Abstract
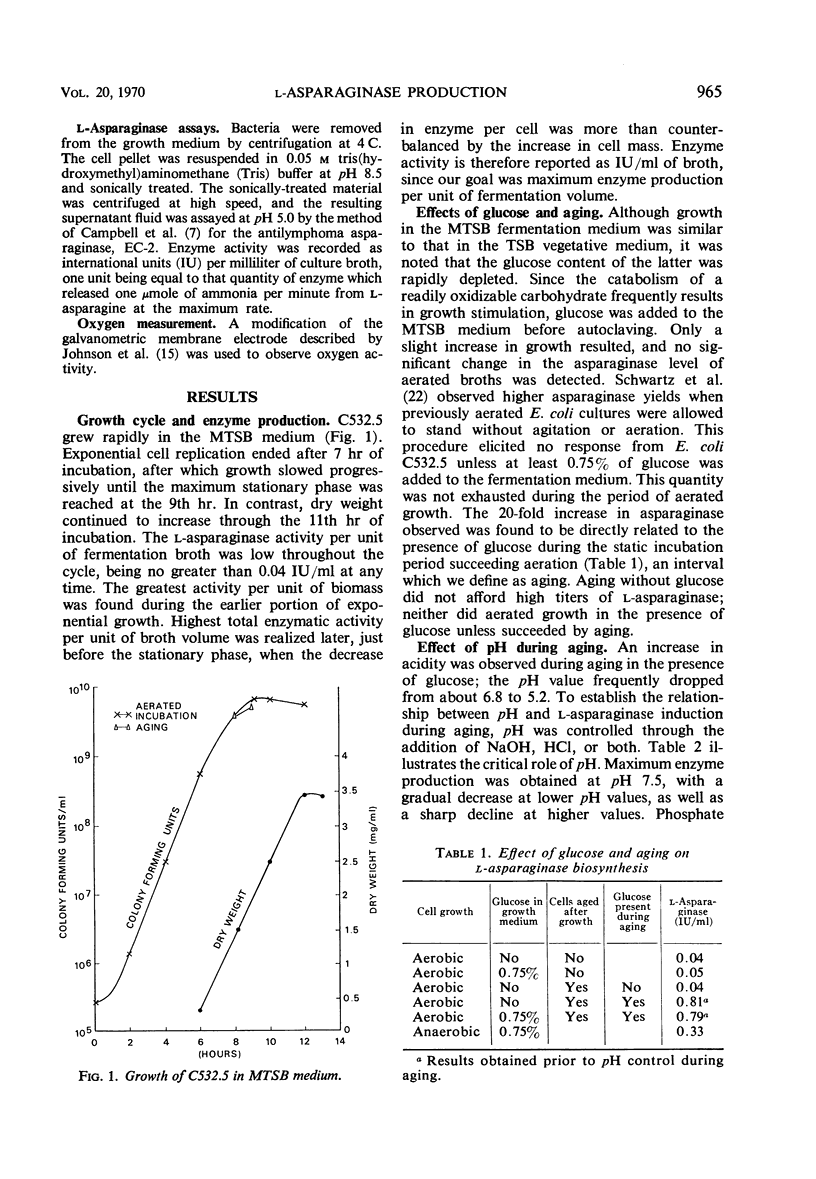
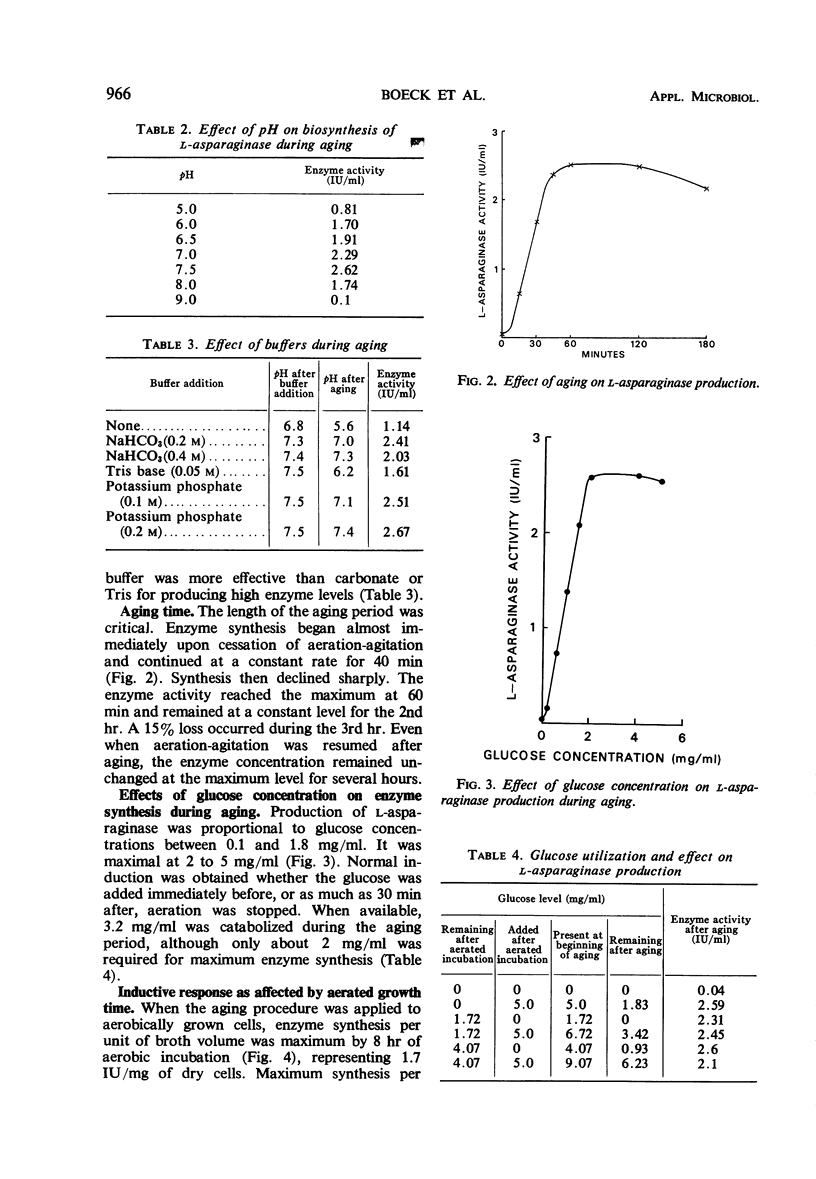
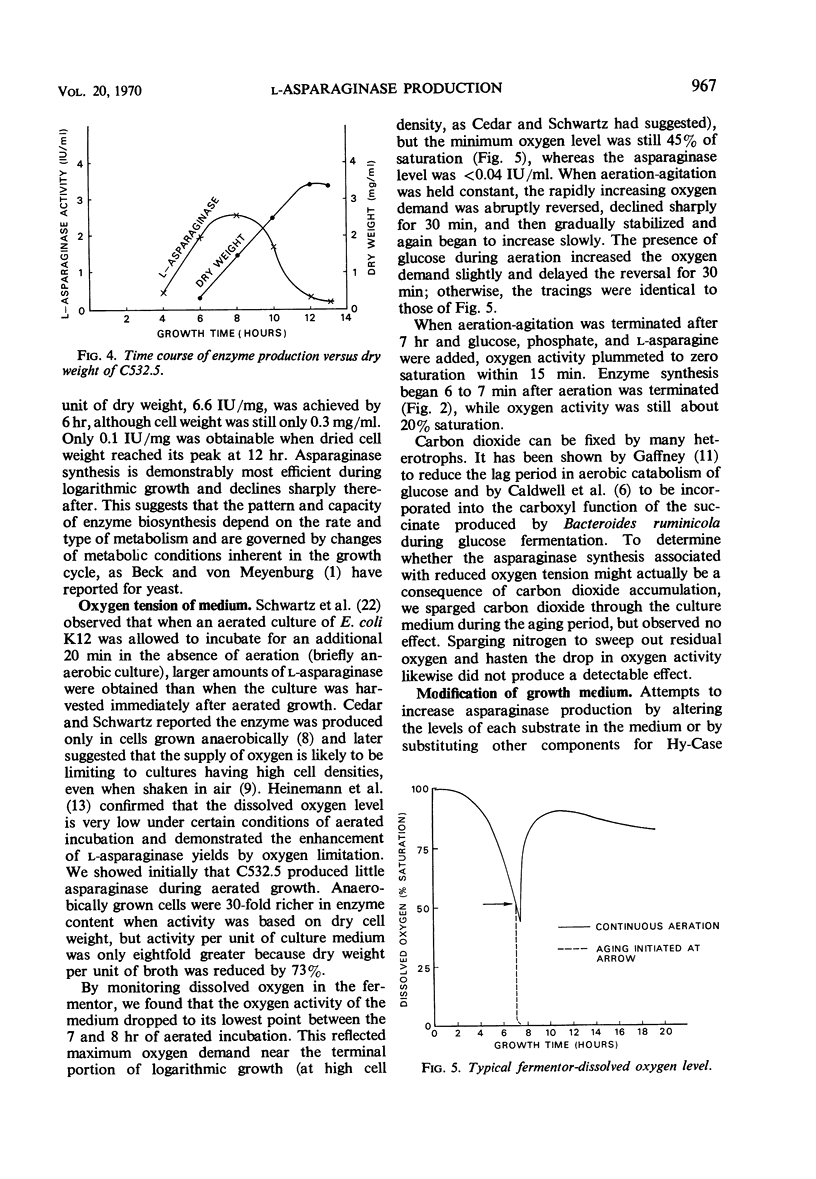
Cultural and nutritional requirements for maximum l-asparaginase synthesis were determined. Conventional aerobic and anaerobic fermentations were not satisfactory. The former yielded larger quantities of cells containing minimal amounts of l-asparaginase, whereas the latter supplied only minute amounts of bacteria that contained an abundance of enzyme. However, the combination of these classical methods, i.e., allowing growth to proceed aerobically until the mid to late exponential phase and then forcing the facultative microbial cells toward anaerobic metabolism by static incubation, produced 2.6 international units of enzyme per ml of fermentation broth when glucose was present. Enzyme synthesis was not induced by terminating aeration-agitation in the absence of glucose, nor was it induced in the presence of glucose when aeration was continued. Use of 0.2 m phosphate buffer resulted in a constant pH near the optimum value of 7.5 during l-asparaginase formation. Addition of 0.05% l-asparagine prior to induction was also beneficial, but other amino acids or their catabolites failed to increase biosynthesis of l-asparaginase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEGGS W. H., LICHSTEIN H. C. REPRESSION OF TRYPTOPHANASE SYNTHESIS IN ESCHERICHIA COLI. J Bacteriol. 1965 Apr;89:996–1004. doi: 10.1128/jb.89.4.996-1004.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C., von Meyenburg H. K. Enzyme pattern and aerobic growth of Saccharomyces cerevisiae under various degrees of glucose limitation. J Bacteriol. 1968 Aug;96(2):479–486. doi: 10.1128/jb.96.2.479-486.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs W. H., Rogers P. Galactose repression of beta-galactosidase induction in Escherichia coli. J Bacteriol. 1966 May;91(5):1869–1874. doi: 10.1128/jb.91.5.1869-1874.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome J. D. Antilymphoma activity of L-asparaginase in vivo: clearance rates of enzyme preparations from guinea pig serum and yeast in relation to their effect on tumor growth. J Natl Cancer Inst. 1965 Dec;35(6):967–974. [PubMed] [Google Scholar]
- Caldwell D. R., Keeney M., Van Soest P. J. Effects of carbon dioxide on growth and maltose fermentation by Bacteroides amylophilus. J Bacteriol. 1969 May;98(2):668–676. doi: 10.1128/jb.98.2.668-676.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell H. A., Mashburn L. T., Boyse E. A., Old L. J. Two L-asparaginases from Escherichia coli B. Their separation, purification, and antitumor activity. Biochemistry. 1967 Mar;6(3):721–730. doi: 10.1021/bi00855a011. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Localization of the two-L-asparaginases in anaerobically grown Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3753–3755. [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Production of L-asparaginase II by Escherichia coli. J Bacteriol. 1968 Dec;96(6):2043–2048. doi: 10.1128/jb.96.6.2043-2048.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey R. J., Kiszkiss D. F., Nuner J. H. Influence of oxygen on development of nitrate respiration in Bacillus stearothermophilus. J Bacteriol. 1969 Jun;98(3):1056–1062. doi: 10.1128/jb.98.3.1056-1062.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAFFNEY P. E. CARBON DIOXIDE EFFECTS ON GLUCOSE CATABOLISM BY MIXED MICROBIAL CULTURES. Appl Microbiol. 1965 Jul;13:507–510. doi: 10.1128/am.13.4.507-510.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann B., Howard A. J., Palocz H. J. Influence of dissolved oxygen levels on production of L-asparaginase and prodigiosin by Serratia marcescens. Appl Microbiol. 1970 May;19(5):800–804. doi: 10.1128/am.19.5.800-804.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann B., Howard A. J. Production of tumor-inhibitory L-asparaginase by submerged growth of Serratia marcescens. Appl Microbiol. 1969 Oct;18(4):550–554. doi: 10.1128/am.18.4.550-554.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. P., Frank B. H., Burck P. J. Crystalline L-asparaginase from escherichia coli B. Science. 1969 Aug 1;165(3892):510–512. doi: 10.1126/science.165.3892.510. [DOI] [PubMed] [Google Scholar]
- KIDD J. G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med. 1953 Dec;98(6):565–582. doi: 10.1084/jem.98.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laishley E. J., Bernlohr R. W. Regulation of arginine and proline catabolism in Bacillus licheniformis. J Bacteriol. 1968 Aug;96(2):322–329. doi: 10.1128/jb.96.2.322-329.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASHBURN L. T., WRISTON J. C., Jr TUMOR INHIBITORY EFFECT OF L-ASPARAGINASE FROM ESCHERICHIA COLI. Arch Biochem Biophys. 1964 May;105:450–452. doi: 10.1016/0003-9861(64)90032-3. [DOI] [PubMed] [Google Scholar]
- Mindich L. Pathway for oxidative dissimilation of glycerol in Bacillur subtilis. J Bacteriol. 1968 Aug;96(2):565–566. doi: 10.1128/jb.96.2.565-566.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. E., Ciegler A. L-asparaginase production by various bacteria. Appl Microbiol. 1969 Jun;17(6):929–930. doi: 10.1128/am.17.6.929-930.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. V., Jayaram H. N., Sirsi M., Ramakrishnan T. Inhibitory activity of L-asparaginase from Mycobacterium tuberculosis on Yoshida ascites sarcoma in rats. Arch Biochem Biophys. 1969 Jun;132(1):262–267. doi: 10.1016/0003-9861(69)90361-0. [DOI] [PubMed] [Google Scholar]
- Roberts J., Burson G., Hill J. M. New procedures for purification of L-asparaginase with high yield from Escherichia coli. J Bacteriol. 1968 Jun;95(6):2117–2123. doi: 10.1128/jb.95.6.2117-2123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Reeves J. Y., Broome J. D. Two L-asparaginases from E. coli and their action against tumors. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1516–1519. doi: 10.1073/pnas.56.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade H. E., Elsworth R., Herbert D., Keppie J., Sargeant K. A new L-asparaginase with antitumour activity? Lancet. 1968 Oct 5;2(7571):776–777. doi: 10.1016/s0140-6736(68)90977-x. [DOI] [PubMed] [Google Scholar]


