Abstract
Rationale
MicroRNAs modestly suppress their direct mRNA targets and these direct effects are amplified by modulation of gene transcription pathways. Consequently, indirect mRNA modulatory effects of microRNAs to increase or decrease mRNAs greatly outnumber direct target suppressions. Because microRNAs are products of transcription, the potential exists for microRNAs that regulate transcription to regulate other microRNAs.
Objective
Determine if cardiac-expressed microRNAs regulate expression of other cardiac microRNAs, and measure the impact of microRNA-mediated microRNA regulation on indirect regulation of non-target mRNAs.
Methods and Results
Transgenic expression of pre-microRNAs was used to generate mouse hearts expressing 6-16 fold normal levels of miR-143, miR-378, and miR-499. Genome-wide mRNA and microRNA signatures were established using deep sequencing; expression profiles provoked by each microRNA were defined. miR-143 suppressed its direct cardiac mRNA target hexokinase 2, but exhibited little indirect target regulation and did not regulate other cardiac microRNAs. Both miR-378 and miR-499 indirectly regulated hundreds of cardiac mRNAs and 15-30 cardiac microRNAs. MicroRNA overexpression did not alter normal processing of either transgenic or endogenous cardiac microRNAs, and microRNA-mediated regulation of other microRNAs encoded within parent genes occurred in tandem with parent mRNAs. MicroRNA regulation by miR-378 and miR-499 was stimulus-specific, and contributed to observed mRNA downregulation.
Conclusions
MicroRNAs that modulate cardiac transcription can indirectly regulate other microRNAs. Transcriptional modulation by microRNAs, and microRNA-mediated microRNA regulation, help explain how small direct effects of microRNAs are amplified to generate striking phenotypes.
Keywords: microRNA, deep sequencing, transcriptional regulation, translational regulation, myocardial, genetics, transgenic models
Introduction
MicroRNAs are important regulators of cardiac homeostasis and stress responses. By destabilizing messenger RNAs that encode proteins within cell metabolism, growth, calcium signaling, and programmed death pathways, microRNAs exert “nodal” control over critical biological processes 1. Individual microRNA-mRNA interactions typically only fractionally reduce levels of the target mRNA, leading to the notion that microRNAs are “fine tuners” of cell functions 2, 3. However, striking phenotypes can be induced when some microRNA levels are artificially manipulated, and especially when all forms of a given microRNA are genetically ablated 4, 5. The disparity between modest individual effects of a microRNA on its direct mRNA targets and the dramatic end-organ changes that can be provoked by microRNA gain- or loss-of-function 6 have been partially explained by targeting of transcriptional regulators by stress-regulated cardiac microRNAs. We designated the orchestration of non-target mRNA levels through regulation of transcription pathways as “epitranscriptional regulation” 7.
Like all RNAs, microRNAs are products of transcription. It is therefore almost inescapable that microRNAs which regulate gene transcription also regulate the expression of some other microRNAs. An example of this type of microRNA-mediated microRNA regulation is the cross-regulation that occurs between members of the “myomiR” family of microRNAs that are encoded within myosin heavy chain genes 8, 9. Despite a strong logical foundation and the example of myomiRs, other microRNAs have been almost completely overlooked as microRNA targets. Yet, microRNA-mediated microRNA regulation is being detected when properly assayed 10. Thus, there is a critical need to determine whether cardiac microRNA-mediated microRNA regulation is unique to myomiRs. If such regulation is common, then these events illuminate a mechanism by which specific microRNAs can recruit to RISC complexes and suppress mRNAs that are not their direct targets, i.e. “indirect” mRNA targeting.
Here, we investigated the potential for three structurally and functionally distinct cardiac-expressed microRNAs to direct microRNA-mediated cardiac microRNA regulation. We applied quantitative, unbiased, and genome-wide analytical techniques to novel mouse models in which miR-143, miR-378, and miR-499 were expressed at levels comparable to those described in diseased hearts 11, 12. We observe that these microRNAs differ in their individual capacities to regulate expression of other cardiac-expressed microRNAs (cardiomiRs) in proportion to their modulation of gene transcription pathways. These results uncover a largely overlooked consequence of natural, artificial, or therapeutic microRNA regulation, microRNA-mediated microRNA regulation, that helps to explain unpredictable microRNA functions and that may contribute to “off-target” effects of antagomiR/antimiRs.
Methods
Mouse models and deep sequencing assays
Mice with Myh6-driven expression of pre-miR-143 and pre-miR-378 were created as previously described for miR-499 11, 13. The late-developing cardiomyopathy phenotype of the miR-499 transgenic mice utilized herein has been reported (TG15; 11), but its RNA expression signature was not characterized. Procedural and analytical techniques for deep mRNA and microRNA sequencing of mouse hearts have been previously described 7, 14. Genome-wide significance for multiple mRNA and microRNA comparisons was established as +/- 25% regulation at a false discovery rate (FDR) of 0.05.
microRNA annotation using miRBase 19
Previous nomenclature for microRNAs often described the minor product (passenger strand) of a microRNA stem-loop structure as a miR* form. In this manuscript, we have annotated mouse microRNAs according to the nomenclature used by miRBase 19, released in August 2012 (http://www.mirbase.org/) 15, 16. microRNAs are designated as -5p or -3p forms according to their site of origin in the microRNA stem-loop precursor; the terms ‘major’ or ‘minor’ forms refer to abundance measured from our cardiac deep sequencing data.
Other detailed procedures and protocols are in the electronic supplement.
Results
Characteristics of miR-143, miR-378, and miR-499 in mouse hearts
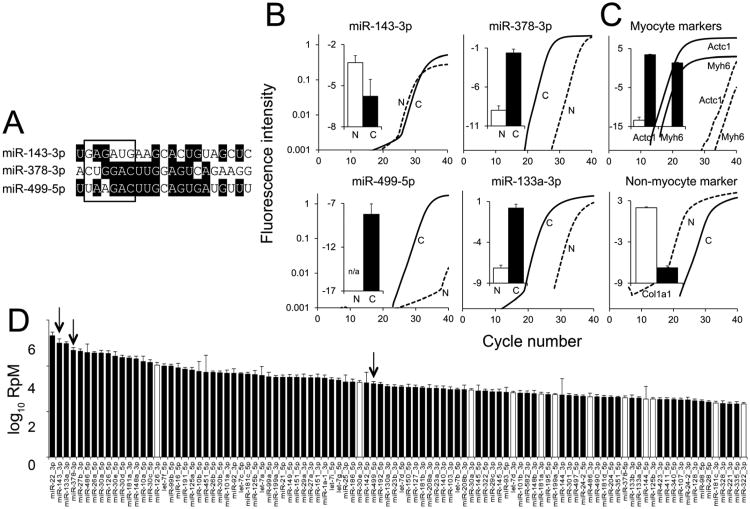
We interrogated three abundant cardiac microRNAs (cardiomiRs) previously reported to be increased in heart disease: miRs-143, -378(a), and -499. The regulatory and structural diversity (Figure 1a) of these microRNAs suggest different actions (reviewed in 17). The muscle-specific myomiR miR-499 is upregulated in human and experimental heart failure 11, 12, and is implicated in transcriptional and post-translational regulation of pathological hypertrophy 11. Ubiquitous miR-378 is upregulated in human heart failure and decreased or unchanged in murine cardiac hypertrophy 7, 12, 18, and has been implicated in cardiac regulation of systemic metabolism 19. miR-143 is upregulated in human heart failure 12, but not in murine pressure-overload hypertrophy 7. miR-143 is highly expressed in vascular smooth muscle 20 and in cardiomyocytes of the developing heart 21-23. Because the relative expression levels of mature microRNAs for these three cardiomiRs have not been determined in adult heart myocyte and non-myocyte cell populations, we isolated cardiomyocytes and nonmyocytes from hearts of normal adult mice. As shown in Figure 1b, all three microRNAs are measurable in cardiac myocyte RNA. miR-143 was slightly more abundant, and miR-499 was not detectable, in the non-myocyte myocardial fraction.
Figure 1. Characteristics of three cardiac-expressed microRNAs.
A. Sequence alignment of guide strands for miR-143, -378, and -499. Box denotes seed sequences. B. MicroRNA RT-qPCR from cardiomyocytes, denoted as C (solid lines, amplification curves; black bars, summary graphs) and nonmyocytes, denoted as N (dashed lines, representative amplification curves; white bars, summary graphs). C. mRNA RT-qPCR from cardiomyocytes and nonmyocytes (represented as for B). For B and C, fluorescence intensity data are all on the same scale and bar graphs show the difference in Ct between target and control RNAs (U6 snRNA for microRNAs, Gapdh for mRNAs); n=3 biological replicates, mean ± s.e.m. D. Contextual abundance of cardiomiR guide (black) and passenger (white) strands in 8 wk mouse hearts; the most abundant 100 cardiomiRs are shown (Reads per Million, log10 scale). Black bars designate guide strands (major forms), white bars designate passenger strands (minor forms); further discussed in Figure 5. Abundances of the remaining cardiomiRs are shown in Online Figure III. Arrows indicate positions of guide strands for miR-143 -378, and -499.
To quantify these three cardiomiRs in context, we performed deep sequencing of microRNAs from wild-type, 8 week mouse hearts. The 100 most abundant cardiomiRs are shown in Figure 1d (and data on a further 200 cardiomiRs are shown in Online Figure III). Similarly to previous determinations using a separate cohort of mice 7, miR-143 was one of the two must abundant cardiomiRs, miR-378 was the 4th most abundant, and miR-499 was the 47th most abundant cardiomiR.
Cardiac overexpression of miR-143, miR-378, and miR-499
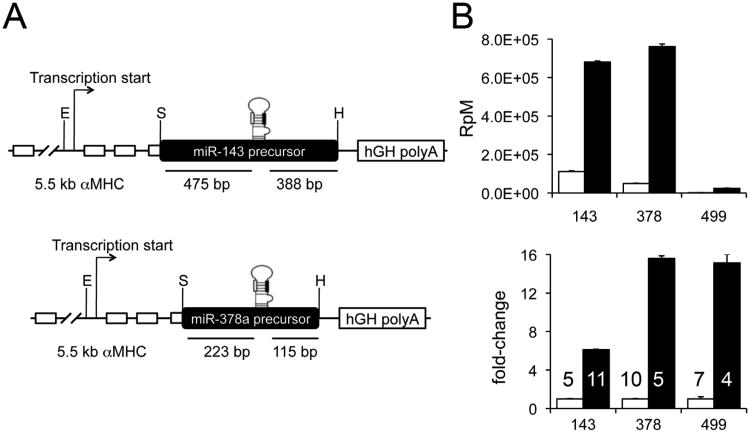
To overexpress miR-143 and -378, DNA fragments flanking the respective precursor microRNAs (premiR-143: 404 base pairs; premiR-378: 926 base pairs) were amplified from mouse genomic DNA and expressed using the Myh6 promoter (Figure 2a). This system drives cardiac miR expression shortly after birth 24, which avoids potentially confounding developmental effects of embryonic microRNA expression. miR-499 was expressed using the same system. The cardiac phenotypes of different miR-499 cardiac transgenic mouse lines have been described 11, 13, but current studies used a line (TG 15) in which the transcriptional and epitranscriptional characteristics were not previously determined. Each of the three transgenic microRNAs were expressed at levels ranging from 6 to 16 times their respective endogenous levels in nontransgenic lettermates (Figure 2b), which is within the range observed in cases of human heart failure 11, 12. All transgenic mice were born at expected frequencies. miR-143 and miR-378 transgenic hearts were functionally normal by echocardiographic examination at 8 weeks of age, and were studied at that age (Online Figure I). Because miR-499 induces cardiac hypertrophy and failure after 8 weeks, and hypertrophy/failure can primarily alter microRNA and mRNA expression 7, we studied functionally normal hearts from 4 week-old miR-499 transgenic mice and age-matched littermate controls. Comparing the results of miR-499 mice at 4 weeks and miR-378 and miR-143 mice at 8 weeks was uncomplicated because the levels of each microRNA used for transgenic overexpression are stable between those ages (see Figure 5a).
Figure 2. Forced expression of miR-143, -378, and -499 and effects on parent and guide strand abundance.
A. Schematic diagram of constructs for miR-143 and miR-378(a) cardiac transgenic mice. Black and white bars in stem loop structures indicate positions of guide and passenger strands, respectively. B. Overexpression of miR-143, -378, and -499 in their respective transgenic lines. Top panel is absolute quantity in reads per million microRNA reads (RpM). Bottom panel shows relative fold-increase in expression of transgenic (black) compared to respective littermate nontransgenic controls (white). Numbers of hearts used for each determination are shown in lower panel. All are significant at FDR <1E-20.
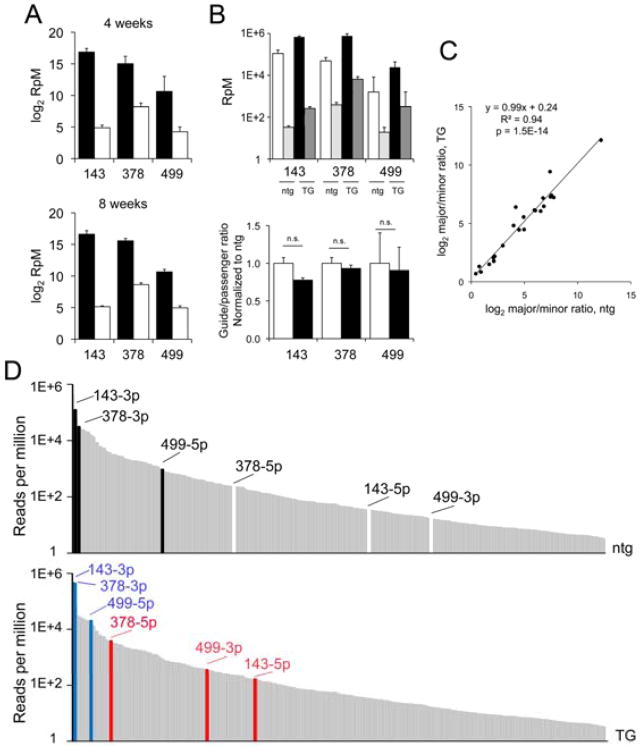
Figure 5. A. microRNA guide and passenger strand processing in normal and microRNA transgenic hearts.
Quantitation by miR-Seq of guide (black) and passenger (white) strands for miR-143, -378, and -499 in 4 and 8 week old normal mouse hearts. Reads per million microRNA reads (RpM) are plotted on log2 scale. n= 6 (4 wk) and 15 (8 wk) mouse hearts. B. Comparison of guide and passenger strand abundance in nontransgenic (ntg) and transgenic (TG) mice for miR-143, -378, and -499 mouse lines. Top panel is absolute quantity shown on log10 scale. Bottom panel is relative change in guide/passenger abundance in TG (black) normalized to respective ntg controls (white). C. The ratio (log2) of major/minor strand of miR-378 and miR-499 regulated cardiomiRs in transgenic mice is plotted (vertical axis) vs nontransgenic controls. D. Top: Abundance (log10 RpM) of miR-143, -378, and -499 guide (black) and corresponding passenger (white) strands in nontransgenic mouse hearts in context of normal microRNA abundance distribution. Bottom: Abundances of guide (blue) and passenger (red) strands in transgenic mouse hearts.
Transcriptional redirection by cardiac-expressed miR-143, miR-378, and miR-499
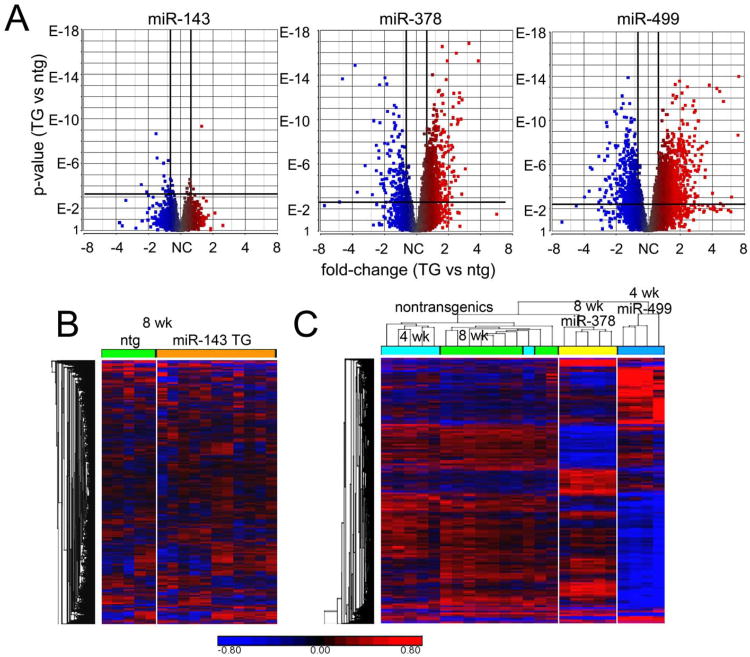
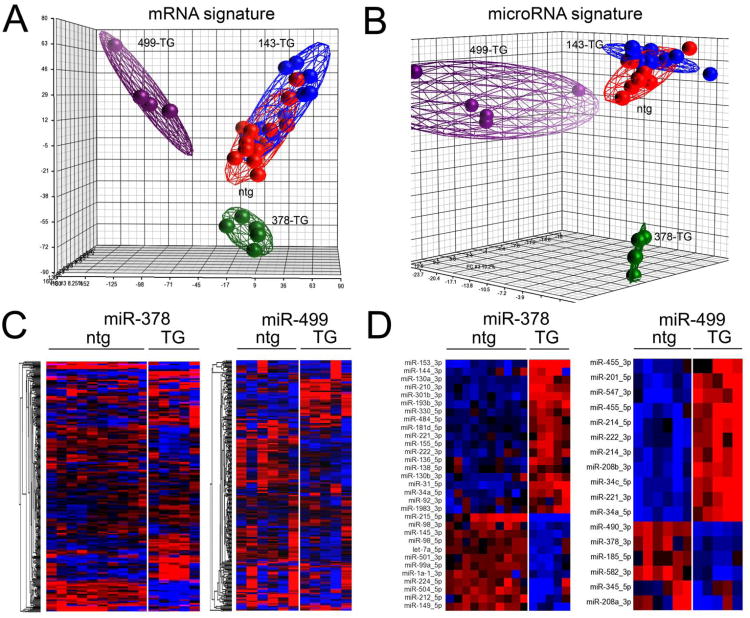
We interrogated the effects of miR-143, -378, and -499 on cardiac mRNA levels using deep RNA-sequencing 14. A total of 1,903 cardiac mRNAs were regulated by any expressed microRNA. Volcano plots depicting regulated mRNAs in each of the microRNA transgenic mouse lines are shown in Figure 3a. It is evident that miR-143 overexpression had little effect on cardiac mRNA levels at our pre-specified levels of significance (25%-fold change, false discovery rate (FDR) < 0.05). By contrast, miR-378 and miR-499 each regulated large numbers of cardiac mRNAs (Online Table I). A heat map of cardiac mRNA levels in miR-143 mice (Figure 3b) confirms that the transcript profile is virtually indistinguishable from that of nontransgenic littermates, with only 17 mRNAs significantly regulated (one of these is the previously determined miR-143 target, hexokinase 2 25-28) (Online Table I). By contrast, unsupervised hierarchical clustering of miR-378 and miR-499 transgenic mice and their respective nontransgenic controls reveals distinct mRNA signatures induced by miR-378 (954 regulated mRNAs) and miR-499 (1,636 regulated mRNAs) (Figure 3c and Online Table I). Thus, these three microRNAs display varying abilities to regulate cardiac mRNA levels. The observation that roughly 50% of all miR-378-regulated mRNAs and 40% of miR-499-regulated mRNAs were increased (rather than destabilized and decreased) reveals epitranscriptional effects of these two cardiac microRNAs (Online Table I) 7, 11, 19.
Figure 3. Effects of miR-143, -378, and -499 on cardiac gene expression.
A. Volcano plots of cardiac-expressed mRNA regulation (n∼9,500) in microRNA overexpressing hearts, compared to littermate controls. Vertical and horizontal bold lines depict threshold levels for +/- 25% fold-change (horizontal axis) and p-value corresponding to FDR of 0.05 (vertical axis). B. Heat map of normalized expression levels for 1,903 microRNA-regulated mRNAs in miR-143 transgenic and littermate nontransgenic (ntg) mice at 8 weeks of age. C. Heat map depicting unsupervised hierarchical clustering of normalized expression for 1,903 microRNA-regulated mRNAs in miR-378 and miR-499 transgenic mice and littermate controls (8 weeks of age for miR-378 transgenic and nontransgenic; 4 weeks of age for miR-499 transgenic and nontransgenic). Distinct regulatory patters are induced by miR-378 and miR-499. For heat maps, each column depicts mRNA expression data from one mouse heart; blue designates low expression, red designates high expression relative to mean level across all samples for each mRNA.
MicroRNA regulation of cardiac microRNAs
To further assess gene expression control by miR-143, miR-378, and miR-499 we performed a systems analysis of the transcriptional signatures they invoke in mouse hearts. Principal components analysis of transcriptomes from each transgenic line validated the conclusion that miR-378 and miR-499 cardiac mRNA signatures are not only different from controls, but are remarkably distinct from each other (Figure 4a). By contrast, the miR-143 mRNA signature clustered together with the nontransgenic transcriptomes (Figure 4a). Thus, cardiomyocyte miR-143 overexpression does not meaningfully impact overall cardiac gene expression whereas miR-378 and -499 do, but with different consequences.
Figure 4. Transcriptome-wide mRNA and microRNA effects of miR-143, -378, and -499.
A. Principal components analysis of ∼9,500 cardiac-expressed mRNAs in microRNA transgenic (TG) hearts and nontransgenic (ntg) controls. B. Principal components analysis of 300 cardiac-expressed microRNAs in microRNA transgenic (TG) hearts and nontransgenic (ntg) controls. In each data set the overexpressed microRNA was excluded from analysis. C. Unsupervised hierarchical clustering of expression levels for the 300 cardiomiRs in miR-378 and miR-499 transgenic hearts, compared to littermate controls. D. Exploded heat maps depicting unsupervised clustering according to expression levels of microRNAs regulated at genome-wide significance in miR-378 and miR-499 transgenic hearts, compared to littermate controls. All microRNAs shown represent guide (major) strands of the respective microRNAs, with the exception of miRs-214 and -455, for which both guide and passenger strands were regulated and sufficiently abundant for inclusion within the set of 300 cardiomiRs. For C and D, blue indicates downregulation while red indicates upregulation relative to mean level for each microRNA.
MicroRNAs are encoded in the genome either as independent non-protein-coding genes (∼56% of all mouse miRs) or within “miRtrons”, i.e. microRNA-containing introns of protein-coding genes 29. Regardless, microRNA expression is directed by cis elements that bind transcription factors. Therefore, microRNAs that directly or indirectly modulate transcriptional activity have the potential to regulate the expression of genes encoding other microRNAs. Accordingly, both miR-378 and miR-499 should regulate other microRNAs because they have the capacity to modulate pathways that direct cardiac gene expression. By comparison, miR-143 should have minimal effects on other cardiac microRNAs. We tested this notion by deep sequencing cardiac microRNAs in miR-143, miR-378, and miR-499 transgenic mice. Principal components analysis of the cardiac microRNA expression signatures (absent the three overexpressed microRNAs) shows a pattern of clustering that is strikingly similar to the respective mRNA profiles (compare Figure 4b to Figure 4a). Unsupervised hierarchical clustering of 300 cardiac-expressed microRNAs (cardiomiRs) according to expression level revealed individual differences in cardiomiR expression profiles invoked by miR-378 and miR-499 (Figure 4c, Online Table II). The same analysis for miR-143 transgenic mice was indistinguishable from nontransgenic mouse hearts (Online Figure II, Online Table II). Using our prespecified thresholds for genome-wide significance in microRNA regulation (25%-fold change at FDR<0.05), miR-378 regulated 31 cardiomiRs (18 up, 31 down) and miR-499 regulated 17 cardiomiRs (11 up, 6 down), with only miRs-34a, 221and 222 regulated by both (Figure 4d). These findings indicate that microRNA-mediated microRNA regulation in hearts is a specific response.
MicroRNA processing is unaffected by overexpression, but passenger strands are rendered more likely to have biological effects
MicroRNAs are transcribed, processed, and exported from the nucleus as hairpin pre-microRNAs, and then further processed into a duplex encoding two microRNA strands with different sequences. In most instances the mature dominant miR (guide strand) is incorporated into RNA-induced silencing complexes (RISCs) where it targets mRNAs with compatible sequences; the other (passenger) strand is rapidly degraded and consequently is far less abundant. Some microRNA -5p and -3p strands are both inserted into RISCs 30, 31 where they each target their respective complementary mRNAs (reviewed in 32). A recent report described retention of both miR-378 strands, and therefore dual functionality, in heart and other tissues 19.
We determined levels of miR-143-3p, -378-3p, and -499-5p (the microRNA guide strands) and passenger strands in normal 4 week-old and 8 week-old hearts from our deep sequencing data. The 5p/3p ratio of each of these cardiomiRs was constant; the dominant guide strands are expressed at levels ∼ one order of magnitude greater than passenger strands (Figure 5a). An expanded analysis comprising the 300 most abundant cardiomiRs revealed that the pattern of a highly expressed guide strand and a less abundant passenger strand applies across all the cardiomiRs (Online Figure III). Remarkably, 85 of the 300 most abundant cardiomiRs are “passenger strands”, and 12 passenger strands are expressed at levels that place them within the 100 most abundant cardiomiRs (Figure 1d), suggesting contextual functionality for at least some cardiomiR passenger strands.
As with normal microRNA transcription, transgenic overexpression of microRNA precursors produces both guide and passenger microRNAs (see Figure 2a). Thus, transgenic passenger microRNA strands have the potential to be expressed, incorporated within RISCs, and to suppress mRNAs having complementary sequences within their 3′ untranslated regions. We tested whether forced microRNA expression perturbs normal elimination of passenger microRNA strands by quantifying miR-143, -378, and -499 passenger and guide strand levels in our transgenic mouse hearts. The respective minor strand microRNAs were preferentially eliminated, maintaining normal ratios of guide and passenger strand even with overexpression (Figure 5b). Furthermore, the strong correlation of major-to-minor microRNA ratio for microRNAs regulated by miR-378 or miR-499 (Figure 5c) shows that these two cardiomiRs do not significantly perturb normal processing or stability of the cardiomiRs they regulate and support a mechanism that involves regulation of transcription.
Nevertheless, the absolute levels of transgenically overexpressed miR-143, -378 and -499 passenger strands move them to the 102nd, 23rd and 91st most abundant cardiomiRs in their respective transgenic hearts (Figure 5d). While overexpression of the already highly abundant miR-143 and -378 guide strands barely changed their ranking amongst cardiomiRs, the somewhat less abundant miR-499 guide strand moved from the 51st to the 11th most abundant (Figure 5d). The law of mass action dictates that the proportional effect of overexpressed minor microRNAs will be small relative to the respective co-expressed major microRNAs, but transgenic minor microRNAs may nevertheless have measurable effects. We therefore identified passenger strand effects according to sequence complementarity and comparison to biological data (Online Table III).
Higher order consequences of microRNA-mediated cardiomiR regulation
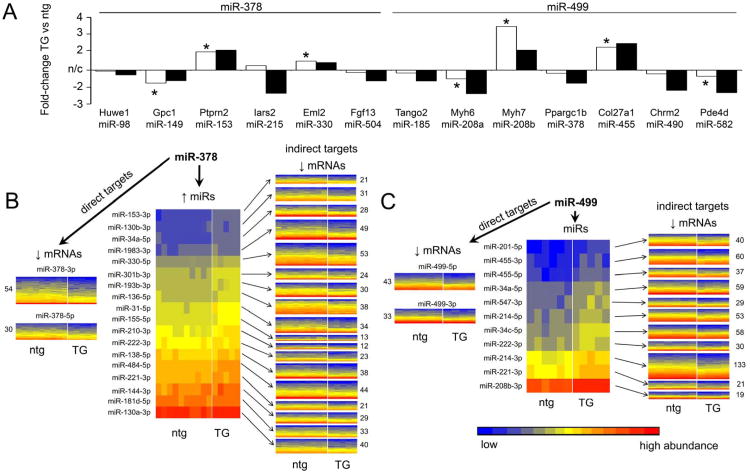
We identified 13 miR-378 or miR-499 regulated microRNAs that are encoded within introns of known parent genes (Figure 6a). Considering that a transcriptional mechanism of microRNA regulation should exert similar modulatory influences on the regulated cardiomiR and its parent mRNA, we compared the relative change induced by miR-378 and miR-499 on parent gene mRNA to that of the respective daughter cardiomiR. In almost every instance the parent mRNA and daughter microRNA are co-regulated (Figure 6a). Since the parent mRNA and daughter microRNA are products of the same transcriptional event, their co-regulation supports transcriptional regulation.
Figure 6. Regulated microRNA-mRNA interactomes in miR-378 and miR-499 hearts.
A. Parent gene and encoded microRNA levels are regulated similarly by microRNAs. Fold-changes of parent gene (white bars) and encoded microRNA (black bars) are shown for miR-378 and miR-499 regulated microRNAs. * indicates significant regulation of parent mRNA at genome-wide significance for multiple comparisons. B. and C. Heat maps of microRNA and mRNA regulation in miR-378 (B) and miR-499 (C) transgenic hearts and secondary consequences of regulated microRNAs on cardiac transcript levels. Left panels: mRNAs downregulated in transgenic hearts by the primary microRNA. Center panels: microRNAs secondarily upregulated in transgenic hearts. Right panels: mRNAs predicted by FastamiRs to be targeted by each secondarily regulated microRNA (details in Online Table III). Colors represent abundances of microRNAs and mRNAs.
To determine the extent to which microRNA-regulated microRNAs contribute to the altered transcriptomic profiles observed in miR-378 and miR-499 transgenic hearts, the FastamiRs algorithm 7 was used at high stringency (see Online Methods) to predict binding between the upregulated microRNAs (including the transgenic microRNAs) and downregulated mRNAs. A total of 468 mRNAs were downregulated in miR-378 transgenic hearts compared to controls (Online Table I); 290 of these are predicted targets of one or more upregulated microRNAs (Figure 6b, left and center). 74 different mRNAs (16% of all downregulated mRNAs) are targeted by miR-378-3p or miR-378-5p, while a further 216 different mRNAs (46%) are targets of the other three upregulated microRNAs (Figure 6b, right; Online Table III). In miR-499 transgenic hearts (Figure 6c), 969 mRNAs were downregulated, with 376 of these being predicted targets of upregulated microRNAs. 76 different mRNAs (7.8%) are targets of miR-499-5p or miR-499-3p, while 298 different mRNAs (31%) are targeted by the other 12 microRNAs (Figure 6c, right; Online Table III). Thus, secondary mRNA regulation by microRNA-regulated microRNAs accounts for ∼75% of the microRNA-dependent transcript downregulation in these transgenic models.
Discussion
Here, we describe two microRNAs that regulate different sets of other cardiac microRNAs. MicroRNA regulation by other microRNAs has been almost completely ignored when studying mechanisms of microRNA effects. Indeed, the one example of microRNA-mediated microRNA regulation within the family of myomiRs seems, since its description in 2007 8, 9, to have been treated as a unique case rather than as an indication of a broader and more general function of microRNAs. Certainly microRNA-mediated microRNA regulation has not previously been assigned mechanistic importance outside of the special case of the myomiR family. The current studies show that, together with direct mRNA suppression 1, 32, indirect regulation of mRNA transcription 7, and regulation of post-translational protein modification 11, microRNA-mediated microRNA regulation can be a major consequence for some microRNAs. These findings provide further insight into how microRNAs with modest direct effects can provoke dramatic end-organ phenotypes 6.
In designing experiments to assess microRNA-mediated microRNA regulation in the in vivo mouse heart, we chose to study three highly expressed and disease-regulated cardiac microRNAs. MicroRNA-143 is one of the most abundant mouse cardiomiRs and is highly expressed in vascular smooth muscle, but virtually nothing is known about its actions in cardiac myocytes. Based on our previous studies examining microRNA expression and regulation in early cardiac hypertrophy, we classified miR-143 as one of the “housekeeping” microRNAs because it is constitutively expressed at high levels, it regulates essential cellular functions (in other tissues), and was not regulated during early cardiac hypertrophy 7. Abnormal miR-143 activity has been linked to several cancers via destabilization of hexokinase 2 25-27. In our studies, forced expression of miR-143 suppressed its direct target hexokinase 2 by ∼30%, but had virtually no effect on other cardiac mRNAs. Consistent with absence of epitranscriptional activity, cardiomyocyte-specific miR-143 overexpression did not regulate the expression of other cardiomiRs. Of course, miR-143 might have epitranscriptional effects on mRNAs and other microRNAs in other cell types 28, 33, 34.
In contrast to miR-143, miR-378 and miR-499 each regulated hundreds of cardiac-expressed mRNAs and a dozen or so cardiac microRNAs. These two stress-modulated cardiomiRs direct biological pathways broadly orchestrating either metabolic remodeling (miR-378; 19) or hypertrophic remodeling (miR-499; 11). Indeed, we included miR-499 as the most likely candidate to prove our hypothesis that microRNAs directing transcriptional signaling would also regulate expression of other microRNAs 11. While both miR-378 and miR-499 were capable of regulating other cardiomiRs, the pattern of their microRNA regulation showed almost no overlap. As illustrated by their respective principal components analyses, the different microRNA expression signatures induced by these two microRNAs mirrored their different transcriptional signatures. For example, two microRNAs that contribute to the molecular signature of doxorubicin toxicity, miR-34c and miR-208b 35, are induced by miR-499 but not by miR-378. As previously reported by the Olson laboratory 9, we detected regulation of miRs-208a and 208b by miR-499, and greatly expanded the number of miR-499-modulated cardiomiRs beyond interactions within this specific microRNA family.
In the absence of evidence for differential processing, and with data showing parallel regulation of microRNA and parent mRNA, our findings reveal microRNA-mediated microRNA regulation to be an epitranscriptional event 7. Based on these findings, we would anticipate that other microRNAs with modulatory effects on cardiac gene expression, such as miR-1 and miR-22 36, also have significant effects mediated via their regulation of other cardiac microRNAs. An important consequence of uncovering microRNA-mediated microRNA regulation is the recognition that RISC-enriched transcripts in microRNA-programmed hearts/tissues/cells represent direct mRNA targets of both the primary (transgenic) microRNA and any secondarily increased microRNAs 7, 11, 13, 37. This does not represent a flaw in the approach of measuring RISC-associated mRNAs to define microRNA targets, but does suggest that concomitant knowledge of microRNA expression signatures is essential to properly interpreting RISC-Seq data. Thus, since the indirect effects of microRNAs include regulation of both mRNA transcription and microRNA transcription, we believe that formal assessment of microRNA-mediated microRNA regulation is essential to understand microRNA effects and phenotypes.
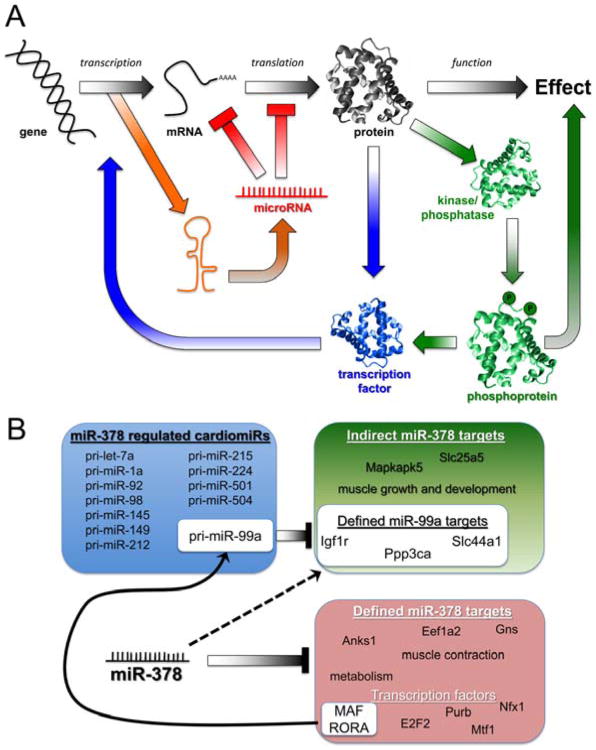
Our observation that some microRNAs regulate other microRNAs adds another layer of complexity to the mechanisms by which these simple molecules direct biological processes. Indeed, some authorities in the field have expressed consternation that the actions of microRNAs are so complex that they can never be fully understood. While correct from a purely reductionist perspective, we would argue that a linear view of molecular cause and effect ignores the obvious non-linearity of microRNA signaling. MicroRNAs directly suppress a few mRNAs within target biological pathways. Positive or negative modulation of those pathways induces secondary effects that reverberate throughout the system, amplifying some responses and inhibiting others. miR-499, for example, directly targets phosphatases that post-translationally regulate transcriptional pathways 11, thereby broadly regulating cardiac gene expression. Indeed, transcriptional pathways seem to be preferentially targeted by cardiomiRs induced during hemodynamic stress 7. Now, we must include microRNA-mediated microRNA regulation as another higher order event that contributes to the ultimate effects of a given microRNA (Figure 7a). Based on the current results, we can apply this schema to real-world problems of how cardiac genes and microRNAs are epitranscriptionally regulated by miR-378 and miR-499: We bioinformatically identified putative transcription factor binding sites in the promoters of microRNA-regulated microRNAs and parent genes (see Figure 4d, Online Tables IV and V) using MAPPER 38, and delineated trans-activating factors targeted by the respective microRNAs. Thus, miR-99a appears to be indirectly downregulated by miR-378 via its direct targeting of MAF and RORA. In turn, miR-99a directly targets 31 cardiac-expressed mRNAs (according to TargetScan predictions), accounting for their indirect regulation by miR-378 (Figure 7b). Further examples are in Online Table V. Given that the majority of microRNA effects are not the direct result of mRNA suppression, we suggest that terms such as “off-target” and “non-specific” be abandoned in favor of “indirect” or “higher-order” to reflect our observations that these effects are normal and real, but simply not predicted by available analytics.
Figure 7. A. Schematic depiction of the complexity and inter-connectivity of microRNA-mediated effects.
Traditional linear paradigm is in black and white; mechanisms of microRNA modulation are color-coded according to pathway. B. Example of miR-378 regulation of microRNA expression via transcription factor suppression. miR-378 is predicted to suppress several transcription factors (Online Table IV); the MAF and RORA families are predicted to activate transcription of the miR-99a pri-miR (Online Table V). Procedures for the definition of miR-99a and miR-378a targets are given in Detailed Methods.
The complexity and non-linearity of microRNA effects does not preclude comprehension of their actions. Rather, like a Mandelbrot set in fractal geometry where visual complexity results from simple mathematical rules 39, understanding simply requires a different approach than standard reductionism. Biological systems are sufficiently complex that they cannot be adequately described in terms of individual functioning units. But if the rules that govern the behavior of these units are understood, then the system-wide responses are predictable. It is the complexity of microRNA actions that makes them therapeutically attractive, because parallel effects on multiple targets bypass the usual resiliency of biological systems to interventions that target a single factor. In this context, microRNA-mediated microRNA regulation is one of the consequences of microRNA-directed therapeutics to consider as they move toward clinical applications.
Supplementary Material
Novelty and Significance.
What Is Known?
MicroRNAs capture their direct mRNA targets via complementary nucleotide sequences and recruit them into RNA-induced silencing complexes (RISCs) to be rendered translationally incompetent.
-
The overall effect of some microRNAs on the mRNA signature is driven by regulation of “indirect” mRNA targets that are not directly bound, rather than direct target suppression.
The mechanisms for indirect mRNA regulation by microRNAs are unclear, but are thought to contribute substantially to observed phenotypes.
What New Information Does This Article Contribute?
In mouse hearts two microRNAs with numerous indirect mRNA targets, but not a microRNA with little indirect target activity, regulated expression of numerous other cardiac myocyte microRNAs.
MicroRNA-mediated microRNA regulation greatly amplified microRNA-mediated signal redirection.
For miR-378 and miR-499, approximately 30-50% of indirect cardiac mRNA target regulation is attributable to higher order effects from secondarily regulated microRNAs.
MicroRNAs directly target specific mRNAs, but indirect “epitranscriptional” regulation of non-targeted mRNAs is a hallmark of microRNA signaling for which a mechanistic basis is unclear. We examined whether some microRNAs are indirectly regulated by other microRNAs via regulated transcription of microRNA and host genes in which microRNAs are intronically encoded. Using transgenic mice overexpressing one of three different microRNAs in the heart (miR-143, miR-378 or miR-499), coupled with transcriptome-wide deep sequencing of microRNAs and mRNAs, we observed that other microRNAs did undergo alterations in their abundance levels in a manner specific to the programming microRNA. While miR-143 overexpression did not regulate any cardiac microRNAs, miR-378 and miR-499 each altered the levels of hundreds of mRNAs and 15-30 cardiac microRNAs. Secondary regulation of microRNAs by miR-378 and miR-499 contributed substantially to the total number of modulated mRNAs. These findings indicate that microRNA-mediated microRNA regulation should be considered when evaluating microRNA-mediated signaling mechanisms and phenotypic outcomes.
Acknowledgments
Sources Of Funding: Funded by NIH R01 HL108943 and by a Fondation Leducq Transatlantic Network of Excellence in Cardiovascular Research Program.
Nonstandard Abbreviations
- antagomiR / antimiR
synthetic molecule designed to inhibit microRNA function
- cardiomiR
cardiac-expressed microRNA
- FDR
false discovery rate
- miRtron
microRNA-containing intron of protein-coding gens
- myomiR
microRNA originating from myosin heavy chain gene
- RISC
RNA-induced silencing complex
- RISC-Seq
deep sequencing of mRNAs present in the RISC
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dorn GW., 2nd MicroRNAs: Redefining mechanisms in cardiac disease. J Cardiovasc Pharmacol. 2010;56:589–595. doi: 10.1097/FJC.0b013e3181f8d173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon C, Han Z, Olson EN, Srivastava D. MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling. Proc Natl Acad Sci USA. 2005;102:18986–18991. doi: 10.1073/pnas.0509535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Ransom JF, Li A, Vedantham V, von DM, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladka MM, da Costa Martins PA, De Windt LJ. Small changes can make a big difference - microRNA regulation of cardiac hypertrophy. J Mol Cell Cardiol. 2012;52:74–82. doi: 10.1016/j.yjmcc.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Matkovich SJ, Hecker PA, Zhang Y, Edwards JR, Dorn GW., 2nd Epitranscriptional orchestration of genetic reprogramming is an emergent property of stress-regulated cardiac microRNAs. Proc Natl Acad Sci USA. 2012;109:19864–19869. doi: 10.1073/pnas.1214996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 9.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zisoulis DG, Kai ZS, Chang RK, Pasquinelli AE. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature. 2012;486:541–544. doi: 10.1038/nature11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matkovich SJ, Hu Y, Eschenbacher WH, Dorn LE, Dorn GW., 2nd Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ Res. 2012;111:521–531. doi: 10.1161/CIRCRESAHA.112.265736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA, Margulies KB, Dorn GW., 2nd Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009;119:1263–1271. doi: 10.1161/CIRCULATIONAHA.108.813576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn GW, 2nd, Matkovich SJ, Eschenbacher WH, Zhang Y. A human 3′ miR-499 mutation alters cardiac mRNA targeting and function. Circ Res. 2012;110:958–967. doi: 10.1161/CIRCRESAHA.111.260752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matkovich SJ, Zhang Y, Van Booven DJ, Dorn GW., 2nd Deep mRNA sequencing for in vivo functional analysis of cardiac transcriptional regulators: application to Gaq. Circ Res. 2010;106:1459–1467. doi: 10.1161/CIRCRESAHA.110.217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condorelli G, Latronico MV, Dorn GW., 2nd microRNAs in heart disease: putative novel therapeutic targets? Eur Heart J. 2010;31:649–658. doi: 10.1093/eurheartj/ehp573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 19.Carrer M, Liu N, Grueter CE, Williams AH, Frisard MI, Hulver MW, Bassel-Duby R, Olson EN. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc Natl Acad Sci USA. 2012;109:15330–15335. doi: 10.1073/pnas.1207605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deacon DC, Nevis KR, Cashman TJ, Zhou Y, Zhao L, Washko D, Guner-Ataman B, Burns CG, Burns CE. The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development. 2010;137:1887–1896. doi: 10.1242/dev.050526. [DOI] [PubMed] [Google Scholar]
- 22.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW, 2nd, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S, Zhang LF, Zhang HW, Hu S, Lu MH, Liang S, Li B, Li Y, Li D, Wang ED, Liu MF. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012;31:1985–1998. doi: 10.1038/emboj.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregersen LH, Jacobsen A, Frankel LB, Wen J, Krogh A, Lund AH. MicroRNA-143 down-regulates Hexokinase 2 in colon cancer cells. BMC Cancer. 2012;12:232. doi: 10.1186/1471-2407-12-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peschiaroli A, Formosa A, Markert EK, Bongiorno-Borbone L, Levine AJ, Candi E, D'Alessandro A, Zolla L, Finazzi Agro A, Melino G. miR-143 regulates hexokinase 2 expression in cancer cells. Oncogene. 2012 doi: 10.1038/onc.2012.100. [DOI] [PubMed] [Google Scholar]
- 28.Takaoka Y, Shimizu Y, Hasegawa H, Ouchi Y, Qiao S, Nagahara M, Ichihara M, Lee JD, Adachi K, Hamaguchi M, Iwamoto T. Forced expression of miR-143 represses ERK5/c-Myc and p68/p72 signaling in concert with miR-145 in gut tumors of Apc(Min) mice. PLoS One. 2012;7:e42137. doi: 10.1371/journal.pone.0042137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutter D, Marr C, Krumsiek J, Lang EW, Theis FJ. Intronic microRNAs support their host genes by mediating synergistic and antagonistic regulatory effects. BMC Genomics. 2010;11:224. doi: 10.1186/1471-2164-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 31.Yang JS, Phillips MD, Betel D, Mu P, Ventura A, Siepel AC, Chen KC, Lai EC. Widespread regulatory activity of vertebrate microRNA* species. RNA. 2011;17:312–326. doi: 10.1261/rna.2537911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorn GW., 2nd Decoding the cardiac message: the 2011 Thomas W. Smith Memorial Lecture. Circ Res. 2012;110:755–763. doi: 10.1161/CIRCRESAHA.111.256768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai VK, Ashraf M, Jiang S, Haider K. MicroRNA-143 is a critical regulator of cell cycle activity in stem cells with co-overexpression of Akt and angiopoietin-1 via transcriptional regulation of Erk5/cyclin D1 signaling. Cell Cycle. 2012;11:767–777. doi: 10.4161/cc.11.4.19211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguchi S, Yasui Y, Iwasaki J, Kumazaki M, Yamada N, Naito S, Akao Y. Replacement treatment with microRNA-143 and -145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Vacchi-Suzzi C, Bauer Y, Berridge BR, Bongiovanni S, Gerrish K, Hamadeh HK, Letzkus M, Lyon J, Moggs J, Paules RS, Pognan F, Staedtler F, Vidgeon-Hart MP, Grenet O, Couttet P. Perturbation of microRNAs in rat heart during chronic doxorubicin treatment. PLoS One. 2012;7:e40395. doi: 10.1371/journal.pone.0040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorn GW., 2nd Resolving a catch-22 in cardiac gene regulation. Circulation. 2012;125:2695–2697. doi: 10.1161/CIRCULATIONAHA.112.110767. [DOI] [PubMed] [Google Scholar]
- 37.Matkovich SJ, Van Booven DJ, Eschenbacher WH, Dorn GW., 2nd RISC RNA sequencing for context-specific identification of in vivo microRNA targets. Circ Res. 2011;108:18–26. doi: 10.1161/CIRCRESAHA.110.233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marinescu VD, Kohane IS, Riva A. The MAPPER database: a multi-genome catalog of putative transcription factor binding sites. Nucleic Acids Res. 2005;33:D91–97. doi: 10.1093/nar/gki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandelbrot B. Fractals: Form, Chance and Dimension. W.H.Freeman & Company; 1977. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.