Abstract
The cytokine-inducible isoform of nitric oxide synthase (NOS2) is constitutively expressed in human respiratory epithelia and is upregulated in inflammatory lung disease. Here, we sought to better define the protein interactions that may be important for NOS2 activity and stability, as well as to identify potential targets of NOS2-derived NO, in the respiratory epithelium. We overexpressed Flag-tagged, catalytically-inactive NOS2 in A549 cells and used mass spectrometry to qualitatively identify NOS2 co-immunoprecipitating proteins. Stable isotope labeling of amino acids in cell culture (SILAC) was used to quantify the coordinate effects of cytokine stimulation on NOS2-protein interactions. Multi-protein networks dominated the NOS2 interactome, and cytokine-inducible interactions with allosteric activators and with the ubiquitin-proteasome system were correlated with cytokine-dependent increases in NO metabolites and in NOS2 ubiquitination. The ubiquitin ligase scaffolding protein, FBXO45, was identified as a novel, direct NOS2 interactor. Similar to the SPRY domain-containing SOCS box (SPSB) proteins, FBXO45 requires Asn27 in the 23DINNN27 motif of NOS2 for its interaction. However, FBXO45 is unique from the SPSBs in that it recruits a distinct E3 ligase complex containing MYCBP2 and SKP1. Collectively, these findings demonstrate the general utility of interaction proteomics for defining new aspects of NOS2 physiology.
Keywords: nitric oxide synthase, nitric oxide, B30.2/SPRY domain, ubiquitination, proteomics
INTRODUCTION
All three NO synthases (NOS1, NOS2 and NOS3) are expressed in the lung, and NOS-derived NO is vital to many processes such as lung development, beta-adrenergic receptor signaling and immunoregulation [1–3]. The cytokine-inducible isoform (NOS2) is constitutively expressed in the airway epithelium [4], and inflammatory stimuli further increase NOS2 expression both in normal and neoplastic lung epithelial cells [5–8]. In asthma, airway NOS2 expression is increased, and high NOS2 levels are correlated with asthma severity, exhaled NO and lung eosinophilia [9]. Airway NOS2 expression is also increased in chronic obstructive pulmonary disease (COPD) [10] and in acute lung injury, in which we have found it functions to deactivate pro-inflammatory pathways [11]. Findings also suggest that NOS2 plays a crucial role in the pathogenesis of lung cancer [12–15].
Compared to NOS1 and NOS3, NOS2 binds calmodulin (CAM) constitutively and with high affinity and has a 10-fold larger Vmax, resulting in higher, and more sustained, NO output. Under these conditions, the potential cytotoxicity of NO mandates that the expression, activity and stability of NOS2 be tightly regulated. Activators of toll-like receptor (TLR) signaling, as well as numerous cytokines, induce NOS2 via transcription factors such as nuclear factor kappa b (NF-κB) and interferon regulatory factor 1, and NOS2 is regulated by numerous other transcriptional and post-transcriptional mechanisms [16]. NOS2 activity is also modulated by availability of substrate (L-arginine) [17,18] and cofactors (NADPH, tetrahydrobiopterin), phosphorylation [19–21], and protein-protein interactions (PPIs) that affect subcellular localization [22,23]. PPIs of NOS2 are also central to its ubiquitination and proteasomal degradation [24,25]. NO and its congeners can affect feedback regulation through a variety of mechanisms (e.g. S-nitrosylation and inhibition of NF-κB) [26–28]. Despite this wealth of data, the interplay between these many regulatory pathways in the respiratory epithelium is not well understood.
While NOS2 was initially regarded as an indiscriminant source of reactive nitrogen species (RNS), accumulating evidence now indicates that NOS2-derived NO is utilized in signal transduction via precisely orchestrated PPIs. This paradigm is exemplified in the interaction of NOS2 with cyclooxygenase 2 (COX-2), which results in S-nitrosylation and enzyme activation [29]. Similarly, NOS2 also binds to, S-nitrosylates and activates cytosolic phospholipase A2α (cPLA2α), an enzyme that lies upstream of COX-2 in the arachidonic acid pathway [30]. Interestingly, the interaction of NOS2 and cPLA2α is potentiated by COX-2 [30]. This suggests that a protein complex involving NOS2, COX-2 and cPLA2α is important for NOS2-dependent activation of prostaglandin E2 biosynthesis. These findings support a new paradigm for NOS2-based signaling in which PPIs are critical for targeting of NOS-derived NO.
Historically, the identification of NOS2-specific interacting proteins has come via a number of binary and/or hypothesis-based approaches, including yeast-2-hybrid (Y2H) analysis and co-immunoprecipitation [22,25,31–36]. Mass spectrometry (MS) is now recognized as an essential tool for the unbiased characterization and quantitative analysis of protein interaction networks [37–39]; however, thus far, MS-based efforts have revealed only a few NOS2-interacting proteins [23,35]. Here, we sought to adapt modern MS-based methods for large scale and unbiased identification of the NOS2-interacting proteins in human airway epithelial cells, and we sought to quantify whether, and to what end, cellular stimuli (i.e. inflammatory cytokines) might shape this “NOS2 interactome”.
MATERIALS AND METHODS
Plasmid and virus constructs
The E377Q mutant of human hepatocyte NOS2 was amplified from pLP –E377Q-NOS2 [40] by PCR using the primers 5′-NOS2_NotI: 5′-TAC TTA TAG CGG CCG CAC CAT GGC CTG TCC TTG G-3′ and 3′-NOS2_Flag_SalI 5′-TTA AGT CGA CTT ACT TGT CGT CAT CGT CTT TGT AGT CGA GCG CTG ACA TCT CCA GGC TGC-3′ and cloned into pShuttle-IRES-EGFP to produce a C-terminal FLAG-tagged E377Q-NOS2. Adenoviruses expressing E377Q-NOS2-Flag (Ad.E377Q-NOS2-Flag) and the wild-type human hepatocyte NOS2 (Ad.wt-NOS2) [41] were producing using the AdEasy system (Stratagene) according to the manufacturer’s instructions and purified by CsCl gradient as previously described [42].
For transient overexpression in HEK-293 cells, wild-type NOS2-Flag was made by reverse mutation of E377Q-NOS2-Flag-pShuttle using Quickchange XL (Stratagene) using the following primers: iNOS_Q377E_for 5′-GTA CAT GGG CAC AGA GAT CGG AGT CC-3′ and iNOS_Q377E_rev 5′-GGA CTC CGA TCT CTG TGC CCA TGT AC-3′. Similarly, N27A-NOS2-Flag was constructed from the wild-type vector using the primers: N27A_NOS2_for 5′-GGG AAA AAG ACA TCA ACA ACG CTG TGG AGA AAG CC-3′ and N27A_NOS2_rev 5′-GGC TTT CTC CAC AGC GTT GTT GAT GTC TTT TTC CC-3′. The expression plasmid for N-terminal myc-tagged human FBXO45 was previously described [43].
Cell culture
A549 cells were cultured in RPMI containing 10% FBS, and HEK-293 or Ad-293 cells were cultured in DMEM containing 10% FBS. Media containing 2% FBS was utilized for viral infection. For SILAC analyses, A549 cells were cultured in Lys- and Arg-deficient RPMI (Pierce) containing 10% dialyzed FBS (Sigma), Penicillin/Streptomycin/Fungizone (Gibco), supplemented with 10 mg/l L-proline and either 50 mg/l L-arginine and L-lysine (light) or 50 mg/l 15N413C6-arginine and 15N213C6-lysine (heavy). Cells were conditioned in SILAC media for a minimum of 6 doublings. Adenoviral infections were performed at 70% confluency using RPMI containing 2% FBS. The E377Q-NOS2-Flag adenovirus was used at ~1E9 particles/ml.
Immunoprecipitations
The qualitative NOS2 IP utilized 10 × 150 mm dishes of A549 cells infected with Ad.E377Q-NOS2-Flag and stimulated with 20 ng/ml tumor necrosis factor alpha (TNFα) for 16 h. The SILAC NOS2 IP utilized 10 × 150 mm dishes of light (control) or heavy (+cytomix) SILAC-labeled A549 cells infected with Ad.E377Q-NOS2-Flag. Cytomix (CM) was added to media at a final concentration of 500 ng/ml lipopolysaccharide (LPS), 100 pg/ml interferon gamma (IFNγ), 20 ng/ml TNFα, 10 ng/ml interleukin-1 beta (IL-1β) for 16 h [26]. The control Flag IP utilized 10 × 150 mm dishes of uninfected, CM-stimulated A549 cells. Cells were washed with cold phosphate-buffered saline (PBS) and lysed by scraping on ice with 1 ml/dish of lysis buffer (PBS containing 10% glycerol, 0.5% NP-40, Complete protease inhibitors (Roche), 1 mM NaF, and 1 mM ortho-vanadate). After tumbling for 20 min at 4 °C, lysates were centrifuged at 20,000 ×g for 10 min. For SILAC analysis, equal amounts of protein (~2 mg/ml protein in ~ 15 ml of lysis buffer) were incubated separately overnight with 100 μl anti-FlagM2 agarose (Sigma), and beads were combined after washing 2x with lysis buffer. Beads were washed an additional 3x with 20 mM Tris, pH 8.0 containing 100 mM NaCl and 0.2 % NP-40 followed by Tris/NaCl (20 mM Tris, pH 8.0 containing 100 mM NaCl). Beads were centrifuged between washes at 200 × g for 10 s. Finally, proteins were eluted by tumbling for 1 h at 4 °C with 500 μl elution buffer containing 0.25 mg/ml Flag peptide (Sigma) in Tris/NaCl. This step was repeated, and combined eluents were concentrated and exchanged with 50 mM ammonium bicarbonate, pH 8.0 (AMBIC) using a Millipore 5 kDa-cutoff centrifugal concentrator.
Sample preparation
For in-solution digestion of the qualitative NOS2 IP, 10 μg of immunoprecipitated protein was reduced in AMBIC containing 0.1% w/v Rapigest (Waters) and 10 mM DTT at 80 °C for 15 min followed by alkylation with 20 mM iodoacetamide in the dark for 30 min and digestion with 0.2 μg Sequencing Grade Modified Trypsin (Promega) overnight at 37 °C. Finally, samples were acidified by addition of 1% TFA/2% acetonitrile and heated at 70 °C for 1 h, to degrade the Rapigest, followed by centrifugation and transfer of supernatant to a Maximum Recovery LC Vial (Waters).
For GeLC analysis, up to 50 μg of immunoprecipitates were separated by SDS-PAGE on a 4–12% SDS-PAGE gel (Invitrogen NuPage). After staining with Colloidal Blue (Invitrogen), the entire lane was excised using a 2 mm × 7 mm gridcutter (GelCompany) into 32 bands, and except for the NOS2 band, every two contiguous bands were combined to reduce sample number. In-gel tryptic digestions were performed as previously described [44]. Finally, peptides were extracted with ddH2O containing 1% formic acid (FA) and 2% acetonitrile (ACN) followed by 100% ACN. After lyophilization, peptides were resuspended in 12 μl 0.2% FA, 2% ACN in ddH2O.
1D-LC-MS/MS analysis
Peptides (1 μg of in-solution digests or one-half of reconstituted peptides from in-gel digest) were analyzed by 1D-LC-MS/MS using a nanoAcquity UPLC system coupled to a Synapt G1 HDMS mass spectrometer (Waters). Samples were trapped on a 20 μm × 180 mm Symmetry C18 column (Waters) at 20 μl/min for 2 min in water containing 0.1% FA and were further separated on a 75 μm × 250 mm column with 1.7 μm C18 bridged ethane-silicone hybrid (BEH) particles (Waters) using a gradient of 5 to 40% ACN/0.1% FA over 90 min at a flow rate of 0.3 μl/min and a column temp of 45 °C. Samples were analyzed in data-dependent (DDA) mode using a 0.9 s precursor scan followed by MS/MS product ion scans on the top 3 most intense ions using a dynamic exclusion window of 120 s.
SILAC-encoded NOS2 IPs were analyzed on a nanoAcquity UPLC coupled to a Orbitrap XL mass spectrometer. LC conditions were as described above except that 5 μl of in-gel digested peptide was separated over a 60 min gradient. The Orbitrap MS/MS method used DDA in the Orbitrap. Briefly, the precursor scan method used profile mode and 60000 resolution with AGC target of 1e6 and 1 microscan. MS/MS acquisition was performed on the top three precursor ions above a 5000-count threshold using CID with a 3 Da isolation window, normalized collision energy of 35%, and 1 microscan. Product ion spectra were collected in profile mode in the Orbitrap mass analyzer with a resolution of 7500 and AGC target setting of 2e5. Dynamic exclusion settings were: repeat count = 3, repeat duration = 30 s, exclusion list = 250, and exclusion time = 120 s.
Control Flag IPs were analyzed by nano-ESI-Chip system interfaced to a 6520 QTof (Agilent). The large-capacity Chip contained a 160 nl C18 trapping column and a 0.75 × 150 mm 300 Å C18 analytical column. Digests were first trapped at 3.5 μl/min and were separated on the analytical column at 300 nl/min using a linear gradient of 5–40% ACN containing 0.1% FA over 50 min. Samples were analyzed by data-dependent analysis (DDA) with MS scans acquired at 4 scans/s from 300–1800 Da and MS/MS scans at 2 scans/s from 59–2000 Da. The top 5 most intense ions were selected for MS/MS analysis and each precursor ion was excluded after 1 scan and released after 0.4 min. Precursor ion selection was based on abundance and charge state with doubly and triply charged ions preferred. Collision energy (CE) followed a linear equation of CE = (3.6 V per 100 daltons) − 4.6V.
Database searching and SILAC quantitation
For qualitative proteome and interactome analyses, data was processed using Mascot Distiller and searched with Mascot v2.2 (Matrix Sciences) against the Swissprot database v57.1, with human taxonomy and containing a reverse decoy database. Mascot search parameters were 10 ppm (for Agilent and Thermo data) and 20 ppm precursor (for Waters data) and 0.04 Da product ion tolerance, with oxidation (M) and deamidation (NQ), as variable modifications. Data was visualized using Scaffold 3.0 (Proteome Software) and annotated using a 1% peptide false-discovery rate (FDR). Instructions for downloading a Scaffold data containing these data are given in Supporting Information.
SILAC-based quantitation was performed using Rosetta Elucidator v3.3 (Rosetta Biosoftware), and Mascot searches were performed within Elucidator as described above except that Label:13C615N2 (K) and Label:13C615N4 (R) were included as additional variable modifications. Peptide identifications were accepted if they had a <1% FDR as determined by decoy database searching and the PeptideTeller algorithm in Elucidator [45,46]. Heavy and light Arg- and/or Lys-containing forms of the same peptide (H/L SILAC pairs) were located in Elucidator with a minimum m/z tolerance of 30 ppm and retention time tolerance of 0.2 min and a maximum of three labeled amino acids per peptide. H/L SILAC pair ratios were calculated using intensity normalization in Elucidator, using the peak height of the highest abundance isotopomer, and p-values were calculated as previously described [47,48]. High-confidence quantifications were based on the H/L peak height ratios of 2 or more unique SILAC pairs for a given protein as previously described [49].
RESULTS
MS-based identification of NOS2-interacting proteins
NOS2 is expressed at relatively low levels in the respiratory epithelium, and high intracellular levels of NO can be cytotoxic. To overcome these obstacles, we developed a tractable model system for in-depth identification of NOS2-interacting proteins in A549 respiratory epithelial cells by utilizing adenoviral expression of a C-terminal flag-tagged, catalytically-inactive NOS2 protein (E377Q-NOS2) [40]. Targeting of this amino acid was based on previous findings in NOS3 where mutation of the analogous active site Glu361 to Asn prevents binding of substrate L-Arg by removing the requisite salt bridge between the NOS active site and the guanidine moiety of L-Arg [50]. Importantly, this mutation does not impact other redox properties of the heme active site or NOS dimerization. The E377Q mutation is therefore a simple strategy to prevent NO synthesis without affecting other known properties of NOS2.
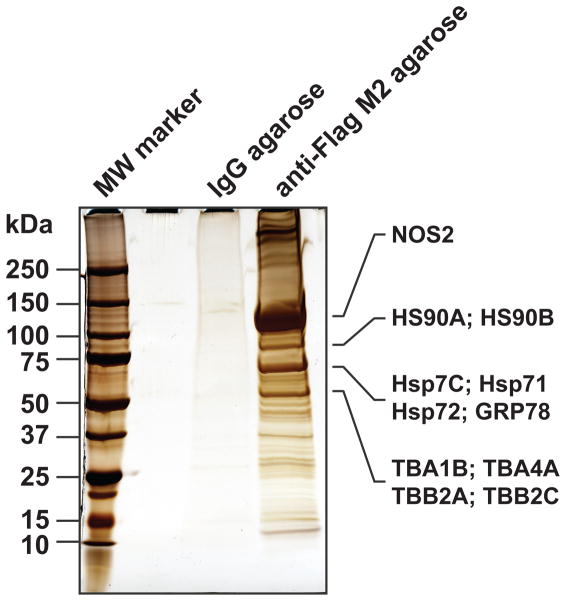
To recapitulate the cytokine-induced conditions under which NOS2 is normally expressed, E377Q-NOS2-overexpressing cells were also stimulated for 16 h with TNFα (10 ng/ml) in our initial screen. Immunoprecipitates (IPs) were prepared from whole cell lysates using control IgG- or anti-Flag agarose beads followed by protein elution with Flag peptide. Anti-Flag agarose enriched numerous proteins compared to the IgG control, including a prominent NOS2 band, demonstrating the feasibility and specificity of the IP (Fig. 1). In-solution digestion and subsequent replicate LC-MS/MS analyses of the anti-Flag IP eluates identified forty-six proteins (2+ peptides to match) which constitute the most abundant co-immunoprecipitated proteins. NOS2-derived peptides dominated the analysis (Fig. 1 and Supplemental Data), but other abundant species included the known NOS2-interacting proteins HSP60, HSP70, HSP90 and calmodulin [33,51,52]. Based on comparison of the SDS-PAGE gel image to the results of the LC-MS/MS analysis (Supplemental Data), multiple HSP90, HPS70 and tubulin isoforms likely comprised several of the prominent bands (Fig. 1).
Figure 1.
Qualitative analysis of NOS2 interactome. A549 cells were infected with an adenovirus expressing E377Q-NOS2-Flag. After 32 h, cells were stimulated with TNFα. Cleared lysates were immunoprecipitated o/n with control IgG-agarose or anti-Flag-M2-agarose, bound proteins were eluted with Flag peptide, separated by SDS-PAGE and visualized by silver staining. The NOS2 IP was subsequently digested in solution with trypsin and analyzed by LC-MS/MS. Based on the relative abundance of identified proteins, several of the abundant bands were assigned to NOS2, HSP90, HSP70 and tubulin isoforms (indicated at right).
To further increase depth of coverage, the protein eluate was separated by 1D-SDS-PAGE, and 16 fractions (2 gel bands slices per fraction) were subjected to in-gel digestion followed by LC-MS/MS (GeLC-MS/MS). Greater than 300 NOS2-associated proteins were confidently identified using this strategy (Supplemental Data), including the previously-characterized interactors: STIP1 homology and U box-containing protein 1 (STUB1/CHIP) [52], the ubiquitin C-terminal hydrolase UCHL5 [36], and SPRY domain-containing SOCS box proteins 1 and 2 (SPSB1 and SPSB2) [25,53]. Collectively, the identification of a wide repertoire of known NOS2-interacting proteins within a single experiment lent confidence to the validity and power of this approach.
Quantification of the cytokine-inducible interactome
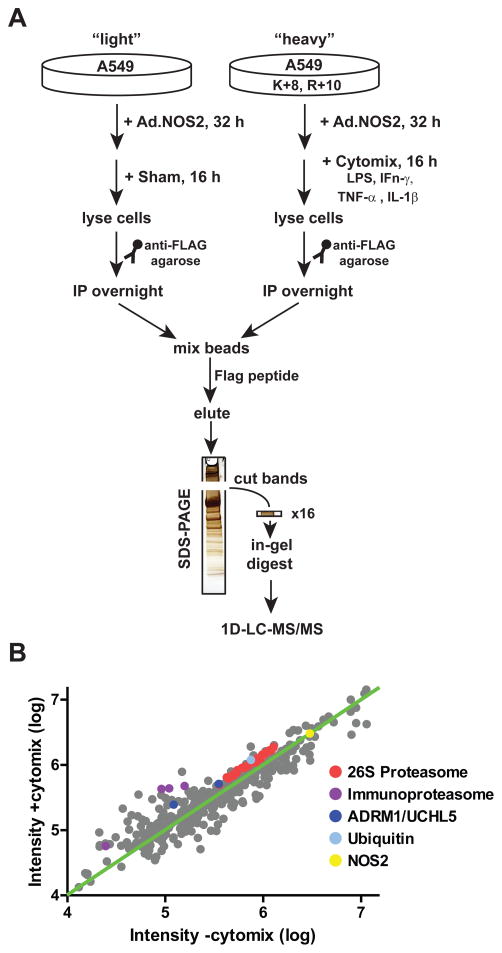
Several cytokine-inducible proteins, including intracellular adhesion molecule 1 (ICAM1) and TNFα-induced protein 2 (TNFAIP2) were identified in the qualitative NOS2 proteome, which raised the question of whether cytokines that induce NOS2 expression might also more generally affect NOS2-protein interactions. To quantify cytokine-dependent NOS2 interactions, we utilized stable isotopic labeling of amino acids in cell culture (SILAC), an approach readily adaptable to A549 cells and amenable to the GeLC-MS/MS methodology (Fig. 2A) [54–57]. Light and heavy isotope-conditioned cells were infected with Ad.E377Q-NOS2-Flag, and the heavy isotope-labeled cells were further stimulated with cytomix (LPS, IFNγ, TNFα and IL-1β [26]; CM). Lysates from light (unstimulated) and heavy (CM-stimulated) cells underwent separate immunoprecipitations using Flag-agarose, and following washing, were combined prior to Flag peptide elution and analysis by GeLC-MS/MS (Fig. 2A). Quantification was performed using Rosetta Elucidator, with confidence assigned to proteins with heavy/light ratios derived from at least two unique SILAC pairs [49]. 350 proteins were quantified in unstimulated versus CM-stimulated cells based on this criterion (Table S1–S2). Relative levels of NOS2 did not significantly change in heavy versus light IPs (fold-change = 1.01; 135 SILAC pairs), demonstrating that the ectopically-expressed NOS2 (E377Q-NOS2) was not altered by cytokine stimulation.
Figure 2.
Workflow for quantitative analysis of NOS2 interactome. (A) SILAC workflow for quantification of the cytokine-inducible interactome. (B) Intensity plot of the quantified light (control) versus heavy (+CM) forms of proteins that were quantified in the NOS2 IPs (fold-changes between ±6; Table S2). The expression of proteasome subunits and associated proteins are highlighted.
One-hundred and five of the 350 quantified proteins were enriched greater than ±1.4-fold after cytokine stimulation (p<0.001; Table S2). Of these, 11 proteins were >100-fold lower in the IP from CM-stimulated cells, although all of these represented probable contaminants (e.g. keratins, bovine serum albumin) which would not be expected to have an associated heavy isotope form. On the other hand, ICAM1 and TNFAIP2 were identified almost exclusively in their heavy isotope forms, consistent with their expression only under cytokine-induced conditions. The remainder of proteins quantified by 2+ SILAC pairs fell within the range of ±6-fold in NOS2 IPs from CM-stimulated versus unstimulated cells. Notable cytokine-induced proteins on this list include tryptophanyl-tRNA synthase, immunoproteasome subunits (PSB9 and PSB10) and sirtuin 1 (SIRT1; Table S2).
Numerous multiprotein complexes were quantified in the CM-regulated NOS2 interactome, and the individual members of many of these complexes trended similarly in response to cytokine stimulation (Table S2). These included: the survival motor neuron (SMN) complex (Table S2), which had seven associated proteins (DDX20, GEMI2, GEMI4, GEMI5, LG3BP, SMN1, and STRAP) [58–61] that were enriched ~2–4-fold; the 80S ribosome (quantified by at least twenty-nine 40S and forty-three 60S subunits), which was essentially unchanged; and numerous mRNP complex-associated proteins, which were less abundant in Flag IPs after cytokine treatment (Table S2).
The 26S proteasome and associated proteins were also abundant in the NOS2 interactome (Table S2). The 29 quantified subunits of the 26S proteasome were tightly clustered together and had a mean-fold enrichment in CM-stimulated cells of 1.41 ± .01 (s.e.m.; Fig. 2B). The proteasome-associated proteins ADRM1 and UCHL5 were similarly enriched, while the cytokine-inducible 20S beta “immunoproteasome” subunits (PSB9, PSB10 and PSB11) and the interferon-inducible proteasome-associated activator PSME2 were appropriately enriched relative to the proteasome itself.
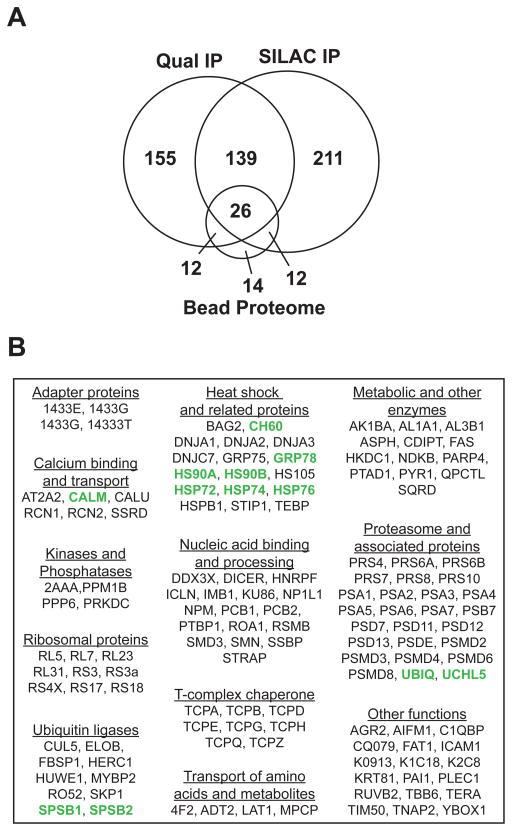
It is recognized that proteins can bind non-specifically to Flag-agarose [62], and to account for any potential artifacts, we also analyzed a Flag IP from uninfected, cytokine-stimulated, A549 cells using the GeLC-MS/MS approach as a control for the NOS2 IPs (Supplemental Data). Fifty of the 64 proteins identified in the “bead proteome” were also identified in the qualitative and/or quantitative NOS2 IPs and were thus classified as non-specific interactors; of these, 26 proteins were common to all three proteomes (Fig. 3A). Among the proteins that were specific to the NOS2 IP datasets, 139 were identified in both NOS2 IPs and were classified as specific and reproducible interactors (Table S3). These proteins were further classified based on their known cellular functions (Fig. 3B).
Figure 3.
Specificity and classification of NOS2 interactors. (A) Venn diagram comparing numbers and overlap of proteins (each with two or more unique peptides) identified in the qualitative and SILAC-encoded NOS2 IPs versus bead proteomes (i.e. control Flag IP), analyzed once each (B) The Software Tool for Rapid Annotation of Proteins [85] was used to gather gene ontology and functional annotations for the 139 proteins that overlapped NOS2 IPs (Table S3), and proteins were manually classified based on their known functions. Previously-described NOS2-interacting proteins are highlighted in green.
Cytokines increase NOS2 activity and protein-bound NO species
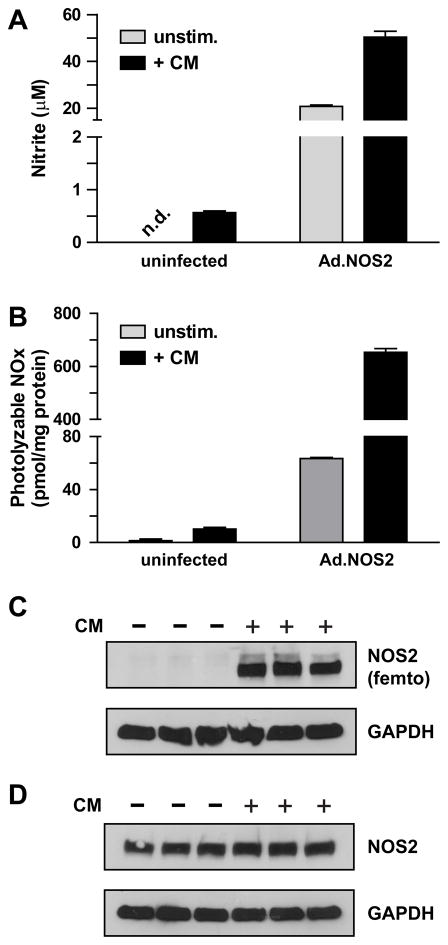
We next assessed the potential functional consequences of NOS2 interactions that were altered by cytokine treatment. We found that CM increased association with numerous known allosteric activators of NOS2. For example, the association with α-actinin-4, which is thought to be important for NOS2 plasma membrane localization and activity [23], was increased >6-fold with CM. The association of NOS2 with heat shock proteins HSP90α, HSP90β and calmodulin was also increased ~1.5-fold (Table S2). Calmodulin is a constitutively bound activator of NOS2 and HSP90 is a known allosteric activator [33,63] that also facilitates heme insertion into the NOS2 apoenzyme [64]. Collectively, these results suggest that CM stimulation might increase NOS2 activity independent of NOS2 expression. To further test this hypothesis, we measured cytokine-dependent formation of nitrite (NO2−) in cell culture supernatants as well as photolyzable NO (i.e. nitrosyl-protein and nitroso-protein) in cell lysates from uninfected versus wild-type NOS2 adenovirus-infected A549 cells.. As previously observed [5], cytokines increased NO metabolites (Fig. 4A–B) and NOS2 protein (Fig. 4C) in uninfected A549 cells. Compared to cells infected with NOS2 adenovirus (Fig. 4A–B, 4D), these levels were modest. In NOS2-overexpressing cells, cytomix affected a 2-fold increase in NO2− (Fig. 4A), and a 10-fold increase in protein-bound NO species (Fig. 4B), while NOS2 expression levels were unchanged (Fig. 4D). These changes could not be due to the modest contibution of endogenous NOS2 (Fig. 4A–B). This regulation of NOS2 activity by cytokines independent of its expression has not been previously described in the respiratory epithelium.
Figure 4.
Cytokine-dependent increase in NOS2 activity and cellular protein-bound NO. A549 cells were infected ± an adenovirus expressing wild-type NOS2 (Ad.wt-NOS2). After 32 h, cells were stimulated ± CM for 16 h, and (A) NO2− was quantified by Greiss assay in cell culture supernatants (mean ± S.E.M.; n=3), (B) photolyzable NO was quantified in lysates by photolysis chemiluminescence (mean ± S.E.M.; n=3). C–D) NOS2 and GAPDH expression were visualized by western blotting of 25 μg of lysates from (C) uninfected A549 cells (D) and from A549 cells infected with NOS2 adenovirus. Note that NOS2 was detected in (C) using Supersignal West Femto Chemiluminescent Substrate (Pierce) while all other proteins in (C–D) were detected with standard ECL reagent.
Cytokines induce NOS2 ubiquitination
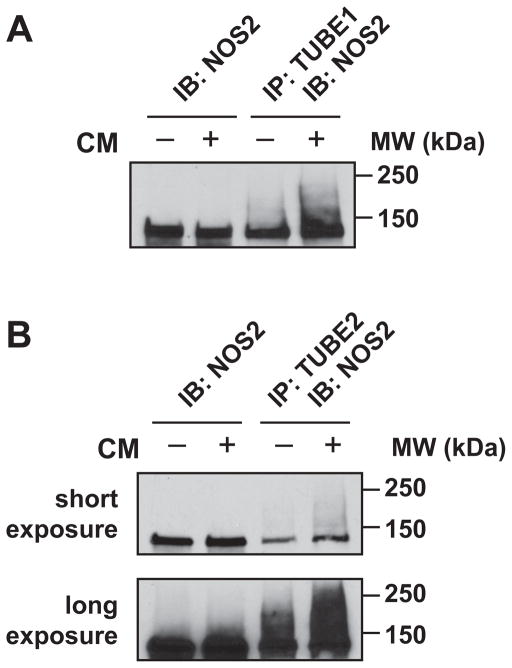
Ubiquitinated NOS2 forms a ternary complex with the proteasome-associated ubiquitin receptor ADRM1, and the ubiquitin hydrolase UCHL5, both of which are important mediators of NOS2 proteasomal degradation [24]. Based on the cytokine-dependent association of NOS2 with these proteins, and with the proteasome itself (Fig. 2B), we reasoned that cytokines should also increase NOS2 ubiquitination. Using agarose-conjugated tandem ubiquitin-binding entities (TUBE1- and TUBE2-agarose) [65], we compared the precipitation of NOS2 and ubiquitin following cytokine treatment in A549 cells overexpressing wt-NOS2. NOS2 was enriched in TUBE pulldowns after cytokine stimulation (Fig. 5A–B), and this coincided with an increase in high MW NOS2 indicative of post-translational modification by ubiquitination. TUBE1 is reported to have a 10-fold higher affinity for K63- versus K48-linked ubiquitin, and TUBE2 has an equal affinity for both conjugates. However, while NOS2 is known to be ubiquitinated and degraded via K48-linked polyubiquitin [66], we did not observe a difference in NOS2 precipitation with the two matrices.
Figure 5.
Cytokine-dependent ubiquitination of NOS2. (A) and (B) A549 cells were infected with Ad.wt-NOS2 for 32 h followed by stimulation ± CM for an additional 16 h. Polyubiquitin-conjugated proteins were precipitated from cell lysates with (A) TUBE1-agarose and (B) TUBE2-agarose and probed for NOS2 by western blotting.
Association of NOS2 with ubiquitin ligases, including a novel NOS2 interactor
We next considered whether our data might better define the scope, and possible regulation, of NOS2-specific ubiquitin ligases in the respiratory epithelium. A spate of recent studies have demonstrated a role for the adapter proteins SPSB1, SPSB2 and SPSB4 in NOS2 ubiquitination and degradation in macrophages [25,67–69], although these proteins have not been characterized in the respiratory epithelium. In our analysis, SPSB1 was upregulated in the NOS2 IP from CM-treated A549 cells, consistent with its induction by LPS in murine macrophages [69].
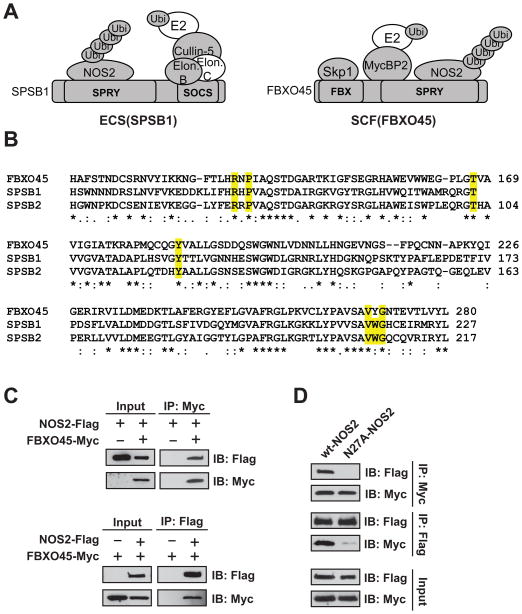
Besides the ECS complexes, a number of other ubiquitin E3 ligases were identified; of these, RO52, HERC1, HUWE1 and Myc-binding protein 2 (MYCBP2) were specific to both the qualitative and quantitative interactomes (Fig. 3B and Table S3). MYCBP2 forms part of a SCF-type E3 ligase complex, SCF(FBXO45), and we also reproducibly identified two other components of SCF(FBXO45) in the NOS2 IPs, F-box/SPRY domain-containing protein 1 (FBXO45) and S-phase kinase-associated protein 1 (SKP1; Fig. 6A) [70]. Interestingly, a BLAST search revealed that SPSB1, SPSB2 and SPSB4, previously described NOS2-interacting proteins, are the closest human paralogues of FBXO45, with FBXO45 and SPSB2 sharing ~45% identity in their B30.2/SPRY domains (Fig. 6B) Most of the residues critical for SPSB1/2 substrate binding are conserved in FBXO45 (Fig. 6B) [53], and homology modeling has predicted that the SPRY domains of FBXO45 and SPSB proteins have nearly identical substrate-binding pockets [71].
Figure 6.
FBXO45 has shared function with SPSB1/SPSB2 as a NOS2-interacting protein. (A) Putative NOS2 complex containing the ECS(SPSB) versus SCF(FBX045) E3 ligases. Shaded proteins were identified in NOS2 co-IPs. (B) Alignment of conserved B30.2/SPRY domains of human FBXO45, SPSB1 and SPSB2. Residues implicated in substrate interaction are highlighted in yellow. (C) 5 μg each of plasmids expressing wt-NOS2-Flag and myc-FBXO45 were transiently transfected separately, or in combination, in HEK-293 cells. After 24 h, total cell lysates were co-IP’d overnight with either M2-Flag or c-Myc agarose, and eluents were analyzed by western blotting. (D) Wild-type or N27A NOS2-Flag was co-transfected with myc-FBXO45 and interactions were analyzed by forward and reverse IP as in (C).
To assess the specificity of the NOS2-FBXO45 interaction, we confirmed the association of FBXO45 and NOS2 by forward and reverse IP in HEK-293 cells overexpressing myc-tagged FBXO45 and wt-NOS2-FLAG (Fig. 6C). We then examined the specificity of the interaction using NOS2 harboring a mutation in the 23DINNN27 motif (N27A) which has previously been shown to abolish binding between NOS2 and the SPSB proteins. The N27A mutant of NOS2 failed to IP with FBXO45, indicating a conserved mechanism for the binding of NOS2 with FBXO45 (Fig. 6D). FBXO45, SPSB1 and SPSB2 were all identified in the same gel band in the NOS2 IPs, allowing us to estimate (based on average of top 2–3 most intense peptides) [72] that these proteins were all within an order of magnitude of relative abundance in NOS2 IPs. Collectively, these data identify FBXO45 as a novel NOS2-interacting protein and suggest that these B30.2/SPRY domain proteins are potential critical regulators of NOS2 proteasomal degradation in the airway epithelium.
DISCUSSION
The importance of PPIs in the regulation of NOS2 cellular localization, enzyme activity and proteasomal degradation is well-described, and it is increasingly appreciated that PPIs are also critical for post-translational modifications (e.g. S-nitrosylation) of proteins by NOS2-derived NO. Nonetheless, a system-wide investigation of NOS2-protein interactions has not previously been reported, nor has MS-based interaction proteomics been applied to the large scale study of any NOS isoform. In this analysis, we have identified a new and direct NOS2-interactor (FBXO45) that likely regulates NOS2 degradation in the respiratory epithelium. Furthermore, using a SILAC-based approach, we have uniquely correlated stimulus-coupled changes in PPIs with the regulation of NOS2 activity and ubiquitination. The idea that inflammatory stimuli may coordinately affect NOS2 homeostasis independently of NOS2 gene transcription is not well-established, and our data likely identify the pathways involved in stimulus-coupled regulation of NOS2. The CM-dependent enrichment of some of the interacting proteins in NOS2 IPs may be correlated with cytokine-inducible changes in protein expression or alternatively may be affected by post-translational modifications (PTMs), including phosphorylation of NOS2 [19,21] or of its associated proteins (e.g. SPSB1) Thus, future integration of quantitative interactome and PTM analyses [73] may help to identify additional mechanisms by which NOS2 PPIs are regulated.
The 26S proteasome and its associated factors comprise a significant part of the NOS2 interactome, highlighting the importance of the ubiquitin-proteasome pathway in NOS2 homeostasis. While the degradation of NOS2 through this pathway was first described ten years ago [66], the interacting proteins and associated protein complexes that facilitate NOS2 ubiquitination, including HSP70/CHIP and ECS(SPSB) have only recently been described [25,52,67]. Association with HSP70-associated E3 ligase CHIP is conserved across NOS isoforms and (as demonstrated for nNOS) [74] occurs with the substrate- and heme-binding cleft of the NOS oxygenase domain. CHIP has been shown to ubiquitinate dozens of proteins and is thought to play a role in clearance of damaged (e.g. oxidized, misfolded) NOS proteins [74]. On the other hand, NOS2 is one of only two mammalian proteins known to interact with the ECS (SPSB) proteins through the [D/E]-[I/L]-N-N-N consensus motif, indicating a higher level of specificity
In addition to harboring the site of interaction with the B30.2/SPRY domain, the N-terminus of NOS2 (specifically, residues 1–77) is necessary and sufficient for the interaction of NOS2 with ADRM1, the proteasome subunit that is a receptor for ubiquitinated proteins [24,31]. These data are consistent with a central role for the N-terminus, and not necessarily the HSP70/CHIP-interacting catalytic domain, in NOS2 ubiquitination and proteasomal degradation. It is also interesting to note that although we reproducibly identified B30.2/SPRY domain proteins in NOS2 IPs, none of the B30.2/SPRY domain proteins were quantified in a recent in-depth proteomic analysis of A549 cells, in which >5000 proteins were identified [75]. While this may reflect subtle differences in analytical approach or cell state, it stands to reason that the high affinity of the B30.2/SPRY-NOS2 interaction facilitates the enrichment of these low-abundance complexes. Finally, a recent study has implicated the ECS(SPSB) ligase (and not HSP70/CHIP) in the ubiquitination and degradation of NOS2 in mouse macrophages [68]; and overexpression of SPSB proteins can drive the subcellular localization of NOS2 [67,68], while CHIP does not affect a similar response [68]. Collectively, these data lend support for a catalytic function of the ECS(SPSB) and SCF(FBXO45) complexes in ubiquitination and proteasomal degradation of NOS2 versus HSP70/CHIP, which may play a crucial role in “quality control”. Furthermore, our identification of the B30.2/SPRY proteins within the respiratory epithelial NOS2 interactome also demonstrate for the first time that these proteins are likely important for NOS2 stability across cell types other than the macrophage. Further work is needed to determine whether NOS2 ubiquitination by ECS(SPSB) or SCF(FBXO45) is important for cytokine-stimulated NOS2 ubiquitination.
The large and complex nature of the NOS2 interactome makes the task of de novo identification of novel NOS2-regulated proteins (e.g. targets of S-nitrosylation) a major challenge. However, we propose that novel NOS2-regulated proteins may be identified based on several criteria including proteins that: preferentially interact with NOS2 under cytokine-inducible conditions; proteins that do not fall into previously characterized networks (e.g ubiquitin-proteasome); and proteins known to be regulated by NO or other redox-modifying agents. A number of proteins in our NOS2 interactome meet one or more of these criteria. DICER, ICAM-1, SIRT1 and TNFAIP2 are intriguing since none associates with a readily definable protein network that was enriched in NOS2 IPs, and all are induced by inflammatory stimuli [76,77]. In addition, SIRT1, HSC70/HSP90-organizing protein (HOP; stress-induced phosphoprotein 1) [78], HSP90 [79,80] and sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) [81,82] are among proteins with reactive Cys thiols that can be modified by NO and thus are candidates for proximity-directed S-nitrosylation by NOS2. The SMN complex is also inactivated by ROS [83], and we hypothesize that this complex may contain redox-sensitive thiols that are targets for S-nitrosylation. Collectively, these proteins warrant further investigation as possible targets of NOS2-mediated S-nitrosylation.
Interestingly, several previously characterized NOS2-interacting proteins were not identified in our analysis, including COX2 [29], cPLA2α [30], glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [84] and Rac1 [32]. Of these, GAPDH was found to be abundant in our A549 cell proteome, and COX2 as well as Rac1 were previously detected in a more in-depth analysis of A549 cells [75]. However, the interactions of these proteins with NOS2 have been primarily characterized in mouse RAW264.7 macrophages, indicating perhaps unique properties of human and murine NOS2 isoforms or differences in the regulation and function of NOS2 between epithelia and macrophages. Given that our system utilized catalytically inactive (E377Q), NO-dependent protein interactions may also factor to explain these discrepancies. Finally, the fact that the A549 cell line is derived from lung adenocarcinoma may suggest differences in neoplastic versus non-transformed cells. Given the importance of respiratory epithelial NOS2 in the pathogenesis of inflammatory lung disease and lung cancer, we believe that further study of NOS2 protein interactions, in particular the contrast of normal and pathological conditions, will provide further insight into the wide ranging role of NOS2 in cellular pathophysiology.
Supplementary Material
Table S1. SILAC labeled pair data. This table contains fold changes and p-values for each unique identified SILAC pair from Rosetta Elucidator workup.
Table S2. SILAC protein expression data. This table contains protein-level fold changes and p-values, # of quantified SILAC pairs and # of identified peptides per protein from Rosetta Elucidator workup.
Table S3. Gene ontology and functional annotation of specific, reproducible NOS2-interacting proteins. This table shows proteins that were identified in both NOS2 IPs (with at least 2+ unique peptides per protein) and absent in the NOS2 IP, as determined from the Scaffold file (Supplemental Data) followed by further analysis using the Venn diagram tool at http://bioinforx.com/free/bxarrays/venndiagram.php. The resulting 139 proteins were analyzed using the Software Tool for Rapid Annotation of Proteins [85] to gather gene ontology and functional annotations for the 139 proteins that overlapped NOS2 IPs (Table S3).
Highlights.
Proteomics identifies hundreds of NOS2-associated proteins in airway epithelial cells
Cytokines modulate the NOS2 interactome
Cytokines modulate NOS2 activity and ubiquitination
The ubiquitin ligase adapter FBXO45 is identified as a novel specific interactor with NOS2.
Acknowledgments
The authors thank Edith Tzeng (University of Pittsburg) for the pAdlox-iNOS vector, Michele Pagano (New York University) for the Myc-FBXO45 expression plasmid, and Carol Ball for assistance in assistance in acquiring Agilent data. This work was supported in part by National Institutes of Health grants HL106121 (M.W.F and H.E.M.) and HL092994 (H.E.M.)
Footnotes
Supplemental Data. A supplemental dataset, “NOS2_MSMS.sf3” containing all assigned MS/MS spectra is available for download at https://proteomecommons.org using hash Pl3Vw1AxtSl162TnDVOOyYnUzYEx83ai6VaFATf7PiA6ms5+3+BqnYWUF1DJK7p2XRibogcElwtIgWuFeC8mbGzgcxIIAAAAAAAADJA=. The file “NOS2_MSMS.sf3” can be viewed using the Scaffold Free Viewer (Proteome Software).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol. 2002;282:L379–85. doi: 10.1152/ajplung.00462.2000. [DOI] [PubMed] [Google Scholar]
- 2.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–22. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, Ma B, Baluk P, Lin MI, McDonald DM, Homer RJ, Sessa WC, Elias JA. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci U S A. 2006;103:11021–6. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A. 1995;92:7809–13. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano K, Chee CB, Gaston B, Lilly CM, Gerard C, Drazen JM, Stamler JS. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A. 1994;91:10089–93. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo FH, Uetani K, Haque SJ, Williams BR, Dweik RA, Thunnissen FB, Calhoun W, Erzurum SC. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100:829–38. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suresh V, Mih JD, George SC. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2007;37:97–104. doi: 10.1165/rcmb.2006-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chibana K, Trudeau JB, Mustovich AT, Hu H, Zhao J, Balzar S, Chu HW, Wenzel SE. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38:936–46. doi: 10.1111/j.1365-2222.2008.02969.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Tochino Y, Chibana K, Trudeau JB, Holguin F, Wenzel SE. Nitric oxide and related enzymes in asthma: relation to severity, enzyme function and inflammation. Clin Exp Allergy. 2012;42:760–8. doi: 10.1111/j.1365-2222.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, Nikam S, Roth M, Sydykov A, Medebach T, Klepetko W, Jaksch P, Dumitrascu R, Garn H, Voswinckel R, Kostin S, Seeger W, Schermuly RT, Grimminger F, Ghofrani HA, Weissmann N. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher ZT, Potts EN, Brahmajothi MV, Foster MW, Auten RL, Foster WM, Marshall HE. NOS2 regulation of LPS-induced airway inflammation via S-nitrosylation of NF-{kappa}B p65. Am J Physiol Lung Cell Mol Physiol. 2011;301:L327–33. doi: 10.1152/ajplung.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kisley LR, Barrett BS, Bauer AK, Dwyer-Nield LD, Barthel B, Meyer AM, Thompson DC, Malkinson AM. Genetic ablation of inducible nitric oxide synthase decreases mouse lung tumorigenesis. Cancer Res. 2002;62:6850–6. [PubMed] [Google Scholar]
- 13.Wei D, Richardson EL, Zhu K, Wang L, Le X, He Y, Huang S, Xie K. Direct demonstration of negative regulation of tumor growth and metastasis by host-inducible nitric oxide synthase. Cancer Res. 2003;63:3855–9. [PubMed] [Google Scholar]
- 14.Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, Wiltrout RH. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med. 2010;207:2455–67. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okayama H, Saito M, Oue N, Weiss JM, Stauffer J, Takenoshita S, Wiltrout RH, Hussain SP, Harris CC. NOS2 enhances KRAS-induced lung carcinogenesis, inflammation and microRNA-21 expression. Int J Cancer. 2013;132:9–18. doi: 10.1002/ijc.27644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang WY, Takiguchi M, Koshiyama Y, Gotoh T, Nagasaki A, Iwase K, Yamamoto K, Takeshima H, Negi A, Mori M. Expression of citrulline-nitric oxide cycle in lipopolysaccharide and cytokine-stimulated rat astroglioma C6 cells. Brain Res. 1999;849:78–84. doi: 10.1016/s0006-8993(99)01987-3. [DOI] [PubMed] [Google Scholar]
- 18.Nussler AK, Billiar TR, Liu ZZ, Morris SM., Jr Coinduction of nitric oxide synthase and argininosuccinate synthetase in a murine macrophage cell line. Implications for regulation of nitric oxide production. J Biol Chem. 1994;269:1257–61. [PubMed] [Google Scholar]
- 19.Tyryshkin A, Gorgun FM, Abdel Fattah E, Mazumdar T, Pandit L, Zeng S, Eissa NT. Src kinase-mediated phosphorylation stabilizes inducible nitric-oxide synthase in normal cells and cancer cells. J Biol Chem. 2010;285:784–92. doi: 10.1074/jbc.M109.055038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Brovkovych V, Brovkovych S, Tan F, Lee BS, Sharma T, Skidgel RA. Dynamic receptor-dependent activation of inducible nitric-oxide synthase by ERK-mediated phosphorylation of Ser745. J Biol Chem. 2007;282:32453–61. doi: 10.1074/jbc.M706242200. [DOI] [PubMed] [Google Scholar]
- 21.Hausel P, Latado H, Courjault-Gautier F, Felley-Bosco E. Src-mediated phosphorylation regulates subcellular distribution and activity of human inducible nitric oxide synthase. Oncogene. 2006;25:198–206. doi: 10.1038/sj.onc.1209030. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Kuncewicz T, Yu ZY, Zou L, Xu X, Kone BC. Protein-protein interactions involving inducible nitric oxide synthase. Acta Physiol Scand. 2003;179:137–42. doi: 10.1046/j.1365-201X.2003.01119.x. [DOI] [PubMed] [Google Scholar]
- 23.Daniliuc S, Bitterman H, Rahat MA, Kinarty A, Rosenzweig D, Lahat N. Hypoxia inactivates inducible nitric oxide synthase in mouse macrophages by disrupting its interaction with alpha-actinin 4. J Immunol. 2003;171:3225–32. doi: 10.4049/jimmunol.171.6.3225. [DOI] [PubMed] [Google Scholar]
- 24.Mazumdar T, Gorgun FM, Sha Y, Tyryshkin A, Zeng S, Hartmann-Petersen R, Jorgensen JP, Hendil KB, Eissa NT. Regulation of NF-kappaB activity and inducible nitric oxide synthase by regulatory particle non-ATPase subunit 13 (Rpn13) Proc Natl Acad Sci U S A. 2010;107:13854–9. doi: 10.1073/pnas.0913495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuang Z, Lewis RS, Curtis JM, Zhan Y, Saunders BM, Babon JJ, Kolesnik TB, Low A, Masters SL, Willson TA, Kedzierski L, Yao S, Handman E, Norton RS, Nicholson SE. The SPRY domain-containing SOCS box protein SPSB2 targets iNOS for proteasomal degradation. J Cell Biol. 2010;190:129–41. doi: 10.1083/jcb.200912087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282:30667–72. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 27.Yu Z, Kuncewicz T, Dubinsky WP, Kone BC. Nitric oxide-dependent negative feedback of PARP-1 trans-activation of the inducible nitric-oxide synthase gene. J Biol Chem. 2006;281:9101–9. doi: 10.1074/jbc.M511049200. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell DA, Erwin PA, Michel T, Marletta MA. S-Nitrosation and regulation of inducible nitric oxide synthase. Biochemistry. 2005;44:4636–47. doi: 10.1021/bi0474463. [DOI] [PubMed] [Google Scholar]
- 29.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–70. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Han C, Lim K, Wu T. Activation of cytosolic phospholipase A2alpha through nitric oxide-induced S-nitrosylation. Involvement of inducible nitric-oxide synthase and cyclooxygenase-2. J Biol Chem. 2008;283:3077–87. doi: 10.1074/jbc.M705709200. [DOI] [PubMed] [Google Scholar]
- 31.Ratovitski EA, Bao C, Quick RA, McMillan A, Kozlovsky C, Lowenstein CJ. An inducible nitric-oxide synthase (NOS)-associated protein inhibits NOS dimerization and activity. J Biol Chem. 1999;274:30250–7. doi: 10.1074/jbc.274.42.30250. [DOI] [PubMed] [Google Scholar]
- 32.Kuncewicz T, Balakrishnan P, Snuggs MB, Kone BC. Specific association of nitric oxide synthase-2 with Rac isoforms in activated murine macrophages. Am J Physiol Renal Physiol. 2001;281:F326–36. doi: 10.1152/ajprenal.2001.281.2.F326. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida M, Xia Y. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J Biol Chem. 2003;278:36953–8. doi: 10.1074/jbc.M305214200. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto A, Comatas KE, Liu L, Stamler JS. Screening for nitric oxide-dependent protein-protein interactions. Science. 2003;301:657–61. doi: 10.1126/science.1079319. [DOI] [PubMed] [Google Scholar]
- 35.Navarro-Lerida I, Martinez-Moreno M, Ventoso I, Alvarez-Barrientos A, Rodriguez-Crespo I. Binding of CAP70 to inducible nitric oxide synthase and implications for the vectorial release of nitric oxide in polarized cells. Mol Biol Cell. 2007;18:2768–77. doi: 10.1091/mbc.E06-12-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazumdar T, Gorgun FM, Sha Y, Tyryshkin A, Zeng S, Hartmann-Petersen R, Jorgensen JP, Hendil KB, Eissa NT. Regulation of NF-{kappa}B activity and inducible nitric oxide synthase by regulatory particle non-ATPase subunit 13 (Rpn13) Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0913495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pflieger D, Gonnet F, de la Fuente van Bentem S, Hirt H, de la Fuente A. Linking the proteins--elucidation of proteome-scale networks using mass spectrometry. Mass Spectrom Rev. 2011;30:268–97. doi: 10.1002/mas.20278. [DOI] [PubMed] [Google Scholar]
- 38.Gavin AC, Maeda K, Kuhner S. Recent advances in charting protein-protein interaction: mass spectrometry-based approaches. Curr Opin Biotechnol. 2011;22:42–9. doi: 10.1016/j.copbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Dunham WH, Mullin M, Gingras AC. Affinity-purification coupled to mass spectrometry: Basic principles and strategies. Proteomics. 2012;12:1576–90. doi: 10.1002/pmic.201100523. [DOI] [PubMed] [Google Scholar]
- 40.Forrester MT, Seth D, Hausladen A, Eyler CE, Foster MW, Matsumoto A, Benhar M, Marshall HE, Stamler JS. Thioredoxin-interacting protein (Txnip) is a feedback regulator of S-nitrosylation. J Biol Chem. 2009;284:36160–6. doi: 10.1074/jbc.M109.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kibbe MR, Tzeng E, Gleixner SL, Watkins SC, Kovesdi I, Lizonova A, Makaroun MS, Billiar TR, Rhee RY. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. Journal of Vascular Surgery. 2001;34:156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 42.Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nature Protocols. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 43.Peschiaroli A, Scialpi F, Bernassola F, Pagano M, Melino G. The F-box protein FBXO45 promotes the proteasome-dependent degradation of p73. Oncogene. 2009;28:3157–66. doi: 10.1038/onc.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996;379:466–9. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- 45.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 46.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 47.Nittis T, Guittat L, LeDuc RD, Dao B, Duxin JP, Rohrs H, Townsend RR, Stewart SA. Revealing novel telomere proteins using in vivo cross-linking, tandem affinity purification, and label-free quantitative LC-FTICR-MS. Mol Cell Proteomics. 2010;9:1144–56. doi: 10.1074/mcp.M900490-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng L, Dai H, Zhan Y, He Y, Stepaniants SB, Bassett DE. Rosetta error model for gene expression analysis. Bioinformatics. 2006;22:1111–21. doi: 10.1093/bioinformatics/btl045. [DOI] [PubMed] [Google Scholar]
- 49.Foster MW, Yang Z, Gooden DM, Thompson JW, Ball CH, Turner ME, Hou Y, Pi J, Moseley MA, Que LG. Proteomic characterization of the cellular response to nitrosative stress mediated by s-nitrosoglutathione reductase inhibition. J Proteome Res. 2012;11:2480–91. doi: 10.1021/pr201180m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen PF, Tsai AL, Berka V, Wu KK. Mutation of Glu-361 in human endothelial nitric-oxide synthase selectively abolishes L-arginine binding without perturbing the behavior of heme and other redox centers. J Biol Chem. 1997;272:6114–8. doi: 10.1074/jbc.272.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suliman HB, Babiker A, Withers CM, Sweeney TE, Carraway MS, Tatro LG, Bartz RR, Welty-Wolf KE, Piantadosi CA. Nitric oxide synthase-2 regulates mitochondrial Hsp60 chaperone function during bacterial peritonitis in mice. Free Radic Biol Med. 2010;48:736–46. doi: 10.1016/j.freeradbiomed.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sha Y, Pandit L, Zeng S, Eissa NT. A critical role for CHIP in the aggresome pathway. Mol Cell Biol. 2009;29:116–28. doi: 10.1128/MCB.00829-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filippakopoulos P, Low A, Sharpe TD, Uppenberg J, Yao S, Kuang Z, Savitsky P, Lewis RS, Nicholson SE, Norton RS, Bullock AN. Structural basis for Par-4 recognition by the SPRY domain- and SOCS box-containing proteins SPSB1, SPSB2, and SPSB4. J Mol Biol. 2010;401:389–402. doi: 10.1016/j.jmb.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann M. Functional and quantitative proteomics using SILAC. Nature Reviews Molecular Cell Biology. 2006;7:952–958. doi: 10.1038/nrm2067. [DOI] [PubMed] [Google Scholar]
- 55.Hilger M, Mann M. Triple SILAC to Determine Stimulus Specific Interactions in the Wnt Pathway. Journal of Proteome Research. 2012;11:982–994. doi: 10.1021/pr200740a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammond DE, Hyde R, Kratchmarova I, Beynon RJ, Blagoev B, Clague MJ. Quantitative Analysis of HGF and EGF-Dependent Phosphotyrosine Signaling Networks. Journal of Proteome Research. 2010;9:2734–2742. doi: 10.1021/pr100145w. [DOI] [PubMed] [Google Scholar]
- 57.Doherty MK, Hammond DE, Clagule MJ, Gaskell SJ, Beynon RJ. Turnover of the Human Proteome: Determination of Protein Intracellular Stability by Dynamic SILAC. Journal of Proteome Research. 2009;8:104–112. doi: 10.1021/pr800641v. [DOI] [PubMed] [Google Scholar]
- 58.Fuller HR, Man NT, Lamle T, Thanhle T, Keough RA, Asperger A, Gonda TJ, Morris GE. The SMN interactome includes Myb-binding protein 1a. J Proteome Res. 2010;9:556–63. doi: 10.1021/pr900884g. [DOI] [PubMed] [Google Scholar]
- 59.Carissimi C, Saieva L, Baccon J, Chiarella P, Maiolica A, Sawyer A, Rappsilber J, Pellizzoni L. Gemin8 is a novel component of the survival motor neuron complex and functions in small nuclear ribonucleoprotein assembly. J Biol Chem. 2006;281:8126–34. doi: 10.1074/jbc.M512243200. [DOI] [PubMed] [Google Scholar]
- 60.Gubitz AK, Mourelatos Z, Abel L, Rappsilber J, Mann M, Dreyfuss G. Gemin5, a novel WD repeat protein component of the SMN complex that binds Sm proteins. J Biol Chem. 2002;277:5631–6. doi: 10.1074/jbc.M109448200. [DOI] [PubMed] [Google Scholar]
- 61.Charroux B, Pellizzoni L, Perkinson RA, Yong J, Shevchenko A, Mann M, Dreyfuss G. Gemin4. A novel component of the SMN complex that is found in both gems and nucleoli. J Cell Biol. 2000;148:1177–86. doi: 10.1083/jcb.148.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trinkle-Mulcahy L, Boulon S, Lam YW, Urcia R, Boisvert FM, Vandermoere F, Morrice NA, Swift S, Rothbauer U, Leonhardt H, Lamond A. Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes. Journal of Cell Biology. 2008;183:223–239. doi: 10.1083/jcb.200805092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ying WZ, Sanders PW. Accelerated ubiquitination and proteasome degradation of a genetic variant of inducible nitric oxide synthase. Biochem J. 2003;376:789–94. doi: 10.1042/BJ20031058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh A, Chawla-Sarkar M, Stuehr DJ. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J. 2011;25:2049–60. doi: 10.1096/fj.10-180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hjerpe R, Aillet F, Lopitz-Otsoa F, Lang V, England P, Rodriguez MS. Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Rep. 2009;10:1250–8. doi: 10.1038/embor.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolodziejski PJ, Musial A, Koo JS, Eissa NT. Ubiquitination of inducible nitric oxide synthase is required for its degradation. Proc Natl Acad Sci U S A. 2002;99:12315–20. doi: 10.1073/pnas.192345199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishiya T, Matsumoto K, Maekawa S, Kajita E, Horinouchi T, Fujimuro M, Ogasawara K, Uehara T, Miwa S. Regulation of inducible nitric-oxide synthase by the SPRY domain- and SOCS box-containing proteins. J Biol Chem. 2011;286:9009–19. doi: 10.1074/jbc.M110.190678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsumoto K, Nishiya T, Maekawa S, Horinouchi T, Ogasawara K, Uehara T, Miwa S. The ECS(SPSB) E3 ubiquitin ligase is the master regulator of the lifetime of inducible nitric-oxide synthase. Biochem Biophys Res Commun. 2011;409:46–51. doi: 10.1016/j.bbrc.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 69.Lewis RS, Kolesnik TB, Kuang Z, D’Cruz AA, Blewitt ME, Masters SL, Low A, Willson T, Norton RS, Nicholson SE. TLR regulation of SPSB1 controls inducible nitric oxide synthase induction. J Immunol. 2011;187:3798–805. doi: 10.4049/jimmunol.1002993. [DOI] [PubMed] [Google Scholar]
- 70.Saiga T, Fukuda T, Matsumoto M, Tada H, Okano HJ, Okano H, Nakayama KI. Fbxo45 forms a novel ubiquitin ligase complex and is required for neuronal development. Mol Cell Biol. 2009;29:3529–43. doi: 10.1128/MCB.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woo JS, Suh HY, Park SY, Oh BH. Structural basis for protein recognition by B30.2/SPRY domains. Mol Cell. 2006;24:967–76. doi: 10.1016/j.molcel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Silva JC, Gorenstein MV, Li GZ, Vissers JP, Geromanos SJ. Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Mol Cell Proteomics. 2006;5:144–56. doi: 10.1074/mcp.M500230-MCP200. [DOI] [PubMed] [Google Scholar]
- 73.Yachie N, Saito R, Sugiyama N, Tomita M, Ishihama Y. Integrative features of the yeast phosphoproteome and protein-protein interaction map. PLoS Comput Biol. 2011;7:e1001064. doi: 10.1371/journal.pcbi.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng HM, Morishima Y, Pratt WB, Osawa Y. Modulation of heme/substrate binding cleft of neuronal nitric-oxide synthase (nNOS) regulates binding of Hsp90 and Hsp70 proteins and nNOS ubiquitination. J Biol Chem. 2012;287:1556–65. doi: 10.1074/jbc.M111.323295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geiger T, Wehner A, Schaab C, Cox J, Mann M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol Cell Proteomics. 2012;11:M111 014050. doi: 10.1074/mcp.M111.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiesen JL, Tomasi TB. Dicer is regulated by cellular stresses and interferons. Mol Immunol. 2009;46:1222–8. doi: 10.1016/j.molimm.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang HN, Li L, Gao P, Chen HZ, Zhang R, Wei YS, Liu DP, Liang CC. Involvement of the p65/RelA subunit of NF-kappaB in TNF-alpha-induced SIRT1 expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 2010;397:569–75. doi: 10.1016/j.bbrc.2010.05.160. [DOI] [PubMed] [Google Scholar]
- 78.Marozkina NV, Yemen S, Borowitz M, Liu L, Plapp M, Sun F, Islam R, Erdmann-Gilmore P, Townsend RR, Lichti CF, Mantri S, Clapp PW, Randell SH, Gaston B, Zaman K. Hsp 70/Hsp 90 organizing protein as a nitrosylation target in cystic fibrosis therapy. Proc Natl Acad Sci U S A. 2010;107:11393–8. doi: 10.1073/pnas.0909128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Retzlaff M, Stahl M, Eberl HC, Lagleder S, Beck J, Kessler H, Buchner J. Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Rep. 2009;10:1147–53. doi: 10.1038/embor.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez-Ruiz A, Villanueva L, Gonzalez de Orduna C, Lopez-Ferrer D, Higueras MA, Tarin C, Rodriguez-Crespo I, Vazquez J, Lamas S. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci U S A. 2005;102:8525–30. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008;44:361–9. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ying J, Tong X, Pimentel DR, Weisbrod RM, Trucillo MP, Adachi T, Cohen RA. Cysteine-674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arterioscler Thromb Vasc Biol. 2007;27:783–90. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- 83.Wan L, Ottinger E, Cho S, Dreyfuss G. Inactivation of the SMN complex by oxidative stress. Mol Cell. 2008;31:244–54. doi: 10.1016/j.molcel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chakravarti R, Aulak KS, Fox PL, Stuehr DJ. GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 2010;107:18004–9. doi: 10.1073/pnas.1008133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhatia VN, Perlman DH, Costello CE, McComb ME. Software tool for researching annotations of proteins: open-source protein annotation software with data visualization. Anal Chem. 2009;81:9819–23. doi: 10.1021/ac901335x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. SILAC labeled pair data. This table contains fold changes and p-values for each unique identified SILAC pair from Rosetta Elucidator workup.
Table S2. SILAC protein expression data. This table contains protein-level fold changes and p-values, # of quantified SILAC pairs and # of identified peptides per protein from Rosetta Elucidator workup.
Table S3. Gene ontology and functional annotation of specific, reproducible NOS2-interacting proteins. This table shows proteins that were identified in both NOS2 IPs (with at least 2+ unique peptides per protein) and absent in the NOS2 IP, as determined from the Scaffold file (Supplemental Data) followed by further analysis using the Venn diagram tool at http://bioinforx.com/free/bxarrays/venndiagram.php. The resulting 139 proteins were analyzed using the Software Tool for Rapid Annotation of Proteins [85] to gather gene ontology and functional annotations for the 139 proteins that overlapped NOS2 IPs (Table S3).