Abstract
Inter-specific hybridization between Pleurotus pulmonarius and P. florida was attempted through PEG-induced protoplast fusion to select a fusant. The protocol for protoplast release, regeneration and fusion in these two Pleurotus species was standardized using the variables controlling the process. The mixture of mycolytic enzymes, i.e. commercial cellulase, crude chitinase and pectinase, KCl (0.6 M) as osmotic stabilizer, pH 6 of the phosphate buffer and an incubation time of 3 hours resulted in the maximum release of protoplasts from 3-day-old mycelia of P. florida (5.3~5.75 × 107 protoplasts/g) and P. pulmonarius (5.6~6 × 107 protoplasts/g). The isolated protoplasts of P. florida regenerated mycelium with 3.3% regeneration efficiency while P. pulmonarius showed 4.1% efficiency of regeneration. Polyethyleneglycol (PEG) - induced fusion of protoplasts of these two species resulted in 0.28% fusion frequency. The fusant produced fruiting bodies on paddy straw but required a lower temperature of crop running (24 ± 2℃) than its parents which could fruit at 28 ± 2℃. The stable fusant strain was selected by testing for the selected biochemical markers i.e. Carbendazim tolerance and utilization of the lignin degradation product, vanillin.
Keywords: Carbendazim, P. florida, Pleurotus pulmonarius, Polyethyleneglycol (PEG), Protoplast fusion
The fungal protoplast limited by the cell membrane can be isolated by mechanical or enzymatic removal of the cell wall. A viable protoplast is capable of cell wall regeneration, cell division, growth and it also reverts to the parent culture in an osmotically balanced medium. The isolation and regeneration of protoplasts has been considered a very useful tool in the biotechnological manipulations of higher plants and fungi (Peberdy, 1989). Protoplasts are also required for the genetic map studies using electrophoretic karyotype analysis as well as for the development of gene transformation systems and for the isolation of homokaryotic regenerates for their use in systematic breeding programme (Kaul, 2002). Protoplasts have been studied and prepared in a large number of edible mushrooms but their yields have been found poor as compared to non-basidiomycetes fungi (Zhao and Chang, 1993; Gupta et al., 1997).
Protoplast fusion technology is applied for developing inter specific, intra specific and inter generic, intra generic supra hybrids with higher potentiality than their parental strains. Through protoplast fusion technique, improved strains with enhanced potential for the production of antibiotics, enzymes, useful myco products, and high yielding mushrooms could effectively be developed (Lalithakumari, 2000).
Oyster (Pleurotus sp.) mushrooms are commonly cultivated in Tamilnadu, India, as their cultivation technique is simple and as they can be grown under a wide range of temperatures (25~35℃). Strain improvement in mushrooms is carried out by induced mutations through UV irradiation or chemical mutagens. Protoplast fusion is yet to be developed as a successful strain improvement technique in mushrooms. This paper reports the standardization of the optimum conditions for protoplast release and regeneration in a native cultivated strain, Pleurotus florida and an exotic strain Pleurotus pulmonarius and for isolating a fusion product through PEG-induced protoplast fusion which showed the desirable characteristics of both the parents.
Materials and Methods
Fungal strains and growth conditions
A lyophilized culture of an exotic strain, P. pulmonarius (M42), procured from the National Institute for Agro-Environmental Sciences, Japan and a slant culture of native cultivated strain of P. florida (PF1) obtained from Tamilnadu Agricultural University, Coimbatore, India, were used in the present investigation. The cultures were maintained on Potato Dextrose Agar (PDA) slants at 15℃. Mating type compatibility between these two strains was checked by growing them as dual cultures in PDA plates with a distance of 4 cm between the two inocula and checking for the presence of a thick mycelial barrage at the zone of contact.
The parental strains were tested for their tolerance or sensitivity to the fungicide carbendazim in the range of concentration, 0.1 mM, 0.5 mM and 1 mM and for their potential to utilize vanillin, a degradation product of lignin, in the range of concentration, 0.01%~0.05%. The protoplast fusants were screened and segregated from the parental self fusants by the biochemical marker characteristics of carbendazim tolerance (Lalithakumari, 2000) and vanillin utilization (Cai et al., 1993).
Isolation and purification of protoplasts
Agar blocks (8 mm) from actively growing 6-day-old cultures of the selected fungi were inoculated individually in Erlenmeyer flasks (250 ml) containing sterile potato dextrose broth (100 ml) and incubated at 28 ± 2℃ on a rotary shaker (Remi, India) at 120 rpm for 3 days. The mycelia were harvested by filtration through sterile muslin cloth and washed twice with sterile water (Lalithakumari, 2000).
The culture filtrates of 3-day-old Trichoderma harzianum grown in Czapek broth substituted with chitin or pectin as the sole carbon source instead of sucrose were used as the source of lytic enzymes (Benitez et al., 1975). The culture broth was filtered successively through glass wool and Geena glass filter (G3) to remove the hyphal fragments. The lytic enzyme mixture (3 ml/study) used in the protoplast fusion studies contained equal volumes of chitinase and pectinase enzyme extracts and commercial cellulase (Celluclast, India). Potassium chloride (0.6 M) in 0.1M phosphate buffer (pH 6.0) was used as the osmotic stabilizer.
The harvested mycelium (100 mg) was aseptically transferred to 1 ml of osmotic stabilizer (in 0.1M phosphate buffer, pH 6.0) and the enzyme mixture (3 ml) was added to it. The mycelium in enzyme mixture was incubated on a rotary shaker (120 rpm) (Remi, India) at room temperature (28 ± 2℃) for 3 h (Lalithakumari, 2000). After the incubation period, hyphal fragments were removed by filtration through a column of cotton wool packed up to the 0.5 ml mark of a 5 ml syringe. Protoplasts were collected from the filtrate by centrifugation (1400 g, 10 min). Pellets were washed twice in 0.6M KCl and then suspended in the same osmotic stabilizer. Protoplast yield was determined with a haemocytometer.
Effect of physico-chemical parameters
The different factors studied to standardize the protocol for protoplast isolation and regeneration were: different enzymes (Celluclast-commercial cellulase, crude pectinase, crude chitinase and their combination), osmotic stabilizers of different molarities (MgSO4, KCl, sorbitol - 0.2 to 1M), age of the culture (3, 5, 7 days), pH of incubation medium (pH 4, 6, 8) and incubation time (1~4 h).
Regeneration and growth of protoplasts
Regeneration of protoplasts was checked in solid medium (Mukherjee and Sengupta, 1986). For regeneration, 0.1 ml of suitably diluted protoplast suspension (about 104/ml) was plated on MYG medium (malt extract, yeast extract, glucose medium) containing 0.6M KCl as an osmotic stabilizer. The plates were incubated at room temperature (28 ± 2℃) for 3 to 4 days. The regeneration frequency was calculated as the ratio of number of protoplasts regenerated to the number of protoplasts incubated in the regeneration medium. Regeneration in liquid phase was checked by suspending aliquots of protoplasts in the regeneration medium both at 28 ± 2℃ and observed under microscope at different intervals.
PEG-induced protoplast fusion
Protoplasts were fused following the method of Stasz et al. (1988) in the presence of polyethyleneglycol (PEG, mol.wt.4000, 30%), CaCl2 (0.05M) and glycine (0.05M) at pH 7.5. To the purified protoplasts of the two test organisms in the osmotic stabilizer, an equal volume of PEG mixture was added and the mixture was shaken slightly for 10 min for fusion to take place. The sample was diluted with equal volume of osmotic stabilizer and a small volume was observed under the compound microscope for fusion. An aliquot (0.1 ml) of the fused protoplasts sample was plated on non-selective medium (MYG-malt extract 10 g/l; yeast extract, 4 g/l; glucose 4 g/l; agar 14 g/l, pH 5.5 with osmotic stabilizer) and checked for regeneration. Protoplasts from the same strains were also fused as controls. Fusion frequency was determined as the ratio of the number of regenerated colonies in regeneration minimal medium (RMM) to the number of regenerated colonies in regeneration complete medium. The isolation, regeneration and fusion of the protoplasts were observed and photographed using photomicrographic equipment (Nikon, Japan). Hyphal tips of regenerated colonies developing on RMM were transferred to minimal medium (MM-asparagine 2 g/l; glucose 20 g/l; thiamine - HCl 0.12 g/l; K2HPO4 1 g/l; KH2PO4 0.46 g/l MgSO4. 7H2O 0.5 g/l; agar 14 g/l. RMM contained in addition the osmotic stabilizer). This procedure excluded the possibility of a dual culture. Only those progeny that continued to grow on MM were considered to be fusion hybrids.
Selection of fusion products
The parent and fusant strains were subjected to fruiting trials using paddy straw (100 g with 70% moisture content) as the substrate. The fusant strains were selected by testing for sensitivity/resistance to the fungicide carbendazim and for the ability to utilize vanillin, a lignin derived phenolic compound.
Results and Discussion
Parental characteristics
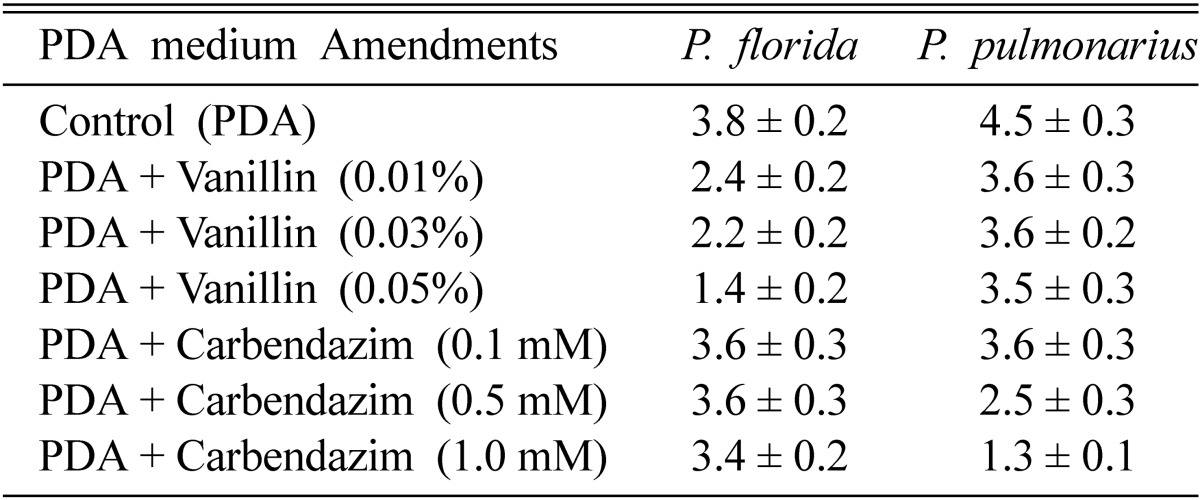
The parental strains, P. pulmonarius and P. florida showed compatibility in dual culture plates with a thick barrage of intermingled hyphae at the zone of contact. In PDA plates amended with different concentrations of carbendazim (0.1~1.0 mM), the growth rate of P. florida was not affected, while the growth of P. pulmonarius was significantly inhibited at the higher concentration. In PDA plates amended with different concentrations of vanillin (0.01~0.05%), the growth rate of P. florida was significantly inhibited at the higher concentration, while the growth of P. pulmonarius was not affected indicating its potential to utilize the lignin break-down product, vanillin (Table 1). The biochemical marker characters of carbendazim tolerance and vanillin utilization were used to characterize the parents and to identify the fusion products as suggested by Lalithakumari (2000) who reported that when wild - type parent strains were used as fusion partners, some type of selective medium must be employed to ensure that only fusion products regenerate into colonies.
Table 1.
Radial growth (cm) of 6-day-old cultures of P. florida and P. pulmonarius on PDA plates amended with vanillin/carbendazim (values are mean ± S.E of three replicates)
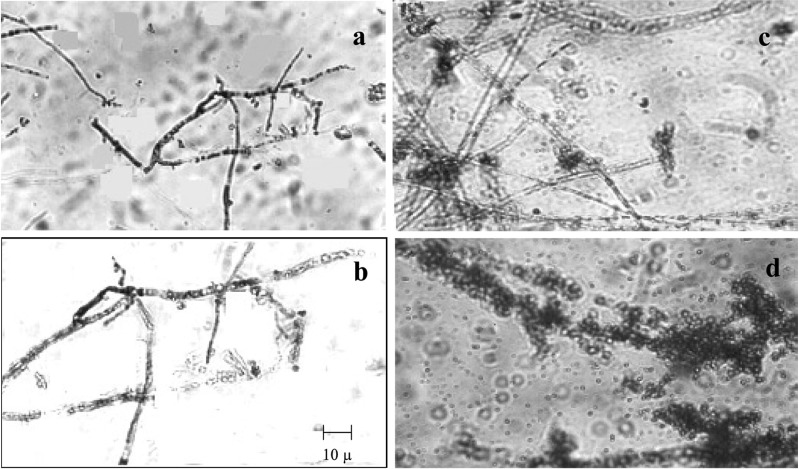
Release of protoplasts
The mixture of lytic enzymes (commercial cellulase, crude pectinase, and crude chitinase) was more efficient in the release of maximum yield of protoplasts from the cultures of P. pulmonarius and P. florida mycelia (6.0 × 107 and 5.42 × 107 protoplasts g-1 respectively) than the individual enzyme. Observations similar to this study were reported by Lalithakumari (1996) who observed the maximum release of protoplasts in Venturia inaequalis using a mixture of enzymes containing cellulase, chitinase, pectinase and β-glucuronidase (Table 2, Fig. 1).
Table 2.
Effect of various factors on the protoplast yield (× 107) of Pleurotus florida and Pleurotus pulmonarius (values are mean ± S.E of three replicates)
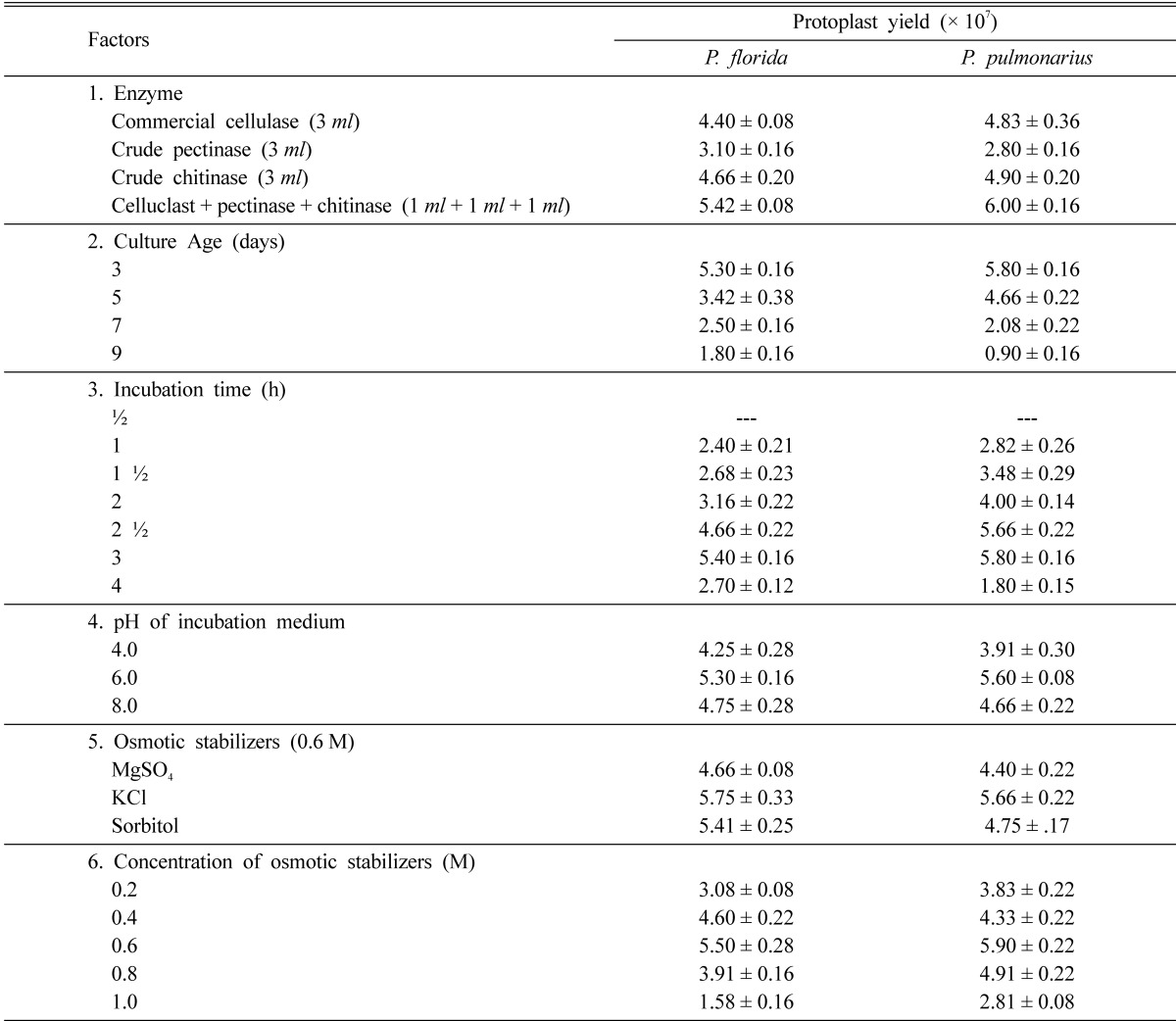
Fig. 1.
Time course of protoplasts release from the hyphae of P. pulmonarius and P. florida. a. Rounding up of protoplasts with in the hyphae (½ h), b. Protoplast emerging from hyphal tips (1 h), c. Protoplast with digested mycelial debries (1½ h), d. Chain of protoplast after complete digestion. Experimental conditions as in materials and methods.
Potassium chloride (0.6 M) used as the osmotic stabilizer was the best to yield the maximum 5.75 × 107 protoplasts g-1 in P. florida and 5.66 × 107 protoplasts g-1 in P. pulmonarius mycelia respectively. Several other osmotic stabilizers like sucrose (Kim et al., 2000), magnesium sulphate (Gupta et al., 1997), potassium chloride (Iijima and Yanagi, 1986), mannitol (Wakabayashi et al., 1985) and sorbitol (Vijaya palani, 1995) had been successfully used for maximum protoplast yield in fungi.
pH of the incubation medium was found to affect protoplast yield. Phosphate buffer at pH 6.0 was more effective in releasing the maximum number of protoplasts from the cultures of P. florida and P. pulmonarius (5.3 × 107 and 5.6 × 107 protoplasts g-1 mycelium respectively) than at other pH values. Several workers had recorded the optimum pH of the incubation medium for the maximum release of protoplasts in different white rot fungi i.e. citrate buffer at pH 4.0 for Agaricus bisporus (Gupta et al., 1997) and pH 5.0 for Lentinus lepideus (Kim et al., 2000); malate buffer pH 5.5 for auxotrophic mutants of Coprinus macrorhizus (Kiguchi and Yanagi, 1985).
The yield of protoplasts depended on the age of the mycelium. When hyphae of various ages were used for protoplast isolation, the highest number of protoplasts was derived from the youngest hyphae of P. florida and P. pulmonarius (3-day-old cultures) which yielded 5.3 × 107 protoplasts g-1 mycelium and 5.8 × 107 protoplasts g-1 mycelium respectively. The results are in accordance with these of various researchers who observed high yield of protoplasts from 2-day-old cultures of Volvariella volvaceae and in several mushrooms and other fungal cultures which are 3~6 days old (Gupta et al., 1997; Kim et al., 2000). The four-day-old cultures of P. florida and P. pulmonarius showed a drastic decrease in the yield of protoplasts. Zhao and Chang (1993) related the decrease in protoplasts yield from older mycelia to the changes in composition and thickness of hyphal cell wall associated with aging process.
The release of protoplasts was observed to start with the lysis of hyphal walls at 1.5 hours of incubation followed by the rounding up of the protoplasts at 2 h and their release through the hyphal tips at 2.5 h. The highest yield of protoplasts from P. florida and P. pulmonarius were obtained after 3 h of incubation with the complete digestion of the mycelium (Fig. 1) using the mixture of lytic enzymes (5.4 × 107 protoplasts g-1 P. florida mycelium and 5.8 × 107 protoplasts g-1 P. pulmonarius mycelium). Prolonged incubation resulted in a decrease in number of protoplasts from both the mushroom fungal mycelia (Table 2). Different white rot fungi were found to differ in the duration of incubation time required for maximum yield of protoplasts. A minimum incubation time of 2~3 h was required by Volvariella volvaceae (Mukherjee and Sengupta, 1988) but Lentinus lepideus exceptionally needed 6 h of incubation for maximum release of protoplasts (Kim et al., 2000).
Regeneration of protoplasts
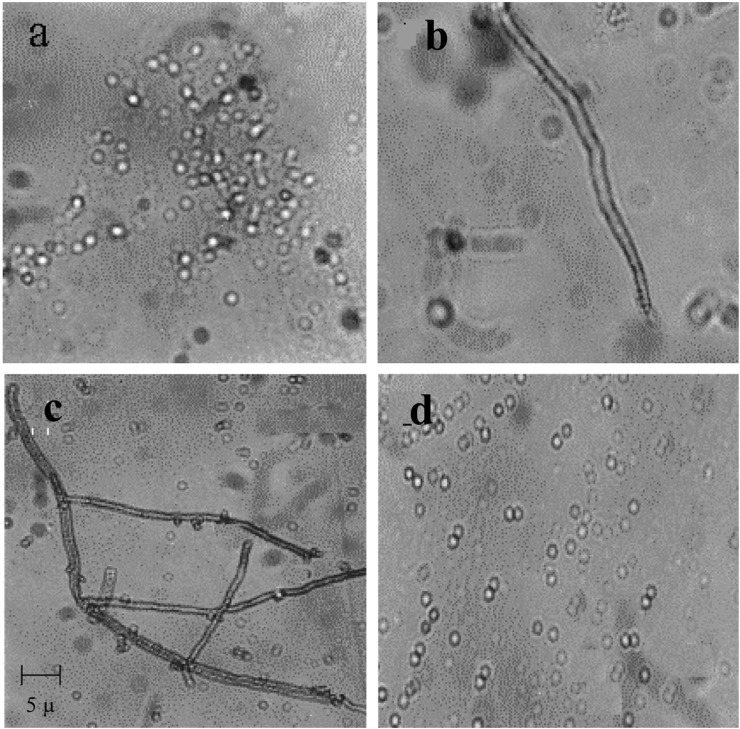
Protoplasts of P. florida and P. pulmonarius showed a regeneration frequency of 3.3% and 4.1% respectively. Similar observations have been made by Zhao and Chang (1993) who noted that P. florida and P. sajor-caju required 3 and 4 days respectively and 30℃ for regeneration. They observed the highest regeneration rate (9.6%) in P. florida and much lower frequencies of 2.4% and 0.96% in P. sajur-caju and L. edodes respectively. Two types of regeneration i.e. a chain of cells or a normal hypha directly emerging from the protoplasts were observed (Fig. 2). Lalithakumari (2000) reported similar patterns of hyphal regeneration (including a third type of a germ tube like hypha germinating from the protoplasts) in several filamentous fungi.
Fig. 2.
Isolation regeneration and fusion of protoplasts P. pulmonarius and P. florida. a. Isolated protoplast, b, c. Regeneration of protoplasts into chain of cells and hyphae; d. PEG-induced protoplast fusion. Experimental conditions as in materials and methods.
Isolation of fusion products
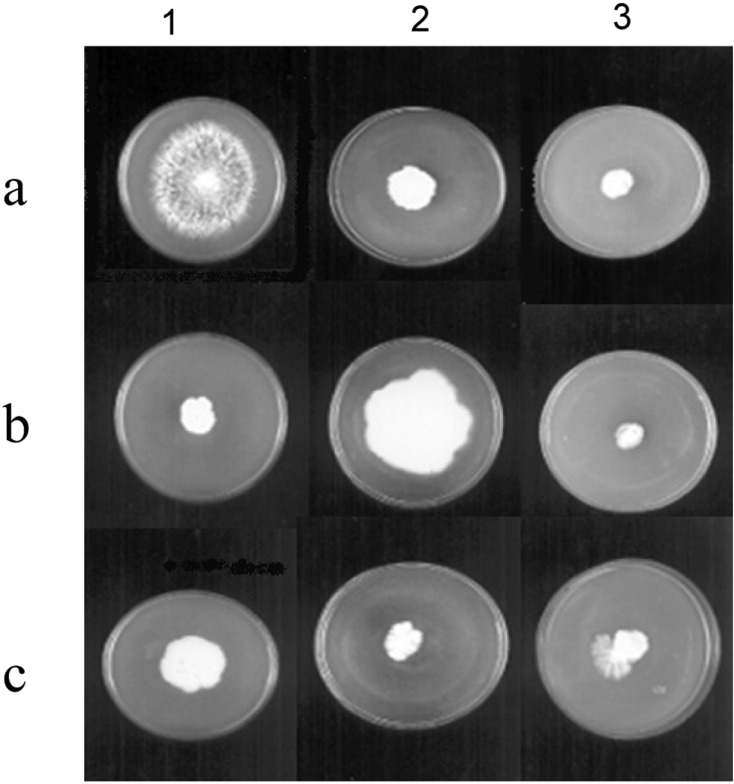
The protoplast fusion between P. florida and P. pulmonarius resulted in fusion frequency of 0.28%. Self fusants of the parents, P. florida and P. pulmonarius, in the control experiments showed nearly the same fusion frequency of 0.26% and 0.29% respectively. Six fusant colonies were isolated after eliminating self fusants by their growth on PDA plates amended with carbendazim (1 mM) and vanillin (0.05%). Only fusant No. 2 could be retrieved by transport through hyphal tips on minimal medium (MM) and on PDA plates with the amendments carbendazin and vanillin. It was stable even after five successive subculturings. By the definition of Zhao and Chang (1996), the fusant No. 2 was not a dual culture but should be a fusion hybrid of P. florida and P. pulmonarius as it could continue to grow on minimal medium. Lalithakumari (2000) reported that colony morphology can be used to identify interspecific and intergeneric fusion products especially if the species differ greatly in colony morphology. The fusant No. 2 showed mixed morphology acquired from its parents (P. pulmonarius with stringy hyphae and P. florida with fluffy hyphae) on PDA plates amended with carbendazim and vanillin (Fig. 3: c3).
Fig. 3.
Selection of fusion product of P. pulmonarius and P. florida. a. Vanillin tolerant, Carbendazim sensitive P. pulmonarius, b. Vanillin inhibited, Carbendazim resistant P. florida, c. Vanillin tolerant, Carbendazim resistant fusant on 1. Vanillin (0.05%) + PDA, 2. Carbendazim (1mM) +PDA, 3. Vanillin (0.05%) + Carbendazim (1mM) + PDA.
Zhao and Chang (1996, 1997) obtained very low fusion frequencies of 0.0036 to 0.007% in intergeneric fusion between P. ostreatus and S. commune, while interspecific hybridization between Volvariella volvaceae and V. bombycina gave a much higher percentage of 0.032%~0.33%.
The fusant could not fruit at the crop running temperature required by its parents (28 ± 2℃) but produced fruiting bodies at a lower temperature of 24 ± 2℃ (data not shown). Fusants of mushroom strains of compatible mating types produce fruiting bodies but those of incompatible mating types are sterile as they are either aneuploids or heteroploids. There are reports on the fusants exhibiting novel nutrient and biochemical characteristics even though they resembled any one or both the parents morphologically (Zhao and Chang, 1996). Non-parental interspecific fusion products were also obtained in Pleurotus (Go et al., 1989). Yoo et al. (1987) mentioned that the induced markers like auxotrophic and drug resistant mutants were often unstable and resulted in slow growth, while the naturally occurring property of tannin tolerance was stable and did not inhibit the growth rate. Specific tolerance to tannin and the ability to utilize vanillin, a lignin derived phenolic compound had been used as selective markers for mushrooms like L. edodes in protoplast fusion experiments (Cai et al., 1993). Among the parent strains, P. pulmonarius was sensitive to the fungicide carbendazim (1 mM) but it showed the ability to utilize vanillin (0.05%), while P. florida showed resistance to carbendazim (1 mM) and growth inhibition in the presence of vanillin (0.05%). In PDA plates amended with both carbendazim (1 mM) and vanillin (0.05%), the growth of both parents was inhibited. But the fusant showed uniform growth rate in all the plate assays indicating that it had acquired the characters of both the parents (Fig. 3). As it showed biparental morphology (Fig. 3: c3), it could be a recombinant as suggested by Lalithakumari (2000). Further work is in progress to study the ligno cellulolytic potential and the nutrient use efficiency of the protoplast fusant.
Acknowledgement
The gift of a lyophilized culture of P. pulmonarius from Prof. Y. Fujii, National Institute for Agro-Environmental Sciences, Japan is gratefully acknowledged.
References
- 1.Benitez T, Ramos S, Garcia Acha I. Protoplasts of Trichoderma viride: Formation and regeneration. Arch Microbiol. 1975;103:199–204. doi: 10.1007/BF00436350. [DOI] [PubMed] [Google Scholar]
- 2.ai YJ, Buswell JA, Chang ST. Effect of lignin - derived phenolic monomers on the growth of the edible mushrooms Lentinus edodes, Pleurotus sajor - caju and Volvariella volvacea. World J Microbiol Biotechnol. 1993;9:503–507. doi: 10.1007/BF00386283. [DOI] [PubMed] [Google Scholar]
- 3.Go SJ, You CH, Shin GC. Effects of incompatibility on protoplast fusion between intra species and inter species in Basidiomycete. Korean J Mycol. 1989;17:137–144. [Google Scholar]
- 4.Gupta U, Cheema GS, Sodhi HS, Phutela RP. Protoplast isolation and regeneration in Agaricus bisporus strain MS 39. Mush Res. 1997;6:59–62. [Google Scholar]
- 5.Iijima Y, Yanagi SO. A method for high yield preparation and high frequency regeneration of basidiomycete, Pleurotus ostreatus (Hirabke), protoplasts using sulphate pulp waste components. Agri Biol Chem. 1986;50:1855–1861. [Google Scholar]
- 6.Kaul TN. Biology and conservation of mushrooms. New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd.; 2002. pp. 35–39. [Google Scholar]
- 7.Kiguchi T, Yanagi SO. Intra specific heterokaryon and fruit body formation in Coprinus macrorhizus by protoplast fusion of auxotrophic mutants. Appl Microbiol Biotechnol. 1985;22:121–127. [Google Scholar]
- 8.Kim BK, Kang JH, Jin M, Kim HN, Shim MJ, Choi EC. Mycelial protoplast isolation and regeneration in Lentinus lepideus. Life Sciences. 2000;66:1359–1367. doi: 10.1016/s0024-3205(00)00444-6. [DOI] [PubMed] [Google Scholar]
- 9.Lalithakumari D. Protoplasts - A biotechnological tool for plant pathological studies. Ind Phytopath. 1996;49:199–212. [Google Scholar]
- 10.Lalithakumari D. Fungal protoplast - A Biotechnological tool. New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd.; 2000. pp. 101–112. [Google Scholar]
- 11.Mukherjee M, Sengupta S. Isolation and regeneration of protoplasts from Termitomyces clypeatus. Can J Microbiol. 1988;34:1330–1332. [Google Scholar]
- 12.Peberdy JF. Fungi without coats-protoplasts as tools for mycological research. Mycol Res. 1989;93:1–20. [Google Scholar]
- 13.Stasz TE, Harman GE, Weeder NF. Protoplast preparation and fusion in two biocontrol strains of Trichoderma harzianum. Mycologia. 1988;80:141–150. [Google Scholar]
- 14.Vijaya palani P. Biochemical, physiological and molecular aspects of penconazole and carbendazim resistance in mutants and protoplast fusants of Venturia inaequalis (Cooke) Wint. Chennai, India: University of Madras; 1995. Ph.D. Thesis. [Google Scholar]
- 15.Wakabayashi SH, Magae Y, Cashiwagi Y, Sasaki T. Formation of giant protoplasts from protoplast of Pleurotus cornu-copiae by the cell wall lytic enzyme. Appl Microbiol Biotechnol. 1985;121:328–330. [Google Scholar]
- 16.Yoo YB, You CH, Shin PG, Park YH, Chang KY. Transfer of isolated nuclei from Pleurotus florida into protoplasts of Pleurotus ostreatus. Korean J Mycol. 1987;15:250–253. [Google Scholar]
- 17.Zhao J, Chang ST. Monokaryotization by protoplasting heterothallic species of edible mushrooms. World J Microbiol Biotechnol. 1993;9:538–543. doi: 10.1007/BF00386290. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Chang ST. Inter generic hybridization Pleurotus ostreatus and Schizophyllum commune by PEG - induced protoplast fusion. World J Microbiol Biotechnol. 1996;12:573–578. doi: 10.1007/BF00327717. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Chang ST. Inter specific hybridization between Volvariella volvacea and Volvariella bombucina by PEG - induced protoplast fusion. World J Microbiol Biotechnol. 1997;13:145–151. [Google Scholar]