Abstract
We have synthesized the unnatural amino acid (UAA), 4-azidomethyl-Lphenylalanine (pN3CH2Phe), to serve as an effective vibrational reporter of local protein environments. The position, extinction coefficient, and sensitivity to local environment of the azide asymmetric stretch vibration of pN3CH2Phe are compared to the vibrational reporters: 4-cyano-L-phenylalanine (pCNPhe) and 4-azido-L-phenylalanine (pN3Phe). This UAA was genetically incorporated in a site-specific manner utilizing an engineered, orthogonal aminoacyl-tRNA synthetase in response to an amber codon with high efficiency and fidelity into two distinct sites in superfolder green fluorescent protein (sfGFP). This allowed for the dependence of the azide asymmetric stretch vibration of pN3CH2Phe to different protein environments to be measured. The photo-stability of pN3CH2Phe was also measured relative to the photoreactive UAA, pN3Phe.
Keywords: 4-Azidomethyl-L-phenylalanine, 4-Cyano-L-phenylalanine, 4-Azido-Lphenylalanine, Unnatural amino acids, Vibrational reporters, IR spectroscopy, Green fluorescent protein
Introduction
The ability to probe local protein environments has been significantly increased by the use of unnatural amino acids containing vibrational reporters that can be genetically incorporated into proteins with site-specificity.1,2 For instance, the unnatural amino acids, 4-cyano-L-phenylalanine (pCNPhe) and 4-azido-L-phenylalanine (pN3Phe), which contain the nitrile and azide vibrational reporters, respectively, have been successfully incorporated with high efficiency and fidelity into proteins in a site-specific manner utilizing an engineered, orthogonal aminoacyl-tRNA synthetase to study local protein environments.1–8 The nitrile and azide groups are effective vibrational reporters in part due to the position of the nitrile symmetric stretch vibration and the azide asymmetric stretch vibration, which occur in a relatively clear region of the infrared; the oscillator strengths of these vibrations; and the sensitivity of these vibrations to local environment.2,9,10 These two groups also represent two-atom (nitrile) and three-atom (azide) probes, which have the potential to be minimally invasive.2
Previous work with the modified nucleoside, 2'-azido-5-cyano-2'-deoxyuridine (N3CNdU), that contains both the nitrile and azide vibrational reporters allowed these two probes to be directly compared.10 The azide asymmetric stretch vibration of N3CNdU was found to have an extinction coefficient that was approximately twice as large as the nitrile symmetric stretch vibration and the azide asymmetric stretch vibration demonstrated a greater sensitivity to local environment as shown by a larger frequency shift between solvents selected to mimic environments of biological significance.10 However, previous work has also demonstrated that the azide modified UAA, pN3Phe, is photoreactive,11–13 while the nitrile modified UAA, pCNPhe, is not, which is a significant factor for the increased use of pCNPhe as a vibrational reporter compared to pN3Phe, as well as the ability of pCNPhe to serve as a fluorescence probe by forming a FRET pair with tryptophan.4,14–17
Here, we have proposed a new vibrational reporter unnatural amino acid that has the aforementioned advantages of both the nitrile and azide vibrational reporters, while being able to be genetically incorporated into proteins site-specifically using an engineered, orthogonal aminoacyl-tRNA synthetase. Specifically, we have synthesized 4- azidomethyl-L-phenylalanine (pN3CH2Phe, Figure 1B, Scheme 1), which contains the azide vibrational reporter that is expected to have a larger oscillator strength compared to the nitrile vibrational reporter of pCNPhe and have a greater sensitivity to local environment compared to the nitrile probe. The addition of the methylene group between the azide and phenyl groups of pN3CH2Phe is expected to greatly diminish the photoreactivity of pN3CH2Phe relative to pN3Phe, thus allowing pN3CH2Phe to serve as a stable, sensitive azide modified UAA vibrational reporter of local protein environments. The ability of pN3CH2Phe to serve as an effective probe of local protein environment was investigated by measuring the position of the azide asymmetric stretch vibration of pN3CH2Phe in various solvents and by incorporating this UAA site-specifically into two distinct sites of the 247-residue monomeric protein, superfolder green fluorescent protein18 (sfGFP, Figure 1A) with high efficiency and fidelity. The photo-stability of this UAA compared with pN3Phe incorporated into sfGFP was also examined.
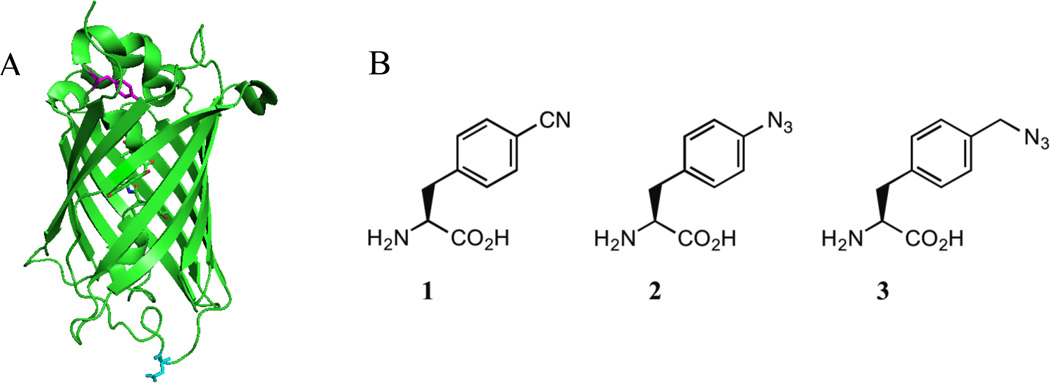
Figure 1.
A. Structure of wt-sfGFP (PDB ID 2B3P) with site 75 (magenta) and site 134 (cyan) highlighted. B. Structure of 4-cyano-L-phenylalanine (pCNPhe, 1), 4-azido-Lphenylalanine (pN3Phe, 2), and 4-azidomethyl-L-phenylalanine (pN3CH2Phe, 3).
Scheme 1a.
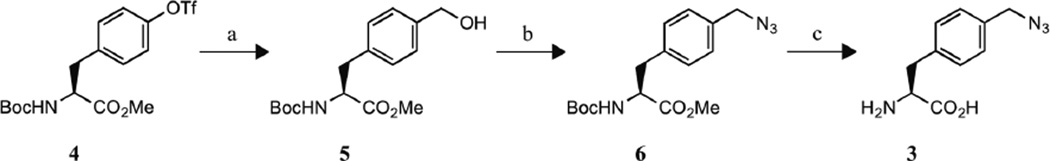
a Conditions: (a) (i) Pd(OAc)2, dppp, Et3N, DMF, CO, Oct3SiH, 70°C; (ii) NaBH4, CH3OH, 0°C to RT; (b) (i) MsCl, Et3N, DMF, 0°C to RT; (ii) MeOH, RT; (iii) NaN3, RT; (c) (i) LiOH·H2O, THF/H2O, RT; (ii) HCl, C4H8O2, RT.
Experimental
General Information
Chemical reagents were purchased from Sigma-Aldrich, Strem Chemicals, Gelest, and Praxair and used without further purification. Deuterated chloroform (98.8% D enrichment) and deuterium oxide (99.9% D enrichment) were purchased from Cambridge Isotope Labs. DH10B cells and pBadA were purchased from Invitrogen. All aqueous solutions were prepared with 18 MΩ-cm water.
Reactions were stirred with a magnetic stir bar and conducted under a dry argon atmosphere unless otherwise noted. Reactions performed above or below ambient temperature were carried out in an oil bath or an ice bath, respectively. Analytical thin layer chromatography (TLC) was performed on 0.2 mm silica plastic coated sheets (Selecto Scientific) with F254 indicator. Flash column chromatography was performed on 230–400 mesh silica gel.
1H (499.7 MHz) NMR spectra were obtained at 499.7 MHz with a Varian INOVA 500 multinuclear Fourier transform NMR spectrometer. Chemical shifts are reported in parts per million (ppm) and coupling constants (J) are reported in hertz (Hz). 1H spectra in CDCl3 were referenced to the solvent peak at 7.26 ppm and 1H spectra in D2O were referenced to the solvent peak at 4.65 ppm.
Synthesis of N-(tert-butoxycarbonyl)-4-hydroxymethyl-L-phenylalanine methyl ester (5)
5 was synthesized based upon previous literature procedures with minor modifications.19 A mixture of N-(tert-butoxycarbonyl)-4-((trifluoromethyl)sulfonyl)-Lphenylalanine methyl ester (4) (3.36 g, 7.86 mmol) synthesized as previously described,20–22 palladium (II) acetate (159 mg, 0.24 mmol), 1,3-Bis(diphenylphosphino)propane (97 mg, 0.24 mmol), and triethylamine (2.74 mL, 19.7 mmol) in anhydrous DMF (39.3 mL) was purged with carbon monoxide for 10 min. Trioctylsilane (7.1 mL, 15.7 mmol) was then added in one portion and the reaction mixture was stirred under CO gas for 16 hr at 70°C. The mixture was subsequently flushed with N2 gas and cooled to 0°C. Sodium borohydride (446 mg, 11.8 mmol) was added in one portion followed by the drop wise addition of anhydrous methanol (7.86 mL) at 0°C. The reaction mixture was then stirred at room temperature for 1 hr and subsequently treated with 10% aqueous acetic acid for 10 min. The mixture was extracted three times with ethyl acetate and the combined organic layers were washed twice with water, dried over magnesium sulfate, filtered through a Celite cake and concentrated in vacuo to an oil. The oil was purified by flash chromatography (4:6 ethyl acetate:petroleum ether) to give 1.01 g (42%) of 5 as a pale yellow oil that crystallized upon standing. 1H NMR (CDCl3): δ 1.41 (s, 9H, C(CH3)3), 3.04 (dd, 1H, Cβ-H1, J = 5.8 Hz, J = 13.7 Hz), 3.12 (dd, 1H, Cβ-H2, J = 5.6 Hz, J = 13.9 Hz), 3.72 (s, 3H, OCH3), 4.57 (m, 1H, Cα-H), 4.66 (s, 2H, CH2OH), 4.97 (d, 1H, NH, J = 7.8 Hz), 7.11 (d, 2H, ArH, J = 8.4 Hz), 7.29 (d, 2H, ArH, J = 8.3 Hz).
Synthesis of N-(tert-butoxycarbonyl)-4-azidomethyl-L-phenylalanine methyl ester (6)
A mixture of 5 (850 mg, 2.75 mmol) and triethylamine (498 µL, 3.57 mmol) in anhydrous DMF (2.2 mL) was cooled to 0°C. Methanesulfonyl chloride (276 µL, 3.57 mmol) was added drop wise at 0°C and the reaction mixture was stirred for 1 hr at room temperature. Anhydrous methanol (111 µL, 2.75 mmol) was then added in one portion and the reaction mixture was stirred for 20 min at room temperature. Sodium azide (268 mg, 4.12 mmol) was subsequently added and the reaction mixture was stirred for an additional 16 hr at room temperature. The mixture was diluted with water and extracted three times with ethyl acetate. The combined organic layers were washed with water, 1 M ammonium chloride, water, brine (2×), dried over magnesium sulfate, filtered through a Celite cake, and concentrated in vacuo to an oil that crystallized upon standing to give 6 as a white solid (833 mg, 91%). 1H NMR (CDCl3): δ 1.42 (s, 9H, C(CH3)3), 3.05 (dd, 1H, Cβ-H1, J = 6.4 Hz, J = 13.7 Hz), 3.13 (dd, 1H, Cβ-H2, J = 6.2 Hz, J = 13.5 Hz), 3.71 (s, 3H, OCH3), 4.32 (s, 2H, CH2N3), 4.59 (m, 1H, Cα-H), 4.98 (d, 1H, NH, J = 7.3 Hz), 7.15 (d, 2H, ArH, J = 7.8 Hz), 7.25 (d, 2H, ArH, J = 7.8 Hz).
Synthesis of 4-azidomethyl-L-phenylalanine hydrochloride (3·HCl)
Lithium hydroxide monohydrate (124 mg, 2.95 mmol) was added to a solution of 6 (760 mg, 2.27 mmol) in THF/H2O (3:1, 26.2 mL) and stirred for 16 hr at room temperature. The pH of the reaction mixture was adjusted to ~2.5 with 0.5 M sodium bisulfate and the mixture was extracted three times with ethyl acetate. The combined organic layers were washed with water and brine, dried over magnesium sulfate, filtered through a Celite cake, and concentrated in vacuo to yield N-(tert-butoxycarbonyl)-4-azidomethyl-L-phenylalanine as a pale yellow oil. N-(tert-butoxycarbonyl)-4-azidomethyl-L-phenylalanine was subsequently dissolved in 2.5 M HCl in 1,4-dioxane (5.7 mL) and the reaction mixture was stirred at room temperature for 4 hr. The reaction mixture was concentrated and pentane was added to precipitate 3·HCl. The white solid product was isolated by filtration to give 510 mg (87%). 1H NMR (D2O): δ 3.11 (dd, 1H, Cβ-H1, J = 7.9 Hz, J = 14.7 Hz), 3.24 (dd, 1H, Cβ-H2, J = 5.9 Hz, J = 14.7 Hz), 4.17 (dd, 1H, Cα-H, J = 5.4 Hz, J = 7.8 Hz,), 4.32 (s, 2H, CH2N3), 7.25 (d, 2H, ArH, J = 8.3 Hz), 7.31 (d, 2H, ArH, J =7.9 Hz); MS: 221.0 (M+1).
Expression and Purification of sfGFP Constructs
The codon-optimized gene containing a C-terminal 6-His affinity tag for wild-type sfGFP (wt-sfGFP)18,23,24 was inserted into pBadA generating pBad-sfGFP. The codons for Y75 and D134 were individually replaced by site-directed mutagenesis with the amber stop codon (TAG) generating pBad-sfGFP-75TAG and pBad-sfGFP-134TAG, respectively. The aminoacyl-tRNA synthetase for the incorporation of pN3Phe and pN3CH2Phe was inserted into pDule generating pDule-pN3/pN3CH2Phe.5,17 Each of these plasmids, except pBadsfGFP-75TAG, was obtained from Dr. Ryan A. Mehl (Oregon State University).
pBad-sfGFP was transformed into DH10B E. coli cells, while pBad-sfGFP-75TAG and pBad-sfGFP-134TAG were individually co-transformed with pDule-pN3/pN3CH2Phe into DH10B E. coli cells. The transformed cells were used to inoculate 5 mL of noninducing media that was grown to saturation while shaking (250 rpm) at 37°C. A portion (2.5 mL) of the cultured cells was used to inoculate 250 mL of autoinduction media containing pN3Phe or pN3CH2Phe at 1 mM except for negative control experiments where the UAAs were excluded from the autoinduction media. The cells from the autoinduction media were collected by centrifugation after shaking at 37°C for 24 – 30 hrs and the expressed protein was purified using TALON cobalt ion-exchange chromatography (Clontech) similar to previous procedures.5,23,24
Site-specific incorporation of pN3Phe or pN3CH2Phe into site 75 or 134 with high efficiency and fidelity was verified by SDS-PAGE and electrospray ionization quadrupole time-of-flight (ESI-Q-TOF) mass analysis. Typical yields for the sfGFP constructs containing pN3Phe or pN3CH2Phe were 240 mg and 130 mg of purified protein per liter of autoinduction media, respectively. The protein expression yields were calculated using the extinction coefficient of sfGFP at 488 nm.18
Equilibrium FTIR Measurements
Equilibrium FTIR absorbance spectra were recorded on a Bruker Vertex 70 FTIR spectrometer equipped with a globar source, KBr beamsplitter, and a liquid nitrogen cooled mercury cadmium telluride (MCT) detector. The spectra were recorded using a temperature-controlled transmission cell consisting of calcium fluoride windows with a path length of ~100 µm or using a Harrick BioATRcell II accessory. The temperature was measured using an embedded thermocouple in the cells. The spectra were the result of 1024 scans recorded at a resolution of 1.0 cm−1. The spectra were analyzed in Igor Pro (Wavemetrics).
Results and Discussion
Comparison of pCNPhe, pN3Phe, and pN3CH2Phe
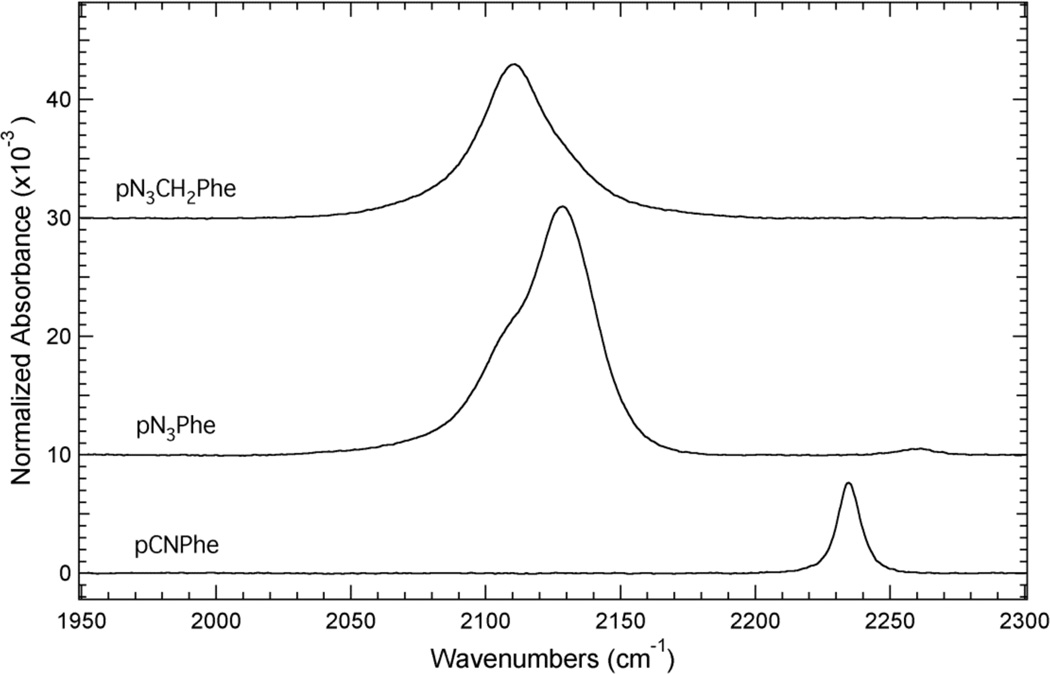
The nitrile symmetric stretch vibration of pCNPhe (2234.6 cm−1) and the azide asymmetric stretch vibration of pN3Phe (2128.6cm−1) and pN3CH2Phe (2110.7cm−1) in water appear in a relatively clear region of the infrared as shown in Figure 2. These spectra show that the IR absorbance band resulting from the nitrile symmetric stretch vibration of pCNPhe is relatively sharp and symmetric compared to the IR absorbance band of pN3Phe and pN3CH2Phe in this region (1950 – 2300 cm−1). The IR absorbance band for pN3Phe has at least two spectral components. The more intense high frequency component is assigned as resulting from the azide asymmetric stretch vibration based upon literature precedent,6–8 while the low frequency component is likely the result of anharmonic coupling such as accidental Fermi resonance. The IR absorbance band for pN3CH2Phe in this region is more symmetric than the absorbance band of pN3Phe although it is slightly asymmetric towards higher frequencies, potentially due to anharmonic effects. This band is assigned as arising from the azide asymmetric stretch vibration of pN3CH2Phe since this is the expected region for this vibration,6,7,10,25–29 although 2D IR spectroscopy30–40 would be required to determine if the observed asymmetry is due to anharmonic coupling.
Figure 2.
ATR-FTIR absorbance spectra of pCNPhe, pN3Phe, and pN3CH2Phe dissolved in water. The unnatural amino acids were dissolved to a concentration of ~50 mM in either an acidic or basic aqueous solution. The spectra were recorded at 25°C and were baseline corrected.
Figure 2 also shows that the IR absorbance band of pN3Phe and pN3CH2Phe in this region is more intense than the IR absorbance band of pCNPhe. The azide IR absorbance band of pN3Phe and pN3CH2Phe is ~2.8 and ~1.7 times more intense than the nitrile IR absorbance band of pCNPhe, respectively. The higher extinction coefficient of the azide asymmetric stretch vibration compared to the nitrile symmetric stretch vibration observed here is consistent with the previous literature comparisons of these two vibrational reporters.2,10 The observed lower extinction coefficient for the azide asymmetric stretch vibration of pN3CH2Phe compared to pN3Phe is in contrast with the reported higher extinction coefficient for this vibration in the nicotinamide adenine dinucleotide (NAD+) analog 3-picolyl azide adenine dinucleotide (PAAD+) compared to azido-NAD+.41,42
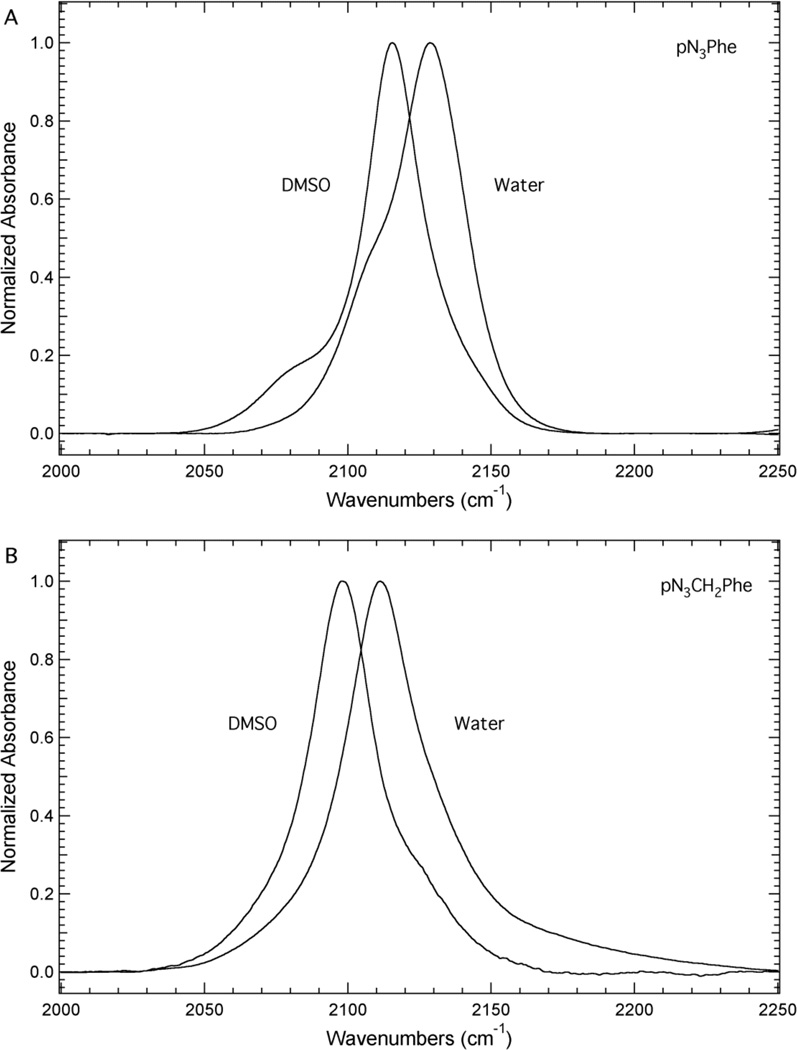
Figure 3 illustrates the sensitivity of the azide asymmetric stretch vibration of pN3Phe and pN3CH2Phe to solvent. The solvents DMSO and water were selected to mimic hydrophobic and hydrophilic environments present in proteins, respectively. Figure 3 shows that the azide asymmetric stretch vibration of pN3Phe shifts from 2115.5 cm−1 to 2128.6 cm−1 upon going from DMSO to water as the solvent, resulting in a blue shift of 13.1 cm−1. Similarly, the azide asymmetric stretch vibration of pN3CH2Phe shifts from 2097.7 cm−1 to 2111.2 cm−1 upon going from DMSO to water as the solvent, resulting in a blue shift of 13.5 cm−1. The full-width at half-maximum (fwhm) of this band increases from 28 cm−1 to 33 cm−1 for pN3CH2Phe upon going from DMSO to water as the solvent. These solvent-induced frequency shifts are significantly larger than the shift of the nitrile symmetric stretch vibration of pCNPhe in similar solvents. For instance, the nitrile symmetric stretch vibration was found to shift from 2228.5 cm−1 for Fmoc-pCNPhe in THF to 2237.2 cm−1 for pCNPhe in water,9 a blue shift of 8.7 cm−1, which is roughly two-thirds the size of that observed for pN3Phe and pN3CH2Phe. The direction and magnitude of the solvent-induced frequency shift of the azide asymmetric stretch vibration of pN3Phe and pN3CH2Phe is similar to the previously reported solvent-induced frequency shifts observed for this vibration in 5-azido-1-pentanoic acid.43
Figure 3.
Transmission FTIR absorbance spectra of pN3Phe (Panel A) and pN3CH2Phe (Panel B) dissolved in either DMSO or water. The unnatural amino acids were dissolved to a concentration of ~50 mM. The spectra were recorded at 25°C, baseline corrected, and intensity normalized.
Structure of wt-sfGFP
Figure 1A shows the structure of the 247 residue β-barrel protein wt-sfGFP with residues 75 (magenta) and 134 (cyan) highlighted. This monomeric protein consists of 47% β-sheet and 10% helical structure. Residues 75 and 134 are both located in loop regions of the protein, however these two residues represent two distinct local environments in the protein as illustrated by the solvent accessible surface area (SASA) of these two residues. The SASA for Y75 and D134 in wt-sfGFP was calculated to be 4 Å2 and 100 Å2, respectively, using the software GETAREA44 with a probe radius of 1.4 Å. These results illustrate that site 75 represents a buried position in the protein, while site 134 represents a fully solvated position in the protein.
Incorporation of pN3Phe and pN3CH2Phe into Site 75 or Site 134 of sfGFP
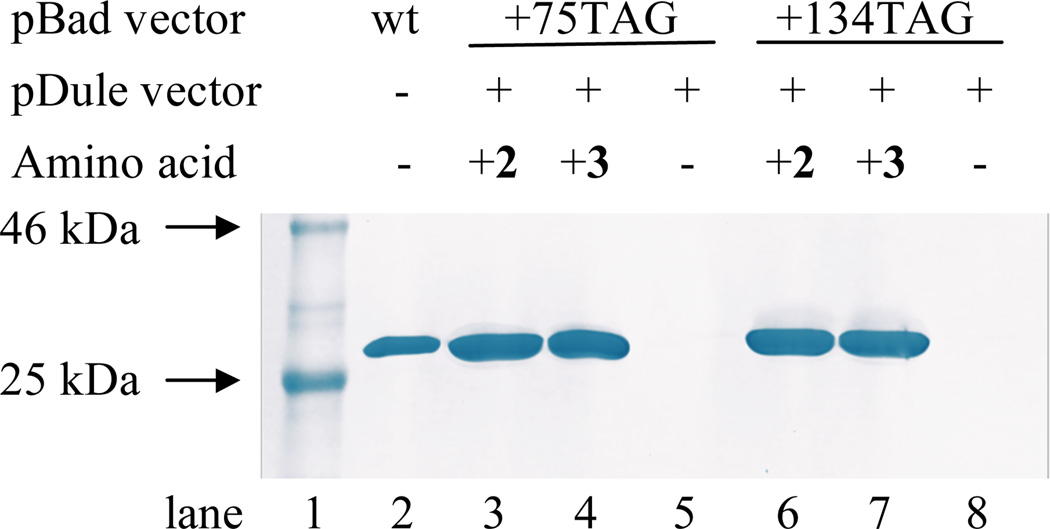
The unnatural amino acids, pN3Phe and pN3CH2Phe, were genetically incorporated into the buried (site 75) or the fully solvated position (site 134) in sfGFP in response to an amber codon in an efficient, site-specific manner with high fidelity utilizing an engineered, orthogonal aminoacyl-tRNA synthetase. The incorporation of pN3Phe into site 75 or site 134 in sfGFP resulted in the production of the protein constructs sfGFP-75-pN3Phe and sfGFP-134-pN3Phe, while the incorporation of pN3CH2Phe into site 75 or site 134 in sfGFP resulted in the production of the protein constructs sfGFP-75-pN3CH2Phe and sfGFP-134-pN3CH2Phe. The production of these constructs was verified by SDS-PAGE (see Figure 4) and ESI-Q-TOF mass analysis (see the Supporting Information). The high fidelity of the UAA incorporation was also confirmed by SDS-PAGE (Figure 4, lanes 5 and 8).
Figure 4.
Coomassie blue stained tris-glycine SDS-PAGE illustrating efficient, site-specific incorporation of 2 and 3 with high fidelity into sfGFP. The protein constructs were expressed from pBad-sfGFP (wt-sfGFP, lane 2); pBad-sfGFP-75TAG and pDulepN3/pN3CH2Phe (lanes 3 – 5) in the presence (lanes 3 and 4) or absence (lane 5) of 2 or 3, respectively; or pBad-sfGFP-134TAG and pDule-pN3/pN3CH2Phe (lanes 6 – 8) in the presence (lanes 6 and 7) or absence (lane 8) of 2 or 3, respectively.
Sensitivity of pN3CH2Phe to Local Protein Environments
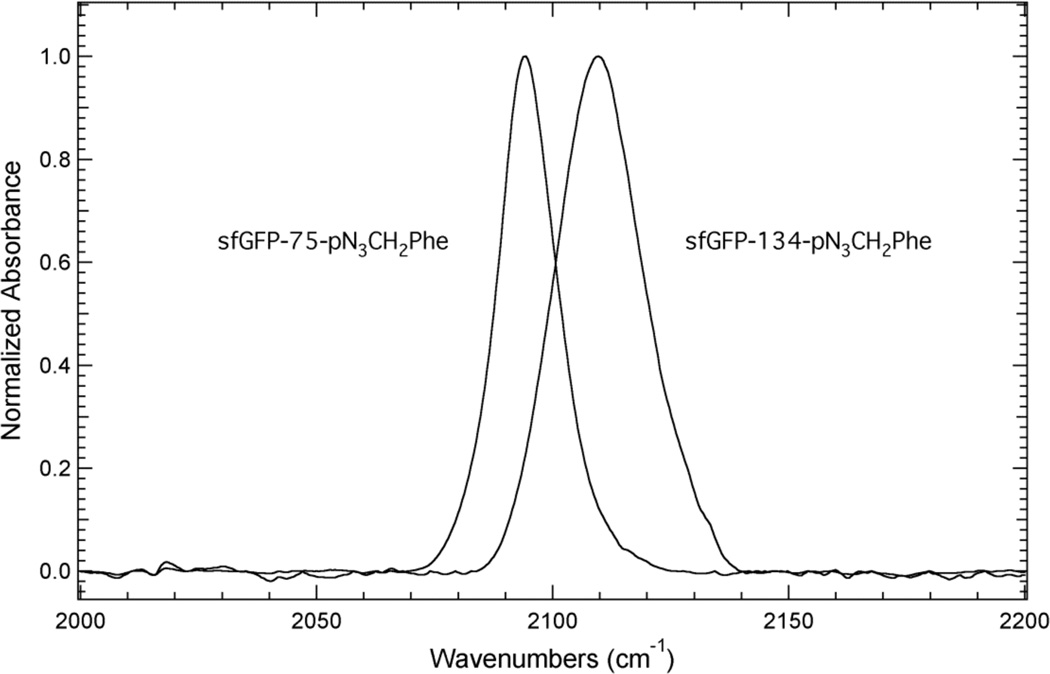
Figure 5 shows the linear IR absorbance spectra of sfGFP-75-pN3CH2Phe and sfGFP-134-pN3CH2Phe in the azide asymmetric stretch vibration region (2000 – 2250 cm−1). The IR spectrum of sfGFP-75-pN3CH2Phe shows a single, fairly symmetrical absorbance band at 2094.3 cm−1. This frequency suggests that the azide group of pN3CH2Phe is in a buried, hydrophobic position in the protein due to the similarity of this frequency to the position of the azide IR absorbance band of pN3CH2Phe dissolved in DMSO (Figure 3B) and is in agreement with the SASA calculated for site 75 in sfGFP.
Figure 5.
Transmission FTIR absorbance spectra of sfGFP constructs containing 3 at either site 75 or site 134 in the protein. The protein samples were dissolved to a concentration of ~1 mM in a pH 7.3 aqueous buffer containing 50 mM sodium phosphate and 150 mM sodium chloride. The spectra were recorded at 25°C, baseline corrected, and intensity normalized.
The IR spectrum of sfGFP-134-pN3CH2Phe also shows a single, fairly symmetrical IR absorbance band, which is centered at 2109.8 cm−1. This frequency is similar to the frequency of pN3CH2Phe dissolved in water (Figure 3B) suggesting that the azide group of pN3CH2Phe is in a solvated position in the protein, which is in agreement with the SASA calculated for site 134 in sfGFP. The position of the azide IR absorbance band in these two protein constructs represents a 15.5 cm−1 blue shift in the azide asymmetric stretch vibration from site 75 to site 134 and illustrates a high sensitivity of this vibration to local protein environment. This result was expected based upon the direction and magnitude of the shift of the azide asymmetric stretch vibration of pN3CH2Phe when dissolved in DMSO and water (Figure 3B).
Photo-Stability of pN3CH2Phe in sfGFP
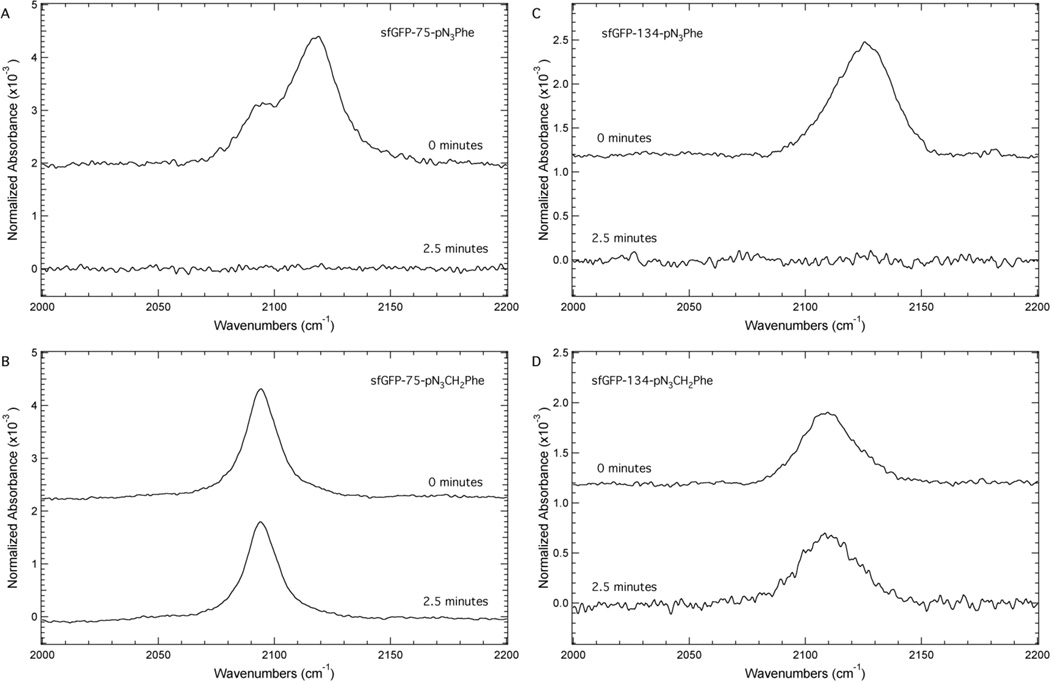
Figure 6 illustrates the significantly decreased photoreactivity of pN3CH2Phe relative to pN3Phe. This figure shows the IR absorbance spectra of sfGFP-75-pN3Phe (Panel A), sfGFP-75-pN3CH2Phe (Panel B), sfGFP-134-pN3Phe (Panel C), and sfGFP-134-pN3CH2Phe (Panel D) in the region 2000 – 2200 cm−1 before and after irradiation of the samples for 2.5 minutes with a handheld UV lamp (254 nm). The irradiation conditions were selected to be consistent with previous work11 involving pN3Phe and due to the position of the UV absorbance band of pN3Phe at 251 nm (see the Supporting Information).
Figure 6.
ATR-FTIR absorbance spectra of sfGFP containing either 2 or 3 at site 75 (Panels A and B) or at site 134 (Panels C and D) in the protein. The protein samples were dissolved to a concentration of ~1 mM in a pH 7.3 aqueous buffer containing 50 mM sodium phosphate and 150 mM sodium chloride. The protein samples were irradiated for either zero or 2.5 minutes with a handheld UV lamp (254 nm, 4 W). The spectra were recorded at 25°C, baseline corrected, and normalized to the Amide I band.
Panels A and C show an azide IR absorbance band for sfGFP-75-pN3Phe and sfGFP-134-pN3Phe at 2118.9 cm−1 and 2125.7 cm−1, respectively, before irradiation. These frequencies correspond to the maximum intensity of the absorbance band. The position of the bands and the direction of the shift between the bands were expected based upon the effect of solvent on the position of this band in pN3Phe (Figure 3A). After irradiation at 254 nm for 2.5 minutes the azide IR absorbance band is absent from the spectra as expected based upon previous studies of pN3Phe.11–13
In contrast, the azide IR absorbance band of sfGFP-75-pN3CH2Phe (Panel B) and sfGFP-134-pN3CH2Phe (Panel D) at 2094.3 cm−1 and 2109.8 cm−1, respectively, remain after irradiation at 254 nm for 2.5 minutes, exhibiting only a slight (~10%) decrease in intensity after the irradiation. This significantly decreased photoreactivity of pN3CH2Phe relative to pN3Phe was expected due to the significant decrease in absorbance at 254 nm in pN3CH2Phe compared to pN3Phe (see the Supporting Information).
Conclusions
The unnatural amino acid 4-azidomethyl-L-phenylalanine (pN3CH2Phe) has been successfully synthesized and represents a much improved vibrational reporter of local protein environments compared to the unnatural amino acid vibrational reporters 4-cyano-L-phenylalanine (pCNPhe) and 4-azido-L-phenylalanine (pN3Phe). Similar to the nitrile symmetric stretch vibration of pCNPhe, the azide asymmetric stretch vibration of pN3CH2Phe occurs in a relatively clear region of the infrared. However, the extinction coefficient of the azide asymmetric stretch vibration of pN3CH2Phe is ~1.7 times greater than the nitrile symmetric stretch vibration of pCNPhe, which will ultimately allow lower protein concentrations to be utilized when pN3CH2Phe is incorporated into protein constructs compared to pCNPhe. Additionally, the azide asymmetric stretch vibration of pN3CH2Phe is more sensitive to local environment than the nitrile symmetric stretch vibration of pCNPhe as illustrated by the blue shift of 13.5 cm−1 of this vibration from DMSO to water.
pN3CH2Phe was also successfully genetically incorporated individually into two distinct sites of sfGFP in response to an amber codon in an efficient, site-specific manner with high fidelity utilizing an engineered, orthogonal aminoacyl-tRNA synthetase. The azide asymmetric stretch vibration of pN3CH2Phe was found to blue shift 15.5 cm−1 upon going from a buried position (site 75) in the protein to a solvated position (site 134), illustrating the high sensitivity of this vibrational mode to local protein environment.
The selection of pN3CH2Phe as the target azide analogue of pCNPhe was made to take advantage of the favorable azide vibrational properties, compared to that of the nitrile, while attempting to minimize the unfavorable photoreactivity of pN3Phe (the direct azide analogue of pCNPhe). Our results show that the inclusion of the methylene spacer between the azide group and the phenyl ring of pN3CH2Phe, although reducing the intensity of the azide asymmetric stretch vibration of pN3CH2Phe relative to pN3Phe, greatly reduced the photoreactivity of pN3CH2Phe compared to pN3Phe, while minimizing the increase in size of pN3CH2Phe relative to pN3Phe. Thus the photostability of pN3CH2Phe will permit the azide vibrational reporter to be utilized to effectively study local protein environments in a relatively non-invasive manner.
In addition to serving as effective vibrational reporters, azides can also undergo bioorthogonal click cycloaddition reactions with terminal alkynes.45–47 Thus pN3CH2Phe can serve as both a vibrational reporter of local protein environments and a conduit to many other probes such as fluorescent dyes, nitroxide spin labels or metal carbonyl vibrational probes.
Supplementary Material
Acknowledgments
We thank Dr. Edward E. Fenlon and Dr. Ryan A. Mehl for helpful conversations; Lisa Mertzman for obtaining materials and supplies; Beth Buckwalter for acquiring NMR spectra; and Jacob Lipkin for experimental contributions. This work was supported by F&M Hackman funds, NSF (CHE-1053946) to SHB, and NIH (R15GM093330) to SHB/EEF.
Footnotes
Supporting Information
Sequence of wt-sfGFP, ESI-Q-TOF mass analysis of the sfGFP protein constructs, equilibrium UV/Vis absorbance spectra of pN3Phe and pN3CH2Phe in water. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Xie J, Schultz PG. A Chemical Toolkit for Proteins - An Expanded Genetic Code. Nat. Rev. Mol. Cell Biol. 2006;7:775–782. doi: 10.1038/nrm2005. [DOI] [PubMed] [Google Scholar]
- 2.Waegele MM, Culik RM, Gai F. Site-Specific Spectroscopic Reporters of the Local Electric Field, Hydration, Structure, and Dynamics of Biomolecules. J. Phys. Chem. Lett. 2011;2:2598–2609. doi: 10.1021/jz201161b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz KC, Supekova L, Ryu Y, Xie J, Perera R, Schultz PG. A Genetically Encoded Infrared Probe. J. Am.Chem. Soc. 2006;128:13984–13985. doi: 10.1021/ja0636690. [DOI] [PubMed] [Google Scholar]
- 4.Taskent-Sezgin H, Chung J, Patsalo V, Miyake-Stoner SJ, Miller AM, Brewer SH, Mehl RA, Green DF, Raleigh DP, Carrico I. Interpretation of p-Cyanophenylalanine Fluorescence in Proteins in Terms of Solvent Exposure and Contribution of Side-Chain Quenchers: A Combined Fluorescence, IR and Molecular Dynamics Study. Biochemistry. 2009;48:9040–9046. doi: 10.1021/bi900938z. [DOI] [PubMed] [Google Scholar]
- 5.Bazewicz CG, Lipkin JS, Smith EE, Liskov MT, Brewer SH. Expanding the Utility of 4-Cyano-L-Phenylalanine as a Vibrational Reporter of Protein Environments. J. Phys. Chem. B. 2012;116:10824–10831. doi: 10.1021/jp306886s. [DOI] [PubMed] [Google Scholar]
- 6.Ye SX, Huber T, Vogel R, Sakmar TP. FTIR Analysis of GPCR Activation Using Azido Probes. Nat. Chem. Biol. 2009;5:397–399. doi: 10.1038/nchembio.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye SX, Zaitseva E, Caltabiano G, Schertler GFX, Sakmar TP, Deupi X, Vogel R. Tracking G-Protein-Coupled Receptor Activation Using Genetically Encoded Infrared Probes. Nature. 2010;464:1386–1389. doi: 10.1038/nature08948. [DOI] [PubMed] [Google Scholar]
- 8.Thielges MC, Axup JY, Wong D, Lee HS, Chung JK, Schultz PG, Fayer MD. Two-Dimensional Ir Spectroscopy of Protein Dynamics Using Two Vibrational Labels: A Site-Specific Genetically Encoded Unnatural Amino Acid and an Active Site Ligand. J. Phys. Chem. B. 2011;115:11294–11304. doi: 10.1021/jp206986v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Getahun Z, Huang CY, Wang T, De Leon B, DeGrado WF, Gai F. Using Nitrile-Derivatized Amino Acids as Infrared Probes of Local Environment. J. Am.Chem. Soc. 2003;125:405–411. doi: 10.1021/ja0285262. [DOI] [PubMed] [Google Scholar]
- 10.Gai XS, Coutifaris BA, Brewer SH, Fenlon EE. A Direct Comparison of Azide and Nitrile Vibrational Probes. Phys. Chem. Chem. Phys. 2011;13:5926–5930. doi: 10.1039/c0cp02774j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin JW, Santoro SW, Martin AB, King DS, Wang L, Schultz PG. Addition of p-Azido-L-Phenylalanine to the Genetic Code of Escherichia Coli. J. Am.Chem. Soc. 2002;124:9026–9027. doi: 10.1021/ja027007w. [DOI] [PubMed] [Google Scholar]
- 12.Carrico IS, Maskarinec SA, Heilshorn SC, Mock ML, Liu JC, Nowatzki PJ, Franck C, Ravichandran G, Tirrell DA. Lithographic Patterning of Photoreactive Cell-Adhesive Proteins. J. Am.Chem. Soc. 2007;129:4874–4875. doi: 10.1021/ja070200b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang KC, Diehl MR, Tirrell DA. Artificial Polypeptide Scaffold for Protein Immobilization. J. Am.Chem. Soc. 2005;127:10136–10137. doi: 10.1021/ja051457h. [DOI] [PubMed] [Google Scholar]
- 14.Tucker MJ, Oyola R, Gai F. Conformational Distribution of a 14-Residue Peptide in Solution: A Fluorescence Resonance Energy Transfer Study. J. Phys. Chem. B. 2005;109:4788–4795. doi: 10.1021/jp044347q. [DOI] [PubMed] [Google Scholar]
- 15.Aprilakis KN, Taskent H, Raleigh DP. Use of the Novel Fluorescent Amino Acid p-Cyanophenylalanine Offers a Direct Probe of Hydrophobic Core Formation During the Folding of the N-Terminal Domain of the Ribosomal Protein L9 and Provides Evidence for Two-State Folding. Biochemistry. 2007;46:12308–12313. doi: 10.1021/bi7010674. [DOI] [PubMed] [Google Scholar]
- 16.Glasscock JM, Zhu YJ, Chowdhury P, Tang J, Gai F. Using an Amino Acid Fluorescence Resonance Energy Transfer Pair to Probe Protein Unfolding: Application to the Villin Headpiece Subdomain and the Lysm Domain. Biochemistry. 2008;47:11070–11076. doi: 10.1021/bi8012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyake-Stoner SJ, Miller AM, Hammill JT, Peeler JC, Hess KR, Mehl RA, Brewer SH. Probing Protein Folding Using Site-Specifically Encoded Unnatural Amino Acids as FRET Donors with Tryptophan. Biochemistry. 2009;48:5953–5962. doi: 10.1021/bi900426d. [DOI] [PubMed] [Google Scholar]
- 18.Pedelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Engineering and Characterization of a Superfolder Green Fluorescent Protein. Nat. Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- 19.Morera E, Ortar G, Varani A. An Improved Preparation of 4-Hydroxymethyl-L-Phenylalanine. Synthetic Commun. 1998;28:4279–4285. [Google Scholar]
- 20.Bazewicz CG, Lipkin JS, Lozinak KA, Watson MD, Brewer SH. Synthesis of Isotopomers of N-(tert-Butoxycarbonyl)-4-Cyano-L-Phenylalanine Methyl Ester: Choice of Cyanation Solvent. Tetrahedron Lett. 2011;52:6865–6868. [Google Scholar]
- 21.Kim BC, Hwang SY, Lee TH, Chang JH, Choi HW, Lee KW, Choi BS, Kim YK, Lee JH, Kim WS, Oh YS, Lee HB, Kim KY, Shin H. Development of a Scalable Synthetic Route Towards a Thrombin Inhibitor, Lb30057. Org. Process Res. Dev. 2006;10:881–886. [Google Scholar]
- 22.Shieh WC, Carlson JA. A Simple Asymmetric-Synthesis of 4-Arylphenylalanines Via Palladium-Catalyzed Cross-Coupling Reaction of Arylboronic Acids with Tyrosine Triflate. J. Org. Chem. 1992;57:379–381. [Google Scholar]
- 23.Miyake-Stoner SJ, Refakis CA, Hammill JT, Lusic H, Hazen JL, Deiters A, Mehl RA. Generating Permissive Site-Specific Unnatural Aminoacyl-tRNA Synthetases. Biochemistry. 2010;49:1667–1677. doi: 10.1021/bi901947r. [DOI] [PubMed] [Google Scholar]
- 24.Smith EE, Linderman BY, Luskin AC, Brewer SH. Probing Local Environments with the Infrared Probe: L-4-Nitrophenylalanine. J. Phys. Chem. B. 2011;115:2380–2385. doi: 10.1021/jp109288j. [DOI] [PubMed] [Google Scholar]
- 25.Oh KI, Lee JH, Joo C, Han H, Cho M. β-Azidoalanine as an IR Probe: Application to Amyloid Aβ(16–22) Aggregation. J. Phys. Chem. B. 2008;112:10352–10357. doi: 10.1021/jp801558k. [DOI] [PubMed] [Google Scholar]
- 26.Taskent-Sezgin H, Chung JA, Banerjee PS, Nagarajan S, Dyer RB, Carrico I, Raleigh DP. Azidohomoalanine: A Conformationally Sensitive IR Probe of Protein Folding, Protein Structure, and Electrostatics. Angew. Chem., Int. Ed. 2010;49:7473–7475. doi: 10.1002/anie.201003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gai XS, Fenlon EE, Brewer SH. A Sensitive Multispectroscopic Probe for Nucleic Acids. J. Phys. Chem. B. 2010;114:7958–7966. doi: 10.1021/jp101367s. [DOI] [PubMed] [Google Scholar]
- 28.Nagarajan S, Taskent-Sezgin H, Parul D, Carrico I, Raleigh DP, Dyer RB. Differential Ordering of the Protein Backbone and Side Chains During Protein Folding Revealed by Site-Specific Recombinant Infrared Probes. J. Am.Chem. Soc. 2011;133:20335–20340. doi: 10.1021/ja2071362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloem R, Koziol K, Waldauer SA, Buchli B, Walser R, Samatanga B, Jelesarov I, Hamm P. Ligand Binding Studied by 2D IR Spectroscopy Using the Azidohomoalanine Label. J. Phys. Chem. B. 2012;116:13705–13712. doi: 10.1021/jp3095209. [DOI] [PubMed] [Google Scholar]
- 30.Cho MH. Coherent Two-Dimensional Optical Spectroscopy. Chem. Rev. 2008;108:1331–1418. doi: 10.1021/cr078377b. [DOI] [PubMed] [Google Scholar]
- 31.Ganim Z, Chung HS, Smith AW, Deflores LP, Jones KC, Tokmakoff A. Amide I Two-Dimensional Infrared Spectroscopy of Proteins. Acc. Chem. Res. 2008;41:432–441. doi: 10.1021/ar700188n. [DOI] [PubMed] [Google Scholar]
- 32.Shim SH, Gupta R, Ling YL, Strasfeld DB, Raleigh DP, Zanni MT. Two-Dimensional IR Spectroscopy and Isotope Labeling Defines the Pathway of Amyloid Formation with Residue-Specific Resolution. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6614–6619. doi: 10.1073/pnas.0805957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YS, Hochstrasser RM. Applications of 2D IR Spectroscopy to Peptides, Proteins, and Hydrogen-Bond Dynamics. J. Phys. Chem. B. 2009;113:8231–8251. doi: 10.1021/jp8113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucker MJ, Kim YS, Hochstrasser RM. 2D IR Photon Echo Study of the Anharmonic Coupling in the OCN Region of Phenyl Cyanate. Chem. Phys. Lett. 2009;470:80–84. doi: 10.1016/j.cplett.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baiz CR, Mcrobbie PL, Anna JM, Geva E, Kubarych KJ. Two-Dimensional Infrared Spectroscopy of Metal Carbonyls. Acc. Chem. Res. 2009;42:1395–1404. doi: 10.1021/ar9000263. [DOI] [PubMed] [Google Scholar]
- 36.Urbanek DC, Vorobyev DY, Serrano AL, Gai F, Hochstrasser RM. The Two-Dimensional Vibrational Echo of a Nitrile Probe of the Villin Hp35 Protein. J. Phys. Chem. Lett. 2010;1:3311–3315. doi: 10.1021/jz101367d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nydegger MW, Dutta S, Cheatum CM. Two-Dimensional Infrared Study of 3-Azidopyridine as a Potential Spectroscopic Reporter of Protonation State. J. Chem. Phys. 2010;133:134506. doi: 10.1063/1.3483688. [DOI] [PubMed] [Google Scholar]
- 38.Chung JK, Thielges MC, Fayer MD. Dynamics of the Folded and Unfolded Villin Headpiece (Hp35) Measured with Ultrafast 2D IR Vibrational Echo Spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3578–3583. doi: 10.1073/pnas.1100587108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucker MJ, Gai XS, Fenlon EE, Brewer SH, Hochstrasser RM. 2D IR Photon Echo of Azido-Probes for Biomolecular Dynamics. Phys. Chem. Chem. Phys. 2011;13:2237–2241. doi: 10.1039/c0cp01625j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagchi S, Boxer SG, Fayer MD. Ribonuclease S Dynamics Measured Using a Nitrile Label with 2D IR Vibrational Echo Spectroscopy. J. Phys. Chem. B. 2012;116:4034–4042. doi: 10.1021/jp2122856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutta S, Li YL, Rock W, Houtman JCD, Kohen A, Cheatum CM. 3-Picolyl Azide Adenine Dinucleotide as a Probe of Femtosecond to Picosecond Enzyme Dynamics. J. Phys. Chem. B. 2012;116:542–548. doi: 10.1021/jp208677u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutta S, Rock W, Cook RJ, Kohen A, Cheatum CM. Two-Dimensional Infrared Spectroscopy of Azido-Nicotinamide Adenine Dinucleotide in Water. J. Chem. Phys. 2011;135:055106. doi: 10.1063/1.3623418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfshorndl MP, Baskin R, Dhawan I, Londergan CH. Covalently Bound Azido Groups Are Very Specific Water Sensors, Even in Hydrogen-Bonding Environments. J. Phys. Chem. B. 2012;116:1172–1179. doi: 10.1021/jp209899m. [DOI] [PubMed] [Google Scholar]
- 44.Fraczkiewicz R, Braun W. Exact and Efficient Analytical Calculation of the Accessible Surface Areas and Their Gradients for Macromolecules. J. Comput. Chem. 1998;19:319–333. [Google Scholar]
- 45.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int.. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 46.Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 47.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-Free Click Chemistry for Dynamic in Vivo Imaging. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.