Abstract
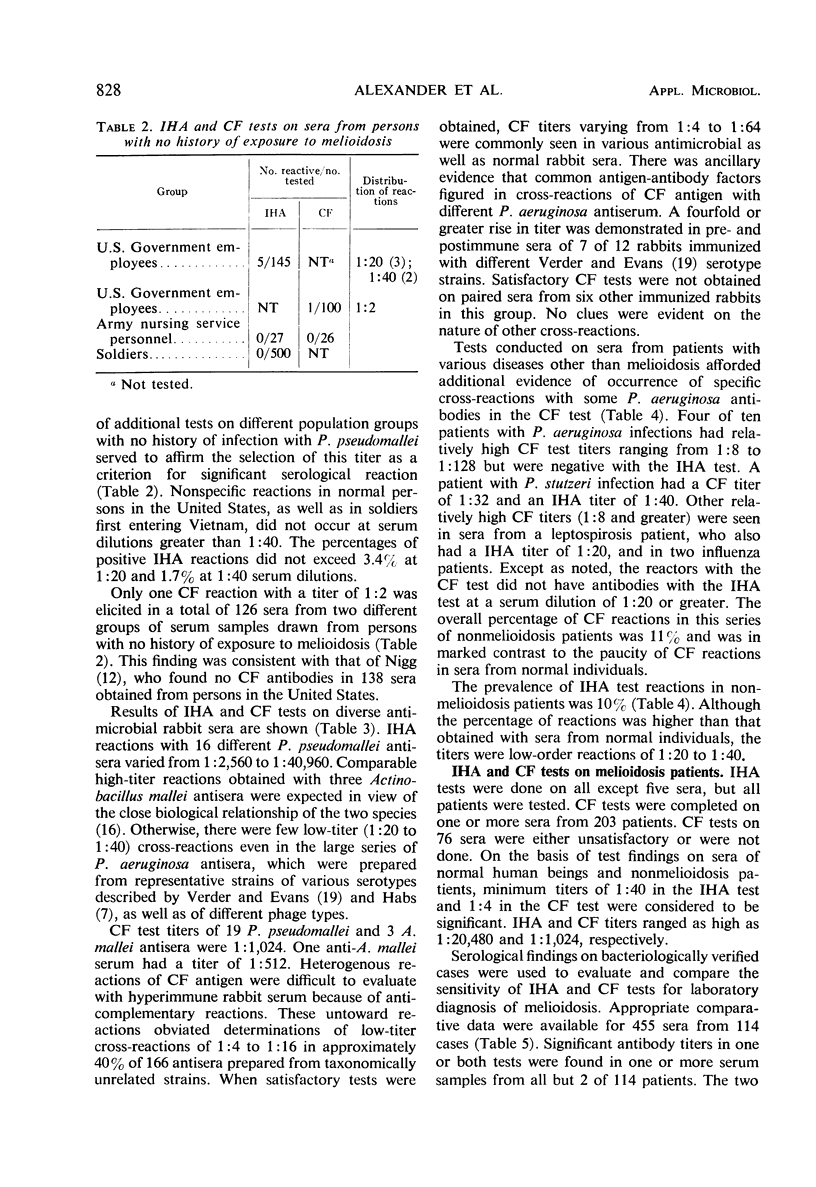
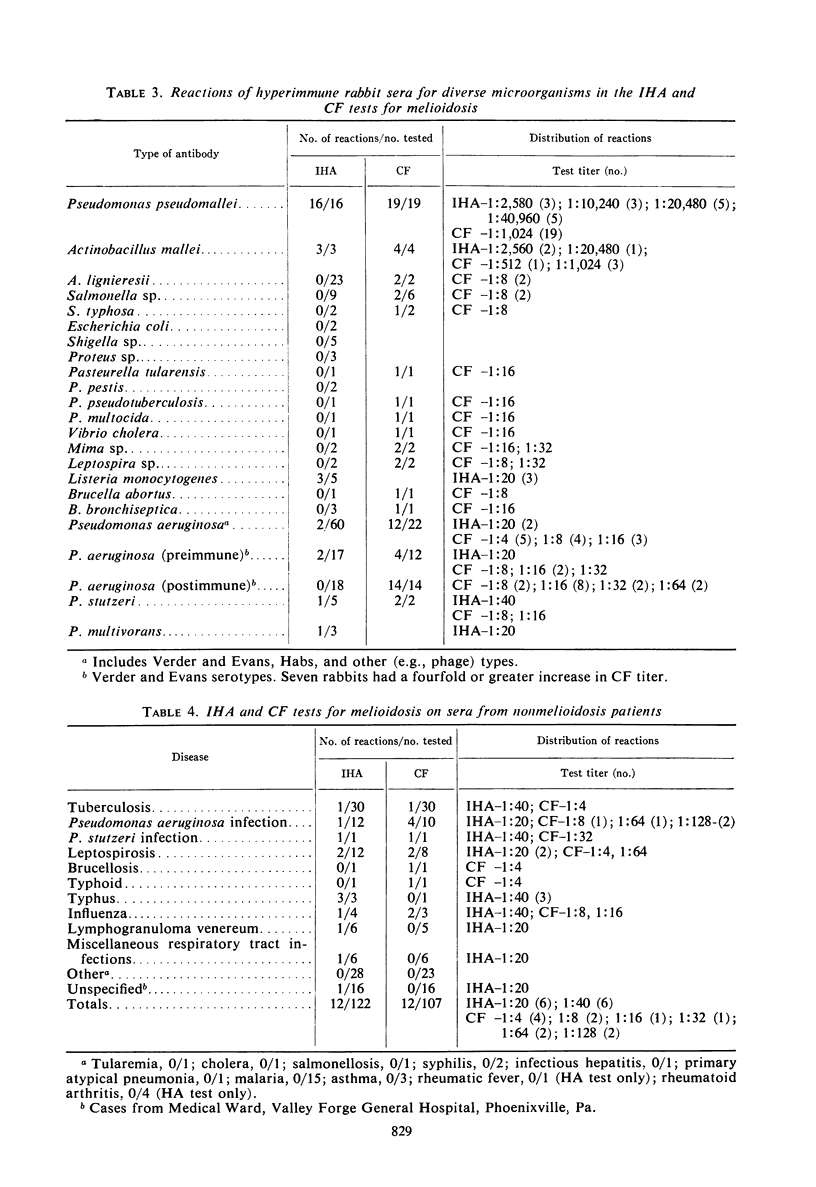
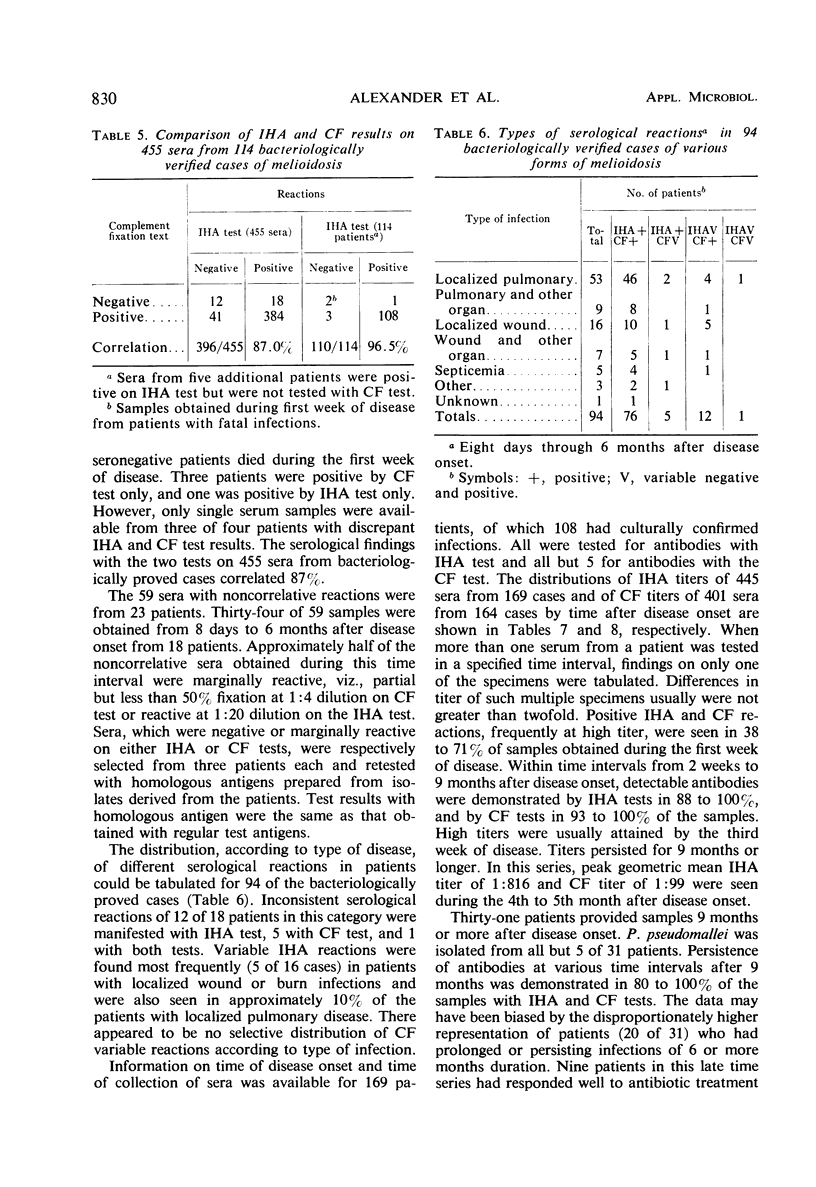
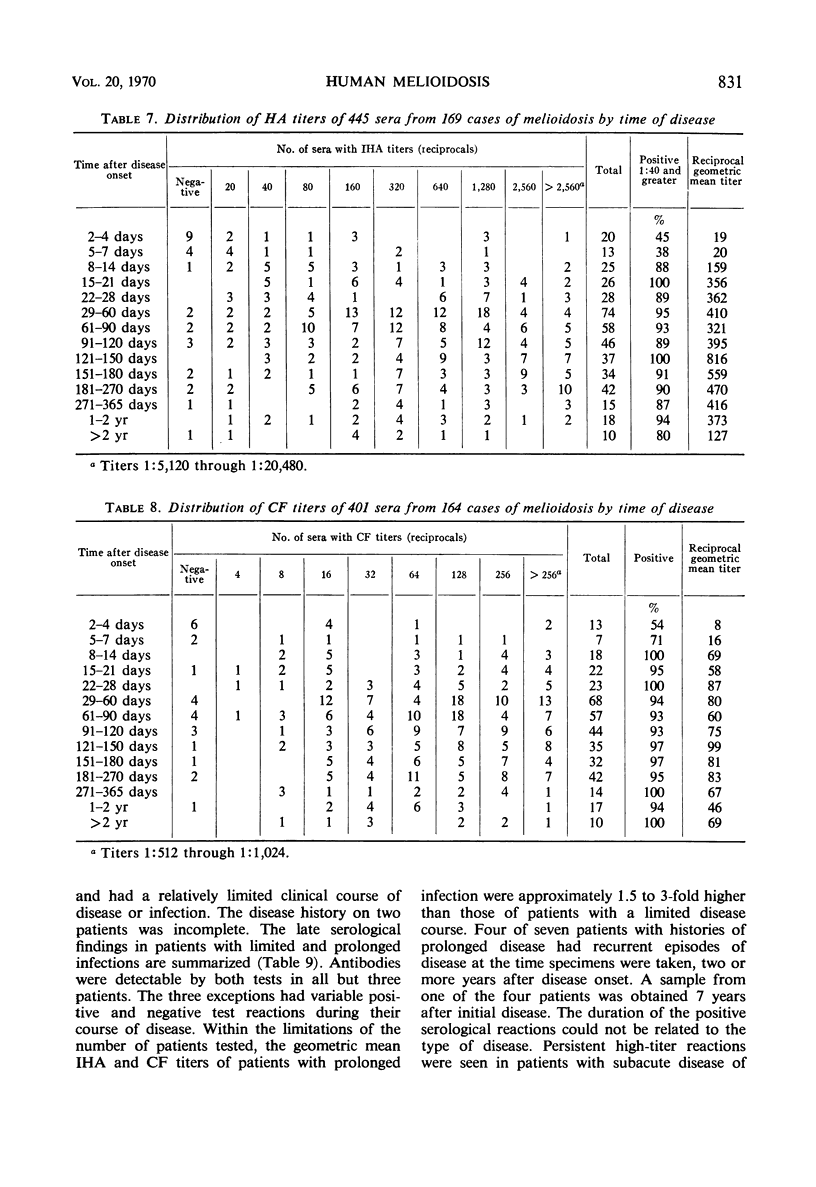
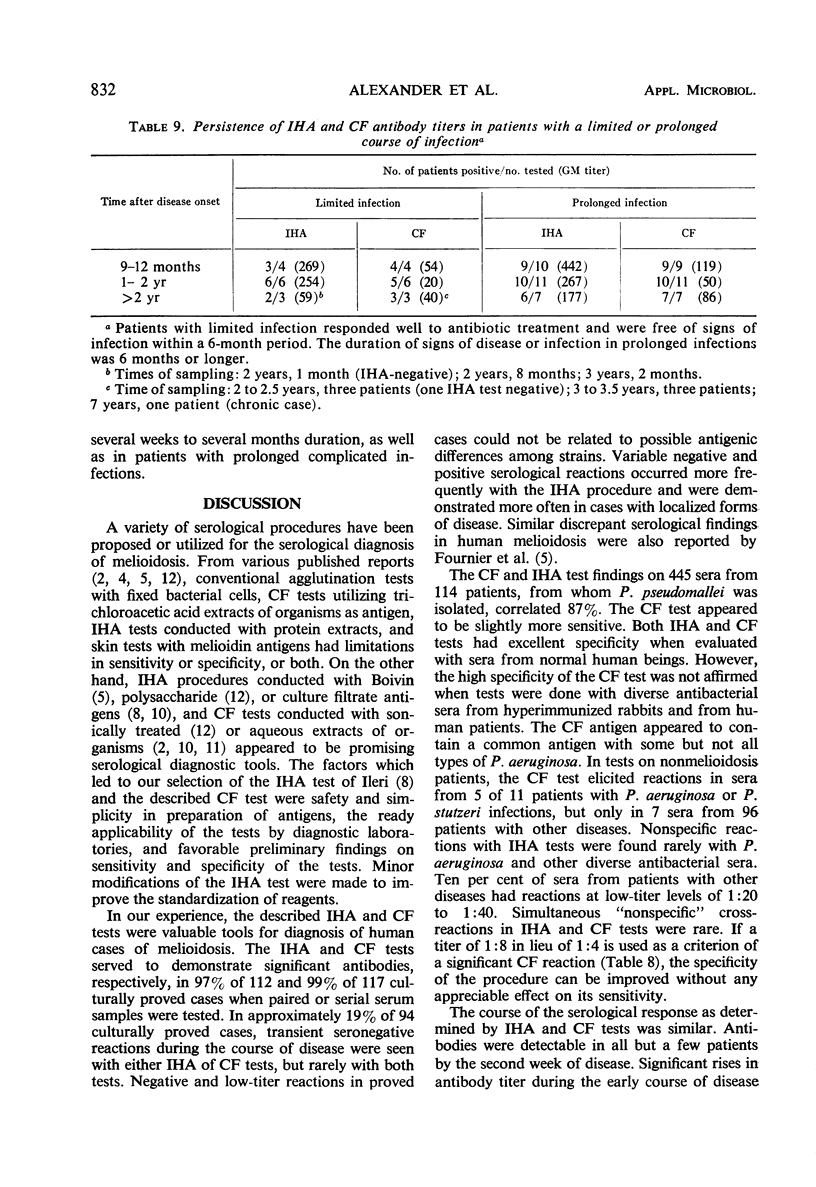
An indirect hemagglutination (IHA) test and a complement fixation (CF) test were evaluated from test results on sera from 212 human melioidosis patients of which 119 were culturally proved cases. Significant antibody titers (IHA titers of 1:40 or greater and CF titers of 1:4 or greater) were demonstrated with either test in all except five patients. IHA and CF titers ranged as high as 1:20,480 and 1:1,024, respectively. Antibodies were usually demonstrated by both tests 1 week after onset of disease. Transient seronegative reactions during the course of disease were seen in sera of approximately 19% of the patients with either IHA and CF but rarely with both tests. High titers in either test were obtained by the third week of disease and reached maximum levels in 4 to 5 months. Titers usually were detectable for 9 or more months. Antibodies were detected by IHA and CF tests in 80 to 100% of the sera obtained at various time intervals from 9 months to 2 or more years after disease onset. Antibody persistence occurred in patients who had a short disease course, as well as in patients with prolonged, complicated infections. The IHA test had excellent specificity when evaluated with normal human sera and diverse antimicrobial sera from hyperimmunized rabbits and human patients. The CF antigen appeared to contain common antigens with some but not all types of Pseudomonas aeruginosa. The specificity of the CF antigen could be enhanced without appreciable effect on its sensitivity by use of a titer of 1:8 in lieu of 1:4 as a criterion for a significant reaction. Either test could be used advantageously for the laboratory diagnosis of melioidosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAVITZ L., MILLER W. R. Immunologic studies with Malleomyces mallei and Malleomyces pseudomallei, agglutination and complement fixation tests in man and laboratory animals. J Infect Dis. 1950 Jan-Feb;86(1):52–62. doi: 10.1093/infdis/86.1.52. [DOI] [PubMed] [Google Scholar]
- Cooper E. B. Melioidosis. JAMA. 1967 May 8;200(6):452–453. [PubMed] [Google Scholar]
- FOURNIER J., DE LAJUDIE P., CHAMBON L. Recherches sur les procédes de laboratoire applicables au diagnostic de la melioidose. Med Trop (Mars) 1953 Sep-Oct;13(5):683–697. [PubMed] [Google Scholar]
- Flemma R. J., DiVincenti F. C., Dotin L. N., Pruitt B. A., Jr Pulmonary melioidosis; a diagnostic dilemma and increasing threat. Ann Thorac Surg. 1969 Jun;7(6):491–499. doi: 10.1016/s0003-4975(10)66386-2. [DOI] [PubMed] [Google Scholar]
- Greenawald K. A., Nash G., Foley F. D. Acute systemic melioidosis. Autopsy findings in four patients. Am J Clin Pathol. 1969 Aug;52(2):188–198. doi: 10.1093/ajcp/52.2.188. [DOI] [PubMed] [Google Scholar]
- HABS I. Untersuchungen über die O-Antigene von Pseudomonas aeruginosa. Z Hyg Infektionskr. 1957;144(3):218–228. [PubMed] [Google Scholar]
- ILERI S. Z. THE INDIRECT HAEMAGGLUTINATION TEST IN THE DIAGNOSIS OF MELIOIDOSIS IN GOATS. Br Vet J. 1965 Apr;121:164–170. doi: 10.1016/s0007-1935(17)41254-1. [DOI] [PubMed] [Google Scholar]
- KENT J. F., FIFE E. H., Jr Precise standardization of reagents for complement fixation. Am J Trop Med Hyg. 1963 Jan;12:103–116. doi: 10.4269/ajtmh.1963.12.103. [DOI] [PubMed] [Google Scholar]
- NIGG C., JOHNSTON M. M. Complement fixation test in experimental clinical and subclinical melioidosis. J Bacteriol. 1961 Aug;82:159–168. doi: 10.1128/jb.82.2.159-168.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGG C. SEROLOGIC STUDIES ON SUBCLINICAL MELIOIDOSIS. J Immunol. 1963 Jul;91:18–28. [PubMed] [Google Scholar]
- PREVATT A. L., HUNT J. S. Chronic systemic melioidosis; review of literature and report of a case, with a note on visual disturbance due to chloramphenicol. Am J Med. 1957 Nov;23(5):810–823. doi: 10.1016/0002-9343(57)90382-0. [DOI] [PubMed] [Google Scholar]
- Rice C. E., Konst H., Duthie R. C. Studies by Complement-Fixation Methods of Malleins Produced in Broth and Synthetic Media. 1. Relative Immunizing Activities in Horses and Rabbits. Can J Comp Med Vet Sci. 1951 Dec;15(12):284–291. [PMC free article] [PubMed] [Google Scholar]
- Rogul M., Brendle J. J., Haapala D. K., Alexander A. D. Nucleic acid similarities among Pseudomonas pseudomallei, Pseudomonas multivorans, and Actinobacillus mallei. J Bacteriol. 1970 Mar;101(3):827–835. doi: 10.1128/jb.101.3.827-835.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spotnitz M., Rudnitzky J., Rambaud J. J. Melioidosis pneumonitis. Analysis of nine cases of a benign form of melioidosis. JAMA. 1967 Dec 4;202(10):950–954. doi: 10.1001/jama.202.10.950. [DOI] [PubMed] [Google Scholar]
- Strauss J. M., Alexander A. D., Rapmund G., Gan E., Dorsey A. E. Melioidosis in Malaysia. 3. Antibodies to Pseudomonas pseudomallei in the human population. Am J Trop Med Hyg. 1969 Sep;18(5):703–707. [PubMed] [Google Scholar]
- VERDER E., EVANS J. A proposed antigenic schema for the identification of strains of Pseudomonas aeruginosa. J Infect Dis. 1961 Sep-Oct;109:183–193. doi: 10.1093/infdis/109.2.183. [DOI] [PubMed] [Google Scholar]
- Weber D. R., Douglass L. E., Brundage W. G., Stallkamp T. C. Acute varieties of melioidosis occurring in U. S. soldiers in Vietnam. Am J Med. 1969 Feb;46(2):234–244. doi: 10.1016/0002-9343(69)90008-4. [DOI] [PubMed] [Google Scholar]


