Abstract
The pregnane X receptor (PXR) and constitutive androstane receptor (CAR) have been known to play a role in xenobiotic metabolism by regulating the expression of drug-metabolizing enzymes and transporters. In addition, PXR agonists were found to exert therapeutic effects through multiple mechanisms, such as detoxification of bile acids and inhibition of inflammation. In this study, we first investigated the effects of three natural product compounds, carapin, santonin and isokobusone, on the activity of PXR and CAR. These compounds activated both PXR and CAR in transient transfection and luciferase reporter gene assays. Mutagenesis studies showed that two amino acid residues, Phe305 of the rodent PXR and Leu308 of the human PXR, are critical for the recognition of these compounds by PXR. Importantly, the activation of PXR and CAR by these compounds induced the expression of drug-metabolizing enzymes in primary human and mouse hepatocytes. Furthermore, activation of PXR by these compounds inhibited the expression of inflammatory mediators in response to lipopolysaccharide (LPS). The effects of these natural compounds on drug metabolism and inflammation were abolished in PXR−/− hepatocytes. These natural compounds can be explored for their potential in the treatment of diseases where the PXR activation has been shown to be beneficial, such as inflammatory bowel disease, cholestasis, and hyperbilirubinemia.
Keywords: nuclear receptor, gene regulation, natural products, drug metabolism
Introduction
The nuclear receptors pregnane X receptor (PXR, NR1I2) (1, 2) and constitutive androstane receptor (CAR, NR1I3) (3, 4) are transcriptional factors that regulate the expression of genes that are involved in the metabolism and disposition of xenobiotics and endogenous substances. PXR is primarily expressed in the liver, intestine and kidney, whereas CAR is highly expressed in the liver and small intestine. PXR and CAR have been characterized as xenobiotic sensors, because they can recognize a wide array of xenobiotic and endogenous chemicals. The human cytochrome P450 3A4 (CYP3A4)/mouse Cyp3a11 and human CYP2B6/mouse Cyp2b10 are primary target genes of PXR and CAR, respectively (3, 5). As such, activation of PXR and CAR has been shown to regulate drug metabolism. In addition to their xenobiotic functions, PXR and CAR also play essential roles in various physiological and pathophysiological processes, such as bile acid metabolism, lipid metabolism, glucose homeostasis, and inflammation (6). Both receptors have been suggested as useful targets for pharmacological interventions of several diseases, such as hepatic steatosis, cholestatic liver disease, hyperbilirubinemia, osteoporosis, and inflammatory bowel disease (IBD) (7–9). For the anti-inflammatory function of PXR, Zhou and colleagues demonstrated that ligand activation of PXR inhibited the activity of NF-κB, a key regulator of inflammatory responses. Activation of PXR also suppressed the expression of several pro-inflammatory genes, such as prostaglandin-endoperoxide synthase 2 (COX-2), tumor necrosis factor alpha (TNF-α), intercellular adhesion molecule 1 (ICAM-1), and several interleukins. In contrast, the expression of NF-κB target genes in hepatocytes derived from the PXR−/− mice was substantially upregulated compared to that of the wild-type mice, suggesting that PXR plays an important role in suppressing the NF-κB signaling (10).
Both PXR and CAR form obligate heterodimers with a common partner, the retinoic X receptor (RXR, NR1B2), after which the PXR-RXR or CAR-RXR heterodimers bind to the specific response elements termed PXR response elements or CAR response elements (also called phenobarbital response element) in the target gene promoters. PXR and CAR share some ligands as well as target genes. In the meantime, PXR and CAR also have their own unique ligands and both receptors exhibit considerable species specificity in their ligand recognition. In addition to their activation by prescription drugs such as antibiotics, anti-neoplastic drugs and anti-inflammatory agents, insecticides, pesticides, and nutritional compounds, PXR and CAR have also been reported to be activated by natural compounds. For example, herbal medicines such as Schisandra chinensis (Wu Wei Zi), Glycyrrhiza uralensis (Gan Cao), Piper methysticum (kava kava) and Hypericum perforatum (St. John’s Wort) were reported to activate PXR, whereas Allium sativum (Garlic) and Yin Zhi Huang were found to activate CAR (11, 12). Quercetin and kaempferol, on the other hand, can activate both PXR and CAR (13–16). There is a continued need to identify PXR and CAR ligands, especially from traditional medicines, in order to harness the therapeutic potential of these receptors.
In this study, we examined the effects of three natural product compounds carapin, santonin and isokobusone on the activities of PXR and CAR. Carapin, an extracted compound from Carapa procera, Carapa guyanensis and Carapa indica, has been shown to have antifungal/antibacterial activity and wound healing activity (17, 18). Santonin, commonly found in the plants of the Compositae family, has been widely used as an antiparasitic drug (19). Santonin was also found to have significant anti-inflammatory activity on acute inflammatory processes (20, 21). Isokobusone, a compound that can be isolated from crude extract of tubers of Cyperus rotundus Linn, has long been used as a traditional Chinese medicine to treat nausea, pain reduction, muscle relaxation, fever and inflammation (22, 23). We found that these compounds can activate PXR and CAR and subsequently increase the expression of drug-metabolizing enzymes. The same natural compounds also exhibit anti-inflammatory activities.
Materials and Methods
Chemicals
The natural product compounds were purchased from MicroSource (Gaylordsville, CT). Each compound has a purity of greater than 95%. We did not use crude extracts or fractions from plants in this study. Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Plasmids and Transient Transfection
The reporter plasmids (tk-CYP3A23-Luc and tk-PBRE-Luc) and the expression vectors (hPXR, mPXR, hCAR and mCAR) used in this study have been described previously (24, 25). The rat PXR (rPXR) F305L and hPXR L308F mutants are kind gifts from Dr. Richard Kim (The University of Western Ontario, Canada) and these two mutants have been described previously (26). The CMV2-hCAR3 and CMV2-mCAR3 vectors were obtained from Dr. Curtis Omiecinski (Pennsylvania State University, USA). The monkey kidney-derived fibroblast (CV-1) cells and human embryonic kidney HEK293 cells were seeded on 48-well plates and maintained in Dulbecco’s Modified Essential Medium (DMEM) containing 10% fetal bovine serum, 100 U/ml penicillin G, and 100 g/ml streptomycin. After being transfected overnight, cells were treated with either vehicle (0.1% DMSO) or appropriate compounds (10 μM) for 24 h before luciferase assay. Luciferase activity was normalized against the co-transfected β-galactosidase activity. All transfections were performed in triplicate.
Human and Mouse Primary Hepatocyte Preparation
Human livers were obtained through the Liver Tissue Procurement and Distribution System, University of Pittsburgh, and hepatocytes were isolated by three-step collagenase perfusion (27). Mouse primary hepatocytes from C57BL6 wild-type, PXR−/− mice (28), and “humanized” hPXR transgenic mice (28) were isolated by standard collagenase perfusion. Cells were plated on collagen-coated 6-well plates at a density of 5×105 cells/well and maintained in hepatocyte maintenance medium from Cambrex (Walkersville, MD) supplemented with 0.1 mM dexamethasone, 0.1 mM insulin, 50 g/ml gentamicin, 50 ng/ml amphotericin, and incubated overnight. Cells were then treated with either vehicle or test compounds for 24 h before RNA isolation.
RNA Isolation and Real-Time PCR
Total RNA was isolated by using the TRIZOL reagent from Invitrogen (Carlsbad, CA). After DNase I treatment, 1 μg of RNA was reverse-transcribed by using iScript cDNA synthesis kit (Bio-Rad). Amplifications were performed with the ABI 7500 real-time PCR (Applied Biosystem) by using the SYBR Green Master Mix (29). The primer sets used in this study are presented in Table 1. The GADPH or cyclophilin were used as an internal control for normalization.
Table 1.
Primer sequences for real-time PCR
| Gene Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| Human: | ||
| hCYP3A4 | CACCCTGATGTCCAGCAGAA | TGCCTTATTGGGTAAAACTGCAT |
| hCYP2B6, | AGACGCCTTCAATCCTGACC | CCTTCACCAAGACAAATCCGC |
| hiNOS | CAGCGGGATGACTTTCCAA | AGGCAAGATTTGGACCTGCA |
| hMCP-1 | GATCTCAGTGCAGAGGCTCG | TGCTTGTCCAGGTGGTCCAT |
| hGAPDH | CCCATCACCATCTTCCAGGAG | GTTGTCATGGATGACCTTGGC |
| hCyclophilin | TGGTGTTTGGCAAAGTGAAA | TCGAGTTGTCCACAGTCAGC |
| Mouse: | ||
| mCyp3a11 | AAACTGCAGGATGAGATCGATGA | TCCAGGTATTCCATCTCCATCAC |
| mCyp2b10 | TCCTGACCAGTTCCTGGATG | CTGGAGGATGGACGTGAAGAA |
| miNOS | GTGACGGCAAACATGACTTCAG | GCCATCGGGCATCTGGTA |
| mMCP-1β | CAACTCTCACTGAAGCCAGCTCT | CAGGCCCAGAAGCATGACA |
| mIL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| mGAPDH | CAGTGCCAGCCTCGTCCCGTAGA | CTGCAAATGGCAGCCCTGGTGAC |
| mCyclophilin | GGAGATGGCACAGGAGGAA | GCCCGTAGTGCTTCAGCTT |
Measurements of Cytochrome P450 Activity
After the hepatocytes were treated with compounds for 24 h, cells were washed and incubated with a medium containing testosterone (200 μM) for 6 h. Then, the medium was collected and the reaction was terminated by the addition of an equal volume of cold methanol. The precipitated proteins were separated by centrifugation at 13,000g for 15 min. The formation of 6β- and 16β-hydroxytestosterone in the medium were used as markers for CYP3A and CYP2B activities, respectively, and were measured by HPLC as described previously (30, 31) with some modifications. One hundred microliters of the culture medium was injected into a LiChrospher 100 RP-18 column (4.6 × 250 mm, 5 m) from Merck (Darmstadt, Germany). A mobile phase of methanol/water (60:40, v/v) at a flow rate of 1.2 ml/min was used to separate various components. The eluents were measured using a UV detector at a wavelength of 242 nm. The concentrations of 6β- and 16β-hydroxytestosterone were quantified by comparing the peak area of the samples to a standard curve containing a known amount of metabolites.
Statistical Analysis
Statistical analyses were performed by the unpaired Student’s t-test. For comparison of more than two groups of data, One-way ANOVA with Student–Newman-Keuls post-hoc test was performed. P values of less than 0.05 were considered to be statistically significant.
Results
The effects of carapin, santonin and isokobusone on PXR activation
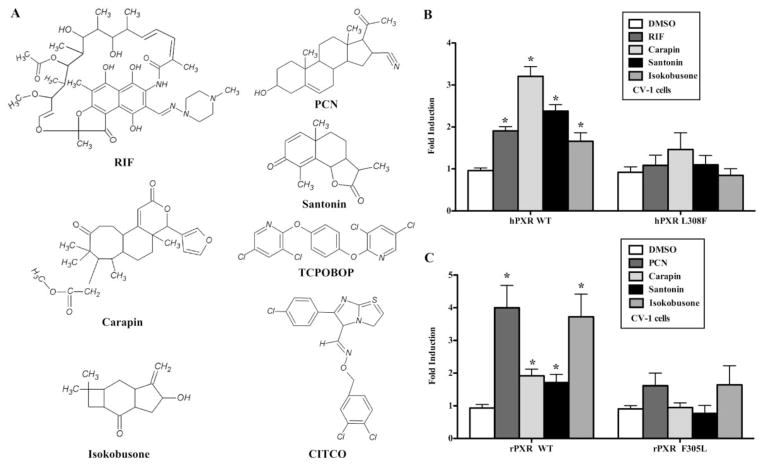
Carapin, santonin and isokobusone were three candidate PXR activators identified in a screen of the GENPLUS natural product library from MicroSource (Gaylordsville, CT) using a transient transfection and reporter gene assay (data not shown). The chemical structures of these three compounds, along with those of the typical PXR and CAR agonists RIF, PCN, TCPOBOP and CITCO, are shown in Fig. 1A. We first validated the effect of these three compounds on PXR activity using the PXR transfection assay in CV-1 cells. Cells were transfected with the human PXR (hPXR) and rat PXR (rPXR) expression vectors. The activity of PXR after drug treatment was determined using a PXR-responsive tk-CYP3A23-Luc reporter. As shown in Fig. 1B–C, carapin, santonin and isokobusone at the dose of 10 μM activated both hPXR and rPXR compared to that of the vehicle controls. In these experiments, RIF and PCN were included as hPXR-specific and rPXR-specific ligands, respectively. We next determined the specific amino acid determinants of PXR in its activation by carapin, santonin and isokobusone. Two amino acid residues, Phe305 of rPXR and Leu308 of hPXR, have been reported to be critical for determination of the species-specific ligand activation (26). We used the rPXR F305L and hPXR L308F mutants to determine whether these residues are important for the activation of PXR by these compounds. The results showed that the activation of hPXR by carapin, santonin and isokobusone was abolished in hPXR L308F mutant transfected cells. The activation of rPXR activation by these compounds was also abolished by the rPXR F305L mutation.
Figure 1. Carapin, santonin and isokobusone activated the human and rat PXR.
(A) Chemical structures of carapin, santonin and isokobusone, along with those of the typical PXR and CAR agonists rifampicin, PCN, TCPOBOP, and CITCO. (B and C) CV-1 cells were cotransfected with the tk-CYP3A23-Luc reporter gene, together with the wild-type and mutant hPXR (B) or rPXR (C). Transfected cells were treated with the vehicle or the indicated compounds (10 μM each) for 24 h before luciferase assay. Rifampicin (RIF) and prenenolon-16α-carbonitrile (PCN) were included as the positive control ligands for hPXR and rPXR, respectively. Results are shown as fold induction over vehicle control and represent the average from triplicate assays. *, P < 0.05, vs the vehicle control group of each WT and mutant receptors.
The effects of carapin, santonin and isokobusone on CYP3A4 expression in primary human hepatocytes and Cyp3a11 expression in primary mouse hepatocytes
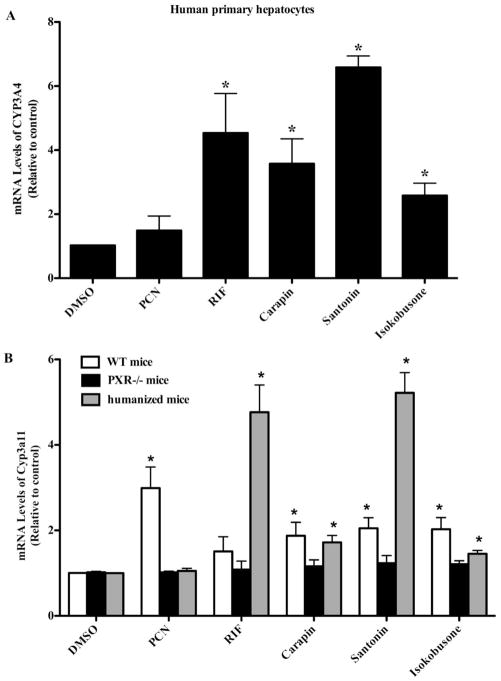
We then determined whether carapin, santonin and isokobusone could induce the expression of drug-metabolizing enzymes in human primary hepatocytes. PXR activation is known to increase the mRNA expression of CYP3A4. Consistent with the result obtained from hPXR reporter gene assay, all of the test compounds at the dose of 10 μM effectively induced CYP3A4 mRNA expression in primary human hepatocytes. We also determined the effect of carapin, santonin and isokobusone on the expression of Cyp3a11, a mouse PXR target gene, in primary mouse hepatocytes. As shown in Fig. 2B, these compounds induced the expression of Cyp3a11 mRNA in WT mouse hepatocytes, and this induction was abolished in PXR−/− mice, suggesting that the induction was PXR dependent. We previously generated “humanized” hPXR transgenic mice in which a liver-specific hPXR transgene was introduced into the PXR−/− background (28). Consistent with the results from human primary hepatocytes, we found Cyp3a11 mRNA expression was increased after treatment of humanized primary mouse hepatocytes with these compounds (Fig. 2B). Again, RIF and PCN were included as hPXR-specific and mPXR-specific ligands, respectively.
Figure 2. Carapin, santonin and isokobusone induced the expression of PXR target gene CYP3A in human and mouse primary hepatocytes.
(A) Treatment with carapin, santonin and isokobusone induced the mRNA expression of CYP3A4 in primary human hepatocytes. (B) The induction of Cyp3a11 mRNA by carapin, santonin and isokobusone in primary hepatocytes was abolished in hepatocytes isolated from PXR−/− mice. In contrast, the inducibility was restored in hepatocytes isolated from hPXR humanized mice. The cells were treated with the compounds at the dose of 10 μM for 24 h before harvesting total RNA and real-time PCR analysis. RIF was included as positive control for CYP3A4 induction in human hepatocytes, whereas PCN was used as positive control for Cyp3a11 induction in mouse hepatocytes. Results normalized to GAPDH are shown as fold induction over vehicle control and represented the averages from triplicate assays. *, P < 0.05, vs respective vehicle controls of each genotype.
The effects of carapin, santonin and isokobusone on human and mouse CAR activation
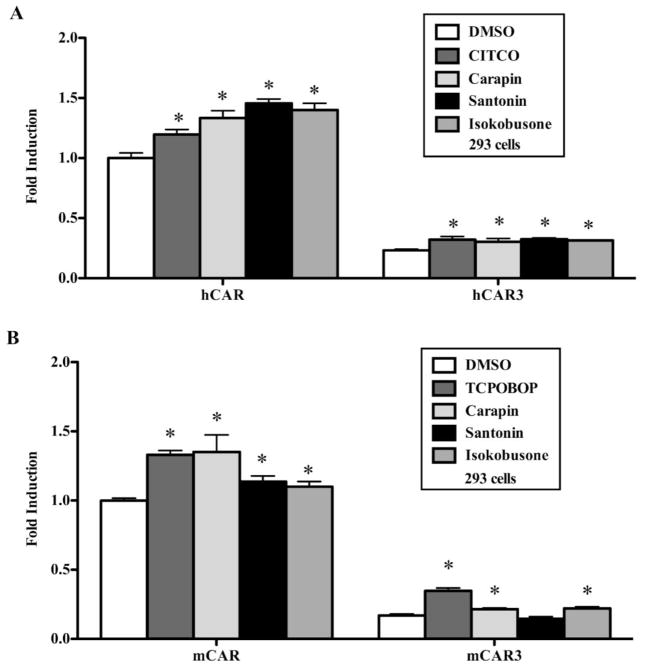
Since CAR is a sister xenobiotic receptor of PXR and shares some of its ligands as well as target genes, we determined the effect of carapin, santonin and isokobusone on CAR activation using luciferase reporter assay. HEK293 cells were transiently transfected with human CAR (hCAR) and the CAR-responsive tk-PBRE reporter gene. As shown in Fig. 3A, carapin, santonin and isokobusone activated hCAR with potency similar to that of the positive control CITCO. The WT CAR (also termed CAR1) is known to possess a high level of constitutive activity in cell-based assays, obscuring the detection of ligand activators. Previous studies showed that the CAR gene produces a number of differentially spliced mRNAs encoding structurally diverse proteins (32). A human splice variant of CAR, termed CAR3, functions as a ligand-dependent receptor transcript and exhibits substantially lower constitutive activity. In addition, CAR3 is activated by ligands with similar specificity as the reference CAR. Therefore, we used hCAR3 expression vector to determine the effect of these three compounds on CAR activation using luciferase reporter assay. As expected, carapin, santonin and isokobusone could also activate hCAR3 in this assay (Fig. 3A). We then used mCAR and mCAR3 to repeat the experiments, and TCPOBOP was included as a positive control. As shown in Fig 3B, carapin, santonin and isokobusone also activated mCAR. Interestingly, although carapin and isokobusone were effective in activating mCAR3, santonin showed little activity on mCAR3. As expected, CAR3s showed lower basal activities compared to their WT counterparts.
Figure 3. Carapin, santonin and isokobusone activated human and mouse CAR.
(A and B) HEK293 cells were co-transfected with hCAR (hCAR1) or hCAR3 (A) and mCAR or mCAR3 (B), together with the tk-PBRE-Luc reporter gene. Transfected cells were treated with the vehicle or the indicated compounds (10 μM each) for 24 h before luciferase assay. CITCO (1 μM) and TCPOBOP (250 nM) were included as the positive control ligands for hCAR and mCAR, respectively. Results are shown as fold induction over vehicle control and represent the averages from triplicate assays. *, P < 0.05, vs vehicle control of each receptor or its mutant variant.
The effects of carapin, santonin and isokobusone on CYP2B6 and Cyp2b10 expression in human and mouse primary hepatocytes
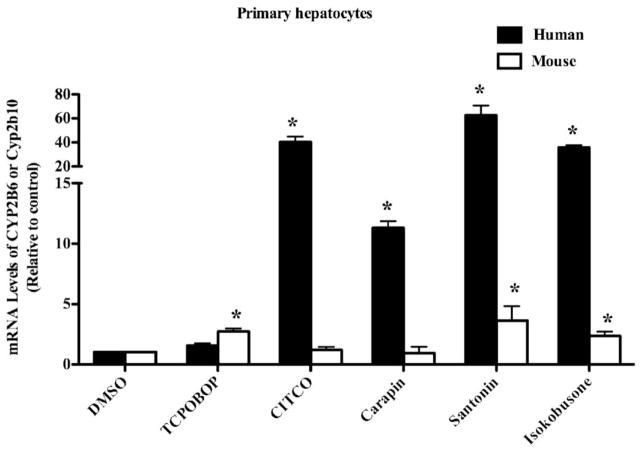
CYP2B6 and Cyp2b10 are primary CAR target genes in mice and humans, respectively. Therefore, we evaluated the effects of carapin, santonin and isokobusone on the expression of CYP2B6 and Cyp2b10 genes in human and mouse primary hepatocytes, respectively. We found that all three compounds were effective in inducing CYP2B6 in human hepatocytes. When the mouse hepatocytes were evaluated, santonin and isokobusone, but not carapin, were effective in inducing Cyp2b10 (Fig 4). Again, CITCO and TCPOBOP were included as hCAR-specific and mCAR-specific ligands, respectively.
Figure 4. Carapin, santonin and isokobusone induced the expression of CAR target gene CYP2B in human and mouse primary hepatocytes.
Human and mouse primary hepatocytes were treated vehicle DMSO), CITCO (1 μM), TCPOBOP (250 nM), carapin, santonin, or isokobusone (10 μM each) for 24 h. CYP2B6 and Cyp2b10 mRNA expression was measured by real-time PCR analysis. Data were expressed as fold change over the control, normalized to GAPDH expression. *, P < 0.05, vs the vehicle controls of each genotype.
The effects of carapin, santonin and isokobusone on CYP3A and CYP2B activities in human and mouse hepatocytes
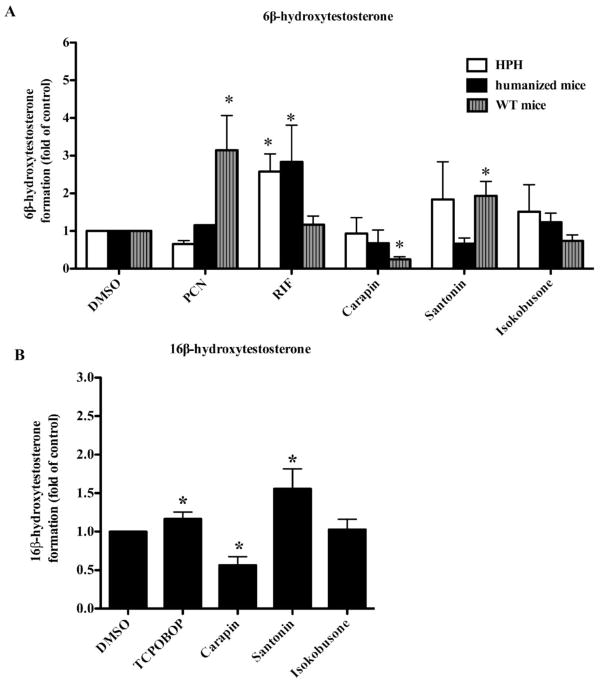
The data presented above implied that carapin, santonin and isokobusone activate PXR, leading to an increased expression of CYP3A. To assess whether carapin, santonin and isokobusone increased the CYP3A activity, we determined the metabolism of testosterone by measuring 6β-hydroxytestosterone levels in the medium of hepatocyte cultures. RIF and PCN were included as hPXR-specific and rPXR-specific control ligands, respectively. Surprisingly, the results showed that these compounds did not affect the 6β-hydroxytestosterone level in either the primary human hepatocytes or hepatocytes isolated from the humanized mice. In the WT primary mouse hepatocytes, only santonin, but not isokobusone, was effective in inducing 6β-hydroxytestosterone level. In contrast, the 6β-hydroxytestosterone level was significantly decreased by carapin (Fig 5A). As positive controls, RIF was effective in inducing 6β-hydroxytestosterone formation in primary human hepatocytes and hepatocytes isolated from humanized mice, whereas PCN was effective in inducing 6β-hydroxytestosterone formation in primary mouse hepatocytes.
Figure 5. Effects of carapin, santonin and isokobusone on CYP3A and CYP2B activities.
(A and B) 6β-hydroxytestosterone level in primary human hepatocytes as well as wild-type and humanized mice (A) and 16β-hydroxytestosterone level in primary mouse hepatocytes (B). Cells were treated with test compounds at the concentration of 10 μM or vehicle for 24 h. Then, they were washed with medium followed by the addition of 200 μM testosterone for 6 h. Aliquots of medium were analyzed for 6β-and 16β-hydroxytestosterone by HPLC. RIF and PCN were included as the control ligands for hPXR and mPXR, respectively. CITCO (1 μM) and TCPOBOP (250 nM) were included as the positive control ligand for hCAR and mCAR, respectively. Data are expressed as means ± SD from triplicate experiments. *, P < 0.05, vs DMSO-treated cells of each genotype.
As CYP2B mediates the 16β-hydroxylation of testosterone, we next determined the CYP2B activity by measuring 16β-hydroxytestosterone level in the medium after treatment of the cells with carapin, santonin and isokobusone. Similar to CYP3A activity in WT hepatocytes, 16β-hydroxytestosterone level after treatment with santonin, but not isokobusone, was significantly increased. On the other hand, carapin decreased 16β-hydroxytestosterone level in mouse primary hepatocytes (Fig 5B). TCPOBOP induced 16β-hydroxytestosterone formation as expected.
Inhibitory effect of carapin, santonin and isokobusone on the LPS responsive expression of pro-inflammatory genes
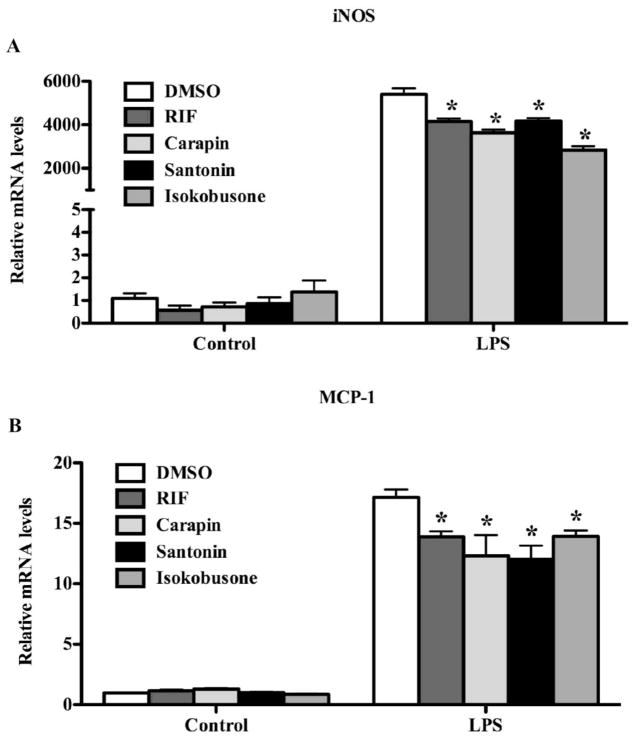
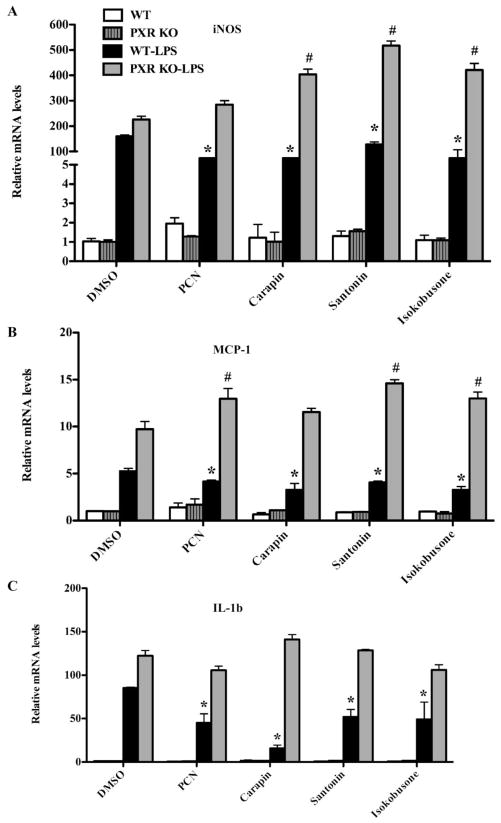
It has been reported that PXR has an anti-inflammatory activity by antagonizing the NF-κB pathway (33). We next investigated whether activation of PXR by carapin, santonin and isokobusone has an effect on the LPS responsive expression of pro-inflammatory genes in hepatocytes. In primary human hepatocytes, treatment with LPS induced the expression of inducible nitric oxide synthase (iNOS) (Fig. 6A) and monocyte chemoattractant protein-1 (MCP-1) (Fig. 6B) as expected. Pre-treatment of cells with carapin, santonin or isokobusone significantly lowered the LPS-responsive expression of iNOS and MCP-1 (Fig 6A and 6B). A similar inhibition of the LPS-responsive expression of iNOS, MCP-1 and IL-1β by the pre-treatment of carapin, santonin and isokobusone was observed in primary mouse hepatocytes (Fig. 7A–C). The inhibitory effect of the three compounds on the LPS-responsive expression of iNOS, MCP-1 and IL-1β was PXR dependent, because the inhibitions were abolished in hepatocytes isolated from PXR−/− mice (Fig. 7A–C).
Figure 6. Carapin, santonin and isokobusone inhibited the LPS-responsive expression of pro-inflammatory genes in human primary hepatocytes.
(A and B) Human primary hepatocytes were pre-treated with vehicle, RIF, carapin, santonin or isokobusone (10 μM each) for 24 h before the addition of 20 μg/ml LPS for 6 h. Total RNA was isolated and the expression of iNOS (A) and MCP-1 (B) was determined by real-time PCR. Data were normalized to cyclophilin expression and expressed as fold change over controls. *, P < 0.05, vs LPS alone treatment groups. N = 3 for each group.
Figure 7. Carapin, santonin and isokobusone suppressed the LPS-responsive expression of pro-inflammatory genes in mouse hepatocytes in a PXR-dependent manner.
(A–C) Real-time PCR analysis of the expression of iNOS (A), MCP-1 (B) and IL-1β (C) in WT and PXR−/− mouse hepatocytes that were pre-treated with vehicle, PCN, carapin, santonin, or isokobusone (10 μM each) for 24 h before treatment with LPS (20 μg/ml) for 6 h. Results were normalized to cyclophilin mRNA levels and expressed as fold change over controls. *, P < 0.05, vs LPS alone treatment groups in WT mice; # P < 0.05, vs LPS alone treatment groups in PXR KO mice. N = 3 for each group.
Discussion
In this study, we reported the characterization of three natural product compounds carapin, santonin and isokobusone as ligands for the xenobiotic receptors PXR and CAR. These three compounds activated PXR and CAR in transient transfection and reporter gene assays. Mutagenesis analysis revealed Phe305, a residue located in the flexible loop that forms part of the pore to the ligand-binding cavity of the rPXR (26), and its hPXR counterpart Leu308, are essential for the recognition of these three compounds by PXR.
Treatment of human and mouse primary hepatocytes with carapin, santonin, or isokobusone induced the expression of endogenous CYP3A and 2B genes. Moreover, pre-treatment of hepatocytes with these compounds inhibited the LPS-responsive induction of pre-inflammatory genes. The induction of drug-metabolizing enzymes by carapin, santonin and isokobusone was abolished in primary hepatocytes isolated from PXR−/− mice, suggesting that the induction was PXR dependent. Although it remains to be determined whether the parent compounds or their metabolites are responsible for the activation of PXR and CAR, the use of HEK293 and CV-1 cells, in which the endogenous expression of drug-metabolizing enzymes and transporters was very low or absent (34), implied that the activations may have resulted from the parent compounds instead of their metabolites.
Having shown that carapin, santonin, or isokobusone activated PXR and CAR and induced the expression of CYP3A and CYP2B, we were surprised to find that treatment of primary human hepatocytes with these compounds failed to increase the production of 6β- and 16β-hydroxytestosterone. The pattern of effect on mouse hepatocytes was also not quite consistent with the pattern of receptor activation. A possible explanation is that the inhibition of CYP3A4 catalytic activity in the hepatocyte might be due to either these compounds acting as competitive inhibitors for CYP enzymes, or they cause irreversible inactivation of the enzymes. The first possibility seemed unlikely, because the hepatocytes were washed before the addition of the testosterone substrate in the experiments. Alternatively, the induction at mRNA level might not have been translated into increases in enzyme proteins. It has been reported that the changes in transcription and activity of CYP3A4 may not be in parallel because certain PXR-activating chemicals could also directly affect the activity of CYP3A4 through mechanism-based inactivation (35). The disconnection between PXR activation/P450 induction and drug metabolism has also been reported for meclizine, a piperazine-derived histamine H1 antagonist that increased CYP3A4 mRNA expression but decreased CYP3A4 catalytic activity (36).
The inhibition of LPS-responsive induction of pre-inflammatory genes by carapin, santonin and isokobusone was consistent with the notion that PXR has an anti-inflammatory activity through the inhibition of the NK-kB signaling. The expression of NK-kB target genes was found to increase in the small bowel of PXR−/− mice (10). In contrast, treatment of primary hepatocytes with PXR agonists inhibited LPS responsive activation of pre-inflammatory genes (11). Treatment of hPXR humanized mice with rifaximin, an hPXR agonist, alleviated symptoms in the DSS model of inflammatory bowel disease (IBD) (37). We found that pre-treatment of human or mouse primary hepatocytes with carapin, santonin and isokobusone decreased the LPS-responsive expression of iNOS, MCP-1 and IL-1β. The anti-inflammatory effect of santonin was consistent with a previous report that this compound exhibited anti-inflammatory activity closely resembling that of diclofenac sodium, a non-steroidal anti-inflammatory drug (21). The inhibition of LPS-induced pro-inflammatory gene expression by these compounds was abolished in PXR−/− mice, suggesting that the activation of PXR by these compounds may have accounted for the inflammatory effect.
In summary, our results show that the three novel natural compounds, carapin, santonin and isokobusone, activate PXR and CAR and induce drug-metabolizing enzymes. In addition, these compounds inhibited the expression of inflammatory mediators in response to LPS through the activation of PXR. It remains to be tested whether these compounds can be used for other diseases where the PXR activation has been shown to be beneficial, such as cholestasis, hyperbilirubinemia, and IBD.
Acknowledgments
Grant Support: This work was supported in part by an NIH grants ES019629 (to W.X.), ES010337 (to R.M.E.), and the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant PHD/0204/2549 to S.K.). R.M.E. is an investigator of the Howard Hughes Medical Institute and March of Dimes Chair in Molecular and Developmental Biology at the Salk Institute. Normal human hepatocytes were obtained through the Liver Tissue Procurement and Distribution System, Pittsburgh, Pennsylvania, which was funded by NIH Contract [N01-DK-7-0004/HHSN267200700004C].
We thank Yanhong Shi for her assistance in the initial library screen. We also thank Lushan Yu for his assistance in the P450 enzymatic assay. Wen Xie is the Joseph Koslow Endowed Chair in Pharmaceutical Sciences at the University of Pittsburgh School of Pharmacy.
Abbreviations
- CAR
constitutive androstane receptor
- IBD
inflammatory bowel disease
- LPS
lipopolysacharide
- PCN
pregnenolone-16α-carbonitrile
- PXR
pregnane X receptor
- RIF
rifampicin
- TCPOBOP
1,4-bis [2-(3,5-Dichloropyridyloxy)] benzene
References
- 1.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998 Oct 15;12(20):3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998 Jan 9;92(1):73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 3.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998 Oct;18(10):5652–8. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000 Oct 19;407(6806):920–3. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999 Dec;56(6):1329–39. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- 6.Moreau A, Vilarem MJ, Maurel P, Pascussi JM. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm. 2008 Jan-Feb;5(1):35–41. doi: 10.1021/mp700103m. [DOI] [PubMed] [Google Scholar]
- 7.Saini SP, Mu Y, Gong H, Toma D, Uppal H, Ren S, et al. Dual role of orphan nuclear receptor pregnane X receptor in bilirubin detoxification in mice. Hepatology. 2005 Mar;41(3):497–505. doi: 10.1002/hep.20570. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Zhou J, Xie W. PXR and LXR in hepatic steatosis: a new dog and an old dog with new tricks. Mol Pharm. 2008 Jan-Feb;5(1):60–6. doi: 10.1021/mp700121u. [DOI] [PubMed] [Google Scholar]
- 9.Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, et al. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2006 Feb;130(2):341–8. doi: 10.1053/j.gastro.2005.12.008. quiz 592. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Tabb MM, Nelson EL, Grun F, Verma S, Sadatrafiei A, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006 Aug;116(8):2280–9. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, et al. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007 Jun;35(6):995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004 Jan;113(1):137–43. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, et al. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Ther. 2006 Mar;316(3):1369–77. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- 14.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, et al. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang TK, Waxman DJ. Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR) Drug Metab Rev. 2006;38(1–2):51–73. doi: 10.1080/03602530600569828. [DOI] [PubMed] [Google Scholar]
- 16.Chang TK. Activation of pregnane X receptor (PXR) and constitutive androstane receptor (CAR) by herbal medicines. AAPS J. 2009 Sep;11(3):590–601. doi: 10.1208/s12248-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak BS, Kanhai J, Milne DM, Swanston WH, Mayers S, Eversley M, et al. Investigation of the wound healing activity of Carapa guianensis L. (Meliaceae) bark extract in rats using excision, incision, and dead space wound models. J Med Food. 2010 Oct;13(5):1141–6. doi: 10.1089/jmf.2009.0214. [DOI] [PubMed] [Google Scholar]
- 18.Miranda RN, Dolabela MF, da Silva MN, Povoa MM, Maia JG. Antiplasmodial activity of the andiroba (Carapa guianensis Aubl., Meliaceae) oil and its limonoid-rich fraction. J Ethnopharmacol. 2012 Aug 1;142(3):679–83. doi: 10.1016/j.jep.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Souto N, Lynch RJ, Measures G, Hann JT. Use of high-performance liquid chromatographic peak deconvolution and peak labelling to identify antiparasitic components in plant extracts. J Chromatogr. 1992 Feb 28;593(1–2):209–15. doi: 10.1016/0021-9673(92)80288-6. [DOI] [PubMed] [Google Scholar]
- 20.Tariq M, Mossa JS, Al-Yahya MA, Parmar NS, Ageel AM. Evaluation of Artemisia inculta for anti-inflammatory activity in rats. Am J Chin Med. 1987;15(3–4):127–32. doi: 10.1142/S0192415X87000163. [DOI] [PubMed] [Google Scholar]
- 21.al-Harbi MM, Qureshi S, Ahmed MM, Raza M, Miana GA, Shah AH. Studies on the antiinflammatory, antipyretic and analgesic activities of santonin. Jpn J Pharmacol. 1994 Mar;64(3):135–9. doi: 10.1254/jjp.64.135. [DOI] [PubMed] [Google Scholar]
- 22.Dang GK, Parekar RR, Kamat SK, Scindia AM, Rege NN. Antiinflammatory activity of Phyllanthus emblica, Plumbago zeylanica and Cyperus rotundus in acute models of inflammation. Phytother Res. 2011 Jun;25(6):904–8. doi: 10.1002/ptr.3345. [DOI] [PubMed] [Google Scholar]
- 23.Daswani PG, Brijesh S, Tetali P, Birdi TJ. Studies on the activity of Cyperus rotundus Linn. tubers against infectious diarrhea. Indian J Pharmacol. 2011 May;43(3):340–4. doi: 10.4103/0253-7613.81502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie W, Barwick JL, Simon CM, Pierce AM, Safe S, Blumberg B, et al. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000 Dec 1;14(23):3014–23. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honkakoski P, Moore R, Gynther J, Negishi M. Characterization of phenobarbital-inducible mouse Cyp2b10 gene transcription in primary hepatocytes. J Biol Chem. 1996 Apr 19;271(16):9746–53. doi: 10.1074/jbc.271.16.9746. [DOI] [PubMed] [Google Scholar]
- 26.Tirona RG, Leake BF, Podust LM, Kim RB. Identification of amino acids in rat pregnane X receptor that determine species-specific activation. Mol Pharmacol. 2004 Jan;65(1):36–44. doi: 10.1124/mol.65.1.36. [DOI] [PubMed] [Google Scholar]
- 27.Strom SC, Pisarov LA, Dorko K, Thompson MT, Schuetz JD, Schuetz EG. Use of human hepatocytes to study P450 gene induction. Methods Enzymol. 1996;272:388–401. doi: 10.1016/s0076-6879(96)72044-x. [DOI] [PubMed] [Google Scholar]
- 28.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000 Jul 27;406(6794):435–9. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, et al. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006 May 26;281(21):15013–20. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crespi CL, Penman BW. Use of cDNA-expressed human cytochrome P450 enzymes to study potential drug-drug interactions. Adv Pharmacol. 1997;43:171–88. doi: 10.1016/s1054-3589(08)60205-7. [DOI] [PubMed] [Google Scholar]
- 31.Waxman DJ, Ko A, Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983 Oct 10;258(19):11937–47. [PubMed] [Google Scholar]
- 32.Auerbach SS, Ramsden R, Stoner MA, Verlinde C, Hassett C, Omiecinski CJ. Alternatively spliced isoforms of the human constitutive androstane receptor. Nucleic Acids Res. 2003 Jun 15;31(12):3194–207. doi: 10.1093/nar/gkg419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mencarelli A, Migliorati M, Barbanti M, Cipriani S, Palladino G, Distrutti E, et al. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol. 2010 Dec 1;80(11):1700–7. doi: 10.1016/j.bcp.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Ahlin G, Hilgendorf C, Karlsson J, Szigyarto CA, Uhlen M, Artursson P. Endogenous gene and protein expression of drug-transporting proteins in cell lines routinely used in drug discovery programs. Drug Metab Dispos. 2009 Dec;37(12):2275–83. doi: 10.1124/dmd.109.028654. [DOI] [PubMed] [Google Scholar]
- 35.Fahmi OA, Kish M, Boldt S, Obach RS. Cytochrome P450 3A4 mRNA is a more reliable marker than CYP3A4 activity for detecting pregnane X receptor-activated induction of drug-metabolizing enzymes. Drug Metab Dispos. 2010 Sep;38(9):1605–11. doi: 10.1124/dmd.110.033126. [DOI] [PubMed] [Google Scholar]
- 36.Lau AJ, Yang G, Rajaraman G, Baucom CC, Chang TK. Differential effect of meclizine on the activity of human pregnane X receptor and constitutive androstane receptor. J Pharmacol Exp Ther. 2011 Mar;336(3):816–26. doi: 10.1124/jpet.110.175927. [DOI] [PubMed] [Google Scholar]
- 37.Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, et al. Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther. 2010 Oct;335(1):32–41. doi: 10.1124/jpet.110.170225. [DOI] [PMC free article] [PubMed] [Google Scholar]