Abstract
The activation of the Akt signalling in response to cytokine receptor signalling promotes protein synthesis, cellular growth and proliferation. To determine the role of Akt in interleukin-3 (IL-3) signalling, we generated IL-3-dependent myeloid cell lines from mice lacking Akt1, Akt2 or Akt3. Akt1 deletion resulted in accelerated apoptosis at low concentrations of IL-3. Expression of constitutively active Akt1 was sufficient to delay apoptosis in response to IL-3 withdrawal, but not sufficient to induce proliferation in the absence of IL-3. Akt1 prolonged survival of Bim- or Bad-deficient cells, but not cells lacking Puma, indicating that Akt1-dependent repression of apoptosis was in part dependent on Puma and independent of Bim or Bad. Our data show that a key role of Akt1 during IL-3 signalling is to repress p53-dependent apoptosis pathways, including transcriptional upregulation of Puma. Moreover, our data indicate that regulation of BH3-only proteins by Akt is dispensable for Akt-dependent cell survival.
Keywords: Akt, Bcl-2, interleukin-3, puma
In myeloid progenitor cells, interleukin-3 (IL-3) maintains cell viability and promotes proliferation.1 When IL-3 signalling is lost, cells activate intrinsic cell death pathways that are regulated by the Bcl-2 family of proteins.2, 3 An inappropriate repression of cell death and activation of proliferation cooperate to cause haematological malignancies, and the signalling pathways that maintain cell viability therefore stand out as important therapeutic targets in these diseases.
Apoptosis induced by IL-3 withdrawal is blocked by the overexpression of anti-apoptotic Bcl-2 family members and the combined loss of the pro-apoptotic multi-BH domain Bcl-2 family members Bax and Bak2, 3, 4, 5 or the combined loss of the pro-apoptotic BH3-only Bcl-2 family members Puma and Bim.6 In response to a loss of IL-3/IL-3 receptor (IL-3R) signalling, the pro-apoptotic BH3-only proteins directly and indirectly activate Bax and Bak. In the presence of IL-3/IL-3R signalling the activity of BH3-only proteins is repressed.3, 4, 7 Akt activation has been thought to have a key role in the regulation of BH3-only proteins8, 9 and maintain the levels of anti-apoptotic proteins, such as Mcl-1.10 However, the requirement for direct regulation of BH3-only proteins by Akt remains unclear.
Akt (Protein Kinase B) is an AGC kinase sub-family member, related to PKA and PKC. Three mammalian isoforms of Akt exist (Akt1, Akt2 and Akt3), each containing a kinase domain and a Pleckstrin Homology (PH) domain. Akt is activated by the lipid kinase phosphoinositide-3 kinase (PI3K).11, 12 In IL-3/IL-3R signalling, Akt activation is thought to be a key signalling pathway that represses apoptosis pathways, in part by regulation of members of the Bcl-2 family of proteins. Understanding how Akt regulates cell survival will provide important insights into the mechanisms underpinning the oncogenic functions of Akt.
To investigate the role of Akt in IL-3/IL-3R signalling, we generated IL-3-dependent myeloid progenitor cell lines from mice lacking one of each of the three Akt isoforms, Akt1, Akt2 or Akt3. Moreover, we generated IL-3-dependent myeloid progenitor cell lines from mice lacking BH3-only proteins, in which we overexpressed constitutively active Akt. We found that, compared with control cells, more Akt1-deficient cells underwent apoptosis when IL-3 was at limiting concentrations. Akt1 overexpression delayed apoptosis after IL-3 deprivation but was not sufficient to maintain clonogenic proliferation. Our data indicate that the important mechanism by which Akt1 maintains cell viability during IL-3 signalling is by suppressing p53-dependent apoptosis pathways, including p53-dependent upregulation of the BH3-only protein Puma, and not by directly controlling Bcl-2 family members. This mechanism may contribute to the oncogenic functions of activated Akt.
Results
Akt1 is required to maintain viability of myeloid progenitor cells in low IL-3 concentrations
Akt is activated by IL-3/IL-3R signalling and contributes to maintenance of the viability of myeloid progenitor cells. To investigate the specific role of each Akt isoform in IL-3/IL-3R-mediated cell survival, we generated multiple IL-3-dependent myeloid progenitor-derived cell lines (hereafter called factor-dependent myeloid (FDM) cells) from wild-type (WT), Akt1−/−, Akt2−/− or Akt3−/− mice,13, 14, 15 by immortalising haematopoietic progenitor cells from E14.5 embryos with Hoxb8.16 Deletion of Akt1, Akt2 or Akt3 did not prevent the generation of FDM cells, indicating that no individual Akt isoform is absolutely required for IL-3-dependent survival and proliferation.
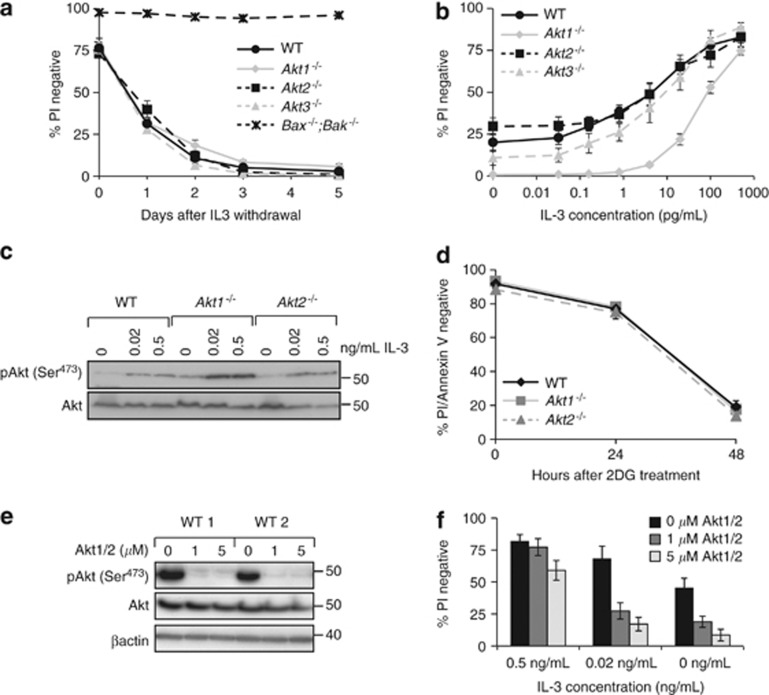
The impact of IL-3 deprivation on the viability of WT, Akt1−/−, Akt2−/− or Akt3−/− FDM cell lines was assayed over 5 days using flow cytometry to determine propidium iodide (PI) exclusion. Bax−/−;Bak−/− FDM cells lines were used as controls, as these cells are resistant to IL-3 withdrawal-induced apoptosis.3 In the absence of IL-3, WT, Akt1−/−, Akt2−/− and Akt3−/− FDM cells underwent apoptosis at comparable rates (Figure 1a). The loss of any individual Akt isoform did not accelerate apoptosis in response to complete IL-3 deprivation. We next tested whether loss of Akt1, Akt2 or Akt3 enhanced apoptosis in limiting IL-3 concentrations. The same WT, Akt1−/−, Akt2−/− and Akt3−/− FDM cell lines were maintained in IL-3 concentrations ranging from 0 to 0.5 ng/ml for 48 h before viability was determined. At IL-3 concentrations between 1 and 100 pg/ml, significantly more Akt1−/− FDM cells underwent apoptosis compared with WT, Akt2−/− or Akt3−/− FDM cells (Figure 1b). We next tested the impact of Akt1 or Akt2 deletion on the total levels of Akt and phosphorylated Akt in response to IL-3 stimulation. Lysates from WT, Akt1−/− and Akt2−/− FDM cells cultured in the indicated concentrations of IL-3 were probed with antibodies against Akt phosphorylated on Serine 473 and total Akt (Figure 1c). Total and phosphorylated Akt levels were not reduced in either knockout cell line. Indeed, phosphorylated Akt was generally more abundant in FDM cells lacking Akt1 compared with WT cells, which may indicate a compensatory mechanism in expression that does not reduce the amount of Akt. Thus, deletion of Akt1 specifically reduces viability in limiting IL-3 concentrations, independently of the total levels of activated Akt.
Figure 1.
Deletion of Akt1 reduces viability in limiting concentrations of IL-3. (a) Multiple independently generated clones of FDM cells of the indicated genotypes (n=number of clones; Akt1−/− n=3, Akt2−/− n=4, Akt3−/− n=4, WT n=4 and Bax−/−;Bak−/− n=2) were starved of IL-3 over 5 days. At the indicated time points, cell viability was determined by propidium iodide (PI) exclusion using flow cytometry. Data represent mean and standard error from three independent experiments using multiple clones. (b) Multiple independent clones of FDM cells of the indicated genotypes (Akt1−/− n=5, Akt2−/− n=4, Akt3−/− n=4 and WT n=4) were cultured at the indicated concentrations of IL-3 for 48 h. Viability was then determined by PI uptake using flow cytometry. Data represent mean and standard error, from three independent experiments over which all clones were tested. (c) Lysates from WT, Akt1−/−or Akt2−/− FDM cells cultured for 24 h in the indicated concentrations of IL-3 were probed with antibodies to phosphorylated Akt (serine 473) and total Akt. (d) WT, Akt1 or Akt2 null cells were treated with 3 mM 2-DG for 48 h. Cell viability was determined by PI uptake and Annexin V staining using flow cytometry. Data represent mean and standard error, from two independent experiments over which three clones of each genotype were tested. (e) Two independent WT clones were cultured in the presence of IL-3 and the AKT1/2 inhibitor (AKT1/2). Cell lysates were resolved by SDS-PAGE and western blots probed with antibodies to detect phosphorylated Akt (serine 473), total AKT and β-actin as a loading control. (f) Three independent WT clones were cultured at the indicated IL-3 concentrations in the presence of AKT1/2. After 24 h, cell viability was determined by PI uptake using flow cytometry. Data represent mean and standard error, from two independent experiments using two clones in each experiment
To establish the sensitivity of Akt1−/− FDM cells to related apoptotic stimuli, we cultured WT, Akt1−/− and Akt2−/− cells with 2 deoxyglucose (2DG) to simulate glucose deprivation, as Akt can maintain glucose import and reduce apoptosis after IL-3 deprivation.17 WT, Akt1−/− and Akt2−/− FDM cells were equally susceptible to 2DG-induced apoptosis (Figure 1d), indicating that deletion of Akt1 or Akt2 does not accelerate apoptosis caused by glucose deprivation. Akt1 is phosphorylated downstream of PI3K activation in response to IL-3R signalling.18 To determine how Akt1 deletion effects apoptosis induced by PI3K inhibitors, we treated WT and Akt1−/− FDM cells with one of three PI3K inhibitors and measured viability in reducing concentrations of IL-3 (Supplementary Figure S1). In the absence of IL-3 or in low IL-3 (0.02 ng/ml), the PI3K inhibitors induced apoptosis. In the presence of IL-3 at 0.5 ng/ml (normal culture conditions), apoptosis was inhibited. These data show that Akt1 deletion does not enhance or inhibit apoptosis induced by PI3K inhibitors, and that Akt1 is not required for IL-3R signalling to block apoptosis induced by PI3K inhibitors. This further defines the specific conditions under which Akt1 functions as a regulator of IL-3R-dependent survival signalling.
We reasoned that because Akt1 was required for cell viability at low IL-3 concentrations, Akt inhibition would induce more apoptosis in low IL-3 concentrations. To test this, we measured the viability of cells cultured in high (0.5 ng/ml), low (0.02 ng/ml) or no IL-3 in the absence or presence of 1 or 5 nM of the Akt inhibitor, Akt1/2 (Calbiochem, Billerica, MA, USA)19 (Figures 1e and f). Akt inhibition significantly reduced WT FDM viability in low IL-3, consistent with Akt functioning to maintain viability at limiting IL-3 concentrations. In the absence of IL-3, Akt1/2 increased the population of cells undergoing apoptosis, whereas high IL-3 concentrations blocked Akt1/2-dependent apoptosis (Figure 1f). These data emphasise that Akt contributes to IL-3/IL-3R-mediated cell survival when IL-3 concentrations are low but not absent.
Akt1 overexpression partially blocks apoptosis in low IL-3 concentrations
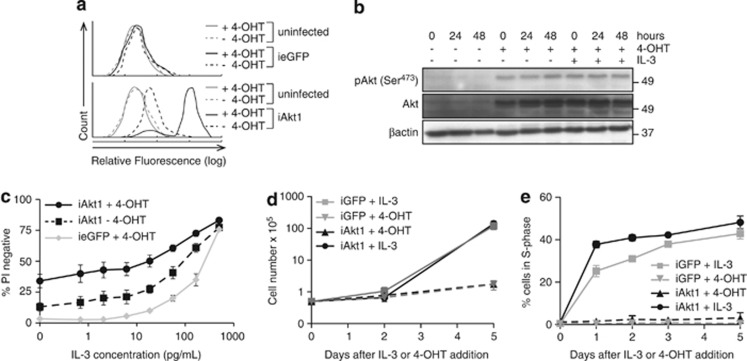
We next examined whether overexpression of constitutively active Akt1 increased survival of FDM cells in low IL-3 concentrations. We used a 4-Hydroxytamoxifen (4-OHT) inducible lentiviral system to overexpress HA-myristoylated-(ΔPH)Akt1 (iAkt1) or enhanced green fluorescent protein (ieGFP). In this system, the induced protein is not oestrogen-receptor tagged.20 For each independent clone, induced Akt1 or GFP expression in the presence or absence of IL-3 was confirmed (Figures 2a and b). Four independent clones expressing iAkt1 or ieGFP were induced with 4-OHT for 24 h before being replated in IL-3 at concentrations ranging from 0 to 0.5 ng/ml, in the presence of 4-OHT. After 72 h, cell viability was determined by PI exclusion. Overexpression of iAkt1 increased survival at low IL-3 concentrations compared with ieGFP (Figures 2c and d). Approximately 25% of cells expressing iAkt1 remained viable in the complete absence of IL-3. Uninduced cells expressing iAkt1 had detectable background expression (Figure 2a), which was sufficient to promote an intermediate increase in cell viability. Thus, expression of constitutively active Akt1 delayed apoptosis induced by IL-3 deprivation.
Figure 2.
Enforced expression of constitutively active Akt increases survival of FDM cells in low IL-3 concentrations. (a) Expression of inducible Akt1 as detected by intracellular FACS analysis using FITC-conjugated anti-HA antibody. Representative histograms of Akt expression compared with uninduced and ieGFP expressing cells are shown. (b) Lysates of iAkt1 expressing cells cultured in the presence or absence of 4-OHT and IL-3 were probed with antibodies to detect phosphorylated Akt (serine 473), total AKT and β-actin as a loading control. (c) The viability of multiple independent WT clones of FDM cells expressing inducible myr-HA-(ΔPH)Akt1 (iAkt1; n=4) cultured in the presence or absence of 4-hydroxytamoxifen (4-OHT) was compared with inducible GFP (ieGFP) expressing cells cultured in the indicated concentrations of IL-3 for 72 h. The results represent means and standard error of the mean of three independent experiments. (d and e) FDM cells expressing iAkt1 were starved of IL-3 overnight, and either IL-3 or 4-OHT added, the later to induce expression of activated Akt. Cell number was counted using haemocytometer (d) and the proportions of cells in S phase (e) were determined by using intracellular PI staining and flow cytometry over a 5-day period
We also tested whether iAkt1 expression was sufficient to induce proliferation of FDM cells in the absence of IL-3. Cells starved of IL-3 were treated with either IL-3 or 4-OHT to induce Akt expression, and the proportions of cells in S phase and the number of viable cells were measured over 5 days (Figure 2e). IL-3 induced rapid reentry into S phase and proliferation. In contrast, constitutively active Akt1 was not sufficient to drive cell division, indicating that overexpressed Akt1 is not sufficient for proliferation independent of IL-3R signalling.
Overexpressed Akt1 does not increase survival of Puma −/− FDM cells
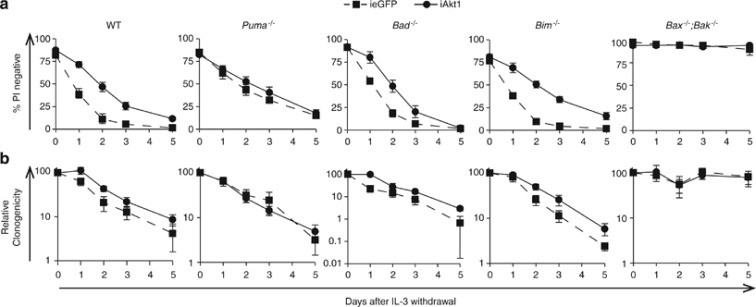
Evidence suggests that Akt represses activation of certain pro-apoptotic BH3-only Bcl-2 family proteins to promote survival in response to cytokine signalling. The mechanisms include direct phosphorylation of Bad,8, 21 or indirect regulation of the expression of Bim and Puma.22 We reasoned that if Akt1 directly or indirectly regulated a specific BH3-only protein to maintain cell viability during IL-3/IL-3R signalling, then any survival advantage derived from overexpression of Akt would be observed only when that BH3-only protein was expressed. We generated Bad−/−, Bim−/− or Puma−/− FDM cells that expressed iAkt1 or ieGFP (negative control). At least four independent clones of each genotype were generated and tested for inducible expression of each protein. FDM cell lines were then cultured in the presence or absence of 4-OHT for 24 h before being deprived of IL-3 over the next 5 days. Cell viability was determined by PI exclusion (Figure 3a; Supplementary Figure S2). Cells from these same deprivation experiments were replated in soft agar with IL-3 and the numbers of colonies formed were counted after 14 days (Figure 3b). iAkt1 expression delayed apoptosis of WT, Bim−/− and Bad−/− FDM cells, but not Puma−/− FDM cells. In clonogenic assays, iAkt1 cells formed more colonies than ieGFP expressing cells, but these differences were not significant. These data show that the capacity of constitutively active Akt1 to promote survival of FDM cells in the absence of IL-3 in part depends on Puma, but not Bim or Bad expression. Importantly, iAkt1 was not sufficient to enhance clonogenic survival of FDM cells, indicating that Akt1 activation alone was not sufficient to permit cytokine-independent proliferation in FDM cells.
Figure 3.
Enforced expression of constitutively active Akt1 only minimally enhances viability of Puma-deficient cells in the absence of IL-3. (a) Multiple independent clones of FDM cells of the indicated genotypes expressing iAkt1 or ieGFP were cultured in the presence of 4-OHT for 24 h before being starved of IL-3 over a 5-day time course. Cell viability was determined by PI exclusion using flow cytometry. (b) The same cells shown in (a) were removed from liquid culture and plated in semi-solid medium containing 0.3% soft agar and 0.5 ng/ml IL-3. The numbers of colonies were counted after 10 days and expressed relative to the numbers of colonies from time 0 day. Data represent means and standard errors of at least four clones of each genotype, from at least three independent experiments
Akt1 delays Puma upregulation in response to IL-3 deprivation
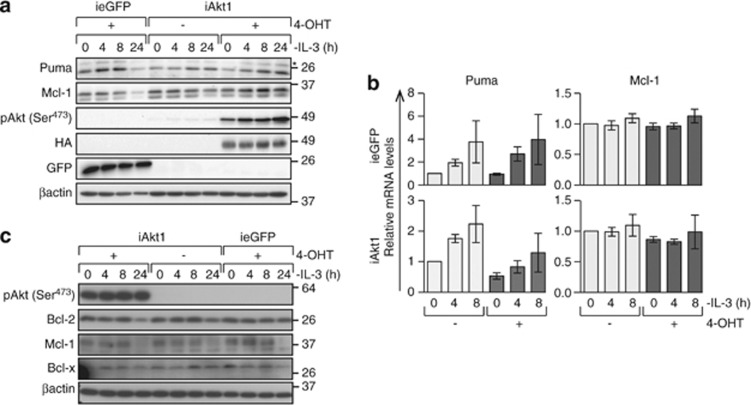
The ability of iAkt1 to delay apoptosis of WT but not Puma−/− FDM cells after IL-3 withdrawal suggested overexpressed Akt1 may delay or prevent upregulated Puma expression in response to IL-3 deprivation. WT FDM cells expressing iAkt1 or ieGFP were starved of IL-3 over 24 h and protein lysates were analysed for Puma expression by western blotting (Figure 4). iAkt1 delayed but did not prevent increased Puma expression over the time course (Figures 4a and b). In ieGFP expressing cells, elevated Puma expression was evident 4 h after IL-3 withdrawal and peaked between 4 and 8 h. By 24 h of IL-3 deprivation, Puma levels had significantly declined because many cells had undergone apoptosis. In cells expressing iAkt1, Puma expression peaked considerably later at 24 h (Figures 4a and b). The level of background iAkt1 expression in the absence of 4-OHT appeared sufficient to delay the increase in Puma expression. To determine whether Akt overexpression delayed Puma transcription, we used qRT-PCR to measure Puma mRNA levels in the presence and absence of IL-3 (Figure 4c). iAkt1 induction delayed the increase in Puma transcription that followed IL-3 deprivation. Thus, constitutively active Akt1 inhibits Puma transcription in response to IL-3 deprivation.
Figure 4.
Enforced expression of constitutively active Akt1 delays Puma upregulation in FDM cells response to IL-3 deprivation. (a) WT cells expressing iAkt1 or ieGFP were cultured in the presence or absence of 4-OHT for 24 h and then deprived of IL-3 for the indicated times. Cell lysates were resolved on SDS-PAGE and western blots probed with the indicated antibodies. Anti-HA antibody was used to detect the presence of induced Akt. (b) WT cells expressing iAkt1 or ieGFP were cultured in the presence or absence of 4-OHT for 24 h before IL-3 was withdrawn for the indicated times. RNA was extracted and reverse transcribed and Q-PCR was performed to measure Puma or Mcl-1 mRNA levels. mRNA levels are expressed relative to the mRNA levels of two reference genes, Hmbs and Polr2a, using the ΔΔCT method. Data represent means and standard errors of three independent clones assayed in three independent experiments. (c) Bax−/−;Bak−/− FDM cells expressing iAkt1 or ieGFP were treated as described in (a). Proteins in cell lysates were resolved on SDS-PAGE and western blots probed with the indicated antibodies
The maintenance of Mcl-1 levels is regulated by cytokine receptor signalling and is critical for haematopoietic cell survival.10, 23 Mcl-1 protein expression was maintained in the presence of iAkt1 but declined in cells expressing ieGFP over 24 h of IL-3 deprivation (Figure 4a). Interestingly, Mcl-1 mRNA expression was stable over this time (Figure 4b), indicating that decreased Mcl-1 levels were a result of protein degradation. Mcl-1 protein levels in cells expressing Akt1 could reflect more viable cells or be a result of a direct function of Akt1 to maintain Mcl-1. To address this, we expressed iAkt1 or ieGFP in FDM cells lacking both Bax and Bak, which are completely resistant to apoptosis induced by IL-3 deprivation, so excluding apoptosis as a cause for changes in Mcl-1 levels. Western blots of induced Akt1 or ieGFP expression in Bax−/−;Bak−/− FDM cells showed that Mcl-1 protein levels declined over 24 h in the absence of IL-3, even when iAkt1 was overexpressed (Figure 4c), thus demonstrating that activated Akt1 was not sufficient to maintain Mcl-1 protein levels following IL-3 deprivation.
Constitutively active Akt1 does not enhance survival of p53 −/− cells
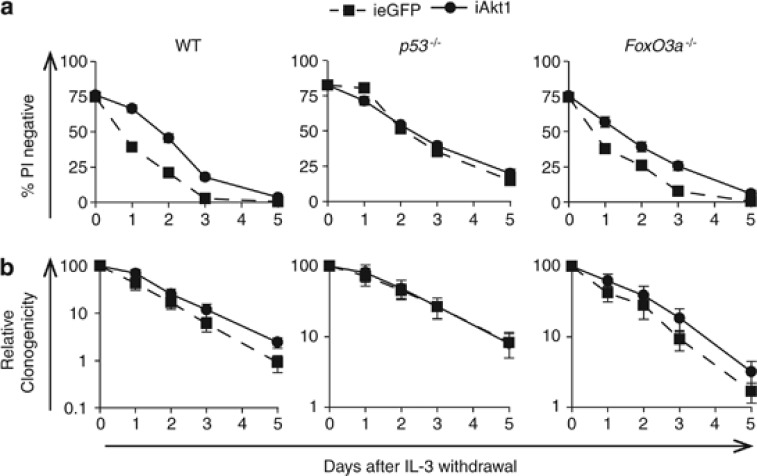
Transcriptional upregulation of Puma in FDM cells after IL-3 deprivation is p53 dependent4 but may also be regulated by the forkhead transcription factor FoxO3a.6, 22 To determine whether p53 or FoxO3a was required for enforced iAkt expression to repress apoptosis, we generated p53−/− and FoxO3a−/− FDM cells expressing iAkt1 or ieGFP and subjected these to IL-3 deprivation in the presence or absence of 4-OHT. Cell viability was determined by PI exclusion (Figure 5a; Supplementary Figure S3a). iAkt1 expression prolonged WT FDM survival after IL-3 withdrawal, but did not prevent their ultimate death. iAkt1 enhanced the survival of FoxO3a−/− but not p53−/− FDM cells. The clonogenic potential of p53−/− or FoxO3a−/− FDM cells was measured in these same experiments using soft agar cultures (Figure 5c; Supplementary Figure S3b). Neither p53−/− nor Foxo3a−/− FDM cells expressing iAkt1 formed more colonies compared with control cells. We used a p53-GFP reporter assay we developed4 to show that iAkt expression repressed p53-dependent transcriptional activity, in the presence of IL-3 (Supplementary Figure S4). These data indicate that iAkt1 repressed p53-dependent apoptotic responses to IL-3 deprivation, which include upregulated Puma expression. However, expression of constitutively active Akt1 did not enhance clonogenic survival above the baseline level that results from p53 deletion, further emphasising that Akt1 activation alone is insufficient to promote proliferation.
Figure 5.
Constitutively active Akt1 does not enhance viability or clonogenicity of p53-deficient FDM cells in the absence of IL-3. (a) Multiple independent clones of FDM cells of the indicated genotypes expressing iAkt1 or ieGFP were cultured in the presence of 4-OHT for 24 h before being starved of IL-3 over a 5-day time course. Cell viability was determined by PI exclusion using flow cytometry. (b) The same cells shown in (a) were removed from liquid culture and plated in medium containing 0.3% soft agar and 0.5 ng/ml IL-3. The numbers of colonies were counted after 10 days and expressed relative to the numbers of colonies from time 0 day. Results from (a) and (b) represent the mean and standard errors of three independent experiments. The numbers (n) of independent clones were as follows: WT iAkt1 n=5, WT ieGFP n=4, p53−/− iAkt1 n=5, p53−/− ieGFP n=5, FoxO3a−/− iAkt1 n=5, FoxO3a−/− ieGFP n=5
Akt inhibition induces apoptosis in WT but not in p53 −/− FDM cells
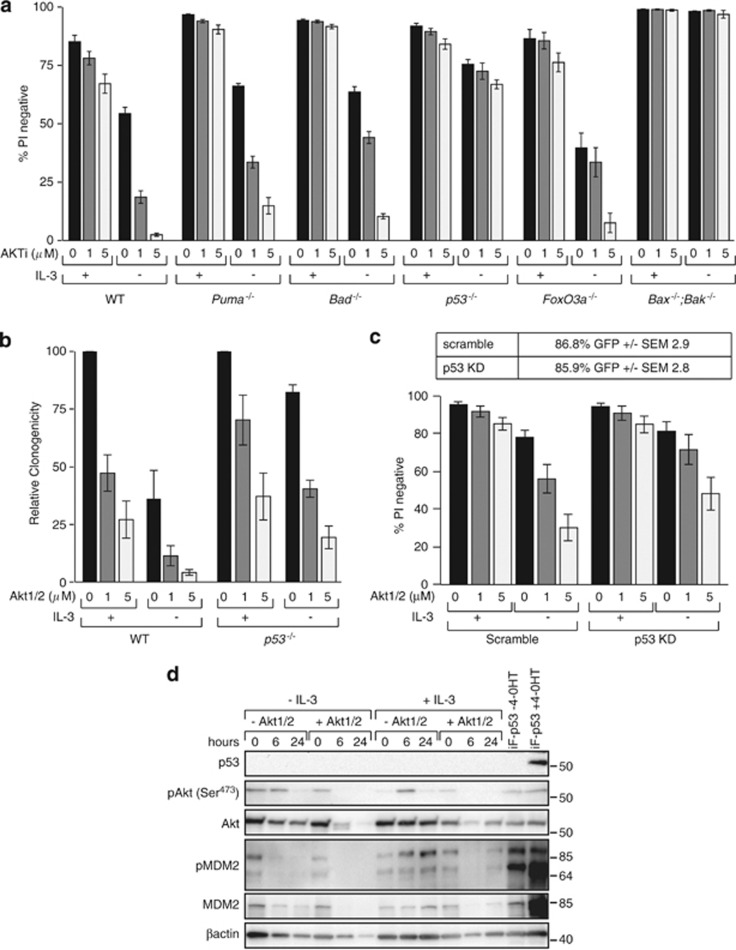
We previously showed that p53−/− FDM cells resist death induced by Akt inhibition.24 We therefore compared how much apoptosis occurred after Akt inhibition in FDM cells lacking Puma, Bad and FoxO3a with cells lacking p53. Multiple independent WT, Bad−/−, Puma−/−, FoxO3a−/−, p53−/− and Bax−/−;Bak−/− FDM cell lines were cultured for 24 h in the presence or absence of IL-3 and the increasing doses of the Akt1/2 inhibitor with cell viability were determined by PI exclusion (Figure 6a). In the absence of IL-3, similar numbers of WT, Bad−/−, FoxO3a−/− and Puma−/− cells died in response to Akt inhibition. Cell death was substantially blocked by IL-3. p53−/−FDM cells were resistant to apoptosis induced by the Akt inhibitor, in the presence or absence of IL-3. To determine if surviving p53−/− cells proliferated when IL-3 was restored, WT and p53−/− FDM cells from these experiments were replated in soft agar containing 0.5 ng/ml IL-3 (Figure 6b). Akt inhibitor-treated WT cells had decreased clonogenic potential. Fewer p53−/− FDM cells formed colonies after Akt inhibitor exposure, although the baseline clonogenicity was much greater than WT. To assess this further, we used a shRNA construct to diminish p53 expression in WT FDM cells, and then treated cells with the Akt inhibitor (Figure 6c). When compared with a scrambled shRNA, fewer cells expressing the p53 shRNA underwent apoptosis in response to Akt inhibition, in the presence or absence of IL-3. The contrast between the susceptibility of Puma−/− and p53−/− FDM cells to the Akt inhibitor indicated that p53-dependent, Puma-independent processes contributed to apoptosis and loss of clonogenicity observed in response to Akt inhibition. p53 protein levels are below detectable limits using western blotting, in the presence or absence of IL-3 (Figure 6d). However, the Akt inhibitor decreased MDM2 levels in cells cultured in IL-3 (Figure 6d). When IL-3 is removed, MDM2 levels also decline, whether treated or not with the Akt inhibitor. These data support the hypothesis that in the context of IL-3/IL-3R signalling, Akt functions to repress p53 activation.
Figure 6.
Apoptosis induced by Akt inhibition requires p53 but not Puma. (a) FDM cells of the indicated genotypes were cultured in the presence or absence of IL-3 and the AKT inhibitor (AKT1/2) at the indicated doses. Cell viability was measured by PI exclusion by flow cytometry. The numbers of independent clones (n) tested were as follows: WT n=5, Puma−/− n= 3, Bad−/− n=2, p53−/− n=3, FoxO3a−/− n=3, Bax−/−;Bak−/− n=2. The results represent means±S.E.M. of all clones tested in at least two independent experiments. Not all clones were tested in all experiments. (b) Multiple WT clones (n=4) or p53−/− clones (n=3) of FDM cells were cultured as in (a) before being washed and replated into medium containing soft agar and IL-3 (0.5 ng/ml). The numbers of colonies were counted after 10 days and expressed relative to the numbers of colonies in cultures not exposed to AKT1/2. Data represent means and standard errors of three independent experiments. (c) WT clones were transduced with retroviral constructs encoding a p53 shRNA or a scrambled control. Infected cells expressed GFP and the percentage of infected cells (±S.E.M.) is shown in the table. Viability was determined by PI exclusion in the presence or absence of IL-3 and the indicated doses of Akt inhibitor (AKT1/2). Data are means±S.E.M. of three independent clones tested in two independent experiments. (d) Western blot of WT FDM cells cultured in the presence or absence of IL-3 and the absence or presence of 5 μM AKT1/2 for 0, 6 and 24 h were probed with antibodies to detect p53, pAkt (Ser473), total Akt, MDM2, pMDM2 and β-actin as a loading control. WT FDM clone infected with lentiviral construct encoding FLAG-tagged p53 under the control of a 4-OHT inducible promoter, in the presence and absence of 4-OHT, is shown as a control for p53 expression
Discussion
IL-3/IL-3R signalling maintains myeloid progenitor cell viability by repressing intrinsic apoptosis pathways. PI3K/Akt activation is thought to have a critical role in this cell survival signalling process. We have shown that Akt1 has a non-redundant role in maintaining FDM cell viability, primarily when concentrations of IL-3 are limiting. Akt represses apoptosis activation by regulating the p53-dependent response to IL-3 deprivation, including upregulation of the expression of the pro-apoptotic Bcl-2 family protein, Puma.
IL-3-dependent cell lines derived from Akt1-, Akt2- or Akt3-deficient mice are a unique model and show that Akt1 is the predominant isoform involved in IL-3-dependent survival signalling through the IL-3R. This is consistent with the increased susceptibility to apoptosis observed in thymocytes and MEFs from Akt1-deficient animals.25 However, it remains an open question as to whether Akt isoforms have non-redundant roles in controlling cell survival. The different phenotypes of Akt1 and Akt2 gene-deleted mice clearly show non-redundant functions of Akt1 and Akt2.26 Akt1−/− mice are prone to growth retardation including pulmonary hypoplasia.25 In contrast, Akt2-deficient mice have abnormalities in insulin receptor signalling and glucose homeostasis.13 With respect to the regulation of apoptosis, it has been suggested that the total level of activated Akt determines whether cells survive or commit to apoptosis. Any apparent non-redundant role of Akt isoforms reflects cell type-specific expression patterns.26 Our data indicate that Akt1 has a specific role in IL-3-dependent FDM cells because deletion of Akt1 (but not Akt2 or Akt3) is sufficient to reduce viability in low concentrations of IL-3 without diminishing total Akt levels. We cannot, however, conclude that only Akt1 can regulate p53-dependent Puma expression, but that in myeloid cell lines, Akt1 is the dominant Akt isoform that regulates cytokine-dependent survival.
Our experiments suggest a specific functional link between Akt1 and Puma because expression of constitutively active Akt1 was unable to further increase survival of Puma−/− FDM cells. Puma was shown to be required for the efficient induction of apoptosis in IL-3 starved myeloid cells3, 17 and Akt1 expression delayed Puma upregulation after cytokine deprivation. This contrasted with the ability of Akt1 to protect the viability of IL-3-dependent FDM cells lacking Bad and Bim, showing that Akt must promote survival independently of these BH3-only proteins. Bim or Bad phosphorylation and inactivation by Akt8, 21, 27 are not required for Akt to repress apoptosis in response to cytokine deprivation. Activated Akt blocks proteasomal degradation of Mcl-1 by inhibiting glycogen synthase kinase-3 (GSK-3), a kinase responsible for Mcl-1 phosphorylation with consequent targeting for ubiquitination.10 Mcl-1 degradation occurs in response to IL-3 (or other cytokine) deprivation. In WT cells and in Bax−/−; Bak−/− FDM cells (which are completely resistant to IL-3 deprivation-induced apoptosis), Mcl-1 expression levels still decline. It is therefore unlikely that in these cells Akt1 has any direct biochemical function to prevent Mcl-1 degradation.
A notable feature of our results was the relatively restricted circumstances under which deletion of Akt1 reduced cell viability, and the relatively weak effect enforced expression of constitutively active Akt1 had in maintaining cell survival. In contrasts to Bcl-2 overexpression or combined loss of Bax and Bak,2, 3 supraphysiological Akt activation was sufficient only to delay apoptosis and not sufficient to maintain proliferation and to promote clonogenic survival. This is consistent with recent observations that the oncogenic effects of Akt activation are most apparent when the tumour suppressor function of p53 is lost.28 Interestingly, in our hands, the deletion of p53 also renders cells resistant to apoptosis triggered by Akt inhibitory drugs. Although Akt activation is a frequent feature of many malignancies, including acute myeloid leukaemia,29 our data indicate that Akt activation is not sufficient to promote leukaemiagenesis. Furthermore, Akt inhibition in the context of mutations in tumour suppressor genes may not prove an effective treatment strategy.
Enforced Akt1 activation regulated apoptosis by delaying p53-mediated induction of Puma after IL-3 withdrawal. This emphasises the central role p53 has in the response to cytokine deprivation in myeloid cells,4, 17, 30 but not in lymphoid cells.31 Several mechanisms may link cytokine-mediated Akt activation to p53. First, the p53-regulator MDM2 is a substrate of Akt,4, 28, 32 and Akt can control the abundance of p53 by regulating this E3 ligase responsible for p53 turnover. Second, at least in the context of enforced Akt activation, Akt may maintain glucose uptake in the face of IL-3 derivation, perhaps by maintaining the activity of specific glucose transporters.17, 33 Consistent with this are data showing that enforced expression of Glut1 or Hexakinase maintains glucose import and represses Puma expression in FL5.12 cells deprived of IL-3,17 and data that the capacity of constitutively active Akt to block Puma upregulation after IL-3 deprivation requires glucose.33 Third, a recent model showed that p53-dependent Puma expression in response to DNA damage requires GSK-3.34 Since GSK-3 is a substrate of Akt, it is possible that this pathway also operates in the presence of IL-3 or activated Akt1 expression. Finally, loss of p53 is associated with an altered kinase signalling environment in IL-3-dependent FDM cells that permits survival and proliferation in an Akt-independent manner.24
In conclusion, we have shown that Akt1 regulates the viability of FDM cells in limiting concentrations of cytokine. Akt1 represses Puma expression, to promote viability, although there are clearly Puma-independent mechanisms that are also controlled by Akt. Our data strongly support the hypothesis that Akt represses the activation of apoptosis pathways after cytokine deprivation principally through repressing p53-dependent responses that accompany cytokine deprivation rather than by the direct regulation of Bcl-2 family members. It may be that Akt-dependent repression of apoptosis in conditions of limiting cytokine concentrations permits survival of cells that then acquire mutations that drive unrestricted proliferation.
Materials and Methods
Cell lines and culture
FDM cells of the indicated genotypes were generated from Puma−/−, Bim−/−, Bad−/−, Bax−/−;Bak−/−, Akt1−/−, Akt2−/− and Akt3−/− mice that are described as referenced.13, 14, 15, 35, 36, 37, 38 All animals had been crossed for at least 10 generations onto a C57Bl/6 background having been generated 129svJ embryonic stem cells. Haematopoietic stem/progenitor cells from E14.5 embryos were infected with a Hoxb8 retrovirus, as previously described.16 FDM cells were cultured in low-glucose (LG) DMEM (Invitrogen, Mulgrave, VIC, Australia) supplemented with 10% (v/v) Heat-Inactivated Fetal Calf Serum (JRH Laboratories) and 0.25 ng/ml murine rIL-3 (0.5 ng/ml IL-3 in Akt1−/−, Akt2−/−, Akt3−/− and corresponding WT cell lines) (R&D Systems, Minneapolis, MN, USA) at 10% CO2 at 37oC. ψ2 fibroblasts (producing Hoxb8 retrovirus) and 293T cells were maintained in high-glucose DMEM (Invitrogen) supplemented with 10% (v/v) FCS.
Cloning
The mouse Akt1 cDNA, encoding myristoylated Akt, lacking the PH domain with an N-terminal HA tag (myr-HA-AKT1(ΔPH) (gift from D James, Garvan Institute of Medical Research, Sydney), Flag-tagged p53 or eGFP was cloned into the pF5xUAS-SV40-puromycin lentiviral vector.20 Cells were co-infected with GEV16 and pF5xUAS-SV40. Infected cells were selected using hygromycin (400 μg/ml) and puromycin (4 μg/ml). Clones were selected and tested for expression of the retrovirally encoded proteins by intracellular staining. Expression of HA-myr-Akt1 or eGFP was induced by treatment with 1 μM 4-OHT for 24 h before the start of all experiments.
Lentiviral and retroviral production and infection
293T cells were grown to 70% confluence before being transfected with either a 5 : 3 : 2 mixture of pCMVδR.8, GEV16 or pF5xUAS-SV40 and VSVG plasmids for lentiviral production or 3 : 5 : 2 mixture of GAGpol, LMP shRNA and Env for retroviral production using Effectene as per manufacturer's instructions (Qiagen, Chadstone, VIC, Australia). Viral supernatants were harvested at 24 and 48 h and either used fresh or stored at −80oC. Lentiviral infection of FDM cells was performed by plating FDM cells at 50% confluence in viral supernatant, supplemented with 10% (v/v) FCS, 0.25 ng/ml rIL-3, with 5 μg/ml polybrene (Sigma-Aldrich, St Louis, MO, USA). Cells were then centrifuged for 90 min at 2500 r.p.m. at 30oC. Cultures were left overnight before fresh medium was added. After 1 week, cells were selected with either 400 μg/ml Hygromycin (Roche, Melbourne, VIC, Australia) for 7 days or 4 μg/ml Puromycin (Sigma-Aldrich) for 4 days. Clones were selected by growing aliquots of cells in DMEM containing 0.5 ng/ml and 20% FCS in semi-solid media of DMEM, 10% FCS, 0.5 ng/ml IL-3 and 0.03% soft agar. Retroviral infection was performed after non-tissue culture 12-well plates were coated with 30 μg/ml retronectin in PBS for 1 h at room temperature. Wells were washed gently with PBS before a 2% BSA/PBS solution was added to wells for 30 min. After incubation, wells were again washed in PBS before viral supernatant was added to wells in DMEM, 10% FCS, 0.25 ng/ml IL-3 and plates spun at 4000 r.p.m. for 1 h at 22oC. Cells were then added to wells in the presence of 5 μg/ml polybrene overnight at 37 oC 10% CO2. Cells were then cultured as normal and viral transduction confirmed by GFP expression.
Intracellular staining
To detect Akt expression, cells were induced for 24 h with 100 μM 4-OHT before being washed in PBS and fixed with 80% methanol on ice for 20 min. Cells were washed in PBS again and 1 : 200 anti-HA (Covance) was added to cells in antibody buffer (0.03% Saponin, 10% FCS in PBS). Cells were incubated for 1 h on ice before being washed and 1 : 100 anti-mouse-PE was added in antibody buffer. Cells were incubated for 1 h at room temp before being washed, resuspended in PBS and analysed by LSRII. Comparative histograms were generated using FCSExpress (DeNovo Software, Los Angeles, CA, USA).
Immunoblotting
Cell lysates were generated using RIPA buffer (150 mM NaCl, 50 mM TrisHCl pH 7.4, 0.5% NaDOC, 0.1% SDS, 1% NP-40) or Onyx Buffer (20 mM TrisHCl pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 10% Glycerol, 1% Triton X-100) with a Protease Inhibitor Cocktail (Roche) and Phosphatase Inhibitors (2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM Na3VO4) at a cell density of 5 × 104 cells/μl of buffer. Lysates were boiled in the presence of loading buffer (2% SDS, 10% Glycerol, 60 mM Tris pH 6.8, 5% 2-mercaptoethanol and 0.01% bromophenol blue) for 5 min before proteins were resolved on 12 or 15% SDS-PAGE gels. Proteins were detected by western blotting using chemiluminescence (ECL reagent; Thermo Scientific (Scoresby, VIC, Australia) or ECL Plus reagent; Amersham (Rydalmere, NSW, Australia)). The following antibodies were used: anti-AKT, anti-phosphoAKT, anti-phosphoAKT XP, anti-AKT1, anti-phosphoMDM2 (Cell Signaling, Boston, MA, USA), anti-Bcl2 (Pharmingen, North Ryde, NSW, Australia), anti-Bcl-XL (R&D Systems, Minneapolis, MN, USA), anti-GFP (Molecular Probes, Mulgrave, VIC, Australia), anti-HA (Chemicon, Billerica, MA, USA), anti-Mcl-1 (Rockland, Gilbertsville, PA, USA), anti-MDM2 (Calbiochem, Billerica, MA, USA), anti-mouse-HRP, anti-β-actin (Sigma-Aldrich), anti-PUMA (ProSci, Poway, CA, USA), anti-Rabbit-HRP (Amersham), anti-p53 (Pharmingen).
Viability and clonogenic assays
Viability was determined using PI exclusion as assessed by flow cytometry. At the end point of the experiment, cells were washed in PBS and propidium iodide (Sigma-Aldrich) added at 1 μg/ml in PBS. Cells were analysed using a Becton Dickson LSRII and FACSDiva. Clonogenic assays were performed as previously described.16 Briefly, 500, 2500 or 12 500 cells from viability assays were plated in DMEM supplemented with 20% FCS and 0.5 ng/ml IL-3 and 0.03% soft agar. After 14 days, the number of colonies was then counted and the clonogenicity calculated relative to the same clone at time zero or untreated.
Cell-cycle analysis
Cell-cycle analysis was performed by staining nuclear DNA of fixed cells with PI (using hypotonic solution) followed by FACS analysis as previously described.4
Real-time PCR
RNA was extracted from 1 × 106 cells using the RNeasy RNA extraction kit (Qiagen), as per manufacturer's instructions. In all, 500 ng of RNA was reverse transcribed using MMLV RVTase H minus mutant (Promega, Sydney, NSW, Australia) and random primers (Promega) following manufacturer's instructions. TaqMan gene expression assays using FAM (6-carboxy-fluorescein)-labelled probes (Applied Biosystems, Mulgrave, VIC, Australia) were used to quantitate Puma (Bbc3, Mm00519268_m1) or Mcl-1 (Mm00725832_s1) mRNA levels and compared with two reference genes, Hmbs (Mm01143545_m1) and Polr2a (00839502_m1) using the Viia7 Real-Time PCR system (Applied Biosystems). The ΔΔCT method was used to calculate mRNA levels relative to control genes.
Acknowledgments
We thank Professor Andreas Strasser and Dr. Philippe Bouillet for the provision of mice and advise. We thank Dr. Matthew Burton for help with flow cytometry. This work is supported by project grants from the National Health and Medical Research Council of Australia (NHMRC). PGE is supported by the Viertel Senior Medical Research Fellowship. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Glossary
- 4-OHT
4-hydroxytamoxifen
- PI3K
phosphoinositide-3 kinase
- IL-3R
interleukin-3 receptor
- IL-3
interleukin-3
- FDM cells
factor-dependent myeloid cells
- WT
wild type
- iAkt1
4-OHT inducible Akt14-OHT
- ieGFP
inducible enhanced Green Fluorescent Protein
- GSK-3
glycogen synthase kinase-3
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by C Borner
Supplementary Material
References
- Brumatti G, Salmanidis M, Ekert PG. Crossing paths: interactions between the cell death machinery and growth factor survival signals. Cell Mol Life Sci. 2010;67:1619–1630. doi: 10.1007/s00018-010-0288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Ekert PG, Jabbour AM, Manoharan A, Heraud JE, Yu J, Pakusch M, et al. Cell death provoked by loss of interleukin-3 signaling is independent of Bad, Bim, and PI3 kinase, but depends in part on Puma. Blood. 2006;108:1461–1468. doi: 10.1182/blood-2006-03-014209. [DOI] [PubMed] [Google Scholar]
- Jabbour AM, Daunt CP, Green BD, Vogel S, Gordon L, Lee RS, et al. Myeloid progenitor cells lacking p53 exhibit delayed upregulation of Puma and prolonged survival after cytokine deprivation. Blood. 2010;115:344–352. doi: 10.1182/blood-2009-07-230730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T, Thompson CB. Cell death in the absence of Bax and Bak. Cell Death Differ. 2006;13:1272–1276. doi: 10.1038/sj.cdd.4401953. [DOI] [PubMed] [Google Scholar]
- Ekoff M, Kaufmann T, Engstrom M, Motoyama N, Villunger A, Jonsson JI, et al. The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood. 2007;110:3209–3217. doi: 10.1182/blood-2007-02-073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Altman BJ, Coloff JL, Herman CE, Jacobs SR, Wieman HL, et al. Glycogen synthase kinase 3alpha and 3beta mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol. 2007;27:4328–4339. doi: 10.1128/MCB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert PG, Read SH, Silke J, Marsden VS, Kaufmann H, Hawkins CJ, et al. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J Cell Biol. 2004;165:835–842. doi: 10.1083/jcb.200312031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Coloff JL, Ferguson EC, Jacobs SR, Cui K, Rathmell JC. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J Biol Chem. 2008;283:36344–36353. doi: 10.1074/jbc.M803580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu AL, Gonin S, Leverrier Y, Blanquier B, Thomas J, Dantin C, et al. Activation of the phosphatidylinositol 3-kinase/Akt pathway protects against interleukin-3 starvation but not DNA damage-induced apoptosis. J Biol Chem. 2001;276:10935–10942. doi: 10.1074/jbc.M007147200. [DOI] [PubMed] [Google Scholar]
- Chan JC, Hannan KM, Riddell K, Ng PY, Peck A, Lee RS, et al. AKT promotes rRNA synthesis and cooperates with c-MYC to stimulate ribosome biogenesis in cancer. Sci Signal. 2011;4:ra56. doi: 10.1126/scisignal.2001754. [DOI] [PubMed] [Google Scholar]
- Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O'Reilly LA, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 2009;16:555–563. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu HA, Gotoh Y, et al. Akt phosphorylation of bad couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coloff JL, Macintyre AN, Nichols AG, Liu T, Gallo CA, Plas DR, et al. Akt-dependent glucose metabolism promotes mcl-1 synthesis to maintain cell survival and resistance to bcl-2 inhibition. Cancer Res. 2011;71:5204–5213. doi: 10.1158/0008-5472.CAN-10-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour AM, Gordon L, Daunt CP, Green BD, Kok CH, D'Andrea R, et al. p53-dependent transcriptional responses to interleukin-3 signaling. PLoS One. 2012;7:e31428. doi: 10.1371/journal.pone.0031428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Xu P, Gottlob K, Chen M, Sokol K, Shiyanova T, et al. Growth retardation and increased apoptosis in mice with homozygous disruption ofthe akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shi Y, Birnbaum MJ, Ye K, De Jong R, Oltersdorf T, et al. Quantitative analysis of anti-apoptotic function of Akt in Akt1 and Akt2 double knock-out mouse embryonic fibroblast cells under normal and stressed conditions. J Biol Chem. 2006;281:31380–31388. doi: 10.1074/jbc.M606603200. [DOI] [PubMed] [Google Scholar]
- Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- Astle MV, Hannan KM, Ng PY, Lee RS, George AJ, Hsu AK, et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene. 2012;31:1949–1962. doi: 10.1038/onc.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini J, Elie C, Bardet V, Chapuis N, Park S, Broet P, et al. Constitutive phosphoinositide 3-kinase/Akt activation represents a favorable prognostic factor in de novo acute myelogenous leukemia patients. Blood. 2007;110:1025–1028. doi: 10.1182/blood-2006-12-061283. [DOI] [PubMed] [Google Scholar]
- Lotem J, Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood. 1993;82:1092–1096. [PubMed] [Google Scholar]
- Strasser A, Harris AW, Jacks T, Cory SDNA. damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–1303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- Coloff JL, Mason EF, Altman BJ, Gerriets VA, Liu T, Nichols AN, et al. Akt requires glucose metabolism to suppress puma expression and prevent apoptosis of leukemic T cells. J Biol Chem. 2011;286:5921–5933. doi: 10.1074/jbc.M110.179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet C, Wissler M, Brauns-Schubert P, Wang SJ, Tang Y, Sigloch FC, et al. Phosphorylation of Tip60 by GSK-3 determines the induction of PUMA and apoptosis by p53. Mol Cell. 2011;42:584–596. doi: 10.1016/j.molcel.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2003;22:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton J, DI G, Zhang L-C, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.