Abstract
Objective
Childhood deprivation is inimical to health throughout the life-course. Early experiences of stress could play a role in health inequalities. An important aspect of childhood poverty that has not received much attention is cardiovascular reactivity and recovery to acute stressors.
Methods
Piecewise, multi-level growth curve regression was used to examine blood pressure reactivity and recovery to mental arithmetic among late adolescents (M(SD) = 17.3 (1.0) years; n = 185) as a function of early childhood poverty (9 years). We also tested whether exposure to family conflict at age 13 mediated expected linkages between childhood poverty and adolescent blood pressure reactivity and recovery to an acute stressor.
Results
Blood pressure reactivity was unaffected by household income during childhood, but late adolescents with lower household income during childhood showed slower systolic (b=−0.29, p=.004) and diastolic (b=−0.19, p=.002) recovery. These results include age and gender as statistical covariates. The significant poverty impact on systolic but not on diastolic blood pressure recovery was mediated by exposure to family conflict [95% confidence interval −0.1400, −0.0012].
Conclusions
We show that late adolescents who grew up in poverty have delayed blood pressure recovery from an acute stressor. Furthermore, childhood exposure to family conflict, a well-documented component of early childhood deprivation, accounted for some of the adverse effects of childhood poverty on stressor recovery among these adolescents. We discuss the importance of considering physiological stress accompanying early experiences of deprivation in thinking about health inequalities.
Keywords: Childhood poverty, stress reactivity and recovery, blood pressure
Socioeconomic status (SES) is inversely related to a wide range of mental and physical health outcomes (1–4). Moreover, these health inequalities may have their origins in early childhood. Low SES children appear to be placed on trajectories leading to ill health as adults (5,6,7) with prospective relations between early childhood deprivation and adult health maintained independently of adult SES (8,9). One pathway that may account for early low SES and poorer lifetime health is childhood stress (10,11,12). Perhaps exposure to psychosocial and physical stressors that often accompany early childhood poverty influences the body in ways that eventually elevate cardiovascular and neuroendocrine processes that proffer disease. Two primary domains of evidence have been offered to support a stress related explanation of health inequalities. First, psychosocial stressors (e.g., family conflict, harsh, insensitive parenting) and physical stressors (e.g., substandard housing, exposure to toxins) are robustly associated with childhood poverty (13,14). Second, cardiovascular and neuroendocrine markers of chronic physiological stress are elevated in low-income children (5,8,9,13,15). In this paper we examine dynamic physiological stress responses, specifically blood pressure, among 17 and 18 year olds as a function of childhood poverty. We then investigate exposure to family conflict as an underlying mechanism that might explain some of the expected childhood poverty – dynamic cardiovascular linkages.
Childhood SES and Dynamic Cardiovascular Processes
A large literature reveals that lower SES is associated with higher resting blood pressure in children (5,6,7,8,9). However, an important omission in the SES and cardiovascular health literature has been inattention to dynamic physiological stress responses, specifically reactivity and recovery to acute stressors. This is an important limitation in research on childhood SES and health because stressor reactivity and recovery may be more sensitive indicators of early pathogenesis in the cardiovascular system compared to resting, baseline cardiovascular functioning (16,17,18,19,20,21). In a meta-analysis of prospective studies, adults with greater cardiovascular reactivity and slower recovery to acute stressors (most typically, mental arithmetic) were significantly more likely to have elevated cardiovascular disease risk factors (22). As an illustration, persons with greater blood pressure reactivity were 23% more likely to develop hypertension compared to those with relatively lower reactivity to acute stressors.
A few investigators have examined blood pressure reactivity in relation to acute stressor exposure as a function of childhood SES. Primary school aged children with lower SES backgrounds manifest elevated blood pressure reactivity to acute psychosocial stressors (23,24,25). A similar pattern has been uncovered among adolescents (23,25,26). Two studies, however, found the opposite pattern, showing muted blood pressure reactivity among lower SES adolescents (27,28). One possible reason for the mixed SES and blood pressure reactivity data could be moderating variables. Jackson and colleagues (29) found an inverse relationship between neighborhood SES and blood pressure reactivity for white adolescents but the opposite pattern among black youth. Wilson and colleagues (30) also found that neighborhood and family SES interacted to affect blood pressure reactivity among adolescents. For adolescents from low SES families, neighborhood SES was inversely related to blood pressure reactivity; whereas for those from high SES backgrounds, neighborhood SES was positively associated with reactivity. In sum the relationship between childhood SES and blood pressure reactivity is mixed with some studies uncovering a positive and others a negative association between childhood SES and blood pressure reactivity.
Fewer studies have examined cardiovascular recovery following exposure to an acute stressor in relation to SES. Walter and Hofman (31) contrasted heart rate recovery following a standard exercise protocol in fourth graders living either in an affluent New York City suburb or a low SES borough of the city. White but not Black, low SES neighborhood children had less efficient heart rate recovery relative to their middle SES counterparts. Two studies, however, found no association between SES and blood pressure recovery to psychosocial stressors among 13 year olds (27,29).
Summarizing, there are few studies on childhood SES and dynamic blood pressure changes to acute stressors, and the results that do exist are mixed, particularly for cardiovascular reactivity. This likely reflects both methodological and conceptual reasons; for example to obtain reliable estimates of resting cardiovascular functioning, six or more measurements, following an initial assessment, are recommended (32, 33). Most of the prior studies are cross-sectional and none of the above studies on SES and cardiovascular responses to stressors capitalized on recent analytic techniques for modeling blood pressure changes over time.
Childhood SES, Conflict and Stress
One reason why early childhood poverty might adversely affect the developing cardiovascular system is because of exposure to conflict and hostility. Children in lower SES families are more likely to be exposed to violence both inside (34) and outside (35) the home. Lower SES children interact more with aggressive peers (36,37) and are exposed to more conflictual family relationships (38). Their parents also tend to use harsher, more punitive parenting practices (39,40). Finally, in a meta-analysis, Grant and colleagues (41) documented moderate to large effect sizes between familial SES and harsh parenting practices.
Thus it is plausible that altered physiological stress responses to environmental demands might explain how early childhood experiences of conflict could get under the skin, leading to elevated morbidity and mortality in adulthood (9,42). Conflictual interactions among adults precipitate elevated sympathetic arousal in children (43) and in another study, adolescent girls with more frequent, naturalistic negative social interactions showed elevated trajectories of resting blood pressure, over a two year period (44). Finally, college students who reported more strained family relationships growing up evidenced greater blood pressure reactivity to an acute stressor (45). Thus there is evidence to show that: 1) lower SES children experience higher levels of conflict and harsh parenting; and 2) exposure to higher levels of conflict and negative social interactions can precipitate elevated physiological stress, including greater cardiovascular reactivity to acute stressors.
Three studies have examined more directly the potential interplay among SES, conflict, and physiological stress responses. Wright and colleagues (46) found inverse relations between SES and resting blood pressure in a sample 11-year-olds and this association was stronger for children whose mothers were higher in trait hostility. In an adult sample, the Whitehall study of British civil servants (47) plus an American study of children and adolescents (25), demonstrated that significant associations between SES and blood pressure reactivity were mediated by individual levels of hostility.
Summary and Hypotheses
Lower SES children have higher resting blood pressure and other indices of elevated chronic physiological stress compared to their more advantaged counterparts. However, few investigators of childhood SES have examined dynamic physiological processes in response to acute stressors or examined their possible longitudinal sequencing later on in the life course. The present paper builds upon earlier work both methodologically and conceptually. We assess resting blood pressure levels over a longer period of time to better ensure reliable assessment and leverage advanced analytic techniques to model blood pressure reactivity and recovery. We then test whether expected childhood SES – late adolescent cardiovascular dynamic relations are explained, in a longitudinal design, by exposure to family conflict.
We hypothesize that childhood poverty will be associated with slower returns to baseline, resting levels of blood pressure during a standard cardiovascular reactivity protocol among late adolescents. Because both hypo- and hyper- blood pressure reactivity to acute stressors have been uncovered among low- relative to middle-SES children and adults, we have no clear hypothesis for the relations between childhood poverty and later cardiovascular reactivity. We predict that SES alterations of blood pressure dynamic stress responses will be at least partially mediated by the higher levels of family conflict exposure that are more common in lower SES households.
Method
Participants
One hundred and eighty five late adolescents (M (SD) = 17.3 (1.0) years; 53 % male) who were part of a longitudinal study of rural poverty were included in the sample. These individuals were initially recruited when they were 8–10 years old (M (SD) = 9.2 (1.1) years) in 1995–1998 from rural areas in upstate New York with approximately half of the sample from families at or below the federal poverty line (income-to- needs ratio = 1) and the other half from families 2–4 times the poverty line, the economic segment of the majority of Americans. Income-to-needs is an annually adjusted, per capita index of household income used throughout the United States to determine poverty statistics as well as eligibility for various social service programs. At Wave 1, the mean (SD) income-to-needs ratio of the sample was 1.88 (1.10). The sample is predominantly white (95.1%) which is representative of the rural counties from which the sample was drawn.
Procedure
Information from three waves of data collection at ages 9, 13, and 17 are included herein. Because of the size and complexity of data collection, details are provided only on the measures of family conflict exposure and blood pressure. More details on the entire protocol are available from the first author. The study was approved by the Cornell University IRB and participants provided assent and their parents informed consent at each wave of data collection.
Measures
Income-to-needs was measured at each wave and is an annually adjusted, per capita index administered by the federal government to index poverty. An income-to-needs ratio of 1 equals the official US poverty line.
To assess blood pressure, the participant was restricted from vigorous physical activity for one hour prior to data collection and resting blood pressure was taken while he or she sat quietly reading with their non-dominant arm supported at heart height. Seven readings were taken at two minute intervals with an automated Critikon Dinamap Model 1846SXP. The mean of the second through seventh reading was used as the indicator of resting blood pressure in accordance with measurement data on blood pressure reliability (32,33).
The measurements of reactivity and recovery in response to the acute stressor are described next. Without warning, the participant was informed she or he would now be given a math test. Individuals heard a four-digit number and were instructed to mentally subtract aloud by a two-digit number for a period of 12 minutes (reactivity phase). The starting four-digit number was changed at regular intervals. Participants were then told the math test was completed, encouraged to relax and return to their reading, and assured no additional testing would occur. Thirty seconds following completion of the math test, the first of five blood pressure recovery measures (total time = 10 minutes) was taken. Automated readings were taken every two minutes throughout the entire blood pressure protocol. The same dynamic cardiovascular procedure was completed at Waves 2 and 3 of data collection.
Exposure to family conflict was assessed at all waves of data collection with information from Wave 2 considered herein as a mediator. The participant’s mother evaluated family conflict within the household with a subscale from the Family Environment Scales (48). The family conflict subscale consists of nine true/false items. A sample item is “Family members often criticize each other.” Scores ranged from 1.00 – 2.00, M = 1.65 (0.25) with higher scores indicative of less conflict exposure. This scale has undergone extensive psychometric development with multiple validation evidence. The internal reliability for this subscale herein (α = .74) is similar to psychometric information in the Family Environment Scales Manual (48).
Covariates included were Wave 1 age, gender (0 = male), and race (0 = non-White).
Data Analysis Plan
Univariate and bivariate statistics were examined for the independent variable income-toneeds ratio and individual level covariates (age, gender, race). Variables that were related to the outcome (diastolic/systolic blood pressure) at the p < .20 level were retained for inclusion in multivariate models.
Piecewise growth curve modeling
To investigate blood pressure dynamics during the reactivity and recovery periods, we used a two-level, piecewise growth curve model to estimate change during the reactivity and recover periods (49,50,51). The piecewise or spline approach allows growth estimation to be separated into distinct linear components according to specific time periods (50,51). Since the dependent variable in this study, cardiovascular activity, was by design divided into two distinct stages (reactivity and recovery), piecewise modeling was appropriate.
Following data exploration and initial model fits, we determined that a non-linear growth curve for both the reactivity and recovery periods provided the best fit to these data. Thus both linear and quadratic terms were included in our final estimates of cardiovascular growth. The first piece of this model covers growth during the reactivity period (Stage 1), and the second piece covers growth during the recovery period (Stage 2). The transition from reactivity to recovery occurred at the start of the recovery period (Table 1).
Table 1.
Coding of Piecewise Parameters
| BL | RE1 | RE2 | RE3 | RE4 | RE5 | RE6 | RC1 | RC2 | RC3 | RC4 | RC5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| π0i | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| π1i | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 6 | 6 | 6 | 6 | 6 |
| π2i | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | 4 | 5 |
| π3i | 0 | 1 | 4 | 9 | 16 | 25 | 36 | 36 | 36 | 36 | 36 | 36 |
| π4i | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 9 | 16 | 25 |
BL = resting blood pressure level (M of six resting readings); RE# = reactivity period reading number (every two minutes) during acute stressor; RC# = recovery period reading number (every two minutes) beginning 30 seconds after the cessation of the acute stressor.
Specifically, growth during the reactivity and recovery periods at time t for person i was modeled at level 1 (i.e., intra-individual level) as:
where Yti is the systolic or diastolic blood pressure at time t for person i; π0i, the intercept, represents resting blood pressure for person i at baseline; π1i represents the expected linear change in blood pressure for person i during Stage 1 (i.e., reactivity); π2i represents the expected linear change in blood pressure during recovery for person i during Stage 2 (i.e., recovery); π3i represents the quadratic change (i.e., acceleration) in blood pressure for person i during Stage 1 (reactivity); π4i represents quadratic change in blood pressure for person i during Stage 2 (recovery) and εti is the residual term. In sum, the level 1 model has an initial status parameter that is constant over time, and four growth parameters, two for the reactivity period and two for the recovery period.
It is important to note that the interpretation of π0i, the intercept, depends on the scaling of our time variable. Since we have chosen baseline as the point at which all growth weights are set to 0 (Table 1), we interpret the intercept as the predicted value of blood pressure at baseline (52). Also, since this model includes quadratic terms, the interpretation of the lower-order linear parameters are also conditional on our chosen time coding, and represent the instantaneous rate of change at the beginning of each time period (52). In this model, the quadratic parameters represent acceleration in growth over time (33). Specifically, 2*π3i represents the rate of change in linear reactivity for a 1-unit change in time, and 2*π4i represents the rate of change in linear recovery for a 1-unit change in time (52).1 Given the chosen coding, a 1-unit change in time is two minutes.
Our level 2 (i.e., inter-individual) model represents inter-individual variability in initial status and linear and quadratic growth during the two growth stages. The optimal random and fixed structure at Level 2 was determined using methods described by Zuur, Ieno, Walker, Savaliev and Smith (53) through the use of nested log-likelihood tests, and comparing the AIC and BIC for candidate models. Using these methods for both systolic and diastolic blood pressure, we determined that the best fit model included two individual level predictors, gender and Wave 1 income-to-needs and a random structure as specified below. Gender and Wave 1 income-to-needs are used to assess variability in randomly varying slopes and intercepts (i.e., differences in growth trajectories between individuals).
where β00 is the average resting blood pressure level at baseline; β10 is the average instantaneous linear growth during Stage 1 (reactivity); β20 is the average instantaneous linear growth during Stage 2 (recovery); β30 is the average acceleration during Stage 1 (reactivity); and β40 is the average acceleration during Stage 2 (recovery). The error terms in the equations for the intercept, for linear growth during recovery and for quadratic growth during reactivity (r0i, r2i and r3i, respectively) represent inter-individual variability in these three parameters. Continuous covariates were grand-centered in all models, but gender was left uncentered.
Mediation
Mediation of the effect of Wave 1 income-to-needs on Wave 3 cardiovascular reactivity by Wave 2 family conflict was assessed using a 2-2-1 mediation model (54). This model combines ordinary least squares (OLS) and MLMs in order to investigate if an inter-individual variable (i.e., exposure to family conflict) mediates the association between an inter-individual predictor (i.e., income-to-needs) and an intra-individual outcome (e.g., instantaneous linear growth in systolic blood pressure during recovery). An OLS model is estimated with the inter-individual predictor of interest (income-to-needs) predicting the inter-individual mediator (family conflict exposure). If a significant relationship exists between the mediator and outcome in the OLS model, the mediator is then entered into the multilevel model at level 2 to test for mediation of the relationship of interest. To determine a point estimate of the mediated effect, the coefficient from the OLS model is multiplied by the coefficient of the mediator from the multilevel model. To test the significance of this effect, we derived a 95% confidence interval by using the Monte Carlo Method for Assessing Mediation (MCMAM) (55,56). Our final mediation model is presented below:
Multilevel analyses were performed in HLM 6.08 with robust standard errors under restricted maximum likelihood (REML), unless otherwise stated. All other analyses were conducted in SPSS. All results were evaluated at p < .05 unless otherwise specified.
Missing data
Of the 241 Wave 3 respondents, 211 provided cardiovascular reactivity and recovery data (i.e., 12.4% missing data rate). The 30 participants who did not provide cardiovascular data did not differ from non-missing individuals on Wave 2 family conflict exposure, race, age or gender at Wave 1. Missingness on covariates in the HLM analyses reduced the final analytic sample to 185.
Results
Descriptive and Zero Order Correlational Data
Average resting systolic blood pressure at Wave 3 was 114.86 (13.23), and average resting diastolic blood pressure was 64.32 (6.79). For descriptive purposes, the average systolic blood pressure reactivity at Wave 3 (calculated as the baseline value subtracted from the mean over the reactivity period) was 5.67 (6.57), and average diastolic reactivity was 3.72 (4.17). Average systolic blood pressure recovery (calculated as the mean over the recovery period subtracted from the mean over the reactivity period) was 6.52 (6.53), and average diastolic recovery was 3.61 (4.12). Correlations among the outcome variables (reactivity and recovery), primary predictor variables and individual level covariates are presented in Table 2. At the cutoff level of p < .20, Wave 3 resting systolic blood pressure was associated with Wave 1 age and Wave 1 income-to-needs, and Wave 3 resting diastolic blood pressure was associated with Wave 1 income-to-needs (Table 2). Average Wave 3 systolic and diastolic reactivity and recovery were related to Wave 2 family conflict and Wave 1 income-to-needs, and were all significantly inter-correlated (Table 2). There was also a significant difference in systolic, but not diastolic, blood pressure by gender (tsys(183)=6.47, p < .001) with males having higher resting blood pressure than females (M(SD)= 120.22 (13.06) vs. M(SD)=108.81 (10.60)). At the cut-off level of p < .20, there was also a difference in systolic, but not diastolic, reactivity and recovery by gender, with males having greater reactivity and recovery than females. There was no difference in either resting systolic or diastolic blood pressure, or systolic or diastolic reactivity or recovery, by race. On the basis of these bivariate results, age, gender, and income-to-needs were retained for inclusion in multivariate models (i.e., these three variables were initially entered in all randomly varying equations for our growth model). However, on the basis of nested-log likelihood tests and the AIC and BIC, we removed several of these variables from our model equations. Final models are presented in Tables 3 and 4 for systolic and diastolic blood pressure, respectively.
Table 2.
Zero order correlations
| Resting SBP, Wave 3 | Resting DBP, Wave 3 | Family conflict, Wave 2 | Income-to-needs, Wave 1 | Age, Wave 1 | Gender, Wave 1 | Race, Wave 1 | SBP reactivity difference score, Wave 3 | SBP recovery difference score, Wave 3 | DBP reactivity difference score, Wave 3 | DBP recovery difference score, Wave 3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Resting SBP, Wave 3 | 1.00 | ||||||||||
| Resting DBP, Wave 3 | .57*** | 1.00 | |||||||||
| Family Conflict, Wave 2 | .03 | .05 | 1.00 | ||||||||
| Income-to-needs, Wave 1 | −.21** | −.15* | .28*** | 1.00 | |||||||
| Age, Wave 1 | .11^ | .07 | −.01 | .07 | 1.00 | ||||||
| Gender, Wave 1 | −.43*** | −.04 | −.04 | .06 | −.14^ | 1.00 | |||||
| Race, Wave 1 | −.01 | .04 | −.10^ | −.11^ | −.09 | .04 | 1.00 | ||||
| SBP reactivity difference score, Wave 3 | −.01 | −.03 | .19* | .25** | .08 | −.11^ | .08 | 1.00 | |||
| SBP recovery difference score, Wave 3 | .04 | −.06 | .21** | .21** | .06 | −.10^ | .04 | .72*** | 1.00 | ||
| DBP reactivity difference score, Wave 3 | .004 | −.11^ | .24** | .26*** | .004 | −.05 | −.06 | .63*** | .47*** | 1.00 | |
| DBP recovery difference score, Wave 3 | .03 | .02 | .18* | .20** | −.02 | −.01 | .003 | .51*** | .53*** | .73*** | 1.00 |
p <.20;
p < .05;
p < .01;
p < .001
Table 3.
Parameter Estimates from Multivariate Models with Wave 3 SBP as the Dependent Variablea
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Fixed Effects, b (SE) | |||
| Resting blood pressure | |||
| Mean resting blood pressure | 116.83 (0.98)*** | 122.86 (1.33)*** | 122.82 (1.32)*** |
| Gender (female) | -- | −12.84 (1.85)*** | −12.74 (1.84)*** |
| Wave 2 family conflict | -- | -- | 5.07 (3.38) |
| Instantaneous growth during reactivity | |||
| Mean growth | 2.86 (0.32)*** | 2.86 (0.32)*** | 2.86 (0.32)*** |
| Instantaneous growth during recovery | |||
| Mean growth | −2.24 (0.41)*** | −2.51 (0.42)*** | −2.50 (0.42)*** |
| Gender (Female) | 0.58 (0.24)* | 0.56 (0.24)* | |
| Wave 1 income-to-needs | −0.29 (0.10)** | −0.24 (0.11)* | |
| Wave 2 family conflict | -- | −0.96 (0.48)* | |
| Acceleration during reactivity | |||
| Mean acceleration | −0.44 (0.05)*** | −0.44 (0.05)*** | −0.44 (0.05)*** |
| Acceleration during recovery | |||
| Mean acceleration | 0.25 (0.08)** | 0.25 (0.08)** | 0.25 (0.08)** |
| Random Effects | |||
| σ2b, intercept | 197.55*** | 157.22*** | 156.00*** |
| σ2b, linear recovery | 2.17*** | 2.08*** | 2.08*** |
| σ2b, quadratic reactivity | 0.013*** | 0.013*** | 0.013*** |
| σ2w | 35.32 | 35.32 | 35.32 |
p < .05;
p < .01;
p < .001
All models estimated under restricted maximum likelihood.
Table 4.
Parameter Estimates from Multivariate Models with Wave 3 DBP as the Dependent Variablea
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Fixed Effects, b (SE) | |||
| Resting blood pressure | |||
| Mean resting blood pressure | 65.54 (0.51)*** | 66.14 (0.77)*** | 66.10 (0.75)*** |
| Gender (female) | -- | −1.27 (1.03) | −1.18 (1.01) |
| Wave 2 family conflict | -- | -- | 4.39 (2.07)* |
| Instantaneous growth during reactivity | |||
| Mean growth | 1.89 (0.22)*** | 1.89 (0.22)*** | 1.89 (0.22)*** |
| Instantaneous growth during recovery | |||
| Mean growth | −1.30 (0.24)*** | −1.37 (0.26)*** | −1.36 (0.26)*** |
| Gender (Female) | -- | 0.14 (0.16) | 0.13 (0.15) |
| Wave 1 income-to-needs | -- | −0.19 (0.06)** | −0.18 (0.07)* |
| Wave 2 family conflict | -- | −0.38 (0.33) | |
| Acceleration during reactivity | |||
| Mean acceleration | −0.29 (0.04)*** | −0.29 (0.04)*** | −0.29 (0.04)*** |
| Acceleration during recovery | |||
| Mean acceleration | 0.17 (0.05)*** | 0.17 (0.05)*** | 0.17 (0.05)*** |
| Random Effects | |||
| σ2b, intercept | 53.53*** | 53.64*** | 52.68*** |
| σ2b, linear recovery | 0.85*** | 0.82*** | 0.82*** |
| σ2b, quadratic reactivity | 0.0029** | 0.0028** | 0.0028** |
| σ2w | 15.95 | 15.96 | 15.96 |
p < .05;
p < .01;
p < .001
All models estimated under restricted maximum likelihood
Multivariate Models and Mediation
Systolic blood pressure
In the systolic blood pressure (SBP) model with no level 2 predictors (i.e., including time at level 1 only), we found that the mean (SE) resting SBP level was 116.83 (0.98), the within-person variance (σ2w) was 35.31, the between-person variance for resting blood pressure (σ2b, intercept) was 197.55, the between-person variance for the linear recovery slope (σ2b, recovery) was 2.17, and the between-person variance for the quadratic reactivity slope (σ2b, quadratic reactivity) was 0.01 (Table 3). At the start of the reactivity period, SBP instantaneously increased by 2.86 mmHg, and then continued to increase at an average rate of 0.89 mmHg over the remainder of this period (Table 3). At the beginning of the recovery period, SBP instantaneously decreased by 2.24 mm Hg, and then continued to decrease at an average rate of 0.49 mm HG over the remainder of this period (Table 3).
Adding predictors to this empty model, we found that gender predicted initial status; specifically, females had lower average resting SBP than males (b01=−12.84, p<.001). We also found that gender and Wave 1 income-to-needs predicted instantaneous recovery (Table 3), with females showing slower instantaneous recovery than males on average (b21=0.58, p=.017), and individuals with higher Wave 1 income-to-needs (i.e., wealthier individuals) showing faster instantaneous recovery (b22=−0.29, p=.004). Adding these predictors explained 4.1% of the between-person variance in linear recovery, and 20.4% of the between-person variance in resting blood pressure.
Finally, we tested whether exposure to family conflict at Wave 2 mediated the relationship between Wave 1 income-to-needs and instantaneous Wave 3 SBP recovery. In the OLS model, income-to-needs was a significant predictor of exposure to family conflict at Wave 2 (b(SE)=0.063 (0.016), p<.001). When we included Wave 2 family conflict exposure in the multilevel model, the Wave 1 income-to-needs parameter decreased by 0.051 units (Table 3). Finally, Wave 2 conflict also significantly predicted variability in instantaneous recovery, wherein individuals with less conflict exposure showed faster instantaneous SBP recovery at Wave 3 (Table 3). In the Wave 2 conflict mediation model, the MCMAM 95% confidence interval indicated a significant effect (i.e., did not contain zero; [−0.1400, −0.0012]). Thus we conclude that family conflict exposure at Wave 2 partially mediated the relationship between income-to-needs at Wave 1 and instantaneous systolic recovery at Wave 3.
To assess effect size, we calculated the unstandardized indirect effect (57). For SBP, this indirect effect was −0.060 (95% CI, −0.1400, −0.0012). This indicates that the instantaneous rate of recovery is expected to change by 0.060 mmHg for every one-unit increase in income-to-needs, if only the indirect effect through family conflict exposure is considered.
Diastolic blood pressure
In the diastolic blood pressure (DBP) model with no level 2 predictors (i.e., including time at level 1 only), we found that the mean (SE) resting DBP level was 65.54 (0.51), the within-person variance (σ2w) was 15.94, the between-person variance for resting blood pressure (σ2b, intercept) was 53.53, the between-person variance for the linear recovery slope (σ2b, recovery) was 0.85, and the between-person variance for the quadratic reactivity slope (σ2b, quadratic reactivity) was 0.0029 (Table 4). At the start of the reactivity period, DBP instantaneously increased by 1.89 mm Hg, and then continued to increase at an average rate of 0.58 mmHg over the remainder of this period (Table 4). At the beginning of the recovery period, DBP instantaneously decreased by 1.30 mm Hg, and then continued to decrease at an average rate of 0.35 mm Hg over the remainder of this period (Table 4).
Adding predictors to this empty model, we found that, unlike for SBP, gender did not predict either initial status or instantaneous recovery. However, similar to the SBP model, Wave 1 income-to-needs did predict instantaneous recovery (Table 4), wherein individuals with higher Wave 1 income-to-needs (i.e., wealthier individuals) showed faster instantaneous recovery (b22=−0.19, p=.002). Adding these predictors explained 4.5% of the between-person variance in linear recovery, but none of the between-person variance in resting blood pressure.
Finally, as with SBP, we tested whether exposure to family conflict at Wave 2 mediated the relationship between Wave 1 income-to-needs and instantaneous Wave 3 DBP recovery. However, Wave 2 conflict was not a significant predictor of DBP recovery, and the MCMAM 95% confidence interval [−0.073, 0.018] was not significant, so we conclude that exposure to family conflict at Wave 2 does not mediate the relationship between Wave 1 income-to-needs and Wave 3 instantaneous recovery for diastolic blood pressure in this sample.
Discussion
The well documented SES – health gradient has instigated many studies examining possible underlying processes that could account for SES-related health inequalities. Increasingly researchers are finding that early childhood poverty portends life-long problems across a wide range of physical and mental health outcomes (6,7,8,9,12). The present study examined blood pressure reactivity and recovery as candidate mechanisms for health inequalities. We found that early childhood poverty was prospectively associated with slower, less complete blood pressure recovery from exposure to a standard laboratory stressor, mental arithmetic, among a sample of healthy, 17 and 18 year olds (see Figures 1 and 2). This result extends earlier studies on cardiovascular recovery and SES in several important respects. First, our data show that childhood poverty is related prospectively to late adolescent blood pressure dynamics. Prior studies on SES and cardiovascular recovery examined either children (27,29,31) or adults (58) only, and none were prospective from childhood to adulthood. Second, we used newer growth curve modeling techniques to better estimate dynamic stress physiological activity than was possible in the prior studies. The third contribution of the present study is our demonstration that the childhood poverty → stress recovery association appears to be mediated, in part, by childhood exposure to elevated family conflict. Late adolescents who grew up in more conflictual households, an ecological covariate of poverty, are more apt to manifest slower systolic blood pressure recovery.
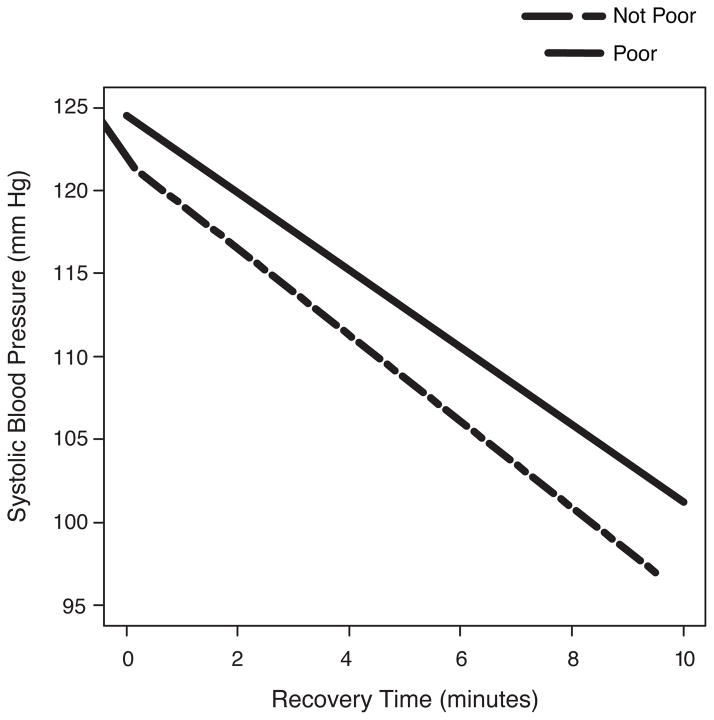
Figure 1.
Average change in systolic blood pressure over the recovery period. X axis indicates the recovery period, in minutes (5 readings taken every two minutes). The slope (SE) estimates for poor and not poor are −2.33 (0.44) and −2.60 (0.44), respectively. All inferential analyses used the continuous measure of income to needs. The slope data are for descriptive purposes only.
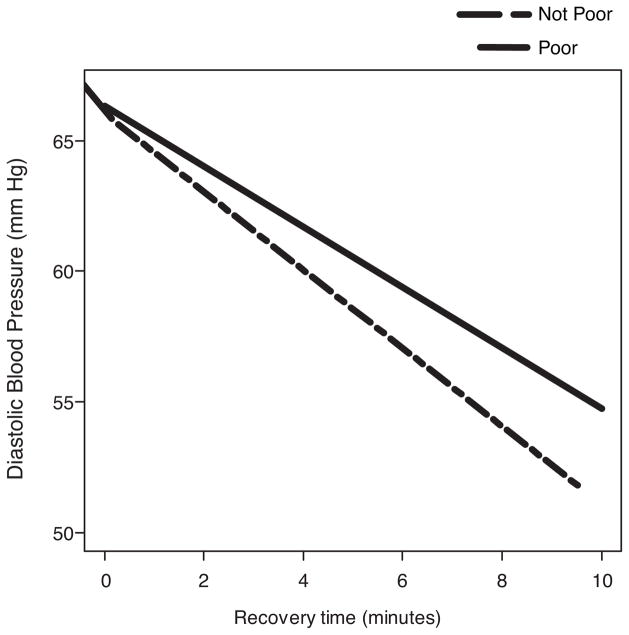
Figure 2.
Average change in diastolic blood pressure over the recovery period. X axis indicates the recovery period, in minutes (5 readings taken every two minutes). The slope (SE) estimates for poor and not poor are −1.16 (0.26) and −1.49 (0.28), respectively. All inferential analyses used the continuous measure of income to needs. The slope data are for descriptive purposes only.
The operation of exposure to family conflict as a psychosocial mechanism linking childhood poverty to slower cardiovascular recovery is in accord with prior studies on family conflict and hostility and cardiovascular responses, although most of the prior work has focused on reactivity rather than recovery. Children respond with elevated sympathetic activity in response to actual as well as simulated interpersonal conflicts (43). Children experience interpersonal conflict, especially among family members, as stressful and this is manifested in elevated sympathetic arousal. Furthermore, individual differences in trait hostility partially explain some of the links uncovered in previous studies between SES and blood pressure reactivity (25,47). Specifically, the inverse link between SES and blood pressure reactivity appears to be accentuated among more hostile individuals. The role of early life experiences of family conflict in these linkages has not been explored but would be a worthwhile question for future investigations. Chen and Matthews in their program of research have documented that low-income children and adults are more likely to perceive ambiguous interpersonal situations as threatening with hostile intent (23,26,59). Moreover, the inverse relationship between SES and blood pressure reactivity is mediated, in part, by such attributions. It is also noteworthy that young adults from lower SES backgrounds reveal greater amygdala reactivity to threatening facial expressions suggesting that early deprivation may influence how the brain processes emotional information (60). Thus both blood pressure reactivity and amygdala findings in relation to threat when considered with the present data suggest an interesting narrative to explain how poverty and other forms of deprivation early in life can get under the skin to ultimately damage health over the lifecourse: disadvantaged children, relative to their more affluent peers, experience more stress and strain, including aggression, conflict, and hostility. This may lead to greater vigilance and sensitivity to negative, interpersonal interactions, including the tendency to perceive neutral interpersonal interactions as hostile and threatening. The latter, in turn, influences dynamic physiological stress responses to environmental demands.
Although both systolic and diastolic recovery from exposure to the acute stressor were associated with early childhood poverty in our sample of 17 and 18 year olds, neither systolic or diastolic reactivity in these youth were related to childhood poverty. As reviewed in the Introduction, the literature on SES and cardiovascular reactivity is mixed with studies finding both elevated and muted reactivity among low SES children and youth relative to their more advantaged counterparts. However, none of this prior work used growth curve modeling and these prior studies did not take six or more baseline blood pressure readings which is necessary to establish maximum reliability of measurement (33,34). Why cardiovascular recovery seems to be a more robust index than reactivity to childhood poverty is unclear. One of the outcomes of chronic, early childhood adversity is elevated allostatic load (61) which reflects dysregulation across multiple physiological response systems including the sympathetic nervous system, the hypothalamic pituitary adrenal axis, the metabolic system, and immune function (62,63,64). Prior work has shown that childhood poverty is associated with elevated allostatic load (11,65,66,67). However it is important to note that physiological reactivity or sensitivity to environmental conditions should not be considered inherently maladaptive; rather the extent to which increased reactivity may be indicative of good or bad outcomes is likely dependent upon context. Specifically while more reactive individuals may be more vulnerable to suboptimal environmental conditions, this same sensitivity may confer greater capacity to take advantage of optimum environmental conditions (68,69,70). On the other hand, once the threat is removed, it would seem universally adaptive to recover to baseline as quickly as possible. Taking longer to recover physiologically from an environmental demand may be uniformly maladaptive. This is an important topic worthy of further investigation.
Although our findings suggest a pathway from childhood poverty to slower systolic blood pressure recovery through exposure to family conflict during childhood, our data are correlational and causal attributions are not tenable. For example, it is possible that the specification of our mechanistic pathway is incorrect. In light of this, we have taken reasonable steps to examine alternative plausible models of mediation. We examined the possibility that income-to-needs at Wave 2 mediated the association of Wave 1 exposure to family conflict and Wave 3 cardiovascular activity. However, for both systolic and diastolic blood pressure, there was no significant mediation in this reverse pathway. We also tested for various alternative variables to poverty such as parental education, parental marital status, maternal teenage pregnancy, etc., and found no associations with reactivity and recovery, but nonetheless the data should be interpreted as correlational evidence for an association that has not been seen previously. Ideally we would have collected information on cardiovascular dynamics during the initial wave of data collection so our prospective, mediational analyses could have also been longitudinal. It is also relevant to consider that our findings are from a sample of young, healthy 17 and 18 year olds. None suffered from hypertension, diabetes or clinical symptoms of heart disease or other major chronic diseases at the time of data collection. With the passage of time, we would expect the prospective relations uncovered herein to grow stronger as disease processes accelerate later in life.
Finally, there are some limitations of the mediation findings. Methodologically, they reflect the rate of linear change at a specific time (i.e., onset of Stage 2, the recovery period). If time were coded differently, the mediational findings might vary. We also found that the childhood poverty effects on systolic but not diastolic blood pressure were mediated by exposure to family conflict. Prior work on social factors including childhood SES (71,72,73) as well as early family interpersonal relationships (74) reflect similar trends. It is not clear why systolic relative to diastolic blood pressure may be a more sensitive marker of stress outcomes to undesirable, early social conditions in life. One possibility might be that systolic blood pressure reflects myocardial functioning more directly in relation to effort whereas diastolic blood pressure is more closely aligned with vascular structure and function (75).
Despite these limitations, the findings herein provide evidence for slower blood pressure recovery to an acute stressor in individuals who experienced childhood poverty and suggest that such dysregulated recovery could serve as a potential explanatory mechanism for some health inequalities. Several major disorders that reflect an income gradient have been linked to altered cardiovascular processes, particularly slower, incomplete recovery following exposure to acute environmental demands (16,17,18,19,20,21). We also found that a psychosocial risk factor associated with childhood deprivation, exposure to family conflict, may account for some of the association between childhood poverty and late adolescents’ cardiovascular recovery. These data are provocative and beg for further, long term followup among SES heterogeneous samples of older adults. Future studies should also examine in greater depth the possible interrelationships among early experiences of chronic stress, particularly within the family, among disadvantaged children and how this, in turn, may affect the ways in which children comprehend interpersonal interactions and its consequences for health.
Acknowledgments
We are grateful to the children and families who have participated in this longitudinal research program. We thank Anthony Ong for critical feedback on earlier drafts. Jana Cooperman, Kim English, Missy Globerman, Matt Kleinman, Rebecca Kurland, Melissa Medoway, Tina Merilees, Chanelle Richards, Adam Rohksar, and Amy Schreier assisted in data collection. This work was supported by the W.T. Grant Foundation, the John D. and Catherine T. MacArthur Foundation Network on Socioeconomic Status and Health, and the National Institute for Minority and Health Disparities, 5RC2MD00467 (GWE).
Abbreviations
- SES
socioeconomic status
- MLM
multilevel modling
- OLS
ordinary least squares
- MCMAM
Monte Carlo method for assessing mediation
- REML
restricted maximum likelihood
- HLM
hierarchical linear modeling
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- M
mean
- SD
standard deviation
- SE
standard error
Footnotes
Interpetation of slopes is determined by taking the first and second derivatives of the level 1 equation with respect to either reactivity or recovery. For example, the instantaneous (linear) rate of change for reactivity is described by 2*π1i + 2*π3i (Reactivity time)ti when reactivity time = 0, and the acceleration in growth (quadratic) is described by 2*π3i. Identical equations hold for recovery. When time is not set to 0, the equation for instantaneous rate of change becomes a conditional slope equation (33).
Conflicts of Interest: None declared.
References
- 1.Adler NE, Rehkopf DHUS. disparities in health: Descriptions, causes, and mechanisms. Ann Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 2.Adler NE, Stewart J. The biology of disadvantage: Socioeconomic status and health. Ann NY Acad Sci. 2010;1186:1–4. doi: 10.1111/j.1749-6632.2009.05385.x. [DOI] [PubMed] [Google Scholar]
- 3.Braveman PA, Cubbin C, Egerter S, Williams DR, Ramuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. Am J Public Health. 2010;100:S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Ann Rev Psychol. 2010;61:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psych Bull. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 6.Hertzman C, Boyce WT. How experience gets under the skin to create gradients in developmental health. Ann Rev Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- 7.Shonkoff JP, Boyce WT, Mc Ewen BS. Neuroscience, molecular biology, and the childhood roots of health disparities. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann NY Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 9.Evans GW, Chen E, Miller GE, Seeman TE. How poverty gets under the skin: A life course perspective. In: Maholmes V, King R, editors. The Oxford handbook of poverty and child development. NY: Oxford University Press; 2012. pp. 13–36. [Google Scholar]
- 10.Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- 11.Evans GW, Kim P. Childhood poverty and young adult allostatic load: The Mediating role of childhood cumulative risk exposure. Psych Sci. 2012;23:979–983. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- 12.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psych Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans GW. The environment of childhood poverty. Am Psych. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 14.Evans GW, Kim P. Childhood poverty, chronic stress, self-regulation and coping. Child Dev Perspectives. 2013;7:43–48. [Google Scholar]
- 15.Seeman T, Epel E, Gruenewald T, Karlamangla A, Mc Ewen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann NY Acad Sci. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- 16.Krantz DS, Manuck S. Acute physical reactivity and risk for coronary heart disease: A review and methodologic critique. Psych Bull. 1984;96:435–464. [PubMed] [Google Scholar]
- 17.Matthews KA, Weiss S, Detre T, Dembrowski TM, Falkner B, Manuck SB, Williams RB. Handbook of stress, reactivity, and cardiovascular disease. NY: Wiley; 1987. [Google Scholar]
- 18.Pickering T, Gerin W. Cardiovascular reactivity in the laboratory and the role of behavioral factors in hypertension: A critical review. Ann Behav Med. 1990;12:3–16. [Google Scholar]
- 19.Hocking-Schuler J, O’Brien W. Cardiovascular recovery from stress and hypertension: A meta-analytic review. Psychophysiology. 1997;34:649–659. doi: 10.1111/j.1469-8986.1997.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 20.Manuck SB, Kasprowicz AL, Muldoon M. Behaviorally-evoked cardiovascular reactivity and hypertension: Conceptual issues and potential associations. Ann Behav Med. 1990;12:17–29. [Google Scholar]
- 21.Schuler J, O’Brien W. Cardiovascular recovery from stress and hypertension risk factors: A meta-analytic review. Psychophysiology. 1997;34:649–659. doi: 10.1111/j.1469-8986.1997.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 22.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 23.Chen E, Matthews KA. Cognitive appraisal biases: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann Behav Med. 2001;23:101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- 24.Gump BB, Reihman J, Stewart P, Lonky E, Darvill T, Matthews KA. Blood lead (Pb) levels: A potential environmental mechanism explaining the relation between socioeconomic status and cardiovascular reactivity in children. Health Psych. 2007;26:296–304. doi: 10.1037/0278-6133.26.3.296. [DOI] [PubMed] [Google Scholar]
- 25.Gump BB, Matthews KA, Raikkonen K. Modeling relationships among socioeconomic status, hostility, cardiovascular reactivity, and left ventricular mass in African American and White children. Health Psych. 1999;18:140–150. doi: 10.1037//0278-6133.18.2.140. [DOI] [PubMed] [Google Scholar]
- 26.Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: The role of stress interpretations. Child Dev. 2004;75:1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 27.Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psych Sci. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 28.Musante L, Treiber FA, Kapuku GK, Moore D, Davis H, Strong WB. The effects of life events on cardiovascular reactivity to behavioral stressors as a function of socioeconomic status, ethnicity, and sex. Psychosomatic Med. 2000;62:760–767. doi: 10.1097/00006842-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Jackson RW, Treiber FA, Turner JR, Davis H, Strong WB. Effects of race, sex, and socioeconomic status upon cardiovascular stress responsivity and recovery in youth. Int J of Psychophysiology. 1999;31:111–119. doi: 10.1016/s0167-8760(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 30.Wilson DK, Kliewer W, Plybon L, Sica DA. Socieconomic status and blood pressure reactivity in healthy black adolescents. Hypertension. 2000;35:496–500. doi: 10.1161/01.hyp.35.1.496. [DOI] [PubMed] [Google Scholar]
- 31.Walter HJ, Hofman A. Socioeconomic status, ethnic origin, and risk factors for coronary heart disease in children. Am Heart J. 1987;113:812–818. doi: 10.1016/0002-8703(87)90724-1. [DOI] [PubMed] [Google Scholar]
- 32.Kamarck T, Jennings R, Debski T, Glicksman-Weis E, Johnson P, Eddy M, Manuck S. Reliable measures of behaviorally evoked cardiovascular reactivity from a PC-based test battery. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 33.Llabre MM, Ironson GH, Spitzer SB, Gellman MD, Weidler DJ, Schneiderman N. How many blood pressure measurements are enough? An application of generalizability theory to the study of blood pressure reliability. Psychophysiology. 2007;25:97–106. doi: 10.1111/j.1469-8986.1988.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 34.Emery RE, Laumann-Billings L. An overview of the nature, causes, and consequences of abusive family relationships. Am Psych. 1998;53:121–135. doi: 10.1037//0003-066x.53.2.121. [DOI] [PubMed] [Google Scholar]
- 35.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 36.Sinclair J, Pettit G, Harrist A, Dodge K, Bates J. Encounters with aggressive peers in early childhood: Frequency, age differences, and correlates of risk for behavior problems. Int J Behav Dev. 1994;17:675–696. [Google Scholar]
- 37.Brody GH, Ge X, Conger RD, Gibbons FW, Murry VM, Gerrard M, Simons RL. The influence of neighborhood disadvantage, collective socialization, and parenting on african american children’s affiliation with deviant peers. Child Dev. 2001;72:1231–1246. doi: 10.1111/1467-8624.00344. [DOI] [PubMed] [Google Scholar]
- 38.Conger RD, Elder GH., Jr . Families in troubled times. New York: Aldine de Gruyter; 1994. [Google Scholar]
- 39.Bradley RH, Corwyn RF, McAdoo HP, Garcia-Coll C. The home environments of children in the United States Part I: Variations by age, ethnicity, and poverty status. Child Dev. 2001;72:1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- 40.Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Ann Rev Psych. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- 41.Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: Moving from markers to mechanisms of risk. Psych Bull. 2003;129:447–466. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- 42.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psych Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 43.Ballard M, Cummings E, Larkin K. Emotional and cardiovascular responses to adults’ angry behavior and to challenging tasks in children of hypertensive and normotensive parents. Child Dev. 1993;64:500–515. [PubMed] [Google Scholar]
- 44.Ross K, Martin T, Chen E, Miller GE. Social encounters in daily life and 2-year changes in metabolic risk in young women. Dev Psychopath. 2011;23:897–906. doi: 10.1017/S0954579411000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosomatic Med. 1998;60:765–772. doi: 10.1097/00006842-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Wright LB, Treiber F, Davis H, Bunch C, Strong W. The role of maternal hostility and family environment upon cardiovascular functioning among youth two years later: Socioeconomic and ethnic differences. Ethnicity Dis. 1998;8:367–376. [PubMed] [Google Scholar]
- 47.Carroll D, Davey-Smith G, Sheffield D, Shipley MJ, Marmot MG. The relationship between socioeconomic status, hostility, and blood pressure reactions to mental stress in men: Data from the Whitehall II study. Health Psych. 1997;16:131–136. doi: 10.1037//0278-6133.16.2.131. [DOI] [PubMed] [Google Scholar]
- 48.Moos RH, Moos BS. Family environment scale. 4. Menlo Park, CA: Mind Garden, Inc; 2009. [Google Scholar]
- 49.Chou C, Yang D, Pentz MA, Hser Y. Piecewise growth curve modeling approach for longitudinal prevention study. Computational Stat Data Analysis. 2004;46:213–225. [Google Scholar]
- 50.Li F, Duncan TW, Duncan SC, Hops H. Piecemeal growth curve mixture modeling of adolescent alcohol use data. Structural Equation Modeling. 2001;8:175–204. [Google Scholar]
- 51.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Los Angeles: Sage; 2002. [Google Scholar]
- 52.Biesanz JC, Deeb-Sossa N, Papadais AA, Bollen KA, Curran PJ. The role of coding time in estimating and interpreting growth curve models. Psych Methods. 2004;9:30–52. doi: 10.1037/1082-989X.9.1.30. [DOI] [PubMed] [Google Scholar]
- 53.Zuur AG, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. NY: Springer; 2009. [Google Scholar]
- 54.Krull JL, MacKinnon DP. Multilevel modeling of individual and group-level mediated effects. Multivariate Behav Res. 2001;36:249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- 55.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selig JP, Preacher KJ. Monte Carlo method for assessing mediation: An interactive tool for creating confidence intervals for indirect effects. Jun, 2008. [Google Scholar]
- 57.Preacher KJ, Kelley K. Effect size measures for mediation model: Quantitative strategies for communicating indirect effects. Psych Methods. 2011;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 58.Steptoe A, Feldman PJ, Kunz S, Owen N, Willemsen G, Marmot M. Stress responsivity and socioeconomic status: A mechanism for increased cardiovascular disease risk? Euro Heart J. 2002;23:1757–1763. doi: 10.1053/euhj.2001.3233. [DOI] [PubMed] [Google Scholar]
- 59.Chen E, Matthews KA. Development of cognitive appraisal and understanding of social events (CAUSE) videos. Health Psych. 2003;22:106–110. doi: 10.1037//0278-6133.22.1.106. [DOI] [PubMed] [Google Scholar]
- 60.Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Scan. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physio Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 62.Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psych Rev. 2010;117:134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McEwen BS. Protective and damaging effects of stress mediators. NEJM. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 64.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann NY Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Nat Acad Sci. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodman E, Mc Ewen BS, Huang B, Dolan LM, Adler NE. Social inequalities in biomarkders of cardiovascular risk in adolescence. Psychosomatic Med. 2005;67:9–15. doi: 10.1097/01.psy.0000149254.36133.1a. [DOI] [PubMed] [Google Scholar]
- 67.Worthman CM, Panter-Brick C. Homeless street children in Nepal: Use of allostatic load to assess the burden of childhood adversity. Dev Psychopathology. 2008;20:233–255. doi: 10.1017/S0954579408000114. [DOI] [PubMed] [Google Scholar]
- 68.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 69.Ellis BJ, Essex MJ, Boyce WT. Biological sensitvity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- 70.Pluess M, Belsky J. Vantage selectivity: Individual differences in response to positive experiences. Psych Bull. 2013 doi: 10.1037/a0030196. Online first publication, October 1, 2012. [DOI] [PubMed] [Google Scholar]
- 71.Chan M, Chen E, Hibbert AS, Wong JHK, Miller GE. Implicit measures of early-life family conditions: Relationships to psychosocial characteristics and cardiovascular disease risk in adulthood. Health Psych. 2011;30:570–578. doi: 10.1037/a0024210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schreier HMC, Chen E. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain, Behav, Immunity. 2010;24:1324–1331. doi: 10.1016/j.bbi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 73.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psych. 2009;28:338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luecken LJ, Rodriguez BS, Appelhans BM. Cardiovascular stress responses associated with family of origin relationship experiences. Psychosomatic Med. 2005;67:514–521. doi: 10.1097/01.psy.0000160466.10397.18. [DOI] [PubMed] [Google Scholar]
- 75.Krantz DS, Falconer JJ. Measurement of cardiovascular responses. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress. NY: Oxford University Press; 1995. pp. 193–212. [Google Scholar]