Abstract
Type IV P-type ATPases (P4-ATPases) are a large family of putative phospholipid translocases (flippases) implicated in the generation of phospholipid asymmetry in biological membranes. P4-ATPases are typically the largest P-type ATPase subgroup found in eukaryotic cells, with five members in Saccharomyces cerevisiae, six members in Caenorhabditis elegans, 12 members in Arabidopsis thaliani and 14 members in humans. In addition, many of the P4-ATPases require interaction with a noncatalytic subunit from the CDC50 gene family for their transport out of the endoplasmic reticulum (ER). Deficiency of a P4-ATPase (Atp8b1) causes liver disease in humans, and studies in a variety of model systems indicate that P4-ATPases play diverse and essential roles in membrane biogenesis. In addition to their proposed role in establishing and maintaining plasma membrane asymmetry, P4-ATPases are linked to vesicle-mediated protein transport in the exocytic and endocytic pathways. Recent studies have also suggested a role for P4-ATPases in the nonvesicular intracellular trafficking of sterols. Here, we discuss the physiological requirements for yeast P4-ATPases in phospholipid translocase activity, transport vesicle budding and ergosterol metabolism, with an emphasis on Drs2p and its noncatalytic subunit, Cdc50p.
Keywords: P4-ATPase, flippase, Drs2p, Cdc50p, membrane asymmetry, clathrin, Kes1p
1. Introduction
Phospholipid asymmetry appears to be a fundamental feature of the eukaryotic cell plasma membrane [1]. In animal cells, the aminophospholipids phosphatidylserine and phosphatidylethanolamine (PE) are primarily restricted to the cytosolic leaflet of the plasma membrane [2], but regulated exposure of these lipids on the extracellular leaflet plays a role in cell signaling [3, 4], cytokinesis [5], blood clotting [6], apoptotic cell corpse removal [7] and host-viral interactions [8]. The budding yeast plasma membrane is also asymmetric with an enrichment of PS and PE on the inner leaflet [9–11], and it is generally assumed that most eukaryotic cells have a similarly organized plasma membrane. Precisely how this asymmetric membrane structure is formed is not known. However, type IV P-type ATPases (P4-ATPases), a large family of putative flippases, are proposed to establish and maintain membrane asymmetry by pumping PS and PE from the exofacial leaflet to the cytosolic leaflet of the bilayer [12].
The human genome contains 14 genes encoding P4-ATPases, including ATP8B1 (also called FIC1), in which mutations can cause familial intrahepatic cholestasis, an inherited liver disease. Loss of Atp8b1 function causes a defect in bile flow through the liver resulting in liver damage [13]. The mouse ATP10A gene (also called ATP10C in the literature) is linked to diet-induced obesity and type II diabetes phenotypes [14], and an ATP10D mutation shows linkage to a fat-prone phenotype in certain strains of mice [15]. These observations imply an important role for P4-ATPases in lipid metabolism and human health. The P4-ATPase Atp8a1 is a PS and PE-stimulated ATPase and is thought to catalyze the aminophospholipid translocase (flippase) activity present in the red blood cell plasma membrane and chromaffin granules [16–18]. A phospholipid flippase activity has not been reconstituted with purified Atp8a1, or any other P4-ATPase, and so definitive biochemical evidence that these enzymes are sufficient to catalyze flippase activity is still lacking. However, genetic studies in yeast imply that P4-ATPases are necessary for phospholipid flippase activities detected in the plasma membrane, secretory vesicles and the trans-Golgi network [19–22].
2. P4-ATPases in Saccharomyces cerevisiae
The Saccharomyces cerevisiae genome contains five P4-ATPase genes: DRS2, NEO1, DNF1, DNF2 and DNF3 [23]. As with most P-type ATPases, the P4-ATPases are predicted to contain 10 transmembrane segments with the N- and C-termini facing the cytosol. By analogy to the crystal structure of the Ca++ ATPase (SERCA1), the cytosolic N-terminal tail and the loop between transmembrane segments 2 – 3 should form the actuator (A) domain, and the large cytosolic loop between membrane segments 4 and 5 should form the phosphorylation (P) and nucleotide-binding (N) domains (Fig. 1) [24, 25]. Several of the P4-ATPases form a complex with a noncatalytic subunit from the CDC50 (also called ROS3) family, which also includes LEM3 and CRF1. Cdc50 family proteins are predicted to have two transmembrane segments and a large, intervening exoplasmic domain that is glycosylated [26]. For Drs2p, interaction with Cdc50p is required for exit of the complex from the endoplasmic reticulum (ER). Similarly, Cdc50p is retained in the ER of drs2Δ cells and so these two proteins are co-dependent for their exit from the ER [11, 26]. Lem3p chaperones both Dnf1p and Dnf2p out of the ER while Crf1p chaperones Dnf3p [26, 27]. No comparable noncatalytic subunit for Neo1p is known.
Figure 1.
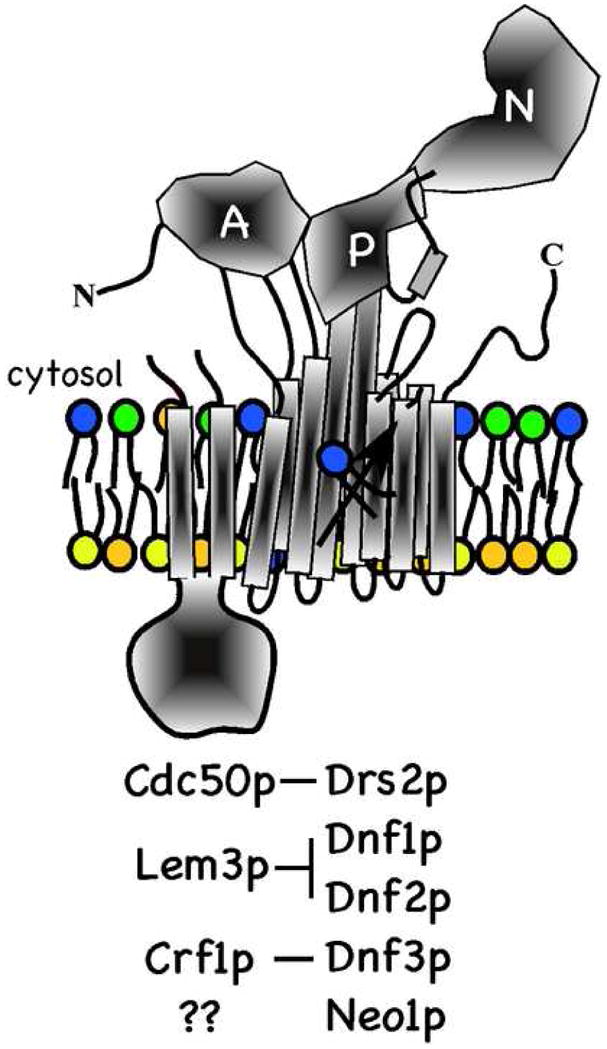
P4-ATPases in Saccharomyces cerevisiae. The catalytic subunit (Drs2p, Dnf1p, Dnf2p, Dnf3p or Neo1) is modeled after the structure of SERCA1 with 10 transmembrane segments and distinct actuator (A), phosphorylation (P) and nucleotide binding (N) domains. The noncatalytic subunit is predicted to have two transmembrane segments and a large ectoplasmic domain that is glycosylated. Interactions between catalytic and noncatalytic subunits required for transport of the complex out of the ER are indicated. It is not known if Neo1p requires interaction with a noncatalytic subunit. The yeast P4-ATPases are proposed to flip phospholipid (PS, PE and PC) to the cytosolic leaflet to generate membrane asymmetry.
P4-ATPases are required for the viability of Saccharomyces cerevisiae. Individually, NEO1 is the only essential gene in this family although disruption of DRS2 causes a severe growth defect at temperatures below 23°C. Disruption of the DNF genes individually or in combination (e.g. dnf1,2,3Δ) has no significant consequence to growth. However, disruption of DNF genes individually from a drs2Δ strain exacerbates the cold-sensitive growth defect and the quadruple mutant (drs2Δ dnf1,2,3Δ) is inviable [28]. These observations imply some degree of functional redundancy between DRS2 and the DNF genes, and that the DNF genes can compensate for the loss of DRS2 at high growth temperatures better than at low temperatures. Consistently, disruption of CDC50 causes a cold-sensitive growth defect (phenocopy of drs2Δ) and the cdc50Δ lem3Δ crf1Δ triple mutant is inviable (phenocopy of drs2Δ dnf1,2,3Δ) [26].
3. Role of Dnf1p and Dnf2p in phospholipid translocation and membrane asymmetry
The P4-ATPase mutants display phenotypes consistent with their proposed biochemical function as phospholipid flippases that control membrane asymmetry. Of the five yeast P4-ATPases, Dnf1p and Dnf2p are most abundantly found at the plasma membrane [28] and the dnf1,2Δ and lem3Δ mutants are deficient in the uptake of fluorescent (6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoic acid), (NBD)) PE and phosphatidylcholine (PC) analogues across the plasma membrane [10, 19, 29], implying a deficit in flippase activity. The distribution of endogenous PC across the plasma membrane of yeast has not been measured. However, the ability of wild-type yeast to take up NBD-PC may suggest that PC is restricted to the inner leaflet of the plasma membrane and that the outer leaflet is primarily composed of sphingolipids, as NBD-sphingomyelin is not taken up [19]. There is some discrepancy in the literature as to whether NBD-PS uptake is also perturbed in Lem3- or Dnf1,2-deficient yeast, although most of the data indicate that NBD-PS is taken up normally [10, 19, 30]. The dnf1,2Δ and lem3Δ cells are also deficient for the uptake and utilization of lysoPE, suggesting a nutrient scavenger role for these pumps [31]. In addition, these cells are defective for the uptake of alkylphosphocholine drugs used for treatment of leishmaniasis, and are therefore resistant to the toxic effects of these drugs [29]. Concordantly, Leishmania donovani strains deficient for Dnf1 or Lem3 orthologs are also alkylphosphocholine drug resistant [32].
A deficiency in the uptake of NBD-PC, -PE and lysoPE across the plasma membrane of dnf1,2Δ and lem3Δ mutants is consistent with the proposed flippase function for these P4-ATPases [19, 29], but does not constitute proof. It is formally possible that disruption of the DNF and LEM3 genes indirectly influences the expression, localization or activity of an undefined lipid transporter. As described below, the P4-ATPases are intimately coupled to protein trafficking in the exocytic and endocytic pathways [23]. Thus, the concern about P4-ATPase mutants displaying pleiotropic defects caused by mislocalization of other proteins is not trivial. Our group has taken a combined genetic and biochemical approach designed to circumvent the known pleiotropic consequences of disrupting DRS2 to test the potential flippase function of this P4-ATPase.
4. Role of Drs2p in phospholipid translocation and membrane asymmetry
Drs2p primarily localizes to the TGN and TGN membranes purified from wild-type cells contain an ATP-dependent flippase that translocates NBD-PS from the luminal leaflet to the cytosolic leaflet. A weaker activity was also detected for NBD-PE, but no ATP-dependent translocation of NBD-PC was observed. To test if Drs2p is required for this flippase activity, TGN membranes were purified from a strain carrying a temperature-sensitive for function allele of drs2 (drs2-ts). The encoded protein, Drs2p-ts, is active at low, permissive temperatures (i.e. 27°C) and can be inactivated by temperature-shift to 37°C. Cells expressing Drs2p-ts were grown at a permissive temperature to maintain the normal function of the TGN, thus minimizing pleiotropic defects, before the cells were lysed and the TGN purified. These membranes displayed an ATP-dependent NBD-PS flippase activity at permissive temperature, but this activity was lost when the same membranes were shifted to 37°C. The NBD-PS flippase activity was observed at both temperatures with TGN membranes containing wild-type Drs2p. Thus, inactivation of a single enzyme, Drs2p-ts, in the Golgi preparation was sufficient to eliminate flippase activity [21].
The NBD-PS flippase studies using purified Golgi membranes indicate that Drs2p is required to drive transport of NBD-PS across the TGN membrane to the cytosolic leaflet. However, this experimental approach does not distinguish between a primary and secondary transport mechanism. It is formally possible that Drs2p pumps an ion into the lumen of the TGN, which is secondarily coupled to lipid translocation as the ion moves back down its concentration gradient through an undefined ion/lipid symporter. The Na+/K+ and H+ ATPases are known to drive a number of active transport processes by this secondary transport mechanism [24]. P4-ATPases are Mg++-dependent, although this requirement appears to be for ATP hydrolysis rather than an indication that Mg++ is a transport substrate. Substitution of ions in the buffers used in the TGN flippase assays, from Na+ and Cl− to K+ and acetate−, had no influence on NBD-PS translocation [21]. Thus far, no ion or heavy metal transport substrate has been detected for P4-ATPases, arguing against a specific ion requirement to drive lipid translocation by a secondary mechanism. However, ultimate proof that P4-ATPases have evolved the ability to directly translocate phospholipid across the bilayer will require reconstitution of this activity with a pure enzyme.
Another incompletely resolved issue with regard to P4-ATPase transport substrate is the extent to which NBD-labeled or spin-labeled substrates mimic endogenous phospholipids. P4-ATPase-dependent NBD-phospholipid transport shows distinct headgroup specificity, arguing that the NBD probe is not being recognized by the lipid transporter. Moreover, ATP-induced translocation of endogenous PE across the bilayer has been detected in yeast exocytic vesicles, and this activity is abolished in vesicles lacking Drs2p and Dnf3p [20]. An elegant series of studies using changes in the shape of erythrocytes induced by phospholipid translocation, without NBD or spin-labeled probes, was used to assess the lipid specificity of the aminophospholipid translocase [33, 34]. As with the spin-labeled probes, a strong preference for the PS headgroup and stereospecificity for the glycerol backbone was observed [34]. The ATPase activity of Atp8a1 is stimulated with a similar headgroup and glycerol backbone stereospecificity [16], suggesting that this stimulation reflects a transport substrate interaction. As PS is pumped towards the cytosol, this potential substrate interaction should induce an E2~P → E1 transition in the translocase. Consistently, Atp8a1 is phosphorylated upon incubation with ATP in the absence of PS, and is rapidly dephosphorylated upon subsequent addition of PS [17].
It is not known if the P4-ATPases transport any substrate during the E1 → E2~P transition. With cation pumps, countertransport of two different cations appears to be an essential component of the transport process [35, 36]. For example, in the Ca++ ATPase, two protons replace the two Ca++ ions as they leave their binding site in the transmembrane domain, and the protons are pumped in the opposite direction as Ca++. Cation interaction with negatively charged amino acids in the transmembrane domain appears to be required to stabilize the structure of the membrane domain [36]. However, instead of the charged residues that form the Ca++/H+ binding site (e.g E309, E771, D800, E908) in the center of the Ca++ ATPase transmembrane domain, Drs2p has nonpolar residues in the analogous positions (I508, L1022, V1055, G1148). In addition, assuming the membrane domain structures are similar, it seems unlikely that a bulky phospholipid could follow the same path through a P4-ATPase as Ca++ moves through the Ca++ ATPase. Another possibility is that the phospholipid moves at the protein/lipid interface of the P4-ATPases, in which case there may not be a structural requirement for countertransport of a an ion. However, PS carries a net charge of −1 and so there may be a requirement for ion movement to balance the electrogenic charge displacement induced by a PS flippase.
The P4-ATPase deficient yeast strains exhibit a loss of plasma membrane asymmetry and expose endogenous PS and PE on the outer leaflet of the plasma membrane [11, 19]. This phenotype and the observations described above are consistent with their proposed flippase function and suggest that endogenous phospholipids are substrates for these pumps. However, a number of mutations in genes linked to protein trafficking, cell polarity or cell wall biogenesis also cause a similar loss of phospholipid asymmetry of the plasma membrane [11, 37]. Thus, loss of phospholipid asymmetry could be a general consequence of perturbing membrane systems rather than a specific consequence of disrupting a flippase, which complicates interpretation of these results. For example, loss of PS asymmetry in dnf1,2Δ or drs2Δ strains does not demonstrate that these enzymes can pump endogenous PS across the plasma membrane. Thus, while it seems likely that the P4-ATPases are primarily required to establish and maintain plasma membrane asymmetry, it will be difficult to prove this point by genetic analyses.
5. Roles for Drs2p-Cdc50p in protein transport
Insertion of exogenous phospholipid into the outer leaflet of erythrocytes and its subsequent translocation to the inner leaflet by the aminophospholipid translocase induces substantial changes in membrane structure consistent with the bilayer couple hypothesis [33, 38]. This seminal observation suggested that flippases could drive membrane bending towards the cytosol by expanding the cytosolic leaflet of the bilayer while reducing phospholipid number in the exofacial leaflet. Thus, it was proposed that flippases might contribute to vesicle-mediated protein transport by facilitating the generation of tight membrane curvature inherent in this process [39]. Indeed, application of transport substrates into the outer leaflet of the plasma membrane stimulated endocytosis concomitant with translocation of the “extra” phospholipid to the cytosolic leaflet [40].
The first indication that P4-ATPases might be part of the normal protein trafficking machinery came from a genetic screen designed to discover proteins that function with the small GTP-binding protein Arf in vesicle budding from the Golgi complex [41]. Arf-GTP plays an essential role in recruiting vesicle coat proteins from the cytosol to induce their assembly on the Golgi membrane surface. COPI and clathrin with associated adaptors were well-characterized coat proteins recruited by Arf [42]. For reasons that are unclear, this arf1 synthetic lethal screen appears to have been biased towards defects in clathrin function. For example, the clathrin heavy chain gene and SWA2, encoding the yeast auxilin ortholog required for uncoating clathrin-coated vesicles, were recovered [43, 44]. DRS2 and CDC50 also came out of this screen [11, 41], although at the time there was no indication that a P-type ATPase, or even an integral membrane protein, might be involved in clathrin function. Genetic interactions were found between drs2, arf and clathrin mutant alleles, but not between drs2 and COPI or COPII mutations [41]. Consistent with these genetic interactions, COPI and COPII-dependent protein transport pathways are unaffected by loss of Drs2p, but clathrin-dependent pathways are strongly perturbed [28, 41, 45].
Drs2p localizes predominantly to the TGN and Drs2p-deficient cells are morphologically very similar to clathrin mutants. Both clathrin and drs2 mutants accumulate large (200 – 250nm diameter) cup-shaped membranes, which would be expected for mutants with a defect in vesicle budding [41]. In contrast, mutants with a defect in vesicle targeting or fusion accumulate 50 –100 nm vesicles. For example, perturbation of the actin cytoskeleton disrupts polarized exocytosis causing an accumulation of post-Golgi exocytic vesicles carrying the secretory cargo invertase. Drs2p and clathrin are both required for budding of these post-Golgi vesicles as inactivation of temperature-conditional mutants (drs2-ts or chc1-ts) prevents their formation (Fig. 2) [46]. At least two classes of exocytic vesicles, distinguished by density and cargo, have been described in yeast [47, 48]. Blocking of one vesicle class leads to rerouting of cargo into the second, and so secretion of invertase is slowed, but not blocked in clathrin or Drs2p deficient cells [28]. In addition, subcellular fractions containing clathrin from drs2Δ cells are deficient for clathrin-coated vesicles, particularly when cells were shifted to a nonpermissive growth temperature (15°C) before lysis, and instead contain empty clathrin baskets and clathrin lattices [41]. These observations support a model whereby Drs2p facilitates formation of clathrin-coated vesicles. But clathrin is required for several different protein transport pathways and so which pathways require Drs2p?
Figure 2.
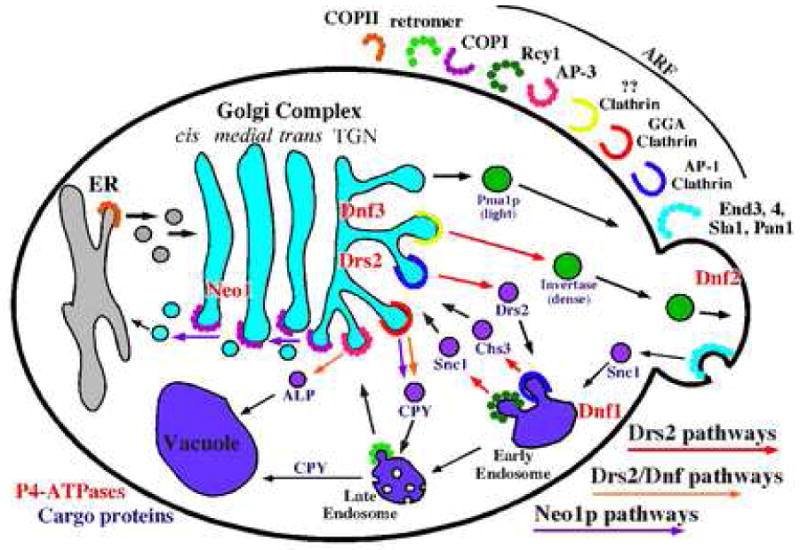
P4-ATPase requirements for vesicle-mediated protein transport in the secretory and endocytic pathways. Transport pathways are defined by cargo protein (purple) traveling the route and the vesicle coat protein (labeled in the upper right quadrant) required for sorting and transport of the cargo. The coat requirement for early endosome to TGN recycling pathway traveled by Snc1 is indicated by Rcy1, although it is not known if Rcy1 is a vesicle coat constituent. Pathways with known P4-ATPase requirements are indicated with colored arrows.
Clathrin-coated vesicles bud from the TGN, early endosomes and plasma membrane [49]. Instead of binding cargo proteins directly, clathrin uses adaptor proteins to sort cargo into different pathways. The tetrameric clathrin adaptor protein AP-1 (β1-, γ-, μ1-, σ1-adaptin) is recruited by Arf to TGN and early endosome membranes, and mediates bidirectional traffic between these organelles [45, 50]. A set of functionally overlapping, monomeric clathrin adaptor proteins, Gga1p and Gga2p (Golgi associated, Gamma-ear containing, Arf-binding), localize to the TGN and sort cargos into a late endosome pathway [51, 52]. Perhaps functioning as clathrin co-adaptors, the epsin-related proteins, Ent3p and Ent5p, bind to the common gamma-ear motif in AP-1 and GGA, and appear to facilitate the function of these clathrin adaptors [53, 54]. AP-3 (β3-, δ-, μ3-, σ3-adaptin) is also recruited by Arf and in yeast mediates transport of cargo from the Golgi directly to the vacuole. Surprisingly, AP-3 functions independently of clathrin in yeast as cargos that require AP-3 for vacuolar transport, such as alkaline phosphatase (ALP), are sorted normally in clathrin mutants [55]. In mammals, the AP-2 clathrin adaptor acts in receptor-mediated endocytosis at the plasma membrane [49], although there is no known function for AP-2 in yeast [56].
Drs2p plays a major role in driving bidirectional transport between the TGN and early endosome in pathways mediated by AP-1/clathrin and the less well-defined Rcy1 pathway (Fig 2). Loss of Drs2p function mimics AP-1 deficiency in several ways. Disruption of AP-1 subunit genes or GGA genes does not perturb growth of yeast. However, combining AP-1 mutations with gga1Δ gga2Δ causes a severe growth defect [51], supporting the model that AP-1 and GGAs mediate parallel pathways to endosomes and at least one of these pathways must be functional to sustain wild-type growth rates. Similarly, drs2Δ gga1Δ gga2Δ strains (or cdc50Δ gga1Δ gga2Δ strains) are severely compromised for growth [45, 57], but combining an AP-1 subunit (γ-adaptin) deletion with drs2Δ causes no additional growth defect [45]. These observations imply that AP-1 function is already lost in the drs2Δ single mutant, and so deletion of AP-1 in this background is inconsequential. Conversely, GGA function must be retained in drs2Δ, at least at 30°C, as the single mutant grows well at this temperature and the GGA genes become essential in this background. However, as described below, it appears that GGA/clathrin function is perturbed in drs2Δ single mutants at nonpermissive growth temperatures of 20°C or below [45].
Chitin synthase III (Chs3p) is a cargo protein in yeast that requires AP-1 for trafficking in the TGN-endosomal system. In wild-type cells, it appears that Chs3p is primarily retained intracellularly by cycling between the TGN and early endosomes. As cells progress into the G1 phase of the cell cycle, a novel coat complex sorts Chs3p to incipient bud sites at the plasma membrane. Disruption of AP-1 perturbs the normal intracellular cycling pattern of Chs3p causing enhanced and unregulated cell surface expression and increased trafficking into the late endosomes [58–60]. Consistent with a requirement for Drs2p in budding AP-1/clathrin-coated vesicles, Drs2-deficient cells exhibit these same defects in Chs3p trafficking [45].
Drs2p itself appears to be a cargo of AP-1/clathrin-coated vesicles (AP-1/CCVs) that bud from the TGN (Fig. 2). Because the TGN is reported to be a transient organelle with a half-life of only a few minutes [61, 62], it is likely that Drs2p also cycles through the endosomal system to maintain its steady-state TGN localization. Unlike other TGN resident proteins, Drs2p does not appreciably traffic through the late endosomes and so its primary trafficking pattern is probably between the TGN and early endosome. In wild-type cells, a relatively small percentage of Drs2p escapes to the plasma membrane, but the presence of multiple endocytosis signals ensures a rapid retrieval back to the TGN [63]. However, disruption of AP-1 subunit genes causes wholesale rerouting of Drs2p to the plasma membrane, where it can be trapped behind an endocytosis block [45]. This observation indicates that AP-1 is needed to remove Drs2p from the exocytic pathway, presumably by its incorporation into AP-1/CCVs targeted to the early endosome. However, another interesting possibility is that AP-1/CCVs mediate retrieval of Drs2p to earlier Golgi cisternae. Removal of the endocytosis block allows efficient retrieval of Drs2p back to the TGN in AP-1 mutants without an apparent increase in trafficking into the late endosome. Therefore AP-1 is not required for early endosome to TGN transport of Drs2p, even though both Drs2p and AP-1 appear to be required for early endosome to TGN transport of Chs3p [45].
Importantly, the enzymatic activity of Drs2p appears to drive its own transport in AP-1/CCVs. As described above, AP-1 function is lost in drs2Δ cells and so one would predict that inactivation of Drs2p activity would lead to the missorting of the dead enzyme to the plasma membrane. To test this prediction, GFP was appended onto the N-terminus of Drs2-ts to assess the trafficking of the mutant protein before and after inactivation of its enzymatic activity. At the permissive temperature, GFP-Drs2-ts was retained normally in the TGN, but a shift to the nonpermissive temperature caused enhanced transport to the plasma membrane, comparable to inactivation of AP-1 [45]. These observations support the model that Drs2p-dependent flippase activity drives formation of AP-1/CCVs. Interestingly, formation of the post-Golgi exocytic vesicles described above is partially perturbed by loss of AP-1, but is unaffected by AP-2, AP-3 or GGA mutations [46]. The influence of AP-1 on this pathway is probably an indirect effect of perturbing Drs2p trafficking, based on the assumption that the vesicle formation defect would be more severe in AP-1 mutants (comparable to clathrin or drs2 mutants) if they were bona fide AP-1/CCVs.
The role of Drs2p in supporting the function of AP-1 and clathrin is independent of coat recruitment to the TGN. This was a surprising result because Drs2p interacts directly with Gea2p, a cytosolic Arf guanine nucleotide exchange factor (ArfGEF) [64]. This interaction suggested that Drs2p could potentially recruit Gea2p to the TGN, where Gea2p would catalyze exchange of GDP on Arf for GTP. ArfGTP would then associate with the TGN and recruit AP-1 and clathrin to initiate assembly of the clathrin coat. However, this model is clearly wrong as Gea2p, AP-1 and clathrin are efficiently recruited to the TGN in drs2Δ cells [45, 64]. Yet it appears that AP-1/CCVs are not generated in drs2Δ cells. This observation implies that the assembly of clathrin is insufficient to drive vesicle budding in the absence of Drs2p. It also seems unlikely that vesicle budding is blocked at the late stage of membrane scission because no budding profiles are seen on accumulated membranes in electron micrographs of drs2Δ cells. By comparison, the shibire (dynamin) Drosphila mutant displays a temperature-conditional block in clathrin-mediated endocytosis and accumulates deeply invaginated clathrin-coated pits [65]. We favor a model whereby Drs2p imparts curvature to the TGN membrane by pumping phospholipid to the cytosolic leaflet. Clathrin would then capture the curved membranes by a Brownian ratchet mechanism and mold the membrane into a vesicle [23].
Recycling of proteins from the early endosome to the TGN is strongly dependent on Drs2p and Cdc50p (Fig. 2). The SNARE protein Snc1p continuously cycles between the TGN, plasma membrane, early endosome and back to the TGN [66]. In wild-type cells, the kinetically slow step in this path is endocytosis and so most of GFP-Snc1 is observed primarily at the plasma membrane of the bud, as exocytosis is polarized to the bud membrane. Both drs2Δ and cdc50Δ mutants accumulate GFP-Snc1p in cytoplasmic punctate organelles, indicating that an internal trafficking step has become rate-limiting [27, 28]. For cdc50Δ mutants, GFP-Snc1 was shown to accumulate in early endosomes [27] and this is likely to be the case for drs2Δ as well. The F-box protein Rcy1p, the sorting nexin Snx4p, Ypt31/32p (rab11 homologues), Arf and the Arf GTPase activating protein (ArfGAP) Gcs1p are all required at this same early endosome to TGN transport step traveled by Snc1p. Moreover, Cdc50p uses this route as well since rcy1Δ causes accumulation of Cdc50p in the early endosomes [27].
There is suggestive evidence that this Rcy1 pathway may be mediated by COPI [67], although more studies are needed to determine if these vesicles are COPI-coated, clathrin-coated, or use a novel coat structure. Rcy1p binds Skp1 of the Skp1-cullin-F-box (SCF) complex, but does not appear to form an active ubiquitin ligase complex [68]. The precise function of Rcy1p in protein transport is uncertain, but it is seems to be a downstream effector of the Rab proteinsYpt31/32p [69] and is closely linked to Drs2p/Cdc50p function [27]. Rcy1 co-immunoprecipitates with Drs2p and Cdc50p, and the rcy1Δ mutant has a cold-sensitive growth defect, reminiscent of drs2Δ or cdc50Δ. Comparable to the situation with AP-1 and Drs2p, deleting RCY1 in the cdc50Δ background does not exacerbate the cdc50Δ growth defect [27]. These observations strongly imply that Drs2p/Cdc50p acts in a common trafficking pathway with Rcy1p, and that Drs2p/Cdc50p activity drives formation of the “Rcy1” vesicles that bud from early endosomes and are targeted to the TGN. Drs2p/Cdc50p is likely a cargo of the Rcy1 vesicles it helps generate.
The AP-1, Rcy1 and exocytic vesicle pathways are disrupted in drs2Δ cells in spite of the presence of four other P4-ATPases with partially overlapping function [45]. Thus, it appears that the Dnf or Neo1p ATPases cannot compensate for the loss of Drs2p in these pathways. However, functional overlap between P4-ATPases is observed in the trafficking of proteins from the TGN to late endosome, a GGA-dependent pathway, and the AP-3-dependent transport of ALP from the Golgi to the vacuole (Fig. 2). Transport of ALP to the vacuole occurs efficiently in drs2Δ or dnf1Δ single mutants, but is strongly perturbed in the drs2Δ dnf1Δ double mutant [28]. Dnf1p normally cycles between the plasma membrane and the TGN/endosomal system and so it is a transient occupant of the Golgi. Mutation of an NPFXD endocytosis signal in Dnf1p increases its occupancy at the plasma membrane and disrupts its ability to support ALP transport to the vacuole [63]. This observation argues for a direct role for Dnf1p at the Golgi in forming the AP-3 vesicles, because providing the plasma membrane function of Dnf1p is not sufficient to allow normal trafficking of ALP to the vacuole.
The influence of P4-ATPases on protein trafficking through the late endosome is more complex. Carboxypeptidase Y (CPY) is a vacuolar protein that is captured by a receptor (Vps10p) in the TGN and sorted to the late endosome prior to vacuolar delivery. Upon arrival in the vacuole, the Golgi modified precursor form of CPY (p2 CPY) is processed to its mature form (mCPY). Deletion of the GGA genes causes partial secretion of p2 CPY and kinetically delays the maturation of the remainder of p2 CPY that is retained in the cell. It is thought that the kinetic delay in maturation reflects the rerouting of the p2 CPY-Vps10 complex through either the early endosome or the plasma membrane, and its longer route to the vacuole through the endocytic pathway [51, 70, 71]. Deletion of DRS2 also causes a three-fold kinetic delay in CPY maturation, but only when cells are shifted to a nonpermissive growth temperature. However, drs2Δ dnf1Δ cells display a similar CPY delay at permissive growth temperatures [28]. One interpretation of these results is that budding of GGA/clathrin-coated vesicles requires either Drs2p or another P4-ATPases at 30°C, but at lower temperatures the other P4-ATPases cannot support this pathway in the absence of Drs2p. This could provide an explanation for why drs2Δ cells exhibit an unusually strong cold-sensitive growth defect. The constitutive loss of the AP-1 and Rcy1p pathways in drs2Δ cells can be tolerated as long as the GGA pathway is functioning. However, additional perturbation of the GGA pathway when drs2Δ cells are shifted to low temperature would abrogate growth.
6. Neo1p pathways
Neo1p is also linked to the GGA pathway for CPY transport (Fig. 2). Inactivation of temperature conditional neo1 alleles (neo1-ts) slows the kinetics of CPY transport to the vacuole [28, 72]. In addition, Gga2p accumulates on punctate membrane structures in neo1-ts cells more so than in wild-type cells. This phenotype could be caused by normal recruitment of the GGA/clathrin coat and a defect in the budding of vesicles. Neo1p is physically linked to GGA through Mon2p (also called Ysl2p), a cytosolic protein with sequence similarity to ArfGEFs. Mon2p binds to Neo1p and the Arf-related protein Arl1p, although it is not known if Mon2p can mediate nucleotide exchange on Arl1p. Mon2p also binds directly to Gga1p and Gga2p through their VHS (cargo-binding) domains. In contrast to neo1-ts, Arl1p- or Mon2p-deficient yeast show a decreased association of Gga proteins with membranes, implying that Arl1p cooperates with Arf1p to recruit Gga proteins to the Golgi [72].
Neither the neo1-ts nor the drs2Δ dnf1Δ strains exhibit quite as strong of a CPY transport defect as the gga1Δ gga2Δ strain. Thus, it is possible that all three of these P4-ATPases can contribute to the budding of GGA vesicles. Neo1p appears to have a steady-state localization to Golgi membranes [28, 73], but trafficks through the late endosome where it can be trapped by mutations that block egress out of this compartment (class E vps mutations) [74]. It will be interesting to determine if Neo1p requires Mon2p and Gga proteins for it’s trafficking into the late endosome, and if Neo1p is incorporated into the GGA/CCVs it helps generate. In contrast to the essential NEO1 gene, GGA, MON2 and ARL1 genes are all nonessential, and their disruption in the neo1-ts background has the surprising consequence of suppressing the neo1-ts temperature-sensitive growth defect [72]. One possible interpretation of this result is that a nonproductive Neo1p-ts interaction with Mon2/Arl1p/Gga depletes Neo1p-ts from sites where it is needed for an essential function. Neo1p is also implicated in COPI-dependent protein transport, which is an essential pathway in yeast.
COPI mediates retrograde transport of proteins from the Golgi to the ER and the COPII coat mediates the forward ER to Golgi step [75]. Inactivation of temperature-sensitive alleles of COPI subunits (e.g. sec21-1) perturbs retrograde transport and COPI cargo is swept to downstream compartments (the plasma membrane or vacuole) [76]. Rer1p is a Golgi-localized protein that constantly cycles between the ER and the Golgi, and is therefore a cargo of both COPI and COPII vesicle. COPII mutants accumulate Rer1-GFP in the ER while in COPI mutants, Rer1-GFP is mislocalized to the vacuole [77, 78]. The neo1-ts mutant also mislocalizes Rer1-GFP to the vacuole when these cells are shifted to the nonpermissive temperature [74, 79]. This result implies that anterograde transport steps are not perturbed by neo1-ts inactivation, but COPI-dependent retrograde transport is disrupted. Consistent with this observation, no genetic interactions were observed between neo1-ts and COPII mutations (sec12-4, sec23-1) or mutations perturbing later anterograde transport steps (sec7, sec14, sec1), but neo1-ts COPI (sec21-1, ret1-1) double mutants were either inviable or very slow growing [79].
7. Links between P4-ATPases and sterol metabolism or localization
Several recent reports have linked P4-ATPase function to sterol metabolism or localization [80–84]. A synthetic lethal screen uncovered a strong genetic interaction between cdc50Δ and mutations affecting the late steps of ergosterol biosynthesis (in order of action in ergosterol synthesis: erg6, erg2, erg3, erg5). Disruption of DRS2 or CDC50 has no effect on ergosterol levels or the distribution of accumulated biosynthetic intermediates in an erg3Δ cdc50 mutant (using a galactose regulated promoter to shut off CDC50 expression) [82]. Thus, Drs2p-Cdc50p does not contribute to ergosterol synthesis. Addition of glucose to shut off expression of PGal1-CDC50 slowly induces a defect in Snc1p recycling as Cdc50p is depleted and Drs2p accumulates in the ER. This Snc1p recycling defect appears much faster after glucose addition to erg3Δ PGal1-CDC50 cells, implying that alteration of cellular sterol has a repressive effect on Drs2p-Cdc50p function [82]. The synthetic lethality between cdc50Δ and erg3Δ also implies that alteration of sterol also has a repressive effect on Dnf function, or a parallel trafficking pathway (for example, cdc50Δ erg3Δ phenocopies cdc50Δ dnf1,2,3Δ or cdc50Δ gga1Δ gga2Δ).
A recent study indicates that the sterol-binding protein Kes1p (also called Osh4p) mediates the repressive effect of erg mutations on Drs2p-deficient cells [84]. Kes1p is a member of the oxysterol binding protein family, which includes seven members in yeast (Osh1p-Osh7p) [85]. The oxysterol binding proteins have been implicated in nonvesicular sterol transport, sterol-dependent signal transduction, and repression of vesicle-mediated protein transport [86]. A screen for suppressors of the cold-sensitive growth defect of drs2Δ uncovered loss of function kes1 mutations. The drs2Δ kes1Δ double mutant grows as well as wild-type cells at 17°C, a temperature that inhibits growth of drs2Δ cells. Disruption of KES1 also suppresses the synthetic lethality between drs2Δ and erg6Δ. Therefore, removal of Kes1p completely alleviates the repressive affect that altering sterol structure has on drs2Δ cells [84].
Loss of function kes1 alleles were previously isolated as suppressors of kre11 and sec14 [87, 88]. Kre11p is a subunit of a nucleotide exchange factor for Ypt31p and Ypt32p, the yeast Rab11 homologues implicated in the Snc1p recycling pathway and the formation of exocytic vesicles [69, 89]. Sec14p is a phospholipid transfer protein that is required for budding exocytic vesicles [90]. These discoveries implied that Kes1p has a repressive effect on vesicle budding from the TGN/early endosome system. kes1Δ suppresses the sec14 defect in budding exocytic vesicles [91], but surprisingly kes1Δ does not suppress the drs2Δ defect in budding post-Golgi exocytic vesicles. Moreover, kes1Δ does not suppress the Snc1p recycling or AP-1 defects of drs2Δ. However, kes1Δ does suppress the cold-sensitive delay in CPY maturation (GGA pathway defect) observed in drs2Δ. Strikingly, kes1Δ only suppresses a trafficking pathway in drs2Δ where other P4-ATPases can act. In addition, kes1Δ will not suppress drs2 dnf1,2,3Δ lethality, suggesting that Dnf P4-ATPases must be present to allow kes1Δ suppression of drs2Δ [84]. These results suggest that Kes1p inhibits P4-ATPase activity at the Golgi complex.
While direct evidence that Kes1p represses Dnf or Neo1p activity at the Golgi is lacking, there is evidence that Kes1p represses Drs2p activity at the TGN. kes1Δ TGN membranes display about twice the NBD-PS flippase activity of wild-type membranes and the enhanced NBD-PS flippase activity in membranes lacking Kes1p appears to be catalyzed by Drs2p. Remarkably, addition of recombinant Kes1p inhibited the “extra” NBD-PS flippase activity to wild-type levels with a half-maximal inhibitory concentration in the range of only 10 – 100 pM. This represents a stoichiometry of 1 – 10 Kes1p molecules for every 1000 Drs2p molecules, suggesting that Kes1p is acting through an enzymatic intermediate, perhaps a kinase or phosphatase, to attenuate Drs2p activity. Drs2p is not known to be subject to regulatory phosphorylation; however, a recent report suggests that a pair of novel protein kinases, Fpk1p and Fpk2p, regulate the activity of Dnf1p and Dnf2p [92]. Kes1p also appears to repress Drs2p activity in vivo. While kes1Δ will not allow growth of drs2Δ dnf1,2,3Δ cells, it will suppress the temperature-sensitive growth defect of drs2-ts dnf1,2,3Δ cells, suggesting that removal of Kes1p improves the activity of Drs2-ts [84].
Another surprising observation is that Drs2p antagonizes the influence of Kes1p on nonvesicular sterol transport in vivo. Kes1p binds a variety of sterols, including oxysterols (25-hydroxycholesterol) and cholesterol, and can mediate a slow exchange of sterols between separate lipid bilayers in vitro [93]. Kes1p- and Osh-deficient cells also display a defect in the nonvesicular transport of exogenously applied cholesterol from the plasma membrane to the ER, where it is esterified by acyl-coenzyme A: cholesterol acyltransferase [94]. The rate of exogenously applied cholesterol esterification is ~5-fold faster in drs2Δ cells relative to wild-type cells. However, drs2Δ kes1Δ cells display a wild-type rate of cholesterol esterification. These results imply that Kes1p is hyperactive in drs2Δ cells and facilitates an enhanced rate of sterol transport from the plasma membrane to internal membranes. Consistent with this possibility, filipin staining of drs2Δ cells shows a substantial reduction of plasma membrane fluorescence and enhanced intracellular staining. This defect is suppressed by deletion of kes1Δ, suggesting that Drs2p and Kes1p have a significant impact on the distribution of endogenous ergosterol between cellular membranes [84].
Our speculative model to explain these results is that Drs2p-Cdc50p helps establish a membrane phospholipid organization in the TGN-endosomal system that traps ergosterol in the membrane, and these sterol rich membrane domains (or “rafts”) are selected for export to the plasma membrane. Sterol molecules in a disorganized membrane would have a greater tendency to escape and associate with Kes1p (the most abundant Osh protein in yeast). Sterol-bound Kes1p would then inhibit P4-ATPase activity in the TGN-endosomal system and would also have the potential to redistribute sterol to other membranes (i.e. the ER). Consistent with this possibility, scrambling of erythrocyte plasma membrane phospholipids has been shown to increase the escape tendency of cholesterol as measured by the rate of transfer to cyclodextrin [95]. Why would cells evolve a mechanism to downregulate the activity of P4-ATPases? Inherent in this model is the assumption that there is an ideal setpoint for the rate of phospholipid translocation by P4-ATPases that is optimal for both vesicle biogenesis and establishment of sterol-rich domains. Increasing P4-ATPase activity beyond this setpoint could induce too much curvature in the membrane placing stress on the bilayer detrimental to membrane organization and sterol retention. Perhaps Kes1p evolved the ability to sense membrane defects associated with hyperactivity of P4-ATPases to provide homeostatic feedback control of flippase activity.
8. Concluding remarks
The antagonistic relationship between Drs2p and Kes1p suggests an intricate regulatory network that couples multiple events in membrane biogenesis: vesicle-mediated protein transport, the establishment of phospholipid asymmetry and the control of membrane sterol content. This review has focused on advances made on understanding the function of P4-ATPases in the budding yeast system. However, the P4-ATPase family is highly conserved among eukaryotes and evidence is accumulating that P4-ATPase deficiency causes phenotypes in plants and animals similar to those observed with yeast. These include growth defects of plants at low temperature [96], loss of PS asymmetry of the plasma membrane [7, 97], defects in sterol trafficking and/or metabolism [81, 83], and perturbation of vesicle-mediated protein transport [98, 99].
Acknowledgments
Studies on the function of P4-ATPases in our laboratory is supported by NIH grant RO1 GM62367.
References
- 1.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 2.Op den Kamp JA. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok VA. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 4.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 5.Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J Cell Biol. 2000;149:1215–1224. doi: 10.1083/jcb.149.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwaal RF, Comfurius P, van Deenen LL. Membrane asymmetry and blood coagulation. Nature. 1977;268:358–360. doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]
- 7.Darland-Ransom M, Wang X, Sun CL, Mapes J, Gengyo-Ando K, Mitani S, Xue D. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science. 2008;320:528–531. doi: 10.1126/science.1155847. [DOI] [PubMed] [Google Scholar]
- 8.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 9.Cerbon J, Calderon V. Generation, modulation and maintenance of the plasma membrane asymmetric phospholipid composition in yeast cells during growth: their relation to surface potential and membrane protein activity. Biochim Biophys Acta. 1995;1235:100–106. doi: 10.1016/0005-2736(94)00311-c. [DOI] [PubMed] [Google Scholar]
- 10.Kato U, Emoto K, Fredriksson C, Nakamura H, Ohta A, Kobayashi T, Murakami-Murofushi K, Kobayashi T, Umeda M. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. J Biol Chem. 2002;277:37855–37862. doi: 10.1074/jbc.M205564200. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Wang J, Muthusamy BP, Liu K, Zare S, Andersen RJ, Graham TR. Roles for the Drs2p-Cdc50p complex in protein transport and phosphatidylserine asymmetry of the yeast plasma membrane. Traffic. 2006;7:1503–1517. doi: 10.1111/j.1600-0854.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 12.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 13.Paulusma CC, Oude Elferink RP. The type 4 subfamily of P-type ATPases, putative aminophospholipid translocases with a role in human disease. Biochim Biophys Acta. 2005;1741:11–24. doi: 10.1016/j.bbadis.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Dhar MS, Sommardahl CS, Kirkland T, Nelson S, Donnell R, Johnson DK, Castellani LW. Mice heterozygous for Atp10c, a putative amphipath, represent a novel model of obesity and type 2 diabetes. J Nutr. 2004;134:799–805. doi: 10.1093/jn/134.4.799. [DOI] [PubMed] [Google Scholar]
- 15.Flamant S, Pescher P, Lemercier B, Clement-Ziza M, Kepes F, Fellous M, Milon G, Marchal G, Besmond C. Characterization of a putative type IV aminophospholipid transporter P-type ATPase. Mamm Genome. 2003;14:21–30. doi: 10.1007/s00335-002-3032-3. [DOI] [PubMed] [Google Scholar]
- 16.Paterson JK, Renkema K, Burden L, Halleck MS, Schlegel RA, Williamson P, Daleke DL. Lipid specific activation of the murine P4-ATPase Atp8a1 (ATPase II) Biochemistry. 2006;45:5367–5376. doi: 10.1021/bi052359b. [DOI] [PubMed] [Google Scholar]
- 17.Ding J, Wu Z, Crider BP, Ma Y, Li X, Slaughter C, Gong L, Xie XS. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J Biol Chem. 2000;275:23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 18.Soupene E, Kuypers FA. Identification of an erythroid ATP-dependent aminophospholipid transporter. Br J Haematol. 2006;133:436–438. doi: 10.1111/j.1365-2141.2006.06051.x. [DOI] [PubMed] [Google Scholar]
- 19.Pomorski T, Lombardi R, Riezman H, Devaux PF, van Meer G, Holthuis JC. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alder-Baerens N, Lisman Q, Luong L, Pomorski T, Holthuis JC. Loss of P4 ATPases Drs2p and Dnf3p disrupts aminophospholipid transport and asymmetry in yeast post-Golgi secretory vesicles. Mol Biol Cell. 2006;17:1632–1642. doi: 10.1091/mbc.E05-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natarajan P, Wang J, Hua Z, Graham TR. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc Natl Acad Sci U S A. 2004;101:10614–10619. doi: 10.1073/pnas.0404146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elvington SM, Bu F, Nichols JW. Fluorescent, acyl chain-labeled phosphatidylcholine analogs reveal novel transport pathways across the plasma membrane of yeast. J Biol Chem. 2005;280:40957–40964. doi: 10.1074/jbc.M507926200. [DOI] [PubMed] [Google Scholar]
- 23.Graham TR. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 2004;14:670–677. doi: 10.1016/j.tcb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 25.Toyoshima C, Mizutani T. Crystal structure of the calcium pump with a bound ATP analogue. Nature. 2004;430:529–535. doi: 10.1038/nature02680. [DOI] [PubMed] [Google Scholar]
- 26.Saito K, Fujimura-Kamada K, Furuta N, Kato U, Umeda M, Tanaka K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:3418–3432. doi: 10.1091/mbc.E03-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuta N, Fujimura-Kamada K, Saito K, Yamamoto T, Tanaka K. Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol Biol Cell. 2007;18:295–312. doi: 10.1091/mbc.E06-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hua Z, Fatheddin P, Graham TR. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol Biol Cell. 2002;13:3162–3177. doi: 10.1091/mbc.E02-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson PK, Malone L, Birchmore JL, Nichols JW. Lem3p is essential for the uptake and potency of alkylphosphocholine drugs, edelfosine and miltefosine. J Biol Chem. 2003;278:36041–36050. doi: 10.1074/jbc.M305263200. [DOI] [PubMed] [Google Scholar]
- 30.Stevens HC, Malone L, Nichols JW. The Putative Aminophospholipid Translocases, DNF1 and DNF2, Are Not Required for 7-Nitrobenz-2-oxa-1,3-diazol-4-yl-phosphatidylserine Flip across the Plasma Membrane of Saccharomyces cerevisiae. J Biol Chem. 2008;283:35060–35069. doi: 10.1074/jbc.M802379200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riekhof WR, Wu J, Gijon MA, Zarini S, Murphy RC, Voelker DR. Lysophosphatidylcholine metabolism in Saccharomyces cerevisiae: the role of P-type ATPases in transport and a broad specificity acyltransferase in acylation. J Biol Chem. 2007;282:36853–36861. doi: 10.1074/jbc.M706718200. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Victoria FJ, Sanchez-Canete MP, Castanys S, Gamarro F. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J Biol Chem. 2006;281:23766–23775. doi: 10.1074/jbc.M605214200. [DOI] [PubMed] [Google Scholar]
- 33.Daleke DL, Huestis WH. Incorporation and translocation of aminophospholipids in human erythrocytes. Biochemistry. 1985;24:5406–5416. doi: 10.1021/bi00341a019. [DOI] [PubMed] [Google Scholar]
- 34.Smriti EC, Nemergut, Daleke DL. ATP-dependent transport of phosphatidylserine analogues in human erythrocytes. Biochemistry. 2007;46:2249–2259. doi: 10.1021/bi061333x. [DOI] [PubMed] [Google Scholar]
- 35.Niggli V, Sigel E. Anticipating antiport in P-type ATPases. Trends Biochem Sci. 2008;33:156–160. doi: 10.1016/j.tibs.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Obara K, Miyashita N, Xu C, Toyoshima I, Sugita Y, Inesi G, Toyoshima C. Structural role of countertransport revealed in Ca(2+) pump crystal structure in the absence of Ca(2+) Proc Natl Acad Sci U S A. 2005;102:14489–14496. doi: 10.1073/pnas.0506222102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsons AB, Lopez A, Givoni IE, Williams DE, Gray CA, Porter J, Chua G, Sopko R, Brost RL, Ho CH, Wang J, Ketela T, Brenner C, Brill JA, Fernandez GE, Lorenz TC, Payne GS, Ishihara S, Ohya Y, Andrews B, Hughes TR, Frey BJ, Graham TR, Andersen RJ, Boone C. Exploring the mode-of-action of bioactive compounds by chemical-genetic profiling in yeast. Cell. 2006;126:611–625. doi: 10.1016/j.cell.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 38.Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 40.Farge E, Ojcius DM, Subtil A, Dautry-Varsat A. Enhancement of endocytosis due to aminophospholipid transport across the plasma membrane of living cells. Am J Physiol. 1999;276:C725–733. doi: 10.1152/ajpcell.1999.276.3.C725. [DOI] [PubMed] [Google Scholar]
- 41.Chen CY, Ingram MF, Rosal PH, Graham TR. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J Cell Biol. 1999;147:1223–1236. doi: 10.1083/jcb.147.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donaldson JG, Jackson CL. Regulators and effectors of the ARF GTPases. Curr Opin Cell Biol. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 43.Chen CY, Graham TR. An arf1Delta synthetic lethal screen identifies a new clathrin heavy chain conditional allele that perturbs vacuolar protein transport in Saccharomyces cerevisiae. Genetics. 1998;150:577–589. doi: 10.1093/genetics/150.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gall WE, Higginbotham MA, Chen C, Ingram MF, Cyr DM, Graham TR. The auxilin-like phosphoprotein Swa2p is required for clathrin function in yeast. Curr Biol. 2000;10:1349–1358. doi: 10.1016/s0960-9822(00)00771-5. [DOI] [PubMed] [Google Scholar]
- 45.Liu K, Surendhran K, Nothwehr SF, Graham TR. P4-ATPase requirement for AP-1/clathrin function in protein transport from the trans-Golgi network and early endosomes. Mol Biol Cell. 2008;19:3526–3535. doi: 10.1091/mbc.E08-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gall WE, Geething NC, Hua Z, Ingram MF, Liu K, Chen SI, Graham TR. Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr Biol. 2002;12:1623–1627. doi: 10.1016/s0960-9822(02)01148-x. [DOI] [PubMed] [Google Scholar]
- 47.Mulholland J, Wesp A, Riezman H, Botstein D. Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretary vesicle. Mol Biol Cell. 1997;8:1481–1499. doi: 10.1091/mbc.8.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 50.Hinners I, Tooze SA. Changing directions: clathrin-mediated transport between the Golgi and endosomes. J Cell Sci. 2003;116:763–771. doi: 10.1242/jcs.00270. [DOI] [PubMed] [Google Scholar]
- 51.Costaguta G, Stefan CJ, Bensen ES, Emr SD, Payne GS. Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol Biol Cell. 2001;12:1885–1896. doi: 10.1091/mbc.12.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black MW, Pelham HR. A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J Cell Biol. 2000;151:587–600. doi: 10.1083/jcb.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duncan MC, Payne GS. ENTH/ANTH domains expand to the Golgi. Trends Cell Biol. 2003;13:211–215. doi: 10.1016/s0962-8924(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 54.Copic A, Starr TL, Schekman R. Ent3p and Ent5p exhibit cargo-specific functions in trafficking proteins between the trans-Golgi network and the endosomes in yeast. Mol Biol Cell. 2007;18:1803–1815. doi: 10.1091/mbc.E06-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colours. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- 56.Yeung BG, Phan HL, Payne GS. Adaptor complex-independent clathrin function in yeast. Mol Biol Cell. 1999;10:3643–3659. doi: 10.1091/mbc.10.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakane H, Yamamoto T, Tanaka K. The functional relationship between the Cdc50p-Drs2p putative aminophospholipid translocase and the Arf GAP Gcs1p in vesicle formation in the retrieval pathway from yeast early endosomes to the TGN. Cell Struct Funct. 2006;31:87–108. doi: 10.1247/csf.06021. [DOI] [PubMed] [Google Scholar]
- 58.Wang CW, Hamamoto S, Orci L, Schekman R. Exomer: A coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J Cell Biol. 2006;174:973–983. doi: 10.1083/jcb.200605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trautwein M, Schindler C, Gauss R, Dengjel J, Hartmann E, Spang A. Arf1p, Chs5p and the ChAPs are required for export of specialized cargo from the Golgi. Embo J. 2006;25:943–954. doi: 10.1038/sj.emboj.7601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valdivia RH, Baggott D, Chuang JS, Schekman RW. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 61.Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- 62.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- 63.Liu K, Hua Z, Nepute JA, Graham TR. Yeast P4-ATPases Drs2p and Dnf1p are essential cargos of the NPFXD/Sla1p endocytic pathway. Mol Biol Cell. 2007;18:487–500. doi: 10.1091/mbc.E06-07-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chantalat S, Park SK, Hua Z, Liu K, Gobin R, Peyroche A, Rambourg A, Graham TR, Jackson CL. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci. 2004;117:711–722. doi: 10.1242/jcs.00896. [DOI] [PubMed] [Google Scholar]
- 65.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson M, Poon PP, Schindler C, Murray LE, Kama R, Gabriely G, Singer RA, Spang A, Johnston GC, Gerst JE. The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast. Mol Biol Cell. 2006;17:1845–1858. doi: 10.1091/mbc.E05-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galan JM, Wiederkehr A, Seol JH, Haguenauer-Tsapis R, Deshaies RJ, Riezman H, Peter M. Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol Cell Biol. 2001;21:3105–3117. doi: 10.1128/MCB.21.9.3105-3117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen SH, Chen S, Tokarev AA, Liu F, Jedd G, Segev N. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol Biol Cell. 2005;16:178–192. doi: 10.1091/mbc.E04-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirst J, Lui WW, Bright NA, Totty N, Seaman MN, Robinson MS. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J Cell Biol. 2000;149:67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deloche O, Schekman RW. Vps10p cycles between the TGN and the late endosome via the plasma membrane in clathrin mutants. Mol Biol Cell. 2002;13:4296–4307. doi: 10.1091/mbc.02-07-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singer-Kruger B, Lasic M, Burger AM, Hausser A, Pipkorn R, Wang Y. Yeast and human Ysl2p/hMon2 interact with Gga adaptors and mediate their subcellular distribution. Embo J. 2008;27:1423–1435. doi: 10.1038/emboj.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 74.Wicky S, Schwarz H, Singer-Kruger B. Molecular interactions of yeast Neo1p, an essential member of the Drs2 family of aminophospholipid translocases, and its role in membrane trafficking within the endomembrane system. Mol Cell Biol. 2004;24:7402–7418. doi: 10.1128/MCB.24.17.7402-7418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 76.Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 77.Sato K, Nishikawa S, Nakano A. Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER): characterization of the RER1 gene product as a component involved in ER localization of Sec12p. Mol Biol Cell. 1995;6:1459–1477. doi: 10.1091/mbc.6.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boehm J, Letourneur F, Ballensiefen W, Ossipov D, Demolliere C, Schmitt HD. Sec12p requires Rer1p for sorting to coatomer (COPI)-coated vesicles and retrieval to the ER. J Cell Sci. 1997;110(Pt 8):991–1003. doi: 10.1242/jcs.110.8.991. [DOI] [PubMed] [Google Scholar]
- 79.Hua Z, Graham TR. Requirement for neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum. Mol Biol Cell. 2003;14:4971–4983. doi: 10.1091/mbc.E03-07-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fei W, Alfaro G, Muthusamy BP, Klaassen Z, Graham TR, Yang H, Beh CT. Genome-wide analysis of sterol-lipid storage and trafficking in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:401–414. doi: 10.1128/EC.00386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groen A, Kunne C, Jongsma G, van den Oever K, Mok KS, Petruzzelli M, Vrins CL, Bull L, Paulusma CC, Oude Elferink RP. Abcg5/8 independent biliary cholesterol excretion in Atp8b1-deficient mice. Gastroenterology. 2008;134:2091–2100. doi: 10.1053/j.gastro.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 82.Kishimoto T, Yamamoto T, Tanaka K. Defects in structural integrity of ergosterol and the Cdc50p-Drs2p putative phospholipid translocase cause accumulation of endocytic membranes, onto which actin patches are assembled in yeast. Mol Biol Cell. 2005;16:5592–5609. doi: 10.1091/mbc.E05-05-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyssenko NN, Miteva Y, Gilroy S, Hanna-Rose W, Schlegel RA. An unexpectedly high degree of specialization and a widespread involvement in sterol metabolism among the C. elegans putative aminophospholipid translocases. BMC Dev Biol. 2008;8:96. doi: 10.1186/1471-213X-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muthusamy BP, Raychaudhuri S, Natarajan P, Abe F, Liu K, Prinz WA, Graham TR. Control of protein and sterol trafficking by antagonistc activtities of a P4-ATPase and oxysterol binding protein homologue. 2009. Manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beh CT, Cool L, Phillips J, Rine J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 2001;157:1117–1140. doi: 10.1093/genetics/157.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schulz TA, Prinz WA. Sterol transport in yeast and the oxysterol binding protein homologue (OSH) family. Biochim Biophys Acta. 2007;1771:769–780. doi: 10.1016/j.bbalip.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MK, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. Embo J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang B, Brown JL, Sheraton J, Fortin N, Bussey H. A new family of yeast genes implicated in ergosterol synthesis is related to the human oxysterol binding protein. Yeast. 1994;10:341–353. doi: 10.1002/yea.320100307. [DOI] [PubMed] [Google Scholar]
- 89.Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, Lipatova Z, Sciorra VA, Emr SD, Segev N. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol. 2006;8:1263–1269. doi: 10.1038/ncb1489. [DOI] [PubMed] [Google Scholar]
- 90.Mousley CJ, Tyeryar KR, Vincent-Pope P, Bankaitis VA. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim Biophys Acta. 2007;1771:727–736. doi: 10.1016/j.bbalip.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakano K, Yamamoto T, Kishimoto T, Noji T, Tanaka K. Protein kinases Fpk1p and Fpk2p are novel regulators of phospholipid asymmetry. Mol Biol Cell. 2008;19:1783–1797. doi: 10.1091/mbc.E07-07-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–158. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol. 2006;173:107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lange Y, Ye J, Steck TL. Scrambling of phospholipids activates red cell membrane cholesterol. Biochemistry. 2007;46:2233–2238. doi: 10.1021/bi6023397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gomes E, Jakobsen MK, Axelsen KB, Geisler M, Palmgren MG. Chilling tolerance in Arabidopsis involves ALA1, a member of a new family of putative aminophospholipid translocases. Plant Cell. 2000;12:2441–2454. [PMC free article] [PubMed] [Google Scholar]
- 97.Paulusma CC, Groen A, Kunne C, Ho-Mok KS, Spijkerboer AL, Rudi de Waart D, Hoek FJ, Vreeling H, Hoeben KA, van Marle J, Pawlikowska L, Bull LN, Hofmann AF, Knisely AS, Oude Elferink RP. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195–204. doi: 10.1002/hep.21212. [DOI] [PubMed] [Google Scholar]
- 98.Poulsen LR, Lopez-Marques RL, McDowell SC, Okkeri J, Licht D, Schulz A, Pomorski T, Harper JF, Palmgren MG. The Arabidopsis P4-ATPase ALA3 localizes to the golgi and requires a beta-subunit to function in lipid translocation and secretory vesicle formation. Plant Cell. 2008;20:658–676. doi: 10.1105/tpc.107.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruaud AF, Nilsson L, Richard F, Larsen MK, Bessereau JL, Tuck S. The C. elegans P4-ATPase TAT-1 regulates lysosome biogenesis and endocytosis. Traffic. 2008 doi: 10.1111/j.1600-0854.2008.00844.x. [DOI] [PubMed] [Google Scholar]


