Abstract
Objective
Inflammatory cell activation plays a key role in atherosclerotic plaque growth and acute complications. While secretion of proteases and inflammatory cytokines are likely involved in the development of plaque instability, the precise mechanistic pathways are not well understood.
Methods and results
Based on our previous study, we crossed Toll-like receptor 3 (Tlr3)−/− mice with a unique BALB-Apoe−/−Npc1−/− plaque complication-susceptible mouse model, as well as the widely-used B6-Ldlr−/− atherosclerosis model, to test the role of TLR3 signaling in the development of plaque instability. TLR3-deficient mice showed no change in aortic root lesion area, but displayed a marked increase in collagen and smooth muscle cell (SMC) content of lesions. Notably, Apoe−/−Npc1−/−Tlr3−/− mice exhibited a 50% reduction in the incidence of medial destruction, a precursor to aortic aneurysm formation. MMP-2 activity was markedly reduced in aortic extracts from Apoe−/−Npc1−/−Tlr3−/− compared to controls, while both MMP-2 and -9 activities were reduced in Ldlr−/−Tlr3−/− extracts. Consistent with the in vivo data, TLR3 deficiency suppressed MMP-2 activity induced by TNF-α or polyinosine–polycytidylic acid in macrophages from Apoe−/−Npc1−/− mice.
Conclusions
TLR3 plays a critical role in regulating the degradation of extracellular matrix in lesions, in part by modulation of macrophage MMP-2 and -9 activities.
Keywords: Atherosclerosis, Metalloproteinase, Macrophage, Toll-like receptor
1. Introduction
While hypercholesterolemia is an important initiating factor in atherosclerotic plaque formation, plaque growth and acute complications involve the infiltration and activation of inflammatory cells including macrophages and lymphocytes [1,2]. Rupture-prone vulnerable plaques and aneurysm formation are thought to occur downstream of protease secretion from inflammatory cells, including matrix metalloproteinases (MMPs) and cathepsins [3–8]. Control of hypercholesterolemia by diet or lipid-lowering drugs reduces macrophage numbers, decreases expression and/or activity of MMP-1/2/9 and increases collagen content in a rabbit model of atherosclerosis [9]. However, the molecular events operating at the interface between lipoprotein cholesterol uptake by macrophages, MMP expression and development of plaque instability and complications are not well understood.
A major limitation to the study of plaque complications is the fact that standard mouse models of atherosclerosis, including apolipoprotein E knockout (Apoe−/−) and low-density lipoprotein receptor knockout (Ldlr−/−) mice, show only limited features of atherosclerotic plaque instability [10,11]. We recently reported spontaneous occurrence of plaque complications in Apoe−/− mice bearing a mutation in the Niemann-Pick C1 (Npc1) gene [12], which regulates the exit of LDL-derived FC from late endosomes [13]. Apoe−/−Npc1−/− mice develop large foam cell rich lesions with thin collagen caps and a significant incidence of medial destruction and athero-thrombosis. Mechanistically, late endosomal accumulation of FC in macrophages led to activation of various Toll-like receptor (TLR) signaling pathways, especially TLR3, and sustained activation of p38 mitogen-activated protein kinase (MAPK) signaling, leading to induction of various Mmps and cathepsin K [14]. To study the specific role of TLR3 in plaque complications, we crossed Apoe−/−Npc1−/− mice with Tlr3−/− mice and discovered a role for TLR3 in determining the collagen content of lesions and integrity of the underlying tunica media, likely acting via Mmp2 and possibly Mmp9.
2. Methods
Mice
BALB-Apoe−/−Npc1−/− mice have been previously described [12]. B6.129S1-Tlr3tm1Flv/J (Tlr3−/−) and B6.129S7-Ldlrtm1Her/J (Ldlr−/−) mice were obtained from The Jackson Laboratory. Following backcrossing of the Tlr3 mutation into the BALB background, Apoe−/−, Apoe−/−Npc1−/− and Apoe−/−Npc1−/−Tlr3−/− littermates were fed standard chow diet for 12 weeks. Ldlr−/− and Ldlr−/−Tlr3−/− mice were weaned onto chow and switched to western-type diet (WTD) (TD88137; Harlan Teklad) at 5–6 weeks of age, for 10 weeks.
Additional methods can be found in the Online Supplement.
3. Results
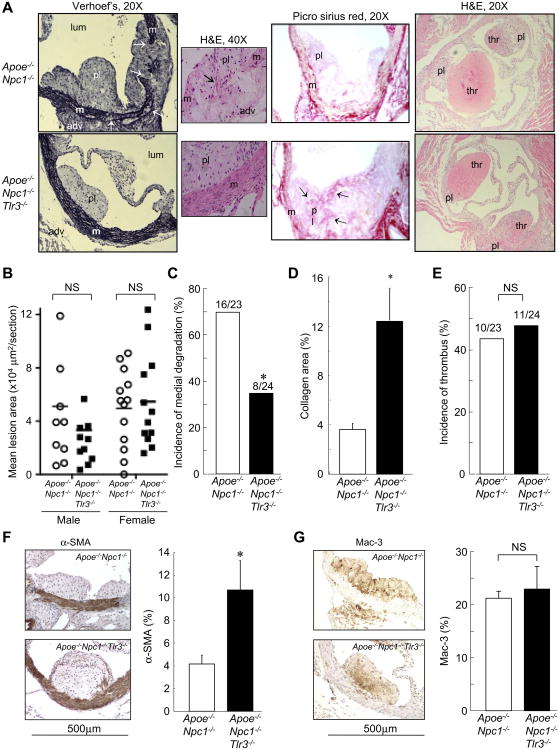
TLR3 deficiency inhibits medial destruction, increasing plaque collagen and SMC content, in plaque complication-susceptible BALB-Apoe−/−Npc1−/−mice. Although wild-type BALB/c and BALB-Apoe−/− mice are highly resistant to atherosclerotic lesion formation, NPC1 deficiency in this background leads to spontaneous lesion formation and acute complications [12]. Thus, we crossed the Tlr3−/− mutation into the BALB-Apoe−/−Npc1−/− background to evaluate effects on medial destruction and athero-thrombosis. Apoe−/−Npc1−/− and Apoe−/−Npc1−/− mice showed no significant differences in body weight, plasma total cholesterol or triglyceride levels (Supplemental Table 1), lipoprotein profiles (Supplemental Fig. 1A), or atherosclerotic lesion area in the proximal aorta (Fig. 1B). However, there was a significant reduction of en face lesional staining area in the aortic arch in the latter group (Supplemental Fig. 1B). Notably, Apoe−/−Npc1−/−Tlr3−/− mice exhibited a 50% reduction in the incidence of medial destruction with medial/adventitial infiltration of foam cells compared to Apoe−/−Npc1−/−controls (Fig. 1A, left panel, and C). This was accompanied by increased collagen content in Apoe−/−Npc1−/−Tlr3−/− lesions, as shown by both Masson's trichrome (Fig. 1A, middle panel, and 1D) and Picro Sirius red staining (Supplemental Fig. 1C). The incidence of athero-thrombosis was unchanged between the two groups (Fig. 1A right panel, and E). There was a 2-fold increase in intimal SMC, but not macrophage, area in Apoe−/−Npc1−/−Tlr3−/− compared to Apoe−/−Npc1−/− mice (Fig. 1F, G). Together, the data indicate that TLR3 regulates medial degradation, collagen content, and SMC accumulation in atherosclerotic lesions in Apoe−/−Npc1−/− mice.
Fig. 1.
TLR3 deficiency results in dramatic inhibition of medial degradation, with increased collagen and SMC content of atherosclerotic plaques in BALB-Apoe−/−Npc1−/−mice. (A) Representative sections from the proximal aorta of twelve-week-old, chow-fed, Apoe−/−Npc1−/− and Apoe−/−Npc1−/−Tlr3−/− mice. Left panel, Verhoef's staining for elastin (black). Arrows indicate medial destruction. Small insert, high-power field of medial area. Arrow indicates foam cells in the region of destructed media. Middle panel, Picro Sirius red staining for collagen (red). Arrows indicate intimal collagen accumulation. Right panel, H&E staining shows thrombi associated with atherosclerotic plaques. pl, plaque; lum, lumen; m, media; thr, thrombus. (B) Quantification of lesion area by morphometric analysis (n = 23−24 mice/genotypic group). Horizontal bars represent mean values. (C, E) Incidence of medial degradation (C) and thrombus formation (E) associated with atherosclerosis in the proximal aorta. (D) Quantification of collagen-positive area relative to total lesion area (n = 12 mice/group). (F, G) Immunostaining for SMCs (α-SMA, F) and macrophages (Mac-3, G) and quantification of positively-stained areas (brown) relative to total intimal lesion area (n = 8 mice/group). NS, not significant. *p < 0.05 vs. Apoe−/−Npc1−/− mice. Data are mean ± SD.
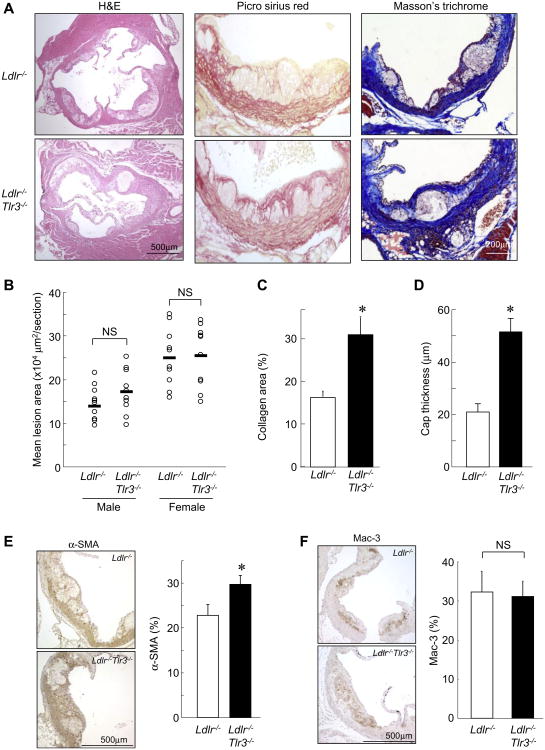
TLR3 deficiency increases collagen and SMC content, and cap thickness, of plaques in B6-Ldlr−/− mice
To determine whether TLR3 deficiency affects matrix composition in a more standard atherosclerosis model, we crossed the Tlr3−/− mutation into the B6-Ldlr−/− background. Following 10-week WTD feeding, there were no significant differences in body weight, plasma total cholesterol or triglyceride levels (Supplemental Table 2), lipoprotein profiles (Supplemental Fig. 2A), or lesion area in the aortic valve (Fig. 2A, B) or aortic arch (Supplemental Fig. 2B) between littermate Ldlr−/− and Ldlr−/−Tlr3−/− mice. However, a striking > 2-fold increase in collagen content and thickness of lesional caps was observed in lesions from Ldlr−/−Tlr3−/− compared to Ldlr−/− mice (Fig. 2A, C, D and Supplemental Fig. 2C). This was accompanied by significantly increased intimal SMC, but not macrophage, area in Ldlr−/−Tlr3−/− compared to Ldlr−/− mice (Fig. 2E, F). The results confirmed that TLR3 regulates collagen and SMC accumulation in hypercholesterolemia-induced atherosclerotic plaques.
Fig. 2. TLR3 deficiency increases collagen and SMC content of plaques in B6-Ldlr−/−mice.
(A) H&E, Picro Sirius red, and Masson's trichrome staining of sections from the proximal aorta of 10wk WTD-fed Ldlr−/− and Ldlr−/−Tlr3−/− mice. (B) Quantification of lesion area by morphometric analysis (n = 10 mice/group). Horizontal bars represent mean values. (C, D) Quantification of collagen-positive area (red staining) relative to total lesion area (n = 8 mice/group) and fibrous cap thickness (n = 10 mice/group). (E, F) Immunostaining for SMCs (α-SMA, E) and macrophages (Mac-3, F) and quantification of positively-stained areas (brown) relative to total intimal lesion area (n = 8 mice/group). NS, not significant. *p < 0.05 vs. Ldlr−/− mice. Data are mean ± SD. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
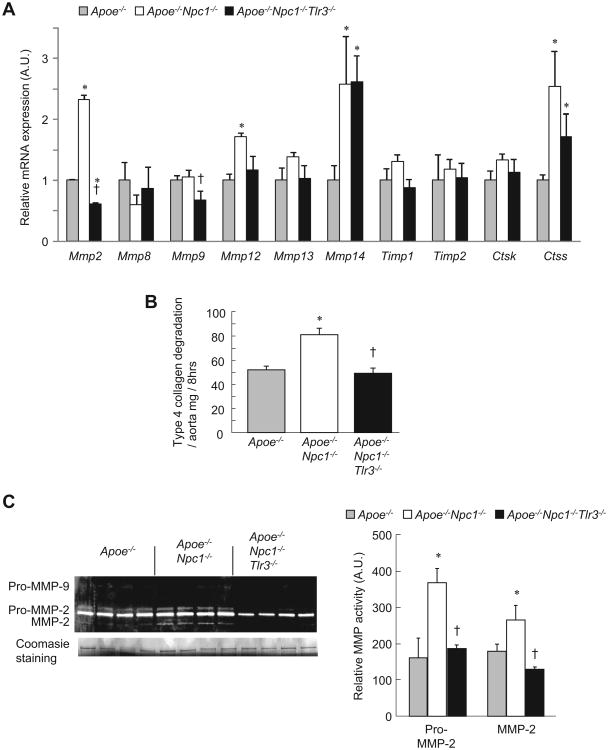
TLR3 deficiency suppresses MMP-2 protease activity in atherosclerotic lesions of BALB-Apoe−/−/Npc1−/− and B6-Ldlr−/− mice
In contrast to TLR4/Myd88 deficiency, and consistent with a recent report [15], TLR3 deficiency did not change inflammatory cytokine expression levels in aorta from BALB-Apoe−/−Npc1−/− or B6-Ldlr−/− mice (Supplemental Fig. 3). Because MMPs and cathepsins promote collagen degradation and features associated with plaque instability [4,10], we analyzed aortic gene expression and activity levels. In the BALB-Apoe−/−Npc1−/− model, Mmp2 expression was induced in Apoe−/−Npc1−/− and repressed in Apoe−/−Npc1−/−Tlr3−/− aorta (Fig. 3A). Mmp9 was not induced in Apoe−/−Npc1−/−, but was repressed in Apoe−/−Npc1−/−Tlr3−/− aorta. Mmp14 and Ctss were increased in both Apoe−/−Npc1−/− and Apoe−/−Npc1−/− aorta, showing no difference with TLR3 deficiency. Mmp12 was increased in Apoe−/−Npc1−/−, but not significantly repressed in Apoe−/−Npc1−/−Tlr3−/− aorta. In summary, TLR3 deficiency in the BALB-Apoe−/−Npc1−/− background resulted in decreased expression of both Mmp2 and Mmp9, encoding gelatinases known to cleave collagen type 4 fibrils [16].
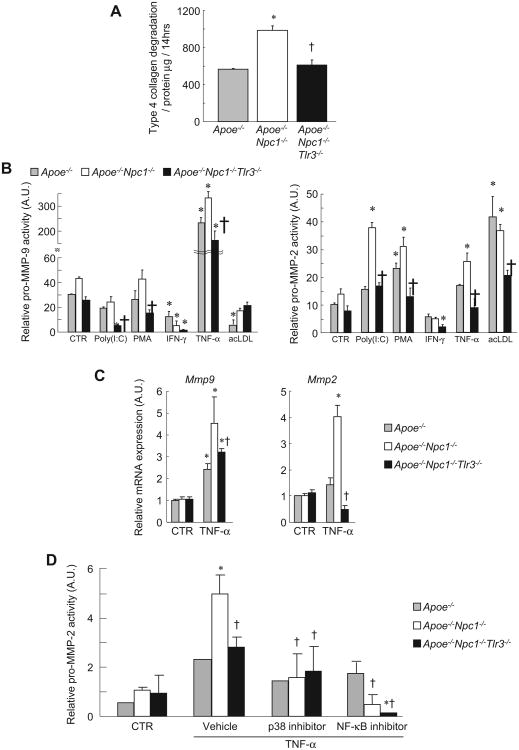
Fig. 3. Suppression of aortic collagen degradation and MMP-2 activity in TLR3-deficient Apoe−/−Npc1−/−mice.
(A) Analysis of gene expression by real-time PCR in aorta from twelve-week-old, chow-fed, Apoe−/−, Apoe−/−Npc1−/− and Apoe−/−Npc1−/−Tlr3−/− mice (n = 4 mice/group). (B) Collagen degradation in aortic extracts incubated with FITC-labeled native type IV collagen and measured as the release of soluble fluorescent material after the indicated time. (C) Relative MMP-2 activity measured by densitometric analysis of gelatin zymographs. Coomasie blue staining is for standard control. *p < 0.01 vs. Apoe−/− group. †p < 0.01 vs. Apoe−/−Npc1−/− group. AU, arbitrary unit Data are mean ± SEM of three individual experiments.
Next, protease activity was assessed via collagen type 4 degradation and gelatin zymography assays. Enhanced collagenolytic activity was observed in Apoe−/−Npc1−/− aortic extracts, and this was suppressed in Apoe−/−Npc1−/−Tlr3−/− extracts (Fig. 3B). By zymography, MMP-2 activity was increased in Apoe−/−Npc1−/− aortic homogenates and reversed by concomitant TLR3 deficiency (Fig. 3C), while the activity of MMP-9 was too low for accurate quantification. The decrease in MMP-2 activity reflected an overall decrease in protein as the ratio of mature form to total MMP-2 protein did not differ between Apoe−/−Npc1−/− and Apoe−/−Npc1−/−Tlr−/− aortic extracts (Apoe−/−, 55.8 ± 9.9%; Apoe−/−Npc1−/−, 41.3 ± 3.0%; Apoe−/−Npc1−/−Tlr3−/−, 41.2 ± 0.9%, mean ± SD).
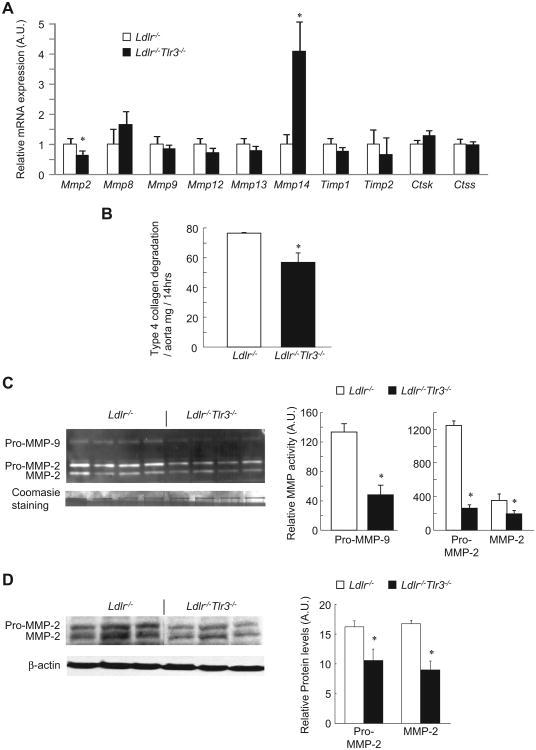
In the B6-Ldlr−/− model, TLR3 deficiency repressed Mmp2 expression, increased Mmp14. and had no effect on the expression of other Mmps in aorta (Fig. 4A). Decreased collagenolytic activity was observed in Ldlr−/−Tlr3−/− protein extracts compared to Ldlr−/− controls (Fig. 4B). By zymography, both MMP-2 and -9 activities were substantially lower in Ldlr−/−Tlr3−/− compared to Ldlr−/− aortic homogenates (Fig. 4C). Again, the ratio of the mature form to total MMP-2 was unchanged between groups (Ldlr−/−, 35.4 ± 2.9%; Ldlr−/−Tlr3−/−, 43.8 ± 4.0%, mean ± SD) indicating a decrease in total protein, and this was confirmed by Western blotting (Fig. 4D). Thus, decreased MMP-2 and -9 activities may contribute to the effect of TLR3 deficiency on lesional collagen content in Ldlr−/− mice.
Fig. 4. Suppression of aortic collagen degradation and MMP-2 activity in TLR3-deficient Ldlr−/−mice.
(A) Analysis of gene expression by real-time PCR in aorta from 10wk WTD-fed Ldlr−/− and Ldlr−/−Tlr3−/− mice (n = 4 mice/group). (B) Collagen degradation in aortic extracts incubated with FITC-labeled native type IV collagen and measured as the release of soluble fluorescent material after the indicated time. (C) Relative MMP-2 and -9 activities measured by densitometric analysis of gelatin zymographs. Coomasie blue staining is for standard control. (D) Western blot analysis of aortic MMP-2 (n = 3 mice/group). *p < 0.01 vs. Ldlr−/−. AU, arbitrary unit. Data mean ± SEM of three individual experiments.
TLR3 deficiency represses macrophage pro-MMP-2 activity induced by inflammatory cytokines present in atherosclerotic plaques. As observed in aortic homogenates (Fig. 3B), collagen degradation was increased in media from Apoe−/−Npc1−/− macrophages, and this effect was reversed using media from Apoe−/−Npc1−/−Tlr3−/− cells (Fig. 5A). Next, we assessed cytokine-induced MMP2/9 activity in BM-derived macrophages using gelatin zymography (Fig. 5B). Treatment with TLR3 ligand (polyinosine–polycytidylic acid [Poly(I:C)]), phorbol-12-myristate-13-acetate (PMA) and tumor necrosis factor (TNF)-α increased pro-MMP-2 activity in Apoe−/−Npc1−/− macrophages, but not in Apoe−/−Npc1−/−Tlr3−/− cells. Pro-MMP-9 activity was induced by TNF- α stimulation in Apoe−/−Npc1−/−macrophages and modestly reduced in Apoe−/−Npc1−/−Tlr3−/− macrophages. Interferon (IFN)-γ reduced both pro-MMP-2 and -9 activities in a non-genotypic fashion, consistent with a previous report [5]. Since these stimuli are likely relevant to the inflammatory milieu of atherosclerotic plaques [2], these data suggest that the effect of TLR3 deficiency on collagenolytic activity in aortic homogenates is mediated, at least partly, by decreased activity of MMP-2 and possibly MMP-9 in macrophages.
Fig. 5.
Macrophage MMP2 activity and gene expression is induced by NPC1 deficiency and suppressed by TLR3 deficiency. (A) Collagen degradation in macrophage media incubated with FITC-labeled native type IV collagen and measured as the release of soluble fluorescent material after 14 h (n = 3/group). (B) Relative activities of pro-MMP-2 and -9 quantified by densitometric analysis of gelatin zymographs. BM-derived macrophages stimulated by indicated cytokines, Poly(I:C) (5 μg/ml), PMA (40 ng/ml), IFN-γ (10 ng/ml), TNF-α (50 ng/ml), or acLDL (50 μg/ml). (C) Mmp2 and Mmp9 expression by real-time PCR in Apoe−/−, Apoe−/−Npc1−/− and Apoe−/−Npc1−/−Tlr3−/− macrophages incubated in TNF-α (50 ng/ml) for 4h. (D) Relative activities of pro-MMP-2 quantified by densitometric analysis of gelatin zymographs. BM-derived macrophages stimulated in serum-free medium containing TNF-α (50 ng/ml) and indicated inhibitor (10 μmol/L) for 24 h p38 inhibitor, SB202190; NF-κB inhibitor, BAY11-7085. *p < 0.05 vs. Apoe−/− CTR. †p < 0.05 vs. Apoe−/−Npc1−/−. AU, arbitrary unit. Data are mean ± SEM of three individual experiments.
Next, we analyzed Mmp2 and Mmp9 expression http://circ.ahajournals.org/cgi/content/full/117/7/931 - R33-188785in basal and TNF-α-stimulated macrophages (Fig. 5C). In TNF-α-stimulated macrophages, NPC1 deficiency enhanced Mmp2 and Mmp9 expression and these were inhibited by TLR3 deficiency. Genotypic regulation of macrophage Mmp2 expression paralleled differences in MMP-2 activity under similar conditions (Fig. 5B), suggesting that altered gene expression regulates MMP-2 activity in media of cultured macrophages and aortic homogenates.
MMP-2 is also secreted by SMCs and endothelial cells [17,18]. In vitro, pro-MMP-2 activity was readily detected in basal SMCs (Supplemental Fig. 4A). However, pro-MMP-2 activity was similar in all genotypes, and was not affected by inflammatory mediators. TNF-α stimulation induced pro-MMP-9 activity, but to a similar level in all genotypes. Moreover, intimal MMP-2-positive immunostaining overlapped F4/80-positive macrophages in BALB-Apoe−/−Npc1−/− and B6-Ldlr−/− mice (Supplemental Fig. 4B). It has been proposed that macrophages activate SMC-derived pro-MMP-2 in response to local cytokine activation of macrophage MMP-14 [19]. In our model, macrophage MMP-14 protein level was unchanged by TLR3 deficiency (Supplemental Fig. 4C) and there was no effect of macrophage genotype on MMP-2 activity in media derived from SMCs (Supplemental Fig. 4D). These data are consistent with an effect of TLR3 on pro-MMP2 activity in macrophages but SMCs.
Macrophage MMP-2 activity induced by TNF-α is suppressed by p38 MAPK and NF-κB inhibitors
TLR3 signals through the adaptor protein, TRIF, leading to activation of MAPKs and nuclear factor (NF)-κB [20]. We previously reported increased basal and stimulated p38 MAPK phosphorylation in Npc1−/− macrophages compared to wild-type [14]. Herein, we assessed the role of MAPKs and NF-κB in regulating macrophage MMP-2 activity. Inhibitors of both p38 MAPK (SB202190) and NF-κB (IκBα phosphorylation inhibitor, BAY11-7085) reduced TNF-α–stimulated MMP-2 activity to a similar low level in all genotypes (Fig. 5D), consistent with a recent report [21]. This suggests that activation of p38 MAPK and NF-κB by endosomal cholesterol loading is responsible for induction of Mmp2 expression.
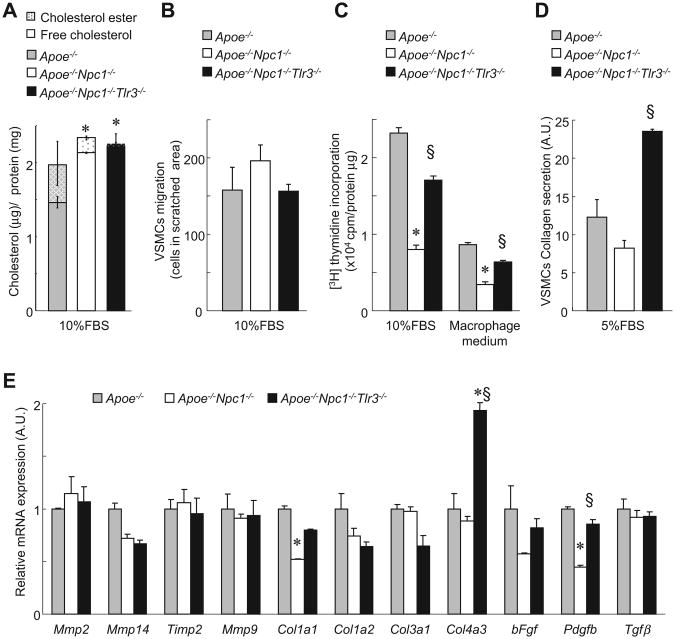
TLR3 deficiency enhances SMC proliferation and collagen secretion, but not migration
To understand the mechanism of increased SMC content in TLR3-deficient lesions, we performed a series of studies in SMCs. Like macrophages, Apoe−/−Npc1−/−-derived SMCs displayed increased cellular FC compared to Apoe−/− controls, and this was not affected by TLR3 deficiency (Fig. 6A). Cell migration was similar in Apoe−/−, Apoe−/−Npc1−/− and Apoe−/−Npc1−/−Tlr3−/− SMCs (Fig. 6B). However, cell proliferation was decreased in Apoe−/−Npc1−/− SMCs, while both proliferation and collagen secretion were increased in Apoe−/−Npc1−/−Tlr3−/− SMCs (Fig. 6C and D). Many cytokines secreted from macrophages regulate SMC proliferation, migration and collagen secretion [22]. Although media from macrophages reduced SMC proliferation in all groups, the pattern of SMC proliferation did not change between genotypes (Fig. 6C), suggesting a cell autonomous effect of TLR3 deficiency. Thus, we looked at SMC gene expression by quantitative real-time PCR. Mmp gene expression was similar between Apoe−/−, Apoe−/−Npc1−/− and Apoe−/−Npc1−/−Tlr3−/− SMCs (Fig. 6E). Pro-collagen Col1a1 gene expression was significantly reduced in Apoe−/−Npc1−/− SMCs, while Col4a3 gene expression was significantly increased in Apoe−/−Npc1−/−Tlr3−/− SMCs. PDGF–B is known to increase SMC proliferation in atherosclerotic lesions [22], and Pdgfb gene expression is down-regulated by TLR3 activation in macrophages [23]. Accordingly, Pdgfb gene expression was decreased in Apoe−/−Npc1−/− SMCs, and increased in Apoe−/−Npc1−/−Tlr3−/− SMCs (Fig. 6E). These results suggested that induction of Pdgfb by TLR3 deficiency facilitates cell proliferation and collagen secretion in SMCs, contributing to increased collagen accumulation and SMC number in lesions of Tlr3−/− mice.
Fig. 6. SMC proliferation and collagen secretion are induced by TLR3 deficiency.
(A) Cellular cholesterol content of SMCs derived from Apoe−/− and Apoe−/−Npc1−/−Tlr3−/− incubated in SMC medium. (B) SMC migration measured by wound healing assay after incubation in 10% FBS for 24 h (C) SMC proliferation measured by thymidine incorporation assay in 10% FBS or macrophage medium. (D) SMC collagen secretion measured by Sircol collagen assay. (E) mRNA expression by real-time PCR in SMCs derived from Apoe−/−, Apoe−/−Npc1−/−, and Apoe−/−Npc1−/−Tlr3−/− mice and incubated in medium for 24 h *p < 0.05 vs. Apoe−/−. fp<0.05 vs. Apoe−/−Npc1−/−. Results are mean ± SEM of triplicate experiments.
4. Discussion
We have used a unique plaque complication-susceptible mouse model, involving NPC1 -related endosomal accumulation of cholesterol, to reveal an effect of TLR3 signaling on medial destruction, collagen degradation, and SMC cell content in atherosclerotic lesions. In humans, collagen degradation is associated with plaque vulnerability, and medial destruction with loss of SMCs is a precursor for aortic aneurysms. The effects of TLR3 deficiency on collagen and SMC content were also observed in the more commonly used B6-Ldlr−/− model. The effects were associated with decreased macrophage Mmp2, and possibly Mmp9, expression. Together with earlier studies, these findings suggest that cholesterol accumulation in the macrophage endosomal system leads to TLR3 signaling via p38 MAPK and NF-κB, and increased Mmp2 and Mmp9 expression. We also observed SMC-mediated effects of Pdgf expression on cell proliferation, collagen secretion and collagen gene expression.
TLRs play a pivotal role in the sterile inflammation associated with atherogenesis. Deletions of Tlr2, Tlr4, and the shared TLR-signaling adaptor protein, Myd88, have been shown to significantly reduce atherosclerotic lesion area in Apoe-deficient mice by decreasing macrophage content and expression of inflammatory and chemokine genes [24–26]. Consistent with a recent report [15], we observed no effect of TLR3 deficiency on aortic cytokine/chemokine expression (Supplemental Fig. 3). Other studies have implicated combinatorial signaling of CD36/TLR4/6 in macrophage inflammatory effects of oxidized LDL [27], as well as inflammasome activation by small cholesterol crystals in the endosomal system [28]. Although we cannot rule out an effect of inflammasome activation in the Apoe−/−Npc1−/− background, our findings suggest that TLR3 may also be an important player at the lipoprotein/macrophage interface, sensing signals derived from endosomal cholesterol deposition and regulating MMP activity, lesional collagen and SMC content, as well as medial degradation. Moreover, TLR3 ligand inhibits cholesterol efflux from macrophages by inhibiting liver × receptor-dependent gene expression, including Abca1 and Abcg1 [29].
Medial destruction, with loss of SMCs and macrophage infiltration into the adventitia, is a hallmark of atherosclerotic aortic aneurysms [30,31]. Mmp9 and Mmp2 have been previously implicated in the destruction of medial elastic fibers. Increased expression of both genes has been observed in aneurysm walls [32–34] and targeted disruptions of both genes suppress the development of experimental abdominal aneurysms [35,36]. In addition, targeted Mmp9 disruption suppressed atherosclerosis-related medial destruction in one study [37] but not another [38]. The disparate findings may have been due to differences in experimental design such as diet and site of plaque characterization. Although we observed a strong protective effect of TLR3 deficiency on atherosclerotic medial destruction in the BALB-Apoe−/−Npc1−/− model, Cole et al. reported [39] the opposite effect on elastic lamina damage following carotid collar-induced injury. However, injuryinduced neointima formation primarily involves SMC proliferation and the underlying mechanism may be different. In our model, the effect of TLR3 deficiency on pro-MMP2 activity was observed in macrophages but not SMCs. It is notable that aortic mRNA expression of Mmp 14 and Timp2, known mediators of MMP2 activation in other models, was not affected by TLR3 deficiency.
The impact of TLR3 signaling on collagen type 4 degradation observed in our study may have clinical relevance in humans. Collagen type 4 is the major structural component of basement membranes. Genome-wide association studies have reported associations of common polymorphisms of COL4a1 and COL4a2 structural genes with arterial stiffness [40], coronary artery calcification/myocardial infarction [41], CAD [42], and intracranial aneurysms [43]. Three other genes involved in ECM integrity – ADAMTS7 and HSPG2/CSPG2 – have been associated with CAD [42] and intracranial aneurysms [43], respectively, further supporting the importance of the ECM in atherogenesis and vascular complications. Although collagen type I is an important structural component of plaque caps, this protein was not studied herein and we cannot rule out a potential role of TLR3-deficiency in collagen type I degradation.
The emerging role of inflammation on plaque growth and complications suggests that anti-inflammatory therapies could effect plaque stabilization. Our study suggests that decreasing TLR3/TRIF signaling in macrophages might lead to an increase in plaque collagen content and cap thickness, and a decrease in medial destruction and aneurysm formation.
Supplementary Material
Footnotes
Appendix A. Supplementary data: Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.atherosclerosis.2013.03.035
References
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006 Jul;6(7):508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009 Jun;6(6):399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 3.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006 Jan;116(1):59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006 Feb 15;69(3):625–35. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Newby AC, George SJ, Ismail Y, Johnson JL, Sala-Newby GB, Thomas AC. Vulnerable atherosclerotic plaque metalloproteinases and foam cell phenotypes. Thromb Haemost. 2009 Jun;101(6):1006–11. [PMC free article] [PubMed] [Google Scholar]
- 6.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008 Dec;28(12):2108–14. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 7.de Nooijer R, Verkleij CJ, von der Thusen JH, et al. Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol. 2006 Feb;26(2):340–6. doi: 10.1161/01.ATV.0000197795.56960.64. [DOI] [PubMed] [Google Scholar]
- 8.Schneider F, Sukhova GK, Aikawa M, et al. Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation. 2008 Feb 19;117(7):931–9. doi: 10.1161/CIRCULATIONAHA.107.707448. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003 Feb 20;91(4A):4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol. 2007 Apr;27(4):705–13. doi: 10.1161/01.ATV.0000261709.34878.20. [DOI] [PubMed] [Google Scholar]
- 11.Jackson CL, Bennett MR, Biessen EA, Johnson JL, Krams R. Assessment of unstable atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007 Apr;27(4):714–20. doi: 10.1161/01.ATV.0000261873.86623.e1. [DOI] [PubMed] [Google Scholar]
- 12.Welch CL, Sun Y, Arey BJ, et al. Spontaneous atherothrombosis and medial degradation in Apoe−/−, Npc1−/− mice. Circulation. 2007 Nov 20;116(21):2444–52. doi: 10.1161/CIRCULATIONAHA.107.701276. [DOI] [PubMed] [Google Scholar]
- 13.Liscum L, Ruggiero RM, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann-Pick type C fibroblasts. J Cell Biol. 1989 May;108(5):1625–36. doi: 10.1083/jcb.108.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Ishibashi M, Seimon T, et al. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res. 2009 Feb 27;104(4):455–65. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards MR, Black AS, Bonnet DJ, et al. The LPS2 mutation in TRIF is atheroprotective in hyperlipidemic low density lipoprotein receptor knockout mice. Innate Immun. 2012 May 25; doi: 10.1177/1753425912447130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gioia M, Monaco S, Van Den Steen PE, et al. The collagen binding domain of gelatinase A modulates degradation of collagen IV by gelatinase B. J Mol Biol. 2009 Feb 20;386(2):419–34. doi: 10.1016/j.jmb.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006 May;26(5):1120–5. doi: 10.1161/01.ATV.0000218496.60097.e0. [DOI] [PubMed] [Google Scholar]
- 18.Kuzuya M, Kanda S, Sasaki T, et al. Deficiency of gelatinase a suppresses smooth muscle cell invasion and development of experimental intimal hyperplasia. Circulation. 2003 Sep 16;108(11):1375–81. doi: 10.1161/01.CIR.0000086463.15540.3C. [DOI] [PubMed] [Google Scholar]
- 19.Stawowy P, Meyborg H, Stibenz D, et al. Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-promatrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation. 2005 May 31;111(21):2820–7. doi: 10.1161/CIRCULATIONAHA.104.502617. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003 Aug 1;301(5633):640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 21.Lin ML, Lu YC, Chung JG, et al. Down-regulation of MMP-2 through the p38 MAPK-NF-kappaB-dependent pathway by aloe-emodin leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol Carcinog. 2010 Sep;49(9):783–97. doi: 10.1002/mc.20652. [DOI] [PubMed] [Google Scholar]
- 22.Raines EW, Ferri N. Thematic review series: the immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. J Lipid Res. 2005 Jun;46(6):1081–92. doi: 10.1194/jlr.R500004-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Chow EK, O'Connell RM, Schilling S, Wang XF, Fu XY, Cheng G. TLR agonists regulate PDGF-B production and cell proliferation through TGF-beta/type I IFN crosstalk. EMBO J. 2005 Dec 7;24(23):4071–81. doi: 10.1038/sj.emboj.7600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004 Apr;10(4):416–21. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 25.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005 Nov;115(11):3149–56. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010 Feb;11(2):155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010 Apr 29;464(7293):1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castrillo A, Joseph SB, Vaidya SA, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003 Oct;12(4):805–16. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 30.Davies MJ. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation. 1998 Jul 21;98(3):193–5. doi: 10.1161/01.cir.98.3.193. [DOI] [PubMed] [Google Scholar]
- 31.MacSweeney ST, Powell JT, Greenhalgh RM. Pathogenesis of abdominal aortic aneurysm. Br J Surg. 1994 Jul;81(7):935–41. doi: 10.1002/bjs.1800810704. [DOI] [PubMed] [Google Scholar]
- 32.Newman KM, Ogata Y, Malon AM, et al. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler Thromb. 1994 Aug;14(8):1315–20. doi: 10.1161/01.atv.14.8.1315. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RW, Holmes DR, Mertens RA, et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995 Jul;96(1):318–26. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huusko T, Salonurmi T, Taskinen P, et al. Elevated messenger RNA expression and plasma protein levels of osteopontin and matrix metalloproteinase types 2 and 9 in patients with ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2012 May 7; doi: 10.1016/j.jtcvs.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Pyo R, Lee JK, Shipley JM, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000 Jun;105(11):1641–9. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002 Sep;110(5):625–32. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luttun A, Lutgens E, Manderveld A, et al. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004 Mar 23;109(11):1408–14. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- 38.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005 Oct 25;102(43):15575–80. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole JE, Navin TJ, Cross AJ, et al. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci U S A. 2011 Feb 8;108(6):2372–7. doi: 10.1073/pnas.1018515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarasov KV, Sanna S, Scuteri A, et al. COL4A1 is associated with arterial stiffness by genome-wide association scan. Circ Cardiovasc Genet. 2009 Apr;2(2):151–8. doi: 10.1161/CIRCGENETICS.108.823245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donnell CJ, Kavousi M, Smith AV, et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation. 2011 Dec 20;124(25):2855–64. doi: 10.1161/CIRCULATIONAHA.110.974899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schunkert H, Konig IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011 Apr;43(4):333–8. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruigrok YM, Rinkel GJ, van't Slot R, Wolfs M, Tang S, Wijmenga C. Evidence in favor of the contribution of genes involved in the maintenance of the extracellular matrix of the arterial wall to the development of intracranial aneurysms. Hum Mol Genet. 2006 Nov 15;15(22):3361–8. doi: 10.1093/hmg/ddl412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.