Abstract
Niacin (nicotinic acid) has been used for decades as a lipid-lowering drug. The clinical use of niacin to treat dyslipidemic conditions is limited by its side effects. Niacin, along with fibrates, are the only approved drugs which elevate high density lipoprotein cholesterol (HDLc) along with its effects on low density lipoprotein cholesterol (LDLc) and triglycerides. Whether niacin has a beneficial role in lowering cardiovascular risk on the background of well-controlled LDLc has not been established. In fact, it remains unclear whether niacin, either in the setting of well-controlled LDLc or in combination with other lipid-lowering agents, confers any therapeutic benefit and if so, by which mechanism. The results of recent trials reject the hypothesis that simply raising HDLc is cardioprotective. However, in the case of the clinical trials, structural limitations of trial design complicate their interpretation. This is also true of the most recent Heart Protection Study 2-Treatment of HDLc to Reduce the Incidence of Vascular Events (HPS2-THRIVE) trial in which niacin is combined with an antagonist of the D prostanoid (DP) receptor. Human genetic studies have also questioned the relationship between cardiovascular benefit and HDLc. It remains to be determined whether niacin may have clinical utility in particular subgroups, such as statin intolerant patients with hypercholesterolemia or those who cannot achieve a sufficient reduction in LDLc. It also is unclear whether a potentially beneficial effect of niacin is confounded by DP antagonism in HPS2-THRIVE.
Keywords: cholesterol, prostaglandin, cardiovascular
Niacin is also known as vitamin B3. Chronic dietary deficiency of niacin can cause pellagra (1). It is a precursor to NAD+/NADH and NADP+/NADPH, both of which play essential metabolic roles in living cells (2). Niacin has been used for more than half a century in the treatment of dyslipidemia. When Altschul, Hoffer, and Stephen (3) discovered that niacin could lower plasma levels of cholesterol, it was the only approved drug with such an effect (1). It has proven to be an attractive option for those intolerant of statin treatment. It has been demonstrated to reduce risk of atherosclerotic cardiovascular disease (4). The clinical use of niacin has been markedly limited by cutaneous flushing. The molecular target of niacin responsible for lipid metabolic changes and flushing was unknown until 2003 (5–7), when the niacin receptor, namely HM74A in humans and PUMA-G in mice, was discovered. The niacin receptor, also known as GPR109A, was shown to be activated by niacin and to mediate its antilipolytic effect as well as the flushing effect (8–10). Interest in niacin has risen because there is still significant residual risk in patients with intensive statin therapy (11). However, recent outcome trials (11, 12) failed to show niacin's benefit on top of statin therapy in lipid-targeted approaches to reduce major cardiovascular events in high-risk patients. In this review we will summarize the pharmacology of niacin, with a particular emphasis on recent outcome trials, including discussion of their limitations.
NIACIN'S LIPID EFFECTS
Niacin has long been shown to have effects on lipids (13). Niacin increases high density lipoprotein cholesterol (HDLc), but reduces low density lipoprotein cholesterol (LDLc), VLDL cholesterol, and triglycerides (TGs) (14). Niacin also decreases the plasma concentration of lipoprotein a [Lp(a)], which has been suggested to play an independent role in the pathogenesis of coronary heart disease (15, 16).
Effect on LDL and VLDL
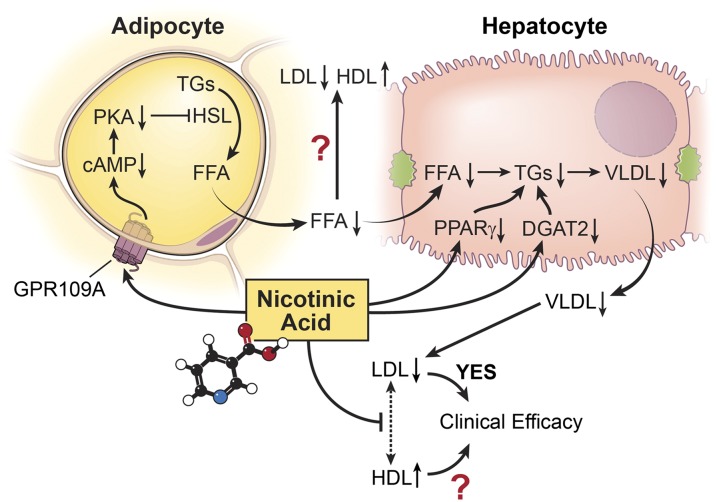
Niacin's lipid effects are poorly understood. It was believed that niacin acts as an inhibitor of FFA mobilization from adipocytes (17–19). Carlson and Oro (17) found that niacin lowered the arterial plasma concentration of FFAs in fasting humans within minutes. This effect is dose dependent (20) and is not desensitized after 6 months of regular treatment (21), in contrast with niacin's flushing effect which undergoes tachyphylaxis within a week (22). This suppression of plasma FFAs reduced the supply of substrate for the hepatic synthesis of TGs, VLDL and LDL particles (23). However, the suppression of FFAs is niacin receptor dependent but the lipid effect is not, which is inconsistent with the FFA hypothesis (24). Niacin can also inhibit diacylglycerol acyltransferase 2 in liver, accelerating the intracellular degradation of apoprotein B, which results in decreased hepatic VLDL and TG synthesis (25, 26) (Fig. 1).
Fig. 1.
FFA hypothesis of niacin's lipid effects. Niacin acts on its receptor GPR109A on adipocytes, lowering cAMP and protein kinase A levels. PKA phosphorylates hormone sensitive lipase (HSL). Therefore, niacin decreases HSL and TG hydrolysis in adipocytes, which causes a suppressed level of plasma FFAs. This suppression of plasma FFAs reduces the supply of substrate for the hepatic synthesis of TGs and VLDL and LDL particles. Niacin can also inhibit diacylglycerol acyltransferase 2 (DGAT2) and reduce hepatic expression of the PPARγ in liver, which results in decreased hepatic VLDL and TG synthesis. Suppressed levels of VLDLs and LDLs reduce the exchange of cholesterol esters and TGs between LDLs and HDLs, resulting in an increase in plasma HDLc levels. Suppression of FFAs is niacin receptor dependent, but the lipid effect is not, which casts doubt on this FFA hypothesis.
Effects on HDL
Several mechanisms by which niacin may increase HDLc have been proposed. The most popular one is based on the FFA hypothesis. Decreased FFA mobilization from adipocytes directly results in decreased TG concentrations in VLDLs and LDLs (27). Generally, there is a strong negative correlation between plasma TG levels and the concentration of HDLc, related to the cholesteryl ester exchange between VLDLs and HDLs mediated by cholesteryl ester transfer protein (CETP). Inhibition of FFA mobilization from adipocytes depletes TGs in VLDLs and LDLs, which, in turn, reduces the exchange of cholesterol esters and TGs, resulting in an increase in plasma HDLc levels (Fig. 1). This suggests that niacin acts like a CETP inhibitor. Interestingly, both CETP inhibitors and niacin cause an elevation of the HDL2 fraction (28). However, CETP inhibition and niacin have different effects on the ability of HDLs to promote net cholesterol efflux. Niacin treatment caused a moderate increase in the ability of HDLs to promote net cholesterol efflux, whereas inhibition of CETP, at least with anacetrapib, led to a more dramatic increase in association with enhanced particle functionality at higher HDLc concentrations (29). A recent study of the niacin receptor also casts doubt on this once popular hypothesis (24).
Another mechanism proposed is that niacin reduces hepatic expression of the peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1β and apolipoprotein C-III (apoC-III) (30), which results in accelerated clearance of triglyceride-rich lipoproteins (31). This, in turn, would elevate HDLc as less cholesterol would be transferred via CETP. This model is supported by the observation that niacin-induced augmentation of HDLc in animal models requires the presence of CETP (32). It has also been found that niacin selectively increases apoA-I-containing HDL particles through inhibition of their uptake and catabolism by hepatocytes (25, 33, 34). However, these effects were observed at niacin concentrations far higher than those attained during chronic treatment with niacin (35).
Effects on Lp(a)
Genetic and epidemiologic studies indicate that high Lp(a) is an independent risk factor for myocardial infarction and stroke (36–40). Statins and other lipid-lowering medications do not affect elevated plasma concentrations of Lp(a) (41). By contrast, treatment with 4 g of niacin for 5–7 weeks reduces plasma concentrations of Lp(a) by nearly 40% (15). While no outcome studies have addressed this possibility, it is not clear whether niacin as an intervention would result in a favorable clinical outcome in patients with high Lp(a) levels.
Niacin receptor
Several pharmaceutical companies began developing selective agonists of GPR109A (42–47). Walters et al. (48) suggested that the effects of niacin on FFAs and flushing could be segregated. Thus, β-arrestin1-null mice displayed reduced cutaneous flushing in response to niacin, although the improvement in serum FFA levels was retained and was similar to that observed in wild-type mice. A partial agonist was developed in an effect to mimic niacin's lipid efficacy bypassing its flushing side effect (49).
However, the effects of niacin on HDLc seem unrelated to either GPR109A or FFA suppression (24). In CETP transgenic mice, niacin increases HDLc and decreases LDLc and TGs. This effect is not abolished by deletion of GPR109A. In humans, two GPR109A agonists lower FFAs acutely; however, neither had the expected effects on serum lipids (24, 49). In addition, chronic FFA suppression via GPR109A agonism is not attained either by niacin or other agonists.
Is simply raising HDLc level cardioprotective?
Recent outcome trials (11, 12, 50) and a study of human genetics (51) have questioned the HDL hypothesis (52). Epidemiological data reveal a strong inverse relationship between cardiovascular risk and HDLc (52). However, given the many potential confounding factors, such as exercise, which might elevate HDLc and be causative of this relationship by other mechanisms, a beneficial effect from pharmacological manipulation of HDLc could never be assumed from such evidence. Perhaps the strongest argument against this theory is the study of Voight et al. (51) in which polymorphisms related to elevations of plasma LDLc were associated directly with the risk of myocardial infarction, whereas an inverse relationship with variants related to elevations of plasma HDLc was not apparent. Multiple lipid-independent effects of niacin have been proposed. There is growing evidence that niacin restrains inflammation, independent of its effects on HDLc (53–56). Niacin retards atherogenesis in hyperlipidemic mice in the absence of significant changes in lipids. This effect may in part reflect a GPR109A-mediated restraint of immune cell infiltration of the vessel wall (54). Niacin may influence the inflammatory process by a variety of mechanisms including the expression of cell adhesion molecules (57), nuclear and scavenger receptors (58, 59), and the release of adipokines (60, 61) and bioactive lipids (62, 63).
However, there are abundant data supporting the possibility that HDL function may be the more relevant issue to cardioprotection than the HDLc levels per se (64, 65). Several experiments on genetically manipulated mouse models are consistent with the suggestion that apolipoprotein A-I (apoA-I) promoting reverse cholesterol transport from macrophages in vivo with resultant attenuation of atherogenesis (66–68). Furthermore, infusion of apoA-I in animal models and also in humans has been reported to reduce lesion burden (69–71). However, despite their promise, these results have yet to be confirmed by the results of clinical outcome trials.
SIDE EFFECTS
Flushing
Niacin-induced flushing is characterized by redness and warmth due to vasodilation of dermal blood vessels, and is associated with a sensation of tingling and burning. It lasts typically for an hour. Most patients (∼70%) receiving niacin experience flushing and about 20% of niacin-treated subjects drop out of clinical trials for this reason (14, 72). Morrow and colleagues first noted that prostaglandin D2 (PGD2) played a central role in the flushing that constrains the use of niacin (73, 74). In mice, niacin-induced flushing results from an early phase of COX-1-dependent formation of PGD2 and prostaglandin E2 (PGE2) in Langerhans cells, followed by delayed COX-2-dependent production of PGE2 by keratinocytes (10). However, niacin also evokes COX-1-derived PGD2 release from platelets in both mice and humans (61). It seems likely that niacin-induced flushing is mediated by prostaglandins produced initially by bone marrow-derived cells such as platelets and dendritic cells (Langerhans), perhaps contributing to subsequent induction of COX-2-dependent lipids by dermal or epidermal cells. During chronic drug administration, niacin-induced flushing and prostaglandin release are subject to tachyphylaxis within a week (22, 75).
Reduction of niacin-induced flushing by pretreatment with aspirin is well established (76). However, despite complete suppression of niacin-evoked PGD2 release by low doses of aspirin (81 mg/day), there are still significant residual symptoms of flushing, even after pretreatment with higher doses (325 and 650 mg) (62, 77, 78) of aspirin which would suppress residual prostaglandin formation. A recent study has shown that aspirin only blocks vasodilation in hairy but not in glabrous skin (79). These findings raised the question of whether niacin-induced flushing is mediated solely by prostaglandins.
PGD2 mediates niacin-evoked flushing via its D prostanoid (DP)1 receptor. Antagonism of DP1 suppresses niacin-induced vasodilation significantly in mice and humans (80). For example, laropiprant (LRPT) (74), a DP1 antagonist, reduces flushing induced by niacin (81–84) without affecting niacin's effects on lipids. This combination was developed as Cordaptive® in the USA and Tredaptive® in Europe. The combination was approved in Europe in 2008, while the US Food and Drug Administration requested more safety data before approval of the drug combination in the USA (85–90). Interestingly, despite tachyphylaxis of niacin-induced PGD2 within a week (75), LRPT's anti-flushing effect is reportedly sustained for months (82, 83). These observations are confusing. First, prostaglandins other than PGD2 may contribute to flushing, as has been shown in mice (10). Such compounds might resist the rapid tachyphylaxis observed with PGD2. However, if this is the case, why is the efficacy of LRPT sustained? It has been reported to block, at concentrations attained clinically, T prostanoid (TP) receptors, albeit at higher concentrations than DP1, but not the I or E prostanoid receptors, more likely relevant to vasodilation. Thus, the combination might disrupt prostanoid-independent mechanisms of niacin-induced flushing. One approach to addressing this possibility would be to compare the relative efficacy of prostanoid suppression with higher doses of aspirin with that of LRPT in restraining niacin-induced flushing.
Diabetes
Niacin has long been known to reduce insulin sensitivity (12, 91). The recent Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) trial (see below) also found that in the niacin/LRPT group, diabetic complications (typically hyperglycemia) were about twice as common as in the control group as a reason for dropping out of the trial (0.9% vs. 0.4%) (12). A proposed mechanism is the rebound increase in FFA levels following the transient FFA suppression induced by niacin (92), although this has been disputed (17).
Despite niacin's impact on insulin sensitivity, the cardiovascular benefits from niacin were similar in patients with or without impaired glucose tolerance in the Coronary Drug Project (CDP) (93). Statins present a similar case. Despite their cardioprotective effects, also evident in diabetic patients, statins have been associated with an increased risk of diabetes (94, 95). Statins and niacin may be interacting with other risk factors to reveal a predisposition to diabetes (96).
Gastrointestinal
Gastritis-like symptoms such as heartburn, indigestion, nausea, diarrhea, or stomach pain can occur after niacin administration. This has been widely observed in experimental and outcome studies (11, 97). The mechanism for these gastrointestinal symptoms is not clear. Niacin can also cause hepatotoxicity (98, 99), which may be more common when the drug is administered in a sustained-release form (99, 100). It has been suggested that this effect may be mediated by niacin's metabolites (101).
Gout
Niacin can also occasionally increase plasma uric acid levels and induce gout (102). This effect may be due to the inhibition effect of niacin on uricase, an oxidizing enzyme of uric acid (103), or due to a decrease in uric acid excretion (104).
RANDOMIZED CONTROLLED TRIALS
The CDP
The CDP (105) was the first clinical trial to show the cardiovascular benefits of any lipid-modifying agent. It was conducted between 1966 and 1975 to assess the long-term efficacy and safety of five lipid-influencing drugs in 8,341 men aged 30 to 64 years with electrocardiogram-documented previous myocardial infarction. Patients were randomized to placebo or clofibrate, dextrothyroxine, two doses of oral estrogen, or to niacin (3,000 mg/day). The two estrogen regimens and dextrothyroxine were discontinued early because of adverse effects. Hence, only the effects of clofibrate and niacin were examined. Clofibrate yielded no evidence of efficacy. Niacin treatment showed modest benefit in decreasing definite nonfatal recurrent myocardial infarction (10.2 vs. 13.8%), but no decrease in total mortality. However, a follow-up study revealed that 9 years after termination of the CDP study, patients originally randomized to niacin had a mortality rate 11% lower than those originally randomized to placebo. Presumably, patients stopped taking the drug after an average of 6.2 years in the trial (106). The cumulative motility rate curves didn't start to diverge until ∼65 months post treatment, raising the possibility that drug-related impact on mortality might be delayed. However, as this comparison no longer fell within the structure of a randomized trial, it did no more than frame a hypothesis.
Surrogate endpoint trials
There have been a few trials assessing niacin efficacy utilizing surrogate endpoints. All are small and some of them were not designed to study niacin efficacy. A couple of them also had prespecified clinical endpoints, although probably none of them were suitably controlled or powered (107–113).
The open-labeled Stockholm trial followed the CDP trial. Patients with prior myocardial infarction were randomized to receive either placebo (N = 276) or the combination of clofibrate and nicotinic acid (N = 279) (107). The concentrations of both serum cholesterol and TGs were lowered by 13 and 19%, on average, respectively, in the treatment versus control groups. The combination resulted in an average 26% reduction in mortality, but with greater effect in those patients with hypertriglyceridemia. However, aside from its open-label design, this trial was far underpowered to assess mortality.
In the Familial Atherosclerosis Treatment Study (FATS) (108), male patients (N = 146) with angiographic evidence of coronary artery disease and elevated apoB levels were treated with either lovastatin-colestipol, niacin-colestipol, or placebo. Lovastatin-colestipol reduced the frequency of the progression of coronary lesions, increased the frequency of regression, and reduced the incidence of cardiovascular events. It was based on a methodology utilizing angio-catheterization. Experienced observers blindly assessed the change in the severity of coronary disease both visually and quantitatively. Again, this was underpowered to assess clinical outcomes.
In the HDL Atherosclerosis Treatment Study (HATS) (109), patients (N = 160) with prior coronary events and low HDL (mean 32 mg/dl) and LDL (mean 130 mg/dl) were randomized to treatment with simvastatin-niacin or placebo, with or without antioxidants. Simvastatin-niacin decreased LDLc by 42% and increased HDLc by 26% on average, compared with placebo. Angiographically, the average coronary stenosis progressed by 0.4% in the simvastatin-niacin group versus 3.9% in the placebo group. In addition, there were fewer clinical events in the simvastatin-niacin group than in the placebo group (9 vs. 1).
The Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2 study (110) tested first the incremental impact of adding niacin to background statin therapy. Patients (N = 167) with known coronary heart disease and low HDLc (<45) were treated with niacin (1,000 mg) or placebo on top of a statin. Niacin significantly reduced the rate of progression of carotid intima-media thickness (IMT), although only in subjects without insulin resistance. A follow-up open-labeled observation found regression of IMT in the niacin group (111).
The Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis (ARBITER 6-HALTS) trial was designed to compare the effect of niacin and ezetimibe on the progression of IMT, on the background of statin therapy (112). Patients who had coronary heart disease or a coronary heart disease risk equivalent with low LDL (<100 mg/dl) and moderate HDL (HDL <50 mg/dl in male and <55 mg/dl in female) were randomized to niacin or ezetimibe treatment. While niacin and ezetimibe had divergent effects on HDLc, a greater reduction in LDLc was attained with ezetimibe. Despite this, progression of atherosclerosis, as reflected by the IMT, was only observed in the patients receiving niacin, leading to premature termination of this study.
The Oxford Niaspan study treated patients (N = 71) with coronary heart disease or risk equivalent with low LDL (∼85 mg/dl) and low HDL (∼38 mg/dl) with either niacin (2,000 mg) or placebo, on top of statin (113). At 12 months, regression was observed in the niacin group versus progression in the placebo group.
Aside from the variable fidelity of the surrogate endpoints in predicting clinical outcomes (114), these studies suffer from a series of limitations ranging from open-label design to being seriously underpowered to assess clinical outcomes which nonetheless are both reported and interpreted.
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes trial
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial was designed to compare niacin versus placebo in patients with established atherosclerotic disease on statin therapy (11). The primary endpoint was the composite of the first event of death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization (for >23 h) for an acute coronary syndrome, or symptom-driven coronary or cerebral revascularization.
Patients with established cardiovascular disease with low LDLc (mean 71 mg/dl) and low HDLc (34 mg/dl) entered an open-label niacin run-in phase. Patients in whom a dose of at least 1,500 mg of niacin per day was associated with a tolerable side-effect profile were randomly assigned to niacin or matching placebo. Patients in the active treatment group received niacin at a dose of 1,500 to 2,000 mg per day plus simvastatin. Patients in the placebo group received simvastatin plus a small dose (50 mg) of immediate-release niacin to induce flushing and to facilitate blinding of patients and observers to drug induced flushing. In both groups, the dose of simvastatin could be adjusted and subjects could receive ezetimibe to achieve and maintain the LDLc within a prespecified target range.
AIM-HIGH was ended after a mean follow-up of 3 years, when an interim analysis suggested the futility of the comparison. There was no incremental benefit of niacin in reducing cardiovascular events, despite significant increases in HDLc and decreases in TG levels in the niacin-treated group.
The outcome and design of the AIM-HIGH trial prompted considerable controversy. The design of the trial allowed liberal use of hypolipidemic agents other than niacin in both groups and, as a consequence, there was unbalanced use of both statins (25% on 80 mg statin in the placebo group vs. 18% in the niacin group) and ezetimibe (22% in the placebo group vs. 10% in the niacin group).
This study was attempting to answer the question of whether once LDLc is reduced, is there an incremental benefit from raising HDLc? However, the absolute difference in HDLc achieved by niacin was only 4 mg/dl (42 vs. 38 mg/dl). This was largely because of a paradoxical increase in HDLc in the placebo group, which might be a result of higher statin doses or of the small doses of immediate-release niacin.
Besides achieving such a modest change in HDLc, the follow up time of 3 years post randomization might have been too short to see a benefit in mortality. For example, the ultimate mortality benefit from statins was not apparent in the 4S (Scandinavian Simvastatin Survival Study) study after 3 years of dosing (115), nor was the divergence in mortality rates in the posthoc analysis of the CDP . For these reasons, the apparently null effect afforded by the results of AIM HIGH left many questions unanswered.
HPS2-THRIVE
The HPS2-THRIVE trial hoped to answer these outstanding questions. It was much larger than the antecedent clinical trials (25,673 patients) and suitably powered to detect a reasonable expectation of benefit. However, this was a trial to assess the usefulness of a drug combination, extended-release niacin (ERN) (2 g) and the DP1 antagonist, LRPT (40 mg), in the reduction of major vascular events (composite of nonfatal myocardial infarction, coronary heart disease death, stroke, or arterial revascularization) (12). The study was not designed to answer the questions of how either drug alone acted or how they compared with the combination.
Prior to randomization, patients diagnosed with occlusive arterial disease received standardized open-labeled LDLc lowering therapy with simvastatin 40 mg (± ezetimibe) daily (to a total cholesterol target of 135 mg/dl). The ability to remain compliant with ERN/LRPT for about 1 month was then assessed in 38,369 patients. About one-third were excluded (mainly due to combination drug-related side effects). A total of 25,673 patients were randomized between ERN/LRPT daily versus placebo on a background of 38,369 and were followed for a median of 3.9 years.
Average baseline lipids were LDLc (63 mg/dl), HDLc (44 mg/dl), and TGs (125 mg/dl). ERN/LRPT treatment reduced LDLc by an average 10 md/dl, and increased HDLc by 6 mg/dl. The primary endpoint was no different between the two groups (relative risk 0.96, [confidence interval 0.90–1.03]) (116).
After 3.9 years of follow-up, 25.4% of the participants allocated active ERN/LRPT had stopped their treatment compared with 16.6% of those on placebo. The most common medical reasons for stopping ERN/LRPT were previously recognized side effects of niacin, such as flushing, gastrointestinal complaints, and the onset of diabetes. Other reasons for stopping the drug combination had not previously been ascribed to niacin. These included myopathy, infection, and bleeding (12).
Myopathy.
Myopathy is a common side effect of statins (117). It had never been recognized as a common side effect of niacin. However, in the HPS2-THRIVE study, the risk of myopathy was increased four times (0.16%/year vs. 0.04%/year) by adding ERN/LRPT to simvastatin (40 mg daily) (12), particularly in Chinese patients which comprised 42.6% of subjects, and whose myopathy rates on simvastatin are also higher than in Europeans (0.13% vs. 0.04%) (12).
Infection.
Similar to myopathy, infection had never been recognized as a side effect of niacin. However, in the HPS2-THRIVE study, treatment with niacin resulted in a 1.4% (8.0% in treatment group vs. 6.6% in placebo group) higher risk of infection (116). PGD2, acting through DP1, has been known to have potent anti-inflammatory effects, especially relating to resolution of acute inflammatory processes (118, 119). Whether LRPT contributed to the excess infection in the HPS2-THRIVE study is unclear.
Bleeding.
In the HPS2-THRIVE study, treatment with niacin/LRPT resulted in 0.7% (2.54% in treatment group vs. 1.85% in placebo group) higher risk of bleeding (116), including an increased risk of hemorrhagic stroke. It is unclear whether this relates to niacin, LRPT, or the combination. Niacin decreases the synthesis of plasminogen activator inhibitor 1 and potentiates fibrinolysis (120). Another study found that niacin reduces fibrinogen and a prothrombin fragment (121). However, higher bleeding events with niacin were not observed in previous large trials like CDP and AIM-HIGH. LRPT antagonizes the TP receptor at concentrations higher than those that antagonize DP1. Deletion of TP in mice predisposes them to bleeding; however, it is unknown whether TP antagonism was asymmetrically attained due to variability in kinetics and consequent drug exposure in patients receiving niacin/LRPT who suffered bleeding episodes.
HPS2-THRIVE was a much larger trial than its antecedents. Besides its effects on HDLc and LDLc, the combination reduced Lp(a) and TGs and reduced average systolic blood pressure by 2 mmHg. These effects might be expected to result in differences that could translate into an ∼17% reduction in vascular events by ERN/LRPT, based on previous observational studies and randomized trials (12, 122). On the other hand, such assumptions are based on trials in which patients had higher baseline levels of LDLc than in HPS2-THRIVE, so the room for an LDLc-based improvement in outcomes would be expected to be diminished.
Recently (62), we found that PGD2, like prostaglandin I2, may function as a limiting homeostatic response to thrombogenic and hypertensive stimuli and may have particular relevance as a constraint on platelets during niacin therapy (61). Moreover, DP1 antagonism may restrain the anti-inflammatory effects and hence the putative atheroprotective benefit of niacin (54); it may further undermine the efficacy of niacin by failing to mediate direct platelet-inhibitory effects or by removing a restraint on niacin-evoked platelet thromboxane A2 formation, which increases dramatically (∼80-fold) after niacin administration. DP1 deletion in mice augments aneurysm formation and the hypertensive response to angiotensin II, and modestly accelerates atherogenesis (62, 123). However, the design of HPS2-THRIVE does not let us draw conclusions about whether concomitant DP1 antagonism limited any actual benefit from niacin with respect to cardiovascular outcomes, as control groups administered niacin or LRPT alone were not included in this study.
Similarly, it is impossible to assign cause for some of the unexpected adverse effects to one or the other component of the combination. As mentioned, bleeding may have derived from TP antagonism in some patients by LRPT, and it is biologically plausible that DP1 antagonism could have contributed to the excessive and infection observed in the combination, as there is some evidence that PGD2 contributes to resolution of inflammation (118, 119). On the other hand, although bleeding and infection were not reported as adverse effects of niacin in AIM-HIGH, post hoc analysis of the data by some has reported an excess of infections. Either way, a definitive conclusion cannot be reached from concordance with a post hoc analysis of another trial; HPS2-THRIVE was a trial of niacin/LRPT, not of niacin.
CONCLUSIONS
HPS2-THRIVE is a well-designed and powered clinical trial that has provided a definitive answer as to the efficacy/adverse response profile of a combination of niacin with a DP1 antagonist. However, it is impossible to parse with confidence the differential impact of the two elements of this combination. At face value, the results of this study and AIM-HIGH are concordant with HPS2-THRIVE and fail to support the hypothesis that raising HDLc by niacin would confer any cardiovascular benefit. However, the increment in HDLc was very modest in AIM-HIGH, which was relatively underpowered by comparison with HPS2-THRIVE; it also included niacin in the control group, albeit at a lower dose, and it was prematurely concluded. Adding to this impression, and perhaps more persuasive with respect to HDLc, is the study of human genetics, where variants associated with rising levels of HDLc are not related inversely to cardiovascular risk. However, even here, controversy lingers around the nature of the HDL that is elevated and, with respect to niacin, potentially beneficial mechanisms of action that have nothing to do with raising HDLc.
How will this all play out? To some degree, the results of ongoing trials of CETP inhibitors will influence the HDL discussion. Further, the impact of novel therapeutics, such as inhibitors of PCSK9, may shift the goal posts. Such results are likely to determine whether the performance of a sufficiently large and well-controlled study of niacin in more defined populations, say those relatively resistant to benefit from statins, seems like a cost-effective initiative to a commercial sponsor. Such pragmatic reasoning may drive the progressive abandonment of niacin, a drug that has long been a mainstay of cardiovascular therapy, while we still poorly understand its many potentially relevant mechanisms of action and have an incomplete picture of its clinical utility.
Footnotes
Abbreviations:
- AIM-HIGH
- Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes
- CDP
- Coronary Drug Project
- CETP
- cholesteryl ester transfer protein
- DP
- D prostanoid receptor
- ERN
- extended-release niacin
- HDLc
- high density lipoprotein cholesterol
- HPS2-THRIVE
- Heart Protection Study 2-Treatment of HDLc to Reduce the Incidence of Vascular Events
- IMT
- intima-media thickness
- LDLc
- low density lipoprotein cholesterol
- Lp(a)
- lipoprotein a
- LRPT
- laropiprant
- PGD2
- prostaglandin D2
- PGE2
- prostaglandin E2
- PPARγ
- peroxisome proliferator-activated receptor γ TG, triglyceride
- TP
- T prostanoid receptor
- VLDLc
- very low density lipoprotein cholesterol
This work was supported in part by a National Institutes of Health Grant HL-062250. G.A.F. is the McNeil Professor of Translational Medicine and Therapeutics.
REFERENCES
- 1.Carlson L. A. 2005. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 258: 94–114 [DOI] [PubMed] [Google Scholar]
- 2.Niehoff I. D., Huther L., Lebzien P. 2009. Niacin for dairy cattle: a review. Br. J. Nutr. 101: 5–19 [DOI] [PubMed] [Google Scholar]
- 3.Altschul R., Hoffer A., Stephen J. D. 1955. Influence of nicotinic acid on serum cholesterol in man. Arch. Biochem. Biophys. 54: 558–559 [DOI] [PubMed] [Google Scholar]
- 4.Meyers C. D., Kamanna V. S., Kashyap M. L. 2004. Niacin therapy in atherosclerosis. Curr. Opin. Lipidol. 15: 659–665 [DOI] [PubMed] [Google Scholar]
- 5.Tunaru S., Kero J., Schaub A., Wufka C., Blaukat A., Pfeffer K., Offermanns S. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9: 352–355 [DOI] [PubMed] [Google Scholar]
- 6.Wise A., Foord S. M., Fraser N. J., Barnes A. A., Elshourbagy N., Eilert M., Ignar D. M., Murdock P. R., Steplewski K., Green A., et al. 2003. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 278: 9869–9874 [DOI] [PubMed] [Google Scholar]
- 7.Soga T., Kamohara M., Takasaki J., Matsumoto S., Saito T., Ohishi T., Hiyama H., Matsuo A., Matsushime H., Furuichi K. 2003. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 303: 364–369 [DOI] [PubMed] [Google Scholar]
- 8.Tunaru S., Kero J., Schaub A., Wufka C., Blaukat A., Pfeffer K., Offermanns S. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9: 352–355 [DOI] [PubMed] [Google Scholar]
- 9.Benyó Z., Gille A., Kero J., Csiky M., Suchánoková M. C., Nüsing R. M., Moers A., Pfeffer K., Offermanns S. 2005. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J. Clin. Invest. 115: 3634–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson J., Gille A., Zwykiel S., Lukasova M., Clausen B. E., Ahmed K., Tunaru S., Wirth A., Offermanns S. 2010. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J. Clin. Invest. 120: 2910–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W.; AIM-HIGH Investigators 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267 [DOI] [PubMed] [Google Scholar]
- 12.HPS2-THRIVE Collaborative Group 2013. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 34: 1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons W. B., Jr., Flinn J. H. 1959. Reduction of serum cholesterol levels and beta-lipoprotein cholesterol levels by nicotinic acid. AMA Arch. Intern. Med. 103: 783–790 [DOI] [PubMed] [Google Scholar]
- 14.Birjmohun R. S., Hutten B. A., Kastelein J. J., Stroes E. S. 2005. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 45: 185–197 [DOI] [PubMed] [Google Scholar]
- 15.Carlson L. A., Hamsten A., Asplund A. 1989. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J. Intern. Med. 226: 271–276 [DOI] [PubMed] [Google Scholar]
- 16.Berglund L., Ramakrishnan R. 2004. Lipoprotein(a): an elusive cardiovascular risk factor. Arterioscler. Thromb. Vasc. Biol. 24: 2219–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson L. A., Oro L. 1962. The effect of nicotinic acid on the plasma free fatty acid; demonstration of a metabolic type of sympathicolysis. Acta Med. Scand. 172: 641–645 [DOI] [PubMed] [Google Scholar]
- 18.Carlson L. A. 1963. Studies on the effect of nicotinic acid on catecholamine stimulated lipolysis in adipose tissue in vitro. Acta Med. Scand. 173: 719–722 [DOI] [PubMed] [Google Scholar]
- 19.Butcher R. W., Baird C. E., Sutherland E. W. 1968. Effects of lipolytic and antilipolytic substances on adenosine 3′,5′-monophosphate levels in isolated fat cells. J. Biol. Chem. 243: 1705–1712 [PubMed] [Google Scholar]
- 20.Carlson L. A., Oro L., Ostman J. 1968. Effect of a single dose of nicotinic acid on plasma lipids in patients with hyperlipoproteinemia. Acta Med. Scand. 183: 457–465 [DOI] [PubMed] [Google Scholar]
- 21.Carlson L. A., Oroe L. 1965. Persistence of the inhibitory effect of nicotinic acid on catecholamine-stimulated lipid mobilization during prolonged treatment with nicotinic acid. J. Atheroscler. Res. 5: 436–439 [DOI] [PubMed] [Google Scholar]
- 22.Stern R. H., Spence J. D., Freeman D. J., Parbtani A. 1991. Tolerance to nicotinic acid flushing. Clin. Pharmacol. Ther. 50: 66–70 [DOI] [PubMed] [Google Scholar]
- 23.Lewis G. F. 1997. Fatty acid regulation of very low density lipoprotein production. Curr. Opin. Lipidol. 8: 146–153 [DOI] [PubMed] [Google Scholar]
- 24.Lauring B., Taggart A. K., Tata J. R., Dunbar R., Caro L., Cheng K., Chin J., Colletti S.L., Cote J., Khalilieh S., et al. 2012. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci. Transl. Med. 4: 148ra115. [DOI] [PubMed] [Google Scholar]
- 25.Jin F. Y., Kamanna V. S., Kashyap M. L. 1997. Niacin decreases removal of high-density lipoprotein apolipoprotein A-I but not cholesterol ester by Hep G2 cells. Implication for reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 17: 2020–2028 [DOI] [PubMed] [Google Scholar]
- 26.Ganji S. H., Tavintharan S., Zhu D., Xing Y., Kamanna V. S., Kashyap M. L. 2004. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res. 45: 1835–1845 [DOI] [PubMed] [Google Scholar]
- 27.Szapary P. O., Rader D. J. 2001. Pharmacological management of high triglycerides and low high-density lipoprotein cholesterol. Curr. Opin. Pharmacol. 1: 113–120 [DOI] [PubMed] [Google Scholar]
- 28.Le Goff W., Guerin M., Chapman M. J. 2004. Pharmacological modulation of cholesteryl ester transfer protein, a new therapeutic target in atherogenic dyslipidemia. Pharmacol. Ther. 101: 17–38 [DOI] [PubMed] [Google Scholar]
- 29.Yvan-Charvet L., Kling J., Pagler T., Li H., Hubbard B., Fisher T., Sparrow C.P., Taggart A. K., Tall A. R. 2010. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol. 30: 1430–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez C., Molusky M., Li Y., Li S., Lin J. D. 2010. Regulation of hepatic ApoC3 expression by PGC-1beta mediates hypolipidemic effect of nicotinic acid. Cell Metab. 12: 411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamon-Fava S., Diffenderfer M. R., Barrett P. H., Buchsbaum A., Nyaku M., Horvath K. V., Asztalos B. F., Otokozawa S., Ai M., Matthan M. R., et al. 2008. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler. Thromb. Vasc. Biol. 28: 1672–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Offermanns S. 2012. It ain't over ’til the fat lady sings. Sci. Transl. Med. 4: 148fs130. [DOI] [PubMed] [Google Scholar]
- 33.Blum C. B., Levy R. I., Eisenberg S., Hall M., 3rd, Goebel R. H., Berman M. 1977. High density lipoprotein metabolism in man. J. Clin. Invest. 60: 795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepherd J., Packard C. J., Patsch J. R., Gotto A. M., Jr., Taunton O. D. 1979. Effects of nicotinic acid therapy on plasma high density lipoprotein subfraction distribution and composition and on apolipoprotein A metabolism. J. Clin. Invest. 63: 858–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gille A., Bodor E. T., Ahmed K., Offermanns S. 2008. Nicotinic acid: pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol. 48: 79–106 [DOI] [PubMed] [Google Scholar]
- 36.Nordestgaard B. G., Chapman M. J., Ray K., Borén J., Andreotti F., Watts G. F., Ginsberg H., Amarenco P., Catapano A., Descamps O. S., et al. 2010. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur. Heart J. 31: 2844–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamstrup P. R., Tybjaerg-Hansen A., Nordestgaard B. G. 2011. Lipoprotein(a) and risk of myocardial infarction–genetic epidemiologic evidence of causality. Scand. J. Clin. Lab. Invest. 71: 87–93 [DOI] [PubMed] [Google Scholar]
- 38.Danesh J., Collins R., Peto R. 2000. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 102: 1082–1085 [DOI] [PubMed] [Google Scholar]
- 39.Smolders B., Lemmens R., Thijs V. 2007. Lipoprotein (a) and stroke: a meta-analysis of observational studies. Stroke. 38: 1959–1966 [DOI] [PubMed] [Google Scholar]
- 40.Schreiner P. J., Morrisett J. D., Sharrett A. R., Patsch W., Tyroler H. A., Wu K., Heiss G. 1993. Lipoprotein[a] as a risk factor for preclinical atherosclerosis. Arterioscler. Thromb. 13: 826–833 [DOI] [PubMed] [Google Scholar]
- 41.Berglund L. 1995. Diet and drug therapy for lipoprotein (a). Curr. Opin. Lipidol. 6: 48–56 [DOI] [PubMed] [Google Scholar]
- 42.Tornvall P., Walldius G. 1991. A comparison between nicotinic acid and acipimox in hypertriglyceridaemia–effects on serum lipids, lipoproteins, glucose tolerance and tolerability. J. Intern. Med. 230: 415–421 [DOI] [PubMed] [Google Scholar]
- 43.Fuccella L. M., Goldaniga G., Lovisolo P., Maggi E., Musatti L., Mandelli V., Sirtori C. R. 1980. Inhibition of lipolysis by nicotinic acid and by acipimox. Clin. Pharmacol. Ther. 28: 790–795 [DOI] [PubMed] [Google Scholar]
- 44.Soudijn W., van Wijngaarden I., Ijzerman A. P. 2007. Nicotinic acid receptor subtypes and their ligands. Med. Res. Rev. 27: 417–433 [DOI] [PubMed] [Google Scholar]
- 45.Wang M., Fotsch C. 2006. Small-molecule compounds that modulate lipolysis in adipose tissue: targeting strategies and molecular classes. Chem. Biol. 13: 1019–1027 [DOI] [PubMed] [Google Scholar]
- 46.van Herk T., Brussee J., van den Nieuwendijk A. M., van der Klein P. A., IJzerman A. P., Stannek C., Burmeiter A., Lorenzen A. 2003. Pyrazole derivatives as partial agonists for the nicotinic acid receptor. J. Med. Chem. 46: 3945–3951 [DOI] [PubMed] [Google Scholar]
- 47.Semple G., Skinner P. J., Cherrier M. C., Webb P. J., Sage C. R., Tamura S. Y., Chen R., Richman J. G., Connolly D. T. 2006. 1-Alkyl-benzotriazole-5-carboxylic acids are highly selective agonists of the human orphan G-protein-coupled receptor GPR109b. J. Med. Chem. 49: 1227–1230 [DOI] [PubMed] [Google Scholar]
- 48.Walters R. W., Shukla A. K., Kovacs J. J., Violin J. D., DeWire S. M., Lam C. M., Chen J. R., Muehlbauer M. J., Whalen E. J., Lefkowitz R. J. 2009. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J. Clin. Invest. 119: 1312–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai E., Waters M. G., Tata J. R., Radziszewski W., Perovozskaya I., Zhen W., Wenning L., Connolly D. T., Semple G., Johnson-Levonas A. O., et al. 2008. Effects of a niacin receptor partial agonist, MK-0354, on plasma free fatty acids, lipids, and cutaneous flushing in humans. J. Clin. Lipidol. 2: 375–383 [DOI] [PubMed] [Google Scholar]
- 50.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099 [DOI] [PubMed] [Google Scholar]
- 51.Voight B. F., Peloso G. M., Orho-Melander M., Frikke-Schmidt R., Barbalic M., Jensen M. K., Hindy G., Hólm H., Ding E. L., Johnson T., et al. 2012. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 380: 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rader D. J., Tall A. R. 2012. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat. Med. 18: 1344–1346 [DOI] [PubMed] [Google Scholar]
- 53.Wu B. J., Yan L., Charlton F., Witting P., Barter P. J., Rye K. A. 2010. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler. Thromb. Vasc. Biol. 30: 968–975 [DOI] [PubMed] [Google Scholar]
- 54.Lukasova M., Malaval C., Gille A., Kero J., Offermanns S. 2011. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Invest. 121: 1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holzhäuser E., Albrecht C., Zhou Q., Buttler A., Preusch M. R., Blessing E., Katus H. A., Bea F. 2011. Nicotinic acid has anti-atherogenic and anti-inflammatory properties on advanced atherosclerotic lesions independent of its lipid-modifying capabilities. J. Cardiovasc. Pharmacol. 57: 447–454 [DOI] [PubMed] [Google Scholar]
- 56.Yu B. L., Zhao S. P. 2007. Anti-inflammatory effect is an important property of niacin on atherosclerosis beyond its lipid-altering effects. Med. Hypotheses. 69: 90–94 [DOI] [PubMed] [Google Scholar]
- 57.Tavintharan S., Lim S. C., Sum C. F. 2009. Effects of niacin on cell adhesion and early atherogenesis: biochemical and functional findings in endothelial cells. Basic Clin. Pharmacol. Toxicol. 104: 206–210 [DOI] [PubMed] [Google Scholar]
- 58.Knowles H. J., te Poele R. H., Workman P., Harris A. L. 2006. Niacin induces PPARgamma expression and transcriptional activation in macrophages via HM74 and HM74a-mediated induction of prostaglandin synthesis pathways. Biochem. Pharmacol. 71: 646–656 [Erratum. 2006. Biochem. Pharmacol. 71: 1662.] [DOI] [PubMed] [Google Scholar]
- 59.Rubic T., Trottmann M., Lorenz R. L. 2004. Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin. Biochem. Pharmacol. 67: 411–419 [DOI] [PubMed] [Google Scholar]
- 60.Plaisance E. P., Lukasova M., Offermanns S., Zhang Y., Cao G., Judd R. L. 2009. Niacin stimulates adiponectin secretion through the GPR109A receptor. Am. J. Physiol. Endocrinol. Metab. 296: E549–E558 [DOI] [PubMed] [Google Scholar]
- 61.Westphal S., Luley C. 2008. Preferential increase in high-molecular weight adiponectin after niacin. Atherosclerosis. 198: 179–183 [DOI] [PubMed] [Google Scholar]
- 62.Song W. L., Stubbe J., Ricciotti E., Alamuddin N., Ibrahim S., Crichto I., Prempeh M., Lawson J. A., Wilensky R. L., Rasmussen L. M., et al. 2012. Niacin and biosynthesis of PGD(2)by platelet COX-1 in mice and humans. J. Clin. Invest. 122: 1459–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saareks V., Mucha I., Sievi E., Riutta A. 1999. Nicotinic acid and pyridoxine modulate arachidonic acid metabolism in vitro and ex vivo in man. Pharmacol. Toxicol. 84: 274–280 [DOI] [PubMed] [Google Scholar]
- 64.Degoma E. M., Rader D. J. 2011. Novel HDL-directed pharmacotherapeutic strategies. Nat. Rev. Cardiol. 8: 266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y., Zanotti I., Reilly M. P., Glick J. M., Rothblat G. H., Rader D. J. 2003. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 108: 661–663 [DOI] [PubMed] [Google Scholar]
- 67.Pászty C., Maeda N., Verstuyft J., Rubin E. M. 1994. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J. Clin. Invest. 94: 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weng W., Breslow J. L. 1996. Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility. Proc. Natl. Acad. Sci. USA. 93: 14788–14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badimon J. J., Badimon L., Fuster V. 1990. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 85: 1234–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badimon J. J., Badimon L., Galvez A., Dische R., Fuster V. 1989. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab. Invest. 60: 455–461 [PubMed] [Google Scholar]
- 71.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300 [DOI] [PubMed] [Google Scholar]
- 72.Dunbar R. L., Gelfand J. M. 2010. Seeing red: flushing out instigators of niacin-associated skin toxicity. J. Clin. Invest. 120: 2651–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrow J. D., Parsons W. G., 3rd, Roberts L. J., 2nd 1989. Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins. 38: 263–274 [DOI] [PubMed] [Google Scholar]
- 74.Morrow J. D., Awad J. A., Oates J. A., Roberts L. J., 2nd 1992. Identification of skin as a major site of prostaglandin D2 release following oral administration of niacin in humans. J. Invest. Dermatol. 98: 812–815 [DOI] [PubMed] [Google Scholar]
- 75.Lauring B., Dishy V., Luo W. L., Laterza O., Patterson J., Cote J., Chao A., Larson P., Gutierrez M., Wagner J. A., et al. 2009. Laropiprant in combination with extended-release niacin does not alter urine 11-dehydrothromboxane B2, a marker of in vivo platelet function, in healthy, hypercholesterolemic, and diabetic subjects. J. Clin. Pharmacol. 49: 1426–1435 [DOI] [PubMed] [Google Scholar]
- 76.Truelove L. H., Duthie J. J. 1959. Effect of aspirin on cutaneous response to the local application of an ester of nicotinic acid. Ann. Rheum. Dis. 18: 137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cefali E. A., Simmons P. D., Stanek E. J., McGovern M. E., Kissling C. J. 2007. Aspirin reduces cutaneous flushing after administration of an optimized extended-release niacin formulation. Int. J. Clin. Pharmacol. Ther. 45: 78–88 [DOI] [PubMed] [Google Scholar]
- 78.Jungnickel P. W., Maloley P. A., Vander Tuin E. L., Peddicord T. E., Campbell J. R. 1997. Effect of two aspirin pretreatment regimens on niacin-induced cutaneous reactions. J. Gen. Intern. Med. 12: 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parson H. K., Harati H., Cooper D., Vinik A. I. 2013. Role of prostaglandin D2 and the autonomic nervous system in niacin-induced flushing. J. Diabetes. 5: 59–67 [DOI] [PubMed] [Google Scholar]
- 80.Cheng K., Wu T. J., Wu K. K., Sturino C., Metters K., Gottesdiener K., Wright S. D., Wang Z., O'Neill G., Lai E., et al. 2006. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc. Natl. Acad. Sci. USA. 103: 6682–6687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lai E., De Lepeleire I., Crumley T. M., Liu F., Wenning L. A., Michiels N., Vets E., O'Neill G., Wagner J. A., Gottesdiener K. 2007. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin. Pharmacol. Ther. 81: 849–857 [DOI] [PubMed] [Google Scholar]
- 82.Maccubbin D., Koren M. J., Davidson M., Gavish D., Pasternak R. C., Macdonell G., Mallick M., Sisk C. M., Paolini J. F., Mitchel Y. 2009. Flushing profile of extended-release niacin/laropiprant versus gradually titrated niacin extended-release in patients with dyslipidemia with and without ischemic cardiovascular disease. Am. J. Cardiol. 104: 74–81 [DOI] [PubMed] [Google Scholar]
- 83.Paolini J. F., Mitchel Y. B., Reyes R., Kher U., Lai E., Watson D. J., Norquist J. M., Meehan A. G., Bays H. E., Davidson M., et al. 2008. Effects of laropiprant on nicotinic acid-induced flushing in patients with dyslipidemia. Am. J. Cardiol. 101: 625–630 [DOI] [PubMed] [Google Scholar]
- 84.Kush D., Hu D. Y., Ye P., Kim H. S., Chen E., Sirah W., McCrary Sisk C., Paolini J. F., Maccubbin D. 2009. Flushing profile of extended-release niacin/laropiprant at initiation of therapy in Asian lipid clinic patients. Cardiology. 114: 192–198 [DOI] [PubMed] [Google Scholar]
- 85.Maccubbin D., Bays H. E., Olsson A. G., Elinoff V., Ellis A., Mitchel Y., Sirah W., Betteridge A., Reyes R., Yu Q., et al. 2008. Lipid-modifying efficacy and tolerability of extended-release niacin/laropiprant in patients with primary hypercholesterolaemia or mixed dyslipidaemia. Int. J. Clin. Pract. 62: 1959–1970 [DOI] [PubMed] [Google Scholar]
- 86.Kush D., Kim H. S., Hu da Y., Liu J., Sirah W., Sapre A., McCrary Sisk C., Paolini J. F., Maccubbin D. 2009. Lipid-modifying efficacy of extended release niacin/laropiprant in Asian patients with primary hypercholesterolemia or mixed hyperlipidemia. J. Clin. Lipidol. 3: 179–186 [DOI] [PubMed] [Google Scholar]
- 87.Bays H., Shah A., Dong Q., McCrary Sisk C., Maccubbin D. 2011. Extended-release niacin/laropiprant lipid-altering consistency across patient subgroups. Int. J. Clin. Pract. 65: 436–445 [DOI] [PubMed] [Google Scholar]
- 88.Bays H. E., Shah A., Lin J., McCrary Sisk C., Paolini J. F., Maccubbin D. 2010. Efficacy and tolerability of extended-release niacin/laropiprant in dyslipidemic patients with metabolic syndrome. J. Clin. Lipidol. 4: 515–521 [DOI] [PubMed] [Google Scholar]
- 89.Ballantyne C., Gleim G., Liu N., Sisk C. M., Johnson-Levonas A. O., Mitchel Y. 2012. Effects of coadministered extended-release niacin/laropiprant and simvastatin on lipoprotein subclasses in patients with dyslipidemia. J. Clin. Lipidol. 6: 235–243 [DOI] [PubMed] [Google Scholar]
- 90.Chen F., Maccubbin D., Yan L., Sirah W., Chen E., Sisk C. M., Davidson M., Blomqvist P., McKenney J. M. 2013. Lipid-altering efficacy and safety profile of co-administered extended release niacin/laropiprant and simvastatin versus atorvastatin in patients with mixed hyperlipidemia. Int. J. Cardiol. 167: 225–231 [DOI] [PubMed] [Google Scholar]
- 91.Miettinen T. A., Taskinen M. R., Pelkonen R., Nikkila E. A. 1969. Glucose tolerance and plasma insulin in man during acute and chronic administration of nicotinic acid. Acta Med. Scand. 186: 247–253 [DOI] [PubMed] [Google Scholar]
- 92.Poynten A. M., Gan S. K., Kriketos A. D., O'Sullivan A., Kelly J. J., Ellis B. A., Chisholm D. J., Campbell L. V. 2003. Nicotinic acid-induced insulin resistance is related to increased circulating fatty acids and fat oxidation but not muscle lipid content. Metabolism. 52: 699–704 [DOI] [PubMed] [Google Scholar]
- 93.Canner P. L., Furberg C. D., Terrin M. L., McGovern M. E. 2005. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project). Am. J. Cardiol. 95: 254–257 [DOI] [PubMed] [Google Scholar]
- 94.Ridker P. M., Danielson E., Fonseca F. A., Genest J., Gotto A. M., Jr., Kastelein J. J., Koenig W., Libby P., Lorenzatti A. J., MacFadyen J. G., et al. 2008. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359: 2195–2207 [DOI] [PubMed] [Google Scholar]
- 95.Sattar N., Preiss D., Murray H. M., Welsh P., Buckley B. M., de Craen A. J., Seshasai S. R., McMurray J. J., Freeman D. J., Jukema J. W., et al. 2010. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 375: 735–742 [DOI] [PubMed] [Google Scholar]
- 96.Shah R. V., Goldfine A. B. 2012. Statins and risk of new-onset diabetes mellitus. Circulation. 126: e282–e284 [DOI] [PubMed] [Google Scholar]
- 97.Andersson S., Carlson L. A., Oro L., Richards E. A. 1971. Effect of nicotinic acid on gastric secretion of acid in human subjects and in dogs. Scand. J. Gastroenterol. 6: 693–698 [DOI] [PubMed] [Google Scholar]
- 98.Lawrence S. P. 1993. Transient focal hepatic defects related to sustained-release niacin. J. Clin. Gastroenterol. 16: 234–236 [DOI] [PubMed] [Google Scholar]
- 99.Dalton T. A., Berry R. S. 1992. Hepatotoxicity associated with sustained-release niacin. Am. J. Med. 93: 102–104 [DOI] [PubMed] [Google Scholar]
- 100.Etchason J. A., Miller T. D., Squires R. W., Allison T. G., Gau G. T., Marttila J. K., Kotke B. A. 1991. Niacin-induced hepatitis: a potential side effect with low-dose time-release niacin. Mayo Clin. Proc. 66: 23–28 [DOI] [PubMed] [Google Scholar]
- 101.McKenney J. 2004. New perspectives on the use of niacin in the treatment of lipid disorders. Arch. Intern. Med. 164: 697–705 [DOI] [PubMed] [Google Scholar]
- 102.Gershon S. L., Fox I. H. 1974. Pharmacologic effects of nicotinic acid on human purine metabolism. J. Lab. Clin. Med. 84: 179–186 [PubMed] [Google Scholar]
- 103.Fitzpatrick D. A., FitzGerald O., McGeeney K. F. 1969. Nicotinic acid inhibition of uricase. Ir. J. Med. Sci. 8: 531–534 [DOI] [PubMed] [Google Scholar]
- 104.Gaut Z. N., Pocelinko R., Solomon H. M., Thomas G. B. 1971. Oral glucose tolerance, plasma insulin, and uric acid excretion in man during chronic administration of nicotinic acid. Metabolism. 20: 1031–1035 [DOI] [PubMed] [Google Scholar]
- 105.1975. Clofibrate and niacin in coronary heart disease. JAMA. 231: 360–381 [PubMed] [Google Scholar]
- 106.Canner P. L., Berge K. G., Wenger N. K., Stamler J., Friedman L., Prineas R. J., Friedewald W. 1986. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J. Am. Coll. Cardiol. 8: 1245–1255 [DOI] [PubMed] [Google Scholar]
- 107.Carlson L. A., Rosenhamer G. 1988. Reduction of mortality in the Stockholm Ischaemic Heart Disease Secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Med. Scand. 223: 405–418 [DOI] [PubMed] [Google Scholar]
- 108.Brown G., Albers J. J., Fisher L. D., Schaefer S. M., Lin J. T., Kaplan C., Zhao X. Q., Bisson B. D., Fitzpatrick V. F., Dodge H. T. 1990. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N. Engl. J. Med. 323: 1289–1298 [DOI] [PubMed] [Google Scholar]
- 109.Brown B. G., Zhao X. Q., Chait A., Fisher L. D., Cheung M. C., Morse J. S., Dowdy A. A., Marino E. K., Bolson E. L., Alaupovic P., et al. 2001. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345: 1583–1592 [DOI] [PubMed] [Google Scholar]
- 110.Taylor A. J., Sullenberger L. E., Lee H. J., Lee J. K., Grace K. A. 2004. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 110: 3512–3517 [DOI] [PubMed] [Google Scholar]
- 111.Taylor A. J., Lee H. J., Sullenberger L. E. 2006. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr. Med. Res. Opin. 22: 2243–2250 [DOI] [PubMed] [Google Scholar]
- 112.Taylor A. J., Villines T. C., Stanek E. J., Devine P. J., Griffen L., Miller M., Weissman N. J., Turco M. 2009. Extended-release niacin or ezetimibe and carotid intima-media thickness. N. Engl. J. Med. 361: 2113–2122 [DOI] [PubMed] [Google Scholar]
- 113.Lee J. M., Robson M. D., Yu L. M., Shirodaria C. C., Cunnington C., Dylintireas I., Digby J. E., Bannister T., Handa A., Wiesmann F., et al. 2009. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J. Am. Coll. Cardiol. 54: 1787–1794 [DOI] [PubMed] [Google Scholar]
- 114.Michos E. D., Sibley C. T., Baer J. T., Blaha M. J., Blumenthal R. S. 2012. Niacin and statin combination therapy for atherosclerosis regression and prevention of cardiovascular disease events: reconciling the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial with previous surrogate endpoint trials. J. Am. Coll. Cardiol. 59: 2058–2064 [DOI] [PubMed] [Google Scholar]
- 115.1994. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 344: 1383–1389 [PubMed] [Google Scholar]
- 116.Rosenson R. S., Gotto A. M., Jr 2013. When clinical trials fail to address treatment gaps: the failure of niacin-laropiprant to reduce cardiovascular events. Curr. Atheroscler. Rep. 15: 332. [DOI] [PubMed] [Google Scholar]
- 117.Thompson P. D., Clarkson P., Karas R. H. 2003. Statin-associated myopathy. JAMA. 289: 1681–1690 [DOI] [PubMed] [Google Scholar]
- 118.Rajakariar R., Hilliard M., Lawrence T., Trivedi S., Colville-Nash P., Bellingan G., Fitzgerald D., Yagoob M. M., Gilroy D. W. 2007. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc. Natl. Acad. Sci. USA. 104: 20979–20984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murata T., Aritake K., Tsubosaka Y., Maruyama T., Nakagawa T., Hori M., Hirai H., Nakamura M., Narumiya S., Urade Y., et al. 2013. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc. Natl. Acad. Sci. USA. 110: 5205–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown S. L., Sobel B. E., Fujii S. 1995. Attenuation of the synthesis of plasminogen activator inhibitor type 1 by niacin. A potential link between lipid lowering and fibrinolysis. Circulation. 92: 767–772 [DOI] [PubMed] [Google Scholar]
- 121.Chesney C. M., Elam M. B., Herd J. A., Davis K. B., Garg R., Hunninghake D., Kennedy J. W., Applegate W. B. 2000. Effect of niacin, warfarin, and antioxidant therapy on coagulation parameters in patients with peripheral arterial disease in the Arterial Disease Multiple Intervention Trial (ADMIT). Am. Heart J. 140: 631–636 [DOI] [PubMed] [Google Scholar]
- 122.Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent C., Blackwell L., Emberson J., Holland L. E., Reith C., Bhala N., Peto R., Barnes E. H., Keech A., Simes J., et al. 2010. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376: 1670–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strack A. M., Carballo-Jane E., Wang S. P., Xue J., Ping X., McNamara L. A., Thankappan A., Price O., Wolff M., Wu T. J., et al. 2013. Nicotinic acid and DP1 blockade: studies in mouse models of atherosclerosis. J. Lipid Res. 54: 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]