Abstract
Background
Interferon-alpha (IFNα)–induced thyroid dysfunction occurs in up to 20% of patients undergoing therapy for hepatitis C. The diversity of thyroid disease presentations suggests that several different pathological mechanisms are involved, such as autoimmunity and direct toxicity. Elucidating the relationships between risk factors and disease phenotype provides insight into the mechanisms of disease pathophysiology.
Methods
We studied 869 euthyroid patients from the ACHIEVE 2/3 trial, a randomized international clinical trial comparing pegylated-IFNα2a weekly or albumin-IFNα2b every 2 weeks for up to 24 weeks in patients with hepatitis C, genotype 2 or 3, from 136 centers. The study population was 60% male and 55% white. Serum thyrotropin (TSH) and free thyroxine were measured before therapy, monthly during treatment from week 8, and at 4- and 12-week follow-up visits.
Results
Overall, 181 (20.8%) participants had at least one abnormal TSH during the study. Low TSH occurred in 71 (8.2%), of whom 30 (3.5%) had a suppressed TSH below 0.1 mU/L. Hypothyroidism occurred in 53 patients (6.1%), with peak TSH above 10 mU/L in 12 patients (1.4%). Fifty-seven patients had a biphasic thyroiditis (6.6%), with extreme values for the nadir and/or peak TSH in all but one. Medical therapy was given to one thyrotoxic patient, four hypothyroid patients, and 26 biphasic thyroiditis patients. Multivariate logistic regression analysis demonstrated that biphasic thyroiditis is associated with being female and higher pretreatment serum TSH, whereas being Asian or a current smoker decreased the risk of thyroiditis. Hypo- and hyperthyroidism are most strongly predicted by the pretreatment TSH.
Conclusions
Biphasic thyroiditis accounted for the majority (58%) of clinically relevant IFNα-induced thyroid dysfunction. We confirmed our recent findings in a related cohort that female sex is a risk factor for thyroiditis but not hypothyroidism. Further, in this large multiethnic study, the risk of thyroiditis is dramatically increased, specifically for white women. Smoking was found to be protective of thyroiditis. These results support closer monitoring of women and those with a serum TSH at the extremes of the normal range during therapy so that prompt intervention can mitigate the consequences of thyroid dysfunction associated with IFNα treatment.
Introduction
Interferon-alpha (IFNα) is a highly effective therapy for chronic hepatitis C infection, especially for infections with hepatitis C genotypes 2 or 3, but its use can be complicated by side effects with significant morbidity. Among these, thyroid dysfunction is a frequent and potentially severe and/or permanent complication. The rate of IFNα-induced thyroid dysfunction (IITD) depends on the definition, with 3% of treated patients diagnosed with symptom-triggered screening (1,2), while recent studies with systematic hormonal assessments report up to a 20% incidence of any abnormal serum thyrotropin (TSH) (3). IITD can present with diverse phenotypic patterns, including thyrotoxicosis, hypothyroidism, and biphasic thyroiditis, in which thyrotoxicosis is followed by hypothyroidism. Defining key prognostic factors for these patterns of thyroid dysfunction would help clinicians to identify prospectively high-risk patients who would benefit from increased monitoring during IFNα exposure, and provide mechanistic insight that could be useful in developing preventive strategies.
Female sex, thyroid peroxidase (TPO) antibody positivity, and higher pretreatment TSH are the IITD risk factors most consistently reported (1,4–23). Since these three variables are correlated in the general population (24,25), multivariate analysis of large studies is necessary to determine whether these are in fact independent predictors. Two groups have reached opposite conclusions using multivariate analyses that included sex and antibody status, finding in one study that only sex was a predictor of any IITD (21), while in the other, TPO antibodies predicted hypothyroidism (23). This contradiction may arise from a common limitation of most studies on IITD, the lack of stratification by phenotypic presentation. This results from both intentionally grouping distinct presentations, because of the need to have an adequate cell size in smaller studies, and unintentionally grouping outcomes by using 12-week intervals for assessment, which can misclassify a substantial proportion of biphasic thyroiditis by missing one of the transient abnormalities. This matters because, as several groups have proposed (3,26), the distinct phenotypes (thyrotoxicosis, hypothyroidism, and biphasic thyroiditis) could have distinct pathophysiologies, and therefore different risk factors.
We recently conducted a large multivariate analysis of over 1200 euthyroid patients treated with IFNα for hepatitis C genotype 1 (8). Although antibody status was not available, both sex and baseline TSH were important risk factors, and they conferred differential risks between the phenotypic subtypes of IITD. Both thyrotoxicosis and hypothyroidism were most strongly associated with pretreatment serum TSH levels such that risk increased as TSH increased or decreased, respectively. In contrast, female sex was associated with an almost ninefold increase in risk for biphasic thyroiditis only. This supports the hypothesis that different pathophysiological processes underlie the different phenotypes.
We undertook the current investigation to analyze additional risk factors using a more ethnically diverse cohort from the ACHIEVE 2/3 study. Half of the participants in ACHIEVE 2/3 were white, and over 40% were Asian, which allows us to address the finding in one prior study of an increased risk among Asians (21). In addition, the ACHIEVE 2/3 study was geographically diverse, with almost half of the subjects living in areas defined as having mild iodine deficiency and half in iodine-excess environments according to the World Health Organization (27,28). This study population is quite complex, with multiple subgroups; however, the large size allows for an investigation of statistical interaction effects to identify distinct subgroups with different risk profiles.
Materials and Methods
ACHIEVE study design and patient characteristics
Details of the original clinical trial design have been previously described (29). In brief, otherwise healthy adults with liver biopsy–proven chronic hepatitis C genotype 2 or 3 infection without prior IFNα therapy were recruited between 2007 and 2008 at 136 sites in India, Asia/Pacific, North and South America, and Europe. Informed consent was provided by all subjects and was monitored by the institutional review boards of the participating study sites. Exclusion criteria included pre-existing, overt untreated thyroid disease, psychiatric illnesses, or co-infection with HIV or hepatitis B. Patients were centrally randomized to one of three treatment arms with either pegylated-IFNα2a or albumin-IFNα2b at two doses, stratified by genotype and baseline viral load. All patients also received weight-based ribavirin dosing. Serum TSH and free thyroxine (FT4) were measured at an initial screening, generally a month before therapy, at the time of the first dose, and then every 4 weeks from weeks 8 through 24. Patients also had follow-up thyroid function assessments 4 and 12 weeks after the last delivered dose. A total of 932 patients were treated during the trial with at least one dose of interferon. The original report found a small but statistically significantly increased rate of sustained virological response using Peg-IFNα2a only in Asians, attributed to 100% compliance within this group. In addition to the variables collected during the original trial, we categorized each country into one of three categories of general iodine environment—deficient, sufficient, or excess—using the World Health Organization data (27,28).
Our analysis was restricted to the 869 patients who were not on thyroid hormone replacement or antithyroid medications, and who had normal serum TSH at both the one-month pretreatment screening and at the baseline drawn for the first injection. This subgroup was not different from the cohort as a whole (Table 1). The 63 excluded patients were slightly older (mean 47 vs. 44 years old) and more likely to be female (52% vs. 41%), but these differences were not statistically significant; the excluded patients were not statistically different from the euthyroid cohort by ethnic background, smoking status, iodine environment, genotype, severity or duration of hepatitis C infection, length of therapy, or achievement of remission. Mean pretreatment serum TSH was significantly increased among those excluded, at 2.88 mU/L versus 1.75 mU/L in the study population (p<0.001), as expected based on the exclusion criteria.
Table 1.
Demographics of Included and Excluded Subpopulations
| Included (869) | Excluded (63) | p | |
|---|---|---|---|
| Pretreatment TSH (mU/L) | 1.75±0.94 | 2.88±4.2 | <0.0001 |
| Sex (male) | 59.3% | 47.7% | 0.07 |
| Ethnicity (white/other) | 54.9%/45.1% | 55.6%/44.4% | 0.92 |
| Smoking (current) | 37.2% | 38.1% | 0.88 |
| Iodine excess | 58.9% | 61.9% | 0.64 |
| Age (years) | 44.3±11.3 | 46.9±10.3 | 0.08 |
| Body mass index | 25.9±4.9 | 26.9±5.5 | 0.17 |
| Genotype 2 | 45.7% | 47.6% | 0.77 |
| Sustained viral response | 81.2% | 85.7% | 0.38 |
| Fibrosis (none/severe) | 80.3%/6.5% | 87.1%/3.2% | 0.19 |
| Duration of infection (years) | 4.5±5.5 | 5.0±5.3 | 0.52 |
| Duration of therapy (weeks) | 23.1±3.6 | 23.2±3.2 | 0.90 |
Excluded subjects were not different from the included euthyroid subjects except in having a higher rate of abnormal TSH (a specific exclusion criteria). Values are proportions, or means and standard deviation. All p-values represent the results of a proportion test or two-sided T-test as appropriate.
TSH, thyrotropin.
Missing data for demographic or clinical attributes were less than 1%. Only 9 (1.1%) patients had one interval measurement of TSH that was missing while on therapy, and half of those had an FT4 value available for that visit. Incomplete follow-up was observed for 104 subjects (11.9%), although only 15 (1.7%) had no follow-up beyond the last interferon injection.
Thyroid outcomes
Definitions of three main IITD subtypes were developed based on serum TSH levels relative to the normal range of 0.35–5.5 mU/L in the assay used. Thyrotoxicosis was examined using two cutoffs for dysfunction: any depression of TSH below the lower limit of normal, 0.35 mU/L, and full suppression with a TSH less than 0.1 mU/L. Hypothyroidism was defined as a TSH greater than 5.5 mU/L. Patients were classified as having biphasic thyroiditis if both biochemical hypothyroidism and thyrotoxicosis were observed. Overt disease for any category was defined as an FT4 that also went outside of the normal range.
Multivariate logistic regression
All statistics were performed using STATA 11 (StataCorp, College Station, TX). Independent variables included age, sex, ethnicity, body mass index, smoking history, pretreatment TSH, iodine environment (deficient, sufficient, or excess), genotype of hepatitis C infection, duration of hepatitis C infection, severity of liver disease, length of IFN-α therapy, and the primary treatment outcome of sustained virological response. None of these variables exhibited pervasive co-linearity and thus all were included in the final models. Because of the small numbers of non-Asian nonwhite subjects, our multivariate models considered ethnicity as a binary variable white versus other. We completed a sensitivity analysis excluding nonwhite non-Asian subjects and results were not statistically different. Rates of each IITD subtype were not different by treatment arm (χ2=7.1, p=0.3). This was expected because randomization was effective and the originally published safety analysis found that the treatment arm did not impact rates of thyroid disease by several different definitions (29). Thus, all further investigations were collapsed across treatment arm.
Preliminary investigations with ANOVA and chi-squared techniques confirmed that multiple variables had different patterns of association between the distinct IITD phenotypes. Univariate regression analysis was performed to quantify these associations. We then proceeded to develop multivariate regression models stratified by disease phenotype. We used a stepwise approach to the multivariate regression, with variables added sequentially based on our experience with the ACHIEVE 1 study, which found that baseline TSH was a factor in all disease phenotypes and sex played a large role in biphasic thyroiditis (8). Interaction terms for sex, ethnicity, genotype, and iodine environment were tested in the full model, and significant interaction effects were examined further with additional stratified models.
Results
Incidence of IITD
Overall, 181 (20.8%) participants had an abnormal serum TSH concentration at some time during the treatment or follow-up periods, with the abnormal value occurring after the last dose of IFNα in 78 subjects. To categorize IITD subtypes, we initially defined phenotypes based on TSH pattern: thyrotoxicosis (TSH <0.35 mU/L), hypothyroidism (TSH >5.5 mU/L), and biphasic thyroiditis (sequential thyrotoxicosis and hypothyroidism). We defined overt disease for each subtype where the FT4 was also out of range at least once. Because nonthyroidal illness can cause mild TSH depression, we also stratified the analysis of thyrotoxicosis into two categories: mild TSH depression (TSH 0.35–0.1) and TSH suppression (<0.1).
Low TSH occurred in 71 patients, with a TSH suppressed to less than 0.1 mU/L in 30 patients (Fig. 1A–B). Overt disease with elevated FT4 was found in eight patients, of whom four demonstrated persistent thyrotoxicosis (Fig. 1A). Among the 41 patients with mild TSH depression, approximately twothirds (28 patients) had a pattern in which the concurrent FT4 was either stable or rising (Fig. 1C), while one-third (13 patients) appeared to have FT4 decreasing in parallel to the TSH (Fig. 1D). Cases accumulated linearly throughout the study period (data not shown). The first low TSH occurred after the end of therapy in 31 of the 71 thyrotoxic subjects, with TSH suppression below 0.1 mU/L in half of these post-treatment cases (15 patients). Twenty-one of the late thyrotoxicosis cases were known to be transient, while 10 had a suppressed TSH at the end of follow-up, 12 weeks after the end of therapy, and it is not known which of these were in fact thyroiditis patients.
FIG. 1.
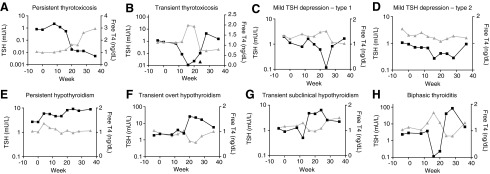
Representative patterns of IITD: TSH (mU/L, log scale, left axis, black squares) and FT4 (ng/dL, right axis, grey triangles) are graphed over time for representative patients with each analyzed form of IITD. Last therapy injection was given at 24 weeks in all cases. Patterns are: (A) persistent thyrotoxicosis; (B) transient thyrotoxicosis; (C) mild TSH depression type 1 (subclinical thyrotoxicosis); (D) mild TSH depression type 2 (euthyroid sick syndrome); (E) persistent hypothyroidism; (F) transient overt hypothyroidism; (G) transient subclinical hypothyroidism; (H) biphasic thyroiditis. IITD, interferon-alpha-induced thyroid dysfunction; TSH, thyrotropin; FT4, free thyroxine.
Hypothyroidism, varying in degree and duration (Fig. 1E–G), occurred in 53 subjects (6.1%). Similar to the situation with thyrotoxicosis, hypothyroidism also occurred in some patients exclusively after the last interferon injection (32/53; 60%). TSH rose above 10 mU/L in 12 patients (1.4%), 4 while on therapy and in 8 during follow-up. A total of four patients were treated with hormone replacement for monophasic hypothyroidism, two of whom had overt disease.
Fifty-seven subjects had biphasic thyroiditis (Fig. 1H), again accumulating a stable rate over time, with 15 cases occurring exclusively after the final dose of IFNα. The majority of overt disease was associated with this subtype of IITD. All but 7 cases had a nadir TSH <0.1 mU/L, while a peak TSH greater than 10 mU/L occurred in 53 out of 57 subjects. In fact, only one patient with biphasic thyroiditis had neither of these more severe TSH derangements. Medical therapy was instituted in 26 biphasic patients, mostly hormone replacement therapy. Out of six treated with antithyroid medication, four resolved to normal after discontinuing treatment, while two remained hypothyroid on methimazole at the end of the study. One subject with overt thyrotoxicosis was treated with 8 mCi 131I, but the FT4 had normalized by the time of the treatment, and therefore this case was included as biphasic thyroiditis. Therapy was ongoing at the end of the study in all 19 of the patients who initiated thyroid hormone replacement therapy during their hypothyroid phase.
Risk factors for IITD
An unadjusted analysis was performed to examine the relationships between variables and the three main phenotypes of IITD. Because overt disease was rare for subtypes other than biphasic thyroiditis, where it was essentially universal, we were unable to substratify the analysis by this feature. Pretreatment TSH, sex, age, ethnicity, smoking history, iodine environment, and hepatitis C genotype were significantly associated with specific thyroid outcomes in univariate regression analysis (Table 2). Median pretreatment TSH in the euthyroid patients was 1.48 mU/L (mean 1.69 mU/L). In those with any degree of TSH suppression, the median baseline TSH was 1.08 mU/L (mean 1.19 mU/L, p<0.001); in those becoming hypothyroid, median baseline TSH was 2.65 mU/L (mean 2.80 mU/L, p<0.001); and in subjects with biphasic thyroiditis, the median baseline TSH was 1.94 mU/L (mean 2.13 mU/L, p<0.001). The pretreatment TSH was thus significantly associated differentially with each thyroid outcome with odds ratios (ORs) of 0.32 ([95% confidence interval (CI) 0.20–0.51], p<0.001) for thyrotoxicosis, 2.60 ([CI 2.02–3.35], p<0.001) for hypothyroidism, and 1.59 ([CI 1.23–2.05], p<0.001) for biphasic thyroiditis. Both biphasic thyroiditis and thyrotoxicosis were enriched for females, 66.7% and 50.7%, respectively, compared with 36.9% in the euthyroid group, representing increased ORs of 3.42 ([CI 1.93–6.06], p<0.001) and 1.76 ([CI 1.08–2.87], p<0.05), respectively. Those with monophasic thyrotoxicosis were somewhat younger, 41.1 years old on average, compared with the euthyroid group, with an average age of 44.7 years (p<0.05). Biphasic thyroiditis was significantly less common in nonwhites than in whites: while 45.1% of the study cohort is nonwhite, only 28.1% of those with biphasic thyroiditis were nonwhite. Smokers were also protected from biphasic thyroiditis, representing only 19.3% of those affected compared with 38.5% of the euthyroid group. Iodine excess was associated with increased risk of hypothyroidism, with 84.9% of hypothyroid subjects living in areas designated as having iodine excess, compared with 59.3% of the euthyroid subjects, and 58.9% of the cohort overall. Finally, hepatitis C genotype was also weakly associated with outcome, with 54.3% of the cohort overall carrying a genotype 3 infection, compared with 68.4% of those with biphasic thyroiditis and 33.9% of those developing hypothyroidism (p<0.05). Length of interferon therapy, sustained virological response, hepatitis disease severity, duration of infection, and BMI were not associated with thyroid outcomes.
Table 2.
Odds Ratios for Risk Factors in Each IFNα-Induced Thyroid Dysfunction Phenotype
| |
Biphasic thyroiditis |
Hypothyroidism |
Thyrotoxicosis |
|||
|---|---|---|---|---|---|---|
| Unadjusted | Multivariate | Unadjusted | Multivariate | Unadjusted | Multivariate | |
| Pretreatment TSH | 1.59±0.21*** | 1.67±0.25** | 2.60±0.34*** | 2.54±0.35*** | 0.32±0.08*** | 0.31±0.08*** |
| Sex (male vs. female) | 3.42±1.00*** | 3.04±0.96*** | 1.65±0.47 | 1.5±0.50 | 1.76±0.44* | 1.99±0.54* |
| Ethnicity (white vs. other) | 0.44±0.13** | 0.36±0.13** | 1.17±0.33 | 0.61±0.22 | 0.65±0.17 | 0.93±0.28 |
| Smoking (current) | 0.38*±0.13* | 0.35±0.13** | 0.89±0.26 | 1.30±0.48 | 1.04±0.27 | 0.78±0.23 |
| Iodine sufficient | 0.57±0.24 | 0.60±0.28 | 1.57±1.12 | 1.43±1.07 | 0.78±0.28 | 0.87±0.33 |
| Iodine excess | 0.51±0.15* | 0.77±0.31 | 4.72±2.50* | 3.24±1.96 | 0.51±0.14* | 0.81±0.28 |
| Age | 0.98±0.01 | 0.99±0.02 | 1.01±0.1 | 0.98±0.02 | 0.97±0.01* | 0.97±0.01 |
| Body mass index | 0.98±0.03 | 1.00±0.03 | 1.00±0.03 | 1.00±0.03 | 0.98±0.03 | 1.00±0.03 |
| Genotype | 1.87±0.55* | 1.73±0.70 | 0.44±0.13* | 0.64±0.26 | 1.59±0.41 | 1.14±0.38 |
| Sustained response | 1.51±0.59 | 0.87±0.40 | 1.62±0.67 | 1.12±0.56 | 1.21±0.40 | 1.01±0.40 |
| Severity | 0.73±0.12 | 0.77±0.13 | 1.08±0.11 | 1.12±0.14 | 0.88±0.10 | 0.98±0.12 |
| Duration | 0.97±0.03 | 0.97±0.03 | 1.00±0.03 | 1.00±0.03 | 0.99±0.02 | 1.00±0.03 |
| Weeks of IFNα | 1.08±0.07 | 1.08±0.08 | 1.10±0.07 | 1.10±0.08 | 1.00±0.03 | 0.98±0.04 |
Odds ratios (±the standard error) for the univariate and multivariate analyses were similar for most variables, except iodine, age, and genotype, which lost significance in the fully adjusted models. Pretreatment TSH is associated with risk of all forms, while sex is a strong predictor only for biphasic thyroiditis and a weak predictor for thyrotoxicosis. Nonwhites and current smokers were at a decreased risk of biphasic thyroiditis.
Statistically significant findings are indicated: ***p<0.001, **p<0.01, *p<0.05.
IFNα, interferon-alpha; IITD, IFNα-induced thyroid dysfunction.
We then analyzed the significant variables in pairs and explored interaction terms. This demonstrated a significant interaction between ethnicity and sex, such that the OR for biphasic thyroiditis among white women compared with the reference group of white men was estimated at 5.30 ([CI 2.57–10.93], p<0.001). In contrast, for nonwhite women, the OR was not significant, OR=1.35 [CI 0.53–3.44] versus the reference group. This is illustrated by the cross-tabulated rates (Table 3), which shows that the rate of biphasic thyroiditis is over 16% in white women, compared with 4.7% in nonwhite (primarily Asian) women and 3.6–3.7% for men of any ethnicity.
Table 3.
Unadjusted Rates of IFNα-Induced Thyroid Dysfunction by Sex and Ethnicity
| |
Women (n=354) |
Men (n=515) |
||
|---|---|---|---|---|
| White | Nonwhite | White | Nonwhite | |
| Euthyroid | 67.8% (124) | 76% (130) | 82% (241) | 87.3% (193) |
| Thyrotoxicosis | 11.4% (21) | 8.8% (15) | 8.2% (24) | 5.0% (11) |
| Hypothyroidism | 4.4% (8) | 10.5% (18) | 6.1% (18) | 4.1% (9) |
| Biphasic thyroiditis | 16.4% (30) | 4.7% (8) | 3.7% (11) | 3.6% (8) |
Percent of each subpopulation diagnosed with biphasic thyroiditis (n cases) demonstrates that biphasic thyroiditis was much more common in white women, while hypothyroidism was more common in nonwhite women. Rates for men are similar to the low-risk groups throughout. For women the Pearson chi-square=17.34, p=0.001 across outcomes, while for men the racial differences are not significant (p=0.34).
Multivariate regression models
Fully adjusted multivariate regression models were developed in a stepwise manner to yield estimates of the ORs associated with each risk factor for each form of IITD (Table 2). Compared with the unadjusted analysis, genotype, age, and iodine intake were no longer significant risk factors in the adjusted models.
Pretreatment serum TSH level represents an important risk factor in all forms of IITD. However, baseline thyroid function is associated in different ways with each phenotype of IITD. Increasing baseline TSH increased the risk of hypothyroidism with an OR=2.54 per 1 mU/L increase in the baseline TSH ([CI 1.94–3.33], p<0.001). In contrast, thyrotoxicosis has an OR=0.31 as TSH increases 1 mU/L ([CI 0.19–0.51], p<0.001), meaning that lower pretreatment TSH is a risk factor for this phenotype. This risk estimate was essentially unchanged for those with TSH suppression but was more pronounced among those with mild TSH depression (OR=0.18 [CI 0.09–0.39], p<0.001). Finally, biphasic thyroiditis is associated with an OR=1.67 per 1 mU/L increase in pretreatment TSH ([CI 1.25–2.24], p<0.01).
Female sex was associated with the development of biphasic thyroiditis, with an OR=3.04 ([CI 1.64–5.63], p<0.001) and less strongly with thyrotoxicosis (OR=1.98 [CI=1.17–3.37], p<0.05). To try and separate subjects with euthyroid sick syndrome from other possible phenotypes, we stratified the thyrotoxicosis into two fairly equal-sized subgroups based on the degree of TSH depression. Those with TSH suppressed below 0.1 mU/L were more likely to be women, with an OR=4.1 ([CI 1.8–9.3], p<0.01), while there was not a significant risk difference by sex among those with milder TSH depression (OR=1.1 [CI 0.54–2.24], NS). Women were not more likely than men to have monophasic hypothyroidism (OR=1.51 [CI 0.78–2.89]).
Ethnicity was a significant risk factor for biphasic thyroiditis, with nonwhites at lower risk compared with whites (OR=0.36 [CI 0.17–0.74], p<0.01). As discussed above, we had observed that the rates of IITD varied by ethnicity among women but not men. Therefore, an interaction term for sex and ethnicity was derived and found to be potentially significant for biphasic thyroiditis (p=0.05). In a multivariate model stratified by ethnicity for biphasic thyroiditis, sex remained a statistically significant risk factor in white women, with an OR=4.47 ([CI 2.03–9.86], p<0.001). In contrast, the estimated OR=1.35 for nonwhite women compared with men, was not significant [CI 0.48–3.84]. Again, the final cell counts are quite small, with only 8 men and 8 women with biphasic thyroiditis among 392 nonwhite subjects, which decreases the precision of the estimate of the risk attributable to sex in the nonwhite subpopulation.
Current smokers were significantly less likely to be affected by biphasic thyroiditis, with an OR=0.35 compared with never and former smokers ([CI 0.17–0.73], p<0.01). This relationship has not been previously reported among interferon-exposed subjects.
Iodine intake was not significantly related to each IITD phenotype in the multivariate models, although a trend was observed with hypothyroidism more common among those living in countries with dietary iodine excess compared with borderline sufficiency (OR=3.24 [CI=0.99–10.58], p=0.05) while thyrotoxicosis was less common (OR=0.81 [CI 0.41–1.59]).
Discussion
Interferon-induced thyroid disease can present as any of the common thyroid dysfunction phenotypes—thyrotoxicosis, hypothyroidism, and biphasic thyroiditis—with a range of severity and duration. The relationships of disease mechanisms to phenotype are unknown, although several proposals have been advanced that hypothyroidism is autoimmune while biphasic thyroiditis is a direct toxic effect (26,30,31). To better understand the factors that distinguish the IITD phenotypes, we have performed an analysis stratified by IITD subtype in a large, closely monitored, multiethnic cohort of euthyroid patients treated with long-acting preparations of IFNα for 24 weeks for hepatitis C, genotypes 2 or 3. Two dominant patterns observed here confirm our recent observations in a cohort of mostly white patients treated for 48 weeks for hepatitis C genotype 1 (8). First, the ACHIEVE 2/3 cohort demonstrates again that the pretreatment thyroid function is strongly associated with IITD, such that a higher or lower pretreatment TSH even within the normal range increases the odds of developing thyroid dysfunction in that same direction. Second, female sex was a strong risk factor for biphasic thyroiditis but was not associated with hypothyroidism. The relationship of female sex to thyrotoxicosis over all was found in this cohort to be concentrated among those with full TSH suppression.
A unique feature of this cohort is the relatively large Asian subgroup, which allowed us to investigate whether ethnicity plays a role as a risk factor for distinct forms of IITD. Two other studies have examined the rates of IITD between Asians and whites; in one, Asians appeared to be at an increased risk (21) and there was no effect in the other (13). In the current investigation, the unadjusted rates of hypothyroidism and biphasic thyroiditis in women were significantly different in nonwhite women, 72.4% of whom are Asian, compared with white women. In the multivariate regression models, we found an interaction between sex and ethnicity for biphasic thyroiditis, and an ethnicity-stratified multivariate regression analysis demonstrated that female sex was a strong risk factor for biphasic thyroiditis only among whites.
A second novel finding is that smoking is protective against biphasic thyroiditis. There are mixed reports in the literature about the interaction of smoking with sporadic forms of thyroid disease. Several reports demonstrate an increased risk for Graves' disease, and especially for eye involvement in those with Graves' disease (32). Several other studies have suggested a protective effect of smoking against autoimmune hypothyroidism (33–35), and a recent report found that smoking was associated with a lower risk of papillary thyroid cancer (36). One group has recently reported that anatabine, a minor nicotinic alkaloid in the tobacco leaf, can decrease disease prevalence and severity in a mouse model of autoimmune thyroiditis (37).
We examined the hypothesis that iodine exposure influences the patterns of thyroid disease in this international cohort. In particular, iodine excess has been reported to increase the risk of hypothyroidism (38–40). Dietary iodine intake in this study was measured on a country-wide basis and provides only an ecological estimate rather than individual exposures, which considerably limits both its power as a variable and also the conclusions that can be drawn from an association. We observed a nonsignificant trend toward more hypothyroidism and less thyrotoxicosis among patients from areas of iodine excess. This finding, which is consistent with the literature, provides a rationale for including measures of iodine exposure in future studies to further understand the importance of this factor.
The major limitation of this study is the fact that TPO and Tg antibody measurements were not available. Since these thyroid antibodies are more prevalent in women in the general population, antibodies are likely to also be associated most strongly with thyroiditis. However, without data on anti-thyroid antibody status, we cannot distinguish whether TPO antibodies are (1) an independent predictor of the IITD phenotype; (2) able to fully explain the sex bias; and/or (3) account for the racial differences in IITD risk among women. Additional studies that are able to analyze these interactions will help to clarify the role of autoimmunity in interferon-induced thyroiditis.
We have used TSH excursions to define the IITD subtypes, as this is the standard in the literature and so our categories are comparable to prior studies. However, inspection of the trends in TSH and FT4 together over time suggests that we are still at risk of misclassifying some subjects. As can be seen from Figure 1A, the most common transient pattern of thyrotoxicosis itself is similar to the biphasic thyroiditis tracings with a smaller amplitude in the second phase that does not cross the threshold for hypothyroidism. This could explain the relationship to baseline TSH, and misclassifying thyroiditis may also explain the finding of an increased risk of thyrotoxicosis among women, and particularly that this association is stronger when euthyroid sick syndrome is definitely excluded by using a more stringent cut off for TSH suppression. Similarly, some transient hypothyroidism may also be thyroiditis with a blunted thyrotoxic phase (e.g., Fig. 1G). However, observer categorization of graphical data is not sufficiently rigorous to provide a basis for stratification. Future assessments that use life-cycle analysis may be able to sort IITD with improved accurately into biphasic thyroiditis, thyrotoxicosis, and nonthyroidal illness patterns.
In sum, our results continue to support the hypothesis that there are distinct mechanisms for different subtypes of IITD. Future studies should aim at including a detailed characterization of patients in order to shed light on the pathophysiology of these thyroid disorders, and in particular to help determine which subtypes are due to enhanced autoimmunity. For current clinical practice, this study suggests that biphasic thyroiditis is the most likely of the IITD subtypes to be biochemically overt and symptomatic for patients. Supportive therapy may therefore be the appropriate first step for most symptomatic thyrotoxicosis. Similarly, symptomatic hypothyroidism may be transient in most cases and the need for hormone replacement therapy should be reassessed in euthyroid patients after completing IFN.
The identification in this study of specific demographic and biochemical risk factors for specific patterns of thyroid dysfunction has clinical importance in targeting of thyroid monitoring to high-risk subgroups. We believe that women and those with a serum TSH at the extremes of the normal range should be assessed more often and with a lower threshold of suspicion for thyroid dysfunction during interferon therapy in order to provide prompt intervention to mitigate the consequences of thyroid dysfunction associated with IFNα treatment.
Acknowledgments
We wish to thank Dr. G. Mani Subramanian (Gilead Sciences, Foster City, CA), Dr. Mark Sulkowski (Division of Infectious Disease, Johns Hopkins University School of Medicine), Dr. Erik Pulkstanis (formerly of Human Genome Sciences, Rockville, MD), Dr. David Thomas (Division of Infectious Disease, Johns Hopkins University School of Medicine), and the ACHIEVE-2/3 study team for their work in the original trial, which has made this study possible. This work was supported by the Stabler Foundation and the Legato Foundation for Gender Medicine and by Grant Number P30AR053503 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
Author Disclosure Statement
J.S.M., S.R.G., A.R., and P.W.L. have nothing to disclose.
References
- 1.Lisker-Melman M. Di Bisceglie AM. Usala SJ. Weintraub B. Murray LM. Hoofnagle JH. Development of thyroid disease during therapy of chronic viral hepatitis with interferon alfa. Gastroenterology. 1992;102:2155–2160. doi: 10.1016/0016-5085(92)90348-3. [DOI] [PubMed] [Google Scholar]
- 2.Kakizaki S. Takagi H. Murakami M. Takayama H. Mori M. HLA antigens in patients with interferon-alpha-induced autoimmune thyroid disorders in chronic hepatitis C. J Hepatol. 1999;30:794–800. doi: 10.1016/s0168-8278(99)80131-7. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli A. Ferri C. Fallahi P. Hepatitis C: thyroid dysfunction in patients with hepatitis C on IFN-alpha therapy. Nat Rev Gastroenterol Hepatol. 2009;6:633–635. doi: 10.1038/nrgastro.2009.168. [DOI] [PubMed] [Google Scholar]
- 4.Pavan MH. Pavin EJ. Goncales FL., Jr Wittmann DE. Virus C genotype predisposes to primary hypothyroidism during interferon-alpha treatment for chronic hepatitis C. Braz J Infect Dis. 2011;15:449–456. doi: 10.1016/s1413-8670(11)70226-4. [DOI] [PubMed] [Google Scholar]
- 5.Vasiliadis T. Anagnostis P. Nalmpantidis G. Soufleris K. Patsiaoura K. Grammatikos N. Orfanou-Koumerkeridou E. Kargiotis K. Slavakis A. Deliyiannidis A. Eugenidis N. Thyroid dysfunction and long-term outcome during and after interferon-alpha therapy in patients with chronic hepatitis C. Ann Acad Med Singapore. 2011;40:394–400. [PubMed] [Google Scholar]
- 6.Huang JF. Huang CK. Yu ML. Dai CY. Huang CF. Hung WW. Yeh ML. Hsieh MH. Yang JF. Hsieh MY. Lin ZY. Chen SC. Wu SS. Chuang WL. Thyroid autoantibodies and dysfunction do not impact the treatment efficacy of peginterferon and ribavirin combination therapy in chronic hepatitis C. Hepatol Int. 2012;6:613–619. doi: 10.1007/s12072-011-9308-5. [DOI] [PubMed] [Google Scholar]
- 7.Costelloe SJ. Wassef N. Schulz J. Vaghijiani T. Morris C. Whiting S. Thomas M. Dusheiko G. Jacobs M. Vanderpump MP. Thyroid dysfunction in a UK hepatitis C population treated with interferon-alpha and ribavirin combination therapy. Clin Endocrinol (Oxf ) 2010;73:249–256. doi: 10.1111/j.1365-2265.2010.03785.x. [DOI] [PubMed] [Google Scholar]
- 8.Mammen JS. Ghazarian SR. Pulkstenis E. Subramanian GM. Rosen A. Ladenson PW. Phenotypes of interferon-alpha-induced thyroid dysfunction among patients treated for hepatitis C are associated with pretreatment serum TSH and female sex. J Clin Endocrinol Metab. 2012;97:3270–3276. doi: 10.1210/jc.2012-1026. [DOI] [PubMed] [Google Scholar]
- 9.Tran HA. Reeves GE. Ianna EA. Leembruggen N. Thyroid function outcomes after pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Endocr Pract. 2010;16:934–939. doi: 10.4158/EP10036.OR. [DOI] [PubMed] [Google Scholar]
- 10.Gelu-Simeon M. Burlaud A. Young J. Pelletier G. Buffet C. Evolution and predictive factors of thyroid disorder due to interferon alpha in the treatment of hepatitis C. World J Gastroenterol. 2009;15:328–333. doi: 10.3748/wjg.15.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamil KM. Leedman PJ. Kontorinis N. Tarquinio L. Nazareth S. McInerney M. Connelly C. Flexman J. Burke V. Metcalf C. Cheng W. Interferon-induced thyroid dysfunction in chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:1017–1023. doi: 10.1111/j.1440-1746.2008.05690.x. [DOI] [PubMed] [Google Scholar]
- 12.Nadeem A. Aslam M. Khan DA. Hussain T. Khan SA. Effects of combined interferon alpha and ribavirin therapy on thyroid functions in patients with chronic hepatitis C. J Coll Physicians Surg Pak. 2009;19:86–89. [PubMed] [Google Scholar]
- 13.Tran HA. Malcolm Reeves GE. Gibson R. Attia JR. Development of thyroid diseases in the treatment of chronic hepatitis C with alpha-interferon may be a good prognosticator in achieving a sustained virological response: a meta-analysis. J Gastroenterol Hepatol. 2009;24:1163–1168. doi: 10.1111/j.1440-1746.2009.05874.x. [DOI] [PubMed] [Google Scholar]
- 14.Andrade LJ. Atta AM. D'Almeida A., Jr Parana R. Thyroid dysfunction in hepatitis C individuals treated with interferon-alpha and ribavirin—a review. Braz J Infect Dis. 2008;12:144–148. doi: 10.1590/s1413-86702008000200009. [DOI] [PubMed] [Google Scholar]
- 15.Masood N. Ghori R. Memon A. Memon S. Memon KI. Memon I. Jaffri M. Baloch GH. Frequency of thyroid disorders during interferon and ribavirin therapy in chronic hepatitis C infection. J Coll Physicians Surg Pak. 2008;18:347–351. [PubMed] [Google Scholar]
- 16.Kabbaj N. Guedira MM. El Atmani H. El Alaoui M. Mohammadi M. Benabed K. Lachkar H. Benaissa A. Thyroid disorders during interferon alpha therapy in 625 patients with chronic hepatitis C: a prospective cohort study. Ann Endocrinol (Paris) 2006;67:343–347. doi: 10.1016/s0003-4266(06)72609-9. [DOI] [PubMed] [Google Scholar]
- 17.Kee KM. Lee CM. Wang JH. Tung HD. Changchien CS. Lu SN. Wang PW. Thyroid dysfunction in patients with chronic hepatitis C receiving a combined therapy of interferon and ribavirin: incidence, associated factors and prognosis. J Gastroenterol Hepatol. 2006;21:319–326. doi: 10.1111/j.1440-1746.2005.03947.x. [DOI] [PubMed] [Google Scholar]
- 18.Caraccio N. Giannini R. Cuccato S. Faviana P. Berti P. Galleri D. Dardano A. Basolo F. Ferrannini E. Monzani F. Type I interferons modulate the expression of thyroid peroxidase, sodium/iodide symporter, and thyroglobulin genes in primary human thyrocyte cultures. J Clin Endocrinol Metab. 2005;90:1156–1162. doi: 10.1210/jc.2004-1173. [DOI] [PubMed] [Google Scholar]
- 19.Moncoucy X. Leymarie F. Delemer B. Levy S. Bernard-Chabert B. Bouche O. Jolly D. Diebold MD. Cadiot G. Thiefin G. Risk factors and long-term course of thyroid dysfunction during antiviral treatments in 221 patients with chronic hepatitis C. Gastroenterol Clin Biol. 2005;29:339–345. doi: 10.1016/s0399-8320(05)80778-x. [DOI] [PubMed] [Google Scholar]
- 20.Bini EJ. Mehandru S. Incidence of thyroid dysfunction during interferon alfa-2b and ribavirin therapy in men with chronic hepatitis C: a prospective cohort study. Arch Intern Med. 2004;164:2371–2376. doi: 10.1001/archinte.164.21.2371. [DOI] [PubMed] [Google Scholar]
- 21.Dalgard O. Bjoro K. Hellum K. Myrvang B. Bjoro T. Haug E. Bell H. Thyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: no association with either interferon dosage or efficacy of therapy. J Intern Med. 2002;251:400–406. doi: 10.1046/j.1365-2796.2002.00974.x. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh MC. Yu ML. Chuang WL. Shin SJ. Dai CY. Chen SC. Lin ZY. Hsieh MY. Liu JF. Wang LY. Chang WY. Virologic factors related to interferon-alpha-induced thyroid dysfunction in patients with chronic hepatitis C. Eur J Endocrinol. 2000;142:431–437. doi: 10.1530/eje.0.1420431. [DOI] [PubMed] [Google Scholar]
- 23.Deutsch M. Dourakis S. Manesis EK. Gioustozi A. Hess G. Horsch A. Hadziyannis S. Thyroid abnormalities in chronic viral hepatitis and their relationship to interferon alfa therapy. Hepatology. 1997;26:206–210. doi: 10.1002/hep.510260127. [DOI] [PubMed] [Google Scholar]
- 24.Hollowell JG. Staehling NW. Flanders WD. Hannon WH. Gunter EW. Spencer CA. Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 25.Spencer CA. Hollowell JG. Kazarosyan M. Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007;92:4236–4240. doi: 10.1210/jc.2007-0287. [DOI] [PubMed] [Google Scholar]
- 26.Tomer Y. Hepatitis C and interferon induced thyroiditis. J Autoimmun. 2010;34:J322–J326. doi: 10.1016/j.jaut.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson M. Takkouche B. Egli I. Allen HE. de Benoist B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005;83:518–525. [PMC free article] [PubMed] [Google Scholar]
- 28.de Benoist B. McLean E. Andersson M. Rogers L. Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull. 2008;29:195–202. doi: 10.1177/156482650802900305. [DOI] [PubMed] [Google Scholar]
- 29.Nelson DR. Benhamou Y. Chuang WL. Lawitz EJ. Rodriguez-Torres M. Flisiak R. Rasenack JW. Kryczka W. Lee CM. Bain VG. Pianko S. Patel K. Cronin PW. Pulkstenis E. Subramanian GM. McHutchison JG. ACHIEVE-2/3 Study Team 2010 Albinterferon Alfa-2b was not inferior to pegylated interferon-alpha in a randomized trial of patients with chronic hepatitis C virus genotype 2 or 3. Gastroenterology. 139:1267–1276. doi: 10.1053/j.gastro.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akeno N. Smith EP. Stefan M. Huber AK. Zhang W. Keddache M. Tomer Y. IFN-alpha mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. J Immunol. 2011;186:4693–4706. doi: 10.4049/jimmunol.1002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menconi F. Hasham A. Tomer Y. Environmental triggers of thyroiditis: hepatitis C and interferon-alpha. J Endocrinol Invest. 2011;34:78–84. doi: 10.1007/BF03346699. [DOI] [PubMed] [Google Scholar]
- 32.Vestergaard P. Smoking and thyroid disorders—a meta-analysis. Eur J Endocrinol. 2002;146:153–161. doi: 10.1530/eje.0.1460153. [DOI] [PubMed] [Google Scholar]
- 33.Belin RM. Astor BC. Powe NR. Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2004;89:6077–6086. doi: 10.1210/jc.2004-0431. [DOI] [PubMed] [Google Scholar]
- 34.Asvold BO. Bjoro T. Nilsen TI. Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med. 2007;167:1428–1432. doi: 10.1001/archinte.167.13.1428. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen IB. Laurberg P. Knudsen N. Jorgensen T. Perrild H. Ovesen L. Rasmussen LB. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol. 2008;158:367–373. doi: 10.1530/EJE-07-0595. [DOI] [PubMed] [Google Scholar]
- 36.Kitahara CM. Linet MS. Beane Freeman LE. Check DP. Church TR. Park Y. Purdue MP. Schairer C. Berrington de Gonzalez A. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control. 2012;23:1615–1624. doi: 10.1007/s10552-012-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caturegli P. De Remigis A. Ferlito M. Landek-Salgado MA. Iwama S. Tzou SC. Ladenson PW. Anatabine ameliorates experimental autoimmune thyroiditis. Endocrinology. 2012;153:4580–4587. doi: 10.1210/en.2012-1452. [DOI] [PubMed] [Google Scholar]
- 38.Teng W. Shan Z. Teng X. Guan H. Li Y. Teng D. Jin Y. Yu X. Fan C. Chong W. Yang F. Dai H. Yu Y. Li J. Chen Y. Zhao D. Shi X. Hu F. Mao J. Gu X. Yang R. Tong Y. Wang W. Gao T. Li C. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–2793. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- 39.Teng X. Shan Z. Chen Y. Lai Y. Yu J. Shan L. Bai X. Li Y. Li N. Li Z. Wang S. Xing Q. Xue H. Zhu L. Hou X. Fan C. Teng W. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943–950. doi: 10.1530/EJE-10-1041. [DOI] [PubMed] [Google Scholar]
- 40.Markou K. Georgopoulos N. Kyriazopoulou V. Vagenakis AG. Iodine-induced hypothyroidism. Thyroid. 2001;11:501–510. doi: 10.1089/105072501300176462. [DOI] [PubMed] [Google Scholar]


