Abstract
It was experimentally demonstrated that two strains of Arthrobacter 37, one growing at 25 C and the other at 5 C, could catalyze MnII oxidation at hydrostatic pressures well in excess of the pressure encountered by the parent culture in its original habitat in the ocean (80 atm). The strain grown at 5 C showed an increase in temperature optimum for manganese oxidation with increase in pressure. It was like-wise experimentally shown that induced Bacillus 29 without added ferricyanide and uninduced Bacillus 29 with added ferricyanide could catalyze MnO2 reduction at hydrostatic pressures in excess of the pressure encountered by this organism in its original habitat (187 atm). The uninduced Bacillus 29, in the presence of ferricyanide, was active over a wider range of pressures (1 to 1,000 atm) than the induced Bacillus 29 in the absence of ferricyanide (1 to 467 atm). At corresponding pressures, the uninduced culture was also considerably more active than the induced culture. Special techniques were developed for measuring MnII-oxidizing and MnO2-reducing activity under pressure.
Full text
PDF


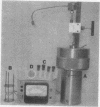
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ehrlich H. L. Bacteriology of Manganese Nodules: I. Bacterial Action on Manganese in Nodule Enrichments. Appl Microbiol. 1963 Jan;11(1):15–19. doi: 10.1128/am.11.1.15-19.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. L. Bacteriology of manganese nodules. II. Manganese oxidation by cell-free extract from a manganese nodule bacterium. Appl Microbiol. 1968 Feb;16(2):197–202. doi: 10.1128/am.16.2.197-202.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM J. W. Metabolically induced precipitation of trace elements from sea water. Science. 1959 May 22;129(3360):1428–1429. doi: 10.1126/science.129.3360.1428. [DOI] [PubMed] [Google Scholar]
- HAIGHT R. D., M ORITA R. Y. Interaction between the parameters of hydrostatic pressure and temperature on aspartase of Escherichia coli. J Bacteriol. 1962 Jan;83:112–120. doi: 10.1128/jb.83.1.112-120.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau J. V. Protein and nucleic acid synthesis in Escherichia coli: pressure and temperature effects. Science. 1966 Sep 9;153(3741):1273–1274. doi: 10.1126/science.153.3741.1273. [DOI] [PubMed] [Google Scholar]
- MORITA R. Y., HAIGHT R. D. Malic dehydrogenase activity at 101 C under hydrostatic pressure. J Bacteriol. 1962 Jun;83:1341–1346. doi: 10.1128/jb.83.6.1341-1346.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Ehrlich H. L. Bacteriology of manganese nodules. IV. Induction of an MnO2-reductase system in a marine bacillus. Appl Microbiol. 1970 Jun;19(6):966–972. doi: 10.1128/am.19.6.966-972.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Ehrlich H. L. Bacteriology of manganese nodules: III. Reduction of MnO(2) by two strains of nodule bacteria. Appl Microbiol. 1968 May;16(5):695–702. doi: 10.1128/am.16.5.695-702.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOBELL C. E., OPPENHEIMER C. H. Some effects of hydrostatic pressure on the multiplication and morphology of marine bacteria. J Bacteriol. 1950 Dec;60(6):771–781. doi: 10.1128/jb.60.6.771-781.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]