of dead cardiomyocytes with fibrous tissue [118]. A growing body of evidence suggests that, under conditions of stress, viable cardiomyocytes may promote interstitial fibrosis by activating interstitial fibroblasts; however, the molecular cascades responsible for these effects are poorly understood. ATP release through pannexin-1 channels may be one of the early cardiomyocyte-derived signals that activate fibroblast responses following cardiac injury [119]. Loss of mineralocorticoid receptors in cardiomyocytes attenuates deoxycorticosterone/salt-mediated cardiac fibrosis in mice, suggesting that cardiomyocyte-specific aldosterone signaling triggers the fibrogenic response [120]. Moreover, cardiomyocyte-selective TGF-β receptor II (TβRII) knockdown significantly attenuated fibrosis of the pressure-overloaded heart, suggesting a crucial role for cardiomyocyte-specific TGF-β signaling in the pathogenesis of fibrotic remodeling [121]. On the other hand, modulation of cardiomyocyte-specific signaling may also exert anti-fibrotic actions. Cardiomyocyte-specific overexpression of the angiotensin II type 2 receptor (AT2) inhibits angiotensin-induced cardiac fibrosis through activation of the kinin/NO system [122].
The extracellular matrix in the fibrotic heart
The fibrillar collagens
Increased accumulation of fibrillar collagen in the cardiac interstitium is the hallmark of cardiac fibrosis. Synthesis of both type I and type III collagen is markedly increased in the remodeling fibrotic heart regardless of the etiology of fibrosis [10, 123]. In models of hypertensive cardiac fibrosis and of myocardial infarction, type I collagen exhibits more intense and prolonged upregulation than collagen III [124, 125]. However, in patients with ischemic cardiomyopathy, the ratio of collagen I:collagen III synthesis was decreased [126], suggesting that expression patterns of various collagen isoforms in the fibrotic heart may depend on contextual factors. Activated myofibroblasts are the main cellular sources of collagens in the fibrotic heart; once outside the cell, procollagen chains are processed, assembled into fibrils, and cross-linked. Collagen cross-linking is associated with the development of diastolic dysfunction in the fibrotic heart [127], but may also contribute to the integrity of the cardiac matrix preventing chamber dilation [128].
In addition to the deposition of fibrillar collagens, the extracellular matrix in the remodeling heart exhibits dynamic alterations in its composition that serve to facilitate proliferation and migration of fibroblasts and transduce signals necessary for fibroblast activation. The extent and time course of these alterations are dependent on the underlying etiology of fibrosis. In replacement fibrosis associated with myocardial infarction, the sudden death of a large number of cardiomyocytes triggers an intense inflammatory reaction that dramatically alters the composition, inducing degradation of the normal interstitial matrix and generation of matrix fragments, followed by formation of a fibrin/fibronectin-based provisional matrix network and by the deposition of newly-synthesized “matricellular” macromolecules, which are incorporated into the matrix and modulate cell phenotype [45, 129].
Non-fibrillar collagens
In the fibrotic heart, deposition of non-fibrillar collagens (such as collagen VI) may play an important role in fibroblast activation. The role of collagen VI has been studied primarily in infarctive fibrosis; whether it is involved in other cardiac fibrotic conditions remains unknown [49]. In vitro, collagen VI potently stimulates myofibroblast transdifferentiation, but has no significant effects on fibroblast proliferation [49]. In an experimental model of myocardial infarction, collagen VI disruption reduced fibrosis and attenuated dysfunction. Whether collagen VI acts primarily by enhancing fibrosis remains unknown, because collagen VI disruption also appears to attenuate cardiomyocyte apoptosis in the infarcted heart [130].
Fibrin and fibronectin: components of the provisional matrix that regulate fibroblast phenotype and function
During the early stages of the fibrotic response, extravasation of plasma proteins (such as fibrinogen and plasma fibronectin) through the hyperpermeable vessels results in the formation of a provisional matrix network comprised of fibrin and fibronectin [131]. This dynamic matrix network facilitates fibroblast migration and stimulates fibroblast proliferation [132] and activation through interactions that involve α5β1 and αvβ3 integrins [133] and syndecan-4 [134]. Lysis of the plasma-derived provisional matrix by granulation tissue cells is followed by generation of an organized cell-derived “second order” provisional matrix that contains cellular fibronectin and hyaluronan [135]. In addition to its role as a conduit for migrating fibroblasts, the cell-derived provisional matrix also promotes myofibroblast transdifferentiation. The splice variant ED-A of cellular fibronectin is known to co-operate with TGF-β in mediating acquisition of the myofibroblast phenotype [27, 47, 48]. ED-A fibronectin is consistently upregulated in the infarcted and pressure-overloaded fibrotic heart and in models of chronic cardiac rejection [46, 136–138]. Although in vitro studies have suggested the role of ED-A fibronectin in cardiac myofibroblast transdifferentiation [139], direct in vivo evidence documenting its involvement in cardiac fibrotic conditions is lacking.
The matricellular concept: incorporation of macromolecules into the matrix transduces signals that modulate fibrotic responses and matrix remodeling
One of the most important alterations observed in the cardiac extracellular matrix in fibrotic conditions is the induction and secretion of “matricellular proteins” into the interstitial space [129]. Matricellular proteins are a family of structurally unrelated extracellular macromolecules that are not part of the normal tissue matrix, but are transiently upregulated following injury and bind to the structural extracellular matrix. Matricellular proteins generally do not play a structural role but function as “molecular bridges” between matrix proteins and cells, transducing or modulating cytokine and growth factor responses [140]. The family includes the thrombospondins (TSPs), tenascin-C, osteopontin (OPN), SPARC (secreted protein acidic and rich in cysteine), periostin, and members of the CCN family; new members and proteins that exhibit both matricellular and non-matricellular functions are being increasingly recognized. Fibroblasts, macrophages, and vascular cells, the major cellular effectors of fibrosis, are major targets of matricellular proteins.
TSP-1, -2, and -4 are upregulated in cardiac fibrotic conditions and play distinct, but important, roles in the pathogenesis of the fibrotic response [141–143]. TSP-1, a potent angiostatic mediator with an essential role in TGF-β activation [144], is upregulated in the infarcted and pressure-overloaded myocardium [141, 145], and is localized in areas with abundant myofibroblasts. In the pressure-overloaded myocardium, TSP-1 disruption is associated with increased MMP activity, impaired myofibroblast transdifferentiation, and reduced fibroblast-derived collagen synthesis [141]. As an important activator of TGF-β, TSP-1 may promote a matrix-preserving phenotype in cardiac fibroblasts enhancing matrix deposition, while preventing chamber dilation. Because TSP-1 inhibits MMP activation, its effects on the fibrotic heart may also be mediated through direct matrix-stabilizing actions [146, 147]. TSP-2 also exerts matrix-preserving actions on the remodeling myocardium. In TSP-2 null mice, angiotensin infusion induces fatal cardiac rupture associated with marked increases in MMP-2 and MMP-9 expression and activity [148]. TSP-4, on the other hand, appears to inhibit the fibrotic response: TSP-4 null animals had increased collagen deposition in the pressure-overloaded heart [143]. Recent evidence suggests that TSPs may also modulate cardiomyocyte function in the remodeling heart. TSP-2 absence is associated with an age-associated dilated cardiomyopathy, in part due to the loss of TSP-2-activated survival signals in cardiomyocytes [142]. TSP-4 acts as a mechano-signaling molecule, necessary for augmentation of contractility in hearts subjected to pressure overload [149].
The prototypical matricellular protein tenascin-C is consistently induced in remodeling fibrotic hearts regardless of the underlying etiology (Fig. 4) [150–152] and is localized in areas with heavy myofibroblast infiltration [152, 153]. Studies using tenascin-C null mice have suggested pro-fibrotic effects of tenascin-C following cardiac injury. In a model of electrical myocardial injury [154], tenascin-C loss was associated with delayed recruitment of myofibroblasts in the site of injury. In an experimental model of myocardial infarction, tenascin-C null animals had significantly reduced cardiac remodeling and attenuated diastolic dysfunction associated with less pronounced fibrosis [155]. Although the cellular and molecular basis for the pro-fibrotic effects of tenascin-C have not been dissected, in vitro experiments suggest that tenascin-C promotes a deadhesive state and may facilitate migration of fibroblasts and other reparative cells in the remodeling myocardium.
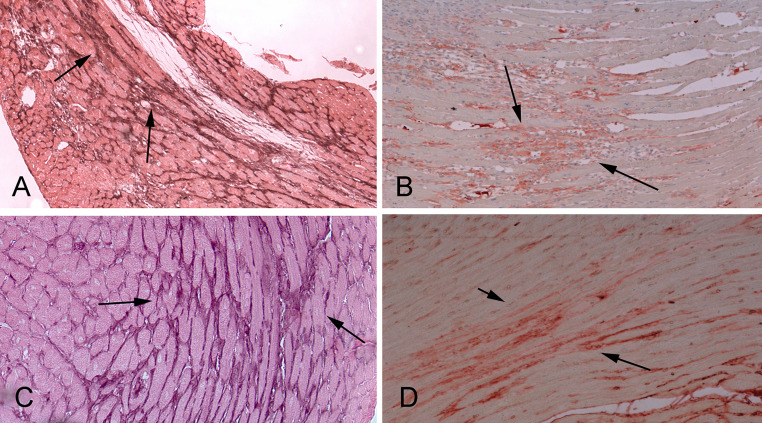
Fig. 4.
Matricellular proteins are induced in the fibrotic heart and modulate cellular responses. Upregulation and deposition of matricellular proteins in the cardiac interstitium is a hallmark of the fibrotic response. a Immunohistochemical staining shows deposition of the prototypical matricellular protein tenascin-C (arrows) in the border zone and remodeling myocardium in reperfused mouse infarcts. b Periostin is also expressed in myofibroblasts and deposited in the infarct matrix (arrows). c, d Both tenascin-C (c) and periostin (d) are upregulated in the murine pressure overloaded heart (arrows)
SPARC and OPN are also well-documented regulators of fibroblast responses in cardiac remodeling. SPARC loss was associated with defective repair following myocardial infarction due to disorganized granulation tissue formation and impaired scar maturation. These defects resulted in increased mortality and a high incidence of heart failure [156]. In the remodeling heart, SPARC may act primarily by promoting or facilitating TGF-β signaling in cardiac fibroblasts. OPN is also upregulated in cardiac fibrosis [157], and can act both as a matricellular protein (when bound to the matrix) and as a cytokine (when secreted in a soluble form). Loss-of-function studies suggested that OPN plays an important role in cardiac fibrosis. Absence of OPN in a model of myocardial infarction resulted in increased left ventricular dilatation associated with reduced collagen deposition in the area of the infarct [158]. Moreover, OPN loss attenuated cardiac fibrosis in models of aldosterone [159] and angiotensin-induced cardiomyopathy [160], and in a genetic model of dilated cardiomyopathy [161], but not in the pressure-overloaded heart [162]. Although the mechanistic basis for the pro-fibrotic effects of OPN has not been dissected, OPN may act by accentuating growth factor signaling in cardiac fibroblasts, by directly exerting pro-survival effects on cardiac fibroblasts [163], by modulating MMP expression and activity, or by directing macrophages towards a pro-fibrotic phenotype.
Periostin expression is markedly upregulated in fibrotic hearts, localized almost exclusively in activated myofibroblasts and in the interstitial matrix (Fig. 4). Periostin absence results in decreased recruitment of fibroblasts, associated with impaired collagen fibrillogenesis; perturbed fibroblast function predisposes to cardiac rupture, but also attenuates late remodeling of the infarcted heart [164, 165]. The pathways mediating the pro-fibrotic actions of periostin have not been investigated.
Of the members of the CCN subfamily of matricellular proteins, CCN2/CTGF is consistently upregulated in the fibrotic heart and may be involved in fibrotic remodeling of the myocardium [166]. However, cardiac overexpression of CCN2 did not result in significant cardiac fibrosis [167], suggesting that CCN2 by itself may not be sufficient to induce fibrotic changes. As a TGF-β-inducible protein, CCN2 may contribute to the pathogenesis of fibrosis by accentuating TGF-β-mediated actions.
Molecular pathways involved in cardiac fibrosis
The wide range of molecular signals implicated in the fibrotic response and the complexity of their interactions have hampered understanding of the mechanistic basis of cardiac fibrosis. Recently, high-throughput genomic and transcriptomic strategies have been used to identify new pathways and molecular signals implicated with initiation, progression, and regression of the fibrotic response [168]. Although such approaches generate large amounts of interesting information, they cannot distinguish mediators with critical involvement in the fibrotic process from the large number of genes that may be differentially regulated but do not play an essential regulatory role [169]. From a pathophysiologic perspective, mechanism-oriented research using animal models, and cell biological studies, remain the most effective and important tools in understanding the biology of cardiac fibrosis.
Several molecular pathways have been implicated in the pathogenesis of cardiac fibrosis; their relative significance is dependent on the underlying cause of the fibrotic reaction. Inflammatory signals seem to be more important in reparative and ischemic fibrosis, which are associated with intense activation of cytokine and chemokine cascades [2, 42]. On the other hand, the angiotensin/aldosterone axis and fibrogenic growth factors, such as TGF-β and PDGF, appear to be involved in most fibrotic cardiac conditions regardless of etiology.
Reactive oxygen species
Oxidative stress has been implicated in the pathogenesis of cardiac fibrosis both through direct actions and through its involvement in cytokine and growth factor signaling. Reactive oxygen species (ROS) directly regulate the quantity and quality of interstitial extracellular matrix by modulating both matrix protein expression and metabolism. Both matrix-preserving and matrix-degrading effects of ROS have been reported. Increased oxidative stress activates MMPs and decreases fibrillar collagen synthesis in cardiac fibroblasts [170]. On the other hand, the TGF-β activating effects of ROS may enhance extracellular matrix deposition in the cardiac interstitium [171]. Beyond their direct actions, ROS are also key mediators of cytokine- and angiotensin II-induced effects on fibroblasts [172]. Inflammatory cytokine-mediated activation of mitogen-activated protein kinases and stress-responsive protein kinases is redox-sensitive. Subsequent activation of transcription factors such as AP-1, Ets, and NF-κB leads to enhanced MMP transcription [173]. The effects of angiotensin appear to be in part dependent on ROS. Angiotensin II activates downstream ROS-sensitive kinases that are critical in mediating fibrotic remodeling of the heart [174]. In adult rat, cardiac fibroblasts angiotensin II-stimulated collagen production is mediated through ROS generation [175].
In addition to the effects of oxidative stress on cardiac fibroblasts, a growing body of in vivo evidence supports the significance of ROS-mediated effects in the pathogenesis of cardiac fibrosis. Mice that overexpress catalase targeted to mitochondria are resistant to cardiac hypertrophy and fibrosis after angiotensin infusion, suggesting that mitochondrial oxidative stress mediates angiotensin-induced fibrotic cardiomyopathy [176]. Moreover, in a model of fibrotic ischemic cardiomyopathy due to brief repetitive myocardial ischemia and reperfusion, extracellular superoxide dismutase (EC-SOD) overexpression attenuated interstitial fibrosis [177]. Experiments in a rat model demonstrated that the profibrotic actions of aldosterone infusion are mediated at least in part through ROS generation [178].
Chemokines
Chemokines are chemotactic cytokines involved in leukocyte trafficking [179]. Several members of the chemokine family have been implicated in the fibrotic process through recruitment of pro-fibrotic leukocyte subpopulations, through chemotactic attraction of fibroblast progenitors and through direct actions on fibroblasts [180]. The CC chemokine CCL2/MCP-1 is the best-studied chemokine in heart disease [181] and appears to play a role in ischemic and pressure overload-induced cardiac fibrosis. Pressure overload due to suprarenal aortic constriction induced myocardial MCP-1 mRNA expression followed by macrophage accumulation, reactive fibrosis, and cardiomyocyte hypertrophy. Treatment with the angiotensin II type I (AT-1) receptor antagonist candesartan significantly attenuated MCP-1 upregulation, suggesting that AT-1 signaling may play a key role in MCP-1 induction in the pressure-overloaded heart [67]. Chronic treatment with a monoclonal neutralizing anti-MCP-1 antibody not only inhibited interstitial macrophage accumulation but also attenuated fibroblast proliferation and TGF-β induction. Furthermore, MCP-1 inhibition reduced myocardial fibrosis, but not cardiomyocyte hypertrophy, and ameliorated diastolic dysfunction without affecting blood pressure and systolic function [182]. Experiments from our laboratory demonstrated that MCP-1 also plays a critical role in the pathogenesis of a fibrotic ischemic cardiomyopathy due to brief repetitive ischemia and reperfusion [36].
MCP-1 may mediate its pro-fibrotic effects through several distinct mechanisms. First, mononuclear cells chemotactically attracted through MCP-1/CCR2 signaling may be an important source of fibrogenic mediators, such as TGF-β and fibroblast growth factors. MCP-1 selectively recruits CCR2+ monocytes; whether these cells are capable of driving the fibrotic response has not been systematically investigated. In addition to its chemotactic properties, MCP-1 may enhance the fibrogenic potential of macrophages by inducing TGF-β1 and collagen synthesis [183] and may accentuate macrophage OPN expression [59]. Second, MCP-1 may directly modulate fibroblast phenotype and activity. In vitro studies have demonstrated that MCP-1 enhances portal fibroblast proliferation and myofibroblast differentiation [184], upregulates collagen and TGF-β1 expression by rat pulmonary fibroblasts [185], and stimulates production of MMP-1 and tissue inhibitor of metalloproteinases (TIMP)-1 by human skin fibroblasts [186]. However, experiments in murine cardiac fibroblasts did not demonstrate significant effects of MCP-1 on fibroblast-derived MMP expression [36]. Third, MCP-1 may be an important mediator in the recruitment of fibroblast progenitors [187, 188]. Experiments in a model of ischemic fibrotic cardiomyopathy and in angiotensin-induced cardiac fibrosis identified recruitment of monocytic fibroblast progenitors as an important mechanism mediating the pro-fibrotic actions of MCP-1 [37, 189].
Certain chemokines may be involved in negative regulation of fibrosis. Our experiments have identified the CXC chemokine IP-10/CXCL10 as a potent antifibrotic mediator in healing myocardial infarction [115, 116]. The antifibrotic effects of IP-10 may be primarily due to inhibition of growth-factor-induced fibroblast migration [116].
Cytokines
Expression of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6 is consistently induced in fibrotic hearts [190–193]. Circulating TNF-α and IL-6 levels correlate with markers of collagen turnover in patients with dilated cardiomyopathy [194], suggesting an association between cytokine activation and matrix remodeling. In vitro, pro-inflammatory cytokines are potent regulators of collagen metabolism and profoundly affect fibroblast phenotype and gene expression [173]. TNF-α, IL-1β, and IL-6 decrease collagen synthesis in isolated cardiac fibroblasts and increase MMP expression and activity [195], while reducing synthesis of inhibitors of metalloproteinases [196]. These actions promote matrix degradation [197]. IL-1β and (to a lesser extent) TNF-α, but not IL-6, stimulate concentration-dependent increases in cardiac fibroblast migration [198]. Furthermore, IL-1β exerts potent antiproliferative effects on cardiac fibroblasts [199], altering expression of fibroblast cyclins, cyclin-dependent kinases, and their inhibitors [200], and enhances fibroblast sphingosine kinase activity [201]. In addition to their direct effects on cardiac fibroblasts, proinflammatory cytokines also induce expression and release of a wide variety of mediators that may modulate the fibrotic process. Due to their highly pleiotropic actions, the role of cytokine signaling in mediating fibrous tissue deposition in vivo is likely to be complex and context-dependent. However, it is generally accepted that the activation of pro-inflammatory cytokines in the myocardium promotes matrix-degrading responses that ultimately lead to ventricular dilation.
Transgenic mice with cardiac-specific overexpression of TNF-α developed heart failure [202] associated with increased collagen synthesis, deposition, and denaturation, and significantly enhanced MMP-2 and MMP-9 activity [203]. Enhanced fibrosis in TNF-α overexpressing animals is associated with increased expression of TGF-β isoforms [204] and may involve fibroblast–mast cell interactions [205]. On the other hand, TNF-α null mice exhibited reduced fibrosis and decreased MMP-9 activity in a model of cardiac pressure overload, highlighting the important role of the cytokine in the pathogenesis of cardiac fibrosis and remodeling [206]. The pro-fibrotic effects of TNF signaling in the myocardium appear to be due to interactions involving the type 1 TNF receptor (TNFR1) [207]; in contrast, TNFR2 signaling may reduce fibrosis [208].
The renin–angiotensin–aldosterone system
Extensive evidence implicates neurohormonal pathways in the pathogenesis of cardiac fibrosis. Activation of the renin–angiotensin–aldosterone system (RAAS) is consistently found in fibrotic hearts regardless of etiology. Macrophages and fibroblasts infiltrating the injured heart produce renin and angiotensin-converting enzyme (ACE), molecules necessary for generation of angiotensin II [209]. Locally released angiotensin II serves as a potent stimulant for cardiac fibroblasts, both through direct actions and through TGF-β-mediated effects. In vitro studies have demonstrated that angiotensin II stimulates cardiac fibroblast proliferation and enhances their collagen-synthetic activity through AT1 receptor-dependent interactions [210–212]. In contrast, AT2 signaling may inhibit AT1-mediated actions, suppressing fibroblast proliferation and matrix synthesis [213], and thus serving as a negative regulator of angiotensin II-mediated pro-fibrotic responses [122]. In vivo, extensive evidence supports the pro-fibrotic actions of AT1 signaling. AT1 blockade significantly reduced interstitial fibrosis in models of myocardial infarction [214] and fibrosis due to cardiac pressure overload [215]. The marked beneficial effects of ACE inhibition and AT1 blockade in patients with chronic heart failure or acute myocardial infarction may be due, at least in part, to inhibition of angiotensin-induced fibrogenic actions.
Aldosterone is also capable of inducing fibrotic changes in the myocardium [216], as suggested by experimental animal studies and by the development of reactive myocardial fibrosis in patients with adrenal adenomas [217]. Several distinct mechanisms may mediate the pro-fibrotic actions of aldosterone in the heart. First, aldosterone may act by promoting pro-inflammatory effects on vascular cells, thus accentuating expression of cytokines and chemokines [68]. Second, aldosterone may drive macrophages towards a fibrogenic phenotype [69]. Third, aldosterone may activate cardiomyocyte-derived fibrogenic signals [120]. Fourth, aldosterone may exert direct effects on fibroblasts, stimulating proliferation [218] and increasing collagen synthesis [219]. Whether the beneficial effects of aldosterone antagonism in patients with heart failure [220] are, at least in part, due to anti-fibrotic actions remains unknown.
TGF-β
Perhaps the best-characterized fibrogenic growth factor [221], TGF-β is markedly and consistently activated in experimental models of cardiac fibrosis [222] and in fibrotic human hearts [223, 224]. In mammals, TGF-β is found in three isoforms (TGF-β1, 2, and 3), encoded by three distinct genes. [225]. Although the three TGF-β isoforms signal through the same cell surface receptors and share common cellular targets, they exhibit distinct patterns of expression. TGF-β1 is the predominant isoform in the cardiovascular system and is ubiquitously expressed, whereas the other isoforms are found in a more limited spectrum of cells and tissues. Although the three isoforms likely have distinct in vivo functions, most of our knowledge on their role in cardiac fibrosis is limited to TGF-β1.
TGF-β1 is present in the normal heart as a latent complex, unable to associate with its receptors. Following cardiac injury, the extracellular concentration of TGF-β activity is regulated primarily through conversion of the latent to the active form; activation of a relatively small amount of latent TGF-β is sufficient to induce a maximal cellular response [226]. A wide range of mediators have been described as TGF-β “activators” and play a role at different stages of the activation process. Proteases, such as plasmin, MMP-2, and MMP-9, are capable of activating TGF-β, thus linking matrix degradation with activation of a molecule that preserves matrix integrity and stability [226–228]. The matricellular protein TSP-1 is a key TGF-β activator with an important role in cardiac remodeling. ROS generation [229] and a mildly acidic environment [230] can also trigger TGF-β activation. The specific signals responsible for TGF-β activation in the fibrotic heart may be in part dependent on the type and intensity of the initial cardiac injury.
Pro-fibrotic actions of TGF-β have been suggested by generation of mice with myocardial overexpression of TGF-β1. Rosenkranz and co-workers [231, 232] demonstrated that cardiac TGF-β1 overexpression induced ventricular fibrosis associated with accentuated collagen deposition and inhibition of interstitial collagenases. On the other hand, Nakajima and co-workers [233] showed that transgenic mice with a large proportion of constitutively active TGF-β1 in the heart (due to a mutation that blocks covalent tethering of the TGF-β1 latent complex to the extracellular matrix) exhibited only atrial and not ventricular fibrosis [233]. Loss-of-function approaches using several distinct experimental models of cardiac fibrosis suggested the involvement of TGF-β in fibrotic ventricular remodeling. Heterozygous TGF-β1±-deficient mice exhibited attenuated age-associated fibrosis and improved left ventricular compliance when compared to control wild-type animals [234]. Moreover, TGF-β blockade prevented myocardial fibrosis in a rat model of cardiac pressure overload [235].
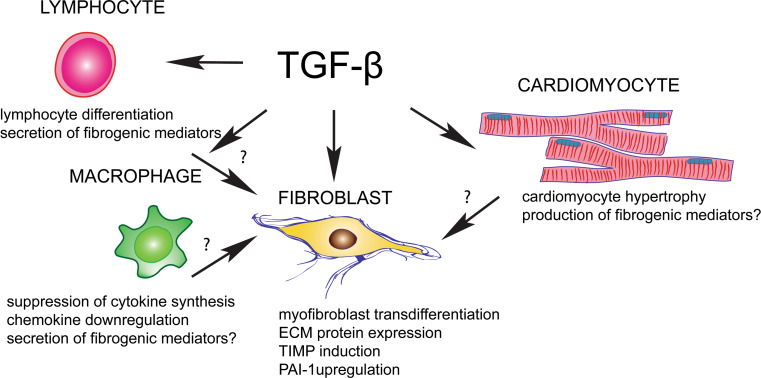
The profound and consistent activating effects of TGF-β on cardiac fibroblasts may provide the cell biological basis for TGF-β-induced cardiac fibrosis (Fig. 5) [236]; TGF-β-mediated actions on other cell types (such as macrophages, lymphocytes, and cardiomyocytes) may also contribute to the fibrotic response in a paracrine manner. TGF-β stimulation induces myofibroblast transdifferentiation [237] and enhances extracellular matrix protein synthesis. Moreover, the matrix-preserving effects of TGF-β are due to induction of expression of protease inhibitors, such as plasminogen activator inhibitor (PAI)-1 and TIMPs [225]. TGF-β-mediated CCN2 induction may also contribute to its fibrogenic actions. The combined action of TGF-β1 and CCN2 may be responsible for a sustained fibrotic response [238].
Fig. 5.
Cellular effects of TGF-β in cardiac fibrosis. TGF-β is a key fibrogenic mediator that may affect all cell types involved in the cardiac fibrotic response
Which TGF-dependent signaling pathways are involved in the pathogenesis of cardiac fibrosis? Once released and activated, TGF-β binds to the constitutively active type II receptor (TβRII) on the cell surface, then the resulting complex transphosphorylates the cytoplasmic domain of the type I receptor (TβRI). Subsequently, TGF-β signals through downstream intracellular signals, the Smads, or by activating Smad-independent pathways. Experiments from our laboratory have demonstrated that Smad3 signaling plays an essential role in fibrotic remodeling of the infarcted ventricle [239]. The anti-fibrotic effects of Smad3 deficiency were not due to reduced fibroblast infiltration into the infarct or to perturbed myofibroblast differentiation. In fact, myofibroblast density in the infarcted myocardium was significantly higher in Smad3 null infarcts, a finding that may be due to Smad3-dependent antiproliferative actions of TGF-β on cardiac fibroblasts. However, TGF-β-mediated induction of extracellular matrix proteins (such as collagen and tenascin-C) in cardiac fibroblasts was dependent on Smad3, suggesting that decreased fibrotic remodeling in infarcted Smad3 null hearts may be due to abrogation of the pro-fibrotic TGF-β responses. Although the role of Smad-independent pathways in fibrotic cardiac remodeling has not been systematically studied, development of transgenic mice with an activating mutation of the TGF-β activated kinase (TAK)-1 expressed in myocardium was sufficient to produce marked cardiac hypertrophy and interstitial fibrosis, suggesting that TGF-β/TAK-1 signaling may exert profibrotic actions.
Negative regulation of TGF-β signaling may play an important role in restraining cardiac fibrosis. Recently published studies have suggested two potential mechanisms that may inhibit fibrogenic TGF-β actions in the infarcted or failing myocardium. Cleavage and release of a soluble form of endoglin may limit and inhibit TGF-β signaling in heart failure [240]. Moreover, expression of the TGF-β pseudo-receptor BAMBI (bone morphogenetic protein/BMP and activin membrane-bound inhibitor) following pressure overload may downmodulate TGF-β signaling, attenuating its profibrotic actions [241]. Because prevention of uncontrolled TGF-β responses in the myocardium may be crucial to maintain structure and function, multiple distinct pathways may co-operate for negative regulation of the TGF-β system [242].
Endothelin (ET)-1
Both in vitro and in vivo studies suggest that ET-1 is a potent fibrogenic mediator that may act downstream of TGF-β and angiotensin [166], and may serve as a link between inflammation and fibrosis [243]. Both TGF-β and angiotensin are capable of inducing ET-1 in various cell types [244]; ET-1 is known to be secreted in failing human hearts [245] and is upregulated in experimental models of hypertensive cardiac fibrosis [246]. In vitro, ET-1 enhances cardiac fibroblast proliferation [247], promotes matrix protein synthesis, decreases collagenase activity [248], and induces an apoptosis-resistant fibroblast phenotype [249]. In vivo, cardiac specific overexpression of ET-1 induced myocardial fibrosis associated with biventricular systolic and diastolic dysfunction [250]. Moreover, endothelin antagonism attenuated fibrotic myocardial remodeling in animal models of hypertensive and infarctive cardiac fibrosis [251, 252].
Platelet-derived growth factor
The platelet-derived growth factor (PDGF) family is comprised of homo- or hetero-dimeric growth factors (including PDGF-AA, -BB, AB, CC, and DD) that signal through two different receptors: PDGFR-α and PDGFR-β. PDGF isoform and receptor expression is upregulated in fibrotic cardiac conditions [253]; however, because of the pleiotopic effects of PDGF signaling, its role in mediating the cardiac fibrotic response remains poorly understood. In vitro, PDGF-AA potently stimulates cardiac fibroblast proliferation and matrix synthesis [254]. In vivo, PDGFR-α and PDGFR-β neutralization reduced collagen deposition in healing myocardial infarcts [253]; however, PDGFR-β inhibition also prevented mural cell recruitment by infarct neovessels suppressing vascular maturation [253]. In a model of chronic allograft rejection, adenoviral-mediated delivery of PDGF-A, -C, and -D, but not PDGF-B, accelerated cardiac fibrosis, enhancing TGF-β expression [255]. In pressure-overloaded hearts, PDGFR-α neutralization attenuated atrial fibrosis and reduced the incidence of atrial fibrillation [81].
Pathophysiologic conditions associated with cardiac fibrosis
Although pathogenetic mechanisms in various cardiac fibrotic conditions share common cellular effectors and molecular pathways, the relative contribution of each pathway is often dependent on the underlying cause of fibrotic remodeling. The distinct cellular alterations that occur with each type of injury have important pathophysiologic and therapeutic implications:
Replacement fibrosis in myocardial infarction is dependent on an inflammatory reaction
Because the adult mammalian heart has negligible regenerative capacity, the death of a large number of cardiomyocytes following myocardial infarction results in their replacement with fibrous tissue. The cellular response to infarction can be divided in three overlapping phases: the inflammatory, the proliferative, and the maturation phase. Although this clearly represents an oversimplification, it is a useful approach to better understand the process of reparative fibrosis. Cardiomyocyte death rapidly activates innate immune pathways that trigger cytokine, chemokine, and adhesion molecule expression initiating the inflammatory phase and leading to infiltration of the infarct with leukocytes. As the wound is cleared of dead cells, inflammatory leukocytes become apoptotic, and activation of a inhibitory mediators suppresses the inflammatory reaction leading to transition to the proliferative phase. Differentiation and activation of macrophages may stimulate transdifferentiation of myofibroblasts, the key effector cells in scar formation and the main source of extracellular matrix proteins in the healing infarct. At the same time, activation of angiogenic pathways results in the formation of a rich microvascular network in the healing infarct, necessary to provide oxygen and nutrients to the reparative cells. The end of the proliferative phase may be associated with activation of anti-fibrotic signals, in order to prevent uncontrolled fibrosis. Transition to the maturation phase follows, as extracellular matrix proteins in the infarct are cross-linked, while most fibroblasts and vascular cells in the scar undergo apoptosis. Activation of fibroblasts and inflammatory cells may persist in the infarct border zone and in the remote remodeling myocardium, as pressure and volume loads may provide stimulatory signals [256–258].
The extracellular matrix during the inflammatory phase of infarct healing
During the inflammatory phase of reparative fibrosis, stimulation of cytokine and chemokine signaling enhances protease expression and activity, leading to extensive degradation of the cardiac matrix [259, 260]. Matrix alterations in the early stages of replacement fibrosis are characterized by two major events. First, cardiac extracellular matrix constituents exhibit extensive fragmentation that is not limited to fibrillar collagen but also involves glycosaminoglycans (such as hyaluronan). Generation of low molecular weight hyaluronan fragments in the infarct may exert pro-inflammatory actions and activate fibroblasts through CD44-mediated pathways [131, 261]. Second, a highly dynamic provisional matrix is formed, comprised of fibrin and fibronectin, and creates a scaffold for infiltration, migration, and proliferation of leukocytes and mesenchymal cells, thus facilitating the reparative process [115, 131]. Migrating cells use integrin receptors to interact with fibrin and fibronectin within the matrix network; integrin-mediated interactions may also provide signals that modulate macrophage and fibroblast phenotypes and stimulate gene expression [258, 262].
Matrix:fibroblast interactions during the proliferative phase of cardiac repair
The transition from the inflammatory to the proliferative phase is associated with activation of “stop signals” that inhibit inflammation while promoting fibrous tissue deposition and angiogenesis. Induction of interleukin receptor-associated kinase (IRAK)-M in infarct macrophages suppresses their inflammatory activity and serves as one of many inhibitory pathways involved in restraining and containment of post-infarction inflammation [263]. During the proliferative phase of the reparative response, both secreted mediators and matrix-derived signals stimulate activation of myofibroblasts in the healing infarct. As the plasma-derived provisional matrix is cleared by the plasminogen system [264], cellular fibronectin is produced by macrophages and fibroblasts and serves as a “second order” provisional matrix [265]. The ED-A isoform of fibronectin co-operates with TGF-β to induce myofibroblast transdifferentiation [27, 48, 266]. Locally generated angiotensin II, PDGF, TGF-β, and mast cell-derived chymase and tryptase, activate fibroblasts, enhancing their matrix synthetic capacity. Spatially-restricted deposition of matricellular proteins in the infarct border zone results in fine regulation of growth factor responses, preventing inappropriate expansion of the inflammatory and fibrotic processes.
Formation of a mature scar
As the scar matures, increased expression of lysyl-oxidase induces cross-linking of the matrix in the infarcted myocardium [267]. Matricellular proteins are cleared and the mature scar, comprised of dense cross-linked collagen, enhances tensile strength of the infarct while increasing passive stiffness and contributing to diastolic dysfunction [268]. In the mature scar, deprivation of growth factors, stress-shielding, and removal of matricellular proteins result in apoptotic death of most myofibroblasts and vascular cells in the infarct [253, 269]. Although myofibroblasts are cleared from the mature scar, in the viable remodeling myocardium, fibroblasts may exhibit persistent activation due to increased hemodynamic loading. These chronic fibrotic changes may play an important role in the pathogenesis of chronic cardiac remodeling and heart failure.
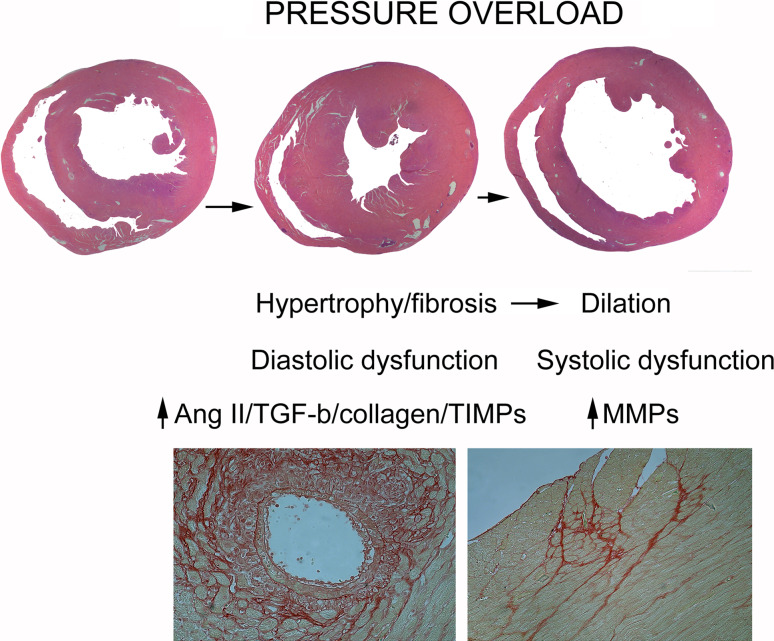
Fibrotic remodeling of the pressure-overloaded heart
Pressure overload is a common pathophysiologic condition in cardiac remodeling and plays a dominant role in the pathogenesis of fibrotic cardiomyopathy in patients with hypertension and aortic stenosis. In animal models, pressure overload induces early hypertrophy, fibrosis, and diastolic dysfunction, followed by decompensation, dilative cardiomyopathy, and the development of systolic dysfunction (Fig. 6) [270]. Induction of inflammatory mediators is consistently observed in experimental models of pressure overload cardiomyopathy; however, the intensity of the inflammatory reaction is much lower than in infarcted hearts [270, 271]. The basis for initiation of the inflammatory reaction in the pressure-overloaded myocardium remains poorly understood and may involve activation of innate immune signals due to cardiomyocyte death, reactive oxygen generation, or angiotensin-mediated pro-inflammatory actions. Both chemokines (such as MCP-1/CCL2) and cytokines (such as TNF-α) have been implicated in the pathogenesis of pressure overload-induced myocardial fibrosis [67, 206], Pro-inflammatory signals may promote fibrosis by inducing recruitment of fibrogenic monocytes subsets and fibroblast progenitors, by driving macrophages towards a fibrogenic phenotype, and by activating resident cardiac fibroblasts. Early alterations in the cardiac interstitial matrix may play an important role in the transition from inflammation to fibrosis. Upregulation of matricellular proteins (including periostin, OPN, SPARC, TSP-1, and -2) in the pressure-overloaded heart is well documented and contributes to activation of growth factor signaling responses [129, 272].
Fig. 6.
Remodeling and fibrosis in the pressure-overloaded heart. Animal models of cardiac pressure overload exhibit rapid development of concentric myocardial hypertrophy and fibrosis associated with diastolic dysfunction, followed by chamber dilation and systolic dysfunction. The protease/antiprotease balance plays an important role in remodeling of the pressure overloaded heart. During the early stages of the response to pressure overload, angiotensin II and TGF-β may promote matrix preservation, stimulating collagen and TIMP synthesis. Although the events associated with decompensation are poorly understood, increased MMP synthesis may be involved in transition to the dilative phase. Lower panel The histopathological images show Sirius red staining in a mouse model of transverse aortic constriction to illustrate the typical alterations of the cardiac interstitium in the pressure-overloaded heart: perivascular (left) and interstitial (right) fibrosis
Generation of angiotensin II and activation of TGF-β are central events in the fibrotic response in the pressure-overloaded myocardium and are capable of modulating phenotype and function of all cells involved in the fibrotic process. Although both angiotensin and TGF-β are potent activators of fibroblasts, whether their effects in cardiac fibrosis are mediated directly through fibroblast-specific actions remains unknown. Angiotensin- or TGF-β-mediated effects on macrophages and cardiomyocytes may also contribute to activation of fibrogenic signaling through paracrine mechanisms.
Because late development of cardiac dilation in the pressure-overloaded ventricle marks progression to decompensated heart failure and systolic dysfunction, the molecular signals involved in transition from the hypertrophic/pro-fibrotic stage to dilative remodeling are of outstanding interest. MMP activation has been reported in dilated pressure-overloaded hearts [20], and may be in part responsible for chamber dilation. Thus, in the pressure-overloaded heart, geometry of the ventricle may depend on the balance between matrix-degrading and matrix-preserving signals.
Volume overload is associated with extensive matrix degradation
Volume overload is the predominant pathophysiologic condition in patients with valvular regurgitant lesions and results in chamber dilation and development of systolic dysfunction. Matrix metabolism appears to play a key role in remodeling of the volume-overloaded heart. In contrast to the increase in collagen content observed in pressure-overloaded hearts, volume overload is associated with marked loss of interstitial collagen [273], associated with induction of MMP-2, -9, and -3 [273, 274]. The basis for volume overload-induced protease upregulation remains unknown. In experimental models of volume overload, a marked increase in cardiac mast cell numbers has been implicated in the pathogenesis of dilative remodeling [74, 275]. As important sources of MMPs and pro-inflammatory cytokines, mast cells may induce degradation of extracellular matrix proteins driving a dilative response and promoting systolic dysfunction. Mast cell numbers are also increased in fibrotic conditions associated with increased matrix deposition [33]. Whether volume overload drives mast cell phenotype towards a matrix-degrading direction remains unknown. Moreover, effects of volume overload on other cell types implicated in matrix remodeling (such as fibroblasts and macrophages) have not been systematically studied.
The fibrotic changes in the aging heart may contribute to the development of diastolic dysfunction
Aging is associated with cardiac fibrosis; in the absence of other conditions, normal aging does not induce systolic dysfunction but promotes matrix deposition in the cardiac interstitium and progressively increases ventricular stiffness [276]. Descriptive studies have suggested that aging-associated fibrosis may be due to reduced matrix degradation, rather than to increased collagen synthesis [277]. ROS generation, AT1 signaling, activation of TGF-β-mediated pathways, and induction of inflammatory chemokines have been implicated in the pathogenesis of age-associated cardiac fibrosis [278]. However, most of the evidence is derived from associative studies; investigations documenting the significance of specific pathways are lacking. Although aging is associated with a basal increase in myocardial collagen deposition, in reparative responses, senescent animals exhibit suppressed collagen synthetic capacity and impaired scar formation [279]. The age-related defects in cardiac repair appear to be mediated, at least in part, through reduced responsiveness of senescent fibroblasts to growth factors [279].
Targeting cardiac fibrosis
Because fibrotic cardiac remodeling is associated with both systolic and diastolic dysfunction, prevention and reversal of cardiac fibrosis is an important goal for cardiovascular researchers and clinicians. Identification of therapeutic targets will require understanding of the mechanistic basis of the fibrotic disease; however, the effectiveness of anti-fibrotic strategies will likely depend on the underlying etiology and the severity and extent of disease. In the presence of pro-fibrotic pathophysiologic conditions (such as pressure or volume overload), protection of the myocardium from fibrosis is best achieved by treating the underlying pathophysiologic process. For example, anti-hypertensive treatment, or valve surgery, would be the ideal strategies for myocardial protection in patients with hypertension or valvular disease, respectively.
Whether established cardiac fibrosis is reversible depends on the etiology and extent of disease, the age of the fibrotic lesions, and the amount of protease-resistant cross-linked matrix. In an experimental model of fibrotic interstitial cardiomyopathy due to brief repetitive ischemia/reperfusion, discontinuation of the ischemia protocol resulted in reversal of fibrosis [177]. Both experimental and clinical studies have suggested that hypertensive fibrosis is reversible upon treatment with ACE inhibitors. Lisinopril induced regression of fibrosis in spontaneously hypertensive rats with advanced fibrotic cardiomyopathy [280]. Moreover, in a small clinical study, 35 patients with hypertension, left ventricular hypertrophy, and diastolic dysfunction had significant regression of fibrosis (assessed through endomyocardial biopsy) after a 6-month course of lisinopril. Attenuated fibrosis was associated with improved diastolic function [281].
Reversibility of cardiac fibrosis has also been documented in experimental models of genetic cardiomyopathy. In a mouse model of calcineurin-dependent cardiomyopathy, fibrosis was in part reversed when calcineurin was turned off [282]. In a rabbit model of hypertrophic cardiomyopathy, statin therapy induced regression of cardiac fibrosis and hypertrophy [283]. Moreover, AT1 blockade with losartan reversed fibrosis and attenuated TGF-β expression in a transgenic mouse model of human hypertrophic cardiomyopathy [284]. Clearly, established fibrotic lesions due to replacement of a large amount of myocardium are less likely to be reversible. Regression of myocardial scars due to large infarcts would require extensive myocardial regeneration, a major visionary goal of modern cardiovascular research. Significant regression may be impossible even with smaller, but established and cross-linked, fibrotic lesions. Patients with severe aortic stenosis showed no reversal of myocardial fibrosis 9 months after aortic valve replacement [285].
Although regression of fibrosis has been documented in several cardiac conditions, the mechanisms responsible for the reversal of fibrotic disease remain unknown. Clearance of collagen and other matrix proteins from the fibrotic heart likely requires activation of proteases. Whether specific subpopulations of “anti-fibrotic” macrophages and lymphocytes are involved in driving the resolution of fibrotic lesions remains unknown. Moreover, the functional characteristics and molecular profile associated with a pro-regression phenotype in cardiac fibroblasts have not been investigated.
Acknowledgments
Supported by NIH grants R01 HL76246 and R01 HL85440, and the Wilf Family Cardiovascular Research Institute.
References
- 1.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borer JS, Truter S, Herrold EM, Falcone DJ, Pena M, Carter JN, Dumlao TF, Lee JA, Supino PG. Myocardial fibrosis in chronic aortic regurgitation: molecular and cellular responses to volume overload. Circulation. 2002;105:1837–1842. doi: 10.1161/01.CIR.0000014419.71706.85. [DOI] [PubMed] [Google Scholar]
- 4.Ashrafian H, McKenna WJ, Watkins H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res. 2011;109:86–96. doi: 10.1161/CIRCRESAHA.111.242974. [DOI] [PubMed] [Google Scholar]
- 5.Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med. 2009;19:247–252. doi: 10.1016/j.tcm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc Pathol. 2010;19:308–311. doi: 10.1016/j.carpath.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 8.Bharati S, Lev M. Cardiac conduction system involvement in sudden death of obese young people. Am Heart J. 1995;129:273–281. doi: 10.1016/0002-8703(95)90008-X. [DOI] [PubMed] [Google Scholar]
- 9.Leonard BL, Smaill BH, LeGrice IJ. Structural remodeling and mechanical function in heart failure. Microsc Microanal. 2012;18:50–67. doi: 10.1017/S1431927611012438. [DOI] [PubMed] [Google Scholar]
- 10.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 11.Shirwany A, Weber KT. Extracellular matrix remodeling in hypertensive heart disease. J Am Coll Cardiol. 2006;48:97–98. doi: 10.1016/j.jacc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 13.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eghbali M, Blumenfeld OO, Seifter S, Buttrick PM, Leinwand LA, Robinson TF, Zern MA, Giambrone MA. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989;21:103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 16.Gersch C, Dewald O, Zoerlein M, Michael LH, Entman ML, Frangogiannis NG. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol. 2002;118:41–49. doi: 10.1007/s00418-002-0425-z. [DOI] [PubMed] [Google Scholar]
- 17.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 18.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 19.Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail. 2002;8:S319–S325. doi: 10.1054/jcaf.2002.129260. [DOI] [PubMed] [Google Scholar]
- 20.Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002;39:1384–1391. doi: 10.1016/S0735-1097(02)01756-4. [DOI] [PubMed] [Google Scholar]
- 21.Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284:H122–H132. doi: 10.1152/ajpheart.00233.2002. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Hoshijima M, Lam J, Zhou Z, Jokiel A, Dalton ND, Hultenby K, Ruiz-Lozano P, Ross J, Jr, Tryggvason K, Chien KR. Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J Biol Chem. 2006;281:213–220. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- 23.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.CIR.89.1.151. [DOI] [PubMed] [Google Scholar]
- 24.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 26.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie KO, Taatjes DJ, Schwarz J, vonTurkovich M, Low RB. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol. 1991;139:207–216. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–H1881. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 31.Szardien S, Nef HM, Troidl C, Willmer M, Voss S, Liebetrau C, Hoffmann J, Rolf A, Rixe J, Elsasser A, et al. Bone marrow-derived cells contribute to cell turnover in aging murine hearts. Int J Mol Med. 2012;30:283–287. doi: 10.3892/ijmm.2012.995. [DOI] [PubMed] [Google Scholar]
- 32.Law BA, Levick SP, Carver WE. Alterations in cardiac structure and function in a murine model of chronic alcohol consumption. Microsc Microanal. 2012;18:453–461. doi: 10.1017/S1431927612000372. [DOI] [PubMed] [Google Scholar]
- 33.Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687–698. doi: 10.1161/01.CIR.98.7.687. [DOI] [PubMed] [Google Scholar]
- 34.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000;48:89–100. doi: 10.1016/S0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 35.Pichler M, Rainer PP, Schauer S, Hoefler G. Cardiac fibrosis in human transplanted hearts is mainly driven by cells of intracardiac origin. J Am Coll Cardiol. 2012;59:1008–1016. doi: 10.1016/j.jacc.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 36.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 37.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 40.Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, Kaye DM. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol. 2010;176:1735–1742. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol. 2005;14:241–246. doi: 10.1016/j.carpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–595. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- 43.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–747. [PubMed] [Google Scholar]
- 44.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 45.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS, Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest. 1992;89:1060–1068. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 48.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, Folkesson HG, Horne WI, Doane KJ, Meszaros JG. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;290:H323–H330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- 50.Carracedo S, Lu N, Popova SN, Jonsson R, Eckes B, Gullberg D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem. 2010;285:10434–10445. doi: 10.1074/jbc.M109.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA. α11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res. 2012;96:265–275. doi: 10.1093/cvr/cvs259. [DOI] [PubMed] [Google Scholar]
- 52.Herum KM, Lunde IG, Skrbic B, Florholmen G, Behmen D, Sjaastad I, Carlson CR, Gomez MF, Christensen G. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J Mol Cell Cardiol. 2013;54:73–81. doi: 10.1016/j.yjmcc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Matsui Y, Ikesue M, Danzaki K, Morimoto J, Sato M, Tanaka S, Kojima T, Tsutsui H, Uede T. Syndecan-4 prevents cardiac rupture and dysfunction after myocardial infarction. Circ Res. 2011;108:1328–1339. doi: 10.1161/CIRCRESAHA.110.235689. [DOI] [PubMed] [Google Scholar]
- 54.Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- 55.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frangogiannis NG, Mendoza LH, Ren G, Akrivakis S, Jackson PL, Michael LH, Smith CW, Entman ML. MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H483–H492. doi: 10.1152/ajpheart.01016.2002. [DOI] [PubMed] [Google Scholar]
- 57.Frangogiannis NG, Shimoni S, Chang SM, Ren G, Shan K, Aggeli C, Reardon MJ, Letsou GV, Espada R, Ramchandani M, et al. Evidence for an active inflammatory process in the hibernating human myocardium. Am J Pathol. 2002;160:1425–1433. doi: 10.1016/S0002-9440(10)62568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 59.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 60.Behr TM, Wang X, Aiyar N, Coatney RW, Li X, Koster P, Angermann CE, Ohlstein E, Feuerstein GZ, Winaver J. Monocyte chemoattractant protein-1 is upregulated in rats with volume-overload congestive heart failure. Circulation. 2000;102:1315–1322. doi: 10.1161/01.CIR.102.11.1315. [DOI] [PubMed] [Google Scholar]
- 61.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blyszczuk P, Berthonneche C, Behnke S, Glonkler M, Moch H, Pedrazzini T, Luscher TF, Eriksson U, Kania G. Nitric oxide synthase 2 is required for conversion of pro-fibrogenic inflammatory CD133+ progenitors into F4/80+ macrophages in experimental autoimmune myocarditis. Cardiovasc Res. 2013;97(2):219–229. doi: 10.1093/cvr/cvs317. [DOI] [PubMed] [Google Scholar]
- 63.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 65.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Yang M, Zheng J, Miao Y, Wang Y, Cui W, Guo J, Qiu S, Han Y, Jia L, Li H, et al. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32:1675–1686. doi: 10.1161/ATVBAHA.112.248732. [DOI] [PubMed] [Google Scholar]
- 67.Tokuda K, Kai H, Kuwahara F, Yasukawa H, Tahara N, Kudo H, Takemiya K, Koga M, Yamamoto T, Imaizumi T. Pressure-independent effects of angiotensin II on hypertensive myocardial fibrosis. Hypertension. 2004;43:499–503. doi: 10.1161/01.HYP.0000111831.50834.93. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 70.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, Lumeng CN, Mortensen RM. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, Iredale JP. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 72.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levi-Schaffer F, Rubinchik E. Mast cell/fibroblast interactions. Clin Exp Allergy. 1994;24:1016–1021. doi: 10.1111/j.1365-2222.1994.tb02737.x. [DOI] [PubMed] [Google Scholar]
- 74.Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2012;89:12–19. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sperr WR, Bankl HC, Mundigler G, Klappacher G, Grossschmidt K, Agis H, Simon P, Laufer P, Imhof M, Radaszkiewicz T, et al. The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood. 1994;84:3876–3884. [PubMed] [Google Scholar]
- 76.Somasundaram P, Ren G, Nagar H, Kraemer D, Mendoza L, Michael LH, Caughey GH, Entman ML, Frangogiannis NG. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol. 2005;205:102–111. doi: 10.1002/path.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patella V, Marino I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, Marone G. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–978. doi: 10.1161/01.CIR.97.10.971. [DOI] [PubMed] [Google Scholar]
- 78.Shiota N, Rysa J, Kovanen PT, Ruskoaho H, Kokkonen JO, Lindstedt KA. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens. 2003;21:1935–1944. doi: 10.1097/00004872-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 79.Wei CC, Lucchesi PA, Tallaj J, Bradley WE, Powell PC, Dell’Italia LJ. Cardiac interstitial bradykinin and mast cells modulate pattern of LV remodeling in volume overload in rats. Am J Physiol Heart Circ Physiol. 2003;285:H784–H792. doi: 10.1152/ajpheart.00793.2001. [DOI] [PubMed] [Google Scholar]
- 80.Hara M, Ono K, Hwang MW, Iwasaki A, Okada M, Nakatani K, Sasayama S, Matsumori A. Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med. 2002;195:375–381. doi: 10.1084/jem.20002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liao CH, Akazawa H, Tamagawa M, Ito K, Yasuda N, Kudo Y, Yamamoto R, Ozasa Y, Fujimoto M, Wang P, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120:242–253. doi: 10.1172/JCI39942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 83.Zhang W, Chancey AL, Tzeng HP, Zhou Z, Lavine KJ, Gao F, Sivasubramanian N, Barger PM, Mann DL. The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions. Circulation. 2012;124:2106–2116. doi: 10.1161/CIRCULATIONAHA.111.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Card Fail. 2005;11:548–556. doi: 10.1016/j.cardfail.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Kolck UW, Alfter K, Homann J, Kugelgen I, Molderings GJ. Cardiac mast cells: implications for heart failure. J Am Coll Cardiol. 2007;49:1107–1108. doi: 10.1016/j.jacc.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 86.de Almeida A, Mustin D, Forman MF, Brower GL, Janicki JS, Carver W. Effects of mast cells on the behavior of isolated heart fibroblasts: modulation of collagen remodeling and gene expression. J Cell Physiol. 2002;191:51–59. doi: 10.1002/jcp.10071. [DOI] [PubMed] [Google Scholar]
- 87.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.CIR.98.7.699. [DOI] [PubMed] [Google Scholar]
- 88.Kanellakis P, Ditiatkovski M, Kostolias G, Bobik A. A pro-fibrotic role for interleukin-4 in cardiac pressure overload. Cardiovasc Res. 2012;95:77–85. doi: 10.1093/cvr/cvs142. [DOI] [PubMed] [Google Scholar]
- 89.Jordana M, Befus AD, Newhouse MT, Bienenstock J, Gauldie J. Effect of histamine on proliferation of normal human adult lung fibroblasts. Thorax. 1988;43:552–558. doi: 10.1136/thx.43.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hatamochi A, Fujiwara K, Ueki H. Effects of histamine on collagen synthesis by cultured fibroblasts derived from guinea pig skin. Arch Dermatol Res. 1985;277:60–64. doi: 10.1007/BF00406482. [DOI] [PubMed] [Google Scholar]
- 91.Kunzmann S, Schmidt-Weber C, Zingg JM, Azzi A, Kramer BW, Blaser K, Akdis CA, Speer CP. Connective tissue growth factor expression is regulated by histamine in lung fibroblasts: potential role of histamine in airway remodeling. J Allergy Clin Immunol. 2007;119:1398–1407. doi: 10.1016/j.jaci.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 92.Kim J, Ogai A, Nakatani S, Hashimura K, Kanzaki H, Komamura K, Asakura M, Asanuma H, Kitamura S, Tomoike H, Kitakaze M. Impact of blockade of histamine H2 receptors on chronic heart failure revealed by retrospective and prospective randomized studies. J Am Coll Cardiol. 2006;48:1378–1384. doi: 10.1016/j.jacc.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 93.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 94.Zhao XY, Zhao LY, Zheng QS, Su JL, Guan H, Shang FJ, Niu XL, He YP, Lu XL. Chymase induces profibrotic response via transforming growth factor-beta 1/Smad activation in rat cardiac fibroblasts. Mol Cell Biochem. 2008;310:159–166. doi: 10.1007/s11010-007-9676-2. [DOI] [PubMed] [Google Scholar]
- 95.Urata H, Healy B, Stewart RW, Bumpus FM, Husain A. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res. 1990;66:883–890. doi: 10.1161/01.RES.66.4.883. [DOI] [PubMed] [Google Scholar]
- 96.Fang KC, Raymond WW, Blount JL, Caughey GH. Dog mast cell alpha-chymase activates progelatinase B by cleaving the Phe88-Gln89 and Phe91-Glu92 bonds of the catalytic domain. J Biol Chem. 1997;272:25628–25635. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- 97.Stewart JA, Jr, Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell’Italia LJ. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003;35:311–319. doi: 10.1016/S0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 98.Matsumoto T, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in the progression of heart failure. Circulation. 2003;107:2555–2558. doi: 10.1161/01.CIR.0000074041.81728.79. [DOI] [PubMed] [Google Scholar]
- 99.Oyamada S, Bianchi C, Takai S, Chu LM, Sellke FW. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. J Pharmacol Exp Ther. 2011;339:143–151. doi: 10.1124/jpet.111.179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanemitsu H, Takai S, Tsuneyoshi H, Nishina T, Yoshikawa K, Miyazaki M, Ikeda T, Komeda M. Chymase inhibition prevents cardiac fibrosis and dysfunction after myocardial infarction in rats. Hypertens Res. 2006;29:57–64. doi: 10.1291/hypres.29.57. [DOI] [PubMed] [Google Scholar]
- 101.Ruoss SJ, Hartmann T, Caughey GH. Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest. 1991;88:493–499. doi: 10.1172/JCI115330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997;99:1313–1321. doi: 10.1172/JCI119290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McLarty JL, Melendez GC, Brower GL, Janicki JS, Levick SP. Tryptase/Protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. 2011;58:264–270. doi: 10.1161/HYPERTENSIONAHA.111.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Joseph J, Kennedy RH, Devi S, Wang J, Joseph L, Hauer-Jensen M. Protective role of mast cells in homocysteine-induced cardiac remodeling. Am J Physiol Heart Circ Physiol. 2005;288:H2541–H2545. doi: 10.1152/ajpheart.00806.2004. [DOI] [PubMed] [Google Scholar]
- 105.Barron L, Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastrointest Liver Physiol. 2011;300:G723–G728. doi: 10.1152/ajpgi.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol. 2011;50:248–256. doi: 10.1016/j.yjmcc.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 110.Kanellakis P, Dinh TN, Agrotis A, Bobik A. CD4(+)CD25(+)Foxp3(+) regulatory T cells suppress cardiac fibrosis in the hypertensive heart. J Hypertens. 2011;29:1820–1828. doi: 10.1097/HJH.0b013e328349c62d. [DOI] [PubMed] [Google Scholar]
- 111.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 112.Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 113.Adiarto S, Heiden S, Vignon-Zellweger N, Nakayama K, Yagi K, Yanagisawa M, Emoto N. ET-1 from endothelial cells is required for complete angiotensin II-induced cardiac fibrosis and hypertrophy. Life Sci. 2012;91:651–657. doi: 10.1016/j.lfs.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 114.Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y, Suzuki T, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–2418. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 115.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 2001;15:1428–1430. doi: 10.1096/fj.00-0745fje. [DOI] [PubMed] [Google Scholar]
- 116.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wei H, Bedja D, Koitabashi N, Xing D, Chen J, Fox-Talbot K, Rouf R, Chen S, Steenbergen C, Harmon JW, et al. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-beta signaling. Proc Natl Acad Sci USA. 2012;109:E841–E850. doi: 10.1073/pnas.1202081109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Duffy HS. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol. 2012;303:H1208–H1218. doi: 10.1152/ajpheart.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rickard AJ, Morgan J, Bienvenu LA, Fletcher EK, Cranston GA, Shen JZ, Reichelt ME, Delbridge LM, Young MJ. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension. 2012;60:1443–1450. doi: 10.1161/HYPERTENSIONAHA.112.203158. [DOI] [PubMed] [Google Scholar]
- 121.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kurisu S, Ozono R, Oshima T, Kambe M, Ishida T, Sugino H, Matsuura H, Chayama K, Teranishi Y, Iba O, et al. Cardiac angiotensin II type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension. 2003;41:99–107. doi: 10.1161/01.HYP.0000050101.90932.14. [DOI] [PubMed] [Google Scholar]
- 123.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 124.Mukherjee D, Sen S. Alteration of cardiac collagen phenotypes in hypertensive hypertrophy: role of blood pressure. J Mol Cell Cardiol. 1993;25:185–196. doi: 10.1006/jmcc.1993.1021. [DOI] [PubMed] [Google Scholar]
- 125.Whittaker P, Boughner DR, Kloner RA. Analysis of healing after myocardial infarction using polarized light microscopy. Am J Pathol. 1989;134:879–893. [PMC free article] [PubMed] [Google Scholar]
- 126.Mukherjee D, Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest. 1991;88:1141–1146. doi: 10.1172/JCI115414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lopez B, Querejeta R, Gonzalez A, Larman M, Diez J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension. 2012;60:677–683. doi: 10.1161/HYPERTENSIONAHA.112.196113. [DOI] [PubMed] [Google Scholar]
- 128.Woodiwiss AJ, Tsotetsi OJ, Sprott S, Lancaster EJ, Mela T, Chung ES, Meyer TE, Norton GR. Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation. 2001;103:155–160. doi: 10.1161/01.CIR.103.1.155. [DOI] [PubMed] [Google Scholar]
- 129.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luther DJ, Thodeti CK, Shamhart PE, Adapala RK, Hodnichak C, Weihrauch D, Bonaldo P, Chilian WM, Meszaros JG. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ Res. 2012;110:851–856. doi: 10.1161/CIRCRESAHA.111.252734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 132.Rybarczyk BJ, Lawrence SO, Simpson-Haidaris PJ. Matrix-fibrinogen enhances wound closure by increasing both cell proliferation and migration. Blood. 2003;102:4035–4043. doi: 10.1182/blood-2003-03-0822. [DOI] [PubMed] [Google Scholar]
- 133.Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997;110(Pt 7):861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- 134.Lin F, Ren XD, Doris G, Clark RA. Three-dimensional migration of human adult dermal fibroblasts from collagen lattices into fibrin/fibronectin gels requires syndecan-4 proteoglycan. J Invest Dermatol. 2005;124:906–913. doi: 10.1111/j.0022-202X.2005.23740.x. [DOI] [PubMed] [Google Scholar]
- 135.Welch MP, Odland GF, Clark RA. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Samuel JL, Barrieux A, Dufour S, Dubus I, Contard F, Koteliansky V, Farhadian F, Marotte F, Thiery JP, Rappaport L. Accumulation of fetal fibronectin mRNAs during the development of rat cardiac hypertrophy induced by pressure overload. J Clin Invest. 1991;88:1737–1746. doi: 10.1172/JCI115492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol. 1992;262:H1861–H1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- 138.Franz M, Grun K, Richter P, Brehm BR, Fritzenwanger M, Hekmat K, Neri D, Gummert J, Figulla HR, Kosmehl H, et al. Extra cellular matrix remodelling after heterotopic rat heart transplantation: gene expression profiling and involvement of ED-A+ fibronectin, alpha-smooth muscle actin and B+ tenascin-C in chronic cardiac allograft rejection. Histochem Cell Biol. 2010;134:503–517. doi: 10.1007/s00418-010-0750-6. [DOI] [PubMed] [Google Scholar]
- 139.Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, Bedosky KM, Freed DH, Kardami E, Dixon IM. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 140.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal. 2009;3:163–165. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–911. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D’Hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- 143.Frolova EG, Sopko N, Blech L, Popovic ZB, Li J, Vasanji A, Drumm C, Krukovets I, Jain MK, Penn MS, et al. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J. 2012;26:2363–2373. doi: 10.1096/fj.11-190728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. The critical role of endogenous thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 146.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hogg PJ. Thrombospondin 1 as an enzyme inhibitor. Thromb Haemost. 1994;72:787–792. [PubMed] [Google Scholar]
- 148.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, et al. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 149.Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee DI, Ramirez-Correa G, Bedja D, Barth AS, Moens AL, Kass DA. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res. 2011;109:1410–1414. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chablais F, Jazwinska A. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development. 2012;139:1921–1930. doi: 10.1242/dev.078543. [DOI] [PubMed] [Google Scholar]
- 151.Imanaka-Yoshida K, Hiroe M, Yoshida T. Interaction between cell and extracellular matrix in heart disease: multiple roles of tenascin-C in tissue remodeling. Histol Histopathol. 2004;19:517–525. doi: 10.14670/HH-19.517. [DOI] [PubMed] [Google Scholar]
- 152.Frangogiannis NG, Shimoni S, Chang SM, Ren G, Dewald O, Gersch C, Shan K, Aggeli C, Reardon M, Letsou GV, et al. Active interstitial remodeling: an important process in the hibernating human myocardium. J Am Coll Cardiol. 2002;39:1468–1474. doi: 10.1016/S0735-1097(02)01792-8. [DOI] [PubMed] [Google Scholar]
- 153.Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. J Pathol. 1996;179:321–325. doi: 10.1002/(SICI)1096-9896(199607)179:3<321::AID-PATH555>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 154.Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, Imanaka-Yoshida K. Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2010;298:H1072–H1078. doi: 10.1152/ajpheart.00255.2009. [DOI] [PubMed] [Google Scholar]
- 156.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d’Hooge J, Van de Werf F, Carmeliet P, Pinto YM, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994;145:1450–1462. [PMC free article] [PubMed] [Google Scholar]
- 158.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88:1080–1087. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 159.Sam F, Xie Z, Ooi H, Kerstetter DL, Colucci WS, Singh M, Singh K. Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens. 2004;17:188–193. doi: 10.1016/j.amjhyper.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 160.Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D, Law RE, Nicholas S, Ross RS, Hsueh WA. Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J Am Coll Cardiol. 2004;43:1698–1705. doi: 10.1016/j.jacc.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 161.Psarras S, Mavroidis M, Sanoudou D, Davos CH, Xanthou G, Varela AE, Panoutsakopoulou V, Capetanaki Y. Regulation of adverse remodelling by osteopontin in a genetic heart failure model. Eur Heart J. 2012;33:1954–1963. doi: 10.1093/eurheartj/ehr119. [DOI] [PubMed] [Google Scholar]
- 162.Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension. 2004;44:826–831. doi: 10.1161/01.HYP.0000148458.03202.48. [DOI] [PubMed] [Google Scholar]
- 163.Zohar R, Zhu B, Liu P, Sodek J, McCulloch CA. Increased cell death in osteopontin-deficient cardiac fibroblasts occurs by a caspase-3-independent pathway. Am J Physiol Heart Circ Physiol. 2004;287:H1730–H1739. doi: 10.1152/ajpheart.00098.2004. [DOI] [PubMed] [Google Scholar]
- 164.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 167.Ahmed MS, Gravning J, Martinov VN, von Lueder TG, Edvardsen T, Czibik G, Moe IT, Vinge LE, Oie E, Valen G, Attramadal H. Mechanisms of novel cardioprotective functions of CCN2/CTGF in myocardial ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300(4):H1291–H1302. doi: 10.1152/ajpheart.00604.2010. [DOI] [PubMed] [Google Scholar]
- 168.Teufel A, Becker D, Weber SN, Dooley S, Breitkopf-Heinlein K, Maass T, Hochrath K, Krupp M, Marquardt JU, Kolb M, et al. Identification of RARRES1 as a core regulator in liver fibrosis. J Mol Med (Berl) 2012;90:1439–1447. doi: 10.1007/s00109-012-0919-7. [DOI] [PubMed] [Google Scholar]
- 169.Mehal WZ, Iredale J, Friedman SL. Scraping fibrosis: expressway to the core of fibrosis. Nat Med. 2011;17:552–553. doi: 10.1038/nm0511-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 171.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 172.Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ. Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol. 2003;42:1845–1854. doi: 10.1016/j.jacc.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 173.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 174.Ohtsu H, Frank GD, Utsunomiya H, Eguchi S. Redox-dependent protein kinase regulation by angiotensin II: mechanistic insights and its pathophysiology. Antioxid Redox Signal. 2005;7:1315–1326. doi: 10.1089/ars.2005.7.1315. [DOI] [PubMed] [Google Scholar]
- 175.Lijnen P, Papparella I, Petrov V, Semplicini A, Fagard R. Angiotensin II-stimulated collagen production in cardiac fibroblasts is mediated by reactive oxygen species. J Hypertens. 2006;24:757–766. doi: 10.1097/01.hjh.0000217860.04994.54. [DOI] [PubMed] [Google Scholar]
- 176.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci USA. 2003;100:2700–2705. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction wtih the renin-angiotension system. Am J Hypertens. 2004;17:597–603. [PubMed] [Google Scholar]
- 179.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 180.Dobaczewski M, Frangogiannis NG. Chemokines and cardiac fibrosis. Front Biosci (Schol Ed) 2009;1:391–405. doi: 10.2741/s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xia Y, Frangogiannis NG. MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm Allergy Drug Targets. 2007;6:101–107. doi: 10.2174/187152807780832265. [DOI] [PubMed] [Google Scholar]
- 182.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43:739–745. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 183.Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, Yamahana J, Okumura T, Matsushima K, Yokoyama H, Kaneko S. MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14-positive monocytes. J Leukoc Biol. 2006;79:555–563. doi: 10.1189/jlb.0305127. [DOI] [PubMed] [Google Scholar]
- 184.Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2006;290:G765–G771. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 185.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–17784. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 186.Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol. 2000;164:6174–6179. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- 187.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 188.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.CIR.93.4.704. [DOI] [PubMed] [Google Scholar]
- 191.Habib FM, Springall DR, Davies GJ, Oakley CM, Yacoub MH, Polak JM. Tumour necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet. 1996;347:1151–1155. doi: 10.1016/S0140-6736(96)90610-8. [DOI] [PubMed] [Google Scholar]
- 192.Francis SE, Holden H, Holt CM, Duff GW. Interleukin-1 in myocardium and coronary arteries of patients with dilated cardiomyopathy. J Mol Cell Cardiol. 1998;30:215–223. doi: 10.1006/jmcc.1997.0592. [DOI] [PubMed] [Google Scholar]
- 193.Plenz G, Song ZF, Reichenberg S, Tjan TD, Robenek H, Deng MC. Left-ventricular expression of interleukin-6 messenger-RNA higher in idiopathic dilated than in ischemic cardiomyopathy. Thorac Cardiovasc Surg. 1998;46:213–216. doi: 10.1055/s-2007-1010227. [DOI] [PubMed] [Google Scholar]
- 194.Timonen P, Magga J, Risteli J, Punnonen K, Vanninen E, Turpeinen A, Tuomainen P, Kuusisto J, Vuolteenaho O, Peuhkurinen K. Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int J Cardiol. 2008;124:293–300. doi: 10.1016/j.ijcard.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 195.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li J, Schwimmbeck PL, Tschope C, Leschka S, Husmann L, Rutschow S, Reichenbach F, Noutsias M, Kobalz U, Poller W, et al. Collagen degradation in a murine myocarditis model: relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc Res. 2002;56:235–247. doi: 10.1016/S0008-6363(02)00546-1. [DOI] [PubMed] [Google Scholar]
- 197.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–1265. doi: 10.1161/01.RES.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 198.Mitchell MD, Laird RE, Brown RD, Long CS. IL-1beta stimulates rat cardiac fibroblast migration via MAP kinase pathways. Am J Physiol Heart Circ Physiol. 2007;292:H1139–H1147. doi: 10.1152/ajpheart.00881.2005. [DOI] [PubMed] [Google Scholar]
- 199.Palmer JN, Hartogensis WE, Patten M, Fortuin FD, Long CS. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J Clin Invest. 1995;95:2555–2564. doi: 10.1172/JCI117956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Koudssi F, Lopez JE, Villegas S, Long CS. Cardiac fibroblasts arrest at the G1/S restriction point in response to interleukin (IL)-1beta. Evidence for IL-1beta-induced hypophosphorylation of the retinoblastoma protein. J Biol Chem. 1998;273:25796–25803. doi: 10.1074/jbc.273.40.25796. [DOI] [PubMed] [Google Scholar]
- 201.Kacimi R, Vessey DA, Honbo N, Karliner JS. Adult cardiac fibroblasts null for sphingosine kinase-1 exhibit growth dysregulation and an enhanced proinflammatory response. J Mol Cell Cardiol. 2007;43:85–91. doi: 10.1016/j.yjmcc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 202.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.CIR.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 203.Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci USA. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 205.Zhang W, Chancey AL, Tzeng HP, Zhou Z, Lavine KJ, Gao F, Sivasubramanian N, Barger PM, Mann DL. The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions. Circulation. 2011;124:2106–2116. doi: 10.1161/CIRCULATIONAHA.111.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 207.Duerrschmid C, Crawford JR, Reineke E, Taffet GE, Trial J, Entman ML, Haudek SB. TNF receptor 1 signaling is critically involved in mediating angiotensin-II-induced cardiac fibrosis. J Mol Cell Cardiol. 2013;57:59–67. doi: 10.1016/j.yjmcc.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2012;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 210.Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993;72:1245–1254. doi: 10.1161/01.RES.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 211.Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.RES.73.3.413. [DOI] [PubMed] [Google Scholar]
- 212.Crabos M, Roth M, Hahn AW, Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J Clin Invest. 1994;93:2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ohkubo N, Matsubara H, Nozawa Y, Mori Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Tsutumi Y, Shibazaki Y, et al. Angiotensin type 2 receptors are reexpressed by cardiac fibroblasts from failing myopathic hamster hearts and inhibit cell growth and fibrillar collagen metabolism. Circulation. 1997;96:3954–3962. doi: 10.1161/01.CIR.96.11.3954. [DOI] [PubMed] [Google Scholar]
- 214.Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, Drexler H. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. doi: 10.1161/01.CIR.89.5.2273. [DOI] [PubMed] [Google Scholar]
- 215.Regan CP, Anderson PG, Bishop SP, Berecek KH. Pressure-independent effects of AT1-receptor antagonism on cardiovascular remodeling in aortic-banded rats. Am J Physiol. 1997;272:H2131–H2138. doi: 10.1152/ajpheart.1997.272.5.H2131. [DOI] [PubMed] [Google Scholar]
- 216.Lijnen P, Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- 217.Campbell SE, Diaz-Arias AA, Weber KT. Fibrosis of the human heart and systemic organs in adrenal adenoma. Blood Press. 1992;1:149–156. doi: 10.3109/08037059209077510. [DOI] [PubMed] [Google Scholar]
- 218.Neumann S, Huse K, Semrau R, Diegeler A, Gebhardt R, Buniatian GH, Scholz GH. Aldosterone and d-glucose stimulate the proliferation of human cardiac myofibroblasts in vitro. Hypertension. 2002;39:756–760. doi: 10.1161/hy0302.105295. [DOI] [PubMed] [Google Scholar]
- 219.Brilla CG, Zhou G, Matsubara L, Weber KT. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994;26:809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- 220.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 221.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL, Kuhl U, Schultheiss HP. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999;99:2750–2756. doi: 10.1161/01.CIR.99.21.2750. [DOI] [PubMed] [Google Scholar]
- 224.Li RK, Li G, Mickle DA, Weisel RD, Merante F, Luss H, Rao V, Christakis GT, Williams WG. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 1997;96:874–881. doi: 10.1161/01.CIR.96.3.874. [DOI] [PubMed] [Google Scholar]
- 225.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 226.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 227.Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 228.Rifkin DB, Mazzieri R, Munger JS, Noguera I, Sung J. Proteolytic control of growth factor availability. APMIS. 1999;107:80–85. doi: 10.1111/j.1699-0463.1999.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 229.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Seeland U, Haeuseler C, Hinrichs R, Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K, Bohm M. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. 2002;32:295–303. doi: 10.1046/j.1365-2362.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 232.Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schluter KD, Bohm M. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1) Am J Physiol Heart Circ Physiol. 2002;283:H1253–H1262. doi: 10.1152/ajpheart.00578.2001. [DOI] [PubMed] [Google Scholar]
- 233.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.RES.86.5.571. [DOI] [PubMed] [Google Scholar]
- 234.Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol. 2000;32:187–195. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- 235.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.CIR.0000020689.12472.E0. [DOI] [PubMed] [Google Scholar]
- 236.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 237.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 239.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 240.Kapur NK, Wilson S, Yunis AA, Qiao X, Mackey E, Paruchuri V, Baker C, Aronovitz MJ, Karumanchi SA, Letarte M, et al. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation. 2012;125:2728–2738. doi: 10.1161/CIRCULATIONAHA.111.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Villar AV, Garcia R, Llano M, Cobo M, Merino D, Lantero A, Tramullas M, Hurle JM, Hurle MA, Nistal JF. BAMBI (BMP and activin membrane-bound inhibitor) protects the murine heart from pressure-overload biomechanical stress by restraining TGF-beta signaling. Biochim Biophys Acta. 2013;1832:323–335. doi: 10.1016/j.bbadis.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 242.Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176–184. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 243.Alvarez D, Briassouli P, Clancy RM, Zavadil J, Reed JH, Abellar RG, Halushka M, Fox-Talbot K, Barrat FJ, Buyon JP. A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. J Biol Chem. 2011;286:30444–30454. doi: 10.1074/jbc.M111.263657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shi-wen X, Kennedy L, Renzoni EA, Bou-Gharios G, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 2007;56:4189–4194. doi: 10.1002/art.23134. [DOI] [PubMed] [Google Scholar]
- 245.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, et al. Transcardiac extraction of circulating endothelin-1 across the failing heart. Am J Cardiol. 2000;86:524–528. doi: 10.1016/S0002-9149(00)01006-7. [DOI] [PubMed] [Google Scholar]
- 246.Yamamoto K, Masuyama T, Sakata Y, Mano T, Nishikawa N, Kondo H, Akehi N, Kuzuya T, Miwa T, Hori M. Roles of renin-angiotensin and endothelin systems in development of diastolic heart failure in hypertensive hearts. Cardiovasc Res. 2000;47:274–283. doi: 10.1016/S0008-6363(00)00101-2. [DOI] [PubMed] [Google Scholar]
- 247.Piacentini L, Gray M, Honbo NY, Chentoufi J, Bergman M, Karliner JS. Endothelin-1 stimulates cardiac fibroblast proliferation through activation of protein kinase C. J Mol Cell Cardiol. 2000;32:565–576. doi: 10.1006/jmcc.2000.1109. [DOI] [PubMed] [Google Scholar]
- 248.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res. 1993;27:2130–2134. doi: 10.1093/cvr/27.12.2130. [DOI] [PubMed] [Google Scholar]
- 249.Kulasekaran P, Scavone CA, Rogers DS, Arenberg DA, Thannickal VJ, Horowitz JC. Endothelin-1 and transforming growth factor-beta1 independently induce fibroblast resistance to apoptosis via AKT activation. Am J Respir Cell Mol Biol. 2009;41:484–493. doi: 10.1165/rcmb.2008-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mueller EE, Momen A, Masse S, Zhou YQ, Liu J, Backx PH, Henkelman RM, Nanthakumar K, Stewart DJ, Husain M. Electrical remodelling precedes heart failure in an endothelin-1-induced model of cardiomyopathy. Cardiovasc Res. 2011;89:623–633. doi: 10.1093/cvr/cvq351. [DOI] [PubMed] [Google Scholar]
- 251.Ammarguellat F, Larouche I, Schiffrin EL. Myocardial fibrosis in DOCA-salt hypertensive rats: effect of endothelin ET(A) receptor antagonism. Circulation. 2001;103:319–324. doi: 10.1161/01.CIR.103.2.319. [DOI] [PubMed] [Google Scholar]
- 252.Mulder P, Richard V, Derumeaux G, Hogie M, Henry JP, Lallemand F, Compagnon P, Mace B, Comoy E, Letac B, Thuillez C. Role of endogenous endothelin in chronic heart failure: effect of long-term treatment with an endothelin antagonist on survival, hemodynamics, and cardiac remodeling. Circulation. 1997;96:1976–1982. doi: 10.1161/01.CIR.96.6.1976. [DOI] [PubMed] [Google Scholar]
- 253.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 254.Simm A, Nestler M, Hoppe V. Mitogenic effect of PDGF-AA on cardiac fibroblasts. Basic Res Cardiol. 1998;93(Suppl 3):40–43. doi: 10.1007/s003950050209. [DOI] [PubMed] [Google Scholar]
- 255.Tuuminen R, Nykanen AI, Krebs R, Soronen J, Pajusola K, Keranen MA, Koskinen PK, Alitalo K, Lemstrom KB. PDGF-A, -C, and -D but not PDGF-B increase TGF-beta1 and chronic rejection in rat cardiac allografts. Arterioscler Thromb Vasc Biol. 2009;29:691–698. doi: 10.1161/ATVBAHA.108.178558. [DOI] [PubMed] [Google Scholar]
- 256.Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, Dutta P, Iwamoto Y, Ueno T, Begieneman MP, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–163. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833:945–953. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dobaczewski M, de Haan JJ, Frangogiannis NG. The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. J Cardiovasc Transl Res. 2012;5:837–847. doi: 10.1007/s12265-012-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cannon RO, 3rd, Butany JW, McManus BM, Speir E, Kravitz AB, Bolli R, Ferrans VJ. Early degradation of collagen after acute myocardial infarction in the rat. Am J Cardiol. 1983;52:390–395. doi: 10.1016/0002-9149(83)90145-5. [DOI] [PubMed] [Google Scholar]
- 260.Whittaker P, Boughner DR, Kloner RA. Role of collagen in acute myocardial infarct expansion. Circulation. 1991;84:2123–2134. doi: 10.1161/01.CIR.84.5.2123. [DOI] [PubMed] [Google Scholar]
- 261.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol. 2008;180:2625–2633. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- 262.Corbett SA, Schwarzbauer JE. Fibronectin-fibrin cross-linking: a regulator of cell behavior. Trends Cardiovasc Med. 1998;8:357–362. doi: 10.1016/S1050-1738(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 263.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32:2598–2608. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Creemers E, Cleutjens J, Smits J, Heymans S, Moons L, Collen D, Daemen M, Carmeliet P. Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction. Am J Pathol. 2000;156:1865–1873. doi: 10.1016/S0002-9440(10)65060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van de Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993;142:793–801. [PMC free article] [PubMed] [Google Scholar]
- 266.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 267.Lerman RH, Apstein CS, Kagan HM, Osmers EL, Chichester CO, Vogel WM, Connelly CM, Steffee WP. Myocardial healing and repair after experimental infarction in the rabbit. Circ Res. 1983;53:378–388. doi: 10.1161/01.RES.53.3.378. [DOI] [PubMed] [Google Scholar]
- 268.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 269.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem. 2002;50:71–79. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 270.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–481. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nicoletti A, Heudes D, Mandet C, Hinglais N, Bariety J, Michel JB. Inflammatory cells and myocardial fibrosis: spatial and temporal distribution in renovascular hypertensive rats. Cardiovasc Res. 1996;32:1096–1107. doi: 10.1016/S0008-6363(96)00158-7. [DOI] [PubMed] [Google Scholar]
- 272.Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res. 2004;64:24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 273.Zheng J, Chen Y, Pat B, Dell’italia LA, Tillson M, Dillon AR, Powell PC, Shi K, Shah N, Denney T, et al. Microarray identifies extensive downregulation of noncollagen extracellular matrix and profibrotic growth factor genes in chronic isolated mitral regurgitation in the dog. Circulation. 2009;119:2086–2095. doi: 10.1161/CIRCULATIONAHA.108.826230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nagatomo Y, Carabello BA, Coker ML, McDermott PJ, Nemoto S, Hamawaki M, Spinale FG. Differential effects of pressure or volume overload on myocardial MMP levels and inhibitory control. Am J Physiol Heart Circ Physiol. 2000;278:H151–H161. doi: 10.1152/ajpheart.2000.278.1.H151. [DOI] [PubMed] [Google Scholar]
- 275.Janicki JS, Brower GL, Gardner JD, Forman MF, Stewart JA, Jr, Murray DB, Chancey AL. Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload. Cardiovasc Res. 2006;69:657–665. doi: 10.1016/j.cardiores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 276.Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 277.Robert V, Besse S, Sabri A, Silvestre JS, Assayag P, Nguyen VT, Swynghedauw B, Delcayre C. Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab Invest. 1997;76:729–738. [PubMed] [Google Scholar]
- 278.Chen W, Frangogiannis NG. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010;15:415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Brilla CG, Matsubara L, Weber KT. Advanced hypertensive heart disease in spontaneously hypertensive rats. Lisinopril-mediated regression of myocardial fibrosis. Hypertension. 1996;28:269–275. doi: 10.1161/01.HYP.28.2.269. [DOI] [PubMed] [Google Scholar]
- 281.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.CIR.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 282.Berry JM, Le V, Rotter D, Battiprolu PK, Grinsfelder B, Tannous P, Burchfield JS, Czubryt M, Backs J, Olson EN, et al. Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ Res. 2011;109:407–417. doi: 10.1161/CIRCRESAHA.110.228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, Quinones MA, Zoghbi WA, Entman ML, Roberts R, Marian AJ. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation. 2001;104:317–324. doi: 10.1161/hc2801.094031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.CIR.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]