Abstract
While mobility strategies are considered important in understanding selection pressures on individuals, testing hypotheses of such strategies requires high resolution datasets, particularly at intersections between morphology, ecology and energetics. Here we present data on interactions between morphology and energetics in regards to the cost of walking for reproductive women and place these data into a specific ecological context of time and heat load. Frontal loads (up to 16% of body mass), as during pregnancy and child-carrying, significantly slow the optimal and preferred walking speed of women, significantly increase cost at the optimal speed, and make it significantly more costly for women to walk with other people. We further show for the first time significant changes in the curvature in the Cost of Transport curve for human walking, as driven by frontal loads. The impact of these frontal loads on females, and the populations to which they belong, would have been magnified by time constraints due to seasonal changes in day length at high latitudes and thermoregulatory limitations at low latitudes. However, wider pelves increase both stride length and speed flexibility, providing a morphological offset for load-related costs. Longer lower limbs also increase stride length. Observed differences between preferred and energetically optimal speeds with frontal loading suggest that speed choices of women carrying reproductive loads might be particularly sensitive to changes in heat load. Our findings show that female reproductive costs, particularly those related to locomotion, would have meaningfully shaped the mobility strategies of the hominin lineage, as well as modern foraging populations.
Keywords: Cost of transport, Speed, Sexual dimorphism, Walking, Pelvis, Energetics
Introduction
When studying selection pressures on human morphology, mobility strategies are often recognized as a key aspect of fitness (Leonard and Robertson, 1997; Binford, 2001; Kramer, 2004; Wallace and Shea, 2006; Daujeard and Moncel, 2010). In essence, mobility strategies illuminate niche adaptation – the suite of physiological processes (metabolism, thermoregulation, water balance), morphologies (body size and proportions), and behaviors (finding/handling food, water, raw materials) that enable individuals to sustain an appropriate body composition while gaining mates and successfully raising their young. Mobility can be defined in a number of ways but is commonly estimated as the distance traversed by a population or individual during some fairly long (day, season, year) time period of interest (Kelly, 1983; Eder, 1984). These overall movement patterns are at least partially determined by travel speeds over a combination of tasks and terrains typically covered by individuals.
The travel duration and speed of progression for individual locomotor tasks, and thus the mobility strategies emerging from them, are primarily constrained by the rate of energy use (Steudel, 1994; Wall-Scheffler et al., 2007; Kramer and Sylvester, 2009), the rate of heat dissipation (Blurton Jones and Sibly, 1978; Stroud,1993; Ulijaszek, 2001; Carey and Crompton, 2005) and the time available for travel (season-limited day length or temperature-limited activity periods) (Torrence, 1983; Foley, 1993; Ulijaszek, 2001; Hill, 2005). All three of these interdependent factors are tightly linked to travel speed. We expect that tasks that must be accomplished daily, such as walking to find food and water, should be done in a manner that minimizes costs, allowing resources to be used to maximize reproduction (Foley and Elton, 1998; Ellison, 2003; Gibson and Mace, 2006; Kramer and Sylvester, 2009). For human walking, the metabolic cost per distance travelled (Cost of Transport, CoT) describes a ‘U’ shaped curve as a function of speed, with a minimum value around intermediate walking speeds (Ralston, 1958; Bastien et al., 2005). Numerous studies have found that unloaded people who are walking alone prefer walking speeds at or near the speed that costs them the fewest calories per distance (near their gross CoT minimum) (Ralston, 1958; Browning and Kram, 2005; Browning et al., 2006; O’Connor and Donelan, 2012; Peyrot et al., 2012). While it remains unresolved what biomechanical or physiological criteria allow people to detect their ‘optimal’ speed for a given set of conditions, muscular activation levels (Ackermann and Van den Bogert, 2010; Miller et al., 2012), heat load (Ulijaszek, 2001), and fatigue indicators (Maughan et al., 2007) are likely factors. Heat strain, for example, has systematically been shown to decrease exercise performance and speed as the result of increased sensations of fatigue, likely caused by changes in brain neurotransmission and the integrity of the blood–brain barrier (Maughan et al., 2007). Factors such as heat load are strongly correlated with the rate of activity (energy used/time) as compared to the rate of heat dissipation, and not necessarily the duration of the activity or the distance travelled. Under more challenging conditions, such as carrying loads or travelling in hot environments, an individual might thus ‘choose’ a speed that is slower than the optimal speed (the speed at which cost per distance is minimized) because the physiological mechanisms allowing the body to detect the speed at which minimum cost typically occurs (e.g., sensory receptors for muscular force, temperature, pH) are triggered at an earlier point (the slower speed). Thus, the cost per distance probably always remains an important influence on preferred speed, but this criterion might be compromised when the rate of activity is limited for homeostatic reasons. If a mobility strategy does not minimize cost for a given distance, then we might expect that an upper limit on cost per time is modulating speed choices in order to maintain homeostasis. Beyond balancing these considerations, advantages related to reproductive success, predator avoidance, or food or water payoff may at times be other important determinants of the speed of travel.
The fertility of females in natural birth populations is sensitively tuned to both energetic expenditure and associated heat load. Energy-sparing mechanisms during pregnancy have been well-documented in women whose nutrition is limited and/or whose work load is substantial (Prentice et al., 1989; Heini et al., 1991; Poppitt et al., 1993; Ellison, 2008). Greater work intensity and energetic expenditure have been linked to suppressed ovarian function (Jasienska and Ellison, 2004; Ellison, 2008) and early pregnancy loss (Vitzthum et al., 2009) – all contributing to longer interbirth intervals. Though reproduction, and baby carrying in particular, dramatically increases the cost of mobility (Kramer, 1998; Wall-Scheffler et al., 2007 ; Watson et al., 2008), due to increased mass and different postural costs (Gruss et al., 2009), this is an obviously necessary cost to increase fitness. At least since the mid-Pleistocene, and probably for the entire hominin lineage, keeping babies on the mother’s body was the likely strategy, as it is with the other extant apes (Ross, 2001; Rosenberg et al., 2004). The high costs of carrying position hominin females squarely at the reproductive success-locomotor strategy nexus; increases in energy expenditure for females come with a high premium in terms of reproductive success. The options for pregnant or lactating females for dealing with the higher costs of walking include: slowing down but maintaining daily movement distances, limiting daily movements while maintaining walking speed, or some compromise between these two. Clearly, effective mobility strategies would also be constrained by the economic and ecological circumstances of a population (Foley, 1993). For instance, during the dry season, Dobe ! Kung females must walk long distances (10 km), carrying both children and food burdens, between mongongo nut groves and camp (near permanent water sources) (Blurton Jones et al., 1989). The Pumé of Venezuela balance trips to and from mango patches based on the distance to the patch and the number of mangoes being carried (Hilton and Greaves, 2008).
With the movement into northern latitudes at around 1.77 million years ago (Ma) (Gabunia et al., 2000), populations also found themselves encountering new significant time pressures; as daylight decreases during the winter months, the amount of time available to gain access to resources is reduced (Hill et al., 2003). Under these circumstances, the option of slowing down simultaneously limits daily movement distances, putting reproductive females in the situation of dramatically limiting access to resources, or paying a substantial energetic burden of fast walking to access the same amount of resources throughout the annual cycle. A reduction of mobility and movement distances could imply a number of features in the archaeological record of mid-Pleistocene northern latitude populations (Macdonald et al., 2009), most particularly, sites used in the winter months should be closer together in a given geographic area than sites used in the summer months or on a more annual basis.
In order to carefully assess the costs and benefits of alternative mobility strategies for female hominins, we need to understand how speed, reproductively relevant loads, and energetic costs are interrelated. To these ends, we have ascertained preferred speeds, and developed CoT versus speed curves for females walking unloaded and with frontal loads mimicking second trimester pregnancy (8% of body mass) and full-term pregnancy or post-pregnancy slinging (16% of body mass). We use these relationships to explore conditions where we would expect, as part of maximizing reproductive success, changes in the mobility strategies of pregnant women and groups containing pregnant or lactating females.
Methods
We recruited 20 non-smoking, physically active women (age range 19–64 years, mean 36 years). All participants signed written informed consent approved by the St. Catherine University IRB Committee. Full body anthropometrics were collected (Table 1), including mass, stature, lower limb length (greater trochanter to lateral malleolus), bitrochanteric breadth, biiliac breadth, and biacromial breadth. Using the segment endpoints and conversion equations described in Porter (1996), crural index was determined from external measures and adjusted to be comparable to bony landmarks.
Table 1.
Participant anthropometrics (N = 20).
| Anthropometric measures | Mean | SD |
|---|---|---|
| Body mass (kg) | 63.9 | 10.1 |
| Stature (cm) | 165.7 | 6.5 |
| Lower limb length (cm) | 81.3 | 4.5 |
| Crural index | 0.82 | 0.04 |
| Bitrochanteric breadth (cm) | 30.1 | 2.7 |
| Biiliac breadth (cm) | 24.9 | 2.8 |
| Waist circumference (cm) | 85.8 | 10.1 |
| Biacromial breadth (cm) | 37.9 | 2.0 |
The protocol consisted of participants walking on a treadmill for 5 min periods at each of 12 different, randomly ordered, speed and frontal-load combinations while we measured steady-state metabolic rate (SensorMedics Vmax 29C). The 12 experimental conditions constitute a three by four factorial design: three loading conditions (0, 8, and 16% of body mass carried at the belly) and four walking speeds (one slow, two medium, and one fast). All 12 speed-load combinations were performed by each participant on three different days. Trials were videotaped to determine stride frequency and stride length (see Kinematic Data section for details).
Loading and speed conditions
Loads were applied in layers to the participant’s abdominal area (belly) by means of small packages of lead shot placed in the pouches of two overlapping carpenter aprons, one tied at the level of the base of the sternum and one tied around the waist. The aprons were then supported, to avoid excessive movement during walking, by nestling the loads in a commercially available ‘prenatal cradle’ that was wrapped with a large elastic band and ace bandages (Best Cradle, size medium, manufactured by It’s You Babe, LLC, Michigan, USA). For the 0% loading condition, only the supporting cradle and wraps were worn.
At the point of study enrollment and consent before the first test session, we established each participant’s four self-selected walking speeds, which were then maintained throughout the experiment (i.e., same speeds used during all three days of testing). We used participant-selected speed options to accommodate potential existing differences between individuals in preferred walking speed. During speed selection, participants were asked to walk on the treadmill with the nose plug worn during metabolic data collection. To determine the slow speed, participants were fitted with the 8% load and asked to select (from 0.6, 0.8, or 1.0 m/s) the slowest speed at which they felt they were still ‘walking’ (fluidly, without undue hesitation). Still wearing the 8% load, participants were asked to select a medium speed (from 1.0, 1.2, 1.4 or 1.6 m/s) at which they could comfortably walk for an hour or more, and then a fast speed (from 1.6, 1.8, or 2.0 m/s) that was the fastest walking speed they could maintain aerobically for a minimum of 5 min without ‘getting short of breath’. While the speed chosen as the ‘fastest’ speed was likely not the fastest speed each subject could have maintained in an unloaded state, it was always substantially faster than the medium speeds chosen. Lastly, participants were wrapped up without a load (0%) and asked to pick a second medium speed (1.0, 1.2, 1.4 or 1.6 m/s) at which they could comfortably walk for an hour or more. If participants selected the same ‘comfortable’ speed at 0% load as for the 8% condition, they were asked to select a speed that was the second most comfortable for them as their second medium speed. The twelve combinations of these four speeds and three loading conditions were then randomly sequenced for each of the three testing sessions for each participant.
On the third and final day of testing, when participants were the most familiar with the loading conditions, from amongst the slow and moderate speed options (0.6, 0.8, 1.0, 1.2, 1.4 or 1.6 m/s), each participant selected a preferred walking speed (speed at which they felt they could walk ‘with the least effort’ for hours at a time) at each of the three loading conditions.
Metabolic data
Experiments were conducted in an air-conditioned lab where ambient temperatures ranged from 22 to 24 °C and relative humidity from 50 to 65%. Each trial produced 5 min of breath by breath values for the rate of oxygen consumption and carbon dioxide production. These rates were used to calculate metabolic power (Watts) using the Weir (1949) equation. Steady-state metabolic power (Cost of Locomotion, CoL) was calculated as the average of the last 3 min of each trial; all three testing sessions of a participant were averaged to determine the CoL for each of the twelve speed-load combinations. Cost of Transport (CoT) for each condition was computed by dividing CoL by walking speed. For each participant’s three loading conditions, we generated a CoT equation by fitting a second-order polynomial to the CoT versus walking speed curve. From these equations, for each loading level we determined a measure of the acuteness of the CoT curve – the x2 coefficient for the CoT equation (x2CoT), the minimum cost of transport (MinCoT), and the speed at which the MinCoT occurred (SPMinCoT or energetically optimal speed). The percentage increase in loaded CoT over unloaded (0%) CoT was calculated using the equation 100*(CoT Loaded − CoT Unloaded)/CoT Unloaded.
With respect to metabolism, resting (thermoregulation, ion pumping) and standing (resting plus postural costs of standing still) represent different physiological states than the dynamic state of even steady-speed walking (e.g., costs related to maintaining lateral stability during walking are quite different than the costs of balancing while standing). There is no basis to argue that either of these one point measures represents a consistent portion of the cost during walking that could be meaningfully subtracted, or that what they represent is fixed as a function of speed or load. On a practical level, even authors who present net costs disagree about how to calculate net values: i.e., by subtracting resting metabolic rate (Weyand et al., 2009), standing metabolic rate (Donelan et al., 2002), or extrapolated zero-speed metabolic rate (Malatesta et al., 2003). Furthermore, it is the total metabolic rate during a given time period that must be fueled calorically and that generates heat; two potential locomotor constraints with which we are concerned. Lastly, numerous studies have found that people and other animals prefer walking speeds at or near the speed associated with their gross CoT minimum, not their significantly slower net CoT minimum (Wickler et al., 2001; Browning and Kram, 2005; Browning et al., 2006; Peyrot et al., 2012). Thus, we believe gross (absolute) CoL and gross (absolute) CoT are the appropriate ways to express the costs we are modeling in this study. However, we understand that a number of other workers in this area have published net metabolic costs (often in addition to gross metabolic costs) and that a reader may wish to make comparisons. In fact, we did measure standing metabolic rate each day for our subjects. The mean value was 79.3 watts, SD = 13.7.
Kinematic data
From rear-view video taken of the feet of our subjects during each trial, we used a motion analysis program (Kinematic Analysis) to determine the time of heel strike for both left and right feet. Videotape was analyzed at 60 fields/s. Stride frequency, the number of strides (e.g., left foot heel strike to left foot heel strike) per minute, was calculated as the inverse of the average of five consecutive intervals between heel strikes of the same foot (left and right feet were averaged). Stride length, the distance between successive contacts of the same foot, was calculated by dividing calibrated treadmill speed by stride frequency.
Statistical analysis
Two-tailed, paired t-tests were used to test for differences between loading conditions in MinCoT, SPMinCoT, and x2CoT. Linear regression models were developed to determine which anthropometric measures explained significant fractions of the variation in MinCoT, x2CoT, stride length, and stride frequency. Because there was a high degree of collinearity among the independent anthropometric variables, to create the most robust, appropriately parameterized model, we entered all variables shown to be biomechanically relevant in previous work into the model (subject, load, speed, stride frequency, stride length, body mass, stature, lower limb length, biiliac breadth, bitrochanteric breadth) and only retained variables whose entry or removal from the model did not unduly influence the regression coefficients of other model factors. All statistics were done using PASW, SPSS 17.0.
Results
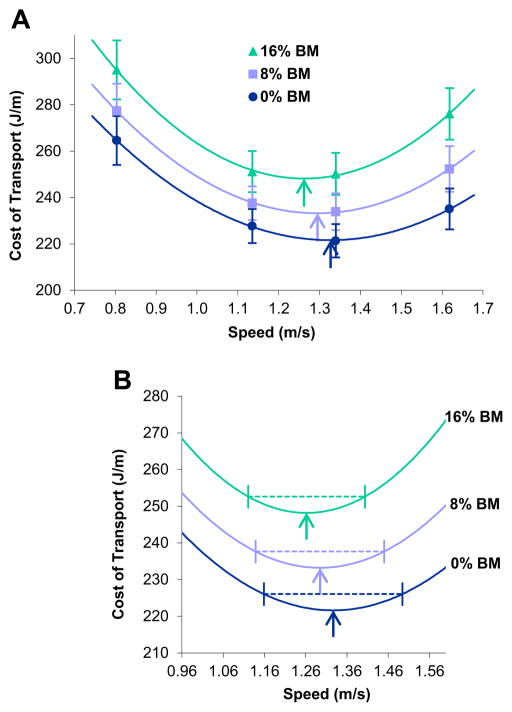
The (gross) Cost of Transport (CoT) curves generated in our experiment (Fig. 1A) document three systematic differences as a function of the size of frontal loads (0, 8, and 16% of body mass): an increase in the minimum CoT (MinCoT), a decrease in the speed at which the MinCoT occurs (SPMinCoT, or the ‘optimal’ speed), and an increase in the degree of curvature of the CoT curve (x2CoT) (Table 2). Compared with the unloaded (0%) condition, the MinCoT for females was 5% higher at the 8% loading condition (p < 0.001) and 12% higher with 16% loads (p < 0.001) (Fig. 1A). The SPMinCoT showed the reverse pattern (arrows in Fig. 1A), dropping by 3% at the 8% loading level (mean = 1.30 ± 0.09 m/s, p = 0.025) and by 5% at the 16% loading condition (mean = 1.27 ± 0.08 m/s, p = 0.004), compared with unloaded walking (mean = 1.34 ± 0.14 m/s). Our participants demonstrated an even steeper drop in preferred speed as a function of frontal load amount (Fig. 2), choosing speeds at the 8% and 16% loaded levels that were 6% (mean = 1.14 ± 0.22 m/s, p = 0.012) and 17% (mean = 1.01 ± 0.18 m/s, p < 0.001) slower, respectively, than the unloaded preferred speed (mean = 1.21 ± 0.19 m/s). The acuteness of the CoT curve (x2CoT) increased directly with loading level (Fig. 1A), becoming 20% (p = 0.05) and 47% (p < 0.001) steeper than for unloaded walking at the 8% and 16% loading conditions, respectively. The significant load-related increase in x2CoT resulted in a progressively smaller and left-shifted speed range at which the CoT was reasonably flat (Fig. 1B, shown for a CoT interval within 2% of the unloaded MinCoT).
Figure 1.
Average cost of transport (CoT) as a function of walking speed systematically shifted as frontal loads (equal to 0, 8, and 16% of body mass) increased. The minimum CoT (MinCoT) for each loading level is noted with arrows (A, B); a broken line demarks the range of speeds within 2% of the unloaded MinCoT for each loading level (B). Results are expressed as mean ± SEM; n = 20 women per speed-load combination. Second-order polynomial regressions lines were fit to averages for each loading condition to generate the CoT curves (R2 = 1.0 for all lines); the speed of the MinCoT (SPMinCoT) was determined from these regression lines, as was the degree of curvature (x2CoT). MinCoT increased, SPMinCoT decreased, and x2CoT increased significantly with load level (p = 0.05 or less for all comparisons; two-tailed, paired t-tests).
Table 2.
Mean and standard deviation of the cost of transport, speed, and kinematic variables (N = 20) at each of the three loading conditions.
| Locomotor measures | Unloaded
|
8%
|
16%
|
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| MinCoT (Jm−1) | 218.4 | 31.9 | 230.3 | 32.9 | 244.7 | 39.8 |
| x2CoT (Js2 m−3) | 148.3 | 55.8 | 178.6 | 61.7 | 218.6 | 73.5 |
| SpMinCoT (ms−1) | 1.34 | 0.14 | 1.30 | 0.09 | 1.27 | 0.08 |
| Preferred speed (ms−1) | 1.21 | 0.19 | 1.14 | 0.22 | 1.01 | 0.18 |
| Stride length (m) | 1.32 | 0.08 | 1.32 | 0.08 | 1.30 | 0.07 |
| Stride frequency (str min−1) | 54.7 | 3.5 | 55.0 | 3.4 | 55.6 | 3.3 |
Figure 2.
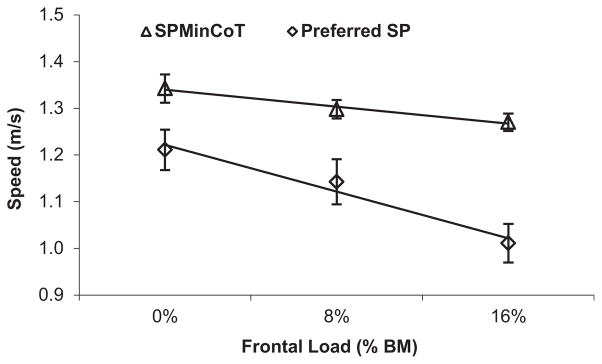
Average speed of the MinCoT (SPMinCoT) and preferred walking speed decreased as frontal loads (equal to 0, 8, and 16% of body mass) increased. Results are expressed as mean ± SEM; n = 20 women per loading condition. Both SPMinCoT and preferred speed decreased significantly with load level (p = 0.05 or less for all comparisons; two-tailed, paired t-tests). The drop in preferred speed (17%) approximated the increase in load as a percent of body mass (16%).
The pattern of frontal load-related changes observed in the CoT curves has consequences for time and speed tradeoffs during travel. For a fixed travel distance (such as 10 km from camp to a resource patch), as travel time is progressively limited (e.g., fewer usable daylight hours), travel speed must increase nonlinearly (Fig. 3). Likewise, constraints on travel speed (e.g., needing to keep pace with slower travelers), increase the time involved in the task. The effect of these trade-offs on the CoT depends upon an interaction between the frontal load and how far the resultant travel speed is from the SPMinCoT; the increased CoT curvature at higher loading levels yields a higher cost penalty for a given change in travel speed or time (Fig. 3A–C progression).
Figure 3.
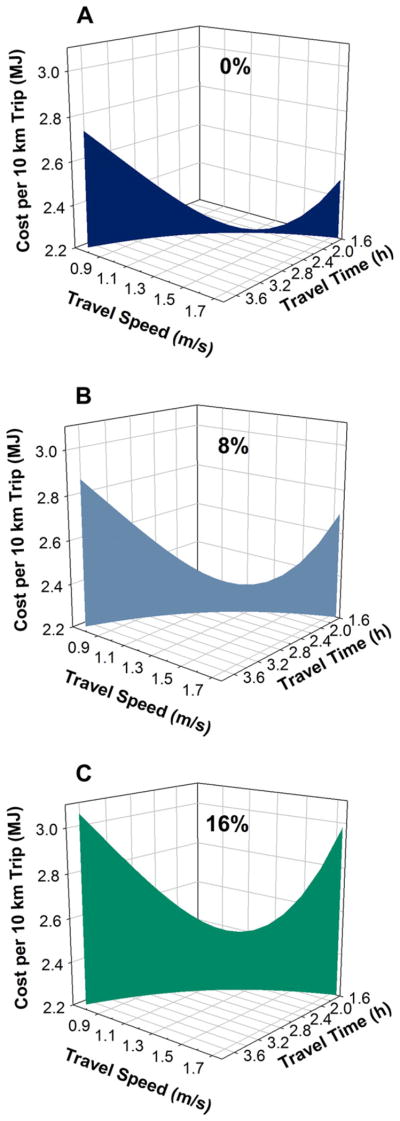
Cost for a 10 km trip as a function of travel speed, travel time and frontal load level: 0% (A), 8% (B), and 16% (C) of body mass. Cost curves were generated from aggregate (n = 20) CoT equations for each loading level and cover the range of reasonable walking speeds. Average body mass for participants was 63.9 kg.
In order to distinguish which aspects of morphology impact CoT curve characteristics and gait kinematics (stride length and stride frequency), we developed linear regression models that included participant and design factors (speed and/or load level) along with biomechanically-relevant anthropometric measures (listed above) that explained significant additional parts of the variation in these dependent variables (Tables 3 and 4). No stable model existed for morphological variables and SpMinCoT across the loaded conditions. In a regression model which explained 70.7% of the variation in MinCoT (Table 3), both load level (9.1% of model R2) and body mass (51.9% of model R2) had a highly significant positive effect on MinCoT. Neither lower limb length, nor stature, nor either pelvic variable was statistically significant in the model, nor any combination of the above. The most robust regression model for x2CoT (Table 3) explained 36.9% of total variation; load level (17.3% of model R2), body mass (11.9% of model R2), and lower limb length (1.3% of model R2) positively influenced x2CoT (increased curvature), while wider biiliac breadth (5.2% of model R2) decreased the acuteness of the CoT curve. Gait variables, stride length (SL) and stride frequency (SF), both changed systematically with loading condition (Table 2). In regression models which explained 95.0% and 91.4% of the variation in stride length and stride frequency, respectively (Table 4), both gait variables were highly and positively correlated with walking speed (SL: 90.2%, SF: 83.4% of model R2), while larger frontal loads (with speed accounted for) decreased stride length and increased stride frequency a small but statistically significant amount (SL: 0.2%, SF: 0.3% of model R2). Conversely, both longer lower limbs (SL: 4.3%, SF: 7.2% of model R2) and wider bitrochanteric breadth (SL: 0.3%, SF: 0.5% of model R2) significantly increased stride length and reduced stride frequency.
Table 3.
Effects of design and anthropometric measures on cost of transport (CoT) variables.
| Dependent variable | Expt design variable | Anthropometric variable | Coefficient | Model R2 |
|---|---|---|---|---|
| Minimum | Load level | Positive** | 18.8% | |
| CoT (MinCoT) | Body mass | Positive** | 70.7% | |
| CoT x2 | Load level | Positive** | 18.5% | |
| Coefficient (x2CoT) | Body mass | Positive** | 30.4% | |
| Biiliac breadth | Negative** | 35.6% | ||
| Lower limb length | Positive* | 36.9% |
A ‘participant’ term was included in all regression models. The Coefficient column shows the sign of each regression coefficient and associated level of statistical significance (*, p <= 0.05; **, p <= 0.001). Model R2 column is % of variation explained cumulatively by the regression model as each variable is added.
Table 4.
Effects of design and anthropometric measures on kinematic variables.
| Dependent variable | Expt design variable | Anthropometric variable | Coefficient | Model R2 |
|---|---|---|---|---|
| Stride length | Speed | Positive** | 90.2% | |
| Load level | Negative* | 90.4% | ||
| Lower limb length | Positive** | 94.7% | ||
| Bitrochanteric breadth | Positive** | 95.0% | ||
| Stride frequency | Speed | Positive** | 83.4% | |
| Load level | Positive* | 83.7% | ||
| Lower limb length | Negative** | 90.9% | ||
| Bitrochanteric breadth | Negative** | 91.4% |
A ‘participant’ term was included in all regression models. The Coefficient column shows the sign of each regression coefficient and associated level of statistical significance (*, P <= 0.05; **, p <= 0.001). Model R2 column is % of variation explained cumulatively by the regression model as each variable is added.
Discussion
Our experiment reveals that females walking with reproductively relevant frontal loads experience systematic alterations in the relationship between metabolic cost and speed; changes reflected in their Cost of Transport (CoT) curves (Fig.1), walking speed options (Fig. 2), and travel time per task (Fig. 3). We believe the ramifications of female reproductive loads on the interdependent factors of time, speed, distance, and energy have shaped fundamental aspects of individual and group mobility in the evolution of the hominin lineage. Given that human newborns are (nearly universally) loaded to the front, either in the arms or within clothing, sling, or shawl (Whiting, 1994; Konner, 2005), between the time spent negotiating pregnancy loads and the time spent carrying young infants, women in most hunter-gatherer populations go through a significant fraction of their life walking with frontal loads.
Based on our aggregate results, compared with an unloaded counterpart, a female with a frontal load equal to 16% of her body mass has a 12% (somewhat less than proportional) increase in CoT if she walks at or near the speed at which her minimal CoT occurs (SPMinCoT) – on average 5% slower than when unloaded (Fig. 1A). Additionally, due to a significantly steeper CoT curve, the same reproductively loaded female has a smaller range of speed options where CoT is minimized (Fig. 1B). Interestingly, the load-related drop in preferred speed appears to approximately maintain the rate at which energy is used (Cost of Locomotion) across loading conditions (Fig. 4); a 16% increase in load resulted in a 17% drop in preferred speed (Fig. 2). Numerous back-loading studies have demonstrated decreases in self-selected walking speed in proportion to added mass, resulting in a fairly constant CoL and heat production independent of loads (Myles and Saunders, 1979; Haisman, 1988; Demura and Demura, 2010). Reducing preferred walking speed in response to loads may be explained by the fact that preferred speed appears to be selected partly based on minimizing lower limb muscle activation (Ackermann and Van den Bogert, 2010; Miller et al., 2012), as the extra muscle force required to carry loads is produced by the lower limb muscles (Ghori and Luckwill, 1985; Griffin et al., 2003; Demura and Demura, 2010). Here, preferred speed for unloaded walking was reasonably close to the SPMinCoT (within the fairly flat part of the CoT curve); as frontal loads increased, the preferred speed chosen by our participants was reduced significantly compared to SPMinCoT (Fig. 2). In a study on preferred speed and SPMinCoT in trotting horses, Wickler et al. (2001) found that back loads equal to 19% of the animal’s body mass reduced both preferred speed and SPMinCoT. As in the present study, although the preferred speed was close to the SPMinCoT in both the unloaded and loaded situations, there was a larger gap between the preferred and minimum CoT speeds for loaded trials.
Figure 4.
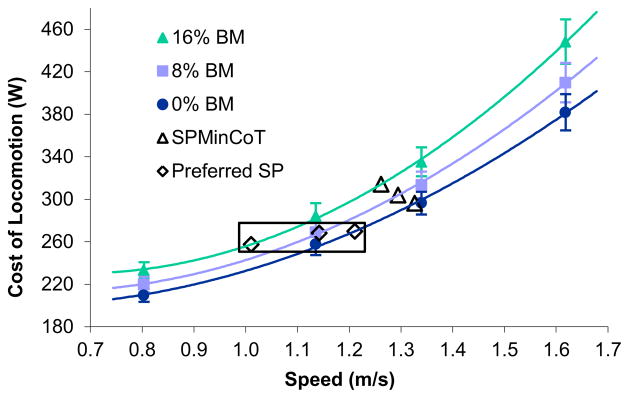
On average, subjects chose preferred speeds (open diamonds) at comparable Cost of Locomotion (CoL) values across loading levels (equal to 0, 8, and 16% of body mass). Load-related shifts in the speed of the MinCoT (SPMinCoT, open triangles) also modulated the CoL compared to the cost for maintaining a constant speed across loads. CoL values are expressed as mean ± SEM; n = 20 women per speed-load combination.
The discrepancy between preferred speeds and the SPMinCoT when carrying loads suggests one of three obvious possibilities. Possibility one is that minimizing the energy used in locomotion is not an important influence on speed selection. This seems unlikely due to the habitual nature of locomotion; even small differences in cost per time or distance would accumulate quickly. Numerous researchers across disciplines have suggested that not only are energy saving strategies during locomotion important, but that behavioral responses to real-time sensory input, such as speed adjustments, probably evolved in ways that tended to minimize the cost of transport (Bertram, 2005; Miller et al., 2012). The second possibility is that despite the three-day nature of the study, the participants were not sufficiently physiologically or biomechanically habituated to the loads to accurately assess the speed they would prefer over a long period of time or distance. Testing this hypothesis would require a study of the energetically optimal and preferred walking speeds of pregnant or habitually loaded (e.g., Maloiy et al., 1986) women. A third possibility is that the physiological and biomechanical cues that mediate speed selection during load-carrying are significantly influenced by the rate of energy consumption (Cost of Locomotion) and/or the rate of heat generation. In addition to heat load, variables such as proprioceptive input relating to muscle forces have been implicated in cost of transport minimizing behavior (Wickler et al., 2001; Ackermann and Van den Bogert, 2010; Miller et al., 2012). The evidence consistently shows that humans and animals ‘tune’ their preferred speed of locomotion to the energetic (Wickler et al., 2001; Browning et al., 2006; Peyrot et al., 2012; this study), muscular (Ackermann and Van den Bogert, 2010; Miller et al., 2012), and thermoregulatory (Blurton Jones and Sibly, 1978; Ulijaszek, 2001; Wall-Scheffler and Myers, 2012) constraints of a given locomotor task. The results of this study may provide evidence that the speed choices of women carrying reproductive loads are likely to be particularly sensitive to changes in heat load, presumably due to the negative consequences of hyperthermy for ova health. For pregnant females, accurately assessing the rate of energy usage, and limiting work intensity in order to maintain core body temperature out of the hyperthermic range, is particularly important. Hyperthermy has been shown to cause preterm delivery and preeclampsia (Molvarec et al., 2010), as well as contributing to birth defects. !Kung women have been shown to tune the size of loads (food and children) based on heat strain produced by the interaction of increased work load and hot, dry conditions (Blurton Jones and Sibly, 1978). Thus, we predict that female hominins with sizeable loads would either reduce their daily travel distance (walking less) or increase their work day (walking slowly), unless the latter option was not available due to day length limitations and/or conflicts with other tasks (Dunbar, 1992; Hilton and Greaves, 2008). Such behavior allows for a reduction of energy used per unit time as well as protecting fecundity and fertility. Further studies should assess the heat gains of both males and females as well as speed choices males make in response to loading.
There is good ethnographic evidence that female foragers adjust (tune) their speed to the loads they carry and the task to be accomplished. !Kung females traveling seven miles to collect mongongo nuts walked at 1.25 m/s on the way to the grove, while carrying their children and water, but slowed to 0.9 m/s on the way back, when they were carrying an additional 13.6 kg of nuts (Bentley, 1985). Xhosa women with 10 years or more of experience carrying heavy head-loads, chose 0.9 m/s when asked to walk at speeds appropriate for carrying loads (Lloyd et al., 2010). The average moving speed of Hadza females on foraging trips has been calculated at 0.97 m/s (Marlowe, 2006). In their almost daily job of moving camp to a new location in the forest, Ache women travel, with their family’s belongings loaded on their backs, either carrying or walking with their children, at an average speed of 0.8 m/s (Hurtado et al., 1985). These reported traveling speeds are at or below the preferred or ‘tuned’ speed women chose here for carrying loads of 16% of their body mass, but the situations likely involve loads proportionally greater than those used in our study (larger absolute loads and/or smaller women) or walking with slower-moving children.
Most female foragers travel as members of a group, often a group of primarily women, or women and children. Our results also have implications for how walking speed is chosen within a traveling cohort. The interaction between loading condition and speed, as represented by the systematic increase in x2CoT, means that the penalty for walking at a speed away from the SPMinCoT invokes a significantly more costly penalty for women carrying frontal loads. Whereas non-carrying females could feasibly walk either more slowly or more quickly with less of an increase in cost, frontal loads change these conditions, reducing speed flexibility. Both the energetic cost and preferred speed data presented here imply the speed of women walking together when at least one is pregnant or lactating is going to be slower than estimates of walking speed based on unloaded women walking alone (Wall-Scheffler, 2012a).
Once hominin populations moved into northern latitudes – into central Asia by 1.7 Ma and into Europe by 1.2 Ma (Gabunia et al., 2000; Carbonell et al., 2008) – the significantly shorter day times during the winter began to impose a time constraint on foraging activities. Both the sites of Dmanisi (1.7 Ma) and Atapuerca (1.2 Ma) occur at around 41–42° latitude, which gets approximately eight to nine hours of daylight during the winter. Given that hominins have historically been limited to daylight hours for gaining access to food and water (though not necessarily for processing these resources), all mobility activities must be accomplished in this shorter time frame during part of the year. In winter, groups which included frontally loaded females should have been walking shorter distances than at other times of the year to avoid the large increases in cost detailed here. This inevitably has consequences for the mobility and productivity of hominin groups living at higher latitudes. Taphonomic studies on Dmanisi and Atapuerca are still in their early stages and have yet to outline detailed settlement strategies of early European and Eurasian hominins (Bermúdez de Castro et al., 1999; Tappen et al., 2007) however, later European hominins have been well studied for mobility and site use. In particular, it has been suggested that among high latitude Neanderthal populations, site locations were used for short periods of time and included only a small foraging radius (Macdonald et al., 2009). Macdonald et al. (2009) further suggest that Neanderthals moved often, but because they moved short distances, did not actually move a cumulatively large distance over the course of the year. The question is whether these patterns of mobility can actually be interpreted as responses to changes in seasonality and daylight availability. The results of this study suggest that we should expect to see larger group ranges during the summer months in higher latitude locales, and distinctly smaller group ranges during winter months. Seasonal studies do exist for marine oxygen isotope stage (MIS) 3 sites at high latitudes. These studies suggest shorter ranges for winter periods, as evidenced by shorter distances between sites utilized during the winter periods (Pike-Tay et al., 1999; Wall, 2005).
In terms of selection acting upon locomotor morphology, mass has previously been shown to increase the MinCoT and the curvature around it during unloaded walking (Wall-Scheffler, 2012a) suggesting selection for increased size may relate to other selection pressures (e.g., increased fecundity or increased infant size) and also suggesting that populations that maintain some amount of sexual dimorphism of size may have individuals with different mobility strategies (Wall-Scheffler, 2012a). Because absolutely longer lower limbs increase stride length and reduce steps taken (this study, Grieve and Gear, 1966), and relatively (to body mass) reduce energetic costs of walking (Steudel-Numbers and Tilkens, 2004), the appearance of longer lower limbs in the hominin lineage should be expected to reduce the energetic cost of bipedal walking (Pontzer et al., 2010). Furthermore, larger individuals with longer lower limbs should be able to move more quickly for less energy than larger individuals with shorter lower limbs, which has implications for daily movement distances of hominin populations.
Recent reconstructions (Simpson et al., 2010) have emphasized that for much of hominin evolution a broad pelvis was a characteristic piece of morphology. Generally this has been shown to be the result of flaring ilia. Our findings show that biiliac breadth can mitigate some load-related costs by means of enhanced speed flexibility. A relatively wider pelvis reduces x2CoT (Table 3), offering a broader base to the CoT walking curve, and thus more flexibility in speeds around the SPMinCoT. This allows individuals to change their speed without a substantial increase in cost, in order to accommodate those with whom they are walking, and to accommodate their own changes in body shape during reproduction. Another mechanism by which a wider pelvis reduces cost is evidenced by the observed relationship between bitrochanteric breadth and stride length (Table 4). Our observation that a significant positive increment in stride length can be explained by bitrochanteric breadth is consistent with hypothesized mechanisms (Rak, 1991) and empirical evidence (Wall-Scheffler et al., 2007; Whitcome et al., 2012) of increased stride length (for a given lower limb length) due to the greater translation of a wider pelvis as it rotates. This consequence of wider bitrochanteric breadth counters the reduction in stride length associated with frontal loading (Table 4).
These data have two key implications for the evolution of the hominin lineage. Prior to Homo sapiens, both males and females of the hominin lineage had wide pelves (Pycraft, 1930; Rak and Arensburg, 1987; Arsuaga et al., 1999; Rosenberg et al., 2006; Simpson et al., 2008, 2010), suggesting the pelvic morphology of both sexes allow for flexibility in walking speed. Because people have universally been found to walk with others and to experience variable tasks (different walking cohorts, different loads, different environmental conditions, different resource patches), those individuals who can adjust their speed (without undue energetic penalty) to walk with other individuals and/or can walk different distances in the same amount of time (e.g., seasonal shifts in light and temperature) will be able to reduce the energetic burden of mobility and be able to use that energy instead for reproduction. Since evidence from australopithecines suggest differently sized individuals walking together (e.g., Leakey and Hay, 1979), such flexibility may have been a feature early in the hominin lineage. Evidence from studies on muscle activity during locomotion (Carrier et al., 2011) shows that people have the neuromuscular flexibility for moving at different speeds near their preferred speed range; the fact that different muscle groups operate optimally at somewhat different speeds suggests speed flexibility. Because the effect of biiliac breadth on speed flexibility (x2CoT) is significant even with load in the regression model, we might expect that even if males were not carrying frontal loads, if they were walking with pregnant or front-slinging females, it would be equally important for them to maintain reasonable speed flexibility to avoid higher costs. We expect that the substantially more narrow pelvis in H. sapiens, particularly in males, is indicative of changes in group mobility (Wall-Scheffler, 2012b), tool use (Kuhn and Stiner, 2006), or a relationship between pelvis width and lower limb length that is not yet well understood.
Secondly, for hominins moving into higher latitudes the implications of these findings are substantially different selection pressures (from lower latitudes) for reproductive females in the form of day length time constraints. Even in the absence of a seasonally-shortened day, low latitude women have been shown to have reduced foraging productivity whilst lactating (Marlowe, 2003), both because of reduced foraging and because of lower return rates. We can expect seasonal shifting of time constraints on female mobility to be magnified as latitude increases (Altman, 1984; Dunbar, 1992; Hill et al., 2003). Kuhn and Stiner (2006) suggest that high latitude women are often involved in work that is only minimally mobility-dependent (finding of water being the key mobility-necessary task). It is possible that the broader pelves documented for high latitude women, though generally attributed to thermo-regulatory constraints (Ruff, 1994), may have the additional benefit of allowing speed flexibility during travel.
Conclusion
Our findings show that female reproductive costs, particularly those related to locomotion, would have meaningfully shaped the mobility strategies employed by early Homo, as well as modern foraging populations. Persistent reproductive loads during pregnancy and child-carrying would have slowed females, tempered the speed of group travel, and increased the frequency of camp moves. The impact of reproductive loads on females, and the populations to which they belonged, would have been magnified by time constraints due to seasonal changes in day length at high latitudes and thermoregulatory limitations at low latitudes. Increased costs and decreased speeds with frontal loads is a non-negotiable corollary of reproduction, which according to archaeological evidence at high latitudes, appears to induce mobility shifts. Wider pelves increase both stride length and speed flexibility, providing some hominin populations with a morphological offset for load-related costs.
Acknowledgments
We acknowledge J. McCafferty, A. Hokanson, S. Meissner, and C. Hayes for their extensive assistance in data collection and are grateful to our participants for generously giving of their time. We thank K.L. Steudel for helpful discussions and M. Teaford and two anonymous reviewers for their thoughtful and apt suggestions that improved this paper. This research was funded by grants from the National Institutes of Health (#G11HD039786) and the Center of Excellence for Women, Science, and Technology at St. Catherine University (3M Faculty/Student Collaborative Grants # 800707 & 800718).
References
- Ackermann M, Van den Bogert AJ. Predictive simulation of gait in rehabilitation. Conf Proc IEEE Eng Med Biol Soc. 2010:5444–5447. doi: 10.1109/IEMBS.2010.5626512. [DOI] [PubMed] [Google Scholar]
- Altman J. Foragers subsistence: production in Arnhemland: the original affluent society re-examined. Mankind. 1984;14:179–190. [Google Scholar]
- Arsuaga JL, Lorenzo C, Carretero JM, Gracia A, Martínez I, García N, Bermúdez de Castro JM, Carbonell E. A complete human pelvis from the Middle Pleistocene of Spain. Nature. 1999;399:255–258. doi: 10.1038/20430. [DOI] [PubMed] [Google Scholar]
- Bastien GJ, Willems PA, Schepens B, Heglund NC. Effect of load and speed on the energetic cost of human walking. Eur J Appl Physiol. 2005;94:76–83. doi: 10.1007/s00421-004-1286-z. [DOI] [PubMed] [Google Scholar]
- Bentley GR. Hunter–gatherer energetics and fertility: a reassessment of the !Kung San. Hum Ecol. 1985;13:79–109. [Google Scholar]
- Bermúdez de Castro JM, Carbonell E, Cáceres I, Díez JC, Fernández-Jalvo Y, Mosquera M, Ollé A, Rodríguez J, Rodríguez XP, Rosas A, Rosell J, Sala R, Vergés JM, van der Made J. The TD6 (Aurora stratum) hominid site. Final remarks and new questions. J Hum Evol. 1999;37:695–700. doi: 10.1006/jhev.1999.0334. [DOI] [PubMed] [Google Scholar]
- Bertram JE. Constrained optimization in human walking: cost minimization and gait plasticity. J Exp Biol. 2005;208:979–991. doi: 10.1242/jeb.01498. [DOI] [PubMed] [Google Scholar]
- Binford LR. Constructing Frames of Reference: an Analytical Method for Archaeological Theory Building using Hunter–Gatherer and Environmental Data Sets. University of California Press; Berkeley: 2001. [Google Scholar]
- Blurton Jones NG, Sibly RM. Testing adaptiveness of culturally determined behaviour: do Bushman women maximize their reproductive success by spacing births widely and foraging seldom? In: Reynolds V, Blurton Jones NG, editors. Human Behaviour and Adaptation. Taylor and Francis; London: 1978. pp. 135–157. [Google Scholar]
- Blurton Jones N, Hawkes K, O’Connell JF. Modelling and measuring costs of children in two foraging societies. In: Standen V, Foley RA, editors. The Behavioural Ecology of Humans and Other Mammals. Blackwell Scientific Publications; Oxford: 1989. pp. 367–390. [Google Scholar]
- Browning RC, Kram R. Energetic cost and preferred speed of walking in obese versus normal weight women. Obes Res. 2005;13:891–899. doi: 10.1038/oby.2005.103. [DOI] [PubMed] [Google Scholar]
- Browning RC, Baker EA, Herron JA, Kram R. Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol. 2006;100:390–398. doi: 10.1152/japplphysiol.00767.2005. [DOI] [PubMed] [Google Scholar]
- Carbonell E, Bermúdez de Castro JM, Parés JM, Peréz-Gonzalez A, Cuenca-Bescos G, Ollé A, Mosquera M, Huguet R, van der Made J, Rosas A, Sala R, Vallverdú J, García N, Granger DE, Martinón-Torres M, Rodríguez XP, Stock GM, Vergès JM, Allué E, Burjachs F, Cáceres I, Canals A, Benito A, Díez C, Lozano M, Mateos A, Navazo M, Rodríguez J, Rosell J, Arsuaga JL. The first hominin of Europe. Nature. 2008;452:465–469. doi: 10.1038/nature06815. [DOI] [PubMed] [Google Scholar]
- Carey TS, Crompton RH. The metabolic costs of ‘bent hip, bent knee’ walking in humans. J Hum Evol. 2005;48:25–44. doi: 10.1016/j.jhevol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Carrier DR, Adners C, Schilling N. Musculoskeletal system of humans is not tuned to maximize the economy of locomotion. Proc Natl Acad Sci. 2011;108:18631–18636. doi: 10.1073/pnas.1105277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daujeard C, Moncel MH. On Neanderthal subsistence strategies and land use: a regional focus on the Rhone Valley area in southeastern France. J Anthropol Archaeol. 2010;29:368–391. [Google Scholar]
- Demura T, Demura S. Relationship among gait parameters while walking with varying loads. J Physiol Anthropol. 2010;29:29–34. doi: 10.2114/jpa2.29.29. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J Exp Biol. 2002;205:3717–3727. doi: 10.1242/jeb.205.23.3717. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. Time: a hidden constraint on the behavioural ecology of baboons. Behav Ecol Sociobiol. 1992;31:35–49. [Google Scholar]
- Eder JF. The impact of subsistence change on mobility and settlement pattern in a tropical forest foraging economy: some implications for archaeology. Am Anthropol. 1984;86:837–853. [Google Scholar]
- Ellison PT. Energetics and reproductive effort. Am J Hum Biol. 2003;15:342–351. doi: 10.1002/ajhb.10152. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Energetics, reproductive ecology and human evolution. Paleoanthropology. 2008:172–200. [Google Scholar]
- Foley R. The influence of seasonality on hominid evolution. In: Ulijaszek SJ, Strickland SS, editors. Seasonality and Human Ecology. Cambridge University Press; Cambridge: 1993. pp. 17–37. [Google Scholar]
- Foley RA, Elton S. Time and energy: the ecological context for the evolution of bipedalism. In: Strasser E, Fleagle J, Rosenberger A, McHenry H, editors. Primate Locomotion: Recent Advances. Plenum Press; New York: 1998. pp. 419–433. [Google Scholar]
- Gabunia L, Vekua A, Lordkipanidze D, Swisher CC, III, Ferring R, Nioradze M, Tvalchrelidze M, Antón SC, Bosinski G, Jöris O, de Lumley MA, Majsuradze G, Mouskhelishvili A. Earliest Pleistocene cranial remains from Dmanisi, Republic of Georgia: taxonomy, geological setting, and age. Science. 2000;288:1019–1025. doi: 10.1126/science.288.5468.1019. [DOI] [PubMed] [Google Scholar]
- Ghori GM, Luckwill RG. Responses of the lower limb to load carrying in walking man. Eur J Appl Physiol. 1985;54:145–150. doi: 10.1007/BF02335921. [DOI] [PubMed] [Google Scholar]
- Gibson MA, Mace R. An energy-saving development initiative increases birth rate and childhood malnutrition in rural Ethiopia. PLoS Med. 2006;3:476–484. doi: 10.1371/journal.pmed.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve DW, Gear RJ. The relationships between length of stride, step frequency, time of swing and speed of walking for children and adults. Ergonomics. 1966;9:679–399. doi: 10.1080/00140136608964399. [DOI] [PubMed] [Google Scholar]
- Griffin TM, Roberts TJ, Kram R. Metabolic cost of generating muscular force in human walking: insights from load-carrying and speed experiments. J Appl Physiol. 2003;95:172–183. doi: 10.1152/japplphysiol.00944.2002. [DOI] [PubMed] [Google Scholar]
- Gruss LT, Wall-Scheffler CM, Malik N. Infant carrying in humans: interactions between morphometric and gait parameters. Am J Phys Anthropol. 2009;S48:182–183. [Google Scholar]
- Haisman MF. Determinants of load carrying ability. Appl Ergon. 1988;19:111–121. doi: 10.1016/0003-6870(88)90004-x. [DOI] [PubMed] [Google Scholar]
- Heini A, Schutz Y, Diaz E, Prentice AM, Whitehead RG, Jequier E. Free-living energy expenditure measured by two independent techniques in pregnant and nonpregnant Gambian women. Am J Physiol. 1991;261:E9–E17. doi: 10.1152/ajpendo.1991.261.1.E9. [DOI] [PubMed] [Google Scholar]
- Hill R. Day length seasonality and the thermal environment. In: Brockman DK, van Schaik CP, editors. Seasonality in Primates: Studies of Living and Extinct Human and Non-human Primates. Cambridge University Press; Cambridge: 2005. pp. 197–213. [Google Scholar]
- Hill RA, Barrett L, Gaynor D, Weingrill T, Dixon P, Payne H, Henzi SP. Day length, latitude and behavioural (in)flexibility in baboons (Papio cynocephalus ursinus) Behav Ecol Sociobiol. 2003;53:278–286. [Google Scholar]
- Hilton CE, Greaves RD. Seasonality and sex differences in travel distance and resource transport in Venezuelan foragers. Curr Anthropol. 2008;49:144–153. [Google Scholar]
- Hurtado AM, Hawkes K, Hill K, Kaplan H. Female subsistence strategies among Ache hunter–gatherers of Eastern Paraguay. Hum Ecol. 1985;13:1–28. [Google Scholar]
- Jasienska G, Ellison PT. Energetic factors and seasonal changes in ovarian function in women from rural Poland. Am J Hum Biol. 2004;16:563–580. doi: 10.1002/ajhb.20063. [DOI] [PubMed] [Google Scholar]
- Kelly RL. Hunter–gatherer mobility strategies. J Anthropol Res. 1983;39:277–306. [Google Scholar]
- Konner M. Hunter–gatherer infancy and childhood: the !Kung and others. In: Hewlett BS, Lamb ME, editors. Hunter–Gatherer Childhoods. Aldine Transaction; New Brunswick: 2005. pp. 19–64. [Google Scholar]
- Kramer PA. The costs of human locomotion: maternal investment in child transport. Am J Phys Anthropol. 1998;107:71–85. doi: 10.1002/(SICI)1096-8644(199809)107:1<71::AID-AJPA6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kramer PA. The behavioral ecology of locomotion. In: Meldrum DJ, Hilton CE, editors. From Biped to Strider: the Emergence of Modern Human Walking, Running and Resource Transport. Plenum Publishers; New York: 2004. pp. 101–115. [Google Scholar]
- Kramer PA, Sylvester AD. Bipedal form and locomotor function: understanding the affects of size and shape on velocity and energetics. Paleoanthropology. 2009:238–251. [Google Scholar]
- Kuhn SL, Stiner MC. What’s a mother to do? The division of labor among Neanderthals and modern humans in Eurasia. Curr Anthropol. 2006;47:953–980. [Google Scholar]
- Leakey MD, Hay RL. Pliocene footprints in the Laetolil beds at Laetoli, northern Tanzania. Nature. 1979;278:317–323. [Google Scholar]
- Leonard WR, Robertson ML. Comparative primate energetics and hominid evolution. Am J Phys Anthropol. 1997;102:265–281. doi: 10.1002/(SICI)1096-8644(199702)102:2<265::AID-AJPA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lloyd R, Parr B, Davies S, Cooke C. Subjective perceptions of load carriage on the head and back in Xhosa women. Appl Ergon. 2010;41:522–529. doi: 10.1016/j.apergo.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Roebroeks W, Verpoorte A. An energetics perspective on the Neandertal record. In: Hublin J-J, Richards MP, editors. The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence. Springer; Dordrecht: 2009. pp. 211–220. [Google Scholar]
- Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani R, Prefaut C, Caillaud C. Energy cost of walking and gait instability in healthy 65 and 80 yr olds. J Appl Physiol. 2003;95:2248–2256. doi: 10.1152/japplphysiol.01106.2002. [DOI] [PubMed] [Google Scholar]
- Maloiy GM, Heglund NC, Prager LM, Cavagna GA, Taylor CR. Energetic cost of carrying loads: have African women discovered an economic way? Nature. 1986;319:668–669. doi: 10.1038/319668a0. [DOI] [PubMed] [Google Scholar]
- Marlowe FW. A critical period for provisioning by Hadza men: implications for pair bonding. Evol Hum Behav. 2003;24:217–229. [Google Scholar]
- Marlowe FW. Central place provisioning: the Hadza as an example. In: Hohmann G, Robbins M, Boesch C, editors. Feeding Ecology in Apes and Other Primates. Cambridge University Press; Cambridge: 2006. pp. 359–377. [Google Scholar]
- Maughan RJ, Shirreffs SM, Watson P. Exercise, heat, hydration and the brain. J Am Coll Nutr. 2007;26:6045–6125. doi: 10.1080/07315724.2007.10719666. [DOI] [PubMed] [Google Scholar]
- Miller RH, Umberger BR, Hamill J, Caldwell GE. Evaluation of the minimum energy hypothesis and other potential optimality criteria for human running. Proc R Soc Lond B Biol Sci. 2012;279:1498–1505. doi: 10.1098/rspb.2011.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvarec A, Tamasi L, Losonczy G, Madach K, Prohaska Z, Rlgo J. Circulating heat shock protein 70 (HSPA1A) in normal and pathological pregnancies. Cell Stress Chaperon. 2010;15:237–247. doi: 10.1007/s12192-009-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles WS, Saunders PL. The physiological cost of carrying light and heavy loads. Eur J Appl Physiol. 1979;42:125–131. doi: 10.1007/BF00421911. [DOI] [PubMed] [Google Scholar]
- O’Connor SM, Donelan JM. Fast visual prediction and slow optimization of preferred walking speed. J Neurophysiol. 2012;107:2549–2559. doi: 10.1152/jn.00866.2011. [DOI] [PubMed] [Google Scholar]
- Peyrot N, Thivel D, Isacco L, Morin JB, Belli A, Duche P. Why does walking economy improve after weight loss in obese adolescents? Med Sci Sports Exerc. 2012;44:659–665. doi: 10.1249/MSS.0b013e318236edd8. [DOI] [PubMed] [Google Scholar]
- Pike-Tay A, Valdes VC, de Quiros FB. Seasonal variations of the Middle-Upper Paleolithic transition at El Castillo, Cueva Morin and El Pendo (Canta-bria, Spain) J Hum Evol. 1999;36:283–317. doi: 10.1006/jhev.1998.0271. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Rolian C, Rightmire GP, Jashashvili T, Ponce de León MS, Lordkipanidze D, Zollikofer CPE. Locomotor anatomy and biomechanics of the Dmanisi hominins. J Hum Evol. 2010;58:492–504. doi: 10.1016/j.jhevol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Poppitt SD, Prentice AM, Jequier E, Schutz Y, Whitehead RG. Evidence of energy sparing in Gambian women during pregnancy: a longitudinal study using whole-body calorimetry. Am J Clin Nutr. 1993;57:353–364. doi: 10.1093/ajcn/57.3.353. [DOI] [PubMed] [Google Scholar]
- Porter AMW. PhD Dissertation. University of London; 1996. Physique and the Skeleton. [Google Scholar]
- Prentice AM, Goldberg GR, Davies HL, Murgatroyd PR, Scott W. Energy-sparing adaptations in human pregnancy assess by whole body calorimetry. Br J Nutr. 1989;62:5–22. doi: 10.1079/bjn19890004. [DOI] [PubMed] [Google Scholar]
- Pycraft WP. The pelvis of Rhodesian man. Man. 1930;30:117–121. [Google Scholar]
- Rak Y. Lucy’s pelvic anatomy: its role in bipedal gait. J Hum Evol. 1991;20:283–290. [Google Scholar]
- Rak Y, Arensburg B. Kebara 2 Neanderthal pelvis: first look at a complete inlet. Am J Phys Anthropol. 1987;73:227–231. doi: 10.1002/ajpa.1330730209. [DOI] [PubMed] [Google Scholar]
- Ralston HJ. Energy-speed relation and optimal speed during level walking. Eur J Appl Physiol. 1958;17:277–283. doi: 10.1007/BF00698754. [DOI] [PubMed] [Google Scholar]
- Rosenberg KR, Golinkoff RM, Zosh JM. Did australopithecines (or early Homo) sling? Behav Brain Sci. 2004;27:522. [Google Scholar]
- Rosenberg KR, Zuné L, Ruff CB. Body size, body proportions, and encephalization in a Middle Pleistocene archaic human from northern China. Proc Natl Acad Sci. 2006;103:3552–3556. doi: 10.1073/pnas.0508681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. Park or ride? Evolution of infant carrying in primates. Int J Primatol. 2001;22:749–771. [Google Scholar]
- Ruff CB. Morphological adaptation to climate in modern and fossil hominids. Yearb Phys Anthropol. 1994;37:65–107. [Google Scholar]
- Simpson SW, Quade J, Levin NE, Butler R, Dupont-Nivet G, Everett M, Semaw S. A female Homo erectus pelvis from Gona, Ethiopia. Science. 2008;322:1089–1092. doi: 10.1126/science.1163592. [DOI] [PubMed] [Google Scholar]
- Simpson SW, Spurlock LB, Lovejoy CO, Latimer B. A new reconstruction of the KNM-WT 15000 juvenile male pelvis. Am J Phys Anthropol. 2010;217(Suppl 50) [Google Scholar]
- Steudel KL. Locomotor energetics and hominid evolution. Evol Anthropol. 1994;3:42–48. [Google Scholar]
- Steudel-Numbers K, Tilkens M. The effect of lower limb length on the energetic cost of locomotion: implications for fossil hominins. J Hum Evol. 2004;47:95–109. doi: 10.1016/j.jhevol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Stroud MA. Environmental temperature and physiological function. In: Ulijaszek SJ, Strickland SS, editors. Seasonality and Human Ecology. Cambridge University Press; Cambridge: 1993. pp. 38–53. [Google Scholar]
- Tappen M, Lordkipanidze D, Bukhsianidze M, Ferring R, Vekua A. Are you in or out (of Africa)? Site formation at Dmanisi and actualistic studies in Africa. In: Pickering TR, Schick K, Toth N, editors. Breathing Life in Fossils: Taphonomic Studies in Honor of C.K. (Bob) Brain. Stone Age Institute Press; Gosport: 2007. pp. 119–135. [Google Scholar]
- Torrence R. Time budgeting and hunter–gatherer technology. In: Bailey G, editor. Hunter–Gatherer Economy in Prehistory. Cambridge University Press; Cambridge: 1983. pp. 11–22. [Google Scholar]
- Ulijaszek SJ. Potential seasonal ecological challenge of heat strain among Australian Aboriginal people practicing traditional subsistence methods: a computer simulation. Am J Phys Anthropol. 2001;116:236–245. doi: 10.1002/ajpa.1119. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ, Thornburg J, Spielvogel H. Seasonal modulation of reproductive effort during early pregnancy in humans. Am J Hum Biol. 2009;21:548–558. doi: 10.1002/ajhb.20936. [DOI] [PubMed] [Google Scholar]
- Wall CM. The seasonality of site deposition of Gibraltar Neanderthals: evidence from Gorham’s and Vanguard Caves. J Iberian Archaeol. 2005;7:9–22. [Google Scholar]
- Wall-Scheffler CM, Geiger K, Steudel-Numbers K. Infant carrying: the role of increased locomotory costs in early tool development. Am J Phys Anthropol. 2007;133:841–846. doi: 10.1002/ajpa.20603. [DOI] [PubMed] [Google Scholar]
- Wall-Scheffler CM. Size and shape: morphology’s impact on human speed and mobility. J Anthropol. 2012a;2012:1–9. [Google Scholar]
- Wall-Scheffler CM. Energetics, locomotion and female reproduction: implications for human evolution. A Rev Anthropol. 2012b;41:71–85. [Google Scholar]
- Wall-Scheffler CM, Myers MJ. Is female morphology selected for economy and male morphology selected for efficiency? Evidence from studies on humans. Am J Phys Anthropol. 2012;54:296. [Google Scholar]
- Wallace IJ, Shea JJ. Mobility patterns and core technologies in the Middle Paleolithic of the Levant. J Archaeol Sci. 2006;33:1293–1309. [Google Scholar]
- Watson JC, Payne RC, Chamberlain AT, Jones RK, Sellers WI. The energetic costs of load-carrying and the evolution of bipedalism. J Hum Evol. 2008;54:675–683. doi: 10.1016/j.jhevol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Weir JdV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand PG, Smith BR, Sandell RF. Assessing the metabolic cost of walking: the influence of baseline subtractions. IEEE Eng Med Biol Mag Conf Proc. 2009:6878–6881. doi: 10.1109/IEMBS.2009.5333126. [DOI] [PubMed] [Google Scholar]
- Whitcome KK, Lopez J, Miller EE, Burns JL. Revisiting the human obstetrical dilemma: effect of pelvic rotation stride length. Am J Phys Anthropol. 2012;54:302. [Google Scholar]
- Whiting J. Environmental constraints on infant care practices. In: Chasdi EH, editor. Culture and Human Development: The Selected Papers of John Whiting. Cambridge University Press; Cambridge: 1994. pp. 107–134. [Google Scholar]
- Wickler SJ, Hoyt DF, Cogger EA, Hall KM. Effect of load on preferred speed and cost of transport. J Appl Physiol. 2001;90:1548–1551. doi: 10.1152/jappl.2001.90.4.1548. [DOI] [PubMed] [Google Scholar]