Abstract
Purpose
To determine the effect of dietary fat and oxalate on fecal fat excretion and urine parameters in a rat model of Roux-en-Y gastric bypass (RYGB) surgery.
Materials and Methods
Diet-induced obese Sprague Dawley rats underwent sham (Control, n=16) or RYGB (n=19) surgery. Once recovered, animals were fed ad lib normal calcium, high fat (40%) diet with (Ox) or without (No Ox) 1.5% potassium oxalate for 5 weeks, then normal (10%) fat diet for 2 weeks. Stool and urine were collected after each period. Fecal fat was determined by gas chromatography and urine metabolites by assay spectrophotometry.
Results
Daily fecal fat excretion remained low in controls on either diet. RYGB animals, however, ingested similar food quantity as controls yet had 8-fold higher fecal fat excretion (p<0.001) and heavier stools (p=0.02). On high fat, RYGB Ox had 5-fold increase in urine oxalate excretion (p<0.001) while RYGB No Ox had 2-fold increase in urine calcium (p<0.01) versus controls. Lowering dietary fat in RYGB Ox animals led to a 50% decrease in oxalate excretion (p<0.01), a 30% reduction in urinary calcium, and an increase in urine pH by 0.3 units (p<0.001).
Conclusions
In this RYGB model, high fat feeding resulted in steatorrhea, hyperoxaluria, and low urine pH, partially reversible by lowering dietary fat and oxalate content. RYGB animals on normal fat and no oxalate diets excreted twice as much oxalate as age-matched, sham controls. Although RYGB-hyperoxaluria appears primarily gut and diet-mediated, secondary causes of oxalogenesis from liver or other mechanisms deserve further exploration.
Keywords: morbid obesity, steatorrhea, gastric bypass, calcium oxalate
INTRODUCTION
Because medical weight loss is difficult to attain and maintain, the bariatric procedure Roux-en-Y gastric bypass (RYGB) has been recommended by NIH consensus guidelines as the most effective weight loss therapy for morbidly obese (BMI > 40 kg/m2) and severely obese patients (BMI > 35 kg/m2) with medical complications, curing obesity-related diabetes and hypertension as well as lowering cardiovascular and overall mortality risk1. RYGB is purported to work by reduced food intake through a smaller stomach pouch, by malabsorption of food components that move from the stomach to the distal jejunum (Figure 1), and through alterations in gut hormones responsible for glucose and lipid metabolism. The effect of these three events is a durable 25–30% reduction in total body weight for a large majority of RYGB patients1.
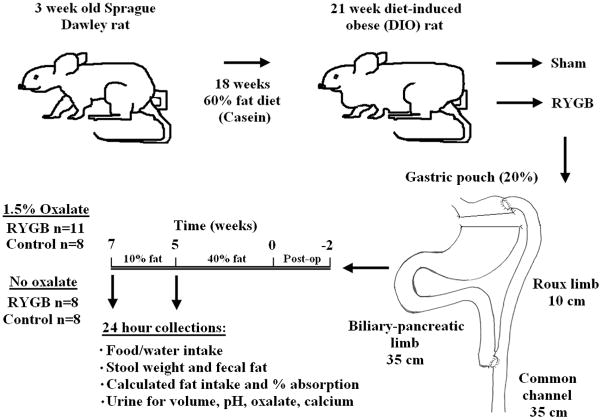
Figure 1. Animal flow diagram.
Following 18 weeks on high fat diet, rats underwent RYGB or sham. Once recovered, animals were randomized to 5 weeks of high (40%) fat chow with or with 1.5% potassium oxalate. Diet pellets were then changed to normal (10%) fat with or without 1.5% potassium oxalate for two weeks. Stool and urine were collected at each period end.
Despite its metabolic benefits, RYGB has been linked to increased post-operative kidney stone risk and hyperoxaluria2–4, falling into a spectrum of gastrointestinal disorders characterized by malabsorption of bile salts and/or fatty acids. Mechanistically, instead of calcium and oxalate precipitating within the gut lumen, fatty acids are thought to bind intestinal calcium, leading to increased unbound oxalate luminal availability, exaggerated enteric responses to oxalate, and ultimately hyperoxaluria5–7. Additionally, the permeability of the colon, the major site of oxalate absorption8, can be dramatically increased by exposure to unconjugated bile salts and long chain fatty acids9, both of which are believed to occur after RYGB. While this hypothesis fits well within oxalate and stone paradigms, few prospective RYGB human studies have been published on fecal fat excretion10, 11 or on adaptive responses following an oxalate load7, 10. Clinical malabsorption is not usually reported as a feature of modern RYGB12, and, in fact, most of the bariatric literature argues that RYGB weight loss occurs through increased metabolic energy expenditure, not through fat or caloric malabsorption13–15.
Bariatric studies in rats have demonstrated similar weight-loss patterns, insulin resistance, and neuropeptide changes as gastric bypass surgery in humans, making this an extremely reliable model to investigate the effect of RYGB on different organ systems13, 14, 16, 17 We sought to establish and characterize a diet-induced obese (DIO) rat model of RYGB surgery to study the long-term effect of dietary fat and oxalate on fecal fat excretion and 24 hour urine parameters.
MATERIALS AND METHODS
Animals and Surgical Protocols
All protocols involved in this study were approved by the Institutional Animal Care and Use Committee in accordance with guidelines established by the National Institutes of Health. Male Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed in individual shoe-box cages at a constant temperature of 21–23°C with a 12-hour light-dark cycle. To produce diet-induced obesity (DIO), 3 week male pups were given ad lib access to 18 weeks of a 60% fat (casein-based), 20% protein, 20% carbohydrate diet without added oxalate (D12492, Research Diets, New Brunswick, NJ), providing 5.2 kcal/gm (Figure 1).
Once DIO was established, rats were randomly assigned to RYGB (n=25) or sham procedure (Control, n=16) by random number tables. For RYGB animals, a 4 cm mid-line incision was made below the xyphoid process. The terminal ileum was identified at the ileocecal valve and followed orally 35 cm where a 4 mm enterotomy was made (common channel, Figure 1). Bowel was followed another 10 cm proximally and completely transected (Roux limb). Hand-sewn interrupted end-to-side anastomosis was created by sewing the proximal portion of biliopancreatic limb (25–35 cm) to the enterotomy using 5-0 PDS. The gastric artery and vagal nerves of the stomach were identified and mobilized laterally. A 45 mm ETS Laparoscopic Endo-GIA stapler with 2.5 mm reload (Ethicon Endo-Surgery, Cincinnati, OH) was used to transect the stomach 3 mm below the level of the gastro-esophageal junction, creating a small stomach pouch. A 4 mm incision was then made on the anterio-lateral stomach above the staple line and hand-sewn gastrojejunostomy was performed using 5-0 PDS. Fascia was closed using a running 4-0 Vicryl suture, and skin re-approximated. All sham animals received similar incisions, stomach mobilization, operative time, and closure as RYGB animals.
Diet Protocols and weight distribution
Following their procedure, rats were allowed 2 weeks for return of bowel function (Figure 1 “post-op”) and were then randomized to ad lib 0.6% calcium, high fat diet (40% fat, 40% carbohydrate, 20% protein; D11021101, Research Diets, New Brunswick, NJ) with 1.5% potassium oxalate (Ox) or without added oxalate (No Ox), providing 4.6 kcal/gm. Weekly weights and daily food and water intake were recorded. After five weeks, rats were switched to ad lib 0.6% calcium, normal fat diet (10% fat, 70% carbohydrate, 20% protein; D11032601, Research Diets, New Brunswick, NJ) for an additional 2 weeks without changing oxalate content, providing 3.8 kcal/gm.
To determine weight distribution at study end, whole body adiposity was assessed with a Minispec lean fat analyzer (Bruker Optics, The Woodlands, TX) using previously validated time-domain nuclear magnetic resonance (TD-NMR) methodology18. Lean-body mass (LBM) and fat mass (FM) was calculated for each animal by multiplying body weight by % lean body mass or % fat mass respectively, and totals were averaged.
Urine and Fecal Samples
At each study period end, rats were placed individually in metabolic cages and 24-hour urines collected under mineral oil into 70 ml vessels containing 30 μl of 2% sodium azide preservative as previously described19. Urine pH was determined immediately following collection using a pH meter. In acidified (HCl) samples, urine oxalate was determined using a kit assay (Trinity Biotech, St. Louis, MO) at wavelength of 590 nm. Urine calcium concentration was determined using kit assay (Point Scientific, Canton, MI) at wavelength of 575nm.
Fecal pellets, collected twice over a 48 hour period from a single animal, were slurried, and three separate slurry aliquots were saponified, methylated, and hexane extracted on an Agilent 6890N gas chromatograph at the Wake Forest School of Medicine Lipoprotein Analysis Laboratory (Winston Salem, NC)20. Total fatty acid identification was made by comparison of retention times with those of internal heptadecanoic acid (C17:0) standard, and replicate analyses in duplicate were averaged. Fat absorption estimates were based on the difference between animal intake and excretion: 100% × [(Daily Fat Intake − Daily Fat Excreted in the Feces)/Daily Fat Intake].
Statistical Calculations
Animal numbers were determined by power analysis using data from a pilot study. Based on an estimated 70% survival for RYGB groups, we calculated that at least 6 animals in each group would provide 85% power to detect a 33% difference in urinary oxalate levels with a p=0.01. All data expressed as mean ± standard error. End of study points were compared with the use of 2-tailed, paired Student t test or one-way ANOVA (Statistical Analaysis Software Version 9.2; Cary, NC). P < 0.05 was accepted for significant differences.
RESULTS
Weight loss and distribution, food intake
After 18 weeks on high fat diets, DIO rats weighed 742 ± 29 gm. No deaths occurred in sham controls whereas 3 animals in RYGB No Ox and 3 in RYGB Ox died post-operatively, leaving RYGB Ox (n=11) and RYGB Ox (n=8) for analysis. By post-operative day 16, control animals were back to pre-operative weight (Figure 2) while RYGB continued to lose 30–35 gm/week until a weight loss nadir at week 7. At study week 8, LBM (Figure 2) was slightly higher in controls vs. RYGB animals (452.4 ± 38.2 vs 403.2 ± 33.6, p=0.16), and FM was significantly higher in controls vs. RYGB (248.6 ± 23.5 vs 106.4 ± 11.8, p<0.001).
Figure 2. Body weight, lean-body mass (LBM), and fat mass (FM) in obese male rats after sham or RYGB.
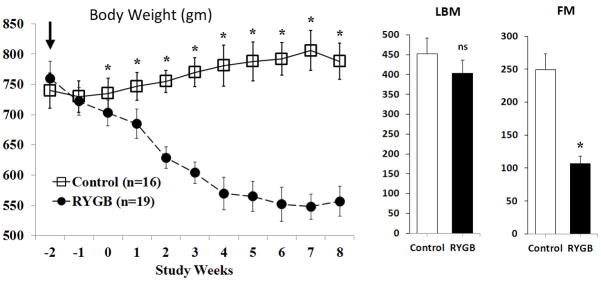
Control and RYGB animal weights over time (weeks) starting on procedure day (dark arrow). Weight loss became significantly different ~2 weeks after surgery. Horizontal bracket represents the weeks animals transitioned from 40% to 10% fat diet. LBM and FM were measured at study end. Data are shown as mean values ± SEM. ns – not significant; * - p<0.05
By 4 weeks post-operative, RYGB animals had similar mean food intake as controls regardless of diet fat content (Table 1). In contrast, water consumption increased significantly in RYGB rats compared to controls (p<0.01), and RYGB rats on high fat diet tended to drink more water than those on 10% fat, regardless of oxalate content (p<0.02, Table 1).
Table 1.
Fecal fat and food, water, and fat intake on high and normal fat diets.
| Food Intake (gm/day) | Water Intake (gm/day) | Fat Intake (gm/day) | Fecal Fat (mg/24 hr) | |
|---|---|---|---|---|
|
| ||||
| RYGB Ox | ||||
| High fat (N=11) | 23.7 ± 9.6 | 41.8 ± 24.6† | 4.7 ± 1.9 | 1484 ± 589† |
| Normal fat (N=11) | 24.6 ± 5.6 | 32.4 ± 28.9*† | 0.9 ± 0.2* | 632 ± 347*† |
|
| ||||
| RYGB No Ox | ||||
| High fat (N=8) | 25.4 ± 8.3 | 38.5 ± 42.7† | 4.8 ± 1.8 | 1164 ± 480† |
| Normal fat (N=8) | 22.9 ± 7.6 | 31.4 ± 41.5*† | 0.8 ± 0.2* | 524 ± 265*† |
|
| ||||
| Control Ox | ||||
| High fat (N=8) | 21.5 ± 3.7 | 20.9 ± 18.8. | 4.4 ± 0.7 | 218 ± 185 |
| Normal fat (N=8) | 23.6 ± 5.0 | 16.3 ± 23.5. | 0.9 ± 0.2* | 12 ± 4* |
|
| ||||
| Control No Ox | ||||
| High fat (N=8) | 22.3 ± 7.7 | 16.8 ± 21.3 | 4.8 ± 2.0 | 143 ± 96 |
| Normal fat (N=8) | 24.9 ± 9.2 | 12.7 ± 17.9 | 0.9 ± 0.2* | 16 ± 5* |
p-value <0.05 within group comparison
p-value <0.05 compared to sham age-matched controls
Fat intake and fecal fat
Across all groups and regardless of oxalate content, animals on high fat ingested 5-fold more fat than animals on normal fat diet (p<0.001). Stool was heavier (Figure 2A, p<0.001) in RYGB animals compared to controls on both high and normal fat diets. Additionally, RYGB rats had higher stool weights on high fat vs. normal fat diet (p<0.01), a finding not associated with dietary food oxalate content.
Regardless of fat content, RYGB animals excreted more total fecal fat (mg/day) than controls (Table 1, p<0.001). While % fat absorption remained consistently above 95% for controls (Figure 2B), RYGB animals on normal fat absorbed less % dietary fat (mean 41.8% ± 24.7) compared to high fat diet (mean 61.7% ± 25.3, p<0.01). Dietary oxalate content did not contribute to differences in % fat absorption.
Urine parameters
RYGB animals, regardless of dietary oxalate or fat content, had more than 2-fold increase in urine volume (Figure 4A) compared to controls (p<0.01). An increasing urine pH trend was noted for all animals when switched from 40% to 10% fat (Table 4B) but found to be only significant within RYGB No Ox (6.30±0.17 vs 6.53±0.19, p<0.01) and RYGB Ox groups (6.33±0.21 vs 6.67±0.13, p<0.01).
Figure 4. Urine volume and pH.
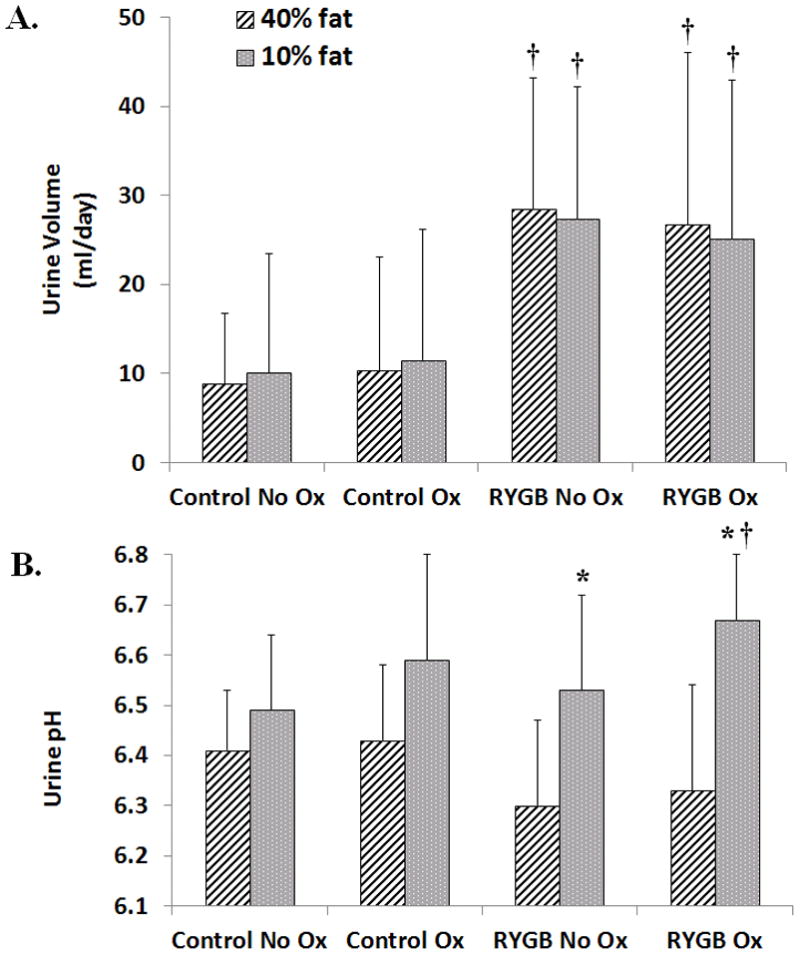
A) Urine volume was higher for RYGB animals compared to sham, age-matched controls. Urine volume was not affected by dietary fat or oxalate content. B). An increased urine pH trend was noted for all animals when switched from 40% to 10% fat but was significant only in RYGB animals. Data are shown as mean values ± SEM. * - p<0.05 within group; † - p<0.05 compared to sham, age-matched controls.
Urine oxalate excretion (Figure 5A) was highest in RYGB Ox rats on high fat diet compared to all other groups (21.57 ± 3.26 μmol/day, p<0.001). When these animals were switched to normal fat, oxalate excretion decreased ~40% (13.05 ± 2.19 μmol/day, p<0.01). RYGB No Ox animals on high fat diet had slightly higher urine oxalate than controls (6.32 ± 1.73 μmol/day vs. 4.8 ± 1.35 μmol/day, p=0.34) but had increased urine oxalate on normal fat diet (9.93 ± 1.86 μmol/day, p=0.031). No differences were noted in oxalate excretion within or between control groups.
Figure 5. 24 hour urinary oxalate and calcium excretion.
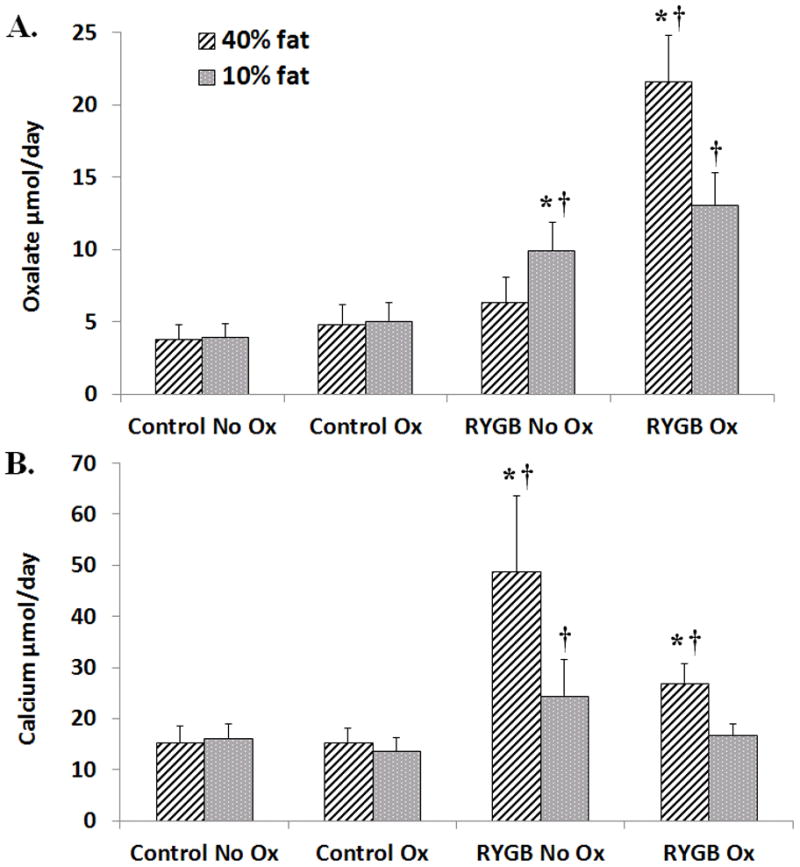
A) Urine oxalate excretion was highest in RYGB Ox rats on 40% fat diet compared to all other groups. On 10% fat, oxalate excretion decreased in RYGB Ox rats but remained higher than sham, age-matched controls. A modest increase was seen RYGB No Ox. B) On high fat diet, urine calcium excretion was highest in RYGB No ox rats and RYGB Ox animals compared to all other groups. On 10% fat, calcium excretion decreased but remained significantly higher in RYGB No ox rats than controls and RYGB Ox rats. Data are shown as mean values ± SEM. * - p<0.05 within group; † - p<0.05 compared to sham, age-matched controls.
Compared to controls on high fat diet, both RYGB No Ox animals (Figure 3B, 48.82 ± 14.73 μmol/day vs. 15.23 ± 3.26 μmol/day, p<0.001) and RYGB Ox animals (26.79 ± 3.96 μmol/day vs. 15.3 ± 2.79 μmol/day, p<0.01) had higher urine calcium excretion. When fat content was lowered, calcium excretion halved in RYGB No Ox rats (24.51 ± 6.96 μmol/day, p<0.03) but remained higher than RYGB Ox rats (16.76 ± 2.26 μmol/day, p<0.01) and Control No Ox (16.06 ± 3.93 μmol/day, p<0.01). No statistically significant differences were noted in calcium excretion within or between control groups.
Figure 3. Stool weight and % fat absorption.
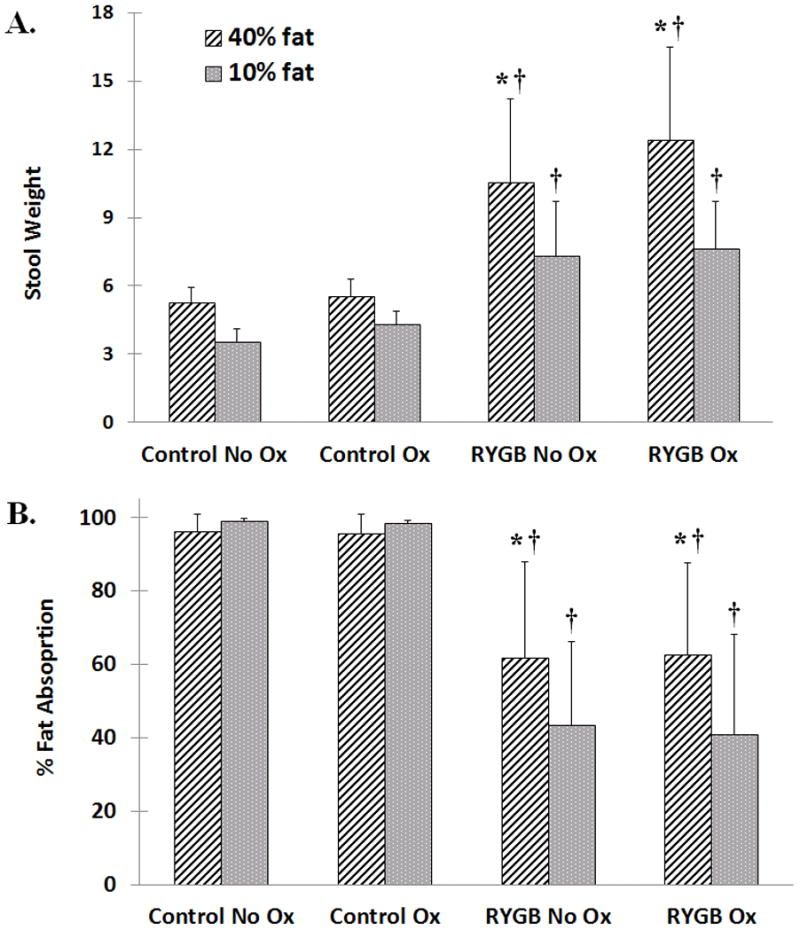
A) Stool weight was highest in RYGB animals on 40% fat diet compared to all other groups. Even on normal fat, RYGB animals had significantly heavier stools than sham aged-matched controls. B) Control animals consistently maintained higher % fat absorption than RYGB animals whereas RYGB animals absorbed less % fat on high fat diet. Oxalate content did not affect % fat absorption. Data are shown as mean values ± SEM. * - p<0.05 within group; † - p<0.05 compared to sham, age-matched controls.
DISCUSSION
The typical American diet as well as that of RYGB patients contains 50% of total calories from carbohydrates, 35% from fat, and 15% from protein7. After inducing obesity on very high fat diet, we imitated this fat pattern in our diet pellets, maintaining protein content at 20% and changing only fat and carbohydrate ratios at clearly defined intervals. Both the 25% total body weight loss at 7 weeks and a higher RYGB fat-mass weight loss compared to lean-mass weight loss are similar to other RYGB publications14, 15. Additionally, RYGB animals ate similar amounts of food as controls, a finding corroborated by others in this model14, 21. If RYGB rats lost weight but ate similar food quantity, weight loss must be occurring either through caloric malabsorption, increased energy expenditure, or combinations of the two. Despite reports citing that energy expenditure is the primary weight loss method in both animals and humans11, 13–15, 21, our findings support that malabsorption may play other roles, particularly in oxalate absorption.
Bueter et al (2010) used a very similar 30 cm common channel in non-obese (mean 460 gm) Wistar rats but found no fat differences by fecal bomb calorimetry when fed low fat (3%) diet15. Stylopoulos et al (2009), using a 50 cm common channel in obese SD rats (mean 550 gm), reported an increase in RYGB fecal fat from 0.1 → 0.2 gm/day when given a 60% high fat diet14. Lastly, le Roux et al13 evaluated feces from 12 non-obese rats (mean 380 gm) that had jejunoileal bypass (JIB) surgery and found no increase in calorie content within fecal material. Our fecal fat analysis contests these studies, as our 35 cm common channel created significant steatorrhea on both high and low fat compared to sham age and food-matched controls. Our animals averaged more than 740 gm, so weight may have contributed to this difference. Another variation was collection technique. Rats are coprophagic (eat their own stool), a nutritional behavior essential for nutrient recovery. Previous reports of fecal collections within metabolic cages (unlike our shoebox cages) may have altered nutritional and fat components, especially since rats are known to increase coprophagic activities when nutritionally deprived22. Bomb calorimetry may overestimate nutritional fecal content, as it includes calories of non-digestable bedding and fibrous materials. Our fecal fat results include semi-quantification by an internal heptadecanoic acid standard that has not been previously used in this model. A low coefficient of variation across replicates and high correlation in duplicate analyses supports the test’s validity. Finally, in controlled diet studies, human RYGB patients excrete roughly 25% more fat after gastric bypass, a finding validated by fecal fat measurement in our model (Figure 3)11.
A number of clinical studies have linked RYGB to increased kidney stone risk and hyperoxaluria2, 4, 23, 24. The three most rigorous prospective cohort studies done before and after RYGB surgery with no history of stone disease found, on average, a 40% increase in urine oxalate (from 34 →58 mg/day) and a two-fold increase in calcium oxalate supersaturation4, 23, 24. To examine this phenomenon more closely, Kumar and collegues10 tested plasma and urinary oxalate, fecal fat excretion, and response to oral oxalate load in 9 pre- and post-RYGB morbidly-obese patients. At 12 months post-op, they found a 25% increase in urine oxalate (from 26 → 33 mg/day, p=0.185), a two-fold increase in calcium oxalate supersaturation (p=0.003) and fecal fat excretion (p=0.26), and a 50% increase in urine oxalate following oxalate load (p<0.02)10. Although this study was well done, it has been criticized for allowing patients to have “choice” diet during the study (unknown fat content), for supplementing calcium aggressively (1,600 mg/day), for lacking controls, and for enrolling a small number of patients, some of whom had prior stone history. These previous limitations led us to characterize and further investigate these questions in an animal model, and our urinary findings support that of enteric hyperoxaluria.
It is well known that urine oxalate can be augmented in rats by adding oxalate to food. In a thorough study of escalating potassium oxalate concentration in rodent chow, Wiessner and colleagues25 detected an increase in urinary oxalate levels in control rats when dietary oxalate supplementation exceeded 2%. To avoid this confounder, we chose 1.5% potassium oxalate in our chow, and indeed urinary oxalate excretion in control animals remained low and constant throughout the study (range 3.75–5.05 μmol/day, Figure 5) regardless of fat content. The 60% increase in urine oxalate in the high fat, RYGB Ox group (from 13.05 to 21.57 μmol/day) can be explained by the established GI pattern of fatty acid/calcium saponification theory, resulting in increased passive oxalate absorption. In contrast, RYGB No Ox animals on 10% fat had a small yet significant 3 μmol/day increase in urinary oxalate excretion (p=0.04) when given a normal fat diet. Because oxalate is the end product of liver glyoxylate and ascorbic acid metabolism, it is possible that increased systemic oxalate production or enhanced renal oxalate excretion may be responsible for this urinary oxalate. Enhanced oxalate uptake due to slow GI transit time is another possibility, as both small bowel transection and elevated peptide YY hormones (acting on the myenteric plexus) have been noted to delay bowel activity26. All of these seem plausible as many RYGB patients suffer with hyperoxaluria despite strict low oxalate and fat diets4, 7. Alternatively, an unknown dietary precursor may have be metabolized and excreted in these animals. This seems unlikely as control urine oxalate is changed. Future flux studies are planned to investigate active transcellular oxalate transport in this model.
Other urinary changes included slightly lower RYGB urine pH (pH ~6.3) on high fat diet when compared to controls (~6.42). When dietary fat content was lowered, significant increases were seen in urine pH, particularly within the RYGB Ox group (6.33 → 6.67, p=0.017). We speculate that the lower urine pH in RYGB animals eating high fat diet may be due to higher GI bicarbonate loss associated with fecal output and renal compensation through urine acidification. Within RYGB groups, urinary calcium excretion is increased on high fat diet compared to the normal fat. Although this seems counter-intuitive, this mild urine calcium elevation could be due to increased bone resorption secondary to chronic GI bicarbonate loss, a phenomenon documented in a small group of RYGB patients prior to administration of potassium alkali therapy27. More detailed studies of calcium homeostasis are underway in this model.
Finally, compared to controls, RYGB rats had a 250% increase in water intake and urine volume, minimally affected by fat or oxalate content. Increased RYGB animal drinking activity has been noted by other authors16, 21 including one group who noted increased water and ethanol consumption following RYGB28. This diuresis may be due to altered thirst mechanisms secondary to gut hormones ghrelin29 and obestatin30. We are intensely studying these diuretic mechanisms, as this may be one of several pathways for RYGB’s beneficial effect on hypertension.
CONCLUSIONS
In our DIO model of gastric bypass surgery, high fat feeding resulted in significant steatorrhea and hyperoxaluria only partially reversible by lowering dietary fat and oxalate content. Although this model supports the theory that RYGB-associated hyperoxaluria is primarily gut and diet-mediated, there are likely RYGB patients with hyperoxaluria and kidney stones with secondary causes for their systemic oxalate production, such as metabolism or slow GI transit time. These mechanisms deserve further exploration, especially in symptomatic RYGB patients who already maintain a low fat and low oxalate diet.
Acknowledgments
Shannon Moore, Amandeep Chadda, Bryce Bergeron, and Zachary Marmetschke for laboratory assistance; George Sarosi MD and Carolina Goncalves MD for bypass model development
Funding: NIH K08 DK089000-03, AUA Care Foundation Rising Star in Urology Research Award, Astellas Global Development, Inc., and Ethicon Endo-Surgery.
References
- 1.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 2.Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol. 2009;181:2573. doi: 10.1016/j.juro.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Maalouf NM, Tondapu P, Guth ES, Livingston EH, Sakhaee K. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol. 2010;183:1026. doi: 10.1016/j.juro.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel BN, Passman CM, Fernandez A, Asplin JR, Coe FL, Kim SC, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181:161. doi: 10.1016/j.juro.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann AF, Laker MF, Dharmsathaphorn K, Sherr HP, Lorenzo D. Complex pathogenesis of hyperoxaluria after jejunoileal bypass surgery. Oxalogenic substances in diet contribute to urinary oxalate. Gastroenterology. 1983;84:293. [PubMed] [Google Scholar]
- 6.Andersson H, Jagenburg R. Fat-reduced diet in the treatment of hyperoxaluria in patients with ileopathy. Gut. 1974;15:360. doi: 10.1136/gut.15.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froeder L, Arasaki CH, Malheiros CA, Baxmann AC, Heilberg IP. Response to dietary oxalate after bariatric surgery. Clin J Am Soc Nephrol. 2012;7:2033. doi: 10.2215/CJN.02560312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freel RW, Hatch M, Earnest DL, Goldner AM. Oxalate transport across the isolated rat colon. A re-examination. Biochim Biophys Acta. 1980;600:838. doi: 10.1016/0005-2736(80)90486-1. [DOI] [PubMed] [Google Scholar]
- 9.Dobbins JW, Binder HJ. Effect of bile salts and fatty acids on the colonic absorption of oxalate. Gastroenterology. 1976;70:1096. [PubMed] [Google Scholar]
- 10.Kumar R, Lieske JC, Collazo-Clavell ML, Sarr MG, Olson ER, Vrtiska TJ, et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149:654. doi: 10.1016/j.surg.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odstrcil EA, Martinez JG, Santa Ana CA, Xue B, Schneider RE, Steffer KJ, et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92:704. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 12.Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr. 1990;52:87. doi: 10.1093/ajcn/52.1.87. [DOI] [PubMed] [Google Scholar]
- 13.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009;17:1839. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Bueter M, Ashrafian H, Frankel AH, Tam FW, Unwin RJ, le Roux CW. Sodium and water handling after gastric bypass surgery in a rat model. Surg Obes Relat Dis. 2011;7:68. doi: 10.1016/j.soard.2010.03.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stylopoulos N, Davis P, Pettit JD, Rattner DW, Kaplan LM. Changes in serum ghrelin predict weight loss after Roux-en-Y gastric bypass in rats. Surg Endosc. 2005;19:942. doi: 10.1007/s00464-004-8825-x. [DOI] [PubMed] [Google Scholar]
- 18.Matheny M, Zhang Y, Shapiro A, Tumer N, Scarpace PJ. Central overexpression of leptin antagonist reduces wheel running and underscores importance of endogenous leptin receptor activity in energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1254. doi: 10.1152/ajpregu.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol. 2011;300:G461. doi: 10.1152/ajpgi.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 2004;127:139. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Furnes MW, Tommeras K, Arum CJ, Zhao CM, Chen D. Gastric bypass surgery causes body weight loss without reducing food intake in rats. Obes Surg. 2008;18:415. doi: 10.1007/s11695-007-9392-8. [DOI] [PubMed] [Google Scholar]
- 22.Sukemori S, Kurosawa A, Ikeda S, Kurihara Y. Investigation on the growth of coprophagy-prevented rats with supplemented vitamin B12. J Anim Physiol Anim Nutr (Berl) 2006;90:402. doi: 10.1111/j.1439-0396.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 23.Duffey BG, Alanee S, Pedro RN, Hinck B, Kriedberg C, Ikramuddin S, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg. 2010;211:8. doi: 10.1016/j.jamcollsurg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Park AM, Storm DW, Fulmer BR, Still CD, Wood GC, Hartle JE., 2nd A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol. 2009;182:2334. doi: 10.1016/j.juro.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 25.Wiessner JH, Garrett MR, Hung LY, Wille DF, Mandel NS. Improved methodology to induce hyperoxaluria without treatment using hydroxyproline. Urol Res. 2011;39:373. doi: 10.1007/s00240-011-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cullen JJ, Eagon JC, Hould FS, Hanson RB, Kelly KA. Ectopic jejunal pacemakers after jejunal transection and their relationship to transit. Am J Physiol. 1995;268:G959. doi: 10.1152/ajpgi.1995.268.6.G959. [DOI] [PubMed] [Google Scholar]
- 27.Sakhaee K, Griffith C, Pak CY. Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg Obes Relat Dis. 2012;8:67. doi: 10.1016/j.soard.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Thanos PK, Subrize M, Delis F, Cooney RN, Culnan D, Sun M, et al. Gastric Bypass Increases Ethanol and Water Consumption in Diet-Induced Obese Rats. Obes Surg. 2012 doi: 10.1007/s11695-012-0749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto H, Fujihara H, Kawasaki M, Saito T, Shibata M, Otsubo H, et al. Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology. 2007;148:1638. doi: 10.1210/en.2006-0993. [DOI] [PubMed] [Google Scholar]
- 30.Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am J Physiol Regul Integr Comp Physiol. 2007;292:R637. doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]